Companion Quarterly
OFFICIAL NewsLetter OF the COmpANION A NIm AL veter INA r IAN s br ANC h OF the NzvA


Volume 34, No. 3 | September 2023
The importance of physical rehabilitation
Giardia infection in dogs and cats

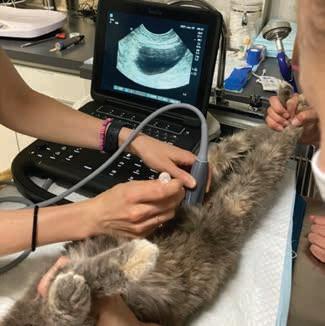
POCUS: ultrasoundguided procedures in cats
Neurological examination and neurolocalisation
Anaesthetic considerations for cardiac patients

E XECut IVE Comm I tt EE 2023 cav@vets.org.nz

President
Natalie Lloyd
Vice President
Becky Murphy
Secretary
Sally Aitken
treasurer
Kevanne McGlade
Committee members
Toni Anns
Shanaka Sarathchandra
Maddie Jardine
Nathan Wong
Head of Veterinary Services

Sally Cory
EDI to RIAL Comm I tt EE
Sarah Fowler (Editor)
Ian Millward
Juliet Matthews
Aurore Scordino
Shanaka Sarathchandra
Liz Foo
Address for submitting copy/ correspondence
Sarah Fowler
66 Callum Brae Drive, Rototuna, Hamilton 3210
T (H) 07 845 7455 | M 027 358 4674
E sarah.fowler@gmail.com
Advertising manager
Tony Leggett
NZ Farmlife m edia Ltd
Agribusiness Centre
8 Weld St, Feilding
T 027 4746 093
E tony.leggett@nzfarmlife.co.nz
NZVA website

www.nzva.org.nz
CAV website
www.nzva.org.nz/cav
Copyright
t he whole of the content of the Companion Quarterly is copyright, t he Companion Animal Veterinarians Branch of the NZVA (CAV) and t he New Zealand Veterinary Association (NZVA) Inc.


Cover credit
Genna Ackroyd's Cocker Spaniel t hompson enjoys a day out at the beach
Newsletter design and setting
Penny May
T 021-255-1140
E penfriend1163@gmail.com
Disclaimer
t he Companion Quarterly is a non peer reviewed publication. It is published by the Companion Animal Veterinarians Branch of the NZVA (CAV), a branch of the New Zealand Veterinary Association Incorporated (NZVA). t he views expressed in the articles and letters do not necessarily represent those of the editorial committee of the Companion Quarterly, the CAV executive, the NZVA, and neither CAV nor the editor endorses any products or services advertised. CAV is not the source of the information reproduced in this publication and has not independently verified the truth of the information. It does not accept legal responsibility for the truth or accuracy of the information contained herein. Neither CAV nor the editor accepts any liability whatsoever for the contents of this publication or for any consequences that may result from the use of any information contained herein or advice given herein. t he provision is intended to exclude CAV, NZVA, the editor and the staff from all liability whatsoever, including liability for negligence in the publication or reproduction of the materials set out herein.
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 1 CON teN t S 2 Editorial 6 Updates from CAV Executive Committee and NZVA Head of Veterinary Services (CA) 8 CAV Noticeboard 10 News in brief 14 What is your diagnosis? Connor Heap 16 WSAVA Regional Members Meeting Natalie Lloyd 18 Evolution of treatment of FIP in cats – an update Ryan Cattin 20 The importance of physical rehabilitation Nina Field 26 Giardia infection in cats and dogs Natalie Lloyd 34 Point-of-care ultrasound for cats: ultrasound-guided procedures Søren Boysen, Serge Chalhoub, Julie Menard 40 Neurological examination and neurolocalisation in cats and dogs Wen-Jie Yang 44 Cardio Corner: Anaesthetic considerations for the cardiac patient Megan Yeung, Keaton Morgan, Joana Chagas 48 Institute of Health Care Communications Train the Trainer Workshop Meg Irvine 49 Snippets from the NZVA 2023 Centenary Conference 50 ISFM Research Roundup 52 What is your diagnosis? The answers 56 Companion Animals NZ update 58 Healthy Pets NZ update 60 Committee biographies Companion Quarterly Helps you solve personal and work problems, including: Relationship problems Drug and alcohol issues Work issues Change Stress Grief 0508 664 981 24-Hour Freephone Confidential Counselling Service Vets in Stress Programme Volume 34 | No. 3 | September 2023 ISSN No. 2463-753X
20 34 26
Menopause – It’s everyone’s business
This month the NZVA hosted a webinar by Dr linda Dear on menopause. This is a condition that affects at least 50% of veterinarians. There is no doubt that my husband and kids will tell you it directly affected them, so it is likely a much greater number than 50% of us are impacted by this condition. CAV have talked about writing an article on this topic for a while, but with the excellent content provided by linda at her webinar in July, we thought it was a great opportunity to take some notes and share them here with those of you who missed it. Thank you to toni Anns for putting this together.
 Natalie lloyd, CAV President
Natalie lloyd, CAV President
Dr Linda Dear, mBBS BA(Hons) RNZCGP mNCP DRCoG, GP, certified menopause practitioner (NAmS) personal fitness trainer, Asthanga yoga teacher and psychology graduate/AC t therapist is highly qualified to inform on menopause! We were very grateful to have her agree to speak at an NZVA Zoom meeting on the 26 July 2023, a talk very well attended by close to 200 attendees. Her talk was engaging, easy to understand, practical and generated multiple questions. Here I share some notes and insights from listening on the night.
menopause in general is neglected. menopause is not just about women; it impacts everyone in some way and by 2030 it is estimated there will be 1.2 billion women experiencing menopause. menopause is inevitable, it affects us both physically and psychologically, and every woman experiences it at some point. It is not always a natural process and can happen with brutality overnight such as during cancer treatment. Whether it occurs naturally or is induced, it is not the same experience for everyone. It happens at different ages, and importantly it is often missed. t he changes associated with menopause can be blamed on other things and the impact that hormones may be having are often not considered. t his can be a
blind spot for GP doctors. At least 75% of women notice symptoms and in 25% of women the symptoms negatively impact their lives.
Definitions
t he formal definition of menopause is the point at which a woman has her final ever period. It is the ending of the fertile years, the loss of ovarian function including both eggs and hormones. Even though ovaries are tiny organs, they have big important jobs, but in menopause they decide to quit.
Perimenopause occurs when the ovaries are starting to shut down. Perimenopause can start in the late 30s and last for 4–8 years. In this stage, periods are still happening, hormones are fluctuating, and pregnancy is still possible. Egg production starts to change from age 35.
menopause is the day when the ovaries officially retire, however this time-point is only defined one whole year after the last period. t he average age of menopause is 51. If early menopause (under 45) occurs, treatment is recommended. For some women, menopausal symptoms can last for 10 years. Everything after menopause is post-menopause.
Signs of menopause
Determining when menopause is occurring is a challenge and GP doctors often find it difficult to diagnose. Perimenopausal periods can be regular or irregular, longer or shorter, heavier or lighter or less or more painful which may add to the confusion. Fatigue, mood changes, insomnia, body pains and headaches are common symptoms. Hot flushes can be particularly troubling and are thought to be due to changes in the hypothalamus. t hey can be embarrassing and distracting and really debilitating. However, 20% of women never have a single hot flush or night sweat.
o varies are more than fertility organs as they make both eggs and hormones (oestrogen, progesterone, and testosterone). o varian hormones conduct a lot of processes in the brain, heart, liver, bones, skin, joints and muscles, nerves, bladder, and vagina. With menopause, women lose the function of their gonads. t his is a huge physiological change and differs from men who don’t generally experience their gonads just falling off one day.
testosterone doesn’t get enough attention in women; it is often considered a male hormone, but in fact women make more testosterone than
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 2 eDI tOR IA l

oestrogen over their reproductive life. testosterone has many roles in the body with the main role being libido. It can affect mood, sleep, cognitive function, cardiovascular health, bone and muscle health and metabolism.
Progesterone prepares the body for pregnancy and supports bone function. Falling levels can affect fertility, menstruation, sleep, anxiety, bone density and headaches. taking micronised progesterone can help keep the uterine lining healthy, improve sleep, improve anxiety, lessen migraine attacks, reduce hot flushes, and may improve blood pressure. However, response may vary: either there is no effect, or you feel amazing, or you hate it.
t here are a multitude of symptoms associated with menopause and it’s often difficult to know if it’s due to hormonal changes or other disease/s, for example heart palpitations can occur due to changing hormone levels. menopause can mimic diseases such as fibromyalgia, depression/anxiety, stress, chronic fatigue, IBS, arthritis, thyroid issues, long CoVID, oSA, ADHD or lymphoma.
Hormone tests
Wouldn’t it be nice if there was a simple test to know where you are at? unfortunately, there is no test for perimenopause because you can’t measure ‘a mess’ and we are ‘too complicated to be just a number in our spit!’ Hormone tests are just a snapshot in time and with a wide ‘normal’ range, there is no ‘perfect’ hormone level to measure. t he receptors are where the action is, and we can’t measure at a receptor level.
t he concentration of FSH in blood may be checked in some circumstances, for example in women under 40 whose periods stop, women without a uterus, women not responding to hormone patches, or to rule out causes of other symptoms.
Health risks associated with menopause
After menopause women’s risk of heart attack goes up and there is an increased risk of high blood pressure. ‘Bad’ cholesterol goes up and ‘good’ cholesterol goes down. t he risk of cardiovascular disease is the same as men’s once women reach menopause, and then it increases after menopause.
It is thought that women with more severe flushes may be more at risk of cardiovascular disease. oestrogen helps coordinate the actions of insulin. With menopause comes increased body fat mostly around the abdomen. Cholesterol levels in blood and blood pressure may both increase. menopause increases the risk of osteoporosis and therefore bone fractures.
to counteract these changes in metabolism it is highly advised that women reduce stress levels, get a good sleep, eat well and regularly exercise, particularly strength-building exercise as strength and increased muscle mass increase metabolism.
Managing menopause
t here are lots of treatment choices: hormone replacement therapy (HRt ), supplements, non-HRt medications and herbal remedies. t hey all may have some benefits depending on what is right for each individual woman.
t he aim of HRt (more recently named ‘mHt ’, menopause hormone therapy) is to replace what your ovaries used to do with bio identical hormones. t here are more benefits than risks. Dermal patches are preferred over oral oestrogen supplementation because they have less risk of blood clots. t here is no time limit when taking HRt. HRt will probably not be prescribed for health benefits only; you must have some symptoms to deal with before it will be prescribed.
A study 20 years ago linked HRt and breast cancer. t his was the WHI Women’s Health Initiative clinical trial and was a study in older women with older treatments. t he study found an increase of six cases of breast cancer per 10,000 women over the age of 60 taking combined (oestrogen+progesterone) HRt. However, life expectancy was improved overall in women on HRt – in fact there was a reduction in death from any cause. t here is some research on HRt and dementia – it probably helps and it’s good for weak bones.
HRt (mHt ) should be a choice for all women. Don’t compare yourself to other women, everyone is different. Don’t suffer in silence, there is no prize for pushing through. Don’t fear HRt Learn about this life stage – read books, read websites, listen to podcasts.
Diet: menopausal bodies won’t put up with nonsense anymore, so eat a low sugar diet and avoid processed foods that your body doesn’t know what to do with. t he mediterranean diet is very good.
What about brain fog? Lifestyle changes: reduce stress and simplify life, don’t try and do too many things. Find time to meditate – this is powerful, it’s almost as good as sleep. Also, get a good sleep.
What can employers do? Employers can encourage learning and talking. t his is a concept for everyone to understand. Reduce stigmatisation.
Please seek medical advice if you have any questions regarding menopause or treatments, these notes are not intended as medical advice.
Useful websites
https://linkpop.com/menodoctor https://www.menopause.org/ https://www.menopause.org.au/ BVA menopause hub: https://www.bva. co.uk/take-action/good-veterinaryworkplaces/menopause-hub/menopausehub-support-for-you/
Toni Anns, CAV Executive Committee
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 4

WOR k INg tO PROMO te AND SUPPOR t COMPANION ANIMA l PRAC t ICe IN N e W Z e A l AND Activities of the CAV e xecutive Committee
Meeting highlights
t he CAV Committee met on 27 June at the NZVA offices in Wellington. t he Annual General meeting was conducted by Zoom following this meeting at 4.30pm. members of the committee then attended the NZVA Centenary Conference in Wellington 28–30 June and hosted the CAV Dinner on the evening of the 28th where Dr Nicholas Cave was presented with the CAV Service Award for his huge contribution to the profession (see page 10).
t he CAV Committee were also thrilled to see Dr Craig Irving awarded Honorary Life m embership of the NZVA, to a standing ovation, at the NZVA Gala Dinner. t his follows the nomination CAV submitted on behalf of the membership (see page 10). It has been a busy time preparing for these events and awards, with the committee putting in many volunteer hours of work to ensure companion animal veterinarians were recognised and well catered for during the 3 days.
Changes to the e xecutive Committee
At the AGm, the committee welcomed two enthusiastic new members: maddie Jardine and Nathan Wong (for profiles page 11), with Simon Clark and Nina Field stepping down. on behalf of the CAV membership we thank Nina and Simon for their many years of service to the CAV Executive Committee and we wish them well.
Continuing in their roles are: Kevanne mcGlade as treasurer, Sally Aitken as Secretary, and Simon Clark as CAV mAG representative, mAG Chair, and mAG representative on the NZVA Board. Simon Clark has stepped down officially from the committee but will continue to liaise with us as CAV mAG representative until his term as mAG Chair ends January 2024.
Recent activities
topics discussed most recently at CAV Executive Committee level include financial planning and consulting with an independent financial advisor on the best type of investment for the CAV Specified Fund, which has a balance of approximately $600,000. t he NZVA have been investing the specified funds of other SIBs into a Forsyth Barr managed fund, and CAV are investigating the best option for their fund, balancing returns with use promoting members’ interests. t he latter requires availability of at least some of the funds which affects how we invest. For example, a portion of this fund will be needed for the revamp of the Vet Refresher Scheme in the near future.
Sally Cory, NZVA Head of Veterinary Services-Companion Animals, has been introducing the committee to the NZVA’s Policy, Position Statement and technical Notes review system. t he plan is to systematically review these documents over the coming years. t his will require committee members to review and update the documents that members have access to via the NZVA website.
Hans Anderson and trish t horpe have reviewed NZVA’s Best Practice Guidelines, and this was discussed at a recent WSAVA regional meeting (South East Asia) on hospital standards in Bangkok, t hailand in June by CAV President Natalie Lloyd (see page 16).
Vet Refresher Scheme update
t he Vet Refresher Scheme revamp continues, with the NZVA Education team coordinating the work alongside a working group from the CAV committee. Course co-ordinators have been set for some of the courses and meetings with an instructional designer and a platform provider have taken place. t he refresher scheme is essential for supporting veterinarians who have been away from
practice and are looking to enter the workforce again, or those looking to change their area of practice. It is also important for overseas veterinarians to set context for the New Zealand work environment.
Sub-gingival dental procedure update
Following our presentation to the Regulations Review Committee (RRC; https://www.parliament.nz/en/pb/sc/ scl/regulations-review/) in June 2022, representatives of CAV, NZVA, and other stakeholders (VCNZ, New Zealand Veterinary Nursing Association, Allied Veterinary Professional Council) met with the Animal Welfare team at mPI to draft the new regulation. t he regulation was sent out for public consultation and shared with members via a Survey monkey survey in February– march 2023. t he draft regulation was strongly supported by our membership; feedback that was given to mPI. t he regulation has been passed on to the Associate minister of Agriculture (Animal Welfare), Jo Luxton, and at the time of writing we were waiting on an update as to whether this proposal will be passed on to cabinet prior to Parliament rising on 31 August 2023. CAV have been in touch with both mPI and the RRC (12 July) asking for an update and reiterating the need for urgency on progressing this regulation on behalf of our members.
The CAV Committee
l World Small Animal Veterinary Association
l World Small Animal Veterinary Association – Congress Steering Committee
l World Small Animal Veterinary Association – Hereditary Disease Committee
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 6
continue to represent vets on the following organisations
l Federation of Asian Small Animal Veterinary Association
l New Zealand Companion Animal trust
l Healthy Pets New Zealand
l Companion Animals New Zealand
l National Cat management Strategy Group
l Veterinary Council of New Zealand – Professional Standards Committee
l NZVA Policy Advisory Committee
Health-Focused Breeding Project
During 2023 the committee are working on a Health-Focused Breeding Project providing education for members in areas such as genetic diseases, testing and counselling, the university of Cambridge/Kennel Club Respiratory
Function Grading Scheme and general advice on decision-making when faced with breeding, whelping and neonatal care. Work on this project will start to become available to members later this year and include a dedicated issue of CQ.
Hill’s/CAV educating the educators grant
t he Educating the Educators
Award (sponsored by Hill’s Pet Nutrition and CAV) received four applications for the 2023 award, and at our June committee meeting $8,500 was awarded to the applicants. Further details will be announced once they have formally accepted their awards. CAV thanks Hill’s for their ongoing support of this award.

t he CAV Committee thank members for your continuing support of our special interest branch. We welcome your ideas
and feedback, so please feel free to contact us by email at any stage (cav@ vets.org.nz). NZVA and CAV are asked to represent veterinarians’ views to various stakeholder groups, which provides opportunities to members who may want to participate at a greater level. We welcome your interest and would also encourage you to participate through connecting with your Regional Networks and SIBs. l
NZVA Head of Veterinary Services (Companion Animals) update


Work over the last few months has involved progressing some key projects. Following great survey responses on both after-hours and parents returning to work, data obtained was collated and analysed to identify and prioritise the areas in which the NZVA can focus on in providing resources and guidance for both employers and employees. Data collected was also integral in preparing for sessions delivered on both these topics at last month’s conference. t hese sessions were well received, and some great discussions were generated. t his has provided further direction for this work.
t he NZVA and CAV have provided representation on a rewriting group for the Code of Welfare for Dogs. t his is the first step in the process prior to the code being submitted to NAWAC for review this year.
Another valuable piece of work has been the creation of a canine leptospirosis factsheet which is available to members (https://nzva.org.nz/resource/general/specific/). We are grateful to those who contributed to this document and hope that it provides a clear summary regarding what we currently know about canine leptospirosis in New Zealand.
Sally Cory, HoVS-CA
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 7
The CAV Noticeboard

Companion Quarterly – a call for contributions

Do you have a clinical case to share, need to tick off a task on your CPD plan or want to earn some pocket money?
CQ publishes case studies, clinical updates, reviews etc. on topics that are of interest to companion animal veterinarians. An award of $300 is paid for all published articles, with the chance to win the Best Article of the Issue and Best Article of the Year (thanks to tCI Glenbred).



Please send your contributions to the Editor at sarah.fowler@gmail.com

microsoft Word format is preferred and photographs/images are welcome, preferably 2 megapixels or higher, and sent as a separate attachment (rather than embedded within the Word document).
Healthy Pets NZ Project Grant 2023
Healthy Pets NZ is a charitable trust that acts as the research funding arm for CAV. Funding applications are invited in march and September for research projects that will enhance companion animal health and welfare.

See the Healthy Pets NZ website (www. healthypets.org.nz) to find out how we are

WINNER
Article of the Issue

supporting projects on analgesia for ovariohysterectomy, treatment of squamous cell carcinoma and FIV prevalence. Any queries on how to make an application or donate please email healthypetsnz@gmail.com.
June 2023 | Volume 34(2) | Pp 30–34
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 8
"Rat bait is not always an anticoagulant! Cholecalciferol rat bait poisoning in a dog."
Neil Stuttle
Craig Irving awarded Honorary life Membership of the NZVA

Craig Irving’s career started in 1969 after graduating with a Bachelor of Veterinary Science (BVSc) with Distinction. After graduation, Craig worked in a large animal practice before spending a year as a small animal intern at the university of melbourne animal hospital in Werribee. Craig gained credentials for the Royal Veterinary College Certificate in ophthalmology before travelling to Australia, the uK and the uS to gain experience. After this, he successfully took his Royal Veterinary College examinations. Craig proceeded to develop his ophthalmology practice with the purchase of necessary specialist equipment. t his allowed him to provide an essential and up-to-date service. Inherited eye disease was common, and Craig worked with breed clubs to help eradicate particular conditions by having successful eye clinics around New Zealand. His contribution was immeasurable for the effort and
knowledge he provided at the time, and he had a significant effect on improving canine welfare in New Zealand. Being based in Palmerston North, it was not long before Craig was asked to deliver ophthalmology lectures to students. He was an excellent lecturer who provided extensive notes and had a comprehensive library of slides to illustrate his lectures. most massey university
veterinary graduates have Craig to thank for their knowledge and skills with eye examinations.
A man with a unique style and charm, despite his infamous sense of humour, most veterinarians in New Zealand probably have a Craig story to tell. Craig’s generous sharing of his professional expertise is legendary.
t hroughout Craig’s 54 years of service, he has been a stalwart for the veterinary profession, providing exceptional service to the New Zealand Veterinary Association (NZVA) through dedication to his work, to ophthalmology, to teaching, and to raising the bar with professional standards.
Craig has dedicated his life to veterinary science and has been an active and respected member of the profession, which makes him a worthy recipient of an NZVA Life membership. l
Winner of CAV Service Award for 2023: Nick Cave
t he CAV Service Award is granted to someone who has gone above and beyond in their support of companion animal veterinarians in NZ. t his year’s recipient has worked tirelessly in his role helping to nurture and develop many of New Zealand’s veterinarians. He is internationally well respected and has earned New Zealand recognition on the global stage of companion animal health research. He has been a huge part of the fabric of massey university’s veterinary school where he is incredibly well liked by veterinary students and teaches with a passion.
Nick Cave graduated from massey university in 1991 as part of the class that spawned many academics (Wendi Roe, Janet Paterson-Kane, Susan tomlin, Stefan Smith and Andrew Worth) and private specialists (Andrea Ritmeester, Jenny Donald, Angus Fechney, Alexander macLachlan, Richard mcKee). After graduation Nick left massey for private mixed practice which was followed by a stint in small animal practice working alongside the late Frazer Allan and
todd Halsey in Hamilton. He joined the medicine team at massey’s Veterinary teaching Hospital as a resident under ex-Head-of-Institute Grant Guilford during a very productive period of gastroenterology-based clinical research. He then headed to the university of
California, Davis and completed a second residency and PhD in small animal nutrition before being invited in 2005 to come back to massey to join the medicine department as a Lecturer. on his return to massey Nick established a research programme in small animal immunology and nutrition, and created residency positions which have led to two further specialists in this field. During this time he has been intimately involved with the Feline Nutrition unit and Canine Research Colony at massey. He has been a huge part of the academic life of the BVSc degree at massey and supervised masters and doctoral students. many former students with be able to attest to how Nick enlivened their learning with his passion for teaching. Students always found him to be an entertaining and engaging teacher. A question was never answered with a simple yes/no but became a leap onto the ladder of further understanding of the complexities of veterinary medicine. He is always at his best with an audience, captivating and witty, and a favourite on the CPD circuit as well as with undergraduates.

Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 10
Ne WS IN BRI eF
[Photo credit: Mark Coote for the NZVA]
[Photo credit: Nick Cave]
Nick gives his time to support the academic and veterinary community. He is always willing to spend time discussing cases with colleagues and offering valuable advice. During the first Covid lockdown he was on call every evening and weekend for veterinary medicine emergencies. He did this without complaint as a service to the wider companion animal community and to his other colleagues at massey university Veterinary teaching Hospital. He also likes a bit of fun and hosted daily hospital staff zoom meetings during lockdown, instigating the Formal Fridays dress up zoom meetings for staff morale during this time.

Nick has recently resigned from his role as an Associate Professor at massey university and so we are looking forward to hearing
where life will take him next; hopefully he will continue to remain an active part of the veterinary community in New Zealand. to finish, here are some wise words that Nick shared with the graduating class of 2020 in Ihenga, that esteemed record of veterinary life, which we hope will provide an insight into how he has helped to shape the veterinarians of New Zealand in his capacity as a teacher……
“In the painfully stupid film, t he Phantom menace, Senator Palpatine says to Darth maul, ‘You have been well prepared, my young apprentice, they will be no match for you.’ Do you feel well prepared? or do you fear that, like Darth maul, you are about to be cleft in twain by obi Wan Kenobi's light sabre named ‘Lack of Experience’? If our
experiences with you in the clinic since lockdown are anything to go by, you are still pretty extraordinary. Perhaps it is testament to the work you put in during lockdown, or to what is still fundamentally a really good curriculum taught by people that care, or it is due to the value of the condensed experiences concocted for you at the end of the year, where lymph node aspirates on a dog become an exercise the whole family can enjoy. or perhaps, just perhaps, it is that old chestnut, that ultimately, you are all rather special human beings, whom many of us feel privileged to teach.”
(with thanks to Natalie Lloyd, Susan tomlin and Andrew Worth) l
Welcome to the new members of the CAV executive committee
Simon Clark and Nina Field have recently resigned from their positions on the CAV Executive Committee and we thank them for their efforts and input. Following the AGm in June, their places were taken up by two fresh faces, Nathan Wong and maddie Jardine. Below Nathan and maddie introduce themselves:
Auckland with my fiancé and three cats; Jeffrey, Jellybean and Juniper.
Maddie Jardine
Expressing feline anal gland contents into my face on a Friday evening during my first year of work was a life-changing experience. t he caustic sensation on my mucous membranes was unforgettable, and the indifference expressed by the owner of said felid left me yearning to understand how clients perceive us, our efforts, and our advice.
Nathan Wong
I am a 2014 massey university graduate and started out working in general practice for the first 6 years of my career. I've since transitioned to working at the SPCA (plus the occasional moonlight shift at afterhours clinics) and loving the different and rewarding nature of shelter medicine. I'm passionate about animal welfare and making the veterinary profession more sustainable for us all. I currently reside in
After almost nine years of clinical practise, I craved that thrill. Enter: CAV AGm 2022. I gently accosted the attendees about my frustrations surrounding the remuneration of support staff in our industry, and found a strong sense of shared frustration, and empathy. my hope is that by being a part of this committee, I can be a part of the growth this industry needs to embrace, and lend my voice and enthusiasm to such causes. I have a passion for communication, and I think it’s vital that we strive for better communication with ourselves, between ourselves, and between our industry and the public. I graduated from massey and have worked in various places around NZ and uK, including a stint as a teaching associate at Bristol Veterinary School. I work at Aldwins Road Vet Clinic in Christchurch and relish our team. I advocate for diversity, equity and environmental sustainability in our profession and am also a member of the NZVA Climate Champions crew. In semester two of 2023 I will be studying with te Wānanga o Raukawa towards Poupou Huia te Reo 2. l

Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 11
[Photo courtesy N. Wong]
[Photo courtesy M. Jardine]
WSAVA news: launch of Certificate in Pain Management
t he World Small Animal Veterinary Association (WSAVA) is to launch a Certificate in Pain management, supported by Zoetis. t he WSAVA Certificate in Pain management is based on the updated Global Guidelines for the Recognition, Assessment and treatment of Pain, unveiled by the WSAVA Global Pain Council (GPC) at the end of 2022. Despite rapid advances in pain management, the GPC is concerned that pain in companion animals is underdiagnosed and under-treated.
In offering this new certificate in conjunction with its Global Pain management Guidelines, the GPC aims to raise awareness of the importance of pain management for patient health and welfare and provide practical help
to veterinary professionals who wish to refresh or deepen their knowledge in this area.
Globally relevant and available online for ease of access, the WSAVA Certificate course comprises three modules, with tailored content for veterinarians and veterinary nurses/technicians, covering:

l understanding and assessing pain
l Preventing and treating pain
l Pain management in practice
Each module contains recorded lectures from members of the GPC and other global experts, together with links to mandatory and suggested reading materials. Quizzes are included at the end of each lecture. t he course is available free of charge to all companion
animal veterinarians and veterinary nurses/technicians.
t he WSAVA Certificate in Pain management is available in English and is being submitted for RACE accreditation. Interest in the Certificate can be registered here: https://wsava.org/pain-certificate/
t he WSAVA’s Global Guidelines for the Recognition, Assessment and treatment of Pain are available for free download from the WSAVA website in a range of languages. t he work of the Global Pain Council is generously supported by Zoetis. l
Would you like to see your pet on the cover of Companion Quarterly?
We now have a new cover photo for each issue of Companion Quarterly. This means we are always on the lookout for suitable photos. Photos selected for the cover must be landscape orientation (or able to be cropped to this), crisp and well focused, and of high resolution (at least 300 DPI). They must also be well composed and interesting.
Please send any suitable images to the Editor (sarah.fowler@gmail.com). If however you have a favourite snap of your furfamily that’s not quite up to cover standards, please send that in too: photos that are not selected for the cover may be used elsewhere within the magazine..

Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 12

What is your diagnosis?
t He QU eSt IONS…
CONNOR He AP, BVSc, 2023 Zoetis Intern, Veterinary Specialists Auckland

Case history:
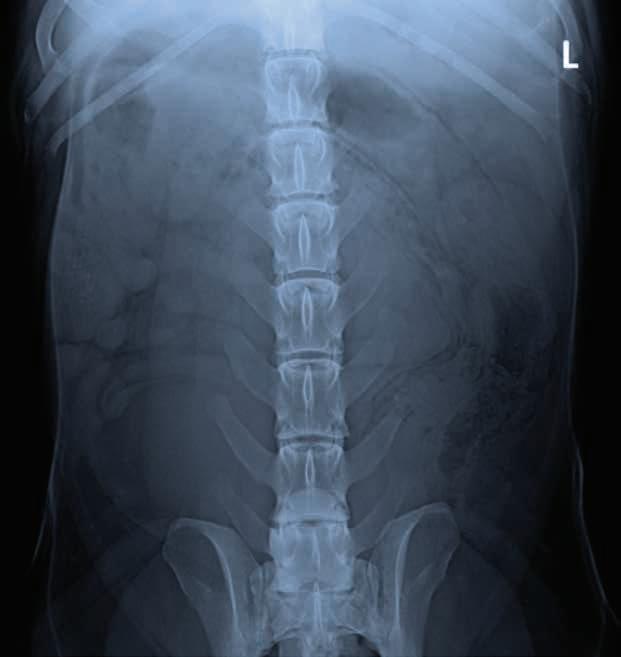
A 3-year-old, male neutered Labrador Retriever presented for chronic diarrhoea. t he patient had a history of inflammatory bowel disease and had undergone multiple previous surgeries for foreign body removal. A colopexy had also been previously performed. t he patient had completed a course of metronidazole on the day of presentation and was also receiving vitamin B12, psyllium husk (metamucil) and probiotics. He was up to date with de-worming treatment and core vaccinations. A three-view abdominal radiograph series was taken as part of the diagnostic workup, and two cropped projections are provided below (Figure 1).
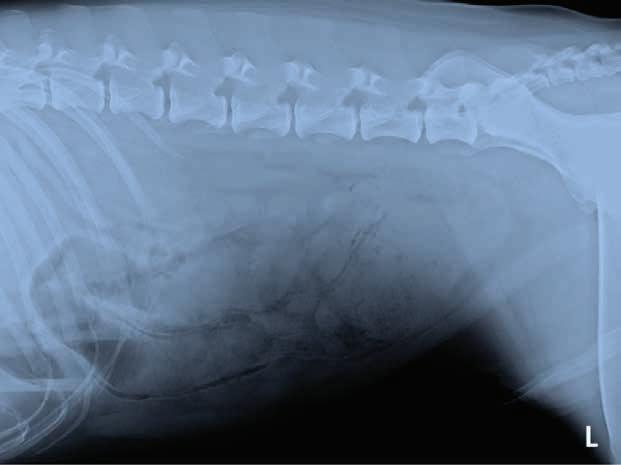
Questions
1. What are your radiographic findings?
2. What are your radiographic differentials?
3. What would be your next steps?
Answers on page 52
Contact: connor.heap@vsnz.co.nz
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 14
Figure 1. l eft lateral (a) and ventrodorsal (b) abdonimal radiographics of a dog with chronic diarrhoea.
a b

CAV UPDAte
WSAVA Regional Members Meeting
NAtA l I e l l Oy D, CAV president
Background
t he WSAVA represents a global veterinary community of over 125 countries. During a guided interview on Needs and Capacity Assessment carried out in 2019, the top guidelines project determined from our membership worldwide was Animal Hospital Standards/Veterinary Practice Management to create guidelines that can be relevant to our global community a more in-depth discussion to understand challenges, obstacles and opportunities and input needed to be collated from our membership. It was therefore the proposed topic for the Asia Regional members’ Forum that I attended earlier this year in Bangkok. t his event was hosted by the Veterinary Practitioners Association of t hailand (VPAt ) Regional Veterinary Congress.
Purpose
l to identify needs and obstacles/challenges for animal hospital/veterinary practice standards
l to find out the vision that WSAVA membership has for a guidelines framework
l Exchange information on standards and accreditation process currently used worldwide and within each country
June 12–14 2023 Bangkok, Thailand Programme
l Collate information and resources for a potential guidelines working group
Attendees
l WSAVA Executive Board representatives including the current WSAVA President, Vice President, Immediate Past President and Executive Board members.
l Representatives from countries with a scheme for animal hospital standards already in place (New Zealand, Australia, united States, t hailand)
l Representatives from WSAVA member associations and or local organisations (including but not limited to Singapore, Philippines, China, India, malaysia, Vietnam, Cambodia).
Contact: CAV@vets.org.nz
Morning session – All member representatives were asked to invite members who were present at the VPAt Regional Veterinary Congress to attend this session and contribute to the discussions. t he session started with presentations from organisations currently running animal hospital standards schemes (i.e. AAHA (united States), ASAV (Australia) and tAHSA ( t hailand)) followed by sharing from other countries. t he ensuing discussion part was a very vibrant and open session that allowed concepts and concerns to be included in the afternoon members’ forum.
Afternoon session – member’s Forum was open only to WSAVA member representatives. t he session was facilitated by Dr. t hitirat Chaimee (Zoetis t hailand)

Discussion
t he afternoon session was a workshop style session covering a range of topics which have been summarised into the main points below.
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 16
[Photo courtesty of the author]
Benefits of developing guidelines:
l Establishing benchmarks for high quality practice
l Better animal welfare
l Improve standards of care
l Improve working environment
l Enhancement of workplace health and safety conditions
l motivates and empowers the entire veterinary team
l Financial benefit
Identification of groups or individuals who benefit from the development of guidelines
l Veterinarians
l Pets and pet caregivers
l Associations
l Government
l Improves social status of vets
l General public
Obstacles to the development of guidelines were identified as being
l money, financial investment
l Awareness, acceptance, opinion, mindset
l Human resource and time
l Assessor objectivity, transparency, governance
l Government, though this factor was determined to not be within the scope of our influence
t he session concluded with a discussion of what members felt was WSAVA’s role in the development of these guidelines or standards.
Broadly, it was decided that WSAVA should look to initiate the development of a set of minimum clinical standards. It may be that many of these standards already exist in the form of current WSAVA guidelines. WSAVA may also be able to provide a central repository for storage of this information for member associations.
t he broad and variable range of requirements identified across different organisations means that WSAVA are more likely to develop a set of minimal clinical standards rather than guidelines as such. t hese standards may well then serve as a platform for each country to base their own scheme upon.
The New Zealand perspective
I found this a very valuable conversation to be part of. New Zealand is ahead of many countries with respects to having a well-established set of hospital guidelines (Best Practice) in place. Recently the Best Practice team have completed a thorough review of the guidelines, and a personal review of these was part of my preparation for this meeting. I was interested to note that New Zealand and Australia have a similar number of practices accredited (approximately 80–90) despite a marked difference in number of clinics in each country.
t here are some notable differences in the structure and verification component of the schemes between the countries. For example, New Zealand has quite a unique approach
to verification and assessment of practices, and Australia has a unique case based assessment component. As far as I am aware, from the countries that were present at the meeting, New Zealand is the only country to have a wellbeing component built into their scheme.
these differences provide learning opportunities for those who currently have schemes to refine and improve, as well as providing insight to those countries looking to set something up.

t here does seem to be a trend that in countries where the standard of practice is reasonably high, uptake for the schemes has plateaued. on the other hand, in countries where the service being provided by veterinarians is markedly variable, there was a strong demand for standards or guidelines. t he activity of the local regulator (and indeed whether there even is a regulator in a country) plays a significant role in this.
However, the immense benefits of hospital or clinical standards for the whole veterinary team were widely agreed upon, and ongoing development and support of some type of standards in any country was something that the group wholly supported.
Where to from here?
A taskforce was put together which New Zealand will be part of to further discuss the outcomes of the workshop. t his group will meet again in Lisbon at the WSAVA Annual Congress in September 2023. one of the first topics that the taskforce will look to develop will be to understand if the need for these standards is limited to members in South East Asia, or whether this is something required by the wider WSAVA membership.
I look forward to updating members on my return from this meeting. l
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 17
[Photo courtesty of the author]
Cl INICA l UPDAte evolution of treatment of FIP in cats – an update

RyAN CAtt IN, BVSc (dist) MANZCVS FANZCVS
Feline infectious peritonitis (FIP) is a common infectious disease of cats. t his disease occurs when the feline enteric coronavirus acquires a mutation which allows multiplication within macrophages. Historically the marked inflammatory response that ensues was a life-ending disease.
Fortunately, there are now two nucleoside analogues available for cats in New Zealand that appear highly effective, Remdesivir and molnupiravir. Nucleoside analogues are similar molecules to the ‘true’ nucleosides that are the building blocks of DNA and RNA. Because of their structural similarities, they interfere with key enzymes in DNA or RNA replication, or are integrated into DNA or RNA strands, leading to chain termination or non-functional nucleic acids. one of the limiting factors is these analogues are not only used by viral enzymes, but also by mammalian enzymes, which largely accounts for their potential toxicity.
t he treatment of FIP has evolved significantly from the initial reports of success by Peterson et al. in 2019. t his initial study investigated the use of GS-441524 in 31 cats with FIP. o verall, 80% of these cats survived long term. Four of the nonsurviving cats were initially severely affected, and were either euthanised, or passed away in the initial days of the treatment regime. t he treatment regime in this study involved daily subcutaneous injections for 12 weeks. A small proportion of cats required repeat protocols following relapse. Considering this disease was almost universally fatal, long-term survival in 80% of cats is remarkable.
GS-441524 has never been available as a legal treatment option in cats. t he publication from Peterson et al. (2019) resulted in the establishment of a black market for GS-441524 around the world. t his medication became surprisingly easy to order online, getting through the border disguised as cosmetics. As a result of this widespread use, there are some large studies emerging on treatment outcomes. Jones et al. (2021) published the results of an online survey of outcomes of almost 400 cats. t here are some obvious limitations of an online survey, however the results still provide some useful insights. only 8.7% of owners reported receiving significant help from their veterinarian – no doubt due the legalities of this. Almost 90% of the owners reported noticing improvement after 1 week. 12.7% of cats experienced a relapse, with only 3.3% of cats dying despite treatment.
As GS-441524 is not able to be obtained legally in New Zealand, its use cannot be supported. t his is especially the case now
Contact: Ryan.Cattin@arcvets.co.nz
legal options are available that are substantially cheaper than the black-market options. Remdesivir is a prodrug of GS441524, that we now know is rapidly metabolised into GS441542 in cats, so other than different dose rates they are very similar medications.
As noted above, there are large numbers of cats that have been treated without veterinary support. t his, and the everchanging research provides opportunities for vets to help cats in the future. Veterinarians in New Zealand need to re-take a central role in treating FIP as our knowledge and skills will help to ensure more cats are treated and survive long term. I think the main way we can do this as a profession is to keep up with the current literature and treatment protocols. As a result of these past events, there are large social media groups where owners are proving advice of variable accuracy. t here are frequent comments by owners that their vet did not know about FIP treatment, which of course drives the owners to seek information elsewhere. While these groups are a great source of support, the treatment information provided by other
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 18
Ollie, one of the adorable cats treated at the Animal Referral Centre [Photo courtesy of the author]
owners is often out of date or incorrect. I hope that some of the insights below will help to start some more conversations.
the severity of the cat at presentation seems to be the biggest factor influencing survival with treatment. overall, around 85% of treated cats will survive. If the cat survives the first week of treatment, this survival rate increases to over 95%. In a rapidly progressive disease, clearly early diagnosis and prompt treatment are key. there are some very useful recent guidelines on how to reach a clinical diagnosis of FIP ( thayer et al. 2022). unfortunately, there is inevitably a delay in reaching a clinical diagnosis while awaiting histology, or a PCR (of effusion for wet FIP, or CSF for dry FIP). We routinely start treatment when we have a very strong index of suspicion of FIP while awaiting the results of the diagnostic tests. treatment trials without diagnostic tests should be discouraged if possible as the development of resistance of these vital medications is a big concern. there has not yet been much investigation into the mechanisms of resistance development, but we do see it already. A small proportion of cats appear to be resistant to medications from the onset. more often we suspect resistance develops during treatment which can be partial requiring a higher dose, or complete. We have anecdotally observed a cluster of resistant cats from one location with dubious use of remdesivir which has raised some alarm bells.
treatment protocols have changed significantly with time. Initially we started parenteral remdesivir in every cat as the oral absorption was presumed to be poor. Subcutaneous injections at home for 12 weeks were a big challenge for most owners, especially as the solution is acidic, so stings when administered. t he recommended starting dose has crept up with time as remdesivir was shown to be safe. A study from uC Davis recently investigated the absorption of oral remdesivir, and it was found to be around 40–50% (Cook et al. 2022). So, the transition to oral doses has necessitated a doubling of the administered dose. unfortunately, under-dosing remains a very big problem with both out of date doses administered, and parenteral doses given orally. t here is concern this may lead to resistance to treatment.
t he Animal Referral Centre is currently offering two main treatment options for cats with FIP; starting with 2 weeks of injections, followed by 10 weeks of oral capsules, or oral capsules alone. It is unknown whether any parenteral treatment is needed. t he pharmacokinetics of remdesivir has only been studied in healthy cats. We strongly suspect that sick cats, especially with effusive disease, will have altered absorption of oral medications. We are currently evaluating the pharmacokinetics and treatment outcomes of these two groups. molnupiravir closely resembles GS- 441524, but is an analog of cytidine rather than adenine. t here are no published comparative studies comparing the efficacy of remdesivir compared to molnupiravir. t he optimal dose of molnupiraivir remains unknown. t his medication has a narrower safety margin compared to remdesivir with higher doses seem more likely to result in a severe leukopenia and other adverse effects. t here is currently one study evaluating the use of molnupiravir in cats that noted broadly similar success rates and relapse rates to those published with remdesivir in 30 cats that had failed remdesivir (Roy et al. 2022). Remdesivir/ GS-441542 has been in use for a longer period of time resulting in substantially more published literature, so this remains our current initial treatment of course. Fortunately, the costs of treatment have fallen dramatically recently, especially with the use of oral capsules. Per capsule, the cost of remdesivir is currently very similar to molnupiravir. As molnupiravir is usually a twice-daily medication, a treatment protocol is typically more expensive at the current prices. more information will come in the future, with comparative studies underway. However, with the current information available molnupiravir is probably best reserved for treating cats that have failed remdesivir.
monitoring during treatment is vital to help determine which cats need an increased dose, or duration of treatment. t his is one of the areas veterinarians can help most. one of the secondary aims of our research is to evaluate “milestones” that should be met during the treatment, so these can be used to help predict the response to treatment and make early adjustments. We routinely monitor for
resolution of the clinical signs, effusions, and biochemical markers at roughly monthly intervals during and after treatment.
Cats with dry/neurologic/ocular FIP remain a challenge, both for reaching a diagnosis, and for monitoring treatment. Dry cases tend to have much less dramatic (and often no) biochemical changes and will often retain some mild-moderate neurologic deficits even when cured which presents a challenge. We need a high index of suspicion for these cases to ensure they are promptly diagnosed. Fortunately, there is a relatively limited list of common differential diagnosis for neurologic signs in this signalment (i.e. young often purebred cats), with FIP being by far the most common diagnosis.
It is very difficult to keep up with the rapidly changing literature, but we are here to help! t he Animal Referral Centre is offering free phone consultation to veterinarians in New Zealand to help provide the most up to date treatment advice as part of our studies, so please do not hesitate to get in touch.
References
Cook S, Wittenburg L, Yan VC, Theil JH, Castillo D, Reagan KL, Williams S, Pham CD, Li C, Muller FL, Murphy BG. An optimized bioassay for screening combined anticoronaviral compounds for efficacy against feline infectious peritonitis virus with pharmacokinetic analyses of gS-441524, remdesivir, and molnupiravir in Cats. Viruses 14, 2429, 2022
Jones S, Novicoff W, Nadeau J, Evans S. Unlicensed gS-441524-like antiviral therapy can be effective for at-home treatment of feline infectious peritonitis. Animals 11, 2257, 2021
Pedersen NC, Perron M, Bannasch M, Montgomery E, Murakami E, Liepnieks M, Liu H. efficacy and safety of the nucleoside analog gS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. Journal of Feline Medicine and Surgery 21, 271–81, 2019
Roy M, Jacque N, Novicoff W, Li E, Negash R, Evans SJ. Unlicensed Molnupiravir is an effective rescue treatment following failure of unlicensed gS-441524-like therapy for cats with suspected feline infectious peritonitis. Pathogens 11, 1209, 2022
Thayer V, Gogolski S, Felten S, Hartmann K, Kennedy M, Olah GA. 2022 AAFP/ everyCat feline infectious peritonitis diagnosis guidelines. Journal of Feline Medicine and Surgery 24, 905–33, 2022 l
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 19
The importance of physical rehabilitation
N INA F I el D BVSc CCRt
We all want to live our best lives, physically and mentally, and most pet guardians want the same for their animals even if they are unaware of how to provide it! Whether the pet is a lieon-the-couch companion, an essential assistant dog or a “vital” staff member, their owners generally want the best for them or the best financial outcome after a surgery to promptly restore a dog back to work.
Because of human nature, motivation will differ and the ability to complete an entire physical rehabilitation programme to regain 100% function is rare, as human physiotherapists will attest to! However, this shouldn’t stop veterinarians from recommending a rehabilitation programme because even a halfcompleted rehab programme is better than nothing.
A rehabilitation programme aims to restore the patient to normal function, or as near to normal function as possible, after a health issue. t hese health issues could be an injury, surgery, illness, or just general aging. It is a physiotherapy programme but the reason I refer to this as rehabilitation is because only people trained through a human physiotherapy programme can call themselves physiotherapists. Veterinarians providing these services should have completed a post-graduate training course and will refer to themselves as Veterinary Rehabilitation t herapists. most veterinary surgical specialist centres in New Zealand will offer rehab programmes from their premises and there are other private providers dotted about the country.
Reasons to recommend a rehabilitation programme t he most common reasons that an animal is referred to me by another veterinarian are: post-surgical recover, an injury for which surgery is not an option, or lame/sore/unbalanced without a clear diagnosis. Some owners whose dogs perform agility will bring these dogs to me for maintenance therapy or because they themselves are unhappy with the way their dog is moving. o thers are looking to help their arthritic dogs be more comfortable.
The first visit on the first visit I’ll get a verbal history from the owner and watch the animal moving. You can assess how physically comfortable a dog is by their posture, whether they continue to stand in a consult room or lie down. How they sit and how quickly they move from sit to stand will give you an indication of pain or weakness. It is amazing how few dogs will walk or trot next to their owners in a straight line but usually after a few attempts with some circling and changes of direction, an assessment can be made on their gait.
t hen I perform a physical examination including orthopaedic and neurological examination depending on the issue. treatment begins with the examination as I’m usually massaging as I go. my idea is the dog relaxes and gets used to my touch. What I love about these cases is the ‘incidental’ things that can be found especially in older animals. If you are a 7-year-old, active dog that has ruptured a cranial cruciate ligament, chances are you also have some stiffness in your spine and maybe a front leg. t his is especially true if you have been lame for a while before receiving surgery or visiting the vet in the first place. t his is also a good time to discuss weight control, pain relief, diet supplements, home modifications and other health issues. t his holistic approach is a great way to educate owners on providing their pets with management they may need for the rest of their lives.
Alongside the examination with the massage comes other manual therapies such as joint manipulation and muscle stretching and modalities to help with inflammation and healing (see Box 1).
Box 1. Manual therapies and treatment modalities used for rehabilitation
Manual therapies and treatment modalities
Notes
Joint manipulation t his encourages the joint through a normal range of motion. t his is often restricted by joint injury or surgery, muscle shortening or tightness. Joint mobilisation helps to stretches the joint capsule or ligament, allowing a good range of motion.
Stretching
Increases the flexibility of the muscles, allowing the fibres to realign for better healing and allowing the joints to have a good range of motion.
Laser Helps with inflammation and healing. uses light energy to stimulate tissues. Red light is absorbed by mitochondria and this increases At P production. Physiologically this results in a decrease in inflammation, oedema and pain and an increase in wound healing. t herapeutic ultrasound Sound waves that cause microscopic air bubbles that stimulate cell membranes and promote healing.
Contact: ninathevet@gmail.com
Believed to stimulate the central nervous system to release chemicals into the muscles, spinal cord and brain. t hese biochemical changes stimulate the body's natural healing abilities. Neuromuscular electrical stimulation. (NmES, E-Stim) used to preserve or recover muscle mass, particularly in totally immobilised patients.
Acupuncture
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 20
Cl INICA l UPDAte

