5 Latest Innovations of Significance Highlighted in Today’s Emerging Science Session 24 It’s a Snap to be Included in Inclusive Interactive Photo Wall 9 It’s National Institutes of Health Day! Monday, April 24, 2023
DAYBUE ™ is now FDA approved to treat Rett syndrome 1
DAYBUE is the first and only FDA-approved treatment for Rett syndrome (RTT) in adult and pediatric patients 2 years and older.1,2 RTT is a complex neurodevelopmental disorder with symptoms that can impact the ability to function.3-5
1 pm to 1:20 pm in-booth presentation
1 pm to 1:20 pm
Emerging Neurologic Care Presentation Stage (in the Exhibit Hall)
1 pm to 1:20 pm in-booth presentation
Indication
Come see us at Booth #1384 to learn more about Rett syndrome and DAYBUE
DAYBUE is indicated for the treatment of Rett syndrome in adults and pediatric patients 2 years of age and older.
Important Safety Information
• Warnings and Precautions
– Diarrhea: In a 12-week study and in long-term studies, 85% of patients treated with DAYBUE experienced diarrhea. In those treated with DAYBUE, 49% either had persistent diarrhea or recurrence after resolution despite dose interruptions, reductions, or concomitant antidiarrheal therapy. Diarrhea severity was of mild or moderate severity in 96% of cases. In the 12-week study, antidiarrheal medication was used in 51% of patients treated with DAYBUE.
– Patients should stop taking laxatives before starting DAYBUE. If diarrhea occurs, patients should notify their healthcare provider, consider starting antidiarrheal treatment, and monitor hydration status and increase oral fluids, if needed. Interrupt, reduce dose, or discontinue DAYBUE if severe diarrhea occurs or if dehydration is suspected.
– Weight Loss: In the 12-week study, 12% of patients treated with DAYBUE experienced weight loss of greater than 7% from baseline, compared to 4% of patients who received placebo. In long-term studies, 2.2% of patients discontinued treatment with DAYBUE due to weight loss. Monitor weight and interrupt, reduce dose, or discontinue DAYBUE if significant weight loss occurs.
• Adverse Reactions: The common adverse reactions (≥5% for DAYBUE-treated patients and at least 2% greater than in placebo) reported in the 12-week study were diarrhea (82% vs 20%), vomiting (29% vs 12%), fever (9% vs 4%), seizure (9% vs 6%), anxiety (8% vs 1%), decreased appetite (8% vs 2%), fatigue (8% vs 2%), and nasopharyngitis (5% vs 1%).
• Drug Interactions: Effect of DAYBUE on other Drugs
– DAYBUE is a weak CYP3A4 inhibitor; therefore, plasma concentrations of CYP3A4 substrates may be increased if given concomitantly with DAYBUE. Closely monitor when DAYBUE is used in combination with orally administered CYP3A4 sensitive substrates for which a small change in substrate plasma concentration may lead to serious toxicities.
– Plasma concentrations of OATP1B1 and OATP1B3 substrates may be increased if given concomitantly with DAYBUE. Avoid the concomitant use of DAYBUE with OATP1B1 and OATP1B3 substrates for which a small change in substrate plasma concentration may lead to serious toxicities.
• Use in Specific Population: Renal Impairment
– DAYBUE is not recommended for patients with moderate or severe renal impairment.
DAYBUE is available as an oral solution (200mg/mL).
Read the Brief Summary of full Prescribing Information on the following page.
1. Acadia Pharmaceuticals Inc. DAYBUE [Package Insert]. San Diego, CA, 2023. 2. Acadia Pharmaceuticals announces U.S. FDA approval of DAYBUE™ (trofinetide) for the treatment of Rett syndrome in adult and pediatric patients two years of age and older. [press release]. Acadia Pharmaceuticals Inc. March 10, 2023.
References:
3. Fu C, Armstrong D, Marsh E, et al. Consensus guidelines on managing Rett syndrome across the lifespan. BMJ Paediatr Open. 2020;4(1):e000717. 4. Neul JL, Kaufmann WE, Glaze DG, et al for the RettSearch Consortium. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68(6):944-950. 5. Anderson A, Wong K, Jacoby P, et al. Twenty years of surveillance in Rett syndrome: what does this tell us? Orphanet J Rare Dis. 2014;9:87. 6. International Rett Syndrome Foundation. IRSF expands center of excellence network. Accessed March 14, 2023. https://www.rettsyndrome.org
®
©2023 Acadia Pharmaceuticals Inc. Acadia is a registered trademark and DAYBUE is a trademark of Acadia Pharmaceuticals Inc. All rights reserved. DAY-0082 03/23
DAYBUE presentations with Caroline (Carrie)
of
a Rett syndrome Center of Excellence 6 4/23 4/24 4/25
Attend
Buchanan, MD,
the Greenwood Genetic Center,
DAYBUE™ (trofinetide) oral solution
Rx Only
Brief Summary: This information is not comprehensive. Visit www.DAYBUEhcp.com to obtain the full Prescribing Information or call 1-844-422-2342
1 INDICATIONS AND USAGE
DAYBUE is indicated for the treatment of Rett syndrome in adults and pediatric patients 2 years of age and older.
2 DOSAGE AND ADMINISTRATION
Administer DAYBUE orally twice daily, in the morning and evening, according to patient weight as shown in the table below. DAYBUE can be taken with or without food.
Recommended Dosage of DAYBUE in Patients
2 years of age and older
Patient Weight DAYBUE Dosage DAYBUE Volume
9 kg to <12 kg 5,000 mg twice daily 25 mL twice daily
≥12 kg to <20 kg 6,000 mg twice daily 30 mL twice daily
≥20 kg to <35 kg
8,000 mg twice daily 40 mL twice daily
≥35 kg to <50 kg 10,000 mg twice daily 50 mL twice daily
≥50 kg 12,000 mg twice daily 60 mL twice daily
Administration Information
Administer DAYBUE orally or via gastrostomy (G) tube; doses administered via gastrojejunal (GJ) tubes must be administered through the G-port. A calibrated measuring device, such as an oral syringe or oral dosing cup, should be obtained from the pharmacy to measure and deliver the prescribed dose accurately. A household measuring cup is not an adequate measuring device. Discard any unused DAYBUE oral solution after 14 days of first opening the bottle.
Missed Dose or Vomiting After Administration
If a dose of DAYBUE is missed, the next dose should be taken as scheduled. Doses should not be doubled.
If vomiting occurs after DAYBUE administration, an additional dose should not be taken. Instead, continue with the next scheduled dose.
Dose Modification for Diarrhea or Weight Loss
Advise patients to stop laxatives before starting DAYBUE. Interrupt, reduce the dosage, or discontinue DAYBUE if severe diarrhea occurs, if dehydration is suspected, or if significant weight loss occurs.
5 WARNINGS AND PRECAUTIONS
Diarrhea
In a 12-week randomized, double-blind, placebocontrolled study (Study 1) and in long-term studies, 85% of patients treated with DAYBUE experienced diarrhea. In those treated with DAYBUE, 49% either had persistent diarrhea or recurrence after resolution despite dose interruptions, reductions, or concomitant antidiarrheal therapy. Diarrhea severity was of mild or moderate severity in 96% of cases. In Study 1, antidiarrheal medication was used in 51% of patients treated with DAYBUE.
Advise patients to stop laxatives before starting DAYBUE. If diarrhea occurs, patients should notify their healthcare provider, consider starting antidiarrheal treatment, and monitor hydration status and increase oral fluids, if needed. Interrupt, reduce dose, or discontinue DAYBUE if severe diarrhea occurs or if dehydration is suspected.
Weight Loss
In Study 1, 12% of patients treated with DAYBUE experienced weight loss of greater than 7% from baseline, compared to 4% of patients who received placebo. In long-term studies, 2.2% of patients discontinued treatment with DAYBUE due to weight loss. Monitor weight and interrupt, reduce dose, or discontinue DAYBUE if significant weight loss occurs.
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
• Diarrhea [see Warnings and Precautions]
• Weight Loss [see Warnings and Precautions]
Clinical trial experience
In controlled and uncontrolled trials in patients with Rett syndrome, 260 patients ages 2 to 40 years were treated with DAYBUE, including 109 patients treated for more than 6 months, 69 patients treated for more than 1 year, and 4 patients treated for more than 2 years.
Adult and Pediatric Patients with Rett Syndrome 5 Years of Age and Older
The safety of DAYBUE was evaluated in a randomized, double-blind, placebo-controlled, 12-week study of patients with Rett syndrome (Study 1). In Study 1, 93 patients received DAYBUE, and 94 patients received placebo. All patients were female, 92% were White, and the mean age was 11 years (range 5 to 20 years).
Adverse Reactions Leading to Discontinuation of Treatment
Eighteen patients (19%) receiving DAYBUE had adverse reactions that led to withdrawal from the study. The most common adverse reaction leading to discontinuation of treatment with DAYBUE was diarrhea (15%).
Common Adverse Reactions
Adverse reactions that occurred in Study 1 in at least 5% of patients treated with DAYBUE and were at least 2% more frequent than in patients on placebo are presented in the table below.
Adverse Reactions in at Least 5% of Patients
Treated With DAYBUE and at Least 2% Greater than Placebo in Study 1
mother’s clinical need for DAYBUE and any potential adverse effects on the breastfed infant from DAYBUE or from the underlying maternal condition.
Pediatric Use
The safety and effectiveness of DAYBUE for the treatment of Rett syndrome have been established in pediatric patients aged 2 years and older. The safety and effectiveness of DAYBUE for the treatment of Rett syndrome in pediatric patients 5 years of age and older was established in Study 1, which included 108 pediatric patients age 5 to less than 12 years of age and 47 pediatric patients age 12 to less than 17 years of age. Use of DAYBUE in patients 2 to 4 years of age is supported by evidence from Study 1 and pharmacokinetic and safety data in 13 pediatric patients 2 to 4 years of age treated with DAYBUE for 12 weeks.
Safety and effectiveness in pediatric patients less than 2 years of age have not been established.
Geriatric Use
Clinical studies of DAYBUE did not include patients 65 years of age and older to determine whether or not they respond differently from younger patients. This drug is known to be substantially excreted by the kidney. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function.
Renal Impairment
No dedicated clinical study has been conducted to evaluate the pharmacokinetics of DAYBUE in subjects with renal impairment. Since the drug is eliminated mainly through the kidney, administration of DAYBUE to patients with moderate or severe renal impairment is not recommended.
16 Storage and Handling
Store DAYBUE in an upright position refrigerated at 2°C to 8°C (36°F to 46°F). Do not freeze. Keep the child-resistant cap tightly closed. Discard any unused DAYBUE oral solution after 14 days of first opening the bottle.
17 PATIENT COUNSELING INFORMATION
Advise the caregiver or patient to read the FDAapproved patient labeling (Patient Information).
Pediatric Patients With Rett Syndrome 2 to 4 Years of Age
In an open-label study in pediatric patients 2 to 4 years of age with Rett syndrome, a total of 13 patients received DAYBUE for at least 12 weeks and 9 patients received DAYBUE for at least 6 months. Adverse reactions in pediatric patients 2 to 4 years of age treated with DAYBUE were similar to those reported in adult and pediatric patients 5 years of age and older with Rett syndrome in Study 1.
7 DRUG INTERACTIONS
Effect of DAYBUE on Other Drugs
Trofinetide is a weak CYP3A4 inhibitor; therefore, plasma concentrations of CYP3A4 substrates may be increased if given concomitantly with DAYBUE. Closely monitor when DAYBUE is used in combination with orally administered CYP3A4 sensitive substrates for which a small change in substrate plasma concentration may lead to serious toxicities.
Plasma concentrations of OATP1B1 and OATP1B3 substrates may be increased if given concomitantly with DAYBUE. Avoid the concomitant use of DAYBUE with OATP1B1 and OATP1B3 substrates for which a small change in substrate plasma concentration may lead to serious toxicities.
8 USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
There are no adequate data on the developmental risks associated with the use of DAYBUE in pregnant women. No adverse developmental effects were observed following oral administration of trofinetide to pregnant animals at doses associated with plasma exposures below those used clinically.
Lactation
Risk Summary
There is no information regarding the presence of trofinetide or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the
DAYBUE Administration
Advise the caregiver or patient that DAYBUE may be given orally or via gastrostomy (G) tube; doses administered via gastrojejunal (GJ) tubes must be administered through the G-port. DAYBUE may be taken with or without food.
Instruct the caregiver or patient to obtain a calibrated measuring device, such as an oral syringe or oral dosing cup, from the pharmacy to measure and deliver the prescribed dose accurately. A household measuring cup is not an adequate measuring device. Instruct the caregiver or patient to discard any unused DAYBUE after 14 days of first opening the bottle.
Diarrhea
Advise the caregiver or patient that DAYBUE can cause diarrhea. Instruct the patient to stop taking laxatives before starting DAYBUE. If diarrhea occurs, patients should notify their healthcare provider, consider starting antidiarrheal treatment, and monitor hydration status and increase oral fluids, if needed [see Warnings and Precautions]
Weight Loss
Inform the caregiver or patient that DAYBUE may cause weight loss and to notify their healthcare provider if weight loss occurs [see Warnings and Precautions]
Vomiting
Advise the caregiver or patient that DAYBUE can cause vomiting and if vomiting occurs after DAYBUE administration, do not take an additional dose, but continue with the next scheduled dose.
Storage
Keep bottles of DAYBUE oral solution upright and refrigerated before and after opening. Do not freeze [see Storage and Handling]
Marketed by: Acadia Pharmaceuticals Inc. San Diego, CA 92130 USA
©2023 Acadia Pharmaceuticals Inc. DAYBUE is a trademark of Acadia Pharmaceuticals Inc. All rights reserved. DAY-0040 03/23
Adverse Reaction DAYBUE (N=93) % Placebo (N=94) % Diarrhea 82 20 Vomiting 29 12 Fever 9 4 Seizure 9 6 Anxiety 8 1 Decreased appetite 8 2 Fatigue 8 2 Nasopharyngitis
1
5
Meeting-at-a-Glance
Monday, April 24, 2023
The Vision of the American Academy of Neurology is to be indispensable to our members.
The Mission of the American Academy of Neurology is to promote the highest quality patient-centered neurologic care and enhance member career satisfaction.
American Academy of Neurology 201 Chicago Avenue Minneapolis, MN 55415 USA
Phone: (800) 879-1960 (Toll Free) or (612) 928-6000 (International)
Fax: (612) 454-2744
Email: memberservices@aan.com
Website: AAN.com
AAN Chief Executive Officer: Mary E. Post, MBA, CAE
Managing Editor: Angela M. Babb, MS, CAE, APR
Editor: Tim Streeter
Writers: Ryan Knoke, Sarah Parsons
Designer: Andrew Imholte
Photography: Will Evans
Annual Meeting Daily is published by the American Academy of Neurology.
The American Academy of Neurology’s registered trademarks and service marks are registered in the United States and various other countries around the world.
“American Brain Foundation” is a registered service mark of the American Brain Foundation and is registered in the United States.
7 a.m. 8 a.m. 9 a.m. 10 a.m. 11 a.m. 12 p.m. 1 p.m. 2 p.m. 3 p.m. 4 p.m. 5 p.m. 6 p.m. 7 p.m. 8 p.m. 9 p.m. 10 p.m.
Java, Juice, and Jobs 7:00–9:00 a.m. Poster Session 4 8:00–9:00 a.m. Courses C73–C83 7:00–9:00 a.m. Hubs 7:00–9:00 a.m. Contemporary Clinical Issues Plenary Session 9:15–11:00 a.m. Exhibit Hall 11:30 a.m.–6:00 p.m. Networking: Crawl 4:00–6:00 p.m. Hubs 11:00 a.m.–5:30 p.m. Poster Session 5 11:45 a.m–12:45 p.m. Courses C84–C89 11:15 a.m–12:15 p.m. Emerging Science Session 1 11:15 a.m–12:45 p.m. Scientific Session S11-S13 11:15 a.m–12:15 p.m. Industry Therapeutic Updates 11:45 a.m–12:45 p.m. Courses C90–C113 | Health Care Equity Symposium 1:00–5:30 p.m. Neuroscience in the Clinic Session | Scientific Sessions S14–S17 1:00–3:00 p.m. Invited Science | Scientific Sessions S18–S22 3:30–5:30 p.m. Poster Session 6 5:30–6:30 p.m. Trainee and Faculty Networking Reception 6:00–8:00 p.m. Industry Therapeutic Updates 6:00–9:00 p.m. *Start and end times may vary. Check with each hosting company for further information.
Networking Crawl 4:00–6:00 p.m.
Latest Innovations of Significance Highlighted in Today’s Emerging Science Session
If you’re looking for the most timely, significant, and innovative research in neurology, then look no further than the abstracts set to be showcased in today’s Emerging Science program beginning at 11:15 a.m. in room 210C. Abstracts selected for Emerging Science presentations include key aspects of research conducted after the October 2022 abstract submission deadline and must be new and of sufficient scientific importance to warrant expedited presentation and publication. These previously unpublished abstracts contain timely, significant, and innovative content.

