Guide













Europea n Biotechnolog y Science &
Industry
Guide
© BIOCOM AG, Berlin 2025
European Biotechnology Science & Industry Guide 2025 (Vol. 15)
Published by:
BIOCOM Interrelations GmbH
Jacobsenweg 61 13509 Berlin, Germany
Tel. +49-30-264921-0
Fax +49-30-264921-11 service@biocom.de www.biocom.de
In cooperation with the European Biotechnology Network www.european-biotechnology.net
Editor: Andreas Mietzsch
Executive Producer: Andreas Macht
Production: Martina Willnow
Graphic Design: Michaela Reblin
Printed at: Druckhaus Sportflieger GmbH, Berlin
This book is protected by copyright. All rights including those regarding translation, reprinting and reproduction reserved. No part of this book covered by the copyright hereon may be processed, reproduced, and proliferated in any form or by any means (graphic, electronic, or mechanical, including photocopying, recording, taping, or via information storage and retrieval systems, and the Internet).
The copyright for the printed photos is held by the presented companies.
ISBN: 978-3-928383-95-0
Biotechnology is the magic key
An editorial in a European company guide with a look at the global political situation? That’s very unusual. But we live in strange times. On the one hand, we enjoy freedom, peace and historically unrivalled prosperity. On the other hand, we feel threatened. Not so much in the short term and in our personal environment, but noticeably. Democracy is suddenly perceived as exhausting. In addition, there is the impression that it is mostly only crazy dictators and simplifiers or rather trivial things that cause a splash, while the real problems are not being tackled. What about the threat to our biosphere from too much CO2 in the air? We are facing a natural disaster of almost biblical proportions and what do we do? Half the world is forced to invest its scarce resources in armaments to defend itself from an atavistic would-be tsar. And the USA is now governed by a man who can be described as ‘erratic’, which is already a sign of great self-restraint.
Is the risk aversion and fear of loss of the ageing population in many European countries behind the omnipresent suppression of the necessary? Or is it the dumbing down – ahem – blinding of the younger generation by the Unsocial Media? Or both? I recently read in a letter to the editor on the political situation: ‘No hope, nowhere!’ However, I would like to strongly disagree with this. Mankind already has all the means at its disposal to turn the tide. The only question is whether everything has to get much worse before the shackles of the past can be cast off enough for us to turn our attention to the future. And that brings me to the crux of the matter: the future can only consist of the masses of people adapting to the conditions of our planet again! Economising in cycles and consuming only as much as our ‘spaceship Earth’ can provide. The key to this is biotechnology – something that has unfortunately been largely forgotten. It has already established itself as the central technology in the pharmaceutical sector, but CRISPR/Cas and the New Genomic Techniques (NGT) are not being let off the leash, especially in the EU. One day in the not-too-distant-future, however, this will/must happen, and then we will have climate protection, growth, a unique selling point and a future all in one.
Biotechnologists are incorrigible optimists – otherwise we would be doing something different, faster and more profitable. And although the general conditions in Europe are not always the best (see above), the biotechnology sector in Europe is very successful and is growing in many places even against the trend. In this book, dear readers, you will find many optimistic messages. Companies large and small are focussing on biotechnology and life sciences. However, biotechnological research and later the transfer of the results to consumers require freedom, peace and a lot of money. But what will happen if the transatlantic community of values crumbles? What will be left of the free markets? As optimists, however, we continue undeterred because we are convinced that we are doing the right job.
With this in mind, I hope you all enjoy reading this 15th European Biotechnology Guide published with enthusiasm by BIOCOM®

Andreas Mietzsch President of the European Biotechnology Network
european-biotechnology.net
Let’s save the planet!

Biodiversity loss, climate apocalypse, billions of people in turmoil. New strategies are desperately needed. Biotechnology creates confidence and solutions. The European Biotechnology Network is a non-profit organisation that aims to facilitate cooperation between all professionals in biotechnology and life sciences on the European continent. Find out about (free) membership on our website www.european-biotechnology.net









Introduction
Biotechnology: From green opportunity to wallflower
In politics, priorities can shift rapidly - much to the chagrin of the economy, which depends on longterm strategic planning. A notable example occurred in 2010, when the Renewable Energy Directive set the target of achieving 10% biofuel usage by 2020. However, support for biodiesel and bioethanol was swiftly abandoned in favour of emobility, culminating in the decision to ban new registrations of cars with combustion engines –including those using climate-neutral fuels – after 2035. A similar policy reversal is now unfolding under European Commission President Ursula von der Leyen (EPP). Her first legislative period (20192024) was characterised by the Green Deal, which emphasised regulatory measures to curb greenhouse gas emissions – particularly CO 2 – and a shift from fossil fuels to renewable resources as part of a bio(mass)-based bioeconomy. But in light of geopolitical tensions, particularly Russia’s aggression against Ukraine, von der Leyen has pivoted toward policies aimed at making Europe more competitive, more self-sufficient in raw materials as well as energy and more resilient to crises – at the expense of the Green Deal. This shift could have a negative impact on biotechnology, a key cross-sectoral and enabling technology.
in Europe and hinted at a future EU biotech law. The strategic paper outlines how to solve the challenges that the biotech and biomanufacturing sector in the EU faces. These include accelerating technology transfer to market, reducing regulatory complexity, improving access to financing, attracting skilled workers, eliminating value chain obstacles, enhancing IP protection, boosting public acceptance of new technologies, and strengthening economic security. Vestager’s EU initiative for biotechnology and bioproduction proposes several measures “to address these challenges in a timely manner”.
Leveraging research and boosting innovation: To help identify drivers and bottlenecks of innovation and technology adoption, the Commission ordered a study to investigate the EU’s position compared to other global leaders in industrial biotechnology. On top, the Commission is looking at how to speed up the development and use of biobased innovations within the Industrial Biotechnology Innovation and Synthetic Biology Accelerator (EU IBISBA) as a digital hub and service network for the sector.
European lip service
Credible studies indicate that the 2015 Paris Agreement target of permanently limiting global warming to 2°C (or better yet 1.5°C) above the 1850-1900 preindustrial average temperature was exceeded for the first time in 2024, reaching 1.67°C. Current forecasts project 2.5°C and above for the year 2100.
In March 2024, the previous EU Commission, represented by Executive Vice-President Margrethe Vestager, stated that biotechnology could help counteract both climate change and the increasing shortage of raw materials. As part of an ‘EU Biotech and Biomanufacturing Initiative’, she then presented eight measures designed to speed up technology transfer
Stimulating market demand: For bio-based products to gain a competitive edge, they want to assess their environmental impact compared to fossil-based alternatives. To ensure fair assessment, the European Commission will review the evaluation criteria for both fossil- and bio-based products, incorporating methods for carbon storage in construction materials. To accelerate the substitution of fossil feedstock and to stimulate the demand and market uptake of bio-manufactured products, the Commission will conduct an in-depth impact assessment on the feasibility of biobased content requirements for specific product categories and in public procurement, while also eliminating subsidies making the fossil-based industry more cost-effective. Furthermore, the Commission said it will explore how bio-manufactured non-food products can enhance their market positioning through improved labeling.
Streamlining regulatory pathways: The Commission has committed to assessing how EU legislation and its implementation can be streamlined to shorten the time-to-market for biotech innovations. A separate
EuropaBio

study is set to lay the foundations for a potential EU Biotech Act. To further support the sector, the Commission plans to establishing an EU Biotech Hub, designed to help biotech companies navigate regulatory complexities and access resources to scale up. Furthermore, so-called regulatory sandboxes are being considered. These controlled environments would allow novel solutions to be tested under the supervision of regulators for a limited amount of time, facilitating faster market entry.
Fostering public and private investments: The EU names several existing financing instruments to support biotechnology and biomanufacturing such as Horizon Europe; the Innovation Fund; and STEP. To foster the development and scale-up of innovations with the potential to create new markets, a key policy objective is to address specific challenges for biotech and biomanufacturing in the European Innovation Council (EIC) Accelerator Work Programme 2025. Further- Source:
more, the Commission has initiated a study to identify barriers and propose measures to support the consolidation of investment funds, stock exchanges and post-trading infrastructure. This effort aims to enable the development of the necessary scale, enhance the knowledge base, create deeper pools of liquidity and help lower the cost of financing for high-growth companies.
Strengthening biotech-related skills: According to the initiative presented by Vestager, large-scale and regional skills partnerships could play a key role in providing upskilling and reskilling opportunities in biotech and biomanufacturing. The Commission will explore the creation of a dedicated large-scale partnership for industry players in these fields, with potential co-financing through the Blueprint Alliances initiative under the Erasmus+ programme.
Updating standards: The Commission has stated that it will promote the elaboration and revision of Euro -
pean standards for biotech and biomanufacturing to facilitate market access.
Supporting collaboration and fostering international cooperation: The Commission has committed to promoting the deployment of biotech-related technologies across EU regions through so-called Regional Innovation Valleys. It will also explore the possibility of establishing biotech and biomanufacturing partnerships with international partners to foster collaboration on research and tech transfer, and strategic cooperations on regulatory and market access issues.
Artificial intelligence: The Commission aims to accelerate the adoption of AI and generative AI in biotech and biomanufacturing in the context of GenAI4EU, a €500m initiative designed to enhance Europe’s competitiveness in artificial intelligence (AI).
Reviewing the bioeconomy strategy: Finally, the Commission will review its EU Bioeconomy Strategy by the end of 2025 and explore the potential implementation of an EU Bioeconomy Act.
EuropaBio’s Director, Dr Claire Skentelbery welcomed Vestager’s initiative emphasising that a global technology and market race is underway in biotechnology and biomanufacturing. “Successful regions will lead global trade and supply chains for sectors that Europe considers vital and grow next-gen economies based on biotech innovation,” Skentelbery stressed. “It is vital that the proposed initiative recognises the need for tangible industrial impact during the next Commission mandate and is bold in its ambition.”
A 2029 vision for EU biotech
“Biotechnology plays an essential and significant role for the delivery of EU goals for competitive, healthy, resilient and sustainable economies and societies,“ Vestager promised according to a new EuropaBio vision paper of the EU Biotech Act. “The EU Biotech Act must maximise Europe’s global impact and position this strategic technology, with an ambitious, cross-sectoral approach […]. A strong EU Biotech Act will amplify the effectiveness and impact of initiatives including the EU Life Sciences Strategy, Bioeconomy Strategy and Clean Industrial Deal (see Fig. 1)”, EuropaBio stated at
the end of January 2025. The EU lobby group is hopeful that the EU Biotech Act will strengthen value chains, from R&D to end-users, create coherent regulatory pathways, and foster a next-generation ecosystem built on skilled employment, resilient supply chains, and full integration across all Member States.
New realities
As of 2025, Vestager’s plan remains relevant, with both a biotech law and a bioeconomy law included in the new EU Commission’s 2025 work programme for the fourth quarter. However, insiders suggest the biotech law will be delayed and fall under the leadership of DG Health led by controversial Hungarian anti-GMO commissioner Olivér Várhelyi. Meanwhile, the bioeconomy law is expected to be unveiled in Copenhagen at the end of the year. Behind the scenes in Brussels, proponents of the bio-based bioeconomy express frustration that, five years after the adoption of the EU Bioeconomy Strategy 2020, there is still no action plan. They argue that while the deep-tech bioeconomy concept has gained strong support outside Europe, it has not been realised in the EU. In contrast to first-generation approaches, the deep tech bioeconomy utilises new genomic technologies (NGT) to breed climate-resilient crops and synthetic biology to enable cell or enzymebased bioproduction - a cross-sector market that is expected to be worth hundreds of billions in the medium term. This approach promises to significantly reduce dependence on agricultural imports and provide sustainable, climate-neutral resources to replace fossil raw materials and products.
Furthermore, a new plan for “Europe’s sustainable prosperity and competitiveness” has been added to the EU Commission’s 2025 work programme, reflecting a priority shift among EU decision-makers. Driven by the loss of competitiveness in Europe, the war in Ukraine and the resulting energy price and supply problems, this plan –just like the Competitiveness Compass presented this January – prioritises strengthening Europe’s competitiveness, skilled workforce, raw material resilience and military autonomy. If the European People’s Party (EPP), has its way, this will be at the expense of the Green Deal.
BIOCOM CARD
Europe Individual
› European Biotechnology Magazine Subscription
› European Biotechnology Guide 2025
› Guide to German Biotech Companies 2025
› conveniently accessible ePapers
› Discount for BIOCOM events for 1 person
› additional partner offers
Price: 80 Euro p.a. (incl. VAT)
Students 50% discount (subject to proof of enrolment)
BIOCOM CARD
Europe Corporate
› European Biotechnology Magazine Subscription
› European Biotechnology Guide 2025
› Guide to German Biotech Companies 2025
› conveniently accessible ePapers
› Discount for BIOCOM events for 2 persons
› additional partner offers
Price: 120 Euro p.a. (plus VAT)



In January 2025, the EPP decision-makers adopted a resolution paper aimed at suspending central Green Deal regulations on supply chain law, taxonomy, and sustainability reporting, which are considered too bureaucratic, for an initial period of two years. In addition, the Christian Democrats have decided to delay the climate target of reducing CO2 emissions by 90% by five years, pushing the deadline to 2045. As a result, there is a real risk that the importance of biotechnology – despite its potential for new economic growth based on decarbonisation technologies – will be drastically diminished in Europe by 2029. In the meantime, China and the US will likely follow the same pattern they have in the past with European technological breakthroughs, such as bispecific antibodies: they will license, replicate, finance, and profit from these innovations.
EU insiders argue that anyone who believes that Donald Trump, following his renewed withdrawal from the Paris Agreement, will abandon the commercialisation of the sustainable bioeconomy, is mistaken. The sector, which was expanded under Joe Biden to include synthetic biology and biomanufacturing and supported with billions, is firmed backed by the governors who helped Trump win the presidency. States like Maine, Michigan, Indiana, Nebraska and Wyoming are committed to strengthening the bioconomy. The EPP’s belief that
sustainability is at odds with economic growth in the EU is misguided. As we see in the US and the UK, both of which are rapidly advancing in the deep-tech bioeconomy, biotech investment is proving to be a catalyst for economic growth and job creation. In contrast, the EU continues to focus on first-generation, low-tech bioeconomy models relying on biomass-derived resources instead of modern biotechnological processes that reduce atmospheric CO2 levels, such as archaeal CO2 fixation to produce amino acids for feed and food (Arkeon Biotechnologies GmbH, Vienna, Austria).
German bioeconomy pioneers
In late February, just before the German parliamentary elections, bioengineers and tech transfer experts from the German innovation agency SPRIN-D presented measures to implement a deep-tech bioeconom. These include expanding biomanufacturing capacities to accelerate up-scaling, commercialisation and regulatory approval of previously piloted biotech processes. According to a brand-new market analysis commissioned by the Good Food Institute (GFI) Europe, the German market for cultivated meat and diary products alone could potentially reach revenues of €65bn by 2045. “With sufficient political support, up to 250,000 futureproof jobs could be created in Germany by 2045. With an annual investment of (only) €260m in production infrastructure, an annual turnover of €25bn including €15bn from technology and product exports and the creation of 95,000 new jobs would be possible by 2030,” estimate market analysts of Systemiq, who conducted the study for GFI Europe. Their findings are reinforced by a 2024 survey conducted by Cap Gemini Research and its UK arm Cambridge Consultants. The survey, which gathered information from 1,100 industry leaders across eleven industry sectors, concludes that synthetic biology (SynBio) and artificial intelligence (AI) are at the core of a future-proof economy. Nearly three out of four industry decision-makers surveyed believe that Synbio will help them achieve their corporate sustainability goals significantly faster while also improving product performance. A striking 100% of the 1,100 CEOs from leading companies in cosmetics, food, pharmaceutical, material sciences, chemical industries and agriculture said that Syn-
bio will transform their industries by 2033 at the latest. A World Economic Forum white paper concludes that the bioeconomy is no longer limited to bioconversion of organic waste or biomass. Industry leaders in no way view sustainable production as a barrier to competitiveness, but rather as a solution to the EU’s economic crisis. For years, EU member states have used public reservations about genetic engineering and ‘synthetic imitations of nature’ or ‘meat plagiarism’ to justify lengthy approvel processes. These delays have slowed the commercialisation of new genomic technologies (NGTs) in breeding research and cell-based or microbial meat substitutes, allowing China and the USA to take the lead in these emerging markets. However, public sentiment may be shifting. A mid-February survey by Leaps by Bayer of 13,000 citizens across 13 countries reveals that 56% of respondents support the use of NGTs in agriculture, especially when these technologies improve climate resilience or enable self-fertilising crops.
In Europe, 47% of respondents have a positive view of NGTs, 34% are neutral, while only 12% explicitly reject them. Scepticism towards synthetic foods is higher, with 19% disapproving. To address this, experts rec-
ommend introducing a product-specific positive labelling – similar to the “No genetic engineering” seal – such as “Cholesterol-free thanks to biotech”. The study found that trust in biotech-related high-tech innovations – including cell and gene therapy, NGTs, and cultured meat – is higher in China and the USA than in Europe. And for the 9.1 million farms across the EU, the constantly optimised lab meat in particular is fuelling growing existential fears. In response, the farright governments of Italy and Hungary have already imposed marketing bans, while other agricultural producers such as Austria are advocating for similar restriction – even though they would violate EU law.
It will be interesting to see which direction the EU Commission will take in the promising field of biologisation of manufacturing. Will it side with optimistic economic leaders pushing for innovation or with farmers fearful about the future? And more importantly, will its approach be consistent across all sectors? The Commission’s decisions in the coming months may well define its role on the world stage for decades to come.
Thomas Gabrielczyk


Profiles

Name ›
3PBIOVIAN
Address/P.O. Box › Postal Code/City › Country ›
Manufacturing site Polígono Mocholi, Plaza Cein, 1 31110 Noáin Spain
Address/P.O. Box › Postal Code/City › Country ›
Manufacturing site Tykistökatu 6A, Biocity 20520 Turku Finland
Commercial office
Address/P.O. Box › Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media › Number of Employees › Areas of Activity ›
53 State Street, Suites 503 504 02109 Boston MA (USA)
Elena Erroba (Group Chief Commercial Officer)
+34-948-34-64-80 +35-8-50-3650973 businessdevelopment@3pbiovian.com
www.3pbiovian.com
I 550+
| Biotechnology | Biomedicine | CDMO | GMP | Biologics | Recombinant Proteins | Advanced Therapies
| Viral Vectors
| Plasmid DNA
| Fill & Finish
Affinity in the making
3PBIOVIAN is a globally operating Contract Development and Manufacturing Organisation (CDMO), providing unparalleled end-to-end development and manufacturing services for biologics and advanced therapies.
Collaboration is at the core of how we work. We partner closely with our clients, adapting to their unique needs and guiding them through every stage: from clinical development and trials to commercial production. Our client-oriented approach ensures seamless project execution and efficient progression.
Our service offering spans multiple platforms, including microbial and mammalian expression systems, adenoviruses, adeno-associated viruses (AAV), cell therapy, and plasmids. With a diverse range of bioreactor sizes, we offer flexible production scaling to meet the needs of biopharmaceutical companies worldwide.
Leveraging decades of expertise and extensive capabilities, we position ourselves as a leading pan-European independent biologics CDMO, dedicated to advancing therapies that address patients’ unmet clinical needs, making advanced biologics and tomorrow’s life transforming therapies.
GMP certified
3PBIOVIAN offers GMP manufacturing services for microbial and mammalian protein expression platforms, viral vector production for adenoviruses and AAVs, cell therapy, and plasmid DNA production.
Summary of services
› Plasmid DNA production
› Cell line development
› GMP cell banking and Virus Seed Stock production
› Process development and optimisation
› Technology transfer
› Analytical methods development, qualification, and validation
› Scale-up and cGMP manufacturing




› Formulation development
› Fill and Finish
› Quality Assurance (QA) and Quality Control (QC)
› Drug Substance (DS) & Drug Product (DP) release
GMP manufacturing capacities
› Mammalian, single use bioreactors: 50L, 200L, 400L, 2000L
› Microbial, stainless-steel bioreactors: 10L, 100L, 200L, 500L, 1000L
› Viral Vector manufacturing:
• Adherent: Up to 500 m2
• Suspension: 10L, 25L, 40L, 200L, 500L, 1000-2000L
› Plasmid DNA, stainless steel bioreactors: 25L, 40L, 200L
Our manufacturing sites
Our manufacturing sites in Pamplona-Noáin, Spain, and Turku, Finland, seamlessly support the diverse needs of our clients, covering everything from pre-clinical and clinical supply to full-scale commercial manufacturing.
In Turku, Finland, our manufacturing site boasts a workforce of around 200 employees and spans 5,100 m2, with an on-going expansion to 6,300 m2 within the GeneCity manufacturing facility. Our facilities feature dedicated capacities for GMP manufacturing, analytical methods development, quality control services, and fully automated aseptic fill-and-finish processes.
In Pamplona, Spain, our manufacturing facility employs +350 people and encompasses an expansive area of 10,500 m2. It houses GMP manufacturing capabilities, state-of-the-art equipment and advanced technology for analytical method development and quality control assessments.
Our flexible facilities, certified by EMA and inspected by the FDA, are continually expanding to accommodate the evolving landscape of cutting-edge biopharmaceutical manufacturing requirements.

Name ›
4base lab AG advanced molecular analysis
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website ›
Founded (year) › Type of Laboratory ›
Areas of Activity ›
Aspenhaustr. 25 72770 Reutlingen Germany
Dr Johannes Ries
+49-7121-317878-0 pharma@4base-lab.com https://en.4base-lab.com/pharma/ 1995
GMP, S1/L2
| Identity/quality assessment of mRNA/ DNA vaccines
| Contamination analysis of residual DNA/RNA
| Vaccine quality analysis by Nanopore direct RNA-sequencing
| DNA/cDNA-sequencing by Sanger
| Determination of mRNA poly(A) tail length
| Sequencing the CDS in mRNA
| Direct colony full-length sequencing
| Contract research/outsourcing
| Viral- and microbial detection
The Experts
For almost three decades, 4base lab has been at the forefront of molecular analysis, maintaining GMP-compliant laboratories that consistently meet the highest industry standards. As a reliable partner of mRNA/DNA vaccine manufacturers including leading pharmaceutical companies, 4base lab offers safety at every stage of the manufacturing process.
Excellence through collaboration is our guiding principle. Our team of experienced scientists and qualified technicians partners closely with you to comprehend your unique objectives. We leverage this understanding to develop customized assays and validation methods that are tailored to your specific requirements and ensure optimal results.
Customized advice and flexible adaptation of our test systems are our strengths.
The Services
DNA sequencing: The confirmation of sequence identity is an essential part of the production of therapeutic nucleic acids. 4base lab offers reliable non-GMP and GMP sequencing services according to Sanger, covering all steps necessary for the determination of complete DNA sequences. Even samples with ultra-low DNA concentrations, limited volume or colonies can be sequenced in full-length using validated Rolling Circle Amplification (RCA) methods.
RNA sequencing: The assessment of therapeutic mRNA vaccines directly, without reliance on intermediate molecules, is increasingly in focus of regulatory quality control requirements regarding the sequence coding identity, modifications, and structural motifs. 4base lab offers a qualified analysis pipeline to address these issues directly on the RNA.




Determination of poly(A) tail length: for determination of poly(A) length of mRNA vaccines, qualified sequencing strategies utilizing Sanger adapter sequencing as well as Oxford Nanopore Technologies (ONT) direct RNA sequencing are available.
Residual nucleic acid analysis: Detection of residual DNA contamination. Safety at every step of your manufacturing process of mRNA vaccines. Various validated qPCR/RT-PCR protocols are established for the detection of residual E. coli genomic DNA, RNA and plasmid DNA contaminant in mRNA vaccines.
Development and Validation: New detection systems can be developed at any time according to customer requirements and validated in accordance with ICH Q2.
Viral and microbial detection: Please inquire for available systems.
Quality assurance
4base lab has been maintaining GMP compliant laboratories for molecular analysis for almost three decades. Furthermore, 4base lab is certified according to DIN EN ISO 9001, DIN EN ISO 14001 and accredited according to DIN EN ISO 17025.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees › Founded (year) › Areas of Activity › External › Collaborations
ABclonal Germany GmbH
Emanuel-Leutze-Straße 8 40547 Dusseldorf Germany Dr Jing Lu +49-211-1584-9841 info@eu-abclonal.com abclonal.com
F I I 500 2011
| Develop and manufacture high quality biological reagents, including antibodies, molecular enzymes, NGS library preparation kits, recombinant proteins, and raw materials for in vitro diagnostic.
| CRO service for antibody discovery, engineering, and production using hybridoma, single B cell, or phage display strategies.
| CRO service for recombinant protein expression and peptide synthesis
| The Michael J.Fox Foundation | YCharOS
ABclonal
Germany
GmbH –Your European Partner in Global Life Sciences Innovation
ABclonal is an innovative growth company aiming to provide reliable and cost-effective products and services for both basic and translational research in cutting edge biomedical science. Innovation is our DNA, with worldwide R&D centres focusing on technical innovation and product development for protein science and molecular biology.
ABclonal Germany GmbH, a strategic branch of ABclonal Technology Co., Ltd., embodies the global vision and scientific excellence of its parent company while delivering localised expertise to researchers, biotech companies, and healthcare institutions across Europe. As part of ABclonal’s international network – supported by R&D, production, and commercial hubs spanning North America, Asia, and Europe – we are committed to advancing life sciences innovation worldwide.
Global Strength, Local Precision
Leveraging ABclonal’s global infrastructure, ABclonal Germany provides European clients with direct access to cutting-edge solutions in biological reagents, molecular enzymes, NGS automation, and antibody discovery services.
Why Partner with ABclonal Germany?
› Trusted Globally, Rooted Locally: Join 100,000+ international customers who rely on ABclonal’s products, backed by our German team’s commitment to responsiveness and regulatory excellence.
› End-to-End Solutions: From research antibodies to diagnostic materials, we provide products validated for global reproducibility, with logistics optimised for European timelines.
› Sustainability & Compliance: Aligned with EU regulations and sustainability goals, we prioritise ethical practices and eco-friendly innovation.

Our Milestones
› Antibody Production Services
ABclonal was founded with a strong focus on providing customised antibody production services. Our mission has always been to enhance life science research by delivering better, high-quality antibodies.
› Every Protein Should Have an Antibody
We launched the Human Genome Antibody Project, aiming to document and generate an antibody for every protein encoded in the human genome. By utilising recombinant protein antigens, we have successfully developed over 20,000 rabbit polyclonal and monoclonal antibodies.
› Next-Generation Sequencing (NGS)
Leveraging the expertise of our molecular biologists and geneticists, we are developing cutting-edge NGS technology. Our goal is to produce innovative NGS library preparation kits while integrating advanced bioinformatics platforms. This will accelerate biomedical research and aid in the discovery of biomarkers for genetic and epigenetic diseases.
Our Mission
To become a global leader in biomolecular solutions. Our Values:
› Customers Come First – We prioritise our customers’ needs and success.
› Collaboration Over Individualism – We believe in teamwork and shared achievements.
› Clarity & Transparency – Honesty and openness drive our innovation.
› Embracing Challenges – We see obstacles as opportunities for growth.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees › Founded (year) › Type of Laboratory ›
AGC Biologics
Czernyring 22 69115 Heidelberg Germany
Ben Clauberg +49-1515-7528832 bclauberg@agcbio.com www.agcbio.com
F I
2,500+ 2018
Technology Transfer, Process Development, Analytical Development, cGMP Manufacturing, Quality Control and Quality Assurance, and Process Validation
Areas of Activity ›
Mammalian and Microbial-based Therapeutic Proteins, Recombinant DNA, Plasmid DNA (pDNA), Messenger RNA (mRNA), Viral Vectors, and Genetically Engineered Cells.
Request for › Further Collaborations
We are currently seeking additional collaborations and have capacity available at all of our sites in Europe.
AGC Biologics is a global CDMO (Contract Development and Manufacturing Organisation) providing pharmaceutical development and manufacturing services for protein-based biologics and cell and gene therapies. With eight locations and teams of scientists across three continents, we have the resources and available capacity you need to bring your next project to life. From development to clinical trials to full-scale commercialisation, we can help you reach your goals at any stage in your drug product’s journey.
We specialise in the following modalities and substances: mammalian and microbial-based therapeutic proteins, recombinant DNA, plasmid DNA (pDNA), messenger RNA (mRNA), viral vectors, and genetically engineered cells.
Our sites offer services you need at any stage of product development and manufacturing, including Technology Transfer, Process Development, Analytical Development, cGMP Manufacturing, Formulation Development, Quality Control and Quality Assurance, and Process Validation.
We have several sites near Germany that can service small, medium, and large biotech companies, global pharmaceutical organisations, and emerging advanced therapies organisations working on cell and gene therapies.
European Facilities
Heidelberg, Germany
The Heidelberg facility serves as a hub for microbial systems and services for our global customers. Our microbial expression systems support projects from pre-clinical through commercial – with scales as high as 2,000 L for protein refolding. The site has scientists with several decades of protein expression experience, which has enabled AGC Biologics to establish a centre for custom pDNA and mRNA service lines at this site. We have a new new single-use line offering more pDNA and mRNA projects, at a faster pace.




The facility’s segregated line design allows for capacity and technological flexibility while ensuring compliance with the current global guidelines required for cGMP compliance.
Copenhagen, Denmark
The Copenhagen site is one of the most active biologics facilities in the AGC Biologics network. Here, we operate multiple mammalian and microbial cGMP manufacturing lines at a variety of scales, and the facility also offers a great depth of technological flexibility. The core teams of scientists have been working together to develop and manufacture biologics for two decades. The site completed an expansion in 2024, which more than doubled its single-use bioreactor mammalian cell culture capacity. The new building is operational and taking on new projects.
Milan, Italy
AGC Biologics Milan specialises in cell therapy and viral vector development and manufacturing. The site works with any cell type and lentiviral, retroviral, and adenoassociated viral vectors.
The facility was the first cell and gene therapy site approved in Europe for GMP manufacturing of clinical and commercial supplies. The core scientific team has 30 years of expertise in the field and unique knowledge you will not find anywhere else.
The Milan team has guided five viral vectors and four cell therapy products from development to commercialization and produced hundreds of batches for clinical and commercial use, demonstrating unparalleled late-stage and commercial manufacturing expertise.
Sites in Japan and U.S.A.
With two locations in Japan and three in the U.S., one of the unique things about AGC Biologics is we can produce products on three continents. If you need something done outside of Europe, we have the network and global supply chain to meet your needs.
The company is building a new state-of-the-art cell therapy, mRNA and mammalian facility in Yokohama, Japan, which will be fully operational in 2027. Cell Therapy PD work begins in 2025.

Name ›
Address/P.O. Box ›
Postal Code/City › Country › Email › Social Media › Number of Employees › Founded (year) ›
Areas of Activity ›
ALPX
71 rue des Martyrs 38000 Grenoble France contact@alpx-services.com I 18 2019 | Structural biology | Protein crystallography | Cryo-EM | Fragment and compound screening | Epitope mapping
ALPX: Advancing Structural Biology for Biotech and Pharma
ALPX, a spin-off from EMBL and a French CRO, provides transparent structural biology services to biotech and pharmaceutical companies. We accelerate highquality structural insights, enabling partners to optimise small molecule or biologic projects into promising lead candidates. With automated protein crystallography and cryo-EM platforms, we offer a comprehensive, one-stop solution for structural biology needs.
Automated Protein Crystallography Services
Our platform is centred around two proprietary technologies:
› CRIMS (Crystallography Information Management System): A web-based LIMS that integrates and automates the entire protein crystallography workflow, from crystallisation up to sample harvesting. Partners can monitor project progress and resource allocation in real time, enhancing collaboration and flexibility.
› CrystalDirect™: A laser-based automated sample harvesting system using the proprietary CrystalDirect™ plate format. This technology enables precise and efficient harvesting of protein crystals grown on a thin film.
Coupled with weekly access to synchrotron facilities and an in-house processing pipeline based on the Global Phasing suite, and Pipedream, our entire workflow supports:
› Rapid, iterative structure determination to accelerate medicinal chemistry efforts
› Compound and fragment screening (in-house or with external libraries)
› Structural studies of membrane protein targets
› Data collection services
› Structure solutions and refinement



Single-Particle Cryo-EM Services
Our cryo-EM platform provides an integrated workflow from grid preparation to structure determination. With access to leading local and European imaging centers and robust cloud-based processing, we ensure streamlined, reliable structural insights to confidently advance projects.
Our flexible, phased workflow adapts to project and sample needs:
› Rapid sample screening: Quick assessment of sample preparation on cryo-EM grids.
› In-depth sample screening: Optimisation of grids preparation for high-resolution data collection.
› Data collection: Capturing movies using TEM 200kV or TEM 300kV microscopes for medium- to highresolution 3D map generation.
› Cloud-based data processing and model building: Secure, scalable solutions for efficient cryo map determination and structure determination.
By integrating automation, advanced technologies, and expert-driven workflows, ALPX accelerates drug discovery and structural biology research, delivering critical insights to drive innovation in biotech and pharma.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees › Founded (year) › Type of Laboratory ›
Areas of Activity › Altasciences
575 Armand-Frappier Blvd. Laval, QC H7V 4B3 Canada Sophie Dubois +1-450-973-6077 contact@altasciences.com www.altasciences.com I
+1,800 1992
Full-service CRO/CDMO offering pharmaceutical and biotechnology companies a proven, flexible approach to preclinical and clinical pharmacology studies, including bioanalysis, formulation, manufacturing, analytical services, and the full array of CRO services.
CRO, CDMO, preclinical safety testing, clinical pharmacology and proof of concept, bioanalysis, formulation, drug manufacturing, programme management, medical writing, biostatistics, data management, small and large molecules, biologics, CNS, ophthalmology, gene therapy, GLP-1 RAs.
What We Do
Altasciences is a fully integrated Contract Research Organisation (CRO) and Contract Development and Manufacturing Organisation (CDMO) dedicated to advancing early-phase drug development. We partner with pharmaceutical and biotechnology companies to provide a seamless solution from lead candidate identification to clinical proof of concept and beyond.
Who We Are
Altasciences is built on experience that delivers results, driven by the power of accumulated knowledge put into action to facilitate the outsourcing of early-phase drug development.
With nine facilities across North America and an office in the UK, we provide a comprehensive range of preclinical and clinical pharmacology services, including bioanalysis, formulation, manufacturing, and analytical support, all with a focus on delivering a positive customer experience.
Our mission is to eliminate barriers, accelerate timelines, and turn challenges into success stories, ensuring lifechanging therapies reach patients faster.
Integrated Drug Development Solutions
Proactive Drug Development is Altasciences’ innovative approach that aligns over three decades of expertise around delivering a streamlined, synchronised early-phase drug development pathway, supported by proprietary designed communication and scheduling platforms, processes, and a unique organisational structure. Our services and teams are fully integrated for optimal communication, collaboration, and scheduling to safely save sponsors up to 40% in early-phase drug development time.
Altasciences’ Solutions
Whether for one study or a complete programme, Altasciences offers sponsors a customised and synchronised approach to CRO and CDMO services for their IND/CTA and NDA/Marketing Authorisation submissions.




