Delivering cutting edge design
We combine electronics, software, mechanical engineering and brand led industrial design with rigour and creativity to generate innovative, successful products.


Designing beautiful products that people want to use
DCA has significant real-world experience in creating market leading devices through all stages of development, industrialisation and in-market support.


Experts in developing robust and precise delivery devices
Our design and analysis work with global device companies includes ENDS devices, pressurised metered dose and dry powder inhalers for asthma, COPD and inhaled insulin.
Combination dry powder inhaler for major pharmaceutical company - development of mathematical model and early design verification to understand the lid foil compensation mechanism for an inhaler.


Vaping is personal
As products get closer to you, they increasingly become a reflection of your personality and character. The demand for individuality and personalisation drives the need for a range of ENDS products and services.


We research the market and identify opportunities
We explore areas of opportunity to improve products and services aligned to the evolving expectations of users, elevating consumer ENDS experiences.


We help to design for a sustainable future
We are all conscious consumers, aware and concerned about our impact on the world. Therefore we must put sustainability at the centre of the design of new products and services.

Reducing the risk
We are passionate about developing safer alternatives to smoking and embrace the emerging new regulations and legislation.
At DCA we strive, through the intelligent application of user research, engineering, materials and technology, to deliver more sustainable, reduced risk products that address the needs of the consumer.


Pushing the boundaries
Building on our experience and capabilities we can develop the core technology or the delivery format at the heart of the device, to enhance the user experience and reduce risk.

Medical device development
With our team of inhouse experts, combined with our robust development procedures and methods, we develop world class drug delivery systems that users want.

The successful development of products and services is a balance of technology and user needs in the context of the market and the environments that they exist in.
We understand the ingredients for a successful product development

Our team generate creative concepts

Concept generation is a fundamental step in the development process.
We use structured brainstorming sessions with targeted insights and opportunities identified during market research, to conceive and evaluate new solutions.

We explore the difficult problems
At DCA we quickly build development rigs and prototypes to explore new concepts and fundamentally prove the viability of solutions to address unmet user needs early in development cycles.

We are experts in first principles mathmodelling. Class leading analysis software is applied intelligently in combination with physical testing to benchmark predictions and improve understanding.
Intelligent application of computational analysis to create better designs, faster



Efficient design implementation

As devices become more feature rich and connected, an increasing demand is put on the battery and device footprint. At DCA we specialise in designing low cost, ultra low powered devices, to maximise battery performance.
Supporting this specialism our design, electronics and mechanical teams work together to craft the technical packaging with the device design.



Connecting devices opens the opportunity for vendors to monitor user behaviour and consumption to provide a curated, managed service for their customers. Successful connected implementations are unobtrusive and provide an intelligent balance of usability and user benefit.


We help connect it all together
A highly creative team with in-depth IP experience
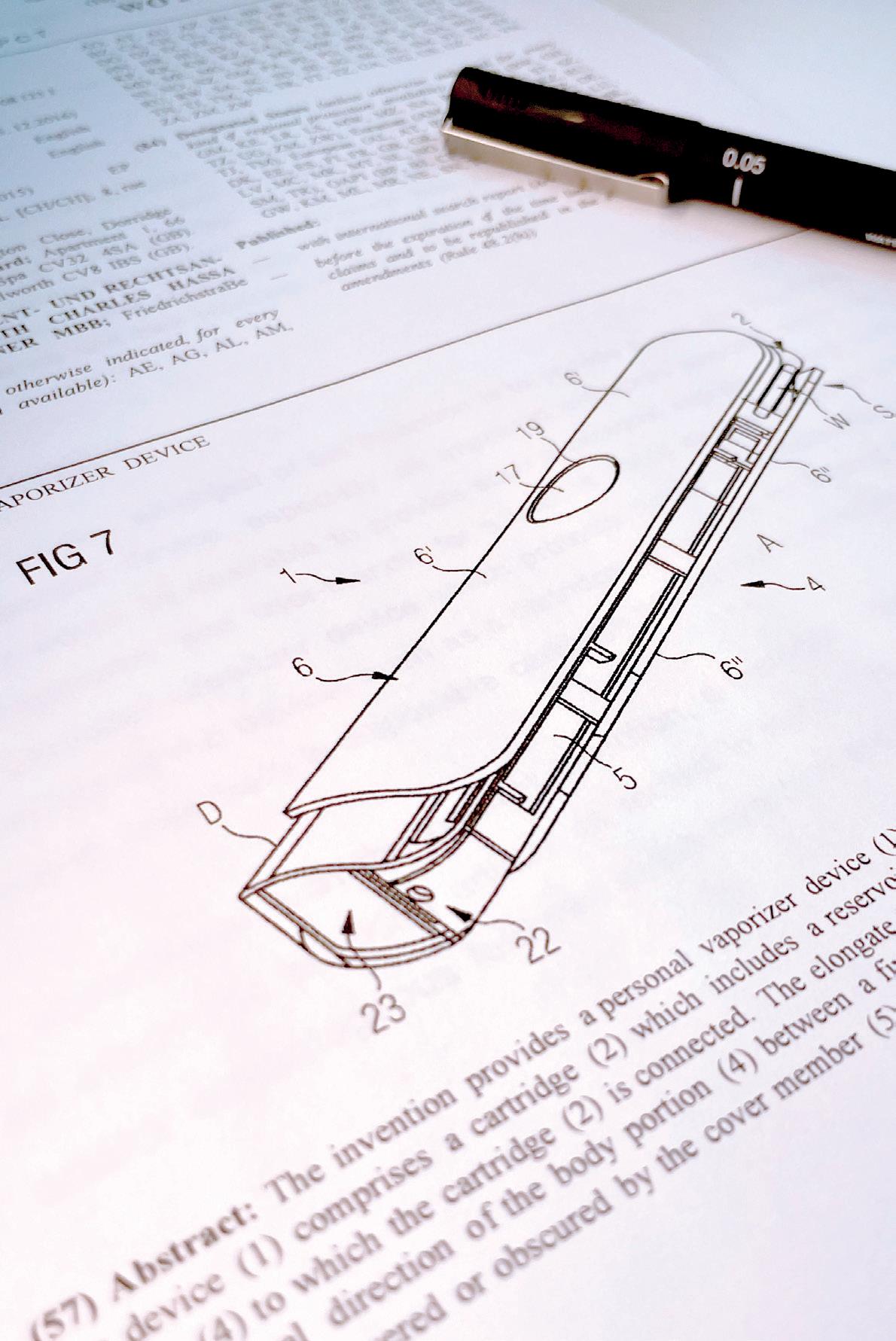
Over 4000 patent applications and over 1700 granted patents since 2000.
Our in-house prototyping and test facilities enable us to rapidly validate designs and analyses

A team of multidisciplinary product development experts
Mechanical Engineering
Interaction Design Design Research & Planning
Software Engineering
Prototyping
Electronic Engineering
Industrial Design
Human Factors & Usability
Rigorous device development procedures
Aligned with international standards.
QMS General Procedures
ISO 9001
Medical Device Development Procedure
Software Development Procedure - Medical Devices
ISO 13485
21 CFR part 820 & EN 60601:1 clause 14
EN 62304
Usability Engineering for Medical Devices
Risk Management Procedure - Medical Devices
EN 62366
ISO 14971
A proven track record
100+ products launched, 50+ full-scale drug delivery projects and 100 major design awards in the last 10 years.









Contact
2023
