


And more: Pests & Diseases Factographic Grower’s Tip Puzzle & Win Facts Questions & Answers Powered by WATER Quality Considerations for your grow Grow it yourself THE VERSATILY SPICE GINGER IND UCTION COOKING Is Heating Up Grower’s Circle MAGAZINE FOR SERIOUS GROWERS ISSUE 40 | 2023































2| Contents CANNA COCO Perfected through experience cannagardening.com
Water, water everywhere, nor any drop to drink – Samuel Taylor Coleridge (1834). Albeit a line from an old poem, this remains true today. Fresh potable water is getting scarcer every year. Because humanity is a poor steward of our natural resources, we continue to contaminate our freshwater resources. Our rivers, lakes, dams, and underground water continue to be contaminated. Trusting our authorities to provide potable water or relying on “fresh ground” water can be a recipe for disaster, especially if your lively hood and health depend on it.
When I was younger, I worked for a company that specialized in consulting and selling equipment to greenhouse producers in Africa. I once visited a newly established greenhouse farm that cost millions to set up. Having purchased a new plot of land and built new greenhouses and infrastructure for his new venture, the farmer failed to test the property’s water. He was well into his first crop of tomatoes and cucumbers and struggled with keeping them watered and fed. After finally getting his groundwater tested, he discovered the iron levels were extremely high. The iron in his water would oxidize and clog his filters and drippers. Ultimately, the cost was significant to clean up his water source before being able to irrigate his crops. A cheap water test would have saved this farmer hundreds of thousands of dollars in extra equipment and filtration systems. He said he would have never purchased the property if he knew the groundwater was not ideal.
While this story eventually ended with the farmer turning his situation around, it could have been worse. This issue of CANNATalk touches on the quality of water. On page for CANNA Research discusses pairing the ideal water treatment with your system, and in the Grower Circle, we discuss “In water we trust.”
David Hill
CANNA
Pairing the ideal water treatment with your system Grow it Yourself

Questions & Answers
Your questions answered!
Grower’s circle
In water we trust Factographic
Blue Fire - Story behind the glow
What’s Happening?
Pests & Diseases Viral
vectors and prevention
4 9 12 14 17 18 20
What’s your watertype?
Grower’s tip
The Free Radical Harnessing the power of oxygen Puzzle

Win a 1 liter bottle of CALMAG Facts

Myths about wheat, the importance of Nitrogen


22 27 28 29 30 Research

CANNAtalk|3
Contents
Foreword
CANNA Research
What’s next
The versatily of ginger
Induction Cooking is Heating
Up
TO BE A WELL-PREPARED GROWER, IT’S ESSENTIAL TO HAVE A BASIC UNDERSTANDING OF WATER QUALITY CONCEPTS (EC, HARDNESS, PH) AND TO TEST YOUR WATER SOURCE, BEFORE YOU GET STARTED. ONCE YOU KNOW YOUR WATER QUALITY, YOU NEED TO CONSIDER THE NEEDS OF THE PARTICULAR SYSTEM IN WHICH YOU ARE GROWING AND DETERMINE IF YOU NEED TO SUPPLEMENT OR TREAT YOUR WATER. COCO, SOIL, AND HYDRO SYSTEMS ARE EACH BASED WITH THEIR OWN, UNIQUE
SUBSTRATE AND FERTILIZER DEMANDS. LET’S WALK THROUGH THE WATER, FERTILIZER, AND SUBSTRATE CONSIDERATIONS FOR EACH OF THESE SYSTEMS ONE BY ONE AND LEARN HOW THEY AFFECT YOUR WATER MANAGEMENT.
 By Abha Gupta, MS Horticulture, Assistant Horticulturist
By Abha Gupta, MS Horticulture, Assistant Horticulturist
PAIRING THE IDEAL WATER TR

Coco to the coir
WATER MANAGEMENT BY SYSTEM
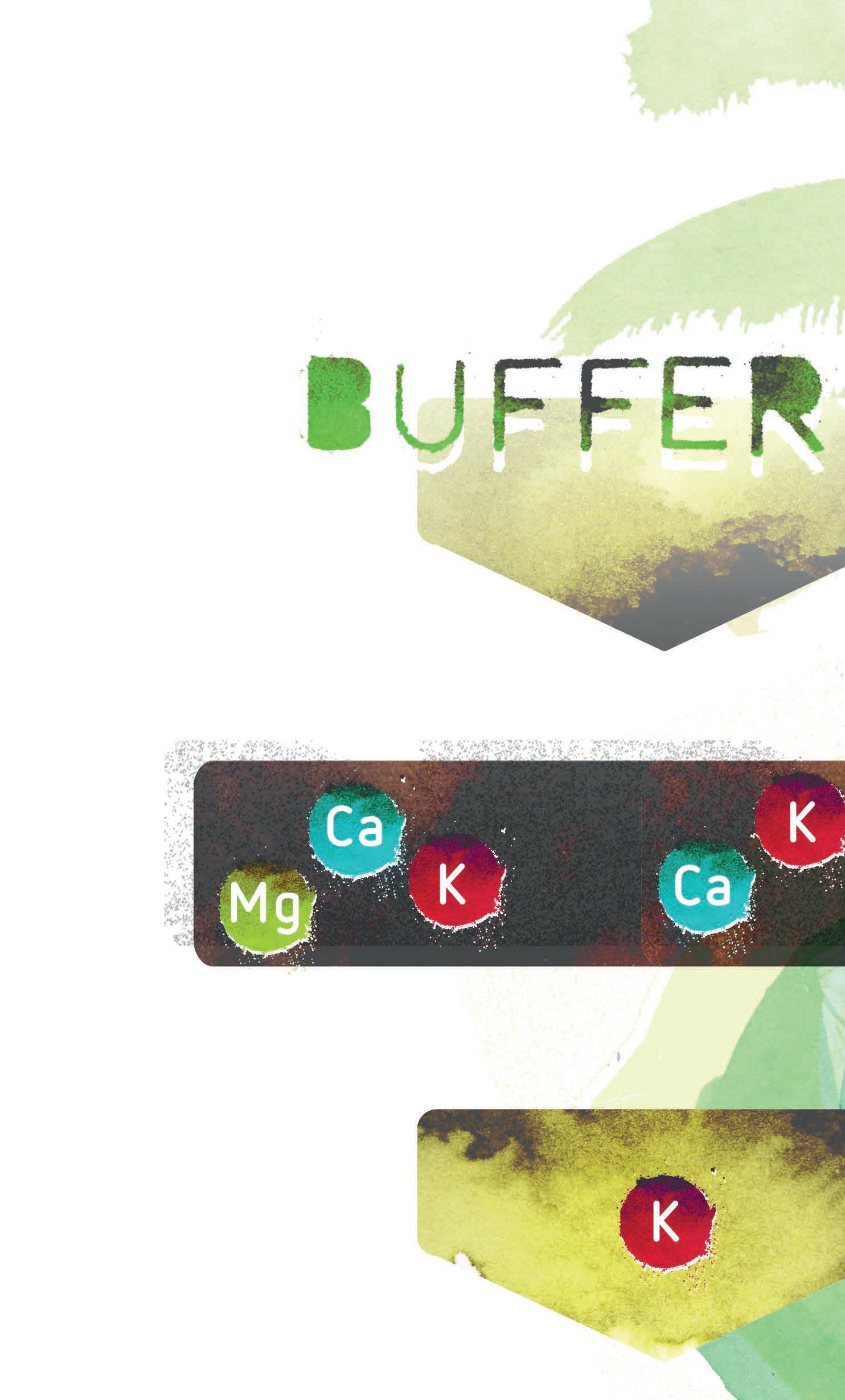
Coco substrate has limited cation exchange sites, which are essentially docking stations for cations, or positively charged ions, like calcium (Ca+2), magnesium (Mg+2), and potassium (K+). In addition, coco comes with a high concentration of potassium, since it is part of the material itself. These nutrients will rest in these exchange sites and then are used by the plant when needed. It’s important to balance out the ratio of calcium, magnesium, and potassium found on the coco so that

CANNARESEARCH 4|CANNAtalk
Figure 1: Properly buffered coco will be appropriately balanced with calcium, magnesium, and potassium. Coco that has not been buffered or has had damage to its buffer will have a greater load of potassium on the substrate. This causes the coco substrate to draw calcium and magnesium out from the nutrient solution and replace it with potassium, as it attempts to even out the concentration of potassium on the substrate as compared to that which is in the nutrient solution.

EATMENT WITH YOUR SYSTEM
the plant receives the correct balance for successful growth and to prevent deficiencies. In order to ensure that the substrate has come equipped with the proper ratio between these competitive cations, a good coco substrate will arrive already buffered with a nutrient solution or with directions on how to buffer it yourself. After this point, the nutrient buffer on the coco needs to be maintained by always applying a nutrient solution, designed specifically for coco, and by avoiding applying
plain water or a poor nutrient solution. It is also important to water with sufficient drainage, every time.
When plain water is poured over the coco, the coco nutrient buffer becomes damaged as some of the calcium and magnesium on the substrate is displaced from the coco medium and is replaced with potassium. This movement happens due to the nutrient elements on the coco substrate striving for a concentration balance with

CANNAtalk|5
the nutrient elements in the water. As a result, the plant does not receive the correct balance of nutrients that it needs and the substrate is also left imbalanced. During subsequent feedings with a proper nutrient solution, the coco substrate will attract some of the nutrients in order to restore its nutrient balance and, as a result, continue to leave a nutrient shortage for the plant. To fix the issue, a balanced, complete nutrient solution would need to be thoroughly flushed through the coco and some time would be needed for the coco buffer to repair.

In a completely coco system (coco fiber substrate, along with coco-specific nutrients), moderately hard to hard water is generally best. This is 60-180 ppm calcium carbonate equivalence. (Note this is not total dissolved solids. This is a measure of dissolved calcium and magnesium. See the article, ‘What’s your water type?’ in this issue to learn more.) Some water hardness is helpful for ensuring that the entire system has sufficient calcium and magnesium. In comparison, soft water by definition has a low supply of calcium and magnesium. If you are working with soft or reverse osmosis (RO) water (which, if filtered correctly, is rendered to become soft water) on coco, you may consider adding a calmag supplement to increase water hardness. Wellmade coco fertilizers should come with a good load of calcium already, in consideration of the coco fiber and plant needs. If working with RO or soft water, another treatment option is to use a higher rate of quality cocodesigned fertilizers.
If your original water source has an EC greater than 0.6 mS/cm or is very hard (greater than 180 ppm calcium carbonate equivalence), it is safest to treat your water through RO and add calmag. Alternatively, you can mix your original water back in with the RO treated water till you reach an EC of 0.2-0.4 mS/cm, so long as your source water does not contain an excess of toxins. It is not advised to use high EC source water without treatment, which may cause challenges in maintaining a proper watering technique. Get your water tested and review your water’s hardness, total dissolved solids, and elemental concentrations. Many university labs provide guidelines for interpreting irrigation water test results and levels of concern. Plants need their 13 macro and micronutrients delivered in the correct ratios and an

6|CANNAtalk CANNARESEARCH
Figure 2: Well-made potting mixes have plenty of cation exchange sites that attract nutrients as a type of nutrient bank and releases the nutrients when needed by the plant.
excess of one nutrient could lead to the plant absorbing less of another nutrient, creating a deficiency, reducing yield and quality.
Earthy, complex potting mix
The expectation behind a good potting mix is that it contains nutrients that will be released over time and that it is well-limed to prevent fluctuations in acidity. Soil and soilless mixes are often pre-fertilized and have the ability to hold onto nutrients as a sort of nutrient bank. This is due to the fact that these materials provide a high cation exchange capacity, meaning that they are loaded with plenty of docking stations where cations (Ca+2, Mg+2, K+, Mn+2, Fe+2) can land, temporarily, until they are needed by the plant, at which point they are released and made
available. Peat and potting mixes contain a combination of quick release, mineralized nutrients and slow release, organically-bound nutrients that are released over time to your plants.
Most potting mixes contain a peat moss base, which is naturally acidic. Good soilless mixes are limed to bring the pH up to around 5.5-7.0. The lime serves as a pH buffer, so that many soil nutrients such as phosphate, iron, manganese, and zinc remain in an available form to your plant. Beware that repeatedly using the same soil will cause the lime to become depleted. The lime buffer is used over time as nutrient solutions tend to be acidic and contain compounds like ammonium which also increases acidity. If you’re working create a ‘living soil’ that is built over time or simply want to reuse your soil, take a soil test to check in on the pH and nutrient load. Soil testing is generally pretty cheap and state-funded universities often have testing labs.

