A FAIR WORKPLACE FOR ALL
The Florey forges ahead

A highlight of the communications group was the successful Women in Neuroscience “Wikibomb” or Wikipedia edit-a-thon, led by Dr Michele Veldsman and Dr Katherine Jackman, pictured left. In partnership with Wikimedia Australia, women and men from the Florey and beyond came together to create Wikipedia pages to profile the achievements of prominent Australian women who have worked or are currently working in neuroscience. The aim? To overcome the poor representation of this group. On the day, the 20 participants created 20 new pages. Amongst these was one dedicated to the achievements of our own Professor Ingrid Scheffer who has won several awards for her ground-breaking achievements in the genetics of epilepsy, including the 2014 Prime Minister’s Prize for Science.
At the Florey we are committed to harnessing the full complement of talent within the organisation. We believe that supporting and promoting diversity will help accelerate scientific discovery and cures. The Equality in Science Committee (EqIS) collaborates to create conditions in which diverse people can both operate to their full potential and also work together cohesively. This committee has broad representation from professional and academic staff of the Florey to ensure relevant equity and staff development issues are canvassed. Our working groups tackled important issues across the institute and this year’s report celebrates many achievements
The parenting group continued to work on issues important to staff with young families. A key achievement in 2014 was the creation of two family-friendly workspaces (parenting rooms) at each campus of the Florey. This is in addition to supporting new policies to help increase flexible work practices such as family-friendly meeting times, flexible working hours and part-time positions. The familyfriendly workspaces will allow scientists with young children to bring them to work when necessary, meet with colleagues or conduct other business in an environment that is safe for children.
At the policy level we continue to work to gain more equitable representation of our female scientists on committees across the Institute. We recognise that diversity in committees can change conversations and that opportunities to participate in decision-making groups builds skills and confidence. We have also been working to increase transparency of committee appointments to ensure that everyone at the Florey gets a chance to contribute to the life and culture of the organisation.
The value of mentoring is often underappreciated by scientists, yet mentoring can create enormous opportunities for growth for both the mentee and the mentor. In 2014 we relaunched our mentoring program with a view to creating an even stronger culture of mentorship for Florey staff.
Another exciting development this year was our successful partnership with the Baker Foundation, supported by Kerry Kornhauser from Women in Rotary, to develop a new Women in Science Fellowship. Following an unusual selection process which considered both the quality and potential impact of the candidate’s science (judged relative to opportunity), and their ability to communicate their science to a lay audience, we selected Dr Despina Ganella as the preferred Florey candidate for this award. An application for the Fellowship was then made to the Baker Foundation Board and we were delighted to have the Fellowship approved in December. This three-year fellowship will support Despina as she works to find new treatments for young people suffering from anxiety disorders.
“To be the best at research and translation by providing an environment that allows more women to lead and excel.”
Parkville hub - collective vision
Further progress was made with Women in Science Parkville Precinct (WISPP) collaboration throughout 2014. The Florey acts as the backbone (support) organisation for the collaboration of some 4000 researchers in the Parkville hub. Following a number of meetings, stakeholder interviews and workshops, we created the collective vision for the group: “To be the best at research and translation by providing an environment that allows more women to lead and excel.” We launched the initiative in November, which now includes researchers from some the largest medical research institutes in Australia: The Florey, Walter and Eliza Hall Institute (WEHI), Peter Mac, Murdoch Children’s Research Institute and the Doherty Institute.
The work of the group will continue with the support of funds from the Trust Company Australia as we move to develop agreed ways of measuring institute performance and achievements. The challenge of supporting more women to excel in science and the work of our WiSPP initiative was highlighted across the ABC network in late 2014.
It has been an extremely successful year for the EqIS committee and we thank the efforts of our committee and our very active subgroup members who have helped to develop and implement policies and practices that encourage diversity at all levels of our Institute. We’d also like to thank supporters from outside the organisation for their donations to the Women in Science corpus. At the end of year function we were delighted to have a young male scientist say “your committee is really helping to change the culture of the Florey for the better.” That kind of feedback is deeply gratifying and motivating.
We will continue to find new ways of building equity for all into the fabric of our Institute.
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 34 35
Pictured: Dr Michele Veldsman and Dr Kath Jackman.

PROFESSOR ANDREW LAWRENCE
Behavioural Neuroscience
Professor Lawrence is the Co-Division Head of Behavioural Neuroscience, running the Addiction Neuroscience laboratory. His primary research interest is in the development of robust animal models of drug-seeking, drug-taking and drug-induced neural adaptation. In addition, his group uses these models to define new potential therapeutic targets for drug and alcohol abuse disorders. He has published over 180 original articles and reviews. Professor Lawrence is currently Senior Editor of The British Journal of Pharmacology and also sits on the editorial boards of Neurochemical Research, the Journal of Pharmacological Sciences & Addiction Biology. In 2009, Professor Lawrence was awarded the Australian Neuroscience Society medallion for services to the society. In his spare time, Andrew is a keen cyclist and a surf life guard.
DIVISION HEADS

Behavioural Neuroscience
Dr Amy Brodtmann is Co-Division Head of Behavioural Neuroscience, consultant neurologist at Austin Health and Director of the Eastern Cognitive Disorders clinic, Eastern Health, Box Hill Hospital. She is a past recipient of a National Health and Medical Research Council Australian Training Research Fellowship, and previous National Brain School Co-ordinator for the Australian and New Zealand Association of Neurologists. She sits on the editorial board of Neurology, the research board of Alzheimer’s Australia Victoria, and is the founding director of the Australian Frontotemporal Dementia Association. Her research focuses on imaging correlates of cognitive decline in stroke, the neural basis of neglect, and the diagnosis and management of focal onset dementias.

PROFESSOR GRAEME JACKSON
Epilepsy and Imaging
Professor Graeme Jackson is the Deputy Director of the Florey, a practising clinical neurologist specialising in epilepsy at the Austin Hospital, and a Professorial Fellow of the University of Melbourne. He is recognised as an expert and world authority in understanding brain function and structure using new MR technologies, particularly as they apply to understanding and treating epilepsy. The National Health and Medical Research Council of Australia (NHMRC) awarded him the Outstanding Achievement Award for research excellence.
DIVISION HEADS

Brain Development and Regeneration
Professor Tan is the Head of the Division of Brain Development and Regeneration, NH&MRC Senior Principal Research Fellow, and Adjunct Professor at the Melbourne Neuroscience at The University of Melbourne, and University of Queensland Brain Institute. He is interested in understanding how the brain is assembled during development, and what mechanisms protect brain cells from death following brain injury such as trauma and stroke. Professor Tan has published over 100 papers and was awarded the Amgen Australia Medical Research Award (1997). He is on the Editorial Boards of the Journal of Neuroscience (USA) and Experimental Neurology. Professor Tan is a keen swimmer and a member of the Brighton Icebergers. He recently gave a TEDX Brisbane talk on “Why women have different brains.”

PROFESSOR STEVEN PETROU
Epilepsy
Professor Petrou is the Co-Division Head of the Division of Epilepsy, and heads the Laboratory of Ion Channels and Human Disease, a multidisciplinary team of researchers with a focus on revealing fundamental mechanisms of disease genesis in the central nervous system. Current major areas of investigation centre on the development and characterisation of genetically engineered mouse models for the study of human familial epilepsy. He works closely with industry and has several patents for his discoveries. In addition, he is the Deputy Director of the Centre for Neural Engineering at University of Melbourne, serves on the editorial board of the Journal Neurobiology of Disease and the Basic Science Committee for the International League Against Epilepsy and is Editor of the Australian and New Zealand Society for Neuroscience.

Professor Alan Connelly is an NHMRC Principal Research Fellow. He is the Co-Division Head of the Imaging Division, and Head of the Advanced MRI Development group. He also heads the MRI imaging Facility at the Florey Austin (including two research-only 3T MRI scanners) and the Florey Parkville Small Animal Imaging Facility. Prof Connelly is a development MRI physicist whose work has encompassed a range of MR methods, with current focus primarily on diffusion and perfusion MRI and their application to the investigation of epilepsy, stroke, and cognitive function. His group is internationally recognised as leaders in the field of diffusion MRI fibre tractography, and has developed novel methods to characterise the complex white matter fibre connections in the brain. He has published widely in magnetic resonance, general scientific, and neuroscientific journals, and is a member of the editorial board of Epilepsia.

LAUREATE PROFESSOR COLIN MASTERS
Mental Health
Professor Colin Masters is the Head of the Division of Mental Health, a Fellow of the Royal College of Pathologists, the Royal Australian College of Pathologists and the Australian Academy of Science. Professor Masters is a world leader in Alzheimer’s disease research. His research helped to isolate and characterise elements of the primary pathway causing Alzheimer’s disease. This discovery has been important in defining the mechanisms that contribute to the nerve cell degeneration which is the main feature of Alzheimer’s disease. His findings are now the subject of intense world-wide research for diagnosis and drug discovery. Professor Masters has also won many national and international prizes and awards.
02 04 01 04
DR AMY BRODTMANN
PROFESSOR ALAN CONNELLY Imaging
PROFESSOR SEONG-SENG TAN
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 36 37

PROFESSOR TREVOR KILPATRICK
Multiple Sclerosis
Trevor Kilpatrick leads the MS Division at the Florey Institute of Neuroscience and Mental Health and is a neurologist and Head of the MS Unit at the Royal Melbourne Hospital, in addition to being a Professor of Neurology and Director of the Melbourne Neuroscience Institute at The University of Melbourne. His research interests include the neurobiology of multiple sclerosis, neural precursor cell biology and the study of genetic and environmental factors that contribute to MS as well as the translation of basic research discoveries to the clinic.
Professor Kilpatrick has been the recipient of the Sunderland Award (1994), AMRAD Postdoctoral Award (1995), inaugural Leonard Cox Award (2000), Bethlehem Griffiths Research Foundation Award for Medical Research (2004), the Australian Museum’s Jamie Callachor Eureka Prize for Medical Research (2008), the Stephen C. Reingold Research Award by the US MS National Multiple Sclerosis Society (2010) and in 2013 Professor Kilpatrick was awarded the Bethlehem Griffiths Research Foundation Medal for outstanding leadership in medical research.

PROFESSOR PHILIP BEART
Neurodegeneration
DIVISION HEADS

PROFESSOR ROSS BATHGATE
Neuropeptides
Professor Ross Bathgate is the Head of the Division of Neuropeptides, an NHMRC Senior Research Fellow (Level B) and an Honorary Professorial Fellow in the Department of Biochemistry and Molecular Biology at the University of Melbourne. His work focuses on understanding the interactions of peptide ligands with their G protein-coupled receptor (GPCR) targets for the development of peptidebased drugs and utilizing structure based drug design to develop novel therapeutics. One of the peptide targets his lab is investigating is the peptide hormone relaxin which has recently completed a successful Phase III clinical trial for the treatment of acute heart failure. He works closely with Novartis who are conducting the clinical trial on relaxin as well as a number of other pharmaceutical companies interested in the clinical potential of drugs targeting peptide GPCRs. He currently serves on the editorial boards of Molecular and Cellular Endocrinology, Frontiers in Molecular and Structural Endocrinology and Journal of Pharmacological Sciences.

PROFESSOR JULIE BERNHARDT Stroke
Professor Philip Beart (PhD ANU, DSc Melbourne) has been a NH&MRC Research Fellow for 30 years and is currently Professorial Fellow in the Florey and Adjunct Professor in Pharmacology, University of Melbourne. He worked at Cambridge and Harvard Universities, before holding positions at the Austin Hospital and Monash University. Professor Beart has published >200 papers, served on numerous editorial boards and has diverse involvements in learned societies. Awards include the Bethlehem Griffiths Medal (2010), the Lawrie Austin Lectureship of the Australian Neuroscience Society (2009) and in pharmacology the Michael Rand Medal (2009). He has trained numerous honours and post-graduate students. He has a long-term commitment to promoting medical research, particularly neuroscience, in the wider community, and has provided extensive service to the NH&MRC. Professor Beart is currently Chair of the Scientific Promotions Committee and past President of the International Society for Neurochemistry (2011-13).
Professor Julie Bernhardt is the Co-Division Head of the Division of Stroke and leads the AVERT Early Intervention Research Program, which includes a multidisciplinary team of researchers committed to the development and testing of new, rehabilitation interventions that can reduce the burden of stroke related disability. AVERT, the largest, international, acute stroke rehabilitation trial ever conducted, sits at the core of the program. Advancing understanding of how exercisebased interventions alter bone, muscle and brain function after stroke is another aim of the program. Julie’s clinical career has been devoted to working with people with stroke and other neurological diseases. As a strong proponent of evidence-based care, another key theme within her program is the synthesis and translation of evidence into practice. Julie sits on the Board of the National Stroke Foundation and on a number of national and international stroke advisory groups.

Associate Professor David Howells is the Co-Division Head of the Division of Stroke and began his career investigating the biochemical and genetic basis of dopamine and serotonin deficits in children. He went on to describe a new population of dopaminergic neurons, demonstrated that BDNF depletion can cause parkinsonism and that Parkinson’s disease patients are deficient in BDNF. His other research interest is in stroke: his studies of neuroprotection in stroke have led to improved modelling of stroke in animals, the development of new methods of imaging, and development of systematic review and analysis as tools for rigorously evaluating basic science literature. The latter have led three leading stroke journals to publish guidelines for Good Laboratory Practice.
DIVISION HEADS

PROFESSOR RICHARD MACDONELL Systems Neurophysiology

PROFESSOR ROBIN MCALLEN Systems Neurophysiology
Professor Robin McAllen is the Co-Division Head of the Division of Systems Neurophysiology and a NHMRC Principal Research Fellow. He trained in Physiology in London and Birmingham and in Medicine at Birmingham (UK) before moving to the Florey in 1988. He is a neurophysiologist with an interest in the central nervous regulation on cardiovascular and autonomic functions, and has published extensively of this topic. More recently he has collaborated with Florey colleagues in neuroimaging experiments that aim to translate lessons learned from animal studies to the human brain. He currently serves on the editorial board of the American Journal of Physiology, is a section editor for Clinical and Experimental Pharmacology and Physiology, and is a member of the Faculty of 1000.
Professor Richard Macdonell is Director of Neurology at Austin Health, a Co-Division Head of Systems Neurophysiology and an Honorary Professorial Fellow. He trained in Neurology and Clinical Neurophysiology at Austin Health, Massachusetts General and the London Hospitals and has been in charge of the Neurophysiology and Neuroimmunology services at Austin Health since 1991. His research interests include multiple sclerosis, peripheral nerve and muscle disorders and using transcranial magnetic stimulation to study the pathophysiology of epilepsy.
04 04 03 04
ASSOCIATE PROFESSOR DAVID W HOWELLS Stroke
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 38 39
BRAIN DEVELOPMENT AND REGENERATION
Leader: Professor Seong-Seng Tan
A QUICK SNAPSHOT
Our group is interested in the self-defence mechanisms that operate in the brain when something goes wrong. This may take the form of degenerative disease (Parkinson’s, Alzheimer’s) or cancer (brain tumours) due to gene mutations and ageing. As a result, mutant or toxic proteins accumulate in brain cells, causing them to degenerate or proliferate. We have been working with one system of self-defence called protein ubiquitination which allows harmful proteins in brain cells to be removed and in the process, halt or reverse the disease process. We are particularly interested in finding how to accelerate beneficial ubiquitination in neurones using the Nedd4/Ndfip1 proteins. Our studies so far demonstrate that these proteins can halt cell death following injury and stroke, and slow down the division of brain cancer cells.
RESEARCH HIGHLIGHT
We have made some progress in understanding how the natural brain protein Ndfip1 is capable of improving the survival of brain cells in stress environments which occur in traumatic brain injury and stroke. We have shown that artificially increasing the concentrations of Ndfip1 in brain cells can protect against metal poisoning. We found that Ndfip1 concentrations in human post-mortem brains is strongly correlated with neurones affected by Parkinson’s disease. In animal models, we have shown that Ndfip1 is increased in surviving neurones after stroke. To study the consequences of removing Ndfip1 from neurones, we have deleted the Ndfip1 gene by mouse knockout technology. These animals demonstrate a greater sensitivity to brain damage from stroke, compared to normal animals that still carry the Ndfip1 gene.
We have found that Ndfip1 protects brain cells from death by hijacking an anti-cancer brain protein called PTEN. This protein is normally utilized as a defensive mechanism against tumour formation in brain cells. We found that after brain injury, this mechanism is activated to bolster the longevity of brain cells. The implication of this discovery exposes the PTEN anti-cancer pathway for therapeutic manipulation. In a separate discovery, we found that the anti-cancer protein PTEN can be secreted by cells and be carried in micro vesicles for transport to other cells. The secreted PTEN can then be internalised by recipient cells for physiological benefit, for example, for slowing down the proliferation of cancerous cells. This discovery opens up new ways of administering anticancer PTEN for the control of brain tumours.
AN IDEA LIKELY TO CHANGE LIVES BY 2035
We are working on using natural body transport vesicles called exosomes. We have discovered a way to load useful proteins into exosomes to bolster the survival of neurones under stress following injury. These exosomes can also be loaded with anti-cancer proteins to treated brain cancer.
OUR LAB
Our mission is to translate discoveries from our laboratory to help patients with brain diseases. Our experiments make use of a variety of modern techniques to study how brain cells are able to resist death in a hostile environment. Our experiments are framed around hypotheses that may be answerable using novel tools developed by our team. These tools include creation of new mouse strains with inserted genes, molecular cloning of brain genes and manipulation of brain proteins. We have a democratic team structure, where every scientist including students and technicians all have an equal voice.
• Seong-Seng Tan • Joanne Britto • Jason Howitt • Ulrich Putz • Ley-Hian Low • Choo-Peng Gohr • “
SENIOR STAFF
We have found that Ndfip1 protects brain cells from death by hijacking an anti- cancer brain protein called PTEN. ”

Leader: Joanne Britto
In order for brain cells in the adult brain to function properly, it is important that they are first found in the correct locations. Failure of brain cells to migrate to their destinations is a frequent cause of many developmental disorders such as autism and schizophrenia. For many years, our group has performed experiments to understand how brain cells are able to correctly migrate to their final positions.
Research highlights for 2014
We recently focussed on a class of brain cells called interneurones. Although they are in the minority, this class of neurones controls much of our mental processing and sensory experience. Therefore studying the behaviour of interneurones holds the key to understanding a number of developmental brain disorders. Previously, we showed that interneurones are capable of migrating vast distances to reach their final positions in highly specific addresses. Over the last year, we have been tracking the migration of these neurones, using genetic mice engineered to paint brain cells with artificial colours. This technology allows us to follow the movements of different interneurone classes under a laser


microscope. The information from these studies allows us to pinpoint which types of interneurones are most at risk when something goes wrong. Our work has showed that different interneurones destined for different layers have separate rules for movement, dispelling previous notions that all interneurones behave alike.
 BRAIN DEVELOPMENT GROUP
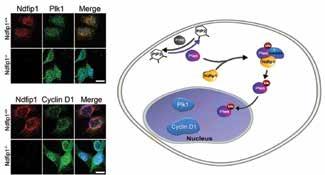
Binding of PTEN to Ndfip1 in the cell can be visualized by protein fluorescence –work by PhD student Yijia Li and published on the cover of Traffic.
The Brain Development team: Choo-Peng Goh, Ley-Hian Low, Anh Doan, Yijia Li, Jason Howitt, Seong-Seng Tan, Sophia Mah, Ulrich Putz, Yuh-Lit Chow, Michelle Tang, Ulrich Sterzenbach. Absent: Joanne Britto, Hui Xuan Ng, Ean Phing Lee.
Ndfip1 controls cell proliferation by driving PTEN into the cell nucleus. Work performed by Jason Howitt and published in Journal of Molecular Cell Biology
BRAIN DEVELOPMENT GROUP
Binding of PTEN to Ndfip1 in the cell can be visualized by protein fluorescence –work by PhD student Yijia Li and published on the cover of Traffic.
The Brain Development team: Choo-Peng Goh, Ley-Hian Low, Anh Doan, Yijia Li, Jason Howitt, Seong-Seng Tan, Sophia Mah, Ulrich Putz, Yuh-Lit Chow, Michelle Tang, Ulrich Sterzenbach. Absent: Joanne Britto, Hui Xuan Ng, Ean Phing Lee.
Ndfip1 controls cell proliferation by driving PTEN into the cell nucleus. Work performed by Jason Howitt and published in Journal of Molecular Cell Biology
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 40 41
Brain trauma directs the survival protein PTEN in to the cell nucleus to keep neurons alive. Work by Choo-Peng Goh (published in Experimental Neurology) shows that this process requires Ndfip1.
BRAIN SURVIVAL GROUP
Leader: Jason Howitt
When the brain is injured, such as in an accident or in stroke, the main area of the brain injury is not recoverable due to massive bleeding and swelling. However, what is not commonly known is that dying brain cells (called neurones) can transmit death signals from the injured area into surrounding healthy tissues, this ripple effect causes death of neighbouring brain cells in large numbers. Right now, there is no drug treatment to reduce the rate of brain death after trauma. Therefore medical strategies to prevent neurones from succumbing to trauma-induced death is an unmet need.
Research highlights for 2014
Our brain survival team has identified a survival protein in human brain cells called Ndfip1. This survival protein is present in human brains following road trauma, and Ndfipi1 is massively increased in surviving cells, indicating its role as a protective agent.
Ndfip1 behaves like a bar-coding tool by putting a mark on harmful proteins that are produced during injury. These bar-coded harmful proteins are then recognised by the waste disposal system called ubiquitination in the neurone for removal.
This protein is also able to wake up a survival mechanism in brain cells using cancer pathways in neurones without causing tumours, taking advantage of the survival mechanisms that cancer cells are famous for.
We have also discovered that Ndfip1 is an important defender against metal accumulation in brain cells. This is important for degenerative brain diseases such as Parkinson’s and Alzheimer’s disease. We showed that post-mortem brains from Parkinson’s disease contain deficient levels of Ndfip1. This deficiency is associated with increased iron accumulation in diseased brain cells. This work opens up the manipulation of Ndfip1 as a way of controlling onset and progress of Parkinson’s disease.
BRAIN COMMUNICATION GROUP
Leader: Ulrich Putz
This group is interested in studying ways of using natural vesicles called exosomes to behave as cargo systems. This comes on top of our discovery that the anti-cancer protein PTEN is exported by cells in exosomes, and the anti-cancer protein can be transferred into other cells with anti-cancer consequences. This group is excited to use this technology to try and target anti-cancer protein PTEN into brain tumour cells to arrest the disease.
Research highlights for 2014
Over the last year, we have identified a peptide that can be used to coat exosomes so that they specifically recognise and target brain tumour cells. In cell culture experiments, we showed that brain tumour cells with mutations in the epidermal growth factor receptor are targeted by our engineered exosomes.
Realising the need for accurate and effective delivery of exosomes, we have invested our resources to understand how to more effectively transfer exosomes into recipient cells. We have discovered that centrifugation using special conditions and special media can increase the uptake of exosomes by recipient cells.
EDITORIAL POSITIONS
SEONG-SENG TAN
ҩ Experimental Neurology (USA)
ҩ Journal of Anatomy (UK)
ҩ Journal of Cell Biology (Korea)
ҩ Frontiers in Neuroscience
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES 2014
INVITED SPEAKER
ҩ Asian-Pacific Society Neurochemistry, Kaohsiung, Taiwan. August 2014. “Role of Ndfip1 during brain development.”
ҩ Brain Disorders Institute, Beijing Capital Medical University, Beijing, China. August 2014. “Potential use of PTEN for therapy in gliomas.”
ҩ Japan Neuroscience Society Annual Meeting, Yokohama. September 2014. “How PTEN can save neurones from death following stroke and brain injury.”
ҩ Invited Speaker, TEDx Brisbane, October 2014. “Why women have different brains.”

“The process of discovery is the most exciting, intellectually stimulating and challenging of activities. The painstaking and arduous work culminates in the pleasure of finding something new that no-one has known or understood before. When this is applied to medical research then there is the added reward that it may improve the lives and reduce the suffering of people with illness. I am determined to discover better treatments for schizophrenia and related disorders and develop markers to better diagnose and manage people with these illnesses.
”
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 42 43
Associate Professor Suresh Sundram, head, Molecular psychopharmacology laboratory and Northern Psychiatry Research Centre.
I
CHASING A CURE FOR MOTOR NEURONE DISEASE
Dr Bradley Turner
t’s no secret that funding for medical research is critically short. So it was a ‘dream come true’ when the Florey’s Dr Brad Turner spoke to a community group and unwittingly attracted the attention of a ‘scout’ who would radically change his future – and hopefully the lives of people living with motor neurone disease
Dr Bradley Turner is renowned for his community outreach activities and his relentless quest to cure motor neurone disease (MND). As the head of the Florey’s MND group, Brad was recently speaking to an Inner Wheel Club in Pakenham 70km south east of Melbourne.
Brad enthusiastically described his work to the group, outlining a project he believed promised great hope for a new treatment. This alerted Rotary Pakenham and a short time later, a phone call arrived from a trustee of a philanthropic foundation, recommending he submit a research proposal as quickly as possible.
A few days later, Brad was told he would receive $3 million over five years, thanks to the Stafford Fox Medical Research Foundation, a fund that does not accept requests but, rather, seeks worthy projects and invites applications.
“This could change the course of MND. Until now, I haven’t had the funding to achieve it. It is truly, utterly amazing,” Brad says. It is a relentless disease, with nerves controlling movement (motor neurones) degenerating and rapidly wasting muscles. It strips away the independence of people living with it, who lose their ability to walk, feed themselves, talk and breathe. The average lifespan from diagnosis is 27 months.
So the need to find effective treatment is urgent, Brad says.
The grant will allow Brad and his team to fast-track some significant findings they made last year, using a form of gene therapy to substantially increase the lifespan of MND mice.
The research builds on previous work Brad has done at Oxford University, looking at a gene involved in a childhood form of MND called Spinal Muscular Atrophy (SMA).
Children born with the condition are missing a gene and become weak at six months and die within two years. Significantly, Brad found the gene was also missing in MND mice and in tissue from patients.
He experimented by putting the gene back in the MND mice – with dramatic results: the lifespan of the mice increased by two months. MND mice usually only live for four months. The process of replacing the gene also measurably saved motor neurones.
“The sad thing about MND is that by the time people are diagnosed with it, 50 per cent of motor neurones are gone so that they are already at crisis point,” he says.
“We want to prolong a person’s lifespan and save their motor neurones – they are the two key objectives for an effective treatment.”
“Conventionally, a clinical trial can take 10 to 15 years to happen but in the case of MND it can be sooner due to accelerated approval and fast track status of promising drug candidates. Within five years we could potentially have something.”
Dr Bradley Turner
For the next phase of research, Brad and his team will collaborate with Flinders University scientists using a specially devised tool – a gene therapy – to deliver the SMA gene to motor neurones in the brains of mice.
He says that once the team has demonstrated that the tool brings about the same effect in the mice – a two-month increase in survival – they will adapt it to a clinical trial in humans.
Brad says the MND patients he talks to are heartened by the findings and particularly by a graph showing the prolonged lifespan of the mice.
“When people are diagnosed with MND they know precisely what it is, they know the course it will take and that they often have no or little hope. Part of their hope comes from the knowledge that people in lab coats are beavering away working on their disease, passionately, and as a fulltime commitment – and that actually lifts their spirits.”
Brad will continue to share his work with the public – going out to speak to interest groups and hosting an annual “Ask the Expert” day when patients and their families hear talks and see demonstrations of lab techniques.
“They love it!” he says, “and they ask some impressive questions.”
The 36-year-old has researched MND for more than a decade and has succumbed to the Ice Bucket Challenge four times.
“When first read about MND, I thought ‘it’s tragic and terminal’. Then I thought ‘I need to help solve this.’ tell people that I have a lifelong commitment to work on this disease.”
“The Florey’s a great place to work. It has prestige. It’s unique. I don’t know of many places that are working on so many neurological disorders under the one roof. That means great collaboration.”
“At the start of my PhD, people would ask ‘is there going to be a cure’ and I’d say I wasn’t sure. Now I tell people that it’s not a matter of ‘if’ but ‘when’. An effective treatment or cure is on the horizon.”
Brad Turner has a strong ally. Ian Davis, a medico living with MND, is driving a highprofile campaign to raise awareness and funds for research into a cure for the disease.
Ian was in his early thirties, engaged to be married to his now wife Mel, and researching childhood leukaemia as a lab researcher when he was diagnosed with MND – but only after recognising the symptoms himself. Since then Ian has made the most of his limited time. He has been skydiving, has been on stage in a wheelchair with Pearl Jam (his favourite band), and completed an epic tandem bike ride from Brisbane to Sydney, raising money for MND research.
Last year he and Mel became parents to baby Archie. Ian has inspired public figures including cricketer Shane Watson, Masterchef winner Julie Goodwin, and Australian Open champion Serena Williams to speak out about the cause and to donate to it. He says medical research into MND is “absolutely crucial” because there is so little that can be done for people living with the disease.
TO DONATE, PLEASE VISIT FLOREY.EDU.AU

THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 44 45
COGNITIVE NEUROSCIENCE
Leaders: Professor Andrew Lawrence and Dr Amy Brodtmann
A QUICK SNAPSHOT
The Division of Behavioural Neuroscience focuses on the use and development of animal models that reflect aspects of human disorders such as addiction, anxiety, depression, schizophrenia, autism and neurodegenerative conditions such as Huntington’s disease. The Cognitive Neuroscience group additionally studies cognitive disorders caused by diseases such as stroke (cerebrovascular disease), Alzheimer’s disease and other dementias from a clinical perspective.
RESEARCH HIGHLIGHT
There have been a number of highlights within the Division during 2014. A long-term collaboration between Professor Andrew Lawrence and Professor Alon Chen (Max Planck Institute, Munich) came to fruition with the finding that CRF1 receptors within the ventral tegmental area are critically implicated in both cue- and stress-induced relapse to cocaine-seeking. This study was performed predominantly by a PhD student, Nicola Chen, and published in the prestigious Journal of Neuroscience.
SENIOR STAFF
• Professor Andrew Lawrence
• Associate Professor
Anthony Hannan
• Associate Professor John Drago
• Dr Amy Brodtmann
• Associate Professor David Darby
Dr Jhodie Duncan
• Dr Jess Nithianantharajah
•
• Dr Jee Hyun Kim
“
We showed that CRF1 receptors within the ventral tegmental area are critically implicated in both cue- and stress-induced relapse to cocaine-seeking.
RESEARCH OVERVIEW
2014 saw the Division strengthened by the addition of new laboratory heads, Dr Jhodie Duncan and Dr Jess Nithianantharajah. Moreover, 2014 was a highly successful year for the Division in terms of competitive grants. Dr Nithianantharajah was awarded an ARC Future Fellowship and Dr Jee Hyun Kim was awarded a Career Development Fellowship from the NHMRC; Professors Andrew Lawrence and Tony Hannan were both awarded two NHMRC project grants each; Dr Nithianantharajah received an NHMRC New Investigator project grant and Dr Kim received an ARC Discovery project. Dr Heather Madsen returned from her postdoctoral training in France to join the Developmental Psychobiology lab. Dr Despina Ganella won a highly competitive Baker Fellowship. Dr Kim won a Victoria Young Tall Poppy Award. In 2014 Professor Lawrence was appointed President of the Asian-Pacific Society for Neurochemistry (APSN).
AN IDEA LIKELY TO CHANGE LIVES BY 2035
By 2035, dementia will be a rare and treatable disease. Patients admitted with stroke will receive dementia risk stratification and be treated with therapies that will recover all functions, including cognition. People at risk of Alzheimer’s disease will be identified via online testing or following a sentinel risk event, and treated prior to the development of brain atrophy.
ADDICTION NEUROSCIENCE GROUP
Leader: Andrew Lawrence
The Addiction Neuroscience Group studies how alcohol and other drugs change the brain’s chemistry, structure and function. During 2014 we made a number of important findings, many of which are still being actively pursued. As part of her PhD, Hanna Kastman continues to unravel the circuitry implicated in stress-induced relapse to alcohol-seeking. In addition, she has also demonstrated for the first time that the neuropeptide, relaxin-3, interacts with both orexin and CRF to regulate relapse-like behaviour. This project has been expanded with the recruitment of two new PhD students (Sarah Sulaiman Ch’ng and Leigh Walker) plus an overseas placement student (Jan Koeleman). Together, these students are interrogating how and where in the brain these peptide systems interact to regulate reward-seeking. In parallel to this, Nicola Chen (co-supervised by Professor Andrew Lawrence & Dr Jee Hyun Kim) showed that CRF1 receptors within the ventral tegmental area are critically implicated in both cue- and stress-induced relapse to cocaine-seeking. Her studies are now concentrating on aversive learning paradigms. In 2014 Professor Andrew Lawrence was awarded an NHMRC project grant to continue these important studies.
We also continued with our longstanding project on mGlu5 receptors. The process of behavioural extinction is a form of inhibitory learning that can help to protect against relapse. Dr Christina Perry and Ms Felicia Reid are currently assessing the role of mGlu5 signalling in the extinction of drug cues and contexts. This is potentially translatable by using mGlu5 positive modulators to enhance the rehabilitation of addicts undergoing behavioural therapy.
Another project involves examining the link between salt appetite and opiate addiction. In collaboration with Professors Derek Denton and Michael McKinley, we have shown that opioid receptor antagonists that are used clinically to treat alcohol and heroin addicts reduce salt appetite. More intriguingly opiate dependent mice show an exaggerated salt appetite compared to normal mice. We are now in the process of establishing where in the brain this regulation occurs. Professor Andrew Lawrence received an NHMRC project grant to pursue these studies.
COGNITIVE NEUROSCIENCE GROUP
Leader: Amy Brodtmann
We are a clinically-based research group examining the cognitive trajectories of patients. These include patients with stroke and dementia, using advanced imaging techniques in concert with novel testing paradigms developed by our team members. This also includes charting cognition in community dwelling healthy participants, via Associate Professor Darby’s online testing, which now includes over 1500 people.
Cognitive disorders can develop from a range of brain diseases, including stroke, Alzheimer’s disease, and other less well known dementias such as frontotemporal dementia. In 2013, there were 44.4 million people living with dementia; this number will increase to 135 million by 2050. Dementia care is estimated to cost $698 billion per year: one per cent of global GDP.
2014 was a productive year for the Cognitive Neuroscience group. The international significance of the Cognition and Neocortical Volume After Stroke (CANVAS) study continued to grow, with conference proceedings and the strengthening of important collaborations with groups in Germany and the United States. The CANVAS project is an NHMRC study where we aim to establish whether stroke patients have increased atrophy (reductions in brain volume) in the first three years post-stroke compared to control subjects, perhaps answering whether stroke can be associated with neurodegenerative mechanisms. This NHMRC-funded project continues to be assisted by help from the Sidney and Fiona Myer Family Foundation.
Funding from the Mason Foundation was utilised to develop and compare imaging techniques in patients with dementia, and with half
the patients recruited for this Florey-Royal Melbourne project, and the final patients booked for the first three months of 2015. Support from the Collie Trust allowed us to recruit a new post-doctoral research fellow in 2014, Dr Michele Veldsman, who been enormously productive, publishing an influential review paper in Human Brain Mapping on the challenges of functional imaging in stroke populations.
After Associate Professor Darby launched a study, more than 1500 people logged-in monthly for online cognitive testing. This study was covered by many of the major newspapers nationally, and was a feature on national television. He launched a similar project in Brazil, and is working with a long-term collaborator in Oxford, Professor Kia Nobre, with a large community of volunteers based in the UK.
Dr Brodtmann continued her appointment with the Wicking Strategic Panel, a position which, along with her appointments to the Australian Frontotemporal Dementia Association and Alzheimer’s Australia – Victoria Dementia Research Grants committee and panel will allow her to lobby and advocate for people with cognitive disorders. She was involved with organisation of a very successful Wicking Symposium. She developed further productive collaborations with Monash University’s Professor John McNeil and the Florey’s Lachlan Thompson, with two new successful NHMRC project grants in 2014 with these colleagues.
NEURAL PLASTICITY GROUP
Leader: Anthony Hannan
Many neurological and psychiatric disorders, including schizophrenia and autism spectrum disorders (ASD), involve abnormal development of the brain. We are interested in the mechanisms whereby specific genes regulate maturation of the brain and are dynamically regulated by interaction with the environment in conditions like ASD and schizophrenia.
Autism spectrum disorders affect approximately one per cent of children. Dr Emma Burrows has discovered abnormalities of social interaction and communication in a mouse model of ASD carrying a human gene mutation. Ongoing investigations focus on identifying key molecules and cellular changes involved in ASD and testing new therapeutic approaches in this model. With collaborators at the University of Melbourne, we have new evidence that both the central and peripheral nervous system is disrupted in these mice, contributing to a broad array of ASD symptoms.
Schizophrenia is another neurodevelopmental disorder involving a complex combination of genetic and environmental factors which disrupt normal maturation and function of the brain. Using a mouse model of schizophrenia, Dr Emma Burrows has shown that enhanced mental and physical activity can ameliorate cognitive deficits and other behavioural symptoms and benefit specific areas of the brain. Identifying molecules modulated by environmental stimulation has paved the way for future development of new therapeutic approaches. Furthermore, Dr Burrows and graduate student Faith Lamont have been the first researchers in Australia to generate data using our new automated touchscreen tests (which are directly translatable to human neuropsychological test batteries). They have identified specific aspects of cognitive dysfunction and are testing a therapeutic intervention in this model of schizophrenia. Huntington’s disease (HD) is an inherited single-gene abnormality that involves a triad of psychiatric symptoms (e.g. depression), cognitive deficits (culminating in dementia) and a movement disorder. Dr Terence Pang, along with PhD student Xin Du, discovered an abnormal stress response associated with depression-like behaviours (which respond to antidepressants) in the mouse model of HD and identified key molecules involved in the disease, the response to environmental stimuli and the increased vulnerability of females. The effects of a major environmental factor, stress, on HD has been explored by Dr Thibault Renoir and a PhD student (Christina Mo) who demonstrated for the first time that stress can accelerate the onset of HD, in particular the cognitive deficits (modelling dementia). These findings will have
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 46 47
”
implications not only for HD, but for depression and dementia in the wider community. Further study of gene-environment interactions and experience-dependent changes in the nervous system may lead to new therapeutic approaches for HD and other brain disorders.
Another PhD student, Dean Wright, with Dr Renoir and Melbourne collaborators, has discovered a new treatment for HD, and shown that it works via mitochondria, the cellular ‘batteries’ that provide energy. Furthermore, their findings have implications not only for HD, but depression in the wider community.
These findings contributed to an NHMRC Project Grant won by Professor Hannan, Dr Nithianantharajah, Dr Renoir, in collaboration with the University of Melbourne. Furthermore, another new NHMRC Project Grant exploring the effects of paternal lifestyle on the mental health of offspring was won by Professor Hannan, Dr Pang and a collaborator from University of Queensland.
DEVELOPMENTAL PSYCHOBIOLOGY GROUP
Leader: Jee Hyun Kim
Our vision is to understand the role of memory and forgetting across development in mental disorders, namely anxiety and substance abuse. We believe the key to finding effective treatments lies in how we remember and forget emotionally significant events formed at various stages of our development. A number of important discoveries have been made in 2014. Dr Despina Ganella showed that disruption of the communication between brain regions during inhibitory learning can permanently reduce the strength of a fear memory during adulthood. In collaboration with Melbourne Neuropsychiatry Center, she also showed in humans that childhood maltreatment was significantly associated with accelerated rate of growth in pituitary gland volume across adolescence in females but not males.
Dr Despina Ganella and Dr Heather Madsen, with PhD students Isabel Zbkuvic and Sophia Lukinga demonstrated that the popular bipolar medication, Aripiprazole, can lead to changes in dopamine signalling in the prefrontal cortex during inhibitory learning in adolescent animals. This finding explains why Aripiprazole may enhance inhibitory learning and reduce relapse in anxiety and drug seeking. Dr Heather Madsen is using transgenic mice that show green florescence in dopamine receptor 1 (D1) or D2, and PhD student Isabel Zbkuvic is measuring changes in D1/D2 mRNA profile across adolescence to understand the mechanisms behind adolescent vulnerability to anxiety and addiction.
Lastly, PhD student Nicola Chen discovered that reduction of corticotropin releasing factor 1 receptor in the ventral tegmental area leads to a stronger fear memory formation in mice, which suggests reductions in the stress signalling during learning may be beneficial.
INHALANT ABUSE LABORATORY
Dr. Jhodie Duncan
The abuse of inhaled chemical vapours to produce self-intoxication is a significant concern, especially among adolescent and Indigenous populations. In 2014 Dr Jhodie Duncan and Mr Alec Dick published work demonstrating that exposure to inhalants during adolescence results in specific impairments in aspects of instrumental learning, without altering motor function and spatial learning. Our data suggest that exposure to inhalants affects corticostriatal processes specifically and does not involve global toxicity to white matter pathways in the brain as was originally thought.
Using this model we have also shown that exposure to inhalants during adolescence results in long-term metabolic dysfunction. This includes altering dietary preference and glycemic control. This novel finding suggests that adolescent inhalant abuse may increase the risk of adult onset disorders such as diabetes and has significant implications for our understanding of the long-term consequences, especially in Indigenous populations where there is a high degree
of overlap between inhalant abuse and nutrition related illness. The next step in our studies is to determine the underlying mechanisms, including both changes to central or peripheral mediated processes that drive the adverse outcomes observed in human abusers.
SYNAPSE BIOLOGY AND COGNITION GROUP
Jess Nithianantharajah
2014 saw the addition of the Synapse Biology and Cognition laboratory to the Division of Behavioural Neuroscience led by Dr. Jess Nithianantharajah, who was recruited back to Melbourne after six years in the UK (Cambridge and Edinburgh). Dr. Nithianantharajah was awarded a highly competitive Australian Research Council Future Fellowship in 2014, the only application out of 150 successful proposals awarded under the theme of Neurosciences.
Sensory information from the environment is ultimately processed at the level of synapses, the connection between neurons that form the most fundamental information-processing units in the nervous system. A central research focus of the lab is to understand the role of synaptic genes in cognition and disease.
Vertebrate synapses contain a large yet intricately organised signalling complex of proteins encompassing neurotransmitter receptors, scaffold proteins and cell adhesion proteins. In recent years, human genetic studies have increasingly highlighted that disruption of over 200 genes that encode postsynaptic proteins result in over 130 brain diseases. While it is accepted that postsynaptic proteins are fundamental for synaptic function, plasticity and thus behaviour, very little is actually known about the impact of postsynaptic gene mutations in regulating complex cognition and higher order processing.
Moreover, modelling the complex cognitive processes that are routinely assessed in the clinical setting has been challenging in animal models. Towards bridging the gap between mouse and human cognitive testing, the lab is employing novel behavioural technologies such as the recently developed rodent touchscreens as an innovative tool for dissecting higher cognitive functions in rodents. Dr. Nithianantharajah, along with PhD student Rebecca Norris, is beginning to investigate how a family of cell adhesion proteins (neuroligins) are involved in complex cognitive behaviours. This is of particular interest as human mutations in neuroligins have been reported in autism spectrum disorders and schizophrenia. The group is also developing novel touchscreen tests for mice to be able to further model and probe distinct cognitive capacities that are clinically relevant.
ANDREW LAWRENCE
EDITORIAL POSITIONS
ҩ The British Journal of Pharmacology, Senior Editor
ҩ Neurochemical Research, Associate Editor
ҩ The Journal of Pharmacological Sciences, Associate Editor
ҩ Addiction Biology, Editorial board member
ҩ The Open Neuropsychopharmacology Journal, Editorial board member
ҩ ISRN Addiction, Editorial board member
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES 2014
INVITED SPEAKER
ҩ Japanese Pharmacological Society Annual Conference. Invited symposium speaker “Relaxin-3 Regulates Stress-Induced AlcoholSeeking in Rats”. Sendai, Japan.
ҩ 3rd Asia-Pacific Society for Alcohol and Addiction Research Conference. Plenary Lecture “Ascending Relaxin-3 systems regulate stress-induced reinstatement of alcohol-seeking”. Shanghai, China
ҩ 12th Conference of Asia-Pacific Society for Neurochemistry (APSN). Symposium speaker “mGlu5 & extinction of drug-seeking”, Kaohsiung, Taiwan.
ҩ Joint Scientific Conference of The Hong Kong Society of Neurosciences and HK Biophysical Society. Plenary lecture “Neuropeptides & Reward-Seeking”.
ҩ 8th International Meeting on Metabotropic Glutamate Receptors, Sicily, Italy – invited symposium speaker “mGlu5 Receptors and Extinction of Drug-Seeking”.
AMY BRODTMANN
EDITORIAL POSITIONS
ҩ Neurology, Editorial Board Member, vascular division
ҩ International Journal of Stroke, Co-Section Editor
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES 2014
INVITED SPEAKER
ҩ Hypertension and Dementia, World Congress of Neurology, Melbourne.
TERENCE PANG
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES 2014
INVITED SPEAKER
ҩ Invited Symposium Speaker, ‘Behavioral manipulation and alcohol misuse’, 37th Annual Research Society on Alcoholism (RSA) Scientific Meeting and 17th Congress of the International Society for Biological Research on Alcoholism (ISBRA), Bellevue, Washington, USA.
THIBAULT RENOIR
EDITORIAL POSITIONS
ҩ Frontiers in Neuropharmacology, Associate Editor
ҩ 16th International Society of Addiction Medicine Annual Meeting, Yokohama, Japan - invited symposium speaker “Neuropeptides as therapeutic targets for drug-seeking”.
ҩ 2nd International Anniversary KBRI Symposium, Korea Brain Research Institute Daegu, Korea – invited speaker.
ҩ ANS Annual Conference in Adelaide, invited symposium speaker “The many faces of the orexin system”
ҩ ASCEPT-MPGPCR Meeting, invited speaker “Peptides & RewardSeeking”, Melbourne.
OTHER ACADEMIC INVITATIONS
ҩ Cass Memorial Lecture, Dundee University Medical Research Institute.
ҩ Faculty member, APSN Advanced School in neurochemistry, Center for Translational Research in Biomedical Research, Chang Gung Memorial Hospital, Kaohsiung, Taiwan.
ҩ President, Asian-Pacific Society for Neurochemistry (APSN).
ҩ Member scientific programming committee International Society for Neurochemistry (ISN) conference in Cairns, Australia.
ҩ Ambassador for the 2016 CINP World Congress of Neuropsychopharmacology, Seoul, Korea.
ҩ Neuropsychiatry Neurobehavioural Meeting, Frontotemporal Dementia Update, Melbourne.
ҩ Public Lecture Florey: Stroke and Dementia.
ҩ FTD support group: Update from the International FTD Meeting, Vancouver.
OTHER ACADEMIC INVITATIONS
ҩ Neurology Trainee lectures: Frontal lobe syndromes; Memory and H.M.
DESPINA GANELLA
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES 2014
INVITED SPEAKER
ҩ “The partial agonist of the dopamine receptor 2, Aripiprazole, facilitates extinction in adolescent rats.” The 12th meeting of APSN. Kaohsiung, Taiwan.
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES 2014
INVITED SPEAKER
ҩ Invited Symposium Speaker, Society for Mental Health Research (SMHR; formerly known as ASPR), Adelaide.
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 48 49
ANTHONY HANNAN
EDITORIAL POSITIONS
ҩ Journal of Huntington’s Disease, Associate Editor
ҩ European Journal of Neuroscience, Scientific Review Associate
ҩ CNS & Neurological Disorders - Drug Targets, Editorial board member
ҩ Neural Plasticity, Editorial board member
ҩ Neuroscience Letters, Associate Editor
ҩ Frontiers in Neuropharmacology, Review Editor
ҩ Neuroepidemiology, Editorial board member
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES 2014
INVITED SPEAKER
ҩ Integrative Center for Learning and Memory, Brain Research Institute, UCLA, USA.
ҩ Invited Symposium Introduction and Discussant, ‘Behavioral manipulation and alcohol misuse’, 37th Annual Research Society on Alcoholism (RSA) Scientific Meeting and 17th Congress of the International Society for Biological Research on Alcoholism (ISBRA), Bellevue, Washington, USA.
ҩ Invited Lecturer, European Molecular Biology (EMBL) PhD Course, ANU, Canberra.
ҩ Invited Speaker, 12th International Conference on Cognitive Neuroscience (ICON) satellite meeting, ‘Mission MMN Workshop’, Newcastle.
ҩ Symposium Speaker, 12th International Conference on Cognitive Neuroscience (ICON), Brisbane.
JEE HYUN KIM
EDITORIAL POSITIONS
ҩ Frontiers in Behavioral Neuroscience, Review Editor
ҩ International Scholarly Research Network: Neuroscience, Associate Editor
ҩ Neurotransmitter, Associate Editor
ҩ Pharmacology Research & Perspectives, Associate Editor
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES 2014
INVITED SPEAKER
ҩ “Adolescent sensitivity to cocaine-associated cues: a dopamine story”. 2014 Annual Pavlovian Society Meeting. Seattle, USA.
ҩ “Extinction of fear and drug-seeking during adolescence.” 37th Annual meeting of the Japan Neuroscience Society (JNS). Yokohama, Japan
ҩ “Extinction of fear and drug-seeking during adolescence.” The 12th meeting of the Asian-Pacific Society for Neurochemistry (APSN). Kaohsiung, Taiwan.
JHODIE DUNCAN
EDITORIAL POSITIONS
ҩ Journal of Reward Deficiency Syndrome, Associate Editor
ҩ Brain Pathology, Editorial board member
ҩ Neurochemical Research, Editorial board member
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES 2014
INVITED SPEAKER
ҩ “Glue sniffing sucks! The long-term consequences of inhalant abuse during adolescence on the brain and body” Department of Neuroscience Research, Strathclyde University, Glasgow, Scotland, UK.
ҩ Invited Keynote Speaker, ‘The neurobiology of environmental enrichment and experience-dependent plasticity in animal models of mental illness’, Society for Mental Health Research (SMHR; formerly known as ASPR), Adelaide.
ҩ Invited Speaker, SMHR Symposium, ‘Lifestyle approaches to mental health: The role of physical activity’, Adelaide.
ҩ Invited Keynote Speaker, National Huntington’s Conference, UWA, Perth.
OTHER ACADEMIC INVITATIONS
ҩ Member, National Committee for Brain and Mind, Australian Academy of Science.
ҩ State Representative for Victoria and Tasmania, National Association of Research Fellows (NARF).
ҩ Scientific Committee Member, 12th International Conference on Cognitive Neuroscience (ICON).
ҩ Scientific Committee Member, 5th International Congress on Neurology and Epidemiology (ICNE).
ҩ Monash Clinical Imaging Neuroscience, Monash Biomedical Imaging, Monash University – Invited lecture.
ҩ Leaders in Science Seminar, Garvan Institute of Medical Research, Sydney.
ҩ Stroke Division Seminar Series, Melbourne Brain Centre, Austin Campus.
OTHER ACADEMIC INVITATIONS
ҩ Department of Psychology, Korea University, Seoul, South Korea –Invited lecture.
ҩ RIKEN Brain Science Institute, Tokyo, Japan – Invited lecture.
ҩ School of Psychology, Australian Catholic University, Melbourne, Australia – Invited lecture.
ҩ School of Medical Sciences, The University of Sydney, Sydney, Australia – Invited lecture.
ҩ Department of Cell Biology and Neuroscience, Sapienza University of Rome, Italy – Invited lecture.
ҩ Academy of Sciences of the Czech Republic, Prague, Czech Repulic. –Invited lecture.
ҩ 12th meeting of Asian-Pacific Society for Neurochemistry, Kaohsiung, Taiwan - Symposium Chair.
ҩ International Society for Developmental Psychobiology – Board Member.
ҩ “Glue sniffing sucks! The long-term consequences of inhalant abuse during adolescence on the brain and body” Department of Pharmacology, The University Of Melbourne, Vic., Aust.
ҩ “Why glue sniffing sucks! The long-term consequences of inhalant abuse during adolescence on the brain and body” Monash Institute of Medical Research –Prince Henry’s Institute, Vic., Aust.
JESS NITHIANANTHARAJAH
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES 2014 INVITED SPEAKER
ҩ International Conference on Cognitive Neuroscience (ICON), Brisbane Australia (Chair of workshop)
ҩ Evolution Symposium, Victorian Department of Environment and Primary Industries, Melbourne Australia
EXTERNAL COLLABORATIONS FOR ADDICTION NEUROSCIENCE GROUP
Professor Alon Chen (Weizmann Institute, Israel), Professor Jian-Hui Liang (NIDD, Beijing, China), Professor Bernard Balleine (University of Sydney), Professor Peter Kalivas (MUSC, USA), Professor Rainer Spanagel (ZIMH, Germany), Professor Caroline Rae (UNSW), Professor Sarah Dunlop (UWA), Assoc Professor Peter Dodd (UQ), Dr Kevin Pfleger (UWA), Dr Amir Rezvani (Duke, USA), Dr. Masa Funada (National Institute of Mental Health, Kohnodai, Japan), Dr. Tim Bredy (The Queensland Brain Institute), Dr. Zane Andrews (Monash), Professor Dan Lubman (Turning Point / Monash), Professor Michael Cowley (Monash), Professor Danny Winder (Vanderbilt, USA).
ҩ Neuropsychiatric diseases Symposium, Monash Institute of Pharmaceutical Sciences, Melbourne Australia
ҩ Centre for Neural Engineering, University of Melbourne, Australia
ҩ National Institute on Alcohol Abuse and Alcoholism, Rockville USA.
EXTERNAL COLLABORATIONS FOR INHALANT ABUSE LABORATORY
Professor Andrew Lawrence (Florey), Professor Bernard Balleine (University of Sydney), Professor Caroline Rae (UNSW), Dr. Masa Funada (National Institute of Mental Health, Kohnodai, Japan), Dr. Tim Bredy (The Queensland Brain Institute), Dr. Zane Andrews (Monash), Professor Dan Lubman (Turning Point / Monash), Mr. David Wright and Dr. Leigh Johnston (Florey and Melb. Uni), Dr. Joseph Nicolazzo (Monash University), Dr. Michael Mathai (Vic. Uni), Dr Sarah MacLean (Turning Point Alcohol and Drug Center, Melb), Associate Professor Sheree Cairney (Menzie’s and Flinders Uni), Deanne Skelly (Uni Melb), Professor Hannah Kinney and Dr. David Paterson (Harvard Medical School/ Children’s Hospital Boston), Prof. Roger Byard (Uni Adelaide), Drs. Marianne Garland, Michael Myers, William P. Fifer, and Raymond Stark (Primate Center and Departments of Pediatrics and Psychiatry, Columbia University College of Physicians and Surgeons, New York).
EXTERNAL COLLABORATIONS FOR COGNITIVE NEUROSCIENCE GROUP
AMY BRODTMANN
Dr Marina Boccardi and Dr Giovanni Frisoni, LENITEM, Fatebenefratelli, IRCSS, Brescia, Italy, Dr Marco Catani, King’s College, London, UK, Dr Charles DeCarli, University of California Davis, USA, Professor Trish Desmond, Royal Melbourne Hospital, Dr Martin Dichgins, Institute for Stroke and Dementia Research (ISD), Ludwig-Maximilians-University, Munich, Professor Vladimir Hachinski, Case Western University, London, Ontario, Canada, Professor John Hodges (FRONTIER, Sydney, Cambridge), Dr Tobias Loetscher (Flinders University, Adelaide), Professor Terence O’Brien, Royal Melbourne Hospital, Professor John McNeil, Monash University.
DAVID DARBY
Professor Kia Nobre, Oxford University,UK, Dr Moacir Silva Neto, Brasilia, Brazil.
EXTERNAL COLLABORATIONS FOR DEVELOPMENTAL PSYCHOBIOLOGY GROUP
Professor Steven Collins (The University of Melbourne); Professor Antonio Paolini (RMIT); Professor Terry Speed (Walter and Eliza Hall Institute); Dr Sarah Whittle (Melbourne Neuropsychiatry Center).
EXTERNAL COLLABORATIONS FOR SYNAPSE BIOLOGY AND COGNITION GROUP
Prof. Seth Grant (Edinburgh University), Dr. Noboru Komiyama (Edinburgh University), Prof. Timothy Bussey (Cambridge University), Dr. Lisa Saksida (Cambridge University), Dr. Max Kopanitsa (Synome, Cambridge), Dr. Andrew McKechanie (Patrick Wild Centre, Edinburgh University), Dr. Mandy Johnston (Royal Edinburgh Hospital), Prof. Douglas Blackwood (Royal Edinburgh Hospital), Prof. Chris Pantelis (Melbourne Neuropsychiatry), Prof. Arthur Christopoulos (Monash Institute of Pharmaceutical Sciences), Dr. Chris Langmead (Monash Institute of Pharmaceutical Sciences).
2014 STUDENTS:
PhD: • Nicola Chen • Alec Dick • Rose Chesworth • Hanna Kastman • Andrew Walker • Danay Baker-Andresen • Isabel Zbukvic
• Sophia Luikinga• Christina Mo • Annabel Short • Dean Wright • Jake Rogers • Shlomo Yushuren • Sarah Sulaiman Ch’ng • Leigh Walker
• Jirawoot Srisontiyakul • Fiona Bright (Adelaide) • Rebecca Norris • Matthew Poole • Kirrily Rogers • Karen Borschmann (primary supervisor Julie Bernhardt) • MASTERS: • Shawn Tan • Faith Lamont • Emma Giles • Felicia Reid • Katherine Beringer • HONOURS: • Rosa Heller • Ashleigh Qama • Katherine Drummond • Johnny Park •
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 50 51

FOUR KEY WAYS TO IMPROVE YOUR BRAIN HEALTH
By Professor Anthony Hannan
The human brain is the most extraordinary and complex object in the known universe, a kilogram and a half of soft tissue that, at its peak, leaves computers behind with its endless capacity for problem solving, innovation and invention
If the body is a “temple”, then surely the brain must be the “high altar” as it generates all of our thoughts, feelings and movements. Indeed, it is fundamental to all of our conscious experience.
Brain diseases such as Huntington’s, Alzheimer’s and other forms of dementia demonstrate how devastating it is when the brain degenerates, dragging the mind and its many wonderful capacities down with it.
It’s time we all focused more on this most important organ, to improve brain health throughout our lives.
FIRST: STAY PHYSICALLY ACTIVE
This is an obvious idea, however not everyone realises that physical activity is not only good for the body, it also boosts brain health.
There are many ways this could occur, as the brain and body are in constant, dynamic communication. Muscles release beneficial molecules that reach the brain, and exercise also stimulates the heart and other body systems which can beneficially impact the brain. Enhanced physical activity may stimulate the generation of new brain cells and connections.
Evidence is growing that such healthy lifestyle choices may help protect against Alzheimer’s, depression and other brain disorders.
SECOND: STAY MENTALLY ACTIVE
Two cardinal rules of brain plasticity are “use it or lose it” and “neurones that fire together wire together”. People who maintain higher levels of cognitive activity may, in fact, be protected from Alzheimer’s and other forms of dementia.
Cognitive stimulation may help build a “brain reserve” to protect from, and compensate for, the brain ageing. So what mentally stimulating activities could you do? Each individual will know what cognitively challenges and interests them and making positive lifestyle choices that can be maintained for months and years are more likely to lead to long-term benefits.
THIRD: EAT A HEALTHY DIET
Did you realise a balanced nutritious diet is good for your brain?
Most of the nutrients from food circulate through your brain via the bloodstream. So a healthy diet can directly improve the health of brain cells and may even slow down brain ageing.
What’s more, by improving body health, the brain may benefit via the heart and
cardiovascular system, as well as the immune system, that impact on the nervous system.
FOURTH: DON’T STRESS TOO MUCH!
Seldom do we need the stress response (“fight or flight”) that protected cave dwellers thousands of years ago.
Excessive chronic stress may be toxic for the brain which is loaded with sensitive “stress receptors.”
Stress-reducing strategies such as “mindfulness” and meditation are increasingly popular. Other lifestyle choices, such as a good diet, plenty of physical activity, as well as healthy sleep patterns, may also contribute to resilience.
TAKE-HOME MESSAGE
The phrase “mens sana in corpore sano” (a sound mind in a sound body) is thousands of years old, so the concept is clearly not new. The good news is that we can all do something to improve the health of our brains and bodies.
Each of us is dealt a genetic deck of cards at conception that we can do nothing about. Through development and adulthood, our genes interact constantly with environmental factors to regulate how our brains and bodies function, as well as dysfunction when they put us at risk of specific diseases.
With a positive mental attitude supporting a healthy lifestyle, we may be able to maintain soundness of body and mind for as long as possible. And hopefully, brain research at the Florey and elsewhere will deliver new treatments for devastating disorders such as Alzheimer’s disease and other forms of dementia.
Courtesy: TheConversation.com
Pictured: Professor Anthony Hannan, head of the Florey’s Neural Plasticity lab.
Four key ways to improve your brain health
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 52 53
By Professor Anthony Hannan
EPILEPSY
Leaders: Professor Graeme Jackson and Professor Steve Petrou
A QUICK SNAPSHOT
The Florey Epilepsy Division is a world-leading centre for epilepsy research. The division has major groups at both the Florey’s Austin and Parkville campus. The group studies mechanisms that cause epilepsy from cells to the function of the whole brain. We use technologies including advanced MRI and cutting edge cellular physiology techniques to allow us to understand genetic and acquired mechanisms that give rise to epilepsy. Together with our colleagues from The University of Melbourne and across Australia, we are working towards finding a cure for epilepsy.
RESEARCH HIGHLIGHTS
Our neuroimaging studies of epilepsy, including Lennox-Gastaut Syndrome, a particularly severe form of epilepsy, are helping to explain why epilepsy can be associated with intellectual disability. Abnormal electrical activity has been detected in the brains of patients with this syndrome. It occurs frequently, even when the patients are not having a seizure and the activity is widespread, involving many of the brain’s major functional networks, and is therefore likely disrupting normal cognition. Yet in some patients with these symptoms we have located a small brain lesion. After surgical removal of the lesion, the widespread abnormal activity is eliminated and the patients are seizure-free.
AN IDEA LIKELY TO CHANGE LIVES BY 2035
We have shown that abnormal activity involving much of the brain can be triggered by a small focal brain lesion. We have also shown that if such a lesion can be found and surgically removed, seizures can be stopped. We will continue to help drive neuroimaging advances and we hope in coming years this will enable us to routinely locate all such lesions, providing many more epilepsy patients a surgical option to stop their seizures.
MAIN AREAS OF RESEARCH
ҩ Epilepsy genetics
ҩ Epilepsy imaging
With the recent advances in genetics and technology it is now possible to devise and deliver therapies based specifically on the fundamental disease mechanism in individual patients.”
ҩ Human brain structure and function
ҩ Functional modelling of gene mutations in epilepsy
ҩ Multimodal imaging in epilepsy disease models
ҩ Purinergic signalling
SENIOR STAFF
EPILEPSY IMAGING
• Professor Graeme Jackson (Division Head) • Dr David Abbott • Associate Professor Peter Brotchie • Dr Patrick Carney • Associate Professor Paul McCrory • Dr Saul Mullen • Professor Ingrid Scheffer • ION CHANNELS AND EPILEPSY LAB
• Professor Steve Petrou • Dr Alison Clarke • Dr Carol Milligan • Dr Elena Gazina • Dr Kay Richards • Dr Robert Richardson • Dr Verena Wimmer • SYNAPTIC PHYSIOLOGY
• Dr Christopher Reid • Dr Marie Phillips • PURINERGIC SIGNALLING
• Dr Ben Gu • Professor James Wiley •
EPILEPSY – ION CHANNELS AND HUMAN DISEASE
Leader: Steve Petrou
Our research focuses on understanding the pathology of ion channel disorders, in particular epilepsy, using in vitro and in vivo models to reveal opportunities for developing novel therapies. We use a multidisciplinary approach spanning ion channel biophysics, mouse transgenesis, genetic analysis, computational modeling and in vitro and in vivo electrophysiology.
Research highlights for 2014
In 2014 we have accelerated our efforts towards delivering precision medicine outcomes in genetic epilepsy. We showed that quinidine, a drug related to the quinine of tonic water, is effective in controlling the function of an aberrant gene in a severe epilepsy.
Subsequent clinical trials showed efficacy of this treatment paving the way for disease mechanism specific therapies – the backbone of precision medicine approaches.
We continued to seek to find markers of the disease state in epilepsy, from fundamental biophysical changes in ion channel function, to structure of neurones and brains studied with light microscopy and MRI. This year alone contributed to the characterisation of four different epilepsy genes.
These markers of the disease state are critical for accurate diagnosis, but even more importantly can be used to track the efficacy of new therapeutics emerging from our disease mechanism targeted drug programs. We have fully embraced state-of-theart optical imaging approaches for structure and function of neurones to enhance our ability to tease out disease mechanisms.
NEUROIMAGING
Leader: Graeme Jackson
Research highlights for 2014
This group undertakes activities across both the Imaging Division and the Epilepsy Division and has published over 50 peer reviewed papers in 2014. This is a reflection of its origins as an advanced imaging centre with methods development and application to the problems of epilepsy. Through the use of cutting-edge MRI methods such as functional connectivity, tractography, simultaneous electroencephalography and functional MRI (EEG/fMRI) and other advanced imaging, we continue to achieve greater understanding of epilepsy mechanisms. The new knowledge is rapidly translated to improved patient care through the Victorian Epilepsy Centre’s comprehensive epilepsy program at the Austin Hospital in Heidelberg, and through Epilepsy Melbourne, which integrates centres across University of Melbourne teaching hospitals.
This group is primarily based at the Austin campus of the Florey. Our basic work is to understand brain development and function, particularly the disturbances in distributed networks that underlie epileptic disorders. We are using EEG/fMRI, advanced diffusion techniques including tractography, functional connectivity and network analysis to investigate the underlying brain mechanisms responsible for the clinical symptoms. This is done in a translational environment that is immediately relevant to patient care.
In addition to the obvious symptomatology of epilepsy (i.e. seizures) cognition, memory and language disturbances are key cognitive areas affected by the disease. For example, we recently studied language in a group of patients with focal epilepsy, some with and some without reading difficulty, in an attempt to identify brain regions that may have disturbed function. We imaged the brain using functional MRI in the patients and also in healthy control subjects.

“
”
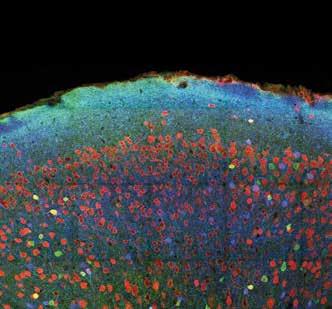
A: mouse somatosensory cortex.
B: part of the hippocampus and overlying cortex.
Investigating subtle changes in the neuronal anatomy of the cortex in epilepsy. Red: neurones stained with an antibody against the neuronal marker NeuN. Blue: staining for the inhibitory neurotransmitter GABA, showing inhibitory neurones. Green: an inhibitory subclass of neurones identified by staining against the calciumbinding protein parvalbumin.
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 54 55
B A
We discovered a functional abnormality in the left frontal region of the brain in patients with and without reading difficulties, and a further functional abnormality specific to the reading difficulty group localized to right temporo-occipital cortex—a region previously implicated in lexicosemantic processing (figure 3). Our observations suggest a failure of left hemisphere specialisation among focal epilepsy patients with reading difficulties, perhaps arising from abnormalities in brain development.
The epilepsy imaging group is also developing new neuroimaging analysis methods to improve sensitivity to detect abnormal brain activity. For example, we have developed a novel processing method
to remove unwanted noise from functional MRI data. The algorithm, called “SOCK”, is an objective and fully automated procedure. An example of its effectiveness in a simultaneous EEG-fMRI study of Rolandic epilepsy is shown in figure 4. SOCK increases power to detect real effects in functional neuroimaging providing a more complete picture of the brain networks of interest.


Generalized paroxysmal fast activity detected on EEG is simultaneously imaged with fMRI to obtain spatial activation maps. The average activity of six subjects is shown here in colour overlayed on an anatomical template. Despite the common pattern of widespread activity, each subject has a different type and location of brain lesion. Adapted from Archer JS, Warren AEL, Stagnitti MR, Masterton RAJ, Abbott DF and Jackson GD, Lennox-Gastaut Syndrome and Phenotype: Secondary network epilepsies. Epilepsia 55(8):1245-54 (2014).
Preoperative and postoperative EEG studies in 38-year-old man with Lennox-Gastaut Syndrome, a lesion, and intractable seizures since childhood. Prior to resection of a left frontal cortical dysplasia (arrowed), the patient had daily tonic seizures. Preoperative interictal EEG showed bursts of slow spike-and-wave (SSW) and generalized paroxysmal fast activity (PFA). Day 3 postoperative EEG shows persistence of SSW, whereas day 30 EEG shows complete normalization, consistent with a winding down of the epileptic process. The patient is 2 years seizure-free, consistent with LGS being a potentially reversible “secondary network epilepsy”. Adapted from Archer JS, Warren AEL, Stagnitti MR, Masterton RAJ, Abbott DF and Jackson GD, Lennox-Gastaut Syndrome and Phenotype: Secondary network epilepsies. Epilepsia 55(8):1245-54 (2014).
Brain activity during performance of a language task (thinking of words beginning with a given letter). Three brain slices are shown for each subject group, with significant activity associated with the task overlayed in colour (increases in warm colours, decreases in blue). Top row: healthy controls. Second row: focal epilepsy patients without reading difficulty. Bottom row: Focal epilepsy patients with reading difficulty. Relative to healthy controls, both patient groups showed reduced activation in left inferior frontal cortex. However the reading difficulty group also exhibited greater activation in the right temporooccipital cortex (see dashed ellipse), indicating a failure of normal specialisation to the left hemisphere in this region. Adapted from Tailby C, Weintrob DL, Saling MM, Fitzgerald C, Jackson GD. Reading difficulty is associated with failure to lateralize temporooccipital function. Epilepsia. 2014;55(5):746-753.


