Tech and Public Policy To Save The Brain

Neurodegenerative Diseases are a Silent Emergency That Requires Imminent Solutions Driven by Technology and Public Policy
Lead Author:
Giani, Luca
Priyamvada Saraf
Mathew Alexander
Sohaib Nasim
Hardy Ding
REPORT MAY 2023 TECHNOLOGY AND PUBLIC PURPOSE PROJECT
Technology and Public Purpose Project Belfer Center for Science and International Affairs
Kennedy School
JFK Street Cambridge, MA 02138
and views expressed in this report are solely those of the author(s) and do not imply endorsement by Harvard University, Harvard Kennedy School, or the Belfer Center for Science and International Affairs.
2023, President and Fellows of Harvard College
Harvard
79
www.belfercenter/TAPP Statements
Copyright
Tech and Public Policy To Save The Brain
Neurodegenerative Diseases are a Silent Emergency That Requires Imminent Solutions Driven by Technology and Public Policy
Lead Author:
Giani, Luca
Priyamvada Saraf
Mathew Alexander
Sohaib Nasim
Hardy Ding
REPORT MAY 2023 TECHNOLOGY AND PUBLIC PURPOSE PROJECT
Acknowledgments
In a year full of turmoil, characterized by a post-pandemic regression to the norm, instability from the Ukraine War, and a very volatile market, the biotech field has had funding shortages and several layoffs. It has also had a lot of innovation and giant leap-forwards, with various drugs being developed and companies developing breakthrough biotech advancing steadily.
This fellowship year marked a very much unexpected transition for the Belfer Center, with the sad premature passing of Secretary Ash Carter, Harvard University Belfer’s Center Director and Professor of Technology and Global Affairs, and former Secretary of Defense. Even if I could only enjoy his presence as mentor and director for a few months, I am thankful for the great guidance, teachings, and mentorship that will surely remain for the rest of our lives. A special thanks to my Fellowship Mentor, Dr. Bob Langer, MIT Institute Professor and Director of the Koch Institute for Brain Research, whose constant support, advice, and leadership has opened so many avenues for my personal and professional development. Thanks to Dr. Miyoung Chun, the Director of the MIT Alzheimer’s Innovation Hub, for her deep expertise in the field of neurodegenerative diseases, Alzheimer’s, and drug discovery in general.
Another huge thanks to the TAPP team, particularly our Director Amritha Jayanti, who supported us with constant tactical and operational advice, and Victoria Burnham, with all her administrative and program-related organization. Additionally, I am particularly thankful to all the people that have agreed to speak and work with us, from Academia, Industry, and the Government, including: Dr. Larry Steinman from Stanford, Drs. Li-Huai Tsai, Ann Graybiel and Alice Stanton from MIT, Dr. Al Sandrock from Voyager Therapeutics, Drs. Judith Steen, Jonathan Darrow and Amitabh Chandra from Harvard, Dr. Walter Koroshetz from NINDS, Mike Thomas from ARPA-H, Livio Valenti from Vaxess Technologies, Dr. Robbie Barbero from Ceres Nanoscience, Dr. Jim Ray from The Neurodegeneration Consortium, and Drs. Amy Rommel and Glen Harris from the Rainwater Foundation.
I would also like to thank the TAPP project funders for their generous support. Finally a special thanks to my family for the constant support and love that makes every work I do simpler, more joyful, and full of purpose.
iii Belfer Center for Science and International Affairs | Harvard Kennedy School
About the Author
Luca Giani brings years of experience at the intersection of Business and Government, with a focus on Biotech Entrepreneurship, Finance and Management, and Investments.
He started his career in the Investment Banking Division of Credit Suisse London, where he assisted companies with financing strategy, initial public offerings, and mergers and acquisitions. Later, at Bain & Company, Luca worked on several strategy projects in operations, go-to-market, and organization design.
Luca was on the founding team of a medical device startup focused on snoring and sleep apnea, and he later co-founded Innbiotec Pharma and Ilios Therapeutics – biotech companies that translate university IP into novel molecules for neurodegenerative diseases such as Alzheimer’s and dementia.
Luca is a Forbes under 30 awardee, he holds a Master of Public Policy from Harvard Kennedy School, and a B.S. from Georgetown University.
iv Tech and Public Policy To Save The Brain
Priyamvada Saraf is a dual-degree candidate pursuing an MBA at the MIT Sloan School of Management and an MPA at Harvard Kennedy School. With experience in management consulting, product management, venture capital, and impact investing, Priyamvada has held roles at Innovaccer and Owl Ventures. Previously, she contributed to the COVID-19 vaccine delivery strategy at Gavi, the Vaccine Alliance, and implemented strategic initiatives at Pratham Education Foundation. Priyamvada began her career at McKinsey & Company, serving clients across various sectors in India, Africa, and the US.
Mathew Alexander is a dual MD/MPP student at Virginia Commonwealth University and Harvard University, where he is a Jerome Grossman Fellow in Healthcare Policy. He is an aspiring primary care physician and has previously spent time in federal and state government, consulting, tech, and academia. His scholarly work has been published in Health Affairs, Health Policy, and Academic Medicine and his opinion writing has been published in media outlets like CNN, Newsweek, and The Wall Street Journal.
Sohaib Nasim is a first-year student in the MPA/ID program at the Harvard Kennedy School. Having worked on a research project about the lives of the elderly population back home in his home state in India, Sohaib saw how the lives of the elderly were affected by neurodegenerative diseases like Alzheimer’s and Dementia. This led to a passion for helping solve the problems in this field and motivated him to join this research project.
Hardy Ding is currently pursuing dual degrees in Medicine (MD) and Public Health (MPH) at Virginia Commonwealth University and the Harvard T.H. Chan School of Public Health. With aspirations of becoming a neurologist, Hardy has accrued a wealth of experience by collaborating with municipal, provincial, and federal government agencies in Canada. His research interests lie in promoting healthcare equity and evaluating the cost-effectiveness of emerging therapeutic approaches for the management of neuromuscular diseases.
v Belfer Center for Science and International Affairs | Harvard Kennedy School
About the Technology and Public Purpose Project (TAPP)
The arc of innovative progress has reached an inflection point. It is our responsibility to ensure it bends towards public good.
Technological change has brought immeasurable benefits to billions through improved health, productivity, and convenience. Yet as recent events have shown, unless we actively manage their risks to society, new technologies may also bring unforeseen destructive consequences.
Making technological change positive for all is the critical challenge of our time. We ourselves - not only the logic of discovery and market forces - must manage it. To create a future where technology serves humanity as a whole and where public purpose drives innovation, we need a new approach.
Founded by former U.S. Secretary of Defense Ash Carter, the TAPP Project works to ensure that emerging technologies are developed and managed in ways that serve the overall public good.
TAPP Project Principles:
• Technology’s advance is inevitable, and it often brings with it much progress for some. Yet, progress for all is not guaranteed. We have an obligation to foresee the dilemmas presented by emerging technology and to generate solutions to them.
• There is no silver bullet; effective solutions to technology-induced public dilemmas require a mix of government regulation and tech-sector self-governance. The right mix can only result from strong and trusted linkages between the tech sector and government.
• Ensuring a future where public purpose drives innovation requires the next generation of tech leaders to act; we must train and inspire them to implement sustainable solutions and carry the torch.
For more information, visit: www.belfercenter.org/TAPP
vi Tech and Public Policy To Save The Brain
Neurodegenerative diseases (NDDs) represent a global health emergency, affecting millions of individuals and imposing significant economic burdens. Despite increased funding and government initiatives, effective therapies remain elusive, and NDD research lags behind other fields. The problem is not limited to funding but extends to the complex and cumbersome landscape of numerous programs and initiatives, which are often not well-publicized. In this report, we argue for a simplified, accelerated, and transparent unified approach to NDD research, building on lessons from other fields and industries. We discuss key pain points in NDD research, from intellectual property and education to data management and collaboration, and propose fostering cross-sector collaboration, increasing public awareness, and leveraging innovative strategies to accelerate knowledge creation and therapy development. Drawing on insights from the COVID-19 pandemic, we emphasize the importance of collective action and a streamlined approach in addressing the urgent challenge of NDDs, with implications for policy and technology usage.
vii Belfer Center for Science and International Affairs | Harvard Kennedy School Abstract
viii Belfer Center for Science and International Affairs | Harvard Kennedy School Table of Contents The NDD Emergency ......................................................................................... 1 What are NDDs .................................................................................................. 3 Alzheimer’s Disease (AD)................................................................................................. 3 Parkinson’s Disease (PD) 3 Multiple Sclerosis (MS) 4 Frontotemporal Dementia (FTD) .................................................................................. 4 Huntington’s Disease (HD)............................................................................................... 5 Amyotrophic Lateral Sclerosis (ALS) ............................................................................ 5 Mapping the Space ........................................................................................... 6 The Drug Development Process ..................................................................................... 7 Stakeholders Involved in NDD Drug Discovery ..........................................................8 Stakeholders Mapping 12 The Funding Ecosystem for NDDs 14 How Policy Affects NDD Drug Development ........................................................... 22 Basic Science as a Pillar for Drug Development ........................................... 26 Case Study on Basic Science Research: Schizofrenia 27 Pain Points Hindering Drug Discovery for NDDs and the Role of Technology and Public Policy ....................................................................................... 29 Short IP for Long Development Needs 32 Case Study on GAIN: Government Efforts to Combat Antibacterial Drug Resistance ............................................................................................................... 35 Increase and Retain Neuroscience Talent ................................................................. 36 Geographical Concentration of Research ................................................................. 38 Case Study: Alzheimer’s Research Centers are Not Located Where Most Needed 41 Lack of Idea Diversification (and Risk-taking) ........................................................ 43
ix Belfer Center for Science and International Affairs | Harvard Kennedy School Dearth of Reliable Biomarkers ..................................................................................... 45 Case Study: Groundbreaking ALS Research Collaborative (ARC) To Accelerate Global Research in ALS 46 Extended Developmental Timelines 48 ROI Metrics Focused on Financial Returns 49 Lack of Non-Financial Incentives ................................................................................. 51 Insufficient Allocation of Philanthropic Funding .................................................... 53 Data - Funding Trap 54 Fragmented and Tedious Journal Publication Requirements 55 Case Study: DeSci - A Decentralized Science Platform Based on Web3 and DAO ................................................................................................................. 58 Limited Publication of Unsuccessful Studies ............................................................ 61 Case Study: Embracing Failure in Scientific Research .......................................... 62 Irreproducibility of Research Studies 64 Case Study: Open Science in Physics 66 Lack of Knowledge Sharing and Collaboration ....................................................... 69 Conclusion: NDDs as Testing Ground for Drug Discovery Changes............. 71

x Tech and Public Policy To Save The Brain
The NDD Emergency
Neurodegenerative diseases (NDDs) such as Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis (ALS), affect over 9 million Americans, with this number expected to increase to 21 million by 20501. The economic burden of NDDs is staggering, with estimates exceeding $900 billion per year in direct and indirect costs in the United States alone. These costs do not include the emotional burden to society, both from the patients and their families, as well as their caregivers. As the global population continues to age, these costs will only increase, placing a significant strain on healthcare systems and the broader economy. It is essential to recognize NDDs as a pressing emergency, allocate resources accordingly, and create policies to mitigate future costs and suffering, and importantly collaborate across all stakeholders involved to create effective diagnostics and therapies.
Despite increased efforts from governments and other institutions to fund drug discovery, there is currently no effective therapy for these conditions. Experts consider NDD research at least a decade behind cancer research due to several factors, including the complexity of NDDs and challenges in studying their progression. NDDs primarily affect older populations and are characterized by the progressive loss of neuronal function, leading to cognitive and motor impairment. These diseases arise from a complex interplay of genetic, environmental, and age-related factors, making them particularly challenging to study and treat.
One major obstacle in NDD research is the difficulty in observing the early stages of these diseases. Often, scientists can only study the aftermath of the disease, akin to analyzing a train wreck rather than witnessing the accident itself. This limitation hampers our understanding of the mechanisms underlying disease progression and makes the development of effective therapies somewhat of a guessing game that has not yielded the most effective results yet.
Addressing the NDD emergency requires a multi-faceted approach involving government, scientific communities, and the general public. The recent COVID-19 pandemic demonstrated the power of collective action in tackling

1 Belfer Center for Science and International Affairs | Harvard Kennedy School
1 2023 Alzheimer’s Disease Facts and Figures. (2023). Alzheimer’s Dementia, 19(4), 1598-1695. doi:10.1002/alz.13016
an imminent crisis, with science, technology, policy, and public engagement working in concert to understand the disease, and later develop and distribute vaccines. A similar concerted effort is necessary to make strides in NDD research and treatment.
Accelerating progress in NDD research calls for a shift in the way we approach the problem. We must learn from the successes of other fields, such as cancer research, or other industries such as aerospace and defense, and apply innovative strategies to study and treat NDDs. This will involve fostering collaboration between academia, industry, and government institutions to leverage the expertise and resources of various sectors.
Moreover, increasing public awareness of the NDD emergency is crucial for generating support and advocacy. By educating the public about the human and economic costs of these diseases, we can cultivate a sense of urgency that will drive policy changes and investment in research.
Neurodegenerative diseases represent an urgent global health crisis that demands immediate attention and action. By embracing collaboration across sectors, investing in research, and raising public awareness, we can accelerate progress in understanding and treating these devastating conditions. The lessons learned from the COVID-19 pandemic provide a blueprint for collective action, emphasizing the need for society to unite in tackling the NDD emergency. Only through such concerted efforts can we hope to alleviate the suffering of millions of individuals affected by these debilitating diseases.
2 Tech and Public Policy To Save The Brain
What are NDDs2
Neurodegenerative diseases (NDDs) are characterized by the progressive deterioration and loss of neurons and motor neurons in the brain and spinal cord. Although each NDD is unique in its clinical presentation and underlying biology, they often have overlapping features3. The exact cause of neuronal loss varies across diseases, but common factors include misfolded protein aggregates, oxidative stress, and brain inflammation. Currently, no effective treatments exist for NDDs, which are ultimately fatal. Most treatments offer palliative relief from symptoms, and sometimes a slight increase in life expectancy or improved daily functionality for a limited time. The most prevalent NDDs include Alzheimer’s disease (AD), Parkinson’s disease (PD), Frontotemporal Dementia (FTD), Huntington’s disease (HD), and Amyotrophic lateral sclerosis (ALS).
Alzheimer’s Disease (AD)
AD is the most common form of dementia, affecting 6 million Americans and accounting for approximately 60% of all NDD cases4. Although AD can present in younger individuals, it predominantly affects the elderly, with an incidence as high as 50% among those over 85 years of age. AD patients typically experience insidious onset and gradual progression of memory loss, accompanied by deterioration in other cognitive domains such as visuospatial or executive function. This decline eventually leads to a complete loss of functional independence. Medical management of AD primarily aims to improve patient symptoms and optimize the quality of life for both patients and caregivers.
Parkinson’s Disease (PD)
PD is the second most common NDD, following AD. It affects 1.5-2 million Americans, with a prevalence of 0.3% for the general population, 1% for those over 60, and 3% for those over 80. The hallmark symptom of PD is bradykinesia, a condition characterized by resting tremor, rigidity, and postural instability. Other motor symptoms include limited facial expression, vision changes, abnormally small handwriting, and stooped posture.
2 Giani, L. (2022). Combat Neurodegenerative Diseases Crisis with Technology and Public Policy. Retrieved April 25, 2023, from https://www.belfercenter.org/publication/combat-neurodegenerative-diseases-crisistechnology-and-public-policy
3 Erkkinen, M. G., Kim, M., & Geschwind, M. D. (2017). Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harbor Perspectives in Biology, 10(4). doi:10.1101/cshperspect.a033118.
4 Evans, D. A. (1989). Prevalence of alzheimer’s disease in a community population of older persons. JAMA, 262(18), 2551. doi:10.1001/jama.1989.03430180093036.
3 Belfer Center for Science and International Affairs | Harvard Kennedy School
PD can also manifest as nonmotor symptoms, such as autonomic dysfunction, sleep disturbances, mood disorders, and cognitive disturbances, all of which can significantly impact a patient’s quality of life.
Multiple Sclerosis (MS)
MS is a progressively debilitating disease that affects the brain and spinal cord. In MS, the immune system attacks the myelin sheath covering nerve fibers, disrupting communication between the brain and the rest of the body. The disease eventually results in permanent nerve damage and loss of function. Severe disabilities may necessitate treatment, with some individuals losing their ability to walk independently or with assistance. Others may experience prolonged periods of remission without new symptoms. Approximately 400,000 Americans suffer from MS5.
Frontotemporal Dementia (FTD)
FTD is an umbrella term for a group of clinically heterogeneous conditions arising from neurodegeneration primarily in the anterior and frontal temporal lobes and subcortical structures. FTD is characterized by early changes in emotion, behavior, language, and motor skills. An estimated 60,000 Americans suffer from some form of FTD6. FTD is a common form of early-onset dementia in patients under 65, with an average age of onset at 56. However, it can be detected in patients as young as the second decade of life7, with 13% of cases occurring before the age of 50.
5 Feigin, V. L., Abajobir, A. A., Abate, K. H., Abd-Allah, F., Abdulle, A. M., Abera, S. F., . . . Vos, T. (2017). Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the global burden of disease study 2015. The Lancet Neurology, 16(11), 877-897. doi:10.1016/s1474-4422(17)30299-5.
6 Knopman, D. S., & Roberts, R. O. (2011). Estimating the number of persons with frontotemporal lobar degeneration in the US population. Journal of Molecular Neuroscience, 45(3), 330-335. doi:10.1007/s12031-011-9538-y
7 Dobson-Stone, C., Hallupp, M., Shahheydari, H., Ragagnin, A. M., Chatterton, Z., Carew-Jones, F., . . . Kwok, J. B. (2020). CYLD is a causative gene for frontotemporal dementia – amyotrophic lateral sclerosis. Brain, 143(3), 783-799. doi:10.1093/brain/awaa039.
4 Tech and Public Policy To Save The Brain
Huntington’s Disease (HD)
HD is a rare inherited genetic neurodegenerative disorder affecting approximately 60,000 Americans8. It causes involuntary movements, personality changes, and dementia due to excessive repeats of sequences in huntingtin genes9. The worldwide prevalence of HD is estimated at 2.7 cases per 100,000, with higher rates observed in Asia, North America, and Europe. The median age of HD diagnosis is 40 years.
Amyotrophic Lateral Sclerosis (ALS)
ALS affects around 20,000 Americans and is characterized by degeneration of the upper and lower motor neurons in the brain and spinal cord, leading to respiratory paralysis and death. Approximately 10% of patients have a family history of ALS and are classified as familial ALS.
ALS is a fatal disease resulting in severe disability and eventual death due to ventilatory failure. It has a prevalence of 5 in 100,000, with an incidence of 1.7 per 100,000, reflecting the short average survival10
8 Pringsheim, T., Wiltshire, K., Day, L., Dykeman, J., Steeves, T., & Jette, N. (2012). The incidence and prevalence of Huntington’s disease: A systematic review and meta-analysis. Movement Disorders, 27(9), 1083-1091. doi:10.1002/ mds.25075.
9 Pringsheim, T., Wiltshire, K., Day, L., Dykeman, J., Steeves, T., & Jette, N. (2012). The incidence and prevalence of Huntington’s disease: A systematic review and meta-analysis. Movement Disorders, 27(9), 1083-1091. doi:10.1002/ mds.25075.
10 Oskarsson, B., Gendron, T. F., & Staff, N. P. (2018). Amyotrophic lateral sclerosis: An update for 2018. Mayo Clinic Proceedings, 93(11), 1617-1628. doi:10.1016/j.mayocp.2018.04.007
5 Belfer Center for Science and International Affairs | Harvard Kennedy School
Mapping the Space
In the early-stage research conducted by our team, we explored the complex landscape of neurodegenerative diseases to identify the critical components that drive progress and innovation. Our goal was to understand the space to then analyze how technology and policy can enhance the efficiency of NDD research networks, expedite drug discovery, and propel the development of novel treatments.
Our investigation focused on three main research areas:
1. The Stakeholders Involved in the Neurodegenerative Disease Drug Development Process. We examined the key stakeholders involved in NDD drug discovery and development, encompassing academia, industry, government institutions, and patient advocacy groups. By understanding their roles, interactions, and differentiation within their respective therapeutic areas, we looked to elucidate the dynamics that shape the NDD research ecosystem.
2. The Funding Ecosystem for Neurodegenerative Disease. Lastly, we investigate the distribution of funds across NDDs and their sources, shedding light on the financial landscape that underpins research and development efforts. This information is crucial for recognizing potential gaps and opportunities for investment, ensuring that resources are allocated strategically to maximize the chances of breakthrough discoveries.
3. How Policy Affects Neurodegenerative Disease Drug Development. Our analysis covers various policies, programs, and non-governmental actions that are generating momentum and effectiveness in the fight against neurodegenerative diseases. We assess their impact on NDD research and treatment, identifying successful strategies that can serve as a blueprint for future initiatives.
By comprehensively mapping the space of NDD research, we aimed to provide valuable insights that can guide stakeholders in their pursuit of innovative solutions to tackle the pressing emergency of neurodegenerative diseases.
6 Tech and Public Policy To Save The Brain
The Drug Development Process11
The drug development and discovery process is a complex and multi-step process that involves many different stages and activities, often requiring various stakeholders to take part in each stage of the process, especially when focusing on difficult diseases such as neurodegenerative ones.
Basic Research:
The initial phase, basic research, comprises molecular biology, pathophysiology identification, and genetic mapping studies. The objective is to improve the understanding of the underlying biology of specific diseases or conditions, thereby providing valuable insights into potential therapeutic targets and strategies. This aspect of the drug discovery process carries the highest importance, since understanding what causes a disease makes researching a solution much more feasible.
Research and Development (R&D):
Upon completion of basic research, the process advances to the research and development (R&D) stage, which entails target identification, compound screening, and lead identification and optimization. These activities aim to identify potential therapeutic compounds and assess their effectiveness in treating particular diseases or conditions.
11 Giani, L., & Saraf, P. (2022). The stakeholders involved in the Neurodegenerative Disease Drug Development Process. Retrieved April 25, 2023, from https://www.belfercenter.org/publication/stakeholders-involved-neurodegenerativedisease-drug-development-process

