
Review
Polycystic Ovary Syndrome: An Evolutionary Adaptation to Lifestyle and the Environment
Jim Parker, Claire O’Brien, Jason Hawrelak and Felice L. Gersh
Special Issue
Polycystic Ovary Syndrome (PCOS)
Edited by Dr. Michał Kunicki

4.5
https://doi.org/10.3390/ijerph19031336
International Journal of Environmental Research and Public Health
Review

PolycysticOvarySyndrome:AnEvolutionaryAdaptationto LifestyleandtheEnvironment
JimParker 1,* ,ClaireO’Brien 2,JasonHawrelak 3 andFeliceL.Gersh 4
1 SchoolofMedicine,UniversityofWollongong,Wollongong2500,Australia
2 FacultyofScienceandTechnology,UniversityofCanberra,Bruce2617,Australia; Claire.obrien@canberra.edu.au
3 CollegeofHealthandMedicine,UniversityofTasmania,Hobart7005,Australia;Jason.Hawrelak@utas.edu.au
4 CollegeofMedicine,UniversityofArizona,Tucson,AZ85004,USA;felicelgersh@yahoo.com
* Correspondence:jimparker@ozemail.com.au
Citation: Parker,J.;O’Brien,C.; Hawrelak,J.;Gersh,F.L.Polycystic OvarySyndrome:AnEvolutionary AdaptationtoLifestyleandthe Environment. Int.J.Environ.Res. PublicHealth 2022, 19,1336. https://doi.org/10.3390/ ijerph19031336
AcademicEditor:MichałKunicki
Received:3December2021
Accepted:21January2022
Published:25January2022
Publisher’sNote: MDPIstaysneutral withregardtojurisdictionalclaimsin publishedmapsandinstitutionalaffiliations.

Copyright: ©2022bytheauthors. LicenseeMDPI,Basel,Switzerland. Thisarticleisanopenaccessarticle distributedunderthetermsand conditionsoftheCreativeCommons Attribution(CCBY)license(https:// creativecommons.org/licenses/by/ 4.0/).
Abstract: Polycysticovarysyndrome(PCOS)isincreasinglyrecognizedasacomplexmetabolic disorderthatmanifestsingeneticallysusceptiblewomenfollowingarangeofnegativeexposuresto nutritionalandenvironmentalfactorsrelatedtocontemporarylifestyle.ThehypothesisthatPCOS phenotypesarederivedfromamismatchbetweenancientgeneticsurvivalmechanismsandmodern lifestylepracticesissupportedbyadiversityofresearchfindings.Theproposedevolutionarymodel ofthepathogenesisofPCOSincorporatesevidencerelatedtoevolutionarytheory,geneticstudies, inuterodevelopmentalepigeneticprogramming,transgenerationalinheritance,metabolicfeatures includinginsulinresistance,obesityandtheapparentparadoxofleanphenotypes,reproductive effectsandsubfertility,theimpactofthemicrobiomeanddysbiosis,endocrine-disruptingchemical exposure,andtheinfluenceoflifestylefactorssuchaspoor-qualitydietandphysicalinactivity. Basedonthesepremises,thediverselinesofresearcharesynthesizedintoacompositeevolutionary modelofthepathogenesisofPCOS.Itishopedthatthismodelwillassistcliniciansandpatientsto understandtheimportanceoflifestyleinterventionsinthepreventionandmanagementofPCOSand provideaconceptualframeworkforfutureresearch.Itisappreciatedthatthistheoryrepresentsa synthesisofthecurrentevidenceandthatitisexpectedtoevolveandchangeovertime.
Keywords: polycysticovarysyndrome;evolution;insulinresistance;infertility;toxins;endocrinedisruptingchemicals;environment;lifestyle;diet
1.Introduction
Polycysticovarysyndromeisareversiblemetabolicconditionthatmakesasignificant contributiontotheglobalepidemicoflifestyle-relatedchronicdisease[1–3].Manyof thesechronicdiseasesshareasimilarpathogenesisinvolvingtheinteractionofgeneticand environmentalfactors[4–6].TherevisedInternationalGuidelinesfortheassessmentand managementofwomenwithPCOSemphasizethattheassociatedmetabolicdysfunction andsymptomsshouldinitiallybeaddressedvialifestyleinterventions[7].Aunified evolutionarymodelproposesthatPCOSrepresentsamismatchbetweenourancientbiology andmodernlifestyle.
Evolutionarymedicineisanemergingdisciplineinvolvingthestudyofevolutionary processesthatrelatetohumantraitsanddiseasesandtheincorporationofthesefindings intothepracticeofmedicine[8].Evolutionarymedicinebringstogetherinterdisciplinaryresearchtoinformclinicalmedicinebasedontheinfluenceofevolutionaryhistoryonhuman healthanddisease[9].Previousutilizationoftheprinciplesofevolutionarymedicinehas beenlimitedtomonogeneticdiseases(cysticfibrosis,sicklecellanemia,phenylketonuria andmanyothers),drugresistanceofmicroorganisms,tumorgrowthandchemoresistance[8].Futureinsightsintotheapplicationofevolutionaryresearchoffersthepotential
Int.J.Environ.Res.PublicHealth 2022, 19,1336.https://doi.org/10.3390/ijerph19031336https://www.mdpi.com/journal/ijerph
toimproveandpersonalizetheestablishedmedicalandscientificapproachestocomplex chronicdiseasessuchastype2diabetes,metabolicsyndromeandPCOS[5,9].
Theevolutionaryoriginsofcomplexchronicdiseasesincorporateconsiderationsofrelativereproductivefitness,mismatchbetweenourbiologicalpastandmodernenvironment, trade-offsinvolvingcombinationsofgenetictraits,andevolutionaryconflicts[8,10].These evolutionaryfactorsarerelevantwhenanalyzingthecontributorstothepathogenesisof PCOSinmodernandmodernizingsocietiesthatresultinamismatchbetweenourrapid culturalevolutionwithourslowbiologicalevolution[11,12].Theuniqueculturalevolution ofhumansdoesnothaveaplausibleanalogueinmostotherspeciesandisincreasingly recognizedtoplayasignificantroleinthepathogenesisofmetabolicdiseasessuchas PCOS[5,13–17].
Polycysticovarysyndromeisacomplexmultisystemconditionwithmetabolic,endocrine,psychological,fertilityandpregnancy-relatedimplicationsatallstagesoflife[7,18]. ThemajorityofwomenwithPCOSmanifestmultiplemetabolicfeaturesincludingobesity, insulinresistance(IR),hyperlipidemiaandhyperandrogenism[19,20].PCOSresultsin anincreasedriskofdevelopingmetabolicdisease(type2diabetes,non-alcoholicfatty liverdisease[NAFLD]andmetabolicsyndrome),cardiovasculardisease,cancer,awide arrayofpregnancycomplications(deepvenousthrombosis,pre-eclampsia,gestational diabetes[GDM],macrosomia,growthrestriction,miscarriage,stillbirthandpretermlabor)andpsychologicalproblems(anxiety,depression)[6,21–25].PCOSispartofacluster ofinter-relatedmetabolicconditionsandmakesasignificantcontributiontothechronic diseaseepidemic.
ExtensiveresearchsuggeststhattheetiologyofPCOSinvolvesaninteractionbetween environmentalfactorsandgenevariants,althoughithasbeensuggestedthatgeneticfactors contributelessthan10%todiseasesusceptibility[26–28].Alargenumberofgeneticand genome-wideassociationstudies(GWAS)haveidentifiedcommongenelociassociated withPCOSphenotypesindifferentethnicpopulations[29–31].Theseappeartobenormal genevariantsorpolymorphisms,giventhefrequencyandtypeofgenesthathavebeen identified.PCOSisthereforeviewedasapolygenictraitthatresultsfromaninteraction betweensusceptiblegenomicvariantsandtheenvironment.
PCOSeffectsupwardof10%ofreproductive-agedwomen,estimatedatover 200million womenworldwide[32,33].PCOSisthoughttobeincreasinginincidenceinbothdevelopinganddevelopednationsasaresultoflifestyle-relatedchangesindietquality,reduced physicalactivity,ubiquitousenvironmentalendocrine-disruptingchemicals(EDC),altered lightexposures,sleepdisturbance,heightenedlevelsofstressandotherenvironmental factors[11,34–38].Thesefactors,andthehighprevalenceofPCOS,suggestthattherecould beanevolutionarybasisforthesyndrome[15,16,39].Evolutionarymedicinehaschanged theparadigmforunderstandingPCOS,acknowledgingmanyofthecontributinglifestyle andenvironmentalfactorsthatfacilitatetheobservedmetabolicandclinicalfeaturesand thatarealsosharedwithrelatedmetabolicdiseases[8].These“mismatchdisorders”are estimatedtomakeasignificantcontributiontochronicdiseaseindevelopedcountriesand agrowingproportionofdisabilityanddeathindevelopingnations[3].Accordingtothe GlobalBurdenofDiseaseStudy,thehumandietisnowtheleadingriskfactorformorbidity andmortalityworldwide[3].Inkeepingwiththesefindings,dietisrecognizedasoneof themajorcontributorstothegrowingprevalenceofPCOSglobally[7,40].
DietaryandenvironmentalfactorsarehypothesizedtohaveanimpactondevelopmentalprogrammingofsusceptiblegenevariantsinwomenwithPCOS[41–43].Extensive experimentalevidencesuggeststhatprenatalandrogenexposuremayplayaroleinthe pathogenesisofPCOS-likesyndromesinanimalmodels[19,44–46].ThediscoveryofnaturallyoccurringPCOSphenotypesinnon-humanprimatessupportsasurvivaladvantage ofahyperandrogenic,insulinresistantphenotypewithdelayedfertility[47].Inhumans, theoriginofexcessandrogensmaybefrommaternal,fetalorplacentalsources.Inaddition,emergingandconcerningevidencesuggeststhatEDCmaycontributetoalteredfetal programmingandplayaroleinthepathogenesisofPCOS[41,48].
Int.J.Environ.Res.PublicHealth 2022, 19,1336 2of25
Inuterogenomicprogrammingofmetabolicandendocrinepathwayscanincreasethe susceptibilityofoffspringtodevelopPCOSfollowingexposuretospecificnutritionaland environmentalconditions[45].ThisviewofthepathogenesisofPCOSisconsistentwith theDevelopmentalOriginsofHealthandDisease(DOHaD)modelproposedbyNeel[49]. Postnatalexposuretolifestyleandenvironmentalfactors,suchaspoor-qualitydietand EDC,mayactivateepigeneticallyprogrammedpathwaysthatfurtherpromotetheobserved featuresofPCOS.Dietaryandlifestyleinterventionshavedemonstratedthatmanyofthe clinical,metabolicandendocrinefeaturesofPCOScanbereversed[7,50,51].
Lifestyle-inducedchangesinthegastrointestinaltractmicrobiomeareanothersignificantfactorintheetiologyofPCOS[52,53].Dysbiosisofthegutmicrobiotahasbeen hypothesizedtoplayaroleinincreasedgastrointestinalpermeability,initiatingchronic inflammation,insulinresistance(IR)andhyperandrogenism[40].Numerousstudieshave reportedreducedalphadiversityofthemicrobiomethathasbeenassociatedwiththe metabolic,endocrineandclinicalfeaturesobservedinwomenwithPCOS[54,55].The resultingdysbiosishasbeenshowntobereversibleafterinterventionsaimedatimproving dietqualityortreatmentwithprobioticsorsynbiotics[50,51,56–58].
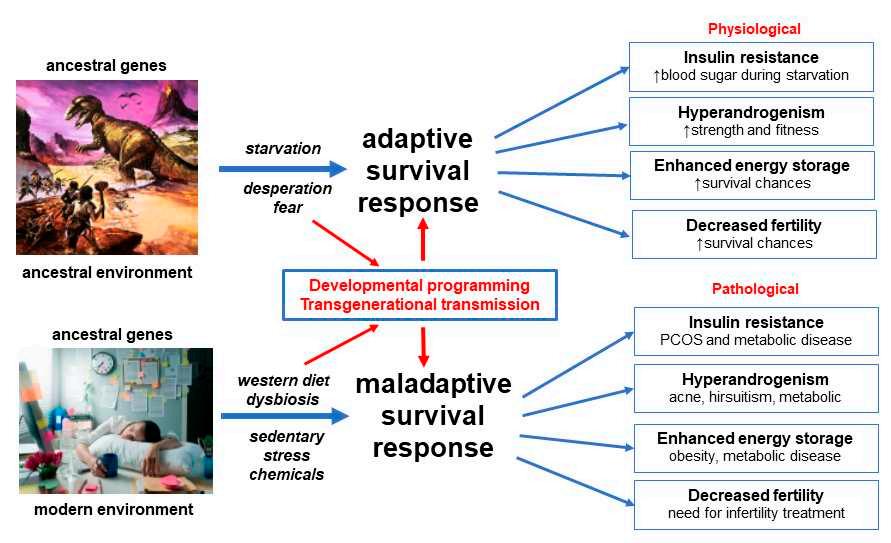
AunifiedevolutionarytheoryofthepathogenesisofPCOSproposesthatancient geneticpolymorphismsthatwerealignedwiththeenvironmentofthatera,resultedin anadaptivesurvivaladvantageinoffspringinancestralpopulations[14–16,28].When thesesamegeneticvariantsareexposedtomodernlifestyleandenvironmentalinfluences, maladaptivephysiologicalresponsesoccur.Theprioradvantagesofinsulinresistance, hyperandrogenism,enhancedenergystorageandreducedfertilityinancestralpopulationsbecomepathologicalandresultintheobservedfeaturesofPCOSincontemporary women(Figure 1).
2.MaterialsandMethods
Theliteraturesearchfocusedonresearchpublicationsrelatedtothepathogenesisof PCOSusingthekeywordslistedaboveandrelatedmeshtermsfordataontheevolutionary aspectsofPCOS,geneticstudies,inuterodevelopmentalepigeneticprogramming,transgenerationalinheritance,metabolicfeaturesincludinginsulinresistance,obeseandlean PCOSphenotypes,reproductivechangesandsubfertility,impactofthemicrobiomeand dysbiosis,possibleeffectsofendocrine-disruptingchemicalexposureandtheinfluenceof lifestylefactorssuchasdietandphysicalactivity.ThedatabasessearchedincludedPubMed,
Int.J.Environ.Res.PublicHealth 2022, 19,1336 3of25
Figure1. Evolutionarymodelofthepathogenesisofpolycysticovarysyndrome.Adaptedwith permissionfromRef.[12].2021JournalofACNEM.
Scopus,CochraneandGoogleScholar.Relevantpaperswereselected,andcitationsearches wereperformed.
Thepresentmanuscriptsynthesizesthefindingsintoaunifiedevolutionarymodel. Thefollowingtextispresentedasanarrativereviewoffactorsinvolvedinthepathogenesis ofPCOSandisdiscussedintenmainsubjectareasthatprovidetherationaleforthe developmentofaunifiedmodel.1.Evolution2.Genetics3.DevelopmentalEpigenetic Programming4.MicrobiomeandDysbiosis5.Insulinresistance6.Obesityandthe leanparadox7.Endocrine-DisruptingChemicalExposure8.Lifestylecontributorsto thepathogenesisofPCOS9.CircadianRhythmDisruptionandPCOS10.Conceptual FrameworkandSummaryoftheUnifiedEvolutionaryModel.
3.PathogenesisofPCOS
3.1.Evolution
ThedescriptionofPCOSphenotypescanbefoundinmedicalrecordsfromantiquity andthemodernsyndromewasdescribedover80yearsago[17,59].Nevertheless,there isongoingdebateregardingtheevolutionaryoriginsofPCOS[15–17,39,60–64].PCOS susceptibilityallelesmayhaveariseninourphylogeneticancestors,inthehunter–gatherer PaleolithicperiodoftheStoneAge,aftertheNeolithicAgriculturalRevolutionorfollowing theIndustrialRevolution[16,17].Fromanevolutionaryperspective,nearlyallgenetic variantsthatinfluencediseaseriskhavehuman-specificorigins,butthesystemstheyrelate tohaveancientrootsinourevolutionaryancestors[8].Regardlessoftheprecisetiming oftheoriginofPCOSinhumans,thecomplexmetabolicandreproductivegenevariants identifiedinwomenwithPCOSrelatetoancientevolutionary-conservedmetabolicand reproductivesurvivalpathways[15,29].Althoughevolutionaryhypothesesaboutdisease vulnerabilityareimpossibletoprovetheyhavethepotentialtoframemedicalthinkingand directscientificresearchfortheproximatecausesofdisease[15,60].
MultiplehypotheseshavebeenproposedregardingtheevolutionaryoriginsofPCOS andrelatedmetabolicdiseases[8,60,63].Thesehypothesesarefocusedontherelative importanceofmetabolicsurvivaladaptationsversusimprovedreproductivesuccess,ora combinationofboth.Adetailedanalysisofthesehypotheses,andthecomplexitiesofthe evolutionaryconsiderations,havebeenreviewedelsewhereandisbeyondthescopeofthe presentreview[8,60].OnecommonthemeisthatPCOSmaybeviewedasa“conditional phenotype”whereaspecificsetofconditionshasunmaskednormallyunexpressedor partlyexpressedgeneticpathways,whichthenprovideasurvivaladvantageundercertain environmentalconditions[14,16].
Allorganismshavephysiologicaladaptiveresponsestodealwithchangingenvironmentalconditions(starvation,fasting,physicalthreat,stressandinfection)andthevarying demandsofinternalphysiologicalstates(pregnancy,lactationandadolescence)[14,65]. IthasbeenproposedthatthePCOSphenotypemayhavebeeninvokedinspecificenvironmentalconditionsinancestralpopulationsasashort,mediumorevenlong-term adaptivesurvivalmechanism[15–17].TheviewofPCOSasaconditionalphenotypeproposesthatthesephysiologicalresponsesbecomepathologicalinourmodernenvironment duetofactorssuchasfoodabundance,reducedphysicalactivity,circadiandisruption, stressandenvironmentalchemicalexposure.Thetransgenerationalevolutionarytheory ofthepathogenesisofPCOSencompassesalloftheaboveideastoexplaintheobserved pathophysiologicalandclinicalfeaturesofPCOS[28].
Itisgenerallyacceptedthatalmostallpre-industrialsocietiesandanimalpopulations experiencedseasonalorunpredictableepisodesoffoodshortagethatappliedevolutionary pressuretodevelopmetabolicandreproductiveadaptivesurvivalresponses[17,49].It isalsoappreciatedthatmetabolicandreproductivepathwaysareinterconnectedand involvereciprocalfeedbackcontrolmechanisms[66–68].Duringperiodsofstarvation, anorexiaorexcessiveweightgain,reproductionisdown-regulatedandovulationbecomes irregularorceases[69,70].Similarly,metabolicfunctioniscoordinatedwiththemenstrual cycletoensureoptimalphysiologicalconditionsforfertilization,implantation,pregnancy,
Int.J.Environ.Res.PublicHealth 2022, 19,1336 4of25
parturitionandlactation[71].Recentresearchhaselaboratedonthedetailsofhowsomeof thesecomplexregulatorymechanismsinteractusingspecifichormonal,nutrientsensing andintracellularsignalingnetworks[72–74].
Detailsofthemechanismsunderlyingtheproposedadaptivesurvivaladvantages ofIR,hyperandrogenism,enhancedenergystorageandsubfertilityhavebeenobtained frompaleolithicrecords,animalmodelsandhumanpopulationsexposedtoadverseenvironmentalconditionssuchaswarandfamine-inflictedstarvation[14,16,62,63].Multiple linesofevidencesupportthemaladaptiveresponseofhumanpopulationstorapidly changingnutritional,physical,psychologicalandculturalenvironments,inthemodern world[5,11,14,75].These“adaptations”resultinpathologicalresponsestoIR,hyperandrogenism,enhancedenergystorageandovulation(Figure 1).
Theoriesofevolutionarymismatchhavealsobeenadvancedtoexplainallofthe clusterofmetabolicdiseasesassociatedwithPCOS(type2diabetes,metabolicsyndrome, NAFLDandcardiovasculardisease)andfollowthesamesetofbasicprinciplesandexplanations[14,76].Thiscommonbodyofevolutionaryevidenceissupportedbytheincreasing incidenceofmetabolic-relateddisease,suchasdiabetesandobesity,indevelopedcountries andindevelopingnationsadoptingaWesterndietandlifestyle[11,77].Inaddition,the demonstratedreversibilityofPCOSandrelatedmetabolicandbiochemicalfeaturesfollowingchangesindiet,increasedphysicalactivityandotherlifestyleinterventions,adds furthersupporttoatransgenerationalevolutionarymodel[50,51].
3.2.Genetics
TheheritablenatureofPCOShasbeenproposedsincethe1960′ sfollowingarange offamilial,twinandchromosomalstudies[78–80].Cytogeneticstudiesfailedtoidentify karyotypicabnormalitiesandgeneticstudiesdidnotshowamonogenicinheritancepattern followingexaminationofcandidategenes[81,82].Inaddition,twoormorephenotypes canbepresentinthesamefamilysuggestingthatsomeofthephenotypicdifferencescould beaccountedforbyvariableexpressionofthesamesharedgenes[81,83].
Themappingofthehumangenomein2003[84]andthepublicationofthehuman haplotypemap(morethanonemillionsinglenucleotidepolymorphismsofcommon geneticvariants)in2005[85],leadtotherealizationthatmostDNAvariationissharedby allhumansandisinheritedasblocksoflinkedgenes(linkagedisequilibrium)[86].These advancesenabledarevolutionincase-controlstudiesandthedevelopmentofGWASwhich maptheentirehumangenomelookingforsusceptibilitygenesforcomplextraitssuchas obesity,type2diabetesandPCOS[81].
ThefirstPCOSGWASwaspublishedin2010anddemonstrated11genelociassociated withPCOS[87].Additionallocihavesubsequentlybeenfoundinseveraldifferentethnic groups[86,88].ThefirstGWASanalysisofquantitativetraitswaspublishedin2015and showedthatavariant(rs11031006)wasassociatedwithluteinizinghormonelevels[88]. ThelargestGWASincludedameta-analysisof10,074PCOScasesand103,164controls andidentified19locithatconferriskforPCOS[29].Thegenesassociatedwiththese lociinvolvegonadotrophinaction,ovariansteroidogenesis,insulinresistanceandtype 2diabetessusceptibilitygenes.ThefirstGWASusingelectronichealthrecord-linked biobankshasintroducedgreaterinvestigativepowerandidentified2additionalloci[89]. Thesevariantswereassociatedwithpolycysticovariesandhyperandrogenism(rs17186366 near SOD2)andoligomenorrhoeaandinfertility(rs144248326near WWTR1)[89].In additiontoidentifyingcommongenevariantsforPCOSphenotypes,findingthesame signals(THADA,YAP1andc9orf3)inChineseandEuropeanpopulationssuggeststhat PCOSisanancienttraitthatwaspresentbeforehumansmigratedoutofAfrica[81].
MorerecentlyMendelianrandomization(MR)studieshavebeenusedtoexplorethe potentialcausativeassociationbetweengenevariantsidentifiedinGWASand PCOS[90,91] ManyofthegenevariantsidentifiedinGWASarelocatedinnon-codingregionsof DNA[92].ThegenesorfunctionalDNAelementsthroughwhichthesevariantsexert theireffectsareoftenunknown.Mendelianrandomizationisastatisticalmethodology
Int.J.Environ.Res.PublicHealth 2022, 19,1336 5of25
usedtojointlyanalyzeGWASandquantitativegenelocitotestforassociationbetween geneexpressionandatrait,duetoasharedorpotentiallycausalvariantataspecific locus[93].AdetailedanalysisofMRmethodologyandthelimitationsofthisstatistical toolisbeyondthescopeofthepresentreview.AlthoughMRstudieshavethepotential toinfercausationitisrecognizedthattheyalsohavelimitationsin PCOSresearch[90]. Nevertheless,preliminaryevidencesuggeststhatseveralgenesrelatedtoobesity,metabolic andreproductivefunction,mayplayacausalroleinthepathogenesisofPCOS[90,91].
DecadesofgeneticresearchhasthereforecharacterizedPCOSasapolygenictraitthatresultsfrominteractionsbetweentheenvironmentandsusceptiblegenomic traits[27,29,79,88] Thefailuretoidentifyaqualitativeormonogenicinheritancepatternandthefindings fromGWAS,MR,familialandtwinstudies,suggeststhattheheritabilityofPCOSislikely tobeduetothecombinationofmultiplegeneswithsmalleffectsize,ashasbeenfound withobesityandtype2diabetes[79,80,94–96].Polygenictraitsaretheresultofgenevariantsthatrepresentoneendofthebell-shapednormaldistributioncurveofcontinuous variationinapopulation[97].Fromanevolutionaryperspective,womenwithPCOS mayrepresentthe“metabolicelite”endofthenormaldistributioncurve,beingableto efficientlystoreenergyinperiodsoffoodabundanceanddown-regulatefertilityintimes offoodscarcity,oreveninanticipationofreducedseasonalfoodavailabilityasapredictive adaptiveresponse[16,17,60].
TherealizationthatPCOSisaquantitativetrait(phenotypedeterminedbymultiple genesandenvironmentalfactors)hasfar-reachingimplicationsforthediagnosis,treatment andpreventionofsymptomsandpathologyassociatedwithPCOS.Theimplications requireashiftinthinkingaboutPCOSasa“disease”toavariationofnormalmetabolic andreproductivefunction.Thisshiftinvitesachangeinvocabularyfromtalkingabout “disorder”and“risk”totalkingabout“expression”and“variability”[97].Thisnew understandingsupportsandreinforcesanevolutionarymodelofthepathogenesisofPCOS. Inkeepingwiththismodel,multiplelinesofevidencesuggestthatinheritedPCOSgene variantsaredevelopmentallyprogrammedinawaythatprimesthemforactivationby nutritionalandenvironmentalfactorsinpostnatallife[41,42,98].
3.3.DevelopmentalEpigeneticProgramming
ThedevelopmentalprogrammingofPCOSrepresentschangesingeneexpression thatoccurduringcriticalperiodsoffetaldevelopment[99].Followingfertilization,most parentalepigeneticprogrammingiserasedanddramaticepigenomicreprogrammingoccurs[100].Thisresultsintransformationoftheparentalepigenometothezygoteepigenome anddeterminespersonalizedgenefunction.Compellingevidenceshowsthatawiderange ofmaternal,nutritionalandenvironmentalfactorscaneffectfetaldevelopmentduring thesecriticalperiodsofprogramming[44,98,99,101,102].Theseincludehormones,vitamins, diet-derivedmetabolitesandenvironmentalchemicals[48,98,103,104].Inaddition,epigeneticreprogrammingofgerm-linecellscanleadtotransgenerationalinheritanceresulting inphenotypicvariationorpathologyintheabsenceofcontinueddirectexposure[98].
Experimentalstudiesinprimates,sheep,ratsandmiceshowthatPCOS-likesyndromescanbeinducedbyarangeoftreatmentsincludingandrogens,anti-Mullerian hormoneandletrozole[19,44,46].Nevertheless,thereissignificantdebateregardingwhen ananimalmodelqualifiesasPCOS-like[105].Themodelusedandthemethodofinduction ofPCOSphenotypesthereforeneedstobecarefullyscrutinizedwhengeneralizingfindings fromanimalresearchtowomenwithPCOS.Mostoftheanimalandhumanresearchon thedevelopmentaloriginsofPCOShasfocusedontheroleofprenatalandrogenexposure.Thishasbeenextensivelyreviewedinnumerouspreviouspublications[41,46].This researchhasresultedinaproposed“twohit”hypothesisforthedevelopmentofPCOS phenotypes[43,45].The“firsthit”involvesdevelopmentalprogrammingofinheritedsusceptibilitygenesandthe“secondhit”arisesduetolifestyleandenvironmentalinfluences inchildhood,adolescenceandadulthood[41,106].
Int.J.Environ.Res.PublicHealth 2022, 19,1336 6of25
IfPCOSisaquantitativetraitinvolvingnormalgenevariants,assuggestedbytheevolutionaryconsiderationsandfindingsfromgeneticresearch,thenthe“firsthit”mayresult fromnormaldevelopmentalprogrammingeventsasoccurswithothergenevariants[102]. Accordingtothishypothesis,thepolygenicsusceptibilitygeneswouldbenormally“activated”and“primed”torespondtofuturematernalandenvironmentalconditionsand exposures,aswouldbethecasewithmanyothernormalgenes[28].Inaddition,the susceptibilityallelesmaybe“activated”or“functionallyenhanced”byarangeofmaternal andenvironmentalfactors,asisusuallypresumedtobethecaseinPCOS[5,14,102].This developmentalplasticitywouldprovideamechanismforapredictiveadaptiveresponse, basedoninputsfromthematernalenvironmentthatcouldbeusedtoprogrammetabolic andreproductivesurvivalpathways,tobetterpreparetheoffspringforthefutureworldin whichtheymaybeexpectedtolive[107].
Parentallifestylefactorsincludingdiet,obesity,smokingandendocrine-disrupting chemicals,haveallbeenshowntomodulatediseaserisklaterinlife[104,108,109].The originaldescriptionofthefetalorigin’shypothesisproposedthatpoormaternalnutrition wouldincreasefetalsusceptibilitytotheeffectsofaWestern-styledietlaterinlife[49]. Subsequentstudieshaveconfirmedthatmaternalexposuretoeithernutrientexcessor deficit,canhavelong-termconsequencesforthehealthoftheprogeny[104].Evidence fromhumanandanimalstudiessuggeststhatmaternalobesityprogramstheoffspringfor increasedriskofdevelopingobesity,hyperglycemia,diabetes,hypertensionandmetabolic syndrome[108].
ThedevelopmentaloriginsofPCOSmayhavebeenduetodifferentfactorsinancestral andmodernpopulations[17,60].Ithasbeenhypothesizedthatenvironmentalstress, infection,nutrientdeprivation,fetalgrowthrestrictionandstresshormoneresponses mayhaveresultedinmaternallymediatedmodulationofgeneexpressioninancestral offspring[17,110].Someofthesefactorshavebeeninvestigatedandconfirmedinmodern populationssubjecttostarvationandextremeenvironmentalconditions[111].Incontrast, alteredfetalprogramminginmodernsocietiesmaybesecondarytomaternalovernutrition, sedentarybehavior,obesity,emotionalstress,circadianrhythmdisruption,poorguthealth orenvironmentalchemicalexposure[35,101,112,113].Thepreconceptionandpregnancy periodsthereforeprovideauniqueopportunityforlifestyleinterventionsthatpromote optimalfuturehealthforboththemotherandtheoffspring(Figure 2).

Int.J.Environ.Res.PublicHealth 2022, 19,1336 7of25
Figure2. Nutritionalandenvironmentalinfluencesthroughoutthelifecourseandtheperpetuation ofthetransgenerationalinheritanceofpolycysticovarysyndrome.ReprintedfromRef.[28].
3.4.MicrobiomeandDysbiosis
Thegastrointestinalmicrobiomeisnowappreciatedtoplayacentralroleinhuman healthanddisease[114,115].Themicrobiomeisknowntoco-regulatemanyphysiological functionsinvolvingtheimmune,neuroendocrineandmetabolicsystemsviacomplex reciprocalfeedbackmechanismsthatoperatebetweenthemicrobialecosystemandthe host[116,117].EvidencefromstudiesinWesternpopulations,hunter–gatherersocieties andphylogeneticstudiesinotherspecies,haveattemptedtoplacethehumanmicrobiome intoanevolutionarycontext[118].Althoughmicrobesclearlyimpacthostphysiologyand havechangedalongbranchesoftheevolutionarytree,thereisongoingdebateregarding whetherthemicrobiomecanevolveaccordingtotheusualevolutionaryforces[119,120]. Nevertheless,ithasbeenarguedthatfocusingonfunctionalpathwaysandmetabolicroles ofmicrobialcommunities,ratherthanonspecificmicrobes,providesabettermodelfor understandingevolutionaryfitness[118].Theco-evolutionofthemicrobiomeandhuman physiologymaythereforebeimportantinunderstandingthedifferencesbetweenancient adaptivephysiologicalsurvivalmechanismsandmodernlifestyle-relatedpathological responses,inwomenwithPCOS(Figure 1).
TwinstudiesandGWASshowthathostgeneticscaninfluencethemicrobiomecomposition,andmicrobescanexerteffectsonthehostgenome,althoughtheenvironmenthas animportantrole[121,122].Humansareconstantlyadaptingtothegutmicrobiometotry todeterminewhichmicroorganismsarebeneficialorharmful.Immunegenesinvolved inthisprocessarethemostrapidlyevolvingprotein-encodinggenesinthemammalian genome[123,124].Diversificationofmicrobesallowshumanstoaccessdietarynichesand nutritionalcomponentstheyotherwisewouldnotbeabletoaccess,whichmaybebeneficialandultimatelyleadtotheintegrationofspecificmicrobesintotheecosystem[125]. Althoughnolivingpopulationtodaycarriesanancestralmicrobiome,comparisonstudiesofnon-WesternandWesternpopulationsshowsignificantdifferencesintherelative abundancesofcommonphylaandamuchgreaterspeciesdiversityinnon-Westernpopulations[126,127].Areviewofnon-humanprimateandhumangutmicrobiomedatasets, revealedachangingmicrobiomeinresponsetohosthabitat,seasonanddiet,although thereappeartobecommonspecies-specificsymbioticcommunities[118].
Rapidhumanculturalchangeshaveresultedinsignificantdietarymodificationsin urban-industrializedcommunitiesandshiftedthemicrobiomeatanunprecedentedrate. Theresulthasbeenthedevelopmentofamismatchbetweenhumanmetabolicgenesand bacteriathatenhancefatstorage[128].Inourevolutionarypast,whennutrientswerescarce, ithasbeentheorizedthathostselectionledtothemaintenanceofmicrobesthatenhance nutrientuptakeorhostenergystorage.However,inthemodernenvironment,wherea high-fat,high-sugar,low-fiberdiethasbecomecommonandeasilyaccessible,integration ofthesemicrobesleadstomaladaptivephysiologicalresponses[40].Formetabolically thriftyindividualswithPCOS,harboringmicrobesthatenhanceenergystorageescalates theevolutionaryconflict,furtheringthedevelopmentofinsulinresistanceandtherefore progressiontoobesityandtype2diabetes[12,129].Furthercompoundingthismaladaptive responseisthelossofmicrobesthatarerequiredtoaccessotherdietaryniches.One exampleisthelossofsymbioticspeciesofTreponemainindividualslivinginurbanindustrializedcommunities[130].Achangefromtheancestralhunter–gathererdiet,where foodsconsumedchangedseasonallyandawidevarietyoffoodcomponentswereeaten,to adietthatissimilaracrossseasonsandsignificantlylessvaried,isanotherlikelycontributor toreduceddiversityofthemicrobiomesofindividualslivinginurbanized–industrialized communities[131].
ThemajorityofwomenwithPCOSareoverweightorobeseandevidenceindicates thatthemicrobiomeofobeseindividualsiscapableofextractingmoreenergyfromthehost dietcomparedwiththemicrobiomeofleanindividuals[132].Thisisthoughttobedriven byanexpansioninpro-inflammatoryspeciesofbacteria,suchas E.coli,andadepletionof anti-inflammatorybacteriasuchas Faecalibacteriumprausnitzii [133,134].Chroniclow-grade
Int.J.Environ.Res.PublicHealth 2022, 19,1336 8of25
‘metabolic’inflammation,ormeta-inflammation,isaresultofanimbalancedgutmicrobiomethatpromotesthedevelopmentofinsulinresistanceandtype2diabetes[135–137].
ThedysbiosisofgutmicrobiotatheoryofPCOS,proposedbyTremellenin2012,accountsforthedevelopmentofallofthecomponentsofPCOS(multipleovarianfollicles, anovulationormenstrualirregularityandhyperandrogenism)[40].Thetheoryproposes thatapoor-qualitydietandresultingimbalancedmicrobiome,inducesintestinalpermeabilityandendotoxemia,exacerbatinghyperinsulinemia.Increasedinsulinlevelspromote higherandrogenproductionbytheovariesanddisruptsnormalfollicledevelopment. Metabolic,endocrineandenvironmentalfactorsassociatedwithPCOSarenotmutually exclusive,andthereforetheirrelativecontributionstodysbiosisinPCOSremainsuncertain[138].Consumingabalanceddietthatislowinfatandhighinfiber,canalsorestore balancetotheecosystem(termedeubiosis)[50].Arecentstudyshowedthatdietaryintake offiberandvitaminDwassignificantlydecreasedinbothleanandobesewomenwith PCOS,comparedtohealthycontrols,andcorrelatedwithlowerdiversityofthegutmicrobiome[139].Dysbiosisisreversiblewithimprovementindietqualityaugmentedbythe additionofprobioticsorsynbiotics[51,56–58].
Dysbiosisisaconsistentfindingwhenlookingatthemicrobiomeofwomenwith PCOS[140–143].Althoughmoststudiesaresmall,dysbiosishasconsistentlybeenfound tocorrelatewithdifferentphysiologicalparameters,suchasobesity,sexhormonesand metabolicdefects[140,141,143].Similartomicrobiomesassociatedwithobesity,themicrobiomesofindividualswithPCOShavegenerallybeenfoundtohaveloweralphadiversity (lowernumbersofbacterialtaxa)thancontrols,andmoststudiesdescribeanaltered compositionoftaxarelativetocontrols[140,143].However,thebacterialtaxaobserved tobeeitherincreased,depletedorabsentinPCOSdiffersfromstudytostudy.Thisis likelyduetoboththeimmenseinter-individualvariationinmicrobiotas,aswellthefact thatPCOSisaquantitativetraitwithwomenwithvariousdegreesandlevelsofobesity andsexhormones.
Inkeepingwiththedevelopmentaloriginshypothesispreviouslydiscussed,maternal androgensmayalterthecompositionandfunctionofthemicrobiome,thereforefacilitating thepathogenesisofPCOS[140].Onestudyshowedthatbetadiversity,whichisusedto measuredifferencesbetweengroups,wasnegativelycorrelatedwithhyperandrogenism, suggestingthatandrogensplayasignificantroleindysbiosis[140].The‘firsthit’in uteromaythereforecombinewithverticaltransmissionofadysbioticmicrobiomefroma motherwithPCOS,resultingindysbiosisintheoffspring.Preconceptionandpregnancy provideauniqueopportunitiesforlifestyleanddietaryinterventionsaimedatrestoring eubiosis,toenablethetransferenceofabalancedecosystemtotheoffspring,viavertical transmission[118].
Theaccumulatingscientificevidencestronglysupportsthesignificantroleplayedby themicrobiomeinthepathogenesisandmaintenanceofPCOS,consistentwithresearchin otherrelatedmetabolicconditions.Theroleofdysbiosisissupportedbyover30proof-ofconceptstudiesthathaverecentlybeenreviewed[144].Dysbiosisisthereforeasignificant factorinthepathogenesisofPCOSandanimportantcomponentofaunifiedevolutionary model.Dysbiosisrepresentsamaladaptiveresponseofthemicrobiometomodernlifestyle influencesandisamodifiablefactorinthetreatmentofwomenwithPCOS.
3.5.InsulinResistance
ThereareseveraldilemmaswhenassessingtheroleofIRinwomenwithPCOS. ThereisnoconsensusonthedefinitionofIR[145,146],measurementisdifficult[147,148], whole-bodyIRisusuallymeasuredalthoughitisrecognizedthatIRcanbeselective beingeithertissue-specificorpathway-specificwithincells[149–151],normalvaluesare categoricalanddeterminedbyarbitrarycut-offs(4.45mg/kg/min)[145],testingisnot recommendedinclinicalpractice[38],reportedprevalenceratesinobeseandleanwomen varywidely[147,152],andthesignificanceofIRasapathognomoniccomponentofPCOS isanareaofdebate[153–155].
Int.J.Environ.Res.PublicHealth 2022, 19,1336 9of25
Despitetheselimitations,itishypothesizedthatIRisasignificantproximatecause ofPCOSandisintrinsictotheunderlyingpathophysiology[44,156].Inaddition,itis recognizedthatIRplaysamajorroleinthepathophysiologyofallofthemetabolicdiseases, cardiovasculardisease,someneurodegenerativediseases,andselectedcancers[22,157]. Insulinresistanceisthereforeconsideredtobethemaindriverformanydiseasesand makesasignificantcontributiontothechronicdiseaseepidemic[158].Nevertheless, beingabletovarythesensitivityandphysiologicalactionofinsulinisthoughttohave conferredasignificantadaptivesurvivalroleinmanyanimalsthroughoutevolutionary history[146,159].IthasbeenproposedthatIRmayhaveevolvedasaswitchinreproductive andmetabolicstrategies,sincethedevelopmentofIRcanresultinanovulationandreduced fertility,inadditiontodifferentialenergyrepartitioningtospecifictissues[159].
Insulinreceptorsarelocatedonthecellmembranesofmosttissuesinthebody[160]. Ligandbindingtothealpha-subunitinducesautophosphorylationofspecifictyrosine residuesonthecytoplasmicsideofthemembrane[160,161].Theactivatedinsulinreceptorinitiatessignaltransductionviathephosphatidylinositol-3kinase(PI-3K)metabolic pathwayandthemitogen-activatedproteinkinasepathway(MAPK)whichisinvolved incellgrowthandproliferation[161].Insulinisananabolichormonethatfacilitates glucoseremovalfromtheblood,enhancesfatstorageandinhibitslipolysisinadipose tissue,stimulatesglycogensynthesisinmuscleandliverandinhibitshepaticglucoseoutput[161].IRcanbedefinedasastatewherehighercirculatinginsulinlevelsarenecessary toachieveanintegratedglucose-loweringresponse[146].IRresultsfromalterationsto cellularmembraneinsulin-receptorfunctionorintracellularsignaling,enzyme,metabolic orgenefunction[146,160,161].
Insulinresistancecanbecausedbyawidevarietyofmechanismsthathavethe abilitytodisruptanypartofthismetabolicsignalingsystem[53,161].Theseinclude autoantibodies,receptoragonistsandantagonists,hormones,inflammatorycytokines, oxidativestress,nutrientsensorsandmetabolicintermediates[160–163].Physiological regulationofinsulinfunctioncanbeviewedasanadaptivemechanismtoregulatethe metabolicpathwayofinsulinsignaling(PI-3K),inresponsetochangingenvironmental conditions[starvation,fear,stress][164,165]orduringnormalalterationsofinternalstates (pregnancy,lactation,adolescence)[65,146,152].
ThephysiologicalactivationofIRallowstheorganismtoswitchfromananabolic energystoragestatetoacatabolicorenergymobilizingstate.Thisallowsfreefattyacids tobemobilizedfromadiposetissue,whicharethenconvertedtoglucoseintheliver andreleasedintothecirculation[161].Asaresultofthismetabolicchange,bloodsugar levelsaremaintainedforvitalmetabolicprocessesandbrainfunction[14].Thisadaptive protectivemechanismcanbepathway-specificduringperiodsofgrowth,suchaspregnancy, lactationandadolescence,sothatonlythemetabolicsignaling(PI-3K)isinhibitedandnot themitogenicpathway(MAPK),whichmayevenbeup-regulated[30,65,160].
Whenthephysiologyofinsulinfunctionisconsideredtobeaquantitativeorcontinuousvariablefromanevolutionaryperspective,itislikelythatallwomenwithPCOS, whetherobeseorlean,havereducedinsulinsensitivity[152,155,166].Asystematicreview andmeta-analysisofeuglycemic-hyperinsulinemicclampstudiesfoundthatwomenwith PCOShavea27%reductionininsulinsensitivitycomparedtobodymassindex(BMI)and age-matchedcontrols[155].Inevolutionaryterms,womenwithaPCOSmetabolicphenotypewouldhaveincreasedsurvivalchancesduringtimesofenvironmentalorphysiological demandforalteredenergymetabolism,butbemorevulnerabletothepathologicaleffects ofIRwhenexposedtomodernlifestylefactors[14,17,159].Inparticular,apoor-quality, high-glycemic,high-fat,low-fiberdiethasbeenshowntocauseIR[40,167].Asdiscussed inthedysbiosissection,diet-relatedchangesinthegastrointestinalmicrobiomehavealso beenshowntocauseIRinwomenwithPCOS[53,55].Numerousstudieshaveshownthat dietarymodification[168–170],ortreatmentwithprobioticsorsynbiotics,hasthepotential torestorenormalinsulinfunction[57,171].
Int.J.Environ.Res.PublicHealth 2022, 19,1336 10of25
Consumptionofahigh-glycemic-loaddietresultsinrapidincreasesinbloodsugar levelsthatcausecompensatoryhyperinsulinemia[167,172].Excessivedietaryintakeof glucoseandfructoseareconvertedtofattyacidsbydenovolipogenesisintheliver, transportedtoadipocytesvialipoproteins,releasedasfattyacidstoadipocytesandstored infatglobulesastriglycerides[161].Asaresultofnutrientoverload,diacylglycerol,the penultimatemoleculeinthesynthesisoftriglyceride,accumulatesinthecytoplasmand bindswiththethreonineaminoacidinthe1160positionoftheinsulinreceptor.Thisinhibits autophosphorylationanddown-regulatesthemetabolicPI-3KpathwayandcausesIR[161]. Thisprocesshasthepotentialtobereversiblefollowingchangesindietquantityandquality, ashasbeenshowntooccurwithcalorierestriction,fasting,time-restrictedeating,gastric bypasssurgery,lowsaturatedfatandlowglycemicdiets[168,170,173].Dietshighinanimal proteinorsaturatedfatcanalsocauseIRindependentofBMI[174,175].Thesemechanisms providetherationalefortheprincipalrecommendationoftheInternationalGuidelines thatwomenwithPCOSshouldbeadvisedaboutdietarymodificationasthefirstlineof managementinallsymptompresentations[38].
3.6.ObesityandtheLeanPCOSParadox
InsightcanbeobtainedintotheroleofobesityinwomenwithPCOSbyexaminingtheevolutionaryhistory,geneticstudiesandpathologicaldisordersofadipose tissue[151,176,177].Theabilitytostoreenergyisabasicfunctionoflifebeginningwith unicellularorganisms[176].Inmulticellularorganisms,fromyeasttohumans,thelargest sourceofstoredenergyisastriglyceridesinlipiddropletsinordertoprovideenergyduring periodswhenenergydemandsexceedcaloricintake[176].Understandingthebiological functionsofadiposetissuehasprogressedfromenergystorageandthermalinsulationto thatofacomplexendocrineorganwithimmuneandinflammatoryeffectsandimportant reproductiveandmetabolicimplications[176,178].
Adiposetissueisorganizedintobrownadiposetissue(BAT)andwhiteadiposetissue (WAT),bothwithdifferentfunctions[178].AlthoughtheevolutionaryoriginsofBATand WATarethesubjectofongoingdebate[176],BATislocatedinthesupraclavicularand thoracicprevertebralareasandisprimarilyinvolvedincoldthermogenesisandregulation ofbasalmetabolicrate[179].WATisdistributedinmultipleanatomicalareassuchas visceraladiposetissue(VAT)andsubcutaneousadiposetissue(SAT)andfunctionsasa fatstoragedepotandanendocrineorgan[178,179].AnadditionallayerofSATisthought tohaveevolvedasinsulationagainstcoolnighttemperaturesinthePleistoceneopenSavanah[180].ThelowerbodydistributionofSATinwomenishypothesizedtohaveevolved toprovideadditionalcaloriestorageforpregnancyandlactationandisuniquetohuman females[14].LowerbodySAThasametabolicprogramthatmakesitlessreadilyavailable forevery-dayenergyneeds,butitcanbemobilizedduringpregnancyandlactation[14]. Inaddition,excessaccumulationofSATismuchlesslikelytocauseIRandmetabolic dysfunctionandexplainswhyIRisnotobservedinallobese individuals[151,181].Visceral WATisassociatedwithIRinwomenwithPCOSleadingtobothmetabolicandreproductive problems[182].
Multiplelinesofevidencefromevolutionaryhistory,geneticandtwinstudies,support ageneticbasisforobesityanddifferencesinobeseandleanphenotypesinwomenwith PCOS[183–186].ThemajorityofwomenwithPCOSareoverweightorobese,withreports rangingfrom38–88%[152,186].StudiescomparingobeseandleanwomenwithPCOShave severalmethodologicalproblemsincludingsmallsamplesize,overlapofPCOScharacteristicswithnormalpubertalchanges,non-standardizeddiagnosticcriteria,andlimited generalizabilitytotheentirepopulationduetoafocusonaspecificethnicgroup[166,182]. Inaddition,mostofthestudiesexaminingbodycompositioninPCOShavereliedon anthropomorphicmeasurements(BMI,waistcircumference,waist-to-hipratio)whichare consideredinaccuratecomparedwiththecurrentgold-standardofmagneticresonance imaging[182].Consequently,thereiswideheterogeneityinreportsexaminingtherelation-
Int.J.Environ.Res.PublicHealth 2022, 19,1336 11of25
shipbetweenbodycompositionmeasures,includingextentofVATandmetabolicchanges suchasIR[186].
Inhumans,thereislargeindividualvariationinthefatstoragecapabilityandexpandabilityofdifferentadiposetissuedepots[151].Ithasbeenhypothesizedthatonce thegeneticallydeterminedlimitofexpandabilityofSATisreached,thereisexpansion ofVATandexcesslipidaccumulationinmuscle,liverandotherorgans,resultinginIR, inflammationandmetabolicdysregulation[151].Wehypothesizethatleanwomenwith PCOShaveageneticallydeterminedlimitedabilitytostoreexcesslipidinSAT,butdevelop increasedlipiddepositioninVATandorganssuchastheliver,resultinginmetabolic dysregulationandIRinasimilarmannertowhatoccursinobesewomenwithPCOS.The widevariationinthegeneticlimitationofSATexpansionisalsosupportedbystudiesin individualswithlipodystrophy.
Lipodystrophiesareaheterogenousgroupofrareinheritedandacquireddisorders characterizedbyaselectivelossofadiposetissue[177,187].Theyareclassifiedonthebasis oftheextentoffatlossasgeneralized,partialorlocalized[187].Patientswithcongenital generalizedlipodystrophyhaveageneralizeddeficiencyoffatfrombirth,usuallyhave severeIRanddevelopdiabetesatpuberty.Asaconsequenceofgeneticallylimitedability forSATlipidstorage,lipidscanonlybestoredectopicallyinnon-adipocytesresultingin majorhealthconsequencesincludingIR,fattyliver,diabetesandPCOS[188].Incontrast togeneralizedlipodystrophy,patientswithfamilialpartiallipodystrophyhavenormal fatdistributionatbirthbutlooseSATinthelimbs,buttocksandhips,atpuberty.Fifty percentofwomendevelopdiabetesand20–35%developirregularperiodsandpolycystic ovaries[177].Despitetherarenatureofthesesyndromesmuchhasbeenlearnedaboutthe underlyinggeneticvariantsinvolved[187].
