




















































































Kiaora, and welcome to the June Edi on of Supplyline. We are half way through the year already, and coun ng down to our 50th Anniversary Conference in September and beyond that to Christmas 2024. The year is just passing by.
We have a huge amount of informa on in this edi on. Ar cles, adverts and updates rela ng to on-going projects that the Execu ve Body is involved in. More importantly, in this issue of Supplyline is the informa on around vo ng for the NZSSA President and Execu ve member’s roles for the next term. Paul Moody our Secretary, has done a lot of work around this. The vo ng will be available on-line for NZSSA Members so please read the informa on carefully.
Donna Dador from Southern Cross Hospital has shared some photos of her staff celebra ng World Sterilisa on Day. The cake made to look like a wrapped set is amazing. Very crea ve and in keeping with what we do. Thank you for Donna and her team for sharing these photos.

If there are any other units that have celebratory occasions and would like to share photos and informa on, please send it through to me and I’ll include it into Supplyline. Would love to see what other areas are doing and celebra ng.
As I men oned above, it is our 50th Anniversary this year. Such a huge achievement, we should be extremely proud of this. We have come a long way in this profession from being thought of as ‘glorified cleaners’, to being the professional body for reprocessing RMDs. Our 50th Anniversary conference in September is an opportunity to acknowledge and celebrate how far we have come.
Take care and hope to see many of you at the NZSSA Conference in September.
Ngā mihi
Aileen Derby Editor NZSSA Supplyline
Welcome to the winter edi on of your journalSupplyline.
This edi on is our largest yet, therefore lots for you to read.
I am excited, who else is excited to celebrate 50 years of the NZSSA. Not many professions can claim they have been around that long. When I started in theatre many moons ago CSSD staff were effec vely the “dishwashers or cleaners” No one was expected to have knowledge and qualifica ons and did what they were told without knowing why. Even myself as a theatre nurse never really knew what CSSD was about un l I took over the manager role. Look at you all now. You are educated professionals, you have a voice and can make decisions on what is best prac ce. The profession and each of you have come a long way.
This year we are celebra ng those 50 years in style in Auckland. Are trades are all coming on board to join us and we would love you the technicians to make this the best conference ever.
As you may all be aware this is the year that you the members have the opportunity to vote for your execu ve
to lead the profession through the next three years. All details of nominees and how and when to vote are in this edi on. I am very pleased to see that we have some new members who are willing to step up and join the execu ve as well as some exis ng members of the team.
In order to vote you will need your membership number which is available to you on line on the NZSSA website. Please make sure that your email address is current in order for vo ng informa on to be sent to you.
The NZSSA is s ll receiving applica ons for equivalency cer fica on from overseas applicants as well as from those overseas origina ng technicians who are working in New Zealand and are seeking equivalency in order to obtain a residency visa. We expect that there will be a lull in the process while Health NZ/Te Whatu Ora are taking longer to approve vacancies.
On that note it is important to state that Sterile Technicians may be Allied Health professionals, however we remain as clinical front line staff. This was affirmed to be by the NZ nursing council when I had to submit my senior nurse por olio to them a year ago. I asked the ques on “as a qualified sterile technician and a registered nurse am I considered clinical”?
The response from the NZNC was that my CSSD role
was clinical and that through the reprocessing of surgical instruments I was providing indirect clinical care to the pa ents. Therefore remember if anyone tries to tell you otherwise, correct them, as without CSSD staff in the periopera ve clinical arena there will be no surgeries occurring.
Aileen Derby and I recently a ended a workshop presented by Pharmac. They had leaders from relevant professions a ending to discuss reusable medical devices including instruments, sterilisers, washers etc. Going forward they will seek our input on new devices that companies wish to register and hospitals may wish to use. They are being very proac ve. Unfortunately it appears from that mee ng that a TGA equivalent for NZ is s ll a way off
Aileen and I also a ended one of the regular scheduled mee ngs with the Ministry of Health for self-regula ng professions. These are chaired by The Chief Allied Professions Officer for NZ Dr Mar n Chadwick and Lauren Hancock, Senior Advisor to the CAPO. At this mee ng we had a presenta on rela ng to the HPCA and poten al changes that could occur. They are looking at regulated and self- regulated professions and it could mean we become regulated or they will introduce new categories of regula on. The work is only in the early stages but it looks promising.
Take care of yourselves
Shelagh Thomas President/NZSSA
We care for each other, showing kindness and empathy in all that we do.
We are commi ed to fi nding future focused solu ons and take personal responsibility to be be er every day.
Our diversity is our strength, we back each other and work together in partnership.
We are commi ed to doing the right thing by ensuring equity and hauora are at the heart of everything we do.
The current execu ve term is due to end in September of this year, and we have a host of new candidates looking to join the Na onal Execu ve and take on the role of President. Our cons tu on defines the execu ve as comprising ten members: the President, the Secretary and eight other members and they serve for a period of three years so please take the me to read the biographies below and learn a li le about each volunteer before exercising your vo ng rights!
This year vo ng papers will be sent out via an email link and will also be available through the associa on website (www.nzssa.org). You have two weeks to complete the vo ng form (but don’t wait, make an informed decision now) and will need to supply your name, email address and associa on membership number to ensure your vote is valid.
You will be asked to vote on two independent categories:
Category 1 – Associa on President
There are three candidates running for the role of
President. You have ONE vote for this category. Place your vote alongside the name of the candidate you believe will best serve the associa on over the next three years.
Category 2 – Members of the Execu ve
There are ten candidates wan ng to join the next execu ve team. You have NINE votes for this category. Place a vote alongside the names of up to nine candidates you believe will best serve the associa on over the next three years. You do not have to use all nine votes.
Note that votes in category one have no bearing on outcomes in category two and vice versa. If you want a candidate to be President but also think they should be an execu ve member then you need to vote for them in both categories.
To help with your decision each candidate has supplied a short statement as to why they wish to serve on the Na onal Execu ve. These are presented below in alphabe cal order on a first name basis.
Choose one of the following three candidates.

I have worked in the sterilising field for over 40 years now star ng as a CSSD Assistant in a small public hospital in Te Kui . During the 40 years I have had a variety of roles, including Technician, Deputy Manager, Educator and Manager.
Currently I am the Manager of the CSSD at Coun es Manukau District in Auckland. There are two CSSD’s in Coun es Manukau District, one at Middlemore Hospital and one at Manukau Health Park (MHP). We are currently part of a project where four more theatres are being built at MHP, as well as a new PACU and a new CSSD.
I have had the privilege of having been on the NZSSA Execu ve body previous to my current tenure, and served as both Secretary and President. I then had a break before being nominated as an Execu ve Member in our last elec on process.
For the last 2 ½ years I have served on the NZSSA Execu ve Body as the Vice President. I’ve been involved in a number of interes ng projects including being the Editor of Supplyline, se ng up a complaints process, self-regula on and looking at competencies for sterilising personnel to name a few. This year I’m on the Conference commi ee for what will be an exci ng occasion – our 50th Anniversary conference.
The world of sterilising prac ces and standards has come a long way and it’s been a honour to have been part of that. I’m looking forward to seeing what comes next, both in my own workplace and with the NZSSA.
Thank you

Hello to everyone reading this. I have wri en a li le bit about my vision for moving the NZSSA forward and why I think I would make a good NZSSA President.
My vision for the NZSSA moving forward
Currently the NZSSA is moving forward in the right direc on with the self regula on process, I would like to see this con nue and the NZSSA become the regulatory body for all reprocessing of reusable medical devices ac vi es throughout New Zealand. I would also like to strengthen our rela onship with government departments to help our industry get more recogni on for the work that we do, which in turn may help with pay parity discussions in the future. I belive it is important that the rest of the healthcare industry understands and respects our contribu on to the pa ent journey and understands how important our role and industry is.
The NZSSA has been established for 50 years and in that me it has grown to become what it is today, the NZSSA has moved the direc on of this industry forward since its establishment and I want to do my part to help it con nue to move forward.
I have been in this industry for 15 years now and I am here to stay, I am here to help grow the industry, I am here to help it gain the respect it deserves, I am here to ensure the pai ents receive the best possible care that we can provide.
My name is Mar n Bird. I am the manager of Sterile Science Department at Dunedin Hospital, and I have been an execu ve member for 4 terms, and with nearly 30 years prac ce experience behind me I have networked extensively throughout New Zealand and Australia which has enabled me to accumulate great resources, mentors and specialist knowledge.
I am confident that I have the leadership skills, qualifica ons, experience and commitment that the role of President of the New Zealand Sterile Science Associa on requires, and I believe that this is the next progressive step within the Sterile Sciences industry.
Thank you for considering me for the Sterile Science Execu ve.
Choose up to nine of the following ten candidates to form the remainder of the Execu ve commi ee.

I have worked in the sterilising field for over 40 years now star ng as a CSSD Assistant in a small public hospital in Te Kui . During the 40 years I have had a variety of roles, including Technician, Deputy Manager, Educator and Manager.
Currently I am the Manager of the CSSD at Coun es Manukau District in Auckland. There are two CSSD’s in Coun es Manukau District, one at Middlemore Hospital and one at Manukau Health Park (MHP). We are currently part of a project where four more theatres are being built at MHP, as well as a new PACU and a new CSSD.
I have had the privilege of having been on the NZSSA Execu ve body previous to my current tenure, and served as both Secretary and President. I then had a break before being nominated as an Execu ve Member in our last elec on process.
For the last 2 ½ years I have served on the NZSSA Execu ve Body as the Vice President. I’ve been involved in a number of interes ng projects including being the Editor of Supplyline, se ng up a complaints process, self-regula on and looking at competencies for sterilising personnel to name a few. This year I’m on the Conference commi ee for what will be an exci ng occasion – our 50th Anniversary conference.
The world of sterilising prac ces and standards has come a long way and it’s been a honour to have been part of that. I’m looking forward to seeing what comes next, both in my own workplace and with the NZSSA.
Thank you

Kia Ora, my name is Antony Owens, and I have been involved in the Sterilisa on Sciences field since January 2002. I have a keen interest in educa on and following comple on of my Degree in Management in 2004, I a ained the Cer ficate in Sterilisa on Technology in 2006, and completed the Diploma in Sterilisa on Technology in 2022.
I have been the Sterilisa on Department Team Lead at Southern Cross Gillies Hospital since 2015, and fully enjoy the challenges the role encompasses and the interdepartmental rela onships it provides, especially with the Theatre Services Manager and wider Hospital Team.
I am passionate about Sterilisa on, its importance in the pa ent’s journey, the work we do, and the con nual professional development for those who work in the area each day. My passion is quality management within sterilisa on, and ensuring the educa onal pathway provides opportuni es and challenges to encourage people to choose sterilisa on as a career, whilst ensuring those who work in sterilisa on have a visible pathway to progress.
Outside of sterilisa on I have been Chairman of a Football Club in Auckland for the past several years and hold a number of OFC/NZF coaching qualifica ons. Being involved in the running of a football club, and many hundred members, always presents a challenge but it is a fantas c way to contribute to my community. I am also very pa ently learning the Samoan language, thanks to my very pa ent partner!

I am currently employed as SSD Coordinator and Health & Safety Coordinator at Evolu on Healthcare Royston Hospital in the lovely Hawkes Bay. I have obtained the NZ Level 4 Cer ficate in Sterilising Technology through Toi Ohomai in 2021 and am currently studying towards obtaining my NZ Level 5 Diploma in Sterilising Technology. I hold a degree in Social Sciences, and a post-graduate (Honores) degree in Criminology, have a cer ficate as Ambulance A endant and am a qualified Enrolled Nurse.
My main objec ve in the working environment is to con nually be er myself and grow, gaining knowledge through experience in a wide variety of fields, to enjoy what I do and use all my skills and talents. I have a passion for health & safety, problem solving, audi ng, processes, learning and training people.
Personally, I am friendly, love animals, socialising, fundraising for chari es and watching rugby. I value respect, integrity, honesty, empathy, kindness and fairness.
I would appreciate the opportunity to serve as Execu ve Member for the NZSSA - to learn and specialise in the profession, contribute to the growth of the profession and the wellbeing of members, advoca ng for them and informing them.
Thank you for the opportunity.

I am Charanjeet Kaur Sidhu and I am currently working as a Quality Facilitator for CSSD, Health New Zealand Coun es Manukau, covering two sites, Middlemore Hospital CSSD and Manukau Health Park CSSD. I have about 15 years of experience working in CSSD.
I started as a CSSD technician at Auckland City Hospital, and have held roles of Ac ng Shi Co-ordinator, CSSD Supervisor, Ac ng CSSD Manager, and now CSSD Quality Facilitator. I have grown step by step in my career by learning through courses and by experience.
Being in a quality role, I go through the standards and take quality implementa on and quality improvement ini a ves for the unit.
NZSSA is a sterile sciences organisa on for the whole country. Being on the execu ve commi ee, I would be able to share my knowledge around the standards and the challenges with the quality improvement ini a ves. It would also give me an insight how NZSSA works, understand the changes happening in sterile sciences and think big for the country as a whole.

Life is too short to be anything but happy, the more you enjoy and love your work ,you become a be er version of yourself. A lot of great outcomes are by-product of loving what we do.
Hi, I’m Donna, A highly mo vated SSD Manager with change maker mindset and passion for quality improvement ini a ves.
A Nurse by heart and an SSD technician by choice. I have come to terms that although I am not working beside a pa ent assis ng them personally, I am s ll taking part in their journey to recovery by providing safe, sterile, and quality instruments for their surgery.
I wish to join the NZSSA to be able to connect to more people and further my professional development. To be able to learn informa on, share and promote educa on to my fellow SSD professionals.
I have a bachelor’s degree in nursing, holder of level 7 Health Services Management diploma, Completed Level 5 steriliza on cer ficate and am currently enrolled for the Diploma in Sterilizing technology Level 5. I genuinely believe that educa on is the key to achieving a safe and quality service for every pa ent.
My skills include a proac ve approach to change and quality improvements, sensible and resilient, able to blend in and adapt to diverse cultures and environment as evidence by previous employments in other countries such as Philippines and Singapore.

Kia Ora
Hello, my name is Jenny Carston and I work in the Periopera ve department of our hospital as the team leader for the CSSD department, Hauora a Toi Bay of Plenty. Although I am based on the Tauranga site, I provide professional advice and support for the team on the Whakatane site. I have been on the Execu ve Commi ee for the last I think 17 years and have been in the sterilising field for 27 years. I enjoy what I do and have no ced that over the last few years, we are finally ge ng a voice, especially through Pay Equity and now a lot of the hospitals realize that without us they cannot perform their cases in theatre and as I remember back when I first started 27 years ago and a ending a wage nego a on HR saying anyone can do our job we are just cleaners. I would like to see that person now and ask him does he s ll feels the same a er all these years.
We are finally ge ng the voice we need and with everyone behind NZSSA with more members obtaining registra on, I see greater recogni on of our specialty and a be er understanding of the importance of our role.

I am the Manager for the Sterile Sciences Unit- Acute & Elec ve Surgical Services, here at Palmerston North Hospital. I started working in the SSU back in 2004.I wanted a change, and a trainee technician role came available. I found the work interes ng and rewarding and it quickly changed from “a job” to something I could see becoming a career.
I became the Manager 3 days before the Covid lockdown back in 2020. Naviga ng a new posi on in an unknown and changing health environment wasn’t without its challenges, but with help from a great and suppor ve team – here I am- s ll here four years later. With the support from the NZSSA, our Sterile Science Technicians are now recognised as professionals within the Surgical Services family. I would like to see the encouragement of con nued educa on so Technicians can con nue to build the important profile we deserve within healthcare.
Outside of work, when I am not being a taxi to my 5 children, I work for MPI as a Fishery Officer, conduc ng recrea on fishery compliance inspec ons.