7 Belfer Center for Science and International Affairs | Harvard Kennedy School
Figure 1. The Stages of the Drug Discovery Process
Figure 1 depicts the various stages of the drug discovery process schematically, showing the progression from basic research to pre-clinical studies, clinical trials, and review and approval.
Pre-Clinical Studies:
The third phase, pre-clinical studies, involves in-vitro and in-vivo efficacy studies, proof of concept and mechanism of action investigations, and Investigational New Drug (IND)-enabling studies. These studies evaluate the safety and effectiveness of potential therapeutic compounds in animal models, gathering essential data and information to support subsequent clinical trials in humans.
Clinical Trials:
The fourth phase, clinical trials, tests potential therapeutic compounds in humans to determine their safety and efficacy. Clinical trials typically progress through three stages: Phase I, involving a small number of healthy volunteers or patients; Phase II, encompassing a larger patient population with the target condition; and Phase III, designed to confirm the efficacy and safety of the therapeutic compound in a substantial patient cohort.
Review and Approval:
After a potential therapeutic compound completes clinical trials, the final phase of drug development is review and approval. This phase requires submitting a New Drug Application (NDA) to the Food and Drug Administration (FDA), which evaluates the compound’s safety and efficacy based on data and information collected during clinical trials. If the FDA approves the NDA, the compound can be manufactured and made available to patients. However, the process extends beyond this point, as post-release monitoring is a crucial aspect, ensuring the therapeutic compound’s ongoing safety and effectiveness in the long term.
Stakeholders Involved in NDD Drug Discovery12
The drug discovery and development process for neurodegenerative diseases is an intricate, multifaceted endeavor that engages a diverse array of stakeholders across the United States. These include government agencies, private enterprises, research institutions, academic establishments, and non-profit organizations. The involvement of these stakeholders spans the cycle of drug development, encompassing numerous
12 Giani, L., & Saraf, P. (2022). The stakeholders involved in the Neurodegenerative Disease Drug Development Process. Retrieved April 25, 2023, from https://www.belfercenter.org/publication/stakeholders-involved-neurodegenerativedisease-drug-development-process
8 Tech and Public Policy To Save The Brain
activities such as pre-clinical research, clinical trials, regulatory approval, and commercialization.
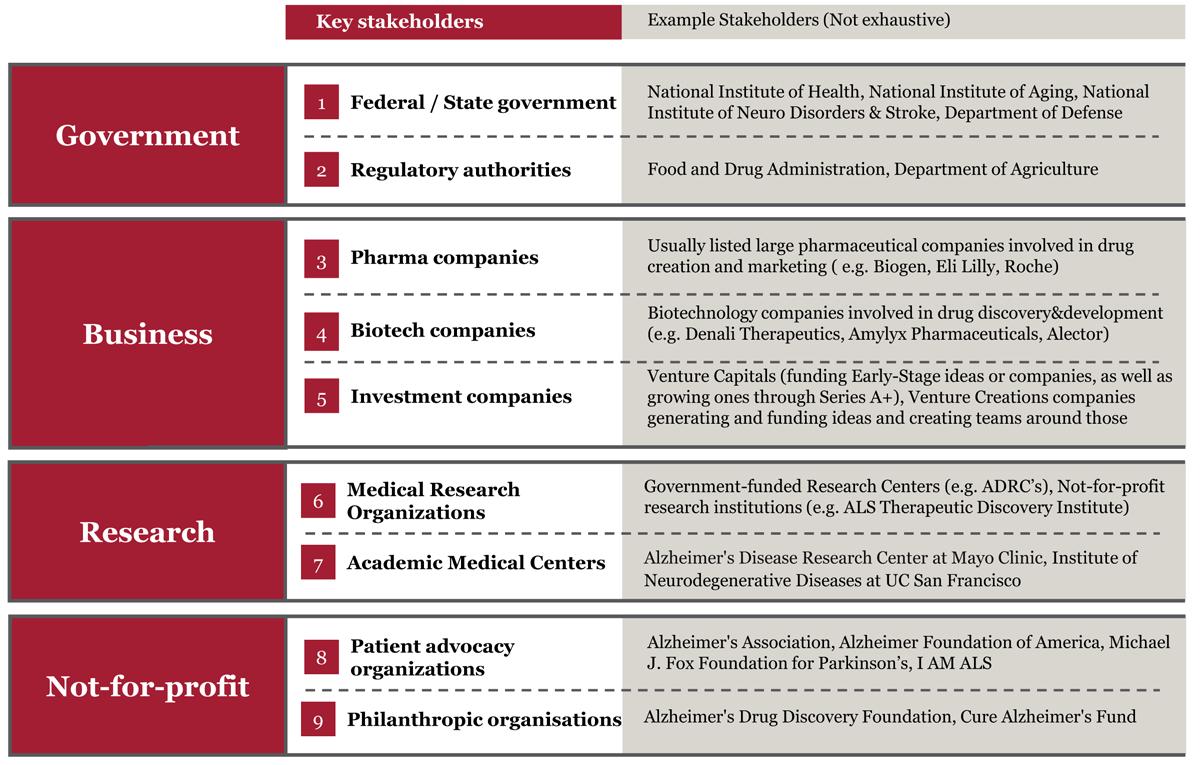
Because of the complexity of this process and the inherent challenges in devising effective therapies for NDDs, it is crucial to gain a comprehensive understanding of the roles of different stakeholders and the potential obstacles they encounter. Below, we undertake an in-depth exploration of the various stakeholders engaged in NDD drug discovery and development, striving to delineate key challenges that might contribute to the paucity of disease-modifying treatments for these conditions.
Government:
A crucial government stakeholder is the National Institutes of Health (NIH), one of the largest funders of neurodegenerative disease (NDD) research in the United States. The NIH encompasses three notable institutes conducting and supporting NDD research: the National Institute on Aging (NIA), the National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute of Mental Health (NIMH). The Department of Defense (DoD) is another significant funder of
9 Belfer Center for Science and International Affairs | Harvard Kennedy School
Figure 2. Stakeholders in NDD Drug Discovery
Figure 2 provides a table highlighting the various stakeholders involved in the drug discovery process for neurodegenerative diseases.
NDD research programs. Moreover, certain states support NDD-related research, such as California’s funding of the California Alzheimer’s Disease Centers.
Regulatory authorities like the Food and Drug Administration (FDA) also play a vital role in the drug discovery process. The FDA is responsible for reviewing drug applications and assessing risks and benefits before approving new treatments for use.
Industry:
Pharmaceutical and biotech companies are prominent industry stakeholders in the NDD drug discovery process, funding research and development efforts and clinical trials often in collaboration with other stakeholders, such as the NIH. As opposed to Academic institutions and Government labs, which are mostly focused on basic science research, Biotech companies, and larger Pharmaceuticals are typically involved in translational and clinical research. These are typically funded by venture capital, private equity, or other investors, also through the public markets.
Research:
Research organizations, including NDD-focused research centers, are pivotal stakeholders in drug discovery. These centers offer resources and opportunities for research participation and the development of novel patient care strategies. This category also comprises research centers within hospitals and medical institutes, such as Massachusetts General Hospital’s Mass General Institute for Neurodegenerative Disease (MIND). Leading academic institutions host NDD research centers, including UC San Francisco’s Institute of Neurodegenerative Diseases, the University of Pennsylvania’s Center for Neurodegenerative Disease Research (CNDR), and the Mayo Clinic’s Alzheimer’s Disease Research Center.
Investors:
Venture capital firms, private equity firms, and private debt providers significantly contribute to NDD drug discovery, supplying critical funding to support the research and development of new treatments, particularly during early stages when risks are higher and potential returns are uncertain. In addition to funding, investors often possess expertise and experience in the pharmaceutical and biotech industries, aiding the development and commercialization of new treatments.
10 Tech and Public Policy To Save The Brain
Public markets also participate in NDD drug discovery, with publicly traded pharmaceutical and biotech companies raising funds through stock or bond sales to support new treatment research and development. The stock market can indicate the potential value of new treatments, guiding research and development efforts.
Investors play a vital role in NDD drug discovery, providing necessary funding and expertise for new treatment development and market introduction. However, returns on these investments may vary across different drug discovery stages, a topic discussed later in this article.
Patient Advocacy Groups:
Patient advocacy groups represent patients and their families, ensuring their needs and concerns are addressed. These groups can significantly advance NDD treatment development by raising research and development awareness and pushing for progress. The Alzheimer’s Association is among the largest non-corporate funders of Alzheimer’s disease-focused research.
Beyond raising awareness and advocating for patients, patient advocacy groups offer essential support and resources for patients and their families, including information on available treatments and clinical trials, and support for accessing care and managing NDD-related challenges.
Philanthropic Organizations:
Philanthropic organizations are critical to NDD drug discovery, mobilizing financial commitments and addressing gaps in drug development by supporting early-phase clinical studies and research. For instance, the Alzheimer’s Drug Discovery Foundation (ADDF) has generated commitments worth $50 million from partners such as Bill Gates, Leonard A. Lauder, the Dolby family, the Charles and Helen Schwab Foundation, and additional partners including Jeff Bezos and MacKenzie Scott.
11 Belfer Center for Science and International Affairs | Harvard Kennedy School
Stakeholders Mapping13
The above stakeholders participate in the NDD drug development process across various stages.

