
9 minute read
EAU meets the EU’s health chief, Commissioner Kyriakides
Mrs. Sarah Collen EAU Policy Coordinator Brussels (BE)
s.collen@uroweb.org
Advertisement
On 24 March 2021, Prof. Christopher Chapple (GB), Prof. Hein Van Poppel (BE) and Prof. James N’Dow (GB) from the EAU Executive team and Mrs. Sarah Collen (BE), EAU Policy Manager, met with EU Commissioner for Health, Stella Kyriakides.
The focus of the meeting was to introduce the work of the EAU and our members and to discuss two particular EU initiatives which are of keen interest to us: Europe’s Beating Cancer Plan and the European Health Data Space.
Prof. Chapple led the discussion by introducing the EAU and our many initiatives. He also touched upon ERN eUROGEN, the European Reference Network for rare and complex urorectogenital diseases and conditions, and the invaluable network of clinical expertise it has established across Europe. care. This led nicely into a discussion on a new initiative under the Cancer Plan called the InterSpecialty Training Programme, where we think we could add our expertise through our multidisciplinary training and guidelines.
Prof. N’Dow was then able to introduce the EAU-led Prostate PIONEER project on harnessing the power of big data for better prostate cancer outcomes, which has been driven by the EAU Guidelines Office as it strives for better and more personalised guidelines for prostate cancer patients. This project will be important for Europe’s Beating Cancer Plan and the European Health Data Space. We discussed how valuable we find the project of digitalisation and enhanced secure and ethical sharing of health data. We will work with the European Commission to ensure this will be a successful endeavour, which means better use of data for better patient outcomes.
Further news from the European Union As the headlines have been quite dominated by COVID-19 vaccine rollout (or delays), you may have missed some of the other developments happening at EU level.
This month has seen the approval of a major EU funding programme, EU4Health, which will have 5.1 billion Euros available for funding health projects across the EU over the next 7 years.
Priorities for this programme will be:
• Crisis preparedness and management of crossborder health threats. This will include funding for surveillance, preparedness and response, antimicrobial resistance, medicines shortages and security of supply. • Health promotion and disease prevention. A major chunk of this funding is likely to go to implementing Europe’s Beating Cancer Plan and additionally to prevention of other noncommunicable diseases and related risk factors, mental health, transfers of best practices, and health information. The programme is obliged to spend at least 20% on prevention programmes. • Strengthening health systems and improving their resilience and resource efficiency. Funding will be available for innovative health care models, resilience testing methodology, planning, and forecasting health workforce. • Digital transformation of healthcare and health systems. The European Health Data Space and projects on health data sharing will benefit from this funding. • Strengthening the implementation of the health legislation. Besides regulations applying to the clinical practice, this also includes regulations on in-vitro diagnostics, tobacco control and the pharmaceutical legislation.
The first work programme in 2021 will have €316 million available and we are likely to see calls for proposals for projects being launched in summer 2021. There will be increasing amounts available throughout the years, rising to over €900 million available in 2027.
Europe’s Beating Cancer Plan was published by the European Commission on World Cancer Day, 4 February 2021. The plan is wide-ranging: it covers all phases of cancer, from prevention and early diagnosis to treatment and care and survivorship. The EAU has been encouraging the European Commission to address risk-stratified early detection of prostate cancer through guidelines and qualityassurance work at EU level. In order to do this, the 2003 EU Council Recommendations on Cancer Screening will need to be revised to add new cancers as they currently only address breast, colorectal and cervical cancer. This is exactly what Europe’s Beating Cancer Plan has promised to do: the European Commission will review these recommendations this year and look to add prostate, lung, and gastric cancer to the list of cancers addressed.
During the meeting with the Commissioner, Prof. Van Poppel was able to say that what prostate cancer needs is a risk-stratified and targeted approach, and this starts with well-informed men. This was well received by the Commissioner, who confirmed that the EAU will be one of the key stakeholders as the review gets underway.
We also discussed important initiatives under Europe’s Beating Cancer Plan such as the Comprehensive Cancer Centres. We were able to feed in our experience with the Prostate Cancer Centres of Excellence, Prostate Cancer Units and the work conducted with the European Cancer Organisation on essential quality requirements for prostate cancer EAU’s leading role in implementation of EU Medical Device Regulation On 26 May 2021, the EU’s Regulation on Medical Devices will be applied across the European Union. This Regulation was agreed upon in 2017 and the implementation was delayed by one year as the anticipated date last year was during the early days of the COVID-19 crisis. The implementation of the corresponding In-Vitro Diagnostic Regulation has also been pushed back by a year and will be implemented on 26 May 2022.