Thank you for considering my nomina on for a posi on on the execu ve commi ee of the New Zealand Sterile Sciences Associa on (NZSSA). I am honored to have the opportunity to con nue serving in this capacity.
As a current execu ve member, I have found great sa sfac on in my role, par cularly in assessing registra on applica ons. This responsibility allows me to gain insight into the ongoing educa on efforts of sterilising staff across New Zealand. Addi onally, I am privileged to witness the exemplary work of talented technicians, which underscores the dedica on of our profession within the healthcare sector.
With over 30 years of experience in Sterile Services, encompassing both public and private sectors, I bring a wealth of prac cal knowledge to the table. My journey has seen me serve as a sterilising technician and, currently, as the manager of the sterilising facility at the University of Otago Faculty of Den stry.
This role has been fascina ng and fulfilling, as it has afforded me the opportunity to bridge the gap between den stry and reprocessing standards, ensuring alignment with both office-based and hospital standards.
In conclusion, I am driven by a passion for advancing the field of sterile services and empowering others to deliver the highest quality of care. I am eager to collaborate with fellow commi ee members to address challenges, share best prac ces, and elevate the standards of our profession. Thank you for considering my candidacy, and I look forward to the opportunity to contribute to the NZSSA’s mission.
Ngã mihi Kelly Swale

My name is Mar n Bird. I am the manager of Sterile Science Department at Dunedin Hospital, and I have been an execu ve member for 4 terms, and with nearly 30 years prac ce experience behind me I have networked extensively throughout New Zealand and Australia which has enabled me to accumulate great resources, mentors and specialist knowledge.
I am confident that I have the leadership skills, qualifica ons, experience and commitment that the role of Execu ve of the New Zealand Sterile Science Associa on requires, and I believe that this is the next progressive step within the Sterile Sciences industry.
Thank you for considering me for the Sterile Science Execu ve.
Nga mihi, Mar n Bird

I am seeking to be re-elected to the execu ve team of the NZSSA.
Why? The answer is PASSION
I am passionate about our profession and ensuring that it con nues to grow and develop and move forward into the future.
I am passionate about educa on for sterile technicians and for them to be recognised for their hard work both professionally and financially.
I am passionate about every hospital in New Zealand achieving and maintaining levels of excellence in sterilising technology and adhering to the standards without compromise.
I am keen to carry on working on projects that I am involved with for the associa on and see them come to frui on e.g. the scope of prac ce and competencies document for sterile technicians.
In my tenure on the execu ve to date we have achieved a lot including:
• Close links with the Ministry of Health
• Recogni on as a self-regulated profession
• Involvement in Pay-equity process
• Sterile Technicians added to the immigra on green list
• Introduc on of level 5 Diploma of Sterilising Technology
I have 17 years’ experience working as an SSD manager and my experience in that role has given me skills which I can use as an execu ve team member.
I have been the president of the NZSSA since I was thrust into the role in 2012. It is now me for someone else to take the lead and I am happy to work in the background offering support to whoever takes my place as president.
Please vote for me to be an execu ve team member.
Thank you

Steriking Reinforced Rolls and Roll Holder



Original Article
Brian Kirk
Corresponding author: Dr Brian Kirk
Brian Kirk Sterilization Consultancy Group Ltd 10 Harcourt Place Castle Donington Derby DE74 2XJ U.K.
Bkirk0256@outlook.com
Conflict of interest:
The author is an independent researcher and director of Brian Kirk Sterilization Consultancy Group Ltd. Prior to establishing his company he worked for 3M Health Care. This study was carried out by Brian Kirk Sterilization Consultancy Group Ltd and funded by a research grant provided by 3M St Paul, USA.
Citation: Kirk B. Detecting vaporised hydrogen peroxide sterilization (VH2O2) process failures in clinical settings using chemical indicators. Zentr Steril 2020; 28 (6): 334–343
Manuscript data:
Submitted: 8 September 2020
Revised version accepted: 10 November 2020
Summary
Background: VH2O2 sterilizers are used to process heat sensitive medical devices. Chemical Indicators (CIs) are often used to monitor such processes.
Aim: Determine the ability of VH2O2 CIs to indicate when sterilizers were operated according to recommendations (pass) and when operated with unsuitable loads or processing cycles (fails).
Method: Studies were conducted in sterile processing departments (SPD) in -
tance colourimetry were used to assess CI colour change when placed inside loads which were within or exceeded the weight limit for a STERRAD® NX100 EXPRESS process (SPD1) and then a different load in EXPRESS or STANDARD NX100 processes (SPD2). Findings: Shinva®, gke® and Terragene® CIs gave slight differences in colour change in different test conditions. The Shinva and Terragene CIs were interpreted as passes. The gKe CI gave an aquamarine colour which could have been interpreted as Pass (chosen) or Fail. Differences in col-
observed in the three indicators, some
Steris-Celerity®, Steris-Verify®, 3MTrimetric® and SPS® CIs differentiated recommended from not recommended loads and processes giving visual pass or fail results and differences in E but with differing levels of accuracy and precision. The ASP® CI gave similar observable and measurable differences but all were passes when compared to the reference colour.
The Steris-Celerity and Steris-Verify CIs showed observable and measur-ferent positions inside one pack but not the others. The Trimetric and SPS CIs showed observable and measurable positions in all packs.
The ASP CI showed similar differentia-
The 3M-Trimetric had the greatest accuracy and precision indices.
Conclusion: CIs help provide an assurance of sterility for VH2O2 sterilization processes. New loads or processes introduced into an SPD should be subject to help identify non-uniform conditions within packs. Routine monitorof incorrect loads or processes and can help detect process failures in clinical settings.
Introduction
Sterile medical devices, either single use or re-usable, must be sterilized by a validated sterilization process [1,2]. High temperature steam is used for re-usable metal surgical instruments.
Keywords
EN ISO 11140-1
Type 1 and 4 chemical indicators
VH2O2 sterilization
Sterilization routine monitoring
Sterilization performance qualification
Detecting process failures
Low temperature processes employing ethylene oxide gas, low temperature steam with formaldehyde vapour and more recently vaporised hydrogen peroxide (VH2O2) are used for medical devices made of heat sensitive materials [3]. International standards describe validation and routine control for some sterilization processes [4,5 and 6]
VH2O2 processes; these are in development [7]. In the absence of a specific process standard, EN ISO 14937 applies [8]. However, VH2O2 is now one of the most popular processes employed in health care facilities.
ed on a substrate, which respond to de-
Table 1: A list of CIs tested in the study.
ProductType
A – ASP® STERRAD® Chemical Indicator Strip (14100)
Stated Values (SV) VH2O2 conc (mg/l) / T (°C) / t (s)
12.3 / 50 / 360 Dark Red to Orange /Yellow Orange printed on CI
B – Shinva® vH2O2 label (010104) 1a N/ARed to Yellow Six reference colours from Red to Yellow in IfU
C – Steris® Celerity® HP Chemical Indicator (PCC074)
D – Steris® Verify® HPU Chemical Indicator (PCC061)
E - 3M® Attest®
VH2O2 Tri-Metric® Indicator (1348)
F – gke® Steri-Record® CI for VH2O2 (C-V-P-6)
G – SPS Medica®l
VH2O2 Indicator Strip (GPS-250R)
12.3 / 50 / 360 b Red to Orange/ Yellow Orange printed on CI
12.3 / 50 / 360 Magenta to Yellow Yellow printed on CI
45.1 / 50 / 60Blue to PinkNone
42.3 / 50 / 360 Light Blue to Light Green blue to light green in IfU
42.3 / 50 / 360Dark Pink to Bluenone
H – Terragene® Chemdye® VH2O2 CI (CD40) c 42.3 / 50 / 360 Purple to dark green / blue to greenc
Green printed on CI. Five ref colours from purple to light green in IfU (not the same green)
Interpretation instructions
“Bar changes from red to colour indicated by comparator bar [orange] or lighter” printed on CI.
Orange or lighter (towards yellow) is a Pass (ref chart)
An arrow pointing from the indicator strip to the reference colour printed on the indicator. “If the indicator colour matches the orange reference colour or is lighter…” in the IfU.
“Accept only if magenta circle turns yellow” printed on CI
“Any pink in accept zone is a pass” printed on the CI
Blue to green. “If the indicator dot remains purple/blue or has not turned to the final colour completely” in IfU.
“Indicator turns BLUE when exposed to VH2O2 during the sterilization process” printed on CI
“The indicator must turn to the reference colour for considering that the indicated conditions were met” in IfU.
A list of CIs tested in the study including their stated values, specified colour change, presence or absence of a colour reference on the CI or in the Instructions for Use (IfU) and a description of the manner in which the colour change should be interpreted as printed on the CI or contained in the IfU.
a: The Shinva packaging and accompanying technical sheets did not specify which type therefore it was assumed that because it was an adhesive label for attachment to the outside of packs it would be a process indicator of type 1.
b; The Celerity indicator is claimed to be a type 1 which according to EN ISO 11140-1 has defined SVs as shown.
c The Terragene Chemdye tested were provided in a foil pouch and had a purple starting colour (see 15),
® Acknowledges registered trade marks
The requirements for six types of CIs
Type 1, process indicators, usually placed on the outside of a pack to indicate that it has been processed. Type 2 for special tests. Type 3 are single variable indicators having limited utility. Type 4, 5 and 6 are multi-variable, integrating and emulating indicators, having different performance requirements according to EN ISO 11140-1 and are related to manufacturer’s claims. This latter group are used as internal indicators, placed inside individual load items to assess attainment of sterilizing conditions at the point of placement. Some guidance recommends the routine use of such indicators [11,12] and the WHO surgical safety check list [13] mentions their use as part ofthe informaof every pack” before use.
Unlike steam sterilization, which provides a high overkill, and wide utility, VH2O2 processes are designed for loads. It is vital that manufacturers recommendations are carefully followed. However, in a busy sterile processing department it is possible to inadvertently process a device or load in a sterilization cycle which is not recommended by the manufacturer. It is also possible to use inappropriate accessory items and sterile barrier systems which can, individually or in combination compromise process ef-strument sets can potentially detect process failures when used routinely or when conducting performance qualis compatible with the intended sterilization cycle.
An earlier study [15] characterised eight CIs for VH2O2 sterilization processes to give a pass or fail result when tested according to the methods in EN ISO 11140-1 and also to detect changes in the individual process variables time, temperature and VH2O2 concentration. In this study the same cohort of CIs were used in clinical settings to characterise their performance when sterilizers were operated according to recommendations or when operated with unsuitable loads or sterilization processes. In addition, the ability to
Zentralsterilization | Volume 28 | 6/2020
detect differences in processing conditions inside individual packs was also evaluated.
Methods
Chemical Indicators.
CIs, purchased from commercial sources, are shown in Table 1 along withour change characteristics, colour of any reference present and a summary of the instructions for interpretation. All CIs were stored in their original packaging equilibrated to ambient conditions before use (ca. 20 to 25 °C and 30 to 50% rh).
Products A to D and F to H had indicator ink printed on a substrate. Product E had indicator ink printed on a substrate covered with a polymer sheet with gaps cut along its length. Beyond the “accept line” the overlying sheet enclosed the ink creating a small gap through which VH2O2 penetrated to effect colour change.
Evaluation of CI colour change.
The methods described previously [15] were used to evaluate colour change.
eter (X-Rite eXact Standard spectrophotometer,https://www.xrite.com/categories/portable-spectrophotometers/ exact ) with an aperture of 1.5mm and white light illumination and expressed as International Commission on Illumination, (CIE) L*,a*,b* coordinates [16].
The variable E, which is a measure of the visible appearance of a colour, was then calculated for each CI sampleferences (p=<0.05) between values of E for sample groups of CI products exposed to different conditions was then determined using ANOVA and t-test calculations (the values of E from different products not being directly comparable). The values of E were then plotted
Colourchange was also judged by visual observation in bright daylight by a west facing window and categorised as pass or fail according to the manufacturer’s instructions, and, if provided, by comparison with a colour reference. From these observations two values were calculated [17]. Accuracy, representing a measure of whether the CI reported a correct result whether pass or fail and precision, the proportion of
true passes as a percentage of the total of true passes plus false passes (maximum=1).
The variability of the measurementstometer were found to have a standard deviation of <0.1 units of L*, a* and b*.
Test Conditions Employed in Clinical Settings
Tests were carried out in ASP® STERRAD® NX100® VH2O2sterilizers (https://www.asp.com/product/terminal-sterilization/STERRAD-100nx) operating either EXPRESS (4.85 kg /10.7lb maximum loading weight) or STANDARD (9.7kg/21.4lb) cycles at two different sterilization processing departments in hospitals in the USA. During exposure the VH2O2 concentration was measured using an on-board UV spectrophotometer and then reported as an integrated variable, area under the curve (AuC or dose) with dimensions mg.s/L.
Three standard loads were employed in department 1 (Xi, DV1, DV2) each exposed to an EXPRESS cycle. A fourth load was used in department 2 exposed to either an EXPRESS (StE) or STANDARD (StS) process as follows;
Xi – DaVinci Xi Endoscope set with mounting tray and lid (Intuitive Surgical Inc, USA https://www.intuitive.com/en-us/products-and-services/da-vinci), double wrapped in a non-woven polypropylene sterilization wrap secured with a 15 cm length of VH2O2 indicator tape (3M
1A)
DV1 – A DaVinci Si probe mounted on pillars within an Aescuand 2 bottom) container, weighing
DV2 – As for DV1 but with two DaVinciSiprobes,weighing
StE or StS – A Stryker camera system (Stryker 1588 AIM Pendulum Camera Head with Integrated Coupler) in an Aesculap rigid half DIN
1D) (three replicate sets) weighing 5kg/11lb exposed to either an EXPRESS or STANDARD process
The instructions for the STERRAD sterilizer recommend maximum loading weights for the EXPRESS and STAND-
ARD cycles. Based on this information and the information in the instructions for the medical devices tested the loads processed in test condition Xi and StS are recommended whereas those processed in test conditions DV1 and DV2 (excess weight) and StE (inadequate exposure time) are not recommended. One might expect CIs to provide a pass indication in the Xi and StS and a fail in DV1, DV2 and StE.
Triplicate samples of each CI were included in each
were conditioned for 45–60 minutes to ambient conditions (ca. 24 °C, 30%rh) between uses. Test loads were placed on the lower shelf in the sterilizer (random orientation unre-
ther EXPRESS or STANDARD processes as described above. Immediately after processing the cycle record was recovered and relevant data noted, the load temperature measured and the CI samples recovered, placed between tissue paper and secured in sealable polythene bags. Colour measurements of CIs were made within 3 days of exposure.
Results
Table 2 shows the test conditions employed and the VH2O2 AuCs reported by the sterilizer. The average AuC when using test condition Xi in the EXPRESS cycle in department 1 was
when using test condition StE and StS in the EXPRESS and STANDARD cycles in department 2 were statistically differ-
ible interpretation, as a fraction of the total number of CIs used in the respective test condition and the accuracy and precision of all observations.
Table 3 shows examples of each of the indicators colour change when exposed to the conditions described in table 2.
Figure 1 shows the location and notation of each group of CIs within load Xi, DV1, DV2 and StE/StS. Figure 2 shows the value of E for combined data sets (left+middle+right and edge+centre) in each test condition (table 2). Figure 3 shows the data sets for CIs placed in the left, middle and right po-
which were exposed to the ASP STERRAD EXPRESS process. (middle line) the range of values (whisker extremes) and theterns indicate statistical differences (SD) in values of E but not visible differences. Shaded boxes show both SD in values of E and observable differences in CI colour (see table 3).
Discussion
The response of the CIs.
Any given colour can be expressed by three coordinates L*, a* and b* and the addition of these three coordinates to give a value termed E is a numerical representation of the appearance of that colour (16). L* represents the lightness or darkness of a colour but the coordinates a* and b* represent the actual colour in terms of red (+a*), yellow (+b*), green (-a*), and blue (-b*). CIs will often have a dominant a* or b* coordinate and changes to these values may repre-


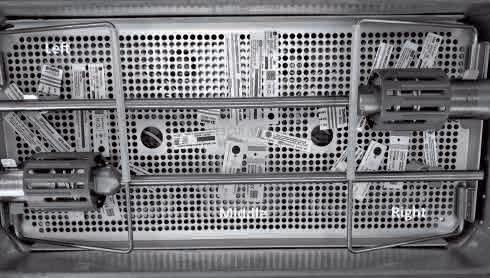

Table 2 : Visual interpretation of the colour change indicated by CIs exposed to the test conditions shown. Results are expressed as the number of Fail results within each replicate group when interpreted according to the manufacturers instructions ie comparison with a colour reference printed on the CI or in IfUs or as instructed by IfUs. The accuracy and precision, expressed as a percentage, of each set of indicators expresses a value related to the proportion of false and true pass and fails (see text)
Test Condition Location Within Tray AuCd (mean, range) (mg.s/l)
Xi. Xi Probe / ASP STERRAD EXPRESS cycle Left 1237 (9211453) 0/100/101/101/101/100/100/100/10
Middle0/100/102/101/102/100/101/100/10
Right0/100/101/104/102/100/100/100/10
DV1. One DaVinci probe / ASP STERRAD EXPRESS cycle Left 874 (7631146) 0/100/107/1010/1010/100/106/100/10
Middle0/100/107/1010/1010/100/107/102/10
Right0/100/105/1010/1010/100/106/100/10
DV2. Two DaVinci probes / ASP STERRAD EXPRESS cycle Left 838 (695965) 0/100/107/1010/1010/100/107/100/10
Middle0/100/108/1010/1010/100/107/100/10
Right0/100/107/1010/1010/100/108/100/10
StE. Stryker Camera probe / ASP STERRAD EXPRESS cycle Centre 1760 (15971971) 0/100/10 2/1010/1010/100/108/100/10
Outer Edges 4/200/2017/206/2020/200/2020/200/20
StS. Stryker Camera probe / ASP STERRAD Standard cycle Centre 6902 (66427371)
0/100/100/100/100/100/100/100/10 Outer Edges
0/200/200/200/100/200/200/200/20
a. Product F was extremely difficult to interpret with all CI colours being a similar turquoise / aquamarine /blue-green and therefore could be interpreted as a pass or fail.
b. The reference colour printed on CI H varied in depth of green some being much darker than others thereby giving rise to possible misinterpretation.
c. CIs C, D and E gave colour changes very close to their endpoint when exposed to test condition Xi making absolute interpretation of the result difficult. The following observations were interpreted as fails. Product D had a very slightly observable magenta ring around the central yellow portion. Product C was a red/yellow colour rather than the reference orange. Product E had a slightly mauve colour in the accept zone rather than the described pink.
d. AuC is the area under the VH2O2 concentration curve measured from within the chamber. Test condition Xi was significantly higher (p≤0.05) than test condition DV1 and DV2 which were equal.
sent a more sensitive means of evaluating change. However in terms of visual appearance changes in individual coordinates cannot be observed. For this reason expression of colour change using values of E and their differences is more representative of observed colour
Products B and H gave visual passes (except for 2 fails with H) in all test conditions with slight or no differentiation by visual interpretation (table 2 -
cult to interpret with all CI colours being a similar aquamarine which could be interpreted as pass (chosen) or fail. Some statistical differences in the values of E between loads and processes recommended by manufacturers and those not recommended were observed.
in low accuracy and precision values (table 2). Product B was a self adhesive label which was presumed to be catego-
rised as a process indicator for adhesion to external surfaces of packs. The green reference colour printed on product H varied in colour from one indicator to another giving rise to potential misinterpretation (see table 3).
Some samples of products C, D, E and G gave a colour change very close to their endpoint when exposed to test condition Xi making visual interpreta-
had a very faint but observable magenta ring around the central yellow portion interpreted as fail. Product C was a red/ yellow colour rather than the reference orange. In some cases product E had a slightly mauve colour in the accept zone rather than the described pink. Product G had inconsistent colour change across its indicator strip making interpretation -
solved by instructional material with examples.
Products C and D, were in general able to differentiate loads and processes recommended by manufacturers (Xi and StS), giving mostly visual passes, from loads and processes not recommended (DV1, DV2 and StE) giving a high proportion of, or all, visual fails (table 2 and
higher values for accuracy and precision. Numerically, SDs in values of E were not-
entiate between the conditions occurring at location left, middle right within load Xi, being visually similar and numerically not SD. However the group both visually and numerically differentiated edge to centre differences in test condition StE, although differences in E
Products E and G, were able to differentiate recommended loads and processes (Xi and StS) giving a high pro-
Table 3: Examples of the colour change indicated by CIs exposed to the test conditions shown (also see table 2). For product A the indicator colour in the final two rows had changed to a very light yellow which photographic reproduction has not detected.
Xi. Xi Probe / ASP STERRAD EXPRESS cycle Left
