The complex landscape of neurodegenerative disease (NDD) drug development necessitates the involvement of a diverse array of stakeholders, each contributing their unique expertise and resources to various stages of the process. In the figure above, we attempted mapping their participation across the drug discovery and development continuum. By examining the interactions and contributions of these diverse stakeholders, we aim to elucidate the multifaceted nature of NDD drug development, highlighting the importance of synergistic collaborations in driving advancements in the field.
In the realm of neurodegenerative disease (NDD) treatment development, federal agencies have consistently held a pivotal role by endorsing and financing fundamental research as well as clinical trials. The private sector frequently concentrates on later-stage clinical trials that have a greater probability of success, while early-stage research is frequently regarded as excessively high-risk, thus receiving minimal private investment.
13 Giani, L., & Saraf, P. (2022). The stakeholders involved in the Neurodegenerative Disease Drug Development Process. Retrieved April 25, 2023, from https://www.belfercenter.org/publication/stakeholders-involved-neurodegenerativedisease-drug-development-process
12 Tech and Public Policy To Save The Brain
Figure 3. Stakeholders Roles in NDD Drug Discovery
Figure 3 provides a table highlighting the role that various stakeholders play in the drug discovery process for neurodegenerative diseases.
Public-private partnerships (PPPs) have emerged as an innovative financial model, enabling the distribution of costs and risks throughout all stages of drug development.
One prominent example of a PPP is the Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU), which specifically targets a rare form of Alzheimer’s disease impacting younger individuals. DIAN-TU is spearheaded by the Washington University School of Medicine in St. Louis, and it receives financial support from a diverse consortium of organizations. These include the University of Washington, the Alzheimer’s Association, the National Institute on Aging, and major pharmaceutical companies such as Biogen, Eisai, Janssen, Eli Lilly, and Roche/Genentech.
Although the successful development of NDD treatments can yield significant financial rewards, the inherent high risk often restricts the extent of venture capital available for supporting novel drug and treatment ideas pursued by biotechnology companies. Research organizations and academic medical centers (AMCs) stand at the forefront of innovation in the NDD domain, offering essential backing for research that may culminate in the development of groundbreaking drugs and treatments. AMCs predominantly rely on funding from federal agencies, such as the National Institutes of Health (NIH), and occasionally receive support from industry stakeholders.
Advocacy organizations hold an equally critical role in sustaining patients and their families, promoting increased investment in NDD research, and collaborating with both federal and private stakeholders to secure research funding. Philanthropic individuals frequently contribute to these advocacy organizations or research centers, supporting high-risk projects and bridging funding gaps.
It is essential to acknowledge that various stakeholders may participate in different stages of the drug development process and frequently engage with one another. For instance, the Food and Drug Administration (FDA) review committee may comprise experts and representatives from pharmaceutical companies and government agencies, such as the NIH. This collaborative approach ensures a well-rounded perspective and facilitates the integration of diverse expertise, ultimately contributing to more effective and efficient drug development processes for NDD treatments.
13 Belfer Center for Science and International Affairs | Harvard Kennedy School
The Funding Ecosystem for NDDs14
Funding constitutes a crucial element in the advancement of therapeutic interventions, and an understanding of its dynamics is essential for comprehending the trajectory of NDD research. Historically, the allocation of financial resources for NDDs has been comparatively lower than that dedicated to other diseases, such as Cancer and AIDS, and this has significantly impacted the progress in this area.
The landscape of funding for NDD research is multifaceted, with the National Institutes of Health (NIH) being a primary source. However, other stakeholders, including industry players, venture capital and private equity investors, as well as non-governmental organizations and philanthropic entities, also contribute to the financial support system. It is important to note that the distribution of funding varies considerably across different neurodegenerative diseases, further highlighting the complexity of the funding ecosystem.
Over the years, the National Institutes of Health (NIH) has predominantly allocated most of its funding for disease research to cancer. This trend can be traced back to the inception of the “War on Cancer” in 1971, a national endeavor aimed at increasing funding for cancer research and expediting progress in the field. The War on Cancer has been instrumental in driving significant increases in funding for cancer research, cementing its position as the disease with the highest level of NIH support.
There was a notable upswing in funding for AIDS research in the late 1980s. However, in recent years, the amount of funding allocated for AIDS research has remained relatively stable.
In stark contrast, funding for neurodegenerative diseases (NDDs) has historically been meager, potentially due to lower levels of public awareness and pressure concerning these diseases, as well as their biologically intricate nature. Nevertheless, there has been a marked increase in funding for NDDs, specifically Alzheimer’s research, since 2016. This shift occurred when the US Congress directed the NIH to begin earmarking a specific amount of funding for Alzheimer’s disease research.
14 Giani, L., & Nasim, S. (2022). The funding ecosystem for Neurodegenerative Disease. Retrieved April 25, 2023, from https://www.belfercenter.org/publication/funding-ecosystem-neurodegenerative-disease
14 Tech and Public Policy To Save The Brain
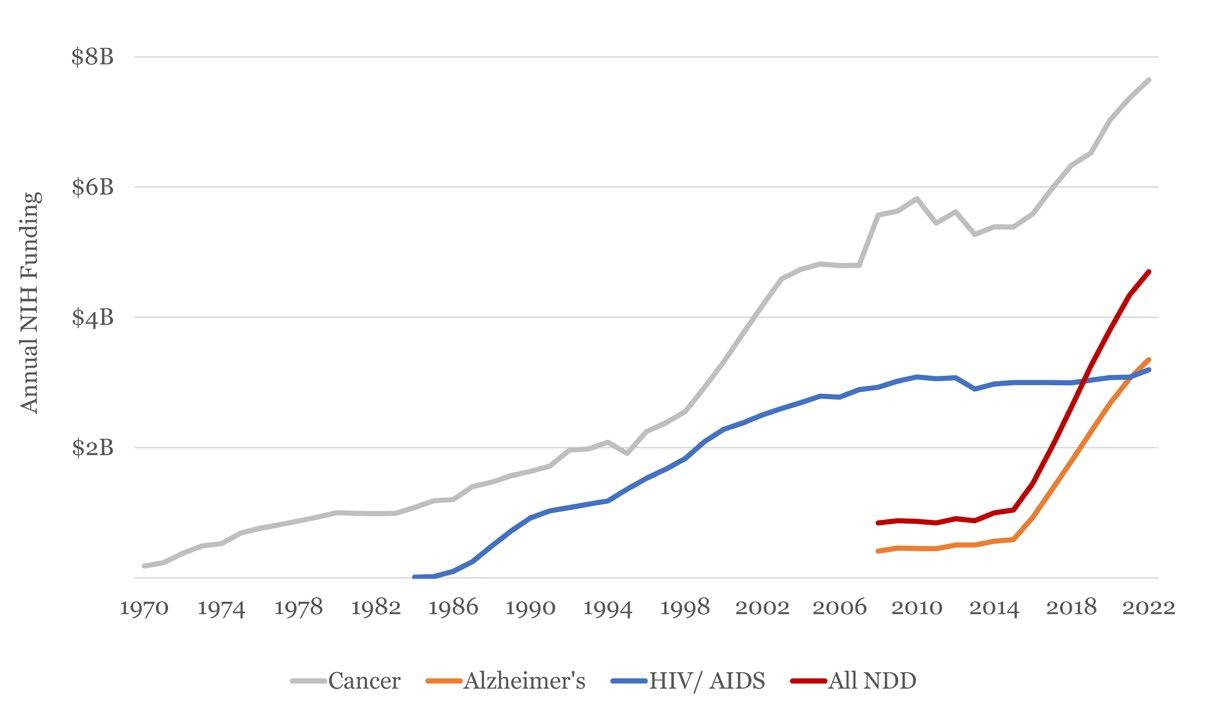
This chart illustrates the historical funding trends of the National Institutes of Health (NIH) for cancer, HIV/AIDS, Alzheimer’s disease (AD), and the aggregate of neurodegenerative diseases (NDDs) from 1970 to 2022. The chart highlights the steady increase in funding for cancer research since the 1970s, with a notable milestone of reaching $4 billion in funding in 2000. HIV funding has experienced a steady rise until 2008, after which it plateaued. It is important to note that NIH began tracking Alzheimer’s disease funding in 2008, and the figures presented do not include related dementias. Between 2008 and 2015, AD and NDDs funding remained relatively stable and low. However, starting in 2015, there was a sharp increase in funding for these areas, with an annual growth of around $500 million, reflecting the government’s prioritization of these diseases. Despite the increased attention and nearly $4 billion in annual funding for AD and NDDs, these areas still lag behind cancer research by approximately 20 years, in terms of financial investment. The chart provides a clear visual representation of the funding landscape and the progress made in recent years, as well as the disparities between the prioritization of different diseases in terms of research funding..
15 Report. (2023). Retrieved April 25, 2023, from https://report.nih.gov/funding/categorical-spending#/
15 Belfer Center for Science and International Affairs | Harvard
School
Kennedy
Figure 4. NIH Funding by Disease Area15
The Different Sources of Funding
As mentioned, there are various funding sources that support NDD research, including government agencies, non-governmental organizations (NGOs), large pharmaceutical companies, and traditional investors through private capital (such as venture capital and private equity funds) or public markets for traded companies.
Government agencies, such as the National Institutes of Health (NIH) and the Department of Defense (DOD), are significant sources of funding for research on NDDs and biotechnology in general. The NIH, for instance, has allocated $3.5 billion specifically for Alzheimer’s research. These agencies typically provide funding through grants and contracts to support research projects at universities, hospitals, and other institutions.
NGOs working in particular disease areas are another vital source of funding for NDD and biotech research. Examples include the Michael J. Fox Foundation for Parkinson’s and the Alzheimer’s Association, which raise funds from donors and use them to support research and other initiatives related to their specific disease focus.
Large pharmaceutical companies also play a role in funding NDD and biotech research. These companies often have their own internal research and development (R&D) programs and may also collaborate with academic and other institutions on joint research projects through grants or Public-Private Partnerships (PPPs). In addition to internal funding, pharmaceutical companies can raise funds through public markets, such as by being listed on the NASDAQ, and through debt markets by issuing bonds.
Traditional private investors, such as venture capital and private equity funds, can also provide funding for NDD and biotech research. These investors typically focus on early-stage biotech companies or companies looking to expand and scale up their operations. The interplay of these diverse funding sources creates a dynamic and multifaceted ecosystem that drives research and innovation in the field of neurodegenerative diseases.
16 Tech and Public Policy To Save The Brain
NIH Allocations of Funding
The major source of funding for neurodegenerative disease research is the Government through the Department of Defense (DOD) and the National Institutes of Health (NIH).
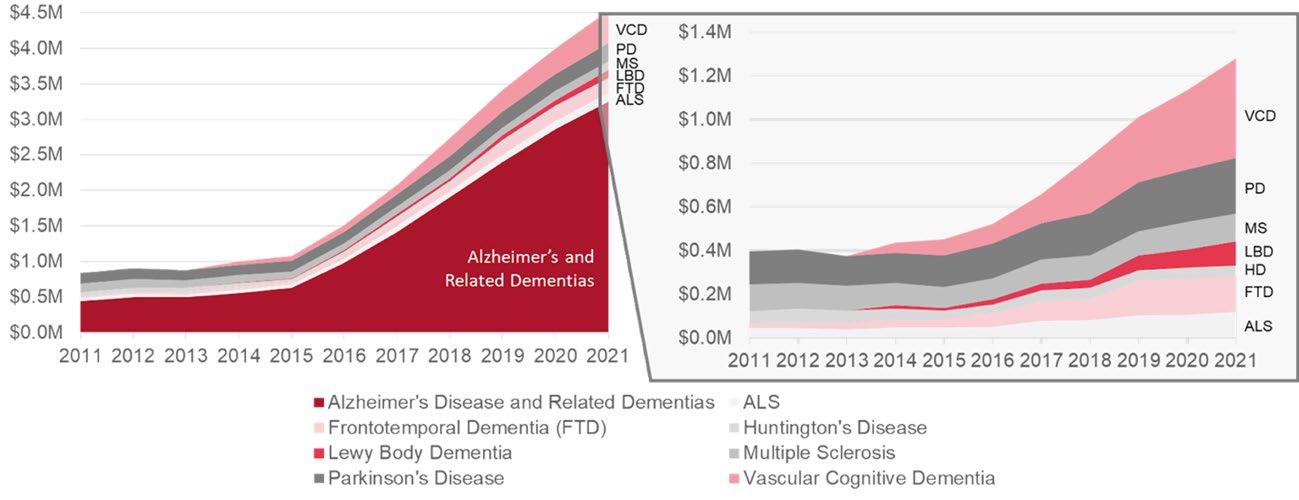
The left side of Figure 5 shows the prevalence of neurodegenerative diseases and the right side shows corresponding NIH funding in 2021 per disease. Prevalence data from latest available years: ALS (2017), FTD (2022), Huntington’s Disease (2020), Vascular Dementia (2022), Alzheimer’s Disease (2022), Parkinson’s Disease (2020), Multiple Sclerosis (2020), Lewy Body Dementia (2021).

Alzheimer’s disease and related dementias are the most common NDDs, affecting more than 6.5 million people in the United States alone, Parkinson’s disease impacts 1.2 million people, and amyotrophic lateral sclerosis (ALS) affects approximately 31,000 people. However, the allocation of National Institutes of Health (NIH) funding per disease does not necessarily correspond to the prevalence of the disease. In fact, some diseases, such as ALS and Huntington’s disease, receive a higher proportion of NIH funding compared to their prevalence.
16 Report. (2023). Retrieved April 25, 2023, from https://report.nih.gov/funding/categorical-spending#/
17 Belfer Center for Science and International Affairs | Harvard Kennedy School
Figure 5. Prevalence vs. NIH Funding per Disease16
Figure 6. NIH Research Funding per Patient per Disease17
This discrepancy becomes even more pronounced when we compare NIH research funding for these diseases as a proportion of their prevalence, as illustrated in Figure 6. Diseases like ALS and Huntington’s disease receive significantly more funding per person affected. Several possible explanations can account for this.

Firstly, rare diseases like ALS and Huntington’s disease often garner high public awareness through high-profile cases, leading to increased funding. Furthermore, advances in the basic science understanding of these diseases, thanks to ongoing research, have resulted in a clearer identification of genetic causal pathways, which can also attract more funding. Secondly, it is possible that a minimal threshold of funding is required for any drug discovery exercise, and dividing the funding by much lower prevalence rates creates this stark difference. Finally, some diseases, such as Parkinson’s disease and multiple sclerosis, already have approved and effective treatment drugs. This may have contributed to lower amounts of funding being allocated for these diseases. As we continue to explore funding allocation for NDDs, it is essential to consider these factors and their implications for the development of therapies and treatments.
The National Institutes of Health (NIH) allocates funding for various diseases based on various factors, including policy action and public pressure. As an example, as shown in Figure 4 below, NIH funding for Alzheimer’s research saw a sharp change of directions in 2016. This started a steady increase in funding in response to a directive from the US Congress, culminating with the NIH allocating a significant amount of funding specifically for Alzheimer’s disease research, resulting in a $3.5B allocation for the year 2022.
Similarly, the increase in funding for ALS research after the viral Ice Bucket Challenge in 2014 highlights the role that public awareness and pressure can play in directing funding towards lesser-known diseases.5 This illustrates the positive effects of raising public awareness and applying pressure on policymakers to prioritize funding for specific diseases.
17 Report. (2023). Retrieved April 25, 2023, from https://report.nih.gov/funding/categorical-spending#/
18 Tech and Public Policy To Save The Brain
The previous section discussed the increased funding for Alzheimer’s research, which is supposed to translate into more clinical trials, results and hopefully effective therapies soon. This case study compares the number of AD clinical trials to other diseases, the changes in the NIA-AD clinical drug development portfolio, and the need for open science and data sharing to develop new biomarkers and targets.
Comparison of Clinical Trials by Disease: While funding for Alzheimer’s research is steadily increasing, the number of clinical trials for the disease is still lagging behind other diseases, particularly cancer. Comparing the number of clinical trials for cancer and neurological diseases highlights the diversity and constancy of cancer research, with AD trailing behind.
19 Belfer Center for Science and International Affairs | Harvard Kennedy School
Figure 7. NIH Research Funding per Disease Over Time (2011-2021)18
Figure 7 shows NIH research spending per NDD per year. The left side of the figure presents the overall spending trend, while the right side presents a zoomed-in version of the same data, with Alzheimer’s disease excluded Case Study: Current State of Alzheimer’s Research19
18 Report. (2023). Retrieved April 25, 2023, from https://report.nih.gov/funding/categorical-spending#/ 19 Clinical Trials on Alzheimer’s Disease CTAD San Diego, CA December 5, 2019 Overview of the NIA portfolio in AD clinical trials: Which new targets could be explored? Eliezer Masliah Division of Neuroscience, National Institute on Aging, NIH
Diversify the Portfolio: Over the past few years, there have been significant changes in the NIA-AD clinical drug development portfolio, with more targets being explored. These emerging targets move beyond the mostly studied amyloid-beta and tau proteins, with neuroinflammation, synaptic dysfunction, neurovascular dysfunction, and metabolic and mitochondrial dysfunction emerging in the number of clinical trials. The expansion of the research portfolio demonstrates a growing effort to understand the complex nature of Alzheimer’s disease and the need for diverse therapeutic approaches.

20 Tech and Public Policy To Save The Brain
Figure 8. Comparison of Clinical Trials by Disease
Despite the investments, the chart above clearly shows the much higher number of clinical trials for different disease areas, namely Cardiovascular Diseases, Cancer, HIV and Alzheimer’s.
The graph shows the change in composition of clinical trials by target in Alzheimer’s research over the years, emphasizing the encouraging efforts to diversify the pipeline. The graph demonstrates that as time progresses, the variety of targets being explored in Alzheimer’s clinical trials has broadened, reflecting an increased willingness to understand the disease’s complexity. This diversification showcases the scientific community’s commitment to exploring novel therapeutic avenues and the potential for discovering more effective treatments for Alzheimer’s disease. However, as shown in previous graphs, it is important to note that the number of clinical trials remains around 50, highlighting the need for further investment in more trials to accelerate the development of new treatments and deepen our understanding of Alzheimer’s disease. Expanding the number of clinical trials can help ensure that the diversification of targets continues, ultimately increasing the likelihood of finding successful therapies for this debilitating disease.

Open Science and Data Sharing: Developing new biomarkers and targets for Alzheimer’s research requires collaboration and data sharing among researchers. Several programs are currently underway to facilitate this collaborative effort:
• ACTC: Alzheimer’s Disease Clinical Trials Consortium
• ADSP: Alzheimer’s Disease Sequencing Program
• ADGC: Alzheimer’s Disease Genomic Center
• ADNI: Alzheimer’s Disease Neuroimaging Initiative
• ADCs: Alzheimer’s Disease Centers
• AMP-AD: Accelerating Medicines Partnership - Alzheimer’s Disease
• MODEL-AD: Model Organism Development and Evaluation for Late-onset
Alzheimer’s Disease
• NACC: National Alzheimer’s Disease Coordinating Center
• NCRAD: National Centralized Repository for Alzheimer’s Disease
21 Belfer Center for Science and International Affairs |
Harvard Kennedy School
Figure 9. Changes in NIA-AD Clinical Drug Development Portfolio from 2014 to 201820
20
NIH - International Alzheimer’s and Related Dementias Research Portfolio (IADRP). (n.d.). Retrieved April 25, 2023, from https://iadrp.nia.nih.gov/
These programs aim to bring together researchers, clinicians, and other stakeholders to share data, resources, and knowledge, ultimately accelerating the development of effective treatments for Alzheimer’s disease. In future chapters, we will discuss the potential to improve efficiency in these programs, as the spirit and intention are already in place, and the NIA’s efforts are commendable. However, there may be opportunities to create simpler processes that are more accessible, further bolstering the fight against Alzheimer’s and advancing the search for effective treatments.

How Policy Affects NDD Drug Development22
The development of curative or disease-altering therapies for neurodegenerative diseases (NDDs) has been historically hindered by factors such as limited understanding of the pathophysiology of NDDs and comparatively lower investment in comparison to other disease areas. However, policy instruments hold the potential to drive progress and improve NDD drug development, especially if geared towards promoting greater investment in basic science research.
21 Masliah, E. (2019). CTAD Alzheimer. Retrieved April 26, 2023, from https://www.ctad-alzheimer.com/files/files/ CTAD%202020%20Abstracts%20final.pdf
22 Giani, L., & Alexander, M. (2022). Understanding how policy affects Neurodegenerative Disease Drug Development. Retrieved April 25, 2023, from https://www.belfercenter.org/publication/understanding-how-policy-affectsneurodegenerative-disease-drug-development
22 Tech and Public Policy To Save The Brain
Figure 10. NIA Resources for Data Sharing21
Figure 11. Example Policy/Programs by Year and Area of Impact
Policy/Program
Orphan Drug Act
FDA Accelerated Approval Program
Prescription Drug User Fee Act of 1992
NIH Blueprint for Neuroscience Research
FDA Expanded Access Program
National Alzheimer’s Project Act
FDA Patient-Focused Drug Development Program
BRAIN Initiative
NIH Accelerated Medicines Program
21st Century Cures Act
FDA Reauthorization Act
Accelerating Access to Critical Therapies for ALS Act
Brief Description
Incentivizes drug development for rare diseases (diseases that affect <200,000 people like ALS)6
Allows for expedited drug approval using endpoints that may predict clinical benefit7
Permits the FDA to collect user fees from drug developers and created the Priority Review which help expedite drug approval8
Collaboration between multiple NIH Institutes/Centers supporting early-stage small molecule drug discovery through phase 19
A pathway by which patients can request access to investigational drugs outside of clinical trials10
Created a national plan for addressing AD and related dementias11
Focused on enhancing patient voice in drug development and evaluation, including improving clinical trial enrollment, better identifying endpoints and tradeoffs, and being cognizant of patient preferences12
Public-private partnership with goal of increasing understanding of brain function and diseases, including through leveraging new technologies
Public-private partnership with goal of jointly identifying and validating promising biological targets for therapeutics13
Bipartisan bill that allocated $1.5 billion to BRAIN Initiative and streamlined drug approval process
Implemented changes to drug user fee programs and clarified clinical trial inclusion/exclusion criteria guidelines14
Required creation of a public-private partnership to advance understanding of and develop treatments for ALS & other rare NDDs and 5-year action plan
FDA’s ALS Guidance Provided formal guidance on drug development and clinical trial design for developers targeting ALS15
ARPA-H
New federal agency focused on high-risk, high-reward drug development for diseases like AD16
Year Area of Impact
1983 Drug Development
1992 Approval & Access
1992 Approval & Access
2004 Drug Development
2009 Approval & Access
2011 Funding
2012 Drug Development
2013 Funding
2014 Drug Development
2016 Funding, Drug Development, Approval & Access
2017 Drug Development
2021 Drug Development
2019 Drug Development
2022 Drug Development
23 Belfer Center for Science and International Affairs | Harvard Kennedy School
Policy can serve as a tool to signal priorities, as evidenced by the changes in funding allocation for Alzheimer’s disease research by the US Congress in recent years. For instance, from 2001 to 2014, Congress encouraged the National Institutes of Health to prioritize AD research, although no specific dollar amount was designated. In contrast, from 2016 to 2018, Congress specified that the NIH must allocate a certain portion of the budget to AD research, indicating the increasing importance of addressing AD and other NDDs to the federal government.
Policies can also create opportunities for rapid, transformative change in healthcare. For example, the FDA’s Accelerated Approval Program, developed in 1992, expedited the approval process for drugs that treat serious conditions and fill an unmet medical need based on surrogate endpoints. Although this program primarily benefited oncology drugs, it has also shortened the median approval time of non-oncology drugs by 53 months.
In recent years, policymakers have expressed interest in accelerating NDD drug development and streamlining the approval process. The House Subcommittee on Health hearing “The Path Forward: Advancing Treatments and Cures for Neurodegenerative Diseases” reaffirms this sentiment, suggesting that the policy window for NDD drug development is more critical now than ever.
An analysis of the FDA CDER drug approval database reveals that a total of 46 new drug approvals for neurodegenerative diseases (NDDs) have been granted since January 1970. The majority of these approvals are for multiple sclerosis and Parkinson’s, which is likely due to a better understanding of the underlying pathophysiology of these conditions compared to other NDDs.

