Tuesday, April 25, 2023
5 Landmark Clinical Trials Showcased in This Morning’s Plenary
20 Movement Disorders Highlighted in Invited Science Session

21 Second Emerging Science Session to Take Place Tonight

DAYBUE ™ is now FDA approved to treat Rett syndrome 1
DAYBUE is the first and only FDA-approved treatment for Rett syndrome (RTT) in adult and pediatric patients 2 years and older.1,2 RTT is a complex neurodevelopmental disorder with symptoms that can impact the ability to function.3-5
Attend
DAYBUE presentations
1 pm to 1:20 pm in-booth presentation
1 pm to 1:20 pm
Emerging Neurologic Care Presentation Stage (in the Exhibit Hall)
Indication
DAYBUE is indicated for the treatment of Rett syndrome in adults and pediatric patients 2 years of age and older.
Important Safety Information
• Warnings and Precautions
– Diarrhea: In a 12-week study and in long-term studies, 85% of patients treated with DAYBUE experienced diarrhea. In those treated with DAYBUE, 49% either had persistent diarrhea or recurrence after resolution despite dose interruptions, reductions, or concomitant antidiarrheal therapy. Diarrhea severity was of mild or moderate severity in 96% of cases. In the 12-week study, antidiarrheal medication was used in 51% of patients treated with DAYBUE.
– Patients should stop taking laxatives before starting DAYBUE. If diarrhea occurs, patients should notify their healthcare provider, consider starting antidiarrheal treatment, and monitor hydration status and increase oral fluids, if needed. Interrupt, reduce dose, or discontinue DAYBUE if severe diarrhea occurs or if dehydration is suspected.
– Weight Loss: In the 12-week study, 12% of patients treated with DAYBUE experienced weight loss of greater than 7% from baseline, compared to 4% of patients who received placebo. In long-term studies, 2.2% of patients discontinued treatment with DAYBUE due to weight loss. Monitor weight and interrupt, reduce dose, or discontinue DAYBUE if significant weight loss occurs.
• Adverse Reactions: The common adverse reactions (≥5% for DAYBUE-treated patients and at least 2% greater than in placebo) reported in the 12-week study were diarrhea (82% vs 20%), vomiting (29% vs 12%), fever (9% vs 4%), seizure (9% vs 6%), anxiety (8% vs 1%), decreased appetite (8% vs 2%), fatigue (8% vs 2%), and nasopharyngitis (5% vs 1%).
• Drug Interactions: Effect of DAYBUE on other Drugs
– DAYBUE is a weak CYP3A4 inhibitor; therefore, plasma concentrations of CYP3A4 substrates may be increased if given concomitantly with DAYBUE. Closely monitor when DAYBUE is used in combination with orally administered CYP3A4 sensitive substrates for which a small change in substrate plasma concentration may lead to serious toxicities.
– Plasma concentrations of OATP1B1 and OATP1B3 substrates may be increased if given concomitantly with DAYBUE. Avoid the concomitant use of DAYBUE with OATP1B1 and OATP1B3 substrates for which a small change in substrate plasma concentration may lead to serious toxicities.
• Use in Specific Population: Renal Impairment
– DAYBUE is not recommended for patients with moderate or severe renal impairment. DAYBUE is available as an oral solution (200mg/mL).
Read the Brief Summary of full Prescribing Information on the following page.
References: 1. Acadia Pharmaceuticals Inc. DAYBUE [Package Insert]. San Diego, CA, 2023. 2. Acadia Pharmaceuticals announces U.S. FDA approval of DAYBUE™ (trofinetide) for the treatment of Rett syndrome in adult and pediatric patients two years of age and older. [press release]. Acadia Pharmaceuticals Inc. March 10, 2023. 3. Fu C, Armstrong D, Marsh E, et al. Consensus guidelines on managing Rett syndrome across the lifespan. BMJ Paediatr Open. 2020;4(1):e000717. 4. Neul JL, Kaufmann WE, Glaze DG, et al for the RettSearch Consortium. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68(6):944-950. 5. Anderson A, Wong K, Jacoby P, et al. Twenty years of surveillance in Rett syndrome: what does this tell us? Orphanet J Rare Dis. 2014;9:87. 6. International Rett Syndrome Foundation. IRSF expands center of excellence network. Accessed March 14, 2023. https://www.rettsyndrome.org
©2023 Acadia Pharmaceuticals Inc. Acadia is a registered trademark and DAYBUE is a trademark of Acadia Pharmaceuticals Inc. All rights reserved. DAY-0082 03/23
with
1 pm to 1:20 pm in-booth presentation 4/23 4/24 4/25
Caroline (Carrie) Buchanan, MD, of the Greenwood Genetic Center, a Rett syndrome Center of Excellence 6
Come see us at Booth #1384 to learn more about Rett syndrome and DAYBUE
DAYBUE™ (trofinetide) oral solution
Rx Only
Brief Summary: This information is not comprehensive. Visit www.DAYBUEhcp.com to obtain the full Prescribing Information or call 1-844-422-2342
1 INDICATIONS AND USAGE
DAYBUE is indicated for the treatment of Rett syndrome in adults and pediatric patients 2 years of age and older.
2 DOSAGE AND ADMINISTRATION
Administer DAYBUE orally twice daily, in the morning and evening, according to patient weight as shown in the table below. DAYBUE can be taken with or without food.
Recommended Dosage of DAYBUE in Patients
2 years of age and older
Patient Weight DAYBUE Dosage DAYBUE Volume
9 kg to <12 kg 5,000 mg twice daily 25 mL twice daily
≥12 kg to <20 kg 6,000 mg twice daily 30 mL twice daily
≥20 kg to <35 kg
8,000 mg twice daily 40 mL twice daily
≥35 kg to <50 kg 10,000 mg twice daily 50 mL twice daily
≥50 kg 12,000 mg twice daily 60 mL twice daily
Administration Information
Administer DAYBUE orally or via gastrostomy (G) tube; doses administered via gastrojejunal (GJ) tubes must be administered through the G-port. A calibrated measuring device, such as an oral syringe or oral dosing cup, should be obtained from the pharmacy to measure and deliver the prescribed dose accurately. A household measuring cup is not an adequate measuring device. Discard any unused DAYBUE oral solution after 14 days of first opening the bottle.
Missed Dose or Vomiting After Administration
If a dose of DAYBUE is missed, the next dose should be taken as scheduled. Doses should not be doubled. If vomiting occurs after DAYBUE administration, an additional dose should not be taken. Instead, continue with the next scheduled dose.
Dose Modification for Diarrhea or Weight Loss
Advise patients to stop laxatives before starting DAYBUE. Interrupt, reduce the dosage, or discontinue DAYBUE if severe diarrhea occurs, if dehydration is suspected, or if significant weight loss occurs.
5 WARNINGS AND PRECAUTIONS
Diarrhea
In a 12-week randomized, double-blind, placebocontrolled study (Study 1) and in long-term studies, 85% of patients treated with DAYBUE experienced diarrhea. In those treated with DAYBUE, 49% either had persistent diarrhea or recurrence after resolution despite dose interruptions, reductions, or concomitant antidiarrheal therapy. Diarrhea severity was of mild or moderate severity in 96% of cases. In Study 1, antidiarrheal medication was used in 51% of patients treated with DAYBUE.
Advise patients to stop laxatives before starting DAYBUE. If diarrhea occurs, patients should notify their healthcare provider, consider starting antidiarrheal treatment, and monitor hydration status and increase oral fluids, if needed. Interrupt, reduce dose, or discontinue DAYBUE if severe diarrhea occurs or if dehydration is suspected.
Weight Loss
In Study 1, 12% of patients treated with DAYBUE experienced weight loss of greater than 7% from baseline, compared to 4% of patients who received placebo. In long-term studies, 2.2% of patients discontinued treatment with DAYBUE due to weight loss. Monitor weight and interrupt, reduce dose, or discontinue DAYBUE if significant weight loss occurs.
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
• Diarrhea [see Warnings and Precautions]
• Weight Loss [see Warnings and Precautions]
Clinical trial experience
In controlled and uncontrolled trials in patients with Rett syndrome, 260 patients ages 2 to 40 years were treated with DAYBUE, including 109 patients treated for more than 6 months, 69 patients treated for more than 1 year, and 4 patients treated for more than 2 years.
Adult and Pediatric Patients with Rett Syndrome 5 Years of Age and Older
The safety of DAYBUE was evaluated in a randomized, double-blind, placebo-controlled, 12-week study of patients with Rett syndrome (Study 1). In Study 1, 93 patients received DAYBUE, and 94 patients received placebo. All patients were female, 92% were White, and the mean age was 11 years (range 5 to 20 years).
Adverse Reactions Leading to Discontinuation of Treatment
Eighteen patients (19%) receiving DAYBUE had adverse reactions that led to withdrawal from the study. The most common adverse reaction leading to discontinuation of treatment with DAYBUE was diarrhea (15%).
Common Adverse Reactions
Adverse reactions that occurred in Study 1 in at least 5% of patients treated with DAYBUE and were at least 2% more frequent than in patients on placebo are presented in the table below.
Adverse Reactions in at Least 5% of Patients
Treated With DAYBUE and at Least 2% Greater than Placebo in Study 1
mother’s clinical need for DAYBUE and any potential adverse effects on the breastfed infant from DAYBUE or from the underlying maternal condition.
Pediatric Use
The safety and effectiveness of DAYBUE for the treatment of Rett syndrome have been established in pediatric patients aged 2 years and older. The safety and effectiveness of DAYBUE for the treatment of Rett syndrome in pediatric patients 5 years of age and older was established in Study 1, which included 108 pediatric patients age 5 to less than 12 years of age and 47 pediatric patients age 12 to less than 17 years of age. Use of DAYBUE in patients 2 to 4 years of age is supported by evidence from Study 1 and pharmacokinetic and safety data in 13 pediatric patients 2 to 4 years of age treated with DAYBUE for 12 weeks.
Safety and effectiveness in pediatric patients less than 2 years of age have not been established.
Geriatric Use
Clinical studies of DAYBUE did not include patients 65 years of age and older to determine whether or not they respond differently from younger patients. This drug is known to be substantially excreted by the kidney. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function.
Renal Impairment
No dedicated clinical study has been conducted to evaluate the pharmacokinetics of DAYBUE in subjects with renal impairment. Since the drug is eliminated mainly through the kidney, administration of DAYBUE to patients with moderate or severe renal impairment is not recommended.
16 Storage and Handling
Store DAYBUE in an upright position refrigerated at 2°C to 8°C (36°F to 46°F). Do not freeze. Keep the child-resistant cap tightly closed. Discard any unused DAYBUE oral solution after 14 days of first opening the bottle.
17 PATIENT COUNSELING INFORMATION
Advise the caregiver or patient to read the FDAapproved patient labeling (Patient Information).
Pediatric Patients With Rett Syndrome 2 to 4 Years of Age
In an open-label study in pediatric patients 2 to 4 years of age with Rett syndrome, a total of 13 patients received DAYBUE for at least 12 weeks and 9 patients received DAYBUE for at least 6 months. Adverse reactions in pediatric patients 2 to 4 years of age treated with DAYBUE were similar to those reported in adult and pediatric patients 5 years of age and older with Rett syndrome in Study 1.
7 DRUG INTERACTIONS
Effect of DAYBUE on Other Drugs
Trofinetide is a weak CYP3A4 inhibitor; therefore, plasma concentrations of CYP3A4 substrates may be increased if given concomitantly with DAYBUE. Closely monitor when DAYBUE is used in combination with orally administered CYP3A4 sensitive substrates for which a small change in substrate plasma concentration may lead to serious toxicities.
Plasma concentrations of OATP1B1 and OATP1B3 substrates may be increased if given concomitantly with DAYBUE. Avoid the concomitant use of DAYBUE with OATP1B1 and OATP1B3 substrates for which a small change in substrate plasma concentration may lead to serious toxicities.
8 USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
There are no adequate data on the developmental risks associated with the use of DAYBUE in pregnant women. No adverse developmental effects were observed following oral administration of trofinetide to pregnant animals at doses associated with plasma exposures below those used clinically.
Lactation
Risk Summary
There is no information regarding the presence of trofinetide or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the
DAYBUE Administration
Advise the caregiver or patient that DAYBUE may be given orally or via gastrostomy (G) tube; doses administered via gastrojejunal (GJ) tubes must be administered through the G-port. DAYBUE may be taken with or without food.
Instruct the caregiver or patient to obtain a calibrated measuring device, such as an oral syringe or oral dosing cup, from the pharmacy to measure and deliver the prescribed dose accurately. A household measuring cup is not an adequate measuring device. Instruct the caregiver or patient to discard any unused DAYBUE after 14 days of first opening the bottle.
Diarrhea
Advise the caregiver or patient that DAYBUE can cause diarrhea. Instruct the patient to stop taking laxatives before starting DAYBUE. If diarrhea occurs, patients should notify their healthcare provider, consider starting antidiarrheal treatment, and monitor hydration status and increase oral fluids, if needed [see Warnings and Precautions]
Weight Loss
Inform the caregiver or patient that DAYBUE may cause weight loss and to notify their healthcare provider if weight loss occurs [see Warnings and Precautions]
Vomiting
Advise the caregiver or patient that DAYBUE can cause vomiting and if vomiting occurs after DAYBUE administration, do not take an additional dose, but continue with the next scheduled dose.
Storage
Keep bottles of DAYBUE oral solution upright and refrigerated before and after opening. Do not freeze [see Storage and Handling]
Marketed by: Acadia Pharmaceuticals Inc. San Diego, CA 92130 USA
©2023 Acadia Pharmaceuticals Inc. DAYBUE is a trademark of Acadia Pharmaceuticals Inc. All rights reserved. DAY-0040 03/23
Adverse Reaction DAYBUE (N=93) % Placebo (N=94) % Diarrhea 82 20 Vomiting 29 12 Fever 9 4 Seizure 9 6 Anxiety 8 1 Decreased appetite 8 2 Fatigue 8 2 Nasopharyngitis 5 1
Meeting-at-a-Glance
Tuesday, April 25, 2023
The Vision of the American Academy of Neurology is to be indispensable to our members.
The Mission of the American Academy of Neurology is to promote the highest quality patient-centered neurologic care and enhance member career satisfaction.
American Academy of Neurology
201 Chicago Avenue
Minneapolis, MN 55415 USA
Phone: (800) 879-1960 (Toll Free) or (612) 928-6000 (International)
Fax: (612) 454-2744
Email: memberservices@aan.com
Website: AAN.com
AAN Chief Executive Officer: Mary E. Post, MBA, CAE
Managing Editor:
Angela M. Babb, MS, CAE, APR
Editor: Tim Streeter
Writers: Ryan Knoke, Sarah Parsons
Designer: Andrew Imholte
Photography: Will Evans
Annual Meeting Daily is published by the American Academy of Neurology.
The American Academy of Neurology’s registered trademarks and service marks are registered in the United States and various other countries around the world. “American Brain Foundation” is a registered service mark of the American Brain Foundation and is registered in the United States.
7 a.m. 8 a.m. 9 a.m. 10 a.m. 11 a.m. 12 p.m. 1 p.m. 2 p.m. 3 p.m. 4 p.m. 5 p.m. 6 p.m. 7 p.m. 8 p.m. 9 p.m. 10 p.m.
Run/Walk for Brain Research 6:30–8:00 a.m. Poster Session 7 8:00–9:00 a.m. Courses C114–C122 7:00–9:00 a.m. Hubs 7:00–9:00 a.m. Clinical Trials Plenary Session 9:15–11:30 a.m. Exhibit Hall 11:30 a.m.–4:00 p.m. Hubs 11:00 a.m.–5:30 p.m. Poster Session 8 11:45 a.m–12:45 p.m. Industry Therapeutic Updates 11:45 a.m–12:45 p.m. Courses C123–C149 1:00–5:30 p.m. Invited Science | Scientific Sessions S23–S27 1:00–3:00 p.m. Attendee Happy Hour 4:30–5:30 p.m. Industry Therapeutic Updates 6:00–9:00 p.m.
and end times may vary. Check with each hosting company for further information.
*Start
Poster Session 9 | Emerging Science Session 2 5:30–7:00 p.m. Neuroscience in the Clinic Session | Scientific Sessions S28–S32 3:30–5:30 p.m.
Clinical Trials Frontiers
Landmark Clinical Trials Showcased in This Morning’s Plenary