11:15 a.m.–11:21 a.m.
The Effect of Catheter Ablation on Cognitive Outcomes in Elderly Patients with Atrial Fibrillation: SAGE-AF
Presenter: Bahadar Singh Srichawla, DO, MS
11:21 a.m.–11:27 a.m.
Efficacy and Safety of Continuous Subcutaneous ND0612 Infusion Compared with Oral Immediate-release LevodopaCarbidopa in Patients with Parkinson’s Disease and Motor Fluctuations
Presenter: Alberto J. Espay, MD, FAAN
11:27 a.m.–11:33 a.m.
Abstract withdrawn
11:33 a.m.–11:39 a.m.
RELIEF-PHN1: A Phase 2, Double-blind, Randomized, Placebocontrolled Trial of LX9211 in the Treatment of Postherpetic Neuralgia Pain
Presenter: Anand Patel, MD
11:39 a.m.–11:45 a.m.
UB-313, an Investigational CGRP Vaccine for the Prevention of Migraine
Presenter: Jean-Cosme Dodart, PhD
11:45 a.m.–11:51 a.m.
Deoxycytidine/Deoxythymidine Combination Therapy Safety and Efficacy in Treatment of POLG-related Disorders: Results After 6 Months of Treatment
Presenter: Ken Myers, MD, PhD
11:51 a.m.–11:57 a.m.
First-in-Human Trial of NRTX-1001 GABAergic Interneuron Cell Therapy for Treatment of Focal Epilepsy—Emerging Clinical Trial Results
Presenter: Robert Beach, MD, PhD, FAAN
11:57 a.m.–12:03 p.m.
Eplontersen in Hereditary ATTR-polyneuropathy: Week 66 Final Analysis of the Phase 3 NEURO-TTRansform Study
Presenter: Sami L. Khella, MD, FAAN
12:03 p.m.–12:09 p.m.
Effects of EDG-5506, a Fast Myosin Modulator, on Proteomic Biomarker Profile of Muscle Damage in Adults with Becker Muscular Dystrophy (BMD)
Presenter: Joanne Donovan, MD, PhD
12:09 p.m.–12:15 p.m.
Peripheral Blood Gene Expression Transcriptional Profiling Predicts Disease Progression in Primary Progressive Multiple Sclerosis
Presenter: Michael Gurevich, MD
Monday, April 24, 2023 • Annual Meeting Daily 5
Contemporary Clinical Issues
Experts to Discuss Critical Practice Issues in Today’s Contemporary Clinical Issues Plenary








The issues most critical to practicing neurologists will be explored by leading researchers as they discuss abstracts related to new therapeutic developments, clinical applications of basic and translational research, and innovative technical developments already affecting the practice of neurology during this morning’s Contemporary Clinical Issues Plenary. Set to take place between 9:15 a.m. and 11:00 a.m. in the Grand Ballroom: Level 3.
The session will be moderated by:
Amy Brodtmann, PhD, FRACP, MBBS


AAN Science Committee Member













Monash University, Melbourne, Australia
CE-VST01-JC: a Novel Allogeneic T-cell Based Immunotherapy for the Treatment of Progressive Multifocal Leukoencephalopathy (PML)

Presenter: George Ambalathingal, PhD, NP


QIMR Berghofer Medical Research Institute, Brisbane, Australia
Discussant: Carolyn B. Britton, MD Columbia University, New York, NY
Deep Machine Learning Algorithms in Glioblastoma (GBM) MRI Evaluation

Presenter: Jay-Jiguang Zhu, MD, PhD, FAAN

University of Texas Health Science Center, Houston, TX
10 kHz SCS Provides Durable Pain Relief and Neurological Improvements for Patients with Painful Diabetic Neuropathy: 24-Month RCT Results
Presenter: Erika Petersen, MD University of Arkansas, Little Rock, AR


Discussant: Narayan R. Kissoon, MD Mayo Clinic, Rochester, MN

Safety and Efficacy of Continuous Subcutaneous Levodopa/Carbidopa Infusion for Parkinson’s Disease: Three-year Data from the Open-label BeyoND Study



Presenter: Aaron Ellenbogen, DO Michigan Institute for Neurological Disorders, Farmington Hills, MI

Discussant: Jaishri Blakeley, MD
Johns Hopkins University School of Medicine, Baltimore, MD
Live “Fireside Chat” to Follow Contemporary Clinical Issues Plenary Session
Head over to the Research Hub in the North Lobby after the session to engage with and ask questions of plenary moderators and presenters during the live, 30-minute “Fireside Chat.”
Discussant: Deborah Hall, MD, PhD, FAAN Rush University, Chicago, IL

Seizure Forecasting and Detection with Wearable Devices and Subcutaneous EEG – Outcomes from the My Seizure Gauge Trial
Presenter: Benjamin Henry Brinkmann, PhD Mayo Clinic, Rochester, MN

Discussant: Vikram Rao, MD UCSF, San Francisco, CA
What was your greatest takeaway from the Contemporary Clinical Issues Plenary? Join the conversation at #AANAM.
6 Monday, April 24, 2023 • Annual Meeting Daily
Associate Professors: Don’t Miss Today’s Exclusive Networking Event






Calling all associate professors! You are assigned to enjoy a relaxed networking reception this afternoon from 4:30 p.m. to 5:30 p.m. in the Southeast Lobby B2: Level 1. Meet new contacts, reconnect with old friends, and enjoy an array of refreshments. This event, organized by the Associate Professor Work Group, honors the importance of networking and developing relationships. Brian D. Berman, MD, MS, FAAN; Samantha K. Holden, MD, MS, FAAN; Babar Khokhar, MD, FAAN; Jennifer J. Majersik, MD, FAAN; and Sarah Elizabeth Zauber, MD, FAAN, will be your hosts as you learn what your colleagues are doing in 2023.
Excellence works here.
Ranked 4th in the nation by U.S. News and World Report, the Department of Neurological Sciences at Rush University Medical Center is dedicated to excellence in clinical care, education and research. Our program is currently recruiting for the following positions:
• General Neurology
• Neurohospitalist
• Neurocritical Care
• Epilepsy
• Interventional Movement Disorders
Learn more and apply at joinrush.org.
Berman Holden Khokhar
Majersik Zauber
1980s–1990s



The AAN Annual Meeting





8 Monday, April 24, 2023 • Annual Meeting Daily
Victor Potamkin (left) and Robert D. Terry, MD, recipient of the first Potamkin Prize for Research in Pick’s, Alzheimer’s, and Related Diseases. Cincinnati 1988
AAN Nerve Center. San Diego 1992
Robert C. Griggs, MD, FAAN. San Diego 1992
Stanley B. Prusiner, MD; Roger N. Rosenberg, MD, FAAN; Francis Crick, PhD; William H. Oldendorf, MD. San Diego 1992
Foundation Minority Scholar Program, Washington, DC 1994
Logo, 50th Annual Meeting, Minneapolis 1998
Minneapolis 1998
Francis Kittredge, Jr, MD, FAAN. Toronto 1999
1980s–1990s

It’s National Institutes of Health Day!
Did you know that AAN founder President A.B. Baker, MD, FAAN, and then-AAN President Elect Pierce Bailey were instrumental in lobbying for the 1950 establishment of what we know today as the National Institute of Neurological Disorders and Stroke within the National Institutes of Health? Bailey was appointed as its first director in 1951 while he served as AAN president. Since NINDS received its first congressional appropriation as a line item of $4.5 million in the NIH’s 1953 budget to its 2022 fiscal year allocation of more than $2.6 billion, the AAN has been NINDS’ strongest advocate for neurologic research funding on Capitol Hill.