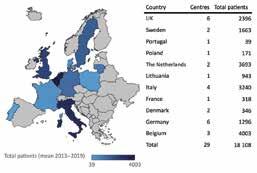
An important new feature of both pieces of legislation is the creation of expert panels to support the scientific assessment and advices. The EAU team meets with Stella Kyriakides, the European Commissioner for Health and Food Safety These expert panels will provide opinions on assessments from EU notified bodies of clinical evaluations of certain high-risk medical devices. We are very excited to announce that Prof. Jens Rassweiler (DE) has been appointed as the vice-chair of the expert panel on urology and nephrology. This is a great recognition of Prof. Rassweiler’s expertise and is a significant appointment as it means that Prof. Rassweiler will have a leading oversight role to play as the new high-risk medical devices in urology come onto the market. The advice of the expert panels will play an important role in ensuring the coherent approach of notified bodies across the EU and in ensuring that patient safety is paramount. It will be very Figure 1: Distribution of patients between countries, with the difficult for notified bodies to number of full members displayed. The total mean patient ignore a negative assessment of numbers requiring long-term care between 2013 and 2019 are a device by the expert panel. We shown. From: W.F.J. Feitz, L. Oomen, E. Leijte, D E. Shilhan, congratulate Prof. Rassweiler on M. Battye, Members of ERN eUROGEN. Rare and Complex his appointment, and we look Urology: Clinical Overview of ERN eUROGEN. European forward to supporting him in Urology, 2021, ISSN 0302-2838. https://doi.org/10.1016/j. Prof. Jens Rassweiler his role as we move forward. eururo.2021.02.043. ERN eUROGEN update ERN eUROGEN is very proud to announce that their first peer-reviewed article “Rare and Complex Urology: Clinical Overview of ERN eUROGEN” has been accepted and published in European Urology. This important paper provides an overview and identifies challenges in data collection from the network’s patient population treated by their Healthcare Provider (HCP) members. ERN eUROGEN analysed the patient population between 2013 and 2019, and the data show that ERN eUROGEN’s HCPs and expert multidisciplinary teams are treating an increasing number of patients and performing an increasing number of complex surgical procedures. However, despite a well-structured continuous monitoring system, challenges persist regarding definitions of diagnostic codes, extraction of patient numbers and procedures, and validation of these data; therefore, improvements are needed in patient registration. The network is expanding in 2021 (with an expected 30 new full HCP members and new disease areas), which will also facilitate the provision of equal care for all patients suffering from rare urorectogenital diseases and complex conditions in Europe. ERN eUROGEN will continue to follow this up with a view to further publications. Future actions will include re-evaluation of network members and current practices to maintain expertise levels, continuation of patient registry development, and research aimed at providing new insights and optimal care studies on rare diseases. Knowledge sharing is also expanding with more frequent webinars, development of clinical guidelines, and the start of the ERN Mobility Programme facilitating expertise exchange between HCPs. The network is very grateful to all who contributed to and helped with the landmark publication in European Urology, and they encourage readers to share it widely with their networks. The Open Access paper can be viewed on Elsevier’s ScienceDirect platform here: http://tiny.cc/eUROGENpaper
European Parliament’s Special Committee on Beating Cancer tackles PCa early detection In a public hearing on early detection and screening of cancer on 18 March 2021, Members of the European Parliament (MEPs) heard from Prof. Van Poppel, the EAU Adjunct Secretary General, on the issue of early detection of prostate cancer. Union (breast, cervix, and colorectal cancer) and to address the three additional cancers that are to be considered by the European Commission this year in a review of the 2003 EU Council Recommendations on Cancer Screening (prostate, lung, and gastric cancer).
Prof. Van Poppel reminded the participants that prostate cancer is the most common male cancer and a condition that is on the rise with ageing populations across Europe. It is also a killer of too many men, and in Germany it has overtaken colorectal cancer to become the second-highest cancer killer in men behind lung cancer. It is a condition that can cause much suffering for men and their families.
In the past, 1 out of 2 men who were diagnosed with prostate cancer died. Using prostate-specific antigen (PSA) testing allowed for a lot more cancers to be diagnosed. Prostate cancer mortality had fallen more in comparison with any other cancer, but at the cost of overdiagnosis and overtreatment, which led for PSA testing to be discouraged. As a result of this, while prostate cancer mortality had declined for a long time, it is now on the increase again due to reduced testing.
Prof. Van Poppel said that science has moved on since 2003, and doctors now have a way of reducing the risk of overdiagnosis and overtreatment. Risk calculators are used to avoid doing too many biopsies, along with MRI. On Europe’s Beating Cancer Plan, he stressed that information to men on prostate cancer and early detection saves lives, especially for a cancer such as prostate which could be easily detected and cured in its early stages. Nobody should die from prostate cancer anymore, he underlined. Europe’s Beating Cancer Plan has a unique opportunity to add prostate cancer to the list of cancers that benefit from EU guidelines and quality assessment work.
Véronique Trillet-Lenoir, rapporteur for the file in the European Parliament, summarised the event in her concluding remarks, and she agreed with Prof. Van Poppel’s analysis that screening programmes for other types of cancer such as prostate should be included in the EU recommendations. We look forward to the Parliament’s final report including this important issue.