The Clinical Trials Plenary Session is set to take place this morning from 9:15 a.m. to 11:30 a.m. ET in the Grand Ballroom. Experts will highlight the latest landmark results from clinical trials that are impacting the landscape of patient care.
The session will be moderated by:
H. E. Hinson, MD, MCR, FAAN
Reena Parada Thomas, MD

Safety and Efficacy of Ataluren in nmDMD
Patients from Study 041, a Phase 3, Randomized, Double-blind, Placebocontrolled Trial

Jeffrey Statland, MD
University of Kansas Medical Center, Kansas City, KS
Results from the First Four Regimens of the HEALEY ALS Platform Trial


Sabrina Paganoni, MD, PhD

Massachusetts General Hospital, Boston, MA
Efficacy and Safety of Zavegepant Nasal Spray for the Acute Treatment of Migraine:
Results of a Phase 3 Double-blind, Randomized, Placebo-controlled Trial

Richard B. Lipton, MD, FAAN Albert Einstein College of Medicine, Bronx, NY
VISIONARY-MS Top Line Results: A Phase 2, Randomized, Double-blind, Parallel Group, Placebo-controlled Study to Assess the Safety and Efficacy of CNM-Au8, a Catalytically Active Gold Nanocrystal Suspension in Relapsing Multiple Sclerosis

Michael Barnett, MBBS
University of Sydney, Camperdown, Australia
A Study to Confirm Safety and Efficacy of Lecanemab in Participants with Early Alzheimer’s Disease (Clarity AD)














Christopher Van Dyck, MD Yale University, New Haven, CT

Endovascular Treatment for Acute Basilar Artery Occlusion: A Multicenter Randomized-controlled Trial (ATTENTION)
Raul Nogueira, MD
Grady Memorial Hospital, Atlanta, GA

Topline Results of the PROOF-HD Pivotal Phase 3 Trial: Pridopidine’s Outcome on Function in Huntington Disease

Andrew S. Feigin, MD





NYU Langone Health
A Randomized Trial of Endovascular Thrombectomy Versus Medical Management for Ischemic Stroke with a Large Core Infarct on Non-contrast CT or Perfusion Imaging
Amrou Sarraj, MD
Live “Fireside Chat” to Follow Clinical Trials Plenary Session
Head over to the Research Hub in the North Lobby after the session to engage with and ask questions of plenary moderators and presenters during the live, 30-minute “Fireside Chat.”
University Hospitals, Cleveland, OH
Continue the conversation on social using #AANAM




Contemporary
Tuesday, April 25, 2023 • Annual Meeting Daily 5
Daily Reminders
For easy access to meeting links, visit AAN.com/QuickLinks
Attendee Lunches and Happy Hour
Attendee lunches are served from 11:00 a.m. to 1:00 p.m. in the Poster Hall, Exhibit Hall B2. Happy hour is the best hour! Eat, drink, and mingle with colleagues from 4:30 p.m. to 5:30 p.m. in the North Lobby. Beverages and light appetizers provided.
Program Slides Available Online
Slides are available online only at AAN.com/materials or through the AAN Conferences mobile app. You can access program materials through March 1, 2024. (Please note that availability of materials is at the discretion of the specific speaker. Not all sessions will have materials.)
Access Past Content
Submit Evaluations for Annual Meeting CME
Complete your evaluations to get your CME credits by May 15, 2023 (or March 1, 2024, with Annual Meeting On Demand) by using the AAN Conferences mobile app or by visiting AAN.com/AMCME. Transcripts will be available upon evaluation submission. AAN members can also access their transcript via NeuroTracker™ at AAN.com/NeuroTracker.
Get Your Certificate of Attendance
Share Your Feedback to Help Us Improve the Annual Meeting!
Please take five minutes to complete the overall evaluation for the Annual Meeting at AAN.com/AMCME. Your feedback will help us to continue to improve this conference in the future.
Conference attendees have access to the virtual platform through May 15, 2023. Visit AAN.com/VirtualAM and use your 6-digit ID and password to log in. It may take up to 48 hours after course completion for content to become available online.
Visit Registration or the Information Booth in the North Lobby for a printed copy of your Certificate of Attendance. You can also download a Certificate of Attendance at AAN.com/ QuickLinks. You will need to know your AAN ID or registration confirmation number to access it.
Want More Time to View Programs?
Add Annual Meeting On Demand to your registration to extend your access to session recordings through March 1, 2024, at a 50-percent discount Check the back of your badge to see if you already have Annual Meeting On Demand. If not, head to Registration or email aanamsupport@cmrus.com by May 15, 2023, to upgrade.
Safety and COVID-19 Protocols
The AAN continues to encourage attendees to practice safe measures to stop the spread of COVID-19, including using social distancing, testing when appropriate, taking recommended actions when symptomatic or having tested positive, and wearing a mask if you choose. We will continue to follow best practices recommended by the Centers for Disease Control and Prevention and abide by any legal mandates and recommendations from government officials.
View all conference guidelines at AAN.com/ConfGuidelines.
AANTV Studio
Stop by the AANTV Studio in the North Lobby, where you can witness live interviews being recorded for later broadcast accessible on the virtual platform, TV monitors around the convention center, and on AAN.com and YouTube. The AANTV Studio is sponsored by the AAN Family of Publications.
6 Tuesday, April 25, 2023 • Annual Meeting Daily
AAN Conferences Mobile App Daily Tip
Trying to decide which session to attend? The AAN Conferences mobile app is here to help.
• Log in and go to your profile to add interests to get tailored program recommendations.
• Use the search function to find sessions or attendees. Filter your search by program type, topic, and more to help find sessions most relevant to you.
• Visit 2023 Highlights on the main menu for can’t-miss events, programs, exhibitors, and other offerings.
• Visit Today’s Highlights on the main menu to view your upcoming sessions (if you’ve added to your agenda) for the day ahead.
Be sure to allow push notifications in the AAN Conferences mobile app for timely updates throughout the 2023 Annual Meeting. Need mobile app support?
Visit the Information Booth by Registration or in the North Lobby.
DISCOVER THE POWER OF PROOF
The only skin-based test to help diagnose Parkinson’s disease and other synucleinopathies
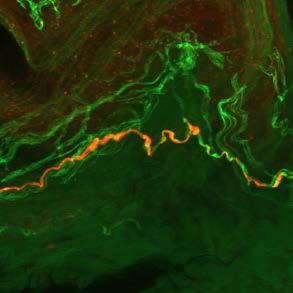
Pathological evidence of misfolded synuclein In office, minimally invasive procedure Visit us at Booth #1877 To learn more and order free Syn-One Test Biopsy Kits, visit SynOneTest.com Scan to DOWNLOAD app
2000s–2010s
The AAN Annual Meeting