Therapeutic exercise
t hen it’s time for some therapeutic exercise. A limb either needs strengthening, or to maintain its strength whilst the injured area heals. t he animal still needs to move to toilet etc., so this needs to be in a controlled fashion so the injury is not exacerbated, or the surgical site compromised. t he mental health of the patient (and the owner) also needs consideration – crate resting a 12-year-old dog is much easier than a dog of 12 months!
Exercises suggested will depend on the time since surgery, the age and overall mobility of the animal and also what the owner wants to achieve. I usually send my clients home with three exercises to be performed daily or 2–3 times per day. Following surgery the animal is usually standing, or being supported to do so and the exercise might be just putting a small amount of weight on a limb for a few seconds. t his will be repeated 2–3 times and maybe again later in the day. Small and often is usually the key. I will see the patient again in 1–2 weeks and normally increase the difficulty or the duration of the exercises. Increasing difficulty might be adding a different surface (that is less stable) or more weight by, for example taking two legs off the ground (see Figure 1).
So a home exercise programme might look something like this; massage spine and both hind limbs. Perform range of motion of all hindlimb joints, starting with toes up to the hip. t hen the three exercises: e.g. front legs raised (this increases the weight taken by the rear end), 3-leg stance (one limb is taken off the ground to make the other three hold more weight) and proper sit (Figure 2a). For a proper sit the stifles should be over the feet – nice and straight rather than slouched to the side (Figure 2b). t his is often due to a stifle issue: the dog doesn’t want to flex the straight stifle as it’s uncomfortable – watch for this with cruciate disease. t his is often something you may notice initially in the consult room whilst the dog is moseying about whilst you talk to the owner.


It always amazes me how everyone adapts to the new regime. o wners that are invested in their animal’s future and are fully engaged get good results. I guess those that aren’t I don’t see again and maybe the pet does well enough. Pets have been recovering from surgery and injury for many decades without specific rehabilitation but with it the results are better and/or faster. Gone are the days when the dog is confined to a kennel for 6 weeks……arrgggg! t his is not necessary and can, in fact, be deleterious as the owner will often let the dog out to toilet and let it run about madly for a few moments undoing days of healing, if not causing damage to implants or healing tissues.
A home exercise programme can require a fair amount of commitment from the owner but I will try and make life for all as easy as possible by finding ways to make it work with the facilities and environment available. Where there’s a will, there is a way. o ften floors need to be made less slippery with, for example, carpet mats over lino or tiles for post operative patients or geriatric dogs. Ramps may need to put over steps and harnesses required to help the owner support a large dog down steps or into a vehicle. A crate so the dog is restricted when not supervised makes life a lot easier for everyone. A harness is a much easier way to control the dog on a lead so it walks slowly using all four limbs.
My rehabilitation practice
At present I offer rehabilitation services from the veterinary practice I do GP work at. I have some equipment which is brought out as necessary but it is also a good idea to be creative with what you have around you: for example, I have the street outside with its curbs, plus a set of six concrete steps across the road. Perfect! Hills are not plentiful in Ashburton but there are a few slopes that count for ‘hill’ work, especially in the initial stages and many irrigation ponds for later hydrotherapy! Garden stakes on squashed coke cans can be used for caveletti work and inflatable camping mattresses for ‘wobbly’ surfaces.
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 22
a
Figure 1. e xamples of therapeutic exercises. [Image courtesy of the author]
b

Dogs may be a bit anxious initially, but I get a real buzz from the waggy tails that great me on the second or third visit, and of course seeing the progress that is made from visit to visit. With acute injury or post-surgery cases, I may see them 2–3 times in the first 2 weeks to get the ball rolling so to speak, but then it’s generally every 2 weeks. t his is mainly due to my availability (vetting is busy – anyone else noticed!) and also the client’s time and finances. Geriatric/maintenance treatments are 4–8 weeks apart.
I encourage you to find your closest rehab provider and refer patients to them. Your clients will thank you and this will increase their bond with you and everyone will see improvement in outcomes. Even with one visit the client will be given tools and strategies to help their pet.

Recommended reading
WI Baltzer. Rehabilitation of companion animals following orthopaedic surgery. New Zealand Veterinary Journal 68, 157–67, 2020, https://doi.org/10.1080/00480169.2020.1722271 l



Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 24
Figure 2. good sit (a) and bad sit (b) [Image courtesy of the author]
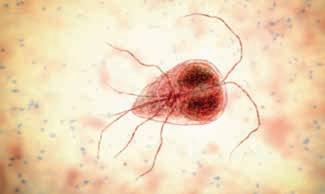
Cl INICA l UPDAte Giardia infection in cats and dogs
NAtA l I e l l Oy D, BVSc, MANZCVS (Medicine of Cats), Companion Animal Veterinary Adviser, Zoetis New Zealand
Introduction
Giardia duodenalis (syn G.lamblia, G.intestinalis; (henceforth called Giardia) is a gastrointestinal parasite of mammals found worldwide (uiterwijk et al. 2019). Global prevalence rates of Giardia are between 5 and 15% for dogs and cats (Carlin et al. 2006). However, there is high heterogeneity between populations. Young animals were more likely to be infected than older animals and symptomatic cats and dogs are more likely to be infected than those that are asymptomatic.
t he average annual incidence of Giardia in people living in New Zealand is one of the highest among the industrialised countries (Canterbury DHB 2022). t here is a dearth of published information however, regarding prevalence of infection in dogs and cats in New Zealand. A study from 1991 reported prevalence rates of 3–25% among asymptomatic dogs and cats from Palmerston North and Hamilton (Brown et al. 1991). In addition to this data, a New Zealand reference laboratory has published figures of 25% (canine) and 13% (feline) positive Giardia tests among faecal samples submitted to the lab for diarrhoea panels (Idexx 2019).
u p-to-date published data for cats and dogs is needed to provide New Zealand practitioners with accurate prevalence information on Giardia to help inform their approach to symptomatic animals. t he purpose of this article is to provide practitioners with a summary of the current recommendations on zoonotic potential, diagnosis and treatment of this parasite in New Zealand dogs and cats.
Zoonotic potential
Giardia duodenalis comprises eight molecularly identified genotype strains or “assemblages” designated A through H. While the specific assemblages that cats and dogs are infected with (C,D and F) are often different from that which infects human (A and B), assemblage A and B have been seen in both cats and dogs hence there is a small potential for zoonotic transmission between humans and dogs and cats (Ballweber et al. 2010).
An infected human can be a source of infection to a cat and dog which then, in turn, may represent a zoonotic risk. While
the zoonotic risk is low, people in contact with infected pets should consult their doctor if they have relevant clinical signs.
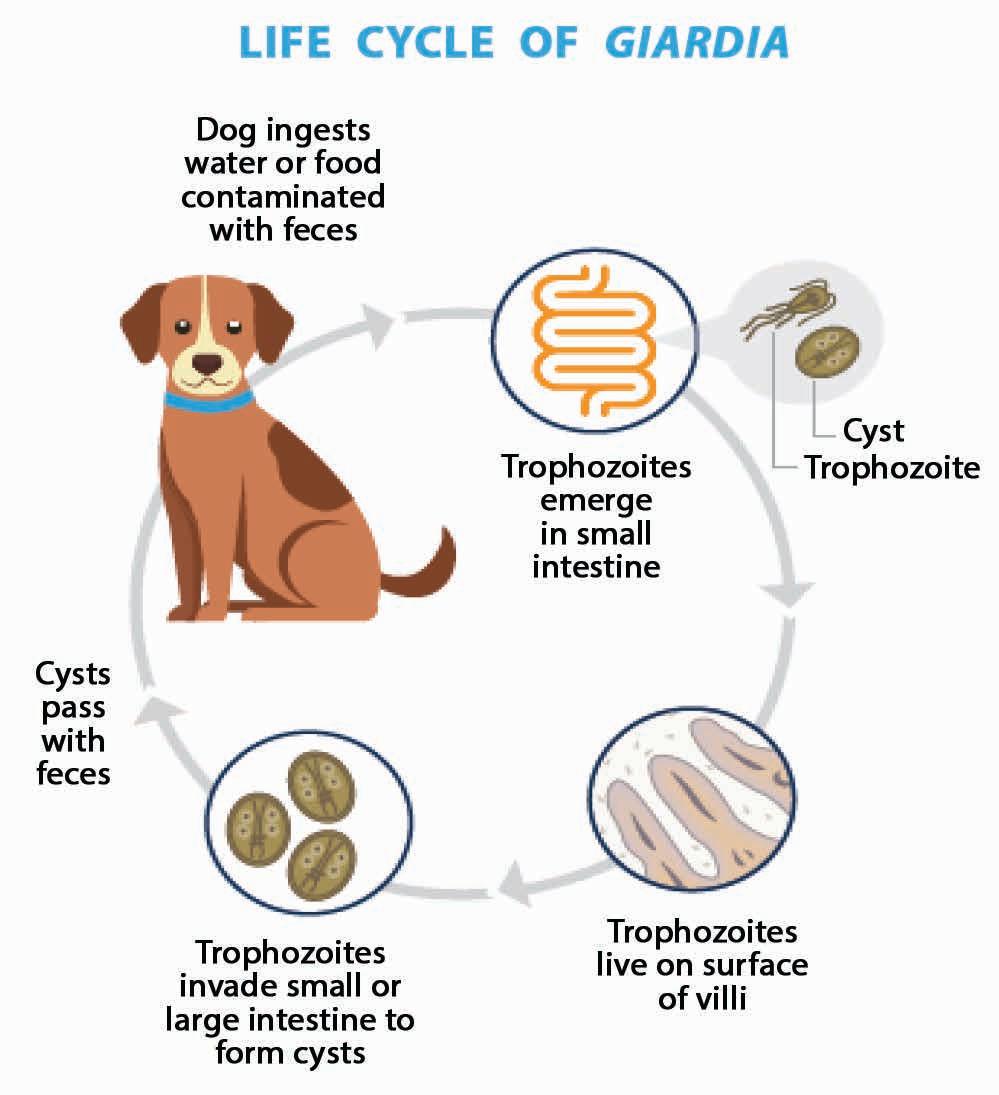
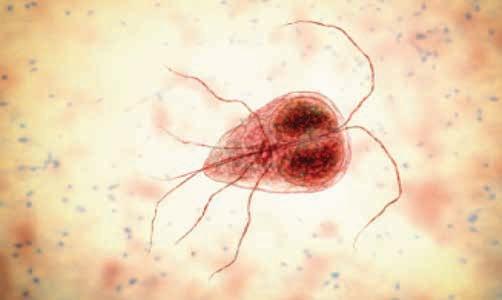
life cycle
Giardia exists in two stages described below (Nagamori and Penn 2021). See Figure 1 for an illustration of the lifecycle. Trophozoite: t his is the motile stage in the small intestine. trophozoites are usually 12–17 μm x 7–10 μm in size. t hey are motile, flagellated organisms that originate from cysts and appear teardrop or pearshaped. trophozoites are bilaterally symmetrical and have two nuclei, each
Contact: natalie.lloyd@zoetis.com
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 26
Figure 1. life cycle of Giardia (Nagamori and Penn 2021) [Image courtesy of Dr. yoko Nagamori]

with a large endosome. t hey also have a pair of transverse, dark-staining median bodies. trophozoites inhabit the mucosal surfaces of the small intestine where they attach to the brush border, absorb nutrients and multiply by binary fission. t hey usually live in the proximal portion of the small intestine.
Giardia can also be underdiagnosed, as cysts may be shed intermittently and can deteriorate in faecal solutions very quickly, making detection and identification more difficult. Given these complexities, there is no easy, quick test with 100% accuracy when it comes to diagnosing a Giardia infection, but there are many tests that can be used in conjunction to detect its presence (Saleh et al 2019; CAPC 2019).
Veterinary patients may be asymptomatic and infected with Giardia. Interpretation of a positive test in an animal with diarrhoea requires careful assessment of the animal’s history, signalment and physical exam in order to understand the significance of the test result and avoid interpreting a positive result incorrectly.
When should we be testing for Giardia?
Veterinarians should test cats or dogs for Giardia when they show symptoms such as chronic or intermittent diarrhoea (CAPC 2019). Patients at higher risk for Giardia infection, such as in contact, young animals or those at higher risk of contracting Giardiasis due to lifestyle, may benefit from Giardia screening at routine wellness examinations (Calvin et al. 2006; uiterwijk et al. 2019).
testing methods for Giardia
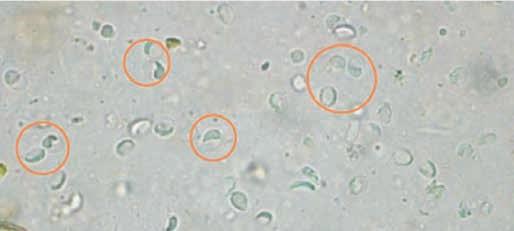
Cyst: t his is the infective stage responsible for environmental contamination trophozoites invade the small or large intestine to form cysts. Cysts are ellipsoidal, nonmotile and contain 2–4 nuclei with long- and short-curved rods. t hey are 9–13 μm x 7–9 μm in size and possess a thick refractile wall (see Figure 3 above). Newly formed cysts pass in the faeces. Cyst shedding may be continual over several days and weeks but is often intermittent, especially in the chronic phase of infection. Cysts can survive for several weeks to months in the environment, whereas trophozoites cannot.
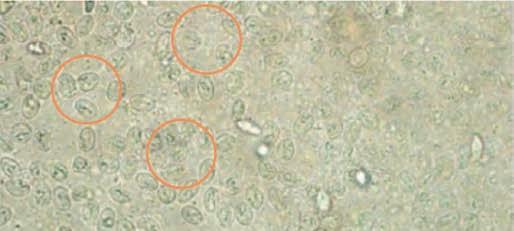
Clinical signs
upon infection, dogs and cats may be asymptomatic or present with symptoms such as weight loss and diarrhoea. Diarrhoea may be continuous or intermittent and is seen more commonly in puppies and kittens. Where the pet is symptomatic, faeces are usually soft, poorly formed, pale, malodorous, contain mucous and appear “fatty”. Watery diarrhoea is unusual in uncomplicated cases and blood is usually not present in faeces. occasionally vomiting occurs. minimum database clinical laboratory findings are usually normal (Calvin et al. 2006).
Diagnosis testing for Giardia presents challenges
It can be challenging to correctly diagnose a Giardia infection, and in fact, Giardia can be both over and under diagnosed. Giardia can easily be mistaken for many other objects (pseudoparasites) within the faeces such as yeast, that can lead to potential overdiagnosis.
t here are a number of different testing methods available to diagnose Giardiasis in symptomatic animals based on direct observation of the organism in faeces, or detection by ELISA or PCR. testing methods can be used alone, or in combination.
In one study, combination of faecal floatation with a commercially available Giardia spp. antigen assay had a combined sensitivity of 97.8% (mekaru et al. 2007)
multiple tests performed over several (usually alternating) days may be necessary to identify infection because of the possibility of intermittent shedding of cysts (Calvin et al. 2006; CAPC 2019; ESCCAP 2019; Guffydd-Jones et al. 2013).
testing for a broader range of parasites by faecal floatation with centrifugation using both 33% zinc sulfate solution and sugar solutions may also be warranted at wellness visits in patients who are asymptomatic but have high-risk factors for giardiasis (Zajac et al. 2012; CAPC 2019). See table 1 and Figure 3 below for comparison between floatation methods.
1. Faecal examination
Faecal examination is simple, quick and inexpensive. It remains one of the most important procedures to perform when evaluating gastrointestinal disorders in pets (matz and Guilford 2003). Despite this, routine faecal testing is not widely practiced amongst companion animal practitioners in New Zealand. Barriers cited to performing in-house faecal examinations are time constraints (for sample preparation and evaluation), reluctance to handle patient’s faeces, and concerns around accuracy in technical interpretation of samples. For this reason, Giardia infections in New Zealand are most often diagnosed at external
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 28
Figure 2. 3-D illustration of trophozoite (Nagamori and Penn 2021) [Image courtesy of Dr. yoko Nagamori]
Figure 3. Use of 33% ZnSO4 for faecal floatation improves detection of Giardia compared to sugar solution [Image courtesy of Dr. yoko Nagamori]
Giardia cysts in sugar solution
Giardia cysts in 33% ZnSO4 solution
reference laboratories. Diagnosis at the point-of-care can provide a quick, reliable and inexpensive option but is often overlooked.
a. Detection of Giardia by a direct faecal smear (Gruffydd-Jones et al. 2013)
l mix a small amount of fresh (<30 minutes old) faeces with a drop of saline (not water) on a slide to make a suspension, place coverslip and examine under a microscope at a magnification of 100x and 400x.
l motile trophozoites can be seen showing a “falling leaf” motion
b. Detection of Giardia using zinc sulfate centrifugal floatation (CAPC 2019; Gruffydd-Jones et al. 2013; t RoCAP n.d.)
l test of choice for the visualisation of Giardia cysts in solid/semisolid faeces.
l Solutions concentrate and separate intestinal parasite eggs and cysts from faecal debris.
l 33% ZnSo4 solution (SG =1.018) is recommended for examination for Giardia cysts.
l Avoid use of saturated salt or sucrose solutions as they may cause distortion of the cysts.
l Cysts are oval, 10–12 µm long and surrounded by a thin wall (Figure 3).
c. Artificial Intelligence at the point of care
In clinic diagnostic platforms (such as Vetscan Imagyst; Zoetis, Auckland, NZ) are now available to practitioners in New Zealand. t hese platforms can help to overcome some of the recognised barriers to in house faecal testing through their clean and userfriendly sample preparation processes and accurate interpretation of results (Nagamori et al. 2020, 2021).
Vetscan Imagyst uses an automatic scanner and cloud-based, deep learning algorithms to locate, classify and identify parasite eggs and cysts including Giardia found on faecal microscope slides. Vetscan Imagyst sample preparation is comparable to reference methods and showed good sensitivity and specificity across different parasites tested when compared to experts (parasitologist) review (Nagamori et al. 2021). Both sugar and ZnSo4 solutions are available to clinicians to provide a complete diagnostic evaluation of most common internal parasites of cats and dogs (i.e. Toxocara, Toxascaris, Trichuris,
33% ZnSo4 1.18 Floats common helminth and protozoa eggs and cysts
Preferred for Giardia
Sheather’s sugar solution 1.25 Floats common helminth and protozoa eggs and cysts
Causes less damage to parasite eggs and cysts than salt solutions
table
Less effective for floatation of tapeworm eggs than others
Does not float some fluke and unusual tapeworm and nematode eggs
Less sensitive than 33% Zn So4 for Giardia
Creates sticky surfaces
Drug Route Dosage Fenbendazole oral 50 mg/kg once daily for 3–5 days
Pyrantel+praziquantel+febantel oral 56 mg/kg (based on febantel component) once daily for 3 days metronidazole benzoatea oral 10–25 mg/kg once or twice daily for 7 days
Furazolidoneb (cats only) oral 4 mg/kg twice daily for 7–10 days
a Neurological toxicity may develop following either chronic therapy or acute high doses b Furazolidone causes inappetence and vomiting
Ancylostoma, Taenia, Cystoisospora and Giardia spp.) (Nagamori et al. 2020, 2021).
2. Faecal ELISA Test (Giardia antigen)
Identification of Giardia in faeces using an ELISA may be performed in-house or at an external reference laboratory.
l Giardia ELISA assays are approved and commercially available for patientside testing in dogs and cats (e.g. SNAP Giardia test, IDEXX Laboratories, Hamilton, NZ; Witness Giardia Antigen testkit, Zoetis; Vetscan Giardia Rapid test (canine only), Zoetis).
l ELISA techniques do not appear to be more sensitive than careful faecal screening (Gruffydd-Jones et al. 2013).
l t hese tests are helpful to use alongside faecal floatation to eliminate false negatives due to shedding of low numbers of cysts ( t RoCCAP 2019).
l Positive faecal ELISA results should be interpreted in relation to clinical presentation as many clinically healthy dogs and cats will test positive but do not require treatment (ESCCAP 2019).
l ELISA tests may remain positive even after treatment for variable periods of time and should not be used as a guide to determine reinfection or failure of treatment (CAPC 2019).
3. PCR testing for Giardia
PCR testing is performed on faeces at an external reference laboratory. PCR testing is available in New Zealand at IDEXX laboratories.
l t hese tests amplify Giardia DNA in faecal material.
l DNA sequencing can allow determination of Giardia assemblages.
l Some PCR methods can identify assemblages which may provide information about zoonotic potential of infection.
l Faeces may contain PCR inhibitors affecting amplification of Giardia genetic material.
treatment of Giardia in cats and dogs
See table 2 for recommended treatment regime for dogs and/or cats (GruffydJones et al. 2013; CAPC 2019; ESCCAP 2019;
t RoCCAP 2019). Please note:
No treatment is required for asymptomatic animals that test positive unless testing is part of an overall control programme in breeding establishments, kennels or shelters.
environmental control
Environmental control is also important in managing Giardia infections in cats and dogs as large numbers of cysts can be intermittently shed for long periods of time.
l Dog and cat faeces should be disposed of promptly and caregivers’ hands should be washed immediately after contact with any faeces from an infected pet.
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 30
Floatation solution Specific gravity Advantages Disadvantages
table 1. Advantages and disadvantages of solutions for faecal floatation (Zajac et al. 2012)
2. Protocols for treatment of giardiasis in symptomatic dogs and cats

