
13 minute read
A New Generation for Regenerative Medicine
— Dr. Joshua Hare
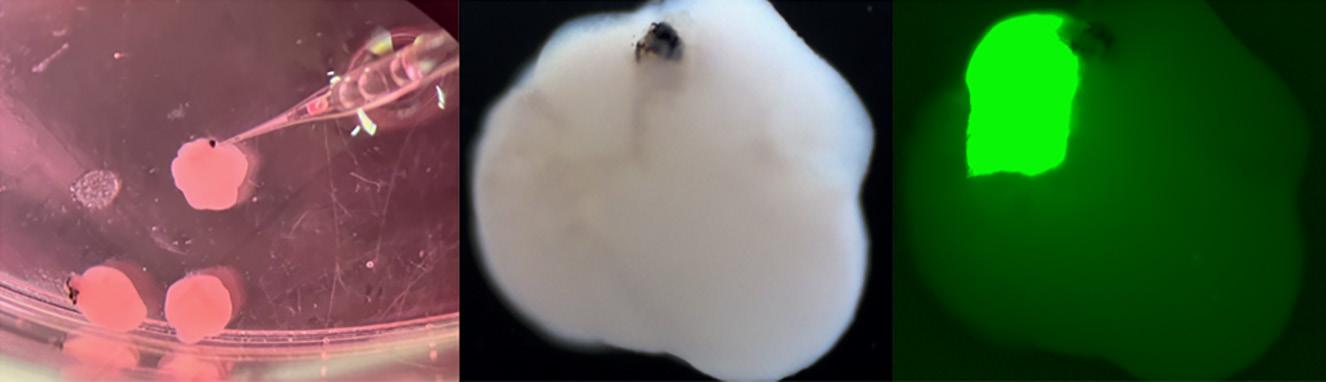
Joshua Hare, M.D. At left: Image showing injection of fluorescent retinoblastoma cells into forebrain organoids with primitive eyes as well as massive growth of the tumor in the forebrain organoid.
Above: Image showing staining of the heart organoids as well as a heart invaded by green fluorescent neural crest cells.
Dr. Hare and his colleagues work with medical researchers across the country to develop ground-breaking protocols using a variety of approaches that include using stem cells such as induced pluripotent stem cells, adult cells that can be expanded and potentially used as medicines, extra cellular vesicles or exosomes and peptides or synthetic drugs that stimulate the cell cycle and promote endogenous healing and physiologic tissue growth. A recent area of research focuses on how stem cell injections benefit patients with hypoplastic left heart syndrome. Hypoplastic left heart disease is a rare congenital heart condition where a child is born with only one ventricle instead of two. It’s an extremely serious condition that requires extensive cardiac surgery that can save the patient, at least initially. The complex procedure, however, can cause further damage and ultimately leads to heart failure. Cell-based therapy is a promising adjunct to surgery. “We developed the idea working together with our colleagues at the University of Maryland,” says Dr. Hare. “Through their own research they developed information suggesting that this could boost the function of the heart in the neonates suffering from hypoplastic left heart. They collaborated with us to get the cell types they wanted to pilot this approach in the babies they were treating.”
Dr. Hare says the results of this trial will enable him and his colleagues to initiate a phase II trial. “We have extended research to Chicago and a consortium of seven centers across the country, including Children’s Healthcare of Atlanta, University of Michigan and Lauri Children’s Hospital. The research has further expanded to involve different kinds of cell-based therapy.”
Research into cell-based therapy and other uses of stem cell investigations falls under the jurisdiction of the Interdisciplinary Stem Cell Institute (ISCI), which Hare founded at the Miller School in 2008. Though relatively young, the institute has overseen several pivotal studies. At a recent symposium, for instance, Dr. Hare and his team presented findings of a study involving stem cells to treat adult heart failure.
The two leading causes of heart failure in adults are ischemic cardiomyopathy, where a patient experiences a heart attack that damages part of the heart, and nonischemic cardiomyopathy, where heart failure is caused by something already wrong with the heart muscle. Dr. Hare and his colleagues conducted extensive research on both populations and have reported exciting results.
“We’ve learned that about 40 percent of nonischemic patients have a genetic defect responsible for their heart failure. Those who don’t have a genetic cause are likely to have inflammation underlying their cardiac dysfunction,” says Dr. Hare.
Working with Dr. Robert Myerburg and Dr. Raul Mitrani, Dr. Hare and colleagues developed information and basic preliminary findings suggesting that those individuals with the genetic defect don’t respond well to cell-based therapy. the ones who don’t have the genetic defect respond extremely well. “It’s an issue of personalized medicine, figuring out which types of patients should get which type of treatment,” says Dr. Hare.
