AtomicClusterswithUnusual Structure,BondingandReactivity
TheoreticalApproaches,Computational AssessmentandApplications
Editedby PratimKumarChattaraj
IndianInstituteofTechnologyKharagpur,Kharagpur,India
SudipPan
InstituteofAtomicandMolecularPhysics,JilinUniversity,Changchun,China
GabrielMerino UniversidaddeMerida,Merida,Mexico
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2023ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthe Publisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearance CenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecome necessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusing anyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethods theyshouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhavea professionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliability foranyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,or fromanyuseoroperationofanymethods,products,instructions,orideascontainedinthematerialherein.
ISBN:978-0-12-822943-9
ForinformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionsEditor: CharlesBath
EditorialProjectManager: JudithClarissePunzalan
ProductionProjectManager: KumarAnbazhagan
CoverDesigner: MarkRogers
TypesetbySTRAIVE,India
GuruduttDubeyandPrasadV.Bharatam
theoryandallmetalaromaticity
DebolinaPaulandUtpalSarkar
6.Structuralevolution,stability,and spectraofsmallsilverandgold clusters:Aviewfromtheelectron shellmodel
PhamVuNhat,NguyenThanhSi, andMinhThoNguyen
1.Introduction
2.Equilibriumstructuresandgrowth
3.Thermodynamicstabilities
4.Phenomenologicalshellmodel
5.Electronicabsorptionspectra
7.Opticalresponsepropertiesof somemetalclustersupported host-guestsystems
ArpitaPoddarandDebduttaChakraborty
1.Introduction
3.1Geometricalstructuresand thermodynamicfeasibilityof obtainingthecorrespondinghostguestmoieties
3.2Opticalandelectronicpropertiesof theselectedmetalcluster-host complexes
8.GroupIII–Vhexagonalpnictide clustersandtheirpromisefor graphene-likematerials
EshaV.ShahandDebeshR.Roy
5.GroupIII–Vgraphene-likematerialsfrom potentialclusterunits
thermoelectrics
5.2Mono-andmultilayerthalliumnitride forthermoelectrics
5.3Othertwo-dimensionalgroupIII–V
9.M(L)8 complexes(M=Ca,Sr,Ba; L=PH3,PF3,N2,CO):Actofan alkaline-earthmetalasa conventionaltransitionmetal
Hai-XiaLi,Zhong-HuaCui,DandanJiang, LiliZhao,andSudipPan
6.M(Bz)3:20-electroncomplex
10.Structures,reactivity,and propertiesoflowionization energyspeciesdopedfullerenes andtheircomplexeswith superhalogen
AbhishekKumar,AmbrishKumarSrivastava, GargiTiwari,andNeerajMisra
1.Introduction
3.LowIEspeciesdoped endofullerenes
3.1Li@C60 vsSA@C60 endofullerene (SA=FLi2,OLi3,andNLi4)
3.2Li@C60 vsLr@C60 endofullerene 176
4.Endofullerene-superhalogen complexes 177
4.1Li@C60 PF6 endofullerene complex 177
4.2SA@C60 BF4 endofullerene complex 179
5.Conclusionsandperspectives
11.Generationofglobalminimum energystructuresofsmall molecularclustersusingmachine learningtechnique
GourhariJanaandRanitaPal
1.Introduction
2.Ourproposedmethodologyand algorithm(parallelimplementation) 187
2.1Particleswarm optimization 187
2.2Fireflyalgorithm 188
2.3ADMP-CNN-PSOapproach 190
3.Computationaldetails 191
4.Experimentalsetup 191
4.1PSO,FA,andADMP-CNN-PSO 192
5.Resultsanddiscussion 192
5.1PSO:Boronclusters,Bn (n =5,6) 192
5.2CNNandPSO:N4 2 ,N6 4 ,Aun (n =2–8)andAunAgm (2 n + m 8) clusters
5.3Fireflyalgorithmwithdensity functionaltheory 202
6.Conclusion 206 Acknowledgments 206
12.Studiesonhydrogenstoragein molecules,cages,clusters,and materials:ADFTstudy
K.R.Maiyelvaganan,M.Janani,K.Gopalsamy, M.K.Ravva,M.Prakash,andV.Subramanian
1.Introduction
2.H-storageinvariousmotifs—Theroad maprepresentation
2.1H-storageinsmallmolecules 215
2.2Hydrogenstorageinmolecular cages
2.3H-storageinmolecularclusters
2.4H-storageinmaterials
13.Adensityfunctionaltheorystudy ofH3+ andLi3+ clusters:Similar structureswithdifferentbonding, aromaticity,andreactivity properties
DongboZhao,XinHe,MengLi, ChunnaGuo,ChunyingRong, PratimKumarChattaraj,andShubinLiu 1.Introduction
14.Designingnanoclustersfor catalyticactivationofsmall molecules:Atheoreticalendeavor
AnupPramanik,SouravGhoshal, andPranabSarkar
1.Introduction
2.N2 activation
3.H2 activation
4.ActivationandreductionofCO2
4.1Specificroleofmetalhydrideforthe reductionofCO2 254
5.ActivationofO2 andoxidationofCOon Aun nanoclusters 255
5.1EffectofdopinginAun nanoclusters 256
5.2Aln anionicnanoclusters:Effectof electronspin 257
6.H2Oactivation 258
7.C–XandC–Hbondsactivation 260
7.1C–XbondactivationonAln nanoclusters 260
7.2CompetitiveH–Xeliminationon aluminananoclusters 260
7.3Selectivityofaluminananoclusters duringelimination 262
7.4SelectiveC–Hbond activation 262
8.Summaryandfutureoutlook
15.Molecularelectrides:Anoverview oftheirstructure,bonding,and reactivity
RanajitSahaandPrasenjitDas
1.Introduction
1.1Electrides
1.2Confinementoftheelectron
1.3Developmentof organic electrides
1.4Developmentof inorganic electrides 276
1.5Towardthe molecularelectride 277
2.Normsandconditionsofbeing a molecularelectride 278
3.Computationalmethodology 279
4.Examplesofmolecularelectrides 281
4.1Alkalimetal-doped electrides 281
4.2Mg2EP,molecularelectrideandsmall moleculeactivation 283
4.3Bondingin[Mg4(HDippL)2]2 complex anditselectridenature 285
4.4Mg2@C60 anditselectride characteristics 286
4.5BinuclearSandwichcomplexesof alkalineearthmetalsaselectrides 287
4.6Li3@Cg(Cg=B40 andC60)andtheir electridenature 288
5.Conclusion
16.Hydrogentrappingpotentialofa fewnovelmolecularclusters andions
SukantaMondal,PrasenjitDas,and SantanabGiri
1.Introduction
2.Theoreticalbackground
3.Computationaldetails
4.Atomicandmolecularclusters
4.1MgandCaclusters
4.2B2LiandB2Li2 moieties
4.3C12N12 cage
5.Ionicclusters
5.1N4Li2 andN6Ca2 clusters
5.2Li3 + andNa3 + ions
5.3B2Li+ andB2Li2 + ions
5.4M5Li7
17.Polarizabilityofatomsandatomic clusters
SwapanK.Ghosh
1.Introduction 313
2.Basicsofresponsepropertiesand polarizability 314
3.DFT-basedapproachtocalculationof polarizability 314
4.Polarizabilityofsphericallysymmetric systems:Atomsandatomicclusters withinthejelliummodel 317
5.Chemicalreactivityindices-basedroute topolarizability 318
6.Discussiononpolarizabilityvaluesof atomicclusters
18.Advancesinclusterbonding: Bridgingsuperatomicbuilding blocksviainterclusterbonds
NikolayV.Tkachenko,Zhong-MingSun, AlexanderI.Boldyrev,andAlvaro Munoz-Castro
1.Introduction
2.Interclusterbondingofgoldclusters
3.InterclusterbondingofZintlclusters
4.Extendednetworks
19.Zintlclusterasabuildingblockof superalkali,superhalogen, andsuperatom
SwapanSinha,RuchiJha,SubhraDas,and SantanabGiri
1.Introduction
20.Metallicclustersforrealizing planarhypercoordinatesecondrowmaingroupelementsand multiplebondedspecies
