6 minute read
THE EVOLUTIONARY BASIS OF DISEASE
By Sanjana Rao Esther J Beck
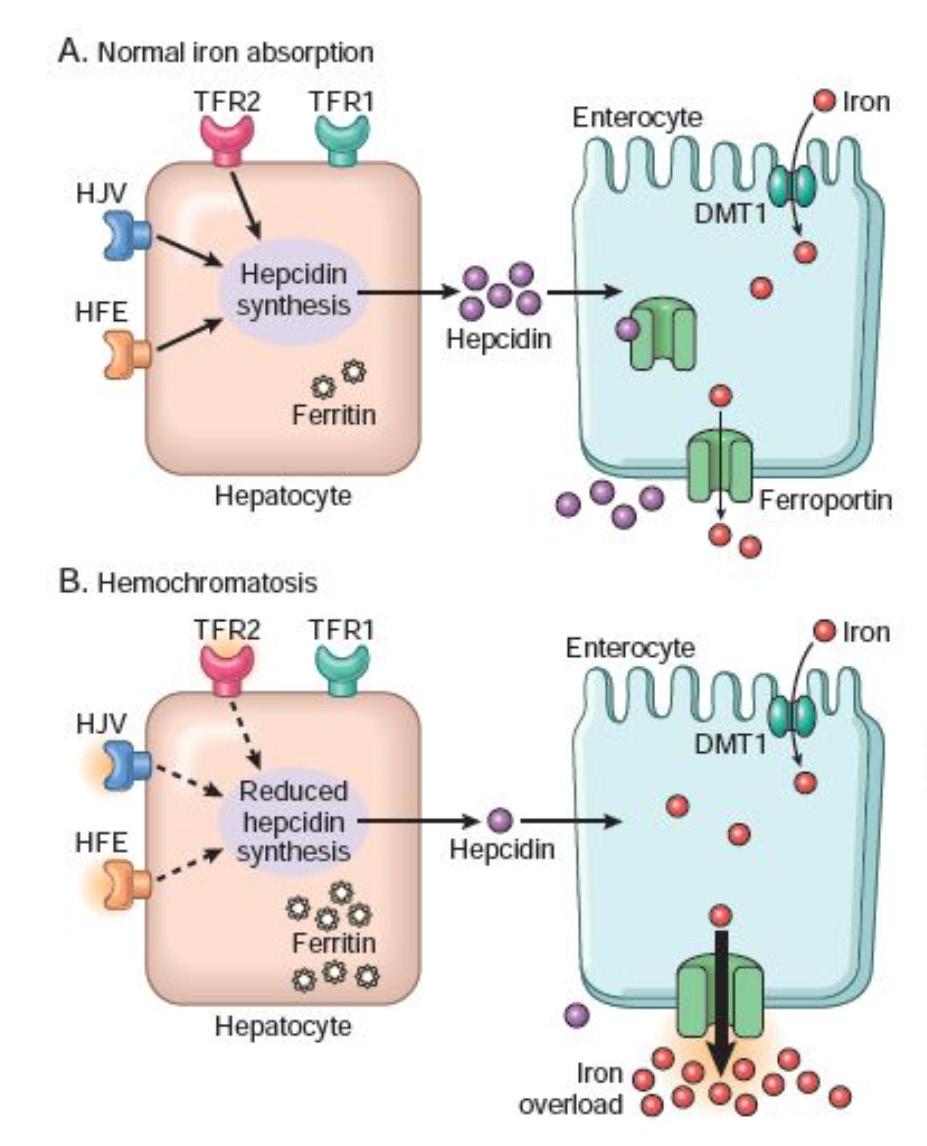
A phlebotomy, or the act of ‘blood letting’, was a common treatment for most illnesses in medieval times, as it was believed that the withdrawal of blood from the patient would help balance their humors and cure them. This practice, said to be the most common treatment used in Europe, was relatively commonly used until the beginning of the 19th century. Modern medicine has since dismissed the practice as pseudoscience, however, there is at least one disease that it does effectively treat: Haemochromatosis. Haemochromatosis is an inherited condition where your body absorbs too much iron, leading to excess iron buildup in your organs. This can have potentially fatal effects, leading to liver disease, heart problems, and diabetes, and patients often suffer from joint pain and fatigue. Those afflicted with this condition often unknowingly treat their disease by becoming blood donors, using phlebotomies to get rid of the excess iron in their body and alleviate their symptoms.
Advertisement
Nearly 6% of individuals of Caucasian descent carry or are afflicted with the inheritable form of haemochromatosis. But how did it arise and become so prevalent? Natural selection, or the survival of the fittest, is the idea that traits that make an organism better adapted to their environment are more likely to be passed on and therefore increase in prevalence in a population. Individuals with traits that increase their ‘fitness’ survive longer or have a reproductive advantage, leading to more individuals with that trait. These traits arise via mutations, which lead to altered phenotypes. The mutation that leads to hereditary haemochromatosis is found in the HFE gene, and is called C282Y.
So what evolutionary benefit could a mutation that results in excess iron uptake give us? There are at least two possible explanations. The first is that it is a ‘thrifty’ mutation, allowing our ancestors to make the most of the resources available to them. During the time this mutation that leads to hemochromatosis is hypothesized to have arisen, the northern Euro-
pean population’s diet changed. With the advent of the agricultural revolution, they shifted from a meat-rich paleo diet to a more iron deficient cereal based diet. This mutation may have helped them adapt to this change and prevent anemia, especially menstruating women. The other explanation surrounds the immune system of afflicted individuals. Micro-organisms such as bacteria depend heavily on iron for their growth and proliferation, suggesting that individuals with hemochromatosis would be especially susceptible to infection. However, that does not appear to be the case. Instead, macrophages in affected patients have a lower iron content than those of healthy patients, protecting them from bacteria that rely on macrophage iron. One such bacterium has been responsible for billions of deaths worldwide, Myobacterium tubercolosis, the cause of tubercolosis.
