MembraneContactorTechnology
WaterTreatment,FoodProcessing,GasSeparation,and CarbonCapture
EditedbyMohammadYounasandMashallahRezakazemi
Editors
ProfessorMohammadYounas UniversityofEngineering&Technology DepartmentofChemicalEngineering No.2UniversityRd 25120Peshawar Pakistan
AssociateProfessorMashallahRezakazemi ShahroodUniversityofTechnology ChemicalandMaterialsEngineering Shahrood Iran
CoverImage:©amixstudio/GettyPixabay
Allbookspublishedby WILEY-VCH arecarefully produced.Nevertheless,authors,editors,and publisherdonotwarranttheinformation containedinthesebooks,includingthisbook, tobefreeoferrors.Readersareadvisedtokeep inmindthatstatements,data,illustrations, proceduraldetailsorotheritemsmay inadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor
BritishLibraryCataloguing-in-PublicationData Acataloguerecordforthisbookisavailable fromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailedbibliographic dataareavailableontheInternetat <http://dnb.d-nb.de>.
©2022WILEY-VCHGmbH,Boschstr.12, 69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).Nopartof thisbookmaybereproducedinanyform–by photoprinting,microfilm,oranyother means–nortransmittedortranslatedintoa machinelanguagewithoutwrittenpermission fromthepublishers.Registerednames, trademarks,etc.usedinthisbook,evenwhen notspecificallymarkedassuch,arenottobe consideredunprotectedbylaw.
PrintISBN: 978-3-527-34861-9
ePDFISBN: 978-3-527-83102-9
ePubISBN: 978-3-527-83104-3
oBookISBN: 978-3-527-83103-6
Typesetting Straive,Chennai,India
Printedonacid-freepaper 10987654321
Dedicatedtomywholefamily,teachers,andfriendswhomIowealot.
Contents
Preface xv
AbouttheAuthors xvii
1IntroductiontoMembraneTechnology 1
MohammadYounasandMashallahRezakazemi
1.1OverviewofMembraneTechnology 1
1.2ConventionalMembraneSeparationProcesses 2
1.2.1Microfiltration(MF) 2
1.2.2Ultrafiltration(UF) 2
1.2.3Nanofiltration(NF) 3
1.2.4ReverseOsmosis(RO) 3
1.2.5Electrodialysis(ED) 4
1.2.6Pervaporation(PV) 5
1.3MolecularWeightCutoff(MWCO) 8
1.4ConcentrationPolarization 9
1.5MembraneFouling 10
1.6Diafiltration 11
1.7HistoricalPerspective 11
1.8ConcludingRemarksandFutureChallenges 12 References 14
2IntroductiontoMembraneContactorTechnology 17
MohammadYounasandMashallahRezakazemi
2.1MembraneContactorSeparationProcesses 17
2.1.1MembraneContactors 17
2.1.2HistoryandBackgroundofMembraneContactors 20
2.1.3TypesofMembraneContactorSystems 21
2.1.3.1SolidPorousMembraneasMediumofContactinMembrane Contactors 21
2.1.3.2LiquidMembraneContactors 30
2.1.4MembraneContactorIntegratedSystems 34
2.1.5PotentialofMembraneContactorinConcentration,Temperature Polarization,Wetting,andFoulingofMembranes 35
2.2ConclusionandFutureTrendsofMembraneContactors 37 References 38
3TransportTheoryinMembraneContactor:Operational Principle 45
MohammadYounas,WaheedUrRehman,andMashallahRezakazemi
3.1DiffusionalMassandHeatTransferModeling 45
3.2MembraneCharacterizationModels 46
3.2.1ContactAngleandLiquidEntryPressure 46
3.2.2LiquidEntryPressure(LEP) 49
3.2.3Permporometry(PoreSizeDistribution) 52
3.2.4ElectronMicroscopy 52
3.3TransportModelsinLiquid–LiquidContactor 52
3.3.1ResistanceinSeriesModel 55
3.3.1.1ModelApproach 56
3.3.1.2TwoFilmTheory 56
3.3.1.3PhaseEquilibriuminLiquid–LiquidSystem 58
3.3.1.4OverallMassTransferCoefficient 59
3.4TransportModelinGas–LiquidSystems 60
3.4.1PhaseEquilibriumforGas–LiquidSystem 61
3.4.2ResistanceinSeriesModel 61
3.5ReactiveDiffusioninLiquid-SideBoundaryLayer 62
3.6MassTransferResistanceAnalysis 63
3.7CorrelationsforMassTransferCoefficients 65
3.7.1CorrelationforFlowinShellSide 66
3.7.2CorrelationforFlowinTubeSide 66
3.7.3CorrelationforMassTransferinMembranePores 68
3.8CorrelationsforHeatTransferCoefficients 69
3.9InterfacialTransferArea 70
3.10AxialPressureDropinMembraneContactorModule 71
3.11DynamicModeling 71
3.12TransferUnitsandModuleDesignLength 72
3.13NumericalModelingofMassTransportinMembraneContactor Modules 73
3.13.1MassTransferinShellSide 75
3.13.2MassTransferInsideFibers 77
3.13.3MassTransferinMembranePores 78
3.13.4NumericalModelingTermintheCaseofMembraneWetting 79
3.14NumericalModelingofHeatTransportinMembraneContactor Modules 81
3.14.1GoverningEquationinColdandHotChannels 82
3.14.2GoverningEquationInsideMembrane 82
3.15ModelSolutionAlgorithm 83
3.16ConclusionsandPerspectives 84
3.AMembraneTransportTheory:OperationalPrinciple 84
3.A.1Steady-StateResistance-in-SeriesModelAcrossLiquid–Liquid Contactor 84
3.A.1.1HydrophobicMembraneBasedonAqueous-PhaseSide(Species TransfersfromAqueousPhasetoOrganicPhase) 84
3.A.1.2HydrophobicMembraneBasedonOrganic-PhaseSide(SpeciesTransfers fromAqueousPhasetoOrganicPhase) 85
3.A.1.3HydrophobicMembraneBasedonOrganic-PhaseSide(SpeciesTransfers fromOrganicPhasetoAqueousPhase) 85
3.A.1.4HydrophobicMembraneBasedonAqueous-PhaseSide(Species TransfersfromOrganicPhasetoAqueousPhase) 85
3.A.1.5HydrophilicMembraneBasedonAqueous-PhaseSide(SpeciesTransfers fromAqueousPhasetoOrganicPhase) 86
3.A.1.6HydrophilicMembraneBasedonOrganic-PhaseSide(SpeciesTransfers fromAqueousPhasetoOrganicPhase) 86
3.A.1.7HydrophilicMembraneBasedonOrganic-PhaseSide(SpeciesTransfers fromOrganicPhasetoAqueousPhase) 86
3.A.1.8HydrophilicMembraneBasedonAqueous-PhaseSide(SpeciesTransfers fromOrganicPhasetoAqueousPhase) 87
3.A.1.9CompositeMembraneBasedonAqueous-PhaseSide(SpeciesTransfers fromAqueousPhasetoOrganicPhase) 87
3.A.1.10CompositeMembraneBasedonOrganic-PhaseSide(SpeciesTransfers fromAqueousPhasetoOrganicPhase) 87
