POLYMER THERAPEUTICS



With this guiding theme in mind, Iris Biotech’s mission is to support researchers by supplying
• innovative technologies, rare compounds, as well as a broad portfolio on standard consumables, available in flexible quantities from small scale to bulk quantities. To fulfill our dedication “Empowering Peptide Innovation”, we are attending various conferences, symposia, and exhibitions each year. This allows us to remain in direct contact with scientists all over the world, both from academia and industry, to exchange knowledge, and to gather new ideas to tackle your current challenges.
Guided by our dedication to provide competent service, as well as novel substances and latest technologies,
Iris Biotech is your trusted partner for the world of peptides, while having strong expertise in associated disciplines. Thus, our portfolio comprises reagents and tools for the synthesis and modification of peptides, e.g. amino acids, resins and solvents but also for related technologies such as Drug Delivery, Linkerology® and Life Sciences.
Owed to the growing demand for tailor-made compounds, our portfolio is fine-tuned by our Custom Synthesis Service at Iris Biotech Laboratories. Our skilled scientists offer profound expertise in
• de novo route development,
• upscaling towards larger scale production,
• as well as synthesis optimization for increased efficiency.
Examples are the synthesis of rare chiral building blocks, unnatural amino acid derivatives, sophisticated orthogonal protecting groups, heterocycles, building blocks for nucleotides, PEGs and PEG-analogues as well as specific linkers for controlled drug delivery and release.
(Protected) Amino Acids
Standards such as Fmoc-D/L-AAA and Boc-D/L-AAA, Smoc amino acids for peptide synthesis in water, variety of protecting groups (e.g. Pbf, Trt, tBu, Bzl, Acm, Mob, SIT, Phacm, Allocam, Mmt), unusual amino acids, fluorinated derivatives, substituted prolines, arginine analogues
Building Blocks
Amino alcohols, amino aldehydes, diamines and hydrazines, (pseudoproline) dipeptides, polyamines and spermines, fatty acid derivatives
Reagents
Coupling reagents, solvents and scavengers, protecting groups
Resins
Preloaded resins (e.g. based on Trityl, TCP, TentaGel, Methoxybenzhydryl, Merrifield, PAM, Rink, Wang), scavenger resins, hydrazone resins
Linkers for Solid Phase Peptide Synthesis
Cleavable Linkers
Val-Ala based, Val-Cit based, disulfide based, Dde-helping hands
Photo-Activatable Linkers
Functionalized Linkers
Clickable linkers, trifunctional linkers, linkers with maleimide function, cross-linkers, selective N-term acylation and biotinylation
PROTACs
Ligands, linkers & modules
Fullerenes & Poly(2-oxazolines)
Poly-Amino Acids
Poly-Arg, Poly-Glu, Poly-Lys, Poly-Orn, Poly-Sar
PEGylation
Branched PEGylating reagents, (amino-)PEG-acids, PEG-amines & hydrazides & guanidines, reagents for Click-conjugation, Biotin-PEG-reagents, PEG-thiols, PEG-maleimides, other PEGylating reagents
Carbohydrates
Galactose, Glucose, Maltose, Mannose, Xylose and others
Drug Metabolites
Peptides
Substrates & Inhibitors
E.g. protein kinase inhibitors, substrates for fusion (Halo/ Snap/Clip)-tagged proteins
Natural Products
Dyes and Fluorescent Labels
E.g. ICG, AMC, DAPI
Maillard & Amadori Reaction Products
Large portfolio of derivatives useful as standards for food, pharma and cosmetics industry
Vitamins
• Customer’s demands
Your project requires a compound not listed in our portfolio? Get in contact and inquire about our custom synthesis capabilities. Our experienced scientists are excited to accept your synthetic challenge! In such cases, your request undergoes the following stages:
Detailed literature review
• Synthetic possibilities
Protocol development
• Method development and validation
• Customized synthesis
Identity confirmation
• Purity verification
All our services are based on high standards, transparency & documentation, trust, honesty & confidentiality, as well as the required know-how.
• Values: sustainability & responsibility
• State-of-the-art equipment & latest technologies
High quality standards
• Qualified suppliers & regular audits
Intergenerational business valuing partnerships
• Meeting the customer‘s expectations Integrity towards our customers
• Talk to our specialists – customer care
• Certificates of analysis & impurity profiling
Analytical and process reports
One-step reactions & complex multi-step synthesis
• Scalability from mg to kg quantities
Route scouting

Modern drug development technologies such as combinatorial chemistry and automated high-throughput screening have led to the identification of numerous potential new active pharmaceutical ingredients (APls).
However, many of those promising new molecules never reach market approval because they are not sufficiently soluble, cannot reach the desired target, are attacked by the immune system, are degraded by endogenous enzymes, or suffer from rapid renal clearance. To overcome these restrictions, first attempts with polymers were made already in the 1960s - either by attaching the therapeutic agent covalently to a polymer or by entrapping it non-covalently in a polymer nanoparticle.
The first polymer-drug conjugates that showed promising results contained poly(ethylene glycol) „PEG“, and until today, PEG is the most widely used gold standard for stealth polymers in the continuously emerging field of polymer-based drug delivery.
The pharmacological effects of PEG and many other first generation polymer attachments are mainly of physical nature:
PEG and many other polymers are very hygroscopic and hydrophilic and thus improve the plasma solubility of hydrophobic pharmaceuticals. By this, higher therapeutic concentrations are accessible.
Polymer chains are covering the surface of a pharmaceutical, thus efficiently shielding it against attacks by the immune system or degrading enzymes such as proteases. The polymeric shield has characteristics rather like a solvent than like a protein. This prevents uptake by the macrophage system. Recognition by the immune system (antibodies, proteases, and other degradation enzymes etc.) is significantly reduced. The drug stays intact and is not destroyed (degraded or metabolized) during its presence in the body and journey through the physiological system.
Hygroscopic polymers are surrounded by a large solvating sphere of water molecules. The hydrodynamic radius of the polymer-drug conjugate can be increased to a size larger than the diameter of the kidney‘s glomerular capillaries (6 to 12 nm).
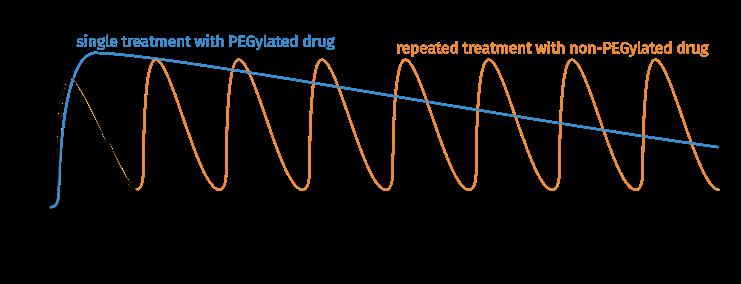
Retarded renal filtration prolongs plasma half-life of the biological drug by means of a purely biophysical size effect, without any receptor interactions that may influence pharmacodynamics or lead to side effects.
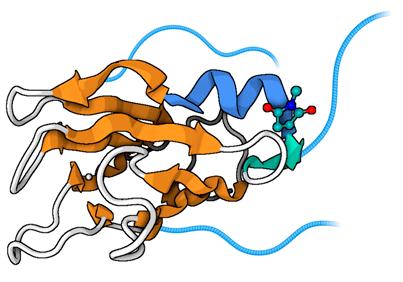
Fig. 1a: PEGylation increases the hydrodynamic radius and aqueous solubility of proteins (example: PEGylated plastocyanin, adapted from Cattani et al . 2015).
References:
Fig. 1b: Pharmacokinetic properties of a PEGylated drug in comparison with a non-PEGylated drug.
→ Drug delivery systems for RNA therapeutics; K. Paunovska, D. Loughrey, J. E. Dahlman; Nat. Rev. Genetics 2022; 23: 265-280. https://doi.org/10.1038/s41576-021-00439-4
→ Drug delivery systems in cancer therapy; B. Qorri, A. DeCarlo, M. Mellon, M. R. Szewczuk; in Drug Delivery Devices and Therapeutic Systems 2021; 423-454. https://doi.org/10.1016/b978-0-12-819838-4.00016-x
→ Chapter One - Molecular platforms for targeted drug delivery; K. Maso, A. Grigoletto, M. J. Vicent, G. Pasut; in International Review of Cell and Molecular Biology; edited by L. Galluzzi; Academic Press 2019; 346: 1-50. https://doi.org/10.1016/bs.ircmb.2019.03.001
→ Polymer-drug conjugate therapeutics: advances, insights and prospects; I. Ekladious, Y. L. Colson, M. W. Grinstaff; Nat. Rev. Drug discovery 2019; 18: 273-294. https://doi.org/10.1038/s41573-018-0005-0
→ Polymer Therapeutics: Design, Application, and Pharmacokinetics; B. A. Aderibigbe, H. E. Mukaya; in Nano- and Microscale Drug Delivery Systems; edited by A. M. Grumezescu; Elsevier 2017; 33-48. https://doi.org/10.1016/b978-0-323-52727-9.00003-0
→ Structure of a PEGylated protein reveals a highly porous double-helical assembly; G. Cattani, L. Vogeley, P. B. Crowley; Nat Chem 2015; 7: 823-8. https://doi.org/10.1038/nchem.2342
→ An Overview Of Polymer Therapeutics; G. Srinivasan, M. Vaishnavi; World Journal of Pharmaceutical Research 2014; 3: 1446-1467.
→ Polymer therapeutics: Top 10 selling pharmaceuticals — What next?; R. Duncan; Journal of Controlled Release 2014; 190: 371-380. https://doi.org/10.1016/j.jconrel.2014.05.001
→ Polymer therapeutics-prospects for 21st century: The end of the beginning; R. Duncan, M. J. Vicent; Advanced Drug Delivery Reviews 2013; 65: 60-70. https://doi.org/10.1016/j.addr.2012.08.012
→ Bioconjugate Techniques (Third Edition); G. T. Hermanson; Academic Press; Boston 2013; 1146 https://doi.org/10.1016/C2009-0-64240-9
→ Polymer-Based Therapeutics; S. Liu, R. Maheshwari, K. L. Kiick; Macromolecules 2009; 42: 3-13. https://doi.org/10.1021/ma801782q
→ The dawning era of polymer therapeutics; R. Duncan; Nat. Rev. Drug discovery 2003; 2: 347-60. https://doi.org/10.1038/nrd1088
With the decades, several further polymers have entered the field of drug delivery, natural and nonnatural ones, biodegradable and non-degradable ones, for example proteins, poly(amino acids), peptides, peptoids, polysaccharides, modified cellulose derivatives, polyesters, polyamides, polyanhydrides, polyphosphonates, polyacrylates, and many more.
back to content arrow-up
Independent of their chemical nature, polymer therapeutics can be classified by their mode of application:
Here, the polymer itself is the active pharmaceutical ingredient. One market approved example is Copaxone®, a random copolymer of Alanine, Glutamate, Lysine, and Tyrosine for the treatment of multiple sclerosis.
Here, a polymer backbone carries one or several active moieties, as for example in Opaxio®, a poly(glutamic acid)-Paclitaxel conjugate for cancer treatment.
Here, one or several polymer chains are attached to a protein, peptide, or antibody, as for example in Pegasys®, a PEGylated Interferon alpha 2a for the treatment of Hepatitis C.
Some polymers can be used to form hydrogels of defined pore size, water content, life-time and many more properties. Such hydrogels can be used to cage active ingredients, keep them perfectly hydrated, regulate their skin or tissue permeation, dose their release, and thus optimize their function. Often, such hydrogels are used in cosmetic applications.
These nanoparticles are typically formed by a mixture of different lipid and polymer types and can form - among others - micelles (consisting of a monolayer), Liposomes (consisting of a bilayer), or lipid nanoparticles „LNPs“ (composed of multiple lipid layers as well as microdomains of lipid and nucleic acid). Examples for such nanoparticles are the latest mRNA-based SARS-CoV-2 vaccines from Moderna, BioNTech/Pfizer or CureVac.
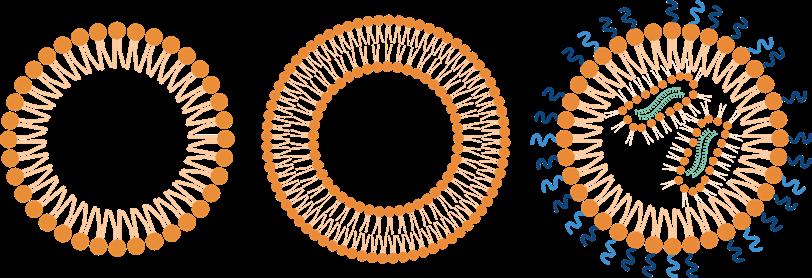
These different application modes already point out that polymer therapeutics offer far more possibilities than the simple shielding and enlarging effects previously mentioned:
A large polyvalent polymeric carrier can enable the attachment of several active moieties on one macromolecule. If, for example, the pharmaceutical role of the active ingredient is to bind and block a surface receptor, the presence of several binding ligands will significantly increase the binding energy and thus lead to a more efficient receptor binding. One can also explain this kinetically: non-covalent receptor binding is a dynamic equilibrium of association and dissociation. A released single small molecule can be washed away easily by bloodstream and thus be prevented from re-binding. However, if it is part of a polymer that is already fixed to a surface by several other of its binding moieties, it will not diffuse away, but will re-bind quickly to any free receptor on the surface.
A polymer carrier can be equipped both with a targeting moiety and with active molecules. For example, an RGD peptide can guide the polymer to cancer cells. After internalization, several bound cytotoxic molecules such as Paclitaxel can be released to kill the abnormal cell.
An intelligent design of the drug delivery system can help transporting a payload to destinations where it could not go without the polymer‘s help. There are systems that help crossing the skin barrier, some poly(amino acids) can help small molecules to cross cell membranes, LNPs as nonviral vectors can even transport DNA and RNA into living cells, and other polymers help crossing the blood-brain-barrier.
Some cells develop resistance to a pharmaceutical treatment. If this happens, the treatment must be changed to a new strategy and cells may subsequently also develop resistance to the altered therapy. However, in polymer therapeutics, the two different active ingredients can be attached to one polymer. The simultaneous delivery of two different active cargos strongly suppresses the formation of resistances, because cells would have to develop both resistances at the same time, which is almost impossible.
back to content arrow-up
→ Drug delivery systems for RNA therapeutics; K. Paunovska, D. Loughrey, J. E. Dahlman; Nat. Rev. Genetics 2022; 23: 265-280. https://doi.org/10.1038/s41576-021-00439-4
→ Polymer-based non-viral vectors for gene therapy in the skin; L. Tortajada, C. Felip-León, M. J. Vicent; Polymer Chemistry 2022; 13: 718-735. https://doi.org/10.1039/D1PY01485D
→ Polyamide/Poly(Amino Acid) Polymers for Drug Delivery; S. H. S. Boddu, P. Bhagav, P. K. Karla, S. Jacob, M. D. Adatiya, T. M. Dhameliya, K. M. Ranch, A. K. Tiwari; Journal of Functional Biomaterials 2021; 12: 58. https://doi.org/10.3390/jfb12040058
→ Polypeptides as building blocks for image-guided nanotherapies; I. Conejos-Sánchez, S. Đorđević, M. Medel, M. J. Vicent; Current Opinion in Biomedical Engineering 2021; 20: 100323. https://doi.org/10.1016/j.cobme.2021.100323
→ Carbohydrate-Derived Metal-Chelator-Triggered Lipids for Liposomal Drug Delivery; T. Holmstrom, M. Galsgaard Malle, S. Wu, K. J. Jensen, N. S. Hatzakis, C. M. Pedersen; Chemistry 2021. https://doi.org/10.1002/chem.202005332
→ From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases; E. H. Pilkington, E. J. A. Suys, N. L. Trevaskis, A. K. Wheatley, D. Zukancic, A. Algarni, H. Al-Wassiti, T. P. Davis, C. W. Pouton, S. J. Kent, N. P. Truong; Acta Biomater 2021; 131. https://doi.org/10.1016/j.actbio.2021.06.023
→ Drug-induced phospholipidosis confounds drug repurposing for SARS-CoV-2; T. A. Tummino, V. V. Rezelj, B. Fischer, A. Fischer, M. J. O’Meara, B. Monel, T. Vallet, K. M. White, Z. Zhang, A. Alon, H. Schadt, H. R. O’Donnell, J. Lyu, R. Rosales, B. L. McGovern, R. Rathnasinghe, S. Jangra, M. Schotsaert, J.-R. Galarneau, N. J. Krogan, L. Urban, K. M. Shokat, A. C. Kruse, A. García-Sastre, O. Schwartz, F. Moretti, M. Vignuzzi, F. Pognan, B. K. Shoichet; Science 2021; 373: 541-547. https://doi.org/10.1126/science.abi4708
→ The evolution of commercial drug delivery technologies; A. M. Vargason, A. C. Anselmo, S. Mitragotri; Nature Biomedical Engineering 2021; 5: 951-967. https://doi.org/10.1038/s41551-021-00698-w
→ A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy; W. Mu, Q. Chu, Y. Liu, N. Zhang; Nano-Micro Letters 2020; 12. https://doi.org/10.1007/s40820-020-00482-6
→ Polysarcosine-Functionalized Lipid Nanoparticles for Therapeutic mRNA Delivery; S. S. Nogueira, A. Schlegel, K. Maxeiner, B. Weber, M. Barz, M. A. Schroer, C. E. Blanchet, D. I. Svergun, S. Ramishetti, D. Peer, P. Langguth, U. Sahin, H. Haas; ACS Applied Nano Materials 2020; 3: 10634-10645.
https://doi.org/10.1021/acsanm.0c01834
→ Polymer Therapeutics: Biomarkers and New Approaches for Personalized Cancer Treatment; S. P. Atkinson, Z. Andreu, M. J. Vicent; J Pers Med 2018; 8: 6. https://doi.org/10.3390/jpm8010006
→ Polymer Therapeutics: Design, Application, and Pharmacokinetics; B. A. Aderibigbe and H. E. Mukaya; in Nano- and Microscale Drug Delivery Systems; edited by A. M. Grumezescu; Elsevier 2017; 33-48. https://doi.org/10.1016/b978-0-323-52727-9.00003-0
→ A review of solute encapsulating nanoparticles used as delivery systems with emphasis on branched amphipathic peptide capsules; S. M. Barros, S. K. Whitaker, P. Sukthankar, L. A. Avila, S. Gudlur, M. Warner, E. I. Beltrao, J. M. Tomich; Archives of biochemistry and biophysics 2016; 596: 22-42. https://doi.org/10.1016/j.abb.2016.02.027
→ Peptide-Based Polymer Therapeutics; A. Duro-Castano, I. Conejos-Sánchez, M. Vicent; Polymers 2014; 6: 515-551. https://doi.org/10.3390/polym6020515
→ Factors influencing in vivo disposition of polymeric micelles on multiple administrations; E. Hara, M. Ueda, A. Makino, I. Hara, E. Ozeki, S. Kimura; ACS Med Chem Lett 2014; 5: 873-7. https://doi.org/10.1021/ml500112u
→ An Overview Of Polymer Therapeutics; G. Srinivasan, M. Vaishnavi; World Journal of Pharmaceutical Research 2014; 3: 1446-1467.
→ Do HPMA copolymer conjugates have a future as clinically useful nanomedicines? A critical overview of current status and future opportunities; R. Duncan, M. J. Vicent; Adv Drug Deliv Rev 2010; 62: 272-82. https://doi.org/10.1016/j.addr.2009.12.005
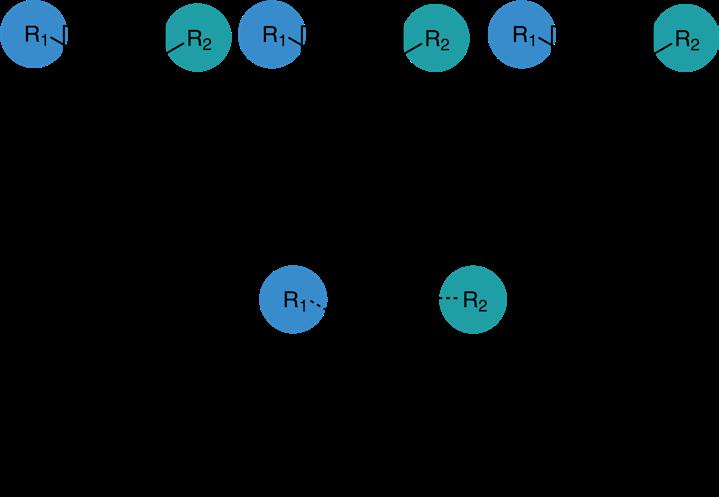
The most important parameter describing a polymer is the degree of polymerization „DP“. DP describes the number of monomeric units in a polymer molecule. If a polymer consists of only one single molecular weight species (a defined number n of repeating units), the polymer is called „homopolymer“ or „uniform“. The quite common term „monodisperse“ should no longer be used as it is an antithesis.

