As we conclude a remarkable year, we are proud to present the 2024 annual report for the Translational Resource Center, formerly known as the Michigan-PittsburghWyss Regenerative Medicine Resource Center. This year has seen significant growth and notable achievements.
Our commitment to advancing technologies in dental, oral, and craniofacial care has driven our progress. Key milestones have been reached by our project teams, crucial for moving products towards clinical implementation and commercialization. These accomplishments highlight the collective dedication of everyone involved, from our Interdisciplinary Translational Project teams to our technology translation experts. We hope this report conveys the excitement and pride we feel about our progress. Together, we continue to push boundaries and strive for innovative solutions that enhance quality of life.
We are thrilled to unveil our refreshed branding, marking an exciting chapter for the Resource Center. Our new look and feel embodies our commitment to advancing technology translation, and represents the dynamic nature of our work in translating cutting-edge technologies as well as our vision for the future.
Thank you for your continued support and partnership. We look forward to another year of shared successes and transformative achievements.
With sincere gratitude,
David H. Kohn, PhD – University of Michigan
William V. Giannobile, DDS, DMSc – Harvard University
David J. Mooney, PhD – Wyss Institute
Charles S. Sfeir, DDS, PhD – University of Pittsburgh
William R. Wagner, PhD – University of Pittsburgh


























The “Translational Resource Center”, formerly known as the Michigan-Pittsburgh-Wyss Regenerative Medicine Resource Center, is one of the two national Resource Centers established by the National Institute of Dental and Craniofacial Research (NIDCR)’s Dental Oral and Craniofacial Tissue Regeneration Consortium (DOCTRC) initiative, under award numbers U24DE026915 & U24DE029462. With the overarching goal of developing clinical trial-ready tissue engineering/ regenerative medicine products and protocols, the DOCTRC initiative provides funding and resources through the Interdisciplinary Translational Project (ITP) program administered by the two national Resource Centers.
The Translational Resource Center brings together a multi-disciplinary team of clinicians, engineers, scientists, and technology commercialization and regulatory experts from academia and industry. This team supports the
the
its
and
the
and
and
or
of


Fueling Innovation, Bridging Realities: The Translational Resource Center empowers visionary technologies with unparalleled support, expertise, and funding to help navigate the product development ‘valley of death’ - ensuring that new therapies reach the market and improve patient lives.
We are excited to introduce our new look which celebrates our continued dedication to translating innovations into impactful solutions. The rebranding initiative has galvanized support from a wide range of stakeholders, fostering a dynamic and collaborative atmosphere that drives enthusiasm and collective action. As we continue to evolve,
we remain committed to sharing the valuable lessons learned and insights gained with a wider audience. Positioned for growth, the Translational Resource Center is poised to amplify its impact and continue to strengthen its role in advancing patient care through technology translation towards clinical adoption.
Year of Resource Center Inception
Number of ITP Proposals Reviewed
Number of ITPs Funded to Date
Number of Currently Active ITPs
Investigators Trained







• ITP Team, RevBio, awarded NIH grant for its novel regenerative bone adhesive to treat complex fractures
• DOCTRC hosts Innovator Networking Series with Dr. Gary D. Hammer and Dr. Charles Hart on pivoting from basic discovery to translational research
• ITP Team, Ostiio, Awarded NSF SBIR Phase II grant on the miniaturization of a magnetic craniofacial distraction system
• 4th Annual DOCTRC Retreat in Los Angeles, CA
• ITP Team, RevBio, receives FDA approval to conduct a first-in-human clinical trial for its regenerative bone adhesive for cranial flap fixation


• Resource Center member, Dr. Hom-Lay Wang, on Legends of Oral Regeneration Podcast
• ITP PI, Dr. Pamela C. Yelick, named to the 2024 class of senior members of the National Academy of Inventors
• Resource Center co-hosts Symposium at 2024 IADR/AADOCR: NIDCR75: Accelerating Product Innovation and Development – From Research to Commercialization
• ITP Team, Amend Surgical, submits 510(k) application
– Dr. Jadia and Dr. Yelick share their ITP journeys