The lime added to soils also serves as a source of calcium and magnesium for the plant and should be accounted for when considering additional fertilizer requirements. Fertilizers that are designed specifically for soil-based systems should take into account the calcium and


CANNAtalk|7
Figure 4: Mineral lime.
Figure 3: Potting mixes are limed in order to balance the pH.
magnesium already supplied by the soil and prevent potentially supplying an excess of calcium and magnesium, which could throw off the nutrient ratios given to the plant.
A soil based system that is properly limed will be able to tolerate a wide range of water quality, due to lime’s buffering capability. Both hard and soft water are generally acceptable for this system and there is no need for additional calmag, since again the expectation is that the soil has been well-limed. At the same time, the liming agent helps to buffer the pH in the soil zone and for that reason, a wide pH range for the nutrient solution is also acceptable. If the nutrient solution is going to be stored in a tank, it is best to keep the pH between 5.0-7.0 in order to protect and maintain nutrient quality over time.
Inert materials: rockwool, clay pebbles



Inert materials like rockwool and clay pebbles are exactly that – inert! These substrates do not bind nutrients nor do they release nutrients. However, rockwool does require special treatment due to its natural characteristics. Rockwool is made from molten basaltic rock that is spun into fibers. This material is alkaline, meaning that it is basic with a relatively high pH. In order to prime rockwool, before growing it needs to be buffered in a mildly acidic solution.
In nutrient recirculating systems, soft water or RO water is often the best choice to use alongside a well-made
fertilizer designed specifically for this system. In drain to waste (DTW) systems it’s important to adjust the pH of the nutrient solution and maintain the correct pH as the solution sits in the tank. As for water hardness with DTW systems, there are fertilizers in the market specifically suited to hard or soft water for use in hydro systems. Lastly in deep water culture systems, beware that aerating pumps will introduce carbon dioxide (CO2) into the nutrient solution that your plants sit in. This additional CO2 will potentially react with elements in fertilizers and hard water and cause pH fluctuations, so choose your fertilizers wisely and you are welcome to ask us for assistance.
Know before you grow
The more knowledge you have on your water, the dynamics of your substrate, and the fertilizers or supplements truly needed for your system, the likelier you are to achieve success. Every part of your system should be included based on good reasoning and understanding. Use the right fertilizer designed specifically for your substrate, water quality, and growing system. Monitor your plants, measure the EC and pH of your nutrient solution, and get your water and soil tested every couple years or if your source changes. In case of doubt, feel free to contact the CANNA team, your local garden store, or a university advisor. Better to know what you are working with, what you are trying to achieve, and plan specifically for that.•

8|CANNAtalk CANNARESEARCH
Figure 5: Rockwool substrate.
GrowIT
GINGER
FOR CENTURIES, GINGER HAS BEEN A POPULAR CULINARY SPICE THROUGHOUT THE WORLD. IT CAN ALSO BE USED AS A TREATMENT FOR NAUSEA AND STOMACHPROBLEMS, COLDS, AND HIGH BLOOD
PRESSURE. THE GINGER PLANT IS NATIVE TO TROPICAL CLIMATES. IT HAS ELONGATED, AROMATIC LEAVES, BUT IT IS THE TUBERS, WITH THEIR LUMPS AND FINGERS, THAT WE ARE MOST FAMILIAR WITH AND ARE THE EDIBLE PART OF THE PLANT.

Ginger is very versatile and is used in products ranging from spicy cakes, breads, to drinks (ginger ale) and sweets. Ginger is also widely used in Asian dishes. That’s no coincidence, because the tropical plant originated in
By Marco Barneveld, www.braindrain.nu
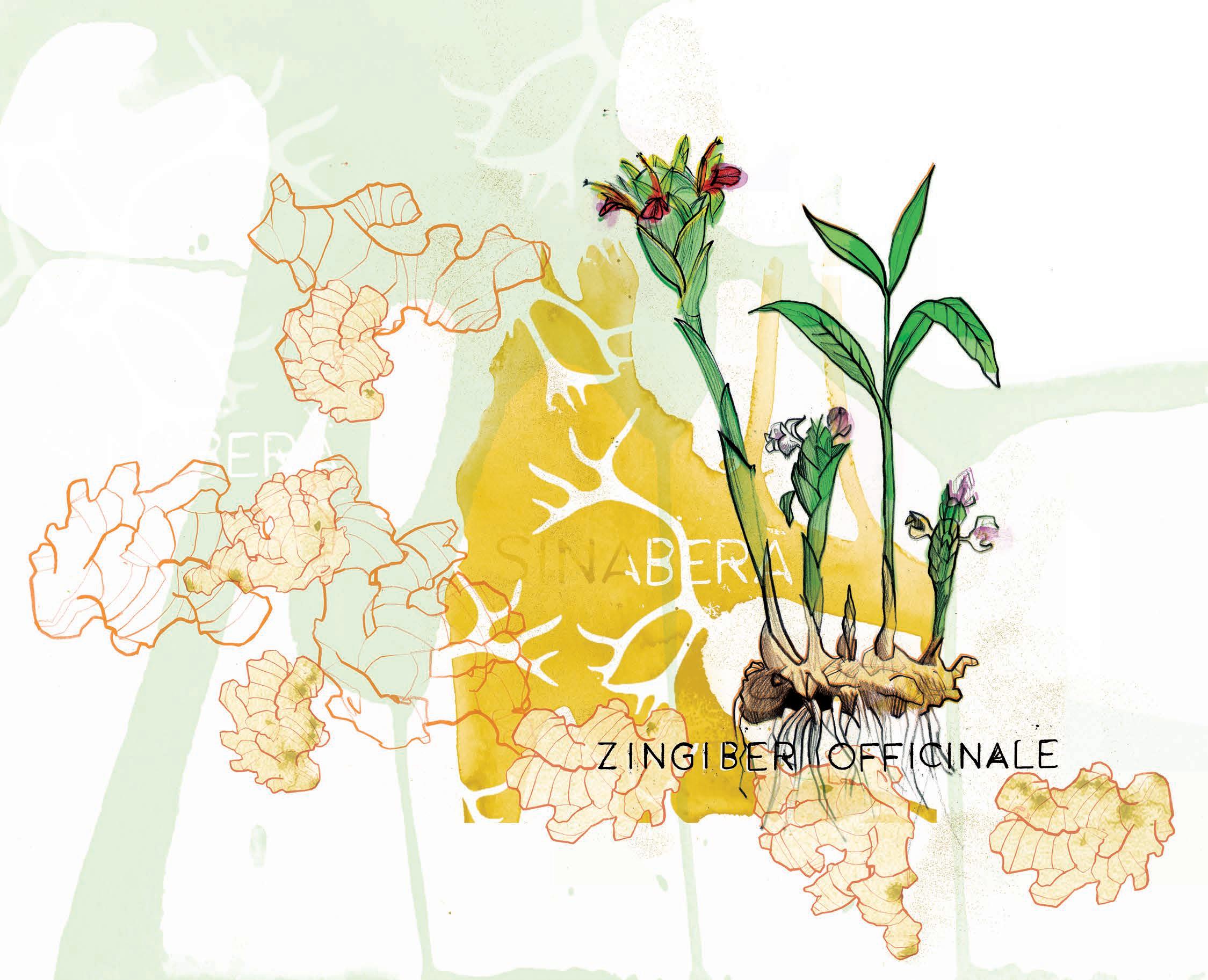
south-east Asia. It has pretty yellow-red flowers, and ginger plants are now grown ornamentally too. So let’s take a closer look at this versatile, exotic plant with a fascinating history.
CANNAtalk|9
Figure 1: The ginger plant, in its entirety, including its beautiful ornamental flowers.
Both our everyday name ‘ginger’ and the botanical name Zingiber Officinale are derived from the Sanskrit word ‘sinabera’, which translates as ‘horn-shaped’. The first growers of ginger thought that the rhizome of the plant – the lumpy tuber to which the rest of the root system is attached – resembled antlers. It’s the rhizome of the plant that you see sold as ‘root ginger’ in supermarkets, and with a bit of imagination you can see the resemblance to an antler.

The spice to cure all ills
Little is known about how ginger first came to be cultivated. Historians think that the plant did not exist naturally in its current form, but was bred by humans. The ginger plant has been known in Chinese culture for over 3000 years. Dried ginger roots found their way from China and India to the Middle East, and eventually to Europe. It was transported via the trade caravans of the ‘Silk Route’, along with other luxurious spices, gold and precious stone. These days, most ginger still comes from Asia. India produces the largest quantity, followed by Nigeria, China, and Indonesia. Other top ginger-producing countries include Nepal, Thailand, and Cameroon.. Around 2000 years ago, ginger roots were rarer than diamonds in the Roman Empire and few could afford to buy even a single stem. Ginger was especially prized for its healing properties, which made it a valuable commodity. It was reputed to be a miracle cure for all sorts of ills and ailments. It was a painkiller, a relaxant, a breath-freshener, a decongestant and an anti-septic. And as if that wasn’t enough, it was also a remedy for ‘flu, colds, catarrh, fatigue, headaches, migraine, nausea, fever, bowel problems, diarrhea, menstrual pain and even impotence.