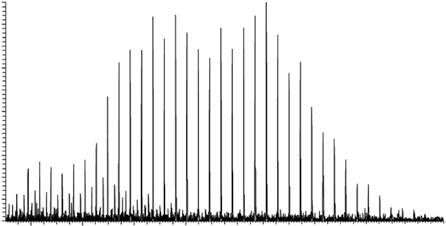
In contrast, most polymers consist of a range of species with an average mass and a distribution of n around a mean value. These polymers are referred to as „polydisperse“, „disperse“, or „non-uniform“. If the polymer is polydisperse, its mass spectrum will show a range of different molecular weights:
A measure of the distribution of molecular weights in a polymer is given by the Dispersity Đ, which is defined as the ratio between the weight average molecular weight M w and the number average molecu lar weight M n :
The weight average M w does not „count“ species just by their number but considers the total weight of each species and is therefore a much more realistic indicator of the gross mechanical properties of a polymer.
In case of a homopolymer with a defined chain length, M w is equal to M n , thus the dispersity Đ equals 1.00 and the compound is referred to as uniform.
Whenever there is a distribution of molecular weights, the weight average M w is always higher than the number average M n , and consequently the dispersity Đ is greater than 1.00. The dispersity of polymers typically used in polymer therapeutics ranges between 1.01 and 1.20.
Generally, a rather low Đ value close to 1.00 is aspired as it is an indication for a well-controlled and mastered polymerization process. However, such a narrow weight distribution is not always of advantage in practice. For the formation of some nanostructures, it may be necessary to have a rather broad distribution of molecule sizes, resulting in a rather high dispersity Đ. For example, some polymers can form spheric nanoparticles when used at high dispersity but will form fibrils if used in a dispersity close to 1.00. In other applications, it may be necessary to have material with very low dispersity.
Anyway, to reach reproducible results, it is crucial to work with highly pure polymers of good batch-to batch reproducibility. Always obey an old rule: Don‘t waste clean thoughts on dirty polymers!
back to content arrow-up
→ Polymer-drug conjugate therapeutics: advances, insights and prospects; I. Ekladious, Y. L. Colson, M. W. Grinstaff; Nat. Rev.. Drug discovery 2019; 18 : 273-294. https://doi.org/10.1038/s41573-018-0005-0
→ Polysarcosine-containing copolymers: Synthesis, characterization, self-assembly, and applications; A. Birke, J. Ling, M. Barz; Progress in Polymer Science 2018; 81: 163-208. https://doi.org/10.1016/j.progpolymsci.2018.01.002
→ Fundamentals of Polymer Science: An Introductory Text; P. C. Painter, M. M. Coleman; 1997. https://doi.org/10.1201/9780203755211
For a small molecule, typically a rather limited set of analytical parameters (for example: purity by HPLC, identity by NMR or mass spectroscopy, and residual solvents content) is sufficient to describe the quality of each material batch.
For a polymer, a much more sophisticated analysis is necessary to describe the quality of the material comprehensively. Depending on the customer‘s needs (and also on authorities‘ demand), the certificate of analysis for a therapeutic polymer may contain dozens of different analytical parameters.
The development of analytical methods and processes requires an extensive understanding of the polymer itself and about the expressiveness of the various analytical parameters.
For our therapeutic polymers, the analytical laboratory is staffed by highly-qualified personnel who use equipment specially focused, but not limited, to the quality control of polymers. All instruments are qualified, and the validated analysis conforms to international and European standards.
Our scientists support the development of polymer therapeutic substances and their analysis through the entire project life cycle from first R&D batches through pre-clinical material to final GMP- & GLPcertified commercial production and testing. Equipment and skills areparticularly suited but not limited to method development, validation, and stability studies.
Please ask for a hardcopy or download from www.iris-biotech.de!