Today, you can hear from NINDS Director Walter J. Koroshetz, MD, FAAN, and other experts from the NIH about their current work, initiatives, and funding opportunities. NIH Day will be held in the Research Hub located in the North Lobby.
Agenda
Coffee Connect: 8:00 a.m.–9:00 a.m.
Grab a cup of coffee and connect with NIH staff about these exciting initiatives:
• Programs to Enhance Neuroscience Workforce Diversity
• Understanding NIH and Training Programs
• Navigating Clinical Trials
• Advancing Therapeutic Developments
• Engaging with Trans-NIH Initiatives
Afternoon Sessions: 1:00 p.m.–5:00 p.m. 1:00 p.m.–1:20 p.m. NIH Overview Presentations (will cover translational updates and opportunities)
Speaker: Walter J. Koroshetz, MD, FAAN

Trainee Research and Development (this will also incorporate aspects of diversity efforts specific to trainees)
FLASHBACK: AAN and NINDS
 (Front) AAN President Kenneth M. Viste, Jr., MD, FAAN; (Rear from left) President Elect Steven P. Ringel, MD, FAAN; Past President Jack P. Whisnant, MD, FAAN; NINDS Director Zach Hall, PhD; Sid Gilman, MD, FAAN. Circa 1995.
Pearce Bailey, MD, FAAN
(Front) AAN President Kenneth M. Viste, Jr., MD, FAAN; (Rear from left) President Elect Steven P. Ringel, MD, FAAN; Past President Jack P. Whisnant, MD, FAAN; NINDS Director Zach Hall, PhD; Sid Gilman, MD, FAAN. Circa 1995.
Pearce Bailey, MD, FAAN
1:20 p.m.–1:45 p.m. Q&A
1:45 p.m.–2:05 p.m.
2:05 p.m.–2:30 p.m. Q&A / Open Discussion with NINDS staff 2:30 p.m.–2:50 p.m.
2:50 p.m.–3:15 p.m. Q&A / Open Discussion with NINDS staff 3:15 p.m.–3:35 p.m. Clinical
3:35 p.m.–4:00 p.m. Q&A
Discussion
NINDS staff 4:00 p.m.–4:20 p.m. Special
4:20 p.m.–5:00 p.m. Q&A / Open Discussion with NINDS staff
/ Open Discussion with NINDS leadership
Health Disparities and Diversity Programming: Updates and Opportunities
Supporting
Research: Updates and Opportunities
/ Open
with
Programs in Neurodegeneration (including AD/ADRD, ALS, Parkinson’s, etc. updates/opportunities)
Koroshetz
Minneapolis 1998
Helen and Francis M. Forster, MD, FAAN. Minneapolis 1998
Industry Therapeutic Update from argenx at the 2023 AAN Annual Meeting
Individualizing gMG Treatment: VYVGART, an FcRn Inhibitor for the Treatment of Adult Patients Who Are Anti-Acetylcholine Receptor Antibody Positive
Join us as we discuss treatment individualization and present clinical data for efgartigimod in adult patients with generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor antibody positive.
gMG is a rare but debilitating disease in which pathogenic immunoglobulin G (IgG) autoantibodies cause failure of signal transmission at the neuromuscular junction. The neonatal Fc receptor (FcRn) is a molecule that recycles IgG antibodies, including pathogenic autoantibodies, rescuing them from lysosomal degradation and perpetuating the underlying pathophysiology behind gMG. Efgartigimod is a human IgG1-derived Fc fragment that blocks and binds to FcRn, resulting in a reduction of circulating IgG (including levels of pathogenic autoantibodies). As gMG treatment goals take into consideration for the fluctuating nature of the disease and a heterogenous patient population, we will discuss the potential for efgartigimod to provide an individualized treatment approach for patients.
INDICATION
VYVGART® (efgartigimod alfa-fcab) is indicated for the treatment of generalized myasthenia gravis in adult patients who are anti-acetylcholine receptor (AChR) antibody positive.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Infection
VYVGART may increase the risk of infection. The most common infections observed in Study 1 were urinary tract infection (10% for VYVGART vs 5% for placebo) and respiratory tract infection (33% for VYVGART vs 29% for placebo). Patients on VYVGART vs placebo had below normal levels for white blood cell counts (12% vs 5%, respectively), lymphocyte counts (28% vs 19%, respectively), and neutrophil counts (13% vs 6%, respectively). The majority of infections and hematologic abnormalities were mild to moderate in severity. Delay VYVGART administration in patients with an active infection until the infection is resolved; monitor for clinical signs and symptoms of infections. If serious infection occurs, administer appropriate treatment and consider withholding VYVGART until the infection has resolved.

Immunization
Immunization with vaccines during VYVGART treatment has not been studied; the safety with live or live-attenuated vaccines and the response to immunization with any vaccine are unknown. Because VYVGART causes a reduction in immunoglobulin G (IgG) levels, vaccination with live-attenuated or live vaccines is not recommended during VYVGART treatment. Evaluate the need to administer age-appropriate vaccines according to immunization guidelines before initiation of a new treatment cycle with VYVGART.
Hypersensitivity Reactions
Hypersensitivity reactions, including rash, angioedema, and dyspnea, were observed with VYVGART. In clinical trials, hypersensitivity reactions were mild or moderate, occurred within 1 hour to 3 weeks of administration, and did not lead to treatment discontinuation. Monitor patients during administration and for 1 hour thereafter for
Please see Important Safety Information within and full Prescribing Information (PI) at VYVGART.com/PI. The full PI will also be available at the symposium. This program is NOT accredited for continuing education by any organization. Additionally, Industry Therapeutic Updates program content and the views expressed herein are those of the presenting corporate entity and not of the AAN. These programs are not an official part of the 2023 AAN Annual Meeting education or scientific programs, nor are they endorsed by the AAN. The AAN cannot affirm claims pertaining to FDA off-label medication, research use of pre-FDA drugs, or other research information that might be discussed. Industry Therapeutic Updates are industry events. argenx complies with all applicable laws, regulations, ordinances, and industry standards that relate to interactions with health care professionals, including transparency disclosure requirements.
Mondayat6:00 pm
Session Faculty
Professor
Monday, April 24 6:00 PM to 9:00 PM Dinner
Hypersensitivity Reactions (cont’d)
Professor and Associate Chairman, Department of Neurology, the Director for the Center for Restorative Neurology at Loma Linda, Director for Neuromuscular/ALS Programs at Loma Linda University Health Systems



Harbor Ballroom 1 Westin Boston Waterfront
clinical signs and symptoms of hypersensitivity reactions. If a hypersensitivity reaction occurs during administration, discontinue VYVGART infusion and institute appropriate supportive measures if needed.
ADVERSE REACTIONS
The most common (≥10%) adverse reactions with VYVGART were respiratory tract infection, headache, and urinary tract infection.
USE IN SPECIFIC POPULATIONS
Pregnancy
As VYVGART is expected to reduce maternal IgG antibody levels, reduction in passive protection to the newborn is anticipated. Risk and benefits should be considered prior to administering live or live-attenuated vaccines to infants exposed to VYVGART in utero.
Lactation
There is no information regarding the presence of VYVGART in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VYVGART and any potential adverse effects on the breastfed infant from VYVGART or from the underlying maternal condition.
Please see the full Prescribing Information.
You may report side effects to the US Food and Drug Administration by visiting http://www.fda.gov/medwatch or calling 1-800-FDA-1088. You may also report side effects to argenx US, Inc, at 1-833-argx411 (1-833-274-9411).
will be served
Neelam Goyal, MD
Clinical Associate Professor, Division of Neuromuscular Medicine, Stanford University
James F. Howard Jr, MD, FAAN
of Neurology & Medicine, Former Distinguished Professor of Neuromuscular Diseases, Former Chief of Neuromuscular Disorders Division, Department of Neurology, University of North Carolina School of Medicine at Chapel Hill
Jeffrey Rosenfeld, PhD, MD, FAAN, FANA
©2023 argenx, US, Inc MED-US-VYV-2300005 v1.0 January 2023
Check out Today’s Hub Highlights for Collaborative Learning
Step outside the traditional classroom and into the world of unconventional learning with eight Hub areas. Each Hub offers unique educational and networking opportunities. Engage in collaborative learning, gain actionable tools for your patients and career, and find your community. Here are today’s highlights:
ACADEMIC HUB
Southeast Lobby: Level 1
Exploring the Victories and Defeats of Feeling Like an Imposter
11:00 a.m.–11:45 a.m.
With vignettes about their own struggles with imposter phenomena, Charlene E. Gamaldo, MD, FAAN, FAASM; Lynn Liu, MD, FAAN; and Rachel Marie E. Salas, MD, MEd, FAAN, hope to normalize the emotion and create an environment that fosters honest dialogue amongst attendees. Attendees will practice implementing strategies (values and strengths) for both recognizing and celebrating the positive elements while preventing or mitigating the negative influence.
Neurology Today Panel Discussion and Q&A: Career Consult





4:00 p.m.–4:45 p.m.
Move from printed page to in-person conversation by joining members of the Neurology Today ® editorial board, including Joseph E. Safdieh, MD, FAAN, editor-in-chief, in this career development discussion. The board will answer your questions related to making informed decisions about your neurology career. Gather to discuss solutions, connect with new collaborators, learn about emerging opportunities, and network with peers.
HEADTALKS
Northwest Lobby A: Level 1
Lost in Translation
12:00 p.m.–12:45 p.m.
Joseph I. Sirven, MD, FAAN, and Omar A. Danoun, MD, will highlight and show solutions to dealing with neurologic care in situations where the neurologist and the patient are of different cultures or speak different languages. Through live interviews and storytelling, neurologists will share their experiences, both successful and tragic, when different cultures meet.