Brain activation associated with abnormal epileptic activity in an individual is shown in colour at two slice positions. The images on the left were created using conventional analysis, whilst the images on the right were created after noise-filtering with SOCK. The plots underneath are the associated estimates of the time-course of signal change. The shape of the time-course after applying SOCK is qualitatively smoother (less noisy), however the most dramatic improvement is evident in the spatial maps. Arrows in green point to areas of increased activation in expected regions when SOCK is employed. From Bhaganagarapu K, Jackson GD, Abbott DF. Frontiers in Neuroscience 8(285):1-9 (2014).
EPILEPSY RD EPILEPSY NO-RD CONTROL MEAN LI = 0.82 MEAN LI = 0.62 MEAN LI = 0.47
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 56 57
EPILEPSY – ION CHANNELS AND HUMAN DISEASE
EDITORIAL POSITIONS
ҩ Neurobiology of Disease
ҩ PLOS Genetics
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES 2014
ҩ Mechanism of proton modulation of neuronal excitability. International Biophysics Congress. Brisbane, Australia.
ҩ Functional correlates of severity in Alternating Hemiplegia of Childhood. 3rd International Meeting of the AHC in Amsterdam September.
ҩ Epilepsy Society of Australia. Microstructure and networks
ҩ Translational opportunities in genetic epilepsies: Are we there yet? Duke University. USA.
ҩ Clear thinking in epilepsy: CLARITY imaging of short and long range neuronal networks. Hertie Institute, University of Tubingen.
ҩ Direct and emergent pathologies reveal therapeutic opportunities in EIMFS and Dravet. Hertie Institute, University of Tubingen.
ҩ pH modulation of neuronal excitability in epilepsy. Hertie Institute, University of Tubingen.
ҩ Translational opportunities in the genetic epilepsies: Something old, something new, something borrowed, something blue. Aikenhead medical research week. St Vincent’s Hospital.
ҩ Precision therapeutics in epilepsy. Epilepsy Genetics in the Era of Precision Medicine, San Francisco, USA.
ҩ Translational opportunities in the epileptic encephalopathies. Hertie Institute, University of Tubingen.
ҩ Mechanisms of pH modulation of neuronal function, Epilepsy Research Retreat, Yarra Valley Lodge.
ҩ Biophysical consequences of SCN2A pathology in Epileptic Encephalopathies, Epilepsy Research Retreat, Yarra Valley Lodge.
ҩ University of Newcastle, NSW. September.
ҩ Multi-modal approaches to understand brain functions. Interactive Brain Function Workshop, Monash University.
ҩ Modulation of neural excitability in health and disease. Pain Program Grant Retreat. University of Queensland.
ҩ CLARITY imaging in genetic epilepsy models. Epilepsy retreat, Austin Hospital.
ҩ Novel peptide therapy in Dravet Syndrome. Dravet round table meeting. Seattle, USA.
ҩ Challenges and opportunities in delivering precision therapies in genetic epilepsy. The Epi4k annual meeting. Seattle, USA.
ҩ Modulation of neural excitability in health and disease. Pain Program Grant Retreat. University of Queensland.
ҩ CLARITY imaging in genetic epilepsy models. Epilepsy retreat, Austin Hospital.
ҩ Novel peptide therapy in Dravet Syndrome. Dravet round table meeting. Seattle, USA.
ҩ Challenges and opportunities in delivering precision therapies in genetic epilepsy. The Epi4k annual meeting. Seattle, USA
EPILEPSY - NEUROIMAGING
EDITORIAL POSITIONS
ҩ Annals of Neurology – Ingrid Scheffer
ҩ Epileptic Disorders - Ingrid Scheffer
ҩ Frontiers in Neurology – David Abbott, Patrick Carney, David Vaughan, Graeme Jackson
ҩ Frontiers in Neuroscience – David Abbott, Patrick Carney, David Vaughan, Graeme Jackson
ҩ Journal of Neuroimaging – Graeme Jackson
ҩ Progress in Epileptic Disorders series - Ingrid Scheffer
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES 2014
ҩ nvited speaker: “Malformations of cortical development” Center for Integrative Brain Research Seattle Children’s Research Institute Seattle, 9th Dec 2014.
ҩ Plenary speaker: “From the bedside to the bench and back” 3rd annual National Health and Medical Research symposium Parkville Melbourne 13th November 2014.
ҩ Chair: UCB Educational meeting: Management of epilepsy in women across the life cycle. Melbourne, 28th August 2014.
ҩ Invited speaker & Co-chair: “Structural MRI; visual inspection versus automated assessment” 10th Asian & Oceanian Epilepsy Congress, Singapore 7th August 2014.
ҩ Featured Speaker: “The near future of imaging in epilepsy”, Third International Epilepsy Research Center Symposium, Newport Beach, California 21st Feb 2014.
ҩ Plenary lecture: “EEG-fMRI in Epilepsy”, EEG-fMRI turns 21, Kiel, Germany 8th June 2014.
ҩ Keynote lecture: “Finding epilepsy networks in functional magnetic resonance imaging of the human brain” and participation in the mathematics Study Group Workshop (SGW 2014), Kyushu University, Fukuoka, Japan & University of Tokyo, Tokyo, Japan. 30th July – 5th August 2014.
ҩ Invited seminar, “Data-driven artefact removal and analysis of fMRI and simultaneous EEG-fMRI”, Advanced Brain Signal Processing, RIKEN Brain Science Institute, Wako, Japan. 6th August 2014.
ҩ Presentation: “Age-related structural changes to the human visual pathway determined using in vivo clinical imaging techniques”. Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO), Denver, Colorado, USA (May 3-7, 2015).
ҩ Presentations: 20th Annual Meeting of the Organization for Human Brain Mapping, Hamburg, Germany, June 2014.
ҩ Presentations: 12th International Conference on Cognitive Neuroscience, Brisbane, Australia. July 27-31, 2014.
ҩ Presentations: 68th American Epilepsy Society annual meeting, Seattle, WA, USA, December 2014.
ҩ Presentations: Section for Magnetic Resonance Technologists (SMRT) 23rd Annual Meeting Milan 10-11th May 2014.
ҩ Presentations: Joint Annual Meeting ISMRM-ESMRMB, Milan, Italy, 10-16 May 2014.
ҩ Presentation (Best Scientific Poster 2014): Australia & New Zealand Spinal Cord Society (ANZSCoS), Auckland, New Zealand, 19 – 21 November 2014.
ҩ Invited talk: “Mild head injury and flying”, Aviation Medical Society of Australia Annual Meeting, Melbourne 19 July 2014.
ҩ Invited Lecture: “Concussion issues for medical staff”, AFL Medical Education Seminar, 24 June 2014.
ҩ Keynote: IOC Injury Prevention and Athlete Health meeting Monaco, 8-12 April 2014.
ҩ Invited Lectures: “What’s new in concussion – the Zurich consensus” and “The difficult concussion patient”, IOC Advanced Team Physician Course Mandelieu, France 12-13 April 2014.
ҩ Invited Lecture: “Concussion in sport”, Aspetar Hospital Scientific Research Meeting, Doha, Qatar 7-14 March 2014.
ҩ Invited Lecture: “Concussion and Medico-legal issues”, NRL CEO annual meeting, Auckland, New Zealand. 14 February 2014.
ҩ Invited Lecture: “Chronic Traumatic Encephalopathy”, Australasian Neuroscience Society Annual Scientific Meeting, Adelaide January 2014.
ҩ Invited Lecture: “Genetic testing in epilepsy: the key to precision medicine”, Dravet Syndrome Foundation Affiliate Meeting, Seattle, USA, 6 December 2014.
ҩ Invited Lecture: “Dravet syndrome – where does the ketogenic diet sit in practice”, Global symposium for the dietary therapies for epilepsy and other neurological disorders. Liverpool, UK, 7-10 October 2014.
ҩ Invited Chair: “Translation to the clinic”, Epilepsy Genetics in the new era of Precision Medicine San Francisco, US, 29-30 September 2014.
ҩ Invited Lecture: “KCNQ2 encephalopathy: an emerging disease within the epileptic encephalopathies.” KCNQ2 summit Denver, US, 20 Septermber 2014.
EXTERNAL COLLABORATIONS FOR EPILEPSY IMAGING GROUP
Ann & Robert H. Lurie Children’s Hospital of Chicago, USA, Austin Health, Melbourne, Boston Children’s Hospital & Harvard Medical School, Boston, USA, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Columbia University, NY, USA, Department of Molecular Genetics and Cytogenetics, Women’s and Children’s Hospital, North Adelaide, South Australia., Foothills Medical Centre, Canada, Harvard Medical School, Boston, USA, Hôpital Necker Enfants Malades, Paris, INSERM, Marseilles, France, Monash University, Melbourne, Murdoch Children’s Research Institute, Melbourne, New York University School of Medicine, USA, Northwestern University Feinberg School of Medicine, USA, Royal Children’s Hospital, Melbourne, Royal Hospital for Sick Children, Glasgow, Royal Melbourne Hospital, The Hospital for Sick Children, Great Ormond Street, London, UK, The University of Melbourne, The University of Queensland, University of Antwerp, Belgium, University of Bologna, Italy, University of Calgary, Canada, University of California, San Francisco, University of Otago, Wellington, New Zealand, University of Washington, Seattle, Walter and Eliza Hall Institute of Medical Research, Melbourne.
MAJOR AWARDS
The seminal contributions to epilepsy research of Professor Ingrid Scheffer continue to be recognised with several major awards bestowed in 2014:
ҩ 2014 Prime Minister’s Prize for Science
Most prestigious science prize in Australia
ҩ 2014 Officer of the Order of Australia (AO)
Awarded for distinguished service to medicine as a clinician, academic and mentor, and for her research identifying new epilepsy syndromes and genes
ҩ 2014 Australian Academy of Science Fellowship (FAA)
Elected as a Fellow for paradigm-shifting research into the genetic causes of epilepsy and defining new epilepsy syndromes.
ҩ 2014 Founding Fellow and Vice-President of the Australian Academy of Health and Medical Sciences (FAHMS)
ҩ 2014 Bethlehem Griffiths Research Foundation Medal In recognition of outstanding leadership and international contribution to medical research
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 58 59
STROKE
Leaders: Professor Julie Bernhardt and Associate Professor David Howells
A QUICK SNAPSHOT
15 million strokes and six million stroke deaths occur each year leaving 55 million survivors suffering the consequences of stroke. The Stroke Division of the Florey Institute of Neuroscience and Mental Health comprises a team of 80 basic and clinical scientists whose aim is to understand the pathophysiology of stroke, optimise existing therapies and develop new treatments and techniques to both prevent and reduce the impact of stroke.
The division has five research teams: Stroke Preclinical Science, the AVERT Early Intervention Research Program, the Neurorehabilitation and Recovery group, Public Health and Epidemiology including the Stroke Telemedicine program and the Australian Stroke Clinical Registry, and Clinical Trials.
RESEARCH HIGHLIGHT
The Florey Public Health and Epidemiology group is responsible for the integrity of the Australian Stroke Clinical Registry (AuSCR). This important national infrastructure provides the ability for hospitals around Australia to enter standardised data about their patients and compare their clinical care to a national standard. Currently, 51 hospitals are approved to use AuSCR. The registry is about to provide information on how many patients are receiving clotbusting thrombolysis medication within the first 4.5 hours of stroke and receiving access to stroke units. Both these treatment offer patients the best opportunity for a good outcome after stroke, however not all hospitals provide these treatments consistently for a variety of reasons. Through use of AuSCR, for the first time in Australia, we are able to understand that factors that influence access to these treatments and also how this impacts on longer term outcomes within 180 days of stroke.
AN IDEA LIKELY TO CHANGE LIVES BY 2035
Our Stroke Telemedicine program is improving equity of access to the best available care for Victorians, whether they live in the city or country. It is the most comprehensive program of its kind that has been developed for acute stroke care in Australia. By 2035, we’d hope to see the program used across Australia.
SENIOR STAFF
• Associate Professor David Howells • Professor Julie Bernhardt • Associate Professor Dominique Cadilhac
• Professor Christopher Bladin • Professor Geoff Donnan
• Professor Leeanne Carey • “
Recruitment to the AVERT trial, an international, 56 multicentre, randomised controlled trial of very early rehabilitation, completed in October 2014. Participants were recruited from five countries. This single blind, Phase III trial, tests whether starting out-of-bed mobility training within 24 hours of stroke is superior to current care. The results are eagerly awaited by the international stroke community and the first results of the trial will be reported at the European Stroke Organisation Conference in Glasgow, April 2015. ”
STROKE PRECLINICAL SCIENCE
Leader: David Howells
The 17 member preclinical stroke laboratories comprise Australia’s leading translational stroke research team with expertise in therapeutic assessment in animal models of stroke, the use of human stem cell derived neurons and glia to explore stroke biology and identify novel drugs and the use of systematic review and meta-analysis to understand why translation to the clinic is so difficult and to provide evidence based selection of agents for clinical trial.
Research highlights for 2014
In both Stroke and SCI our analyses show that stem cell transplants improve outcome. However, in both circumstances facets of the disease modelling account for more of detected effect size than stem cell biology. This led us to concentrate on using stem cells as a source of human neurons for drug testing rather than implantation and the demonstration that hypothermia protects human neurons against ischemia via an oxidative and glucose dependent mechanism. This has led to a new collaboration to expand the use of human stem cell derived neurons and glia to explore stoke biology (Dr Cooper-White, UQ; Dr Dottori, UoM; Dr Wilson, CSIRO).
Evidence from meta analysis of the impact of hypothermia and hypertension on outcome in animal models of stroke contributed to the design of experiments which confirm that hypothermia is effective in animal models of stroke and that pethidine, used to supress shivering in human clinical trials of hypothermia, does not interfere in provision of this benefit.
Cumulative evidence from our analyses of the stroke literature and experimentation in co-morbid animals have contributed to the launch of the Multi-PART consortium (Multicentre Preclinical Animal Research Team; www.multi-part.org), an international in vivo collaboration designed to develop the capacity to undertake international multicentre animal studies to improve the validity, and generalizability of current preclinical research to improve the prospects of success for translation of efficacy to human clinical trials. The internal validity of experiments designed to examine candidates prioritized for in vivo testing will be assured by centralized statistical planning, randomization, outcome adjudication, and quality control to reduce the risk of bias. Initial “proof of concept” experiments designed to confirm in vivo activity will be followed by robust exploration of the limits to efficacy (time to treatment, age and sex of animals, efficacy in hypertensive or diabetic animals, efficacy in different models of stroke). The consortium is now established and funded by the EU. A/Prof Howells chair the project management committee of this EU funded multinational, multidisciplinary consortium and provided guidance for the NINDS call for US researchers to join the initiative (http://tinyurl.com/p6mvjfm).
AVERT EARLY INTERVENTION RESEARCH PROGRAM
Leader: Professor Julie Bernhardt
We believe that the best way to advance science is by collaboration between individuals from a wide range of backgrounds who work together to tackle complex problems. Finding more effective ways to help stroke patients to recover is a key goal the world over. Our group of six post-doctoral researchers, seven research staff and students are working to develop, test and implement new ways of treating people with stroke. Starting active rehabilitation very early after stroke is a relatively new concept with enormous potential to reduce death and chronic disability. While there is some evidence that exercise and purposeful, task specific activity aids recovery after stroke, currently there are no specific recommendations about post stroke exercise. Pilot work by our group suggests that commencing activity within 24 hours of stroke may lead to faster recovery of
function, less disability and better long term quality of life at a lower cost than current care. To test these hypotheses, we are conducting the first randomised controlled trial of very early rehabilitation (AVERT - A Very Early Rehabilitation Trial). Over 2000 patients with stroke have been recruited to this international, multicentre, Phase III efficacy and cost effectiveness clinical trial. The findings of this study have the potential to change clinical practice around the world. Our group also aims to identify key mechanisms by which exercise may improve the physical, cognitive, mood and fatigue outcomes of people affected by stroke.
Research highlights for 2014
Completing recruitment to the AVERT trial, was a highlight for 2014. We witnessed a magnificent effort by hundreds of investigators from five countries (Australia, New Zealand, Malaysia, Singapore, and the UK; Northern Ireland, Wales, England, Scotland) who have been involved in this study over the course of its eight years. The most important time point for review of patient outcomes is at three months post stroke, so we expect to have all of our data in and ready to present for the first time in April 2015. The results are eagerly awaited by the international stroke community. We follow all patients over the first year of their life after stroke, so final results will not be known until 2016. Further information about the trial can be found at the ANZCRT trials register: http://www.anzctr.org.au or on the Florey website.
Another highlight for 2014 was the development of our new acute exercise laboratory at the Austin Campus. This lab will support our acute exercise testing and training of people affected by stroke, with much of the equipment making bedside assessment and testing a possibility. We are now uniquely positioned to interrogate and develop treatments to help the profound deconditioning that occurs quickly after stroke.
Optimising health environments forum
We held our second optimising health environments forum in 2014, which brings together clinicians, architects, researchers and consumers of health care. The purpose of the forum is to build strong linkages across the different sectors to improve the science that underpins decisions about the environments in which health care is delivered. Julie has the view that hospitals are expensive to build and the built environment (as well as the care services within) influences patient outcomes, so, if we can improve the evidence base for decision making about the health care environment itself, everyone benefits. This is an emerging area of interest in Australia and the Florey is taking a role in advancing the science of design and building strong collaborative partnerships. Julie’s vision is to design the best rehabilitation building and service model in the world, and then one day see that design become a reality.
Key research projects and staff
Toby Cumming: The energy cost of walking in the first week after stroke: Stroke can have a major impact on walking ability, fitness and muscle strength, and these impairments may make being physically active more demanding. We are measuring the energy used by patients during steady-paced walking in the first week after stroke, and comparing this to energy used by healthy controls. Data from an unobtrusive electronic activity monitor are compared to ‘gold standard’ measurement of energy expenditure, which involves wearing a mask to measure oxygen intake. Understanding how hard patients can work early after stroke will inform treatment and rehabilitation programs.
Julie Bernhardt: Dose escalation study for stroke survivors with impaired mobility: In partnership with Royal Talbot Rehabilitation Centre, Austin Health, we conducted a dose escalation study of exercise in stroke survivors with significant difficulty with walking. In clinical practice and clinical trials the selection of treatment dose (amount, frequency, intensity) is rarely based on solid data. We aimed to challenge this approach and develop a new way of identifying the best dose of training to get the best results.
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 92 93
Computer games as a tool for rehabilitation: In collaboration with Melbourne Health and gaming company Current Circus, we have conducted a pilot study with 40 stroke affected individuals in rehabilitation to explore whether computer games (using the Kinect system) can be a useful tool for training in this population. We found that the training was fun, well tolerated and engaging.
Evaluating the effect of ward redesign on patient behaviour: In collaboration with Royal Talbot hospital, we conducted a study of the activity patterns of patients in rehabilitation in an old rehabilitation ward, and then repeated the study following a redevelopment of the Mellor Ward. This study piloted a method for exploring how the environment in which care is delivered affects patients behaviour and we found some interesting differences between old and new ward environments.
Karen Borschmann: Understanding changes in bone, glucose and muscle after stroke and their relationship to physical activity. Having a stroke often leads to physical inactivity, and the combination of these two things can have a range of negative physiological effects. In this study we track changes in bone density, glucose metabolism and lean muscle mass which occur over six months after stroke, and investigate the effect of physical activity on these physiological outcomes, with a sub-study to two years. The final six-month assessment of participants has recently been undertaken, results will be presented internationally in 2015.
Coralie English: Sitting time and physical activity in people with stroke: Sitting for long periods each day is an independent risk factor for cardiovascular disease and recurrent stroke. New advances in wearable activity monitors provide us with the tools to be able to accurately and precisely measure sitting time, physical activity and energy expenditure of people with stroke living in the community. We completed an observational study of sitting time and physical activity in stroke survivors and healthy controls, and a pilot RCT testing a novel intervention to reduce sitting time in stroke survivors.
Julie Luker: Exploring the implementation of early stroke rehabilitation in acute settings. The barriers and enablers of providing early rehabilitation in acute settings are poorly understood. This qualitative study explores the experiences of 41 allied health and nursing staff at 17 acute stroke units where the AVERT early mobilisation protocol was implemented.
Patients’ experiences of physical rehabilitation after stroke. This systematic review synthesises the best available evidence from 31 studies on the perspectives and preferences of stroke survivors undertaking physical rehabilitation as inpatients. The review’s findings inform patient centred care and future research.
Liam Johnson: This is my first year with AVERT and we spent 2014 establishing the exercise laboratory and started to establish methods for measuring cardiovascular fitness in people early after stroke. Planning for an observational study of fitness after stroke was a key focus on 2014.

NEUROREHABILITATION AND RECOVERY
Lead: Professor Leeanne Carey
The potential to drive adaptive neural plastic changes through rehabilitation is enormous and likely to lead to major changes in rehabilitation practice and better outcomes for stroke survivors. However, we do not have effective means of identifying individuals who may benefit from therapy nor how to select the most optimal therapy for an individual. The Neurorehabilitation and Recovery research program focuses on stroke recovery: in particular how the brain adapts and how we can harness that potential in rehabilitation. The research involves development of novel rehabilitation approaches and personalised stroke rehabilitation informed by neuroscience. MRI is used to investigate how changes in the brain can help target rehabilitation most optimally to individual stroke survivors. Our research also examines factors, such as depression and cognition, that impact on stroke recovery. An important focus is to translate these discoveries into clinical practice and better outcomes for stroke survivors.
Research highlights for 2014
In 2014 the Neurorehabilitation and Recovery group achieved major steps forward in advancing its program of research focused on harnessing real world drivers of neuroplasticity. The major objective of our group is to translate knowledge from neuroscience into evidence-based clinical practice protocols and better outcomes for stroke survivors. This goal represents a paradigm shift for stroke rehabilitation and better outcomes for the one in six people who experience a stroke. Our research is focussed on: Targeting rehabilitation to the individual based on accessible brain networks; Neurobiology of recovery and impact of depression on outcome; and Implementation of evidence-based practice as outlined below.
In 2014, we achieved 100% recruitment to the START longitudinal stroke cohort study and continued to lead the advanced imaging and clinical arm of the study. We received final ethics approval and initiated the first site for our new study SENSe Implement. Our aim here is to develop a template and clinical practice guidelines for implementation of evidence-based rehabilitation in clinical practice settings. Our national and international standing in translational neuroscience and stroke rehabilitation, and scope for accelerated influence in the field, has been affirmed by our success in 2014 as lead site and Principal Investigator of an international James S. McDonnell Collaborative Award to Translate Neuroscience to Rehabilitation and Everyday Life (2015-2017; $528,000 USD; a consortium of 70 researchers worldwide) and lead of the clinical discovery and neuroimaging stream on the NHMRC Centre for Research Excellence in Stroke Rehabilitation and Brain Recovery (20152019; $2,500,000).
Targeting rehabilitation to the individual based on viable brain networks
Few rehabilitation interventions are based on robust principles of neuroscience. With a rare combination of expertise in clinical rehabilitation and neuroscience our program of research is designed to build stroke rehabilitation evidence: from clinical discovery to implementation. Our group has demonstrated changes in the brain associated with rehabilitation and how therapy may be used to drive neuroplastic changes. However, as yet, we do not have effective means of identifying individuals who may benefit from therapy nor how to select the most optimal therapy for an individual. In our current study we examine for the first time, how different training conditions (task-specific vs transfer-enhanced) and different lesion sites (cortical vs subcortical) impact on reorganization of brain networks involved in recovery of touch sensation. This project Effective sensory rehabilitation after stroke: Targeting viable brain networks is funded by an NHMRC Project grant (2012-2015; CIA Carey). In 2014 we expanded our recruitment sites and increased our recruitment to the study. We continue to collaborate with expert neurologists in Germany and Newcastle (Aus) and with the Advanced MRI Development team at the Florey. We are also involved
in the program of research into neuroimaging outcomes of motor recovery after stroke conducted in Newcastle at the Hunter Medical Research Institute. Our findings will guide therapists in choosing the best therapy for the right individual, based on knowledge of brain networks that have capacity to adapt. Translation: We developed a video animation to depict the translation of neuroscience to an effective rehabilitation intervention: i.e. Connect: Neurogenesis to Neurorehabilitation. The video is available at: http://youtu.be/ G9V3I30pn68 and has been sought after by therapists and stroke survivors alike. Our systematic approach to development of the SENSe neuroscience-base intervention now provides a template for future development of interventions and is the topic of keynote presentations (see below). Expected outcomes from this program of research include: (i) First-ever knowledge of how brain networks alter under different sensory training conditions; and (ii) Evidencebased guidelines to better target sensory rehabilitation to individuals, informed by knowledge of brain networks with capacity to adapt. The clinical value is on identifying strengths of an individual, i.e. neurobiological potential, to use in therapy, and best use of limited resources. Targeting therapy according to viable brain networks is a paradigm shift for stroke rehabilitation.
Neurobiology of recovery and impact of depression on outcome after stroke
One barrier to successful rehabilitation and a major factor impacting recovery and quality of life is post-stroke depression. Despite evidence that depression and stroke are epidemiologically linked, our recent meta-analyses indicate few reliable associates. Our group are using a multimodal approach to investigate the neurobiological associates of recovery and post-stroke depression in a longitudinal stroke cohort. This cohort study is influential in its measurement of a profile of outcomes from mechanisms to participation, and includes blood-based biomarkers, changes in brain structure and function, mood, cognition, lifestyle, and activity participation over 12 months. The cohort, known as START: STroke imAging pRevention and Treatment, is funded by a CSIRO preventative health flagship ($3,000,000; 2010-2015). START PrePARE (Prediction and Prevention to Achieve optimal Recovery Endpoints), the longitudinal cohort arm with advanced imaging and clinical outcomes is led through the Neurorehabilitation and Recovery group. 100% of the sample was recruited as of 2014 and the final data will be collected in April 2015. The imaging and biomarker work is supported by teams from CSIRO (Biomedical Imaging and Molecular Biomarkers), the Florey Advanced MRI Development group, and the La Trobe Institute for Molecular Science. Findings are contributing new insights to stroke rehabilitation that is founded in neuroscience. Expected outcomes include: (i) Novel insight into the neurobiology of post-stroke depression and functional outcome; (ii) Identification of imaging and molecular associates of post-stroke depression to help identify people ‘at risk’ of depression early, inform personalised approach to rehabilitation, and guide more targeted interventions; (iii) Identification of individual patient characteristics that impact on outcome during the 12-month period; (iv) Quantification of the impact of stroke-associated depression on participation in household, social, and leisure activities.
Implementation of evidence-based practice in clinical practice settings
The main objective of this research theme is to develop a template and clinical practice guidelines for implementation of evidence-based rehabilitation in clinical practice settings. This will be achieved in the first instance through implementation of the SENSe intervention. This therapy has been described as ‘life changing’ from stroke survivors. The SENSe Implement study will address one important knowledge-practice gap in stroke rehabilitation: treatment of sensory loss after stroke. Our national survey of current evidencebased assessment and treatment of sensory impairment after stroke, accepted for publication in 2014, highlighted this important evidence-practice gap. This gap now needs to be systematically and urgently addressed. We have produced specialised therapeutic tools, manuals and training videos to teach therapists to effectively
use SENSe therapy with their clients. These tool are now available for use in clinical practice settings nationally and internationally. We will now implement this effective neuroscience-based sensory intervention in clinical practice settings using recommended ‘knowledge-to-action’ methods. The SENSe Implement project will be funded in-kind from hospitals and a dedicated SENSe fund. Three experienced therapists who will be involved in the study have enrolled in a PhD and Masters research program. All have now received competitive scholarships to support their work in this field. The team also includes a rehabilitation physician/director, senior therapist and 5-10 therapists at each site. Professor Marion Walker and Dr Louise Connell will support implementation in the UK. Expected outcomes include: (i) Improved outcomes for stroke patients receiving evidence-based SENSe rehabilitation; (ii) A community of ‘up-skilled’ champion therapists; (iii) New evidence of the success of knowledge-transfer methodologies in stroke rehabilitation; and (iv) Dissemination of the implementation template, with application to interventions for other functions.
Application of SENSe in children
The SENSe intervention is also being applied to children with cerebral palsy using randomised controlled methodology. A research group (n=8) comprising a paediatrician, therapists and PhD students are conducting this research in Perth in collaboration with us.
PUBLIC HEALTH AND EPIDEMIOLOGY
Leader: Associate Professor Dominique Cadilhac
Our group conducts various projects related to improving the clinical management and outcomes of stroke. Research on the quality of stroke care in public hospitals continues to be a major objective, as well as designing and evaluating systems to improve the delivery of evidence-based care and achieve better health outcomes after stroke.
Research highlights for 2014
In 2014, our team continued to lead the expansion of the Australian Stroke Clinical Registry and the Victorian Stroke Telemedicine Program. In addition, there was terrific progress with the Stroke123 NHMRC partnership project whereby AuSCR data were linked to the National Death Index and the acute hospital quality improvement intervention, facilitated by the Stroke Foundation, was delivered across 20 hospitals in Queensland. These are our major flagship projects. We also continued to provide research support for the National Stroke Foundation ‘Know your numbers program’ with approximately 190,900 registrants data from 1,519 health check stations (93 per cent were from pharmacy locations) processed for analysis. The ‘Know your numbers’ program promotes the importance of regular blood pressure checks and the awareness of other stroke risk factors in the community. People who are identified as potentially being at high risk of stroke or cardiovascular disease are referred to their doctor for advice on how to lower their risk and stay healthy. The research program associated with this initiative is being conducted in collaboration with Monash University, where Associate Professor Dominique Cadilhac is also based. Assoc Prof Cadilhac was also successful in obtaining an NHMRC grant ($475K) to undertake a comprehensive cost-effectiveness analysis of the Victorian Stroke Telemedicine program with Monash and Deakin Universities. In 2014, our team also co-hosted the second national workshop with the Stroke Foundation on stroke data, quality and telemedicine which was attended by over 100 delegates.
Stroke Telemedicine (leaders Professor Chris Bladin and Associate Professor Dominique Cadilhac)
The Victorian Stroke Telemedicine initiative is a ground breaking program of work that has the potential to transform the way clinicians collaborate across organisational boundaries to deliver the best care possible to stroke patients irrespective of their location.
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 94 95
Dr Liam Johnson, a postdoctoral research fellow, putting a stroke patient through his paces.
The objective of the program is to improve the care of acute stroke patients across Victoria through the use of cutting edge technology. We aim to do this by implementing a seamless videoconference service to 16 regional hospitals providing 24 hour access to a stroke neurologist roster. Professor Chris Bladin and Assoc Prof Cadilhac, who co-lead this work, have secured over $8.96 million in funding for this program with an additional $475K for a more complete costeffectiveness analysis with project funding from the NHMRC.
The Victorian Stroke Telemedicine Program includes three projects. The initial project was the VST – Bendigo, a successful single site telemedicine project undertaken at Bendigo Health which concluded in 2013. The VST - Loddon Mallee project was commenced in 2013 and is funded by the Victorian Government under the Broadband Enabled Innovation Program (BEIP) grant. This project was the basis for expanding support using the Bendigo model to include Echuca Regional Health, Swan Hill District Health and Mildura Base Hospital in 2014. This work has been project managed by Michelle Vu who joined our team in 2014. At the conclusion of the twoyear BEIP funding, Loddon Mallee health services will continue to receive support under the VicStroke Project. The VicStroke Project is funded by the Australian Government Health and Hospital Fund and will continue to support the VST Loddon Mallee project whilst increasing the scope to include a further 12 hospitals across the other regions of Victoria. This will enable state wide coverage of the teleneurology service and which is funded until 2018. These projects form the largest acute stroke telemedicine program in Australia. Our project partners include Victorian Stroke Clinical Network (Victorian Department of Health), Monash University, Telstra, Polycom, Ambulance Victoria, National Stroke Foundation and the Loddon Mallee Rural Health Alliance. Boehringer Ingleheim has also provided an educational grant to support adaptations to the AuSCR web-tool and education.
In the Victorian Stroke Telemedicine program there are three key research themes: i) clinical ii) implementation science, and iii) health economics. The data collection has been developed to use the infrastructure available through the Australian Stroke Clinical Registry (AuSCR) (Assoc Prof Cadilhac and The Florey are national data custodians) which has been expanded to include stroke telemedicine variables. In this way, ongoing benefits of the program can be captured to measure sustainability impacts beyond 2018.
Australian Stroke Clinical Registry and Stroke123 NHMRC partnership project (leader Associate Professor Dominique Cadilhac)
Our team leads expansion of the Australian Stroke Clinical Registry (AuSCR), mainly across Queensland and Victoria where these respective State governments have invested in its use for monitoring and improving stroke care. The data from AuSCR are also being used as part of a NHMRC Partnership grant whereby a non-randomised, multi-centre, historical, controlled cohort substudy in Queensland that involves quality improvement interventions is being undertaken. Through this work, Assoc Prof Cadilhac is also leading efforts to advance cross-jurisdictional data linkage in Australia and, as such, has established a highly imminent working party that includes members of the Australian Institute of Health and Welfare, the Population Health Research Network and lead researchers from various states who have undertaken data linkage projects, as well as representatives of various State Health Departments. In 2014, she convened a national workshop to progress collectively these efforts to achieve cross-jurisdictional data linkage).
In 2014, there were 51 hospitals with ethical approval to collect data in AuSCR which is an increase from 47 in 2013 (AuSCR 2013 annual report available at www.auscr.com.au). At the end of 2014, there were over 23,000 registered patients of which 5435 were added in 2014. Overall, 2.7 per cent have opted out information from the registry. The Florey AuSCR team, in particular registry manager Brenda Grabsch, data managers Francis Kung and Kate Paice, Queensland coordinator Enna Salama and Victorian Coordinator Kasey Wallace have been the driving force behind the expansion of
AuSCR in 2014. At the end of 2014, the team were informed that the Victorian Department of Health, through the Victorian Stroke Clinical Network, had committed about $785,000 to support greater establishment of AuSCR within Victorian hospitals. The Victorian government has provided, to date, the largest commitment we have received from government to support this initiative.
CLINICAL TRIALS
Leader: Professor Geoffrey Donnan
The Clinical Trials Platform crosses an array of clinical trial initiatives in stroke. The platform services of Neuroscience Trials Australia (NTA), housed within the Florey, are utilised. These include project management, regulatory and monitoring issues, statistical and data storage.
Research highlights for 2014
The main focus of clinical trial activity continues to be around the extended time window for neurological deficits (EXTEND) series of trials. This is in partnership with Professor Stephen Davis at the Melbourne Brain Centre@RMH and his colleagues. This family of trials is designed to extend the time window for the most commonly used stroke intervention, thrombolysis (clot dissolving). We are testing the hypotheses that the time window may be extended out to nine hours from 4.5 hours and that clot removal with the SOLITAIRE device may improve outcomes. This trial has now been completed and the results will be presented at the International Stroke Congress in the US in February 2015. A further study in which the hypothesis that administration of tranexamic acid (a clot promoting agent) may improve outcomes in patients with intracerebral haemorrhagic by minimising further bleeding into the brain. Analysis has been completed on an earlier trial (ARCH trial - prevention of second stroke events in patients with stroke caused by clots coming from the main artery from the heart. Although the trial was stopped early because of the inability of the European arm to continue, the combination of the antiplatelet agents aspirin and clopidogrel were found to be safe and as effective as the anticoagulant warfarin. Given the simplicity of use of the combination antiplatelet agents, these would be the obvious ones to use in clinical practice. The results were published in the journal Stroke.
Clinical trial activity is built upon national and international collaboration. Of the numerous trials conducted at the Florey, the majority are conducted by collaborating with numerous academic colleagues throughout Australia and commonly with those in other countries. Particular collaborations occur with Professors Stephen Davis at the Royal Melbourne Hospital, Christopher Levi and Mark Parson at Newcastle, Greg Albers at Standford University USA, Pierre Amarenco Paris France, and Werner Hacke Heidelberg Germany.
Commercial projects conducted through Neuroscience Trials Australia (NTA)
Clinical trial activity in stroke also includes very important partnerships and collaborations with pharmaceutical and biotechnology companies (both local and global). The trials range from Phase II to III and include drug as well as devices. One of the products assessed aims to assist in dissolving clots that are associated with stroke. Another trial is assessing the use of aspirin compared to another agent in the prevention of TIAs.
STROKE PRECLINICAL SCIENCE
COLLABORATIONS
ҩ International cooperation in preclinical stroke research: Correcting the problems of bias, lack of statistical power and lack of generalizability in preclinical research requires larger, more rigorously controlled and replicated experiments. Our approach to achieve this is establishment of the Multi-PART (mentioned above). This platform has the potential to transform preclinical animal research across the life sciences, similar to the tremendous improvements in clinical research that occurred through the introduction of multicenter clinical trials. This program is now being geared up for the conduct of the first multi-center pre-clinical studies.
ҩ NHMRC Program (Donnan, Davis, Hankey, Parsons & Howells). Our NHMRC program (AppID’s 251525, 454417, 1013612) established a unique vertically integrated program with basic and clinical science elements to select neuroprotectants for clinical trial. We then expanded our basic science so that we had the capacity to identify novel targets for therapy and study the role of long term plasticity and are now exploring the use of stem cell derived human neurons as a drug screening tool and the use of blood biomarkers to select stroke patients for treatment.
ҩ Stroke on a chip: Since our understanding of human stroke pathophysiology is incomplete, we may not have targeted the right cells or the right molecular processes within these cells in our attempts to develop drugs for stroke. A new collaboration with colleagues from University of Queensland (Prof Justin CooperWhite, A/Prof Ernst Wolvetang), University of Melbourne (Dr Mirella Dottori) and CSIRO (Dr William Wilson, Dr Dadong Wang) will use human neurons, astroglia and oligodendroglia derived from embryonic stem cells grown on and microbioreactor arrays, to better define the human ischemic cascade. The multifactorial capacity of the MBAs will allow rapid and reproducible examination of complex interactions between the cells and of the effects of ischemia and of the pharmacological injuries, previously used to identify the key steps in the rodent ischemic cascade, on the biology of these cells. This data will allow us to build the first quantitative and statistically robust map of the human ischemic cascade. The nodes of the map most easily disrupted by ischemia and related insults and those that allow for the greatest degree of normalization when treated will be identified as therapeutic targets for future drug testing.
ҩ Eng Lo, Harvard, USA, Novel lipoxygenase inhibitors to reduce oxidative injury after stroke (US Patent 2012/0053220 A1);
ҩ Dave Lambeth, Emory University, USA, Novel NOX inhibitors to treat stroke.
ҩ William Wilson & Lance Macaulay, CSIRO, Biomarkers to generate a stroke clock (Patent PCT/AU2012/000071).
EDITORIAL POSITIONS
ҩ Evidence based preclinical medicine (Editor in chief)
ҩ International Journal of Stroke (Associate Editor)
ҩ Virtual Medical Centre (Editorial board member)
ҩ Journal of Experimental Stroke Translational Medicine (Editorial board member)
ҩ Translational Stroke Research (Editorial board member).
STAFF
ҩ Dr Katherine Jackman
ҩ Dr Ana Antonic
ҩ Dr Sarah McCann
ҩ Dr Sarah Rewell
ҩ Dr Taryn Wills
ҩ Dr Marie Dagonnier
AVERT EARLY INTERVENTION RESEARCH PROGRAM
KEY COLLABORATIONS
Gothenburg University, Sweden; St Olav’s University Hospital, Norway; Glasgow University, UK; Edinburgh University, UK; Hunter Medical Research Institute, NSW; Austin Health, Vic; Melbourne Health, Victoria; Linköping University, Sweden.
EDITORIAL POSITIONS
ҩ Stroke
ҩ International Journal of Stroke, Section editor Rehabilitation
ҩ Topics in Stroke Rehabilitation
ҩ International Journal of Therapy and Rehabilitation
ҩ Journal for Neurologic Physical Therapy
ҩ American Heart Association writing group “Physical Activity and Exercise Recommendations for Stroke Survivors.” (2014)
ҩ Editorial board member, BMC Neurology
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES
ҩ World Stroke Conference, Invited speaker. Istanbul.
ҩ Physiotherapy New Zealand Conference, Linking the Chain, Auckland. Keynote Speaker.
ҩ World Federation for Neurorehabilitation, Invited speaker & Plenary chair – Advancing Clinical Trials in Neurorehabilitation. Istanbul.
ҩ Singapore Rehabilitation Conference. Invited speaker. Singapore.
ҩ International Stroke Conference, San Diego.
ҩ NTRI Forum. (National Trauma Research Institute) Using Evidence Briefs and Structured Stakeholder Dialogues to address challenges in trauma care, Melbourne.
ҩ University of Melbourne, Melbourne. Evidence based medicine –views from a researcher, clinician and guideline developer.
ҩ Stroke Society of Australasia Scientific Meeting, Hamilton Island.
ҩ The Florey Institute of Neuroscience and Mental Health, Stroke Division. Annual Scientific Meeting. Nelson Bay.
ҩ Occupational Therapy Conference, Adelaide.
ҩ Australasian Nursing & Allied Health Stroke Conference, Sydney.
ҩ Australasian Faculty of Rehabilitation Medicine (AFRM) 2014 ASM, Adelaide.
ҩ International Cognitive Neuroscience Conference, Brisbane.
ҩ Australian Faculty of Rehabilitation Medicine Conference (invited speaker).
KEY STAFF
ҩ Fiona Ellery
ҩ Dr Janice Collier
ҩ Jan Chamberlain
ҩ Tara Purvis
ҩ Dr Toby Cumming
ҩ Dr Coralie English
ҩ Charlotte Krenus
ҩ Alice Liu
ҩ Heidi Ho
ҩ Katie Ardipradja
ҩ Kate Sidon
ҩ Dr Julie Luker
ҩ Dr Liam Johnson
ҩ Karen Borschmann
ҩ Sharon Kramer
ҩ Anne Hokstad
ҩ Nat Fini
NEUROREHABILITATION AND RECOVERY COLLABORATIONS
INTERNATIONAL
ҩ Washington Uni. St. Louis. USA
ҩ Heinrich Heine University, Germany
ҩ University of Haifa, Israel
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 96 97
ҩ University of Nottingham, UK
ҩ Department of Psychological and Brain Sciences, Indiana Uni, USA
ҩ Department of OT Education, Kansas Medical Centre, USA
ҩ A/Prof Thomas Linden. Neurologist/psychiatrist, Gothenburg, Sweden.
ҩ Dept. of Occupational Science and Occupational Therapy and Graduate Dept. of Rehabilitation Science, University of Toronto, Canada
ҩ Dept. of Clinical and Biological Neurosciences, University. Hospital, Grenoble, France
ҩ McDonnell consortia: Advancing the Science of Rehabilitation. 70 scientists from North America, Israel/Europe and Australasia.
ҩ Implementation Science consortia (UK, Canada, Aus).
ҩ International Post Stroke Upper Extremity Working Group.
ҩ National Institute of Health (NIH) Toolbox: Assessment of Neurological and Behavioural Function group.
NATIONAL
ҩ CSIRO preventative Health Flagship. START program of research.
ҩ Hunter Medical Research Institute, Newcastle, Centre for Brain and Mental Health Research
ҩ University of Newcastle, NSW
ҩ Advanced MRI Development, Florey Institute
ҩ La Trobe University: School of Allied Health; Research Focus Area, Sport Exercise and Rehabilitation; and Living with Disability research group (LiDS).
ҩ La Trobe Institute of Molecular Sciences (LIMS)
ҩ School of Paediatric and Child Health, University of Western Australia
ҩ Australian National University, Canberra
ҩ Allied and Public Health, Australian Catholic University, Melbourne.
ҩ Neurology, Austin Health
EDITORIAL POSITIONS
ҩ Neurorehabilitation and Neural Repair
ҩ Occupational Therapy International
ҩ Australian Occupational Therapy Journal
ҩ Brain Impairment.
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES 2014
INTERNATIONAL – KEYNOTE AND INVITED
ҩ Cross-Cultural Issues in Stroke: Policy, Population and Clinical Comparisons. Global Ageing Research Network Meeting. McDonnell International Scholars Academy 5th International Symposium. The Role of Research Universities in Addressing Global Challenges. St Louis. MO.
ҩ Neuroscience makes Sense for Occupational Therapy. Washington University at St Louis St Louis. 8th October 2014.
ҩ Post-Stroke Rehabilitation; Translating Neuroscience to neurorehabilitation. 11th International Conference on Low Vision. Advancing Research, Upgrading Practice, Improving Participation. Melbourne.
ҩ A learning based model of recovery and rehabilitation post-stroke in the Symposium: Rehabilitation of Neurological Vision Impairment. 11th International Conference on Low Vision. Advancing Research, Upgrading Practice, Improving Participation. Melbourne.
ҩ Imaging associates of post-stroke depression and functional outcome: A longitudinal cohort study. 12th International Conference on Cognitive Neuroscience. Symposia. Brisbane.
ҩ The Stroke Profile and Recovery Journey: Across Countries and Over time. McDonnell International Scholars Academy 5th International Symposium. St Louis. MO.
ҩ Health indicators, incidence, prevalence and mortality rates, temporal
trends and gender differences in stroke between United States, Australia and Singapore: An In-depth cross-country comparison. McDonnell International Scholars Academy 5th International Symposium. St Louis. MO.
ҩ Stroke rehabilitation and services in late stages of recovery and return to the community: Comparisons from three corners of the globe. McDonnell International Scholars Academy 5th International Symposium. St Louis. MO.
ҩ Same intervention-Different reorganization: Impact of lesion location on training touch after stroke. 2014 Organisation of Human Brain Mapping. Hamburg, Germany.
ҩ Brain Regions Activated during Tactile Stimulation in Healthy Individuals: An ALE meta-analysis. 2014 Organisation of Human Brain Mapping. Hamburg, Germany.
ҩ Functional and structural connectivity in the brain associated with depression after stroke. 8th World Congress for NeuroRehabilitation. Istanbul, Turkey.
ҩ SENSe: Individual patient characteristics that predict favorable outcomes for sensory rehabilitation after stroke. 8th World Congress for NeuroRehabilitation Istanbul, Turkey.
ҩ The Path through Life project. ICNE 2014. International Conference of Neurology and Epidemiology.
ҩ Automated detection of brain regions associated with post-stroke depression: A hypothesis. ISMRM 2014. Milan, Italy.
ҩ Automated lesion detection from multimodal brain MRI using Markov random fields and random forest. ISMRM 2014. Milan, Italy.
ҩ Fish oil diet associated with acute reperfusion related haemorrhage, and with reduced stroke-related sickness behaviours and motor impairment. Modulating the Brain: Facts, Fiction, Future Conference. Belgian Brain Council.
ҩ Sensory discrimination training in children with hemiplegic cerebral palsy: 2 case studies. 8th World Congress for NeuroRehabilitation. Istanbul, Turkey.
ҩ Advancing the Science of Rehabilitation: Translating Neuroscience and Rehabilitation into Everyday Life. 16th World Federation of Occupational Therapy Congress. Yokohama, Japan.
ҩ Understanding occupational/activity participation after stroke. 16th World Federation of Occupational Therapy Congress. Yokohama, Japan.
ҩ Extending the Time for Thombolysis in Emergency Neurological Deficits - The Extend Trial. International Stroke Conference 2014. San Diego, CA.
PUBLIC HEALTH AND EPIDEMIOLOGY
COLLABORATIONS
Stroke and Ageing Research, School of Clinical Sciences at Monash Health, Monash University; The George Institute for Global Health; Stroke Society of Australasia, La Trobe University; Hunter Stroke Service, NSW Australia; New South Wales Health, Australia; National Stroke Foundation, Australia; Australian Catholic University, Australia; Centre for Translational Excellence in Neuroscience, Melbourne Health; Queensland Statewide Stroke Clinical Network; Victorian Stroke Clinical Network; Victoria; Victorian Department of Health, Edith Cowan University, Western Australia; University of Western Australia; South Australian Statewide Stroke Clinical Network; NSW Statewide Stroke Clinical Network; Queensland Health; Michigan State University, USA; Ottawa Hospital Research Institute, Canada;; Telehealth Connect, Australia; NBNCo, Australia; Loddon Mallee Rural Health Alliance, Australia; Loddon Mallee Rural Health Alliance; Bendigo Health, Echuca Hospital, Swan Hill Hospital, Wangaratta Hospital, Albury and Wodonga Hospitals, Victoria Australia; Ambulance Victoria; Telstra, Australia; Polycom, Australia; School of Population Health, University of Melbourne; University of South Australia; Menzies Institute, University of Tasmania; University of Newcastle, NSW; Hunter Medical Research Institute, NSW;
Nursing Research Institute, NSW; Population Health Research Network, Eastern Health, Monash University; Health Outcomes Institute, Arizona
US; University of Tennessee Health Science Center, Memphis US; School of Health, University of Central Lancashire, Preston, UK
EDITORIAL POSITIONS
ҩ Cadilhac: International Journal of Stroke, Clinical Audit, Dove Medical Press and World Journal of Hypertension
ҩ Dewey: Stroke journal and International Journal of Stroke
ҩ Bladin: Current Neurology and Neuroscience Reports and British Journal of Sports Medicine
STAFF
ҩ Dr Adele Gibbs
ҩ Alison Dias
ҩ Brenda Grabsch
ҩ Prof Christopher Bladin
ҩ Enna Salama
ҩ Francis Kung
ҩ Gary Eaton
ҩ Prof Helen Dewey (honorary)
ҩ Karen Moss
ҩ Kasey Wallis
ҩ Kate Paice
ҩ Dr Katie Bagot
ҩ Linda Francis
ҩ Michelle Vu
ҩ Monique Kilkenny (honorary)
ҩ Nancy Capitanio
ҩ Sally Berger
ҩ Tara Purvis (honorary)
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES
CADILHAC
ҩ Reviewing the options for performance benchmarks for acute stroke care in Australia NHMRC Research Translation faculty conference, Melbourne, November 2014
ҩ Update on the Victorian Stroke Telemedicine (VST) Project. Smart Strokes, Sydney. August 2014
ҩ Stroke data collection in the Australian Stroke Clinical Registry –Progress with a purpose, Smart Strokes, Sydney August 2014
ҩ Introduction to Research Principles, Smart Strokes, Sydney August 2014 (Invited)
ҩ Variances in hospital mortality: Experiences from the Australian Stroke Clinical Registry (AuSCR). Stroke Society of Australasia, Hamilton Island. August 2014
ҩ NSW Stroke Services Clinical Network, panel discussion with Agency for Clinical Innovation on stroke data May 2014 (Invited)
ҩ The economic implications of practice gaps in stroke care: an Australian case study. European Stroke Conference, Nice, France, May 2014
ҩ Co-convenor National Stroke Data, Quality and Telemedicine workshop, Florey, Melbourne March 2014
BLADIN
ҩ Co-convenor National Stroke Data, Quality and Telemedicine workshop, Florey, Melbourne March 2014
DEWEY
ҩ Generalisability of imaging selection for intra-arterial therapy in ischaemic stroke – up to 30% of tPA eligible patients are potentially eligible. European Stroke Conference, Nice, France May 2014.
ҩ Early mobilisation after thrombolysis (rt-PA) in acute stroke: are rtPA treated patients enrolled in a trial of early mobilisation (AVERT) different from those that are not? International Stroke Conference, San Diego, USA, February 2014.
VU
ҩ Experience with scaling up the Victorian Stroke Telemedicine Programme, Successes and Failures in Telehealth (SFT-14), Adelaide November 2014
GRABSCH
ҩ Australian Stroke Clinical Registry: progress and future plans. Registry Special Interest Group, Melbourne, September, 2014.
KILKENNY
ҩ Quality of life and readmission after stroke: the Australian Stroke Clinical Registry experience, Stroke Society of Australasia, Hamilton Island, July 2014
ҩ Influence of area-level socioeconomic status and risk factors for stroke: impact of the New South Wales (NSW) Know your numbers program, Stroke Society of Australasia, Hamilton Island, July 2014
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 98 99
SYSTEMS NEUROPHYSIOLOGY
Leader: Professor Robin McAllen & Professor Richard Macdonell
A QUICK SNAPSHOT
In Systems Neurophysiology we seek to learn how the nervous system controls various bodily functions and how that control is altered in disease. Our disease focus includes not only neurological disorders such as epilepsy and multiple sclerosis, but also how the nervous system impacts on non-neurological diseases such as heart failure and inflammatory diseases. A clear understanding of basic mechanisms is crucial in developing better therapies and reducing the impacts of illness.
OUR FOCUS
Professor Robin McAllen heads the Systems Neurophysiology group at Parkville, which researches brain function in health and disease. A particular focus is on how the brain controls basic bodily functions such as blood pressure, body temperature, body fluids and breathing.
AN IDEA LIKELY TO CHANGE LIVES BY 2035
We have shown that a key area for upper airway control can be identified by a single gene-marker. This maker will allow us to investigate critical cell populations in animals, but importantly also in the human brain. These finding will significantly help us understand and treat neurodegenerative upper airway disorders.
We’ve made a very exciting finding about a primitive part of the brain called the nucleus of the solitary tract or NTS. We have found that adult neurogenesis occurs in the NTS and, importantly, that this process plays a critical role in the regulation of blood pressure. Using a synthetic analogue of the building blocks of DNA, our group found that adult neurogenesis in the solitary tract is increased in both genetically and experimentally induced hypertensive models. The main challenge now is to understand how increased neurogenesis within the NTS contributes to hypertension. We believe this research will help us better understand the neurogenic origins of high blood pressure and reveal a new target for treating hypertensive patients.
SENIOR STAFF
• Professor Robin McAllen • Emeritus Professor Derek Denton • Professor Richard Macdonell • Associate Professor Mathias Dutschmann • Dr David Farmer • Dr Davide Martelli • Associate Professor Clive May • Professor Michael McKinley • Dr Glenn Pennington • Dr Rohit Ramchandra • Dr Davor Stanić • Dr Song Yao • Dr Stuart McDougall • Dr Tara Bautista • Dr Lindsay Booth • Dr Tony Shafton • Dr Yugeesh Lankadeva • Dr Junko Kosaka •
AUTONOMIC NEUROSCIENCE GROUP
Leader: Robin McAllen
We investigate the central nervous pathways that regulate basic bodily functions such as blood pressure and body temperature, as well as their transmission to bodily targets via autonomic nerves.
Research highlights for 2014
It has long been known that, to control body temperature and maintain it within a narrow range, the brain uses temperature signals from the skin and from deep body structures as well as from the brain itself. Tony Shafton, Michael McKinley and Robin McAllen are tracking down the brain’s intrinsic temperature sensors but in order to do this, we need to eliminate temperature signals arriving at the brain from the rest of the body. Others have shown that skin temperature signals relay in brainstem nuclei called the parabrachial nuclei: eliminating those structures prevents the influence of skin temperature on thermoregulatory responses such as increasing or decreasing blood flow to the skin. We hypothesised that temperature signals from deep body structures, such as the stomach and abdominal organs, are transmitted by to the brain by pathways closely similar to those that transmit skin temperature signals. In anaesthetised rats, we first showed that the nerves to blood vessels in the tail (its major organ of heat exchange) responded to temperature changes in the abdomen, and did so independently of brain or skin temperatures. Next, we made lesions of the parabrachial nuclei, and confirmed that they blocked any influence of skin temperature on the thermoregulatory response of the rat’s tail nerves. Finally, we showed that these same lesions also blocked the effects of temperature changes in the abdomen. But the influence of brain temperature was preserved. Now we can remove the influence of temperature signals from the rest of the body and investigate the actions and neural pathways of the brain temperature sensors acting alone.
The research focus of the team hasn’t changed over the last 12 months and most of the time has been used to investigate the neural control of immune function. During 2014, we published a study (Martelli D., Yao S.T., McKinley M.J., McAllen R.M. Reflex control of inflammation by sympathetic nerves, not the vagus. J. Physiol., 592, 2014:1677-1686.
This was recommended in Faculty of 1000 and as the editor’s choice of the published issue), where we described a neural reflex, termed the inflammatory reflex, that controls inflammation. This study contradicts the current dogma, that considers the vagus nerve as the main player of the inflammatory reflex, and shows how inflammation is controlled by the splanchnic sympathetic nerves. We also performed a thorough investigation on the target organ of the inflammatory reflex. Our results (still unpublished), once again, contradict the common view that it’s the spleen the principal organ where nerves physiologically interact with white cells to inhibit inflammation. We now have data that demonstrate how this interaction is generalised to all the organs innervated by the greater splanchnic nerves (spleen, adrenal glands, liver, stomach and intestine).
on their body fat levels. These findings show that fat can be metabolised in dehydrated animals to produce water. We suspect that this process is controlled by osmoreceptors in the brain.
RESPIRATORY NEUROBIOLOGY LAB
Leader: Mathias Dutschmann
We study the basic neural mechanisms underlying breathing, how these patterns of nerve activity adjust to accommodate other behaviours such as swallowing, and how they are modified during development and in neurodegenerative disease.
Research highlights for 2014
Our main research focus is how the central nervous system controls upper airway muscles to modulate respiratory airflow during normal breathing (eupnoea). We also want to understand how the same brain circuitry adapts during important oro-pharyngeal behaviours, such as swallowing and breathing. The upper airway control circuits are prone to neurodegeneration including symptoms such as breath and vocal disorders, and also dysphagia (swallowing disorders). We are showing that a key area for upper airway control can be identified by a single gene-marker (foxp2). This maker will allow us to investigate critical cell populations in animals, but importantly also in the human brain. These finding will significantly facilitate our translational research program which aims to understand and treat neurodegenerative upper airway disorders.
NEUROCARDIOVASCULAR GROUP
Leader: Clive May
The Neuro-cardiovascular group is investigating the effects of the sympathetic nervous system in diseases including heart failure and septic shock.
Research highlights for 2014
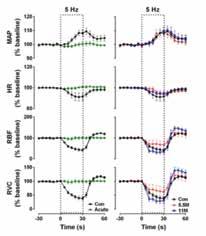
Professor Michael McKinley has been investigating the relationship between fat and hydration. When fat is broken down by metabolism, it generates water as well as energy. We wondered whether the body uses this process to generate water when it is needed to offset dehydration. We tested this idea in rats. Using an MRI instrument designed for the purpose, we measured their total body fat and total body water before and after 24 hours of water deprivation. After this, their total body water fell by eight per cent and their body fat by 16 per cent. The fat levels returned to normal within 48 hours of restoring access to water. Although dehydrated rats ate less food, reducing the food intake of normally hydrated rats by the same amount had only a very small effect
Effects of electrical stimulation of the renal nerve on mean arterial pressure (MAP), heart rate (HR), renal vascular conductance (RVC) and renal blood flow (RBF). The left panel shows that the responses in the control situation (black) were abolished acutely after renal denervation (green).
The right panel shows that the response at 5.5 (red) and 11 months (blue) after denervation were similar to the control responses (black).
Heart Failure
Heart failure is a complex syndrome resulting from structural changes in the heart that reduce cardiac output and thus perfusion of tissues. It is a major public health problem in all Western countries where, in addition to the human cost in terms of morbidity and mortality, it is estimated to cost more than five per cent of health budgets. Disturbingly, the incidence of new cases of heart failure is increasing due to the ageing of the population and the conversion of acute cardiac problems into chronic disorders. A novel technique that has been used for the treatment of hypertension, denervation of the renal nerves, has been proposed as a treatment for heart failure. It is however unclear how effectively this technique denervates the renal nerves and whether the nerves subsequently regrow.
Studies conducted by Dr Lindsea Booth demonstrated that renal denervation with a clinically used radiofrequency catheter effectively denervated both the renal afferent and efferent renal nerves. This was demonstrated by a loss of renal sympathetic nerve activity, lack of responses to electrical stimulation of the renal nerve and absence of anatomical markers for afferent and efferent nerves in the kidney. We then demonstrated that at five months after denervation the level of renal nerve activity, the responses to electrical stimulation and level of anatomical markers were close to normal, and had fully returned to normal by 11 months
“
”
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 100 101
after denervation. These findings demonstrate that radiofrequency catheter-based renal denervation is effective, but raise questions regarding the mechanism by which renal denervation causes a prolonged fall in blood pressure in hypertensive patients.
Septic shock
Sepsis is the major cause of death in intensive care units with a high mortality rate of 30-70 per cent. Sepsis causes more deaths than prostate cancer, breast cancer and AIDS combined and the annual number of cases of sepsis is increasing. Despite the high costs in terms of morbidity, mortality and health budgets, few advances have been made in understanding of the pathophysiological mechanisms of sepsis, and consequently new therapeutic strategies have not been developed.
One of the hallmarks of sepsis is a large fall in blood pressure that can lead to multi-organ failure and death. A primary treatment is to give vasopressor drugs to increase blood pressure, but in sepsis the responsiveness to the drugs is reduced, leading to severe hypotension. One theory to explain this desensitisation is that the large increase in sympathetic nerve activity, that helps maintain blood pressure in sepsis, also has a detrimental action to reduce responsiveness of blood vessels. Dr Yugeesh Lankadeva and Dr Junko Kosaka have therefore investigated the effect of treatment with a drug, clonidine, that acts in the brain to inhibit sympathetic nerve activity. Although clonidine might be expected to cause a greater degree of hypotension, it in fact reduced the decrease in blood pressure during sepsis. An explanation for this is our finding that in sepsis treatment with clonidine fully restored the responsiveness of blood vessels to drugs that increase blood pressure. Clonidine might therefore be a useful treatment for septic patients who are resistant to vasopressor drugs and it is now important to confirm these experimental findings in a clinical trial in septic patients.