Preclinical Services:
› 4 facilities for a total of 585K sq. ft.
› 700+ safety studies conducted annually
› 800+ team members
› Full range of in-vivo non-GLP and GLP safety evaluation studies, for large and small molecules
› Experience with both rodent and non-rodent species
› Multiple routes of administration
Clinical Services:
› 3 facilities in the United States and Canada
› 580+ beds
› 285+ clinical trials completed annually
› 400k+ participants in our database
› First-in-human to end of Phase II
› Clinical pharmacology/NDA-enabling trials
› Healthy normal volunteers (HNVs), patients, and special populations
› Pharmacy on-demand
Bioanalytical Services:
› 3 Laboratories across North America
› From discovery to preclinical to Phase IV
› Small and large molecules
› 260+ highly trained regulatory bioanalysis specialists
› 25+ research and development scientists
› Capacity for 720,000+ study samples per year
› Method Development, Validation and Analysis
› 685+ validated methods for a wide range of compounds
› Mass spectrometry, ligand binding, or hybrid assays
Manufacturing and Analytical Services:
› Small molecules
› Formulation development
› Phase I through commercial manufacturing
› ICH stability testing
› DEA License for Schedule I-V
CRO Services:
› Clinical Trial Site Identification and Selection
› Biostatistics and Data Management
› Scientific Guidance and Regulatory Affairs
› Pharmacokinetics/Pharmacodynamics/Toxicokinetics
› Clinical Trial Site Management and Monitoring
› Project Management
› Medical Writing
› Clinical Sample Management Kits
› Full-Time Equivalent (FTE)

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees › Founded (year) › Type of Laboratory ›
Areas of Activity ›
Aminoverse B.V.
Daelderweg 9
6361 HK, Nuth The Netherlands
David Schönauer
+31-45-2084-815 info@aminoverse.com www.aminoverse.com I 18 2020
Biosafety ML-1 laboratory, GMO allowance
| Contract Research
| Enzyme discovery, enzyme profiling, enzyme engineering, enzyme libraries, and enzyme production
| In silico enzyme design
| AI-powered enzyme search and engineering
| Enzyme kits for biocatalysis: Unspecific Peroxygenases (UPO) enzyme panel and Fe(II)/αketoglutarate-dependent oxygenases (KGO) enzyme panel
Aminoverse – Exploring Enzyme
Innovation across Industries
Aminoverse is all about enzymes.
Starting from a target reaction or application, our CRO activities cover all enzyme development steps from
› Enzyme identification (Enzyme Discovery)
› Enzyme application testing with custom assay development (Enzyme Profiling)
› Enzyme development powered by AI (Enzyme Engineering)
› Enzyme supply from mg to kg scale (Enzyme Production)
Customers in the pharma, F&F, agrochemical, food, feed, laundry, home care, cosmetics, diagnostics, biomaterials, and recycling sectors benefit from 70+ cumulative years of enzyme expertise and a track record spanning more than 30 different enzyme types.
Aminoverse’s unique approach combines the strengths of wet lab enzyme R&D with state-of-the-art in silico design and the latest advancements in machine learning to maximise success chances and minimise development risks and budget.
The fee-for-service model with full IP rights for customers ensures 100 % client-owned enzymes in a royaltyfree model.
Enzyme Discovery –“Finding the Needle in the Haystack”
The efficiency of a product or a biocatalytic process highly depends on selecting the right enzyme. Aminoverse helps customers find the optimal enzyme among >4 billion candidates with its proprietary EnzyNAV AI workflow fusing bioinformatics, biophysical simulations, and powerful AI filters. The most promising candidates are curated for freedom-to-operate, Nagoya, and producibility/solubility to ensure the identification of enzymes that pass future commercialisation criteria.


Enzyme Engineering –“Going beyond Nature”
To achieve or optimise economic targets, Aminoverse assists customers in tailoring the enzyme to the desired process or application via Enzyme Engineering. Overall, 10x-1,000x economic efficiency gains are obtained by customised R&D services carefully balancing wet lab research, biophysical simulations, and machine learning algorithms to design the ideal enzyme while minimising risk and budget. Highlights of Aminoverse’s approach are:
› EnzyMAP AI: identifies the true best possible mutations in a given enzyme
› EnzyREC AI: combines the best mutations to predict enzyme variants with maximised performance and stability for the target application among trillions of possible combinations.
Development targets go beyond typical enzyme properties like activity and thermal stability, including pH resistance, protease resilience, reduced inhibition, altered regio- and/or stereoselectivity, solubility – or any combination of these factors.
Enzymes for Oxyfunctionalisation –“Putting the ‘O’ in Biocatalysis”
Particularly for challenging and environmentally harmful oxygenation chemistry, Aminoverse offers a portfolio of >100 off-the-shelf scalable biocatalysts – marking the world’s first and largest collection of such enzymes to obtain regio- and enantioselective products at >95% yields under physiological conditions.
The selected enzymes are active on various substrates such as
› amino acids, antibiotics, nucleosides, and nucleotides
› alkanes and alkenes, fatty acids, heterocycles, steroids, and terpenes
› novel, unnatural, and complex targets (e.g. HPAPIs)
Additionally, the biocatalysts are suitable for epoxidation, demethylation, sulfoxidation, and halogenation reactions. Biocatalytic process scale-up is facilitated by Aminoverse’s supply of all biocatalysts up to kg quantities to achieve metric ton scale production. All biocatalysts are free of 3rd party IP.

Name ›
Contact Person › Telephone › Fax › Email › Website › Social Media › Number of Employees › Founded (year) › Areas of Activity › antibodies-online.com
Address/P.O. Box ›
Postal Code/City › Country ›
Schloss-Rahe-Str. 15 52072 Aachen Germany Sonja Puhl
+49-241-95-163-153 +49-241-95-163-155 info@antibodies-online.com www.antibodies-online.com
I 130 2006 | Research Reagents | Antibody Development | Custom Antibody Solutions
| Immunoassay Development
| HCP Assay Development
| Critical Reagents
| ELISA Kits
| Protein Expression & Characterisation
We Make the Complex Convenient When it Comes to Buying Research Reagents
In the rapidly advancing field of Life Science Research, precision tools such as research reagents are critical for driving innovation and discovery. Antibodies, in particular, have become indispensable due to their high specificity and affinity, enabling diverse applications like quantifying, localising, or modulating the activity of drug targets.
At antibodies-online.com, we tackle the challenge of complexity, offering scientists a streamlined solution for sourcing the most relevant products for their research needs. With access to over 4 million reagents targeting 90,000+ proteins across >2,000 species, and validated for >300 applications, we provide an unparalleled selection. Our robust product offering empowers researchers to accelerate breakthroughs in fields such as oncology, immunology, and molecular biology.
antibodies-online.com is your single-source partner for research at the protein level. In addition to our extensive catalog, we continue to build exclusive, cutting-edge products that meet the evolving needs of the scientific community. Our dedication to advancing the life sciences ensures researchers have access to tools that support both basic and applied research endeavors.
Our Vision: To be an invaluable partner to the life science community, advancing the development and delivery of therapies and diagnostics worldwide.
Our Mission: We aim to refine scientific expertise and operational excellence continually, developing and delivering fit-for-purpose products – from antibodybased tools to biologic critical reagents – that propel research forward.

Catalog Products
› Monoclonal Antibodies
› Polyclonal Antibodies
› Recombinant Antibodies
› Antibody Arrays
› Proteins & Peptides
› ELISA & Assay Kits
› Lysates, Serum & Plasma
› Accessory Reagents
Custom Services
› Custom Antibody Production and Modification
› Recombinant Antibody Design
› Analytical Tools for RNA Therapeutics
› Assay Development
› Services for Impurity Detection
› Protein Expression, Purification & Characterisation
› Cell Culture Services
› Immunisation Studies
Introducing AccuSignal™ E. coli HCP ELISA Kit
E. coli is a widely utilised organism in scientific research and biomanufacturing of therapeutic biologic drug substances. Monitoring the concentrations of impurities, particularly E. coli Host Cell Proteins (HCP), is of paramount importance in biomanufacturing processes. The primary objective of purification processes is to eliminate HCPs to minimise immunogenicity, enhance potency, and ensure the stability of a pure drug substance.
Our AccuSignal™ E. coli HCP ELISA Kit is a robust sandwich enzyme-linked immunosorbent assay (ELISA) designed to detect contaminating E. coli HCPs in therapeutic products throughout their purification processes, including early, mid, late, and final stages. The kit’s unique combination of sensitivity, speed, and compatibility with both B and K-12 E. coli strains makes it an invaluable tool for biomanufacturers.
Learn more at: www.antibodies-online.com/hcp-elisa-kit

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email
Website
Number of Employees
Founded (year)
Type of Laboratory
Areas of Activity
Axolabs GmbH
Fritz-Hornschuch-Str. 9
95326 Kulmbach
Germany
Dr Ingo Röhl
+49-9221-82762-0
+49-9221-82762-99 info@axolabs.com www.axolabs.com
~230
2000 (as Ribopharma; acquired by LGC in 2017) S1, S2
Research solutions for preclinical and clinical development with a focus on nucleic acid therapeutics (NAT), including bioinformatics-guided sequence selection, high quality oligonucleotide manufacturing, analytics (GMP), bioanalytics (GLP, GCP), lead molecule identification, and in vitro and in vivo pharmacology
Proprietary bioanalytical assay for the detection and quantification of oligonucleotides in blood and tissue; proprietary lipid platform for LNPmediated cellular delivery of nucleic acids
Collaborations
Axolabs collaborates with research groups around the world in the field of oligonucleotide therapeutics, including > 15 Big Pharma enterprises, > 200 biotech companies, and > 30 academic research institutes
Axolabs is the leading custom research organisation focused on the discovery, development and manufacture of oligonucleotide and nucleic acid therapeutics. Founded firmly on our world-leading know-how and more than 20 years of experience, we provide high-end preclinical and clinical development solutions tailored to our clients’ needs.
At our branches in Petaluma, California, and Berlin we have manufacturing and purification capabilities for oligonucleotide drug substance under the standards of GMP.
Services and Products
Manufacturing
› High-quality non-GMP oligonucleotide synthesis in quantities of up to several 100 g
› GMP production of oligonucleotides (in Berlin and Petaluma)
› Custom-tailored process optimisation
› Chemical synthesis of long RNA/DNA sequences
› Wide range of chemically modified oligonucleotides and conjugates
› Potency and stability improvement
› Process-related impurity marker synthesis and reference standards
› Lipid synthesis
Analytics
› Broad capabilities for CMC analytical method development
› Physicochemical and thermodynamic characterisation
› Stability determination in biological matrices
› Identification of impurities and metabolites by LC/MS
GMP-compliant analysis of oligonucleotide APIs
› Release testing for Drug Substances and Drug Products
› ICH-compliant stability studies

Analyses specific to mRNA therapeutics
› Cap structure by uHPLC with ESI-MS
› Poly-A tail by uHPLC with ESI-MS
› Purity by uHPLC with UV
› Release testing of mRNA-LNP Drug Products
PK and biodistribution
› GLP-and GCP-certified test site
› Proprietary assay system for sensitive detection of oligonucleotides
› Quantification of mRNA
Oligonucleotide lead identification
› Bioinformatics assessment for sequence pre-selection
› Oligonucleotide design and synthesis
› High-throughput in vitro screening
› Lead characterisation and optimisation
› Efficacy and early safety assessment
Biological and pharmacological tests and analyses
› Safety and toxicology
› Cell-based assays for cell function, proliferation and toxicity
› Ligand-receptor interaction and uptake studies/ histology
› Flow cytometry
Platform for functional cell type-specific delivery of oligonucleotides and mRNAs
› In vitro functional analysis and in vivo models
› Rational oligonucleotide designs tailored for specific delivery systems
› Proprietary lipid nanoparticle formulations
Gene-editing therapeutics
› Unprecedented purity of therapeutic sgRNAs for CRISPR Cas and prime editing applications
› Superior biological editing efficiency

Name ›
Address/P.O. Box ›
Postal Code/City › Country › Contact Person › Telephone › Fax › Email › Website ›
Social Media › Number of Employees › Founded (year) › Type of Laboratory › Areas of Activity › External › Collaborations
Request for › Further Collaborations
Biaffin GmbH & Co KG
AVZ 2, Heinrich-Plett-Str. 40 34132 Kassel Germany Dr Bastian Zimmermann +49-561-8044668 +49-561-8044665 info@biaffin.com www.biaffin.com www.proteinkinase.biz
Company overview
Analytical, ISO 9001:2015 certified
Service provider for biomolecular interaction analysis using SPR Biacore technology; supplier of recombinant proteins and reagents for kinase research and signal transduction
Collaborations with academic research groups and industrial partners
Biaffin is looking for strategic partners interested in complementing their analytical portfolio with SPR Biacore technology.
Biaffin GmbH & Co KG is an ISO 9001:2015 certified bioanalytical service provider specialised in the application of biosensors based on surface plasmon resonance (SPR) for biomolecular interaction analysis (BIA). BIA technology is ideally suited for highly accurate kinetic characterisation of any interacting molecules in real time in a label-free state. Biaffin has extensive knowledge and many years of experience in various application areas and supports biopharmaceutical drug development programme of pharma, biotech, and generic drug companies as well as scientific institutions worldwide as a competent partner. Our services are performed by a highly specialised team (PhD level scientists) in a laboratory well equipped with several state-of-the art SPR instruments (5x Biacore T200, 1x Biacore 8K+). Sufficient capacity of SPR instruments allows Biaffin to start interaction studies on short notice, quickly run multiple projects in parallel, and receive results in a timely manner. A qualified Biacore T200 instrument is available for SPR assay validation and GMP-like SPR analysis. The company’s expertise is documented by many peerreviewed publications in renowned scientific journals.
Analytical services for biopharmaceutical development
For the development of therapeutic antibodies, biopharmaceuticals, and biosimilars, Biaffin offers reliable SPR services for a highly accurate, quantitative kinetic characterisation, semi-quantitative kinetic ranking, or rapid qualitative screening of drug candidates. SPR assays have already been established for many target proteins and can be developed quickly by application of established protocols for reversible and site-directed coupling of biomolecules using specific capture surfaces. These assays can be applied for comparability and stability studies and batch-to-batch comparison during process optimisation. Available assay formats include dual-potency assays, pair-wise epitope mapping, and determination of active concentration based on binding activity (calibration-free methods). Assays can be validated and conducted under GMP-like conditions on a qualified Biacore instrument.

ADCC SPR assays
ADCC SPR assays are established for interaction analysis of antibodies with Fc gamma receptors (CD16, CD32, CD64) including receptor variants and different species, neonatal Fc receptor (FcRn), and complement C1q binding.
Small molecule drug development
Biaffin’s services in small molecule drug development comprise customised SPR assay development for defined target proteins, fragment screening, hit validation, secondary screening, kinetic profiling, competition analy sis, and lead optimisation of compounds to improve specificity, selectivity, and potency. Early ADME studies (serum protein binding) and studies on mode of action, cofactor requirements, and thermodynamic binding analysis further support the drug development process.
Catalogue products
Apart from analytical services, Biaffin offers a range of products serving customers’ needs in kinase research and exploring cellular signal transduction pathways. Biaffin’s product portfolio, available from the online shop www.Proteinkinase.biz, comprises recombinant proteins (kinases, cytokines, receptors, CD antigens), peptide substrates, specific inhibitors, and a biochemical ATP determination assay for customers’ in-house research.
Quality statement
Biaffin has successfully implemented and maintains a Quality Management System certified according to the ISO 9001:2015 international standard, enhancing our ability to supply high-quality products and provide reliable, innovative services according to the highest quality standards, which consistently meet the needs and expectations of our clients.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Address/P.O. Box ›
Postal Code/City › Country › Email › Website › Social Media ›
Number of Employees › Founded (year) ›
Areas of Activity › BiBo Pharma
225 Turnpike Road Southborough, MA 01772 USA
Building 4, No.647 Songtao Road, Zhangjiang 201210 Shanghai China
BD@bibo-pharma.com www.bibo-pharma.com I ~400 2014
| Bioengineering
| Biomanufacturing
| Monoclonal Antibodies
| Recombinant Therapeutic Proteins
| Cell Line & Strain Development
| Microbial Fermentation & Mammalian Cell Culture
| Bioconjugation
| Aseptic Fill and Finish
| GMP Facility Engineering Services
A rapidly-growing full spectrum biopharmaceutical CDMO
Founded in 2014 in the USA in Boston, Massachusetts, BiBo Pharma is a rapidly growing full-spectrum Contract Development and Manufacturing Organisation (CDMO). With two R&D centres and three GMP facilities around the world, BiBo Pharma offers comprehensive services from DNA sequence to global commercial supply.
Expertise & track record
BiBo Pharma’s team consists of experts from leading global biologics CDMOs and biopharmaceutical companies across the US, Europe, and China. With a proven track record in over 50 biologics molecules including more than 8 in Phase III, BiBo offers a comprehensive range of CMC services, including
› Cell line development and strain development
› R&D services including process development, analytical development, formulation development, and toxicology material supply
› cGMP Drug Substance/Drug Product supply for PhI II/III clinical study and BLA submission, advancing to commercial stage
› Process characterisation and process validation
› Scale up and ultra-large-scale production with competitive cost efficiency
› Rapid capacity expansion through strong engineering service*
*Record of greenfield to ready-for-GMP-operation in just 18 months
cGMP compliance ready for commercial supply in 2026
BiBo obtained EU QP audit approval in October/November 2022. In Q1 and Q2 of 2024, BiBo completed technology transfer and scale-up leading to the very first 30kL GMP run for US Phase III clinical trial material supply. All of these efforts contribute to readying BiBo’s cGMP system for commercial supply to the regulated market in 2026.
Pioneering innovation in the “fourth wave” of biomanufacturing
› Creator of the world’s first and largest 30,000L ultralarge mammalian production line, achieving a 100%




success rate in all scale-up projects through our “Success on the First Time” system.
› N-1 perfusion processes as well as intensified fedbatch production, expertise and infrastructure to effectively manage batch, fed-batch, perfusion, or intensified fed-batch processes.
› High cooling capacity to support extremely high cell density fermentation for Pichia, E. coli, Saccharomyces cerevisiae, and other various yeast or bacterial strains, exceeding industry limits of 2kL or 5kL, up to the 30kL scale and beyond.
Competitive production cost and risk-free engineering solution
BiBo’s proprietary Epic-CHO ® cell line achieves expression levels over 10g/L, while its Grand-CHO ® process, specifically designed for ultra-large-scale production, combines perfusion with intensified fed-batch technology. The integration of BiBo’s PanFlex® -Engineering and the Grand-CHO ® process has led to the development of a new biologics production model: Pulse Continuous Manufacturing (PCM). PCM enhances equipment and facility utilisation, increasing productivity by 50-300% compared to conventional methods. BiBo moment, a combination of BiBo’s breakthroughs in Epic-CHO® cell line, Grand-CHO ® process and PanFlex ® -Engineering empower BiBo to achieve a significantly reduced monoclonal antibody production cost – from hundreds of dollars per gram to US$10 per gram
BiBo’s “Blue Whale” model offers clients a customisable, cost-effective production capacity expansion solution for biologics with refundable investment and no financial risk, effectively mitigating regulatory, engineering, market, and financial challenges.
Meet BiBo in 2025
Booth #3035 | BIO International Convention | June 16-19 | Boston, USA
Booth #6.0B92 | CPHI Frankfurt | October 28-30 | Frankfurt, Germany

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website ›
Number of Employees › Founded (year) ›
Areas of Activity ›
BIO4 LTD Belgrade
Nemanjina Street 11 11000, Belgrade Republic of Serbia
Anja Matejic +38169-5951-754 office@bio4.rs www.bio4.rs 10+ 2023
Biotechnology, Biomedicine, Bioinformatics & AI, Biodiversity
A New Bioeconomy Hub in Europe
BIO4 Campus is an emerging center of excellence in Serbia, dedicated to advancing biotechnology, biomedicine, bioinformatics & AI, and biodiversity. As a unique research and innovation hub in Southeast Europe, it fosters collaboration between academia, industry, and government, positioning itself as an important player in the European life sciences landscape.
State-of-the-Art Infrastructure for Next-Gen Research
With its ongoing infrastructure development, BIO4 Campus is set to become a leading hub for life sciences , bringing together key scientific institutions, including seven life-science faculties of the University of Belgrade, nine research institutes, and biotech R&D centers.A dedicated section of the Campus will function as an innovation hub for biotech start-ups, fostering entrepreneurship, technology transfer, and the commercialization of scientific breakthroughs. At the heart of BIO4 Campus will be the Minglarium, the Campus’s landmark building and a state-of-the-art convention center. Designed as a central meeting point for collaboration, innovation, and networking, the Minglarium will provide a dynamic space for researchers, industry leaders, and policymakers to engage, exchange ideas, and drive interdisciplinary cooperation.
Shaping the Future of Biotech Innovation
Through Nature Masterclasses, the Nature Conference in Belgrade, and the Biotech Future Forum, along with start-up incubation programmes, BIO4 Campus is nurturing talent, fostering entrepreneurship, and accelerating commercialisation.
BIO4 Campus offers unparalleled opportunities for researchers, start-ups, and industry leaders to collaborate, innovate, and drive the future of life sciences.



Strategic Global Partnerships
BIO4 Campus is an emerging biotech R&D hub with over 1,500 researchers engaged in pre-clinical and clinical drug development, synthetic biology, and AIdriven applications in medical, agri-food, and industrial biotechnology.
BIO4 Campus is strengthening its position within the European scientific ecosystem, collaborating with institutions across multiple countries, with particularly strong ties to Spain’s advanced biomedical sector. Serbia’s recent prospect membership in EMBL (European Molecular Biology Laboratory) provides new opportunities for collaboration in molecular biology, access to advanced infrastructure, and deeper integration into Europe’s life science networks. Also, researchers from BIO4 actively participate in EU-funded programs like Horizon Europe.
Beyond Europe, BIO4 Campus is expanding its global partnerships with leading pharmaceutical companies, biotech firms, and innovation clusters across North America, Europe, and Asia. Through joint R&D MoUs, BIO4 collaborates with Roche, Takeda, BGI, MSD, SwissRockets, SK Bioscience, Zdravlje, Pfizer, AstraZeneca, Ginkgo Bioworks, Merck, Zhongguancun Science Park & ZGC Group, Medtronic, Arexpo & FITT, and Novartis, among others, advancing drug discovery, diagnostics, and next-generation biotechnologies. These collaborations establish a foundation for long-term cooperation, knowledge exchange, and Serbia’s growing role in the global bioeconomy
Request for Further Collaborations
BIO4 Campus is actively seeking:
› Medical and non-medical biotech companies that need cutting-edge and efficient pre-clinical and clinical services, as well as research partners in agricultural and industrial applications.
› Further collaborations with EU scientific institutions and universities, as well as members of ERIC research infrastructures , to expand joint research initiatives, technology transfer, and innovation-driven partnerships within the European biotech ecosystem.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website › Social Media ›
Number of Employees › Founded (year) › Areas of Activity ›
BioConcept Ltd.
Paradiesrain 14 4123 Allschwil Switzerland
Soheyl Baban
+41-61-486-80-80
+41-61-486-80-00 baban@bioconcept.ch www.bioconcept.ch
I 45 1992
Production of customised cell culture media, defined media for recombination protein production, animal component free media, standard (classical) media, buffers, solutions, sterile QC liquids, HPW and WFI.
Established in 1978, BioConcept AG is a premium Swiss manufacturer of bioprocessing media and buffers for life sciences, biotech, and biopharma industries. BioConcept strives to combine its long experience with an innovative spirit to support its customers’ needs with the best possible solutions.
During the past 45 years, BioConcept has established close relationships with its customers to understand their needs and to respond to their stringent requirements for their specific applications. BioConcept products are of the highest quality standard and are manufactured according to GMP guidelines in an ISO certified facility. BioConcept productions sites for liquid and powder media are located in the Basel Life Science area (Switzerland), in close proximity to Germany and France.
BioConcept’s proprietary media platforms include CHO, HEK, Hybridoma and Baculovirus platforms, comprise a range of various media, supplements, and feed components, all of which are routinely used in various aspects of biologics and biosimilar bioproduction by global biopharmaceutical companies.
Mammalian Artificial Media - Protein Free ACF (MAMPF®) is a protein-free, hydrolysate-free, animal component-free (ACF) and chemically defined medium for high cell density cultivation of various CHO cell lines and highlevel expression of recombinant proteins and mAbs.
HEKxpress and HEKxpress Feed have been developed for the long-term cultivation of HEK cells, enabling maximum production of recombinant proteins and antibodies. HEKxpress supports HEK293 cells in achieving high cell densities and maximum production yields. HEKxpress and HEKxpress Feed are ready-to-use, chemically defined (CD), and animal component-free (ACF) and do not contain serum (SF), proteins (PF) or




hydrolysates. These features allow a seamless transition through research and development stages and for further manufacturing. The HEKxpress platform can be used for the cultivation of HEK293 cells for various purposes, including the production of recombinant proteins, antibodyies, viral vectors and vaccines. It is suiteable for transient transfection and the establishment and cultivation of stable cell lines. HEKxpress supports all common transfection methods, indlucing chemical methods (Polyethylenimine, Lipofection, Calcium Phosphate etc.), physical methods (electroporation), and biological methods (viral transduction).
Hybridoma Growth Media (HYGM6 & HYGM7) allows for the growth and maintenance of hybridoma cell lines and expression of recombinant mAbs.
Custom-made bioprocessing media and buffers (USP &DSP): We routinely manufacture various USP & DSP media and buffers for our biopharma customers. Our options include:
› Contract manufacturing (OEM) of sterile liquids and powder formulations
› Custom-designed media for upstream bioprocessing applications (biologics, biosimilar, vaccines)
› Variable packaging sizes and packaging systems (PET / glass bottles, sterile 2D/3D bags, customised tubing systems)
› Standard products & their modifications
› Specialty waters (WFI USP/EP, nuclease free waters, etc)
› Specialty buffers & salt solutions for upstream & downstream bioprocessing
› Serum-free and animal component free (ACF)
› Standard and animal origin (AO) media for primary and stems cells
› Supplements and auxiliary reagents
For further info and details contact: baban@bioconcept.ch

Name ›
Address/P.O. Box ›
BioCopy AG
Switzerland Innovation Park Basel Area AG
The BioCopy Group
Postal Code/City › Country ›
Contact Person › Email › Website › Social Media › Number of Employees ›
Areas of Activity ›
Novartis-Campus, Gebäude (Building) WSJ-210 Lichtstrasse 35 4056 Basel
Switzerland Dr Matthias Wiedenfels, CEO info@biocopy.com www.Biocopy.com I 40
Drug Discovery and Development Immuno-oncology
TCR-like Antibody Engineering
Development of next-generation Cancer Drug Candidates
AI-powered closed-loop Platform pHLA complexes
BioCopy is a German-Swiss biotech company headquartered in Switzerland with one R&D site in the south of Germany. BioCopy is breaking new ground in the development of next generation cancer drugs with its proprietary technologies joined in a unique AI-powered engineering platform.
The BioCopy platform
The BioCopy platform is designed to streamline and optimise the drug discovery and development process. It combines AI-powered workflows with wet-lab data in a closed-loop feedback system.
The Challenge in Drug Discovery and Development
One of the most promising strategies in fighting cancer is the development of drugs that target specific markers typically concealed within cancer cells. While this approach holds great potential for therapeutic breakthroughs, it also presents significant challenges in identifying the ideal antibody and developing the optimal drug.
BioCopy focuses on the target class of so-called pHLA complexes. These are intracellular proteins presented as peptide fragments via HLA complexes on cell surfaces. Certain pHLA complexes are only found on cancer cells, making them ideal targets for cancer treatment – however, the difference to healthy cells is very small.
The engineering of therapeutic antibodies that target pHLA (so-called TCR-like antibodies) has been challenging and high-risk due to toxicity issues and the difficulties in the later manufacturing process.



The BioCopy Solution
BioCopy unlocks the transformative potential of TCRlike antibodies with a unique solution. We combine expertise in the pHLA space with high-throughput screening technologies, advanced protein engineering, and cutting-edge cell culture. This wet lab framework is integrated with databases and artificial intelligence, enabling accelerated learning through a closed-loop feedback system.
The combination of bioinformatic workflows and advanced AI tools broadens the scope of off-target sequences, ensuring comprehensive coverage and precise analysis. This minimises off-target toxicity, which is one of the biggest challenges in the development of such drugs.
The Unique BioCopy Approach
The BioCopy approach ensures exceptional efficacy and safety. Our platform allows us to work on multiple tasks and processes in parallel.
This paves the way for a streamlined, efficient and highly effective path from molecular concepts to drug candidates that are not only ready for clinical trials, but have a high probability of success.
At BioCopy, we herald a revolution in the discovery and development of drugs with pHLA targeting, and we are redefining the benchmark for time to market.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Bioengineering AG
Sagenrainstrasse 7 8636 Wald Switzerland Roland Durner
+41-55-256-81-11 info@bioengineering.ch www.bioengineering.ch 85 1972
l Engineering & design
l Service
l Plants
l Bioprocess control
l Lab & pilot
l Components
Company pro fi le
Bioengineering AG, founded in Switzerland with subsidiaries in Korea, China, and the USA, has been a trusted partner to the biotech industry for over 50 years. Our global reach and depth of experience make us a valued choice for leading companies. Guided by continuous improvement and a culture of responsibility and solution-focused collaboration, we build trusted relationships that support progress and quality in every project.
What we do
We design and build equipment and systems that improve the efficiency and reliability of bioproduction. Our engineering teams unify technological expertise with a deep understanding of customer needs to create customised bioreactors and fermenters for applications ranging from laboratory scale to production facilities. Committed to quality and partnership, we provide solutions that meet the evolving needs of the biotech and pharmaceutical industries.
Product portfolio
From research to production, Bioengineering offers customised solutions that grow with your requirements. Our product portfolio ranges from 1-liter laboratory reactors to 30,000-liter production plants and includes:
KLF: The only in-situ sterilisable glass benchtop fermenter on the market. Ideal for research and downscaling studies.
RALF: Autoclavable benchtop fermenter for cell culture or microbial fermentation.
NLF & LP/P: Laboratory/pilot fermenter for research or GMP production.
Plants: Customised plants for GMP biopharma, novel food & biotech.
Peripheral solutions: Media vessels, CIP systems, Inversina mixers, dosing systems.



What we provide
From project management and process engineering to automation and installation, we bring your vision to life with precision. Our full set of qualification services makes sure that your systems meet the highest standards and regulations. We also provide ongoing support, including maintenance and replacement parts for any issues. This ensures that your systems perform at their best, from lab reactors to full-scale production plants.
› Project management
› Process engineering
› Automation
› Manufacturing
› Qualification
› On-site installation
› Service
• Spare parts
• Field service
• Preventive maintenance
• Technical support
• Revamping
• Training

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees › Founded (year) › Type of Laboratory ›
Areas of Activity ›
Biomay AG
Ada-Lovelace-Strasse 2 1220 Vienna Austria Dr. Angela Neubauer +43-1-7966296-100 info@biomay.com www.biomay.com I 110+ 1984 CDMO
GMP manufacturing / aseptic filling of plasmid DNA, mRNA, recombinant proteins; supply for clinical phases I-III, market
End-to-End Manufacturing Solutions
Biomay specialises in the manufacturing and aseptic filling of plasmid DNA, LNP formulated mRNA and recombinant proteins, derived from microbial fermentation. With a proven track record of over 200 successfully released GMP products in the past 15 years, the company continues to support clients in the development and production of biopharmaceuticals. The company currently operates two state-of-the-art, fully integrated biomanufacturing facilities, both of which have undergone inspection by the U.S. Food and Drug Administration (FDA).
Extensive Range of Services for Client Projects
Biomay offers fully scalable GMP manufacturing across five parallel production lines, with bioreactor capacities of 30L, 50L, 150L and 750L. Routinely, up to several hundred grams per batch of starting material, drug substance, or drug product are achieved, supporting applications in gene therapy, mRNA, plasmid DNA, and protein vaccines. Notably, Biomay’s manufacturing process is free of solvents, antibiotics and animal components.
Following an increased global demand, Biomay operates five GMP-commissioned clean rooms for mRNA production, LNP formulation and aseptic filling. Stateof-the-art enzymatic run-off transcription is used for mRNA synthesis, with in-house linear DNA template production, various capping and modification options, and a comprehensive GMP-qualified process using single-use equipment. mRNA analytics and stability testing are conducted in-house to ensure regulatory compliance and product quality.
For precision medicine applications, Biomay operates a dedicated facility for personalised tumour vaccine production, using 3L bioreactors in multiple parallel clean rooms. This setup is optimised for rapid turnaround while maintaining strict quality standards.




Ready-to-Use Cas9 Nuclease and Viral Vector Plasmids
Recent advancements in gene-editing and gene-therapy research have led to a growing number of products reaching the market. Supporting these developments, Biomay has introduced a set of off-the-shelf products including CRISPR-Cas9 nuclease for gene-editing applications, as well as starting vectors for adeno-associated virus (AAV) and Lentivirus (LV) mediated gene-therapies. For AAV plasmids, clients have now access to a sizeoptimised AV-Helper and a wide range of rep/capserotypes, allowing for a maximum flexibility in tissue specificity. A fluorescent reporter (eGFP) and transgene vectors complete the portfolio. Mainly used for CAR-T applications and ideal for large payloads, 2nd and 3 rd generation LV plasmids are offered.
AAV and LV Plasmids are available for research use and in GMP quality, including reporter plasmids and transgene vectors. The latest addition to the growing number of catalog products is Cas9-nuclease, available in GMP and research grade.
All off-the-shelf products are immediately accessible and distributed via Biomay’s online platform.
A Reliable Partner for Long-Term Collaboration
Biomay’s commitment to supporting clients throughout the entire project life cycle – from clinical phases I–III to market readiness and commercial supply – has been reinforced by its involvement in the first approved CRISPR/Cas9-based gene editing therapy, CASGEVY, for which the company manufactures the nuclease Cas9. During the COVID-19 pandemic, Biomay contributed a significant quantity of linearised plasmid templates for Pfizer-BioNTech’s mRNA vaccine, Tozinameran/ Comirnaty.
With continued growth and expansion capacity at its facilities, Biomay remains well-positioned to meet both current and future custom manufacturing demands, providing tailored solutions for its clients.

