SCI ENCE BIOTODAY

ISSUE29
supply chain management
•
lab tech & machine learning
• THE BIG
INTERVIEW
•
diagnostics
•
life science



 Karen Southern Editor in chief
Karen Southern Editor in chief
Editor
Karen Southern karen.southern@distinctivegroup.co.uk

Design
Distinctive Media Group Ltd, 3rd Floor, Tru Knit House, 9-11 Carliol Square, Newcastle, NE1 6UF Tel: 0191 580 5990 distinctivegroup.co.uk
Advertising
Distinctive Media Group Ltd, 3rd Floor, Tru Knit House, 9-11 Carliol Square, Newcastle, NE1 6UF Tel: 0191 580 7163
e: helen.flintoff@distinctivegroup.co.uk distinctivegroup.co.uk
foreword
Distinctive Media Group Ltd or BioScience Today cannot be held responsible for any inaccuracies that may occur, individual products or services advertised or late entries. No part of this publication may be reproduced or scanned without prior written permission of the publishers and BioScience Today.
Will the Vision ever become a reality?
‘I will make it easier than ever for firms … to invest in Britain and make us a science and technology superpower’.
So said Ms. Truss shortly before her election as PM. Fast forward a frenetic few weeks and even as this column is being written, her short tenure looks set to come to an unedifying end.
Now more than ever, some form of government stability and a clear sense of direction is desperately needed, not least for the future good of our life sciences sector.
Let’s get straight to the point. The sector is absolutely essential for the future wellbeing of the UK -- not just in terms of investment and innovation, but also for the literal good of the nation’s health.
The Covid-19 vaccination scheme was an undoubted success, demonstrating what UK healthcare plc can achieve when set free from the chains of over-regulation and bureaucracy.
But in an open letter to the leadership contenders prior to the PM vote, Richard Torbett, chief executive of the ABPI, exposed some worrying trends.
While he praised the 2019 Tory manifesto pledge to make the UK a ‘leading global hub’ - along with groundwork already laid
- he also flagged up major signs that we are slipping behind in the race to become a life sciences superpower.
For instance, the decline in clinical research, with the UK falling from 5th to 7th globally for Phase 3 clinical trials since 2017.1
And since 201, the UK has fallen from 4th to 98th place in overall trade balance in pharmaceuticals. 2 (Yes, you read that right!)
On the plus side, if our PM does fully commit to the previous government’s Life Sciences Vision, an extra £68bn could be added to the UK economy over 30 years through R&D investment, while the disease burden across the UK could significantly fall by up to 40%.
Major stakeholders like the ABPI and many, many others in industry and academia are fully committed to working with the government to reverse current negative trends and deliver on the Vision.
Prime Minister (whoever that may be), it’s over to you.
1. Clinical research in the UK: an opportunity for growth, Autumn 2021
2. Selling less and buying more: the worsening trade balance of the UK pharmaceutical industry, June 2022
| BIOSCIENCE TODAY | 3 www.biosciencetoday.co.uk | foreword |
Neurons are caught rapidly switching gearsfeatures
10Photonics: shining a light on the future of healthcare
Synthace: defining the future of experiment design

| BIOSCIENCE TODAY | 4 | contents |
14
8-9
biosciencetoday.co.uk
Foreword
Contents
SUPPLY CHAIN MANAGEMENT
Healing the pharma supply chain: how the industry can outsmart disruption. Visibility & transparency are key to optimum performance, says Allen Jacques at Kinaxis.
12 & 13
LABRATORY TECH & MACHINE LEARNING
Researchers at Queen’s University Belfast have developed a ground-breaking plastic film that can kill viruses on its surface with room light and Health WildCard winner builds library of biological profiles.

14-17 THE BIG INTERVIEW
Synthace is a biotech start-up accelerating biological discovery and optimisation through computer-aided biology. CEO Guy LevyYurista tells Bioscience Today why this is the decade when life sciences’ biggest challenges will be solved - in a matter of days.
18-19 GENETIC INSIGHTS
Techbio company PrecisionLife has unveiled the first detailed genetic insights into the pathophysiological mechanisms underpinning Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS).
22-23 bio-informatics
Plain-speaking research summaries could help the public make more informed decisions in major societal issues like COVID-19, says Charlie Rapple, co-founder of Kudos.
24 & 25 clinical trials & disease focus
Pain or gain? How the brain chooses and how four chemistry students from the University of St Andrews have helped discover a class of molecules that can help accelerate DNA repair which could help treat diseases such as Alzheimer’s.
30-31 diagnostics
Long-term neurological conditions like Alzheimer’s Disease could be treated using a completely new drug strategy.
32-33 life science
Imperial College Healthcare NHS Trust has set out its vision for a new life sciences cluster in Paddington, founded on its growing partnerships with research, industry and community organisations around St Mary’s Hospital.
| BIOSCIENCE TODAY | 5 | contents | 3
4-5
26 36
Synthetic mouse embryo models created from stem cells
contents
/
/ issue 29 /
Guy Levy-Yurista CEO of biotech start-up Synthace
Synthace has developed unique capabilities around Design of Experiments (DOE).

Guy is an accomplished executive with deep multidisciplinary experience across science, technology and business, he specialises in commercialising advanced technologies in fast-changing market environments and has previously served as the Chief Strategy Officer for Sisense, a hyper-growth big data company where he played a pivotal role in their success as a market-leader for data analytics. His personal superpower is finding product-market fit for technical innovations, then delivering on it.
Guy holds a PhD in Physics from the Weizmann Institute of Science in Israel, an MBA from The Wharton School at the University of Pennsylvania, and is a graduate of the Talpiot program at the Hebrew University of Jerusalem. He lives in the Washington DC area with his wife, three daughters, and his guitar (which he never seems to get enough practice with).
Rosie Casey
Vice President of High Growth Companies & Life Sciences UK, IDA Ireland, the investment promotion and development agency for Ireland

Ireland’s life sciences sector has a global reputation for operational and innovational excellence, and the country is the world’s third largest exporter of pharmaceuticals, accounting for 39% of all annual exports.
Charlie Rapple
Chief customer officer and co-founder of Kudos


A research communication platform ‘to bring storytelling to science’ and bridge the gap between research producers and users.
Charlie’s role is to help shape the strategic direction of Kudos, identify and build relationships with organisational partners, and help manage the development and delivery of its web-based services.
Allen Jacques Industry Thought Leader at Kinaxis
Kinaxis is a provider of cloud-based supply chain management solutions.
Allen was previously VP of Global Technical Operations and Biologics and External Manufacturing for Leo Pharma, and VP of Network Supply Planning at Pfizer, as well as a variety of roles at Wyeth and Baxter Healthcare.

Allen has worked across the life sciences spectrum in pharma, biotech, hospital, and consumer divisions in the US and Europe. He has a BS in Microbiology from the University of Maryland and doctoral studies in Biochemistry at Johns Hopkins.
to advertise or contribute to the next edition
advertising: liz.hughes@ distinctivegroup.co.uk
editorial: karen.southern@ distinctivegroup.co.uk
| BIOSCIENCE TODAY || industry contributors | | BIOSCIENCE TODAY 6 SCI ENCE BIOTODAY
Novel synthetic polymers could lead to greater crop yields
The breakthrough is expected to result in stronger, healthier plants and higher crop yields in agriculture.
In nature, the roots of seedlings form mutually beneficial relationships with communities of microbes (fungi, bacteria, viruses) in soil, and exchange nutrients, allowing both the plant and the microbes to flourish. This is particularly critical in the early stages of a plant’s life when the seedling is in a race against time to reach self-sufficient growth before the nutrients and energy stores in the seed run out.
Dr Tim Overton, an applied microbiologist from the University’s School of Chemical Engineering, and Dr Francisco Fernandez-Trillo from the School of Chemistry led a team to develop novel synthetic polymers that stimulate the formation of these bacterial communities, in a way that mirrors a natural process known as biofilm formation.
A biofilm is a finely orchestrated community of microbes, supported by matrix of biological polymers that forms a protective microenvironment and holds the community together.

The researchers worked jointly on a four-year project on how polymers interact with bacteria, which resulted in the synthesis of a group of acylhydrazone-based polymers.
These new polymers were designed to act as an adhesive scaffold, “seeding” the formation of a microorganismpolymer complex to initiate and expedite biofilm formation. Once the biofilm is formed, the bacteria become a selfsufficient and self-organizing community, and produce their own matrix to allow the transmission of nutrients and water, and the discharge of waste products.
The project was funded by Biotechnology and Biological Sciences Research Council (BBSRC) through their Midlands Integrative Biosciences Training Partnership.
It involved PhD students Pavan Adoni and Omar Huneidi, who subsequently progressed research showing the polymers aggregate bacteria, and improve biofilm formation. Critically, they also showed the process is fully reversible, and the biofilm can be dispersed by changing the environmental conditions. The results of these experiments and further studies will be published in 2022.
Pavan Adoni commented: “We anticipate that the polymer will ultimately be used as a seed coating, perhaps in conjunction with bacteria such as B. Subtilis, which is naturally present in soil, increases the stress tolerance of plants, and is currently used as a soil inoculant. We envisage a more targeted approach that only treats the seed, so that when it germinates the bacteria are ready to grow in the safe harbour environment provided by a microorganism polymer complex. Ultimately this should result in stronger plants, which grow more quickly, & have greater resilience to disease.”
University of Birmingham Enterprise filed a broadbased patent application covering the novel polymers, the method of forming the biofilm and the method of polymer cleaving, and its use to promote growth of a biofilm with any micro-organism including those that can produce or deliver chemical or biological molecules.
The patent has now been licensed to specialist life science company PBL Technology, which invests in, protects and promotes emerging innovations from public research sources worldwide. In agriculture, PBL’s technologies include crop genetics, crop treatments, precision agriculture and promoters and R&D tools.
| BIOSCIENCE TODAY | 7 | news |
Scientists at the University of Birmingham have invented a new method to encourage bacteria to form growth-promoting ecosystems that could be used to coat the roots of plant seedlings.
“These new polymers were designed to act as an adhesive scaffold, “seeding” the formation of a microorganism-polymer complex to initiate and expedite biofilm formation. ”
Healing the pharma supply chain: how the industry can outsmart disruption

The pharmaceutical industry fared relatively well through the pandemic, with few manufacturing sites being closed due to infections. It may come as some surprise, then, that the sector has recently hit rockier ground. A supply chain shortage has come to light, leaving many patients struggling to access pain relief, oncology medication, antihistamines, hormone replacement therapy, anticlotting agents, and other important drugs.
The ramifications of this supply chain issue could be severe. Already, over half (54%) of UK pharmacists surveyed fear that patients have been put at risk in the past six months due to shortages. With the government now urging hospitals to ration stocks of certain drugs, action must be taken to minimise the impact on end consumers needing medicines. However, the picture is not all bleak. The pharmaceutical industry is robust enough to heal its supply chains – if it leverages the right tools. By using technology to improve supply chain visibility and transparency, companies can plan for volatility and identify potential disruption before it occurs, thereby restoring consumer wellbeing and trust.
WHAT IS FUELLING THE SUPPLY CHAIN SHORTAGE?
Supply chain shortages have been a recurring problem over recent months, leaving very few industries unscathed. But for pharma, a number of factors have combined to create a unique challenge. Firstly, demand for hormone replacement therapy has doubled over the last five years – largely due to increased awareness of the menopause – while capacity has failed to keep up. Seasonal demand for certain hay fever medications was similarly left unmet recently, this time due to shortages in stocks of chlorphenamine maleate.
Current trade tensions have only exacerbated the issue. Raw materials and active pharmaceutical ingredients sourced

