14 minute read
Effects of Polypropylene on Tetrahymena Cell Counts, Swim Speed, and Vacuole Formation
from Scientia 2020
Noah Mendoza, Madison Ambrose, Abel Thomas, Rithvik Bartham, Samantha Hodges, Tamarah Adair, Ph.D.
Abstract
Advertisement
Nanoplastics, plastic fragments smaller than 1 μm, are prevalent throughout the environment. The effect of nanoplastics on terrestrial ecosystems is largely unknown. Tetrahymena pyriformis is a single-cell model organism frequently used for analyzing toxicity which also represents a major group of predators (ciliates) in the soil ecosystem. The goal of this study is to shed light on the impact of polypropylene (PP) nanoplastics on eukaryotic cells by examining the effects of polypropylene treatments on Tetrahymena pyriformis. To generate potential breakdown products from the polypropylene, treatment solutions were made by shredding and adding heat to polypropylene baling twine in proteose-peptone-tryptone (PPT) media. To test the null hypotheses that polypropylene nanoplastics do not impact reproduction, swim speed, and vacuole formation of Tetrahymena pyriformis, assays were conducted in both the control and the polypropylene treatment cultures after 48 hours. The results disprove the null hypotheses and indicated that polypropylene had a significant impact indicated by an increase in all three variables. These results indicate that nanoplastics do have an effect on single-cell organisms in culture. Further studies will focus on the effect of nanoplastics on terrestrial ecosystems. The soil ecosystem is the foundation for a healthy biotic environment and investing in research within this scope will help determine the system effects of plastics on the overall ecosystem.
Introduction
Plastics have found their way into many aspects of our lives, and with an ever-growing population, the presence of plastics and more specifically, nanoplastics, in our lives will continue to grow. The increasing presence of nanoplastics has gained attention over recent decades and is currently gaining the attention of many media outlets, such as the National Geographic article We Depend On Plastic. Now, We’re Drowning in It (Parker, 2018). The term “nanoplastics” refers to particles of plastic that are smaller than 1 μm in diameter. Currently, humans are producing 245 million tons of plastics annually to keep up with the growing population, and this number will only continue to rise (Andrady, 2011). The increase in plastic usage requires an increase in the disposal and recycling of plastics currently consumed. This is problematic because, when not recycled, plastics may take decades or even centuries to fully decompose, leaving an abundance of nanoplastics in the environment. Without an effective way to decompose nanoplastics, this could eventually bring forth negative health effects on humans and organisms in the environment. Nanoplastics are often ingested by organisms in the environment and become embedded in their intestines, leading to digestive blockages and appetite suppression (Galloway & Thompson, 2013).
Unfortunately, not all plastics are disposed of properly, resulting in plastics being incorporated into agriculture through mulch and fertilizers. Many fields, however, are being impacted by a type of baling twine that is often left behind to be decomposed, resulting in the introduction of nanoplastics directly into the soil and indirectly into agricultural goods. This polypropylene (PP)-based twine is often treated with various chemicals that enhance its ability to withstand weather and UV light.
With this being said, limited research has been done regarding the impact of plastics on terrestrial ecosystems and organisms (Rillig, 2012). In order to understand the impact of nanoplastics within ecosystems, Tetrahymena pyriformis was exposed to polypropylene (PP) that was derived from baling twine. In order to study its effects on terrestrial organisms, PP was administered to Tetrahymena pyriformis that was cultured within the lab. Tetrahymena pyriformis was specifically chosen due to its credibility as a model organism in laboratory studies and its presence within various terrestrial ecosystems. The effects of PP on the Tetrahymena pyriformis were examined through asexual reproduction rate (cell counts), swim speed, and food vacuole formation.
Tetrahymena pyriformis has been used in countless research studies due to its consistency and easily identifiable developmental traits (Cole et al., 2012; Sauvant et al., 1999). Tetrahymena pyriformis uses phagocytosis to engulf food, in a non-discriminatory manner. This characteristic is observed when feeding on laboratory substances, such as India ink,
allowing researchers to measure consumption rates of various food sources (Aijaz & Koudelka, 2017; Kaminskaya et al., 2007). In this experiment nanoplastics found in baling twine will be used to test the hypothesis that nanoplastics affect the reproduction, swim speed, and vacuole formation of Tetrahymena pyriformis.
