Yonsei Student Pharmacist
THE BLUE VANGUARD
MAIN ARTICLES
Column
Seoyeon Choi, Abuse of opioid painkiller Pharmaceuticals Dawon lee, Polypharmacy Management Program
National issues
Yoonah Jung, The Application and Prospect of AI Technology in Korea's Pharmaceutical Industry

International issues
Jaeeun Park, NASH Treatment Breakthrough, Rezdiffra

Editor's note

yoonah jung
Hello readers!
It is my utmost pleasure to introduce the 25th edition of The Blue Vanguard. In this issue, we delve into a diverse array of topics, offering insights into the latest advancements and trends within the pharmaceutical and healthcare industries. From the management of polypharmacy and the development of cancer drugs to the pioneering work in mRNA vaccine delivery and the transformative power of big data in drug discovery, we aim to bring you the most relevant and engaging content.
Our focus this semester is particularly on the integration of technology with medicine, an area that is rapidly reshaping the future of healthcare. We explore various technological applications, such as advances in mRNA vaccine delivery using lipid nanoparticles, the impact of big data in pharma and its role in future drug discovery, and the application and prospects of AI technology in Korea’s pharmaceutical industry. Through a series of thoroughly researched and meticulously written articles, we examine the implications, challenges, and potential of these advancements.
As pharmacists, we are committed to being effective communicators and lifelong learners. Our team at The Blue Vanguard exemplifies these principles, demonstrating a strong desire to explore cuttingedge pharmaceutical research and share their knowledge with our readers. It has been a privilege to work alongside such passionate and dedicated individuals.
We hope that this issue of The Blue Vanguard provides you with valuable knowledge and inspiration. Enjoy the read!
Warm regards, Yoonah Jung, The Blue Vangaurd

Contents
Pharmaceuticals
• Polypharmacy Management Program
• Cancer drug development in the past, present, future
• Use of Lipid Nanoparticles for mRNA Vaccine Delivery
• Process of the pharmaceutical industry’s production line
• Use of Big Data in Pharmaceutical Marketing
Column
• Abuse of opioid painkiller
• How to Improve Adherence to Medication for Clinical Trial Subjects
National issues
• The Application and Prospect of AI Technology in Korea’s Pharmaceutical Industry
• The development of Korean Sports pharmacy
• Fixed-dose combination therapy in type 2 diabetes
• Shifting Trends in the Pharmaceutical Market Due to Korea’s Aging Population Growth
International issues
• NASH Treatment Breakthrough, Rezdiffra
• The Impact of Online Platforms in Pharmacies
•The Rise of Telemedicine in Pharmaceutical Care: Benefits and Drawbacks
• Current status and controversies about Neura
• Global Acceleration in Obesity Treatment Develop
PolyPharmacy management program

Journalist | Dawon Lee | dalee5451@yonsei.ac.kr
Designer | Jeongwoo Shin | steven1020@yonsei.ac.kr
Apatient who has one or more chronic diseases and takes more than 10 types of oral medications for 60 days or more is referred to as a polypharmacy user. According to prescription data in South Korea in 2019 related to patient safety, the rate of polypharmacy prescriptions was 70.2%, significantly higher than the OECD average of 46.7%, and has been steadily increasing since 67.2% in 2013. With the recent increase in the elderly population and patients with chronic diseases, the number of polypharmacy users increased significantly from 815,000 in 2019 to 1,175,000 in 2022. As of the first half of 2023, it has been reported that 1.29 million people among the South Korean population regularly take more than 10 types of medications. According to a study on prescriptions and outcomes related to underlying diseases of polypharmacy users at the Ilsan Hospital in 2018, the risk of hospitalization for elderly patients prescribed polypharmacy increased by 18%, and the risk of mortality increased by 25%.
Managing polypharmacy can be challenging due to various factors related to healthcare providers, institutional factors, and patient-related factors. Firstly, there may be a lack of relevant regulations or incentives for integrated medication management services regarding healthcare providers and institutional factors. Additionally, healthcare professionals may not always be motivated to track the medication history of patients who have not been prescribed by themselves. Furthermore, while individual prescriptions for each condition may seem appropriate, patients with multiple diseases may receive prescriptions from different specialties, leading to unnecessary prescriptions. On the patient side, there may be a lack of awareness regarding the increased risk of side effects associated with a higher number of medications. Patients may also exhibit a high level of dependency on their medications, leading to resistance to changing their existing medication regimen. The complexity of managing polypharmacy arises from factors including institutional gaps, challenges in coordinating care among multiple healthcare providers, and lack of awareness among patients.
In South Korea, many elderly individuals receive prescriptions for numerous medications from various healthcare institutions, leading to increased risks of hospitalization and mortality. The National Health Insurance Service initiated the Polypharmacy Management Program in 2018 to resolve this issue. This program involves experts such as physicians and pharmacists reviewing medications for citizens taking multiple types of drugs, providing education, and adjusting prescriptions to prevent side effects and enhance the effectiveness of medication use, ultimately protecting public health. Unlike Drug Utilization Review (DUR) services, which provide real-time information on medication safety during the prescribing and dispensing stages, the Polypharmacy Management Program directly reviews all medications taken by patients to identify drug interactions and adverse reactions, providing personalized medication counseling. It ensures proper medication use through prescription adjustments.
The program commenced with 99 pharmacists advising 684 individuals over six months in 2018, and by December 2023, consultation services had been provided to a total of 24,153 people. Currently, the program serves more than 6,000 individuals annually. It can be seen as a significant achievement that has driven substantial quantitative growth. This program operates in two models: community-based and hospital-based.
The hospital-based model, initiated in 2020, facilitates collaboration between pharmacists and physicians, leading to seamless cooperation. The outcomes of polypharmacy consultations are integrated into physician consultations, resulting in prescription adjustments. Patients who received Polypharmacy Management Program services showed a 50% decrease in emergency room visits and a 21% decrease in rehospitalizations compared to those who did not. The effectiveness of comprehensive medication review and adjustment between admission and discharge was demonstrated. Additionally, the controlled environment of inpatient settings facilitates thorough medication review and adjustment.

However, the community-based model revealed limitations in pharmacist-physician collaboration. In this model, pharmacists review patients’ medications and provide consultation results to physicians through the patient, rather than directly collaborating with physicians. As a result, it is challenging for pharmacist consultation results to be reviewed during physician consultations, and there is a lack of information sharing among medical staff, resulting in low continuity. To address this, a pilot program was initiated in Seoul’s Dobong-gu in 2023, where local community physicians and pharmacists collaborated on polypharmacy management. This demonstrated the feasibility of multidisciplinary medication management through collaboration between physicians and pharmacists in the community. Among the 184 individuals who received Polypharmacy Management Program services, 54.9% had their consultation results linked to physician prescription adjustments. Consequently, there are plans to expand this collaborative model to other regions with an interest in pharmacist-physician collaboration. In 2024, Gangbukgu in Seoul, Ilsan in Gyeonggi-do, Gwangju, Jeonju, and Uiseong are discussing implementing this model.
In efforts to reduce the risks associated with polypharmacy, smooth implementation adjustments and expansions of the Polypharmacy Management Program are underway across various regions. In this process, the role of pharmacists in providing medication therapy is becoming increasingly significant. The risks of polypharmacy are not exclusive to South Korea. Still, they are also actively addressed in countries such as Europe, Australia, the United States, Canada, the United Kingdom, and Japan, where pharmacists’ expertise is utilized to resolve medication-related issues. Through this process, it is believed that the diversification of pharmacists’ roles will contribute to the enhancement of their professional capabilities. B