circle-arrow-right
All the following PEGylation products are not listed in this brochure but can be found in our separate PEG catalogue.
PEGylated proteins were the first polymer therapeutic drugs reaching market approval around 1990. PEGs show a spectrum of unique physical and chemical properties which have been described in literature extensively.
Here are summarized the most common ones:
PEG fragments can be attached to many different positions in a protein. Amino groups of any solvent accessible lysines as well as the N-termini are the most prominent candidates for conjugation together with thiol functions of available cysteines. The C-terminus or carboxylic groups from aspartic acid and glutamic acid are also possible for conjugation, however, are less frequently used.
PEG can serve as spacer or cross linker between two moieties.
• PEG provides high solubility and does not contain charged side chains.
• PEG is FDA-approved for internal application, is non-toxic, lacks T-cell epitopes, and shows no signs of immunogenicity in most animal experiments.
PEG derivatives are available from uniform molecules with short chain lengths (down to two ethylene oxide units only), to long disperse constructs, allowing regio-specific chemical conjugation with small molecules, proteins, peptides, and biopharmaceuticals through their broad variety of available terminal chemical groups.
• Good solubility in BOTH hydrophilic AND hydrophobic solvents as water, toluene, methylene chloride, and many other organic solvents.
Insoluble in diethyl ether, hexane, ethylene glycol. Insoluble in water at elevated temperature.
• The solubility is influenced by formed derivatives.
• Highly mobile in water with high exclusion volume; large hydrodynamic radius.
• Complex formation with metal cations is possible.
• Can be used to precipitate proteins and nucleic acids. Form two-phase systems with aqueous solutions of other polymers. Non-toxic and FDA-approved for use in drug products.
Improves solubility of conjugated molecules. Renders proteins non-immunogenic and tolerogenic.
• Reduces the rate of renal clearance through the kidney and alters pharmacokinetics.
• Alters electroosmotic flow.
• Increases cell permeability.
back to content arrow-up
The increasing use of PEG and PEGylated products in pharmaceutical research and on the market not only provides new insight into the underlying mechanism of the beneficial properties of PEG, but it also increased the likelihood of encountering potentially unfavorable effects. These can be divided into several groups:
PEG, which was originally thought to be non-immunogenic, turned out to provoke immune reactions in several individuals.
• Adverse side effects in the body can be provoked by the polymer itself or by side products formed during synthesis that may lead to hypersensitivity.
Unexpected changes in the pharmacokinetic behavior can occur with PEG-based carriers.
PEGs are not biodegradable in the human body and can lead to PEG accumulation in cytoplasmic vacuoles, especially in kidneys:
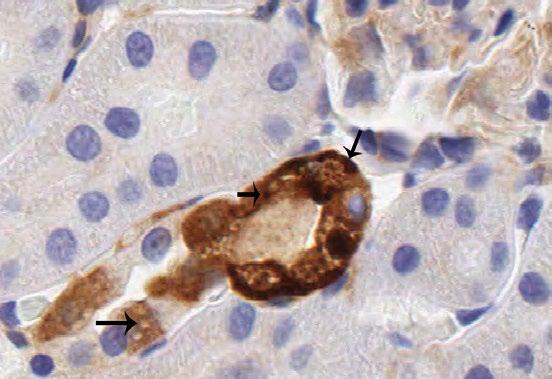
Fig.
immunoreactivity in kidney from rats given 100 mg/kg 10kPEG IV. There is variable but diffuse PEG staining in tubular epithelial cells throughout the renal cortex. PEG staining is not associated with epithelial cell vacuolation (Magnification 230x).
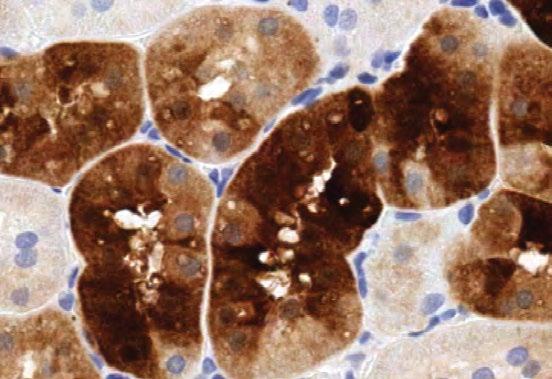
(Right) Cytoplasmic vacuoles (arrows) are associated with variable levels of PEG immunoreactivity in the kidney (300x) in rats given 100 mg/kg 40kPEG. The cortical tubule and choroid plexus had both darkly stained and clear vacuoles. (pictures adapted from Rudman et al . 2013).
Despite all sporadic drawbacks as mentioned above, PEG is still the most widely applied polymer in drug delivery.
Iris Biotech offers a portfolio of a thousand different PEG chemicals with a length ranging from only two ethylene glycol units (with a molecular weight around 100 g/mol) up to long chain PEGs of molecular weights beyond 20,000 g/mol. Further to this standard portfolio, we can produce for you also customized PEG variants according to your personal needs.
References:
→ Efficacy of PEGylated ciliary neurotrophic factor superagonist variant in diet-induced obesity mice; M. R. Battista, A. Grigoletto, T. Tedeschini, A. Cellucci, F. Colaceci, R. Laufer, G. Pasut, A. Di Marco; PLOS ONE 2022; 17: e0265749. https://doi.org/10.1371/journal.pone.0265749
→ Conjugation to PEG as a Strategy to Limit the Uptake of Drugs by the Placenta: Potential Applications for Drug Administration in Pregnancy; A. Dodd, A. A. Natfji, A. Evangelinos, A. Grigoletto, G. Pasut, F. Beards, L. Renshall, H. M. I. Osborn, F. Greco, L. K. Harris; Molecular pharmaceutics 2022; 19: 345-353.
https://doi.org/10.1021/acs.molpharmaceut.1c00498
→ Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNAbased SARS-CoV-2 vaccines; J. Szebeni, G. Storm, J. Y. Ljubimova, M. Castells, E. J. Phillips, K. Turjeman, Y. Barenholz, D. J. A. Crommelin, M. A. Dobrovolskaia; Nature Nanotechnology 2022; 17: 337-346.
https://doi.org/10.1038/s41565-022-01071-x
→ The role and impact of polyethylene glycol on anaphylactic reactions to COVID-19 nano-vaccines; P. Bigini, M. Gobbi, M. Bonati, A. Clavenna, M. Zucchetti, S. Garattini, G. Pasut; Nature Nanotechnology 2021; 16: 1169-1171. https://doi.org/10.1038/s41565-021-01001-3
→ Allergic Reactions and Anaphylaxis to LNP-Based COVID-19 Vaccines; S. M. Moghimi; Molecular Therapy 2021; 29: 898-900. https://doi.org/10.1016/j.ymthe.2021.01.030
→ Polyethylene glycol-based linkers as hydrophilicity reservoir for antibody-drug conjugates; T. Tedeschini, B. Campara, A. Grigoletto, M. Bellini, M. Salvalaio, Y. Matsuno, A. Suzuki, H. Yoshioka, G. Pasut; Journal of Controlled Release 2021; 337: 431-447. https://doi.org/10.1016/j.jconrel.2021.07.041
→ PEG hydration and conformation in aqueous solution: Hints to macromolecular crowding; S. Di Fonzo, B. Bellich, A. Gamini, N. Quadri, A. Cesàro; Polymer 2019; 175: 57-64. https://doi.org/10.1016/j.polymer.2019.05.004
→ A head-to-head comparison of poly(sarcosine) and poly(ethylene glycol) in peptidic, amphiphilic block copolymers; D. Huesmann, A. Sevenich, B. Weber, M. Barz; Polymer 2015; 67: 240-248. https://doi.org/10.1016/j.polymer.2015.04.070
→ On the biodegradability of polyethylene glycol, polypeptoids and poly(2-oxazoline)s; J. Ulbricht, R. Jordan, R. Luxenhofer; Biomaterials 2014; 35: 4848-61. https://doi.org/10.1016/j.biomaterials.2014.02.029
→ Bioconjugate Techniques (Third Edition); G. T. Hermanson; Academic Press; Boston 2013: 1146. https://doi.org/10.1016/C2009-0-64240-9
→ Chapter 6 - Heterobifunctional Crosslinkers; G. T. Hermanson; in Bioconjugate Techniques (Third Edition); edited by G. T. Hermanson; Academic Press; Boston 2013; 299-339
https://doi.org/10.1016/B978-0-12-382239-0.00006-6
→ High molecular weight polyethylene glycol cellular distribution and PEG-associated cytoplasmic vacuolation is molecular weight dependent and does not require conjugation to proteins; D. G. Rudmann, J. T. Alston, J. C. Hanson, S. Heidel; Toxicol Pathol 2013; 41: 970-83. https://doi.org/10.1177/0192623312474726
→ Poly(ethylene glycol)-Prodrug Conjugates: Concept, Design, and Applications; S. S. Banerjee, N. Aher, R. Patil J. Khandare; J Drug Deliv 2012; 103973 . https://doi.org/10.1155/2012/103973
→ Overcoming the PEG-addiction: well-defined alternatives to PEG, from structure–property relationships to better defined therapeutics; M. Barz, R. Luxenhofer, R. Zentel M. J. Vicent; Polymer Chemistry 2011; 2: 1900-1918. https://doi.org/10.1039/c0py00406e
→ Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives; K. Knop, R. Hoogenboom, D. Fischer, U. S. Schubert; Angew Chem Int Ed Engl 2010; 49: 6288-308. https://doi.org/10.1002/anie.200902672
→ Peptide and Protein PEGylation III: Advances in Chemistry and Clinical Applications; F. M. Veronese, J. M. Harris; Advanced Drug Delivery Reviews 2008; 60: 1-88.
→ PEGylation - The Magic Wand. Turning Proteins and other Biopharmaceuticals into Super Performing Block Busters; T. Bruckdorfer; PharManufacturing 2007; 1: 34-41.
→ PEGylation, successful approach to drug delivery; F. M. Veronese, G. Pasut; Drug Discov Today 2005; 10: 1451-8. https://doi.org/10.1016/S1359-6446(05)03575-0
→ PEGylated antibodies and antibody fragments for improved therapy: a review; A. P. Chapman; Adv Drug Deliv Rev 2002; 54: 531-45. https://doi.org/10.1016/S0169-409X(02)00026-1
→ Chemistry for peptide and protein PEGylation; M. J. Roberts, M. D. Bentley, J. M. Harris; Adv Drug Deliv Rev 2002; 54: 459-76. https://doi.org/10.1016/S0169-409X(02)00022-4
→ Peptide and protein PEGylation: a review of problems and solutions; F. M. Veronese; Biomaterials 2001; 22: 405-17. https://doi.org/10.1016/S0142-9612(00)00193-9
→ Synthesis and characterization of poly(ethylene glycol) derivatives; J. M. Harris, E. C. Struck, M. G. Case, M. S. Paley, M. Yalpani, J. M. Van Alstine, D. E. Brooks; Journal of Polymer Science: Polymer Chemistry Edition 1984; 22: 341-352. https://doi.org/10.1002/pol.1984.170220207
→ Functionalization of poly(ethylene glycol) and monomethoxy-poly(ethylene glycol); A. F. Bückmann, M. Morr, G. Johansson; Die Makromolekulare Chemie 1981; 182: 1379-1384. https://doi.org/10.1002/macp.1981.021820509
back to content arrow-up
PEG is made of monomer units connected via an ether bond. This is a rather rare chemical bond in living nature and not used in natural biopolymers. Consequently, the human body does not possess suitable enzymes to degrade polyether molecules - leading to an undesired accumulation of large PEG molecules in cells upon long-term treatment with high doses of PEG.
Nature uses for its biopolymers primarily three types of covalent bonds: esters (e.g., in DNA and RNA), amides (e.g., in peptides and proteins) and glycosidic bonds (e.g., in cellulose, starch, or glycogen). Therefore, most metabolizing enzymes are specialized to break bonds of this type. To achieve biodegradable drug delivery and polymer therapeutic molecules, it was therefore consequent to choose polymers that are chemically closer to those typical natural polymers.
Biodegradable poly(amino acids) appear very attractive in this context. However, their availability in sufficient quality and quantity for broad pharmaceutical application was a bottleneck in the first years: on the one hand, the production of defined polymers by either recombinant bioproduction or by peptide synthesis is extremely expensive and makes sufficient amounts for commercial application unaffordable. On the other hand, the first poly(amino acids) made by synthetic polymerization were not homogeneous, pure, and reproducible enough to use them in pharma applications. Long years of research were necessary to finally develop clean and robust production strategies for synthetic polypeptides and polypeptoids. Nowadays, we can proudly provide them from R&D to pharma grade, from milligrams to kilograms. Herein, we are presenting biodegradable and biocompatible polypeptides of both canonical and non-canonical amino acids, and polypeptoids such as poly(sarcosine) for drug delivery:
Depending on the particular monomer, polymer chain lengths are accessible with the degrees of polymerization (DP) ranging from five to more than 1,000. Thus, molar masses from 500 g/mol to 100,000 g/mol are feasible.
As with PEG, the termini of these polymers can be equipped with various functionalizations (monofunctional, homo- and heterobifunctional) to make the polymer more hydrophilic or more hydrophobic, inert, or reactive. For example, we can provide amines, azides, alkynes, and thiols for bioconjugation or synthetic click-linking to drugs, proteins, and surfaces of your choice.
Additionally, and different from PEG, poly(amino acids) of monomers with reactive side chains open the field of polymer therapeutics to small molecules which can be conjugated to the polymer backbone through both terminal AND plenty of side chain conjugations. Multiple loading can be achieved, and also loading with different drug compounds or additional analytical or therapeutic agents. Combination therapy, personalized medicine, and diagnostics are applications that are easily accessible through these new carriers.
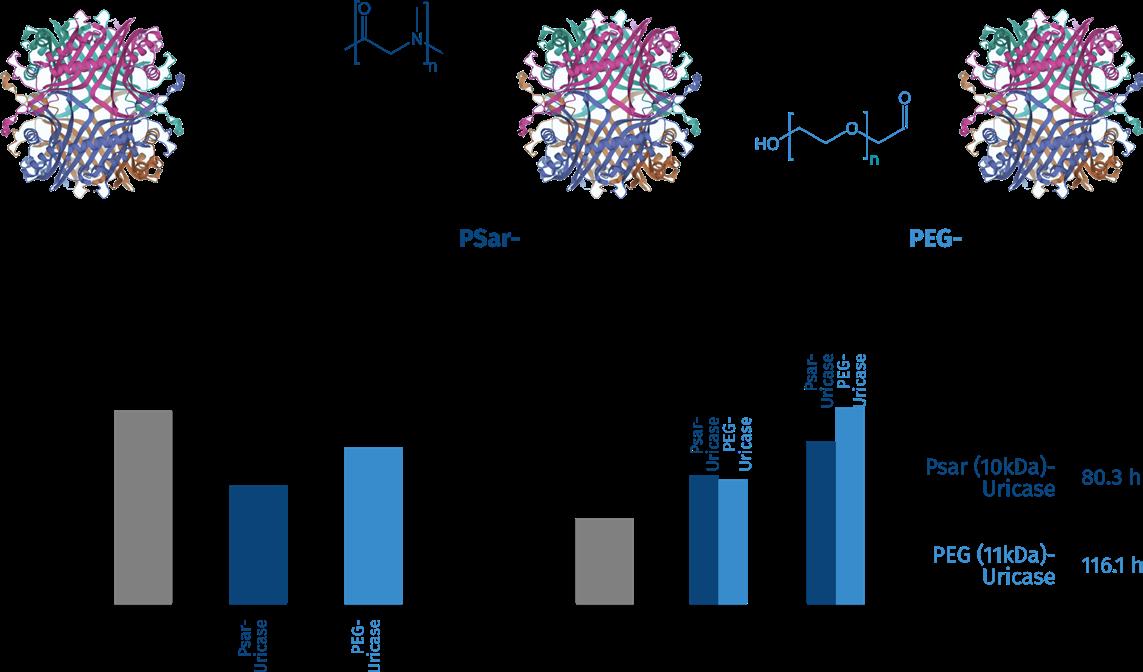
Poly(sarcosine) „PSar“ - originating from the natural, non-toxic amino acid sarcosine (N-methylglycine)is the simplest polypeptoid and a newly rediscovered biocompatible and degradable polymer. PSar is hydrophilic and shows excellent non-fouling properties, leading to protein-repellent surfaces and longcirculating polymers or polymer nanoparticles. Functional poly(sarcosine) offers a great possibility to create innovation and opportunities in many different fields of applications.
