a carboxyl group.
d) It is prepared through the reaction of salicylic acid with CH COOH


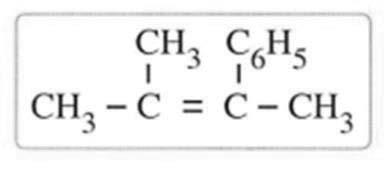
d) Acetic acid with methyl alcohol 20) The aqueous solution of sulphurous acid contains................... a) H2SO3 H3O , SO32- HSO - OHb) H2SO3 H3O , OHc) H3O , HSO - OHd) H2SO3 H3O , SO3 HSO3- , OH21) 3.4 g of impure potassium chloride dissolved in water, and excess of silver nitrate solution is added to the solution 6.7 g of silver chloride precipitated. So the percentage of chlorine in
a) 1-Nitro-2-bromo-3-chlorobenzene
b) 2-Nitro-3-chloro-4-bromobenzene

c) 1-Bromo-2-chloro-3-nitrobenzene
d) 3-Bromo-2-chloro-1-nitrobenzene
44) What is the IUPAC name of the following aromatic compound?

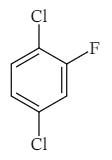

a) 6-Fluoro-1,4-dichlorobenzene
b) 2-Fluoro-1,4-dichlorobenzene
c) 1,4-Dichloro-2-fluorobenzene
d) 1,4-Dichloro-6-fluorobenzne
45) Calcium carbide could be converted into meta-chloro benzene sulfonic acid through multiple steps of chemical reactions. Which of the following sequences of reactions would be suitable for this conversion?


a) Polymerization, hydration, sulfonation, and then halogenation
b) Halogenation, hydration, polymerization, and then sulfonation
c) Hydration, polymerization, sulfonation, and then halogenation
d) Hydration, polymerization, sulfonation, and then nitration

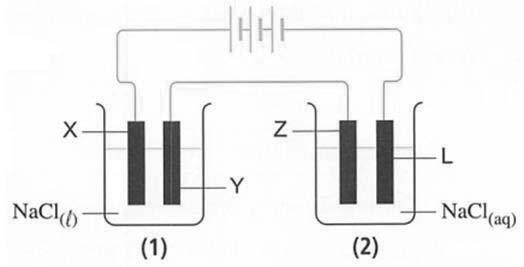
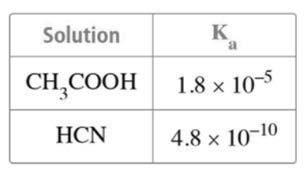
صﺎﺧ ﻒﻠﻣ 06 ـﻫ1444 ةﺪﻌﻘﻟا وذ 4 - 2023 ﻮﻳﺎﻣ 24 ءﺎﻌﺑرﻷا 4376 دﺪﻌﻟا Wednesday - 24 may 2023 - Issue NO 4376 8 53) n the opposite figure .. What are the substances formed at the graphite electrodes (1) and (2) ? 54) By knowing the following electrodes potentials: Mg2+ + 2e → Mg E=-2.37 v V + 2e → V E=-1.19 V Cu2+ + e → Cu E= + 0.16 V Which of the following equations represents a spontaneous reaction? a) V + 2Cu2+ → V2+ + 2Cu b) Mg2+ + V → V2+ + Mg c) V2+ + 2Cu → V + 2Cu2+ d) Mg2+ + 2Cu → 2Cu2+ + Mg 55) n the equilibrium reaction: A2(g)+ 2B(g)= C(g)+ Q KJ More of the product C is formed by: a) Raising the temperature and decreasing the pressure b) Raising the temperature and increasing the pressure c) Lowering the temperature and increasing the pressure d) Lowering the temperature and decreasing the pressure 56) In terms of the opposite table..which of the following solutions its solute has the highest ionization degree? a 0.01 M HCN solution b) 0.01 M CH3COOH solution c) 0.1 M HCN solution d) 0.001 M CH3COOH solution 57) Which of the following aromatic hydrocarbons its molar mass is 128 g/mol? [C=12 H=1] a) Aromatic benzene b) Naphathlene c) Toluene d) Anthracene 5 30) An element (X) its electronic configuration ends by (3d7 ), so the compound XCl3 is ................... a) Uncolored, has zero unpaired electrons. b) Colored, has two unpaired electrons. c) Colored, has four unpaired electrons. d) Uncolored, has three unpaired electrons. 31) When 1 mole of an alkane (X),and an alkene (Y) are completely combusted separately. The number of water vapour moles which are produced from (X) and (Y) are ................... a) from X n + 1), from Y (n). b) from X (n - 1), from Y (n + 1). c) from X (3���� 1) 2 , from Y (3n). d) from X (3n + 1), from Y (3n). 32) Which of the following is the correct order of boiling point? a) Pyrogallol > catechol > phenol > ethanol b) Pyrogallol > catechol > ethanol > phenol c) Ethanol > Pyrogallol > catechol > phenol d) ethanol > phenol > catechol > Pyrogallol 33) Which of the following is correct about the solution obtained by mixing equal volumes of KOH and H3PO4 solutions, each with a concentration of 2 M? a) The obtained solution will be acidic. b) The obtained solution will be alkaline. c) The concentration of the obtained solution will be 4 M. d) The obtained solution will be neutral. 34) n the equilibrium process N2O4(g) + 58KJ ↔ 2NO2(g) The reaction shifts to right by ……………. a) Adding NO2 b) Withdrawing N O c) Lowering the temperature d) Increasing the system volume. 35) If you know that : • AI0 → AI3+ + 3e- E = 1.67 V • Cu2+ + 2e- → Cu0 E = 0.34 V The diagram of the cell composed of these two electrodes is ................... a) Al / Al // Cu / Cu b) 3Cu / 3Cu // 2Al / 2AI c) 2Al / 2Al // 3Cu / 3Cu d) Cu / Cu // Al /Al 36) Arrange the following compounds according to the boiling point. a) CH COOH > CH OH > CH OHCH OH > C H b) CH OHCH OH > CH OH > CH COOH > C H c) CH OH > CH COOH > CH OHCH OH > C H d) CH OHCH OH > CH COOH > CH OH > C H 37) At constant temperature, on dilution of a weak electrolyte................... a) the degree of ionization decreases, and the solution concentration increases b) The degree of ionization increases, and the solution concentration increases. c) The degree of ionization increases and the solution concentration decreases. d) the degree of ionization decreases and the solution concentration decreases 7 46) Which of he figures shown represents the relation between the pH and the pOH of the same solution? a) B b) C c) A d) D 47) What happens to the mass of the anode and that of the cathode during the electrolysis process of copper (II) sulfate using two copper electrodes? a) The mass of the anode increases, while the mass of the cathode decreases. b) The mass of the anode decreases, while the mass of the cathode remains unchanged. c) The mass of the anode remains
d)
cathode
48) What
which represents
graduation
transition metals ? a) Co > Ni > V >Sc b) Ni > Co > V >Sc c) V > Co > Ni>Sc d) Sc > V > Ni > Co 49) ln two electrolysis experiments, the same quantity of electricity was passed in twodifferent solutions, hence,16 g of copper were deposited from copper ( II) nitratesolution, and 6 g of titanium from the solution (x) ..what is the oxidation number oftitanium in salt solution (X) ? [Cu=63.5 , Ti= 47.91] a) +1 b) +2 c) +3 d) +4 50) AII the following are isomers of ethyl ethanoate .. Except a) butanoic acid. b) Methyl propanoate. c) propanone. d) Propyl methanoate. 51) Which of the following statements is correct ? a) Angles between the bonds in cyclic C H O are larger than those in C H b) The general formula of cyclobutane differs from that of butene. c) Boiling point of cyclobutane is higher than that of cyclopropane. d) Cyclopropane is more stable than cyclobutane. 52) Aspirin is an analgesic (pain reliever) which is produced from salicylic acid ." Which of the following statements does not represent aspirin correctly ? a) It is prepared through the reaction of salicylic acid with CH OH b) It reacts with NaHCO3 c) A molecule of it contains an ester group and
unchanged, while the mass of the cathode increases.