24 Tech and Public Policy To Save The Brain
Figure 12. NDA and BLA Approvals Since 1970 by Disease
Figure 12 depicts the number of new drug application (NDA) and biologics license application (BLA) approvals issued by the US Food and Drug Administration (FDA) since 1970, according to FDA CDER drug approval database
The pattern of drug approvals suggests that some policies and programs may have had a more significant impact than others. For example, the 21st Century Cures Act, passed in December 2016, potentially facilitated the development and approval of drugs for a wider range of NDDs, benefiting patients and the healthcare system as a whole. Additionally, the Priority Review process introduced by the Prescription Drug User Fee Act (PDUFA) in 1992 appears to have been particularly helpful for the approval of drugs for rarer NDDs such as ALS and SMA.
While it is challenging to determine the causal effects of these policies and programs on NDD drug development, these insights can inform future strategies and approaches in this field, ultimately emphasizing the crucial role of policy in shaping the landscape of NDD drug development.
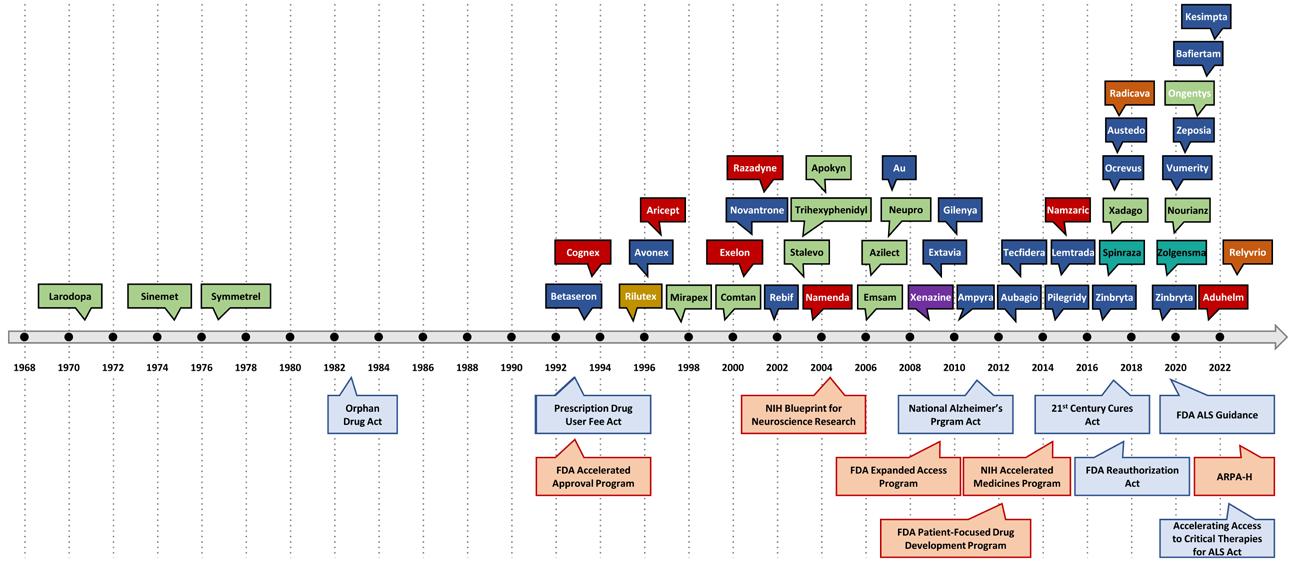
25 Belfer Center for Science and International Affairs | Harvard Kennedy School
Figure 13. Timeline of FDA Drug Approvals and Policies Since 1970
Figure: Timeline of FDA drug approvals since 1970, with selected policies and programs indicated at the bottom. Diseases are color-coded as such: green = PD, dark blue = MS, red = AD, orange = ALS, purple = HD, teal blue = SMA. Programs = pink, and policy = blue.
Basic Science as a Pillar for Drug Development23
Basic science research serves as a fundamental pillar in the development of effective treatments for neurodegenerative diseases (NDDs). FDA’s Dr. Patrizia Cavazzoni emphasized the importance of enhancing our basic scientific understanding of NDDs in a 2021 House hearing, stating that such progress is critical for developing treatments. To achieve similar advancements in NDD drug development as in oncology and HIV, we must first attain comparable progress in disease characterization. This sentiment is echoed by numerous experts in the field.
Both Dr. Richard Hodes, the Director of the National Institute of Aging, and Dr. Walter Koroshetz, the Director of the National Institute of Neurological Disorders and Stroke, have identified the largest barrier to developing effective NDD treatments as our incomplete understanding of NDD pathophysiology. Additionally, leadership from the Biotechnology Innovation Organization, the largest advocacy organization for the biotech industry, agrees that upstream interventions are necessary to identify better drug targets and novel approaches.
Despite its importance, basic science research faces significant challenges that create a major upstream bottleneck. This challenge further disincentivizes funding for NDD research, especially in the private sector, where investment is often driven by potential profit and shorter timelines. Marginal returns on investment are a key driver for why the private sector and larger pharmaceutical companies have historically focused on the later, more de-risked stages of drug development, where there is a higher rate of success.
While basic science research is inherently challenging and risky, often discouraging non-governmental funds, it remains crucial for developing effective disease-modifying therapies. As such, identifying ways to promote more effective basic science research is essential.
Despite the challenges it faces, prioritizing and promoting basic science research is indispensable for making significant strides in the understanding and treatment of these debilitating diseases. By recognizing and attempting to address the existing bottlenecks
23 Giani, L., & Alexander, M. (2022). Understanding how policy affects Neurodegenerative Disease Drug Development. Retrieved April 25, 2023, from https://www.belfercenter.org/publication/understanding-how-policy-affectsneurodegenerative-disease-drug-development
26 Tech and Public Policy To Save The Brain
Case Study on Basic Science Research: Schizophrenia
There is evidence that investing in basic science research works. Previous literature looking at new drug approvals in the 2010s has shown how investment by the NIH in basic science has directly contributed to drug development.24,25
This is very apparent and well-documented for HIV/AIDS and cancer. In the case of HIV/AIDS, the first breakthrough was discovery of retroviral etiology and identification of the virus that causes this disease. Increased federal investment in basic science led to advances in molecular virology including identification of structural and regulatory genes encoding HIV viral proteins, furthering our understanding of the pathogenesis and providing targets for potential antiretroviral drugs.26 The translation of this basic science research into drug development, eventually led to creation of new drug classes like protease inhibitors and has allowed us to reach a point where patients can have a undetectable HIV viral load by taking antiretroviral therapy.27 Similarly, in oncology, advances in basic science have directly led to new therapies. For instance, improved understanding of DNA repair pathways has led to treatments like olaparib, a treatment for ovarian cancer that works by inactivating DNA repair pathways and promoting cell death.28 And basic science research can “work” even when it’s not targeted. Basic science research into bacteria, for instance, ultimately laid the groundwork for CRISPR and gene-editing technologies that hold promise to revolutionize cancer treatment.29
The success of basic science research has also been seen in brain-related disorders like schizophrenia. In the 1950s, the discovery of a new class of medications named chlorpromazine and its unexpected effects on schizophrenia unleashed a flurry of research into the pharmacology of this specific class of drugs and how it intersects with
24 Galkina Cleary, E.,
D. (2018). Contribution of NIH funding to New Drug Approvals 2010–2016. Proceedings of the National Academy of Sciences, 115(10), 2329-2334. doi:10.1073/pnas.1715368115
25 Cleary, E., & Jackson, M. (2020). US tax dollars funded every new pharmaceutical in the last decade. Retrieved April 25, 2023, from https://www.ineteconomics.org/perspectives/blog/us-tax-dollars-funded-every-new-pharmaceutical-in-thelast-decade
26 Fauci, A. S. (2003). HIV and AIDS: 20 Years of science. Nature Medicine, 9(7), 839-843. doi:10.1038/nm0703-839
27 HIV/AIDS. (2015, October 07). Retrieved April 25, 2023, from https://www.nih.gov/about-nih/what-we-do/nih-turningdiscovery-into-health/hiv/aids
28 Lowy, D. R. (n.d.). Progress against cancer: The role of basic science. Retrieved April 25, 2023, from https://www.cancer. gov/news-events/cancer-currents-blog/2015/bypass-basic-science
29 Doudna, J., & Marson, A. (2017). Federal funding for basic research led to the gene-editing revolution. don't cut it. Retrieved April 25, 2023, from https://www.vox.com/the-big-idea/2017/4/22/15392912/genes-science-march-nihfunding-basic-research-doudna
27 Belfer Center for Science and International Affairs | Harvard Kennedy School
and fostering a collaborative and supportive environment, we can pave the way for the development of novel, effective therapies for NDDs.
Beierlein, J. M., Khanuja, N. S., McNamee, L. M., & Ledley, F.
the pathophysiology of schizophrenia. This wave of basic science research led to further understanding of the pathophysiologic mechanisms of schizophrenia, ultimately resulting in the unveiling of the D-2 receptor as an important step in the underlying mechanism of schizophrenia and a target for future therapeutics. This discovery has led to generations of new antipsychotics including aripiprazole. In fact, no drug without a modicum of antagonism of DA at the D-2 receptor has yet been approved by the FDA as an antipsychotic. In addition, the so-called “dopamine hypothesis” has laid the groundwork for more extensive research into the pathophysiology of schizophrenia, producing newer theories such as the “glutamate hypothesis” (emphasizing the importance of N-methyl-D-aspartate (NMDA) receptors). This new discovery has introduced a new potential target for the development of antipsychotic drugs and suggests a promising new horizon for the development of newer, more effective antipsychotic medications.30
The timeline of clinical drug development in Schizophrenia is depicted in the graphic32, following the discovery of chlorpromazine’s antipsychotic activity in 1952. The pharmacological mechanisms of individual compounds are listed in the boxes. Several mechanisms have been targeted with multiple compounds, as indicated by the value in parentheses. The dates provided are approximations based on publication or drug approval dates. The list also includes allosteric modulators.
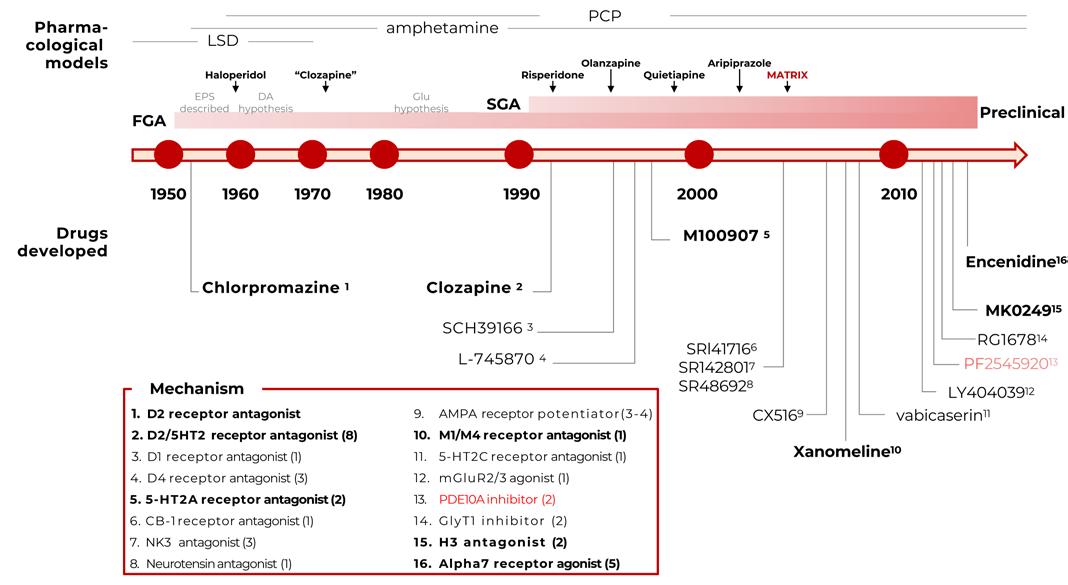
30 Lieberman, J. A., & Dishy, G. (2021). Milestones in the history of schizophrenia. A comprehensive chronology of schizophrenia research: What do we know and when did we know it. Psychiatric News, 56(01). doi:10.1176/appi.pn.2021.1.7.
31 Menniti, F. S., Chappie, T. A., & Schmidt, C. J. (2021). PDE10A inhibitors—clinical failure or window into antipsychotic drug action? Frontiers in Neuroscience, 14. doi:10.3389/fnins.2020.600178.
32 Menniti, F. S., Chappie, T. A., & Schmidt, C. J. (2021). PDE10A inhibitors—clinical failure or window into antipsychotic drug action? Frontiers in Neuroscience, 14. doi:10.3389/fnins.2020.600178
28 Tech and Public Policy To Save The Brain
Figure 14.
Clinical Drug Development in Schizophrenia31
Pain Points Hindering Drug Discovery for NDDs and the Role of Technology and Public Policy
Basic research is essential for developing new therapies and treatments to address the growing NDD crisis. The drug discovery process for NDDs, however, faces numerous challenges that hinder progress. While some pain points are shared across the entire drug discovery space, certain challenges are particularly significant for NDDs. Understanding these pain points and exploring how technology and public policy can help overcome them is crucial for accelerating drug development.
Addressing the pain points specific to NDD drug discovery requires a targeted approach that leverages technology and public policy to expedite the research process, promote diversification and risk-taking, enhance the human capital pipeline, balance innovation and access in intellectual property, develop reliable biomarkers, and increase the number of research hubs around the US. By overcoming these challenges, the field of NDD research can make significant strides in developing new therapies and treatments for these debilitating diseases.
The NDD drug research process is characterized by long timelines, which can hinder the development of new therapies. Technology can help expedite the research process by facilitating data sharing, interdisciplinary collaboration, and adopting advanced technologies like artificial intelligence (AI) and machine learning (ML) to accelerate science (such as target identification, validation, and drug development) as well as lower the workload for administrative, publishing and funding-related tasks. Public policy can support these efforts by implementing milestone-driven funding schemes and streamlining IP granting and negotiation processes.
The drug discovery space, particularly in NDDs, suffers from a lack of idea diversification and risk-taking. Public policy can help address this by offering high-risk funding programs, seed funding, and encouraging the development of non-traditional approaches. Technology can further support diversification by promoting open sharing of research and facilitating virtual collaboration platforms.
Attracting and retaining talent in the field of neuroscience research is essential for progress in NDD drug discovery. Public policy can improve the human capital pipeline by increasing
29 Belfer Center for Science and International Affairs | Harvard Kennedy School
funding for basic science research, providing competitive salaries, promoting diversity, and reforming graduate education. Technology can assist through remote learning platforms, virtual conferences, and mentorship programs, connecting researchers worldwide and fostering a more inclusive research environment.
Striking a balance between incentives for medical innovation and access to low-cost medications is crucial in the NDD drug development process. Governments can explore non-patent exclusivities and vouchers as alternatives to incentivize drug development, ensuring a more equitable landscape for NDD research.
Lastly, the development of reliable translational biomarkers for NDDs is a significant challenge that hinders accurate disease recapitulation. Technology can offer potential solutions by enabling human-based models, promoting interdisciplinary collaboration and data sharing, and exploring alternative preclinical models.
Geographic inequality is a challenge in NDD research, with a concentration of research around specific hubs along the coasts. Public policy can address this by incentivizing regional development, ensuring inclusivity in research hubs, and developing outreach programs. Technology can support these efforts through virtual collaboration platforms, enabling researchers from diverse locations to work together and share knowledge effectively.
Other pain points shared by most diseases and common to drug discovery include the limited resources and coordination in philanthropic funding. Current funding models often struggle to allocate resources effectively, and a lack of coordination between stakeholders can lead to inefficiencies. Public policy can help address these challenges by promoting awareness campaigns, incentivizing donations, fostering relationships, and encouraging greater collaboration between stakeholders. Furthermore, technology can facilitate virtual collaboration platforms, allowing researchers to work together seamlessly, regardless of location.
Rethinking the definition of successful drug development and creating new measures that assess more than academic production or IP creation can help identify high-quality projects and allocate funding more effectively. Public policy can encourage changes to incentive structures, academic advancement criteria, and private-public partnerships to better support NDD research.
30 Tech and Public Policy To Save The Brain
Increasing transparency and collaboration between stakeholders is essential for overcoming the challenges in the basic research process for NDD drug development. Technology can facilitate this by establishing data sharing platforms, interdisciplinary consortia, and targeted incentives for researchers and universities. Streamlining IP granting and negotiation processes can further reduce time taken for technology transfer.
Innovative financing vehicles are needed to support NDD drug development, given the limited resources available for basic research. Public policy can encourage the use of Public-Private Partnerships (PPPs), Social Impact Bonds (SIBs), and government financial incentives, such as tax breaks and concessional loans, to attract private sector investment.
The academic journal publication process can be improved by utilizing technology to create a single submission service, reducing publication costs, and promoting idea diversification. Publicizing research failures, celebrating those and creating standards to make study results replicable is also paramount to ensure success in drug discovery.
In conclusion, the unique challenges faced by neurodegenerative disease (NDD) drug discovery and the related emergency provide an ideal testing ground for the implementation of novel public policies and technology applications. By addressing these pain points and fostering innovative approaches, NDD research can pave the way for better understanding of these complex diseases, leading to more translational science and therapeutic development. This not only benefits the NDD research field but can also serve as a model for the broader drug discovery landscape. The remainder of this report delves deeper into the various pain points, offers insightful case studies, and highlights potential alternatives to consider, emphasizing the importance of a collaborative approach to accelerate NDD drug discovery and improve patient outcomes.
31 Belfer Center for Science and International Affairs | Harvard Kennedy School
Short IP for Long Development Needs
Can we balance Innovation and Access while promoting a new paradigm?
A key trade-off in drug development is balancing incentives for medical innovation with access to low-cost medications.33 Intellectual property (IP) rights - patents and regulatory exclusivities - are at the heart of this debate. Given the high costs, failure risks, and time needed to bring a potential drug from conception to approval, pharmaceutical manufacturers claim IP rights provide a reward for drug development and spur medical innovation. Currently, IP law provides inventors with exclusive rights to their product/invention for 20 years from the date of filing.
On the one hand, a one-size-fits-all patent length for both a life-saving ALS drug and a washing machine may seem ludicrous. Additionally, the clock often starts ticking far before a drug reaches humans - one study found that post-approval patent lengths for drugs were only 13 years.34 Concerningly, this may incentivize pharmaceutical manufacturers to prioritize the development of drug candidates with potentially faster timelines, rather than focusing on disease areas of need including the neurodegenerative disease space.35 However, IP rights also prevent competition from generic and biosimilar manufacturers, often leading to higher prices and limiting access for patients.
Aside from patents, some drugs may also be eligible for regulatory exclusivity after receiving FDA approval or licensure. The exclusivity prevents the FDA from approving a generic or biosimilar version of the drug if the original drug is used as a reference. Exclusivities can range in length from six months to 12 years.36 Drugs that have an active chemical component that has never been approved, for instance, can be eligible for five years of exclusivity.37
33 Saxell, T., Takalo, T., & Izhak, O. (2020, August 25). Optimal patent policy for pharmaceuticals: Balancing innovation and access to new drugs. Retrieved April 25, 2023, from https://cepr.org/voxeu/columns/optimal-patent-policypharmaceuticals-balancing-innovation-and-access-new-drugs
34 Grabowski, H., Long, G., & Mortimer, R. (2013). Recent trends in brand-name and Generic Drug Competition. Journal of Medical Economics, 17(3), 207-214. doi:10.3111/13696998.2013.873723
35 Ricks, D. (2019). Changing the Math on Alzheimer's. Retrieved April 25, 2023, from https://www.lilly.com/news/stories/ changing-the-math-on-alzheimers
36 Ward, E. H., Hickey, K. J., & Richards, K. T. (2021). Drug Prices: The Role of Patents and Regulatory Exclusivities. Retrieved April 26, 2023, from https://crsreports.congress.gov/product/details?prodcode=R46679
37 Lal, R. (2015). Inside this issue patents and exclusivity - Food and Drug Administration. Retrieved April 26, 2023, from https://www.fda.gov/media/92548/download
32 Tech and Public Policy To Save The Brain
Though increasing patent lengths will likely lead to less competition, higher prices, and decreased access, there are other IP incentives that could potentially be used to incentivize increased neurodegenerative drug development. The federal government has previously experimented with regulatory exclusivities for different types of diseases including orphan diseases, pediatric diseases, and antimicrobials.