AmlanJ.Kalita,ShahnazS.Rohman, ChayanikaKashyap,LakhyaJ.Mazumder, IndraniBaruah,RitamRajBorah, FarnazYashmin,KangkanSarmah, andAnkurK.Guha
1.Introduction
2.Planarhypercoordinatemaingroup elements
3.Planarpentacoordinatenitrogen 348
4.Metalclustersupportedmultiplebonded second-rowmaingroupelement
5.Conclusionsandfutureaspects
21.Planarhypercoordinatecarbon
PrasenjitDas,SudipPan, andPratimKumarChattaraj
1.Introduction
2.Planartetracoordinatecarbon(ptC) 357
3.Planarpentacoordinatecarbon (ppC) 361
4.Planarhexacoordinatecarbon(phC) 365
5.Highercoordinatecarbon
6.Conclusion
22.Transformationofnanoclusters withoutco-reagent
SaniyaGratious,SayaniMukherjee, andSukhenduMandal
1.Introduction 373
2.Co-reactant-freetransformations 373
2.1pH-inducedtransformation 373
2.2Solvent-inducedtransformation 375
2.3Photo-inducedtransformation 379
2.4Temperature-induced transformation 381
3.Perspectivesandconclusions
23.ApplicationoffrustratedLewis pairsinsmallmoleculeactivation andassociatedtransformations
DandanJiang,ManasGhara,SudipPan, LiliZhao,andPratimKumarChattaraj
1.Introduction 387
2.ThechemistryofLewisacidsand bases 387
3.IdentificationofFLPreactivity 389
4.MechanismofH2 activationbyFLPs 389
5.ThermodynamicsonH2 activationby FLP 392
6.Activationofothersmallmolecules 393
7.Aromaticity-enhancedsmallmolecule activation 397
8.Catalytichydrogenation 398
9.Boron-ligandcooperation 401 10.Polymerizationreaction 403 11.Summaryandoutlook 407 References 407
24.Ligand-protectedclusters
YukatsuShichibuandKatsuakiKonishi
1.Introduction 411
2.Representativeexamplesoftheoretical studies 411
3.Diphosphine-ligatedgoldclusters 411 3.1Jelliummodelsandcoreshapes 411
3.2Geometricstudies 413
3.3Electronicstudies 414
3.4Effectsofligandsongeometricand electronicstructures 416 4.Conclusion
Contributors
Numbersinparenthesesindicatethepagesonwhichtheauthors’ contributionsbegin.
IndraniBaruah (345),AdvancedComputationalChemistryCentre,DepartmentofChemistry,CottonUniversity,Guwahati,Assam,India
PrasadV.Bharatam (61),DepartmentofMedicinal Chemistry,NationalInstituteofPharmaceuticalEducationandResearch,S.A.S.Nagar,Punjab,India
AlexanderI.Boldyrev (321),DepartmentofChemistry andBiochemistry,UtahStateUniversity,Logan,UT, UnitedStates
RitamRajBorah (345),AdvancedComputationalChemistryCentre,DepartmentofChemistry,CottonUniversity,Guwahati,Assam,India
DebduttaChakraborty (123),DepartmentofChemistry, BirlaInstituteofTechnology,Mesra,Ranchi,Jharkhand,India
PratimKumarChattaraj (237,357,387),Departmentof Chemistry,IndianInstituteofTechnology,Kharagpur, India
Zhong-HuaCui (157),InstituteofAtomicandMolecular Physics,KeyLaboratoryofPhysicsandTechnologyfor AdvancedBatteries(MinistryofEducation),JilinUniversity,Changchun,China
PrasenjitDas (275,297,357),DepartmentofChemistry, IndianInstituteofTechnologyKharagpur,Kharagpur, India
SubhraDas (333),SchoolofAppliedSciencesand Humanities,HaldiaInstituteofTechnology,Haldia; DepartmentofChemistry,CoochBeharPanchanan BarmaUniversity,CoochBehar,WestBengal,India
GuruduttDubey (61),DepartmentofMedicinalChemistry,NationalInstituteofPharmaceuticalEducation andResearch,S.A.S.Nagar,Punjab,India
ManasGhara (387),DepartmentofChemistryandCentre forTheoreticalStudies,IndianInstituteofTechnology Kharagpur,Kharagpur,India
SwapanK.Ghosh (313),UM-DAE-CentreforExcellence inBasicSciences,UniversityofMumbai,Mumbai, India
SouravGhoshal (247),DepartmentofChemistry,VisvaBharatiUniversity,Santiniketan,India
SantanabGiri (297,333),SchoolofAppliedSciencesand Humanities,HaldiaInstituteofTechnology,Haldia, India
K.Gopalsamy (213),CenterforHighComputingandInorganicPhysicalChemistryLaboratory,CentralLeather ResearchInstitute,CouncilofScientificandIndustrial Research,Chennai,TamilNadu,India
SaniyaGratious (373),SchoolofChemistry,Indian InstituteofScienceEducationandResearchThiruvananthapuram,Trivandrum,Kerala,India
AnkurK.Guha (345),AdvancedComputationalChemistryCentre,DepartmentofChemistry,CottonUniversity,Guwahati,Assam,India
ChunnaGuo (237),KeyLaboratoryofChemicalBiology andTraditionalChineseMedicineResearch(Ministry ofEducationofChina),HunanNormalUniversity, Changsha,Hunan,PRChina
XinHe (237),KeyLaboratoryofChemicalBiologyand TraditionalChineseMedicineResearch(Ministryof EducationofChina),HunanNormalUniversity, Changsha,Hunan,PRChina
GourhariJana (185),DepartmentofChemistry,Indian InstituteofTechnologyBombay,Mumbai,India
M.Janani (213),DepartmentofChemistry,Faculty ofEngineeringandTechnology,SRMInstituteof ScienceandTechnology,Chengalpattu,TamilNadu, India
RuchiJha (333),AdvancedTechnologyDevelopment Center(ATDC),IndianInstituteofTechnologyKharagpur,Kharagpur,WestBengal,India
DandanJiang (157,387),InstituteofAdvancedSynthesis,SchoolofChemistryandMolecularEngineering,JiangsuNationalSynergeticInnovation CenterforAdvancedMaterials,NanjingTechUniversity,Nanjing,China
AmlanJ.Kalita (345),AdvancedComputationalChemistryCentre,DepartmentofChemistry,CottonUniversity,Guwahati,Assam,India
ChayanikaKashyap (345),AdvancedComputational ChemistryCentre,DepartmentofChemistry,Cotton University,Guwahati,Assam,India
KatsuakiKonishi (411),GraduateSchoolofEnvironmentalScience,HokkaidoUniversity,Sapporo,Japan
AbhishekKumar (173),DepartmentofPhysics,UniversityofLucknow,Lucknow,UttarPradesh,India
Hai-XiaLi (157),InstituteofAtomicandMolecular Physics,KeyLaboratoryofPhysicsandTechnology forAdvancedBatteries(MinistryofEducation),Jilin University,Changchun,China
MengLi (237),KeyLaboratoryofChemicalBiologyand TraditionalChineseMedicineResearch(Ministryof EducationofChina),HunanNormalUniversity, Changsha,Hunan,PRChina
ShubinLiu (237),ResearchComputingCenter; DepartmentofChemistry,UniversityofNorthCarolina, ChapelHill,NC,UnitedStates
K.R.Maiyelvaganan (213),DepartmentofChemistry, FacultyofEngineeringandTechnology,SRMInstitute ofScienceandTechnology,Chengalpattu,TamilNadu, India
SukhenduMandal (373),SchoolofChemistry,Indian InstituteofScienceEducationandResearchThiruvananthapuram,Trivandrum,Kerala,India
LakhyaJ.Mazumder (345),AdvancedComputational ChemistryCentre,DepartmentofChemistry,Cotton University,Guwahati,Assam,India
JoseM.Mercero (19),KimikaFakultatea,Euskal HerrikoUnibertsitatea( UPV/EHU)andDonostia InternationalPhysicsCenter(DIPC),Donostia, Euskadi,Spain
NeerajMisra (173),DepartmentofPhysics,Universityof Lucknow,Lucknow,UttarPradesh,India
M.Molayem (41),PhysicalandTheoreticalChemistry, SaarlandUniversity,Saarbrucken,Germany
SukantaMondal (297),DepartmentofEducation, AshutoshMukhopadhyaySchoolofEducational Sciences,AssamUniversity,Silchar,Assam,India
SayaniMukherjee (373),SchoolofChemistry,Indian InstituteofScienceEducationandResearchThiruvananthapuram,Trivandrum,Kerala,India
AlvaroMun ˜ oz-Castro (321),GrupodeQuı´micaInorga ´ nicayMaterialesMoleculares,FacultaddeIngenierı´a,UniversidadAutonomadeChile,ElLlano Subercaseaux,Santiago,Chile