DV1. 1 DaVinci probe / ASP STERRAD EXPRESS cycle Left Middle
DV2. 2 DaVinci probes / ASP STERRAD EXPRESS cycle Left Middle Right
StE. Stryker Camera probe / ASP STERRAD EXPRESS cycle Centre Outer Edges
StS. Stryker Camera probe / ASP STERRAD Standard cycle Centre Outer Edges
For footnotes a to c see table 2
















































































Figure 2: The values of E for CI sample sets A to H (table 1) when used in different test conditions.
Xi – Xi Probe in bespoke case exposed to EXPRESS Cycle
DV1 - One DaVinci Probe mounted in a metal sterilization container exposed to a STERRAD EXPRESS cycle
DV2 - Two DaVinci Probes mounted in a metal sterilization container exposed to a STERRAD EXPRESS cycle
StE - Stryker Camera Probe mounted in a sterilization container exposed to a STERRAD EXPRESS cycle
StS - Stryker Camera Probe mounted in a sterilization container exposed to a STERRAD STANDARD cycle
Open boxes showing different fill patterns indicate statistically significant differences in values of E but not visible differences . Shaded boxes show significantly different values of E and observable differences.
When the values of a* for product B were tested for significant differences all the indicators were statistically different. All other indicators had the same levels of statistical difference irrespective of comparing E, a* or b*.
When the values of a* for product D were tested for significant differences indicators from load Xi were equal but differed from indicators in load StE.
Zentralsterilization | Volume 28 | 6/2020

Figure 3: The values of E for CI sample sets A to H (table 1) when placed in positions left (L), middle (M) and right (R) of the Xi Probe container (figure 1A) and in positions edge (E) and centre (C) within the Stryker Camera probe container (figure 1D) and then exposed to an ASP EXPRESS cycle.
Open boxes showing different fill patterns indicate statistically significant differences in values of E but not visible differences . Shaded boxes show significantly different values of E and observable differences.
When the values of b* for product H were tested for significant differences the indicators located in the edge position in the StE cycle were statistically different to the values for indicators located in the centre position.
When the values of a* for product B were tested for significant differences the indicators located in the Xi load were significantly different to those located in the edge and centre positions of the StE load. Zentralsterilization | Volume 28 | 6/2020 341
In such circumstances the monitoring system associated with the sterilizer may show a reduction in chamber VH2O2 concentration but might not
conditions at location left, middle and had numerical SDs) and edge to centre
tiate between the different test conditions and the different locations with-
from 4 fails in the edge location of StE
reference orange colour. This was relighter in hue.
Colour Interpretation
colour blindness or stress levels, and environmental factors such as the lev-
might aid, but can also confuse inter-
these factors may lead to an incorrect-
within each instrument set, which has
ance documents [11, 20]. The results of using a range of monitoring systems, rather than relying on one, the information from which add to the overall sterility.
The value of type 4 versus type 1 and are designed to show that the load
Sterilizers using VH2O2 usually meas-
vs 7s;[10]) they will offer limited val-
to bring about a colour change in the the AuC measured in the chamber and
cantly different E values in different loinhomogeneous conditions. This may be due to changes in VH2O2 concentra-
rower window in the test conditions
have been encountered.
assurance of sterility for VH2O2 sterili-
tion involved very close scrutiny to de-
lowed observation of differences in E
the instruments and might be in subdued light so many indicators exhibit-
than ideal conditions are therefore more valuable than others which do not.
of
when routinely monitoring VH2O2 sterilizers and medical devices give recommen-
Zentralsterilization | Volume 28 | 6/2020
A, E and G showed visual and/or SD
tion in load Xi. CIs A, C, D, E and G all
middle clearly indicating differences in
surance associated with a load and in-
vices, sterile barrier systems or acces-
es between conditions measured in thecluding non-uniformity. Routine moni-
of VH2O2 is discussed in national guid-
to recommendations. Of the CIs exhibitthey were a valuable aid in detecting
cating failures due to incorrect loading
ble of indicating non-uniformity of ster-
ilizing conditions within instrument sets and would prove useful in PQ studies in addition to routine use for detecting sterilization failures. Of the CIs tested, the 3M-Trimetric had the highest accuracy and precision indices in terms of correctly detecting failures.
Acknowledgements
Thanks to Dr Marco Bommarito, Mr Sandy Riley and Mr Lawrence Talapa of 3M USA for their support.
References
1.Regulation (EU) 2017/745 of the European Parliament and of the council of 5 April 2017 on medical devices , amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC, 5th May 2017
2.Submission and Review of Sterility In(510(k)) Submissions for Devices Labelled as Sterile. Guidance for Industry and Food and Drug Administration Staff, January 21 2016, Food and Drug Administration, Washington, USA
3.Block, S.S. (ed), Sterilization with ethylene oxide and other gases. In Disinfection, Sterilization and Preservation (4th ed). London, Lea and Febiger, 1991: 580–95.
4.EN ISO 17665, Sterilization of healthcare products – Moist Heat – Part 1: Requirements for the development, validation and routine control of a sterilization process for medical devices, 2006, CEN-CENELECManagementCentre: Avenue Marnix 17, B-1000 Brussels
5.ENISO 11135, Sterilization of healthcare products – Ethylene Oxide – Requirements for the development, valida-
tion and routine control of a sterilization process for medical devices, 2014, , CEN-CENELECManagementCentre: Avenue Marnix 17, B-1000 Brussels
6.ENISO 25424, Sterilization of healthcare products – Low temperature steam and formaldehyde – Requirements for the development, validation and routine control of a sterilization process for medical devices, 2014, CEN-CENELEC Management Centre: Avenue Marnix 17, B-1000 Brussels
7.ISO/CD 22441 Sterilization of health care products – Low temperature vaporized hydrogen peroxide – Requirements for the development, validation and routine control of a sterilization process for medical devices, 2019, International Standards Organisation, Geneva.
8.EN ISO 14937, Sterilization of healthcare products – General requirements for characterization of a sterilizing agent and the development, validation and routine control of a sterilization process for medical devices, 2009. CEN-CENELEC Management Centre: Avenue Marnix 17, B-1000 Brussels
9.EN ISO 11138 Sterilization of health care products – Biological indicators –Part 1: General requirements, 2017, CEN-CENELECManagementCentre: Avenue Marnix 17, B-1000 Brussels
10.EN ISO 11140-1, Sterilization of health care products – Chemical indicators –Part 1: General requirements, 2014, , CEN-CENELECManagementCentre: Avenue Marnix 17, B-1000 Brussels
11.ANSI/AAMI ST 58.:2013 Chemical sterilization and high-level disinfection in health care facilities , 2013, Association for the Advancement of Medical Instrumentation, 901 N. Glebe Road, Suite 300, Arlington, VA 22203 USA.
12.UK Dept Health, National Decontamination Program, Theatre Support Pack, 2009. Dept Health London.
13.World Health Organisation, WHO surgical safety checklist, 2009. WHO, Avenue Appia 20, 1211 Geneva
14.Talapa, L, Vaporised Hydrogen Peroxide V H2O2 Sterilization; riding atop a new wave, presentation at IAHCSMM 2019 annual conference April 27th to May 1st, Anaheim, CA2019.
15.Kirk, B. Evaluation of a number of chemical indicators for monitoring vaporized hydrogen peroxide (VH2O2) sterilization processes. Zentr Steril, 2020; 28 (4): 208–217.
16.The International Commission on Illumination(Commissioninternationale de l’éclairage), CIE L*,a*,b* colour standard, 1976, CIE Central Bureau, Babenbergerstraße 9/9A, 1010 Vienna, Austria
Precision–https://en.wikipedia.org/
18.Hurlbert A, Pearce B, Aston S. All illuminations are not created equal: The limits of colour constancy. In: 38th European Conference on Visual Perception (ECVP). 2015, Liverpool, UK: Sage Publications Ltd.
19.Van Doornmalen, J.P.C.M, Hermsen R-J, Kopinga, K. Six commercially available class 6 chemical indicators tested against their stated values. Central Service 2012 (6): 400.
20.Scottish Health Technical Memorandum 01-01, Decontamination of Medical devices in a Central Decontamination Unit: Part E Sterilization by Hydrogen Peroxide or Ethylene Oxide. NSS Health Facilities Scotland, 2019.
Zentralsterilization | Volume 28 | 6/2020 343

Conference Dinner: Thursday 26th September Dinner theme: Fashion, Style, Music, History, and Events of 1974.
Right items, delivered to the right place, at the right time
Torin & T-DOC, you can ensure availability of items for each surgery as well as ensure surgical case carts or setup trolleys are delivered with the right RMD’s, implants, and consumables at the right place and at the right time.
With Torin and T-DOC working together as one you can improve delivery for surgeries whilst:
• Reducing strain on hospital staff
• Keeping track of your equipment
• Providing real time updates and progress
• Reducing impacts on patient while improving the patient experience
www.getinge.com/anz For more information email sales.nz@getinge.com or call 0800 143 8464




Donna Dador and her team celebrated SSD Sterilisa on Day back in April 2024. Above are some pictures from that celebra on.
By JeyJeyarajah
SterileServicesUnit,WaikatoHospital,Hamilton
ThereprocessingstandardAS5369:2023requiresthefollowingasmanagement responsibilitiesofaSterileSciencesUnit[SSU].Theresponsibilitytoimplementrequirements ofAS5369:2023andrelevantnominativestandards,implementingpoliciesandprocedures, supervisionofdailyactivities,ensuringthestaffeducationbyformalorientation,training,and development,andensuringappropriateresourcesareavailableforreprocessingtheReusable MedicalDevices[RMD]accordingtothestandardrequirementsaretheresponsibilitiesofthe personwhoisresponsibleforthefacility.Thus,therolesofaSSUmanagerincludeoperational management,strategicmanagement,andleadership.
TheoperationalmanagementskillsofanSSUManagerinvolveconvertinginputsintoproducts andservicesbyaneffectiveandefficientprocess.Itensuresthataprocessdeliversaproduct orserviceatanexpectedqualityatthelowestcost.Thus,theoperationalmanagementofa reprocessingserviceincludesplanning,organizing,directing,andcontrolling.Effectiveplanning canbeachievedbyimplementingpolicies,educatingandempoweringstaff,monitoringto ensuretherequiredtargetsaremet,andensuringqualityimprovementachieved.Thetasksfor organizingincludeorganizingsufficientqualifiedstaffandadjustingsufficientresource requirements.Thetasksfordirectingincludeallocatingstafftoensuresmoothoperationsofthe processandallocatingrequiredresourcestomanagechanges.Finally,tasksforcontrolling includeverificationofthecosts,andauditingtoensuretheprocessadherestotheQuality ManagementSystem[QMS](Theopenuniversity).Therefore,itisessentialfortheoperations managertoeffectivelycarryoutthedutiestoestablishandmaintainacomprehensivestaff traininganddevelopmentprogram,effectiveleadershipandteamcommunication,teamwork, andstrategicallymanagedHumanResourceManagement[HRM].
Traininganddevelopmentareprovidedbyanorganizationtoimprovetheknowledge,skills,and attitudeoftheemployeeswiththeexpectationtoimprovegrowth,productivity,stability,talent pool,andjobsatisfaction,andreduceaccidents,mistakes,waste,andsupervision(Surbhi, 2019).SSUtechniciansworktirelesslytopreventnosocomialinfectionsinhealthcare.The outcomeofsurgicalproceduresfundamentallydependsonthetaskscarriedoutbytheSSU technicians.Theymustbeeducated,trained,andempowered(Ricupito2009).Trainingis short-termanddesignedtoeducateanemployeetoperformaspecifictask.Ontheotherhand, developmentistopromoteanindividual’sleadershipskillsthroughalong-termlearningprocess (Murugesan,2011).TraininganddevelopmentmustbeaContinuousQualityImprovement [CQI]goalforthesuccessandenrichmentofanSSU.Theymustmeetthecurrentandfuture needsofanSSU.Strengths,Weaknesses,Opportunities,andThreats [SWOT]analysisisan excellenttoolforanalyzingthetrainingrequirementsforrefreshertraining(Milhem& Abushamsieh,2014).OthertypesoftrainingcanbeprovidedbytheAnalyze,Design,Develop, Implement,andEvaluate[ADDIE]model.HRMmustrecognizethestrategicgoalsoftheSSU
withtheaimofContinuousQualityImprovement(CQI)andproductivity(Milhem& Abushamsieh,2014).
Softskillsareequallyimportantasformaleducationandlearningskillsfortoday’sjobmarket. Employeeswithexcellentsoftskillscanincreaseproductivitytoadifferentlevel.Softskillsare notpartofthelearningstrategy.Theyarepartofabusinessperformancestrategythatenables anemployeetoacquireanddevelopknowledgeandskillsthroughsocialcapital(DeakinCo, 2017).
EffectiveleadershipcommunicationisrequiredtomaintainandcontinuouslyimproveSSU.A supportiveleadershipstyle,behaviors,andcommunicationinfluenceorganizationalculture,the learningclimateofanSSU,processimplementation,adherencetoQMS,andrelevant standards(Knoblochetal.,2019).Themostimportantsoftskillsofleadershipcommunication areearningtrust,empathy,andgoodlisteningtoengagewiththeteammemberseffectivelyto implementchanges(SpriggHR,2021).Listeningisimportantforrelationshipsforboth managersandnonmanagers.Manageriallisteningcontributestoemployeemotivation, recognition,andproduction.Managerswhohavegoodlisteningskillscanpromote organizationalcultureamongemployees.However,nationalcultureinfluencesthelistening behaviorofmanagersaswellasnonmanagers.Therefore,thereisaneedtounderstandthe nationalculturetoimprovegoodlisteningbehavior.Theglobeleadershipprojectidentifiednine leadershipdimensionsamongdifferentnations(Roebuckal.,2016).Thefollowingtable summarizesNewZealand’spositionin6dimensionsbasedonthe“6-DModel”(Theculture factorgroup).
Teamcommunicationisimportantfororganizationaltransformationandperformance.Team communicationisanexchangeofallinformationandinteractionstoachievetheteam’sgoal.It enablesteammembersnotonlytocollect,identify,interpret,andevaluatethedataand informationgeneratedduringthereprocessingprocessesbutalsoenablethemtointeractand exchangetheinformationtoachievetheirgoals(SpriggHR,2021).Acohesiveteamwith excellentleadershipandteamcommunicationworkinginalearningenvironmentwillmeetthe majorgoalsoftheHRMandorganizationalculture.Effectiveteamcommunicationinfluences performanceandmorale,betterteamcohesion,providesanidealenvironmentforCQI,and helpstoachievetheorganization’sgoals,highperformance,andproductivity(Hassall,2009).
Inconclusion,anSSUthatsupportscontinuoustraininganddevelopmentandlearning environment,excellentleadershipandteamcommunication,teamwork,andstrategically managedHRMwillenhanceproductivityandorganizationalculture.
TheStrategicmanagementskillsofanSSUManagerinvolveprovidingavisionforthe organization.Ourworldischangingrapidly.Thedemandtoimproveproductivityis ever-increasing.Theneedfor“rapidimprovementinefficiencybyaveryeffectiveprocess” requiresaclearvisionforchange.Strategicmanagementprovidesadirectiontotransforman organization’svisionintoreality.Itprovidescleargoalsforthestakeholders,enablesthe organizationtofulfilltheregulatoryrequirements,enablestheC-suitedecision-makersto understandtheenvironmentdoesnotchangeannuallyorperiodicallyhavetoberegularly reviewedandmadeadjustments,andprovidesleadership.Moreover,aclearvisionfromgood leadershipempowerseveryemployee(NewZealandManagement,2010).
Leadershipskillsarerequiredtoprovidechange.TheHealthQuality&SafetyCommissionNew Zealand,2016requiresthatleadersmustsetanexamplebydoingwhatisright,implementand supporttheimprovementofqualityandsafety,andguidethequalityandsafetyimprovements basedontheorganizationalandnationalgoals.Moreover,leadershiprolesinalllevelsofthe healthcaresystemhaveincreasinglybecomeimportanttoimproveefficiencyandproductivity. Thecontemporaryleadershiptheoriesofhealthcareincludegreatmantheory,traittheory, behavioraltheory[includesautocratic,democratic,andlaissez-fairestyles]contingency leadershiptheory,transactionalleadership,andtransformationalleadershiptheory(Kumarand Khiljee,2016).ThesearchforidealleadershipinSSUisdebatable.Thereareeightmajor attributesforSterileServicesleadership.Firstly,Leadershiprequiresanunderstandingoffuture trends.Theymustbeabletomakechangesthroughinnovation,insightfulness,andconfidence. Continuousimprovementrequireswinningthesupportandcommitmentofthestaff.Secondly, leadershiprequiresanunderstandingoftherolesoftheotherstaffandmakingchangesby qualified,competent,andskillfulleaders[credibility].Thirdly,thebestandbrightesttechnicians mustbemanagedbyconnectingandengaging.Leadersmustfulfilltheneedsofthe technicians,shareknowledgeandskillsandsomotivateandinspirethem(Jelks2019). Fourthly,leadersmustbeabletoinspireateamduringdifficultsituations.Theymustbeableto understandtheiremotionsandothers.“Peoplemayhearyourwords,buttheyfeelyourattitude” (Jelks2019).Tounderstandandmanageemotions,leadersmustcommunicatepositivelyand
possessexcellentinterpersonalskills,suchasemotionalandsocialintelligence.Fifthly,conflicts mayarisewhenmakingchanges,becausestaffmembersmaynotagreewiththechanges. Leadersmustbeabletoresolveconflictsbyunderstandingthereasonsfortheemotional discomfort.Theymustacknowledgeandexpresstheirconcerns,identifythemeanstoresolve conflicts,discusstheoutcome,andunderstandbygettingfeedback.Managingachangeand resolvingconflictsrequirepatient,persistent,andinsightfulleadership(Jelks2019).Sixthly, leadersmustinvolveallstaffmemberswhenmakingchangesbyusingqualitytoolssuchas brainstorming.Tosolveproblemsduringchangesleadersmustthinkcriticallywithanalytical skills.Seventhly,leadersmustactasrolemodelstobetrustedandrespected.Finally, leadershiprequiresadministrativeskillsforplanning,organizing,directing,andcontrolling (Ricupito2009).Themainattributesoftransformationalleadershiparetomotivateandinspire employees,overcomesocialandemotionaldiscomforts,andengagewithemployees. Transformationalleadersarecharismaticaswell.Therefore,anidealleadershipforanSSU manageristransformationalleadership.
Inconclusion,themainobjectiveofSSUmanagementistomaintainandcontinuouslyimprove theunit’sLeadership,Human,Physical,Social,andCulturalcapital(organizationalculture). Theorganizationalculturedependsonthevalues,beliefs,andbehaviorsofstaff,andinteraction ofemployeesandthemanagementstaff,andtheviewofthepublicoftheorganization.Agood organizationalcultureisstrongemployeerelationships,evidenceofmotivation,trustand integrity,excellentteamwork,innovation,andemployeeresilienceforchange(Jain&Jain, 2013).Moreover,theorganizationalculturemustmeettherequiredactionslistedundersection 3ofthe“frameworkforbuildingqualityandsafetycapabilityintheNewZealandhealthsystem” (HealthQuality&SafetyCommissionNewZealand,2016).Theorganizationalcultureofan SSUcouldbecomparedtoaplant.Thenitsleaves,flowers,andbranchesaretheunit’s mission,strategicplans,goals,website,anditsrecognizedstaffmemberswhomadesignificant contributionstoSterileservices.Thesearetheartifactsoftheorganizationwhicharevisibleto membersofthepublic.Itstrunkisthevaluesoftheunit,whicharethepolicies,procedures, records,andQMS.ThesearevisibletomembersofthepublicandSSUstaff.Itsrootsarethe assumptionsthatincludebehaviors,andbeliefsoftheemployeesthatarevisibleonlyto employeesonly.