Elucidationofclinicalsubtypesandthegeneticbackgroundofpatientswithlipodystrophiesmaypavethewaytonewinsightsintotheroleoffatpartitioningandobesity,and hasimplicationsforunderstandingthepathogenesisofinsulinresistance,diabetesand PCOS[177].LeanwomenwithPCOSmayhaveageneticpredispositionforlimitedSAT fatstorage,coupledwithunderlyingmetabolicpredispositionsthatresultindeposition ofexcesslipidinVATandliverandtheobservedmetabolicfeaturesofIR,fattyliverand diabetes.IftheextentofIRandectopicfatdepositionisexcessive,theresultinghormonal changesmaybesufficienttocauseoligomenorrhoeaandsubfertilityasoccurswithsecondaryfamilialpartiallipodystrophytype2[188,189].Ifthisunderlyingmechanismis confirmedinfuturestudies,themaindifferencebetweenwomenwithleanorobesePCOS maybethecombinedeffectsofmetabolicprogrammingandthegeneticallydetermined extentofSCTfatdeposition.Thiswouldexplainwhyleanwomenhaveallthesameclinical, biochemicalandendocrinefeatures,althoughpossiblylesssevere,thanoverweightand obesewomenwithPCOS[186].
3.7.Endocrine-DisruptingChemicalExposure
Anthropomorphicchemicalexposureisubiquitousintheenvironmentandhaspossibleeffectsonmanyaspectsrelatedtowomen’shealthandPCOS[36,190–192].The identificationofmorethan1000EDCinfood,air,water,pesticides,plastics,personalcare products,andotherconsumergoods,raisesspecificconcernsforpregnantwomenand womenwithincreasedsusceptibilitytometabolicdiseasessuchasPCOS[36,172,192–194]. AccumulatingevidencesuggeststhatEDCmaybeinvolvedinthepathogenesisofPCOS giventheirknownandpotentialhormonalandmetaboliceffects[36,190,195].Thisincludes manyoftheareasthathavebeenconsideredintheunifiedevolutionarymodel,suchas developmentalepigeneticprogramming,microbiomecompositionandfunction,metabolic processessuchIR,andregulationofbodyweight.
ManyobservationalstudieshavedemonstratedthepresenceofEDCinmaternaland fetalserumandurine,amnioticfluid,cordbloodandbreastmilk[196–198].Sixclasses ofEDChavebeenshowntocrosstheplacentaconfirmingthatthefetusisexposedatall stagesofdevelopment[109,196].Althoughitisimpossibletoperformexperimentalstudies
Int.J.Environ.Res.PublicHealth 2022, 19,1336 12of25
inhumans,evidencefromepidemiological,moleculartoxicologyandanimalstudies providecompellingevidenceofadversedevelopmentaleffectsandtransgenerational toxicity[172,190,192,199].TherealizationofthetragiceffectsofDESinthe1970′ swasfirst exampleofaninuteroexposurecausingserioustransgenerationalhealtheffects[192].
SeveralestrogenicEDChavebeenassociatedwithbirthoutcomesthatarethoughttobe associatedwiththedevelopmentofPCOS[190].Theseincludedecreasedbirthweight(perfluoroakylsubstances[PFAS],perfluorooctanoicacid)andpretermbirth(di-2-ethylhexyl phthalate)[190].PrenatalexposuretoandrogenicEDC(triclosan,glyphosate,tributyltin, nicotine)isofincreasingconcern,giventhesuspectedepigeneticroleofinuteroandrogen exposureinthepathogenesisofPCOS[48,200,201].
Asaresult,implementationoftheprecautionaryprincipleisahighpriorityincounsellingwomenwithPCOS[202].Internationalprofessionalbodies(TheRoyalCollegeof ObstetriciansandGynecologists,EndocrineSociety,FIGO)haverecommendedthatallpregnantwomenshouldbeadvisedofthepossiblerisksofEDCandthateducationprogramsbe developedtoinformhealthprofessionals[203–205].Anexplanationofthepathogenesisof PCOSshouldincludereferencetoenvironmentalchemicalexposureandopenthewayfor moredetaileddiscussionofspecificpersonalizedadviceandlifestylerecommendations.
3.8.LifestyleContributorstothePathogenesisofPCOS
Severallifestylefactorshavebeeninvestigatedfortheirroleinthepathogenesis ofPCOS.Theseincludediet,exercise,stress,sleepdisturbance,circadiandisruptionand exposuretoenvironmentalchemicals[28,41,206].Recentadvancesingenomics,epigenetics, metabolomics,nutrigenomics,evolutionarybiology,computertechnologyandartificial intelligence,areprovidingmanyinsightsintothemechanismsofhowlifestylefactors impactthepathogenesisofPCOS[9,90,207,208].Nutritionalstudiesbasedondietindices, dietcompositionandmetabolomicshaveidentifieddietarycomponentsthatcontribute toahealthyeatingpattern[51,207,209,210].Healthydietpatterns,orwholefooddiets, havebeenfoundtobeeffectiveincontrollingandreversingmanyofthesymptomsand metabolicalterationsassociatedwithPCOS[50].
Aspreviouslydiscussed,themodernWesterndietandlifestyleisatoddswithour evolutionarybackground.Onedietarycomponentthatdifferssignificantlyinancestral andmodernpopulationsisdietaryfiberintake.Assessmentofdietaryfiberintakeisalsoa goodsurrogatemarkerforahealthywholefooddiet.Ingeneral,ourtraditionalhunter–gathererancestorsconsumedsignificantlymorefiberthanmodernpopulations.Studies thathaveinvestigatedthedietarypatternsofremainingcontemporaryhunter–gatherer societies,havefoundtheirdietaryfiberintaketobearound80–150gperday[211].This contrastswiththecontemporaryWesterndiet,wheretheaveragefiberintakeis18.2gper dayinchildrenand20.7gperdayinadults[212].Adequatedietaryfiberconsumptionis importantasithasseveralbenefits,suchasimprovedinsulinsensitivity,reducedblood glucoselevels,decreasedsystemicinflammation,lowerserumlevelsofandrogensandLPS, allofwhichhavebeenlinkedtothepathogenesisofPCOS[213–216].
Recentsystematicreviewsofobservationalstudiesandrandomizedcontrolledtrialshave founddietaryfiberconsumptiontobeinverselyrelatedtoriskofobesity, type2diabetes, andcardiovasculardisease[217,218].ArecentcohortstudyfromCanadafoundthat obesewomenwithPCOSconsumedsignificantlylessdietaryfiberthannormalweight womenwithoutPCOS[219].Inaddition,fiberintakeofwomenwithPCOSwasnegatively correlatedwithIR,fastinginsulin,glucosetoleranceandserumandrogens[219].Hence, themismatchbetweentheamountoffibertraditionallyconsumedandthefibercontentof Westerndiets,maybeanimportantdietarycomponentcontributingtotheincreasedrates ofPCOSseenindevelopedanddevelopingnations.
3.9.CircadianRhythmDisruptionandPCOS
Thecircadianrhythmisamechanismwithwhichlivingorganismscansynchronize theirinternalbiologicalprocesseswiththeexternallightanddarkpatternoftheday[220].
Int.J.Environ.Res.PublicHealth 2022, 19,1336 13of25
Circadianrhythmshaveformedacentralcomponentoftheevolutionaryadaptationofall organismstoavarietyofenvironmentalconditions,fromprocaryotestocomplexmulticellularorganisms[221–223].Mostorganismsexperiencedailychangesintheirenvironment, includinglightavailability,temperatureandfood.Hundredsofthousandsofyearsof evolutionhavesynchronizedtherhythmicdailyprogrammingofinternalmetabolic,endocrineandbehavioralsystemstotheexternalenvironmentalconditions[222].Circadian clocksanticipateenvironmentalchangesandconferapredictiveadaptivesurvivalbenefit toorganisms.
Thenormalfunctionofthecircadiansystemisbasedonahierarchicalnetworkof centralandperipheralclocks[224].Thecentral,ormasterclock,isinthesuprachiasmicnucleusintheanteriorhypothalamus.Itisstrategicallyplacedtocommunicatewithmultiple physiologicalhomeostaticcontrolnuclei(bodytemperature,metabolicrate,appetite,sleep), pituitaryhormonalsystems(gonadal,thyroid,somatotrophic,adrenal),theautonomic nervoussystem(digestion,heartrate),andconsciouscorticalcenters(behavior,motivation, reward,reproduction)[225].Humansareprogrammedforspecificdayandnight-timesurvivalbehaviorsthatareregulatedbytheavailabilityoftemperature,feedingandsunlight. Photonsoflightstimulatespecializedphotoreceptorsintheretinalganglionlayerwhich transmitanelectricalimpulsetothecellsofthemasterclockviatheretinohypothalamic tract[226].Thecentralclockcanthenconveyrhythmicinformationtoperipheralclocks inothertissuesandorgansthroughoutthebody[224].Feedingandfastingcyclesarethe primarytimecuesforcircadianclocksinperipheraltissues[227].
Circadianclocksexistinallcells,includingthemicrobiome,andfunctionasautonomoustranscriptional-translationalgeneticfeedbackloops[228,229].Thechanging lengthofdaylight,determinedbytherotationoftheearthonitsaxis,requiresthatthe autonomousclocksarereset,orentrained,onadailybasis[230].Themolecularmechanismsofcircadianclocksaresimilaracrossallspeciesandareregulatedbygenetic enhancer/repressorelements,epigeneticmodulationbymethylationandacetylation,posttranslationmodificationofregulatoryproteins,andavarietyofhormonalandsignaling molecules[220,229,231].Thiscomplexinterconnectedregulatoryframework,ensuresthat thesamemoleculesthatregulatemetabolismandreproduction,alsocontributetoabidirectionalfeedbacksystemwiththeautonomouscircadiancircuits[224,231].Thisresultsin synchronicityofinternalphysiologywithenvironmentalcues,tooptimizebothindividual andspeciessurvival.Evolutionhasthereforeprovidedamechanismforhumanstoadapt andsurviveundertheselectivepressuresoffoodscarcity,seasonalchangesinsunlightand arangeoftemperatureexposures.
Theevolutionaryadaptivesurvivalbenefitofsynchronizedcircadiansystemsin ancientpopulationsisinmarkedcontrasttothemultiplecircadiandisruptionsthatare associatedwithmodernlifestyle.Theseincludepoor-qualitydiet[232],impropermeal timingandalteredfeeding-fastingbehavior[233,234],sub-optimalexercisetiming[235],disruptedsleep-wakecycles[236],shiftwork[237],EDC[238],andstress[239,240].Changes inalloftheseparametersarecorrelatedwithsignificantincreasesinobesity,diabetes, cardiovasculardisease,andsomecancers[222].Notsurprisingly,lifestyle-relateddisturbancesofcircadianrhythmshavealsobeeninvestigatedfortheirroleinthepathogenesisofPCOS[35,241,242].Theavailableevidencesuggeststhatcircadiandisruptionhas detrimentaleffectsoninuterodevelopment[243],alteredmetabolismandinsulinresistance[241,244],bodyweightandobesity[245],andfertility[34].Alltheseinfluencesare relevanttoanevolutionarymodelofthepathogenesisofPCOS.
Recognitionoftheimpactoflifestylebehaviorsoncircadiandysregulationand metabolicandreproductivefunction,opensthewayfortargetedinterventionstrategies tomodulateandreversetheseeffects[246].Theseincluderegularmealtiming[222,247], time-restrictedfeeding[248,249],restorationofnormalsleepcycles[250],optimalexercisetiming[235],limitationofexposuretobrightlightatnight[251],andimproveddiet quality[227].RecognitionofcircadiandysfunctionandtheinvestigationoflifestyleinterventionsshouldbeapriorityinbothclinicalmanagementandfutureresearchinPCOS.
Int.J.Environ.Res.PublicHealth 2022, 19,1336 14of25
3.10.ConceptualFrameworkandSummaryoftheUnifiedEvolutionaryModel
TheevolutionarymodelproposesthatPCOSisaconditionthatarisesfromtheinheritanceofgenomicvariantsderivedfromthematernalandpaternalgenome.Inuterofetal metabolic,endocrineandenvironmentalfactorsmodulatedevelopmentalprogrammingof susceptiblegenesandpredisposetheoffspringtodevelopPCOS.Postnatalexposureto poor-qualitydiet,sedentarybehavior,EDC,circadiandisruptionandotherlifestylefactors activateepigeneticallyprogrammedpathways,resultingintheobservedfeatures.
Dietaryfactorscausegastrointestinaldysbiosisandsystemicinflammation,insulin resistanceandhyperandrogenism.Continuedexposuretoadverselifestyleandenvironmentalfactorseventuallyleadstothedevelopmentofassociatedmetabolicconditionssuch asobesity,GDM,diabetes,NAFLDandmetabolicsyndrome(Figure 1).
Balancedevolutionaryselectionpressuresresultintransgenerationaltransmissionof susceptiblegenevariantstoPCOSoffspring.Ongoingexposuretoadversenutritionaland environmentalfactorsactivatedevelopmentallyprogrammedgenesandensuretheperpetuationofthesyndromeinsubsequentgenerations.TheDOHaDcyclecanbeinterrupted atanypointfrompregnancytobirth,childhood,adolescenceoradulthoodbytargeted interventionstrategies(Figure 2).
Insummary,weproposethatPCOSisanenvironmentalmismatchdisorderthat manifestsafterinuterodevelopmentalprogrammingofaclusterofnormalgenevariants. Postnatalexposuretoadverselifestyleandenvironmentalconditionsresultsintheobserved metabolicandendocrinefeatures.PCOSthereforerepresentsamaladaptiveresponseof ancientgeneticsurvivalmechanismstomodernlifestylepractices.
ComprehensiveInternationalGuidelineshavemade166recommendationsforthe assessmentandmanagementofPCOS[38].Webelievethecurrentunifiedevolutionary theoryofthepathogenesisofPCOSprovidesaconceptualframeworkthatmayhelp practitionersandpatientsunderstandthedevelopmentofPCOSsymptomsandpathology inthecontextofourmodernlifestyleandenvironment.Itwillhopefullycontributeto improvedcommunication,resultinimprovedfeelingsofempowermentoverthepersonal manifestationsofPCOS,improvecompliance,reducemorbidity,increasequalityoflifeand informfutureresearch(Figure 3).

4.Conclusions
Substantialevidenceanddiscussionsupportanevolutionarybasisforthepathogenesisofpolycysticovarysyndrome,althoughmanyofthemechanisticdetailsareyetto
Int.J.Environ.Res.PublicHealth 2022, 19,1336 15of25
Figure3. Impactoftheunifiedtheoryonthemanagementofpolycysticovarysyndrome.Reprinted fromRef.[28].
bedetermined.Nevertheless,multiplelinesofevidencefromevolutionarytheory,comparativebiology,genetics,epigenetics,metabolismresearch,andcellbiology,provide supportiveevidenceandhypothesis-generatingdata.Theabilityofanimalstosynchronize internalphysiology,metabolismandreproductivefunction,withourchangingexternal environmentandhabitat,areanecessaryrequirementforindividualandspeciessurvival. Theco-operativeandsometimescompetitiveevolutionofmetabolismandreproduction providedadaptivesurvivalmechanismsinancestralenvironmentsthatappeartobemaladaptiveinmodernenvironments.Anevolutionarymodelthereforeprovidesaframework toenhancepractitionerandpatientunderstanding,improvecompliancewithlifestyleinterventions,reducemorbidity,improvequalityoflifeandwillevolveandchangeovertime.
AuthorContributions: J.P.conceptualized,designedandwrotetheoriginaldraftofthemanuscript; C.O.conceptualization,writing,reviewedandeditedthemanuscript;J.H.conceptualization,writing, reviewedandeditedthemanuscript;F.L.G.conceptualized,reviewed,editedandsignificantly improvedthemanuscript.Allauthorscriticallyrevisedthemanuscript.Allauthorshavereadand agreedtothepublishedversionofthemanuscript.
Funding: Thisresearchreceivednoexternalfunding.
InstitutionalReviewBoardStatement: Notapplicable.
InformedConsentStatement: Notapplicable.
DataAvailabilityStatement: Notapplicable.
Acknowledgments: PermissiontoreprintFigures 1–3 wereobtainedfromtheJournaloftheAustralasianCollegeofNutritionalandEnvironmentalMedicine.
ConflictsofInterest: Theauthorsdeclarenoconflictofinterest.
References
1. Bodai,B.I.;Nakata,T.E.;Wong,W.T.;Clark,D.R.;Lawenda,S.;Tsou,C.;Liu,R.;Shiue,L.;Cooper,N.;Rehbein,M.;etal.Lifestyle Medicine:ABriefReviewofItsDramaticImpactonHealthandSurvival. Perm.J. 2017, 22,17–25.[CrossRef][PubMed]
2. McMacken,M.;Shah,S.Aplant-baseddietforthepreventionandtreatmentoftype2diabetes. J.Geriatr.Cardiol. 2017, 14, 342–354.[CrossRef][PubMed]
3. Gakidou,E.;Afshin,A.;Abajobir,A.A.;Abate,K.H.;Abbafati,C.;Abbas,K.M.;Abd-Allah,F.;Abdulle,A.M.;Abera,S.F.; Aboyans,V.;etal.Global,regional,andnationalcomparativeriskassessmentof84behavioural,environmentalandoccupational, andmetabolicrisksorclustersofrisks,1990–2016:AsystematicanalysisfortheGlobalBurdenofDiseaseStudy2016. Lancet 2017, 390,1345–1422.[CrossRef]
4. Parker,J.NEM:ANewParadigmforUnderstandingtheCommonOriginsoftheChronicDiseaseEpidemic. ACNEMJ. 2018, 37,6–11.
5. Glastras,S.J.;Valvi,D.;Bansal,A.Editorial:Developmentalprogrammingofmetabolicdiseases. Front.Endocrinol. 2021, 12. [CrossRef]
6. Zore,T.;Joshi,N.V.;Lizneva,D.;Azziz,R.PolycysticOvarianSyndrome:Long-TermHealthConsequences. Semin.Reprod.Med. 2017, 35,271–281.[CrossRef]
7. Teede,H.;Misso,M.;Costello,M.;Dokras,A.;Laven,J.;Moran,L.;Piltonen,T.;Norman,R. InternationalEvidence-BasedGuideline fortheAssessmentandManagementofPolycysticOvarySyndrome2018;NationalHealthandMedicalResearchCouncil[NHMRC]: Canberra,Australia,2018;pp.1–198;ISBN9780646554709.
8.Benton,M.L.Theinfluenceofevolutionaryhistoryonhumanhealthanddisease. Nat.Rev.Genet. 2021, 22,269–283.[CrossRef]
9. Painter,D.Theevolutionofevolutionarymedicine.In TheDynamicsofScience:ComputationalFrontiersinHistoryandPhilosophyof Science;PittsburghUniversityPress:Pittsburgh,PA,USA,2020.
10.Fay,J.C.Diseaseconsequencesofhumanadaptation. Appl.Transl.Genom. 2013, 2,42–47.[CrossRef]
11. Pathak,G.;Nichter,M.PolycysticovarysyndromeinglobalizingIndia:Anecosocialperspectiveonanemerginglifestyledisease. Soc.Sci.Med. 2015, 146,21–28.[CrossRef]
12. Parker,J.;O’Brien,C.Evolutionaryandgeneticantecedentstothepathogenesisofpolycysticovarysyndrome[PCOS]. J.ACNEM 2021, 40,12–20.
13. Stearns,S.C.Evolutionarymedicine:Itsscope,interestandpotential. Proc.R.Soc.BBiol.Sci. 2012, 279,4305–4321.[CrossRef] [PubMed]
14. Tsatsoulis,A.;Mantzaris,M.D.;Sofia,B.;Andrikoula,M.Insulinresistance:Anadaptivemechanismbecomesmaladaptiveinthe currentenvironment—Anevolutionaryperspective. Metabolism 2013, 62,622–633.[CrossRef][PubMed]
Int.J.Environ.Res.PublicHealth 2022, 19,1336 16of25
15. Shaw,L.M.A.;Elton,S.Polycysticovarysyndrome:Atransgenerationalevolutionaryadaptation. BJOGInt.J.Obstet.Gynaecol. 2008, 115,144–148.[CrossRef][PubMed]
16. Charifson,M.A.;Trumble,B.C.Evolutionaryoriginsofpolycysticovarysyndrome:Anenvironmentalmismatchdisorder. Evol.Med.PublicHealth 2019, 2019,50–63.[CrossRef]
17. Azziz,R.;Dumesic,D.A.;Goodarzi,M.O.Polycysticovarysyndrome:Anancientdisorder? Fertil.Steril. 2011, 95,1544–1548. [CrossRef]
18. Teede,H.;Deeks,H.;Moran,L.Polycysticovarysyndrome:Acomplexconditionwithpsychological,reproductiveandmetabolic manifestationsthatimpactsonhealthacrossthelifespan. BMCMed. 2010, 8,41.[CrossRef]
19. Sanchez-Garrido,M.A.;Tena-Sempere,M.Metabolicdysfunctioninpolycysticovarysyndrome:Pathogenicroleofandrogen excessandpotentialtherapeuticstrategies. Mol.Metab. 2020, 35,100937.[CrossRef]
20. Glueck,C.J.;Goldenberg,N.Characteristicsofobesityinpolycysticovarysyndrome:Etiology,treatment,andgenetics. Metabolism 2019, 92,108–120.[CrossRef]
21. Reyes-Muñoz,E.;Castellanos-Barroso,G.;Ramírez-Eugenio,B.Y.;Ortega-González,C.;Parra,A.;Castillo-Mora,A.;DeLa Jara-Díaz,J.F.TheriskofgestationaldiabetesmellitusamongMexicanwomenwithahistoryofinfertilityandpolycysticovary syndrome. Fertil.Steril. 2012, 97,1467–1471.[CrossRef]
22. Rodgers,R.J.;Avery,J.C.;Moore,V.M.;Davies,M.J.;Azziz,R.;Stener-Victorin,E.;Moran,L.J.;Robertson,S.A.;Stepto,N.K.;Norman,R.J.;etal.Complexdiseasesandco-morbidities:Polycysticovarysyndromeandtype2diabetesmellitus. Endocr.Connect. 2019, 8,R71–R75.[CrossRef]
23. Wu,J.;Yao,X.Y.;Shi,R.X.;Liu,S.F.;Wang,X.Y.Apotentiallinkbetweenpolycysticovarysyndromeandnon-alcoholicfattyliver disease:Anupdatemeta-analysis. Reprod.Health 2018, 15,77.[CrossRef][PubMed]
24. Yumiceba,V.;López-Cortés,A.;Pérez-Villa,A.;Yumiseba,I.;Guerrero,S.;García-Cárdenas,J.M.;Armendáriz-Castillo,I.;GuevaraRamírez,P.;Leone,P.E.;Zambrano,A.K.;etal.OncologyandPharmacogenomicsInsightsinPolycysticOvarySyndrome:An IntegrativeAnalysis. Front.Endocrinol. 2020, 11,840.[CrossRef][PubMed]
25. Li,G.;Hu,J.;Zhang,S.;Fan,W.;Wen,L.;Wang,G.;Zhang,D.ChangesinResting-StateCerebralActivityinWomenWith PolycysticOvarySyndrome:AFunctionalMRImagingStudy. Front.Endocrinol. 2020, 11,981.[CrossRef][PubMed]
26. Cooper,H.;Spellacy,W.N.;Prem,K.A.;Cohen,W.D.HereditaryfactorsintheSteinleventhal1968. Am.J.Obstet.Gynecol. 1968, 100,371–387.[CrossRef]
27. Diamanti-Kandarakis,E.;Piperi,C.Geneticsofpolycysticovarysyndrome:Searchingforthewayoutofthelabyrinth. Hum.Reprod.Update 2005, 11,631–643.[CrossRef][PubMed]
28. Parker,J.UnderstandingthePathogenesisofPolycysticOvarySyndrome:Atransgenerationalevolutionaryadaptationtolifestyle andtheenvironment. ACNEMJ. 2020, 39,18–26.
29. Day,F.;Karaderi,T.;Jones,M.R.;Meun,C.;He,C.;Drong,A.;Kraft,P.;Lin,N.;Huang,H.;Broer,L.;etal.Large-scale genome-widemeta-analysisofpolycysticovarysyndromesuggestssharedgeneticarchitecturefordifferentdiagnosiscriteria. PLoSGenet. 2018, 14,e1007813.[CrossRef]
30. Crespo,R.P.;Bachega,T.A.S.S.;Mendonça,B.B.;Gomes,L.G.AnupdateofgeneticbasisofPCOSpathogenesis. Arch.Endocrinol.Metab. 2018, 62,352–361.[CrossRef]
31. Jones,M.R.;Goodarzi,M.O.Geneticdeterminantsofpolycysticovarysyndrome:Progressandfuturedirections. Fertil.Steril. 2016, 106,25–32.[CrossRef]
32. Varanasi,L.C.;Subasinghe,A.;Jayasinghe,Y.L.;Callegari,E.T.;Garland,S.M.;Gorelik,A.;Wark,J.D.Polycysticovariansyndrome: PrevalenceandimpactonthewellbeingofAustralianwomenaged16–29years. Aust.N.Z.J.Obstet.Gynaecol. 2018, 58,222–233. [CrossRef]
33. Ding,T.;Hardiman,P.J.;Petersen,I.;Wang,F.F.;Qu,F.;Baio,G.Theprevalenceofpolycysticovarysyndromeinreproductiveaged womenofdifferentethnicity:Asystematicreviewandmeta-analysis. Oncotarget 2017, 8,96351–96358.[CrossRef][PubMed]
34. Shao,S.;Zhao,H.;Lu,Z.;Lei,X.;Zhang,Y.Circadianrhythmswithinthefemalehpgaxis:Fromphysiologytoetiology. Endocrinology 2021, 162,bqab117.[CrossRef][PubMed]
35. Wang,F.;Xie,N.;Wu,Y.;Zhang,Q.;Zhu,Y.;Dai,M.;Zhou,J.;Pan,J.;Tang,M.;Cheng,Q.;etal.Associationbetweencircadian rhythmdisruptionandpolycysticovarysyndrome. Fertil.Steril. 2021, 115,771–781.[CrossRef][PubMed]
36. Piazza,M.J.;Urbanetz,A.A.Environmentaltoxinsandtheimpactofotherendocrinedisruptingchemicalsinwomen’sreproductivehealth. J.Bras.Reprod.Assist. 2019, 23,154–164.[CrossRef][PubMed]
37. Basu,B.;Chowdhury,O.;Saha,S.Possiblelinkbetweenstress-relatedfactorsandalteredbodycompositioninwomenwith polycysticovariansyndrome. J.Hum.Reprod.Sci. 2018, 11,10–18.[CrossRef]
38. Teede,H.J.;Misso,M.L.;Costello,M.F.;Dokras,A.;Laven,J.;Moran,L.;Piltonen,T.;Norman,R.J.;Andersen,M.;Azziz,R.;etal. Recommendationsfromtheinternationalevidence-basedguidelinefortheassessmentandmanagementofpolycysticovary syndrome. Fertil.Steril. 2018, 110,364–379.[CrossRef]
39. Casarini,L.;Simoni,M.;Brigante,G.Ispolycysticovarysyndromeasexualconflict?Areview. Reprod.Biomed.Online 2016, 32, 350–361.[CrossRef]
40. Tremellen,K.;Pearce,K.DysbiosisofGutMicrobiota[DOGMA]—AnoveltheoryforthedevelopmentofPolycysticOvarian Syndrome. Med.Hypotheses 2012, 79,104–112.[CrossRef]
Int.J.Environ.Res.PublicHealth 2022, 19,1336 17of25
41. Parker,J.;O’Brien,C.;Gersh,F.L.Developmentaloriginsandtransgenerationalinheritanceofpolycysticovarysyndrome. Aust.N.Z.J.Obstet.Gynaecol. 2021, 61,922–926.[CrossRef]
42. Abbott,D.H.;Dumesic,D.A.;Franks,S.Developmentaloriginofpolycysticovarysyndrome—Ahypothesis. J.Endocrinol. 2002, 174,1–5.[CrossRef]
43. Rosenfield,R.L.;Ehrmann,D.A.ThePathogenesisofPolycysticOvarySyndrome[PCOS]:ThehypothesisofPCOSasfunctional ovarianhyperandrogenismrevisited. Endocr.Rev. 2016, 37,467–520.[CrossRef]
44. Stener-Victorin,E.;Padmanabhan,V.;Walters,K.A.;Campbell,R.E.;Benrick,A.;Giacobini,P.;Dumesic,D.A.;Abbott,D.H. AnimalModelstoUnderstandtheEtiologyandPathophysiologyofPolycysticOvarySyndrome. Endocr.Rev. 2020, 41,538–576. [CrossRef][PubMed]
45. Abbott,D.H.;Dumesic,D.A.;Abbott,D.H.Fetalandrogenexcessprovidesadevelopmentaloriginforpolycysticovarysyndrome. ExpertRev.Obs.Gynecol. 2009, 4,1–7.[CrossRef]
46. Abbott,D.H.;Kraynak,M.;Dumesic,D.A.;Levine,J.E.InuteroAndrogenExcess:ADevelopmentalCommonalityPreceding PolycysticOvarySyndrome? Front.Horm.Res. 2019, 53,1–17.[CrossRef][PubMed]
47. Abbott,D.H.;Rayome,B.H.;Dumesic,D.A.;Lewis,K.C.;Edwards,A.K.;Wallen,K.;Wilson,M.E.;Appt,S.E.;Levine,J.E. ClusteringofPCOS-liketraitsinnaturallyhyperandrogenicfemalerhesusmonkeys. Hum.Reprod. 2017, 32,923–936.[CrossRef] [PubMed]
48. Hewlett,M.;Chow,E.;Aschengrau,A.;Mahalingaiah,S.PrenatalExposuretoEndocrineDisruptors:ADevelopmentalEtiology forPolycysticOvarySyndrome. Reprod.Sci. 2017, 24,19–27.[CrossRef]
49.Neel,J.V.DiabetesMellitis:A“thrifty”genotyperendereddetrimentalby“progress”? Am.J.Hum.Genet. 1962, 14,353–362.
50. Parker,J.;Hawrelak,J.;Gersh,F.L.Nutritionalroleofpolyphenolsasacomponentofawholefooddietinthemanagementof polycysticovarysyndrome. J.ACNEM 2021, 40,6–12.
51. Tremellen,K.P.K.Nutrition,Fertility,andHumanReproductiveFunction.In Nutrition,Fertility,andHumanReproductiveFunction; CRCPress:Adelaide,Astralia,2015;pp.27–50.
52. Rizk,M.G.;Thackray,V.G.IntersectionofPolycysticOvarySyndromeandtheGutMicrobiome. J.Endocr.Soc. 2021, 5,bvaa177. [CrossRef]
53. He,F.F.;Li,Y.M.Roleofgutmicrobiotainthedevelopmentofinsulinresistanceandthemechanismunderlyingpolycysticovary syndrome:Areview. J.OvarianRes. 2020, 13,73.[CrossRef]
54. Chen,F.Analysisofthegutmicrobialcompositioninpolycysticovarysyndromewithacne. ZigongMatern.ChildHealthHosp. 2019, 35,2246–2251.
55. Zhou,L.;Ni,Z.;Cheng,W.;Yu,J.;Sun,S.;Zhai,D.;Yu,C.;Cai,Z.Characteristicgutmicrobiotaandpredictedmetabolicfunctions inwomenwithPCOS. Endocr.Connect. 2020, 9,63–73.[CrossRef][PubMed]
56. Tabrizi,R.;Ostadmohammadi,V.;Akbari,M.;Lankarani,K.B.;Vakili,S.;Peymani,P.;Karamali,M.;Kolahdooz,F.;Asemi,Z. TheEffectsofProbioticSupplementationonClinicalSymptom,WeightLoss,GlycemicControl,LipidandHormonalProfiles, BiomarkersofInflammation,andOxidativeStressinWomenwithPolycysticOvarySyndrome:ASystematicReviewand Meta-analysisofRa. ProbioticsAntimicrob.Proteins 2019,1–14.[CrossRef][PubMed]
57. Darvishi,S.;Rafraf,M.;Asghari-Jafarabadi,M.;Farzadi,L.SynbioticSupplementationImprovesMetabolicFactorsandObesity ValuesinWomenwithPolycysticOvarySyndromeIndependentofAffectingApelinLevels:ARandomizedDouble-Blind Placebo-ControlledClinicalTrial. Int.J.Fertil.Steril. 2021, 15,51–59.[CrossRef][PubMed]
58. Karimi,E.;Moini,A.;Yaseri,M.;Shirzad,N.;Sepidarkish,M.;Hossein-Boroujerdi,M.;Hosseinzadeh-Attar,M.J.Effectsof synbioticsupplementationonmetabolicparametersandapelininwomenwithpolycysticovarysyndrome:Arandomised double-blindplacebo-controlledtrial. Br.J.Nutr. 2018, 119,398–406.[CrossRef][PubMed]
59. Stein,I.F.;Leventhal,M.L.Amenorrheaassociatedwithbilateralpolycysticovaries. Am.J.Obstet.Gynecol. 1935, 29,181–191. [CrossRef]
60. Corbett,S.;Morin-Papunen,L.ThePolycysticOvarySyndromeandrecenthumanevolution. Mol.Cell.Endocrinol. 2013, 373, 39–50.[CrossRef]
61. Rodgers,R.J.;Suturina,L.;Lizneva,D.;Davies,M.J.;Hummitzsch,K.;Irving-Rodgers,H.F.;Robertson,S.A.Ispolycysticovary syndromea20thCenturyphenomenon? Med.Hypotheses 2019, 124,31–34.[CrossRef]
62. Holte,J.Polycysticovarysyndromeandinsulinresistance:Thriftygenesstrugglingwithover-feedingandsedentarylifestyle? J.Endocrinol.Investig. 1998, 21,589–601.[CrossRef]
63. Corbett,S.J.;McMichael,A.J.;Prentice,A.M.Type2diabetes,cardiovasculardisease,andtheevolutionaryparadoxofthe polycysticovarysyndrome:Afertilityfirsthypothesis. Am.J.Hum.Biol. 2009, 21,587–598.[CrossRef]
64. Dinsdale,N.L.;Crespi,B.J.Endometriosisandpolycysticovarysyndromearediametricdisorders. Evol.Appl. 2021, 14,1693–1715. [CrossRef][PubMed]
65.Sonagra,A.D.NormalPregnancy—AStateofInsulinResistance. J.Clin.DiagnosticRes. 2014, 8,CC01.[CrossRef][PubMed]
66. Lipovka,Y.;Chen,H.;Vagner,J.;Price,T.J.;Tsao,T.S.;Konhilas,J.P.Oestrogenreceptorsinteractwiththe α-catalyticsubunitof AMP-activatedproteinkinase. Biosci.Rep. 2015, 35,e00264.[CrossRef]
67. López,M.;Tena-Sempere,M.EstradioleffectsonhypothalamicAMPKandBATthermogenesis:Agatewayforobesitytreatment? Pharmacol.Ther. 2017, 178,109–122.[CrossRef][PubMed]
Int.J.Environ.Res.PublicHealth 2022, 19,1336 18of25
68. Rettberg,J.R.;Yao,J.;Brinton,R.D.Estrogen:Amasterregulatorofbioenergeticsystemsinthebrainandbody. Front.Neuroendocrinol. 2014, 35,8–30.[CrossRef][PubMed]
69. Alaaraj,N.;Soliman,A.;Hamed,N.;Alyafei,F.;DeSanctis,V.Understandingthecomplexroleofmtorcasanintracellularcritical mediatorofwhole-bodymetabolisminanorexianervosa:Aminireview. ActaBiomed. 2021, 92,e2021170.[CrossRef][PubMed]
70. Seif,M.W.;Diamond,K.;Nickkho-Amiry,M.Obesityandmenstrualdisorders. BestPract.Res.Clin.Obstet.Gynaecol. 2015, 29, 516–527.[CrossRef]
71. Draper,C.F.;Duisters,K.;Weger,B.;Chakrabarti,A.;Harms,A.C.;Brennan,L.;Hankemeier,T.;Goulet,L.;Konz,T.;Martin,F.P.; etal.Menstrualcyclerhythmicity:Metabolicpatternsinhealthywomen. Sci.Rep. 2018, 8,14568.[CrossRef]
72. Roh,E.;Song,D.K.;Kim,M.S.Emergingroleofthebraininthehomeostaticregulationofenergyandglucosemetabolism. Exp.Mol.Med. 2016, 48,e216.[CrossRef]
73.Ong,Q.;Han,W.;Yang,X.O-GlcNAcasanintegratorofsignalingpathways. Front.Endocrinol. 2018, 9,599.[CrossRef]
74. Gnocchi,D.;Bruscalupi,G.Circadianrhythmsandhormonalhomeostasis:Pathophysiologicalimplications. Biology 2017, 6,10. [CrossRef][PubMed]
75. Ludwig,D.S.;Aronne,L.J.;Astrup,A.;deCabo,R.;Cantley,L.C.;Friedman,M.I.;Heymsfield,S.B.;Johnson,J.D.;King,J.C.; Krauss,R.M.;etal.Thecarbohydrate-insulinmodel:Aphysiologicalperspectiveontheobesitypandemic. Am.J.Clin.Nutr. 2021, 114,1873–1885.[CrossRef]
76. Gluckman,P.D.;Hanson,M.A.Developmentalandepigeneticpathwaystoobesity:Anevolutionary-developmentalperspective. Int.J.Obes. 2008, 32,S62–S71.[CrossRef][PubMed]
77. Balakumar,P.;Maung-U,K.;Jagadeesh,G.Prevalenceandpreventionofcardiovasculardiseaseanddiabetesmellitus. Pharmacol.Res. 2016, 113,600–609.[CrossRef]
78. Crosignani,P.G.;Nicolosi,A.E.Polycysticovarydisease:Heritabilityandheterogeneity. Hum.Reprod.Update 2001, 7,3–7. [CrossRef][PubMed]
79. Vink,J.M.;Sadrzadeh,S.;Lambalk,C.B.;Boomsma,D.I.HeritabilityofpolycysticovarysyndromeinaDutchtwin-familystudy. J.Clin.Endocrinol.Metab. 2006, 91,2100–2104.[CrossRef][PubMed]
80. Kahsar-Miller,M.D.;Nixon,C.;Boots,L.R.;Go,R.C.;Azziz,R.Prevalenceofpolycysticovarysyndrome[PCOS]infirst-degree relativesofpatientswithPCOS. Fertil.Steril. 2001, 75,53–58.[CrossRef]
81. Dunaif,A.Perspectivesinpolycysticovarysyndrome:Fromhairtoeternity. J.Clin.Endocrinol.Metab. 2016, 101,759–768. [CrossRef]
82.Kosova,G.;Urbanek,M.Geneticsofthepolycysticovarysyndrome. Mol.Cell.Endocrinol. 2013, 373,29–38.[CrossRef]
83. Legro,R.S.;Driscoll,D.;Strauss,J.F.;Fox,J.;Dunaif,A.Evidenceforageneticbasisforhyperandrogenemiainpolycysticovary syndrome. Proc.Natl.Acad.Sci.USA 1998, 95,14956–14960.[CrossRef]
84. Lander,E.S.;Linton,L.M.;Birren,B.;Nusbaum,C.;Zody,M.C.;Baldwin,J.;Devon,K.;Dewar,K.;Doyle,M.;Fitzhugh,W.;etal. Initialsequencingandanalysisofthehumangenome:InternationalHumanGenomeSequencingConsortium. Nature 2001, 409, 860–921,Erratumin Nature 2001, 411,720.[CrossRef]
85. Belmont,J.W.;Boudreau,A.;Leal,S.M.;Hardenbol,P.;Pasternak,S.;Wheeler,D.A.;Willis,T.D.;Yu,F.;Yang,H.;Gao,Y.;etal.A haplotypemapofthehumangenome. Nature 2005, 437,1299–1320.[CrossRef]
86. Welt,C.K.GeneticsofPolycysticOvarySyndrome:WhatisNew? Endocrinol.Metab.Clin.N.Am. 2021, 50,71–82.[CrossRef] [PubMed]
87. Chen,Z.J.;Zhao,H.;He,L.;Shi,Y.;Qin,Y.;Shi,Y.;Li,Z.;You,L.;Zhao,J.;Liu,J.;etal.Genome-wideassociationstudyidentifies susceptibilitylociforpolycysticovarysyndromeonchromosome2p16.3,2p21and9q33.3. Nat.Genet. 2011, 43,55–59.[CrossRef] [PubMed]
88. Hayes,M.G.;Urbanek,M.;Ehrmann,D.A.;Armstrong,L.L.;Lee,J.Y.;Sisk,R.;Karaderi,T.;Barber,T.M.;McCarthy,M.I.;Franks, S.;etal.Genome-wideassociationofpolycysticovarysyndromeimplicatesalterationsingonadotropinsecretioninEuropean ancestrypopulations. Nat.Commun. 2015, 6,7502.[CrossRef][PubMed]
89. Zhang,Y.;Ho,K.;Keaton,J.M.;Hartzel,D.N.;Day,F.;Justice,A.E.;Josyula,N.S.;Pendergrass,S.A.;Actkins,K.E.;Davis,L.K.;etal. Agenome-wideassociationstudyofpolycysticovarysyndromeidentifiedfromelectronichealthrecords. Am.J.Obstet.Gynecol. 2020, 223,559.e1–559.e21.[CrossRef]
90. Zhu,T.;Goodarzi,M.O.Causesandconsequencesofpolycysticovarysyndrome:InsightsfromMendelianRandomization. J.Clin.Endocrinol.Metab. 2021.[CrossRef]
91. Sun,Q.;Gao,Y.;Yang,J.;Lu,J.;Feng,W.;Yang,W.MendelianRandomizationAnalysisIdentifiedPotentialGenesPleiotropically AssociatedwithPolycysticOvarySyndrome. Reprod.Sci. 2021,1–10.[CrossRef]
92. Visscher,P.M.;Brown,M.A.;McCarthy,M.I.;Yang,J.FiveyearsofGWASdiscovery. Am.J.Hum.Genet. 2012, 90,7–24.[CrossRef]
93. Zhu,Z.;Zhang,F.;Hu,H.;Bakshi,A.;Robinson,M.R.;Powell,J.E.;Montgomery,G.W.;Goddard,M.E.;Wray,N.R.;Visscher, P.M.;etal.IntegrationofsummarydatafromGWASandeQTLstudiespredictscomplextraitgenetargets. Nat.Genet. 2016, 48, 481–487.[CrossRef]
94. Rung,J.;Cauchi,S.;Albrechtsen,A.;Shen,L.;Rocheleau,G.;Cavalcanti-Proença,C.;Bacot,F.;Balkau,B.;Belisle,A.;BorchJohnsen,K.;etal.GeneticvariantnearIRS1isassociatedwithtype2diabetes,insulinresistanceandhyperinsulinemia. Nat.Genet. 2009, 41,1110–1115.[CrossRef][PubMed]
Int.J.Environ.Res.PublicHealth 2022, 19,1336 19of25
95. Udler,M.S.;McCarthy,M.I.;Florez,J.C.;Mahajan,A.GeneticRiskScoresforDiabetesDiagnosisandPrecisionMedicine. Endocr.Rev. 2019, 40,1500–1520.[CrossRef][PubMed]
96. Khera,A.V.;Chaffin,M.;Wade,K.H.;Zahid,S.;Brancale,J.;Xia,R.;Distefano,M.;Senol-Cosar,O.;Haas,M.E.;Bick,A.;etal. PolygenicPredictionofWeightandObesityTrajectoriesfromBirthtoAdulthood. Cell 2019, 177,587–596.e9.[CrossRef]
97. Plomin,R.;Haworth,C.M.A.;Davis,O.S.P.Commondisordersarequantitativetraits. Nat.Rev.Genet. 2009, 10,872–878. [CrossRef][PubMed]
98. Dumesic,D.A.;Hoyos,L.R.;Chazenbalk,G.D.;Naik,R.;Padmanabhan,V.;Abbott,D.H.Mechanismsofintergenerational transmissionofovarysyndrome. Reproduction 2020, 159,R1–R13.[CrossRef][PubMed]
99. Sloboda,D.M.;Hickey,M.;Hart,R.Reproductioninfemales:Theroleoftheearlylifeenvironment. Hum.Reprod.Update 2011, 17, 210–227.[CrossRef][PubMed]
100. Xu,R.;Li,C.;Liu,X.;Gao,S.Insightsintoepigeneticpatternsinmammalianearlyembryos. ProteinCell 2021, 12,7–28.[CrossRef] [PubMed]
101. Glastras,S.J.;Chen,H.;Pollock,C.A.;Saad,S.Maternalobesityincreasestheriskofmetabolicdiseaseandimpactsrenalhealthin offspring. Biosci.Rep. 2018, 38,BSR20180050.[CrossRef][PubMed]
102. Simeoni,U.;Armengaud,J.B.;Siddeek,B.;Tolsa,J.F.PerinatalOriginsofAdultDisease. Neonatology 2018, 113,393–399.[CrossRef]
103. Risnes,K.;Bilsteen,J.F.;Brown,P.;Pulakka,A.;Andersen,A.M.N.;Opdahl,S.;Kajantie,E.;Sandin,S.MortalityAmongYoung AdultsBornPretermandEarlyTermin4NordicNations. JAMANetw.Open 2021, 4,e2032779.[CrossRef]
104. Behere,R.V.;Deshmukh,A.S.;Otiv,S.;Gupte,M.D.;Yajnik,C.S.MaternalVitaminB12StatusDuringPregnancyandIts AssociationWithOutcomesofPregnancyandHealthoftheOffspring:ASystematicReviewandImplicationsforPolicyinIndia. Front.Endocrinol. 2021, 12,288.[CrossRef][PubMed]
105.Azziz,R.Animalmodelsofpcosnottherealthing. Nat.Rev.Endocrinol. 2017, 13,382–384.[CrossRef][PubMed]
106. Poon,K.BehavioralFeedingCircuit:DietaryFat-InducedEffectsofInflammatoryMediatorsintheHypothalamus. Front.Endocrinol. 2020, 11,905.[CrossRef][PubMed]
107. Bateson,P.;Gluckman,P.;Hanson,M.ThebiologyofdevelopmentalplasticityandthePredictiveAdaptiveResponsehypothesis. J.Physiol. 2014, 592,2357–2368.[CrossRef][PubMed]
108. Catalano,P.M.;Presley,L.;Minium,J.;Mouzon,S.H.DeFetusesofobesemothersdevelopinsulinresistanceinutero. DiabetesCare 2009, 32,1076–1080.[CrossRef][PubMed]
109. Rosenfeld,C.S.TranscriptomicsandOtherOmicsApproachestoInvestigateEffectsofXenobioticsonthePlacenta. Front.CellDev.Biol. 2021, 9,723656.[CrossRef]
110. DeMelo,A.S.;Dias,S.V.;DeCarvalhoCavalli,R.;Cardoso,V.C.;Bettiol,H.;Barbieri,M.A.;Ferriani,R.A.;Vieira,C.S.Pathogenesis ofpolycysticovarysyndrome:Multifactorialassessmentfromthefoetalstagetomenopause. Reproduction 2015, 150,R11–R24. [CrossRef]
111. Schulz,L.C.TheDutchhungerwinterandthedevelopmentaloriginsofhealthanddisease. Proc.Natl.Acad.Sci.USA 2010, 107, 16757–16758.[CrossRef]
112. Gaillard,R.Maternalobesityduringpregnancyandcardiovasculardevelopmentanddiseaseintheoffspring. Eur.J.Epidemiol. 2015, 30,1141–1152.[CrossRef]
113. Ishimwe,J.A.Maternalmicrobiomeinpreeclampsiapathophysiologyandimplicationsonoffspringhealth. Physiol.Rep. 2021, 9, e14875.[CrossRef]
114.Gilbert,J.A.;Blaser,M.J.;Caporaso,J.G.;Jansson,J.K.;Lynch,S.V.;Knight,R.Currentunderstandingofthehumanmicrobiome. Nat.Med. 2018, 24,392–400.[CrossRef][PubMed]
115. Valdes,A.M.;Walter,J.;Segal,E.;Spector,T.D.Roleofthegutmicrobiotainnutritionandhealth. BMJ 2018, 361,36–44.[CrossRef]
116. Rinninella,E.;Raoul,P.;Cintoni,M.;Franceschi,F.;Miggiano,G.A.D.;Gasbarrini,A.;Mele,M.C.Whatisthehealthygut microbiotacomposition?Achangingecosystemacrossage,environment,diet,anddiseases. Microorganisms 2019, 7,14. [CrossRef][PubMed]
117. Schmidt,T.S.B.;Raes,J.;Bork,P.TheHumanGutMicrobiome:FromAssociationtoModulation. Cell 2018, 172,1198–1215. [CrossRef]
118. Davenport,E.R.;Sanders,J.G.;Song,S.J.;Amato,K.R.;Clark,A.G.;Knight,R.Thehumanmicrobiomeinevolution. BMCBiol. 2017, 15,127.[CrossRef]
119. Theis,K.R.;Dheilly,N.M.;Klassen,J.L.;Brucker,R.M.;Baines,J.F.;Bosch,T.C.G.;Cryan,J.F.;Gilbert,S.F.;Goodnight,C.J.;Lloyd, E.A.;etal.GettingtheHologenomeConceptRight:AnEco-EvolutionaryFrameworkforHostsandTheirMicrobiomes. mSystems 2016, 1,e00028-16.[CrossRef][PubMed]
120. Douglas,A.E.;Werren,J.H.Holesinthehologenome:Whyhost-microbesymbiosesarenotholobionts. mBio 2016, 7,e02099-15. [CrossRef]
121. Goodrich,J.K.;Davenport,E.R.;Beaumont,M.;Jackson,M.A.;Knight,R.;Ober,C.;Spector,T.D.;Bell,J.T.;Clark,A.G.;Ley,R.E. GeneticDeterminantsoftheGutMicrobiomeinUKTwins. CellHostMicrobe 2016, 19,731–743.[CrossRef]
122. Wang,J.;Thingholm,L.B.;Skieceviˇcie,J.;Rausch,P.;Kummen,M.;Hov,J.R.;Degenhardt,F.;Heinsen,F.A.;Rühlemann,M.C.; Szymczak,S.;etal.Genome-wideassociationanalysisidentifiesvariationinVitaminDreceptorandotherhostfactorsinfluencing thegutmicrobiota. Nat.Genet. 2016, 48,1396–1406.[CrossRef]
Int.J.Environ.Res.PublicHealth 2022, 19,1336 20of25
123. Liston,A.;Humblet-Baron,S.;Duffy,D.;Goris,A.Humanimmunediversity:Fromevolutiontomodernity. Nat.Immunol. 2021, 22,1479–1489.[CrossRef]
124. Meyerson,N.R.;Sawyer,S.L.Two-steppingthroughtime:Mammalsandviruses. TrendsMicrobiol. 2011, 19,286–294.[CrossRef] [PubMed]
125. Greene,L.K.;Williams,C.V.;Junge,R.E.;Mahefarisoa,K.L.;Rajaonarivelo,T.;Rakotondrainibe,H.;O’Connell,T.M.;Drea,C.M.A roleforgutmicrobiotainhostnichedifferentiation. ISMEJ. 2020, 14,1675–1687.[CrossRef][PubMed]