The Miller School of Medicine also received a large grant from the U.S. Department of Defense (DOD) to test these results further. Hare is the principal investigator for the cell-based therapy program trial, known as the DCM II trial. Hare is the first cardiologist conducting dilated cardiomyopathy clinical trial using the DOD funding platform. Of the four centers conducting similar research using donor cells, the Miller School has seen the most significant enrollment. The unique feature of this trial is that the GMP cell manufacturing laboratory, led by Aisha Khan, is manufacturing and supplying stem cells to all participating centers.
“We don’t only manufacture these cells, we equip and train these sites to handle cellular products while maintaining compliance and quality,” says Dr. Hare. “This is how we make progress.”
Another exciting area of cross-campus interdisciplinary work is the longstanding collaboration between the ISCI and the Miami Project to Cure Paralysis led by Aisha Khan, Dr. Dalton Dietrich, and Dr. Allan Levi. As part of this work, ISCI and the Miami Project investigators, have long collaborated on developing Schwann cells and Schwann cell-derived exosomes as investigation agents for testing in clinical trials for spinal cord injury and even possibly Lou Gehrig’s Disease also known as Amyotrophic Lateral Sclerosis (ALS).
Khan, along with her Miami Project team, is investigating whether Schwann cells and their exosome products may be used as therapeutic tools in neurodegenerative disease states like ALS. Preliminary studies have shown outgrowth to dorsal root ganglion (DRG) when treated with exosomes.
The institute also is investigating a fascinating area of stem cell research called induced pluripotent stem (iPS) cells. This new program is a giant leap forward in medical research. Physician-scientists have learned how to take adult tissue and genetically modify it into self-renewing cells. Those cells have the potential to then grow into an actual human organ – a pancreas, for example, or a brain.
“These iPS cells aren’t quite yet ready for the clinic. Research at the bench [in the lab] is still being conducted and that’s what we’re very involved in. It’s fascinating what we’ve been able to do. We’ve been able to grow miniature versions of human organs, or organoids, in a dish,” says Dr. Hare.
In work led by Dr. Stefan Kurtenbach at the ISCI, organoids resembling hearts and brains have been produced. From the brain organoids, researchers can also grow eyes, leading to an amazing opportunity for sensorineural research. These tiny organs, however, are still confined to the dish since they aren’t yet ready for ‘prime time.’
“Known as modeling or what we call a disease in a dish, this very powerful technique is emerging as a way to conduct future research, understand what causes diseases and how to treat them,” says Dr. Hare.
Another piece of the heart regeneration puzzle fell into place ths year when Hare and Khan used human stem cell-derived exosome technology to show efficacy of cell treatments for heart disease. The use of stem cell-derived exosomes for heart conditions is another exciting developing area of cardiac research.
“Researchers are developing stem cellderived exosomes therapy to improve cardiac function after myocardial infarction. Progress in pre-clinical research will open the doors to research funding,” says Dr. Hare.
A long-standing focus of the institute has been the translation of stem cell research into therapies that can be used to treat disease.
“We are proud that researchers at all career levels and from many scientific and cultural backgrounds have made pivotal contributions to discoveries that are closer than ever to the clinic – and yet we know there’s much more to do to achieve our full potential,” says Dr. Hare. “Thanks to those who have supported the ISCI and contributed to the progress you’ve made possible.”


Researchers Develop Promising Treatment for Rare Genetic Syndrome
Department of Medicine researchers have developed a promising treatment for a rare, neurodegenerative genetic syndrome that impacts the development of children and limits their lifespan.
Endocrinologist Roy E. Weiss, M.D., Ph.D., Chair of the Department of Medicine, and Khemraj Hirani, M.Pharm., Ph.D., M.B.A, R.P.H., Associate Vice Chair, Research and Regulatory Compliance of the Department of Medicine, joined forces to test a drug in the treatment of Allan-Herndon-Dudley Syndrome (AHDS).
Drs. Weiss and Refetoff looked to a drug formerly known as DITPA (diiodothyropropionic acid) as a solution. Results of the first-in-human compassionate clinical trial were significant.

Samuel Refetoff, M.D.
SRW101 DEVELOPMENT MILESTONES
“I have always been interested in investigating orphan diseases. These are diseases that are very rare and for which there are no treatments and can have tremendous impact on the lives of these patients,” said Dr. Weiss. “These patients are eternally grateful if you can do anything to help them because they’re not in the main crosshairs of large pharmaceutical companies that treat diseases like diabetes, hypertension, heart disease or cancer.”
