
5 minute read
In-Situ Forming Plants: The Key to HIV Prevention
Jane Oberhauser
By the end of 2018, an estimated 38 million people worldwide were living with HIV: a retrovirus that cripples its host’s immune system by subjecting it to a state of constant infection. 1 The resulting condition, known as Acquired Immunodeficiency Syndrome, or AIDS, leaves the infected body vulnerable to a wide range of other infectious diseases and proves lethal if left untreated. Today, a daily, single tablet antiretroviral treatment regimen has replaced the cocktail of medications required to sustain life with HIV in the past. The focus of ongoing research has shifted from treating AIDS symptoms as they appear to preventing HIV infection in the first place. The effort begins with protecting the populations most at risk for contracting HIV. No viable HIV vaccine currently exists. As a result, protection comes in the form of a treatment known as preexposure prophylaxis, or PrEP. Clinical studies have demonstrated full protection from HIV infection for individuals who take PrEP daily: the same regimen required of those who have actually contracted the disease. 2 However, adherence becomes a serious issue when prescribing a strict pattern of regular medication as a preventative measure for an otherwise healthy population. “If people have HIV, they know that they will die if they do not take the pill,” points out Dr. Martina Kovarova, an associate professor at UNC-Chapel Hill’s School of Medicine. “But for people who are healthy, taking the drug becomes more difficult.” 2 Dr. Kovarova and a team of researchers from Gillings School of Global Public Health, the Eshelman School of Pharmacy, and the School of Medicine at UNC-Chapel Hill, along with the UNC-NC State Joint Department of Biomedical Engineering and Case Western Reserve University hope to improve PrEP adherence with the development of a long-acting, injectable drug delivery implant. Such an implant would eliminate the need for daily medication. Instead, patients would receive injections in 6-month cycles that would not need to
Advertisement
correspond with a pre-existing pattern of doctor’s visits. 2 According to Dr. Kovarova, a pharmaceutical company, Viiv Healthcare, has already begun the clinical development of another long-acting injectable with a different formulation. This product, known as Cabotegravir involves the direct injection of a drug suspension into the patient’s muscular tissue. 2 However, this method comes with a serious disadvantage: once the injection is applied, there exists no mechanism for its removal. A lack of reversibility presents serious issues in instances of patient pregnancy or adverse reaction to treatment. With this type of intramuscular injection, traces of the long-acting formulation of the drug can linger in the body for up to a year after the discontinuation of PrEP treatment. 2 As a result, the UNC group looks to take a different approach. “The system we have... is an injectable formulation that can be administered under the skin, and in a hydrophilic tissue environment it becomes solid,” Dr. Kovarova explains. 2 This type of injectable is known as an in-situ forming implant, (ISFI). 2 Using a process known as phase inversion, ISFI’s solidify only after injection into the body. The injectable formulation contains drug and biodegradable polymer dissolved in a biocompatible solvent. After solidification, implant releases the drug steadily over time as the polymer breaks down. 2 Moreover, the injectable designed by the UNC group remains ultrasonically detectable beneath the skin while it dispenses the drug. As a result, patients may stop PrEP therapy at any time. Withdrawal from
Dr. Martina Kovarova
represent a four-month improvement from the treatment windows offered injectables already in development. 3 However, EFdA also presents a unique challenge. The drug is significantly more soluble than any other compound the group has previously formulated for a long-acting injectable. 2 More soluble drugs tend to release faster from the solidified ISFI implant. As a result, Dr. Kovarova and the UNC group must find an EFdA composition capable of overcoming this problem. 3 The grant also requires that the formulation be tested in two different animal models. Dr. Kovarova and the UNC group plan to first test the formulation in rats, which represent a preferred point of reference in clinical development. Once the injectable reaches a certain threshold of success in rat models, the group plans to proceed to testing in mini pigs, whose skin more closely resembles humans. 2 The development of any drug in animal models presents the problem of translating results into predictions about human therapeutic outcomes. However, Dr. Kovarova remains optimistic that the UNC group will be able to innovate their way around any issues that might arise. 2 The development of a long-acting in-situ injectable to replace PrEP in its tablet form would vastly improve the quality of life for individuals at risk for HIV. Such a formulation would cut down on the period during which a patient must adhere to a temporary tablet regimen after ceasing treatment by injection, and would improve the odds of patient adherence during treatment. 2 Better protecting at-risk individuals from HIV infection would represent a significant step forward in the long-term effort to eradicate the HIV epidemic at its roots.