Sex life
Sometimes, the stories of ginger’s miraculous properties took on mythical proportions. One anecdote told of an Arab prince who, although he had a harem full of beautiful women, was unable to produce an heir. He had tried all the remedies that the doctors could offer him, but all in vain. One day, a traveling merchant paid a visit to his palace,
and he presented the prince with a mysterious ginger drink. Whether it was down to the ginger or one of the other mysterious ingredients is not clear, but whatever it was, the prince was suddenly cured of all his inhibitions in the bedroom. He spent seven days and seven nights uninterrupted in his harem, so the story goes. The prince was so grateful to the merchant that he thanked him with his own weight in gold!
Cooking with ginger
There is a grain of truth in all these ancient legends. Ginger does indeed contain active substances that can have a beneficial effect on the human body. One of these is gingerol, which is converted to shogaol as the root dries out, and counteracts nausea and other stomach complaints.
Of course, ginger is also the perfect way to spice up your cooking. The intensity of the flavor varies according to when the ginger is harvested. The older the plant, the hotter the root will taste. Young ginger roots are softer and more succulent, and have a milder flavor. These young tubers can be eaten fresh or preserved in vinegar, sugary water or sherry, for example. Young ginger is also perfectly suited for making ginger tea. Just add honey and lemon to taste! The juice of older tubers, by contrast, has a very strong flavor and is often used to flavor Asian recipes. The hotter varieties of the ginger root are an indispensable kitchen ingredient in China, Japan and many other South Asian countries. For example, fresh

10|CANNAtalk
Figure 2: Ginger has distinct fibers in its tubers. These tubers will sprout new growth as well.
Figure 3: Ginger-based teas have been especially popular in Asian cultures, including the Indonesian drink wedang jahe.
Figure 4. Ginger bud sites are where stems will grow.
ginger is one of the most important ingredients in Indian curries and it is widely used in Burmese dishes, too. The Indonesian ginger tea drink, wedang jahe is made from ginger, spices like cardamom, lemongrass and cinnamon, and palm sugar and is popular during the colder months. In Bangladesh, ginger is finely chopped or ground into a paste with garlic and shallots. The paste is then used as a basis for chicken and meat dishes.
Grow it yourself!
Ginger is a tropical plant that you can easily grow yourself and does not require much expert knowledge. You start with a piece of fresh root ginger (actually the rhizome of the plant), which you can buy at any supermarket. Choose a piece which has some well-developed ‘growth buds’. The shoots will develop from these buds. The next step is to break the root into pieces with a growth bud on each piece, and to plant these pieces in a container filled with moist potting mix, with a good nutrient base and good drainage. The usual time of year to do this is around the end of winter or the beginning of spring. Keep the ginger at around 75 F, positioned so it receives at least 5 hours of preferably filtered light. It’s also a good idea to place the container in a tray lined with stones and filled with water. This will help to increase the humidity around the plant. Central heating can make the air a little too dry, so you can also spray the plants with a mister once in a while. We are trying to recreate a tropical, humid, partly jungle shaded environment here. Avoid cold, wind or drafts at all costs. When the weather warms, you can bring your plants outdoors to enjoy a natural setting. The growing tips at the end of each ‘finger’ of the rhizome will sprout quickly. Long, slim leaves will grow from the end, which look much like sprouting grass. As the plant grows larger, transplant it into a larger container. Within eight to ten months, the ginger plant will be fully grown, although you can start harvesting small pieces at around 3-4 months. The plant can grow up to around 3 feet all so you should allow some space to accommodate it. •
Eat it yourself:
After all that talk about ginger, we couldn’t finish without giving you a recipe for a sumptuous Thai stir-fry with ginger! no need to worry it won’t take you long to prepare this.
It’s really quite simple and excluding the time needed for the marinade, it only takes about ten minutes!
GINGER STIR FRY SHRIMP

You will need:
• 3 spring onions cut into rings
• 1 red chili pepper cut into rings, use less for less spice
• 2 cloves of garlic, finely sliced
• 1 tablespoon grated ginger

• A few drops of fish sauce
• 2 tablespoons of sesame oil
• 2 tablespoons of vegetable oil
• 1 pound of large shrimp or prawns, raw and peeled
Preparation
Throw all the ingredients together in a bowl and let them marinade for an hour. Then heat up a wok or your next best, simple pan on medium heat and stir-fry the whole lot for about five minutes or until the prawns are pink and curled. Serve with rice or noodles.
ENJOY
CANNAtalk|11
I’ve been growing on a small scale
QuestionsAnswers &
You can use an irrigation system with

We receive a lot of questions about growing. Of course, our researchers are more than happy to answer them! Just go to the contact page on our website, www.cannagardening.com, to submit your question.
Question
Hi there. I’m getting set up at a new location and planning to grow in coco. I’m considering either dechlorinating my water or filtering it through reverse osmosis. If I just dechlorinate, do I still need to use CalMag? Also, I read in your directions that I should wait at least 24 hours after adding CalMag before using my nutrient solution. Can I go ahead and feed my plants sooner?
Let’s start with talking through the quality of your base water supply. Generally, water with a chloride level below 70 ppm is ok to use on all types of plants and a chloride level of 71-140 ppm can present problems. If you have too much chloride in your water, I could see the desire to dechlorinate it. You could achieve this by using a carbon filter or you could let the water sit out in an open container for 24 hrs, over which time the chloride will volatilize out
If your water contains toxins, has too high concentration of sodium or chlorine, is very hard, or has a high EC (above 0.6), I could see the desire to put it through reverse osmosis. Note that most reverse osmosis systems come with a carbon filter, which acts to dechlorinate the water before it goes through the reverse osmosis filter. If chlorine was allowed to build up on the RO filter, it would damage it. Also, water above 0.6 EC could be used, so long as it is clear of toxins, however, you may need to adjust your watering frequency.
Our coco line pairs best with moderately hard to hard water, which is 60-180 ppm of calcium carbonate equivalence. Calcium carbonate equivalence is determined by the concentration of multivalent cations in the water, generally made up of calcium and magnesium. If you put your water through an RO filter, that renders your water into soft water, which is defined as having less than 60 ppm of calcium carbonate equivalence. Our Calmag is designed specifically for soft water in coco systems. If you put your water through a dechlorinator only, you would not have removed any of the calcium or magnesium (all the stuff that makes water ‘hard’). So, if your water was moderately hard or hard to begin with, then you do not need to treat it with Calmag. However, if you have soft water, then you would want to use Calmag.
As you noted, when using our CANNA Calmag we recommend a 24-48 hour wait period and this is to allow our Calmag to condition the water, which is unique to our Calmag. This wait period allows time for water hardening and buffering the pH. Do not add pH up, as the pH will naturally drop and then increase over the course of 24-48 hours. If you use your nutrient solution sooner, that is fine and it will work as a typical Calmag supplement rather than a conditioner. Another option for how to use CANNA Calmag is to add it to the water, allow it to sit for 24-48 hours, and then add the nutrients and additives.
especially
I’ve been growing on a small scale on coco, with Coco A and B, Cannazym, and Boost. I’ve just been hand watering but I’m planning to expand my grow and set up an irrigation system. Can I run your bases and additives through a drip irrigation system?
Answer
You can use an irrigation system with our fertilizers, however, since you are using an organic component (Boost), spray stakes would be a better fit compared to drip emitters. Boost has an organic base (as do BioCANNA fertilizers, for example), meaning that it has thicker substances within it. A spray stake can effectively irrigate with little clogging issues as compared to a drip system. All irrigation systems do clog periodically and require regular monitoring and flushing, so with whichever option you choose remember that maintaining your irrigation system will need to be a regular component of your management tasks. Also, screen filters can help keep your irrigation system running smoothly, and these would need to be checked regularly as well.
Hi there. I’m getting set up at a new location and Let’s start with talking through
12|CANNAtalk
Question
Hey, I’ve used your line for years and love it. Recently, I switched to LEDs in my Substra, rockwool system and my plants are looking great. They are definitely getting bigger faster than usual. How much should I increase the rate I’m using? Seems like I should feed more since they are thriving. I’ve noticed higher rates from other product lines as well, so just wondering what do you guys recommend?
Answer
There are several factors that come into play in affecting a plant’s metabolism and optimal fertilization. Plants have a light saturation point, above which greater intensity light does not bring greater plant development. LEDs can provide high intensity lighting, especially since they can be placed close, without overheating the plant. In order for a plant to effectively use greater light intensity beyond its saturation point, additional carbon dioxide and a slightly higher temperature is needed to support this increase in metabolism and plant development. The light intensity, carbon dioxide, and temperature trifecta works in concert to support plant gains. When considering an increase in your fertilizer rate, a good starting point is to follow the ‘heavy feeding’ recommendations listed in our grow guide. Observe your plants, make minor adjustments to your fertilizer rates, observe again, and take notes on your environmental conditions and lighting arrangement.

Do be aware that as the nutrient solution becomes more concentrated, it becomes harder for plants to take up water. So, if the EC becomes too high, especially under high temperatures, plants may not be able to take up enough water to meet basic needs. The leaves could start to curl and turn brown around the edges, a condition known as fertilizer burn. If the nutrient solution becomes even more concentrated, it could pull water out of the roots, causing plants to wilt and die. Monitor for any ill effects, as described. Also, be sure you continue to monitor and tweak your system so you best understand where the sweet spot is for fertilizing. Beyond a certain point and plants will either suffer, see no added benefit, or are unable to utilize a greater supply of nutrients. No one wants to waste money, potentially cause environmental harm, or overdraw on natural resources by over-fertilizing their plants. Good luck tending to those robust plants!
Question
I’m working through the grow chart that I downloaded off of the website and am looking for clarification on EC+. What does this represent?


LEDs can provide high intensity lighting, especially since they can be
There are several factors that come into play in affecting a especially since they can be placed close,
at
Hey, I’ve used your line for years and love it. Recently, I switched to
Grower’s circle
Every grower loves getting new gear. Whether it´s the latest state-of-the-art lighting system, an upgrade to the next level environmental controller, or even a fresh set of trays, it all sparks excitement about what these fancy toys can bring to the garden. Since this CANNAtalk is all about water this is a great opportunity to dive into one of the smaller, and most misunderstood, pieces of equipment in the hydro store- the TDS meter. By
George Hetfield
INWATER WE TRUST

As essential to many growers as the pH meter, this paragon of parameters is not as straight forward as one would think. It provides some extremely valuable information about our water and fertilizer solutions, while at the same time raising many more questions about how we should be approaching our garden systems. Join in the Growers Circle and let´s take a closer look at this classic hydro store tool and how it can lead us to unlocking the secrets surrounding water quality and the way we feed our plants.
As we learned in the previous technical section of this issue, TDS, or Total Dissolved Solids is the measurement of the electrical conductivity (EC) of a solution. The more mineral nutrients we put into our water, the greater the ability to conduct electricity. This results in our meter reading a higher value. Measured in parts per million (PPM) in some models and EC in others, values can range from .1 up to 5 EC (anything higher is not ideal for horticultural
applications). Simply put, by monitoring the EC of a solution we can accurately measure the concentration of the solution we are preparing to feed to our plants.
If you are a frugal grower you may be asking ¨Why can´t I just follow the grow guides that are provided from nutrient companies? Why do I need this extra step? I already measure my pH, isn’t that good enough?¨ Yes and No. Think of it like this, if you bought a car and the speedometer was broken you would probably have a general sense of how fast you were going, but without really knowing your true speed you are just asking for a ticket (or taking forever to get to your destination). In our case with plant health, it is just a matter of time before an imbalance will occur if you don´t know your nutrient ¨speed¨. Too much or too little fertilizer can cause serious problems in our crop if we don’t closely monitor the inputs of our garden systems. Many factors go into
14|CANNAtalk
how concentrated a nutrient solution should be and none are more important than knowing all the facts about your water- the foundation of our gardens. So now that we know the basics let´s check out our cleaned and calibrated TDS meter. With our reservoir or bucket filled with fresh water and settled to the proper temp ( around 60-70 F), we take our first reading and it shows 0.2 EC (140 PPM on a 700 scale). Over the years, many of us have been schooled that the proper way to treat this lower
can perform a water test for a modest fee (usually $40$100 $35-55 dollar range). This is simple and quick. You can collect your own samples, mail them to the science guys and obtain a full readout of what is going on in your water. Growers using well water will need to have this test done and probably should consider sampling at least twice a year to monitor fluctuations when ground water levels change. Below is an example of a standard water test to get an idea of what is being measured.