126. Schnorr,S.L.;Candela,M.;Rampelli,S.;Centanni,M.;Consolandi,C.;Basaglia,G.;Turroni,S.;Biagi,E.;Peano,C.;Severgnini,M.; etal.GutmicrobiomeoftheHadzahunter-gatherers. Nat.Commun. 2014, 5.[CrossRef][PubMed]
127. Clemente,J.C.;Pehrsson,E.C.;Blaser,M.J.;Sandhu,K.;Gao,Z.;Wang,B.;Magris,M.;Hidalgo,G.;Contreras,M.;Noya-Alarcón, Ó.;etal.ThemicrobiomeofuncontactedAmerindians. Sci.Adv. 2015, 1,e1500183.[CrossRef][PubMed]
128.Shen,J.;Obin,M.S.;Zhao,L.Thegutmicrobiota,obesityandinsulinresistance. Mol.Asp.Med. 2013, 34,39–58.[CrossRef]
129. Zhao,X.;Jiang,Y.;Xi,H.;Chen,L.;Feng,X.ExplorationoftheRelationshipbetweenGutMicrobiotaandPolycysticOvary Syndrome[PCOS]:AReview. GeburtshilfeFrauenheilkd. 2020, 80,161–171.[CrossRef]
130. Obregon-Tito,A.J.;Tito,R.Y.;Metcalf,J.;Sankaranarayanan,K.;Clemente,J.C.;Ursell,L.K.;Xu,Z.Z.;Treuren,W.;VanKnight, R.;Gaffney,P.M.;etal.Subsistencestrategiesintraditionalsocietiesdistinguishgutmicrobiomes. Nat.Commun. 2015, 6,6505. [CrossRef]
131. Smits,S.A.;Leach,J.;Sonnenburg,E.D.;Gonzalez,C.G.;Lichtman,J.S.;Reid,G.;Knight,R.;Manjurano,A.;Changalucha,J.; Elias,J.E.;etal.SeasonalcyclinginthegutmicrobiomeoftheHadzahunter-gatherersofTanzania. Science 2017, 357,802–806. [CrossRef]
132.Kallus,S.J.;Brandt,L.J.Theintestinalmicrobiotaandobesity. J.Clin.Gastroenterol. 2012, 46,16–24.[CrossRef]
133. Ridaura,V.K.;Faith,J.J.;Rey,F.E.;Cheng,J.;Duncan,A.E.;Kau,A.L.;Griffin,N.W.;Lombard,V.;Henrissat,B.;Bain,J.R.;etal. Gutmicrobiotafromtwinsdiscordantforobesitymodulatemetabolisminmice. Science 2013, 341,1241214.[CrossRef]
134. Backhed,F.;Ding,H.;Wang,T.;Hooper,L.V.;Koh,G.Y.;Nagy,A.;Semenkovich,C.F.;Gordon,J.I.Thegutmicrobiotaasan environmentalfactorthatregulatesfatstorage. Proc.Natl.Acad.Sci.USA 2004, 101,15718–15723.[CrossRef][PubMed]
135. Karlsson,F.H.;Tremaroli,V.;Nookaew,I.;Bergstrom,G.;Behre,C.J.;Fagerberg,B.;Nielsen,J.;Backhed,F.Gutmetagenomein Europeanwomenwithnormal,impairedanddiabeticglucosecontrol. Nature 2013, 498,99–103.[CrossRef][PubMed]
136. Chatelier,E.;LeNielsen,T.;Qin,J.;Prifti,E.;Hildebrand,F.;Falony,G.;Almeida,M.;Arumugam,M.;Batto,J.M.;Kennedy,S.; etal.Richnessofhumangutmicrobiomecorrelateswithmetabolicmarkers. Nature 2013, 500,541–546.[CrossRef][PubMed]
137. Qin,J.;Li,Y.;Cai,Z.;Li,S.;Zhu,J.;Zhang,F.;Liang,S.;Zhang,W.;Guan,Y.;Shen,D.;etal.Ametagenome-wideassociation studyofgutmicrobiotaintype2diabetes. Nature 2012, 490,55–60.[CrossRef][PubMed]
138. Rocha,A.L.;Oliveira,F.L.;Azevedo,R.Recentadvancesintheunderstandingandmanagementofpolycysticovarysyndrome. F1000Research 2019, 8,1–11.[CrossRef]
139. Liang,Z.;Di,N.;Li,L.;Yang,D.Gutmicrobiotaalterationsrevealpotentialgut–brainaxischangesinpolycysticovarysyndrome. J.Endocrinol.Investig. 2021, 44,1727–1737.[CrossRef]
140. Torres,P.J.;Siakowska,M.;Banaszewska,B.;Pawelczyk,L.;Duleba,A.J.;Kelley,S.T.;Thackray,V.G.GutMicrobialDiversityin WomenwithPolycysticOvarySyndromeCorrelateswithHyperandrogenism. J.Clin.Endocrinol.Metab. 2018, 103,1502–1511. [CrossRef]
141. Insenser,M.;Murri,M.;DelCampo,R.;Martínez-García,M.Á.;Fernández-Durán,E.;Escobar-Morreale,H.F.Gutmicrobiota andthepolycysticovarysyndrome:Influenceofsex,sexhormones,andobesity. J.Clin.Endocrinol.Metab. 2018, 103,2552–2562. [CrossRef]
142. Zhu,X.;Li,Y.;Jiang,Y.;Zhang,J.;Duan,R.;Liu,L.;Liu,C.;Xu,X.;Yu,L.;Wang,Q.;etal.PredictionofGutMicrobialCommunityStructureandFunctioninPolycysticOvarySyndromeWithHighLow-DensityLipoproteinCholesterol. Front.CellInfect.Microbiol. 2021, 11,665406.[CrossRef]
143. Lindheim,L.;Bashir,M.;Münzker,J.;Trummer,C.;Zachhuber,V.;Leber,B.;Horvath,A.;Pieber,T.R.;Gorkiewicz,G.;Stadlbauer, V.;etal.Alterationsingutmicrobiomecompositionandbarrierfunctionareassociatedwithreproductiveandmetabolicdefects inwomenwithpolycysticovarysyndrome[PCOS]:Apilotstudy. PLoSONE 2017, 12,e0168390.[CrossRef]
144. Parker,J.;O’Brien,C.;Hawrelak,J.Anarrativereviewoftheroleofgastrointestinaldysbiosisinthepathogenesisofpolycystic ovarysyndrome. Obstet.Gynecol.Sci. 2022, 65,14–28.[CrossRef][PubMed]
145. Tam,C.S.;Xie,W.;Johnson,W.D.;Cefalu,W.T.;Redman,L.M.;Ravussin,E.Defininginsulinresistancefromhyperinsulinemiceuglycemicclamps. DiabetesCare 2012, 35,1605–1610.[CrossRef][PubMed]
146. Nolan,C.J.;Prentki,M.Insulinresistanceandinsulinhypersecretioninthemetabolicsyndromeandtype2diabetes:Timefora conceptualframeworkshift. DiabetesVasc.Dis.Res. 2019, 16,118–127.[CrossRef][PubMed]
147. Diamanti-Kandarakis,E.;Kouli,C.;Alexandraki,K.;Spina,G.Failureofmathematicalindicestoaccuratelyassessinsulin resistanceinlean,overweight,orobesewomenwithpolycysticovarysyndrome. J.Clin.Endocrinol.Metab. 2004, 89,1273–1276. [CrossRef][PubMed]
148.Singh,B.Surrogatemarkersofinsulinresistance:Areview. WorldJ.Diabetes 2010, 1,36.[CrossRef]
149. Wu,X.K.;Zhou,S.Y.;Liu,J.X.;Pöllänen,P.;Sallinen,K.;Mäkinen,M.;Erkkola,R.Selectiveovaryresistancetoinsulinsignalingin womenwithpolycysticovarysyndrome. Fertil.Steril. 2003, 80,954–965.[CrossRef]
150. Brown,M.S.;Goldstein,J.L.SelectiveversusTotalInsulinResistance:APathogenicParadox. CellMetab. 2008, 7,95–96.[CrossRef]
Int.J.Environ.Res.PublicHealth 2022, 19,1336 21of25
151. Hardy,O.T.;Czech,M.P.;Corvera,S.Whatcausestheinsulinresistanceunderlyingobesity? Curr.Opin.Endocrinol.DiabetesObes. 2012, 19,81–87.[CrossRef]
152. Toosy,S.;Sodi,R.;Pappachan,J.M.Leanpolycysticovarysyndrome[PCOS]:Anevidence-basedpracticalapproach. J.DiabetesMetab.Disord. 2018, 17,277–285.[CrossRef]
153. Wang,J.;Wu,D.;Guo,H.;Li,M.Hyperandrogenemiaandinsulinresistance:Thechiefculpritofpolycysticovarysyndrome. LifeSci. 2019, 236.[CrossRef]
154. Cibula,D.IsinsulinresistanceanessentialcomponentofPCOS?Theinfluenceofconfoundingfactors. Hum.Reprod. 2004, 19, 757–759.[CrossRef][PubMed]
155. Cassar,S.;Misso,M.L.;Hopkins,W.G.;Shaw,C.S.;Teede,H.J.;Stepto,N.K.Insulinresistanceinpolycysticovarysyndrome: Asystematicreviewandmeta-analysisofeuglycaemic-hyperinsulinaemicclampstudies. Hum.Reprod. 2016, 31,2619–2631. [CrossRef][PubMed]
156. Stepto,N.K.;Cassar,S.;Joham,A.E.;Hutchison,S.K.;Harrison,C.L.;Goldstein,R.F.;Teede,H.J.Womenwithpolycysticovary syndromehaveintrinsicinsulinresistanceoneuglycaemic-hyperinsulaemicclamp. Hum.Reprod. 2013, 28,777–784.[CrossRef] [PubMed]
157. Rubin,K.H.;Glintborg,D.;Nybo,M.;Abrahamsen,B.;Andersen,M.Developmentandriskfactorsoftype2diabetesina nationwidepopulationofwomenwithpolycysticovarysyndrome. J.Clin.Endocrinol.Metab. 2017, 102,3848–3857.[CrossRef]
158.Crofts,C.A.P.Hyperinsulinemia:Aunifyingtheoryofchronicdisease? Diabesity 2015, 1,34.[CrossRef]
159. Watve,M.G.;Yajnik,C.S.Evolutionaryoriginsofinsulinresistance:Abehavioralswitchhypothesis. BMCEvol.Biol. 2007, 7,61. [CrossRef]
160. Khalid,M.;Alkaabi,J.;Khan,M.A.B.;Adem,A.Insulinsignaltransductionperturbationsininsulinresistance. Int.J.Mol.Sci. 2021, 22,8590.[CrossRef]
161.Petersen,M.C.;Shulman,G.I.Mechanismsofinsulinactionandinsulinresistance. Physiol.Rev. 2018, 98,2133–2223.[CrossRef]
162. DaSilvaRosa,S.C.;Nayak,N.;Caymo,A.M.;Gordon,J.W.Mechanismsofmuscleinsulinresistanceandthecross-talkwithliver andadiposetissue. Physiol.Rep. 2020, 8,e14607.[CrossRef]
163. Schenk,S.;Saberi,M.;Olefsky,J.M.Insulinsensitivity:Modulationbynutrientsandinflammation. J.Clin.Investig. 2008, 118, 2992–3002.[CrossRef]
164. Soeters,M.R.;Soeters,P.B.;Schooneman,M.G.;Houten,S.M.;Romijn,J.A.Adaptivereciprocityoflipidandglucosemetabolism inhumanshort-termstarvation. Am.J.Physiol.-Endocrinol.Metab. 2012, 303,1397–1407.[CrossRef][PubMed]
165. Li,L.;Li,X.;Zhou,W.;Messina,J.L.Acutepsychologicalstressresultsintherapiddevelopmentofinsulinresistance. J.Endocrinol. 2013, 217,175–184.[CrossRef][PubMed]
166. Morciano,A.;Romani,F.;Sagnella,F.;Scarinci,E.;Palla,C.;Moro,F.;Tropea,A.;Policola,C.;DellaCasa,S.;Guido,M.;etal. Assessmentofinsulinresistanceinleanwomenwithpolycysticovarysyndrome. Fertil.Steril. 2014, 102,250–256.[CrossRef] [PubMed]
167. Small,L.;Brandon,A.E.;Turner,N.;Cooney,G.J.Modelinginsulinresistanceinrodentsbyalterationsindiet:Whathavehigh-fat andhigh-caloriedietsrevealed? Am.J.Physiol.-Endocrinol.Metab. 2018, 314,E251–E265.[CrossRef]
168. Hallberg,S.J.;Gershuni,V.M.;Hazbun,T.L.;Athinarayanan,S.J.ReversingType2Diabetes:ANarrativeReviewoftheEvidence. Nutrients 2019, 11,766.[CrossRef][PubMed]
169. Rabbani,N.;Xue,M.;Weickert,M.;Thornally,P.ReversalofInsulinResistanceinOverweightandObeseSubjectsbytransResveratrolandHesperetinCombination-LinktoDysglycemia,BloodPressure,Dyslipidemia,andLow-GradeInflammation. Nutrients 2021, 13,2374.[CrossRef][PubMed]
170. Shang,Y.;Zhou,H.;Hu,M.;Feng,H.Effectofdietoninsulinresistanceinpolycysticovarysyndrome. J.Clin.Endocrinol.Metab. 2020, 105,3346–3360.[CrossRef]
171. Kazemi,A.;Soltani,S.;Ghorabi,S.;Keshtkar,A.;Daneshzad,E.;Nasri,F.;Mazloomi,S.M.Effectofprobioticandsynbiotic supplementationoninflammatorymarkersinhealthanddiseasestatus:Asystematicreviewandmeta-analysisofclinicaltrials. Clin.Nutr. 2020, 39,789–819.[CrossRef]
172.Street,M.E.;Bernasconi,S.Endocrine-disruptingchemicalsinhumanfetalgrowth. Int.J.Mol.Sci. 2020, 21,1430.[CrossRef]
173. Patterson,R.E.;Laughlin,G.A.;LaCroix,A.Z.;Hartman,S.J.;Natarajan,L.;Senger,C.M.;Martínez,M.E.;Villaseñor,A.;Sears, D.D.;Marinac,C.R.;etal.IntermittentFastingandHumanMetabolicHealth. J.Acad.Nutr.Diet. 2015, 115,1203–1212.[CrossRef]
174. Adeva-Andany,M.M.;González-Lucán,M.;Fernández-Fernández,C.;Carneiro-Freire,N.;Seco-Filgueira,M.;Pedre-Piñeiro, A.M.Effectofdietcompositiononinsulinsensitivityinhumans. Clin.Nutr.ESPEN 2019, 33,29–38.[CrossRef][PubMed]
175. González,F.;Considine,R.V.;Abdelhadi,O.A.;Xue,J.;Acton,A.J.Saturatedfatingestionstimulatesproatherogenicinflammation inpolycysticovarysyndrome. Am.J.Physiol.Metab. 2021, 321,E689–E701.[CrossRef][PubMed]
176. Ottaviani,E.;Malagoli,D.;Franceschi,C.Theevolutionoftheadiposetissue:Aneglectedenigma. Gen.Comp.Endocrinol. 2011, 174,1–4.[CrossRef][PubMed]
177. Knebel,B.;Müller-Wieland,D.;Kotzka,J.Lipodystrophies—disordersofthefattytissue. Int.J.Mol.Sci. 2020, 21,8778.[CrossRef]
178. Scheja,L.;Heeren,J.Theendocrinefunctionofadiposetissuesinhealthandcardiometabolicdisease. Nat.Rev.Endocrinol. 2019, 15,507–524.[CrossRef]
179. Choe,S.S.;Huh,J.Y.;Hwang,I.J.;Kim,J.I.;Kim,J.B.Adiposetissueremodeling:Itsroleinenergymetabolismandmetabolic disorders. Front.Endocrinol. 2016, 7,30.[CrossRef]
Int.J.Environ.Res.PublicHealth 2022, 19,1336 22of25
180. Pawłowski,B.; Zela´zniewicz,A.Theevolutionofperenniallyenlargedbreastsinwomen:Acriticalreviewandanovelhypothesis. Biol.Rev. 2021, 2798,2794–2809.[CrossRef]
181. McLaughlin,T.;Lamendola,C.;Liu,A.;Abbasi,F.Preferentialfatdepositioninsubcutaneousversusvisceraldepotsisassociated withinsulinsensitivity. J.Clin.Endocrinol.Metab. 2011, 96,1756–1760.[CrossRef]
182. Barrea,L.;Frias-Toral,E.;Verde,L.;Ceriani,F.;Cucalón,G.;Garcia-Velasquez,E.;Moretti,D.;Savastano,S.;Colao,A.;Muscogiuri, G.PCOSandnutritionalapproaches:Differencesbetweenleanandobesephenotype. Metab.Open 2021, 12,100123.[CrossRef]
183. Sellayah,D.Theimpactofearlyhumanmigrationonbrownadiposetissueevolutionanditsrelevancetothemodernobesity pandemic. J.Endocr.Soc. 2019, 3,372–386.[CrossRef][PubMed]
184. Locke,A.E.;Kahali,B.;Berndt,S.I.;Justice,A.E.;Pers,T.H.;Day,F.R.;Powell,C.;Vedantam,S.;Buchkovich,M.L.;Yang,J.;etal. Geneticstudiesofbodymassindexyieldnewinsightsforobesitybiology. Nature 2015, 518,197–206.[CrossRef][PubMed]
185. Bouchard,C.;Tremblay,A.;Després,J.P.;Nadeau,A.;Lupien,P.J.;Thériault,G.;Dussault,J.;Moorjani,S.;Pinault,S.;Fournier,G. Theresponsetolong-termoverfeedinginidenticaltwins. N.Engl.J.Med. 1990, 322,1477–1482.[CrossRef][PubMed]
186. Barber,T.M.;Hanson,P.;Weickert,M.O.;Franks,S.ObesityandPolycysticOvarySyndrome:ImplicationsforPathogenesisand NovelManagementStrategies. Clin.Med.InsightsReprod.Health 2019, 13,1179558119874042.[CrossRef][PubMed]
187.Hussain,I.;Garg,A.LipodystrophySyndromes. Endocrinol.Metab.Clin.N.Am. 2016, 45,783–797.[CrossRef][PubMed]
188. Gambineri,A.;Zanotti,L.Polycysticovarysyndromeinfamilialpartiallipodystrophytype2[Fpld2]:Basicandclinicalaspects. Nucleus 2018, 9,392–397.[CrossRef][PubMed]
189. Garg,A.Genderdifferencesintheprevalenceofmetaboliccomplicationsinfamilialpartiallipodystrophy[Dunniganvariety]. J.Clin.Endocrinol.Metab. 2000, 85,1776–1782.[CrossRef]
190. Kahn,L.G.;Philippat,C.;Nakayama,S.F.;Slama,R.;Trasande,L.Endocrine-disruptingchemicals:Implicationsforhumanhealth. LancetDiabetesEndocrinol. 2020, 8,703–718.[CrossRef]
191. Whitmee,S.;Haines,A.;Beyrer,C.;Boltz,F.;Capon,A.G.;DeSouzaDias,B.F.;Ezeh,A.;Frumkin,H.;Gong,P.;Head,P.;etal. SafeguardinghumanhealthintheAnthropoceneepoch:ReportoftheRockefellerFoundation-LancetCommissiononplanetary health. Lancet 2015, 386,1973–2028.[CrossRef]
192. Schug,T.T.;Johnson,A.F.;Birnbaum,L.S.;Colborn,T.;Guillette,L.J.;Crews,D.P.;Collins,T.;Soto,A.M.;VomSaal,F.S.;McLachlan, J.A.;etal.Minireview:Endocrinedisruptors:Pastlessonsandfuturedirections. Mol.Endocrinol. 2016, 30,833–847.[CrossRef]
193. TEDXListofPotentialEndocrineDisruptorstheEndocrineDisruptorExchange.2018.Availableonline: https:// endocrinedisruption.org/interactive-tools/tedx-list-of-potential-endocrine-disruptors/search-the-tedx-list (accessedon 15November2021).
194. Eskenazi,B.;Chevrier,J.;Rauch,S.A.;Kogut,K.;Harley,K.G.;Johnson,C.;Trujillo,C.;Sjödin,A.;Bradman,A.In uteroandchildhoodpolybrominateddiphenylether[PBDE]exposuresandneurodevelopmentintheCHAMACOSstudy. Environ.HealthPerspect. 2013, 121,257–262.[CrossRef]
195.Parker,J.GlyphosateinducedintestinalpermeabilityinthepathogenesisofPCOS. ACNEMJ. 2015, 34,3–7.
196. Mitro,S.D.;Johnson,T.;Zota,A.R.CumulativeChemicalExposuresDuringPregnancyandEarlyDevelopment. Curr.Environ.HealthRep. 2015, 2,367–378.[CrossRef][PubMed]
197. Barr,D.B.;Bishop,A.;Needham,L.L.Concentrationsofxenobioticchemicalsinthematernal-fetalunit. Reprod.Toxicol. 2007, 23, 260–266.[CrossRef][PubMed]
198. Starling,A.P.;Adgate,J.L.;Hamman,R.F.;Kechris,K.;Calafat,A.M.;Ye,X.;Dabelea,D.Perfluoroalkylsubstancesduring pregnancyandoffspringweightandadiposityatbirth:Examiningmediationbymaternalfastingglucoseinthehealthystart study. Environ.HealthPerspect. 2017, 125,067016.[CrossRef][PubMed]
199. Mohajer,N.;Du,C.Y.;Checkcinco,C.;Blumberg,B.Obesogens:HowTheyAreIdentifiedandMolecularMechanismsUnderlying TheirAction. Front.Endocrinol. 2021, 12,1503.[CrossRef][PubMed]
200. DeAraújo,J.F.P.;Podratz,P.L.;Sena,G.C.;Merlo,E.;Freitas-Lima,L.C.;Ayub,J.G.M.;Pereira,A.F.Z.;Santos-Silva,A.P.;MirandaAlves,L.;Silva,I.V.;etal.Theobesogentributyltininducesabnormalovarianadipogenesisinadultfemalerats. Toxicol.Lett. 2018, 295,99–114.[CrossRef][PubMed]
201. Abbott,D.H.;Dumesic,D.A.;Levine,J.E.;Angeles,L.Hyperandrogenicoriginsofpolycysticovarysyndrome-Implicationsfor PathophysiologyandTherapy. ExpertRev.Endocrinol.Metab. 2019, 14,131–143.[CrossRef]
202.Resnik,D.B.Theprecautionaryprincipleandmedicaldecisionmaking. J.Med.Philos. 2004, 29,281–299.[CrossRef]
203. DiRenzo,G.C.;Conry,J.A.;Blake,J.;Defrancesco,M.S.;Denicola,N.;Martin,J.N.;McCue,K.A.;Richmond,D.;Shah,A.;Sutton, P.;etal.InternationalFederationofGynecologyandObstetricsopiniononreproductivehealthimpactsofexposuretotoxic environmentalchemicals. Int.J.Gynecol.Obstet. 2015, 131,219–225.[CrossRef][PubMed]
204. Gore,A.C.;Chappell,V.A.;Fenton,S.E.;Flaws,J.A.;Nadal,A.;Prins,G.S.;Toppari,J.;Zoeller,R.T.EDC-2:TheEndocrine Society’sSecondScientificStatementonEndocrine-DisruptingChemicals. Endocr.Rev. 2015, 36,E1–E150.[CrossRef]
205. ChemicalExposuresDuringPregnancy:DealingwithPotential,butUnproven,RiskstoChildHealth.Availableonline: https://eprints.gla.ac.uk/97765/ (accessedon3August2021).
206. Shishehgar,F.;RamezaniTehrani,F.;Mirmiran,P.;Hajian,S.;Baghestani,A.R.;Moslehi,N.ComparisonofDietaryIntakebetween PolycysticOvarySyndromeWomenandControls. Glob.J.HealthSci. 2016, 8,302.[CrossRef][PubMed]
207. Rajska,A.;Buszewska-Forajta,M.;Racho´n,D.;Markuszewski,M.J.Metabolomicinsightintopolycysticovarysyndrome—An overview. Int.J.Mol.Sci. 2020, 21,4853.[CrossRef][PubMed]
Int.J.Environ.Res.PublicHealth 2022, 19,1336 23of25
208. Lim,S.S.;Hutchison,S.K.;VanRyswyk,E.;Norman,R.J.;Teede,H.J.;Moran,L.J.Lifestylechangesinwomenwithpolycystic ovarysyndrome. CochraneDatabaseSyst.Rev. 2019.[CrossRef][PubMed]
209. Jankovic,N.;Geelen,A.;Streppel,M.T.;DeGroot,L.C.P.G.M.;Orfanos,P.;VanDenHooven,E.H.;Pikhart,H.;Boffetta,P.; Trichopoulou,A.;Bobak,M.;etal.Adherencetoahealthydietaccordingtotheworldhealthorganizationguidelinesand all-causemortalityinelderlyadultsfromEuropeandtheUnitedStates. Am.J.Epidemiol. 2014, 180,978–988.[CrossRef]
210. Kim,H.;Rebholz,C.M.Metabolomicbiomarkersofhealthydietarypatternsandcardiovascularoutcomes. Curr.Atheroscler.Rep. 2021, 23,26.[CrossRef][PubMed]
211.Pontzer,H.;Wood,B.M.;Raichlen,D.A.Hunter-gatherersasmodelsinpublichealth. Obes.Rev. 2018, 19,24–35.[CrossRef]
212. Fayet-Moore,F.;Cassettari,T.;Tuck,K.;McConnell,A.;Petocz,P.Dietaryfibreintakeinaustralia.Paperi:Associationswith demographic,socio-economic,andanthropometricfactors. Nutrients 2018, 10,599.[CrossRef]
213.Satija,A.;Hu,F.B.Cardiovascularbenefitsofdietaryfiber. Curr.Atheroscler.Rep. 2012, 14,505–514.[CrossRef]
214. Müller,M.;Canfora,E.E.;Blaak,E.E.Gastrointestinaltransittime,glucosehomeostasisandmetabolichealth:Modulationby dietaryfibers. Nutrients 2018, 10,275.[CrossRef]
215. Lattimer,J.M.;Haub,M.D.Effectsofdietaryfiberanditscomponentsonmetabolichealth. Nutrients 2010, 2,1266–1289.[CrossRef]
216. Ghanim,H.;Batra,M.;Abuaysheh,S.;Green,K.;Makdissi,A.;Kuhadiya,N.D.;Chaudhuri,A.;Dandona,P.Antiinflammatory andROSSuppressiveEffectsoftheAdditionofFibertoaHigh-FatHigh-CalorieMeal. J.Clin.Endocrinol.Metab. 2017, 102, 858–869.[CrossRef][PubMed]
217. Thompson,S.V.;Hannon,B.A.;An,R.;Holscher,H.D.Effectsofisolatedsolublefibersupplementationonbodyweight,glycemia, andinsulinemiainadultswithoverweightandobesity:Asystematicreviewandmeta-analysisofrandomizedcontrolledtrials. Am.J.Clin.Nutr. 2017, 106,1514–1528.[CrossRef][PubMed]
218. Veronese,N.;Solmi,M.;Caruso,M.G.;Giannelli,G.;Osella,A.R.;Evangelou,E.;Maggi,S.;Fontana,L.;Stubbs,B.;Tzoulaki,I. Dietaryfiberandhealthoutcomes:Anumbrellareviewofsystematicreviewsandmeta-analyses. Am.J.Clin.Nutr. 2018, 107, 436–444.[CrossRef][PubMed]
219. Cutler,D.A.;Pride,S.M.;Cheung,A.P.Lowintakesofdietaryfiberandmagnesiumareassociatedwithinsulinresistanceand hyperandrogenisminpolycysticovarysyndrome:Acohortstudy. FoodSci.Nutr. 2019, 7,1426–1437.[CrossRef][PubMed]
220.Chaix,A.;Zarrinpar,A.;Panda,S.Thecircadiancoordinationofcellbiology. J.CellBiol. 2016, 215,15–25.[CrossRef]
221. Bhadra,U.;Thakkar,N.;Das,P.;PalBhadra,M.Evolutionofcircadianrhythms:Frombacteriatohuman. SleepMed. 2017, 35, 49–61.[CrossRef]
222.Gerhart-Hines,Z.;Lazar,M.A.Circadianmetabolisminthelightofevolution. Endocr.Rev. 2015, 36,289–304.[CrossRef]
223. Palm,D.;Uzoni,A.;Simon,F.;Fischer,M.;Coogan,A.;Tucha,O.;Thome,J.;Faltraco,F.Evolutionaryconservations,changesof circadianrhythmsandtheireffectoncircadiandisturbancesandtherapeuticapproaches. Neurosci.Biobehav.Rev. 2021, 128,21–34. [CrossRef][PubMed]
224. Hastings,M.;O’Neill,J.S.;Maywood,E.S.Circadianclocks:Regulatorsofendocrineandmetabolicrhythms. J.Endocrinol. 2007, 195,187–198.[CrossRef]
225.Rosenwasser,A.M.;Turek,F.W.Neurobiologyofcircadianrhythmregulation. SleepMed.Clin. 2015, 10,403–412.[CrossRef]
226. Lucas,J.A.;Schmidt,T.M.Cellularpropertiesofintrinsicallyphotosensitiveretinalganglioncellsduringpostnataldevelopment. NeuralDev. 2019, 14,8.[CrossRef][PubMed]
227. Potter,G.D.M.;Cade,J.E.;Grant,P.J.;Hardie,L.J.Nutritionandthecircadiansystem. Br.J.Nutr. 2016, 116,434–442.[CrossRef] [PubMed]
228. Patke,A.;Young,M.W.;Axelrod,S.Molecularmechanismsandphysiologicalimportanceofcircadianrhythms. Nat.Rev.Mol.CellBiol. 2020, 21,67–84.[CrossRef][PubMed]
229. Voigt,R.M.;Forsyth,C.B.;Green,S.J.;Engen,P.A.;Keshavarzian,A. CircadianRhythmandtheGutMicrobiome,1sted.;ElsevierInc.: Amsterdam,TheNetherlands,2016;Volume131.
230. Julius,A.A.;Yin,J.;Wen,J.T.Timeoptimalentrainmentcontrolforcircadianrhythm. PLoSONE 2019, 14,e0225988.[CrossRef] [PubMed]
231.Bass,J.;Takahashi,J.S.Circadianintegrationofmetabolismandenergetics. Science 2010, 330,1349–1354.[CrossRef]
232. Milanova,I.V.;Kalsbeek,M.J.T.;Wang,X.L.;Korpel,N.L.;Stenvers,D.J.;Wolff,S.E.C.;DeGoede,P.;Heijboer,A.C.;Fliers,E.;La Fleur,S.E.;etal.Diet-inducedobesitydisturbsmicroglialimmunometabolisminatime-of-daymanner. Front.Endocrinol. 2019, 10,424.[CrossRef]
233. Jakubowicz,D.;Barnea,M.;Wainstein,J.;Froy,O.HighCaloricintakeatbreakfastvs.dinnerdifferentiallyinfluencesweightloss ofoverweightandobesewomen. Obesity 2013, 21,2504–2512.[CrossRef]
234.Oike,H.;Oishi,K.;Kobori,M.Nutrients,ClockGenes,andChrononutrition. Curr.Nutr.Rep. 2014, 3,204–212.[CrossRef]
235. Gabriel,B.M.;Zierath,J.R.Circadianrhythmsandexercise—Re-settingtheclockinmetabolicdisease. Nat.Rev.Endocrinol. 2019, 15,197–206.[CrossRef]
236.Sridhar,G.R.;Sanjana,N.S.N.Sleep,circadiandysrhythmia,obesityanddiabetes. WorldJ.Diabetes 2016, 7,515.[CrossRef]
237. Ganesan,S.;Magee,M.;Stone,J.E.;Mulhall,M.D.;Collins,A.;Howard,M.E.;Lockley,S.W.;Rajaratnam,S.M.W.;Sletten,T.L.The ImpactofShiftWorkonSleep,AlertnessandPerformanceinHealthcareWorkers. Sci.Rep. 2019, 9,4635.[CrossRef][PubMed]
238. Kopp,R.;Martínez,I.O.;Legradi,J.;Legler,J.Exposuretoendocrinedisruptingchemicalsperturbslipidmetabolismandcircadian rhythms. J.Environ.Sci. 2017, 62,133–137.[CrossRef][PubMed]
Int.J.Environ.Res.PublicHealth 2022, 19,1336 24of25
239. Tahara,Y.;Aoyama,S.;Shibata,S.Themammaliancircadianclockanditsentrainmentbystressandexercise. J.Physiol.Sci. 2017, 67,1–10.[CrossRef][PubMed]
240. Papalou,O.;Diamanti-Kandarakis,E.TheroleofstressinPCOS. ExpertRev.Endocrinol.Metab. 2017, 12,87–95.[CrossRef] [PubMed]
241. Simon,S.L.;McWhirter,L.;DinizBehn,C.;Bubar,K.M.;Kaar,J.L.;Pyle,L.;Rahat,H.;Garcia-Reyes,Y.;Carreau,A.M.;Wright, K.P.;etal.MorningCircadianMisalignmentIsAssociatedwithInsulinResistanceinGirlswithObesityandPolycysticOvarian Syndrome. J.Clin.Endocrinol.Metab. 2019, 104,3525–3534.[CrossRef]
242. Zhou,X.;Huddleston,H.Lettherebelight:Doescircadianrhythmdisruptioncausepolycysticovarysyndrome? Fertil.Steril. 2021, 115,607–608.[CrossRef]
243. Amaral,F.G.;Castrucci,A.M.;Cipolla-Neto,J.;Poletini,M.O.;Mendez,N.;Richter,H.G.;Sellix,M.T.Environmentalcontrolof biologicalrhythms:Effectsondevelopment,fertilityandmetabolism. J.Neuroendocrinol. 2014, 26,603–612.[CrossRef]
244. Gurusinghe,D.;Gill,S.;Almario,R.U.;Lee,J.;Horn,W.F.;Keim,N.L.;Kim,K.;Karakas,S.E.Inpolycysticovarysyndrome, adrenalsteroidsareregulateddifferentlyinthemorningversusinresponsetonutrientintake. Fertil.Steril. 2010, 93,1192–1199. [CrossRef]
245. Roelfsema,F.;Kok,P.;Pereira,A.M.;Pijl,H.Cortisolproductionrateissimilarlyelevatedinobesewomenwithorwithoutthe polycysticovarysyndrome. J.Clin.Endocrinol.Metab. 2010, 95,3318–3324.[CrossRef]
246. Bravo,R.;Ugartemendia,L.;Cubero,J.CurrentOpinionsinChrononutritionandHealth. J.Clin.Nutr.Diet. 2017, 3,3–5. [CrossRef]
247. Adafer,R.;Messaadi,W.;Meddahi,M.;Patey,A.;Haderbache,A.;Bayen,S.;Messaadi,N.FoodTiming,CircadianRhythmand Chrononutrition:ASystematicReviewofTime-RestrictedEating’sEffectsonHumanHealth. Nutrients 2020, 12,3770.[CrossRef] [PubMed]
248.Challet,E.;Kalsbeek,A.Editorial:Circadianrhythmsandmetabolism. Front.Endocrinol. 2017, 8,201.[CrossRef][PubMed]
249. Jamshed,H.;Beyl,R.A.;MannaDella,D.L.;Yang,E.S.;Ravussin,E.;Peterson,C.M.EarlyTime-RestrictedFeedingImproves 24-Hourglucoselevelsandaffectsmarkersofthecircadianclock,aging,andautophagyinhumans. Nutrients 2019, 11,1234. [CrossRef][PubMed]
250. Touitou,Y.;Touitou,D.;Reinberg,A.Disruptionofadolescents’circadianclock:Theviciouscircleofmediause,exposuretolight atnight,sleeplossandriskbehaviors. J.Physiol.Paris 2016, 110,467–479.[CrossRef][PubMed]
251. Tähkämö,L.;Partonen,T.;Pesonen,A.K.Systematicreviewoflightexposureimpactonhumancircadianrhythm. Chronobiol.Int. 2019, 36,151–170.[CrossRef]
Int.J.Environ.Res.PublicHealth 2022, 19,1336 25of25

Pathophysiological Effects of Contemporary Lifestyle on Evolutionary-Conserved Survival Mechanisms in Polycystic Ovary Syndrome
Jim Parker
Special Issue
Polycystic Ovary Syndrome: Current Knowledge and Future Perspectives
Edited by Dr. Cristina Preda, Prof. Dr. Dumitru Branisteanu and Prof. Dr. Simona Fica
Review https://doi.org/10.3390/life13041056

3.253

Review
PathophysiologicalEffectsofContemporaryLifestyleon Evolutionary-ConservedSurvivalMechanismsinPolycystic OvarySyndrome
JimParker
Citation: Parker,J.
PathophysiologicalEffectsof ContemporaryLifestyleon Evolutionary-ConservedSurvival MechanismsinPolycysticOvary Syndrome. Life 2023, 13,1056. https://doi.org/10.3390/ life13041056
AcademicEditors:CristinaPreda, DumitruBranisteanu andSimonaFica
Received:28March2023
Revised:15April2023
Accepted:18April2023
Published:20April2023

Copyright: ©2023bytheauthor. LicenseeMDPI,Basel,Switzerland. Thisarticleisanopenaccessarticle distributedunderthetermsand conditionsoftheCreativeCommons Attribution(CCBY)license(https:// creativecommons.org/licenses/by/ 4.0/).
SchoolofMedicine,UniversityofWollongong,Wollongong,NSW2522,Australia;jimparker@ozemail.com.au
Abstract: Polycysticovarysyndrome(PCOS)isincreasinglybeingcharacterizedasanevolutionary mismatchdisorderthatpresentswithacomplexmixtureofmetabolicandendocrinesymptoms.The EvolutionaryModelproposesthatPCOSarisesfromacollectionofinheritedpolymorphismsthat havebeenconsistentlydemonstratedinavarietyofethnicgroupsandraces.Inuterodevelopmental programmingofsusceptiblegenomicvariantsarethoughttopredisposetheoffspringtodevelop PCOS.Postnatalexposuretolifestyleandenvironmentalriskfactorsresultsinepigeneticactivation ofdevelopmentallyprogrammedgenesanddisturbanceofthehallmarksofhealth.Theresulting pathophysiologicalchangesrepresenttheconsequencesofpoor-qualitydiet,sedentarybehaviour, endocrinedisruptingchemicals,stress,circadiandisruption,andotherlifestylefactors.Emerging evidencesuggeststhatlifestyle-inducedgastrointestinaldysbiosisplaysacentralroleinthepathogenesisofPCOS.Lifestyleandenvironmentalexposuresinitiatechangesthatresultindisturbanceof thegastrointestinalmicrobiome(dysbiosis),immunedysregulation(chronicinflammation),altered metabolism(insulinresistance),endocrineandreproductiveimbalance(hyperandrogenism),and centralnervoussystemdysfunction(neuroendocrineandautonomicnervoussystem).PCOScan beaprogressivemetabolicconditionthatleadstoobesity,gestationaldiabetes,typetwodiabetes, metabolic-associatedfattyliverdisease,metabolicsyndrome,cardiovasculardisease,andcancer.This reviewexploresthemechanismsthatunderpintheevolutionarymismatchbetweenancientsurvival pathwaysandcontemporarylifestylefactorsinvolvedinthepathogenesisandpathophysiology ofPCOS.
Keywords: polycysticovarysyndrome;evolution;inflammation;insulinresistance;hyperinsulinemia;immune;infertility;endocrinedisruptingchemicals;environment;microbiome;lifestyle;diet
1.Introduction
ThereisgeneralagreementthatPCOSisapolygenicmultisystemdisorderarisingfrom aninteractionbetweengeneticandenvironmentalfactors[1].ComprehensiveInternational Guidelinesrecommendarangeoflifestyle-basedinterventionsasfirst-linemanagementfor allwomendiagnosedwithPCOS[2].Theserecommendationsarebasedonevidencethat lifestyletherapies,suchasdietandexercise,cancontrolandreversemanyofthebiochemicalandendocrinefeaturesofPCOS[2,3].Ithasbeenhypothesizedthatcontemporary lifestyleandenvironmentalexposuresareinstrumentalinthepathogenesisofPCOSdueto amismatchbetweenourancientandmodernlifestylesandtheenvironment[1,4–6].
PCOSaffects8–13%ofreproductiveagedwomen,isthoughttobeincreasinginprevalenceglobally,andisestimatedtoaffectupto200millionwomenworldwide[6,7].Women affectedwithPCOSpresentwithawidevarietyofsymptoms(menstrualdisturbance,acne, hirsutism,alopecia,subfertility,anxiety,anddepression)thatreflecttheunderlyingmultisystempathophysiology[8–10].WomenwithPCOShaveanincreasedriskofpregnancy complications(deepvenousthrombosis,pre-eclampsia,macrosomia,growthrestriction, miscarriage,stillbirth,andpretermlabour)[11],psychologicalproblems(anxietyanddepression)[12],andcanprogresstoarangeofothermetabolic-relatedconditions(obesity,
life
Life 2023, 13,1056.https://doi.org/10.3390/life13041056 https://www.mdpi.com/journal/life
gestationaldiabetes,typetwodiabetes(T2DM),metabolic-associatedfattyliverdisease, chronickidneydisease,metabolicsyndrome,cardiovasculardisease,andcancer)[13–16]. Thepopulation-attributableriskofPCOStoT2DMalonehasbeenestimatedat19–28% ofwomenofreproductiveage[17].PCOScanbeaprogressivemetabolicconditionthat makesasignificantcontributiontothechronicdiseaseepidemic[18].
Genome-wideassociationstudies(GWAS)haveidentifiedcommonPCOSriskalleles inwomenfromChineseandEuropeanpopulations,suggestingPCOSisanancientinheriteddisorderthatwaspresentbeforehumansmigratedoutofAfrica[19,20].Familialand twinstudies[21,22],morerecentMendelianrandomization[23,24],andtranscriptome-wide associationstudies(TWAS)[25],alsosupportageneticbasisforPCOS.Fromanevolutionaryperspective,decadesofresearchhavecharacterizedPCOSasaninheritedpolygenic traitthatmanifestsafterexposuretolifestyleandenvironmentalriskfactors [1,26,27] Nevertheless,itisthoughtthatgeneticfactorscontributelessthan10%todiseasesusceptibility,ashasbeenfoundwithotherlifestyle-relatedchronicdiseases,suchasobesityand T2DM[28].ThegeneticbasisofPCOShaspreviouslybeencomprehensivelyreviewedand isnotthefocusofthecurrentreview[1,7,29].
Theprimedirectiveofalllifeistooptimizereproductionandspeciessurvival[30]. Reproductionandmetabolismareintimatelylinkedsooptimalreproductivefitnessrequiresoptimalmetabolism[31,32].Thereisalwaysanevolutionarytradeofftooptimize metabolismand/orreproduction,dependingonthespeciesandprevailingenvironmentalconditions[33].Thisisachievedbyacomplexnetworkofhormonalandsignalling moleculesthatlinkmetabolismtoreproductivecyclesviahormonalregulatoryprocesses, post-translationalmodificationofenzymes,substrate-levelinhibitionofmetabolicpathways,andepigeneticregulationofgeneexpression[34–36].Ithasbeenhypothesizedthat PCOSmayrepresentanevolutionarymetabolicadaptationtobalanceenergysubstrate availability(glucoseandfattyacid)andoptimizereproduction[5].Thisshiftinfocustothe importanceofmetabolicadaptationanddysregulationhasalsobeenemphasizedinthe InternationalGuidelines[2,37].Anumberofevolutionaryhypotheseshavebeendeveloped totrytoexplainthepathogenesisofPCOSandwillbediscussedinthefollowingsections ofthisreview[1,5,26,27,38].
Avarietyoflifestyleandenvironmentalexposureshavebeenfoundtocontributeto thepathogenesisofPCOS[1,2,5,39].Theseincludedietandnutritionalfactors,exercise andsedentarybehaviour,sleepandcircadiandisruption,endocrine-disruptingchemicals, stress,directandindirecteffectsofclimatechange,andcommunitysupportsystems[1]. Contemporarylifestyleexposuresaresignificantlydifferentfromtheenvironmentalconditionsthatexistedthroughoutmostofhumanevolution.Namely,starvation,predation,fear, increasedmaternalmortality,andexposuretodifferentclimaticconditions[4,27,40].
Thereductionofchronicdiseasefollowinglifestyleinterventions,suchasdiet,exercise, andsmokingcessation,hasprovidedstrongevidenceforcontemporarylifestyleasthe primary“cause”ofmanydiseases,includingPCOS[41].Optimalhealthandwell-beingare achievedwhenmetabolic,cellular,andwhole-bodyhomeostasisissynchronizedwiththe prevailingenvironmentalconditions[34].Multipleadaptivesurvivalmechanisms(immune, metabolic,neurological,andhormonal)haveevolvedtoensurethere-establishmentof homeostasisduringperiodsofenvironmentalandpersonalstressandworksynergistically tomaintainhealth[4,5,27,42,43].Rapidsocialevolutionhascreatedacontemporarylifestyle andenvironmentthatisoutofstepwithourevolutionarypast[1,4].Chronicdisturbance toadaptivehomeostaticregulatorynetworks,disruptthe“HallmarksofHealth”[44], resultinginmaladaptivemetabolic,immune,andphysiologicalresponses,thatcausethe observedfeaturesofPCOS[45,46].
Itisnowappreciatedthatmorethanonepathophysiologicalmechanismisinvolvedin thedevelopmentofPCOS[47,48].Thisnarrativereviewoutlinestherelationshipbetween lifestyleandenvironmentalriskfactorsandtheunderlyingpathophysiologicalmechanismsidentifiedinwomenwithPCOS.Theseincludeimmunedysregulation(chronic systemicinflammationandoxidativestress),metabolicdysfunction(insulinresistance
Life 2023, 13,1056 2of35
(IR),hyperglycaemia,andhyperinsulinemia),hormonaldysregulation(hyperandrogenism, estrogen,follicle-stimulatinghormone,andluteinizinghormone),andgastrointestinal dysbiosis(decreasedalphadiversityandincreasedgastrointestinalmucosalpermeability) (Figure 1).ThepathologicalprocessesarediscussedinthecontextoftheEvolutionary ModelofPCOS[1].

Figure1. PathophysiologyofPolycysticOvarySyndrome.Depictsthemulti-directionalinteractions betweennutritionalandenvironmentallifestyle-relatedriskfactorsandtheidentifiedpathophysiologicalprocessesandsymptomsinPCOS.Healthisaresultofthesuccessfulintegrationofmultidimensionalsubcellular,cellular,andsystemic,integratedcircuitsandnetworks.Disturbances inthesenetworksduetodysbiosis,chronicinflammation,insulinresistance,andneuroendocrine deregulationinisolationorincombinationcanleadtolossofhomeostaticresilienceinthesystem. Combinationsofadversenutritionalandenvironmentalfactorscandisturbthisnetworkinamyriad ofways,atmultipledifferentsites,andareresponsibleforthepathogenesisofPCOS.Theinfluence oftheexposomeondevelopmentallyprogrammedsusceptibilitygenesprogramstheembryo/foetus toexpressthephenotypicmanifestationsofPCOSduringchildhood,adolescence,andadulthood, followingexposuretolifestyle,dietary,andenvironmentalfactors.QOL,qualityoflife.
2.MaterialsandMethods
Theliteraturesearchfocusedonresearchpublicationsrelatedtothepathophysiology andpathogenesisofPCOSusingthekeywordslistedaboveandrelatedmeshtermsfor dataontheevolutionaryaspectsofPCOS,chronicsystemicinflammation,in-uterodevelopmentalepigeneticprogramming,insulinresistance,hyperinsulinemia,hyperandrogenism, reproductivechanges,infertility,microbiome,dysbiosis,endocrinedisruptingchemicals, lifestyle,diet,andphysicalactivity.ThedatabasessearchedincludedPubMed,Scopus, Cochrane,andGoogleScholar.Theliteraturehasbeensearchedrepeatedlyoverthepast 10years.Aglossaryofabbreviationsisincluded.
Life 2023, 13,1056 3of35
ff
Thepresentmanuscriptprovidesasummaryofthepathogenesisandpathophysiology ofPCOSinthecontextoftheEvolutionaryModel[1]andtheHallmarksofHealth[44].
3.ChronicSystemicInflammation
3.1.EvolutionandtheAdvantagesofaProinflammatoryDesign
Inflammationisanormalphysiologicalprocessthatisanevolutionaryconserved homeostaticmechanismincellsandtissuesthroughoutthebody[49].Optimalhealthis achievedwhenabalancebetweenpro-andanti-inflammatoryprocessesremovesaging, damagedorinfectedcells,andrestoresnormalcellularfunction[50].Inflammationis aprotectivemechanisminresponsetospecificenvironmentalconditionsandoccursat acosttonormaltissuefunction[51,52].Localanti-inflammatorymediatorsattemptto limitthesystemicspreadofinflammationandcontaintheinflammatoryresponse.The resolutionofinflammationisfacilitatedbytheremovaloftheprimarycause,localnegative feedbackloops,systemicregulationbytheautonomicnervoussystem(ANS)[53],and glucocorticoids[44].Chroniclow-gradesystemicinflammationcanoccurasaresult ofthefailureofanyofthesehomeostaticmechanismsandisacornerstoneofPCOS pathophysiology[54].Largesystematicreviewsconfirmtheimportantroleofchronic systemicinflammationinthepathogenesisofPCOS[8,46].
Rapidchangesinthecontemporaryhumanenvironmenthaveoutpacedgeneticadaptation,leadingtoamismatchbetweenourmodernexposures,andselectedmetabolic andreproductivetraits.Thismismatchhasresultedinadysregulatedinflammatoryresponsethathasincreasedsusceptibilitytomanycommonchronicdiseases,including obesity,typetwodiabetes,metabolicsyndrome,cardiovasculardisease,neuroinflammatorydiseases,andPCOS[50].Chroniclow-gradeinflammationormetaflammation,caused bypoor-qualitydiet,nutritionalexcess,andotherenvironmentalfactors,ismaintained atasubacuteleveloverlongperiodsoftime,enhancinginflammatoryandmetabolic signaltransductionpathwaysthatleadtothesymptomsanddiseasesassociatedwith PCOS[55].Theconceptofmetaflammationreferstothepathophysiologicalassociation betweenmetabolicdisordersandtheimmunesystemandisproposedtooriginatefromthe evolutionarycrosstalkbetweenimmuneandmetabolicpathways[52].
Thecentralroleofchronicsystemicinflammationandmetabolicdysregulationand theirrelationshiptoevolution,obesity,themicrobiota,endocrine-disruptingchemicals, andtherecentlydescribed“HallmarksofHealth”aredescribedinthefollowingsectionsof thisreview.Thereisaparticularfocusonthemoleculardetailsandmechanismsinvolved inthepathophysiologyofPCOS.
3.2.OverviewoftheInflammatoryResponse
Thehumanbodyhastwoparallelsystemsofcellulardefence(innateandadaptiveimmunity)thatworkco-operativelytoprotectcells,individuals,andultimatelythe species[56].Billionsofyearsofevolutionhaveequippedunicellularorganismswitha rangeofstressresponsestoabioticenvironmentalthreats,suchastemperature,salinity, sunlightexposure,heavymetals,andoxygen[57].Inaddition,multicellularorganisms possesselaborateinnateandadaptiveimmuneresponsestodefendagainstexposuretononinfectious(reactiveoxygenspecies,uricacid,cholesterol,microparticles,andexosomes) andinfectiousagents(bacteria,viruses,protozoa,parasites,fungi)[58].Theseresponses areactivatedbyarangeofbiologicalmechanisms,includingoxidativestressandreactive oxygenspecies(ROS),advancedglycationend-products(AGE),andviapatternrecognition receptors(PRR)[59].
