A Shifting Spirochetosis: Renewed Approaches to Leptospirosis
Jane E. Sykes, BVSc (Hons) Ph.D., MBA, GCPH, DACVIM (SAIM) Professor of Small Animal Internal Medicine (Infectious Diseases) University of California-Davis
Davis, CA, USA
INTRODUCTION
Leptospirosis is caused by infection with various serovars of Leptospira interrogans sensu lato. Organisms are transmitted by direct contact with infected urine, bite wounds or ingestion of infected tissues, or indirectly, through contact with infected water, soil, food or bedding. Survival of leptospires is promoted by stagnant warm water, a neutral or slightly alkaline pH, and temperatures between 0 and 25°C, although the spirochete can survive outside these conditions, and even survive freezing. The seasonality of the disease is variable depending on local climatic conditions, especially rainfall. In areas with year-round rainfall, the disease may occur throughout the year.
There are over 200 pathogenic serovars, which are grouped into antigenically-related serogroups.
Classification of leptospires is gradually moving from predominantly serovar-based classification to that based on genetic typing (genotype-based classification). Each serovar (and more accurately, each genotype) is adapted to a one or more mammalian host species (maintenance hosts). Other hosts act as incidental hosts. Disease in incidental hosts tends to be more severe and the duration of shedding is generally shorter. Although we previously used to assign serovars/serogroups to certain reservoir hosts (such as rats and Icterohaemorrhagiae), it is becoming apparent that many serovars can be found in multiple reservoir host species, and sequence type associations may be more important. The prevalence of infection with a sequence type in dogs depends on the degree of contact between the dog population and the maintenance host for that sequence type.
Despite the problems with classification, we still need to know about serovars because vaccine-induced immunity is serovar-specific. Based on serology and culture, the most common serovars thought to infect dogs before the introduction of the Leptospira vaccines several decades ago were Icterohaemorrhagiae and Canicola. After the introduction of the bivalent bacterins containing these two serovars, in North America and Europe, there were decreasing reports of disease associated with seroconversion to Canicola and Icterohaemorrhagiae, and increasing reports of disease associated with seroconversion to serovars Pomona, Grippotyphosa, Autumnalis and Bratislava (in North America) and Sejroe, Australis and Grippotyphosa (in Europe). Vaccine pressure, increasing contact between dogs and certain wildlife reservoir hosts and increased testing have been suggested as reasons for this change.
9
In truth, the actual serovars causing disease in dogs worldwide still remain poorly characterized because the disease is diagnosed by serology and PCR, and these test results cannot accurately identify the infecting serovar. Recent studies using culture have begun to shed light on the true infecting serovars.
Pathogenic leptospires penetrate abraded skin or mucus membranes and multiply rapidly in the bloodstream and tissues, causing renal failure, hepatic injury (usually not hepatic failure) and vasculitis. The disease is multisystemic and may also involve the pancreas (pancreatitis), gastrointestinal tract (gastroenteritis), eye (uveitis) and lungs (leptospiral pulmonary hemorrhage syndrome, or LPHS). In humans, Leptospira can also cause meningitis, which is most commonly manifest as a severe headache. Clinical manifestations may also depend on the age of the host, the infectious dose, and the strain of Leptospira involved.
CLINICAL MANIFESTATIONS
Most infections are subclinical. The disease can occur in any dog breed and at any age; dogs that live in cities may become infected as a result of exposure to rodent reservoir hosts. There has been a widely recognized increase in the percentage of small breed dogs diagnosed with leptospirosis. Younger animals tend to be more severely affected, and disease can occur in dogs as young as 4-6 months of age. Male dogs may be predisposed.
Lethargy, anorexia, vomiting, pyrexia, dehydration, abdominal pain and increased thirst and urination are common signs of acute leptospirosis. Reluctance to move due to myositis, icterus, and uveitis may be noted. Respiratory difficulty may result from pulmonary hemorrhage, which is often associated with the development of moderate anemia.
LABORATORY FINDINGS
Leukocytosis, thrombocytopenia, azotemia, hypoalbuminemia and mild to moderately elevated liver enzyme activities are common. Although hyperkalemia has been reported, normokalemia or hypokalemia are more common because of the effect of Leptospira on the renal medullary thick ascending limb tubular Na+-K+-Cl- cotransporter. The hepatopathy is a cholestatic hepatopathy and liver failure is not typically present. It is uncommon to see hepatopathy in the absence of renal failure, but it has been described in some outbreaks. Urinalysis may reveal isosthenuria, proteinuria, glucosuria and casts. Although it occurs with other causes of renal tubular damage, glucosuria in addition to azotemia can be a “red flag” for a diagnosis of leptospirosis.
Proteinuria is typically low-level (urine protein:creatinine ratio < 5), which helps differentiate leptospirosis (interstitial nephritis) from Lyme nephritis (which involves the glomerulus).
Thoracic radiography may reveal a focal, nodular or diffuse interstitial to bronchointerstitial pattern; alveolar patterns may represent pulmonary hemorrhage.
10
Occasionally mild pleural effusion is evident. Hepatomegaly, splenomegaly, renomegaly and/or peritoneal effusion may be evident from abdominal radiography. Hyperechoic renal cortices and mild renal pelvis dilation are occasionally seen with abdominal ultrasound.
DIAGNOSIS
Canine leptospirosis is an underdiagnosed disease in some regions/practices and an over diagnosed disease in other regions/practices. Identification of leptospirosis requires a high clinical suspicion for the disease based on knowledge of the range of clinical presentations that suggest leptospirosis. Currently available diagnostic tests include PCR, serology using the microscopic agglutination test (MAT), and in-clinic serologic assays that detect IgG/IgM (SNAP Lepto, IDEXX Laboratories), or IgM (WITNESS Lepto, Zoetis).
In the MAT, respective titers are provided for each of several different serovars in order to increase the chance of antibody detection. Studies in humans and dogs have shown that the serovar with the highest titer can vary over time and that paradoxical cross-reactivity to multiple serovars occurs after exposure to a single serovar. Thus, the MAT does not accurately predict the infecting serovar, and therefore should not be used for this purpose. Titers with any serologic test may be negative in the first week of illness because of the short incubation period and delay in antibody production. Low positive or negative titers after at least one week of illness make leptospirosis less likely. Overdiagnosis results from misinterpretation of positive serologic test results. Positive titers early in the course of an illness may reflect residual post-vaccination titers or prior subclinical infection, and are not diagnostic for the disease. Demonstration of a fourfold rise in titer is required over a 1-2 week interval. In acutely ill dogs (< 1 week of illness), it is the author’s opinion that leptospirosis serology should only be performed in a paired fashion or not at all, because of the limited utility of a single positive titer, regardless of its magnitude. Post vaccinal titers occasionally rise as high as 1:6400 for a few months after vaccination, and these can interfere with interpretation. The results can also vary dramatically between laboratories. Use of a laboratory with a high level of quality control is recommended, or a laboratory that participates in the International Leptospirosis Society’s proficiency testing scheme.
In-clinic serologic assays yield qualitative (positive or negative) results, and are useful for assessing for the presence or absence of antibodies. Should these kits yield negative results, then the clinician should consider whether it may be too early for the animal to have developed antibodies (as can occur with the MAT).
Another test should be performed one week later to see if the animal seroconverts. Should these kits yield positive results, then the clinician should consider whether recent previous vaccination for leptospirosis has occurred.
11
The WITNESS Lepto (Zoetis) is an IgM-based assay that has a high clinical specificity for diagnosis of leptospirosis. Thus, if this test is positive in conjunction with clinical signs supportive of disease, it usually means leptospirosis is present. If this test is negative, leptospirosis cannot be ruled out.
Interpretation of IgG-based assays such as SNAP Lepto (IDEXX) is more difficult as positive results can be due to previous exposure without clinical disease. Clinicians should consider reflex testing with MAT in order to obtain a quantitative titer if positive results occur using in-clinic serologic tests, followed by convalescent serology 1-2 weeks later in order to document a change in titer. Additional clinical validation of these assays in different regions of the United States would be helpful to confirm their sensitivity and specificity.
Darkfield microscopy of the urine is not recommended as sole test for diagnosis because of the large number of false positives and false negatives. Culture is difficult because of the fastidious growth requirements of leptospires and the need for specialized media, but there have been recent advancements in methods that accelerate growth of leptospires and may overcome these hurdles. PCR assays do not provide information about the infecting serovar, although they have been used to provide information on genotype. The author’s experience is that PCR may be insensitive for diagnosis of canine leptospirosis, but the sensitivity and specificity may vary geographically depending on the serovars present and shedding patterns that occur for those serovars. The sensitivity may also be higher very early in the course of illness and in dogs that have not received any treatment with antimicrobials. Positive results have been detected in the urine of some healthy dogs, especially those that are free-roaming, from kennels, or shelters (acting as reservoir hosts). PCR assays are best performed on blood AND urine concurrently because urinary shedding begins 10 days after the onset of infection.
TREATMENT
Specific treatment involves initial use of parenteral penicillin derivatives for leptospiremia. Ampicillin is recommended (20 mg/kg IV q6-8h, adjusting dose down if severe azotemia is present) for up to 14 days or as long as the patient is vomiting or appears nauseated. It is recommended that treatment then be changed to doxycycline (5 mg/kg PO q12h) for 2 weeks, in order to eliminate the carrier phase. Doxycycline can be used instead of penicillins if vomiting does not occur after administration, or intravenous doxycycline can be used. Supportive therapy is also indicated for acute kidney injury (e.g. IV fluids, H2 blockers, antihypertensives, gastric protectants, antiemetics, phosphate binders, packed red cells and nutritional support). The use of hemodialysis can improve survival in dogs with severe renal failure. Approximately 50% of the patients with leptospirosis at the author’s institution are dialyzed, and the average number of treatments required before polyuria and recovery occur is 3. Euthanasia or death due to leptospirosis is recorded in about 20% of our leptospirosis patients.
12
PREVENTION
In North America, vaccines are available that contain serovars Canicola, Icterohaemorrhagiae, Pomona and Grippotyphosa and are in widespread use. The vaccines are generally safe and efficacious and studies suggest they provide a minimum of a 1-year duration of immunity. Vaccination of dogs with 4serovar vaccines has been associated with reduction in the prevalence of disease in dogs worldwide, although disease can still occasionally occur in vaccinated dogs (usually caused by serovars that are not included in the vaccine). A 2015 study that examined over 130,000 dogs seen at a mobile vaccine clinic showed that although administration of a Leptospira vaccine increased the risk of adverse reactions, the risk of a reaction was still extremely low (0.45%, compared with 0.28% for all vaccines). Moreover, when broken down by type of adverse event, the rate of hypersensitivity-type events (most severe) increased from 7.2/10,000 dogs to 9.1/10,000 dogs with administration of Leptospira vaccine and this increase was not significant.
Leptospira bacterins have been associated with occasional acute, severe allergic reactions, but the incidence of these reactions has decreased dramatically, and reaction rates appear to be approaching those of distemper-hepatitis-parvovirus vaccines, even in small breed dogs. Vaccination against pathogenic leptospires is strongly recommended throughout the US and are recommended even for small breed dogs that are confined to urban backyards, because of the possibility of infection as a result of direct or indirect rodent exposure. There have been recent outbreaks of leptospirosis in dog populations from environments not typically associated with leptospirosis (including dog daycare environments and urban concrete backyards). Minimizing access to rodents, farm animals and other wild animals also should help to prevent infection.
PUBLIC HEALTH RISK
Leptospirosis remains an important zoonosis, although most documented human leptospirosis in North America results from recreational activities that involve water, rather than contact with dogs. Because dogs are generally incidental hosts they may not shed for significant periods of time, although more studies are required to confirm this, and there are anecdotal reports of leptospirosis in staff that work in veterinary hospitals. Human leptospirosis is typically a ‘flu-like illness’, but in some cases may be associated with vomiting, diarrhea, shock, jaundice, renal failure, pneumonia, meningitis, or abortion. Any animal with acute renal failure should be treated as a suspect.
Warnings should be placed on cages, gloves should be worn while handling these dogs and bleach or iodine-based disinfectants should be used to clean areas soiled with urine. Owners should be warned that without specific treatment, leptospires may be shed in the urine for months despite clinical recovery. Contact precautions can be reasonably lifted after 72 hours of specific antimicrobial therapy. Where there are populations of humans, rodents, and domestic/stray dogs intermingling, vaccination of dogs may reduce risk of human infection from rodents
13
Antimicrobial Use Guidelines: Why, When, How Much
‡ This course satisfies the one hour of California CE requirement on the judicious use of medical important antimicrobial drugs.
