TheResearchonSemaglutide.
SemaglutideisamedicationthatwasoriginallydevelopedtotreatdiabetesHowever,ithas gainedpopularityasaweightlossdrugafteritwasfoundtoeffectivelyhelpindividualslose weight,includingtheworld'ssecondrichestman,ElonMusk.Recently,the study----Once-WeeklySemaglutideinAdolescentswithObesity----publishedintheNew EnglandJournalofMedicine(NEJM)hasfoundthatsemaglutidemayalsohelpobese adolescentsreduceweightandpromotehearthealth.
②KeyFindings
AASrawBiochemicalTechnologyCo.,ltd www.aasraw.com king@aasraw.com
①Introduction
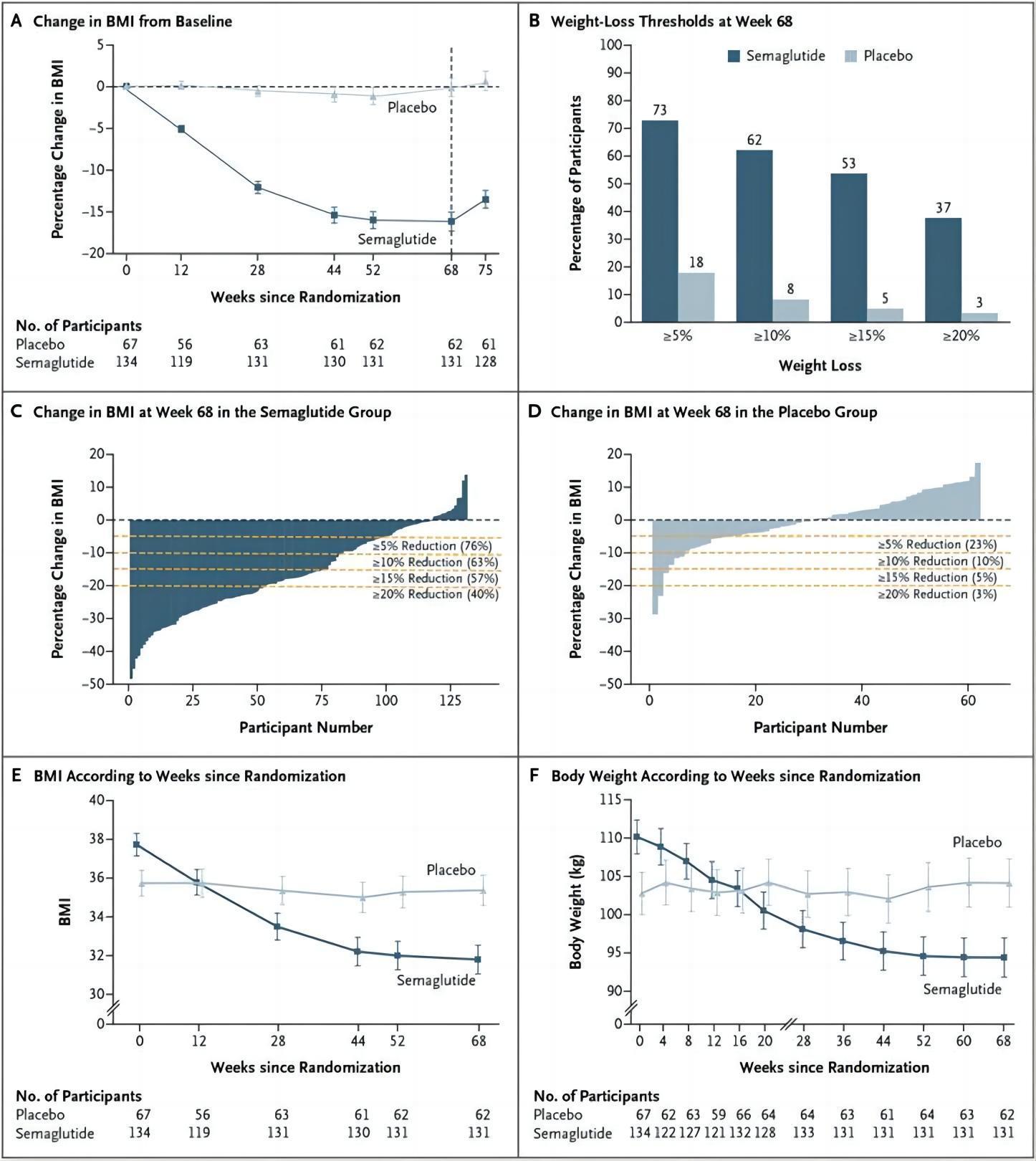
Obeseadolescentswhotookweeklysemaglutidesawa16.1%increaseinbodymassindex (BMI)inaninternationalphase3aclinicaltrial,comparedtoa0.6%increaseintheplacebo group.
Semaglutideisaglucagon-likepeptide1(GLP-1)receptoragonistthatcanreduceappetite, diet,andcalorieintake,makingiteffectiveforweightloss.
Alarge-scaleclinicaltrialshowedthatsemaglutidehasanamazingeffectasaweightloss medicine,withthetreatedgrouplosinganaverageof15.3kg.
InJune2021,theU.S.FoodandDrugAdministration(FDA)approvedthemarketingof semaglutide(Semaglutide),aweight-lossdrugwithatradenameofWegovy.
Arecentstudyhasfoundthatsemaglutideoutperformedplacebointermsofweightloss andimprovementofcardiometabolicriskfactorsinobeseadolescents
Aftertakingsemaglutide,cardiovascularriskfactorssuchaswaistcircumference,blood glucoseindexHbA1c,totalcholesterol,low-densityandverylow-densitylipoprotein cholesterol,triglycerides,andtriglyceridesimproved.
Thesemaglutidegroupoutperformedtheplacebogrouponweight-relatedqualityoflife measures,owingmostlytohigherphysicalcomfortscores.
③Conclusion
Semaglutidehasbeenshowntobeaneffectiveweightlossmedication,andrecentresearch hasdemonstratedthatitmayalsobebeneficialforobeseadolescents.Whilethe medicationisassociatedwithsomegastrointestinalsideeffects,ithasbeenfoundto improvecardiovascularriskfactorsandqualityoflifemeasures.
king@aasraw.com