Cancer drug development in the past, present, and future
Journalist Jungbin Shin | jungbin@yonsei.ac.kr Designer Inhyeok Kim | inhyeok206@gmail.com
As science and technology have developed over multiple generations, there has been a drastic change in the biomedical field. Different types of drugs are being researched, and not only limited to drugs but also gene tools such as CRISPR and targeted drug delivery are being widely exploited in patient treatment. However, not all diseases are fully cured by the current biomedical technology. Cancer is one example of a disease that does not have a treatment method that can fully cure the patient.
According to the CDC, the percentage of adults who have been diagnosed with cancer is 9.6% as of 2022 data surveyed in the U.S.
As we can see from this statistic, cancer is a common thing that anyone regardless of gender or age can get. In terms of cancer mortality, it has been shown by CDC that there have been 146.2 deaths per 100,000 population in 2019. This is a drastic improvement compared to the mortality data in 1950, which showed 193.9 deaths per 100,000 population.
How was this possible? The drastic change in drugs used to treat cancer led to this reduced mortality. Even though the mortality rate has dropped exponentially, the war on cancer is still ongoing. In this article, we will be exploring cancer drugs in the past, present, and future.
Timeline for drug development (Past and Present)
The timeline for proper cancer drug development began in the modern era. Starting from the 1820s, it was known that cryotherapy was employed to combat cancer. Cryotherapy is a treatment that uses extremely cold temperatures delivered by needle-like instruments to destroy abnormal cells, such as cancer cells.
Dr. James Arnott, the father of cryosurgery, initiated this. Although this medical treatment had the huge advantage of not requiring surgery, it had side effects such as swelling and skin irritation. Cryotherapy is not commonly used in cancer treatment due to its low success rate: 4% 5-year survival rate for stage 4 lung cancer patients.
In 1896, radiation therapy was first used to treat cancer. The first person who introduced X-rays to treat cancer was Dr.Victor Despeignes, who is known to be the father of radiotherapy. High doses of X-rays kill the cancer cells by damaging their DNA. This will limit the ability of the cancer cells to repair themselves or to divide. As a result, cancer cells will be removed from the body.
The 1900s was the period where another significant change in the history of cancer drug treatment. Most chemotherapy drugs were developed and employed to treat cancer during this period. In 1942, the first chemotherapy drug, mustine, was used to treat cancer. The US FDA approved many chemotherapeutic drugs in this period, such as mechlorethamine, mercaptopurine, and others. Since then, many different types of chemotherapy drugs have been researched, and the success rate for chemotherapy is exponentially rising.
After chemotherapy, new therapies to treat cancer are being researched. Nowadays, targeted drug therapy, immunotherapy, and personalised medicine have been continuously researched and employed by doctors to treat cancer. Although these types of therapies are thought to be the future of cancer therapy, the current cancer treatment that is commonly employed is known to be chemotherapy. We will be looking into chemotherapy: its effectiveness and drawbacks in the next chapter.
Current drugs used and drawbacks
As mentioned before, chemotherapy is currently the most popular medical therapy used to treat cancer. Then how does chemotherapy work? What drawbacks does it have? First, we should know the precise definition of chemotherapy. Chemotherapy is a treatment that uses strong chemical substances to destroy cancer cells. There are many different types of drugs used in chemotherapy. We can separate the drug into 5 big types.
1. Alkylating agents, such as Altretime, damage cell DNA to prevent the cancer cells from dividing.
2. Antimetabolites, such as 5-fluorouracil, stop cancer cells from making genetic materials, which is crucial for the cancer cells to replicate
3. Topoisomerase inhibitors, such as Etoposide, prevent the enzyme (topoisomerase) from making DNA to replicate
4. Miotic inhibitors, such as Cabazitaxel, affect the cell’s ability to carry out mitosis. As a result, no more cancer cells will be produced.
5. Antitumor antibiotics, such as Doxorubicin, prevent the genetic material in the cancer cells from replicating.
After these types of drugs are employed in chemotherapy, the survival rate of patients appears to be very high. Different cancers have different survival rates, but most of the cancers, such as Prostate cancer and Bladder cancer, have a firstyear survival rate of over 90%. Even though chemotherapy has a very high success rate, the drawbacks and side effects of the treatment are significant. According to the MD Anderson Cancer Centre, most patients suffer from many side effects: nausea, hair loss, fatigue, nerve damage, and others.
Because of these side effects, some patients are not willing to take chemotherapy. Not only because of side effects but also because the price of chemotherapy is expensive, some patients are not willing to take the treatment. To combat this, researchers are working hard to create drugs that are cheaper and have a higher success rate.
Hopes for future cancer drugs
Which therapy will come after chemotherapy? In the future, many therapies such as personalised cancer vaccines, immunotherapy, and targeted therapy seem to take the lead in future cancer treatment. Personalised cancer vaccines will be given in the form of mRNA, and will stimulate immune response against the cancer cell. The fact that it does not directly interfere with human DNA and is cheaper, personalised cancer vaccines possess huge advantages over other therapies. Immunotherapy, on the other hand, uses a technology called CAR-T cell therapy to target specific cancer antigens. This therapy is still in the clinical trials and is very pleasing due to its high efficacy and success rate. Last but not least, targeted therapy, using CRISPR cas9 gene editing seems promising. It can be used with the CAR-T treatment to have a better effectiveness against cancer. Since it only targets particular genes, it can have fewer side effects compared to other medical therapies. B

Use of Lipid Nanoparticles for mRNA Vaccine Delivery
Journalist | Siyun Hwang | siyun8097@naver.com Designer | Seonu Hong | hsw5020@gmail.com
n contrast to traditional cationic polymeric carriers, lipid nanoparticles (LNPs) formulated with ionisable lipids offer numerous benefits. These include excellent biocompatibility, efficient encapsulation of nucleic acids, successful transfection, enhanced tissue penetration for therapeutic agent delivery, controlled drug release, minimal offtarget effects, and reduced cytotoxicity and immunogenicity. Consequently, LNPs have emerged as the predominant third-generation mRNA delivery platform, significantly advancing the mRNA industry and clinical applications. Notably, LNP-assisted mRNA delivery holds promise for potent cancer immunotherapy. Comparative analyses reveal that LNPs outperform other delivery systems in terms of nucleic acid encapsulation, expression efficiency, and safety both in vitro and in vivo, establishing them as the current leading delivery system.
The mechanism behind lipid-based particles for mRNA delivery
LNPs are typically composed of four main components: mRNA payload, lipids, cholesterol, and a polyethylene glycol (PEG) lipid. The mRNA, which encodes the target antigen, is encapsulated within the core of the LNP. Lipids and cholesterol form the lipid bilayer surrounding the mRNA, providing stability to the nanoparticle structure. PEG lipids help to increase the stability and circulation time of the LNPs in the bloodstream. Upon administration, LNPs are taken up by cells at the injection site, often by endocytosis. The exact mechanism of cellular uptake can vary depending on factors such as LNP size, surface charge, and composition. Once inside the cell, LNPs must escape from endosomes to release the mRNA payload into the cytoplasm where protein translation occurs.

To facilitate endosomal escape, LNPs may exploit the proton sponge effect. This effect involves the influx of protons into endosomes due to the acidic environment, leading to osmotic swelling and rupture of the endosomal membrane. LNPs containing ionisable lipids can buffer the acidic pH within endosomes, causing an influx of ions and water, ultimately leading to endosomal disruption and the release of mRNA into the cytoplasm. Once released into the cytoplasm, the mRNA payload is translated by the host cell’s ribosomes to produce the target antigen protein. This protein can then undergo post-translational modifications and be presented on the cell surface via major histocompatibility complex (MHC) molecules to activate the adaptive immune response.
The synthesized antigen protein serves as a foreign antigen, triggering the immune system to mount an immune response. This response typically involves the activation of both cellular (T cell-mediated) and humoral (antibodymediated) immunity, leading to the generation of antigen-specific immune responses and memory.


The Process of the Pharmaceutical Industry’s Production Line
Journalist | Si Yun Park | gracepark02@naver.com
Designer | Jin Yeong Kim | kimjin02000@yonsei.ac.kr
When we visit pharmacies, we usually see various types of medicines and the pharmacist gives us the medicine that the doctor had prescribed. However, people are unaware of how the medicine we receive is produced. Hence, this article will go over the process of how chemists transform raw materials into finished products, and how they release new drugs in the pharmacy.
The process of Drug Development
First, university researchers have to find the target genes that cause the typical disease. Then, they make the lists of candidate groups that suppress the function of the target genes. They design the new material and take multiple clinical trials until they find the material that is safe enough to use for humans. Since the FDA only recognizes the results of safety tests conducted by authorized agencies, these tests must be conducted by internationally recognized agencies. After the approval for the sale of new drugs, you can go to the next step which is manufacturing the drugs.
How the drugs are Manufactured
The manufacturing process is different between the types of drugs, which are synthetic drugs and biomedicines. A typical example of a synthetic drug is a pill. The process of this type is mixing, setting, tablet, coating, and packaging. First, measure the amount of main and sub-materials, which are ingredients of drugs, and mix them according to the determined order, time, and speed. Then, granulate the mixture using a binding solution or lubricant. The granulated form can bind more easily during tablet than fine particles. After making granulated form, set them evenly, and tablet the form into familiar pill shape. Lastly, they are coated, packaged and sold.
For biomedicines, it undergoes cell culture, harvesting, purification, filtration, and packaging. First, we get the cells from cell banks and culture the cells that contain target proteins. After cell culture, separate the cells through a centrifuge, and extract the target proteins by using chromatography. Lastly, it goes through a filtration process to remove impurities, and inject the medicine into syringes or vials.