3.A.1.11CompositeMembraneBasedonOrganic-PhaseSide(SpeciesTransfers fromOrganicPhasetoAqueousPhase) 87
3.A.1.12CompositeMembraneBasedonAqueous-PhaseSide(SpeciesTransfers fromOrganicPhasetoAqueousPhase) 88
3.A.2Steady-StateResistance-in-SeriesModelAcrossGas–Liquid Contactor 88
3.A.2.1HydrophobicMembraneBasedonGas-PhaseSide(SpeciesTransfers fromGasPhasetoLiquidPhase) 88
3.A.2.2HydrophobicMembraneBasedonLiquid-PhaseSide(SpeciesTransfers fromGasPhasetoLiquidPhase) 88
3.A.2.3HydrophobicMembraneBasedonLiquid-PhaseSide(SpeciesTransfers fromLiquidPhasetoGasPhase) 89
3.A.2.4HydrophobicMembraneBasedonGas-PhaseSide(SpeciesTransfers fromLiquidPhasetoGasPhase) 89
3.A.2.5HydrophilicMembraneBasedonGas-PhaseSide(SpeciesTransfersfrom GasPhasetoLiquidPhase) 89
3.A.2.6HydrophilicMembraneBasedonLiquid-PhaseSide(SpeciesTransfers fromGasPhasetoLiquidPhase) 90
3.A.2.7HydrophilicMembraneBasedonLiquid-PhaseSide(SpeciesTransfers fromLiquidPhasetoGasPhase) 90
3.A.2.8HydrophilicMembraneBasedonGas-PhaseSide(SpeciesTransfersfrom LiquidPhasetoGasPhase) 91
3.A.2.9CompositeMembraneBasedonGas-PhaseSide(SpeciesTransfersfrom GasPhasetoLiquidPhase) 91
3.A.2.10CompositeMembraneBasedonLiquid-PhaseSide(SpeciesTransfers fromGasPhasetoLiquidPhase) 91
3.A.2.11CompositeMembraneBasedonLiquid-PhaseSide(SpeciesTransfers fromGasPhasetoLiquidPhase) 91
3.A.2.12CompositeMembraneBasedonGas-PhaseSide(SpeciesTransfersfrom LiquidPhasetoGasPhase) 92
3.A.3DynamicModelingAcrosstheStorageTank 92 References 93
4ModuleDesignandMembraneMaterials 99 NabilahFazil,SidraSaqib,AhmadMukhtar,MohammadYounas,and MashallahRezakazemi
4.1Introduction 99
4.2MembraneModuleDesignConfiguration 100
4.2.1Plate-and-FrameModules 100
4.2.2SpiralWoundModules 103
4.2.3TubularModules 104
4.2.4HollowFiberModules 106
4.3MembraneContactorModuleHousing 111
4.4MembraneModuleFlowConfiguration 116
4.5MembraneMaterials 116
4.5.1PolymerMaterials 118
4.5.2InorganicFillers 125
4.6MembraneandMembraneModuleforMembraneDistillation(MD)and OsmoticMembraneDistillation(OMD) 126
4.7SolventsUsedinMembraneSynthesis 128
4.8MembraneSynthesisTechniques 128
4.9Conclusions 130
4.10FuturePerspective 131 References 131
5ModeofOperationinMembraneContactors 143 WaheedUrRehman,ZarrarSalahuddin,SarahFarrukh,MuhammadYounas, andMashallahRezakazemi
5.1MembraneDistillation(MD) 143
5.1.1BasicPrinciplesofMDProcess 143
5.1.2MDConfigurations 144
5.1.3OverallDrivingForce 145
5.1.4OverallMassTransferCoefficient, K147
5.1.4.1Feed-SideMassTransfer 148
5.1.4.2MembraneMassTransfer 150
5.1.4.3Strip-SideMassTransfer 151
5.1.5VaporPressurePolarizationCoefficient, �� v 152
5.1.5.1DCMD 152
5.1.5.2Feed–SideandStrip–SideHeatTransfer 153
5.1.5.3MembraneHeatTransfer 153
5.1.6AGMD 154
5.1.6.1SGMD 156
5.1.7VMD 157
5.1.8MembranesforMDProcess 157
5.1.9ProsandConsofMDProcess 158
5.1.10FutureProspectsofMDProcess 161
5.2OsmoticMembraneDistillation(OMD) 161
5.2.1BasicPrinciplesofOMDProcess 161
5.2.2OverallMassTransfer 163
5.2.2.1MassTransferAcrossFeedBoundaryLayer 163
5.2.2.2MassTransferAcrossStripperBoundaryLayer 163
5.2.2.3MassTransferAcrossMembrane 164
5.2.2.4MassTransferCoefficientforFeedandStripperSide 164
5.2.2.5MassTransferCoefficientAcrossMembrane 164
5.2.3StrippingSolutionsforOMD 165
5.2.4MembranesforOMDProcess 166
5.2.5ProsandConsofOMDProcess 166
5.3ForwardOsmosis 167
5.3.1BasicPrinciplesofFOProcess 167
5.3.2CalculationoftheOsmoticPressures 167
5.3.3ReverseSoluteFluxinFO 170
5.3.4MembranesforFOProcess 170
5.3.5DrawSolutesforFOProcess 171
5.3.6ProsandConsofFOProcess 172
5.4Pressure-RetardedOsmosis 172
5.4.1BasicPrinciplesofPROProcess 172
5.4.2MembranesforPROProcess 175
5.4.3ProsandConsofPROProcess 176
5.5Conclusions 176 References 176
6ApplicationsofMembraneContactorTechnologyin WastewaterTreatment 185 AyeshaRehman,XianhuiLi,SarahFarrukh,MohammadYounas,and MashallahRezakazemi
6.1Introduction 185
6.2CommonToxicSubstancesinWastewater 187
6.2.1Phenols 187
6.2.2HeavyMetals 188
6.2.3Ammonia 188
6.2.4HydrogenSulfide 188
6.2.5CarbonDioxide 188
6.2.6PetroleumHydrocarbons 188
6.2.7PolycyclicAromaticHydrocarbons 189
6.2.8Nitrobenzene 189
6.3EnvironmentalRisksofWastewater 189
6.4MembraneTechnologyforWastewaterTreatment 190
6.5MembraneContactorTechnologyforRemovalofOrganicContaminants fromWastewater 193
6.6RemovalofInorganicContaminantsfromWastewater 200
6.7Polymer-BasedAdsorptionMembranes 202
6.8Ion-ExchangeNanoporousMembrane 204
6.9Micellar-EnhancedUltrafiltrationMembrane 204
6.10MembraneMaterialsforWaterTreatment 205
6.11MembraneMaterialsforMicrofiltration(MF)andUltrafiltration (UF) 206
6.12MembraneMaterialsforNanofiltration(NF) 206
6.13MembraneMaterialsforReverseOsmosis(RO) 207
6.14MembraneMaterialsforForwardOsmosis(FO) 207
6.15ChallengesinMembraneMaterialstoPreventFouling 208
6.16ConclusionsandPerspectives 209 References 210
7ApplicationsofMembraneContactorsinFoodIndustry 219 WaheedUrRehman,BazlaSarwar,SidraSaqib,AhmadMukhtar, MohammadYounas,andMashallahRezakazemi
7.1Introduction 219
7.2MembraneDistillation(MD)ApplicationsinFoodIndustry 219
7.2.1MDintheConcentrationofAppleJuice 221
7.2.2MDintheConcentrationofOrangeJuice 222
7.2.3MDintheConcentrationofMilk 222
7.2.4MDintheTreatmentofSalineDairyWasteWater 223
7.2.5MDintheConcentrationofMuscadineGrapePomace 224
7.2.6MDintheRecoveryofPhenolsfromOliveMillWastewater 225
7.2.7MDintheConcentrationofSucroseSolution 225
7.2.8EffectofOperatingParametersonMDFlux 225