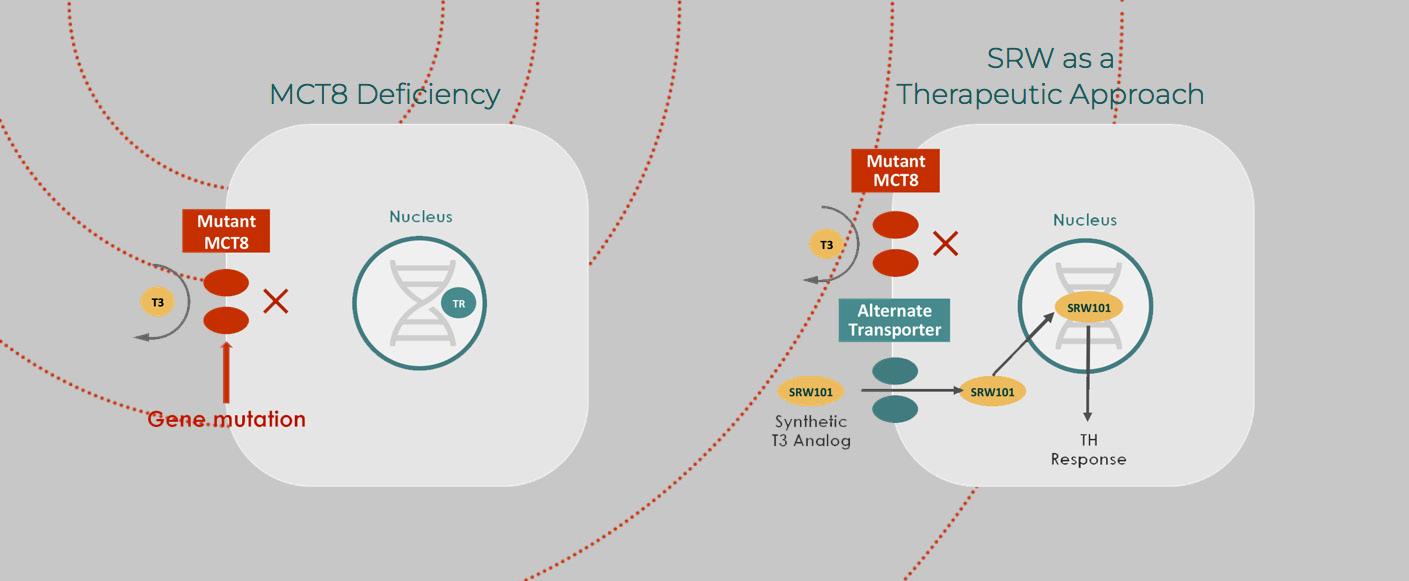
AHDS is just such a disease. It manifests predominantly in males and severely impairs communication, movement, increased heart rate and the ability to gain weight. The syndrome is caused by a defect in the transport of the thyroid hormone T3 into the brain but results in an excess of the hormone in the peripheral organs.
Dr. Weiss, who holds the Kathleen and Stanley Glaser Distinguished Chair in Medicine and the Rabbi Morris I. Esformes Endowed Chair in Medicine and Endocrinology, has worked to find a treatment for AHDS for nearly 35 years. His work started at the University of Chicago School of Medicine (UChicago Medicine) where he worked closely with acclaimed endocrinologist Dr. Samuel Refetoff.
“These children are severely delayed in terms of psychomotor activity. They don’t walk; they don’t talk. They can’t feed themselves. They can’t hold their heads up. It’s a very devastating disease, for them and for their families as well,” Dr. Weiss said.
A genetic mutation inhibits the T3 hormone from crossing the brain-blood barrier and reaching the central nervous system. The lack of thyroid hormone during fetal and early development causes universal delays, including intellectual disabilities, low muscle tone, limited speech and motor abilities, feeding difficulties and spasticity.
Conversely, an overabundance of T3 builds up in the organs, creating a metabolic imbalance that results in growth dysfunction and cardiovascular issues.
The average life expectancy for males with this syndrome is 35 years of age, and to this point, there has been no effective therapy to treat it.
Drs. Weiss and Refetoff looked to a drug formerly known as DITPA (diiodothyropropionic acid) as a solution. It is a thyroid hormone analogue that mimics thyroid hormone, but bypasses the mutant receptor to get into the brain. Tested successfully first in animals, the doctors then administered it to four patients with ADHS under the Food and Drug Administration’s “compassionate use” provision. This allows researchers to test investigational medical products on patients with immediately life-threatening illnesses that have no available comparable treatment.