MinhThoNguyen (99),InstituteforComputational ScienceandTechnology(ICST),QuangTrungSoftware City,HoChiMinhCity,Vietnam
PhamVuNhat (99),DepartmentofChemistry,CanTho University,CanTho,Vietnam
EdisonOsorio (1),FacultyofNaturalSciencesand Mathematics,UniversityofIbague,Ibague,Colombia
RanitaPal (185),AdvancedTechnologyDevelopment Centre,IndianInstituteofTechnologyKharagpur, Kharagpur,India
SudipPan (157,357,387),InstituteofAtomicand MolecularPhysics,JilinUniversity,Changchun,China
DebolinaPaul (87),DepartmentofPhysics,Assam University,Silchar,India
ArpitaPoddar (123),DepartmentofChemistry,Indian InstituteofTechnologyKharagpur,Kharagpur,West Bengal,India
M.Prakash (213),DepartmentofChemistry,Facultyof EngineeringandTechnology,SRMInstituteofScience andTechnology,Chengalpattu,TamilNadu,India
AnupPramanik (247),DepartmentofChemistry,SidhoKanho-BirshaUniversity,Purulia,India
M.K.Ravva (213),DepartmentofChemistry,SRM University—AP,Amaravati,AndhraPradesh,India
ShahnazS.Rohman (345),AdvancedComputational ChemistryCentre,DepartmentofChemistry,Cotton University,Guwahati,Assam,India
ChunyingRong (237),KeyLaboratoryofChemical BiologyandTraditionalChineseMedicineResearch (MinistryofEducationofChina),HunanNormal University,Changsha,Hunan,PRChina
DebeshR.Roy (139),MaterialsandBiophysicsGroup, DepartmentofPhysics,SardarVallabhbhaiNational InstituteofTechnology,Surat,India
RanajitSaha (275),InstituteforChemicalReaction DesignandDiscovery(WPI-ICReDD),Hokkaido University,Sapporo,Japan;DepartmentofChemistry, IndianInstituteofTechnologyKharagpur,Kharagpur, India
PranabSarkar (247),DepartmentofChemistry,VisvaBharatiUniversity,Santiniketan,India
UtpalSarkar (87),DepartmentofPhysics,Assam University,Silchar,India
KangkanSarmah (345),AdvancedComputational ChemistryCentre,DepartmentofChemistry,Cotton University,Guwahati,Assam,India
EshaV.Shah (139),MaterialsandBiophysicsGroup, DepartmentofPhysics,SardarVallabhbhaiNational InstituteofTechnology,Surat,India
YukatsuShichibu (411),GraduateSchoolofEnvironmentalScience,HokkaidoUniversity,Sapporo,Japan
NguyenThanhSi (99),DepartmentofChemistry,CanTho University,CanTho,Vietnam
SwapanSinha (333),SchoolofAppliedSciencesand Humanities,HaldiaInstituteofTechnology,Haldia, India
M.Springborg (41),PhysicalandTheoreticalChemistry, SaarlandUniversity,Saarbr€ ucken,Germany
AmbrishKumarSrivastava (173),Departmentof Physics,DeenDayalUpadhyayaGorakhpurUniversity, Gorakhpur,UttarPradesh,India
V.Subramanian (213),CenterforHighComputingand InorganicPhysicalChemistryLaboratory,Central LeatherResearchInstitute,CouncilofScientificand IndustrialResearch;AcademyofScientificandInnovativeResearch(AcSIR),Chennai,TamilNadu,India
Zhong-MingSun (321),StateKeyLaboratoryof Elemento-OrganicChemistry,TianjinKeyLabofRare EarthMaterialsandApplications,SchoolofMaterials ScienceandEngineering,NankaiUniversity,Tianjin, China
GargiTiwari (173),DepartmentofPhysics,PatnaUniversity,Patna,Bihar,India
NikolayV.Tkachenko (321),DepartmentofChemistry andBiochemistry,UtahStateUniversity,Logan,UT, UnitedStates
JesusM.Ugalde (19),KimikaFakultatea,Euskal HerrikoUnibertsitatea(UPV/EHU)andDonostiaInternationalPhysicsCenter(DIPC),Donostia,Euskadi, Spain
FarnazYashmin (345),AdvancedComputationalChemistryCentre,DepartmentofChemistry,CottonUniversity,Guwahati,Assam,India
DongboZhao (237),InstituteofBiomedicalResearch, YunnanUniversity,Kunming,Yunnan,PRChina
LiliZhao (157,387),InstituteofAdvancedSynthesis, SchoolofChemistryandMolecularEngineering, JiangsuNationalSynergeticInnovationCenterfor AdvancedMaterials,NanjingTechUniversity,Nanjing, China
Describingchemicalbondinginexotic systemsthroughAdNDPanalysis
EdisonOsorio FacultyofNaturalSciencesandMathematics,UniversityofIbague,Ibague,Colombia
1.Introduction
Thechemicalbondingtheoryisoneofthemostimportantconceptsinchemistryanditsobjectiveistoexplainthestability ofthousandsofcompoundspresentinnature.However,theseconceptsareconstantlyevolvinginordertosatisfythemajor challengesofresearchthroughtheformulationofnewandincreasinglysophisticatedbindingmodels.TheLewischemical bondingtheory,formulatedthroughlonepairsandtwo-centertwo-electron(2c-2e)covalentbonds,hasbeenusedto explainthenatureandbehaviorofchemicalbondingattheundergraduateandresearchlevelsformorethan90years. Thesuccessisthesimplicityofmodel,theavailabilityandsimplicityofrulesforbuildingstructures,andthegraphical representationofchemicalbondingpattern.Successfully,thesemodelscouldbeassociatedwiththepropertiesofspecies andtheirreactivity,thusprovidingadescriptiveandpredictivemodel. [1,2] Nevertheless,therearesituationsinwhichthe chemicalbondandpropertiesofcertainspeciescannotbesatisfactorilydescribedbythistheory.Withinthesegroups,we emphasizetheclusters,aggregatesofatomsormoleculesbondedtogetherbydifferenttypesofinteractions.Studiesofthese systemsbeganinthe1950sandareoftensynthesizedusingmassspectrometerionsourcesorlaservaporizationtechniques. Thelattertechniqueallowstheresearcherto“assemble”speciesofanycompositionandtogobeyondstudiesofaggregates ofvolatilematerials [3–5].Structurally,clustersareanintermediateformofmatterbetweentheatomiclevelandthesolid phase,andtheirpropertiesareextremelysensitivetocompositionandchargeandcanbedrasticallyalteredbytheaddition orabstractionofaslittleasasingleatomorelectron,socreatingspecieshomologoustothoseobservedinmolecularbeams isextremelydifficult [6,7].OneofthechemicalsystemsinwhichchemicalbondingcannotbeexplainedbyLewisbond theoryareboronhydrides,systemscharacterizedbyatypical3c-2e,4c-2e,etc.,chemicalbonds [8–10]
TheAdaptiveDensityNaturalPartitioning(AdNDP)isatheoreticaltooldevelopedin2008byZubarevandBoldyrevto determinethechemicalbondingpatternsindifferentsystemsofinterest. [11] Thisapproachhasbeensuccessfullyapplied formorethanadecadetodescribethechemicalbondinginaromaticandantiaromaticorganicmolecules [12] anddifferent atomicclusters,includingboronclustersandcombinationsofdifferent chemical elementssuchasC,Si,Ge,Sn,Mg,Ca,Sr Ba,Be,andamongothers [13–22].AdNDPisbasedontheconceptofelectronpairasthemainelementofchemicalbonding modelandallowsrepresentingtheelectronicstructureintermsof nc-2ebonds,where n includestheintervaloftotalnumber ofatomsinaparticularatomicensemble.ThisapproachrecoverstheLewischemicalbondingmodelanddelocalized bondingelementsassociatedtotheconceptsofaromaticityandantiaromaticity.Inthisperspective,AdNDPprovidesa perfectdescriptionofsystemswithlocalizedanddelocalizedbonds,withoutinvolvingtheresonanceconcept.Essentially, AdNDPisapowerfulvisualapproachforinterpretateofwavefunctionsbasedonmolecularorbitals(MOs);nevertheless, MOswillnotbeconsideredproperlyasachemicalbondingmodel,unlesstheyareusedasapartofaromaticity/antiaromaticityconceptfordelocalizedbonds.