3.2.1.OxidativeStressinPCOS
Theevolutionoflifechemistryandmetabolismdatesback3.5billionyears[60].The firstcellularlifeformsaroseinananaerobicenvironmentandmostofthepathwaysof intermediatemetabolism(glycolysis,fattyacidsynthesisandoxidation,pentosephosphate pathway,Krebscycle,electrontransport,andmanymore),developedinanenvironment
Life 2023, 13,1056 4of35
whereoxygenwastoxic[61].Approximately2.4billionyearsago,cyanobacteriastarted producingoxygenfromphotosynthesis,raisingtheatmosphericoxygento2–4%.Abillionyearslater,duringthePre-Cambrianperiod,oxygenlevelsrose,andmulticellular organismsflourished.Thisresultedintheabilityofcellstouseoxygentomakeadenosinetriphosphate(ATP)andproduceROS(superoxide,hydroxylradical)asametabolic by-product,andforthepurposesofcellsignallinganddefence,anddetoxifyingoxygen withavarietyofantioxidantsystems[62].ROScontainanunpairedelectronthatmakes themextremelyunstableandreactive.ROSattempttostabilizethemselvesbyscavenging electronsfromhealthycellsandcauseoxidativedamage.
Livingcellscanbedifferentiatedfromdeadcellsbecauseofthecessationofthe coordinatedflowofenergythatoccursduetoelectrontransferfromonemoleculeto anotherduringmetabolism,followingfailureofadaptiveresponsestorestorecellular homeostasis[63].Whentheflowofelectronstothemitochondrialelectrontransportchain isdisruptedbyenvironmentalfactors,suchasmicrobialinfection,chemicaltoxins,accumulationofmetabolicintermediates,duetonutritionalexcess,andothercellularstressors, metabolicmismatchoccurs[62].Electronsaredivertedawayfrommitochondria,mitochondrialoxygenconsumptionfalls,andcytoplasmicoxygenrises.Thisredoximbalance createsROSandreactivenitrogenspecies(RNS)thatinitiateinnateimmuneresponses designedtodefendandprotectthecell[64].Mitochondrialstructure,dynamics,biogenesis, andmembranepotentialarealteredinwomenwithPCOS[65].
Thebodyhasanin-builtsystemofantioxidantstostabilizeandneutralizeROSand protectthecell.Antioxidantsarehighlystablemoleculesthathavetheuniqueabilityto serveaselectrondonorstohelpstabilizefreeradicalswithoutbecomingreactivethemselves.Oxidativestressreferstotheimbalancebetweentheproductionofoxidantspecies andantioxidantdefences,andthegenerationofexcessiveamountsofROSthatunderlie thevariousformsofcelldeath[66].Oxidativestresscanarisefromendogenous(leakage ofROSfrommitochondrialoxidativephosphorylation,cytochromeP-450detoxification enzymesystems,peroxisomaloxidases,andnicotinamidedinucleotideadeninephosphate oxidases)orexogenoussources(environmentalchemicals,cigarettesmoke,alcohol,ionizingradiation,microbialinfection,stress,andsleepdeprivation)[66,67]andisapotent stimulatorofinflammation[68,69].Oxidativestresshasbeenfoundtoplayacentralrolein thepathogenesisofPCOS[66].Oxidativestresscanimpairinsulinsignallingandcause IR[70]anddysregulatefollicularcalciumwhichresultsinreproductiveandmenstrual dysfunction[71],oxidizeplasmaproteinsthatmayactaspro-inflammatorymediators[72], causelipidperoxidation[73],andinduceDNAdamage[74]inwomenwithPCOS.
CumulativestudiesshowanassociationbetweenoxidativestressandPCOS[64,66]. Inaddition,oxidantandantioxidantstatushasbeenfoundtovarybetweenindividuals becauseofdifferencesindiet,lifestyle,andenzymaticanddietaryantioxidants[64,68].A recentcase-controlstudyshowedthatplant-baseddietarypatternisassociatedwithalower oddsratioofPCOSandsuggestedthatantioxidant-richfoodsmayprotectthebodyagainst oxidativedamage[75].Dietarytotalantioxidantcapacitywassubsequentlyassessedusing theNutrientDataLaboratoryoftheUnitedStatesDepartmentofAgriculturereference values.TheinvestigatorsreportedasignificantreductionintheoddsofPCOSinwomen thatconsumedahightotalantioxidantcontainingplant-baseddiet[76].Thesefindings supporttheexistingbodyofdietarypatternresearchthatrecommendhealthydietsforthe managementofPCOS[2,77].
3.2.2.AdvancedGlycationEndProductsandPCOS
Advancedglycationend-productsarereactivemoleculesthatareformedbynonenzymaticreactionsofcarbohydrateswithproteins,lipids,ornucleicacids[78].Advanced glycationendproductsresultintheirreversiblecross-linkingofproteinsandlossofprotein structureandfunctionandcaninitiateapoptosis[79].Advancedglycationendproducts canbegeneratedendogenouslyundernormalconditions,andcanbeingestedinfood, particularlyacookedfast-fooddiet,andwithcigarettesmoking[80].Advancedglycation
Life 2023, 13,1056 5of35
endproductscancauseoxidativestressandinflammation,resultingincellularandtissue damagewhenproducedoringestedinexcessiveamounts[81].Protectivecirculatingantiinflammatoryreceptorscalledsolublereceptorsforadvancedglycationendproductsand membrane-boundreceptorsofadvancedglycationend-products(RAGE)areassociated withprotectionagainstAGE[82].
TheinteractionofAGEwiththeirmembranereceptorsactivatesintracellularsignallingpathwaysthatleadtoincreasedoxidativestress,inflammation,IR,diabetes,hyperandrogenism,obesity,andovulatorydysfunction,allofwhichhavebeenassociatedwith PCOS[83,84].RecentdatahaveshownelevatedcirculatinglevelsofAGEandincreased expressionofRAGEreceptorsinovariantissue[85,86].ProinflammatoryAGE–RAGE signallinghasbeenfoundtocausealteredsteroidogenesisandfollicledevelopmentin ovariangranulosacellsinPCOS[87,88].Inaddition,hyperandrogenisminducesendoplasmicreticulumstressingranulosacells,resultinginincreasedaccumulationofAGEinthe ovary[86].
ModernWesterndietsarerichinAGEwhichisabsorbedthroughtheintestine[84]. Ahigh-glycaemicdietandexcessiveglucoseingestionresultinelevatedbloodglucose levelsandthegenerationofAGEthatbindwithcellmembrane-boundRAGEandactivate inflammation[89,90].LigandbindingbyglycatedproteinsandlipidstoRAGEstimulates intracellularsignallingeventsthatactivatenuclearfactorkappa-light-chain-enhancerofactivatedB(NF-κB).NuclearfactorkappaBcontrolsseveralgenesinvolvedininflammation, andRAGEitselfisupregulatedbyNF-κB,establishingapositivefeedbackcyclethatleads tochronicinflammation[90].
Studieshavedemonstratedthattheintakeofthelow-AGEcontainingdietisassociatedwithfavourablemetabolicandhormonalprofilesaswellasfeweroxidativestress biomarkersinpatientswithPCOS[91].Onestudyemployedalow-AGEdiet,consistingof Mediterranean-stylefoodscookedattemperaturesbelow180degreesbyboiling,poaching, stewing,orsteaming[91].High-temperaturecookingabove220degreesCelsiusbyroasting,grilling,andbakingwasavoided.Dietaryrecommendationsforminimisingingestion ofAGEincludesincreasingconsumptionofawholefoodsthatincludevegetables,fruits, seafood,andwholegrainswhilereducingtheconsumptionofhighAGEcontainingfoods. Theseincludehighlyprocessedfoods(packagedmeats,cheese,andsnackfoods),excessive sugarinsweetsandbeverages,andfriedfoods[77,84].Adoptionofotherhealthylifestyle behaviours,suchasexercise,maintainingnormalbodyweight,andcessationoftobacco consumption,arealsoimportantforreducingAGE[84,92].
3.2.3.PatternRecognitionReceptorsandtheInnateImmuneSystem
Theexistenceofreceptorsexpressedbyinnateimmunecellsthatwereresponsiblefor detectingmicrobialproductswasfirstproposedbyCharlesJanewayin1989[93].Polly Matzingersubsequentlyproposedthe“DangerTheory”,suggestingthattheimmunesystemproducedmoleculesthatinitiateandpropagateinflammationinresponsetotissue stress,damage,orinfection[94].In2013,RobertNaviauxfurtherexpandedthisconcepttothe“CellDangerResponse”(CDR)[95].Naviauxproposedthatevolutionary selectionhaspreservedasimilarresponsetoavarietyofthreatsascellshavealimited numberofwaystheycanmobilizeexistingcellularmachineryandenergy.TheCDRis, therefore,anevolutionary-conservedcellularprotectiveresponsethatisactivatedwhen acellencountersachemical,physical,ormicrobialthreatthatcouldinjureorkillthe cell[95].Morerecentresearchhascharacterizedthemoleculardetailsofanelaborate dangerdetectionsystem,involvingPRR,damage-associatedmolecularpatterns(DAMPS), pathogen-associatedmolecularpatterns(PAMPS),inflammasomes,andanintricatesystem ofsignallingmoleculesthatactivateasystem-widenetworkofinnateandadaptiveimmune responses[59].Thishasbeencalledthe“IntegratedStressResponse”andisreviewedin detailinpreviousreports[96,97].
TherearefivetypesofPRRthatcanbeclassifiedintotwomaingroupsbasedontheir cellularlocalization[98].Toll-likereceptors(TLR)andC-typelectinreceptors(CLR)are
Life 2023, 13,1056 6of35
transmembranereceptorsthatallowsensingofhost-derivedDAMPSandPAMPSatthe cellsurfaceorwithinmembrane-boundintracellularcompartments.SpecificcytoplasmicbasedreceptorsalsoprovideanintracellularrecognitionsystemforsensingDAMPSand PAMPS.Patternrecognitionreceptorsarealsoexcretedextracellularlyandcanbefound ininterstitialfluidandthebloodstream,wheretheyplayanimportantroleinpathogen recognition[59].Patternrecognitionreceptorsalsoactivatemultipletypesofcelldeath pathways,suchasapoptosisandpyroptosis(arapidpro-inflammatoryformofcelldeath), ifcellulardefenceagainstPAMPSandDAMPSisunsuccessful[52,59].
Therearethreemaintypesofmoleculesinvolvedinsignaltransductionfollowing infectiousordanger-relatedligandbindingtoPRR:proteinkinases,adaptorproteins, andtranscriptionfactors[98].Signaltransductionoccursviaseveralcommonpathways, includingNF-κB,mitogen-activatedproteinkinase(MAPK),andinflammasomes[52,98]. Thesignalstheygeneratecancrosstalkwitheachotherandcanconvergeintoseveral commonpathways.EachofthePRRinitiatesignallingcascadesthatcauseepigenetic modulationofgeneexpressionandposttranslationalmodificationofcytokineprecursors. Thisresultsinactivationoftheinnateimmuneresponseandleadstodegradationof microbes,productionofinflammatorycytokines,andrecruitmentoftheadaptiveimmune response[59,99].OncePAMPSandDAMPSbindwithTLRandnod-likereceptors,they activatetheformationofinflammasomecomplexesthatformanessentialelementofthe innateimmuneresponse[52,100].
Unlikeadaptiveimmunity,innateimmunitydoesnotrecognizeeverypossibleantigen. InnateimmunityrecognizesPAMPSsharedbyrelatedgroupsofmicrobesthatarenot foundinmammaliancells,suchaslipopolysaccharide(LPS)fromgram-negativebacterialcellwalls.Thisearlyinducedinnateimmunity(4–96h)involvestheformationof inflammasomesthatleadtothereleaseofchemokinesandrecruitmentofdefencecells. Therecruiteddefencecellsincludephagocyticleukocytes,suchasneutrophils,eosinophils, andmonocytesandtissuephagocyticcells,suchasmacrophages,macrophages,andmast cells,thatreleaseinflammatorymediatorsandbasophils,eosinophils,andnaturalkiller cells[59].
Patternrecognitionreceptorsrepresentevolutionarilyconservedpathogenanddamagerecognitionmechanismsthatconstitutethestartingpointfortheinflammatoryresponse.Thesecell-autonomousstressresponseshaveevolvedtoformthebasisofthe non-antigen-dependentdefencemechanismsthatcharacterizeinnateimmunity[94,101]. Lipopolysaccharidereleasedbygram-negativebacteriainthedysbioticgastrointestinal microbiomebindswithTLRonsub-mucosalmacrophages,resultingintheactivationof NF-κBandinflammatorycytokineproductionandsecretion[102].Thismechanismis thoughttobeamajorcontributortochronicinflammationinwomenwithPCOSandplays asignificantroleinthepathogenesis(discussedinSection 6)[54,103].
3.2.4.InflammasomesinPCOS
Inflammasomesaremultiproteinself-assemblingcomplexesinthecytoplasmthat formanintegralpartoftheinnateimmuneresponse[104].Theyareproducedinresponse toavarietyofdangersignalsandarealsoinvolvedincellularapoptosisandpyroptosis[52]. Inflammasomesactasfinelytunedalarmsystemsthattriggerandamplifyinnatedefence mechanismsinresponsetocellularstressesandinfection[100].Theinflammasomecomplexcontainsasensormolecule,anadaptorprotein,andapro-inflammatorycaspase-1 enzyme[104].OnceactivatedbyDAMPS(suchasLPS)andPAMPS,theinflammasome complexconvertsprocaspase-1totheactivecaspase-1enzymewhichsubsequentlyactivates pro-inflammatorycytokines(IL-1B,IL-18)thatarereleasedintotissuesandcirculation[105].
InflammasomesformanintegralpartofthecommonCDRpathwayforcellularprotectionfrommultipletypesofthreateningstimuli[51,94].Interruptiontotheflowofelectrons inmetabolism,generationofROS,andothermechanismsdiscussedabove,activateinnate intracellulardefencemechanismsinanattempttocontainandeliminatecellularthreats[62]. TheCDRalsoincludesthereleaseofpurinergicsignallingtoneighbouringcellsandim-
Life 2023, 13,1056 7of35
munecellsthatactivateinflammation[106].Ifthethreatiscontained,theCDRresolvesand normalcellularfunctionisrestored[51].IftheCDRisunsuccessful,pyroptosispathways areactivatedandthecellissacrificedinafurtherattempttocontainthethreatandprotect theorganism[52].Iftheincitingstimuluspersists,activationofchronicinflammationcan resultintissuedamageanddisease,suchasPCOS.
Inflammasomesandtheirpro-inflammatorycytokinesandchemokineshavebeen investigatedfortheirroleininflammation,oxidativestress,ovulation,fertilization,steroidogenesis,glucosemetabolism,IR,andadipogenesisandmaybeinvolvedinthepathogenesis ofPCOS[100,107,108].Theseandotherinflammatorymediators,suchasadipokines(leptin, adiponectin,vaspin,resistin,visfatin,andomentin-1),cyclophilinA,vasculardysfunction mediators(endothelin-1,vascularcelladhesivemolecule-1),NF-κB,andepigeneticregulators(microRNAs)arethoughttohavearoleinthepathogenesisofPCOSandhaverecently beenreviewed[105].
3.2.5.AdaptiveImmuneResponseinPCOS
Theadaptiveimmunesysteminvolvesantigen-specificdefencemechanismsthatare designedtoreacttoandremovespecificantigens[109].Theadaptivesysteminvolves humeralandcell-mediatedimmunityandmaytakeseveraldaystobecomeeffective.The bodyrecognizesanantigenasforeignwhenepitopes(fragmentsofanantigenthatreactwithantibodiesorlymphocytereceptors)bindtospecificreceptorsonthesurfaceof B-lymphocytesand/orT-lymphocytes[110].Itisestimatedthatthehumanbodycanrecognize107 epitopesandmakeupto109 differentantibodies[111].Nevertheless,activation ofboththeinnateandadaptiveimmunesystemswasprimarilydesignedtobeacuteand short-livedinordertocontainandeliminateamultitudeofenvironmentalthreats.
ThemajorityoftheresearchontheroleofadaptiveimmunityinPCOShasbeen conductedonT-cellsandtheirsubpopulations[109].T-cellsplayacrucialroleinmediating inflammationandIRbysecretingproinflammatorycytokines[112,113].T-cellspromote follicledevelopmentandselectionbyreleasingspecificchemokinesandgrowthfactorsand producingcytotoxicsignalstoinduceapoptosisofgranulosacells[114].Availableevidence suggeststhattheremaybeageneraldeclineinadaptiveimmunityandregulatoryT-cell functioninPCOS[109].Recentstudieshavesuggestedthatimmunesystemdysregulation, includingT-celldysfunction[115,116],mayplayaroleinthepathogenesisofPCOS[117].
Thegoaloftheinnateandadaptiveimmuneresponseistoprovideabalancebetween pro-andanti-inflammatorytissueturnoverandprotectivemeasures,inordertoachieve whole-bodyinflammatoryhomeostasis[118].Asisthecasewithmetabolism,theinternal inflammatoryresponseneedstobematchedtoprevailingexternalenvironmentalconditions[34].TheevolutionarytheoryofPCOSproposesthatthereisamismatchbetween ourevolutionaryconservedmetabolicandimmunesignallingsystemsandourmodern environment[1,119,120].Accumulatingevidenceoverthepast25yearssuggeststhat systemicinflammationismodulatedbyneuralandhumoralcommunicationpathwaysthat connectthebraintotheimmunesystem[121,122].Neuroimmunomodulationisawayfor organismstoregulatewhole-bodyinflammatoryhomeostasisandoptimizesurvival[118]. Thisinterconnectedregulatorysystemhasevolvedovermillionsofyearsofevolutionary pressurefromexposuretopathogensandotherenvironmentalthreats.
3.3.NeuroimmunomodulationandtheLinkbetweentheNervousSystemandPCOS
Boththeimmuneandnervoussystemssharemanysimilaritiesandhaveunique qualitiesthatallowthemtosensechangesintheinternalandexternalenvironments andcounteractdeviationsinhomeostasis[118].Communicationbetweenthenervous, endocrine,andimmunesystemsinvolvesevolutionary-conservedmechanismsthatare essentialforhostdefenceandsurvival[121].TheimmunesystemandANScanrespond tonumerouscommonregulatorymolecules,includingcytokines,neurotransmitters,and glucocorticoids[123].Thebrainistheultimateregulatorofwhole-bodyhomeostasisof allphysiologicalparameters.Thisincludesbloodglucose,thermoregulation,hydration,
Life 2023, 13,1056 8of35
electrolytelevels,bloodpressure,stressresponses,feeding,behaviour,reproduction,body weight,andwhole-bodyinflammatorybalance[34,48].
Regulationoftheinflammatoryresponsehaspreviouslybeenthoughttobeautonomous[124].Substantialevidencenowsuggeststhatthenervoussystemexertsan activeroleinmaintaininginflammatoryhomeostasis[48,125,126].Thebrainparticipatesin abidirectionalnetworkofmediators,includinghormones,cytokines,andneurotransmitters,thatmonitor,coordinate,andregulatethesystemicinflammatoryresponse[121,126]. Themagnitudeoftheinflammatoryresponseiscrucialtoadaptationandsurvival.An inefficientresponsecanresultinimmunodeficiency,infection,andcancer,andanexcessive responsecanleadtomorbidityandmortality[127].Abnormalitiesintheneuroendocrineimmuneresponseareimplicatedinthepathogenesisofmanychronicdiseases,including obesity,atherosclerosis,autoimmunedisease,depression,andPCOS[48,109,122,123].
3.3.1.AnatomyofNeuroendocrine-ImmuneConnections
Animalandhumanresearchoverthepastsixdecadeshaveinvestigatedtheextensive networkofafferentandefferentcommunicationmechanismsthatcoordinatethesystemic inflammatoryresponse[121,122,128].Thereisnowaconsensusthat“theinflammatory reflex”iscomposedof(1)asystemofsensors(thatidentifyPAMPSandDAMPS),(2)an afferentarmwhichconveysinformationaboutsystemicinflammatorystatustothecentral nervoussystem(CNS),(3)processingcentresinthebrainthatintegrateandinterpret incomingsignals(hypothalamicnucleiandbrainstemautonomicneurons),(4)andan efferentarmwhichexertsimmunomodulatoryfunctions(Hypothalamic-Pituitary-Adrenal (HPA)-axis,ANS)[125].Thebrainexertsstrongimmunomodulatoryeffectsonavarietyof componentsoftheimmunesystembyactivationoftheHPA-axisandANS(Figure 2).
Thehypothalamusplaysacentralroleinsensingandcoordinatingneuralandhumoral factorsandcanmodulateinflammatorypathways[48].Circulatingcytokines,suchasIL-1SS andTumourNecrosisFactor-alpha(TNF-α),cancrosstheblood–brainbarrierbyacarriermediatedmechanism[129]orviathecircumventricularorgans[130].Thehypothalamus canalsoreceiveinputfromvisceralvagusafferentfibresaftertheysynapsewiththedorsal motornucleusofthevagusnerveinthebrainstem.Thishasbeentermedthe“cholinergic anti-inflammatorypathway”[121].Ascendingconnectionsreachthehypothalamusvia thenucleustractussolitarius[131].Theeffectorarmorefferentsystemismodulatedby theHPA-axisandthesympathetic(SNS)andparasympathetic(PNS)componentsofthe ANS[121,132].
TheHPA-axisisaneurohormonalpathwaythathasclassicallybeenstudiedforits roleinregulatingtheimmunesystem[133](Figure 2).TheSNSmodulatesbothproandanti-inflammatoryactivities[121].TheSNSinnovatesprimary(thymusandbone marrow)andsecondary(spleen,lymphnodes,andtissues)lymphoidorgans.Sympatheticneuronsreleasenoradrenalinandadrenalinthatinteractwithadrenoreceptorson lymphocytesandmacrophagesandstimulatetheproductionofcytokinesthatresultinantiinflammatoryeffects[134].ThePNSalsoplaysasignificantroleinmodulatingimmune cellsandinflammatoryactivity[121].Evidencethatvagalafferentfibresrelaymessages totheCNSthatinflammationispresentinotherbodysiteshasbeendemonstratedin animalmodels,althoughevidenceforthemechanismsofactivationisstillnotclear[122]. Theparasympatheticnervoussystemhasananti-inflammatoryeffectthroughreleaseof acetylcholinethatinteractsdirectlywithnicotinicreceptorsonmacrophages[135],andalso indirectlywiththespleen[122].Overall,theSNSandPNSappeartoactsynergisticallyto downregulateinflammation.
Life 2023, 13,1056 9of35
Figure2. Hypothalamic-Pituitary-Adrenal-ImmuneAxis(HPA).Thehypothalamusreleasescorticotropinreleasinghormonethatstimulatesproductionofadrenocorticotropichormone(ACTH)from theanteriorpituitary.ACTHstimulatesthesynthesisofimmunosuppressiveglucocorticoids(cortisol) fromtheadrenalcortex[133].Pro-inflammatorycytokinesandneuralinputsactivatetheHPA-axisto releaseACTH,andtheHPA-axisissubjecttoaclassicnegativefeedbackloopbycortisolthatinhibits bothcorticotropinreleasinghormoneandACTH[136].Sympatheticneuralactivationofchromaffin cellsintheadrenalmedullaleadstoanincreasedreleaseofcatecholaminesintothecirculation. Sympatheticinnervationofcorticalcellsleadstothereleaseofglucocorticoids.CNS-controlledSNS outputis,therefore,convertedtohormonalimmunoregulationinperipheraltissues.ANS,Autonomic NervousSystem;ParasympatheticNervousSystem(PNS);SympatheticNervousSystem(SNS);CRH, CorticotropinReleasingHormone;AdrenocorticotropicHormone(ACTH);IL-1SS,interleukin-1SS; TNF-α,tumournecrosisfactor-α.©Designua|Dreamstime.com.
3.3.2.NeuroimmunomodulationinPCOS
Severalstudieshaveexaminedthebi-directionconnectionsbetweenthenervousand immunesystemsinPCOS.WomenwithPCOShavebeenfoundtohaveincreasedSNS activitybymuscleandskinmicroneurography,heartratevariability,measurementofnerve growthfactor,andcatecholaminemetabolites[126,137].Insulinhasbeenfoundtostimulate SNSoutput,andthereisacomplexbidirectionalrelationshipbetweenIRandsympathetic
Life 2023, 13,1056 10of35 ff ffi ẞ ẞ α α
activityinboththehypothalamusandovary[137].Althoughmostoftheresearchhas focusedonSNSoveractivity,reducedPNSactivityhasalsobeendemonstrated[132]. ImpairedANSfunctionhasbeenproposedasacontributortohyperandrogenemiavia mechanismsinthehypothalamus,adiposetissue,andovary[138,139].
SNSactivitycanbereducedinwomenwithPCOSbyelectroacupuncture,treatmentof obstructivesleepapnoeawithpositiveairwaypressure,renaldenervationinrefractoryhypertension,pharmacotherapy,exercisetraining,andweightloss[137,140].Takentogether, thesedatasuggestthatanimbalanceoftheANSislikelytoplayareversibleroleinthe pathophysiologyofPCOS.
3.4.HyperandrogenismandChronicInflammation
Chronicsystemicinflammationcancausehyperandrogenism[46]andelevatedandrogenscanaffectimmunecells,resultinginpredominatelyanti-inflammatoryeffectsonthe immunesystem[141].Thereisdebateintheliteratureregardingthedirectionofcausation, physiologicalimportance,andevolutionarysignificanceoftheseprocesses.Thepreponderanceoftheevidenceandthemostwidelyacceptedcurrentviewisthathyperandrogenism issecondarytothesynergisticactionsofchronicsystemicinflammationandIRthrough upregulationofovarianthecacellandrogensynthesis(discussedinSection 4.5)[142].The evidencepresentedinthecurrentreviewalsosupportsthisparadigm.
Serumandrogenscanbederivedfrommultipletissuesincludingtheadrenalgland, adiposetissue,andovaries[143].MostserumandrogensinPCOSarethoughttobeproducedbytheovaries.Nevertheless,itislikelythatmultiplemechanismsareinvolvedin excessiveandrogenproductionindifferenttissuesinwomenwithPCOS[143,144].LowgradechronicsystemicinflammationiscommonlyfoundinpatientswithPCOSexhibiting hyperandrogenism,andovarianinflammationandfibrosisarepartofthehistopathology[145].Ovarianinflammatorymechanismshavebeenlinkedtosystemicmarkers ofinflammation(CRPandwhitecellcount)[144–146]independentofbodymassindex (BMI)[147].Anextensiverangeofinflammatorycytokineshavebeenassociatedwith impairedfolliculogenesisandandrogensynthesisinwomenwithPCOS[144].Cytokines caninfluencetheinflammatoryresponsebyepigenicmechanismsthataltergeneexpression orbyposttranscriptionalregulatoryprocesses[46].Oxidativestresshasbeenreported toenhanceovariansteroidogenicenzymesandincreaseandrogenlevelsinwomenwith PCOS[67].Endoplasmicreticularstresspathwaysareactivatedintheovariesinmouse modelsandinhumansandmaycontributetothepathophysiologyofPCOSthrough multipleeffectsingranulosacells[148,149].
Asdiscussedpreviously,AGEandRAGEhavebeenfoundtocausealteredsteroidogenesisingranulosacellsinPCOS[87].Dehydroepiandrosteroneisacirculatingpre-androgen producedintheadrenalcortexthathasbeenfoundtostimulateinflammationandimpair ovarianfunctioninPCOS[150].Increasedluteinizinghormone(LH)levels,commonly foundinPCOS,canamplifytheabnormalitiesdescribedinthecacellsteroidogenesis[105]. Thecombinedeffectsoftheseprocessesresultinexcessfollicularandrogenswhichcombine withincreasedlevelsofinsulintodownregulatearomataselevelsingranulosacells.This createsacontinuousfeedbackloopbetweeninflammation,IR,andhyperandrogenismwith noapparentbeginningorend.Despitetheseuncertainties,diet-inducedinflammationcan invokehyperandrogenism[142],andeffectivetreatmentofinflammationcannormalize androgenlevelsandrestorefertilityinwomenwithPCOS[151].Insummary,accumulating evidencesuggeststhattheovariesarenottheprimarycauseofhyperandrogenisminmost womenwithPCOS(seeSection 4.5).
TheinfluenceofandrogensinthepathogenesisandpathophysiologyofPCOSis undisputable,despitethedebateregardingtheprimarymechanismofcausation.Data derivedmostlyfromanimalmodelsofPCOSclearlyindicatethatintrauterineexposureto elevatedandrogenlevelsinducesthedevelopmentofPCOStraitsinadultfemales.PCOS canalsodevelopinwomenwithotherhyperandrogenicsyndromes,suchascongenital adrenalhyperplasia,lipodystrophy,andovarianoradrenaltumours.Inaddition,therehas
Life 2023, 13,1056 11of35
alsobeendebateregardingtheevolutionary“advantage”ofhyperandrogenisminPCOS. Inflammation-inducedhyperandrogenismhasbeenproposedasapossiblecompensatory mechanismtorestorehomeostasiswithintheimmunesystemgiventheanti-inflammatory effectsofandrogensinwomen[46].Itisalsopossiblethathyperandrogenism(dueto inflammationand/orhyperinsulinemia)mayrepresentanadaptivephysiologicalmechanismtodown-regulatereproductionduringperiodsofenvironmental(infection,starvation, andclimatic)andpersonalstress.Inaddition,theremaybeotherindividualadvantages, suchasincreasedstrengthandfitness[1].
3.5.SummaryoftheRoleofInflammationinPCOSfromanEvolutionaryPerspective Asignificantbodyoftheliteraturesupportstheroleofchronicinflammationinthe pathogenesisofPCOS.Billionsofyearsofevolutionhaveconstructedacooperativesystem ofsensors,receptors,hormones,cytokines,chemokines,andothersignallingmolecules thatinvokeintracrine,autocrine,paracrine,andneuroendocrinemechanismsdesigned toregulatecellular,tissue,andwhole-bodyinflammatoryresponses.Thisprocessforms partoftherepertoireofadaptivesurvivalresponsesthatareintimatelyconnectedwith totalbodymetabolichomeostasistoensure individual survival.Inflammationinvokesa seriesofadaptivemetabolicsurvivalmechanisms,suchasIR,toensureadequateenergy supplytoimmunecells.Neuroendocrinemechanismsactasacounter-regulatoryantiinflammatorymechanismtocontrolinflammationandrestorehomeostasis.Inaddition, immuneandmetabolicphysiologyareintimatelylinkedtoreproduction,tooptimize fertilityandensure species survival.Asaresult,bothchronicinflammationandIRcan contributetohyperandrogenemia,whichalsohasadaptivesurvivaladvantagesthatrestore immunehomeostasisandtemporarilydownregulatereproduction.
4.InsulinResistanceandReducedInsulinSensitivity
4.1.PhysiologicalActionsofInsulin
Insulinisapeptidehormone(51aminoacids)producedbythebetacellsinthe isletsofLangerhansinthepancreaswhereitisstoredincytoplasmicgranulesasan inactivehexamerwithzincandcalcium[152].Insulinreleaseischaracterizedbylowlevelbasalsecretioninthefastingstateandanoscillatingpulsatileultradianpattern (1–2-hcycle),whenstimulated.Insulinbindswithitsreceptorwhichisalarge(320kDa) transmembraneproteinwithanintracellulardomainthatactsasatyrosinekinase.The insulinreceptorishighlyconservedfromanevolutionaryperspective,andispresentinall mammaliancells,althoughthedistributionisunequal(40perredbloodcell,300,000per adipocyte)[153].Insulinbindstothealpha-subunitandinducesautophosphorylationof specifictyrosineresiduesonthecytoplasmicsideofthemembrane[43,154].Theactivated insulinreceptorinitiatessignaltransductionviathephosphatidylinositol-3kinase(PI-3K) metabolicpathwayandthemitogen-activatedproteinkinasepathway(MAPK)whichis involvedincellgrowthandproliferation[154].Therearemultiplephysiologicalstimulators (vagalPNS,growthhormone,chroniccortisol,prolactin,andgonadotropins)andinhibitors ofinsulin(adrenaline,noradrenaline,SNS,parathyroidhormone,somatostatin,acute cortisol,andpancreaticpolypeptide)[155].Glucoseisbyfarthemostpotentstimulatorof insulinandalltheothersareregulatoryandmuchlesseffective.
Insulinhaspleiotropicmetabolicandgrowth-promotinganaboliceffectsthroughout thebody[155].Importantly,thecellulareffectsofinsulinaretissuespecific,andnot allcellsrequireinsulintotransportglucoseintocells.Insulinsignallingincreasesthe availabilityofGLUT-4glucosetransportproteinstothesurfaceofinsulin-dependent cells(skeletalandcardiacmuscle,adiposetissue,andvascularendothelium).Theliver andbrainareinsulin-independentanduseotherGLUTtransportersinadditiontousing insulin-responsiveGLUT-4transporters.Insulinmodulatesawiderangeoftissue-specific physiologicalprocessesinadditiontofacilitatingglucoseremovalfromtheblood[34]. Insulinenhancesfatstorageandinhibitslipolysisinadiposetissue,stimulatesglycogen synthesisinmuscleandliver,inhibitshepaticglucoseoutput(gluconeogenesis),causes
Life 2023, 13,1056 12of35
vasodilationinvascularendothelium,andtheheart(viaproductionofnitricoxide)and enhancessodiumreabsorptioninthekidney[155,156].
Insulinalsohasadirectanti-inflammatoryrolebypreventinghyperglycaemia-related generationofROSandAGE,inhibitingNF-kB(byreducingtheproductionofinflammatorycytokines),inducingvasodilation(vianitricoxiderelease),reducingleukocyte adhesiontotheendothelium[157],andbyinhibitingformationoftheNLRinflammasomecomplex[158].Asaresult,hyperglycaemiaandIRarepro-inflammatorystates. Insulinresistancecancauseinflammation,andinflammationcancauseIRinwomenwith PCOS[157,159].MostoftheliteraturehasfocusedontheevolutionarybenefitoftheproinflammatoryeffectsofIRinstarvation,trauma,andinfection(seeSection 4.4)[159].In contrast,theanti-inflammatoryeffectsofinsulinmayhaveanevolutionaryprotectiveeffect againstexcessiveactivationofthesystemicimmuneresponsefollowingexposuretosmall amountsofingestedantigensinasimilarmannertothegastrointestinalmucosalimmune system[160,161].
Insummary,insulin’soverallroleistocontrolenergyconservationandutilizationduringfeedingandfastingstates[152].Insulinactsasametabolicswitchbetweenanabolicand catabolicprocessestoregulatebloodglucoselevelsandhasasignificantanti-inflammatory effect.Insulinprovidesadirectlinkbetweenmetabolismandimmuneregulationandis paramountinactivatingnumerousadaptivesurvivalmechanisms[162].Dysregulationof theprotectivephysiologicalfunctionsofinsulinisinstrumentalinthepathogenesisand progressionofmanychronicdiseases,includingPCOS[152,163,164].
4.2.ReducedInsulinSensitivityversusInsulinResistanceinPCOS
Diminishedtissuesensitivitytoinsulinhasbecomecharacterizedasapathological conditionknownasIRasaresultoftheassociationbetweenIR,metabolicconditions,and chronicdisease[17,164].Beingabletovarythesensitivityofthecellularandtissueresponse toinsulinisanevolutionarilyconservedprotectivemechanismusedbymanyspecies (insects,worms,vertebrates,andhumans)toenhancesurvival[165].Insulinresistance isacategoricalvariablethathasanarbitrarydefinitioninresearchstudiesbuthasno agreeddefinitionornormalrangeinclinicalpractice[2,166].Reducedinsulinsensitivity isacontinuousvariablethatisconsideredaquantitativetrait(interactionofmultiple geneswiththeenvironmentthatresultsinacontinuousdistributionofphenotypes),in evolutionarymedicine[167].
ThegoldstandardforassessingIRisthehyperinsulinemic-euglycemicclamp(clamp) test.Theclamptestisperformedusinginsulinandglucoseinfusionsandistime-consuming, labour-intensive,expensive,andhasassociatedrisks[168].Ahigh-doseinsulininfusion (80mU/m2/min)isusedtosuppresshepaticgluconeogenesisandcreateasteady-state bloodglucoseconcentration.Therateofglucoseinfused,inordertomaintainasteady state,isequaltowhole-bodyglucosedisposal.Decreasedinsulinsensitivityisdefinedas IRwhentheglucosedisposalrateislessthananarbitrarycutoffof4.45mg/kg/min[168].
ItisimportanttodistinguishbetweenreducedinsulinsensitivityandIRasmost womenwithPCOShavereducedinsulinsensitivity,butnotallwomenwithPCOSmeet theexperimentalcriteriaforinsulinresistance.ThereportedprevalenceofIRinPCOS hasvariedwidelyduetotheheterogeneityofdiagnosticcriteriaforPCOS,thevarietyof assessmentmethods,andthearbitrarydefinitionofIRselectedfordifferentstudies[169]. Nevertheless,IRhasbeenconsideredacentralfeatureinthemajorityofwomenwith PCOS[170].Asystematicreviewofhyperinsulinemic-euglycemicclampstudiesfound thatwomenwithPCOShavea27%reductionininsulinsensitivitycomparedtomatched controls[163].
Whenconsideredfromanevolutionaryperspective,womenwithaPCOSphenotype wouldhaveimprovedsurvivalchancesduringtimesofincreasedphysiologicaldemand orimposedenvironmentalstressbutbemorevulnerabletothepathologicaleffectsof IRwhenexposedtocontemporarylifestylefactors[1,5].Whenviewedasacontinuous
Life 2023, 13,1056 13of35
variable,itislikelythatallwomenwithPCOS,whetherobeseorlean,havereducedinsulin sensitivity[163,171,172].
4.3.MechanismsofInsulinResistanceinPCOS
Insulinresistancecanbecausedbynumerousmechanisms,includinghyperinsulinemia,insulinreceptorvariants,receptorantagonistsandagonists,autoantibodies,oxidative stress,advancedglycationend-products,hormones,nutrientsensors,inflammatorycytokines,andmetabolicintermediates[155,173].Itislikelythatmorethanonemechanismis involvedinanyindividual,giventheinteractivenatureofmanyofthepathophysiological processesandsignallingpathways.
InsulinresistancehasbeenrelatedtotheWesterndietviaavarietyofmechanisms[174]. Theseincludehigh-glycaemicdiet-relateddysbiosis[54,103],chronicinflammation,and intracellularaccumulationofmetabolicintermediates[155].Thesemechanismsmayact individuallyortogethertoproducetheobservedfeaturesofIR.Agraphicaldescriptionis giveninFigure 3 tt
Figure3. Pathophysiologyofinsulinresistance.Anyofthecauseslistedabovecanimpactinsulin signallingpathwaysandleadtotissue-selectiveimpairmentofinsulinaction.Onceinsulinresistance occursinmuscle,glycogensynthesisandglucoseuptakefromthecirculationarereduced.Since muscleconstitutesapproximately50%ofbodymass,insulinresistanceinmusclemakesasignificant contributiontohyperglycaemia.Insulinresistanceinadiposetissueleadstoimpairedlipogenesisand continuedlipolysis,withreleaseofglycerolandfreefattyacidsintothecirculation.Hepaticinsulin resistanceimpairsglycogensynthesisandpreventsinsulinfrominhibitinggluconeogenesis.Adipose lipolysissuppliessubstratesforcontinuedhepaticgluconeogenesis.Together,theeffectsofdecreased muscleglucoseuptake,adiposelipolysis,andhepaticgluconeogenesis\resultinhyperglycaemia. Thiscausesthecompensatoryreleaseofinsulinfromthepancreasandhyperinsulinemia.DNL, denovolipogenesis;DAG,diacylglycerol;FFA,freefattyacid;IR,insulinresistance;VLDL,very low-densitylipoprotein.
Life 2023, 13,1056 14of35
ff tt ff
4.4.EvolutionaryAdaptiveRoleofInsulinResistanceinPCOS
Humansurvivalhasreliedontheabilitytoalterourphysiologyaccordingtothe changingdemandsoftheenvironmentortodifferentinternalstatesduringvariouslife stages[175].Inevolutionaryterms,physiologicalIRisanadaptivesurvivalmechanism thatallowsorganismstoselectivelymodulatecellularandtissueresponsestoavariety ofenvironmentalchallenges(infection,starvation,dehydration,psychologicalstress,and physicalstressfrominjury)[156,176,177]andinternalstates(pregnancy,puberty,andadolescence)[178,179].PathologicalIRisadetrimentalconditionassociatedwithmetabolic syndrome,otherchronicdiseases,andPCOS[173].Thesuggestedmechanismsandevolutionaryadaptiverolesofinsulinresistancearediscussedinthefollowingsections.
4.4.1.InsulinResistanceandInfection
Bidirectionalimmune-endocrineinteractionsregulatemetabolisminthecontextof infectiontoensurepathogenclearance,providetheappropriatemetabolicrequirements, andrestorehomeostasis[175,176].TheimmunesystemusesPRR-activatedcytokines(see Section 3.2.3)tocommunicatewiththeendocrinesystemandmodifytheresponsiveness ofperipheraltissuestoendocrinesignals.Immunecellsarehighlydependentonglucose formetabolismandareresponsiblefornearly20%oftotalbodyenergyconsumption[180]. Thiscanriseto30%duringinfectionandneedsaneffectiveandrapidresponsesystemto facilitatetheswitchfromoxidativephosphorylationtomorenutrient-intensiveglycolytic metabolism[176].Inaddition,inflammatorycytokinescaninfluenceprocessesnormally regulatedbytheendocrinesystem,suchashunger,temperature,insulinsensitivity,and glucoseuptake.Similarly,theendocrinesystemcanregulateimmunecellsviaacomplex systemofhormones(leptin,adiponectin,andinsulin),receptors,nutrientsensors,and metabolicsignallingpathways[176,180].
Whenaninfectiousthreatisdetectedbytheimmunesystem,thebodyactivates neuroendocrinecomponentsoftheHPA-axisandSNS(seeSection 3.3)toincreaseglucocorticoidandcatecholaminerelease[180].TheHPA-axisandSNSacttogetherwithastate ofcytokine-mediatedIR,tomobilizefreefattyacidsfromadiposetissue,andglucosefrom hepaticglycogentoensureanappropriatesupplyofenergytoimmunecells.Theabilityto regulateinsulinsensitivityandselectivelyredistributeglucoseandenergy,isanadaptive survivalmechanismconservedinmanyspeciesthroughoutevolutionaryhistory[175].
Incontemporarysociety,stressismorelikelytobepsychologicalratherthanphysical orinfectious,butthesystemicstressresponseissimilar[175].TheHPA-axisandSNSare activated,IRisimplemented,butthemetabolicdemandismuchless,andthemobilized energyisre-storedinadiposetissue.Thestressisoftenprotractedresultinginanxiety, increasedappetitefromcortisolexcessandleptinresistance,stresseating,andcentral obesity[181].Inaddition,sustainedadversenutritionalandenvironmentalexposurescan resultin“trainedimmunity”(adaptivechangestoinnateimmuneresponses)thatmay partlyexplainIR-relatedpathologies[182].Inevolutionaryterms,chronicIRrepresentsan evolutionarymismatchbetweenancestralsurvivalresponsesandmodernculturaldemands andlifestyles.WomenwithPCOSexperienceincreasedlevelsofanxiety,depression, andstress,andtheInternationalGuidelinesrecommendscreeningforemotionalwell being[2,183].
4.4.2.InsulinResistance,StarvationandDehydration
Insulinisananabolichormonethatpromotescellulargrowthandenergystorage inadiposetissue,liver,andmuscle[155].Prolongedcalorierestrictionduringstarvation activatesadaptivesurvivalmechanismsthatreduceenergyexpenditureandpreventmuscleloss[184].Clampstudieshavedemonstratedthatstarvation-relatedIRiscausedby decreasedinsulinsignallingandreducedglucoseuptake[185].TheimplementationofIR instarvationstatesdiminishestheoxidationofglucose,providesenergybymobilizing fattyacidsfromadiposetissue,limitsproteinloss,andredirectsenergytoin-demand organs[184].
Life 2023, 13,1056 15of35
Hyperinsulinemiamayhaveplayedaroleinpreventingdehydrationinancestralpopulations[156].Insulinregulatessodiumchannelsandincreasesthereabsorptionofsodium andwaterintherenaltubules[186].Hyperinsulinemiamayincreasebloodvolumeand bloodpressurebythismechanismorviaactivationoftherenin-angiotensin-aldosteronesystem[187].Insulinalsostimulatesnitricoxide,resultinginvasodilationanddecreasedblood pressure.Insulinresistanceresultsinselectiveimpairmentofthenitricoxidepathway, andhyperinsulinemiamayactivatetheMAPKsignallingpathway,causevasoconstrictionandelevatedbloodpressure[188].Thecombinedeffectsofthesemechanismsmay contributetothemaintenanceofbloodvolumeandincreasedcerebralperfusionduring starvation.Pathologicalactivationofthesemechanismscouldcontributetotheincreased riskofhypertensionandmetabolicsyndromeobservedinwomenwithPCOS[189].
WomenwithaPCOSphenotypemayhavehadanevolutionaryadvantagefromhaving abetterresponsetoinfection,dehydration,andstarvationbutarenowmoresusceptibleto stress,hypertension,metabolicsyndrome,andPCOSasaresultofcontemporarylifestyle andenvironmentalexposures[1,5].
4.4.3.InsulinResistanceandPregnancy
Insulinsensitivitydecreasesthroughoutpregnancyandisanevolutionary-conserved mechanismtolimitmaternalglucoseuseandshuntenergytothefoetus,particularly duringthesecondhalfofgestation[178].Insulinsensitivitygraduallydecreasesupto 20weeksgestation,followedbyamorerapiddecreaseto50%ofthenon-pregnantvalues by40weeksinnormalpregnancy[190].Thepancreaticbeta-cellsrespondbyincreasing insulinsecretionbyupto250%tomaintaineuglycaemia[191].Aninabilitytosecrete adequateamountsofinsulinresultsinelevatedbloodsugarlevelsandGestationalDiabetes Mellitis(GDM).
MaternalIRresultsintheuseofmorelipidsforenergybythemotherandsparescarbohydratesforthefoetus.Thedecreasedmaternalresponsetoinsulinismediatedbyoestrogen,progesterone,humanplacentallactogen,humanplacentalgrowthhormone,cortisol, prolactin,inflammatorycytokines,adipokines,exosomes,andthemicrobiome [178,192,193]. Clampstudiesinlatepregnancyshowthathepaticgluconeogenesisisonly80%suppressed inlatepregnancyinwomenwithGDMcomparedtonon-diabeticpregnantwomen[191]. Insulinsensitivityisalsoaffectedbyobesity,andfatmassincreasesinbothleanandobese womenduringpregnancy.WomenwithPCOShavehighergestationalweightgainand proportionatelymorevisceraladiposity,whichisassociatedwithgreaterincreasesinIR andGDM[178].
WomenwithPCOShavea25–50%chanceofdevelopingGDMinpregnancy[194,195]. WomenwithGDMhaveuptoa50%riskofdevelopingT2DMinthe5–10yearsfollowing pregnancy[196,197].Thepopulation-attributableriskofPCOStoT2DMhasbeenestimated at19–28%[17].Therefore,upto28%ofadultwomenwithT2DMhavepre-existingPCOS thatprogressestoT2DM.Upto50%ofindividualsdiagnosedwithT2DMhavecomplications(retinopathy,nephropathy,neuropathy,andvascular)atthetimeofdiagnosis[198]. TheInternationalDiabetesFederationestimatesthatthereareapproximately537million diabeticsintheworld,andwomenwithPCOS,therefore,makeasignificantcontributionto thisglobalepidemic[199].ThesedatasupportthecharacterizationofPCOSasaprogressive metabolicdisorder.RecentstudieshavedemonstratedthattheriskofprogressionofGDM toT2DMcanbereducedbygreaterthan90%bydietaryandlifestyleinterventions[200]. PCOS,therefore,appearstobeaprogressivemetabolicdisorderthatispreventable.
Insummary,thedownregulationofinsulinsignallingandimplementationofIR canbeviewedasaphysiologicaladaptivemechanismthatfacilitatestheswitchfroman anabolictoacatabolicstate.Thisresultsinthemobilizationoffattyacidsfromadipose tissue,increasedgluconeogenesis,releaseofglucosefromtheliver,andredistributionof energytotheimmunesystemandbrain.Thisprocessismediatedbythecooperative interactionoftheimmune,nervous,andendocrinesystems.Chronicoveractivationof anyofthesemechanismscreatesadisturbancetowhole-bodyhomeostasisthatresultsin
Life 2023, 13,1056 16of35
chronicinflammationandIR.WomenwithPCOSappeartohaveageneticallydetermined proinflammatorydesignandincreasedinsulinsensitivitythatwouldbeprotectiveinan ancestralenvironmentbutbecomesmaladaptiveinmodernsociety.
4.5.InsulinResistanceandHyperandrogenism
Androgensplayanessentialroleinmanyaspectsofmaleandfemalephysiology, metabolism,sexdifferentiation,andreproductivebiology[201].Optimalhealthisachieved whenbioactivemetabolites(testosterone,dihydrotestosterone)aremaintainedinadefinedhomeostaticrange.Inwomen,lowlevelsofandrogenshavebeenassociatedwith endometriosisandhighlevelswithPCOS[202].AswithallotherpathophysiologicalprocessesinPCOS,thereappearstobeabidirectionalrelationshipbetweenhyperinsulinemia andhyperandrogenism.Hyperinsulinemiacancausehyperandrogenismviamechanisms discussedbelow,andhyperinsulinemiahasbeenshowntooccuraftertheadministration ofandrogensinclampstudies[203,204].Thismayrepresentaself-reinforcingpositive feed-forwardmechanismtoaugmenttheeffectsofIR.
Ovarianandrogensynthesisoccursinacooperativetwo-stepprocessinvolving luteinizinghormone(LH)stimulationofsteroidogenesisinthethecacell,transferof androstenedionetothegranulosacells,andfollicle-stimulatinghormone(FSH)induced productionoftestosterone[205].Androgensareirreversiblyconvertedtooestrogeningranulosacellsbytherate-limitingaromataseenzyme.Aromatasebelongstothecytochrome P450superfamilyandistheproductoftheCYP19A1gene.Theprincipalroleofaromatase istoconvertandrogenstooestrogen.Disruptionofaromatasefunctioncanleadtoelevated orreducedlevelsofandrogensorestrogen,whichareassociatedwithawidevarietyof commonpathologies(endometriosis,osteoporosis,hypogonadism,Alzheimer’sdisease, cancer,andPCOS)[201].Aromataseiscrucialformanymetabolicfunctionsduetoitsrole asanestrogenbiosyntheticenzyme(glucoseandlipidhomeostasis,bonemineralization, andbrainfunction)[206].Ovarianaromataseactivityisanimportantregulatorofovulation andsequentialendometrialchangesduringthenormalmenstrualcycle.Dysregulation ofaromatasehasbeenlinkedtoalteredsteroidogenesis,hyperandrogenism,impaired folliculogenesis,andanovulation,andisthoughttoplayacentralroleinthepathogenesis ofPCOS[207,208].
Structuralmodelsusedtoinvestigatethemolecularevolutionofaromatasehave characterizedtheamino-acidsequencesandconfigurations,substraterecognitionsites, catalyticmechanisms,andinhibitorspecificities[209–211].Thereisacomplexmolecular networkthatregulatesthenormalphysiologyofaromataseactivity,andsubsequentandrogenandestrogenproductiontomaintaintissue-specifichomeostasisandfunction[211]. TheCYP19A1geneandaromataseenzymearepresentinvirtuallyallvertebratesand havebeenidentifiedinmanyinvertebrates.Thecorestructure,activesiteaminoacid sequences,andsubstraterecognitionsiteshavebeenhighlyconservedthroughoutevolutionaryhistory[209].Inhumans,thereareelevenpromotersthatregulatetissue-specific aromatasegeneexpression.Post-translationalmodificationbyphosphorylationallows rapidmodulationofaromataseactivitycomparedtogeneregulation.