The use of poly(sarcosine) with functional head- and tail groups for bioconjugation is comparable to the well-known PEGylation technology. In a study, poly(sarcosine)- and PEG-conjugated Uricase were compared, and it was shown that the PSar conjugation is efficient in extending Uricase half-life in vivo more than 20-fold. Furthermore, PSar-Uricase is less immunogenic compared to either native or PEGylated Uricase in vivo, and PSar did not affect the enzymatic activity. Most importantly, the whole large PSar conjugate can finally be degraded to „natural“ and biocompatible small molecules.
back to content arrow-up
In brief, our polypeptides and polypeptoids are characterized by the following properties:
Biobased, degradable and non-immunogenic.
Excellent solubility in water and in organic solvents (depending on the kind of amino acid monomer).
• Highly defined polymers with narrow distribution of the degree of polymerization.
• Monofunctional, homo- or heterobifunctional at the polymer termini.
• Multiple further possible functionalities in the side chains.
• Custom-designed functionalities according to your particular needs.
• Excellent shelf-life, analytical purity, and batch-to-batch reproducibility.
References:
→ Polymer-based non-viral vectors for gene therapy in the skin; L. Tortajada, C. Felip-León, M. J. Vicent; Polymer Chemistry 2022; 13: 718-735. https://doi.org/10.1039/D1PY01485D
→ Polyamide/Poly(Amino Acid) Polymers for Drug Delivery; S. H. S. Boddu, P. Bhagav, P. K. Karla, S. Jacob, M. D. Adatiya, T. M. Dhameliya, K. M. Ranch, A. K. Tiwari; Journal of Functional Biomaterials 2021; 12: 58. https://doi.org/10.3390/jfb12040058
→ Polypeptides as building blocks for image-guided nanotherapies; I. Conejos-Sánchez, S. Đorđević, M. Medel, M. J. Vicent; Current Opinion in Biomedical Engineering 2021; 20: 100323. https://doi.org/10.1016/j.cobme.2021.100323
→ α-Amino acid N-carboxyanhydride (NCA)-derived synthetic polypeptides for nucleic acids delivery; Y. Liu, L. Yin; Advanced Drug Delivery Reviews 2021; 171: 139-163.
https://doi.org/https://doi.org/10.1016/j.addr.2020.12.007
→ Designing peptide nanoparticles for efficient brain delivery; A. Duro-Castano, D. Moreira Leite, J. Forth, Y. Deng, D. Matias, C. Noble Jesus, G. Battaglia; Advanced Drug Delivery Reviews 2020; 160: 52-77.
https://doi.org/10.1016/j.addr.2020.10.001
→ Therapeutic potential of polypeptide-based conjugates: Rational design and analytical tools that can boost clinical translation; T. Melnyk, S. Đorđević, I. Conejos-Sánchez, M. J. Vicent; Advanced Drug Delivery Reviews 2020; 160: 136-169. https://doi.org/10.1016/j.addr.2020.10.007
→ Investigation of α-amino acid N-carboxyanhydrides by X-ray diffraction for controlled ring-opening polymerization; O. Schäfer, D. Schollmeyer, A. Birke, R. Holm, K. Johann, C. Muhl, C. Seidl, B. Weber, M. Barz; Tetrahedron Letters 2019; 60: 272-275. https://doi.org/10.1016/j.tetlet.2018.12.028
→ Polysarcosine-containing copolymers: Synthesis, characterization, self-assembly, and applications; A. Birke, J. Ling, M. Barz; Progress in Polymer Science 2018; 81: 163-208. https://doi.org/10.1016/j.progpolymsci.2018.01.002
→ Peptide-Based Polymer Therapeutics; A. Duro-Castano, I. Conejos-Sánchez, M. Vicent; Polymers 2014; 6: 515-551. https://doi.org/10.3390/polym6020515
→ Suppressive immune response of poly-(sarcosine) chains in peptide-nanosheets in contrast to polymeric micelles; E. Hara, M. Ueda, C. J. Kim, A. Makino, I. Hara, E. Ozeki, S. Kimura; J Pept Sci 2014; 20: 570-7. https://doi.org/10.1002/psc.2655
→ Introducing PeptoPlexes: polylysine-block-polysarcosine based polyplexes for transfection of HEK 293T cells; P. Heller, A. Birke, D. Huesmann, B. Weber, K. Fischer, A. Reske-Kunz, M. Bros, M. Barz; Macromol Biosci 2014; 14: 1380-95. https://doi.org/10.1002/mabi.201400167
→ On the biodegradability of polyethylene glycol, polypeptoids and poly(2-oxazoline)s; J. Ulbricht, R. Jordan, R. Luxenhofer; Biomaterials 2014; 35: 4848-61. https://doi.org/10.1016/j.biomaterials.2014.02.029
→ Sintesis controlada de poliglutamatos con baja polidispersidad y arquitecturas versátiles; M. J. Vicent Docon, M. Barz, F. Canal, I. Conejos Sanchez, A. Duro Castano, R. M. England; Patent 2013: WO2013060919 A1, EP2772497 A1.
→ Surface-grafted polysarcosine as a peptoid antifouling polymer brush; K. H. Lau, C. Ren, T. S. Sileika, S. H. Park, I. Szleifer, P. B. Messersmith; Langmuir 2012; 28 : 16099-107. https://doi.org/10.1021/la302131n
→ Overcoming the PEG-addiction: well-defined alternatives to PEG, from structure–property relationships to better defined therapeutics; M. Barz, R. Luxenhofer, R. Zentel, M. J. Vicent; Polymer Chemistry 2011; 2: 1900-1918. https://doi.org/10.1039/c0py00406e
→ Integrin-assisted drug delivery of nano-scaled polymer therapeutics bearing paclitaxel; A. Eldar-Boock, K. Miller, J. Sanchis, R. Lupu, M. J. Vicent, R. Satchi-Fainaro; Biomaterials 2011; 32: 3862-74.
https://doi.org/10.1016/j.biomaterials.2011.01.073
→ Multifunctional synthetic poly(L-glutamic acid)-based cancer therapeutic and imaging agents; M. P. Melancon, C. Li; Mol Imaging 2011; 10: 28-42.
→ Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives; K. Knop, R. Hoogenboom, D. Fischer, U. S. Schubert; Angew Chem Int Ed Engl 2010; 49: 6288-308. https://doi.org/10.1002/anie.200902672
→ Thermoresponsive release from poly(Glu(OMe))-block-poly(Sar) microcapsules with surface-grafting of poly(N-isopropylacrylamide); T. Kidchob, S. Kimuram, Y. Imanishi; J Control Release 1998; 50: 205-14. https://doi.org/10.1016/S0168-3659(97)00135-1
→ Non-immunogenic polypeptides; F. F. Davis, T. Van Es, N. C. Palczuk; Patent 1979: US4179337.
back to content arrow-up
At the first sight very similar to poly(amino acids) and poly(sarcosines) are the so-called poly(2-oxazolines), commonly abbreviated as PAOx, POx, POXA or POZ. However, their polymer backbone is not a polyamide, but a polyamine. Due to the missing amide bonds, the poly(2-oxazolines) are typically not biodegradable by human enzymes and thus these polymers have a much longer plasma half-life in vivo
Further different to poly(amino acids) is the fact that the branching to the polymer side-chains is not via carbon atoms, but through tertiary amines. Thus, the side chains are rather at the positions of a polypeptoid than of a poly(amino acid). Here is a short example of available poly(2-oxazolines):
As you can see from the examples, poly(2-oxazolines) also make up an extraordinary polymer platform with numerous tunable properties and possible variations in chain length, functionalization of termini and structure of side chains. As with poly(amino acids), this allows a high degree of functionalization while the properties of the polymer can be accurately tuned by modifying the polymer side-chainsperfectly fitting to your particular API.
References:
→ Poly(2-oxazoline)s: A comprehensive overview of polymer structures and their physical properties; M. Glassner, M. Vergaelen, R. Hoogenboom; Polymer International 2018; 67: 32-45.
https://doi.org/10.1002/pi.5457
→ Microwave-assisted cationic ring-opening polymerization of 2-oxazolines; K. P. Luef, R. Hoogenboom, U. S. Schubert, F. Wiesbrock; Advances in polymer science 2015; 274: 183-208.
https://doi.org/10.1007/12_2015_340
→ On the biodegradability of polyethylene glycol, polypeptoids and poly(2-oxazoline)s; J. Ulbricht, R. Jordan, R. Luxenhofer; Biomaterials 2014; 35: 4848-61. https://doi.org/10.1016/j.biomaterials.2014.02.029
→ Poly(2-oxazoline)s--are they more advantageous for biomedical applications than other polymers?; O. Sedlacek, B. D. Monnery, S. K. Filippov, R. Hoogenboom, M. Hruby; Macromol Rapid Commun 2012; 33: 1648-62. https://doi.org/10.1002/marc.201200453
→ Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives; K. Knop, R. Hoogenboom, D. Fischer, U. S. Schubert; Angew Chem Int Ed Engl 2010; 49: 6288-308.
https://doi.org/10.1002/anie.200902672
→ Poly(2-oxazoline)s: a polymer class with numerous potential applications; R. Hoogenboom; Angew Chem Int Ed Engl 2009; 48: 7978-94. https://doi.org/10.1002/anie.200901607
An attractive feature of the polymers presented above is the fact that they can be combined with each other to form copolymers. This allows us to modify parameters such as
• Size
• Conformation
• Charge
• Solubility
• Geometry
Topology
and opens a toolbox to an almost unlimited number of combinations specifically tailored to your particular needs.
References:
→ Polymer-based non-viral vectors for gene therapy in the skin; L. Tortajada, C. Felip-León, M. J. Vicent; Polymer Chemistry 2022; 13: 718-735. https://doi.org/10.1039/D1PY01485D
→ α-Amino acid N-carboxyanhydride (NCA)-derived synthetic polypeptides for nucleic acids delivery; Y. Liu, L. Yin; Advanced Drug Delivery Reviews 2021; 171: 139-163. https://doi.org/https://doi.org/10.1016/j.addr.2020.12.007
→ Drug delivery systems in cancer therapy; B. Qorri, A. DeCarlo, M. Mellon, M. R. Szewczuk; in Drug Delivery Devices and Therapeutic Systems 2021; 423-454. https://doi.org/10.1016/b978-0-12-819838-4.00016-x
→ Therapeutic potential of polypeptide-based conjugates: Rational design and analytical tools that can boost clinical translation; T. Melnyk, S. Đorđević, I. Conejos-Sánchez, M. J. Vicent; Advanced Drug Delivery Reviews 2020; 160: 136-169. https://doi.org/10.1016/j.addr.2020.10.007
→ A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy; W. Mu, Q. Chu, Y. Liu, N. Zhang; Nano-Micro Letters 2020; 12. https://doi.org/10.1007/s40820-020-00482-6
→ Chapter One - Molecular platforms for targeted drug delivery; K. Maso, A. Grigoletto, M. J. VicentG. Pasut; in International Review of Cell and Molecular Biology; edited by L. Galluzzi; Academic Press 2019; 346: 1-50. https://doi.org/10.1016/bs.ircmb.2019.03.001
→ Polysarcosine-containing copolymers: Synthesis, characterization, self-assembly, and applications; A. Birke, J. Ling, M. Barz; Progress in Polymer Science 2018; 81: 163-208. https://doi.org/10.1016/j.progpolymsci.2018.01.002
→ Peptide-Based Polymer Therapeutics; A. Duro-Castano, I. Conejos-Sánchez, M. Vicent; Polymers 2014; 6: 515-551. https://doi.org/10.3390/polym6020515
→ Do HPMA copolymer conjugates have a future as clinically useful nanomedicines? A critical overview of current status and future opportunities; R. Duncan, M. J. Vicent; Adv Drug Deliv Rev 2010; 62: 272-82. https://doi.org/10.1016/j.addr.2009.12.005
Linear Polymers with functional side chains can be modified in the side chain. This can be done with a certain percentage of side chains or even with all - depending mainly on the size of the modification.
The most common modification is the loading of active pharmaceutical (small) molecules to the side chain, either with identical APls, or with a combination of different APls, or a combination of API and a targeting or detecting moiety.
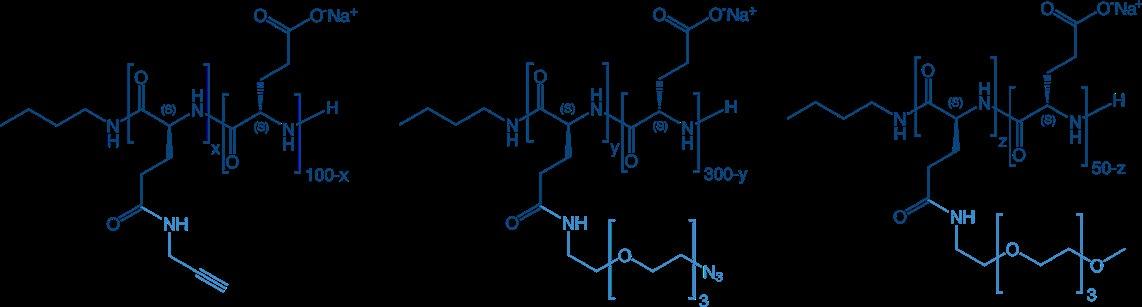
To enable further modification, a certain percentage of side chains can be „pre-activated“ with molecules for coupling reactions, e.g. with propargyl or with PEG-N 3 for Click Chemistry, or simply be equipped with an inert PEG to improve solubility:
Functional side chains can also be used for cross-linking of polymer strands to introduce rigidity and stability or to fine-tune physical properties.
References:
→ Design, synthesis and biological applications of glycopolypeptides; Z. S. Clauss, J. R. Kramer; Advanced Drug Delivery Reviews 2021; 169: 152-167. https://doi.org/10.1016/j.addr.2020.12.009
→ Surface-grafted polysarcosine as a peptoid antifouling polymer brush; K. H. Lau, C. Ren, T. S. Sileika, S. H. Park, I. Szleifer, P. B. Messersmith; Langmuir 2012; 28 : 16099-107. https://doi.org/10.1021/la302131n
→ Integrin-assisted drug delivery of nano-scaled polymer therapeutics bearing paclitaxel; A. Eldar-Boock, K. Miller, J. Sanchis, R. Lupu, M. J. Vicent, R. Satchi-Fainaro; Biomaterials 2011; 32: 3862-74.
https://doi.org/10.1016/j.biomaterials.2011.01.073
→ Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer; C. J. Langer, K. J. O‘Byrne, M. A. Socinski, S. M. Mikhailov, K. Lesniewski-Kmak, M. Smakal, T. E. Ciuleanu, S. V. Orlov, M. Dediu, D. Heigener, A. J. Eisenfeld, L. Sandalic, F. B. Oldham, J. W. Singer, H. J. Ross; J Thorac Oncol 2008; 3: 623-30. https://doi.org/10.1097/JTO.0b013e3181753b4b