7.3ApplicationofOsmoticMembraneDistillation(OMD)inFood Industry 227
7.3.1EffectofOperatingConditionsonOMDWaterFlux 228
7.3.2OMDintheConcentrationofAppleJuice 231
7.3.3OMDintheConcentrationofGrapeJuice 232
7.3.4OMDintheConcentrationofPomegranateJuice 233
7.3.5OMDintheConcentrationofOrangeJuice 235
7.3.6OMDintheConcentrationofCranberryandNoniJuices 235
7.3.7OMDintheConcentrationofKiwiandPineappleJuices 236
7.3.8OMDintheConcentrationofTeaExtracts 236
7.3.9DealcoholizationofBeerandWine 237
7.4CoupledOperationofOsmoticDistillationandMembrane Distillation 238
7.5Conclusions 239
7.6FuturePerspectives 239 References 240
8ApplicationsofMembraneContactorTechnologyfor Pre-combustionCarbonDioxide(CO2 )Capture 247 ZarrarSalahuddin,SarahFarrukh,MohammadYounas,and MashallahRezakazemi
8.1Introduction 247
8.2WhyPre-combustionCarbonCapture? 250
8.3MembranesforPre-combustionCarbonCapture 250
8.3.1Hydrogen(H2 )-SelectiveMembranes 250
8.3.2CO2 -SelectiveMembranes 255
8.4AdvantagesandLimitationsofPre-combustionCarbonCaptureUsing MembraneTechnology 262
8.5ApplicationsofPre-combustionCarbonCapture 263
8.6CurrentTrendsandFutureProspects 263
8.7ConcludingandFutureDirections 269 References 269
9ApplicationofMembraneContactorTechnologyfor Post-combustionCarbonDioxide(CO2 )Capture 281 MuhammadB.Wazir,MuhammadDaud,MohammadYounas,and MashallahRezakazemi
9.1Introduction 281
9.2MembranesforPost-combustionCO2 Capture 282
9.2.1MembraneTypes 282
9.2.2MembraneModules 285
9.3ExperimentalMembraneMaterialsforPost-combustionCO2 Sequestration 285
9.4CommercialMembranesforPost-combustionCO2 Separation 288
9.5CostofPost-combustionCO2 CaptureinMembraneContactors 289
9.6AbsorbentsforPost-combustionCO2 Separation 291
9.6.1Amine-BasedAbsorbents 291
9.6.2Ammonia 293
9.6.3SaltSolutions 294
9.6.4IonicLiquids 295
9.7ConclusionandFuturePerspective 295 References 296
10MarketProspectsofMembraneContactors 305 ZahraPezeshki,MohammadYounas,andMashallahRezakazemi
10.1MembraneContactorMarketDynamics 305
10.2MarketOverview 306
10.3MembraneContactorMarketbyApplication 313
10.3.1WaterandWastewaterTreatmentMarket 313
10.3.2FoodProcessingMarket 315
10.3.3GasSeparationMarket 318
10.3.4CarbonCaptureMarket 321
10.4MembraneContactorMarket,byMembrane 321
10.5MembraneContactorMarket,byRegion 325
10.6RecentDevelopmentsofMembraneContactorCompanies 328
10.6.13MCompany 328
10.6.2CobetterFiltrationEquipmentPvt.Ltd. 329
10.6.3EUROWATER 329
10.6.4JU.CLA.SSrl 329
10.6.5VeoliaEnvironnementSA 329
10.6.6PTIPacificPty.Ltd. 330
10.6.7KværnerASA 330
10.6.8LenntechB.V. 330
10.6.9PureWaterGroup 330
10.6.10TNOCompany 330
10.6.11EuwaH.H.EumannGmbH(Euwa) 330
10.6.12Hydro-ElektrikGmbH 331
10.6.13KHTECGmbH 331
10.6.14RomfilGmbH 331
10.7FutureDirections 331
10.8Conclusion 332 References 332
11ConclusionsandPerspective 337 MohammadYounasandMashallahRezakazemi
11.1FutureDirections 340
Index 342
Preface
Membranetechnologyhasbeenemergingasasustainabletechnologyinseparation scienceandengineeringforthelasttwodecades.Membraneshavebecomethe centerpointinwatertreatmentanddesalination,CO2 capturetechnology,and foodandpharmaceuticalindustry.Manypartsoftheworldarenowincritical needofcleanwater,energy,andfoodsecurity.Amongthemembranetechnology, themembranecontactortechnologyisgainingincreasingimportanceinwater treatmentanddesalination,gasseparation,andjuiceconcentrationbecauseitis workingatloworatmosphericpressureandtakingothernaturalforcesasdriving forces.Membranecontactorshaveabrightfutureindiverseapplicationareas. Tremendousworkhasbeencarriedoutonmembranecontactortechnologyand isstillinprogressindifferentworldregionstofurtherimprovethetechnology thanitscurrentstatus.However,ifobservedclosely,onecanrealizethatresearch anddevelopmentactivitieshavemostlyfocusedonmembranematerialssynthesis anddevelopmentratherthanfocusingonmembranecontactormoduledesignand development.Itappearsthereisatimelyneedforabookthatillustratesthebasic theory,applications,andpracticesofmembranecontactortechnologyinwater treatment,gasseparation,andjuiceconcentration.
Toundertakethetask,wearecomposedofyoungandenthusiasticengineers andscientiststohelpusindraftingthecontentsofthebookthatcancoverup thetheory,applications,andpracticeofmembranecontactorsinasuccinctbut easy-to-understandwayforanoviceworkinginmembranecontactortechnology. Together,weprepared11chaptersstartingfromthepresentationofthemembrane technologyingeneralandtheillustrationoftheneedformembranecontactor technology,followedbythedetaileddescriptionoftheoryandprocessesusedin membranecontactortechnology.Chapters2,3,and5coverthetheoreticalaspects andworkingprinciplesofmembranecontactorsusedasliquid–liquid,gas–liquid, andgas–gascontactors.Designaspectsofmembranecontactorweredescribedin Chapter4.Chapters6–9focusedonillustratingimportantapplicationsofmembrane contactorsinwatertreatment,juiceconcentration,andpre-andpost-combustion CO2 capture.Chapters6–9indeedcoveravastfieldofapplicationssuccinctly andbriefly.Marketaspects,conclusions,andperspectiveofthistechnologywere presentedattheendofthechapters.
Webelievethattheuniquefeatureofthisbookisthepresentationoftheory,design, practice,andapplicationsofmembranecontactortechnologyverybrieflyinone book.Thisis,ofcourse,arelativecomparison,ingeneral,withtheotherpublished booksonmembranecontactors.Anotherdistinctivefeatureofthebookisthata verystrongcoverageofalltheseparationprocessesusingmembranecontactorsfrom micro-tomacrolevelispresentedforthereasonsdescribedabove.