l Pets should be shampooed regularly to remove faecal material.
l Clipping the hair of long-haired cats around the perianal area and the tail and legs may help to prevent reinfection from self-grooming (Little 2018).
l Environmental areas (e.g., soil, grass, standing water) can be difficult to decontaminate, but surfaces can be sanitised by steam-cleaning or use of commercially available disinfectants. Allow surfaces to dry thoroughly after cleaning (CAPC 2019).
l Pet lifestyle habits may need management until the infection is resolved – particularly for those dogs that visit dog parks or swim in areas frequented by other dogs (Little 2018).
Duration of treatment
Follow-up testing after diagnosis and treatment is very important, particularly if symptoms have not been resolved after the conclusion of therapy. testing may be conducted 24–48 hours after the completion of therapy (CAPC 2019) and can be accomplished with centrifugal faecal floatation with ZnSo4; if centrifugation is negative and the animal seems to be healthy, no further testing is needed (Carlin et al. 2006; CAPC 2019).
It is important to remember that Giardia antigen tests may provide a false-positive result in an animal that is no longer infected with Giardia as antigen excretion may persist for several weeks after treatment (CAPC 2019; t RoCCAP n.d.).
Conclusion
Giardia is a common parasite infecting cats and dogs globally. t here is a need for new research to better understand the prevalence of the infection in New Zealand pets. t he risk of zoonotic spread to humans is minimal, but clinicians should be aware of the low potential, particularly in households with immune suppressed individuals.
Diagnostic testing for Giardia should be performed in at-risk or symptomatic patients and a combination of testing methods frequently proves to be the most successful way to identify infection with the parasite. treatment is usually successful using therapies that are readily available to practitioners but should also involve environmental management and follow up testing to ensure the pet has fully recovered.
References
Ballweber LR, Xiao L, Bowman DD, Kahn G, Cama VA. giardiasis in dogs and cats: update on epidemiology and public health significance. Trends in Parasitology 26, 180–9, 2010 Brown TJ, Brown TJ, Ionas G. giardia infection of cats and dogs in New Zealand. New Zealand Veterinary Journal 39, 33–4, 1991, doi:10.1080/00480169.1991.35654 Canterbury DHB. Communicable Diseases Protocol – Giardia . https://intel.cph. co.nz/media/47410/giardiasis-protocol. pdf (accessed 22 July 2023). Canterbury District Health Board, Christchurch, NZ, 2022
Carlin EP, Bowman DD, Scarlett JM, Garrett J, Lorentzen L. Prevalence of giardia in symptomatic dogs and cats throughout the United States as determined by the IDe XX SNAP giardia test. Veterinary Therapeutics 7, 199–206, 2006
CAPC. Guidelines: Giardia – Dog and Cat. https://capcvet.org/guidelines/giardia (accessed 22 October 2019), Companion Animal Parasite Council, USA, 2019 ESCCAP. Giardia infection in dogs and cats https://www.esccap.org/uploads/docs/ aos09363_1008_ eSCCAP_giardia_ Fact_Sheet__ english_v2.pdf (accessed 1 August 2023). european Scientific Council Companion Animal Parasites, Malvern, U k , 2019
Gruffydd-Jones T, Addie D, Belák S, Boucraut-Baralon C, Egberink H, Frymus T, Hartmann K, Hosie MJ, Lloret A, Lutz H, et al giardiasis in cats: ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery 15 650–2, 2013 doi:10.1177/1098612X13489232
Idexx. New Zealand and Australian RealPCRTM Results Indicate CoInfections with Multiple Pathogens are Common in Companion Animals. https:// www.idexx.co.nz/files/RealPCRdiagnostic-update-co-infection-anz. pdf.(accessed 22 July 2023). IDe XX l aboratories Pty. ltd., Hamilton, NZ, 2019
Little SE. How I treat Giardiasis. www.vin. com/members/cms/project/defaultadv1. aspx?pid=22915&catId=&id=8896559& said=&meta=&authorid=&preview= World Small Animal Veterinary Association Congress Proceedings, 2018 Matz ME, Guilford WG. l aboratory procedures for the diagnosis of gastrointestinal tract diseases of dogs and cats. New Zealand Veterinary Journal 51, 292–301, 2003
Mekaru SR, Marks SL, Felley AJ, Chouicha N, Kass PH. Comparison of direct immunofluorescence, immunoassays, and fecal flotation for detection of Cryptosporidium spp. and Giardia spp. in naturally exposed cats in 4 Northern California animal shelters. Journal of Veterinary Internal Medicine 21, 959–5, 2007
Nagamori Y, Hall Sedlak R, DeRosa A, Pullins A, Cree T, Loenser M, Larson BS, Smith RB, Goldstein R. evaluation of the V et SCAN
IMAgyS t: an in-clinic canine and feline fecal parasite detection system integrated with a deep learning algorithm. Parasites and Vectors 13, 346, 2020, doi:10.1186/s13071-020-04215-x.
Nagamori Y, Penn C. Accurate Testing and Diagnosis of Giardia . https://www. vetscanimagyst.com/assets/pdf/ kOlWhite-Paper.pdf (accessed 22 July 2023).
Zoetis ll C, Parsippany, NJ, USA, 2021
Nagamori Y, Sedlak RH, DeRosa A, Pullins A, Cree T, Loenser M, Larson BS, Smith RB, Penn C, Goldstein R. Further evaluation and validation of the V et SCAN
IMAgyS t: in-clinic feline and canine fecal parasite detection system integrated with a deep learning algorithm. Parasites and Vectors 14, 89, 2021, doi.10.1186/ s13071-021-04591-y
Sahatchai T, Scorza V. Update on the diagnosis and management of Giardia spp infections in dogs and cats. Topics in Companion Animal Medicine 25, 155–62, 2010, doi:10.1053/j.tcam.2010.07.003
Saleh MN, Heptinstall JR, Johnson EM, Ballweber LR, Lindsay DS, Werre S, Herbein JF, Zajac AM. Comparison of diagnostic techniques for detection of Giardia duodenalis in dogs and cats. Journal of Veterinary Internal Medicine 33, 1272–7, 2019, doi:10.1111/jvim.15491.
Tangtrongsup S, Scorza V. Update on the diagnosis and management of Giardia spp infections in dogs and cats. Topics in Companion Animal Medicine 25, 155–62, 2010, doi:10.1053/j.tcam.2010.07.003
TroCCAP. Giardia . www.troccap.com/ canine-guidelines/gastrointestinalparasites/canine-giardia/ (accessed 22 July 2023). tropical Council for Companion Animal Parasites, n.d. TroCCAP. Guidelines for the Diagnosis, Treatment and Control of Feline Endoparasites in the Tropics. https://www. troccap.com/2017press/wp-content/ uploads/2019/06/ troCCAP_Feline_ endo_guidelines_ english_Ver2.pdf (accessed 22 July 2023). tropical Council for Companion Animal Parasites, 2019 Uiterwijk M, Nijsee R, Kooyman F. Host factors associated with Giardia duodenalis infections in dogs across multiple diagnostic tests. Parasites and Vectors 12, 556, 2019, doi:10.1186/s13071-0193810-3.
Zajac AM, Conboy GA, Greiner EC, et al Fecal examination for the diagnosis of parasitism. In: Zajac AM, Conboy gA, (eds). Veterinary Clinical Parasitology. 8th edtn. Wiley-Blackwell;, Chichester, U k , 2012 l
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 32

Fe At UR e AR t ICle
Point-of-care ultrasound for cats: ultrasound-guided procedures
Veterinary point-of-care ultrasound (POCUS) is a rapid, clinically- and often binary question-driven diagnostic modality that serves as an extension of the physical examination, particularly in triage settings. It can be used to quickly identify abdominal, pleural and pericardial effusions, pneumothorax, lung parenchymal diseases and several abdominal pathologies including generalised ileus and ureteral obstructions. This is the second of two articles from iCatCare discussing the use of POCUS in cats. The first article described POCUS techniques of assessment of the pleural space and lung and can be found in the March 2023 issue of Companion Quarterly.
R eN
Veterinary point-of-care ultrasound (PoCuS) is a real-time diagnostic modality, performed by attending personnel in conjunction with the clinical examination to answer specific clinically-driven (often binary) questions (e.g., is the vessel thrombosed?) or to guide interventions (e.g., centesis, vascular guided access). Its advantages lie in bringing the ultrasound machine to the patient without disrupting the triage examination, stabilisation efforts or the physical examination (mcmurray et al 2016).
t he use of ultrasound-guided procedures by veterinarians is gaining popularity in multiple clinical settings. Although not well documented in veterinary medicine, using ultrasound during standard procedures has been shown to increase patient safety, save time, and improve chances of success and accuracy in multiple human studies. In this article ultrasound-guided interventions will be discussed, with an emphasis on ultrasound-guided centesis, blood sampling and IV catheter placement, all techniques that can be life-saving in the emergency and critical care setting.
Contact: ellen.marcinkiewicz@icatcare.org
Probe axis and orientation
t he choice of probe axis orientation depends on the operator’s experience and preferences, the anatomic structures to be assessed/aspirated and the surrounding structures. Vessels and other elongated structures can be visualised in long- or short-axis planes.
In the short-axis orientation, the ultrasound probe is placed transverse and perpendicular to the structure of interest and swept along it. For example, if vessels are assessed in short-axis orientation the vessel appears as a circular area of hypoechoic to anechoic blood within the ultrasound image, which the probe is swept along as the needle or catheter is advanced.
In the long-axis view, the ultrasound transducer is placed along the course of the vessel, which produces an elongated, hypoechoic to anechoic structure, the probe is kept stationary over the vessel and the needle or catheter is ultrasonographically guided into the structure.

Non-elongated structures such as the urinary bladder, gall bladder, masses, lymph nodes, and so on, are typically assessed and sampled in long-axis orientation. Recall that ultrasound probes have an indicator marker on one side of the transducer. t his indicator marker is often a dot, light or a raised line, and corresponds with the orientation marker on the ultrasound screen. By convention, for most ultrasound-guided procedures, this
Box 1. Machine settings and functions
many ultrasound-guided centesis procedures can be performed with a microconvex probe. In cats, because of the superficial nature of many structures and fluid accumulations, a linear probe can also be used. For ultrasound-guided vascular access and blood sampling techniques, a linear probe (Figure 1) improves visualisation of small superficial vessels compared with a microconvex probe. It has a higher frequency than microconvex probes and provides better resolution of superficial structures.
central marker is located in the centre of the probe which facilitates orientation to where the needle will appear in the ultrasound image when using out-ofplane ultrasound guided techniques. This central marker should not be confused with the indicator marker of the probe, which is always located on one side of the probe. Many ultrasound machines also have a central marker on the corresponding ultrasound image, which aligns with the central marker on the probe. If the needle is advanced under the central marker of the probe using outof-plane techniques, the needle tip will appear as a white dot directly below the central marker on the ultrasound image. (Image courtesy of Dr J Menard)
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 34
Sø
BOySeN, DVM DACV eCC, SeRge CHA l HOUB, DVM DACVIM (SAIM), J U l I e MeNARD, DVM DACV eCC
a b
Figure 1. (a and b) linear probe. (b) A
indicator marker should be directed towards the patient’s right side when using short-axis views, or toward the patient’s head when using long-axis views. t he corresponding orientation marker on the ultrasound screen should be set to the upper left side of the image.
Ultrasound-guided procedures
Cystocentesis
Equipment required
l 20–22 gauge, 1–2 inch hypodermic needles;
l red (plain) topped tubes for sterile fluid collection if samples are to be sent for analysis;
l 3 or 6 mL syringes;
l ultrasound machine and appropriate ultrasound probe.
Patient positioning
t he cat is placed in lateral or dorsal recumbency. t he fur is parted over the caudal ventral aspect of the abdomen, at the expected site centesis is to be performed, and alcohol applied to the skin surface.

Probe orientation using a longitudinal orientation to the body (long axis to the bladder) and with the indicator marker directed cranially towards the head, the probe is placed on the skin surface and manipulated with sweeping and sliding motions until the urinary bladder becomes visible. Fanning and rocking motions are then used to centre the urinary bladder within the ultrasound image. too much pressure applied to the probe tends to compress the urinary bladder and should be avoided. However, gentle pressure may be required to displace small intestines from the ultrasonographic field of view.
Procedure
l using the non-dominant hand, place the probe perpendicular to the skin with the ultrasound indicator marker directed cranially (Figure 2).
l With the dominant hand and keeping the needle in plane to the ultrasound probe, angle the needle 45–60° to the skin (depending on the depth and size of the urinary bladder) and 0.5 cm cranial to the side of the ultrasound probe that contains the indicator marker. t his creates a 30–45° angle between the probe and the needle
Figure 2.
The probe is placed in a longitudinal orientation over the urinary bladder, so that the bladder fills the centre of the screen. The needle is placed cranial to the marker side of the probe at a 45° angle and advanced in real time until the needle is visible within the lumen of the urinary bladder.
insertion site of the skin. Keep the eyes focused on the needle insertion site until the needle tip fully enters the skin (i.e., the bevel of the needle is no longer visible), then redirect the eye focus back to the ultrasound screen. t his will decrease the chances of accidently damaging the probe surface as the needle tip is well situated below the skin surface before advancing the needle cranially below the probe.
l If the needle is difficult to visualise, rocking the probe slightly (without changing the angle of the needle) to create an angle closer to 90° between the needle and probe will often improve needle visualisation.
l using real-time ultrasound-guided assistance, the tip of the needle can be visualised as it passes through the SC tissues and into the peritoneal cavity.
l Look for an indentation of the peritoneal lining, which helps locate the needle tip before it enters the peritoneal cavity.
ideally placed in line with the probe and the operator’s line of vision to allow easy focus between the probe skin interface, needle tip and ultrasound image without the operator having to turn or lift their head. Shaving is not necessary. Alcohol is used as a coupling agent. The probe is in longitudinal orientation to the body and bladder with the marker (visible as a lighter grey line) directed cranially. The depth has been decreased to maximise the size of the bladder (which is centred in the ultrasound image) and to allow the needle to be more easily tracked in real time. The needle is situated at a 45° angle to the patient and the probe has been rocked slightly cranially (roughly 35° in this case), which creates an angle of approximately 80° between the probe and the needle. (Image courtesy of Dr J Menard)
(Figure 3). t he needle should be orientated such that the bevel of the needle is directed upwards towards the ultrasound probe surface. t he bevel of the needle is rough where it is cut and therefore reflects the most ultrasound beams, making it more visible than the shaft of the needle.
l t he needle should not be placed too close to the probe as it may damage the probe.
l once the urinary bladder is centred in the ultrasound image, focus attention on the probe and needle
l Failure to identify the needle ultrasonographically usually indicates the needle is not directly in plane with the ultrasound beams; the ultrasound probe should be fanned or swept slightly to either side of the needle to try and visualise it. Rapid movement of the needle by advancing and retracting it a couple of mm may also help localise the needle by creating tissue movement in proximity to the needle.
l track the trajectory of the needle tip until it traverses the urinary bladder wall and is lying within the lumen of the urinary bladder.
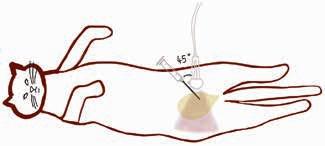
l Again, looking for an indentation of the bladder wall helps locate the needle tip before it enters the urinary bladder.
l If the bladder is very small, the tip of the needle may bounce off the bladder wall and a small ‘jab’ may be required to traverse the bladder wall. Avoid losing sight of the needle tip when ‘jabbing’ or there is a risk of traversing the distal bladder wall.
l once the tip of the needle is confirmed within the urinary bladder, gentle negative pressure should be applied to the syringe. t he presence of urine in the needle hub and syringe confirms proper location of the needle tip.
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 35
Schematic image of ultrasoundguided cystocentesis.
(Image courtesy of Dr J Menard)
Figure 3. A cat placed in dorsal recumbency for cystocentesis. The ultrasound machine is
l Release negative pressure on the syringe before withdrawing the needle from the cat.
l t he probe can be released if both hands are needed to aspirate the syringe prior to its removal.
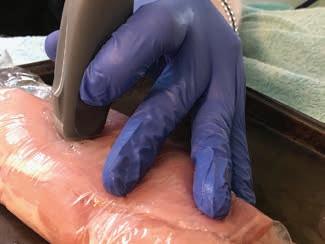

Abdominocentesis
ultrasound guidance is very helpful when performing abdominocentesis as it allows visualisation of the needle, ensuring fluid can be collected with confidence and with fewer complications.

Equipment required
l 20–22 gauge, 1–2 inch hypodermic needles;
l 3 or 6 mL syringes (an IV extension set, 3-way stopcock and larger 10–60 mL syringes are helpful if large volumes of abdominal fluid are to be removed);
l clippers, antimicrobial scrub and solution (if a sterile sample is to be collected);
l red (plain) and lavender (EDtA) topped tubes for sterile fluid collection and analysis;
l sterile culturettes if sepsis is suspected;
l ultrasound machine and appropriate ultrasound probe.
Patient positioning
Abdominocentesis may be performed with the cat in left or right lateral recumbency, dorsal recumbency (if stable) or standing (if unstable), depending on patient comfort and clinician preference. typically, cats are placed in lateral recumbency. Some sedation is generally beneficial to calm the patient, decrease the need for restraint and lessen the effort of breathing in respiratory compromised patients. t he fur is parted over the expected centesis site, which is usually the gravity dependent abdominal region, the largest pocket of fluid is identified on PoCuS and alcohol applied to the skin surface.
Probe orientation using a longitudinal or short-axis orientation to the body, depending on where the largest pocket of fluid is located and so as to avoid any intraabdominal organs/structures, the probe is placed on the skin surface and manipulated with sweeping, sliding, fanning, rocking and rotating motions until the largest abdominal fluid pocket
a
Figure 4. The afternoon tea technique being demonstrated on a chicken phantom vascular access model. (a) The ultrasound probe is held similar to a cup of tea, with the fingers (ring to baby finger) being spread out and away from the ultrasound probe. (b) Stabilisation of the probe using the ‘afternoon tea technique’, which helps facilitate ultrasound-guided procedures by immobilising the probe against the surface of the object to be aspirated using ultrasound guidance. This helps to stabilise the probe to keep the structure of interest (such as a fluid pocket or blood vessel) visible and central within the ultrasound image throughout the guided procedure while still permitting small subtle probe manipulations to be performed as needed. (c) Comparison of the probe being held similar to a pencil, which has much less stability when performing ultrasoundguided procedures. The syringe and needle are held in the dominant hand (here the right hand) with probe held by the nondominant hand (left hand shown). (Images courtesy of Drs S Boysen, S Chalhoub and J Menard)
in the absence of abdominal organs or structures between the skin surface and fluid pocket is identified.
Procedure
l t he abdomen should be rapidly scanned using abdominal PoCuS to identify the largest pocket of peritoneal fluid, to ascertain the location of other abdominal organs
in proximity to the fluid and to help determine the possible cause of the abdominal fluid.
l once the largest fluid pocket has been identified, real-time ultrasoundguided abdominocentesis can be performed, most often in a longitudinal orientation to the body.
l Verify that the indicator maker of the probe is directed towards the head of the patient or to the patient’s right for long- and short-axis views, respectively. t his ensures proper orientation of the ultrasound probe relative to the needle.
l Avoid applying too much pressure to the probe as it may displace and prevent fluid from being detected.
l A ‘teacup’ approach can be used to steady the hand (Figure 4).
l t he ultrasound-guided abdominocentesis technique is very similar to that described for cystocentesis.
l the needle should enter the skin at the cranial aspect of the probe and at a 45–60° angle to the skin using an in-plane technique. the needle tip will appear as a white anechoic point on the top left side of the ultrasound screen.
l t he needle tip is advanced until it can be visualised within the effusion (Figure 5).

l once the needle tip is visualised within the fluid pocket, gentle negative pressure (not more than 1–2 cm) can be applied to the syringe (the probe can be released, and two hands used to aspirate the plunger of the syringe if needed).
probe
The probe
placed above the larger pocket of abdominal effusion seen, centring it in the middle of the screen. The needle enters the skin at a 45° angle to the probe, 0.5 cm from the probe, and is advanced under ultrasound guidance over the deepest pocket of fluid that can be identified using abdominal POCUS. Once the tip of the needle is visualised as a bright echogenic point in the effusion, the fluid can be aspirated. The probe can be released if both hands are needed to aspirate the syringe prior to its removal.
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 36
b c
Figure 5. Schematic image of ultrasoundguided abdominocentesis. The syringe is held in the dominant hand, the
in the non-dominant hand.
is
(Image courtesy of Dr J Menard)
a larger gauge needle may be necessary.
l t he length of the needle required depends on the cat’s body fat. most often a ¾ inch needle is sufficient.
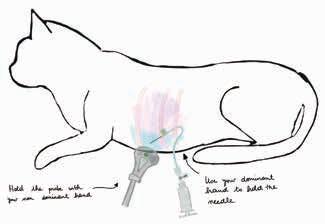
l If a butterfly catheter is not available, a 20–22 G IV catheter or hypodermic needle can be used instead with an IV extension set.
l Attach the 3-way stopcock and syringe to a butterfly needle or attach an IV extension set, threeway stopcock and syringe to the hypodermic needle or stylet of the IV catheter depending on the clinician’s preference.