Neuro-Jeopardy: Telencephalon Twisters

5:00 p.m.–5:45 p.m.
Looking to relax after today’s courses and sessions? Grab a glass of wine to watch your colleagues test their neurologic fortitude fortitude in this favorite game!
12 Monday, April 24, 2023 • Annual Meeting Daily
Gamaldo Liu Salas
Safdieh
Sirven Danoun
INNOVATION HUB
Exhibit Hall B1
Build Your Own Board Games and Card Games to Teach Neurology
11:30 a.m.–12:00 p.m.
Zachary N. London, MD, FAAN, will discuss lessons he learned from self-publishing a growing library of neurology-themed tabletop games, and help you figure out how to get your out-of-the-box ideas into an actual box.
TRAINEE AND EDUCATOR HUB
Room 155
Learning Neurology Through Small Bytes: Where to Go for Learning as a Medical Student
3:00 p.m.–4:00 p.m.
Hear results of a research study of how AAN microlearning products help to improve the learning outcomes of medical students. Learn about AAN microlearning products NeuroBytes, NeuroBytes Medical Student Series, and Neurology Question of the Day, and how to best leverage these resources as educators and medical students.
London
Brainstorm Competition
4:00 p.m.–6:00 p.m.
Watch as your neurology colleagues compete in front of a panel of judges as they present their ideas about transforming health care systems and innovations in patient care. A $1,500 prize is on the line!
LEADERSHIP UNIVERSITY
Northeast Lobby A: Level 1
Leadership of a Culturally Intelligent Organization
12:00 p.m.–1:00 p.m.
Room 154
Culturally intelligent leaders create an environment where diversity and culture flourish, and where conflicting values can be safely expressed and explored through dialogue. Organizations and leaders that embrace this model ask probing questions on whether their organization’s culture really accepts the differences it invites, and whether they really embrace the different perspectives that come from increasing our commitment to recruiting. Roy H. Hamilton, MD, MS, FAAN, will explore culturally intelligent organizations within medicine, and strategies to develop this model.
Introduction to Leadership Circles: Imposter Syndrome
2:00 p.m.–3:00 p.m.
Attend this Leadership University program to learn about the exciting new opportunity of Leadership Circles. Cynthia L. Comella, MD, FAAN, and Madhu Soni, MD, FAAN, will take you through the topic of imposter syndrome and you’ll also hear about the ongoing engagement opportunities of Leadership Circles.
PRACTICE AND POLICY HUB
Northwest Lobby: Level 2
Leveraging Data Science and Value-based Methods to Improve Physician and Care Experience





2:45 p.m.–3:45 p.m.
Jeffrey R. Buchhalter, MD, FAAN; Lidia Maria Veras Rocha Moura, MD, PhD, MPH, FAAN; and Susan T. Herman, MD, FAAN, will introduce frameworks to address common inefficiencies encountered by system administrators and providers to help you achieve value-based health outcomes for patients while decreasing physician burnout rates. Learn how to leverage data science and value-based methods to improve documentation, patient outcomes, and overall providerpatient experience.
on page 14 ⊲ Monday, April 24, 2023 • Annual Meeting Daily 13
Continued
Hamilton
Comella Soni
Lidia Maria Veras Rocha Moura, MD, PhD, MPH, FAAN, and Anup Patel, MD, FAAN, at the 2022 Annual Meeting.
Check out Today’s Hub Highlights for Collaborative Learning continued from page 13

RESEARCH HUB
North Lobby
National Institutes of Health (NIH) Day
1:00 p.m.–5:00 p.m.
Join NINDS Director Walter J. Koroshetz, MD, FAAN, and others from NINDS/NIH providing updates on opportunities, information on health disparity and diversity programming, supporting trainee research and development and special programs in neurodegeneration.

WELLNESS HUB
Northeast Lobby: Level 2
Wellness Hub Social

3:00 p.m.–4:30 p.m.
All attendees are invited to this informal gathering featuring a musical performance, member art showcase, and other activities to promote connection and well-being. Light refreshments will be served.
Catalyst Pharmaceuticals now proudly serves the Epilepsy and LEMS* communities. Visit the Catalyst booth to learn more. Acquisisiton Details *Lambert - Eaton myasthenic syndrome @CatalystForRare | CatalystPharma.com © 2023 Catalyst Pharmaceuticals, Inc. All Rights Reserved. Printed in USA. 23LEM0968. March 2023 23LEM0968_Catalyst Half Page Ad_horizontal_V3.pdf 1 3/13/23 1:20 PM
Olajide Abiola, MD, showcased his drawing skills in a rousing game of Neurology Pictionary at the HeadTalks stage.
From left, AAN Committee on Public Engagement Chair David A. Evans, MBA; AAN President Orly Avitzur, MD, MBA, FAAN; World Federation of Neurology President Wolfgang Grisold, MD, FAAN; European Academy of Neurology President Paul A.J.M. Boon, MD, PhD, FEAN; and Committee on Public Engagement Co-chair Natalia S. Rost, MD, MPH, FAAN, FAHA, led a HeadTalks discussion on building the future of brain health worldwide.
Today’s NeuroPanels Taking Place Live on the HeadTalks Stage
Make your way to the HeadTalks stage today between 2:30 p.m. and 3:15 p.m. for the AAN’s new, popular webinar series—live and in person!

Recipients of New Award Honored at Today’s Health Care Equity Symposium



The AAN debuts a new award at the Health Care Equity Symposium this afternoon from 1:00 p.m. to 4:05 p.m. in room 253AB.
Posas
José H. Posas, MD, FAAN, will be joined by a panel of experts for an open, in-person, casebased discussion on neurogenetics and autism in adolescents, and its far-reaching impacts into adulthood, that every neurologist needs to know. Guest experts Madeline A. Chadehumbe, MD, FAAN, and Rujuta Bhatt Wilson, MD, will first discuss two challenging cases followed by an audience Q&A session.
The Health Care Equity Research Award recognizes a neurologist or neuroscientist who has demonstrated their commitment to health equity and addressing health disparities through their clinical research, service, or leadership role. The 2023 recipients are Bruce I. Ovbiagele, MD, MSc, FAAN; Jordan Janae Cole, MD; and Nicole Rosendale, MD. The recipients will receive their award and give a 15-minute presentation on their research with a panel discussion following the presentations. All are welcome to attend.
PANELS
AAN Conferences Mobile App Daily Tip
The AAN Conferences mobile app makes finding your way to sessions easier than ever. Tap “Maps” on the main menu to help navigate the convention center to get to your next session.

Far away from your next session? Use the watch live feature to view your next session on the go. Navigate to the Schedule view and select the “play” icon. (Available for most sessions.)
Need mobile app support? Visit the Information Booth by Registration or in the North Lobby.
Scan to DOWNLOAD app
Ovbiagele Cole Rosendale
Revelers Celebrate 75th Anniversary of Their AAN
The mood was festive at the 75th Anniversary Celebration at Boston’s Museum of Science, as attendees took a journey through the decades, enjoying food and beverages, music, exhibitions, and more.



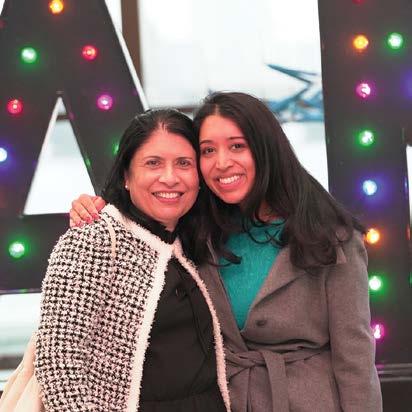



16 Monday, April 24, 2023 • Annual Meeting Daily
Top Science Draws Media Coverage in Press Conference

Natalia S. Rost, MD, MPH, FAAN, FAHA, chair of the Science Committee, spoke to reporters during a press conference on research from the 2023 Annual Meeting. Presenters were Marianna Spatola, MD, PhD, of Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology, and Harvard Medical School in Boston; George Ambalathingal, PhD, NP, of QIMR Berghofer Medical Research Institute, Queensland, Australia; and David C. Spencer, MD, FAAN, Oregon Health & Science University, Portland.
Spatola’s abstract is titled “Serum and Cerebrospinal Fluid Antibody Signatures Track with Outcome of Neurologic Post-Acute Sequelae of SARS-Cov-2 Infection (NeuroPASC).”
Ambalathingal presented on “CE-VST01-JC: a Novel Allogeneic T-cell Based Immunotherapy for the Treatment of Progressive Multifocal Leukoencephalopathy (PML).”

Spencer’s abstract is “First-in-human Trial of NRTX-1001 GABAergic Interneuron Cell Therapy for Treatment of Focal Epilepsy–Emerging Clinical Trial Results.”


A recording of the press conference, which was held on April 12 via video conference, is available on the AAN YouTube channel at YouTube.com/AANChannel.
 Rost Spatola
Ambalathingal Spencer
Rost Spatola
Ambalathingal Spencer
prescription medicine for the treatment of Friedreich’s ataxia in adults and adolescents aged 16 years and older1
Get Started With SKYCLARYS:
Submit a Start Form today at ReataREACH.com. Reata REACH is here to support your eligible patients with FA. Scan the QR code to start now.

IMPORTANT SAFETY INFORMATION WARNINGS AND PRECAUTIONS
Elevation of Aminotransferases: Treatment with SKYCLARYS can cause an elevation in hepatic transaminases (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]). Monitor ALT, AST, and total bilirubin prior to initiation of SKYCLARYS, every month for the first 3 months of treatment, and periodically thereafter.
Elevation of B-Type Natriuretic Peptide: Treatment with SKYCLARYS can cause an increase in B-type natriuretic peptide (BNP), a marker of cardiac function. Elevations in BNP may indicate cardiac failure and should prompt an evaluation of cardiac function. Check BNP prior to initiation of SKYCLARYS. Monitor patients for the signs and symptoms of fluid overload.
Lipid Abnormalities: Treatment with SKYCLARYS can cause changes in cholesterol. Assess lipid parameters prior to initiation of SKYCLARYS and monitor periodically during treatment.
ADVERSE REACTIONS
Adverse reactions reported in 10% or more of patients and greater than placebo were elevated liver enzymes (AST/ALT) (37%), headache (37%), nausea (33%), abdominal pain (29%), fatigue (24%), diarrhea (20%), musculoskeletal pain (20%), oropharyngeal pain (18%), influenza (16%), vomiting (16%), muscle spasms (14%), back pain (13%), decreased appetite (12%), rash (10%).
To report SUSPECTED ADVERSE REACTIONS, contact Reata Pharmaceuticals, Inc. at 1-800-314-3934 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.