level is to reach for our trusty bottle of Cal-Mag and add it into our starting water until the meter reads .4 EC (about 280 PPM). … but do we truly understand what we are adjusting with the addition of this supplement? Is it high iron? Calcium? Manganese? Lead (hopefully not!)? All of these and more could be present in the solution. If we don’t know what levels we are adjusting in the program, how do we know that we are treating the water properly? So many unknowns make things difficult to go forward responsibly. Slowly, very slowly, we take our hand off our Cal-Mag bottle and step away to reassess the situation. We started with plain water, nothing has been added to it, but the meters are picking up the presence of elements. Like we touched on above, how can we be sure that these are the elements we want in our solution and that they are at the proper levels for optimal plant health? Luckily, all our answers can be found within a couple of key strokes or quick phone call. We need a water test. City folk are fortunate enough to look up their local municipality and find out the annual report of the water in their area. There will be seasonal fluctuations so growers preferring to monitor their systems with greater accuracy will need to find a local water quality professional, University, state department of agriculture, or lab that
Once you have your results, it is important to make sure that all your components fall within the ranges that most testing reports provide. Even with the guides and explanations, reading the test is no easy task. There are some great references to help you determine where your results fall. One of these is a website created by Penn State University entitled, ‘Interpreting Irrigation Water Tests’. This can be accessed with a quick web search to see it live. Should you need more assistance most horticultural professionals that work with water quality testing will be happy to provide recommendations. For our discussion though, let´s pay special attention to a couple specific lines regarding calcium, magnesium, water hardness, and what information we can gather from them.
First, a quick review of some basic chemistry so we can understand the testing better. Water hardness accounts for the total concentration of dissolved multivalent cations in the solution and primarily consists of calcium and magnesium.
Multivalent cations are positive ions with a charge of at least +2 (the periodic table is a great refresher to see the different classifications).
CANNAtalk|15
ANALYSIS RESULTS UNIT IDEAL RANGE pH 7.6 mg/L (PPM) 5.0-7.0 Total Alkalinity as CaCo3 34.9 mg/L (PPM) 30-100 - Bicarbonate Alkalinity (HCO3) 41.6 mg/L (PPM)- Carbonate Alkalinity ( CO3) 0.5 mg/L (PPM)Hardness as CaCO3 15.8 mg/L (PPM) 50-100 Electrical Conductivity (EC) 0.08 mmhos/cm Above 1.0 Total Dissolved Solids (TDS) 48.9 mg/L (PPM) Above 640 Nitrate- Nitrogen (NO3-N) 0.4 mg/L (PPM) fertility program dependent Ammonium- Nitrogen (NH4-N) 0.97 mg/L (PPM) fertility program dependent Phosphorus (P) 1.12 mg/L (PPM) Below 5.0
Watertest table
You’ll notice on the results in the periodic table above it’s written as, ‘Hardness as CaCO3’. Labs will sometimes use CaCO3, or calcium carbonate, as a means of expressing hardness, but the real reference we want to take notice of is the concentration of dissolved calcium and magnesium (primarily- there can be other things present in small amounts). So this line is important as a general overview of how hard our water is but we still need to look at the whole picture of the test results.
The other line to note is ‘Total Alkalinity as CaCO3’. Similarly to hardness, labs will sometimes use CaCO3 (calcium carbonate) as a means of expressing alkalinity- or pH buffering capability. The presence and concentration of these carbonates or bicarbonates account for the dissolved ions that buffer or neutralize acids and keep the pH of your solution from having heavy fluctuations.
Why is CaCO3 being referenced for both hardness and alkalinity? Well, the two concepts are very closely related. CaCO3 isn’t necessarily physically present in your water (it’s a solid), but, it’s components – calcium, bicarbonate, and carbonate ions, could be found dissolved in your water. The calcium (or magnesium) ion contributes to hardness and the bicarbonate and carbonate ions contribute to alkalinity. Labs just sometimes choose to express hardness and alkalinity in terms of CaCO3 because, in theory, water has the capability of forming CaCO3 and we want to use these factors to get a sense of our water’s true potential. The more we know about our water chemistry the better choices we can make going forward.

Now here is the big kicker- our TDS meter does NOT measure water hardness specifically but instead indicates the total amount of mineral elements in the solution! This makes sense because it’s in the name – it measures the total dissolved solids, not just the elements that contribute to hardness. So if we look at our example water from the start of our discussion (not the water test example) of 0.2 EC (140 PPM) it is possible that most of that reading is comprised of calcium and magnesium. This means that you’ve got adequately hard water and would not require Cal-Mag even though it has a relatively lower reading on the TDS pen. Also important to note that the inverse could be true as well. Starting water with a 0.6 EC (560ppm) could have elevated levels of iron and manganese making the reading high but still having low levels of dissolved calcium and magnesium resulting in soft water which
would need to be treated! Much of this depends on your area of the country and what types of rocks your water has traveled through to get to you. Making even more of a case that each gardens´ water is unique and needs to be monitored. In settings where the readings are outside of the recommended parameters, water hardness can be adjusted through a chemical process or by physical filters such as reverse osmosis machines.
At this point you’re most likely wondering, ¨Whats the deal with my ol’ reliable Cal-Mag bottle? I have been using it for years and I thought you said these elements are what give water its hardness….. so why can´t I just add some in after I get my water test? In one word…...Balance. Over applying calcium and magnesium to achieve hardness risks throwing our nutrient program out of wack in a couple ways. An abundance of any element in our systems is obviously detrimental but we now know hardness and pH stability are connected so you begin to mess with nutrient availability as well if we over do one thing. Knowing your water hardness level, while paying attention to overall TDS, is an essential component to getting the most out of our fertilizer programs and maintaining proper plant health.
Even though we went deep into the abyss of water quality chemistry it is time to swim up to the surface and regroup. It may seem like our TDS meter has been eclipsed by our water test but this is where we will bring it back into the spotlight. After our water test has confirmed we have appropriate hardness levels and we have assessed what elements are present we are ready to add our favorite balanced fertilizer. Our meter now becomes the essential odometer to our car analogy from the beginning. Depending on the crop and the production system we are implementing (coco, soil, hydro in LED, DE or CMH rooms) we may want higher or lower levels and can use the TDS meter to adjust our nutrient concentrations. As always, it is extremely important that we make proper adjustments to your feeding programs to match the conditions of the environment and the growing system we have in place (refer back to the Primed for Success article for more on this!)
Armed with our water test in one hand and our trusty TDS meter in the other we can beam with confidence going into our next grow that our results will be up to the high quality standards we have in the Growers Circle! •
16|CANNAtalk
Periodic table
BLUE FIRE DID YOU KNOW THAT...?
• This impressive photo was taken at the Kawah Ijen volcano on the Indonesian island of Java. The blue glow that you can see is not lava, but light created by the combustion of sulfurous gases.

• These gases escape through cracks in the volcano and are released at high pressure and high temperatures (up to 1,112°F). When the gases come into contact with the air, they spontaneously combust, sending blue flames up to 16 feet high into the atmosphere.
• Some of the gas condenses into liquid sulfur and this also burns as it continues to flow down the sides of the volcano. This makes it look like there is molten lava flowing down its sides.

• This phenomenon occurs twenty-four hours a day, but only becomes visible at night. The Kawah Ijen volcano is the largest ‘blue flame area’ in the world. The locals also call it ‘the blue fire’.
• The Kawah Ijen volcano is Indonesia’s main sulfur-producing area. Every day, people here, gather large quantities of the yellow crystallized lumps of sulfur, which they sell or make souvenirs from.
• The Kawah Ijen crater lake is the largest acidic lake in the world. In 2008, George Kourounis managed to measure the lake’s pH – which was just 0.5. The reason that the lake is so acidic is because in the past the volcano gave out hydrogen chloride gas. This reacted with the water and formed highly concentrated hydrochloric acid with a pH close to 0. The acid makes the water in the lake appears green.
• The Kawah Ijen volcano is officially dormant. The last eruption of magma was in 1817, and the last phreatic eruption (steam, ash and rocks) was in 2011. However, at times the volcano still gives out so much smoke that it is inaccessible to tourists.
• The blue fire, the crater lake and sulfur mines all go to make Kawah Ijen a real must-see for visitors, but a visit to the volcano is not without its risks. It’s a tough climb to reach it and the sulfur fumes are not good for your health. It is advisable to take a tour with a guide and you should also cover your mouth with a scarf or wear a mask or a respirator.
CANNAtalk|17 Facto
Photo courtesy of Olivier Grunewald,
oliviergrunewald.com
HAPPENING What ,s


Induction stovetops are rare in the U.S., but that could soon change. In an effort to fight climate change, many cities are pushing buildings and homes to go all-electric. Induction’s powerful cooking technology might be the tool to get people to give up their gas stoves.
By Andres O’Hara, www.andresohara.com

INDUCTION COOKING
IS HEATING UP
Here’s a fun party trick, if you have an induction stove at home. Turn one of the burners up as high as it goes, and then give it a minute. Put your hand right on the burner, and then your tongue. The burner stays cold, and your guests will assume that the stove’s defective, and the burner isn’t even on. But then, with some flourish, put a pan on the stove, watch it heat up instantly, and scramble some eggs.
That’s the magic of induction. There’s no heating element inside an induction cooktop. Instead, under the flat glass top lies a ring of copper coils. Turn it on, and an alternating current zips through the coils, creating a magnetic field that induces an electric current in the pot or pan sitting on the cooktop. The molecules inside the cookware are churning and vibrating, and the friction creates heat. The cookware is actually heating itself from the inside.


