




















Kiaora, and welcome to the October Edition of Supplyline. Spring is finally with us although some days it still feels like it is Winter.
The NZSSA 50th Anniversary Conference was held in the last week of September 2024 and was well attended by both delegates and company representatives. The conference was held in the Aotea Centre in Auckland which turned out to be a very good venue for a conference.
In this issue of Supplyline you will find information and photos from this year’s annual conference including photos from the Wearable Art’s competition organised and supported by Intermed Medical. A huge thank you to Intermed Medical for bringing back Wearable Arts to

Kia Ora
I hope you all enjoyed the conference in Auckland last month, a massive thank you to the organisers and Britta form Mtanz. The 70s theme was a big hit! Next year’s conference will be in Rotorua.
I would like to thank everybody who voted for me and putting their trust in me for the next three years.
A big thank to Shelagh for all her hard work over the last nine-years, I am pleased to let everyone know that Shelagh is still on the executive as a member.
I would like to introduce you to the new executive, and they are: Shelagh Thomas, Donna Dador, Kelly Swale, Aileen Derby, Jenny Carstens, Antonio Owens, Charanjeet Sidhu, John Barnacott Carla Coetzee
our national conference.
The entries for the Wearable Art’s competition were all amazing and the amount of thought and effort that had gone into each entry was obvious. My congratulations to all those that took part.
You will find the NZSSA Annual Report included in this edition of Supplyline. The Annual Report has information relating to our membership numbers as well as the President’s, Secretary’s and Treasurer’s reports for the year. Also included is an updated list of the people who make up the NZSSA Executive Body along with their contact details.
Ngā mihi
Aileen Derby Editor NZSSA Supplyline
- Donna Dador is the new Vice President,
- Carla Coetzee is the Secretary and
- Alison Stewart is the Treasurer.
The new Executive will meet in Wellington on the 1st of November 2024 as an opportunity to meet and greet.
Registration is one of the thing that will become compulsory in the year or so, we are still working on the finer detail.
We as an executive, are looking forward to the challenges within the Health Care sector that we will work towards resolving within the next coming months and we will continue to be there to support the Sterile Science community.
Ngā mihi
Martin Bird NZSSA President
We care for each other, showing kindness and empathy in all that we do.
We are committed to finding future focused solutions and take personal responsibility to be better every day.
Our diversity is our strength, we back each other and work together in partnership.
We are committed to doing the right thing by ensuring equity and hauora are at the heart of everything we do.
Eveland et al. 3D Printing in Medicine (2024) 10:6 https://doi.org/10.1186/s41205-024-00206-1
Randal Eveland1* , Kathleen Antloga1, Ashley Meyer1 and Lori Tuscano1
Abstract
Background Low temperature vaporized hydrogen peroxide sterilization (VH2O2) is used in hospitals today to sterilize reusable medical devices. VH2O2 sterilized 3D printed materials were evaluated for sterilization, biocompatibility and material compatibility.
Materials & methods Test articles were printed at Formlabs with BioMed Clear™ and BioMed Amber™, and at Stratasys with MED610™, MED615™ and MED620™. Sterilization, biocompatibility and material compatibility studies with 3D printed materials were conducted after VH2O2 sterilization in V-PRO™ Sterilizers. The overkill method was used to evaluate sterilization in a ½ cycle. Biocompatibility testing evaluated the processed materials as limited contact (< 24-hours) surface or externally communicating devices. Material compatibility after VH2O2 sterilization (material strength and dimensionality) was evaluated via ASTM methods and dimensional analysis.
Results 3D printed devices, within a specific design window, were sterile after VH2O2 ½ cycles. After multiple cycle exposure, the materials were not cytotoxic, not sensitizing, not an irritant, not a systemic toxin, not pyrogenic and were hemo-compatible. Material compatibility via ASTM testing and dimensionality evaluations did not indicate any significant changes to the 3D printed materials after VH2O2 sterilization.
Conclusion Low temperature vaporized hydrogen peroxide sterilization is demonstrated as a suitable method to sterilize 3D printed devices. The results are a subset of the data used in a regulatory submission with the US FDA to support claims for sterilization of 3D printed devices with specified materials, printers, and device design 1
Keywords 3D printing, Sterilization, Vaporized hydrogen peroxide
Background
The hospital production of 3D printed, or additive manufacturing (AM), devices is becoming more prevalent. Patient specific 3D printed anatomical models are of tremendous value to medical practitioners for patient education, pre-operative surgical planning, surgeon training as well as interoperative and surgical use [2, 3]. With the increase in the number of evidence-based use cases,
*Correspondence: Randal Eveland randal_eveland@steris.com 1STERIS, 5960 Heisley Road, Mentor, OH 44060, USA