The Challenges and Future of Pharmaceutical Production Line
In order to develop a biomedicine, pharma companies require spending a significant amount of time and money to ensure the drug meets the desired safety profile and therapeutic efficacy. The emergence of AI will bring efficiency to every step of the process. So, the production will be cheaper by using predictive analytics. For example, these analytics could be used to predict the optimal production schedule for a drug based on inventory levels, current demand, and the factory’s capacity.
Also, organic compounds are highly fragile drugs that could potentially fail at any point in the manufacturing process. Proteins, for example, are susceptible to degradation, if not kept under optimal conditions such as temperature control or stable pH levels. As a solution, single-use technology is rapidly emerging in the pharmaceutical industry, which reduces cross-contamination risk. This type of equipment can reduce costs by eliminating the need for in-house sterilization. Examples include chromatography devices, bioreactors, and ion exchange membranes. B
Use of Big Data in Pharmaceutical Marketing
Journalist | Chanyoung Kim | kkimchanyoung@gmail.com
Designer | Soyun Kang | soyun525@yonsei.ac.kr
In a society where information is easily accessible, harnessing that information to suit our needs is essential.
Analyzing the given data, finding tendencies and variation points, and creating new strategies and amendments are key to survive in various industries nowadays. Pharmaceutics, and specifically, pharmaceutical marketing is not an exception. Pharmaceutical companies will exploit various types of Big Data including digital health records, social media, search history, and other digital sources to find optimal marketing strategies for optimally targeted consumers. Thus, the marketing of pharmaceutical companies not only focuses on inventing and advertising pharmacologically excellent medication but also strategically accessing Big Data records of people who could be potential customers. Throughout this article, we will focus on the methods pharmaceutical companies utilize Big Data for marketing purposes and its connotations. Additionally, the challenges of analyzing Big Data for pharmaceutical marketing will be further discussed.
Methods
One of the purposes of marketing departments of Pharmaceutical companies making use of Big Data is to understand the audience- or demand of certain medications. The data of patients who currently use medication, ordinary people who have no illness, and people who fear of getting the illness, all are useful samples when predicting the demand; both in terms of the amount of medication itself or deciding the people being advertised. For instance, conversations on social media could be a hint to identify the concerns of the patients and points that could be amended from earlier medications. Also, by analyzing digital health records, the prescribing habits and formulation preferences of patients could be understood. All these data are then sub-categorized into factors such as age, sex, social/economic status, etc, which are used as algorithms for individually targeted advertisements.
Additionally, utilizing Big Data for marketing is also beneficial within the drug development process. For one, finding adequate participants for clinical trials would be less time-consuming. By analyzing data such as health records, companies could easily identify the patients in need of new treatment and those who fit the criteria of clinical trials (age, genetic factor, etc). Also, competition in the generic market in the pharmaceutical industry is fierce, and thus, finding promising candidate drugs is essential to gain sufficient profit. This is where Big Data comes along, because it reveals opportunities for both cost-effective and lucrative products for the company to invest. It allows the company to stay up-to-date on the latest trends and current issues to remain relevant. Lastly, another side of Big Data utilized in the drug development process is by analyzing the data of other previous clinical trials. By doing so, analytics could gain insights on into how the clinical trials for their product would lead to.

Challenges
Propitious the future of Big Data analysis for pharmaceutical marketing seems, there are some significant challenges. One of those challenges is patient privacy. Securing the privacy of patients is crucial as more data are being accumulated and individuals could easily be endangered without protection. Not complying with these regulations, the company could be fined and more importantly, harm their reputation; a vital component in marketing. Additionally, the data they use must be accurate and reliable, because inaccurate data could lead to improper marketing strategies and extravagant expenditures.
Ironically, although using Big Data could save time for areas such as the drug development process, to some degree it could also be time-consuming. Big Data has accumulated numerous amount of information in time, and its sheer volume is expected to increase more rapidly. Within this massive ocean of data, finding the right information could be demanding and costly. This problem, however, could be aided by the development of AI (Artificial Intelligence), which could identify data trends and patterns within seconds to minutes and is still improving. Lastly, the company ought to ensure that the data being used is both relevant and actionable for their purpose. Whether the data results could be applied in real life and a different setting is a difficult task.
Overall, it is evident that Big Data is a valuable tool for marketers to comprehend customer needs and seek marketing strategies accordingly. The decisions made by these data analysis could ultimately invent drugs that save millions of lives. Not to mention its’ ability to aid those feeling discomfort from their medication, and cure orphan diseases that some thought their lives were hopeless. The analysis of Big Data gains huge significance in the pharmaceutical world, and it is my longing for those in the field to be responsible for their decisions and bring happiness to patients around the world. JB
ABUSE OF OPIOID PAINKILLER
Journalist | Seo Yeon Choi
Designer | Dawon Han
Prescription Opioids
Opioids are a type of narcotics that refer to opiate analgesics. Opioids act on opioid receptors to produce morphine-like effects. Since opiates contain chemical compounds that can relax the body and alleviate pain, they are commonly used as medications. Common prescription opioids include hydrocodone (Vicodin), oxycodone (OxyContin), oxymorphone (Opana), morphine (Kadian), codeine, and fentanyl.
The seriousness of opioid misuse has been recognized for several years in countries including the United States, Europe, Canada, and Australia. According to the WHO, approximately 115,000 people died from opioid overdose in 2017, and in 2019, it was reported that globally about 275 million people (which is 5.5% of the population aged 15 to 65) have tried opioids at least once. Additionally, the Centers for Disease Control and Prevention(CDC) estimated that three-quarters of approximately 100,000 drug overdose deaths in the United States from 2020 to 2021 were due to opioids.

| sychoi0@yonsei.ac.kr | handw3639@naver.com
The Mechanism of Action of Opioids in Human Body
‘Opioids act on the brain, spinal cord, and peripheral nervous system to suppress pain. Opiate-like substances exert their analgesic effects by acting on one or more of the opioid receptors: mu, kappa, or delta. The primary distribution sites of opioid receptors are in the brain and spinal cord. Morphine and fentanyl primarily act on mu receptors, enkephalin binds to delta receptors, and dynorphin binds to kappa receptors. Narcotic analgesics act on receptors on the dorsal horn of the spinal cord to inhibit the release of neurotransmitters from presynaptic nerve terminals, blocking the transmission of pain signals to the brain. Peptide neurotransmitters that transmit pain signals include substance P and calcitonin gene-related peptide (CGRP), while amino acid neurotransmitters include glutamate and aspartate. When the release of these neurotransmitters is inhibited, the transmission of pain signals to the brain is blocked. Alternatively, they may act on the postsynaptic membrane of the spinal cord synapses to induce excessive depolarization, interfering with depolarization blockade and inhibiting the transmission of pain signals to the brain.

Why are Prescription Opioids Problematic?
To obtain opioids, patients need a doctor’s prescription. Essentially, this means that opioids, despite being narcotics, can be legally purchased with a doctor’s prescription. In hospitals in the United States, if a patient undergoing major surgery or suffering from pain states that their pain medication is ineffective, opioids are often prescribed without much difficulty. However, the problem arises when opioid painkillers lead to dependence and addiction, even though they are properly used as prescribed. This becomes even more serious when individuals do not follow the proper usage instructions and misuse them. Some patients crush prescribed pills for snorting or mix them with liquids for injection, which results in faster and more intense effects. Moreover, prescription opioids and heroin are chemically similar and produce similar euphoria, leading some individuals to eventually use heroin. If you obtain heroin through illegal means, you don’t need a prescription, and it’s often cheaper and easier to get in crime hotspots.

The opioid crisis is widely considered to have its roots in the mid-to late-1990s. As part of Purdue Pharma’s marketing efforts for OxyContin, the U.S. Food and Drug Administration began the ‘Pain as a 5th Vital Sign’ campaign, which sparked the notion that pain must be eradicated. People began quantifying their pain, leading to a lowered threshold for pain recognition than before. Over the next decade, the number of deaths from opioid overdoses steadily rose, but it wasn’t until 2011 that the CDC officially labeled it as an “epidemic”. In response, efforts were made to reduce the overprescription of opioids, with the CDC issuing guidelines and many states implementing legal
Around 2011, there was a shift in the opioid crisis as deaths from prescription opioids stabilized, while fatalities from heroin began to rise rapidly. This was followed by a surge in deaths from synthetic opioids like fentanyl, which quickly became a major concern. Drug traffickers turned to fentanyl due to its ease of production and higher potency compared to heroin. Consequently, while deaths from heroin started to decline, fatalities from fentanyl continued to climb, exacerbating the crisis further. In the past decade, the opioid crisis has evolved significantly, with fentanyl emerging as the most deadly opioid. Despite efforts to address the crisis, overdose death rates have continued to rise, driven by the increasing availability and use of potent synthetic opioids. Most states have seen significant

How to improve adherence to medication for clinical trial subjects
Journalist | Hoyeon Dam |
sjk28ho@gmail.com
Designer | Soyun Kang | soyun525@yonsei.ac.kr
Importance in new drug development with AI
As AI technologies have improved drastically, technical convergence has been introduced in diverse industries nowadays. This circumstance led us to a more convenient life to live. Even the pharmaceutical industry couldn’t be spared from this. To develop new drugs, patients with similar symptoms must go through a clinical trial stage to confirm the treatment’s effectiveness. The development of new drugs requires lots of money and time. There are various steps in the development of new drugs. Among them, the clinical trial is the most important because it is the last step before getting approved by the Ministry of Food and Drug Safety. Clinical research is the process of proving the effectiveness and safety of any trial or new drugs under research and development that is conducted using samples extracted from a person or information about a person, before the use of a new drug. The clinical trial stages are divided into non-clinical, Phase 1, Phase 2, Phase 3, and Phase 4 trials. Clinical trials can be expensive, depending on several factors. To process the trial precisely, subjects of trials need to take medicine as the pharmacists indicate.
Example of company utilizing AI in a trial stage
InhandPlus is a company that has developed smartwatches and AI-based medication management solutions to address the low drug compliance of subjects in pharmaceutical clinical trials. It optimizes global clinical trials by providing accurate practical medication data (RWD) based on an AI smartwatch that can analyze when and which drugs patients are taking. Their solutions are considered to be innovative solutions that can overcome existing limitations with only wearable devices. It has the advantage of being easy to use and having high data accuracy and is profitable because it can analyze a wide variety of medicines. The problem of the lack of an accurate solution for drug administration in the existing market was the compliance of clinical trial subjects was poor. In addition, the need for the collection of RWD has emerged as remote clinical trials or decentralized clinical trials have emerged as a trend after the COVID-19 pandemic. InhandPlus emphasized that it has been working to develop a solution that fully responds to this demand. InhandPlus’s solution is already being used in domestic clinical trial sites, and it has also achieved the result of attracting large clinical trial trustees (CRO) companies as investors. It is also partnering with a variety of pharmaceutical companies and hospitals to sustain growth potential.