Nevertheless,insystemswherenotinvolvethesharingofelectronssuchashydrogenbridges,electrostaticinteractions andVanderWaalsforcesareprevalent,thereisnoelectrondensitysharinginthetargetregionandAdNDPtoolfailsto describethesetypesofinteractionscorrectly.However,thismethodologyallowsdescribingacorrectlocalizationofcore electronsandlonepairsresponsibleofstabilityforcertainmolecules,validatingtheresultsobtainedbyothermethodologies,whicharebettertodescribethistypeofinteractions.Anexampleofthisalliancewasappliedinthestudychemical bondingschemeinEC3+,EC4+,EC5+,andEC6+ species(E ¼ Sc,Y,andLa),workpublishedbyOsorioetal.,indepthusinga combinationofdifferenttheoreticalstrategies [23].Inafirstinstance,theexhaustiveexplorationsofrelevantpotential energysurfaces(PESs)providedafan-likestructuresasthemostenergeticallystableconfigurations.Thechemicalbonding
analysisusingthenaturalbondorbital(NBO)analysisindicatedthatthemetal-carboninteractionhasstrongioniccharacter,increasingwhengoingfromSctoLa.Besides,NBOpredictedthepresenceofsomedegreeofcovalentmetal-carbon interaction,resultverifiedbymeansofenergydecompositionanalysis(EDA) [24].TheEDAresultsshowedthatinall studiedcases,bothelectrostaticandcovalentcomponentssignificantlycontributetobondinginteractionbetweenthe carbonfragment(Cn 1 )andmetal(E2+).Additionally,thetopologicalanalysisofelectrondensityshowedthatmetal-carbon interactionsaremainlyofaclosed-shellnature(ionic-likeinteractions).However,theyalsohaveadegreeofcovalentcharacter.Finally,theAdNDPresultssupportthecovalentcomponentintheseinteractionsand,inturn,describemetal-carbon bondsasdelocalizedforms.
Inorganicmoleculesarea,anexamplewhereacombinationofdifferenttheoreticalmethodologiesisusedtoexplain chemicalbondingcanbefoundinthetheoreticaldescriptionofmechanismforthewalkrearrangementinDewarthiophenesclarifiedbyRestrepoetal. [25],wheretheresultsobtainedbyNBOandAdNDPtoolsshowedainterestingevolutionpictureofbondingduringtherearrangements,fullyconsistentandcomplementarytotheBader’stheory [26–28], wherethenatureofbondinginteractionsandevolutionofbondingusingdescriptorscalculatedatthebondcriticalpoints (BCPs)showedanexpectedincrease(decrease)intheelectrondensityattheBCPsassociatedtochemicalbondsinthe processofbeingformed(broken).Anotherexampleisthetheoreticalstudyofreactionstepsduringthebiosynthesisof suicidalclavulanicacid(coformulatedwith b-lactamantibioticsandusedtofightbacterialinfections) [29].Inthiswork, Restrepoetal.,providedevidenceofareactionchannelforthedoubleinversionofconfigurationthatinvolvesatotalofsix reactionsteps.Themoleculargeometriesandelectronicstructurescalculationsshowedasubstantialreorganizationof electrondensityrightattheonsetofreaction,mostlyinvolvingacyclicevolution/involutionoflargeregionsof p delocalizationusedtostabilizetheexcesschargeleftaftertheinitialprotonabstraction.Anumberofbondingdescriptors derivedfromanalysisofelectrontopologydistributionsshowedtheevolutionofbondordersandarequiteconsistentwith theplotsofevolutionofelectrondensityandbondingorbitalslocalizedbyAdNDPanalysis.
1.1AdNDPimplementation
TheAdNDPmethodologyisaNBOanalysisgeneralizationbasedontheoptimaltransformationofamultielectronwave functiontoalocalizedform,consistentwiththetheoreticalLewischemicalbondingmodel.Thefirstorderreducedmatrix operatorforaclosedshellsystem,independentofspin,isdefinedas:
where1and10 aretheabbreviationsfor w1 and w1 0 ,respectively,andthematrixelementis
So, g(1 j 10 )canbeexpressedasanorthonormalbasissetofatomicorbitals{wk}
Thediagonalelements Pkl ofmatrixdensity P ¼ {Pkl}correspondtooccupancynumber(ON)of wk orbitals.If wk arethe bondingorbitalswithamaximumoccupancy,thesetofhybridorbitalsshouldbeconsideredasoptimal,inthesensein whichtheapproximatewavefunctionconstructedusingthe wk orbitalswillhaveabetteroverlapwiththeoriginalwave function.Itisnecessarytoperformsomeapproximationsinthesearchforthesehybridorbitalswithmaximumoccupancy, sincethisprocedureiscomputationallydemanding.Thedensitymatrix P isrepresentedinblockformasfollows:
whereblock Pjj correspondstothe jthatomiccenter.Thenaturalspinorbitalswithmaximumoccupancyareeigenvectorsof completedensitymatrix P.Itispossibletoobtainhybridorbitalsmaximizingtheoccupationoveranatomiccenter,this means,diagonalizingthesubblocks P whichinvolvethisatomiccenter.Theprocedurecorrespondstosolvingthefollowing eigenvalueproblem:
where Pij isthedensitymatrixofsubblockonthe jthcenter, Sij istheoverlapmatrix,and hl(j) and nl(j) correspondtothefirst eigenvectorandeigenvalueof Pij,respectively,where nl(j) iscloseto2.00.Thealgorithmimplementationiscalledadaptive naturaldensitypartitioning(AdNDP)andisbasedonthediagonalizationof n-atomsubblocksofdensitymatrixforan n-atomicmolecularsystemwritteninthebasesofnaturalatomicorbitals(NAO).NAOsare1-centerorbitalsofmaximum occupancyforagivenmolecularwavefunctionderivedfromtheatomicsubblocksofdensitymatrix.Thegoalofalgorithm istorevealthemostprobableregionsinwhichlocalizedelectronpairsexist [11,12].
Inthenextsections,areviewofsomeapplicationofAdNDPmethodologytoboronchemistryandthecombinationof thesewithotherelementsoftheperiodictablewillbepresented.Withthisknowledge,itisexpectedthatthescientific communitywillbemotivatedtoexploresystemsthatdifferinasignificantwayfromclassicalchemistryandunderstand thechemicalbondingconceptspresentedinthem.
2.Boronhydrides
Boronisoneofthelightestchemicalelementsintheperiodictablewhichcanformcovalentbondswithhydrogenand, therefore,canbeusefulinformingunitsorbuildingblocksfordesigningandconstructinghydrogenstoragematerials, orinotherareassuchascatalysis [9].Understandingtheelectronicstructure,chemicalbondingandstabilityofdifferent conformationsorboronhydridesserieswithdifferentstoichiometrieswillallowtheestablishmentofboron-hydrogenratios usefulfordifferentkindsofapplications.