Application of an amino acid mixture instead of using only one single monomer starting material allows the formation of random copolymers. The first market-approved pharmaceutical of this kind is Copaxone®, a random copolymer of Alanine, Glutamic acid, Lysine, and Tyrosine for the treatment of multiple sclerosis.
References:
→ Glatiramer Acetate: from Bench to Bed and Back; R. Arnon, R. Aharoni; Isr Med Assoc J 2019; 21: 151-157.
→ A Pilot Trial of Cop 1 in Exacerbating–Remitting Multiple Sclerosis; M. B. Bornstein, A. Miller, S. Slagle, M. Weitzman, H. Crystal, E. Drexler, M. Keilson, A. Merriam, S. Wassertheil-Smoller, V. Spada, W. Weiss, R. Arnon, I. Jacobsohn, D. Teitelbaum, M. Sela; New England Journal of Medicine 1987; 317: 408-414. https://doi.org/10.1056/nejm198708133170703
→ Studies on the chemical basis of the antigenicity of proteins. 5. Synthesis, characterization and immunogenicity of some multichain and linear polypeptides containing tyrosine; M. Sela, S. Fuchs, R. Arnon; Biochemical Journal 1962; 85: 223-235. https://doi.org/10.1042/bj0850223
Block copolymers are combinations of two or more types of polymers in a structured order. The synthesis of the blocks happens one after the other and not simultaneously as with the random copolymers.
The differing solubility properties of the polymer blocks makes them ideal structure builders for the formation of nanoparticles like micelles or LNPs.
back to content arrow-up
If you have any idea or need for a novel mixed polypeptide, please do not hesitate to contact us!
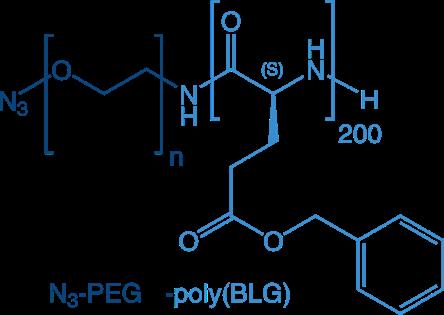
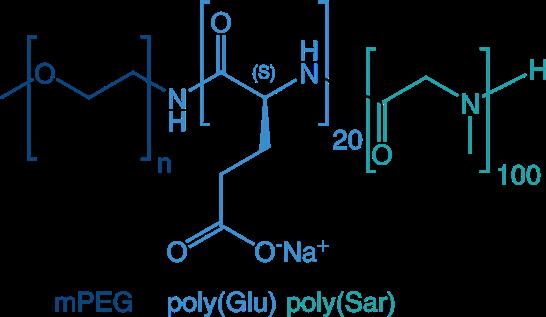
The most frequently applied block copolymers in drug delivery are combinations of PEG plus poly(amino acid), poly(peptoid), or poly(2-oxazoline), but of course also more sophisticated combinations are feasible such as triple-blocks:
References:
→ Conjugate of Bio-related substance and block polymer, and block polymer derivative for obtaining said conjugate; H. Yoshioka, M. Hirai, M. Kamiya, G. Pasut; 2022; US20220185969A1.
→ α-Amino acid N-carboxyanhydride (NCA)-derived synthetic polypeptides for nucleic acids delivery; Y. Liu, L. Yin; Advanced Drug Delivery Reviews 2021; 171: 139-163. https://doi.org/https://doi.org/10.1016/j.addr.2020.12.007
→ Designing peptide nanoparticles for efficient brain delivery; A. Duro-Castano, D. Moreira Leite, J. Forth, Y. Deng, D. Matias, C. Noble Jesus, G. Battaglia; Advanced Drug Delivery Reviews 2020; 160: 52-77. https://doi.org/10.1016/j.addr.2020.10.001
→ Preparation and Evaluation of PEGylated Poly-L-ornithine Complex as a Novel Absorption Enhancer; Y. Kamiya, T. Yamaki, M. Uchida, T. Hatanaka, M. Kimura, M. Ogihara, Y. Morimoto, H. Natsume; Biol Pharm Bull 2017; 40: 205-211. https://doi.org/10.1248/bpb.b16-00781
→ Secondary-Structure-Driven Self-Assembly of Reactive Polypept(o)ides: Controlling Size, Shape, and Function of Core Cross-Linked Nanostructures; K. Klinker, O. Schafer, D. Huesmann, T. Bauer, L. Capeloa, L. Braun, N. Stergiou, M. Schinnerer, A. Dirisala, K. Miyata, K. Osada, H. Cabral, K. Kataoka, M. Barz; Angew Chem Int Ed Engl 2017; 56: 9608-9613. https://doi.org/10.1002/anie.201702624
→ A head-to-head comparison of poly(sarcosine) and poly(ethylene glycol) in peptidic, amphiphilic block copolymers; D. Huesmann, A. Sevenich, B. Weber, M. Barz; Polymer 2015; 67: 240-248. https://doi.org/10.1016/j.polymer.2015.04.070
→ Factors influencing in vivo disposition of polymeric micelles on multiple administrations; E. Hara, M. Ueda, A. Makino, I. Hara, E. Ozeki and S. Kimura; ACS Med Chem Lett 2014; 5: 873-7. https://doi.org/10.1021/ml500112u
→ Introducing PeptoPlexes: polylysine-block-polysarcosine based polyplexes for transfection of HEK 293T cells; P. Heller, A. Birke, D. Huesmann, B. Weber, K. Fischer, A. Reske-Kunz, M. Bros, M. Barz; Macromol Biosci 2014; 14: 1380-95. https://doi.org/10.1002/mabi.201400167
→ Methodologies for preparation of synthetic block copolypeptides: materials with future promise in drug delivery; T. J. Deming; Adv Drug Deliv Rev 2002; 54: 1145-55. https://doi.org/10.1016/S0169-409X(02)00062-5
→ Thermoresponsive release from poly(Glu(OMe))-block-poly(Sar) microcapsules with surface-grafting of poly(N-isopropylacrylamide); T. Kidchob, S. Kimura, Y. Imanishi; J Control Release 1998; 50: 205-14.
https://doi.org/10.1016/S0168-3659(97)00135-1
circle-arrow-right Please contact us with your ideas and we will do our best to make the idea come true!
Almost all polymers discussed by now are water soluble - for good reason: most drug delivery systems are designed to transport their payload in the bloodstream and thus they must be highly water soluble. However, once the delivery complex has reached its destination, the picture turns to the opposite: if a cargo should enter a cell through the cell membrane (which mainly consists of a hydrophobic lipid bilayer), a highly hydrophilic transport system is rather counterproductive. Here, a lipophilic moiety should rather be helpful. Therefore, many drug delivery systems also contain hydrophobic blocks that help to form barrier-overbearing nanostructures such as micelles, liposomes, or lipid nanoparticles (LNPs).
Especially in context of the worldwide SARS-CoV-2 pandemic, the rapid development of mRNA vaccines emphasized the importance of tools for secured nucleic acid delivery. Free nucleic acids like DNA or RNA in the bloodstream are regarded by the organism as foreign, infectious, and harmful and are thus degraded immediately by endogenous nucleases. A delivery system must therefore shield them well from recognition. Later, it must help the RNA to cross the physiological barrier of the cell membrane.
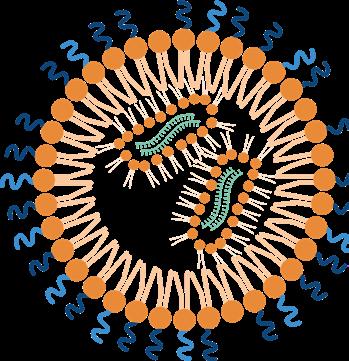
For successful RNA drug or vaccine delivery, polymer-conjugated LNPs capable to permeate plasma membranes were developed and numerous ones are reported in literature. Here, we would like to introduce to you a few very interesting and efficient conjugates for LNP formation:
Poly(sarcoines) of various length combined with Vitamin E as lipid moiety
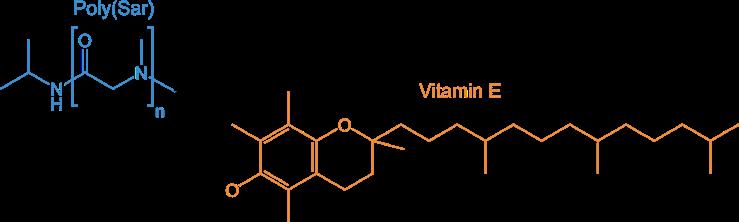
and poly(Glu diols) with one- and two-arm lipophilic terminus
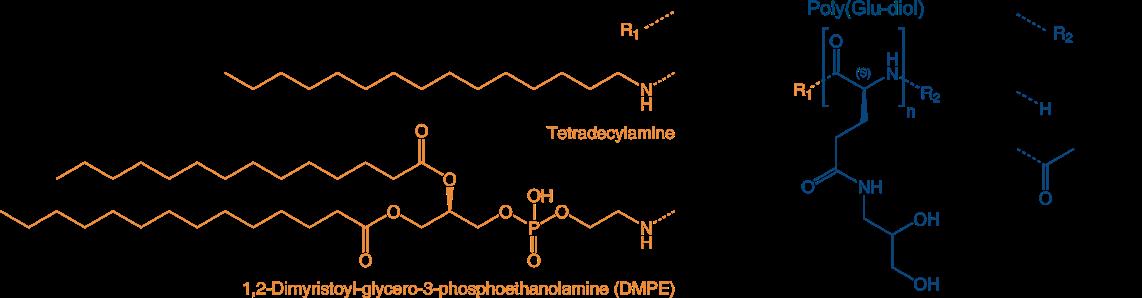
Reference:
→ Polysarcosine-Functionalized Lipid Nanoparticles for Therapeutic mRNA Delivery; S. S. Nogueira, A. Schlegel, K. Maxeiner, B. Weber, M. Barz, M. A. Schroer, C. E. Blanchet, D. I. Svergun, S. Ramishetti, D. Peer, P. Langguth, U. Sahin, H. Haas; ACS Applied Nano Materials 2020; 3: 10634-10645.
https://doi.org/10.1021/acsanm.0c01834
back to content arrow-up
The following PSar-lipids and PGA diol-lipids and combinations are currently available at Iris Biotech:
PSR1830 VitE-PSar 10
N-alpha-isopropyl polysarcosine(10) N-omega-(Vitamine E)-4-oxobutanoate
Mol. weight 1300 Da
PSR1820 VitE-PSar20
N-alpha-isopropyl polysarcosine(20) N-omega-(Vitamine E)-4-oxobutanoate
Mol. weight 2000 Da
PGA1880 C14-[PGA(DIOL)]10 -H