Patient position
b Figure 6. e quipment that can used to perform thoracocentesis. (a) A butterfly needle or (b) a hypodermic needle with an intravenous extension and set. A butterfly needle is most often used for cats. It is connected to a three-way stopcock and a syringe. During aspiration of fluid or air, the stopcock is closed to the atmosphere and open between the syringe and the cat (butterfly needle). During evacuation of the syringe, the three-way stopcock is closed towards the cat (butterfly needle) to prevent refilling of the pleural space. The needle/ catheter should always be handled in an aseptic fashion. The syringe and three-way stopcock can be handled with non-sterile gloves and/or clean hands. (Image courtesy of Dr J Menard)
Thoracocentesis
t horacocentesis can be performed using a regular hypodermic needle, butterfly catheter, or a peripheral IV catheter. Ideally, use the smallest gauge catheter/ needle (Figure 6).
Equipment required
l 18–22 gauge, 1–2 inch butterfly needle, IV catheter or hypodermic needle;
l IV extension set if using an IV catheter or hypodermic needle;
l 3-way stopcock;
l 10–20 mL syringe dependent on patient size and the amount of pleural effusion present (diagnostic vs. therapeutic);
l receptacle for fluid collection (i.e., kidney bowl);
l clippers and antimicrobial scrub;
l red (plain) and lavender (EDtA) topped tubes for sterile fluid collection and analysis;
l sterile culturettes;
l lidocaine for a local anaesthetic block as needed;
l sedation, if needed;
l ultrasound machine and appropriate ultrasound probe.
Equipment Tips
l A 22 G needle is often sufficient for thoracocentesis, although in some high viscosity fluids such as pyothorax or feline infectious peritonitis effusion,
l Cats are usually placed in sternal recumbency, with minimal restraint. Sedation and analgesia are used as needed to decrease the need for restraint and lessen the effort of breathing in respiratory compromised patients. A local anaesthetic block using lidocaine may be given but is rarely, if ever, necessary.
l With the assistance of ultrasound, identify the deepest pocket of pleural effusion that is not in proximity to vital structures such as the heart and lungs.
l once the site of thoracocentesis has been identified (deepest/safest pocket), the skin should be clipped and aseptically prepped at that site.
l In cases of overt life-threatening respiratory distress, the fur can be parted and alcohol applied to the skin surface to allow for rapid urgent thoracocentesis.
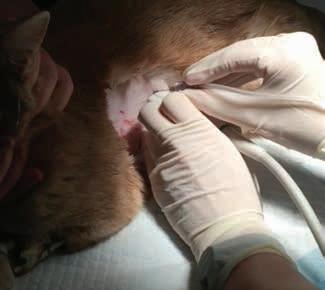
Probe orientation
thoracocentesis can be performed with the probe in a perpendicular orientation to the ribs, using an out-of-plane technique (see later), or a parallel orientation to the ribs using an in-plane technique. In-plane techniques with the probe parallel to the ribs are most commonly used for thoracocentesis in cats.
Procedure
l orientate the probe parallel to the ribs, over the widest accumulation of fluid with the indicator marker of the probe directed dorsally (Figure 7).
l Care should be taken to avoid inserting the needle at the caudal rib margins as the nerves and vessels are located here. t he needle should enter the centre to caudal third of the intercostal space.
ultrasound-guided thoracocentesis. The probe is usually held with the non-dominant hand. The dominant hand is used to manipulate and advance the needle/catheter. The ultrasound probe is placed between ribs where the deepest ultrasonographically visible pocket of pleural effusion is localised. The needle is inserted at an angle and advanced using ultrasound guidance until the tip is well situated within the pleural effusion. (Images courtesy of Drs J Menard and S Boysen)
l once the site of thoracocentesis has been identified the skin should be clipped and aseptically prepped over the site of needle insertion.
l Attach the 3-way stopcock and syringe to the butterfly needle or attach an IV extension set, threeway stopcock and syringe to the hypodermic needle or stylet of the IV catheter, depending on the clinician’s preference.
l using sterile gloves, the needle is placed dorsal to the ultrasound probe at an angle of about 30–45° to the skin, bevel towards the ultrasound probe, starting about 0.5 cm dorsal to the probe.
l A lower needle angle will keep the needle more perpendicular to the ultrasound probe, increasing visualisation of the needle/needle tip as it traverses the SC tissues and enters the pleural space, although the distance travelled by the needle to enter the pleural space will be longer.
l t he needle should be advanced under the tip of the probe at the probe marker (Figure 8).
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 37
a
Figure 7. (a) Schematic and (b) photographic image of
a b
l t he tip of the needle will typically indent the parietal pleura before piercing it and entering the pleural cavity. t he tip of the needle is advanced until it is visible within the effusion.
l An assistant will draw back on the plunger of the syringe to evacuate pleural effusion.
l No more than 1–2 cm of pressure should be applied to the syringe.
Figure
The needle or catheter is positioned roughly 0.5 cm from the probe, and advanced at roughly a 45° angle below the probe skin interface until it is visualised passing through the skin, subcutaneous tissues and entering the pleural effusion.
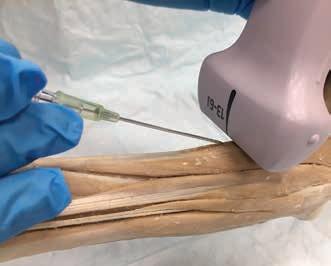
courtesy of Dr J Menard)
l t he needle will appear as a white anechoic point on the top left angle of the ultrasound screen when the indicator marker of the probe is directed towards the needle.
l once the needle/needle tip is seen to enter the SC tissues in real time, an assistant may apply gentle negative pressure while the needle continues to be advanced through the intercostal muscles to the level of the pleura.
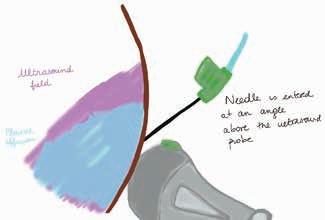
Box 3. Out-of-plane technique
l As the needle tip is seen entering the pleural effusion, fluid should simultaneously enter the needle hub and extension set, with the exception of viscous exudates.
l once the needle tip is visible within the pleural effusion, the angle of the needle should be decreased so it is more parallel to the skin and lying closer to the parietal surface of the thoracic wall. t he bevel of the needle should face towards the pleural effusion and away from the thoracic wall. t his will potentially decrease the risk of accidental lung puncture and allow more pleural effusion to be evacuated from the pleural space.
l once the needle is situated within the pleural effusion, the ultrasound probe is no longer required and can be placed on the table.
l once filled, the 3-way stopcock is closed towards the cat and the fluid/ air is ejected into the fluid receptacle. A sample should be saved for complete fluid analysis, including culture if pyothorax is suspected.
Ultrasound-guided vascular access
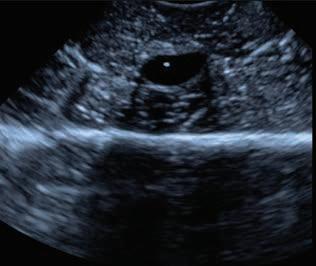
ultrasound-guided vascular access is very useful for patients with oedema (and vessels that cannot be palpated), overweight patients, or patients where their vessels have been sampled multiple times and may have thrombosed or developed perivascular haematomas. An advantage of ultrasound-guided catheter placement is that the depth of the vessel, radius of the vessel (which may guide selection of the catheter size) and the patency of the vessel (presence of thrombus formation) can be assessed prior to placing a catheter.
t he out-of-plane technique described here is for ultrasound-guided vascular IV catheter placement (Figure 10), although the technique is similar for blood sampling.
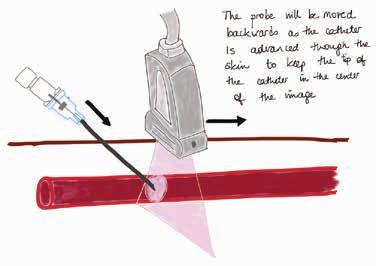
l using the non-dominant hand, the probe is placed perpendicular and in a short-axis orientation to the vessel of interest. t he vessel appears as a black anechoic circle on the screen.
l Find and assess the vessel of interest to determine its size and depth, ensure thrombosis is not present and determine if perivascular oedema or haematomas are present.
l Following preliminary vessel assessment, remove the probe and aseptically prepare the skin.
l Cover the probe with a sterile glove, if desired.
l Relocate the vessel with the probe in short-axis orientation to the vessel.
l Set the depth of the probe to maximise vessel size. With the probe perpendicular (at a 90° angle to the vessel) use probe manipulations (sweeping, sliding, rocking and fanning) to situate the vessel within the horizontal and vertical centre of the ultrasound display. Note: if the operator wishes to continue to visualise the procedure with ultrasound, an assistant is needed to advance the catheter off the stylet and down the lumen of the vessel.
l using the dominant hand, insert the catheter and stylet into the skin at a 30–45° angle, just distal to the probe. Identify the needle tip as a ‘white dot’ on the display between the skin and the vessel. Stop advancing the catheter/needle the second the ‘white dot’ is visible.
l Slowly sweep the ultrasound probe proximally along the vessel (away from the catheter tip) until the catheter stylet tip disappears.
l Hold the probe stationary and advance the catheter and stylet until the hyperechoic tip is again visible.
l Repeat the above two steps in alternating order until the stylet tip is visible as a ‘white dot’ in the vessel lumen.
l A flash of blood will be visible in the stylet hub.
l once the stylet tip is visible within the vessel lumen, decrease the catheter angle and advance it another 1–2 mm to ensure both the catheter and stylet are within the vessel.
l t he remainder of the technique is the same as for non-ultrasound guided percutaneous IV catheter placement.
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 38
8.
(Image
a b c
Figure 9. Out-of-plane ultrasound-guided vascular access. (a) Schematic diagram showing the probe perpendicular to the target vessel. (b) Cadaveric model showing the probe orientation to the limb using a linear probe and the IV catheter angle and insertions site relative to the probe. (c) Ultrasound still image. When using out-of-plane techniques the vessel appears as a black anechoic circle while the needle tip appears as a white hypoechoic dot (shown within the lumen of the vessel in this still image). (Images courtesy of Drs J Menard and S Boysen)
Box 4. In-plane technique
It may be helpful to start a procedure with an out-of-plane view to ensure that the needle is centred over the middle of the vessel and then rotate the probe to an in-plane view as the needle is advanced.
l For the in-plane technique (Figure 11) the probe is placed above and parallel to vessel. t he procedure is very similar to techniques described above for other in-plane centesis procedures.
l t he tip of the catheter/needle appears as a bright hyperechoic point and line. Advance the catheter and stylet in real time until the stylet tip and catheter can be visualised with the vessel lumen.
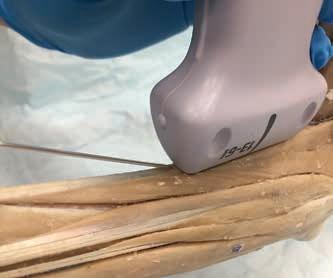
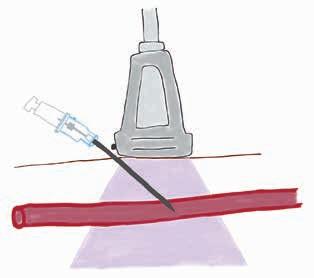
l Decrease the angle of the catheter, advance it 1–2 mm within the vessel lumen and then advance the catheter off the stylet as per percutaneous IV catheter placement.
tip more white and
Equipment required
l clippers;
l sterile scrub;
l tourniquet;
l sterile gel or a gel-filled sterile glove that can be placed over the ultrasound probe;
l a high frequency linear array probe (8–13 mHz) is preferred, although a microconvex probe can be used if a linear probe is unavailable;
l sterile IV catheter (1.8 or 2.5 inch);
l bandage material.
Patient position
l Set up the machine in front of you when performing ultrasound guided procedures to avoid having to look over your shoulder when manipulating the catheter/sliding the probe.
l For jugular catheter placement and/ or blood sampling the cat is typically placed in lateral recumbency.
l For femoral or saphenous catheter placement the cat is typically placed in lateral recumbency.
l For cephalic vein sampling the cat is placed in sternal recumbency.
l A stand-off ultrasound pad can be used for more superficial structures, which is particularly helpful for peripheral vessels in cats.
tip is
of the
Probe orientation
A linear array probe is preferred, with the gain set higher (bright image) than usual to enhance catheter tip visualisation. In the absence of a linear array probe a microconvex probe can also be used. In-plane or out-of-plane techniques can be used. t he authors have the greatest success using an out-of-plane technique and sweeping the probe along the vessel as the catheter is advanced. t his way, the catheter tip is always visible and accidental puncture of the far wall of the vessel is avoided. Another advantage of out-of-plane techniques is the catheter tip can be readjusted to centre it over the vessel if the vein deviates left or right from where the ultrasound probe is first situated.
Procedure
l t he technique to place a catheter with ultrasound guidance can be performed in both out-of-plane, in-plane and using an oblique angle technique (See Box 3, 4). Sterile techniques should be followed for ultrasound-guided catheter placement. Sterile ultrasound gel should be used in combination with alcohol and the probe covered with a sterile glove if desired. Stabilise the probe by resting your hand on the patient using the ‘afternoon tea technique’.
l Depth should be set to maximise the size of the vessel.
l t he vessel should be centred in the screen when using out-of-plane techniques.
Conclusions
ultrasound-guided techniques are extremely useful and applicable for veterinary technicians and nurses for obtaining diagnostic samples and also in therapeutic applications in cats presenting for triage.
Reference
McMurray J, Boysen S, Chalhoub S. Focused assessment with sonography in non-traumatized dogs and cats in the emergency and critical care setting. Journal of Veterinary Emergency and Critical Care (San Antonio) 26, 64–73, 2016 l
t his article was generously shared by International Cat Care
Learn more about ISFm at https://isfm-members.net and follow us on social media
@iCatCare @ISFmcats

Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 39
a b c
Figure 10. In-plane ultrasound-guided vascular access. (a) Schematic diagram showing the probe parallel to the target vessel. (b) Cadaveric model showing the probe orientation to the limb using a linear probe and the IV catheter angle and insertions site relative to the probe. (c) Ultrasound still image. When using in-plane techniques the vessel appears as a black tube, the needle appears as a white line and the needle
hyperechoic. The needle
shown traversing the near wall
vessel in this image while only a portion of the shaft of the catheter is visible as a white line within the soft tissue above the vessel, which appears to change direction slightly as it passes from a soft tissue to fluid interface at the near vessel wall. (Images courtesy of Drs J Menard and S Boysen)
A R t ICle
Neurological examination and neurolocalisation in cats and dogs
WeN-J I e yANg BVSc, gradDiped, MVetClinStud, MVetIntMed, DeCVIM-CA, Registered Specialist Small Animal Internal Medicine, Veterinary Specialists Aotearoa
Introduction
Neurological diseases are a relatively common presenting problem in general practice and especially common in referral practice. Dogs and cats can present with gait abnormalities, seizures, behavioural changes (including circling, dullness), neck pain or have non-specific lethargy, inappetence or weakness. A neurological examination is indicated in animals with seizures or ataxia. It can also help increase or decrease the suspicion of primary neurological disease in animals with vague clinical signs (e.g. lethargy, fever, neck pain).
In the beginning, practicing more neurological examinations will make the process easier and more informative in differentiating normal from abnormal. Similar to a general physical examination, it helps to have a set routine.
t his article describes a simplified version of a full neurological exam which is aimed at busy general practitioners. t he emphasis is to narrow down to a broad neurological region to help guide differential diagnoses and further diagnostics. For readers interested in further details, I suggest either the Textbook of Veterinary Internal Medicine (Ettinger et al. 2016) or the Practical Guide to Canine and Feline Neurology (Dewey 2015). t here are also many online resources with video demonstrations and explanations e.g. https://vetmed. illinois.edu/demo-sa-orthopedics/ postural-exam/
Contact: Wen-jie.yang@vsnz.co.nz
It is important to understand some basic neurophysiology to enable interpretation of abnormal findings. For example, the physiology of a withdrawal reflex (which is a local reflex to the spinal intumescence) is very different to proprioceptive testing (which is a long reflex arc that travels from the foot, to the spinal cord, to the brainstem, cerebral cortex and back down to the foot). Further details of pathophysiology are again available in textbooks (e.g. BSAVA Manual of Canine and Feline Neurology by Platt and olby, 2014).
Procedure
Step 1
It is first important to differentiate whether the primary presenting sign is neurological or non-neurological in origin. A careful history and general physical examination should always be performed to document other problems. For example, in dogs and cats presenting with collapse or weakness, a clinician should consider cardiovascular, respiratory, and systemic (e.g. sepsis, hypoglycaemia, acute abdomen, anaemia, thromboembolism) diseases, and orthopaedic conditions (e.g. bilateral cruciate disease).
Findings from physical examination can help increase the index of suspicion, e.g. tachycardia, weak pulses and delayed capillary refill time suggest shock; pale mucous membranes suggest anaemia; vomiting, diarrhoea or abdominal pain suggest an acute abdomen; cold extremities suggest a thromboembolism; diffuse joint swelling or pain suggest a polyarthropathy.
A complete blood count, serum biochemistry panel and urinalysis are recommended to screen for systemic disease. measurement of blood pressure is also recommended as hypotension can cause weakness, and hypertension can be an extra-cranial cause of
neurological dysfunction. In some cases, abdominal imaging may be beneficial to screen for an acute abdomen.
t here is also overlap with nonneurological disease causing neurological signs, e.g. hypoglycaemia or hepatic encephalopathy can cause seizures. Disease categories do not exist in isolation and clinicians should try to identify all existing problems.
Step 2
If neurological disease is still suspected, then the main locations to consider for the source of the problem are intracranial, spinal or peripheral nervous system. See table 1 for signs that can be used to determine where a neurological lesion may be located. Reaching this level of differentiation is often enough to help narrow differential diagnoses and choose appropriate diagnostic tests.
Step 3 Performing the neurological examination.
Following a set routine every time you perform a neurological exam helps you to avoid missing steps in the examination. Items needed to perform the examination include a light, reflex hammer, haemostat and a sling if the dog is not walking.
1. Assess mentation, stance and gait while off-leash in consult room and then on-leash while the owner walks the dog in a straight line.
l Decreased/altered mentation is very supportive of intra-cranial disease.
l Allow the opportunity for the animal to display circling or blindness.
l If an animal presents as nonambulatory, sling walking is recommended to allow assessment of motor function and proprioception.
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 40 Fe At UR
e
table 1. Clinical signs that can be used to localise neurological lesions to different parts of the nervous system. Common signs are highlighted in bold.
Intracranial
Location
Forebrain/proencephalon (cerebrum, diencephalon (thalamus, hypothalamus) Seizures Circling
mid-to-caudal brainstem
midbrain
Common clinical findings
Behavioural changes (e.g. aggression, loss of training)
Compulsive pacing
Head turn (not head tilt)
Central blindness
Reduced /altered consciousness
Paresis/paralysis
Proprioceptive deficits
Deficits in cranial nerves III and IV
Decerebrate rigidity
Pons and medulla
Cerebellum
Spinal
C1-C5
C6-t 2
t 3-L3
L4-S3
Peripheral nervous system
Paresis/gait abnormalities
Proprioceptive deficits
Deficits in cranial nerves V-XII
Hypoventilation
Dysmetria (goose-stepping gait)
Intention tremors
Vestibular signs
Wide base stance
truncal sway
Upper motor neuron signs in fore and hindlimbs
Tetraparesis/paralysis
Proprioceptive deficits in fore and hindlimbs
Lower motor neuron signs in forelimbs
Upper motor neuron signs in hindlimbs
Tetraparesis/paralysis
Proprioceptive deficits in fore and hindlimbs
Upper motor neuron signs in hindlimbs
Paraparesis/paralysis
Proprioceptive deficits in hindlimbs
Normal forelimbs
Lower motor neuron signs in hindlimbs
Paraparesis/paralysis
Proprioceptive deficits in hindlimbs
Normal forelimbs
Lower motor neuron bladder if lesion is caudal to L7
t hree main differential diagnoses (neuropathy, junctionopathy, and myopathy) present similarly:
Lower motor neuron signs in fore and/or hindlimb(s)
• May have flaccid paralysis
• Decreased/absent spinal reflexes
• Decreased muscle tone
Severe, rapid, muscle atrophy
2. Assess cranial nerve function – the main reflexes of interest include menace response, palpebral, pupillary light and oculocephalic reflexes, eye position, vision test (do their eyes follow a dropped cotton ball? can they navigate around the room?), and facial sensation (placing forceps gently into nostrils, ear canals and running them along the face). t hese tests assess the main cranial nerves (see table 2). Additional testing can be undertaken as required (e.g. gag test if there is concern for dysphagia, positional nystagmus if there is concern for vestibular dysfunction).
3. Assess for neck and spinal pain. I like to firmly squeeze the wings of the atlas, gently manipulate the neck through range of motion dorsoventrally and side-to-side. t hen firmly push on each spinous process
of the vertebrae. take care not to over manipulate the neck in dogs predisposed to cervical instability (e.g. Chihuahuas).
4. Assess proprioception:
l Postural reaction testing or proprioceptive placing (flexing and placing the paw onto its dorsal surface) is the most common and basic test. It is important to support the animal’s weight, especially if they are weak or nonambulatory.
l Hopping, wheelbarrowing and hemi-walking are also useful complementary tests of proprioception.
5. Assess spinal reflexes:
l Withdrawal reflexes are the most reliable spinal reflex and easiest to test. A withdrawal can be tested
by firmly pinching the webbing between toes and seeing if there is a full flexion of the limb.
l t he other spinal reflexes (patella, cranial tibial, sciatic and gastrocnemius reflex) require a reflex hammer. Further details can be found in many online videos e.g. https://vetmed.illinois.edu/ demo-sa-orthopedics/forelimbreflexes/
6. If there is no motor function, assessment of deep pain is appropriate. See ‘Common pitfalls’.
7. o ther tests that may be of interest depending on relevant presenting problems:
l Reflexes: perineal reflex, panniculus reflex, and anal tone.
l Bladder: in non-ambulatory dogs it is useful to determine whether it is overflowing and hard to express (upper motor neuron bladder) or easy to express (lower motor neuron bladder).
l Examination of the ocular fundus is also useful, if an ophthalmoscope is available, to look for granulomas, haemorrhage, and neoplastic lesions.
It is acceptable to tailor the neurological exam to the patient and target the main regions of interest based on history and/ or clinical signs.
Step 4 Assessing the clinical findings
Based on the neurological findings, can a lesion in one location explain all the clinical signs of the animal? (See table 1). If so, then one lesion is most likely.
If a lesion in one location can’t explain all the signs (e.g. cranial nerve deficits and seizures), then multi-focal disease should be suspected. one of the more common causes of multi-focal diseases is meningoencephalitis (immunemediated or infectious).
t he mnemonic “DAmNIt-V” is helpful when considering differential diagnoses. It prompts consideration for degenerative, anomalous, metabolic, neoplastic, infectious/inflammatory/ immune-mediated, toxic, and vascular causes. Differential diagnoses can then be prioritised after considering patient signalment and history. Neoplasia is more likely in an older animal with gradual onset and progressive signs. A cerebrovascular accident usually presents with a history of acute onset and is nonprogressive.
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 41
table 2. Cranial nerve function and testing. The main cranial nerves of interest and commonly used tests are highlighted in bold. Based on ettinger et al. (2016).
Cranial nerve Function Dysfunction Testing
I (o lfactory) Smell Anosmia
II (Optic) Vision
III (Oculomotor) motor to extraocular muscles
Parasympathetic innervation to pupil (constriction of pupils)
Blindness, dilated pupils
Ventrolateral strabismus
Dilated pupils
IV ( trochlear) motor ocular muscle (dorsal oblique muscle) Strabismus (only in cats)
V (Trigeminal) motor to masticatory muscles
Facial sensation
Sympathetic innervation travels with ventral to trigeminal nerve
VI (Abducent) motor to ocular muscles (lateral rectus and retractor bulbi)
VII (Facial) motor to face
Parasympathetic to lacrimal glands
VIII (Vestibulocochlear) Balance
Hearing
IX (Glossopharyngeal) Sensory and motor to pharynx
X (Vagus)
Sensory and motor to pharynx and larynx
XI (Accessory) motor to trapezius muscle
XII (Hypoglossal) motor to tongue
t he differential diagnoses to consider for generalised lower motor neuron signs include polyradiculoneuritis, botulism, myasthenia gravis, and less likely polyneuropathy or polymyopathy (e.g. immune-mediated or infections with Toxoplasma gondii or Neospora caninum). t ick paralysis and snake envenomation are possibilities in Australia. Animals with mild cases of lower motor neuron disease may still be ambulatory (but will typically have what has classically been described as a short and choppy gait).
Step 5 Planning your next steps
After differential diagnoses have been considered, appropriate testing and/or treatment can be chosen. For example, if central nervous system disease is suspected then the next diagnostic step is often advanced imaging of the brain or spine and/or analysis of cerebrospinal fluid. t his often requires referral to a specialist centre.
If advanced imaging is financially impossible, then treatment for the most likely causes could be discussed if appropriate, e.g. medical management for suspected intervertebral disc disease, antibiotic therapy for Neospora caninum infection, or supportive care for idiopathic vestibular syndrome.
masticatory muscle atrophy, dropped jaw
Absent facial sensation
Horner’s syndrome
m edial strabismus
Facial paralysis
Dry eye
Head tilt, spontaneous nystagmus
Deafness
Dysphagia
Dysphagia
Laryngeal paralysis
Atrophy of trapezius
Abnormal tongue position
If peripheral nervous system disease is suspected, testing for myasthenia gravis or polymyopathy may be pursued. Polyradiculoneuritis and botulism do not have a specific confirmatory tests, so these are often diagnosed on the basis of consistent clinical signs and exclusion of other differential diagnoses, and treatment is supportive.
Common pitfalls
Withdrawal reflex vs. testing for deep pain
A normal withdrawal reflex confirms the function of the local reflex arc, which tests only the local spinal cord segment (i.e. the afferent nerve, spinal intumescence (C6-t2 for forelimbs, L4-S3 for hindlimbs) and the efferent nerve). A withdrawal reflex should be tested by firmly pinching the interdigital skin. A normal withdrawal is seen when there is full flexion of the joints in response to the pinch. A withdrawal test does not provide information about spinal cord function cranial or caudal to the intumescence.
A deep pain test should be reserved for non-ambulatory, motor-negative animals. Spinal injuries cause damage in a predictable order based on
table 3. Features of upper and lower motor neuron disease
Reaction to a noxious stimulus such as an alcohol soaked swab
Menace response, pupillary light response, tracking objects, obstacle course
Oculocephalic reflex
Pupillary light response
Resting eye position (only in cats)
Jaw tone
Sensation to face (palpebral reflex, nasal sensation)
Oculocephalic reflex
Palpebral reflex
Schirmer tear test
Oculocephalic reflex
Gag reflex, swallowing
Gag reflex , laryngeal examination
trapezius muscle mass assessment
tongue movement assessment
increasing severity: firstly, there is loss of proprioception, then motor function, bladder function and lastly nociception. t hus animals with motor function will always have a deep pain response present due to location of the nerve tracts. to test for deep pain, the periosteum needs to be stimulated. In practice, I like to use forceps to squeeze one of the digits and a positive response consists of a behavioural response to the pain (yelping, trying to move away, looking at the source of pain). A positive withdrawal reflex is does not represent the presence of deep pain.
Upper motor neuron vs. lower motor neuron signs in a tetraparetic animal Differentiating upper vs. lower motor neuron signs can be remembered with the mnemonic ARt = atrophy, reflex and tone. See table 3 for features that can be used to distinguish lower motor neuron disease from upper motor neuron disease.
References
Ettinger SJ, Feldman EC, Cote E. Textbook of Veterinary Internal Medicine, Eighth Edtn (E-book). Saunders, Philadelphia, PA, USA. 2016
DeLahunta A. Veterinary Neuroanatomy and Clinical Neurology, Second Edtn Saunders, Philadelphia, PA, USA, 1983
Upper motor neuron disease
Lower motor neuron disease muscle atrophy Slow (as a result of disuse) Rapid and severe due to denervation atrophy
Dewey C, da Costa RC. Practical Guide to Canine and Feline Neurology, Third Edtn Wiley Blackwell, Hoboken, N y, USA, 2015
Spinal reflexes Present and can be hyper-reflexive
Decreased/absent muscle tone Increased due of the absence of inhibition from upper motor neurons
Decreased muscle tone (i.e. muscles are flaccid)
Platt SR, Olby NJ. BSAVA Manual of Canine and Feline Neurology, Fourth Edtn. British Small Animal Veterinary Association. Quedgeley U k , 2014 l
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 42