Results of the first-in-human compassionate clinical trial were significant. Each of the four patients took DITPA daily for 26-40 months and saw fully normalized T3 levels, did not develop seizures and were able to forego a gastric feeding tube. Half the patients gained weight
2004
Identification of the first child with mutation in the gene encoding MCT8. 2006
Production of the first MCT8-deficient mouse (Mct8KO) showing that it manifests the same thyroid abnormalities as humans 2009-2012
First In-Human Study: Compassionate use of SRW101 in children with MCT8 deficiency for a period of 26-40 months
2006-2016
NIH MERIT award to UM’s scientific co-founders to study MCT8 deficiency and therapeutics for 10 years. NIH funding without interruption for 48 years to study inherited thyroid diseases. 2009 2010
Publication showing that the use of SRW101 (DITPA) normalizes key chemical abnormalities equally in induced hypothyroidism of normal and Mct8KO mice Expression of MCT8 in thyroid cell membrane promotes hormone secretion, which partially accounts for the low blood T4 in MCT8 deficiency Khemraj Hirani, M.Pharm., Ph.D., M.B.A, R.P.H . and Roy E. Weiss, M.D., Ph.D.

2013
Prenatal and postnatal changes in thyroid status of Mct8KO mice 2014
MCT8-deficient human embryo has brain abnormalities 2015
SRW-101 corrects hypermetabolism of Mct8KO mice by reducing D1 and blood T3
2013
Demonstration that Mct8KO mice have increased energy consumption because of high T3 in blood 2014
Diiodothyropropionic acid (DITPA) in pregnant mice crosses the placenta reaching 8-fold higher levels in the MCT8-deficient fetus; significant potential for prenatal treatment without affecting the mother 2015
Meeting with the FDA to use DITPA in treatment of MCT8deficient subjects before and after birth (PIND)

–Dr. Roy Weiss
SRW101 DEVELOPMENT MILESTONES
and saw normalized brain development, while 75% experienced a decline in heart rate, decreasing the work of the heart, as DITPA also improves peripheral thyrotoxicosis, a type of permanent congenital hypothyroidism.
The researchers were also able to show that DITPA can cross the placenta in pregnant women and increase the amount of the T3 hormone in the developing embryo. In 2016, the drug was authorized by the FDA under an emergency investigational new drug (IND) for use in pregnant women carrying a fetus suspected to have ADHS. Since women are carriers of the genetic mutation, if her first male baby was born with ADHS, the next male baby will have it as well. Screening to identify female carriers is also underway.
Today, the drug is called SRW101 and is ready to enter phase III trials. PriZm, a late-stage clinical biotech company co-founded by Drs. Weiss, Hirani and Refetoff, will conduct the trial on some 40 patients. The results will be evaluated for clinical effectiveness and submitted to the FDA.
The team secured both an Orphan Drug Designation (ODD) in the United States and European Union as well as a Rare Pediatric Disease (RPD) designation from the FDA. This allows research to move forward on a faster track than other pharmaceutical trials.
“We believe the EMA (European Medicines Agency) and the FDA’s granting of Orphan Drug Designation to DITPA reflects the agencies’ recognition that effective treatment options are needed for AHDS, a serious endocrine disorder with an unmet medical need. An End of Phase 2 (EOP2) meeting with the FDA further led to concurrences on key topics of the development pathway. These decisions add further credibility to our existing clinical evidence that DITPA has the potential to bring a new essential therapy option to children with AHDS,” said Dr. Hirani.
Dr. Weiss said he’s confident the next phase of trials will begin soon and have the potential to make a substantial difference in both patients’ and caregivers’ lives.
“It’s the job of a physician-scientist to recognize the challenges faced by patients. By using our medical expertise to address problems, we can affect real change in the world,” he said. “The University of Miami has a culture of team science and the resources at hand to spark discovery across all disciplines. Whether it’s engineering, physics or biology, we are uniquely situated because of the community of scholars that reside here. Working together, we can take discovery to a new level and impact humankind.”
2016
FDA DITPA Treatment Study (IND# 127859) may proceed to treat MCT8deficient embryos from 10 weeks pregnancy until 3 years after birth 2017
MCT8-deficient mice have decreased postnatal bone mineralization and linear growth 2020
US FDA orphan drug designation
2016-present
Stability testing of cGMP clinical lot # 024K7278 reports to the FDA via CDMO (Syngene Pharmaceuticals) 2018-present
Screening of carrier mothers 2020
US FDA rare pediatric disease (RPD) designation 2020
UM acquires technology from UM and UC via assignment and exclusive license agreement 2021
EU orphan drug designation SRW101 UPCOMING MILESTONES 2022
2023
• Drug product manufacturing and single P3 clinical trial filing
• Phase III trial
• 12-month interim analysis phase III trial
• Filing EU/US • US approval and launch • US rare pediatric disease (RPD) priority review voucher
• EU approval and launch
2024 2025
2026
2021
Neonatal diagnosis of MCT8 deficiency using mass spectrometry analysis on routinely collected dried blood spots
2020
Selective fetal administration of thyroid hormone in human with prenatal diagnosis of MCT8 deficiency ameliorates outcome 2021
Successful EOP2/Pre-Phase 3 meeting for single registration and NDA agreement