Wearetrulythankfulforthestrongcooperationfromallthecontributingauthors. Asamatteroffact,westillhavequitemanyideasthathavebeenpartofthebookbut havenotfinishedduetothefactthatwealwaysboundourselvestobeverybriefand toaddressthereaderswhoarenewtothisfield.Meanwhile,weareindeeddelighted tohavethisbookpublishedforbeginnersinmembranecontactortechnology.We alsowishtothankallthereviewersandchaptercontributors.
July2021
Peshawar,Pakistan MashallahRezakezemi
MohammadYounas
AbouttheAuthors
Dr.MohammadYounas obtainedtheMaster(II)by researchanddoctoratedegreesfromtheUniversitéde Montpellier2,France,during2006–2011.Healsoworked intheUniversityofTechnology,Eindhoven,Netherlands, during2011–2012asapartofEuropeanappliedresearch projectFP7(DemoCLOCK).Currently,Dr.Younasisworkingasprofessorandleaderofthe“MembranesandProcess Intensification”groupintheDepartmentofChemical EngineeringatUniversityofEngineeringandTechnology, Peshawar,Pakistan.HeisalsoengagedwithStateKeyLaboratoryofSeparation MembranesandMembraneProcesses,SchoolofMaterialsScienceandEngineering,TianjinPolytechnicUniversity,Tianjin300387,China,andtheUniversityof Toledo,OH,USA,asvisitingresearchscholar.
Hisresearchfocusisinthegeneralareaofthewater–energy–foodnexus,CO2 capture,gasseparation,anddesalination,totheserviceofthebroadareasoflearningandtraining.Specifically,hisresearchinengineeredandnaturalenvironmental systemsinvolvesmembrane-basedprocessesforenergy-efficientdesalination,environmentalapplicationsandimplicationsofnanomaterials,watertreatment,and juiceconcentration.Hehascoauthoredinmorethan70highlycitedjournalpublications,conferenceproceedings,andbookchapters.
Dr.MashallahRezekazemi receivedhisBScandMSc degreesin2009and2011,respectively,bothinChemicalEngineeringfromtheIranUniversityofScienceand Technology(IUST)andhisPhDfromtheUniversityof Tehran(UT)in2015.Inhisfirstappointment,Rezakazemi hasbeenservedasprofessoroftheFacultyofChemical andMaterialsEngineeringattheShahroodUniversityof Technologysince2016.Hehasalsoreceivedhisdegreepromotiontoassociate professorin2019.
Dr.Rezakazemi’sresearchisinthegeneralareaofthewater–energynexus,CO2 capture,gasseparation,anddesalination,totheserviceofthebroadareasoflearningandtraining.Specifically,hisresearchinengineeredandnaturalenvironmental
systemsinvolves(i)membrane-basedprocessesforenergy-efficientdesalination, CO2 capture,gasseparation,andwastewaterreuse;(ii)sustainableproduction ofgasstream,water,andenergygenerationwiththeengineeredmembrane;(iii) environmentalapplicationsandimplicationsofnanomaterials;and(iv)waterand sanitationindevelopingcountries.
Hehascoauthoredinmorethan100highlycitedjournalpublications,conferencearticles,andbookchapters.Hehasreceivedmajorawardsandgrantsfrom variousfundingagenciesinrecognitionofhisresearch.Notableamongtheseisthe KhwarizmiYouthAwardfromtheIranianResearchOrganizationforScienceand Technology(IROST)in2012andOutstandingYoungResearcherAwardinChemicalEngineeringfromAcademyofSciencesofIran.Heisbeingnamedastop1most HighlyCitedResearcherbyWebofScience(ESI).
IntroductiontoMembraneTechnology
MohammadYounas 1 andMashallahRezakazemi 2
1 UniversityofEngineeringandTechnology,DepartmentofChemicalEngineering,Peshawar25120,Pakistan
2 ShahroodUniversityofTechnology,FacultyofChemicalandMaterialsEngineering,Shahrood,Iran
1.1OverviewofMembraneTechnology
Membranetechnologyisageneraltermusedforarangeofdifferentseparation processes.Membraneseparationprocesseshavebeenproventobewell-established technologiesinawiderangeofwater,energy,food,andenvironmentalapplications throughouttheproduction,purification,andformulationofusefulproducts[1–4]. Thus,themembraneseparationprocesseshavebecometheleadingseparation technologyoverthepasttwodecades.Themembraneisdefinedasaselectivethin layerofasemipermeablematerialthatactsasaselectivebarrierandseparates undesiredspeciesfromafeedsolutionbasedontheirsizesoraffinitybyexerting apotentialgradient,suchaspressure,temperature,electrical,orconcentration difference(Figure1.1).Separationisaccomplishedifonespeciesofamixturemoves throughthemembranefasterthananotherspeciesinthemixture.Themainadvantageofmembranetechnology,whichdifferentiatesitfromtraditionalseparation, purification,andformulationprocesses,isthatitproducesstableproductswithout addingchemicalswitharelativelylowenergyconsumptionwitharemarkable potentialforanenvironmentalimpact.Otherbenefitsincludemodularandeasy toscale-up,well-arranged,compact,andstraightforwardprocessinconceptand operation,decreasedcapitalandoperationalcostoftechnologyapplicationsusing membrane,andenvironmentfriendly.
Ingeneral,membranesareclassifiedbasedontheiraverageporesize,driving force,morphology,andmaterials.Theporesizeofthemembranematerialorsurface isaparamountfactorinitsfirstdifferentiation.Nevertheless,membranematerials canbeorganicandinorganic.Allofthemembraneseparationprocessesareeffective methodsoftreatingthefeedmixture,e.g.water,gas,andfoodthathardlyistreated usingconventionalseparationmethods.
MembraneContactorTechnology:WaterTreatment,FoodProcessing,GasSeparation,andCarbonCapture, FirstEdition.EditedbyMohammadYounasandMashallahRezakazemi. ©2022WILEY-VCHGmbH.Published2022byWILEY-VCHGmbH.
Permeate
Figure1.1 Typicalmembraneseparationprocess.
1.2ConventionalMembraneSeparationProcesses
1.2.1Microfiltration(MF)
Microfiltration(MF)isthefirstclassificationofmembraneseparationtechniques basedonporesize.TheMFmembranewasfirstdevelopedtoanalyzethebacteria inthewater.Inthe1960s,thefirstcommercialMFmembranewasalsodeveloped inbiologicalandpharmaceuticalapplications.Sincethen,MFmembraneshave beenwidelyappliedinwastewatertreatmentandjuicetechnologytoremove microorganisms,clarifyciderandotherjuices,andsterilizebeerandwine.The separationmechanisminMFmembranesisgovernedbythesievingeffector sizeexclusiontechnique.Thus,thespeciesareseparatedaccordingtotheirsize. LargeporesofMFremovesuspendedsolids,whileevenproteinscanpassthrough theMFmembraneeasily.TheMFmembranescanalsobeusedtoseparatesand, clays,algae,andsomebacteriafromaqueousfeedstreams.Theyarerecommended toseparatespecieswithadiameterlargerthan0.1 μm.Theappliedpressurein MFislow(usually <2bar),whilethisisthelowestappliedpressureinother pressure-drivenmembraneseparationprocesses[5,6].