WhileFSHhasbeenidentifiedasthemostimportantfactorthatregulatestheexpressionofaromatase[207],otherhormonessuchasmelatoninandleptinalsoplayanimportant stimulatoryrole[212,213].Inaddition,hormonalcontraceptivesandendocrine-disrupting chemicals(BisphenolA)havebeenshowntoincreasearomataseactivity[214,215].Multipleendogenous(insulin,leptin,adiponectin,cortisol,vitaminD,AGE,andinflammatory cytokines)[201]andexogenousmolecules(glyphosate,phytoestrogens,resveratrol,curcumin,nicotine,alcohol,andazoleagriculturalantifungals)[216–221]canactasaromatase inhibitorsandpotentiallycontributetohyperandrogenisminPCOS.Insummary,aromataseappearstobeasignificantphysiologicalintersectionpointforregulatorymolecules involvedinimmune,metabolic,hormonal,andreproductivefunctions.Inevolutionary terms,varyingaromataseactivityadjuststhehomeostaticbalancebetweenoestrogen
Life 2023, 13,1056 17of35
andandrogens,modulatingadaptivesurvivalpathwaysinmultipletissuesthroughout thebody.
Aspreviouslydiscussed,IRisthoughttohaveevolvedasanadaptationtoenvironmentalstressors,suchasstarvation,infection,andfear[1].Varyinglevelsofinsulin sensitivitypermitstheredistributionoftotalbodyenergytoorgansofgreaterneed,such asthebrain,immunesystem,andfoetus[175].Inaddition,ithasbeenproposedthat thedevelopmentofselectiveinsulinresistanceisamechanismthatactivatesspecificbehaviouralandreproductivesurvivalstrategies[43].Specificcellsandtissuesareprotected fromdevelopingIR,includingthebrain,immunecells,placenta,andovaries[222,223]. AreasofthebraindonotdevelopIRandbenefitfromtheredistributionofglucosefrom muscleandfattissue.Inpregnancy,theplacentaisaninsulin-independentorgan,andthe developmentofmaternalIRisexpectedtodivertmorenutrientsthroughtheplacenta.The ovarydoesnotdevelopIRandremainssensitivetothehighlevelsofinsulinthatoccurin IRandhyperinsulinemia[224,225].Thismaybeamechanismtodownregulatefertilityat timesofphysiologicalorpsychologicalstress.Insummary,boththephysiologicalactions ofinsulinandthedevelopmentofinsulinresistancearetissuespecificandmayfacilitatea varietyofadaptivesurvivalresponsesinwomenwithPCOS.
Inourmodernenvironment,insulinresistanceandhyperinsulinemiaarethoughttobe primaryfactorsinthedevelopmentofhyperandrogenisminPCOS,inadditiontochronic inflammation(discussedinSection 3.4)[225].Insulinhasseveralknownmechanismsthat canincreaseandrogenlevelsintheserum,liver,andovaries[67,225].Insulinisreported tostimulateovarianandrogenproductiondirectly(viathePI3KandMAPKpathways)or indirectlybyaugmentingLH-stimulatedandrogensynthesis[67].Insulinincreasesthe availabilityofinsulin-likegrowthfactoranddecreasesitsbindingprotein,resultingin increasedandrogenstimulation[226].Insulincanincreasetheamplitudeofgonadotropinreleasinghormone-stimulatedLHpulses[227]thatareknowntooccurinPCOS.Both insulinandtestosteronedecreasehepaticproductionofsexhormone-bindingglobulin, resultinginincreasedfreetestosterone[228].Inaddition,hyperinsulinemiastimulatesthe HPAaxisleadingtoincreasedadrenalandrogenproduction[229].
Accumulatingevidencesuggeststhattheovariesarenottheprimaryabnormalityin PCOS[225,230,231].WomenwithPCOSoftenrespondpromptlytogonadotropinstimulationwithclomipheneandgonadotropin-releasinghormoneagonistsorantiandrogenic compounds[232].Furthermore,lifestyleinterventions,suchasweightlossandexercise, reduceIRandinsulinlevels,normalizegonadotropinsecretion,andregulatemenstrualcyclesinwomenwithPCOS[233].Therapywithmetforminresultsinreducedandrogensand restoresovulationbyreducinginsulinlevelsandalteringtheeffectofinsulinonandrogen biosynthesisandthecacellproliferation[234].Asdiscussedpreviously,inflammationalso contributestothepathophysiologyofhyperandrogenism.Lifestyleinterventionsreduce bothinsulinlevelsandinflammation,highlightingthelikelihoodthathyperinsulinemia andchronicinflammationacttogethertoalterovariansteroidogenesis,increaseandrogen levels,impairfolliculardevelopment,andreduceovulation[10,235].Fromanevolutionary perspective,inhibitionofaromatasemayactasanotherphysiologicalmechanism(inadditiontohypothalamiceffectsdiscussedinSection 3.3)todownregulatereproductionuntil inflammatoryandmetabolicstressorsarecontained.
5.EvolutionarySignificanceofAdiposeTissueinPCOS
Hundredsofmillionsofyearsofevolutionhaveshapedadiposetissue(AT)into itscurrentform[236–239].Takinganevolutionaryperspectiveprovidesinsightintothe complexrangeofAT-relatedadaptivesurvivalfunctionsthatformpartofthenetwork ofinterdependenthomeostaticsystemspreviouslydiscussed.Adiposetissueisinvolved inavarietyoffunctions,includingimmuneresponses(innate,adaptive,andinflammatory),metabolism(glucoseandlipidmetabolism,appetiteregulation,maintenanceofbody weight,andinsulinresistance),andreproduction(pregnancy,lactation,andhyperandrogenism)[236,240].Adiposetissuehasabidirectionalrelationshipwiththeneuroendocrine,
Life 2023, 13,1056 18of35
immune,metabolic,andreproductivesystemsthatfacilitatethesefunctions.Thiscommunicationisachievedviaavarietyofcellularreceptorsandthesecretionofalargenumber ofsignallingmolecules.Theseincludeadipokines(adiponectin,leptin,resistin,visfatin, retinol-bindingprotein4,pigmentepithelium-derivedfactor,endocannabinoids,andmany more),cytokines(50cytokineshavebeenidentified),metabolites,lipids,non-codingRNAs orextracellularvesicles,andchemokines[239,241].
Adiposetissuecontainsadiverserangeofcelltypes,suchasimmunecells(macrophages, monocytes,granulocytes,andT-cells),stromalcells,fibroblasts,preadipocytes,and adipocytes[240,241]andisdividedintobrownAT(BAT)andwhiteAT(WAT).WATisclassifiedbyitsanatomicallocationassubcutaneousAT(SAT)orvisceralAT(VAT)[242,243]. BrownATisprimarilyinvolvedinthermoregulationandtherearedistinctdifferences betweenthelocation,structure,andfunctionofVATandSAT.
SubcutaneousATfunctionsasanendocrineorganandenergystoragedepotand representsanormalphysiologicalbuffertostoreexcessconsumedenergy.Thisenergycan bereleasedduringperiodsoflimitedfoodavailabilityandisanimportantevolutionaryconservedadaptivesurvivalmechanism[5].Carbohydrates(glucoseandfructose)that arenotconvertedtoglycogenaremetabolizedtotriglycerides,andalongwithdietary lipids,aretransportedtoAT,andstoredinlipiddroplets[237].SubcutaneousATiswidely distributedbutpredominantlylocatedintheabdominalwall(males)andfemurogluteal region(females).ThelowerbodydistributionofSATinwomenisthoughttohaveevolved toprovideinsulationinthecoolertemperaturesofthePleistoceneopenSavana[244].This fatdistributionisuniquetohumanfemalesandmayhaveprovidedextraenergystorage duringlactationandpregnancy[175].
GeneticdifferencesintheabilitytostorelipidsinSATmaypartlyexplaintheapparent leanparadoxin20%ofwomenwithPCOS(seereference[1]fordetaileddiscussion)[1]. Accordingtothe“adiposeexpandabilityhypothesis”,whenthegeneticallydetermined storagecapacityisexceeded,ortheabilitytogeneratenewadipocytesisimpaired,fat beginstoaccumulateinareasoutsideSAT[245].Inaddition,excesslipidaccumulationin SATleadstoadipocytehypertrophy,hypoxia,insulinresistance,inflammation,andrelease ofinflammatorycytokinesintothecirculation[246].Chronicstress,hyperandrogenism, chronicinflammation,andinsulinresistancefoundinwomenwithPCOSmaycontribute totheaccumulationofadditionalVAT[241,247,248].
Upto80%ofwomenwithPCOSareoverweightorobese[249].Bothleanand obesewomenwithPCOShavebeenfoundtohaveanincreasedproportionofVATusing avarietyofbodycompositionassessmentmethods[250].Inaddition,therearemany anatomicalandphysiologicaldifferencesbetweenVATandSAT.VisceralATcontains largeradipocytes,ismorevascular,hasmoreglucocorticoid,androgen,SS-adrenoreceptors, angiotensinogen,adiponectinandleptin,haslessoestrogenreceptors,andreducedfatty aciduptake[239–241].VisceralATalsohasincreasedcatecholamine-inducedlipolysis, greaterinsulinsensitivity,andproducesmoreinflammatorycytokines[236,240].Ithas beenproposedthathavinganincreasedproportionofVATprovidesanadaptivesurvival advantageagainstinfection[236].Thishasbeencalledthe“VATprioritizationhypothesis” andissupportedbymultiplelinesofevidence.
MostVATisintheomentumandmesentery[236].MesentericVATsurroundsthe smallintestineandisthenextlineofdefenceagainstpathogensandendotoxins(LPS) translocatedfromtheintestine,followingpassagethroughthesubmucosalimmunesystem[251].ThemesentericVATcontainslargenumbersofimmunecellsthatcanmounta rapidenergy-intensiveimmuneresponseincaseofinfection.Visceraladipocytes,such aslymphnodes,arespecializedtostorediet-derivedpolyunsaturatedfattyacidsthat arerequiredforanimmuneresponse.MesentericVATcontainslymphoidclustersthat expandinresponsetoinflammationandinfection[251].Bloodfromthemesentericand omentalVATdrainsintotheportalveinandismoveddirectlytotheliver.Thejunction oftheportalveinandlivercontainsanotherclusterofimmunologicallyimportantcells, stellatecells(specializedfatcells),andKupffercells(macrophages)thatprovideafurther
Life 2023, 13,1056 19of35
layerofimmunologicalprotection[252].TheVATprioritizationhypothesisproposesthat theseevolutionaryadaptivechangesaredevelopmentallyprogrammedinasimilarway totheproposeddevelopmentalprogrammingofPCOS.Readersarereferredtoarticlesby West-Eberhard[236]andParker[253]fordetaileddiscussion.
Insummary,adiposetissueandobesityhaveasignificantbidirectionalroleinthe pathophysiologyofchronicsystemicinflammationandinsulinresistanceinwomenwith PCOS.Theanatomicalandfunctionalredistributionofadiposetissueinwomenwith PCOSmayhaveadaptivesurvivalbenefitsinanancestralenvironment(improvedenergy storagecapacityandgreaterprotectionfrominfection)thatbecomemaladaptiveinresponse tocontemporarylifestyleandenvironmentalexposures(diet,inactivity,andstress).In addition,adiposetissueiscloselylinkedtothereproductivesystemandclearlyplaysa significantroleinthepathophysiologyofPCOS.
6.CentralRoleoftheMicrobiomeinthePathogenesisofPCOS
Themicrobiotaisthesumofmicrobialorganisms(bacteria,viruses,archaea,and fungi)thatinhabittheinterfacebetweentheexternalenvironmentorhabitatandtheinternalenvironmentofthehumanbody[254].Thisinterfacemainlyoccursatmucosal surfaces(nasal,oral,respiratory,gastrointestinal,andgenitourinary),eyes,andskin.The gastrointestinal(GI)microbiome(microbialorganismsandtheirgeneticmaterial)makes up80%ofthemicrobiotaandisnowconsideredtohaveacentralroleinhumanhealthand disease,includingPCOS[103,255,256].Thefunctionsofthemicrobiomeincludeinhibition ofpathogencolonization,regulationofthemucosalandsystemicimmunesystems,alterationofmetabolism,energybalance,hormonalaction,maintenanceoftheintegrityofthe GIbarrier,andbidirectionalsignallingwithmostorgansandtissuesthroughoutthebody (gut-brain,gut-bone,gut-immune,gut-liver,etc).TheGImicrobiome,therefore,formspart ofthewhole-bodyhomeostaticregulatoryframeworkthatmaintainshealthandisnow appreciatedtobepartofthehuman-microbemeta-organism[44].
Microbiomeeubiosis(abalancedmicrobialecosystem)canbedisruptedbyexternal factors(lifestyle,diet,environmentalchemicals,pathogenicmicrobes,andmedications),or endogenousfactors(hormones,circadian,exercise,andstress).Manyofthesefactorscan causedysbiosis(animbalanceofthecomposition,metabolism,ordistributionofthemicrobiomeassociatedwithanegativehealthoutcome)andhavebeeninvestigatedfortheir roleinawiderangeofhumandiseases[257].Theseincludeobesity,diabetes,gestational diabetes,metabolicsyndrome,cardiovasculardisease,inflammatoryboweldisease,multiplesclerosis,dementia,PCOS,andmanyothers[258,259].Lifestyle-relateddisturbanceto thebalancedmicrobialecosystemcontributestoavarietyofdiseases,dependingonthe levelofexposure,lengthofexposure,combinationofexposures,andindividualdisease susceptibility[257].Accumulatingevidencesuggeststhatdysbiosisplaysafundamental roleinthepathogenesisofPCOS[54,103].
ThedysbiosisofgutmicrobiotatheoryofPCOSproposesthatpoor-qualityWesternstylediets(high-glycaemic,highfat,highcalorie,highlyprocessed,lownutrient,low fibre),resultinanimbalancedmicrobiomewhichinducesincreasedGIpermeabilityand resultsinendotoxin-mediatedchronicinflammation[54].Dysbiosisresultsinbreakdown oftheGIbarrierfunction(lossofprotectivemucous,activationofthezonulinpathway, andbreakdownofintercellulartightjunctions),andreleaseoflipopolysaccharidefrom thecellwallsofgram-negativebacteriawhichcantraversethe“leakygut.”LipopolysaccharidebindswithlipopolysaccharidebindingproteinwhichtogetherbindwithToll-like receptor4onthesurfaceofsubmucosalmacrophages.ThisactivatestheNF-κBinflammatorypathway,resultinginreleaseofinflammatorycytokines.Continuingingestionofa poor-qualitydietresultsinchronicinflammation,insulinresistance,hyperandrogenism, ovulatorydysfunction,andtheclinicalfeaturesofPCOS[1,54].
Arecentreviewof31proof-of-conceptstudiesthatspecificallyinvestigatedthismechanism,concludedthatpreliminaryevidencesupportsthistheory[103].Moststudiesshowed reducedalpha-diversity(lowernumbersofbacterialtaxa)ofthemicrobiomeinwomen
Life 2023, 13,1056 20of35
withPCOScomparedtohealthyindividuals.Despitethisfinding,nospecificmicrobial signaturehasbeenfoundinwomenwithPCOS[255].Thisislikelyduetoimmenseindividualvariabilityinmicrobiomes,theexistenceoffunctionalredundancy(microbiotas containmanyspeciesandstrainsthatcanperformthesamefunction)[260],aswellasthe factthatPCOSisaquantitativetrait(interactionbetweenenvironmentandindividual). Individualvariationinthecompositionofthemicrobiomehaslikelycontributedtothe abilityofhumanstoadapttosomanyvariedenvironmentsandtypesofdiets,throughout evolutionaryhistory[254].
Manyotherdysbiosis-relatedpathophysiologicalmechanismshavebeenproposed andinvestigatedsincethedysbiosismodelwasproposedin2012.Theseincludealtered cholinepathways,changesinbileacidmetabolitesthataffectinflammation,glucoseand lipidmetabolism,processesinvolvedinenergyabsorption,short-chainfattyacids,effects ongastrointestinalhormones,andotherhostmetabolites(trimethylamineN-oxide,lactate, primarybileacids)[255,261].Manyfoodcomponentsarenotdigestedinthesmallintestine andtransittothecolonwheretheyarefermentedbythecolonicmicrobiota.Bacterial conversionofthesecompoundsresultsinawidevarietyofmetabolitesthatenterthe host,alterimmunity,metabolism,hormonesandreproduction,andcaninfluencetherisk ofdisease[77].Fermentationofundigestedcarbohydrates(solublefibre),proteinsand plant-derivedphytochemicals(polyphenols)resultsinarangeofbioactivemetabolites, suchasshort-chainfattyacids,amines,secondarymetabolitesofpolyphenols,andgases (hydrogen,hydrogensulphide,andmethane).Itislikelythatfutureresearchwillidentifya rangeofpathophysiologicalmechanismsrelatedtotheroleofdysbiosisinthepathogenesis ofPCOS.
Insummary,aswiththerestofthebiologicalcomponentsinvolvedinPCOS,themicrobiomeappearstohaveasignificantbidirectionalroleinthepathophysiologyofchronic systemicinflammation,insulinresistance,hyperandrogenism,andovulatorydysfunction. Dysbiosisisanimportantpartoftheevolutionarymodelandrepresentsamaladaptive responseofthemicrobiometocontemporarylifestyleandtheenvironment.Dysbiosisisa modifiablechangethatcanbereversedwithlifestyle,diet,prebiotics,andprobiotics[103].
7.EnvironmentalandEndocrineDisruptingChemicalsinPCOS
Endocrine-disruptingchemicalsareaglobalproblemforhumanhealthandtheenvironment[262].Thereisnodoubtthatanthropomorphicchemicalsinterferewithhuman physiologyandhaveadversehealtheffects[263–266].Humanexposuremainlyoccurs throughmucosalsurfaces(oral,gastrointestinal,respiratory,andgenitourinary)orvia dermalabsorption.EDCexposureisubiquitousandnumerousinternationalorganizations haveissuedwarningstodoctorsandpatients,regardingthepossibledangerstohuman healthandpregnantwomen.TheseincludeTheRoyalCollegeofObstetriciansandGynaecologists[264],theInternationalFederationofGynecologyandObstetrics[265],andthe EndocrineSociety[266].Theyhaverecommendedthatallpregnantwomenbeadvisedof thepossiblerisksofEDC,andthateducationprogrammesbedevelopedtoinformhealth professionalsoftherisks.
Endocrinedisruptingchemicalshaveasignificantroleinchronicsystemicinflammation[267],insulinresistance[268],hyperandrogenism[269],microbiomedisruption[270] neuroendocrineimbalance[271],obesity[272],oxidativestress[273],AGE[274],andinflammasomes[275],allofwhichareinvolvedinthepathophysiologyofPCOS.Intervention trials,randomized-controlledtrials(RCT),andsystematicreviewsofRCTwillneverbe possibleorethicalinhumans.Hypothesesaboutmechanismsofcausationneedtobe basedonobservationalstudiesinhumans,humanbio-monitoringstudies,intervention studiesinanimals,anddisastersfrominadvertenthumanexposure[262].Largeamounts ofdataarealreadyavailablethatprovide“evidence”ofthepotentialharmfuleffectsof EDCinhumans[267–276].Theimplementationofthe“PrecautionaryPrinciple”[277]is supportedbyalonglistofpreviousknowndisastersthathaveoccurredduetodelayed
Life 2023, 13,1056 21of35
actionornegligentinaction(diethylstilboestrol,thalidomide,nicotine,dioxin,asbestos,and mesh)[278].
ManyEDChasbeeninvestigatedandimplicatedinthepathogenesisandpathophysiologyofPCOS.Theseincludeheavymetals,persistentorganicpollutants,polychlorinated biphenyls,organochloridepesticides,airpollutants,pesticides,herbicides,nanomaterials, andplastics[271,279–283].EDCcanactindividually,buthumansareusuallyexposed tomixturesofchemicalsonaregularbasis[284].EDChasbeenidentifiedinmostbody fluidsinwomenwithPCOS[285,286].SixclassesofEDChavebeenshowntocross theplacenta[285,287],andEDCmaybeinvolvedintransgenerationaltransmissionof PCOS[103,288].TheanthropometricproductionofEDCisintimatelyconnectedtoclimate changeandrelatedadversehealthoutcomes,andclimatechangecanexacerbatetheeffects ofEDC[284].Improvedregulatoryprocesses,bio-detectionmethods,climatechangemitigationstrategies,anddietaryinterventions,canresultinreducedexposuretoEDCand otherenvironmentalchemicals[289].
WomenwithageneticsusceptibilitytoPCOSmaybeatincreasedriskofadverseeffects ofEDCduetohavingaheightenedproinflammatorydesign(Section 3.1)andincreased metabolicsensitivity(Section 4.2).Inaddition,thein-uterodevelopmentaleffectsofEDC canbeinheritedbytransgenerationaltransmission[253,278].Everyeffortshouldbemade toinformwomenwithPCOSaboutthepotentialrisksofenvironmentalchemicalsand discusswaystoavoidorminimizeexposure.Adetaileddiscussionofthemechanismsof actionofEDC,animal,andepidemiologicalstudiesrelatedtoPCOSisbeyondthescopeof thisreview.Readersarereferredtopublishedreportsforfurtherinformation[280–282,290].
8.EvolutionaryModelofPCOSandtheHallmarksofHealth
Therehasbeenagradualparadigmshiftinconceptualizingthecausesofhealth anddiseaseoverthepast20years.Seminalpublicationsonthe“HallmarksofCancer” summarizethepropertiesofmalignantcellsandtheirinteractionwiththeirnon-malignant environment[291].The“HallmarksofAging”focusontheinteractionsofmolecular, cellular,andsystemicprocessesthatexplainthedeteriorationoforganismsovertime[292]. Thisisaparadigmshiftthatsignalsamoveawayfromconventional,anatomical,and physiological-basedconceptionsofdisease(individualcells,tissues,organs,andsystems) toamore“organizational”structurefocusedonfactorsthatarecausativelyinvolvedin maintaininghomeostasisandequilibrium.
The“HallmarksofHealth”hasendeavouredtodefinehealthasa“compendium oforganizationalanddynamicfeaturesthatmaintainphysiology[44].”Thiscontrasts withtheusualdefinitionofhealthasthe“absenceofdisease.”Thecurrentconception ofPCOSasapolygenicdisorder(collectionofnormalalleles)thatisprogrammedinuteroandthenmanifestsinadolescencefollowingexposuretolifestyle,nutritional,and environmentalinfluences,seemstobeanexcellentexampleofthisnewparadigm[1]. Manyoftheinter-relatedcomponentsofthehallmarksofhealtharedysregulatedin womenwithPCOS.Therearedisturbancesatthemolecular(ROS,AGE,andmetabolites), organelle(mitochondriaandendoplasmicreticulum),cellular(immune,endocrine,and neural),supracellular(gastrointestinalmucosaandmucosalimmunity),organ(ovaryand pancreas),systemic(endocrine,reproductive,andimmune),andmeta-organismlevels (host–microbiotainteraction)(Table 1).Theprevioussectionsofthismanuscripthave attemptedtodescribesomeofthedetailedinteractionsthatdisrupttheorganizational dynamicsofthehallmarksofhealthinwomenwithPCOS.
TheEvolutionaryModelofPCOSisconsistentwiththehallmarksofhealth paradigm [1,5].Seenfromthisnewperspective,PCOSisaprogressivedisturbanceof theoverallorganisminvolvingmultiplelevelsthatusuallyoperatetomaintaininternal homeostasisandequilibriumwiththeenvironment.Multiplecontemporarylifestylefactors canderailtheoverallorganizationofthesystem,resultingincomplexpathophysiological interactionsandfeedbackmechanisms,thathavenoapparentbeginningorend.Allthe componentsareinter-relatedandinterdependent,inasystemwheremultiplelifestyle
Life 2023, 13,1056 22of35
andenvironmental“causative”factorsoperatesimultaneouslyandsynergistically.DefiningPCOSinapositiveway,withanevolutionaryandhallmarksofhealthperspective, opensnewopportunitiesforunderstandingthiscomplexcondition,improvingpatient communicationandcompliance,andinformingfutureresearch,prevention,andinterventionstrategies.
Table1. DysregulationoftheHallmarksofHealthinPolycysticOvarySyndrome.
HallmarkPolycysticOvarySyndrome
Barrierintegrity
Containmentoflocalperturbations
Recyclingandturnover
Integrationofcircuitries
Rhythmicoscillations
Homeostaticresilience
Hormeticregulation
Repairandregeneration
Gastrointestinalpermeability
Respiratorymucosa
Mucosalandadaptiveimmunity ROS,AGE,DAMPS,PAMPS
Autophagy,apoptosis,pyroptosis GIstemcellturnover
Metabolic,reproductive neurological,circadian
Menstrualcycle,circadian GIperistalsis
Endocrine,ANS
metabolic,immune,microbiome
oxidativestress,inactivity
xenohormesis #
Endoplasmicreticularstress mitochondrialstress
# xenohormesis=dietaryphytochemical-induced;ANS=autonomicnervoussystem;DAMPS=danger-associated molecularpatterns;ROS=reactiveoxygenspecies;PAMPS=pathogen-associatedmolecularpatterns;GI=Gastrointestinal;AGE=advancedglycationend-products.
9.Conclusions
Polycysticovarysyndromeisacommonconditionthataffectswomenatallstages ofthelifecycle.Thepathogenesisisrelatedtoacombinationofnutritionalandenvironmentalexposuresinageneticallysusceptibleindividual.Polycysticovarysyndromecan becharacterizedasanevolutionarymismatchdisorderresultingfromadisturbanceto thehallmarksofhealth.Chronicsystemicinflammationandinsulinresistancearecore mechanismsthatoperateatanorganismallevelinthephysiologyofsurvivalandplay acentralroleinthepathophysiologyofPCOS.Polycysticovarysyndromeisusuallydiagnosedinadolescencebythecombinationofoligomenorrhoeaandhyperandrogenism andisaprogressiveconditionthatisassociatedwithsignificantmetabolic,hormonal, reproductive,andpsychologicalproblems.Thesymptomsandchronicsequelaecanbe controlledandpreventedbylifestyleinterventions,andthediagnosisofPCOSprovidesthe idealopportunityforthepreventionofchronicdisease.Thisnarrativereviewhasoutlined somecomponentsofthecomplexwebofbiologicalandpathophysiologicalchangesthat contributetothepathogenesisofPCOS.
Funding: Thisresearchreceivednoexternalfunding.
InstitutionalReviewBoardStatement: Notapplicable.
InformedConsentStatement: Notapplicable.
Acknowledgments: IwouldliketothankGeoffreyReid,HelenRienits,EugeneCheah,andClaire O’Brienfortheirhelpfulcommentsandsuggestionsinreviewingthismanuscript.Iwouldalsolike tothankHannahToogoodforherassistancewiththegraphics.
ConflictsofInterest: Noconflictsofinteresttodeclare.
Life 2023, 13,1056 23of35
Abbreviations
ACTHAdrenocorticotropicHormone
AGEAdvancedGlycationEnd-Products
ANSAutonomicNervousSystem
ATAdiposeTissue
BATBrownAdiposeTissue
CDRCellDangerResponse
DAMPSDangerAssociatedMolecularPatterns
DNADeoxyriboseNucleicAcid
EDCEndocrineDisruptingChemical
GDMGestationalDiabetes
GIGastrointestinal
GLUTGlucoseTransporter
HPAHypothalamic-Pituitary-Adrenal
IRInsulinResistance
LHLuteinizingHormone
LPSLipopolysaccharide
PAMPSPathogenAssociatedMolecularPatterns
PCOSPolycysticOvarySyndrome
PNSPeripheralNervousSystem
PRRPatternRecognitionReceptors
RAGEReceptorofAdvancedGlycationEnd-Products
RNARiboseNucleicAcid
RNSReactiveNitrogenSpecies
ROSReactiveOxygenSpecies
SATSubcutaneousAdiposeTissue
SNSSympatheticNervousSystem
TLRToll-likeReceptor
TNFTumourNecrosisFactor
T2DMType2DiabetesMellitis
WATWhiteAdiposeTissue
VATVisceralAdiposeTissue
References
1. Parker,J.;O’brien,C.;Hawrelak,J.;Gersh,F.L.PolycysticOvarySyndrome:AnEvolutionaryAdaptationtoLifestyleandthe Environment. Int.J.Environ.Res.PublicHealth 2022, 19,1336.[CrossRef][PubMed]
2. Teede,H.J.;Misso,M.L.;Costello,M.F.;Dokras,A.;Laven,J.;Moran,L.;Piltonen,T.;Norman,R.J.;Andersen,M.;Azziz,R.;etal. Recommendationsfromtheinternationalevidence-basedguidelinefortheassessmentandmanagementofpolycysticovary syndrome. Fertil.Steril. 2018, 110,364–379.[CrossRef]
3. Cowan,S.;Lim,S.;Alycia,C.;Pirotta,S.;Thomson,R.;Gibson-Helm,M.;Blackmore,R.;Naderpoor,N.;Bennett,C.;Ee,C.;etal. Lifestylemanagementinpolycysticovarysyndrome—Beyonddietandphysicalactivity. BMCEndocr.Disord. 2023, 23,14. [CrossRef][PubMed]
4. Pei,Z.;Lu,W.;Feng,Y.;Xu,C.;Hsueh,A.J.W.OutofstepsocietalandDarwinianadaptationduringevolutionisthecauseof multiplewomen’shealthissues. Hum.Reprod. 2022, 37,1959–1969.[CrossRef][PubMed]
5. Dumesic,D.A.;Padmanabhan,V.;Chazenbalk,G.D.;Abbott,D.H.Polycysticovarysyndromeasaplausibleevolutionary outcomeofmetabolicadaptation. Reprod.Biol.Endocrinol. 2022, 20,12.[CrossRef][PubMed]
6. Pathak,G.;Nichter,M.PolycysticovarysyndromeinglobalizingIndia:Anecosocialperspectiveonanemerginglifestyledisease. Soc.Sci.Med. 2015, 146,21–28.[CrossRef]
7. Parker,J.;O’Brien,C.Evolutionaryandgeneticantecedentstothepathogenesisofpolycysticovarysyndrome(PCOS). J.ACNEM 2021, 40,12–20.
8. Aboeldalyl,S.;James,C.;Seyam,E.;Ibrahim,E.M.;Shawki,H.E.D.;Amer,S.Theroleofchronicinflammationinpolycystic ovariansyndrome—Asystematicreviewandmeta-analysis. Int.J.Mol.Sci. 2021, 22,2734.[CrossRef][PubMed]
9. Giampaolino,P.;Foreste,V.;DiFilippo,C.;Gallo,A.;Mercorio,A.;Serafino,P.;Improda,F.P.;Verrazzo,P.;Zara,G.;Buonfantino,C.;etal.MicrobiomeandPCOS:State-of-artandfutureaspects. Int.J.Mol.Sci. 2021, 22,2048.[CrossRef][PubMed]
10. Wang,J.;Wu,D.;Guo,H.;Li,M.Hyperandrogenemiaandinsulinresistance:Thechiefculpritofpolycysticovarysyndrome. Life Sci. 2019, 236,116940.[CrossRef][PubMed]
11. Palomba,S.;DeWilde,M.A.;Falbo,A.;Koster,M.P.H.;LaSala,G.B.;Fauser,B.C.J.M.Pregnancycomplicationsinwomenwith polycysticovarysyndrome. Hum.Reprod.Update 2015, 21,575–592.[CrossRef]
Life 2023, 13,1056 24of35
12. Brutocao,C.;Zaiem,F.;Alsawas,M.;Morrow,A.S.;Murad,M.H.;Javed,A.Psychiatricdisordersinwomenwithpolycysticovary syndrome:Asystematicreviewandmeta-analysis. Endocrine 2018, 62,318–325.[CrossRef][PubMed]
13. Zore,T.;Joshi,N.V.;Lizneva,D.;Azziz,R.PolycysticOvarianSyndrome:Long-TermHealthConsequences. Semin.Reprod.Med. 2017, 35,271–281.[CrossRef][PubMed]
14. Wu,J.;Yao,X.Y.;Shi,R.X.;Liu,S.F.;Wang,X.Y.Apotentiallinkbetweenpolycysticovarysyndromeandnon-alcoholicfattyliver disease:Anupdatemeta-analysis. Reprod.Health 2018, 15,77.[CrossRef]
15. Yumiceba,V.;Lopez-Cortes,A.;Perez-Villa,A.OncologyandPharmacogenomicsInsightsinPolycysticOvarySyndrome:An IntegrativeAnalysis. Front.Endocrinol. 2020, 11,5130.[CrossRef][PubMed]
16. Du,Y.;Li,F.;Li,S.;Ding,L.;Liu,M.Causalrelationshipbetweenpolycysticovarysyndromeandchronickidneydisease:A Mendelianrandomizationstudy. Front.Endocrinol. 2023, 14,119.[CrossRef]
17. Rodgers,R.J.;Avery,J.C.;Moore,V.M.;Davies,M.J.;Azziz,R.;Stener-Victorin,E.;Moran,L.J.;Robertson,S.A.;Stepto,N.K.; Norman,R.J.;etal.Complexdiseasesandco-morbidities:Polycysticovarysyndromeandtype2diabetesmellitus. Endocr. Connect. 2019, 8,R71–R75.[CrossRef]
18. Parker,J.NEM:ANewParadigmforUnderstandingtheCommonOriginsoftheChronicDiseaseEpidemic. ACNEMJ. 2018, 37,6–11.
19. Dunaif,A.Perspectivesinpolycysticovarysyndrome:Fromhairtoeternity. J.Clin.Endocrinol.Metab. 2016, 101,759–768. [CrossRef]
20. Day,F.;Karaderi,T.;Jones,M.R.;Meun,C.;He,C.;Drong,A.;Kraft,P.;Lin,N.;Huang,H.;Broer,L.;etal.Large-scale genome-widemeta-analysisofpolycysticovarysyndromesuggestssharedgeneticarchitecturefordifferentdiagnosiscriteria. PLoSGenet. 2018, 14,e1007813.[CrossRef]
21. Vink,J.M.;Sadrzadeh,S.;Lambalk,C.B.;Boomsma,D.I.HeritabilityofpolycysticovarysyndromeinaDutchtwin-familystudy. J.Clin.Endocrinol.Metab. 2006, 91,2100–2104.[CrossRef][PubMed]
22. Kahsar-Miller,M.D.;Nixon,C.;Boots,L.R.;Go,R.C.;Azziz,R.Prevalenceofpolycysticovarysyndrome(PCOS)infirst-degree relativesofpatientswithPCOS. Fertil.Steril. 2001, 75,53–58.[CrossRef][PubMed]
23. Sun,Q.;Gao,Y.;Yang,J.;Lu,J.;Feng,W.;Yang,W.MendelianRandomizationAnalysisIdentifiedPotentialGenesPleiotropically AssociatedwithPolycysticOvarySyndrome. Reprod.Sci. 2021, 29,1028–1037.[CrossRef]
24. Zhu,T.;Goodarzi,M.Causesandconsequencesofpolycysticovarysyndrome:InsightsfromMendelianRandomization. J.Clin. Endocrinol.Metab. 2021, 107,e899–e911.[CrossRef]
25. Lyle,S.M.;Ahmed,S.;Elliott,J.E.;Stener-Victorin,E.;Nachtigal,M.W.;Drögemöller,B.I.Transcriptome-wideassociationanalyses identifyanassociationbetweenARL14EPandpolycysticovarysyndrome. J.Hum.Genet. 2023 Onlineaheadofprint.[CrossRef]
26. Charifson,M.A.;Trumble,B.C.Evolutionaryoriginsofpolycysticovarysyndrome:Anenvironmentalmismatchdisorder. Evol. Med.PublicHealth 2019, 2019,50–63.[CrossRef]
27. Shaw,L.M.A.;Elton,S.Polycysticovarysyndrome:Atransgenerationalevolutionaryadaptation. BJOGAnInt.J.Obstet.Gynaecol. 2008, 115,144–148.[CrossRef]
28. Diamanti-Kandarakis,E.;Piperi,C.Geneticsofpolycysticovarysyndrome:Searchingforthewayoutofthelabyrinth. Hum. Reprod.Update 2005, 11,631–643.[CrossRef]
29. Khan,M.J.;Ullah,A.;Basit,S.Geneticbasisofpolycysticovarysyndrome(PCOS):Currentperspectives. Appl.Clin.Genet. 2019, 12,249–260.[CrossRef][PubMed]
30.Chodasewicz,K.Evolution,reproductionanddefinitionoflife. TheoryBiosci. 2014, 133,39–45.[CrossRef]
31. Corbett,S.;Morin-Papunen,L.ThePolycysticOvarySyndromeandrecenthumanevolution. Mol.Cell.Endocrinol. 2013, 373,39–50.[CrossRef][PubMed]
32. Benton,M.L.Theinfluenceofevolutionaryhistoryonhumanhealthanddisease. Nat.Rev.Genet. 2021, 22,269–283.[CrossRef] [PubMed]
33. Brady,S.P.;Bolnick,D.I.;Barrett,R.D.H.;Chapman,L.;Crispo,E.;Derry,A.M.;Eckert,C.G.;Fraser,D.J.;Fussmann,G.F.; Gonzalez,A.;etal.Understandingmaladaptationbyunitingecologicalandevolutionaryperspectives. Am.Nat. 2019, 194,495–515.[CrossRef][PubMed]
34. Parker,J.Glucosemetabolism,energyproductionandregulationofcellularandwhole-bodymetabolism. ACNEMJ. 2020, 39,29–33.
35. Chantranupong,L.;Wolfson,R.L.;Sabatini,D.M.Nutrient-sensingmechanismsacrossevolution. Cell 2015, 161,67–83.[CrossRef]
36. Holly,J.M.P.;Biernacka,K.;Perks,C.M.Systemicmetabolism,itsregulators,andcancer:Pastmistakesandfuturepotential. Front. Endocrinol. 2019, 10,65.[CrossRef]
37. Norman,R.;Teede,H.Anewevidence-basedguidelineforassessmentandmanagementofpolycysticovarysyndrome. Med.J. Aust. 2018, 209,299–300.[CrossRef]
38. Casarini,L.;Simoni,M.;Brigante,G.Ispolycysticovarysyndromeasexualconflict?Areview. Reprod.Biomed.Online 2016, 32,350–361.[CrossRef]
39. Parker,J.EmergingConceptsinthePathogenesisandTreatmentofPolycysticOvarySyndrome. Curr.Womens.HealthRev. 2016, 10,107–112.[CrossRef]
40. Azziz,R.;Dumesic,D.A.;Goodarzi,M.O.Polycysticovarysyndrome:Anancientdisorder? Fertil.Steril. 2011, 95,1544–1548. [CrossRef]
Life 2023, 13,1056 25of35
41. Gluckman,P.D.;Low,F.M.;Hanson,M.A.Anthropocene-relateddisease. Evol.Med.PublicHealth 2020, 2020,304–310.[CrossRef]
42. Rubio-Ruiz,M.E.;Peredo-Escárcega,A.E.;Cano-Martínez,A.;Guarner-Lans,V.AnEvolutionaryPerspectiveofNutritionand InflammationasMechanismsofCardiovascularDisease. Int.J.Evol.Biol. 2015, 2015,1–10.[CrossRef]
43. Watve,M.G.;Yajnik,C.S.Evolutionaryoriginsofinsulinresistance:Abehavioralswitchhypothesis. BMCEvol.Biol. 2007, 7,61. [CrossRef]
44.López-Otín,C.;Kroemer,G.HallmarksofHealth. Cell 2021, 184,33–63.[CrossRef][PubMed]
45.Ling,C.;Rönn,T.EpigeneticsinHumanObesityandType2Diabetes. CellMetab. 2019, 29,1028–1044.[CrossRef]
46. Szukiewicz,D.;Trojanowski,S.;Kociszewska,A.;Szewczyk,G.ModulationoftheInflammatoryResponseinPolycysticOvary Syndrome(PCOS)—SearchingforEpigeneticFactors. Int.J.Mol.Sci. 2022, 23,14663.[CrossRef][PubMed]
47. Shorakae,S.;Ranasinha,S.;Abell,S.;Lambert,G.;Lambert,E.;deCourten,B.;Teede,H.Inter-relatedeffectsofinsulinresistance, hyperandrogenism,sympatheticdysfunctionandchronicinflammationinPCOS. Clin.Endocrinol. 2018, 89,628–633.[CrossRef] [PubMed]
48. Barlampa,D.;Bompoula,M.S.;Bargiota,A.;Kalantaridou,S.;Mastorakos,G.;Valsamakis,G.Hypothalamicinflammationasa potentialpathophysiologicbasisfortheheterogeneityofclinical,hormonal,andmetabolicpresentationinpcos. Nutrients 2021, 13,520.[CrossRef][PubMed]
49.Okin,D.;Medzhitov,R.Evolutionofinflammatorydiseases. Curr.Biol. 2012, 22,R733–R740.[CrossRef][PubMed]
50. Furman,D.;Campisi,J.;Verdin,E.;Carrera-Bastos,P.;Targ,S.;Franceschi,C.;Ferrucci,L.;Gilroy,D.W.;Fasano,A.; Miller,G.W.;etal.Chronicinflammationintheetiologyofdiseaseacrossthelifespan. Nat.Med. 2019, 25,1822–1832.[CrossRef] [PubMed]
51. Naviaux,R.K.Metabolicfeaturesandregulationofthehealingcycle—Anewmodelforchronicdiseasepathogenesisand treatment. Mitochondrion 2019, 46,278–297.[CrossRef][PubMed]
52. Yu,S.Y.;Li,X.L.Pyroptosisandinflammasomesinobstetricalandgynecologicaldiseases. Gynecol.Endocrinol. 2021, 37,385–391. [CrossRef]
53. Katayama,P.L.;Leirão,I.P.;Kanashiro,A.;Luiz,J.P.M.;Cunha,F.Q.;Navegantes,L.C.C.;Menani,J.V.;Zoccal,D.B.; Colombari,D.S.A.;Colombari,E.Thecarotidbodydetectscirculatingtumornecrosisfactor-alphatoactivateasympathetic anti-inflammatoryreflex. BrainBehav.Immun. 2022, 102,370–386.[CrossRef][PubMed]
54. Tremellen,K.;Pearce,K.DysbiosisofGutMicrobiota(DOGMA)—Anoveltheoryforthedevelopmentofpolycysticovarian syndrome. Med.Hypotheses 2012, 79,104–112.[CrossRef][PubMed]
55. Xiong,P.;Zhang,F.;Liu,F.;Zhao,J.;Huang,X.Metaflammationinglucolipidmetabolicdisorders:Pathogenesisandtreatment. Biomed.Pharmacother. 2023, 161,114545.[CrossRef]
56. Netea,M.G.;Balkwill,F.;Chonchol,M.;Cominelli,F.;Donath,M.Y.;Giamarellos-Bourboulis,E.J.;Golenbock,D.;Gresnigt,M.S.; Heneka,M.T.;Hoffman,H.M.;etal.Aguidingmapforinflammation. Nat.Immunol. 2017, 18,826–831.[CrossRef][PubMed]
57.Zhang,H.;Zhu,J.;Gong,Z.;Zhu,J.K.Abioticstressresponsesinplants. Nat.Rev.Genet. 2022, 23,104–119.[CrossRef]
58. Khan,R.N.;Hay,D.P.Aclearandpresentdanger:InflammasomesDAMPingdowndisordersofpregnancy. Hum.Reprod.Update 2015, 21,388–405.[CrossRef]
59. Amarante-Mendes,G.P.;Adjemian,S.;Branco,L.M.;Zanetti,L.C.;Weinlich,R.;Bortoluci,K.R.Patternrecognitionreceptorsand thehostcelldeathmolecularmachinery. Front.Immunol. 2018, 9,2379.[CrossRef]
60. Cavalier-Smith,T.CellevolutionandEarthhistory:Stasisandrevolution. Philos.Trans.R.Soc.BBiol.Sci. 2006, 361,969–1006. [CrossRef]
61. Tang,K.H.;Blankenship,R.E.BothforwardandreverseTCAcyclesoperateingreensulfurbacteria. J.Biol.Chem. 2010, 285,35848–35854.[CrossRef]
62.Naviaux,R.K.Oxidativeshieldingoroxidativestress? J.Pharmacol.Exp.Ther. 2012, 342,608–618.[CrossRef]
63. Galluzzi,L.;Vitale,I.;Aaronson,S.A.;Abrams,J.M.;Adam,D.;Agostinis,P.;Alnemri,E.S.;Altucci,L.;Amelio,I.; Andrews,D.W.;etal.Molecularmechanismsofcelldeath:RecommendationsoftheNomenclatureCommitteeonCellDeath 2018. CellDeathDiffer. 2018, 25,486–541.[CrossRef]
64. Dabravolski,S.A.;Nikiforov,N.G.;Eid,A.H.;Nedosugova,L.V.;Starodubova,A.V.;Popkova,T.V.;Bezsonov,E.E.;Orekhov,A.N. Mitochondrialdysfunctionandchronicinflammationinpolycysticovarysyndrome. Int.J.Mol.Sci. 2021, 22,3923.[CrossRef] [PubMed]
65. Zhao,H.;Zhao,Y.;Li,T.;Li,M.;Li,J.;Li,R.;Liu,P.;Yu,Y.;Qiao,J.Metabolismalterationinfollicularniche:Thenexusamong intermediarymetabolism,mitochondrialfunction,andclassicpolycysticovarysyndrome. FreeRadic.Biol.Med. 2015, 86,295–307. [CrossRef][PubMed]
66. Mohammadi,M.OxidativeStressandPolycysticOvarySyndrome:ABriefReview. Int.J.Prev.Med. 2017, 8,86.[CrossRef] [PubMed]
67. Zuo,T.;Zhu,M.;Xu,W.Rolesofoxidativestressinpolycysticovarysyndromeandcancers. Oxid.Med.Cell.Longev. 2016, 2016,8589318.[CrossRef][PubMed]
68. Sharifi-Rad,M.;AnilKumar,N.V.;Zucca,P.;Varoni,E.M.;Dini,L.;Panzarini,E.;Rajkovic,J.;TsouhFokou,P.V.;Azzini,E.; Peluso,I.;etal.Lifestyle,OxidativeStress,andAntioxidants:BackandForthinthePathophysiologyofChronicDiseases. Front. Physiol. 2020, 11,694.[CrossRef]
Life 2023, 13,1056 26of35
69. Herman,R.;Jensterle,M.;Janež,A.;Goriˇcar,K.;Dolžan,V.Geneticvariabilityinantioxidativeandinflammatorypathways modifiestheriskforPCOsandinfluencesmetabolicprofileofthesyndrome. Metabolites 2020, 10,439.[CrossRef]
70. Diamanti-Kandarakis,E.;Dunaif,A.Insulinresistanceandthepolycysticovarysyndromerevisited:Anupdateonmechanisms andimplications. Endocr.Rev. 2012, 33,981–1030.[CrossRef][PubMed]
71. Rashidi,B.;Haghollahi,F.;Shariat,M.;Zayerii,F.TheEffectsofCalcium-VitaminDandMetforminonPolycysticOvary Syndrome:APilotStudy. Taiwan.J.Obstet.Gynecol. 2009, 48,142–147.[CrossRef][PubMed]
72. Kaya,C.;Erkan,A.F.;Cengiz,S.D.;Dünder,I.;Demirel,Ö.E.;Bilgihan,A.Advancedoxidationproteinproductsareincreasedin womenwithpolycysticovarysyndrome:Relationshipwithtraditionalandnontraditionalcardiovascularriskfactorsinpatients withpolycysticovarysyndrome. Fertil.Steril. 2009, 92,1372–1377.[CrossRef][PubMed]
73. Enechukwu,C.I.;Onuegbu,A.J.;Olisekodiaka,M.J.;Eleje,G.U.;Ikechebelu,J.I.;Ugboaja,J.O.;Amah,U.K.;Okwara,J.E.; Igwegbe,A.O.OxidativestressmarkersandlipidprofilesofpatientswithpolycysticovarysyndromeinaNigeriantertiary hospital. Obstet.Gynecol.Sci. 2019, 62,335–343.[CrossRef][PubMed]
74. Dincer,Y.;Akcay,T.;Erdem,T.;IlkerSaygili,E.;Gundogdu,S.DNAdamage,DNAsusceptibilitytooxidationandglutathione levelinwomenwithpolycysticovarysyndrome. Scand.J.Clin.Lab.Investig. 2005, 65,721–728.[CrossRef]
75. Noormohammadi,M.;Eslamian,G.;Malek,S.;Shoaibinobarian,N.;Mirmohammadali,S.N.Theassociationbetweenfertilitydiet scoreandpolycysticovarysyndrome:ACase-Controlstudy. HealthCareWomenInt. 2022, 43,70–84.[CrossRef]
76. Shoaibinobarian,N.;Eslamian,G.;Noormohammadi,M.;Malek,S.;Rouhani,S.;Mirmohammadali,S.N.DietaryTotalAntioxidantCapacityandRiskofPolycysticOvarySyndrome:ACase-ControlStudy. Int.J.Fertil.Steril. 2022, 16,200–205. [CrossRef]
77. Parker,J.;Hawrelak,J.;Gersh,F.NutritionalRoleofPolyphenolsasaComponentofaWholefoodDietintheManagementof PolycysticOvarySyndrome. Australas.Coll.Nutr.Environ.Med.J. 2021, 40,6–12.Availableonline: https://search.ebscohost. com/login.aspx?direct=true&db=rzh&AN=154032844&lang=ja&site=ehost-live (accessedon21June2021).