Prospect of Korea’s pharmaceutical using AI
The new drug development with AI is a very segmented and highly competitive market. As a result, global pharmaceutical companies are actively investing in the development of AI for new drugs that can speed up new drug development and dramatically increase the probability of success. AI technology is being introduced in a variety of ways, including building its own AI technology platform or collaborating with AI drug development companies, partnerships, and joint development. There are still no worldwide cases of developing new drugs using AI from start to finish. By leveraging different platforms step by step, companies are actively accelerating AI development through collaboration with other companies. Recently, Nvidia, a U.S. semiconductor company, drew attention by invested $5 million (about 64 billion won) in bio-company Recursion. In the case of overseas companies such as Pfizer and AstraZeneca, the size of R&D investment is so large that they are actively focusing on securing AI professional manpower or actively investing in ventures.
In Korea, governments and associations are supporting the development of new AI drugs. First of all, the AI New Drug Development Support Center is conducting LAIDD, an online education platform for new AI drug development. LAIDD is the first Korean comprehensive education platform for AI new drug development that aims to nurture convergence talents in the field of AI and pharmaceutical bio. In addition, it has revitalized the AI new drug development ecosystem through cooperation networks. Currently, the ‘AI-based New Drug Development Advisory Committee’ is running, in which 18 experts from industry, academia, and research institutions participate. The introduction of AI by pharmaceutical bio companies is also spreading. Pharmaceutical bio companies that establish an AI team or carry out collaborative research with AI startups have expanded from 5 companies to 6 in 2019 to 30 in 2023. Thus, the acceptance of AI in the process of the development of new drugs is now inevitable. It’s time for pharmaceutical companies to positively accept AI in various aspects. The more actively the company accepts, the higher the probability that the experiment will succeed. JB

The Application and Prospect of AI Technology in Korea’s Pharmaceutical Industry
Journalist | Yoonah Jung | nyoonah@yonsei.ac.kr
Designer | In Jeong | jeong.in@yonsei.ac.kr
Domestic pharmaceutical companies are actively using AI (artificial intelligence) to develop new drugs. The convergence of pharmaceuticals and AI is expanding worldwide, and Korean pharmaceutical companies are expected to follow this trend.
In August 2023, the Korea Pharmaceutical Bio Association established a new AI drug development team, and the number of domestic pharmaceutical bio companies conducting cooperative research with AI companies has increased by 35 over the past five years.
Application of AI (Artificial Intelligence) to Drug Discovery
The term ‘AI new drug development’ refers to the development of a new drug using clinical data and an AI algorithm suitable for the development of a new drug. The AI algorithm used in the pharmaceutical industry is an ‘AI platform’ that utilizes deep learning. This AI platform analyzes data based on the vast amount of research data accumulated so far and hospital treatment records. It also contributes to the stable and efficient process of new drug development by using data to find innovative new drug development candidates and predict the efficacy of the drug. Pharmaceutical companies are adopting AI technology because it drastically reduces the time and cost required for developing new drugs. The average time required to develop new drug without AI is more than 10 years and the cost is 2 to 3 trillion won, the probability of failure reached 92%. However, if AI is incorporated into this process, the new drug development cycle could be shortened from 15 years to 7 years, and the development cost could be reduced to about 600 billion won.
‘Federated Learning-based New Drug Development Acceleration Project’ in Korea
The ‘Federated learning-based new drug development acceleration project’ aims to shorten the development period of new drugs and reduce costs by using a Federated Learning model that learns AI from individual institutions, without mixing data held by various companies and institutions.
Since ‘federated learning’ learns data held by each institution without leaking it to the outside, there is little risk of information leakage, so it is possible to ‘protect’ and ‘use’ sensitive information at the same time. Through this, an artificial intelligence (AI)-based new drug development system is expected to be created by jointly utilizing data from domestic pharmaceutical companies.

Korea’s Pharmaceutical Industry Signing MOU with AI Platform
As the importance of applying AI to the pharmaceutical industry is emphasized, domestic pharmaceutical companies are actively signing MOUs with AI platforms. For example, ‘Oncocross’ is using their development platform ‘RAPTOR AI’ to discover new indications. According to the company, the platform is used to search for new indications for new drugs under discovery or already approved drugs and to derive combinations of drugs for combination therapy. ‘Oncocross’ signed an MOU with ‘Boryeong Pharmaceutical’ last year to confirm a new indication for its hypertension drug, ‘Kanav’. The company is also collaborating with ‘Daewoong Pharmaceutical’ and ‘Jeil Pharmaceutical’.
Difficulties of using AI Technology in Korea’s Pharmaceutical Industry
Korea is facing difficulties in developing new AI drugs. While global pharmaceutical companies are using AI in all areas of new drug development, Korean companies are focusing on discovering candidate substances, and are not using it in target discovery or in preclinical/clinical fields.
In addition, there is a lack of data that AI can learn in Korea. Domestic pharmaceutical companies have less than one-hundredth of the data compared to global pharmaceutical companies. Accordingly, domestic AI companies are creating AI models based on public data, so which inevitably has limitations.
Lastly, the lack of experience cannot be ignored. AI is advanced through deep learning based on various experiences, and domestic AI companies currently lack experience compared to global companies due to the lack of recognition and research funds from pharmaceutical companies.
Prospects of Korean Pharmaceutical Companies Using AI
It has only been four to five years since AI introduction has been activated in Korea, so few results have been made compared to investment costs. However, it is difficult to judge AI as an unhelpful technology. Korea Pharmaceutical and Bio-Pharma Manufacturers Association’s master said “Now is the time to think a lot about which direction drug development should proceed in the future. Also, it is time to decide which step AI should be used to find new drugs.” In Korea, AI new drug development technology has not yet matured and seems to be developing. Korean Pharmaceutical needs to show a lot in terms of performance that AI technology is developing well. B

The development of korean sports pharmacy
Journalist | Myungjun Kim Designer | Dahyun Ryu
| flytothejune@naver.com | dahyunryu@yonsei.ac.kr
Recently, as athletes’ doping problems and the importance of medical support are emerging, the role of pharmacists in sports has never been greater. Sports pharmacy can be divided into two categories; doping prevention and sports medicine. ‘Doping’ is the usage of prohibited drugs that help to enhance sports ability and athletic performance. Doping should be eradicated because it undermines fundamental sports values such as fair play and integration. Also, it can cause severe health issues. As the origin of doping goes back to the first creation of sports itself, it is clear that doping has been a serious problem for a very long time. In this area, the role of sports pharmacists is to prevent doping by providing accurate guidelines for sports medications to athletes. Furthermore, athletes need proper healthcare and injury management. Pharmacists can help sports conditioning by providing appropriate nutritional supplements, medication, and treatment.
Many foreign countries have activated sports pharmacy policies. For example, JADA(Japan Anti Doping Agency) introduced an authentication policy including strict compulsory steps such as both theoretical and practical examinations, annual seminars, and additional renewal tests. Japanese sports pharmacy policy was able to be stabilized through wholehearted support from the government and the active participation of pharmacists. However, despite its importance, sports pharmacy activities have been started not long ago in Korea. In this article, I would like to introduce issues in Korea related to sports pharmacy.
Society of Sports Pharmacy
The Society of Sports Pharmacy was established in 2023 based on The Sports Nutrition Pharmacy Organization. Its goal is to develop the national role of pharmacists in the field of sports; working in various areas including doping prevention, sports conditioning, sports physiology, biochemistry, and dietetics. They have conducted diverse training courses and seminars for sports organizations, serialized journals, and participated in health-related symposiums. They are also planning various activities in tandem with organizations such as The Korean Pharmaceutical Association and KADA(Korea Anti Doping Agency).