1.2.2Ultrafiltration(UF)
Ultrafiltration(UF)isalsoincludedinsizeexclusion-basedpressure-driven membraneseparationprocesses.TheporesizeofUFmembranesisaround 0.01 μm.Thesemembranescanpreventspeciesinthemolecularweightrangeof 300–500000Datopassthrough.UFrejectsproteinandsuspendedsolids.However,
1.2ConventionalMembraneSeparationProcesses 3 dissolvedsubstancescouldnotberemovedbyUFunlesstheyarefirstpretreatedin anadsorptioncolumnlikewithactivatedcarbonorcoagulatedwithalumoriron salts.Similarly,UFmembranescannotretainthemono-anddisaccharides,salts, aminoacids,organics,inorganicacids,orsodiumhydroxide.Theyexhibitsmall osmoticpressuredifferentialsduetotheirinabilitytorejectsalts,ascomparedwith reverseosmosis(RO).UFprocessesoperateat2–10bars.Separationefficiencywill furtherbeaugmentedifthedifferenceinthesizesofthespeciesishighenough.UF isconsiderednowadaystobethedominantpartofmembraneseparationprocesses duetoitsdiverseapplicationsinwater,energy,food,andtheenvironment.UF processesareconsideredthemostusedmembraneseparationprocessnextto dialysisandMF[7].
1.2.3Nanofiltration(NF)
Nanofiltration(NF)isanotherpressure-drivenmembraneprocessbetweenROand UFporesizeofaround0.001 μm.NFmembranesremovemostorganicmolecules, viruses,andarangeofsalts.Thesemembranesareoftenappliedtosoftenthe hardwaterbyremovingdivalentions.NFmembranespossessanegativecharge onthesurface.Itdemonstratestheanionrepulsion,whichmainlycausesthe speciesrejection.Lowrejectioniswitnessedforsaltswithmonovalentanionand nonionizedorganicswithamolecularweightbelow150.However,highrejection canbeobservedforsaltswithdi-andmultivalentanionsandorganicswitha molecularweightabove300.NFisadvantageousoverROindifferentaspects, suchasbeingoperatedatlowpressure,givinghighpermeateflux,retentionof multivalentsaltandorganicsolutes,andhavinglowinvestmentandoperationand maintenancecosts.
NFmembraneismoresuitableforionswithmorethanonenegativechargein singlechargedionspass,suchassulfateorphosphate.However,NFmembranesalso rejectunchargedandpositivelychargedionsaccordingtothemolecule’ssizeand shape.Forexample,thesamerejectionofcalciumchlorideandsodiumchloridecan beobservedwhiletherejectionofsodiumsulfateisthesameformagnesiumsulfate. Instead,therejectionofdi-andmultivalentanionsishighcomparedwiththatfor monovalentions.Thespeciesrejectiondecreaseswithincreasingconcentration. TheDonnanexclusionmodelcanexplainthisphenomenon.Thehigherthespecies concentration,themorecationsavailabletoshieldthenegativechargesonthe membranesurface,makingiteasierfortheanionstopassthroughthemembrane pores.Ontheotherhand,thechargedensityofionsalsoplaysanimportantrole initsrejection.Forexample,thesulfateionhasahigherchargedensitythanthe chlorideionandisalmostcompletelyrepelledbytheNFmembraneeveninahigh ionicstrengthsolutionsuchasseawater[8].
1.2.4ReverseOsmosis(RO)
ROdemonstrates,inprinciple,theleastpossibleporestructureamongthe membranes.WateristheonlyspeciesthatcanpassthroughtheROmembrane;
essentially,alldissolvedandsuspendedspeciesarerejected.ROmembraneshavea poresizeofaround0.0001 μm.Thepermeateisessentiallythepurewaterbecause ROalsoremovesmosthealthymineralssuchascalcium,zinc,magnesium,etc.that arepresentinthewaterandareusefulinacertainquantityfordrinkingwaterespeciallyforpeoplewithinadequatedietsandpeoplelivinginhotclimates.Thewater canbemadehealthybypassingtheROwaterthroughcalciumandmagnesium beds.ROremovesmonovalentionstodesalinatethesalinewater.BothNFandRO arealsotermedasdensemembraneseparationprocessesbecauseseparationrelies tosomeextentonphysicochemicalinteractionsbetweenthepermeate(species) andthemembranematerial.Inwastewatertreatmentandreclamation,ROsystems aretypicallyusedasthelaststepforremovingtotalorganiccarbon(TOC).ROhas beenproventoremovedissolvedspecieseffectively,microbes,andneutralbase compounds[9,10].
TounderstandtheworkingprincipleofRO,itishelpfultounderstandfirstosmosis.Osmosisreferstothemigrationofwaterfromaweakersolutiontothestronger solutionwhenasemipermeablemembraneseparatestwosaltsolutionsofdifferent concentrations.Themigrationofsaltscontinuesuntilthetwosolutionsreachthe sameconcentrations,achievingtheosmoticequilibrium.Thesemipermeablemembraneallowsthewaterspeciestopassthroughnaturally,butnotthesalt.InRO,the twosolutionsarestillseparatedbyasemipermeablemembrane,butthepressure isappliedtoreversethewater’snaturalflow.Thisforcesthewaterspeciestomove fromthemoreconcentratedsolutiontotheweaker.Thus,thesoluteaggregateon onesideofthesemipermeablemembraneandthepurewaterpassthroughthemembraneontheotherside.TheconceptofosmosisandROisdescribedschematically inFigure1.2where(a)and(b)illustratetheprocessofosmosisand(c)represents theRO.Ifacertainpressure(ΔP)appliedtotheconcentratedsolutionequalsthe osmoticpressuredifferencebetweenthetwosolutions(Δ�� ),thesystemreachesthe osmoticequilibrium,andwaterflowstops.Iftheappliedpressureexceedsosmotic pressure(ΔP > Δ�� ),waterflowsfromtheconcentratedsolutiontothedilute solution.Asummaryofpressure-drivenprocessesisoutlinedinTables1.1and1.2.
1.2.5Electrodialysis(ED)
Electrodialysis(ED)referstoanelectricallydrivenmembraneseparationprocess inwhichchargedionsareseparatedfromafeedsolutionthroughselectively
1.2ConventionalMembraneSeparationProcesses 5
Table1.1 Pressuredrivensize-basedmembraneprocessesfortheremovaloftypical pollutants.
Membraneseparationprocess
Feedcomponent
Water
Monovalentions
Multivalentions
Dissolvedsubstances
Viruses
Bacteria,protozoa
Suspendedsolids ion-permeablemembranes.InanEDprocessconfiguration,cationicandanionic membranesarealternatelyarrangedbetweenananodeandacathodeplate.By applyinganelectricalpotential,theionsmigratetowardtheanodeandcathode, andconsequently,thewatermoleculeisdeionized.AtypicalEDcellconsistsof electrodesandion-permeablemembranes,asshowninFigure1.3.Whenanelectric fieldacrossthemembranesisapplied,thecationsmovetowardthecathode,and theanionsmigratetowardtheanode.Thecationspassthroughthecation-selective membrane,whileanionspassthroughtheanion-selectivemembrane.Thus,the feedbecamedilutedinonesideandconcentratedintheelectrolyteontheother side.BestperformanceinEDmembranescouldbeachievedbyselectingthehighly permselective,physicallystrong,andlowelectricalresistancemembranes[5].