References
DeakinCo,.(2017). Howmicro-credentialscanpreparepeopleforthefutureofwork https://www.deakinco.com›Resources
HealthQuality&SafetyCommissionNewZealand.(2016). Fromknowledgetoaction.
Hassall,S.L.(2009).Therelationshipbetweencommunicationandteamperformance:testing moderatorsandidentifyingcommunicationprofilesinestablishedworkteams.PhDthesis, QueenslandUniversityofTechnology.https://eprints.qut.edu.au
Jain,A.,&Jain,S.(2013).Understandingorganizationalcultureandleadership-enhance efficiencyandproductivity.TheJournalofManagementAwareness,16,(2),43 https://www.indianjournals.com/ijor.aspx?target=ijor:pr&volume=16&issue=2&article=004
Jelks,L.(2019).Employeeengagement!Managingoutbestandbrightestinsterileprocessing. HealthcarePurchasingNews,43,(12),34-37.
Knobloch,M.J.,Musuuza,J.,Safdar,N.,&Thomas,K.V.(2019).Exploringleadershipwithina systemsapproachtoreducehealthcare–associatedinfections:Ascopingreviewofonework systemmodel. AmericanJournalofInfectionControl, 47(6), 633–637.https://doi-org.ezproxy.toiohomai.ac.nz/10.1016/j.ajic.2018.12.017
Kumar,R.D.C,&Khiljee,(2016). Leadershipinhealthcare. AnaesthesiaandIntensiveCare Medicine, 17(1),DOI:63-65.10.1016/j.mpaic.2015.10.012
Milhem,W.,&Abushamsieh,K.(2014).TrainingStrategies,TheoriesandTypes. Journalof Accounting–Business&Management,21(1),12-26. https://www.researchgate.net/profile/Khalil-Abushamsieh/publication/269165999
Murugesan,G.(2011). Humanresourcemanagement.
NewZealandManagement.(2010). Strategicmanagement:thestrategicplan–isitrelevant anymore? https://management.co.nz/article/strategic-management%C2%A0-strategic-plan-it-rel evant-any-more
Openuniversity.(n.d.). Understandingoperationsmanagement. https://www.open.edu DeborahBrittRoebuck1,ReginaldL.Bell2,
Roebuck,D.B.,Bell,R.L.,Raina,R.,&Lee,C.E.(2016).Comparingperceivedlistening behaviordifferencesbetweenmanagersandnonmanagersLivingintheUnitedStates,India, andMalaysia. InternationalJournalofBusinessCommunication,53(4),485-518.
Ricupito,G.(2009). Buildqualityleadershipforastrongerdepartment,organization. Materials managementinhealthcare, 18(1), https://www.researchgate.net/scientific-contributions/Gene-Ricupito-36621456
StandardsAustralia.(2023). Reprocessingofreusablemedicaldevicesandotherdevicesin healthandnon-healthrelatedfacilities (AS5369:2023). https://store.standards.org.au/product/as-5369-2023
SpriggHR,(2020).Theimportanceofleadershipcommunicationin2021. https://sprigghr.com/blog/leaders/the-importance-of-leadership-communication-in-2021/
Surbhi,J.(2019). HRsolutionsforexcellenceintrainingdevelopment. TheCultureFactorGroup.(2016).(n.d.). CountryComparisonTool.



