
5 minute read
Too much and not enough:
Hypoxemia and pulmonary capillary blood flow in COVID-19
Andrew Marquis PhD Student, Molecular & Integrative Physiology and Daniel Beard, PhD Carl J Wiggers Collegiate Professor of Cardiovascular Physiology, Professor of Molecular & Integrative Physiology
Despite the robust academic and industry research efforts across the globe, there are still many gaps in our understanding of the pathology of severe acute respiratory syndrome coronavirus 2 (SARS CoV2). Arguably the most concerning symptom of severe SARS CoV-2 infection is hypoxemia—inadequate oxygen delivery to the body’s tissues. Hypoxemia in Coronavirus disease 2019 (COVID-19) has been compared to Acute Respiratory Distress Syndrome (ARDS), a form of respiratory failure characterized by the rapid onset and widespread inflammation throughout the lungs. This impairs the lungs’ ability to exchange oxygen and carbon dioxide, also resulting in hypoxemia. In ARDS, abnormal ventilation is typically explained by a decrease in lung compliance, or the ability of the lungs to stretch and expand. The primary treatment involves mechanical ventilation with low volumes, low pressures, and supplementary oxygen. While at first glance it may appear that COVID-19 causes ARDS, critically ill COVID-19 patients requiring mechanical ventilation surprisingly tend to have normal lung compliance. So, if COVID-19 patients are suffering from hypoxemia but they do not have reduced lung compliance, what then is the mechanism underlying their hypoxemia?
Efficient gas exchange in the lungs requires effective matching of airflow and blood flow—referred to as ventilation-perfusion (V/Q) matching. Because V/Q ratios are influenced by gravity, a patient’s posture can influence their V/Q ratios. Indeed, moving a patient into a prone position (laying face down), has been shown to improve V/Q matching and oxygenation in critically ill COVID-19 patients. V/Q matching is physiologically regulated through hypoxic pulmonary vasoconstriction (HPV). HPV is a regulatory mechanism where in response to low oxygen in the alveolar space of the lung, upstream pulmonary arteries vasoconstrict, redirecting blood flow away from areas of the lung that have poor oxygen supply. In ARDS, fluid accumulation in the alveoli and alveolar walls can prevent gas exchange, resulting in poorly oxygenated blood returning to the left side of the heart. HPV counteracts this effect by limiting blood flow through poorly ventilated or fluidfilled alveoli. Yet in COVID-19 patients with severe hypoxemia, lung imaging via chest CT suggests that their alveoli remain well aerated while severe capillary microthrombosis is present. We hypothesize that the hypoxemia observed in COVID-19 is possibly explained by dysregulated pulmonary capillary blood flow distribution. In simpler terms, some pulmonary capillaries have too much blood flow, while others have not enough. Some studies have suggested COVID-19 disrupts normal HPV function. Combined with the obstruction of blood flow by microthrombi, this could create a perfect storm to disrupt the normal pulmonary
Figure 1: Schematic of our multi-scale multi-physics model of ventilation-perfusion matching. Block (A) illustrates the whole-lobe vascular network model. Black lines represent blood vessels, and colored regions represent discrete zones of perfusion. Block (B) shows how the mechanics of each vessel segment is represented as an equivalent circuit. Intravascular pressure (Pv) and flow into the vessel (qin) are state variables; inlet pressure (Pin) and flow out of the vessel (qout) are the initial conditions at boundaries for a given vessel segment; outlet pressure (Pout) is an algebraic constraint; alveolar pressure (Palv) is a dynamic pressure source; and hydraulic resistance (R), inertance (L), compliance (C), and vessel wall resistance (RD) are anatomical parameters calculated from the geometry of the vessel segment (length and radius) and can be modulated by vasoregulation (purple boxes). Block (C) depicts a representative gas exchange unit. Gases flowing through the capillary tube are exchanged with an alveolar compartment. Block (D) portrays the oxygen-sensitive vasoregulatory mechanism hypoxic pulmonary vasoconstriction (HPV). Hypoxia in the alveolar space induces conducted vasoconstriction. Conducted vascular responses are spatially propagated upstream through the endothelium - these responses modulate the values of the anatomical parameters (R, C, and RD) in the arterial network.