Haemochromatosis isn’t the only genetic disease that provides resistance to disease. Cystic fibrosis is another genetic disorder that causes buildup of mucus in the airways and lungs. It too is caused by a recessive mutation, requiring affected individuals to inherit both copies of the mutated gene (CTFR). Carriers, or heterozygotes, lack one functioning copy, and thus do not have many functioning CTFR proteins. As these are required for the entry of S. typhi (the cause of typhus) into the cell, it is hypothesized that heterozygotes may have a resistance to typhus. In addition, heterozygotes have been shown to have some level of tuberculosis resistance, as carriers lack sufficient activity in arylsulphatase, an enzyme essential to the virulence of M. tuberculosis.
Heterozygote advantage is a common phenomenon, where individuals who have only one mutated copy of the gene reap the benefits (such as disease resistance) without the often fatal downsides that affect those with both mutated copies (homozygotes). Another example of this is sickle cell anemia, where a point mutation in the 12th amino acid of the hemoglobin gene leads to brittle, warped red blood cells that resemble sickles. In the homozygous form, patients have a low life expectancy as these cells can get stuck in arteries and cause vaso-occlusive crises, and have lowered oxygen carrying capacity. In heterozygotes, however, there is enough normal hemoglobin present such that red blood cells are not overly warped. However, there is enough defective haemoglobin to give carriers resistance to malaria. The protozoan that causes malaria, Plasmodium, spends part of its life cycle in red blood cells. In carriers, the defective hemoglobin causes the cells to rupture prematurely, preventing the proliferation of Plasmodium.

This resistance to malaria explains the pattern of sickle cell trait prevalence in the population, as it is most common in regions where malaria is endemic as it is selected for in these regions. In regions where malaria is uncommon, the trait is purely disadvantageous, and is thus less prevalent.
How can we use what we’ve learnt from evolutionary medicine to our advantage? Asian glow, or alcohol flush syndrome is a condition that mostly affects approximately 30 to 50% of Chinese, Japanese, and Koreans. It results from a deficiency in an enzyme that is integral to the metabolism of alcohol, acetaldehyde dehydrogenase 2, resulting in a buildup of (toxic) acetaldehyde in one’s system. There are multiple possible theories as to how this arose, including the methods of purification of water in Asia versus Europe. In Europe, fermentation and the production of alcohol was a common method to purify and store beverages. In Asia, tea was more common, and water was purified by boiling. In addition, it has been hypothesized that the elevated levels of acetaldehyde seen in individuals with alcohol flush syndrome may protect against some types of parasitic infections, such as Entamoeba histolytica. The mechanism of action behind alcohol flush syndrome has been used by industry in the form of disulfiram. Disulfiram, brand name Antabuse, inhibits acetaldehyde dehydrogenase 2, leading to the buildup of acetaldehyde and the adverse symptoms seen in individuals with alcohol flush syndrome. This drug is used to treat alcoholism by inducing the effects of a hangover immediately upon consumption of alcohol, negatively conditioning the individual.
By studying the evolutionary basis of disease, we can better understand the pathophysiology of the condition and find potential avenues of treatment for other diseases. In addition, with the advent of gene therapy, it may be possible to alleviate the burden of many genetic diseases on homozygotes while still preserving their evolutionary advantage. By understanding historical problems and the traits that arose to solve them, we can find out of the box solutions to epidemics that still plague us today — from tuberculosis to addiction.
Neil D. Theise .Liver and Gallbladder. In:Robbins and Cotrans Pathologic Basis of Disease. 9th edition. "Sickle Cell Disease Overview". 2021.
Practical Pain Management. https://www. practicalpainmanagement.com/patient/ conditions/sickle-cell-disease/sickle-celldisease-overview. "How Does Disulfiram (Antabuse) Interact With
Alcohol (Ethanol) To Cause Patients To Get So
Sick?". 2021. Ebmconsult.Com. https://www. ebmconsult.com/articles/disulfiram-antabusealcohol-ethanol-mechanism-interaction. Wertheim, Bradley. 2021. "The Iron In Our Blood
That Keeps And Kills Us". The Atlantic. https:// www.theatlantic.com/health/archive/2013/01/ the-iron-in-our-blood-that-keeps-andkills-us/266936/. Hollerer, Ina, and Andre Bachmann. 2017. "Pathophysiological Consequences And
Benefits Of HFE Mutations: 20 Years Of
Research". Haematologica. https://www. haematologica.org/article/view/8060. Rühli, F.J., Henneberg, M. New perspectives on evolutionary medicine: the relevance of microevolution for human health and disease. BMC Med 11, 115 (2013). https://doi. org/10.1186/1741-7015-11-115 "Haemochromatosis". 2021. Nhs.Uk. https://www. nhs.uk/conditions/haemochromatosis/. Stephen C. Stearns, Randolph M. Nesse, Diddahally
R. Govindaraju, Peter T. Ellison. “Evolutionary perspectives on health and medicine”.
Proceedings of the National Academy of
Sciences Jan 2010, 107 (suppl1) 1691-1695;
DOI: 10.1073/pnas.0914475107 "Hemochromatosis - Symptoms And Causes". 2021. Mayo Clinic. https://www.mayoclinic. org/diseases-conditions/hemochromatosis/ symptoms-causes/syc-20351443. Bosch, Lander et al. “Cystic fibrosis carriership and tuberculosis: hints toward an evolutionary selective advantage based on data from the
Brazilian territory.” BMC infectious diseases vol. 17,1 340. 12 May. 2017, doi:10.1186/s12879017-2448-z