Cookingand climate change
The induction stove isn’t just a cool new appliance, it’s the latest tool in a bigger fight by climate activists who are pushing to make gas-powered homes all-electric. Natural gas production is now a bigger source of pollution than burning coal, and natural gas leaks release methane, a

powerful greenhouse gas, and these leaks occur at a far higher rate than was previously realized. If you have a gas stove at home, then natural gas is also powering your furnace, water heater, and clothes dryer. Most people don’t have a problem switching all of those appliances from gas to electric, except for one: the gas stove.
People have a strong emotional connection to their stove. Cherished childhood memories often involve the kitchen and the stove. Gas stoves also have the reputation as being superior to electric. They seem more professional. When was the last time you saw an electric stove on a cooking show? Aaron Fairchild, co-CEO of the green building company Green Canopy NODE, told me that they’ve lost sales of all-electric houses because they didn’t have gas stoves.
That’s where induction comes in. These stoves are far more precise than gas and conventional electric ones. They can cook at lower temperatures, but also get cookware hotter, faster. They look futuristic, and won’t burn you if you accidentally put your hand on it. There’s also a growing awareness about the potential health risks of gas stoves. Using a gas stove can produce
18|CANNAtalk
increased levels of Nitrogen Dioxide, and this air pollution disproportionately affects children. Climate activists hope that the benefits of induction will convince people to switch away from gas stoves, because, like it or not, change is coming. Cities across the country, including New York and Los Angeles, have banned gas hookups in new construction in an effort to fight climate change. This means that new houses and apartments will be all-electric, and induction might be a lot more popular over the next few years.
A chef makes the switch
What’s it like to make the switch? I visited Francisco Anton, chef and owner of the restaurant La Ñapa in Brooklyn, NY, to find out. His kitchen is all electric, and uses induction stoves and burners for a lot of the cooking. One big adjustment for Francisco was cooking with buttons instead of knobs. Instead of getting to high heat by turning a knob all the way, you press a button ten times. The other difference is the lack of a visual cue of a gas flame. The induction burner never heats up, only the pan does. So in a busy kitchen, you need to pay attention to which pan is heating up, and not accidentally grab it with a bare hand.
But there are plenty of positives, like the ability to heat a pot of water super-fast, or go from high to low temperatures much quicker. But one of the biggest reasons why Francisco went all electric was so that he wouldn’t have to deal with a gas line. He’ll never have to worry about his restaurant getting shut down because of a faulty gas line, or worry that a gas leak could lead to an explosion at his restaurant. This all-electric restaurant has put Francisco ahead of the curve, now that New York has banned gas lines in new buildings.
If you are curious about trying induction, but aren’t ready to switch out your stove yet, you can get an induction single-burner cooktop for around 100 bucks. Wirecutter, Cooks Illustrated, and Consumer Reports all have roundups of their favorites. And if you live in California, a growing number of cities-- including San Diego, the East Bay, and San Jose-- are offering induction checkout programs, where you can borrow an induction burner for a few weeks. These programs are part of larger campaigns to educate the public about what induction actually is, and why it’s worth trying. The hope is that cooking on induction will start feeling more natural than cooking with natural gas. •
CANNAtalk|19
Pests & DISEASES

Over the last two years we all have become much more familiar with how viruses can spread among people, move quickly, evolve into new variants, and continue to disrupt lives. Unfortunately, viruses behave much the same way in the plant world. Both viruses and viroids have become a significant problem and are often much more common in our environment than we realize, considering that they often have a latent, or unseen, nature. As much as we try to avoid a viral infection to our plants, there is real potential that some of our most prized varieties can become exposed. The best way to protect our valued plants is to gear up with knowledge on what viruses and viroids are, how they spread, and how they can be prevented.
Viruses and viroids
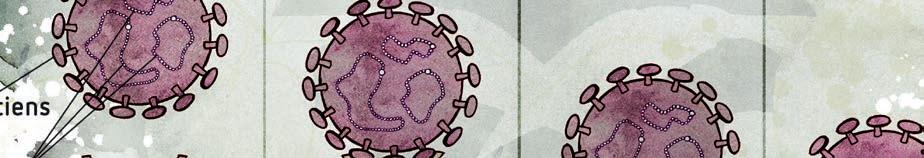
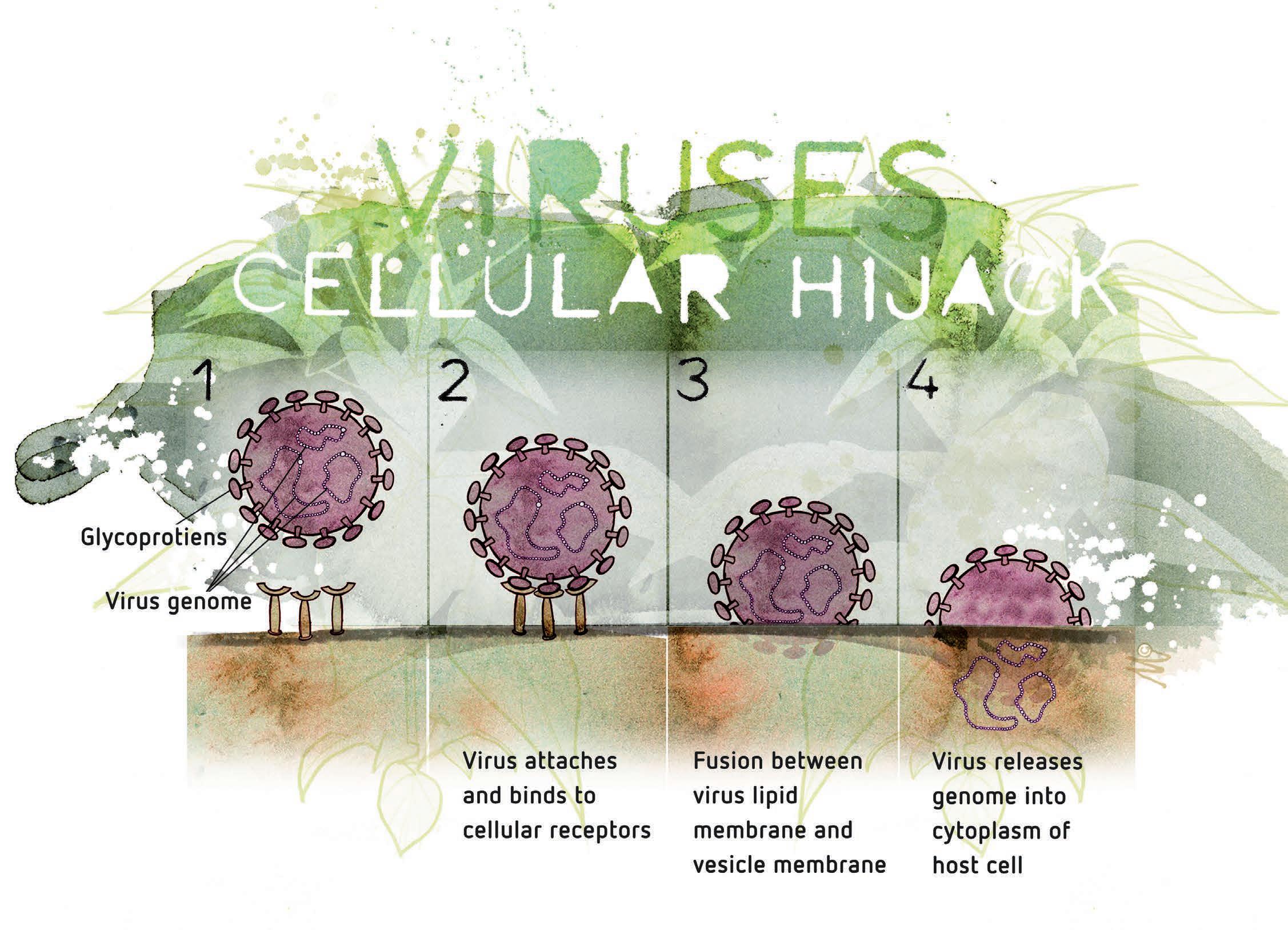
Viruses consist of genetic material made from DNA or RNA that is coated with a protective layer called a capsid, which is made out of protein. The capsid is covered with lipids (fatty cells) to provide further protection. Viroids
By Abha Gupta, MS Horticulture
are single-stranded RNA molecules, not protected with a protein capsid, and are smaller in size compared to viruses. Viruses and viroids both hijack the host’s nucleic acid replication system to reproduce themselves. Generally, infection does not kill the plants, as it’s also to the
20|CANNAtalk
Figure 1: Viruses incorporate into the plant and use the plant’s metabolism to replicate themselves.
disease’s advantage to continue to have a live host whose metabolism can fuel their own proliferation. However, the damage can be significant. Symptoms can be confused with abiotic stresses, which are stresses that come from the environment like exposure to strong light, drought, or windburn. Symptoms can also be confused with herbicide damage, resembling the mottled, streaking pattern that viruses can display.
We tend to see viruses like lettuce chlorosis virus, which can show symptoms such as stunted growth and yellow, brittle, rolling leaves. These symptoms are prompted by a hormonal response within the plant. Phytohormones like salicylic acid and jasmonic acid trigger defenses such as necrosis and cell death – with the goal being to limit cell to cell transfer of the virus. The infection generally does not kill plants; however, it can significantly affect yield. Another unfortunate virus in circulation is hemp streak virus, which shows symptoms such as interveinal chlorosis and leaf wrinkling. The most common viroid currently is the hop latent viroid disease, which causes stunting, tighter internodal spacing during the vegetative state, and less developed flowers with reduced quality. Hop latent viroid disease can also be latent, showing no symptoms until appropriate conditions occur between the plant host and the environment.
How do viruses and viroids spread?
Viruses and viroids can spread through open wounds, such as when taking cuttings. Tools can become infected from one plant and then spread to subsequent plants as a grower goes down the line. For that reason, it’s very important that cultivators sterilize equipment before starting to work on a new plant and to keep their hands clean or wear fresh gloves between plants.