Name ›
Boehringer Ingelheim Biopharmaceuticals GmbH
Address/P.O. Box › Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website › Number of Employees › Founded (year) › Type of Laboratory ›
Areas of Activity › Net Sales › Relevant R&D Budget › Request for › Further Collaborations
Binger Strasse 173 55216 Ingelheim Germany
Rebekka Wuester
+49-6132-77-0 +49-6132-72-0 bioxcellence@boehringer-ingelheim.com www.bioxcellence.com around 53,000 in 2023 1885 in Ingelheim, Germany S1
Focus on human pharmaceuticals, animal health and biopharmaceutical contract manufacturing
25.6 billion in 2023 5.8 billion in 2023
Boehringer Ingelheim BioXcellence™ is a dedicated partner for biopharma companies, providing comprehensive development and GMP manufacturing services. Renowned for its reliability, the company supports clients throughout the lifecycle of their products, thus transforming biologic innovations into commercial realities.
Company profile
Boehringer Ingelheim is a biopharmaceutical company active in both human and animal health. As one of the industry’s top investors in research and development, the company focuses on developing innovative therapies that can improve and extend lives in areas of high unmet medical need. Independent since its foundation in 1885, Boehringer takes a long-term perspective, embedding sustainability along the entire value chain. More than 53,500 employees serve over 130 markets to build a healthier, more sustainable, and equitable tomorrow.
BioXcellence Manufacturing – Tailored Capacities. Reliable Supply. Your Success, Our Commitment. Boehringer Ingelheim BioXcellence™ is a leading biopharmaceutical contract manufacturer – a reliable partner that accompanies customers throughout the life cycle of their products. We provide E2E biopharmaceutical manufacturing based on mammalian cell culture and microbial fermentation.
We have extensive experience with a variety of different host cells, producing a broad range of molecules in accordance with cGMP manufacturing guidelines and covering all stages of development, including aseptic filling. Our focus is on creating value for our customers through robust processes, effective preparation for launch, and global clinical and commercial supplies of high-quality biopharmaceuticals.
We are committed to maintaining high-quality standards in all operations, with robust systems designed to meet stringent regulatory requirements, and strong quality control capabilities that ensure product quality at every manufacturing stage. Our team’s extensive regulatory experience and impressive inspection track record demonstrate our ability to navigate complex regulations and integrate in-depth technological knowledge into our operations.
World-Class Expertise
With over 40 years of biotechnology experience and a team of dedicated and highly trained employees, we have helped our customers bring more than 45 biopharmaceutical medicines to the market, serving patients all




over the world. Our expertise spans across various host cells, enabling us to produce a wide array of molecules. Our commercial portfolio comprises monoclonal antibodies, recombinant proteins, antibody fragments, and recombinant vaccines, and is complemented by intensive experience in bi- and multispecific antibodies, Fc fusion proteins, scaffolds, peptide hormones, and pDNA.
World-class Facilities
Our network of manufacturing facilities spans the globe, from Fremont, California in the United States to Biberach in Germany, Vienna in Austria, and Shanghai in China. With more than 440 kL for mammalian cell culture and 12 kL for microbial fermentation in fully licensed multiproduct facilities we are your right partner for high-quality global supplies of biopharmaceuticals to patients. This is evidenced by our regulatory excellence. Our operations are based on fully licensed multi-product facilities for all major markets. One-quality, one-compliance, and oneregulatory form the foundation of our global network.
Your World-class Production Partner
For many of our customers, we are the preferred partner for reliable supplies. Seven of the top 10 pharmaceutical companies already trust us due to our extensive commercialisation experience. At Boehringer Ingelheim, we are deeply invested in the success of your product. Our shared passion is reflected in our commitment to providing the highest quality services and solutions to ensure your product reaches its full potential. Aside from our extensive experience and technical expertise, which complements yours, there are several other compelling reasons to choose us as your trusted contract manufacturing partner. At Boehringer Ingelheim, we have learned over the generations that the challenging path towards health innovation requires long-term commitment, resilience, and transformative action.
Let’s build a future together based on our history. We have capabilities to complement your expertise and are committed to turning your biologic innovation into commercial reality.

Name ›
Address/P.O. Box ›
Postal Code/City › Country › Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees ›
Founded (year) › Type of Laboratory ›
Areas of Activity ›
YMC ChromaCon
Technoparkstrasse 1
8005 Zurich Switzerland
Thomas Müller-Späth +41-44-445-20-10 info@chromacon.com www.ChromaCon.com I 11 2006
Process Development and Modelling
Multi-Column Chromatography Systems:
| Development & Manufacturing Scale | API and Biologics Purification | Continuous Chromatography
Advanced Automated Chromatographic Processes: | MCSGP | CaptureSMB | N-Rich
Chromatography Services:
| Feasibility and Scale-up Studies | Process Modelling | Chromatography Consulting
Request for › Further Collaborations
Pharma and CDMO manufacturers looking to reduce the cost of goods and PMI for purification of high value assets (AAV, Oligonucleotides, Polysaccharides, Peptides, mAbs, RNA and Proteins)
With a vision to improve manufacturing efficiency in the pharmaceutical industry, YMC ChromaCon has been a center of excellence for continuous chromatographic technologies since 2006. We offer chromatography systems from laboratory to manufacturing scale, enabled with advanced process technologies. Our solutions offer 30-60% cost reduction compared to conventional purification methods.
Process Automation Technologies
MCSGP – Increase purification yield to 90% while reducing PMI. MCSGP is a key technology for improving process economics and sustainability in TIDES and biologics manufacturing. For therapeutic peptides, such as GLP-1 agonists, and oligonucleotides, high purity requirements coupled with high synthesis costs mean that product losses during manufacturing are very costly. Standard processes using single-column chromatography are inherently inefficient due to unavoidable yield/purity tradeoffs.
MCSGP overcomes yield/purity tradeoffs by implementing automated side-cut recycling between two columns to increase yield and eliminate the need for side-fraction processing and re-chromatography. Moreover, as a continuous process, MCSGP is highly automated and reduces the need for manual handling. Typical yield improvements of MCSGP range from 60% to 90%, reducing costs and improving the sustainability of the manufacturing process (PMI).
MCSGP is supported by UV-based dynamic process control (AutoPeak ® ), an essential feature to provide robustness against process variation.
MCSGP was recently a finalist in the 2024 Swiss Technology Award for industry innovation. Multiple largescale manufacturing systems are now installed around the globe.




CaptureSMB - Save 40-60% costs in affinity capture. Affinity capture chromatography is a crucial step in mAb production. However, traditional single column chromatography is limited by an inherent trade-off capacity utilisation and throughput. To overcome this tradeoff, YMC ChromaCon developed CaptureSMB, a simple and efficient twin-column capture process that maximises capacity utilisation, reduces affinity resin costs by 40-60%, increases throughput by a factor of 2-3x, while reducing buffer consumption by 40-60%. Together with UV-based dynamic process control (AutomAb®), CaptureSMB operates robustly in response to resin aging and feed titer fluctuations.
N-Rich – Reduces project time and handling by 90% in preparation of analytical standards. Traditional preparation of analytical standards is cumbersome work requiring many chromatography runs and fraction analyses. N-Rich is a continuous chromatography technology to automate this process and reduce handling and analytical effort. N-Rich uses internal recycling between two columns to target, accumulate, and purify low abundance products such as product related impurities or other difficult to isolate compounds. N-Rich saves up to 90% time and effort for the most difficult purification challenges.
Systems, Software and Services
Advanced chromatographic processes are only accessible with the right development tools. We make it easy with our bench scale Contichrom® CUBE system, enabled with unique software especially designed for transferring traditional batch processes to continuous mode. Processes developed on the Contichrom® CUBE are fully scalable to our manufacturing scale Contichrom TWIN, a GMP capable continuous manufacturing system line.
Interested in continuous chromatography?
We support you with workshops, feasibility studies and system rentals.

Name ›
Number of Employees › Founded (year) › Areas of Activity › Collaborative Drug Discovery, Inc.
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Nine Hills Road
CB2 1GE Cambridge United Kingdom Dr Mariana Vaschetto +44-1223-803830 mariana@collaborativedrug.com www.cddvault.com
>100 2004
| Drug discovery collaboration
| Collaborative data management
| Cloud-based research tools
| Data analysis and management
| Workflow automation
| AI/ML integration in drug discovery
| Cross-team collaboration
Introduction to Collaborative Drug Discovery (CDD)
Collaborative Drug Discovery (CDD) is a pioneering software company dedicated to advancing pharmaceutical research by providing innovative tools for the effective management and analysis of chemical and biological data. Founded in 2004, CDD empowers researchers to accelerate drug discovery processes while fostering secure and collaborative environments for data sharing across teams, organisations, and disciplines.
Specialised focus on biologics and chemical data
CDD’s unique platform supports both small molecule and biologics research, enabling seamless integration and analysis of diverse datasets. This dual focus ensures researchers can efficiently manage chemical structures, reaction pathways, and biological assay data, while simultaneously handling complex biologics such as peptides, antibodies, and nucleic acids. By addressing the specific needs of chemical and biological research, CDD bridges a critical gap in drug discovery workflows.
The flagship product: CDD Vault
At the core of CDD’s offering is CDD Vault, a user-friendly, cloud-based platform for managing, visualising, and sharing drug discovery data. Designed with researchers in mind, CDD Vault provides a secure environment to store and analyse chemical and biological data. Key features include:
› Structure-Activity Relationship (SAR) Tools: Advanced tools for correlating chemical structures with biological activities.
› Biologics Management: Specialised functionalities for organising and analysing biologics-related data.
› Data Visualisation: Intuitive visualisations to interpret complex datasets.
› Collaboration: Role-based access ensures secure data sharing within and across organisations.



Driving collaboration across disciplines
CDD is committed to breaking down silos in drug discovery by enabling seamless collaboration between medicinal chemists, biologists, and computational scientists. The platform facilitates shared insights and transparent communication, fostering innovation and efficiency. Its compatibility with other tools and systems ensures easy data integration for comprehensive analyses.
Industry applications and impact
CDD Vault is widely adopted by pharmaceutical companies, academic institutions, and research organisations. It supports:
› Target Identification and Validation
› Lead Optimisation
› Preclinical Research
› Biologics and Small Molecule Development
Commitment to data security and compliance
Recognising the sensitive nature of drug discovery data, CDD maintains stringent security measures and adheres to regulatory standards, ensuring confidentiality and data integrity. The platform’s compliance with guidelines such as 21 CFR Part 11 further enhances its reliability for regulated environments.
Future-focused innovation
CDD continues to innovate by integrating advanced technologies like artificial intelligence and machine learning into its platform. These capabilities further empower researchers to derive actionable insights from vast chemical and biological datasets, reducing the time and cost of bringing new therapeutics to market.
Conclusion
Collaborative Drug Discovery is redefining how research teams manage and collaborate on chemical and biological data. Through its intuitive platform and specialised focus, CDD is helping to accelerate breakthroughs in drug discovery, enabling better therapies and improving global health outcomes.

Name ›
comprei Reinraum-Handel und Schulungs GesmbH
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media › Number of Employees › Founded (year) ›
Europastraße 10 9524 Villach Austria Simon Fiala Head of training and learning solutions +43-4242-44075 office@comprei.eu www.comprei.eu I 120 1999
comprei – contamination in control
Contamination-critical high-tech industries are faced with the challenge of maintaining the highest level of awareness for appropriate movement and action in the cleanroom environment daily. The successful implementation of a contamination control strategy (CCS) depends largely on solidly trained people who are sensitised on a recurring basis.
This is exactly where comprei learning solutions come in. As a tailor-made service, they are characterised by
› a distinct process orientation in coordination with manufacturing and quality management
› the focus on clear individual learning objectives with maximum real-world relevance
› a high proportion of impressive visualisations and simulations
comprei training courses motivate learners to do more than just adhere to procedures and implement rules. We allow learners to clearly comprehend the background and understand the reasons in depth - for always acting confidently and appropriately in highly clean environments.
What can your individual learning solution look like?
Continuous learning support
› Ongoing learning impulses in a playful and lowthreshold digital way
› Practical training, simulation, and reflection in person
› Tracking of learning progress and intervention by training officers
› Flexible adaptation of learning content and addressing trends
Classroom training on site or at the comprei training center
› Individualisation of the learning content
› Impressive visualisation and practical training

Live online training / webinar
› Efficient use of time and minimal organisational effort
› Option as a refresher and for small learning units without hands-on training



Examples for content packages
Safe and confident in the GMP cleanroom for operators in biotech and pharma
Sustainable motivation and awareness of correct behaviour in the GMP environment. Experiment in our simulation cleanroom, experience the effects of your behaviour and become aware of critical factors influencing your processes and products.
Special package: Aseptic handling in manufacturing and sterile fill-and-finish for operators in the aseptic manufacturing and filling of pharmaceuticals
Raising awareness of aseptic movement and handling. Simulation of movement in the cleanroom, interventions on the filling line/RABS and handling on the isolator. Visualisation of risks and best practice based on SOPs and protocols. Assessment and training of aseptic practices such as gowning, movement, handling/interventions.
Certification programme cleanroom expert for hygiene managers, production managers, QA officers, cleanroom managers, training officers, etc. 9 days of instruction in 3 modules, including project work and final exam for the certificate
Certifications
› ISO 21001:2018 Management systems for educational organisations
› ISO 29993:2017 Learning services outside formal education
› ISO 9001:2015 Quality management systems


Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Email › Website › Social Media ›
Number of Employees ›
Founded (year) › Type of Laboratory ›
Areas of Activity ›
Coriolis Pharma Research GmbH
Fraunhofer Straße 18b 82152 Martinsried/Munich Germany
Bettina von Klitzing-Stückle
Bettina.Klitzing@coriolis-pharma.com www.coriolis-pharma.com I ~200
2008
Contract research and development
Contract Research and Development for biopharmaceuticals, including development, analytical and manufacturing services for biologics, gene and cell therapies and vaccines.
Coriolis Pharma – Drug Development, Analytical and Manufacturing Services
Coriolis Pharma is a global contract research and development organisation (CRDO) and the premier partner for the development of liquid and lyophilised drug products, analytical services, and manufacturing services across a vast array of therapeutic modalities, including biologics, gene and cell therapies and vaccines.
Our expert scientists design and execute platform and custom services to accelerate and derisk your programme right from the start and throughout the entire product lifecycle.
Partner with Us – Right from the Start
Accelerate Preclinical Development
We help you establish a robust formulation foundation from the outset, reducing risks and avoiding costly delays later in development. Our collaborative approach and deep scientific expertise ensure that your formulations are optimised for stability and efficacy from day one.
Reliable Early-Phase Support
Transitioning through the mid-phases of drug development involves complex analytical and validation requirements. We provide consistent, reliable support through these critical stages, delivering robust analytical methods that streamline development and ensure compliance.
Efficient Late-Phase Solutions
As your drug approaches commercialisation, managing the complexities of process development and regulatory submission becomes paramount. Our development support in this phase provides the necessary insight and expertise to navigate these challenges efficiently.
Commercial Services
We support your drug’s journey to market with proven GMP processes and meticulous attention to detail. We excel at process optimisation and release testing alike, providing comprehensive support throughout the final stages of development.



CORIOLIS
Expert Guidance Throughout
Navigating biologics development can be daunting, particularly when faced with unique formulation challenges or complex regulatory landscapes. Coriolis acts as a knowledgeable guide, providing specialised insights and collaborative support to address these issues effectively.
Our Services Available under R&D and GMP
Drug Product Development
› Developability assessment
› Pre-formulation screening
› Formulation development, optimisation and robustness
› Stability studies
› Primary packaging and container closure selection
› Forced degradation studies
› Clinical/commercial in-use and compatibility studies
› Life cycle management and reformulation
Manufacturing Services
› Tox study manufacturing
› Filing of reference material
› Lead lot stability material
› Lyophilisation process development, optimisation, transfer and scale-up
› Comparability, pump, headspace & CCIT studies
› Process development
› Manufacturing troubleshooting
Analytical Services under R&D and GMP
› Method development, qualification, and transfer
› Phase-appropriate method validation
› Compendial method verification
› Release testing / Tech transfer support
› Stability studies
› Fast track analytics
› Particle characterisation
› Particle identification

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone
Website › Social Media ›
Number of Employees › Founded (year) › Areas of Activity › External › Collaborations
Cytosurge AG
Sägereistrasse 25 8152 Opfikon Switzerland Sabina Packeiser, PhD +41-43-544-87-37 marketing@cytosurge.com www.cytosurge.com F I 40+ 2009
Nano-injection, Single-cell applications, Biophysical and biomechanical analysis, Single-cell gene editing, Single-cell transcriptomics (e.g. Live-seq)
Nanosurf AG, Bruker Corporation, Central Scientific Commerce, Inc., Lexogen GmbH, Lab Automation Network (LAN), Cenibra GmbH, MEMS AG, Quantum Design China (QDC), a subsidiary of Quantum Design International (QDI), Altium International Inc. (US & Canada)
Cytosurge: Revolutionising Single-Cell Research
Founded in 2009 as a spin-off of ETH Zurich, Cytosurge AG specialises in developing cutting-edge solutions for single-cell manipulation. Using its proprietary FluidFM® technology, featuring the smallest needle in the world, the company has become a leader in advancing precision biology and unlocking new possibilities in life sciences research. Next to the commercialisation of the FluidFM OMNIUM platform, in 2022, Cytosurge launched the groundbreaking CellEDIT Service, delivering advanced gene editing solutions that redefine cell-line engineering.
FluidFM Technology for Single-Cell Manipulation
Cytosurge’s patented FluidFM technology combines microfluidics and force microscopy, enabling researchers to manipulate cells with unparalleled precision. FluidFM is available as:
Request for › Further Collaborations
We are actively seeking collaborators to further develop both the CellEDIT Service and Live-seq applications, as well as investors to drive innovation and growth.
› FluidFM OMNIUM: A standalone semi-automated single-cell manipulation platform for precise and nondestructive injection and extraction. A standalone semi-automated single-cell manipulation platform for precise and non-destructive injection and extraction (first image on the top). This versatile system supports single-cell RNA analysis and gene editing techniques, combining precision, ease of use, and cell gentleness – all while maintaining cell viability.
› FluidFM ADD-ON: An upgrade for Bruker or Nanosurf AFMs, to extend their capabilities with FluidFM’s microfluidic features for advanced applications like faster single-cell force spectroscopy and nanoprinting.
CellEDIT, CRISPR Cell Line Engineering Service
The CellEDIT Service harnesses FluidFM’s gentle intranuclear CRISPR RNP injections to perform precise, vector-free genetic edits with minimal off-target effects. By injecting controlled volumes of CRISPR-Cas9 RNP




directly into the nucleus, the residence time is minimised, ensuring greater accuracy and reduced risk of off-target effects, making it ideal for developing cell lines in hard-to-transfect cells. CellEDIT delivers knockout monoclonal cell lines within 10 weeks.
Global Reach and Impact
Cytosurge has installed 140+ FluidFM systems worldwide, with its technology featured in 150+ scientific publications since its invention. By enabling researchers to explore new frontiers in single-cell analysis, Cytosurge continues to make a significant global impact across academic and industrial research sectors.
Collaborations Driving Innovation
Collaborations are at the core of Cytosurge’s success. The company partners with academic institutions, industry leaders, and technology innovators to expand the possibilities of single-cell research. Recent partnerships include Lexogen, with whom Cytosurge is developing a scalable workflow for live-cell sequencing (Live-seq), enabling transcriptome analysis from live cells without disrupting their viability.
Advancing Research Through Innovation
Cytosurge is committed to driving advancements in single-cell manipulation and gene editing, providing researchers with tools and services that empower groundbreaking discoveries. Its dedication to precision and innovation continues to shape the future of cellular research.
Join Us in Shaping the Future
We actively seek partners to advance the CellEDIT Service and Live-seq applications, along with investors to fuel innovation and growth.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees › Founded (year) ›
Areas of Activity ›
CZ VACCINES
A Relva s/n Torneiros
36410 O Porriño Spain
Mercedes Rodriguez +34-986-330400 mercedes.rodriguez@czvaccines.com czvaccines@czvaccines.com https://czvaccines.com I
600+ (Zendal Group) 1993
| Biotechnology
| CDMO
| Development
| Manufacturing
| Vaccines
| Viral & microbial vaccines and cell therapy
| Aerobes and anaerobes, yeast
| Covid-19 vaccines
| Tuberculosis vaccines
| One-Stop Shop
| Biomedicine
| Aseptic filling
| Lyophilisation
| GMP upstream & downstream processes
| Biotech production sites in Spain and Portugal
Relevant R&D Budget ›
11% revenue
Full-service biologics CDMO
EU GMP certification
CZ VACCINES is a full service provider. We cover the entire value chain from contract development through clinical phases I to III to commercial production of vaccines and biological products.
30 years in vaccines
CZ VACCINES has extensive scientific experience in developing new and ambitious projects that include different categories and vaccine platforms:
› Human vaccines and animal vaccines
› Against viral, bacterial, and parasite diseases
› Conventional vaccines
› Genetic engineering of live attenuated vaccines
› Subunit vaccines (recombinant protein)
› Latest generation DNA vaccines
End-to-End CDMO
› Live and inactivated bacterial and viral vaccines up to BSL-2
› Roller bottles, cell factory and wave bioreactor
› Single-use and stainless steel bioreactors
› Aseptic processing systems
› Centrifugation, and ultrafiltration and diafiltration
› Chromatography (ÄKTA ready)
› Aseptic filling into vials
› Lyophilisation (clinical trial material and large scale)
› Process development / optimisation
› Quality control testing: microbiological, sterile / nonsterile, chemical-physical, and biological
› Storage: (2º-8ºC), (-20ºC), (-30ºC), (-80ºC)




CZ VACCINES
Large-scale biotech manufacturing
CZ VACCINES has large manufacturing capacity located in several GMP certified biotech production plants in Spain and Portugal
cGMP-production capabilities
Upstream:
› Single-use bioreactors: 2x50L + 3x250L + 3x2,000L
› Stainless steel bioreactors: 100L + 300L+ 2x2,500L
Downstream:
› Single-use mixers: 500L, 650L and 1500L
Media:
› Single-use and stainless-steel bioreactors: From 200L up to 3,000L
Purification:
› State-of-the-art purification processes: chromatography (ÄKTA ready), centrifugation, ultrafiltration, diafiltration, tangential flow filtration
cGMP fill and finish capabilities
› Liquid and lyophilised products up to BSL-2
› 4 filling machines: 6,000 vials/hour - 2x12,000 vials/ hour – 24,000 vials/hour
› 2 freeze dryers: up to 24,000 vials per run, and up to 180,000 vials per run with automatic loading and unloading system
› Component size: 2R-10R-20R
› Manual and automatic visual inspection
› Labeling and packaging

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees › Founded (year) ›
Areas of Activity › Dr. Paul Lohmann GmbH & Co. KGaA
Hauptstrasse 2 31860 Emmerthal Germany Dr Joswin Kattoor +49-5155-630 sales@lohmann-minerals.com www.lohmann-minerals.com I
Approx. 650 1886
Raw materials for biopharma, biotech food, Industrial biotech processes
Dr. Paul Lohmann® is a manufacturer and developer of high quality Mineral and Metal Salts for food, food supplements, pharma, biopharma, cosmetics and industrial applications.
Since 1886, a high level of flexibility and an innovative spirit enables Dr. Paul Lohmann® to respond to changing needs. Customers profit from the broadest portfolio available of high value Minerals. Our strength lies in customised solutions tailored to your exact needs. We work closely with you to develop and optimise products to meet your specific requirements. This personalised approach ensures that you receive the perfect solution for your application – giving you a distinct competitive advantage through innovation, reliability, and superior performance.
Salts for Biopharma
We are experts in high quality Salt manufacturing and provide DPL-BioPharm Salts for upstream, downstream and fill & finish processes of biopharmaceutical industry. Our Salts are an indispensable part of bioproduction and act as nutrients, processing aids or excipients in the final formulation. Our multi-compendial DPL-BioPharm grade convinces with a constant and high-quality including Bioburden and heavy metal profile according to ICH Q3D.
Minerals for Food Biotech
Biotechnology is one of our forward-looking segments, transforming renewable resources into valuable products in novel ways. Among the various techniques used, fermentation plays a crucial role in the food industry. Biomass fermentation and precision fermentation are biotechnological processes for producing a wide range of foods and food ingredients, including cultured meats, plant proteins and flavourings.



In this context, Minerals can fulfil multiple roles, including:
› Macronutrients such as Magnesium, Calcium, or Ammonium
› Micronutrients such as Iron, Manganese, or Copper
› Buffering agents such as Acetates, Formates, and Phosphates
We offer high value Salts specifically designed for food biotechnological applications creating an optimal environment for microorganism to grow robustly. Made in Germany through carefully defined processes, our Salts meet the highest quality standards and can be tailored to customers specifications.
Lohtragon ® BT Portfolio for Industrial Biotechnology
The increasing role of Biotechnology (BT) is linked to the sustainable approach of producing value-added products from renewable sources with reduced waste, energy consumption and greenhouse gas emission, avoiding fossil resources and hazardous intermediates.
The Lohtragon® BT product range for industrial biotechnology focuses on large-scale fermentation processes considering all steps along the biotechnological valuechain. We thereby permanently support the formulation of defined synthetic fermentation media. Our experts are keen to adjust our inorganic components and their blends according to your requirements. Our range of metal salts combined with our expertise in product modification contribute to a properly defined culture media.
Salts From Your Trusted Partner
At our ISO- and GMP-certified production sites in Germany we use state-of-the-art equipment and this, combined with our many years of extensive high-level manufacturing experience, ensures the highest and reliable quality. Our commitment to supply chain stability, along with full transparency and traceability, ensures that you benefit from a dependable partnership. Let’s shape the future together and develop innovative solutions for your challenges.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website › Social Media ›
Number of Employees › Founded (year) › Areas of Activity ›
Enzymaster Deutschland GmbH
Neusser Straße 39 40219 Dusseldorf Germany
Dr Thomas Daussmann
+49-211-158-216-10 +49-211-158-216-12 info-europe@enzymaster.de https://enzymaster.de/ I Q 255 2013
| Biocatalysis Research
| Enzyme Panel Screening
| Enzyme Engineering: Computer-Aided Directed Evolution of Enzymes
| General and Customised Enzyme Screening Kits
| Immobilisation of Enzymes
| Reaction Process and DSP Development
| Fermentative Enzyme Preparation and Formulation
| Biocatalytic Manufacturing of Fine Chemicals, Intermediates, APIs, and other Compounds (Lab to Industrial Scale)
Background Enzymaster
Biological Patents › External › Collaborations
Comprehensive international patent portfolio mainly in the field of novel engineered enzymes for biocatalysis
| Austin Chemicals
| Lake Chemicals and Minerals
| ThermoFisher Scientific
| Research Centre Jülich
| Z&S Handel
| VIRIDA BioCat
| Cluster Industrielle Biotechnologie
Enzymaster provides a one-stop solution for the development and commercialisation of innovative and sustainable enzyme catalysis technologies. With our proprietary BioEngine ® and BioNavigator ® platforms as well as our long-term experience, we offer R&D services combined with the establishment of complete technology transfer packages and manufacturing collaborations to fine chemical, pharmaceutical, and other industries. Our portfolio includes enzyme identification, enzyme panel screening, smart enzyme engineering, process development, enzyme preparation by fermentation, and biocatalytic manufacturing. In addition to these services, we also offer general enzyme kits that represent various enzyme classes such as ketoreductases, transaminases, imine reductases, aldolases and decarboxylases as well as customized enzyme kits that fit to the individual biotransformation needs of our customers. To better serve our global customers, we have established a joint venture in Germany (VIRIDA BioCat). This facility provides contract research (CRO) services and supports smaller-scale production campaigns (up to pilot), offering a fast and flexible solution for enzyme and process development. At the same time, we continue to leverage cost-effective and rapid scale-up solutions in China for enzyme manufacturing and chemical production, ensuring efficiency at every stage of the process. Enzymaster Deutschland GmbH, a subsidiary of Enzymaster (Ningbo) Bio-Engineering Co. Ltd., is your reliable partner in the international market for enzyme applications and products manufactured by biocatalytic processes.
Enzymaster strives to contribute to a greener environment and improve manufacturing processes by helping organisations leverage the benefits of biocatalysts, decreasing dependence on toxic and often inefficient, purely chemical syntheses. Flexible fee-for-service business models moving in lockstep with R&D programmes, high success rates, commitment to confidentiality, and the avoidance of one-size-fits-all IP policies allow Enzymaster to advance biocatalysis innovation rather than impede it, ultimately fuelling the future of manufacturing. Green Magic Happens Here!

BioEngine ®
Enzymaster has developed BioEngine ®, a proprietary directed evolution platform with integrated computational enzyme engineering. The implementation of computational hot spot identification, in silico enzyme library screening, and in silico recombination into the directed evolution cycle allows us to shift most of the screening efforts from the laboratory into the computer and cover a large sequence space of the target enzyme, all while accounting for the real chemical process conditions. Based on the computational results, we construct smart, focused, and small libraries of enzyme variants, which can conveniently be screened via HPLC or GC to characterise a comprehensive range of desired properties for these variants. This enables us to reach the desired biocatalytic process target in typically only 3-6 rounds of combined in silico and experimental directed evolution. Therefore, BioEngine ® can significantly speed up the process of bringing your idea to the market.
BioNavigator ®
The BioNavigator ® platform is part of BioEngine ® and has been developed by Enzymaster as a proprietary in-house computational toolbox for sequence- and structure-guided combinatorial library design and enhanced diversity generation. BioNavigator ® is a set of state - of-the -art methods for computational enzyme identification, in silico activity and selectivity prediction, and stability calculation under process conditions. This includes bioinformatics-driven hot spot identification, focused combinatorial library design, and in silico loop design. Artificial intelligence (AI) and structure - based recombination algorithms are applied to in silico prescreening to enhance the combinatorial sequence diversity coverage beyond traditional random and sitesaturation mutagenesis approaches, thereby increasing the sequence space coverage and identifying all important sequence areas for fast and efficient enzyme engineering.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees › Founded (year) › Type of Laboratory › Areas of Activity ›
Enzymicals AG
Walther-Rathenau-Str. 49b 17489 Greifswald Germany
Dr Rainer Wardenga +49-3834-515470 info@enzymicals.com www.enzymicals.com I 20 2009
| S1 Biotech
| Chemistry & Analytics
| Process Engineering
| Kilo Lab & Small-Scale Manufacturing
| Development and scale-up of chemoenzymatic processes incl. DSP
| Customer-specific solutions in enzyme technology and biotransformation
| Development and production of enzymes
| Applications in pharma, fine chemicals, Flavors & Fragrances
Patents › External › Collaborations
IP protected by international patent applications and granted patents
| Broad international academic R&D collaborations, most notably Greifswald University, Bornscheuer group
| 4Synth Network
| Cluster Industrielle Biotechnologie
| Aminoverse B.V.
| ChiralVision B.V.
| Candidum GmbH
| SpinChem AB
| Allozymes Pte Ltd.
| Bio-Prodict B.V.
| CMO and CDMO network
Enzymicals AG: Industrial Biocatalysis from gram to ton
With 15 years of industry experience, Enzymicals is your trusted partner for industrial biocatalysis, offering end-to-end solutions from catalyst lead-finding to process optimisation and scale-up, spanning from gram- to ton-scale.
Our Commitment: At Enzymicals, we champion in vitro biosynthesis technologies for industrial implementation, driving a shift towards biology-inspired production concepts, which is a global trend. Being part of a strong EU-based partner network ensures highest regard for your IP and supply chain security. We are a CRO that offers our clients with an attractive business model, granting them full ownership of all new intellectual property. This enables clients to devise strategic IP plans and ensures freedom of action, without cumbersome license agreements.
Our Expert Team: Backed by a highly skilled interdisciplinary team of 20 chemists and biologists, Enzymicals specialises in complex chemical synthesis and performs comprehensive services encompassing biocatalysis, enzymology, tailor-made cloning and expression in a variety of hosts, along with fermentation development for protein manufacturing.
Comprehensive Enzyme Services: Enzymicals is a premier source of enzymes for diagnostics, research, and commercial production. With access to one of the world’s largest off-the-shelf collections of biocatalysts, totalling over 7,000 enzymes from 50 classes, we offer catalyst screening services to identify high-performing enzymes tailored to specific research needs. What is not found in these collections, we can access by expression of suitable genes in various microbial host systems centered around E. coli, Pichia and Bacillus, but we have experience with other, more exotic systems. This also allows us to supply almost any biocatalyst of interest on an analytical and commercial scale.
Your Experienced Partner for Biocatalytic Processes




Seamless Transition from Screening to Synthesis: Our chemical expertise seamlessly translates screening hits into optimised processes and kilogram-scale syntheses through bespoke development programmes. We employ scalable strategies that include feasibility studies, DOEbased process intensification (4x 0.5 L for chemistry and 4x 1.0 L for fermentation) and scale-up of both enzyme production (up to 30 L) and the chemo-enzymatic process (up to 10 L glass) including downstream processing, ready for tech transfer. Our industry-exclusive approach ranges from traditional batch reactions to enzymatic flow and rotating bed reactors.







Enzyme Technology Alliance: Enzymicals is a founding member of the Enzyme Technology Alliance, a collaborative effort involving seven independent partners. This alliance addresses complex development projects, integrating in-silico catalyst discovery, AI-supported enzyme engineering, biocatalyst immobilisation, process intensification, and scale-up of both enzyme production and chemo-enzymatic processes.
Network Capabilities: Within our network, we offer processes in fermenters up to 85 m³ or multi-kilogram- to ton-scale for chemical processes, both under non-GMP or GMP. The combination of superior catalysts, optimised chemistry, and integrated processing enables us to accelerate the delivery of effective solutions.



Let’s Create Value Together: Join us in creating value for you through innovative, sustainable, and efficient biocatalysis. Enzymicals is your dedicated partner for shaping a future where industrial processes align with the principles of green chemistry.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website › Social Media ›
Number of Employees › Founded (year) ›
Areas of Activity ›
EuroscreenFast
47 rue Adrienne Bolland, Gosselies 6041 Charleroi Belgium
Laurent Meeus
+32-71- 348-508 +32-71-348-519 info@euroscreenfast.com euroscreenfast.com I 9 1994
GPCRs, High Throughput Screening, Assay Development, Deorphanisation, Cell Line Development, Hit-to-lead support & profiling
Based in Charleroi, Belgium, EuroscreenFast has been a pioneer in GPCR science for more than 30 years.
GPCR Screening
Since establishing as the first company to offer access to recombinant GPCR assays, EuroscreenFast’s evergrowing catalogue has expanded to include over 1,000 functional assays representing more than 530 GPCR and other cell membrane targets. Orthologs are available for many species including human & primates, mouse, rat, dog, cat, pig and rabbit. Therapeutic developers worldwide trust EuroscreenFast with their compounds to determine affinity, potency, efficacy and functional selectivity.
Aequorin Technology
While there are many methods used to monitor calcium signaling, EuroscreenFast specialises in Aequorin technology due to its high sensitivity, specificity, and sub-cellular compartment targeting. In contrast to some other methods using fluorescent dyes, Aequorin’s unique properties make it a precise and versatile reporter system with a larger assay window and a improved signal-to-noise ratio. Another critical benefit of Aequorin is its ability to be expressed in various subcellular compartments, such as mitochondria. Aequorin’s signature blue luminescence is due to a unique photoprotein originally derived from the jellyfish species Aequorea victoria.
Deorphanisation
EuroscreenFast’s expertise reaches beyond binding & functional GPCR screening. Of the 800 known human GPCRs, more than 100 still remain orphan receptors. The identification of endogenous ligands and surrogate agonists is a huge opportunity for drug development and EuroscreenFast boasts an impressive track record in this



domain having identified 17 natural receptor-ligand pairs, resulting in 12 patents. EuroscreenFast’s world leading deorphanisation platform is based on a unique collection of natural tissue extracts, a large library of cellular tools and more than three decades in the field. EuroscreenFast has one of the largest libraries of orphan GPCR cell lines too, verified for receptor expression.
Custom Cell Line & Assay Development
Custom cell line and assay development is also core to the offering at EuroscreenFast. Using a proprietary high-copy, bicistronic expression vector, cell lines from a range of species can be engineered to express the client’s target of choice and validated for use in functional or binding assays, or as a tool for high throughput screening. EuroscreenFast has also developed specialized assays to bridge the gap between in vitro and in vivo data. Custom cell lines and assays are development to fit the client’s robustness and sensitivity criteria with rapid turn-around times and can be established on an exclusive or non-exclusive basis. Non-GPCR cell lines and assay development is also possible, as is development in a range of cell backgrounds including, but not limited to, CHO, HEK293, U2 OS and 1321N1.
EuroscreenFast also has a licence to import and work with controlled substances.
With a team of highly skilled scientists and decades of experience, EuroscreenFast strategically designs each program based on the latest advancements in GPCR biology. Whether supporting early discovery efforts, optimizing lead compounds, or unlocking new therapeutic targets, EuroscreenFast’s expertise and application of core technologies ensure robust, reliable, and translatable data – EuroscreenFast is your unrivalled GPCR development partner.
EuroscreenFast is a business unit of EPICS Therapeutics SA.