8 | supply chain management | | BIOSCIENCE TODAY |
Allen Jacques, Kinaxis.
Visibility and transparency are key to optimum performance, says Allen Jacques, Industry Thought Leader at Kinaxis.
from countries like China can be caught in log jams due to international disputes. Meanwhile, some believe that Brexit has created importation complexity, significantly slowing down the entry of externally sourced drugs.
In some cases, pharma manufacturing sites have had to be closed for periods of time due to non-compliance with regulatory requirements. This was recently seen in the US when Abbott Laboratories shut down its Michigan plant in February. Unsanitary, non-compliant conditions had led to the contamination of Abbott’s baby formula, worsening national shortages of the product. These business interruptions can have substantial and long-lasting downstream effects.
Inevitably, questions of profitability can also come into play. Pharmaceutical companies often prioritise patent protected drugs, where profit margins are high, over drugs with generic competition, where profit margins are much lower. This does little to help tackle shortages of non-patented painkillers, blood pressure pills, and antidepressants.
TECHNOLOGY: AN ANTIDOTE TO THE SUPPLY CHAIN CRUNCH
Of course, some factors – like Brexit and geopolitical trade tensions – are outside of the pharmaceutical industry’s control. But the time has come for companies to focus on what they can control – and they can do this by bolstering

the resiliency of their supply chains. Technological solutions have now come to the fore as key enablers of supply chain performance and agility.
The pharma supply chains that are in the best current shape are those that can see and assess events quickly and drive actions to mitigate any ensuing disruption. This involves having consistent visibility and transparency, not only internally but also into suppliers, contract manufacturers, and downstream distribution. While this may initially sound complex, innovations in technology mean that companies can now work from a single platform to intuitively manage, link, align, share, and collaborate with data insights across the supply chain network.
Having this capability allows companies to respond much more quickly to potential disruptions in the pharmaceutical supply chain. For example, the visibility provided by data analytics will help the industry to anticipate and pre-empt changes in consumer demand, so that a targeted, timely response can be put into motion. Technology can also facilitate better planning for factors such as manufacturing problems, late supply of raw materials and components, and compliance issues.
Everybody benefits from greater supply chain visibility, transparency, and resilience. For pharmaceutical companies, the agility to make fast, confident decisions across the entire supply chain will undoubtedly drive business growth and help to protect revenues. But even more importantly, it ensures that end consumers can get the medicines they need, exactly when they need them. With technology, the pharma industry can futureproof its supply chains to better serve the needs of companies, healthcare providers, and the public at large.
9 | supply chain management || BIOSCIENCE TODAY |
“The time has come for companies to focus on what they can control.”
Photonics: shining a light on the future of healthcare

With increasing demand for healthcare innovation, Rosie Casey, Vice President of High Growth Companies & Life Sciences UK, IDA Ireland, explores the future of photonics in the medical healthcare industry and the necessary requirements for such innovation to thrive.
10 | technology | | BIOSCIENCE TODAY |
The healthcare industry is going through a transformation as technological advancements such as artificial intelligence, robotics and nanotechnology continue to improve healthcare across the world.
These technologies are increasingly being embedded into medical technology (MedTech) through medical devices, which has set the industry in a prime position for innovation and improvements. With a market value of half a trillion dollars – expected to increase to over $600 billion in 2023 – medical device companies are looking to apply these innovations with agility to their production lines in order to meet current needs, as well as increasing predicted demand years down the line.
One particularly monumental transformation in the MedTech industry is the utilisation of photonics – the science and technology of light and, distinctly, the ability to generate, manipulate, amplify, guide and detect light. Whilst not necessarily a household term, photonics is an ubiquitous technology in everyday life and has been recognised by the European Commission as one of Europe’s key enabling technologies (KETs) of this century.
The utility of photonics has become omnipresent in everyday life, in telecommunications, manufacturing processes, construction, space exploration and aviation and agriculture to name a few.

As photonics and other emerging technologies are increasing embedded into healthcare, they will help provide cheaper, faster and more effective solutions for illnesses and diseases. So, the question in the next few years is: how can technological advancements like photonics be developed efficiently and applied most effectively to the healthcare industry?
REVOLUTIONISING THE HEALTHCARE INDUSTRY
Although the incorporation of photonics into the healthcare system has been gradual, results so far have already brought significant changes to medical practice. One of them includes the widespread popularity of laser eye surgery that has replaced glasses and contact lenses to mitigate eye conditions such as myopia, hyperopia or astigmatism.
Perhaps more monumental is the accurate detection and treatment of cancer and diseases. This has come at a time of continuing reports of failed, wrong or late diagnosis.
For instance, it was documented by England’s National Health Service (NHS) that in the three years before 2019, there were 4,097 successful compensation claims arising out of an incorrect or delayed diagnosis and £583 million paid out to claimants. One major contributor to these occurrences were cancer misdiagnosis. Due to human error, doctors cannot always be 100% accurate with a diagnosis, but this becomes an issue when someone receiving this misdiagnosis could face life-changing problems. As a result, medical professionals are increasingly looking to photonics’ diagnosis technologies to ensure they are giving the patient the finest, most accurate service.
Photonics also play an important role in detecting, mitigating and treating cancers, as they allow doctors to study the structure of biological tissues at a drastically smaller scale and allow real-time and dynamic visualisation of complex macromolecules, like DNA and proteins.
This has proved pivotal for creating new solutions for clinical diagnosis and therapies. Whilst these photonic nanomedicines are undergoing clinical trials, their effectiveness in cancer treatment have already been highlighted in pre-clinical trials and will help shape the future of medicine and medical procedures. Not only will photonics be crucial for ophthalmology and oncology by detecting and treating diseases and illnesses, but it will also revolutionise the health monitoring sector.
Due to the non-evasive nature of photonics and its unprecedented resolution and depth, detailed information can be gathered, including a wide range of biomarkers from glucose trends to blood alcohol. Popular smart watches could offer health monitoring far beyond the current “sleep, steps and heart” standard. What’s more, this technology could flag up serious diseases and other common health concerns – and doctors could then use these applications to inform their diagnosis, monitoring and treatment.
DEVELOPING A VIBRANT PHOTONICS ECOSYSTEM

In order to reach the potential of photonics, strong research and development, talent and investment is an absolute requirement. Facilitating these inroads is Ireland, which is quickly becoming a MedTech hotspot for companies due to its highly interconnected ecosystem, talent pool and regulatory regime that supports a thriving MedTech industry. Ireland contains more than 300 MedTech companies supported by academic, clinical, industry, and government agencies. Well-known enterprises such as Johnson & Johnson, Boston Scientific and Stryker have long-established operations in the country. Particularly, the Science Foundation Ireland Advanced Materials and Bioengineering Research Centre (AMBER) has the ability to co-fund projects with the collaborating company, which makes it attractive to early-stage high risk research. With support from the Irish Government and IDA Ireland, more companies are noticing the exciting prospect to help them kickstart their potential.
The rich research ecosystem Ireland has developed is opportune for innovation. This involves the close collaboration between Ireland’s top tier universities, its 14 Institutes for technology, and its seven clinical research facilities to ensure the evolving skill needs of this fastgrowing sector are met. This makes Ireland ranked number one globally for the exchange of technology and ideas, which explains why 14 of the world’s top 15 medical device companies have a base in Ireland. Boston Scientific has recently announced a $30 million investment into its Cork facility to accelerate its research and development of medical technologies to treat patients suffering from cancer and peripheral arterial diseases around the world.
Finally, world-leading companies establishing operations in the country gain access to Ireland’s young talent. Whilst Ireland’s universities are ranked number one globally for the highest employable graduates, Ireland also bolsters a strong business network of companies that share best practices and identify specific skill needs of the sector. For instance, the Irish Medtech Skillnet is co-funded by Skillnet Ireland and network companies such as the Nation Training Fund and the Department of Further and Higher Education, Research, Innovation and Science. All this, as a result, has generated a highly skilled and knowledgeable, cross-sectoral ecosystem.
Looking ahead, it’s clear: the future of healthcare lies in working hand-in-hand with technology, and healthcare workers have to embrace emerging healthcare technologies in order to stay relevant in the coming years. With the optimal environment and support, companies that continue to create and deliver innovative technologies, such as photonics, are perfectly positioned to makes waves in the future of healthcare.
11 | technology || BIOSCIENCE TODAY |
Rosie Casey, Vice President of High Growth Companies & Life Sciences UK
Plastic film can kill viruses using room lights
The self-sterilising film is the first of its kind; it is low-cost to produce, can be readily scaled and could be used for disposable aprons, tablecloths, and curtains in hospitals. It is coated with a thin layer of particles that absorb UV light and produce reactive oxygen species – ROS. These kill viruses, including SARS2.
The technology used to create the film also ensures it is degradable (unlike the current disposable plastic films it would replace), making it much more environmentally friendly.
The breakthrough could lead to a significant reduction in the transmission of viruses in healthcare environments but also in other settings that uses plastic films, for example, food production factories.

The Queen’s researchers tested the film for anti-viral activity using four different viruses – two strains of influenza A virus, a highly-stable picornavirus called EMCV and SARS2 –exposing it to either UVA radiation or with light from a cool white light fluorescent lamp.
They found that the film is effective at killing all the viruses, even in a room lit with simple white fluorescent tubes.
The research, which has been published in the Journal of Photochemistry and Photobiology B: Biology, was carried out by Professor Andrew Mills, Dr Ri Han and Dr Christopher O’Rourke in the School of Chemistry and Chemical Engineering at Queen’s University Belfast and Dr Connor Bamford and Dr Jonathon D. Coey at the Wellcome-
Wolfson Institute for Experimental Medicine in the School of Medicine, Dentistry and Biomedical Sciences at Queen’s.
Professor Andrew Mills said: “This film could replace many of the disposable plastic films used in the healthcare industry as it has the added value of being self-sterilising at no real extra cost. Through rigorous testing we have found that it is effective at killing viruses with just room light. This is the first time that anything like this has been developed and we hope that it will be a huge benefit to society.”
Dr Connor Bamford added: “Pathogenic viruses like SARS2 and influenza will continue to be global problem for years to come. In developing self-sterilising thin plastic films, we have created a low-cost technology that could have a significant impact on the transmission of such concerning viruses in a healthcare environment and other sectors where they are used.”
The project was funded by the Engineering and Physical Research Council, which is part of UK Research and Innovation.
EPSRC Director for Cross Council Programmes, Dr Kedar Pandya, said: “This is a hugely exciting development which has the potential to dramatically reduce the transmission of viruses across a wide range of settings while being environmentally sustainable.
“It is an excellent example of adventurous, innovative research which has the potential to improve the lives of millions of people.”
12 | BIOSCIENCE TODAY || labratory tech |
Researchers develop plastic film that can kill viruses using room lights. Credit: Queen’s University Belfast
Researchers at Queen’s University Belfast have developed a ground-breaking plastic film that can kill viruses on its surface with room light.
Health WildCard winner builds library of biological profiles
Initially backed by EIT Health, part of the European Institute of Innovation and Technology, a body of the European Union, iLoF’s intelligent platform is collecting vast amounts of data to build a library of biological profiles, bringing together world-class physicists, biologists, and data scientists to deliver life-saving personalised treatments to patients faster.
Using cutting-edge optical lasers, the company first analyses blood samples from patients before using AIpowered technology to create a unique digital molecular profile of a patient. Patients’ digital profiles are then compared to iLoF’s cloud-based library of biological profiles in search of potential matches. Matches ultimately facilitate the development of personalised treatment, which informs patient monitoring, diagnosis and screening plans.
With over $8M in funding to date, iLoF looks set to help cut time and costs of recruiting patients for clinical trials. By helping to remove the barriers to the development of personalised, effective, and life-saving therapeutics, iLoF promises to usher in a new generation of personalised therapies and patient-centric treatment.
Luis Valente, co-founder and CEO of iLoF, said, “For hundreds of years, treatments have been developed with the assumption they will work for everyone. However, each person is different, and for many severe diseases such as Alzheimer’s, multiple factors can contribute to the effectiveness of a treatment on a given patient.
“This means millions of patients currently live without access to an effective, disease-modifying treatment - which sparked the vision behind iLoF. We collect vast amounts of data to create digital twins of biological profiles and disease subtypes, which we store in our digital library. Different patient profiles can be selected and screened using our platform to speed up the development of effective and
personalized treatments while enabling humane, patientcentric clinical trials.”