2.1ChemicalbondingschemeinB3Hy complexes
Althoughthestructureandpropertiesofalargenumberofboronhydridecompoundsarewellknown [10,30–32],thereare stillhundredsofunexploredsystemsthatcouldbetheoreticallydesignedfromtheBxHy0 0/n+/n generalformula.Inorderto understandthestructuralrelationshipbetweenthreeboronatomsand n hydrogens,theBoldyrev’sgroupexploredthePES ofneutralandanionicclustersofB3Hy series(y ¼ 4–7)usingtheGradientEmbeddedGeneticAlgorithm(GEGA)program [33].ThechemicalbondingschemerevealedbyAdNDPanalysisallowedtoexplaincorrectlythegeometricalandnotvery particularshapeofthesesystems:presenceof2c-2eB BandB H,3c-2eB H Bbonds,andfinallyoneB B B3c2esigmabondonB3 triangle [34]
InrelationtotheimportanceofB3Hn systems,ithasbeenexperimentallydemonstratedthattheoctahydrotriborane anionB3H8 occupiesanintermediatepositionintherankingofboronhydridecompoundsoflowercomplexity,and duetothetriangular(deltahedral)geometry,thisanioncanbeconsideredasabuildingblockforthepreparationofpolyhedralboronhydrides [35].Informationrelatedtosynthesisofthesecompoundscanbereviewedandconsultedintheliterature [36–39].InordertounderstandandestablishthebindingschemeandstabilityofB3 aggregates,theBoldyrev’s groupconductedanexplorationonthePEStounderstandthereversibledehydrogenationofMg(B3H8)2 system,which occursexperimentallyundercertainspecialconditions [40].ThestudywasperformedusingtheCoalescenceKick (CK)algorithm [41,42] andlocalizedthemoststableconformationforB3H8 anionandanadditionalstructureveryclose inenergywhichexplainsthefluxionalbehaviorofthisanion.TheAdNDPanalysisrevealedthepresenceofclassicalB H andB Bbonds(2c-2e),B H B3c-2ebondsandoneB B B3c-2edelocatedbondonB3 [40]
2.2IsostructuralrelationshipsinBnHn series
Istherearelationshipbetweencarbonandboronchemistry?Toanswerthisquestion,itisnecessarytoreviewtheestablishedconceptstaughtinundergraduatechemistrycourses.Carbonandboronareneighborsonperiodictable,buttheir chemicalbondingisdifferent.Moleculesthatpossesscarboncanform2c-2ebondswithothercarbonatomsorwithother elementssuchashydrogen.AspecificexampleisthesaturatedhydrocarbonswithCnH2n+2 stoichiometry,compounds characterizedbythepresenceof2c-2ebondsformedbyhybridorbitalsdenominatedassp3 [2].Accordingtotheperiodicity trendsandthenumberofvalenceelectronsofchemicalelements,theBnHn+2 serieswouldbeexpectedtoconsistofclassical 2c-2ebonds,whichshouldbeformedbysp2 hybridorbitals.AnexplorationofPESonBnHn+2 (n ¼ 2–5)series,usingthe CKprogram,revealedthattheclassicalstructurescomposedofsp2 hybridbondsbecomeprogressivelylessstableasthe seriesbecomeslarger,i.e.,geometricallymorecompactstructuresarecreatedanddifferentchemicalbondslike3c-2e, 4c-2e,etc.appear [43].Theconformationsandchemicalbondinganalysisareshownin Fig.1.

FIG.1 GlobalminimaandchemicalbondsidentifiedbyAdNDPanalysis. (PictureobtainedfromE.Osorio,J.K.Olson,W.Tiznado,A.I.Boldyrev, Analysisofwhyboronavoidssp2hybridizationandclassicalstructuresintheBnHn+2 series,Chem.AEur.J.18(2012)9677–9681. https://doi.org/ 10.1002/chem.201200506 withthepermissionofChemistryAEuropeanJournal.)
Thisperformanceoccursbecausetheboronatomsinthemoleculesstudiedtrytoavoidsp2 hybridization,sincean empty2patomicorbitalwouldbehighlyunfavorable.Thisaffinityofborontohaveacertainelectrondensityinall2p atomicorbitalsisoneofthemainreasonswhyclassicalstructuresarenotthemoststableconfigurations [43]
Ontheotherhand,Tiznadoetal.performedaPESscanonLinBnH2n series(n ¼ 3–6)andshowedthatboronavoids adoptingstructuressimilartothoseoforganiccycloalkanes(CnH2n),wherecyclopentane(C5H10)andcyclohexane (C6H12)arethemoststablesystems.However,theauthorsreportedthedesignofsmallestanalogofaromaticcarbocations (C3H3+),theLi3B3H3+ system,wheretheglobalminimumhasatriangularB3H32 shapewithstructuralfeaturesand chemicalbondingpatternssimilartoitsorganiccounterpart.Theauthorsconcludethataromaticityisakeyfactorfor designinganalogsofcyclicorganiccompoundsbasedonlithiumboronhydrides [44].
2.3Electronictransmutation
Oneofthemostimportantobjectivesofalchemistsatthebeginningofhistorywasthetransmutation(transformation)of basemetalsintogoldorsilver.Toachievethisgoal,thescientistsoftimelearnedtoextractcertainmetalsfrommineralsand toproducedifferenttypesofinorganicacidsandbases,whichestablishedthefundamentalsofmodernchemistry [45,46]. Alreadyinthe20thcentury,scientistsdemonstratedthatalthoughnucleartransmutationispossibleandoneelementcanbe transformedintoanotheronlybyanuclearreaction,suchreactionsrequiresignificantlyhighenergiescomparedtoanormal chemicaltransformation.Recentinvestigationsshowthattheoldalchemist’sideaofchemicaltransmutationisnot completelydead.Theoreticalanalysesshowthat,inparticularsystems,whenaboronatomacquiresanadditionalelectron akindofelectronictransmutationoccurs,andthechemicalbondandgeometricalstructureofresultingspeciebehavelikea carbonatom [47].TheworkreportedbyOlsonetal.showedthatthemoststablegeometricshapeofLi2B2H6 systemcontainsaLi2B2H6 nucleuswhichisisostructuraltoC2H6 ethanemolecule.Theauthorsproposethatthisconceptmayhavea significanteffectonpredictionofnewchemicalcompounds [47].
