


















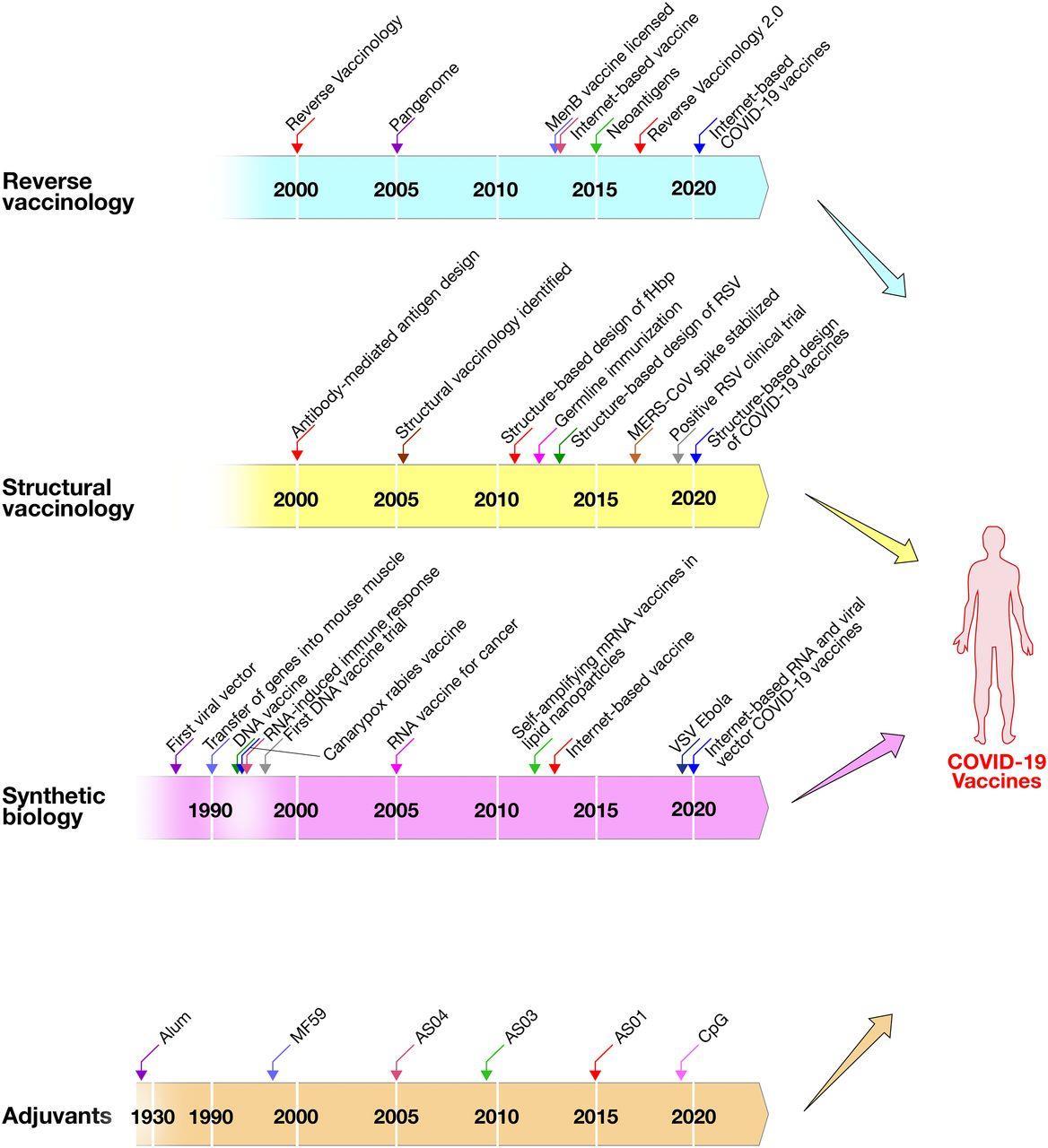
Rino Rappuoli et al. PNAS 2021;118:3:e2020368118
©2021 by National Academy of Sciences