Mehak Mumtaz, co-ounder and COO of iLoF, added, “Our ultimate vision is to go from a next-generation bioinformatics platform to a breakthrough disease screening one. Moving from supporting researchers and scientists to directly helping patients around the world will vastly deepen our understanding of diseases and help physicians detect the world’s more severe and impactful diseases, such as Ovarian Cancer or Alzheimer’s Disease.”
Consisting of 20 international scientists, entrepreneurs, and investors, the renowned iLoF team was initially formed during the EIT Health WildCard programme in 2019 – which provides world-class mentorship and training to innovators with life-changing ideas to improve healthcare. iLoF has also previously won EIT’s Jumpstarter pre-accelerator programme, which boosts innovation and entrepreneurship within Central-Eastern and Southern-European regions.
Jean Marc Bourez, CEO of EIT Health, said, “We are thrilled about the news of iLoF’s well-deserved funding success. The company has come a long way since its inception at the EIT Health WildCard programme in 2019. With its grand vision to improve patient outcomes using pioneering technology, we couldn’t be prouder of our role in nurturing iLoF and we look forward to watching as the company continues to grow and transform the lives of patients living with degenerative diseases such as Alzheimer’s.”
The seed funding will be used to accelerate the development of iLoF, progress further partnerships and scale the team. Currently, the company is hiring internationally across physics, data science, biology, and product management profiles.
More information at ilof.tech, eithealth.eu, and wildcard. eithealth.eu
13 | BIOSCIENCE TODAY | | machine learning |
Digital digital health company iLoF has received $5m in funding to accelerate its mission of improving access to personalised medicine for millions of people living with complex diseases, such as Alzheimer’s.

14 | BIOSCIENCE TODAY || the big interview |
Synthace: defining the future of experiment design
Synthace is a biotech start-up accelerating biological discovery and optimisation through computer-aided biology. CEO Guy Levy-Yurista tells Bioscience Today why this is the decade when life sciences’ biggest challenges will be solved - in a matter of days.

15 | BIOSCIENCE TODAY | | the big interview |
If you wanted to create the world’s best cappuccino, how would you start?
The usual scientific way would entail a taste test with five cups of coffee heated at different temperatures, and made with different amounts and types of coffee, sugar, milk etc. At every point in time, one parameter would be tested everything else is kept constant. That is the paradigm of science.
A better way is to try five different parameters of ingredients, temperature etc, across five different trails. That would mean 3,125 different cups of coffee to be prepared and tasted.
But there is a different way - Design of Experiments (or DOE), which allows you to smartly and automatically test a subset of this permutation to get the best solution.
Synthace has developed unique capabilities around DOE. Its cloud-based digital experiment platform, based in London and Boston, digitises the entire process end to end. With minimal training, scientists can design and plan experiments, simulate them in silico, schedule them on their automation equipment, and aggregate experimental data in one place.
Put simply, it means experiments are enabled that were previously thought impossible.
Synthace claims to give scientists a really powerful edge when it comes to finding the best disease treatment, the best drug, the best way to grow food, and so on.
Currently digitilisation of life science R&D is very fragmented, with experiment design using a range of platforms and scientific collaborations which are neither efficient nor cost-effective. Imagine then adding coding to the mix. There’s no need for coding with our operating platform, say the Synthace team… it’s as easy as dragging and dropping!
Guy tells us more:
HOW AND WHY DID SYNTHACE ORIGINATE?
We started out as CRO with our own lab in North London. Because our founding team spent a lot of time in the lab, they knew the pain of manual, repetitive tasks of biological experimentation and limitations of science when it’s done this way. Scientists spend most of their time doing tedious, boring, manual tasks, and little on science itself. More so, so much information from experiments is lost because it requires a scientist to record all this data manually. It is a huge burden on scientists and limits and slows down scientific progress.
We thought “what if we could alleviate some of that pain? What if we could represent all of this labwork with code, and lift a lot of this burden from scientists? How much would that free scientists to do breakthrough discoveries not thought possible before?”
Thus we’ve created an experiment platform that enables scientists to design and run more effective and insightful experiments, accelerating scientific progress, and increasing the lifetime value of laboratory operations.
WHAT DID THE PLATFORM’S CREATION ENTAIL IN TERMS OF MAN HOURS, RESOURCES ETC? WHAT WAS THE ‘EUREKA’ MOMENT?
I think most people think of ‘eureka’ as finding the right answer. For us, our biggest eureka was when we realised the correct question - it went deeper than just how to represent lab work with code. We started asking what it was that biologists actually needed to build a deeper understanding of biology. What would that look like?
Our platform today is the product of many years of difficult work by a group of amazing people. We are a life science SaaS company working on a deep tech problem to build a digital interface with laboratories, and that’s hard. It’s also why it’s so hard to put a number on it in terms of hours.
DIGITALISATION IN LIFE SCIENCES IS ENTERING A GOLDEN ERA. WHAT PART HAS SYNTHACE PLAYED IN THIS TRANSFORMATIVE PROCESS?

I wouldn’t say we’re “entering” it. I’d say the golden age for life science digitalisation is already here…it’s just unevenly distributed. At Synthace, we are focused entirely on digitalisation of the experiment.
The reason we’re leading a digital transformation in labs all around the world is that we’re approaching the problem from first principles. Most are focused on improving the individual “point solutions” we find in labs—automation, ELNs, LIMS—but nobody else is bringing everything together the way we are.
Want to automate? You’ve got to use an automation tool. Want to write up your protocol? You’ve got to use an ELN. Want to bring all your data together? You’ll need a LIMS. They’re all separate, isolated islands. What Synthace does, it brings it all together to run a better experiment. Better experiments lead to better discoveries. That’s the power of our experiment platform, that’s what nobody else can do.
HOW DO YOU SEE YOUR PLATFORM EVOLVING? ANY OTHER INNOVATIONS IN THE PIPELINE?
We’re constantly rolling out improvements. For example, we’ve recently made it possible for scientists to accelerate their manual pipetting, meaning the burden of calculating and planning pipetting volumes is completely gone for the scientists using it. This also includes the ability to switch between manual and automated execution with a single click, whenever they want.
One of our biggest innovations right now is built on top of that core DOE capability, and we’ve already seen it produce incredible results for our customers. It’s something we’re calling ‘High Dimensional Experimentation,’ or HDE. It’s
16 | BIOSCIENCE TODAY || the big interview |
Guy Levy-Yurista, SYNTHACE
a capability that combines automation-enabled DOE with high throughput liquid handling/dispensing. You won’t find this anywhere else—it’s not something we’ve seen anyone else get remotely close to. There’s one customer of ours who has used it to knock months off the critical path in their assay development for drug discovery, and that’s just the beginning. We’re talking hundreds of millions of dollars in value created, all while helping people get drugs into the market much faster.
WHAT’S THE BIGGEST ADVANCE IN YOUR FIELD THAT WOULD HAVE BEEN CONSIDERED TOTALLY IMPOSSIBLE, SAY, FIVE YEARS AGO.
I think the biggest advance in our field is actually a change in mindset and the realisation that those “point solutions” in the lab aren’t going to cut it anymore. This has worked for many for a long time, and I’m sure there’s more value to get out of it, but I think sticking to this old mindset is going to get riskier every day.
The new mindset is all about looking at things from the perspective of the experiment and asking how scientists can do better science. If you get a better ELN, all you get is a better ELN. That can definitely be helpful, but does it let you run better experiments, does it help you do better science? No, not even close.
WE’RE LIVING IN UNPARALLELED TIMES: DOES SYNTHACE REGARD THE CURRENT ECONOMIC TURMOIL AS AN OPPORTUNITY OR OBSTACLE FOR THE COMPANY AND ITS CUSTOMERS?
The current turmoil we’re seeing in the world -- whether that’s the price of food, the effects of climate change, or the continuing knock-on effects of the pandemic -- is a stark reminder of how important the life sciences are, and
the impact it can have on our future. After all, these are all issues that are fundamentally about biology and our ability to understand and make use of it, whether that’s developing vaccines, growing hardier crops, or finding better ways to capture carbon and maybe even store energy.
I would hate to call this an opportunity, more as a necessity for us and our customers. A necessity to embrace technology and move away from the status quo of doing science as we’ve done it.
SYNTHACE IS CLASSED AS A GREAT PLACE TO WORK, PARTICULARLY FOR WOMEN. HOW HAS THIS SHAPED THE COMPANY’S SUCCESS?
I wouldn’t say that it’s shaped our success, I’d say it’s one of the main reasons for our success. The old adage is true: culture eats strategy for breakfast. If you’ve got a bad culture, neither the very best talent nor an unlimited budget will save you from failure. A strong culture is the key to solving difficult problems and making progress.
I think the difference at Synthace is that we believe a company is not the sum of the roles it has, but the people that we go to work with. We all have complex lives outside of our work life: children to take care of, ageing parents to worry about, house moves to organise, diagnoses to understand, interests to explore, dreams to strive for—the detailed reality of life in total. When our people come to work, they bring all of themselves, and so we must celebrate all of them, their authenticity, their uniqueness.
This means we very deliberately make decisions about diversity, maternity, salaries, vacation, remote working, and everything else to do right by the individual—not some wrongheaded idea of “what a company should do.”
HOW WILL LIFE SCIENCES LOOK IN THE FUTURE?

The short answer is it will be unrecognisable. The longer answer is more difficult and depends entirely on how big we’re willing to dream. I think, at the very least, we’ll see dramatically improved AI models for biology, informing exceptionally powerful and well-designed experimentation in the lab.
There won’t just be a lab-to-human loop, it will become a lab-to-digital-to-human loop. Every aspect of what is done in biological R&D will be linked to the digital world, with every experiment run in vastly more sophisticated laboratory environments, and aided by digital tools. Tools that, crucially, draw a line through the entire process and build a robust digital interface with the reality of lab work. Find out more at synthace.com
17 | BIOSCIENCE TODAY | | the big interview |
“we’ll see dramatically improved AI models for biology, informing exceptionally powerful and well-designed experimentation in the lab.”
First genetic links revealed in ME and Chronic Fatigue Syndrome study
This is the first time that replicable genetic findings have been reported in over 30 years of study into the disease, offering new approaches for better diagnosis and treatment of patients.
PrecisionLife, based in Oxford, specialises in generating deep insights into disease biology to create novel precision medicines for chronic diseases.
The results of their ME/CFS study were presented at the M.E. Genetics Research Summit organised by ME charity, Action for M.E. and the MRC Human Genetics Unit, University of Edinburgh. The data has also been submitted for peer reviewed publication and is available on prepublication site medRxiv.
The use of a new hypothesis-free combinatorial analytics approach using the PrecisionLife™ platform enabled the
study to identify 14 novel genetic associations with ME/CFS from the UK Biobank cohort. Specifically, the combinatorial analysis revealed 199 SNPs, mapping to these 14 genes, that were significantly associated with 91% of the cases in the ME/CFS population. Many of these genes are involved in highly plausible cellular mechanisms associated with ME/CFS disease including vulnerabilities to stress and infection, mitochondrial dysfunction, sleep disturbance and autoimmune development.