AASrawBiochemicalTechnologyCo.,ltd www.aasraw.com
SourceOrigin:https://wwwncbinlmnihgov/pmc/articles/PMC9997064/

AASrawBiochemicalTechnologyCo.,ltd www.aasraw.com king@aasraw.com
BenefitsofUsingSemaglutidePowder
Semaglutidepowderisaversatilemedicationthatoffersarangeofbenefitsforindividuals withvariousmedicalconditions.Someofthemostcommonconditionsthatcanbenefit fromtheuseofAASrawSemaglutidepowderincludingType2DiabetesMellitus, caidiovascularrisks,obesityandAlzheimer.

①LoweringBloodGlucoseLevels
SemaglutidepowderisaGLP-1receptoragonistthatiseffectiveinloweringbloodglucose levelsinpeoplewithtype2diabetesbyseveralmechanisms.Itstimulatesinsulinsecretion, whichhelpstomoveglucosefromthebloodintothecellswhereitcanbeusedforenergy.
Semaglutidealsosuppressesglucagonsecretion,whichreducesglucoseproductionbythe liver.Additionally,itslowsdowngastricemptying,whichreducestherateatwhichglucose entersthebloodstreamafterameal.
Inclinicaltrials,semaglutidehasbeenshowntosignificantlyreduceHbA1clevels,whichis anindicatoroflong-termbloodglucosecontrolIntheSUSTAIN-1trial,semaglutidereduced HbA1clevelsby15%comparedtoplacebo,andintheSUSTAIN-10trial,itreducedHbA1c levelsby1.8%comparedtoplacebo.Semaglutidehasalsobeenshowntobeeffectivein reducingfastingplasmaglucoselevelsandpostprandialglucoseexcursions.
②ReducingRiskofCardiovascularEvents
AASrawBiochemicalTechnologyCo.,ltd www.aasraw.com king@aasraw.com
Semaglutidepowderhasbeenshowntoreducetheriskofmajoradversecardiovascular events(MACE)suchascardiovasculardeath,nonfatalmyocardialinfarction,andnonfatal strokeinadultswithtype2diabetesandestablishedcardiovasculardisease.Thisbenefit wasobservedintheSUSTAIN-6andPIONEER-6trials,whichshowedasignificantreduction inMACEwithsemaglutidetreatmentcomparedtoplacebo.Moreover,Semaglutidepowder hasbeenshowntoimproveseveralcardiovascularriskfactorssuchasbloodpressure,lipid profile,andmarkersofinflammationinpeoplewithtype2diabetes.IntheSUSTAIN-6trial, semaglutidewasassociatedwithasignificantreductioninsystolicbloodpressureand improvementinlipidprofile,includingreductionsintotalcholesterol,LDLcholesterol,and triglycerides.
③LosingWeight
Semaglutidepowderisapotentweightlossagent,eveninpeoplewithoutdiabetes. Semaglutideworksbyreducingappetiteandcalorieintake,leadingtoadecreaseinbody weight.Thisisachievedthroughitseffectsonthecentralnervoussystemandthe gastrointestinaltract.Semaglutideactsonthehypothalamus,whichregulateshungerand satiety,andreducesthedesiretoeatbyincreasingfeelingsoffullness.Additionally, semaglutideslowsdowngastricemptying,whichprolongsthefeelingoffullnessaftera mealandreducestheurgetoeat.
Clinicaltrialshavedemonstratedtheweightlossbenefitsofsemaglutide.IntheSTEP program,whichevaluatedtheuseofsemaglutideforweightmanagementinpeople