• 2024 MPWRM Resource Center Annual Stakeholder Summit. Event included a panel session on “Regenerative Therapies in the Dental, Oral, and Craniofacial Space: Opportunities, Challenges, and Trends”, featuring leaders from the DOC industry and ITP Pitching Hour, with awards to Amend Surgical, Dr. Lombaert, and Ostiio
• GreenMark, ITP team, partners for co-branded launch of their CrystLCare™ Biorestorative, Fluoride-Free sensitivity strips
• ITP Cycle 9 launched
• Article from an ITP PI, Dr. Luiz Bertassoni, regarding work on microfluidics and lab-on-a-chip featured on the Portland Tribune!
• Resource Center PI, Dr. William Giannobile, presents a webinar on AI in dentistry on Osteology Foundation


• RevBio: TETRANITE® space mission shared, awarded NINDS SBIR to conduct first-in-human study for Cranial Flap Fixation and enrolls first 5 patients
• ITP PI Dr. Feinberg’s, work on functional human lips published in Frontiers in Bioengineering and Biotechnology
• SBIR awarded to VIC Foundry, based on ITP work led by Dr. Juan Taboas
• Dr. Isabelle Lombaert receives 2nd translational award from U-M Frankel Innovation Initiative
• 5th Annual DOCTRC Retreat in Bethesda, MD
The mission of the Translational Resource Center is to strategically partner with scientists, engineers, and clinicians in the public and private sectors to translate dental, oral, and craniofacial tissue engineering and regenerative medicine technologies to the clinical marketplace.
The Translational Resource Center consists of more than 60 members whose expertise spans the spectrum of translational research and commercialization. With the majority of the members serving on an Advisory Team, each project receives broad and customized guidance.
The Interdisciplinary Translational Project (ITP) program seeks to identify promising technologies that address clear unmet clinical need with market potential in the dental, oral, and craniofacial space, and to catalyze the clinical translation of these technologies towards FDA submissions. The long-term goal is to achieve highimpact outcomes in the clinical marketplace. To date, we have reviewed over 100 projects from around the world.
510(k) f iled
SBIRs and STTRs Issued/ pending patents 2
Number of formal interactions with the FDA (pre-sub/ INTERACT meetings, 510(k) submission, etc.)
1 14 >46 >15 >$ 47M 89 %
Total number of follow-on grants and award recognitions
External funding garnered
DOC Indications
Bone Grafting
Craniofacial Distraction
Trigeminal Nerve Wrap
Oral Wound Care
Periodontal Regeneration
Pulp Regeneration
Tooth Remineralization
Xerostomia
INSTITUTION TYPE
The Translational Resource Center plays a pivotal role in advancing promising regenerative technologies by helping its project teams navigate and overcome the ‘valley of death’ in early-stage product development. Throughout its tenure, the Resource Center has fostered a paradigm shift among researchers, encouraging a stronger focus on moving technology from the lab to commercialization and, ultimately, to clinical adoption. This shift in focus underscores the Center’s significant support, innovative infrastructure, and commitment to translational success.
– Grayson Allen, Joseph Fiorellini, Brian Hess, Rahul Jadia, George Kay (RevBio)
Support from the Resource Center helped establish the target market profile to highlight the unmet clinical need and identified a strong value proposition for our product as a bone graft material for complex procedures like ridge augmentation.
We started with a literature review, identifying suitable animal models, and designing a screening strategy that included formulation optimization and safety and efficacy test plans. FDA engagement was critical, providing data-driven justification in the pre-sub meetings. Through an iterative process, the pre-clinical model was modified, and a validation study was later conducted. This approach allowed us to refine our product, optimizing formulations and improving design. The funding and support from the Resource Center enabled us to lay a strong foundation for future development and emphasized Tetranite’s potential in complex clinical needs.
–
Juan Taboas, Herb Ray (University of Pittsburgh)
This project highlighted key lessons in pre-clinical study design that are essential for a successful product development process. Key takeaways include evaluating development and adoption costs and focusing on customer/ end-user value. Effective communication is crucial for understanding what investors and technology adopters care about, such as market size and product safety and efficacy.
Here, we share valuable insights and experiences from our project teams in three key areas in pre-clinical product development, with the goal to further advance technology translation and accelerate progress across the broader DOC and regenerative medicine ecosystem.
– Luiz Bertassoni (Oregon Health & Science University, RegendoDent, Inc.), Pamela Yelick (Tufts University, RegendoDent, Inc.), Anissa Bartolome (Oregon Health & Science University)
Conducting endodontic treatments on pre-clinical models posed challenges due to anatomical differences and the scarcity of clinicians familiar with the models. With support from and connections through the Resource Center, we collaborated with experienced clinicians and regulatory experts who guided our strategy. As a result, we shifted our focus from conducting more pre-clinical tests to directly pursuing a small first-in-human study, streamlining the product development process.
Key lessons learned include the importance of close collaboration with the Resource Center teams and experts in product development, and considering alternatives to traditional pre-clinical models. The Resource Center expedited our timeline to FDA submission, and its support facilitated the formation of RegendoDent, Inc.