The study also identified similarities with genes that PrecisionLife has found to be associated with multiple sclerosis (MS) and long COVID.
ME/CFS is a debilitating chronic disease affecting around 17 million people worldwide, which presents with diverse symptoms including post-exertional malaise, chronic pain,
18 | BIOSCIENCE TODAY || genetic insights |
Techbio company PrecisionLife has unveiled the first detailed genetic insights into the pathophysiological mechanisms underpinning Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS).
“MS and ME/CFS patients share a number of similar symptoms and there is increasing evidence that many long COVID patients also share similar symptoms.”
and cognitive impairment. There are currently no approved disease modifying therapies for ME/CFS, and patients are managed via prescription of drugs and other therapies for symptomatic relief, including pain relief, anti-depressants, and cognitive behavioural therapy.
Sonya Chowdhury, Chief Executive, Action for M.E., said: “For too long, people with ME/CFS have struggled to get their condition diagnosed, understood, and acknowledged. These are exciting findings from PrecisionLife that may be used to develop diagnostic biomarkers and discover novel drug targets and precision repositioning opportunities in the future. If successful, these could be used to create the first therapeutic options for this debilitating disease.”
Dr Steve Gardner, CEO, PrecisionLife, added: “These groundbreaking results provide new hope of developing effective precision medicines for people living with ME/ CFS around the world. It is further validation for our unique approach to the analysis of complex disease biology. PrecisionLife now has this unprecedented level of insight across over 40 diseases that we have so far studied, which creates wonderful opportunities for us and our partners to bring novel solutions for patients with unmet medical needs.”
MS and ME/CFS patients share a number of similar symptoms and there is increasing evidence that many long COVID patients also share similar symptoms. It is also believed that some patients may be developing ME/CFS symptoms as a direct result of having a COVID-19 infection.
PrecisionLife is in the process of analysing long COVID-19 and MS populations to identify shared genes and biological mechanisms underpinning ME/CFS, MS and long COVID-19 development. Establishing the similarities and differences between them in more detail is likely to have profound implications for patients and the development of novel diagnostic and therapeutic tools.

Preliminary findings from the PrecisionLife long COVID analysis have indicated that three of the genes identified its ME/CFS study are also significant in the long COVID patient group. These will be subject of further validation in a new publication later this year.
Next, PrecisionLife aims to replicate and extend the results of this UK Biobank study with combinatorial analysis of the DecodeME study, the largest genetic ME/ CFS study, with over 20,000 participants. This is likely to allow further insights into the different subgroups and targets involved with the disease.
19 | BIOSCIENCE TODAY | | genetic insights |
Disease architecture diagram demonstrating 15 groups of SNPs that make up the structure of ME/CFS patient subpopulations identified by the PrecisionLife study. Each circle represents a disease-associated SNP genotype, edges represent their co-association in patients in disease signature(s), and colours represent distinct patient sub-populations. On the right: the same disease architecture view coloured to show the critical SNPs associated with each community.
Copyright: PrecisionLife
UKRI unveils plan for outstanding research and innovation

UKRI has published a corporate plan for the world-class research and innovation needed to drive economic, social, environmental and cultural benefits in the future.
The plan sets out how UK Research and Innovation (UKRI) will spend its annual £7.9 billion budget and support the government’s ambitions for the UK to be: a global leader in research and innovation the best place in the world to innovate and invest in or grow a business.
UKRI considers the plan’s publication to be a significant milestone.
BUILDING ON FIVE-YEAR STRATEGY
It builds on UKRI’s five-year strategy, transforming tomorrow together, and provides more detail on how UKRI will deliver its ambition for UK research and innovation set out through six strategic objectives.
It will be followed by publication of strategic delivery plans for UKRI’s nine councils.
Through this full suite of documents UKRI will set its path for the forthcoming years, delivering on the promises of the Spending Review settlement.
FOSTERING OUTSTANDING RESEARCH AND INNOVATION
UKRI Chief Executive Professor Dame Ottoline Leyser said: “Our corporate plan sets out the key actions UKRI will take over the course of our three-year budget settlement to foster an outstanding research and innovation system for the UK.
This collective programme of work across UKRI is guided by our ambitious strategy, which outlines our strategic objectives and four principles for change: diversity, connectivity, resilience and engagement.
We are committed to ensuring that UKRI realises its full potential, working with our many partners to convene, catalyse and invest in the UK’s world-class research and
innovation endeavour, enriching lives locally, nationally and globally.
Business Secretary Kwasi Kwarteng added: “esearch and innovation is crucial to the UK’s future; it will drive prosperity in every part of the country and deliver benefits for all.
UKRI’s corporate plan outlines how it will support the UK’s world-leading researchers and innovators, strengthening its position as a science superpower and the best place in the world to innovate.
INVESTING IN TALENT AND TECHNOLOGIES
Commitments include:
increased investment in technologies of the future, including building on investments in artificial intelligence, quantum technologies and engineering biology.
a £2 billion talent programme.
increasing research and innovation infrastructure investment to £1.1 billion a year by 2024 to 2025.
supercharging innovation, including through Innovate UK with a budget increase to more than £1 billion by 2024 to 2025.
piloting Innovation Accelerators in Greater Manchester, West Midlands and Glasgow City Region.
an extra £185 million across UKRI to target global and national challenges, including the move to net zero through a new ‘Building a Green Future’ programme.
a pilot £65 million investment in new and emerging areas that reach beyond disciplinary boundaries.
UKRI becoming a more open and agile organisation. Read the UKRI corporate plan 2022 to 2025
20 | BIOSCIENCE TODAY || news |
The virtual reality environment seen by mice.

Switch tells brain when to learn and when to remember
These essential brain functions seem to compete against each other, but a fine balance is actually at play.
As we go about our lives, our brain continually remembers information that we have learnt in the past and uses it to make sense of the world in the present.
However, we also often encounter objects and events that we have never experienced before, and then the brain needs to be ready to learn. These two essential functions of our brain’s memory system, learning and remembering, seem to constantly compete against each other.
“How the brain finds the balance between these two opposing processes is a question that has fascinated neuroscientists for a long time,” explains Christoph Schmidt-Hieber, head of the Neural Circuits for Spatial Navigation and Memory Laboratory at the Institut Pasteur.
Christoph Schmidt-Hieber’s research group has recently tackled this problem by designing an experiment in which mice explore virtual reality environments as their brains are recorded.
“We realised that the main obstacle to studying how the brain reacts to novelty was physical reality itself!” explains Ruy Gómez-Ocádiz, a PhD student in the laboratory and first author of the study.
It is almost impossible to study the effect that absolute novelty has on the brain in a traditional experiment, because one would need to instantly change everything that an animal is perceiving.
“We could easily overcome this problem if only we could ‘teleport’ a mouse to a new room as we record its brain. This might sound like science fiction, but virtual reality technology allowed us to do precisely that,” continues Ruy Gómez-Ocádiz.
The scientists designed a video game in which mice learn to explore a virtual ‘world’ and get sugar rewards when they correctly follow the rules of a simple game. While mice were engaged in playing the video game, the researchers recorded the activity of neurons in the hippocampus, a brain region that is essential for forming and recalling memories.
Using this innovative approach, they discovered an electric signal in the hippocampus that appears at the precise
moment when the animal is teleported to a new virtual world. The signal is emitted by granule cells and triggered by novelty. It induces a transition from a neural state of memory to a state of learning.
Teaming up with physicists from the École normale supérieure, Université PSL and the CNRS, the scientists then developed a computational model suggesting how such a novelty signal may work as a switch to enable the brain to alternate between remembering and learning modes depending on the information present in the environment.
“The discovery of this novelty signal in the hippocampus provides exciting new clues to understand how the brain finds the necessary equilibrium between formation of new memories and recall of familiar ones,” concludes Christoph Schmidt-Hieber.
The study was funded by the institutions mentioned above, the European Research Council (ERC) and the French National Research Agency (ANR).
REFERENCES
A synaptic signal for novelty processing in the hippocampus, Nature Communications, 15 juillet 2022
Ruy Gómez-Ocádiz 1,2,4, Massimiliano Trippa3, Chun-Lei Zhang 1, Lorenzo Posani 1,5, Simona Cocco 3, Rémi Monasson3 & Christoph Schmidt-Hieber 1
1. 1nstitut Pasteur, Université Paris Cité, Neural Circuits for Spatial Navigation and Memory, Department of Neuroscience, F-75015 Paris, France.
2. Sorbonne Université, Collège Doctoral, F-75005 Paris, France.
3. Laboratory of Physics of the École Normale Supérieure, PSL Research and CNRS UMR 8023, Sorbonne Université, Université de Paris, F-75005 Paris, France.
4. Present address: Department of Neuroscience, Karolinska Institutet, 17177 Stockholm, Sweden.
5. Present address: Center for Theoretical Neuroscience, Mortimer B. Zuckerman Mind Brain Behavior Institute, Columbia University, New York, NY, USA.
21 | news || BIOSCIENCE TODAY |
Credit: © RUY GÓMEZ-OCÁDIZ, CHRISTOPH SCHMIDT-HIEBER, INSTITUT PASTEUR
Putting scientific research to better use
the public make more informed decisions
like COVID-19, says Charlie Rapple, co-founder of Kudos.
Misinformation about science and healthcare spreads quickly – and acting on the wrong information can have drastic consequences. Research from The American Journal of Tropical Medicine and Hygiene found that, of the 2,311 reports related to COVID-19 prevention or treatment written between January and March 2020, 88.7 per cent were classified as rumours and 7.8 per cent were conspiracy theories. So, how can we support the wider public in understanding what represents rigorous science research?
When we face emerging global challenges, such as extreme weather or COVID, there are typically knowledge gaps — something particularly evident at the beginning of the pandemic. These can be made worse by the misconception that scientists deal in absolute truths. In reality, science is an ongoing process of discovery that expands our understanding of topics, but may not always provide definitive answers on what each of us should do in relation to global issues. Public trust in scientific research can be undermined by the complexity of findings. However, the pandemic has led to an increase in the public appetite for science. While it might be easy to find information — as of July 2022 there are 3,820,000,000 search results for the term COVID — it’s not always clear which information is trustworthy. When reviewing reports for their paper in The American Journal of Tropical Medicine and Hygiene, for example, researchers found articles offering a range of unfounded COVID prevention measures, from eating garlic to spraying chlorine.
So how do we ensure that the public can find, understand and act on reliable, scientific research?
SCIENCE NEEDS STORIES