there are more clinical scenarios where anatomic models are beneficial in the procedure or operating room and this use requires sterilization. Patient specific 3D printed surgical guides improve patient outcomes via shorter procedure times and better post-operative results, and they are a prerequisite for many procedures. Sterilization prevents contamination of an established sterile field, and it mitigates patient risk when the medical team uses the surgical guide intraoperatively or interacts with the model during a procedure.
When a hospital produces a 3D printed medical device for patient use, [4, 5] the provider assumes the role as the medical device’s manufacturer, including the design,
© The Author(s) 2024. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
fabrication, sterilization, and surgical use. The process to create patient specific medical devices from patient radiological data has been well discussed [3, 6]. One area of this process that has been less studied and documented is the impact of sterilization on devices and the resulting data to support a general approach to sterilization within a healthcare setting.
For sterilization, steam is the most common method identified for hospital manufactured 3D printed devices. Sterilization using low temperature vaporized hydrogen peroxide (VH2O2) has recognized benefits for temperature and moisture sensitive medical devices. The operating temperature for vaporized hydrogen peroxide sterilizers is typically about 50 °C while typical steam sterilization exposure temperatures can range from 121 to 134 °C and devices can reach these temperatures during the sterilization cycle.
The purpose of this study is to report test data for microbicidal efficacy, material compatibility, and biocompatibility testing performed in the low temperature vaporized hydrogen peroxide VPRO maX 2 Sterilizer Specialty Cycle.
Methods
Test articles
Test-specific 3D printed samples used as test articles were with vat polymerization (Form3B, Formlabs, Cambridge, MA) using BioMed Clear and BioMed Amber resins and with material jetting (J750, Stratasys, Israel) with MED610, MED615, and MED620 resins. The test articles were printed, cured, cleaned, and support material removed in accordance with printer instructions for use (contact printer manufacturers or see their websites at www.formlabs.com and www.stratasys.com).
The Formlabs vat polymerization printers employ a laser beam to cure liquid resin into hardened plastic via photopolymerization. The vat polymerization creates bonds within and between print layers, and in conjuncture with the final curing process, creates an anisotropic part that should not contain voids. The Stratasys material jetting printers layer photo-polymeric materials from printer heads where they are subsequently cured on exposure to UV light creating isotropic materials without voids.
Microbicidal efficacy and biocompatibility evaluations used a proprietary test article (3DTA or 3-Dimensionally Printed Test Article) that contained worst-case features of surgical guides and anatomical models to allow evaluation in accordance with the sterilization cycle design limits. The design included a lumen (tubular, hollow) feature that established the 3D printed medical device design limitation within efficacy evaluations (3 mm ID x 20 mm length or 3 mm ID x 30 mm length, dependent on material) and a variety of surface features present on
surgical guides and anatomical models. For ASTM material compatibility evaluations, a 3D printed test article was printed to meet each method’s specific test article requirement.
Sample processing
3D printed test samples were exposed to either a V-PRO maX 2 Sterilizer Specialty Cycle, a V-PRO maX 2 Specialty ½ Cycle, or to a worst-case chemical exposure of three [3]. VPRO s2 Sterilizer Lumen Cycles. VAPROX HC Sterilant was used for all evaluations. Specialty Cycle selection (D, E, or F) is based on the material used as each material is qualified for use in a specific cycle as shown in Table 1
The worst-case chemical exposure condition is determined in terms of hydrogen peroxide (H2O2) theoretical sterilant dose (mg-min/L). The dose is calculated by multiplying the theoretical concentration (in mg/L; 9.1 mg/L H2O2 for the V-PRO maX 2 Sterilizer and 10.8 mg/L H2O2 for V-PRO s2 Sterilizer) by the sterilant exposure time (min). The sterilant exposure phase of each Specialty Cycle is the same, with two [2] sterilization pulses and a total sterilant exposure time of 7.5 min for a theoretical hydrogen peroxide sterilant dose of 68 mg/L x min (7.5 min x 9.1 mg/L = 68 mg/L x min). The V-PRO s2 Sterilizer Lumen Cycle has the highest theoretical sterilant dose (346 mg-min/L; 32 min x 10.8 mg/L) of the V-PRO Sterilizers’ cycles [7]. For the worst-case chemical exposure, unpackaged 3D printed test samples were placed in a tray bottom without a lid or any additional load, and exposed to three back-to-back V-PRO s2 Sterilizer Lumen Cycles for a total dose of 1038 mg/L x min (3 × 346 mg-min/L). The 1038 mg/L x min dose equates to a 15-fold higher chemical exposure than the Specialty Cycle.
Sterilization efficacy
When designing experiments to demonstrate device sterility following manufacturing, one method considers the known bioburden of the manufacturing process while another method uses an overkill approach (see ISO 22441:2022 Sterilization of health care products — Low temperature vaporized hydrogen peroxide — Requirements for the development, validation and routine control of a sterilization process for medical devices Annex B and D). For this study, the overkill method was used. The overkill method is common for devices to be processed in hospitals and for sterilization of single-use devices in industry. The method used ≥ 106 of the most resistant organism to the low temperature vaporized hydrogen peroxide process, Geobacillus stearothermophilus, in a ½ cycle under worst-case processing conditions. Verification in a ½ cycle, with half the sterilization exposure time,
et al. 3D Printing in Medicine (2024) 10:6
validates the full-cycle sterilization process with a 106 sterility assurance factor.
The 3DTA was used to evaluate for surface and lumen sterilization in triplicate trials. Each test site was challenged with ≥ 1 × 106 colony forming units (CFU) Geobacillus stearothermophilus spores and dried. The test articles were pouched along with a sterilization load and exposed to a Specialty ½ Cycle. The ½ cycle uses the same Condition and Aeration Phase as the standard cycle, but ½ the sterilant exposure. After sterilization, the test articles were aseptically cultured into tryptic soy broth, incubated for 14 days at 55–60 °C, then evaluated for growth.
Biocompatibility
3DTAs of each material were pouched, placed in a tray bottom without a lid or any additional load and exposed to three back-to back Specialty Cycles. Following processing, the 3DTA were extracted with a mixed polarity solvent (cell culture test sample media) in accordance with ISO 10993-5 and ISO 10993-12. A cytotoxicity evaluation in accordance with ISO 10993-5 was conducted. For the remaining biological evaluations, material coupons were pouched, placed in a tray bottom without a lid or any additional load and exposed to three Specialty Cycles, then evaluated by NAMSA test laboratories for sensitization (ISO 10993-10), intracutaneous irritation testing (ISO 10993-23), systemic toxicity (ISO 1099311), material mediated pyrogenicity (ISO 10993-11) and hemocompatibility (ISO 10993-4 and ASTM F756).
Chemical evaluations
For the chemical analysis of 3D printed materials postprocessing, test articles were processed via a worst-case chemical exposure (see Sample Processing section). Exposed and unexposed samples were then analyzed by Fourier Transform Infrared Spectroscopy (FTIR), Gas Chromatography/ Mass Spectroscopy (GC-MS), and Inductively Coupled Plasma (ICP) atomic emission spectroscopy. The FTIR evaluated sample surfaces while the GC-MS and ICP evaluated 24-hour (37 °C) test article water extracts in accordance with ISO 10993-12 extraction recommendations. GC-MS was conducted on the chloroform soluble components of the water extract.
The residual hydrogen peroxide sterilant remaining post sterilization was evaluated after exposure of the pouched 3DTA without any additional load to three Specialty Cycles. The test articles were extracted at 37 °C in sterile water for 24 to 72 h and analyzed for hydrogen peroxide residue by a validated (in accordance with USP < 1225>) xylenol orange spectrophotometric assay. The basis of the assay is the complexing of ferric ion (Fe 2+) by H202 in the presence of xylenol orange (CAS Number 3618-43-7).
Peroxides in the sample oxidize Fe 2 + to Fe 3+, and the Fe 3 + forms a colored complex with xylenol orange that is read at 525 nm.
ASTM test-specific 3D printed test articles for tensile strength, flexural strength, compressive strength, Izod notched impact, and Shore hardness were processed via a worst-case chemical exposure (see Sample Processing section). The number of test articles processed and tested was in accordance with ASTM test-specific requirements. Additionally, a single Specialty Cycle exposure was used to evaluate a subset of test articles in a simulated use exposure. For all material evaluation exposures, unpackaged test articles (to allow for maximum exposure to sterilant) were placed in a tray base without any additional load. Post-exposure, the exposed and unexposed test articles were sent to Westmoreland Mechanical Testing & Research, Inc. for evaluation.
ASTM test results were evaluated via ANOVA, General Linear Model analysis and by Tukey Pairwise Comparison for statistical significance (p < 0.05). Statistical analyses were conducted with Minitab 19.2020.
Pre- and post-sterilization dimensional analysis was conducted with a 3DTA printed with each Formlabs and Stratasys material. Each 3DTA was exposed to the Specialty Cycle identified for the material. Dimensional analyses were conducted via physical measurements (calipers) and via scanning with a Faro inspection arm/ digital scanner before and after sterilization. Twenty to twenty-seven physical measurements were made for each 3DTA. Scanner data was processed with PolyWorks™ 2019 Inspector Essentials software and evaluated for differences.
The temperature of plastic medical devices was evaluated before and after sterilization to determine the impact of the longest cycle, Specialty F Cycle, on temperature. An infrared thermometer was used to determine temperature before and after sterilization in three independent evaluations.
Sterilization efficacy
3DTAs of each material were evaluated over triplicate trials for surface and lumen sterilization efficacy. In each trial, two lumen sites and six total combined surface features were tested. All test articles were sterile after exposure to Specialty ½ Cycles (Table 1). These results demonstrate that the Specialty Cycle effectively sterilizes 3D printed surgical guides and anatomical models made with the tested Formlabs and Stratasys materials within the design limitations evaluated. The lumen results qualify devices with equivalent or larger ID and equivalent
Table 1 Specialty ½ cycle microbicidal efficacy evaluation results Material
Formlabs BioMed Amber F 3 mm ID x 30 mm length 6/6 18/18
Formlabs BioMed Clear D 3 mm ID x 30 mm length 6/6 18/18
Stratasys MED610 E 3 mm ID x 20 mm length 6/6 18/18
Stratasys MED615 E 3 mm ID x 20 mm length 6/6 18/18
Stratasys MED620 E 3 mm ID x 20 mm length 6/6 18/18
Table 2 Biocompatibility results after 3x specialty cycle exposure
Material Evaluation
Cytotoxicity* ISO 10993-5
Sensitization** ISO 10993-10
Intracutaneous Irritation testing** ISO 10993-23
Systemic Toxicity** ISO 10993-11
Material Mediated Pyrogenicity** ISO 10993-11
Hemocompatibility** ISO 10993-4 and ASTM F756
Formlabs BioMed Amber Not cytotoxic Not sensitizing Not an irritant Not a systemic toxin Not pyrogenic Hemo-compatible
Formlabs BioMed Clear Not cytotoxic Not sensitizing Not an irritant Not a systemic toxin Not pyrogenic Hemo-compatible
Stratasys MED610 Not cytotoxic Not sensitizing Not an irritant Not a systemic toxin Not pyrogenic Hemo-compatible
Stratasys MED615 Not cytotoxic Not sensitizing Not an irritant Not a systemic toxin Not pyrogenic Hemo-compatible
Stratasys MED620 Not cytotoxic Not sensitizing Not an irritant Not a systemic toxin Not pyrogenic Hemo-compatible
* Testing conducted at STERIS in accordance with ISO 10993-5 standard under Good Laboratory Practice (GLP) regulations as provided in 21 CFR § 58 ** Testing conducted at NAMSA in accordance with the identified ISO 10,993 standards. NAMSA is certified to ISO 9001:2015 and is accredited to ISO/IEC 17025:2017
or shorter length, e.g., ≥ 3 mm ID x ≤ 30 mm length for Formlabs BioMed Amber.
Biocompatibility
The Formlabs BioMed Clear and BioMed Amber and the Stratasys MED610, MED615, and MED620 materials used in this study are identified as biocompatible by Formlabs and Stratasys. For sterilization in low temperature vaporized hydrogen peroxide, test devices and materials were processed in a sterilization cycle designed to ensure biocompatibility. Sterilization cycles from approximately 1 to 20 h were evaluated to determine the aeration required for the 3D printed devices to be safe for immediate use after sterilization. Once the aeration for each material was determined, biological evaluations were conducted. The Sterilize Phase was kept constant to ensure the same exposure of hydrogen peroxide sterilant (7.5 min). The importance of hydrogen peroxide sterilant removal through aeration is known in sterilization and in other vaporized hydrogen peroxide applications such as room decontamination [8]. It was apparent that 3D printed devices from these materials required more aeration than reusable medical devices processed in hospitals, which is typically 3 or 6 min aeration for similar sterilant exposure [7]. The Specialty D, E, and F Cycles identified for each material (Table 1) are approximately 8, 16, and 20 h, respectively, with the bulk of the time in the aeration phase (approximately 16 min of each cycle are used for conditioning and sterilization).
