9 minute read
MEDBULLETINS
from COVID-19 Mini Issue
MEDBULLETIN
COVID-19 INFECTS BRAIN ORGANOIDS
Advertisement
ADRIAN WONG
While COVID-19 is most known for causing respiratory illness, neurological symptoms have also arisen among infected patients. In April, Mao et al. reported that out of 214 patients in Wuhan, China, 36.4% also suffered from neurological manifestations.1 These symptoms can range from the common headaches, dizziness, and loss of smell or taste, to more severe conditions such as acute ischemic stroke, encephalitis, and Guillain-Barré syndrome.2 Yet, to date, the connection between COVID-19 infection and pathologies of the nervous system remains incomplete.
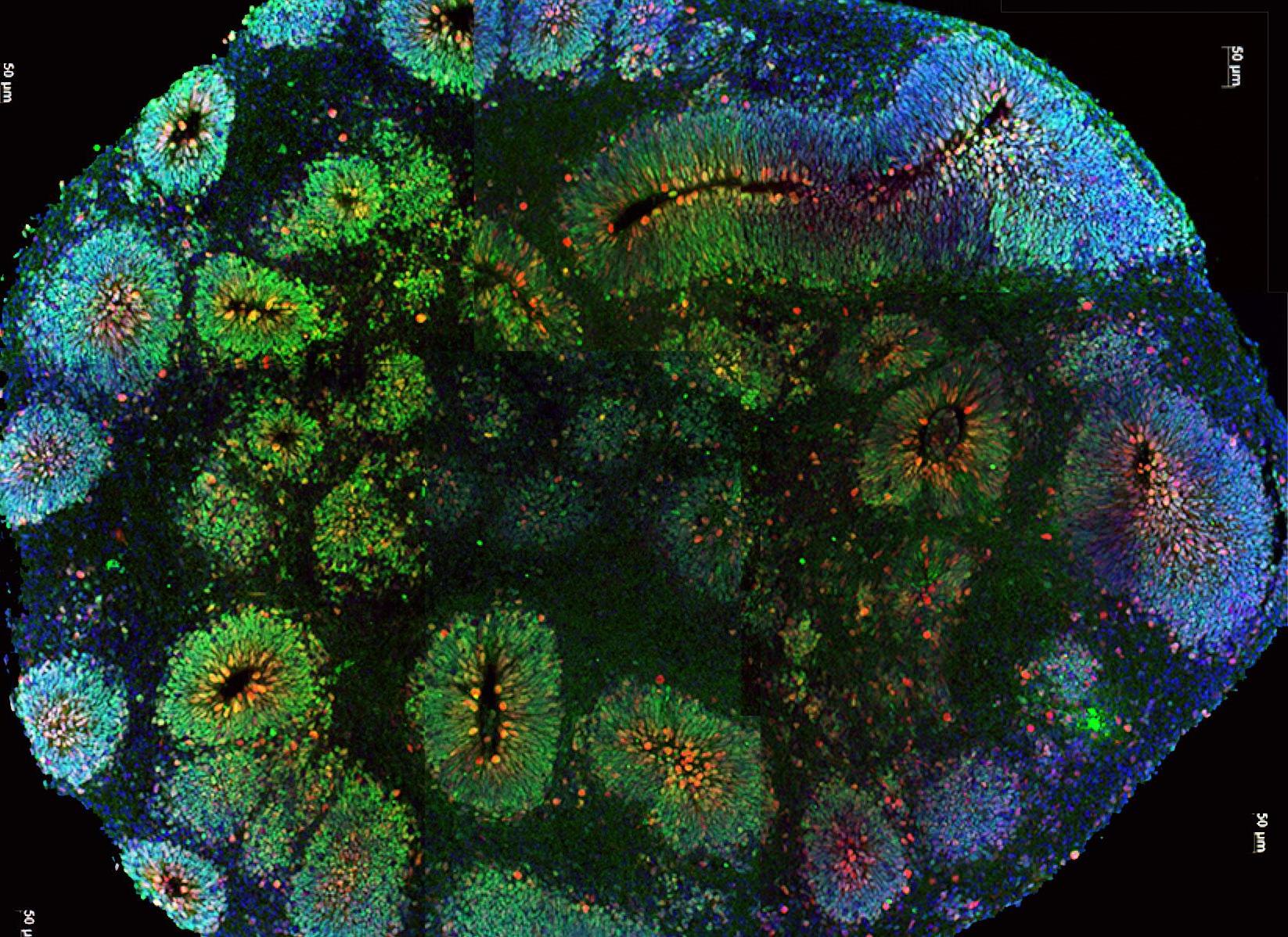
New research from the University of Hong Kong has revealed that the SARS-CoV-2 virus can directly infect neuronal progenitor cells (NPCs) and brain organoids. While NPCs may differentiate into various neuronal cell types, brain organoids mimic the structure and development of the human brain and have been used to understand how viruses can cause encephalopathy.3,4 Zhang et al. first infected NPCs with SARS-CoV-2, using SARS-CoV virus as a control. It was found that only SARS-CoV-2 was capable of replication in NPCs, and that the quantity of NPCs was reduced to 4.7% of its original value following SARS-CoV-2 infection.3 The researchers then infected a brain organoid with SARS-CoV-2 and discovered significant amounts of viral antigen 72 hours after infection, indicating direct infection by the virus.