ECOLAB HEALTHCARE ANZ
7A Pacific Rise, Mount Wellington, Auckland 1060, New Zealand
NZ: 0800 425 529 www.healthcare-nz.ecolab.com E: salesanzhc@ecolab.com
“Exploring
Hafiz Abdul Mannan
CSSD Technician
Hamad Medical Corpora on, Qatar
Abstract
For appropriate disinfec on and steriliza on, following professional recommenda ons and manufacturer's instruc ons is crucial in order to prevent crosstransmission of pathogens. Neglec ng this crucial step can lead to dire consequences, par cularly with the emergence of new pathogens and the use of complex medical devices. A search was directed using different search engines including google scholar, and PubMed to find ar cles on novel disinfec on and steriliza on methods. A number of promising disinfec on methods and steriliza on technologies have recently been iden fied. These include electrosta c spraying, which uses an electrically charged mist to evenly distribute disinfectant over surfaces, and new sporicides, which are specifically designed to kill bacterial spores that are especially resistant to tradi onal disinfectants. Addi onally, colorized disinfectants colorized disinfectants, “no touch” room and “con nuous room” decontamina on have been developed. Finally, a new steriliza on method for endoscopes has been developed. It is expected that these new technologies would greatly lower the risk of infec on for both pa ents and medical staff.
Keywords: steriliza on, disinfec ons and current trends.
Introduc on
Since the inven on of the germ hypothesis of disease by Louis Pasteur, medical science has advanced significantly. Since that me, preven ng and controlling of different microorganisms everywhere, par cularly in healthcare ins tu ons, has become the primary job of physician (1).
Hospital acquired infec ons are a global health concern. Hospital infec ons are es mated to affect about 1.4 million people worldwide, with 80000 fatali es annually. Due to microbial contamina on in the hospital specialized units, the occurrence of nosocomial infec ons rises. Pa ents are seriously threatened by the illnesses brought on by bacteria resistant to an bio cs, which are acquired in the opera ng rooms and intensive care units. The occurrence and dissemina on of microbial resistance are commonly associated with the presence of hot zones in ICU (2).
Nosocomial infec ons, par cularly surgical site infec ons (SSIs), are a significant contributor to morbidity and mortality following surgery. They rank as the second most prevalent type of infec on a er urinary tract infec ons (UTIs) and pose a primary challenge in the field of surgery. . Infec ons can originate from both external and internal sources, including the air, soil, dust and surfaces found in OTs. Microbiological environmental and procedural surveillance play crucial roles in infec on control programs. It provides the data according to the types and number of microbial flora (3).
Hospital acquired infec on effect five to ten % of all pa ents enlisted to the hospital in developed countries. They have an impact on quarter of hospitalized pa ents in low-income countries. Therefore, it is obvious that examining the hospital se ng is a central sec on of aver ng nosocomial infec on. Infected personnel, staff movement and outsider loads are the most important cause of droplet genera on. Inappropriate use of methods of microbial control is a substan al risk factor of SSI (4).
Implemen ng steriliza on and disinfec on measures is of utmost important to prevent the emergence and spread of microbial resistance in the health care areas. These areas are frequently recognized as cri cal zones owing to invasive procedures, extensive an bio c usage, and the risk of bacterial transmission among pa ents. To effec vely monitor the presence of microbial flora and detect any changing trends, it is beneficial to conduct microbiological tes ng of surface and equipment, as well as biological air sampling to assess the types and quan es of microorganisms present in the indoor air. By implemen ng proper measures to control air borne pathogens, we not only ensure the wellbeing of pa ents but also subsidize to the complete wellbeing of the hospital environment. Furthermore, it is essen al to establish a surveillance system and early warning mechanisms at the local, regional, and na onal levels in order to promptly iden fy signs of emerging or increasing microbial resistance (5).
Disinfec ng and sterilizing of medical instruments and environmental surfaces are crucial to avoid the spread of infec ons. Innova ve techniques and trends are con nuously evolving in healthcare setup. Processes for disinfec on and steriliza on are categorized into different types: cleaning, low to high-level disinfec on, and steriliza on (6).
Steriliza on eliminates all microorganisms from an object such as surgical instruments and implants that must be
sterile before coming into contact with sterile ssue or vascular system to prevent disease transmission. These objects should be bought sterile or sterilized using steam steriliza on, ethylene oxide, hydrogen peroxide gas plasma, vaporized H2O2, H2O2 vapor with ozone, or aqueous chemical sterilants if other techniques are ineffec ve (6).
A number of promising disinfec on methods and steriliza on technologies have recently been iden fied. These include electrosta c spraying, which uses an electrically charged mist to evenly distribute disinfectant over surfaces, and new sporicides, which are specifically designed to kill bacterial spores that are especially resistant to tradi onal disinfectants. Addi onally, colorized disinfectants colorized disinfectants, “no touch” room and “con nuous room” decontamina on have been developed. Finally, a new steriliza on method for endoscopes has been developed (6,7). This paper provides an overview of the latest technologies and developments of these processes.
New developments in disinfec on Electrosta c spraying
Within healthcare services, surfaces are disinfected using disposable wipes or disinfectant applied with a cloth. However, electrosta c sprayer devices that deliver electrosta cally charged droplets of disinfectant can be more effec ve. These droplets, a racted to surfaces, contain 0.25% sodium hypochlorite and reduce contamina on by microbes on uneven surfaces, such as
mobility aids, handheld devices, and wai ng area seats. The sprayer requires minimal precleaning/disinfec on, and chlorine has been found to reduce spores and plaque-forming units with short contact mes. Electrosta c sprayers can be used as supplemented technology to surface disinfec on with wipes in pa ent accommoda ons and for decontamina ng portable equipment on contact precau ons or all pa ent rooms. A trial found that adjunc ve UV-C light treatment with a room decontamina on device, and sodium hypochlorite delivered via electrosta c sprayer were all opera ve in lowering remaining health care-associated infec ous agents on floors and high-touch surfaces a er manual disinfec on. Instead of environmental services workers, research staff has conducted the current effec veness evalua ons (8,9).
Given that this technique relies on me culous applica on, it is plausible that if environmental service professionals use the sprayer and apply it less thoroughly than desirable, the electrosta c sprayer's effec veness might be inferior to that of UV-C (9).
Furthermore, more research is required to determine the level of cleaning and disinfec on required to guarantee efficacy in lowering microbial contamina on. This degree of cleaning and disinfec on may vary depending on the usage of disinfectants and electrosta c spray technology (9).
Figure 1: Summary of issues that should be considered when purchasing or using an electrosta c sprayer
-Disinfectant should be tested and approved by the EPA for use with an electrostatic sprayer(i.e. system consist of sprayer and disinfectant)
-Disinfectant safety (i.e. sporicicdal or non-sporicidal)
-Disinfectant safety (i.e. EPA category 2,3 or 4)
-Well-suited for irregular devices with multiple angles
-Spray distance
-Spray Application speed
-Spray Coverage
-Spray method (i.e. S-pattern with overlap)
-Contact time
-Quantity of water used and sprayer on surfaces
-Uniformity of spray or spray coverage
-Odor and persistence
Gram-posi ve, anaerobic bacteria called Clostridium difficile establish colonies the human diges ve tract when an bio cs disturb the balance of the gut microbiota. C difficile infec on (CDI) is a common infec on associated with health care, with 202,600 cases and 11,500 deaths in the U S in 2019 (10).
This spreads through the fecal-oral route. A study shows that hospital rooms where CDI pa ents are treated, and healthcare personnel who take care for those pa ents, are infected. A pa ent's chance of developing CDI increases when they are admi ed to a room where a pa ent with CDI has previously resided (11% prior occupant with CDI, 4% prior occupant without CDI, or 239% greater risk). The main measures to prevent CDI include decreasing use of CDI-precipita ng medica ons, placing CDI pa ents in isola on with gloves and proper hand cleanness, and using sporicidal agents for improved room disinfec on (11).
Several disinfec on strategies have been successful in reducing cases of CDI. Mayfield and colleagues found that room disinfec on with 1:10 hypochlorite directed to a significant reduc on in these infec on cases from 8 to 3 per 1,000 days in a transplant unit. However, when they reverted to using a quaternary ammonium compound, the infec on rate increased to 8.1 cases per 1,000 days (12).
Similarly, another study reported a noteworthy lower in CDI on one of two wards while using hypochlorite. Another study by Orenstein et al that used bleach wipes (0.55% ac ve chlorine) for both daily and terminal cleaning, demonstrated a reduc on in C. difficile on two wards for which C. difficile was hyper-endemic (13).
According to the recommenda ons of the Centers for Disease Control and Preven on (CDC), in the case of a C. difficile infec on, sporicidal disinfectants are o en used in pa ent rooms; nonsporicidal disinfectants are typically used in all other pa ent rooms (6).
However, numerous sporicidal chemicals have proper es that deject their use. For instance, sodium hypochlorite is destruc ve and may develop respira onal irrita on in personnel and pa ents. Perace c acid-hydrogen peroxide products are more stable in terms of materials compa bility but have been reported to cause eye, upper and lower airway irrita on in working staff (14).
A new disinfectant with 4% hydrogen peroxide was found effec ve in killing C difficile and other pathogens. It has good material compa bility, a 24-month shelf life, and a ready-to-use form. It can kill vegeta ve bacteria in 1 minute and C difficile spores in 5 minutes, but a study showed it can reduce C. difficile spores by 4.7-log10 in just one minute (15).
Colorized disinfectant
Opera ve disinfec on of surface is important in aver ng spreading of healthcare-associated pathogens. However, subop mal disinfec on is common, and studies show less than fi y% of high-touch surfaces are disinfected. Thoroughness monitoring can expand disinfec on, but it is difficult to endure. Surface disinfec on is crucial to prevent transmission of pathogens and is an important infec on preven on strategy. Although disinfec on interven ons have shown to be effec ve in dropping microbial load and healthcare-associated infec ons, many surfaces remain ineffec vely disinfected and contaminated (16).
A new disinfec on method involves adding a blue color indicator to the disinfectant. This helps visualize surface coverage and contact me for thorough disinfec on. The blue color fades a er 4 minutes. It can be dispensed through a lid device on any standard commercially available wipes container. This provides real- me visual feedback for staff and ensures complete surface coverage (17).
One study found that a color addi ve decreases disinfec on failure rates constructed on fluorescent marker removal from 15 % to 4.5% (18). In other research conducted, the use of a colorized disinfectant resulted in a 29% increase in fluorescent marker removal, indica ng an improvement in the thoroughness of disinfec on. However, A small sample size, the use of a prototype dispensing device, and possibly less vigorous wiping were among the limita ons listed in a pilot study that used fluorescent markings and microbial evalua ons to compare a bleach product with a new colorant addi ve. The study did not find a significant difference between the two products (17).
“No-touch” room
Numerous researches have shown that surfaces and items in healthcare facili es are o en not cleaned adequately, which can lead to the spreading of microbes that cause infec ons related to health care system. However, interven ons designed to improve disinfec on methods, such as subs tu ng disinfectant products or using "no-touch" room decontamina on techniques, have been effec ve in elimina ng microbial load on surfaces and preven ng infec ons (19).
A number of companies have produced room disinfec on devices that are capable of inocula ng test surfaces and/ or successfully disinfec ng substances and surfaces in the surrounding environment. The technologies that are most frequently men oned include electrosta c sprayers, ultraviolet C, pulsed xenon UV, aerosolized hydrogen peroxide, and hydrogen peroxide vapor. The two systems that have been thoroughly examined are the hydrogen peroxide and ultraviolet light systems (20,21).
Hospitals ought to consider about using a "no touch" equipment for terminal room cleaning following pa ent
discharge based on contact restric ons, according to these sta s cs. These "no touch" room decontamina on systems eliminate, or at least reduce, the possibility that infec ous agents le on contaminated environmental surfaces from the preceding pa ent may infect the next pa ent (22).
“Con nuous room” decontamina on
Because "no touch" room need the evacua on of all people from the room, which limit this to use exclusively in terminal rooms. Furthermore, pa ents, visitors, and staff are constantly contamina ng noncri cal pa ent care items and surrounding surfaces with microbes. Con nuous room decontamina on techniques are being inves gated as no touch" room is usually insufficient and recontamina on happens fast a er disinfec on (23).
This technology aims to make surfaces clean by reducing or elimina ng pathogens present on them, which helps prevent disease transmission. Different technologies are available, including visible light disinfec on, hydrogen peroxide, far-UV light, ioniza on, mul jet plasma, and self-disinfec ng surfaces. Some of these have explained their ability to minimize infec ons, making them promising tools to control disease spread from inanimate objects (24,25,26).
New developments in steriliza on
A verified procedure called steriliza on is used to remove all traces of live germs, including spores, from a product. As previously indicated, items that come into contact with vascular systems or sterile ssue are deemed vital and need to be kept sterile to prevent the spread of illness. Implants, cardiac and urogenital catheters, and surgical equipment fall under this group of cri cal goods. This category's products ought to be bought as sterile or, if that's not feasible, steam sterilized. In the event that the object is heat-sensi ve, other techniques such as ethylene oxide, hydrogen peroxide gas plasma, vaporized H2O2, hydrogen peroxide vapor with ozone, or liquid chemical sterilants may be used (6).
New steriliza on development for endoscope
Both non intact mucous membranes and sterile ssue are encountered by gastrointes nal (GI) endoscopes and bronchoscopes (when biopsies are acquired). Consequently, there exists a possibility of poten al pathogens spreading from one pa ent to another, thereby eleva ng the risk of infec on. A empts to lower this risk by reprocessing endoscopes (by doing double high-level disinfec on, for example) have not been effec ve (27).
There is a 6-log10 safety margin when switching from high-level disinfec on to steriliza on, which means that bacteria are decreased by a factor of one million. Vegeta ve bacteria are reduced by ≥6 log10 in highlevel disinfec on, but spores are reduced by ≥12 log10 in steriliza on. This change would enable adherence
to the Spaulding scheme, which states that devices like cystoscopes, bronchoscopes and duodenoscopes that penetrate a mucous membrane and indirectly touch normally sterile ssue should be categorized as cri cal (28).
Due to their interac on with intact mucous membranes, endoscopes such as gastrointes nal and bronchoscopes are classified as semi cri cal goods. These equipments are usually cleaned and subjected to high-level disinfec on, which can reduce the number of bacteria by up to 6-log10. It's crucial to remember, though, that an endoscope may harbor up to 7–10 logs—that is, 10 million–10 billion microorganisms. It is important to ensure pa ent safety when dealing with medical equipment such as duodenoscopes, bronchoscopes, and cystoscopes. These instruments commonly come into contact with non-intact and sterile ssue, are classified as cri cal items. As per the scheme, some semi-cri cal items come into contact with sterile ssue must followed high-level disinfec on to steriliza on. This is necessary to maintain the highest standards of pa ent safety (29,30).
The medical industry is shi ing towards the use of steriliza on methods to minimize the risk of infec on during gastrointes nal (GI) endoscopies and other endoscopic procedures. This shi is likely to occur through one of five technologies:
• new low-temperature steriliza on technologies,
• disposable sterile endoscopes or its accessories,
• steam steriliza on for GI endoscopes,
• use of non endoscopic methods to diagnose or treat disease (such as capsular endoscopy)
• bmodified endoscope design (30).
A new sterilizer for endoscopes has been developed which uses a unique, low-temperature steriliza on technology. This technology ensures that the steriliza on process is safe, effec ve, and rapid, making it ideal for terminal steriliza on of endoscopic devices. The process offers a noteworthy development in safety margins linked with endoscopic procedures. This sterilizer is capable of terminally sterilizing a broad range of endoscopes, including those with as many as 7 channels and a lumen inner diameter of 0.8 mm or larger x 1600 mm, and 1.2 mm or larger x 4 m. New steriliza on tech uses hydrogen peroxide gas plasma to diffuse vaporized hydrogen peroxide into endoscope channels. Achieves required efficacy concentra on in all channels (up to 4m) in <20 secs. Low HP concentra on & shorter exposure mes minimize endoscope damage (30). Manufacturers are sa sfying the current steriliza on demand as follows: two endoscope steriliza on methods approved by the food and drug Administra on (FDA); Three producers of sterile disposable GI endoscopes makers of disposable sterile bronchoscopes (31).
Advanced technologies can help reduce the risk of infec ons caused by pathogens on medical equipment or environmental surfaces. A number of promising disinfec on methods and steriliza on technologies have recently been iden fied. Researchers must inves gate these technologies, and if proven effec ve, they should be used in infec on preven on prac ces. Con nuous research and assessment are necessary to determine their effec veness in reducing environmental contamina on and ul mately decrease HAIs.
References
1. Shukla A, Srivastava S, Srivastava A, Srivastava T. Surveillance of Microbiological Environment of Opera on Theaters. Cureus. 2021 Dec 20;13(12).
2. Alber ni R, Alessia C, Colucci ME, Zoni R, Affanni P, Veronesi L, Pasquarella CI. An overview of the studies on microbial air contamina on in opera ng theatres and related issues over me: a useful tool for a mul disciplinary approach. ACTA BIOMEDICA. 2023;94(Supplement 3: e2023149).
3. Najotra DK, Malhotra AS, Slathia P, Raina S, Dhar A. Microbiological surveillance of opera on theatres: Fiveyear retrospec ve analysis from a Ter ary Care Hospital in North India. Interna onal Journal of Applied and Basic Medical Research. 2017 Jul 1;7(3):165-8.
4. Laxmi RV, Ramya A, Vanaja S, Vijayalakshmi P. Microbiological surveillance of hospital environment in Chevella, India. J Pure Appl Microbiol. 2021 Sep 1;15(3):1449-54.
5. Panjwani DM, Lakhani SJ, Mehta SJ, Kikani KM, Madaan K. A Study of Hospital Acquired Bacterial Infec ons and its An microbial Suscep bility Pa ern in a Teaching Hospital of Gujarat, India. Int. J. Curr. Microbiol. App. Sci. 2020;9(2):1399-408.
6. Rutala WA, Donskey CJ, Weber DJ. Disinfec on and steriliza on: New technologies. American Journal of Infec on Control. 2023 Nov 1;51(11):A13-21.
7. Bhar B, Li H, Ren Z, Zhu R, Zhu Z. Recent advances in steriliza on and disinfec on technology: A review. Chemosphere. 2022 Dec 1;308:136404.
8. Cadnum JL, Jencson AL, Livingston SH, Li DF, Redmond SN, Pearlmu er B, Wilson BM, Donskey CJ. Evalua on of an electrosta c spray disinfectant technology for rapid decontamina on of portable equipment and large open areas in the era of SARS-CoV-2. American journal of infec on control. 2020 Aug 1;48(8):951-4.
9. Carlisle MG, Rutala WA, Cadnum JL, Wilson BM, Deshpande A, Donskey CJ. A randomized trial of ultraviolet-C (UVC) light versus sodium hypochlorite delivered by an electrosta c sprayer for adjunc ve decontamina on of
hospital rooms. Infec on Control & Hospital Epidemiology. 2023 Jun;44(6):1025-8.
10. Bainum TB, Reveles KR, Hall RG, Cornell K, Alvarez CA. Controversies in the preven on and treatment of clostridioides difficile infec on in adults: a narra ve review. Microorganisms. 2023 Feb 3;11(2):387.
11. Shaughnessy MK, Micielli RL, DePestel DD, Arndt J, Strachan CL, Welch KB, Chenoweth CE. Evalua on of hospital room assignment and acquisi on of Clostridium difficile infec on. Infec on Control & Hospital Epidemiology. 2011 Mar;32(3):201-6.
12. Mayfield JL, Leet T, Miller J, Mundy LM. Environmental control to reduce transmission of Clostridium difficile. Clinical Infec ous Diseases. 2000 Oct 1;31(4):995-1000.
13. Orenstein R, Aronhalt KC, McManus JE, Fedraw LA. A targeted strategy to wipe out Clostridium difficile. Infec on Control & Hospital Epidemiology. 2011 Nov;32(11):1137-9.
14. Hawley B, Casey M, Virji MA, Cummings KJ, Johnson A, Cox-Ganser J. Respiratory symptoms in hospital cleaning staff exposed to a product containing hydrogen peroxide, perace c acid, and ace c acid. Annals of work exposures and health. 2018 Jan 1;62(1):28-40.
15. Cadnum JL, Pearlmu er BS, Haq MF, Jencson AL, Donskey CJ. Effec veness and real-world materials compa bility of a novel hydrogen peroxide disinfectant cleaner. American Journal of Infec on Control. 2021 Dec 1;49(12):1572-4.
16. Carling PC, O’Hara LM, Harris AD, Olmsted R. Mi ga ng hospital-onset Clostridioides difficile: the impact of an op mized environmental hygiene program in eight hospitals. Infec on Control & Hospital Epidemiology. 2023 Mar;44(3):440-6.
17. Rathod SN, Beauvais K, Sullivan LK, Sudikoff SN, Peaper DR, Mar nello RA. The effec veness of a novel colorant addi ve in the daily cleaning of pa ent rooms. Infec on Control & Hospital Epidemiology. 2019 Jun;40(6):721-3.
18. Tyan K, Zuckerman JM, Cutler C, Modupe K, Ray D, Marmolejo L, Kang J. A mul phase interven on of novel color addi ve for bleach disinfectant wipes improves thoroughness of cleaning in an academic medical center. American Journal of Infec on Control. 2022 Apr 1;50(4):469-72.
19. Beswick AJ, Fry C, Bradley CR, Po age T, Sharpe S, Haill CF, Mugglestone MA, Bak A, Marsden GL, Benne A, Garvey M. Automated room decontamina on: report of a Healthcare infec on Society Working Party. Journal of Hospital Infec on. 2022 Jun 1;124:97-120.
20. McGinn C, Sco R, Ryan C, Donnelly N, Cullinan MF, Becke M. Rapid disinfec on of radiology treatment rooms using an autonomous ultraviolet germicidal irradia on robot. American Journal of Infec on Control. 2022 Aug 1;50(8):947-53.
21. Diab-El Schahawi M, Zingg W, Vos M, Humphreys H, LopezCerero L, Fueszl A, Zahar JR, Presterl E, ESCMID Study Group on Nosocomial Infec ons “The decontamina on research working group”. Ultraviolet disinfec on robots to improve hospital cleaning: Real promise or just a gimmick?. An microbial Resistance & Infec on Control. 2021 Dec;10:1-3.
22. Weber DJ, Rutala WA, Anderson DJ, Chen LF, SickbertBenne EE, Boyce JM. Effec veness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamina on: focus on clinical trials. American journal of infec on control. 2016 May 2;44(5):e77-84.
23. Rutala WA, Kanamori H, Gergen M, Weber DJ. Microbial assessment of recontamina on with Acinetobacter in pa ent room environment in burn units. American Journal of Infec on Control. 2020 Aug 1;48(8):S20.
24. Schmidt MG, Fairey SE, A away HH. In situ evalua on of a persistent disinfectant provides con nuous decontamina on within the clinical environment. American Journal of Infec on Control. 2019 Jun 1;47(6):732-4.
25. Warren BG, Barre A, Graves A, King C, Turner NA, Anderson DJ. An enhanced strategy for daily disinfec on in acute care hospital rooms: a randomized clinical trial. JAMA Network Open. 2022 Nov 1;5(11):e2242131-.
26. Cahill OJ, Claro T, Cafolla AA, Stevens NT, Daniels S, Humphreys H. Decontamina on of hospital surfaces
with mul jet cold plasma: A method to enhance infec on preven on and control?. infec on control & hospital epidemiology. 2017 Oct;38(10):1182-7.
27. Heuvelmans M, Wunderink HF, van der Mei HC, Monkelbaan JF. A narra ve review on current duodenoscope reprocessing techniques and novel developments. An microbial Resistance & Infec on Control. 2021 Dec 23;10(1):171.
28. Rutala WA, Weber DJ. Reprocessing semicri cal items: Outbreaks and current issues. American journal of infec on control. 2019 Jun 1;47:A79-89.
29. Rutala WA, Weber DJ. Outbreaks of carbapenemresistant Enterobacteriaceae infec ons associated with duodenoscopes: what can we do to prevent infec ons?. American journal of infec on control. 2016 May 2;44(5):e4751.
30. Rutala WA, Kanamori H, Sickbert-Benne EE, Weber DJ. What's new in reprocessing endoscopes: Are we going to ensure “the needs of the pa ent come first” by shi ing from disinfec on to steriliza on?. American Journal of Infec on Control. 2019 Jun 1;47:A62-6.
31. Molloy-Simard V, Lemyre JL, Martel K, Catalone BJ. Eleva ng the standard of endoscope processing: Terminal steriliza on of duodenoscopes using a hydrogen peroxide–ozone sterilizer. American journal of infec on control. 2019 Mar 1;47(3):243-50.












































InlovingmemoryofLeonieJackandherloveofthewearablearts v i n g m e m o
InterMedInvitesYOU

r y o f L e o n i e J a c k a n d h e r l o v e o f t h e w e a r a b l e

Getateamtogetherandgetthosecreativejuicesflowing!
Thethemeforthisyearis
GREENNZ-Sustainability
Reduce-Reuse-Recycle
®
MakeawearableartpiecemadefromSteriking WrapsandrepurposedCSSDproductsavedfromlandfill. (wewillprovideyouwithastarterpack)
Toregisterorformoreinformationemail claire.smith@intermed.co.nz beforethe1stJuly2024.





Saturday 27th July 2024
09.30 hrs to 13.30 hrs - TBC
Venue: Waipuna Hotel and Conference Centre - Auckland
Companies: Intermed
NZ Valida on Services
Please contact Aileen Derby if interested in a ending. Further details will be on the NZSSA Website.
Aileen Derby
021 221 6984
Aileen.Derby@middlemore.co.nz
Further informa on will be made available on the NZSSA Website as it is confirmed.