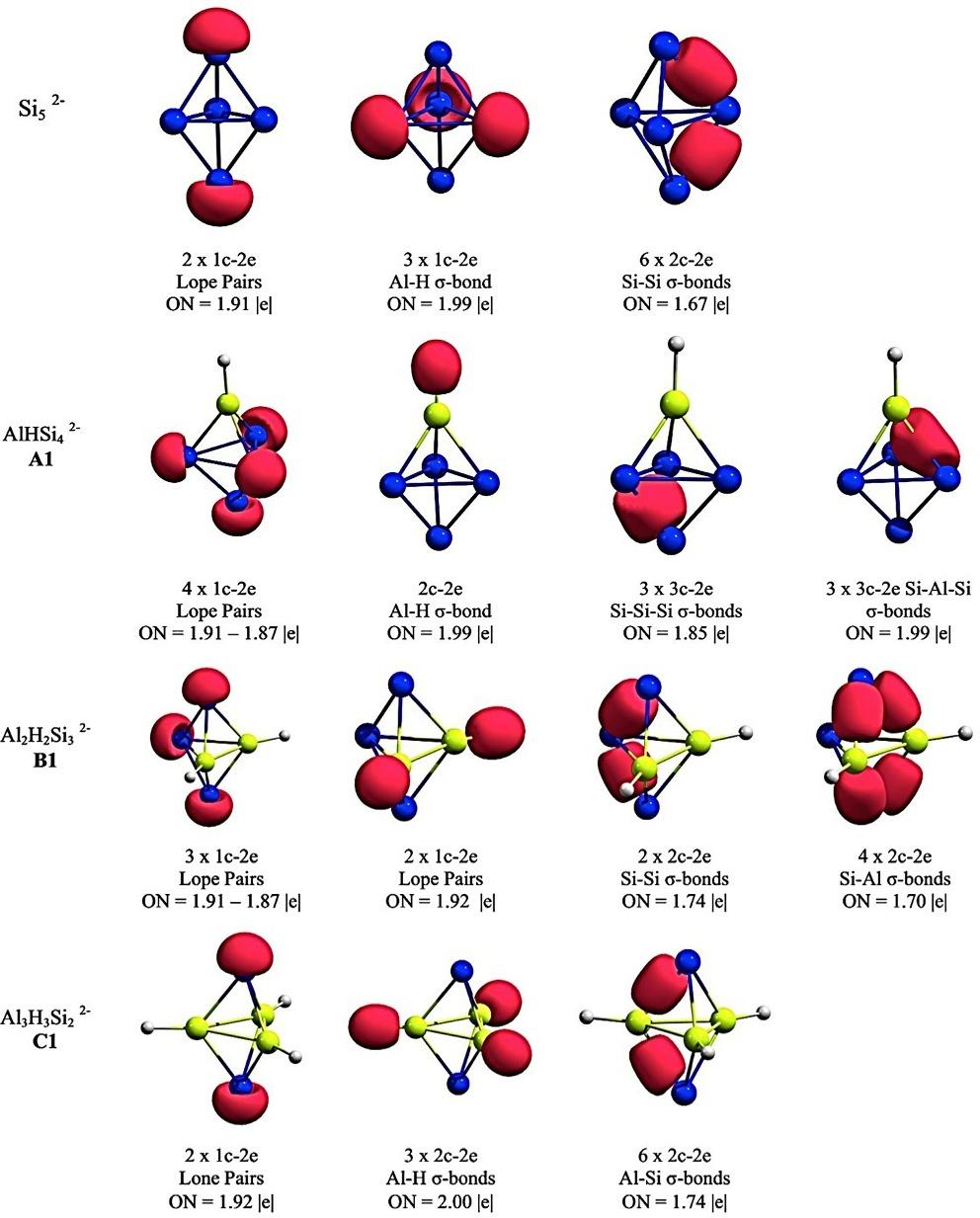
Accordingtotheelectronictransmutationconcepts,itcouldbegeneralizedtodifferentsystems.Theexplorationon PESsofSi5-n(BH)n 2 andNa(Si5 n(BH)n) systems,with n ¼ 0–5,showedthatanisoelectronicsubstitutionofaSiatom foraB HunitalongthetransformationfromSi52 toB5H52 ispossibleandthedeltahedralshapeofglobalminimaisnot affectedasonemovesuptheseries [48].ThechemicalbondingschemefortheSi5 n(BH)n 2 seriesispresentedin Fig.2. TheAdNDPanalysesshowedthattheconservationofstructureisduetovalenceelectronscombiningtoformsix2c-2e Xeq-Xaxbonds(X ¼ SiorB),5-n lonepairsonthesiliconatomand n B H2c-2ebonds;aparticularfactistheclosenessin electronegativityvaluesofboronandsiliconatoms(2.04vs.1.90,onthePaulingscalerespectively),whichexplainsthe prevalenceofstructuresatseverallevelsofisoelectronicsubstitution.

FIG.2 ChemicalbondingpictureofSi5 2 (A),BHSi4 2 (B),B2H2Si3 2 (C),B3H3Si2 2 (D),B4H4Si2 (E),andB5H5 2 (F)revealedbytheAdNDPanalysis. (PictureobtainedfromreferenceE.Osorio,A.P.Sergeeva,J.C.Santos,W.Tiznado,TheoreticalstudyoftheSi5-n(BH)n2-andNa(Si5-n(BH)n)-(n ¼ 0-5) systems,Phys.Chem.Chem.Phys.14(2012)16326–16330. https://doi.org/10.1039/c2cp42674a withthepermissionofPhysicalChemistryChemical PhysicsJournal.)
ElectronictransmutationonSi52 systemcanbeexploredwithotherelementsofperiodictablesuchasaluminum, whichhasthesameelectronnumberasboron.Osorioetal.investigatedthetransformationofSi52 toAl5H52 through thesuccessivesubstitutionofsiliconatomsbyAl Hunits,exploringthePESsforSi5 n(AlH)n 2 (n ¼ 0–5)systems [49].Theresultsshowedhowtheglobalminima,with n ¼ 1–3,keepthesamedeltahedralstructureofSi52 clusterand thesamechemicalbondingscheme.ThechemicalbondinganalysisforSi5 n(AlH)n 2 (n ¼ 1–3)ispresentedin Fig.3 Nevertheless,inthecaseof n ¼ 4(Al4H4Si2 )thedeltahedralconformationiscompletelydestroyedandAl4Sifragment adoptsaplanarconformationwith C2V symmetry.Thisresultshowshowthisconceptofelectronictransmutationisnot alwaysapplicabletoagivensystem [49]
Regardingthenatureofaluminum,itiswellknownthataluminum-hydrogenatomicclustersarestabilizedbytheconventionalAl AlbondsandAl H Almulticentricbonds,however,informationabouttheexistenceofdoubleAl]Alor tripleAl^Albondsislimited.Olsonetal.reportedthroughcombinedstudiesofphotoelectronspectroscopyandabinitio simulationsthepresenceofanAl]AldoublebondwithintheLiAl2H4 cluster,whichwasproposedthroughthetheoreticalmodelofelectronictransmutation [50].ExhaustivesearchesforthemoststablestructuresofLiAl2H4 cluster showedthattheglobalminimumpossessesageometricalstructuresimilartoSi2H4,thusdemonstratingthatanelectronic transmutationphenomenonoccursfromAltoSithroughelectronicdonation.Theoreticalsimulationsofphotoelectron spectrumallowedtoestablishthecoexistenceoftwoisomersandconfirmedthepresenceofaAl]Aldoublebond [50]
Unfortunately,theelectronictransmutationmodelisonlyfeasibleforsomeparticularsystems,i.e.,itcannotbe extendedtoallatomsoftheperiodictable.ThisbehaviorwasdescribedbyOlsonandetal.whoperformedabinitiostudies forelectronictransmutationofberylliumatomintoboron.ExhaustivesearchesoflowestenergystructuresforLinBen and Bn (n ¼ 3–5)showedhowthestructurecorrespondingtotheglobalminimumofLi3Be3 possessesachemicalbonding schemeandgeometricalstructuresimilartoB3 system.However,inthecaseofserieswith n ¼ 4and5,theminimumenergy structuresdonotresembletheirBn counterparts [51].
2.4ChemicalbondingindeltahedralBnHn 2 systems
Withintheenormousexistinginformationonboron-hydrogensystems,theBnHn 2 areparticularlyinterestingsincethey arecharacterizedbytheirhigh3Dsymmetry,bybeingstablearomaticspeciesandalsobecausetheypossess n +1valence electronpairs,accordingtoWade’srules [52–55].However,theelectronicstructurescannotbeexplainedbytheclassical Lewisstructurepictureduetotheelectrondeficientcharacter.ThemulticentricbondingschemeforBnHn 2 systems,with n ¼ 2–17,hasbeenrevealedthroughAdNDPanalysisinordertoobtaininformationonstabilityandaromaticity.
FIG.3 ChemicalbondingschemeforSi52 ,AlHSi42 (A1),Al2H2Si32 (B1),andAl3H3Si22 (C1)revealedbytheAdNDPanalysis. (Pictureobtained fromI.Fuenzalida-Valdivia,M.J.Beltran,F.Ferraro,A.Vasquez-Espinal,W.Tiznado,E.Osorio,IsoelectronicsubstitutionfromSi52 toAl5H52 : explorationoftheseriesSi5 n(AlH)n2 (n ¼ 0–5),Chem.Phys.Lett.647(2016)150–156. https://doi.org/10.1016/j.cplett.2016.01.062 withthepermissionofChemicalPhysicsLettersJournal.)