In accordance with ISO 10993-1, 3D printed surgical guides and anatomical models were categorized for use as surface and external communicating medical devices
with limited duration (< 24 h) patient contact via mucosal membrane, breached or compromised surface, blood path (indirect), circulating blood, or tissue/bone/dentin.
Biological evaluations were conducted in accordance with the ISO 10,993 standard series for the biological evaluation of medical devices and were selected via a risk-based approach. As shown in Table 2, this included evaluation of Cytotoxicity, Sensitization, Intracutaneous Irritation, Systemic Toxicity, Material Mediated Pyrogenicity, and Hemocompatibility. The evaluations included both in-vitro (cell) and in-vivo (animal) testing.
All test controls responded as expected in these evaluations and there were no anomalous observations. The results in Table 2 show that the materials are not a biological safety concern after sterilization in the Specialty Cycle identified for each material.
evaluations
Chemical evaluations were completed to understand potential differences in materials after vaporized hydrogen peroxide sterilization. The testing included an extensive chemical analysis of materials pre- and poststerilization. As a worst-case, the materials were exposed to vaporized hydrogen peroxide at 15-times higher concentration than that used for sterilization (see Sample Processing section). The intent of these evaluations was not to characterize the material, but instead to look for any differences caused by the extreme worst-case exposure.
A detailed evaluation of FTIR surface spectrum of the materials did not identify any differences caused by the chemical exposure. In the GC-MS and ICP analyses,
10:6
Stratasys
exposed and unexposed materials were extracted for analysis. Analysis of the GC-MS and ICP results similarly did not identify that any new materials were created from reaction with the hydrogen peroxide sterilant.
Sterilant residue
To further evaluate the safety of vaporized hydrogen peroxide exposed Formlabs and Stratasys materials, test devices processed for three sterilization cycles (as identified in Table 1) were extracted and tested for hydrogen peroxide residuals. Table 3 shows the residual hydrogen peroxide was less than 0.3 mg hydrogen peroxide per gram per device. These material residual levels were determined to be less than the tolerable exposure limits for mucosal and internal tissue contact established according to ISO 10993-17. As a relative comparison, a 3% hydrogen peroxide solution (30,000 ppm H2O2 or 30 mg H2O2 per gram of water) is identified as a topical solution per USP (US Pharmacopeia). These evaluations support that test articles produced with Formlabs BioMed Clear and BioMed Amber and Stratasys with MED610, MED615 and MED620 retain biocompatibility after vaporized hydrogen peroxide sterilization.
Material evaluations
ASTM testing
Materials evaluations in this study were conducted in consideration of ISO/ASTM 52910:2018(E) Additive manufacturing — Design — Requirements, guidelines and
recommendations. Shore Hardness, a common material test, was also evaluated. For each ASTM test evaluated, a test article was 3D printed to meet the method’s test article requirement. To understand the potentially differing impacts of worst-case hydrogen peroxide sterilant exposure versus the sterilization process (e.g. pressure, temperature, and sterilant) on the material, evaluations were conducted with a worst-case chemical exposure to hydrogen peroxide and, for select tests, with a simulated use exposure after processing in the Specialty Cycle identified for each material (Table 1).
Exposed test articles and unexposed control test articles were evaluated in accordance with ASTM methods at an external contract laboratory. There were no unusual observations or non-conformities identified in any of the contract laboratory test reports. Test results were analyzed for statistical differences using Minitab statistical analysis software. When test articles were required to be printed in two test configurations, e.g., in both lengthwise and crosswise print configurations per the ASTM test method, an ‘H’ indicates the sample was printed in a horizontal/lengthwise configuration (the XY plane) and a ‘V’ indicates the sample was printed 90° to this configuration in the vertical/crosswise configuration (the XZ plane).
Tables 4 and 5 show ASTM test results as a percent difference comparison of exposed test articles to the unexposed control test articles for the worst-case chemical exposure and simulated use exposure respectively. Percent change was calculated as follows: (exposed – unexposed)/unexposed x 100%. As identified in Tables 4 and 5 by an asterisk, many exposed test article results were not statistically different from the unexposed control test article. Increases in strength after processing are not considered practically significant. The remaining results, where material changes were negative post-processing, are not considered practically significant as the overall data does not suggest any gross negative material effects. Practical significance could vary depending on the
Hardness
† Testing was conducted by Westmoreland Mechanical Testing & Research, Inc., an A2la ISO 17,025 accredited and NADCAP accredited laboratory * The exposed sample result is Not Statistically Significant compared to the unexposed control
Table 5 Mechanical property evaluations after simulated use exposure in the specialty cycle Test Name
† Testing was conducted by Westmoreland Mechanical Testing & Research, Inc., an A2la ISO 17,025 accredited and NADCAP accredited laboratory * The exposed sample result is Not Statistically Significant compared to the unexposed control
Table 6 Dimensional analysis pre- and post-sterilization differences
Material 3D Scan Differences Measured Differences
Formlabs BioMed Amber ≤ 0.5 mm ≤ 0.01 mm
Formlabs BioMed Clear ≤ 0.1 mm ≤ 0.01 mm
Stratasys MED610 ≤ 0.2 mm ≤ 0.1 mm
Stratasys MED615 ≤ 0.5 mm ≤ 0.1 mm
Stratasys MED620 ≤ 0.5 mm ≤ 0.1 mm
specific device design, so the device manufacturer must ensure the printed device design will meet its intended use.
Overall, the single simulated use exposure (Table 5) resulted in smaller percent changes than the worst-case chemical exposure (Table 4) for the test methods evaluated. For the isotropic Formlabs vat polymerization produced materials, minor differences were observed in horizontal versus vertical printing for compressive strength and Izod notched impact. For the anisotropic Stratasys material jetting produced materials, there were differences in tensile strength, compressive strength, and Izod notched impact based on print orientation. The results support the material compatibility of the 3D printed material for vaporized hydrogen peroxide sterilization, and the differences are not considered practically significant. Further, these results support the 15x chemical exposure as a worst-case compared to single, simulated use exposure.
For this study, the effect of a sterilization process on the dimensional accuracy of a 3D printed item has also been evaluated. 3DTAs manufactured with the Formlabs and Stratasys materials were evaluated before and after sterilization then analyzed for differences. After the post-processing analysis was complete, the data sets for the pre- and post-processing analyses were overlaid and compared for difference via a heat map generated by the PolyWorks Inspector Essentials software. Physical measurements of the test article were taken with calipers before and after processing. As shown in Table 6, vaporized hydrogen peroxide processed 3D printed devices are dimensionally stable as little to no changes were observed after sterilization.
Lastly, plastic devices were processed in the Specialty F Cycle (the longest Specialty Cycle) and had their temperatures taken immediately afterwards. A total of nine temperature measurements ranged from 48 to 53 °C immediately after sterilization. The maximum plastic device temperature had previously been 43 °C for similar cycles used to process reusable medical devices. Therefore, even with the slightly higher temperatures after the additional aeration of the longest Specialty Cycle, device temperatures maintained a temperature range considered ‘low temperature’ (below 60 °C) for medical device processing.
Discussion
Sterilization efficacy
Medical devices used in the surgical field must be sterilized to prevent nosocomial infections. Patient-specific devices that are 3D printed within a hospital have considerations beyond the devices that a hospital routinely sterilizes. For example, device contamination during and post-manufacture coupled with the potential of material voids formed within the device are novel concerns for 3D printed device sterilization. Importantly though, and unlike reusable medical devices, single-use 3D printed devices are not exposed to patient soils and clinically relevant, pathogenic organisms prior to sterilization.
While the vat polymerization and material jetting produced parts without voids, other printing methods can contain significant porosity that may be of concern for sterilization. Research by Popsecu et al. [9] studied the disinfection and decontamination of devices 3D printed with ABS filament via material extrusion which creates parts layer by layer. Despite build optimization and solvent treatment with acetone vapor post-production, significant porosity remained on the devices and allowed liquid infiltration.
The potential for viable microorganisms within 3D printed device voids is a concern as the printing process can theoretically seal microorganisms within the void. Should a device be damaged during use, as shown by Shea et al., [10] there is a risk for patient exposure to an entrained microorganism. This raises questions about the potential bioburden on a 3D manufactured device as well
et al. 3D Printing in Medicine (2024) 10:6
as the post-processing sterility of those devices. Wangsgard and Winters reported that for 3D printed devices, bioburden levels would be low due to heat in the 3D print process [11]. Neches et al. identified that parts printed via material extrusion were sterile post-processing when taken from the printer and immersed within a growth media [12]. This effect was attributed to the temperature of the process, which, at 190–240 °C, is hotter than many decontamination and sterilization processes. When contamination was observed, it was determined to have been caused by post-print handling as the contaminants, Staphylococcus epidermidis and Propionibacterium acnes, are common to human skin. Lastly, in a study by AguadoMaestro et al., five material extrusion printed cylinders were directly inoculated during a halt in the printing process with > 108 CFU Staphylococcus epidermidis [13]. The print process was resumed sealing the cylinders, and the cylinders were incubated in growth media to evaluate for organism growth. Four of the five cylinders showed growth of 2–12 CFU of organism while one cylinder had no growth; a greater than 7-log reduction.
To limit risk of ineffective sterilization, 3D medical device manufacturers need to (1) select materials/print methods that minimize the presence of voids and (2) develop a system to control the microbial quality of the device prior to sterilization.
Biocompatibility
3D printed medical devices must be demonstrated to be biocompatible to protect patients from biological risks from the device. Medical devices have been 3D printed for many years [[14]. More recently it is hospitals that are manufacturing the 3D printed medical devices [4, 15, 16]. It is the device manufacturer’s responsibility to establish biocompatibility of the finished device. There are a multitude of factors than can affect biocompatibility of a 3D printed medical device including the material, curing, cleaning, support removal, and sterilization process.
The importance of following printer manufacturer printing and processing guidelines cannot be minimized. The material selection is straightforward as printer manufacturers identify specific materials qualified as biocompatible for different applications. The instructions for use of solvents for residual resin removal, support removal, and cleaning is validated by the manufacturer. All processing instructions must be strictly followed to ensure biocompatibility. One risk to not following manufacturer’s instructions is the risk of insufficiently cured or polymerized acrylates (the resin used to create the part) as acrylates are a recognized health concern [17–19].
The impact of sterilization on biocompatibility has not been as well characterized as other process steps. With steam sterilization identified as the final processing step, there may be an assumption that sufficient
biocompatibility data exists to support that the sterilization process had no effect on the biocompatibility of the processed devices; hence, biocompatibility testing may not have been conducted. While a change in physical properties or appearance after steam sterilization can be identified in a visual inspection process, a change in biocompatibility cannot, so an understanding of the device biocompatibility after sterilization should be considered.
Steam sterilization (at 121 °C, 132 °C, or 134 °C) is the most common method used to sterilize 3D printed parts in a hospital today [20–22]. The impact of steam sterilization on a variety of printed device materials has been evaluated by many researchers with widely varying results. For example, while Shaheen et al. and Marei et al. found steam sterilization to be less reliable and cause physical changes to the device, Torok et al. did not identify any difference after steam sterilization [20–22]. The benefit of vaporized hydrogen peroxide sterilization is that it is a low temperature method with a maximum temperature of less than 60 °C. While a lower process temperature is generally less damaging to materials, there is limited data for comparing the VH2O2 sterilization results presented in this report to other sterilization modalities.
