5 minute read
Hospital medical devices and Te Pātaka Whaioranga – Pharmac
Megan Nagel
Medical Devices Strategy and Development Engagement Lead Pharmac
Members from the Sterile Sciences executive and staff from Pharmac’s devices team have been meeting recently. Pharmac have pulled together some information about their role in the health and disability system. As well as an update on their hospital medical devices work programme and what this means for staff working in sterile services.
Te Pātaka Whaioranga – Pharmac’s role in the health system
We are a crown entity that was created in 1993, in response to rising prices of medicines through the 1980s.
Our job is to get the best health outcomes from pharmaceuticals (defined by Pae Ora, as “a medicine, therapeutic medical devices, or related products”) for New Zealanders, while staying within the fixed budget set out by the government.
We do not approve pharmaceuticals – that is Medsafe’s role to make sure pharmaceuticals are safe and work as they’re supposed to.
Our Board is accountable to the Minister of Health, who on behalf of the Crown, is accountable to Parliament for our performance.
Manatū Hauora – the Ministry of Health monitors how we are performing on the Minister’s behalf.
Each year the Minister outlines their priorities for Te Pātaka Whaioranga – Pharmac in a Letter of Expectation and we agree on our annual Statement of Performance Expectations. We also report to the Minister quarterly on actions and changes that we need to make, and we table an annual report in Parliament each year.
Managing the pharmaceutical budget
The Pae Ora (Healthy Futures) Act 2022 says Pharmac must decide which pharmaceuticals to fund for the best health outcomes for New Zealanders, while staying within our budget.
In 2022/23, we managed $2.4 billion in spending on pharmaceuticals.
We currently fund approximately 1,000 different pharmaceuticals (in over 2,000 different forms). These are the pharmaceuticals available in hospitals and from your local pharmacies.
Our budget has increased over time to enable us to fund new pharmaceuticals, widen access to pharmaceuticals already funded, and meet other costs related to inflation and population growth.
Our decisions are informed by evidence, internationally recognised assessment methods, and expert advice. We also use commercial tools (such as competitive procurement) to get as much value from the budget as possible so that we can fund more pharmaceuticals for more New Zealanders.
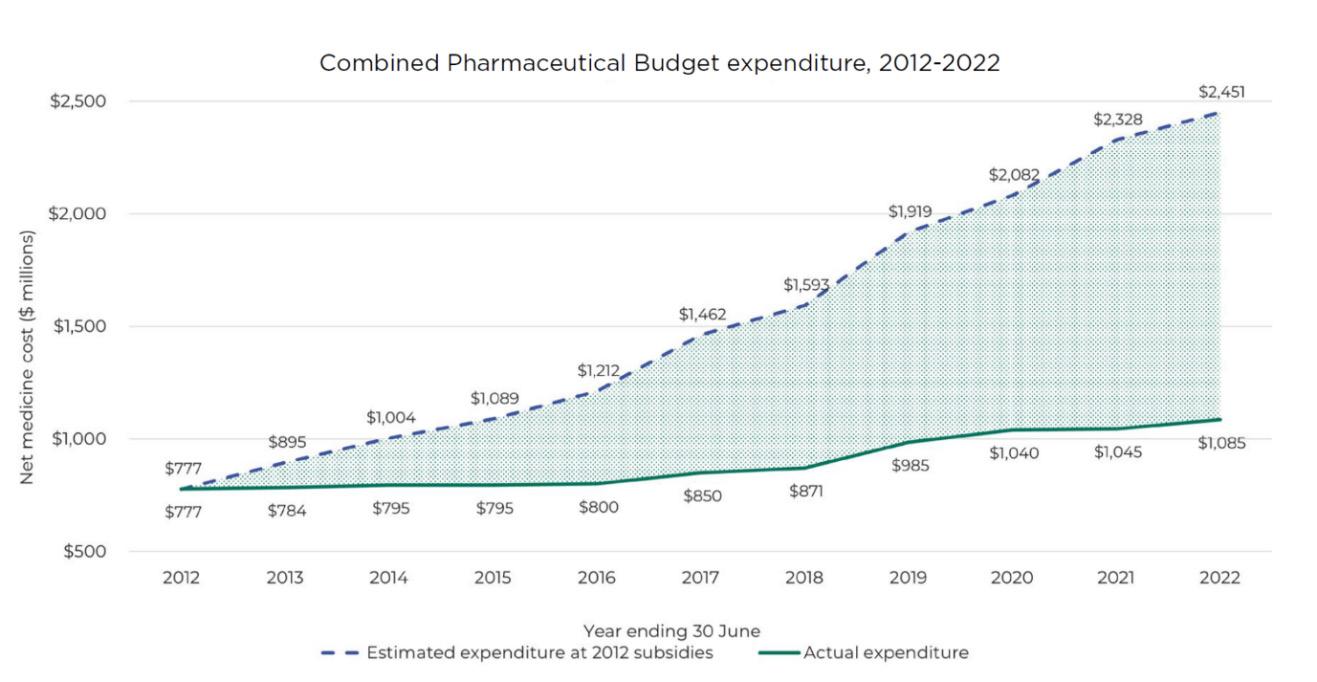
This graph shows our impact on New Zealand’s spending over the past decade, using 2012 prices as a baseline.
