
14 minute read
Unicystic Ameloblastoma Presenting as Dentigerous Cyst: A Case Report
Brinda Suhas Godhi, MDS; Raghavendra Shanbhog, MDS; Usha Hegde, MDS; and Suhas S. Godhi, MDS
ABSTRACT Unicystic ameloblastoma (UA) is a subtype of the common odontogenic tumor ameloblastoma. The clinical and radiological presentation of UA can present a confusing picture of an odontogenic cyst. Several reports in the literature claim that a UA arises from a dentigerous cyst (DC), but there is no conclusive evidence to support such a contention. With these conflicts, we report a case of UA in a 13-year-old patient that mimicked a DC on clinical, radiological and incisional-biopsy examination.
Advertisement
Odontogenic cysts and tumors constitute a considerable percentage of pathologies involving jaws. [1] Unicystic ameloblastomas (UA) are variants of ameloblastomas, which refer to those cystic lesions that show clinical and radiological characteristics of odontogenic cysts, but on histological examination show typical ameloblastomatous epithelium that lines part of the cyst cavity with or without luminal or mural tumor proliferation. [2] Dentigerous cysts (DC) are common developmental odontogenic cysts of the jaws and account for approximately 20% to 24% of all epithelium-lined jaw cysts. [1]
In the literature, a number of authors have claimed that ameloblastoma arises in a dentigerous cyst. But according to Shear, there is no evidence to support such a contention. [3] Much of the confusion has probably arisen for three reasons. Firstly, ameloblastoma, which is similar to an odontogenic keratocyst, may involve an unerupted tooth and may incorrectly be interpreted as a DC on radiographs. The second reason is that the biopsy in an ameloblastoma may be taken from an expanded locule lined by a thin layer of epithelium and thus mimicking a dentigerous cyst. Third, as Lucas pointed out, apparently isolated islets or follicles of epithelium are sometimes found in the cyst wall some distance from the epithelial lining. [3] These have been interpreted as ameloblastoma, although they bear only a superficial resemblance to the tumor. With these conflicts, herewith we report a case of a 13-year-old female patient with a unicystic ameloblastoma that mimicked a dentigerous cyst on clinical, radiological and incisional biopsy examination.
The importance of examining the complete excised biopsy specimen before arriving at a conclusive diagnosis in such cases has been highlighted.
Case Report
A 13-year-old female reported with the chief complaint of swelling on the lower left side of the face for a year. There was no history of pain or discharge associated with the swelling. The swelling gradually increased to the present size.
On extraoral examination, diffuse swelling was seen on the lower border of the mandible extending from the angle of the mouth to 1 cm anterior to the angle of the mandible. The overlying skin was normal; no visible pulsations or discharge were seen. On palpation, the swelling was afebrile, nontender and firm in consistency. No appreciable paresthesia of the lower lip and chin regions were found.
On intraoral examination, all permanent teeth were present except the left second premolar with a retained deciduous mandibular second molar. Nontender diffuse swelling was seen in the mandibular left buccal vestibular region extending from the lower left canine to first permanent molar. ( FIGURES 1 ) The swelling was firm in consistency with classic eggshell crackling concerning the deciduous mandibular second molar region. Past dental history and medical history were unremarkable. Her physical examination revealed no abnormality other than those related to the chief complaint.