Materials and Methods
General Experimental Design
The following experiment was designed to test the effect of nanoplastics, specifically polypropylene from baling twine, on Tetrahymena pyriformis. Black baling twine, which has added chemicals to provide UV protection, longevity, and resistance to moisture, was used in the experiments.
Preparation of Polypropylene
To prepare the polypropylene, baling twine was cut into small pieces. After shredding the baling twine into small pieces, 0.5 g of twine was combined with 50 mL of protease-peptonetryptone (PPT) media in a sterile glass jar. The contents of the glass were then placed into a microwave for approximately an hour to simulate environmental breakdown. The solution was kept overnight in a 55-degree Celsius water bath before filtering, so that the solution contained nanoplastics less than 5 µm in diameter. PPT media was added so that the concentration of filtered solution was 0.5 g/50 mL or 1%. The liquid was autoclaved for 15 minutes to eliminate any remaining bacteria and microbes in the solution.
Preparation of Tetrahymena pyriformis Culture
After the solution was sterilized in the autoclave, 5 mL of a 6.1 x 10 4 cells/mL Tetrahymena pyriformis culture was added to either 45 mL of PPT media for the control solution or 45 mL of the filtered Polypropylene (PP) solution for the treatment solution, resulting in a 1:10 dilution ratio for both solutions.
The Effect of Polypropylene Treatment on Tetrahymena pyriformis Cell Counts
5 mL samples of the treatment and control were aseptically transferred from the 50 mL flask, which was swirled before pipetting, to separate sterile test tubes. To examine cell counts following exposure of PP to Tetrahymena pyriformis, 2 µL of either the control or treatment culture of Tetrahymena pyriformis was combined with 1 mL of iodine. The iodine was used to stain the Tetrahymena pyriformis and pictures were captured of cells to provide an accurate count. The counts were performed in triplicate for both the control and treatment solutions to ensure accuracy.
The Effect of Polypropylene Treatment on the Swim Speed of Tetrahymena pyriformis
To conduct the swim speed assay, a 20 µL sample of either the control or treatment media was placed onto a flat slide. A millimeter marked ruler was placed under the slide to measure the distance traveled by Tetrahymena pyriformis. Only Tetrahymena pyriformis swimming in a straight line during the entire length of their swimming path was recorded. Swim speed was expressed as millimeter (mm)/ seconds (s). This was repeated 10 times for both the control and the treatment cultures. While collecting this data, the samples were videotaped to ensure the correct data was recorded.
The Effect of Polypropylene Treatment on the Vacuole Formation of Tetrahymena pyriformis
A 10 µL sample of either the treatment or control media were placed on concavity slides. A 2 µL sample of India ink, which helped to identify food vacuoles, was mixed into the media. A 2 µL sample of methylcellulose was added to slow down the speed of Tetrahymena pyriformis. A compound microscope was then used to observe the number of food vacuoles formed after 5 minutes and 15 minutes. This was repeated for 10 cells.
Statistical Analysis
Statistical analyses for the three assays (cell count, vacuole formation, and swim speed) were conducted using the “Data Analysis” ToolPak program in Excel. The data analysis was used to conduct descriptive statistics, which calculated the means and standard errors of each of the data sets. An F-test was conducted to compare the variances of the control and the treatment group and determine the type of t-test that should be conducted to examine the differences in the control and treatment means, a t-test was conducted to test the null hypothesis.