The mass of the anode decreases, while the mass of the
increases.
is he choice
the correct
in the density of the
3 15) If you know that the solubility product of silver chloride salt in saturated solution its volume is (0.1 L) at a certain temperature equals 2.56 x 10 -6, the mass of silver chloride dissolving in the solution equals ................... [Ag = 108 , Cl = 35.5] a) 0.023 g b) 0.0115 g c) 2.3 X 10 - 6 g d) 1.15 x 10 - 6 g 16) All of the following happen during charging a car battery except a) the density and pH value of the sulfuric acid increasing b) lead(II) sulfate being converted to lead (Pb) at the anode c) sulfuric acid restoring its concentration d) lead(II) sulfate being converted to lead dioxide (PbO2) at the cathode 17) How many benzene derivative isomers do have the molecular formula C 8H10? a) 2 b) 3 c) 4 d) 5 18) The following table illustrates the standard reduction potentials of the elements W, X, Y and Z One of the following choices expresses anodic protection, ................... a) the element (Y) is plated by element (Z) b) the element (Y) is plated by element (X) c) the element (W) is plated by element (Z) d) the element (W) is plated by element (X) 19) The ester which is an isomer of C6H5COOCH can be prepared by the reaction of ……………. a) Benzoic acid with methyl alcohol b) Benzoic acid with ethyl alcohol c) Acetic acid with phenol.
the sample = ................... [K = 39 , Ag = 108 , Cl = 35.5] a) 24.5% b) 48.7% c) 46.7% d) 94.1 % 22) Heating iron (II) oxalate in air strongly gives a solid compound (X),when concentrated hot sulphuric acid is added to compound (X) another compound (Y) will be formed. In comparing the properties of compounds (X) and (Y) it is found that ................... a) The compound (X) has higher magnetic moment than (Y) and one of them is coloured. b) The compounds (X) and (Y) have equal magnetic moment and both of them are uncoloured. c) The compounds (X) and (Y) have equal magnetic moment and both of them are coloured. d) The compound (Y) has higher magnetic moment than (X) and both of them are coloured. 6 38) A solid mixture its mass 4 g of calcium hydroxide and calcium chloride is titrated by 100 mL of hydrochloric acid 0.5 M, So the percentage of calcium hydroxide in the mixture is ................... [Ca = 40, O = 16 H =I , CI = 35.5] a) 7.5 % b) 46.25 % c) 53.57 % d) 92.5 % 39) a rod of an element (A) is placed in a solution of ions of an element (B) If you know that (A) is divalent, (B) is monovalent. Which of the following is true ? a) Number of dissolved moles of (A) is double the number of precipitated moles of (B). b) Number of dissolved moles of (A) is half the number of precipitated moles of (B). c) Number of dissolved moles of (A) equals the number of precipitated moles of (B). d) Number of dissolved moles of (A) is three times the number of precipitated moles of (B). 40) All the following compounds can be oxidized by normal oxidizing agents ,except …………… a) CH3CH2CH2CHO b) CH3CH2CH2OH c) (CH ) COH d) (CH ) CHOH 41) Formation of ROR compound from ROH compound is accomplished through …………… a)
c)
c)
Dehydration reaction b) Dehydrogenation reaction
hydration reaction d) Hydrogenation reaction 42) What is the effect of adding sodium acetate to 0.1 M acetic acid? a) pOH decrease b) pOH increase
pH decrease d) pH doesn’t change 43) Using IUPAC convention, what name does the trisubstituted benzene have?