Companion Animal Quarterly: Anaesthetic considerations for the cardiac patient
Dr MegAN y eUNg BVSc, VSA rotating intern, Dr k e AtON MORgAN BVSc (Hon) DACVIM (Cardiology), VSA Veterinary Cardiologist, Dr JOANA CHAgAS BVSc, DACVAA, VSA Clinical Anaesthesiologist
Assessment and planning are vital before placing any patient under general anaesthesia. t his starts with obtaining a thorough patient history and performing a physical examination (with particular focus on the cardiorespiratory system), in order to detect any potential abnormalities. t his helps with the selection of anaesthetic drugs, anticipation of potential complications and strategies to support patient management during anaesthesia. In this article we will discuss some anaesthetic considerations for the most common cardiac conditions. our disclaimer is not to ignore the entire picture of a patient’s status and comorbidities, which should be taken into account during anaesthetic planning. Additionally, the best way to assess the heart is via echocardiography, which should always be recommended when there is concern for cardiac disease.
Following the ACVIm staging system for cardiac disease (Crosland et al. 2021), patients at stage A (predisposed) and B1 (disease present without cardiac remodelling) are at minimal risk of cardiac complications, though should be closely monitored under anaesthesia. Stage B2 indicates hemodynamically significant disease due to the presence of cardiac remodelling, and for these patients greater attention is required for drug selection and intraoperative monitoring. Patients with clinical signs of congestive heart failure such as tachypnoea, exercise intolerance or syncope (stage C) should have additional diagnostics performed (such as thoracic radiographs) and be stabilised before
Contact: megan.yeung@vsnz.co.nz
general anaesthesia is considered. once stable, there is still an increased risk of cardiac-related complications and the reduced lifespan from the underlying cardiac disease needs to be evaluated. Nevertheless, careful general anaesthesia can be performed for conditions affecting quality of life, but supervision by a veterinary anaesthesiologist is recommended. Stage D patients (endstage heart failure that is refractory to treatment) have a remarkably high risk of complications and general anaesthesia should be avoided, or performed by a veterinary anaesthesiologist.
Anaesthetic considerations for dogs with cardiac disease
Myxomatous mitral valve disease (MMVD)
t his disease is characterised by the degeneration of cardiac valves, most commonly, the mitral valve, causing leakage of blood backwards from the left ventricle to the left atrium during ventricular contraction. t his is the most commonly acquired heart disease in
dogs, comprising 75% of cardiovascular disease cases seen in practice (mcCauley et al. 2020). t his disease has a 30% prevalence in dogs >10 years of age, and small to medium-sized dog breeds are predisposed, including Cavalier King Charles Spaniels, Dachshunds, Beagles, Chihuahuas and miniature Poodles (Crosland et al. 2021). As this is the most common cardiac disease in dogs, mmVD will be the greatest consideration when anaesthetising older dogs with murmurs, particularly those <15kg.

Anaesthetic considerations
If anaesthetising dogs with mmVD, it is important to maintain cardiac output by supporting forward blood flow and minimising regurgitant flow. t his can be achieved through the following considerations (Scarabelli et al. 2016; Crosland et al. 2021):
l maintain normal or slightly increased heart rate;
l maintain or slightly reduce afterload, which helps promote forward flow and reduces regurgitant blood flow;
CARDIO CORN eR
[Photo credit: Fran@thisisfranpatel from Pixabay]
44 Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023
l Avoid acute increases in afterload, by avoiding increases in systemic vascular resistance.
Oral medications
Dogs may be on medications prior to general anaesthesia for stage B2, C or D heart disease and a decision needs to be made whether to discontinue the medications prior to the anaesthetic event. Pimobendan should be given at the patient’s usual dose on the day of anaesthesia. ACE inhibitors can cause significant, non-responsive hypotension in response to vasodilatory effects of inhalational anaesthetic agents. Excessive vasodilation can decrease arterial blood pressure, causing poor tissue perfusion (Crosland et al. 2021). A study by Coleman et al. (VAA 2016) suggested avoiding administration at least 24 hours before general anaesthesia, however, they can be discontinued up to 5 days before.
Administration of diuretics is only advised in patients with previous episodes of heart failure. t he decision on whether to administer on the day of anaesthesia is dependent on the patient’s clinical status. Electrolyte levels should be closely monitored in patients given diuretics.
Premedication
Premedication is often used to decrease stress and anxiety of the patient during handling and restraint. Pre-oxygenation with a tight-fitting face mask after administration of sedative medication is ideal. However, it should only be performed if it does not cause distress to the patient. Flow-by oxygen is an acceptable alternative.
opioids (full mu agonists e.g., methadone and morphine) are commonly used due to their analgesic properties and minimal cardiovascular effects. For patients that are not compliant or compromised enough, they can be administered in conjunction with sedatives. Dosedependent bradycardia can be seen due to elevated parasympathetic activity to the heart. Anticholinergics (atropine and glycopyrrolate) can be used to counteract these effects in the absence of tachyarrhythmias, with preference for glycopyrrolate. It is important to monitor blood pressure throughout, since some dogs can tolerate a lower heart rate and maintain normotension. t herefore, anticholinergics are typically only required in dogs with hypotensive bradycardia.
Benzodiazepines (midazolam and diazepam) are often used in conjunction with opioids in patients with mmVD. t hey have minimal effects on the cardiovascular
system. However, when used as a sole pre-medication agent, unreliable sedative effects and paradoxical excitation may be seen. Acepromazine can also be considered a useful pre-medication for mmVD as it has calming and sedative effects. It has vasodilatory effects through inhibition of α1 adrenergic receptors. When used at low doses (0.005–0.015 mg/kg), mild vasodilation helps reduce afterload and decrease regurgitant blood flow. However, at higher doses, there is risk of profound vasodilation leading to hypotension.
α2 adrenergic receptor agonists (medetomidine and dexmedetomidine) should be avoided in mmVD patients as it causes peripheral vasoconstriction, which leads to considerable increases in cardiac afterload. t his results in a reduced stroke volume and increased regurgitant blood flow. Additionally, a baroreceptormediated reflex bradycardia is also seen in response to peripheral vasoconstriction. Both effects lead to a marked reduction in cardiac output that could lead to acute cardiogenic pulmonary oedema.
Induction
Induction agents such as propofol and alfaxalone are commonly used. Some studies have shown that alfaxalone has an advantage compared to propofol as it increases heart rate and maintains arterial blood pressure (muir et al. 2008; Crosland et al. 2021). Both agents should be administered slowly to reduce the dose needed to achieve adequate anaesthetic depth, hence reducing dose-dependent cardiovascular effects.
Benzodiazepines can also be used as a co-induction agent (following an initial dose of propofol or alfaxalone). midazolam has been shown to decrease the dose of induction agent needed for intubation, but with no substantial improvement of cardiovascular parameters (miller et al. 2019; Crosland et al. 2021).
Ketamine has indirect sympathomimetic effects and causes increase in heart rate, arterial blood pressure and cardiac output. In patients with stage B1–2 of disease, these effects can be beneficial in maintaining normal cardiac output. Ketamine (5–10 mg/kg) should be administered in combination with midazolam or diazepam (both at 0.25–0.5 mg/kg) due to its lack of muscle relaxant effects (Ferreira et al. 2015). Alternatively, ketamine (2 mg/kg) can be administered in association with propofol (2 mg/ kg, titrated to effect). t his combination is reported to increase heart rate and
blood pressure, but also is more likely to induce apnoea in comparison to a sole propofol induction (martinez-taboada and Leece 2014). t he increased myocardial oxygen consumption caused by ketamine can become detrimental in dogs with advanced mitral valve disease. It should be avoided in patients with tachyarrhythmias due to increased sympathetic activity and risk of the condition worsening.
Maintenance of anaesthesia
Isoflurane and sevoflurane are inhalation agents typically used for maintenance of general anaesthesia. t hey cause dose-dependent hypotension due to a vasodilatory effect and reduced myocardial contractility (Crosland et al 2021). t herefore, it is important to keep the amount of inhalation agent used at a minimum. multimodal and balanced anaesthetic protocols can help reduce the amount of inhalation agent required to maintain anaesthesia. t hese include loco-regional techniques (local blocks to reduce painful stimuli) and administration of systemic analgesics (fentanyl, ketamine) as a constant rate infusion intraoperatively.
Fluid therapy
Fluid therapy should be administered with care for patients with mmVD (particularly, stage B2 or higher patients), as they are less tolerant of high rates of IV fluids, which increases preload.
A fluid rate of 3–5 mL/kg/hour with crystalloid solutions is considered acceptable in most stable dogs to meet metabolic requirements, while decreasing risk of cardiac overload and progression into heart failure (Crosland et al. 2021). Rates between 2–3 mL/kg/hour become applicable with more advanced disease and occasionally no fluids are given. Importantly, repeat fluid boluses should be avoided. Hypotension experienced during anaesthesia is likely related to vasodilation or poor cardiac output from the underlying cardiac disease, which is best treated with inotropes. Fluid deficits should be corrected before the patient undergoes anaesthesia.
Dilated cardiomyopathy (DCM)
Dilated cardiomyopathy is the second most common cardiac disease in dogs, with a prevalence of 0.5–1.3% in North American and European referral hospitals (mcCauley et al. 2020). It is often an idiopathic disease characterised by poor myocardial contractility and dilation, with genetic predisposition in large and giant breed dogs (particularly, the Doberman Pinscher, Irish Wolfhound and Great Dane).
45 Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023
Dilation of the left ventricle increases risk of mitral valve regurgitation, which contributes to volume overload of the left atrium.
Anaesthetic considerations for patients with DCm are similar to those with mVmD, however, anaesthesia can be more unstable due to systolic dysfunction contributing to hypotension and increased incidence of cardiac arrhythmias (Perkowski et al. 2015; Scarabelli et al 2016). Anaesthetic drugs that promote maintenance of myocardial contractility and cause minimal changes in systemic vascular resistance should be selected. Patients with systolic dysfunction are prone to hypotension and may benefit from inotropic support (dobutamine or dopamine) during anaesthesia.
Dogs with DCm are prone to tachyarrhythmias (ventricular premature complexes and atrial fibrillation).
tachyarrhythmias should be treated and controlled before general anaesthesia, as they can lead to profound hypotension and decrease in cardiac output (Perkowski et al. 2015)
Puppy or congenital cardiovascular disease considerations
Congenital cardiac disease comprises approximately 21% of cardiac cases diagnosed in small animal referral hospitals (oliveira et al. 2011; Robinson & Borgeat 2016). It is less commonly diagnosed in general practice.
Normal, soft murmurs are often identified in clinically healthy puppies at their first puppy wellness exam, with a prevalence of between 15–31% in puppies <6 months old. t hese murmurs are non-pathological. Alternatively, pathological murmurs indicate congenital heart disease. Features of pathological murmurs include loud cardiac murmurs (grade III or higher), continuous or diastolic murmurs, rightsided murmurs and/or murmurs that don’t disappear with age. Possible clinical signs associated with pathological murmurs include exercise intolerance, increased respiratory rate and effort, cyanosis, ascites or syncopal episodes.
If concerned for a pathological murmur, echocardiography is the gold standard for definitive diagnosis of the cause of the murmur and congenital heart disease present, alongside severity evaluation. Early detection enables early treatment, preventing clinical signs and decreasing cardiac morbidity and mortality (Rovroy and Szatmari 2021). Furthermore, it allows proper anaesthetic planning, careful drug
selection and peri-anaesthetic monitoring of patients. Given the variety of congenital cardiac diseases that are possible, it is beyond the scope of this article to describe best anaesthetic protocols for each condition. Rather, obtaining a definitive diagnosis by echocardiography should be performed prior to general anaesthesia in puppies with pathological heart murmurs.
Anaesthetic considerations for cats
Hypertrophic cardiomyopathy (HCM)
Cardiomyopathy affects approximately 15% of the general feline population, with hypertrophic cardiomyopathy as the most common form of myocardial disease in cats.
Hypertrophic cardiomyopathy is characterised by concentric hypertrophy of the left ventricle, increased myocardial oxygen demand, diastolic dysfunction and reduced cardiac output. one third of HCm cats have left ventricular outflow tract obstruction, due to the systolic anterior motion of the anterior leaflet of the mitral valve (Gil- ortuno et al. 2019).
t he cause of HCm in the majority of cats is unknown, but genetic mutations have been identified in maine Coons, Ragdolls and Sphynx breeds. o ther causes of left ventricular hypertrophy include systemic hypertension and hyperthyroidism in older cats (less commonly, acromegaly, myocarditis and dehydration). t hese secondary causes must be excluded before a final diagnosis of HCm is made (Kittleson and Cote 2021). treatment of secondary causes can lead to reversal of the myocardial thickening.
Pre-anaesthetic assessment
Cats can have no significant findings on physical examination despite having substantial underlying cardiac disease. Alternatively, up to 70% of murmurs in cats are normal/physiological (Payne et al 2015). t herefore, it is important to identify which cats are at-risk of having underlying cardiac disease during the pre-anaesthesia assessment. t he prevalence of cardiac disease significantly increases with age in cats, with a prevalence of up to 29% in cats >9 years old compared to 15% in the general cat population (Payne et al. 2015). Cats with abnormal auscultation findings, including murmurs, but particularly a gallop rhythm or arrhythmia, are also at-risk of underlying cardiac disease compared to cats with normal cardiac auscultation. Lastly, predisposed breeds (maine coons,
Ragdolls and Sphynx in particular) and cats with siblings diagnosed with heart disease are in the at-risk group, despite age or auscultation findings. t herefore, if you believe a cat is at-risk of having underlying cardiac disease in your pre-anaesthetic evaluation, an echocardiogram should be considered as the gold standard for diagnosis and evaluation of severity. If echocardiography is unavailable, an initial screening test (N t-proBNP) should be performed. A normal result does not rule out cardiac disease but indicates that moderate to severe HCm is highly unlikely (machen et al. 2014). An abnormal N t-proBNP warrants a strong recommendation for echocardiography prior to general anaesthesia. Anaesthesia should be avoided altogether, or ‘cardiacsafe’ anaesthetic agents (more information below) should be used, with warnings of potential risk to the owner. Remember that false-positive elevations of N t-proBNP are possible with systemic hypertension, hyperthyroidism, and IRIS stage III renal disease. t herefore, positive results should be followed up by assessing blood pressure, tt4 concentration and serum biochemistry.
Anaesthetic considerations
Cats that are stressed or anxious should be identified before the day of anaesthesia, as stress can be detrimental to anaesthetic protocols and the underlying cardiac disease. Administration of gabapentin (20 mg/kg) before the visit can facilitate stress reduction and enable ease of patient handling. When anaesthetising cats with HCm, it is important to consider the following (Perkowski et al. 2015; Scarabelli et al. 2016):
l maintain normal (or slightly low) heart rate to optimise diastolic filling time;
l optimise preload and ventricular filling;
l maintain or slightly decrease contractility to reduce myocardial oxygen consumption;
l maintain or slightly increase systemic vascular resistance to decrease outflow obstruction presence or severity.
Oral medications
t he decision to continue clopidogrel should be decided on a case-by-case basis. It is dependent on the surgeon’s experience, surgical tools available and type/invasiveness of the surgery performed. For instance, an experienced surgeon with cautery may perform more invasive procedures without an increased haemorrhage risk. If clopidogrel is discontinued, it should be stopped at least
46 Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023
5 days prior to general anaesthesia due to prolonged activity in the body.
Anaesthetic drugs
t he use of α2 adrenergic agonists (medetomidine and dexmedetomidine) as a premedication in HCm patients is controversial. t here are documented benefits associated with vasoconstriction. It increases afterload, hence reducing outflow tract obstruction severity while increasing preload, consequently improving diastolic filling (Steinbacher and Doerflet 2012; Scarabelli et al. 2016). A study by Lamont et al. (2002) further demonstrated that medetomidine administration in cats with outflow tract obstructions led to the elimination of outflow obstruction altogether. t hese benefits can make α2 agonists useful in patients with stage B1 HCm, particularly in the presence of an outflow tract obstruction. However, in patients with more advanced disease (stage B2 or higher) indicating severe left atrial enlargement, an increase in afterload and myocardial wall stress can reduce cardiac output, leading to cardiogenic pulmonary oedema. t herefore, the severity of disease must be considered.
opioids and benzodiazepines are good premedication options for patients with HCm as they have minimal cardiovascular effects. When administering opioids as a single agent, the sedative effects are not as effective in cats as compared to dogs. However, in combination with sedatives, there are synergistic effects resulting in a more pronounced sedation.
Acepromazine has unreliable sedative effects in cats. It also induces vasodilation (due to α1 antagonist effects) which will worsen an outflow tract obstruction severity and reduce preload. Consequently, acepromazine is best avoided in cats with cardiac disease. Ketamine should not be used as an induction agent in HCm patients at stage B2 or higher, or in the presence of an outflow tract obstruction. It causes increased sympathetic activity, leading to tachycardia and increased myocardial contractility which will heighten outflow tract obstruction severities (Perkowski et al. 2015). t hese effects also increase myocardial oxygen consumption and can predispose to tachyarrhythmias.
Propofol and alfaxalone should be administered slowly to dose to effect, reducing dose-dependent cardiovascular effects. Alfaxalone is beneficial in cats as it can be used as a sedative Im with minimal cardiovascular effects.
Fluid therapy
A maintenance fluid rate of 2–3 mL/kg/ hour is recommended intraoperatively for cats. t his can vary depending on the patient’s hydration status and requirements. Cats with advanced cardiac disease may require no fluids. Importantly, repeated fluid boluses should be avoided in cats as they are sensitive to acute changes in intravascular blood volume that can lead to iatrogenic pulmonary oedema.
References
Côté E, Edwards NJ, Ettinger SJ et al Management of incidentally detected heart murmurs in dogs and cats. Journal of Veterinary Cardiology 17, 245–61, 2015 Crosland A, Love B, Trimble T. Anaesthetic management of dogs with myxomatous mitral valve disease. Companion Animal 26, 81–6, 2021 Ferreira JP, Dzikit TB, Zeiler GE, Buck R, Nevill B, Gummow B, Bester L. Anaesthetic induction and recovery characteristics of a diazepamketamine combination compared with propofol in dogs. Journal of the South African Veterinary Association 86, 1258, 2015
Gil-Ortuño, C, Sebastián-Marcos, P, Sabater-Molina, M, NicolasRocamora, E, Gimeno-Blanes, JR, Fernández del Palacio, MJ. genetics of feline hypertrophic cardiomyopathy. Clinical Genetics 98, 203–14, 2020
Kittleson MD, Côté E. The feline cardiomyopathies: 2. Hypertrophic cardiomyopathy. Journal of Feline Medicine and Surgery 23, 1028–51, 2021 Lamont LA, Bulmer BJ, Sisson DD, Grimm KA, Tranquilli WJ. Doppler echocardiographic effects of medetomidine on dynamic left ventricular outflow tract obstruction in cats. Journal of the American Veterinary Medical Association 221, 1276–81, 2002
Luis Fuentes V, Abbott J, Chetboul V, et al . ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. Journal of Veterinary Internal Medicine 34, 1062–77, 2020
Machen MC, Oyama MR, Gordon SG, et al . Multi-centered investigation of a point-of-care N t-proBNP el ISA assay to detect moderate to severe occult (pre-clinical) feline heart disease in cats referred for cardiac evaluation. Journal of Veterinary Cardiology 16:,245–55, 2014
Martinez-Taboada F, Leece EA. Comparison of propofol with ketofol, a propofol-ketamine admixture, for induction of anaesthesia in healthy dogs. Veterinary Anaesthesia and Analgesia 41, 575–82, 2014
McCauley SR, Clark SD, Quest BW, Streeter RM, Oxford EM. Review of canine dilated cardiomyopathy in the wake of diet-associated concerns. Journal of Animal Science 98, skaa155, 2020
Miller C, Hughes E, Gurney M. Coinduction of anaesthesia with alfaxalone and midazolam in dogs: a randomized, blinded clinical trial. Veterinary Anaesthesia and Analgesia 46, 613–9, 2019
Muir W, Lerche P, Wiese A, Nelson L, Pasloske K, Whittem T. Cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in dogs. Veterinary Anaesthesia and Analgesia 35, 451–62, 2008
Oliveria P, Domenech O, Silva J, et al Retrospective review of congenital heart disease in 976 dogs. Journal of Veterinary Internal Medicine 25, 477–83, 2011
Payne JR, Brodbelt DC, Luis Fuentes V. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). Journal of Veterinary Cardiology 17 Suppl 1, S244–57, 2015
Perkowski, SZ, Oyama MA. Pathophysiology and anesthetic management of patients with cardiovascular disease. In: grimm k A, l amont l A. tranquilli WJ, greene SA, Robertson SA. (eds). Veterinary Anesthesia and Analgesia. 5th e dtn. Pp 496–510. John Wiley & Sons Inc, Ames, IA, USA, 2015
Pugliese M, Biondi V, La Maestra R et al Identification and clinical significance of heart murmurs in puppies involved in puppy trade. Veterinary Science 8, 139, 2021
Rovroy LB, Szatmari V. Age of puppies at referral to veterinary cardiology specialists for murmur investigation. Acta Veterinaria Scandinavica 63, 1-12, 2012
Scarabelli S, Bradbrook C. Anaesthesia of the patient with cardiovascular disease part 2: Anaesthesia for specific disorders. Companion Animal 21, 337–44, 2016
Steinbacher R, Dörfelt R. Anaesthesia in dogs and cats with cardiac disease – An impossible endeavour or a challenge with manageable risk? European Journal of Companion Animal Practice 23, 4–22, 2013
White KL, Yates D. Clinical comparison of alfaxalone, ketamine and propofol following medetomidine and methadone in dogs. Veterinary Anaesthesia and Analgesia 44, 1027–34, 2017 l
47 Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023
Institute of Health Care Communications train the trainer Workshop
Athens, gA, USA, May 2023
Meg I RVIN e BVSc, Vet lifeskills ltd
After being awarded an Educate the Educators Scholarship in early 2023, I applied and was accepted into the train the trainer course run by the Institute for Healthcare Communication (IHC), being held at the university of Georgia (Athens, GA, uSA), at the end of may 2023.
t he IHCare Communication is a notfor-profit organisation started in 1987, originally called the Bayer Institute as it was funded by Bayer Pharmaceuticals. It was founded with the purpose of creating highly interactive educational programmes, assisting clinicians and clinical assistants in human health to enhance their communication skills. t he programmes are based around a patientcentered model that recognises the impact that collaborative, relationship-centred care has on health and emotional outcomes in human patients.
In 2002 the IHC expanded to include communication training in veterinary medicine, again, sponsored by Bayer Animal Health. t he programme recognised that there were many gaps in the communication training given to veterinary students in many veterinary schools across the world. t he organisation produced 16 modules, resources and accompanying video content, and set about training faculty members who met certain criteria, in how to train other veterinarians in these communication skills. Since then, over 500 people have attended train the trainer courses and become faculty members of the IHC and hold the license to use and teach their materials.
Contact: meg.irvine@vetlifeskills.co.nz, www.vetlifeskills.com
t he course is now incorporated into schools of veterinary medicine across the united States, Canada, Japan and Australia. t he course was weeklong and held at the university of Georgia College of Veterinary medicine in Athens, Georgia, uSA. t here were 11 participants, most of whom were from various uSA veterinary schools, plus me and one other veterinarian who was in private practice. t he week consisted of learning to teach communication skills, exploring the 16 modules, clinical interviewing (with simulated clients/ owners) and working one-on-one with learners in a coaching role.
t he modules we covered in detail were ‘Building trust’, ‘ t he power of feedback’, ‘Conflict management within veterinary teams’ and ‘Disclosing medical errors’. t his content included how to use the resources and video material to teach these skills, run small group exercises and facilitate discussions. t he video content is extensive and covers examples of communication skills used (and mis-used) in everyday practice, acted and filmed professionally as a teaching resource.
t he other modules included in the course content are:
l Creating teams that work
l Eliciting and understanding the client’s perspective
l Guiding clients through difficult decisions
l Enhancing adherence
l Nonverbal communication
l Addressing conflict
l talking about money
l Sharing bad news
l Ethics
l Communication within the veterinary Health care team
l Compassion fatigue
Each of these modules includes written content, team-based exercises, and video examples.
t here is strong evidence that experiential learning is the most effective way to teach communication skills to veterinarians. t his is where the IHC course differs from many other communications training courses, in that it is largely experiential. We had small groups of 3–4 participants and one facilitator. Each participant had a turn at being the learner (the veterinarian who was seeing the client and imaginary pet) and a turn being the coach to the learner. As the learner (veterinarian) we practiced skills such as eliciting an agenda, showing empathy, reflective listening, summarising, asking permission, and skills such as ‘asktell-ask’. t he coach’s job was to elicit from the learner what they hoped to practice, how they planned to practice and then observe and record their findings. t he coach then gave feedback using a series of what-worked-well and some even-betteryet suggestions. All sessions were videoed and retrospectively assessed.
Learning how to run simulated client learning sessions, facilitate teaching sessions and practicing coaching skills were the highlights of the experience.
Access to this content (now that I am a faculty member of the Institute and a license holder) is also going to be extremely beneficial. I hope to use the material to create collaborative workshops that will be available as CPD opportunities to veterinarians across New Zealand over the latter half of 2023 and 2024. It also will allow me to use this content to tailor in-clinic workshops for full teams based on their specific challenges as well as educational webinars.
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 48 WOR k SHOP
R ePOR t
t his was such a fantastic opportunity to spend time in an environment with those at the forefront of communications training in the uSA. t his course has consolidated my belief and passion for the impact that communications training can have on the success of a veterinary professional and that mastering these skills requires knowledge of the skills, practice, and reflection.
I would like to acknowledge and thank the Companion Animal Veterinarians Branch of the NZVA and Hill’s Pet Nutrition for their contribution to my attendance. l