8 Tuesday, April 25, 2023 • Annual Meeting Daily
Poster Exhibit. Philadelphia 2001
Actor and Parkinson’s disease activist Michael J. Fox. Philadelphia 2001
New AAN President Sandra F. Olson, MD, FAAN, takes the gavel from outgoing President Stanley Fahn, MD, FAAN. Hawaii 2003
Run/Walk for Research Toronto 2010
NeuroBowl. Hawaii 2011
Science Press Conference. Washington, DC 2015
70th Anniversary Party, Universal Studios. Los Angeles 2018
Visit Leadership University Area to Discover Oncein-a-Lifetime Career Development Opportunities

The value of great leadership is more important than ever in today’s challenging health care environment and the AAN is committed to investing in its members now to develop tomorrow’s leaders.
There’s no better time than this week to stop by the Leadership University area in the Northeast Lobby A-Level 1 to discover how you can make a big investment in yourself and your career with AAN Leadership Programs. Staff will be on hand to share information about and answer all your questions regarding these all-expenses-paid, intensive, and rewarding opportunities that incorporate executive leadership education, 1:1 leadership coaching, and mentoring not found anywhere else.
Learn about these leadership opportunities that are currently accepting applications at the Leadership University area or online:
DIVERSITY LEADERSHIP
The AAN is committed to building leadership reflective of our diverse member and patient demographics. This empowering and inspirational program provides a meaningful developmental experience that fully engages members from underrepresented neurology groups. Learn more visit the Leadership University Area on Level 1 of the Northeast Lobby A or at AAN.com/DLP.
EMERGING LEADERS
This program is designed to identify, engage, and mentor talented early-career members less than seven years out of residency who have the disposition to lead and are interested in future roles within the AAN and the field of neurology. Learn more at AAN.com/ELP.

PRACTICE LEADERSHIP
A unique opportunity designed specifically to identify and engage US neurologists in a solo or small practice who are interested in helping to shape the future of neurology with the AAN and their communities. The program’s convenient format was designed to provide high-quality training while accommodating the demanding schedules of busy practitioners. Learn more at AAN.com/PLP.
23 Annual Meeting Demand On

2000s–2010s
Actor, author, and American Brain Foundation Public Leadership in Neurology Award recipient Julie Andrews. Boston 2007
American Brain Foundation Booth. New Orleans 2012
It’s Not Too Late: Upgrade Your Registration to Include On Demand to Save 50%! There’s still time to bundle your Annual Meeting registration with Annual Meeting On Demand to save 50% on regular On Demand pricing and get extended access to most session recordings and the ability to claim CME through March 1, 2024. You’ll also get six months of access to the poster gallery. Visit Registration or email aanamsupport@cmrus.com to upgrade now.
Poster Hall. Seattle 2022
prescription medicine for the treatment of Friedreich’s ataxia in adults and adolescents aged 16 years and older1
Get Started With SKYCLARYS:
Submit a Start Form today at ReataREACH.com. Reata REACH is here to support your eligible patients with FA. Scan the QR code to start now.

IMPORTANT SAFETY INFORMATION WARNINGS AND PRECAUTIONS
Elevation of Aminotransferases: Treatment with SKYCLARYS can cause an elevation in hepatic transaminases (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]). Monitor ALT, AST, and total bilirubin prior to initiation of SKYCLARYS, every month for the first 3 months of treatment, and periodically thereafter.
Elevation of B-Type Natriuretic Peptide: Treatment with SKYCLARYS can cause an increase in B-type natriuretic peptide (BNP), a marker of cardiac function. Elevations in BNP may indicate cardiac failure and should prompt an evaluation of cardiac function. Check BNP prior to initiation of SKYCLARYS. Monitor patients for the signs and symptoms of fluid overload.
Lipid Abnormalities: Treatment with SKYCLARYS can cause changes in cholesterol. Assess lipid parameters prior to initiation of SKYCLARYS and monitor periodically during treatment.
ADVERSE REACTIONS
Adverse reactions reported in 10% or more of patients and greater than placebo were elevated liver enzymes (AST/ALT) (37%), headache (37%), nausea (33%), abdominal pain (29%), fatigue (24%), diarrhea (20%), musculoskeletal pain (20%), oropharyngeal pain (18%), influenza (16%), vomiting (16%), muscle spasms (14%), back pain (13%), decreased appetite (12%), rash (10%).
To report SUSPECTED ADVERSE REACTIONS, contact Reata Pharmaceuticals, Inc. at 1-800-314-3934 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.

