THE CLINICAL ADVISOR • DECEMBER 2013
A F O RU M F O R N U R S E P R AC T I T I O N E R S
NEWSLINE
■■CVD prevention guidance ■■Too few treated for hep C ■■Malaria cases on the rise
ADVISOR FORUM
■■Testing for multiple STDs ■■The life span of mono ■■Relief of oral mucositis
| D E C E M B E R 2 013 | www.ClinicalAdvisor.com
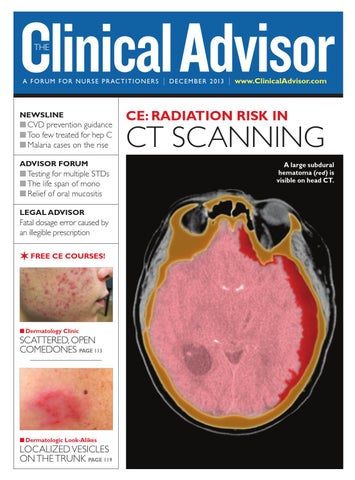
CE: RADIATION RISK IN
CT SCANNING A large subdural hematoma (red) is visible on head CT.
LEGAL ADVISOR
Fatal dosage error caused by an illegible prescription
✶ FREE CE COURSES!
n Dermatology Clinic
SCATTERED, OPEN COMEDONES PAGE 113
VOLUME 16, NUMBER 12
n Dermatologic Look-Alikes
LOCALIZED VESICLES ON THE TRUNK PAGE 119
CA1213_Cover.indd 1
11/27/13 12:33 PM