The Resource Center played a pivotal role in advancing this project, accelerating its development and helping avoid potential pitfalls from uninformed market assumptions. Their assistance was essential in crafting a compelling narrative for prospective investors and partners. Without this support, the team would have faced challenges with strategically identifying the target indication and insufficient market clarity.
The product development of Vital-Dent benefited from various services from the Resource Center, including market research, regulatory expertise, and technical services like histology and microCT. The integrative approach of the Resource Center support has been vital in pushing the project forward, tackling market and regulatory challenges, and enabling the team to present a strong case to potential investors and adopters.
– Isabelle Lombaert (University of Michigan)
Embarking on the journey to secure non-dilutive funding has taught us several invaluable lessons. Persistence is key: keep applying until you reach the elusive 1% ‘YES.’ Throughout this process, effective negotiations with Contract Research Organizations prove paramount, requiring both patience and strategy. Keeping meticulous records of everything, from emails to contracts, is a habit that pays off. Thinking like a corporation early on can help navigate the complexities of the funding process. Staying flexible is crucial, especially when milestone timelines change unexpectedly. And finally, be prepared to juggle multiple roles – taking on the responsibilities of CEO, CTO, and CSO all at at once.
–
An “early bet” on Ostiio: SBIR grants will fund early-stage work, but robust demonstration of technical viability is necessary. ITP support made this possible for Ostiio.
Bridging the funding gap: it takes a long time to receive an SBIR award; ITP support was critical to keeping things progressing while going after SBIR grants. Without ITP there may not have been a company by the time we received SBIR grants.

Investing in company success: the program supported Ostiio’s holistic vision through intimate knowledge of the company and technology, helping tee up follow-on SBIR funding. Company success depends on solutions, not projects. ITP’s continued investment in Ostiio’s technology solution positioned us to win follow-on SBIR funding with aims we would not have been ready to propose otherwise.
Bio-inspired, Long-lasting
– Jamie Shaikoski, Jessica Boehlein, Robby Lane (Amend Surgical, Inc.)
Regulatory lessons learned:
Choose your consultant wisely, clearly define roles, and focus discussions on specific questions
Regulatory Lessons Learned:
• OWN your submission and clearly define roles and responsibilities with your regulatory consultant
• Pre-submission meeting should be limited to targeted questions
• Be cautious in communicating any potential feature or claims for your technology
• Without a good predicate, consider pursuing a clinical strategy rather than a simple 510(k)
• Competent, technical leadership and some clinical experience of the lead reviewer matter
• Attempt to connect, communicate and clarify if possible (especially important as a company with platform or novel technologies)
– Nathan Jones, Wendy Bloembergen, Steven Bloembergen (GreenMark Biomedical Inc.)
Developing a preventive dental product highlighted the vital need for expert collaboration and strategic navigation of regulatory and financial complexities. Expert teams played crucial roles in driving the project forward, focusing on necessary regulatory milestones.
Understanding the regulatory landscape and addressing risk assessment early were critical lessons. The funding support from and access to resources made available through the Resource Center accelerated our progress. SBIR grants from NIH/NIDCR were pivotal, underscoring the importance of securing such funds.

Strategic pivots, like focusing from caries treatment to prevention, and selective regulatory pathways expedited our product development journey. With the right expertise and resources, overcoming major obstacles is achievable: “Where there is a will, there is a way!”