1.2.6Pervaporation(PV)
Pervaporation(PV)isamembraneseparationprocessusedtorecovermorevolatile componentsinliquidmixturethroughadensemembrane.ThePVisgovernedby apartialpressuredifferenceacrossthemembraneasthedrivingforcebyapplyingavacuumatthepermeateside[11–14].Thesolution–diffusionmodelgenerally describesthetransportofspeciesacrossnonporousmembranesinPV.Becauseof thenegativepressureonpermeateside,theosmoticpressureisnotalimitingfactor,asisthecaseforRO.Thepartialpressuredifferenceatfeedandpermeatesides causesthemorevolatileliquidtovaporizewithinthemembrane.Thevaporpasses throughthemembraneandfinallycondensesatthepermeateside(Figure1.4).PV
Table1.2 Comparativeanalysisofconventionalmembraneprocesses.
Microfiltration(MF)Ultrafiltration(UF)Nanofiltration(NF)Reverseosmosis(RO)Electrodialysis(ED) Membrane pervaporation(MPV)
F/PL/LL/L,G/LL/LL/LL/LL/G
Membrane material PolymericPolymeric/ ceramic
Polymeric/ ceramic/ mixedmatrix
PolymericPolymericPolymeric;polyvinyl alcoholcomposites, silicones,cellulose acetates
Membrane structure Symmetrical/ asymmetrical AsymmetricalAsymmetricalAsymmetricalAsymmetricalAsymmetrical Membrane morphology/ thickness PorousPorousPorous/denseDenseDenseDense
Supportlayer10–150 μm150–250 μm150 μm150 μm
Thinfilm1 μm1 μm1 μm1 μm
Poresize0.05–10
Drivingforce ΔP ΔP,activitydifference, concentration difference,temperature difference
Separation principle Sieving mechanism
SievingmechanismDonnanexclusion/ solution–diffusion/ capillaryflow
ΔP vacuum, chemicalpotential gradient
Solution–diffusionSolution–diffusion/ion migration Donnan exclusion/solution–diffusion
Operating pressure <2bar2–5bar5–15bar15–100barElectrical potential Partialpressure difference
Membrane moduletype
Tubular, hollowfiber
Plateandframe,spiral wound,tubular,hollow fiber
Plateandframe, spiralwound, tubular
Plateandframe, spiralwound, tubular
Plateandframe, spiralwound, tubular,hollowfiber
MWCO300–500000Da200–1000Da <500Da
ApplicationsSeparationof macromolecularto cellularsize particles (bacteria,fat, proteins, whey industry)
Fruitjuiceclarification andconcentration, milkseparation,food, beverage,anddairy, biotechnology,medical applications
Watersoftening, removalofcolor, hardness,TOC, sulfatefromwater, concentrationof organicswith molecularweightof 300–1000inthe foodand pharmaceutical
Permeateflux150l/m2 /h43I/m2 /h/bar
Solute rejection (type) Particles, clay,bacteria Macromolecules, proteins, polysaccharides, sugars,biomolecules, polymers,colloidal particles
HMWC,mono-,di-, and oligosaccharides, polyvalentions ( ive),MgSO4 , glucose,sucrose
SWRO,BWRO desalination
Separationof ionsmostlyin desalinationof water
Dehydrationofliquid organic,ethanol, isopropylalcohol, ethyleneglycol
HMWC,LMWC, sodium chloride, glucose,amino acids
Solute rejection(%) >90%Upto100% >90%
Issuesand problems Membranefoulingand concentration polarization
F/P,feed/permeate;TMP,transmembranepressuredifference;HMWC,high-molecular-weightcompounds;LMWC,low-molecular-weightcompounds;SWRO, seawaterreverseosmosis;BWRO,brackishwaterreverseosmosis;MWCO:molecularweightcutoff.
Figure1.3 Abasicelectrodialysissystem.
ischaracterizedbytheimpositionofabarrierlayerbetweentwophases.Masstransferoccursselectivelyacrossthemembranefromonesidetotheothersideofthe membrane.TheuniquephenomenonofPVisthephasechangerequiredoftheone phase(feed)diffusingacrossthemembrane[17–19].Sincedifferentspeciespresent inthefeedmixturepermeatethroughthemembraneatdifferentrates,alowconcentrationcomponentinthefeedmixturecanbehighlyenrichedinthepermeate. Thus,themembrane’sselectivitybecomesthedefiningfactorintherelativeflowof thedifferentspecies.PVhasgainedmoreattentionfromthechemicalindustryin thepastdecadeduetotheeffectiveseparationprocessforrecoveringvolatilecomponentsinliquidmixtures.Itiscurrentlyconsideredmoreeffectivefordehydration ofliquidhydrocarbonstoyieldhigh-purityorganics,mostnotablyethanol,isopropyl alcohol,andethyleneglycol.PV,duetoitssimplicityandeasyinstallation,isused asanintegratedprocesswithdistillation[20,21].
1.3MolecularWeightCutoff(MWCO)
Molecularweightcutoff(MWCO)isausefultoolforcharacterizingfiltrationmembranes.Inearlydevelopment,UFmembraneswereusedtopurifymacromolecules inbioseparationprocessessuchastoretaintheproteins.Sincetheirmolecular weightcharacterizesmacromolecules,themembranesarealsocharacterized bywhetherthemacromoleculesuptocertainmolecularweightsareretained.It dependsonthesizeoftheporeofthemembranes.MWCOisindicatedinDaltonthat referstotheMWCOofspeciesorsolutewith90%rejection.MWCO500describes
1.4ConcentrationPolarization 9
Figure1.4 Membranepervaporationprocess.Source:BasedonWinzelerandBelfort[15] andShirazietal.[16].
thatthemoleculeswithmolecularweight(MW)above500arerejected,andthose withbelow500arepassedthroughthemembrane.TheMWCOofanymembrane canbealteredfromthechemistryofthesolutewithmembraneinteraction,their molecularorientationandconfiguration,andtheoperatingconditions[22].
1.4ConcentrationPolarization
Concentrationpolarizationinmembranefiltrationisoneofthesignificantproblems thathindersthesolventfluxandsoluterejection.Concentrationpolarizationis animportantfeatureinmembraneseparationprocesses.Speciesrejectedbythe membraneaccumulateatthemembranesurface.Thisaccumulationofspecieson thesurfaceofthemembraneiscalledconcentrationpolarization.Itproducesa concentrationgradientinthezonewherethespeciesaccumulate.Thereremains
abalancebetweenspeciesbroughttothemembranesurfacebyconvectiveflowof thesolventandback-diffusestothebulk.Attimes,however,thebalanceinspecies concentrationatthemembranesurfacediminishes.Itreachesitssolubilitylimit, whichislowerthanthatpredictedbythefluidhydrodynamicsofthesystem.
Consequently,themembraneeffectivelyexperiencesahigherfeedsideconcentrationatitsinterface,resultinginreducedfluxandreducedapparentsolute rejection.Oftentheseverityofconcentrationpolarizationcanbecontrolledby operatingconditions,modulegeometry,andfluidhydrodynamics.Concentration polarizationpracticestoasmallerincreaseintransmembranesolventfluxwiththe riseinoperatingfeedpressureuntilagellayerisformedatthemembrane’ssurface. Itcanalsobelessenedbyincreasingthefluidshareatthesurfaceofthemembrane orproducingtheturbulencebyintroducingthechannelspacersinthemodules. Thus,thetransmembranesolventfluxshowsnofurtherincreasewiththepressure andistermedaslimitingflux[15,23].