One study that can be compared to data from this evaluation was published by Van Dal, who evaluated Formlabs BioMed Clear tensile bars printed at angles of 0° and 45° [23]. After cleaning with an automated washer, the materials were steam sterilized at 134 °C for 3.5 min. The percent change pre- and post-sterilization is shown in Table 7 where % change is calculated in same manner as for Tables 4 and 5: [(exposed-unexposed)/unexposed x 100%]. The steam processed tensile strength bars printed at 0° were warped after processing and showed some delamination; both defects were attributed to peel forces from the 0° print orientation. Note that no materials defects were observed for the materials evaluated in this report, inclusive of the BioMed Clear tensile bars processed in the Specialty Cycle.
The Van Dal results in Table 7 are best compared to single sterilization cycle (simulated use) results in Table 5 for BioMed Clear for tensile strength where the tensile strength was very similar after vaporized hydrogen peroxide and steam. For the flexural and impact strength evaluations, comparing 0° print (Table 7) to the horizontally printed (H) BioMed Clear results (Table 4), shows that flexural strength did not significantly change after vaporized hydrogen peroxide but increased after steam while impact strength increased after vaporized hydrogen peroxide but decreased after steam.
A separate study by Torok et al., evaluated Stratasys MED610 in a surgical guide configuration for Tensile
Strength, Flexural Strength, and Hardness after steam sterilization at 121 °C (20 min) and 134 °C (10 min) [22]. The % change results are shown in Table 8 with % change calculated from report data in same manner as for Tables 4 and 5. The authors further determined that there was no significant difference pre- and post-steam sterilization via scanning electron and stereomicroscopic examinations. Steam sterilization at 134 °C for 10 min was noted to cause deformations while no materials defects were observed for the materials evaluated in this report, inclusive of the MED610 ASTM coupons processed in the Specialty Cycle.
The Torok et al. 121 °C steam data is most directly compared to MED610 data in Table 5 where tensile strength after sterilization was similar. Comparing 121 °C steam data (Table 8) to the MED610 results (Table 4), flexural strength did not significantly change after steam but decreased after vaporized hydrogen peroxide while hardness changed minimally after steam or vaporized hydrogen peroxide sterilization.
The ability to terminally sterilize 3D printed devices at lower processing temperatures than steam, e.g., with vaporized hydrogen peroxide, may allow for new materials selection, in particular for materials than cannot withstand the high temperatures of steam sterilization.
Understanding the impact a sterilization process has on device dimensions is critical as a 3D printed anatomical model may be used diagnostically and a surgical guide will be used intraoperatively. The critical considerations and contributions to consider when measuring and evaluating the accuracy of 3D printed medical models has been detailed by George et al. [24].
The contribution of sterilization to the workflow process, although not directly considered by George et al., is especially important with plastics when considering
a low temperature sterilization method like vaporized hydrogen peroxide (temperatures 60 °C or lower) in contrast to steam sterilization (temperatures of 121–134 °C). Many studies have evaluated the effect of steam sterilization on the dimensionality of 3D printed items with mixed results dependent on material and exposure temperatures as discussed within the physical properties results (Torok, Marei, and Shaheen).
This study demonstrated that low temperature vaporized hydrogen peroxide processed 3D printed devices are dimensionally stable; little to no changes were observed after sterilization: ≤ 0.5 mm for pre- and post-processing scans overlaid and compared for difference via a heat map. Similarly, other researchers have identified only minimal dimensional changes on devices after vaporized hydrogen peroxide. Toro et al. evaluated the dimensionality of material extrusion printed ABS anatomical models and guides before and after evaluation with vaporized hydrogen peroxide [25]. Their dimensional analysis found that post-sterilization the mean differences between the printed pieces and original design were within the 95% confidence interval of -0.096 to -0.094 mm for models and 0.140 to 0.141 mm for guides; thus maintaining dimensional stability after sterilization. The biological evaluation showed that after sterilization these devices were not cytotoxic, pyrogenic, or sensitizers, and had no acute systemic toxicity.
A device manufacturer is responsible for ensuring that each device meets its intended design requirements (form, fit, and function). This responsibility spans the entire life cycle of the medical device; that is, from manufacture, including post-production processing (e.g., cleaning, curing and sterilization), through use in a patient procedure.
et al. 3D Printing in Medicine (2024) 10:6
Hospital-based3D printed medical device is relatively new when compared to medical devices 3D printed by companies and sold to hospitals. Authors have reviewed the concept of 3D printing within the context of current regulations [5, 26–29]. In December 2021, the US FDA published a discussion paper for comment as they seek to create a regulatory framework for this new application [30]. As a practical matter, organizations such as Radiological Society of North America (RSNA) have worked collaboratively to identity and define what the 3D device quality system process in a hospital would entail [4, 15, 31]. Regardless of the eventual regulatory and quality system particulars, there will be an obligation upon the 3D printed medical device manufacturer to establish within that process a sterilization method that ensures sterilization efficacy, biocompatibility, and material compatibility. Within the context of a quality system, data supporting sterilization may prove useful, as would the methodologies used to support FDA clearance of this particular workflow.
Conclusion
A vaporized hydrogen peroxide sterilization method with the Specialty Cycle was used to evaluate sterilization, biocompatibility and material compatibility of select Formlabs and Stratasys materials. The test results, and a comparison to other relevant sterilization methodology results, support the use of low temperature vaporized hydrogen peroxide to sterilize 3D printed surgical guides and anatomical models. The test result data was used in support of regulatory validation and clearance [1].
Trademarks
V-PRO™ and VAPROX™ are trademarks of STERIS, its affiliates or related companies.
All other product and company names referenced are trademarks of their respective owners.
Acknowledgements
Formlabs and Stratasys provided material samples and extensive assistance throughout the evaluations.
Author contributions
Randal Eveland was responsible for study design, supervising experiments, and drafting the manuscript. Kathleen Antloga, Ashley Meyer and Lori Tuscano were responsible for experimental design, execution, and analysis.
Funding Not applicable.
Declarations
Consent for publication
Not applicable.
Competing interests
Authors are employees of STERIS.
Page 9 of 10
Received: 1 September 2023 / Accepted: 12 February 2024
References
1. K223476 at FDA 510(k) Premarket Notification website. (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm).
2. Wake et al. Creating patient-specific anatomical models for 3D printing and AR/VR: a supplement for the 2018 Radiological Society of North America (RSNA) hands-on course. 3D Printing in Medicine (2019) 5:17 https://doi. org/10.1186/s41205-019-0054-y
3. Mertz L. Dream it, design it, print it in 3-D: what can 3-D printing do for you? IEEE Pulse. 2013;4(6):15–21.
4. Chepelev L, Althobaity W, Gupta A, Mitsouras D, Christensen A, Rybicki FJ, Sheikh A. Radiological Society of North America (RSNA) 3D printing Special Interest Group (SIG): guidelines for medical 3D printing and appropriateness for clinical scenarios. 3D printing in medicine, (2018 Nov 21) Vol. 4, No. 1, pp. 11. Electronic Publication Date: 21 Nov 2018 Journal code: 101721758. E-ISSN: 2365–6271. L-ISSN: 2365–6271. Report No.: PMC-PMC6251945. https://doi. org/10.1186/s41205-018-0030-y
5. Rybicki FJ. The impact of regulation, reimbursement, and research on the value of 3D printing and other 3D procedures in medicine. 3D Print Med. 2022;8:6. https://doi.org/10.1186/s41205-022-00132-0
6. Christensen R. Maintaining safety and efficacy for 3D printing in medicine. 3D Printing in Medicine (2017) 3:1 https://doi.org/10.1186/s41205-016-0009-5
7. STERIS document M3644 rev C., Trust the Material Compatibility of V-PRO® Sterilizers.
8. Eveland R. Disinfection and sterilization with hydrogen peroxide in Hanson and McDonnell, editor disinfection, sterilization and preservation, 6th Ed. Wolters Kluwer Chapter 32, 671–83.
9. Popescu D_Effect Disinfect Absorption Med Decontam of 3D ABS. parts_ Polymers_2021 13 4249. https://doi.org/10.3390/polym13234249
10. Shea G et al.; A review of the manufacturing process and infection rate of 3D-printed models and guides sterilized by hydrogen peroxide plasma and utilized intra-operatively. 3D Printing in Medicine, 2020 6:7. https://doi. org/10.1186/s41205-020-00061-w
11. Wangsgard W, Winters M. Validation of a sterilization dose for products manufactured using a 3D printer. Radiat Phys Chem. 2018;143:38–40.
12. Neches RY, Flynn KJ, Zaman L, Tung E, Pudlo N. On the intrinsic sterility of 3D printing. PeerJ. 2016;4:e2661. https://doi.org/10.7717/peerj.2661
13. Aguado-Maestro M, De Frutos-Serna A, González-Nava, Injury et al. https:// doi.org/10.1016/j.injury.2020.09.014).
14. Ricles L, Coburn J, Di Prima M, Oh S. Regulating 3D-printed medical products, SCIENCE TRANSLATIONAL MEDICINE, 3 Oct 2018, Vol 10, Issue 461, https:// doi.org/10.1126/scitranslmed.aan6521
15. Mitsouras D, Liacouras P, Wake N, Rybicki FJ, RadioGraphics, Update. Medical 3D Printing for the Radiologist, RadioGraphics 2020 40:4, E21–E23. https://doi. org/10.1148/rg.2020190217
16. Mitsouras D, Liacouras P, Imanzadeh A, Giannopoulos AA, Cai T, Kumamaru KK, George E, Wake N, Caterson EJ, Pomahac B, Ho VB, Grant GT, Rybicki FJ. Medical 3D Printing for the Radiologist. Radiographics. 2015;35:1965–88.
17. Leggat P, Smith D, Kedjarune U. Surgical Applications of Methyl Methacrylate: a review of toxicity. Arch Environ Occup Health, 64:3, 207–12, https://doi. org/10.1080/19338240903241291
18. Final Report on the Safety Assessment of Acrylates Copolymer and 33 Related Cosmetic Ingredients. Int J Toxicol. 2002;21(3suppl):1–50. https://doi. org/10.1080/10915810290169800
19. Münker TJAG, van de Vijfeijkenb SECM, Muldera CS, Vespasianoa V, Beckingb AG, Kleverlaana CJ. On behalf of the CranioSafe Group, effects of sterilization on the mechanical properties of poly(methylmethacrylate) based personalized medical devices. J Mech Behav Biomed Mater. 2018;81:168–72. https:// doi.org/10.1016/j.jmbbm.2018.01.033
20. Shaheen E, et al. Evaluation of dimensional changes of 3d printed models after sterilization: a pilot study. Open Dent J. 2018;12:72–9. https://doi.org/10. 2174/1874210601812010072
21. Marei HF, Alshaia A, Alarifi S, Almasoud N, Abdelhady A. Effect of Steam Heat Sterilization on the Accuracy of 3D Printed Surgical Guides. IMPLANT DENTISTRY / VOLUME 28, NUMBER 4. 2019.
22. Török G, et al. Effects of disinfection and sterilization on the dimensional changes and mechanical properties of 3D printed surgical guides for implant
Eveland et al. 3D Printing in Medicine (2024) 10:6
therapy– pilot study. BMC Oral Health. 2020;20:19. https://doi.org/10.1186/ s12903-020-1005-0
23. van Dal V. H.A.M.,Effect of sterilization on 3D printed patient-specific surgical guides. Technical Medicine– Master Thesis, Delft University of Technology 25-02-2021.
24. George E, Liacouras P, Rybicki FJ, Mitsouras D. Measuring and establishing the accuracy and reproducibility of 3D printed medical models. Radiographics. 2017;37:1424–50.
25. Toro M, Cardona A, Restrepo D, et al. Does vaporized hydrogen peroxide sterilization affect the geometrical properties of anatomic models and guides 3D printed from computed tomography images? 3D Print Med. 2021;7:29. https://doi.org/10.1186/s41205-021-00120-w
26. Pettersson Ante BV et al.; Legal issues and underexplored data protection in medical 3D printing: A scoping review., Frontiers in bioengineering and biotechnology, (2023) Vol. 11, pp. 1102780. Electronic Publication Date: 27 Feb 2023 Journal code: 101632513. ISSN: 2296–4185. L-ISSN: 2296–4185. Report No.: PMC-PMC10009255. https://doi.org/10.3389/fbioe.2023.1102780
27. Carl A, Hochmann D.; Comparison of the regulatory requirements for custom-made medical devices using 3D printing in Europe, the United States, and Australia., Biomedizinische Technik1 Apr, (2022) Vol. 67, No. 2, pp. 61–69. Refs: 35 ISSN: 0013-5585 CODEN: BMZTA7. https://doi.org/10.1515/ bmt-2021-0266
28. Beitler B et al. December; Interpretation of regulatory factors for 3D printing at hospitals and medical centers, or at the point of care. 3D Printing in
Medicine, (2022) Vol. 8, No. 1. arn. 7. Refs: 22 E-ISSN: 2365–6271, https://doi. org/10.1186/s41205-022-00134-y
29. Horst A, et al. A clarion call for understanding regulatory processes for additive manufacturing in the health sector. Expert Rev Med Dev. May 2019;4(5):405–12. https://doi.org/10.1080/17434440.2019.1609353. Refs: 33 ISSN: 1743–4440; E-ISSN.
30. FDA. 3D Printing Medical Devices at the Point of Care.: Discussion Paper. 12/10/2021. Available from: https://www.fda.gov/medical-devices/3d-printing-medical-devices/3d-printing-medical-devices-point-care-discussionpaper
31. Matsumoto JS, Morris JM, Foley TA, Williamson EE, Leng S, McGee KP, Kuhlmann JL, Nesberg LE, Vrtiska TJ. Three-dimensional Physical Modeling: Applications and Experience at Mayo Clinic. Radiographics. 2015 Nov-Dec;35(7):1989–2006. https://doi.org/10.1148/rg.2015140260. PMID: 26562234.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.


































