7 minute read
A Survey of Clinicians: Bringing Tissue Adhesive to the Neonatal Population
For more information on the PediNeoSig and how to join: www.avainfo.org/pedineosig
Lori Kaczmarek, MSN, RN, VA-BC™ | 2021 AVA Presidential Advisor Vascular Access Clinical Specialist, Adhezion Biomedical, LLC
Perhaps the most exciting recent advancement in vascular access care and technology has come in the form of glue. Glue, or tissue adhesive (TA), has been around for many years, but it was not until September 2017 when the Food and Drug Administration (FDA) approved a unique formula specifically for vascular access devices (VADs) and with no age restrictions. And in just three short years, TA has been adopted by the Infusion Nurses Society (INS) in the recently published (January 2021) Infusion Therapy Practice Standards as a standard of care for all VADs.
For context and disclosure, I am a consultant for Adhezion Biomedical, LLC, makers of SecurePortIV® (SPIV) TA for vascular access. Prior to my tenure with the company, I practiced as the director of a vascular access team in southeast Wisconsin. Our 25-member team shared the same challenges all clinicians do securing VADs, protecting them, and, of course, minimizing the unscheduled dressing changes and migration issues. I cared for primarily adult patients in my 10 years with the team, so I brought no direct, hands-on insertion or maintenance experience with the neonatal ICU (NICU) VADs. As luck would have it, my first clinical education support assignment for TA landed me right in the heart of a busy NICU. I was amazed by the skill and dedication NICU vascular access and bedside clinicians demonstrated as they place and maintain VADs that to me looked like a piece of thread. The babies were so tiny that I quickly appreciated the unique challenge that came with securing and dressing the VAD. Unlike adults, there is zero tolerance for line migration and a limited surface area makes securement a far greater challenge. Over the last couple of years, I have traveled and engaged with clinicians from NICU, infant, and pediatric centers across the United States (US). My interest and desire to support the most fragile babies led me to conduct a comprehensive survey with clinicians about their experiences using TA in this population. What prompted them to explore TA? How does it contribute to VAD care in the NICU? And what changes came about because of glue? The healthcare landscape has certainly changed over the past 10-15 years. The advent of value analysis teams and material management require a different approach to trial and implementation of new products. While I understood the process of adding new products to the adult population, neonates and infants were a new ballgame. The unique needs of infants and pre-term babies require multiple
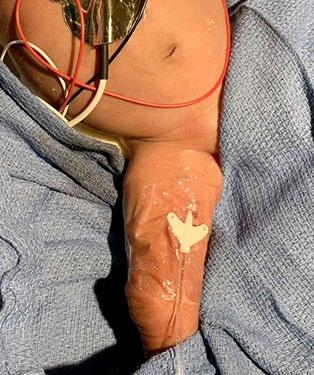
Photo credit: Adhezion Biomedical, LLC CONTINUED ON NEXT PAGE
A SURVEY OF CLINICIANS, CONTINUED FROM PREVIOUS PAGE
stakeholder input prior to product trials. I wanted to better understand what process each used and who was involved in their product selection? What factors were considered? And how long did it take to trial this product and adopt it as part of their VAD toolbox? This qualitative survey explored many variables that every NICU clinician wants answered when they consider TA for their patients. Twenty-one NICU clinicians randomly selected from a database of consumers who implemented or were currently trialing TA were sent an electronic survey on Dec. 1, 2020. I received 11 responses, each representing a unique facility, by the survey close date Dec. 31, 2020. The title or position of respondents included Peripherally Inserted Central Catheter Registered Nurse (PICC-RN), Clinical Nurse Specialist (CNS), Neonatal CNS/Nurse Practitioner (NP), and Vascular Access Managers. The majority (n=6) of respondents said they first learned about TA from a colleague, four through conference attendance, and one from a vendor. The primary reasons for seeking a trial with TA was improve securement (n=9), manage line migration issues (n=9), and address bleeding or oozing of lines (n=7). Other issues include dissatisfaction with previous securement device (n=1) and skin integrity issues with previous securement device (n=1). Most (n=10) applied TA to PICC lines during their trial. Three also included central venous catheters (CVC), two also used the TA on umbilical catheters, three