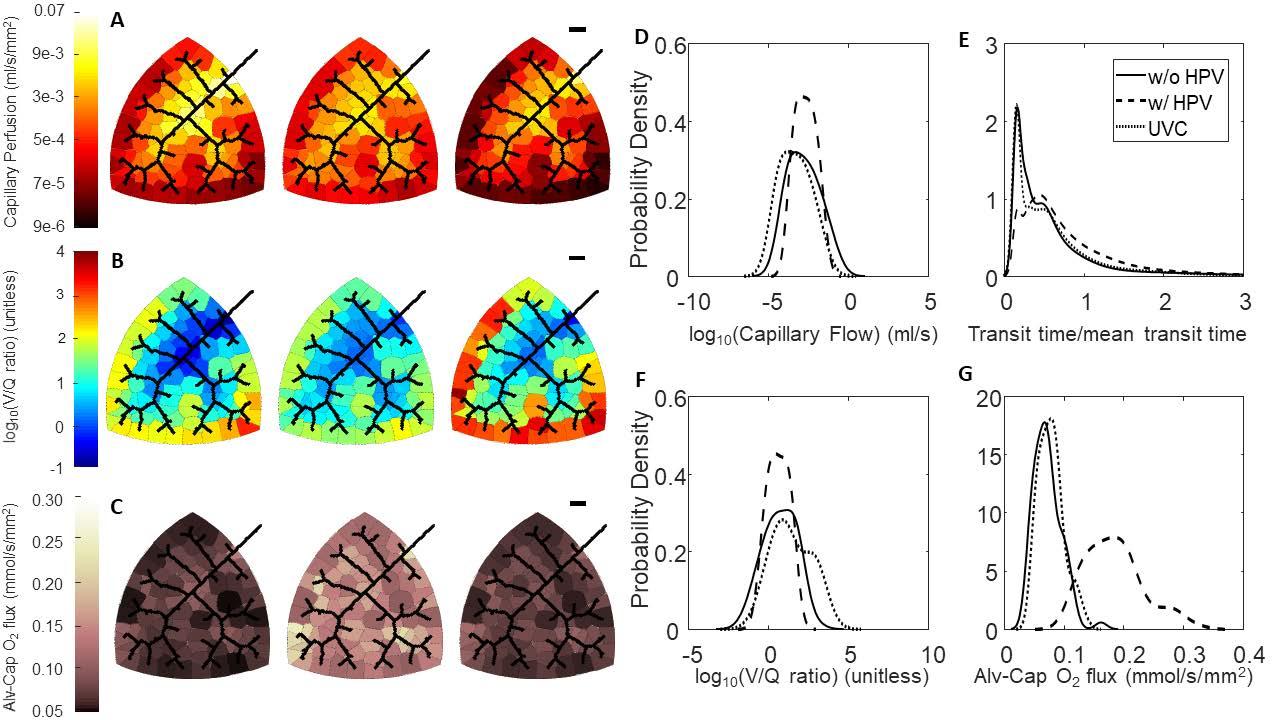
Figure 2: Effect of HPV and uniform vasoconstriction on regional perfusion, V/Q matching, and oxygen flux. (A-C) Leftmost column of networks are without regulation from HPV, middle column of networks are with regulation from HPV, and the rightmost column of networks are with uniform vasoconstriction. The black scale bar is 1000 um. (A) Perfusion in the capillary compartments; (B) Ventilation-perfusion (V/Q) ratios in the capillary compartments; (C) Oxygen flux in the capillary compartments. (D-G) Blood flow, RBC transit times, V/Q ratios, and oxygen flux expressed as probability densities. (D) Distribution of flow; (E) RBC transit times normalized to mean transit time; (F) Distribution of V/Q ratios; (G) Distribution of oxygen flux.
capillary blood flow distribution.
Despite decades of research, we still do not understand how HPV affects the distribution of blood flow throughout the lungs. Moreover, the molecular governing pathways underlying HPV remain elusive. To understand how HPV influences pulmonary blood flow distribution at the system level and to analyze the role of HPV in disease, we have developed a multi-scale multi-physics computational model of V/Q matching in rat lungs (Figure 1). The major components of our model are: (a) morphometrically realistic pulmonary vascular networks; (b) a tileable lumped-parameter model of vascular fluid and wall mechanics; (c) oxygen transport accounting for oxygen bound to hemoglobin and dissolved in plasma; and (d) an empirical model of HPV based on conducted vascular response mechanics.
Model simulations predict that HPV functions to match perfusion to ventilation by more evenly distributing capillary blood flow throughout the organ. This homogenizes the distribution of V/Q ratios. Moreover, a more homogenous distribution of V/Q ratios leads to a more homogenous distribution of regional alveolarcapillary oxygen flux and thus increases whole-organ oxygen uptake (Figure 2). Simulation with regulation from HPV increases the venous outflow oxygen tension from 81 mmHg to 100 mmHg, a favorable outcome. This is in contrast to the uniform vasoconstriction simulation where venous outflow oxygen tension decreased slightly to 78 mmHg.
Our model represents a flexible platform to understand many diseases states characterized by blood gas derangements such as collapsed airways, asthma, pulmonary fibrosis, thromboembolic diseases, and COVID-19. The significance of this model is that it can capture function/dysfunction at a variety of observable and unobservable scales. We are currently working to compare the effects of a single large acute pulmonary embolism (PE) versus a large number microthrombi. Much like COVID-19, acute PE frequently results in hypoxemia. While the lethal threat of not having enough oxygen in a patient’s blood is certainly an obvious similarity between these two disease states, the optimal therapeutic strategy to treat each condition are different. For acute PE, current clinical guidelines recommend a surgical approach to remove the thromboembolism as a primary intervention. For many microthrombi, this approach is exceptionally challenging or infeasible due to the limitations of current technology such as the size of intravenous catheters. Our model may also have more practical use for diagnostic purposes. For example, perhaps microthrombi alter the red blood cell transit time distribution in a manner that is wholly distinct from large acute PE and healthy controls,our model could potentially be used to predict how to differentially diagnose these conditions. •