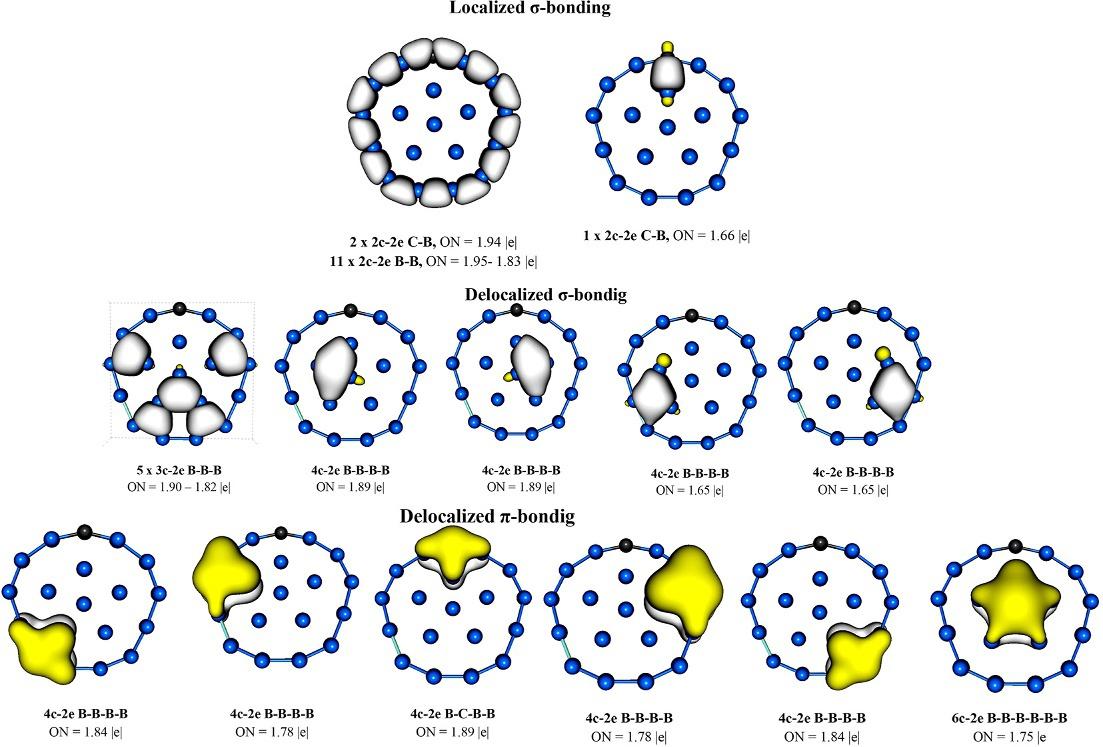
DifferenttheoreticalanalysesusedtodescribechemicalbondingshowhowallsandpvalenceelectronsinBnHn 2 are involvedintheformationofmulticentricbondsonthecagesurface,whichcoincideswithdifferentsymmetricconfigurations.Inthissense,AdNDPisabletodetectfivedifferenttypesofmulticentricbonds,includinganopen3c-2eBBB bond,atriangle-shaped3c-2eBBBbond,adiamond-shaped4c-2ebond,an8c-2ebondintheformofadoublering, andabondtotallydelocalizedovertheentiresurfaceofboroncage [52]
TheAdNDPtoolhasalsobeenusefulforstudyingthestabilityoflargerclusters,suchastheB80H20,C80H20 and Al80H20 systems,whichcontaintetrahedralB4H,C4H,andAl4HfragmentsinplaceofC Hfragmentsatthevertices ofdodecahedralscaffold.TheAdNDP,NBOandELFanalysesdemonstratethechemicalbondinginC80H20 canbe describedintermsofclassical2c-2eC C s bonds,whiletheelectron-deficientB80H20 andAl80H20 analogsshowthe presenceof2c-2eand3c-2e s bondsasresponsibleforthebondingbetweenandwithinthetetrahedralfragments, respectively [56].
3.Boronnanowheels
Thissectionpresentsananalysisofchemicalbondinginboronwheelstounderstandtheirbehaviorandhowtheincorporationofcertainmetalsintotheirstructureproceeds.
3.1Dynamicbehaviorinsmallboronclusters
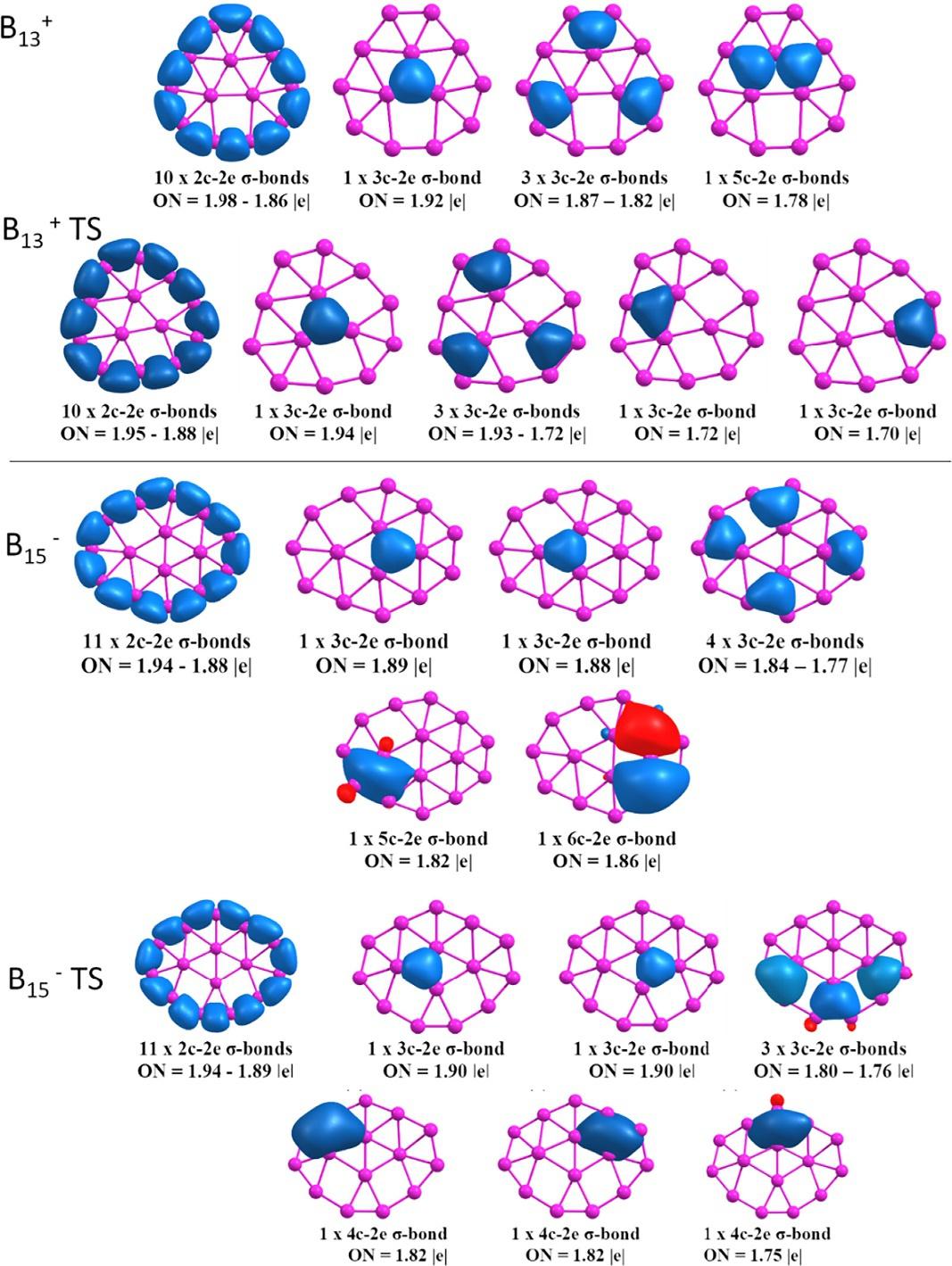
Atpresent,itispossibletofindintheliteratureinnumerableinformationaboutthelowerenergystructuresofsmalland medium-sizedboronclusters,mostofwhicharemadeupofapairofconcentricringsandpresenttwo-dimensionalshapes [41,57,58].However,thecurrentresearchneedstoaddeffortstounderstandthedynamicbehaviorofthesesystemsandto findapplicationsforthem.AstudycarriedoutbyMerino’sgroupusingBorn-Oppenheimermoleculardynamics(BO-MD) simulationstools [59,60] showedhowinclustersB11 ,B13+,B15 ,andB19 therotationbarriersofoneoftheseringswith respecttootherareremarkablylow [61].Inthesesystems,theouterringsubunitssurroundtheinnerfragment,whichina sequenceofrandommotionsmakesitswayaroundthemainaxisinonedirectionortheother,allowingpartialrotationof innerfragment.TheAdNDPanalysispresentedin Fig.4 showshowthesesystemspossessanetworkof2c-2esinglebonds linkingtheboronatomsintheouterrings.