Juan Taboas, PhD
University of Pittsburgh

Herb Ray, DMD
University of Pittsburgh



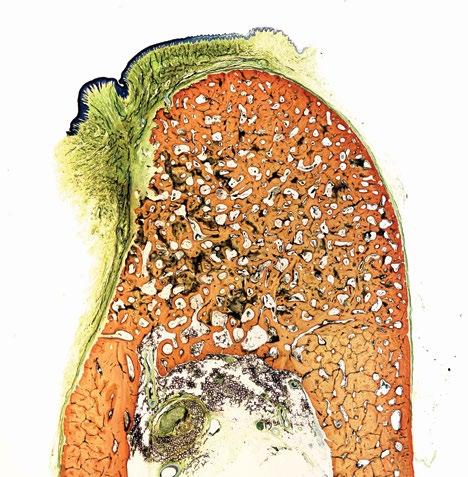
Approximately 5 million procedures are performed in the USA each year to treat pulpitis of permanent teeth in the pediatric population. Children are often subject to multiple procedures because the standard of care is to stabilize the tooth until more definitive treatment can be performed when the child’s growth stops. The outcomes are unideal, because the whole tooth is not revitalized after pulp removal, resulting in tooth discoloration, loss of tooth structure, and limited (if any) tooth growth.
Vital-Dent is an acellular, resorbable hydrogel scaffold intended for revitalization of tooth pulp and maintenance of tooth vitality in immature permanent teeth treated with endodontic therapies. It is supplied as a powder in a single-use kit with sterile saline. The powder is rehydrated at the chairside using kit tools to make a clear liquid. The liquid is inserted into the instrumented canal space as would conventional sealers and sets in three minutes with a dental curing lamp to form the colorless Vital-Dent hydrogel. The tooth is then sealed with a bioceramic and restored with conventional techniques.
Unlike current obturating materials, Vital-Dent is resorbable and promotes continued development of immature teeth, pulp revitalization and regenerative dentin, root strengthening, and long-term survival of the tooth. Vital-Dent eliminates difficulties of the only available revitalization procedure, revascularization therapy, with more consistent outcomes including root development, and a better fit with conventional clinic workflows including no phlebotomy and faster delivery.
• Zaky et al. Effect of the periapical “inflammatory plug” on dental pulp regeneration: A histologic in vivo study. J Endod 2020
• PCT/US2019/023132 Regeneration of Vital Tooth Pulp
• Awarded Direct to Phase II SBIR for $1.7 M in August 2024
• Executed option to license with VIC Foundry in September 2024, a partner in commercialization
• 510(k)
• Seeking corporate partner to back fundraising efforts for first-in-human studies and facilitate market adoption
• Academic partnership in studies that promote adoption and advance therapy development
Based on market research conducted by RevBio, almost half the patients that seek a dental implant supported crown suffer from chronic edentulism and require extensive bone grafting to rebuild their alveolar ridge. Over 30% of the time, these grafting procedures achieve suboptimal results and require some form of re-grafting adding to the overall cost, treatment time, and morbidity for these patients.
RevBio has developed Tetranite® Adhesive Dental Bone Scaffold (TN-ADBS), a synthetic, porous, cohesive organic-mineral bone scaffold with adhesive properties that resorbs and is replaced by bone on a timescale commensurate with existing graft materials but does not require ancillary fixation or containment devices.
Currently available particulate bone grafting products require significant surgical skill to apply. In contrast, TN-ADBS is both cohesive and adhesive which makes it easy to use. The product will reduce the overall time necessary to perform ridge augmentation procedures, better maintain graft volume over time, and minimize the need for re-grafting.
• Kesseli et al. Identification of a calcium phosphoserine coordination network in an adhesive organoapatitic bone cement system. Acta Biomater 2020
• Kirillova et al. Bioinspired mineral–organic bioresorbable bone adhesive. Adv Healthc Mater 2018
• US8,232,327 Tetra Calcium Phosphate Based Organophosphorus Compositions and Methods
• US8,765,189 Organophosphorous & Multivalent Metal Compound Compositions and Methods
• Received FDA approval to conduct and SBIR to fund a first-in-human clinical trial for cranial flap fixation, and enrolled 5 patients
• IDE
• Distribution of the product: Strong industry distribution partner that can represent the product globally
• Investment opportunity: Currently seeking Series A financing of $20M to initiate multiple clinical studies