Insect pests are also responsible for spreading viruses and viroids. Insects in the order Homoptera, such as
aphids, whiteflies, leafhoppers, planthoppers, and thrips – that have piercing sucking mouthparts – are common viral vectors. They spread viruses and other pathogens by first feeding on an infected plant, and then feeding on an uninfected plant. Whiteflies in particular are associated with lettuce chlorisis virus
Virus and viroid prevention
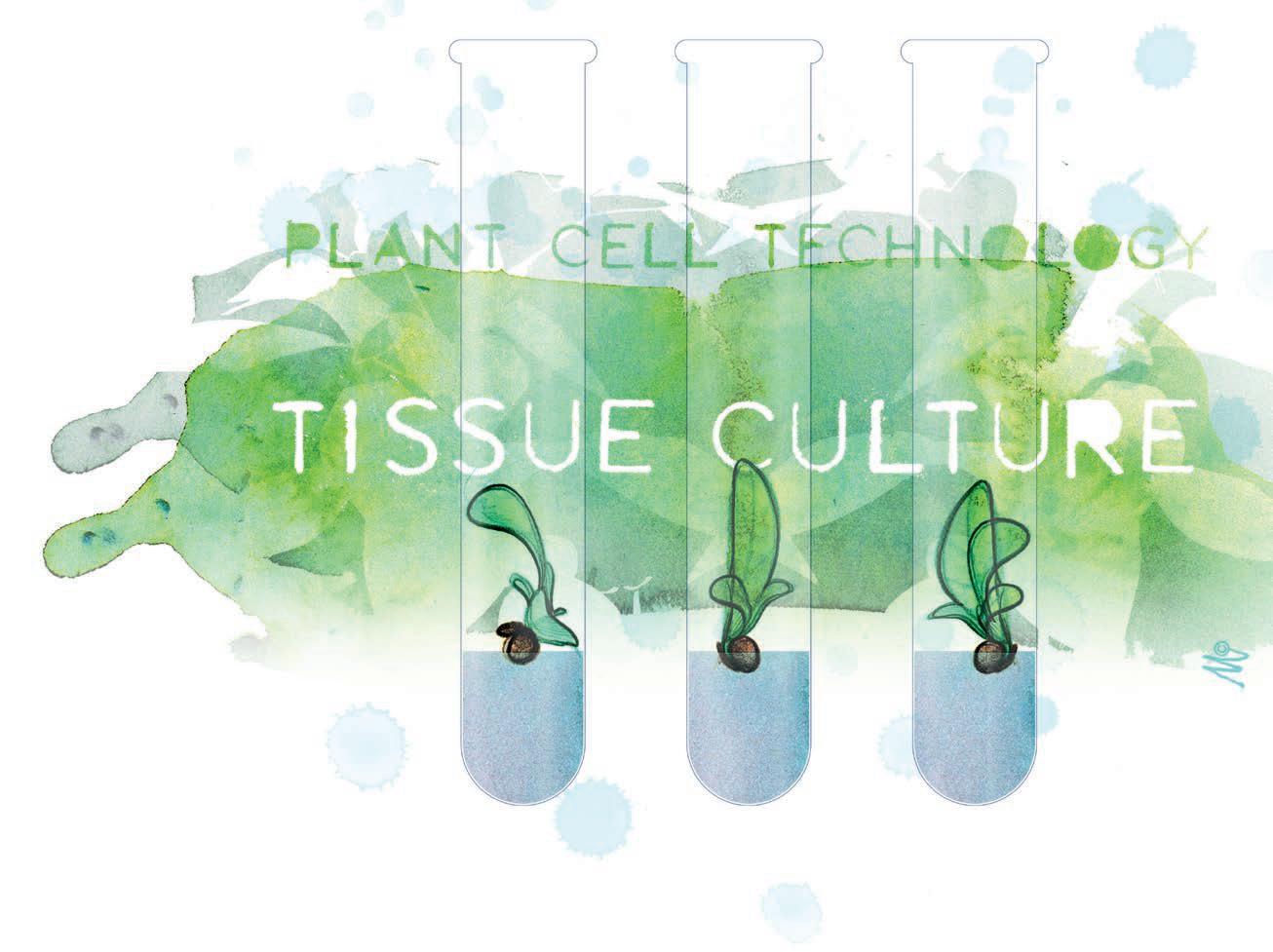
Unfortunately, once a virus or viroid infects a plant it cannot be removed from the live plant itself. That is why prevention is extremely important when it comes to managing viruses. Be very careful with your plant materials once they are on site. Cleanliness of tools, equipment, and staffers is essential. Take every effort to ensure that new plant stock being brought in is free from diseases. Similarly, make sure that stock, or mother, plants are kept clean. The best way to verify cleanliness is to have these plant materials tested by either a private lab or a public university that provides testing services. There are many, many testing labs located throughout the country and most allow shipping of plant samples. Regular testing is advised especially for certain diseases, like hop latent viroid disease, due to their latent nature. Testing just once does not necessarily ensure that the entire plant is clean or that symptoms will not arise in the future. If the worst case scenario occurs, where one of your most desired cultivars is infected – the cultivar can be saved using lab techniques. Meristematic or nodal tissue sample can be treated using a heat or cold therapy (the exact treatment is protected by each lab) until it is rendered disease-free. This material can then be used to generate new, clean plants using tissue culture technology.
It can be challenging to ensure that your plant production remains completely free from diseases, but every effort made towards this will be worth saving future headache and lost value. Good luck while protecting your plants and as always, feel free to reach out as questions come up.
CANNAtalk|21
curled, mottled, or streaked leaves.
Figure 3: Tissue culturing allows lab specialists to generate a new plant from a small sample of plant material.
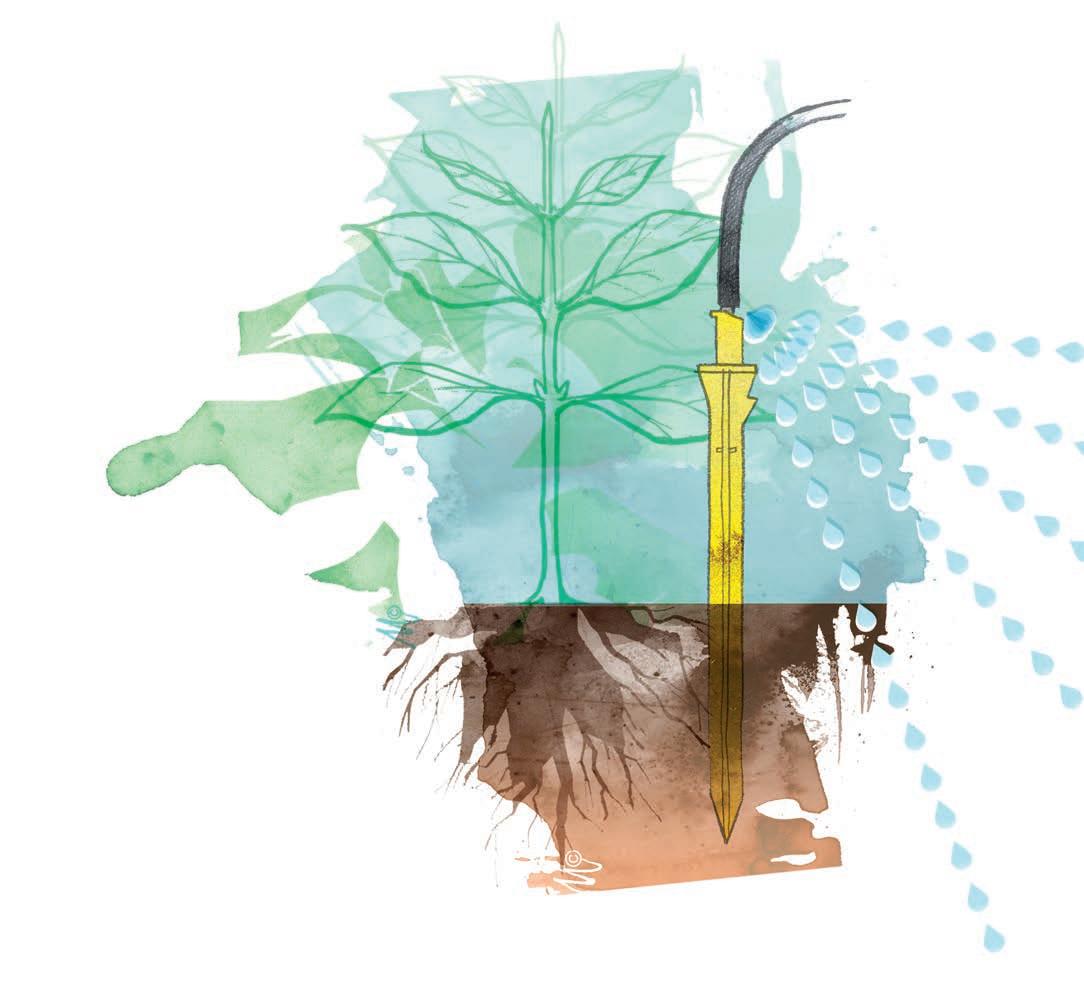
EC, HARDNESS, PH AND HOW WATER QUALITY AFFECTS YOUR MANAGEMENT
The principles of water quality are even more impactful in a hydroponic system, whereas well-designed soils-based systems are less sensitive. Regardless of how you grow, there are key elements of water quality that you need to know – like EC, hardness, and pH, and how these factors will impact your management decisions
If your water were electrified… don’t try this at home
As the universal solvent, water dissolves salts and minerals to become ions in solution. If we were to pass an electrical
current through mineral solutions of various concentrations, the more concentrated solutions would be able to conduct more electricity. Under this reasoning, ion concentration is measured as electrical conductivity (EC) expressed in milliSiemens per centimeter (mS/cm). So, the more ions in solution means a higher EC. Ion concentration can also be measured as a physical concentration, expressed in parts per million (ppm). When it comes to a nutrient solution, this means that 1 ppm is equivalent to one part by weight or
water. So,
per