Name ›
Address/P.O. Box › Postal Code/City › Country › Contact Person › Telephone › Email › Website › Social Media › Number of Employees › Founded (year) › Type of Laboratory › Areas of Activity › External › Collaborations
Request for › Further Collaborations
EVONIK Operations GmbH
Kirschenallee 64293 Darmstadt Germany
Dr Ingo Klement
+49-6151-18 3414 ingo.klement@evonik.com www.evonik.com F I I
>1,000 1982 L1/L2/S1/S2,
Strain and process development, analytics, quality control, target validation, piloting and scale-up USP + DSP, commercial routine production
CDMO, Joint Development, Supply Agreements, Joint Venture, Venture Capital
Long-term business orientation
A global precision fermentation leader from strain development to large-scale production
The division Nutrition & Care at Evonik operates a global network of biotechnological production sites at different scales with up to 250 m 3 fermentors. This network includes a versatile multi-product site in Slovakia, designed for process scale-up and market launch of new and innovative products, which is available to our industrial partners.
Evonik has leveraged its expertise in fermentation and biocatalysis technologies, including strain development, process optimisation, piloting, and large-scale manufacturing to bring a wide range of products to market. These products span various application fields, such as APIs, pharma intermediates, biomaterials, food ingredients, excipients, and cosmetics. With a total CDMO fermentation capacity exceeding 4,000 m³, Evonik’s network of sites in the U.S. and Europe is well positioned to support customer projects and products, regardless of scale or process complexity.
Evonik CDMO services – a one-stop shop
In addition to development and production services, Evonik can support customers in techno-economical assessments (TEA) and life cycle assessments (LCA). Leveraging Evonik’s extensive engineering experience, Evonik can also facilitate the realisation of brownfield or greenfield concepts in case the volume demand should exceed current capacities.
As such, Evonik positions itself as a comprehensive, one-stop shop and full-service provider.



Sustainability as a growth driver for biotechnology services
Industrial biotechnology is a crucial driver for growth and innovation, enabling the development of novel, natureinspired products that are inaccessible through chemical synthesis, thereby complementing Evonik’s existing technologies and services. Advances in synthetic biology and a shift towards sustainable manufacturing methods are increasing the demand for Contract Development and Manufacturing Organizations (CDMOs) with bestin-class microbial fermentation capabilities. For over four decades, Evonik has been a dependable partner to pharmaceutical companies and other innovators aiming to industrialise disruptive biofabrication technologies.
The Evonik biotechnology platform
The Evonik Biotech Hub serves as a central hub for various scientific disciplines within the global biotechnological network. With more than 100 scientists at three international sites, the Biotech Hub is the competence centre for industrial biotechnology at Evonik, providing everything from groundbreaking ideas to industrial bioprocesses, facilitating the transformation of business opportunities into commercially feasible processes.
The profound understanding of biological systems, like the interactions in different target systems or the elucidation of the mode of action, as well as the differentiating capability in designing and operating efficient and sustainable manufacturing processes for biomolecules and microbial solutions, enables the Biotech Hub to develop tailored and competitive solutions allowing its customers to assume a leading position in their respective markets.

Name ›
Address/P.O. Box › Postal Code/City › Country › Contact Person › Telephone › Email › Website › Social Media › Number of Employees › Founded (year) › Areas of Activity › External › Collaborations
Request for › Further Collaborations
FGK Clinical Research GmbH
Heimeranstr. 35 80339 Munich Germany
Martin Krauss
+49-89-893-119-0 martin.krauss@fgk-cro.com rfp@fgk-cro.com www.fgk-cro.com
I 240 2002
Full service CRO for conduct of clinical studies, offering support in | Regulatory Affairs | Project Management/Monitoring | Medical Safety | Data Management/Biostatistics | Medical Writing | eSolutions | Quality Assurance all over Europe and US.
Our daughter companies create special added value for our clients: | “FGK Representative Service”: EU Representation Services which are legally required for non-European sponsors conducting clinical studies in the EU (EEA). | “FGK Pharmacovigilance”: Post-Market PV services including QPPV and PMSF.
BVMA - Federal Association of Contract Research Organisations
Small to mid-sized biotech, device or pharmaceutic seeking flexible support in their clinical development
European Biotechnology Network Member of
Company overview
FGK Clinical Research GmbH is a well-established and 100% privately owned full-service CRO with offices in Munich (HQ) and Berlin (branch office), Poland, Czech Republic, Hungary, United Kingdom and US (Boston). We provide full service for all phases of clinical studies. Our client base from all over the world includes biotechnology, medical device and pharmaceutical companies. FGK has experience in conducting international, multicenter studies with hundreds of patients as well as single country studies with few patients. We have already conducted clinical trials in all major indication areas and have experience in rare diseases and orphan drugs, advanced new therapies, cell and gene therapies, pediatric diseases as well as first in human studies. With our highly qualified medical, scientific and regulatory experts, we guide our customers through the clinical trial process, from planning and approval to the final report. We also provide legal representation services to our non-European customers. As an ownerdriven company, our flat hierarchies, extremely stable teams, and yearlong study expertise are the basis for a sponsor-oriented, flexible approach to conduct every project in the highest quality for an optimal outcome throughout the complete clinical development.
Our approach to a project
The main philosophy for FGK is to prepare and conduct studies in close cooperation with the sponsor. Our operational team works hand-in-hand with all other departments involved. This also applies for the project manager, who is the central contact person delivering all required information to the sponsor.
Timely approvals and efficient troubleshooting are achieved through a combination of centralized project management and local monitoring, as well as local expertise and regulatory submissions within the country of study conduct.







Services
Clinical Operations
› Project management, primary liaison for sponsor communication, status reports, etc.
› Feasibility, site management, monitoring, etc.
Regulatory Affairs
› Consulting on regulatory topics
› Review of study documents (e.g. protocol, informed consent form, labels)
› Clinical Trial Application with submission to EU and non-EU regulatory bodies
› Legal representative – also visit www.fgk-rs.com
Medical Safety/Pharmacovigilance
› Adverse Event Management and assessment/reporting
› Drug safety, medical monitoring and coding of medical terms
› Pharmacovigilance – also visit www.fgk-pv.com
Medical Writing
› Investigator’s brochures, study protocols, ICF and subject information
› Clinical expert reports, clinical publications, IMP and submission dossiers
Data Management
› CRF design and review, clinical trial databases
› Data validation, processing and cleaning, external data handling, CDISC SDTM
Biostatistics and Programming
› Study design, sample size calculations
› Statistical consultancy, analysis plan, programming and reporting
› CDISC ADaM
Quality Assurance
› Audits of investigator site, database and system audits, internal audits
eSolutions
› eCRF/ePRO, RTSM, eTMF, CTMS, DCT elements
Name ›
Address/P.O. Box ›
Postal Code/City › Country › Contact Person › Telephone › Fax › Email › Website › Social Media › Number of Employees › Founded (year) › Areas of Activity › External › Collaborations
Formycon AG
Fraunhoferstr. 15 82152 Planegg Germany
Sabrina Müller
+49-89-864-667-149
+49-89-864-667-110
sabrina.mueller@formycon.com www.formycon.com
I Q
250 2012
Biosimilar Development
Formycon aims to develop its biosimilar candidates to near-market development stages before transferring them fully or partially into partnerships for global commercialisation.
Project and marketing rights for the biosimilar candidates FYB206, FYB208, FYB209 and FYB210 are fully owned by Formycon.
There are different partnership models in place for the biosimilars that have already been approved: For FYB202, Formycon entered into a global commercialisation partnership with Fresenius Kabi. FYB203 is in a licensing partnership with Klinge Biopharma GmbH. Formycon owns a 50% stake in FYB201, which is marketed by Sandoz in the US, by Teva Pharmaceutical Industries Ltd. in the EU and other Territories as well as MS Pharma in the MENA region.
Who we are:
Formycon is a globally operating, independent biosimilar specialist with a highly attractive product pipeline and a fully scalable development platform in the fields of ophthalmology, immunology, immuno-oncology and other major indications. With its biosimilars – follow-on products for approved biopharmaceutical drugs –Formycon is making a significant contribution to improving patient access to highly effective and affordable medicines.
Biosimilars – Medicines for the Future
A biosimilar is a biological medicine highly similar to another already approved biological medicine. Biosimilars are approved in highly regulated markets such as the EU, the USA, Canada, Japan and Australia in accordance with strict regulatory procedures. Biosimilars create competition and thus give more patients access to biopharmaceutical therapies. At the same time, they reduce costs for healthcare systems.





Products and Pipeline:
In addition to FYB201 (reference product Lucentis ®), which has already been approved and launched in key global markets, Formycon’s development pipeline includes two additional biosimilars as well as four biosimilar candidates with attractive market potential.
The biosimilars FYB202 (reference product Stelara®) and FYB203 (reference product Eylea®) are approved in the US and the EU. FYB202 has also received approval in Canada and the UK. The biosimilar candidate FYB206 for the immuno-oncology blockbuster drug Keytruda® is currently undergoing clinical evaluation. The two undisclosed biosimilar candidates FYB208 and FYB209 are in advanced preclinical development. In addition, the new biosimilar development project FYB210 was started in November 2024.
Our Expertise:
Formycon is a “pure-play” biosimilar company. Given its specialised area of focus, the company can cover the entire technical-pharmaceutical development chain, from drug candidate selection, cell line development, comparative analytics, process development and preclinical and clinical development. The team also has extensive expertise in the preparation of approval documents and management of approval procedures in highly regulated markets as well as in establishing and managing all supply chain and product logistics.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website › Social Media ›
Number of Employees › Founded (year) › Type of Laboratory › Areas of Activity ›
GALAB Laboratories GmbH
Am Schleusengraben 7 21029 Hamburg
Germany
Dr Jürgen Kuballa
+49-40-36-80-77-0
+49-40-36-80-77-401 info@galab.com www.galab.com
F I I
250
1992
Research and contract laboratory for bio-analytics, S1 according to GenTSV, S2 according to BioStoffV
| Synthetic biology | Biocatalysis
| Enzyme production | Enzyme immobilisation
| Cofactor regeneration | Process development
GALAB Biotechnology –Next level biocatalysis
GALAB Biotechnology is specialised in the biocatalysis of valuable products for a broad range of industrial applications. One of our main focused products are human milk oligo-saccharides (HMOs). Our interdisciplinary experts develop customised bioprocesses with synthetic biology and complex multi enzyme cascades for sustainable and high-yield productions. Moreover, with our GlycoKat ® and NucleoKat ® technologies, we provide innovative, high-quality products and services for our customers.
Synthetic Biology for in vivo production processes
We apply synthetic biology and metabolic engineering for precise modifications of cells to initiate and enhance the production of target compounds. One of our competencies at GALAB Biotechnology is the in vivo synthesis of valuable HMOs with our patented strains. Our engineered Corynebacterium glutamicum and Escherichia coli strains produce high titer of fucosyllactose and sialyllactose. CRISPR/Cas9 is one of our powerful tools for precise genome editing and modifying metabolic pathways. It allows the fast addition of new genes, modification of existing genes or the knockout of specific genes to optimise the synthesis of our target products.
In vitro synthesis – from genes over stable biocatalysts to products
We offer a complete process development service for in vitro synthesis, from genes to products. Our proprietary expression strains are used in high cell density fermentation, which have been developed and optimised over the last 10 years to achieve high enzyme yields. In order



to develop a tailor-made production process for each enzyme, we offer a variety of enzyme purification and preservation methods. Furthermore, our unique enzyme immobilisation technology enables us to produce highly stable enzymes that can be used in different reactor systems. We have developed a technology that simplifies the implementation of multi-enzyme cascades by using our stable immobilised enzymes in packed bed reactors in continuous flow reactor setups.
Modular process design –
GlycoKat ® and NucleoKat ®
Modular process design makes it easy to implement multi-enzyme cascades. Our GlycoKat ® technology enables the continuous synthesis of a wide range of glycosylated oligosaccharides, with simple product changes by replacing individual modules. The addition of our NucleoKat ® technology takes glycosylation to a whole new level. The NucleoKat® technology is a module that enables the regeneration of expensive nucleotide cofactors and can be integrated into other processes for all types of nucleotides.
Services and expertise
› Synthetic Biology to produce HMOs in efficient E. coli and C. glutamicum strains
› CRISPR/Cas9 technology for precise genome editing and modifying metabolic pathways
› Production of pure, active, and stable biocatalysts through formulation- and immobilisation techniques
› Process development in different reactor concepts, e.g. continuous flow operation with packed bed reactors
› Model development of multi-enzyme cascades
› GlycoKat ® and NucleoKat ® modular technology for efficient synthesis and cofactor regeneration

Name ›
Address/P.O. Box › Postal Code/City › Country ›
Address/P.O. Box › Postal Code/City › Country › Contact Person › Email › Website › Social Media › Number of Employees › Founded (year) › Areas of Activity › External › Collaborations
Request for › Further Collaborations
Galimedix Therapeutics, Inc.
3704 Calvend Lane Kensington, MD 20895 USA
Am Klopferspitz 19 82152 Planegg/Martinsried Germany
Alexander Gebauer, MD, PhD Cofounder and Executive Chairman info@galimedix.com galimedix.com
Small molecules, amyloid beta neuropathologies, dry age-related macular degeneration, glaucoma, Alzheimer’s disease
Théa Open Innovation
We are looking for licensing/codevelopment partners for GAL-101 (topical/oral) for the treatment of ophthalmic indications in Asia Pacific.
Galimedix is a Phase 2 clinical-stage private company developing novel oral and topical neuroprotective therapies with the potential to revolutionize the treatment of serious eye and brain diseases. Founded by a seasoned and highly dedicated team of bio-entrepreneurs, pharmaceutical executives and scientists, Galimedix’s novel small molecules offer the hope of changing the course of disease where amyloid beta (A β) plays a role, such as in dry age-related macular degeneration (dAMD), glaucoma and Alzheimer’s disease (AD) - Galimedix’s initial areas of focus. Galimedix has a licensing agreement with Théa Open Innovation (TOI), a major ophthalmology player, for the development and commercialization of GAL-101 for the topical and oral treatment of dAMD, glaucoma and other ophthalmic indications with high unmet medical need, in Europe, the Americas, the Middle East and Africa.
Targeting neurodegeneration at its source
Normal A β monomers are important to neuronal function, but misfolding of these monomers can lead to the development of toxic forms of A β – oligomers and protofibrils – that can be highly damaging to retinal and brain cells. Many studies have indicated that these oligomers and protofibrils are an underlying cause of neurodegenerative diseases of the eye, such as dAMD and glaucoma. Recent approvals and promising Phase 3 results of anti-A β drugs also have validated them as a key target in AD. All three diseases are chronic, age-related disorders with no known cure and lead to irreversible loss of function.
Galimedix aims to stop neurodegeneration at its source by blocking a key step in the neurodegenerative process, namely, preventing the formation of toxic A β oligomers and protofibrils. This is in contrast to most products in development and on the market, which aim to remove already formed toxic species.


Small
molecules designed to prevent toxic A β oligomer formation
Galimedix is developing cutting-edge A β aggregation modulators that target the beginning of the A β peptide aggregation cascade. Lead product candidate, GAL-101, and backup candidate, GAL-201, both act upstream to most other A β -targeting approaches on the market and in advanced development. This unique mode of action, which not only removes already formed toxic species, but prevents their formation, too, could enable these small molecules to effectively impact disease progression without disturbing normal neuronal function.
GAL-101: Current stage of development
Compelling pre-clinical data support the potential of Galimedix’s product candidates to slow or stop neurodegeneration and also restore lost neuronal function. A Phase 2 proof-of-concept study in dAMD with GAL-101 eyedrops is underway, and a Phase 1 study with the oral formulation (capsules) of GAL-101 also is ongoing. Clinical studies in other indications are planned.
Galimedix’s approach could offer potentially gamechanging oral and topical neuroprotective therapies for the treatment of serious eye and brain diseases that impact millions of people.
The current market for each of the lead indications –dAMD, glaucoma and AD – in the US and Europe alone is in the several billion dollar range and growing steadily.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website ›
Number of Employees › Founded (year) ›
Areas of Activity ›
Genius Search GmbH & Co. KG
Hauptstrasse 25 69117 Heidelberg Germany
Thomas Winter +49-6221-32176-0 genius@genius-search.com www.genius-search.com 12 2018
Executive Search for the Life Sciences Sector
Genius Search is a leading life sciences executive recruiting firm serving the pharmaceutical, biotechnology, medical devices, and diagnostics industry as well as other related fields, with a focus on filling management positions and supervisory boards. The company’s services and assistance range from executive search and coaching to long-term talent pipelining.
Through years of experience, the skilled team of Life Sciences and Health Care consultants, specialists, and researchers of Genius Search knows the relevant personalities and companies in the industry and understands the special requirements of its customers.
Genius Search advises and supports global players, medium-sized companies, investors, and family offices. The team is in constant personal contact with the leading companies and heads of the industry. In a fast-changing industry, Genius Search analyses the current changes in Human Resources and plans the future for careers and companies in the Life Sciences Sector.
With the best possible networking and an excellent international network, Genius Search can fill management positions with suitable candidates. In addition to providing the resources of a global network, Genius Search has industry-specific expertise and market insight that has been developed and enhanced over decades.
Discretion, trust, respect, and high personal commitment are the foundations for the work of Genius Search.
Genius Search runs offices in Germany (Heidelberg & Wiesbaden), Switzerland (Davos), and in the United Kingdom (London).
References
For many years, we have enjoyed successful collaborations with globally active companies such as BioNTech, Boehringer Ingelheim, Sartorius and Johnson & Johnson, as well as a large number of small biotech and tech start-ups, which we support in their development and accompany or have accompanied through to exit.




Focus, Services & Assignments
Services and sectors:
› Life Sciences Executive Search
› Board Services
› Talent Pipelining
› Coaching Services
› Professional Assessments
Excerpt from Assignments:
› Several Board Functions (CEO, CDO, CFO, COO, CBO, CSO) for stock-listed and venture capitalbacked companies
› Corporate VPs Clinical Development & Medical Directors (several indications)
› Corporate Heads Drug Discovery (several indications)
› Corporate VP Translational Medicine
› Corporate VP Collaborative Research
› Several BU Heads & Franchise Heads (several indications)
› Senior Director BD & KAM Europe
› VP Corporate Business Strategy & PM BU Biopharmaceuticals
› Corporate Head of Controlling
› Head Global Procurement
› Senior Legal Counsel
› Head of Global Patents & Trademarks
› Country Manager Germany
› General Manager DACH
› Senior VP Global Operations
› Corporate Head Regulatory Affairs
› Head Medical Affairs and Regulatory Affairs
› Head Portfolio/BD/Licensing & Launch Management
› Global Head HR & VP HR (different companies)
› Corporate VP Global Talent Management
› Corporate Head Market Access, Pricing & Reimbursement


Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website ›
Founded (year) ›
Areas of Activity ›
Genosphere Biotechnologies
52 rue du Moulin de Pierre 92140 Clamart France
Dr M. Benamira, CEO +33 -142-717-021 info@genosphere-biotech.com www.genosphere-biotech.com www.biomodul.de 1996
| Custom peptide synthesis | Custom polyclonal antibody development | Custom monoclonal antibody development | Custom recombinant protein expression
Genosphere Biotechnologies is an independent and trusted service organisation for the development and production of customised peptides, custom polyclonal and monoclonal antibodies, and custom recombinant proteins. Customers from universities, research & development industrial groups, and high-tech startup companies benefit from our expertise and from our individual and flexible solutions for each customer requirement.
We support our customers in their success. Our goal is to provide reliable services at competitive prices. With over 25 years of experience in organic chemistry, peptide chemistry, immunochemistry, and recombinant technologies, we are able to provide excellent service with highly reproducible quality.
Custom peptide synthesis
Peptides are essential in a large variety of biological processes. A number of peptides have been shown to play a key role in specific biochemical processes as well as disease. Hence, a trustworthy partner in chemical peptide synthesis has become critical to many laboratories across the life sciences in both basic and industrial R&D.
Over the past 25 years, Genosphere Biotechnologies has produced complex peptides using total chemical synthesis, incorporating any of the 20 standard L- amino acids or other unnatural amino acids (D-form, glycosylated, azide-containing, methylated, etc). Additionally, we can engineer structural modifications, such as gamma-peptide bond branching or epsilon-peptide bond branching. Other popular structural changes to the linear peptide chain include disulfide bridges or Nter to Cter lactam cyclisations.
Genosphere Biotechnologies has been synthesising custom peptides for research and development laboratories since 1996 from proof-of-concept to preclinicalmass production steps and is one of the most trusted group of peptide chemists in the industry. The company’s peptide synthesis group will support you in the design of your peptides and peptide derivatives.






Custom polyclonal antibody development
Genosphere Biotechnologies offers complete polyclonal antibody production services packages, including peptide or protein antigen synthesis, choice of host immunisation, antibody purification, and quality control analyses.
Our polyclonal antibody production services are designed to meet a variety of experimental requirements. Multiple purification methods are available, including protein A and antigen affinity purification.
Our polyclonal antibody development services are based on years of process optimisation and a strong reputation for reliable antibody production services. We have been a preferred partner to various institutions worldwide for antibody development and antibody production services for over 25 years.
Monoclonal antibody development
Mouse monoclonal antibodies have broad applications as therapeutics, diagnostic tools, and research reagents. Genosphere Biotechnologies provides comprehensive mouse monoclonal antibody development services based on our well-established hybridoma platform. We provide complete support for all steps of mouse monoclonal antibody development, from antigen preparation to animal immunisation, fusion and screening, subcloning, and antibody production, which allows the identification of clones with the desirable qualities. We provide free consultation for target antigen design and expression, and our experienced project managers and scientists will support you directly throughout the process.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website ›
Founded (year) › Type of Laboratory › Areas of Activity ›
glyXera GmbH
Brenneckestraße 20 – ZENIT 39120 Magdeburg Germany
Dr Erdmann Rapp (CEO & CSO)
+49-391-6117-251 +49-391-6117-255 info@glyxera.com www.glyxera.com 2011 | Glycomics | Glycoproteomics | Proteomics
High Performance Glycoanalytical & (Glyco)Proteinanalytical Services & Products
glyXera GmbH is a Max Planck Society spin-off, specialised in high performance glycoanalysis. We are utilising separation- and mass-spectrometry-based glycoanalytical tools and have substantial experience in providing glycoanalytical products & services tailored to the specific needs of our customers. Worldwide exclusively glyXera provides the high-performance glycoanalysis system glyXboxTM
Our clients include leading pharmaceutical, biotechnology and food companies throughout the world. We offer you our sophisticated technology platforms and our expertise with respect to glycoanalytical services, tailored to your specific needs. We guide you for better decisions and help you to perform better and faster during discovery phase, R&D, (clinical) trials and approval of your innovative products (biopharmaceuticals (originators & biosimilars), vaccines, food additives, functional food, etc.), and QA/QC in your production stages.
Expertise & pace
glyXera GmbH is specialised in glycoanalysis, utilising state-of-the-art separation- and mass-spectrometrybased glycoanalytical tools and has a strong background in providing glycoanalytical services. We run modern analytical and synthesis labs, equipped with state-of-the-art instrumentation.
glyXera offers high performance glycoanalysis to its clients, providing sophisticated technology platforms and expertise with respect to glycoanalytical services, tailored to the specific needs of the variety of its customers from academia, clinics, pharmaceutical and food industries. We have substantial experience in glycomics, glycoproteomics and proteomics of biotechnological (e.g. recombinant glycoproteins (mAbs, fusion proteins, factors, hormones, etc) or viral membrane glycoproteins) and clinical (blood, milk and other body fluids) samples.
High-performance glycoanalysis
glyXera provides exclusively worldwide fast and reliable glycoanalysis utilising a “real” high-throughput system (method/software/database) with superior performance and capacity compared to other glycoanalysis tools available on the market.




Our patented system glyXboxTM, based on multiplexed capillary gelelectrophoresis with laser induced fluorescence detection (xCGE-LIF) enables generation and comparison of glyco-fingerprints from large sets of samples and structural analysis of glycans within these samples.
High throughput, high resolution, high sensitivity, high reproducibility, high reliability:
› Up to 96 capillaries in parallel enable “real” high throughput
› Up to 3500x more sensitive and 450x faster, compared to LC
› An order of magnitude higher separation power, compared to LC
High-performance glycoanalysis on 3 levels:
› Glycofingerprinting: Glycosylationpattern analysis & comparison
› Standard Glycoprofiling: Identification of glycans in complex mixtures via database matching
› Extended Glycoprofiling: Extended structural analysis of glycans in complex mixtures using exoglycosidase sequencing in combination with repeated glycoprofiling.
Our services
› Glycofingerprinting & Glycoprofiling by xCGE-LIF & HILIC-FLR
› MALDI-MS based Glycoprofiling
› LC-MS based profiling of N- and/or O-glycans
› Monosaccharide (Composition) Analysis
› Site Occupancy Analysis
› Proteomics
› N- & O-Glycopeptide Mapping
› Intact Mass Protein Analysis (Glycoform Determination)
› General (Glyco)-Protein Characterisation by Protein Gel Electrophoresis Pattern Analysis
Our products
› glyXbox CE : High performance glycoanalysis system based on xCGE-LIF (incl. glyXtoolCE software)
› glyXprep: Sample preparation kit for glycoanalysis
› Tailored standards & kits for glycoanalysis
Name ›
HealthCapital – Cluster Healthcare Industries Berlin-Brandenburg
The Berlin-Brandenburg BioRegion –a Leading Hub for Life Sciences
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Areas of Activity › External › Collaborations
Fasanenstr. 85 10623 Berlin Germany
Dr Kai Bindseil, Cluster management HealthCapital Berlin-Brandenburg +49-30-46302-463 info@healthcapital.de www.healthcapital.de I #HealthCapitalBB
l Technology transfer between science and industry
l Initiation and support of networks
l Support for technology-oriented start-ups
l Funding support for innovative project concepts
l Providing and presenting regional life sciences information
l Building and coordinating of scientific and interdisciplinary networks
l Establishing contacts between experts from all disciplines
l Organisation of events and seminars, Public Relations work for the life sciences region
l Member of the Council of European Bioregions (CEBR)
l Member and contact point in Berlin for Enterprise Europe Network (EEN)
l Collaborations with European Life Science clusters and SMEs in several European projects and other activities
l Bio Deutschland
l Global Health Hub
The Berlin-Brandenburg region is one of the leading, international locations for life sciences. The area concentrates on clinical research via a dense and compact healthcare network, and boasts a state-of-the-art IT infrastructure. It is no coincidence that the region is Germany’s ‘health capital’ as it is home to both the German government, as well as the centre for healthcare industries.
The region’s distinction is anchored in its unique research and clinical landscape, as well as its ability to closely link the key players in the life sciences and healthcare. Biotechnology, in particular, is a strong driving force within the Berlin-Brandenburg healthcare industries cluster – HealthCapital – generating innovation and growth there and beyond. Through networking the pharmaceutical, diagnostics and medical technology sectors completely new fields of business are created. More than 300 biotechnology and 35 pharmaceutical companies are located in the German capital region. They include market leaders like Bayer, BERLIN-CHEMIE (Menarini), Daiichi Sankyo, Pfizer, Bausch + Lomb, Sanofi, and Takeda. Along with the sectors many small and mid-sized companies, they benefit from close cooperation, both with science and with more than 150 hospitals – above all, one of Europe´s largest university hospitals: Charité – Universitätsmedizin Berlin. Customers from research and industry have access to patient cohorts of urban and rural populations of about 180 ethnicities, covering all medical indications. The many technology parks and networks in the various branches of modern biotechnology create the excellent infrastructure and technological support that characterize the BerlinBrandenburg life sciences area. Focus of biotech activity within the region are biomedicine and diagnostics, therapeutics and regenerative medicine as well as industrial biotechnology.

Connected to the European Hotspots
Active cooperation in the networks of Europe’s BioRegions has played a central role in the internationalisation efforts in the region. The region is a member of the Council of European Bioregions (CEBR).
Offering Service and Support for Life Sciences in the Capital Region
The central contact and coordination office for all issues concerning life sciences in the German Capital Region is HealthCapital. At the interface of business, science and clinics, the HealthCapital cluster management drives networking and the technology transfer and supports companies interested in relocating to the region. Berlin Partner for Business and Technology and the Economic Development Agency Brandenburg (WFBB) are responsible for managing the cluster.
Meet us 2025 at
› BIO Europe Spring | March 17-19, Milan, Italy
› DMEA | April 8-10, Berlin, Germany
› BIONNALE | May 14-15, Berlin, Germany
› BIO International Convention | June 16-19, Boston, MA, USA
› BIO Europe | November 3-5, Vienna, Austria
› MEDICA | November 17-20, Düsseldorf

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website › Social Media ›
Number of Employees › Founded (year) › Type of Laboratory ›
Areas of Activity › Biological Patents › External › Collaborations
IBA Lifesciences GmbH
Rudolf-Wissell-Str. 28 37079 Göttingen Germany Dr Fabian Mohr
+49-551-50672-0 +49-551-50672-181 info@iba-lifesciences.com www.iba-lifesciences.com
l Protein Production & Assays l Strep-Tactin®XT l Twin-Strep-tag®
Numerous in- and out-licensing contracts
Strep-tag ® - the leading affinity tag in recombinant protein production
IBA Lifesciences GmbH is a biotechnology company that develops innovative products for life science research in both academic and industrial settings. Our unique Strep-tag® technology opens up a wide range of application possibilities in the purification and analysis of recombinant proteins.
Strep-tag ® technology
The Strep-tag ® technology exploits one of the strongest non-covalent interactions in nature; the interaction of biotin and streptavidin. Strep-tag ® II and its tandem equivalent Twin-Strep-tag ® are peptide sequences exhibiting intrinsic affinity towards the biotin-binding pocket of two specifically engineered streptavidin variants, Strep-Tactin ® and its high affinity version StrepTactin ®XT. The wide range of affinities as well as the reversibility of the binding interaction make Strep-tag ® the leading affinity chromatography system.
From protein purification...
Strep-Tactin®XT is the high affinity variant for the purification of strep-tagged fusion proteins, providing binding affinities in the picomolar range for Twin-Strep-tag® while still maintaining binding reversibility and mild recovery of immobilized proteins. It is suitable for efficient protein purification independent of protein class and size, including challenging proteins as well as low abundant proteins.
Fused to magnetic beads, MagStrep ® Strep-Tactin®XT, is the ideal tool for complex identification via pull-downs and fast small-scale batch purification in reaction tubes or 96-well plates.
In particular, Strep-Tactin ® XT 4Flow ® high capacity provides superior performance for the purification of strep-tagged proteins from diluted cell extracts and facilitates intensive wash procedures with large volumes of wash buffer. Strep-Tactin ®XT 4Flow ® high capacity resin is stable over a wide range of pH and compatible with various buffer conditions, and can be reused at



least 100 times without losing performance. Due to this frequent reusability, experimental costs can be significantly reduced.
... to analytical applications
The near covalent affinity of Strep-Tactin ®XT to TwinStrep-tag ® expands the range of use towards analytical applications such as high throughput screening, assay development and protein kinetic studies. You can obtain Strep-Tactin ® XT conjugated to microplates, fluorophores, or chips.
Strep-Tactin®XT coated microplates ensure convenient diagnostic assay and high-throughput screenings with high stability and antibody-free protein immobilization. Moreover, immobilized biomolecules are presented to interaction partners in a uniform manner, which results in reliable and highly reproducible assay formats.
The picomolar affinity is particularly valuable for surface plasmon resonance (SPR) analysis and bio-layer interferometry (BLI) and supports measurements with long dissociation times and slow off-rates. Moreover, Strep-Tactin®XT biosensors can be easily regenerated.
Key features:
› Highly selective binding properties leading to unparalleled protein purity (> 95 %)
› Bioactive target proteins due to rapid one-step purification under target specific conditions
› Variability in buffer conditions, e.g. high salts, detergents, metal ions, chelators or reducing agents
› Favorable for protein-protein interaction studies due to mild elution conditions that preserve natural protein structure
› Products covering the entire workflow from cloning, purification to analytical applications (ELISA, FACS, SPR, Microscopy, Western Blot, etc.)