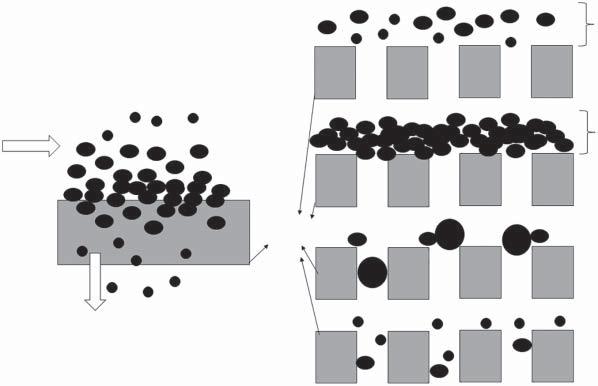
1.5MembraneFouling
Foulingreferstothedepositionofsoluteoranyotherspeciesinfeedonthemembranesurfaceorinsidethemembranepores.Forexample,ifthebalanceinspecies concentrationatthesurfaceduetoconvectiveflowandfeedbulkconcentration reachesthepointwherespeciesprecipitatesorformsathixotropicgel,thesituation istermedasfouling.Theformedgellayercausesanadditionalmasstransfer resistanceinconjunctionwiththemembraneitself.Insuchcases,increasedapplied feedpressuremaynotimprovethetransmembraneflux;ratheritwillincreaseor densifythegellayer[24].
Foulingmaybecausedbytheporegeometry/tortuosityorspecies–porewall interactions.Consequently,theporesareblockedentirelyorbemarginallyreduced indiameter,causingadeclineintransmembranefluxwhiletherejectionmay beeitherconstantormayincrease.Properandscheduledmembrane/module cleaningmayreversethefouling;however,irreversiblefoulingmayalsooccur overtime,permanentlydeterioratingthemembranesurfaceandpores.Insuch cases,themembrane’sreplacementbecomesindispensabletoregaintheactual transmembranefluxandthespeciesrejection.Althoughbothconcentration polarizationandfoulingreducetransmembranesolventflux,theyhaveopposite effectsonspeciesrejection.Forexample,concentrationpolarizationisafunctionof operatingparameterslikepressure,temperature,feedconcentration,andvelocity butisindependentoftime.Incontrasttothat,foulingispartiallydependenton operatingparameters,particularlyfeedconcentration,butisalsotimedependent. ThesephenomenahavebeendescribedschematicallyinFigure1.5[15,16].
InUF,feed-sidemasstransferresistanceandresistanceduetothegel/cake layerformationonthemembranesurfacebecauseoffoulingplayanessential roleintransmembranefluxandspeciesrejection.Usually,thepropermembrane processandmaterialselectionarechosentodecreasethefoulingtendenciesofthe membranesurface.Thebasepolymersurfacechemistrycanbemodifiedtoincrease
1.7HistoricalPerspective
Concentration polarization
Figure1.5 Membranefoulingandconcentrationpolarization.(a)Membraneseparation process.(b)Concentrationpolarization.(c)Gel/cakelayer.(d)Poreblocking/plugging. (e)Poreadsorption.
hydrophilicityofthemembranewiththecontactingfluidtoincreasethefluxand reducethefoulinginmostaqueousapplications.Fouling,scaling,orchemical interactiongreatlyaffectstheNFandROsystemswhileMFandUFaremildly affectedintheireffectiveoperation.Thus,extensivepretreatmentisoccasionally mademandatorybeforeNFandROtoavoidconditionsleadingtofouling,scaling, orchemicalinteraction[16,23].
1.6Diafiltration
Insomecases,duringfiltration,recoveryofspeciesmaybehinderedduetoreduced flux,highfeedviscosity,solubilitylimitsofnonpermeatingsolutes,etc.Forsuch cases,theconcentrateisdilutedbysolvent(water)duringcontinuousfiltrationuntil satisfactoryrecoveryofthepermeablespecies,whichistermeddiafiltration.
1.7HistoricalPerspective
Thehistoryofmembraneseparationtechnologydatesbackto1748whentheFrench AbbeNolletpublishedhisobservationsonosmoticphenomena[25].Thestudyof UFhasbeencloselyassociatedwiththatofdialysis.Dialysisexperimentsthrough artificialmembranesofcollodionwererecordedbyFick[26].Similarobservations ondialysisweremadebyHoppe-Seyler[27]andSchumacher[5].Thepioneering studyUFprocesswasreportedbySchmidt[28],whoinvestigatedthefiltrationofa solutionofproteinorgumArabicthroughananimalmembrane.
Inthethirddecadeoftwentiethcentury,membraneswereregardedasmechanical sieves,andpermeabilitywasconsideredasthesoledependentonparticleandpore dimensions.Theconceptofsemipermeabilityofthemembraneandthetheoryof partialsolubilitywasalsointroducedinthisdecade,whichdescribesmembrane’s permeabilityassolventdissolvinginthemembranefromonesidetotheother.For thefirsttime,themembranewasusedinseawaterdesalinationtoproducesources forfreshwater,putforthbyHassler[29].Later,ReidandBretonintroducedtheRO membraneswhentheydevelopedthecellulosediacetatefilmshowingsaltrejection upto96%[30].However,thebreakthroughwasachievedwhenLoebandSourirajan developedacellulosediacetateasymmetricmembraneandsuccessfullytestedfor highfluxandsaltrejection.
DuPontdevelopedahollowfibercapillarymembranefromaromaticpolyamide. However,in1985,Cadottepreparedhigh-performancemembranesusinginsitu interfacialpolycondensationbetweenpoly/monomericamineandpoly/monomeric functionalacidhalide[31,32].Thisopenedanewerafortheresearcherstoexplore thepolyamidefilmsbycrosslinking,whichgavehighpermeatefluxthatcellulose acetate(CA)membranes[9].
FilmTecintroducedthetwo-layerdesignmembranemodulesforwaterdesalinationattheindustrialscale,whichisstilldominatingthedesalinationindustry. Undoubtedly,themembranematerialandthemoduleshavebeenimproved overtheyears,butthebasicconceptadoptedbyFilmTecisstillwidelyaccepted. DesalinationSystems,Inc.(DSI)introducedthree-layercompositemembranesfor NFandRO.Inthetwenty-firstcentury,membraneseparationprocessessuchasUF, NF,andROemergedasreliabletechnologyinwater,food,environment,andfood.