INDICATION
SKYCLARYS is indicated for the treatment of Friedreich’s ataxia in adults and adolescents aged 16 years and older.
For additional information about SKYCLARYS, please see the Brief Summary on the following page and the full Prescribing Information at hcp.SKYCLARYS.com. US-SKY-2300056
Reference: 1. Skyclarys. Prescribing information. Reata Pharmaceuticals, Inc; 2023. © 2023 Reata Pharmaceuticals, Inc. All rights reserved. SKYCLARYS, REATA, and their logos are trademarks of Reata Pharmaceuticals, Inc. US-SKY-2200001 03/2023
v2.0
BRIEF SUMMARY
SKYCLARYS™ (omaveloxolone) capsules, for oral use
1 INDICATIONS AND USAGE
SKYCLARYS is indicated for the treatment of Friedreich’s ataxia in adults and adolescents aged 16 years and older.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Testing Before Initiating SKYCLARYS and Monitoring to Assess Safety
Obtain ALT, AST, bilirubin, BNP, and lipid parameters prior to initiating SKYCLARYS and during treatment [see Warnings and Precautions (5.1, 5.2, 5.3)]
2.2 Recommended Dosage
The recommended dosage of SKYCLARYS is 150 mg (3 capsules) taken orally once daily.
• Administer SKYCLARYS on an empty stomach at least one hour before eating [see Clinical Pharmacology (12.3)]
• Swallow SKYCLARYS capsules whole. Do not open, crush, or chew.
2.3 Missed Doses
If a dose of SKYCLARYS is missed, take the next dose at its scheduled time the following day. A double dose should not be taken to make up for a missed dose.
2.4 Recommendations for Concomitant Use with Strong or Moderate CYP3A4 Inhibitors and Inducers
The recommended dosage for concomitant use of SKYCLARYS with cytochrome P450 (CYP) 3A4 inhibitors and inducers are described in Table 1 [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]
Table 1: Recommended Dosage of SKYCLARYS with Concomitant Use of CYP3A4 Inhibitors and Inducers
5.3 Lipid Abnormalities
Treatment with SKYCLARYS can cause changes in cholesterol. In Study 1, 29% of patients treated with SKYCLARYS reported elevated cholesterol above ULN at one or more time points. Mean increases were observed within 2 weeks of initiation of SKYCLARYS and returned to baseline within 4 weeks of discontinuing treatment. A total of 16% of patients treated with SKYCLARYS had an increase in low-density lipoprotein cholesterol (LDL-C) from baseline, compared to 8% of patients who received placebo. The mean increase in LDL-C for all SKYCLARYS-treated patients was 23.5 mg/dL at 48 weeks. A total of 6% of patients treated with SKYCLARYS had decreases in high-density lipoprotein cholesterol (HDL-C) from baseline compared to 4% of patients who received placebo.
The mean decrease in HDL-C for all SKYCLARYS-treated patients was 5.3 mg/dL at 48 weeks.
Assess lipid parameters prior to initiation of SKYCLARYS and monitor periodically during treatment.
Manage lipid abnormalities according to clinical guidelines.
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described in greater detail in other labeling sections:
• Elevation of aminotransferases [see Warnings and Precautions (5.1)]
• Elevation of BNP [see Warnings and Precautions (5.2)]
• Lipid abnormalities [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of SKYCLARYS 150 mg once daily has been evaluated in 165 patients with Friedreich’s ataxia, including 137 patients exposed for at least 48 weeks, and 125 patients exposed for at least 96 weeks.
The most common adverse reactions in Study 1 (≥20% and greater than placebo) were elevated liver enzymes (AST/ALT), headache, nausea, abdominal pain, fatigue, diarrhea, and musculoskeletal pain. Table 3 shows the adverse reactions that occurred in 10% or more of patients treated with SKYCLARYS and greater than placebo.
Table 3: Adverse Reactions Reported in 10% or More of Patients Treated with SKYCLARYS and Greater than Placebo (Study 1)
dose range-finding study, oral administration of omaveloxolone at doses up to 30 mg/kg/day to pregnant rats throughout organogenesis produced increases in post-implantation loss and resorptions, resulting in a decrease in viable fetuses, and reduced fetal weight at the highest dose tested. At the highest dose tested in the pivotal study (10 mg/kg/day), plasma exposure (AUC) was approximately 5 times that in humans at the recommended human dose (RHD) of 150 mg/day.
Oral administration of omaveloxolone (0, 3, 10, or 30 mg/kg/day) to pregnant rabbits throughout organogenesis resulted in increased embryofetal mortality and skeletal variations and reduced fetal weight at the highest dose tested, which was associated with maternal toxicity. At the no-effect dose for adverse effects on embryofetal development (10 mg/kg/day), plasma exposure was less than that in humans at the RHD.
Oral administration of omaveloxolone (0, 1, 3, or 10 mg/kg/day) throughout pregnancy and lactation resulted in an increase in stillbirths and impaired neurobehavioral function (increased locomotor activity and learning and memory deficits) in offspring at all doses, reduced body weight in offspring at all but the lowest dose tested, and delayed sexual maturation (males), increased postnatal mortality, and impaired reproductive performance in offspring at the highest dose tested. A no-effect dose for adverse effects on pre- and postnatal development was not identified. Plasma exposure (AUC) at the lowest dose tested was less than that in humans at the RHD.
8.2 Lactation
Risk Summary
There are no data on the presence of omaveloxolone or its metabolites in human milk. The effects on milk production and the breastfed infant are unknown. Omaveloxolone was excreted in the milk of lactating rats following oral administration. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SKYCLARYS and any potential adverse effects on the breastfed infant from SKYCLARYS or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
SKYCLARYS may decrease the efficacy of hormonal contraceptives [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)]. Advise patients to avoid concomitant use with combined hormonal contraceptives (e.g., pill, patch, ring), implants, and progestin only pills. Counsel females using hormonal contraceptives to use an alternative contraceptive method (e.g., non-hormonal intrauterine system) or additional non-hormonal contraceptive (e.g., condoms) during concomitant use and for 28 days after discontinuation of SKYCLARYS.
8.4 Pediatric Use
• If adverse reactions emerge, coadministration with strong CYP3A4 inhibitors should be discontinued.
• Reduce the dosage of SKYCLARYS to 100 mg once daily with close monitoring for adverse reactions.
• If adverse reactions emerge, further reduce the dosage of SKYCLARYS to 50 mg once daily. Strong or Moderate CYP3A4 inducer Recommended to avoid concomitant use.
2.5 Recommended Dosage for Patients with Hepatic Impairment
The recommended dosage for patients with hepatic impairment are described in Table 2 [see Use in Specific Populations (8.6)]
Table 2: Recommended Dosage in Patients with Hepatic Impairment
• Consider lowering to 50 mg once daily if adverse reactions emerge
3 DOSAGE FORMS AND STRENGTHS
SKYCLARYS capsules contain 50 mg of omaveloxolone, and are supplied as opaque hard capsules having a light green body and blue cap, imprinted with “RTA 408” in white ink on the body and “50” in white ink on the cap.
4 CONTRAINDICATIONS
None.
5 WARNINGS AND PRECAUTIONS
5.1 Elevation of Aminotransferases
Treatment with SKYCLARYS can cause an elevation in hepatic transaminases (ALT and AST). In Study 1 [see Clinical Studies (14)], the incidence of elevations of ALT or AST above 5 times and 3 times the upper limit of normal (ULN) was 16% and 31%, respectively, in patients treated with SKYCLARYS. There were no cases of concomitant elevation of transaminases and total bilirubin observed in Study 1. Maximum increases in ALT and AST occurred within 12 weeks after starting SKYCLARYS. Increases in serum aminotransferases were generally asymptomatic and reversible following discontinuation of SKYCLARYS. Patients with clinically significant liver disease were excluded from Study 1.
Monitor ALT, AST, and total bilirubin prior to initiation of SKYCLARYS, every month for the first 3 months of treatment, and periodically thereafter. If transaminases increase to levels greater than 5 times the ULN, or greater than 3 times the ULN with evidence of liver dysfunction (e.g., elevated bilirubin), immediately discontinue SKYCLARYS and repeat liver function tests as soon as possible. If transaminase levels stabilize or resolve, SKYCLARYS may be reinitiated with an appropriate increased frequency of monitoring of liver function [see Adverse Reactions (6.1) and Use in Specific Populations (8.6)]
5.2 Elevation of B-Type Natriuretic Peptide
Treatment with SKYCLARYS can cause an increase in BNP, a marker of cardiac function. In Study 1, a total of 14% of patients treated with SKYCLARYS had an increase from baseline in BNP and a BNP above the ULN (100 pg/mL), compared to 4% of patients who received placebo. The incidence of elevation of BNP above 200 pg/mL was 4% in patients treated with SKYCLARYS. Cardiomyopathy and cardiac failure are common in patients with Friedreich’s ataxia. Patients were excluded from Study 1 if they had BNP levels > 200 pg/mL prior to study entry, or a history of clinically significant left-sided heart disease and/or clinically significant cardiac disease, with the exception of mild to moderate cardiomyopathy associated with Friedreich’s ataxia [see Adverse Reactions (6.1)] Whether the elevations in BNP in Study 1 are related to SKYCLARYS or cardiac disease associated with Friedreich’s ataxia is unclear.
Elevations in BNP may indicate cardiac failure and should prompt an evaluation of cardiac function. Check BNP prior to initiation of SKYCLARYS. Monitor patients for the signs and symptoms of fluid overload, such as sudden weight gain (3 pounds or more of weight gain in one day, or 5 pounds or more of weight gain in a week), peripheral edema, palpitations, and shortness of breath. If signs and symptoms of fluid overload develop, worsen, or require hospitalization, evaluate BNP and cardiac function, and manage appropriately. Management of fluid overload and heart failure may require discontinuation of SKYCLARYS.
Laboratory Abnormalities
In addition to elevated liver enzymes, additional laboratory abnormalities include elevation of BNP and lipid abnormalities [see Warnings and Precautions (5.1, 5.2, 5.3)]
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on SKYCLARYS
CYP3A4 Inhibitors
Omaveloxolone is a CYP3A4 substrate. Concomitant use of SKYCLARYS with moderate or strong CYP3A4 inhibitors is expected to result in clinically significant increased exposure of omaveloxolone [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions. Avoid concomitant use of SKYCLARYS with moderate or strong CYP3A4 inhibitors. If use cannot be avoided, dosage modifications are recommended [see Dosage and Administration (2.4)]
CYP3A4 Inducers
Omaveloxolone is a CYP3A4 substrate. Concomitant use of SKYCLARYS with moderate or strong CYP3A4 inducers may significantly decrease exposure of omaveloxolone [see Clinical Pharmacology (12.3)], which may reduce the effectiveness of SKYCLARYS. Avoid concomitant use of SKYCLARYS with moderate or strong CYP3A4 inducers.
7.2 Effect of SKYCLARYS on Other Drugs
CYP3A4 and CYP2C8 Substrates
Omaveloxolone is a weak inducer of CYP3A4 and CYP2C8. Concomitant use with SKYCLARYS can reduce the exposure of CYP3A4 and CYP2C8 substrates which may reduce the activity of these substrates [see Clinical Pharmacology (12.3)]. Refer to the prescribing information of substrates of CYP3A4 and CYP2C8 for dosing instructions if used concomitantly with SKYCLARYS and monitor for lack of efficacy of the concomitant treatment.
Hormonal Contraceptives
Omaveloxolone is a weak CYP3A4 inducer [see Clinical Pharmacology (12.3)]. Concomitant use with SKYCLARYS may reduce the efficacy of hormonal contraceptives. Advise patients to avoid concomitant use with combined hormonal contraceptives (e.g., pill, patch, ring), implants, and progestin only pills [see Use in Specific Populations (8.3)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risks associated with the use of SKYCLARYS in pregnant women. In animal studies, administration of omaveloxolone during pregnancy or throughout pregnancy and lactation produced evidence of developmental toxicity (embryofetal mortality and growth impairment, and mortality, growth impairment, and neurobehavioral deficits in offspring) at plasma exposures similar to or less than exposures in humans (see Animal Data). In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
Oral administration of omaveloxolone (0, 1, 3, or 10 mg/kg/day) to pregnant rats throughout organogenesis resulted in no adverse effects on embryofetal development; however, in a
The safety and effectiveness of SKYCLARYS for the treatment of Friedreich’s ataxia have been established in pediatric patients aged 16 years and older. Use of SKYCLARYS for this indication is supported by evidence from one adequate and well-controlled study (Study 1) in adults and in pediatric patients aged 16 years and older [see Clinical Studies (14)]
Safety and effectiveness of SKYCLARYS have not been established in pediatric patients less than 16 years of age.
8.5 Geriatric Use
Clinical studies of SKYCLARYS in Friedreich’s ataxia did not include patients aged 65 and over. No data are available to determine whether they respond differently than younger adult patients.
8.6 Hepatic Impairment
Omaveloxolone plasma exposure is increased in patients with moderate or severe hepatic impairment (Child-Pugh Class B and C) [see Clinical Pharmacology (12.3)]. Avoid treatment with SKYCLARYS in patients with severe hepatic impairment, including those who develop severe hepatic impairment. If hepatic function improves to moderate impairment, mild impairment, or normal function, initiation of SKYCLARYS treatment at the approved recommended dosage may be considered. For patients with moderate hepatic impairment, a reduced dosage is recommended with close monitoring for adverse reactions [see Dosage and Administration (2.5)]. For patients with mild hepatic impairment (Child-Pugh Class A), no dose adjustments are recommended.
9 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Elevation of Aminotransferases
Inform patients that elevation in aminotransferases have occurred in patients treated with SKYCLARYS. Liver function tests will be performed prior to initiating SKYCLARYS, every month for the first 3 months of treatment, and periodically thereafter as needed [see Warnings and Precautions (5.1)]
Fluid Overload
Inform patients that elevations in BNP, a marker of cardiac function, have occurred in patients treated with SKYCLARYS. BNP will be performed prior to initiating SKYCLARYS and if signs and symptoms of fluid overload occur, such as sudden weight gain, peripheral edema, palpitations, and shortness of breath. Advise patients to contact their healthcare provider if signs and symptoms of fluid overload develop [see Warnings and Precautions (5.2)]
Lipid Abnormalities
Inform patients that treatment with SKYCLARYS has been associated with increases in LDL cholesterol and decreases in HDL cholesterol. Cholesterol will be assessed prior to starting SKYCLARYS and monitored periodically during treatment [see Warnings and Precautions (5.3)]
Drug Interactions
Advise patients to discuss all medications they are taking, including other prescription medications, non-prescription medications, or herbal products (e.g., St. John’s Wort) with their healthcare provider [see Drug Interactions (7)]
Pregnancy
Advise women to notify their healthcare provider if they become pregnant or intend to become pregnant during SKYCLARYS therapy [see Use in Specific Populations (8.1)]
Females of Reproductive Potential
Counsel females using hormonal contraceptives to use an alternative contraceptive method (e.g., non-hormonal intrauterine system) or additional non-hormonal contraceptive (e.g., condoms) during concomitant use and for 28 days after discontinuation of SKYCLARYS [see Drug Interactions (7.2) and Use in Specific Populations (8.3)]
Administration
Advise patients to take SKYCLARYS on an empty stomach at least 1 hour before eating [see Dosage and Administration (2.2)]
Swallow SKYCLARYS capsules whole. Do not open, crush, or chew.
Advise patients that if a dose of SKYCLARYS is missed to not to double their dose or take more than the prescribed dose [see Dosage and Administration (2.3)]
Advise patients to avoid grapefruit juice and grapefruit while they are taking SKYCLARYS [see Drug Interactions (7.1)]
Manufactured for Reata Pharmaceuticals, Inc., Plano, TX 75024 USA
SKYCLARYS is a trademark of Reata Pharmaceuticals Holdings, LLC, used under license by Reata Pharmaceuticals, Inc., Plano, TX 75024 USA
Copyright© 2023, Reata Pharmaceuticals, Inc., Plano, TX 75024 USA
All rights reserved
© 2023 Reata Pharmaceuticals, Inc. All Rights Reserved. SKYCLARYS, REATA, and their logos are trademarks of Reata Pharmaceuticals, Inc. US-SKY-2200013 03/2023
Adverse Reaction SKYCLARYS
mg (N=51) Placebo (N=52) Elevated liver enzymes (AST/ALT) 37% 2% Headache 37% 25% Nausea 33% 13% Abdominal pain 29% 6% Fatigue 24% 14% Diarrhea 20% 10% Musculoskeletal pain 20% 15% Oropharyngeal pain 18% 6% Influenza 16% 6% Vomiting 16% 12% Muscle spasms 14% 6% Back pain 13% 8% Decreased appetite 12% 4% Rash 10% 4%
150
Concomitant Drug Class Dosage Strong CYP3A4 inhibitor Recommended to avoid concomitant use. If
be
coadministration cannot
avoided:
• Reduce the dosage of SKYCLARYS to 50 mg once daily with close monitoring for adverse reactions.
Moderate CYP3A4
inhibitor Recommended to avoid concomitant use. If coadministration cannot be avoided:
Impairment Classification (Child-Pugh) Dosage Severe (Child-Pugh
C) Avoid
Class
use Moderate (Child-Pugh Class B) • 100 mg once daily with close monitoring for adverse reactions
Mild (Child-Pugh Class A) 150 mg once daily
Don’t Miss Today’s Publications and Online Learning Talks
TODAY
Utilizing NeuroBytes in Your Clerkship
11:30 p.m.–12:00 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
Jeremy Moeller, MD, FAAN, and Adam Quick, MD
Meet the Editor-in-Chief of Neurology Today ® : Q&A with Dr. Joseph E. Safdieh
1:00 p.m.—1:30 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
Joseph E. Safdieh, MD, FAAN
Disputes & Debates: Pulled from the Pages of Neurology ®: Meet the Editors and Learn More
1:30 p.m.–2:00 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
Steven Galetta, MD, FAAN; Aravind Ganesh, MD, DPhil (OXON), FRCPC; Ariane Lewis, MD, FAAN
Neurology Quiz LIVE
2:00 p.m.–2:30 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
Prasanna Venkatesan Eswaradass, MD
Meet the Editors of Neurology : All Questions Welcome
2:30 p.m.–3:00 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
José G. Merino, MD, MPhil, FAAN; Luca Bartolini, MD; Josep O. Dalmau, MD, PhD, FAAN; Stefan M. Pulst, MD, FAAN; Roy E. Strowd III, MD, MEd, MS, FAAN
Neurology Today Panel Discussion and Q&A: Career Consult
4:00 p.m.–4:45 p.m.
ACADEMIC HUB (SOUTHEAST LOBBY C: LEVEL 1)
Joseph E. Safdieh, MD, FAAN; Neil A. Busis, MD, FAAN; Temitayo Oyegbile-Chidi, MD, PhD; Barney J. Stern, MD, FAAN; and Andrew R. Spector, MD
Editorial Career Path for Educators
4:30 p.m.–5:00 p.m.
TRAINEE AND EDUCATOR HUB (ROOM 155)
Roy E. Strowd III, MD, MEd, MS, FAAN
All the Current Buzz–Meeting the Editors of Practice
Current and Practice Buzz
5:00 p.m.–5:30 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
Aravind Ganesh, MD, DPhil (OXON), FRCPC
TUESDAY
Utilizing NeuroBytes in Your Residency
12:30 p.m.–1:00 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
Kara Stavros, MD, FAAN, and Muhib Khan, MD, FAAN
Everything You Want to Know About the Residency
In-service Training Examination Exam (RITE)
8:00 a.m.–9:00 a.m.
TRAINEE AND EDUCATOR HUB (ROOM 155)
Raymond Price, MD, FAAN; Sudha Kessler, MD; Sashank Prasad, MD; and Lynne Shindoll, MA
The Peer Review Process and Addressing the Comments of the Reviewers
2:00 p.m.–2:45 p.m
RESEARCH HUB (NORTH LOBBY)
José G. Merino, MD, MPhil, FAAN; Olga Ciccarelli, MD, PhD, FRCP; Anthony A. Amato, MD, FAAN; Josep O. Dalmau, MD, PhD, FAAN; Massimo Pandolfo, MD, FAAN; Bradford B. Worrall, MD
Meet New Continuum® Editor-in-Chief, Lyell K. Jones, Jr., MD, FAAN
3:00 p.m.–4:00 p.m.
ACADEMIC HUB (SOUTHEAST LOBBY C: LEVEL 1)
Lyell K. Jones, Jr., MD, FAAN
How to Write a Good Peer Review
5:00 p.m.–5:30 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
Whitley Warfield Aamodt, MD, MPH; Nadia Khalil, MD; Jonathan Read Gaillard, MD, MHS; Galina Gheihman, MD; Stephanie Bridget SycMazurek, MD, PhD; and Joaquin Augusto Vizcarra, MD
20 Monday, April 24, 2023 • Annual Meeting Daily
Today’s Invited Science Session Focuses on Neuro-oncology