Email: secretary@nzssa.org
Website: www.nzssa.org


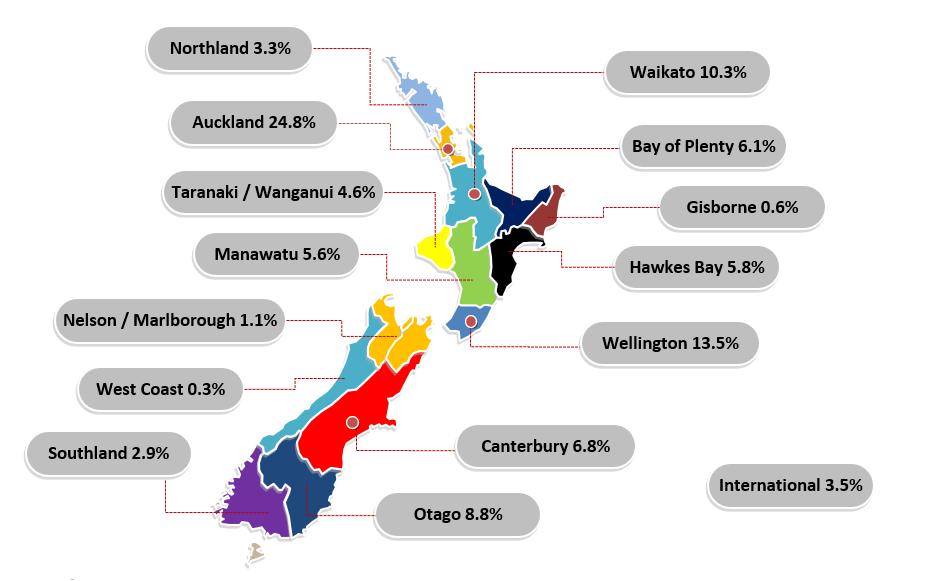

• Our longest membership period is fifty years! Two members joined the association in 1974.
• More than half of our members have been with us for five or less years.