1.8ConcludingRemarksandFutureChallenges
Membranetechnologyhasbecomeanimportantentityofourdailyliferoutine work.Membraneshaveapotentialinthefuture.Waterscarcityandwaterstress, carboncapture,foodsecurity,energyconstraints,environmentalregulations, andnanotechnologyarekeydriverstoboostthemembranetechnologyfurther. However,thedevelopmentinmembranetechnologywouldriseexponentiallyif “engineeringaspects”inallmembraneseparationprocesseswithkeyattentionto “industrialists”and“entrepreneurs”arecorrectlyaddressed.Membranesperform thespecifictaskforwhichtheyaredesigned.Eachtypeofmembranefiltration, e.g.MF,UF,NF,andRO,hasitsownroledependinguponthepores’size,driving force,operatingconditions,membranematerialproperties,andphysicochemical interactionwithfeedcomponents.However,ifaspecificmembranewaschosenfor theparticularapplicationandprocess,itperformswellandachievestherequired objectives.Twofactsshouldbekeptfirmlyinmindbeforedecidinganymembrane process:“Membranesdonotlie.”Thestatementdescribesthatmembranesdo exactlywhattheycandounderthegivencircumstances.Forexample,ifthe membranematerialisnotcompatiblewiththefeedsolutionorcannotwithstand theoperatingparameters,themembranewillnotperformtoexpectations.In
1.8ConcludingRemarksandFutureChallenges 13 suchcases,thiswillnotbethedeficiencyofthemembrane.Theotherfactis: “Membranesaredesignedtorejectdissolvedsolids.”Thismeansthatifthefeed mixturecontainssubstantialanddiverseundesiredcomponentslikesuspended solids,thenthemembranesystemswillperformverypoorly.Soforeachmembrane separationprocess,thefeedcharacteristicshavealsobeendescribed,andtheir protocolshouldbeobeyed.Otherwise,pretreatmentoffeedshouldbeensuredthat thefeedsolutionisfreeofspeciesthatmayprecipitateordegradethemembrane poresandsurfaceduetotheiraggregationduringtheprocess.
Inthetwenty-firstcentury,theworldisfacingmoreseverechallengesthanever towardsustainabledevelopmentintermsofwaterqualityandsourcesindeveloped anddevelopingcountries,meetingincreasingenergydemands,securingthefood shortages,andcontrollingtheadverseeffectsofglobalwarming.Therefore,the demandfortheuseofnovelmembranes,innovativeprocesses,andcompactmodulardesignstoaddresstheseissuesinvariousapplicationswillcontinuetoincrease. Theconventionalmembraneseparationprocesseshavealreadyemergedasa promisingtechnologyindifferentwaterfoodandenvironmentsectorapplications. Still,thereremainedagaptodevelopthemembranetechnologytobedrivenby higherproductivity,lowercostofproduction,andincreaseddevelopmentspeed. Itwaslearnedthatseveralmembranecharacteristicscoulddetermineamembrane’ssuitabilityforaspecificseparationapplication.Theseinclude(i)porosity, (ii)morphology,(iii)surfaceproperties,(iv)mechanicalstrength,(v)chemical resistance,(vi)selectivity,and(vii)drivingforce.Thesecharacteristicsdependon theproperchoiceofmembranematerialandthesynthesistechnique.Furtherto that,moduledesignisalsoessentialtoagreatextenttoachievetheseproperties. Thesecharacteristicsareinterrelated;forexample,ahighlyporousmembrane structurecanbemaintainedonlyifthepolymerhasadequatemechanicalstrength orthemembraneshouldbeoperatedatloworatmosphericpressure.Surface propertiesandporemorphologyarelinkedtofoulingproperties,fluxthroughthe membrane,andsoluteseparation.Thereisaneedtoreduceorevenremovethegas betweenscientistsandindustrialists.Forexample,scientistsandengineers’major challengesareasfollows:(i)membranedesignsshouldbemanufacturerspecific, and(ii)application-specificmembranesshouldbedevelopedtargetingthespecific industry.Membranesystemcostsandapplicationsarecurrentlymateriallylimited, whereasmembraneperformanceismeasuredassolventfluxandselectivitywhich arethelimitingfactorsforscientistsandengineers.However,foranefficientand economicallyfeasibleindustrialapplication,membranesneedtokeeptheirwhole lifetimeintegrity.Unfortunately,theintegrityandfluxorselectivityisoftenin theoppositetrend.Lessintegritywilllessenthemembranelifeandthusismeant forhigherreplacementcostsofthemembrane.Itisalsonotedthatmembrane technologyhasitsowndisadvantages.Forexample,highpressureasadriving forcecauseshighenergyconsumptionandpollutiontotheenvironmentandusesa rangeofchemicalsolventthatcouldbeveryharmfultotheenvironment.Thus,the futuredevelopmentofmembranetechnologyanditsapplicationscouldconform withthesustainabledevelopmentgoals(SDG).Theoretically,0.7kWh/m3 should betheminimumenergyrequiredtoconvertseawatertopurewater[33].Membrane
separationtechnologyiscurrentlyconsideredamongthebestavailabletechnologies (BAT)inthenexusofmanyprocessesandapplicationslikefood,water,energy, andtheenvironment.However,withthecurrentchoiceofmaterials,modules, andtechnology,theenergyconsumptionstillstandsbetween2and5kWh/m3 [34].Thus,theresearchisfocusedonincreasingtheseparationefficiency,reducingenergyconsumption,andmakingitmoreenvironmentfriendlyandfouling resistant.Thegapbetweenscientistsandindustrialistsshouldberemoved.Such objectivescouldbeachievedbyadoptingthemembranecontactortechnology andswitchingovertoconcentrationdifferenceasadrivingforceinsteadofusing pressuredifferenceasthedrivingforce.Thesuccessfuldesignandoperationof membranesystemslieinadeeperunderstandingofprinciples,engineering,and practicalaspectssuchasinterfacialphenomena,rheology,materialscience,and moduledesignofmembraneseparationprocesses.Theresearchanddevelopment (“R&D”)effortsshouldbefocusedratheron“engineeringapplications”suchas water,energy,food,andtheenvironment.Thiswouldalsoleadtocleartheapproach thatanymembranemodulewillresultintheexpectedseparation.
References
1 Sheikh,M.,Pazirofteh,M.,Dehghani,M.etal.(2020).ApplicationofZnO nanostructuresinceramicandpolymericmembranesforwaterandwastewater technologies:areview. ChemicalEngineeringJournal 391:123475.
2 Mirqasemi,M.S.,Homayoonfal,M.,andRezakazemi,M.(2020).Zeoliticimidazolateframeworkmembranesforgasandwaterpurification. Environmental ChemistryLetters 18(1):1–52.
3 Rezakazemi,M.,Sadrzadeh,M.,andMatsuura,T.(2018).Thermallystable polymersforadvancedhigh-performancegasseparationmembranes. Progressin EnergyandCombustionScience 66:1–41.
4 Rezakazemi,M.,Dashti,A.,RiasatHarami,H.etal.(2018).Fouling-resistant membranesforwaterreuse. EnvironmentalChemistryLetters 16(3):715–763.
5 Lonsdale,H.K.(1982).Thegrowthofmembranetechnology. JournalofMembraneScience 10(2–3):81–181.
6 Fane,A.T.,Wang,R.,andJia,Y.(2011).Membranetechnology:past,presentand future.In: MembraneandDesalinationTechnologies (eds.L.K.Wang,J.P.Chen, Y.-T.HungandN.K.Shammas),1–45.Springer.
7 Zeman,L.J.andZydney,A.(2017). MicrofiltrationandUltrafiltration:Principles andApplications.CRCPress.
8 Jye,L.W.andIsmail,A.F.(2016). NanofiltrationMembranes:Synthesis,Characterization,andApplications.CRCPress.
9 Uemura,T.andHenmi,M.(2008).Thin-filmcompositemembranesforreverse osmosis.In: AdvancedMembraneTechnologyandApplications (eds.N.N.Li,A.G. Fane,W.S.W.HoandT.Matsuura),1–19.Wiley.
10 Amjad,Z.(1993). ReverseOsmosis:MembraneTechnology,WaterChemistry,and IndustrialApplications.NewYork:VanNostrandReinhold.