Ari M Wes, MD Ostiio LLC



Each year >200K newborns suffer from conditions that restrict growth of the skull or jawbone. Left untreated, these can have life threatening outcomes in an otherwise healthy child. However, treatments are complex and traumatic. Distraction is a gentler therapy that uses a device to slowly expand abnormal bone, but distractors are semi-buried, increasing complication risk; manual expansion is performed by parents, leading to noncompliance; and surgeons are blinded to treatment, forcing weekly X-rays and exams.
Ostiio’s therapy uniquely solves pain points of distraction to improve patient outcomes and experience. The fully buried implant is expanded magnetically, removing the complication risk of semi-buried devices. The automated driver simplifies expansion, reducing parental noncompliance. The remote monitoring platform allows surgeons to track treatment progress, reducing post-op follow-up.
Distraction is the segment within the CMF device market that offers opportunity to differentiate, but technology advancement has been iterative with competitors focused on providing greater flexibility to the surgeon in the OR. Since treatment is home-based, Ostiio instead will transform how distraction is provided by parents and monitored by surgeons to improve patient outcomes and experience.
• Kalmar et al. Forces exerted in craniofacial distraction osteogenesis. J Craniofac Surg 2022
• PCT/US2020/017918 Systems and Methods for a Smart, Implantable Cranio-maxillo-facial Distractor
• PCT/US2023/060119 Systems and Processes for Distraction Control via Torque Sensing
• Awarded a Phase II SBIR grant from the NIH for a total of $1.74M
• Awarded a Phase II SBIR grant from the NSF for a total of $1M
• 510(k)
• Seeking investment: The company will soon be raising a Seed round of $5M to complete verification and validation studies supporting 510(k) submission and initiate commercialization
Radiotherapy is commonly used to treat head-and-neck cancers. Because of the anatomical proximity, salivary glands often receive secondary radiation damage, resulting in xerostomia. While intensitymodulated radiotherapy significantly reduces the incidence of radiation-induced xerostomia, a need still exists for patients suffering from xerostomia.
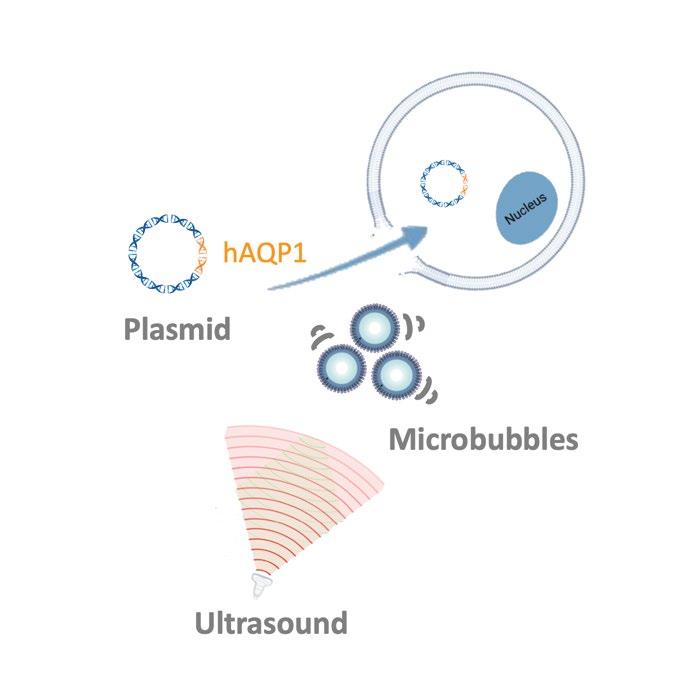
Ultrasound-assisted gene transfer is based on sonoporation generated by ultrasound, enabling gene transfer into cells. The delivery of a water channel to glands in a large animal model restored salivary flow post-radiation to pre-treatment levels, demonstrating efficacy of our non-viral gene transfer approach.
While a recent clinical trial using a viral-based AQP1 gene delivery demonstrated an increase in saliva production, this approach has not advanced beyond Phase I/II trial due to side-effects generated by the adenovirus vector. With our non-viral based approach, it is anticipated that enhanced safety is provided in patients with AQP1 gene therapy throughout their lifetime.
• Wang et al. Ultrasound-assisted nonviral gene transfer of AQP1 to the irradiated minipig parotid gland restores fluid secretion. Gene Ther 2015
• Secured non-diluted funding to support GLP toxicology studies
Regulatory Pathway
• IND
• Seeking investment for first-in-human trial





Steven Bloembergen, PhD GreenMark Biomedical Inc.