NZVA 2023 Centenary Conference
Wellington, 28–30 June 2023
t he 2023 NZVA conference was held over 3 days in late June at tākina, Wellington’s brand new conference and convention centre. Located in the CBD next to te Papa, tākina was an excellent venue for the conference set over multiple levels with first class facilities.
t he CAV stream comprised 2 days of cardiology (feline then canine) and 1 day on topics relating to brachycephaly. All 3 days were well attended with very positive feedback about the content. Special thanks must be given to the specialists who presented during those days:
Joon Seo – veterinary cardiologist (Arcvets, NZ)
Keaton morgan – veterinary cardiologist (Vet Specialists Aotearoa, NZ)
Joanna Chagas – veterinary anaesthesiologist (Vet Specialists Aotearoa, NZ)
Damien Chase – veterinary surgeon (Vet Specialists Aotearoa, NZ)
Dan o’Neill – veterinary epidemiologist (Royal Veterinary College, uK)
Jane Ladlow – veterinary surgeon (Cambridge university, uK)
Jason Gagne – veterinary nutritionist (Nestle Purina institute, uSA)
t he topics were fascinating and there were a lot of take-home learnings. my top 10 (oK, actually 15) would be:
1. 21% of cats presenting in congestive heart failure have normal cardiac auscultation.

2. Reperfusion injury can be a serious complication of aortic thromboembolism.
3. t he N t-proBNP test has been actioned using pleural fluid instead of blood (different cut-off result). Diluting 1:1 with saline improves specificity.
4. temporary heart failure related to transient myocardial thickening can be seen in young cats.
5. minimally invasive transcatheter treatment of PDA has a 97% success rate.
6. 40–50% of anaesthesia-related deaths occur during the recovery period.
t his article was written as part of the requirements for receiving the Hill's Pet Nutrition/CAV Educating the Educators scholarship
7. medium chain triglycerides, amino acids, omega 3 fatty acids, and magnesium and vitamin E blend can reduce oxidative stress and improve cellular metabolism.
8. Hypoxia secondary to pulmonary hypertension can be a cause of syncope in dogs.
9. t he respiratory functional grading scheme can give guidance to breeders on how to lower the risk of producing puppies with severe BoAS (training programme required: watch this space!).
10. Brachycephaly is a human-made problem.
11. t here is ongoing research using Vet Compass – an information platform in the uK that has access to millions of clinical records from primary and tertiary practices.
12. Brachycephalic patients undergoing anaesthesia are automatically classified as stage 2 or greater on the ASA scale.
13. Be prepared for emergency intubation at any time during the recovery phase.
14. Negative intrathoracic pressure generated by increased inspiratory effort is likely a major cause of gastroesophageal reflux.
15. Laryngeal collapse is present in at least 50% of BoAS affected dogs and should not be confused with laryngeal paralysis.
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 49
Shanaka Sarathchandra CAV Executive Committee l
[Photo credit: Bodi Raw for Unsplash]
ISFM Research Roundup
ISFM publishes a monthly summary of the latest feline research which they have generously shared with CQ. In the July 2023 issue of Research Round-up we have an informative review on the feline skin microbiome, a pilot study of non-surgical contraception in female cats and studies of on foreign body removal and outcome and early identification of degenerative joint disease with the owner’s help. We hope you enjoy reading these interesting summaries.


Journal: Nature Communications 14, 3140, 2023.
Background: While surgical neutering of cats is the mainstay of pet population control, to manage free-roaming cat populations around the world, there is the need for efficient, safe and costeffective permanent contraception.

Aims of the study: t he study aimed to determine whether a single intramuscular (Im) treatment with an adeno-associated viral vector (AVV) delivering an anti- müllerian hormone (AmH) transgene can produce long-term contraception in the female domestic cat. High circulating levels of AmH were hypothesised to permanently suppress folliculogenesis, making the cat infertile.
Title: The feline skin microbiome: interrelationship between health and disease
Authors: CE older, A Rodrigues Hoffmann and AB Diesel.
Journal: Journal of Feline Medicine and Surgery 25, doi: 10.1177/1098612X231180231
Are you confused about what a microbiome is? t his review summarises what is currently known about the feline cutaneous microbiome. You will learn about the role of the skin microbiome in health and disease, the current state of research in this area and the potential for future studies to produce targeted interventions for cats.
You can access the full article here: https://journals.sagepub.com/ doi/epdf/10.1177/1098612X231180231
Methods: t he main study used nine female cats (aged 6–12 months); three controls, three low-dose and three high-dose. All cats were sedated with a single Im injection. t he control cats had a low dose of the empty vector. Additionally, there were three cats (6–7 years) that were treated as part of a pilot study which had the same treatment protocol as the low-dose cats but with an earlier version of the transgene. two mating trials with proven tom cats were performed for 4 months, 8 months and 20 months posttreatment.
Results: two of the three cats in the pilot study produced anti-AmH antibodies and circulating levels of AmH fell outside of the target range. No cats in the main study produced anti-AmH antibodies and while circulating AmH levels declined over the first year, they stabilised in all six of the treated cats within the target concentration range. In the breeding trials, all three of the control cats mated and produced litters at both breeding trials. two of the six treated cats allowed mating but none had luteal phases or became pregnant post-mating at both breeding trials.
Limitations of the study: t his is a proof-of-concept study; however, the numbers are very small and further research is needed.
Relevance to clinical practice: While not currently clinically relevant, this is a very interesting article that shows the value of collaborations outside of veterinary medicine, which may have a future benefit to feline welfare. t here are still a lot of questions to be answered before this is a commercially viable option, not least the breeding behaviour that was exhibited by two of the six treated cats.
Title: Durable contraception in the female domestic cat using viral-vectored delivery of a feline anti-Müllerian hormone transgene
Authors: Vansandt Lm, meinsohn mC, Godin P, Nagykery N, Sicher N, Kano m, Kashiwagi A, Chauvin m, Saatcioglu HD, Barnes JL, miller AG.
You can access the full article here: https://doi.org/10.1038/s41467023-38721-0
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 50
Title: Retrospective evaluation of surgical treatment of linear and discrete gastrointestinal foreign bodies in cats: 2009–2021
Authors: Gollnick HR, Schmiedt CW, Wallace mL, Sutherland BJ, Grimes JA.
Journal: Journal of Feline medicine and Surgery 25, doi:10.1177/1098612X231178140, 2023.
Background: Gastrointestinal (GI) foreign bodies (FBs) are common in cats, but little is known about the outcome in feline patients, particularly for linear foreign bodies (LFBs) which may be more common in cats. Variable rates of dehiscence are reported post-discrete foreign body (DFB) removal, and risk factors such as hypoalbuminaemia and neoplasia have been reported. With regard to LFBs, the prognosis is suggested to be poorer, particularly for cats with preoperative septic peritonitis.
Aims of the study: t he study aimed to report outcomes in cats undergoing surgery for GI FBs, identify preoperative risk factors and compare perioperative courses in DFB and LFB cases.
Methods: Records from the university of Georgia Veterinary teaching Hospital were examined and cats with a GI FB undergoing exploratory laparotomy were included in the study. Data from records were extracted, including details of presentation, surgery, anaesthesia, recovery and discharge.
Results: A total of 56 cats were included; 18 with LFB and 38 with DFB. most cats (96%) presented with vomiting, but only 12% had a palpable mass. Nearly a third (31%) of cats had an FB in multiple areas of the gut, with the most common location being the jejunum (44%), followed by the duodenum (24%). LFBs were most frequently anchored at the pylorus (56%) and cats with LFBs had a significantly higher body condition score, higher albumin (likely due to dehydration), longer surgery duration, higher ASA score, higher visit cost, longer duration of fluids and increased likelihood of developing a surgical site infection. All cats survived and none had post-operative septic peritonitis.
Limitations of the study: Limitations were that it is a retrospective study with data from clinical records, it was a referral population cats and there would have been bias in the way that the LFB cases were managed (i.e., more intensively).
Relevance to clinical practice: Cats undergoing surgery for FB removal have a good prognosis in general, whether linear or discrete, with minimal serious complications. o wners can be reassured that, in general, cats will recover after surgery to remove the FB, although more intensive perioperative care may be needed for LFB cases.
You can access the full article here: https://journals.sagepub.com/ doi/full/10.1177/1098612X231178140
Title: Do owner-reported changes in mobility reflect measures of activity, pain and degenerative joint disease in cats?

Authors: maniaki E, murrell J, Langley-Hobbs SJ, Blackwell EJ
Journal: Journal of Feline Medicine and Surgery 25, doi:10.1177/1098612X231178765, 2023
Background: Feline degenerative joint disease (DJD) is a common cause of pain in cats, with clinical signs that are difficult to identify given the tendency for cats to hide signs of pain and illness. Additionally, there can be a lack of agreement between radiographs and orthopaedic examination. t he Feline musculoskeletal Pain Index (FmPI) and Vetmetrica are ownercompleted questionnaires used for assessment of DJD pain and can be useful in identifying affected cats, while accelerometers are activity monitors used to objectively assess mobility.
Aims of the study: the study aimed to determine whether ownerreported mobility changes were indicative of early DJD-related changes as evaluated by the FmPI and orthopaedic evaluation.
Methods: o wners were recruited from the Bristol Cats Study and via an appeal to the public. Cats with unrestricted outdoor access were excluded and owners were asked 12 questions to establish mobility, dividing into control (no impaired mobility) and case groups (owner-reported impaired mobility). Cats were visited at home for orthopaedic assessment and placement of an activity monitor for 2 weeks. Vetmetrica and FmPI questionnaires were completed online by the owners prior to the visit.
Results: t here were 57 cats in the study (30 case, 27 control), and on orthopaedic examination, case cats had significantly higher pain scores than control cats, with pain bilaterally in 82% of cases, and significantly more crepitus and thickening of the joints. Case cats also had significantly lower scores for the FmPI and Vetmetrica domain of comfort, signifying a higher degree of impaired mobility and quality of life related to comfort. Accelerometry data were different between groups but not presented in the study.
Limitations of the study: o wner-reported data may result in reporting bias, cat temperament could affect orthopaedic assessment and there were more purebred cats than the general population in the study.

Relevance to clinical practice: using owners to recognise signs of impaired mobility (e.g., with a questionnaire) could mean earlier identification of cats with DJD. t his could facilitate earlier treatment, therefore improving the quality of life of affected cats. Given the agreement between orthopaedic examination and the FmPI with owner-reported information, we can adjust the questioning of owners of older cats during consultations to find cats in need of analgesia.
You can access the full article here: https://journals.sagepub.com/ doi/10.1177/1098612X231178765
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 51
What is your diagnosis? t He ANSW eRS…
1. What are your radiographic findings?
In the cropped ventrodorsal and left lateral projections (Figure 2) there is a reduction in serosal detail and fine discontinuous linear gas opacities dissecting throughout the colonic wall. t his is most apparent in the descending colon. t he colon is moderate-toseverely distended with fluid, gas, granular mineral opaque foci, and within the terminal descending colon, soft tissue opaque material with a stippled gas trapping appearance.
2. What are your top differential diagnoses for these findings?
Intramural gas opacities throughout the large intestinal tract are pathognomonic for pneumatosis coli (PC). t he fluid and gas distention of the colon are consistent with concurrent colitis, whereas the material with a stippled gas-trapping appearance located within the terminal descending colon may represent a more formed portion of the stool or some non-obstructive indigestible material.
Reduced serosal detail may be artefactual and secondary to radiographic technique or algorithm selection. A true reduction in serosal detail may be seen in patients with poor body condition/emaciation, an abdominal effusion, or generalised peritoneal inflammation.
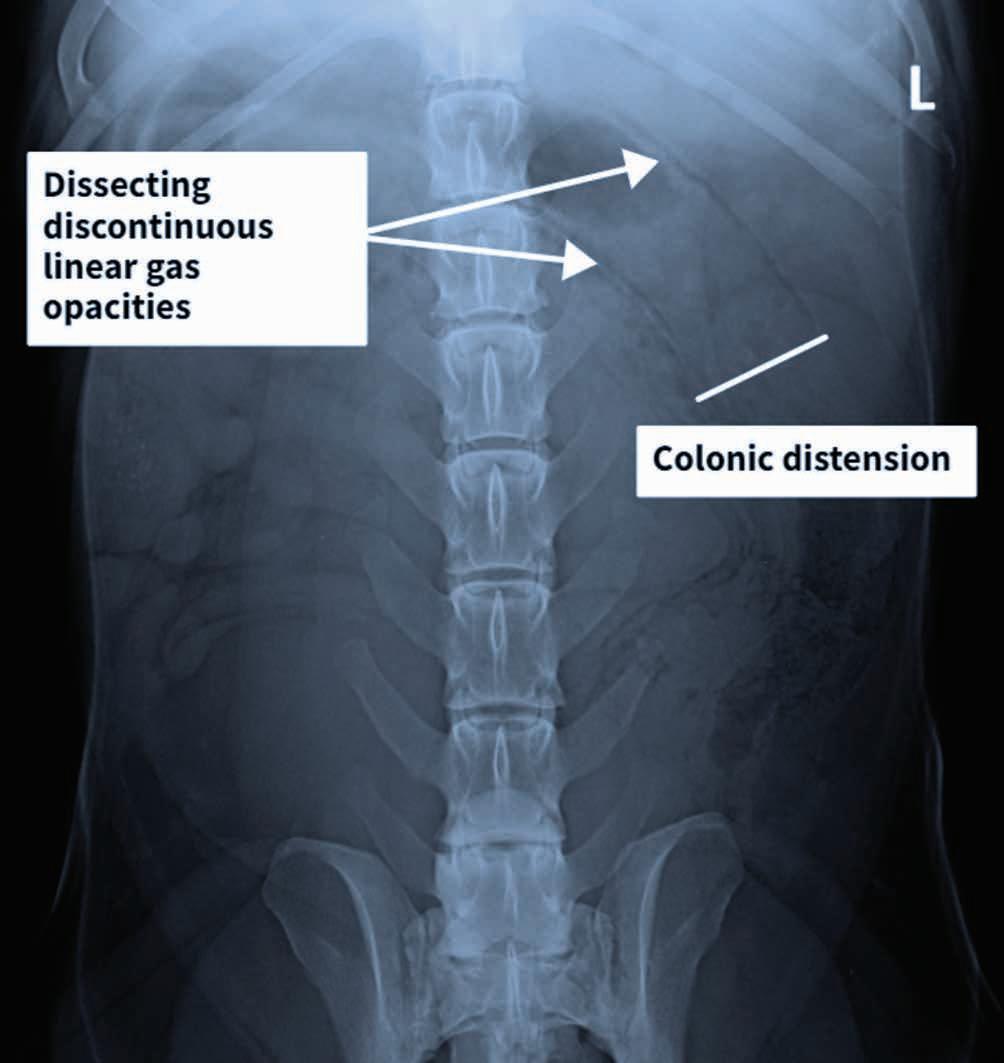