This year has been full on for the NZSSA since our 2023 conference.
The country was awaiting the introduction of AS5369 which finally happened in January of this year. This caused a lot of confusion and a lot of questions to the executive. Much of the confusion arose not from the NZSSA, rather from the Ministry of Health who had not effectively advised the changes to Standards NZ or any other health authority e.g. Dental Council who utilise the standards. Finally this matter has been resolved with all parties concerned. This is the standard that the DAA auditors are now working with and they will use to audit CSSD Departments.
The NZSSA was invited to participate in regular on-line meetings with the Chief Allied Professions Officer at the Ministry of Health Dr Martin Chadwick. The President and Vice President attended. These meetings related to Self-regulated professions. It was stated that the NZSSA was a self- regulated profession and therefore as the regulator has the right to make decisions regarding the profession and what is required from its members. Regular meetings are held with the Ministry of Health and all the self-regulating professions who sit under the Allied Health Scientific and Technical Umbrella.
After much discussion again with the Ministry of Health around the shortage of trained sterile technicians and difficulty employing into vacancies, Sterile Technicians were finally added to the immigration green list. This gives oversea trained technicians whose qualifications are assessed and approved by the NZSSA fast access to NZ and a residency visa. There have been many applicants, however some are put off by the paperwork they have to supply and the cost of applying to INZ for a visa and the NZSSA have found that not all overseas qualifications are equal to NZ qualifications. With the current freeze on hiring in NZ health there may be a downturn in applications.
Another initiative has seen a lot of work put into developing a pilot Sterilisation Programme. This has been a collaboration between the NZSSA, Ministry of Health and the Northern region DHB’s. The aim is to get young people into the profession. However, after the initial conversations where there was hope of funding for additional FTE, hospitals were advised that they can only employ to this if they had the vacancies.

A national educator forum was initiated by an Executive member. It brought together CSSD educators to discuss issues and similarities in their roles and for them collaborate.
Sterile Sciences education days were put on by the NZSSA. These were in Wellington, Christchurch and Auckland. They were very well attended and the take home message was “we want more of these events.”
Education is important to the association and this year we have awarded several scholarships to the NZSSA conference across the categories of trainee technician and qualified registered technician. We also awarded a scholarship to a registered technician to attend the WFHSS congress to be held in Santiago Chile in November of this year.
The NZSSA were invited to participate in a forum held by Pharmac in Wellington. This was a forum for those directly involved with reusable medical devices and brought together a wide group of professionals. Pharmac emphasised that they wished to have closer collaboration with us when it came to all matters affecting CSSD. This included new products coming into the market, all our CSSD equipment, equipment contracts and much more. This was not just a one off meeting. There has been follow up meetings since.
The NZSSA became part of the Asia Pacific Implant Consortium. The aim of this group is to promote safer outcomes for patients through best practice. This group have endorsed a Sterilisation recall policy and procedure, and steps for proper management of loan instruments and implants as well as quality assurance for sterilised items. There is opportunity through this group for technicians to undertake projects relating to the above topics and with support and encouragement via tutorials from members of the WHO.
Finally, this year is election year where we nominated and voted for the executive members we wanted to see carry us through for the next three years. Congratulations to those who were elected and re-elected.
Our new NZSSA executive after ratification at this meeting are:
President - Martin Bird
Executive team membersAileen Derby, Shelagh Thomas, Jenny Carsten, Kelly Swale, Donna Dador, Carla Coetze, Charanjeet Kaur Sidhu, Antony Owens, John Barnacott.

We sadly say farewell to Anthony Valvoi, Maureen Scott and Paul Moody. The team will miss working with you and your dedication to the profession.
Thank you
Shelagh Thomas President NZSSA


Tena koutou katoa,
The final year of this executive term seems to have flown past with the association elections and scholarships (seven in total this year) taking up a big chunk of the time. In the background we’ve continued updating and documenting our systems and processes to help ensure smooth operations as we continue growing towards self-regulation.
As mentioned the executive elections were conducted online this year and whilst it took a bit of work to set up it has made the process a lot more efficient. However, online communications only work if the association has accurate records for each member –especially important is your email address. It would be fantastic if managers could ensure that their team members email addresses are complete. Simply send a list of everyone’s preferred email to secretary@nzssa.com and we’ll update the association records.
Once again congratulations to all those who have taken on additional study and achieved their level 4 and 5 diplomas this year – and thank you to all those who have taken the time to complete their association registrations.
There are a number of challenges on the horizon for the NZSSA, as there are for many in the healthcare field and one of these engaging with the general youth of our membership. As you will see in this Annual report 1 in 5 have been with the association for less than two years and over half for five years or less. This demonstrates a large interest in the sterile sciences and also offers an opportunity to realign with what we want to achieve as an industry body to best serve our members.
Finally, please remember as we welcome in a new executive that our association is run by volunteers, all of whom also have full working lives so be patient with your queries
Nga mihi.
Paul Moody Secretary, NZSSA

The figures presented in this report are as at 31 March 2024 for the financial year 2023 –2024. The accounts were prepared by McIntyre Dick & Partners and audited by Accounting for Charities Trust.
The Association remains in a strong financial position which was boosted by a very successful conference in 2023. The Executive has managed the finances well towards being able to reintroduce face to face education events and scholarships.
The accounts operate on an accrual system in line with the Association reporting as a Tier 3 registered charity. •
Statement of Comprehensive Income (Profits and Losses)
•
•

The audited performance report demonstrates an overall deficit of $915. The year was proposed to be near breakeven based on expenses for conference and increase in liability insurance costs. This was planned so that registration costs for members could be offered without increase. This has been reviewed for future conferences and as the Association endeavours to provide more face-to-face education opportunities in the coming financial year.
The financial trend of the Association is holding in a good space. The Covid years demonstrated the ability of the Association to maintain a sound foundation. The Association is not immune to increases in costs and the Executive carefully consider any expenses against the benefit to members.
Financial year 2020 – 2021
Financial year 2021 – 2022
Financial year 2022 – 2023
Financial year 2023 – 2024
Deficit $ 4,641
Deficit $ 3,894
Profit $ 9,379
Deficit (915)
The Executive is focused on ensuring the members get value for their membership. This is achieved through planning of education events and provision of scholarships. The executive conducts the majority of its business via online meetings to keep costs down. There are only two face-to-face executive meetings per year. All others are online.
Careful monitoring of conference expense versus income is a priority in a time where costs are ever increasing.

The association strategic plan for the financial year 2024 – 2025 is included after the financial summary.
Each year the Executive, as the management arm of the Association, allocate funds to scholarships and activities to members to promote educational opportunities and advocate for the profession.
Congratulations to the following for being awarded scholarships for 2023-2024:
WFHSS Conference Scholarship – Donna Dador
Education Scholarship – Sophia Twinkle Samuel
There continues to be low uptake of the scholarships which cover all upfront costs for and event or education opportunity. These will continue to be promoted through the website, Facebook, Supplyline and to students undertaking study.
The intention is to ensure 2024 onwards sees a return of the regional meetings which have been excellent networking and education sessions for all personnel involved in reprocessing.

Auditor’s Report
• Qualified Opinion Report
o It is the opinion of the auditor that the performance report gives a true and fair view, in accordance with Public Benefit Entity Simple Format Reporting – Accrual (NotFor-Profit).
• Basis for Opinion
o This opinion is based on the belief that the audit evidence obtained was sufficient and appropriate. The audit was conducted in accordance with International Standards on Auditing (ISAs).
• Key Audit Matters
o Key audit matters are those of most significance in the audit of the performance report for the current period (1/4/2023 – 31/03/2024). Those matters were addressed in the context of the audit as a whole and informing the auditors opinion and a separate opinion is not provided on these matters.
• Going Concern
o In the opinion of the Executive Committee the Association is a going concern for the foreseeable future.