ppm
equivalent
parts
1 mg
liter.
CANNARESEARCH 22|CANNAtalk
TO
A
volume of nutrients
one million
by weight or volume of
1
is
to
per
WATER QUALITY HAS A HUGE IMPACT ON YOUR GROW SYSTEM. USING INADEQUATE WATER CAN LEAD TO NUTRIENT DEFICIENCIES IN YOUR PLANTS, CONTRIBUTING TO YIELD LOSS. ON THE OTHER HAND, UNNECESSARILY SUPPLEMENTING YOUR WATER WITH CALCIUM AND MAGNESIUM COULD BE A WASTE OF RESOURCES. EXCESS WATER SUPPLEMENTATION CAN ALSO THROW OFF THE IDEAL NUTRIENT RATIOS DELIVERED
YOUR PLANT AND LEAD TO
DEFICIENCY AS WELL. By Abha Gupta, MS Horticulture, Assistant Horticulturist
Water hardness
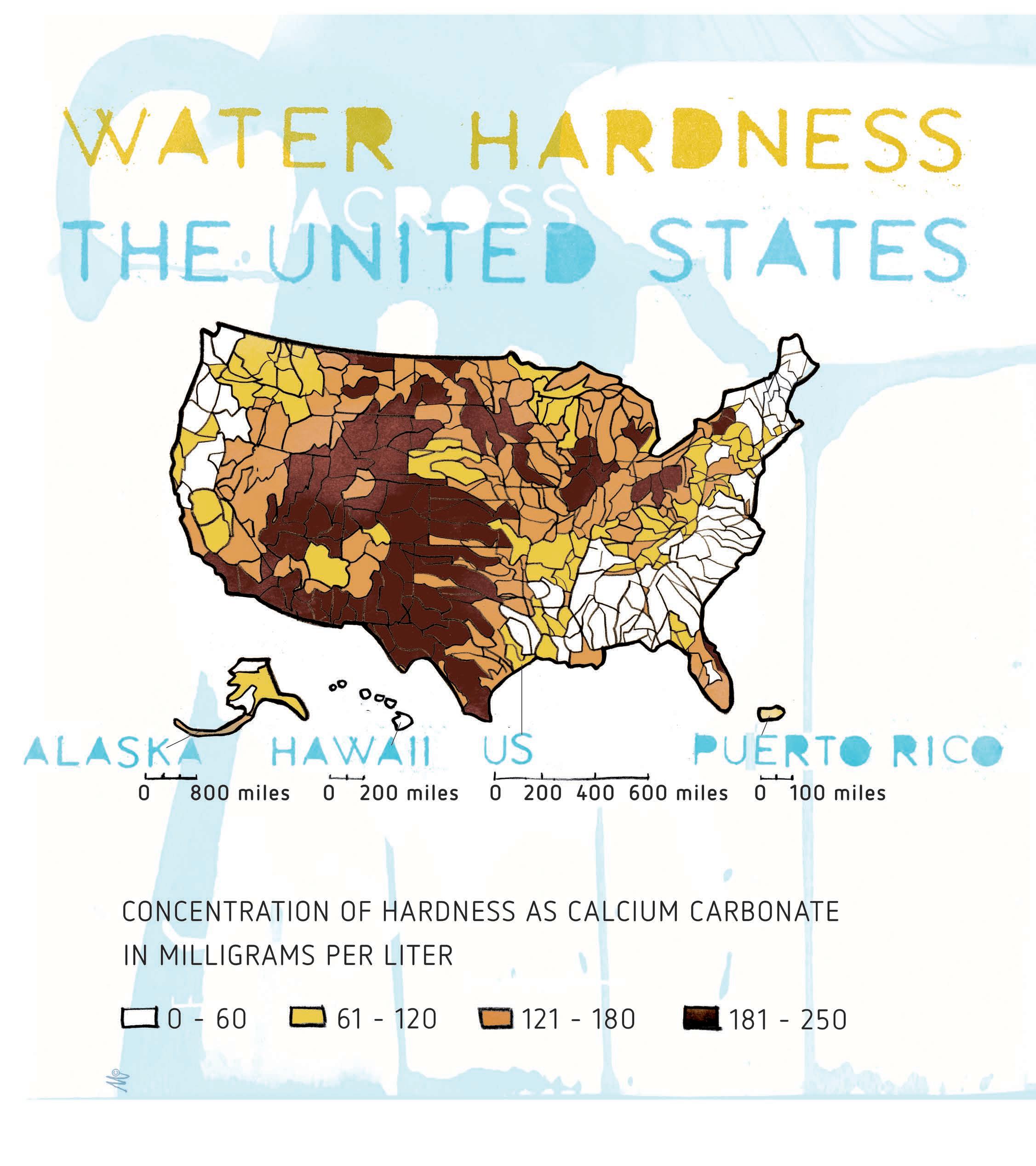
An ion with a positive charge is called a cation. Water hardness is measured by the concentration of cations with a +2 or higher electrical charge, known as a multivalent cation. The multivalent cations of most interest for water hardness are Ca+2 and Mg+2. In nature, hard water is formed when water percolates through deposits of limestone, chalk, or gypsum and picks up minerals along the way. These materials are largely made up of calcium and magnesium carbonates, bicarbonates, and sulfates. Hard water is naturally found
in certain geographical regions like Texas, New Mexico, Arizona, Utah, parts of Colorado, southern Nevada, and southern California. In comparison, rainwater, snow, and precipitation generally have low concentrations of calcium and magnesium and are considered soft water. The softest water is naturally found in parts of New England, the South Atlantic-Gulf, the Pacific Northwest, and Hawaii.
According to the United States Geological Survey, soft water has 0-60 ppm calcium carbonate equivalence,
CANNAtalk|23
Figure 1: Water hardness across the United States.
water with 61-120 is considered moderately hard, 121-180 is hard, and more than 180 ppm is considered very hard..
Hard water can leave deposits, known as scale, in pipes, heat exchangers, or in tea kettles, as some of you may have seen. Scaling in pipes may lead to reduced water flow or heating efficiency. Hard water can be beneficial
to human health since it helps fulfill your dietary need for essential minerals. For some plant production systems, the additional nutrients in hard water can be helpful for growth whereas soft water may require supplementation. See the following article in this issue, ‘Pairing the ideal water treatment with your system,’ for more guidance on water use in plant production.
24|CANNAtalk CANNARESEARCH
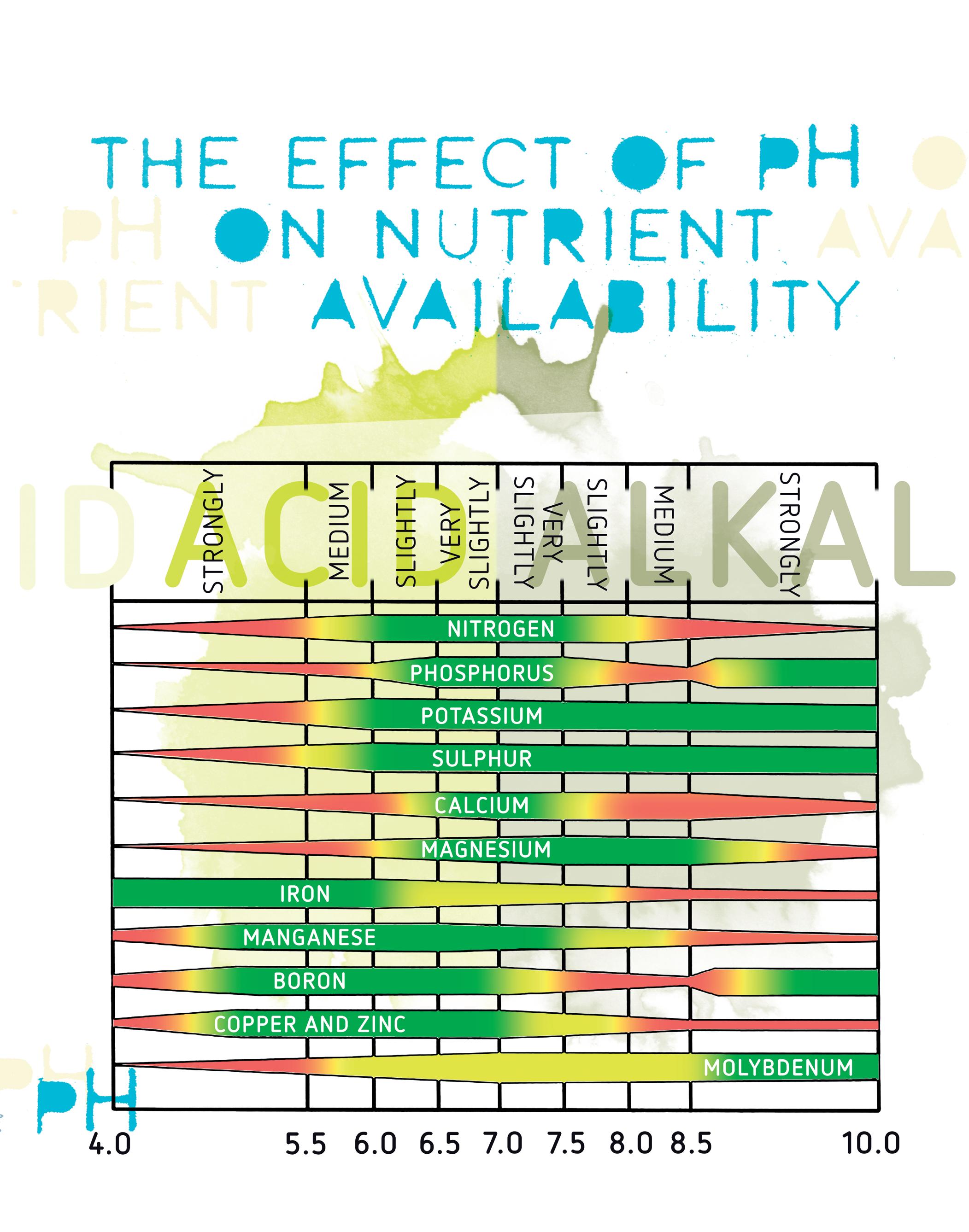
Figure 2: The effect of pH on nutrient availability.
When water moves through rocks and picks up minerals along the way, limestone and dolomite dissolve into ion form. Half of the molecule is the calcium (Ca+2) or magnesium (Mg+2) ion which contributes to hardness and the other half is the bicarbonate (2HCO3-2) ion, which contributes to the alkalinity. The bicarbonate leads to an increase in pH. Therefore, hard water tends to have a higher, or more basic, pH. The pH of your water and nutrient solution has a direct impact on nutrient availability. Some nutrients are more available at a lower pH and others are more available at a higher pH. With each growing medium (coco, hydro, soil) there is a recommended pH range for your nutrient solution and some fluctuation within this range is OK, since that shifting will naturally allow certain nutrients to become more available.
The effect of pH
Maintaining the correct pH range is most important when storing nutrient solution in a reservoir. In such storage, the proper pH will maintain nutrient availability in your solution as intended whereas an incorrect pH may lead to nutrients being less available or combining with other nutrients, rendering them also unavailable.

While in the reservoir, gaseous nitrogen, oxygen, and carbon dioxide will naturally dissolve into the nutrient solution as an effort to create an equilibrium between the gases found in the atmosphere and in liquids. In fact, the ocean is a major sink for carbon dioxide because of this phenomenon. Aerating the nutrient solution with an air stone also adds dissolved carbon dioxide. This added carbon dioxide has the potential to cause pH fluctuations as it reacts with elements in fertilizers and hard water to create pH buffers. For that reason, be sure to only aerate the nutrient solution with a pump if your system truly needs it, such as in a deep water culture system where the submersed roots need to be oxygenated. Also be sure to choose fertilizers designed for submerged root systems and monitor the pH. Nutrient reservoirs that are not aerated will still gain some dissolved gases from the atmosphere, but not in quantities of concern. A simple stirrer can be used as needed to maintain a welldistributed nutrient solution.
Total solids in solution

It is important to be aware that hardness is only a measure of multivalent cations and is not a measure of the total dissolved solids (TDS) in solution. The TDS
includes elements such as phosphorus, potassium, chlorine, sodium, nitrate, ammonium, and trace nutrients in addition to calcium and magnesium, the primary cations that contribute to water hardness. When you measure the EC of water at home using a personal meter, it accounts for the TDS and does not provide direct information on water hardness or if your water contains specific elements, like chlorine or sodium, which can be harmful in excess and qualify your water as ‘bad’. The only way to know if the concentration in your water actually contains calcium carbonate equivalents, the water hardness, and to find out about potential toxicities, is to get your water tested in a lab.
Testing and treatment options

Nobody wants to use the wrong water type for their system so to prevent this – we have to gear up with knowledge! The best way to get to know your water is to get it tested by a local university with a testing lab, state department of agriculture lab, a private lab, or call up your city water supplier and ask them for an analysis. In addition to a formal lab analysis, a pH and EC meter will help you monitor the quality of your water and nutrient solution on your own. If the meter you purchase has no means of calibrating it before using, it probably will not give the best results. It’ll be worth it to have a reliable, at home means of checking your system, including your substrate media. If the water available to you is.
Reverse osmosis
Reverse osmosis (RO) is the most common strategy for treating both hard water and ‘bad’ water. In normal osmosis, solvents like water naturally move from an area of low solids concentration to an area of high concentration, to try to create an equilibrium throughout the solution. With RO, external pressure is applied to reverse this natural osmotic flow and pass the solution through a membrane filter. RO uses a partially permeable membrane to separate ions, unwanted molecules, and larger particles from water. The process can remove many types of chemical elements as well as biological microorganisms, like bacteria. The partial membrane allows solvent molecules like water to pass through, while solids are retained on the pressurized side of the membrane. The resulting purified water is considered very soft, if the RO process is done correctly. Also, note that most reverse osmosis filters contain a carbon filter for dechlorinating water and protecting the RO filter.
Water softening
Water softening is a method where calcium and magnesium ions are typically exchanged for sodium or potassium ions in a resin column. This process only works to soften the water and will increase the total dissolved solids of your water. This is because water softening replaces a divalent ion (Ca+2 or Mg+2) with two monovalent ions (K+ or Na+). While this method
CANNAtalk|25
may be an appealing way to reduce the hardness of water, beware that additional sodium can be potentially undesirable as well. It would be wise to test your water after “softening” it.
Sediment filtration
A sediment filter may be needed if using water from an outdoor source (pond, lake, stream). This filter will remove particles, the size of which will depend on the filter type. However, the filters generally do not remove small, chemical ions.
Distillation
Distillation is another widely used, non-chemical method for water softening. Distilled water is boiled into vapor and condensed back into liquid in a separate container. Any of the solids that do not boil will remain in the original container. The simplest example of distilled water is a solar still which is a basic, survival method for purifying water. Distillation is often considered too expensive for large scale operation. However, the
process is worth the cost when it comes to alcohol production, where fermented liquids are distilled into liquor.
Assess your water for your system