(Tetradecylamine)-poly-L-glutamic acid(gamma-dihydroxypropylamide)acetamide
Mol. weight 2000 Da
PGA1890 C14-[PGA(DIOL)] 20 -H
(Tetradecylamine)-poly-L-glutamic acid(gamma-dihydroxypropylamide)acetamide
Mol. weight 4000 Da
PGA1900 C14-[PGA(DIOL)]10 -COCH3
(Tetradecylamine)-poly-L-glutamic acid(gamma-dihydroxypropylamide)acetamide
Mol. weight 2000 Da
PGA1910 C14-[PGA(DIOL)] 20 -COCH3 (Tetradecylamine)-poly-L-glutamic acid(gamma-dihydroxypropylamide)acetamide
Mol. weight 4000 Da
PGA1920 DMPE-[PGA(DIOL)] 30 -H
(1,2-Dimyristoyl-glycero-3-phosphoethanolamine)-poly-L-glutamic acid(gamma-dihydroxypropylamide) acetamide


Mol. weight 7000 Da
References:
→ Drug delivery systems for RNA therapeutics; K. Paunovska, D. Loughrey, J. E. Dahlman; Nat. Rev. Genetics 2022; 23: 265-280. https://doi.org/10.1038/s41576-021-00439-4
→ Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNAbased SARS-CoV-2 vaccines; J. Szebeni, G. Storm, J. Y. Ljubimova, M. Castells, E. J. Phillips, K. Turjeman, Y. Barenholz, D. J. A. Crommelin, M. A. Dobrovolskaia; Nature Nanotechnology 2022; 17: 337-346. https://doi.org/10.1038/s41565-022-01071-x
→ Carbohydrate-Derived Metal-Chelator-Triggered Lipids for Liposomal Drug Delivery; T. Holmstrom, M. Galsgaard Malle, S. Wu, K. J. Jensen, N. S. Hatzakis, C. M. Pedersen; Chemistry 2021. https://doi.org/10.1002/chem.202005332
→ Allergic Reactions and Anaphylaxis to LNP-Based COVID-19 Vaccines; S. M. Moghimi; Molecular Therapy 2021; 29: 898-900. https://doi.org/10.1016/j.ymthe.2021.01.030
→ From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases; E. H. Pilkington, E. J. A. Suys, N. L. Trevaskis, A. K. Wheatley, D. Zukancic, A. Algarni, H. Al-Wassiti, T. P. Davis, C. W. Pouton, S. J. Kent, N. P. Truong; Acta Biomater 2021; 131. https://doi.org/10.1016/j.actbio.2021.06.023
→ Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines; T. T. H. Thi, E. J. A. Suys, J. S. Lee, D. H. Nguyen, K. D. Park, N. P. Truong; Vaccines (Basel) 2021; 9: 359. https://doi.org/10.3390/vaccines9040359
→ Drug-induced phospholipidosis confounds drug repurposing for SARS-CoV-2; T. A. Tummino, V. V. Rezelj, B. Fischer, A. Fischer, M. J. O’Meara, B. Monel, T. Vallet, K. M. White, Z. Zhang, A. Alon, H. Schadt, H. R. O’Donnell, J. Lyu, R. Rosales, B. L. McGovern, R. Rathnasinghe, S. Jangra, M. Schotsaert, J.-R. Galarneau, N. J. Krogan, L. Urban, K. M. Shokat, A. C. Kruse, A. García-Sastre, O. Schwartz, F. Moretti, M. Vignuzzi, F. Pognan, B. K. Shoichet; Science 2021; 373: 541-547. https://doi.org/10.1126/science.abi4708
→ Polysarcosine-Functionalized Lipid Nanoparticles for Therapeutic mRNA Delivery; S. S. Nogueira, A. Schlegel, K. Maxeiner, B. Weber, M. Barz, M. A. Schroer, C. E. Blanchet, D. I. Svergun, S. Ramishetti, D. Peer, P. Langguth, U. Sahin, H. Haas; ACS Applied Nano Materials 2020; 3: 10634-10645. https://doi.org/10.1021/acsanm.0c01834
back to content arrow-up
Research can be time-consuming, exciting, disappointing, fulfilling. But in the end, research alone is unprofitable if commercial implementation is not possible.
Therefore, efficient drug delivery systems must not only perform nicely in research, they must finally do the same also in large and commercial scale. And they must be available in a quality that satisfies scientists, regulatory authorities, and finally patients.
To guarantee this from the beginning, the development of your poly(amino acids), polypeptoids, and copolymers thereof lies in the same hands that can later scale-up and transfer the production to cGMP certified cleanrooms. In this way, we ensure that even the first tiny research batches have the same physicochemical properties as the later commercial GMP batches in kilogram quantities.
Without deviations, without undesired delays!
We provide narrow molecular weight distribution and reproducible and scalable manufacturing from R&D to final GMP production.
In the beginning, the polymer team can support you with an extensive knowledge on polymer-biology interface to help you designing technologies especially suited for delivering nucleic acids, small drugs, and proteins. If desired, we can also offer biodistribution-, PK-, and PD-studies.
During process development and preclinical phase, we support you with a flexible and agile approach to develop robust, reproducible, and scalable processes meeting your specific product requirements for GLP TOX studies. Polymer production uses high-quality monomers and precise control over the polymerization reaction. This results in a very homogeneous polymer with low dispersity and unbeatable batch-to-batch consistency along all development stages and batch sizes.
In parallel, analytical methods specific for your product can be developed and validated. Polymer QC scientists support the development of drug substances and drug products through the entire project life-cycle. Equipment and skills are especially suited but not limited to method development, validation, and stability studies. Analytical work can be performed using qualified equipment within state-of-the art GMP & GLP certified facilities.
Finally, your commercial product will be manufactured in one of the cleanrooms. If demanded by you or by regulatory authorities, even in one of the GMP suits fully equipped to manufacture any type of poly(amino acid)-based therapeutic. Excellent feedback on the CMC packages delivered to date across the different project stages and scales from both EMA and FDA underlines the high level of the polymer production site and of all scientists involved.
The handling of pharmaceutical polymers is not always simple, although their chemical reactions are in principle the same as with any other organic chemical compound. Even experienced pharmaceutical chemists who previously worked with small API molecules are facing unexpected troubles when combining their small molecules with polymers or when trying to scale-up such reactions.
Our polymer specialists can help you to develop the process, or they can perform the synthetic steps for you - even up to the final polymeric API. The GMP polymer production cleanrooms are fully equipped to also host these production steps.
Reference:
→ Therapeutic potential of polypeptide-based conjugates: Rational design and analytical tools that can boost clinical translation; T. Melnyk, S. Đorđević, I. Conejos-Sánchez, M. J. Vicent; Advanced Drug Delivery Reviews 2020; 160: 136-169. https://doi.org/10.1016/j.addr.2020.10.007
circle-arrow-right
You could not find the product you are
Please contact us for a custom synthesis!
back to content arrow-up

Finding the best formulation for a drug product can be a long and nerve-wracking process - especially if one has not used a certain delivery technology before. Therefore, better benefit from the years of know how of our specialists!
No matter whether your pharmaceutical payload is a small molecule,
• a biologic,
• a nucleic acid
• or even a whole cell,
the formulation team can identify the best delivery technology. This might be a polymer conjugate, a colloidal nanoparticle,
• a hydrophilic particle,
• a polymeric non-viral vector (e.g., LNP),
• or a hydrogel.
Once the best delivery system is found, we can also perform all further formulation screening and optimization for your drug product. These services include optimization of key process parameters such as molar ratios, mixing flow rates, concentrations, temperature, and flow speed.
Finally, we can provide GMP fill & finish of aseptic vials and other non-aseptic formats in small batches (from 500 to 15,000 vials) to support your (pre)clinical development:
• Filled Containers: vials (from below 1 mL to 50 mL) syringes (1 mL - 10 mL)
various bottles
• flexible bags
Batch sizes from 5 L to 50 L.
• Terminal sterilization if material cannot be filter sterilized.
• Testing services before and after fill & finish.
• Release & stability testing.
• Batch certification by a Qualified Person.
Administration routes: intravenous, intramuscular, subcutaneous.
circle-arrow-right
No matter whether you just need a polymer for R&D only or you might be interested in the „whole package“ up to the final aseptic drug product: please do not hesitate to contact us!
In addition to the previously described polymeric drug delivery systems, Iris Biotech provides fullerenes and fullerenols of different core sizes (C60 vs. C70).
Fullerenes consist of fused five- and six-membered carbon rings connected by single and double bonds forming spherical, elliptical, or tubular structures. Intensive studies revealed their unique properties, e.g. nanometric size, tensile strength, thermal/photo conductivity as well as drug loading and delivery. Functionalized fullerenes allow facile conjugation to other (bio-)molecules expanding their range of applications.
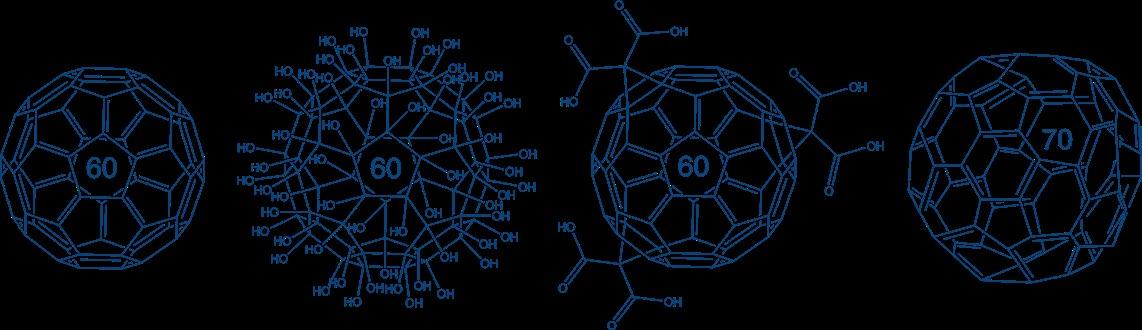
Fullerenes and Fullerenols are suitable as carbon-based nanocages for the targeted delivery of therapeutic molecules, e.g. for nucleic acid delivery, benefiting from their nonimmunological reactions.
In addition, fullerene and its derivatives show high potential in crossing the blood-brain barrier and delivering drugs into the CNS.
Nevertheless, one major issue concerning biomedical applications remains the bad solubility of fullerene itself in aqueous solutions. The most promising classes of water-soluble derivatives are carboxylated or the already mentioned polyhydroxylated fullerenol derivatives, which are both part of Iris Biotech‘s portfolio. Furthermore, the advertised C60 and C70 fullerenes react with nucleophiles, e.g. the amino groups of amino acids, which improves their solubility. The functionalization of fullerenes allows the easy generation of conjugates with other biomolecules, PEGs or linkers, to fulfill your required demands.
References:
→ Water-soluble fullerenes for medical applications; I. Rašović; Materials Science and Technology 2016; 33: 777-794. https://doi.org/10.1080/02670836.2016.1198114
→ Fullerenol Nanoparticles: Toxicity and Antioxidant Activity; R. Injac, M. Prijatelj and B. Strukelj; in Oxidative Stress and Nanotechnology: Methods and Protocols; edited by D. Armstrong, D. J. Bharali; Humana Press; Totowa, NJ 2013; 75-100. https://doi.org/10.1007/978-1-62703-475-3_5
→ Anti-influenza activity of c60 fullerene derivatives; M. Shoji, E. Takahashi, D. Hatakeyama, Y. Iwai, Y. Morita, R. Shirayama, N. Echigo, H. Kido, S. Nakamura, T. Mashino, T. Okutani, T. Kuzuhara; PLoS One 2013; 8: e66337. https://doi.org/10.1371/journal.pone.0066337
→ Medicinal chemistry and pharmacological potential of fullerenes and carbon nanotubes; F. Cataldo, T. Da Ros; Springer Science & Business Media 2008; 1.
→ Medicinal applications of fullerenes; R. Bakry, R. M. Vallant, M. Najam-ul-Haq, M. Rainer, Z. Szabo, C. W. Huck, G. K. Bonn; International journal of nanomedicine 2007; 2: 639-49.
back to content arrow-up
See the latest catalogue portfolio also at www.iris-biotech.de/products/drug-delivery/poly-arginines.html Product details
PAR1060 n Bu-PArg(20)*HCl
n-Butyl-poly-L-Arginine hydrochloride
CAS-No. 26982-20-7
Mol. weight 3800 Da
PAR1020 n Bu-PArg(50)*HCl
n-Butyl-poly-L-Arginine hydrochloride
CAS-No. 26982-20-7
Mol. weight 9600 Da
PAR1030 n Bu-PArg(100)*HCl
n-Butyl-poly-L-Arginine hydrochloride
CAS-No. 26982-20-7
Mol. weight 19000 Da
PAR1050 n Bu-PArg(200)*HCl
n-Butyl-poly-L-Arginine hydrochloride
CAS-No. 26982-20-7



Mol. weight 38500 Da

See the latest catalogue portfolio also at www.iris-biotech.de/products/drug-delivery/poly-glutamic-acids.html
Product details
PGA1005 n Bu-PGA(20)
n-Butyl-poly(L-glutamic acid) sodium salt
CAS-No. 26247-79-0
Mol. weight 3000 Da
PGA1010 n Bu-PGA(50)
n-Butyl-poly(L-glutamic acid) sodium salt
CAS-No. 26247-79-0
Mol. weight 7500 Da
PGA1015 n Bu-PGA(100)
n-Butyl-poly(L-glutamic acid) sodium salt
CAS-No. 26247-79-0
Mol. weight 15100 Da
PGA1020 n Bu-PGA(200)
n-Butyl-poly(L-glutamic acid) sodium salt
CAS-No. 26247-79-0





Mol. weight 30200 Da
PGA1085 Prg-PGA(20)
Propargyl-poly(L-glutamic acid) sodium salt
Mol. weight 3000 Da
PGA1090 Prg-PGA(50)
Propargyl-poly(L-glutamic acid) sodium salt
weight 7500 Da
PGA1095 Prg-PGA(100)
Propargyl-poly(L-glutamic acid) sodium salt
Mol. weight 15000 Da
PGA1100 Prg-PGA(200)
Propargyl-poly(L-glutamic acid) sodium salt
weight 30200 Da
PGA1125 N3 -PGA(20)
Azido-ethyltri(ethylene glycol)-poly(L-glutamic acid)
PGA1130 N3 -PGA(50)
Azido-ethyltri(ethylene glycol)-poly(L-glutamic acid)
weight 7500 Da
PGA1135 N3 -PGA(100)
Azido-ethyltri(ethylene glycol)-poly(L-glutamic acid)





15000 Da

PGA1140 N3 -PGA(200)
Azido-ethyltri(ethylene glycol)-poly(L-glutamic acid)





sodium salt
Mol. weight 30000 Da
PGA1190 n Bu-PGA(20)[Prg(20)]
n-Butyl-poly(L-glutamic acid gamma-propargyl amide)
sodium salt (10-20mol% propargyl substitution)
Mol. weight 3000 Da
PGA1195 n Bu-PGA(50)[Prg(20)]
n-Butyl-poly(L-glutamic acid gamma-propargyl amide)
sodium salt (10-20mol% propargyl substitution)
Mol. weight 7500 Da
PGA1200 n Bu-PGA(100)[Prg(20)]
n-Butyl-poly(L-glutamic acid gamma-propargyl amide)
sodium salt (10-20mol% propargyl substitution)
Mol. weight 15000 Da
PGA1205 n Bu-PGA(200)[Prg(20)]
n-Butyl-poly(L-glutamic acid gamma-propargyl amide)
sodium salt (10-20mol% propargyl substitution)
weight 30000 Da
PGA1290 n Bu-PGA(20)[PEG2-N3 (20)]
n-Butyl-poly(L-glutamic acid gamma-azido-ethyltri(ethylene glycol) amide) sodium salt (10-20mol% azido substitution)
Mol. weight 3600 Da
PGA1295 n Bu-PGA(50)[PEG2-N3 (20)]
n-Butyl-poly(L-glutamic acid gamma-azido-ethyltri(ethylene glycol) amide) sodium salt (10-20mol% azido substitution)
Mol. weight 9100 Da
PGA1300 n Bu-PGA(100)[PEG2-N3 (20)]






n-Butyl-poly(L-glutamic acid gamma-azido-ethyltri(ethylene glycol) amide) sodium salt (10-20mol% azido substitution)
Mol. weight 18300 Da
PGA1305 n Bu-PGA(200)[PEG2-N3 (20)]
n-Butyl-poly(L-glutamic acid gamma-azido-ethyltri(ethylene glycol) amide) sodium salt (10-20mol% azido substitution)
Mol. weight 36700 Da
PGA1490 n Bu-PGA(20)[mPEG(20)]
n-Butyl-poly(L-glutamic acid gamma-(omega-methoxyhepta(ethylene glycol))) sodium salt (10-20mol% mPEG substitution)
Mol. weight 4300 Da
PGA1495 n Bu-PGA(50)[mPEG(20)]
n-Butyl-poly(L-glutamic acid gamma-(omega-methoxyhepta(ethylene glycol))) sodium salt (10-20mol% mPEG substitution)
Mol. weight 10900 Da
PGA1500 n Bu-PGA(100)[mPEG(20)]
n-Butyl-poly(L-glutamic acid gamma-(omega-methoxyhepta(ethylene glycol))) sodium salt (10-20mol% mPEG substitution)
Mol. weight 21800 Da
PGA1505 n Bu-PGA(200)[mPEG(20)]
n-Butyl-poly(L-glutamic acid gamma-(omega-methoxyhepta(ethylene glycol))) sodium salt
weight 40600 Da
PGA1700 Aldehyde-PGA(20)t Bu
(4,4-Diethoxybutyl)-poly(sodium L-glutamato) pivalate
Mol. weight 3000 Da
PGA1710 Aldehyde-PGA(50)t Bu
(4,4-Diethoxybutyl)-poly(sodium L-glutamato) pivalate
Mol. weight 7600 Da
PGA1720 Aldehyde-PGA(100)t Bu
(4,4-Diethoxybutyl)-poly(sodium L-glutamato) pivalate
Mol. weight 15100 Da
PGA1740 Aldehyde-PGA(200)t Bu
(4,4-Diethoxybutyl)-poly(sodium L-glutamato) pivalate