The strategic direction for the current financial year was discussed at Executive meetings and has resulted in the plan presented in this document.
Education is the focus for the coming year. The Association is gaining traction with the return to face-to-face education events, hosting the annual conference and providing scholarships for conference attendance both nationally and internationally. These activities are in addition to scholarships for education and providing knowledge through the Association journal, Supplyline, printed three times per year.

The executive plan of work focuses on putting processes in place to become formally selfregulated and build the Association’s reputation as the national membership body for people involved in reprocessing reusable medical devices.
This plan of work is intended to create a robust framework for the Association to;
• measure overseas qualifications against NZ qualifications to enable immigrating technicians and employers to have clarity for positive and safe employment practices,
• advocate on the behalf of members on appropriate reprocessing practices,
• build relationships with the Ministry of Health (MoH), Ministry of Business, Innovation and Employment (MBIE), and other key entities, and
• ensure members are receiving value as a member of the NZSSA
The following table presents the plan initiated in 2022 and continuing through to 2025
Complaints process
Review of Association constitution and rules
MBIE Immigration Green List requirements
Education plan
within for all NZSSA documentation
Aileen A clear robust process accessible by members for making complaints
Aileen Have a constitution and rules document that conforms with the Incorporated Societies Act 2022 and Charities Act 2005 (although is under review)
Alison Robust process for technician qualifications to be reviewed by the NZSSA for equivalency to NZ qualifications
Jenny, Maureen, Shelagh, Martin
NZSSA Annual Conference
Provide face to face education events for members of NZSSA to develop as professionals.
Aileen, Shelagh, Anthony Delivery of a conference each year which prompts the industry and provides exposure to new and innovative ideas and products
Team for conference delivery reviewed each year.
Account has been established and key email accounts set up.
Folders established for work streams. Access provided as appropriate TEAMS being used for meetings.
The process has been created and tested
Incorporated Societies Act 2022 takes effect October 2023, Reregistration required between 5 October 2023 and 5 April 2026
Review of Constitution & Rules is progressing
Framework established for qualification review May 2023. Applicants are being processed within 6 weeks of submission.
1x Wellington – May 2024
1x Christchurch May 2023
1x Dunedin meeting planned for 2024
1x Auckland Meeting – July 2024
1x Central North Island meeting to be confirmed
2024 Conference – 50th Anniversary, Auckland, 25-27 September.
Planning is going well.

Toi Ohomai Institute of Technology Ltd NZ Certificate in Sterilising Technology (Level 4) Trimester 2 & 3 2023
Jordanne Bernaldez
Dennis Ramirez
Ann Bowen ~ Santhosh George
Samson Chacko Varghese
Glen Davies
Nicholas Gardiner
John Gutierrez
Britney Hamer
Katherine Hije
Jitha Jacob
Gregoria Jap
Timoteo Kakure
Monet Keepa
Alexandra Kerr
Indu Kuniyadathu Mathew
Mandy Labuschagne
Paul Martillana
Caroline McComb
Ella Morison
Jenna-Vee Ou
Sandra Paku
Jaime Jr Pardo
Sachin Paulose
Paul Jr Puguon
Presly Sebastian
Aleshia Sinclair
Sophia Stephens
Angela Summerfield
Paul Susamma Robin
Beau Takarua
Janelle Tamati
Martin Thomas
Megan Toki
Danielle Tom
Quang Tran
Vanessa Tsang
Layane Veras
Frank Villegas
Dylan Watkin
Joanne Wilson
Kris Wu
Shashi Yadav
Sanja Janish
Mary Llobet
Emma Mclean
Toi Ohomai Institute of Technology Ltd NZ Diploma in Sterilising Technology (Level 5) Trimester 2 & 3 2023
Maree Carmen Zulekha Ali
Grace Fermo
Shelley Morrison
Kimberley Saunders
Zoe McMeeken
Margaret O'Quinn
Sophia Samuel
Joey James Fellipp

Toi Ohomai Institute of Technology Ltd NZ Certificate in Sterilising Technology (Level 4) Trimester 1 2024
Fiona Angus
Sonya Birchler
Sephora Carlos
Marx Casaje
Shaju Cheria Chakkalakkal
Ronnie Collamar
Michele Fuller
Glenn Gerebese
Rae Marie Gibb
Sue Marie Grantham
Kenneth Handugan
Rebecca Henderson
Raju Jacob
Jane Kingsbury
Helen Kirk
Steven Klein
Asok Koorpilliyil Varghese
Jingxuan Liu
Julie Logan
Reniel Mangahas
David Manhire
Amanda Mannering
Josephine Mataele
Elizabeth McCully
Heidi McMullen
Sabina Natuituba
Jamie Newdick
Lisa-Maree Potts
John Sapasap
Rachael Scully
Rubilyn Sedo
Subash Sundharan
Maleta Tufele
Wasantha Udaha Ola Gedara
Lenita Usmar
Bryan Valois
Abhilash Varghese
Brite Varghese
Nicola Wilson
Regan Wootton
Toi Ohomai Institute of Technology Ltd NZ Diploma in Sterilising Technology (Level 5) Trimester 1 2024
Anita Castelino
Jennifer Sucich
Michael Owens
Michael Prosser
• Changing to semesters in 2025
• From Semester 1 2025 the Certificate and Diploma will be delivered in 2 semesters per year. This change only applies to new students starting in Semester 1 2025
• All students will need to enrol in two courses in their first semester of the year, then one course in their second semester of the year.
• Fees for both courses in the first semester of study will need to be paid prior to the semester start.
• New students can enrol in Semester 2.
• The Certificate will still be completed in 1 year of study (2 semesters)
• The Diploma will still be completed in 2 years of study (4 semesters)
• Enquiries regarding your application:
• email: SterilisationApps@toiohomai.ac.nz
• Prepare for study:
• Devices for study – smart phones and tablets are alright for the learning, a computer or laptop is needed for the portfolio assessment tasks.
• Study plan - to fit study around work and family – this study is at distance and you will have to do it in your own time. Allow 12 – 16 hours per week
• Check your internet connection – you will need this to access the online learning modules, library and upload portfolio assessment tasks.
• Throughout your study you will have full support from Toi Ohomai in the same way a student coming onto campus does. Your tutor can connect you with the student support services.


As
Contact your local ASP representatives today.

Protecting patients during their most critical moments.™
INTRODUCING ASP’s Steam Sterilization Monitoring Portfolio









Wow I won the scholarship to go to the conference in Auckland, also it was the 50th year as well. To me personally this felt like I had won lotto. The scholarship included flights there and back from my home town, accommodation for three nights and full attendance to conference and dinner.
The first night was a get together and have a few drinks and nibbles and have a look around exhibiting companies. This was interesting to meet colleagues from other CSSD within New Zealand from private to public hospitals. It also gave me insight in what companies were offering to us for our CSSD units.
The next day was a full day of listening to guest speakers. I found this considerably interesting and enhancing my professional development. The first speaker David Bellamy talked about the quality assurance of reprocessing the screw caddy. This highlighted areas we should be aware of. It gave us insights on improving sterility and providing solutions to using the screw caddy. It also made me aware to read new studies that is conducted within our profession to help advance my knowledge within my practice.
Another speaker I found interesting was Lania Coffey from Getinge. Identifying we may need to use warm up cycles in our department, if machines are sitting for a long period of downtime. This highlighted questions I need to ask my manager in regarding this. Also to find out our consistency of our quality of steam. I was also very interested in why we do the weekly tests for our autoclaves and what problems may arise and why.
The next speaker that I found incredibly interesting was Linda Hutchings. Linda spoke of the power of learning, unlearning and relearning. She pointed out how each generation learns and how to relearn information. This made me more aware of how varying age groups learn and I felt this enabled me to understand how my colleagues learn within my department. Linda also made us laugh and be engaged continually within her speech. It was like light bulbs going off all the time when she was speaking and I really enjoyed listening to her.
There were a lot of speakers during the two-day conference that I learnt a lot of information from.
Darran Laydan from Whitely talked about 50 years of chemistry and how advanced we have become. It made me question what do we use in our department. I made notes to ask my manager what we have and also to have a look at all chemistry deliverance system.
Rob Burrell from Middlemore Hospital spoke about sustainability. Which as we know is a continual problem within our field. He gave us food for thought on this topic and highlighted a number of things we can try to become more sustainable within our department.
Jenny Langton talked about safer surgery saves lives by using identification, duty cycles and traceability. Using software data that helps within our department and why. Although we do not have the system in place in our department yet this will be coming in the future and it highlighted how good it is to have in place. Traceability is necessary to enable safer surgery; in which we play a role within this.
Annie Watt talked about Sterilisation of 3D printed devices. This highlighted what is going to happen in the future for our department. Annie raised a number of issues we need to be aware of when we are going to sterilize these new devices.
Another speaker that I really enjoyed was Premsiri Ngeonthongkrajang from Olympus. Premsiri went in great detail on endoscopes and how to clean them and what the channels are for. For further information, she highlighted there is a program on their website to enhance our knowledge on endoscopes.
The last speaker for the conference, was Sven Wurst from Braun. I feel he had the hardest job as he was last speaker of the day and he had a mammoth task in speaking on the past, present and future of surgical instruments. Although he had an hour, his speech was very informative. Sven gave us an understanding on the past of how they developed the medical instruments to now. I really didn’t understand how hands on it took to develop medical instruments. I always had a thought that there was a lot of machines that did the majority of the work. However, this is not how it is done as there is a lot of quality control and hands on still used in manufacturing the surgical instruments. I have a more understanding of