People without academic training are unlikely to know where to start when looking for research articles. However, for public interest or health topics, like COVID or climate change, it’s important that people can form opinions based on quality evidence. To support this, we must make it easier for the public to find and understand scientific research.
This requires us to tackle two main problems. The first is that scientific reports are usually written for other scientists. Recommendations are made in academic language that is hard for non-specialists to act on. The second problem is that science is published
across such a wide range of academic publications and university resources. We can tackle both problems with a central platform designed specifically for plain-language explanations of science. Writing stories about research brings it to life so more people can take on board its implications and recommendations. Armed with better understanding, readers can use the important takeaways from the research to make more informed decisions.
Scientific publishers, societies, universities and funders should be working together to fill the knowledge gap, tackle disinformation, and influence how people behave during public health crises.
STORIES CAN ACCELERATE SCIENTIFIC IMPACT

Our success in tackling future pandemics depends on how well we learn from this one. The global public adopted a wide range of new behaviours based on guidance from researchers. We now need to make sure that follow-up research is clearly explained to maintain trust in science in preparation for potential future pandemics.
The rapid response required for the prevention and treatment of illness during the coronavirus pandemic also accelerated research and development in multiple fields. We’ve seen unprecedented research into vaccines, drug development, mask efficacy and more. For example, research published by the American Institute of Physics has shown how advances in machine learning led to the development of low cost microscopes. Explaining these advancements to the public will build on and maintain trust and interest in science.
The coronavirus pandemic also accelerated developments in cheaper and quicker technologies that enable patients to take some aspects of their healthcare into their own hands. Alternative testing techniques, such as rapid PCR tests and the ability to use mobile applications for selfreporting were vital in curbing disease transmission. As a result, smartphones are emerging as a new tool for patient monitoring, contact tracing, data collation or sharing, and performing point-of-care tests. Improving reporting also means that researchers have much larger datasets, enabling them to make significant developments in modelling to better understand the spread of disease. Providing better access to influential research that is easy to understand ensures that whenever we face major social issues, whether it’s another pandemic, climate change or something else. Equipped with better understanding, everyone who plays a role can understand and act on reliable research.
The Kudos research communication platform provides research summaries on a range of topics, including explanations of trustworthy research on Coronavirus and other respiratory and infectious diseases, at growkudos. com/showcase/collections/coronavirus


22 | bio-informatics | | BIOSCIENCE TODAY |
Plain-speaking research summaries could help
in major societal issues
“As of July 2022 there are 3,820,000,000 search results for the term COVID.”
Charlie Rapple, Kudos





23 | bio-informatics || BIOSCIENCE TODAY |
Pain or gain? How the brain chooses
Imagine having to choose over and over between what you enjoy doing and the pain that it might cause you, whether physical or emotional.
If you live with conditions such as depression, anxiety, or chronic pain, you are probably familiar with making these difficult choices on a daily or weekly basis. But surprisingly little is known about which areas of the brain are involved in decisions of this kind.
In a recent article in PNAS, McGill University researchers show that the ventral striatum plays a crucial role when it comes to choices about future pain versus future profit. Interestingly, this brain region has already been identified as being involved in motivation and rewards, but it has not been associated with pain until now. This discovery could advance treatments for a range of disorders that are characterized by excessive avoidance.
To see which areas of the brain were activated during decisions about future pain and profit, participants in the recent study were asked to make choices, very quickly, that involved a certain (random) amount of pain in exchange for a certain (random) amount of profit – or vice versa.
WATCHING THE BRAIN MAKING CHOICES
As participants were asked to choose repeatedly (there were 100 trials) between succeeding offers of
pain or profit, the researchers used brain scans to monitor areas of cerebral activity. They discovered that, although many different areas of the brain were associated with future pain or money offers, there was one particular region, the ventral striatum, that systematically activated or deactivated as a function of future pain or rewards.
By using machine-learning algorithms, the researchers were able to identify patterns of brain activity that allowed them not only to predict the levels of pain and reward on offer, but also whether the participants would accept or reject these offers. They were, in effect, watching the brain making decisions between future pain and profit.

“It was almost like seeing a dimmer switch moving up or down, depending on whether pain or profit was on offer,” said Mathieu Roy, an Associate Professor in McGill’s Psychology Department and the senior author on the paper. “We found that when money was on offer, as expected, activity in the ventral striatum increased. But what was interesting was that activity in the same area of the brain decreased in proportion to the pain on offer. This suggests that there is a shared representation of pain and profit in the ventral striatum, almost a common currency involved in making decisions where you need to compare the two.”
REFERENCE
“The neural signature of the decision value of future pain” by Michel-Pierre
10.1073/pnas.2119931119

Coll et al in PNAS. DOI:
24 | BIOSCIENCE TODAY || clinical trials |
“there is a shared representation of pain and profit in the ventral striatum”
Students’ discovery could help treat Alzheimer’s
The undergraduate students – Nicholas D’Arcy-Evans, Kirill Mamonov, Josephine Stewart and Marek Varga – were all co-authors on a paper published in leading journal Science based on cutting-edge medicinal chemistry research carried out at the Karolinska Institutet in Stockholm, Sweden.
The discovery opens new avenues for the treatment of diseases which result from DNA damage, such as Alzheimer’s disease, certain cancers and lung conditions, and the compounds they have identified should slow down the ageing process.
The study improved the function of a protein called OGG1, an enzyme that repairs oxidative DNA damage using a method called organocatalysis. The researchers examined how catalyst molecules bind to OGG1 and affect its function in cells. One of the molecules proved to be of particular interest.
“When we introduce the catalyst into the enzyme, the enzyme becomes ten times more effective at repairing oxidative DNA damage and can perform a new repair function,” says the study’s first author Maurice Michel, assistant professor at the Department of OncologyPathology, Karolinska Institutet.
Professor David O’Hagan from the University of St Andrews School of Chemistry said: “I acted as the academic liaison with the students from St Andrews over this period. Despite the pandemic they have conducted impressive and clearly important research and they have been supported by an amazing team of scientists at the Karolinska Institutet. It has been a pleasure to observe what can be achieved even at an early career stage in science when involved in a dynamic team.”
The work was conducted as part of the Industrial Placement year of the students’ MChem degrees and took place over several years in the laboratory of Professor Thomas Helleday.

Three of the students have now graduated, while one –Nicholas D’Arcy-Evans – will resume the final year of their degree in September. Kiril Mamonov graduated in 2021 and is working in Poland, Josephine Stewart will start a PhD in St Andrews in September, and Marek Varga, who worked on placement at the Karolinska when he was a student at Edinburgh University, is now entering the second year of his PhD in St Andrews.
Nicholas said: “To be involved in work like this was far beyond anything I expected during the placement year. It was fantastic to learn from and work alongside leading scientists to answer such a challenging question. We believe that the discovery may shift the way we approach medicinal chemistry – where drugs are designed for enzyme activation, rather than inhibition. I’m excited to see where this goes and how it can help patients in the future.”
Dr Georg Haehner, student placement coordinator, said: “In our placement programme we collaborate with industrial companies and research labs to offer our students the opportunity to work on a scientifically challenging project in an industrial research environment as part of their degree.
“The Helleday Labs in Sweden are among the world leaders in developing novel treatments for cancer using medicinal chemistry. For this reason, we are delighted to engage with their team, where our students gain practical professional experience alongside some of the best researchers in medicinal chemistry, which is reflected by the publication.”
The paper ‘Small molecule activation of OGG1 increases repair of oxidative DNA damage by gaining a new function’ is published in Science and is available online. (DOI: 10.1126/science.abf8980).
25 | BIOSCIENCE TODAY | | disease focus |
Four chemistry students from the University of St Andrews have helped to discover a class of molecules that can help accelerate DNA repair which could in turn help treat of diseases such as Alzheimer’s.
Undergraduates in the School of Chemistry, University of St Andrews.

26 | BIOSCIENCE TODAY || neuroscience |
The neural switch, seen in Egyptian fruit bats, happened within 100 milliseconds. Photo by Brock Fenton
Neurons are caught rapidly switching gears
A new study suggests that neurons are not programmed for a single task but are actually highly dynamic and agile.

27 | BIOSCIENCE TODAY | | neuroscience |
Even during such routine tasks as a daily stroll, our brain sometimes needs to shift gears, switching from navigating direction to avoiding traffic or crossing the street to see a friend. These switches pose a challenge: How do the brain’s circuits deal with such dynamic and abrupt changes in behavior? A Weizmann Institute of Science study on bats, published in Nature, suggests an answer that does not fit the classical thinking about brain function.
“Most brain research projects focus on one type of behavior at a time, so little is known about the way the brain handles dynamically changing behavioral needs,” says Prof. Nachum Ulanovsky of Weizmann’s Brain Sciences Department.

In the study, he and his team designed an experimental setup that mimicked real-life situations in which animals or humans rapidly switch from one behavior to another – for example, from navigation to avoiding a predator or a car crash. Graduate students Dr. Ayelet Sarel, Shaked Palgi and Dan Blum led the study, in collaboration with postdoctoral fellow Dr. Johnatan Aljadeff. The study was supervised by Ulanovsky together with Associate Staff Scientist Dr. Liora Las.
Using miniature wireless recording devices, the researchers monitored neurons in the brains of pairs of bats that had to avoid colliding with one another while flying toward each other along a 135-meter-long tunnel at the high speed of 7 meters per second. This amounted to a relative speed – that is, the rate at which the distance between the bats closed, or the sum of both bats’ speeds – of 14 metres per second, or about 50 kilometres an hour.
To check whether the bats switched their behavioral mode, the researchers took advantage of their unique ability to sense their environment using sonar, or echolocation. Indeed, when spotting another animal flying rapidly toward them, the bats promptly raised their rate of echolocation clicks, signifying elevated attention levels.
As their attention increased, a rapid shift occurred in the neural circuits in the bats’ hippocampus, the main brain

area responsible for navigation, among other functions. The scientists discovered this shift by recording electrical signals from individual neurons in this area, known as place cells.
When the bats were flying solo, their place cells encoded their location in space, but as soon as the animals spotted the other, fast-approaching bat, more than half of the neurons in the hippocampus – about 55 percent – switched modes.
The scientists could tell that the neural switch had taken place because the neurons’ firing pattern changed, indicating that they now encoded not only the bat’s own, absolute location but also a relative measure: the distance to the other bat. The higher the animal’s attention, the more pronounced the neural switch.
To the scientists’ surprise, this switch occurred extremely rapidly, within some 100 milliseconds, or one-tenth of a second. Whether the fast-approaching bat was a regular, familiar partner or a mere ‘acquaintance’ had no effect on the neural coding, suggesting that the switch was intended to avoid a collision and had nothing to do with social behavior.
“Our study suggests that we may need to revise some basic assumptions about the brain’s circuits,” Ulanovsky says. He explains that over the past century, the prevailing view was that each brain region performs its own function, and that different behaviors are encoded in different parts of the brain.
According to this classical notion, one may expect that when a switch occurs in behaviour, for example, from navigation to collision avoidance, different brain regions would ‘light up’ one after another. The new study reveals an entirely different picture: an amazingly fast switch in neural coding not just within the same brain area but in the same neurons.
Ulanovsky adds: “Of course, the division of labour between different brain regions still holds. If the visual cortex, for example, is damaged, the result is visual
28 | BIOSCIENCE TODAY || neuroscience |
Dan Blum and Dr. Johnatan Aljadeff helped lead the Weizmann Institute of Science study.
impairment – not deafness or loss of smell. But we’ve shown in our new study that the brain is much more dynamic than previously thought. Contrary to most models of the brain, which assume that neurons have a stable function, we’ve found that in response to a rapid switch in behavioral needs, neurons can very rapidly switch to performing a totally new function.”
Some parts of the brain have already been shown to be less rigidly determined than thought – a phenomenon known as plasticity – but this occurs on slower timescales,
reflecting longer-term biochemical changes in the brain’s synapses, the connections between neurons. In contrast, the newly revealed switch works much faster, probably reflecting a swift reorganisation in neural network activity.
Future studies may look for switches between different neural coding modes in various brain areas, not only in the hippocampus, and in a wide variety of behaviors and situations. These studies might also ask how prevalent this switching is, and whether it slows down in the aging brain.
Yet another compelling research direction raised by the study is learning how the brain keeps our reality from appearing fragmented. “Now that we know that neurons can change what they do within one-tenth of a second, it would be fascinating to find out how we still perceive the world as smooth and stable,” Ulanovsky says.
Prof. Nachum Ulanovsky is the incumbent of the Barbara and Morris L. Levinson Professorial Chair in Brain Research. The Ben B. and Joyce Eisenberg Foundation Research Fellow Chair in Neuroscience supports a staff scientist in his lab. Prof. Ulanovsky’s research is supported by Dita and Yehuda L. Bronicki.