INDICATION
SKYCLARYS is indicated for the treatment of Friedreich’s ataxia in adults and adolescents aged 16 years and older.
For additional information about SKYCLARYS, please see the Brief Summary on the following page and the full Prescribing Information at hcp.SKYCLARYS.com. US-SKY-2300056
Reference: 1. Skyclarys. Prescribing information. Reata Pharmaceuticals, Inc; 2023. © 2023 Reata Pharmaceuticals, Inc. All rights reserved. SKYCLARYS, REATA, and their logos are trademarks of Reata Pharmaceuticals, Inc. US-SKY-2200001 03/2023
v2.0
BRIEF SUMMARY
SKYCLARYS™ (omaveloxolone) capsules, for oral use
1 INDICATIONS AND USAGE
SKYCLARYS is indicated for the treatment of Friedreich’s ataxia in adults and adolescents aged 16 years and older.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Testing Before Initiating SKYCLARYS and Monitoring to Assess Safety
Obtain ALT, AST, bilirubin, BNP, and lipid parameters prior to initiating SKYCLARYS and during treatment [see Warnings and Precautions (5.1, 5.2, 5.3)]
2.2 Recommended Dosage
The recommended dosage of SKYCLARYS is 150 mg (3 capsules) taken orally once daily.
• Administer SKYCLARYS on an empty stomach at least one hour before eating [see Clinical Pharmacology (12.3)]
• Swallow SKYCLARYS capsules whole. Do not open, crush, or chew.
2.3 Missed Doses
If a dose of SKYCLARYS is missed, take the next dose at its scheduled time the following day. A double dose should not be taken to make up for a missed dose.
2.4 Recommendations for Concomitant Use with Strong or Moderate CYP3A4 Inhibitors and Inducers
The recommended dosage for concomitant use of SKYCLARYS with cytochrome P450 (CYP) 3A4 inhibitors and inducers are described in Table 1 [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]
Table 1: Recommended Dosage of SKYCLARYS with Concomitant Use of CYP3A4 Inhibitors and Inducers
5.3 Lipid Abnormalities
Treatment with SKYCLARYS can cause changes in cholesterol. In Study 1, 29% of patients treated with SKYCLARYS reported elevated cholesterol above ULN at one or more time points. Mean increases were observed within 2 weeks of initiation of SKYCLARYS and returned to baseline within 4 weeks of discontinuing treatment. A total of 16% of patients treated with SKYCLARYS had an increase in low-density lipoprotein cholesterol (LDL-C) from baseline, compared to 8% of patients who received placebo. The mean increase in LDL-C for all SKYCLARYS-treated patients was 23.5 mg/dL at 48 weeks. A total of 6% of patients treated with SKYCLARYS had decreases in high-density lipoprotein cholesterol (HDL-C) from baseline compared to 4% of patients who received placebo.
The mean decrease in HDL-C for all SKYCLARYS-treated patients was 5.3 mg/dL at 48 weeks.
Assess lipid parameters prior to initiation of SKYCLARYS and monitor periodically during treatment.
Manage lipid abnormalities according to clinical guidelines.
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described in greater detail in other labeling sections:
• Elevation of aminotransferases [see Warnings and Precautions (5.1)]
• Elevation of BNP [see Warnings and Precautions (5.2)]
• Lipid abnormalities [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of SKYCLARYS 150 mg once daily has been evaluated in 165 patients with Friedreich’s ataxia, including 137 patients exposed for at least 48 weeks, and 125 patients exposed for at least 96 weeks.
The most common adverse reactions in Study 1 (≥20% and greater than placebo) were elevated liver enzymes (AST/ALT), headache, nausea, abdominal pain, fatigue, diarrhea, and musculoskeletal pain. Table 3 shows the adverse reactions that occurred in 10% or more of patients treated with SKYCLARYS and greater than placebo.
dose range-finding study, oral administration of omaveloxolone at doses up to 30 mg/kg/day to pregnant rats throughout organogenesis produced increases in post-implantation loss and resorptions, resulting in a decrease in viable fetuses, and reduced fetal weight at the highest dose tested. At the highest dose tested in the pivotal study (10 mg/kg/day), plasma exposure (AUC) was approximately 5 times that in humans at the recommended human dose (RHD) of 150 mg/day.
Oral administration of omaveloxolone (0, 3, 10, or 30 mg/kg/day) to pregnant rabbits throughout organogenesis resulted in increased embryofetal mortality and skeletal variations and reduced fetal weight at the highest dose tested, which was associated with maternal toxicity. At the no-effect dose for adverse effects on embryofetal development (10 mg/kg/day), plasma exposure was less than that in humans at the RHD.
Oral administration of omaveloxolone (0, 1, 3, or 10 mg/kg/day) throughout pregnancy and lactation resulted in an increase in stillbirths and impaired neurobehavioral function (increased locomotor activity and learning and memory deficits) in offspring at all doses, reduced body weight in offspring at all but the lowest dose tested, and delayed sexual maturation (males), increased postnatal mortality, and impaired reproductive performance in offspring at the highest dose tested. A no-effect dose for adverse effects on pre- and postnatal development was not identified. Plasma exposure (AUC) at the lowest dose tested was less than that in humans at the RHD.
8.2 Lactation
Risk Summary
There are no data on the presence of omaveloxolone or its metabolites in human milk. The effects on milk production and the breastfed infant are unknown. Omaveloxolone was excreted in the milk of lactating rats following oral administration. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SKYCLARYS and any potential adverse effects on the breastfed infant from SKYCLARYS or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
• Reduce the dosage of SKYCLARYS to 50 mg once daily with close monitoring for adverse reactions.
• If adverse reactions emerge, coadministration with strong CYP3A4 inhibitors should be discontinued.
• Reduce the dosage of SKYCLARYS to 100 mg once daily with close monitoring for adverse reactions.
• If adverse reactions emerge, further reduce the dosage of SKYCLARYS to 50 mg once daily. Strong or Moderate CYP3A4 inducer Recommended to avoid concomitant use.
2.5 Recommended Dosage for Patients with Hepatic Impairment
The recommended dosage for patients with hepatic impairment are described in Table 2 [see Use in Specific Populations (8.6)]
Table 2: Recommended Dosage in Patients with Hepatic Impairment
Table 3: Adverse Reactions Reported in 10% or More of Patients Treated with SKYCLARYS and Greater than Placebo (Study 1)
SKYCLARYS may decrease the efficacy of hormonal contraceptives [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)]. Advise patients to avoid concomitant use with combined hormonal contraceptives (e.g., pill, patch, ring), implants, and progestin only pills. Counsel females using hormonal contraceptives to use an alternative contraceptive method (e.g., non-hormonal intrauterine system) or additional non-hormonal contraceptive (e.g., condoms) during concomitant use and for 28 days after discontinuation of SKYCLARYS.
8.4 Pediatric Use
The safety and effectiveness of SKYCLARYS for the treatment of Friedreich’s ataxia have been established in pediatric patients aged 16 years and older. Use of SKYCLARYS for this indication is supported by evidence from one adequate and well-controlled study (Study 1) in adults and in pediatric patients aged 16 years and older [see Clinical Studies (14)]
Safety and effectiveness of SKYCLARYS have not been established in pediatric patients less than 16 years of age.
8.5 Geriatric Use
Clinical studies of SKYCLARYS in Friedreich’s ataxia did not include patients aged 65 and over. No data are available to determine whether they respond differently than younger adult patients.
8.6 Hepatic Impairment
Omaveloxolone plasma exposure is increased in patients with moderate or severe hepatic impairment (Child-Pugh Class B and C) [see Clinical Pharmacology (12.3)]. Avoid treatment with SKYCLARYS in patients with severe hepatic impairment, including those who develop severe hepatic impairment. If hepatic function improves to moderate impairment, mild impairment, or normal function, initiation of SKYCLARYS treatment at the approved recommended dosage may be considered. For patients with moderate hepatic impairment, a reduced dosage is recommended with close monitoring for adverse reactions [see Dosage and Administration (2.5)]. For patients with mild hepatic impairment (Child-Pugh Class A), no dose adjustments are recommended.
9 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Elevation of Aminotransferases
• 100 mg once daily with close monitoring for adverse reactions
• Consider lowering to 50 mg once daily if adverse reactions emerge
3 DOSAGE FORMS AND STRENGTHS
SKYCLARYS capsules contain 50 mg of omaveloxolone, and are supplied as opaque hard capsules having a light green body and blue cap, imprinted with “RTA 408” in white ink on the body and “50” in white ink on the cap.
4 CONTRAINDICATIONS
None.
5 WARNINGS AND PRECAUTIONS
5.1 Elevation of Aminotransferases
Treatment with SKYCLARYS can cause an elevation in hepatic transaminases (ALT and AST). In Study 1 [see Clinical Studies (14)], the incidence of elevations of ALT or AST above 5 times and 3 times the upper limit of normal (ULN) was 16% and 31%, respectively, in patients treated with SKYCLARYS. There were no cases of concomitant elevation of transaminases and total bilirubin observed in Study 1. Maximum increases in ALT and AST occurred within 12 weeks after starting SKYCLARYS. Increases in serum aminotransferases were generally asymptomatic and reversible following discontinuation of SKYCLARYS. Patients with clinically significant liver disease were excluded from Study 1.
Monitor ALT, AST, and total bilirubin prior to initiation of SKYCLARYS, every month for the first 3 months of treatment, and periodically thereafter. If transaminases increase to levels greater than 5 times the ULN, or greater than 3 times the ULN with evidence of liver dysfunction (e.g., elevated bilirubin), immediately discontinue SKYCLARYS and repeat liver function tests as soon as possible. If transaminase levels stabilize or resolve, SKYCLARYS may be reinitiated with an appropriate increased frequency of monitoring of liver function [see Adverse Reactions (6.1) and Use in Specific Populations (8.6)]
5.2 Elevation of B-Type Natriuretic Peptide
Treatment with SKYCLARYS can cause an increase in BNP, a marker of cardiac function. In Study 1, a total of 14% of patients treated with SKYCLARYS had an increase from baseline in BNP and a BNP above the ULN (100 pg/mL), compared to 4% of patients who received placebo. The incidence of elevation of BNP above 200 pg/mL was 4% in patients treated with SKYCLARYS. Cardiomyopathy and cardiac failure are common in patients with Friedreich’s ataxia. Patients were excluded from Study 1 if they had BNP levels > 200 pg/mL prior to study entry, or a history of clinically significant left-sided heart disease and/or clinically significant cardiac disease, with the exception of mild to moderate cardiomyopathy associated with Friedreich’s ataxia [see Adverse Reactions (6.1)] Whether the elevations in BNP in Study 1 are related to SKYCLARYS or cardiac disease associated with Friedreich’s ataxia is unclear.
Elevations in BNP may indicate cardiac failure and should prompt an evaluation of cardiac function. Check BNP prior to initiation of SKYCLARYS. Monitor patients for the signs and symptoms of fluid overload, such as sudden weight gain (3 pounds or more of weight gain in one day, or 5 pounds or more of weight gain in a week), peripheral edema, palpitations, and shortness of breath. If signs and symptoms of fluid overload develop, worsen, or require hospitalization, evaluate BNP and cardiac function, and manage appropriately. Management of fluid overload and heart failure may require discontinuation of SKYCLARYS.
Laboratory Abnormalities
In addition to elevated liver enzymes, additional laboratory abnormalities include elevation of BNP and lipid abnormalities [see Warnings and Precautions (5.1, 5.2, 5.3)]
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on SKYCLARYS
CYP3A4 Inhibitors
Omaveloxolone is a CYP3A4 substrate. Concomitant use of SKYCLARYS with moderate or strong CYP3A4 inhibitors is expected to result in clinically significant increased exposure of omaveloxolone [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions. Avoid concomitant use of SKYCLARYS with moderate or strong CYP3A4 inhibitors. If use cannot be avoided, dosage modifications are recommended [see Dosage and Administration (2.4)]
CYP3A4 Inducers
Omaveloxolone is a CYP3A4 substrate. Concomitant use of SKYCLARYS with moderate or strong CYP3A4 inducers may significantly decrease exposure of omaveloxolone [see Clinical Pharmacology (12.3)], which may reduce the effectiveness of SKYCLARYS. Avoid concomitant use of SKYCLARYS with moderate or strong CYP3A4 inducers.
7.2 Effect of SKYCLARYS on Other Drugs
CYP3A4 and CYP2C8 Substrates
Omaveloxolone is a weak inducer of CYP3A4 and CYP2C8. Concomitant use with SKYCLARYS can reduce the exposure of CYP3A4 and CYP2C8 substrates which may reduce the activity of these substrates [see Clinical Pharmacology (12.3)]. Refer to the prescribing information of substrates of CYP3A4 and CYP2C8 for dosing instructions if used concomitantly with SKYCLARYS and monitor for lack of efficacy of the concomitant treatment.
Hormonal Contraceptives
Omaveloxolone is a weak CYP3A4 inducer [see Clinical Pharmacology (12.3)]. Concomitant use with SKYCLARYS may reduce the efficacy of hormonal contraceptives. Advise patients to avoid concomitant use with combined hormonal contraceptives (e.g., pill, patch, ring), implants, and progestin only pills [see Use in Specific Populations (8.3)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risks associated with the use of SKYCLARYS in pregnant women. In animal studies, administration of omaveloxolone during pregnancy or throughout pregnancy and lactation produced evidence of developmental toxicity (embryofetal mortality and growth impairment, and mortality, growth impairment, and neurobehavioral deficits in offspring) at plasma exposures similar to or less than exposures in humans (see Animal Data). In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
Oral administration of omaveloxolone (0, 1, 3, or 10 mg/kg/day) to pregnant rats throughout organogenesis resulted in no adverse effects on embryofetal development; however, in a
Inform patients that elevation in aminotransferases have occurred in patients treated with SKYCLARYS. Liver function tests will be performed prior to initiating SKYCLARYS, every month for the first 3 months of treatment, and periodically thereafter as needed [see Warnings and Precautions (5.1)]
Fluid Overload
Inform patients that elevations in BNP, a marker of cardiac function, have occurred in patients treated with SKYCLARYS. BNP will be performed prior to initiating SKYCLARYS and if signs and symptoms of fluid overload occur, such as sudden weight gain, peripheral edema, palpitations, and shortness of breath. Advise patients to contact their healthcare provider if signs and symptoms of fluid overload develop [see Warnings and Precautions (5.2)]
Lipid Abnormalities
Inform patients that treatment with SKYCLARYS has been associated with increases in LDL cholesterol and decreases in HDL cholesterol. Cholesterol will be assessed prior to starting SKYCLARYS and monitored periodically during treatment [see Warnings and Precautions (5.3)]
Drug Interactions
Advise patients to discuss all medications they are taking, including other prescription medications, non-prescription medications, or herbal products (e.g., St. John’s Wort) with their healthcare provider [see Drug Interactions (7)]
Pregnancy
Advise women to notify their healthcare provider if they become pregnant or intend to become pregnant during SKYCLARYS therapy [see Use in Specific Populations (8.1)]
Females of Reproductive Potential
Counsel females using hormonal contraceptives to use an alternative contraceptive method (e.g., non-hormonal intrauterine system) or additional non-hormonal contraceptive (e.g., condoms) during concomitant use and for 28 days after discontinuation of SKYCLARYS [see Drug Interactions (7.2) and Use in Specific Populations (8.3)]
Administration
Advise patients to take SKYCLARYS on an empty stomach at least 1 hour before eating [see Dosage and Administration (2.2)]
Swallow SKYCLARYS capsules whole. Do not open, crush, or chew. Advise patients that if a dose of SKYCLARYS is missed to not to double their dose or take more than the prescribed dose [see Dosage and Administration (2.3)]
Advise patients to avoid grapefruit juice and grapefruit while they are taking SKYCLARYS [see Drug Interactions (7.1)]
Manufactured for Reata Pharmaceuticals, Inc., Plano, TX 75024 USA
SKYCLARYS is a trademark of Reata Pharmaceuticals Holdings, LLC, used under license by Reata Pharmaceuticals, Inc., Plano, TX 75024 USA
Copyright© 2023, Reata Pharmaceuticals, Inc., Plano, TX 75024 USA
All rights reserved
© 2023 Reata Pharmaceuticals, Inc. All Rights Reserved. SKYCLARYS, REATA, and their logos are trademarks of Reata Pharmaceuticals, Inc. US-SKY-2200013 03/2023
Adverse Reaction SKYCLARYS 150 mg (N=51) Placebo (N=52) Elevated liver enzymes (AST/ALT) 37% 2% Headache 37% 25% Nausea 33% 13% Abdominal pain 29% 6% Fatigue 24% 14% Diarrhea 20% 10% Musculoskeletal pain 20% 15% Oropharyngeal pain 18% 6% Influenza 16% 6% Vomiting 16% 12% Muscle spasms 14% 6% Back pain 13% 8% Decreased appetite 12% 4% Rash 10% 4%
Concomitant Drug Class Dosage Strong CYP3A4 inhibitor Recommended to
concomitant use.
avoid
If coadministration cannot be avoided:
CYP3A4
Moderate
inhibitor Recommended to avoid concomitant use. If coadministration cannot be avoided:
Impairment Classification (Child-Pugh) Dosage Severe
Class C) Avoid
(Child-Pugh
use Moderate (Child-Pugh Class B)
Mild (Child-Pugh Class A) 150 mg once daily
Check out Today’s Hub Highlights for Collaborative Learning
Step outside the traditional classroom and into the world of unconventional learning with eight Hub areas. Each Hub offers unique educational and networking opportunities. Engage in collaborative learning, gain actionable tools for your patients and career, and find your community.
Here are today’s highlights:
ACADEMIC HUB
Southeast Lobby: Level 1
HEADTALKS
Northwest Lobby A: Level 1
Academic
Neurology: What Is the AAN Doing to Help Academic Neurology? Conversation Corner