FIGURE 1A. Preoperative clinical picture at the first visit. Extraoral swelling on the lower left side of the face.
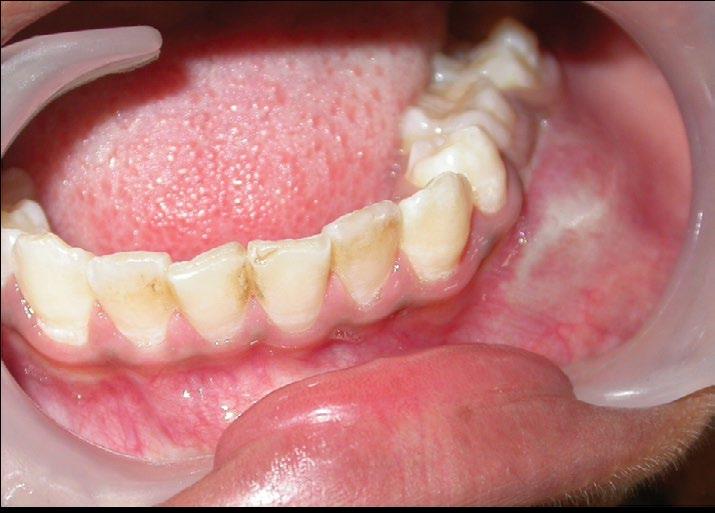
FIGURE 1B. Intraoral swelling in the mandibular left buccal vestibular region.
The panoramic radiograph revealed a well-defined homogeneous unilocular radiolucency measuring 3 cm by 3 cm with a sclerotic border associated with the crown of the impacted left second premolar arising from the cementoenamel junction. The left first premolar and first permanent molar were partially displaced with resorption of the roots of the deciduous second molar. The left second premolar was displaced to the inferior border of the mandible. The distal aspect of the lesion showed a scalloped outline suggestive of two-wall resorption (FIGURE 2). The mandibular cross-sectional occlusal radiograph showed expansion of the buccal cortical plate. Two milliliters of yellow, straw-colored fluid with cholesterol crystals was obtained on aspiration. Biochemical analysis of the fluid showed 8 gm/dl of protein.
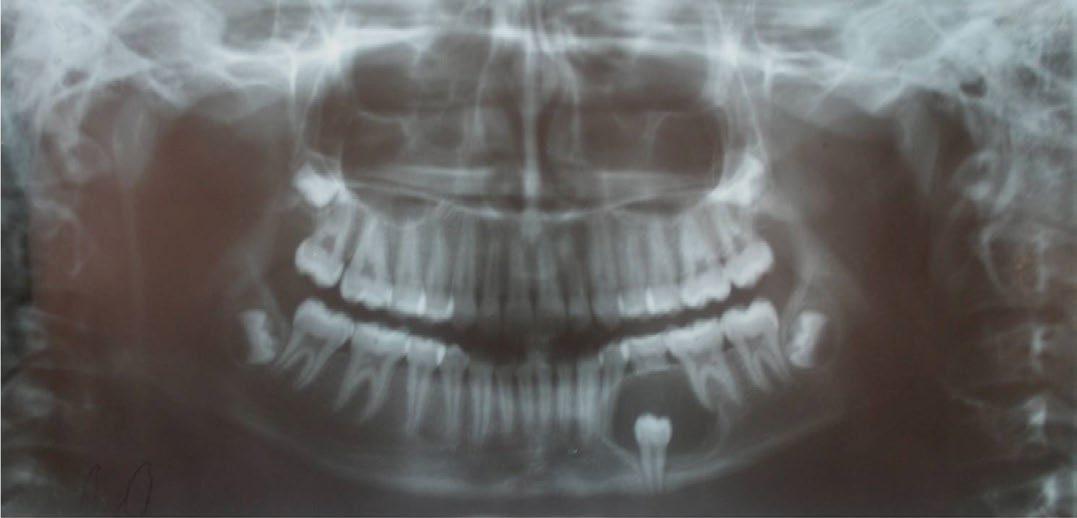
FIGURE 2. Panoramic radiograph showing welldefined homogeneous unilocular radiolucency with sclerotic border associated with the crown of the impacted left second premolar.
Based on these findings, a provisional diagnosis was made of a dentigerous cyst (central type) associated with an impacted left second premolar. Differential diagnoses of odontogenic keratocyst, unicystic ameloblastoma and odontogenic myxoma were considered.
An incisional biopsy was planned and the parents were informed to report back for the hematological investigations; however, the patient did not report back. After 18 months, the patient once again reported with the history of mild pain and increase in size of the extraoral swelling on the left side of the face. Worm’s-eye view showed an increase in the expansion of the left buccal cortex. Intraoral examination showed expansion of the left buccal cortical plate with complete obliteration of the left buccal vestibule. (FIGURES 3) All signs and symptoms were aggressive when compared with the initial visit.
FIGURE 3A. Preoperative clinical picture at the second visit. Extraoral swelling on left side of the face. SEE FULL VERSION OF JOURNAL FOR FIGURE.
FIGURE 3B. Worm’s-eye view showing expansion of the left buccal cortex. SEE FULL VERSION OF JOURNAL FOR FIGURE.

FIGURE 3C. Intraoral photograph showing expansion of the left buccal cortical plate with complete obliteration of the buccal vestibule.
The second panoramic radiograph showed unilocular radiolucency with the migration of the lower left permanent canine and first premolar and resorption of the first permanent molar (FIGURE 4). The impacted left second premolar was displaced further, thinning the inferior border of the mandible and indicating the aggressiveness of the lesion. Considering the aggressive nature of the lesion, a keratocystic odontogenic tumor and ameloblastoma were considered as differential diagnoses.