29 | BIOSCIENCE TODAY | | neuroscience |
“An amazingly fast switch in neural coding can occur not just within the same brain area but in the same neurons.”
Shaked Palgi, Dr. Ayelet Sarel, Dr. Liora Las and Prof. Nachum Ulanovsky.
New treatment target for neurodegenerative diseases
Long-term neurological conditions like Alzheimer’s Disease could be treated using a completely new drug strategy.
Researchers at the University of Birmingham have identified a potential target for drugs to treat serious neurological conditions and foster nerve regeneration in central nervous system injuries.
Their research, published in Science Advances, is part of a workstream exploring the signalling pathways that respond to DNA damage, which is seen in both long-term neurological conditions and after traumatic injury, such as to the spinal cord or optic nerve.
Regardless of the cause, nerve cells appear unable to fully repair this damage, and the cell’s DNA damage response (DDR) system is activated. The persistent activation of this system affects nervous system function and potentially triggers programmed cell death (apoptosis).
Professor Zubair Ahmed, from the University of Birmingham’s Institute of Inflammation and Ageing, and Dr Richard Tuxworth from the Institute of Cancer and Genomic Sciences, set out to elucidate how this takes place.
Professor Ahmed explained: “Our research started out exploring DNA damage pathways activated following nerve injury. However, the same molecular factors featured in
pathways are seen in neurodegenerative diseases, and a full understanding of these mechanisms is an important step towards identifying potential targets for drug treatments.”
The latest research paper follows two recently published studies in spinal cord injury from the same research group. These studies showed an existing drug may reduce damage after spinal cord injury, by blocking the inflammatory response in the spinal cord, and brain-penetrating candidate drug currently in development as a cancer therapy can foster nerve repair after injury.
The current study focussed on pathways that include enzymes called Checkpoint kinase 1 (Chk-1) and Checkpoint kinase 2 (Chk-2), which act as gatekeepers to the DDR system, and can be inhibited using small molecules called Checkpoint kinase inhibitors (Chk-is).
The researchers first looked at a fruit fly model of amyloid toxicity, which occurs in neurological diseases when abnormal levels of this naturally occurring protein clump together and disrupt nerve cell function. Here the researchers found that reducing Chk1 or Chk2 expression had a protective effect.
30 | BIOSCIENCE TODAY || diagnostics |
They then switched their attention to investigating whether these findings could be reproduced in animal neurotrauma models of optic nerve damage and spinal cord injury.



Optic nerve damage was of interest to the researchers because it occurs in patients with glaucoma, multiple sclerosis, Alzheimer’s and Parkinson’s disease.
Here the animal models showed that administration of Chk2is promoted nerve cell survival, and resulted in significant nerve regeneration with improved function of the optic nerve after injury.
These results were mirrored in spinal cord injury, where administration into the spinal canal promoted significant regrowth of nerve cells beyond the site of the injury, and, at three weeks, a full restoration of the previously impaired sensation and movement.
Dr Tuxworth added: “This study raises the possibility of a completely new treatment strategy for a variety of neurodegenerative diseases, that is aimed at supporting nervous system function and slowing the progression of disease.”
University of Birmingham Enterprise has filed patent applications covering pathways and mechanisms disclosed in all three research papers and is now seeking investment, and partners for commercial development or licencing.
REFERENCE
Inhibition of Chk2 promotes neuroprotection, axon regeneration and functional recovery 4 after CNS injury is published in Science Advances, and available at doi. org/10.1126/sciadv.abq2611
ABOUT THE RESEARCH
The researchers examined the effect of inhibitors of the Checkpoint kinase 1 and Checkpoint kinase 2 (Chk-1i and Chk-2i) pathways in cell culture and in vivo models. Results from Drosophila studies showed a protective effect against chronic amyloid toxicity when the Chk1 or Chk2 pathways were targeted, indicating a role for double-stranded DNA break signalling. Results from cell culture showed that several Chk-2 inhibitors including, CCT241533, BML-277 and Prexasertib all had a positive effect on dorsal root ganglion neuron survival and growth, whereas the Chk1i inhibitor, LY2603618, had no effect on cell survival or growth. In vivo studies with all of the Chk2i, including Prexasertib, showed significant axon regeneration and functional recovery in models of spinal cord injury and optic nerve injury.
31 | BIOSCIENCE TODAY | | diagnostics |
Professor Zubair Ahmed, Professor of Neuroscience
Dr Richard Tuxworth, Associate Professor
New life sciences cluster in Paddington will champion health partnerships

Imperial College Healthcare NHS Trust has set out its vision for a new life sciences cluster in Paddington, founded on its growing partnerships with research, industry and community organisations around St Mary’s Hospital.


The Trust unveiled its ‘Paddington Life Sciences’ vision on a new website, including three imminent initiatives:
A new digital collaboration space, opening in October 2022, located at Sheldon Square, next to St Mary’s and Paddington station. Housing the National Institute for Health and Care Research (NIHR) Imperial Biomedical Research Centre’s (BRC) expanding digital health programme team, it will provide space for lectures, training, events and meetings. It will also benefit from additional investment in Imperial College Healthcare’s trusted data environment which is already helping to produce important, new clinical insights.
The creation of a new centre for clinical infection, a specialist clinical and translational research facility to complement Imperial College London’s new Institute of Infection. Together they will be one of only a few resources in the world to offer ‘end-to-end’ innovation, from initial discovery to improved patient outcomes, for the management of infectious diseases as well as antimicrobial resistance.
Paddington Life Sciences Partners, bringing together NHS, academic, local authority and life sciences industry partners with a commitment to the area to help ensure the delivery of major social, health and commercial value as quickly as possible.
For the longer term, the Trust is progressing a full redevelopment of the St Mary’s estate as part of the government’s new hospital programme. As well as delivering a new, state-of-the-art hospital, the redevelopment is intended to create an additional 1.5 million square feet of cross-functional commercial and lab space for life sciences businesses to start, develop and grow.
Imperial College Healthcare chief executive Professor Tim Orchard said: “Research and innovation are fundamental to the clinical excellence our hospitals are renowned for, from the Nobel Prize-winning discoveries of penicillin and the chemical structure of antibodies and the invention of the electrocardiogram, to pioneering robotic surgery, HIV care and the clinical use of virtual reality technology. Most recently, we have played a key role in developing understanding of Covid-19 and trialling a range of new treatments. “We are now entering a new era of discovery, at an even more ambitious scale, by maximising the potential of our existing work areas and joining them up with new opportunities. With Imperial College London, we run one of the largest NIHR biomedical research centres, undertaking hundreds of clinical trials and analysing data from well over a million patient contacts each year.
| BIOSCIENCE TODAY | 32 | life science |
The St Mary’s Hospital/ Paddington site where the cluster is to be based.
“Through the pandemic, many more patients and staff have been encouraged to get involved in research and we are confident this trend will grow as we continue to deepen our relationships with local communities and organisations. We are working together to improve not just healthcare but also health and wellbeing, creating synergies that will boost education, skills development and employment opportunities in some of the most deprived areas of the UK.
“The regeneration of Paddington is also drawing more and more life sciences and technology businesses to the area, attracted by investment in transport infrastructure and excellent national and international travel connections.

This also means strong links to other life sciences hubs, including Imperial College London’s growing campus at White City, adjacent to another of our own campuses, Hammersmith Hospital, and the knowledge quarter in King’s Cross and Euston.
“And this is all on top of all our major investment and redevelopment plans for the St Mary’s campus.”
Dr Bob Klaber, Imperial College Healthcare director of strategy, research and innovation added: “British life sciences firms raised £4.5bn in 2021, up from just £261m in 2012. But London has not yet reached its full potential to attract investment and innovation in the life sciences sector.
“MedCity’s 2021 London Life Sciences Real Estate Demand Report identified an estimated 500,000 square feet shortfall in innovation and lab space. Imperial College Healthcare is ideally placed to help fill that gap.”
Paddington Life Sciences, and the new digital collaboration space, are formally launched in October.
| BIOSCIENCE TODAY | 33 | | life science |
“British life sciences firms raised £4.5bn in 2021, up from just £261m in 2012.”
New algorithm uncovers cell factory secrets
Drug molecules and biofuels can be made to order by living cell factories, where biological enzymes do the job.
The researchers tested their model by simulating metabolism in more than 300 types of yeasts. When compared with measured, pre-existing knowledge, the researchers concluded that models with predicted kcat values could accurately simulate metabolism. The image shows common baker’s yeast, Saccharomyces cerevisiae
Researchers at Chalmers University of Technology have developed a computer model that can predict how fast enzymes work, making it possible to find the most efficient living factories and study difficult diseases.
Enzymes are proteins found in all living cells. Their job is to act as catalysts that increase the rate of specific chemical reactions that take place in the cells. The enzymes thus play a crucial role in making life on earth work and can be compared to nature’s small factories.
They are also used in detergents, and to manufacture, among other things, sweeteners, dyes and medicines. The potential uses are almost endless but are hindered by the fact that it is expensive and time-consuming to study the enzymes.
“To study every natural enzyme with experiments in a laboratory would be impossible, they are simply too many. But with our algorithm, we can predict which enzymes are most promising just by looking at the sequence of amino acids they are made up of”, explains Eduard Kerkhoven, researcher in systems biology at Chalmers University of Technology and the study’s lead author.
ONLY THE MOST PROMISING ENZYMES NEED TO BE TESTED
The enzyme turnover number, or kcat value, describes how fast and efficient an enzyme works and is essential for understanding a cell’s metabolism. In the new study, Chalmers researchers developed a computer model that can quickly calculate the kcat value.
The only information needed is the order of the amino acids that build up the enzyme - something that is often widely available in open databases. After the model makes a first selection, only the most promising enzymes need to be tested in the lab.
Given the number of naturally occurring enzymes, the researchers believe that the new calculation model may be of great importance.
“We see many possible biotechnological applications. As an example, biofuels can be produced when enzymes break down biomass in a sustainable manufacturing process. The algorithm can also be used to study diseases in the metabolism, where mutations can lead to defects in how enzymes in the human body work”, Eduard adds.
MORE KNOWLEDGE ON ENZYME PRODUCTION
Other possible applications are more efficient production of products made from natural organisms, as opposed to industrial processes. Penicillin extracted from a mould is one such example, as well as the cancer drug taxol from yew and the sweetener stevia. They are typically produced in low amounts by natural organisms.
“The development and manufacture of new natural products can be greatly helped by knowledge of which enzymes can be used”, Eduard says.
The calculation model can also point out the changes in kcat value that occur if enzymes mutate, and identify unwanted amino acids that can have a major impact on an enzyme’s efficiency. The model can also predict whether the enzymes produce more than one “product”.
“We can reveal if the enzymes have any ‘moonlighting’ activities and produce metabolites that are not desirable. It is useful in industries where you often want to manufacture a single pure product.”
The researchers tested their model by using 3 million kcat values to simulate metabolism in more than 300 types of yeasts. They created computer models of how fast the yeasts could grow or produce certain products, like ethanol. When compared with measured, pre-existing knowledge, the researchers concluded that models with predicted kcat values could accurately simulate metabolism.
The study Deep learning based kcat prediction enables improved enzyme constrained model reconstruction has been published in Nature Catalysis. The authors are Feiran Li, Le Yuan, Hongzhong Lu, Gang Li, Yu Chen, Martin Engqvist, Eduard Kerkhoven and Jens Nielsen. The researchers are active at Chalmers University of Technology.
34 | BIOSCIENCE TODAY || news |
Digital gulf will widen between ‘leaders and laggards’
A Siemens commissioned study has identified the UK and Irish biopharmaceutical sector’s key areas for priority investment in digitalisation, with the challenges of time to market and making sense of large amounts of data running as a constant thread.



