THE CLINICAL ADVISOR • NOVEMBER 2013
A F O RU M F O R N U R S E P R AC T I T I O N E R S
NEWSLINE
■■Hormones and CV risk ■■Sleep apnea guideline ■■BP screening in children
ADVISOR FORUM
■■Smoking and low libido ■■Hepatits C: When to refer ■■Classifying dysplastic nevi
| N OV E M B E R 2 013 | www.ClinicalAdvisor.com
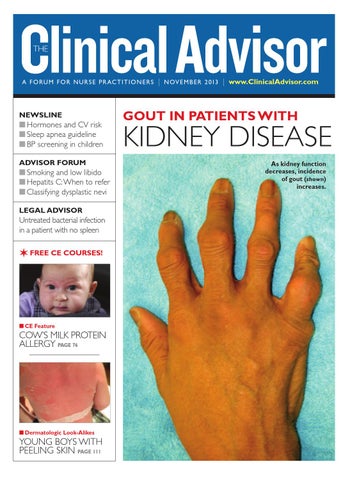
GOUT IN PATIENTS WITH
KIDNEY DISEASE As kidney function decreases, incidence of gout (shown) increases.
LEGAL ADVISOR
Untreated bacterial infection in a patient with no spleen
✶ FREE CE COURSES!
n CE Feature
COW’S MILK PROTEIN ALLERGY PAGE 76
VOLUME 16, NUMBER 11
n Dermatologic Look-Alikes
YOUNG BOYS WITH PEELING SKIN PAGE 111
CA1113_Cover.indd 1
10/30/13 1:58 PM