The gap between estimated expenditure (dotted line) and actual expenditure (solid line) highlights the $2.4 billion the health and disability system would have had to spend on treatments this year without Pharmac’s pharmaceutical management.
Our role in hospital medical devices
Our work in hospital medical devices covers a broad range of medical devices. These devices could be:
• used in a public health setting or part of a clinically driven pathway,
• used to diagnose, monitor, treat, modify, prevent, or support health requirements,
• used to maintain or improve health and/or safety for all people in the context of the NZ healthcare environment,
• part of an integrated system that supports the clinical environment.
This includes a vast range of products from tongue depressors and bandages to implantable devices (such as pacemakers), diagnostic software, and robotic surgery machines, through to rehabilitation equipment and continence support products. With the health and disability sector reforms, Pharmac is working closely with Te Whatu Ora as we continue to build a comprehensive national list of medical devices that are used in Te Whatu Ora hospitals and their community services.
We are building this list by negotiating national contracts. Currently our medical device contracts cover over $530 million of Te Whatu Ora’s annual expenditure on medical devices.
As of May 2023, about 75% of medical devices (~163,000 items) have been contracted and available for Te Whatu Ora to use, saving the health and disability system over $100 million since the work began.
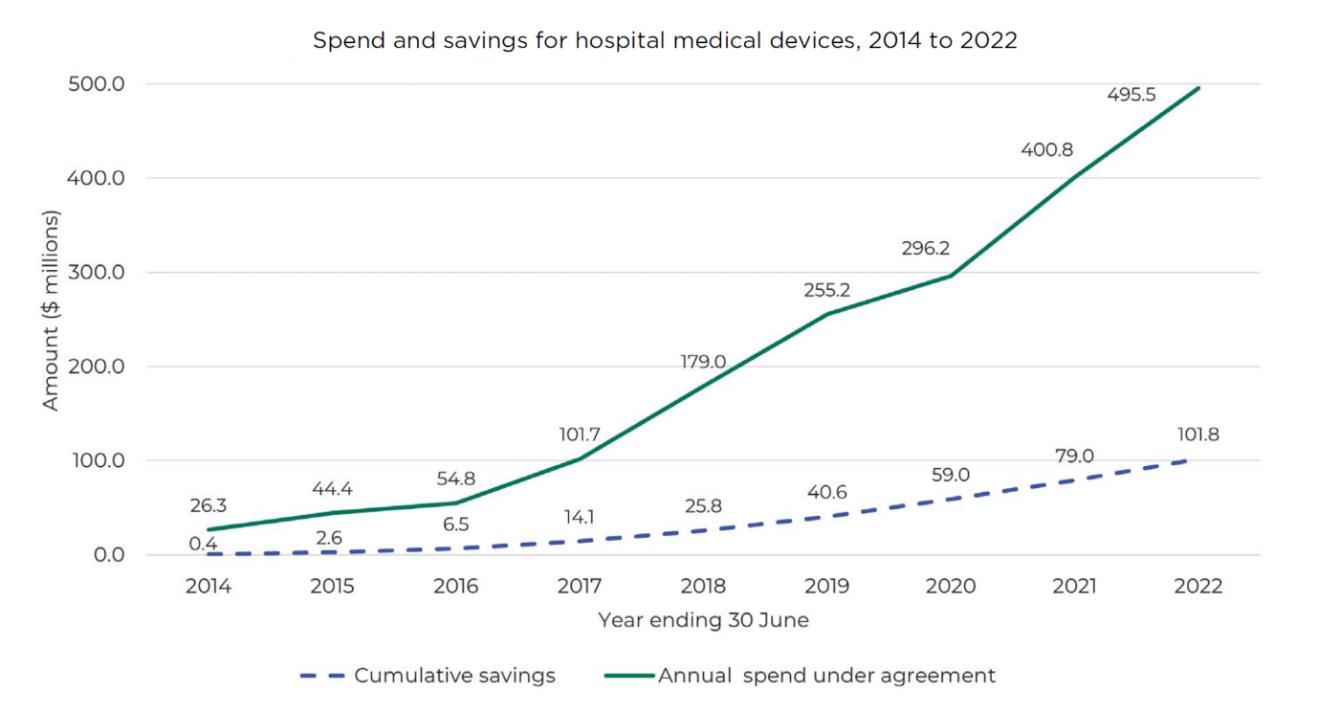
This graph shows our impact on medical devices spending and savings over the past decade. The total annual expenditure under agreement (solid line) shows how much has been spent on devices we have under contract each year. The dotted line shows the estimated savings that have accumulated through Pharmac’s national contracting.