Covid-19 Vaccines: a state of art


Titolo Presentazione
Rino Rappuoli et al. PNAS 2021;118:3:e2020368118








Testing of potential vaccine candidates begins in animals in preclinical trials. Once data supporting the safety are established, the first in human studies begin (phase 1) Most pediatric vaccine clinical trials begin in phase 2. Phase 3 studies provide data supporting the safety in large numbers of individuals and typically demonstrate whether the vaccine works in preventing disease (efficacy). Effectiveness and safety in the general population is evaluated in postlicensure phase 4 studies..






Twelve candidate vaccines currently in Phase III trial. COVID-19 Vaccines based on Spike protein DNA carried by adenoviruses.




state

Twelve candidate vaccines currently in Phase III trial. COVID-19 Vaccines based on Spike protein DNA carried by adenoviruses.
































Electrocardiogram (ECG) and cardiac magnetic resonance imaging (CMR) findings corresponding to a case of adverse events. ECG shows diffuse ST elevations. B. 4 chamber cine. C. T1 imaging demonstrates regional elevation in the lateral wall (between the arrows). D. T2 mapping shows elevated T2 values in the lateral wall (between the arrows). E. Late gadolinium enhancement imaging shows subepicardial enhancement (between the arrows) in the same areas of T1 and T2 elevations.

PredictedbenefitsofreductioninCOVID-19–relatedhospitalizationsand deathandrisksofmyocarditisafterseconddoseofmRNACOVID-19 vaccinationbyagegroup.





PotentialriskofmyocarditiswithCOVID-19mRNAvaccinationinthe120 daysaftervaccinationandpredictedpreventionofCOVID-19cases,COVID19–relatedhospitalizations,intensivecareunitadmissions,anddeaths accordingtoagegroupsandsex.










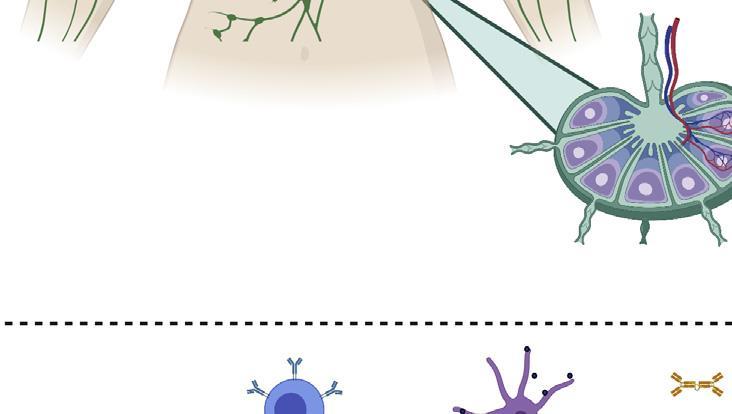
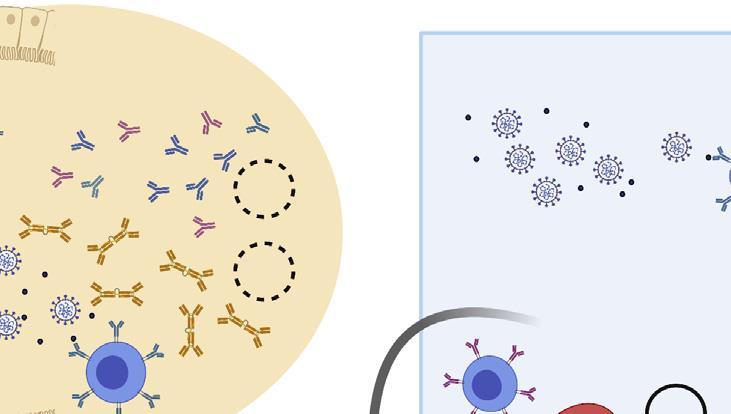
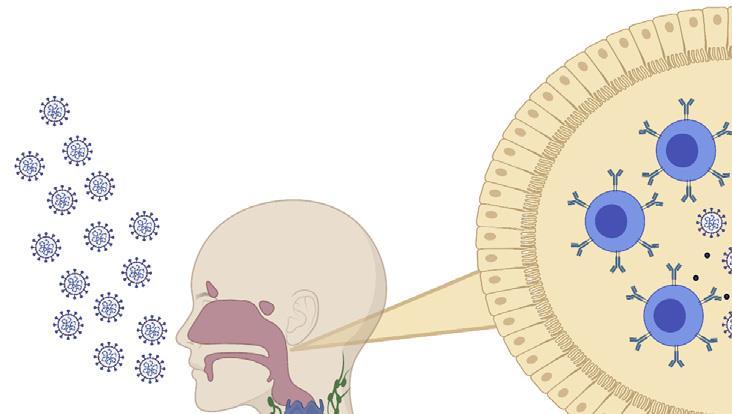
The generation of an immune response to a vaccine.

