78. Piperi,C.;Adamopoulos,C.;Dalagiorgou,G.;Diamanti-Kandarakis,E.;Papavassiliou,A.G.Crosstalkbetweenadvanced glycationandendoplasmicreticulumstress:Emergingtherapeutictargetingformetabolicdiseases. J.Clin.Endocrinol.Metab. 2012, 97,2231–2242.[CrossRef]
79. Inagi,R.Inhibitorsofadvancedglycationandendoplasmicreticulumstress.In MethodsinEnzymology,1sted.;ElsevierInc.: Amsterdam,TheNetherlands,2011;Volume491,pp.361–380.[CrossRef]
80. Goldberg,T.;Cai,W.;Peppa,M.;Dardaine,V.;Baliga,B.S.;Uribarri,J.;Vlassara,H.Advancedglycoxidationendproductsin commonlyconsumedfoods. J.Am.Diet.Assoc. 2004, 104,1287–1291.[CrossRef]
81. Garg,D.;Merhi,Z.Advancedglycationendproducts:LinkbetweendietandovulatorydysfunctioninPCOS? Nutrients 2015, 7,10129–10144.[CrossRef][PubMed]
82. Basta,G.Receptorforadvancedglycationendproductsandatherosclerosis:Frombasicmechanismstoclinicalimplications. Atherosclerosis 2008, 196,9–21.[CrossRef][PubMed]
83.Kathryn,C.;Tan,B.;Sammy,W.;Shiu,M.;Wong,Y.XystusTamSerumadvancedglycationendproducts(AGEs)areassociated withinsulinresistance. Diabetes.Metab.Res.Rev. 2011, 27,1488–1492.[CrossRef]
84. Palimeri,S.;Palioura,E.;Diamanti-Kandarakis,E.Currentperspectivesonthehealthrisksassociatedwiththeconsumptionof advancedglycationendproducts:Recommendationsfordietarymanagement. DiabetesMetab.Syndr.Obes.TargetsTher. 2015, 8,415–426.[CrossRef]
85. Diamanti-Kandarakis,E.;Piperi,C.;Patsouris,E.;Korkolopoulou,P.;Panidis,D.;Pawelczyk,L.;Papavassiliou,A.G.;Duleba,A.J. Immunohistochemicallocalizationofadvancedglycationend-products(AGEs)andtheirreceptor(RAGE)inpolycysticand normalovaries. Histochem.CellBiol. 2007, 127,581–589.[CrossRef][PubMed]
86. Azhary,J.M.K.;Harada,M.;Kunitomi,C.;Kusamoto,A.;Takahashi,N.;Nose,E.;Oi,N.;Wada-Hiraike,O.;Urata,Y.; Hirata,T.;etal.AndrogensIncreaseAccumulationofAdvancedGlycationEndProductsinGranulosaCellsbyActivatingER StressinPCOS. Endocrinology 2020, 161,1–13.[CrossRef]
87. Tatone,C.;DiEmidio,G.;Placidi,M.;Rossi,G.;Ruggieri,S.;Taccaliti,C.;D’Alfonso,A.;Amicarelli,F.;Guido,M.AGEs-related dysfunctionsinPCOS:Evidencefromanimalandclinicalresearch. J.Endocrinol. 2021, 251,R1–R9.[CrossRef]
88. Garg,D.;Merhi,Z.RelationshipbetweenAdvancedGlycationEndProductsandSteroidogenesisinPCOS. Reprod.Biol.Endocrinol. 2016, 14,1–13.[CrossRef]
89. VanDerLugt,T.;Weseler,A.R.;Gebbink,W.A.;Vrolijk,M.F.;Opperhuizen,A.;Bast,A.Dietaryadvancedglycationendproducts induceaninflammatoryresponseinhumanmacrophagesinvitro. Nutrients 2018, 10,1868.[CrossRef]
90. Uribarri,J.;delCastillo,M.D.;delaMaza,M.P.;Filip,R.;Gugliucci,A.;Luevano-Contreras,C.;Macías-Cervantes,M.H.; MarkowiczBastos,D.H.;Medrano,A.;Menini,T.;etal.Dietaryadvancedglycationendproductsandtheirroleinhealthand disease. Adv.Nutr. 2015, 6,461–473.[CrossRef]
91. Tantalaki,E.;Piperi,C.;Livadas,S.;Kollias,A.;Adamopoulos,C.;Koulouri,A.;Christakou,C.;Diamanti-Kandarakis,E.Impact ofdietarymodificationofadvancedglycationendproducts(AGEs)onthehormonalandmetabolicprofileofwomenwith polycysticovarysyndrome(PCOS). Hormones 2014, 13,65–73.[CrossRef]
92. Vlassara,H.;Striker,G.E.AGErestrictionindiabetesmellitus:Aparadigmshift. Nat.Rev.Endocrinol. 2011, 7,526–539.[CrossRef] [PubMed]
Life 2023, 13,1056 27of35
93. Janeway,C.A.Approachingtheasymptote?Evolutionandrevolutioninimmunology. ColdSpringHarb.Symp.Quant.Biol. 1989, 54,1–13.[CrossRef][PubMed]
94.Matzinger,P.Tolerance,danger,andtheextendedfamily. Annu.Rev.Immunol. 1994, 12,991–1045.[CrossRef][PubMed]
95.Naviaux,R.K.Metabolicfeaturesofthecelldangerresponse. Mitochondrion 2014, 16,7–17.[CrossRef][PubMed]
96. Pakos-Zebrucka,K.;Koryga,I.;Mnich,K.;Ljujic,M.;Samali,A.;Gorman,A.M.Theintegratedstressresponse. EMBORep. 2016, 17,1374–1395.[CrossRef]
97. Costa-Mattioli,M.;Walter,P.Theintegratedstressresponse:Frommechanismtodisease. Science 2020, 368,eaat5314.[CrossRef]
98.Li,D.;Wu,M.Patternrecognitionreceptorsinhealthanddiseases. SignalTransduct.Target.Ther. 2021, 6,291.[CrossRef]
99. Moretti,J.;Blander,J.M.Insightsintophagocytosis-coupledactivationofpatternrecognitionreceptorsandinflammasomes. Curr. Opin.Immunol. 2014, 26,100–110.[CrossRef]
100. Zhou,F.;Li,C.;Zhang,S.Y.NLRP3inflammasome:Anewtherapeutictargetforhigh-riskreproductivedisorders? Chin.Med.J. 2020, 134,20–27.[CrossRef]
101.Moretti,J.;Blander,J.M.Cell-autonomousstressresponsesininnateimmunity. J.Leukoc.Biol. 2017, 101,77–86.[CrossRef]
102. Cani,P.D.;Amar,J.;Iglesias,M.A.;Poggi,M.;Knauf,C.;Bastelica,D.;Neyrinck,A.M.;Fava,F.;Tuohy,K.M.;Chabo,C.;etal. Metabolicendotoxemiainitiatesobesityandinsulinresistance. Diabetes 2007, 56,1761–1772.[CrossRef]
103. Parker,J.;O’Brien,C.;Hawrelak,J.Anarrativereviewoftheroleofgastrointestinaldysbiosisinthepathogenesisofpolycystic ovarysyndrome. Obstet.Gynecol.Sci. 2021, 65,14–28.[CrossRef]
104. Murthi,P.;Pinar,A.A.;Dimitriadis,E.;Samuel,C.S.Inflammasomes—Amolecularlinkforalteredimmunoregulationand inflammationmediatedvasculardysfunctioninpreeclampsia. Int.J.Mol.Sci. 2020, 21,1406.[CrossRef]
105. Rostamtabar,M.;Esmaeilzadeh,M.;Tourani,M.;Rahmani,A.;Baee,M.;Shirafkan,F.;Saleki,K.;Mirzababayi,S.;Ebrahimpour,S.; Nouri,H.Pathophysiologicalrolesofchroniclow-gradeinflammationinpolycysticovarysyndrome. J.Cell.Physiol. 2020, 236,824–838.[CrossRef]
106. Eltzschig,H.K.;Sitkovsky,M.V.;Robson,S.PurinergicSignalingduringInflammation. N.Engl.J.Med. 2013, 368,1260.[CrossRef] [PubMed]
107. Rostamtabar,M.;Esmaeilzadeh,S.;Karkhah,A.;Amiri,M.;Rahmani,A.;Bakouei,F.;Nouri,H.R.ElevatedexpressionofIL-18 butnotIL-1β geneisassociatedwithNALP3andAIM2inflammasomeinPolycysticOvarySyndrome. Gene 2020, 731,144352. [CrossRef]
108. Guo,Q.J.;Shan,J.;Xu,Y.F.;Hu,Y.Y.;Huo,C.L.;Song,J.Y.;Wang,C.Q.;Zhou,H.;Yu,C.Q.;Huang,Q.PioglitazoneMetformin ComplexImprovesPolycysticOvarySyndromeComorbidPsychologicalDistressviaInhibitingNLRP3InflammasomeActivation: AProspectiveClinicalStudy. Mediat.Inflamm. 2020, 2020,1–7.[CrossRef][PubMed]
109. Hu,C.;Pang,B.;Ma,Z.;Yi,H.Immunophenotypicprofilesinpolycysticovarysyndrome. Mediat.Inflamm. 2020, 2020,5894768. [CrossRef][PubMed]
110. Mahajan,S.;Vita,R.;Shackelford,D.;Lane,J.;Schulten,V.;Zarebski,L.;Jespersen,M.C.;Marcatili,P.;Nielsen,M.;Sette,A.;etal. EpitopespecificantibodiesandTcellreceptorsintheimmuneepitopedatabase. Front.Immunol. 2018, 9,2688.[CrossRef] [PubMed]
111.Rees,A.R.Understandingthehumanantibodyrepertoire. MAbs 2020, 12,1729683.[CrossRef]
112. AlbertoKöllikerFrers,R.;Otero-Losada,M.;InésHerrera,M.;Porta,S.;Cosentino,V.;Kerzberg,E.;Udovin,L.;Capani,F. Immune-MediatedInflammation:HumanTCD4HelperLymphocyteDiversityandPlasticityinHealthandDisease.In Cells oftheImmuneSystem,1sted.;OtaFuchs,Q.,Athari,S.S.,Eds.;Intechopen:London,UK,2020;pp.1–14.Availableonline: https://www.intechopen.com/chapters/69166 (accessedon17February2023).
113. Sbierski-Kind,J.;Goldeck,D.;Buchmann,N.;Spranger,J.;Volk,H.D.;Steinhagen-Thiessen,E.;Pawelec,G.;Demuth,I.;Spira,D. Tcellphenotypesassociatedwithinsulinresistance:ResultsfromtheBerlinAgingStudyII. Immun.Ageing 2020, 17,1–11. [CrossRef]
114. Wu,R.;Fujii,S.;Ryan,N.K.;VanderHoek,K.H.;Jasper,M.J.;Sini,I.;Robertson,S.A.;Robker,R.L.;Norman,R.J.Ovarian leukocytedistributionandcytokine/chemokinemRNAexpressioninfollicularfluidcellsinwomenwithpolycysticovary syndrome. Hum.Reprod. 2007, 22,527–535.[CrossRef]
115. Krishna,M.B.;Joseph,A.;Subramaniam,A.G.;Gupta,A.;Pillai,S.M.;Laloraya,M.ReducedtregsinperipheralbloodofPCOS patients—AconsequenceofaberrantIl2signaling. J.Clin.Endocrinol.Metab. 2015, 100,282–292.[CrossRef]
116. Ma,M.;Wang,M.;Xu,F.;Hao,S.TheImbalanceinTh17andTregCellsinPolycysticOvarianSyndromePatientswith AutoimmuneThyroiditis. Immunol.Investig. 2022, 51,1170–1181.[CrossRef]
117. Luan,Y.-Y.;Zhang,L.;Peng,Y.-Q.;Li,Y.-Y.;Liu,R.-X.;Yin,C.-H.Immuneregulationinpolycysticovarysyndrome. Clin.Chim. Acta 2022, 531,265–272.[CrossRef]
118. Salvador,A.F.;deLima,K.A.;Kipnis,J.Neuromodulationbytheimmunesystem:Afocusoncytokines. Nat.Rev.Immunol. 2021, 21,526–541.[CrossRef][PubMed]
119. Itoh,H.;Ueda,M.;Suzuki,M.;Kohmura-Kobayashi,Y.DevelopmentalOriginsofMetaflammation;ABridgetotheFuture BetweentheDOHaDTheoryandEvolutionaryBiology. Front.Endocrinol. 2022, 13,9436.[CrossRef][PubMed]
120. Patel,S.Polycysticovarysyndrome(PCOS),aninflammatory,systemic,lifestyleendocrinopathy. J.SteroidBiochem.Mol.Biol. 2018, 182,27–36.[CrossRef][PubMed]
Life 2023, 13,1056 28of35
121. Pavlov,V.A.;Wang,H.;Czura,C.J.;Friedman,S.G.;Tracey,K.J.TheCholinergicAnti-inflammatoryPathway:AMissingLinkin Neuroimmunomodulation. Mol.Med. 2003, 9,125–134.[CrossRef]
122. Bellocchi,C.;Carandina,A.;Montinaro,B.;Targetti,E.;Furlan,L.;Rodrigues,G.D.;Tobaldini,E.;Montano,N.TheInterplay betweenAutonomicNervousSystemandInflammationacrossSystemicAutoimmuneDiseases. Int.J.Mol.Sci. 2022, 23,2449. [CrossRef]
123. Ingegnoli,F.;Buoli,M.;Antonucci,F.;Coletto,L.A.;Esposito,C.M.;Caporali,R.TheLinkBetweenAutonomicNervousSystem andRheumatoidArthritis:FromBenchtoBedside. Front.Med. 2020, 7,589079.[CrossRef]
124. delRey,A.;Besedovsky,H.O.;Sorkin,E.;daPrada,M.;Arrenbrecht,S.Immunoregulationmediatedbythesympatheticnervous system,II. Cell.Immunol. 1981, 63,329–334.[CrossRef]
125.Martelli,D.Theinflammatoryreflexreloaded. BrainBehav.Immun. 2022, 104,137–138.[CrossRef][PubMed]
126. Zangeneh,F.Z.;Bagheri,M.;Naghizadeh,M.M.HyponeurotrophinemiainSerumofWomenwithPolycysticOvarySyndromeas aLowGradeChronicInflammation. OpenJ.Obstet.Gynecol. 2015, 05,459–469.[CrossRef]
127.Tracey,K.J.Theinflammatoryreflex. Nature 2002, 420,853–858.[CrossRef][PubMed]
128. Wexler,B.C.;Dolgin,A.E.;Tryczynski,E.W.Effectsofabacterialpolysaccharide(piromen)onthepituitary-adrenalaxis:Adrenal ascorbicacid,cholesterolandhistologicalterations. Endocrinology 1957, 61,300–308.[CrossRef][PubMed]
129. Banks,W.A.;Kastin,A.J.;Broadwell,R.Passageofcytokinesacrosstheblood-brain-barrier. Neuroimmunomodulation 1995, 2,241–248.[CrossRef]
130. Buller,K.M.Roleofcircumventricularorgansinpro-inflammatorycytokine-inducedactivationofthehypothalamic-pituitaryadrenalaxis. Clin.Exp.Pharmacol.Physiol. 2001, 28,581–589.[CrossRef][PubMed]
131. Berthoud,H.R.;Neuhuber,W.L.Functionalandchemicalanatomyoftheafferentvagalsystem. Auton.Neurosci.BasicClin. 2000, 85,1–17.[CrossRef]
132. Dag,Z.O.;Alpua,M.;Turkel,Y.;Isik,Y.Autonomicdysfunctioninpatientswithpolycysticovarysyndrome. Taiwan.J.Obstet. Gynecol. 2015, 54,381–384.[CrossRef]
133. Webster,J.I.;Tonelli,L.;Sternberg,E.M.Neuroendocrineregulationofimmunity. Annu.Rev.Immunol. 2002, 20,125–163. [CrossRef][PubMed]
134. Kox,M.;vanEijk,L.T.;Zwaag,J.;vandenWildenberg,J.;Sweep,F.C.G.J.;vanderHoeven,J.G.;Pickkers,P.Voluntaryactivation ofthesympatheticnervoussystemandattenuationoftheinnateimmuneresponseinhumans. Proc.Natl.Acad.Sci.USA 2014, 111,7379–7384.[CrossRef][PubMed]
135. Sun,P.;Liu,D.G.;Ye,X.M.NicotinicAcetylcholineReceptor α7SubunitIsanEssentialRegulatorofSeizureSusceptibility. Front. Neurol. 2021, 12,6752.[CrossRef]
136. Shaikh,S.;Verma,H.;Yadav,N.;Jauhari,M.;Bullangowda,J.ApplicationsofSteroidinClinicalPractice:AReview. ISRN Anesthesiol. 2012, 2012,1–11.[CrossRef]
137. Lansdown,A.;Rees,D.A.Thesympatheticnervoussysteminpolycysticovarysyndrome:Anoveltherapeutictarget? Clin. Endocrinol. 2012, 77,791–801.[CrossRef]
138. Chen,M.J.;Yang,W.S.;Yang,J.H.;Chen,C.L.;Ho,H.N.;Yang,Y.S.Relationshipbetweenandrogenlevelsandbloodpressurein youngwomenwithpolycysticovarysyndrome. Hypertension 2007, 49,1442–1447.[CrossRef]
139. Perciaccante,A.;Fiorentini,A.;Valente,R.;Tubani,L.Polycysticovarysyndrome:Androgens,autonomicnervoussystem,and hypertension. Hypertension 2007, 50,91710.[CrossRef]
140. Giallauria,F.;Palomba,S.;Maresca,L.;Vuolo,L.;Tafuri,D.;Lombardi,G.;Colao,A.;Vigorito,C.;Orio,F.Exercisetraining improvesautonomicfunctionandinflammatorypatterninwomenwithpolycysticovarysyndrome(PCOS). Clin.Endocrinol. 2008, 69,792–798.[CrossRef]
141. Trigunaite,A.;Dimo,J.;Jørgensen,T.N.Suppressiveeffectsofandrogensontheimmunesystem. Cell.Immunol. 2015, 294,87–94. [CrossRef]
142. González,F.InflammationinPolycysticOvarySyndrome:Underpinningofinsulinresistanceandovariandysfunction. Steroids 2012, 77,300–305.[CrossRef]
143. Wagner,I.V.;Savchuk,I.;Sahlin,L.;Kulle,A.;Klöting,N.;Dietrich,A.;Holterhus,P.M.;Dötsch,J.;Blüher,M.;Söder,O.DeNovo andDepot-SpecificAndrogenProductioninHumanAdiposeTissue:ASourceofHyperandrogenisminWomenwithObesity. Obes.Facts 2022, 15,281–291.[CrossRef]
144. Velez,L.M.;Seldin,M.;Motta,A.B.Inflammationandreproductivefunctioninwomenwithpolycysticovarysyndrome. Biol. Reprod. 2021, 104,1205–1217.[CrossRef][PubMed]
145. Xiong,Y.L.;Liang,X.Y.;Yang,X.;Li,Y.;Wei,L.N.Low-gradechronicinflammationintheperipheralbloodandovariesofwomen withpolycysticovariansyndrome. Eur.J.Obstet.Gynecol.Reprod.Biol. 2011, 159,148–150.[CrossRef][PubMed]
146. Diamanti-Kandarakis,E.;Alexandraki,K.;Piperi,C.;Protogerou,A.;Katsikis,I.;Paterakis,T.;Lekakis,J.;Panidis,D.Inflammatory andendothelialmarkersinwomenwithpolycysticovarysyndrome. Eur.J.Clin.Investig. 2006, 36,691–697.[CrossRef]
147. Escobar-Morreale,H.F.;Luque-Ramírez,M.;González,F.Circulatinginflammatorymarkersinpolycysticovarysyndrome:A systematicreviewandmetaanalysis. Fertil.Steril. 2011, 95,1048–1058.e2.[CrossRef]
148. Azhary,J.M.K.;Harada,M.;Takahashi,N.;Nose,E.;Kunitomi,C.;Koike,H.;Hirata,T.;Hirota,Y.;Koga,K.;Wada-Hiraike,O.;etal. Endoplasmicreticulumstressactivatedbyandrogenenhancesapoptosisofgranulosacellsviainductionofdeathreceptor5in PCOS. Endocrinology 2019, 160,119–132.[CrossRef]
Life 2023, 13,1056 29of35
149. Koike,H.;Harada,M.;Kusamoto,A.;Xu,Z.;Tanaka,T.;Sakaguchi,N.;Kunitomi,C.;Azhary,J.M.K.;Takahashi,N.;Urata,Y.;etal. Rolesofendoplasmicreticulumstressinthepathophysiologyofpolycysticovarysyndrome. Front.Endocrinol. 2023, 4,1124405. [CrossRef][PubMed]
150. Li,Y.;Zheng,Q.;Sun,D.;Cui,X.;Chen,S.;Bulbul,A.;Liu,S.;Yan,Q.Dehydroepiandrosteronestimulatesinflammationand impairsovarianfunctionsofpolycysticovarysyndrome. J.Cell.Physiol. 2019, 234,7435–7447.[CrossRef]
151. Zhai,Y.;Pang,Y.Systemicandovarianinflammationinwomenwithpolycysticovarysyndrome. J.Reprod.Immunol. 2022, 151,103628.[CrossRef]
152. Rahman,M.S.;Hossain,K.S.;Das,S.;Kundu,S.;Adegoke,E.O.;Rahman,M.A.;Hannan,M.A.;Uddin,M.J.;Pang,M.G.Roleof insulininhealthanddisease:Anupdate. Int.J.Mol.Sci. 2021, 22,6403.[CrossRef]
153. Watanabe,M.;Hayasaki,H.;Tamayama,T.;Shimada,M.Histologicdistributionofinsulinandglucagonreceptors. Braz.J.Med. Biol.Res. 1998, 31,243–256.[CrossRef]
154. Khalid,M.;Alkaabi,J.;Khan,M.A.B.;Adem,A.Insulinsignaltransductionperturbationsininsulinresistance. Int.J.Mol.Sci. 2021, 22,8590.[CrossRef][PubMed]
155. Petersen,M.C.;Shulman,G.I.Mechanismsofinsulinactionandinsulinresistance. Physiol.Rev. 2018, 98,2133–2223.[CrossRef] [PubMed]
156. Zhou,M.S.;Wang,A.;Yu,H.Linkbetweeninsulinresistanceandhypertension:Whatistheevidencefromevolutionarybiology? Diabetol.Metab.Syndr. 2014, 6,12.[CrossRef][PubMed]
157. Sun,Q.;Li,J.;Gao,F.Newinsightsintoinsulin:Theanti-inflammatoryeffectanditsclinicalrelevance. WorldJ.Diabetes 2014, 5,89.[CrossRef][PubMed]
158. Chang,Y.W.;Hung,L.C.;Chen,Y.C.;Wang,W.H.;Lin,C.Y.;Tzeng,H.H.;Suen,J.L.;Chen,Y.H.InsulinReducesInflammationby RegulatingtheActivationoftheNLRP3Inflammasome. Front.Immunol. 2021, 11,7229.[CrossRef]
159. Fernández-Real,J.M.;Ricart,W.Insulinresistanceandinflammationinanevolutionaryperspective:Thecontributionofcytokine genotype/phenotypetothriftiness. Diabetologia 1999, 42,1367–1374.[CrossRef]
160.Wan,Y.Y.RegulatoryTcells:Immunesuppressionandbeyond. Cell.Mol.Immunol. 2010, 7,204–210.[CrossRef]
161. Jacobse,J.;Li,J.;Rings,E.H.H.M.;Samsom,J.N.;Goettel,J.A.IntestinalRegulatoryTCellsasSpecializedTissue-Restricted ImmuneCellsinIntestinalImmuneHomeostasisandDisease. Front.Immunol. 2021, 12,6499.[CrossRef]
162. Makhijani,P.;Basso,P.J.;Chan,Y.T.;Chen,N.;Baechle,J.;Khan,S.;Furman,D.;Tsai,S.;Winer,D.Regulationoftheimmune systembytheinsulinreceptorinhealthanddisease. Front.Endocrinol. 2023, 14,1128622.[CrossRef]
163. Cassar,S.;Misso,M.L.;Hopkins,W.G.;Shaw,C.S.;Teede,H.J.;Stepto,N.K.Insulinresistanceinpolycysticovarysyndrome: Asystematicreviewandmeta-analysisofeuglycaemic-hyperinsulinaemicclampstudies. Hum.Reprod. 2016, 31,2619–2631. [CrossRef]
164. Stepto,N.K.;Cassar,S.;Joham,A.E.;Hutchison,S.K.;Harrison,C.L.;Goldstein,R.F.;Teede,H.J.Womenwithpolycysticovary syndromehaveintrinsicinsulinresistanceoneuglycaemic-hyperinsulaemicclamp. Hum.Reprod. 2013, 28,777–784.[CrossRef] [PubMed]
165.Katic,M.;Kahn,C.R.TheroleofinsulinandIGF-1signalinginlongevity. Cell.Mol.LifeSci. 2005, 62,320–343.[CrossRef]
166. Cibula,D.IsinsulinresistanceanessentialcomponentofPCOS?Theinfluenceofconfoundingfactors. Hum.Reprod. 2004, 19,757–759.[CrossRef]
167. Plomin,R.;Haworth,C.M.A.;Davis,O.S.P.Commondisordersarequantitativetraits. Nat.Rev.Genet. 2009, 10,872–878. [CrossRef]
168. Tam,C.S.;Xie,W.;Johnson,W.D.;Cefalu,W.T.;Redman,L.M.;Ravussin,E.Defininginsulinresistancefromhyperinsulinemiceuglycemicclamps. DiabetesCare 2012, 35,1605–1610.[CrossRef]
169. Dunaif,A.;Segal,K.R.;Futterweit,W.;Dobrjansky,A.Profoundperipheralinsulinresistance,independentofobesity,inpolycystic ovarysyndrome. Diabetes 1989, 38,1165–1174.[CrossRef][PubMed]
170. Teede,H.J.;Hutchison,S.K.;Zoungas,S.Themanagementofinsulinresistanceinpolycysticovarysyndrome. TrendsEndocrinol. Metab. 2007, 18,273–279.[CrossRef]
171. Toosy,S.;Sodi,R.;Pappachan,J.M.Leanpolycysticovarysyndrome(PCOS):Anevidence-basedpracticalapproach. J.Diabetes Metab.Disord. 2018, 17,277–285.[CrossRef][PubMed]
172. Morciano,A.;Romani,F.;Sagnella,F.;Scarinci,E.;Palla,C.;Moro,F.;Tropea,A.;Policola,C.;DellaCasa,S.;Guido,M.;etal. Assessmentofinsulinresistanceinleanwomenwithpolycysticovarysyndrome. Fertil.Steril. 2014, 102,250–256.e3.[CrossRef] [PubMed]
173. Gorjão,R.;Takahashi,H.K.;Pan,J.A.;MassaoHirabara,S.Molecularmechanismsinvolvedininflammationandinsulinresistance inchronicdiseasesandpossibleinterventions. J.Biomed.Biotechnol. 2012, 2012,2012–2014.[CrossRef][PubMed]
174. Fernandez,M.;Murillo,A.DietaryTreatmentstoReduceInsulinResistanceandInflammationinType-2DiabeticPatients. Med.Res.Arch. 2022, 10,1–20.[CrossRef]
175. Tsatsoulis,A.;Mantzaris,M.D.;Sofia,B.;Andrikoula,M.Insulinresistance:Anadaptivemechanismbecomesmaladaptiveinthe currentenvironment—Anevolutionaryperspective. Metabolism 2012, 62,622–633.[CrossRef]
176. Wensveen,F.M.;Šestan,M.;TurkWensveen,T.;Poli´c,B.‘Beautyandthebeast’ininfection:Howimmune–endocrineinteractions regulatesystemicmetabolisminthecontextofinfection. Eur.J.Immunol. 2019, 49,982–995.[CrossRef]
177.Wang,P.;Mariman,E.C.M.Insulinresistanceinanenergy-centeredperspective. Physiol.Behav. 2008, 94,198–205.[CrossRef]
Life 2023, 13,1056 30of35
178. Kampmann,U.;Knorr,S.;Fuglsang,J.;Ovesen,P.DeterminantsofMaternalInsulinResistanceduringPregnancy:AnUpdated Overview. J.DiabetesRes. 2019, 2019,1–9.[CrossRef][PubMed]
179. Sprague,J.E.;Gandica,R.;Kelsey,M.M. InsulinResistanceinPuberty;HumanaPressInc.:Totowa,NJ,USA,2020; ISBN9783030250553.
180. Straub,R.H.;Cutolo,M.;Buttgereit,F.;Pongratz,G.Energyregulationandneuroendocrine-immunecontrolinchronicinflammatorydiseases. J.Intern.Med. 2010, 267,543–560.[CrossRef][PubMed]
181.Rabasa,C.;Dickson,S.L.Impactofstressonmetabolismandenergybalance. Curr.Opin.Behav.Sci. 2016, 9,71–77.[CrossRef]
182. Ieronymaki,E.;Daskalaki,M.G.;Lyroni,K.;Tsatsanis,C.Insulinsignalingandinsulinresistancefacilitatetrainedimmunityin macrophagesthroughmetabolicandepigeneticchanges. Front.Immunol. 2019, 10,1330.[CrossRef]
183. Chaudhari,A.P.;Mazumdar,K.;Deepak,P.Anxiety,Depression,andQualityofLifeinWomenwithPolycysticOvarianSyndrome. IndianJ.Psychol.Med. 2018, 40,239–246.[CrossRef]
184.Soeters,M.R.;Soeters,P.B.Theevolutionarybenefitofinsulinresistance. Clin.Nutr. 2012, 31,1002–1007.[CrossRef]
185. Soeters,M.R.;Sauerwein,H.P.;Dubbelhuis,P.F.;Groener,J.E.;Ackermans,M.T.;Fliers,E.;Aerts,J.M.;Serlie,M.J.Muscle adaptationtoshort-termfastinginhealthyleanhumans. J.Clin.Endocrinol.Metab. 2008, 93,2900–2903.[CrossRef]
186. Horita,S.;Seki,G.;Yamada,H.;Suzuki,M.;Koike,K.;Fujita,T.Insulinresistance,obesity,hypertension,andrenalsodium transport. Int.J.Hypertens. 2011, 2011,1–8.[CrossRef]
187. Velloso,L.A.;Folli,F.;Perego,L.;Saad,M.J.A.Themulti-facetedcross-talkbetweentheinsulinandangiotensinIIsignaling systems. Diabetes.Metab.Res.Rev. 2006, 22,98–107.[CrossRef]
188. Zhou,M.S.;Schulman,I.H.;Raij,L.Vascularinflammation,insulinresistance,andendothelialdysfunctioninsalt-sensitive hypertension:RoleofnuclearfactorkappaBactivation. J.Hypertens. 2010, 28,527–535.[CrossRef][PubMed]
189. Ehrmann,D.A.;Liljenquist,D.R.;Kasza,K.;Azziz,R.;Legro,R.S.;Ghazzi,M.N.;Aronoff,S.;Bernstein,R.;Bodenner,D.; Braithwaite,S.;etal.Prevalenceandpredictorsofthemetabolicsyndromeinwomenwithpolycysticovarysyndrome. J.Clin. Endocrinol.Metab. 2006, 91,48–53.[CrossRef]
190. Sonagra,A.D.NormalPregnancy-AStateofInsulinResistance. J.Clin.DiagnosticRes. 2014, 8,CC01–CC03.[CrossRef][PubMed]
191. Catalano,P.M.;Huston,L.;Amini,S.B.;Kalhan,S.C.Longitudinalchangesinglucosemetabolismduringpregnancyinobese womenwithnormalglucosetoleranceandgestationaldiabetesmellitus. Am.J.Obstet.Gynecol. 1999, 180,903–916.[CrossRef]
192. Jayabalan,N.;Nair,S.;Nuzhat,Z.;Rice,G.E.;Zuñiga,F.A.;Sobrevia,L.;Leiva,A.;Sanhueza,C.;Gutiérrez,J.A.;Lappas,M.;etal. Crosstalkbetweenadiposetissueandplacentainobeseandgestationaldiabetesmellituspregnanciesviaexosomes. Front. Endocrinol. 2017, 8,239.[CrossRef]
193. Koren,O.;Goodrich,J.K.;Cullender,T.C.;Spor,A.;Laitinen,K.;KlingBäckhed,H.;Gonzalez,A.;Werner,J.J.;Angenent,L.T.; Knight,R.;etal.Hostremodelingofthegutmicrobiomeandmetabolicchangesduringpregnancy. Cell 2012, 150,470–480. [CrossRef][PubMed]
194. Li,X.;Liu,X.;Zuo,Y.;Gao,J.;Liu,Y.;Zheng,W.Theriskfactorsofgestationaldiabetesmellitusinpatientswithpolycysticovary syndrome:Whatshouldwecare. Medicine 2021, 100,E26521.[CrossRef]
195. Yan,Q.;Qiu,D.;Liu,X.;Xing,Q.;Liu,R.;Hu,Y.Theincidenceofgestationaldiabetesmellitusamongwomenwithpolycystic ovarysyndrome:Ameta-analysisoflongitudinalstudies. BMCPregnancyChildbirth 2022, 22,1–12.[CrossRef][PubMed]
196. Noctor,E.Type2diabetesaftergestationaldiabetes:Theinfluenceofchangingdiagnosticcriteria. WorldJ.Diabetes 2015, 6,234. [CrossRef][PubMed]
197. Vounzoulaki,E.;Khunti,K.;Abner,S.C.;Tan,B.K.;Davies,M.J.;Gillies,C.L.Progressiontotype2diabetesinwomenwitha knownhistoryofgestationaldiabetes:Systematicreviewandmeta-analysis. BMJ 2020, 369,m1361.[CrossRef]
198. Bonora,E.;Trombetta,M.;Dauriz,M.;Travia,D.;Cacciatori,V.;Brangani,C.;Negri,C.;Perrone,F.;Pichiri,I.;Stoico,V.;etal. Chroniccomplicationsinpatientswithnewlydiagnosedtype2diabetes:Prevalenceandrelatedmetabolicandclinicalfeatures: TheVeronaNewlyDiagnosedType2DiabetesStudy(VNDS)9. BMJOpenDiabetesRes.Care 2020, 8,e001549.[CrossRef]
199. Bolton,A.(Ed.) InternationalDiabetesFederationDiabetesAtlas,10thed.;DiabetesResearchandClinicalPractice;TheIDFDiabetes Atlas:Brussels,Belgium,2021;Volume102,Availableonline: www.diabetesatlas.org (accessedon15December2021).
200. Yang,J.;Qian,F.;Chavarro,J.E.;Ley,S.H.;Tobias,D.K.;Yeung,E.;Hinkle,S.N.;Bao,W.;Li,M.;Liu,A.;etal.Modifiablerisk factorsandlongtermriskoftype2diabetesamongindividualswithahistoryofgestationaldiabetesmellitus:Prospectivecohort study. BMJ 2022, 378,e070312.[CrossRef]
201. Patel,S.Disruptionofaromatasehomeostasisasthecauseofamultiplicityofailments:Acomprehensivereview. J.Steroid Biochem.Mol.Biol. 2017, 168,19–25.[CrossRef]
202. Evans,S.F.;Hull,M.L.;Hutchinson,M.R.;Rolan,P.E.Androgens,EndometriosisandPain. Front.Reprod.Health 2021, 3,2920. [CrossRef][PubMed]
203. Diamond,M.P.;Grainger,D.;Diamond,M.C.;Sherwin,R.S.;Defronzo,R.A.Effectsofmethyltestosteroneoninsulinsecretionand sensitivityinwomen. J.Clin.Endocrinol.Metab. 1998, 83,4420–4425.[CrossRef]
204. Polderman,K.H.;Gooren,L.J.;Asscheman,H.InductionofInsulinResistancebyAndrogensandEstrogens. J.Clin.Endocrinol.Metab. 1994, 79,265–271.Availableonline: https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ jcem/79/1/10.1210_jcem.79.1.8027240/1/jcem0265.pdf?Expires=1498610821&Signature=TRE3mYh6fQ2FNOn4dry9Y~-YoZVmlGINOvJhlM7Lrm2J-l-9VG1X9UeJK1XUU9wSsmEDz4kiZRNOXaeefJFQDNsFOPEIfUp~vqLk91tWtQ (accessedon19 February2023).[PubMed]
Life 2023, 13,1056 31of35
205. Skinner,M.K.;Schmidt,M.;Savenkova,M.I.;Sadler-Riggleman,I.;Nilsson,E.E.Regulationofgranulosaandthecacelltranscriptomesduringovarianantralfollicledevelopment. Mol.Reprod.Dev. 2008, 75,1457–1472.[CrossRef][PubMed]
206. Clegg,D.J.Minireview:Theyearinreviewofestrogenregulationofmetabolism. Mol.Endocrinol. 2012, 26,1957–1960.[CrossRef]
207. Stocco,C.Aromataseexpressionintheovary:Hormonalandmolecularregulation. Steroids 2008, 73,473–487.[CrossRef] [PubMed]
208. Armanini,D.;Boscaro,M.;Bordin,L.;Sabbadin,C.ControversiesinthePathogenesis,DiagnosisandTreatmentofPCOS:Focus onInsulinResistance,Inflammation,andHyperandrogenism. Int.J.Mol.Sci. 2022, 23,4110.[CrossRef]
209. DiNardo,G.;Zhang,C.;Marcelli,A.G.;Gilardi,G.Molecularandstructuralevolutionofcytochromep450aromatase. Int.J.Mol. Sci. 2021, 22,631.[CrossRef][PubMed]
210. Simeon,S.;Spjuth,O.;Lapins,M.;Nabu,S.;Anuwongcharoen,N.;Prachayasittikul,V.;Wikberg,J.E.S.;Nantasenamat,C.Origin ofaromataseinhibitoryactivityviaproteochemometricmodeling. PeerJ 2016, 2016,e1979.[CrossRef]
211. Conley,A.;Mapes,S.;JoCorbin,C.;Greger,D.;Graham,S.Structuraldeterminantsofaromatasecytochromep450inhibitionin substraterecognitionsite-1. Mol.Endocrinol. 2002, 16,1456–1468.[CrossRef][PubMed]
212. Jin,Y.;Choi,Y.J.;Heo,K.;Park,S.J.Melatoninasanoncostaticmoleculebasedonitsanti-aromataseroleinbreastcancer. Int.J. Mol.Sci. 2021, 22,438.[CrossRef]
213. Cheng,J.C.;Fang,L.;Li,Y.;Wang,S.;Li,Y.;Yan,Y.;Jia,Q.;Wu,Z.;Wang,Z.;Han,X.;etal.Melatoninstimulatesaromatase expressionandestradiolproductioninhumangranulosa-luteincells:Relevanceforhighserumestradiollevelsinpatientswith ovarianhyperstimulationsyndrome. Exp.Mol.Med. 2020, 52,1341–1350.[CrossRef]
214. Kim,J.Y.;Han,E.H.;Kim,H.G.;Oh,K.N.;Kim,S.K.;Lee,K.Y.;Jeong,H.G.BisphenolA-inducedaromataseactivationismediated bycyclooxygenase-2up-regulationinrattesticularLeydigcells. Toxicol.Lett. 2010, 193,200–208.[CrossRef]
215. Maia,H.;Haddad,C.;Pinheiro,N.;Casoy,J.TheeffectoforalcontraceptivesonaromataseandCox-2expressioninthe endometriumofpatientswithidiopathicmenorrhagiaoradenomyosis. Int.J.Womens.Health 2013, 5,293–299.[CrossRef] [PubMed]
216. Wang,C.;Mäkelä,T.;Hase,T.;Adlercreutz,H.;Kurzer,M.S.Lignansandflavonoidsinhibitaromataseenzymeinhuman preadipocytes. J.SteroidBiochem.Mol.Biol. 1994, 50,205–212.[CrossRef]
217. Singh,S.;Awasthi,M.;Pandey,V.P.;Dwivedi,U.N.Plantderivedanti-canceroussecondarymetabolitesasmultiprongedinhibitor ofCOX,Topo,andaromatase:Molecularmodelinganddynamicssimulationanalyses. J.Biomol.Struct.Dyn. 2017, 35,3082–3097. [CrossRef]
218. Richard,S.;Moslemi,S.;Sipahutar,H.;Benachour,N.;Seralini,G.E.Differentialeffectsofglyphosateandrounduponhuman placentalcellsandaromatase. Environ.HealthPerspect. 2005, 113,716–720.[CrossRef]
219. Zarn,J.A.;Brüschweiler,B.J.;Schlatter,J.R.Azolefungicidesaffectmammaliansteroidogenesisbyinhibitingsterol14αdemethylaseandaromatase. Environ.HealthPerspect. 2003, 111,255–261.[CrossRef]
220. Rojas,J.;Chávez-Castillo,M.;Torres,W.;Arraiz,N.;Cabrera,M.MetformininCancer:ChemicalPathwaysforTumoralControl IndependentofAmp-DependentKinase. J.Endocrinol.DiabetesObes. 2014, 2,1036.
221. Biegon,A.;Alia-Klein,N.;Fowler,J.S.Potentialcontributionofaromataseinhibitiontotheeffectsofnicotineandrelated compoundsonthebrain. Front.Pharmacol. 2012, 3,185.[CrossRef][PubMed]
222. Lee,S.H.;Park,S.Y.;Choi,C.S.InsulinResistance:FromMechanismstoTherapeuticStrategies. DiabetesMetab.J. 2022, 46,15–37. [CrossRef][PubMed]
223. Baillargeon,J.P.;Nestler,J.E.Commentary:Polycysticovarysyndrome:Asyndromeofovarianhypersensitivitytoinsulin? J.Clin. Endocrinol.Metab. 2006, 91,22–24.[CrossRef]
224. Zhao,H.;Zhang,J.;Cheng,X.;Nie,X.;He,B.Insulinresistanceinpolycysticovarysyndromeacrossvarioustissues:Anupdated reviewofpathogenesis,evaluation,andtreatment. J.OvarianRes. 2023, 16,1–17.[CrossRef]
225. Bremer,A.A.;Miller,W.L.Theserinephosphorylationhypothesisofpolycysticovarysyndrome:Aunifyingmechanismfor hyperandrogenemiaandinsulinresistance. Fertil.Steril. 2008, 89,1039–1048.[CrossRef][PubMed]
226. Ibáñez,L.;Potau,N.;Zampolli,M.;Riqué,S.;Saenger,P.;Carrascosa,A.Hyperinsulinemiaanddecreasedinsulin-likegrowth factor-bindingprotein-1arecommonfeaturesinprepubertalandpubertalgirlswithahistoryofprematurepubarche. J.Clin. Endocrinol.Metab. 1997, 82,2283–2288.[CrossRef]
227. Soldani,R.;Cagnacci,A.;Yen,S.S.C.Insulininsulin-likegrowthfactorI(IGF-I)andIGF-IIenhancebasalandgonadotrophinreleasinghormone-stimulatedluteinizinghormonereleasefromratanteriorpituitarycells invitro Eur.J.Endocrinol. 1994, 131,641–645.[CrossRef][PubMed]
228. Nestler,J.E.;Powers,L.P.;Matt,D.W.;Steingold,K.A.;Plymate,S.R.;Rittmaster,R.S.;Clore,J.N.;Blackard,W.G.Adirecteffectof hyperinsulinemiaonserumsexhormone-bindingglobulinlevelsinobesewomenwiththepolycysticovarysyndrome. J.Clin. Endocrinol.Metab. 1991, 72,83–89.[CrossRef][PubMed]
229. Chan,O.;Inouye,K.;Akirav,E.;Park,E.;Riddell,M.C.;Vranic,M.;Matthews,S.G.Insulinaloneincreaseshypothalamo-pituitaryadrenalactivity,anddiabeteslowerspeakstressresponses. Endocrinology 2005, 146,1382–1390.[CrossRef][PubMed]
230. Kanbour,S.A.;Dobs,A.S.HyperandrogenisminWomenwithPolycysticOvarianSyndrome:PathophysiologyandControversies. Androgens 2022, 3,22–30.[CrossRef]
231. Pateguana,N.B.;Janes,A.Thecontributionofhyperinsulinemiatothehyperandrogenismofpolycysticovarysyndrome. J.Insul. Resist. 2019, 4,3.[CrossRef]
Life 2023, 13,1056 32of35
232. Vyrides,A.A.;ElMahdi,E.;Giannakou,K.Ovulationinductiontechniquesinwomenwithpolycysticovarysyndrome. Front. Med. 2022, 9,2230.[CrossRef]
233. Panth,N.;Gavarkovs,A.;Tamez,M.;Mattei,J.TheInfluenceofDietonFertilityandtheImplicationsforPublicHealthNutrition intheUnitedStates. Front.PublicHealth 2018, 6,211.[CrossRef]
234. Elnashar,A.M.Theroleofmetformininovulationinduction:Currentstatus. MiddleEastFertil.Soc.J. 2011, 16,175–181.[CrossRef]
235. NehirAytan,A.;Bastu,E.;Demiral,I.;Bulut,H.;Dogan,M.;Buyru,F.Relationshipbetweenhyperandrogenism,obesity, inflammationandpolycysticovarysyndrome. Gynecol.Endocrinol. 2016, 32,709–713.[CrossRef]
236. West-Eberhard,M.J.Nutrition,thevisceralimmunesystem,andtheevolutionaryoriginsofpathogenicobesity. Proc.Natl.Acad. Sci.USA 2019, 116,723–731.[CrossRef]
237. Ottaviani,E.;Malagoli,D.;Franceschi,C.Theevolutionoftheadiposetissue:Aneglectedenigma. Gen.Comp.Endocrinol. 2011, 174,1–4.[CrossRef]
238. Kuzawa,C.W.AdiposeTissueinHumanInfancyandChildhood:AnEvolutionaryPerspective. Yearb.Phys.Anthropol. 1998, 41, 177–209.[CrossRef]
239. Parra-Peralbo,E.;Talamillo,A.;Barrio,R.OriginandDevelopmentoftheAdiposeTissue,aKeyOrganinPhysiologyandDisease. Front.CellDev.Biol. 2021, 9,786129.[CrossRef][PubMed]
240. Ibrahim,M.M.Subcutaneousandvisceraladiposetissue:Structuralandfunctionaldifferences. Obes.Rev. 2010, 11,11–18. [CrossRef][PubMed]
241. Spritzer,P.M.;Lecke,S.B.;Satler,F.;Morsch,D.M.Adiposetissuedysfunction,adipokines,andlow-gradechronicinflammation inpolycysticovarysyndrome. Reproduction 2015, 149,R219–R227.[CrossRef]
242. Scheja,L.;Heeren,J.Theendocrinefunctionofadiposetissuesinhealthandcardiometabolicdisease. Nat.Rev.Endocrinol. 2019, 15,507–524.[CrossRef]
243. Choe,S.S.;Huh,J.Y.;Hwang,I.J.;Kim,J.I.;Kim,J.B.Adiposetissueremodeling:Itsroleinenergymetabolismandmetabolic disorders. Front.Endocrinol. 2016, 7,30.[CrossRef]
244. Pawłowski,B.; Zela´zniewicz,A.Theevolutionofperenniallyenlargedbreastsinwomen:Acriticalreviewandanovelhypothesis. Biol.Rev. 2021, 2798,2794–2809.[CrossRef]
245. Tan,C.Y.;Vidal-Puig,A.Adiposetissueexpandability:Themetabolicproblemsofobesitymayarisefromtheinabilitytobecome moreobese. Biochem.Soc.Trans. 2008, 36,935–940.[CrossRef]
246.Villa,J.;Pratley,R.E.Adiposetissuedysfunctioninpolycysticovarysyndrome. Curr.Diab.Rep. 2011, 11,179–184.[CrossRef]
247. Echiburú,B.;Pérez-Bravo,F.;Galgani,J.E.;Sandoval,D.;Saldías,C.;Crisosto,N.;Maliqueo,M.;Sir-Petermann,T.Enlarged adipocytesinsubcutaneousadiposetissueassociatedtohyperandrogenismandvisceraladiposetissuevolumeinwomenwith polycysticovarysyndrome. Steroids 2018, 130,15–21.[CrossRef][PubMed]
248. Arpaci,D.;GurkanTocoglu,A.;Yilmaz,S.;Ergenc,H.;Tamer,A.;Keser,N.;Gunduz,H.Therelationshipbetweenepicardialfat tissuethicknessandvisceraladiposetissueinleanpatientswithpolycysticovarysyndrome. J.OvarianRes. 2015, 8,71.[CrossRef] [PubMed]
249. Glueck,C.J.;Goldenberg,N.Characteristicsofobesityinpolycysticovarysyndrome:Etiology,treatment,andgenetics. Metabolism 2019, 92,108–120.[CrossRef][PubMed]
250. Kałuzna,M.;Czlapka-Matyasik,M.;Bykowska-Derda,A.;Moczko,J.;Ruchala,M.;Ziemnicka,K.Indirectpredictorsofvisceral adiposetissueinwomenwithpolycysticovarysyndrome:Acomparisonofmethods. Nutrients 2021, 13,2494.[CrossRef] [PubMed]
251. Jones,G.W.;Hill,D.G.;Jones,S.A.Understandingimmunecellsintertiarylymphoidorgandevelopment:Itisallstartingtocome together. Front.Immunol. 2016, 7,401.[CrossRef]
252. Dixon,L.J.;Barnes,M.;Tang,H.;Pritchard,M.T.;Nagy,L.E.Kupffercellsintheliver. Compr.Physiol. 2013, 3,785–797.[CrossRef]
253. Parker,J.;O’Brien,C.;Gersh,F.L.Developmentaloriginsandtransgenerationalinheritanceofpolycysticovarysyndrome. Aust. N.Z.J.Obstet.Gynaecol. 2021, 61,922–926.[CrossRef][PubMed]
254. Davenport,E.R.;Sanders,J.G.;Song,S.J.;Amato,K.R.;Clark,A.G.;Knight,R.Thehumanmicrobiomeinevolution. BMCBiol. 2017, 15,1–12.[CrossRef]
255. Rizk,M.G.;Thackray,V.G.IntersectionofPolycysticOvarySyndromeandtheGutMicrobiome. J.Endocr.Soc. 2021, 5,bvaa177. [CrossRef][PubMed]
256. Valdes,A.M.;Walter,J.;Segal,E.;Spector,T.D.Roleofthegutmicrobiotainnutritionandhealth. BMJ 2018, 361,36–44.[CrossRef]
257. Martinez,J.E.;Kahana,D.D.;Ghuman,S.;Wilson,H.P.;Wilson,J.;Kim,S.C.J.;Lagishetty,V.;Jacobs,J.P.;Sinha-Hikim,A.P.; Friedman,T.C.UnhealthyLifestyleandGutDysbiosis:ABetterUnderstandingoftheEffectsofPoorDietandNicotineonthe IntestinalMicrobiome. Front.Endocrinol. 2021, 12,7066.[CrossRef][PubMed]
258. Degruttola,A.K.;Low,D.;Mizoguchi,A.;Mizoguchi,E.Currentunderstandingofdysbiosisindiseaseinhumanandanimal models. Inflamm.BowelDis. 2016, 22,1137–1150.[CrossRef]
259. Shahi,S.K.;Ghimire,S.;Lehman,P.;Mangalam,A.K.Obesityinducedgutdysbiosiscontributestodiseaseseverityinananimal modelofmultiplesclerosis. Front.Immunol. 2022, 13,6417.[CrossRef][PubMed]
260. Moya,A.;Ferrer,M.FunctionalRedundancy-InducedStabilityofGutMicrobiotaSubjectedtoDisturbance. TrendsMicrobiol. 2016, 24,402–413.[CrossRef][PubMed]
Life 2023, 13,1056 33of35
261. Zhao,X.;Jiang,Y.;Xi,H.;Chen,L.;Feng,X.ExplorationoftheRelationshipbetweenGutMicrobiotaandPolycysticOvary Syndrome(PCOS):AReview. GeburtshilfeFrauenheilkd. 2020, 80,161–171.[CrossRef][PubMed]
262. Yilmaz,B.;Terekeci,H.;Sandal,S.;Kelestimur,F.Endocrinedisruptingchemicals:Exposure,effectsonhumanhealth,mechanism ofaction,modelsfortestingandstrategiesforprevention. Rev.Endocr.Metab.Disord. 2020, 21,127–147.[CrossRef]
263. Fabozzi,G.;Rebuzzini,P.;Cimadomo,D.;Allori,M.;Franzago,M.;Stuppia,L.;Garagna,S.;Ubaldi,F.M.;Zuccotti,M.;Rienzi,L. Endocrine-DisruptingChemicals,GutMicrobiota,andHuman(In)Fertility—ItIsTimetoConsidertheTriad. Cells 2022, 11,3335. [CrossRef]
264. Bellingham,M.;Sharpe,R.ChemicalExposuresDuringPregnancy:DealingwithPotential,butUnproven,RiskstoChildHealth. R.Coll.Obstet.Gynaecol. 2013,1–7.