Sterilisation and Decontamination Equipment and Associated Consumables (SDEAC)
Sterilisation and decontamination equipment and associated consumables are included within the scope of this work includes and covers the following products:
Decontamination and sanitation equipment
• Dirty utility sanitisers/macerators
• Medical device washers and disinfectors
Sterilisation equipment
• Low temperature sterilisers
• High temperature sterilisers
• UV light sterilisers
• Pre/post-sterilisation dryers
• Post-sterilisation storage cabinets
Trolleys for sterilisers/washers etc
• Containers for use in sterilisers/washers
• Trays
• Crates
• Baskets
• Tamper proof locks
Process tests/indicators, including but not limited to:
• Indicator tapes
• Air removal and steam penetration tests e.g. Bowie Dick
• Single parameter tests
• Multi-parameter tests
• Critical parameter/Cycle specific indicators
• Process challenge devices
• Artificial test soil products (when testing equipment such as cleaners/disinfectors and sterilisers)
Packaging
• Sterilisation wraps
• Sterilisation pouches/rolls
• Multipurpose towels/tray liners used in association with clinical decontamination and sterilisation processes
The products that have been contracted so far are on the hospital medical devices list which is published in Part III of Section H of the Pharmaceutical Schedule. This is available on our website (pharmac.govt.nz/devices-list).
The future of Hospital Medical Devices
To date, our focus has been on building a comprehensive national list of devices that are used in public hospitals through negotiating national contracts to build a strong foundation for strategic management of hospital medical devices.
The next step is to implement an integrated approach to hospital medical devices with our sector partners, which drives better value and more consistent and equitable access.
We continue to collaborate with Te Whatu Ora on this to ensure that our devices work is aligned and is supporting the broader organisations goals.
We expect that by 2024 this comprehensive list of devices will be finalised, and:
• Te Whatu Ora hospitals will purchase devices only from the national medical devices list,
• Pharmac will use new assessment and decision-making systems and processes to add new devices to the list.
There will be a new process to request changes to the list which will include adding new technologies, upgrading existing technologies, and delisting devices.
We will need feedback from a range of people to make sure we understand the clinical, technical, and operational requirements for a device. We will also need feedback from consumers and others who bring other important perspectives including Māori, Pacific and disabled people to inform decision-making.
The work we’re doing will mean that people, regardless of where they live, will have the same access to medical devices. Hospital staff will all be buying devices from the same list, with the same pricing and terms for supply.
We are supporting Te Whatu Ora to manage New Zealand’s increasing spending on medical devices sustainably and transparently. And by doing so, we will free up funding in hospitals for reinvestment in services and new technologies that will benefit more people.
We are also taking a longer-term view and aligning how we manage devices with the wider goals of the health and disability system.
To find out more
For more information go to www.pharmac.govt.nz/devices. If you have any questions, please email us at enquiry@ pharmac.govt.nz or keep up to date with our progress by signing up online to receive our bi-monthly newsletter Device Advice (www.pharmac.govt.nz/device-advice).
We look forward to continuing getting input from the Sterile Sciences Association and providing ongoing updates as our work continues.