There are many factors to consider when assessing water quality (hardness, contaminants, pH) and growers must do their due diligence in testing and getting to know their water. While there are many courses of action a grower can take to remediate or improve their water quality, ultimately the best water treatment will depend on the system in which you are growing. For more information on water quality recommendations per system, look to the article in this issue, ‘Pairing the ideal water treatment with your system.’ Always keep learning and enjoy your grow. •
26|CANNAtalk CANNARESEARCH
Figure 3: Change to ‘The EC value cannot tell you about the quality of your water. Sometimes hard water with a high EC is still fine to use for growing, while other water with the same EC could be bad or harmful to your plants because it contains too much of the wrong elements.’
Grower,s
Let’s put on our chemistry hats now and think back to the structure of an atom. Atoms are striving to make bonds so that their outer electron shell becomes complete, there’s no missing holes, they’ve all been filled in. A free radical is an atom or group of atoms that have one or more unpaired electrons. Radicals power through their environment on a mission to react, combine, or divide in order to pair up all of their electrons. They are necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc. Oxygen is a particularly reactive, highly oxidizing agent in part because it has two unpaired electrons in its outer shell. This makes oxygen highly susceptible to radical formation and ready to bond with another atom, receiving its electrons. In a human body, exposure to environmental toxins can cause oxygen to form as a free radical, which can then damage DNA and may play a role in the development of cancer. That’s why ‘antioxidants’ founds in blueberries and green tea are promoted for disease prevention.
Growers harness the radical power of oxygen to work as a disinfectant when using materials like hydrogen peroxide, bleach, calcium hypochlorite, and dissolved ozone. These materials are common within the arsenal of tools that many growers use. While oxidizers have their place within a grow operation, the role of keeping clean conditions in the first place cannot be overlooked. The primary pillar for disease management should be prevention. This is achieved by maintaining clean hand tools, stakes, floors, tables, and other equipment. The work crew must adhere to a strict hygiene protocol to prevent pathogens from houseplants and outside plants being brought in on their clothing, their friendly dog, their dirty gloves, etc. Oxidizers help achieve clean conditions, especially when used to sanitize a grow space in between production cycles. The common oxidizers are peroxide based, like hydrogen peroxide, and chlorine based, like bleach and calcium hypochlorite. Let’s take a closer look at how these materials work and how to use the power of oxygen while preventing damage to our beloved plants.
Hydrogen peroxide
Hydrogen peroxide (H2O2) is used in both field and equipment cleaning applications and works by means of an oxidation reaction that works against anything organic. This includes fungal and bacterial pathogens, but also, unfortunately, includes healthy plant matter as well. While this is a non-issue when using hydrogen peroxide in between grow cycles simply to clean equipment, it must be a consideration if choosing to use an oxidizer in systems with living plants. Be sure to weigh your options, consult the product manufacturer on appropriate rates to use, and monitor your grow to see that all is well. Similarly, ozone, while not properly a peroxide, works to disinfect when applied as dissolved ozone and is commonly used as a post-harvest application.
Bleach and calcium hypochlorite
Some growers do not realize that bleach is an oxidizer as well, working off the power of oxygen as a free radical. This oxidation power is what makes bleach a well-known, household disinfectant and goto equipment cleaner. Bleach is a strong base and by chemical definition is a dilute solution of sodium hypochlorite (NaClO). Bleach in water dissolves to hypochlorous acid and this weak acid works as an oxidizer, breaking down cell membranes. Similarly, calcium hypochlorite, which is commonly sold as a cleaner for pools, dissolves to hypochlorous acid and thereafter also works to break down cell membranes. Just like with hydrogen peroxide and any other oxidizer, these materials do not discriminate between your desired plants, beneficial microbes, and harmful pathogens. So, if using oxidizers during your growing period, be aware of this broad effect, balance the benefits vs drawbacks, use the appropriate rate, and closely monitor your production.
THE FREE RADICAL HARNESSING THE POWER OF OXYGEN oxygen
Maintaining clean growing conditions and managing pathogens is a task for which every grower needs to plan. The best way to make this plan is to truly understand the materials used in your grow system, their purpose, and the way they function. From there, it is up to the individual to decide what tradeoffs are suitable for them and what will work best. Good luck and happy gardening!
CANNAtalk|27 TIP
#40
CANNAtalk wouldn’t be complete without a good old Sudoku puzzle. Sit down, relax and train your brain for a moment. It’s not too difficult and you could win an awesome prize! Are you new to this kind of puzzle? Here’s what to do: each row, column and 3x3 grid must contain all the numbers between one and nine, once only.
You might be lucky this time! Another great prize is waiting for one of you. You just have to send us the correct solution (sending the middle part of the puzzle to editor@cannatalk.com and mention CANNAtalk 40.
If we pick your name, a bottle of CALMAG could be coming your way.




The winner of last Sudoku is Mrs. Sally D. Congrats on your 1 liter bottle of CALMAG!

We will contact you as soon as possible to make sure you receive your prize. Enjoy!




Sudoku Puzzle #7 LEVEL: Medium Answer rands, Inc. No other duplication permitted 2 1 5 7 3 2 4 3 9 5 6 4 5 9 1 5 8 2 9 1 6 8 7 5 3 4 3 5 7 4 1 2 9 8 6 4 6 8 3 5 9 2 1 7 1 4 2 9 7 3 6 5 8 5 7 6 2 4 8 3 9 1 8 3 9 5 6 1 7 4 2 6 1 4 7 3 5 8 2 9 9 8 3 1 2 6 4 7 5 7 2 5 8 9 4 1 6 3 &WIN! PUZZLE
&WIN
Puzzle
Winner puzzle#39
x 10 WIN A 1 LITER BOTTLE CALMAG AGENT?
28|CANNAtalk
KNOW YOU!
Plant biologists from Utrecht University working with German colleagues have discovered a detection system in some plants that can recognize a very wide and unrelated range of micro-organisms. These micro-organisms secrete what are known as NLP proteins, a very small piece of which is recognizable to the immune system of the Arabidopsis model plant.

Other plants, such as tomato and potato plants, are unable to recognize the NLP proteins. The next step in the research will therefore be to identify the NLP immune receptor in the Arabidopsis. Then more tests can be done into whether it is also active in other plant species, which could lead to the development of protection for agricultural crops against agents of disease.
NITROGEN SENSOR
WHEAT MYTHS ARE JUST THAT - MYTHS

A review undertaken by scientists at the University of Warwick of the current evidence on the dietary and health impact of consuming whole grain cereals finds that many of the myths attributed to wheatfree diets are just that - myths. They found that whole grains such as wheat are beneficial for the majority of people.
One of the researchers: “Apart from the two percent of the population who suffer from coeliac disease or other sensitivities such as wheat intolerance, there is overwhelming evidence that there are clear health benefits to eating a diet based on whole grains that features store cupboard staples such as bread and cereals made from lightly processed wheat. These benefits are even stronger when the whole grains have undergone relatively little processing.
Other than for the 2% of the population with a specific gluten or wheat intolerance, the scientific evidence behind many of the most popular wheat-free and carbohydrate-free diets is surprisingly thin and is used highly selectively. Some of these diets may result in short-term weight loss but the same results could be achieved in the long term by eating smaller amounts of the higher quality or relatively unprocessed foods.” We can only say: keep growing and eating wheat!
Nitrogen is one of the most important nutrients for plant growth. Scientists from the research group of Professor Karl Forchhammer at the Interfaculty Institute for Microbiology and Infectious Medicine in Tübingen, Germany, have investigated how plants keep track of their nitrogen supply. The researchers have discovered that plants possess a sophisticated glutamine sensor. Proteins known as PII-signalling proteins act as a ‘fuel gauge’ for the amount of available nitrogen by measuring the concentration of glutamine. This information is then used by the plants to regulate their growth with some precision.
“Whether the discovery of the nitrogen sensor will have a significant impact on plant breeding remains to be seen,” says Forchhammer. But the discovery has already taught us a lesson in evolution. The nitrogen sensor is an example of how new characteristics or capabilities can emerge on the basis of existing components. Chloroplasts were originally cyanobacteria that migrated into cells. These bacteria had PII-signalling proteins with no extension. In the plant, it became necessary to measure the availability of nitrogen for metabolic control directly, so the extension was added and the signal transmission was coupled to the glutamine level. This trait was so useful that it was passed on to all descendants in the plant kingdom and –with few exceptions – plants still have it to this day.
!
Photo courtesy of AgriLife Today
CANNAtalk|29 Facts
Photo courtesy of BlueRidgeKitties
WHAT , S CANNA Research Grow it Yourself Questions & Answers Factographic
CANNA
Is published by CANNA Continental,

Email: editor@cannatalk.com
Printed by: PIP printing
Contributors issue 40:
Marco Barneveld, Abha Gupta, George Hetfield, David Hill, Andres O´Hara, David Rosenberg and Mirjam Smit.
CANNAtalk doesn’t just write about nature, it is also committed to preserving our natural environment. Did you know, for example, that this paper comes from sustainably managed forests? And that your favorite magazine is printed in a carbon-neutral printworks?

30|CANNAtalk :
Talk
a
All editorial is copyright. All rights reserved. No part of this publication may be reproduced in any form without prior written permission of the publisher. The publisher is not responsible for any inaccuracies. Material which has been contributed does not necessarily reflect the opinion of the publisher. It is assumed that any image from widely distributed sources, such as the internet are in public domain although these images are often passed on between websites which makes it sometimes impossible to trace the original source. the
company dedicated to making
best solutions for growth and bloom. - Is distributed through CANNA dealers in the USA and on our website. Find the closest dealer near you through www.cannagardening.com.
Editors: Abha Gupta and Adrienne Kellam
MAGAZINE FOR SERIOUS GROWERS ISSUE 41
What’s Happening? Pests & Diseases Grower’s tip Puzzle & Win and more ...
The gods must be crazy

www.cannagardening.com







www.cannagardening.com CANNA CALMAG AGENT Calcium and Magnesium supplement for water conditioning.








































 By Abha Gupta, MS Horticulture, Assistant Horticulturist
By Abha Gupta, MS Horticulture, Assistant Horticulturist


















































