AASrawBiochemicalTechnologyCo.,ltd www.aasraw.com
king@aasraw.com
withoutdiabetes,semaglutidewasassociatedwithsignificantweightlosscomparedto placebo.Participantswhoreceivedonce-weeklysemaglutidelostanaverageof15%oftheir bodyweightover68weeks,whilethosewhoreceivedplacebolostonly2.4%.
Inpeoplewithtype2diabetes,semaglutidepowdercanleadtoadditionalweightloss benefits.IntheSUSTAIN7trial,whichevaluatedtheefficacyandsafetyofsemaglutidein peoplewithtype2diabetes,semaglutidewasassociatedwithasignificantreductionin bodyweightcomparedtoplacebo.Participantswhoreceivedsemaglutidelostanaverageof 4.6kg,whilethosewhoreceivedplacebolostonly1.2kg.
④TreatingAlzheimer’sDiseaseSymptom
Multiplepreclinicalstudieshaveshownthatsemaglutidepowderhasneuroprotective propertiesagainstamyloid-βplaquesinahumanneuroblastoma(SH-SY5Y)cellline, suggestingthatsemaglutidecanalleviateAlzheimer'sdiseasesymptom.Animalmodels havealsoshownthatsemaglutidehasneuroprotectiveeffectsonanimalmodels.According toNovoNordisk’sannouncement,theindicationofsemaglutidetabletsforAlzheimer’s disease(AD)isintheclinicalstage.TwoglobalPhaseIIItrials,EVOKEandEVOKEplus,are underway,andabout3,700volunteersareexpectedtoberecruited.Inthisstudy, comparedwithplacebo,evaluatethesuperiorityofsemaglutidetabletsoncognitive functioninsubjectswithmildcognitiveimpairment(MCI)ormilddementiacausedbyAD.
Precautions:Itiskeytopurchasingsemaglutidepowderfromreputablesources,otherwise,
king@aasraw.com

AASrawBiochemicalTechnologyCo.,ltd www.aasraw.com
www.aasraw.com

youcannotgetthebestefficacyofsemaglutidepowder.Asaprofessionalsemaglutide powdermanufacturerandsupplier,AASrawaimtosupplypuresemaglutidepowder worldwide.Ifyouhaveneeds,AASraw’ssemaglutidepowderisagreatchoiceforyou.
TheSideEffectsofSemaglutidePowder?
Semaglutidepowder,likeanymedication,maycausesideeffects
①Commonsideeffectsareinclude:
Nausea
Vomiting
Diarrhea
Abdominalpain
Lossofappetite
Constipation
Headache
Fatigue
Dizziness
②Lesscommonbutmoreserioussideeffectsmayinclude:
Pancreatitis
Hypoglycemia(lowbloodsugar)
king@aasraw.com
AASrawBiochemicalTechnologyCo.,ltd
Acutekidneyinjury
Diabeticretinopathycomplications
Gallbladderdisease
Allergicreactions
Thyroidtumors
Notes:Thedurationofsideeffectsofsemaglutidepowdercanvarydependingonthe individualandtheseverityofthesideeffects.Inmostcases,thesideeffectsofsemaglutide aretemporaryandwillimproveasyourbodyadjuststothemedication.Commonside effectssuchasnausea,diarrhea,vomiting,constipation,andheadachetypicallyresolve withinafewdaystoaweekIfyouexperienceanyunusualorseveresideeffectswhile takingsemaglutide,gotothedoctorpromptlyAdditionally,buyingsemaglutidepowder withhighqualityfromreliablesupplier,likeAASraw,iscrucial.
DosageandAdministrationofSemaglutidePowderforReference
Thedosageandadministrationofsemaglutidecanvarydependingontheindicationforuse.
Herearesomegeneralguidelines:
①Indication:Type2DiabetesMellitus
SubcutaneousInjection
king@aasraw.com

AASrawBiochemicalTechnologyCo.,ltd www.aasraw.com
Week1-4:0.25mg/week
Week5andonward:0.5mg/week