Dental caries is the most prevalent chronic disease worldwide. The unmet clinical need is noninvasive treatment through subsurface remineralization of enamel, as current fluoride toothpastes & varnishes incompletely repair caries lesions. Our professional-use products treat both dental hypersensitivity and early-stage caries, to reduce discomfort, preserve precious enamel and improve oral health.
We produce tiny positively charged, mineral-loaded starch particles, to target and release inside negatively charged tooth subsurfaces. In-situ degradation forms hydroxyapatite crystals, occluding dentinal tubules and enamel porosities. Our products, CrystLCare™ Biorestorative, Fluoride-Free and Fluoride-Plus, are easy-to-use dissolvable dental strips, 14 single-use doses in a Fliptop Pack which release the targeted particles when placed in contact with teeth.
Current fluoride products merely seal the extreme upper surface layer of enamel lesions, but targeting the subsurface enables effective non-surgical treatment of early-stage caries as well as sensitivity relief. Localized delivery of minerals and fluoride facilitates tooth structure regeneration through formation of apatite crystals, yielding superior treatment outcomes compared to currently available products.
• Jones et al. Targeted Enamel Remineralization with Mineral-Loaded Starch Particles. JADA Foundational Science, 2024 (accepted)
• Jones et al. Nanoparticle-based targeting and detection of microcavities. Adv Healthc Mater 2017
• Amaechi et al. Evaluation of a Novel Caries Detecting Oral Rinse. BDJ Open 2023
• US10,987,434 Detection and Treatment of Caries and Microcavities with Nanoparticles
• CrystLCare™ Biorestorative, Fluoride-Free product, a Class I Medical Device now being sold
• Established partnerships for co-branded product launches with 100,000-dose order from each partner
• CrystLCare™ Biorestorative, Fluoride-PLUS product submitted as a 510(k) Class II Medical Device for dental hypersensitivity indication in April 2024
• class I registration (Fluoride-free product)
• 510(k) class II (dentinal hypersensitivity)
• OTC Fluoride anti-caries drug monograph
• de novo application (remineralization/ regeneration)
• Seeking strategic partnership with global dental supply company and/or distributors
• Raising $4+ million Series A equity investment round
Dental caries is the most common non-transmissible infectious disease in the world. If untreated, caries lesions will progress to the dental pulp, exposing it to infection. Standard of care techniques involve removing infected pulp and capping the defect with inert material, or root canal therapy. Currently, there are no clinically available materials that regenerate the pulp-dentin complex.
A team led by Luiz Bertassoni, DDS, PhD and Pamela Yelick, PhD has developed a novel material, RegendoGEL, intended to be the first-of-its-kind clinical product to promote vital pulp and dentin regeneration. RegendoGEL contains key bioactive molecules present in healthy teeth that naturally promote dental pulp and dentin regeneration and may be used for pulpotomies.
Compared to non-degradable silicate/calcium hydroxide-based products currently used for endodontic treatments, RegendoGEL is a soft, biodegradable hydrogel material. RegendoGEL stimulates cells to migrate into the defect site and regenerate living dental pulp tissue and dentin in 5 days. RegendoGEL is designed as a ready-to-use product that can easily be applied using routine dental procedures.
• Cunha et al. 3D-printed microgels supplemented with dentin matrix molecules as a novel biomaterial for direct pulp capping. Clin Oral Investig 2023
• PCT/US2018/035200 Dental pulp constructs
• US11,278,474 Pulp regeneration compositions and methods of forming and using the same
• 2 confirmatory large-animal studies concluded
• GMP manufacturing started
• Seed round in progress
Regulatory Pathway
• IDE
• Seed Funding for RegendoDent, Inc. to launch RegendoGEL and conduct a clinical trial