FIGURE 4. Panoramic radiograph showing unilocular radiolucency with migration of teeth Nos. 21 and 22 and resorption of No. 19. Impacted tooth No. 20 perforating the inferior border of the mandible is also seen.
A CT scan was advised to assess the nature and extent of the lesion and to plan appropriate surgical treatment. The axial view of the CT showed a welldefined buccal cortical expansion with an intact lingual cortical plate showing little expansion. A 3D reconstruction image also showed expansion and involvement of the lower left permanent canine, first premolar and first permanent molar (FIGURES 5).

FIGURE 5A. CT scan (axial view) shows a welldefined buccal cortical expansion with intact lingual cortical plate showing little expansion.

FIGURE 5B. Sagittal and coronal section 3D reconstruction image shows expansion and involvement of the lower left permanent canine, first premolar and first permanent molar.
Based on the clinical and radiographic examinations, an incision biopsy was planned. The deciduous second molar was extracted and an incision biopsy from deeper tissue was obtained by raising an envelope flap under local anesthesia. Histopathology of the incisional biopsy specimen showed a cystic lumen lined by three to four layers of flat to cuboidal cells with chronic inflammatory cell infiltration in the connective tissue capsule suggestive of a dentigerous cyst ( FIGURE 6).

FIGURE 6. Hematoxylin- and eosin-stained sections reveal reduced enamel epithelium transforming into a multilayered stratified epithelium from left to right (40 times magnification): TOP. cystic lumen, SECOND FROM TOP. reduced enamellike epithelium, THIRD FROM TOP. focal thickening of the lining epithelium and BOTTOM chronic inflammatory cell infiltrate underneath the epithelial lining.
Surgical enucleation of the cyst was planned under general anesthesia with the extraction of the permanent canine, the first premolar, the first permanent molar and the impacted second premolar on the left side of the mandible, followed by prosthetic rehabilitation.
Surgical Phase
The surgical area was exposed by placing a crevicular incision buccally extending from the mandibular left central incisor to the distal of the mandibular left second molar with a releasing incision to raise a mucoperiosteal flap and to expose the surgical field. The lesion was excised and the decision to apply Carnoy’s solution over the defect was made on the surgical table due to the extensive aggressive nature of the lesion. The flap was approximated and wound closure was done using 3-0 Vicryl (Ethicon, Somerville, N.J.) after placing platelet-rich plasma (PRP) in the surgical wound. (FIGURES 7)

FIGURE 7A. Surgical exposure of the lesion.

FIGURE 7B. After surgical excision, peripheral corticotomy.

FIGURE 7C. Excised specimen.

FIGURE 7D. Wound closure using 3-0 Vicryl suture.
The entire excised specimen was sent for histopathological examination. The hematoxylin and eosin (H&E) stained sections of the excised specimen revealed features suggestive of a unicystic ameloblastoma type 1.2 in areas, along with the incisional biopsy findings of a dentigerous cyst in other areas. In a few areas, the epithelium lining of the cyst showed basal cells with a palisaded appearance with a well-polarized nuclei, subnuclear vacuolization and subepithelial hyalinization, suggestive of unicystic ameloblastoma. Apart from areas showing luminal and intraluminal proliferation, there was no evidence of any solid tumor islands or mural proliferations. Hence, a conclusive diagnosis of unicystic ameloblastoma type 1.2 was given (FIGURES 8). Oneyear postoperative radiographic findings showed satisfactory healing with normal bone formation (FIGURES 9).

FIGURE 8A. Hematoxylin- and eosin-stained sections with epithelial lining showing Vickers and Gorlin criteria (40 times magnification).

FIGURE 8B. Hematoxylin- and eosin-stained sections of epithelial lining showing: TOP luminal proliferation, MIDDLE intraluminal proliferation and BOTTOM subepithelial hyalinization.

FIGURE 9A. Follow-up findings after 12 months. Orthopantomogram at 12 months revealed no evidence of recurrence and favorable bone remodeling.