Ifneeded,afteratleast4weeksonthe0.5-mgdose,increaseto1mgsubcutaneouslyonce weekly.
Ifneeded,afteratleast4weeksonthe1-mgdose,increaseto2mgsubcutaneouslyonce weekly;donotexceed2mg/week.
OralTablet
Day1-30:3mg/day
Day31andonward:7mg/day
Ifneeded,afteratleast30daysonthe7-mgdose,increaseto14mgorallyoncedaily.
Note:Donottaketwo7-mgtabletstoachievea14-mgdose
②Indication:ChronicWeightManagement
SubcutaneousInjection
AASrawBiochemicalTechnologyCo.,ltd www.aasraw.com
king@aasraw.com
Week1-4:0.25mg/week
Week5-8:0.5mg/week
Week9-12:1mg/week
Week13-16:1.7mg/week
Week17andonward:2.4mg/week(maintenancedose)
Initiatewithlowdoseandgraduallyescalatetomaintenancedosetominimize gastrointestinaladversereactions. Ifunabletotolerateadoseduringescalation,considerdelayingdoseescalationfor4weeks.

Ifunabletotoleratethemaintenancedoseof2.4mgonceweekly,maytemporarily decreaseto17mgonceweeklyforamaximumof4weeks;after4weeks,increasebackto maintenance24mgonceweekly;discontinueifnottoleratedafterthesecondattempt
Note:Thedosageandadministrationofsemaglutidepowdermayvarydependingonthe indicationforuse.Followtheinstructionsprovidedbyyourdoctororasindicatedonthe medicationlabel.Moreover,tryyourbesttobuypuresemaglutidepowdertoachievethe bestefficacy.
WheretobuySemaglutidePowder?
Semaglutidepowder,ahighlysought-aftermedicationforthetreatmentoftype2diabetes, hasbecomeincreasinglypopularamongpatientsseekingeffectivesolutionstomanage theircondition.Asaresult,onlinemarketplaceshaveemergedasaconvenientplatformfor
AASrawBiochemicalTechnologyCo.,ltd www.aasraw.com
king@aasraw.com
peopletobuysemaglutidepowderonline.Thesedigitalplatformsprovidecustomerswith theopportunitytocompareprices,readreviews,andaccessawiderangeofoptionstosuit theirneeds.However,itisessentialforcustomerstoexercisecautionwhenpurchasing semaglutidepowderonline,ascounterfeitorlow-qualityproductscouldpotentially compromisetheirhealth.Byconductingthoroughresearchandselectingareputableand certifiedseller,individualscanconfidentlypurchasesemaglutidepowderonlineand effectivelymanagetheirdiabeteswithease.
AASrawaimingtomanufacturingabdsupplyingchemicalintermediatesandactive pharmaceuticalingredients(APIs),isatrustedproviderofhigh-qualitysemaglutidepowder andotherrelatedproductsTheircommitmenttorigorousqualitycontrolandadherenceto industrystandardsensuresthatcustomersreceiveonlythebestproductsfortheirhealth andwell-being.ByshoppingatAASraw,individualsseekingsemaglutidepowdercanenjoya convenient,secure,andreliablepurchasingexperience,allwhilebenefitingfromtheir extensiveknowledgeandexpertiseinthefield.
Reference:
WeightLossOutcomesAssociatedWithSemaglutideTreatmentforPatientsWith OverweightorObesity.JAMANetwOpen.2022Sep1;5(9):e2231982.
king@aasraw.com

AASrawBiochemicalTechnologyCo.,ltd
www.aasraw.com
[1]GhusnW,DelaRosaA,SacotoD,CifuentesL,CamposA,FerisF,HurtadoMD,AcostaA.
[2]WildingJPH,BatterhamRL,DaviesM,VanGaalLF,KandlerK,KonakliK,LingvayI, McGowanBM,OralTK,RosenstockJ,WaddenTA,WhartonS,YokoteK,KushnerRF;STEP1 StudyGroup.Weightregainandcardiometaboliceffectsafterwithdrawalof semaglutide:TheSTEP1trialextension.DiabetesObesMetab.2022Aug;24(8):1553-1564.
[3]GarveyWT,BatterhamRL,BhattaM,BuscemiS,ChristensenLN,FriasJP,JódarE,Kandler K,RigasG,WaddenTA,WhartonS;STEP5StudyGroup.Two-yeareffectsofsemaglutidein adultswithoverweightorobesity:theSTEP5trial.NatMed.2022Oct;28(10):2083-2091.
[4]KnudsenLB,LauJTheDiscoveryandDevelopmentofLiraglutideandSemaglutideFront Endocrinol(Lausanne)2019Apr12;10:155
[5]MahapatraMK,KaruppasamyM,SahooBM.TherapeuticPotentialofSemaglutide,a NewerGLP-1ReceptorAgonist,inAbatingObesity,Non-AlcoholicSteatohepatitisand Neurodegenerativediseases:ANarrativeReview.PharmRes.2022Jun;39(6):1233-1248.
king@aasraw.com

AASrawBiochemicalTechnologyCo.,ltd www.aasraw.com