Though the evidence on the GAIN Act, which added five years of market exclusivity to new antibiotics and antifungals, is mixed,38 it is possible that adding a similar new category of non-patent exclusivity for neurodegenerative disease drugs may move the needle. Unlike antimicrobials where a new drug is unlikely to be used as a first-line agent due to antibiotic stewardship and cheaper alternatives, a new, effective drug for Alzheimer’s, for instance, would likely be the new first-line therapy and thus, adding exclusivity may have more positive results compared to antimicrobials. There will likely need to be some nuance. For a deadly neurodegenerative disease like ALS, adding a sunset clause that actually limits data exclusivity (not market exclusivity) could allow for improved data sharing and more drug development.39
The federal government has also experimented with vouchers to encourage drug development in other spaces like tropical diseases and pediatric diseases. While the
38 Gupta, T. (2021). Gain exclusivity. Retrieved April 25, 2023, from https://www.iqvia.com/blogs/2021/01/gain-exclusivity
39 Parker, V. J., Boyer, B., Sheehan, S., & Romine, M. (2021). Collaboration roadmap to advance drug development in ALS. Retrieved April 26, 2023, from https://healthpolicy.duke.edu/sites/default/files/2022-10/Roadmap%20for%20 Collaboration%20to%20Advance%20Drug%20Development%20for%20ALS.pdf
33 Belfer Center for Science and International Affairs | Harvard Kennedy School
Figure 15: Schematic Representation of Drug Development vs. IP Protection
evidence is once again mixed,40,41 some pharmaceutical manufacturers may be more interested to invest in neurodegenerative disease drug development if they can receive the ability to use their priority review voucher on a different drug or profit off selling a voucher. Another revolutionary idea could be to delink the drug development system from an IP-based system entirely. Instead, the government would offer monetary rewards to the pharmaceutical manufacturer in exchange for IP rights, thus allowing generic manufacturers to enter the market earlier. This, however, would require significant government investment and remains an unpopular approach to date.42
On top of the IP protection per se, management of IP in the neurodegenerative disease space should be further analyzed. The slow pace of tech transfer offices at universities in negotiating licensing agreements, particularly for neurodegenerative intellectual properties (IPs), represents a significant obstacle to advancing neurodegenerative drug discovery. Oncology patent agreements tend to be negotiated more quickly, while the protracted process for neuro IPs hinders research and delays the development of potential treatments for conditions such as Alzheimer’s, Parkinson’s, and other neurodegenerative diseases. This sluggish pace can be attributed to several factors, including the complex nature of neurodegenerative diseases, concerns over IP rights, and risk-averse tech transfer offices that may prioritize protecting their institutions’ interests over accelerating research progress. The consequences of this problem are especially concerning given the urgent need for innovative therapies to address the growing prevalence of neurodegenerative disorders.
To address this issue, a potential solution would be to establish standardized terms for licensing agreements specifically for neurodegenerative drug discovery, similar to the National Institutes of Health’s (NIH) model agreements for cooperative research and development projects. By implementing a widely accepted set of standard terms, negotiations could be accelerated, and research progress would no longer be hindered by lengthy legal processes. Additionally, universities could establish internal guidelines that incentivize tech transfer offices to expedite negotiations for neuro IPs. These guidelines could be modeled after best practices in other fields, such as oncology, where agreements are negotiated more rapidly. Another potential solution
40 Lowe, D. (2016). Closer looks at Priority Review Vouchers. Retrieved April 25, 2023, from https://www.science.org/ content/blog-post/closer-looks-priority-review-vouchers
41 Mezher, M., Brennan, Z., & Gaffney, A. (2020). Regulatory explainer: Everything you need to know about FDA's priority review vouchers. Retrieved April 25, 2023, from https://www.raps.org/regulatory-focus/news-articles/2017/12/ regulatory-explainer-everything-you-need-to-know-about-fdas-priority-review-vouchers
42 Philip Stevens, S. (2020). Delinkage debunked: Why replacing patents with prizes for drug development won't work. Retrieved April 25, 2023, from https://itif.org/publications/2020/02/03/delinkage-debunked-why-replacing-patentsprizes-drug-development-wont-work/
34 Tech and Public Policy To Save The Brain
is to increase collaboration between academia, industry, and regulatory agencies to create an ecosystem that fosters efficient knowledge transfer and accelerates the drug discovery process. This could involve establishing public-private partnerships or consortiums focused on neurodegenerative disease research, as has been successfully demonstrated in the field of oncology with initiatives like the Cancer Moonshot program. By adopting these strategies, universities and their tech transfer offices can play a crucial role in advancing neurodegenerative drug discovery and ultimately improving patient outcomes.
Case Study on GAIN: Government Efforts to Combat Antibacterial Drug Resistance43
The Generating Antibiotics Incentives Now (GAIN) Act was passed in 2012 as a part of the Food and Drug Administration Safety and Innovation Act (FDASIA). Its primary objective is to address the public health threat posed by antibacterial drug resistance by stimulating the development and approval of new antibacterial and antifungal drugs. This case study highlights the progress and accomplishments made under the GAIN Act over five years.
Background: Antibacterial drug-resistant infections pose a significant public health threat worldwide, with at least 2 million people in the United States developing serious infections annually, and at least 23,000 dying as a direct result. The emergence of antibacterial drug resistance threatens advances in modern medicine, such as cancer treatment, organ transplantation, and surgery. Developing new antibacterial drugs is scientifically and economically challenging due to various factors, including the identification of new drug targets and the low return on investment in a field with numerous therapeutic options.
GAIN Act Incentives and Implementation: The GAIN Act offers incentives for sponsors to develop and market antibacterial and antifungal drugs intended to treat serious or life-threatening infections. The act provides for the designation of certain antimicrobial drugs as Qualified Infectious Disease Products (QIDPs), which can receive additional marketing exclusivity, Fast Track designation, and priority review. From July 2012 to September 2017, the FDA granted 147 QIDP designations and approved 12 drug products with QIDP designation.
Stewardship Programs and Accomplishments: The Centers for Disease Control and Prevention (CDC) promotes the implementation of effective antibiotic stewardship
43 Department of Health and Human Services. (2017). Generating antibiotic incentives now - Food and Drug Administration. Retrieved April 26, 2023, from https://www.fda.gov/media/110982/download
35 Belfer Center for Science and International Affairs | Harvard Kennedy School
programs and practices to help slow and control the spread of resistant infections. Despite accomplishments in the development and approval of new antibacterial drugs, significant scientific and economic challenges persist.
Conclusion: Although the GAIN Act has contributed to facilitating new antibacterial drug development, the drug pipeline remains fragile. Efforts beyond the GAIN Act are required to build an antibacterial research and development enterprise capable of bringing new drugs to patients in need. Initiatives such as the Combating Antibiotic Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) by the Biomedical Advanced Research and Development Authority (BARDA), CDC’s work on stewardship, and groups evaluating the economic issues for antibacterial drugs are essential components in the overall effort to develop new antibacterial drugs to address patient needs.
Increase and Retain Neuroscience Talent
How can we expand the talent pipeline and offer worthy opportunities for retention?
Addressing the neurodegenerative disease crisis requires an all-hands-on-deck approach. Recruiting talented researchers who can spearhead basic science and clinical research will be key to developing novel and effective neurodegenerative disease drugs.
We’ve previously highlighted that there are more than 1,000 neurobiology and neuroscience Ph.D. graduates every year, even larger than the number of Ph.D. graduates in other disciplines like cancer biology, cell biology, immunology, and others.44 Other studies have similarly described how the number of neuroscience graduates has been increasing, yet the number of neuroscientists going into academia has dropped.45 Instead, there is a growing number of neuroscientists going into non-science (e.g., teaching) or other (e.g., government research, unemployed) roles with industry and science-related roles seeing stagnant rates. The largest barrier to academia remains the lack of funding for such positions. Given that government labs and academic institutions are the catalysts for basic science research, increasing funding for basic science research can help create more positions for potential researchers.
44 Giani, L., Alexander, M., & Ding, H. (2023). Basic science research as the Pillar for NDD drug development. Retrieved April 25, 2023, from https://www.belfercenter.org/publication/basic-science-research-pillar-ndd-drug-development
45 Stix, G. (2016). Are there too many neuroscientists? - scientific American. Retrieved April 26, 2023, from https://www. scientificamerican.com/article/are-there-too-many-neuroscientists/
36 Tech and Public Policy To Save The Brain
Aside from the lack of jobs in academia, it is likely that salary concerns are a major driver. Surveys of those in academia and industry have found that those in industry are paid higher salaries and are more satisfied with their careers.46 Providing competitive salaries and benefits to those in academia along with increasing mentorship and professional development opportunities will help recruit more talent to the field.
Improving diversity and inclusion must be top of mind when addressing education and workforce concerns. The NIH, in particular, has taken steps to better promote diversity and inclusion in its training programs.48 Increasing government-sponsored internships in neuroscience for high school and college students may also drive more younger and diverse talent into the field. Additionally, we must increase interdisciplinary collaboration - including recruiting graduates with backgrounds in computer science and engineering to work in this space.
There is also room for broader graduate education reform:
• Ensuring that Ph.D. training provides translatable, real-world skills.
• Offering non-doctoral level training (e.g., masters, vocational learning) programs that also emphasize real-world skills.
46 Industry scores higher than academia for job satisfaction. (2021). Nature, 600(7887), 8-8. doi:10.1038/d41586-02103567-3
47 Kang, K., & Falkenheim, J. (n.d.). Doctorate Recipients from U.S. Universities - National Center for Science and Engineering Statistics (NCSES). Retrieved April 25, 2023, from https://ncses.nsf.gov/pubs/nsf23300/data-tables
48 Hodes, R. J., & Koroshetz, W. J. (2021). The Path Forward: Advancing Treatments and Cures for Neurodegenerative Diseases. Retrieved April 26, 2023, from https://www.congress.gov/117/meeting/house/113983/witnesses/HHRG-117IF14-Wstate-HodesR-20210729.pdf

37 Belfer Center for Science and International Affairs | Harvard Kennedy School
Figure 16: Supply and Demand of Neuroscience PhDs47
• Removing barriers like unclear program expectations or incentivizing completion to decrease the time needed to graduate.49
• Providing more academic and career support to improve graduation rates.50
• Increasing cross-disciplinary training and collaborative work.51
Geographical Concentration of Research
Is there a case to establish new hubs?
Research hubs are beneficial for a number of reasons. They bring together experts from various fields who may have different perspectives and expertise to work together on a specific research topic. This can lead to new ideas and new collaborative approaches to the research problem that may not have been considered otherwise coupled with accelerating returns. Secondly, research hubs provide access to state-of-the-art equipment and facilities that individual researchers or smaller research teams may not have access to. This can allow for more advanced and efficient research to take place. Finally, research hubs can facilitate the translation of research findings into practical applications. By bringing together researchers, industry partners, and other stakeholders, hubs can create a pathway for research findings to be developed into new treatments, diagnostics, and therapies for patients.
Most research related to Alzheimers is located in the coastal regions of the USA. This leads to geographic inequality, where concentrated research hubs may result in unequal access to resources and opportunities for researchers in other regions. This can limit the diversity of perspectives and approaches, hindering scientific progress. Furthermore, talented researchers in other regions may be drawn to established hubs, leading to a brain drain and exacerbating regional disparities in research capabilities. High costs of living in coastal areas where many hubs are located can also make it challenging for early-career scientists to afford living and working in these regions, further limiting access to talented researchers.Finally, overcrowding in concentrated hubs can lead to competition for limited resources, potentially reducing overall efficiency and effectiveness.
49 Cassuto, L., & Weisbuch, R. (2021). The new Phd: How to build A better graduate education. Retrieved April 25, 2023.
50 Kowarski, I. (2019). How long does it take to get a ph.D.. degree? Retrieved April 26, 2023, from https://www.usnews. com/education/best-graduate-schools/articles/2019-08-12/how-long-does-it-take-to-get-a-phd-degree-and-shouldyou-get-one
51 Phd training is no longer fit for purpose - it needs reform now. (2023). Retrieved April 25, 2023, from https://www. nature.com/articles/d41586-023-00084-3
38 Tech and Public Policy To Save The Brain
Figure 17: Map of Biotech Companies Prevalence by State52 Most companies are concentrated in Massachusetts and California
The figure presents a visual representation of the number of biotech companies by state, revealing a distinct concentration of biotechs around the coastal regions. Notably, California and Massachusetts emerge as major hubs for biotechnology, boasting a significant number of companies in the sector. In addition to these two powerhouse states, New York, New Jersey, and Pennsylvania also exhibit high concentrations of biotech companies. Following closely behind are North Carolina and Washington, further contributing to the coastal prevalence of the industry. Apart from Texas, the central and interior regions of the country exhibit a considerably lower presence of biotech companies.

