Differences between Indian GCP and ICH-GCP by CR Tutor www.crtutor.com
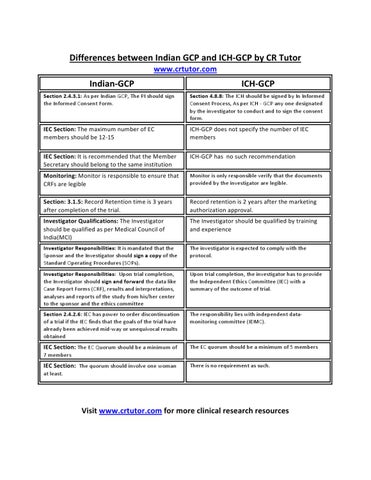
Indian-GCP
ICH-GCP
Section 2.4.3.1: As per Indian GCP, The PI should sign the Informed Consent Form.
Section 4.8.8: The ICH should be signed by In Informed Consent Process, As per ICH - GCP any one designated by the investigator to conduct and to sign the consent form.
IEC Section: The maximum number of EC members should be 12-15
ICH-GCP does not specify the number of IEC members
IEC Section: It is recommended that the Member Secretary should belong to the same institution
ICH-GCP has no such recommendation
Monitoring: Monitor is responsible to ensure that CRFs are legible
Monitor is only responsible verify that the documents provided by the investigator are legible.
Section: 3.1.5: Record Retention time is 3 years after completion of the trial.
Record retention is 2 years after the marketing authorization approval.
Investigator Qualifications: The Investigator should be qualified as per Medical Council of India(MCI)
The Investigator should be qualified by training and experience
Investigator Responsibilities: It is mandated that the Sponsor and the Investigator should sign a copy of the Standard Operating Procedures (SOPs).
The investigator is expected to comply with the protocol.
Investigator Responsibilities: Upon trial completion, the Investigator should sign and forward the data like Case Report Forms (CRF), results and interpretations, analyses and reports of the study from his/her center to the sponsor and the ethics committee
Upon trial completion, the investigator has to provide the Independent Ethics Committee (IEC) with a summary of the outcome of trial.
Section 2.4.2.6: IEC has power to order discontinuation of a trial if the IEC finds that the goals of the trial have already been achieved mid-way or unequivocal results obtained
The responsibility lies with independent datamonitoring committee (IDMC).
IEC Section: The EC Quorum should be a minimum of
The EC quorum should be a minimum of 5 members
7 members
IEC Section: The quorum should involve one woman
There is no requirement as such.
at least.
Visit www.crtutor.com for more clinical research resources