NEUROVASCULAR BIOLOGY LAB
Lead: Song Yao
Research highlights for 2014
Our group has made a very exciting finding in a primitive part of the brain call the nucleus of the solitary tract (NTS). This region of the brain stem is essential for regulating blood pressure. For many decades it was assumed that the adult brain does not acquire more brain cells (or neurones) in adulthood. This was shown to be an incorrect assumption in the 1960’s when an American group at MIT showed that the birth of new brain cells, termed ‘adult neurogenesis’ occurs in only two parts of the adult brain - one responsible for memory and the other responsible for smell.

Newly born brain cells (shown in red) have been discovered in the nucleus of the solitary tract or NTS. This region of the brain is essential for controlling blood pressure. We are currently trying to identify the function of these nascent cells and to understand how they contribute to high blood pressure.
We have recently demonstrated that adult neurogenesis also occurs in the NTS and, importantly, that this process plays a critical role in the regulation of blood pressure. Using a synthetic analogue of the building blocks of DNA our group found that adult neurogenesis in the solitary tract is increased in both genetically and experimentally induced hypertensive models. The main challenge now is to understand how increased neurogenesis within the NTS contributes to hypertension. We believe this research will help us better understand the neurogenic origins of high blood pressure and reveal a new target for treating hypertensive patients.
CLINICAL
BRAIN FUNCTION IN HEALTH AND DISEASE GROUP
Lead: Richard Macdonell
Our lab studies how diseases such as epilepsy and multiple sclerosis change the excitability of neurones. A second focus is on the physiology of neurorehabilitation and neural repair.
VISCEROSENSORY LAB
Lead: Stuart McDougall
Research highlights for 2014
One of the lab’s investigative aims is to understand how the brain coordinates internal organ function with behaviour, during stress and in diseases like depression or hypertension. We take a reductionist approach to study components of the neuronal network involved in these ‘automatic’ functions. With funding from the University of Melbourne Early Career Researcher Grants Scheme, my lab has utilised optogentic technology to pick these circuits apart over the last year. We are the first to demonstrate at the synaptic level how hypothalamic and amygdala neurones alter viscerosensory signals as they first enter the brain. With these fundamental findings the lab can establish how these networks are altered in autonomic related disease states. This work was presented at the meeting Central Cardiovascular and Respiratory Control at the Baker Institute during 2014 and I have been invited to present this work at the a combined meeting of the world’s major Autonomic Societies in 2015.
Research highlights for 2014
We are currently conducting a placebo controlled study investigating whether the drug (4-aminopyridine) can be used to improve upper limb and hand function in patients with multiple sclerosis. The drug has previously been shown to improve walking speed and quality of life in patients suffering from MS. We use clinical measures of hand function which may respond to using the drug, such as strength and dexterity and tactile discrimination.
We are also conducting neurophysiological measures of conduction in central nervous system pathways using the techniques of transcranial magnetic stimulation and somatosensory evoked potentials. The drug is thought to produce benefits by blocking potassium channels in demyelinated axons thus permitting conduction to be through areas damaged by MS. We are on track to have results of this study available in early 2016.
AUTONOMIC NEUROSCIENCE GROUP
EDITORIAL POSITIONS AND AWARDS
MCALLEN
ҩ American Journal of Physiology (Regulatory, Comparative and Integrative Physiology).
ҩ Temperature.
ҩ Faculty of 1000.
ҩ International Presentation.
ҩ Physiology and Pharmacology of Temperature Regulation, Kruger National Park, South Africa, September 2014.
ҩ Best 2014 research article published in the journal Temperature.
MARTELLI
ҩ 2014: Editorial Board of the Journal of Immune Research.
ҩ 2014: “Post-doctoral/Early Career Investigator Award” from the American Physiological Society (Neural Control/Autonomic Regulation section). Presented at EB2014 in San Diego, California, USA.
ҩ 2015: Awarded with a IBRO Research Fellowship.
MCKINLEY
ҩ American Journal of Physiology. (Regulatory, Comparative and Integrative Physiology)
ҩ Faculty of 1000.
RESPIRATORY NEUROBIOLOGY LAB
INTERNATIONAL CONFERENCES & EDITORIAL POSITIONS
DUTSCHMANN
ҩ Oxford breathing meeting, Sydney 2014.
ҩ Session speaker, session chair.
ҩ Tara Bautista, session speaker.
ҩ Editor in chief: Respiratory Physiology & Neurobiology (Elsevier).
NEUROCARDIOVASCULAR GROUP
COLLABORATIONS
ҩ Baker IDI, Melbourne.
ҩ University of Lyon, France.
ҩ University of Iowa, USA.
ҩ Rush University Medical School, Chicago, USA.
EDITORIAL POSITIONS
MAY
ҩ American Journal of Physiology, Regulatory, Integrative and Comparative Physiology.
ҩ Clinical and Experimental Pharmacology and Physiology.
ҩ Frontiers in Integrative Physiology.
ҩ Frontiers in Autonomic Neuroscience.
ҩ Faculty of 1000.
MAJOR NATIONAL AND INTERNATIONAL CONFERENCES 2014
ҩ World Congress of Cardiology, Melbourne.
ҩ PanAmerican Congress of Physiology, Iguassu Falls, Brazil.
ҩ High Blood Pressure Research Council of Australia, Adelaide.
NEUROVASCULAR BIOLOGY LAB
MAJOR INTERNATIONAL CONFERENCES
YAO
ҩ Pan-American Congress of Physiological Societies, Brazil 2014.
COLLABORATIONS
ҩ University of Sao Paulo, Sao Paulo Brazil.
ҩ Roslin Institute, University of Edinburgh, Scotland.
ҩ University of Bristol, United Kingdom.
CLINICAL BRAIN FUNCTION IN HEALTH AND DISEASE GROUP
COLLABORATIONS
MACDONELL
ҩ University of Queensland.
ҩ Monash University.
ҩ Biogen Idec (Australia).
An infected neuron in the amygdala forced to express a light sensitive ion channel and fluorescent protein. Light activates the neuron.
A neuron in the brain stem is inhibited as light evokes neurotransmitter release in amygdala axons.
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 102 103