The Birth of the First Korean Sports Pharmacist
As sports pharmacy at the 2018 Pyeongchang Olympics and 2019 Gwangju FINA World Aquatics Championships got a favorable response and the need for counseling for athletes in local pharmacies arose, the Korean Pharmaceutical Association opened an authorized sports pharmacy qualification certification course to nurture professional sports pharmacists. The course is composed of 12 online lectures about diverse topics such as injuries and rehabilitation, global issues, and local sports pharmacy management. On January 14th, 2024, 1177 pharmacists completed the course and were certified as professional sports pharmacists. The Korean Pharmaceutical Association is planning to give certificates and signboards that can be posted at pharmacies. Also, as types of banned drugs vary every year, they are planning to open an annual training program and mandate participating in the updated program every 3,4 years. Furthermore, opening websites that can help athletes through sports pharmacists’ counseling is a plan.
As this is the first birth of an official Korean pharmacist, there are several deficiencies in the authorization course program compared to other countries. However, it is remarkable in that it can start an expansion of pharmacist expertise area and sports pharmacist promotion.
The Necessity of Sports Pharmacists in Korea
The role of pharmacists in sports is essential because athletes can violate doping unintentionally. Park Taehwan, 2008 Olympic gold medalist in 400-meter freestyle, tested positive for the doping test by WADA. The caught substance was testosterone in Nebido injection. The problem is that he had asked his attending physician whether the injection violated doping, but the doctor told him it was okay. Similarly, Asian game gold medalist Lee Jinil was caught doping, not knowing that his cold medicine contained a prohibited component. Also, doping is a significant problem not only in elite sports but also in sports for all. According to ‘A Survey on the Prevention of Doping of Sportsmen’ by The Ministry of Culture, Sports and Tourism, 34.8% of a beginner in everyday sports applied that they had used forbidden substances. This is because they lack a precise understanding of allowed sports medications. Furthermore, providing accurate pharmaceutical treatment for sports injuries is significant. Pharmacists’ expertise contrary to doctors can effectively help sports conditioning in areas such as dietetics and prescribing appropriate medicine to athletes, who have special requirements as they are a unique group of patients.
Therefore, the settlement of the sports pharmacist system in Korea is urgent. As athletes’ interest in sports pharmacy is increasing and diverse activities are in progress, it is predicted that sports pharmacy in Korea will develop a lot soon. Promotion of local sports pharmacies to professional and amateur athletes, authorized training programs and certification renewal policies, and activation of counseling programs are imperative. Unintended doping should disappear and every athlete should get proper pharmaceutical treatments so that sports in Korea can develop and every athlete receives credit for their great efforts. B

Fixed-Dose Combination Therapy in Type 2 Diabetess
Journalist | Hyemin Park | aeternamarbar@naver.com Designer | Seungwoo Lee | seungwu210@yonsei.ac.kr
People must have been prescribed medicine at the hospital. Was there only one medicine you received at that time? Or did you receive several types? In most cases, people have to take several drugs to cure one disease. When you take different kinds of drugs, wouldn’t it be nice if all these drugs were combined into one? Thanks to pharmacologists who work in clinical pharmacology, the invention of fixed-dose drug combinations has become available, making this into reality.
What is a fixed-dose drug combination?
According to the Food and Drug Administration (FDA), Fixed-dose drug combinations, also called FDCs, are combinations of two or more active drugs in a single dosage form. They have become a significant alternative to monotherapies in the treatment of diseases such as hypertension, diabetes, Helicobacter pylori, AIDS-HIV infections, tuberculosis, asthma, and Chronic Obstructive Pulmonary Disease by offering several advantages, which will be dealt in the next chapter.
Advantages and Disadvantages of FDCs
People are paying attention to FDCs because they have benefits in terms of accessibility to patients and the efficacy of medicine that general drugs do not have.
Benefits in Accessibility to Patients
1. Increase patient compliance by reducing the pill burden;
2. Increase disease adherence
3. Improve patient convenience
4. Simplify disease management by reducing the dose of individual drugs
5. Reduce financial burden; FDCs are cheaper than individual drugs due to reduced costs from packaging to distribution.
Thus, FDCs are especially beneficial for patients with chronic diseases, which have to be managed for the long term.
1. Have pharmacokinetic advantage
2. Decrease the development of resistance
Benefits in Efficacy
3. Reduce risk of adverse events; FDCs reduce toxicity by counteracting another’s adverse reactions.
Just as there are two sides to every coin, there are also disadvantages.
Disadvantages
1. Cannot achieve the individual optimum dose for a patient; All components have to be changed even if there are slight changes in treatment.
2. Have difficulties in discovering new medicines; The action of FDCs in the human body may be diverse in duration and bioavailability. In addition, interactions between different components also have to be considered.

Treating type 2 diabetes by taking FDCs in Korea
Despite the difficulties of discovering safe, effective FDCs, there are plenty of them on the market, one of which is a drug used to treat type 2 diabetes.
Then why have FDCs become one of the notable treatments for type 2 diabetes? In patients with type 2 diabetes, most of them need to take multiple drugs at once, but this may decrease long-term adherence. Due to the nature of diabetes, FDCs are a silver bullet to increase the drug compliance of patients.
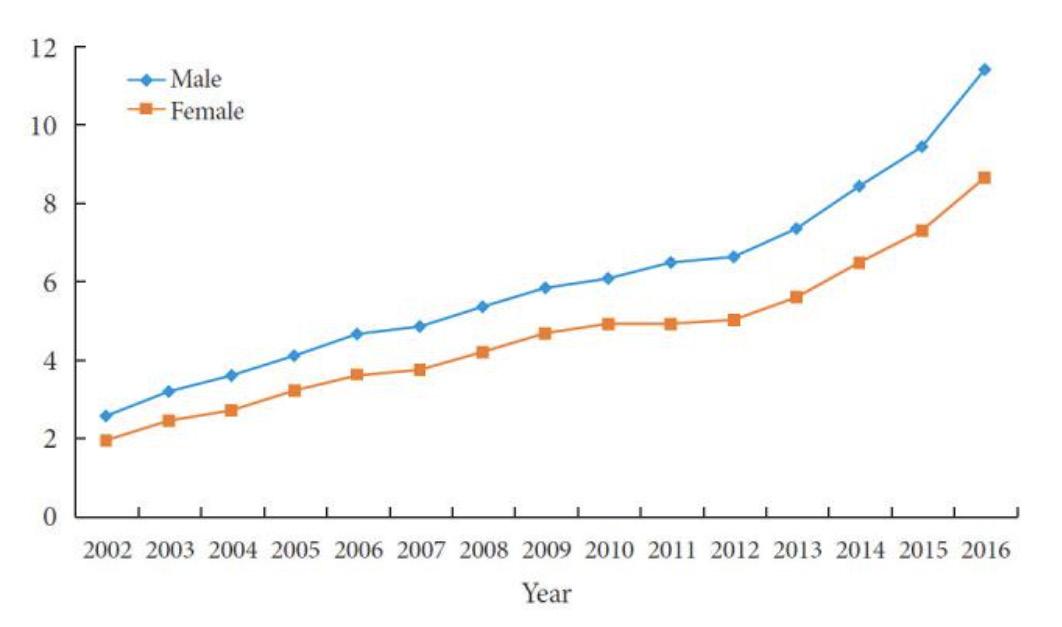
<Fig. 1.> The prevalence of type 2 diabetes mellitus per 10,000 people among Korean children, adolescents, and young adults younger than 30 years of age from 2002 to 2016. A difference in the slope between males and females is confirmed with the test for trend, P (P=0.015).
Recently, the incidence and prevalence of type 2 diabetes have been rapidly increasing in Korea, especially in those under 30 years of age. Fig. 1. presents the gradual and constant increase in the prevalence of type 2 diabetes. The prevalence steadily increased from 2.27 per 10,000 people in 2002 to 10.08 per 10,000 in 2016. As the demand for type 2 diabetes treatments increases, efforts to develop diabetes treatments continue.
In June 2023, Korea AstraZeneca's type 2 diabetes combination drug 'Sidafvia' was approved by the Ministry of Food and Drug Safety. Sidafvia is a combination of Dapagliflozin, an SGLT-2 inhibitor, which has benefits of weight loss, heart failure, and chronic kidney disease (especially in diabetes patients), as well as blood sugar control effects, and Sitagliptin, a DPP-4 inhibitor, which can help manage blood sugar after meals and reduce blood sugar variability.
There are several pros of FDC therapy in treating type 2 diabetes. A DPP-4 inhibitor is characteristic of increasing insulin secretion, and a SGLT-2 inhibitor is characteristic of increasing glucagon secretion. DPP-4 can offset the increase in glucagon secretion and have a complementary effect with SGLT-2. Hence, combination drugs of SGLT-2 and DPP-4 are suitable for improving insulin resistance and secretion ability. Sidafvia has a better glycemic inhibitory effect than taking Dapagliflozin and Sitagliptin separately. In addition to the drug's effect, patients' compliance with the drug was 13% higher than those who had taken the two components separately, and the compliance rate was also 21% higher.
FDC therapy has advantages in terms of effectiveness, safety, and compliance due to the nature of type 2 diabetes treatments, which should show all the effects of weight control, cardiovascular disease effectiveness, postprandial blood sugar management, and blood sugar variability reduction. As the number of diabetic patients increases, I hope that the use of fixed-dose combination drugs will expand as interest in FDC therapies that are effective in treating diabetes increases. B