 Jane Sykes, BVSc (Hons), Ph.D., MBA, DACVIM (SAIM)
Jane Sykes, BVSc (Hons), Ph.D., MBA, DACVIM (SAIM)
‡
15
Antimicrobial Use Guidelines: Why, When, How Much
Jane E. Sykes, BVSc, (Hons), Ph.D., MBA, GCPH, DACVIM (SAIM) Professor of Small Animal Medicine University of California, Davis, CA Davis, CA, USA
The International Society for Companion Animal Infectious Diseases (ISCAID) Antimicrobial Guidelines Working Group was formed to develop guidelines for antimicrobial drug use in dogs and cats, because of concerns that antimicrobial drug resistance has dramatically increased in prevalence among isolates from dogs and cats in the last decade. The guidelines have been published in open access format so that they are widely available. Input has also been obtained from panels of Diplomates of relevant specialty groups.
Guidelines for treatment of urinary tract disease in dogs and cats (updated), respiratory infections in dogs and cats, and superficial pyoderma in dogs have been published (www.iscaid.org).1-3 Recommendations are based on available data, whenever present, along with expert opinion, considering principles of infectious diseases, antimicrobial treatment, antimicrobial resistance, pharmacology, and internal medicine. Funding for studies on antimicrobial resistance in companion animals is badly needed. Clinical trials that evaluate antimicrobial drug regimens for bacterial infections in dogs and cats are encouraged.
Because of the increased prevalence of antimicrobial drug resistance, the need to properly document the presence of an infection before initiating antimicrobial drug treatment is more important than ever. In veterinary medicine, this may be at odds with client financial resources. However, inappropriate use of antimicrobial drugs is wasteful of client resources when an infection is not present or a multidrug resistant pathogen is present, and risks selection for antimicrobial resistant bacteria that may be harmful to the pet, other animals, and also humans that are in contact with the animal. Increasingly, veterinarians need to re-think the empiric use of antimicrobial drugs, especially when the underlying condition is not immediately life-threatening. An emphasis on rational antimicrobial treatment needs to be made to pet owners, as has been made in human medicine. The guidelines do not provide specific recommendations for hygiene and disinfection, but posters describing appropriate measures and guideline documents are available from veterinary associations in North America and in Europe and these should be followed.
Some of the basic recommendations within the urinary and respiratory guidelines that relate to cats are summarized below. Doses of specific antimicrobial drugs are listed in the guidelines themselves.1,2
RECOMMENDATIONS FOR
Sporadic Cystitis
URINARY TRACT DISEASE
Definition: Sporadic bacterial infection of the bladder (< 3 UTIs per year)
The presence of urinary tract infection implies the presence of dysuria, pollakiuria, and/or stranguria. However, diagnosis of UTI cannot be made on the basis of clinical signs alone.
17
Sediment analysis alone is not adequate for diagnosis because of the variable quality of interpretation, although the availability of artificial intelligence and virtual expert review is helping to overcome many problems.
Urinalysis and quantitative aerobic C&S testing should be performed in cats with lower urinary tract signs before starting antibiotics. Free-catch samples should not be used. Use of a novel rapid assay for detection of bacteriuria (RapidBac Vet, Silver Lake Research Corporation, https://www.rapidbacvet.com/) is encouraged for clients that cannot afford culture or when information on the presence or absence of bacteriuria is required at point-of-care. Studies to date have shown that this assay is highly sensitive and specific when compared with traditional culture, although it does not provide susceptibility information.
For cystocentesis specimens following culture, counts ≥ 103 CFU/mL indicate UTI. For catheterized specimens, counts ≥ 104 in males and ≥ 105 CFU/mL in females are significant. Bacterial isolation should only be attempted in clinics with appropriate laboratory facilities, proper biosafety containment and waste management, and adequately trained individuals. In-house “urine paddles” may be useful to rule out the presence of infection but these do not reliably identify bacteria and can generate false negative results.3
Treatment is indicated to relieve patient discomfort while awaiting C&S test results. Recommendations for initial treatment are amoxicillin (11 – 15 mg/kg PO q12h) or trimethoprimsulfonamide (15 mg/kg PO q12h).
Veterinarians are encouraged to document and monitor resistance patterns among isolates from their hospital.
If C&S testing reveals a resistant isolate and there is a lack of clinical response, treatment should be changed to an appropriate antimicrobial drug. Although treatment in the past has often been for 7 to 14 days, it is recommended that it be limited to 3-5 days 4,5
Intra- or post-treatment urinalysis or urine culture are not indicated in the absence of ongoing clinical signs of UTI.
Recurrent UTI
Definition: the presence of 3 or more episodes of UTI during a 12-month period. The same general principles as for sporadic cystitis apply Efforts should be made to identify the underlying cause; consider referral. Treatment should be based on the results of C&S testing. Although 4 weeks has been recommended for treatment, shorter durations are recommended (1014 days), with a focus on clinical cure rather than microbiological cure (clearing the bacteriuria). There is insufficient evidence to recommend “pulse” or chronic low-dose treatment, urinary antiseptics, and nutritional supplements such as cranberry juice extract for prevention of UTIs.
Subclinical Bacteriuria
Definition: presence of bacteria in the urine as determined by positive bacterial culture, in the absence of clinical signs of UTI. This is much more common than UTI.
18
Treatment may not be necessary, but could be considered if there is a high risk of ascending or systemic infection (e g., patients with underlying renal disease) If the significance of the bacteriuria is unclear (e.g., whether it is contributing to lethargy or evidence of kidney disease), a short course of treatment (3-5 days) could be tried.
Urinary Catheters
Clinical signs of UTI are absent and a catheter is in place: no culture or treatment is indicated. Removal of urinary catheters: urine culture is reasonable at the time of catheter removal if the risk and implications of a UTI are high, but in general culture is not recommended. There is no indication for routine use of prophylactic antimicrobials.
Clinical signs of UTI present (gross evidence of hematuria or flocculent urine in the collection system, fever): perform a culture after replacement of the urinary catheter with a new catheter. Several milliliters of urine should be removed to clear the catheter before a specimen is obtained for culture. Alternatively, remove the catheter and perform a cystocentesis. Culture from the collection bag, and culture of the catheter tip after removal are not recommended because biofilm forms on these materials and does not represent clinically meaningful information. Treatment should follow the guidelines for sporadic and recurrent cystitis above, and is more likely to be successful after catheter removal.
Pyelonephritis
C&S testing should always be performed.
Treatment should be initiated while awaiting culture results, using antimicrobials effective against Gram-negative Enterobacterales. A fluoroquinolone is a reasonable first choice, after which treatment should be based on C&S results. If combination treatment was used initially and C&S results indicate that both drugs are not required, the spectrum should be narrowed. Treatment for 2 weeks is recommended until further information becomes available.
Culture is recommended 1 week after starting treatment and 1 week after treatment is discontinued.
RECOMMENDATIONS FOR RESPIRATORY DISEASE
Acute Upper Respiratory Tract Disease (URTD)
Consider an observation period of up to 10 days without antimicrobial treatment for cats with acute URTD that are eating and otherwise systemically well. Antimicrobial therapy should be considered if a mucopurulent nasal discharge is accompanied by fever, lethargy or anorexia. In the latter case, appropriate empiric therapy would be doxycycline (first choice) followed by amoxicillin (the latter is not active against Mycoplasma spp.). The duration should be 7-10 days. Avoid performing C&S on nasal discharge from cats with acute URTD. If empiric antimicrobial therapy is ineffective, a diagnostic work-up is indicated.
19
Chronic Upper Respiratory Tract Disease
A diagnostic work-up is recommended. If treatable causes of nasal discharge are not identified, then nasal lavage or brushings could be submitted for C&S testing, and a nasal biopsy could be submitted for histopathology. Treatment should be based on these results. Should nasal discharge recur, the previously effective antimicrobial drug should be used for a minimum of 48 hours; if this is ineffective, only then a switch to a different class should be considered, provided a diagnostic work-up to rule out other causes of nasal discharge (tumors, fungal infection, foreign bodies etc.) has been performed.
Bacterial Bronchitis
Airway lavage with cytologic examination and C&S testing is indicated if bacterial bronchitis is suspected While awaiting results of the above tests, empiric treatment is recommended with doxycycline for 7 to 10 days. If this results in clinical improvement, treatment should be continued for 1-week past resolution of clinical signs.
Pneumonia
Antimicrobial therapy for pneumonia should be initiated as soon as possible and within 1-2 hours if signs of sepsis exist Antimicrobial therapy should be parenteral while patients with pneumonia are hospitalized. If there is no evidence of systemic sepsis, parenteral administration of a beta-lactam is recommended for empiric therapy; if signs of sepsis are present, then a combination of a fluoroquinolone and a drug that targets gram-positive bacteria and anaerobes (e.g., ampicillin or clindamycin) is recommended pending the results of C&S if possible. Animals should be reevaluated for possible discontinuation of antimicrobials no later than 10 to 14 days after starting treatment.
Pyothorax
Pyothorax should be treated with IV fluids and drainage of pus after placement of chest tubes. Surgical debridement may be required for some animals. Empiric antimicrobial therapy pending the results of C&S should be with a parenteral combination of a fluoroquinolone and a penicillin or clindamycin
It has been recommended that treatment continue for at least 3 weeks and ideally 4-6 weeks, but the optimum duration is unknown. Cats should be re-evaluated 10 to 14 days after starting treatment.
20
References
Hillier A, Lloyd DH, Weese JS, et al. Guidelines for the diagnosis and antimicrobial therapy of canine superficial bacterial folliculitis (Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases). Vet Dermatol 2014; 25(3): 163-e43.
Lappin MR, Blondeau J, Boothe D, et al. Antimicrobial Use Guidelines for treatment of respiratory tract disease in dogs and cats: antimicrobial guidelines working group of the International Society for Companion Animal Infectious Diseases. J Vet Intern Med 2017; 31(2): 279-294.
Weese JS, Blondeau J, Boothe D, et al. International Society for Companion Animal Infectious Diseases (ISCAID) Guidelines for the Diagnosis and Management of Bacterial Urinary Tract Infections in Dogs and Cats. Vet J. 2019;247:8-25.
21
Pathogen Soup: Update on Canine Infectious Respiratory Disease
 Jane Sykes, BVSc (Hons), Ph.D., MBA, DACVIM (SAIM)
Jane Sykes, BVSc (Hons), Ph.D., MBA, DACVIM (SAIM)
23
Immune-Mediated Hemolytic AnemiaTreatment Time! (Part 2)
ACVIM Consensus Statement on the Diagnosis of ImmuneMediated Hemolytic Anemia
To vet the published body of diagnostic evidence for IMHA and refine an evidence-based approach to diagnosis, in 2019 the American College of Veterinary Internal Medicine published a consensus statement on the diagnosis of immune-mediated hemolytic anemia in dogs and cats.1 Based upon the evaluated literature, the consensus panel developed a diagnostic algorithm for IMHA which centers on identifying clinical evidence of: 1) anemia, 2) anti-erythrocyte antibodies, and 3) hemolysis. The algorithm provides a stepwise diagnostic ladder which culminates in 4 possible diagnostic outcomes for patients: Diagnostic for IMHA, Supportive of IMHA, Suspicious for IMHA, and Not IMHA. The algorithm can be accessed by the adjacent QR code. Briefly, the first level is identifying anemia. The second level is identifying either a positive SAT with RBC washing or >2 signs of an immune-mediated component to the anemia (spherocytes, positive SAT without washing, or positive DAT). The third level is evidence of hemolysis (hyperbilirubinemia/icterus/bilirubinuria, hemoglobinemia/hemoglobinuria, or ghost cells). Patients identified as Diagnostic for IMHA or Supportive of IMHA are clinically considered dogs with IMHA. Patients Suspicious for IMHA may be considered a tentative diagnosis – additional diagnostics are strongly recommended in these patients to screen for other diseases before IMHA therapies are instituted. Dogs identified as Not IMHA by the algorithm should be evaluated for other causes of their anemia


Treatment
Following diagnosis, prompt and multifaceted therapy is essential for giving patients best chance for disease remission and survival. Comprehensive treatment regimens should center on three key approaches: 1) immunosuppression, 2) thromboprophylaxis, and 3) blood product transfusion. To refine a more standardized and evidence-based approach to treating canine IMHA, in 2019 the American College of Veterinary Internal Medicine published the consensus statement on the treatment of immune-mediated hemolytic anemia in dogs.2 The consensus statement is the culmination of a critical assessment of the published efficacy of different therapeutic modalities for IMHA. Based upon the available literature, the consensus panel crafted evidence-based treatment algorithms use of a) immunosuppressive therapy, b)
52
Scan to access ACVIM Consensus Diagnostic Algorithm for IMHA
antithrombotic therapy, and c) relapse management. All three algorithms can be accessed by the following QR codes:


The main treatment objectives in dogs with active IMHA are a) stabilization of HCT and b) mitigation of thromboembolic events. Concerning initial monitoring, patient HCT should be evaluated daily – stabilization and then gradual increase of HCT to >20% is considered an appropriate initial response therapy.9 Patients that fail to respond to first line immunosuppressive therapy or rapidly decompensate warrant additional, concurrent therapies, including addition of a second immunomodulatory drugs or transfusion. Dogs that positively respond to interventions can be shifted to weekly, then monthly, monitoring of HCT until red cell mass normalizes. When HCT >30% for at least 2 weeks, gradual withdrawal of immunosuppressive therapies may begin. To mitigate relapse, therapeutic withdrawal occurs over the course of 4-8 months depending upon immunosuppressives used and clinical response.