treatment requires only a simple scan and a small incision. This straightforward procedure stands to save individuals months of post-withdrawal adherence to a PrEP tablet regimen. 2 The UNC group recently received a new threeyear, $2.91 million grant from the Bill and Melinda Gates Foundation for the formulation of the anti-HIV drug EFdA for an ISFI system. 3 With low toxicity and high efficacy at low concentrations, EFdA represents the ideal drug for the long-term prevention of HIV transmission. An injectable ISFI system using EFdA could theoretically be used to sustain six months of controlled medication release. This timescale would
Figure 1: An illustration of the mechanisms behind in-situ phase inversion drug delivery implants. Image courtesy of Sheshala, R. et al. (2019)
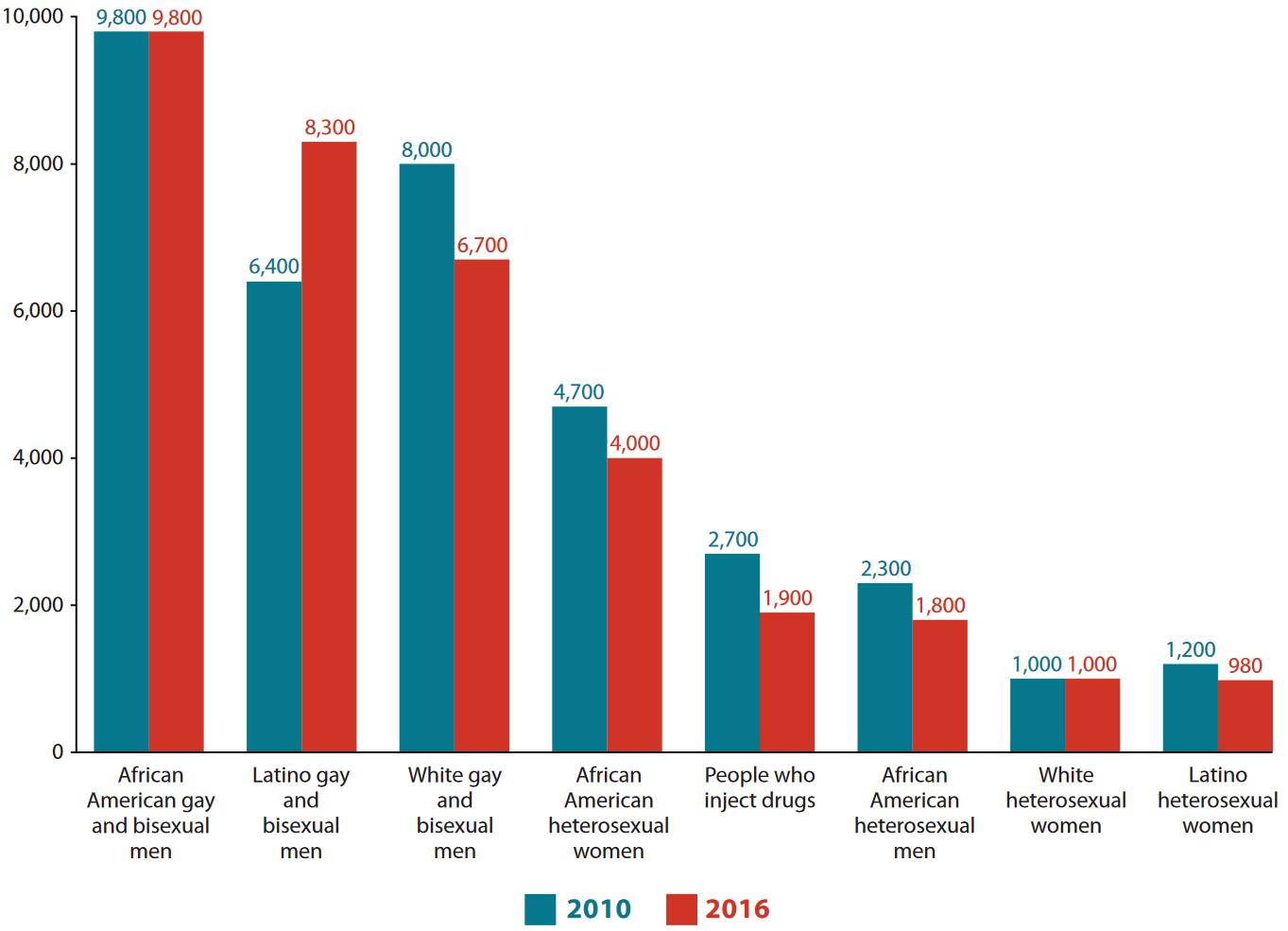
Figure 2: HIV infections in 2010 and 2016 by high-risk demographic group. Image courtesy of the CDC.
References
1. Global HIV & AIDS statistics - 2019 fact sheet. https://www.unaids.org/en/resources/factsheet (accessed Feb 11, 2020). 2. Interview with Martina Kovarova, PhD. 01/28/20. 3. Carolina awarded $2.91 million to create new ultra-long-acting HIV drug delivery implant: UNC-Chapel Hill. https://www.unc.edu/ posts/2019/11/06/carolina-awarded-2-91-million-to-create-new-ultra-long-acting-hiv-drugdelivery-implant/ (accessed Feb 11, 2020).