“
I am passionate about improving the lives of people with Parkinson ’s disease. It’s my life’s work. The thirst for scientific knowledge is important to me but knowing that the field can be directly applied to people, is immensely rewarding. My work gives me a real sense of achievement.
Finding a cure is no small task and it will take a coordinated effort. I would like to see all of the Parkinson’s groups, scientists and clinicians unite and to convince politicians to make this a national health priority. Together, we will win this battle.
Associate Professor David Finkelstein: Head, Parkinson’s disease laboratory
ҩ Mr Andrew Abercrombie
ҩ Mr Charles Allen AO
ҩ Professor James Angus AO
ҩ Professor Etienne Baulieu
ҩ Professor Samuel F Berkovic AM
ҩ Mr Chris Blake
ҩ The Hon Dr Neal Blewett AC
ҩ Mr Graeme Bowker
ҩ Dr Di Bresciani OAM
ҩ Lord Alec Broers
ҩ Mr Graham Brooke AM
ҩ Mr Malcolm Broomhead
ҩ The Hon John Brumby
ҩ Mr Tom Buchan
ҩ Professor Geoffrey Burnstock
ҩ Mr Richard Buxton
ҩ Professor Edward Byrne AO
ҩ The Hon Jim Carlton AO
ҩ Professor Peter Castaldi AO
ҩ Professor Jean-Pierre Changeux
ҩ Mr Trevor Clark OAM
ҩ Mr Peter Clemenger AM
ҩ Professor John Coghlan AO
ҩ Professor David Copolov OAM
ҩ Mr Philip Cornish
ҩ Mr Charles Curwen CVO OBE
ҩ Dr Andrew Cuthbertson
ҩ Mr Tim Daly AM
ҩ Professor Stephen Davis AM
ҩ Mr Jerry de la Harpe
ҩ Mr David de Rothschild
ҩ Dr David de Souza AM
ҩ Emeritus Professor Derek Denton AC
ҩ Emeritus Professor Ralph Doherty AO
ҩ Professor Peter Doherty AC
ҩ Mr Carl Dowd
ҩ Mrs Wendy Dowd
ҩ Mrs Suzanne Downes
ҩ Dr Thomas Hurley AO OBE
ҩ Ms Margaret Jackson AC
ҩ Professor Bevyn Jarrott
ҩ Mr Mark Jones
ҩ The Hon Barry Jones AO
ҩ Mr Peter Jopling QC
FLOREY GOVERNORS
ҩ Mr Craig Drummond
ҩ Professor George Fink
ҩ Dr Alan Finkel AM
ҩ Emeritus Professor John Finlay-Jones
ҩ Mr Roger Flynn
ҩ The Right Hon J Malcolm Fraser AC CH
ҩ Mrs Tamie Fraser AO
ҩ Professor Peter Fuller
ҩ Professor John Funder AC
ҩ Mr Rob Gerrand
ҩ Mr Kelvin Glare AO
ҩ Mr Charles Goode AC
ҩ Baroness Susan Greenfield CBE
ҩ Mrs Helen Groves AO
ҩ Dr Sandra Hacker AO
ҩ Mr Michael Hamson
ҩ Mr Arnold Hancock OBE
ҩ Ms Pamela Hauser
ҩ Ms Caroline Hogg AO
ҩ Emeritus Professor Andrea Hull AO
ҩ Mr Bruce Kean AM
ҩ Mr Graeme Kelly
ҩ Professor Anne Kelso AO
ҩ Professor Bruce Kemp
ҩ Mrs Jennifer Labourne
ҩ Professor Richard Larkins AO
ҩ Emeritus Professor Frank Larkins
ҩ Dr John Lill OAM
ҩ Mr Paul Little AO
ҩ Dr Bernard Lochtenberg
ҩ Dr Raymond Marginson AM
ҩ Ms Lina Marrocco OAM
ҩ Professor Jim McCluskey
ҩ Professor Elspeth McLachlan FAA
ҩ Mr George McMaster
ҩ Professor Frederick Mendelsohn AO
ҩ Ms Naomi Milgrom AO
ҩ Mrs Pamela Miller
ҩ Mr Peter Mitchell AM
ҩ Mr Harold Mitchell AC
ҩ Dr Brendan Murphy
ҩ Mrs Louise Myer
ҩ Mr Martyn Myer AO
ҩ Mr S Baillieu Myer AC
ҩ Mrs Maria Myers AO
ҩ Mr Allan Myers AO QC
ҩ Mr A H (Buck) Myers Jr
ҩ Dr Mark Nelson
ҩ Dr Hugh Niall
ҩ Mr Ross Oakley
ҩ Professor Kerin O’Dea
ҩ Mr Simon Parker Bowles
ҩ Lady Primrose Potter AC
ҩ Professor John Poynter AO
ҩ Mr Ian Renard AM
ҩ Mr Geoffrey Ripper
ҩ Mrs Eda Ritchie AM
ҩ Professor P John Rose AO
ҩ Dr Thomas Schneider
ҩ Professor David Scott
ҩ Professor Richard Smallwood AO
ҩ Mr Stephen Spargo AM
ҩ Mr Andrew Stripp
ҩ Mr Boris Struk
ҩ Mr Robert Trenberth AM
ҩ Dr Gad Trevaks AM
ҩ Ms Anne Ward
ҩ Mr Andrew Wardlaw
ҩ Mrs Elizabeth Wardlaw
ҩ Mr Brian Watson
ҩ Professor Ingrid Winship
ҩ Professor E Marelyn Wintour-Coghlan
ҩ Ms Meredith Woods
ҩ The Hon Dr Michael Wooldridge
ҩ Mr John Wylie AM
ҩ Mr Harrison Young
01 03
”
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 104 105
FINANCE AND INVESTMENT
ҩ Mr Stephen Spargo AM (Chair)
ҩ Professor Geoffrey Donnan AO
ҩ Dr Henry De Aizpurua
ҩ Mr Wayne McMaster
ҩ Dr Ross Macdonald
ҩ Dr Ergad Gold
ҩ Professor Steven Petrou AUDIT
ҩ Mr Craig Drummond (Chair)
ҩ Mrs Jennifer Labourne
ҩ Mr Mark Jones
ҩ Professor Andrea Hull AO
ҩ Professor Geoffrey Donnan AO
ҩ Professor Graeme Jackson
ҩ Mr Andrew Stripp
ҩ Mr Peter Plecher
ҩ Mr Mark Jones (Chair)
ҩ Mrs Jennifer Labourne
ҩ Professor Andrea Hull AO
ҩ Professor Geoffrey Donnan AO
ҩ Professor Graeme Jackson
ҩ Mr Andrew Stripp
ҩ Mr Peter Plecher NOMINATION
ҩ Mr Harold Mitchell AC (Chair)
ҩ Mr Andrew Abercrombie
ҩ Professor James Angus AO
ҩ Professor Geoffrey Donnan AO
ҩ Mr Rob Gerrand
ҩ Professor Andrea Hull AO
ҩ Professor Anne Kelso AO
BOARD COMMITTEES & FOUNDATION COUNCIL
EXTERNAL SCIENTIFIC ADVISORY
ҩ Professor Sam Berkovic AC
ҩ Professor Alastair Buchan
ҩ Professor Seth Grant
ҩ Professor Seong-Seng Tan
ҩ David Abbott
ҩ Paul Adlard
ҩ Timothy Aumann
ҩ Kevin Barnham
ҩ Ross Bathgate
ҩ Philip Beart
ҩ Christopher Bladin
ҩ Joanne Britto
ҩ Ashley Bush
ҩ Dominique Cadihac
ҩ Fernando Calamante
ҩ Leeanne Carey
ҩ Robert Cherny
ҩ Leonid Churilov
ҩ Alan Connelly
ҩ Julie Bernhardt
ҩ Wah Chin Boon
ҩ Nathalie Braussaud
ҩ Amy Brodtmann
ҩ Henry De Aizpurua
ҩ Brian Dean
ҩ Geoffrey Donnan
ҩ John Drago
ҩ Simon Drew
ҩ Jhodie Duncan
ҩ Mathias Dutschmann
ҩ Ben Emery
ҩ David Finkelstein
ҩ Andrea Gogos
ҩ Ben Gu
ҩ Andrew Gundlach
ҩ Anthony Hannan
ҩ Rachel Hill
ҩ Malcolm Horne
ҩ Akhter Hossain
ҩ David Howells
ҩ Jason Howitt
ҩ Graeme Jackson
ҩ Bevyn Jarrott
ҩ Trevor Kilpatrick
ҩ Jee Hyun Kim
ҩ Andrew Lawrence
ҩ Robin McAllen
ҩ Gawain McColl
ҩ Paul McCrory
ҩ Stuart McDougall
ҩ Michael McKinley
ҩ Colin Masters
ҩ Clive May
THE FLOREY FACULTY & SENIOR POSITIONS
ҩ Tobias Merson
ҩ Saul Mullen
ҩ Jess Nithianantharajah
ҩ Lucy Palmer
ҩ Clare Parish
ҩ Steven Petrou
ҩ Chris Reid
ҩ Blaine Roberts
ҩ Ingrid Scheffer
ҩ Daniel Scott
ҩ Karin Sitte
ҩ Suresh Sundram
ҩ Seong-Seng Tan ҩ Lachlan Thompson ҩ Bradley Turner
John Wade
Jim Wiley
FOUNDATION COUNCIL
ҩ Mr Trevor Clark OAM (Chair) (ended Dec 2014)
ҩ Mr Julian Clarke AM
ҩ Mr Graeme Kelly
ҩ Mr Ross Oakley OAM
ҩ Mr Stephen Spargo AM
ҩ Professor Geoffrey Donnan AO
ҩ Mr Ross Johnstone (ended Dec 2014)
Professor Geoffrey Donnan AO ҩ Professor Alan Connelly
ҩ Dr Henry De Aizpurua
ҩ Professor Graeme Jackson
ҩ Professor Colin Masters
ҩ Professor Steven Petrou
Executive
ҩ Associate Professor David Howells
ҩ Mr Peter Plecher
ҩ Professor Andrew Lawrence
ҩ Professor Ashley Bush
ҩ Professor Brian Dean
ҩ Professor Colin Masters
ҩ Professor Geoffrey Donnan AO
ҩ Professor Graeme Jackson
ҩ Dr Henry De Aizpurua
ҩ Professor Ingrid Scheffer AO
ҩ Professor Julie Bernhardt
ҩ Professor Philip Beart
ҩ Professor SeongSeng Tan
ҩ Professor Steve Petrou
ҩ Mr Peter Plecher
03 03
Scientific
ҩ
ҩ
ҩ
Director
Group
Secretary
Florey Deputy
Finance Director and Company
Florey
Directors
THE FLOREY FACULTY SENIOR POSITIONS 02 03
COMMERCIALISATION
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 106 107
ҩ David Abraham
ҩ Marjorie Anne Ada
ҩ Joan Adler
ҩ Craig Adlington
ҩ May Admans
ҩ Stephanie Aeuckens
ҩ Yvonne Ahmet
ҩ Michael Aitken
ҩ Charles Allen AO
ҩ Frances Allen
ҩ Geoff & Lauris Allen
ҩ Jim & Judy Allen
ҩ Rod & Joan Alsop
ҩ Alzheimer’s Drug Discovery Foundation
ҩ Walter Ames
ҩ AMP Foundation
ҩ Keith Anderson
ҩ Pamela Anderson
ҩ Rita Andre
ҩ ANZ Banking Group Limited
ҩ ANZ Trustees Limited
ҩ Frank Archer
ҩ Marilyn Armstrong
ҩ E Arthur
ҩ Barbara Ashton
ҩ Tony Atkinson
ҩ Australia Post
ҩ Australian Communities Foundation - Ralph & Betty Sims Fund
ҩ Australian NPC Disease Foundation Inc
ҩ Philip & Margaret A’Vard
ҩ Christine Bagnara
ҩ Lauren Bailey
ҩ Ursula Baird
ҩ Peter Baird
ҩ Kenneth J Ball
ҩ Rosemary Ball
ҩ William E Bamford
ҩ Kevin Barham
ҩ Ross E Barker
ҩ Rodney Barkman
ҩ Paul Barnett
ҩ Paul Bartlett
ҩ Nick Barton
ҩ John & Lorraine Bates
ҩ Elizabeth Batt
ҩ Vera Baudinette
ҩ William R Baxter
OUR DONORS
We appreciate the generosity of our donors and would like to especially thank those who have given $100 or more during 2014
ҩ Peter & Shanon Beaconsfield
ҩ Lynne Beale
ҩ Walter Beale
ҩ Richard & Mirion Bearman
ҩ Josephine Beatson
ҩ Sally Beavis
ҩ Eva Bedelis
ҩ Roger & Jennifer Beer
ҩ Carmel Behan
ҩ Bell Charitable Fund
ҩ Doreen Bell
ҩ Diana F Benefield
ҩ Alan Benger
ҩ John Benger
ҩ Sandra R Benjamin OAM
ҩ Colin & Barb Benson
ҩ Valerie F Benson
ҩ Berwick Opportunity Shop Incorporated
ҩ Besen Family Foundation
ҩ Shane Bilinski
ҩ Ian Birchall
ҩ Katie Bird
ҩ R D G & M J Birrell
ҩ Evangelo Bledeni
ҩ G R Blair
ҩ Jacqueline A Blake
ҩ Tim Blashki
ҩ The Hon Dr Neal Blewett AC
ҩ Sidney & Felicity Bloch
ҩ Barbara Bonetto
ҩ Jo Bornemissza
ҩ Graeme Bowker
ҩ Loretto & Ray Brady
ҩ Brian J Brand
ҩ B A Brandrup
ҩ William & Marie Brennan
ҩ Breville
ҩ Brian M Davis Charitable Foundation
ҩ John & Nicky Bridges
ҩ Graham Brooke AM
ҩ Janette Broom
ҩ Dennis J Bryant
ҩ Jim A Buchanan
ҩ Hans Bufe
ҩ John Bugg
ҩ Beverley Bull
ҩ Margaret Bullen
ҩ Peter Bullock
ҩ June Burrell
ҩ Norman & Kath Bury
ҩ Elizabeth Butt
ҩ Ronald Butters
ҩ Russell & Sheila Byard
ҩ Normie Bydder
ҩ R I Byers
ҩ Jarrod Byham
ҩ Madeline Byrne
ҩ C R Kennedy & Company Pty Ltd
ҩ Cabrini Health
ҩ CAF Community Fund
ҩ Rosemary Callow
ҩ Ross Campbell
ҩ Suzi Carp
ҩ Janet Carr
ҩ C M Carrigan
ҩ Deirdre Carter
ҩ Rev Robert B Catford
ҩ Keith Cattach
ҩ Suzanne Cecil
ҩ CFMEU
ҩ CGU Foundation
ҩ BNI Chagall
ҩ Anthony E Chaloner
ҩ Jennifer Chambers
ҩ Stephen P Charles
ҩ Sylvia A Charlton
ҩ I G Chenoweth
ҩ Diana M Cherry
ҩ The Chiodo Family
ҩ Helen J Chu
ҩ Frank Clark
ҩ Faye Clarke
ҩ Robert J Clarke
ҩ J L & E S Cleland
ҩ Barbara Clifford
ҩ Arthur C Close
ҩ Kerry Close
ҩ Michael & Jan Cody
ҩ Catherine J Coghlan
ҩ Sally Cohen
ҩ Diane Collacott
ҩ John & Chris Collingwood
ҩ A & S Collins
ҩ Barbara Coltman
ҩ Frank & Dorothy Colwell
ҩ BB & SH Cooling
ҩ Kevin J Cosgrave
ҩ Mark Costello
ҩ Geoffrey Cottrell
ҩ John Coughlan
ҩ Peter Coughlan
ҩ Sue Course
ҩ Helen Cowan
ҩ Damian Coyle
ҩ Richard Coyne
ҩ Patrick Crabb
ҩ Irene Crebbin
ҩ Pamela Crosthwaite
ҩ Wendy Crouch
ҩ Kathy Cunico
ҩ Stephen Curwood
ҩ CWA Noble Park
Branch
ҩ The Daniele Family
ҩ June Danks
ҩ Philip E Darby
ҩ L Gordon Darling
AC CMG & Marilyn
Darling
ҩ Barbara Darvall
ҩ Arthur & Anne Davey
ҩ Geoffrey Davey
ҩ Joan Davidson
ҩ Ann De Paul
ҩ S Deeves
ҩ Eril Deighton
ҩ Matthew Delasey
ҩ Nigel Desousa
ҩ Jacob H Dessauer
ҩ Mark Dick
ҩ Gloria Dickins
ҩ Douglas & Marion Dickinson
ҩ J & D Dixon
ҩ Vanessa Djouma
ҩ Prof Peter C Doherty
AC
ҩ Billie Donaldson
ҩ Elizabeth Donnan
ҩ Julia Donnan
ҩ Sarah Donnan
ҩ Patricia Donovan
ҩ Kiah Doodie
ҩ David Dowdle
ҩ Tania Dowell
ҩ J Michael Dowling
ҩ K & M Doyle
ҩ Rosemary Drenen
ҩ Alison Driver
ҩ Craig M Drummond
ҩ Marlene Dryen
ҩ Jan Dunbar
ҩ Kimiko Duncan
ҩ Marcia Duncan
ҩ P S Durham
ҩ Andrew R Dyer
ҩ Dysons Group of Companies
ҩ East Family Trust
ҩ Geoffrey & Pauline Eastmure
ҩ Ethel Edwards
ҩ Marie Einoder
ҩ Gary & Alex Elliott
ҩ Peg Elmore
ҩ Eltham Wildcats
ҩ Zelman & Diana Elton
ҩ Joseph Epstein
ҩ Equity Trustees Limited
ҩ Andrew Erikson
ҩ Essendon Football Club
Past Players & Officials Association
ҩ Estate of Marian R Eskdale
ҩ Estate of Marjory Mary Jackson
ҩ Estate of Mary Eva Kentish
ҩ Estate of Diane Adrienne Lemaire
ҩ Estate of Joan Mary McPherson
ҩ Estate of Gertrude Silberberg
ҩ John & Carolien Evans
ҩ Genevieve Fahey
ҩ Julie Fairhurst
ҩ Evelyn Fawcett
ҩ David Feldman
ҩ Kevin Feltis
ҩ Janine & Graeme Ferguson
ҩ Tony Fisher
ҩ Roger B Flynn
ҩ Anne Foote
ҩ Robin Foote
ҩ Arthur W Ford
ҩ David Forrest
ҩ Isolde Forstmanis
ҩ Fiona French
ҩ Shirley Fricke
ҩ Fred Frohlich
ҩ Jude Fraser
ҩ A K Fryday
ҩ Prof Peter J Fuller AM
ҩ Gemma Furtado
ҩ Russell & Jill Fynmore
ҩ Joan Gabb
ҩ Patricia Galbraith
ҩ Dorothy M Gannon
ҩ Neilma Gantner
ҩ Garry Martin & Associates
ҩ Rosemary Geer
ҩ R Gehrig
ҩ Geoff & Helen Handbury Foundation
ҩ Lesley Gerrish
ҩ C Norman Geschke OBE
ҩ Roshan Ghadamian
ҩ Tess Gibbs
ҩ Peter Gilbertson
ҩ Brian & Jan Gill
ҩ Jenny Gillin
ҩ Shirley Gionfriddo
ҩ Clare Gleeson-McGuire
ҩ Andrea Goldsmith
ҩ Isabel Goodwin
ҩ R N Gotch
ҩ Louise Gourlay OAM
ҩ Anna Gouttman
ҩ Debbie Grace
ҩ M R Grady
ҩ E M Graham
ҩ Susan V Grant
ҩ Ian Gray
ҩ Peter & Colette Gray
ҩ Janine Greenfield & Peter Coe
ҩ Ken & Ella Griffin
ҩ Lesley Griffin
ҩ Betty Griffiths
ҩ James Guest
ҩ H & K Johnston Family Foundation
ҩ John Haasz
ҩ Ronda Hall
ҩ Shirley Hallows
ҩ Karen Hamilton
ҩ Olive Hamilton
ҩ Kylie Hammon
ҩ Sylvia Hammond
ҩ J Arnold Hancock OBE
ҩ James Hancock
ҩ Justice Hartley R Hansen
ҩ Roy Hardcastle
OUR DONORS
We appreciate the generosity of our donors and would like to especially thank those who have given $100 or more during 2014
ҩ Joan M Harman
ҩ Harold Mitchell Foundation
ҩ Yvonne Harper
ҩ Richard Harrison
ҩ T Hart
ҩ Beth Harvey
ҩ Kathleen Hatch
ҩ Marianne Haughton
ҩ Annette Hayes
ҩ Helen Haynes
ҩ Philip Hayes
ҩ Brian Head
ҩ Michael Healy
ҩ Bill Heape
ҩ Kate Hannan
ҩ Peta Heffernan
ҩ Heidelberg United Soccer Club
ҩ Elizabeth M Heinze
ҩ Gordon Hellsten
ҩ Wilma J Herschell
ҩ Margaret Heselev
ҩ Alison Higgins
ҩ Alistair Hillier
ҩ Patricia Hillman
ҩ W E Hirst
ҩ Pamela Hitchcock
ҩ John & Bev Hodgkisson
ҩ Judith Holden
ҩ Holy Rosary School
ҩ I Y Hoolihan
ҩ Grant Hooper
ҩ Peter Horwood
ҩ Roger Hudson
ҩ Stuart & Donna Hutchinson
ҩ Keith R Hutchison
ҩ Pieter Huveneers
ҩ Joan E Ikin
ҩ Inner Wheel District 62
ҩ George Ioannidis
ҩ Lewis Ioannidis
ҩ Gail Ireland
ҩ Wynnifried Ireland
ҩ Jean Jackson
ҩ David Jacobson
ҩ George R & J M James
ҩ Robert Jamieson
ҩ Elcahnan H Januszewicz
ҩ Andrea Jeffress
ҩ David & Lois Jenkin
ҩ Barbara E Jenkins
ҩ Nola Jennings
ҩ Brian Jessop
ҩ Cecily Johns
ҩ L & R Johnson
ҩ Campbell & Caroline Johnston
ҩ Peter Johnston
ҩ Elizabeth Judd
ҩ H Karsen
ҩ D & M Kaufmann
ҩ Peter Kelly
ҩ Anne Kelso AO
ҩ Jennifer Kelso
ҩ Max & Wilma Kemp
ҩ Stephen Kenmar
ҩ Joseph Kiers
ҩ Wesley Kilham
ҩ Pamela Killer
ҩ Robyn Kingston
ҩ Reinhold Kinkel
ҩ Terry & Janet Kinsella
ҩ A J Kirkham AM
ҩ John Kirkhope
ҩ Paul Kirton
ҩ LyndaJane Kiszczak
ҩ Val Knaggs
ҩ Rodney & Beverley Knight
ҩ John & Rosemary Knorr
ҩ Pam Knott
ҩ Donald E Knox
ҩ Jean Knox
ҩ Michelle Kovacevic
ҩ B C Krkach
ҩ Geoff Kroker
ҩ Marie Ku
ҩ Steven Kunstler
ҩ David V La Fontaine
ҩ Damian La Rosa
ҩ John Landy AC CVO
MBE
ҩ Doug J Lane
ҩ Anne Lang
ҩ Susan Lang
ҩ Gina Langlands
ҩ Fay Langstaff
ҩ Prof Frank P Larkins
ҩ Louise Laskey
ҩ David & Val Lawrence
ҩ Judith Lawrence
ҩ Emilia Lawson
ҩ Alexander Lazare
ҩ James Lees
ҩ Peter Lemon
ҩ W I Leslie
ҩ Lewis Lester
ҩ Alissa Lever
ҩ Patricia Levy
ҩ John Lippmann OAM
ҩ Brian Little
ҩ Algirdas E Liubinas
ҩ Lorna Lockhart
ҩ N Loizou
ҩ Elizabeth Loorham
ҩ Elisabeth N Lord
ҩ Anna Louey
ҩ Pearl Lubansky
ҩ Judith Lukeis
ҩ Frances Luxon
ҩ Doreen Lydeamore
ҩ Heather MacEachern
ҩ Michael Macgeorge
ҩ Christine MacGill
ҩ James MacKenzie
ҩ Macquarie Bank
ҩ Macquarie Group Foundation
ҩ R & M Maeder
ҩ Lescil G Maidment
ҩ Dominic Mammone
ҩ Elaine Mann
ҩ Joan Mann
ҩ Greg Marchio
ҩ Ian J Marks
ҩ Prof Marjory-Dore Martin
ҩ Amanda Mason
ҩ Heather Mason
ҩ Carol Matthews
ҩ David Matthews
ҩ Jean Matthews
ҩ M McAllister
ҩ Darryl McAndrew
ҩ Peter McArdle
ҩ Robert McCallum
ҩ Adele McCarthy
ҩ Vivien McCarthy
ҩ J McCauley
ҩ Ian W McCay
ҩ Prudence McColl
ҩ Andrew McComas
ҩ Karen McComiskey
ҩ V McCormack
ҩ Denys McCullough
ҩ John & Bebe McEnroe
ҩ Zina McEnnally
ҩ Marion McEwin
ҩ Emmeline McFadyen
ҩ David McFarlane
ҩ Ann C McFarling
ҩ Katherine McGloin
ҩ A J McIntosh
ҩ Noeleen McKenry
ҩ Bruce McKenzie
ҩ Matthew McKevitt
ҩ Elizabeth A McLaren
ҩ Muriel Patricia McLaren
ҩ Raymond J McLean
ҩ Betty Meadows
ҩ Janice Medway
ҩ Bernadete Meehan
ҩ Denise Meehan
ҩ Peter Meehan
ҩ R J & W D Meggs
ҩ Graham A Menzies
ҩ John & Marilyn Meyer
ҩ John Michaux
ҩ Judith Middlemass
ҩ Roslynne Milne
ҩ Heather J Mitchell
ҩ John Mitchell
ҩ Ian Moffat
ҩ Graeme & Audrey Moir
ҩ Margaret Moller
ҩ Gillian Montgomery ҩ Judith Moore
ҩ Kate Josephine Moore
ҩ Cynthia Morgan
ҩ G A & C R Morgan ҩ James & Alex Morgan
ҩ Leigh Morgan
ҩ Suzanne Morgan
ҩ Steve Moriarty
ҩ Geoffrey Morley
ҩ Richard Moshinsky
ҩ Toula Mossman
ҩ N Beverley Mower
ҩ Hazel Moyes
ҩ MP Burke Commercial Real Estate
ҩ Jennifer Mullen
ҩ Bruce & Judy Munro
ҩ Richard A Munt
ҩ J M Muntz
ҩ Rupert Murdoch AC
ҩ David Murrie
ҩ My Cause Gift Fund
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 108 109
ҩ Tania Nahum
ҩ Dennis & Fairlie Nassau
ҩ J and S Nathar
ҩ Frank Naylor
ҩ Gary Neilsen
ҩ Nell & Hermon Slade Trust
ҩ Mark A Nelson
ҩ Belinda Nemec
ҩ Danilo Nemec
ҩ Alison Nghiem
ҩ Linda Nguyen
ҩ John & June NixonSmith
ҩ George Nolan
ҩ Helen Nolan
ҩ Robert Nossbaum
ҩ Aileen Oberegger
ҩ Doris Enid O’Brien
ҩ Rosemary O’Connell
ҩ Sue Ohl
ҩ Kerry O’Meara
ҩ One in Five Assoc Inc
ҩ Sue O’Neill
ҩ Mona Osborne
ҩ Christopher O’Shaughnessy
ҩ Damien O’Shea
ҩ Laura & Tom O’Shea
ҩ Marcia O’Shea
ҩ Mary O’Sullevan
ҩ Judith A Overbeek
ҩ Alan Paddon
ҩ Pakenham Opportunity Shop
ҩ Ashley J Parker
ҩ Elizabeth Parker
ҩ Ann C Parris
ҩ Ingrid Parsons
ҩ Dennis Patisteas OAM
ҩ Patsy Patten
ҩ Janet Pavlakis
ҩ K Pearson
ҩ Diana Peatt
ҩ Nigel Peck AM & Patricia Peck
ҩ Perpetual Trustees
Australia Ltd
ҩ Peter Moyes Anglican Community School
ҩ Rhonda Peters
ҩ Kate Phelan
ҩ Mary Phiddian
ҩ Ian R Phillips
OUR DONORS
We appreciate the generosity of our donors and would like to especially thank those who have given $100 or more during 2014
ҩ Catherine Philps
ҩ G C A Pianezzola
ҩ Dorothy Pill
ҩ Gwenda Pillar
ҩ David Pincus
ҩ Donald Pinkerton
ҩ Ron G Pitcher AM
ҩ Margaret Pitt
ҩ Renate Porter
ҩ Norah Powell
ҩ Ed Prendergast
ҩ Preshil School Community
ҩ Joan Preston
ҩ Leonard Price
ҩ Valentine John Price
ҩ Michael Pring
ҩ Katherine Psomas
ҩ Suzanne Pullen
ҩ Kalli Pulos
ҩ Andrew Purcell
ҩ John Quinn
ҩ John T Ralph AC
ҩ Alan Ramsay
ҩ Tannia Recinos
ҩ Reece Australia Ltd
ҩ Lydie Reeve
ҩ Ann Reid
ҩ Ralph & Ruth Renard
ҩ Donald Rewell
ҩ Joyce E Risstrom
ҩ Ritchies Victoria Pty Ltd
ҩ Helen Rix
ҩ Judy Roach
ҩ Rachel Robbins
ҩ Christina Roberts
ҩ Ann Robertson
ҩ RobMeree Foundation
ҩ Sara Roe
ҩ Ivy E Roebuck
ҩ David W Rogers
ҩ Gwen Rogers
ҩ Kitty Rohan
ҩ Nicola Rollerson
ҩ Damien & Katherine Rollinson
ҩ Freda Rossidis
ҩ Rotary Club of Canterbury
ҩ Rotary Club of Gisborne
ҩ Leonard Rouch
ҩ Marie Rundell
ҩ Russell Family Trust
ҩ Jennifer Ruthen
ҩ Ron Sacks-Davis
ҩ Owen James Saleeba
ҩ Mohd Salim
ҩ Salini & Mrzljak Families
ҩ S Samra
ҩ Dorothy Sams
ҩ Samuel & Sadie Mir Foundation
ҩ Donald N Sanders AO
ҩ Paul Sandford
ҩ Lauren Sanford
ҩ Sanitarium
ҩ Norman Sargeant
ҩ Joyce Savage
ҩ Richard Sawers
ҩ Say Family Foundation
ҩ Jane Schmeiszl
ҩ Shirley Scholes
ҩ Donald Scott
ҩ Frances Seidel
ҩ Ian Seymour
ҩ Ed & Judy Shadbolt
ҩ John & Pamela Sharwood
ҩ David Shaw
ҩ Gwen Shaw
ҩ Keith & Frances Shaw
ҩ Andrew Shenton
ҩ Prof Roberta Shepherd
ҩ Neil Shotton
ҩ Siky & Alexandra Siapantas
ҩ Alfonsina Sicari
ҩ Sid & Fiona Myer Family Foundation
ҩ Susan Sims
ҩ Robert & Elisabeth Sinclair
ҩ Steven & Lousje Skala
ҩ Sandra Sloane
ҩ Justine Sloane-Lees
ҩ Isabel M Sloman
ҩ Betty M Smith
ҩ Carol A Smith
ҩ Keith S & M Smith
ҩ Valerie E Smith
ҩ Adrian & Jennifer Smithers
ҩ Annette Smorgon
ҩ Marie Smyth
ҩ Graham Snell
ҩ Joy Sorel
ҩ Southern Peninsula Masters Athletics Venue
ҩ Pauline Speedy
ҩ Shirley Spinks
ҩ Leo Spivak
ҩ Alex Spremic
ҩ Barrie Squires
ҩ Donna Srivastava
ҩ Staff of Level 2 Mercy Place
ҩ Tony & Barbara Standish
ҩ Christine Starcevic
ҩ Ralph & Maureen Stavely
ҩ A W Stephens
ҩ P Stephenson
ҩ Russell Stewart
ҩ Gary Stiliano
ҩ Betty Stinson
ҩ Robert Stinson
ҩ Susie Stock
ҩ Caroline Storm
ҩ Fay Strachan
ҩ Richard Stradwick
ҩ Stephen Stuart
ҩ Takako M Subocz
ҩ Greg Sullings
ҩ Lyndall Sullivan
ҩ Celia Sutterby
ҩ Elizabeth Joan Swain
ҩ Peter Swan OAM
ҩ The late Robyn Swanson
ҩ Christine R Sweeney
ҩ Sylvia and Charles Viertel Charitable Foundation
ҩ Debbie Tacey
ҩ Gregory & Wendy Taggart
ҩ Angelo Taranto
ҩ John Targett
ҩ Ann Tauber
ҩ Anne Taylor
ҩ Bernadette Taylor
ҩ John Taylor
ҩ Margaret Taylor
ҩ Robin Taylor
ҩ Wendy Taylor
ҩ Telstra Community Program
ҩ Texas Biomedical
Research Institute
ҩ Kathryn Thaniel
ҩ The Abercrombie Family Foundation
ҩ The CASS Foundation Ltd
ҩ The Clive & Vera Ramaciotti Foundation
ҩ The Dowd Foundation
ҩ The Eirene Lucas Foundation
ҩ The Finkel Foundation
ҩ The Fox Family Foundation
ҩ The G W Vowell Foundation Ltd
ҩ The Grocer
ҩ The Ian Potter Foundation
ҩ The Ideal Consultancy
ҩ The Isabel & John Gilbertson Charitable Trust
ҩ The Landman Foundation
ҩ The Leo & Mina Fink Fund
ҩ The Marchris Family Charitable Gift
ҩ The Michael J Fox Foundation
ҩ The Miller Foundation
ҩ The Myer Foundation
ҩ The Patricia Dukes Foundation
ҩ The Stuart Leslie Foundation
ҩ The William Angliss (Vic) Charitable Fund
ҩ The Yulgilbar Foundation
ҩ Thiess Pty Ltd
ҩ Jean Thomas
ҩ Linda Thomas
ҩ Peter & Debbie Thomas
ҩ Karlene Thompson
ҩ The late William D Thorn
ҩ Robert C Thrower
ҩ John & Lesley Thrum
ҩ Andrew Tinney
ҩ Arthur Topalidis
ҩ Joan Tornatora
ҩ Lynette Torrens
ҩ Norma Torriero
ҩ Marie Touzeau
ҩ Evelyn Townsend
ҩ Donald Tregonning
ҩ Gad Trevaks AM
ҩ E Trevena
ҩ Michael Troy
ҩ Trust Company Ltd
ҩ Eugenia Tsaclidis
ҩ Kay Tudor
ҩ Joan Tullo
ҩ Bruce Turnbull
ҩ Valerie Turnbull
ҩ Maureen Turner
ҩ Patricia Turner
ҩ U3A Bairnsdale & District Inc
OUR DONORS
We appreciate the generosity of our donors and would like to especially thank those who have given $100 or more during 2014
ҩ UBS Foundation
ҩ David Uhr-Henry
ҩ Margaret J Ullin
ҩ Urbis Pty Ltd
ҩ Eleanor U’Ren
ҩ Robert Utter
ҩ P M van Reesema
ҩ David Vernon
ҩ Victorian Polo Club
ҩ Dorothy Viskovic
ҩ Rain Vojcisek
ҩ Rev Paul R Voumard
ҩ Michael Walker
ҩ Colin & Mary Wallace
ҩ Stan Wallis AC &
Judith Wallis
ҩ Ralph Ward-Ambler AM & Barbara WardAmbler
ҩ Liz & Andy Wardlaw
ҩ Warringal Financial Services Pty Ltd
ҩ Carole Wastell
ҩ Brian Waters
ҩ Barbara Watson
ҩ Donald Watson
ҩ Margaret E Watson
ҩ Kathryn Watty
ҩ Gwen Webb OAM
ҩ Jean M Webster
ҩ Philip Weickhardt
ҩ Marjorie White
ҩ Trevor & C P White
ҩ Helen Whyte
ҩ Kaaren Whyte
ҩ Prof James S Wiley
ҩ Sally Wilkinson
ҩ Paul A Willee QC
ҩ Harry Williams
ҩ Keith Williams
ҩ Noel Williams
ҩ Vera Williamson
ҩ Tony Willigen
ҩ Dennis Willis
ҩ Andrea Wills
SUPPORTING RESEARCH
We gratefully acknowledge the tireless efforts of people in the community who raise funds to support our research, and thank the donors who have supported them
ҩ Cameron Willsher
ҩ Elizabeth Wilson
ҩ Margaret Wilson
ҩ Marie Wilson ҩ Sineke Winter ҩ Timothy Winter ҩ Claire Withers
ҩ Arnold Wittner
ҩ Frances Wood
ҩ Meredith Woods
ҩ Woorinyan Country Womens Association
ҩ Hilary Wright
ҩ Michael Yates
ҩ Peter Zwar
ҩ One in Five
ҩ Alex & Karl Waddell – River’s Gift
ҩ Aaliyah’s Fund
ҩ Crown
ҩ Staging Connections
ҩ Samsung
ҩ WHYBIN\TBWA
ҩ Perfect Events
ҩ Guillaume Brahimi
ҩ Darren Robertson
ҩ Brent Savage
ҩ James Viles Scott Pickett
ҩ WHYBIN\TBWA
ҩ Linklaters
ҩ Andrew and Claire Heenan –Cycling for Ben
ҩ City2Sea runners
ҩ Kingsley Just - Roll for Research
IN-KIND SUPPORTERS
ҩ Prof Mal Horne
ҩ Ian Davis
SUPPORTERS
AND
ҩ Matt Harry – Marathon for the mind
ҩ Annie Smithers – Christmas dinner auction
ҩ Prof Fred Mendelsohn AO
SPONSORS OF OUR CULINARY CHARITY CHALLENGE
ҩ John Lawson
ҩ Stuart McVeigh
ҩ Pierre Reolofs
ҩ Jacques Reymond
ҩ Janne Apelgren
ҩ Anthony Dennis
ҩ Larissa Dubecki
ҩ Joanna Savill
ҩ Michael Harden
ҩ Felice Marandola
ҩ James Madden
ҩ Julia Zemiro
ҩ Daniel Vaughan
ҩ Treasury Wines
ҩ Sanpellegrino
ҩ Ocean Made Seafood
ҩ Felice’s Place
Gourmet Butcher
ҩ Peter Wynne
ҩ Pacific Broadcasting
ҩ Carpet Court and Cavalier Bremworth Carpets
ҩ Will Hanigan
ҩ Signed and Framed
ҩ Southern Star
ҩ Peninsula Hot Springs
ҩ Magenta Shores
ҩ Yering Station
ҩ Karcher
ҩ Neil Perry
ҩ Adele Ferguson & John Silvester
ҩ Sofitel
ҩ Montalto and Pommery
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 110 111
PUBLICATIONS
CHAPTER BRAIN DEVELOPMENT AND REGENERATION
PAGES 40–42
CHAPTER BEHAVIOURAL NEUROSCIENCE
PAGES 46–51
1. Britto, J.M., Tait, K.J., Lee, E.P., Gamble, R.S. Hattori, M. and Tan, S-S (2014) Exogenous reelin modifies the migratory behavior of neurones depending upon cortical location. Cereb. Cortex 24:2835-2847
2. Hammond, V.E., Gunnersen, J.M., Goh, C.P., Low, L.H., Hyakumura, T., Tang, M.M., Britto, J.M., Putz, U., Howitt, J.A., and Tan, S-S (2014) Ndfip1 is required for the development of pyramidal neurone dendrites and spines in the neocortex.Cereb. Cortex 24:3289-3300
3. Goh, C.P., Putz, U., Howitt, J., Low, L.H., Gunnersen, J., Bye, N., Morganti-Kossmann, C. and Tan, S-S. (2014) Nuclear trafficking of Pten after brain injury leads to neurone survival not death Exp. Neurol 252:37-46
4. Nivison-Smith, L., Chua, J., Tan, S-S, and Kalloniatis, M. (2014) Amino acid signatures in the developing mouse retina. Int J. Dev. Neurosci. 33C:62-80
5. Howitt, J., Gybers, A., Ayton, S., Carew-Jones, F., Putz, U., Finkelstein, D., Halliday, G., and Tan, S-S. (2014). Increased Ndfip1 in the substantia nigra of Parkinsonian brains is associated with elevated iron levels. Plos One 9(1):e87119.
6. Altin, J.A., Daley, S.R., Howitt, J., Rickards, H.J., Batkin, A.K., Horikawa, K., Prasad, S.J., Nelms, K.A., Kumar, S., Wu, L.C., Tan, S-S., Cook, M.C., and Goodnow, C.C. (2014) Ndfip1 mediates peripheral tolerance to self and exogenous antigen by inducing cell cycle exit in responding CD4+ T cells. Proc Natl Acad Sci (USA) 111:2067-2074
7. Li, Y., Low, L.H., Putz, U., Goh, C.P., Tan, S-S, and Howitt, J. (2014) Rab5 and Ndfip1 are involved in Pten ubiquitination and nuclear trafficking. Traffic 15:749-761 (cover feature)
1. Murphy, B.P., Pang, T.Y., Hannan, A.J., Proffitt, T-M., McConchie, M., Kerr, M., Markulev, C., O’Donnell, C., McGorry, P.D., Berger, G.E. (2014) Vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) in quetiapine treated first-episode psychosis. Schizophrenia Research and Treatment 2014: 719395 [Epub 2014 Feb 5].
2. Hannan, A.J. (2014) Environmental enrichment and brain repair: Harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathology and Applied Neurobiology 40: 13-25 (invited article for Annual Review Issue).
3. Loy, C.T. and Hannan, A.J. (2014) Neurotoxicity in Huntington Disease. In: Handbook of Neurotoxicity, Springer (invited book chapter).
4. Evans-Galea, M.V., Lockhart, P.J., Galea, C.A., Hannan, A.J., Delatycki. M.B. (2014). Beyond loss of frataxin: the complex molecular pathology of Friedreich ataxia. Discovery Medicine 17:25-35.
5. Mo, C., Pang, T.Y., Ransome, M., Renoir, T., Hannan, A.J. (2014) High stress hormone levels accelerates the onset of memory decline in male Huntington’s disease mice. Neurobiology of Disease 69:248-262 [Epub 2014 May 10].
6. Lim, N.K.H., Pang, T.Y., McLean, C.A., Liddell, J.R., Hilton, J.B., Li, Q.-X., White, A.R., Hannan, A.J., Crouch, P.J. (2014) Localised changes to glycogen synthase kinase-3 and collapsin response mediator protein-2 in the Huntington’s disease affected brain. Human Molecular Genetics 23: 4051-4063 23: 4051-4063 [Epub 2014 Mar 14].
7. Hill, R.A., Klug, M., Von Soly, S.K., Binder, M.D., Hannan, A.J., van den Buuse, M. (2014) Sex-specific disruptions in spatial memory and anhedonia in a ‘two hit’ rat model correspond with alterations in hippocampal brainderived neurotrophic factor expression and signalling. Hippocampus 2014;24(10):1197-211.
8. McOmish, C.E., Burrows, E.L. and Hannan, A.J. (2014) Identifying novel interventional strategies for psychiatric disorders: Integrating genomics, ‘enviromics’ and gene-environment interactions in valid preclinical models. British Journal of Pharmacology 171: 4719-4728 (invited review for Special Issue on ‘Animal models of psychiatric disorders’).
9. Tomas D, Prijanto A, Burrows EL, Hannan AJ, Horne MK, Aumann TD (2014) Environmental modulations of the number of midbrain dopamine neurons in adult mice. J. Vis. Exp. (accepted July 2014).
10. Mo, C., Renoir, T., Hannan, A.J. (2014) Ethological endophenotypes are altered by elevated stress hormone levels in both Huntington’s disease and wildtype mice. Behavioural Brain Research 274: 118-127.
11. Mo, C., Renoir, T., Hannan, A.J. (2014) Effects of chronic stress on the onset and progression of Huntington’s disease in transgenic mice. Neurobiology of Disease 71: 81-94.
12. Hill, R.A, Ratnayake, U., Kiss von Soly, S., Klug, M., Binder, M.D., Hannan, A.J., Van Den Buuse, M (2014) Longterm effects of combined neonatal and adolescent stress on brain-derived neurotrophic factor and dopamine receptor expression in the rat forebrain. Biochim. Biophys. Acta 1842: 2126-2135.
13. Mazarakis NK, Mo C, Renoir T, van Dellen A, Deacon R, Blakemore C, Hannan AJ (2014) ‘Super-enrichment’ reveals dose-dependent therapeutic effects of environmental stimulation in a transgenic mouse model of Huntington’s disease. J. Huntington’s Dis. 3:299-309.
14. Renoir T, Argyropoulos A, Chevarin C, Lanfumey L, Hannan AJ (2014) Sexually dimorphic dopaminergic dysfunction in a transgenic mouse model of Huntington’s disease. Pharmacol. Biochem. Behav. 127C:15-20 [Epub 12 Oct. 2014].
15. Mo, C. Renoir, T., Hannan, A.J. (2014) Novel ethological endophenotypes in a transgenic mouse model of Huntington’s disease. Behavioral Brain Research 276:17-27 (Epub 2014 Apr 18; invited article for Special Issue on ‘Neuropsychiatric spectra’).
16. Du X, Leang L, Mustafa T, Renoir T, Pang TYC, Hannan AJ (2014) The influence of the HPG axis on stress response and depressive-like behaviour in a transgenic mouse model of Huntington’s disease. Exp. Neurol. 263:63-71 [Epub 20 Sep. 2014].
17. Spanagel R, Vengeliene V, Jandeleit B, Fischer WF, Grindstaff K, Zhang X, Gallop M, Krstew EV, Lawrence AJ & Kiefer F (2014) Acamprosate produces its anti-relapse effects via calcium. Neuropsychopharmacology, 39, 783-791.
18. Duncan JR, Gibbs SJ & Lawrence AJ (2014) Chronic intermittent toluene inhalation in adolescent rats alters behavioural responses to amphetamine and MK801. Eur. Neuropsychopharmacol., 24, 480-486
19. Dick AL, Axelsson, M, Lawrence AJ* & Duncan JR* (2014) Specific impairments in instrumental learning following chronic intermittent toluene inhalation in adolescent rats. Psychopharmacology, 231, 1531-1542. * denotes co-corresponding authors.
20. Wang YT, Qin WJ, Liu Q, Li YL, Liang H, Chen F, Lawrence AJ, Zhang XL, Liang JH (2014) Chaperone heat shock protein 70 in nucleus accumbens core: a novel biological target of behavioural sensitization to morphine in rats. Int. J. Neuropsychopharmacol., 17, 469-484.
21. Bird MK, Lohmann P, West B, Brown RM, Kirchhoff J, Raymond CR & Lawrence AJ (2014) The mGlu5 receptor regulates extinction of cocaine-driven behaviors. Drug Alc. Depend., 137, 83-89.
22. Handford CE, Tan S, Lawrence AJ & Kim JH (2014) The effect of the mGlu5 negative allosteric modulator MTEP and NMDA receptor partial agonist D-cycloserine on Pavlovian conditioned fear. Int. J. Neuropsychopharmacol., 17, 1521-1532.
23. Dick AL, Lawrence AJ* & Duncan JR* (2014) Chronic intermittent toluene inhalation initiated during adolescence in rats does not alter voluntary consumption of ethanol in adulthood. Alcohol, 48, 561-569. * denotes cocorresponding authors.
24. Ryan PJ, Krstew EV, Sarwar M, Gundlach AL & Lawrence AJ (2014) Relaxin-3 mRNA levels in nucleus incertus correlate with alcohol and sucrose intake in rats. Drug Alc. Depend., 140, 8-16.
25. Ash BL, Quach T, Williams SJ, Lawrence AJ & Djouma E (2014) Galanin-3 receptor antagonism by SNAP 37889 reduces motivation to self-administer alcohol and attenuates cue-induced reinstatement of alcohol-seeking in iP rats. J. Pharmacol. Sci., 125, 211-216.
26. Li YL, Liu Q, Gong Q, Li JX, Wei SP, Wang YT, Liang H, Zhang M, Jing L, Yong Z, Lawrence AJ & Liang JH (2014) Brucine suppresses ethanol intake and preference in alcohol-preferring Fawn-Hooded rats. Acta Pharmacol Sin., 35, 853-861.
27. Chen NA, Jupp B, Sztainberg Y, Lebow M, Brown RM, Kim JH, Chen A & Lawrence AJ (2014) Knockdown of CRF1 receptors in the ventral tegmental area attenuates cue- and acute food deprivation stress-induced cocaineseeking in mice. J. Neurosci., 34, 11560 –11570 (featured in “This week in the journal”).
28. Darwinkel A, Stanic D, Booth LC, May CN, Lawrence AJ & Yao ST (2014) Distribution of orexin-1 receptor-green fluorescent protein- (OX1-GFP) expressing neurons in the mouse brain stem and pons: co-localisation with tyrosine hydroxylase and neuronal nitric oxide synthase. Neuroscience, 278, 253–264 (front cover illustration).
29. Ganella DE, Thangaraju P, Lawrence AJ & Kim JH (2014) Fear extinction in 17 day old rats is dependent on metabotropic glutamate receptor 5 signaling. Behav. Brain Res. 2014 Dec 10. pii: S0166-4328(14)00795-5.
30. Perry C, Zbukvic I, Kim JH & Lawrence AJ (2014) The role of cues and contexts on drug-seeking behaviour. Br. J. Pharmacol., 171, 4636-4672.
31. Kim JH & Lawrence AJ (2014) Drugs currently in Phase II clinical trials for cocaine addiction. Expert Opin. Investig. Drugs., 23, 1105-1122.
32. Giles EK, Lawrence AJ & Duncan JR (2014) Exploring the modulation of hypoxia-inducible factor (HIF)-1α by volatile anesthetics as a possible mechanism underlying volatile anesthetic-induced CNS injury. Neurochem. Res., 39, 1640-1647.
33. Lawrence AJ & Cryan JF (2014) Found in translation? Commentary on a BJP themed issue about animal models in neuropsychiatry research. Br. J. Pharmacol., 171, 4521-4523.
34. Veldsman M, Cumming T, Brodtmann A. Beyond BOLD: Optimizing functional imaging in stroke populations. Hum Brain Mapp. 2014 Dec 2. doi: 10.1002/hbm.22711. [Epub ahead of print].
35. Li Q, Lichter, R, Raffelt, A, Werden E, Pardoe H, Cumming T, Brodtmann, A Cortical thickness estimation in longitudinal stroke studies: a comparison of 3 measurement methods NeuroImage:Clinical 2014 Available online 23 August 2014.
36. Brodtmann A. Vascular risk, depression, and stroke: Post hoc ergo propter hoc … or not. Neurology. 2014 Nov 4;83(19):1688-9. doi: 10.1212/WNL.0000000000000968. [Epub 2014 Oct 1].
37. Cumming TB, Brodtmann A, Darby D, Bernhardt J. The importance of cognition to quality of life after stroke. J Psychosom Res. 2014 Nov;77(5):374-9. doi: 10.1016/j.jpsychores.2014.08.009. [Epub 2014 Aug 29].
38. Brodtmann A, Werden E, Pardoe H, Li Q, Jackson G, Donnan G, Cowie T, Bradshaw J, Darby D, Cumming Charting cognitive and volumetric trajectories after stroke: protocol for the Cognition And Neocortical Volume After Stroke (CANVAS) study. T. Int J Stroke. 2014 Aug;9(6):824-8.
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 112 113
39. Borschmann K, Pang MY, Iuliano S, Churilov L, Brodtmann A, Ekinci EI, Bernhardt J. Changes to volumetric bone mineral density and bone strength after stroke: A prospective study. Int J Stroke. 2013 Dec 23. doi: 10.1111/ij
40. Louey AG, Cromer JA, Schembri AJ, Darby DG, Maruff P, Makdissi M, Mccrory P. Detecting cognitive impairment after concussion: sensitivity of change from baseline and normative data methods using the CogSport/Axon cognitive test battery. Arch Clin Neuropsychol. 2014 Aug;29(5):432-41. doi: 10.1093/arclin/acu020. [Epub 2014 May 9].
41. Lim YY, Maruff P, Pietrzak RH, Ellis KA, Darby D, Ames D, Harrington K, Martins RN, Masters CL, Szoeke C, Savage G, Villemagne VL, Rowe CC; AIBL Research Group. Aβ and cognitive change: Examining the preclinical and prodromal stages of Alzheimer’s disease. Alzheimers Dement. 2014 Nov;10(6):743-751.e1. doi: 10.1016/j. jalz.2013.11.005. [Epub 2014 Feb 28].
42. Zhou L, Salvado O, Dore V, Bourgeat P, Raniga P, Macaulay SL, Ames D, Masters CL, Ellis KA, Villemagne VL, Rowe CC, Fripp J; AIBL Research Group. MR-less surface-based amyloid assessment based on 11C PiB PET. PLoS One. 2014 Jan 10;9(1):e84777. doi: 10.1371/journal.pone.0084777. eCollection 2014.
43. Ellis KA, Szoeke C, Bush AI, Darby D, Graham PL, Lautenschlager NT, Macaulay SL, Martins RN, Maruff P, Masters CL, McBride SJ, Pike KE, Rainey-Smith SR, Rembach A, Robertson J, Rowe CC, Savage G, Villemagne VL, Woodward M, Wilson W, Zhang P, Ames D; AIBL Research Group. Rates of diagnostic transition and cognitive change at 18-month follow-up among 1,112 participants in the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing (AIBL). Int Psychogeriatr. 2014 Apr;26(4):543-54. doi: 10.1017/S1041610213001956. [Epub 2013 Nov 20].
44. Moore EM, Mander AG, Ames D, Kotowicz MA, Carne RP, Brodaty H, Woodward M, Boundy K, Ellis KA, Bush AI, Faux NG, Martins R, Szoeke C, Rowe C, Watters DA; AIBL Investigators. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care. 2013 Oct;36(10):2981-7. doi: 10.2337/dc130229. [Epub 2013 Sep 5]. Erratum in: Diabetes Care. 2013 Nov;36(11):3850.
45. Harrington KD, Lim YY, Ellis KA, Copolov C, Darby D, Weinborn M, Ames D, Martins RN, Savage G, Szoeke C, Rowe C, Villemagne VL, Masters CL, Maruff P. The association of Aβ amyloid and composite cognitive measures in healthy older adults and MCI. Int Psychogeriatr. 2013 Oct;25(10):1667-77. doi: 10.1017/S1041610213001087. [Epub 2013 Jul 18].
46. Lim YY, Villemagne VL, Laws SM, Ames D, Pietrzak RH, Ellis KA, Harrington KD, Bourgeat P, Salvado O, Darby D, Snyder PJ, Bush AI, Martins RN, Masters CL, Rowe CC, Nathan PJ, Maruff P; Australian Imaging, Biomarkers and Lifestyle (AIBL) Research Group. BDNF Val66Met, Aβ amyloid, and cognitive decline in preclinical Alzheimer’s disease. Neurobiol Aging. 2013 Nov;34(11):2457-64. doi: 10.1016/j.neurobiolaging.2013.05.006. [Epub 2013 Jun 12].
47. Sahathevan R, Mohd Ali K, Ellery F, Mohamad NF, Hamdan N, Mohd Ibrahim N, Churilov L, Cumming TB. A Bahasa Malaysia version of the Montreal Cognitive Assessment: validation in stroke. Int Psychogeriatr. 2014 May;26(5):781-6. doi: 10.1017/S1041610213002615. [Epub 2014 Jan 28].
48. Kramer SF, Cumming T, Churilov L, Bernhardt J. Measuring activity levels at an acute stroke ward: comparing observations to a device. Biomed Res Int. 2013;2013:460482. doi: 10.1155/2013/460482. [Epub 2013 Oct 27].
49. Bernhardt J, Cumming T. The elephant in the single room debate: keeping patients active. BMJ. 2013 Oct 22;347:f6333. doi: 10.1136/bmj.f6333.
50. Vogel AP, Folker J, Poole ML. Treatment for speech disorder in Friedreich ataxia and other hereditary ataxia syndromes. Cochrane Database Syst Rev. 2014 Oct 28;10:CD008953. doi: 10.1002/14651858.CD008953.pub2.
51. Poole ML, Wee JS, Folker JE, Corben LA, Delatycki MB, Vogel AP. Nasality in Friedreich ataxia. Clin Linguist Phon. 2014 Sep 10:1-13. [Epub ahead of print]
52. Ryan NP, Catroppa C, Cooper JM, Beare R, Ditchfield M, Coleman L, Silk T, Crossley L, Rogers K, Beauchamp MH, Yeates KO, Anderson VA. Relationships between acute imaging biomarkers and theory of mind impairment in post-acute pediatric traumatic brain injury: A prospective analysis using susceptibility weighted imaging (SWI). Neuropsychologia. 2014 Nov 6;66C:32-38. doi: 10.1016/j.neuropsychologia.2014.10.040. [Epub ahead of print]
53. Catroppa C, Crossley L, Hearps SJ, Yeates KO, Beauchamp M, Rogers K, Anderson V. Social and Behavioral Outcomes: Pre-Injury to Six Months following Childhood Traumatic Brain Injury. J Neurotrauma. 2014 Apr 28. [Epub ahead of print]
54. J.R. Duncan, S. Gibbs, A. Lawrence (2014) Chronic intermittent toluene inhalation in rats alters behavioural responses to amphetamine and MK801, European Neuropsychopharmacology, 24(3).:480-6 (IF 4.046)
55. A. Dick, M. Axelsson, A. J. Lawrence, J. R. Duncan (2014) Specific impairments in reinforcement learning following chronic intermittent toluene inhalation in adolescent rats, Psychopharmacology 231:1531-42 (IF 4.06)
56. A. Dick, A. J. Lawrence, J. R. Duncan (2014) Chronic intermittent toluene inhalation initiated during adolescence in rats does not alter voluntary consumption of ethanol in adulthood, Alcohol 48(6):561-9 (IF: 2.53)
57. J. R. Duncan, M. Garland, R. Stark, M. Myers, W. Fifer, M. Yang, H. Kinney, Prenatal Nicotine Exposure Selectively Affects Nicotinic Receptor Expression in Primary and Associative Visual Cortices of the Fetal Baboon, Brain Pathology, accepted May 29th, 2014, Jun 5. doi: 10.1111/bpa.12165. [Epub ahead of print]
58. E. Giles, A. Lawrence, J. Duncan (2014) Exploring the modulation of hypoxia-inducible factor (HIF)-1α by volatile anesthetics as a possible mechanism underlying volatile anesthetic-induced CNS injury, Neurochem Res, 39(9):1640-7 (IF 2.55)
59. E. Black, S. MacLean, J. R. Duncan (2014), Inhalants-what you need to know, Facts sheet for the National Drug and Alcohol Research Centre, Australia.
60. Kim JH, & Lawrence AJ (2014). Drugs currently in phase II trials for cocaine addiction. Expert Opinion On Investigational Drugs. 23: 1105-1122.
61. Ganella DE & Kim JH (2014). Developmental rodent models of fear and anxiety: From neurobiology to pharmacology. British Journal of Pharmacology. 171: 4556-4574.
62. Perry C, Zbukvic I, Kim JH, & Lawrence AJ (2014). The role of cues and contexts on drug-seeking behaviour. British Journal of Pharmacology. 171: 4636-4672.
63. Chen N, Jupp B, Sztainberg Y, Brown RM, Kim JH, Chen A, Lawrence AJ (2014). Knockdown of CRF1 receptors in the ventral tegmental area attenuates cue- and acute food deprivation stress-induced cocaine-seeking in mice. Journal of Neuroscience. 34: 11560-11570. (This week in the journal article)
64. Handford CE, Tan S, Lawrence AJ, & Kim JH (2014). The effects of mGlu5 negative allosteric modulator MTEP and NMDA receptor partial agonist D-cycloserine on Pavlovian conditioned fear. International Journal of Neuropsychopharmacology, 17: 1521-1532.
CHAPTER EPILEPSY
PAGES 54–59
AUSTIN CAMPUS –
1. Ahmed OH, Lee H, Schneiders AG, McCrory P, Sullivan SJ. Concussion and Comedy: No Laughing Matter? Pm&R 2014;6:1071-1072.
2. Archer JS, Warren AEL, Jackson GD, Abbott DF. Conceptualizing lennox-gastaut syndrome as a secondary network epilepsy. Frontiers in neurology 2014;5:225-225.
3. Archer JS, Warren AEL, Stagnitti MR, Masterton RAJ, Abbott DF, Jackson GD. Lennox-Gastaut syndrome and phenotype: Secondary network epilepsies. Epilepsia 2014;55:1245-1254.
4. Bagnall RD, Crompton DE, Cutmore C, Regan BM, Berkovic SF, Scheffer IE, Semsarian C. Genetic analysis of PHOX2B in sudden unexpected death in epilepsy cases. Neurology 2014;83:1018-1021.
5. Berkovic SF, Jackson GD. ‘Idiopathic’ no more! Abnormal interaction of large-scale brain networks in generalized epilepsy. Brain 2014;137:2400-2402.
6. Bhaganagarapu K, Jacksonw GD, Abbott DF. De-noising with a SOCK can improve the performance of eventrelated ICA. Frontiers in Neuroscience 2014;8.
7. Brodtmann A, Werden E, Pardoe H, Li Q, Jackson G, Donnan G, Cowie T, Bradshaw J, Darby D, Cumming T. Charting cognitive and volumetric trajectories after stroke: protocol for the Cognition And Neocortical Volume After Stroke (CANVAS) study. International Journal of Stroke 2014;9:824-828.
8. Carney PW, Jackson GD. Insights into the mechanisms of absence seizure generation provided by EEG with functional MRI. Frontiers in neurology 2014;5:162-162.
9. Carvill GL, Weckhuysen S, McMahon JM, Hartmann C, Moller RS, Hjalgrim H, Cook J, Geraghty E, O’Roak BJ, Petrou S, Clarke A, Gill D, Sadleir LG, Muhle H, von Spiczak S, Nikanorova M, Hodgson BL, Gazina EV, Suls A, Shendure J, Dibbens LM, De Jonghe P, Helbig I, Berkovic SF, Scheffer IE, Mefford HC. GABRA1 and STXBP1: Novel genetic causes of Dravet syndrome. Neurology 2014;82:1245-1253.
10. Coe BP, Witherspoon K, Rosenfeld JA, van Bon BWM, Vulto-van Silfhout AT, Bosco P, Friend KL, Baker C, Buono S, Vissers LELM, Schuurs-Hoeijmakers JH, Hoischen A, Pfundt R, Krumm N, Carvill GL, Li D, Amaral D, Brown N, Lockhart PJ, Scheffer IE, Alberti A, Shaw M, Pettinato R, Tervo R, de Leeuw N, Reijnders MRF, Torchia BS, Peeters H, O’Roak BJ, Fichera M, Hehir-Kwa JY, Shendure J, Mefford HC, Haan E, Gecz J, de Vries BBA, Romano C, Eichler EE. Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nature Genetics 2014;46:1063-1071.
11. Culvenor AG, Lai CCH, Gabbe BJ, Makdissi M, Collins NJ, Vicenzino B, Morris HG, Crossley KM. Patellofemoral osteoarthritis is prevalent and associated with worse symptoms and function after hamstring tendon autograft ACL reconstruction. British Journal of Sports Medicine 2014;48:435-U122.
12. Darby D, Moriarity J, Pietrzak R, Kutcher J, McAward K, McCrory P. Prediction of winning amateur boxers using pretournament reaction times. Journal of Sports Medicine and Physical Fitness 2014;54:340-346.
13. Derry CP, Scheffer IE. Autosomal Dominant Nocturnal Frontal Lobe Epilepsy (ADNFLE) (July 2009, November 2010, April 2012, January 2014). In: Gilman S, editor-in-chief. MedLink Neurology. San Diego: Medlink Corporation. Available at www.medlink.com.
14. Euro E-RESC, Epilepsy Phenome/Genome P, Epi KC. De novo mutations in synaptic transmission genes including DNM1 cause epileptic encephalopathies. American journal of human genetics 2014;95:360-370.
15. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J, Jr., Forsgren L, French JA, Glynn M, Hesdorffer DC, Lee BI, Mathern GW, Moshe SL, Perucca E, Scheffer IE, Tomson T, Watanabe M, Wiebe S. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia 2014;55:475-482.
16. Flanagan D, Badawy RAB, Jackson GD. EEG-fMRI in focal epilepsy: Local activation and regional networks. Clinical Neurophysiology 2014;125:21-31
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 114 115
17. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J, Jr., Forsgren L, French JA, Glynn M, Hesdorffer DC, Lee BI, Mathern GW, Moshe SL, Perucca E, Scheffer IE, Tomson T, Watanabe M, Wiebe S. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia 2014;55:475-482.
18. Flanagan D, Badawy RAB, Jackson GD. EEG-fMRI in focal epilepsy: Local activation and regional networks. Clinical Neurophysiology 2014;125:21-31.
19. Franklyn-Miller A, Bilzon J, Wilson C, McCrory P. Can RSScan footscan() D3D software predict injury in a military population following plantar pressure assessment? A prospective cohort study. Foot (Edinburgh, Scotland) 2014;24:6-10.
20. Gardner A, Iverson GL, McCrory P. Chronic traumatic encephalopathy in sport: a systematic review. British Journal of Sports Medicine 2014;48:84-U88.
21. Guandalini M, Butler A, Mandelstam S. Spectrum of imaging appearances in Australian children with central nervous system hemophagocytic lymphohistiocytosis. Journal of Clinical Neuroscience 2014;21:305-310.
22. Hildebrand MS, Damiano JA, Mullen SA, Bellows ST, Oliver KL, Dahl H-HM, Scheffer IE, Berkovic SF. Glucose metabolism transporters and epilepsy: Only GLUT1 has an established role. Epilepsia 2014;55:E18-E21.
23. Hildebrand MS, Damiano JA, Mullen SA, Bellows ST, Scheffer IE, Berkovic SF. Does variation in NIPA2 contribute to genetic generalized epilepsy? Human Genetics 2014;133:673-674.
24. Hinman RS, McCrory P, Pirotta M, Relf I, Forbes A, Crossley KM, Williamson E, Kyriakides M, Novy K, Metcalf BR, Harris A, Reddy P, Conaghan PG, Bennell KL. Acupuncture for Chronic Knee Pain A Randomized Clinical Trial. Jama-Journal of the American Medical Association 2014;312:1313-1322.
25. Int League Epilepsy Consortium C, Grp KS. Genetic determinants of common epilepsies: a meta-analysis of genome-wide association studies. Lancet Neurology 2014;13:893-903.
26. Jamuar SS, Lam A-TN, Kircher M, D’Gama AM, Wang J, Barry BJ, Zhang X, Hill RS, Partlow JN, Rozzo A, Servattalab S, Mehta BK, Topcu M, Amrom D, Andermann E, Dan B, Parrini E, Guerrini R, Scheffer IE, Berkovic SF, Leventer RJ, Shen Y, Wu BL, Barkovich AJ, Sahin M, Chang BS, Bamshad M, Nickerson DA, Shendure J, Poduri A, Yu TW, Walsh CA. Somatic Mutations in Cerebral Cortical Malformations. New England Journal of Medicine 2014;371:733-743.
27. Kim YO, Bellows S, McMahon JM, Iona X, Damiano J, Dibbens L, Kelley K, Gill D, Cross JH, Berkovic SF, Scheffer IE. Atypical multifocal Dravet syndrome lacks generalized seizures and may show later cognitive decline. Developmental Medicine and Child Neurology 2014;56:85-90.
28. Lee H, Sullivan SJ, Schneiders AG, Ahmed OH, Balasundaram AP, Williams D, Meeuwisse WH, McCrory P. Smartphone and tablet apps for concussion road warriors (team clinicians): a systematic review for practical users. Br J Sports Med. 2014 Mar 25. doi: 10.1136/bjsports-2013-092930.
29. Leventer RJ, Jansen FE, Mandelstam SA, Ho A, Mohamed I, Sarnat HB, Kato M, Fukasawa T, Saitsu H, Matsumoto N, Itoh M, Kalnins RM, Chow CW, Harvey AS, Jackson GD, Crino PB, Berkovic SF, Scheffer IE. Is focal cortical dysplasia sporadic? Family evidence for genetic susceptibility. Epilepsia 2014;55:E22-E26.
30. Louey AG, Cromer JA, Schembri AJ, Darby DG, Maruff P, Makdissi M, McCrory P. Detecting Cognitive Impairment After Concussion: Sensitivity of Change From Baseline and Normative Data Methods Using the CogSport/Axon Cognitive Test Battery. Archives of Clinical Neuropsychology 2014;29:432-441.
31. Makdissi M. Sports-related concussion Reply. Australian Family Physician 2014;43:9-10.
32. Makdissi M, Davis G, McCrory P. Updated guidelines for the management of sports-related concussion in general practice. Australian Family Physician 2014;43:94-99.
33. Martin HC, Kim GE, Pagnamenta AT, Murakami Y, Carvill GL, Meyer E, Copley RR, Rimmer A, Barcia G, Fleming MR, Kronengold J, Brown MR, Hudspith KA, Broxholme J, Kanapin A, Cazier J-B, Kinoshita T, Nabbout R, Bentley D, McVean G, Heavin S, Zaiwalla Z, McShane T, Mefford HC, Shears D, Stewart H, Kurian MA, Scheffer IE, Blair E, Donnelly P, Kaczmarek LK, Taylor JC, Consortium WGS. Clinical whole-genome sequencing in severe early-onset epilepsy reveals new genes and improves molecular diagnosis. Human Molecular Genetics 2014;23:3200-3211.
34. Milligan CJ, Li M, Gazina EV, Heron SE, Nair U, Trager C, Reid CA, Venkat A, Younkin DP, Dlugos DJ, Petrovski S, Goldstein DB, Dibbens LM, Scheffer IE, Berkovic SF, Petrou S. KCNT1 Gain of Function in 2 Epilepsy Phenotypes is Reversed by Quinidine. Annals of Neurology 2014;75:581-590.
35. Newton JD, White PE, Ewing MT, Makdissi M, Davis GA, Donaldson A, Sullivan SJ, Seward H, Finch CF. Intention to use sport concussion guidelines among community-level coaches and sports trainers. Journal of Science and Medicine in Sport 2014;17:469-473.
36. Oliver KL, Lukic V, Thorne NP, Berkovic SF, Scheffer IE, Bahlo M. Harnessing Gene Expression Networks to Prioritize Candidate Epileptic Encephalopathy Genes. Plos One 2014;9.
37. Orhan G, Bock M, Schepers D, Ilina EI, Reichel SN, Loeffler H, Jezutkovic N, Weckhuysen S, Mandelstam S, Suls A, Danker T, Guenther E, Scheffer IE, De Jonghe P, Lerche H, Maljevic S. Dominant-negative effects of KCNQ2 mutations are associated with epileptic encephalopathy. Annals of Neurology 2014;75:382-394.
38. Patricios JS, Makdissi M. The sports concussion picture: fewer ‘pixels’, more HD. British Journal of Sports Medicine 2014;48:71-72.
39. Pietersen ANJ, Cheong SK, Solomon SG, Tailby C, Martin PR. Temporal response properties of koniocellular (blue-on and blue-off) cells in marmoset lateral geniculate nucleus. Journal of Neurophysiology 2014;112:14211438.
40. Puskarjov M, Seja P, Heron SE, Williams TC, Ahmad F, Iona X, Oliver KL, Grinton BE, Vutskits L, Scheffer IE, Petrou S, Blaesse P, Dibbens LM, Berkovic SF, Kaila K. A variant of KCC2 from patients with febrile seizures impairs neuronal Cl- extrusion and dendritic spine formation. Embo Reports 2014;15:723-729.
41. Reid CA, Leaw B, Richards KL, Richardson R, Wimmer V, Yu C, Hill-Yardin EL, Lerche H, Scheffer IE, Berkovic SF, Petrou S. Reduced dendritic arborization and hyperexcitability of pyramidal neurons in a Scn1b-based model of Dravet syndrome. Brain 2014;137:1701-1715.
42. Reid CA, Mullen S, Kim TH, Petrou S. Epilepsy, energy deficiency and new therapeutic approaches including diet. Pharmacology & Therapeutics 2014;144:192-201.
43. Reinthaler EM, Lal D, Lebon S, Hildebrand MS, Dahl H-HM, Regan BM, Feucht M, Steinboeck H, Neophytou B, Ronen GM, Roche L, Gruber-Sedlmayr U, Geldner J, Haberlandt E, Hoffmann P, Herms S, Gieger C, Waldenberger M, Franke A, Wittig M, Schoch S, Becker AJ, Hahn A, Maennik K, Toliat MR, Winterer G, Lerche H, Nuernberg P, Mefford H, Scheffer IE, Berkovic SF, Beckmann JS, Sander T, Jacquemont S, Reymond A, Zimprich F, Neubauer BA, Consortium PE, Consortium E, Euro EC. 16p11.2 600 kb Duplications confer risk for typical and atypical Rolandic epilepsy. Human Molecular Genetics 2014;23:6069-6080.
44. Scheffer IE. Epilepsy Genetics Revolutionizes Clinical Practice. Neuropediatrics 2014;45:70-74.
45. Scheffer IE, Dravet C. Transition to adult life in the monogenic epilepsies. Epilepsia 2014;55:12-15.
46. Scheffer IE, Heron SE, Regan BM, Mandelstam S, Crompton DE, Hodgson BL, Licchetta L, Provini F, Bisulli F, Vadlamudi L, Gecz J, Connelly A, Tinuper P, Ricos MG, Berkovic SF, Dibbens LM. Mutations in Mammalian Target of Rapamycin Regulator DEPDC5 Cause Focal Epilepsy with Brain Malformations. Annals of Neurology 2014;75:782-787.
47. Scheffer IE, Mefford HC. EPILEPSY Beyond the single nucleotide variant in epilepsy genetics. Nature Reviews Neurology 2014;10:490-491.
48. Speed D, Hoggart C, Petrovski S, Tachmazidou I, Coffey A, Jorgensen A, Eleftherohorinou H, De Iorio M, Todaro M, De T, Smith D, Smith PE, Jackson M, Cooper P, Kellett M, Howell S, Newton M, Yerra R, Tan M, French C, Reuber M, Sills GE, Chadwick D, Pirmohamed M, Bentley D, Scheffer I, Berkovic S, Balding D, Palotie A, Marson A, O’Brien TJ, Johnson MR. A genome-wide association study and biological pathway analysis of epilepsy prognosis in a prospective cohort of newly treated epilepsy. Human Molecular Genetics 2014;23:247-258.
49. Tailby C, Weintrob DL, Saling MM, Fitzgerald C, Jackson GD. Reading difficulty is associated with failure to lateralize temporooccipital function. Epilepsia 2014;55:746-753.
50. White PE, Newton JD, Makdissi M, Sullivan SJ, Davis G, McCrory P, Donaldson A, Ewing MT, Finch CF. Knowledge about sports-related concussion: is the message getting through to coaches and trainers? British Journal of Sports Medicine 2014;48:119-U127.
51. Williams D, Sullivan SJ, Schneiders AG, Ahmed OH, Lee H, Balasundaram AP, McCrory PR. Big hits on the small screen: an evaluation of concussion-related videos on YouTube. British Journal of Sports Medicine 2014;48:107-111.
52. Williams J, Crowe LM, Dooley J, Collie A, Davis G, McCrory P, Clausen HM, Maddocks D, Anderson VA. Developmental trajectory of information processing skills in children: computer-based assessment. Applied Neuropsychology: Child 2014; published online http://dx.doi.org/10.1080/21622965.2014.939271
53. Zelko FA, Pardoe HR, Blackstone SR, Jackson GD, Berg AT. Regional brain volumes and cognition in childhood epilepsy: Does size really matter? Epilepsy Research 2014;108:692-700.
PARKVILLE CAMPUS
54. Jens Witsch, Daniel Golkowski, Thomas T. G. Hahn, Steven Petrou and Hartwig Spors. Cortical Alterations in a Model for Absence Epilepsy and Febrile Seizures: In Vivo Findings in Mice Carrying a Human GABA(A)R Gamma2 Subunit Mutation Neurobiology of Disease. accepted)
55. Melody Li; Dana Jazayeri; Ben Corry; Melodi McSweeney; Erin L Heinzen; David B Goldstein; Steven Petrou. A functional correlate of severity in alternating hemiplegia of childhood. Neurobiology of Disease, Accepted
56. Mefford, Heather; Allen, Andrew; Berkovic, Samuel; Coe, Bradley; Cook, Joseph; Cossette, Patrick; Delanty, Norman; Dlugos, Dennis; Eichler, E.; Epistein, Michael; Glauser, Tracy; Goldstein, David; Heinzen, E; Johnson, Michael; Krumm, Nik; Kuzniecky, Ruben; Lowenstein, Daniel; Marson, Anthony; Nelson, Ben; Esmaeeli Nieh, Sahar; O’Brien, Terence; Ottman, Ruth; Petrou, Steven; Petrovski, Slave; Poduri, Annapurna; Raja, Archana; Ruzzo, Elizabeth; Scheffer, Ingrid; Sherr, Elliott; Abou-Khalil, Bassel; Allredge, Brian; Andermann, Eva; Andermann, Frederick; Amron, Dina; Bautista, Jocelyn; Boro, Alex; Cascino, Gregory; Consalvo, Damian; Crumrine, Patricia; Devinsky, Orrin; Fiol, Miguel; Fountain, Nathan; French, Jacqueline; Friedman, Daniel; Geller, Eric; Glynn, Simon; Haut, Sheryl; Hayward, Jean; Helmers, Sandra; Joshi, Sucheta; Kanner, Andres; Kirsch, Heidi; Knowlton, Robert; Kossoff, Eric; Kuperman, Rachel; McGuire, Shannon; Motika, Paul; Novotny, Edward; Paolicchi, Julian; Parent, Jack; Park, Kristen; Shellhaas, Renee; Shih, Jerry; Singh, Rani; Sirven, Joseph; Smith, Michael; Sullivan, Joseph; Thio, Liu Lin; Venkat, Anu; Vining, Eileen; Von Allmen, Gretchen; Weisenberg, Judith; Widdess-Walsh, Peter; Winawer, Melodie. CNV analysis from exome data in 349 patients with epileptic encephalopathy”. Annals of Neurology, Accepted.
–
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 116 117
57. Harty RC, Wimmer VC, Richards KL, Phillips AM, Miyazaki A, Nukina N, Petrou S. Sodium channel β1 subunit localizes to axon initial segments of excitatory and inhibitory neurons and shows regional heterogeneity in mouse brain. J Comp Neurol. Accepted 14 Nov 2014
58. Gazina EV, Leaw BTW, Richards KL, Wimmer VC, Kim TH, Aumann TD, Featherby TJ, Churilov L, Hammond VE, Reid CA, Petrou S. Neonatal NaV 1.2 reduces neuronal excitability and affects seizure susceptibility and behavior. Human Mol Genetics (accepted).
59. Epilepsy Phenome/Genome Project; Epi4K Consortium; EuroEPINOMICS-RES Consortium; Epilepsy Phenome/Genome Project; Epi4K Consortium. De Novo Mutations in Synaptic Transmission Genes Including DNM1 Cause Epileptic Encephalopathies. EuroEPINOMICS-RES Consortium. Am J Hum Genet. 2014 Oct 2;95(4):360-70. doi: 10.1016/j.ajhg.2014.08.013. [Epub 2014 Sep 25].
60. Mendis DC, Halgamuge SK, Morrisroe E, Petrou S. Changes in Propagation Delays for Quantifying Pharmacological Effects on Cortical Cultures. Conference paper accepted. Knowledge Discovery in Biological Systems at ICIAfS (International Conference on Information and Automation for Sustainability)
61. Reid CA, Mullen S, Kim TH, Petrou S. Epilepsy, energy deficiency and new therapeutic approaches including diet. Pharmacol Ther. 2014 Nov;144(2):192-201.
62. Oxcarbazepine and its active metabolite, (S)-licarbazepine, exacerbate seizures in a mouse model of genetic generalised epilepsy. Kim TH, Reid CA, Petrou S. Epilepsia. accepted.
63. Muona M, Berkovic SF, Dibbens LM, Oliver KL, Maljevic S, Bayly MA, Joensuu T, Canafoglia L, Franceschetti S, Michelucci R, Markkinen S, Heron SE, Hildebrand M, Andermann E, Andermann F, Gambardella A, Tinuper P, Licchetta L, Scheffer IE, Criscuolo C, Filla A, Ferlazzo E, Ahmad J, Ahmad A, Baykan B, Said E, Topcu M, Riguzzi P, King MD, Ozkara C, Andrade DM, Engelsen BA, Crespel A, Lindenau M, Lohmann E, Saletti V, Massano J, Privitera M, Espay AJ, Kauffmann B, Duchowny M, Møller RS, Straussberg R, Afawi Z, Ben- Zeev B, Samocha KE, Daly MJ, Petrou S, Lerche H, Palotie A, Lehesjoki AE. A recurrent de novo mutation in KCNC1 is a major cause of progressive myoclonus epilepsy. Nature Genetics (Accepted).
64. Milligan CJ, Li MYS, Petrou S. KCNT1 gain-of-function mutations linked to human epilepsy are modulated by quinidine. Molecular & Cellular Epilepsy (Accepted)
65. Wimmer VC, Li MYS, Berkovic SF and Petrou S. Cortical microarchitecture changes in genetic epilepsy. Neurology, Accepted
66. Holien JK, Gazina EV, Elliott RW, Jarrott B, Cameron CE, Williams SJ, Parker MW and Petrou S. Computational analysis of amiloride analogue inhibitors of coxsackievirus B3 RNA polymerase Journal of Proteomics & Bioinformatics Accepted
67. Mendis D, Petrou S, Halgamuge S. Neuromechatronics with In-Vitro Microelectrode Arrays. In Mechatronics Ed Clarence De Silva. 2014
68. Richards K, Calamante F, Tournier JD, Kurniawan ND, Sadeghian F, Retchford AR, Jones GD, Reid CA, Reutens DC, Ordidge R, Connelly A and Petrou S. Mapping somatosensory connectivity in adult mice using diffusion MRI tractography and super-resolution track density imaging. Neuroimage 2014 (accepted)
69. Oliva MK, McGarr TC, Beyer BJ, Gazina EV, Kaplan DI; Cordeiro L, Thomas EA, Dib-Hajj SD, Waxman SG, Frankel WN, Petrou S. Physiological and genetic analysis of multiple sodium channel variants in a model of genetic absence epilepsy. Neurobiology of Disease 2014, Accepted.
70. Puskarjov M, Seja P, Heron SE, Williams TC, Ahmad F, Iona X, Oliver KL, Grinton BE, Vutskits L, Scheffer EI, Petrou S, Blaesse P, Dibbens LM, Berkovic SF, Kaila K. A variant of KCC2 from patients with febrile seizures impairs neuronal Cl- extrusion and dendritic spine formation. EMBO Reports 2014 Accepted
71. Milligan CJ, Li MYS, Gazina EV, Heron SE, Nair U, Trager C, Reid CA, Venkat A, Younkin DP, Dlugos DJ, Petrovski S, Goldstein DB, Dibbens LM, Scheffer IE, Berkovic SF, Petrou S. KCNT1 Gain of Function in Two Epilepsy Phenotypes Is Reversed by Quinidine. Annals of Neurology 2014 Accepted
72. Reid CA, Leaw B, Richards KL, Richardson R, Wimmer VC, Yu C, Hill-Yardin EL, Lerche H, Scheffer IE, Berkovic SF, Petrou S. Reduced dendritic arborisation and hyperexcitability of pyramidal neurons in a Scn1b-based model of Dravet syndrome. Brain 2014 Accepted
73. Hatch RJ, Reid CA and Petrou S. Enhanced in vitro CA1 network activity in a sodium channel SCN1B(C121W) subunit model of genetic epilepsy. Epilepsia 2014 Accepted.
74. Carvill GL, Weckhuysen S, McMahon JM, Hartmann C, Møller RS, Hjalgrim H, Cook J, Geraghty E, O’Roak BJ, Petrou S, Clarke A, Gill D, Sadleir LG, Muhle H, von Spiczak S, Nikanorova M, Hodgson BL, Gazina EV, Suls A, Shendure J, Dibbens LM, De Jonghe P, Helbig I, Berkovic SF, Scheffer IE, Mefford HC.GABRA1 and STXBP1: Novel genetic causes of Dravet syndrome. Neurology. 2014 Mar 12. [Epub ahead of print]
75. TH Kim, S Petrou, CA Reid. Low glycaemic index diet reduces seizure susceptibility in a syndrome-specific mouse model of generalized epilepsy. Epilepsy Research 2014 Jan;108(1):139-43
76. Gu BJ, Sun C, Fuller S, Skarratt KK, Petrou S, Wiley JS. A quantitative method for measuring innate phagocytosis by human monocytes using real-time flow cytometry. Cytometry A. 2014 85(4):313-21
CHAPTER IMAGING
PAGES 60–62
1. S. Groeschel, J-D.Tournier, G.B.Northam, T.Baldeweg, J.Wyatt, B.Vollmer, A.Connelly. Identification and interpretation of microstructural abnormalities in motor pathways in adolescents born preterm. NeuroImage, 87, 209-219 (2014).
2. L.Willats, D.Raffelt, R.E.Smith, J-D.Tournier, A.Connelly, F.Calamante. Quantification of track-weighted imaging (TWI): characterisation of within-subject reproducibility and between-subject variability. NeuroImage, 87, 18-31 (2014).
3. X.Liang, A. Connelly, F.Calamante. Graph analysis of resting-state ASL perfusion MRI data: nonlinear correlations among CBF and network metrics. NeuroImage, 87, 265-275 (2014).
4. J.L.Y.Cheong, A.C.Burnett, K.J.Lee, G.Roberts, D.K.Thompson, S.J.Wood, A.Connelly, P.J.Anderson, L.W.Doyle. Association between postnatal dexamethasone for treatment of bronchopulmonary dysplasia and brain volume at adolescence in infants born very preterm. J Pediatrics, 164, 737-743 (2014).
5. I.E.Scheffer, S.E.Heron, B.M.Regan, S.Mandelstam , D.E.Crompton, B.L.Hodgson, L.Licchetta, F.Bisulli, L.Vadlamudi, A.Connelly, P.Tinuper, M.G.Ricos, S.F.Berkovic, L.M.Dibbens. Mutations in mammalian target rapamycin regulator DEPDC5 cause focal epilepsy with brain malformations. Ann Neurol, 75, 782-787 (2014).
6. C.E Kelly, J.L.Y.Cheong, C.Molloy, P.J Anderson, K.J.Lee, A.C.Burnett, A.Connelly, L.W. Doyle, D.K.Thompson. Neural correlates of impaired vision in adolescents born extremely preterm and/or extremely low birthweight. PLoS ONE, 9, e93188 (2014).
7. J.Mitra, P.Bourgeat, J.Fripp, S.Ghose, S.Rose, O.Salvado, A.Connelly, B.Campbell, S.Palmer, G.Sharma, S. Christensen, L.Carey. Lesion segmentation from multimodal MRI using random forest following ischemic stroke. NeuroImage, 98, 324-335 (2014).
8. K.L.Richards, F.Calamante, J-D.Tournier, N.D.Kurniawan, F. Sadeghian, A.R.Retchford, G.D.Jones, C.A.Reid, D.C.Reutens, R.J.Ordidge, A.Connelly*, S.Petrou*. Mapping somatosensory connectivity in adult mice using diffusion MRI tractography and super-resolution track density imaging. NeuroImage, 102, 381-392 (2014).* equal contribution.
9. X.Liang, A. Connelly, J-D. Tournier, F.Calamante. A variable flip angle-based method for reducing blurring in 3D GRASE ASL. Physics in Medicine and Biology, 18, 5559-5574 (2014).
10. B.Jeurissen, J-D.Tournier, T.Dhollander, A.Connelly, J.Sijbers. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage, 103, 411-426 (2014).
11. Palesi F, Tournier J-D, Calamante F, Muhlert N, Castellazzi G, Chard D, D’Angelo E, Wheeler-Kingshott CAM. Contralateral cerebello-thalamo-cortical pathways with prominent involvement of associative areas in humans in vivo. Brain Struct. Funct. (in press, accepted 21 July 2014).
12. Chappell MA, Mehndiratta A, Calamante F. Correcting for large vessel contamination in DSC perfusion MRI by extension to a physiological model of the vasculature. Magn. Reson. Med. (in press, accepted 7 July 2014).
13. Mehndiratta A, Calamante F, MacIntosh BJ, Crane DE, Payne SJ, Chappell MA. Modeling the Residue Function in DSC-MRI simulations: Analytical Approximation to in vivo data. Magn. Reson. Med. 72:1486–1491 (2014).
14. Mehndiratta A, Calamante F, MacIntosh BJ, Crane DE, Payne SJ, Chappell MA. Modelling and Correction of Bolus Dispersion Effects in DSC-MRI. Magn. Reson. Med. 72: 1762-74 (2014). [Editor’s pick for December 2014 issue]
15. Kurniawan ND, Richards K, Yang Z, She D, Ullmann JFP, Moldrich RX, Liu S, Urriola Yaksic J, Leanage G, Kharatishvili I, Wimmer V, Calamante F, Galloway GJ, Petrou S, Reutens DC. Visualization of Mouse Barrel Cortex using Ex-vivo Track Density Imaging. NeuroImage 87: 465–475 (2014).
16. Jahng G-H, Li K-L, Ostergaard L, Calamante F. Perfusion Magnetic Resonance Imaging: A Comprehensive Update on Principles and Techniques. Korean J. Radiol. 15: 554-577 (2014).
17. Cho ZH, Kang CK, Son YD, Choi SH, Lee YB, Paek SH, Park CW, Chi JG, Calamante F, Law M, Kim YB, Pictorial Review of In Vivo Human Brain: From Anatomy to Molecular Imaging. World Neurosurgery 82: 72–95 (2014).
18. P. Crack, M. Zhang, M.C. Morganti-Kossmann, A.J. Morris, J.M. Wojciak, J.K. Fleming, I. Karve, D. Wright, M. Sashindranath, Y. Goldshmit, A. Conquest, M. Daglas, L.A. Johnston, R.L. Medcalf, R.A. Sabbadini, A. Pebay, Antilysophosphatidic acid antibodies improve traumatic brain injury outcomes, Journal of Neuroinflammation, 11:37, pp.1-11, 2014.
19. N. Bye, O. E. Hutt, T.M. Hinton, D.P. Acharya, L.J. Waddington, B.A. Moffat, D.K. Wright, H.X. Wang, X. Mulet, B.W. Muir, Nitroxide-loaded hexosomes provide MRI contrast in vivo. Langmuir, 30, pp. 8898−8906, 2014.
20. R. Shishegar, J.M. Britto and L.A. Johnston, A method for assessing nonlinear growth in the fetal cortex, Proc. IEEE EMBC, pp.1525-1528, 2014.
21. K.J. Layton, B. Tahayori, I.M.Y. Mareels, P.M. Farrell, L.A. Johnston, Rabi resonance in spin systems: theory and experiment, Journal of Magnetic Resonance, 242:136-142, 2014.
22. S.R. Shultz, X.L. Tan, D.K. Wright, S.J. Liu, A.D. Cook, N.C. Jones, B.D. Semple, L. Johnston, J.A. Hamilton, T.J. O’Brien, Granulocyte-macrophage colony-stimulating factor (GM-CSF) is neuroprotective in experimental traumatic brain injury, Journal of Neurotrauma, vol. 31(10), pp.976-83, 2014.
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 118 119
CHAPTER MENTAL HEALTH
PAGES 66–68
1. Verdile G, Laws SM, Henley D, Ames D, Bush AI, Ellis KA, Faux NG, Gupta VB, Li Q-X, Masters CL, Pike KE, Rowe CC, Szoeke C, Taddei K, Villemagne VL, and Martins RN and for the AIBL Research Group. Associations between gonadotropins, testosterone, and β amyloid in men at risk of Alzheimer’s Disease. Molec Psych 2014; 19: 69-75.
2. Lim YY, Villemagne VL, Laws SM, Ames D, Pietrzak RH, Ellis KA, Harrington K, Bourgeat P, Salvado O, Bush AI, Martins RN, Masters CL, Rowe CC, Maruff P for the AIBL Research Group. Effect of BDNF Val66Met on memory decline and hippocampal atrophy in prodromal Alzheimer’s disease: A preliminary study. PLoS One 2014; 9: e86498. Doi: 10.1371/journal.pone.0086498.
3. Villemagne VL and Masters CL. Alzheimer disease. The landscape of ageing – insights from AD imaging markers. Nat Reviews Neurol. 2014; 10: 678-679.
4. Buckley RF, Saling MM, Irish M, Ames D, Rowe CC, Lautenschlager NT, Maruff P, Macaulay SL, Martins RN, Masters CL, Rainey-Smith S, Rembach A, Savage G, Szoeke C, Ellis KA. Personal memory function in mild cognitive impairment and subjective memory complaints: results from the Australian Imaging, Biomarkers, and Lifestyle (AIBL) study of ageing. J Alz Dis. 2014; 40: 551-561.
5. Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K, Lautenschlager NT, Szoeke C, Martins RN, Masters CL, Villemagne VL, Rowe CC for the AIBL Research Group. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain. 2014; 137: 221-231.
6. Caine JM, Churches Q, Waddington L, Nigro J, Breheney K, Masters CL, Nuttall SD, Streltsov VA. Design of nonaggregating variants of Aβ peptide. Biochem Biophys Res Comm. 2014; 453: 449-454.
7. Klug GM, Boyd A, Sarros S, Stehmann C, Simpson M, McLean C, Masters CL, Collins SJ. Creutzfeldt-Jakob Disease surveillance in Australia, update to December 2013. Commun Dis Intell. 2014; 38: E348-E355.