Immunosuppression
Immunosuppression is the mainstay of managing an IMHA patient, with the aim being to suppress the immune system adequately enough to attenuate or stop immune-mediated erythrolysis. Glucocorticoids are the recommended first line immunosuppressants, being highly effective in attenuating multiple wings of the immune response and the most rapidly acting immunosuppressant. For dogs with IMHA, they can block macrophagic Fc mediated RBC phagocytosis, decrease complement-mediated erythrolysis, lower autoantibody production, and decrease pro-inflammatory cytokine concentrations.9 For dogs in which there is a failure to respond to initial therapy or disease relapse, addition of a second immunomodulatory agent may be required. Unfortunately, most immunosuppressive agents are mechanistically nonspecific therapies and are associated with a spectrum of adverse side effects, some of which can greatly decrease quality of life for IMHA dogs.
Scan to access ACVIM Consensus Immunosuppressive Therapy Algorithm
Scan to access ACVIM Consensus Antithrombotic Therapy Algorithm
53
Scan to access ACVIM Consensus Relapse Therapy Algorithm
For initial glucocorticoid immunosuppression, in relatively clinically stable IMHA dogs the following drugs and doses are recommended:
• Prednisone / prednisolone: 2-3 mg/kg/day PO or 50-60 mg/m2/day for dogs >25kg
o Once daily administration may be associated with fewer adverse effects
• Dexamethasone SP: 0.2-0.3 mg/kg/day IV if vomiting or inappetent
For clinically stable dogs that have started glucocorticoid therapy but do not have controlled disease by 7 days of therapy, addition of a second immunomodulatory disease is advised. In addition, dogs that have life-threatening disease at presentation are recommended to begin glucocorticoid therapy and a second immunomodulatory drug concurrently. Such lifethreatening criteria include a) severe and/or rapidly progressing anemia, b) probable need for multiple transfusions, and c) presence of negative prognostic factors [see section below]. Unlike glucocorticoids, many of the second-line medications take up to two weeks for their immunosuppressive activity to manifest.2 One study in healthy dogs did demonstrate cyclosporine efficacy in 24 hours, but this has not been assessed in sick patients.22 Second-line immunomodulatory drugs to consider include:
• Azathioprine: 2 mg/kg/day PO for 14 days, followed by q48h dosing
• Cyclosporine: 5 mg/kg PO q12h
• Mycophenolate: 10 mg/kg IV or PO q12h
Note, while addition of second line immunomodulatory therapies is recommended in these situations, there is no evidence that these medications improve outcome in dogs with IMHA compared to glucocorticoid therapy alone.2 Likewise, there is inadequate reported data to guide which second-line therapy may be therapeutically superior.2 While some retrospective studies suggest superiority of one agent, others contradict them, and all are marred by the typical shortcomings of retrospective studies. Large, multicenter prospective studies to compare second line immunosuppressive treatments are direly needed. Client acceptance of the potential side effects of each medication and client finances currently help guide selection of second immunosuppressive agents in each case. Addition of a third immunosuppressive agent should be avoided as this is usually associated with increased risk of opportunistic infections and overlapping drug toxicity, and a clear benefit has not been established.2
Thromboprophylaxis
Dogs with IMHA are frequently hypercoagulable and prone to development of TE and DIC. With fatal thrombosis being the leading cause of natural death in IMHA, prompt institution of thromboprophylaxis is essential.2 Anti-thrombotic therapy can be separated into two approaches – anticoagulant therapy or antiplatelet therapy. Since thrombosis from IMHA is primary venous, and such thrombi are typically fibrin rich, the consensus statement prioritized use of anticoagulant medications. However, the cell-based model of hemostasis establishes
54
that platelets play a key role in secondary hemostasis as well as primary hemostasis and likely contribute to venous thrombosis.23,24 Thus, we believe antiplatelet agents are also likely beneficial in dogs with IMHA. 2
Just as with secondary immunosuppressant agents, there is a lack of well-designed prospective studies comparing all available antithrombotics in IMHA dogs. The IMHA consensus statement recommended individually-adjusted, but not constant dose, unfractionated heparin therapy (UFH).2 This recommendation was based on one prospective study of 15 IMHA dogs receiving either constant dose or individually dose-adjusted (based on anti-Xa monitoring) UFH. The outcome of dogs receiving individually-adjusted UFH was superior to that of many other IMHA reports with 88% of those dogs alive at 180 days post-diagnosis; in contrast, only 14% of dogs in the constant dose group were still alive.25 Individualized UFH therapy can be challenging as most clinics do not readily have access to anti-Xa monitoring. Nomograms to adjust UFH therapy using an aPTT assay or thromboelastography have been described.26 The recommended starting dose of UFH is 150 U/kg -300 U/kg SQ q6h or 900 U/kg/24 hours CRI following a 100 U/kg bolus; it is imperative to monitor efficacy ideally using factor Xa inhibition assay and titrate dose based upon results.2,9,25 Constant dose UFH should be avoided. Alternatively, oral direct Xa inhibitors like rivaroxaban (1-2 mg/kg/day) may be equally as effective and can be dosed orally without monitoring.23 Canine vessel occlusion models have demonstrated equal or superior efficacy of Xa inhibitors compared to UFH.27 Rivaroxaban was tolerated in one study of dogs with IMHA, but its efficacy was not compared to UFH.28 Low molecular weight heparins (LMWH) provide another alternative but have not been compared to UFH or rivaroxaban in IMHA.2,9
Antiplatelet agents are also recommended in combination with anticoagulants or can be used alone if anticoagulant therapies are not feasible in a given patient. For dogs exhibiting the highest risk of thromboembolic disease or clinical TE, antiplatelet therapy should be administered concurrently with anticoagulant regimens. Given that thirty percent or more of dogs do not respond to low dose aspirin, clopidogrel (1.1-4 mg/kg PO q24h) therapy is prioritized 2,29,30 If aspirin is utilized it should be dosed at 1 - 2 mg/kg PO q24h, but this dose may be insufficent.2,9,31
Transfusion
While administration of blood products is common for IMHA patients, not all dogs require a transfusion and blood administration should be balanced between the clinical needs of the patient relative to risk of adverse transfusion reactions. Transfusion should be considered when there is clinical evidence of anemia-associated hypoxia compromising physiologic functions – these signs may include weakness, tachypnea, tachycardia, hypothermia, and hypotension. While there is no specific HCT threshold for initiating a transfusion, dogs with HCT >20% are unlikely to have comprised organ function due to anemia and dogs with <12% likely
55
have significant hypoxemic stress.2,9 If transfusion is warranted, administration of packed RBCs (pRBCs) is advised over whole blood since IMHA dogs are usually normovolemic.2 Data from a retrospective study suggests that younger blood improves outcomes in dogs with IMHA 32 In contrast, a more recent prospective study demonstrated no difference in morbidity or mortality in IMHA dogs randomized to receive ≤7 days or ≥ 21 days stored RBC units; however, this study was underpowered.32 The IMHA consensus recommends transfusing with units ≤10 days old if possible.232 Before transfusion administration, dogs should be blood typed preferentially with immunochromatographic typing strips in which agglutination does not interfere with test interpretation. Dogs receiving serial transfusions should also be crossmatched even before 72 hours after the initial transfusion.33
Additional Therapeutics
IMHA dogs with ongoing hemolysis despite glucocorticoid therapy and second-line immunosuppressive agents may warrant either intravenous immunoglobulin (IVIG) therapy or splenectomy. Before considering these therapies, steps should be taken to ensure the diagnosis of IMHA is correct, that drug dose and administration is appropriate, and therapeutic drug monitoring should be performed if available for the patient’s second-line agent.2 IVIG should be considered prior to splenectomy. Mechanistically, administration of human IVIG supersaturates the Fc receptors on macrophages. With receptors saturated, macrophages cannot bind autoantibodies on RBCs and thus cannot phagocytize erythrocytes. Human IVIG product can be administered at dose of 0.5 – 1.5 g/kg over an 8-12 hour period; only a single transfusion is recommended due to concern for risk of immunologic responses to a second unit.2,33 The efficacy of IVIG therapy to blunt hemolysis is variable, but instances of sudden attenuation of erythrolysis are reported.2 As opposed to its established efficacy in ITP, evidence documenting IVIG’s efficacy in IMHA is lacking. This is likely due to the prothrombotic potential of IVIG in an already hypercoagulable condition. If IVIG is to be utilized, it should be combined with anticoagulant therapy.
Splenectomy may be considered in those dogs requiring continuous immunosuppressive therapy to prevent relapse, those suffering from frequent relapses, or those intolerant to medical therapy.2 As splenic macrophages are most implicated in extravascular RBC destruction in IMHA, splenic removal can acutely diminish extravascular hemolysis 9 All published studies assessing splenectomy in dogs with IMHA lack control groups, thus efficacy of splenectomy is hard to gauge. A recent retrospective case series found that of 7 dogs which underwent splenectomy for IMHA, 4 dogs had partial (2) or complete (2) disease remission.34 Before splenectomy is performed, thorough vector-borne disease screening is recommended.
For dogs with associative IMHA, treatment of the underlying cause is also imperative. Resolution of the inciting trigger could potentially lessen the severity of clinical disease and in
56
some cases, especially infectious agents, may promote disease resolution and prevent disease relapse.
Discontinuation of Therapy
In dogs that successfully respond to therapy, deciding when and how to discontinue therapeutics can be challenging as withdrawal of medications too quickly can result in relapse. While there are no uniform metrics for when to begin therapeutic tapering, slow withdrawal could be considered after HCT has been >30% for over 2 weeks without ongoing evidence of hemolysis (spherocytosis, agglutination). Glucocorticoids should be tapered first by reducing dose 20-25% every 3 weeks; patients should be monitored for relapse during this time window. With complete withdrawal of glucocorticoids, secondary immunosuppressive agents can be stopped promptly or tapered, per consensus guidelines, but the authors usually elect to taper these medications. Antithrombotics can also be discontinued with glucocorticoid therapy, but UFH, LMWH, and oral Xa inhibitors should be weaned vs. abruptly discontinued to prevent rebound hypercoagulation.35
Follow-up CBC monitoring is encouraged to screen for early signs of disease recurrence. Reported relapse rates for dogs that survive initial hemolytic crisis range from 11-15%; some dogs may require recurrent or lifelong therapy for disease control.2 Approach to relapse should follow the above algorithm. Caution should be taken to ensure that a true relapse is occurring and that anemia is not now secondary to medication-induced gastrointestinal bleeding.2
Predictive Factors and Prognosis
Many clinical and clinicopathologic variables have been identified as prognostic survival predictors in canine IMHA that can guide earlier institution of second line immunosuppressants.3 In an effort to make a more clinically useful predictive model, Whelan et al. designed the Canine Hemolytic Anemia Objective Score (CHAOS) which includes the following patient metrics: age, temperature, agglutination, albumin concentration, and bilirubin concentration.36 Higher CHAOS is associated with poorer survival, with dogs having CHAOS ≥ 3 being 4x more likely to die before hospital discharge and 3.5x more likely to not survive 30 days post-diagnosis.10,36 Unfortunately even with the most aggressive of therapies, IMHA has an unfavorable prognosis with reported mortality rates ranging from 50-80%.3
Summary
Canine IMHA is a disease with an unacceptably high mortality rate and high treatmentassociated morbidity. Outcome improvement requires a better understanding of disease pathogenesis to allow for development of more targeted immunomodulatory therapy. Furthermore, prospective randomized multi-institutional clinical trials are needed to determine whether secondary immunosuppressive agents improve outcomes, and if so, which agent (if any) is superior. Similar trials are also needed to determine the best antithrombotic regimen.
57
References
1. Garden OA, Kidd L, Mexas AM, et al. ACVIM consensus statement on the diagnosis of immunemediated hemolytic anemia in dogs and cats. Journal of Veterinary Internal Medicine 2019;33:313-334.
2. Swann JW, Garden OA, Fellman CL, et al. ACVIM consensus statement on the treatment of immunemediated hemolytic anemia in dogs. Journal of Veterinary Internal Medicine 2019;33:1141-1172.
3. Piek CJ. Canine idiopathic immune-mediated haemolytic anaemia: a review with recommendations for future research. Vet Q 2011;31:129-141.
4. Friedenberg SG, Buhrman G, Chdid L, et al. Evaluation of a DLA-79 allele associated with multiple immune-mediated diseases in dogs. Immunogenetics 2016;68:205-217.