By Greg S Whiteley
The first major Australian and New Zealand Standard on Sterilisa on was released as AS/NZS 4187:1994 en tled as “Code of prac ce for cleaning, disinfec ng, and sterilizing reusable medical and surgical instruments and equipment, and maintenance of associated environments in healthcare facili es. “, developed by Standards Australia (SA) commi ee HE002.
A later revision in 1998, AS 4187:1998 “Cleaning, disinfec ng and sterilizing reusable medical and surgical instruments and equipment, and maintenance of associated environments in health care facili es” published in January 1998 by SA via the new HE023 technical commi ee and its impact was almost immediate.
A major problem iden fied by the stakeholders, many of whom were now included as members on HE-023, was that the second 1998 edi on was not appropriate for applica on in the smaller end of the broader healthcare market, par cularly for smaller and office-based prac ces.
It was agreed that a separate standard – not a lesser in standard of care, but more lightweight and less onerous in terms of documenta on and traceability was required.
The Australian Dental Associa on (ADA) and the Royal Australian College of General Prac oners (RACGP) and the Australian Dental Industry Associa on, with others, were able to present the case for a second standard aimed par cularly at the Office Based Prac ces. This standard was released in 2001 and AS/NZS 4815:2001 tled as; “Office-based health care facili es not involved in complex pa ent procedures and processes—Cleaning, disinfec ng and sterilizing reusable medical and surgical instruments and equipment, and maintenance of the associated environment”.
Another revision of AS/NZS 4187:2003 was released as an updated version of the earlier standard (this was the third actual version of AS 4187, now AS/NZS 4187:2003).
Therea er, a second edi on of AS/NZS 4815:2006 was published with the new and revised tle as “Office-
based health care facili es— Reprocessing of reusable medical and surgical instruments and equipment, and maintenance of the associated environment”. The tle change was to remove the qualifica on over “…complex medical procedures…” as the public hospital advocates and representa ves of the government agency represented on the commi ee strongly posi oned that in fact any surgical procedure was complex.
Changes to prac ces and technology required further revision to AS/NZS 4187:2003. This was finally released a er an enormous amount of public consulta on over the proposals. The third edi on was released in 2014 tled as; “AS/NZS 4187:2014 Reprocessing of reusable medical devices in health service organisa ons”.
Subsequently the HE-023 commi ee were working on a new standard. This standard, AS 5369:2003 Reprocessing of medical devices and other devices in health and non-health related facili es”, was finally published the month before the prior standard (AS/NZ 4187:2014) was due to come into full effect in December 2023.
Dental prac ces were always going to be covered by the requirements of a standard for sterilisa on. It is also of note that the stakeholder group for the standard has also expanded to include addi onal health professionals (sonographers, podiatrists etc) as well as non-medical organisa ons such as ta ooists and cosme c piercing.
For any medical or non-health related facili es for which this standard will apply, the first step for compliance is to conduct a risk assessment and gap analysis. This will allow those involved in sterilisa on prac ce to iden fy areas for improvement. This might also include capacity building and capital cost for new equipment.
For assistance on understanding the new requirements, we strongly recommend contac ng your relevant trade associa on or a technical advisory group.




























JournalofHospitalInfection131(2023)164 172
Availableonlineat www.sciencedirect.com
journalhomepage: www.elsevier.com/locate/jhin

N.Grae a,A.Singh a,D.Jowitt a,A.Flynn b,E.Mountier b,G.Clendon b , R.Barratt a,B.Gibson a,C.Williams a,S.A.Roberts a, c,A.J.Morris a, c, *
a InfectionPrevention&ControlProgramme,HealthQuality&SafetyCommission,Auckland,NewZealand
b InfectionPrevention&ControlProgramme,HealthQuality&SafetyCommission,Wellington,NewZealand
c AucklandCityHospital,Auckland,NewZealand
ARTICLEINFO
Articlehistory:
Received20August2022
Accepted11October2022
Availableonline19October 2022
Keywords: Hospital-associatedinfection Pointprevalencesurvey
Infectionpreventionand control
Epidemiology
Surgicalsiteinfection
Bloodstreaminfection