The Invited Science Session on neuro-oncology features authors giving encore presentations of top abstracts presented at subspecialty meetings. The session is presented in partnership with the Society for Neuro-Oncology. Select abstracts will emphasize basic, clinical, and translational science as they evolve toward a more complete understanding of neuro-oncology with the overall goal of developing more effective prevention and treatment.
Abstracts and authors include:
Glioma-induced Neuronal Remodeling Promotes Regional Immunosuppression
3:30 p.m.–3:50 p.m.
Takahide Nejo
First-in-children Phase 1 Trial of Indoximod-based Chemo-immunotherapy for Patients with Pediatric Brain Tumors: Analysis of Safety, Tolerability, and 5-year Outcome
3:50 p.m.–4:10 p.m.
Theodore Johnson
Multilamellar mRNA Lipid Particles Induce Immunologic Reprogramming in Canine and Human Glioblastoma Patients
4:10 p.m.–4:30 p.m.
Elias Sayour
Industry Therapeutic Update from Sanofi Medical Livestreaming is available on the AAN virtual platform
Hot Topics in Smoldering Disease and Future Targets in Multiple Sclerosis

Monday April 24th 6:00PM EST
Doors open at 5:30PM EST, Dinner will be served
Westin Waterfront Grand Ballroom AB
Notch3 Drives Meningioma Tumorigenesis and Resistance to Radiotherapy
4:30 p.m.–4:50 p.m.
Martha Cady
Mechanisms of Glioblastoma-induced Cortical Remodeling Identify Therapeutic Vulnerabilities
4:50 p.m.–5:10 p.m.
Saritha Krishna
Selective Targeting of Immune-suppressive Leukocytes to Reprogram the GBM Immune Landscape
5:10 p.m.–5:30 p.m.
Kyuson Yun
You’ll hear from:
Stephen Krieger MD, FAAN (Chair)
Jacqueline Nicholas MD, MPH

Benjamin Greenberg MD, MHS, FANA, FAAN, CRND

Visit
This program is NOT accredited for continuing education by any organization. Additionally, Industry Therapeutic Updates program content and the views expressed herein are those of the presenting corporate entity and not of the AAN. These programs are not an official part of the 2023 AAN Annual Meeting education or scientific programs, nor are they endorsed by the AAN. The AAN cannot affirm claims pertaining to FDA off-label medication, research use of pre-FDA drugs, or other research information that might be discussed. Industry Therapeutic Updates are industry events. ©2023 Genzyme Corporation. All rights reserved. Sanofi is a registered trademark of Sanofi or an affiliate. MAT-US-2300580 v1.0-P Expiration Date 04/30/2023
Monday, April 24, 2023
Annual
to learn more about Sanofi
our Medical Booth (1962)
Neurology.
•
Meeting Daily 21
Don’t Miss This Afternoon’s Brainstorm Competition and Networking Crawl
All Annual Meeting attendees are invited to the Exhibit Hall in Hall A and B1 between 4:00 p.m. and 6:00 p.m. for these not-to-be-missed events:
BRAINSTORM COMPETITION IN THE INNOVATION HUB
Watch as your neurology colleagues compete in front of a panel of judges as they present their innovative ideas in response to the question: “How can neurologists who are just beginning their career use their knowledge/experiences of the world as it is to transform health care systems in a way that enhances physician career satisfaction and improves patient outcomes? Are there innovations being thought of or implemented by your practice that could help improve engagements or outcomes?” This exciting competition will see one neurologist walk away with a grand prize of $1,500!
NETWORKING CRAWL IN THE EXHIBIT HALL
Don’t miss this energizing—and delicious—opportunity to network with colleagues and learn about the latest tools, technologies, products, services, and resources in the industry while enjoying wine and appetizers. Bring a friend, pick up a map, and set out on your funfilled exploration through the hall.
Thank you to the Exhibit Hall Networking Crawl Sponsors: Corium, Grifols, and REATA.
Start Your Neurology Journey at Tonight’s Trainee and Faculty Networking Reception
Medical students, residents, and fellows on their neurology journey won’t want to miss tonight’s Trainee and Faculty Networking Reception where they’re sure to gain new perspectives and insights and make lasting connections that could help further their career development in academic neurology, research, or practice. Taking place from 6:00 p.m. to 8:00 p.m. at the Omni Seaport Hotel, the evening will be an excellent opportunity for trainees to meet one-onone with both peers and seasoned program and clerkship directors

to ask questions and learn about traversing the world of neurology. Program and clerkship directors are also encouraged to attend to connect with peers and discuss their programs and education techniques.
Faculty representatives from more than 90 institutions will be on hand to share information about their programs and discuss the prospects of clerkships, residencies, fellowships, and other neurology career opportunities.
22 Monday, April 24, 2023 • Annual Meeting Daily
Rare Inspiration. Changing Lives.
Alexion is now investigating 2 new treatments for generalized myasthenia gravis (gMG).


For over 30 years, Alexion’s mission has been to deepen our understanding and transform the lives of people who are affected by rare diseases and living with devastating conditions. We bring this same steadfast commitment to patients with generalized myasthenia gravis (gMG).
ALXN2050. The ExpanD Study is a phase 2 study evaluating the oral investigational medication ALXN2050 for the treatment of gMG.
ALXN2050 is an inhibitor of complement factor D, a component of the alternative pathway, which is being investigated as a novel mechanism of action for the treatment of adults with gMG.
ALXN1720. The Prevail Study is a phase 3 study evaluating Alexion’s new complement component C5 inhibitor, gefurulimab (ALXN1720), for the treatment of adults with gMG.
Gefurulimab is developed for selfadministration through once-weekly subcutaneous injection, which would provide patients with more autonomy and greater flexibility to benefit from a mechanism of action that is well established.
NCT05218096
NCT05556096


Visit us at Booth #2361 ALXN1720-MG-301_ALXN2050-MG-201_AAN Ad_V1_06FEB2023
What Are Your Colleagues Saying?
SHARE YOUR THOUGHTS AT #AANAM.
Justin Hoskin, MD Neurologist, Barrow Neurological Institute, Phoenix, AZ

First in-person meeting
What brings you to the Annual Meeting?
I’ve been at the Program Directors meeting. I’m an assistant program director. We’re thinking a lot about our application review process with incoming residents, so I’ve been talking to other people about their process. The collaboration. And a big part is with the pandemic over, starting to make some connections.
It’s a Snap to be Included in Inclusive Interactive Photo Wall
To underscore the AAN’s value of inclusion, this year’s meeting includes a fun, interactive, mosaic display of digital photos of participating attendees.
Both on-site and virtual attendees will be able to access the virtual photo booth using a QR code. Once you arrive at the virtual photo booth, you will be able to take a photo or upload a photo from your mobile device or computer. The virtual photo booth will request your name and email address. You will be able to select a frame and add the AAN logo to your picture.


The uploaded photo will be reviewed by a moderator panel and then will flow into the digital picture mosaic, being a live, interactive art exhibit that depicts we are stronger together. Once your photo is uploaded to the digital picture mosaic, you will receive an email with your photo attached, which you are welcome to post on social media using #NeurologyProud
The digital picture mosaic will be searchable: just input colleague names to see their picture and where they are located within the mosaic.
Find the interactive photo wall in the North Lobby.
Trenley Anderson, BA, MM Medical student, Cleveland Heights, OH Medical Student Essay Award Winner— G. Milton Shy Award

Tell us about your essay.
I was on my clinical rotation and the attending started morning rounds with the question “How are the dragons doing?” The word “dragons” was similar to their name and the patient was non-binary. This was taking a jab at the use of non-binary pronouns. The team also had a difficult relationship with the family and the patient. And the patient had a functional disorder, which is difficult. This insensitive comment really stayed with me. Does your poster provide any resolution?
I give some advice from readings I’ve done on how to improve your cultural competency and sensitivity.
JOIN THE
CONVERSATION!
24 Monday, April 24, 2023 • Annual Meeting Daily
Daily Reminders
For easy access to meeting links, visit AAN.com/QuickLinks.
Attendee Lunches and Networking Crawl
Attendee lunches are served from 11:00 a.m. to 1:00 p.m. in the Poster Hall, Exhibit Hall B2. Join us for the Exhibit Hall Networking Crawl from 4:00 p.m. to 6:00 p.m. in Exhibit Hall A for a walking food tour to different delicious stations allowing you to discover new cuisines and new contacts!
Access Past Content
Conference attendees have access to the virtual platform through May 15, 2023. Visit AAN.com/VirtualAM and use your 6-digit ID and password to log in. It may take up to 48 hours after course completion for content to become available online.
Program Slides Available Online
Slides are available online only at AAN.com/Materials or through the AAN Conferences mobile app. You can access program materials through March 1, 2024. (Please note that availability of materials is at the discretion of the specific speaker. Not all sessions will have materials.)
Shuttles
Shuttles depart and drop off from the Convention Center Southeast entrances on Level 0 (near Registration) or Level 1 (near Academic Hub) every 15 to 30 minutes throughout the day and drop off at AAN conference hotels. See more information in the AAN Conferences mobile app or Room Locator. Questions? Visit the Shuttle Help Desk or Meeting Information. Use the QR code to see where your shuttle is.

Submit Evaluations for Annual Meeting CME
Complete your evaluations to get your CME credits by May 15, 2023 (or March 1, 2024 with Annual Meeting On Demand) by using the AAN Conferences mobile app or by visiting AAN.com/AMCME. Transcripts will be available upon evaluation submission. AAN members can also access their transcript via NeuroTracker™ at AAN.com/NeuroTracker.
Want More Time to View Programs?
Add Annual Meeting On Demand to your registration to extend your access to session recordings through March 1, 2024, at a 50-percent discount. Check the back of your badge to see if you already have Annual Meeting On Demand. If not, head to Registration or email aanamsupport@cmrus.com by May 15, 2023, to upgrade.
AANTV Studio
Stop by the AANTV Studio in the North Lobby, where you can witness live interviews being recorded for later broadcast accessible on the virtual platform, TV monitors around the convention center, and on AAN.com and YouTube. The AANTV Studio is sponsored by the AAN Family of Publications.
Safety and COVID-19 Protocols
The AAN continues to encourage attendees to practice safe measures to stop the spread of COVID-19, including using social distancing, testing when appropriate, taking recommended actions when symptomatic or having tested positive, and wearing a mask if you choose. We will continue to follow best practices recommended by the Centers for Disease Control and Prevention and abide by any legal mandates and recommendations from government officials.
View all conference guidelines at AAN.com/ConfGuidelines.
Monday, April 24, 2023 • Annual Meeting Daily 25

Azurity Pharmaceuticals is pleased to be exhibiting at the 2023 AAN Annual Meeting Visit Booth #1785 to learn more about Azurity’s CNS Portfolio Please visit booth for full Prescribing Information including Indications.