5. Kennedy LJ, Barnes A, Ollier WER, et al. Association of a common dog leucocyte antigen class II haplotype with canine primary immune-mediated haemolytic anaemia. Tissue Antigens 2006;68:502508.
6. Swann JW, Woods K, Wu Y, et al. Characterisation of the Immunophenotype of Dogs with Primary Immune-Mediated Haemolytic Anaemia. PLoS One 2016;11:e0168296.
7. Harkin KR, Hicks JA, Wilkerson MJ. Erythrocyte-bound immunoglobulin isotypes in dogs with immunemediated hemolytic anemia: 54 cases (2001-2010). J Am Vet Med Assoc 2012;241:227-232.
8. Quigley KA, Chelack BJ, Haines DM, et al. Application of a direct flow cytometric erythrocyte immunofluorescence assay in dogs with immune-mediated hemolytic anemia and comparison to the direct antiglobulin test. J Vet Diagn Invest 2001;13:297-300.
9. Haines JM, Mackin A, and Day MJ. Immune-mediated anemia in the dog. In: Brooks MB HK, Seelig DM, Wardrop KJ, and Weiss DJ., ed. Schalm's Veterinary Hematology, 7 edWiley; 2022:278-291.
10. Goggs R, Dennis SG, Di Bella A, et al. Predicting Outcome in dogs with Primary Immune-Mediated Hemolytic Anemia: Results of a Multicenter Case Registry. Journal of Veterinary Internal Medicine 2015;29:1603-1610.
11. Goggs R, Jeffery U, LeVine DN, et al. Neutrophil-Extracellular Traps, Cell-Free DNA, and Immunothrombosis in Companion Animals: A Review. Veterinary Pathology 2020;57:6-23.
12. Lawson C, Smith SA, O'Brien M, et al. Neutrophil Extracellular Traps in Plasma from Dogs with Immune-mediated Hemolytic Anemia. Journal of Veterinary Internal Medicine 2018;32:128-134.
13. Noubouossie DF, Reeves BN, Strahl BD, et al. Neutrophils: back in the thrombosis spotlight. Blood 2019;133:2186-2197.
14. Assenmacher TD, Jutkowitz LA, Koenigshof AM, et al. Clinical features of precursor-targeted immune-mediated anemia in dogs: 66 cases (2004–2013). Journal of the American Veterinary Medical Association 2019;255:366-376.
15. Sun PL, Jeffery U. Effect of dilution of canine blood samples on the specificity of saline agglutination tests for immune-mediated hemolysis. Journal of Veterinary Internal Medicine 2020;34:2374-2383.
16. Caviezel LL, Raj K, Giger U. Comparison of 4 direct Coombs' test methods with polyclonal antiglobulins in anemic and nonanemic dogs for in-clinic or laboratory use. J Vet Intern Med 2014;28:583-591.
17. Klag AR, Giger U, Shofer FS. Idiopathic immune-mediated hemolytic anemia in dogs: 42 cases (19861990). J Am Vet Med Assoc 1993;202:783-788.
18. Balch A, Mackin A. Canine immune-mediated hemolytic anemia: pathophysiology, clinical signs, and diagnosis. Compend Contin Educ Vet 2007;29:217-225.
19. Idalan N, Zeitz JO, Weber CN, et al. Comparative study of immunohematological tests with canine blood samples submitted for a direct antiglobulin (Coombs') test. Canine Med Genet 2021;8:10.
20. Goggs R, Wiinberg B, Kjelgaard-Hansen M, et al. Serial assessment of the coagulation status of dogs with immune-mediated haemolytic anaemia using thromboelastography. Vet J 2012;191:347-353.
58
21. Maggi RG, Birkenheuer AJ, Hegarty BC, et al. Comparison of serological and molecular panels for diagnosis of vector-borne diseases in dogs. Parasit Vectors 2014;7:127.
22. Riggs C, Narayanan L, Mulligan C, et al. Alterations in activated T-cell cytokine expression in healthy dogs over the initial 7 days of twice daily dosing with oral cyclosporine. J Vet Pharmacol Ther 2019;42:385-391.
23. Goggs R, Bacek L, Bianco D, et al. Consensus on the Rational Use of Antithrombotics in Veterinary Critical Care (CURATIVE): Domain 2-Defining rational therapeutic usage. J Vet Emerg Crit Care (San Antonio) 2019;29:49-59.
24. Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest 2012;122:23312336.
25. Helmond SE, Polzin DJ, Armstrong PJ, et al. Treatment of immune-mediated hemolytic anemia with individually adjusted heparin dosing in dogs. J Vet Intern Med 2010;24:597-605.
26. Hanel RM. Heparin monitoring in critically ill dogs. In: 2017 American College of Veterinary Internal Medicine Forum, Washington, D.C. 2017.
27. Rebello SS, Bentley RG, Morgan SR, et al. Antithrombotic efficacy of a novel factor Xa inhibitor, FXV673, in a canine model of coronary artery thrombolysis. Br J Pharmacol 2001;133:1190-1198.
28. Morassi A, Bianco D, Park E, et al. Evaluation of the safety and tolerability of rivaroxaban in dogs with presumed primary immune-mediated hemolytic anemia. J Vet Emerg Crit Care (San Antonio) 2016;26:488-494.
29. Dudley A, Thomason J, Fritz S, et al. Cyclooxygenase expression and platelet function in healthy dogs receiving low-dose aspirin. J Vet Intern Med 2013;27:141-149.
30. Sharpe KS, Center SA, Randolph JF, et al. Influence of treatment with ultralow-dose aspirin on platelet aggregation as measured by whole blood impedance aggregometry and platelet P-selectin expression in clinically normal dogs. Am J Vet Res 2010;71:1294-1304.
31. Blais MC, Bianco D, Goggs R, et al. Consensus on the Rational Use of Antithrombotics in Veterinary Critical Care (CURATIVE): Domain 3-Defining antithrombotic protocols. J Vet Emerg Crit Care (San Antonio) 2019;29:60-74.
32. Hann L, Brown DC, King LG, et al. Effect of duration of packed red blood cell storage on morbidity and mortality in dogs after transfusion: 3,095 cases (2001-2010). J Vet Intern Med 2014;28:1830-1837.
33. Herter L, Weingart C, Merten N, et al. Alloimmunization in dogs after transfusion: A serial crossmatch study. Journal of Veterinary Internal Medicine 2022;36:1660-1668.
34. Bestwick JP, Skelly BJ, Swann JW, et al. Splenectomy in the management of primary immunemediated hemolytic anemia and primary immune-mediated thrombocytopenia in dogs. J Vet Intern Med 2022;36:1267-1280.
35. Brainard BM, Buriko Y, Good J, et al. Consensus on the Rational Use of Antithrombotics in Veterinary Critical Care (CURATIVE): Domain 5-Discontinuation of anticoagulant therapy in small animals. J Vet Emerg Crit Care (San Antonio) 2019;29:88-97.
36. Whelan MF, Rozanski E, O'Tolle TE. Use of the canine hemolytic anemia objective score (CHAOS) to predict survival in dogs with immunemediated hemolytic anemia [abstract]. J Vet Intern Med 2006;20:714-715.
59
Consensus Statement on the Diagnosis and Treatment of Immune Thrombocytopenia in Dogs and Cats
Part 1 & 2
Dana LeVine, DVM, Ph.D., DACVIM (SAIM)
Austin Viall, DVM, MS, DACVP

61
Consensus Statement on the Diagnosis and Treatment of Immune
Thrombocytopenia in Dogs and Cats
Presenters:
Dana LeVine, DVM, Ph.D., DACVIM (SAIM)
Associate Professor of Small Animal Internal Medicine, Auburn University College of Veterinary Medicine, Auburn, AL
Austin Viall, DVM, MS, DACVP
Associate Professor of Clinical Pathology, University of California Davis, Davis, CA
N.B. These notes will provide an overview of the pathogenesis, diagnosis, and treatment of ITP, primarily in dogs since it is a more common disease in dogs with more literature to support its diagnosis and treatment. The American College of Veterinary Internal Medicine (ACVIM) Consensus Statement on diagnosis and treatment of ITP in dogs and cats is currently being drafted by the authors and highlights of our findings will be presented during the Spring Seminar lectures. However, ACVIM does not allow pre-publication of the Consensus guidelines, so they cannot be included in these notes. Any Consensus recommendations that are discussed have not yet been finalized and may be further refined
Introduction
Immune thrombocytopenia (ITP) is the most common acquired disorder of primary hemostasis in dogs. It is a complex autoimmune disorder characterized by both antibody and T-cell mediated platelet and sometimes concurrent megakaryocyte destruction. The resultant thrombocytopenia leads to variable clinical signs ranging from none to a severe mucocutaneous bleeding diathesis. Treatment strategies should aim to restore adequate platelet count to prevent bleeding, but should not necessarily target a normal platelet count. Frontline therapy involves immunosuppressive glucocorticoids combined with adjunctive immunosuppressive therapy as needed. Here we review pathogenesis and diagnosis of ITP and treatment goals and strategies.
What causes ITP?
ITP is an autoimmune disease characterized by both platelet destruction and impaired megakaryocyte and platelet production.1 The immune dysregulation resulting in ITP is incompletely understood, and likely complex. Little is known about ITP pathogenesis in dogs and cats, so we base most of our understanding of the disease on human patients and murine models. Traditionally, ITP has been thought of as a humoral disease.2,3 Autoantibodies targeting platelet surface glycoproteins lead to platelet clearance by the splenic and hepatic macrophages 1 However, it is now recognized that T cells play a central role in platelet
63
destruction in ITP.1,4-6 A proinflammatory T helper cell (Th)1, Th17, and Th22 cytokine milieu predominates in many ITP patients.1,3 T and B regulatory cells that normally serve to maintain self-tolerance are dysfunctional in ITP, enabling the onset of autoimmunity.1,7-11 In some patients, platelet destruction is not mediated by autoantibodies, but instead by autoreactive cytotoxic T lymphocytes.1,6,12,13
Growing evidence indicates that ITP is not only a disorder of destruction, but also one of production. In some patients, antibodies and T-cells attack megakaryocytes, resulting in decreased platelet production.12,14 Megakaryocytes of dogs with ITP display signs of injury including foaminess, vacuolation, and reduction of cytoplasmic granularity.15
In addition to immune targeting of megakaryocytes and platelets, thrombopoietin (TPO) levels are often inappropriately normal in human patients with ITP. TPO is the major regulator of platelet production and should be elevated in response to thrombocytopenia.16-18 As platelets age, they are desialylated and subsequently recognized and cleared by the hepatic AshwellMorell receptor.18 This removal in turn, drives hepatic TPO expression providing a feedback system: as more aged platelets are cleared, more TPO is produced. However, in ITP antibodycoated platelets are cleared by macrophages of the spleen and liver, so it is postulated that the Ashwell-Morell receptor is bypassed and does not trigger hepatic TPO production.18 Circulating TPO levels in people with ITP are often inadequate.16 TPO levels in dogs with ITP are also likely inappropriately low (unpublished data).
Cocker Spaniels and Old English Sheepdogs are predisposed to ITP, which suggests there may be genetic or hereditary variables that contribute to the development of ITP, at least in dogs.1922 ITP likely results from genetic factors predisposing to autoimmunity and some sort of environmental or infectious trigger, which often remains unidentified.
Who gets ITP and how do patients with ITP present?
ITP is the most common cause of severe thrombocytopenia in dogs. Although any dog can develop ITP, affected dogs tend to be young to middle age; Cocker spaniels, Poodles, and Old English Sheepdogs are predisposed.19,21-24 Primary ITP is rarely reported in cats, but has been described.25-30 Interestingly, in people, dogs, and cats, signs of ITP vary widely. When bleeding does occur, it is typically surface bleeding of the skin and mucosal surfaces - cutaneous, oral, and gastrointestinal bleeding is most common in dogs.20,31 However, many patients with severe thrombocytopenia (<30,000 platelets/µl) have no clinical signs of bleeding and platelet count alone is not a reliable predictor of bleeding.31 Why patients demonstrate variable bleeding is not understood and is likely multifactorial. Interference of platelet function by anti-platelet antibodies and the variable impact of thrombocytopenia on endothelial integrity likely play a role in bleeding presentation. 32-36
64
Diagnosis
Because of the variability of ITP pathogenesis, ITP is a diagnosis of exclusion. Unfortunately, there is no one test for the disease, and as such, a systematic approach to excluding other causes of thrombocytopenia must be taken. In human medicine, ITP is defined as a platelet count of under 100,000/µl in the absence of other causes or disorders that may be associated with thrombocytopenia.37 As part of the ITP Consensus, we developed five diagnostic questions in the Population Evaluation Comparison Outcome (PECO) question format to investigate whether in dogs and cats with thrombocytopenia (P), evaluation by a diagnostic test (E) compared to platelet count alone (C) improved differentiation of ITP from non-immune thrombocytopenia (O). The questions were then answered by an extensive process that involved systematic review of the available veterinary literature. The PECO questions, which will be addressed in the seminar, are as follows:
1. In dogs/cats with confirmed thrombocytopenia (P), compared with platelet count alone (C), do platelet indices (e.g. MPV, IPF, reticulated platelets, plateletcrit) (E) improve differentiation of ITP from non-immune thrombocytopenia (O)?