2:30 p.m.–3:00 p.m.
David M. Greer, MD, FAAN, and Brenda Banwell, MD, FAAN, will give a tour of the AAN’s programs and offerings designed to help academic neurology departments and academic neurologists. Principles will apply to both scientists and educators, as well as those serving in an administrative or leadership capacity to help academic neurology departments thrive in the current and changing environment.
Meet New Continuum Editor-in-Chief
Lyell K. Jones, Jr., MD, FAAN
3:00 p.m.–3:45 p.m.
Lyell K. Jones, Jr., MD, FAAN, will present his mission and vision for Continuum: Lifelong Learning in Neurology ® , the AAN’s premier education and CME journal. Share your thoughts and ideas with him on how to make Continuum ® more indispensable to neurology professionals and educators throughout their careers.
Recruitment and Retention of Staff in the Age of the Great Resignation
4:00 p.m.–4:45 p.m.
Steven Galetta, MD, FAAN; David M. Greer, MD, FAAN; and Stacy Miller, MPH, will lead this discussion developed through the Ralph L. Sacco Neurology Chair Summit hosted in December 2022. They will review the challenges faced by academic neurologists to recruit and retain great faculty in the modern age, with increasing financial pressures and challenges finding all types of academicians a clear career path, including both clinician-educators and physician-scientists.
As Seen on TV
2:00 p.m.–2:45 p.m.
Medical dramas are a constant and entertaining presence on television, film, and streaming channels. Many medical-based shows depict neurologic and psychiatric phenomenology, but they rarely get it correct. Katherine Peters, MD, PhD, FAAN, and Andrew Newberg, MD, will present clips of these shows that are centered around neurologic and psychiatric symptoms and conditions, and together determine what is wrong and what can make it right.
Navigating Global Challenges in Neurology
4:00 p.m.–4:45 p.m.
Join us for a panel discussion led by AAN President Orly Avitzur, MD, MBA, FAAN, with leaders of global neurologic societies: European Academy of Neurology, World Federation of Neurology, Mexican Academy of Neurology, Indian Academy of Neurology, and Pan Arab Union of Neurological Societies.
INNOVATION HUB
Exhibit Hall B1
“Patent” Expectations: A Neurologist’s Experience with Intellectual Property Protection
1:00 p.m.–1:30 p.m.
Come learn how your colleagues use their knowledge and experiences of the world to transform health care systems in a way that enhances physician career satisfaction and improves patient outcomes.
Daily Virtual Reality Gaming Stations

11:30 a.m.–4:00 p.m.
Come experience immersive simulations of real or imaginary worlds. There are lots of games to choose from, so try a different one each day!
Alan Chien captivated attendees with his magic performance while showing them how to develop their own magic routine within their practice.
12 Tuesday, April 25, 2023 • Annual Meeting Daily
Check out the AR/VR technology at the Innovation Hub in the Exhibit Hall.
LEADERSHIP UNIVERSITY
Northeast Lobby A: Level 1
An Interactive Workshop on Mission-based Social Media Use: The Why & How
1:30 p.m.–3:00 p.m.
Participants will begin to discover their own “why”: their mission, purpose, and passion and how it relates to social media. Moderators will help participants reflect on their social media identity and alignment with their mission. In an infographic design skills session, participants will create their unique mission-based infographic to be shared on social media. Note: Participants will need a computer or tablet for this session.
Design Thinking as a Leadership Tool
3:00 p.m.–3:30 p.m.
Room 154
Nimish A. Mohile, MD, FAAN, will present on the topic of design thinking which is a novel process that can be used to creatively solve problems, better incorporate innovative approaches in leadership, and provide a structure for change management while developing strategic thinking skills
PRACTICE AND POLICY HUB
Northwest Lobby: Level 2
Increasing Advocacy Engagement Across the Academy: Ways to Effect Change from Clinics to the NIH

1:45 p.m.–2:45 p.m.
Are you wondering what advocacy is and how you can be involved? Join this session to gain a better understanding of AAN advocacy and its applications to research and practice. The session will include information on identifying potential advocacy issues for neurology, sharing the AAN’s approach to advocacy issues at the local, state, and national levels and how you can participate in advocacy as an AAN member.
RESEARCH HUB
North Lobby
Finding a Mentor for Your Research Career
3:00 p.m.–3:45 p.m.
The golden connection is finding the right mentor for your research career. Learn effective strategies and discuss ways to find the mentor who supports and challenges you toward success. Join Shibani Sharon Mukerji, MD; Lama Abdel Wahed, MD; Hugo Javier Aparicio, MD; and Sudha Seshadri, MD, for this engaging conversation.
TRAINEE AND EDUCATOR HUB
ROOM 155
Tweeting to Take Flight as an Educator
3:00 p.m.–3:30 p.m.
Social media is a powerful tool to build a teaching portfolio, network with neurology educators, and reach a global audience. Casey S.W. Albin, MD, focuses on how educators can not only leverage Twitter to become better teachers, but to distinguish their personal style of teaching and develop a robust network. Specifics about how to author a #tweetorial will be included.
WELLNESS HUB
Northeast Lobby: Level 2
Guided Meditation
2:00 p.m.–2:30 p.m.
Sarah Mulukutla, MD, MPH, will provide a five-minute overview of meditation followed by 20 minutes of guided meditation practice. Participants will learn the neuro-anatomical neural networks involved in meditation and begin to identify their own “defaultmode” mind-wandering.
Mulukutla

Tuesday, April 25, 2023 • Annual Meeting Daily 13





What Are Your Colleagues Saying?
Maria Agustina Ruiz Yanzi, MD Neurologist, Madrid, Spain


First-time attendee
What brings you to the Annual Meeting?
I won the International Scholarship in 2020 when I was in my country of Argentina. I was really sad when the meeting couldn’t happen because in my country it is really important to come to the AAN meeting to meet other colleagues and learn the latest updates. When I won it again this year, I was really happy. I’m presenting a poster on the loss of the swallow tail sign, an MRI feature that every neurologist everywhere can apply to their practice as a diagnostic biomarker.
Daniele Urso, MD Neurologist,

Scorrano, Italy
First-time attendee
What brings you to the Annual Meeting?
I’m doing an MPH program in Boston at Harvard in neuroepidemiology—it’s hybrid, both in person and online from Italy. I’m presenting a poster on neuroimaging—“Nucleus basalis of Meynert degeneration predicts cognitive decline in corticobasil syndrome.”
Elena Natera-Villalba, MD Neurologist, Madrid, Spain
First-time attendee
What brings you to the Annual Meeting?
I work in Parkinson’s disease, so I’m most interested here in networking and connecting with other colleagues who work in similar fields.
Jonathan L. Carter, MD Neurologist, Mayo Clinic, Scottsdale, AZ

Attended first Annual Meeting in 1985 or ’86 as a resident
What has been your highlight so far?
The courses are really great. I just attended a course on MOGAD with Dr. Flanagan, Dr. Banwell, and Dr. Palace that is relevant to my specialty field. I really missed this during the pandemic. You can do it online, but it’s easier for me to learn and pay attention in person.
Jasdeep Khurana, MD Neurologist, West Reading, PA

Sixth AAN Annual Meeting
What brings you to the Annual Meeting?
Just to get myself updated. I’m a general neurologist. For us general neurologists, this is the best place to get updated on all the topics. Plus the networking.
Tuesday, April 25, 2023 • Annual Meeting Daily 15 JOIN THE CONVERSATION! SHARE YOUR THOUGHTS AT #AANAM.
JOIN THE CONVERSATION AT #AANAM


Always fun to meet new friends at the #AANAM 75th Anniversary Party! Shout out to my fellow Filipinx fellows @brainattackmd @clareangeli ! Neurologists by day, tearing up the dance floor by night

ndecinstitute



#NDEC gathering ended up full of new ideas #AANAM

I’m so honored and excited to be joining the @AANAM Board of Directors, but I have to admit that my favorite part of the meeting was this note my son left in my phone for me to find before going to bed!