included peripheral IVs (PIV) in their trial while one focused their trial only on arterial lines. The key stakeholders involved in the decision to trial or implement TA included physicians or intensivists, infection prevention, advanced practice providers (APRN/PA), vascular access team (VAT), clinical nurse specialists (CNS) and bedside caregivers including RN’s and respiratory therapists (RT). Four identified value analysis (VA) also being included in the decision. I inquired about the length of their trial and how many applications were used to conclude about the safety and efficacy of TA on addressing their target goals. Interestingly, the trial period ranged from 12-150 days (average 62 days). One box of product includes 50 single-use TA applicators and the respondents averaged 48 applications (range 10-90), or roughly one box to conclude their trial. Given that six of these trials were initiated in 2020 during the COVID-19 pandemic, I expected these numbers might have been higher. Formal approval for implementation happened within 1-3 months at five of the facilities. One responded that they did not require additional approval. Three continued to use TA while awaiting formal approval. Only one required more than three months to receive approval and one facility is conducting a larger scale trial and expanding the device types for use.
A common question that comes with TA use is how or when to remove the glue and what does that process look like? I asked and the clinician comments speak for themselves: • “The adhesive is only removed when needed.
The site is cleaned with CHG and then (in-house standard adhesive remover) is used to remove the remainder of the glue.” • “We don’t use adhesive remover.” • “Use (in-house standard adhesive remover) wipes or spray and remove gently with gauze.” • “Soak the site with remover and let it sit for 30+/- sec.” • “If line adjustment is needed within the first 7 days, apply a couple drops of adhesive remover to dissolve the glue prior to cleaning with CONTINUED ON NEXT PAGE
A SURVEY OF CLINICIANS, CONTINUED FROM PREVIOUS PAGE
chlorhexidine. In most cases, use of adhesive remover is not required; the seal can be broken by gently lifting up on the catheter.” • “Most of the time the glue comes off with the dressing when dressing is removed. If glue remains, (in-house standard adhesive remover) wipe is used, and glue is easily removed with a few seconds of gentle application strokes.”
The majority of respondents (n=6) identified a colleague as their first introduction to TA in the NICU, so I also asked what they would tell another colleague if they were asked about adding TA into their practice. Again, let’s hear it directly from them: • “The product has definitely resolved the line migration issue, and in addition, we are now at month 22 without a single defined catheter line associated bloodstream infection (CLABSI).” • “I think it is a good product. I would encourage them to trial it.” • “To consider its use if they have an existing issue.
Start with trial and track data. Nice option to have.” • “Absolutely love the product. It significantly improved securement for our lines, as well as an added layer of protection to potential bloodstream infections (BSI).” • “Easy to use. Good customer service.” • “I would recommend use based on success in most of our patients but share with them our experience with using it in preterm/neonates.” • “Very pleased with the product and would recommend use in the NICU. We have used down to 500gms without incident.” • “In theory this is a great concept.” • “Absolutely add. Well worth the small price for the added benefits.” • “Primary benefit with (TA) has been reduction in oozing/bleeding at the insertion site for PICC placement. We recently converted to a new EMR platform (Epic); report requests are still pending for how often dressings are being changed.
Intensivists were in-serviced on use of the product for CVCs, but we do not have a mechanism in place for tracking how often it is being used for their line insertions.” • That we have experienced less calls for dressing changes due to bleeding. That we have experienced only 2 dislodged PICC lines since tracking data.
One of which was not using (TA) in home setting and one was patient with terrible eczema and had oils all over skin.”
All of the respondents shared that they realized multiple improvements in their NICU vascular access care and management including:







