Luke Hargrave
Senior Thesis | 2024

Molecular gastronomy in the everyday kitchen
Luke Hargrave
Recipes
Bananas Foster with Cayenne Pecans and Creamy Yogurt
(adapted from Food + Wine’s Banana’s Foster recipe)
Ingredients
1 large egg white
¼ teaspoon kosher salt
½ cup packed brown sugar, divided in half
Zest and juice of 1 large lemon
¾ teaspoon cayenne pepper
¾ teaspoon ground cinnamon
2 cups halved pecans
¼ cup unsalted butter
1 teaspoon vanilla extract (paste will also work)
¼ cup dark rum (optional)
2 large slightly under-ripe bananas, halved lengthwise
1 quart light cream
2 tablespoons yogurt with live and active cultures
Directions
1. Preheat oven to 375℉. Line a rimmed baking sheet with parchment paper. Line a colander with cheesecloth. Cool a heavy-bottomed pot by rubbing it quickly with an ice cube or rinsing it with cold water.
2. Bring cream to a simmer until it begins to bubble around the edges (~180℉), stirring occasionally. Remove from heat and let cool until it feels pleasant for a finger, but not the entire hand (~115℉). Cooling can be accelerated if you’re in a hurry.
3. Whisk together ¼ cup of warm cream with yogurt, and then pour into the rest of the cream.
4. Cover pot and keep warm for 12 to 24 hours, or until it thickens fully. The yogurt will become more flavorful over time, so adjust to your liking. When it is ready, transfer pot to cheesecloth-lined colander, place colander in a dish to collect the liquid as it drains, and let cool in the refrigerator for another 4 hours, or until it reaches the desired thickness.
5. Once yogurt is ready, whisk egg white, salt, ¼ cup brown sugar, 1 teaspoon lemon zest, ¼ teaspoon cinnamon, and cayenne pepper together in a medium bowl. Add pecans and toss to coat. Scoop pecans from bowl to parchmentlined baking sheet, allowing egg white mixture to drain before transferring. Bake until pecans are browned and strongly fragrant, 12 to 15 minutes, stirring halfway through. Remove from oven and let cool while proceeding with the recipe.
6. While pecans cool, combine butter, vanilla extract, and the remaining brown sugar, lemon zest, and cinnamon in a 10inch cast iron skillet over medium-high heat. Whisk constantly until butter is melted, sugar is dissolved, and the mixture is fully smooth; continue to whisk for 1-2 more minutes, or until the mixture has thickened slightly. Add lemon juice and rum, if using, off heat and stir until most of the added liquid has evaporated, 1 minute.
7. Add halved bananas, cut side down, and cook until softened and browned, 1 minute or to your liking. Turn bananas and cook for 30 seconds. Remove from heat.
8. Serve by placing half of the yogurt in a dish, followed by one banana, followed by a handful of pecans and a dash of flaky salt.
Maillard’s Milk
Ingredients
1 quart whole milk
1 teaspoon smoked paprika
½ teaspoon ground mustard
½ teaspoon cracked black pepper
½ teaspoon salt
¼ teaspoon cayenne pepper
Directions
1. Cool a heavy-bottomed pot by rubbing it quickly with an ice cube or rinsing it with cold water.
2. Add whole milk and keep at high heat, stirring frequently, until it reaches a rapid boil (280-300℉). Continue to heat, careful not to exceed 325℉, for 30 seconds.
3. Remove from heat and add spices, salt, and pepper. Let cool to room temperature, and then transfer to refrigerator for 4 hours or until cool.
4. Serve in small glasses with oyster crackers.
Black Mousse with Caramelized White Chocolate (adapted from Cook’s Illustrated’s 2006 Chocolate Mousse recipe)
Ingredients
8 ounces bittersweet chocolate (~70% cacao), chopped fine
2 tablespoons black cocoa powder (dutch-processed works as well)
1 teaspoon instant espresso powder
5 tablespoons water
2 large eggs, separated
1 tablespoon sugar
⅛ teaspoon salt
1 cup plus 2 tablespoons (18 tablespoons) heavy whipping cream, chilled
8 ounces white chocolate, roughly chopped or broken into pieces
3 tablespoons coconut oil
Flaky salt, for topping
Toasted black sesame seeds, chocolate curls, or soft-peak whipped cream, for topping (optional)
Directions
1. Melt chocolate, cocoa powder, espresso powder, and water in medium heatproof bowl set over saucepan with one inch of simmering water, stirring until smooth.
2. Enthusiastically whisk egg yolks together with 1½ teaspoons of sugar and salt in a medium bowl until slightly thickened and light in color, about 1½ minutes. Pour chocolate mixture into egg mixture, whisking constantly to prevent scrambling. Let cool until just above room temperature, 3 to 5 minutes.
3. In the bowl of a stand mixer with whisk attachment, beat egg whites at medium low speed until frothy. Add remaining sugar, increase speed to medium high, and beat until whisk forms soft peaks when lifted.
4. Whisk about a third of the egg whites into chocolate mixture to lighten. Gently fold the remaining egg whites in with a rubber spatula until few white streaks remain.
5. In the bowl of a stand mixer with whisk attachment, whip cream at medium speed until it begins to thicken, about 30 seconds. Increase speed to medium high and beat until soft
peaks. Using rubber spatula, gently fold whipped cream into chocolate mixture until no streaks remain.
6. Spoon into 8 individual serving dishes, cover each with plastic wrap, and chill until set and firm, at least 2 hours but up to 24.
7. About an hour before the mousse is ready, put white chocolate in a Mason jar. Place jar onto a rack in a pressure cooker with an inch of water. Cook at high pressure for 60 to 80 minutes.
8. Carefully to avoid steam burns, open jar. Stir white chocolate together with coconut oil until the mixture is smooth and glossy. Let cool to approximately room temperature.
9. Working quickly, divide white chocolate, pouring an equal amount over each mousse. Once white chocolate is added, transfer each mousse back to the refrigerator to prevent melting. Cool for another two hours, or until white chocolate is fully hardened.
10. Top with flaky salt and other desired toppings and serve.
Baked Eggs with Miso Butternut Squash
Ingredients
1 medium butternut squash
2 tablespoons olive oil
Salt
8 medium egg whites
3 tablespoons miso paste
½ teaspoon ground ginger
¼ teaspoon black pepper
1 tablespoon seasoned rice vinegar
1 tablespoon honey or dark brown sugar
Directions
1. Preheat oven to 425 degrees. Line two baking sheets with parchment paper.
2. Cut squash in half lengthwise. Rub cut faces with olive oil and salt. Season with ginger, pepper, and dark brown sugar. Place squash cut side up on prepared baking sheet and cover tightly with aluminum foil. Bake until squash is soft and easily pierced with a fork, about 50 minutes.
3. When squash is nearly done cooking, beat egg whites and salt to taste to stiff peaks in the bowl of a stand mixer with whisk attachment. Separate into eight mounds on the second prepared sheet. Lower oven temperature to 200 degrees and bake for two and a half hours, or until eggs sound hollow and are a sickly yellow-green color.
4. While eggs bake, let squash cool until just above room temperature, then blend with a food processor or immersion blender until completely smooth. Add miso, rice vinegar, and salt and pepper to taste.
5. Top portioned squash with egg and serve.
Apple-Harissa Jam (adapted from the delish apple jam recipe)
Ingredients
2 pounds apples (ideally a mix), peeled and cored
1 cup water
1 cup sugar
2 tablespoons lemon juice
1 cinnamon stick
1½ tablespoons harissa seasoning
Directions
1. Puree apples in a food processor until mostly smooth, about 1 minute. Spread thinly and evenly on a rimmed baking sheet and let rest for 1 hour or until dark brown.
2. Place a small plate in the freezer.
3. Combine apples with water over medium-high heat. Bring to a simmer and cook until the mixture’s volume is reduced by about ¼, about 15 minutes.
4. Add sugar. Cook over medium heat, stirring frequently, until mixture thickens significantly and looks jammy, about 20 minutes.
5. Add lemon juice, cinnamon stick, and harissa, and simmer for 10 minutes to fully incorporate.
6. When jam appears ready, test consistency by spooning some onto frozen plate and waiting 2 minutes before dragging a finger through it. If it moves and behaves like jam, it’s ready; otherwise, if it appears thin or watery, it needs to be cooked longer. Return the plate to the freezer and continue to simmer.
7. Serve on toasted bread or crackers, or with a soft cheese like Brie or Coulommiers.
Introduction
Molecular gastronomy was named as an independent field in 1988 by Nicholas Kurti and Herve This.1 It was created to rigorously study the science of cooking, and explore ways that knowledge could be applied. Division of the overall subject realm into three distinct areas of study was the motivating factor behind the naming and initial foundation of molecular gastronomy.1 The three focused-on aspects of cooking are the technical, the artistic, and the social.
The technical is what makes a culinary process work. While this can be mechanical, like the beating of egg whites or the churning of ice cream, the field of interest is almost always the biochemical processes motivating a culinary change.1 Molecular gastronomy investigates the organization of lipids and proteins to form air sacs in these; it draws the connection between the similar aerated structures of meringues and vegetable foams.
The second area of study is the artistic. Within this realm lies the non-critical processes that add flavor or other aesthetic property.1 Examples are the mincing, rather than chopping, of an aromatic to further release flavor; spritzing sliced fruit served raw with acid to inhibit enzymatic browning; the addition of extra flavorings such as spices or extracts to a mixture. Depending on the definition of success, the line between technical and artistic can blur somewhat for example, if the goal of a recipe is to be strongly flavored, perhaps mincing an aromatic would be crucial. Because of this, it is important to the study of molecular gastronomy to clearly define recipe success.
Social activity is the third element. The addition of a spice to a recipe because it was taught by an elder, or the regular continuation of a recipe as a tradition, or the effect that food has when shared are all social behaviors centered around food.1 While it is very important, its study leans closer to sociology than biochemistry and so is not the focus of this thesis. Culinary
practices can be split into definitions, artistic elements, and culinary precisions, which roughly correspond with the technique, art, and social studies inherent to molecular gastronomy, respectively.1
While interesting enough in its own right, a major interest of molecular gastronomy is the application of discovered principles to better or otherwise differentiate the field of culinary arts. This application is often restrictive and scientific, as befits a scientific area of study; exercises like note-by-note cooking combine isolated compounds to create new and recognizable forms in a sort of chemical-deconstructivist approach2, but may only be conducted in a lab or professional kitchen where such substances may be found. However, if molecular gastronomy is to be truly influential, it must become accessible to the everyday kitchen. Indeed, much of the field can be executed without scientific precision, as an available alternative to traditional cooking rather than an unattainable realm of obscure methods and rare results. In this project, I endeavored to apply discoveries of molecular gastronomy to an ‘everyday’ context. Of course, this idea came with limitations. Much of the study that has been conducted in the field depends on the precise or specialized techniques and equipment only available in a lab setting. My challenge, therefore, was to determine which research could be applied with the imprecision of the average chef and still function as it does in a formal scientific context.
Maillard + Caramelization Reactions (Non-Enzymatic Browning)
The first recipes that I composed made use of the Maillard reactions. Also known as the ‘browning’ reactions, these are a complicated chemical process in which sugars namely, glucose and fructose are transformed into Maillard reaction products (MRPs) such as Strecker aldehydes, melanoidins, and other advanced glycation endproducts (AGEs). The first steps of the
Maillard reactions are fairly well-established. The amino groups of existing proteins, peptides, and amino acids are condensed with carbonyl groups belonging to sugars to form Schiff bases a type of compound defined by a hydrocarbyl group attached to a specific nitrogen in the compound which then undergo rearrangement to form another intermediate (Fig. 1).3,4 From there, the process becomes extremely complex and variable.
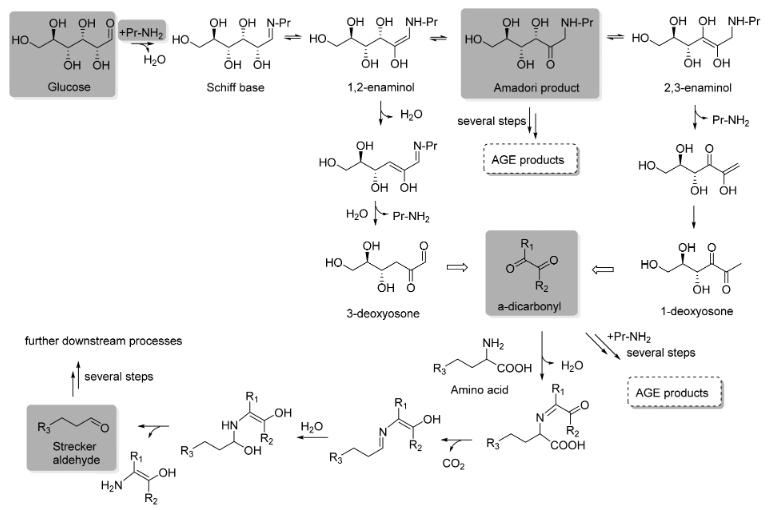
Figure 1. Simplified reaction of a glucose with a protein amine group to trigger a Maillard cascade reaction. Reactants and intermediates thought to control Maillard reactions are highlighted in gray.
Depending on the duration, temperature, and reactants involved, Maillard reactions may yield different products, which might cause a flavor from a light toastiness to a severe char. Despite their complexity, Maillard reactions are quite common. The way I chose to employ them was in milk. Some commercially available milks, especially ultra-high-temperature (UHT) treated milk, has a distinctive browned flavor as a result of unintended
Maillard reactions that occur during its high-temperature processing. I wanted to determine the extent to which this could be exaggerated, and as it turns out, that limit is fairly high. To maintain the liquidity of milk, it is necessary to bring it to the requisite temperature for MRs about 300°F as quickly as possible so as to minimize water loss and milk protein coagulation. Once that is done, however, the product is a unique beverage: slightly thickened, its flavor profile is equally nutty and cheesy. Another way I applied the Maillard Reactions was in white chocolate. Because white chocolate has a much higher glucose content, however, Maillard Reactions occur alongside many other non-enzymatic browning reactions, such as caramelization and lipid oxidation. Like much of food science, however, these pathways are rarely separate and therefore difficult to properly distinguish or model.4 This is why neither the Maillard reaction processes nor the closely related caramelization reactions have not yet been fully distinguished. It is possible for scientists to identify precursors, such as sugars and carbohydrates, and products, such as melanoidins and AGEs, but it is difficult to identify exactly how one leads to the other. Regardless, Maillard and caramelization reactions thought to be the most chemically similar take place under similar conditions. These conditions were the goal I was trying to reach in the creation of both of these recipes. I was concerned about water loss in white chocolate, as well. The best solution I found to this was through the use of a sealed container within a pressure cooker: the pressure cooker raises the temperature quickly, and the seal on the container ensures that any water escaping will condense and can be reintroduced by mechanical mixing once the cooking process is complete. The product of this caramelization shares the sweetness of a white chocolate with the complex, darker flavors of caramelization and MR products. I found this pairs beautifully with a toasted black
cocoa and sesame mousse to form a dessert that is neither too sweet nor too bitter, yet still a delight to eat.
Enzymatic Browning
I also used the enzymatic browning of food. Enzymatic browning, unlike non-enzymatic browning, is a naturally occurring process in many fruits and vegetables linked to the ripening process. Enzymatic browning can be effected to a degree by ethanol, the ‘ripening’ gas emitted by fruits such as apples and bananas. In fruit, the primary enzyme at work in this process is polyphenol oxidase (PPO), which oxidizes phenolic compounds. PPO can be activated either by excess ethylene or by contact with atmospheric oxygen. Phenolic compounds are distinguishable by their astringent, bitter flavor. Oxidized phenolic compounds, called quinones, on the other hand, are flavorless.5 Thus, enzymatic browning shifts the flavor profile of its targets by eliminating astringent and bitter notes. In fruit, this creates a sweetness almost cloying in its solitude; in potatoes, whose flavor is largely determined by bitterness, it nearly eliminates the flavor entirely. The first application of this I used was in apples: first, I pureed apples to maximize the surface area for oxidation, and then I cooked them with sugar and extracted pectin to form a jam. To this, I added harissa, a spice mix originating from Tunisia. Among other things, harissa is composed of dried and ground peppers, cumin, garlic, and coriander. Together, these are tangy and bitter to replace what flavors were lost from the apples. However, the bitterness from harissa, being more herbaceous than fresh and fruity, is distinctly different; in this way, incorporation of the spice into the jam allows for a combination of familiar with unfamiliar, expected with unforeseen. The completed jam is slightly jarring in its aesthetic dissonance, and yet all of my testers liked it. The other way I used it was in potatoes. Like the apples, first I pureed them in order to maximize surface area. Next, I steamed them, in order
to prevent Maillard or caramelization reactions via stovetop cooking. The product is something like mashed potatoes that leave very little recognizable flavor on the palette, yet in its blandness it’s the perfect base for sauces, chutneys, or other additions. I experimented with several toppings to the potatoes mango salsa, muhammara, and several chutneys were all on the list but ultimately, none were sufficient to counteract the absolute blandness of the potatoes. Ultimately, I decided that any recipe that would use these would need to introduce some other flavoring to replace what was lost. While not out of the question, I did feel it was rather counterproductive to neutralize one flavor only to replace it with another both more dilute and texturally uninteresting. For this reason, the potatoes didn’t make the final recipe cut, though I did gather a number of delicious sauce recipes in the testing process.
Thickening
I mentioned the use of extracted pectin in the earlier section. Pectin, a natural thickener, was another subject of interest. Generally, the premise of edible thickeners is that they form a protein matrix in an aqueous context. The pectin family of proteins normally found in plant cell walls happens to be prone to doing so, for reasons too diverse and complex to be practically explored here.6 For this reason, pectins are perhaps the most commonly used plant-derived gelation agent. From jams to jellies, preserves to fillings, the roles of pectins in the kitchen are numerous. However, to thicken properly, pectins must be watersoluble, and solubility depends on a number of factors. Molecule methylation, pH of the mixture, temperature, and size of the pectin all play a role in determining whether a mixture will properly gel. Because pectins are so numerous and varied, though, it is not difficult to sufficiently employ enough to gel a liquid. In the lab, pectins have been extracted in a number of ways: hydrogen
chloride washes, baths in subcritical water, immersion in citric acid, and microwave irradiation were all sufficient.6 While the average home chef should have some access to both citric acid and microwave radiation, the degree of exposure to both required to successfully extract wholly intact pectin is far beyond what most people have the equipment to achieve.6 Therefore, the easiest way for most people to extract pectin was the way it has most commonly been extracted from fruit: through gradual heating and cooling on the stovetop. While I had some concerns that the pureeing of apples would disrupt their pectin structure and require more be added for successful gelation, these were ultimately baseless. I would guess this is because in general, food processors don’t reach a high rate of blending mine hits approximately 1700 rpm on average. This isn’t enough to significantly interfere with pectin structure, so no addition to the jam is necessary.
Another natural thickening agent is chitin. As one of the most common biopolymers second only to cellulose chitin plays a role in maintaining cell structure and organization. Chitin is most commonly found in the exoskeletons of arthropods and the structural components of fungi and bacteria. Chitosan, a compound with parallel structure to chitin but with a deacetylated N-terminus, is also found in all of these sources. It can also be produced from chitosan via chemical treatment encouraging deacetylation. While incredibly significant to the natural world, though, both chitin and chitosan are fiercely insoluble in water. Under acidic conditions, however, chitosan is rendered soluble by the dissolution of free amino groups. Once it is dissolved, chitosan can be used as a natural thickening agent, due to its large, crystalline structure and tendency for enmeshment.7,8 Therefore, the challenge presented to me was how best to create an acidic but edible environment in order to successfully dissolve chitosan for use as a thickener. This has already been done in labs: using subsequent rinses with strong acids and bases, chitosan is dissolved, purified, and then dried for
use in industrial applications as a large-scale natural thickener.8 However, even if strong acids and bases were a common household item, I wouldn’t trust the average chef to treat them with enough caution for the end product to be safely edible. Therefore, I had to resort to the household alternatives: baking soda and vinegar. By blending mushrooms and soaking them in baking soda and vinegar solutions, I was able to successfully produce a mush that, when placed in an acidic broth, fully dissolved. While successful in thickening the mixture, it had the unfortunate side effect of a fairly rancid taste. I tried many different flavor combinations everything from dashi to bone broth to tomato stock but nothing could effectively conceal it. Eventually, I decided to remove the recipe from the collection, as I just couldn’t find a way to make it taste good or even edible. Foams were a significant part of my thinking while composing my recipes. Unconventional foams are a staple of modernist cuisine; I felt that I’d be remiss doing a project on molecular gastronomy without dedicating a portion to foam. In general, foams are composed of three parts: an oil or fat, proteins in aqueous solution, and, of course, a gas. The oil will mix with the aqueous solution, leaving pockets of gas which increase the volume of the mixture and form a distinctive foam structure. Generally, a surfactant is required to reduce the natural repulsion between the oil and aqueous solution. When the tension is reduced, both oil and aqueous solution disperse equally to form the liquidgas boundary. Commonly used surfactants are lecithin and monoacylglycerol.9 However, in order for a surfactant to be helpful the mixture must already be capable of foaming something which not every protein solution is. Like much of molecular gastronomy, which proteins can and cannot foam is poorly understood, both because it is difficult to isolate the proteins involved and because research would offer little practical application. As a result, what studies have been conducted only examine the results of various
surfactant and protein combinations.10 Lecithin and monoacylglycerol both appear to be greatly helpful, but again, it is poorly understood why, and also, the common chef has neither of these ingredients even though diluted lecithin is relatively easy to procure. I at first wanted to make something with a vegetable foam taro and beet both came to mind but eventually came to the conclusion that even if the common chef could acquire the necessary ingredients, they would be unable to measure and create the foam with the degree of care required for it to work.
Then, I remembered that there is an alternative which requires neither which has, in fact, been popular for some time now. Egg whites require neither surfactant nor additional oil, as they contain in themselves the requisite protein structure, fat content, and liquid. This is why meringue is so successful as a mixture of whipped egg whites and sugar, it is nothing more than a simple foam. That isn’t to say it’s unremarkable; rather, the combination is brilliant. Sucrose and glucose together work to stabilize egg foams, which are often partially denatured in the shearing process of mechanical foaming.9 Initially, I wanted to use a nitrous oxide gas injector to form a structurally intact sugarless egg foam, but my supplier failed to deliver. Instead, I decided to explore the consequences of mechanically foaming eggs without sugar, and it is through this exploration that I reached my final result. I found that the eggs held their structure when foamed and then baked slowly at a low temperature, but were crumbly and brittle. In addition, without sugar to offer a strong, primary sweet note, it tasted salty and sulfuric, though not deeply unpleasant. If anything, the savory foam seemed like it had potential, just not as the centerpiece of a dish in my opinion, just like meringue. I determined that it would be best to serve it alongside a smooth, salty-sweet mixture to complement both the flavor and the texture of the egg foam. As an added plus, the butternut squash’s orange color complements well the sulfurous yellow of the eggs and the
umber of miso. This is one of my favorite dishes in the collection, and I’m very pleased with how it came out.
The final dish and perhaps the most difficult for me to work on is bananas foster served over a creamy yogurt. The original vision was to create an especially solid yogurt via the introduction of powdered milk solids, which are readily available in most supermarkets. Yogurt is formed when lactic acid bacteria (LAB) degrade polysaccharides in milk, particularly lactose via hydrolyzation and other processes to form a quantity of lactic acid that will significantly decrease the pH of the solution.11 At a low pH, milk protein has a tendency to coagulate, which forms a relatively thick protein matrix in the solution and solidifies the yogurt. Acidification also affects flavor, but it has been found that the strain of bacteria used is the greatest determinant of flavor compounds produced.12 I assumed that the quantity of milk solids present in the solution would relate proportionally to the thickness of the yogurt, but unsurprisingly, the truth lay elsewhere. When tested, my yogurt became stringy and unpleasant, so I had to take a different tactic: using light cream rather than whole milk. While the protein content may be similar, the fat content in light cream is much different than that of milk. This one performed admirably in testing, holding its shape alongside its lush, fatty mouthfeel. Somewhat reminiscent of a brighter, more zingy sour cream, I find it delightful. Alongside almost-cloying, dense bananas foster, it’s even better. Altogether, the dish is both lush and punchy; the combination works because of its contrast.
Conclusion
Of course, while these recipes are novel and I hope entertaining, much of molecular gastronomy must remain confined to labs and professional kitchens. After all, the field only came into existence once technology had advanced to the point of enabling such focused inquiry. Humans have had millenia to explore every other possible combination of edible ingredients with simple heat and stirring. This was the largest obstacle that I ran into in the process of recipe creation and testing: most things that can be done to make food seem like they’ve already been tried and tested, evaluated and made useful. Nonetheless, I do believe that there is more to cooking than exists today. Molecular gastronomy holds the key to the future of food, and it is my sincere hope that its expansion will see not only Michelin stars and critical acclaim but weeknight dinners and packed lunches as well.
References
1. Burke, R.; This, H.; Kelly, A. L. Molecular Gastronomy. Reference Module in Food Science 2016. DOI:10.1016/b978-0-08-100596-5.03302-3.
2. This, H.; Debevoise, M. Note-By-Note Cooking - The Future of Food; Columbia University Press, 2016.
3. Lund, M. N.; Ray, C. A. Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. Journal of Agricultural and Food Chemistry 2017, 65 (23), 4537–4552. DOI:10.1021/acs.jafc.7b00882.
4. Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases Structure, Importance and Classification. Molecules 2022, 27 (3), 787. DOI:10.3390/molecules27030787.
5. Holderbaum, D. F.; Kon, T.; Kudo, T.; Guerra, M. P. Enzymatic Browning, Polyphenol Oxidase Activity, and Polyphenols in Four Apple Cultivars: Dynamics during Fruit Development. HortScience 2010, 45 (8), 1150–1154. DOI:10.21273/hortsci.45.8.1150.
6. Gawkowska, D.; Cybulska, J.; Zdunek, A. StructureRelated Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10 (7), 762. DOI:10.3390/polym10070762.
7. Ifuku, S.; Nomura, R.; Morimoto, M.; Saimoto, H. Preparation of Chitin Nanofibers from Mushrooms. Materials 2011, 4 (8), 1417–1425. DOI:10.3390/ma4081417.
8. Matyjaszewski, K., et al. Polymer science: A comprehensive reference; Elsevier, 2012.
9. Mcgee, H. On Food and Cooking: The Science and Lore of the Kitchen; Scribner, 2004.
10. Liu, Y.; Binks, B. P. Foams of Vegetable Oils Containing Long-Chain Triglycerides. Journal of Colloid and Interface
Science 2021, 583, 522–534. DOI:10.1016/j.jcis.2020.09.043.
11. Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Frontiers in Bioengineering and Biotechnology 2021, 9. DOI:10.3389/fbioe.2021.612285.
12. Chen, C.; Zhao, S.; Hao, G.; Yu, H.; Tian, H.; Zhao, G. Role of Lactic Acid Bacteria on the Yogurt Flavour: A Review. International Journal of Food Properties 2017, 20 (sup1). DOI:10.1080/10942912.2017.1295988.
13. Quintas, M.; Guimarães, C.; Baylina, J.; Brandão, T. R. S.; Silva, C. L. M. Multiresponse Modelling of the Caramelisation Reaction. Innovative Food Science & Emerging Technologies 2007, 8 (2), 306–315. DOI:10.1016/j.ifset.2007.02.002.
14. Xiang, J.; Liu, F.; Wang, B.; Chen, L.; Liu, W.; Tan, S. A Literature Review on Maillard Reaction Based on Milk Proteins and Carbohydrates in Food and Pharmaceutical Products: Advantages, Disadvantages, and Avoidance Strategies. Foods 2021, 10 (9), 1998. DOI:10.3390/foods10091998.
15. CFR - Code of Federal Regulations Title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/C FRSearch.cfm?fr=163.124 (accessed 2023-012-21).
16. Tamanna, N.; Mahmood, N. Food Processing and Maillard Reaction Products: Effect on Human Health and Nutrition. International Journal of Food Science 2015, 2015, 1–6. DOI:10.1155/2015/526762.
17. Giovannoni, J.; Nguyen, C.; Ampofo, B.; Zhong, S.; Fei, Z. The Epigenome and Transcriptional Dynamics of Fruit
Ripening. Annual Review of Plant Biology 2017, 68 (1), 61–84. DOI:10.1146/annurev-arplant-042916-040906.
18. Espley, R. V.; Leif, D.; Plunkett, B.; McGhie, T.; HenryKirk, R.; Hall, M.; Johnston, J. W.; Punter, M. P.; Boldingh, H.; Nardozza, S.; Volz, R. K.; O’Donnell, S.; Allan, A. C. Red to Brown: An Elevated Anthocyanic Response in Apple Drives Ethylene to Advance Maturity and Fruit Flesh Browning. Frontiers in Plant Science 2019, 10. DOI:10.3389/fpls.2019.01248.
19. Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different Phenolic Compounds Activate Distinct Human Bitter Taste Receptors. Journal of Agricultural and Food Chemistry 2013, 61 (7), 1525–1533. DOI:10.1021/jf304198k.
20. Li, L.; Wu, M.; Zhao, M.; Guo, M.; Liu, H. Enzymatic Properties on Browning of Fresh-Cut Potato. IOP Conference Series: Materials Science and Engineering 2018, 397, 012116. DOI:10.1088/1757899x/397/1/012116.
21. ksdjlfkjsfd
22. Willats, W. G. T.; Knox, J. P.; Mikkelsen, J. D. Pectin: New Insights into an Old Polymer Are Starting to Gel. Trends in Food Science & Technology 2006, 17 (3), 97–104. DOI:10.1016/j.tifs.2005.10.008.
23. and that’s it for pectins
24. Ifuku, S. Chitin and Chitosan Nanofibers: Preparation and Chemical Modifications. Molecules 2014, 19 (11), 18367–18380. DOI:10.3390/molecules191118367.
25. Joseph, S. M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J. A.; Anandharamakrishnan, C. A Review on Source-Specific Chemistry, Functionality, and Applications of Chitin and Chitosan. Carbohydrate Polymer
Technologies and Applications 2021, 2, 100036. DOI:10.1016/j.carpta.2021.100036.
26. Ali, H. M.; El-Gizawy, A. M.; El-Bassiouny, R. E. I.; Saleh, M. A. The Role of Various Amino Acids in Enzymatic Browning Process in Potato Tubers, and Identifying the Browning Products. Food Chemistry 2016, 192, 879–885. DOI:10.1016/j.foodchem.2015.07.100.
27. Fennema, O. R. Damodaran, S.; Parkin, K. L., Eds. Fennema’s Food Chemistry; Taylor & Francis Group, 2017.