Jessica Boehlein Amend Surgical



Micro-suturing is the current standard of care for the repair of trigeminal nerve injuries. Micro-sutured repairs are technically challenging and often inconsistent, with flaws in both fascicular alignment and spacing between severed nerve ends. Scar tissue associated with suture placement can impede axonal regeneration. Clinical need for oral surgeons: a suture-less repair solution.
Tissure™ is a degradable, hydrogel and chitosan-based nerve connector that has the adhesion and mechanical strength required to eliminate the need for suture to reconnect peripheral nerves. The product adheres to wet nerve tissue and provides over five times the adhesion energy of cyanoacrylate while also providing flexibility to stretch through range of motion without damaging nerve healing.
Current competitors in peripheral nerve repair offer largely undifferentiated collagen and polymer materials for connectors, conduits, and wraps. None of the competitors offer a suture-less alternative for a direct (non-connector) nerve repair and all of their product lines require suture to prevent migration of their product.
• Wu DT et al. Tough adhesive hydrogel for intraoral adhesion and drug delivery. J Dent Res 2023
• Freedman, B et al. Degradable and removable tough adhesive hydrogels. Adv Mater 2021
• US9,387,276, US10,383,980 Interpenetrating networks with covalent and ionic crosslinks
• Designed suitable packaging and achieved sterile product through moist heat sterilization while maintaining product integrity
• Modified hydrogel composition to reach desired in vitro degradation timepoints to achieve design freeze by Q4 2024
• Ability to create a fully dried product that rehydrates in 15 seconds prior to application
• Developed study design for non-GLP rat sciatic nerve study to be performed
• 510(k) anticipated
• Ongoing discussions with strategic
An intraoral wound overlay for soft tissue that is easy to apply, remains in place for the duration of wound healing, and can be removed without causing damage to underlying tissue. Current options for oral wound care are limited to difficult to use, uncomfortable, and largely ineffective glues and resins.
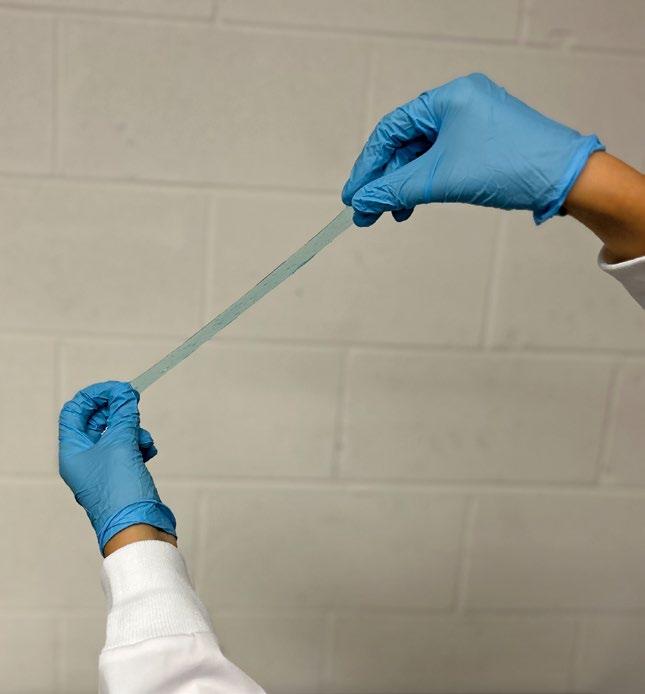
Amend Tissue Tape™ is a hydrogel-based adhesive comprised of two primary elements. The hydrogel consists of an interpenetrating network of alginate and polyacrylamide. The adhesive is composed of chitosan, which forms covalent bonds across the interface. When used together, the hydrogel and adhesive adhere to wet tissue and provide over five times the adhesion energy of cyanoacrylate while also providing a long duration mechanical barrier for the wound and flexibility to stretch with the wound without damaging the underlying tissue.
There are limited products available that will safely adhere to sutured or non-sutured oral wounds. Periacryl, a cyanoacrylate-based product indicated as a dental cement, is often used off-label for wound closure. It requires a dry environment to fully set and is rigid, not stretching to accommodate movement or swelling. Amend Tissue Tape™ adheres to tissue in a wet environment, stretches with the wound, and stays in place for up to one week. This facilitates wound healing and the regeneration process.
• Li et al. Tough adhesives for diverse wet surfaces. Science 2017
• PCT/US2019/055779 Bio-Inspired Degradable Tough Adhesives for Diverse Wet Environments
• Qualifications and validations complete
• Production ready
• In 2024, 31% of our private equity funding round has been secured
• 510(k)
• Continue to pursue strategic partner for distribution and R&D

Your support will help accelerate the translation and commercial ization of technologies into therapies that benefit patients. From research and development to training and education, every aspect of our work is rooted in our commitment to bring ing these therapies to patients around the world. Whether directed toward specific projects, clinical trials, or Center operations, your contribution will continue to drive the clinical translation of promising technologies.
Please join us on this journey – Your partnership is key to turning innovative research into accessible treatments that improve patient outcomes. Together, we can make a lasting impact.
Jeanne Ambruster
The Avenues
Connie Chang
ONL Therapeutics
Kay Fuller
Medical Device Regulatory Solutions LLC
William Giannobile
Harvard University
Darnell Kaigler
University of Michigan
David Kohn
University of Michigan
Paul Kostenuik
Phylon Pharma Services
David Mooney
Wyss Institute at
Harvard University
Katie Moynihan
Blue Ridge Bio
Vicki Rosen
Harvard University
Charles Sfeir
University of Pittsburgh
Tony Torres
University of Pittsburgh
William Wagner
University of Pittsburgh