Serving Overlooked Patients We adapt established medicines for patients who have needs not met by what is currently available Scan here to learn more © 2023 Azurity Pharmaceuticals, Inc. All Rights Reserved. All trademarks referred to are the property of their respective owner(s). PP-HOR-US-0804
Celebrate These 2023 AAN Scientific Award Recipients
The AAN recognizes the 2023 AAN scientific award recipients and their contributions to the art and science of neurology.
AAN NEURO-INFECTIOUS DISEASE AWARD
Sponsored by the American Academy of Neurology.
Mashina Chomba, MBChB, MMed University Teaching Hospital, Lusaka, Zambia
ALLIANCE AWARDS: FOUNDERS
Sponsored by the American Academy of Neurology and endowed by the former American Academy of Neurology Alliance.
Alexander Sandweiss, MD, PhD Baylor College of Medicine and Texas Children’s Hospital, Houston, TX
ALLIANCE AWARDS: S. WEIR MITCHELL
Sponsored by the American Academy of Neurology and endowed by the former American Academy of Neurology Alliance.
John Pluvinage, MD, PhD
University of California, San Francisco, San Francisco, CA
BRUCE S. SCHOENBERG INTERNATIONAL AWARD IN NEUROEPIDEMIOLOGY
Sponsored by the American Academy of Neurology and endowed by GlaxoSmithKline, Inc.
Jitendra Sahu, DM
Postgraduate Institute of Medical Education and Research, Chandigarh, Chandigarh, India
CAREER DEVELOPMENT AWARD
Funded by the American Academy of Neurology.
Jonathan Brent, MD, PhD
Feinberg School of Medicine, Northwestern University, Chicago, IL
Nathan Cohen, MD
Children’s National Medical Center, Washington, DC
CLINICAL RESEARCH TRAINING SCHOLARSHIP
Funded by the American Academy of Neurology.
Andrew Dhawan, MD, PhD Cleveland Clinic, Cleveland, OH
Anthony Linares, MD, PhD
University of California, Los Angeles, Los Angeles, CA
Martineau Louine, MD
University of California, San Francisco, San Francisco, CA
Evan Madill, MD
Brigham and Women’s Hospital, Boston, MA
CLINICAL RESEARCH TRAINING SCHOLARSHIP IN ALS
Funded by The ALS Association and American Brain Foundation in collaboration with the American Academy of Neurology.
Marina Avetisyan, MD, PhD Massachusetts General Hospital, Boston, MA
CLINICAL RESEARCH TRAINING SCHOLARSHIP IN FTD
Funded by Holloway Family Fund of The Association for Frontotemporal Degeneration and American Brain Foundation in collaboration with the American Academy of Neurology.
Daniel Ohm, PhD
University of Pennsylvania, Philadelphia, PA
CLINICAL RESEARCH TRAINING SCHOLARSHIP IN MIGRAINE
Funded by Amgen, Inc. and American Brain Foundation in collaboration with the American Academy of Neurology.
Patricia Olson, MD, PhD
Brigham and Women’s Hospital, Boston, MA
CLINICAL RESEARCH TRAINING SCHOLARSHIP IN NEURODISPARITIES
Funded by the Edgar J. Kenton III, MD Foundation; Eisai; and American Brain Foundation in collaboration with the American Academy of Neurology.
Dominique Popescu, PhD
Massachusetts General Hospital, Boston, MA
CLINICAL RESEARCH TRAINING SCHOLARSHIP IN NEUROMUSCULAR DISEASE
Funded by the Neuromuscular Study Group and American Brain Foundation in collaboration with the American Academy of Neurology.
Natalie Katz, MD, PhD Duke University, Durham, NC
CLINICAL RESEARCH TRAINING SCHOLARSHIP IN PARKINSON’S DISEASE
Funded by the Parkinson’s Foundation and American Brain Foundation in collaboration with the American Academy of Neurology.
Robert Heuermann, MD, PhD Washington University, St. Louis, MO
CLINICAL RESEARCH TRAINING SCHOLARSHIP IN PERIPHERAL NEUROPATHY
Funded by the Foundation for Peripheral Neuropathy through the American Brain Foundation in collaboration with the American Academy of Neurology.
Paula Barreras, MD
Cedars-Sinai Medical Center, Los Angeles, CA
Erika Williams, MD, PhD
Brigham and Women’s Hospital, Boston, MA
DREIFUSS-PENRY EPILEPSY AWARD
Sponsored by the American Academy of Neurology and endowed by members of the American Academy of Neurology Epilepsy Section; Abbott Laboratories, Inc.; Cephalon, Inc.; Cyberonics, Inc.; Elan Corporation; GlaxoSmithKline, Inc.; Novartis; OrthoMcNeil Pharmaceutical, Inc.; Pfizer Inc; Shire Pharmaceuticals Group; and UCB Pharma.
Jonathan Kleen, MD, PhD
University of California, San Francisco, San Francisco, CA
H. RICHARD TYLER AWARD
Sponsored by the American Academy of Neurology and the American Academy of Neurology History Section.
Alison Christy, MD, PhD
Providence Health and Services, Northern Oregon Region, Portland, OR
HEALTH CARE EQUITY RESEARCH AWARD
Sponsored by the American Academy of Neurology.
Jordan J. Cole, MD
Washington University, St. Louis, MO
Bruce Ovbiagele, MD, MSc, MAS, MBA, MLS, FAAN
San Francisco VA Medical Center, San Francisco, CA
Nicole Rosendale, MD
University of California, San Francisco, San Francisco, CA
IRWIN SCHATZ AWARD FOR AUTONOMIC DISORDERS
Sponsored by the American Academy of Neurology and endowed by Lundbeck, Inc.
Paola Sandroni, MD, PhD, FAAN
Mayo Clinic, Rochester, MN
28 Monday, April 24, 2023 • Annual Meeting Daily
JOHN DYSTEL PRIZE FOR MULTIPLE SCLEROSIS RESEARCH
Sponsored by the American Academy of Neurology and National Multiple Sclerosis Society and made possible through a special contribution from the John Dystel Multiple Sclerosis Research Fund at the National Multiple Sclerosis Society.
Roland Martin, MD
University of Zurich, Zurich, Switzerland
JON STOLK AWARD IN MOVEMENT DISORDERS FOR YOUNG INVESTIGATORS
Sponsored by the American Academy of Neurology and endowed by Kyowa Pharmaceutical, Inc., Lineberry Research, Quintiles, Dr. Dennis Gillings, and VelaPharma.
Farwa Ali, MBBS
Mayo Clinic, Rochester, MN
LAWRENCE C. MCHENRY: AN AWARD FOR THE HISTORY OF NEUROLOGY
Sponsored by the American Academy of Neurology.
Kelsey Smith, MD
Mayo Clinic, Rochester, MN
LAWRENCE M. BRASS STROKE RESEARCH AWARD
Funded by the American Heart Association and the American Brain Foundation, in collaboration with the American Academy of Neurology.
Margy McCullough-Hicks, MD
University of Minnesota, Minneapolis, MN
MCKNIGHT CLINICAL TRANSLATIONAL RESEARCH SCHOLARSHIP IN COGNITIVE AGING AND AGE-RELATED MEMORY LOSS
Funded by the McKnight Brain Research Foundation through the American Brain Foundation, and the American Academy of Neurology.
Eva Klinman, MD, PhD
Washington University, St. Louis, MO
Sheena Baratono, MD, PhD
Brigham and Women’s Hospital, Boston, MA
MEDICAL STUDENT ESSAY
AWARDS: G. MILTON SHY
Sponsored by the American Academy of Neurology.
Trenley Anderson, BA, MM
Case Western Reserve University, Cleveland Heights, OH
MEDICAL STUDENT ESSAY
AWARDS: LEWIS P. ROWLAND
Sponsored by the American Academy of Neurology.
Sumita Strander, BA
Harvard Medical School, Brookline, MA
MEDICAL STUDENT ESSAY
AWARDS: ROLAND P. MACKAY
Sponsored by the American Academy of Neurology.
Zabrina Reyes, BS
Tulane University School of Medicine, New Orleans, LA
MEDICAL STUDENT ESSAY AWARDS: SAUL R. KOREY
Sponsored by the American Academy of Neurology.
Alexandra Miner, BA, MS Virginia Tech Carilion School of Medicine, New York, NY
MICHAEL S. PESSIN STROKE LEADERSHIP PRIZE
Sponsored by the American Academy of Neurology and endowed by Dr. Pessin’s family, friends, and colleagues.
Neal Parikh, MD, MS CTNU-Weill Cornell Medicine, New York, NY
MOVEMENT DISORDERS RESEARCH AWARD
Sponsored by the American Academy of Neurology, the Parkinson’s Foundation, and the American Academy of Neurology Movement Disorders Section and endowed by the Parkinson’s Foundation.
Jill Ostrem, MD, FAAN
University of California, San Francisco, San Francisco, CA
NEURO-ONCOLOGY INVESTIGATOR AWARD
Sponsored by the American Academy of Neurology and supported by friends of Dr. Jerome Posner.
Karisa Schreck, MD, PhD Johns Hopkins University, Baltimore, MD
NEURO-ONCOLOGY SCIENTIFIC AWARD
Sponsored by the American Academy of Neurology and supported by friends of Dr. WK Alfred Yung.
Ingo Mellinghoff, MD, FACP
Memorial Sloan Kettering Cancer Center, New York, NY
NEUROSCIENCE RESEARCH PRIZE
Sponsored by the American Academy of Neurology.
Pulkith Paruchuri
Heritage High School, Frisco, TX
Samantha Schaevitz
Byram Hills High School, Bedford, NY
Maxx Yung
Roslyn High School, Roslyn, NY
NEUROSCIENCE RESEARCH PRIZE IN CHILD NEUROLOGY
Sponsored by the American Academy of Neurology and the Child Neurology Society.
Rania Lateef
Charles J. Colgan High School, Manassas, VA
NEUROSCIENCE RESEARCH TRAINING SCHOLARSHIP
Funded by the American Academy of Neurology.
Vishnu Cuddapah, MD, PhD
Children’s Hospital of Philadelphia, Philadelphia, PA
Sharan Srinivasan, MD, PhD
University of Michigan, Ann Arbor, MI
NORMAN GESCHWIND PRIZE IN BEHAVIORAL NEUROLOGY
Sponsored by the American Academy of Neurology and endowed through Dr. Geschwind’s family, friends, and colleagues; Pfizer Inc; and the Society for Behavioral and Cognitive Neurology.
Liliana Ramirez-Gomez, MD
Wang Ambulatory Care Center, Boston, MA
POTAMKIN PRIZE FOR RESEARCH IN PICK’S, ALZHEIMER’S, AND RELATED DISEASES
Sponsored by the American Academy of Neurology and the American Brain Foundation and funded through the philanthropy of the Potamkin family.
Maria Gorno Tempini, MD, PhD
University of California, San Francisco, San Francisco, CA
PRACTICE RESEARCH TRAINING SCHOLARSHIP
Funded by the American Academy of Neurology.
Nirupama Yechoor, MD, MSc
Massachusetts General Hospital, Boston, MA
Continued on page 30 ⊲ Monday, April 24, 2023 • Annual Meeting Daily 29
Celebrate These 2023 AAN Scientific Award Recipients continued from page 29
RICHARD OLNEY CLINICIAN SCIENTIST DEVELOPMENT AWARD IN ALS
Funded by The ALS Association and American Brain Foundation in collaboration with the American Academy of Neurology.
Maurizio Grassano, MD
University of Turin, Turin, Italy
SCIENTIFIC BREAKTHROUGH AWARD
Sponsored by the American Brain Foundation
Josep O. Dalmau, MD, PhD, FAAN
University of Barcelona, University of Pennsylvania, Barcelona, Catalonia, Spain
Vanda Lennon, MD, PhD
Mayo Clinic, Rochester, MN
SHEILA ESSEY AWARD: AN AWARD FOR ALS RESEARCH
Presented by the AAN and the ALS Association and supported through the philanthropy of the Essey family through the American Brain Foundation and the ALS Association.
Virginia Lee, PhD, MBA
University of Pennsylvania, Philadelphia, PA
SLEEP SCIENCE AWARD
Sponsored by the American Academy of Neurology and the Sleep Section and endowed by Cephalon, Inc.
Tiffany Braley, MD, MS
University of Michigan Medical Center, Ann Arbor, MI
SUSAN S. SPENCER, MD, CLINICAL RESEARCH TRAINING SCHOLARSHIP IN EPILEPSY
Funded by the American Epilepsy Society, Epilepsy Foundation, and American Brain Foundation in collaboration with the American Academy of Neurology.
Wesley Kerr, MD, PhD
University of Pittsburgh, Philadelphia, PA
WAYNE A. HENING SLEEP MEDICINE INVESTIGATOR AWARD
Sponsored by the American Academy of Neurology and endowed by UCB, Inc., Lilly USA, Elite Home Medical & Respiratory, Inc., Raleigh Neurology Associates, and friends of Dr. Wayne A. Hening.
Joseph Cheung, MD, MS Mayo Clinic, Jacksonville, FL
INDUSTRY THERAPEUTIC UPDATE FROM UCB
SHARPENING THE LENS

A FOCUS ON MYASTHENIA GRAVIS CARE
Tuesday, April 25, 2023 | 11:45–12:45 ET Room 052B, Boston Convention and Exhibitor Center, 415 Summer Street, Boston, MA

Lunch will be provided
This program is NOT accredited for continuing education by any organization
The program content and the views expressed herein are those of the representing corporate entity and not of the AAN. This program is not an official part of the 2023 AAN Annual Meeting education or scientific program, nor is it endorsed by the AAN. The AAN cannot affirm claims pertaining to FDA off-label medication, research use of pre- FDA drugs, or other research information that might be discussed. Industry Therapeutic Updates are industr y events © 2023 UCB, Inc., Smyrna, GA 30080. All rights reserved. Date of preparation: February 2023. US-N-DA-MG-2300009
JAMES F. HOWARD, JR. MD UNC School of Medicine Chapel Hill, NC USA
NEELAM GOYAL, MD Stanford Health Care Palo Alto, CA USA
MICHAEL SLAMA, MD, PHD Saint Elizabeth’s Medical Center Boston, MA USA
TO CALENDAR
ADD
Today’s Neuroscience in the Clinic Session to Address Biomarkers for Outcome Prediction in Traumatic Brain Injury




Today between 1:00 p.m. and 3:00 p.m. join H. E. Hinson, MD, MCR, FAAN, and Stefania Mondello, along with expert faculty Karnig Kazazian and Nasser Mohammed, MBBS, as they discuss the evolving landscape of fluid-based biomarkers after traumatic brain injury (TBI), what markers may have clinical applications, and what markers are under investigation and not ready for clinical use. Presenters will also discuss how fluid-based biomarkers can be incorporated into multi-modal prognostication after TBI, and recognize emerging methods for new biomarker identification and validation. The session will conclude with a panel discussion along with an opportunity for questions. Attendees may claim two CME credits.

MEM: 22 Member Dues Renewal Ad—Half Page Horizontal> AN Placed in AANnews 8.25 x 5.25 +0.125 bleed, 4C WHEN YOU NEED IT Only AAN membership offers access to the highest quality resources— when you need them—from the world’s largest and most trusted community of neurology professionals. Join or renew today. AAN.com/Membership WHAT YOU NEED Research Advocacy Professional Growth Practice Management Community Education Wellness
Hinson Mondello
Kazazian Mohammed
Kiran Thakur, MD, FAAN, discussed neurological implications of long COVID at a Neuroscience in the Clinic Session at the 2022 Annual Meeting.
Why are clinical trials so slow?

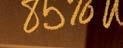


















Ask bigger questions. gene.com/askbiggerquestions










































 (Front) AAN President Kenneth M. Viste, Jr., MD, FAAN; (Rear from left) President Elect Steven P. Ringel, MD, FAAN; Past President Jack P. Whisnant, MD, FAAN; NINDS Director Zach Hall, PhD; Sid Gilman, MD, FAAN. Circa 1995.
Pearce Bailey, MD, FAAN
(Front) AAN President Kenneth M. Viste, Jr., MD, FAAN; (Rear from left) President Elect Steven P. Ringel, MD, FAAN; Past President Jack P. Whisnant, MD, FAAN; NINDS Director Zach Hall, PhD; Sid Gilman, MD, FAAN. Circa 1995.
Pearce Bailey, MD, FAAN



























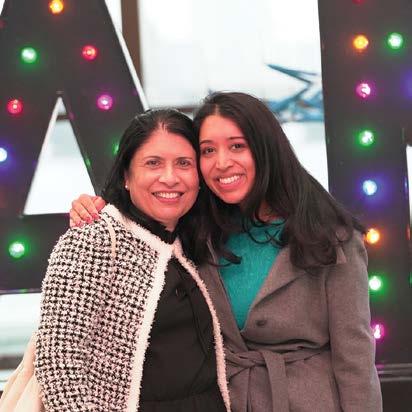






 Rost Spatola
Ambalathingal Spencer
Rost Spatola
Ambalathingal Spencer