FIGURE 9B. Followup findings after 12 months. A satisfactory clinical picture is seen at 12-month follow-up.
Discussion
Cystic lesions of the jaws presenting as small, asymptomatic unilocular radiolucencies enclosing the crown of an unerupted or an impacted tooth with predilection for males and occurrence in second or third decade of life are usually diagnosed as DCs. Sometimes they may become extremely large and cause cortical expansion and erosion. [3] In this case, based on clinical demographics and radiographic findings, a diagnosis of DC was made when the patient presented the first time.
When the patient presented for the second time, based on the clinical findings and the aggressive nature of the lesion on a panoramic radiograph and CT scan, an incision biopsy was planned to rule out the possibility of an aggressive lesion. The results of the incision biopsy revealed a DC. DCs with an extensive nature have the potential for a more aggressive transformation to an ameloblastoma; hence, complete removal of the lining by enucleation rather than conservative procedures like decompression and marsupialization is suggested. [4] Studies have shown that the use of Carnoy’s solution reduces chances of reoccurrence by its penetration into the depth of cancellous bone by 1.5 mm. [5,6] Due to the extensive nature of the lesion in the present case, and awaiting conclusive diagnosis following excisional biopsy, the present case was treated by surgical enucleation followed by use of Carnoy’s solution to decrease the chances of recurrence.
To call a cystic lesion a UA, it should satisfy the Vickers and Gorlin criteria. [7] In the present case, the complete examination of the excised specimen clearly revealed the presence of lining epithelium satisfying the Vickers and Gorlin’s criteria with luminal and intraluminal proliferations, suggestive of UA. The present case also established an increasing growth rate potential of UAs, as there was a definite increase in the size of the lesion from the initial presenting time to that after 18 months later. To aid in treatment planning, the present case was classified as a UA according to the World Health Organization system of 2003 [8,9] and as subgroup 1.2 as per the recently modified classification of Ackermann et al. by Philipsen and Reichart. [8] It is indicated that a tumor in subgroup 1.2 needs not be treated by segmental resection, but treated conservatively. Hence, in the present case, the treatment carried out was appropriate and did not need further intervention. The present case reemphasizes the importance of observation of a complete specimen rather than small incision bits in arriving at a final diagnosis and to intervene with further treatment process if necessary, after the final excisional biopsy report.
According to various theories, UAs may arise from reduced enamel epithelium associated with the developing tooth, or it may develop in a preexisting dentigerous cyst or other types of odontogenic cysts. Also, areas of solid ameloblastoma may undergo cystic degeneration leading to a UA-like appearance. However, satisfactory evidence to prove these theories is difficult and UAs are said to be de novo cystic neoplasms. [9]
Macroscopically, UAs reveal several intraluminal and/or intramural focal thickening nodules, but the absence of these does not contradict the diagnosis of UA. Microscopy of UAs shows the cystic lining with the basal ameloblast-like cells and suprabasal stellate reticulum-like cells. Ameloblastomatous proliferation in the lumen of the cystic cavity is termed as luminal and the same proliferation in the connective tissue as intramural and is considered to be aggressive compared to other variants. [10] Due to its aggressiveness, the treatment of UA continues to be controversial. Determinant factors for treatment planning are age, clinicalradiographic variant, anatomic locations, clinical behavior of the lesion, size, extent of the lesion and the histopathological variant. [5] In the present case, both luminal and intraluminal proliferation was seen. Considering these factors, the treatment of enucleation followed by the use of Carnoy’s solution was justifiable in this case. The use of Carnoy’s solution decreases the chances of recurrence after conservative surgical treatment of UA. [6] After surgical treatment, 93% of UAs with mural invasion have shown a recurrence rate of 10%. This further reinforces the use of Carnoy’s solution after surgical treatment in combating the recurrence of these lesions. [11] PRP is known to aid in fast healing by various mechanisms, [12,13] hence it was placed in our case before wound closure. Interestingly, in the present case the placement of PRP resulted in considerable filling of the defect with bone within a short period. After enucleation, the wound site showed good bone remodeling after six months of treatment without any evidence of recurrence. A long-term follow-up is mandatory, as recurrence of UA may be long delayed. [14] Our case was lost for follow-up after one year.