Mol. weight 30200 Da
PGA1810 n Bu-PGA(20)[Hyd(20)]
n-Butyl-poly(L-glutamic acid gamma-t-butyl carbazate) sodium salt (10-20mol% substitution)
Mol. weight 3700 Da
PGA1770 n Bu-PGA(100)[Hyd(20)]
n-Butyl-poly(L-glutamic acid gamma-t-butyl carbazate) sodium salt (10-20 mol% substitution, MW 20200Da)
Mol. weight 20200 Da
PGA1830 n Bu-PGA(200)[Hyd(20)]
n-Butyl-poly(L-glutamic acid gamma-t-butyl carbazate) sodium salt (10-20mol% substitution)
Mol. weight 40100 Da
PGA1870 n Bu-PGA(50)[Hyd(20)]
n-Butyl-poly(L-glutamic acid gamma-t-butyl carbazate) sodium salt (10-20mol% substitution)
Mol. weight 9900 Da
PGA1880 C14-[PGA(DIOL)]10 -H





(Tetradecylamine)-poly-L-glutamic acid(gamma-dihydroxypropylamide)acetamide
Mol. weight 2000 Da
PGA1890 C14-[PGA(DIOL)] 20 -H
(Tetradecylamine)-poly-L-glutamic acid(gamma-dihydroxypropylamide)acetamide
Mol. weight 4000 Da
PGA1900 C14-[PGA(DIOL)]10 -COCH3
(Tetradecylamine)-poly-L-glutamic acid(gamma-dihydroxypropylamide)acetamide
Mol. weight 2000 Da
PGA1910 C14-[PGA(DIOL)] 20 -COCH3
(Tetradecylamine)-poly-L-glutamic acid(gamma-dihydroxypropylamide)acetamide
Mol. weight 4000 Da
PGA1920 DMPE-[PGA(DIOL)] 30 -H
(1,2-Dimyristoyl-glycero-3-phosphoethanolamine)-po





ly-L-glutamic acid(gamma-dihydroxypropylamide) acetamide
Mol. weight 7000 Da
See the latest catalogue portfolio also at www.iris-biotech.de/products/drug-delivery/poly-lysines.html
Bu-PLys(20)*HCl
Bu-PLys(20)*HBr
Bu-PLys(50)*HBr n-Butyl-poly-L-Lysine hydrobromide
CAS-No. 26124-78-7
weight 10500 Da
PLY1010 n Bu-PLys(100)*HCl
n-Butyl-poly-L-Lysine hydrochloride
CAS-No. 26124-78-7
Mol. weight 16000 Da
PLY1011 n Bu-PLys(100)*HBr
n-Butyl-poly-L-Lysine hydrobromide
CAS-No. 26124-78-7
Mol. weight 20900 Da
PLY1020 n Bu-PLys(200)*HCl
n-Butyl-poly-L-Lysine hydrochloride
CAS-No. 26124-78-7
Mol. weight 32500 Da
PLY1021 n Bu-PLys(200)*HBr
n-Butyl-poly-L-Lysine hydrobromide
CAS-No. 26124-78-7





Mol. weight 42000 Da
Product details
See the latest catalogue portfolio also at www.iris-biotech.de/products/drug-delivery/poly-ornithines.html Product details
POR1060 n Bu-POR(20)*HCl
n-Butyl-poly-L-Ornithine hydrochloride
CAS-No. 26982-21-8
Mol. weight 3000 Da
POR1020 n Bu-POR(50)*HCl
n-Butyl-poly-L-Ornithine hydrochloride
CAS-No. 26982-21-8
Mol. weight 5800 Da
POR1030 n Bu-POR(100)*HCl
n-Butyl-poly-L-Ornithine hydrochloride
CAS-No. 26982-21-8
Mol. weight 15000 Da
POR1040 n Bu-POR(150)*HCl
n-Butyl-poly-L-Ornithine hydrochloride
CAS-No. 26982-21-8
Mol. weight 22600 Da
POR1050 n Bu-POR(200)*HCl
n-Butyl-poly-L-Ornithine hydrochloride





CAS-No. 26982-21-8
Mol. weight 30100 Da
See the latest catalogue portfolio also at www.iris-biotech.de/products/drug-delivery/poly-sarcosines.html
PSR1740 Mal-PSar-OMe (5 kDa)
N-alpha-(3-maleimido)-propanamide polysarcosine omega-methoxyethylamide
PSR1750 Mal-PSar-OMe (10 kDa)
N-alpha-(3-maleimido)-propanamide polysarcosine
PSR1760 Mal-PSar-OMe (15 kDa)
N-alpha-(3-maleimido)-propanamide polysarcosine
PSR1770 NHS-PSar-OMe (5 kDa)
N-alpha-(succinimidylester)-polysarcosine ome
PSR1780 NHS-PSar-OMe (10 kDa)
N-alpha-(succinimidylester)-polysarcosine ome -
PSR1790 NHS-PSar-OMe (15 kDa)
N-alpha-(succinimidylester)-polysarcosine ome -





PSR1830 VitE-PSar 10
N-alpha-isopropyl polysarcosine(10) N-omega-(Vitamine E)-4-oxobutanoate
weight 1300 Da
PSR1820 VitE-PSar20
N-alpha-isopropyl polysarcosine(20) N-omega-(Vitamine E)-4-oxobutanoate
Mol. weight 2000 Da





See the latest catalogue portfolio also at www.iris-biotech.de/products/drug-delivery/poly-2-oxazoline-s.html
Product details
POX1200 Me-PMeOx(50)-N3
alpha-Methyl-poly(2-methyl-2-oxazoline)-omega-azide
CAS-No. 26375-28-0
Formula CH3 (C 4H7NO)50N3
Mol. weight 4300 Da
POX1210 Me-PMeOx(100)-N3
alpha-Methyl-poly(2-methyl-2-oxazoline)-omega-azide
CAS-No. 26375-28-0
Formula CH3 (C 4H7NO)100N3
Mol. weight 8500 Da
POX2200 Me-PEtOx(50)-N3
alpha-Methyl-poly(2-ethyl-2-oxazoline)-omega-azide
CAS-No. 25805-17-8
Formula CH3 (C 5H9 NO)50N3
Mol. weight 5000 Da
POX2210 Me-PEtOx(100)-N3
alpha-Methyl-poly(2-ethyl-2-oxazoline)-omega-azide
CAS-No. 25805-17-8
Formula CH3 (C 5H9 NO)100N3
Mol. weight 5000 Da
POX1220 Me-PMeOx(50)-NH2*HCl
alpha-Methyl-poly(2-methyl-2-oxazoline)-






omega-amine hydrochloride
CAS-No. 26375-28-0
Formula CH3 (C 4H7NO)50NH2*HCl
Mol. weight 4300 Da
POX1230 Me-PMeOx(100)-NH2*HCl
alpha-Methyl-poly(2-methyl-2-oxazoline)omega-amine hydrochloride
CAS-No. 26375-28-0
Formula CH3 (C 4H7NO)100NH2*HCl
Mol. weight 8500 Da
POX2220 Me-PEtOx(50)-NH2*HCl
alpha-Methyl-poly(2-ethyl-2-oxazoline)-ome
CAS-No. 25805-17-8
Formula CH3 (C 5H9 NO)50NH2*HCl
Mol. weight 5000 Da
POX2230 Me-PEtOx(100)-NH2*HCl
alpha-Methyl-poly(2-ethyl-2-oxazoline)-omega-amine hydrochloride
CAS-No. 25805-17-8
Formula CH3 (C 5H9 NO)100NH2*HCl
Mol. weight 10000 Da
POX1240 Me-PMeOx(50)-COOH
alpha-Methyl-poly(2-methyl-2-oxazoline)-omega-succinamic acid
CAS-No. 26375-28-0
Formula CH3 (C 4H7NO)50COCH2CH2COOH Mol. weight 4300 Da
POX1241 HOOC-PMeOx(50)-Pip
alpha-Carboxymethyl-poly(2-methyl-2-oxazoline)-omega-Piperidine
POX2241 HOOC-PEtOx(50)-Pip
alpha-Carboxymethyl-poly(2-ethyl-2-oxazoline)-ome -





Formula HOOC-CH2 (C 5H9 NO)50NC 6 H12
Mol. weight 5000 Da
POX1250 HOOC-PMeOx(50)-N3
alpha-Carboxymethyl-poly(2-methyl-2-oxazoline)-ome
CAS-No. 26375-28-0
Formula HOCOCH2 (C 4H7NO)50N3
Mol. weight 4300 Da
POX2250 HOOC-PEtOx(50)-N3
alpha-Carboxymethyl-poly(2-ethyl-2-oxazoline)-ome
CAS-No. 25805-17-8
Formula HOCOCH2 (C 5H9 NO)50N3
Mol. weight 5000 Da
See the latest catalogue portfolio also at www.iris-biotech.de/products/drug-delivery/fullerenes.html Product
FLL1000
CAS-No. 99685-96-8
Formula C 60
Mol. weight 720,66 g/mol
FLL1040 Fullerene C70
Buckminsterfullerene
CAS-No. 115383-22-7
Formula C 70
Mol. weight 840,77 g/mol
FLL1030 Fullerenol C60
Polyhydroxylated Fullerene
Formula C 60 (OH)n
Mol. weight 720,66+(17,01) n g/mol
FLL1090 Fullerenol C70
Polyhydroxylated Fullerene
Formula C 70 (OH)n
Mol. weight 840,77+(17,01)n g/mol
FLL1020 Fullerene C60 (PBM)
Fulleren-phenyl-(4-phenylbutyric acid methyl ester)
CAS-No. 160848-22-6
Formula C 72H14O 2
Mol. weight 910,9 g/mol
FLL1010 Fullerene C60 (PBM)2
Fulleren-diphenyl-bis(4-phenylbutyric acid methyl ester)
CAS-No. 1048679-01-1
Formula C 84H28 O4
Mol. weight 1104,14 g/mol
FLL1050 Fullerene C70 (PBM)2
Fulleren-diphenyl-bis(4-phenylbutyric acid methyl ester)






Formula C94H28 O4
Mol. weight 1221,25 g/mol
FLL1060 Fullerene C70 (PBM)
Fulleren-phenyl-(4-phenylbutyric acid methyl ester)
CAS-No. 609771-63-3
Formula C 82H14O 2
Mol. weight 1031,01 g/mol
FLL1070 Fullerene C60 (malonic acid) n
Buckminsterfullerene-n-(malonic acid)
Formula C 60 (C 3 H2O4)n
Mol. weight 720,66+(102,05) n g/mol
FLL1080 Fullerene C70 (malonic acid) n
Buckminsterfullerene-n-(malonic acid)
Formula C 70 (C 3 H2O4)n
Mol. weight 840,77+(102,05) n g/mol
back to content arrow-up