The independent research engaged 31 key players in the UK and Irish biopharmaceutical sector to discover key areas for business improvement. The study was conducted between late 2019 and early 2022, covering the period affected by the global pandemic.
The key findings indicate that digital transformation in biopharmaceuticals is expected to pick up speed across the rest of the decade, with an increasing gulf likely to open up between leaders and laggards. Translating digitalisation into value for Pharma and Life Sciences manufacturing remains a key focus for many but the challenges of speed and efficiency need to be addressed with urgency.
The respondents also agreed that ‘Big Data’ and data analytics are crucial for driving quality and efficiency, but it is vital to convert infinite data into valuable insight through solutions like BioMAC from Siemens, while the National Institute of Bioprocessing Research and Training (NIBRT) is helping organisations to translate data into meaningful and actionable information.
The findings also reveal that with much of pharmaceutical manufacturing still employing heavy use of paper-based records, the adoption of electronic batch records can help streamline process operations, improve regulatory compliance and reduce operational costs.
Andrew Matthews, Head of Pharmaceuticals, Siemens, says: “The concept of a Digital Twin for pharma and life sciences is regrettably not widespread. At a recent ISPE event, it surprised me that only about a fifth of the audience had heard of a Digital Twin, which means many have likely never heard of Industry 4.0 or Pharma 4.0! This short research underlines areas for consideration as identified from the wider industry and is designed to spark further discussion.”
Adrian Abbotts, Business Development Manager for Cell and Gene Therapies, Siemens, adds: “Working with our customers to understand their challenges, we have mapped out the sector’s business demands and aligned our solutions to mitigate them. Patients, products, pace of change and performance are areas of focus for our clients as they try to comprehend global influences such as changing demographics, new manufacturing techniques, tech transfer, supply chain complexity and outsourcing.”
The majority of interviewees highlighted the critical importance of compressing the drug discovery process; in particular, using Industry 4.0 technology to accelerate
the speed of new molecule development, such as through computational chemistry and data-driven discovery.
Connecting data ‘silos’ and enabling meaningful data flows is driving moves towards greater standardisation in the industry. One bio-manufacturing technology company noted, “By introducing a new automation platform and further standardising our products, we are making it easy for our customers to integrate their systems into higherlevel automation solutions.”
Visibility of digital data flows in all aspects of biopharma –“from concept to clinic” – is also contributing to regulatory supervision, reducing the burden on biopharma companies, and at the same time increasing hard-pressed regulators’ and notified bodies’ ability to scrutinise the industry. “It’s only a matter of time before we see remote inspections by the regulators becoming the norm,” said one respondent.
The UK market, specifically, is estimated to surpass £12bn in 2022, with worldwide markets expecting an average growth rate of around 10% per year. The UK and Ireland remain the number one destination for life sciences inward investment in Europe, ranks number two globally behind the US, and has also grown a thriving domestic industry with more than 5,600 companies producing some of the strongest research and development capability in Europe.
35 | BIOSCIENCE TODAY | | news |
“At a recent ISPE event … only about a fifth of the audience had heard of a Digital Twin, which means many have likely never heard of Industry 4.0 or Pharma 4.0!” andrew matthews, siemens
Andrew Matthews, Head of Pharmaceuticals at Siemens
Adrian Abbotts, Business Development Manager for Cell & Gene Therapies, Siemens
36 | BIOSCIENCE TODAY || stem cell research |
Synthetic mouse embryo models created from stem cells
An egg meets a sperm -- the necessary first step for life and a common first step in embryonic development research. But now researchers have grown synthetic embryo models of mice outside the womb with stem cells cultured in a petri dish – without using fertilised eggs.
37 | BIOSCIENCE TODAY | | stem cell research |
The Weizmann Institute of Science study published in Cell opens new horizons for studying how stem cells form various organs in the developing embryo, and may one day make it possible to grow tissues and organs for transplantation using synthetic embryo models.
“The embryo is the best organ-making machine and the best 3D bioprinter – we tried to emulate what it does,” says Prof. Jacob Hanna of Weizmann’s Molecular Genetics Department, who headed the research team.
He explains that scientists already know how to restore mature cells to ‘stemness’ – pioneers of this cellular reprogramming had won a Nobel Prize in 2012. But going in the opposite direction, that is, causing stem cells to differentiate into specialised body cells, not to mention form entire organs, has proved much more problematic.
“Until now, in most studies, the specialised cells were often either hard to produce or aberrant, and they tended to form a mishmash instead of well-structured tissue suitable for transplantation. We managed to overcome these hurdles by unleashing the self-organisation potential encoded in the stem cells.”
Hanna’s team built on two previous advances in his lab. One was an efficient method for reprogramming stem cells back to a naïve state – that is, to their earliest stage – when they have the greatest potential to specialise into different cell types.
The other, described in a scientific paper in Nature in March 2021, was the electronically controlled device the team had developed over seven years of trial and error for growing natural mouse embryos outside the womb. The device keeps the embryos bathed in a nutrient solution inside of beakers that move continuously, simulating the way nutrients are supplied by material blood flow to the placenta, and closely controls oxygen exchange and atmospheric pressure. In the earlier research, the team had successfully used this device to grow natural mouse embryos from day 5 to day 11.
In the new study, the team set out to grow a synthetic embryo model solely from naïve mouse stem cells that had been cultured for years in a petri dish, dispensing with the need for starting with a fertilied egg. This approach is extremely valuable because it could, to a large extent, bypass the technical and ethical issues involved in the use of natural embryos in research and biotechnology. Even in the case of mice, certain experiments are currently unfeasible because they would require thousands of embryos, whereas access to models derived from mouse embryonic cells, which grow in lab incubators by the millions, is virtually unlimited.
Before placing the stem cells into the device, the researchers separated them into three groups. In one, which contained cells intended to develop into embryonic organs themselves, the cells were left as they were. Cells in the other two groups were pre-treated for 48 hours to overexpress one of two types of genes: master regulators of either the placenta or the yolk sac. “We gave these two groups of cells a transient push to give rise to extraembryonic tissues that sustain the developing embryo,” Hanna says.
Development of synthetic embryo models from day 1 (top left) to day 8 (bottom right). All their early organ progenitors had formed, including a beating heart, an emerging blood circulation, a brain, a neural tube and an intestinal tract.
Soon after being mixed together inside the device, the three groups of cells convened into aggregates, the vast majority of which failed to develop properly. But about 0.5 percent – 50 of around 10,000 – went on to form spheres, each of which later became an elongated, embryo-like structure.
Since the researchers had labelled each group of cells with a different colour, they were able to observe the placenta and yolk sacs forming outside the embryos and the model’s development proceeding as in a natural embryo. These synthetic models developed normally until day 8.5 – nearly half of the mouse 20-day gestation – at which stage all the early organ progenitors had formed, including a beating heart, blood stem cell circulation, a brain with well-shaped folds, a neural tube and an intestinal tract. When compared to natural mouse embryos, the synthetic models displayed a 95 percent similarity in both the shape of internal structures and the gene expression patterns of different cell types. The organs seen in the models gave every indication of being functional.
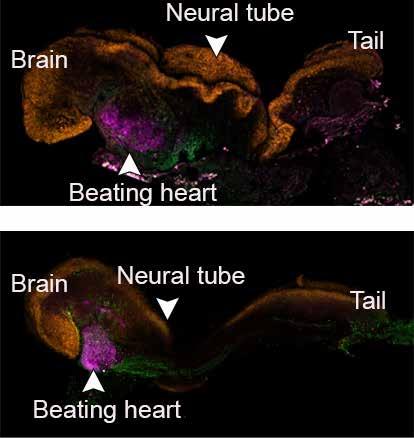
Day 8 in the life of a mouse embryo: a synthetic model (top) and a natural embryo (bottom). The synthetic models displayed a 95 percent similarity in both the shape of internal structures and the gene expression patterns of different cell types

For Hanna and other stem cell and embryonic development researchers, the study presents a new arena: “Our next challenge is to understand how stem cells know what to do – how they self-assemble into organs and find their way to their assigned spots inside an embryo. And because our system, unlike a womb, is transparent, it may prove useful for modelling birth and implantation defects of human embryos.”
As well as helping reduce the use of animals in research,
38 | BIOSCIENCE TODAY || stem cell research |
“As well as helping reduce the use of animals in research, synthetic embryo models might in the future become a reliable source of cells, tissues and organs for transplantation.”
synthetic embryo models might in the future become a reliable source of cells, tissues and organs for transplantation. “Instead of developing a different protocol for growing each cell type – for example, those of the kidney or liver – we may one day be able to create a synthetic embryo-like model and then isolate the cells we need. We won’t need to dictate to the emerging organs how they must develop. The embryo itself does this best.”
This research was co-led by Shadi Tarazi, Alejandro Aguilera-Castrejon and Carine Joubran of Weizmann’s Molecular Genetics Department. Study participants also included Shahd Ashouokhi, Dr. Francesco Roncato, Emilie Wildschutz, Dr. Bernardo Oldak, Elidet Gomez-Cesar, Nir Livnat, Sergey Viukov, Dmitry Lokshtanov, Segev Naveh-Tassa, Max Rose and Dr. Noa Novershtern of Weizmann’s Molecular Genetics Department; Montaser Haddad and Prof. Tsvee Lapidot of Weizmann’s Immunology and Regenerative Biology Department; Dr. Merav Kedmi of Weizmann’s Life Sciences Core Facilities Department; Dr. Hadas Keren-Shaul of the Nancy and Stephen Grand Israel National Center for Personalized Medicine; and Dr. Nadir Ghanem, Dr. Suhair Hanna and Dr. Itay Maza of the Rambam Health Care Campus.