265. DiRenzo,G.C.;Conry,J.A.;Blake,J.;Defrancesco,M.S.;Denicola,N.;Martin,J.N.;McCue,K.A.;Richmond,D.;Shah,A.; Sutton,P.;etal.InternationalFederationofGynecologyandObstetricsopiniononreproductivehealthimpactsofexposureto toxicenvironmentalchemicals. Int.J.Gynecol.Obstet. 2015, 131,219–225.[CrossRef][PubMed]
266. Gore,A.C.;Chappell,V.A.;Fenton,S.E.;Flaws,J.A.;Nadal,A.;Prins,G.S.;Toppari,J.;Zoeller,R.T.EDC-2:TheEndocrine Society’sSecondScientificStatementonEndocrine-DisruptingChemicals. Endocr.Rev. 2015, 36,E1–E150.[CrossRef][PubMed]
267. Liu,Z.;Lu,Y.;Zhong,K.;Wang,C.;Xu,X.Theassociationsbetweenendocrinedisruptingchemicalsandmarkersofinflammation andimmuneresponses:Asystematicreviewandmeta-analysis. Ecotoxicol.Environ.Saf. 2022, 234,113382.[CrossRef][PubMed]
268. Alonso-Magdalena,P.;Morimoto,S.;Ripoll,C.;Fuentes,E.;Nadal,A.Theestrogeniceffectofbisphenoladisruptspancreatic β-cellfunctioninvivoandinducesinsulinresistance. Environ.HealthPerspect. 2006, 114,106–112.[CrossRef]
269. Wang,Y.;Zhu,Q.;Dang,X.;He,Y.;Li,X.;Sun,Y.LocaleffectofbisphenolAontheestradiolsynthesisofovariangranulosacells fromPCOS. Gynecol.Endocrinol. 2017, 33,21–25.[CrossRef]
270. Sun,Y.;Gao,S.;Ye,C.;Zhao,W.Gutmicrobiotadysbiosisinpolycysticovarysyndrome:Mechanismsofprogressionandclinical applications. Front.Cell.Infect.Microbiol. 2023, 13,1142041.[CrossRef]
271. Ananthasubramanian,P.;Ananth,S.;Kumaraguru,S.;Barathi,S.;Santosh,W.;Vasantharekha,R.AssociatedEffectsofEndocrine DisruptingChemicals(EDCs)onNeuroendocrineAxesandNeurotransmitterProfileinPolycysticOvarianSyndromeCondition. Proc.Zool.Soc. 2021, 74,378–386.[CrossRef]
272. Gupta,R.;Kumar,P.;Fahmi,N.;Garg,B.;Dutta,S.;Sachar,S.;Matharu,A.S.;Vimaleswaran,K.S.Endocrinedisruptionand obesity:Acurrentreviewonenvironmentalobesogens. Curr.Res.GreenSustain.Chem. 2020, 3,100009.[CrossRef]
273. Aydemir,D.;Ulusu,N.N.Thepossibleroleoftheendocrinedisruptingchemicalsontheprematureandearlymenopause associatedwiththealteredoxidativestressmetabolism. Front.Endocrinol. 2023, 14,1081704.[CrossRef]
274. Ravichandran,G.;Lakshmanan,D.K.;Raju,K.;Elangovan,A.;Nambirajan,G.;Devanesan,A.A.;Thilagar,S.Foodadvanced glycationendproductsaspotentialendocrinedisruptors:Anemergingthreattocontemporaryandfuturegeneration. Environ. Int. 2019, 123,486–500.[CrossRef]
275. Bansal,A.;Henao-Mejia,J.;Simmons,R.A.Immunesystem:Anemergingplayerinmediatingeffectsofendocrinedisruptorson metabolichealth. Endocrinology 2018, 159,32–45.[CrossRef]
276. Tudurí,E.;Marroqui,L.;DosSantos,R.S.;Quesada,I.;Fuentes,E.;Alonso-Magdalena,P.TimingofexposureandBisphenol-A: Implicationsfordiabetesdevelopment. Front.Endocrinol. 2018, 9,648.[CrossRef][PubMed]
277.Resnik,D.B.Theprecautionaryprincipleandmedicaldecisionmaking. J.Med.Philos. 2004, 29,281–299.[CrossRef][PubMed]
278. Schug,T.T.;Johnson,A.F.;Birnbaum,L.S.;Colborn,T.;Guillette,L.J.;Crews,D.P.;Collins,T.;Soto,A.M.;VomSaal,F.S.;McLachlan, J.A.;etal.Minireview:Endocrinedisruptors:Pastlessonsandfuturedirections. Mol.Endocrinol. 2016, 30,833–847.[CrossRef] [PubMed]
279. More,S.;Bampidis,V.;Benford,D.;Bragard,C.;Halldorsson,T.;Hernández-Jerez,A.;HougaardBennekou,S.;Koutsoumanis,K.; Lambré,C.;Machera,K.;etal.Guidanceonriskassessmentofnanomaterialstobeappliedinthefoodandfeedchain:Human andanimalhealth. EFSAJ. 2021, 19,e06768.[CrossRef][PubMed]
280. Rutkowska,A.Z.;Diamanti-Kandarakis,E.Polycysticovarysyndromeandenvironmentaltoxins. Fertil.Steril. 2016, 106,948–958. [CrossRef][PubMed]
281. Jala,A.;Varghese,B.;Kaur,G.;Rajendiran,K.;Dutta,R.;Adela,R.;Borkar,R.M.Implicationsofendocrine-disruptingchemicals onpolycysticovariansyndrome:Acomprehensivereview. Environ.Sci.Pollut.Res. 2022, 29,58484–58513.[CrossRef]
282. Jozkowiak,M.;Piotrowska-Kempisty,H.;Kobylarek,D.;Gorska,N.;Mozdziak,P.;Kempisty,B.;Rachon,D.;Spaczynski,R.Z. EndocrineDisruptingChemicalsinPolycysticOvarySyndrome:TheRelevantRoleoftheThecaandGranulosaCellsinthe PathogenesisoftheOvarianDysfunction. Cells 2023, 12,174.[CrossRef]
283. Parker,J.Anewhypothesisforthemechanismofglyphosateinducedintestinalpermeabilityinthepathogenesisofpolycystic ovarysyndrome. J.Australas.Coll.Nutr.Environ.Med. 2015, 34,3.
284. Kumar,M.;Sarma,D.K.;Shubham,S.;Kumawat,M.;Verma,V.;Prakash,A.;Tiwari,R.EnvironmentalEndocrine-Disrupting ChemicalExposure:RoleinNon-CommunicableDiseases. Front.PublicHealth 2020, 8,3850.[CrossRef]
285. Mitro,S.D.;Johnson,T.;Zota,A.R.CumulativeChemicalExposuresDuringPregnancyandEarlyDevelopment. Curr.Environ. HealthRep. 2015, 2,367–378.[CrossRef][PubMed]
286. Starling,A.P.;Adgate,J.L.;Hamman,R.F.;Kechris,K.;Calafat,A.M.;Ye,X.;Dabelea,D.Perfluoroalkylsubstancesduring pregnancyandoffspringweightandadiposityatbirth:Examiningmediationbymaternalfastingglucoseinthehealthystart study. Environ.HealthPerspect. 2017, 125,067016.[CrossRef][PubMed]
Life 2023, 13,1056 34of35
287. Rosenfeld,C.S.TranscriptomicsandOtherOmicsApproachestoInvestigateEffectsofXenobioticsonthePlacenta. Front.Cell Dev.Biol. 2021, 9,3656.[CrossRef][PubMed]
288. Hewlett,M.;Chow,E.;Aschengrau,A.;Mahalingaiah,S.PrenatalExposuretoEndocrineDisruptors:ADevelopmentalEtiology forPolycysticOvarySyndrome. Reprod.Sci. 2017, 24,19–27.[CrossRef][PubMed]
289. Corbett,G.A.;Lee,S.;Woodruff,T.J.;Hanson,M.;Hod,M.;Charlesworth,A.M.;Giudice,L.;Conry,J.;McAuliffe,F.M.Nutritional interventionstoamelioratetheeffectofendocrinedisruptorsonhumanreproductivehealth:Asemi-structuredreviewfrom FIGO. Int.J.Gynecol.Obstet. 2022, 157,489–501.[CrossRef][PubMed]
290. Piazza,M.J.;Urbanetz,A.A.Environmentaltoxinsandtheimpactofotherendocrinedisruptingchemicalsinwomen’sreproductivehealth. J.Bras.Reprod.Assist. 2019, 23,154–164.[CrossRef]
291.Hanahan,D.;Weinberg,R.A.TheHallmarksofCancer. Cell 2000, 100,57–70.[CrossRef]
292.López-Otín,C.;Blasco,M.A.;Partridge,L.;Serrano,M.;Kroemer,G.Thehallmarksofaging. Cell 2013, 153,1194.[CrossRef]
Disclaimer/Publisher’sNote: Thestatements,opinionsanddatacontainedinallpublicationsaresolelythoseoftheindividual author(s)andcontributor(s)andnotofMDPIand/ortheeditor(s).MDPIand/ortheeditor(s)disclaimresponsibilityforanyinjuryto peopleorpropertyresultingfromanyideas,methods,instructionsorproductsreferredtointhecontent.
Life 2023, 13,1056 35of35

Review Article
Obstet Gynecol Sci 2022;65(1):14-28
https://doi.org/10.5468/ogs.21185
eISSN 2287-8580
A narrative review of the role of gastrointestinal dysbiosis in the pathogenesis of polycystic ovary syndrome
Jim Parker, FRANZCOG1, Claire O'Brien, PhD2, Jason Hawrelak, PhD3
1School of Medicine, Faculty of Science, Medicine and Health, University of Wollongong, Wollongong; 2Faculty of Science and Technology, University of Canberra, Canberra; 3College of Health and Medicine, University of Tasmania, Tasmania, Australia
Diet-induced gastrointestinal dysbiosis has been hypothesized to play a significant role in stimulating an increase in gastrointestinal permeability and activating systemic inflammation in women with polycystic ovary syndrome (PCOS). We reviewed the current proof-of-concept studies on the proposed mechanism of dysbiosis in the pathogenesis of PCOS. A literature search was performed to identify articles on changes in the intestinal microbiome (dysbiosis) and increased intestinal mucosal permeability involving lipopolysaccharide (LPS), LPS-binding protein (LPS-BP), and zonulin. We also searched for systematic reviews and meta-analyses that synthesized the results of studies on the therapeutic effects of prebiotics, probiotics, or synbiotics in women with PCOS. Our search was confined to human studies between 2012 and 2021 using the PubMed, Scopus, and Cochrane databases. Thirty-one studies met the inclusion criteria (14 microbiota, 1 LPS, 1 LPS-BP, 1 LPS and LPS-BP, 5 zonulin, 9 systematic reviews). Our analysis revealed that most studies reported reduced alpha diversity and dysbiosis in women with PCOS. Preliminary studies suggest that LPS, LPS-BP, and zonulin may be involved in the pathophysiology of increased intestinal permeability. Treatment of PCOS with prebiotics, probiotics, and synbiotics appears to have a range of beneficial effects on metabolic and biochemical profiles. This review highlights the need for continued research into the pathophysiological mechanisms of dysbiosis and the clinical efficacy of prebiotics, probiotics, and synbiotics in women with PCOS.
Keywords: Dysbiosis; Lipopolysaccharides; Polycystic ovary syndrome; Probiotics; Zonulin
Introduction
Polycystic ovary syndrome (PCOS) is both a metabolic and endocrine syndrome that affects 6-15% of reproductive-age women, or approximately 200 million globally, and is believed to be increasing in frequency [1-4]. PCOS is now being recognized as an ecological condition that arises in genetically susceptible women due to a mismatch between ancestral inherited adaptive genetic polymorphisms and modern lifestyle [5]. This evolving ecological view of PCOS takes into account the interaction between lifestyle and environmental factors, such as diet and activity levels, with developmentally programmed metabolic and endocrine pathways [3,5-7]. This view supports the recommendations of the 2018 International Guidelines that the first-line management of women with PCOS should focus on lifestyle-based approaches [8]. The pathogenesis of many chronic diseases, particularly a range of metabolic diseases associated with PCOS, such as obesity,
type-2 diabetes, metabolic syndrome, and gestational diabetes, are also being considered from an evolutionary perspective [5,6,9].
The dysbiosis of gut microbiota theory proposed by Tremellen and Pearce explains the key steps in the development of
Received: 2021.06.10. Revised: 2021.08.17. Accepted: 2021.12.06.
Corresponding author: Jim Parker, FRANZCOG
School of Medicine, Faculty of Science, Medicine and Health, University of Wollongong Australia, Northfields Ave, Wollongong NSW 2500, Australia
E-mail: jimparker@ozemail.com.au
https://orcid.org/0000-0002-5018-5555
Articles published in Obstet Gynecol Sci are open-access, distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons. org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Copyright © 2022 Korean Society of Obstetrics and Gynecology
www.ogscience.org 14
PCOS [10]. This theory proposes that a high-fat, high-sugar, low-fiber diet results in dysbiosis of the gastrointestinal (GI) microbiota, thereby increasing gut permeability and translocation of endotoxins into the circulation. This subsequently leads to pro-inflammatory cytokine release and immunological and metabolic changes, including impaired insulin receptor function. Then, high serum insulin stimulates excessive testosterone production in the ovary, leading to impaired follicle development and the establishment of PCOS [10]. Proofof-concept studies have aimed to elucidate whether shifts in the microbiota of women with PCOS are associated with the pathogenesis of the disease. Studies evaluating changes in lipopolysaccharide (LPS), LPS-binding protein (LPS-BP), zonulin, mucosal permeability, immune system activation, and reversal of dysbiosis with diet, prebiotics, probiotics, or synbiotics have been performed to further investigate the role of dysbiosis in the pathogenesis of PCOS.
The objective of this study was to review the literature related to the potential role of diet-induced gastrointestinal dysbiosis in the pathogenesis of PCOS, summarize the studies investigating specific mechanisms of increased intestinal permeability involving LPS, LPS-BP, and zonulin, and discuss the evidence related to treatment with prebiotics, probiotics, and synbiotics. The results are presented as a narrative review of the published literature.
Methods
We conducted a literature review using the PubMed, Scopus, and Cochrane databases. A combination of medical subject headings and keywords were used, including the search terms polycystic ovary syndrome, PCOS, dysbiosis, dysbiosis of gut microbiota theory, lipopolysaccharide, lipopolysaccharide-binding protein, zonulin, mucosal permeability, endotoxemia, and treatment of polycystic ovary syndrome with prebiotics, probiotics, or synbiotics. We confined our search criteria to research articles on human studies published in English between January 2012 and August 2021. The selection criteria for the narrative review included original articles (randomized and non-randomized controlled trials, prospective observational studies, retrospective cohort studies, and case-control studies) and systematic reviews.
Studies were included if they reported outcomes related to gastrointestinal microbiome and intestinal permeability, LPS,
LPS-BP, zonulin, endotoxemia, and treatment responses following prebiotics, probiotics, or synbiotics in human subjects. Articles were excluded if they were not in English, were performed using animal models, or investigated outcomes related to oral or vaginal microbiomes. This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines. The results are presented as a narrative review of the available evidence examining the role of dysbiosis, increased intestinal mucosal permeability, and treatment of women with PCOS with prebiotics, probiotics, and synbiotics. No attempt was made to combine the results into a formal systematic review or meta-analysis due to the variety of different subject areas reviewed and the degree of heterogeneity in studies reporting similar components of the pathophysiology of the disease.
Results
The initial literature review identified 683 records from the PubMed, Scopus, and Cochrane databases. Thirty-one duplicates were removed, and 552 reports were assessed by screening titles and abstracts. Forty full text articles were reviewed, while nine articles were excluded since they did not meet the inclusion criteria. In all, we identified 31 studies that met the inclusion criteria. Details of the selection process are shown in the PRISMA flow diagram (Fig. 1).
We have summarized the relevant studies and reported the findings in the 9 sections: 1. Role of dysbiosis of gut microbiota in the pathogenesis of PCOS; 2. Dysbiosis; 3. Lipopolysaccharide; 4. Lipopolysaccharide-binding protein; 5. Zonulin treatment and increased mucosal permeability; 6. Role of prebiotics, probiotics, and synbiotics in the treatment of women with PCOS; 7. Prebiotics; 8. Probiotics; and 9. Synbiotics.
1. The role of dysbiosis of gut microbiota in the pathogenesis of PCOS
The human gut microbiota comprises viruses, fungi, parasites, archaea, and bacteria that have adapted to live on the mucosal surface of the intestine or in its lumen [11]. A vast majority of the literature in this field is related to the bacterial component of the microbiota in PCOS [10,12-14]. The human gut bacterial microbiota is largely acquired around
www.ogscience.org 15
Jim Parker, et al. Dysbiosis and the pathogenesis of PCOS
Identification
Records identified through database searching (n=683)
PubMed (n=336)
Scopus (n=311)
Cochrane (n=36)
Records screened (n=552)
Screening Included
Full-text articles assessed for eligibility (n=40)
Studies included in qualitative synthesis (n=31)
the time of birth and stabilizes at approximately 3 years of age [15]. The gut microbiota comprises up to 1,000 different species of bacteria, the majority of which belong to the Firmicutes and Bacteroidetes phyla. There is immense interindividual variation in the taxonomic composition of the gut microbiota, even among healthy individuals; however, the function of the microbiota is similar for all individuals [16]. Humans have evolved an intimate symbiosis with the gut microbiota, which is known to influence the immune system, inflammatory pathways, gastrointestinal epithelial barrier function, endocrine system, and host metabolism [17,18]. These effects on human physiology and metabolism have been found to play a potential role in multiple diseases, including PCOS [10,12,13].
The gut microbiota is relatively stable; however, it may undergo changes due to lifestyle, environmental chemicals, age, antibiotic use, stress, and dietary variation [15,19]. It is necessary to consider antecedents, attributes, and consequences of dysbiosis in order to appreciate the impact of changes in the microbiota on human health [20]. Lifestyle and environmental factors may impair the balance of the microbiota, resulting in compositional and functional alterations [19,21]. This imbalance in the taxonomic composition of the gut microbiota is often referred to as dysbiosis [20,22].
Duplicate records removed (n=131)
Records excluded after title/ abstract screening (n=512)
Full-text articles excluded (n=9)
Not met inclusion criteria (n=8)
Non-English language (n=1)
Studies examining the association between human disease and dysbiosis reveal wide heterogeneity in microbiota profiles, which may be due to confounding host variables, such as alcohol consumption, bowel movement quality, recent antibiotic use, and other physiological and lifestyle characteristics [23]. In addition, the gut microbiota contains numerous species of bacteria that may confer the same benefit to its host, or perform the same function as other species, so that the loss of some species or strains can be compensated for by other species that can perform the same function. This “functional redundancy” may explain why different microbiota compositions are found in individuals with the same pathological condition, such as PCOS [24].
Changes in diet can rapidly and reproducibly shift the composition and diversity of the microbiota [19]. A Western diet is associated with a depletion of bacterial taxa, resulting in reduced alpha diversity, which is a measure of species diversity or richness [25]. A reduction in alpha diversity, which is considered a feature of dysbiosis, could lead to a loss of functional redundancy, resulting in an imbalanced microbiota and pathological changes in the host [24]. Compositional changes arising from reduced alpha diversity often result in a shift toward a greater abundance of pathobionts or proinflammatory bacteria. Reduced alpha diversity is also associ-
www.ogscience.org 16 Vol. 65, No. 1, 2022
Fig. 1. PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow diagram of study selection.
ated with numerous diseases common in Western countries, including inflammatory bowel disease, type 2 diabetes, colorectal cancer, and obesity [26-28]. The dysbiosis of gut microbiota theory proposes that diet-induced dysbiosis triggers the development of PCOS [10].
2. Dysbiosis
We identified 14 human studies that compared the microbiota of patients diagnosed with PCOS with a variety of control groups [29-42]. Ten of the 14 studies reported reduced alpha diversity in the PCOS group compared to the control populations [29-31,35-40,42]. One study reported significant compositional changes between the PCOS and control groups before and after probiotic administration; however, it did not report alpha diversity [31]. Three studies did not find any significant differences in alpha diversity [32-34]. Two of these studies included less than 17 women with PCOS and were statistically underpowered to detect a difference [32,33]. The third study showed decreased beta diversity of PCOS microbiomes and significant differences in species abundance that were found to be related to functional alterations in bile acid metabolism [34]. Taken together, these studies suggest that compositional changes in the gastrointestinal microbiome and dysbiosis are likely to play a significant role in the pathogenesis of PCOS in some women.
Although reduced alpha diversity has been consistently observed in women with PCOS, no single bacterium or causal core change has been identified [32,36-38,42]. Some studies support an expansion of species associated with mucosal inflammation and induction of high levels of pro-inflammatory cytokines and chemokines, such as Prevotella and Escherichia coli, and other LPS-producing gram-negative bacteria [29,37]; however, other studies have identified different compositional changes [36,42]. The lack of standardization of host variables, such as diet, geographic variation and rates of obesity in case and control subjects, functional redundancy, or differences in microbiota assessment techniques, may account for the observed differences [23,24,43].
A dysbiotic microbiota in PCOS may perpetuate and exacerbate systemic dissemination of inflammatory mediators and bacterial products such as LPS, which may in turn modulate the PCOS phenotype by inducing metabolic disturbances, leading to chronic inflammation, insulin resistance (IR), and increased androgen secretion [10,14,37]. No study has concurrently assessed temporal variation in the gut microbiota,
body mass index (BMI), and androgen levels in obese or lean women with PCOS. Indeed, this type of study would be difficult in humans, as many of these factors are non-mutually exclusive. A recent Cochrane review revealed that lifestyle changes associated with modest weight loss were associated with lower male hormone levels and reduced hirsutism, suggesting that weight loss may significantly affect factors contributing to the development of PCOS [44]. Determining whether changes in the microbiota are a cause or effect of obesity, androgen levels, or PCOS is not clinically important if a change in diet and lifestyle results in improvement in all these factors.
Zhao et al. [13] recently reviewed the role of gut microbiota in the pathogenesis of PCOS. They identified several other possible mechanisms in which the gut microbiota may be involved in the pathogenesis of PCOS, in addition to dysbiosisrelated increased gut mucosal permeability. These include processes involving increased energy absorption, multiple possible effects related to alterations of short-chain fatty acid metabolism, changes in bile acid metabolism that affect glucose and lipid metabolism and inflammation, multiple physiological effects of altered choline metabolism pathways, and modulation of gastrointestinal hormones involved in the gut-brain interaction [13]. They suggested that a greater understanding of the diverse microbial metabolic pathways involved in the pathogenesis of PCOS may open the way for more targeted treatments with prebiotics, probiotics, traditional Chinese medicine, and fecal microbiota transplantation.
Rizk et al. [43] reviewed a range of metabolites associated with gastrointestinal dysbiosis, including host-produced metabolites (lactate, trimethylamine N-oxide, and primary bile acids), microbiota-related metabolites (short-chain fatty acids and secondary bile acids), and targeted metabolomic studies. They concluded that our current understanding is limited by significant methodological difficulties, lack of fecal metabolomic studies, and correlational studies between gut metabolites and specific microbial species and strains [43]. Taken together, this preliminary research on the gut microbiota supports the role of dysbiosis in the pathogenesis of PCOS and suggests that there may be multiple mechanistic pathways that are possible, depending on the specific genetic, dietary, environmental, and microbiota characteristics of each individual. When considered within the broader definition of dysbiosis, which includes functional alterations to host
www.ogscience.org 17
Jim Parker, et al. Dysbiosis and the pathogenesis of PCOS
physiology and metabolism [18], in addition to imbalances in the taxonomic composition of the microbiota, it is likely that dysbiosis plays a significant role in the pathogenesis of PCOS in many women with this syndrome.
Dysbiosis of gut microbiota theory proposes two key pathophysiological conditions [10]. First, a high-fat, high-glycemic low-fiber diet results in dysbiosis and release of LPS from the cell wall of gram-negative bacteria, which can traverse the intestinal wall and act as an immunostimulant. Second, dietinduced dysbiosis causes increased mucosal permeability, facilitating the transfer of LPS from the bowel lumen to the circulation, initiating inflammation secondary to metabolic endotoxemia. Several studies have investigated these pathophysiological links in women with PCOS by investigating LPS, LPS-BP, and serum zonulin levels [45-49]. Dysbiosis theory also suggests that treatment with prebiotics, probiotics, and synbiotics may help restore eubiosis, reverse pathophysiological changes, and improve the biochemical and clinical features of PCOS.
3. Lipopolysaccharide
González et al. [45] performed a cross-sectional study to investigate the impact of a single episode of high saturated fat ingestion on circulating LPS, tumor necrosis factor alpha (TNF α ), mononuclear cell (MNC) toll-like receptor-4 (TLR4), and suppressor of cytokine signaling-3 (SOCS-3] in a group of obese and lean women with PCOS compared with matched control groups. All women had similar baseline LPS levels. The data showed increases in LPS and TLR-4 related inflammation in obese patients, with the highest increases in obese women with PCOS. Lean women had no increase in LPS levels, regardless of whether they had PCOS. The investigators commented that the observed increases in LPS and TLR-4 inflammation may be an obesity-related phenomenon that is worsened by PCOS [45]. This finding may also reflect the fact that obese women with PCOS and obese women without PCOS have a similar dysbiosis-related response to a high-fat diet challenge. Both obese and lean women with PCOS and obese women without PCOS had lipid-induced increases in TNFα and SOC-3 gene expression compared with lean controls. The investigators concluded that lipid-induced inflammation may be a potential mechanism of IR in PCOS independent of obesity [45].
A cross-sectional study of 144 women found elevated markers of endotoxemia in women with PCOS compared
with healthy ovulatory women. Women with PCOS had significantly higher mean LPS (P=0.045), LPS to high-density lipoprotein ratio ( P =0.007), and LPS-BP ( P =0.01) [50]. All measures of endotoxemia correlated independently and positively with the inflammatory markers and ovarian volume. Taken together, the findings of these two studies suggest that women with PCOS may exhibit a pro-inflammatory state related to elevated markers of endotoxemia, including LPS and LPS-BP [45,50].
4. Lipopolysaccharide-binding protein
Zhu et al. [46] performed a large cross-sectional study of 238 patients, investigating fasting LPS-BP levels in a mixed group of lean and obese women with PCOS compared with age-matched controls. They reported significantly increased levels of LPS-BP in both lean and obese patients with PCOS compared to age-matched controls. LPS-BP is synthesized by hepatocytes and intestinal epithelial cells in response to inflammatory cytokines [47]. LPS-BP has a dual concentration-dependent role in monocyte-mediated inflammatory responses. At low concentrations, LPS-BP enhances LPSinduced activation of MNCs. In contrast, high levels of LPS and other endotoxins stimulate an acute-phase rise in LPSBP, which inhibits the LPS-induced inflammatory response of monocytes [47]. This appears to be a protective mechanism to prevent an overwhelming inflammatory response to high levels of bacterial endotoxins. In addition, Zhu et al. [46] reported increased levels of IR associated with elevated levels of LPS-BP in women with PCOS. The investigators proposed that endotoxemia stimulated an increase in LPS-BP, and subsequent activation of monocyte-induced inflammation may stimulate hyperinsulinemia. The combined findings of González et al. [45] and Zhu et al. [46] support the dysbiosis hypothesis that endotoxemia may result in increased LPS and LPS-BP, which bind with TLR-4 on monocytes and stimulate systemic inflammation and hyperinsulinemia. Although these initial studies support the involvement of dysbiosis in the pathogenesis of PCOS, further studies are required to investigate the significance of this mechanism.
5. Zonulin and increased mucosal permeability
Zonulin was initially discovered in 2000 as an endogenous human analog to the Vibrio cholerae-derived zonula occludens toxin (ZOT), which was found to induce an enterotoxic increase in intestinal permeability in rabbit and non-
www.ogscience.org 18 Vol. 65, No. 1, 2022
human primate intestinal epithelia [51,52]. Zonulin is a prehaptoglobin protein that regulates small intestinal permeability through its effects on tight junctions (TJ), which are located between gastrointestinal epithelial cells [53]. Small intestinal bacterial membrane-derived LPS, serum inflammatory mediators, and immune cells can stimulate zonulin release [54]. Elevated serum zonulin levels have also been found to correlate with increased intestinal permeability, as demonstrated by the lactulose-mannitol (LA/MA) test [55]. Serum zonulin has subsequently been used as a biomarker for intestinal permeability using a variety of commercially available assay kits.
Zonulin has been extensively studied in celiac and autoimmune diseases [56]. More recent studies show that elevated zonulin levels and presumed altered intestinal permeability have also been observed in a range of other conditions, including obesity, type 2 diabetes, and NAFLD, which are all associated with PCOS [57-59]. A number of studies have investigated the role of zonulin in PCOS using a variety of commercially available ELISA kits [36,56-58]. The following discussion summarizes the results of the identified studies and shows the zonulin assays that are used in parentheses.
A case-control study of 78 women with PCOS and 63 BMIand age-matched controls found significantly higher serum zonulin levels in women with PCOS (P=0.022) (ELISA Kit, Immundiagnostik AG, Bensheim, Germany) [48]. Zonulin levels were also significantly correlated with homeostatic model assessment for IR (HOMA-IR), insulin sensitivity index, and more severe menstrual disorders. A second case-control study of 90 women with PCOS and 45 BMI and age-matched controls found significantly higher zonulin levels in the PCOS group than in controls (P<0.01) (ELISA-unspecified) [49]. Zonulin levels were also positively correlated with HOMA-IR in women with PCOS [49].
Lingaiah et al. [60] reported serum zonulin levels in 104 women from Finland diagnosed with PCOS as part of a longitudinal birth cohort study, compared with 203 BMI-matched non-PCOS controls. All women were aged 46 years at the time of the study. Approximately two-thirds of the study group was diagnosed by questionnaire at the age of 31 years based on a history of oligomenorrhea and hirsutism and one-third at the age of 46 years were diagnosed with PCOS based on a self-report. The investigators reported comparable serum levels of zonulin in both the study and control populations (128.0±17.0 vs. 130.9±14.0 ng/mL, P =0.13) (ELISA
Kit, Immundiagnostik AG). Serum zonulin levels were correlated with BMI, IR, and inflammatory markers [60]. The generalizability of the results of this study is limited by the methodology and similarity of the study and control groups. All patients were aged 46 years and were diagnosed using a questionnaire based on menstrual symptoms and hirsutism without biochemical or ultrasound assessment, and there was no contemporaneous assessment of the microbiome for the presence of dysbiosis. The study group may have been more likely to have mild PCOS and, therefore, were more similar to the control group. Metabolic profiles were comparable in the study and control groups, which may reflect increased age of subjects and the possibility that women may present more similarities with advancing age regarding metabolism.
A small pilot study of 25 women with PCOS and 19 controls reported significantly increased serum zonulin levels in women with PCOS compared to controls (P=0.006) (ELIZAunspecified) [36]. The significance of these findings is limited by the small sample size of the study and the inclusion of 42% of participants with mild PCOS who did not have hyperandrogenemia [36]. Another study did not find a significant difference in serum zonulin levels between women with PCOS and controls [61]. Zonulin levels were found to be low in both groups (PCOS=43.5 ng/mL and control=42.9 ng/mL, P=0.893) (Elabscience Biotechnology Co., Texas, USA) [61]. This study included 45 women with PCOS and 17 controls and was terminated early due to closure of the recruitment hospital. The diagnostic criteria for PCOS were not reported, and the BMI of the PCOS and control groups were both within the normal range (BMI <25 kg/m2). In addition, there were no significant differences in metabolic parameters between subjects and controls (low-density lipoprotein, highdensity lipoprotein, triglycerides, C-reactive protein, HOMAIR) [61].
Although the combined results of these initial studies support the hypothesis that some women with PCOS have elevated zonulin levels, studies in selected subsets have reported conflicting results. These studies highlight the need for further rigorous investigation of the role of zonulin and increased intestinal permeability in women with PCOS.
Studies investigating the validity of the relationship between elevated serum zonulin and increased intestinal permeability have been questioned as a result of recently identified methodological inconsistencies in the results obtained from
www.ogscience.org 19
Jim Parker, et al. Dysbiosis and the pathogenesis of PCOS
some of the commercially available testing assays [62-64].
Ajamian et al. [62] performed a comparative study of two assays with recombinant zonulin protein using immunoprecipitation, mass spectrometry, and gel electrophoresis.The findings indicate that commercial zonulin assays did not detect the actual zonulin protein prehaptoglobin-2. Comparative assays suggested that complement C3 and haptoglobin were the likely proteins detected by the assay kits examined [62]. Although recent comparative studies have increased concerns about the validity of the currently available test kits, there is disagreement regarding the identity of the alternative proteins that are being detected by individual assays [62-64]. Scheffler et al. [63] suggested that some commercial kits identified the same target proteins and Ajamian et al. [62] found that these same assays identified different proteins. Both complement-associated and haptoglobin proteins share similar homology and may also modulate zonulin production and intestinal barrier integrity [53,62,65,66]. Although the commercial kits that were examined may not help detect the actual zonulin protein, they may still contribute to obtaining results that reflect associated molecular biomarkers of intestinal permeability [53,65,66]. Nevertheless, the validity and predictive value of current assays require clarification. These methodological inconsistencies also raise concerns regarding the use of zonulin testing in clinical practice. More specific ELISA detection kits should be developed using reliable monoclonal antibodies against recombinant zonulin/prehaptoglobin-2 [62]. In the meantime, the more invasive LA/MA test should be used in experimental studies to investigate intestinal barrier integrity. The effects of dysbiosis could also be investigated using LPS, LPS-BP, inflammatory mediators, and measures of immune function as surrogate biomarkers for intestinal barrier dysfunction.
6. Role of prebiotics, probiotics, and synbiotics in the treatment of women with
PCOS
Following the proposal that dysbiosis may be a contributor to the pathogenesis of PCOS, it has been hypothesized that modification of the balance of the gastrointestinal microbiota has the potential to be an effective treatment. This has resulted in a large number of studies investigating the therapeutic effects of prebiotics, probiotics, and synbiotics. We identified nine systematic reviews and meta-analyses that have synthesized results from 17 randomized controlled trials (RCTs) published between 2017 and 2021 [67-75]. The focus
of individual systematic reviews has varied and has included outcomes related to anthropomorphic, biochemical, hormonal, inflammatory indices, oxidative stress, and IR. It is not possible to systematically synthesize these reviews due to the heterogeneity of the study aims and reported outcomes. The following sections provide a narrative review of the conclusions of these studies. More detailed results can be obtained from the individual reports.
7. Prebiotics
Prebiotics are substances that are selectively utilized by host microorganisms, conferring health benefits to the host [76]. Substances that are widely considered to meet this definition include inulin-type fructans, galactooligosaccharides, and lactulose [77]. All three of these compounds have demonstrated the capacity to significantly shift the GI microbial ecosystem in beneficial ways [78-90]. Although we were unable to identify specific publications on any of these three prebiotics individually in women with PCOS, other evidence suggests that they could be useful therapeutic options.
Inulin-type fructans have been found to decrease endotoxin absorption [81], enhance intestinal integrity, and improve both blood glucose regulation and fasting insulin in patients with type 2 diabetes and prediabetes [82]. Galactooligosaccharides have been found to improve metabolic markers (e.g., reductions in plasma insulin and C-reactive protein [CRP] levels) in patients with metabolic syndrome [83]. Lactulose has been found to decrease endotoxin absorption [84,85] and improve intestinal hyperpermeability, as well as blood glucose levels and insulin response in type 2 diabetics [86]. More research is needed to evaluate the impact of these prebiotics in PCOS, as the only research to date has been limited to examining prebiotics as components of synbiotic preparations (discussed below), rather than prebiotics in isolation.
Resistant starch (RS) refers to the portion of starch and starch products in foods and food products that resist digestion in the upper gastrointestinal tract [87]. These starches share some characteristics with the prebiotics discussed above, such as indigestibility and a selective impact on the GI ecosystem. Resistant starches have been found to increase the concentrations of butyrate-producing bacteria and bifidobacteria, which result in beneficial shifts in the GI microbiota [88,89]. Studies have also found a consistent beneficial impact of RS on glycemic control and systemic inflammation, both of which are important components in the
www.ogscience.org 20 Vol. 65, No. 1, 2022
pathophysiology of PCOS [90]. A recent RCT compared 20 g per day of RS in women with PCOS to placebo [91]. After 3 months, there were significant improvements in serum triglycerides (P=0.001), total cholesterol (P<0.001), low-density lipoprotein cholesterol ( P <0.001), hsCRP ( P =0.004), free testosterone (P=0.01), menstrual cycle regularity (P<0.001), and hirsuitism ( P <0.001) in women treated with RS [91]. Another publication based on this same trial reported significant reductions in waist circumference, hip circumference, weight, and BMI in the treatment group (all P<0.001) [92]. Unfortunately, this study did not evaluate the impact of RS intervention on the GI microbiota composition; therefore, the impact of RS on the ecosystem itself in women with PCOS is currently unknown.
8. Probiotics
Probiotics are defined as live microbes that, when ingested in adequate amounts, confer health benefits [93]. To date, most of the probiotics that have been studied originate from the genera Lactobacillus and Bifidobacterium . However, a shift has recently been noted [94], in which a range of probiotic preparations and strains have been evaluated in the treatment of PCOS, with a number of recent meta-analyses published in this area showing consistent positive results on a range of PCOS-related parameters [67-70].
In one of the most rigorous and comprehensive metaanalyses, Tabrizi et al. [71] reviewed RCTs of probiotics in women with PCOS to determine their effectiveness on clinical symptoms, glycemic control, weight loss, hormonal and lipid profiles, and markers of inflammation. A wide range of probiotic preparations and dosages were used in the 11 RCTs included in the review. Probiotic supplementation significantly decreased body weight ( P= 0.01), BMI ( P< 0.02), fasting plasma glucose (P<0.001), HOMA-IR (P<0.001), insulin levels (P<0.001), hirsutism (P<0.001), total testosterone levels ( P< 0.001), VLDL cholesterol ( P< 0.001), triglycerides (P<0.001), and C-reactive protein (P<0.001) [71]. Quantitative insulin sensitivity check index (P<0.01), total antioxidant capacity (P<0.001), glutathione levels (P=0.04), and SHBG concentrations (P=0.01) were all found to significantly increase following probiotic supplementation [71]. These data also support the role of dysbiosis in the pathogenesis of PCOS.
There are numerous potential mechanisms of action that may explain the observed beneficial effects of probiotics in
women with PCOS. These include antioxidant [95], antiinflammatory [96], gastrointestinal healing [97], anti-LPS [98], sex hormone-altering [31], and metabolism-regulating actions [99], in addition to the ability of some probiotic strains to significantly impact microbiota composition [32,100,101]. The actions and characteristics of probiotics are strain-specific. The efficacy and mechanisms of action of one probiotic strain, or a combination of strains, cannot be extrapolated to other strains [102]. Taken together, these data suggest that the use of probiotics may be a useful therapeutic adjunct to dietary interventions, as well as other lifestyle interventions, in women with PCOS. Further studies are needed to elucidate strain-specific therapeutic mechanisms of action and to delineate which strains do not confer treatment benefits.
9. Synbiotics
Synbiotics are mixtures of live microorganisms and substrates selectively utilized by host microbes; when consumed, they confer a health benefit to the host [103]. Generally, they contain both probiotic and prebiotic components. Synbiotics can be composed of a wide range of probiotic strains in combination with a variety of prebiotic and prebiotic dosages. As a class of agents, synbiotics have a growing evidence base for the treatment of obesity [104], gestational diabetes [105], type 1 [106], and type 2 diabetes [107]. It is not surprising that synbiotics have also been evaluated in the treatment of women with PCOS, since PCOS is part of this related cluster of metabolic conditions [5,108-110].
A recent RCT investigated the impact of synbiotic preparation on sex hormone profile and glycemic and anthropometric indices in subjects with PCOS. After 8 weeks, subjects who received the synbiotic preparation (Lactobacillus rhamnosus GG in combination with unspecified strains of Bacillus coagulans and Bacillus indicus, as well as the prebiotic inulin) had significant reductions in HOMA-IR, body weight, BMI, waist circumference, fasting blood sugar, insulin, and serum testosterone compared to the placebo group (all P< 0.05) [111]. Darvishi et al. [112] evaluated the efficacy of different synbiotic preparations (containing fructooligosaccharides as the prebiotic and unspecified strains of Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Lactobacillus acidophilus, Bifidobacterium longum, and Streptococcus thermophilus) in the treatment of PCOS. After an 8-week administration period, several metabolic markers were significantly improved, including serum fasting glucose (P=0.02),
www.ogscience.org 21
Jim Parker, et al. Dysbiosis and the pathogenesis of PCOS
HOMA-IR (P=0.001), HDL-cholesterol (P=0.02), BMI (P=0.02), body weight ( P= 0.02), and waist circumference ( P= 0.01), compared to placebo [112].
Given the uniqueness of different synbiotic preparations, which may contain a variety of probiotic strains and different prebiotic compounds, each unique synbiotic preparation needs to be individually evaluated. This is necessary to ascertain both their effectiveness in managing PCOS and their specific mechanisms of action, since it is possible that different synbiotics will act via different mechanisms and that not all synbiotics will be effective [113]. However, research to date suggests that synbiotics are a promising class of agents for the treatment of PCOS. This may be due to several different mechanisms, including microbiota modification, enhanced gastrointestinal integrity, and anti-LPS actions, as previously discussed in the prebiotic and probiotic sections.
Conclusion
Overall, the available research supports the hypothesis that diet-induced dysbiosis of the gastrointestinal microbiota is likely to play a role in the pathogenesis of PCOS. Several lines of emerging evidence suggest that dysbiosis may result in increased gastrointestinal permeability and the development of the observed metabolic, endocrine, and phenotypic features of PCOS. Multiple mechanisms may be involved, in addition to dysbiosis-related permeability changes and LPS-induced systemic inflammation. These include diet-induced microbial metabolic and signaling mechanisms involving luminal nutrient sensing pathways and metabolism of bile acids, hormones, choline, and short-chain fatty acids. These potential mechanisms have not been reviewed in the present study.
It seems likely that a combination of diet and other lifestyle-related factors may be associated with the pathophysiological features of PCOS depending on the composition of the microbiota, microbial metabolism, and underlying genetic predispositions. PCOS represents an opportunity for the early diagnosis of metabolic issues that are predictive of significant future morbidity and mortality. Therefore, the precise details of the wide range of pathogenic mechanisms that may be involved should be the focus of future research on this relevant and common disease. Nevertheless, it is noteworthy that the metabolic, reproductive, and phenotypic features of PCOS are modifiable and reversible in most women with PCOS by
implementing lifestyle-based interventions, regardless of the pathophysiological mechanisms involved.
Conflict of interest
No potential conflict of interest relevant to this article.
Ethical approval
This study did not require approval from the Institutional Review Board because no patient data were included in this study.
Patient consent
Written informed consent and the use of images from patients were not required for publication.
Funding information
None.
References
1. Ding T, Hardiman PJ, Petersen I, Wang FF, Qu F, Baio G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget 2017;8:96351-8.
2. Boyle JA, Cunningham J, O’Dea K, Dunbar T, Norman RJ. Prevalence of polycystic ovary syndrome in a sample of Indigenous women in Darwin, Australia. Med J Aust 2012;196:62-6.
3. Pathak G, Nichter M. Polycystic ovary syndrome in globalizing India: an ecosocial perspective on an emerging lifestyle disease. Soc Sci Med 2015;146:21-8.
4. M. Szmigiera. Proportion of selected age groups of world population in 2021, by region [Internet]. Hamburg (DEU): Statista; c2021 [cited 2021 May 1]. Available form: https://www.statista.com/statistics/265759/worldpopulation-by-age-and-region/.
www.ogscience.org 22 Vol. 65, No. 1, 2022
5. Parker J. Understanding the pathogenesis of polycystic ovary syndrome: a transgenerational evolutionary adaptation to lifestyle and the environment. J ACNEM 2020;39:18-26.
6. Charifson MA, Trumble BC. Evolutionary origins of polycystic ovary syndrome: an environmental mismatch disorder. Evol Med Public Health 2019;2019:50-63.
7. Azziz R, Dumesic DA, Goodarzi MO. Polycystic ovary syndrome: an ancient disorder? Fertil Steril 2011;95:1544-8.
8. Teede H, Misso M, Costello M, Dokras A, Laven J, Moran L, et al. International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2018 [Internet]. NHMRC Canberra (ACT); c2018 [cited 2020 Out 15]. Available form: https:// www.monash.edu/__data/assets/pdf_file/0004/ 1412644/PCOS_Evidence-Based-Guidelines_ 20181009.pdf.
9. Benton ML, Abraham A, LaBella AL, Abbot P, Rokas A, Capra JA. The influence of evolutionary history on human health and disease. Nat Rev Genet 2021;22:26983.
10. Tremellen K, Pearce K. Dysbiosis of gut microbiota (DOGMA)--a novel theory for the development of polycystic ovarian syndrome. Med Hypotheses 2012;79: 104-12.
11. Nuriel-Ohayon M, Neuman H, Koren O. Microbial changes during pregnancy, birth, and infancy. Front Microbiol 2016;7:1031.
12. Yurtdaş G, Akdevelioğlu Y. A new approach to polycystic ovary syndrome: the gut microbiota. J Am Coll Nutr 2020;39:371-82.
13. Zhao X, Jiang Y, Xi H, Chen L, Feng X. Exploration of the relationship between gut microbiota and polycystic ovary syndrome (PCOS): a review. Geburtshilfe Frauenheilkd 2020;80:161-71.
14. He FF, Li YM. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J Ovarian Res 2020;13:73.
15. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature 2012;486:222-7.
16. Human Microbiome Project Consortium. Structure,
function and diversity of the healthy human microbiome. Nature 2012;486:207-14.
17. Tuddenham S, Sears CL. The intestinal microbiome and health. Curr Opin Infect Dis 2015;28:464.
18. García-Montero C, Fraile-Martínez O, Gómez-Lahoz AM, Pekarek L, Castellanos AJ, Noguerales-Fraguas F, et al. Nutritional components in western diet versus mediterranean diet at the gut microbiota-immune system interplay. Implications for health and disease. Nutrients 2021;13:699.
19. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559-63.
20. Perez NB, Dorsen C, Squires A. Dysbiosis of the gut microbiome: a concept analysis. J Holist Nurs 2020;38:223-32.
21. Moschen AR, Wieser V, Tilg H. Dietary factors: major regulators of the gut’s microbiota. Gut Liver 2012;6:411-6.
22. Hawrelak JA, Myers SP. The causes of intestinal dysbiosis: a review. Altern Med Rev 2004;9:180-97.
23. Vujkovic-Cvijin I, Sklar J, Jiang L, Natarajan L, Knight R, Belkaid Y. Host variables confound gut microbiota studies of human disease. Nature 2020;587:448-54.
24. Moya A, Ferrer M. Functional redundancy-induced stability of gut microbiota subjected to disturbance. Trends Microbiol 2016;24:402-13.
25. Partula V, Mondot S, Torres MJ, Kesse-Guyot E, Deschasaux M, Assmann K, et al. Associations between usual diet and gut microbiota composition: results from the Milieu Intérieur cross-sectional study. Am J Clin Nutr 2019;109:1472-83.
26. Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006;55:205-11.
27. O’Brien CL, Pavli P, Gordon DM, Allison GE. Detection of bacterial DNA in lymph nodes of Crohn’s disease patients using high throughput sequencing. Gut 2014;63:1596-606.
28. Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J 2012;6:320-9.
www.ogscience.org 23
Jim Parker, et al. Dysbiosis and the pathogenesis of PCOS
29. Mammadova G, Ozkul C, Yilmaz Isikhan S, Acikgoz A, Yildiz BO. Characterization of gut microbiota in polycystic ovary syndrome: findings from a lean population. Eur J Clin Invest 2021;51:e13417.
30. Haudum C, Lindheim L, Ascani A, Trummer C, Horvath A, Münzker J, et al. Impact of short-term isoflavone intervention in polycystic ovary syndrome (PCOS) patients on microbiota composition and metagenomics. Nutrients 2020;12:1622.
31. Zhang J, Sun Z, Jiang S, Bai X, Ma C, Peng Q, et al. Probiotic bifidobacterium lactis V9 regulates the secretion of sex hormones in polycystic ovary syndrome patients through the gut-brain axis. mSystems 2019;4:e00017-19.
32. Insenser M, Murri M, Del Campo R, Martínez-García MÁ, Fernández-Durán E, Escobar-Morreale HF. Gut microbiota and the polycystic ovary syndrome: influence of sex, sex hormones, and obesity. J Clin Endocrinol Metab 2018;103:2552-62.
33. Eyupoglu ND, Ergunay K, Acikgoz A, Akyon Y, Yilmaz E, Yildiz BO. Gut Microbiota and oral contraceptive use in overweight and obese patients with polycystic ovary syndrome. J Clin Endocrinol Metab 2020;105:dgaa600.
34. Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med 2019;25:122533.
35. Jobira B, Frank DN, Pyle L, Silveira LJ, Kelsey MM, Garcia-Reyes Y, et al. Obese adolescents with PCOS have altered biodiversity and relative abundance in gastrointestinal microbiota. J Clin Endocrinol Metab 2020;105:e2134-44.
36. Lindheim L, Bashir M, Münzker J, Trummer C, Zachhuber V, Leber B, et al. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): a pilot study. PLoS One 2017;12:e0168390.
37. Liu R, Zhang C, Shi Y, Zhang F, Li L, Wang X, et al. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front Microbiol 2017;8:324.
38. Torres PJ, Siakowska M, Banaszewska B, Pawelczyk L, Duleba AJ, Kelley ST, et al. Gut microbial diversity in women with polycystic ovary syndrome correlates
with hyperandrogenism. J Clin Endocrinol Metab 2018;103:1502-11.
39. Chen F, LAI Z, XU Z. Analysis of the gut microbial composition in polycystic ovary syndrome with acne. Zigong Matern Child Heal Hosp 2019;35:2246-51.
40. Zhou L, Ni Z, Cheng W, Yu J, Sun S, Zhai D, et al. Characteristic gut microbiota and predicted metabolic functions in women with PCOS. Endocr Connect 2020;9:63-73.
41. Garcia-Beltran C, Malpique R, Carbonetto B, González-Torres P, Henares D, Brotons P, et al. Gut microbiota in adolescent girls with polycystic ovary syndrome: effects of randomized treatments. Pediatr Obes 2021;16:e12734.
42. Zeng B, Lai Z, Sun L, Zhang Z, Yang J, Li Z, et al. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): a pilot study. Res Microbiol 2019;170:43-52.
43. Rizk MG, Thackray VG. Intersection of polycystic ovary syndrome and the gut microbiome. J Endocr Soc 2020;5:bvaa177.
44. Lim SS, Hutchison SK, Van Ryswyk E, Norman RJ, Teede HJ, Moran LJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2019;3:CD007506.
45. González F, Considine RV, Abdelhadi OA, Acton AJ. Saturated fat ingestion promotes lipopolysaccharidemediated inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab 2019;104:934-46.
46. Zhu Q, Zhou H, Zhang A, Gao R, Yang S, Zhao C, et al. Serum LBP Is associated with insulin resistance in women with PCOS. PLoS One 2016;11:e0145337.
47. Gutsmann T, Müller M, Carroll SF, MacKenzie RC, Wiese A, Seydel U. Dual role of lipopolysaccharide (LPS)binding protein in neutralization of LPS and enhancement of LPS-induced activation of mononuclear cells. Infect Immun 2001;69:6942-50.