Name ›
Address/P.O. Box › Postal Code/City › Country › Contact Person › Telephone › Email › Website › Social Media › Number of Employees › Founded (year) › Type of Laboratory › Areas of Activity ›
Ideogen AG
Hurdnerstrasse 119
8640 Hurden Switzerland
Sanjeet Telang / Murat Goker Office: +41-43 3115252
S Telang: +41-79-277-3626
M Goker: +41-79-676-3628 sanjeet.telang@ideogen.com murat.goeker@ideogen.com www.ideogen.com
I
88 2013 Specialty Pharmaceuticals
Specialty Pharma / Rare & Orphan Diseases / Salvage & Chronic Care Therapies / Managed Access Programmes (MAP) / Oncology, Haematology, Cell & Gene Therapy, Hospital Sales
External › Collaborations
Pre-clinical to clinical development. Multiple ongoing collaborations with research hospitals, disease associations and patient organisations
Ideogen is a Swiss, privately owned, specialty pharmaceutical disease management company focused on providing products that address unmet medical needs in the areas of specialty, salvage , and chronic care in oncology, haematology, and rare/orphan diseases, among numerous other disease areas.
We a commercialisation platform with a robust track record of success in driving the commercialisation of unlicensed products through Managed Access Programmes (MAP) to licensed sales of specific assets, while covering all aspects of the value chain from regulatory pathways, market access, pricing, and reimbursement to commercialisation in Europe, Turkey, and the Middle East.
We are headquartered in Switzerland, and have subregional affiliates in Austria, the Netherlands, and Spain, along with a standalone affiliate in Turkey that caters to the Turkish and Middle East markets.
Our business model and success to date are based on strong in-house medical and clinical expertise and as experienced scientific account management teams, in addition to established partner relations in specialised settings across multiple territories that require geography-specific solutions.
Request for › Further Collaborations
Exclusive in-licensing of specialty innovative assets with/without Orphan Drug Designation (ODD) / unlicensed or licensed commercialisation for the EU & MET regions

Our unique selling proposition is to manage the streamlined roll-out and execution of early and managed access programmes for relevant unregistered assets.
We also commercialise registered assets while managing the market access, pharmacovigilance, supply chain deployment, customer service, data collection, and reporting activities in the geographies in which we operate.
Ideogen is increasingly looking to diversify its scope of activities by entering the high-value, injectables manufacturing space with a view to entering cell and gene therapy, focusing on in vivo gene therapies.
This would cement the company’s position as a vertically integrated, specialty pharmaceutical organisation with relevant CDMO capabilities that can cater to the emerging needs of the next generation of personalised medicine.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media › Number of Employees › Founded (year) ›
Areas of Activity › External › Collaborations
Immunic Therapeutics
Lochhamer Schlag 21 82166 Gräfelfing Germany
Headquarter: New York City, USA
Jessica Breu (Vice President of Investor Relations and Communications) +49-89-2080477-09 info@imux.com www.imux.com
F I 90 2016 | Immunology | Chronic inflammatory and autoimmune diseases
| Ludwig-Maximilians-University (LMU) Munich, Germany: Department of Pharmacy: Prof. Dr. Daniel Merk | Universitätsklinikum Erlangen, Germany: Institute of Clinical and Molecular Virology: Prof. Dr. Manfred Marschall
| University Hospital Frankfurt, Germany: Institute for Infectiology: Prof. Dr. med. Maria J.G.T. Vehreschild | City of Hope Duarte, California, USA: Department of Molecular Imaging & Therapy, Research in Cancer and Immunity, Mechanisms Controlling T-cells: Prof. Zuoming Sun, Ph.D.
Immunic (Nasdaq: IMUX) is developing a clinical pipeline of therapies for chronic inflammatory and autoimmune diseases. The company’s lead asset, vidofludimus calcium (IMU-838), is being developed as a next-generation oral treatment option for patients with relapsing and progressive forms of multiple sclerosis (MS). Immunic’s second clinical-stage asset, IMU-856, has demonstrated clinical proof-of-concept in celiac disease patients and may represent a completely new treatment approach for a variety of gastrointestinal disorders. IMU-381, currently in preclinical testing, is a next generation molecule specifically addressing the needs of gastrointestinal diseases.
Vidofludimus Calcium: Addressing Multiple Drivers of Neurodegeneration in Multiple Sclerosis
Vidofludimus calcium is an orally available, nextgeneration selective immune modulator that acts as a potent, first-in-class nuclear receptor related 1 (Nurr1) activator, in addition to its known mode of action as a dihydroorotate dehydrogenase (DHODH) inhibitor. Nurr1 is a neuroprotective transcription factor and an emerging target in neurodegenerative diseases.
Vidofludimus calcium is currently being investigated in the twin phase 3 clinical ENSURE trials in relapsing multiple sclerosis (RMS) and in the phase 2 CALLIPER trial in progressive multiple sclerosis (PMS). An interim biomarker analysis of the CALLIPER trial demonstrated a clear separation from placebo in serum neurofilament light chain (NfL) levels. Top-line data of the CALLIPER trial is expected in April 2025.
For the ENSURE programme, Immunic announced a positive outcome of an interim analysis, confirming the trials can continue as planned without a need for potential upsizing. Completion of both ENSURE trials is expected in 2026.

Neuroprotective, Anti-Inflammatory, and Anti-Viral Effects
Immunic believes that vidofludimus calcium has the potential to demonstrate medically important advantages versus currently approved MS treatments, due to its combined neuroprotective, anti-inflammatory, and antiviral effects as well as its established favorable safety and tolerability profile.
The molecule has a targeted effect on hyperactive immune cells without suppressing the normal immune function. It also showed a potent MRI lesion suppression and initial signals for improved rates of confirmed disability worsening in phase 2 clinical testing, while also decreasing serum NfL, a biomarker for axonal damage. The broad-spectrum anti-viral effect of vidofludimus calcium may support lowering the rate of viral infections and reactivations, including Epstein-Barr virus (EBV) reactivation, potentially resulting in slowing EBV-related neurodegenerative processes.
IMU-856: Targeted to Restore a Healthy Gut by Renewal of the Gut Wall
IMU-856 is an orally available and systemically acting small molecule modulator that targets Sirtuin 6 (SIRT6), a protein which serves as a transcriptional regulator of intestinal barrier function and regeneration of bowel epithelium. Immunic announced positive results from a phase 1b clinical trial in patients with celiac disease, where IMU-856 demonstrated benefits across four key dimensions of the disease’s pathophysiology: histology, disease symptoms, biomarkers, and nutrient absorption. IMU-856 was also observed to be safe and welltolerated in this trial.
Additionally, in a post hoc analysis of this trial, IMU-856 demonstrated a dose-dependent increase of endogenous glucagon-like peptide-1 (GLP-1) levels and, in preclinical testing, showed a dose-dependent reduction of body weight gain and food consumption, indicating potential as an oral treatment option for weight management. Further clinical testing is in preperation.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website › Social Media ›
Number of Employees › Founded (year) › Type of Laboratory ›
Areas of Activity ›
Indivumed GmbH
Falkenried 88 – Bldg. D 20251 Hamburg
Germany
Prof. Dr. Hartmut Juhl (CEO)
+49-40-4133-83-0
+49-40-4133-83-14 info@indivumed.com www.indivumed-therapeutics.com
~100 2002 S2, S1
Drug development partnerships
| Precision oncology R&D
| Pharma and biotech partners
Proprietary cancer database
| Multi-omics data based on highquality tissue samples
| Extensive clinical data based on longitudinal patient follow-up
Target Discovery
| Multivariate, biomathematical analytics
Target Validation
| 2D and 3D patient-based cellular models
| Cell-free and cell-based assay development
External › Collaborations
Indivumed Global Clinical Network
| Collaboration with leading cancer clinics in Europe, Asia, and North and South America
Academic Partnerships
| Salk Institute
| Georgetown University
| University of Rochester Medical Center
| A*STAR Institute for Molecular and Cell Biology
Moving precision oncology forward
Indivumed Therapeutics is a biotech company with a focus on precision oncology. Our vision is a world in which precise therapeutic treatments exist for every cancer patient. With this goal in mind, we enhance R&D activities and launch new discovery programmes with expertise, capabilities and insight knowledge throughout the drug discovery process. Through a global network of selected partner clinics, we have created and maintain a unique database of proprietary multiomics and longitudinal clinical datasets that reflect the molecular reality of cancer at an unparalleled level. By combining these proprietary datasets with stateof-the-art analytics and comprehensive biochemical approaches, we drive target discovery and development for novel therapies.
Special operation procedures to protect sample integrity
We are convinced that biospecimen quality along with the resulting data is crucial to making all relevant details of a cancer disease transparent. To ensure this, our global clinical partners apply a designed set of standard operating procedures (SOPs) for the collection and processing of biospecimens. By achieving a cold ischemia time for tissue of ≤12 minutes and completing our sets with extensive longitudinal patient data covering around 320 individual data points, we maintain molecular expression profiles and set the gold standard for multi-omics cancer data.
Target identification based on unique data
The excellent quality of our tumour and normal tissue samples enables in-depth multi-omics analysis, resulting in very high-quality data. Our unique and comprehensive cancer database is the foundation of a streamlined process to identifying therapeutically novel



drug targets. By implementing advanced biomathematical, bioinformatic and biophysical algorithms, we have developed a workflow to analyse large amounts of data in a short timeframe. Despite using AI-supported biological research, we rely on an experienced team of scientists to select the right targets to be pursued.
A comprehensive target validation process
We thoroughly validate the selected targets, using 2D and 3D cellular models derived from the original patient cohort used in discovery. These in-house generated models are excellent representations of the characteristic features of the disease and reflect cancer close to physiological conditions. Various state-of-the-art genetic and biochemical techniques are applied to assess target characterisation. Our experts examine the protein class and structure, sub-cellular location and other biological parameters of the target. This enables the establishment of proof-of-concept cell-free and cell-based assays for ligand screening and validation.
Partnering with Indivumed
We work with pharmaceutical and biotech companies to advance new and promising drug targets for innovative cancer treatments. As a trusted R&D partner, we use our expertise, unique insights and deep passion to understand the complexities of cancer. In addition, we offer out-licensing opportunities to enhance our partners’ therapeutic pipelines. The exceptional quality and consistent comparability of our data makes our approach unique in the industry and creates a higher chance of new drugs reaching the market.
“Our targets will have a huge impact on future cancer treatments. We have come a long way, but collaboration is key to make the vision a reality.”
Prof. Dr. Hartmut Juhl, Founder and CEO

Name ›
Address/P.O. Box ›
Postal Code/City › Country › Contact Person › Telephone › Fax › Email › Website › Social Media ›
Number of Employees › Founded (year) ›
Areas of Activity ›
Integris Pharma S.A.
2 Nikitara str. 15232 Chalandri Greece
Agapi Salvara
+30-210-8778-240
+30-210-87-78-242 bd@integris.gr www.integris.gr I 60-80 2007
Hematology, Oncology, Rare and Metabolic Diseases, Wound Care, Primary Care
INTEGRIS Pharma was founded in 2007 as a pharmaceutical company specialising in CNS generics. In 2018, the company successfully relaunched its business model with a clear mission: to address the unmet therapeutic needs of patients with rare, chronic, and life-threatening diseases in Greece, Cyprus, Malta, CEE and the Balkan countries through innovative therapeutics.
Consistently serving this new strategic focus, INTEGRIS Pharma actively seeks innovative medicines throughout the world and is committed to ensuring direct access for all patients in the countries where it operates.
Over the last five years, INTEGRIS Pharma has established 13 new partnerships with leading global biopharmaceutical companies and has built an extensive and diverse product portfolio that addresses more than 17 diseases.
The company’s portfolio includes orphan drugs for rare and ultra-rare diseases, innovative pharmaceuticals, as well as hybrid and generic products in the fields of oncology and hematology. Additionally, it offers first-inclass medical devices for wound healing.
Recognising the significant impact of these diseases on the lives of patients, their families, and caregivers, INTEGRIS Pharma is dedicated to comprehensive disease management – from prevention and diagnosis to product management that meets the highest quality and safety standards, including patient-centric initiatives that aim to address the needs of patients across their journey with the disease.




Our products
We maintain an extensive portfolio of pharmaceutical products and medical devices for more than 16 diseases in the fields of oncology, haematology, rare and genetic metabolic diseases, and wound care.
Our product list includes innovative pharmaceutical products and orphan drugs, hybrid and generic drugs, as well as high-tech medical technology products through our collaboration with international pharmaceutical companies.
Our partnerships
Leading international biopharmaceutical companies trust INTEGRIS Pharma for the overall management of their pharmaceutical products in the Greek market.
Adhering to the highest professional and ethical standards, and drawing on our team’s long expertise and knowledge, we undertake all the steps necessary so that patients in our country gain rapid access to the innovative medicines and high-tech medical devices provided by our partners.
INTEGRIS Pharma oversees all local procedures required to guarantee patient access: from importation, storage, and distribution of products, to pricing and reimbursement processes, regulatory compliance, pharmacovigilance services, and high-quality pharmaceutical detailing for healthcare professionals. Together, we strive to meet the therapeutic needs of patients.
Business development
Our business model is based on establishing long-term collaborations with international pharmaceutical companies that aim to accelerate their product launches following FDA or EMA approval and to rapidly expand their reach to patients in our territories, through a partnership model agreement with us.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees › Founded (year) › Areas of Activity ›
iOmx Therapeutics AG
Fraunhoferstr. 22 82152 Martinsried Germany Dr Apollon Papadimitriou +49-89-8999-7090-0 info@iomx.com www.iomx.com I 46 2016 | Next-generation cancer immunotherapies | Drug development | Immuno-oncology
iOmx is a clinical-stage biotech company that harnesses deep tumour immunology insights to generate novel treatments for the most prevalent solid tumour indications.
The company is translating unexplored immune evasion biology into a growing pipeline of biomarker-enabled drug programmes. Focused on developing drugs with single-agent activity, iOmx is creating potential new backbone therapies in a modality-open fashion. By applying its comprehensive drug discovery & development expertise, iOmx is committed to shaping the future of cancer therapy.
Next generation immuno-oncology programmes
Immunotherapy has become an essential pillar in cancer treatment. Currently, the field is dominated by drugs targeting the PD-1/PD-L1 pathway. However, these agents represent only the beginning of cancer immunotherapy and show variable efficacy across tumour indications. In the majority of cases, they fall short in meeting the needs of cancer patients, leaving a gap in immunooncology treatment options.
iOmx aims to close this gap with a differentiated approach that follows the route of new immune-evasion biology discovery and drug development. The company is developing first-in-class therapies with single-agent efficacy, focusing on targets that are biologically distinguished and orthogonal to the PD-1/PD-L1 suppression pathway. With promising novel targets identified, the company is now advancing their clinical and biomarkerguided development to deliver transformative treatments for patients.
iOmx’s pipeline
The company’s lead candidate, OMX- 0407, is a firstin - class, spectrum - selective inhibitor of SIK (saltinducible kinase) and other oncology-relevant tyrosine kinases, under investigation in a Phase Ia/Ib clinical trial. In a successful Phase Ia dose escalation study,


OMX- 0407 demonstrated a favourable safety profile and signs of anti -tumour activity, including a durable complete response in a heavily pretreated patient. This clinical data has led to the initiation of a Phase Ib expansion study in kidney cancer and angiosarcoma. The expansion phase is currently evaluating the safety and single-agent activity of OMX- 0407 in these tumour types. OMX-0407 has high monotherapy potential in multiple solid tumour indications, e.g. sqNSCLC, UBC, CRC, RCC and soft tissue sarcomas.
The mode of action of OMX- 0407 combines immune sensitisation and modulation of the tumour microenvironment with direct tumour cell - killing effects, thereby driving tumour eradication.
The company’s second programme, IOMX- 0675, is a best- in - class antibody targeting the immunosuppressive receptors LILRB1 and LILRB2, part of the leukocyte immunoglobulin-like receptor (LILR) family – an evolving target class with cumulating clinical validation. This receptor family is expressed on myeloid and immune cells. The fully human, cross-specific, high-affinity antibody neutralises these potent immune - suppressive receptors, while sparing closely related immune-activating members of the LILR family, such as LILRA1 and LILRA3. Neutralisation of these receptors results in a pronounced anti-tumour immune response by retuning the tumour microenvironment and activating T cells against cancer cells. IOMX- 0675, has successfully advanced through IND-enabling studies, with a CTA submitted in Q4/2024. In addition, iOmx is driving discovery programmes to add to its pipeline of next-generation immune-oncology drugs (bispecifics & T cell engagers).
Outlook
iOmx is advancing its clinical programme OMX- 0407 towards clinical proof-of-concept, with key data anticipated in 2026. The second programme, IOMX- 0675, is expected to receive CTA approval in Q2/25. Furthermore, the discovery programmes are expected to deliver clinical candidates in 2025.

Name › Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website ›
Number of Employees › Founded (year) › Type of Laboratory ›
Areas of Activity ›
Number of Firms › External › Collaborations
Fördergesellschaft IZB –Innovations- und Gründerzentrum Biotechnologie mbH
Am Klopferspitz 19 82152 Planegg/Martinsried Germany
Christian Gnam
+49-89-5527948-0 +49-89-5527948-29 info@izb-online.de www.izb-online.de
36 1995 S1
l Biotechnology l Life Sciences
over 40 start-ups
l Collaborations with the scientific faculties on the Campus Martinsried l Munich Life Science Pitch Day with the High-Tech Gründerfonds Management GmbH l IZBrunch - Science meets Sciencs l Law Lab - law for biotech businesses
Hotspot for Life Sciences
The Innovation and Start-up Center for Biotechnology (IZB) with sites in Planegg-Martinsried and FreisingWeihenstephan near Munich is one of the leading biotechnology centres in Europe. The company Fördergesellschaft IZB mbH, founded in 1995, operates the IZB. The IZB hosts over 40 biotech companies with more than 700 employees across a 26,000 m² area. At the main site in Planegg-Martinsried, spanning 23,000 m², resident start-ups primarily focus on medical biotechnology. The IZB in Freising provides ideal conditions for founders in the life sciences sector.
Outstanding infrastructure
The IZB offers young companies a superior environment and infrastructure for realising their product and service ideas. Crucial for successful growth are S1 laboratories and office spaces that flexibly adapt to the companies‘ needs. Additionally, the IZB provides excellent infrastructure. At the Martinsried location, start-ups have access to modern conference rooms, a design hotel, the restaurants „The Bowl“ and „Seven and More“, as well as the Bio Kids kindergarten.
A strong network
Another vital success factor for IZB start-ups is the close proximity of both locations to cutting-edge research. On the Weihenstephan Campus, you’ll find the TUM school of Life Sciences of the Technical University of Munich, as well as the University of Applied Sciences Weihenstephan-Triesdorf. Adjacent to the IZB on the Martinsried/ Großhadern campus, you’ll find, among others, two Max Planck Institutes, faculties, and facilities of LMU, as well as the University Hospital of Munich-Großhadern. The close proximity of the scientific institutes around the Martinsried campus provides an enormous competitive advantage. Young scientists can benefit from incorporating scientific and research expertise into their own companies; short distances promote interaction and cooperation between biotech companies - in the face of globalisation, both factors are essential for successfully entering world markets.





The IZB fosters and supports this strong network through various measures: The Faculty Club offers an exceptional venue for founders and scientists to exchange ideas and know-how. With an array of event formats like the IZBrunch and the Munich Life Science Pitch Day, the IZB has established itself as a key meeting point in the industry, extending far beyond Munich.
IZB – in brief
› 26,000 m2 laboratory and office space for start-ups and growing companies
› Home for nearly 40 start-ups
› Business development support
› In-house estate management
› Center of an impressive research campus
› Access to an international network
› Flexible lab and office structures
› Close contacts with investment partners
› Joint location marketing
› Attractive, modern conference rooms, also for external booking
› IZB Residence CAMPUS AT HOME (42 Rooms)
› Faculty Club G2B
› Restaurants: SEVEN AND MORE and THE BOWL Food Lounge
› 2 day care centres (Bio Kids & Bio Kids2)
Visit us: Get our Newsletter:



Name ›
Address/P.O. Box › Postal Code/City › Country › Contact Person
Telephone
Website › Founded (year)
Areas of Activity
Patents › External › Collaborations Request for › Further Collaborations
Kosmas Therapeutics GmbH
Streitbaumweg 8 91077 Neunkirchen am Brand Germany
Georg Fey +49-151-2236-6573 georg.fey@t-online.de www.kosmastherapeutics.com 2018
Drug development; immunotherapeutics, cancer
https://patents.google.com/patent/ WO2018100139A1 https://patents.google.com/patent/ WO2011070109A1
John F DiPersio, Wash Univ St Louis Med Cntr USA
Matthias Peipp, Univ Schlsw Holst, Med Cntr Kiel, Ger
TA Fehniger, Wash Univ St Louis Med Cntr, USA
Prof E Ullrich, Univ Med Cntr, Frankfurt, Ger
Prof P Lang, Univ Med Cntr, Tübingen, Ger
Overall goals
Kosmas develops immunotherapeutic agents for the treatment of cancer, auto-immune- and inflammatory diseases. Our patent-protected Trilytes are a new class of tri-specific recombinant proteins designed for a safer and more efficient treatment of these diseases. The key innovation is their “dual-targeting” mode of binding to a select subset of target cells, for example Cancer Stem Cells (CSCs). Preferential killing of chosen subsets such as the CSCs is enabled by this novel molecular design and mode-of-action (MOA).
Unique new capability
The new molecular format uniquely permits a single trilyte molecule to bind simultaneously to a pair of 2 different targets, which are expressed on the surface of the select target cell (such as a CSC) in greater density than on other cells, such as bulk cancer cells and non-malignant cells. The trilyte engages Natural Killer (NK) cells of the patient’s immune system and mediates preferential killing of the selected target cells (US patent US11945863B2 approved April 2024; European & Canadian patents EP3548516A1, CA3045385A1 pending; advanced state of application process).
Lead agent to treat Acute Myeloid Leukemia
Our lead agent KT1 was designed to treat Acute Myeloid Leukemia (AML) with an enhanced ability to kill the relapse-inducing Leukemia Stem Cells (LSCs), while also having strong efficacy for bulk AML cells.
NK cells as killers confer a favorable safety profile
NK cells engaged by trilytes have a favourable toxicity profile compared with killer T cells frequently engaged in other immunotherapies. NK cells engaged by KT1 release a less toxic cocktail of inflammatory mediators. These two innovations together, mediated by a single molecule, are expected to achieve greater therapeutic efficacy together with a strongly improved safety profile.



Late preclinical development status of lead agent
Preclinical development of KT1 became possible through an M4 award from the Bavarian Ministry for the Economy and Commerce. Scalable up- & downstream processes for the production of KT1 using standard components and transferable to GMP production, have been developped. Good production yields and excellent long-term storage stability in formulation buffer have been reached. Anti-leukemic efficacy was assessed in collaboration with experts from Washington Univ. St Louis, USA. NSG IL15 mice genetically disabled to reject human cell grafts, were transplanted with human AML cells and treated with KT1 in combination with transfers of Cytokine-Induced Memory Like (CIML) NK cells. The treatment extended mean survival of the mice from 53 to > 75 days, an unusually strong prolongation and an established predictor of clinical efficacy for KT1.
Competent founders team
The expertise of Kosmas’ founders covers the needs for the late preclinical and early clinical development of KT1 (www.kosmastherapeutics.com).
Expert collaborations for clinical trials in place
The collaborative project with leading experts from Wash U St Louis and the U of Schleswig Holstein, Kiel, has received grant support from the US Leukemia and Lymphoma Society in 2024. Kosmas will acquire an exclusive license for the IP portfolio from the Univ of Erlangen, and searches for investor funding to bring KT1 to a first clinical trial to be led by oncologists from the Univ of Erlangen. A first scientific advice visit with the Paul Ehrlich Institute (regulatory authority) has taken place. For the further clinical development of KT1, Kosmas plans to continue the collaboration with Univ Med Centres Erlangen and Kiel, the AML study group Studien-Allianz Leukämie (SAL), two teams from Wash U St Louis, and the US Leukemia & Lymphoma Society.
Name ›
Address/P.O. Box ›
Postal Code/City › Country › Contact Person › Telephone › Fax › Email › Website › Social Media ›
Number of Employees › Founded (year) › Type of Company › Areas of Activity ›
Maiwald Intellectual Property
Elisenstraße 3, Elisenhof 80335 Munich Germany Dr Eva Dörner
+49-89-747266-0
+49-89-776424 info@maiwald.eu www.maiwald.eu
I Q
270 1995 Law Firm
Sectors:
| Pharma & Biotech
| Organic Chemistry & Polymers
| Inorganic & Construction Materials
| Food & Agriculture
| Electrical & Mechanical Engineering
| Communication & Information Technology
| Mobility & Energy
| Displays & Light
| Measuring & Process Technology
| Medical Technology & Imaging
| Artificial Intelligence & Digitalization
Legal Areas:
| Patents & Utility Models
| Supplementary Protection Certificates
| Trademarks & Designs
| Copyright
| Competition & Antitrust Law
| Compliance
| Contract Law
| Employee Invention Law
| Pharmaceutical Law
| Data Protection
| Plant Variety Protection
| UPC
Strategy:
| IP Search
| IP Consulting
As one of Germany’s largest and best-known firms in the field of intellectual property, Maiwald’s foremost aim is to achieve the best possible solution for each client in each particular case. Maiwald employs about 270 people working out of Munich and Düsseldorf. The interdisciplinary teams work closely with each other and with foreign associates, always with an eye to the client’s particular needs, whether start-up, medium-sized firm, or large corporation, across all industrial sectors. Maiwald’s team does its utmost to ensure that all IP matters are handled with competence and care, irrespective of the client’s physical location, whether in Germany, Europe, or beyond.
Client-oriented solutions and personal consultation are at the heart of Maiwald’s professional approach. Our teams comprising 110 highly qualified patent attorneys, attorneys-at-law and technical experts, including search specialists, devote their extensive skills and experience to providing clients with optimal customized IP solutions on a daily basis. Teams of paralegals with client-specific competencies address individual administrative needs. Client satisfaction is evidenced by the long-standing business relationships with many prestigious corporations.
Main areas of practice
Maiwald advises and represents domestic and international clients from all technical fields across the entire spectrum of intellectual property law. When appropriate, an interdisciplinary exchange of information and expertise can take place between various teams, ensuring that the optimal solution is achieved in each particular case.
Maiwald’s patent attorneys
Maiwald’s team of patent attorneys combine their expertise across various technical fields and advise and represent clients across a wide range of industries.
Maiwald’s patent attorneys have extensive experience in the management of international patent portfolios.
Services include drafting and filing patent applications and coordinating worldwide prosecution as well as defending, enforcing, and contesting IP rights. Patent attorneys also prepare freedom-to-operate (FTO) opinions based on thorough research and analysis to establish whether existing IP rights of third parties could potentially stand in the way of a client’s particular product or manufacturing process. Clients also benefit from the exceptional expertise in opposition and appeal proceedings before the German and European Patent Office as well as in infringement and nullity proceedings before the national courts.
Maiwald’s attorneys-at-law
Maiwald’s team of attorneys-at-law can boast many years of experience in trademark, design, copyright, and contract law as well as patent infringement litigation, particularly in the conduct and coordination of international litigation proceedings.
Close cooperation between patent attorneys and attorneys-at-law is especially important in patent infringement litigation, since this type of proceedings generally calls for a comprehensive overview of the various national regulations as well as a thorough grasp of the particular technology. The interdisciplinary teams provide a comprehensive package of services to guarantee the successful conduct of legal proceedings in an international arena, also in cases where speed is of the essence, such as in preliminary injunction proceedings or border seizure procedures.
However, Maiwald’s attorneys-at-law would be the first to acknowledge that litigation is not always the best solution. They are skilled advocates in arbitration and mediation proceedings and are trusted experts when it comes to negotiating licensing contracts

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media › Number of Employees › Founded (year) ›
Areas of Activity ›
Medicon Valley Alliance
Arne Jacobsens Allé 15, 2 2300 Copenhagen Denmark
David Munis Zepernick, Director Member Engagement and Communication +45-2498-1668 dz@mva.org https://mva.org/ I 14 1997
Events, networks, business development, your gateway to the binational Danish-Swedish Medicon Valley region
Medicon Valley – EU’s largest life sciences region
Medicon Valley is the bi-national life sciences cluster spanning the island of Zealand in Eastern Denmark and the Skåne -region of Southern Sweden, with more than 1150 life sciences companies and more than 65,000 privately employed life sciences professionals.
Medicon Valley is the crucible of Scandinavian life sciences. Located at the gateway to Denmark and Sweden it has a vibrant ecosystem and deep talent pool underpinned by world-class life sciences universities and research infrastructure.
Set in a competitive business environment with the Scandinavian quality of life close at hand, Medicon Valley is an attractive location for both businesses and people.
Scandinavian innovation is globally recognised, and our life sciences reflect this. Within Medicon Valley we have a rich life sciences heritage and pioneering spirit that continues to attract many successful companies. Companies like Novo Nordisk, Ferring, Genmab, Baxter Gambro, McNeil AB, PolyPeptide, and recently merged Novozymes and Chr. Nansen – now Novonesis – are representative, but so too are the many smaller and medium-sized innovative Danish and Swedish life sciences companies that continue to energise the area. Many of them choose to become members of the regional network organisation Medicon Valley Alliance and help strengthen the regional life sciences cluster. Furthermore, Medicon Valley is home to five universities, which supply life science-related education, and research facilities such as MAX IV Laboratory and European Spallation Source.

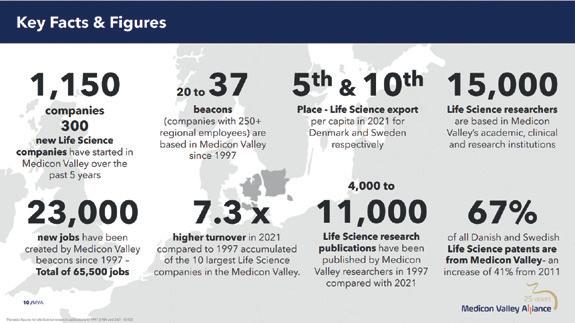

MEDICON VALLEY ALLIANCE
Medicon Valley Alliance
Founded in 1997, Medicon Valley Alliance (MVA) is today a non-profit membership organisation in the Danish-Swedish life sciences cluster Medicon Valley. Our 300+ members – including more than 60 biotech companies – represent the region’s triple helix and include universities, hospitals, human life sciences firms, regional governments, and service providers. We exist to develop Medicon Valley into the leading life sciences cluster in Northern Europe and make the region an attractive destination for the best talent within the field. We create value for our growing number members from both inside and outside the Nordics by launching and driving initiatives that put Medicon Valley firmly ahead in the global race for talent, and we connect, develop, and promote scientific strongholds in the region.
On average MVA hosts and organises 30+ annual network meetings, seminars, conferences, and other events, which serve as meeting and market places for member companies and organisations both within and outside the region or with an interest in exploring the potential of Medicon Valley and reaching out to potential new partners and customers.
Learn more at www.mva.org
Introduce yourself and your expertise to the Medicon Valley region
As a member of Medicon Valley Alliance you can introduce yourself and your company’s expertise to the region using the MVA Good Morning Meeting concept, which allows you to co-organise awareness-raising events with MVA at the reduced member price. MVA Good Morning Meetings are typically 1½ hour events – online or physical – where you get the opportunity to demonstrate your expertise in problem-solving in an educational manner to a relevant audience of potential partners and customers. These events are typically hosted and marketed by MVA, and a qualitatively and quantitatively successful turnout is practically guaranteed.
Learn more on www.mva.org/events/good-morningmeetings/

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website › Social Media ›
Number of Employees › Founded (year) › Type of Laboratory › Areas of Activity ›
Microcoat Biotechnologie GmbH
Am Neuland 3 82347 Bernried Germany
Dr Ingrid Wanninger (Business Development)
Dr Michael Kroder (Business Development)
+49-8158-9981-0
+49-8158-9981-10 info@microcoat.de www.microcoat.de I
> 200 1992
S1/S2, GLP, GCP, GMP, ISO 9001, EN ISO 13485 ISO 14001
| Contract manufacturing | Custom development | Laboratory services | Endotoxin and pyrogen testing service
External › Collaborations Request for › Further Collaborations
Business-to-business with industrial partners in the pharmaceutical industry, diagnostic industry and life science industry
Microcoat seeks joint projects and service partnership with pharmaceutical, biotech and diagnostic companies, as well as with players in the field of food safety testing, crop science and instrumentation (medical devices).
Company profile
Microcoat offers a wide range of individual and specialised services for the diagnostic and pharmaceutical industry. In close cooperation with our customers, we aim for best performance, building on a complete range of advanced technologies and uncompromised quality standards.
For pharmaceutical companies, we leverage our profound expertise in in-vitro diagnostics and assay development in order to become a preferred partner in early development, pre-clinical and clinical phases of drug development and patient sample testing.
For food safety, environmental testing and other specialised diagnostic fields, Microcoat aims to be the preferred OEM-partner for the development and manufacturing of reliable test components and kits.
Contract manufacturing diagnostics
Microcoat is an approved original equipment manufacturer (OEM) for diagnostic test kits, bulk and finished components. We manufacture according to ISO and IVD standards. Since all critical test components are produced and modified in house, we are able to control quality at all stages of the production process.
Production technologies:
› Fermentation (30 litre scale)
› Downstream processing
› Protein chemistry
› Microplate and particle coating
› Dispensing/filling/labelling
› Freeze-drying
› Kit assembly
Product categories:
› Coated microplates
› Lateral flow test strips
› Recombinant proteins/antigens
› Antibody/protein conjugates
› Liquid components
› Dried/lyophilized components
› Diagnostic kits

B2B Diagnostics





Custom development diagnostics
Microcoat offers unique expertise in diagnostic assay and product development. A broad spectrum of methods, skilled staff and intensive communication with the customer are the cornerstones of successful project execution.
Development projects are based on detailed milestone plans and follow standardised development guidelines through the five project phases (feasibility, development, verification, validation and manufacturing).
We develop for you:
› Immunological assays
› Molecular assays
› Sample and matrix preparation protocols
› Depletion protocols
Functional Service Provider Laboratory services
In our certified facilities, we conduct a broad spectrum of GLP/GCP services to support drug development starting from early discovery to post-marketing surveillance. As an independent contract research organisation and preferred provider we serve pharma and life science industries worldwide. Based on long lasting experience in endotoxin and pyrogen testing, Microcoat offers a set of proprietary methods and skilled scientific personnel for GMP release testing as well as non-routine projects. Services are run as flexible customer-specified projects including the search for root causes, exploration of realization alternatives, development of product-specific adaptions and GMP compliant validation of newly established methods.
Our services include:
› Assay development and validation
› Biomarker testing
› Immunogenicity/PK assays
› Endotoxin and pyrogen testing
Business model
Microcoat is focused on business-to-business with industry customers, seeking a reliable and long-lasting partnership for outsourcing projects such as contract manufacturing (OEM), assay and product development, and bioanalytical testing in the context of pre-clinical and clinical studies.
Name ›
Subsidiaries ›
Microsynth AG
Microsynth Seqlab GmbH
Microsynth France SAS
Microsynth Austria GmbH Ecogenics GmbH
Address/P.O. Box › Postal Code/City › Country ›
Contact Person › Telephone › Email › Website ›
Founded (year) › Type of Laboratory ›
Areas of Activity ›
Schützenstrasse 15 9436 Balgach Switzerland
Dr Markus Schmid, Christof Wunderlin (Co-CEOs)
Dr Christoph Grünig (Head of Contract Research)
Johanna Brändli (Head of Quality Management) +41-71-722-83-33 info@microsynth.ch www.microsynth.com www.ecogenics.ch
1989
S1/S2, GMP/GLP
| Oligonucleotide synthesis
| Sanger sequencing
| Oxford nanopore sequencing
| Illumina sequencing
| DNA/RNA isolation
| PCR, qPCR, digital PCR
| Genotyping
| Contract research/outsourcing
Contract Research ›
| Assay development
| Assay validation (ICH guidelines)
l GxP analysis of customer test items (Sanger, NGS, qPCR & dPCR)
| Process outsourcing
| Genomics research
The Company
Microsynth is a leading European company in the field of nucleic acid synthesis and analysis. Its main activities involve oligonucleotide synthesis, DNA/RNA analysis and sequencing, as well as contract research. For over three decades, the company’s goal has been to serve its customers by delivering products and services of the highest quality, on time, while offering outstanding service – and all this at competitive prices. Microsynth has subsidiaries in Germany (Microsynth Seqlab GmbH), France (Microsynth France SAS), Austria (Microsynth Austria GmbH), and Switzerland (Ecogenics GmbH). In total, Microsynth employs a staff of more than 130 people.
Oligonucleotide Synthesis
Microsynth is a premier expert in oligonucleotide manufacturing, providing solutions for diverse applications. From research primers to diagnostics and therapeutic oligos (ASOs, siRNAs) for drug discovery and preclinical testing, we meet your needs. Choose from various backbones (DNA, RNA, 2’-MOE, 2’-OMe, LNA, PTO), >250 modifications (MGB, GalNac, Palmitate), and purification options. Backed by decades of expertise, we drive innovation through technology and protocols. High-throughput platforms and rigorous processes ensure quality, while skilled chemists optimize strategies. Whether it’s a specific project or complex demands, trust Microsynth for tailored oligonucleotide solutions.
DNA/RNA Analysis
With over 30 years of experience, Microsynth is a leading provider of DNA Sanger sequencing services in Europe. Our strategically located Sanger sequencing laboratories in Switzerland, Germany, France and Austria enable efficient and environmentally friendly sample collection. Our commitment to innovation is exemplified by Ecoli NightSeq ®, a groundbreaking service offering faster and more cost-effective E. coli plasmid sequencing. Microsynth’s expertise extends beyond Sanger sequencing. We embrace next-generation sequencing technologies and offer comprehensive support for Illumina and ONT platforms. Our services cover a wide range of applications, from DNA and RNA sequencing
Analytical Testing of Novel Biological Drugs