p
pa of thevaccination schedule,thereare asyet no vaccineeffectivenessdatafor mRNA-1273from theUK.PublicHealth
England reported on theearly impact and effectivenessof COVID-19vaccination
compared with thevaccination coveragein thegeneral population from which thecases arederived).Usingvaccination coverage asthecontrol can producemorereliable information on vaccineeffectiveness,
interventions
Unsurprisingly,both antibody resp and vaccineeffectiveness24 arelow after asingledoseof either BNT16 mRNA-1273than after twodoses. levelsfollowingBNT162b2vaccin wanewithin 12weeksfollowingon
Demographic factors
High levelsof circulating virus
Closeproximityof people living together

Host factors
Old age
Previousinfection
Immunecompromise
Genetic polymorphisms
Underlying health conditions
High levelsof vaccineuptake
High levelsof herd immunity
Vaccine accessfactors
Which vaccineused
Number of doses
Timing between doses
Heterologousprime–boost

national vaccinebodies
Limited accessto vaccines
Thereis,however,protection from vaccinedose, with vaccineeffectiv after asingledoseof BNT162b2o and after asingledoseof AZD122
Viral variant factors
Antigenic mismatch with vaccine
Increased transmissibility
Immune factors
High antibodytitres
Qualityof antibody (neutralization)
Tcells
Fig.1|Factorsinfluencingvaccineeffectiveness.Multiplefactorscanincreaseordecreasevaccine effectiveness(VE)at boththeindividual level andthepopulationlevel.
Covid-19 Vaccines: a state of art.

Oneadvantageof delayingvaccina that antibodyresponsesto theseco might begreater;theantibody res BNT162b2isgreater in individual 80yearsor older when thevaccina isincreased from 3to 12weeks25.U thelength of thedoseinterval com toadecision by national vaccinec asto whether it isbetter to get alo level of immuneprotection in agr number of peopleor moreprotecti fewer people.Thisrisk–benefit pr changeover time,especially once coveragehasbeen achieved in the moreat risk groups,but also in res totheemergenceof new viral vari (TABLE 2).In June2021, individuals 40yearsor older in theUK werein toscheduletheir second vaccinati 8weeksrather than 12weeksasca
Delta(B.1.617.2) variant increased important factor that must beincl any risk–benefit calculationsisthe seriousadverseeffectsassociated w vaccines(BOX1).

626–636 (2021) 20
0123456789();:
Covid-19 Vaccines: a state of art.




Covid-19 Vaccines: a state of art.


















The presence of specific SARS-CoV-2 B &T cell is associated with the development of antibodies and neutralizing activity (PRNT). This methd was used to evaluate immunogenicity of the vaccine







of the
response correlates with viral control and survival.