2. In dogs/cats with confirmed thrombocytopenia (P), does severe thrombocytopenia (E) compared with mild to moderate thrombocytopenia (C), improve differentiation of ITP from non-immune thrombocytopenia (O)?
3. In dogs/cats with confirmed thrombocytopenia (P), compared with platelet count alone (C), does the addition of bone marrow examination (E) help differentiate immune thrombocytopenia from non-immune thrombocytopenia (O)?
4. In dogs/cats with confirmed thrombocytopenia (P), compared with platelet count alone(C), do platelet/megakaryocyte-related antibody assays (E) help differentiate ITP from non-immune thrombocytopenia (O)?
5. In dogs/cats with confirmed thrombocytopenia (P), compared with platelet count alone (C), does the addition of hemostasis testing (e.g. coagulation testing, platelet function testing, viscoelastic testing, fibrinolysis testing, D-dimer concentration) (E) help differentiate immune thrombocytopenia from non-immune thrombocytopenia (O)?
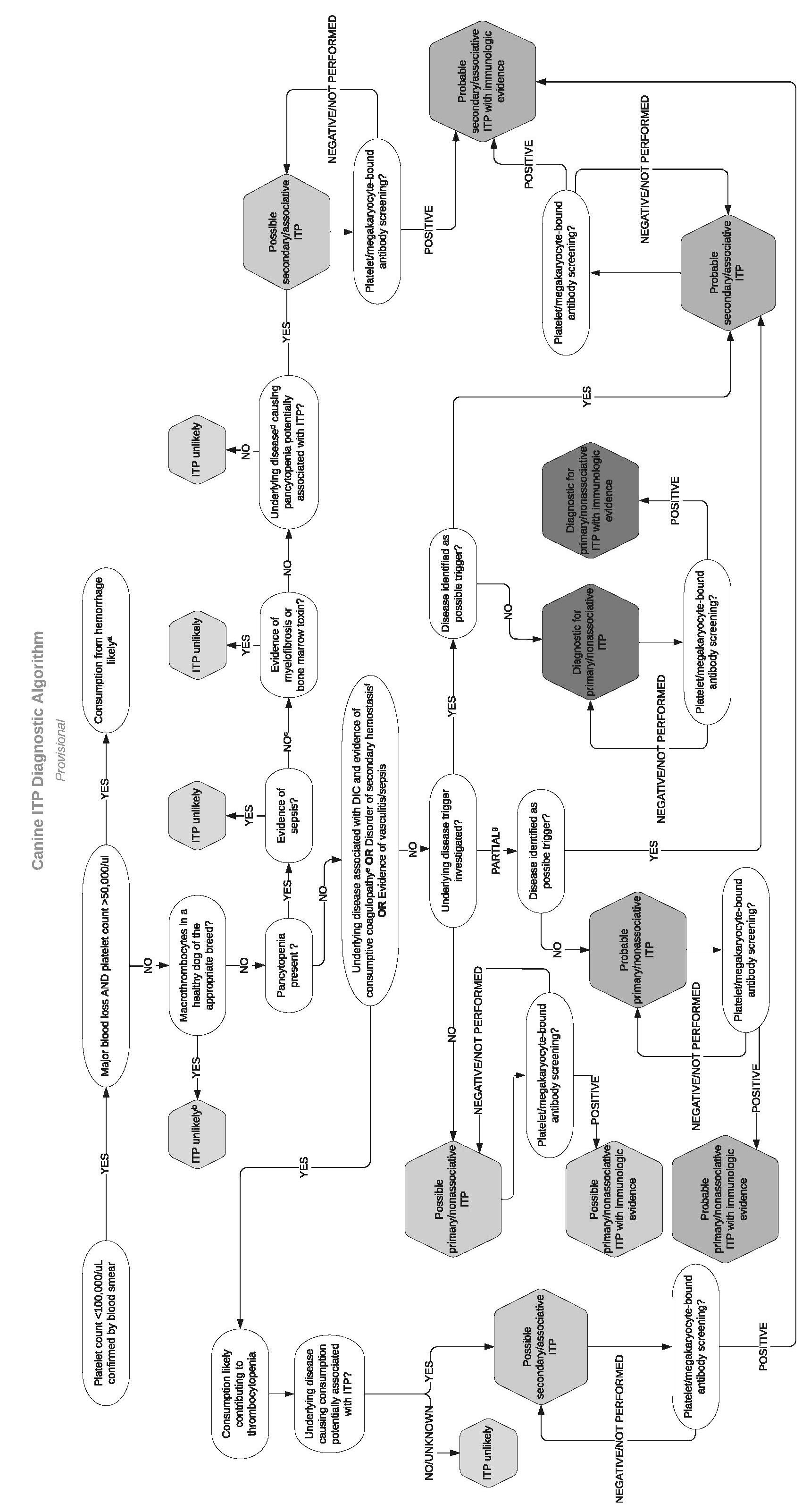
Answers to the PECO questions resulted in the panel’s development of a diagnostic algorithm for ITP shown in Figure 1.
Key take home points for diagnosing ITP include:
1. Pseudothrombocytopenia must be ruled out in any ITP suspect, especially in nonclinical patients and in cats. Cats often have pseudothrombocytopenia due to feline platelet reactivity and difficulty of some hematology analyzers to differentiate them from erythrocytes as they are similarly sized.38 A manual platelet count
65
estimate must be performed before any further workup is pursued. In brief, first assess the slide’s feathered edge under low magnification for clumps, the presence of which suggests the platelet count is falsely low and warrants obtaining a new blood sample. If there are no clumps, a platelet count is estimated by averaging the number of platelets observed in 10 oil immersion fields (100×) and multiplying this by 15,000 to obtain the number of platelets per microliter.39 For example, if there is an average of 4 platelets per 100x field, the estimated platelet count is 60,000/µl.
2. Congenital macrothrombocytopenia should be suspected in dogs with chronic thrombocytopenia in the absence of bleeding signs. Congenital macrothrombocytopenia due to a β1 tubulin gene mutation has been identified in the Cavalier King Charles Spaniel, Norfolk and Cairn Terriers, and several other breeds.40 Auburn University offers DNA testing that can help confirm congenital macrothrombocytopenia.
3. Coagulation testing is essential to rule out consumptive causes of thrombocytopenia like disseminated intravascular coagulation.
4. While severe thrombocytopenia (<20,000 platelets/µl) should make a clinician suspicious of ITP, other causes of thrombocytopenia like consumption can cause equally severe thrombocytopenia. Severity of thrombocytopenia alone cannot confirm an ITP diagnosis.
5. Bone marrow analysis is not routinely recommended unless underlying marrow disease is suspected due to multiple cytopenias, there is a poor response to standard therapy, or if the clinician wants to search for possible lymphoproliferative disease. When needed, a sternal marrow aspirate can be considered as a less invasive alternative to sampling of the humerus or ilium.41
6. Unfortunately, platelet-associated antibody testing is relatively insensitive and nonspecific, thus routine measurement of platelet antibodies is not currently recommended. Positive platelet-associated antibody testing does confirm that there is an immune component to the thrombocytopenia, but it could be primary or secondary.
a. Based on one study, recurrence of platelet-associated antibodies may occur concurrently with disease relapse.42
b. There is new evidence that, at least in human ITP, the platelet glycoprotein that is being targeted by antibodies will determine the pathway of platelet clearance. Antibodies to GPIbα lead to platelet desialylation (premature aging) and clearance of platelets independent of macrophages.43 As a result, patients with anti-GPIbα do not respond well to glucocorticoids or intravenous immunoglobulin but may respond to sialidase inhibitors like oseltamivir (Tamiflu®).3 There may be utility in determining the target of
66
autoantibodies in treatment selection, but no such test is currently available for companion animals. We are currently developing a platelet desialylation assay to further investigate the role of platelet desialylation in canine ITP pathogenesis.
Primary ITP must also be distinguished from secondary ITP due to infections, medications, or neoplastic causes. The consensus panel also systematically evaluated potential secondary triggers of ITP. Triggers for which the most evidence was found in dogs include: Ehrlichia canis, 44,45 Leishmania, 46 Babesia, 47 and some medications (cefazedone and gold salts).48,49 An intermediate level of evidence was found supporting an association between ITP development and Anaplasma, 50,51 solid tumors,52 potentiated sulfonamides.53 Unfortunately, studies assessing ITP triggers in cats are scarce. Overall screening recommendations to rule out secondary ITP should include obtaining a thorough drug history, a minimum database, abdominal and thoracic imaging, and infectious disease testing based on the geographic locale, making sure to include the above-listed agents. To improve the sensitivity of vector borne disease testing, PCR and serology combined are strongly recommended, as one study documented that combining these modalities increased sensitivity by up to 58% 54
Treatment
Treating the Stable ITP Patient
The lack of correlation between platelet count and bleeding severity provides a treatment dilemma. Platelet counts of 30,000/µl are generally considered a threshold for spontaneous hemorrhage, yet some patients with higher platelet counts require extensive transfusion support and many with platelet counts below 10,000/µl have minor petechiae as their only clinical sign. The question then becomes, which patients require aggressive therapy? Clinicians empirically treat all ITP cases with high dose corticosteroids and often include cocktails of immunosuppressive drugs. Much of the overall disease burden of ITP is due to the lack of prognostic criteria and resultant uniform administration of high intensity and long-term immunosuppressive therapy. At least in human patients with severe ITP, mortality results equally from refractory hemorrhage and secondary infections in immunosuppressed patients 55 Very few outcome predictors in ITP have been identified, and more research needs to be performed in this area to help guide individualized therapy so that only higher risk patients are aggressively immunosuppressed. In dogs, elevated BUN and melena are the only markers that have thus far been associated with reduced ITP survival.19 Bleeding scores like the canine bleeding assessment tool or “DOGiBAT,” may also reflect disease severity and help to guide treatment.31
The mainstay of ITP treatment remains immunosuppression, with glucocorticoid therapy being the frontline treatment.
67
Prednisone (prednisolone in cats) is started at 2 mg/kg/day, or 50-60 mg/m2 for dogs over 25 kg. We recommend starting doxycycline therapy pending infectious disease screening results.
The ITP Consensus Panel has systematically reviewed the evidence in the veterinary literature to answer the following PICO questions (Population; Intervention; Comparison; Outcome) questions which will be presented at the seminar:
1. In dogs/cats with primary ITP (P), is treatment with combined glucocorticoids and a 2nd immunosuppressive agent (I) compared with use of glucocorticoids alone (C) associated with different primary or secondary outcomes (O)?
2. In dogs/cats with primary ITP (P), is a maintenance treatment with glucocorticoids and a 2nd immunosuppressive agent (I) superior to glucocorticoids alone (C) in order to prevent relapse (O)?
3. In dogs/cats with primary ITP (P), is treatment with glucocorticoids and any second agent (I) compared to treatment with glucocorticoids and any other second agent (C) associated with different primary or secondary outcomes (O)?
Key highlights of our findings (sneak preview) are:
1. An adjunctive immunosuppressive agent should be considered if
a. The patient does not respond with an adequate platelet count (≥40,000/µl) within 7-10 days of starting glucocorticoids.
b. The patient develops or is expected to develop severe adverse effects related to the use of glucocorticoids. This includes dogs >25 kg.
c. If the patient relapses.
d. Patients with severe bleeding.