8. Burnham SC, Faux NG, Wilson W, Laws SM, Ames D, Bedo J, Bush AI, Doecke JD, Ellis KA, Head R, Jones G, Kiiveri H, Martins RN, Rembach A, Rowe CC, Salvado O, Macaulay SL, Masters CL, Villemagne VL, Alzheimer’s Disease Neuroimaging Initiative and Australian Imaging Biomarker and Lifestyle Research Group. A blood-based predictor for neocortical Aβ burden in Alzheimer’s disease: results from the AIBL study. Molec Psych. 2014; 19: 519-526.
9. Masters CL. Shaken, not sonicated? N Eng J Med. 2014; 371: 571-572.
10. Faux NG, Rembach A, Wiley J, Ellis KA, Ames D, Fowler CJ, Martins RN, Pertile KK, Rumble RL, Trounson B, Masters CL, The AIBL Research Group, Bush AI. An anemia of Alzheimer’s disease. Molec Psych. 2014; 19: 12271234.
11. Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TLS, Ghetti B, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Salloway S, Schofield PR, Sperling RA, Marcus D, Cairns NJ, Buckles VD, Ladenson JH, Morris JC, Holtzman DM. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Science Translat Med. 2014; 6: 226ra30doi10.112b/scitranslmed.3007901.
12. Pham CLL, Kirby N, Wood K, Ryan T, Roberts B, Sokolova A, Barnham KJ, Masters CL, Knott RB, Cappai R, Curtain CC and Rekas A. Guanidine hydrochloride denaturation of dopamine induced α-synuclein oligomers: A smallangle X-ray scattering study. Proteins: Structure, Function and Bioinformatics 2014; 82: 10-21.
13. Villemagne VL, Dorè V, Yates P, Brown B, Mulligan R, Bourgeat P, Veljanoski R, Rainey-Smith SR, Ong K, Rembach A, Williams R, Burnham SC, Laws SM, Salvado O, Taddei K, Macaulay SL, Martins RN, Ames D, Masters CL, Rowe CC. En attendant centiloid. Advances in Research. 2014; 2: 723-729.
14. Roberts BR, Lim NKH, McAllum EJ, Donnelly PS, Hare DJ, Doble PA, Turner BJ, Price KA, Lim SC, Paterson BM, Hickey JL, Rhoads TW, Williams JR, Kanninen KM, Hung LW, Liddell JR, Grubman A, Monty J-F, Llanos RM, Kramer DR, Mercer JFB, Bush AI, Masters CL, Duce JA, Li Q-X, Beckman JS, Barnham KJ, White AR, Crouch PJ. Oral treatment with CuII(atsm) increases mutant SOD1 in vivo but protects motor neurons and improves the phenotype of a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci. 2014; 34: 8021-8031.
15. Adlard PA, Li Q-X, McLean C, Masters CL, Bush AI, Fodero-Tavoletti M, Villemagne V and Barnham KJ. β-amyloid in biological samples: not all Aβ detection methods are created equal. Front Aging Neurosci. 6:203. Doi: 10.3389/ fnagi.2014.00203.
16. Lim YY, Maruff P, Pietrzak RH, Ellis KA, Darby D, Ames D, Harrington K, Martins RN, Masters CL, Szoeke C, Savage G, Villemagne VL, Rowe CC for the AIBL Research Group. Aβ and cognitive change: Examining the preclinical and prodromal stages of Alzheimer’s disease. Alzheimers Dement. 2014; 10: 743-751.
17. Buckley RF, Saling MM, Irish M, Ames D, Rowe CC, Villemagne VL, Lautenschlager NT, Maruff P, Macaulay SL, Martins RN, Szoeke C, Masters CL, Rainey-Smith SR, Rembach A, Savage G, Ellis KA and the Australian Imaging Biomarkers and Lifestyle Study of Ageing (AIBL) Research Group. Autobiographical narratives relate to Alzheimer’s disease biomarkers in older adults. Int Psychogeriatrics. 2014; 26: 1737-1746.
18. Johanssen VA, Johanssen T, Masters CL, Hill AF, Barnham KJ, Collins SJ. C-terminal peptides modelling constitutive PrPC processing demonstrate ameliorated toxicity predisposition consequent to α-cleavage. Biochem J. 2014; 459: 103-115.
19. Rembach A, Hare DJ, Doecke JD, Burnham SC, Volitakis I, Fowler CJ, Cherny RA, McLean C, Grimm R, Martins R, Ames D, Masters CL, Bush AI, Roberts BR. Decreased serum zinc is an effect of ageing and not Alzheimer’s disease. Metallomics: integrated biometal science. 2014; 6: 1216-1219.
20. Moore E, Ames D, Mander A, Carne R, Brodaty H, Woodward M, Boundy K, Ellis K, Bush A, Faux N, Martins R, Masters C, Rowe C, Szoeke C, Watters D, and the AIBL investigators. Among vitamin B12 deficient older people, high folate levels are associated with worse cognitive function: combined data from three cohorts. J Alz Dis. 2014; 39: 661-668.
21. Brown BM, Bourgeat P, Peiffer JJ, Burnham S, Laws SM, Rainey-Smith SR, Bartrés-Faz D, Villemagne VL, Taddei K, Rembach A, Bush A, Ellis KA, Macaulay SL, Rowe CC, Ames D, Masters CL, Maruff P, Martins RN for the AIBL Research Group. Influence of BDNF Val66Met on the relationship between physical activity and brain volume. Neurol 2014; 83: 1345-1352.
22. Thomas JB, Brier MR, Bateman RJ, Snyder AZ, Benzinger TL, Xiong C, Raichle M, Holtzman DM, Sperling RA, Mayeux R, Ghetti B, Ringman JM, Salloway S, McDade E, Rossor MN, Ourselin S, Schofield PR, Masters CL, Martins RN, Weiner MW, Thompson PM, Fox NC, Koeppe RA, Jack CR, Mathis CA, Oliver A, Blazey TM, Moulder K, Buckles V, Hornbeck R, Chhatwal J, Schultz AP, Goate AM, Fagan AM, Cairns NJ, Marcus DS, Morris JC and Ances BM. Functional connectivity in autosomal dominant and late-onset Alzheimer disease. JAMA Neurol. 2014; 71: 1111-1122.
23. Rembach A, Faux NG, Watt AD, Pertile KK, Rumble RL, Trounson BO, Fowler CJ, Roberts BR, Perez KA, Li Q-X, Laws SM, Taddei K, Rainey-Smith S, Robertson JS, Vandijck M, Vanderstichele H, Barnham KJ, Ellis KA, Szoeke C, Macaulay L, Rowe CC, Villemagne VL, Ames D, Martins RN, Bush AI, Masters CL, the AIBL research group. Changes in plasma amyloid beta in a longitudinal study of aging and Alzheimer’s disease. Alz Dement 2014; 10: 53-61.
24. Hare DJ, Lei P, Ayton S, Roberts BR, Grimm R, George JL, Bishop DP, Beavis AD, Donovan SJ, McColl G, Volitakis I, Masters CL, Adlard PA, Cherny RA, Bush AI, Finkelstein DI and Doble PA. An iron-dopamine index predicts risk of parkinsonian neurodegeneration in the substantia nigra pars compacta. Chemical Science. 2014; 5: 2160-2169.
25. Villemagne VL, Fodero-Tavoletti M, Yates P, Masters CL and Rowe CC. Aβ imaging in aging, Alzheimer’s disease and other neurodegenerative conditions. In: PET and SPECT in Neurology. Edited by RAJO Dierckx, A Otte, EFJ de Vries, A van Waarde, and KL Leenders. Springer Verlag (Berlin-Heidelberg-New York). 2014, Chap 10: pp 213-254.
26. Zwan MD, Okamura N, Fodero-Tavoletti MT, Furumoto S, Masters CL, Rowe CC, Villemagne VL. Voyage au bout de la nuit: Aβ and tau imaging in dementias. QJ Nucl Med Mol Imaging. 2014; 58: 398-412.
27. Yates PA, Villemagne VL, Ellis KA, Desmond PM, Masters CL, Rowe CC. Cerebral microbleeds: a review of clinical, genetic and neuroimaging associations. Frontiers in Neurology. 2014; 4: 205.
28. Johnson P, Vandewater L, Wilson W, Maruff P, Savage G, Graham P, Macaulay SL Ellis KA, Szoeke C, Martins RN, Rowe CC, Masters CL, Ames D, Zhang P. Genetic algorithm with logistic regression for prediction of progression of Alzheimer’s disease. BMC Bioinformatics. 2014; 15 (Suppl 16): S11.
29. Coleman BM, Harrison CF, Guo B, Masters CL, Barnham KJ, Lawson VA, Hill AF. Pathogenic mutations within the hydrophobic domain of the prion protein lead to the formation of a protease sensitive prion species with increased lethality. J Virol. 2014; 88: 2690-2703.
30. Rembach A, Watt AD, Wilson WJ, Rainey-Smith S, Ellis KA, Rowe CC, Villemagne VL, Macaulay SL, Bush AI, Martins RN, Ames D, Masters CL, Doecke JD and the AIBL research group. An increased neutrophil-lymphocyte ratio in Alzheimer’s disease is a function of age and is weakly correlated with neocortical amyloid accumulation. J Neuroimmunology. 2014; 273: 65-71.
31. Zhou L, Salvado O, Dore V, Bourgeat P, Raniga P, Macaulay SL, Ames D, Masters CL, Ellis KA, Villemagne VL, Rowe CC, Fripp J, AIBL Research Group. MR-less surface-based amyloid assessment based on 11C PiB PET. PLOS One. 2014; 9: e84777.
32. Hare DJ, George JL, Bray L, Volitakis I, Vais A, Ryan TM, Cherny RA, Bush AI, Masters CL, Adlard PA, Doble PA and Finkelstein DI. The effect of paraformaldehyde fixation and sucrose cryoprotection on metal concentration in murine neurological tissue. J Anal At Spectrom. 2014; 29: 565-570.
33. Yates PA, Desmond PM, Phal PM, Steward C, Szoeke C, Salvado O, Ellis KA, Martins RN, Masters CL, Ames D, Villemagne VL, Rowe CC for the AIBL Research Group. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology. 2014; 82: 1266-1273.
34. Villemagne VL, Furumoto S, Fodero-Tavoletti MT, Mulligan RS, Hodges J, Harada R, Yates P, Piguet O, Pejoska S, Doré V, Yanai K, Masters CL, Kudo Y, Rowe CC, Okamura N. In vivo evaluation of a novel tau imaging tracer for Alzheimer’s disease. Eur J Nucl Med Molec Imag. 2014; 41: 816-826.
35. Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox N, Goate A, Frommelt P, Ghetti B, Langbaum JBS, Lopera F, Martins R, Masters CL, Mayeux RP, McDade E, Moreno S, Reiman EM, Ringman JM, Salloway S, Schofield PR, Sperling R, Tariot PN, Xiong C, Morris JC, Bateman RJ and the Dominantly Inherited Alzheimer Network. Symptom onset in autosomal dominant Alzheimer disease. A systematic review and meta-analysis. Neurology. 2014; 83: 253-260.
36. Ellis KA, Szoeke C, Bush AI, Darby D, Graham PL, Lautenschlager NT, Macaulay SL, Martins RN, Maruff P, Masters CL, McBride SJ, Pike KE, Rainey-Smith SR, Rembach A, Robertson J, Rowe CC, Savage G, Villemagne VL, Woodward M, Wilson W, Zhang P, Ames D and the AIBL research group. Rates of diagnostic transition and cognitive change at 18-month follow-up among 1,112 participants in the Australian Imaging, Biomarkers and Lifestyle flagship study of ageing (AIBL). Int Psychogeriatrics. 2014; 26: 543-554.
37. Fodero-Tavoletti MT, Furumoto S, Taylor L, McLean CA, Mulligan RS, Birchall I, Harada R, Masters CL, Yanai K, Kudo Y, Rowe CC, Okamura N and Villemagne VL. Assessing THK523 selectivity for tau deposits in Alzheimer’s disease and non-Alzheimer’s disease tauopathies. Alzheimer Res & Therapy. 2014; 6:11.
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 120 121
38. Pietrzak RH, Scott JC, Neumeister A, Lim YY, Ames D, Ellis KA, Harrington K, Lautenschlager NT, Szoeke C, Martins RN, Masters CL, Villemagne VL, Rowe CC, Maruff P for the Australian Imaging, Biomarkers and Lifestyle (AIBL) Research Group. Anxiety symptoms, cerebral amyloid burden and memory decline in healthy older adults without dementia: 3-year prospective cohort study. Brit J Psychiat. 2014; 204: 400-401.
39. Rembach A, Watt AD, Wilson WJ, Villemagne VL, Burnham SC, Ellis KA, Maruff P, Ames D, Rowe CC, Macaulay SL, Bush AI, Martins RN, Masters CL, Doecke JD and the AIBL Research Group. Plasma amyloid-β levels are significantly associated with a transition towards Alzheimer’s disease as measured by cognitive decline and change in neocortical amyloid burden. J Alz Disease. 2014; 40: 95-104.
40. Okamura N, Furumoto S, Fodero-Tavoletti M, Mulligan R, Harada R, Yates P, Pejoska S, Kudo Y, Masters C, Yanai K, Rowe C, Villemagne V. Noninvasive assessment of Alzheimer’s disease neurofibrillary pathology using 18F-THK5105 PET. Brain. 2014; 137: 1762-1771.
41. Hung YH, Faux NG, Killilea DW, Yanjamin N, Firnkes S, Volitakis I, Ganio G, Walterfang M, Hastings C, Porter FD, Ory DS, Bush AI. Altered transition metal homeostasis in Niemann-Pick disease, Type C1. Metallomics 2014; 6(3), 542-553.
42. Squitti R, Simonelli I, Ventriglia M, Siotto M, Pasqualetti P, Rembach A, Doecke J, Bush AI. Meta-analysis of serum non-ceruloplasmin copper in Alzheimer’s disease. J Alzheimers Dis 2014; 38(4), 809-22. [DOI: 10.3233/JAD-131247].
43. Ayton S, Zhang M, Roberts BR, Lam LQ, Lind M, McLean C, Bush AI, Frugier T, Crack PJ, Duce JA. Ceruloplasmin and beta amyloid precursor protein confer neuroprotection in traumatic brain injury and lower neuronal iron. Free Radical Biology & Medicine 2014; 69, 331-337.
44. Ayton S, Lei P, Adlard PA, Volitakis I, Cherny RA, Bush AI*, Finkelstein DI*. Iron accumulation confers neurotoxicity to a vulnerable population of nigral neurons: implications for Parkinson’s disease. Molecular Neurodegeneration 2014; 9: 27 [doi: 10.1186/1750-1326-9-27].*co-senior authors
45. Lu Z, Marks E, Chen J, Moline J, Barrows L, Raisbeck M, Volitakis I, Cherny RA, Chopra V, Bush AI, Hersch S, Fox JH. Altered selenium status in Huntington’s disease: neuroprotection by selenite in the N171-82Q mouse model. Neurobiology of Disease 2014; 71, 34-42.
46. Lei P, Ayton S, Moon S, Zhang Q, Volitakis I, Finkelstein DI*, Bush AI*. Motor and cognitive deficits in aged tau knockout mice in two background strains. Molecular Neurodegeneration 2014; 9: 29. [doi:10.1186/1750-13269-29]. *co-senior authors
47. Wong BXW, Ayton S, Lam LQ, Lei P, Adlard PA, Bush AI*, Duce JA*. A comparison of ceruloplasmin to biological polyanions in promoting the oxidation of Fe2+ under physiologically relevant conditions. Biochimica et Biophysica Acta – General Subjects 2014; 1840(12), 3299-3310. [doi: 10.1016/j.bbagen.2014.08.006] *cosenior authors
48. Ciccotosto GD, James SA, Altissimo M, Paterson D, Vogt S, Lai B, de Jonge MD, Howard DL, Bush AI, Cappai R. Quantitation and localization of intracellular redox active metals by X-ray fluorescence microscopy in cortical neurons derived from APP and APLP2 knockout tissue. Metallomics 2014; 6, 1894–1904.
49. Castro PA, Ramirez A, Sepulveda FJ, Peters C, Fierro H, Waldron J, Luza S, Fuentealba J, Munoz JF, De Ferrari JV, Bush AI, Aguayo LG, Opazo CM. Copper-uptake is critical for the down regulation of synapsin and dynamin induced by neocuproine: modulation of synaptic activity in hippocampal neurons. Frontiers in Aging Neuroscience 2014 Dec 3; 6, 319. [doi: 10.3389/fnagi.2014.00319]
50. Wong BX, Tsatsanis A, Lim LQ, Adlard PA, Bush AI*, Duce JA*. β-amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin. PLoS ONE 2014; 9(12), e114174. [doi:10.1371/journal.pone.0114174] *co-senior authors
51. Berk M, Dean OM, Cotton SM, Jeavons S, Tanious M, Kolmann K, Hewitt K, Moss K, Allwang C, Schapkaitz I, Robbins J, Cobb H, Ng F, Dodd S, Bush AI, Malhi GS. The efficacy of adjunctive N-acetylcysteine in major depressive disorder: A double-blind, randomized, placebo-controlled trial. Journal of Clinical Psychiatry 2014; 75(6), 628-636.
52. Takeda A, Nakamura M, Fujii H, Uematsu C, Minamino T, Adlard PA, Bush AI, Tamano H. Amyloid β-mediated Zn2+ influx into dentate granule cells transiently induces a short-term cognitive deficit. PLoS One 2014; 9(12):e115923. [doi:10.1371/journal.pone.0115923]
53. Adlard PA, Parncutt J, Lal V, James S, Hare D, Doble P, Finkelstein DI, Bush AI. Metal chaperones prevent zinc-mediated cognitive decline. Neurobiology of Disease 2014 (online Dec 27, 2014). DOI: 10.1016/j. nbd.2014.12.012
54. Barnard ND, Bush AI, Ceccarelli A, Cooper J, de Jager CA, Erickson KI, Fraser G, Kesler S, Levin SM, Lucey B, Morris MC, Squitti R. Dietary and Lifestyle Guidelines for the Prevention of Alzheimer’s Disease. Neurobiology of Aging 2014; 35S2, S74-S78.
55. Wong BXW, Hung YH, Bush AI*, Duce JA*. Metals and cholesterol: two sides of the same coin in Alzheimer’s disease pathology. Frontiers in Aging Neuroscience 2014; 6, 91 [doi: 10.3389/fnagi.2014.00091].*co-senior authors
56. Opazo CM, Greenough MA, Bush AI. Copper: From neurotransmission to neuroproteostasis. Frontiers in Aging Neuroscience 2014; 6, 143 [doi: 10.3389/fnagi.2014.00143].
57. Barnham KJ, Bush AI. Biological Metals and Metal-targeting Compounds in Major Neurodegenerative Diseases. Chemical Society Reviews 2014; 43, 6727-6749.
58. Adlard P, Tran B, Finkelstein D, Desmond P, Johnston L, Bush AI, Egan G. A review of β-amyloid neuroimaging in Alzheimer’s disease. Frontiers in Neuroscience 2014; 8, 327. [doi: 10.3389/fnins.2014.00327].
59. Bruno Dubois, Howard H Feldman, Claudia Jacova, Harald Hampel, José Luis Molinuevo, Kaj Blennow, Steven T DeKosky, Serge Gauthier, Dennis Selkoe, Randall Bateman, Stefano Cappa, Sebastian Crutch, Sebastiaan Engelborghs, Giovanni B Frisoni, Nick C Fox, Douglas Galasko, Marie-Odile Habert, Gregory A Jicha, Agneta Nordberg, Florence Pasquier, Gil Rabinovici, Philippe Robert, Christopher Rowe, Stephen Salloway, Marie Sarazin, Stéphane Epelbaum, Leonardo C de Souza, Bruno Vellas, Pieter J Visser, Lon Schneider, Yaakov Stern, Philip Scheltens, Jeffrey L Cummings. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG2 criteria. Lancet Neurol 2014; 13: 614–629.
60. Elias A, Woodward M, Rowe CC. Management Impact of FDG PET in a Tertiary Memory Disorders Clinic. J Alzheimer’s Disease, 2014; 42:885-892.
61. Woodward M, Rowe CC, Jones GA, Villemagne VL, Varos T. Differentiating the frontal presentation of Alzheimer’s disease with FDG-PET. J Alzheimer’s Disease. Accepted 21 August 2014.
62. Klunk WE, Robert A Koeppe; Julie C Price; Tammie Benzinger; Michael D Devous Sr.; William Jagust; Keith Johnson; Chester A Mathis; Davneet Minhas; Michael J Pontecorvo; Christopher C Rowe; Daniel Skovronsky; Mark Mintun. The Centiloid Project: Standardizing Quantitative Amyloid Plaque Estimation by PET. Alzheimer’s & Dementia. On-line October 2014. (Impact factor 17)
63. Villemagne VL, Kim SY, Rowe CC, Iwatsubo T. Imago Mundi, Imago AD, Imago ADNI. Alzheimer’s Research and Therapy, 2014; 6:62-72.
64. Kerbler GM, Fripp J, Rowe CC, Villemagne VL, Salvado O, Rose S, Coulson EJ; Alzheimer’s Disease Neuroimaging Initiative. Basal forebrain atrophy correlates with amyloid β burden in Alzheimer’s disease. Neuroimage Clin. 7:105-13, 2014.
65. Ly JV, Singhal S, Rowe CC, Kempster P, Bower S, Thanh G. Convexity Subarachnoid Hemorrhage with PiB Positive Pet Scans: Clinical Features and Prognosis. J Neuroimaging 2014, DOI: 10.1111/jon.12188
66. Hocking DC, Kennedy GA, Sundram S. Mental disorders in asylum seekers: the role of the refugee determination process and employment. J Nerv Ment Dis. 2015; 203(1):28-32. doi: 10.1097/NMD.0000000000000230.
67. Loi S, Sundram S. To flee, or not to flee, that is the question for older asylum seekers. Int Psychogeriatr. 2014 Jun 13:1-4. [Epub ahead of print].
68. Pereira A, Zhang B, Malcolm P, Sugiharto-Winarno A, Sundram S. Quetiapine and aripiprazole signal differently to ERK, p90RSK and c-Fos in mouse frontal cortex and striatum: role of the EGF receptor. BMC Neurosci. 2014 Feb 20;15:30. doi: 10.1186/1471-2202-15-30.
69. Dean B, Tawadros N, Suk Seo MS, Jeon WJ, Everall I, Scarr E, Gibbons A (2014) Lower cortical serotonin 2A receptors in major depressive disorder, suicide and in rats after administration of imipramine. International Journal of Neuropsychopharmacology 17: 895-906.
70. Seo MS, Scarr E, Lai C-Y, Dean B (2014) Potential molecular and cellular mechanism of psychotropic drugs. Clinical Psychopharmacology and Neuroscience 12: 94-110.
71. Seo MS, Scarr E, Brian Dean (2014) An investigation of the factors that regulate muscarinic receptor expression in schizophrenia. Schizophrenia Research 158: 247-254.
72. Gozes I, Merenlender-Wagner A, Malishkevich A, Shemer A, Udawela M, Gibbons A, Scarr E, Dean B, Levine J, Agam G, Gozes I (2015) Autophagy plays a key role in the pathophysiology of schizophrenia. Molecular Psychiatry 20:126-132.
CHAPTER MULTIPLE SCLEROSIS
PAGES 72–75
1. Xing YL, Röth PT, Stratton JA, Chuang BHA, Danne J, Ellis SL, Ng SW, Kilpatrick TJ, Merson TD. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J Neurosci. 2014;34(42), 14128–14146.
2. Merson TD, Bourne JA. Endogenous neurogenesis following ischemic brain injury: insights for therapeutic strategies. Int J Biochem Cell Biol. (2014) pii: S1357-2725(14)00254-4.
3. Jonas A, Thiem S, Kuhlmann T, Wagener R, Aszodi A, Nowell C, Hagemeier K, Laverick L, Perreau V, Jokubaitis V, Emery B, Kilpatrick T, Butzkueven H, Gresle M. Axonally derived matrilin-2 induces proinflammatory responses that exacerbate autoimmune neuroinflammation. J Clin Invest. 2014;124(11):5042-56.
4. Gresle MM, Schulz K, Jonas A, Perreau VM, Cipriani T, Baxter AG, Miranda-Hernandez S, Field J, Jokubaitis VG, Cherny R, Volitakis I, David S, Kilpatrick TJ, Butzkueven H. Ceruloplasmin gene-deficient mice with experimental autoimmune encephalomyelitis show attenuated early disease evolution. J Neurosci Res. 2014;92(6):732-42
5. Kolbe SC, Kilpatrick TJ, Mitchell PJ, White O, Egan GF, Fielding J. Inhibitory saccadic dysfunction is associated with cerebellar injury in multiple sclerosis. Hum Brain Mapp. 2014;35(5):2310-9.
6. McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346(6207):318-22.
7. Shahijanian F, Parnell GP, McKay FC, Gatt PN, Shojoei M, O’Connor KS, Schibeci SD, Brilot F, Liddle C, Batten M; ANZgene Multiple Sclerosis Genetics Consortium, Stewart GJ, Booth DR. The CYP27B1 variant associated with an increased risk of autoimmune disease is underexpressed in tolerizing dendritic cells. Hum Mol Genet. 2014;23(6):1425-34.
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 122 123
CHAPTER NEURODEGENERATION
PAGES 78–81
1. S. Groeschel, J-D.Tournier, G.B.Northam, T.Baldeweg, J.Wyatt, B.Vollmer, A.Connelly. Identification and interpretation of microstructural abnormalities in motor pathways in adolescents born preterm. NeuroImage, 87, 209-219 (2014).
2. Davidson S.M., Lopaschuk G.D., Spedding M., Beart P.M. Mitochondrial pharmacology: energy, injury and beyond. British Journal of Pharmacology 171, 1795–1797 (2014).
3. Lau C.L., Kovacevic M., Tingleff T.S., Forsythe J.S., Cate H.S., Merlo D., Cederfur C., Maclean F.L., Parish C.L., Horne M.K., Nisbet D.R., Beart P.M. 3D Electrospun scaffolds promote a cytotrophic phenotype of cultured primary astrocytes. Journal of Neurochemistry 130, 215-226 (2014).
4. Alexander S.P., Benson H.E., Faccenda E., Beart P.M., Belelli D, Bennett Aj, Birdsall Nj, Boison D, et al. The Concise Guide to Pharmacology 2013/14: overview. British Journal of Pharmacology. 170, pp. 1449-58. (2013).
5. Davidson S.M., Lopaschuk G.D., Spedding M., Beart P.M. (Eds) “Mitochondrial Pharmacology: Energy, Injury and Beyond”. British Journal of Pharmacology 171, pp. 1795–2249 (2014). “Mitochondrial Pharmacology: Energy, Injury and Beyond”.
6. Aumann Td, Prut Y. Do sensorimotor β-oscillations maintain muscle synergy representations in primary motor cortex? Trends Neurosci. 2014 pii: S0166-2236(14) 00219-7.
7. Gazina Ev, Leaw Bt, Richards Kl, Wimmer Vc, Kim Th, Aumann Td, Featherby Tj, Churilov L, Hammond Ve, Reid Ca, Petrou S. Neonatal Nav1.2 reduces neuronal excitability and affects seizure susceptibility and behaviour. Hum Mol Genet. 2014 pii: ddu562.
8. Stanić D., Dubois S., Chua H.K., Tonge B., Rinehart N., Horne M.K., Boon W.C. Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors α and β and androgen receptors. PLoS One. 2014 9: e90451.
9. Bell Jr, Bernasochi Gb, Varma U, Boon Wc, Ellem SJ, Risbridger GP, Delbridge LM. Aromatase transgenic upregulation modulates basal cardiac performance and the response to ischemic stress in male mice. Am J Physiol Heart Circ Physiol. 2014 306: H1265-74.
10. Van Sinderen ML, Steinberg Gr, Jørgensen SB, To SQ, Knower KC, Clyne CD, Honeyman J, Chow JD, Herridge KA, Jones ME, Simpson ER, Boon WC. Hepatic glucose intolerance precedes hepatic steatosis in the male aromatase knockout (ArKO) mouse. PLoS One. 2014 9: e87230.
11. Bird M., Needham K., Frazier A.E., Van Rooijen J., Leung J., Hough S., Denham M., Thornton M.E., Parish C.L., Nayagam B., Pera M., Thornburn D.R, Thompson L.H., Dottori M. Functional characterization of Friedreich Ataxia iPS-derived neuronal progenitors and their integration in the adult brain. PLoS One 2014 7,: e101718
12. Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, Thompson L.H, Halliday G, Kirik D. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain 2014, 137, 2493-508.
13. Sahin G., Thompson L.H., Lavisse S., Ozgur M., Carta M., Rbah L., Dolle F., Hantraye P., Kirik D. Differential dopamine receptor occupancy underlies L-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. PLoS One 2014, 9: e90759.
14. Rodriguez AL, Wang TY, Bruggeman KF, Horgan C, Li R, Williams RJ, Parish CL, Nisbet DR. In vivo assessment of grafted cortical neural progenitor cells and host response to functionalized self-assembling peptide hydrogels and implications for tissue repair. Journal of Materials Chemistry. B, 2014,2, 7771-7778..
15. Bird M, Needham K, Frazier A, Van Rooijen J, Leung J, Hough S, Denham M, Thornton Me, Parish Cl, Pera M, Thorburn D, Thompson Lh, Dottori M. Functional characterization of Friedreich Ataxia iPS-derived neuronal progenitors and their integration in the adult brain. PLoS One (2014) 9: e101718.
16. Fernando CV, Kele J, Bye CR, Blakely BD, Niclis J, Alsanie W, Stenman J, Turner BJ, Parish CL. Wnt7a regulates ventral midbrain neurogenesis and dopaminergic axon morphogenesis in development and embryonic stem cells. Stem Cells and Dev (2014) 23: 1991-2003.
17. Wang TY, Bruggeman KA, Sheean RK, Turner BJ, Nisbet DR, Parish CL. Characterisation of the stability and biofunctionality of tethered proteins on bioengineered scaffolds J Biol Chem (2014) 289: 15044-15051.
18. C.L. Lau, M. Kovacevic, T. Tingleff, J.S. Forsythe, H.S. Cate, D. Merlo, C. Cederfur, F.L. Maclean, C.L. Parish, M.K. Horne, D.R. Nisbet, P.M. Beart. 3D Electrospun scaffolds promote a cytotrophic phenotype of cultured primary astrocytes J Neurochem. (2014) 130: 215-226.
19. Kim HA, Jiang L, Madsen H, Parish CL, Massalas J, Smardencas A, O’leary C, Gantois I, O’tuathaigh C, Waddington JL, Ehrlich ME, Lawrence AJ, Drago J. Loss of striatal D1 dopamine receptor neurons gives a Huntington disease Westphal variant-like model. Neurobiology of Disease (2014); 62: 323-337.
20. Parish CL, Thompson LH. Modulating Wnt signaling to improve cell replacement therapy for Parkinson’s disease. J Mol Cell Biol. (2014) 6: 54-63.
21. Grad LI, Yerbury JJ, Turner BJ, Guest WC, Pokrishevsky E, O’Neill MA, Yanai A, Silverman JM, Zeineddine R, Corcoran L, Kumita JR, Luheshi LM, Yousefi M, Coleman BM, Hill AF, Plotkin SS, Mackenzie IR, Cashman NR (2014) Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosomedependent and -independent mechanisms. Proc Natl Acad Sci USA 111: 3620-3625.
22. Grad LI, Yerbury JJ, Turner BJ, Guest WC, Pokrishevsky E, O’Neill MA, Yanai A, Silverman JM, Zeineddine R, Corcoran L, Kumita JR, Luheshi LM, Yousefi M, Coleman BM, Hill AF, Plotkin SS, Mackenzie IR, Cashman NR (2014) Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosomedependent and -independent mechanisms. Proc Natl Acad Sci USA 111: 3620-3625.
23. Turner BJ, Alfazema N, Sheean RK, Sleigh JN, Davies KE, Horne MK, Talbot K (2014) Overexpression of survival motor neuron improves neuromuscular function and motor neuron surival in mutant SOD1 mice. Neurobiol Aging 35: 906-915.
24. Perera ND, Sheean RK, Scott JW, Kemp BE, Horne MK, Turner BJ (2014) Mutant TDP-43 deregulates AMPK activation by PP2A in ALS models. PLoS One 9: e95549.
25. Polling S, Mok YF, Ramdzan YM, Turner BJ, Yerbury JJ, Hill AF, Hatters DM (2014) Misfolded polyglutamine, polyalanine and superoxide dismutase 1 aggregate via distinct pathways in the cell. J Biol Chem 289: 6669-6680.
26. Roberts BR, Lim NK, McAllum EJ, Donnelly PS, Hare DJ, Doble PA, Turner BJ, Price KA, Lim SC, Paterson BM, Hickey JL, Rhoads TW, Williams JR, Kanninen KM, Hung LW, Liddel JR, Grubman A, Monty JF, Llanos RM, Kramer DR, Mercer JF, Bush AI, Masters CL, Duce JA, Li QX, Beckman JS, Barhnham KJ, White AR, Crouch PJ (2014) Oral treatment with Cu(atsm) increases mutant SOD1 in vivo but protects motor neurons and improves the phenotype of a transgenic mouse model of ALS. J Neurosci 34: 8021-8031.
27. Fernando CV, Kele J, Bye CR, Niclis JC, Alsanie W, Blakely BD, Stenman J, Turner BJ, Parish CL (2014) Diverse roles for Wnt7a in ventral midbrain neurogenesis and dopaminergic axon morphogenesis. Stem Cells Dev 23: 1991-2003.
28. Wang TY, Bruggeman KA, Sheean RK, Turner BJ, Nisbet DR, Parish CL (2014) Characterisation of the stability and bio-functionality of tethered proteins on bioengineered scaffolds: implications for stem cell biology and tissue repair. J Biol Chem 289: 15044-15051.
29. Perera N.D., Sheean R.K., Scott J.W., Kemp B.E., Horne M.K., Turner B.J. Mutant TDP-43 deregulates AMPK activation by PP2A in ALS models. PLoS One. 2014 9: e95549.
30. Kotschet K, Johnson W, Mcgregor S, Kettlewell J, Kyoong A, O’Driscoll DM, Turton Ar, Griffiths RI, Horne MK. Daytime sleep in Parkinson’s disease measured by episodes of immobility. Parkinsonism Relat Disord. 2014 20: 578-83.
31. Stanić D., Dubois S., Chua H.K., Tonge B., Rinehart N., Horne M.K., Boon W.C. Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors α and β and androgen receptors. PLoS One. 2014 9: e90451.
32. Perera N.D., Sheean R.K., Scott J.W., Kemp B.E., Horne M.K., Turner B.J. Mutant TDP-43 Deregulates AMPK activation by PP2A in ALS models. PLoS One. 2014 9: e90449..
33. Lau C.L., Kovacevic M., Tingleff T.S., Forsythe J.S., Cate H.S., Merlo D., Cederfur C., Maclean F.L., Parish C.L., Horne M.K., Nisbet D.R., Beart P.M. 3D Electrospun scaffolds promote a cytotrophic phenotype of cultured primary astrocytes. J Neurochem. 2014 130: 215-26.
34. Turner B.J., Alfazema N., Sheean R.K., Sleigh J.N., Davies K.E., Horne M.K., Talbot K. Overexpression of survival motor neuron improves neuromuscular function and motor neuron survival in mutant SOD1 mice. Neurobiol Aging. 2014 35: 906-15.
35. Atkin J.D., Farg M.A., Soo K.Y., Walker A.K., Halloran M., Turner B.J., Nagley P., Horne M.K. Mutant SOD1 inhibits ER-Golgi transport in amyotrophic lateral sclerosis. J Neurochem. 2014 129: 190-204.
CHAPTER NEUROPEPTIDES
PAGES 84–89
1. Binder, C., Chuang, E., Habla, C., Bleckmann, A., Schulz, M., Bathgate, R., Einspanier A. Relaxins enhance growth of spontaneous murine breast cancers as well as metastatic colonization of the brain. Clin Expl Metastasis 31, 57-65, 2014.
2. Chow, B.S., Kocan, M., Bosnyak, S., Sarwar, M., Wigg, B., Jones, E.S., Widdop, R.E., Summers, R.J., Bathgate, R.A., Hewitson, T.D., Samuel, C.S. Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney Int 86, 75-85, 2014.
3. Diepenhorst, N.A., Petrie, E.J., Chen, C.Z., Wang, A., Hossain, M.A., Bathgate, R.A., Gooley, P.R. Investigation of interactions at the extracellular loops of the relaxin family peptide receptor 1 (RXFP1). J Biol Chem 289, 3493852, 2014.
4. Egloff, P., Hillenbrand, M., Klenk, C., Batyuk, A., Heine, P., Balada, S., Schlinkmann, K. M., Scott, D.J., Schutz, M., Pluckthun, A. Structure of signaling-competent neurotensin receptor 1 obtained by directed evolution in Escherichia coli. Proc Natl Acad Sci USA 111, 655-662, 2014.
5. Grosse, J., Heffron, H., Coll, A.P., Burling, K., Hossain, M.A., Habib, A.M., Rogers, G.J., Richards, P., Larder, R., Rimmington, D., Parton, L., Binda, M., Colledge, W., Doran, J., Toyoda, Y., Wade, J.D., Aparicio, S., Carlton, M., Coll, A.P., Reimann, F., O’Rahilly, S., Gribble, F.M. Insulin-like peptide 5 is an orexigenic gastro-intestinal hormone. Proc Natl Acad Sci USA 111, 11133-11138, 2014.
6. Hossain M.A., Wade, J.D. Synthetic relaxins. Curr Opin Chem Biol, 22, 47-55, 2014.
7. Hojo, K., Shinozaki, N., Hidaka, K., Tsuda, Y., Fukumori, Y., Ichikawa, H., Wade, J.D. Aqueous microwave-assisted solid phase peptide synthesis using Fmoc- strategy. III. Racemization studies and water-based synthesis of histidine-containing peptides. Amino Acids 46, 2347-2354, 2014.
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 124 125
CHAPTER STROKE
PAGES 82–99
8. Jamasbi, E., Batinovic, S., Sharples, R., Sani, M-A., Robins-Browne, R.M., Wade, J.D., Separovic, F., Hossain, M.A. Melittin peptides exhibit different activity on different cells and model membranes. Amino Acids 46, 2759-2766, 2014.
9. Kocan, M., Sarwar, M., Hossain, M.A., Wade, J.D., Summers, R.J. Signalling profiles of H3 relaxin, H2 relaxin and the novel antagonist R3(BΔ23-27)R/I5 acting at the relaxin family peptide receptor 3 (RXFP3). Br J Pharmacol 171, 2827-2841, 2014.
10. Kong, R.C., Bathgate, R.A., Bruell, S., Wade, J.D., Gooley, P.R., Petrie, E.J. Mapping key regions of the RXFP2 lowdensity lipoprotein class-A module that are involved in signal activation. Biochemistry 53, 4537-4548, 2014.
11. Karas, J.A., Scanlon, D.B., Forbes, B.E., Vetter, I., Lewis, R.J., Gardiner, J., Separovic, F., Wade, J.D., Hossain, M.A. 2-Nitroveratryl as a photocleavable thiol protecting group for directed disulfide bond formation in the chemical synthesis of insulin. Chemistry 20, 9549-9552, 2014.
12. Li W., Tailhades, J., O’Brien-Simpson, N., Separovic, F., Otvos, L., Hossain, M.A., Wade, J.D. Proline-rich antimicrobial peptides. Amino Acids 46, 2287-2294, 2014.
13. Otvos, L., Knappe, D., Hoffmann, R., Kovalszky, I., Olah, J., Hewitson, T., Wade, J.D., Surmacz, E., Lovas, S. Development of a second generation peptide agonist and a first generation antagonist of the adiponectin receptor. Front Chem 2, 93, 2014.
14. Otvos, L., Flick-Smith, H., Ostorhazi, E., Dawson, R.M. and Wade, J.D. The designer proline-rich antibacterial peptide A3-APO prevents Bacillus anthracis mortality by deactivating bacterial toxins. Prot Pept Lett 21, 374-381, 2014.
15. Otvos, Jr, L., Vetter, S.W., Koladia, M., Knappe, D., Schmidt, R., Ostorhazi, E., Kovalszky, I., Bionda, N., Cudic, P., Surmacz, E., Wade, J.D., Hoffmann, R. The designer leptin antagonist peptide allo-aca compensates for short serum half-life with very tight binding to the receptor. Amino Acids 46, 873-882, 2014.
16. Otvos, L., Wade. J.D. Current challenges in peptide-based drug discovery. Front Chem 2, 62, 2014.
17. Ryan, P.J., Krstew, E.V., Sarwar, M., Gundlach, A.L., Lawrence, A.J. Relaxin-3 mRNA levels in nucleus incertus correlate with alcohol and sucrose intake in rats. Drug Alcohol Depend 140, 8-16, 2014.
18. Smith, C.M., Chua, B.E., Zhang, C., Walker, A.W., Haidar, M., Hawkes, D., Hossain, M.A., Shabanpoor, F., Wade, J.D., Rosengren, K.J., Gundlach, A.L. Central injection of relaxin-3 receptor antagonist peptides reduces motivated food seeking and consumption in mice. Behav Brain Res 268, 117-126, 2014.
19. Smith, C.M., Walker, A.W., Hosken, I.T., Chua, B.E., Zhang, C., Haidar, M., Gundlach, A.L. Relaxin-3/RXFP3 networks: an emerging target for the treatment of depression and other neuropsychiatric diseases? Front Pharmacol 5, 46, 2014.
20. Scott, D.J., Kummer, L., Egloff, P., Bathgate, R.A., Pluckthun, A. Improving the apo-state detergent stability of NTS1 with CHESS for pharmacological and structural studies. Biochim Biophys Acta 1838, 2817-2824, 2014.
21. Unsworth N.B., Dawson, R.M., Wade, J.D., Liu, C-Q. Synergistic effect of antimicrobial peptides on the in vitro growth of Coxiella burnetti. Prot Pept Lett 21, 115-123, 2014.
1. Amarenco P, Davis S, Jones EF, Cohen AA, Heiss WD, Kaste M, et al. Clopidogrel Plus Aspirin Versus Warfarin in Patients With Stroke and Aortic Arch Plaques. Stroke. 2014 Apr 3.
2. Andrew NE, Kilkenny M, Naylor R, Purvis T, Lalor E, Moloczij N, Cadilhac DA. Understanding long-term unmet needs in Australian survivors of stroke. Int J Stroke, available online 15 Jul 2014 DOI: 10.1111/ijs.12325
3. Andrew NE, Hankey GJ, Cadilhac DA. Evidence-to-practice gaps in post-stroke management: a focus on care in a stroke unit and anticoagulation to prevent death, disability and recurrent stroke. Future Neurology 2014; 9(4):449-459 DOI: 10.2217/fnl.14.30
4. Andrew N, Kilkenny M, Harris D, Price C, Cadilhac D. Outcomes for people with atrial fibrillation in an Australian national audit of stroke care. Int J Stroke 2014 Apr;9(3):270-7 DOI: 10.1111/ijs.12087
5. Askim T, Salveson O, Bernhardt J. Indredavik B. Physical activity early after stroke and its association to functional outcome 3 months later. Journal of Stroke and Cerebrovascular Diseases. 23:5:e305-e312
6. Bath PM, Brainin M, Brown C, Campbell B, Davis SM, Donnan GA, et al. Testing devices for the prevention and treatment of stroke and its complications. Int J Stroke. 2014 Aug;9(6):683-95.
7. Billinger S, Arena, R, Bernhardt J, Eng J, Franklin B, Johnson C, MacKay-Lyons M, Macko R, Mead G, Röth E, Shaughnessy M, Tang A. Physical Activity and Exercise Recommendations for Stroke Survivors: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. http:// stroke.ahajournals.org/content/early/2014/05/20/STR.0000000000000022
8. Bladin C, Cadilhac D. Effect of telestroke on emergent stroke care and outcomes. Stroke 2014;45(6):1876-1880 (Review)
9. Brodtmann A, Werden E, Pardoe H, Li Q, Jackson G, Donnan G, Cowie T, Bradshaw J, Darby D, Cumming T (2014). Charting cognitive and volumetric trajectories after stroke: Protocol for the Cognition And Neocortical Volume After Stroke (CANVAS) Study. International Journal of Stroke, 9, 824-828.
10. Cadilhac DA, Vu M, Bladin C. Experience with scaling up the Victorian Stroke Telemedicine programme, Journal of Telemedicine and Telecare 2014, Vol. 20(7) 413–418.
11. Cadilhac DA and Anderson CS. Pathways to enhancing the quality of stroke care through national data monitoring systems for hospitals [Letter], MJA 2014 Apr 21;200(7):393
12. Cadilhac D, Moloczij N, Denisenko S, Dewey H, Disler P, Winzar B, Mosely I, Donnan G, Bladin C. Establishment of an effective acute stroke telemedicine program for Australia: protocol for the Victorian Stroke Telemedicine project. Int J Stroke 2014 Feb;9(2):252-8, DOI: 10.1111/ijs.12137
13. Cadilhac D.A, Vos T, Thrift A.G. Estimating the annual number of strokes and the issue of imperfect data: an example from Australia. Int J Stroke 2014 Jan;9(1):19-22. DOI: 10.1111/ijs.12246
14. Campbell BCV, Mitchell PJ, Yan B, Parsons MW, Christensen S, Churilov L, Dowling RJ, Dewey H, Brooks M, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Kleinig T, Scroop R, Chryssidis S, Barber PA, Hope A, Moriarty M, McGuinness B, Wong AA, Coulthard A, Wijeratne T, Lee A, Jannes J, Leyden J, Phan TG, Chong W, Holt M, Chandra RV, Bladin C, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Davis SM, Donnan GA.A multicenter, randomized, controlled study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits with Intra-Arterial therapy (EXTEND-IA). International Journal of Stroke 2014; 9:126-132.
15. Campbell BC, Davis SM, Donnan GA. Uric acid for stroke: glimmer of hope or false dawn? Lancet Neurol. 2014 May;13(5):440-1.
16. Campbell BC, Mitchell PJ, Yan B, Parsons MW, Christensen S, Churilov L, et al. A multicenter, randomized, controlled study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits with Intra-Arterial therapy (EXTEND-IA). Int J Stroke. 2014 Jan;9(1):126-32.
17. Carey, L.M. (2014) Core Concepts in Sensory Impairment and Recovery Following Stroke. In Wolf, T. Stroke: Interventions to Support Occupational Performance. AOTA Press. Pp. 95-118. (ISBN: 1569003645)
18. Carey, L.M. (2014). Loss of somatic sensation. In Selzer, N., Clarke, S., Cohen, L., Kwakkel, G., Miller. R. Textbook of Neural Repair and Rehabilitation (2nd ed). Cambridge University Press. (ISBN: 9781107011687)
19. Carey, L., Cusick, A., Hoffman, T., Elliot, C., Froude, E., O’Reilly, N. (2014). Occupational Therapy Australia Research Foundation: Building a Research Foundation for all occupational therapists. Connections 11 (1): 12-13.
20. Churilov L, Arnup S, Johns H, Leung T, Roberts S, Campbell BC, et al. An improved method for simple, assumption-free ordinal analysis of the modified Rankin Scale using generalized odds ratios. Int J Stroke. 2014 Sep 4.
21. Clarey J, Lasserson, D, Levi C, Parsons M, Dewey H, Barber PA, Quain D, McElduff P, Sales M, Magin P. Absolute cardiovascular risk and GP decision-making in TIA and minor stroke. Family Practice 2014 (accepted 4 August 2014).
22. Craig L, Wu O, Bernhardt J. Langhorne P, Approaches to economic evaluations of stroke rehabilitation: a systematic review. International Journal of Stroke. 9:88-100
23. Cumming T, Brodtmann A, Darby D, Bernhardt J. The importance of cognition to quality of life after stroke. Journal of Psychosomatic Research. 77(5):374-9
24. Cumming TB & Mead G (2014). Post-stroke fatigue: Common but poorly understood. In: Management of Poststroke Complications, A Bhalla & J Birns (eds). Springer.
25. Dagonnier M, Howells DW, Donnan GA, Dewey HM. Recruitment to trials of late thrombolysis: Lessons from the EXTEND study. Recruitment to trials of late thrombolysis: Lessons from the EXTEND study. Journal of Clinical Neuroscience 2014;21(7):1215-1219
26. Drury, P, Levi C, D’Este C, McElduff P, McInnes E, Hardy J, Dale S, Cheung N W, Grimshaw J, Quinn C, Ward J, Evans M, Cadilhac D, Griffiths R, Middleton S. Quality in Acute Stroke Care (QASC): Process evaluation of an intervention to improve the management of fever, hyperglycemia and swallowing dysfunction following acute stroke. Int J Stroke 2014;9(6):766-76 DOI: 10.1111/ijs.12202
27. Drury P, Levi C, McInnes L, Hardy J, Ward J, Grimshaw J, D’Este C, Dale S, McElduff P, Cheung W, Quinn C, Griffiths R, Evans M, Cadilhac D, Middleton S. Management of fever, hyperglycaemia and swallowing dysfunction following hospital admission for acute stroke in New South Wales, Australia. Int J Stroke 2014 Jan;9(1):23-31. DOI: 10.1111/ijs.12194
28. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014 Aug 5.
29. English C, Hillier S, Bernhardt J. Circuit class therapy and 7-day week therapy increase physiotherapy time, but not patient activity. Stroke. http://stroke.ahajournals.org/content/early/2014/08/14/STROKEAHA.114.006038
30. Egan K, Janssen H, Sena E, Longley L, Speare S, Howells D, Spratt N, Macleod M, Mead G, Bernhardt J. Exercise reduces infarct volume and facilitates neurobehavioural recovery: a systematic review and meta-analysis of exercise in models of ischaemic stroke. Neurorehabilitation and Neural Repair. (Epub) http://nnr.sagepub.com/ content/early/2014/02/14/1545968314521694
31. English C, Manns P, Tucak C, Bernhardt J, Physical activity and sedentary behaviours in people with stroke living in the community: A systematic review. Physical Therapy 94:2:185-196.
32. Fini N, Holland A, Keating J, Simek J, Bernhardt J. How is physical activity monitored in people following stroke? Disability and Rehabilitation. Online 1-15 http://www.ncbi.nlm.nih.gov/pubmed/25374044
33. Flint AC, Gupta R, Smith WS, Kamel H, Faigeles BS, Cullen SP, et al. The THRIVE score predicts symptomatic intracerebral hemorrhage after intravenous tPA administration in SITS-MOST. Int J Stroke. 2014 Aug;9(6):705-10.
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 126 127
34. Gloede T, Halbach S, Thrift A, Dewey H, Pfaff H, Cadilhac D. Long-term costs of stroke using 10-year longitudinal data from the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke. 2014;45:3389-3394, doi: 10.1161/STROKEAHA.114.006200.]
35. Grimmer K, JM, Milanese S, King E, Beaton K, Thorpe O, Lizarondo L, Luker J, Machotka Z, Kumar S. Efficient clinical evaluation of guideline quality: development and testing of a new tool. BMC Medical Research Methodology 2014; 14(1):63.
36. Hersh D, Godecke E, Armstrong E, Ciccone N, Bernhardt J. “Ward talk”: Nurses’ interaction with people with and without aphasia in the very early period poststroke. Aphasiology. http://dx.doi.org/10.1080/02687038.2014.93 3520
37. Hubbard, I., Carey, LM., Budd, T. W. Levi, C, McElduff, P., Hudson, S., Bateman, G., Parsons, MW. (2014) A randomized controlled trial of early upper limb training on stroke recovery and brain activation. Neurorehabilitation and Neural Repair. (Published online before print; DOI: 10.1177/1545968314562647)
38. Hubbard IJ, Carey LM, Budd TW and Parsons MW (2014). Reorganizing therapy: Changing the clinical approach to upper limb recovery post-stroke. Occupational Therapy International DOI: 10.1002/oti.1381 (early online)
39. Li Q, Pardoe H, Lichter R, Werden E, Raffelt A, Cumming T, Brodtmann A (in press). Cortical thickness estimation in longitudinal stroke studies: A comparison of 3 measurement methods. Neuroimage: Clinical. doi:10.1016/j. nicl.2014.08.017.
40. Liu N, Cadilhac DA, Andrew NE, Zeng L, Li Z, Li J, Li Y, Yu X, Mi B, Li Z, Xu H, Chen Y, Wang J, Yao W, Li K, Yan F, Wang J. Randomized controlled trial of early rehabilitation after intracerebral hemorrhage stroke: difference in outcomes within 6 months of stroke. Stroke 2014, available online October 21.
41. Jackman KA, Cumming T & Miller AA (2014). Stroke, cognitive function and Alzheimer’s disease. In: Genes, Environment and Alzheimer’s Disease, G Tesco & O Lazarov (eds).
42. Kilkenny MF, Johnson R, Andrew NE, Purvis T, Hicks A, Colagiuri S and Cadilhac DA. Comparison of two methods for assessing diabetes risk in a pharmacy setting in Australia. BMC Public Health 2014, 14:1227.
43. Lemmens R, Christensen S, Straka M, De Silva DA, Mlynash M, Campbell BC, et al. Patients with single distal MCA perfusion lesions have a high rate of good outcome with or without reperfusion. Int J Stroke. 2014; 9(2): 156-9.
44. Luker J, Bernhardt J, Grimmer K, and Edwards I. A qualitative exploration of discharge destination as an outcome or a driver of acute stroke care. BMC Health Services Research. 2014, 14:193
45. Mitra, J., Shen, K., Ghose, S., Bourgeat, P., Fripp, J., Salvado, O., Campbell, B., Connelly, A., Palmer, S., Carey, L, Rose, S. (2014) Predicting poststroke depression from brain connectivity. MICCAI (Multimodal Brain Image Analysis (MBIA) 2014 Workshop on Computational Diffusion MRI Lecture Notes in Computer Science, SpringerAccepted 4/7/2014, not online as of 21/01/2015)
46. Lynch L, Luker J, Cadilhac DA, Hillier S. Rehabilitation assessments for patients with stroke in Australian hospitals do not always reflect the patients’ rehabilitation requirements, Archives of Physical Medicine and Rehabilitation, accepted 12 December 2014
47. Lynch E, Cadilhac D, Hillier S. When should physical rehabilitation commence after stroke: a systematic review. Int J Stroke 2014 Jun;9(4):468-478 DOI 10.1111/ijs.12262
48. Ma H, Wright P, Allport L, Phan TG, Churilov L, Ly J, et al. Salvage of the PWI/DWI mismatch up to 48 h from stroke onset leads to favorable clinical outcome. Int J Stroke. 2014 Mar 11.
49. Mackay MT, Chua ZK, Lee M, Yock-Corrales A, Churilov L, Monagle P, et al. Stroke and nonstroke brain attacks in children. Neurology. 2014 Apr 22;82(16):1434-40.
50. Meretoja A, Churilov L, Campbell BC, Aviv RI, Yassi N, Barras C, et al. The spot sign and tranexamic acid on preventing ICH growth--AUStralasia Trial (STOP-AUST): protocol of a phase II randomized, placebo-controlled, double-blind, multicenter trial. Int J Stroke. 2014; 9(4): 519-24.
51. Meretoja A, Keshtkaran M, Saver JL, Tatlisumak T, Parsons MW, Kaste M, et al. Stroke thrombolysis: save a minute, save a day. Stroke. 2014 Apr;45(4):1053-8.
52. Meumann EM, Chuen J, Fitt G, Perchyonok Y, Pond F, Dewey HM. Thromboembolic stroke associated with thoracic outlet syndrome. Journal of Clinical Neuroscience2014;21(5):886-9.
53. Mitra, J., Bourgeat, P., Fripp, J., Ghose, S., Rose, S., Salvado, O., Connelly, A., Campbell, B., Palmer, S., Sharma, G., Christensen, S., Carey, L. (2014). Lesion segmentation from multimodal MRI using random forest following ischemic stroke. NeuroImage 98 (2014) 324–335 (DOI: 10.1016/j.neuroimage.2014.04.056)
54. Mosley I, Nicol M, Donnan G, Thrift AG, Dewey HM. What is stroke symptom knowledge? Int J Stroke. 2014; 9(1): 48-52.
55. Muhl L, Kulin J, Dagonnier M, Churilov L, Dewey H, Linden T, Bernhardt J. Mobilization after thrombolysis (rtPA) within 24 hours of acute stroke: What factors influence inclusion of patients in A Very Early Rehabilitation Trial (AVERT)? A retrospective audit. BioMed Central http://www.biomedcentral.com/1471-2377/14/163
56. O’Halloran P, Blackstock F, Shields N, Holland A, Iles R, Kingsley M, Bernhardt J, Lannin N, Morris M, Taylor N. Motivational interviewing to increase physical activity in people with chronic health conditions: a systematic review and meta-analysis. Clinical Rehabilitation Vol. 28(12) 1159–1171
57. Palmer, S.M., Crewther, S.G., Carey, L.M. and the START project team. (2015) A meta-analysis of changes in brain activation and connectivity in clinical depression. Frontiers in Human Neuroscience, 8, 1045 (DOI: 10.3389/ fnhum.2014.01045)
58. Pascoe, M., Howells, D.W., Crewther, D.P., Carey, L., Rewell, S., Ho, H., Crewther, S.G. (2015). Fish oil supplementation associated with decreased cellular degeneration and increased cellular proliferation six weeks after middle cerebral artery occlusion in the rat. Neuropsychiatric Disease and Treatment, 11, 153-164 (DOI: 10.2147/NDT.572925)
59. Pascoe, M.C., Howells, DW., Crewther, DP, Constantinou, N, Carey, L.M., Rewell, SS, Turchini, GM, Kaur, G, Crewther, S.G. (2014). Fish oil diet associated with acute reperfusion related haemorrhage, and with reduced stroke-related sickness behaviours and motor impairment. Frontiers in Stroke Vol 5, Article 14 (pp1-15). Doi:10.3389/fneur.2014.00014
60. Paul CL, Levi CR,D’Este CA, Parsons MW, Bladin CF, Lindley RI, Attia JR, Henskens F, Lalor E, Longworth M, Middleton S, Ryan A, Kerr E, Sanson-Fisher RW, Anderson C, Bruce I, Buchan H, Burdusel C, Butler E, Cadigan G, Campbell Z, Corbett A, Day S, Denisenko S, Dennett J, Dewey H et.al. Thrombolysis ImPlementation in Stroke (TIPS): Evaluating the effectiveness of a strategy to increase the adoption of best evidence practice – protocol for a cluster randomised controlled trial in acute stroke care. Implementation Science 2014;9(1)article 38.
61. Picanco MR, Christensen S, Campbell BC, Churilov L, Parsons MW, Desmond PM, et al. Reperfusion after 4.5 hours reduces infarct growth and improves clinical outcomes. Int J Stroke. 2014; 9(3): 266-9.
62. Pumpa, L., Cahill, L.S., Carey, L.M. (in press) Somatosensory assessment and treatment after stroke: An evidencepractice gap. Australian Occupational Therapy Journal. (Accepted 18th sept 2014; [Epub 23 January 2015 ahead of print].
63. T Purvis, K Moss, S Denisenko, C Bladin, DA Cadilhac on behalf of the Victorian Stroke Clinical Network. Implementation of evidence-based stroke care: enablers, barriers, and the role of facilitators. Journal of Multidisciplinary Healthcare 2014:7 389–400.
64. Purvis T, Bernhardt J, Indredavik B, Cadilhac DA. Interdisciplinary team interactions in stroke units: can team dynamics influence patient outcomes from a clinician’s perspective. International Journal of Physical Medicine and Rehabilitation 2014 S3:007. DOI: 10.4172/2329-9096.S3-007.
65. Randall, M., Imms, C., Carey, L. & Pallant.J. (2014) Rasch analysis of the Melbourne Assessment of Unilateral Upper Limb Function. Developmental Medicine and Child Neurology, 56 (7), 665-672 (DOI:10.1111/dmcn.12391)
66. Sahathevan R, Ali KM, Ellery F, Mohamad NF, Hamdan N, Ibrahim NM, Churilov L, Cumming TB. A Bahasa Malaysia version of the Montreal Cognitive Assessment: Validation in stroke. International Psychogeriatrics, 2014, 26 (5) 781-786. DOI: 10.1017/S1041610213002615.
67. Sanders L, Cadilhac DA, Srikanth V, Pei Chong C, Phan TG. Is non-admission based care for TIA patients costeffective? A micro-costing study. Neurology: Clinical Practice, accepted 4 March, 2014.
68. Sheedy R, Bernhardt J, Levi C, Longworth M, Churilov L, Kilkenny M, Cadilhac D. Are patients with intracerebral haemorrhage disadvantaged in hospitals? International Journal of Stroke. 9:437-442
69. Sheth KN, Kimberly WT, Elm JJ, Kent TA, Mandava P, Yoo AJ, et al. Pilot Study of Intravenous Glyburide in Patients With a Large Ischemic Stroke. Stroke. 2014 Jan;45(1):281-3.
70. Sheth KN, Taylor Kimberly W, Elm JJ, Kent TA, Yoo AJ, Thomalla G, et al. Exploratory Analysis of Glyburide as a Novel Therapy for Preventing Brain Swelling. Neurocritical care. 2014 Mar 27.
71. Sjöholm A, Skarin M, Churilov L, Nilsson M, Bernhardt J, Linden T. Sedentary Behaviour and Physical Activity of People with Stroke in Rehabilitation Hospitals. Stroke Research and Treatment. http://www.hindawi.com/ journals/srt/2014/591897/
72. Thrift AG, Cadilhac DA, Thayabaranathan T, Howard G, Howard VJ, Rothwell PM, et al. Global stroke statistics. Int J Stroke. 2014 Jan;9(1):6-18.
73. Thrift AG, Srikanth V, Nelson M, Kim J, Fitzgerald S, Gerraty R, Bladin B, Phan T, Cadilhac D. Risk factor management in survivors of stroke: a double-blind, cluster randomised-controlled trial. Int J Stroke 2014, Vol 9, July 2014, 652–657, DOI:10.1111/j.1747-4949.2012.00933.
74. Valentin C, Dones VC, Grimmer K, Thoirs K, Suarez CG, Luker J. The diagnostic validity of musculoskeletal ultrasound in lateral epicondylalgia: a systematic review. BMC Medical Imaging 2014; 14(1):10.
75. Veldsman M, Cumming T, Brodtmann A (in press). Beyond BOLD: Optimizing functional imaging in stroke populations. Human Brain Mapping.
76. Zhang W, Speare S, Thuy M, Churilov L, Bernhardt J. Stroke rehabilitation in China: A systematic review and meta-analysis. International Journal of Stroke. 4.9:494-502 Johnson L. Bridging the evidence-practice divide –will new standards help rehabilitation research? International Journal of Therapy and Rehabilitation 2014; 21(10): 257-258.
77. Zhang W, Cadilhac DA, Churilov L, Donnan GA, O’Callaghan C, Dewey HM. Does abnormal circadian blood pressure pattern really matter in patients with transient ischemic attack or minor stroke? Stroke. 2014; 45(3): 865-7.
78. Zhang WW, Speare S, Churilov L, Thuy M, Donnan G, Bernhardt J. Stroke rehabilitation in China: a systematic review and meta-analysis. Int J Stroke. 2014; 9(4): 494-502.
79. Antonic, A., Dottori, M., Leung, J., Sidon, K., Batchelor, P.E., Wilson, W., Macleod, M.R., and Howells, D.W. (2014). Hypothermia protects human neurons. Int J Stroke 9, 544-552.
80. Ardipradja, K., Yeoh, S.D., Alt, K., O’Keefe, G., Rigopoulos, A., Howells, D.W., Scott, A.M., Peter, K., Ackerman, U., and Hagemeyer, C.E. (2014). Detection of activated platelets in a mouse model of carotid artery thrombosis with 18 F-labeled single-chain antibodies. Nuclear medicine and biology 41, 229-237.
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 128 129
CHAPTER SYSTEMS
NEUROPHYSIOLOGY
PAGES 100–103
81. Chalmers, I., Bracken, M.B., Djulbegovic, B., Garattini, S., Grant, J., Gulmezoglu, A.M., Howells, D.W., Ioannidis, J.P., and Oliver, S. (2014). How to increase value and reduce waste when research priorities are set. Lancet 383, 156-165.
82. Dagonnier, M., Howells, D.W., Donnan, G.A., and Dewey, H.M. (2014). Recruitment to trials of late thrombolysis: Lessons from the EXTEND study. J Clin Neurosci 21, 1215-1219.
83. Egan, K.J., Janssen, H., Sena, E.S., Longley, L., Speare, S., Howells, D.W., Spratt, N.J., Macleod, M.R., Mead, G.E., and Bernhardt, J. (2014). Exercise reduces infarct volume and facilitates neurobehavioral recovery: results from a systematic review and meta-analysis of exercise in experimental models of focal ischemia. Neurorehabil Neural Repair 28, 800-812.
84. Howells, D.W., Sena, E.S., and Macleod, M.R. (2014). Bringing rigour to translational medicine. Nature reviews. Neurology 10, 37-43.
85. Howells, D.W. (2014). Stroke, Animal Models. In Encyclopedia of the Neurological Sciences, Volume Accepted 13th July, 2nd Edition, M.J. Aminoff and R.B. Daroff, eds. (San Diego: Academic Press).
86. McCann, S.K., Irvine, C., Mead, G.E., Sena, E.S., Currie, G.L., Egan, K.E., Macleod, M.R., and Howells, D.W. (2014). Efficacy of antidepressants in animal models of ischemic stroke: a systematic review and meta-analysis. Stroke 45, 3055-3063.
87. O’Collins, V.E., Markus, R., and Howells, D.W. (2014). Neurotoxicity and Stroke. In Handbook of Neurotoxicity, Volume 2, R.A. Kostrzewa, ed. (New York: Springer), pp. 1483-1509.
88. Pascoe, M.C., Howells, D.W., Crewther, D.P., Constantinou, N., Carey, L.M., Rewell, S.S., Turchini, G.M., Kaur, G., and Crewther, S.G. (2014). Fish oil diet associated with acute reperfusion related hemorrhage, and with reduced stroke-related sickness behaviors and motor impairment. Frontiers in neurology 5, 14.
89. Sena, E.S., Currie, G.L., McCann, S.K., Macleod, M.R., and Howells, D.W. (2014). Systematic reviews and metaanalysis of preclinical studies: why perform them and how to appraise them critically. J Cereb Blood Flow Metab 34, 737-742.
90. Vesterinen, H.M., Sena, E.S., Egan, K.J., Hirst, T.C., Churolov, L., Currie, G.L., Antonic, A., Howells, D.W., and Macleod, M.R. (2014). Meta-analysis of data from animal studies: A practical guide. J Neurosci Methods 221, 92-102.
91. Watzlawick, R., Sena, E.S., Dirnagl, U., Brommer, B., Kopp, M.A., Macleod, M.R., Howells, D.W., and Schwab, J.M. (2014). Effect and Reporting Bias of RhoA/ROCK-Blockade Intervention on Locomotor Recovery After Spinal Cord Injury: A Systematic Review and Meta-analysis. JAMA Neurol 71, 91-99.
1. Martelli D, McKinley MJ, McAllen RM. (2014) The cholinergic anti-inflammatory pathway: A critical review. Auton Neurosci. 182:65-9.
2. Martelli D, Yao ST, McKinley MJ, McAllen RM. (2014) Reflex control of inflammation by sympathetic nerves, not the vagus. J Physiol. 592(Pt 7):1677-86.
3. Martelli D, Silvani A, McAllen RM, May CN, Ramchandra R. (2014) The low frequency power of heart rate variability is neither a measure of cardiac sympathetic tone nor of baroreflex sensitivity. Am J Physiol Heart Circ Physiol. 2014 Oct 1;307(7):H1005-12.
4. Martelli D, Yao ST, Mancera J McKinley MJ, McAllen RM. (2014) Reflex control of inflammation by the splanchnic anti-inflammatory pathway is sustained and independent of anesthesia. Am J Physiol Regul Integr Comp Physiol. 2014 Nov 1;307(9):R1085-91.
5. Martelli D, Yao ST, McKinley MJ, McAllen RM. (2014) Neural control of inflammation by the greter splanchnic nerves. Temperature 1 (1) 14-15.
6. Shafton AD, McKinley MJ, Kitchener P, McAllen RM. (2014) Reflex control of rat tail sympathetic nerve activity by abdominal temperature. Temperature 1 (1) 37-41.
7. Farrell MJ, Trevaks D, McAllen RM. (2014) Preoptic activation and connectivity during thermal sweating in humans. Temperature 1 (2) 135-141.
8. Dutschmann M. ‘Big Chief’ - for Respiratory Physiology & Neurobiology. Respir Physiol Neurobiol. 2015 Jan 30. pii: S1569-9048(15)00028-2. doi: 10.1016/j.resp.2015.01.015.
9. Dutschmann M, Jones SE, Subramanian HH, Stanic D, Bautista TG. The physiological significance of postinspiration in respiratory control. Prog Brain Res. 2014;212:113-30. doi: 10.1016/B978-0-444-634887.00007-0.
10. Dick TE, Dutschmann M, Feldman JL, Fong AY, Hülsmann S, Morris KM, Ramirez JM, Smith JC; the respiratory neurobiology consortium. Facts and challenges in Respiratory Neurobiology. Respir Physiol Neurobiol. 2015 Jan 30. pii: S1569-9048(15)00027-0. doi: 10.1016/j.resp.2015.01.014.
11. Bautista TG, Fong AY, Dutschmann M. Spontaneous swallowing occurs during autoresuscitation in the in situ brainstem preparation of rat. Respir Physiol Neurobiol. 2014 Oct 1;202:35-43. doi: 10.1016/j.resp.2014.07.015.
12. Farmer DG, Bautista TG, Jones SE, Stanic D, Dutschmann M. The midbrain periaqueductal grey has no role in the generation of the respiratory motor pattern, but provides command function for the modulation of respiratory activity. Respir Physiol Neurobiol. 2014 Dec 1;204:14-20. doi: 10.1016/j.resp.2014.07.011.
13. Bautista TG, Dutschmann M. Ponto-medullary nuclei involved in the generation of sequential pharyngeal swallowing and concomitant protective laryngeal adduction in situ. J Physiol. 2014 Jun 15;592(Pt 12):2605-23. doi: 10.1113/jphysiol.2014.272468.
14. Bautista TG, Dutschmann M. Inhibition of the pontine Kölliker-Fuse nucleus abolishes eupneic inspiratory hypoglossal motor discharge in rat. Neuroscience. 2014 May 16;267:22-9. doi: 10.1016/j. neuroscience.2014.02.027.
15. Dutschmann M, Bautista TG, Mörschel M, Dick TE. Learning to breathe: habituation of Hering-Breuer inflation reflex emerges with postnatal brainstem maturation. Respir Physiol Neurobiol. 2014 May 1;195:44-9. doi: 10.1016/j.resp.2014.02.009.
16. Langenberg C, Gobe G, Hood S, May CN, Bellomo R. Renal Histopathology during Experimental Septic Acute Kidney Injury and Recovery. Critical Care Medicine 42: e58-67; 2014.
17. Lu Ke Calzavacca P, Bailey M, Wei-qin Li, Bellomo R, May CN. Acid-base changes after fluid bolus: sodium chloride vs. sodium octanoate. Intensive Care Medicine Experimental 1: 4; 2014.
18. Lu Ke, Calzavacca P, Bailey M, Wei-qin Li, Bellomo R May CN. Systemic and renal haemodynamic effects of fluid bolus therapy: sodium chloride versus sodium octanoate-balanced solution. Critical Care and Resuscitation. 16:29-33; 2014.
19. Calzavacca P, Bailey M, Velkoska E, Burrell LM, Ramchandra R, Bellomo R, May CN. Effects of Renal Denervation on Regional Hemodynamics and Kidney Function in Experimental Hyperdynamic Sepsis. Critical Care Medicine 42: e401-9; 2014.
20. Frithiof R, Xing T, McKinley MJ, May CN, and Ramchandra R. Intracarotid hypertonic sodium chloride differentially modulates sympathetic nerve activity to the heart and kidney. American Journal of Physiology 15: R567-75; 2014.
21. Calzavacca P, May CN, Bellomo R. Glomerular hemodynamics, the sympathetic system and sepsis-induced acute kidney injury. Nephrology Dialysis Transplantation 29: 2178-2184; 2014.
22. Booth LC, Ramchandra R, Calzavacca P, May CN. Role of Prostaglandins in Determining the Increased Cardiac Sympathetic Nerve Activity in Ovine Sepsis. American Journal of Physiology 307: R75-81; 2014.
23. Ramchandra R, Hood SG, May CN. Central nitric oxide decreases cardiac sympathetic drive and improves baroreflex control of heart rate in ovine heart failure. American Journal of Physiology 307: R271-80; 2014.
24. Xing DT, May CN, Booth LC, Ramchandra R. Tonic arterial chemoreceptor activity contributes to cardiac sympathetic activation in mild ovine heart failure. Experimental Physiology 99(8):1031-41; 2014.
25. Martelli D, Silvani A, McAllen RM, May CN, Ramchandra R. The low frequency power of heart rate variability is neither a measure of cardiac sympathetic tone nor of baroreflex sensitivity. American Journal of Physiology 307: H1005-12; 2014.
26. Darwinkel A, Stanić D, Booth LC, May CN, Lawrence AJ, Yao ST. Distribution of orexin-1 receptor-green fluorescent protein- (OX1-GFP) expressing neurons in the mouse brain stem and pons: co-localisation with tyrosine hydroxylase and neuronal nitric oxide synthase. Neuroscience 278: 253-64; 2014.
27. Calzavacca P, Lankadeva Y, Bailey SR, Bailey M, Bellomo R, May CN. Effects of Selective β1-Adrenoceptor Blockade on Cardiovascular and Renal Function and Circulating Cytokines in Ovine Hyperdynamic Sepsis. Critical Care 18: 610; 2014.
28. Schneider GA, Calzavacca P, Schelleman A, Huynh T, Bailey M, May CN, Bellomo R. Contrast-enhanced ultrasound evaluation of renal microcirculation in sheep. Intensive Care Medicine Experimental 2: 33; 2014.
29. Calzavacca P, Ishikawa K, Bailey M, May CN, Bellomo R. Systemic and Renal Hemodynamic Effects of Intraarterial Radiocontrast. Intensive Care Medicine Experimental 2: 32; 2014.
30. Ruchaya PJ, Antunes VR, Paton JF, Murphy D, Yao ST. The cardiovascular actions of fractalkine/CX3CL1 in the hypothalamic paraventricular nucleus are attenuated in rats with heart failure. Exp Physiol. 2014 Jan;99(1):11122.
31. Darwinkel A, Stanić D, Booth LC, May CN, Lawrence AJ, Yao ST. Distribution of orexin-1 receptor-green fluorescent protein- (OX1-GFP) expressing neurons in the mouse brain stem and pons: Co-localization with tyrosine hydroxylase and neuronalitric oxide synthase. Neuroscience. 2014 Oct 10;278:253-64.
32. Martelli D, Yao ST, Mancera J, McKinley MJ, McAllen RM. Reflex control ofinflammation by the splanchnic anti-inflammatory pathway is sustained andindependent of anesthesia. Am J Physiol Regul Integr Comp Physiol. 2014 Nov1;307(9):R1085-91.
33. Qiu J, Kleineidam A, Gouraud S, Yao ST, Greenwood M, Hoe SZ, Hindmarch C, Murphy D. The use of proteinDNA, chromatin immunoprecipitation, and transcriptome arrays to describe transcriptional circuits in the dehydrated male rat hypothalamus. Endocrinology. 2014 Nov;155(11):4380-90.
34. Martelli D, Yao ST, McKinley MJ, McAllen RM. Reflex control of inflammation by sympathetic nerves, not the vagus. J Physiol. 2014 Apr 1;592(Pt 7):1677-86.
35. Booth LC, Nishi EE, Yao ST, Ramchandra R, Lambert GW, Schlaich MP, May CN. Reinnervation of renal afferent and efferent nerves at 5.5 and 11 months after catheter-based radiofrequency renal denervation in sheep. Hypertension. 2015 Feb;65(2):393-400.
36. Booth LC, Nishi EE, Yao ST, Ramchandra R, Lambert GW, Schlaich MP, May CN. Reinnervation following catheter-based radio-frequency renal denervation. Exp Physiol. 2015 Jan 8.
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 130 131
37. Brunton PJ, Donadio MV, Yao ST, Greenwood M, Seckl JR, Murphy D, Russell JA. 5α-reduced neurosteroids sexdependently reverse central prenatal programming of neuroendocrine stress responses in rats. J Neurosci. 2015 Jan 14;35(2):666-77.
38. McAllen R.M., Cook A.D., Khiew H.W., Martelli D., Hamilton J.A. The interface between cholinergic pathways and the immune system and its relevance to arthritis. Arthritis Res. Ther. 2015 (accepted).
39. Martelli D., Silvani A., McAllen R.M., May C.N., Ramchandra R. Reply to “Letter to the editor: Does low-frequency power of heart rate variability correlate with cardiac sympathetic tone in normal sheep?”. Am. J. Physiol. Heart Circ. Physiol. 308(2), 2015:H148-9.
40. Cerri M., Del Vecchio F., Mastrotto M., Luppi M., Martelli D., Perez E., Tupone D., Zamboni G., Amici R. Enhanced Slow-Wave EEG Activity and Thermoregulatory Impairment following the Inhibition of the Lateral Hypothalamus in the Rat. Plos One, 9(11), 2014: e112849.
41. Martelli D., Yao S.T., Mancera J., McKinley M.J., McAllen R.M. Reflex control of inflammation by the splanchnic anti-inflammatory pathway is sustained and independent of anaesthesia. Am. J. Physiol. Regul. Int. Comp. Physiol. 307(9), 2014: R1085-91.
42. Martelli D., Silvani A., McAllen R.M., May C.N., Ramchandra R. The low frequency power of heart rate variability is neither a measure of cardiac sympathetic tone nor of baroreflex sensitivity. Am. J. Physiol. Heart Circ. Physiol. 307(7), 2014:H1005-12.
43. Martelli D., Yao S.T., McKinley M.J., McAllen R.M. Neural control of inflammation by the greater splanchnic nerves. Temperature. 2014 Epub: https://www.landesbioscience.com/article/29135/full_text/#load/info/all.
44. Martelli D., Yao S.T., McKinley M.J., McAllen R.M. Reflex control of inflammation by sympathetic nerves, not the vagus. J. Physiol., 592, 2014:1677-1686.
45. Martelli D., Luppi M., Cerri M., Tupone D., Perez E., Zamboni G., Amici R. The Direct Cooling of the PreopticHypothalamic Area Elicits the Release of Thyroid Stimulating Hormone during Wakefulness but Not during REM Sleep. Plos One, 9(2), 2014: e87793.
46. Martelli D., McKinley M.J., McAllen R.M. The cholinergic anti-inflammatory pathway: a critical review. Auton. Neurosci., 182, 2014:65-69.
47. Chambers B, Chambers J, Churilov L, Cameron H, Macdonell R. Internal jugular and vertebral vein volume flow in patients with clinically isolated syndrome or mild multiple sclerosis and healthy controls: results from a prospective sonographer-blinded study. Phlebology. 2014 Sep;29(8):528-35.
48. Confavreux C, O’Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, Wolinsky JS, Bagulho T, Delhay JL, Dukovic D, Truffinet P, Kappos L; TOWER Trial Group. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:247-56
49. Miller AE, Macdonell R, Comi G, Freedman MS, Kappos L, Mäurer M, Olsson TP, Wolinsky JS, Bozzi S, DivePouletty C, O’Connor PW. Teriflunomide reduces relapses with sequelae and relapses leading to hospitalizations: results from the TOWER study. J Neurol. 2014;261:1781-8.
50. Broadley SA, Barnett MH, Boggild M, Brew BJ, Butzkueven H, Heard R, Hodgkinson S, Kermode AG, LechnerScott J, Macdonell RA, Marriott M, Mason DF, Parratt J, Reddel SW, Shaw CP, Slee M, Spies J, Taylor BV, Carroll WM, Kilpatrick TJ, King J, McCombe PA, Pollard JD, Willoughby E. Therapeutic approaches to disease modifying therapy for multiple sclerosis in adults: An Australian and New Zealand perspective. Part 1 Historical and established therapies. J Clin Neurosci. 2014;21:1835-46
51. Broadley SA, Barnett MH, Boggild M, Brew BJ, Butzkueven H, Heard R, Hodgkinson S, Kermode AG, LechnerScott J, Macdonell RA, Marriott M, Mason DF, Parratt J, Reddel SW, Shaw CP, Slee M, Spies J, Taylor BV, Carroll WM, Kilpatrick TJ, King J, McCombe PA, Pollard JD, Willoughby E. Therapeutic approaches to disease modifying therapy for multiple sclerosis in adults: An Australian and New Zealand perspective Part 2 New and emerging therapies and their efficacy. J Clin Neurosci. 2014;21: 1847-56
52. Broadley SA, Barnett MH, Boggild M, Brew BJ, Butzkueven H, Heard R, Hodgkinson S, Kermode AG, LechnerScott J, Macdonell RA, Marriott M, Mason DF, Parratt J, Reddel SW, Shaw CP, Slee M, Spies J, Taylor BV, Carroll WM, Kilpatrick TJ, King J, McCombe PA, Pollard JD, Willoughby E. Therapeutic approaches to disease modifying therapy for multiple sclerosis in adults: An Australian and New Zealand perspective. Part 3 Treatment practicalities and recommendations. J Clin Neurosci. 2014;21: 1857-65
53. Mehndiratta MM, Mehndiratta P, Tsai CP, Kaji R, Gulati NS, Wasay M, Macdonell R.Evolution, current status, and way forward for the Asian Oceanian Association of Neurology. Neurology. 2014;83:1853-5.

“
One in four Australians are diagnosed with an anxiety disorder during their lifetime. This peaks during adolescence and the outcome following therapy is often extremely poor during this age compared to adults. I am trying to understand the neurobiology underlying this impairment while testing novel therapeutics.
The impact of treating anxiety early in life will be tremendous because it will forestall the persistence of anxiety into adulthood and the development of secondary disorders such as depression. My goal is to translate our preclinical findings from the lab bench to the clinic.
”
THE FLOREY INSTITUTE OF NEUROSCIENCE & MENTAL HEALTH ANNUAL REPORT 2014 132 133
Dr Despina Ganell
DIRECTOR’S OFFICE
Director
ҩ Professor Geoffrey Donnan
AO
Executive Administration
ҩ Colleen Buchhorn
DIVISION OF BEHAVIOURAL NEUROSCIENCE
Addiction Neuroscience Laboratory
ҩ Robyn Brown
ҩ Jhodie Duncan
ҩ Despina Ganella
ҩ Morgan James
ҩ Bianca Jupp
ҩ Elena Krstew
ҩ Andrew Lawrence
ҩ Liubov Lee-Kardashyan
ҩ Nathan Marchant
ҩ Christina Perry
ҩ Phillip Ryan
ҩ Craig Smith
PhD Students
ҩ Rose Chesworth
ҩ Nicola Chen
ҩ Alec Dick
ҩ Sophia Luikinga
ҩ Karlene Scheller
ҩ Sarah Sulaiman Ch’ng
ҩ Leigh Walker
ҩ Isabel Zbukvic
Students
ҩ Katherine Beringer
ҩ Katherine Drummond
ҩ Emma Giles
ҩ Rosa Heller
ҩ Jan Koeleman
ҩ Ashleigh Qama
ҩ Felicia Reed
ҩ Jirawoot Srisontiyakul
ҩ Shawn Tan
Developmental Pschobiology
ҩ Despina Ganella
ҩ Jee Hyun Kim
ҩ Heather Madsen
Inhalant Abuse Laboratory
ҩ Jhodie Duncan
Molecular Neurobiology Lab
ҩ John Drago