Background: Therearenocontemporarydataonhealthcare-associatedinfections(HAIs) inNewZealand.
Aims: TodeterminetheepidemiologyofHAIs,prevalenceofmedicaldevices,and microbiologyofHAIsinadultsinpublichospitalsinNewZealand.
Methods: Pointprevalencesurvey.Surveyorsreviewedpatientsaged 18yearsusingthe HAIdefinitionsoftheEuropeanCentresforDiseasePreventionandControl.Deviceuseand microbiologyofHAIswererecorded.
Findings: Intotal,5468patientsweresurveyed;361patients(6.6%)had423HAIs(7.7HAIs per100patients).ThemostcommonHAIswere:surgicalsiteinfections(N¼104,25%), urinarytractinfections(N¼80,19%),pneumonia(N¼75,18%)andbloodstreaminfections (N¼55,13%).Overall,3585patients(66%)hadatleastonedevice,with2922(53%)patients havingaperipheralintravenouscatheter.Sixty-nine(16%)HAIsweredevice-associated. Onmulti-variableanalysis,independentriskfactorsforHAIsincludedthepresenceofa peripheral[oddsratio(OR)2.0]orcentral(OR5.7)intravenouscatheterandclinical service.HAIrateswerehigherinsurgicalpatients(OR1.8),intensivecareunitpatients (OR2.6)andrehabilitation/olderpersons’healthpatients(OR2.4)comparedwithgeneral medicinepatients(P 0.01forallgroups).Intotal,301organismswereidentified. Clostridioidesdifficile infectionwasuncommon,accountingfor1.7%ofallHAIs.Forty-two isolates(14%)weredrug-resistant,andmost(N¼33,79%)wereEnterobacterales.
Conclusion: ThisstudyestablishedthemostcommonHAIsandtheirriskfactorsinNew Zealand.Thehighprevalenceofdeviceuseunderscorestheneedtoensurethatproven multi-modalpreventioninterventionsareinplace.However,aslessthanhalfofHAIsare device-orsurgery-associated,otherinterventionstrategieswillberequiredtoreduce theirburden.
ª 2022TheAuthor(s).PublishedbyElsevierLtd onbehalfofTheHealthcareInfectionSociety.Thisisanopenaccessarticle undertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
*Correspondingauthor.Address:AucklandCityHospital,2Park Road,Grafton,Auckland1023,NewZealand.Tel.: þ64(0)274352727. E-mailaddress: arthurm@adhb.govt.nz (A.J.Morris).
https://doi.org/10.1016/j.jhin.2022.10.002 0195-6701/ª 2022TheAuthor(s).PublishedbyElsevierLtdonbehalfofTheHealthcareInfectionSociety.Thisisanopenaccessarticle undertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Healthcare-associatedinfections(HAIs)areasignificant publichealthproblemassociatedwithincreasedmorbidity, mortality,lengthofhospitalstayandhealthcarecosts[1 8].
InNewZealand,thereislimitedinformationontheprevalence ofHAIs.Previouspointprevalencesurveys(PPSs)inhospitalsin AucklandDistrictHealthBoard(DHB)inthelate1990sreporteda cumulativeincidenceofHAIof6.3%andprevalenceofHAIof9.5% [9,10].ThemostcommontypesofHAI,comprisingmorethan80% ofallHAIs,weresurgicalsiteinfections(SSIs),lowerrespiratory tractinfections(LRTIs),skin/softtissueinfections(SSTIs),urinary tractinfections(UTIs)andbloodstreaminfections(BSIs)[9,10].In 1999,theestimatedcostofHAIsforAucklandDHBwasalmost$19 million($31min2021),andtheestimatedcostforNewZealand was$137million($226min2021)[5].A2013PPSofmedicaland rehabilitationpatientsinanothermetropolitanAucklandDHB hospital,usingadifferentmethodology,foundpointprevalence of5%andcumulativeprevalenceof10.7%[11].
Medicalcarehaschangedoverthepast20yearsandwith contemporaryuseofmedicaldevices,immunosuppressive treatmentsandtransplantprogrammes,olderdata,limitedto larger,urbanAucklandDHBs,maynotrepresentthecurrent rateordistributionofHAIsinthecountry.Inaddition,Health QualityandSafetyCommissionqualityimprovement(QI)programmes,suchasTargetCLABZero,HandHygieneNewZealandandSurgicalSiteInfectionImprovement,havebeenin placeforadecadewiththeaimofreducingHAIs[12 15].
ThisarticlereportsthefirstnationalPPSofallDHBhospitals inNewZealandwiththeaimofobtaininginformationtoinform theselectionofQIinitiativestoreduceHAIs.Thecurrentrate andspectrumofHAIsinNewZealand,prevalenceofdevice use,device-associatedHAIs,andmicrobiologyofHAIs, includingdrugresistance,weredetermined.
ArollingPPSwasperformedacrossallDHBacutecarepublic hospitalsfrom22nd Februaryto23rd June2021usingthe EuropeanCentreforDiseasePreventionandControl(ECDC) methodologyforPPSsonHAIs[16].
Datacollectionandsurveyortraining
Datawerecollectedonmobiledevicesandenteredintoa secureweb-basedsurveytool,ResearchElectronicDataCapture(REDCap)[17].Thecollectiontoolincludedbranching logicsbasedontheECDCHAIdefinitionsusedforthisPPS[16]. Onthesurveyday,areportofpatientspresentoneachdefined wardat08.00hwasgeneratedfromexistingDHBdatawarehouses,anduploadedintoREDCaptoprovidewardlistsof patients.Acensusofthenumberandtypesofinvasivedevices presentinthesepatientswasundertakenbylocalDHBstaffat 08.00honthesurveyday[i.e.peripheralintravenouscatheters (PVCs),centralintravenouscatheters(CVCs),urethralor suprapubiccatheters,andinvasiveventilation(i.e.involving anendotrachealtubeortracheostomy)].
Trainedsurveyors(RB,BG,CW,SR,AM)collectedallthe data.Surveyortrainingwasdeliveredbyacontractedinfection
preventionandcontrol(IPC)expertexperiencedinadulteducation(DJ),usinganin-house60-pagetrainingmanual.Following10daysofclassroomtraining,allfivesurveyorshad2 daysofpracticalexperiencecapturingPPSdataatAuckland CityHospital.
Togainsupportforparticipating inthePPS,presentations toseniorDHBmanagement(i.e.ChiefExecutives,Chief MedicalOfficersandDirectorsofNursing)occurredin NovemberandDecember2020.Onceparticipationwas confirmed,contactwasmadewiththeDHBIPCand/orQI teamstoorganizelogistics,includingscheduling,informationsystemssupportandlocalstafftosupportthesurveyors tocollectdata.KeyDHBstaffparticipatedinmultiplePPS planningmeetings,andotherDHBstakeholderswereinvited toattendtheexitmeetingtoreviewperformanceandprovidefeedback.
Patientsincludedinthisstudywereadultsaged 18years presentindesignatedwardsat08.00handnotdischargedat thetimeofthesurvey,patientstemporarilyofftheward beforeorat08.00handnotdischargedfromthewardatthe timeofthesurvey,andpatientsonshort-termwardleave. Patientsdischargedbeforethesurveyorarrivedontheward, daycases(includingdaysurgery,patientsinoutpatient departments,anddialysisoutpatients),andboarderswere excludedfromthisstudy.Paediatricwards,mentalhealthunits (acuteandnon-acute),neonatalintensivecareunits,longtermrehabilitationwards,palliativecare,andAccidentand Emergency(A&E)departments(exceptforwardsattachedto A&Edepartmentswherepatientsareadmittedandmonitored for >24h)werenotincludedinthisstudy.
TwotriggerswereusedtoscreenforthepresenceofHAIs: presenceoffever(>38.0 Cinprevious24h)and/orcurrent antimicrobialtherapy(excludingsurgicalormedicalprophylaxis).Ifatriggerwasmet,areviewoftheclinicalnotes,and pathologyandradiologyresultswasundertakentodetermine whetherthepatientmettheECDCdefinitionsforHAI.Patient observationandmedicationchartswerealsoreviewed.CategoriesofHAIincluded:BSIs;boneandjointinfections;central nervoussysteminfections;CVC-orPVC-relatedinfections; Clostridioidesdifficile infection(CDI);pneumoniaorother LRTIs(e.g.tracheitisorbronchitiswithoutevidenceofpneumonia);SSTIs;SSIs;sepsis(withoutapositiveculture);ear, eye,nose,throatandmouthinfections;andUTIs.
Multi-drug-resistantorganisms(MDROs)weredefinedas meticillin-resistant Staphylococcusaureus (MRSA),vancomycinresistantenterococci(VRE),carbapenem-resistantGram-negativebacilli(CROs),andEnterobacteralesresistanttothirdgenerationcephalosporinsbutsusceptibletocarbapenems [mostlyextended-spectrumbeta-lactamase(ESBL)-positive;for analysis,called‘ESBL-positiveorganisms’].
ToensurethatHAIsweredefinedandrecordedcorrectly,at theendofeachday,allproposedHAIswerepresentedbythe surveyorsandreviewedbyatleastoneoftheseniorinvestigators (SR,AM).Additionally,arandomsampleofpatients(withand withoutHAIs)wasusedtoassesshowconsistentlythePPSsurveyorsjudgedpatientHAIstatus. Itwaspresumedthatinter-rater reliability(IRR)wouldbehigh, k 0.8,andtheproportionof patientswithHAIsintheoverallsurveysamplewouldbe 0.065. Basedontheseassumptions,theminimumrequiredIRRgroupsize wasestimatedtobe117patientstoyielda95%confidence interval(CI)withlowerbound 0.6.Theagreementcoefficient (AC1),proposedbyGwetforusewhenthereishighagreementfor categoricaldata,wascalculated[18].Patientswereassignedto theIRRgroupbydailyrandomsamplingfrompre-determined wards.IRRwasmeasuredacrossthethreemainsurveyors(BG, CW,RB).Forlargehospitalswhereallmainsurveyorswereonsite, theIRRgroupincludedsixpatientsperday;fewerpatientswere includedwhenonlytwomainsurveyorswereonsite.Theclinical recordsofIRRpatientsweresubjectedtoindependentlyrepeated datacollectionandentrybyallsurveyorsonsite.
Univariateandmulti-variablelogisticregressionwereused toidentifyindependentfactors(demographic,hospital,clinicalserviceanddevicefeatures)associatedwithHAIstatus.All analyseswereundertakenusingRVersion4.1.1(2021-08-10).A twotailed P-valueof <0.05wasconsideredtoindicatestatisticalsignificance.Factorswereincludedinthemulti-variable analysisiftheywereclinicallyplausibleorsignificantlyassociatedwithHAIintheunivariateanalysis.Stepwisemodel selectionwithbackwardeliminationwasperformedbasedon anAkaikeinformationcriterionalgorithm.Thecombinationof variablesprovidingthebestfitofthedatawasretainedinthe finalmodel.Areceiveroperatingcharacteristic(ROC)curve wasusedtotesttheaccuracyofthemodel.Afunnelplotwas generatedtodetectvariationinDHBHAIrates.
Aprivacyimpactassessmentapplicationwasapprovedby theNorthernRegionDHBRegionalPrivacyAdvisoryGroupin August2020.
Asanauditandrelatedactivity,NewZealand’sHealthand DisabilityEthicsCommittee(HDEC)deemedthatthisstudydid notrequireethicalcommitteereview(T.Katz,personalcommunicationtoS.A.Roberts,14th August2020,HDEC).
Results
Studypopulationandriskfactors
ThePPSwasconductedfrom22nd Februaryuntil23rd June 2021,andincluded31hospitals,and313wardsandunits.In total,5468patientswereincluded;ofthese,2189(40%)were medicalpatients,2012(37%)weresurgicalpatients,755(14%)
wereolderpersons’health/rehabilitationpatients,424(8%) wereobstetricsandgynaecologypatients,and87(1.6%)were intensivecareunit(ICU)patients.Theclinicalserviceofone patientwasnotrecorded.
Intotal,2007patients(37%)hadtriggersforfullrecord review;1787werereceivingantimicrobialtreatment,42had fever,and178hadboth.Overall,1965(36%)patientswere receivingantimicrobialtreatment.
Threehundredandsixty-onepatients(6.6%;95%CI6.0 7.3) had423HAIs;ofthese,308patientshadoneHAI,45patients hadtwoHAIs,sevenpatientshadthreeHAIs,andonepatient hadfourHAIs.Therewere7.7HAIsper100patients.One hundredandthirty-twoHAIswerepresentonadmissionin117 patients(2.1%ofallpatients,32.4%ofallpatientswithHAIs): 51SSIs(39%),22BSIs(17%),16UTIs(12%),12casesofpneumonia(9%)and31(23%)otherHAIs.
PatientcharacteristicsandriskfactorsforHAIarepresented in TablesIandII.EthnicitywasnotassociatedwithHAIrate, butHAIratevariedbyclinicalservice.RegionalreferralDHBs didnothavehigherHAIratescomparedwithsmallerDHBs (TableI).Aftermulti-variablelogisticregressionanalysis,the independentriskfactorsforHAIswere:clinicalservicetype; presenceofaPVC;presenceofaCVC;andlengthofhospital stay.TheROCshowedanareaunderthecurveof0.724,indicatinganacceptablemodel.
AlthoughHAIratesvariedbetweenDHBs,funnelplotanalysisshowedthatallrateswerewithinthe98.8%CI,withtwo DHBsoutsidethe95%CI(FigureS1,seeonlinesupplementary material).
Medicaldeviceswerecommon,with3585patients(66%) havingatleastoneinvasivedevice(TableII).Overall,2922 (53%)patientshadatleastonePVC,549(10%)patientshadat leastoneCVC,967(18%)patientshadaurinarycatheter,and 52(1%)patientswereventilated.Twohundredandfifty patientshadmorethanonePVCorCVC.Thetotalnumberof deviceswas4758.Themostcommondevicecombinations were:PVCalone(N¼2278,42%),PVCandurinarycatheter (N¼519,10%),CVCalone(N¼280,5%),andurinarycatheter alone(N¼238,4%)(FigureS2,seeonlinesupplementary material).CVCusewascommoninseveralpatientgroups, including89of191(47%)haematology/oncology/transplant patients,51of181(28%)cardiovascularsurgerypatients,98of 809(12%)generalsurgerypatients,49of609(8%)orthopaedic patients,and86of1558(6%)generalmedicinepatients.
ThedistributionofHAIsispresentedin TableIII.Thefour mostcommonHAIswereSSIs,UTIs,pneumoniaandBSIs, comprising74%ofthetotal.Ofthe104SSIs,34(33%),28(27%) and42(40%)weresuperficial,deepandorganspace,respectively.Themostcommonsourcesofthe55BSIswereintravenous(IV)catheters[N¼14(25%),ofwhich11(20%)and3(5%) wereCVCsandPVCs,respectively],UTIs(N¼8,15%)andSSIs (N¼6,11%).Thesourceof14BSIs(25%)wasnotidentified. Therewereseven(1.7%)casesofCDI.
Device-associatedHAIsaccountedfor69(16%)ofallHAIs; ventilator-associatedpneumoniaaccountedfor13(17%)cases ofpneumonia,andurinarycatheterswereassociatedwith39
TableI
Patientcharacteristicsandriskfactorsforhealthcare-associatedinfections(HAIs)
WithHAIs N ¼ 361(%)
WithoutHAIs N ¼ 5107(%)
CharacteristicsandrisksAllpatients N ¼ 5468(%)
Sex c
Female2889(53)2715(94)174(6)Reference
Male2578(47)2391(92.7)187(7.3)1.220.99
Age,years c,d
653212(58.7)2996(93.3)216(6.7)ReferenceReference < 652256(41.3)2111(93.6)145(6.4)0.950.77
Medianage(IQR)70(53 81)70(53 81)70(57 80)54681.01.00
Ethnicity
NZEuropean3786(69)3527(93.2)259(6.8)Reference M aori766(14)723(94.4)43(5.6)0.810.57
Pacificpeoples410(8)378(92.2)32(7.8)1.150.77
Asian405(7)383(94.6)22(5.4)0.780.49
Other87(2)84(96.6)3(3.4)0.490.12
Emergencyadmission c,d 5468
Yes3815(70)3582(93.9)233(6.1)ReferenceReference No1653(30)1525(92.3)128(7.7)1.291.03
MDROalert c 5467
No5047(92)4724(93.6)323(6.4)Reference Yes420(8)382(91)38(9)1.451.01
RegionalreferralDHB e 5468
No2615(47.8)2455(94)160(6.1)Reference Yes2853(52.2)2652(93)201(7)1.160.94
Clinicalservicetype c,d 5467
Medical2189(40)2094(95.7)95(4.3)ReferenceReference Obstetrics&gynaecology424(8)411(96.9)13(3.1)0.700.37
Rehabilitation/olderpersons’health755(14)691(91.5)64(8.5)2.041.46
Surgical2012(37)1843(91.6)169(8.4)2.021.56 2.63 < 0.0011.751.34
<
ICU87(2)67(77)20(23)6.583.75
Median4(IQR2 9)Median4(IQR2 8)Median9(IQR5 18)54671.021.01 1.02 < 0.0011.011.008 1.019 < 0.001
Admissiontosurveydate/infection date,days c,d
IQR,interquartilerange;MDRO,multi-drug-resistantorganism(e.g.meticillin-resistant Staphylococcusaureus ,extended-spectrumbeta-lactamase-positive);OR,oddsratio;CI,confidence interval;ICU,intensivecareunit;NZ,NewZealand;DHB,districthealthboard.
a Patientswithmissingvaluesforthevariableinquestionwereomittedfromunivariateanalysis.
b Formulti-variableanalysis, N ¼ 5464.Patientswereomittediftheyhadmissingvaluesforanyofthevariablesincludedintheinitialmodel(marked‘ c ’).
c Variableincludedininitialmulti-variablemodel.
d Variableincludedinfinalmulti-variablemodelafterstepwisevariableselection.
e Auckland,CountiesManukau,Waikato,Capital&Coast,andCanterburyDHBs.
TableII
Surgery-anddevice-relatedriskfactorsforhealthcare-associatedinfections(HAIs)
CharacteristicsandrisksAllpatients N¼5468(%) Without HAIs N¼5107(%)
Surgerywithin30days5420
No4294(79)4087(95.2)207(4.8)Reference Yes1126(21)979(86.9)147(13.1)2.962.37 3.70 <0.001
Devices
Anydevice5468
No1,883(34.4)1817(96.5)66(3.5)Reference Yes3585(65.6)3290(91.8)295(8.2)2.471.89 3.27 <
Peripheralvenous catheterc,d 5468
No2546(47)2381(93.5)165(6.5)ReferenceReference
Yes2922(53)2726(93.3)196(6.7)1.040.84 1.290.72.021.55
Centralvenous catheterc,d 5468
No4919(90)4674(95)245(5)ReferenceReference Yes549(10)433(78.9)116(21.1)5.114.0 6.5 <
Urinarycatheterc 5468
No4501(82)4249(94.4)252(5.6)Reference Yes967(18)858(88.7)109(11.3)2.141.69 2.7 <0.001
Mechanicalventilationc 5468
No5416(99)5068(93.6)348(6.4)Reference Yes52(1.0)39(75)13(25)4.852.47 8.94 <0.001
IQR,interquartilerange;OR,oddsratio.
a Patientswithmissingvaluesforthevariableinquestionwereomittedfromunivariateanalysis.
b Formulti-variableanalysis, N¼5464.Patientswereomittediftheyhadmissingvaluesforanyofthevariablesincludedintheinitialmodel (marked‘c’).
c Variableincludedininitialmulti-variablemodel.
d Variableincludedinfinalmulti-variablemodelafterstepwisevariableselection.
(49%)UTIs.Therewere17IV-catheter-relatedHAIs,threelocal insertionsiteinfections,and14BSIs(TableII).
Microbiology
Forthe423HAIs,301pathogenswereisolatedintotal;208 patientshadoneisolaterecoveredfromtheirHAI,37patients hadtwoisolates,fivepatientshadthreeisolates,andone patienthadfourisolates.Onehundredandseventy-two(41%) HAIsdidnothaveanorganismidentified(TableIV). Staphylococcusaureus wasthemostcommonpathogenidentified (N¼63,21%),followedby Escherichiacoli (N¼61,20%).Other Enterobacterales,mainly Klebsiella spp.(N¼32),werethe nextmostcommongroup(N¼59,20%),with Enterococcus spp., otherstaphylococci, Candida spp.and Pseudomonasaeruginosa makingupmostoftheotherisolates(29%)(TableIV). S.aureus wasthemostcommonisolateinthreeofthefour mainHAIgroups,andwasresponsibleforthreePVCBSIsand twoCVCBSIs.EnterobacteralesdominatedUTIs(N¼51,64%), whilenoorganismwasidentifiedformostofthepatientswith pneumonia(83%)andotherHAIs(61%)(TableIV).
Pathogensusceptibility
Therewere42MDROs(14%)(TableIV).Eight(13%)of63 S.aureus wereMRSA.Therewere120Enterobacteralesisolates,ofwhich28(23%)wereESBL-positiveandfive(4%)were
CROs,representingacombinedMDROprevalenceof33(28%) forthisgroup.MDROsaccountedfor12of61(20%) E.coli,11of 32(34%) Klebsiella spp.,and10of27(37%)otherEnterobacterales.TherewerenoVREamongthe36enterococcus isolates,andonlyoneofthe15 P.aeruginosa isolateswas carbapenemresistant(TableIV).
Fourhundredandtwenty(8%)patientshadanMDROor C.difficile alertintheirrecord;243(4.4%)foranESBL-positive organismand188(3.4%)forMRSA.AnMDROalertwasnotan independentriskfactorforHAI(TableI).
IRRonthepresence/absenceofHAIswasmeasuredfroma sampleof316patients(6%ofcohort).For113patientsinthe sample,therewerethreereplicates[i.e.anindependentdata collectionwasperformedbyeachofthethreemainsurveyors (RB,CGandBG)].Fortheremaining203patients,therewere tworeplicates.IRRwashigh[Fleiss k 0.87(95%CI0.76 0.98); Gwet’sAC1 0.987(95%CI0.976 0.999)][18].
RecentPPSresultsfromothercountries[19 24]aresummarizedin TableS1 (seeonlinesupplementarymaterial). ComparisonwithSingaporeisdifficultasthatsurveyattributed ahighproportionofHAIstounspecifiedsepsis(26%)[20],which
TableIII
Distributionof423healthcare-associatedinfections(HAIs)a
Surgicalsite110424.6(20.7 28.9)
Urinarytractb 28018.9(15.5 22.9)
Pneumoniac 37517.7(14.4 21.7)
Bloodstreamd 45513.0(10.1 16.5)
Eye,ear,nose,throat, mouthe 5389.0(6.6 12.1)
Skinandsofttissuef 6¼ 163.8(2.3 6.1)
Systemicg 6¼ 163.8(2.3 6.1)
Gastrointestinalh 8143.3(2.0 5.5)
Cardiovascular981.9(1.0 3.7)
Reproductivetract1061.4(0.7 3.1)
Boneandjoint1151.2(0.5 2.7)
Lowerrespiratorytracti 1240.9(0.4 2.4)
Centralnervoussystem1320.5(0.1 1.7)
CI,confidenceinterval.
a EuropeanCentreforDiseasePreventionandControldefinitions [16].
b Thirty-nineurinarytractinfections(49%)wereassociatedwitha urinarycatheter.
c Thirteenpneumoniaevents(17%)wereassociatedwithmechanical ventilation.
d Fourteenbloodstreaminfections(25%)wereassociatedwithan intravascularcatheter(11central,threeperipheral),eight(15%)dueto urinarytractinfectionsandsix(11%)duetosurgicalsiteinfections.
e Includes33casesoforalcandidiasis.
f Includesthreelocalinfectionsrelatedtoanintravascularcatheter (onecentral,twoperipheral).
g Treatmentinitiatedforseveresystemicinfectionwherenoisolate orsiteofinfectionidentified.
h Includessevencasesof Clostridioidesdifficile infection.
i Infections(e.g.bronchitis,tracheitis)withoutevidenceof pneumonia.
hasnotbeenreportedbyothers[19,21 24].Thepresent resultsforNewZealandareverysimilartothosereportedfrom Australia,theEuropeanUnion(Europe),theUSA,Switzerland andWales,withthetopfourHAIscomprising56 75%ofallHAIs [19,21 24].DirectcomparisonwiththerecentAustralian reportisdifficultbecausethoseresultswerelimitedtoprincipalreferralandgroupAhospitals[24],ratherthanthewide rangeofservicecomplexityprovidedbyNewZealandDHBs.
ThemaindifferencesinthePPSforNewZealandincludea higherproportionofSSIscomparedwiththeEuropeandWales [21,22],ahigherproportionofUTIscomparedwiththeUSA [19],alowerproportionofsystemicinfectionscomparedwith Wales[21],ahigherproportionofsystemicinfectionscomparedwiththeUSA[19],andfewercasesofCDIcomparedwith Europe,theUSAorWales[19,21,22](TableS1,seeonline supplementarymaterial).FewreportsdescribedHAIratesfor differentclinicalservices,butthehigherrateforICUpatients foundinthepresentPPSisconsistentwithotherreports [19 24].TheproportionsofHAIsinthissurveyareverysimilar tothoseobserved20yearsagoinAucklandDHBwiththemain differencebeingthatSSIscurrentlycompriseahigherproportionofHAIs(25%vs18%previously)[10],andalowerproportionofBSIswerefoundtobelinkedtoIVcathetersinthe presentstudy(25%vs40%previously)[25].
Multi-variablelogisticregressionanalysisshowedthatcertainclinicalserviceshadhigherratesthangeneralmedicine, namelyICU,rehabilitation/olderperson’shealthandsurgical specialties.ThepresenceofeitheraCVCorPVCwasanindependentriskfactor,aswaslengthofhospitalstay.
TheorganismscausingHAIsweredominatedby S.aureus andEnterobacterales,ofwhich E.coli and Klebsiella spp.were themostcommon.Theseproportionsareverysimilartothose reportedpreviously(TableS1,seeonlinesupplementary material)[19 24].ThisstudyfoundasimilarrateforMDROsas inSwitzerlandandAustralia[23,24],butalowerproportionof MDROscomparedwiththoseencounteredinEurope[22](14% vs32%).ThehighestproportionofMDROswasencountered amongEnterobacterales,withalmostone-quarterbeing MDROs,andwashighestfor Klebsiella spp.withone-thirdbeing MDROs(TableIV).
MDROor C.difficile alertswerepresentfor8%ofpatients.In arecentAustralianstudy,12%ofpatientswerebeingmanaged asMDRO-positive,and11%ofthemhadactiveHAIs[24].While thepresentstudydidnotspecificallyrecordifpatientswere undertransmission-basedprecautions,manypatientswereand thisrepresentsasignificantresourceissueforhospitals.However,astheuseofMDROalertsdiffersamongDHBs,the observedrateshouldberegardedonlyasanestimateforhow commonlytransmission-basedprecautionsareinplace.
Justoverone-thirdofpatientswerereceivingantibioticsfor treatment.Severalpatientswereonantibioticswithouta statementinthecharttoindicatethereason.Whileauditof antibioticusewasoutwiththescopeofthisPPS,thefrequency ofantibioticuse,MDROprevalence,andadditionalcostof managingMDROinfections[26,27]supporttheneedfora nationalsurveyofantimicrobialprescribingtoidentifyappropriatestewardshipinterventions[28].
ThisPPSwasresourceintensiveinplanning,training,executionandanalysis.CommunicationwascriticaltoPPS implementation,withinitialhigh-levelcontactwithDHBclinicalandmanagementteamsseekingparticipation,followedby planningwiththelocalPPSteamandnotifyingwardstaffabout thesurveyandtheneedforthedevicecensus.
Thisstudyhasseveralstrengths.Comprehensivetraining wasprovidedforsurveyorsontheHAIdefinitionsanddata entry.Datawerecollectedbyalimitednumberofsurveyors, whohadhighinterobserveragreementforrecordingHAIs,and caseswererevieweddailytodecideontheirinclusion.The hospitalsweresurveyedoverarelativelyshortperiodoftime thatavoidedthewinterpeakperiod.
However,thisstudyhassomelimitations.Notallpatients presentonthesurveydaywereassessed,andsomeHAIswould havebeenmissed(e.g.apatientcouldhavebeendischarged homeonoralantibioticsfortheirHAIbeforethesurveillance teamarrivedontheward,orapatientmayhavebeeninthe emergencydepartmentbutnotadmittedbythe08.00hcut-off forinclusion).Somepatienttriggerscouldhavebeenmissed, butthisisconsideredtohavebeenrare.Theapplieddefinitions didnotincludeallpossibleHAIs;forexample,somepatients werereceivingtreatmentfororganspaceSSIs,buttheoriginal operationwasoutsidethe90-dayinclusionperiod.Therefore, theobservedprevalenceofHAIsshouldberegardedasaminimum.ReasonsforantibiotictreatmentforthosewithoutHAIs (e.g.communityinfection)werenotrecorded.Thesusceptibilityresultsofasmallnumberofisolateswerenotknown,and theMDROrateshouldalsoberegardedasaminimum.DHBs
TableIV
Pathogenscausing423healthcare-associatedinfections(HAIs),byinfectiontypea
423HAIsSurgicalsite (N¼104)
Pathogen301isolates N (%)
Rank87isolates N (%)
Urinarytract (N¼80)
Pneumonia (N¼75)
80isolates N (%) 17isolates N (%)
Bloodstream (N¼55)
65isolates N (%)
OtherHAIs (N¼109)
52isolates N (%)
Staphylococcusaureusb 63(21)127(31)3(4)3(18)16(25)14(27)
Escherichiacolic 61(20)211(13)33(41)1(6)8(12)8(15)
Enterococcus spp.d 36(12)39(10)11(14)1(6)8(12)7(13)
Klebsiella spp.e 32(11)45(6)12(15)3(18)9(14)3(6)
OtherEnterobacteralesf 27(9)512(14)6(8)1(6)6(9)2(4)
Otherstaphylococci20(7)69(10)2(3)-6(9)3(6)
Candida spp.g 17(6)73(3)7(9)1(6)3(5)3(6)
Pseudomonasaeruginosah 15(5)85(6)4(5)2(12)2(3)2(4)
OtherGram-negativesi 8(3)91-4(24)3(5)-
Clostridioidesdifficile 7(2)10----7(13)
Streptococcus spp.512(3)-2(3)-
OtherGram-positives43(3)--1(2)-
Otheranaerobes41--1(2)2(4)
Aspergillusfumigatus 1--1(6)-Herpessimplexvirus1----1(2)
Multi-drug-resistant organismsj 42(14)14(16)15(19)2(12)7(11)4(8)
Nopathogenisolatedk 172(41)35(34)9(11)62(83)0(0)66(61)
a Totalof301pathogens:one,two,threeorfourpathogensidentifiedin208,37,fiveandoneHAI,respectively.
b Eight(13%)meticillin-resistant S.aureus
c Eleven(18%)extended-spectrumbeta-lactamase(ESBL)positive,one(1.6%)carbapenem-resistantorganism(CRO).
d Noisolatewasvancomycinresistant.
e Ten(31%)ESBLpositive,one(3%)CRO.
f Seven(26%)ESBLpositive,three(11%)CRO.
g Candidaalbicans (N¼10), C.glabrata (N¼3), C.parapsilosis (N¼1),non-speciated(N¼3).
h One(7%)CRO.
i Noneweredrugresistant.
j Eight(19%)MRSA,28(67%)ESBL,six(14%)CRO.
k NumberofHAIsusedasthedenominator.
havedifferentprocessesformaintainingMDROalertsinpatient records,andthedegreeofvariationisnotknown.Inaddition, theauthorsdidnotcaptureHAIsarisingafterdischargeand managedinthecommunity.Aswithallsuchsurveillance, definitionsmayovercallsomeHAIs[29].
AlthoughthisPPSwasconductedduringthecoronavirus disease2019pandemic,theoperationofhospitalsinNew Zealandwasrelativelyunaffectedcomparedwithmanyother countries.DuringthePPSperiod,electiveandemergency clinicalactivitycloselyresembledthepre-pandemicclinical caseloadforthesehospitals[30].Whiletherewerenocommunitylockdownsduringthesurveillanceperiod,entryinto NewZealandwasessentiallyrestrictedtocitizens,and required14daysofmanagedisolationandtesting.Theborder restrictionsresultedinadramaticreductionintheexpected seasonalrespiratoryvirusactivity,whichcanbegininJune whichwasneartheendofthePPS.
ThisPPSwasconductedasthefirststepinaprocessto developanationalstrategytopreventHAIsinNewZealand. Theobservedprevalencewillenableanestimateofincidence which,withnationaladmissiondata,willenablecalculation ofthelikelynationalburdenofHAIs.Theactionswiththe best-availableevidencetoinformnationalpolicyaremultimodalinterventionsandsurveillance,monitoringandfeedback[31].Themostrecentestimatesforthepreventable
proportionofselectedHAIsinhigh-andmiddle-income countriesareoftheorderof40 60%[32].Well-described bundlesofcaretoreducearangeofHAIsexist[ 33 37]. Choosingwhichinterventionstopromotewillbechallenging becausethedegreetowhichmulti-modalinterventionsare currentlyinplacewithinahospitalandbetweenhospitalsis unclear.Forexample,thenationalCLABZeroimprovement collaborativeforICUsresultedin80%compliancewiththe insertionbundle[13],butCVCsareusedacrossawidespectrumofclinicalservicesoutsideICUs,andcompliance,surveillance,monitoringandfeedbackinthesesettingsare unknown.
Whilenotaformalpartofthesurveillancemethods,variationinrecordingofIVcatheteruseandtheirreviewwas notedinthisstudy.Therewasalsoinconsistentmentionof urinarycathetersinpatientnotes,andinfrequentevidenceof reviewfortheircontinuingneed.Deviceuseandmonitoring areitemstoconsiderforQIfocus.
Inconclusion,thisstudyestablishedthecommonHAIsand theirriskfactorsinNewZealand.Thehighprevalenceof deviceuseunderscorestheneedtoensurethatproven multi-modalpreventioninterventionsareinplace.However, aslessthanhalfofHAIsaredevice-orsurgery-associated, otherinterventionstrategieswillberequiredtoreduce theirburden.
N.Graeetal./JournalofHospitalInfection131(2023)164
TheauthorswishtothankDr.KalisvarMarimuthufromSingaporeandDrs.PhilipRusso,AndrewStewardson,AllenCheng andBrettMitchellfromAustraliaforthesupportprovided duringthedevelopmentphaseofthePPS,andforsharingthe REDCapdatacollectiontool.Theauthorsalsowishtothank HariniSrinivasanforperformingthelogisticregressionandIRR analyses.Theauthorsacknowledgethesignificantcontribution ofthelocalDHBIPCteammembersaswellasthewardstafffor assistingsurveyorsandperformingthedevicecensusonthe surveyday.Inaddition,theauthorswishtothankthewardand unitchargenurseswhofacilitatedaccesstothehospital computersystemandclinicalrecordsontheday.Theauthors alsoacknowledgethetimeandeffortbythelocalDHBbusiness intelligenceunitsfordatauploads.Finally,theauthorswishto thanktheseniormanagementatallDHBs,includingChief ExecutiveOfficers,DirectorsofNursingandChiefMedical Officers,fortheirsupport.
Authorcontributions
NGcontributedtoaspectsofstudydesignandcontributed tothefinalmanuscript.AFcontributedtoaspectsofstudy design,logisticalplanning,andcontributedtothefinal manuscript.AScontributedtostudydesign,logistical planning,andcontributedtothefinalmanuscript.DJ developedthetrainingmaterials,providedsurveyortraining,andcontributedtostudydesignandthefinalmanuscript.EMcontributedtostudydesign,setupelectronic datatransfer,providedoperationalsupportforrunningthe survey,performeddataanalysis,andcontributedtothe finalmanuscript.GCperformedserverprovisioningand configurationfortheelectronicplatformfordatacollection,andcontributedtostudydesign,designofdata transferprocesses,dataanalysisandthefinalmanuscript. RB,BGandCWcontributedtosurveillancedesign,performedthesurveillance,andcontributedtothefinal manuscript.SRandAMaretheprincipalinvestigatorswho conceivedthestudy,contributedtostudydesign,data collection,andmanuscriptpreparationandreview.All authorsapprovedthefinalarticle.
Conflictofintereststatement Nonedeclared.
Fundingsources ThisworkwassupportedbytheHealthQualityandSafety Commission.
AppendixA.Supplementarydata
Supplementarydatatothisarticlecanbefoundonlineat https://doi.org/10.1016/j.jhin.2022.10.002
References
[1] WorldHealthOrganization.Reportontheburdenofendemic healthcare-associatedinfectionworldwide.Asystematicreview oftheliterature.Geneva:WHO;2011
[2] ManoukianS,StewartS,GravesN,MasonH,RobertsonC, KennedyS,etal.Bed-daysandcostsassociatedwiththeinpatient
burdenofhealthcare-associatedinfectionintheUK.JHospInfect 2021;114:43 50
[3] CassiniA,PlachourasD,EckmannsT,SinMA,BlankH-P, DucombleT,etal.Burdenofsixhealthcare-associatedinfections onEuropeanpopulationhealth:estimatingincidence-baseddisability-adjustedlifeyearsthroughapopulationprevalence-based modellingstudy.PLoSMed2016;13:e1002150
[4] LydeamoreMJ,MitchellBG,BucknallT,ChengAC,RussoPL, StewardsonAJ.Burdenoffivehealthcareassociatedinfectionsin Australia.AntimicrobResistInfectControl2022;11:69.
[5] GravesN,NichollsTM,MorrisAJ.Modellingcostsofhospital acquiredinfectionsinNewZealand.InfectControlHospEpidemiol2003;24:214 33
[6] GowM,McGuinnessC,MorrisAJ,McLellanA,HardyAE,MunroJT, etal.Excesscostassociatedwithprimaryhipandkneejoint arthroplastysurgicalsiteinfections:adrivertosupportinvestmentinqualityimprovementstrategiestoreduceinfectionrates. NZMedJ2016;129:51 8
[7] UptonA,SmithP,RobertsS.Excesscostassociatedwith Staphylococcusaureus poststernotomymediastinitis.NZMedJ 2005;118:U1316
[8] BarnacleJ,WilsonD,LittleC,HoffmanC,RaymondN.Excesscost andinpatientstayoftreatingdeepseatedspinalsurgicalsite infections.NZMedJ2018;131:27 34
[9] NichollsTM,MorrisAJ,AucklandHealthcareInfectionControl Service.NosocomialinfectioninAucklandhealthcarehospitals. NZMedJ1997;110:314 7.
[10] GravesN,NichollsTM,WongCGS,MorrisAJ.Theprevalenceand estimatesofthecumulativeincidenceofhospitalacquired infectionsinpatientsadmittedtoAucklandDistrictHealthBoard hospitalsinNewZealand.InfectControlHospEpidemiol 2003;24:56 61
[11] ReadK,BhallyH.‘Real-time’burdenofcommunityandhealthcarerelatedinfectionsinmedicalandrehabilitationpatientsin apublichospitalinAuckland,NewZealand.NZMedJ 2015;128:69 74
[12]HealthQualityandSafetyCommission.Infectionpreventionand controlprogramme.Wellington:HQSC.Availableat: https:// www.hqsc.govt.nz/our-work/infection-prevention-and-control/ [lastaccessedNovember2022].
[13] GrayJ,ProudfootS,PowerM,BennettB,WellsS,SeddonM. TargetCLABZero:anationalimprovementcollaborativeto reducecentralline-associatedbacteraemiainNewZealand intensivecareunits.NZMedJ2015;128:13 21.
[14] FreemanJ,SieczkowskiC,AndersonT,MorrisAJ,KeenanA, RobertsSA.ImprovinghandhygienecomplianceinNewZealand hospitalstoincreasepatientsafetyandreducecosts:resultsfrom thefirstnationalhandhygienecomplianceauditfor2012.NZMed J2012;125:178 81
[15] MorrisAJ,RobertsSA,GraeN,HamblinR,ShukerC,MerryA.The NewZealandSurgicalSiteInfectionImprovement(SSII)Programme:anationalqualityimprovementprogrammereducing orthopaedicsurgicalsiteinfections.NZMedJ2018;131:45 56.
[16] EuropeanCentreforDiseasePreventionandControl.Point prevalencesurveyofhealthcare-associatedinfectionsandantimicrobialuseinEuropeanacutecarehospitals protocolversion 5.3.Stockholm:ECDC;2016.
[17] HarrisPA,TaylorR,PayneJ,GonzalesN,CondeJG.Research electronicdatacapture(REDCap) ametadata-drivenmethodologyandworkflowprocessforprovidingtranslationalresearch informaticssupport.JBiomedInform2009;42:377 81
[18] GwetKL.Computinginter-raterreliabilityanditsvarianceinthe presenceofhighagreement.BrJMathStatPsychol 2008;61:29 48
[19] MagillSS,EdwardsJR,BambergW,BeldavsZG,DumyatiG,KainerMA, etal.Multistatepoint-prevalencesurveyofhealthcare-associated infections.NEnglJMed2014;370:1198 208.
[20] CaiY,VenkatachalamI,TeeNW,TanTY,KurupA,WongSY,etal. Prevalenceofhealthcare-associatedinfectionsandantimicrobial
useamongadultinpatientsinSingaporeacute-carehospitals: resultsfromthefirstnationalpointprevalencesurvey.ClinInfect Dis2017;64(Suppl.2):S61 7.
[21]PublicHealthWales.Nationalpointprevalencesurveyof healthcareassociatedinfection,deviceusageandantimicrobial prescribing2017.Cardiff:PublicHealthWales;2018.Available at: https://www.wales.nhs.uk/news/47633#:w:text¼The% 20National%20Point%20Prevalence%20Survey,3.2%20per%20cent% 20in%202011 [lastaccessedNovember2022].
[22] SuetensC,LatourK,KarkiT,RicchizziE,KinrossP,MoroML,etal. Prevalenceofhealthcare-associatedinfections,estimatedincidence andcompositeantimicrobialresistanceindexinacutecarehospitals fromtwoEuropeanpointprevalencesurveys,2016to2017.Euro Surveill2018;23:pii¼1800516
[23] AlikiM,MiriamV,RamiS,JonasM,CathyV,NicolasT,etal.Point prevalenceofhealthcare-associatedinfectionsandantibioticuse inthreelargeSwissacute-carehospitals.SwissMedWkly 2018;148:w14617
[24] RussoPL,StewardsonAJ,ChengAC,Bucknall,MitchellBG.The prevalenceofhealthcareassociatedinfectionsamongadult inpatientsinnineteenlargeAustralianacute-carepublichospitals:apointprevalencestudy.AntimicrobResistInfectCont 2019;8:114.
[25] NichollsTM,MorganAS,MorrisAJ.Nosocomialbloodstream infectioninAucklandhealthcarehospitals.NZMedJ 2000;113:96 8.
[26] JohnstonKJ,ThorpeKE,JacobJT,MurphyDJ.Theincremental costofinfectionsassociatedwithmultidrug-resistantorganisms intheinpatienthospitalsetting anationalestimate.Health ServRes2019;54:782 92
[27] TechouaketNguemeleuE,BeogoI,SiaD,KilpatrickK,SeguinC, BaillotA,etal.Economicanalysisofhealthcare-associated infectionpreventionandcontrolinterventionsinmedicaland surgicalunits:systematicreviewusingadiscountingapproach. JHospInfect2020;106:134 54
[28] GardinerSJ,DuffyEJ,ChambersST,ThomasMG,AddidleM, ArnoldB,etal.Antimicrobialstewardshipinhumanhealthcarein AotearoaNewZealand:urgentcallfornationalleadershipandcoordinatedeffortstopreserveantimicrobialeffectiveness.NZMed J2021;134:113 28
[29] BearmanG,DollM,CooperK,StevensMP.Hospitalinfection prevention:howmuchcanwepreventandhowhardshouldwe try.CurrInfectDisRep2019;21:2.
[30]HealthQualityandSafetyCommission.Awindowonquality2021: COVID-19andimpactsonourbroaderhealthsystem.Wellington: HQSC;2022.Availableat: https://www.hqsc.govt.nz/resources/ resource-library/a-window-on-quality-2021-covid-19-andimpacts-on-our-broader-health-system-part-1-he-tirohangakounga-2021-me-nga-panga-ki-te-punaha-hauora-whanuiwahanga-1/ [lastaccessedOctober2022].
[31] PriceL,MacDonald,MeloneL,HoweT,FlowersP,CurrieK,etal. Effectivenessofnationalandsubnationalinfectionpreventionand controlinterventionsinhigh-incomeandupper-middle-income countries:asystematicreview.LancetInfectDis2018;18:e159 71
[32] SchreiberPW,SaxH,WolfensbergerA,ClarkL,KusterSP, SwissNoso.Thepreventableproportionofhealthcare-associated infections2005 2016:systematicreviewandmeta-analysis. InfectContHospEpidemiol2018;39:1277 95.
[33] LovedayHP,WilsonJA,PrattRJ,GolsorkhiM,BakA,BrowneJ, etal.epic3:Nationalevidence-basedguidelinesforpreventing healthcare-associatedinfectionsinNHShospitalsinEngland. JHospInfect2014;86(Suppl.1):S1 70
[34] Berrios-TorresSI,UmscheidCA,BratzlerDA,LeusB,StoneER, KelzRR,etal.CentersforDiseaseControlandPrevention guidelineforthepreventionofsurgicalsiteinfection,2017.JAMA Surg2017;152:784 91.
[35] MaN,CameronA,TiveyD,GraeN,RobertsS,MorrisA.Systematicreviewofapatientcarebundleinreducingstaphylococcal infectionsincardiacandorthopaedicsurgery.ANZJSurg 2017;87:239 46
[36] BuettiN,MarschallJ,DreesM,FakidMG,HadawayL, MaragakisLL,etal.Strategiestopreventcentral-lineassociated bloodstreaminfectionsinacute-carehospital:2022update. InfectContHospEpidemiol2022;43:553 69.
[37] KlompasM,BransonR,CawcuttK,CristM,EichenwaldEC, GreeneLR,etal.Strategiestopreventventilator-associated pneumonia,ventilator-associatedevents,andnonventilator hospital-acquiredpneumoniainacute-carehospitals.InfectCont HospEpidemiol2022;43:1 27.






