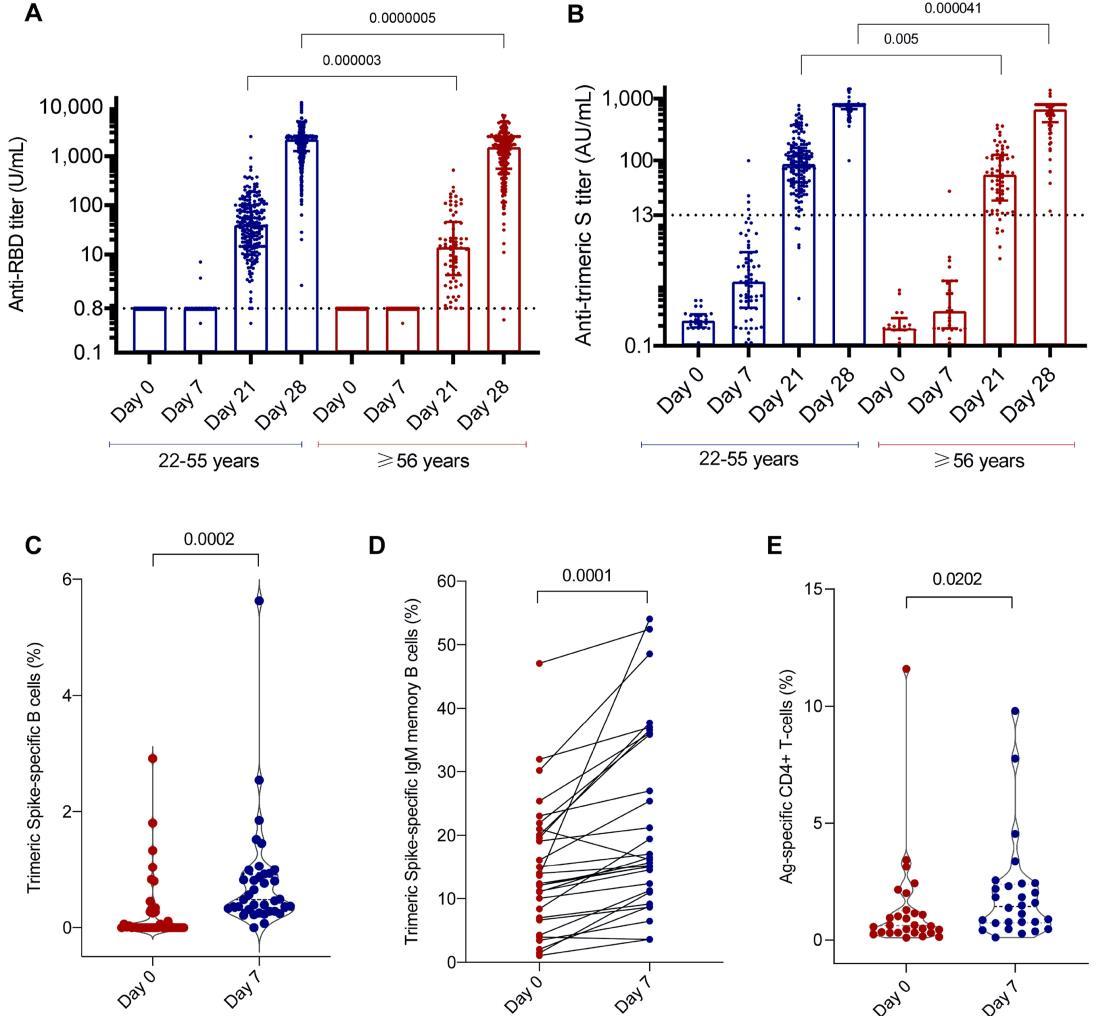





































• Il 70% dei pazienti con titolo anticorpale assente (IgG < 0.8 U/mL) è in terapia con regimi terapeutici che includono

• L’analisi dell’immunofenotipo linfocitario nei pazienti TX con distinta risposta umorale non mostra differenze statisticamente significative (P value CD3+ 0.5129; p value CD4+ 0,4761; p value CD19+ 0,6567).