2. Reasonable options for adjunctive immunosuppressive agents include mycophenolate mofetil, azathioprine, cyclosporine, and leflunomide.
a. Convincing evidence to select one agent over another was not identified.
b. Cyclosporine (5 mg/kg BID) has the advantage of the ability to monitor drug efficacy through the Mississippi State College of Veterinary Medicine’s Pharmacodynamic Laboratory.
c. Cyclosporine has the disadvantage of being associated with increased risk of opportunistic invasive cutaneous fungal infections.56
d. Azathioprine should not be used in cats as they can develop severe, even fatal, drug-induced myelosuppression due to their low concentrations of thiopurine methyltransferase.57
3. If remission is not obtained with two immunosuppressive drugs, we recommend further diagnostic workup for an underlying cause that might have been missed, performing therapeutic drug monitoring, or switching adjunctive
68
immunosuppressant agents. We caution strongly against triple immunosuppressant therapy, as this has been associated with poor outcome in one study.19
The treatment goal in a stable ITP patient should be considered. Does platelet count need to be normalized or should our target be a safe platelet count, as is human medical practice? The American Society of Hematology 2019 ITP guidelines recommend observation only for ITP patients with platelet counts over 30,000 platelets/µl and absence of clinical bleeding.58 Since it would be unrealistic to ask our veterinary patients with ITP to maintain a quiet lifestyle chronically, a higher platelet count goal than 30,000/µl is likely necessary. A provisional treatment goal recommendation of the consensus panel is ≥ 100,000 platelets/µl with no active bleeding
Treating the Scary ITP Patient
The approach to the bleeding ITP patient should be more aggressive. Our approach changes when a patient is bleeding into the gastrointestinal tract or when respiratory or central nervous system bleeding is suspected. There are two medical rescue options:
1. Vincristine: Vincristine is speculated to increase platelet count by preventing microtubule polymerization and thereby accelerating megakaryocyte fragmentation and platelet release from bone marrow.59 Studies have demonstrated more rapid resolution of thrombocytopenia and shorter hospitalization duration in ITP dogs treated with vincristine (0.02 mg/kg) intravenously once in combination with prednisone compared to prednisone alone.60 Some have questioned the hemostatic capacity of vincristine-induced platelets, but one recent study determined by a flow-cytometric assay that vincristine-induced reticulated (young) platelets are functional.61 Evidence for the efficacy of vincristine in feline ITP is less convincing.
2. Intravenous immunoglobulin: IVIg is a human plasma product primarily composed IgG. One of IVIg’s main mechanisms of action in ITP is blocking antibody-mediated platelet clearance by saturating macrophage Fc receptors.12 There are other proposed mechanisms of action such as immunomodulation by increasing regulatory T cell and reduction in autoantibody production.12 A similar study to the vincristine trial demonstrated that treatment of dogs with severe ITP with IVIg combined with prednisone also shortened platelet recovery time and hospitalization duration compared to prednisone alone.62
One PICO question investigated by the consensus panel is:
1. In patients with primary ITP (P), is treatment with combined glucocorticoids and IVIg (I) compared with use of glucocorticoids and vincristine (C) associated with different primary or secondary outcomes (O)?
69
a. One study demonstrated that vincristine and IVIg are equally as effective in reducing time to platelet count recovery and hospital duration in dogs with ITP 59
b. Given that vincristine is less costly and more available than IVIg, the panel will likely recommend its use as a first-line emergency therapy in preference to IVIg in dogs.
c. Evidence from limited case reports may favor IVIg over vincristine as an emergency therapeutic in cats.29,63
Novel Therapies
In people with ITP, frontline treatments include short courses of corticosteroids and IVIg, while second-line therapies include an anti-CD20 antibody (rituximab), splenectomy, and TPO receptor agonists.37
TPO receptor agonists:
Since the identification of inappropriately low to normal TPO levels in human ITP, treatment with TPO receptor agonists has become a standard second-line therapy for patients who do not respond to steroids or IVIg alone. TPO agonists have greatly improved outcomes and reduced side effects in human ITP patients. One such agent, romiplostim (Nplate) is a peptide fusion protein that works at a conserved region of the canine TPO receptor with homology to the human protein. Romiplostim was used successfully in a pilot study of 5 dogs with ITP.64 Since this study was not controlled, further studies of romiplostim in canine ITP are needed. Recent data from the authors’ laboratory suggests that TPO may also be low in canine ITP patients, giving further indication for exploration of this treatment option in dogs (unpublished data).
Splenectomy, another second line therapy in human ITP has a reported response rate of 60% life-long remission.65 Another consensus PICO explores the evidence of splenectomy in dogs and cats with ITP.
1. In patients with primary ITP (P), is treatment with splenectomy (I) compared with no splenectomy (C) associated with different primary or secondary outcomes (O)?
a. Preliminary findings suggest that overall data is equivocal for splenectomy in canine ITP. Splenectomy can be considered in refractory patients, but response rates are variable.66,67 Overall splenectomy appears well-tolerated, but screening for locally relevant infectious agents should be performed prior to splenectomy.
b. There is no data regarding splenectomy in feline ITP with the exception of one case report.68
The final second line therapy in human medicine, rituximab, is not currently an option for our patients. Rituximab is an anti-human CD20 antibody that effectively reduces antibody
70
production by depleting B cells. Its response rate is similar to that of splenectomy in people, though with less sustained remissions.65 However, rituximab does not bind canine or feline B cells and there are currently no available anti-CD20 antibodies for use in veterinary patients.69
Platelet Transfusion Therapy
Platelets transfused to ITP patients will likely have short half-lives and not impact platelet count significantly. However, they may provide essential hemostasis at sites of critical bleeding like the central nervous system or respiratory tract while allowing other treatments time to take effect. Human ITP guidelines recommend that platelet transfusions be reserved for those patients experiencing hemorrhagic bleeding or requiring invasive surgery.37 When these conditions are not present, a recent study determined that platelet transfusions were not associated with improved clinical outcomes in human ITP patients.70 Platelet containing transfusion products include fresh whole blood, fresh platelet-rich plasma, fresh platelet concentrate, cryopreserved platelets, and a lyophilized canine platelet product, StablePlateRx™ (See Hemostatic Hurdles notes).
PICO questions being asked by the consensus panel include:
1. In dogs/cats with primary ITP (P), does treatment with any platelet-containing transfusion product (I), compared to no platelet-containing products (C), improve any outcomes (O)?
2. In dogs/cats with primary ITP (P), does treatment with one platelet-containing product (I), compared to any other platelet-containing products (C), improve any outcomes (O)?
Overall assessment of these questions was similar to human guidelines in that platelet transfusion should be reserved for those patients with severe or life-threatening bleeding. While fresh platelet concentrate is the standard blood product used in human medicine and is routinely available due to an advanced blood banking infrastructure, fresh platelet concentrate is not often an accessible option for our patients. Insufficient numbers of studies have investigated the ideal platelet product in veterinary medicine;71,72 thus there is not enough evidence to determine if one platelet-containing product is superior to another for treatment of dogs/cats with ITP. Product availability, volume, safety and platelet concentration in the available products should factor into transfusion production selection.
Prognosis
The overall prognosis of canine and feline ITP is good with reported survival rates ranging from 70 to 90%.21,22,27,28 We routinely taper patients off immunosuppressant therapy by reducing dose by 25% every 2-4 weeks after confirming a stable platelet count. If a patient is receiving an adjunctive immunosuppressant, the choice to taper glucocorticoids versus the second agent depends on the patient’s tolerance of glucocorticoid side effects and owner finances. If the
71
patient is tolerating steroids and cost is an issue, we may taper the second agent first. However, usually glucocorticoid side effects motivate prednisone taper as the first step. Some patients relapse during taper, with published canine relapse rates ranging from 9 to 58% 19,20,22 Patients that relapse can be maintained on an adjunctive immunosuppressant long term or, alternatively, splenectomy can be considered.
Summary
Due to the complex disease pathogenesis, ITP patients present with variable disease severity and bleeding phenotypes. Therapy should be individualized to the patient’s disease severity as best as possible to balance bleeding risk relative to the risks of immunosuppression. Identification of bleeding predictors will facilitate this approach in the future. Glucocorticoids remain the mainstay of therapy, with second-line immunosuppressants being utilized as needed along with vincristine and/or IVIg in critically bleeding patients. Clinicians should consider a treatment goal of a safe, but not necessarily normal, platelet count. Pathogenesis of human and canine ITP is an area of active research. Improved understanding of the disease pathogenesis will result in better tests and more targeted immunotherapies.
Acknowledgment: Some sections of these notes are extracted from LeVine DN, Brooks MB. Immune thrombocytopenia in Schalm’s Veterinary Hematology, 7th edition. Eds. Weiss DJ, Wardrop J, Harr K, Seelig D, Brooks MB. 2022.
a. The magnitude of thrombocytopenia is consistent with consumption from major hemorrhage. However, it is possible that DIC, vasculitis, sequestration, or ITP may be contributing.
b. Consider genetic testing
c. Sampling of bone marrow by aspiration, core biopsy, or both, is undertaken
d. For example, lymphoreticular neoplasia or ehrlichiosis
e. At least two of five parameters abnormal in addition to thrombocytopenia: PT, aPTT, D-dimer > reference interval (RI); AT, fibrinogen < RI
f. PT or PTT > 25% control value
g. Partial screening (i.e. not exhaustive) for potential trigger factors undertaken and negative
Figure 1. Diagnostic algorithm for canine ITP developed by the ACVIM Consensus Panel for Diagnosis and Treatment of ITP.
72
73
References
1. Consolini R, Legitimo A, Caparello MC. The centenary of immune thrombocytopenia - Part 1: Revising nomenclature and pathogenesis. Front Pediatr 2016;4:102.
2. Harrington WJ, Minnich V, Hollingsworth JW, et al. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J Lab Clin Med 1951;38:1-10.
3. Li J, Sullivan JA, Ni H. Pathophysiology of immune thrombocytopenia. Curr Opin Hematol 2018;25:373-381.
4. Panitsas FP, Theodoropoulou M, Kouraklis A, et al. Adult chronic idiopathic thrombocytopenic purpura (ITP) is the manifestation of a type-1 polarized immune response. Blood 2004;103:2645-2647.
5. Semple JW, Milev Y, Cosgrave D, et al. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood 1996;87:4245-4254.
6. Zhang F, Chu X, Wang L, et al. Cell-mediated lysis of autologous platelets in chronic idiopathic thrombocytopenic purpura. Eur J Haematol 2006;76:427-431.
7. Ji L, Zhan Y, Hua F, et al. The ratio of Treg/Th17 cells correlates with the disease activity of primary immune thrombocytopenia. PloS one 2012;7:e50909.
8. Li X, Zhong H, Bao W, et al. Defective regulatory B-cell compartment in patients with immune thrombocytopenia. Blood 2012;120:3318-3325.
9. Stasi R, Cooper N, Del Poeta G, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood 2008;112:11471150.
10. Bao W, Bussel JB, Heck S, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood 2010;116:4639-4645.
11. Volkmann M, Hepworth MR, Ebner F, et al. Frequencies of regulatory T cells in the peripheral blood of dogs with primary immune-mediated thrombocytopenia and chronic enteropathy: a pilot study. Vet J 2014;202:630-633.
12. Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med 2017;6.
13. Olsson B, Andersson PO, Jernas M, et al. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med 2003;9:1123-1124.
14. Iraqi M, Perdomo J, Yan F, et al. Immune thrombocytopenia: antiplatelet autoantibodies inhibit proplatelet formation by megakaryocytes and impair platelet production in vitro. Haematologica 2015;100:623-632.
15. Joshi BC, Jain NC. Detection of antiplatelet antibody in serum and on megakaryocytes of dogs with autoimmune thrombocytopenia. Am J Vet Res 1976;37:681-685.
16. Aledort LM, Hayward CP, Chen MG, et al. Prospective screening of 205 patients with ITP, including diagnosis, serological markers, and the relationship between platelet counts, endogenous thrombopoietin, and circulating antithrombopoietin antibodies. Am J Hematol 2004;76:205-213.
17. Chang M, Qian JX, Lee SM, et al. Tissue uptake of circulating thrombopoietin is increased in immune-mediated compared with irradiated thrombocytopenic mice. Blood 1999;93:2515-2524.
18. Grozovsky R, Begonja AJ, Liu K, et al. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat Med 2015;21:47-54.
19. O'Marra SK, Delaforcade AM, Shaw SP. Treatment and predictors of outcome in dogs with immunemediated thrombocytopenia. Journal of the American Veterinary Medical Association 2011;238:346352.
20. Williams DA, Maggio-Price L. Canine idiopathic thrombocytopenia: clinical observations and longterm follow-up in 54 cases. J Am Vet Med Assoc 1984;185:660-663.
74
21. Lewis DC, Meyers KM. Canine idiopathic thrombocytopenic purpura. J Vet Intern Med 1996;10:207218.
22. Putsche JC, Kohn B. Primary immune-mediated thrombocytopenia in 30 dogs (1997-2003). J Am Anim Hosp Assoc 2008;44:250-257.
23. Botsch V, Kuchenhoff H, Hartmann K, et al. Retrospective study of 871 dogs with thrombocytopenia. Vet Rec 2009;164:647-651.
24. Lewis DC, Meyers KM, Callan MB, et al. Detection of platelet-bound and serum platelet-bindable antibodies for diagnosis of idiopathic thrombocytopenic purpura in dogs. J Am Vet Med Assoc 1995;206:47-52.
25. Jordan HL, Grindem CB, Breitschwerdt EB. Thrombocytopenia in cats: a retrospective study of 41 cases. J Vet Intern Med 1993;7:261-265.
26. Kohn B, Linden T, Leibold W. Platelet-bound antibodies detected by a flow cytometric assay in cats with thrombocytopenia. J Feline Med Surg 2006;8:254-260.