Lessons learned from this case that could drive a strong message to practitioners encountering such cases are:
■ When a cyst of the jaw is associated with an impacted tooth, the most common provisional diagnosis is a dentigerous cyst. The importance of the radiologist in carefully examining the radiograph to assess the true dentigerous cyst/ impacted tooth relationship needs no emphasis. Yet, unicystic ameloblastoma (dentigerous variant) needs to be considered as one of the differential diagnoses along with other cysts of the jaws until the histopathological examination of the complete excised specimen warrants otherwise.
■ An incisional biopsy cannot be the representation of the total lesion. A conclusive diagnosis can be arrived at only after examining the complete excisional specimen.
■ A thorough macroscopic examination of the excised specimen gives clues to the diagnosis as any nodular growths on the walls of the specimen imply more toward UA with intraluminal/mural growths.
■ Because the incisional biopsy will not give a complete picture and hence the definitive diagnosis, the treatment of an odontogenic lesion with aggressive cystic radiographic picture and not-soagreeable histopathologic findings on incisional biopsy should be treated by enucleation followed by Carnoy’s solution application rather than by the conservative approach.
■ If the final diagnosis favors UA, revision of the treatment based on the type and nature of UA as proposed in the literature with a long-term follow-up of the patient should be mandatory. n
REFERENCES
1. Ikeshima A, Tamura Y. Differential diagnosis between dentigerous cyst and benign tumor with an embedded tooth. J Oral Sci 2002 44(1):13–7. doi: 10.2334/josnusd.44.13.
2. Reddy SK, Rao GS. Unicystic ameloblastoma in 6-year-old child and its significance. World J Dent 2011 2(4):363–366. doi:10.5005/jp-journals-10015-1116.
3. Shear M, Speight P. Cysts of the Oral and Maxillofacial Regions. 4th ed. Oxford: Wiley-Blackwell; 2009:74.
4. Bhushan NS, Rao NM, Navatha M, Kumar BK. Ameloblastoma arising from a dentigerous cyst – a case report. J Clin Diagn Res 2014;8(5):ZD23–25. doi: 1 0.7860/JCDR/2014/5944.4387.
5. Marx RE, Smith BH, Smith BR. Swelling of the retromolar region and cheek associated with limited opening. J Oral Maxillofac Surg 1993 51(3):304–309. doi: 10.1016/ s0278-2391(10)80180-6.
6. Stoelinga PJ, Bronkhorst FB. The incidence, multiple presentations and recurrence of aggressive cysts of the jaws. J Craniomaxillofac Surg 1988 16(4):184–95. doi: 10.1016/s1010-5182(88)80044-1.
7. Vickers RA, Gorlin RJ. Ameloblastoma: Delineation of early histopathologic features of neoplasia. Cancer 1970 Sep 26(3):699–710. doi: doi.org/ 10.1002/1097-0142(197009)26:3<699::aidcncr2820260331>3.0.co;2-k.
8. Philipsen HP, Reichart PA. Unicystic ameloblastoma. A review of 193 cases from the literature. Oral Oncol 1998 Sep 34(5):317–25. doi: 10.1016/s1368- 8375(98)00012-8.
9. Reichart PA, Philipsen HP. Odontogenic Tumors and Allied Lesions. Batavia, Ill.: Quintessence Publishing; 2004:77–86.
10. Gabane M, Kulkarni M, Mahajan A. Unicystic ameloblastoma of mandible: A case report. Indian J Stomatol 2011;2:273–6.
11. Lee PK, Samman N, Ng IO. Unicystic ameloblastoma — use of Carnoy’s solution after enucleation. Int J Oral Maxillofac Surg 2004 Apr;33(3):263–267. doi: 10.1006/ ijom.2003.0496.
12. Forni F, Marzagalli M, Tesei P, Grassi A. Platelet gel: Applications in dental regenerative surgery. Blood Transfus 2013 Jan 11(1):102–7. doi: 10.2450/2012.0007-12.
13. Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: Results of a large multicenter randomized controlled trial. J Periodontol 2005 Dec;76(12):2205–15. doi: 10.1902/ jop.2005.76.12.2205.
14. Ackermann GL, Altini M, Shear M. The unicystic ameloblastoma: A clinicopathological study of 57 cases. J Oral Pathol 1988 Nov;17(9–10):541–6. doi: 10.1111/ j.1600-0714.1988.tb01331.x.
AUTHOR INFORMATION AND DISCLOSURES
Brinda Suhas Godhi, MDS, is a reader in the department of pedodontics and preventive dentistry at the JSS Dental College and Hospital, JSS Academy of Higher Education and Research in Mysuru, India. Conflict of Interest Disclosure: None reported.
Raghavendra Shanbhog, MDS, is a reader in the department of pedodontics and preventive dentistry at the JSS Dental College and Hospital, JSS Academy of Higher Education and Research in Mysuru, India. Conflict of Interest Disclosure: None reported.
Usha Hegde, MDS, is a professor and head of the department of oral pathology at the JSS Dental College and Hospital, JSS Academy of Higher Education and Research in Mysuru, India. Conflict of Interest Disclosure: None reported.
Suhas S. Godhi, MDS, was a professor and head of the department of oral and maxillofacial surgery at the I.T.S Centre for Dental Studies and Research in Ghaziabad, India. He died in 2011. Conflict of Interest Disclosure: None reported.
THE CORRESPONDING AUTHOR, Brinda Suhas Godhi, MDS, can be reached at drbrinda7@yahoo.co.in.