3. What would be your next diagnostic steps?
If available, an abdominal ultrasound would be recommended to look for evidence of an abdominal effusion and further assess the gastrointestinal tract.
Figure 2. l eft lateral (a) and ventrodorsal (b) abdominal radiographic views of a dog with chronic diarrhoea showing reduced serosal detail and gas opacities dissecting the colonic wall (arrows). Distension of the descending colon is visible in the ventrodorsal view.
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 52
a b
o ther diagnostic modalities such as colonoscopy could be used to obtain colonic biopsies.
Diagnosis
Colitis with pneumatosis coli.
Further diagnostics, treatment and outcome
An abdominal ultrasound was performed. t he wall of the colon was markedly thickened (up to ~0.75 cm) with variable loss of normal wall layering. It contained numerous focal-to-diffuse, linear, intramural, hyperechoic interfaces which cast dirty, distal acoustic shadows, consistent with gas (see Figure 3). t hese changes were seen across all aspects of the colon with the descending colon being the most severely affected. t he colonic lumen contained a mix of semi-soft faecal material, fluid and gas. t he colonic lymph nodes were mildly enlarged with a reduced echogenicity and there was a regional increase in echogenicity of the mesocolon. No free peritoneal fluid was detected. t he remaining abdominal organs were unremarkable. Based on these findings, a sonographic diagnosis of
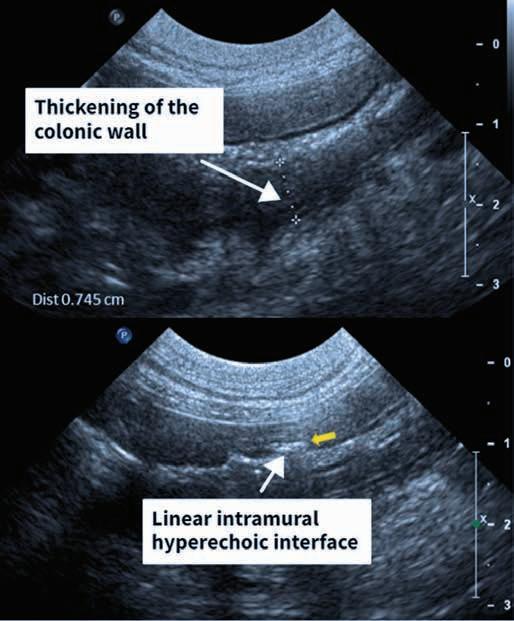
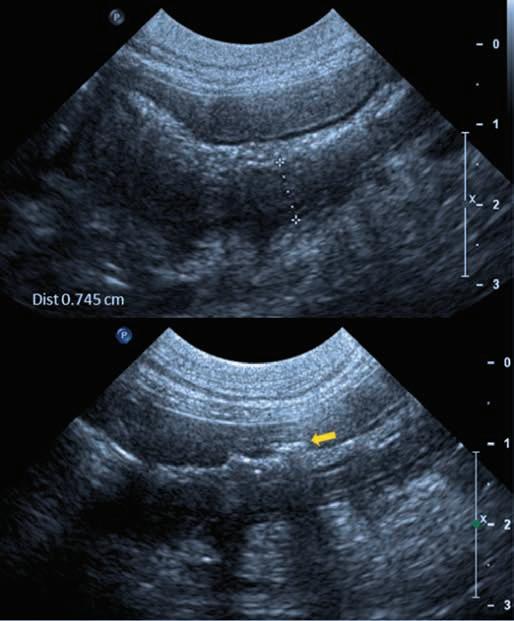
severe diffuse colitis with PC, mild colic lymphadenopathy and regional steatitis was made.
A colonoscopy was then performed, and biopsies taken under endoscopic guidance. on gross appearance, the colonic mucosa was thickened with a cobblestone appearance and sporadic regions of hyperaemia were noted. t he biopsies showed mild hyperplasia and fibrosis with some large rod-shaped bacteria noted within the submucosal layer which could be consistent with clostridial spp. Culture of a colonic biopsy sample yielded a moderate growth of mixed aerobic and anaerobic bacteria. No specific bacterial species were identified on this culture.
t he patient subsequently underwent a faecal microbial transplant (Fmt ) with 100 g of faeces from a young, healthy donor. t he donor had a complete deworming history, had not received antibiotics in the last 3 months and was free from gastrointestinal disease. t he faeces were added to 150 mL of sterile saline, strained to remove vegetable and particulate matter, and administered per rectum via an oesophageal feeding tube. It was observed to be retained for >3 hours.
In a follow-up consultation, the owner commented that the patient's faeces, although still soft at times, were significantly improved. t here were very infrequent bouts of diarrhoea reported, compared to previously when it had been constant.
Discussion
Pneumatosis coli is a rare intestinal disorder in dogs which is characterised by intramural emphysema of the large intestine. PC has a non-specific, largebowel clinical presentation with signs including diarrhoea, haematochezia, weight loss, tenesmus, constipation and abdominal pain. In small animals the possible causes of PC include bacterial overgrowth, foreign bodies, administration of enemas, neoplasia, ulcers and mucosal tears. PC can also be caused by trauma from colonic endoscopic procedures, particularly where colonic biopsies are taken. two hypotheses, bacterial and mechanical, have been proposed and derived from human literature to explain the etiology of PC. t he mechanical theory proposes that an increase in intraluminal pressure due to bowel obstruction causes dissection of gas into the bowel wall
focal,
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 53
a b
Figure 3. Abdominal ultrasound image of the colon with the image on the right showing thickening of the wall of the colon and a
linear, hyperechoic interface.
from either the alimentary tract or the lungs via the mediastinum. t he bacterial theory suggests that gas-producing bacilli lodge in the submucosa due to increased permeability of inflamed mucosa or tears in the mucosa itself.
Commonly in veterinary practice, radiography and ultrasonography are used to diagnose PC. In humans, C t is the modality of choice for definitive diagnosis of PC. on C t and radiography, PC usually presents as linear or bubbly gas patterns in the colonic wall. on ultrasonography, bright echoes within the layers of the large bowel wall are commonly described.
Due to the low prevalence of reported cases, there is no consensus on the best course of treatment. In the reported cases, treatment is typically initiated with metronidazole concurrently with a hypoallergenic diet.
In this case, this patient’s clinical signs were non-responsive to Anallergenic diet (Royal Canin) and metronidazole. t his patient underwent Fmt, a procedure that is well reported in the human literature, and there are emerging applications in small animal medicine. In dogs with acute diarrhoea, a single Fmt can have comparable effects at 1 week and improved effects at 4 weeks when compared to a 7-day course of metronidazole.
In PC, where pathogenic bacteria are buried in the submucosa, antibiotic penetration can be varied. Antibiotic use can decrease the diversity of gut microbiota. t his allows proliferation and mucosal infiltration of pathogenic Clostridium Perfringens C. Perfringens can be cultured from faeces in 80% of dogs, healthy or not. However, heavy overgrowth causes disease and clinical signs and is facilitated by insult to the digestive tract and/or compromised immune status. Faecal microbial transplantion works through increasing the competition for nutrients and space with healthy, normal intestinal bacteria. A Fmt can be a treatment option for PC, as well as other cases of diarrhoea, where antibiotics are ineffective. Surgical intervention has not been reported for PC in small animals. In humans, patients that are refractory to medical therapy or have severe abdominal pain, fever or hematochezia are surgical candidates. Surgery generally involves colonic resection and is often a last resort as mortality rates are high.
Conclusion
Pneumatosis coli is a very rare disorder in dogs that could be the cause of your patient’s large bowel diarrhoea signs that aren’t resolving. Keeping the key radiographic characteristics in your mind when interpreting abdominal radiographs will help you identify
and correctly treat these cases which have a great prognosis with medical management.
Acknowledgements
t hank you to Drs tommy Fluen and Devon t hompson of Veterinary Specialists Aotearoa for their assistance in producing this article.

Relevant reading
Azuma K, Kanemoto H, Ohno K, Fukushima K, Chambers JK, Tsujimoto H. Pneumatosis coli after partial ligation of congenital portosystemic shunt in a dog. Journal of Veterinary Medical Science 80, 1549–52, 2018
Degner DA. Pneumatosis coli in a dog. The Canadian Veterinary Journal 33, 609–11, 1992
Fisk A, Allen-Durrance A. Pneumatosis coli in a Dog. Journal of the American Animal Hospital Association 55, e554-01, 2019
Goodman RA, Riley R. l actuloseinduced pneumatosis intestinalis and pneumoperitoneum. Digestive Diseases and Sciences 46, 2549–53, 2001
Hwang TS, Yoon YM, Noh SA, Jung DI, Yeon SC, Lee HC. Pneumatosis coli in a dog – a serial radiographic study: a case report. Veterinarni Medicina 61, 404–8, 2016
Tuniyazi M, Hu X, Fu Y, Zhang N. Canine faecal microbiota transplantation: current application and possible mechanisms. Veterinary Sciences 9, 396, 2022 l
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 54
[Photo credit: Karen Kennedy]
Companion Animals NZ update
DAVID l l Oy D, general Mgr, CANZ
Significant changes ahead for cats in NZ?
t he Environmental Select Committee requested that we present a submission on the Erica Rowlands petition in June. t he petition called for cats to be desexed, microchipped and registered, to enforce responsible cat ownership and protect wildlife.
You may know by now that, after hearing from a range of organisations, the select committee has recommended parliament implement cat management legislation. t his could have far-reaching consequences for cats and cat guardians in New Zealand. We are excited that cats may require desexing, microchipping and registering. Legislation could also consider breeder registration and limiting cats per household. Discussion will need to be had about the ability of cats to roam off their property.
We know cat management is a contentious issue so we will be doing our bit to encourage sensible, evidence-based debate to ensure New Zealand is a place of responsible companion animal guardianship, where cats can live A Good Life.
Centre for A good life
Companion Animals NZ is creating an exciting new initiative to extend our welfare work. We are establishing a "Good Life Centre" to develop and drive animal welfare research and education programmes.

At the time of writing, we are recruiting Centre Director who will be coordinating and collaborating on research projects, as well as developing human behaviour initiatives to raise the standard of companion animal welfare in New Zealand.
We look forward to working with the companion animal veterinary industry in these projects.
eQuiChip
As part of our contribution to Cyclone Gabrielle recovery, we hosted three Hawke’s Bay equine microchipping days in July and August. our aim was to encourage more horse owners to use microchipping as a permanent means of identification for their horses and ponies.

Horses are under-represented in identification. t hey are harder to evacuate than cats and dogs, don’t wear ear tag identification like many other stock species and as such are more difficult to identify and repatriate when rescued or recovered.
t hanks to Vet Services Hawkes Bay, Vetsone Hastings, mPI, SPCA, Equestrian Sports NZ and Surepet Care who supported the event. All horses received a free t hermochip (meaning their temperature can now be taken with a scanner!) and New Zealand Companion Animal Register registration.

If your team see equine clients, please make microchipping and registration part of your normal callout.
Contact: https://www.companionanimals.nz/contact-us-
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 56
Checking a horse’s microchip at eQuiChip
The eQuiChip vet team from Vet Services Hawkes Bay and VetsOne
Veterinary Behaviour Conference and Accreditation Programme
Companion Animals New Zealand was pleased to sponsor the final day of Kiwi Vet Behaviours’ 2023 conference, The Power of Positive – Building Better Relationships, 4–7 August in Auckland.

Hosting both national and international speakers, including keynote speaker Dr Sarah Heath, (Veterinary Specialist in Behavioural medicine and Clinical Animal Behaviourist) from the uK, the conference was aimed at veterinary professionals, trainers and behaviourists, and anyone interested in the ethics and welfare of training dogs.
our General manager, David Lloyd attended and talked about CANZ’s accreditation programme to standardise behaviour and training using ethical and humane training methods based on an understanding of the way animals learn.
our previous surveys indicated that a national accreditation scheme is 91% of veterinarians and 91% of veterinary nurses (who do not provide their own behaviour services). We know your teams want to refer behavioural cases to a reputable professional.
Please check out www.companionanimals.nz/canzaccreditation to find an accredited trainer near you (or online).




Speaking of accreditation……we are delighted to welcome April Williams, Bridee manning and Rachael Stratton as newly accredited professionals.
l ostpet merging with NZCAR to make things even easier for animal guardians we are migrating our free lost and found pet listing site lostpet.co.nz over to the NZCAR (animalregister.co.nz).
When people lose a pet, they are often unsure what to do first. We are making this easier because soon marking a pet as lost on the NZCAR will automatically generate a LostPet listing.
You can expect to expect some excellent resources from us about how to talk to clients when they have lost a companion animal. Both website addresses will work for the foreseeable future. l
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 57
April Williams – Animal trainer/Animal training Instructor: Masterton, Wairarapa, greater Wellington [April Williams]
Rachael Stratton – Behaviour Veterinarian (and training services): online and lower North Island https://www.tailtherapy.nz/ [Rachel Stratton]
Bridee Manning – Animal trainer/Animal training Instructor: online only at http:// www.dogmechanics.co.nz/ [Bridee Manning]
Healthy Pets NZ update

CAt H WAt SON, Chair Healthy Pets NZ
our mission at HPNZ is “to support the generation and distribution of knowledge about companion animal health and wellbeing in New Zealand.” We achieve this through providing support for projects investigating companion animal health, informing veterinary professionals about industry developments, and through educating the public around advancements in veterinary medicine.
t he first half of 2023 has been a good opportunity to not only look forward to some exciting new research projects we’re funding, but also to take the opportunity to look back at research we’ve funded over the past 20+ years and reflect on the impact this has had on practice here in New Zealand.
In some cases, the research has been very specific to New Zealand – like Bronwyn Smits’ 2012 project which confirmed canine leproid granuloma syndrome as present here; or Kara Dawson’s 2009 study which determined the seroprevalence of Bartonella henselae (cat scratch fever) in cats in New Zealand and its relevance to veterinary professionals.
Recently completed research
With the introduction in 2020 of the Human-Animal Bond grant sponsored by Royal Canin, we’ve been pleased to see a marked increase in the applications we receive to explore the amazing bond we have with our companions. t he very first project we funded in 2020 has recently been completed and the report provided.
t he project by Anne Haase titled: Fostering cats for health: how do cat fostering programmes benefit companion animals and humans? set out to gain a better understanding of the benefits cat fostering programmes bring to the cats and the humans involved.

t he report explore the benefits of cat fostering programmes for both companion animals and humans. It highlights that while fostering of cats is an area that lacks sufficient research compared to fostering of other species such as dogs, it is a popular practice with its unique advantages. t he study consisted of a focus group and a video study to gather insights from volunteer fosterers and observe feline fostering practices.
their deep connection to animals, particularly cats, and their desire to help. Fostering provides an alternative to permanent cat ownership, allowing individuals to contribute without longterm commitment or financial burden. Emotional support from shelter staff and fellow fosterers was identified as crucial in helping fosterers cope with challenges and make difficult decisions. o verall, fosterers experienced personal benefits such as social well-being, mental health improvement, and practical advantages, while the cats benefited from a supportive home environment that reduced stress and prepared them for adoption.
t he video study focused on experienced fosterers’ interactions with the cats and kittens, capturing the skilled work and close relationship between the fosterer and the animals. It emphasised the importance of creating a secure and comfortable environment, understanding cats cues, and adapting the approach to suit individual foster cats needs.
In others, there has been the potential to benefit animals all over the world. t he findings from Jodi Salinski’s 2007 study into behaviour-based indicators of pain in cats may seem obvious now, but this was one of the early studies that has helped us recognise how cats behave when they are in pain and therefore manage it much better than we used to. t hen there is John munday’s research which identified a marker to help predict survival times in cats affected by oral squamous cell carcinoma. t his has led to exploring novel ways of potentially treating these cancers too.
Links to all completed research projects can be found on the HPNZ website: healthypets.org.nz
t he focus group study revealed that fosterers are primarily motivated by Contact: http://healthypets.org.nz/
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 58
[Photo credit: Iryna Bakurskaya from Pixabay]
In summary, cat fostering programmes offer numerous benefits for both humans and companion animals, fostering social well-being, mental health, and practical advantages for fosterers while providing supportive environments for cats and increasing their chances of adoption. Effective fostering practices rely on patience, adaptability, and a strong commitment to the welfare of animals.

New research
1. Professor Andrew Worth: Biomechanical equivalency of 3-D printed titanium bone plates
t his study aims to compare the biomechanical equivalency of 3D printed titanium bone plates with traditional press-manufactured plates used for fracture repair. t itanium plates are commonly used to provide stability during the healing process. While traditional plates are made from titanium ore, 3D printed plates are created by melting titanium alloy powder with a laser based on computer-generated designs. Customisation options offered by 3-D printing technology make it important to determine the equivalency of these plates to
standard recommendations. t he study will assess the strength and performance of 3-D printed plates in comparison to traditional plates, providing valuable insights for their potential use in patients.
2. Professor Carolyn Gates: Strengthening veterinary student communication skills through post-adoption preventive care consultations
t his study aims to address the lack of preventive care knowledge among new pet owners and improve communication skills of veterinary students. By implementing a programme that incorporates motivational interviewing (mI), veterinary students will be trained to effectively communicate with clients and empower them to make positive behavioural changes. t he programme will offer free post-adoption preventive care consultations in partnership with the SPCA, allowing students to gain real-world experience. t he study will collect data to evaluate the impact on adopters’ satisfaction, adherence to preventive care measures, and reduction in subsequent medical and behavioural problems. Additionally,
the programme’s effects on student confidence and competence in conducting preventive care consults will be assessed. ultimately, the findings will contribute to enhancing veterinary education and promoting optimal care for companion animals.
events
t here are a couple of events coming up in october where we’d love to have your support too!
If you are Christchurch-based with a dog and enjoy getting out for exercise together, then sign up for the 4 Paws marathon at https://4pawsmarathon. co.nz/. t his ‘pawsome’ event based in and around Bottle Lake Forest has a range of distances to cater for all abilities, and has partnered with three charities, one of which is Healthy Pets NZ.
If you are based in the Bay of Plenty, then make sure you keep the weekend of 7–8 october free for the Healthy Pets NZ annual Dogs Day out event, with event partner PD Insurance. With a great line up of speakers for the Saturday evening, and a fun family focused dogs’ day of activities on the Sunday, there are plenty of reasons to come along and join the fun. Even better, if you’d like to help out, get in touch. l
Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023 59
Committee
President
Natalie, Companion Animal Veterinary Advisor, Zoetis NZ, Wellington

I have been a companion animal practitioner for over 20 years with a strong interest in feline medicine, preventative health care and veterinary wellbeing. I joined the team at Zoetis in 2018 after selling my practice in Wellington. I enjoy the opportunities my role provides, meeting companion animal veterinarians across New Zealand, talking to them about the issues they face on a day to day basis, and learning where CAV can focus our member support in the most valuable way.
Additional roles: Member of WSAVA Congress Steering Committee
Vice-president
Becky Murphy, owner of TCI Glenbred, Feilding

I am a 2010 graduate from massey university. my practice, tCI GlenBred is devoted to canine inherited disease screening and theriogenology. I have been a member of the CAV Committee since 2018. I enjoy working with dog breeders and veterinarians to promote and improve genetic health, particularly in pedigree dogs. outside of work, my husband and I have two children, one dog, two cats, two bunnies and a very naughty pony, which keeps us very busy!
Additional roles: Member of WSAVA Hereditary Disease Committee
Treasurer
Kevanne McGlade: community veterinarian, Massey University


I work as a companion animal veterinarian in the Community Practice small animal clinic at massey university’s täwharau ora – School of Veterinary Science in Palmerston North. Each year our final year students spend a number of weeks in the clinic. my role is to support and guide them as they take on the role of a veterinarian, conducting first opinion consultations and performing procedures to equip them for practice after graduating.
Shanaka Sarathchandra, Clinical Lead Veterinarian, Pet Doctors Hamilton

I am a 2004 massey graduate working in companion animal practice in Hamilton. I have a special interest in imaging and medicine. I am looking forward to being more involved in the veterinary community.



Toni Anns, National Sales and Key Account Manager for Companion Animals, Zoetis NZ, Auckland
I have been a member of the CAV executive committee since 2016. I currently work for Zoetis as the National Sales and Key Account manager for Companion Animals. In this role I am very privileged to be able to meet and talk with many veterinary professionals. I listen to the trials and tribulations, the challenges, the joy and the angst of veterinary life and I want to help in whichever way I can. ultimately, I would love to see elevation in the reputation of this industry and improved collaboration both nationwide and internationally. my ultimate goal is to help improve the personal satisfaction at the end of each veterinary professional’s day. Additional roles: CAV rep on Healthy Pets NZ Trust
Nathan Wong, Veterinarian, SPCA, Auckland

I am a 2014 massey university graduate and started out working in general practice for the first 6 years of my career. I’ve since transitioned to working at the SPCA (plus the occasional moonlight shift at afterhours clinics) and loving the different and rewarding nature of shelter medicine. I’m passionate about animal welfare and making the veterinary profession more sustainable for us all. I currently reside in Auckland with my fiancé and three cats; Jeffrey, Jellybean and Juniper.
Secretary
Sally Aitken, Lead Companion Animal Veterinarian, VetEnt Turangi Community veterinary work is my passion; caring for animals through their life-stages. I grew up in Greymouth, worked 26 years in taupo in mixed and CA practice and am now based in turangi providing companion animal community veterinary care with VetEnt. Continuing education, ANZCVS, avian medicine, dermatology and internal medicine are my other vet passions, along with mountain-biking, tramping and raising two fantastic teenagers with my husband here in taupo.
Additional roles: CAV rep on Healthy Pets NZ Trust
Maddie Jardine, Companion Animal Veterinarian, Aldwins Road Vet Clinic, Christchurch

Ko maddie au (she/her). I graduated from massey and have worked in various places around NZ and uK, including a stint as a teaching associate at Bristol Veterinary School. I work at Aldwins Road Vet Clinic in Christchurch and relish our team. I advocate for diversity, equity and environmental sustainability in our profession and am also a member of the NZVA Climate Champions crew.
Ex officio members
Sally Cory, NZVA Head of Veterinary Services (Companion Animal) originally from the uK, I gained my veterinary degree from Edinburgh university. I embarked on a working holiday to NZ in 1999 and have been involved in companion animal practice since then, with recent years spent both working in and managing a Wellington based after-hours clinic. t he role of Head of Veterinary Services (Companion Animals) is an exciting new direction for me, and I am looking forward to working alongside CAV to help navigate and support our profession through a period of exciting change and progression.
Additional roles: NZVA rep on Healthy Pets NZ Trust
Sarah Fowler, Editor – Companion Quarterly (and NZVJ), Hamilton I have been the editor of Companion Quarterly (previously CAS Newsletter) since 2013. I am a 2010 massey graduate (after a starter career as a plant scientist) and worked in clinical CA practice before starting in 2016 as an assistant editor at the NZVJ. I am now editor-in-chief of NZVJ and I am stoked to have found a niche in veterinary publishing that allows me to use my previous scientific training and my veterinary qualifications.
Producing Companion Quarterly as a high quality source of CPD for CA vets in NZ gives me a great deal of satisfaction.
60 Companion Quarterly: Official Newsletter of the Companion Animal Veterinarians Branch of the NZVA | Volume 34 No 3 | September 2023
Executive
Members of the NZVA Companion Animal Veterinarians Branch












 Natalie lloyd, CAV President
Natalie lloyd, CAV President





































































