FYI for all my gamer friends out there. #AANAM

I’m taking this as a tacit endorsement of my life choices.
@doctorShawniqua
Just virtually attended an excellent session on recognizing, combatting and harnessing Impostor Syndrome, given by 3 of my @AANmember heroes @RachelSalasMD , Charlene Gamaldo MD, and Lynn Liu MD. Thanks also to the virtual chat participants who spoke up and shared! #AANAM #AAN2023

16 Tuesday, April 25, 2023 • Annual Meeting Daily
Justin Rosati, MD @JustinRosatiMD
Chris Bondoc @The_Bondoctor
Shawniqua Williams Roberson, MEng, MD
Jeffrey McClean @JeffreyMcclean
The first in-person diversity officers mtg at #AANAM - working on #diversity and #culturechange in academic neurology! @wendyvokaty1 @AgSonika @NeuroDrCorrea @jposas
 Lidia Moura, MD, PhD, MPH @LidiaMouraNVL
Lidia Moura, MD, PhD, MPH @LidiaMouraNVL


“it’s never too early to become a leader”!!! Always inspiring everyone around with her patience, persistence, and passion => that’s The Chair of @MGHNeurology at @AANmember #AANAM


@AneeshSinghalMD

At #AANAM with 2 mentors: @NINDSdirector and my very own father Dr. BS Singhal from India, who received the ABF/AAN Mridha Spirit of Neurology Award AmericanBrainFoundation.org/ awards/ for the far-reaching impact of his non-profits NeurologyFoundation.in and ParkinsonsSocietyIndia.com
 Desiree D’Souza @DesireeDSouz
Desiree D’Souza @DesireeDSouz

A healthy dose of dopamine and serotonin! Thank you for a fabulous evening @AANmember @museumofscience #AANAM 75th anniversary
Idha (Joy) Sood @DrIdhaSood


Just #AANAM providing opportunities to meet cool professors! And I get a signed copy!
Thank you, @abudson
@AANmember #NeuroTwitter #NeurologyProud

cooperneuros
Getting our neurology of wine tasting at the AANAM! #aanam

Tuesday, April 25, 2023 • Annual Meeting Daily 17
Aneesh Singhal MD
Nimish Mohile @NimishMohile





18 Tuesday, April 25, 2023 • Annual Meeting Daily
Have an Idea for a Course at the 2024 Annual Meeting?






The Academy is inviting all members to submit course proposals for the 2024 Annual Meeting taking place in Denver, CO, April 13 through 18. The course proposal submission site will open in late summer 2023, and you can sign up to be notified when the site goes live by visiting AAN.com/CourseProposal.

Tuesday, April 25, 2023 • Annual Meeting Daily 19
Today’s Neuroscience in the Clinic Session
Focuses on Bridging Genetics to the Clinic in Neuromuscular Disease

Today between 3:30 p.m. and 5:30 p.m. join Directors Nicholas Elwood Johnson, MD, FAAN, and Monkol Lek, PhD, as they lead expert faculty Melissa Jan Spencer, PhD; Gabriel Haller, PhD; Sandra Terese Cooper, PhD; Divya Jayaraman, MD, PhD; and Preeti Kumari, MD, in a discussion involving the advances in genetic discovery and genetic precision therapy in neuromuscular medicine and the issue of variants of unknown significance and how to resolve them. Presenters will also discuss developing an understanding of genetic precision therapies and provide an update on new breakthroughs in the field. Attendees may claim two CME credits.
Movement Disorders Highlighted in Invited Science Session



Today’s Invited Science Session from 1:00 p.m. to 3:00 p.m. on movement disorders will feature authors giving encore presentations of top abstracts presented at subspecialty meetings. The session is presented in partnership with the International Parkinson and Movement Disorder Society. Select abstracts will emphasize basic, clinical, and translational science as they evolve toward a more complete understanding of movement disorders with the overall goal of developing more effective prevention and treatment.
Abstracts and authors include:
1:00 p.m.–1:20 p.m.
Endocannabinoid Dysfunction in Human Disease: NeuroOcular DAGLA-related Syndrome (NODrS), a Unique Pediatric Condition
Jennifer R.L. Friedman, MD
1:20 p.m.–1:40 p.m.
Cognitive and Functional Trajectories in Prediagnostic Parkinson’s Disease
Meredith Bock, MD

1:40 p.m.–2:00 p.m.
Synaptic Density in Cortical and Subcortical Gray Matter is Associated with Mild Parkinsonian Signs (MPS) in Healthy Older Adults: A [11C] UCB-J PET Study
Margot Van Cauwenberge
2:00 p.m.–2:20 p.m.
Multimodal Mechanistic Disease Stratification in Sporadic Parkinson’s Disease
Thomas William Payne, MBChB, MRCP
2:20 p.m.–2:40 p.m.
Transcranial Alternating Current Stimulation Modulates Motor Network Connectivity in Patients with Blepharospasm
Karishma Anjuli Popli, MD, MBE


2:40 p.m.–3:00 p.m.
Amelioration of Levodopa-induced Dyskinesias Using Genetic Silencing of Striatal CaV1.3 in Parkinsonian Rats: Impact of Sex and Advancing Age
Margaret Caulfield, PhD
20 Tuesday, April 25, 2023 • Annual Meeting Daily
Johnson Lek Spencer
Cooper Jayaraman Kumari
Haller
Second Emerging Science Session to Take Place Tonight
The most timely, significant, and innovative research in neurology will be showcased in the second session of the Emerging Science program this evening from 5:30 p.m. to 7:00 p.m. in 253AB. Abstracts selected for Emerging Science presentations include key aspects of research conducted after the October 2022 abstract submission deadline and must be new and of sufficient scientific importance to warrant expedited presentation and publication. These previously unpublished abstracts contain timely, significant, and innovative content.
5:30 p.m.–5:36 p.m.
Holter of Movement Provides the First Digital Outcome Qualified by a Regulatory Agency
Presenter: Laurent Servais
5:36 p.m.–5:42 p.m.
Interim Results from the NEXUS Open-label Registration Study on the Safety and Efficacy of Leriglitazone in the Treatment of Childhood Cerebral Adrenoleukodystrophy
Presenter: Eric James Mallack, MD
5:42 p.m.–5:48 p.m.
Expansion of an Intronic FGF14 GAA Short Tandem Repeat in Late-onset Cerebellar Ataxia
Presenter: David Pellerin, MD
5:48 p.m.–5:54 p.m.
Continuous Delivery of Levodopa/Carbidopa Using the IntraOral DopaFuse System: A Safety, Tolerability, PK, and Efficacy Trial
Presenter: C. Warren Olanow, MD
5:54 p.m.–6:00 p.m.

MOG-IgA Characterizes a Subgroup of Patients with Central Nervous System Demyelination
Presenter: Anne-Katrin Proebstel, MD
6:00 p.m.–6:06 p.m.
Mitochondrial Dysfunction in Neuron-derived Extracellular Vesicles and Brain Tissues of FXTAS Patients
Presenter: Apostolos Manolopoulos, MD
6:06 p.m.–6:12 p.m.
Atogepant for the Preventive Treatment of Migraine Among Participants with Episodic Migraine with Prior Treatment Failure: Results from the ELEVATE Trial
Presenter: Cristina Tassorelli
6:12 p.m.–6:18 p.m.
MEGAbIT: The Role of Magnetoencephalography in Assessment and Diagnosis in Mild Traumatic Brain Injury
Presenter: Christopher Martin Allen, MBBS
6:18 p.m.–6:24 p.m.
ACHIEVE Trial, a Randomized, Placebo-controlled, Multiple Ascending Dose Study of DYNE-101 in Individuals with Myotonic Dystrophy Type 1 (DM1)
Presenter: Daniel Wolf, PhD
6:24 p.m.–6:30 p.m.
Teriflunomide (Aubagio) Extends the Time to Multiple Sclerosis in Radiologically Isolated Syndrome: The TERIS Study
Presenter: Christine Lebrun Frenay, MD, FAAN
Your Evaluations Count
The AAN continually strives to improve the Annual Meeting experience and highly encourages attendees to complete their overall and session evaluations. The feedback received is used by the various planning committees when they are evaluating the Annual Meeting and planning for the next one. It’s also an opportunity for attendees to claim their muchneeded CME.
Evaluations can be accessed on the mobile app, AAN.com/AMCME, the virtual platform, or scan the QR code. Thank you!

So We’re Counting on You!
Today’s Publications and Online Learning Talks
TODAY
Utilizing NeuroBytes in Your Residency
12:30 p.m.–1:00 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
Kara Stavros, MD, FAAN, and Muhib Khan, MD, FAAN
Everything You Want to Know About the Residency
In-service Training Examination Exam (RITE)
8:00 a.m.–9:00 a.m.
TRAINEE AND EDUCATOR HUB (ROOM 155)
Raymond Price, MD, FAAN, Sudha Kessler, MD, Sashank Prasad, MD, Lynne Shindoll, MA
The Peer Review Process and Addressing the Comments of the Reviewers
2:00 p.m.–3:00 p.m
RESEARCH HUB (NORTH LOBBY)
José G. Merino, MD, MPhil, FAAN; Olga Ciccarelli, MD, PhD, FRCP; Josep O. Dalmau, MD, PhD, FAAN; Massimo Pandolfo, MD, FAAN; Bradford B. Worrall, MD
Meet New Continuum® Editor-in-Chief,
Lyell K. Jones, Jr., MD, FAAN
3:00 p.m.–3:45 p.m.
ACADEMIC HUB (SOUTHEAST LOBBY C: LEVEL 1)
Lyell K. Jones, Jr., MD, FAAN
How to Write a Good Peer Review
5:00 p.m.–5:30 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
Whitley Warfield Aamodt, MD, MPH; Nadia Khalil, MD; Jonathan Read Gaillard, MD, MHS; Galina Gheihman, MD; Stephanie Syc-Mazurek, MD, PhD; Joaquin Augusto Vizcarra, MD
WEDNESDAY
Disputes & Debates: Pulled from the Pages of Neurology ®: Meet the Editors and Learn More
1:30 p.m.–2:00 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
Ariane Lewis, MD
Neurology Quiz LIVE
2:00 p.m.–2:30 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
Sara Maguire Schaefer, MD
What Happens After Peer Review: How Journal Editors Make Decisions
3:00 p.m.–3:30 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
José G. Merino, MD, MPhil, FAAN
Neurology Today ® Panel Discussion: Best Advances in Neurology Research
3:00 p.m.–3:45 p.m.
RESEARCH HUB (NORTH LOBBY)
Joseph E. Safdieh, MD, FAAN; Jacqueline A. French, MD, FAAN; James C. Grotta, MD, FAAN; Melissa J. Nirenberg, MD, PhD, FAAN
Without Borders: Neurology ’s Global Neurology Site— Who We Are and How to Make Your Voice Heard
3:30 – 4:00 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
Deanna Saylor, MD, MHS
Disputes & Debates: Pulled from the Pages of Neurology : Meet the Editors and Learn More
4:00 p.m.–4:30 p.m.
PUBLICATIONS AREA (NORTH LOBBY)
Ariane Lewis, MD
22 Tuesday, April 25, 2023 •
Meeting Daily
Annual
Rare Inspiration. Changing Lives.
Alexion is now investigating 2 new treatments for generalized myasthenia gravis (gMG).