Results
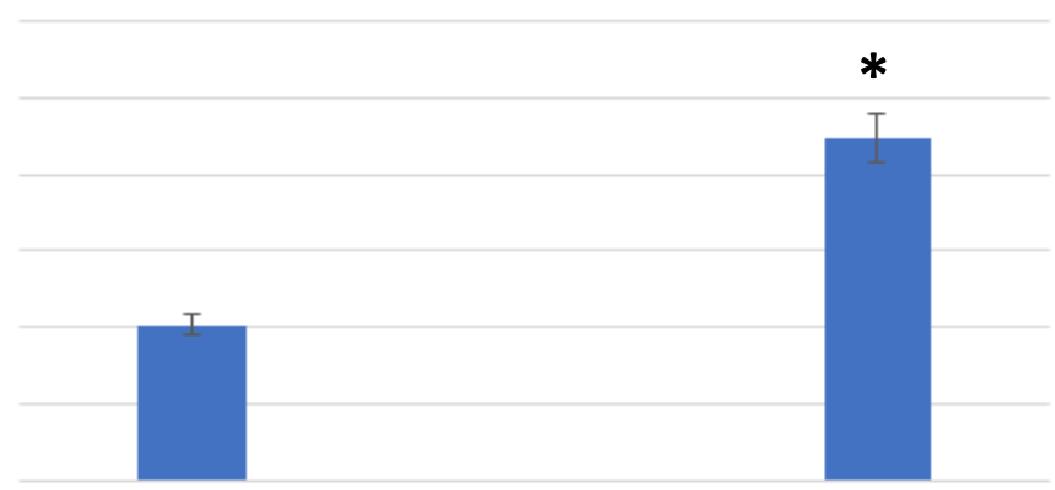
The following discussion reports the results from the reproduction, swim speed, and food vacuole formation assays, overall displaying the impact that the PP had on the Tetrahymena pyriformis by comparing the control group to the treatment group. Figure 1 compares the average cell count values for the control and treatment medias of Tetrahymena pyriformis. The control media showed a mean value of 20,274 cells per milliliter and a standard error of 1,351 cells per milliliter. The treatment
60000
Cell Count per mL 10000 0 40000 30000 20000 50000 Average Cell Count of Control and Treatment Groups
Control Media Sample Condition Treatment Media
Figure 1: Polypropylene significantly increased the Tetrahymena pyriformis cell counts . Control: n = 171, Treatment: n = 174. (* = p < 0.01.) e presented as mean (standard deviation) or frequency (%).
showed a mean value of 44,704 cells per milliliter and a standard error of 3,212 cells per milliliter. An F-test was used to determine the presence of any significant difference in variance between the cell count of the control and treatment medias. The results indicated that the F-critical value was larger than the F-value, allowing for a t-test assuming equal variance to be conducted. The t-test concluded that there was a significant difference in cell counts between the control and treatment medias following the exposure to polypropylene (p = 1.648 x 10 -11 , p < 0.05). More specifically, the polypropylene treatment led to a significant increase in the cell counts of Tetrahymena pyriformis.
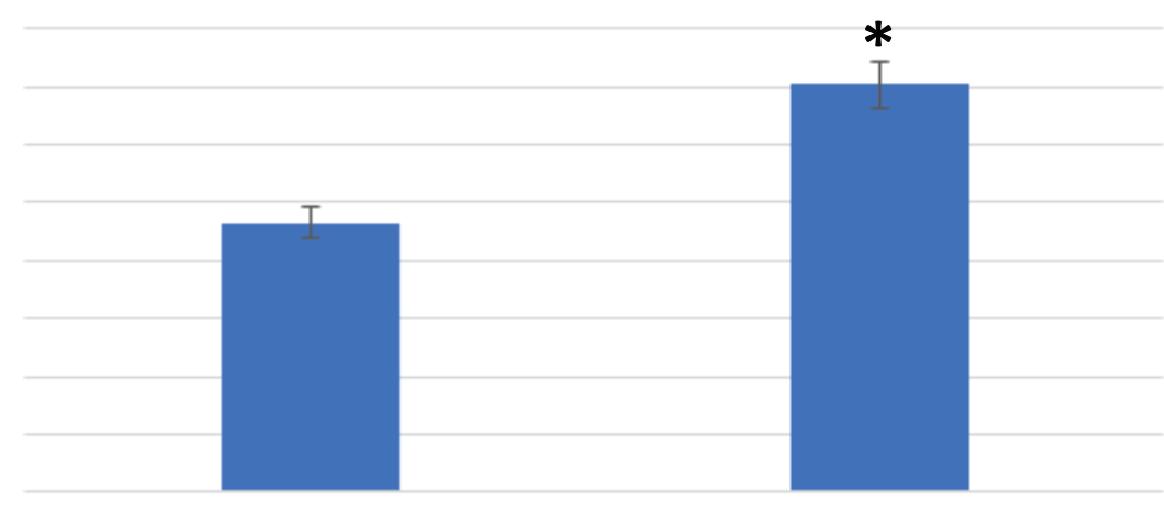