48. Zhang D, Zhang L, Yue F, Zheng Y, Russell R. Serum zonulin is elevated in women with polycystic ovary syndrome and correlates with insulin resistance and severity of anovulation. Eur J Endocrinol 2015;172:2936.
49. Anik Ilhan G, Yildizhan B. Evaluation of zonulin,
www.ogscience.org 24 Vol. 65, No. 1, 2022
a marker of intestinal permeability as a novel biomarker in polycystic ovary syndrome. Fertil Steril 2018;110:e117.
50. Banaszewska B, Siakowska M, Chudzicka-Strugala I, Chang RJ, Pawelczyk L, Zwozdziak, et al. Elevation of markers of endotoxemia in women with polycystic ovary syndrome. Hum Reprod 2020;35:2303-11.
51. Fasano A, Uzzau S, Fiore C, Margaretten K. The enterotoxic effect of zonula occludens toxin on rabbit small intestine involves the paracellular pathway. Gastroenterology 1997;112:839-46.
52. Larkin M. Out with the jab, in with the painless pills. Lancet 1997;349:1676.
53. Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 2011;91:151-75.
54. Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol 2012;10:1096-100.
55. Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006;55:1443-9.
56. Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci 2012;1258: 25-33.
57. Kim AS, Ko HJ. Plasma concentrations of zonulin are elevated in obese men with fatty liver disease. Diabetes Metab Syndr Obes 2018;11:149-57.
58. Aasbrenn M, Lydersen S, Farup PG. Changes in serum zonulin in individuals with morbid obesity after weight-loss interventions: a prospective cohort study. BMC Endocr Disord 2020;20:108.
59. Yuan JH, Xie QS, Chen GC, Huang CL, Yu T, Chen QK, et al. Impaired intestinal barrier function in type 2 diabetic patients measured by serum LPS, zonulin, and IFABP. J Diabetes Complications 2021;35:107766.
60. Lingaiah S, Arffman RK, Morin-Papunen L, Tapanainen JS, Piltonen T. Markers of gastrointestinal permeability and dysbiosis in premenopausal women with PCOS: a case-control study. BMJ Open 2021;11:e045324.
61. Cetin Z, Kosem A, Can B, Baser O, Catak M, Turhan T, et al. Serum zonulin level is not elevated in patients with polycystic ovary syndrome without metabolic syn-
drome. Arch Gynecol Obstet 2019;300:1785-90.
62. Ajamian M, Steer D, Rosella G, Gibson PR. Serum zonulin as a marker of intestinal mucosal barrier function: may not be what it seems. PLoS One 2019;14:e0210728.
63. Scheffler L, Crane A, Heyne H, Tönjes A, Schleinitz D, Ihling CH, et al. Widely used commercial ELISA for human Zonulin reacts with Complement C3 rather than pre-Haptoglobin 2 [Internet]. NY (USA): CSH; c2017 [cited 2021 Apr 17]. Available from: http://www. biorxiv.org/content/biorxiv/early/2017/06/30/157578. full.pdf.
64. Scheffler L, Crane A, Heyne H, Tönjes A, Schleinitz D, Ihling CH, et al. Widely used commercial elisa does not detect precursor of haptoglobin2, but recognizes properdin as a potential second member of the zonulin family. Front Endocrinol (Lausanne) 2018;9:22.
65. Sina C, Kemper C, Derer S. The intestinal complement system in inflammatory bowel disease: shaping intestinal barrier function. Semin Immunol 2018;37:66-73.
66. Sünderhauf A, Skibbe K, Preisker S, Ebbert K, Verschoor A, Karsten CM, et al. Regulation of epithelial cell expressed C3 in the intestine - relevance for the pathophysiology of inflammatory bowel disease? Mol Immunol 2017;90:227-38.
67. Liao D, Zhong C, Li C, Mo L, Liu Y. Meta-analysis of the effects of probiotic supplementation on glycemia, lipidic profiles, weight loss and C-reactive protein in women with polycystic ovarian syndrome. Minerva Med 2018;109:479-87.
68. Heshmati J, Farsi F, Yosaee S, Razavi M, Rezaeinejad M, Karimie E, et al. The effects of probiotics or synbiotics supplementation in women with polycystic ovarian syndrome: a systematic review and meta-analysis of randomized clinical trials. Probiotics Antimicrob Proteins 2019;11:1236-47.
69. Hadi A, Moradi S, Ghavami A, Khalesi S, Kafeshani M. Effect of probiotics and synbiotics on selected anthropometric and biochemical measures in women with polycystic ovary syndrome: a systematic review and meta-analysis. Eur J Clin Nutr 202074:543-7.
70. Shamasbi SG, Ghanbari-Homayi S, Mirghafourvand M. The effect of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indices in women with polycystic ovary syndrome: a systematic review and
www.ogscience.org 25
Jim Parker, et al. Dysbiosis and the pathogenesis of PCOS
meta-analysis. Eur J Nutr 2020;59:433-50.
71. Tabrizi R, Ostadmohammadi V, Akbari M, Lankarani KB, Vakili S, Peymani P, et al. The effects of probiotic supplementation on clinical symptom, weight loss, glycemic control, lipid and hormonal profiles, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Probiotics Antimicrob Proteins 2019 Jun 4 [Epub]. https://doi. ory/10.1007/s12602-019-09559-0.
72. Kazemi A, Soltani S, Ghorabi S, Keshtkar A, Daneshzad E, Nasri F, et al. Effect of probiotic and synbiotic supplementation on inflammatory markers in health and disease status: a systematic review and meta-analysis of clinical trials. Clin Nutr 2020;39:789-819.
73. Cozzolino M, Vitagliano A, Pellegrini L, Chiurazzi M, Andriasani A, Ambrosini G, et al. Therapy with probiotics and synbiotics for polycystic ovarian syndrome: a systematic review and meta-analysis. Eur J Nutr 2020;59:2841-56.
74. Miao C, Guo Q, Fang X, Chen Y, Zhao Y, Zhang Q. Effects of probiotic and synbiotic supplementation on insulin resistance in women with polycystic ovary syndrome: a meta-analysis. J Int Med Res 2021;49:3000605211031758.
75. Li Y, Tan Y, Xia G, Shuai J. Effects of probiotics, prebiotics, and synbiotics on polycystic ovary syndrome: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2021 Jul 21 [Epub]. https://doi.ory/10.1080/1040 8398.2021.1951155.
76. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 2017;14:491-502.
77. Hawrelak J. Prebiotics, synbiotics and colonic foods. In: Murray M, Pizzorno J, editors. Textbook of natural medicine. 5th ed. Amsterdam: Elsevier; 2020. p.797808.
78. Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995;108:975-82.
79. Ballongue J, Schumann C, Quignon P. Effects of lactu-
lose and lactitol on colonic microflora and enzymatic activity. Scand J Gastroenterol Suppl 1997;222:41-4.
80. Vulevic J, Drakoularakou A, Yaqoob P, Tzortzis G, Gibson GR. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr 2008;88:1438-46.
81. Dehghan P, Gargari BP, Jafar-Abadi MA, Aliasgharzadeh A. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized-controlled clinical trial. Int J Food Sci Nutr 2014;65:117-23.
82. Wang L, Yang H, Huang H, Zhang C, Zuo HX, Xu P, et al. Inulin-type fructans supplementation improves glycemic control for the prediabetes and type 2 diabetes populations: results from a GRADE-assessed systematic review and dose-response meta-analysis of 33 randomized controlled trials. J Transl Med 2019;17:410.
83. Vulevic J, Juric A, Tzortzis G, Gibson GR. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J Nutr 2013;143:324-31.
84. Pain JA, Bailey ME. Experimental and clinical study of lactulose in obstructive jaundice. Br J Surg 1986;73: 775-8.
85. Jain L, Sharma BC, Srivastava S, Puri SK, Sharma P, Sarin S. Serum endotoxin, inflammatory mediators, and magnetic resonance spectroscopy before and after treatment in patients with minimal hepatic encephalopathy. J Gastroenterol Hepatol 2013;28:1187-93.
86. Bianchi G, Ronchi M, Marchesini G. Effect of lactulose on carbohydrate metabolism and diabetes mellitus. Scand J Gastroenterol Suppl 1997;222:62-4.
87. Sofi SA, Anjum A, Awsi J. Resistant starch as functional ingredient: a review. Int J Food Sci Nutr 2017;2:195-9.
88. Venkataraman A, Sieber JR, Schmidt AW, Waldron C, Theis KR, Schmidt TM. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 2016;4:33.
89. Bendiks ZA, Knudsen KEB, Keenan MJ, Marco ML. Conserved and variable responses of the gut microbiome to resistant starch type 2. Nutr Res 2020;77:1228.
90. Halajzadeh J, Milajerdi A, Reiner Ž, Amirani E, Kolah-
www.ogscience.org 26 Vol. 65, No. 1, 2022
dooz F, Barekat M, et al. Effects of resistant starch on glycemic control, serum lipoproteins and systemic inflammation in patients with metabolic syndrome and related disorders: a systematic review and metaanalysis of randomized controlled clinical trials. Crit Rev Food Sci Nutr 2020;60:3172-84.
91. Gholizadeh Shamasbi S, Dehgan P, Mohammad-Alizadeh Charandabi S, Aliasgarzadeh A, Mirghafourvand M. The effect of resistant dextrin as a prebiotic on metabolic parameters and androgen level in women with polycystic ovarian syndrome: a randomized, tripleblind, controlled, clinical trial. Eur J Nutr 2019;58:62940.
92. Shamasbi SG, Dehghan P, Charandabi SMA, Aliasgarzadeh A, Mirghafourvand M. Effect of prebiotic on anthropometric indices in women with polycystic ovarian syndrome: a triple-blind, randomized, controlled clinical trial. Iran Red Crescent Med J 2018;20:e67270.
93. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506-14.
94. Perraudeau F, McMurdie P, Bullard J, Cheng A, Cutcliffe C, Deo A, et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res Care 2020;8:e001319.
95. Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J. Probiotics as potential antioxidants: a systematic review. J Agric Food Chem 2015;63:3615-26.
96. Imaoka A, Shima T, Kato K, Mizuno S, Uehara T, Matsumoto S, et al. Anti-inflammatory activity of probiotic Bifidobacterium: enhancement of IL-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of IL-8 secretion in HT-29 cells. World J Gastroenterol 2008;14:2511-6.
97. Guillot CC, Bacallao EG, Dominguez MSC, Garcia MF, Gutierrez PM. Effects of Saccharomyces boulardii in children with chronic diarrhoea, especially cases due to giardiasis. Rev Mex De Puericultura Y Pediatria 1995;2:15.
98. In Kim H, Kim JK, Kim JY, Jang SE, Han MJ, Kim DH.
Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 simultaneously alleviate high-fat diet-induced colitis, endotoxemia, liver steatosis, and obesity in mice. Nutr Res 2019;67:78-89.
99. Kocsis T, Molnár B, Németh D, Hegyi P, Szakács Z, Bálint A, et al. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: a meta-analysis of randomized clinical trials. Sci Rep 2020;10:11787.
100. del Campo R, Garriga M, Pérez-Aragón A, Guallarte P, Lamas A, Máiz L, et al. Improvement of digestive health and reduction in proteobacterial populations in the gut microbiota of cystic fibrosis patients using a Lactobacillus reuteri probiotic preparation: a double blind prospective study. J Cyst Fibros 2014;13:716-22.
101. Odamaki T, Sugahara H, Yonezawa S, Yaeshima T, Iwatsuki K, Tanabe S, et al. Effect of the oral intake of yogurt containing Bifidobacterium longum BB536 on the cell numbers of enterotoxigenic Bacteroides fragilis in microbiota. Anaerobe 2012;18:14-8.
102. Marteau P. Evidence of probiotic strain specificity makes extrapolation of results impossible from a strain to another, even from the same species [Internet]. Ann Gastroentol Hepatol: c2011 [cited 2021 Apr 12]. Available from: http://gastromasterclass2014. s3.amazonaws.com/Session1/Marteau+-+Evidence+of +probiotic+strain+specificity.pdf.
103. Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, et al. The International scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 2020;17:687-701.
104. Hadi A, Alizadeh K, Hajianfar H, Mohammadi H, Miraghajani M. Efficacy of synbiotic supplementation in obesity treatment: a systematic review and meta-analysis of clinical trials. Crit Rev Food Sci Nutr 2020;60:584-96.
105. Ahmadi S, Jamilian M, Tajabadi-Ebrahimi M, Jafari P, Asemi Z. The effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: a randomised, double-blind, placebo-controlled trial. Br J Nutr 2016;116:1394-401.
106. Zare Javid A, Aminzadeh M, Haghighi-Zadeh MH, Jamalvandi M. The effects of synbiotic supplementation on glycemic status, lipid profile, and biomarkers of
www.ogscience.org 27
Jim Parker, et al. Dysbiosis and the pathogenesis of PCOS
oxidative stress in type 1 diabetic patients. A placebocontrolled, double-blind, randomized clinical trial. Diabetes Metab Syndr Obes 2020;13:607-17.
107. Tajabadi-Ebrahimi M, Sharifi N, Farrokhian A, Raygan F, Karamali F, Razzaghi R, et al. A randomized controlled clinical trial investigating the effect of synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Exp Clin Endocrinol Diabetes 2017;125:21-7.
108. Rodgers RJ, Avery JC, Moore VM, Davies MJ, Azziz R, Stener-Victorin E, et al. Complex diseases and co-morbidities: polycystic ovary syndrome and type 2 diabetes mellitus. Endocr Connect 2019;8:R71-5.
109. Zore T, Joshi NV, Lizneva D, Azziz R. Polycystic ovarian syndrome: long-term health consequences. Semin Reprod Med 2017;35:271-81.
110. Wu J, Yao XY, Shi RX, Liu SF, Wang XY. A potential link between polycystic ovary syndrome and non-alcoholic fatty liver disease: an update meta-analysis. Reprod
Health 2018;15:77.
111. Esmaeilinezhad Z, Babajafari S, Sohrabi Z, Eskandari MH, Amooee S, Barati-Boldaji R. Effect of synbiotic pomegranate juice on glycemic, sex hormone profile and anthropometric indices in PCOS: a randomized, triple blind, controlled trial. Nutr Metab Cardiovasc Dis 2019;29:201-8.
112. Darvishi S, Rafraf M, Asghari-Jafarabadi M, Farzadi L. Synbiotic supplementation improves metabolic factors and obesity values in women with polycystic ovary syndrome independent of affecting apelin levels: a randomized double-blind placebo - controlled clinical trial. Int J Fertil Steril 2021;15:51-9.
113. Karimi E, Moini A, Yaseri M, Shirzad N, Sepidarkish M, Hossein-Boroujerdi M, et al. Effects of synbiotic supplementation on metabolic parameters and apelin in women with polycystic ovary syndrome: a randomised double-blind placebo-controlled trial. Br J Nutr 2018;119:398-406.
www.ogscience.org 28 Vol. 65, No. 1, 2022

Nutritional role of polyphenols as a component of a wholefood diet in the management of polycystic ovary syndrome
Jim Parker1 , Jason Hawrelak2 and Felice L Gersh3
Obstetrician, Gynaecologist and Endoscopic Surgeon
Honorary Clinical Senior Lecturer, School of Medicine, University of Wollongong1
Senior Lecturer, College of Health and Medicine, University of Tasmania2
Internal Medicine, University of Arizona College of Medicine, Irvine, California, USA3
ABSTRACT
• Polycystic ovary syndrome (PCOS) affects millions of women worldwide and is thought to be increasing in frequency due to globalisation and the spread of western culture, including the adoption of more Western-style diets. This trend reinforces the view that PCOS is an environmental mismatch disorder where ancient adaptive survival genes become maladaptive due to the effects of modern lifestyle practices.
• In keeping with this emerging paradigm, the International Guidelines recommend lifestyle-based interventions as first-line management for all women diagnosed with PCOS. Diet pattern, diet index and metabolomic studies have identified the common components of a healthy eating pattern.
• The Australian Guide to Healthy Eating recommends that approximately 75% of the food plate is comprised of plant-based wholefood including fruit, vegetables and wholegrains. All of these plant foods contain a range of nutrients, including polyphenols, that have attracted increasing scientific interest because of their possible beneficial effects on human health.
• Most polyphenols undergo extensive gut microbiota-mediated biotransformation prior to being absorbed into the human body. The microbiome is now recognized to play a major role in human health and poor-quality diet and dysbiosis of the microbiota may be a factor in the pathogenesis of PCOS. Emerging evidence suggests that polyphenols have the capacity to beneficially shift the composition of the colonic ecosystem and may be an important dietary component in women with PCOS.
• An understanding of the role of polyphenols obtained from a wholefood diet, may reinforce the value of a healthy diet pattern to women diagnosed with PCOS and contribute to increased compliance and improved outcomes
• This review emphasises the importance of a wholefood diet that contains a range of plant-derived polyphenols in the management of PCOS and the prevention of related metabolic diseases.
Journal of the Australasian College of Nutritional and Environmental Medicine Inc (ACNEM) ACNEM Journal Vol 40 No 2 June 2021
Keywords: polycystic ovary syndrome, wholefood diet, polyphenols, microbiome
Address for correspondence:
Dr Jim Parker
Honorary Clinical Senior Lecturer
School of Medicine
University of Wollongong, NSW, Australia jimparker@ozemail.com.au
ORCID: 0000-0002-5018-5555
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a common metabolic and endocrine problem that affects approximately 10% of women worldwide (1). PCOS is usually diagnosed in adolescence and is the most common cause of anovulatory infertility. Insulin-resistance is a core pathophysiological feature in the majority of women with PCOS (2) and can be caused by a range of dietary factors, including high calorie, high fat and high glycaemic diets (3). Women with PCOS are at increased risk of developing a range of metabolic disorders, including type 2 diabetes, gestational diabetes, metabolic syndrome, non-alcoholic fatty liver disease and cardiovascular disease, all of which are associated with insulin-resistance (4)
PCOS is increasingly being seen in an evolutionary context as an environmental mismatch disorder where ancient genetic adaptive survival metabolic and endocrine pathways become maladaptive when exposed to modern lifestyle practices (5). As a result, PCOS is thought to be increasing in frequency in both the developed and developing world as a more Western-type lifestyle and diet is introduced (6,7). In accordance with this view, the International Guidelines for the management of PCOS have emphasised the importance of lifestyle interventions and dietary modification as the first-line treatment options for all women diagnosed with PCOS (8).
Throughout evolutionary history, humans have likely adapted to a constantly-changing environment by obtaining nutrients from a wide variety of plant and animal food sources (9,10). In order to obtain optimal health, it is necessary to consume a range of essential nutrients from seasonally available dietary foods. These include macronutrients, micronutrients and contingent nutrient factors that contain a range of bioactive components. Contingent nutrients include a variety of compounds that are not essential but may be beneficial to human health (11). Polyphenols comprise a large group of plantderived secondary metabolites that have been found to have beneficial biological and metabolic effects (12). Epidemiological studies suggest that a polyphenol-rich diet may protect against chronic diseases such as diabetes, cardiovascular disease, cancer and PCOS (13).
Journal of the Australasian College of Nutritional and Environmental Medicine Inc (ACNEM) 2 ACNEM Journal Vol 40 No 2 June 2021
The 2013 Australian Dietary Guidelines recommend eating a variety of nutritious foods from the five food groups (14). The guidelines recommend consuming a range of vegetables of different types and colours, fruit and wholegrains. This includes 5-6 standard serves of vegetables (75grams per serve – 100-350kJ), two standard serves of fruit (150grams per serve – 350kJ) and 3-6 servings of wholegrains (500kJ) depending on sex and age. The Australian Guide to Healthy Eating food plate recommends that approximately 75% of the plate is comprised of these plant-based food types. The more recent Canadian Dietary Guidelines recommend that greater than 75% of the food plate be comprised of plant-based foods (figure 1) (15). They recommend, half of the plate are vegetables and fruits, one quarter of the plate are wholegrain foods (whole grain bread and pasta, wild rice, brown rice, red quinoa) and one quarter contains protein foods (plant-derived nuts, seeds, lentils, tofu and beans or animal-derived eggs, yogurt, lean meat, chicken, fish). All of these plant foods contain a range of phytonutrients including polyphenols (12,13).

Source: © All Rights Reserved. Canada’s Food Guide: Plate. Health Canada, 2019. Adapted and reproduced with permission from the Minister of Health, 2021.
Polyphenols are naturally occurring plant secondary metabolites that are derived from shikimic acid or phenylalanine via the Shikimate pathway (12). The Shikimate pathway is only present in micro-organisms and plants and results in the synthesis of the essential amino acids tyrosine, tryptophan and phenylalanine. More than 8,000 polyphenolic compounds have been identified in plants (12). Polyphenols are the most abundant
Journal of the Australasian College of Nutritional and Environmental Medicine Inc (ACNEM) 3 ACNEM Journal Vol 40 No 2 June 2021
Figure 1. Canada’s Food Guide: Plate
antioxidant in the human diet and are classified into a number of groups on the basis of their chemical structure (figure 2). The main classes of polyphenols are phenolic acids (cinnamic acid derivatives such as cinnamon), flavonoids (catechins and quercetin), stilbenes (resveratrol) and lignans (flaxseeds) (12,13).
Many polyphenols have biological functions in plants that are protective against a variety of types of environmental stress (12). They are involved in defense against ultraviolet radiation, contribute to plant healing, possess antimicrobial properties and counteract invasion by pathogens. Polyphenols and their metabolites have been shown to have potent antioxidant and anti-inflammatory activity including anthocyanins (found in bright coloured fruits), flavonoids (spinach, strawberries, blueberries, pomegranate, soy), resveratrol (red grapes) and curcumin (curcuma longa root) (16). These are required to counteract processes such as oxidative stress from ultraviolet radiation and reactive oxygen species that are generated by normal mitochondrial oxidative phosphorylation of macronutrient-derived energy substrates to produce adenosine triphosphate (ATP) (17). The majority of polyphenols are phytoestrogens, which may also explain some of their beneficial effects (18). In addition, dietary phytochemicals can initiate a hormetic response which occurs when ingestion of a small dose results in a mild cellular stress response (such as free radical production, ion fluxes and increased energy demand) that stimulate adaptive stress response pathways that protect cells from more severe stress at a later time (11,19)
Since the pathophysiology of PCOS involves oxidative stress and chronic inflammation, there has been significant interest in investigating the possible health benefits of polyphenols in women with PCOS. The aim of the present narrative review is to summarise the literature on the nutritional role of specific polyphenols as a component of a wholefood diet in women with polycystic ovary syndrome.
Journal of the Australasian College of Nutritional and Environmental Medicine Inc (ACNEM) 4 ACNEM Journal Vol 40 No 2 June 2021
Figure 2. Classification of polyphenols
DIETARY PATTERNS, POLYPHENOLS AND PCOS
The gastrointestinal microbiome and human physiology have co-evolved with plants over thousands of years to take advantage of phytochemicals such as polyphenols, designed to be protective in plants, to aid human physiology and environmental adaptation and improve health (20). Obtaining these contingent nutrients from a wide variety of plants by consuming a wholefood plant-based diet is likely to have reduced the biochemical and genetic requirements of human cells to synthesis these compounds. In addition, plant-based foods provide the necessary micronutrients and fibre now known to be fundamentally important to human health. Numerous World Health Organisation (WHO) expert consultation reports have recommended increased consumption of plant-based nutrients as a strategy to prevent and control chronic diseases for decades (21).
Dietary habits are one of the most important factors for promoting overall health and a number of validated diet quality indices have been developed to assess diet quality. The Healthy Diet Indicator, based on WHO’s nutrient intake goals to prevent chronic diseases, represents a globally applicable diet quality index that has been shown to be associated with all-cause mortality (22,23). Studies using the Healthy Diet Indicator and other diet quality indices have demonstrated that women with PCOS often consume a low nutrient value, poor-quality diet (24–26). A large number of studies have evaluated a variety of diet patterns in women with PCOS and these studies have been comprehensively reviewed in other publications (8,27). No single diet type has been recommended (7,8). New developments in the science of metabolomics are beginning to identify common components of a healthy diet pattern (28,29)
The development of the science of metabolomics has opened new possibilities for studying the role of dietary metabolites in the pathogenesis of PCOS (28) The set of all human metabolites that have been identified so far is stored in the Human Metabolome Database. The Human Metabolome Database currently contains over 114,190 compounds and this is growing each year. In the past PCOS was more commonly viewed as a reproductive and endocrine disorder but is increasingly being seen as a metabolic condition characterised by insulin resistance (4). Recent metabolomic studies have identified common metabolic pathways that are altered in women with PCOS (28,30). These include metabolites related to lipid, amino acid, energy and glucose metabolism and provide further support for the importance of metabolic disturbance in PCOS (28).
Metabolomics has been used to identify biomarkers of healthy dietary patterns and elucidate mechanisms underlying the association of diet with disease (29,30) A review of 17 studies which reported on blood metabolomic signatures of 5 healthy dietary patterns (Healthy Eating Index, Alternative Healthy Eating Index, the Dietary Approaches to Stop Hypertension diet, Mediterranean diet, vegetarian diet) found many common metabolites in different healthy dietary patterns, which suggest that they may represent biomarkers of generally healthy diets (29) These data suggest that there may be a common healthy diet
Journal of the Australasian College of Nutritional and Environmental Medicine Inc (ACNEM) 5 ACNEM Journal Vol 40 No 2 June 2021
pattern that could be recommended for the prevention and treatment of many chronic diseases. In addition, metabolomic studies appear to be reinforcing the findings of previous research using a range of dietary indices. Future research may identify specific dietary components involved in the pathogenesis of PCOS and reinforce the healthy dietary pattern that is emerging from diet index and metabolomic studies.
A variety of diet types have been found to have beneficial effects on the pathophysiology and symptoms of PCOS (8,27). These include Mediterranean, vegetarian, low carbohydrate, low fat, high protein and low-calorie diets (31). Taken together, these apparently different dietary patterns have many features in common. They mostly recommend the intake of wholefood from plant-based sources (fruits, vegetables and wholegrains) and healthy protein from plants (nuts, seeds, soybeans, lentils) or animals (pasture-raised meat, freerange eggs, wild-caught fish) and are low in fat. In addition, these healthy diet patterns exclude many foods that are known to be detrimental to human health. These include packaged, processed and refined food products, seed oils, added salt and sugar-sweetened beverages (15). A diet that includes the commonly recommended and excluded foods has been termed a “wholefood diet” (32,33).
The common healthy eating pattern identified in Healthy Diet Indicator studies and metabolomic research could therefore be called a “wholefood diet”. A wholefood plantbased diet aims to maximise consumption of nutrient-dense plant foods that contain a wide range of micronutrients and contingent nutrients such as polyphenols. Although it is recommended that dietary nutrients are consumed as wholefood, studies of isolated components such as polyphenols provide insights into some of the specific bioactive compounds present in wholefood. Polyphenols have been found to have a number of beneficial effects on the metabolic/endocrine features and symptoms associated with PCOS.
POLYPHENOLS AND THE MICROBIOTA
Polyphenols are plant secondary metabolites that are widely present in fruits, vegetables, legumes, and whole grains, as well as plant-derived foods like green tea, red wine, chocolate and soy (34). Polyphenols vary in structure, size, and complexity and are, in general, poorly absorbed. It has been estimated that less than 5% of ingested polyphenols reach the circulation intact (35). Polyphenols undergo intensive biotransformation by the gastrointestinal microbiota or are metabolised within enterocytes by phase 1 and phase 2 enzymes. A large number of phase 1, phase 2 and microbial products can be detected in plasma compared with extremely low levels of the parent compounds (16). Despite their low bioavailability, numerous studies have reported significant biological effects related to dietary polyphenols (12). This low bioavailability/high bioactivity paradox reinforces the symbiotic nature of the microbiome and human adaptive physiology and the importance of obtaining nutrients as part of a wholefood diet.
Different polyphenols are absorbed at varied sites throughout the gastrointestinal tract. The vast majority of polyphenols reach the colon intact where they interact with the colonic
Journal of the Australasian College of Nutritional and Environmental Medicine Inc (ACNEM) 6 ACNEM Journal Vol 40 No 2 June 2021
microbiota (36). A growing body of evidence is suggesting that polyphenols have the capacity to beneficially shift the colonic ecosystem composition (37). A diversity of dietary polyphenols may therefore contribute to a diverse microbiome. This ability to improve microbiota diversity may also have important implications for the maintenance of normal gastrointestinal barrier function. Dysbiosis and disruption of barrier integrity has been found to be a contributor to the pathogenesis of PCOS in some women (5,38). In addition, polyphenols may offer unique benefits to women with PCOS due to their action as phytoestrogens (18). A number of polyphenol-rich foods and supplements have been trialed in PCOS to date.
Green tea contains a range of polyphenols including catechins from the flavanol group and is produced through processing green leaves right after picking (39). In a 2017 randomised, placebo-controlled trial, 60 overweight women with PCOS (aged 20-40 years) were allocated to receive either placebo or a green tea capsule (500mg powdered green tea leaves twice daily). After a 12-week treatment period, there were significant improvements in mean weight (P=0.03) and fasting insulin (P<0.0001), as well as a significant decrease in mean free testosterone (P<0.0001) in the green tea group compared to controls (39). Although the impact of the green tea supplement on microbiota composition was not assessed in this trial, previous research has found the ingestion of the polyphenols in green tea to significantly increase populations of beneficial colonic bacteria, such as bifidobacteria and lactobacilli (40). One large cohort study of 40,530 adults followed for up to 11 years, found that all-cause mortality decreased incrementally as green tea consumption increased from 1 to 5 cups per day (41).
Soy foods are rich in specific polyphenols called isoflavones from the flavonoid group. These are a class of flavonoid with a wide range of biological activities, including antioxidant, antiinflammatory, and hormone-regulating actions (42). A recent systematic review and metaanalysis found soy isoflavone consumption to significantly decrease serum levels of total testosterone in women with PCOS (P<0.001) (43). Like other polyphenols, the activities of the soy isoflavones (genistein and daidzein) rely on microbial metabolism in the colon to release the biologically-active aglycones. However, in some subjects (20-30% of Americans and 5070% of Asians), daidzein is converted to equol, which has been found to have more potent biological effects (44). Equol binds to both oestrogen receptors and G-protein-coupled oestrogen receptor 1 and may have biological effects via this mechanism (45). The conversion of daidzein to equol is dependent upon bacterial composition of the microbiota, as only some specific species can perform this conversion (such as Asaccharobacter celatus and Slackia isoflavoniconvertens) (46). The pharmacological effects of soy isoflavones may be greater in, and even potentially limited to, individuals who exhibit equol production (47)
In a recent trial, Haudam et al postulated that soy isoflavones could have a prebiotic-like effect in PCOS patients and that therapeutic effects may be greater in equol producers (45). Supplementing 50mg/day of soy isoflavones (from soy drink) for 3 days to subjects with PCOS (or healthy controls) resulted in significant improvements in Homeostasis Model Assessment of Insulin Resistance (HOMA2-IR) (P<0.02), fasting insulin (P<0.01) and fasting glucose
Journal of the Australasian College of Nutritional and Environmental Medicine Inc (ACNEM) 7 ACNEM Journal Vol 40 No 2 June 2021
(P=0.01) compared with pretreatment values. No significant changes in these parameters were noted in the healthy controls. Subjects defined as equol producers were found to produce equol at levels 5-fold larger than non-producers. Equol production (across both PCOS patients and healthy controls) was significantly correlated with improvements in levels of total testosterone (P<0.05), free testosterone (P<0.01), androstenedione (P<0.05), antiMullerian hormone (P<0.01) and waist-to-hip ratio (P<0.05). After 3 days of isoflavone supplementation, the gastrointestinal ecosystem diversity increased significantly in both subjects with PCOS and healthy controls. In the PCOS subjects, this increase in diversity approached that observed in the healthy cohort before isoflavone supplementation (45). Previous research assessing the impact of soy isoflavones over a longer treatment period (2months) in post-menopausal women found prebiotic-like effects with beneficial changes in the gastrointestinal ecosystem observed, such as increases in butyrate-producing genera, Faecalibacterium prausnitzii, and Bifidobacterium spp. (48). These beneficial shifts in the ecosystem may be one of the mechanisms by which soy isoflavones improve PCOS sequalae.
Curcumin is a lipophilic polyphenol contained in the rhizome of the turmeric plant (Curuma longa) that has been recognized for its medicinal properties and is commonly used as a spice in Asian cuisine (49) Curcumin is poorly absorbed and is metabolized by enterocytes and the microbiota resulting in the generation of active metabolites, in common with other polyphenols (20). A variety of beneficial gastrointestinal bacterial species are involved in the biotransformation of curcumin including Blautia spp., E. coli, Bifidobacterium spp,. Lactobacillus spp. and Enterococcus faecalis (20). Animal and human studies have shown that oral curcumin administration can improve the ratio of beneficial to harmful bacteria in the gut microbiota ecosystem (50). Curcumin may therefore have a role in the treatment of dysbiosis that is thought to be involved in the pathogenesis of PCOS (38).
Curcumin is from the phenolic acid group and has been found to have therapeutic effects in a wide range of metabolic conditions including hyperlipidaemia, non-alcoholic fatty liver disease, diabetes and metabolic syndrome. Most of the health benefits of curcumin have been attributed to its antioxidant and anti-inflammatory effects (20,49–51). Curcumin has been investigated in women with PCOS as they exhibit many of these metabolic and pathophysiological features (5,51)
A systematic review and meta-analysis of 3 randomised controlled trials was performed to evaluate the effects of curcumin on glycaemic control and lipid profile in women with PCOS (51). Women with PCOS taking curcumin had significantly greater improvement in glycaemic control than those taking placebo. Curcumin significantly improved the mean difference (MD) of fasting glucose (MD: -2.77, 95% confidence interval (CI): -4.16 to -1.38), fasting insulin (MD: -1.33, 95% CI: -2.18 to -0.49), HOMA-IR (MD: -0.32, 95% CI: -2.18 to -0.49), high-density lipoprotein (MD: 1.92, 95% CI: 0.33-3.51) and total cholesterol (MD: -12,45, 95% CI: -22.05 to -2.85). There was no significant difference in low-density lipoprotein and triglyceride levels between the curcumin and placebo groups. The effects of curcumin on hyperandrogenism were not investigated in this meta-analysis. The curcumin dose used in these trials ranged
Journal of the Australasian College of Nutritional and Environmental Medicine Inc (ACNEM) 8 ACNEM Journal Vol 40 No 2 June 2021
from 500 mg to 1500 mg per day over a treatment period of 6-12 weeks. The authors concluded that curcumin may improve glycaemic control and lipid metabolism in women with PCOS (51).
Pomegranate juice is another food product rich in a variety of polyphenols including phenolic acid and non-phenolic acid compounds (52). The impacts of pomegranate juice (45ml/d) were assessed in a randomised, open-label controlled trial in 44 overweight patients with PCOS (aged 18-40 years). At the end of 8-weeks treatment, subjects who received the pomegranate juice experienced a significant reduction in systolic (P<0.001) and diastolic (P=0.05) blood pressure and serum triglycerides (P=0.01), as well as increases in HDL cholesterol (P=0.03) compared to controls (who received routine care). Once again, the microbiota impact of the polyphenol-rich pomegranate juice was not evaluated in this study. Previous research has, however, found pomegranate polyphenols capable of beneficially altering the composition of the gastrointestinal microbiota when consumed (53).
Pomagranate polyphenols, like those of soy, cannot be efficiently utilized without an optimal microbiome. Gut microbiota-derived metabolites of pomegranate ellagitannins, such as urolithin A, play a direct role in improving insulin sensitivity and preventing high-fat-dietinduced insulin resistance in mice (54). Since insulin resistance is a core pathophysiological feature of PCOS, further investigation of the specific role of pomegranate metabolites in women with PCOS is warranted. Pomegranate polyphenols have also been demonstrated to reduce lipopolysaccharide binding protein, demonstrating a positive impact on endotoxemia, in both overweight subjects and those with newly diagnosed colorectal cancer (55,56). Polyphenols may therefore be involved in the maintenance of gastrointestinal barrier integrity and the prevention of dysbiosis-related endotoxaemia thought to be involved in the pathogenesis of PCOS (38)
In addition, the polyphenols of pomegranate, as from many fruits and vegetables, appear to achieve a significant amount of their metabolic and hormonal benefits because they function as selective oestrogen receptor modulators (57). Since PCOS is characterised by androgen excess and functional oestrogen deficiency, it is important that future research endeavour to understand the possible therapeutic benefits of polyphenols. A wholefood diet containing a wide variety of phytoestrogenic polyphenols and other phytonutrients, may therefore confer a number of benefits to the microbiota, gastrointestinal barrier integrity and metabolic changes thought to be involved in the pathogenesis and maintenance of PCOS.
CONCLUSIONS
The metabolic aspects of PCOS are increasingly being emphasised and have been highlighted in the International Guidelines. Insulin-resistance is a core pathophysiological feature of the metabolic disturbance of PCOS in the majority of affected women. Insulinresistance is usually caused by poor-quality diet and having a healthy diet is now considered the first-line of management for all women diagnosed with PCOS. Nutritional studies based on diet composition, diet indices and metabolomics have identified a number of common
Journal of the Australasian College of Nutritional and Environmental Medicine Inc (ACNEM) 9 ACNEM Journal Vol 40 No 2 June 2021
dietary components that constitute a healthy eating pattern. Healthy diet patterns, or wholefood diets, are predominantly plant-based and have been found to be effective in the treatment of the metabolic alterations and symptoms associated with PCOS. Plant-based foods contain a large number of contingent nutrients, such as polyphenols, that have been shown to be beneficial to women with PCOS in short-term intervention studies and could be used during the transition to a long-term healthy eating pattern. Effective dietary intervention in women with PCOS has the potential to decrease symptoms, restore fertility and prevent the development of the associated metabolic conditions and their complications.
Competing interests: No relevant disclosures
REFERENCES
1. Ding T, Hardiman PJ, Petersen I, Wang FF, Qu F, Baio G. The prevalence of polycystic ovary syndrome in reproductiveaged women of different ethnicity: A systematic review and meta-analysis. Oncotarget. 2017;8(56):96351–8.
2. Cassar S, Misso ML, Hopkins WG, Shaw CS, Teede HJ, Stepto NK. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemichyperinsulinaemic clamp studies. Hum Reprod. 2016;31(11):2619–31.
3. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133–223.
4. Zore T, Joshi N V., Lizneva D, Azziz R. Polycystic Ovarian Syndrome: Long-Term Health Consequences. Vol. 35, Seminars in Reproductive Medicine. 2017. p. 271–81.
5. Parker J. Understanding the Pathogenesis of Polycystic Ovary Syndrome : A transgenerational evolutionary adaptation to lifestyle and the environment. ACNEM J. 2020;39(4).
6. Pathak G, Nichter M. Polycystic ovary syndrome in globalizing India: An ecosocial perspective on an emerging lifestyle disease. Soc Sci Med [Internet]. 2015;146(March 2017):21–8. Available from: http://dx.doi.org/10.1016/j.socscimed.2015.10.007
7. Foley E, Marsh C. Polycystic ovary syndrome: is a Western diet sabotaging our best efforts at management? Fertil Steril [Internet]. 2019;112(4):653–4. Available from: https://doi.org/10.1016/j.fertnstert.2019.06.020
8. Teede H, Misso M, Costello M, Dokras A, Laven J, Moran L, et al. International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2018. National Health and Medical Research Council (NHMRC). 2018. 1–198 p.
9. Andrews P, Johnson RJ. Evolutionary basis for the human diet: consequences for human health. J Intern Med. 2020;287(3):226–37.
10. Mann N. A review of human diet from an evolutionary perspective. ACNEM J. 2015;34(4).
Journal of the Australasian College of Nutritional and Environmental Medicine Inc (ACNEM) 10 ACNEM Journal Vol 40 No 2 June 2021
11. Osiecki H. Contingent nutrient factors. In: Osiecki H, editor. The Nutrient Bible. 9th ed. Queensland: AG Publishing; 2014. p. 217.
12. Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2(5):270–8.
13. Blekkenhorst LC, Sim M, Bondonno CP, Bondonno NP, Ward NC, Prince RL, et al. Cardiovascular health benefits of specific vegetable types: A narrative review. Nutrients. 2018;10(5):1–24.
14. National Health and Medical Research Council. Australian Dietary Guidelines. Canberra [Internet]. Nhmrc. 2013. Available from: https://www.nhmrc.gov.au
15. Health Canada. Canada’s Dietary Guidelines for Health Professionals and Policy Makers [Internet]. Health Canada. 2019. 62 p. Available from: https://foodguide.canada.ca/static/assets/pdf/CDG-EN-2018.pdf
16. Luca SV, Macovei I, Bujor A, Miron A, Skalicka-Woźniak K, Aprotosoaie AC, et al. Bioactivity of dietary polyphenols: The role of metabolites. Crit Rev Food Sci Nutr [Internet]. 2020;60(4):626–59. Available from: https://doi.org/10.1080/10408398.2018.1546669
17. Parker J. Glucose metabolism , energy production and regulation of cellular and whole-body metabolism. ACNEM J. 2020;39(1):29–33.
18. Khani B, Mehrabian F, Khalesi E, Eshraghi A. Original Article Effect of soy phytoestrogen on metabolic and hormonal disturbance of women with polycystic ovary syndrome. 2011;16(3):297–302.
19. Martel J, Ojcius DM, Ko YF, Ke PY, Wu CY, Peng HH, et al. Hormetic Effects of Phytochemicals on Health and Longevity. Trends Endocrinol Metab [Internet]. 2019;30(6):335–46. Available from: https://doi.org/10.1016/j.tem.2019.04.001
20. Shabbir U, Rubab M, Daliri EB, Chelliah R, Javed A, Oh D. Curcumin, Quercetin, Catachins and metabolic diseases: The role of the gut microbiota. Nutrients. 2021;13(206).
21. Amine EK, Baba NH, Belhadj M, Deurenberg-Yap M, Djazayery A, Forrestre T, et al. Diet, nutrition and the prevention of chronic diseases. World Heal Organ - Tech Rep Ser. 2003;(916).
22. Huijbregts, P. Feskens, E. Rasanen L et al. Dietary pattern and 20 year mortality in elderly men in Finland, Italy, and the Netherlands: longitudinal cohort study. Bmj. 1997;315:13–7.
23. Jankovic N, Geelen A, Streppel MT, De Groot LCPGM, Orfanos P, Van Den Hooven EH, et al. Adherence to a healthy diet according to the world health organization guidelines and all-cause mortality in elderly adults from Europe and the United States. Am J Epidemiol. 2014;180(10):978–88.
24. Hosseini MS, Dizavi A, Rostami H, Parastouei K, Esfandiari S. Healthy eating index in women with polycystic ovary syndrome: A case-control study. Int J Reprod Biomed. 2017;15(9):575–82.
Journal of the Australasian College of Nutritional and Environmental Medicine Inc (ACNEM) 11 ACNEM Journal Vol 40 No 2 June 2021
25. Eslamian G, Hekmatdoost A. Nutrient patterns and risk of polycystic ovary syndrome. J Reprod Infertil. 2019;20(3):161–8.
26. Szczuko, M. Sankowska, P. Zapalowska-Chwyc, M. Wysokinski P. Studies on the quality of nutrition in women with polycystic ovary syndrome (PCOS). Rocz Panstw Zakl Hig. 2017;68(1):61–7.
27. Tremellen KPK. Nutrition, Fertility, and Human Reproductive Function. In: Nutrition, Fertility, and Human Reproductive Function. Adelaide: CRC Press; 2015. p. 27–50.
28. Rajska A, Buszewska-Forajta M, Rachoń D, Markuszewski MJ. Metabolomic insight into polycystic ovary syndrome An overview. Int J Mol Sci. 2020;21(14):1–21.
29. Kim, Hyunju Rebholz CM. Metabolomic biomarkers of healthy dietary patterns and cardiovascular outcomes. Curr Atheroscler Rep. 2021;23(6):26.
30. Cree-Green M, Carreau AM, Rahat H, Garcia-Reyes Y, Bergman BC, Pyle L, et al. Amino acid and fatty acid metabolomic profile during fasting and hyperinsulinemia in girls with polycystic ovarian syndrome. Am J Physiol - Endocrinol Metab. 2019;316(5):E707–18.
31. Moran LJ, Ko H, Misso M, Marsh K, Noakes M, Talbot M, et al. Dietary Composition in the Treatment of Polycystic Ovary Syndrome: A Systematic Review to Inform Evidence-Based Guidelines. J Acad Nutr Diet. 2013;113(4):520–45.
32. Ha B. The Power of Plants: Is a Whole-Foods, Plant-Based Diet the Answer to Health, Health Care, and Physician Wellness? Perm J. 2019;23:8–11.
33. Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: A randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes [Internet]. 2017;7(3). Available from: http://dx.doi.org/10.1038/nutd.2017.3
34. Corrêa TAF, Rogero MM, Hassimotto NMA, Lajolo FM. The Two-Way PolyphenolsMicrobiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front Nutr. 2019;6(December):1–15.
35. Cao H, Chen X, Jassbi AR, Xiao J. Microbial biotransformation of bioactive flavonoids. Biotechnol Adv [Internet]. 2015;33(1):214–23. Available from: http://dx.doi.org/10.1016/j.biotechadv.2014.10.012
36. Kawabata K, Yoshioka Y, Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules. 2019;24(2).
37. Moorthy M, Chaiyakunapruk N, Jacob SA, Palanisamy UD. Prebiotic potential of polyphenols, its effect on gut microbiota and anthropometric/clinical markers: A systematic review of randomised controlled trials. Trends Food Sci Technol [Internet]. 2020;99(February):634–49. Available from: https://doi.org/10.1016/j.tifs.2020.03.036
38. Tremellen K, Pearce K. Dysbiosis of Gut Microbiota (DOGMA) - A novel theory for the development of Polycystic Ovarian Syndrome. Med Hypotheses [Internet]. 2012;79(1):104–12. Available from: http://dx.doi.org/10.1016/j.mehy.2012.04.016
Journal of the Australasian College of Nutritional and Environmental Medicine Inc (ACNEM) 12 ACNEM Journal Vol 40 No 2 June 2021
39. Tehrani, HG. Allahdadian, M. Zarre, F. Ranjbar, H. Allahdadian F. Effect of green tea on metabolic and hormonal aspect of polycystic ovary syndrome in overweight and obese women suffering from polycystic ovary syndrome: A clinical trial. J Educ Health Promot. 2017;6(May).
40. Goto, KS. Kanaya, T. Nishikawa T et al. The influence of tea catechins on fecal flora of elderly residents in long-term care facilities. Ann Long-Term Care. 1998;6:43–8.
41. Kuriyama, S. Shimazu, T. Ohmori K et al. Green Tea Consumption and Mortality Due to Cardiovascular Disease, Cancer and all causes in Japan. 2006;296(10):1255–65.
42. Kalaiselvan V, Kalaivani M, Vijayakumar A, Sureshkumar K, Venkateskumar K. Current knowledge and future direction of research on soy isoflavones as a therapeutic agents. Pharmacogn Rev. 2010;4(8):111–7.
43. Zilaee M, Mansoori A, Ahmad HS, Mohaghegh SM, Asadi M, Hormoznejad R. The effects of soy isoflavones on total testosterone and follicle-stimulating hormone levels in women with polycystic ovary syndrome: a systematic review and metaanalysis. Eur J Contracept Reprod Heal Care [Internet]. 2020;25(4):305–10. Available from: https://doi.org/10.1080/13625187.2020.1761956
44. Sekikawa A, Ihara M, Lopez O, Kakuta C, Lopresti B, Higashiyama A, et al. Effect of Sequol and Soy Isoflavones on Heart and Brain. Curr Cardiol Rev. 2018;15(2):114–35.
45. Haudum, Christoph. Lindheim, Lisa. Ascani A. Impact of Short‐Term Isoflavone Intervention in polycystic ovary syndrome (PCOS) patients on microbiota composition and metagenomics. Nutrients. 2020;12:1622.
46. Iino C, Shimoyama T, Iino K, Yokoyama Y, Chinda D, Sakuraba H, et al. Daidzein intake is associated with equol producing status through an increase in the intestinal bacteria responsible for equol production. Nutrients. 2019;11(2):1–14.
47. Mayo B, Vázquez L, Flórez AB. Equol: a bacterial metabolite from the daidzein. Nutrients. 2019;11(9):2231.
48. Clavel T, Fallani M, Lepage P, Levenez F, Mathey J, Rochet V, et al. Isoflavones and functional foods alter the dominant intestinal microbiota in postmenopausal women. J Nutr. 2005;135(12):2786–92.
49. Hewlings S, Kalman D. Curcumin: A Review of Its Effects on Human Health. Foods. 2017;6(10):92.
50. Scazzocchio B, Minghetti L, D’archivio M. Interaction between gut microbiota and curcumin: A new key of understanding for the health effects of curcumin. Nutrients. 2020;12(9):1–18.
51. Chien YJ, Chang CY, Wu MY, Chen CH, Horng YS, Wu HC. Effects of curcumin on glycemic control and lipid profile in polycystic ovary syndrome: Systematic review with meta-analysis and trial sequential analysis. Nutrients. 2021;13(2):1–14.
52. Abedini M, Ghasemi-Tehrani H, Tarrahi MJ, Amani R. The effect of concentrated pomegranate juice consumption on risk factors of cardiovascular diseases in women with polycystic ovary syndrome: A randomized controlled trial. Phyther Res.
Journal of the Australasian College of Nutritional and Environmental Medicine Inc (ACNEM) 13 ACNEM Journal Vol 40 No 2 June 2021
2021;35(1):442–51.
53. Li Z, Henning SM, Lee RP, Lu QY, Summanen PH, Thames G, et al. Pomegranate extract induces ellagitannin metabolite formation and changes stool microbiota in healthy volunteers. Food Funct. 2015;6(8):2487–95.
54. Toney AM, Fan R, Xian Y, Chaidez V, Ramer-tait AE, Chung S. Urolithin A , a Gut Metabolite , Improves Insulin Sensitivity Through Augmentation of Mitochondrial Function and Biogenesis. 2019;00(00):1–9.
55. González-Sarrías A, Núñez-Sánchez MA, Ávila-Gálvez MA, Monedero-Saiz T, Rodríguez-Gil FJ, Martínez-Díaz F, et al. Consumption of pomegranate decreases plasma lipopolysaccharide-binding protein levels, a marker of metabolic endotoxemia, in patients with newly diagnosed colorectal cancer: A randomized controlled clinical trial. Food Funct. 2018;9(5):2617–22.
56. González-Sarrías A, Romo-Vaquero M, García-Villalba R, Cortés-Martín A, Selma MV, Espín JC. The Endotoxemia Marker Lipopolysaccharide-Binding Protein is Reduced in Overweight-Obese Subjects Consuming Pomegranate Extract by Modulating the Gut Microbiota: A Randomized Clinical Trial. Mol Nutr Food Res. 2018;62(11):1–32.
57. Sreeja S, Santhosh TR, Lakshmi BS, Sreeja S. Pomegranate extract demonstrate a selective estrogen receptor modulator profile in human tumor cell lines and in vivo models of estrogen deprivation. J Nutr Biochem [Internet]. 2012;23(7):725–32.
Available from: http://dx.doi.org/10.1016/j.jnutbio.2011.03.015
Journal of the Australasian College of Nutritional and Environmental Medicine Inc (ACNEM) 14 ACNEM Journal Vol 40 No 2 June 2021

