 Prof Ashley Bush & Dr Ya Hui Hung
Prof Ashley Bush & Dr Ya Hui Hung



































 BRAIN DEVELOPMENT GROUP
Binding of PTEN to Ndfip1 in the cell can be visualized by protein fluorescence –work by PhD student Yijia Li and published on the cover of Traffic.
The Brain Development team: Choo-Peng Goh, Ley-Hian Low, Anh Doan, Yijia Li, Jason Howitt, Seong-Seng Tan, Sophia Mah, Ulrich Putz, Yuh-Lit Chow, Michelle Tang, Ulrich Sterzenbach. Absent: Joanne Britto, Hui Xuan Ng, Ean Phing Lee.
Ndfip1 controls cell proliferation by driving PTEN into the cell nucleus. Work performed by Jason Howitt and published in Journal of Molecular Cell Biology
BRAIN DEVELOPMENT GROUP
Binding of PTEN to Ndfip1 in the cell can be visualized by protein fluorescence –work by PhD student Yijia Li and published on the cover of Traffic.
The Brain Development team: Choo-Peng Goh, Ley-Hian Low, Anh Doan, Yijia Li, Jason Howitt, Seong-Seng Tan, Sophia Mah, Ulrich Putz, Yuh-Lit Chow, Michelle Tang, Ulrich Sterzenbach. Absent: Joanne Britto, Hui Xuan Ng, Ean Phing Lee.
Ndfip1 controls cell proliferation by driving PTEN into the cell nucleus. Work performed by Jason Howitt and published in Journal of Molecular Cell Biology











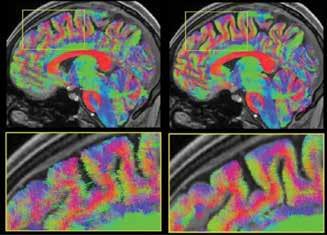





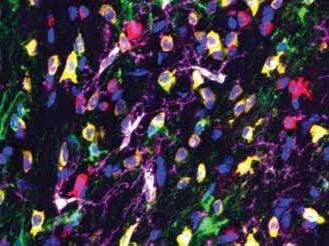




























 Dedication
Dr Alan Rembach
Dedication
Dr Alan Rembach