Figure 2 demonstrates the effect polypropylene had on the swimming speed of Tetrahymena pyriformis. The control media swim speed assay showed that the cells traveled an average of 0.366 millimeters per second with a standard error of 0.012, calculated using the “Data Analysis” ToolPak. The treatment media swim speed assay showed that the cells traveled an average of 0.401 millimeters per second with a standard error of 0.013. A F-test was used to determine the presence of any significant difference in variance between the swimming speed of the control and treatment medias. The results indicated that the F-critical value was larger than the F-value, allowing for a t-test assuming equal variance to be conducted. The t-test concluded that there was a significant difference in swim speed between the control and treatment medias (p = 0.038, p < 0.05). More specifically, the polypropylene treatment led to a significant increase in the swimming speed of Tetrahymena pyriformis.
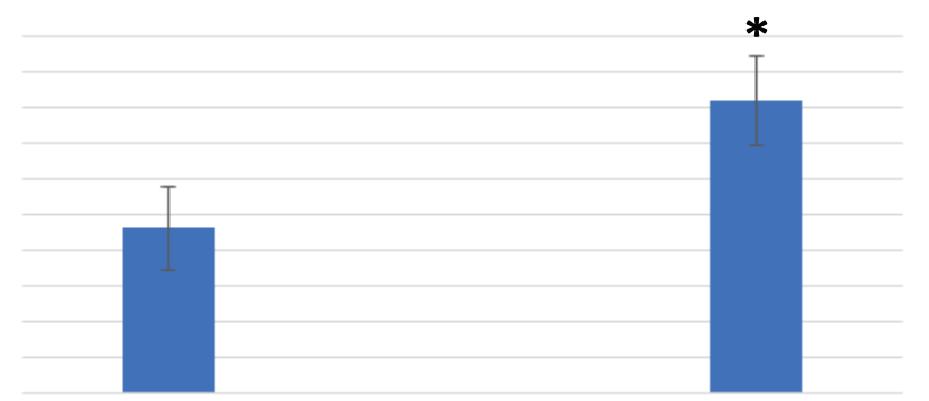
Figure 3 compares the average food vacuole formation of the control and treatment media of Tetrahymena pyriformis after 5 minutes. The average number of food vacuoles formed at 5 minutes for the control media was 2.323 with a standard error of 0.135. The average number of food vacuoles formed at 5 minutes for the treatment media was 3.516 with a standard error of 0.194. An F-test was used to determine the presence of any significant difference in variance between the swimming speed of the control and treatment medias. The results indicated that the F-critical value was larger than the F-value, allowing for a t-test assuming equal variance to be conducted. The t-test concluded that there was a significant difference in food vacuole formation between the control and treatment medias (p = 7.025 x 10 -7 , p < 0.05). Specifically, there was a significant increase in food vacuole formation in the treatment media after 5 minutes.