Key Focus Areas
• Advanced Therapies: Cell and Gene Therapies
• Vaccines: mRNA and DNA Vaccines
• Biologics: Cell Bank Characterization
Overview of Our Analytical Portfolio
• Identity Testing: Sequence and Strain Identity
• Copy Number Analysis: Vector and Transgene Copy Number (VCN)
• CRISPR Analysis: Translocations, On-Target-Assays, and Off-Target- Assays
Key Certificates
• ISO 13485:2016
• EN ISO 17025:2017
• GMP certified (Swissmedic) for testing of TpP/TP/GMO
for various organisms to plasmids and cosmids. We accommodate projects of all sizes, from single loci to complex metagenomic studies. Our commitment to excellence doesn’t end with data generation; our customised bioinformatics pipelines and comprehensive support ensure accurate and user-friendly results. In addition to sequencing, we excel in nucleic acid isolation, PCR (from classic to digital), fragment length analysis, and genotyping by sequencing.
Contract Research
Over the past decade, Microsynth has evolved into a globally renowned contract research organisation, offering cGMP services in assay development, qualification, validation, and sample testing. Our team has extensive knowledge and experience working with scientists, QA/QC professionals, and project managers from top notch pharma and biotech companies to achieve critical milestones cost-effectively and on time. Particularly, in the burgeoning domain of advanced therapy medicinal products, including gene, cell, and RNA therapies, we have carved a distinctive niche. Here, we have excelled in the development and application of nucleic acid-based analytical methods, establishing a noteworthy reputation.
Quality Management System
Microsynth prioritizes continuous production process enhancement with a focus on regulatory compliance. We have ISO 9001:2015 certification across all branches. Moreover, our NGS, Sanger, and fragment length analysis (FLA) departments at Balgach and Vienna (Sanger and FLA) are ISO/IEC 17025:2017 accredited (STS 0429), showcasing our commitment to precision. All our genetic analyses platforms are certified by Swissmedic as GMP-compliant for quality control (chemical, physical, biochemical and biological) of medicinal products as contract laboratory. The scope includes transplant products (TpP), gene therapy drugs (GT), as well as medicinal products involving genetically modified organisms (GMOs) or containing GMOs, all of them with intended use in humans. Lastly, we maintain ISO 13485:2016 certification for production and distribution of nucleic acids and components for medical use and provision of associated activities.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website ›
Number of Employees › Founded (year) › Type of Laboratory
Miltenyi Biotec
Friedrich-Ebert-Straße 68 51429 Bergisch Gladbach Germany
Svea Lübcke
+49-2204-8306-0 MACSsales@miltenyi.com www.miltenyibiotec.com www.miltenyibioindustry.com
4600 worldwide 1989
| S1/S2 | L1/L2 | cGMP
| clean room facilities A/B/C/ | fully automated laboratory (collaboration with Tecan® Group Limited)
Areas of Activity ›
| Sample preparation
| Cell separation
| Flow cytometry
| Cell sorting
| Cell culture and stimulation
| Spatial biology
| Imaging and microscopy
| Lentiviral vector transduction
| Cell analysis
| Cell processing
| Therapeutic apheresis
| Cryopreservation
| Cellular therapy
| Lab automation
External › Collaborations
Customised Applications
Check out our collaborations on our website https://www.miltenyibiotec. com/DE-en/about-us/partners-andcollaborators/grant-projects.html
Miltenyi Biotec is a global provider of products and services that empower biomedical discovery and advance cellular therapy. Our innovative portfolio enables scientists and clinicians to obtain, analyse, and utilise the cell. Our technologies offer solutions for cellular research, cell therapy, and cell manufacturing. Our 35 years of expertise spans research areas including immunology, stem cell biology, neuroscience, cancer, hematology, and graft engineering. In our commitment to the scientific community, we also offer comprehensive scientific support and expert training. Today, Miltenyi Biotec has 4,900 employees in 24 countries.
MACS ® Sample Preparation
Start smart with innovative solutions for your tissue preparation. gentleMACS™ Dissociators and MACS ® Tissue Dissociation Kits enable standardised sample preparation from almost any tissue source.
MACS ® Technology
MACS Technology is the gold standard in manual and automated immunomagnetic cell separation with tens of thousands of publications proving its power in biomedical research and clinical settings. Frequently occurring cells, rare cells, and specific cell subsets can be isolated from blood or tissue samples, from virtually any species.
MACS ® Flow Cytometry
The MACSQuant ® Instruments include ultra-compact flow cytometers and the MACSQuant ® Tyto ®, a microchip-based cell sorter, and provide unrivalled ease of use for multisampling and multiparameter analysis and sorting. Our large portfolio of antibodies, including REAfinity ® Recombinant Antibodies and Vio ® Dyes, comprise a family of conjugates with high fluorescence intensities and stain indices.




MACS ® Imaging and Spatial Biology
The MACSima™ Platform, powered by MICS (MACSima Imaging Cyclic Staining) technology, enables researchers to fully explore spatial biology with its advanced protein multiplexing capabilities. The UltraMicroscope Blaze™ Platform allows automated 3D imaging at a cellular level, from rodent organs or organoids to samples as big as a human kidney or whole mouse models. To complement these technologies, we offer a growing collection of recombinant antibodies that have been pre-tested for fluorescence microscopy.
MACS ® Cell Culture and Stimulation
Our cell culture media, cytokines, growth factors, and reagents for stimulation, expansion, and differentiation of a variety of mammalian cells are also available up to GMP grade, thus enabling GMP-compliant, cellular product manufacturing.
Clinical products and services
Miltenyi Biotec’s comprehensive portfolio for advancing translational research and therapy includes the FDA approved CliniMACS CD34 Reagent System as well as a range of CliniMACS Reagents for cell separation and processing and MACS GMP products for cell culture and stimulation. The CliniMACS Prodigy ® platform is a unique cell processing solution that enables the fully automated and integrated manufacture of a GMPcompliant cell product in a closed environment. Miltenyi Bioindustry is our global CDMO service department helping biopharmaceutical companies and advanced clinical centres from process development into commercial scale, GMP-compliant manufacturing of viral vectors and cell and gene therapies. The CliniMACS Cell Factory ® facilities are located across North America, Europe, and China and in close proximity to customers.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees › Founded (year) ›
Areas of Activity › Molecular Devices
660-665 Eskdale Rd, Winnersh, Triangle Wokingham RG41 5TS
United Kingdom Janet Graystone +44-118-944-8000 infoboxeu@moldev.com www.moleculardevices.com
F I
1,000+ 1983
We are the innovation partner that empowers scientists with nextgeneration technology to advance discoveries, improving the quality of life everywhere.
| Cell Line Development
| CRISPR Gene Editing | Drug Discovery | 3D Biology | Laboratory Automation
Automating Colony Picking for Faster, More Reliable Research
Advancing
scientific discovery through automation
For over 40 years, Molecular Devices has been at the forefront of laboratory automation, providing innovative solutions that accelerate research and discovery. Our QPix® Microbial Colony Pickers offer a powerful, highthroughput solution for scientists in biotechnology, synthetic biology, drug discovery, and microbiome research. By integrating intelligent imaging, precise automation, and robust data tracking, QPix ensures efficient and reproducible microbial screening and selection.
Improving microbial and mammalian screening efficiency
Colony picking is a critical step in microbial strain development, antibody discovery, and protein engineering. Traditional manual methods are time-consuming and prone to errors, leading to inconsistencies in research outcomes. QPix systems streamline this process by automating colony detection, selection, and picking based on customisable parameters such as morphology, fluorescence, and growth rate. This ensures optimal colonies are selected for downstream applications, reducing time-to-result and increasing experimental reproducibility.
Enhancing workflows for drug discovery & synthetic biology
As biopharmaceutical companies and research institutions scale up their discovery efforts, high-throughput automation becomes essential. The QPix platform enables researchers to process thousands of colonies per hour, dramatically reducing bottlenecks in workflows such as CRISPR screening, monoclonal antibody development, and phage display. With integrated data management and electronic traceability, QPix supports regulatory compliance and ensures reproducibility across experiments.

Key benefits of QPix microbial colony pickers
› High-throughput automation: Process up to 30,000 colonies per day with precision and efficiency.
› Advanced imaging & selection: Identify and select colonies based on size, morphology, fluorescence, or growth characteristics.
› Customisable workflows: Adapt to various research applications, from synthetic biology to clinical microbiology.
› Regulatory & data compliance: Ensure traceability and reproducibility with integrated electronic data tracking.
› Seamless integration: Compatible with a wide range of laboratory automation platforms for full workflow optimisation.
Partnering with Researchers Worldwide
Molecular Devices is committed to advancing scientific research through automation. Our expertise in laboratory automation, combined with a deep understanding of the needs of biopharma, biotech, and academic researchers, enables us to deliver solutions that drive meaningful discoveries.

Name ›
Address/P.O. Box › Postal Code/City › Country › Contact Person › Telephone › Fax › Email › Website ›
Number of Employees › Founded (year) › Type of Laboratory ›
Neovii Biotech GmbH
Am Haag 6+7 82166 Gräfelfing Germany
Sebastian Hofmann +49-89-898888-0 +49-89-898888-16 info.germany@neovii.com www.neovii.com 120 2003
BioStoffV S3** cGMP certified production facilities, EU (Annex 1 EU-MP guideline) up to grade A respectively ISO5 (according to EN-ISO 14644-1)
The company
The company Neovii Biotech GmbH – as part of the Neovii Group – is dedicated to delivering targeted biopharmaceutical treatment options in transplantation medicine and haematological oncology. The focus of its activities is the development and commercialisation of immunologically active biopharmaceutical therapeutics based on innovative antibody technologies. All the manu facturing activities are located in Gräfelfing/ Germany.
Neovii has a presence in more than 40 countries worldwide.
For further details please visit: www.neovii.com
Research and Development
Areas of Activity › External › Collaborations
| Transplantation Medicine | Haematological Oncology
Academic research institutions and other companies
Neovii supports research and development activities in the fields of solid organ transplantation, stem cell transplantation, and immune and haemato-oncological disorders. Neovii actively seeks in-licensing and acquisitions opportunities. We are looking to expand our portfolio of products with novel life-transforming therapies that address severe unmet medical needs, in particular in the areas of transplantation, haemato-oncology, and immune disorders.
Areas of interest
Since its inauguration Neovii Biotech has been manufacturing and commercialising a medicinal product for the prevention of graft-versus-host-disease after stem cell transplantation and prevention/treatment of acute organ rejection following solid organ transplantation.


The main fields of our operations are:
› Stem Cell Transplantation
› Solid Organ Transplantation
› Aplastic Anaemia
› Autoimmune Diseases
Transplantation: ATLGs (Anti-human T-lymphocyte immunoglobulins) – polyclonal antibodies
ATLGs are antibody-based medicines for the targeted suppression of immune responses (immunosuppressants).
They suppress immune responses by multiple mechanisms, such as immune cell depletion, inhibition of T-cell function, and induction of regulatory immune cells. Their immunosuppressive properties are used mainly in solid organ transplantation and stem cell transplantation. In stem cell transplantation (SCT) polyclonal antibodies are indicated for the prevention of graft-versus-hostdisease (GVHD).
GVHD is recognised as a severe complication following SCT that negatively impacts on the patient’s quality of life and is a major cause of morbidity and mortality. In GVHD, transplanted immune cells attack the recipient’s body, causing tissue and organ damage. By suppressing these immune reactions, ATLGs significantly lower the incidence of GVHD (Finke J, et al‚ Lancet Oncot 2009;10(9)1855—64).
In solid organ transplantation (SOT), polyclonal antibodies are indicated either for the prevention of organ rejection or to suppress active, acute rejection reactions. The rejection of a transplanted organ is primarily caused by immune cells of the recipient that start attacking the grafted organ, because it is recognised as foreign. By suppressing these immune reactions, ATLGs limit organ damage and prevent graft loss (Kaden J, et al. Ann Transplant. Jan 10 2013;18:9-22).
ID-Number: ATM.00003-2.0

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website › Social Media ›
Founded (year) ›
New England Biolabs GmbH
Brüningstr. 50 – Geb. B852 65926 Frankfurt am Main Germany Dr Carsten Lanwert
+49-69-305-23140
+49-69-305-23149 info.de@neb.com www.neb-online.de F I
I 1981
NEB – Passion for Science
Created by scientists for scientists, New England Biolabs is proud to be a world leader in the discovery and production of enzymes for molecular biology applications. Since our establishment in 1974, we have remained committed to developing high quality, innovative products that not only empower your research but also our own. Our profits have always funded an extensive research program, which we believe is critical for staying connected to our customers and helping to drive scientific breakthroughs. From our founding principles to our unique corporate culture, NEB’s philosophy can be distilled down to three core values: passion, humility and being genuine.
Enzyme Technology Innovations
Our outstanding expertise in protein engineering and evolution has led to the creation of unrivalled enzymes and unique workflows in the library preparation and target enrichment for the NGS space. These products set the benchmarks for optimal performance and ease-of-use in modern molecular biology laboratories. Moreover, NEB’s specialised offerings for alternative DNA assembly, isothermal amplification as well as novel reagents for CRISPR/Cas9 based genome editing makes us a first- choice supplier for today’s molecular biologists.
Customised Solutions/GMP –Grade & Lyophilisation
NEB’s customised solutions unit has been delivering tailor-made enzyme formulations and packaging solutions to biotech, pharma and diagnostic customers for over 30 years. Building on this extensive experience, our GMP-grade manufacturing capabilities offer a range of customised products at scale, enabling commercial customers and partners to use NEB’s world- class products in their specific applications and to support them in their efforts to access regulated markets. The recently established subsidiary NEB Lyophilisation Sciences combines expert knowledge in the design, development and manufacture of innovative enzyme solutions for ambient store products to meet our customers small- to large-scale lyophilisation needs.
THE BRIDGE TO YOUR SUCCESS
Customized Enzymes and Reagents from New England Biolabs
Largest selection of enzymes for genomic research

Research-grade and GMP-grade production
Large to small scale lyophilization capabilities
ISO 9001 and ISO 13485 certified
Formulations without components such as BSA, detergents or glycerol
Enzymes at user defined concentrations
Custom aliquoting, kitting, packaging & private label (OEM)
www.neb-online.de
ISO Certified Quality
NEB is dedicated to providing research products of the highest quality. Our integrated quality management systems are certified to meet the requirements according to the standards ISO13485 and ISO9001.
Social Responsibility & Sustainability
While our passion for science helps us to drive discovery, we continue to be guided by our responsibility to each other and our community. We continuously strive to promote ecologically sound practices and environmental sustainability in order to protect our natural resources. As an additional measure, NEB has received ISO14001 certification, a quality standard for environmental management systems. We also recognise that we must work together to build a more equitable society and improve diversity, equity and inclusion in our workplace.
Basic and Applied Science
At NEB, over 30 labs participate in research projects, which are aided by post-doctoral fellows and students in Masters and Ph.D. programs. NEB researchers have authored or co- authored over 1,450 publications to date. With a dual focus on basic and applied research, NEB’s culture is both collaborative and academic.
Scientific Customer Service
NEB always was conceived to be a part of the scientific community by providing top quality tools and experimental expertise. This philosophy has continued to the present day. Our support model is unique as it utilises technical support scientists, scientist responsible for product development or manufacturing, and product line experts. As such, customers are supported by scientists and often experts in the product or its application.
New England Biolabs GmbH
The NEB subsidiary in Germany represents the service hub for Central Europe. From our location in Frankfurt, we serve scientists and industry customers in Germany, and Austria and support our network of distribution partners across Europe.
For further information please visit our website at www.neb-online.de.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees › Founded (year) › Areas of Activity › External › Collaborations
Northway Biotech
Mokslininkᶙ 4 & Melkio 7 Vilnius Lithuania Prof. Vladas Algirdas Bumelis (CEO) +370-5-210-2247 bd@northwaybiotech.com www.northwaybiotech.com
180-200 (Vilnius sites) 2004
Biopharmaceuticals, Monoclonal Antibodies, Recombinant Therapeutic Proteins, plasmid DNA, Gene Therapy, Viral Vectors, Contract Development, Contract Manufacture, cGMP Manufacture, Analytical Services/ Testing
Northway Biotech cooperates with numerous biotech companies and also with some medium-sized companies and three Big Pharma companies.
A Rapidly Expanding End-to-End Biopharmaceutical CDMO
Northway Biotech is a contract development and manufacturing organisation (CDMO) specialising in biologics and gene therapy, with two out of three facilities located in Lithuania, an EU member state recognised as an emerging hub for biotechnology innovation. The company possesses extensive expertise encompassing the entire biopharmaceutical value chain, providing services that range from cell line development to clinical and commercial production of drug substances and drug products, including antibodies, recombinant therapeutic proteins, plasmid DNA (pDNA), and viral vectors. Throughout its 21-year history, Northway Biotech has partnered with over 95 clients and successfully completed more than 180 projects. These initiatives encompass a wide range of activities, from gene cloning to the manufacturing of final drug products, including numerous technology transfer projects across various clinical phases.
In addition to its second biologics facility in the Greater Boston Area, Northway Biotech is leading the development of Bio City in Vilnius – an ambitious biotechnology campus exclusively created by the company. The total project potential will include two research and experimental development centers and six production buildings. The first gene therapy CDMO centre opened in September 2024. The investment in the entire biotech city is expected to reach around €7bn over the next decade.
Biologics and Gene Therapy CDMO Services
Cell Line Development Capabilities:
› Single-cell cloning with analytical confirmation and stability studies for the development of mammalian cell lines
› Development of bacterial and yeast strains with stable expression
› Full microbial & mammalian cell bank characterisation
Upstream Process Development:
› Optimisation of media and feeding strategy at lab scale
› Small-scale qualification and process characterisation studies




› DOE experiments for yield and quality improvement
› Scale-up and confirmation runs (up to 200 L SUB)
Downstream Process Development:
› Harvesting (centrifugation, depth-filtration) and cell disruption
› Isolation and solubilisation of inclusion bodies followed by refolding of target protein
› Protein purification at different scales using various chromatography techniques
› Small-scale qualification and process characterisation studies
› Rapid technology transfer and scale-up to cGMP manufacturing scale
Analytical and Formulation Development Capabilities:
› Development, qualification, and validation of physicochemical, biochemical, and immunological analytical methods and cell-based bioassays according to ICH guidelines
› Characterisation, monitoring, and optimisation of products’ quality attributes across all stages of development
› Release and in-process control testing
› Formulation development services for liquid and lyophilised drug product in vials and pre-filled syringes
› Stability studies
cGMP Manufacturing:
› GMP certified facilities
› Master and Working Cell Bank (MCB and WCB) manufacturing for microbial and mammalian cell cultures
› Microbial fermentation up to 3,000 L working volume in stainless steel reactors
› Mammalian cell culture up to 4,000 L working volume in single-use reactors
› Viral vectors manufacturing up to 200 L working volume in single-use reactors
› Plasmid DNA production for gene therapy up to 300 L working volume in single-use reactors
› Fill and finish of sterile drug production into vials and pre-filled syringes (PFS)
› Lyophilisation of aseptic drug product into vials

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website › Social Media ›
Number of Employees › Founded (year) › Areas of Activity ›
Nova Biomedical Corp
200 Prospect Street
Waltham, MA 02454-9141
United States
Matt McRae
+1-781-894-0800
+1-781-894-5915 info@novabio.com novabiomedical.com F I Q 1,500 1976
Cell culture analysis, bioprocess monitoring and optimisation, bioprocess automation, in-vitro diagnostics, point-of-care testing, blood gas analysis, critical care, glucose reference
Innovation empowering life science
Nova Biomedical develops, manufactures, and commercialises analytical systems for cell culture and whole blood testing. The company is headquartered in Waltham, MA, USA. Worldwide, more than 1,500 people are employed by Nova, which operates direct subsidiaries in 11 countries and factory-trained distributors in more than 110 countries.
Our commitment to scientific innovation and product quality has made Nova Biomedical a world leader in the development of whole blood analyzers for clinical applications and cell culture/fermentation analyzers for biopharma for more than 40 years.
Cell Culture Analysis
GMP-compliant cell culture analysis made easy Nova offers the latest in maintenance-free cell culture analyzer technology – BioProfile ® FLEX2. FLEX2 provides fully automated analysis of key chemistries and gases, cell density, cell viability, and osmolality with one sample and a single data output stream in under 4.5 minutes. The full 16- test menu includes: Gluc, Lac, Gln, Glu, NH4+, Na+, K+, Ca++, pH, PCO2, pO2, total cell density, viable cell density, viability, cell diameter, and osmolality.
The system can be flexibly configured to meet diverse and changing needs with the optional and field upgradeable pH/gas, CDV and osmolality modules. sampling from 96-well plates, syringes, or a 24-position external “load-and-go” sample tray provides maximum workflow flexibility and efficiency for cell culture monitoring.
FLEX2 is the only cell culture analyzer designed for online sampling from micro-scale vessels through large-scale commercial manufacturing SIP/CIP systemsand integration with the ambr ® 15 and 250 cell culture systems. An optional Sample Retain Collection system automatically collects cell culture samples from the On-Line Autosampler and stores them in a refrigerated environment to enable further offline testing. IQOQ support can be offered by the local Nova representative.




BioPro fi le Analyzers for Fermentation
BioPro fi le 300 series analyzers offer a total of nine measured tests, including glucose, lactate, phosphate or glycerol, acetate, ammonium, pH, sodium, and potassium, plus calculated osmolality. The analyzer is offered in two versions: the BioPro fi le 300A which includes a phosphate assay, and the BioPro fi le 300B with glycerol testing. These systems offer measurement ranges speci fi cally suited to monitor bacterial and yeast cultures. A conversion kit is available for easy interchange between the 300 A and B versions.
BioProfile FAST CDV analyzer
BioProfile FAST CDV is a high-throughput, fully automated viable cell density and viability analyzer with a throughput of 45 samples per hour on 100µL of sample volume. The analyzer performs all sample dilutions internally, enabling cell culture samples up to 140e6 c/mL to be analysed without any external sample dilution. Cell culture samples can be analysed via the external 32 position load-and-go tray or via an innovative 96-well plate option.
BioProfile FLEX2 Basic Analyzers
FLEX2 Basic analyzers provide multiple test menus for chemistry analysis in cell therapy, gene therapy, vaccine development, and alternative foods applications. The FLEX2 Basic A test menu consists of Gluc, Lac, Gln, Glu, NH4+, Na+, K+, Ca++, pH, PCO2, pO2 with automatic dilutions and an extended range. The FLEX2 Basic B provides the same test menu with standard ranges. Flex2 Basic C offers an 8-test menu of Gluc, Lac, Na+, K+, Ca++, pH, PCO2, pO2 with standard ranges.
Glucose Reference Analyzer
Nova Primary Whole Blood Glucose Reference Analyzer
Nova Primary fills the need for a replacement to the discontinued YSI STAT PLUS 2300 Glucose and L-Lactate analyzer. Nova Primary uses a single, reusable glucose electrochemical sensor based on glucose oxidase and has a measurement range of 20-900 mg/dL. It uses a small, 25 µL whole blood or plasma sample and provides results in under 2 minutes.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees › Founded (year) ›
Areas of Activity ›
Numaferm GmbH
Merowingerplatz 1a 40225 Dusseldorf Germany
Dr. Roland Hecht
+49-211-97532900 info@numaferm.com numaferm.com I 25+ 2017
Peptides, Proteins, Biotechnology, Biochemistry, Biologics, R&D, Recombinant Microbial Expression, Strain- and Process Development
Numaferm GmbH, founded in 2017 and based in Düsseldorf, Germany, is a leading biotechnology company focused on strain and process development services for peptide and protein production. To achieve this, Numaferm developed innovative biochemical platforms which offer scalable, cost-effective, and high-quality biomolecule production that meets both R&D and cGMP standards. Our processes are completed within weeks, and we develop sustainable approaches that enable commercial-scale production.
Our Technologies
Numaferm has developed a range of advanced biochemical platforms for discovery, development and production of peptides and proteins:
› Numasec™ – The first secretion technology for gramnegative bacteria. Numasec™ efficiently transports peptides and proteins out of E. coli cells, achieving titers exceeding 30 g/L and initial purities above 95%.
› Numaswitch™ – A biochemical production platform using Switchtags enhances protein expression in E. coli as inclusion bodies. It enables efficient refolding and seamless protein release via enzymatic cleavage (e.g., Numacut TEV protease), overcoming refolding bottlenecks for high yields and purity.
› Numascreen™ – A phage display library featuring over 1 billion peptides enable the discovery and optimisation of high-affinity binders with unique properties.
Our Services
Numaferm provides tailored solutions for peptide discovery, strain and process development, and scale-up to commercial production:
› Cell Line Development – Engineering and optimising microbial strains for peptide and protein expression.
› Process Development – Developing and optimising production workflows to maximise yield, enhance quality, and minimise costs.
› Peptide & Protein Production – Scalable, cost-effective, and sustainable production using Numaswitch™ or Numasec™ technologies.




› Commercial & cGMP Supply – We collaborate with Contract Development and Manufacturing Organisations (CDMOs) worldwide to produce peptides and proteins at commercial and GMP-compliant scales.
› Lead Discovery – Identifying lead candidates with high affinity and specificity for your target molecules using Numascreen™.
Our Product
At Numaferm, we drive innovation through both our services and product development. Our Numacut TEV protease is an advanced variant of the Tobacco Etch Virus (TEV) protease offering improved stability profiles, solubility, and cleavage efficiency. In addition, through artificial intelligence and directed evolution approaches, we have expanded its recognition sequence from ENLYFQ↓S to ENLYFQ↓X (where X represents any amino acid except P). This considerably enhances substrate tolerance, allowing seamless cleavage of any N-terminal target peptide or protein from its fusion partner. It serves as a valuable tool for research and biotechnological applications.
Quality & Industry Commitment
Numaferm is ISO 9001:2015 certified, reflecting our dedication to quality management, efficiency, and customer satisfaction. With over 200 successful peptide and protein projects completed, we have solidified our reputation as a trusted industry partner.
Our Mission
At Numaferm, we make peptides and proteins more affordable and accessible through cost-effective, scalable production. Our broad customer base spans pharmaceuticals, agriculture, veterinary medicine, generics, and cosmetics, highlighting the versatility and impact of our solutions across diverse sectors.
Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website › Social Media › Number of Employees › Founded (year) › Areas of Activity ›
PlasmidFactory GmbH
Meisenstr. 96 33607 Bielefeld
Germany
Dr. Martin Schleef/Dr. Marco Schmeer
+49-521-2997-350 +49-521-2997-355 info@plasmidfactory.com www.plasmidfactory.com
50 2000
| Contract Manufacturing of plasmid and minicircle DNA in different quality grades for research up to clinical applications
| High Quality Grade plasmid and minicircle DNA as starting material for GMP productions, e.g. of mRNA or viral vectors, also in gram scales
| In-Stock Service for AAV Helper & Packaging Plasmids (pDG/pDP) and for further plasmids and minicircles (e.g. reporter genes)
| CGE Analysis of plasmid topology
| GMP quality for late clinical phase and market supply
The better way to DNA!
PlasmidFactory is the leading contract manufacturer of plasmid and minicircle DNA and the driving force in the development of gene vectors for gene and cell therapy and genetic vaccination. PlasmidFactory’s research and development as well as the complete services are located in Bielefeld, Germany.
Customised plasmids and minicircles
PlasmidFactory’s individual manufacturing service is frequently used by researchers from the fields of transfection and drug delivery, virus production, nanobiotechnology, gene therapy, cell or tumour therapy, and RNA or DNA vaccination. The company offers the production of plasmid and minicircle DNA in several quality grades: Research Grade and CCC Grade qualities for research purposes and pre-clinical applications, High Quality Grade as starting material for e.g. GMP production of RNA, viral vectors and CAR-T cells. GMP will be available from Q3 2025.
In-Stock Services
Our In-Stock products are deliverable immediately off-the-shelf – e.g. reporter genes (gfp, lacZ, luc), AAV Helper & Packaging plasmids (2-Plasmid-System, pDG/ pDP family, several serotypes) or pEPI / pEpito plasmids (containing S/MAR elements).
Biological Patents › External › Collaborations
PlasmidFactory owns any relevant know-how, patents and licenses for plasmid and minicircle manufacturing
Fruitful long-term cooperations with renowned academic and industrial institutions in the fields of gene and cell therapy and vaccination
Request for › Further Collaborations
PlasmidFactory is always interested in working in long-term collaborations with pharmaceutical and biotech companies as well as academic insitutes that require plasmid or minicircle DNA for (pre-)clinical applications.
Other services
On request, plasmid DNA linearisation as well as storage and logistics can be organised, supplementing the company’s service portfolio.
PlasmidFactory’s proprietary method for quantification of the structural diversity of plasmid DNA by means of capillary gel electrophoresis (CGE) is the only reliable method able to determine the stability of plasmid DNA, e.g. during storage.

















Plasmid & Minicircle DNA

From Research to GMP grade













Minicircle – a safe vector system


Minicircles (MC) are circular DNA molecules that are generated e.g. by an intramolecular (cis-) recombination from a parental plasmid (PP). The difference between MC and standard plasmid vectors for gene therapy or nucleic acid vaccination is that the MC contains neither the bacterial origin of replication (needed only in bacteria for the amplification of plasmids in cell division) nor any antibiotic resistance markers or other selection systems to keep the plasmid in high amounts within the producer cell.






Hence, minicircles are the most promising tools to achieve both increased efficacy as well as regulatory requirements for future clinical applications.
PlasmidFactory is the exclusive owner of all relevant patents and IP in this field and provides service production of these supercoiled monomeric constructs, according to clients’ requirements.








Starting material for mRNA vaccines in large quantities








In particular, the High Quality Grade production of plasmid DNA as a starting material for the production of RNA vaccines has gained in importance, especially in the context of the COVID-19 pandemic, as RNA is a promising vaccine candidate for the prevention of certain virus infections, with the additional advantage of not integrating into the genome of the cell and thus remaining as a potentially effective molecule in a patient’s body in the long term.
PlasmidFactory has developed and established the process for the production of the corresponding plasmid DNA containing unstable polyA stretches.


Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website › Social Media ›
Number of Employees › Founded (year) › Type of Laboratory
PolyQuant GmbH
Industriestraße 1
93077 Bad Abbach Germany
Dr Werner Deininger
+49-9405-96999-10
+49-9405-96999-28 info@polyquant.com www.polyquant.com
I ~10
2007
S1 Biotechnology Laboratory
ISO 9001:2015 certified
Areas of Activity ›
Services and products for proteomics
| Quantitative and qualitative protein analytics
| Host cell protein (HCP) analysis
| Quality control for biotherapeutics
| Contract research
| Peptide reference standards for quantitative mass spectrometry (QconCAT)
| Calibration and reference standards for proteomics
| Plasma protein quantification kit
| Customised stable isotope labelled (SIL) protein production
External › Collaborations
Academic research institutions, biotech and pharma companies
PolyQuant is a leading company in absolute protein quantification using mass spectrometry.
Based on our QconCAT technology, we provide ISO 9001:2015 certified products, services and supporting bioinformatics for proteomics projects.
Core Technology
Our proprietary QconCAT (Quantification conCATamer) technology enables highly specific detection and absolute quantification of up to hundreds of proteins in parallel.
› Unbiased protein detection
› Antibody-independent
› Scalable from tens to thousands of proteins
We Quantify Your Proteins
From R&D level to validated methods ready for production quality control.
Quality Control
Build on established workflows for analysis of
› Recombinant Proteins
› Biotherapeutics
› Vaccines etc.
Our technology facilitates both detection of all proteins present in a sample as well as targeted detection and quantification of critical host-cell-proteins (HCP) at all production stages. For release testing, production surveillance and optimisation.
Benefit from routine workflows for standard production organisms (E.coli, yeast, CHO cells) and individual solutions for other organisms.