Shifting Trends in the Pharmaceutical Market Due to Korea’s Aging Population Growth
Journalist l Yejoo Lee l minthime04@gmail.com
Designer l Chaerin Oh l cherin02@naver.com
Nowadays, the majority of developed countries have already entered into an aging society, where individuals aged 65 and over represent more than 7% of the total population. In particular, South Korea is experiencing rapid aging due to an increase in life expectancy combined with a significant decrease in birth rates. According to statistics released by the Ministry of the Interior and Safety at the end of 2023, the elderly population aged 65 and above has increased by 5% from the previous year, reaching 9,734,411 and accounting for about 19% of the total population. For the first time since population records began, the population over the age of 70 (6.32 million) has exceeded that of those in their 20s (6.20 million). Future projections by the Statistics Korea suggest that by 2025, the elderly population will make up 20.3% · by 2036, 30.9% · and by 2050, it will exceed 40%. This demographic shift is bound to bring significant changes to the pharmaceutical market.
Characteristics of Elderly Patients in Drug Development and Prescription
When developing new drugs or researching new ways of prescriptions for existing drugs for elderly patients, several factors must be considered. Elderly patients mostly experience a general decline in health and suffer from various chronic diseases. Research from the Asan Medical Center indicates that the prevalence of chronic diseases among those aged 65 and older has doubled from 2008 to 2020, with a steady increase in diseases such as dyslipidemia, diabetes, and cardiovascular diseases. Elderly individuals in Korea suffer from 1.9 chronic diseases on average, with hypertension (56.8%), diabetes (24.2%), excluding hypertension, hyperlipidemia (17.1%), and osteoarthritis or rheumatoid arthritis (16.5%) being the most common. This underscores the importance of thorough research into these diseases. Additionally, the fact that many elderly patients have multiple chronic diseases necessitates careful consideration of the side effects of polypharmacy.
Changes in the Pharmaceutical Market Due to an Increase in the Elderly Population
1. Expansion of the Pharmaceutical Market Size
The elderly population consumes more pharmaceutical products than younger generations due to health deterioration. An estimation by KHIDI in 2021 showed that out of the total pharmaceutical consumption amounting to KRW 23.3669 trillion, the elderly accounted for KRW 10.8517 trillion (46.4%). As the elderly population grows, the market for patient-specific medications grows, leading to a significant increase in health insurance treatment costs and medication expenses.
2. Focus on the Geriatric Disease Market
According to previous statistics by KHIDI, the highest consumption of pharmaceuticals by the elderly was in the cardiovascular system drugs, accounting for about 52% of total cardiovascular drug consumption. Central nervous system medications followed, with 53% being consumed by the elderly. Diseases commonly associated with aging, such as atherosclerotic cardiovascular diseases, arthritis, diabetes, Alzheimer’s disease, stroke, and Parkinson’s disease, are included in the scope of ‘geriatric diseases’. The domestic pharmaceutical market already sees high sales from various treatments targeting these diseases. For example, in the first half of 2022, based on health insurance claims, Prolia (by Chong Kun Dang & Amgen Korea), a treatment for osteoporosis, was the top-selling product. Eylea (by Bayer Korea), a treatment for age-related macular degeneration, ranked fifth. Lots of domestic pharmaceutical companies are actively engaging in research and development in this growing market.
3. Growth of the Digital Healthcare Industry for the Elderly
In an aging society, not only the pharmaceuticals themselves but also ‘how cognitively impaired elderly patients can correctly take medications and improve their environment for better health’ becomes a fundamental issue. Numerous domestic pharmaceutical and biohealth companies are preparing senior-focused businesses incorporating digital technology. Building a non-face-to-face environment to assist many elderly individuals is important. While most programs are still in the trial phase, gradual progress is expected. For instance, from May to September 2023, LifeSemantics provided ICT-based non-face-to-face counseling services, aiming to enhance medical accessibility for elderly patients facing difficulties visiting hospitals. The potential of the elderly digital healthcare industry is immense. The development of remote health management platforms offering medication guidance, exercise, and nutrition management content is possible. Even utilizing AI to review medication adherence instructions in place of medical professionals can be considered.

Conclusion
Considering the pronounced aging phenomenon in Korea alongside the rapid advancements in technology, expected changes in the pharmaceutical market are as outlined above. The elderly population will become a key demographic for the Korean pharmaceutical industry. Pharmaceutical companies should focus on geriatric diseases and digital healthcare to achieve success, while pharmacists must be aware of the characteristics of elderly patients and improve medication counseling for the elderly population, which will soon comprise half of the country’s demographic. B

NASH Treatment Breakthrough, Rezdiffra
Journalist | Jae Eun Park |
ong114164@naver.com
Designer | Jin Yeong Kim | kimjin02000@yonsei.ac.kr
The U.S. Food and Drug Administration (FDA) has approved a treatment for non-alcoholic steatohepatitis (NASH), sold under the brand name RezdiffraTM, for the first time on March 14, 2024. It is considered a historic milestone in the treatment of liver disease and provides NASH patients with a glimmer of hope. Through this article, we would like to find out about NASH and its treatment, as well as the future direction of its development.
What is NASH?
Liver disease has been surging worldwide, and about 24% of U.S. adults have NAFLD. Non-alcoholic fatty liver disease (NAFLD) is a condition in which excess fat builds up in the liver. This buildup of fat is not caused by heavy alcohol use. When heavy alcohol use causes fat to build up in the liver, this condition is called alcohol-associated liver disease.
Two types of NAFLD are non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH). NASH is the form of NAFLD in which inflammation and damage are made within the liver along with the presence of fat. The inflammation and liver damage of NASH can cause fibrosis, or scarring, of the liver. 10 to 29% of NASH patients develop cirrhosis within 10 years, and 4 to 27% of them progress to liver cancer.
The pathogenesis of NASH is complex and diverse. There are various pathophysiological causes such as inflammation, diabetes, and intestinal bacterial imbalance. Hepatocytes are killed by various stimuli, and the resulting inflammatory response and progression of fibrosis are known as the main mechanisms of NASH. Liver fibrosis is the process in which myofibroblastic cells activated by various qualifications produce an extracellular matrix and are excessively deposited in the liver, and hepatic stellate cells are activated to form the majority of myofibroblastic cells. Activated hepatic stellate cells secrete several cytokines to maintain their activity, and increase the activity of other cells to cause continuous inflammation and fibrosis reactions.

Why did the first approval take so long
To synthesize a drug that is resistant to a certain disease, it is necessary to understand the mechanism of onset and identify the target. However, it is difficult to specify only one single cause, as NASH is attributed to various factors, including inflammation mediated by cytokines that transmit intercellular information, endoplasmic reticulum stress caused by vesicles (subcellular organelles) that can lead to diabetes, and imbalances in gut microbiota. Furthermore, NASH is a complex disease characterized by multiple pathophysiological processes that interact simultaneously in the liver. These processes include fat accumulation, inflammation, and fibrosis, among others, and their interplay determines the nature of the disease. Due to this diversity and complexity, a single treatment approach cannot be applied to all patients.
The lack of established indicators or biomarkers for measuring the effectiveness of treatments is also an obstacle to the development of NASH treatments. For diagnosing NASH and assessing treatment responses, liver biopsy, a test that takes a sample of tissue from the liver for examination is required, which is an invasive test and cannot be performed repeatedly. An invasive test refers to a test in which microorganisms like bacteria or testing equipment enter the body’s tissue. Especially for treatments related to liver diseases, evaluation of hepatotoxicity is necessary, and it is important to provide effective treatment while minimizing such side effects. FDA recommends ‘improving NAFLD scores without worsening liver fibrosis’ as an evaluation parameter for NASH treatments.
Lastly, another reason why potential therapeutic agents fail to demonstrate significant effects even in short clinical trials is the time-consuming evaluation of the most crucial aspect of NASH improvement in liver fibrosis. Evaluating this requires a considerable amount of time. Therefore, the development of NASH treatments has been challenging so far, with numerous considerations to address before obtaining the first approval.
The Future of NASH treatment
Rezdiffra, made by Madrigal Pharmaceuticals, is an oral daily pill that activates a thyroid hormone receptor β(THR-β) that reduces liver fat accumulation. Resmetirom, the generic name of Rezdiffra, was tested at two dose strengths and showed resolution of NASH without worsening fibrosis in 26% (low dose) and 30% (high dose) of patients compared to 10% in the placebo group. Also, the trial indicated that the medication improved fibrosis by at least one stage in both low-dose and high-dose groups. NASH fibrosis improvement effects were consistent regardless of age, sex, type 2 diabetes status, and fibrosis stage. However, this approval will be officially approved depending on whether clinical benefits are verified in confirmatory clinical studies undergoing rapid approval.
The global market for NASH treatments is expected to grow from 3.7 billion U.S. dollars in 2021 to 5.8 billion dollars in 2026 and 20 billion dollars in 2030. Some predict that the market will become a big market that exceeds 30 trillion won by 2030.
Then how about the domestic market Representative domestic companies are Hanmi Pharmaceutical and Yuhan Corporation, working hard to develop a treatment for NASH. In addition, clinical trials for the NASH pipeline have been started by Dong-A ST and Olyx. Through these clinical trials, the safety, and effectiveness of the treatment and also a new biomarker should be verified. Since NASH has a large number of patients and has a high demand for drugs, however is one of the representative blue oceans in the bio-industry, efforts to find effective ways to treat NASH should continue. These efforts will provide a glimmer of hope for NASH patients and improve their quality of life. B
The Impact of Online Platforms on Pharmacies