27. Bianco D, Armstrong PJ, Washabau RJ. Presumed primary immune-mediated thrombocytopenia in four cats. J Feline Med Surg 2008;10:495-500.
28. Wondratschek C, Weingart C, Kohn B. Primary immune-mediated thrombocytopenia in cats. J Am Anim Hosp Assoc 2010;46:12-19.
29. Garon CL, Scott MA, Selting KA, et al. Idiopathic thrombocytopenic purpura in a cat. J Am Anim Hosp Assoc 1999;35:464-470.
30. Tasker S, Mackin AJ, Day MJ. Primary immune-mediated thrombocytopenia in a cat. J Small Anim Pract 1999;40:127-131.
31. Makielski KM, Brooks MB, Wang C, et al. Development and implementation of a novel immune thrombocytopenia bleeding score for dogs. J Vet Intern Med 2018;32:1041-1050.
32. Panzer S, Rieger M, Vormittag R, et al. Platelet function to estimate the bleeding risk in autoimmune thrombocytopenia. Eur J Clin Invest 2007;37:814-819.
33. Olsson A, Andersson PO, Tengborn L, et al. Serum from patients with chronic idiopathic thrombocytopenic purpura frequently affect the platelet function. Thromb Res 2002;107:135-139.
34. De Cuyper IM, Meinders M, van de Vijver E, et al. A novel flow cytometry-based platelet aggregation assay. Blood 2013;121:e70-80.
35. Goerge T, Ho-Tin-Noe B, Carbo C, et al. Inflammation induces hemorrhage in thrombocytopenia. Blood 2008;111:4958-4964.
36. LeVine DN, Cianciolo RE, Linder KE, et al. Endothelial alterations in a canine model of immune thrombocytopenia. Platelets 2019;30:88-97.
37. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011;117:4190-4207.
38. Zelmanovic D, Hetherington EJ. Automated analysis of feline platelets in whole blood, including platelet count, mean platelet volume, and activation state. Vet Clin Pathol 1998;27:2-9.
39. Russell K. Platelet kinetics and laboratory evaluation of thrombocytopenia. In: Weiss DJ, Wardrop KJ, eds. Schalm's Veterinary Hematology, 6th edWiley-Blackwell; 2010:276-285.
40. Gelain ME, Bertazzolo W, Tutino G, et al. A novel point mutation in the beta1-tubulin gene in asymptomatic macrothrombocytopenic Norfolk and Cairn Terriers. Vet Clin Pathol 2014;43:317-321.
41. Defarges A, Abrams-Ogg A, Foster RA, et al. Comparison of sternal, iliac, and humeral bone marrow aspiration in Beagle dogs. Vet Clin Pathol 2013;42:170-176.
42. Shropshire S, Dow S, Lappin M. Detection and dynamics of anti-platelet antibodies in thrombocytopenic dogs with and without idiopathic immune thrombocytopenia. J Vet Intern Med 2020;34:700-709.
43. Li J, van der Wal DE, Zhu G, et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat Commun 2015;6:7737.
75
44. Harrus S, Waner T, Weiss DJ, et al. Kinetics of serum antiplatelet antibodies in experimental acute canine ehrlichiosis. Vet Immunol Immunopathol 1996;51:13-20.
45. Grindem CB, Breitschwerdt EB, Perkins PC, et al. Platelet-associated immunoglobulin (antiplatelet antibody) in canine Rocky Mountain spotted fever and ehrlichiosis. J Am Anim Hosp Assoc 1999;35:5661.
46. Cortese L, Sica M, Piantedosi D, et al. Secondary immune-mediated thrombocytopenia in dogs naturally infected by Leishmania infantum. Vet Rec 2009;164:778-782.
47. Wilkerson MJ, Shuman W, Swist S, et al. Platelet size, platelet surface-associated IgG, and reticulated platelets in dogs with immune-mediated thrombocytopenia. Vet Clin Pathol 2001;30:141149.
48. Bloom JC, Blackmer SA, Bugelski PJ, et al. Gold-induced immune thrombocytopenia in the dog. Vet Pathol 1985;22:492-499.
49. Bloom JC, Thiem PA, Sellers TS, et al. Cephalosporin-induced immune cytopenia in the dog: demonstration of erythrocyte-, neutrophil-, and platelet-associated IgG following treatment with cefazedone. Am J Hematol 1988;28:71-78.
50. Gaunt S, Beall M, Stillman B, et al. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasit Vectors 2010;3:33.
51. Chirek A, Silaghi C, Pfister K, et al. Granulocytic anaplasmosis in 63 dogs: clinical signs, laboratory results, therapy and course of disease. J Small Anim Pract 2018;59:112-120.
52. Helfand SC, Couto CG, Madewell BR. Immune-mediated thrombocytopenia associated with solid tumors in dogs. Journal of the American Animal Association 1985;21:787-794.
53. Sullivan PS, Arrington K, West R, et al. Thrombocytopenia associated with administration of trimethoprim/sulfadiazine in a dog. J Am Vet Med Assoc 1992;201:1741-1744.
54. Maggi RG, Birkenheuer AJ, Hegarty BC, et al. Comparison of serological and molecular panels for diagnosis of vector-borne diseases in dogs. Parasit Vectors 2014;7:127.
55. Portielje JE, Westendorp RG, Kluin-Nelemans HC, et al. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood 2001;97:2549-2554.
56. McAtee BB, Cummings KJ, Cook AK, et al. Opportunistic Invasive Cutaneous Fungal Infections Associated with Administration of Cyclosporine to Dogs with Immune-mediated Disease. J Vet Intern Med 2017;31:1724-1729.
57. Foster AP, Shaw SE, Duley JA, et al. Demonstration of thiopurine methyltransferase activity in the erythrocytes of cats. J Vet Intern Med 2000;14:552-554.
58. Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv 2019;3:3829-3866.
59. Balog K, Huang AA, Sum SO, et al. A prospective randomized clinical trial of vincristine versus human intravenous immunoglobulin for acute adjunctive management of presumptive primary immunemediated thrombocytopenia in dogs. J Vet Intern Med 2013;27:536-541.
60. Rozanski EA, Callan MB, Hughes D, et al. Comparison of platelet count recovery with use of vincristine and prednisone or prednisone alone for treatment for severe immune-mediated thrombocytopenia in dogs. J Am Vet Med Assoc 2002;220:477-481.
61. Allen EC, Tarigo JL, LeVine DN, et al. Platelet number and function in response to a single intravenous dose of vincristine. J Vet Intern Med 2021;35:1754-1762.
62. Bianco D, Armstrong PJ, Washabau RJ. A prospective, randomized, double-blinded, placebocontrolled study of human intravenous immunoglobulin for the acute management of presumptive primary immune-mediated thrombocytopenia in dogs. J Vet Intern Med 2009;23:1071-1078.
63. Zini E, Hauser B, Meli ML, et al. Immune-mediated erythroid and megakaryocytic aplasia in a cat. J Am Vet Med Assoc 2007;230:1024-1027.
76
64. Kohn B, Bal G, Chirek A, et al. Treatment of 5 dogs with immune-mediated thrombocytopenia using Romiplostim. BMC Vet Res 2016;12:96.
65. Cooper N. State of the art - how I manage immune thrombocytopenia. Br J Haematol 2017;177:3954.
66. Gettinger MR, Glanemann B, Viall A, et al. Retrospective evaluation of splenectomy in the treatment of canine primary immune thrombocytopenia (Abstract HM01). In: American College of Veterinary Internal Medicine Forum. Phoenix, AZ: 2019.
67. Bestwick JP, Skelly BJ, Swann JW, et al. Splenectomy in the management of primary immunemediated hemolytic anemia and primary immune-mediated thrombocytopenia in dogs. J Vet Intern Med 2022;36:1267-1280.
68. Ano H, Fujino M, Katamoto H. Case of Feline Idiopathic Immune-mediated Thrombocytopenia Effectively Treated with Cyclophosphamide after Splenectomy. . Journal of the Japan Veterinary Medical Association 2014;67:269-273.
69. Impellizeri JA, Howell K, McKeever KP, et al. The role of rituximab in the treatment of canine lymphoma: an ex vivo evaluation. Vet J 2006;171:556-558.
70. Goel R, Chopra S, Tobian AAR, et al. Platelet transfusion practices in immune thrombocytopenia related hospitalizations. Transfusion 2019;59:169-176.
71. Goggs R, Brainard BM, LeVine DN, et al. Lyophilized platelets versus cryopreserved platelets for management of bleeding in thrombocytopenic dogs: A multicenter randomized clinical trial. J Vet Intern Med 2020;34:2384-2397.
72. Davidow EB, Brainard B, Martin LG, et al. Use of fresh platelet concentrate or lyophilized platelets in thrombocytopenic dogs with clinical signs of hemorrhage: a preliminary trial in 37 dogs. J Vet Emerg Crit Care (San Antonio) 2012;22:116-125.
77
Hemostatic Hurdles
Part 1 & 2
Dana LeVine, DVM, Ph.D., DACVIM (SAIM)
Austin Viall, DVM, MS, DACVP

79
Hemostatic Hurdles (Part 1 & 2)
Presenters:
Dana LeVine, DVM, Ph.D., DACVIM (SAIM)
Associate Professor of Small Animal Internal Medicine, Auburn University College of Veterinary Medicine, Auburn, AL
Austin Viall, DVM, MS, DACVP
Associate Professor of Clinical Pathology, University of California Davis, Davis, CA
We thank Dr. Marjory Brooks (Director, Comparative Coagulation Laboratory, Animal Health Diagnostic Center, College of Veterinary Medicine, Cornell University) for graciously providing some of the case/proceedings content.
We will be walking through interactive cases of hemostatic disorders with an intertwining, integrated review of diagnostic testing and treatment relating to bleeding disorders. We have chosen not to provide the cases in note format, as that would reduce the challenge and fun!
We are instead providing relevant background information that will help you in tackling our cases together.
Introduction: Clinical Diagnosis of Bleeding Disorders
Initial patient evaluation is aimed at differentiating hemorrhage from damaged or diseased blood vessels from a failure of normal hemostasis (i.e. bleeding diathesis). Bleeding diatheses are broadly classified as defects of platelet plug formation (primary hemostatic defects), defects of fibrin clot formation (secondary hemostatic defects), or defects of fibrinolysis (tertiary hemostasis).
Tests of Vascular Integrity
Inspection is the primary means for identifying blood loss due vascular defects. Physical examination may be sufficient for definitive diagnosis of acute vascular injury. In other cases, ancillary diagnostics and screening tests are first performed to rule out a hemostatic defect. Vascular defects can be classified as large or small vessel disease.
1. Large vessel (arterial/venous) defects - common clinical signs include blood-loss anemia and localized site of hemorrhage. Tests include physical examination, endoscopy, radiography (contrast studies), ultrasonography, CT scan, and exploratory surgery.
2. Small vessel (vasculopathic) disorders - vasculopathies (inflammatory, toxic, degenerative).
Physical exam may reveal cutaneous erythema, macules, and bruising, and clinical signs of multi-organ involvement. Tests include serology (antibodies against pathogens and nuclear proteins), pathogen detection, endocrine profiles (hypercortisolism, hypothyroidism), and biopsy.
81
Tests of Platelet Plug Formation
Platelet plug formation results from a series of interactions among vessel wall, platelets, and von Willebrand factor. Clinical signs suggestive of primary hemostatic disorders include petechiae, mucosal hemorrhage, and prolonged bleeding from sites of injury. Buccal mucosal bleeding times will be prolonged; laboratory testing differentiates quantitative and qualitative platelet disorders and VWF deficiency/dysfunction.
1. Thrombocytopenia (low platelet number): tests include platelet count or platelet estimate from blood smear. Differentials for thrombocytopenia include production defects, peripheral loss, inappropriate consumption, splenic sequestration, and immune-mediated destruction. Thrombocytopenia is the most common hemostatic defect in most species.
Tests: serology, antigen and PCR tests for infectious agents (viral, arthropod-borne, & bacterial pathogens), coagulation panel, ± bone marrow aspiration cytology, lymph node, splenic aspirate (selected cases), thorough drug history (sulfa drugs, methimazole, chemotherapy), detection of platelet-associated antibodies, genetic testing for macrothrombocytopenia (selected cases).
2. Thrombopathia (platelet dysfunction): tests include in vivo bleeding time, drug history for medications that might inhibit platelets, chemistry panel to look for metabolic causes, flow cytometry, specific platelet function tests (ex. PFA-100 closure time, whole blood and light transmission aggregometry), mutation detection for specific breed-variants of receptor, signaling, and procoagulant defects.
3. von Willebrand Disease: tests include in vivo bleeding time, VWF concentration (VWF:Ag), VWF function (collagen-binding assays), VWF structure (Western blot), mutation detection for specific breed-variants of type 1, 2, and 3 VWD.