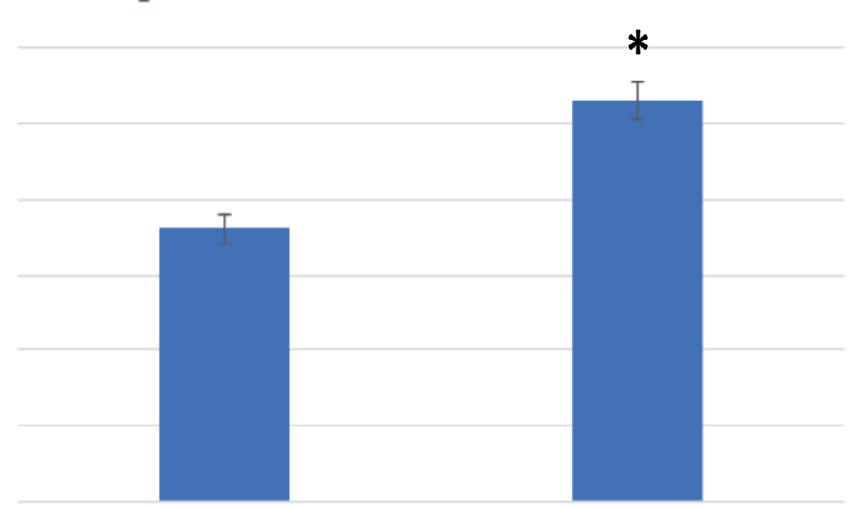
Figure 4 compares the average food vacuole formation of the control and treatment media of Tetrahymena pyriformis, with standard error after 15 minutes. The average number of food vacuoles formed at 15 minutes for the control media was 3.609 with a standard error of 0.195. The average number of food vacuoles formed at 15 minutes for the treatment media was 5.317 with a standard error of 0.246. An F-test was used to determine the presence of any significant difference in variance between the swimming speed of the control and treatment medias. The results indicated that the F-critical value was larger than the F-value, allowing for a t-test assuming equal variance to be conducted. The t-test concluded that there was a significant difference in food vacuole formation between the control and treatment medias (p =1.149 x 10 -7 , p < 0.05). Specifically, there was a significant increase in food vacuole formation in the treatment media after 15 minutes.
Number of Food Vacuole Formed 0 1 1.5 0.5 2 2.5 3.5 4 3
Sample Condition Control Media Treatment Media
Figure 2. Polypropylene significantly increased the Tetrahymena pyriformis swim speed. Control: n = 175, Treatment: n = 194. (* = p < 0.05)
Average Swim Speed of Control and Treatment
Speed (mm/s) 0.37 0.32 0.36 0.41 0.4 0.39 0.38 0.33 0.34 0.35 0.42
Treatment Media Control Media Sample Condition
Figure 3. Polypropylene significantly increased the Tetrahymena pyriformis vacuole formation over 5 minutes. For control at 5 minutes: n = 156. For treatment at 5 minutes: n = 120. (* = p < 0.01)
Number of Food Vacuole Formed 6
4 5
3
2
1
0 Average Food Vacuole Formation: 15 Minutes
Control Media Treatment Media Sample Condition
Figure 4. Polypropylene significantly increased the Tetrahymena pyriformis vacuole formation in 15-minute interval. For control at 15 minutes: n= 161, and treatment for 15 minutes n=85. (* = p < 0.05.)
This experiment examined the effect of polypropylene, a frequently used plastic that is found in baling twine, on Tetrahymena pyriformis, a soil microorganism. Two separate cultures, one containing a control media (PPT) and the other with polypropylene (PP), were used to test the effect of polypropylene on Tetrahymena pyriformis cell counts, swim speeds, and food vacuole formation. Overall, the results disprove the null hypothesis, that nanoplastics do not have an effect on Tetrahymena pyriformis cell counts, swim speed, and food vacuole formation.