39 Belfer Center for Science and International Affairs | Harvard Kennedy School
52 BIO Member Directory |BIO (2023). Available at: https://www.bio.org/
Creating new hubs for NDD (neurodegenerative disease) research in underexplored locations, such as the South or the Midwest, offers numerous advantages and opportunities to revitalize the research landscape. Establishing these research hubs can encourage collaboration, knowledge exchange, and foster innovation, while also overcoming some of the geographical limitations that have traditionally hindered progress in this field. One key benefit of developing new hubs in these regions is the local multiplier effect on attracting talent and boosting the economy. By investing in state-of-the-art facilities, these hubs can draw researchers, academics, and industry professionals, fostering a vibrant community that drives scientific discovery and economic growth.
Moreover, the development of these new hubs presents an opportunity to integrate cutting-edge technologies and embrace remote or cloud-based capabilities. This approach can make research more efficient, cost-effective, and accessible, taking advantage of the increasingly remote working culture and lower costs associated with these locations. For instance, state-of-the-art facilities could incorporate virtual or robot-driven research systems, enabling scientists from around the world to collaborate and contribute to projects without the need for physical presence. By leveraging advanced technologies and remote capabilities, these hubs can facilitate a more inclusive and diverse research environment. This, in turn, can lead to a broader range of perspectives and expertise, ultimately driving innovation and the discovery of more effective treatments for neurodegenerative diseases.
Incentivizing regional development can be a powerful tool to advance NDD research by encouraging biotech companies, universities, and research institutions by promoting research hubs in underrepresented regions. Providing tax breaks, grants, or other incentives can help make this possible. However, it is important to ensure that hubs are inclusive and accessible to researchers from diverse backgrounds and regions, and to promote collaboration and knowledge exchange with institutions and researchers outside of these hubs. This can be achieved by addressing the cost of living and limited resources in these regions to ensure that talented researchers have access to the resources they need.
Developing outreach programs to promote research and STEM education in underrepresented regions can also help cultivate interest and build a talent pipeline to support the growth of new hubs. Additionally, launching innovative programs to train people to be lab technicians and other roles that don’t require very qualified
40 Tech and Public Policy To Save The Brain
workers can ensure an adequate workforce to set up labs. Lastly, virtual collaboration can be utilized to facilitate collaboration between researchers and institutions across different regions, reducing the need for physical proximity. In summary, incentivizing regional development and addressing the associated challenges can help create more opportunities for research in underrepresented regions and improve the overall efficiency and effectiveness of NDD research efforts.
Case Study: Alzheimer’s Research Centers are Not Located Where Most Needed53
The United States is facing a significant challenge in addressing Alzheimer’s disease (AD), with the highest prevalence of diagnosed cases among Medicare recipients found in the southern-eastern part of the country. However, most of the Alzheimer’s research centers are located in more affluent areas, close to universities that have historically conducted such research. This mismatch between the prevalence of AD and the location of research centers creates potential problems and barriers to understanding and treating the disease.
Lack of Access to Research and Treatment Options: The concentration of research centers in affluent areas and around universities leads to a lack of access to research and treatment options for those living in regions with high AD prevalence. Patients in these areas may struggle to participate in clinical trials or receive cutting-edge treatments, which could hinder their ability to manage their condition effectively. 53 McFarling, U. (2022) On the Texas-Mexico border, a bold plan to diversify Alzheimer’s research
41 Belfer Center for Science and International Affairs | Harvard Kennedy School
takes shape, STAT. Available at: https://www.statnews.com/2022/09/28/bold-plan-diversify-alzheimers-research-takes-shape/ (Accessed: 1 April 2023).
Underrepresentation of Diverse Populations: The current distribution of research centers has resulted in a lack of inclusion and representation of diverse populations in clinical trials, including Hispanic populations that are more likely to be diagnosed with AD. This underrepresentation can limit the understanding of the disease’s progression and risk factors among different ethnic and socioeconomic groups, impacting the development of effective treatments tailored to specific populations.
Limited Understanding of Environmental Factors: The existing research centers’ location may also limit the understanding of the role of environmental factors in the development of Alzheimer’s. By focusing on more affluent populations, researchers may miss crucial insights into how factors such as socioeconomic status, access to healthcare, and environmental exposures contribute to AD risk and progression.

42 Tech and Public Policy To Save The Brain
Figure 18: Map of Research Centers vs. Wealth54 Research conducted in more affluent areas
54 National Institute on Aging, US Census
Addressing the Mismatch: New Research Centers in Texas: Recognizing the need to address this mismatch, two new Alzheimer’s research centers have been established in Texas, a region with a high incidence of AD. These centers aim to include a more diverse patient population, particularly Mexican Americans, in their research efforts. By engaging local communities and providing resources for health issues and research opportunities, these new research centers have the potential to greatly impact the understanding and treatment of Alzheimer’s disease. This approach could lead to more patient-researcher derived science, addressing the current shortcomings in AD research and ultimately improving the quality of life for those affected by the disease.