As business activity of Iris Biotech GmbH impacts people’s lives and health, it must be operated in ethical and correct manner and act with integrity and responsibility. To ensure high ethical standards and fair business practices, Iris Biotech GmbH applies an integrated policy known as its Code of Conduct.
In 2001 Iris Biotech GmbH was founded just at the beginning of the Biotech movement and the first remarkable breakthrough of biotech pharma products. Although the biotech field is rather young compared to other industries we believe on long-term business, a good partnership between our business partners and Iris Biotech GmbH and a good reputation. It is our duty as well as our responsibility to maintain and to extend this over the next generations – based on the principles of an honourable and prudent tradesman which based upon the concept of honourable entrepreneurship.
This Code of Conduct has been developed following the “Voluntary Guidelines for Manufacturers of Fine Chemical Intermediates and Active Ingredients” issued by AIME (Agrochemical & Intermediates Manufacturers in Europe) and the requirements of some of our business associates.
Iris Biotech GmbH commits to hold this Code of Conduct and to include and apply its principles in the management system and the company policies.
Iris Biotech GmbH undertakes business in an ethical manner and acts with integrity. All corruption, extortion and embezzlement are prohibited. We do not pay or accept bribes or participate in other illegal inducements in business or government relationships. We conduct our business in compliance with all applicable anti-trust laws. Employees are encouraged to report concerns or illegal activities in the workplace, without threat of reprisal, intimidation or harassment.
Iris Biotech GmbH is committed to uphold the human rights of workers and to treat them with dignity and respect. Child labour, workplace harassment, discrimination, and harsh and inhumane treatment are prohibited. Iris Biotech GmbH respects the rights of the employees to associate freely, join or not join labour unions, seek representation and join workers’ councils. Employees are paid and their working timetable is established according to applicable wage and labour laws. Employees are able to communicate openly with management regarding working conditions without threat of reprisal, intimidation or harassment.
Contracts and Secrecy Agreements are binding and the confidential information received is only used for intended purposes. Clear management and organizational structures exist to provide efficient normal working and to address problems quickly. Know-how is protected and intellectual property isrespected.
Iris Biotech GmbH provides a safe and healthy working environment to the employees and protects them from overexposure to chemical and physical hazards. Products are produced, stored and shipped under the guidelines of the relevant chemical and safety legislation. Risks and emergency scenarios are identified and evaluated, and their possible impact is minimized by implementing emergency plans and written procedures. Safety information regarding hazardous materials is available to educate, train and protect workers from hazards. Preventive equipment and facilities maintenance is performed at suitable periods to reduce potential hazards. Employees are regularly trained in health and safety matters and are informed about product properties and risk classification when it is required.
Iris Biotech GmbH operates in an environmentally responsible and efficient manner, minimizing adverse impacts on the environment. Waste streams are managed to ensure a safe handling, movement, storage, recycling and reuse, before and after being generated. Systems to prevent and mitigate accidental spills and releases to the environment are in place. All required environmental permits and licenses are obtained and their operational and reporting requirements are complied with.
A quality management system following the Good Distribution Practices (GDP rules) of Active Pharmaceutical Ingredients is established covering all the aspects of the worldwide distribution of products. Regular audits are performed to evaluate the efficiency and fulfilling of the quality system. Process controls to provide reproducible product quality are established. There are preventive maintenance procedures to ensure plant reliability and the lowest risk of failure. Staff is trained periodically about GMP and GDP rules. Procedures are established and installations are designed to avoid cross contamination. Batch and analytical records are kept for inspection and audit purposes for suitable periods according guidelines.
Research and development staff education is appropriate to their functional activity and they are trained to develop, optimize and scale-up the processes. Intellectual property is respected and knowhow protected. Development of manufacturing processes reflects the principles of the Green Chemistry according to the American Chemical Society Green Chemistry Institute. Animal testing is not used unless alternatives are not scientifically valid or accepted by regulators. If animal testing is carried out, animals are treated so that pain and stress are minimized.
back to content arrow-up
All orders placed by a buyer are accepted and all contracts are made subject to the terms which shall prevail and be effective notwithstanding any variations or additions contained in any order or other document submitted by the buyer. No modification of these terms shall be binding upon Iris Biotech GmbH unless made in writing by an authorised representative of Iris Biotech GmbH.
Every order made by the buyer shall be deemed an offer by the buyer to purchase products from Iris Biotech GmbH and will not be binding on Iris Biotech GmbH until a duly authorised representative of Iris Biotech GmbH has accepted the offer made by the buyer. Iris Biotech GmbH may accept orders from commercial, educational or government organisations, but not from private individuals and Iris Biotech GmbH reserves the right to insist on a written order and/or references from the buyer before proceeding.
There is no minimum order value. At the time of acceptance of an order Iris Biotech GmbH will either arrange prompt despatch from stock or the manufacture/acquisition of material to satisfy the order. In the event of the latter Iris Biotech GmbH will indicate an estimated delivery date. In addition to all its other rights Iris Biotech GmbH reserves the right to refuse the subsequent cancellation of the order if Iris Biotech GmbH expects to deliver theproduct on or prior to the estimated delivery date. Time shall not be of the essence in respect of delivery of the products. If Iris Biotech GmbH is unable to deliver any products by reason of any circumstances beyond its reasonable control („Force Majeure“) then the period for delivery shall be extended by the time lost due to such Force Majeure. Details of Force Majeure will be forwarded by Iris Biotech GmbH to the buyer as soon as reasonably practicable.
Prices are subject to change. For the avoidance of doubt, the price advised by Iris Biotech GmbH at the time of the buyer placing the order shall supersede any previous price indications. The buyer must contact the local office of Iris Biotech GmbH before ordering if further information is required. Unless otherwise agreed by the buyer and Iris Biotech GmbH, the price shall be for delivery ex-works. In the event that the buyer requires delivery of the products otherwise than ex-works the buyer should contact the local office of Iris Biotech GmbH in order to detail its requirements. Iris Biotech GmbH shall, at its discretion, arrange the buyer‘s delivery requirements including, without limitation, transit insurance, the mode of transit (Iris Biotech GmbH reserves the right to vary the mode of transit if any regulations or other relevant considerations so require) and any special packaging requirements (including cylinders). For the avoidance of doubt all costs of delivery and packaging in accordance with the buyer‘s requests over and above that of delivery in standard packaging ex-works shall be for the buyer‘s account unless otherwise agreed by both parties. Incoterms 2020 shall apply. Any tax, duty or charge imposed by governmental authority or otherwise and any other applicable taxes, duties or charges shall be for the buyer‘s account. Iris Biotech GmbH may, on request and where possible, provide quotations for multiple packs or bulk quantities, and non-listed items. Irrespective of the type of request or means of response all quotations must be accepted by the buyer without condition and in writing before an order will be accepted by Iris Biotech GmbH. Unless agreed in writing on different terms, quotations are valid for 30 days from the date thereof. Payment terms are net 30 days from invoice date unless otherwise agreed in writing. Iris Biotech GmbH reserves the right to request advance payment at its discretion. For overseas transactions the buyer shall pay all the banking charges of Iris Biotech GmbH. The buyer shall not be entitled to withhold or set-off payment for the products for any reason whatsoever. Government/
Corporate Visa and MasterCard (and other such credit cards) may be accepted on approved accounts for payment of the products. Personal credit cards are not acceptable. Failure to comply with the terms of payment of Iris Biotech GmbH shall constitute default without reminder. In these circumstances Iris Biotech GmbH may (without prejudice to any other of its rights under these terms) charge interest to accrue on a daily basis at the rate of 2% per month from the date upon which payment falls due to the actual date of payment (such interest shall be paid monthly). If the buyer shall fail to fulfil the payment terms in respect of any invoice of Iris Biotech GmbH Iris Biotech GmbH may demand payment of all outstanding balances from the buyer whether due or not and/or cancel all outstanding orders and/or decline to make further deliveries or provision of services except upon receipt of cash or satisfactory securities. Until payment by the buyer in full of the price and any other monies due to Iris Biotech GmbH in respect of all other products or services supplied or agreed to be supplied by Iris Biotech GmbH to the buyer (including but without limitation any costs of delivery) the property in the products shall remain vested in Iris Biotech GmbH.
The buyer shall inspect goods immediately on receipt and inform Iris Biotech GmbH of any shortage or damage within five days. Quality problems must be notified within ten days of receipt. Goods must not be returned without prior written authorisation of Iris Biotech GmbH. Iris Biotech GmbH shall at its sole discretion replace the defective products (or parts thereof) free of charge or refund the price (or proportionate price) to buyer. Opened or damaged containers cannot be returned by the buyer without the written prior agreement of Iris Biotech GmbH. In the case of agreed damaged containers which cannot be so returned, the buyer assumes responsibility for the safe disposal of such containers in accordance with all applicable laws.
Products are analysed in the Quality Control laboratories of Iris Biotech GmbH’s production partners by methods and procedures which Iris Biotech GmbH considers appropriate. In the event of any dispute concerning reported discrepancies arising from the buyer’s analytical results, determined by the buyer’s own analytical procedures, Iris Biotech GmbH reserves the right to rely on the results of own analytical methods of Iris Biotech GmbH. Certificates of Analysis or Certificates of Conformity are available at the discretion of Iris Biotech GmbH for bulk orders but not normally for prepack orders. Iris Biotech GmbH reserves the right to make a charge for such certification. Specifications may change and reasonable variation from any value listed should not form the basis of a dispute. Any supply by Iris Biotech GmbH of bespoke or custom product for a buyer shall be to a specification agreed by both parties in writing. Technical information, provided orally, in writing, or by electronic means by or on behalf of Iris Biotech GmbH, including any descriptions, references, illustrations or diagrams in any catalogue or brochure, is provided for guidance purposes only and is subject to change.
All chemicals should be handled only by competent, suitably trained persons, familiar with laboratory procedures and potential chemical hazards. The burden of safe use of the products of Iris Biotech GmbH vests in the buyer. The buyer assumes all responsibility for warning his employees, and any persons who might reasonably be expected to come into contact with the products, of all risks to person and property in any way connected with the products and for instructing them in their safe handling and use. The buyer also assumes the responsibility for the safe disposal of all products in accordance with all applicable laws.
back to content arrow-up
All products of Iris Biotech GmbH are intended for laboratory research purposes and unless otherwise stated on product labels, in the catalogue and product information sheet of Iris Biotech GmbH or in other literature furnished to the buyer, are not to be used for any other purposes, including but not limited to use as or as components in drugs for human or animal use, medical devices, cosmetics, food additives, household chemicals, agricultural or horticultural products or pesticides. Iris Biotech GmbH offers no warranty regarding the fitness of any product for a particular purpose and shall not be responsible for any loss or damage whatsoever arising there from. No warranty or representation is given by Iris Biotech GmbH that the products do not infringe any letters patent, trademarks, registered designs or other industrial rights. The buyer further warrants to Iris Biotech GmbH that any use of the products in the United States of America shall not result in the products becoming adulterated or misbranded within the meaning of the Federal Food, Drug and Cosmetic Act (or such equivalent legislation in force in the buyer‘s jurisdiction) and shall not be materials which may not, under sections 404, 505 or 512 of the Act, be introduced into interstate commerce. The buyer acknowledges that, since the products of Iris Biotech GmbH are intended for research purposes, they may not be on the Toxic Substances Control Act 1976 („TSCA“) inventory. The buyer warrants that it shall ensure that the products are approved for use under the TSCA (or such other equivalent legislation in force in the buyer‘s jurisdiction), if applicable. The buyer shall be responsible for complying with any legislation or regulations governing the use of the products and their importation into the country of destination (for the avoidance of doubt to include, without limitation, the TSCA and all its amendments, all EINECS, ELINCS and NONS regulations). If any licence or consent of any government or other authority shall be required for the acquisition, carriage or use of the products by the buyer the buyer shall obtain the same at its own expense and if necessary produce evidence of the same to Iris Biotech GmbH on demand. Failure to do so shall not entitle the buyer to withhold or delay payment. Any additional expenses or charges incurred by Iris Biotech GmbH resulting from such failure shall be for the buyer‘s account. Save for death or personal injury caused by negligence of Iris Biotech GmbH, sole obligation of Iris Biotech GmbH and buyer‘s exclusive remedy with respect to the products proved to the satisfaction of Iris Biotech GmbH to be defective or products incorrectly supplied shall be to accept the return of said products to Iris Biotech GmbH for refund of the actual purchase price paid by the buyer (or proportionate part thereof), or replacement of the defective product (or part thereof) with alternative product. Iris Biotech GmbH shall have no liability to the buyer under or arising directly or indirectly out of or otherwise in connection with the supply of products by Iris Biotech GmbH to the buyer and/or their re-sale or use by the buyer or for any product, process or services of the buyer which in any way comprises the product in contract tort (including negligence or breach of statutory duty) or otherwise for pure economic loss, loss of profit, business, reputation, depletion of brand, contracts, revenues or anticipated savings or for any special indirect or consequential damage or loss of any nature except as may otherwise be expressly provided for in these terms. All implied warranties, terms and representations in respect of the products (whether implied by statute or otherwise) are excluded to the fullest extent permitted by law. The buyer shall indemnify Iris Biotech GmbH for and against any and all losses, damages and expenses, including legal fees and other costs of defending any action, that Iris Biotech GmbH may sustain or incur as a result of any act or omission by the buyer, its officers, agents or employees, its successors or assignees, its customers or all other third parties, whether direct or indirect, in connection with the use of any product. For the avoidance of doubt and in the event that Iris Biotech GmbH supplies bespoke or custom product to the buyer‘s design or specification, this indemnity shall extend to include any claim by a third party that the manufacture of the product for the buyer or the use of the product by the buyer infringes the intellectual property rights of any third party.
Iris Biotech GmbH shall be entitled to assign or sub-contract all or any of its rights and obligations hereunder. The buyer shall not be entitled to assign, transfer, sub-contract or otherwise delegate any of its rights or obligations hereunder. Any delay or forbearance by Iris Biotech GmbH in exercising any right or remedy under these terms shall not constitute a waiver of such right or remedy. If any provision of these terms is held by any competent authority to be invalid or unenforceable in whole or in part the validity of the other provisions of these terms and the remainder of the provision in question shall not be affected. These terms shall be governed by German Law and the German Courts shall have exclusive jurisdiction for the hearing of any dispute between the parties save in relation to enforcement where the jurisdiction of the German Courts shall be non-exclusive.

Iris Biotech GmbH
Adalbert-Zoellner-Str. 1 95615 Marktredwitz Germany
+49 (0) 9231 97121-0
+49 (0) 9231 97121-99 info@iris-biotech.de www.iris-biotech.de
The list contains the current distributors of Iris Biotech in different regions of the world. The latest list of distribution partners and contact details is available at: www.iris-biotech.de/distribution-partner
China:
Chengdu Yoo Technology Co., Ltd.
Japan:
BizCom Japan, Inc.
Shigematsu & Co., Ltd
Cosmo Bio Co., Ltd.
USA & Canada: Peptide Solutions LLC
India, Bangladesh, Oman, Sri Lanka, United Arab Emirates: Sumit Biosciences Pvt Ltd.
Singapore, Thailand, Malaysia, Indonesia, Vietnam, Philippines, Brunei, Burma (Myanmar), Laos, Cambodia, Timor-Leste:
SciClix Pte. Ltd.