Journalist | Dakyung Baik | dakyungbaik@gmail.com
Designer | Seungwoo Lee | seungwu210@yonsei.ac.kr
The Rise of Online Pharmacies
The traditional brick-and-mortar pharmacies may become a thing of the past. The recent pandemic has ensured that pharmacies find ways to provide clients with easy access and enhanced care. New pharmacy models were brought forth to improve health care. Out of convenience and necessity, online pharmacies have gained more traction throughout the pandemic. With the growth of these digital pharmacies, the benefits and drawbacks of providing health care in such ways have quickly become an issue.
Digital pharmacies have been around for some time. However, recent events regarding the pandemic and the rise of online platforms triggered a dramatic increase in the demand for such services. During the pandemic, patients required access to their medicine without the fear of exposure to the virus. Pharmacies started to provide curbside pickup and delivery services. For patients living in rural areas, ‘mail-order pharmacies’ would pick up their prescriptions and deliver the medicine to their doorstep. Pharmacies also developed management platforms that enable clients to line up for virtual queues and check whether the medicine they are looking for is available via mobile devices. Even after the pandemic, such platforms are expected to continue providing better services to both pharmacists and their patients.
The Benefits and Drawbacks of Online Pharmacies
The benefits of online pharmacies are hard to ignore. Convenience and accessibility are the main perks. Distance and timing are no longer an issue, and clients are given access to a comprehensive range of products. For patients living in rural areas who no longer need to travel long distances for their medicines, this can ensure a significant cost reduction as well. Many online pharmacy platforms provide various other useful functions. Clients can conveniently chat with their pharmacists on the app and get the necessary guidance for their medicines. The platforms also enable pharmacists to set pill reminders for their clients and allow clients to save their health records.

However, online pharmacies come with many risks for both the clients and pharmacists. Lack of counseling that leads to self-medication, self-diagnosis, and substance use disorder (SUD) is the biggest pitfall of online pharmacies. Without the face-to-face interactions of traditional pharmacies, there is a possibility that clients wouldn’t be given enough information on medication use, side effects, and other additional risks. Falsified or substandard medications are also a major threat. Illegal online pharmacies lure customers with discounts, speed, and convenience and provide poor-quality medicines that are considered a public health threat. These medicines lead to a heightened risk of treatment failure and mortality. Low-income countries with poor pharmaceutical regulations and poor supply-chain management are especially at risk of these problems. Internet crime-related losses should also be considered. Healthcare-related crimes like insurance fraud and fabrication of prescription medications will also target online pharmacies.
The Future of Online Pharmacies
Though many pharmacists agree on the potential benefits of online pharmacies, regulatory issues are the major setbacks. Laws regarding online pharmacies vary drastically by territory, and the platforms being online, lack of regulations in one country can affect consumers globally. The International Pharmaceutical Federation stated that regulations that include specific guidelines and enforcement strategies will be necessary to ensure the safety of consumers. Especially, since cross-border operations of online pharmacies are expected to be common in the future, cooperation between countries and states is a must. Pharmacy markets are currently guided by sector-specific laws thus pharmacists must initiate active discussions between regions and countries to ensure a certain agreement on regulations regarding online pharmacies.

The close integration of online platforms with retail pharmacies is a future that can’t be avoided. After COVID-19, there is a growing appreciation for the enhanced role pharmacists can play. With the help of online platforms, pharmacists were able to step up in their roles and provide the necessary clinical services during the pandemic. Even after the pandemic, this trend has continued. Now, the pharmaceutical industry must adapt to the use of technology for its services. Global cooperation and appropriate regulations will be necessary to adapt to this change quickly and appropriately. B

THE RISE OF TELEMEDICINE IN PHARMACEUTICAL CARE :BENEFITS AND DRAWBACKS
Journalist | Yunseo Lee | leeyunseo2003@gmail.com
Designer | Daeun Jeong | jolly1245@yonsei.ac.kr
Telemedicine in pharmaceutical care refers to the use of online platforms and telecommunications to consult with patients without meeting them face to face. Amongst a variety of factors, COVID-19 was a key factor in the accelerated spread of telemedicine. After the pandemic, the increase in the elderly population, the rise of chronic diseases, insufficient healthcare professionals, and improved accessibility to better and cheaper technology promoted the growth of telemedicine (1). With its growing importance, this article focuses on the benefits and drawbacks of telemedicine in pharmaceutical care and how it should be implemented in the future for its best effect in the pharmaceutical industry.
*Telemedicine in pharmaceutical care may be referred to in various terms, including telehealth and telepharmacy.
Types of Telepharmacy
Telepharmacy can be classified in the following four ways: Remote patient counseling, remote dispensing, remote order-entry review, and intravenous admixture verification (2). Order entry-review refers to the reviewing of the medication orders by the pharmacologist before they are administered to the patient (3), and intravenous admixture verification refers to the inspection of the medication, labeling, exact dose, contamination, and such to prevent medication errors (4). These four implementations of telepharmacy facilitate the processes involving pharmaceutical care.
Benefits
This method is extremely beneficial when physical contact becomes difficult or if the patient lives outside the reach of medical supervision. A representative circumstance was COVID-19, when physical contact or face-to-face meetings were strictly restricted by the government at a national and global level. Telehealth was a great way of reaching out to the patients. Moreover, with increased easy access to online platforms and technology, telehealth offers an alternative to those who require extra help to visit pharmacies or drug stores, such as those who have disabilities or are elderly. The use of electronics is also less costly and time-saving. With such clear advantages, telemedicine is paving its way toward a greater public.

Drawbacks
Despite how flawless telemedicine may seem, drawbacks certainly exist. First of all, security and privacy are not guaranteed by telemedicine. Online transfer of patients’ personal information is prone to leakage and hacking. Moreover, telemedicine cannot fully replace in-person visits. “[T]ouch, physical presence, and emotional connection”, which are imperative in the pharmaceutical examination, are absent, which may lead to inaccurate or insufficient delivery of care. Furthermore, those who are elderly or suffer from physical and/or psychological difficulties may have a hard time figuring out how to use digital technologies (5). These factors may be critical to an individual’s interaction with the pharmacologist. Thus, we must remind ourselves of the pros and cons of telemedicine to implement them appropriately in the pharmaceutical industry.
Future Implementation
The spread of telemedicine seems hopeful in a futuristic perspective. According to the findings of the Organisation for Economic Co-operation and Development (OECD), governments have been actively promoting telemedicine through financial support. However, many of these measures are temporary, and it is uncertain whether they will remain as a permanent method of pharmaceutical care. Moreover, in many countries, regulations regarding this matter are not clearly established (6). COVID-19 was clearly the accelerator to the adoption of virtual care, and now that its impact or severity has decreased, or at least perceived so by the public, the future of telemedicine has become unpredictable.
Despite uncertainties in its maintenance and sustainability, telepharmacy has definitively shown its positive outcomes to the pharmaceutical industry. This article suggests the following measures in order to ensure its appropriate application. The building of a secure online platform free of leakage of personal information, efforts to educate and train healthcare professionals in the use of telepharmacy-technology, and the establishment of transparent guidelines and standards. All of the mentioned are crucial in the proper and safe execution of telepharmacy. With proper education and governmental support, telepharmacy, and telehealth in a broader sense, may become a chief factor in the development of worldwide healthcare.

Connecting the Human Brain and Computer: Neuralink
Journalist | Jeonghye Seol | kkum3719@gmail.com
Designer | Su Yeon Kim | sooyeon0112@yonsei.ac.kr
On March 22, 2024, a video of Neuralink’s first clinical trial participant playing chess was released through X (formerly Twitter). The first clinical trial participant was a patient who had been fully paralyzed from the shoulders down for eight years, and according to Neuralink, he underwent surgery in January of this year to implant a chip(N1) into his brain. In the video, he was controlling digital devices using only his thoughts, without using his body, such as playing chess or stopping playing music.
The scenarios often depicted in science fiction movies, such as implanting devices into the living human brain to read information and manipulate computers, have become a reality. While this is not the first time of BMI (or BCI) has been used, the fact that Elon Musk, the CEO of Tesla and SpaceX, known for turning imaginative ideas into reality, is the CEO of this company made this technology even more famous.
Neuralink and its Founder
Neuralink is an American BMI (Brain-machine Interface) development company founded in San Francisco, California, in 2016 by Elon Musk and Max Hodak. The company’s name, Neuralink, a combination of the words “Neural” and “Link”, clearly reflects the company’s mission; to connect the human brain and computers.
Elon Musk has presented two main purposes of the Neuralink project. He has often stated that “Solving human problems is the main goal of his life.” and these two objectives of the Neuralink project reflect this well.
First, overcome human brain-related diseases.
Second, enhance human intelligence so that humans do not lag behind artificial intelligence at the point of ‘Singularity’.
About BCI
BMI (Brain-Machine Interface) technology connects the brain and computer for bidirectional interaction. Originally developed to aid external communication for limb paralysis patients, BMI has expanded to areas such as diagnosing mental disorders and assisting rehabilitation for the elderly and disabled.
BMI technology operates by recognizing and encoding brain signals and then analyzing them to command devices. Brain signal collection typically involves measuring electrical responses or changes in brain blood flow. Devices for signal collection are categorized as invasive or non-invasive, depending on whether they are inserted into the brain inside the skull or placed outside the scalp. While invasive devices enable precise signal measurement, they carry risks of brain damage. Non-invasive devices, on the other hand, pose no risk of brain injury but often collect lower-quality signals due to noise interference.
Processed brain signal information, after removing noise and conducting various analyses, yields insights into the user’s intentions and states, allowing for machine control based on this information. However, invasive BMI technology has been limited in everyday use due to risks of brain injury and constraints on the amount of collectible information, primarily used for therapeutic purposes in neurological patients.