Lack of Idea Diversification (and Risk-taking)
How can we incentivize stakeholders to take more risks?
Neurodegenerative drug discovery has experienced numerous setbacks, partially due to the lack of diversified ideas and the tendency for researchers to explore the same established pathways. The reluctance to venture into uncharted territories is exacerbated by the culture of academia, where entire careers can be built around pursuing the wrong basic science exploration. 55 National Institute on Aging, Centers of Medicare and Medicaid Services
43 Belfer Center for Science and International Affairs | Harvard Kennedy School
Figure 19: Map of Alzheimer’s Research Centers vs. Disease Prevalence55 Research conducted where disease is less prevalent
In order to overcome these challenges and foster innovation, a shift in the incentive structure for researchers and a reevaluation of academic advancement criteria are crucial. Academic research typically favors depth over breadth, with researchers often opting to dive deeper into ideas that have already been established in their field. This approach reduces the risk of failure, as there is a more solid scientific foundation for the work being carried out. However, it also stifles the exploration of more innovative ideas, which may reveal previously unknown knowledge and lead to breakthrough developments.
One of the main reasons researchers shy away from riskier ideas is the difficulty in obtaining funding for such projects. Additionally, the risk of project failure is particularly detrimental to academic researchers, who rely on successful research productivity for career advancement. The current structure of academic advancement, which is based on publications, grants, and contributions to the scientific community, disincentivizes scientists from venturing off the beaten path in search of alternative solutions.
To encourage researchers to pursue more innovative ideas in neurodegenerative drug discovery, changes must be made in the incentive structure and academic advancement criteria. Here are some potential considerations to address these issues:
• Targeted Incentives: Implement targeted incentives that reward researchers for pursuing riskier projects, or at least prevent the loss of funding for such endeavors. This approach would encourage researchers to explore novel ideas without the fear of financial consequences, thereby promoting diversity in research topics and potentially leading to breakthroughs in neurodegenerative drug discovery.
• Rethink Academic Advancement Criteria: Reevaluate the traditional H-index as one of the primary models for measuring academic achievement and determining career development. By introducing alternative methods of evaluating researchers’ contributions, such as their impact on the field or the novelty of their ideas, academia can shift its focus towards fostering innovation rather than merely rewarding productivity.
• Encourage Collaboration and Cross-disciplinary Research: Facilitate collaboration between researchers from different fields and backgrounds, as it can lead to the development of new ideas and approaches in neurodegenerative
44 Tech and Public Policy To Save The Brain
drug discovery. Cross-disciplinary research can help to challenge established notions, promote the exchange of knowledge, and ultimately drive innovation in the field.
• Encourage fast failures and embrace expertise changes: Fosteri an environment where academics are incentivized to quickly terminate projects if results are not promising, and to publish these negative outcomes to inform the wider research community. By doing so, resources can be redirected towards more promising avenues of investigation, mirroring the industry’s focus on efficient research allocation. Furthermore, experts who specialize in niche areas should be encouraged to explore adjacent fields, expanding their skillsets and building diverse careers.
• Improve Funding Allocation: Create funding opportunities specifically designed to support high-risk, high-reward projects. These initiatives can help to ensure that novel ideas receive the necessary financial backing, enabling researchers to explore uncharted territories in neurodegenerative drug discovery.
• Promote Data Sharing and Open Access: Encourage the sharing of research findings, including negative results and data from failed experiments, within the scientific community. This approach can help researchers learn from each other’s mistakes and identify new research avenues, ultimately accelerating progress in the field of neurodegenerative drug discovery.
Dearth of Reliable Biomarkers
Can novel technologies and collaboration help increase disease understanding?
Translational biomarkers in neurodegenerative diseases (NDDs) are challenging to discover and validate due to several factors, including limited access to the brain, inadequate animal models, and the need for a multifaceted approach. Researchers need to focus on bridging the gap between basic science and clinical research to enhance the discovery of relevant biomarkers and improve patient outcomes.
One significant challenge in identifying biomarkers for NDDs is the limited access to the brain, making it difficult to directly analyze brain tissue. This highlights the
45 Belfer Center for Science and International Affairs | Harvard Kennedy School
need for alternative methods to study the underlying biological mechanisms of these diseases. Additionally, animal models often fail to recapitulate the complexity and diversity of human NDDs, leading to poor translation of research findings to humans and high failure rates in clinical trials.
Translating science to humans requires a more thorough combination of multiple assays, including cellular-based ones, animal models, patient-derived cells (iPSCs), and computational models. Integrating these approaches can enhance the identification of relevant biomarkers and improve the likelihood of successful translation to humans. Researchers should focus more on human-based models, such as induced pluripotent stem cells (iPSCs) and organoids, to better represent human biology.
Furthermore, exploring the use of alternative preclinical models, such as computational models and in-silico simulations, can complement traditional animal models and reduce reliance on them, accelerating progress in the development of effective therapies.
To overcome these challenges, researchers should explore how to utilize novel technologies to create a comprehensive model that incorporates various available tools to better predict patient outcomes and recapitulate the disease for faster translation to the clinic and patients. Supporting research into personalized medicine and precision therapies can further improve the likelihood of successful translation to humans.
Promoting data sharing and open access to research findings can aid in the development of reliable biomarkers. For example, researchers can access and upload information to patient databases from around the world and have access to datasets from failed drug trials. This will help in the spread of information and also help scientists decide the kind of biomarkers they want to focus on.
Case Study: Groundbreaking ALS Research Collaborative (ARC) To Accelerate Global Research in ALS
The lack of a widely accessible, shared source of data from people living with the disease has long been a significant challenge for researchers in the quest to discover effective treatments for ALS.
Objective: To overcome this challenge, the ALS Therapy Development Institute (ALS TDI) launched the ALS Research Collaborative (ARC), an ambitious global initiative developed
46 Tech and Public Policy To Save The Brain
to better understand the underlying biology of the disease and significantly accelerate the discovery of ALS treatments.
Method: ARC collects natural history data from people with ALS and layers this with additional data that measures their underlying biological processes through omics. The data is made accessible to researchers worldwide through the ARC Data Commons, an innovative data-sharing platform powered by Google Cloud and Google’s Looker application. The ARC Data Commons houses all the data collected by ALS TDI’s Precision Medicine Program (PMP) since 2014, the longest-running natural history study in ALS.
A New Hope: The ARC Data Commons connects to a massive 35+ terabyte database of information that can catalog and relate thousands of data points on ALS symptoms, genetics, and disease biology. Its key features include the ability to easily filter, sort, visualize, and download all of ARC’s ALS natural history data. This data includes accurate digital measures of movement and speech symptom severity that ALS TDI developed using innovative machine learning technology.
Potential Impact: The ALS Research Collaborative and the associated ARC Data Commons represent a promise to make invaluable data available to researchers worldwide. This unique dataset and the data visualization dashboards will enable ALS researchers to rapidly ask and answer questions about how ALS manifests, accelerating ALS research and sparking new ideas. The ARC Data Commons will expand its dataset by continuing to enroll new study participants across multiple other ALS clinical research protocols at ALS TDI.
Conclusion: The ALS Research Collaborative is a global effort that promises to accelerate the pace of ALS discovery and better equip researchers around the world to end this devastating disease. By providing a large, ever-expanding dataset and powerful tools to filter and visualize the data, the ARC Data Commons will enable scientists to answer complex questions more rapidly, ultimately leading to the discovery and development of new treatments for ALS.
47 Belfer Center for Science and International Affairs | Harvard Kennedy School
Extended Developmental Timelines
How can we use technology and innovative schemes to expedite processes?
The lack of transparency as described above also leads to long-drawn timelines for the drug research and development process. The whole cycle starting from granting research funding until executing the transaction to buy the IP rights can take up to 20 years. This delay can be explained by the time taken by universities to grant IP rights to researchers, time taken by pharma companies to negotiate with universities and then finally time taken by the prospective buyer to raise funds.
This extended drug discovery process not only delays the availability of new and valuable treatments to ailing patients and increases costs, but also leads to inefficiencies due to discontinuity. The process hits roadblocks because often stakeholders change, systems change and partnerships take new form. This also dissuades stakeholders from participating in the process, especially the private sector that is often measured on speed and agility.
Some ways to address the issue are as follows -
• Standardizing IP granting and negotiation process: University tech transfer offices are stricken with long timelines for transferring IP rights to commercial entities. Firstly, universities take significant time in submitting applications and getting patents from the US Patent and Trademark Office (up to 2-5 years56). Once that is done, they take a long time to then transfer these rights to commercial entities. Standardizing and streamlining these processes can go a long way in reducing the time for technology and research transfer. One promising way is to benchmark performance across universities and learn from exemplary institutions who have granted and transferred a considerable number of IPs in the near past. More transparency can also speed up the negotiation process as more data on past transactions is made publicly available and can act as an anchor for similar deals.
• Implementing milestone-driven funding schemes: Another way to speed up the process can be to implement milestone-driven funding schemes so that
48 Tech and Public Policy To Save The Brain
56 University of Minnesota. (2017). Frequently asked questions about IP. Retrieved April 25, 2023, from https://research. umn.edu/units/techcomm/university-inventors/frequently-asked-questions-about-ip
all the stakeholders - researchers, universities, IP buyers - are incentivized to track and report progress of the drug development process, optimize time and reduce time to market. For example, NIH offers the Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs57 that provide support to early-stage small businesses working on high-impact, innovative technologies in healthcare. The programs provide funding in two phases, with the second phase funding contingent upon the achievement of milestones in the first phase. Programs like this in the NDD drug development space can help expedite the process.
• Leveraging advanced technologies to accelerate research: Using new-age technology solutions leveraging Artificial Intelligence and Machine Learning to accelerate scientific processes such as target identification and validation, and administrative processes such as communication and knowledge transfer can also be helpful to expedite the process and make it less tedious. For example, use of Electronic Lab Notebook (ELN)58, digital versions of traditional notebooks that allow researchers to record, organize, and share experimental data more efficiently. This technology allows researchers to collaborate and share data easily. Other examples include High-Throughput Screening (HTS)59 and Computer-Aided Drug Design (CADD)60
ROI Metrics Focused on Financial Returns
Can we rethink how we define successful drug development?
From a purely financial perspective, investors evaluate return on investment (ROI) based on the profit that a particular investment brings. A potential drug candidate’s ROI is calculated using its total sales, though investors likely expect only a small proportion of their entire portfolio of investments to have a large, positive ROI.61
57 Understanding SBIR and sttr. (n.d.). Retrieved April 25, 2023, from https://seed.nih.gov/small-business-funding/smallbusiness-program-basics/understanding-sbir-sttr
58 Electronic Lab Notebooks. (n.d.). Retrieved April 25, 2023, from https://datamanagement.hms.harvard.edu/collectanalyze/electronic-lab-notebooks
59 High throughput screening. (n.d.). Retrieved April 25, 2023, from https://www.sciencedirect.com/topics/biochemistrygenetics-and-molecular-biology/high-throughput-screening
60 Arya, H., & Coumar, M. S. (2021). Lead identification and optimization. The Design & Development of Novel Drugs and Vaccines, 31-63. doi:10.1016/b978-0-12-821471-8.00004-0
61 Toptal Talent Network Experts. (2017). Three core principles of venture capital portfolio strategy: Toptal®. Retrieved April 25, 2023, from https://www.toptal.com/finance/venture-capital-consultants/venture-capital-portfolio-strategy
49 Belfer Center for Science and International Affairs | Harvard Kennedy School
Within the drug development space, the government often subsidizes early-stage, higher-risk research, while the private sector (e.g., venture capital) tends to prioritize later-stage research that tends to be more profitable. While the private sector tends to follow a traditional ROI model, the government and early-stage investors have a more difficult time measuring ROI.
Traditionally, ROI has mostly taken a long-term view, with progression through the preclinical stages (hit-to-lead to lead optimization to preclinical safety and IND-enabling studies) and into the clinical phase considered a success. Other metrics have attempted to capture value creation, including the use of citation counts (i.e., how many times a peer-reviewed publication is cited) and intellectual property rights (e.g., the number of patents filed and granted).62 These measures can serve as a proxy for success in basic science but are still imperfect.
Developing new measures that assess more than academic production or the creation of intellectual property is key to better identifying and assessing the quality of early-stage projects. These could include scoring systems that evaluate projects based on whether the drug candidate targets a priority disease, whether it is disease-modifying or palliative, and whether it targets a priority drug target and/or a novel drug target. Identifying the highest-quality projects will allow us to divert more funding to such projects and provide blueprints that lower-quality projects can emulate. Identifying failures earlier may also generate cost savings as costs increase later in the development process.63
However, while the gold standard of research is to develop a drug candidate that makes it into humans and ultimately receives approval from regulators, there is also value in incentivizing neuroscience research in general. Advancing science through filing patents, creating a novel class/finding a new drug target, or ruling out potential drug targets adds to our knowledge base and spurs the direction of future innovation. Given the major implications of finding a potential disease-modifying therapy for diseases like Alzheimer’s and ALS, being more lenient on success is defined may allow for a lower barrier to entry and encourage more engagement in neurodegenerative disease research. As mentioned in our previous piece, it is likely that increasing the amount of basic science research will lead to spillover effects for all neurodegenerative
62 Lott, M. C. (2011). Measuring "success" in R&D. Retrieved April 25, 2023, from https://blogs.scientificamerican.com/ plugged-in/measuring-success-in-rd/
50 Tech and Public Policy To Save The Brain
63 Cummings, J. L., Goldman, D. P., Simmons-Stern, N. R., & Ponton, E. (2021). The costs of developing treatments for alzheimer's disease: A retrospective exploration. Alzheimer's & Dementia, 18(3), 469-477. doi:10.1002/alz.12450
diseases and increase our understanding of the brain, even if some projects are not eventually approved.64 It may be additionally helpful to take the shotgun approach seen during Operation Warp Speed where the government supported multiple COVID vaccine candidates that used different technologies (e.g., mRNA, recombinant virus) in hopes of producing at least one winner.65
Lack of Non-Financial Incentives
How can we ensure sufficient “rewards” for success?
Stakeholders like pharmaceutical manufacturers and venture capital investors tailor their drug development portfolios largely based on expected revenue. Currently, the highest-grossing drugs are mostly oncology, autoimmune, and COVID drugs,66 suggesting to firms that these disease areas are ripe for investment and potentially driving away investment in the neurodegenerative disease space.
Though the neurodegenerative disease space does not suffer from the lack of a market seen in other spaces like antimicrobials,67 creating stronger incentives will promote increased drug development. Previous studies have described the potential of adding incentives seen in the rare disease space including tax credits, waiving application fees, and strengthening intellectual property rights like market exclusivity.68
Additionally, as discussed in other sections, the government could offer monetary rewards for success. This could take the form of a lump sum reward for drug developers who bring a target drug candidate to market or involve multiple rewards for achieving certain milestones in the drug development process, allowing early-stage actors to also benefit.
Another method could involve obtaining earlier assurance of coverage from payers. Take, for instance, the two new Alzheimer’s drugs, aducanumab and lecanemab, that
64 Giani, L., & Alexander, M. (2022). Understanding how policy affects Neurodegenerative Disease Drug Development. Retrieved April 25, 2023, from https://www.belfercenter.org/publication/understanding-how-policy-affectsneurodegenerative-disease-drug-development
65 Hunter, P. (2021). Lessons from Covid-19. EMBO Reports, 22(10). doi:10.15252/embr.202153834
66 Urquhart, L. (2023). Top companies and drugs by sales in 2022. Nature Reviews Drug Discovery, 22(4), 260-260. doi:10.1038/d41573-023-00039-3
67 Jacobs, A. (2022). Can a federally funded 'netflix model' fix the broken market for antibiotics? Retrieved April 25, 2023, from https://www.nytimes.com/2022/12/16/health/congress-antibiotics-drug-resistance.html
68 Cummings, J. L., Goldman, D. P., Simmons-Stern, N. R., & Ponton, E. (2021). The costs of developing treatments for alzheimer's disease: A retrospective exploration. Alzheimer's & Dementia, 18(3), 469-477. doi:10.1002/alz.12450
51 Belfer Center for Science and International Affairs | Harvard Kennedy School
were approved by the FDA in the last three years. Medicare, under its current policy, does not cover therapeutics that target beta-amyloid that were approved under the accelerated pathway.69 As noted by Anand Shah, a former FDA Deputy Commissioner and CMS Chief Medical Officer, this raises warning signs that we may run into situations where drug developers design trials to meet the requirements of regulators, but are unable to effectively sell the drug until they can gather more data to meet the needs of payers.70 Working with payers upfront to set evidentiary requirements will alleviate this issue and potentially guarantee a market for drug developers.
Given the growing aging population with increasing rates of neurodegenerative diseases, there is further incentive for pharmaceutical manufacturers and payers to work together. Government payers like Medicare and Medicaid pay for 2/3 of all healthcare costs associated with Alzheimer’s Disease in the US.71 Subscription payment models, like the ones used for hepatitis C, would provide rewards to pharmaceutical manufacturers and generate savings for payers, and thus may hold value for more prevalent neurodegenerative diseases like Alzheimer’s.72
It is important to remember that assurance of coverage from payers goes further than ensuring that drug developers are rewarded for their efforts. Others have also noted that earlier engagement with payers on evidentiary requirements can increase access and affordability for patients,73 leading to better health outcomes and more cost savings.
69 Park, A. (2023). Why it's hard to get the new alzheimer's Drug Lecanemab. Retrieved April 25, 2023, from https://time. com/6245670/lecanemab-medicare-costs-alzheimers-drug/
70 Castronuovo, C., & Baumann, J. (2023). Alzheimer's patients' access to new drug hangs on Medicare. Retrieved April 25, 2023, from https://news.bloomberglaw.com/health-law-and-business/medicare-takes-center-stage-in-shapingalzheimers-drug-access
71 Hodes, R. J., & Koroshetz, W. J. (2021). The Path Forward: Advancing Treatments and Cures for Neurodegenerative Diseases. Retrieved April 26, 2023, from https://www.congress.gov/117/meeting/house/113983/witnesses/HHRG-117IF14-Wstate-HodesR-20210729.pdf
72 Lin, P., Cohen, J. T., & Neumann, P. J. (2020). Preparing the health-care system to pay for New Alzheimer's drugs. Alzheimer's & Dementia, 16(11), 1568-1570. doi:10.1002/alz.12155
73 Parker, V. J., Boyer, B., Sheehan, S., & Romine, M. (2021). Collaboration roadmap to advance drug development in ALS. Retrieved April 26, 2023, from https://healthpolicy.duke.edu/sites/default/files/2022-10/Roadmap%20for%20 Collaboration%20to%20Advance%20Drug%20Development%20for%20ALS.pdf
52 Tech and Public Policy To Save The Brain
Insufficient Allocation of Philanthropic Funding
Can incentives and simplified regulations ensure more strategic allocation?
Philanthropic funding plays a pivotal role in advancing neurodegenerative disease (NDD) research, with organizations such as the Rainwater Foundation and the Belfer Foundation supporting numerous researchers and projects across the United States. In fact, philanthropic groups contribute $30 billion annually to science.74 While the distribution of philanthropic funding stacks up to considerable amounts, which sponsor very innovative research, there remain several challenges that limit its effectiveness in the NDD space.
Inefficiencies in resource allocation can be attributed to various factors, including lack of coordination between philanthropic organizations and a limited understanding of the research landscape. To address this issue, philanthropic organizations could collaborate more closely and establish consortiums or networks for sharing information, resources, and expertise. On the other end of the spectrum, researchers could make their information and research goals easier to be accessed and potential research of interest to philanthropic organization found. This could facilitate more strategic allocation of resources and help identify areas where funding could be deployed.
The administrative and legal burden of giving out donations can be a significant barrier for both philanthropic organizations and research institutions. Donors may face complicated regulations and tax implications when making contributions to research projects, while research institutions might struggle with managing grant applications, reporting requirements, and compliance. Legal departments at universities might take long times to negotiate and process donations, which might delay important research. Streamlining the donation process and reducing administrative burdens should be a priority for both philanthropic organizations and research institutions. Developing standardized grant application procedures, reporting templates, and compliance guidelines should be a priority. Additionally, governments can introduce tax incentives and simplified regulations to encourage philanthropic contributions, with particular focus to areas of national priority, such as NDD research.
53 Belfer Center for Science and International Affairs | Harvard Kennedy School
74 Sohn, E (2023) How philanthropy can nurture your research, Nature.com. Retrieved April 26, 2023, from https://www. nature.com/articles/d41586-023-00077-2
Lastly, the fragmentation of philanthropic efforts can result in duplication of research, inefficient use of resources, and a lack of focus on high-priority areas. To tackle this challenge, philanthropic organizations should actively engage with researchers, industry stakeholders, and other funding organizations to align their priorities and coordinate their funding strategies. Creating platforms for communication and collaboration among stakeholders can facilitate information exchange, identify synergies, and foster collective action in addressing critical research gaps.
Data - Funding Trap
How can we break the vicious cycle to promote innovation?
The American research community has been facing a funding crisis for several years, with insufficient resources to support the current demands for research funds. Scientists are dedicating an unprecedented amount of time and effort into competing over the dwindling funds available, leading to a decline in morale, scientific collegiality, and cooperation.
The ongoing funding imbalance has led to several persistent issues, including a mismatch between resources and applicants, an imperfect grant reviewing process, reviewer biases against novelty, and large disparities in funding among investigators. Additionally, the lengthy review process, increasing administrative burden, and wasted time and human power due to grant procurement are all contributing to reduced productivity and a decline in the number of scientific publications. Unless the total level of support for research is dramatically increased, the funding crisis will continue to undermine the recruitment of the best and brightest minds to research and hinder scientific progress.75
The lack of data in novel and unexplored areas of neurodegenerative disease research creates a vicious cycle that limits funding opportunities and impedes progress as funding agencies often require promising preliminary data to justify the allocation of resources while data can only be collected when researchers have funding. The scarcity of research funding makes it challenging for researchers to obtain support for innovative projects, exacerbating the problem.
54 Tech and Public Policy To Save The Brain
75 Fang, F. C., & Casadevall, A. (2009). NIH Peer Review Reform—Change we need, or lipstick on a pig? Infection and Immunity, 77(3), 929-932. doi:10.1128/iai.01567-08
Additionally, funding agencies prioritize projects with a higher likelihood of success, leading to risk aversion and preference for established researchers, higher citations included, and well-known disease mechanisms76. This creates a barrier for researchers with novel ideas but insufficient data. To break this cycle, there is a need to incentivize and invest in innovative and interdisciplinary approaches as well as finding ways to support young researchers who are trying to enter this field.
High-risk, high-reward funding programs to support moonshot ideas should be established, specifically designed to support innovative projects with the potential for groundbreaking discoveries.Seed funding can be provided for early-stage research projects, allowing researchers to generate preliminary data and lay a foundation for larger grants. Policy changes advocating for increased emphasis on supporting innovative research and reducing barriers related to preliminary data requirements can further support novel research projects. Additionally, efforts such as startup packages for professors in engineering, business schools, and policy schools can create opportunities for novel research ideas and drive progress in the field. To support early-career researchers, training programs and mentorship initiatives can equip them with the necessary skills and resources to secure funding, provide initial data, and advance their research. Current researchers in the field must ensure that they provide a conducive environment for new researchers to enter the field and are given adequate credit for their contributions towards research as that is very important in helping the junior researchers progress in their careers.
Fragmented and Tedious Journal Publication Requirements
How can we streamline the process?
Several experts point to the academic journal publication process as a major bottleneck in the neurodegenerative drug development process. Firstly, the journal submission process is fragmented, which requires authors to adapt their manuscripts to conform to different stylistic guidelines (i.e., conflict of interest statements, abbreviation table, data availability statements, etc.). In addition, the process of getting a journal published is extremely tedious and time-consuming, requiring the author to wait for decisions from one journal before moving on to another, and the decision process
55 Belfer Center for Science and International Affairs | Harvard Kennedy School
76 Gallo, S. A., Carpenter, A. S., Irwin, D., McPartland, C. D., Travis, J., Reynders, S., . . . Glisson, S. R. (2014). The validation of peer review through research impact measures and the implications for funding strategies. PLoS ONE, 9(9). doi:10.1371/ journal.pone.0106474
can encompass several revisions. Furthermore, there are oftentimes costs associated with submitting or publishing articles in journals, which can be prohibitive, especially for early-career researchers and those from low-income countries.
Further explanation and potential solutions
• Fragmented journal submission process: Each journal has its own set of publication processes, with specific requirements and formatting instructions. Authors are forced to spend a significant amount of time changing minute details in their articles to fit the published guidelines, of which the time can be better spent elsewhere. Simplifying the submission process can be achieved by adopting standardized templates and no longer insisting on articles being reformatted. Platforms like Authorea and Overleaf provide in-built templates for most journals, which can save time and effort for authors. Moreover, allowing authors to submit manuscripts in any appropriate format could significantly reduce the burden on researchers, as they would not need to reformat their work for each submission.
• Tedious journal submission process: After submission of a journal article, authors are almost always restricted from submitting the same article to another journal simultaneously. This results in authors wasting time waiting to hear back from one journal before even beginning the submission process to another. This wait time can sometimes be further extended when the authors are asked for revisions, which can take a significant amount of time. To address this issue, editors and reviewers should consider any manuscript submitted in any appropriate format before deciding whether to accept or reject it. Rejected articles can be revised and resubmitted to another journal without having to be reformatted, saving time and resources.
• High costs associated with publication: Once a paper is finally accepted for publication, authors often face another difficult dilemma. They can either make their articles “open access”, meaning that everyone will be able to read the article, or not, limiting readers to those who subscribe to the journal or purchase the license. On one hand, making the article open access can increase the influence of the article and spread the knowledge more readily across the scientific community, yet the fee to do so can sometimes reach thousands of dollars. This is especially burdensome for early-stage researchers and those from low-income communities. What is even more troubling is that for all
56 Tech and Public Policy To Save The Brain
the parties involved in the process, the author and reviewers are not paid. Thus, the majority of the payment would be going to the publisher, which is inefficient and should be modified.
Potential solutions we have identified include creating a more streamlined submission service for journals to further simplify the process. Instead of each journal having a separate portal for article submission and having individualized requirements, the submission service will accept submissions on behalf of all journals. The author will have the option of selecting which journals they would ideally like to submit to, and the streamlined service will take on the role of reading the submission to see if it aligns with the journals the authors identified.
Furthermore, having a submission service can better coordinate the reviewer workload, without overburdening some and leaving others with no papers to review. Ideally, there should be three or four standard formats for journals that everyone can use, with trivial house-style requirements abolished. Regarding the cost, while certain scholarships and grants do exist to incentivize publishing by early-career researchers and those from low-income countries, they are not enough to fully eliminate the disparity. In order to fully eliminate this difference, we propose moderating the role of the publisher in the publication process.
Publication of scientific articles should be a way to disseminate and advance knowledge, which should be protected from the intrusion of business and profit. There is no good reason for publishers to make significant amounts of money from the process, so the scientific community should work with journals and policymakers to preserve the sanctuary of science from the business of profit generation.
By implementing a streamlined submission service, the scientific community can simplify the journal submission process, reduce the burden on authors and reviewers, and make the entire system more efficient. Additionally, to address the issue of high costs associated with publication, the community should advocate for more funding opportunities for early-career researchers and those from low-income countries, as well as push for changes in the role of publishers within the publication process. By working together, researchers, journals, and policymakers can help ensure that the publication of scientific articles remains focused on advancing knowledge and making it accessible to all, rather than being driven by profit motives. Furthermore,
57 Belfer Center for Science and International Affairs | Harvard Kennedy School
establishing an in-house factotum to deal with any enquiries can improve the overall experience and efficiency for authors during the submission process.
In conclusion, simplifying the journal submission process and reducing barriers for researchers is essential to accelerate advancements in fields such as neurodegenerative drug discovery. By adopting standardized templates, allowing more flexibility in manuscript formatting, and reevaluating the role of publishers, the academic community can create a more inclusive and efficient environment for sharing knowledge and promoting scientific progress.
Case Study: DeSci - A Decentralized Science Platform
Based on Web3 and DAO
Introduction: Decentralized science (DeSci) is a movement that aims to build public infrastructure for funding, creating, reviewing, crediting, storing, and disseminating scientific knowledge fairly and equitably using the Web3 stack. DeSci is a platform that leverages the power of Web3 and DAO technologies to revolutionize the way scientific research is conducted, shared, and funded. This case study provides an overview of the DeSci platform, discussing how it overcomes challenges faced by traditional academic publishing models and fosters an environment where new and unconventional ideas can flourish.
Platform Overview: DeSci is a decentralized platform that connects researchers, reviewers, and readers from around the world, allowing them to collaborate on scientific projects, share their findings, and access a wealth of knowledge without the barriers imposed by traditional academic publishing. By utilizing blockchain technology, DeSci ensures transparency, security, and data immutability, creating a trustless environment where all participants can contribute and be rewarded for their efforts.
58 Tech and Public Policy To Save The Brain
Table: Comparison of DeSci and Traditional Science77
Decentralized science
Distribution of funds is determined by the public using mechanisms such as quadratic donations or DAOs.
You collaborate with peers from all over the globe in dynamic teams.
Funding decisions are made online and transparently. New funding mechanisms are explored.
Sharing laboratory services is made easier and more transparent using Web3 primitives.
New models for publishing can be developed that use Web3 primitives for trust, transparency and universal access.
You can earn tokens and reputation for peer-reviewing work.
You own the intellectual property (IP) you generate and distribute it according to transparent terms.
Sharing all of the research, including the data from unsuccessful efforts, by having all steps on-chain.
Traditional science
Small, closed, centralized groups control the distribution of funds.
Funding organizations and home institutions limit your collaborations.
Funding decisions are made with a long turnaround time and limited transparency. Few funding mechanisms exist.
Sharing laboratory resources is often slow and opaque.
You publish through established pathways frequently acknowledged as inefficient, biased and exploitative.
Your peer-review work is unpaid, benefiting for-profit publishers.
Your home institution owns the IP you generate. Access to the IP is not transparent.
Publication bias means that researchers are more likely to share experiments that had successful results.
59 Belfer Center for Science and International Affairs | Harvard Kennedy School
77 Ding, W., Hou, J., Li, J., Guo, C., Qin, J., Kozma, R., & Wang, F. (2022). DeSci based on Web3 and Dao: A comprehensive overview and reference model. IEEE Transactions on Computational Social Systems, 9(5), 1563-1573. doi:10.1109/tcss.2022.3204745
Key Features:
• Decentralized Governance: DeSci is governed by a DAO, which allows platform participants to have a say in its development and operation. This democratic approach ensures that the platform remains community-driven and aligned with the best interests of its users.
• Token-based Incentives: The DeSci platform has its native token, which is used to incentivize and reward researchers, reviewers, and other participants for their contributions. This token-based economy encourages active participation and ensures fair compensation for valuable work.
• Open Access and Intellectual Property: DeSci promotes open access to research articles and data, enabling the free flow of information and accelerating scientific progress. The platform also employs blockchain technology to securely timestamp and store intellectual property, ensuring proper attribution and protection for authors. Participants can earn tokens and reputation for peer-reviewing work, and they own the intellectual property (IP) they generate and distribute it according to transparent terms.
• Peer Review and Reputation System: DeSci incorporates a transparent and decentralized peer review process, which allows for rigorous evaluation of research articles by experts in the field. The platform also features a reputation system that rewards quality contributions and helps users identify trustworthy and reliable sources of information.
• Decentralized Funding: DeSci enables more diverse funding sources, such as DAOs, quadratic donations, and crowdfunding, and makes funding decisions online and transparently. New funding mechanisms are explored, breaking away from the traditional, biased, and often slow funding model.
• Decentralized Storage and Data Access: DeSci employs decentralized storage solutions, such as IPFS, Arweave, and Filecoin, to securely store and distribute research articles, data, and other assets, ensuring data persistence and accessibility. This approach supports truly open science, where researchers can create public goods without access permissions or fees.
• Reproducibility and Replicability: DeSci emphasizes the importance of reproducibility and replicability in scientific discovery. Web3-native tools can ensure that reproducibility and replicability are the basis of discovery, creating
60 Tech and Public Policy To Save The Brain
attestations for each analysis component and allowing network participants to reproduce calculations and validate results.
• IP Ownership and Development: DeSci leverages non-fungible tokens (NFTs) to manage intellectual property ownership and development. IP-NFTs can establish transparent value attribution chains, reward researchers, and facilitate the financialization of intellectual property.
Conclusion: DeSci represents a groundbreaking approach to scientific research and publishing, leveraging the power of Web3 and DAO technologies to create a transparent, efficient, and democratic ecosystem. By addressing the challenges faced by traditional academic publishing models, such as centralized control, high costs, and limited access to information, DeSci has the potential to drive innovation and accelerate
Limited Publication of Unsuccessful Studies
Can we incentivize sharing of negative results for collective learning?
Another barrier preventing accelerated neurodevelopmental drug development is the lack of publicizing failures in the field. To discuss this, we must first clarify that we do not mean this in the way of “shaming” scientists for the failure of their experiments. In fact, this would be counterproductive.
On the contrary, we believe that a healthier approach might be to “celebrate” the failures. We must change the mindset from that of seeing failures as a question of competency of the scientist to one where failures are what we need to ultimately achieve greater success. In the current academic climate, positive results are often associated with publications, grants, and resulting career advancements, while negative results can reflect badly on the researcher. As a result, negative results are often internalized by the researcher and rarely shared for the fear of humiliation and a damaged reputation. This lack of transparency, however, can have unintended consequences. Without widespread sharing of this data, other researchers will inevitably come up with similar ideas for research. In most instances, researchers will start with a literature search to see if their idea has been done previously. If they find no results online, then two possible explanations will emerge, 1) no one has done this before,
61 Belfer Center for Science and International Affairs | Harvard Kennedy School
or 2) this idea has been trialed, but it is not a feasible idea and there are no positive results worth publishing. More often than not, we fall into the second category. This problem creates extreme inefficiency in the process, leading to multiple researchers conducting the same experiments on ideas that have already been proven to be unfeasible, while those resources can be better used elsewhere. In addition, this also leads to a lack of learning and progress in the field.
Potential solutions that can be used to address this problem would include having an online suppository that logs all studies, whether successful or unsuccessful. While clinicaltrials.gov may be a good starting point for this, the database does not log the results of the studies, which would be helpful. Of course, in setting up this database, there will be significant security questions that will need to be addressed, including how and how much information will be shared on the platform. This will require a series of specific regulations since the platform will have to be regulated to prevent malicious information falsification (ie. In an attempt to mislead other researchers or to get ahead). A potential model to look at would be the ALS-ARC model, which encourages sharing of negative studies, in the hope of advancing the field of ALS research as a whole.
Furthermore, to address this problem in the long run, we must rework the advancement structure within academia. As previously mentioned, positive studies are closely linked to academic advancement, while negative studies can severely hinder it. Thus, to encourage broader exploration, and this problem being especially dire in the field of neurological diseases where so much is unknown, is so separate the academic career from “positive” research productivity. We have to accept that in the current state of neuroscience, both positive and negative results are valuable, and they are all desperately needed.
Case Study: Embracing Failure in Scientific Research
Introduction: Recognizing the value of sharing failed experiments and negative results, several initiatives have emerged to promote transparency and knowledge sharing in the field. This case study examines three initiatives that encouraged the publication of failures, allowing researchers to learn from one another and advance the scientific process.
The Journal of Negative Results in Biomedicine (JNRBM): This journal was established in 2002 as an open-access, peer-reviewed journal specifically dedicated to publishing negative, null, or contradictory results in the biomedical field. By providing a platform for
62 Tech and Public Policy To Save The Brain
researchers to share their negative results, JNRBM fostered a culture of transparency and knowledge sharing that can prevent unnecessary duplication of failed experiments and promote a more efficient scientific process. Probably for economic reasons and for not finding the right traction, the journal ceased operations in 2017.
The All Results Journals (ARJ): a collection of peer-reviewed, open-access journals that focus on publishing negative and null results across various scientific disciplines, including chemistry, physics, biology, and nanotechnology. ARJ aims to create a repository of valuable information that would otherwise remain hidden, facilitating the exchange of knowledge and preventing the loss of crucial data. By encouraging the publication of negative results, ARJ helps researchers identify potential pitfalls and improve their experimental designs.
The Reproducibility Project: Led by the Center for Open Science, this initiative aims to systematically replicate key findings in various scientific disciplines to assess their reproducibility. By highlighting both successful and unsuccessful replication attempts, this project increases transparency in the scientific process and encourages researchers to learn from failures. The project also promotes open science practices by making replication data and protocols publicly available, fostering a culture of collaboration and knowledge sharing.
Conclusion: The successful initiatives highlighted in this case study demonstrate the value of embracing failures and promoting transparency in the scientific community. By providing platforms for publishing negative results and failed experiments, these initiatives facilitate knowledge sharing, prevent unnecessary duplication of efforts, and ultimately contribute to a more efficient scientific process. The scientific community must continue to support and promote such initiatives to drive progress and innovation in various fields of research.
63 Belfer Center for Science and International Affairs | Harvard Kennedy School
Irreproducibility of Research Studies78
Can community-based best practices and standards help?
The reproducibility crisis in life science research has garnered significant attention due to its implications on the efficiency and validity of drug discovery, particularly for neurodegenerative diseases.
Irreproducible preclinical research has been reported to exceed 50%, in 2015 amounting to around US$28 billion spent annually on irreproducible research in the United States alone. This inefficiency not only delays the discovery of life-saving therapies but also contributes to the considerable costs of drug development.