The findings of Zhang et al. have implications for understanding the link between COVID-19 infection and its neurological manifestations, especially by suggesting the possibility of neuronal infection by SARS-CoV-2. Additionally, the infection and subsequent reduction of NPCs could negatively affect possibilities of recovery.3 Given the increasing importance of the neurological symptoms of COVID-19 —especially the loss of smell and taste —these findings invite further research into the long-term impact of SARS-CoV-2 infection on the central nervous system as well as neurologicallyfocused treatments.3
1.
2.
3.
4. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–90. Available from: doi:10.1001/ jamaneurol.2020.1127. Zhou Z, Kang H, Li S, Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. 2020;1–6. Available from: doi:10.1007/s00415-020-09929-7. Zhang B-Z, Chu H, Han S, Shuai H, Deng J, Hu Y, et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020;1–4. Available from: doi:10.1038/s41422-020-0390-x. Trujillo CA, Muotri AR. Brain organoids and the study of neurodevelopment. Trends Mol Med. 2018;24(12):982–90. Available from: doi:10.1016/j.molmed.2018.09.005. Garcez PP, Loiola EC, Costa RM da, Higa LM, Trindade P, Delvecchio R, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352(6287):816–8. Available from: doi:10.1126/ science.aaf6116.
COVID-19 AND ANOSMIA

NURI SONG
Evidence shows that anosmia, or loss of smell, is a prominent symptom of SARS-CoV-2.1 Identified as one of the earliest signs of viral infection, anosmia is present in 33.9% to 68% of symptomatic individuals, as found by a number of cross-sectional studies.2,3 While the loss of smell is an accepted consequence of SARS-CoV-2, its pathogenic mechanism has been unclear until recently.
Hypothesizing that the loss of smell in patients was due to the binding of angiotensin-converting enzyme 2 (ACE2) receptors by the virus, researchers at Johns Hopkins conducted a notable preliminary study mapping ACE2 receptors in nasal tissue.4 The study results demonstrated a high concentration of ACE2 receptors in the olfactory neuroepithelium. To obtain study findings, Chen et al. collected olfactory epithelium and respiratory epithelial samples from chronic rhinosinusitis patients and control subjects. They conducted an immunohistological analysis, revealing a high concentration of ACE2 receptors on sustentacular cells, which are a type of structural nasal cell in the olfactory neuroepithelium.5 It is likely that SARS-CoV-2 targets these cells as a key point of entry into the body, explaining the onset of anosmia in the earliest stages of infection. As a result, Chen et al. have suggested that when patients are asymptomatic, the olfactory cells of the nasal tissue might be the singular site of infection.
The given findings have various implications for therapeutic approaches for COVID-19. By helping to explain the range of nasal- and taste-related symptoms of COVID-19, the study has advanced the search for the best topical or local antiviral drugs for COVID-19, as well as other therapeutic approaches. Whether saline irrigation, a common treatment for sinonasal conditions, has potential to mitigate spread of infection is still unclear; however, antiviral drug additives such as detergent or povidone iodine directed at nasal viral reservoirs should be further explored.6
1.
2.
3.
4. Meng Z, Deng Y, Dai Z, Meng Z. COVID-19 and anosmia: A review based on up-to-date knowledge. Am J Otolaryngol. 2020;41(5):102581. Available from: doi:10.1016/j.amjoto.2020.102581. Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: A cross-sectional study. Clin Infect Dis. 2020;71(15):889–90. Available from: doi:10.1093/cid/ciaa330. Menni C, Valdes A, Freydin MB, Ganesh S, El-Sayed Moustafa J, Visconti A. Loss of smell and taste in combination with other symptoms is a strong predictor of COVID-19 infection. medRxiv. 2020. Available from: do i:10.1101/2020.04.05.20048421. Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:422. Available from: doi:10.1186/s13054-020-03120-0. Chen M, Shen W, Rowan NR, Kulaga H, Hillel A, Ramanathan M, et al. Elevated ACE2 expression in the olfactory neuroepithelium: Implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur Respir J. 2020;56:2001948. Available from: doi:10.1183/13993003.01948-2020. Higgins TS, Wu AW, Illing EA, Sokoloski KJ, Weaver BA, Anthony BP, Hughes N, PharmD, Ting JY. Intranasal antiviral drug delivery and coronavirus disease 2019 (COVID-19): A state of the art review. Otolaryngol Head Neck Surg. 2020;163(4):682–94. Available from: doi:10.1177/0194599820933170.