Check Monthly































Pseudomonas aeruginosa ( Mycobacterium sp. (

Check Yearly Endotoxin (











































Rinse Assure delivers EWD rinse water that conforms with table 7.3 quickly and easily.




Filtration
















To be compliant with AS5369:2023 Table 7.3 you need to:
























































Reverse osmosis


Chemical dosing



























Compliant water quality




Failing your tests?








Tristel has you covered


























Scan to learn more about Rinse Assure





































































































































President: Shelagh Thomas CSSD Hu Valley Capital, Coast and Hu Valley
Phone: 04 566 6999 ext 2745 Mobile: 027 589 6473
Email: shelagh.thomas@hu valleydhb.org.nz
Maureen Sco Sterile Services Hamilton Waikato District Health Board
Email: Maureen.Sco @waikatodhb.health.nz
Donna Dador Southern Cross Hospital 131 Bealey Avenue, Christchurch, 8013.
Email: donnad@schl.co.nz
Jenny Carston CSSD Manager Tauranga Hauora a Toi Bay of Plenty
Email: Jenny.Carston@bopdhb.govt.nz
Treasurer: Alison Stewart
NZSSA Treasurer 28 Brighton Street Island Bay Wellington 6023
Mobile: 021 209 8127
Email: nzsterilescienceassoc@gmail.com
Secretary: Paul Moody Senior Product Development Manager Fisher & Paykel Auckland
Email: paul.moody@fphcare.co.nz
Anthony Valvoi Sterile Services New Plymouth Taranaki
Email: anthony.valvoi@tdhb.org.nz
Mar n Bird
Sterile Services Dunedin Southern
Email: mar n.bird@southerndhb.govt.nz
Aileen Derby CSSD Manager Manukau Coun es Manukau
Email: Aileen.Derby@middlemore.co.nz
Kelly Swale
Sterile Services Manager Faculty of Den stry, University of Otago Dunedin
Email: kelly.swale@otago.ac.nz

Yesterday’s best isn’t good enough for today’s demanding OR. In fact, when you compare Vaporized Hydrogen Peroxide Sterilizers, you’ll see the truth.
V-PROTM Low Temperature Sterilization Systems are validated on more than 48 device materials compared to just 24 for STERRAD.®*
Outperforming STERRAD Sterilizers in every important category, V-PRO Sterilizers deliver with faster cycles and greater weight and chamber capacity, so you’ll process more trays per shift. Today’s one and only true choice is V-PRO.
*STERRAD is a trademark of ASP Global Manufacturing, GmbH.
steris.com VISIT STERIS.COM or talk to your STERIS representative today