Tests of Fibrin Clot Formation
Generation of a stable fibrin clot is the endpoint of secondary hemostasis. Functional tests include determination of in vitro clotting time, activities of specific procoagulant and anticoagulant factors, and tests of clot strength
1. Coagulation screening tests: aPTT, PT, TCT, fibrinogen
• aPTT (activated partial thromboplastin time): intrinsic and common system screening test
• ACT (activated clotting time): intrinsic and common system screening test
• PT (prothrombin time): extrinsic and common system screening test
• TCT (thrombin clotting time): test of fibrinogen function and concentration
• Fibrinogen: functional or quantitative assay of plasma fibrinogen level
82
2. Factor analyses: specific tests of individual clotting factor activities
3. Inhibitor assays: specific tests of antithrombin, protein C, anticoagulant drugs (i e. unfractionated and low molecular weight heparin and DOAC anti-Xa activity, INR for coumadin monitoring) and pathologic anticoagulants (anti-factor antibody, lupus anticoagulant)
4. Viscoelastic assays (Thromboelastography, thromboelastometry, viscoelastic monitor)qualitative measure of fibrin clot formation, strength, and stability
Tests of Fibrinolysis
Hemorrhage may result from non-localized or too rapid fibrinolysis, whereas delayed or ineffective fibrinolysis may promote thrombosis and fibrotic wound healing. Fibrinolysis tests include quantitative or functional assays of fibrin clot stability, fibrinolytic enzymes, activators, inhibitors and end-products.
1. Enzymes and activators: t-PA (tissue plasminogen activator), plasminogen, plasmin generation
2. End products: D-dimer (terminal, cross-linked fibrin degradation products), FDP (fibrin and fibrinogen degradation products)
3. Inhibitors: antiplasmin, PAI (plasminogen activator inhibitor)
4. Viscoelastic assays (Thromboelastography, thromboelastometry)- qualitative measure of fibrin clot formation, strength, and stability, viscoelastic assays with t-PA for detecting excessive fibrinolysis.
Management of Bleeding Disorders
Thrombocytopenia/Thrombopathia
Thrombopathic animals should be platelet transfused when they are actively bleeding or in preparation for an invasive procedure. It remains somewhat controversial, even in human medicine, when transfusion of a thrombocytopenic patient should be performed. The authors suggest reserving platelet transfusions for thrombocytopenic patients with signs of active or refractory bleeding, especially central nervous system, pulmonary, or gastrointestinal bleeding. Predictors of disease severity are needed to identify those patients with platelet counts less than 30,000 platelets/µl that are most likely to benefit from platelet transfusions. Patients with platelets counts under 50,000/µl that require invasive procedures often benefit from platelet transfusions. Blood product choices and their pros and cons are detailed below.
83
Table 1. Platelet Transfusion Products Product Platelet content Pros Cons
Fresh whole blood (FWB) • 11 x 1010 platelets/450 ml)
• No platelet loss during processing
• Contains pRBC which may be ideal if anemic
• Contains clotting factors if needed
• Extra volume and pRBCs if not needed
• Additional antigens
• Need to use blood rapidly (within 8 hours)
• Time required to collect/call in donor
Packed red blood cells (pRBCs)
• Minimal platelets (~8x107/unit)
• RBC ADP release and NO scavenging may activate recipient’s remaining platelets
• Does not contain a significant amount of platelets indicated for anemia, but may help push platelets to vascular periphery where they can interact with endothelium
Platelet rich plasma (PRP)
• 8-10 x 1010 platelets/ unit
• 1 unit/10 kg will raise platelet count maximally by 40,000/µl
• Small volume of administration
• Near normal platelet function
• Requires specific equipment and staff training to prepare
• 5-day shelf life
• Must be gently agitated during storage
• Bacterial contamination with RT storage
• Reduced platelet recovery compared to FWB (80-90% platelets of FWB)
Fresh platelet concentrate (PC)
• Centrifugation preparation: 5-8 x 1010 platelets/PC unit
• Apheresis preparation: 1.0 x 1011 platelets/100 ml
• Standard of care in human medicine (apheresis)
• Lack of availability
• Centrifugation method results in 25% loss of platelets during processing while apheresis method requires specialized expensive equipment
• 7-day shelf life*
*there may be potential for prolonged cold storage of PC*
Cryopreserved platelet concentrate (CPP)
• Minimum 5 x 1010 platelets/100 ml
• Available immediately
• Long storage time (2 years)
• Pooled platelet product
• Reduced platelet count recovery compared with fresh platelets
• Decreased platelet function
• Short platelet lifespan post transfusion
Lyophilized platelets (StablePlateRX™)
• 1.5 x 109/ml
• Long storage time (up to 2 years)
• Sterile
• Pooled platelet product
• Product consistency in platelet functionality
• Short life span after transfusion
• Expensive
• Currently unavailable
84
von Willebrand Disease
Dogs with von Willebrand Disease can be managed both prophylactically and during active bleeds with a combination of DDAVP (type I VWD only) and plasma products.
1. The ideal plasma product for VWD patients, as described below, is cryoprecipitate, though fresh frozen plasma will also be effective.
2. DDAVP is a synthetic analogue of vasopressin that works by binding to endothelial vasopressin receptors and inducing release of VWF and FVIII, leading to increased circulating plasma VWF and FVIII.1 Although the increase in plasma VWF in dogs with type I VWD is lower (50% increase) following DDAVP administration than in humans with type I VWD (2-5X increase), 1 µg/kg DDAVP given SQ still improved buccal mucosal bleeding times and PFA-100 closure times in Dobermans with type I VWD 1 Furthermore, the VWD type I dogs receiving DDAVP that had subsequent surgery or diagnostic procedures did not have excessive bleeding or require any blood products.1 Two dogs that were already bleeding from another disease (tumor and inflammatory bowel disease) required plasma transfusions and still had ongoing bleeding.1
Secondary Hemostatic Disorders
Acquired and inherited bleeding disorders often respond to transfusion. While red cell transfusion is indicated if clinical signs of anemia (hypoxia) are present, plasma products can be given prophylactically and repeatedly with less risk of volume overload or red cell sensitization. Common indications for plasma components include acute rodenticide toxicity, liver biopsy/shunt correction, hemophilia, and other hereditary factor deficiencies. Patients with hereditary factor deficiencies require factor replacement when they are actively bleeding or if they require an invasive procedure.
Products:
1. Fresh frozen plasma (FFP)- plasma separated from whole blood and frozen within 4 to 6 hours of collection maintains activity of coagulation factors, fibrinogen, VWF, and contains albumin and globulins. Storage at or below -20°C maintains activity of hemostatic factors for up to 1 year. Plasma stored frozen for more than one year (FP) and less than 4 years is still useful for supplying albumin and globulins and heat stable clotting factors (II, VII, IX, and IX).
2. Cryoprecipitate- prepared by slowly thawing FFP and collecting the precipitant fraction. Cryoprecipitate contains factor VIII, von Willebrand factor, and fibrinogen, in approximately 1/10 volume of the starting plasma. Cryoprecipitate is the best product for treating hemophilia A, VWD, and fibrinogen deficiencies because it is effective, lower volume, and is associated with less adverse reactions.2
85
3. Cryosupernatant (cryo-poor plasma)- plasma remaining from cryoprecipitate preparation contains active clotting factors (except FVIII, fibrinogen) and albumin and globulin. Cryosupernatant is used to treat hemophilia B, hereditary factor deficiencies, vitamin K deficiency, and for replacement of albumin and globulin.
Excessive Fibrinolysis
Currently available antifibrinolytic drugs are lysine analogs such as ε-aminocaproic acid (EACA) and tranexamic acid (TXA). These compounds are widely used in human and veterinary medicine to decrease fibrinolysis in diverse applications. In general, they work by reversibly binding to the lysine binding sites of plasminogen, preventing its association with the lysine residues on fibrin, and preventing activation of plasminogen by tPA. The most established use of these agents in veterinary medicine is in greyhound dogs. Investigating delayed postoperative bleeding in greyhounds, a group at Ohio State University hypothesized that these dogs had a hyper fibrinolytic condition and successfully minimized delayed postoperative bleeding in a population of greyhounds following limb amputation for osteosarcoma using aminocaproic acid.3,4 Other hyper fibrinolytic states have been identified in dogs, including hemoperitoneum caused by a ruptured abdominal tumor, and in dogs following trauma.5,6 Anti-fibrinolytic agents may be indicated for use in both of these populations. In addition, canine patients at risk for hemorrhage following routine or emergent surgery may benefit from the use of antifibrinolytic agents. TXA was administered to dogs with Scott syndrome after ovariohysterectomy and castration and EACA was given to a Scott syndrome dog with a pelvic limb hematoma and use has been reported in dogs with severe ITP.7,8 Treatment trials will be need to determine the efficacy of antifibrinolytics to decrease transfusion requirements and prevent rebleeding in such dogs with thrombopathias and perhaps thrombocytopenic patients. Although there is a wide variety of published doses for these drugs, in dogs the authors use 10 mg/kg TXA IV every 3-4 hours and 100 mg/kg EACA IV or PO every 6-8 hours; caution should be taken with TXA in cats as it may induce seizures 9,10 Antifibrinolytics should be avoided in any hypercoagulable condition like DIC.
86
References
1. Callan MB, Giger U. Effect of desmopressin acetate administration on primary hemostasis in Doberman Pinschers with type-1 von Willebrand disease as assessed by a point-of-care instrument. Am J Vet Res 2002;63:1700-1706.
2. Stokol T, Parry B. Efficacy of fresh-frozen plasma and cryoprecipitate in dogs with von Willebrand's disease or hemophilia A. J Vet Intern Med 1998;12:84-92.
3. Lara-Garcia A, Couto CG, Iazbik MC, et al. Postoperative bleeding in retired racing greyhounds. J Vet Intern Med 2008;22:525-533.
4. Marin LM, Iazbik MC, Zaldivar-Lopez S, et al. Retrospective evaluation of the effectiveness of epsilon aminocaproic acid for the prevention of postamputation bleeding in retired racing Greyhounds with appendicular bone tumors: 46 cases (2003-2008). J Vet Emerg Crit Care (San Antonio) 2012;22:332-340.
5. Fletcher DJ, Rozanski EA, Brainard BM, et al. Assessment of the relationships among coagulopathy, hyperfibrinolysis, plasma lactate, and protein C in dogs with spontaneous hemoperitoneum. J Vet Emerg Crit Care (San Antonio) 2016;26:41-51.
6. Hall KE, Holowaychuk MK, Sharp CR, et al. Multicenter prospective evaluation of dogs with trauma. J Am Vet Med Assoc 2014;244:300-308.
7. Jandrey KE, Norris JW, Tucker M, et al. Clinical characterization of canine platelet procoagulant deficiency (Scott syndrome). J Vet Intern Med 2012;26:1402-1407.
8. Kelmer E. MK, Bruchim Y., Klainbart S., Aroch I., Segev G. . Retrospective evaluation of the safety and efficacy of tranexamic acid (Hexakapron) for the treatment of bleeding disorders in dogs. . Israel Journal of Veterinary Medicine 2013;68:94-100.
9. Pellegrini A, Giaretta D, Chemello R, et al. Feline generalized epilepsy induced by tranexamic acid (AMCA). Epilepsia 1982;23:35-45.
10. Letendre J-A, Goggs R. Fibrinolysis and Antifibrinolytics. In: Textbook of Small Animal Emergency Medicine2018:430-439.
87
Events
Live Online Seminar
Sexual Harassment Prevention
Education and Training

March 22, 2023 |
March 26, 2023 | 12:00 PM-2:15 PM
Live Online Seminar
California Animal Blood
Banking Requirements
April
Pacific
October 6-8, 2023
Up to 2 CEUs 1 CEU Up to 28.5
12 CEUs for DVMs

8 CEUs for RVTs
Three half-day CE sessions with time to enjoy the Lake Tahoe area ! Registration opens in May!
Join us for sun, sand, and CE in Long Beach, CA. PacVet offers over 200+ sessions in 15 tracks including small animal medicine, avian/exotics, surgery, equine, technicianspecific CE, and more. Small animal medicine and technician tracks offer both in-person and live, online!
Veterinary Conference Long Beach, CA
5:00 PM-7:15 PM
Visit CVMA.net for more information and registration.
4, 2023
12:30 PM-1:30 PM
June 9-12, 2023 28, 2023 | 5:30 PM-6:30 PM
|
April
CEUs
Fall Seminar
Lake Tahoe, CA


Sponsored by 1400 River Park Drive, Suite 100 Sacramento, California, 95815 800.655.2862 | cvma.net




 Jane Sykes, BVSc (Hons), Ph.D., MBA, DACVIM (SAIM)
Jane Sykes, BVSc (Hons), Ph.D., MBA, DACVIM (SAIM)