B cell phenotype analysis showed similar frequencies of total CD19+ B cells, however the analysis of maturational B cell subsets showed a lower frequency of memory B cell (CD19+CD27+CD21+) in NR compared to NR (p=0.008) (Fig. 4A). This data was also confirmed by a lower frequency of IgD-CD27+ switched memory B cell (p=0.002).









DOI:10.1002/pd.5985

Angsumita Pramanick1,2 | AbhiramKanneganti1 | JingLinJeslynWong1,2 | SarahWeilingLi1,2 | Pooja Sharma Dimri1 | Aniza Puteri Mahyuddin2 | SaileshKumar3,4 | Sebastian Enrique Illanes5 | Jerry Kok YenChan6,7 | LinLinSu1,2 | Arijit Biswas1,2 | Paul Anantharajah Tambyah8 | Ruby Yun‐JuHuang9 | CitraNurfarah Zaini Mattar1,2 | MaheshChoolani1,2
1Department of Obstetricsand Gynaecology, National University Hospital Singapore, Singapore
2Department of Obstetricsand Gynaecology, YongLoo Lin School of Medicine,National University of Singapore,Singapore
3Mater Research Institute‐University of Queensland,South Brisbane,Queensland, Australia

4Faculty of Medicine,TheUniversity of Queensland,Herston,Queensland, Australia
5Department of Obstetrics& Gynaecology, Faculty of Medicine,Universidad delosAndes, Santiago,Chile

6Department of Reproductive Medicine,KK Women'sand Children'sHospital,Singapore
7Academic Clinical Program in Obstetricsand Gynaecology,Duke‐NUSMedical School, Singapore
8InfectiousDiseasesTranslational Research Programme,Department of Medicine,Yong Loo Lin School of Medicine, National University of Singapore,Singapore
9School of Medicine, Collegeof Medicine, National Taiwan University, Taipei,Taiwan
Correspondence
Mahesh Choolani, Department of Obstetrics and Gynaecology, NUHSTower Block,Level 12,1EKent Ridge Road, Singapore 119228. Email: obgmac@nus.edu.sg

Abstract
There are over 50 SARS‐CoV‐2 candidate vaccines undergoing Phase II and III clinical trials. Several vaccines have been approved by regulatory authorities and rolledout for useindifferent countries.Duetoconcernsof potential teratogenicity or adverse effect on maternal physiology, pregnancy hasbeen aspecific exclusion criterion for most vaccinetrialswithonlytwotrialsnot excludingpregnant women. Thus, other than limited animal studies, gradually emerging development and reproductivetoxicity data,andobservational datafromvaccineregistries,thereisa paucityofreliableinformationtoguiderecommendationsfor thesafevaccinationof pregnant women. Pregnancy is a risk factor for severe COVID‐19, especially in women with comorbidities, resulting in increased rates of preterm birth and maternal morbidity.Wediscussthemajor SARS‐CoV‐2 vaccines,their mechanisms of action,efficacy,safety profileandpossiblebenefitstothematernal‐fetal dyadto create a rational approach towards maternal vaccination while anticipating and mitigatingvaccine‐relatedcomplications.Pregnant womenwithhighexposurerisks or co‐morbiditiespredisposing to severe COVID‐19 infection should beprioritised for vaccination. Those with risk factors for adverse effects should be counselled accordingly.It isessential to support patient autonomy by shared decision‐making involving arisk‐benefit discussion with the pregnant woman.
Key points
What isalready known about thistopic?
✏COVID‐19 infection in pregnancy leadsto an increase in adverse maternal outcomes.
✏Owing to paucity of data regarding SARS‐CoV‐2 vaccine use in pregnancy there isuncertainty regarding safety of useand subsequent pregnancy outcomes.