Prof. Jacob Hanna’s research is supported by the Dr. Barry Sherman Institute for Medicinal Chemistry; the Helen and Martin Kimmel Institute for Stem Cell Research; and Pascal and Ilana Mantoux.
A diagram showing the innovative method for growing synthetic mouse embryo models from stem cells – without egg, sperm or womb – developed in the laboratory of Prof. Jacob Hanna

VIDEO LINK
A video showing a synthetic mouse embryo model on day 8 of its development; it has a beating heart, a yolk sac, a placenta and an emerging blood circulation. Click here to watch
39 | BIOSCIENCE TODAY | | stem cell research |
From left: Dr. Noa Novershtern, Prof. Jacob Hanna, Alejandro Aguilera-Castrejon, Shadi Tarazi and Carine Joubran
Science employers want an end to ‘endless changes’ in technical education
Endless changes to apprenticeship and technical education presents a serious risk to the development of future talent, according to a leading group of UK science sector employers.
The Science Industry Partnership (SIP), has published a Skills Manifesto for Technical Education and Workplace Learning which calls for a number of reforms in order to maintain trust, credibility and clarity around the development of technical skills.

Despite the science sector investing heavily in degree-level apprenticeships – with more than 1,070 starts in the three years between 2018/19 and 2020/211. – a third of employers surveyed agreed that changes to degree level apprenticeship policy make them nervous for the future.
Employers are keen to ensure that degree apprenticeship content continues to offer the same breadth and depth of knowledge as a graduate qualification. Employers (67%) also expressed concern about the growing trend to remove qualifications from apprenticeship standards, which may negatively affect the overall perception of apprenticeships. And 69% of employers want greater flexibility on how they spend their Apprenticeship Levy.
Malcolm Skingle, Chair of the SIP board and Academic Liaison Director GSK, said: “Apprenticeships are a key route into the science sector, yet endless changes to
technical education policy presents a serious risk to the development of skills.
“Despite industry concerns, shifting technical education policy has already led to the removal of mandatory qualifications for some apprenticeship standards. This raises the risk of a two-tiered apprenticeship system developing, which could potentially undermine our globally- recognised, accredited qualification system and harm the international transfer of talent, which is so important to our globally competitive science sector.
“A fairer funded and more flexible apprenticeship system would provide a greater incentive for employer investment in future talent pipelines. The ability to use existing levy funds to access additional short course training and qualifications along with a relaxation of off the job training requirements for existing employees needs to be addressed.”
The manifesto also highlights a sharp decline in the number of science apprenticeship starts at SMEs (falling by almost 72% between the 2015/16 and 2020/21 academic years, as highlighted by the Government’s own figures). Reversing this will require a greater use of incentives alongside a dedicated sector specific SME support service to help science-based businesses grow their talent pool.
Read the Manifesto here.
The Science Industry Partnership (SIP) is an influential member-led alliance, representing science industry companies on skills issues.
Details at cogentskills.com/membership/sip
1. Department for Education: Apprenticeships in England by industry characteristics, Academic Year 2020/21
40 | news | | BIOSCIENCE TODAY |
“Despite industry concerns, shifting technical education policy has already led to the removal of mandatory qualifications for some apprenticeship standards.”

https://calendly.com/ cooden https://calendly.com/cooden/dicovery-bus-edge 0300 373 0026
gold nanoparticles screening chemicals uorescent microparticles 99.999% ruthenium spheres process synthesis cryo-electron microscopy transparent ceramics diamond micropowder alternative energy dysprosium pellets sputtering targets 3D graphene foam tungsten carbide refractory metals glassy carbon macromolecules isotopes epitaxial crystal growth drug discovery biosynthetics quantum dots superconductors spectroscopy metamaterials platinum ink Fe3O4 rare earth metalsspintronics thin lm osmium buckyballs zeolites Nd:YAG enantioselective catalysts dielectrics deposition slugs organometallics janus particles optical glass © 2001-2022. American Elements is a U.S.Registered Trademark mesoporous silica chalcogenides laser crystals calcium wiresindicator dyes graphene oxide shift reagents ferro uid metallocenes CVD precursors silver nanoparticles MOFs palladium catalysts nickel foam bioactive compounds III-IV semiconductors metal carbenes ITO pharmacoanalysis nano ribbons nanogels surface functionalized nanoparticles cermet anode excipients ultralight aerospace alloys rhodium sponge nanodispersions cisplatin conjugated nanostructures h-BN MOCVD BINAP InGaAs NMR ZnS AuNPs EuFOD YBCOMBE Now Invent. TM The Next Generation of Material Science Catalogs American Elements opens a world of possibilities so you can Now Invent! Over 35,000 certified high purity laboratory chemicals, metals, & advanced materials and a state-of-the-art Research Center. Printable GHS-compliant Safety Data Sheets. Thousands of new products. And much more. All on a secure multi-language "Mobile Responsive” platform. www.americanelements.com 1.00794 Hydrogen 1 1 H 6.941 Lithium 3 2 1 Li 9.012182 Beryllium 4 2 2 Be 22.98976928 Sodium 11 2 8 1Na 24.305 Magnesium 12 2 8 2Mg 39.0983 Potassium 19 2 8 8 1K 40.078 Calcium 20 2 8 8 2Ca 85.4678 Rubidium 37 2 8 18 8 1Rb 87.62 Strontium 38 2 8 18 8 2Sr 132.9054 Cesium 55 2 8 18 18 8 1Cs 137.327 Barium 56 2 8 18 18 8 2Ba (223) Francium 87 2 8 18 32 18 8 1 Fr (226) Radium 88 2 8 18 32 18 8 2 Ra 44.955912 Scandium 21 2 8 9 2Sc 47.867 Titanium 22 2 8 10 2Ti 50.9415 Vanadium 23 2 8 11 2V 51.9961 Chromium 24 2 8 13 1Cr 54.938045 Manganese 25 2 8 13 2Mn 55.845 Iron 26 2 8 14 2Fe 58.933195 Cobalt 27 2 8 15 2Co 58.6934 Nickel 28 2 8 16 2Ni 63.546 Copper 29 2 8 18 1Cu 65.38 Zinc 30 2 8 18 2Zn 88.90585 Yttrium 39 2 8 18 9 2Y 91.224 Zirconium 40 2 8 18 10 2Zr 92.90638 Niobium 41 2 8 18 12 1Nb 95.96 Molybdenum 42 2 8 18 13 1Mo (98.0) Technetium 43 2 8 18 13 2Tc 101.07 Ruthenium 44 2 8 18 15 1Ru 102.9055 Rhodium 45 2 8 18 16 1Rh 106.42 Palladium 46 2 8 18 18Pd 107.8682 Silver 47 2 8 18 18 1Ag 112.411 Cadmium 48 2 8 18 18 2Cd 138.90547 Lanthanum 57 2 8 18 18 9 2La 178.48 Hafnium 72 2 8 18 32 10 2Hf 180.9488 Tantalum 73 2 8 18 32 11 2Ta 183.84 Tungsten 74 2 8 18 32 12 2W 186.207 Rhenium 75 2 8 18 32 13 2Re 190.23 Osmium 76 2 8 18 32 14 2Os 192.217 Iridium 77 2 8 18 32 15 2Ir 195.084 Platinum 78 2 8 18 32 17 1Pt 196.966569 Gold 79 2 8 18 32 18 1Au 200.59 Mercury 80 2 8 18 32 18 2Hg (227) Actinium 89 2 8 18 32 18 9 2 Ac (267) Rutherfordium 104 2 8 18 32 32 10 2 Rf (268) Dubnium 105 2 8 18 32 32 11 2 Db (271) Seaborgium 106 2 8 18 32 32 12 2Sg (272) Bohrium 107 2 8 18 32 32 13 2 Bh (270) Hassium 108 2 8 18 32 32 14 2 Hs (276) Meitnerium 109 2 8 18 32 32 15 2 Mt (281) Darmstadtium 110 2 8 18 32 32 17 1 Ds (280) Roentgenium 111 2 8 18 32 32 18 1Rg (285) Copernicium 112 2 8 18 32 32 18 2 Cn 4.002602 Helium 2 2 He 10.811 Boron 5 2 3 B 12.0107 Carbon 6 2 4 C 14.0067 Nitrogen 7 2 5 N 15.9994 Oxygen 8 2 6 O 18.9984032 Fluorine 9 2 7 F 20.1797 Neon 10 2 8 Ne 26.9815386 Aluminum 13 2 8 3Al 28.0855 Silicon 14 2 8 4Si 30.973762 Phosphorus 15 2 8 5P 32.065 Sulfur 16 2 8 6S 35.453 Chlorine 17 2 8 7Cl 39.948 Argon 18 2 8 8Ar 69.723 Gallium 31 2 8 18 3Ga 72.64 Germanium 32 2 8 18 4Ge 74.9216 Arsenic 33 2 8 18 5As 78.96 Selenium 34 2 8 18 6Se 79.904 Bromine 35 2 8 18 7Br 83.798 Krypton 36 2 8 18 8Kr 114.818 Indium 49 2 8 18 18 3In 118.71 Tin 50 2 8 18 18 4Sn 121.76 Antimony 51 2 8 18 18 5Sb 127.6 Tellurium 52 2 8 18 18 6Te 126.90447 Iodine 53 2 8 18 18 7I 131.293 Xenon 54 2 8 18 18 8Xe 204.3833 Thallium 81 2 8 18 32 18 3Tl 207.2 Lead 82 2 8 18 32 18 4Pb 208.9804 Bismuth 83 2 8 18 32 18 5Bi (209) Polonium 84 2 8 18 32 18 6Po (210) Astatine 85 2 8 18 32 18 7At (222) Radon 86 2 8 18 32 18 8Rn (284) Nihonium 113 2 8 18 32 32 18 3 (289) Flerovium 114 2 8 18 32 32 18 4 Fl (288) Moscovium 115 2 8 18 32 32 18 5 (293) Livermorium 116 2 8 18 32 32 18 6 Lv (294) Tennessine 117 2 8 18 32 32 18 7 (294) Oganesson 118 2 8 18 32 32 18 8 140.116 Cerium 58 2 8 18 19 9 2Ce 140.90765 Praseodymium 59 2 8 18 21 8 2Pr 144.242 Neodymium 60 2 8 18 22 8 2Nd (145) Promethium 61 2 8 18 23 8 2Pm 150.36 Samarium 62 2 8 18 24 8 2Sm 151.964 Europium 63 2 8 18 25 8 2Eu 157.25 Gadolinium 64 2 8 18 25 9 2Gd 158.92535 Terbium 65 2 8 18 27 8 2Tb 162.5 Dysprosium 66 2 8 18 28 8 2Dy 164.93032 Holmium 67 2 8 18 29 8 2Ho 167.259 Erbium 68 2 8 18 30 8 2Er 168.93421 Thulium 69 2 8 18 31 8 2Tm 173.054 Ytterbium 70 2 8 18 32 8 2Yb 174.9668 Lutetium 71 2 8 18 32 9 2Lu 232.03806 Thorium 90 2 8 18 32 18 10 2 Th 231.03588 Protactinium 91 2 8 18 32 20 9 2 Pa 238.02891 Uranium 92 2 8 18 32 21 9 2 U (237) Neptunium 93 2 8 18 32 22 9 2Np (244) Plutonium 94 2 8 18 32 24 8 2 Pu (243) Americium 95 2 8 18 32 25 8 2 Am (247) Curium 96 2 8 18 32 25 9 2 Cm (247) Berkelium 97 2 8 18 32 27 8 2 Bk (251) Californium 98 2 8 18 32 28 8 2 Cf (252) Einsteinium 99 2 8 18 32 29 8 2 Es (257) Fermium 100 2 8 18 32 30 8 2 Fm (258) Mendelevium 101 2 8 18 32 31 8 2 Md (259) Nobelium 102 2 8 18 32 32 8 2 No (262) Lawrencium 103 2 8 18 32 32 8 3 Lr Nh Mc TsOg




 Karen Southern Editor in chief
Karen Southern Editor in chief



































