Clinical Proteomics
We support development of LC-MS/MS based in-vitro diagnostics, biomarker identification and validation, clinical studies, drug development, monitoring of disease progression, therapeutic response, etc.
Biotechnology
Characterisation of recombinant organisms, proteome analysis, expression profiling, enzyme analytics, etc.
Our Services
Full-service assay development for quantitative proteomics projects and fee for service measurements for projects using established workflows.
Experienced professionals provide protein analysis with highest precision for exploratory or quantitative proteomics projects.
Our technology can be applied to numerous sample types and complex matrices.
Receive tailored services with clear timelines, detailed reporting and support, starting from analysis of a quantification problem, planning of experiments to execution and data analysis.
Products
PolyQuant provides:
› Peptide calibration standards for HPLC and MS
› Recombinant proteins
› Custom peptide reference standards
› Ready-to-use QconCAT kits e.g. for plasma protein quantification
Name ›
Address/P.O. Box ›
Postal Code/City › Country › Contact Person › Telephone › Fax › Email › Website › Social Media › Number of Employees › Founded (year) › Type of Laboratory
Areas of Activity ›
ProBioGen AG
Herbert-Bayer-Str. 8 13086 Berlin Germany
Dr Alfred Merz (CEO)
+49-30-322-935-100 +49-30-322-935-400 info@probiogen.de cdmo@probiogen.de www.probiogen.de I Q
290 1994 S1, S2
l Biopharmaceutical development and GMP production facility
l Proprietary technologies for improving product characteristics and process efficiency
l Designer cell lines
l Vector platforms
Biological Patents › External › Collaborations Request for › Further Collaborations
ProBioGen holds numerous international patents on technologies to improve product quality/potency and enhance cellular productivity.
With many international companies and research organisations, e.g. Boehringer Ingelheim, Bayer, CRISPR, Novartis, etc.
ProBioGen works with biotech and pharma companies that develop (complex) therapeutic proteins and antibodies or viral vaccines and viral vectors.
Intelligent biopharmaceutical solutions
ProBioGen is a Berlin based CDMO, specializing in the development and manufacturing of biopharmaceutical active ingredients, viral vectors and vaccines through proprietary technologies to improve product quality and features. Combining state-of-the-art development services and intelligent product-specific technologies results in biologics with optimized properties. Rapid and integrated cell line and process development, comprehensive analytical development and GMP-compliant manufacturing is performed by a highly skilled and experienced team.
Unique services
Cell line & process development for therapeutic proteins
› CHO.RiGHT® expression system
› DirectedLuck® transposase for efficient gene delivery & high titers
› Proprietary chemically defined (CD) media platform/ multiple suppliers
› Robust and economic platform processes
› Classic and intensified processes
› Reports to support investigational new drug (IND) filing
› Modular services and open source concept
(GMP) Manufacturing for therapeutic proteins
› Cell banking (MCB/WCB)
› Up to 1000 L Tox manufacturing
› Up to 1000 L GMP manufacturing
› Disposable, stirred tank bioreactors
Development services for various viral vectors
› Tailor-made solutions for the production of e.g. lentiviral and adenoviral vectors, AAV and MVA
› Suitable cell substrates for the propagation of various viral vectors in suspension processes using CD medium
› Robust upstream and downstream processing supported by comprehensive analytical tools
› Cost-effective non-cGMP production for preclinical development phase
(GMP) Manufacturing for various viral vectors
› Cell Banking (MCB, WCB) and Virus Banking (MSV/WSV)
› Up to 200L Tox manufacturing

Your Biologics. Elevated.®



› Up to 200L GMP manufacturing (up to biosafety level 2)
› Aseptic filling of drug product and diluent under GMP conditions
Analytics, bioassays & quality management
› Integrated analytical development
› Validation according to bioanalytical guidelines (EMA/FDA)
› Stability studies in accordance with ICH Q1
› Wide range of cell based and binding activity assays
› Various cell based and instrumental analyses for content and identity testing of viral vectors and vaccines
Innovative technologies
GlymaxX® ADCC enhancement & glyco-engineering
› Prevents antibody fucosylation
› Boosts ADCC cell-killing activity against tumors and infected cells
› Applicable to both novel and existing producer cell lines
› Allows adjustment of fucose, galactose, and sialic acid levels in glycoproteins
DirectedLuck® – Transposase system
› Proprietary transposase for efficient gene delivery
› Generates high-titer clones (up to 8 g/L)
› Superior heterodimer rates for bi-specific mAbs
Lenti.RiGHT® packaging and producer cell line
› GMP qualified starter cell bank based on HEK 293
› Efficient LV production in CD medium (107-108 TU/ml)
› No requirement of GMP-grade plasmid DNA or transfection reagent
› Straightforward purification of lentiviral particles for e.g. CAR-T therapies
AGE1.CR® Designer cell line & MVA viral vaccine
manufacturing platform
› Avian suspension cell line permissive for numerous human and veterinary viral vaccine strains
› GMP-compliant chemically defined media and scalable suspension processes
› Proprietary MVA strain, transgene expression design, and technology for rapid generation of recombinants

Name ›
Address/P.O. Box ›
Postal Code/City › Country › Telephone › Fax › Email › Website › Social Media ›
Founded (year) › Type of Laboratory ›
ProJect Pharmaceutics GmbH
Fraunhoferstrasse 22 82152 Martinsried Germany
+49-89-45-22-89-700
+49-89-45-22-89-717 info@project-pharmaceutics.com www.project-pharmaceutics.com
I Q
2010
Contract research and development (CRO); cytotoxic (≤OEL4); BSL-1; BSL-2; fee-for-service business model; 2-4 weeks start up time; slim and reliable timelines; GLP-like quality standards; submission-compliant documentation
Areas of Activity › Biological Patents › External › Collaborations
Analytical characterisation; preformulation screening; formulation development; freeze drying cycle development; lyophilisation process and scale-up; technology transfer Several patents in freeze drying and formulation
| Network for seamless development from pre-clinic to fill/finish and market.
| Formulation specialist for DS and DP CMOs worldwide.
| Partnered with Bi-nex for CDMO services on mAb DS (cell line & process development, manufacturing).
| Cross-country competence hub with business partners in Asia.
| Credited laboratory for Wyatt Technology in Europe.
Request for › Further Collaborations
| Small biotechnology/mid-size pharma companies, large top players in pharma/biotechnology.
| From DSP development to early and late drug product phases up to life cycle management.
| Customised solutions for challenges in formulation, analysis or process.
ProJect Pharmaceutics is a leading independent European CRO specialised in formulation and manufacturing process development for injectables: biologics (rec. proteins, antibodies, fusion proteins), peptides, ADCs, cytotoxics, small molecules, generics, viral therapeutics, ATMPs, VLPs and other nanoparticular drug delivery systems. We apply innovative, quality-by-design based, rational concepts of pharmaceutical development according to ICHQ8 and other guidelines. We support our global customers in developing a consistently high-quality pharmaceutical product that is transferable, scalable and manufacturable under GMP conditions. ProJect Pharmaceutics is managed by experts with >30 years of experience in the pharmaceutical industry. In about 850 m2 we operate special laboratories for biologics, cytotoxics and a BSL-2 unit, equipped with dedicated industrial HOF pilot freeze dryers.
Protein formulation
› Predictive formulation analytics: a QbD-based highthroughput approach for accelerated development
› Pre-formulation, early state & late phase formulation
› Liquid & lyophilised formulation (DoE-based)
High concentration protein formulation
› Low viscosity formulation for s.c. application
› UF/DF process development
› Syringeability evaluation
Cytotoxic small molecules
A deep understanding of the challenges when processing highly potent drugs, such as limited solubility in water and rapid degradation, paired with long-lasting experience and comprehensive know-how of lyophilisation from various organic solvents enables us to provide specific solutions for high potency & cytotoxic drugs.
Antibody-drug conjugates (ADCs)
› Predictive, high-throughput formulation development
› High-end freeze-drying technology
By combining protein formulation and process know-how with cytotoxic drug expertise the company holds unique assets to develop ADCs. On top we offer complementary formulation and process development of intermediate antibody & linker-payload bulk.





PROJECT PHARMACEUTICS
Peptide formulation
› Formulation and lyophilisation cycle development
Innovative liposome technology
› High encapsulation efficiency (hydro- & lipophilic APIs)
› GMP-compatible manufacturability with standard equipment
Viral therapeutics
› Lyophilised formulation and cycle development
In-use stability studies
› Evaluation of DP compatibility with IV line equipment
Freeze drying
› In vials, dual chamber systems, syringes, cartridges, bulk trays or bags, containers in nest&tub configuration
› Out of organic solvents
› Rational cycle development (robust, collapse-safe, cost & time efficient, tailored to the formulation)
› Bulk lyophilisation (solid or powder)
› SEM structural analysis of lyo cake
› Time lapse video & IR thermal camera monitoring
› Aseptic pre-clinical batch manufacturing
› Robustness & design space evaluation
› Smooth technology transfer to GMP
Process development and manufacturing
› DSP: Smart optimisation of UF/DF & purification steps
targeting common BDS/DP formulation
› Container closure system compatibility
› Forced stress, in-use & accelerated stability studies
› Detergent & excipient quantification
› Sterile filtration study
› Manufacturability assessment
› Fill&finish process mock-up
› Aseptic pre-clinical batch manufacturing
Fill&finish
As state-of-the-art development experts we are teamed up with state-of-the-art contract manufacturing experts to provide high-quality parenterals from pre-clinical to clinical and commercial scale.

Name ›
Address/P.O. Box ›
Postal Code/City › Country › Telephone › Fax › Website › Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Promega Corporation
2800 Woods Hollow Road Madison, WI 53711 USA
+1-608-274-4330
+1-608-277-2516
www.promega.com
2,200+
1978 by William A. Linton
| High quality reagents & kits for all types of DNA, RNA and protein analysis; cell-based & biochemical assays for analysis of cellular processes; MSI diagnostics; cell systems for therapeutic antibodies including LOT release assays.
| Integrated Systems including instruments, chemistries and personalized support that simplify the process of automating research in the lab.
| Custom manufacturing
| Onsite Stocking
| Automation Support
| Tailored R&D Solutions
Annual turnover ›
Biological Patents ›
$775m USD
Promega holds hundreds of patents in the areas of:
| Bioluminescence
| Cellular Analysis
| Cell biology
| Drug Discovery

Fuelled by curiosity. Powered by exploration. Promega Corporation is a global leader in biotechnology, biochemistry, and cellular and molecular biology, developing innovative technologies for the Life Sciences. Based on bioluminescent technology innovations, Promega offers a broad portfolio of functional reporter bioassays, primary cell-killing assays, and immunoassays, used for R&D or QC batch release of therapeutic antibodies and cell/gene therapies. Promega’s services are designed to accelerate your biologics drug discovery and development workflows.
Discover Promega
The company’s portfolio of over 4,000 products supports a range of life sciences work across areas such as cell biology; DNA, RNA, and protein analysis; drug development; human identification; and molecular diagnostics. For over 40 years, Promega’s products have been used in labs for academic/government research, forensics, drug discovery, clinical diagnostics, as well as agricultural and environmental testing.
Let’s work together to power ideas for tomorrow and the next 100 years.
Promega supports research and development worldwide with innovative products, technical advice, and service. As a partner of pharmaceutical and biotechnology companies, Promega offers special fillings and formulations of enzymes as well as the production of specialised materials such as reporter cell lines, e.g. CRISPR-modified cell lines based on luminescent Promega technologies. From simple changes in product size or packaging to the development of custom assays and high-throughput solutions, Promega’s custom offerings cover a breadth of capabilities in manufacturing, technology development, assay automation, and inventory management. (www.promega.com/custom-solutions/)
Corporate Responsibility
Since 2008, Promega has reported on its commitment to and progress regarding sustainability. In addition, Promega has been an active participant in the UN Global Compact for almost a decade. Sustainability is a core value across the entire company, from the way Promega




designs its buildings and develops its products, to how Promega cultivates its vision of meaningful business. (www.promega.com/corporate-responsibility-csr/)
Promega is headquartered in Madison, WI, USA with branches in 16 countries and over 50 global distributors, serving over 100 countries.
Our Branches in Europe:
Promega GmbH, Walldorf, Germany
(covering Germany, Austria, Poland, and Eastern-Europe)
Tel: + 49-6227-6906-0
Email: de_custserv@promega.com
Promega Biotech Iberica SL, Madrid, Spain (covering Spain and Portugal)
Tel: +34-916621126
Email: esp_custserv@promega.com
Promega Italia S.R.L., Milan, Italy
Tel: +39-2-54-05-01-94
Email : marketing.it@promega.com
Promega UK Limited, Southampton, UK
Tel: +44-23-8076-0225
Email: ukmarketing@promega.com
Promega AG, Dübendorf, Switzerland (covering Switzerland and Liechtenstein)
Tel: +41-44-878-90-00
Email: ch_custserv@promega.com
Promega Benelux, Leiden, Netherlands (covering Benelux and Rwanda)
Tel: +31-71-532-42-44
Email: benelux@promega.com
Promega Nordic, Nacka, Sweden (covering Nordic countries and Estonia)
Tel: +46-8-452-24-50
Email: sweorder@promega.com
Promega France, Charbonnières-les-Bains, France
Tel: +33-800-488-000
Email: FR.support@promega.com

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees › Founded (year) › Type of Laboratory
Areas of Activity ›
QUANTRO Therapeutics GmbH
Dr.-Bohr-Gasse 7 1030 Vienna Austria Dr Michael Bauer +43-122-66001 contactus@quantro-tx.com www.quantro-tx.com
18 2019
Research and Discovery
| Time resolved transcriptomics
| Measure transcriptional dynamics | R&D pipeline and discovery platform
| Drugging previously undruggable transcription factors
External › Collaborations
| Boehringer Ingelheim Oncology
| Institute for Molecular Pathology IMP, Vienna, Austria
QUANTRO Makes Transcription Factors Druggable
QUANTRO Therapeutics’ proprietary technology, the precise and time-resolved, direct quantitative measure of transcriptional dynamics at any point in time, is defining a new state of the art in transcriptomic drug discovery. We have successfully established a pioneering transcriptomic drug discovery platform technology, which unleashes the potential of drugging previously undruggable transcription factor targets.
Our platform has been validated on >10 different oncology and inflammation targets, is currently running at a capacity of approx. 100´000 compounds tested in our high-throughput dual target screening. Taking drug discovery to a next level we established the first 10-target multiplex assay, moving from individual target screening to compound library profiling for multiple targets simultaneously.
QUANTRO’s technology has been successfully used in several HTS campaigns and created functionally validated and proprietary hit libraries against several key oncogenic transcription factors, thereby successfully building an innovative drug discovery pipeline for our own drug development programmes and our strategic partner Boehringer Ingelheim Oncology.
The Transcription Factor Challenge
Due to the complexity of protein-protein-DNA interactions, the investigation of transcription factors requires cell-based assays. Many of these targets are intrinsically disordered proteins, thereby escape traditional discovery or rational design approaches, hence they have been and still are largely considered undruggable.
Consequently, the discovery of transcription factor targeting drugs has been difficult and transcription factor related therapies remain an unmet medical need for patients, representing untapped opportunity for innovative drug discovery.




QUANTRO Defines a New State of the Art in Transcriptomic Drug Discovery
RNAseq based methods have been used for transcriptomic discovery; however, this technology is limited to quantification of mRNA abundance in absence of understanding transcriptional dynamics. Changes of transcriptional dynamics occur within minutes; therefore, primary transcriptional effects and their molecular targets remain invisible to RNAseq based methods. Reporter assays using green flourescent protein are also too slow and only cover a mixture of primary, secondary and distant pathway effects.
Contrary to these limitations, QUANTRO’s transcriptomic technology precisely measures time-resolved changes of transcriptional dynamics, at any point in time. We capture direct primary on-target effects and differentiate these from downstream signalling-pathway effects.
By matching transcriptional “fingerprints” derived from acute degradation of transcription factor proteins with corresponding “fingerprints” triggered by small molecules, we successfully identify drugs targeting previously undruggable transcription factors.
Our first-in-class oncology pipeline focuses on “undruggable” targets using our time-resolved functional transcriptomics discovery platform to identify modulators, activators, and inhibitors of gene transcription with unmatched precision. We are successfully building our proprietary R&D pipeline and continue advancing our strategic collaboration with Boehringer Ingelheim.
Backed by Boehringer Ingelheim Venture Fund (BIVF) and Evotec as SEED investors, QUANTRO pursues a dual strategy of venture financing and strategic partnering to advance our innovative first-in-class pipeline towards clinical development.

Name ›
Address/P.O. Box ›
Postal Code/City › Country › Contact Person › Telephone › Fax › Email › Website › Social Media › Number of Employees › Founded (year) › Type of Laboratory › Areas of Activity › External › Collaborations
Rentschler Biopharma SE
Erwin-Rentschler-Str. 21 88471 Laupheim Germany
Dr Latika Bhonsle-Deeng (Global Head of Communications) +49-7392-701-0 +49-7392-701-300 communications@ rentschler-biopharma.com www.rentschler-biopharma.com I 1,400 1927 S1
| Contract development and manufacturing organization (CDMO) for biopharmaceuticals
Strategic alliance with Leukocare AG, located in Munich, Germany, for bestin-class formulations
Strategic collaboration Xpert Alliance, with Vetter, headquartered in Ravensburg, Germany, for high quality aseptic filling and secondary packaging
A world-class biopharmaceutical CDMO
Rentschler Biopharma is a leading global contract development and manufacturing organization (CDMO), focusing exclusively on client projects. The company’s end-to-end service offering includes process development and cGMP manufacturing of biopharmaceuticals as well as related consulting activities, project management and regulatory support. The family-owned company is headquartered in Laupheim, Germany, with a second site in Milford, MA, USA. Rentschler Biopharma’s high quality is proven by its long-standing experience and excellence as a solution partner for its clients.
Your trusted partner from concept to market
Rentschler Biopharma is highly experienced in the development and cGMP manufacturing manufacturing of therapeutic proteins, in compliance with international standards.
Request for › Further Collaborations
Rentschler Biopharma offers bioprocess development and cGMP manufacturing solutions, from concept to market, for pharma and biotech companies in long-term collaborations and strategic collaboration settings
Working collaboratively with its clients, Rentschler Biopharma provides customized solutions with optimized work packages for each development stage. State-of-the-art cGMP facilities and cell culture processes are leveraged for manufacturing for both, clinical studies and commercial supply. Consistent monitoring of the international regulatory landscape and in-depth understanding of the necessary regulatory documentation (CMC), ensures that each project is properly documented in accordance with international regulatory requirements. In order to offer excellent solutions across the entire value chain, the company has entered into a strategic alliance with Leukocare for formulation development. Another strategic collaboration is the Xpert Alliance, with Vetter, for aseptic manufacturing and packaging. The desired goal of the collaboration with Vetter is the alignment of manufacturing approaches that enable clients to bring their products to patients more easily and faster.



RENTSCHLER
Bioprocess development
› Fast and efficient supply for multiple candidate screening
› Robust and scalable CHO cell lines for cGMP manufacturing
› Efficient cell culture and purification processes
› Well-established analytical methods
› Advanced formulation development exclusively by Leukocare AG
Best-in-class formulation development
Rentschler Biopharma’s strategic alliance with Leukocare offers best-in-class formulation development considered at every step of the biopharmaceutical development and manufacturing process.
Leukocare’s approach combines formulation expertise with data science to achieve superior drug product formulation. Bioinformatics and Artificial Intelligence are strategically combined to explore a broader design space than conventional approaches and to reach optimal formulations in less time at lower costs.
cGMP biomanufacturing
› Multiple stainless steel bioreactor lines, including a twin system with 2x 3,000 L
› Flexible single-use bioreactor lines up to 2,000 L
› State-of-the-art purification processes
› Guaranteed product quality and purity in accordance with cGMP guidelines
› A new, state-of-the-art production line has added 22,000 square feet of manufacturing cleanroom space and houses four new 2,000L single-use bioreactors to the manufacturing site in Milford, MA, USA
As a world-class solution provider, Rentschler Biopharma ensures optimum time-to-clinic and time-tomarket by accelerating timelines to create competitive advantage for their clients. For seamless market approval, they consult and assist clients with regulatory queries, develop optimised strategies, and generate complete documentation for approval of clinical studies and market launch.
Name ›
Address/P.O. Box ›
Postal Code/City › Country › Contact Person › Telephone › Fax › Email ›
Social Media › Number of Employees › Founded (year) › Areas of Activity ›
Biological Patents ›
External › Collaborations
Request for › Further Collaborations
Richter BioLogics
Suhrenkamp 59 22335 Hamburg Germany Dr Thilo Kamphausen
+49-40-55290-451
+49-40-55290-888 BusinessDevelopment@ richterbiologics.eu
I > 400 1987
Contract development and manufacturing organisation (CDMO)
Several patents on manufacture of proteins and pDNA (pART process)
Biotech and Pharma companies worldwide
Richter BioLogics offers CDMO services from gene to finished product. Our teams provide top level solutions to bring your product to market. Contact us for more details.
High class GMP-compliant microbial production of biopharmaceuticals –Plasmid DNA and Proteins
Richter BioLogics is a leading and expanding Germanybased contract development and manufacturing organisation (CDMO) specialised in a broad range of products derived from bacteria and yeasts. With over 35 years experience in GMP manufacturing, Richter BioLogics provides its clients with a unique knowledge base in process and analytical validation, process performance qualification (PPQ) procedures, commercial production of therapeutic proteins, antibody-like scaffolds (e.g., VHH/ Nanobodies, Fab-fragments), bacterial vaccines, and plasmid DNA (pDNA) products.
Your product – our competence and dedication
Our focus is on our customers, their needs, timelines and quality requirements. Our seasoned, highly educated, and motivated teams support the global pharmaceutical and biotechnological industries with one-stop-shop services ranging from strain/process development to analytical development, manufacturing, QC testing and filling of finished product. In addition to our headquarters, we operate three GMP compliant facilities with biorector capacities of 2x 1,500L and 2x 300L to provide manufacturing solutions for large and commercial scales. Further space and connections exist to install more capacity as needed by the customer.
Quality is our DNA
Richter BioLogics consistently works to the highest standards of pharmaceutical quality as verified by major regulatory bodies including but not limited to EMA, FDA, PMDA, ANVISA, MFDS, and more, as well as by numerous customer audits. Throughout the company teams have excellent expertise in the development and cGMP-compliant production of biopharmaceuticals. To ensure efficient, timely and transparent work, we apply professional project management methods and an integrated Quality Management System. The quality system



at Richter BioLogics meets the strictest standards of quality in biopharmaceutical production.
Products and services
Our process development team possesses extensive experience in developing and optimising fermentation and purification processes. For initial strain development, a variety of proprietary E. coli expression systems are available to support strain selection. Small-scale models of existing processes provide helpful tools for trouble shooting, process characterisation, and validation and ensure a smooth scale-up to large-scale cGMPcompliant manufacture.
Additionally, our in-house QC testing covers development and validation of a variety of analytical methods (incl. biological assays), performance of stability studies, and characterisation of reference standards.
Richter BioLogics offers a full range of biopharmaceutical manufacturing services:
› Establishment of cell banks (MCB/WCB)
› In-house QC testing and release
› Stability studies according to ICH guidelines
› Strain and process development
› pART proprietary process– pDNA for different purposes at scales needed
› cGMP manufacturing for clinical phases I to III
› Commercial cGMP manufacturing
› Process validation and PPQ procedures
Thanks to the broad knowledge of Richter BioLogics’s experts and many decades of experience gained from different kinds of projects, Richter BioLogics works out tailor-made solutions for its customers worldwide, which include large pharmaceutical companies as well as advanced biotech companies.
We manage each of our customer projects individually and with the utmost dedication and flexibility - a guarantee for a successful and ongoing partnership.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media ›
Number of Employees › Founded (year) › Areas of Activity ›
SciRhom GmbH
Am Klopferspitz 19 (IZB) 82152 Martinsried Germany
Dr Jan Poth
+49-89-614241230 info@scirhom.com www.scirhom.com
I 9 2016
Preclinical and early clinical development of therapeutics with novel mode of action, treatment of autoimmune and inflammatory diseases, key inflammation pathways, iRhom2/TACE signal transduction biology
Biological patent › External › collaborations
Comprehensive international patent portfolio
Hospital for Special Surgery, New York, expert academic collaborators, highly reputable Contract Research Organisations
SciRhom and its Vision
SciRhom is a pioneering biotech enterprise with the vision to translate advanced scientific findings into game-changing therapies for the treatment of autoimmune and inflammatory diseases.
Based on a decade of ground-breaking research by Prof. Carl Blobel, M.D., Ph.D., co-founder of SciRhom and Director of the Arthritis and Tissue Degeneration Program at Hospital for Special Surgery, New York, SciRhom is developing first-in-class antibodies against the cellular target protein iRhom2 for the selective inhibition of the inflammatory master switch TACE.
SciRhom’s Approach
TACE (TNFα converting enzyme or ADAM17) controls several major signalling pathways in health and disease, including TNFα, IL-6R, and EGFR signalling, and has therefore been considered a highly attractive target to block pro-inflammatory pathways. However, direct inhibition of TACE causes severe side effects, such as skin and intestinal lesions, since it leads to the blockade also of physiologically important and protective pathways. Very recently, iRhom2 (inactive Rhomboid 2, RHBDF2) was discovered as the exclusive key regulator of TACE for the disease-associated release of TNFα, IL-6R, and HB-EGF from immune cells. In contrast, the activation of TGFα as a crucial mediator of skin and intestinal barrier function is supported by both iRhom2 and its closely related family member iRhom1. SciRhom’s highly specific antibodies against only iRhom2 open the exciting opportunity to selectively and simultaneously target the iRhom2-mediated pro-inflammatory activities of TACE, while preserving its iRhom1-supported protective functions important for normal physiology. This new mode of action allows for a highly efficacious but safe treatment of various disorders, including major debilitating autoimmune diseases such as rheumatoid arthritis, inflammatory bowel disease, and lupus glomerulonephritis.



SciRhom’s Asset
SciRhom’s key asset is a series of first-in-class humanised monoclonal antibodies (mAbs) against iRhom2 with excellent physicochemical, pharmacokinetic, and pharmacological properties. Reflecting the simultaneous blockade of several disease-driving pathways and the restoration of immune-balancing mechanisms, our antibodies have proven superior efficacy over the current standard of care single pathway inhibitors Humira, Remicade, RoActemra, and Skyrizi in various disease mouse models. Further to the unique mode of action, our anti-iRhom2 antibodies promise a better tolerability and a real disease-modifying effect. The CMC development of the clinical candidate SR-878 and its toxicological assessment were successfully completed, and CTA approval of a first clinical study was obtained. Based on a recently closed series A financing round, SciRhom has entered SR-878 into clinical development with the goal to ultimately demonstrate clinical proof-of-concept in two indications. SciRhom’s antibodies are protected by four strong patent families that cover the antibodies themselves, their iRhom2 target epitopes, as well as their therapeutic use until 2039.
SciRhom’s Management
Our management team unites several decades of biotech and big pharma experience in advancing therapeutics into early clinical development and beyond, and consists of
› Jan Poth, Ph.D., Managing Director & CEO
› Jens Ruhe, Ph.D., Co-Founder, Managing Director & COO
› Matthias Schneider, Ph.D., Co-Founder & CSO
› Jürgen Reeß, M.D., Ph.D., CMO

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website ›
Social Media › Founded (year) ›
Areas of Activity › SGS Analytics Germany GmbH
Schwanthaler Str. 115 80339 Munich Germany
Samir Barral El Gaoui +34-664502248 samir.barralelgaoui@sgs.com https://www.sgs.com/en/services/ central-laboratory-services I 1994
Central Lab services / clinical lab testing / Bioanalysis
Leading innovation in clinical trials: Accuracy, reliability, confidence
Driving excellence in your clinical trials through SGS Central Lab services
From our Global Hub in Munich and a network of sites across Germany, Switzerland, Belgium, France, and beyond, we provide top-tier Central Lab, Clinical Testing, and Bioanalitical services. With customized solutions and expert laboratory support, we ensure the success of clinical trials for the biotech, pharmaceutical, and CRO industries.
Our portfolio includes:
Project and data management
› Experienced and dedicated team assumes ownership of our clients projects
› High quality standards
› Local expertise and global oversight
› Lab reports highly customisable
› High flexibility of data transfer formats
› Automated upload for eCRFs
Study services
› Support of study sites by providing customised and easy to read site-specific instructions
› Supply of easy-to-use site and visit-specific kits and ancillary materials to sites
› Worldwide samples logistics at ambient, cooled, and frozen temperatures
› Sample storage: -20°C, -80°C, or in liquid N2
› Global study database
› Secure online access
Customised lab services
› Assay development
› Method validation incl. ICH M10 or FDA guidelines
› PBMC/BMMC cell isolation
› Biomarker screening
Analytical services
› 24/7 lab service for early phase trials (e.g. hematology, biochemistry, serology)
› PK, PD
› Immunogenicity
› Biomarkers
› Immunological analysis



› Genetics incl. NGS
› Histopathology and cytology
› Large allergy testing panel
› Experience with various matrices (e.g. serum, plasma, CSF, ascites, bone marrow)
Quality Assurance
› GCP, GCLP compliant
› Independent QA department
› Periodic audits from customers as well as inspections by the most relevant agencies incl. FDA
› 21 CRF Part 11 compliant software
Why SGS?
› Adaptability – Tailored solutions for every trial, with a strong focus on small and mid-sized companies.
› Streamlining – Simplifying clinical trial management and making lab processes effortless for sites.
› Responsiveness – Fast, proactive project management with direct, human support always.
› Experience & reliability – 30 years of expertise, less than a 5% turnover, and 90% of client retention.
› Best location – HQ in Munich, a key European transport hub for seamless global trial logistics and sites in Germany, Switzerland, Belgium, France and beyond
Additional SGS services for drug development and production control
Clinical CRO services
› Phase I unit, including human challenge studies
› Specialised unit for dermatology/ophthalmology
› Strong expertise in the area of biostatistics and biometric services
› CDMO services
Production control services (GMP)
The clinical trial services are complemented by our contract laboratory services to support our clients during the development and production of the IMP/authorised drug. Our services include CDMO quality control, stability studies, and characterisation as well as impurity analysis, nitrosamines, and Leachables & Extractables.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media › Number of Employees › Founded (year) ›
Areas of Activity ›
Socorex Isba SA
Chemin de Champ-Colomb 7a 1024 Ecublens Switzerland Yves Lachavanne (Marketing & Sales Support Manager) +41-21-651-6000 socorex@socorex.com www.socorex.com
F I 60 1963
| Liquid handling instruments | Laboratory consumables | Calibration services for all brands
Socorex Isba SA, based in Switzerland, manufactures highly reliable liquid handling instruments. Manual and electronic micropipettes, single- or multi-channel pipettors, repeater pipettes, dispensers, pipette controllers, and re-usable syringes, along with consumables, and accessories, constitute the heart of the programme. Each precision instrument bears a unique serial number and undergoes a strict performance control attested by an individual ISO 8655 QC certificate.
With over 60 years in the industry, Socorex distributes its products worldwide via an extended distributor network. Visit our website to find your closest provider and test the products yourself.
Acura ® manual micropipettes –exceptional ergonomics
Acura® manual micropipettes offer comfort and superior performance, along with a 3-year warranty. Key features include:
› Excellent ergonomics
› Patented Justip TM shaft height adjustment system for versatile tip fitting
› Swift-set easy user calibration system with an integrated key and locking mechanism
Handle shape, large-surface tip ejector button and low activation force are designed to eliminate hand stress. Volume adjustment button allows quick setting, even when wearing a glove. The soft springs and tightness ring with PTFE sleeve reduce friction and make plunger activation smoother than ever.
Qualitix ® premium pipette tips and related environmental aspects
Made from virgin polypropylene and free from DNAse, RNAse, endotoxins, proteases, and ATP, the Qualitix® tips offer a certified high purity grade. Available in sterile, filtered, or unfiltered models, packed in bags and racks, they ensure accurate liquid delivery and are compatible with Socorex and all major micropipette brands.

To minimise laboratory plastic waste production, the re-closable tipfill ™ rack filling system provides an effective solution. A stack of 10x96-tip inserts uses 60% less space than the corresponding number of conventional packages and generates significantly less plastic waste by reusing empty racks.
Bottle-top dispensers – excellent chemical resistance
The Calibrex™ dispenser line offers safe, consistent liquid distribution, from 0.1 mL to 100 mL:
› Calibrex™ universal 520 for all common laboratory reagents
› Calibrex™ organo 525 for organics and solvents
› Calibrex™ solutae 530 for salt solutions, weak and strong acids and bases
Volume selection is effortless with a smooth, springloaded cursor. The optional stopcock allows for reagent recycling without loss or contamination. Cleaning the dispenser is simple – it quickly disassembles or can be autoclaved fully assembled at 121°C. Calibration is straightforward with the integrated calibration key located under the plunger cap. A QR code printed on each device provides instant access to the chemical resistance chart.
Your precious instruments always in mint condition
At our state-of-the-art Service Centre, a dedicated team of experts takes care of micropipettes and dispensers regardless the brand. We offer:
› Calibration service in an ISO 17025 accredited laboratory, according to revised ISO 8655:2022 standard
› Quick and comprehensive services - Service Express as fast as 48h (excl. transportation)

Name ›
Address/P.O. Box › Postal Code/City › Country › Contact Person › Telephone › Email › Website › Social Media › Number of Employees › Founded (year) › Areas of Activity ›
SynaptixBio Ltd.
9400 Garsington Road
Oxford OX4 2HN
United Kingdom
Dan Williams PhD +44-61-973-4948 contact_us@synaptixbio.com www.synaptixbio.com I 5 2021 Rare diseases, leukodystrophies, H-ABC, antisense oligonucleotides
About us
Formed in 2021, SynaptixBio is led by a team of experts across drug research, regulatory and corporate development, with a proven track record of growing successful biotechnology companies.
Our mission is to find a therapy for H-ABC, a debilitating neurodegenerative condition that affects mainly babies and young children.
We are the only company licensed to commercialise a treatment for this deadly and currently incurable rare disease. H-ABC is the most severe form of TUBB4A related leukodystrophy. We are now very far advanced in the process of selecting a candidate drug to take into clinical trials, which we hope can begin in 2025. The technology we use is a form of gene silencing; the single-gene mutation that causes H-ABC is targeted by an antisense oligonucleotide (ASO), stopping production of the toxic proteins that cause hypomyelination.
Hypomyelination means depletion of the myelin sheath that surrounds nerve fibres in the brain, disrupting the signals that flow along them.
Progress and achievements
In 2021, seed funding of £2m started the process of identifying candidate ASOs. This work was performed at the Children’s Hospital of Philadelphia, the world’s leading centre for leukodystrophy research.
Private investment of c£11m followed in 2023, then two grants from Innovate UK, the UK Government’s innovation body, amounting to £2.5m.
We hold from the FDA two Rare Paediatric Drug Designations (RPDD) and two Orphan Drug Designations (ODDs), one for H-ABC and another for Isolated Hypomyelination. We are also developing a pipeline in related neurodegenerative disorders.


Gene silencing technology
‘Gene silencing’ is different from gene editing in that the gene itself in untouched; only its expression as a protein is affected.
ASOs are strong candidates for tackling rare diseases, because around 80% are caused by single gene mutations. In addition, ASOs are highly targeted and produce fewer side effects.
Most importantly, ASOs target the molecular causes of disease. This is why they are transformative.
ASOs are in trials for Alzheimer’s, Parkinson’s, and Motor Neuron Disease – amongst others.
According to a report from May last year by Global Market Insights (GMI), the market for ASOs was worth US$4.4bn in 2023 and is predicted to to reach US$19.7 bn in 2032.
Patient voices
Patient advocacy groups have been very active in raising awareness and lobbying for action. Families of rare disease patients face multiple challenges, including getting an early, accurate diagnosis, and accessing support. We highlight those challenges and actively communicate our progress.
In the UK, the H-ABC Foundation maintains a map of patients’ locations, their symptoms, disease stage and other data; this will be a key input to the design of clinical trials.
Next steps
We have come a very long way in just a few short years; now, we are opening up investment opportunities to support us through those vital clinical trials.