COVID-19 RECOVERY IN ADULTS AND CHILDREN

ZAHRA ABDALLAH
In December 2019, a new infectious disease emerged in Wuhan, situated in the Hubei province of China. Since then, SARS-CoV-2 (COVID-19) has rapidly spread across the globe, warranting countries to impose travel restrictions, school closures, and physical distancing.1 As national governments discuss how to approach school reopenings, the need for pediatric-specific data on the impact of COVID-19 has become a topic of focus.2,3
Preliminary results from American, Canadian, Chinese, and Irish studies have shown that cases of COVID-19 may be less severe in children than in adults. One pediatric case series from the United States reported 56% of participants had a fever and 54% had a cough; in contrast, these ratios in adult patients were 71% and 80%, respectively.4 This data is relatively consistent with multiple studies across the globe.1 Interestingly, the largest pediatric case series to date concluded that over 90% of children diagnosed with COVID-19 had asymptomatic, mild, or moderate forms of the disease.4,5
Despite these scoping reviews of COVID-19 in pediatric patients, the underlying cause for the milder symptoms in children is still unclear.4 Several hypotheses have been proposed, often citing superior health outcomes in children’s respiratory tracts as a consequence of minimal exposure to cigarette smoke and air pollution when compared to adults.4 Moreover, children tend to have fewer risk factors for complications, which could reduce the prevalence of severe cases. Finally, it has been proposed that SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2), and that children may have limited ACE2 functionality compared to adults, thus providing protection against the virus.1,5
Although these findings suggest that COVID-19 in children is mild in severity when compared to adults, it is important to monitor trends in its manifestation as new data emerges.1 Additionally, further data indicating the risk of transmission of COVID-19 in a school environment will be essential in determining the appropriate measures to take as children return to classes.2,3
Ho CLT, Oligbu P, Ojubolamo O, Pervaiz M, Oligbu G. Clinical characteristics of children with COVID-19. AIMS Public Health. 2020;7(2):258–73. Available from: doi:10.3934/publichealth.2020022. Kakkar F, Hepburn CM, Drouin O, Morris SK. Canadian Paediatric Surveillance Program commentary on hospitalizations from COVID-19 among children in Canada [Internet]. Canadian Paediatric Surveillance Program, COVID-19 Study Team. 2020 [cited 2020 Oct 9]. Available from: https://www.cpsp.cps.ca/ uploads/publications/CPSP_COVID-19_Commentary_September_2020.pdf. Viner RM, Bonell C, Drake L, Jourdan D, Davies N, Baltag V, et al. Reopening schools during the COVID-19 pandemic: governments must balance the uncertainty and risks of reopening schools against the clear harms associated with prolonged closure. Arch Dis Child. 2020:archdischild-2020-319963. Available from: doi:10.1136/archdischild-2020-319963. Tezer H, Bedir Demirdağ T. Novel coronavirus disease (COVID-19) in children. Turk J Med Sci. 2020;50(3):592–603. Available from: doi:10.3906/sag-2004-174. Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than
COVID-19 AND DEPRESSION

ADRIAN WONG, NURI SONG & ZAHRA ABDALLAH
Depression is a clinical condition marked by reduced day-to-day pleasure, a lack of energy, and low self-esteem and has become an issue of increasing importance in recent years.1 In 2017, over 260 million people worldwide were reported to be suffering from depressive disorders.2 Existing research has established that depression tends to increase throughout and after traumatic events.3 In the wake of its devastating impact on the mental health of individuals globally, the COVID-19 pandemic has been regarded as a traumatic event, prompting researchers from Boston University to hypothesize that depression levels would increase in response to the pandemic.4
Ettman et al.’s study is the first to examine depression symptoms in US adults before and during the COVID-19 pandemic. It collected data from two surveys —one for pre-pandemic depression levels and the other for levels during the pandemic. The researchers found that the prevalence of such symptoms following the onset of the pandemic (at 27.8%) was tripled compared to the prevalence before the pandemic. This increase in prevalence was higher than recorded levels after previous mass traumatic events. Moreover, these symptoms increased in all demographic groups studied, with women being more likely to suffer from depression than men. Importantly, people with lower socioeconomic status and higher exposures to stressors, such as loss of employment, were more likely to suffer from depressive symptoms.5
These findings corroborate existing studies on mental health amid the COVID-19 pandemic in Asia, which demonstrated the extent of the pandemic as a psychological burden.6 While further monitoring depression prevalence throughout the pandemic is necessary, these findings strongly suggest that the negative mental health consequences of COVID-19 will be widespread and long-lasting.7 With these findings in mind, governments may become more informed to implement health policies to alleviate such consequences, especially among individuals in low-resource settings.
1.
2.
3.
4.
5. American Psychiatric Association, (ed.) Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C: American Psychiatric Association; 2013. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. Available from: doi:10.1016/S0140-6736(18)32279-7. Goldmann E, Galea S. Mental health consequences of disasters. Annu Rev Public Health. 2014;35(1):169–83. Available from: doi:10.1146/annurev-publhealth-032013-182435 Kleber RJ. Trauma and public mental health: a focused review. Front Psychiatry. 2019;10:451. Available from: doi:10.3389/fpsyt.2019.00451. Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open. 2020;3(9):e2019686. Available from: doi:10.1001/jamanetworkopen.2020.19686. Lai J, Ma S, Wang Y, Cai Z, Hu J, Wei N, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976. Available from: doi:10.1001/jamanetworkopen.2020.3976. Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med. 2020;180(6):817. Available from: doi:10.1001/jamainternmed.2020.1562.