2 8)
Al O a) 11.2 b) 22.4 c) 33.6 d) 2.24 9) How many H moles are required for the hydrogenation of 3 -phenyl propene to be saturated? a) 2 mole b) 3 mole c) 4 mole d) 5 mole 10) The opposite figure represents an analytical cell for molten iron (III) oxide when 10 amperes passed for two hours through the molten iron (III) oxide, the volume of evolved gas at anode at (S.T.P) is a) 8.34 L b) 16.68 L c) 12.51 L d) 4.17 L 11) Dry distillation for sodium pentanoate salt (C H COONa) in presence of soda lime ................... is produced. a) pentene. b) butene. c) pentane. d) butane. 12) In the opposite equilibrium reaction : Br2(g) + H2(g) ↔ 2HBr(g) If the partial pressure of bromine, hydrogen and hydrogen bromide gases are 0.5 atm , 1 atm ,1.5 atm respectively. So the equilibrium constant for dissociation of hydrogen bromide to its elements equals ............. a) 2.2 b) 0.22 c) 0.45 d) 4.5 13) Three salt solutions (A) (B) and (C), if the solution of the salt (X) is added to all of them separately • A white ppt. is formed turns black by heating in case of (A). • A yellow ppt. does not dissolve in ammonia solution in case of ( B). • A yellow ppt. dissolves in ammonia solution in case of (C). So the anions of the salts (A) (B) , (C) and the reagent (X) are ...... ............. a) (X): AgNO3 , (A) : SO 2- (B) : PO 3- , (C):b) (X): KMnO4 , (A) : I- , (B): SO 2- , (C): PO43c) (X): Na2S4O6 (A) : PO4 (B): Cl- , (C): NO3d) (X ): AgNO3 , (A) : SO3 (B): - (C): PO4 14) Which of the following steps is used to obtain an organic aliphatic acid from calcium carbide? a) Water dropping / polymerization / alkylation / oxidation b) Water dropping / catalytic hydration / oxidation c) Reaction with water / hydrogenation / oxidation d) Catalytic hydration / reduction / dry distillation :داﺪﻋإ ﻒﺻاو ﻰﻧﺎﻫ .أ ءﺎﻴﻤﻴﻜﻟا ﺮﻴﺒﺧ ﻢﻠﻌﻣ ﺮﺼﻧ دﻮﻤﺤﻣ :فاﺮﺷإ 1 1) In the opposite diagram : Cell (1) Contains molten sodium chloride. Cell (2) : Contains aqueous solution of sodium chloride An electrolysis process is made for both of them, the substances formed at the electrodes (X ,Y ,Z and L) are ................... 2) The name of the following compound according to IUPAC is named………….. a) 2-phenyl -3- methyl -2- butene. b) 2,3-dimethyl -2- nonene. c) 2-methyl-3- phenyl butene. d) 2-methyl -3- phenyl -2- butane 3) In the following equilibrium reaction : 2NO2(g) N2O4(g) (KP = 20) The value of Kp for decomposition of 2 mol of N2O4 equals .... ............... a) 40 b) 25 x 10-3 C) 2.5 x 10-3 d) 400 4) When silver nitrate solution is added to two salts solutions (A) and (B), a ppt. is formed with salt solution (A), and no ppt. is formed with salt solution (B). The anions of the two salts respectively are a) (A) : Sulphide (B) Nitrite. b) (A): Nitrite (B): Sulphide. c) (A): Bicarbonate (B): Nitrite. d) (A): Nitrite (B): Bicarbonate. 5) To obtain magnetic iron oxide from iron III chloride the following process are done in sequence a) Reaction with hydrochloric acid – oxidation reduction. b) Reaction with alkaline solution - thermal decomposition- reduction. c) Oxidation reduction- thermal decom position. d) Thermal decom position- oxidation - reaction with alkaline solution. 6) The product of catalytic hydration of propyne is ………… a) CH3CH2CHO b) CH3COCH3 c) CH3CH2CH2OH d) CH3CHOHCH 7) If you know that the ionization constant of period ic acid is (14.44 x 10 ) at 25°C and the concentration of the acid is (3.8 x 10 M). So, its pOH value is ................... a) 2.22 b) 3.13 c) 10.87 d) 11.78 9 58) The opposite figure illustrates the electrolytic cell used in the extraction of aluminum from bauxite ….. which of the following statements is not correct? a) Fluorspar decreases the melting point of bauxite b) Aluminum ions gain electrons during electrolysis so as to be reduced c) The cathode is being replaced from time to time as