For over 30 years, Alexion’s mission has been to deepen our understanding and transform the lives of people who are affected by rare diseases and living with devastating conditions. We bring this same steadfast commitment to patients with generalized myasthenia gravis (gMG).
ALXN2050. The ExpanD Study is a phase 2 study evaluating the oral investigational medication ALXN2050 for the treatment of gMG.
ALXN2050 is an inhibitor of complement factor D, a component of the alternative pathway, which is being investigated as a novel mechanism of action for the treatment of adults with gMG.
ALXN1720. The Prevail Study is a phase 3 study evaluating Alexion’s new complement component C5 inhibitor, gefurulimab (ALXN1720), for the treatment of adults with gMG.
Gefurulimab is developed for selfadministration through once-weekly subcutaneous injection, which would provide patients with more autonomy and greater flexibility to benefit from a mechanism of action that is well established.
NCT05218096
NCT05556096


Visit us at Booth #2361 ALXN1720-MG-301_ALXN2050-MG-201_AAN Ad_V1_06FEB2023
Celebrate These 2023 AAN Scholarship Award Recipients
The AAN recognizes the 2023 AAN scholarship award recipients and their contributions to the art and science of neurology.
EDUCATION RESEARCH GRANT
Catherine Albin, MD
Emory University School of Medicine, Atlanta, GA
Jonathan Attwood, MA (Oxon), BMBCh
University of Oxford, Oxford, United Kingdom
FUTURES IN NEUROLOGICAL RESEARCH SCHOLARSHIP TO THE ANNUAL MEETING
Sponsored by the AAN Clinical Research Subcommittee
Maya Ayoub, MD, EdM
University of California, Los Angeles, Los Angeles, CA
Negin Badihian, MD Mayo Clinic, Rochester, MN
Bryce A. Bagley
Stanford University, Stanford, CA
Mara Bahri
The Ohio State University College of Medicine, Columbus, OH
Shawn Barton, MD, PhD
Emory University School of Medicine, Atlanta, GA
Aaron Eduardo Rodriguez Calienes, MD
University of Iowa Hospitals & Clinics, Iowa City, IA
Kate Dembny
University of Minnesota, Minneapolis, MN
Alyssa Edwards
Case Western Reserve School of Medicine, Cleveland, OH
Karam Gagi, MD
Michigan State University, Lansing, MI
Maria Garcia-Dominguez, MD
UMass Chan Medical School, Worcester, MA
Yung-Tian Gau, MD, PhD
SUNY Upstate Medical University, Syracuse, NY
Joseph Geraghty
University of Illinois College of Medicine, Chicago, IL
Laura Gilbert, DO, MBA
Washington University in St. Louis, St. Louis, MO
Mirna Hennawy, MD
University of British Columbia, Vancouver, BC, Canada
Billie Hsieh
The University of Texas Health Science Center at Houston, Houston, TX
Nimansha Jain
Washington University in St. Louis School of Medicine, St. Louis, MO
Dominic Julian
University of Arizona College of MedicinePhoenix, Phoenix, AZ
Manpreet Kaur
Yale University, New Haven, CT
Angela Kwan
University of Ottawa, Ottawa, ON, Canada
Emile Lemoine, MD, MSc
University of Montreal, Montreal, Quebec, Canada
Yasamin Mahjoub, MD
University of Calgary, Calgary, AB, Canada
Gabriel Marx
Albert Einstein College of Medicine, Bronx, NY
Takahisa Mikami, MD
Tufts Medical Center, Boston, MA
Nicholas Mannix, MD
The Ohio State University Wexner Medical Center, Columbus, OH
Kelli Money, MD, PhD
University of Colorado Anschutz Medical Campus, Aurora, CO
Tanziyah Muqeem, MD, PhD
Duke University Hospital, Durham, NC
George Naratadam
University of Texas Health San Antonio, San Antonio, TX
Ai Ohno
California University of Science and Medicine, San Bernardino, CA
Nikolai Gil Reyes, MD, FPNA University Health Network-Toronto Western Hospital, University of Toronto, Toronto, ON, Canada
Cyprien Rivier, MD, MSc Yale University, New Haven, CT
Nihal Satyadev, MPH
University of Medicine and Health Sciences, Los Angeles, CA
Zachary Weinstock
Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY
Hannah Zhao-Fleming, MD, PhD Mayo Clinic, Rochester, MN
Daniel Zhou, MD, MSE
University of Nebraska Medical Center, Omaha, NE
Zahra Zhu
Penn State College of Medicine, Hershey, PA
INTERNATIONAL SCHOLARSHIP AWARD
Sponsored by the American Academy of Neurology International Subcommittee.
Gianmarco Abbadessa, MD
Naples, Italy
Jeremias Ayerbe, MD
Buenos Aires, Argentina
Solomiia Bandrivska, MD
Kyiv, Ukraine
Benjamin Beland, MD
Calgary, Alberta, Canada
Juliette Brenner, MD
Rotterdam, Netherlands
Gavin Brittain, MBBS, MRCP
Stockport, Great Britain
Nargiza Chekeeva, MD
Bishkek, Chui, Kyrgystan
Mashina Chomba, MBChB, MMed
Lusaka, Zambia
Aline Dias, MD
Campinas, Sao Paulo, Brazil
Adrian Espiritu, MD, FPNA
Toronto, Ontario, Canada
Milagros Galecio-Castillo, MD
Lima, Peru
Tinatin Giuashvili, MD
Tbilisi, Georgia
Enrique Gomez, MD, MSc
Guadalajara, Jalisco, Mexico
Mar Guasp, MD
Barcelona, Catalunya, Spain
Rauan Kaiyrzhanov, MD
Shymkent, Kazakhstan
Eva Kesenheimer, MD, PhD
Basel, Basel Stadt, Switzerland
Nicholas Koluba, MD
Mbarara, Uganda
Aleksandre Kotetishvili, MD
Tbilisi, Georgia
Oreoluwa Lagunju, MD
Ibadan, Oyo, Nigeria
Jimmy Li, MD
Sherbrooke, Quebec, Canada
Salvatore Mazzeo, MD
Florence, Tuscany, Italy
Frighton Mutete, MBChB
Lusaka, Zambia
Amina Nasri, MD
Manouba, Tunis, Tunisia
Christelle Nilles, MD
Paris, France
24 Tuesday, April 25, 2023 • Annual Meeting Daily
Mundih Njohjam, MD
Bameda, Cameroon
Maria Agustina Piedrabuena, MD
Caba, Argentina
Gabriel Pinella, MD, MSc
Cali, Colombia
Valentina Quintana, MD
Cali, Colombia
Saskia Raeuber, MD, FPNA
Dusseldorf, Germany
Lokesh Saini, MD, DM
Jodhpur, India
Kamalesh Tayade, MD
Jalgon, Maharashtra, India
Taras Voloshyn, MD, PhD
Truskavets, Lviv, Ukraine
Maria Janina Wendebourg, MD
Basel, Basel Stadt, Switzerland
Maria Agustina Ruiz Yanzi, MD
Buenos Aires, Argentina
Ramdinal Zairinal, MD
Depok, West Java, Indonesia
MEDICAL EDUCATION RESEARCH TRAINING FELLOWSHIP
Ashley Paul, MD
Johns Hopkins University, Baltimore, MD
MEDICAL STUDENT DIVERSITY ANNUAL MEETING SCHOLARSHIP
Freda Biyaa Assuah
Nova Southeastern University
Connor Bluntson
University of Mississippi Medical Center School of Medicine
Jasmine Shareen Coles
Wayne State University School of Medicine
Hayden Alexander Moses Hatch
Albert Einstein College of Medicine
Jairo Stuart Hernandez
University of Florida College of Medicine
Joy C. Josephs Njiribeako
Texas Tech University Health Sciences
Lubbock
Christine Petit-Frere
Morehouse School of Medicine
Miguel Rodriguez
University of Tennessee Health Science
Center-College of Medicine
Jude Tunyi
The Ohio State College of Medicine
Jasmin Williams
University of Connecticut School of Medicine
MEDICAL STUDENT EXPERIENCE AT THE ANNUAL MEETING SCHOLARSHIP
Abrar Ahmed
University of Western Ontario
Kaitlyn Alleman
Chicago Medical School
Seema Arshed
Noorda College of Osteopathic Medicine
Nadia Azad
Emory University School of Medicine
Alexis Ballew
Medical College of Wisconsin
Robert Cascella
Washington University School of Medicine in St. Louis
Abigail Connor
Long School of Medicine at UTHSCSA
Sierra Cowan
Tilman J. Fertitta Family College of Medicine
JuleLayne Curry
University of Tennessee Health Science Center College of Medicine
Gregory Devine
Creighton University Phoenix Health Sciences
Sarah Doebley
Lewis Katz School of Medicine at Temple University
Lauren Dugan
California University of Science and Medicine
Mahalya Gogerly-Moragoda
Icahn School of Medicine at Mount Sinai
Madison Grindstaff
Lincoln Memorial University
Elijah Hale
University of Colorado School of Medicine
Natasha Hamilton
Rocky Vista University
Mouhamad Hammami
Oakland University
Gregory Heinonen
Columbia University Vagelos College of Physicians and Surgeons
Seungrok Hong
University of Cincinnati College of Medicine
James Johnson
University of Louisville School of Medicine
Jason Li
Indiana University School of Medicine
Moriah Mabry
University of Colorado School of Medicine
Rebecca Merrill
Wake Forest School of Medicine
Cassie Morgan
UNT Health Science Center-TCOM
Daphne Nguyen
UT Health San Antonio, Long School of Medicine
Alexis Notarianni
Geisinger Commonwealth School of Medicine
Riya Parikh
Chicago Medical School at Rosalind Franklin University
Johann Park
California Northstate University College of Medicine
Ihika Rampalli
Baylor College of Medicine
Rui Tang
TTUHSC El Paso
Vani Thirumala
University of Texas Medical Branch
Jaclyn Thoma
Rush Medical College
Allison Valerius
University of North Dakota
Pooja Venkatesh
University of Texas Southwestern Medical Center
Ashley Vincenty-Acosta
Ponce Health Sciences University
Jeremiah Wayne
University of Kentucky
Yunting Yu
Penn State College of Medicine
Andrea Zhang
University of Miami Miller School of Medicine
MEDICAL STUDENT RESEARCH SCHOLARSHIP
John Austin
University of Texas Health Science Center
San Antonio Long School of Medicine, San Antonio, TX
Liam Cooper-Brown
McGill University, Montreal, Quebec, Canada
Nishtha Gupta
Drexel University College of Medicine, Philadelphia, PA
Hunter Hazelwood
University of Kentucky, Lexington, KY
Andrea He