This study showed an increase in cell counts of the ciliate Tetrahymena pyriformis when the nanoplastic polypropylene was added into the media. The increase in cell counts could be due to Tetrahymena pyriformis consuming bacteria in the polypropylene mixture or through absorption of additional unknown nutrients. This increase in nutrients could have provided the energy for the increase in swim speed and reproduction for Tetrahymena pyriformis. Vacuole formation at 5 and 15 minutes was increased by the presence of PP, which could be an indicator that nanoplastics increase the feeding behavior of Tetrahymena pyriformis. But this could also indicate that the solution that we placed Tetrahymena pyriformis in was nutrient rich and allowed for more frequent feeding. This experiment could be repeated using purified nanoplastic which would eliminate the variable of additional nutrients that may have been present in the solution. A recent study performed by Aijaz & Koudelka indicates that Tetrahymena pyriformis’ use of phagocytosis when engulfing substances, like bacteria and other foreign material, allows them to have limited discrimination between the substances they consume (Aijaz & Koudelka, 2017). In return, this ability could help limit the impact that a particular substance may have on the ecosystem by using them to create a barrier between the foreign material and the environment (Aijaz & Koudelka, 2017). The results from our study indicate that one of the potential substances that Tetrahymena pyriformis could consume includes nanoplastics. Additionally, Tetrahymena pyriformis’ ability to withstand the strains of the bacteria within Aijaz and Koudelka’s study displays their high tolerance to unfavorable environments. Future field studies involving terrestrial ciliates could be used to determine if ciliate have the ability to remove nanoplastics from the environment. Future experiments could examine different sources of polypropylene that are common in items that humans use daily. This would make the findings of this study more applicable to humans, as they could directly see how their practices affect microorganisms and their broader impact on the ecosystem. Another direction that could be explored is the effect of nanoplastics on protein production, or also whether there are correlations present between cellular processes such as vacuole formation and increasing concentrations of polypropylene nanoplastics. Since our findings show an increase in ciliate population coinciding with added polypropylene in the ciliate environment, this could cause soil ecosystems to become unbalanced. Since some ciliates are hydrogenosomes and have the ability to produce methane, increases in these organisms in the presence of nanoplastics could lead to an increase in greenhouse gases, such as methane (Lynn, 2009). Overall, the results of the experiment found that polypropylene significantly increased Tetrahymena pyriformis cell counts, swim speed, and vacuole formation.
References
Aijaz, I., & Koudelka, G. B. (2017, March 3). Tetrahymena phagocytic vesicles as ecological micro-niches of phage transfer. Federation of European Microbiological Societies, 93(4), 1-8. Retrieved from https://searchproquest. com.ezproxy.baylor.edu/docview/1992002353?accountid=7014&pq-origsite=summon Andrady, A. L. (2011). Microplastics in the Marine Environment. Marine Pollution Bulletin, 62(8), 1596-1605. doi:https://doi.org/10.1016/j.marpolbul.2011.05.030 Cole, E., & Sugai, T. (2012, March 20). Developmental progression of Tetrahymena through the cell cycle and conjugation. Science Direct, 109, 177-236. Retrieved from https://www.sciencedirect.com/science/article/pii/
B9780123859679000074?via=ihub Galloway, T. S., Thompson, R. C., & Wright, S. L. (2013). The
Physical Impacts of Microplastics on Marine Organisms: A Review. Environmental Pollution, 178, 483-492. doi:https://doi.org/10.1016/j.envpol.2013.02.031 Kaminskaya, A., Pushkareva, V., Moisenovich, M., Stepanova,
T., Volkova, N., Romanova, J., Litvin, V., Gintsburg, A., &Ermolaeva, S. (2007, December). Stimulation of biofilm formation by insertion of Tetrahymena pyriformis wells within Burkholderia cenocepacia biofilms. Pleiades Publishing, 22, 186-194. Retrieved from https:// link-springer-com.ezproxy.baylor.edu/article/10.3103/
S0891416807040088 Lynn, D. H. (2009). Ciliates. Encyclopedia of Microbiology (Third Edition), 578-592. doi:https://doi.org/10.1016/
B978-012373944-5.00248-0 Parker, L. (2018, May 16). We Depend On Plastic. Now, We’re
Drowning in It. Retrieved from https://www.nationalgeographic.com/magazine/2018/06/plastic-planet-waste-pollution-trash-crisis/ Rillig, M. C. (2012, May 31). Microplastic in Terrestrial Ecosystems and the Soil. Environmental Science and Technology, 46, 6453-6454. Retrieved from https://pubs.acs.org/doi/ pdfplus/10.1021/es302011r Sauvant, M. P., Pepin, D., & Piccinni, E. (1999, March 23).
Tetrahymena pyriformis: A tool for toxicological studies. Pergamon, 38(7), 1631-1669. Retrieved from https://www.sciencedirect.com/science/article/pii/ S0045653598003816?via=ihub