History of Neuralink
Neuralink was founded in 2016 and announced its establishment in 2017. In 2023, Neuralink announced plans for clinical trials implanting computer chips into the brains of paralyzed individuals, unveiling company technology and clinical results. In 2019, Neuralink conducted trials on mice, followed by trials on pigs in 2020, and monkeys in 2021. In May 2023, Neuralink received FDA approval for its first human clinical trial. Recruitment for trial participants began in September 2023, and the first surgery targeting a participant took place in January of this year. The video released through X on March 22nd is the result of this first clinical trial.
History of Neuralink
Neuralink uses a method of inserting electrodes, coated threads resembling thin wires, directly into the brain through a sewing-like procedure, allowing precise reading of brain electrical signals near neurons. According to the information brochure available on Neuralink’s official website, Neuralink’s devices consist of the N1 implant, R1 Robot, and N1User App.
- The N1 implant is a device implanted into the brain to read brain activity and operate computers. The N1 Implant has 1024 electrodes, thinner than a person’s hair, to record neural activity, causing minimal damage to the brain and delivering a lot of information precisely.
- The R1 robot is a machine designed to insert the N1 implant into brain areas without blood vessels safely and securely.
- The N1 User App is an app that decodes intentions from brain signals to enable movement.
The steps of the chip insertion surgery are as follows: Firstly, the scalp is incised, and then a circular hole is drilled into the skull. Through this hole, a wire coated with electrodes is inserted into the brain using a machine. Next, the wire is connected to the circular ‘link’ and used to fill the hole in the skull. Once the scalp is covered, the surgery is complete. The inserted chip (N1) can be wirelessly rechargeable, and the measured signals are transmitted to the device via Bluetooth.
The Neuralink product employs a greater number of electrodes for implantation compared to its predecessors, enabling more accurate analysis and interpretation of brain activity. Additionally, it utilizes flexible and biocompatible materials to minimize damage to both equipment and brain tissue. Finally, through the use of surgical robots, electrodes can be inserted while avoiding cerebral blood vessels, thereby reducing brain damage and ensuring the safety of the surgery.
You can learn more about Neuralink’s technology and its purpose at Neuralink’s official website.
Elon Musk has stated that this surgical technique can implant chips into the brain safely without side effects. He also mentioned that he would make this similar to LASIK surgery. Can BCI technology advance to the extent Elon Musk suggests, enabling individuals to implant chips in their brains and control devices like computers, smartphones, and machinery with only thoughts? Furthermore, could this technology expand the boundaries of human physiology and knowledge? While scientific progress is always interesting, BCI technology will raise issues regarding humanity, potential brain damage, and security risks such as hacking. What direction will the continued development of this technology take? We might have to worry a little about this. B

Global Acceleration in Obesity Treatment Development
Journalist | Jiwoo Kang | jiwoo1747@yonsei.ac.kr
Designer | Jini Jeong | 6265mmm@naver.com
Korea’s adult obesity rate increased by 2.6 percentage points to 37.2% in 2022 compared to 34.6% in 2018. The World Obesity Federation predicts that by 2035, half of the global population will be obese. Consequently, as obesity rates rise worldwide, the obesity market is also expanding. Last year, the obesity market grew rapidly, with a surge in demand for obesity treatments globally, leading to shortages of well-known obesity treatments. Although the global market size for obesity treatments was approximately 8 trillion won last year, it is predicted to surpass 100 trillion won by 2030 with an annual growth rate of around 30%. Therefore, domestic pharmaceutical companies are also belatedly entering the market and striving to develop obesity treatments.
Definition of Obesity and Treatment Methods
Obesity refers to a state where there is an excess of adipose tissue in the body, disrupting the hormonal balance of the body’s organs and posing risks of chronic diseases. In 2013, the American Medical Association classified obesity as a disease due to the increasing mortality and morbidity rates associated with it. This classification allows for the possibility of medical prescriptions, reducing patient co-payment rates for treatment. There are three methods for obesity treatment. The first is behavioral therapy, which involves a combination of dieting and exercise. Although it is the healthiest way to treat obesity without relying on medical intervention, it is extremely challenging to adhere consistently. The second is surgical therapy, which involves removing part of the stomach to induce a feeling of fullness even with reduced food intake. While highly effective, it is reserved for severely obese patients due to the burden of surgery on immediate health. The third is pharmacotherapy for weight loss, targeting individuals who wish to lose weight but find behavioral or surgical therapies burdensome. This approach has recently gained prominence in modern Western medicine over the past 20 years.
Emergence of New Obesity Treatments
Before 2014, medications that influenced the appetite center of the brain or inhibited fat absorption were popular. However, in 2014, Saxenda, a medication utilizing liraglutide from the Danish pharmaceutical company Novo Nordisk, entered the market, changing the landscape of obesity treatment. Originally developed as a medication for diabetes treatment and approved by the FDA in 2010, liraglutide was later approved as an obesity treatment through additional clinical trials, demonstrating weight loss effects. Liraglutide acts similarly to glucagon-like peptide-1 (GLP-1) hormones by binding to GLP-1 receptors, increasing insulin secretion from pancreatic beta cells, and decreasing glucagon secretion from pancreatic alpha cells to regulate blood sugar levels. However, there is an enzyme in the blood called DPP-4 that breaks down GLP-1, prompting pharmaceutical companies to develop drugs that mimic GLP-1 while resisting DPP-4 or inhibit DPP-4’s action to prolong the presence of GLP-1 in the body.

Liraglutide is a type of GLP-1 analog that alters the 34th amino acid in GLP-1 and attaches a carbon chain of 16 atoms in length to the 26th amino acid, forming a molecule. As a result, DPP-4 struggles to break it down, prolonging its action and yielding further mechanisms with just one injection per day. Liraglutide not only regulates blood sugar but also delays the passage of food from the stomach to the small intestine and affects the appetite center, making it easier to feel full. However, the fact that daily injections are required has reduced patient compliance.
Development and Current Status of Obesity Treatments
It will come to mind to anyone interested in swimming that the 2019 World Swimming Championships awards ceremony was a hot topic. It was that the gold medalist Sun Yang cursed at Britain’s Scott, who refused to take a picture with him, saying he was a loser. In this situation, Scott deserved criticism for not following the ceremony’s manners, but the arrow of criticism was aimed at Sun Yang. This was because Sun Yang avoided the doping tests of the International Doping Test Management (IDTM) inspectors that he had to take before the competition. Even though the World Anti-Doping Agency filed a complaint with the Court of Arbitration for Sport (CAS), Sun Yang was able to participate in the competition due to the delay in CAS’s ruling. That was the reason why Scott boycotted the award against Sun Yang, who unjustly won the medal. (Actually, it was later revealed that Sun Yang had taken a banned drug called trimetazidine, so he was suspended for eight years!)
Domestic Pharmaceutical Companies Developing Obesity Treatments
With the late entry into the obesity market, domestic pharmaceutical companies are striving to demonstrate differentiation from existing obesity treatments. Previous obesity treatments required ingestion or injections, leading to low patient adherence. In response, Daewoong Pharmaceutical and Daewon Pharmaceutical are developing patches containing the same ingredients as existing obesity treatments. These micro-patches, when applied to the skin, deliver the medication through microvessels via needles. Given that the ingredients are already certified, micro-patches appear to be viable alternatives in terms of convenience and bioavailability.
Some pharmaceutical companies are also taking on new challenges in development. Hanmi Pharmaceutical received approval for its self-developed Epeglenatide (developmental name HM11260C) for Phase 3 clinical trials from the Ministry of Food and Drug Safety in October 2023. It is the fastest among domestic pharmaceutical companies and is scheduled for release in 2025. Yuhan Corporation has transferred its obesity treatment YH25724 to Boehringer Ingelheim for Phase 1 trials and is also developing YH34160, which utilizes a substance similar to GDF-15 to suppress appetite in the brain.
With over a billion obese individuals worldwide and the number expected to continue rising, the demand for obesity treatments is increasing. Shortages of well-known obesity treatments like Wegovy and Mounjaro have led to the synthesis of complex compounds combining semaglutide and illicit trade. It is anticipated that domestically developed obesity treatments from Korean pharmaceutical companies can address such issues. Currently, the market share of domestic obesity treatments is less than 3%, falling short of meeting the demand in the domestic market. If foreign pharmaceutical companies dominate the supply of obesity treatments domestically, they could monopolize the domestic market. However, if domestically developed obesity treatments suit the needs of Koreans better than foreign ones do, it presents an opportunity. The development of obesity treatments by domestic pharmaceutical companies should be expedited to address this. B
Members

Editor



ADMINISRTATOR


Journalist














Designer