Virginia Commonwealth University, Richmond, VA
Seungrok Hong
University of Cincinnati College of Medicine, Cincinnati, OH
Guldamla Kalender
University of Minnesota, Minneapolis, MN
Christina Kargol
University of Cincinnati College of Medicine, Cincinnati, OH
Continued on page 26 ⊲ Tuesday, April 25, 2023 • Annual Meeting Daily 25
Celebrate These 2023 AAN Scholarship Award Recipients continued from page 25
Liliana Ladner
Virginia Tech Carilion School of Medicine, Roanoke, VA
Khashayar Mozaffari
George Washington University, Washington, DC
Tiffany Ong
Wake Forest School of Medicine, WinstonSalem, NC
Casey Orozco-Poore
Harvard Medical School, Boston, MA
Soo Hwan Park
Geisel School of Medicine at Dartmouth, Hanover, NH
Madeline Ross
University of Pittsburgh School of Medicine, Pittsburgh, PA
Ella Sahlas
McGill University, Montreal, Quebec, Canada
Cassandra Seifert
University of Maryland School of Medicine, Baltimore, MD
Natalie Smith
University of Washington School of Medicine, Seattle, WA
Jessica Tang
University of California Davis, Davis, CA
Meesha Trivedi
Texas Tech University Health Science Center
El Paso Paul L. Foster School of Medicine, El Paso, TX
Ethan Zerpa-Blanco, MS, NRP
Rowan University School of Osteopathic Medicine, Stratford, NJ
MEDICAL STUDENT SCHOLARSHIP TO THE ANNUAL MEETING
Nancy Eudalia Abarca, MPH
Michigan State University, East Lansing, MI
Kristen Lynn Adams
Liberty University College of Osteopathic Medicine, Lynchburg, VA
Bakhtawar Amad, MBBS
University of Florida, Gainesville, FL
Nyle Almeida
University of Oklahoma Health Sciences
Center, Oklahoma City, OK
Aisha Bosula
Drexel University College of Medicine, Philadelphia, PA
Valerie Braddick
Larner College of Medicine at the University of Vermont, Burlington, VT
Bobby Bradley
University of Tennessee, Knoxville, TN
Sydney Chatfield
Larner College of Medicine at the University of Vermont, Burlington, VT
Margaret Codispoti
Edward Via College of Osteopathic Medicine, Blacksburg, VA
Alecca Como
Oakland University William Beaumont School of Medicine, Rochester, MI
Elita Rose Delbruck
California University of Science and Medicine, Colton, CA
Victor Ekuta
Xavier University School of Medicine, San Diego, CA
Dafni F.T. Frohman
Renaissance School of Medicine at Stony Brook University, Stony Brook, NY
Mimi Tra Giang
Washington University in St. Louis School of Medicine, St. Louis, MO
Nupur N. Goel
Northeast Ohio Medical University, Rootstown, OH
Brittany Guidos, MS
University of Miami Miller School of Medicine, Miami, FL
Samantha Hao
Johns Hopkins University, Baltimore, MD
Paige Harmon
Emory School of Medicine, Atlanta, GA
Annie Ziyi He
UT Southwestern Medical Center, Dallas, TX
Jeremiah Hyslip
University of Massachusetts Chan Medical School, Worcester, MA
Akansha Jain
University of Iowa, Iowa City, IA
Nora Rano Jandhyala
NYU Grossman School of Medicine, New York City, NY
Hannah Marie Kelly
Case Western Reserve University School of Medicine, Cleveland, OH
Stella Knowlton
California Northstate University College of Medicine, Elk Grove, CA
Nancy Le
California Northstate University College of Medicine, Elk Grove, CA
Nzita Merdi Lutete
Brody School of Medicine, Greenville, NC
Ana Poulette Negron-Garcia
San Juan Bautista School of Medicine, Caguas, PR
Wendy Osei-Bonsu
Penn State College of Medicine, Hershey, PA
Elena Penhos
The Ohio State University, Columbus, OH
Elelia Phillips
University of Mississippi Medical Center, Jackson, MS
Lauren Danielle Pomerantz
Penn State College of Medicine, Hershey, PA
Andres Thomas Pope
University of the Incarnate Word, San Antonio, TX
Padmapriya Radhakrishnan
Edward Via College of Osteopathic MedicineAuburn, Auburn, AL
Maya Mounir Ramy
Texas A&M College of Medicine, College Station, TX
Muslima Sonia Razaqyar
University of Texas Health Science Center at San Antonio, San Antonio, TX
Morgan Nicole Rolon-Newton
Texas Tech Health Sciences Center El Paso, Paul L. Foster School of Medicine, El Paso, TX
Rishi Sharma
University of Minnesota Medical School, Minneapolis, MN
Katherine May Sheu, PhD
University of California, Los Angeles, Los Angeles, CA
Alex David Waldman
Emory University, Atlanta, GA
Emma Wetmore
Medical University of South Carolina, Columbia, SC
Emily Ling Xu
Icahn School of Medicine at Mount Sinai, New York City, NY
Ruiyi Yuan
University of Rochester, Rochester, NY
Chris Zajner
University of Western Ontario, London, ON, Canada
RESIDENT RESEARCH SCHOLARSHIP
Sponsored by the Clinical Research Subcommittee.
Ho Wing Andy Chan, MD
Mount Sinai Hospital, New York, NY
Tanziyah Muqeem, MD, PhD
Duke University Medical Center, Durham, NC
Alexander Sandweiss, MD, PhD
Baylor College of Medicine, Houston, TX
RESIDENT SCHOLARSHIP TO THE ANNUAL MEETING
Albert Aboseif, DO
Cleveland Clinic Foundation
Muhammad Khurram Afzal, MD
Saint Louis University School of Medicine
26 Tuesday, April 25, 2023 • Annual Meeting Daily
Julia Aigbogun, MD Tulane University School of Medicine
Mustafa Al-Chalabi, DO University of Toledo
Pooneh Memar Ardestani, MD, PhD Einstein Healthcare Network
Benjamin Becker, MD University of Michigan
Shivkumar Bhadola, MD Boston Medical Center
John Daniel Bireley, MD Cleveland Clinic
Alexander Buslov, MD
Thomas Jefferson University, Department of Neurology
Kiley Cameron, MD University of Nebraska Medical Center
Jordan Carrier, MD University of Rochester Medical Center
Papul Chalia, MD
Penn State Health Milton S. Hershey Medical Center
Midori Eckenstein, MD University of Utah Medical Center
Aimalohi Esechie, MD, PhD University of Texas Medical Branch
Alexander Seth Fein, MD NYU
Falen Fernandes, MD
Memorial University of Newfoundland and Labrador
Mark Phillips Figgie, Jr., MD University Hospitals/CWRU
Claudia Gambrah-Lyles, MD Children’s Hospital of Philadelphia
Mekka Garcia, MD NYU
Himanshu Gupta, MD Hamilton General Hospital
Patrick Hartnett, MD University of Vermont College of Medicine
Alice Hawkins, MD
Mount Sinai Health System
Katy Anne Helms, MD Wake Forest Baptist
Allyson Heng, MD
UAB Neurology
Lucas Horta, MD
BMC
Allison Courtney Hyland, MD
UCSF Department of Child Neurology
James Im, MD University of Toronto
Dan Tong Jia, MD Northwestern University
Naila Kausar, MD
Texas Tech University Health Sciences Center El Paso
Nadia Khalil, MD
University of South Florida Morsani College of Medicine
Arham Khan, DO Stony Brook Medicine
Nooshin Kiani Nia, MD University of Missouri Kansas City
Ryan Alexander Kollar, DO Hartford Hospital
Sanjith Prahas Krishnam, DO The University of Alabama Hospital
Brianne Kuns, DO Sparrow Hospital
Barbara Kwiecinska, MD University of Louisville Physicians Neurology
Alexus Ludwig, DO University at Buffalo
Daniel Mandel, MD Brown University
Erin McCoy, MD University of Louisville Child Neurology
Brooke McNeilly, DO UMass Memorial Medical Center-University Campus
Amit Mehta, MD Medstar Georgetown University Hospital
Nara Michaelson, MD New York Presbyterian Hospital/Weill Cornell
Hunter Mitchell, MD University of Tennessee
Shalane Alexa Morales-Nunez, MD Augusta University
Jorge Eduardo Patino Murillas, MD
The University of Texas Health Science Center at Houston McGovern Medical School
Ganapathiram Nambi, MD Medical University of South Carolina
George Plummer, MD University of Washington
Kevin Porras, MD
University of Miami
Casey Potts, DO Ohio State University
Min Qiao, MD
Barnes Jewish Hospital
Mathura Ravishankar, MD UPMC
Graham Kiyoshi Reid, DO Wright State University Boonshoft School of Medicine
Elsa Cassandra Rodriguez, MD University of Florida-Neurology
Deborah Kathleen Rose, MD
Duke
Julien Rousseau, MD, CM
University of Montreal
Lekshmi Perringassery Sateesh, MD
SUNY Downstate Medical Center
Benjamin Simpson, MD
Cedars-Sinai
Sandeep Singh, MD
Harbor UCLA Medical Center
Alyssa Smith, MD
Geisinger Medical Center
Jonathan Solomonow, MD
University of Maryland
M. Nelson Starkey, MD
Loma Linda University
Harry William Sutherland, MD
Yale University School of Medicine
Stephanie Syc-Mazurek, MD, PhD
Mayo Clinic
Angel Cadena Tejada, MD
Medical University of South Carolina
Panayiotis Tjionas, MD
BSWH-Temple
Megan Trenz, DO
NYU Langone Hospitals
Rani Priyanka Vasireddy, MBBS
University of Kentucky
Joaquin Augusto Vizcarra, MD
Emory University
Jeremy Wells, MD
University of North Carolina
Connor David Welsh, DO
Barrow Neurological Institute
Anudeep Yelam, MD
University of Missouri
Angela Young, MD
University of Manitoba
Musab Zorlu, MD
UConn Health
Tuesday, April 25, 2023 • Annual Meeting Daily 27
Why are clinical trials so slow?

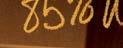


















Ask bigger questions. gene.com/askbiggerquestions




























































 Lidia Moura, MD, PhD, MPH @LidiaMouraNVL
Lidia Moura, MD, PhD, MPH @LidiaMouraNVL





 Desiree D’Souza @DesireeDSouz
Desiree D’Souza @DesireeDSouz






