Irreproducibility is a complex issue resulting from a multitude of factors, including study design, biological reagents and reference materials, laboratory protocols, and data analysis and reporting. The lack of a standards and best practices framework further exacerbates the problem. Errors in study design and biological reagents and materials have been identified as the primary contributors to irreproducibility, making improvements in these areas a priority for addressing the issue.
78 Freedman, L. P., Cockburn, I. M., & Simcoe, T. S. (2015). The economics of reproducibility in Preclinical Research. PLOS Biology, 13(6). doi:10.1371/journal.pbio.1002165
79 Freedman, L. P., Cockburn, I. M., & Simcoe, T. S. (2015). The economics of reproducibility in Preclinical Research. PLOS Biology, 13(6). doi:10.1371/journal.pbio.1002165
64 Tech and Public Policy To Save The Brain
Figure 20: Non-reproducible Preclinical Research Annual Expenditure in the US79
A potential solution to the reproducibility crisis involves the development and adoption of community-based best practices and standards. Drawing from successful examples in other industries, such as the information and communication technology sector, internally organized, dynamic, and self-regulating collaborations among key stakeholders can help establish and enforce rules of engagement. A concerted effort from academia, journals, industry, and government is needed to develop, institutionalize, and reward or sanction behaviors in line with a mutually agreed-upon set of rules and guiding principles.
• For study design, it is crucial to improve training programs at academic institutions, focusing on core skills, methods, technology, and tools. Targeted training, coaching, and certification of established principal investigators should be implemented to reinforce the application of best practices throughout the research process. Research funders, including the National Institutes of Health (NIH) and leading disease foundations, should require successful completion of training courses at all levels.
• In terms of biological reagents and reference materials, efforts should be directed toward promoting the adoption of validated reagents by vendors and their utilization by researchers as a documented best practice. Research funder policies should require the use of validated and non contaminated reagents, annual reagent authentication throughout the research study, and adequate funding to cover these additional costs. Publishers should also require procedures to document reagent validation and lack of contamination. Moreover, incentives should be provided for the development of tools for reagent validation using improved genomics data, and standard operating procedures for biological materials handling should be defined throughout their lifecycle.
Although implementing these changes may initially increase costs in the preclinical research enterprise, the long-term benefits of increased reproducibility will ultimately outweigh these expenses. By recovering even half of the annual US$28 billion spent on irreproducible preclinical research through the application of best practices and standards, the life science research community could save approximately US$14 billion per year. Such improvements in reproducibility could accelerate the discovery of novel therapies for neurodegenerative diseases and alleviate the burden on patients and healthcare systems.
65 Belfer Center for Science and International Affairs | Harvard Kennedy School
Case Study: Open Science in Physics
Introduction: Open science promotes accessibility, reusability, transparency, and fosters trust in scientific results. It aims to accelerate scientific discovery by enabling more people to collaborate and share ideas efficiently and cooperatively. The field of physics has been a pioneer in embracing open science, with initiatives like the arXiv preprint server and SCOAP3 (Sponsoring Consortium for Open Access Publishing in Particle Physics) transforming the way research is disseminated and accessed. This case study examines the impact of open science in physics and its potential implications for other disciplines.
The arXiv Preprint Server and SCOAP3, Catalysts for Open Science: Established in the early 1990s, the arXiv preprint server has enabled researchers in physics and related disciplines to share drafts of their scientific papers publicly before submission to a journal. Similarly, SCOAP3, an initiative coordinated by CERN, has facilitated the transition to an open access publication model for most journals in particle and high-energy physics. These platforms have significantly contributed to the accelerated progress, improved transparency and reproducibility, and enhanced collaboration within the physics community. By the end of 2014, arXiv hosted over one million preprints, with annual growth in new paper uploads exceeding 10% in recent years. Peer-reviewed physics articles published in open access journals also nearly doubled between 2016 and 2019.
Implications of Open Science in Physics:
• Accelerated Progress: Open dissemination of research findings has facilitated faster knowledge dissemination and encouraged collaboration, leading to rapid scientific discoveries. Improved Transparency and Reproducibility: Sharing both positive and negative results enables researchers to reproduce and validate each other’s findings, crucial for advancing scientific knowledge.
• Enhanced Collaboration: Open Science fosters collaboration by breaking down barriers between researchers from different backgrounds and institutions, promoting interdisciplinary research, and encouraging a more diverse array of ideas and approaches.
• Increased Trust in Scientific Results: Open science initiatives help maintain the integrity of the scientific record through rigorous and prompt peer review, improving trust in research outcomes.
66 Tech and Public Policy To Save The Brain
• Greater Inclusivity: Open access models reduce financial and geographical barriers to research, allowing scientists from various regions and economic backgrounds to participate in and benefit from global scientific advancements.
Challenges and Future Directions: Despite the successes of open science in the field of physics, challenges remain in transitioning to a fully and sustainably open landscape in physics and beyond:
• Financial Barriers: The transition to a fully open publishing model may create financial obstacles for researchers from lower-income economies who may struggle to cover the costs of open access article publication charges.
• Uneven Availability of Open Access Venues: While the number of open access journals in the physical sciences has grown, some subdisciplines are better served than others, and many publication venues still do not operate on an exclusively open access model.
• Global Consensus and Coordination: The transition to open science requires consensus on the goal of open science, coordinated action to build the infrastructure, and incentives to create lasting change, all of which will take time.
Conclusion: The field of physics has demonstrated the potential of open science to accelerate progress, improve transparency, and foster inclusivity. To continue the transition to a fully and sustainably open landscape in physics and beyond, to fields like Neurodegenerative diseases, stakeholders must engage in open dialogue, address existing challenges, and explore innovative solutions. By collaborating across disciplines, institutions, and borders, the global scientific community can work together to create a more open, accessible, and effective research environment.
Over the coming years, initiatives such as IOP Publishing’s “open physics” program and other collaborative efforts will play a critical role in expanding open science practices. Through continued engagement with the global physical science community, publishers, funders, and researchers can share insights, resources, and best practices to further advance open science principles. Ultimately, the adoption of open science practices across all scientific disciplines will not only strengthen the integrity and trust in scientific results but also drive innovation, inclusivity, and collaboration. By overcoming the challenges that still exist, the scientific community can move toward a future where scientific knowledge is freely accessible, reproducible, and open to all, ultimately benefiting both science and society.
67 Belfer Center for Science and International Affairs | Harvard Kennedy School

68 Tech and Public Policy To Save The Brain
80 What is SCOAP3 – SCOAP3. (n.d.). Retrieved April 26, 2023, from https://scoap3.org/what-is-scoap3/
Figure 21: SCOAP3 Finances Open Access to Related Research Enabling Free Publishing and Access80
Lack of Knowledge Sharing and Collaboration
How can we encourage and facilitate open sharing?
The basic research process in NDD drug development is characterized as being siloed by various stakeholders in the ecosystem. These universities grant intellectual property (IP) rights which then must be negotiated by interested commercial entities such as pharma companies. The whole process from starting from research funding to selling an IP is extremely opaque and siloed. The challenge is especially pronounced in lack of information sharing about existing IPs within institutions.
Inevitably, the lack of knowledge sharing in this process hinders collaboration between different institutional stakeholders at all levels – it makes it difficult for researchers to share knowledge amongst themselves, and for buyers to acquire IP rights in a cost and time effective way. All of this poses the risk of duplication of effort across research centers leading to an inefficient use of the research funding released by NIH and other agencies.
There is an urgent need for open collaboration to ensure effective use of funding in NDD drug development research. Some proposed alternatives to address the challenge are as follows –
• Establishing data sharing platforms: Trusted platforms that make it easier for universities to structurally come together and share information can help in increasing transparency. Experts indicate that institutions are more open to collaborating in non-profit initiatives and platforms. An example of such an initiative is the not-for-profit platform incubated at MIT Picower Center under the aegis of Dr. Andrew Lo which aimed to bring all Alzheimer’s Disease related IPs on one platform. 16 American universities agreed to participate, share information, and chalk out a future direction together.
• Creating interdisciplinary consortia: Facilitating knowledge exchange across different disciplines and even disease areas within NDD research can foster increased cooperation among stakeholders and lead to shared learnings and insights. The Global Alliance for Chronic Diseases (GACD)81 is an example of such an intervention that aims to bring together major international research
69 Belfer Center for Science and International Affairs | Harvard Kennedy School
81 GACD. (n.d.). About GACD. Retrieved April 25, 2023, from https://www.gacd.org/about
funding agencies to address the growing burden of NCDs. In our context, a similar exclusive platform for NDDs to bring researchers, universities, funders and pharma companies together can increase transparency in the system.
• Encouraging open sharing82: Other ways to encourage open sharing in research should be explored. It might require targeted incentives for researchers and universities to publish their work and progress in open-access journals to increase accessibility. This can include financial incentives in terms of publishing fees, or non-financial incentives such as rewards and recognition.
70 Tech and Public Policy To Save The Brain
82 Smith, E., Haustein, S., Mongeon, P., Shu, F., Ridde, V., & Larivière, V. (2017). Knowledge sharing in global health research – the impact, uptake and cost of open access to scholarly literature. Health Research Policy and Systems, 15(1). doi:10.1186/s12961-017-0235-3
Conclusion: NDDs as Testing Ground for Drug Discovery Changes
Neurodegenerative diseases (NDDs) represent a silent emergency with devastating economic and emotional costs. Despite the complexity of NDD research, the government has recognized its national importance, investing in various programs to accelerate progress. Addressing the numerous pain points in NDD research requires concerted effort from multiple stakeholders, including government, research institutions, and the private sector.
NDDs present a unique opportunity to serve as a testing ground for policy and technological changes in drug discovery. The emergency, size, and incidence of the problem are growing each year, making it a pressing issue that requires immediate attention. The increasing prevalence of NDDs, combined with the complexity of their underlying biology, makes them an ideal candidate for innovative policy and tech interventions. As our population continues to age, the demand for effective treatments and interventions will only grow, placing a greater emphasis on the need for novel approaches in research and drug development. By addressing the unique challenges faced by NDD research, we can not only tackle this urgent health crisis but also pioneer new solutions that have the potential to transform drug discovery across various fields of medicine.
Some of the highest priorities areas identified include:
• Rethinking Intellectual Property: One of the critical pain-points in NDD research is the current Intellectual Property (IP) system, which offers “short” protection for diseases that are chronic and develop over time. This system needs to be rethought to promote more investment in NDD research. By implementing novel protection clauses that are disease-specific and allow for extended and retroactive protection, we can incentivize researchers and companies to endure the long research process. This shift in IP policy would reward innovation and encourage the development of breakthrough treatments for NDDs.
• Improving the Human Capital Pipeline for Neuroscience Research: The field of neuroscience research faces significant challenges in attracting and retaining top talent. We cannot afford to lose neuroscientists to other areas due to a
71 Belfer Center for Science and International Affairs | Harvard Kennedy School
lack of opportunities in the field. To address this issue, we must alleviate administrative processes for funding, provide better career perspectives, and offer more competitive salaries. By creating a supportive environment for neuroscientists to thrive, we can ensure that the best minds are dedicated to finding solutions for NDDs.
• Developing More Research Hubs: To diversify the talent distribution and take advantage of different geographical areas, we need to develop more research hubs focused on NDDs. By providing access to high-tech modern structures that can be used remotely, we can expand patient access and accelerate the research process. Creating these hubs will also promote collaboration and knowledge sharing among researchers from diverse backgrounds, fostering innovation and leading to more effective treatments.
• Encouraging Diversified Ideas and Novel Approaches: NDD research can benefit greatly from the exploration of diverse ideas and novel approaches. By incentivizing the pursuit of sound but unconventional research, we can create a more diversified pipeline of potential treatments. This strategy requires startup capital and support to ensure that innovative ideas have the resources necessary to be tested and developed.
• Investing in Biomarkers and Collaborative Research: Reliable biomarkers are essential for accelerating NDD research. Investing in the development of new biomarkers and promoting research collaboratives that are open and transparent can facilitate global research efforts. The National Institute on Aging (NIA) has created several programs to allow for sharing and access of data; however, these programs are often cumbersome, not intuitive, and require significant effort to be used effectively. To improve these data-sharing initiatives, we can look to other fields, such as Physics’s arXiv preprint server and SCOAP3 open access publishing, for inspiration and best practices. Additionally, we can explore the impact of novel programs such as the ALS Research Collaborative (ARC) and innovative ideas like DeSci (Decentralized Science), which aim to streamline data sharing, review, collaboration, and ownership.
• Long Timeline of NDD Drug Research Process: The lengthy timeline of the NDD drug research process is another significant challenge. Inefficiencies arise from irreproducible data, siloed and replicated research efforts, lack of translational standards, and administrative roadblocks. To overcome these
72 Tech and Public Policy To Save The Brain
obstacles, we must streamline the research process, promote transparency and collaboration, and implement new policies and technologies to accelerate drug discovery.
The challenges faced by NDD research can serve as a testing ground for policy and technological changes that could ultimately reshape drug discovery in various fields. By encouraging multi-stakeholder collaboration and adopting novel approaches, we can accelerate progress in combating NDDs and save millions of brains from the devastating effects of neurodegeneration. The time to act is now, as every moment wasted puts more lives and families at risk, including ours.
73 Belfer Center for Science and International Affairs | Harvard Kennedy School

Technology and Public Purpose Project Belfer Center for Science and International Affairs Harvard Kennedy School 79 JFK Street Cambridge, MA 02138 belfercenter.org/TAPP Copyright 2023, President and Fellows of Harvard College Printed in the United States of America
