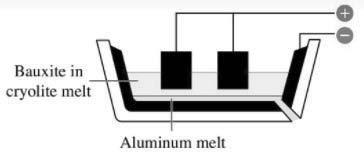
of being burnt d) Both
59) All of the following
methods for preparing red iron oxide, except ……………. a) Oxidizing black iron oxide b) The reaction of red hot iron with air c) Heating iron II oxalate in air d) The thermal decomposition of iron III hydroxide. 60) During the electrolysis of dilute solution of H2SO4 the following reactions occur at the two electrodes: 4OH- →O +2H O +4e4H+ +4e- → 2H What is the ratio between the mass of the evolving gas at the cathode and that of the evolving gas as the anode? [H=1 ,O=16] a) ���� �������� b) �������� ���� c) ���� ���� d) ���� ���� Answers 1) c 2) d 3) c 4) a 5) b 6) b 7) c 8) c 9) c 10) d 11) d 12) b 13) d 14) b 15) a 16) a 17) c 18) a 19) c 20) a 21) b 22) c 23) c 24) c 25) b 26) d 27) b 28) d 29) d 30) c 31) 32) 33) 34) d 35) 36) d 37) 38) b 39) b 40) 41) a 42) a 43) c 44) c 45) c 46) c 47) d 48) b 49) d 50) c 51) c 52) a 53) c 54) a 55) c 56) d 57) b 58) c 59) b 60) d
…………… L oxygen is evolved at anode on passing 6F in molten
a result
the anode and the cathode are made of graphite.
are
ﺔﻣﺎﻌﻟا ﺔﻳﻮﻧﺎﺜﻟا بﻼﻄﻟ ﺔﻴﺋﺎﻬﻨﻟا ﺔﻌﺟاﺮﳌا 5 6 1 2 3 4 23) When copper sulphate solution reacts with gas (A) in acidic medium a black precipitate is formed, and when silver nitrate solution reacts with solution (B) a black precipitates is formed too. So (A) and (B) are............... a) (A): CO2 , (B): NaBr. b) (A) : H2S (B) NaI. c) (A): H2S (B) Na2S d) (A): SO (B): NaCl. 24) Each of the following represents the gaseous mixture which is used in [Fischer – tropsch] process Except that ………….. a) Used in reducing Fe O to iron b) Produced from passing water vapour on methane gas in the presence of a catalyst at 725 °C c) Used in the synthesis of gas fuel at room temperature d) Produced from the reaction of gaseous mixture of methane , carbon dioxide and water vapour 25) which of the following compounds contains a methylene group? a) Propyne b) 1- butyne c) 2 –butyne d) 4,4-dimethyl-2-pentyne 26) A galvanic cel is expressed by the following cell diagram : Fe / Fe // Ni / Ni Fe(s) → Fe2+ (aq) + 2e E = + 0.409 V Ni2+(aq) + 2e → Ni(s) E = - 0.23 V Then the e.m.f of the cell is ................... a) 1.639 V b) 0.936 V c) 0.396 V d) 0.179 V 27) If you know that the solubility degree of silver chromate (Ag CrO ), Is 6.62 x 10-5 M, the solubility product of this compound equals................... a) 0.58 x 10b) 1.16 x 10-12 c) 2.32 x 10-12 d) 3.48 x 10-12 28) What is the products of the alkaline hydrolysis of the compound C 3H COOC3H ? a) Propanoic acid + propyl alcohol b) Sodium propanoate + butyl alcohol c) Butanoic acid + propyl alcohol d) Sodium butanoate + propyl alcohol 29) From the following diagram The chemicals (X) (Y) and (Z) are a) (X): FeSO , (Y) FeCl (Z) Fe(OH) b) (X): FeCO3 , (Y): FeCl3 (Z): Fe(OH)2 c) (X): FeCO3 , (Y) : FeCl2 (Z) Fe(OH)2 d) (X) FeSO (Y) FeCl (Z) : Fe(OH) 4 7 8 9
«تﺎﻐﻟ ءﺎﻴﻤﻴﻜﻟا»
ةدﺎﻣ ﻰﻓ