why they are so expensive due to the intensity it takes to make them.
The Anniversary Dinner was another part of the conference that I really enjoyed. I have always wanted to go into the St Mathews church in Auckland and got the opportunity for our function. It was a beautiful venue and fun night. I recommend all to go to this dinner when they are attending the conference.
Overall, I believe this conference has enhanced my professional development. As a semi newbie to the profession (4 years’ experience) I suggest all sterile technicians should experience going to a conference within their career. It would be great if the conference speakers were recorded so others that cannot go due
to being short staffed within their department got to experience similar knowledge.
I am very lucky that I got to go due to winning a scholarship and feel that this has helped me as a sterile technician to enhance my skills and knowledge. I am very grateful to the New Zealand Sterile Sciences Association to have scholarships available and believe more technicians should apply for this chance.
Kathy King Sterile Science Technician Taranaki Base Hospital



Taranaki CSSD celebrating Allied Professions Day by wearing green scrubs

Anthony Valvoi, Central Sterile Sciences Manager and his team celebrating Allied Professions Day 2024.




The Belimed WD 750 is a large scale washer disinfector, setting new standards in safety, quality and economic efficiency.
Key features include:
EN ISO 15883-1 and EN ISO 15883-2 compliant (complete automatic drainage of piping, pump, tank, valves and fittings; all surfaces coming into contact with the product are inclined).
Most compact large-scale washer disinfector in its class. Easy-to-service, integrated equipment and service compartment offering space for accommodating media connections and detergent containers.

Glass horizontal sliding doors and cabin illumination provides clear visual control of the washing process, as well as the patented process status display and double-door push-through model with interlocking doors for separating contaminated/clean area.
Available in three chamber size options, pre-heating and storage tanks for water reuse and faster cycle times.
PLEASE SPEAK TO YOUR HEALTHCARE MANAGER FOR FURTHER INFORMATION
ECOLAB HEALTHCARE ANZ
4B Pukekiwiriki Place, East Tamaki, Auckland 2013, New Zealand
NZ: 0800 425 529 www.healthcare-nz.ecolab.com


InterMed, in collaboration with New Zealand Sterile Services Association (NZSSA), and in honour of Leonie’s vibrant spirit, were thrilled to resurrect the Wearable Arts Competition at this year’s 50th Anniversary NZSSA conference.
It all started circa 2012 with the amazing Leonie Jack, who worked at InterMed until 2023. Leonie had a knack for connecting with creative minds among her customers and wanted to celebrate their talents. She teamed up with Wipak, who generously provided materials, and got the green light from NZSSA to host a competition at their annual conference. And just like that, the Wearable Arts competition was born!

The event kicked off with a bang at the conference’s welcome soiree, featuring a fantastic display of outfits created by talented participants.
The outfits were judged on originality, creativity, execution, theme, and entertainment value. Drum roll please and the winners were…

People’s Choice: Franklin Hospital (left) Judges’ Award: Southern Cross Gillies Ave (below)




These are a labour of love!
Claire Smith, our InterMed CSSD Product Specialist, drew inspiration from a Greener Aotearoa New Zealand, focusing on sustainability with themes of Reduce, Reuse, and Recycle. Each costume was crafted from recycled materials, reflecting InterMed’s commitment to the environment.
A big shoutout to Medical Technology Association of New Zealand (MTANZ) & NZSSA for contributing to the winners’ vouchers and to everyone who made this event a success, keeping Leonie’s memory shining bright.
What a fantastic celebration of creativity and sustainability!
For more information about the Wearable Arts competition or the Wipak product range contact claire.smith@intermed.co.nz








Press Release - 16th September 2024


Whiteley Corporation were very proud winners of the Excellence in International Business Award (sponsored by Port of Newcastle) and Business of the Year Award (Sponsored by IMB Bank) at the recent 2024 Business Hunter Awards. Whiteley has been the recipient of many business awards over the years; however, this is the first time they have won the major award.
The awards recognise outstanding business in global medical supply and innovative manufacturing Both awards were accepted by Chief Financial Officer, Brooke Cavanagh who recognised the work of all the team at Whiteley and the business’ commitment to growth.
Darran Leyden, Managing Director commented - “Winning the Excellence in International Business Award seemed appropriate as both our Executive Chairman – Dr Greg Whiteley and myself were in the US working on some major projects for the company when we got the fantastic news that we had won the award!”
Whiteley exports to more the 35 markets globally and recently obtained Medical Device Single Audit Program (MDSAP) certification that will allow it to directly export Medical Devices into both the USA and Canada. Whiteley’s products are now certified to leave from their hunter-based manufacturing location of Tomago in the Hunter Region of NSW, directly to the US and Canadian Healthcare markets. This will allow Whiteley to expand and grow hunter-made products globally, while still providing demand nationally.
Managing Director, Darran Leyden also mentioned - “Whiteley have an amazing team that bend over backwards to work through a variety of challenges for our export (and domestic) customers so this is a great award that recognises the hard work of our staff across the company. As a Hunter based manufacturer we are especially delighted to win the Business of The Year Award which we hope reflects the regions “get the job done” attitude as we continue to expand business opportunities across the region, the country and the globe.”
Whiteley also sponsored the ‘Contribution to the Region’ category which was awarded to Out of the Square Media for their significant contribution to the ongoing prosperity and growth of the Hunter Region outside its core business activity. Whiteley would like to thank the great team at Business Hunter and all of the Sponsors. Whiteley’s are excited about both our company’s and the Hunter’s future.
-ENDS-
For media enquiries please contact: Hannah Walmsley
Hannah.walmsley@whiteley.com.au 0411858665
PO Box 1076, North Sydney, NSW 2059 Australia
Manufacturing: 19-23 Laverick Avenue, Tomago NSW 2322 Australia www.whiteley.com.au | whiteley@whiteley.com.au
Press Release - 16th September 2024




PO Box 1076, North Sydney, NSW 2059 Australia
Manufacturing: 19-23 Laverick Avenue, Tomago NSW 2322 Australia www.whiteley.com.au | whiteley@whiteley.com.au















Martin Bird (President)
CSSD Manager
Dunedin Hospital
Dunedin
martin.bird@southendhb.govt.nz
Donna Belle Dador (Vice President) National Sterilising Manager Southern Cross Healthcare Christchurch donna.dador@southerncrosshospitals.co.nz
Carla Coetzee (Secretary) SSD Coordinator Evolution Healthcare Royston Hospital Hastings Email: secretary@nzssa.org Carla.Coetzee@royston.co.nz
Charanjeet Kaur Sidhu
CSSD Quality Facilitator Counties Manukau District
Auckland Charanjeet.Sidhu@middlemore.co.nz
Aileen Derby CSSD Manager Counties Manukau District Auckland Aileen.Derby@middlemore.co.nz
Antony Owens Team Leader Southern Cross Healthcare Southern Cross Gillies Hospital Auckland Antony.Owens@southerncrosshospitals.co.nz
Jenny Carston
Theatre Services Unit
Tauranga Hospital Tauranga Jenny.Carston@bopdhb.govt.nz
John Barnacott
Manager Sterile Services Unit
Mid Central District Health Board Palmerston North Hospital Palmerston North John.Barnacott@midcentraldhb.govt.nz
Kelly Swale (Registration Assessor)
Sterile Services
Otago University Dental Faculty Dunedin kelly.swale@otago.ac.nz
Alison Stewart (Treasurer) NZSSA Treasurer 28 Brighton Street, Island Bay Wellington 6023 Email: accounts@nzssa.org
General Enquiries secretary@nzssa.org Overseas qualification and membership inquiries accounts@nzssa.org Complaints complaints@nzssa.org
Registration AssessorsKelly Swale kelly.swale@otago.ac.nz
Maureen Scott Maureen.Scott@waikatodhb.health.nz

Nice if you like retro, not if you like progress.
V-PRO™ maX 2 Sterilizer is the first and only sterilizer cleared and validated for select 3D materials. Now you can process a broader range of items, including 3D printed anatomical models and patient specific surgical guides.*
Facing the Future Together
VISIT WWW.STERIS.COM/VPROMAX2 for additional information.


steris.com
* Anatomical and surgical guide photos are the design of Synergy3DMED. © 2023 STERIS. All Rights Reserved.