Name ›
Tebubio SAS, Headquarters of Tebubio Group
Facilitating Life Sciences Research Every Day
Address/P.O. Box ›
Postal Code/City › Country
Contact Person
Telephone
Website
Media
Number of Employees › Founded (year)
Areas of Activity ›
39 rue de Houdan – BP 15 78612 Le Perray en Yvelines Cedex France
Karine Lemarchand
+33-1-30-46-39-00 france@tebubio.com www.tebubio.com
| Life Sciences Solutions
| Biological Research
| Contract Research Services
| mRNA Production
| Cell Line Engineering
| Protein Production
| Biomarker Analysis
| E-procurement Solutions
| Supply Chain Management
| Ethical Sourcing
| Custom Research Solutions
| Advanced Biological Tools
| 3D Cell Culture
| Innovative Research Tools
| Scientific Expertise
| Pan-European Support
| Human Biological Samples
| Animal Welfare Policy
We empower Life Sciences Research, contributing to a brighter future for science. Through our local offices across Europe, we support researchers in Life Sciences and Biotechnology with a holistic range of solutions to advance their projects faster:
› Cutting-Edge Biological Solutions: We provide access to biological solutions sourced from renowned global suppliers, carefully selected by our Scientific Team for their ethical and legal compliance.
› Contract Research Expertise: We accelerate research with expertise in mRNA production & delivery, cell line engineering & protein production, cellular studies, and biomarker analysis.
› Streamlined Ordering Process: Our e-procurement solutions and extensive supply chain expertise simplifies ordering processes, saving researchers time and avoiding administrative complexity.
With Tebubio, focus on what matters most: your research.
Who We Are
We are a Pan-European company founded in 1953, family-owned with over 100 employees and local offices throughout Europe.
› Innovation-Driven: Our DNA is rooted in innovation. We are deeply integrated into the EU Life Sciences ecosystem, exemplified by our partnerships like Bio Deutschland in Germany.
› Committed to Ethical Sourcing: We adhere to the highest ethical standards, sourcing only from OEMs. Our Corporate Social Responsibility initiatives include an animal welfare policy, ISO certifications (ISO 14001 / ISO 9001), and a Decarbonation Programme.




A Holistic Range of Solutions
› Biological Solutions Portfolio: Our Scientific Team helps researchers source, select, and use over 1,300,000 references from trusted suppliers, ensuring compliance with ethical and legal standards.
› Custom Solutions: We offer both catalog and custom options tailored to specific research needs.
Areas of Expertise
› Cells & Tissues: Primary cells, melanoma cell lines, human biological samples
› Biomarkers: Profiling, quantification, biostats
› Immuno Tools: Cytokines, antibodies, ELISAs, and arrays
› Live Cell Imaging: Cytoskeleton probes, mitochondrial probes
› Nucleic Acids: mRNA synthesis, CleanCap ® capping technology
› Functional Assays: Reporter cell lines, FRET assays, activity assay kits
› Innovative Tools: 3D matrix, 3D plates, microfluidics devices
› Biochemicals: Inhibitors, compound libraries, FDAapproved compounds
Outsource and Accelerate Your Research
Our Contract Research Services facility in Europe delivers expert support, ensuring project success in areas such as:
› mRNA production & delivery
› Cell line engineering & protein production
› Cellular studies
› Biomarker analysis
Streamline Your Ordering Process
Partner with Tebubio for tailored agreements and benefit from our robust supply chain management, including:
› IATA-certified shipping
› Compliance with human and animal biological solution handling
› Sourcing outside existing suppliers
› Warehousing services

Name ›
Tentamus Pharma & Med Deutschland GmbH, site Bad Kissingen
Address/P.O. Box › Postal Code/City › Country › Telephone › Fax › Name ›
Columbiastraße 14 97688 Bad Kissingen Germany
+49-971-78558-0
+49-971-78558-25
Tentamus Pharma & Med Deutschland GmbH, site Karlsruhe
Address/P.O. Box › Postal Code/City
Country
Telephone
Fax
Name
Address/P.O. Box › Postal Code/City › Country
Telephone
Fax
Contact Persons
Website
Social Media
Number of Employees
Founded (year) › Type of Laboratory
Am Hubengut 3 76149 Karlsruhe Germany
+49-721-957-919-500
+49-721-957-919-900
Tentamus Pharma & Med Deutschland GmbH, site Oldenburg
Würzburger Straße 3 26121 Oldenburg Germany
+49-441-350344-0
+49-441-350344-11
Dr Andreas Nechansky info-tpmd@tentamus.com tentamus-pharma.de I I 100 2021
Contract Laboratory for Pharmaceutical and Medical Device Analytics
Areas of Activity ›
Pharmaceuticals, Medical Devices, Bio-analytics, In-vitro Analytics, Instrumental Analytics (e.g. ASS, ASA, AES, ICP-MS, GC-MS, LC-MS, IC, IR, TOC, TLC), Cell and Immune Analytics, Micro- & Molecular biology, GMP Laboratory
Tentamus Pharma & Med Deutschland GmbH (TPMD) is your Contract Laboratory for Pharmaceutical and Medical Device Analytics .
TPMD offers you the full range of testing for Pharmaceuticals including cytostatics, narcotics and nitrosamines. We are able to determine element impurities according to ICH Q3D. We perform physical chemical analysis according to PH. Eur., USPO, JP, Bp and support you in the whole process ranging from pre-clinical development to market batch release testing (e.g. clinical and pre-clinical samples, analytical method development and validation, stability studies, and release testing).
In respect to safety testing of Medical Devices, we offer a full range of accredited and ISO 10993 compliant analytics including Chemical Characterisation and residual Ethylene Oxide.
In the same context we focus on biocompatibility testing in vitro offering a sophisticated test platform including the evaluation of Cytotoxicity / Genotoxicity / Irritation to skin and eye / Sensitisation / Endotoxin & Pyrogen Analytics / Bioburden and Hemocompatibility.
Our Microbiological Department specialises on guideline compliant purity testing of prokaryotic cell banks in combination with bacteriophage analytics.
In respect to Molecular Biology, we are perfectly equipped for qPCR analytics in accordance with Ph. Eur. 2.6.7.



Within our Department of Cell & Immune Analytics we focus on specialised cell models including the biological safety testing of eukaryotic cell banks (Adventitious Agents in vitro / Viruses / Mycoplasma and more).
TPMD consists of 3 laboratory sites in Germany being in Bad Kissingen, Karlsruhe, and Oldenburg representing different areas of analytical services. We can handle biological samples up to Biosafety level 2, narcotics and high potential samples.
Our laboratories are
› GMP-certified
› Registered with the FDA
› Accredited in accordance with ISO 17025
› Our microbiological laboratory is authorised to handle biosafety level 2 microorganisms (human and animal pathogens) and biosafety level 2 GMOs
› We have a license from the Federal Opium Agency of the German Federal Institute for Drugs and Medical Devices (BfArM) to handle various narcotic drugs.
› We offer batch certification and EU retests performed by qualified personnel.
› We hold a manufacturing license in accordance with Section 13 of the German Drugs Act (AMG), which enables our qualified personnel to monitor pharmaceuticals testing and release products.
› We have a specially equipped cytostatic area in which we can safely process and analyse cytotoxic raw materials and narcotics
› We are specialised in analysing element impurities according to ICH Q3D

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website ›
Number of Employees › Founded (year) ›
Areas of Activity ›
Tosoh Bioscience GmbH
Im Leuschnerpark 4 64347 Griesheim Germany
Regina Roemling
+49-6155-7043700
+49-6155-8357900
Info.tbg@tosoh.com www.tosohbioscience.de >20 1989
Supplier of chromatography media, HPLC and UHPLC columns
Application support in analysis and purification of biomolecules.
Tosoh Bioscience is an acknowledged global leader in the field of liquid chromatography with a focus on bioseparations. For more than 30 years, Tosoh Bioscience is providing cutting-edge solutions to meet the needs of customers developing and producing new biologics, biosimilars or biobetters, such as plasma products, monoclonal antibodies, recombinant proteins, vaccines and oligonucleotides.
Our product portfolio encompasses SEC instruments and a comprehensive line of media and prepacked process development, HPLC, and UHPLC columns. These products are popular in the biotech and biopharmaceutical industry and used in R&D, downstream processing, and quality control. Typical applications comprise the purification of therapeutic proteins in lab, pilot, and commercial scale, as well as their characterisation by HPLC or UHPLC. Latest developments comprise SkillPak columns pre-packed with best-in-class TOYOPEARL resins for quick and reproducible development and scale-up of purification methods and a holistic multicolumn chromatography solution for DSP intensification, the Octave BIO/PRO instrument and dedicated SkillPak BIO/PRO columns. For antibody characterization, we introduced novel TSKgel size exclusion columns suited for native separation with MALS or MS detection and a hydrophobic interaction column for ADC analysis.


Headquartered in Griesheim Germany, in the Frankfurt/ Rhine-Main Metropolitan Region, Tosoh Bioscience’s European operations offer extensive technical support like application development, on-site training and workshops. One of the services that stand out in the industry is the Tosoh Chromatography Workshop Series providing a comprehensive background to the chromatographic purification of biomolecules.
Tosoh Bioscience is part of the Tosoh Group, a Japanese chemical and speciality products and materials group, founded in 1935, which comprises over 100 companies worldwide and a workforce of more than 12,000 people.
Our brands TOYOPEARL® and TSKgel® are renowned for their quality and reliability. The stationary phases cover all common modes of liquid chromatography, including ion exchange, hydrophobic interaction (HIC), mixedmode, reversed phase, hydrophilic interaction (HILIC), size exclusion (SEC) and affinity.

Name ›
Address/P.O. Box › Postal Code/City › Country › Contact Person
Telephone
Email › Website › Social Media › Number of Employees › Founded (year) › Areas of Activity ›
Valicare GmbH
Eschborner Landstrasse 130-132
60489 Frankfurt on the Main Germany
Dr E. Sons-Brinkmann
+49-69-153-293-700/709 info@valicare.com www.valicare.com I > 100 2002
GMP Services for Pharma, Biotech, & Medical Device Industries
Valicare – ONE-STOP SHOP for Quality and GMP Services
Valicare GmbH is a GMP service provider headquartered in Frankfurt/Main since 2002 and a subsidiary of Syntegon Technology GmbH, a leading supplier of process and primary packaging technology for the pharma and food industries. Valicare is ISO 9001:2015 certi fi ed and has a subsidiary (joint venture) in Turna/ Slovakia. Multidisciplinary teams of engineers, scientists, pharmacists, experienced project managers, and senior GMP consultants enables support customers in the pharma, biotech, and medical device industries and particularly manufacturers of Advanced Therapy Medicinal Products (ATMPs). More than 5.000 GMP projects have been successfully completed worldwide thus far, ranging from ISO and GMP compliance consulting to hands-on qualification and validation support.
Our approaches are always risk-based and ef fi cient –according to the motto: as little as possible, as much as necessary.
Together with our quali fi cation, validation, and documentation services, we offer high-level GMP (and GxP) consulting support:
› GMP and ISO compliance consulting based on your needs
› Structuring and moderation of workshops (e.g. risk analysis, risk assessment)
› Design and planning of GMP-compliant project work flows
› De fi nition of high-level process or project specifications (URS), GMP for new and rebuild projects
› Establishment, adaptation, and optimization of comprehensive GMP quality assurance systems
› Individual concepts for topics such as risk, change, or deviation management and validation strategies
› GMP, GAP, and target-performance analyses for every topic in the GxP-regulated environment and for quality systems according to DIN EN ISO 9001 and DIN EN ISO 13485



We offer risk-based, integrated qualification and validation support in compliance with GMP guidelines, especially Annex 15 of EU GMP. Our services include, but are not limited to:
› Design reviews, plant-specific technical and process risk analyses, qualification of equipment from laboratory to large machines and (clean-) rooms, FAT, SAT, preparation of qualification documentation and definition of test conditions, calibration and requalification after modernization, HMI upgrades after format change
› Quali fi cation and validation of biodecontamination processes in isolators as well as systems for optical control (one of our special competences)
› Validation of manufacturing processes, cleaning processes, analytical methods, and computer systems
Our passion: GMP for Advanced Therapy Medicinal Products (ATMPs) –support throughout the whole life cycle
Our service packages offer expert advice on the development, transfer, and implementation of straight and GMP-compliant ATMP manufacturing processes. Our turnkey manufacturing concept (cult.tainer), consisting of fully equipped GMP-compliant cleanroom modules, enables aseptic process development and manufacturing of ATMPs on-site, accompanied and supported in all GMP issues by our experts.
Due to our large number of successfully finalized projects in very different GMP subject areas, we have sound experience with a variety of efficient and pragmatic solution approaches. Benefit from our expertise!

Name ›
VelaLabs GmbH A Tentamus Company
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website › Social Media › Number of Employees › Founded (year) › Type of Laboratory ›
Brunner Strasse 69/3 1230 Vienna Austria
Dr Andreas Nechansky
+43-1-890-597911 +43-1-890-597910 office@vela-labs.at www.vela-labs.at I
95 2006
Analytical services, contract laboratory for pharma and biotech from drug development to product release (GLP, GCLP, GMP)
VelaLabs – a Globally Active Analytical Services Laboratory
VelaLabs combines a focused, customer-oriented approach, a highly motivated team, and broad expertise in analytical development and quality control. VelaLabs supports pharma and biotech companies, starting from early drug development through non-clinical & clinical phases up to and including product commercialisation.
Characterisation / Analytical Portfolio
(GLP, GCLP, GMP)
With our in-depth analytical characterisation expertise, we support the product development of biopharmaceuticals, biosimilars, monoclonal antibodies, hormones, growth factors, and conjugated proteins, as well as RNA and ATMP. VelaLabs offers methods in accordance with relevant ICH guidelines from method development to validation. Our analytical portfolio includes:
Areas of Activity ›
Protein characterisation, ligand binding assays, cell-based assays, microbiological / sterility assays, nonclinical and clinical services (bioanalytics), physicochemical analyses, compendial methods, QP release, storage
Annual turnover › External › Collaborations
€ 9.5-10.5 m
EarlyHealth Group, Gouya Insights, ABF, NBS-C, ProtaGene, EurofinsDiscoverX, MSD MesoScaleDiscovery
› Physico-chemical methods: U/HPLC, CE-SDS, icIEF, excipient testing
› Cell-based (customised) bioassays: potency, cytotoxicity, proliferation; PBMC isolation, whole blood assays, cell banking, expansion and storage; mRNA expression assays
› Reporter gene bioassays (RGA)
› Ligand binding studies: Biacore/SPR, ELISA, ECL/ MSD, multiplexing platforms, host cell proteins, host cell DNA/RNA
› Lectin microarrays / glycoprofiling
› Bioanalytics for non-clinical and clinical testing
› Pharmacokinetic (PK)
› Pharmacodynamic (PD)
› Immunogenicity testing (anti-drug antibodies (ADA))
› Bio-safety (BSL 2) testing capabilities
Biologics and Biosimilars
Standard methods include CE-SDS, Western blot, HPLC, and compendial (Ph. Eur., USP) methods. For complex molecules like antibodies, our extended portfolio covers the Fab part (e.g. target binding by ELISA, SPR, FACS) and the Fc-related part (e.g. Fc gamma receptor binding by SPR, C1q binding by ELISA, and



biological assays related to the mode of action of antiproliferation, CDC, ADCC, apoptosis). For the analysis of glycosylation patterns of proteins, VelaLabs offers an exploratory method using lectin microarray technology.
Microbiological Services
VelaLabs offers sterility testing in accordance with EMA requirements in a H2O2 gassable isolator (A in D), including GMP-level sterility for medical devices. Antibiotic assays, germ identification and bacterial endotoxin testing is part of the portfolio, as well as Bioburden and TOC testing. Our service includes cleanroom qualification and environmental control.
QP Release
Three Qualified Persons at VelaLabs provide audit services for production facilities, QP declaration, batch documentation review and release of both clinical and market material.
Storage and Stability
VelaLabs can carry out stability studies in stability chambers (WEISS) and has qualified equipment for storage ranging from liquid nitrogen (LN2) and ultra-deep freezers (-80°C) to walk-in cold storage rooms (-20°C/ +4°C). All storage capacities are qualified, monitored 24/7 and supported by an uninterrupted power supply with backup energy for 48 hours.
Clinical trial consulting and network integration
VelaLabs offers consulting on current Good Manufacturing Practice (cGMP) and current Good Clinical Laboratory Practice (cGCLP), with a main focus on analytics. Together with our partners, we provide end-to-end support for clinical trials, as well as additional services.
Location and Lab Space
VelaLabs is located in Vienna with a total area of 2,600 m² (laboratory and office spaces), of which nearly half is GxP laboratory space, including a state-of-the-art bio-safety level 2 (BSL2) laboratory.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website ›
Number of Employees › Founded (year) ›
Areas of Activity › Vetter Pharma International GmbH
Eywiesenstrasse 5 88212 Ravensburg Germany
Lars Hahn
+49-751-3700-0
EU and other international inquiries: info@vetter-pharma.com
US inquiries: infoUS@vetter-pharma.com
Asia Pacific inquiries: info@vetter-pharma.com www.vetter-pharma.com
7,000 1950
Drug product development, aseptic clinical and commercial fill and finish, device assembly and packaging, analytical services, regulatory support, logistic services
We place heightened focus on your success as your strategic partner
We produce aseptically prefilled syringes, cartridges, vials and dual-chamber systems as a globally operating CDMO partner. We are a family-owned, independent company rooted in 75+ years of history. We do not manufacture our own drugs – we focus on providing highly skilled support and state-of-the-art manufacturing resources. We support our customers from the initial phases of clinical drug product development and filling to commercial manufacturing, device assembly and packaging, and lifecycle management. Over 80% of our active projects are biologics.
We are a reliable, responsive, and progressive partner
Our portfolio of services includes dedicated resources for clinical development and commercial manufacturing as well as assembly and packaging, and more. We provide tailored solutions to meet your product’s specific market needs.
Services
› Drug Product Development
Support in navigating key early decisions in your product’s evolution with scalability, quality and efficiency in mind.
› Aseptic Filling & Visual Inspection
Comprehensive expertise in manufacturing injectable drug products and related visual inspection systems.
› Device Assembly & Packaging
Strategic, technical and packaging support for patient-friendly combination products.
› Analytical Services
Robust, customizable testing methods for drug products including evaluation and quality verification.
› Regulatory Support
Support to achieve milestones related to compliance during clinical development, market authorization and beyond.
› Logistic Services
Cutting-edge solutions that maximize efficiency, transparency and precision of a vital supply chain.



Fast facts: Vetter-at-a-glance
› Headquartered in Ravensburg, Germany
› Commercial production sites in Ravensburg and Langenargen, Germany
› Dedicated clinical production facilities in Rankweil, Austria, and Chicago, USA
› Branch offices for Asia Pacific in Singapore, Japan, South Korea, and China
› More than 7,000 employees worldwide
› A leading specialist in the development, aseptic production, assembly and secondary packaging of prefilled drug delivery systems
› International expertise with regulatory authorities including FDA, EMA, PMDA (Japan), and RP (Germany)
› Service offerings for pharma and biotech firms of all sizes and locations
› Numerous patents including technologies for protection against tampering and counterfeiting
› Lyophilization (freeze-drying) and siliconization specialist
For more information, please scan the QR code.

Name ›
Address/P.O. Box › Postal Code/City › Country › Contact Person › Telephone › Email › Website › Social Media › Number of Employees › Founded (year) › Areas of Activity ›
Wacker Biotech
Hans-Knöll-Strasse 3 07745 Jena Germany Dr Ilona Koebsch + 49-3641-5348-0 info.biologics@wacker.com www.wacker.com/biologics I ~ 500 2005 (for Wacker Biotech); 1914 (for parent company Wacker Chemie AG) | CDMO | Biologics | cGMP-Compliant Manufacturing | Process Development | R&D and Preclinical to Clinical and Commercial Drug Supply
Who We Are
Wacker Biotech is the all-in-one expert CDMO partner for process development and manufacturing of advanced therapies and vaccines, as well as live microbial products (LMPs) and recombinant proteins based on microbial systems. Our advanced therapies capabilities span the full manufacturing value chain, from the production of plasmid and template DNA as a starting material for mRNA through the IVT of mRNA to lipid nanoparticle (LNP) formulation.
Wacker Biotech is wholly owned by Munich, Germanybased Wacker Chemie AG, a publicly traded company founded in 1914 that has pioneered some of the modern era’s leading chemical and biochemical manufacturing advances.
Our Global Sites & Capacities
A pioneer in microbial production with decades of experience, Wacker Biotech consists of four complementary, state-of-the-art sites: Amsterdam (Netherlands), Halle and Jena (Germany) and San Diego (USA). In Halle, we have our mRNA competence center. Our sites have received approvals from all the major regulatory agencies, including EMA and the FDA. We have the capability to produce at diverse scales, with 10 GMP lines offering capacities ranging from 3 L for research-grade material up to 1500 L for high-quality clinical and commercial GMP supply.
Scientific Research Expertise
Complementing our bioprocess development and manufacturing experts at our four global sites is a team of more than 180 dedicated research scientists at our parent company’s R&D hub in Munich, including approximately 30 focused just on nucleic acids. This team continuously leads new technological innovations and manages more than 200 active and pending patents and 35 active academic and industry collaborations.
Our Track Record
We have led multiple programmes throughout the entire product lifecycle from feasibility runs to commercial manufacturing. Some of the released commercial pro -




ducts manufactured at our sites include Vaxchora ® , an attenuated oral cholera vaccine, and Spectrila®, an injectable for treating acute lymphoblastic leukaemia via the enzyme protein asparaginase. We have managed more than 200 projects for our clients with no major regulatory findings. In Wacker Biotech, you have a flexible partner offering science-driven solutions and guidance from concept to commercialisation.
Products at a Glance
› pDNA -> mRNA -> LNP
› Recombinant Proteins (based on microbial systems)
› Live Microbial Products
› Vaccines (mRNA, protein, polysaccharide, live attenuated, inactivated)
Services at a Glance
› Strain Development & Cell Banking
› Process Characterisation
› Process Development
› Process Validation
› GMP Manufacturing
› Analytical Development & Testing
› Technology Transfer
› Regulatory Filing Guidance
Capacities
› 350 L, 650 L and 1,500 L stainless steel
› 6 – 43 and 250 L single use
› 10 GMP lines
Proprietary Platforms
› ESETEC ® – secretion platform utilising proprietary E. coli strain for expression of soluble proteins that has demonstrated a 3x COGS reduction
› FOLDTEC ® – refolding platform utilising proprietary E. coli strain for expression of insoluble proteins that has demonstrated a 20x increase in productivity
› LIBATEC ® – platform for producing live microbial products including live bacterial therapeutics in a monoseptic environment in both aeorobic and nonaerobic fermentation regimes
› PLASMITEC ® – platform for producing supercoiled/ linearised plasmid DNA (pDNA)

Name ›
Address/P.O. Box ›
Postal Code/City › Country › Contact Person ›
X-act Cologne Clinical Research GmbH
Rudi-Conin-Strasse 4 50829 Cologne Germany
Olivia F. Hoffmann, Sales & Marketing Manager
Jasmin Atarodi, Managing Director
Andreas Fichtner, Head Biostatistical Services
Ilka Strehlau, Head Clinical Data Management
Telephone › Fax › Email › Website › Social Media › Number of Employees › Founded (year) ›
Areas of Activity ›
+49-221-337716-0 +49-221-337716-33
business@x-act-cologne.com x-act-cologne.com I 35+ 1994
Clinical Data Management
Biostatistical Services
Medical Writing
Your Trusted Partner in Clinical Data Management & Biostatistics
From study planning to regulatory submission, we provide comprehensive support to ensure top quality and compliance. Our expert team offers tailored, efficient services that optimise processes, easing our clients’ workload. Committed to excellence and reliability, we support our clients in advancing medical research to deliver groundbreaking therapies globally. Let’s shape the future of healthcare together.
Company Overview
Type: Owner-managed CRO
Founded: 1994
Location: Cologne, Germany
Experience: 480 + successful studies (interventional Phase I – IV, non-interventional, medical device)
Team: 35 + seasoned and technology proficient team members contribute to the success of your project.
Biostatistical Services
› Consulting: Expert statistical advice on scientific study design and sample size determination
› Randomisation: Tailored randomisation strategies and implementation
› Analysis Planning: Statistical Analysis Plans (SAP) complemented by targeted programming
› Data Mapping: Utilising SDTM and ADaM standards to support regulatory submissions
› Reporting: Delivering comprehensive biostatistical analyses for study reports, publications, and dossiers
› Concluding: Interpreting results to address study objectives

Clinical Data Management
› Consulting: Providing strategic, tailor-made eClinical solutions
› CRF Design and Implementation: Aligning with CDISC | CDASH standards
› Documentation: Creating essential data management documents for project success
› Best Practices: Implementing Good Clinical Data Management Practices
› Procedures: Supported by robust and harmonised SOPs
› Collaboration: Work closely with the clinical team and medical experts to review and clean data
› Reporting: Offering comprehensive status updates for clarity and transparency
Interested in our Services?
Contact us at: business@x-act-cologne.com


Name ›
Address/P.O. Box › Postal Code/City › Country › Contact Person › Telephone
Website
Social Media ›
Number of Employees › Founded (year) › Areas of Activity › External › Collaborations
Yggdrasil Bridge Finance GmbH
Berliner Allee 26a 86153 Augsburg Germany
Werner Weiß
+49-89-20300-6353
anfrage@yggdrasil-bridge-finance.de ww@tec7.net www.Yggdrasil-Bridge-Finance.de www.IPO-Mantelgesellschaft.de www.Yggdrasil-SPAC-1.de www.Yggdrasil-SPAC-2.de
I 3 1998
Bridge Finance
Reverse IPO Shell Company Venture Capital
Börse Düsseldorf ICF BANK AG
Who are we?
We are a team of engineers and auditors specialising in IPOs for technology companies. Our approach combines bridge financing with a reverse IPO. The reverse IPO is a lesser-known but widely used international method to execute an IPO quickly and efficiently. Our target group includes companies with at least 30-50 employees, a clear growth story, and headquarters in the DACH region. Preferred industries include BioTech, Life Science and Robotics with strong management and a product featuring a clear USP. Profitability is not a prerequisite, but the company should have a cash runway of at least 8-12 months. If needed, a bridge financing solution from YBF can be utilised.
Advantages of an early stock market listing
A stock market listing expands the business model with a variety of opportunities. The following advantages are particularly targeted:
› Increase in Equity: The reverse IPO immediately increases equity in the consolidated balance sheet. For example, issuing 10 million new shares would raise equity by €10m. Negative equity is balanced out again.
› Access to Equity Capital: Capital increases make it easier to raise new funds. Compared to other options, placement fees are significantly lower.
› No Change in Legal Form Required: There is no need to convert the legal form from a GmbH to an AG.
› Company Valuation: The company’s value becomes visible and transparent. Typically, public valuations are higher.
› Shares as Acquisition Currency: For a buy-and-build strategy, transactions can (partially) be financed with new shares.
› Increased Visibility: A stock market listing serves as a quality seal for employees, customers, and suppliers. International industrial clients see the company as an equal partner.
› Employee Participation and ESOP: Shares as a transaction currency simplify employee participation schemes, making them more transparent and comprehensible.
› Exit for Shareholders: OTC trades enable share sales without impacting the stock price.



What does Yggdrasil Bridge Finance GmbH do?
Yggdrasil Bridge Finance GmbH (YBF) is a provider of bridge financing in the DACH region. Bridge financing refers to short-term funding. In YBF’s case, repayment is mandatory through a reverse IPO followed by a capital increase.
The financing terms are short-term (12-18 months), subordinated, and typically unsecured, structured as a silent participation.
What is a Reverse IPO?
A Reverse IPO is an alternative method for private companies to go public by merging with an already listed company. This process is significantly faster and more cost-effective than a traditional IPO, allowing even smaller companies to benefit from a stock market listing. The process is straightforward: It starts with a publicly listed shell company, which can either be a former operational company or a company specifically created for this purpose.
This shell company then approves a substantial capital increase through a contribution in kind by incorporating the target company. After this transaction is completed via a contribution agreement, all parameters of the new company are redefined: the business purpose is changed, the management board is replaced, the supervisory board is re-elected and the statutes are revised.
Example:
The Yggdrasil SPAC 1 AG is listed on the open market in Düsseldorf with a share capital of €250,000, divided into 250,000 no-par-value shares. The ownership structure consists of 85% held by the initiator (212,500 shares) and 41 other shareholders as the free float. A target company can be incorporated into this shell, for instance, by issuing 10 million new shares. This increases the total share capital to 10.25 million shares, with the target company holding 97.6% of the voting rights. With this majority, the target company can now redefine the management board, supervisory board, and company statutes.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Fax › Email › Website › Social Media ›
Number of Employees › Founded (year) ›
Areas of Activity ›
YMC Europe GmbH
Schöttmannshof 19 46539 Dinslaken Germany Dr Carsten Weber
+49-2064-427-0 +49-2064-427-222 info@ymc.eu www.ymc.eu I Q
50 (Europe) 485 (worldwide) 1993
| Glass Columns for Lab and Pilot Scale | Prepacked Glass Columns | Laboratory Services | Preparative Liquid Chromatography | Analytical Liquid Chromatography
Your resource for chromatography
As a research-driven chromatography specialist, YMC provides chromatographic solutions for compounds such as peptides and proteins, mAbs, and ADCs, as well as oligonucleotides from R&D scale through scale-up to production and final quality control in your lab. We supply innovative bio-chromatography resins and columns for preparative processes, as well as highly reproducible BioLC-(U)HPLC-columns for SEC, IEX, RP, & HIC.
Furthermore, YMC glass columns in laboratory as well as pilot scale are designed to meet bio-chromatographers’ demands. You can choose them empty as well as prepacked according to your requirements by YMC’s inhouse packing service. In addition, you can make use of on-demand services for application support, screening, analytical, or preparative method development. Trainings on different chromatographic topics complement the products and services.
Innovations
The most recent and interesting developments include:
› The new standard for columns for bio-purification suitable for self-packing: YMC PilotPLUS
› Macro-porous AEX resin for the purification of large biomolecules: MacroSep IEX Q
› Innovative solutions for BioLC: YMC-Triart Bio(U HPLC columns, also available in bioinert hardware
The broad and continuously growing portfolio of stationary phases especially designed for the separation of biomolecules provides high flexibility for the chromatographer and ensures successful lab results, day after day. Additionally, the availability of particle sizes from analytical to preparative scale guarantees smooth and easy scale-up processes.

Worldwide availability and support
YMC products are available worldwide through a dedicated support network that guarantees qualified and meaningful practical assistance with regard to method development, method transfer, method validation, purification services, and custom column packing – locally, personally, and efficiently. It is not only product specifications that represent YMC quality, but also the positive energy and dynamics of our employees, who provide competent and consistent performance together with pronounced reliability even for the most difficult and demanding separations. The YMC network consists of highly focused product specialist teams to provide active support for chromatographers.
Regulatory Support
Since all YMC processes and working procedures are thoroughly monitored and documented, YMC resins and glass columns are fully compliant with requirements. Full technical documentation is available to show compliance with all applicable regulations.

Name ›
YUMAB GmbH
Science Campus Braunschweig-Süd
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person › Telephone › Email › Website › Social Media › Number of Employees › Founded (year) › Type of Laboratory ›
Areas of Activity ›
Inhoffenstr. 7 38124 Braunschweig Germany
Dr Inga Hochnadel +49-531-481170-0 info@yumab.com www.yumab.com I 30 2012 S1, S2
| Therapeutic Antibody Development | Broad Immune Repertoires | Libraries & Discovery | Engineering & Lead Optimisation | Humanisation
| New: Animal Health Antibodies | R&D services | Partnered Innovation
Request for › Further Collaborations
YUMAB seeks clients & partners for therapeutic antibody development in human and animal health.
Accelerating Biotherapeutic Innovation
YUMAB is a German biotech company offering contract and partnered research for the development of therapeutic antibodies from target to optimised lead candidates. The company was founded in 2012 with the aim of providing state-of-the-art and next-generation recombinant antibody technologies to researchers from early start-ups to biotech and biopharma industry, helping them to bridge the gap between research innovation and therapeutic application. Since then, the company has evolved into a global player advancing biomedicine by accelerating the development of next-generation biotherapeutics.
Translating Innovation by Technologies
The YUMAB ® antibody platform provides access to challenging targets, supports all antibody drug modalities and accelerates the development of biotherapeutics by addressing the needs of manufacturing and clinical development through advanced protein engineering and lead optimisation technologies. The company delivers fully human and humanised therapeutic antibody candidates that are the closest to the natural human antibody repertoire.
Our highly diverse, fully human antibody libraries contain >1011 natural antibody sequences and specificities to all types of antigens. In addition, YUMAB can also access very broad immune repertoires from different immunised animal species (mouse, rat, rabbit, chicken, llama …), maximising epitope and functional diversity to identify the optimal drug candidate for each target and any modality. Our powerful in-vitro discovery technologies are efficient for rare and difficult target antigens and allow for the pre-design of functional and biophysical properties. Antibody candidates are identified rapidly within weeks. They are highly developable into all antibody drug formats including full-length IgG, Fab, scFv, bi- and multispecific, CAR, fusion proteins, and ADCs. Our advanced protein engineering technologies include in silico and experimental platforms that deliver lead candidates with desirable biophysical properties and lowest immunogenicity optimal for manufacturing and clinical development.
Therapeutic antibody discovery & development
From Target to Lead
Proven Success
Our antibody platform has been proven in over 400 projects with more than 140 customers and partners worldwide, and by several drug candidates in clinical development. In 2020, YUMAB spun out CORAT Therapeutics to bring its proprietary COVID-19 antibody drug into the clinic in just 11 months. The virus-neutralising antibody COR-101 is uniquely designed to treat patients with high viral loads by not inducing unwanted immune responses. In addition, YUMAB is involved in numerous collaborations to develop novel technologies such as AI-assisted discovery and development, target discovery on tumour samples, mammalian display or TCR-like antibodies (TCRm).
Trusted and Collaborative Partner
YUMAB offers customised service and partnering concepts to accelerate the translation of your research innovation into drug development, with affordable entry costs, flexible licensing options, and no additional thirdparty obligations. Our team of experts is committed to close communication with clients and partners, providing advice and guidance throughout the antibody discovery and early development process.
YUMAB at a Glance
Therapeutic antibody platform
› Fully human libraries for low immunogenicity
› Broad immune repertoires of different species including close to human germline humanisation
› Canine & feline antibody libraries for animal health
› Optimised in vitro selection for difficult targets
› Rapid discovery within weeks
Direct link to lead development
› Advanced antibody lead development & optimisation
› All antibody modalities, all disease areas
› Robust, adjustable process, high success rates
YUMAB® platform
› Fully integrated discovery & development platform from target to optimised lead
› Modular projects with flexible entry & exit points
Flexible services, collaborations & partnering
› Fee-for-service & flexible licensing options
› No technology access fee
› Collaborative development
Become partner of the next edition 2026!

Media Contact:
Andreas Macht
Tel. +49-30-264921-54
a.macht@biocom.de