Inthecaseofinnerrings,thebondingisduetoanetworkofmulticenter s bondsinvolvingalmostexclusivelytheinner atoms,andaseparatesetofbondsbetweenthreeand,insomecases,morecenterslinkingtheinnerandouterrings.Asa conclusionofthiswork,theauthorsarguedthatacombinationofelectronicandgeometricfactorsisnecessaryfora decreaseinrotationalbarrierstooccurinthesetwo-dimensionalclusters.Thesefactorscanbesummarizedinthreeitems: (i)asufficientlylargeouterring;(ii)a s-skeletonofindividualringsthatremainsessentiallyintactduringrotation;and(iii) atransitionstateforinnerringrotationthatinvolvesthetransformationfromasquaregeometricshapetoadiamond,arule thatmayberelatedtoamechanismsuggesteddecadesagofortheisomerizationofcarboranesandboranes [61]
3.2BoronwheelsmembersofWankelmotorfamily
TheresearchcarriedoutbyBoldyrevandWang’sgroupsontheexistenceofaboronwheelformedby19boronatoms (B19 ) [41],whichcontainsaninternalpentagonalfragmentofsixboronatomsandsurroundedby13otheratoms,inspired differentauthorstounderstandthemotionofthesetwoconcentricrings.
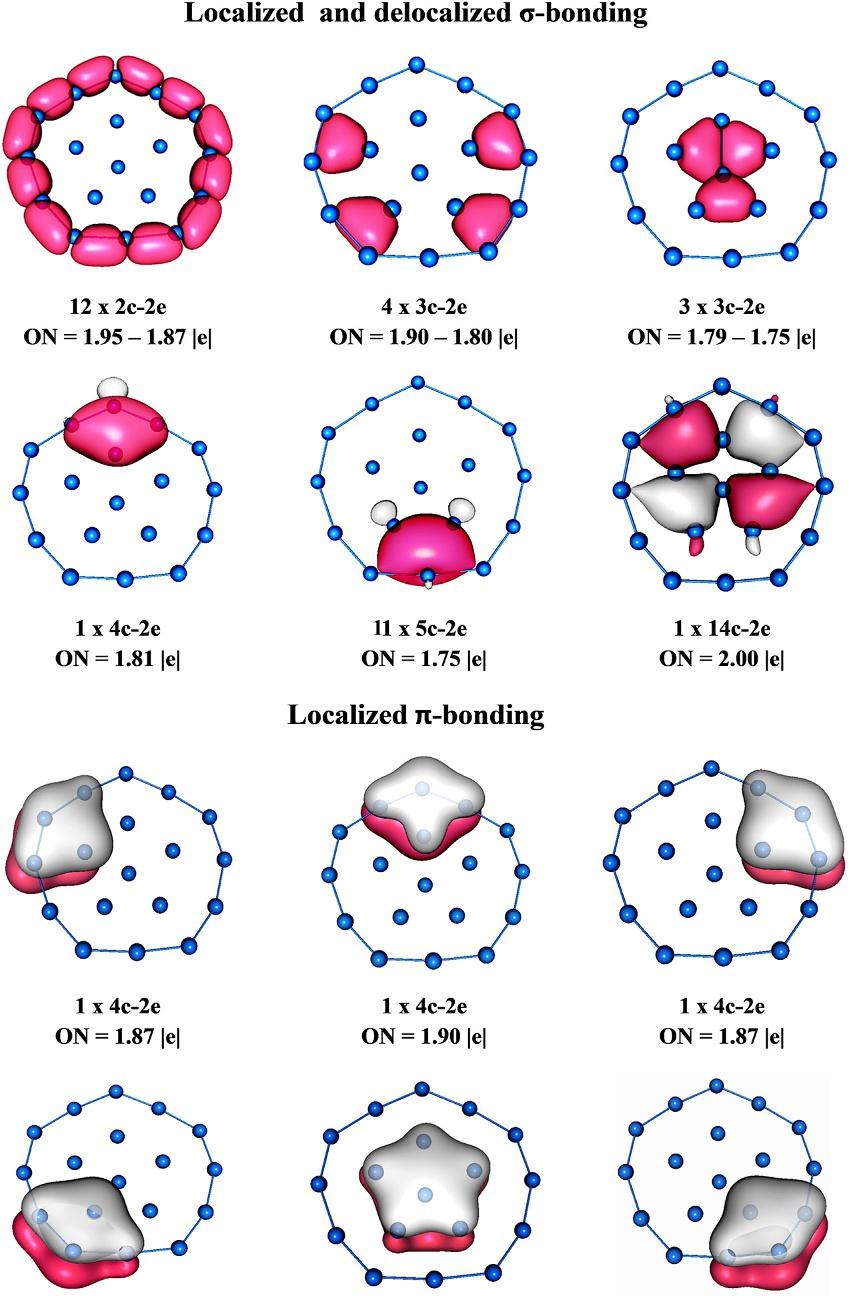
Merino’sgroupdeterminedthroughBO-MDsimulationshowintheB19-clusterthepentagonalfragmentandtheouter boronringcanrotatealmostfreelyinoppositedirections,similartoaWankelmotor [62].Inspiredbytheseresults,thesame researchgroupreportedthetheoreticaldesignofB182 cluster,asystemthathasadynamicbehaviorsimilartoB19 and wouldthereforebehavelikeaWankelmotor [63].Thisdianionicandelectronicallystableclusterhasadoublearomatic concentricsystem, s and p.Theinternalunit,composedofsixboronatoms,undergoesanalmostfreerotationwithinthe perimeterofB12 ring.
TheAdNDPanalysispresentedin Fig.5 showstheabsenceof2c-2esigmabondslocatedbetweentheouterringB12 and innerunitB6;additionally,AdNDPonlydetectsthepresenceofmulticentricdelocalizedbondsas3c-2e,4c-2e,etc.,which migrateeasilyfromonepositiontoanotherduringtherotationofB6 unit.
Similarly,theAdNDPtoollocatedasetof4c-2edelocalized p bondsbetweentheinnerandouterring,andone6c-2e bondlocatedontheinnerring.Theabsenceofanylocalized s bondbetweentheinnerringandperipheralboronatoms makestheB182 systemshowafluxionalbehavior.
TheunderstandingofdynamicperformanceofthesesystemshasallowedtoMerino’sgroupestablishhowtostopor preventonefragmentfromrotatingaroundanother.TheirtheoreticalinvestigationsshowshowthesubstitutionintheB19 systemofacarbonatomforaboronatomcangiveaneutralspecies,CB18, andcancelthefluxionalityofthisanion [64].The AdNDPanalysisreportedin Fig.6 showshowCB18 clusterhasapielectrondistributionanalogoustoB19 ;however,the sigmaelectrondistributionisconsiderablydifferent:elevenperipheralB B2c-2ebonds,twoC B2c-2ebondsandone extra2c-2eC Bbond,whichconnectstheperipheralandinternalpentagonalrings.Thislastlocalizedbondisthemain reasonwhytheCB18 clusterhasaradicallydifferentdynamicbehaviorthantheB19 cluster.
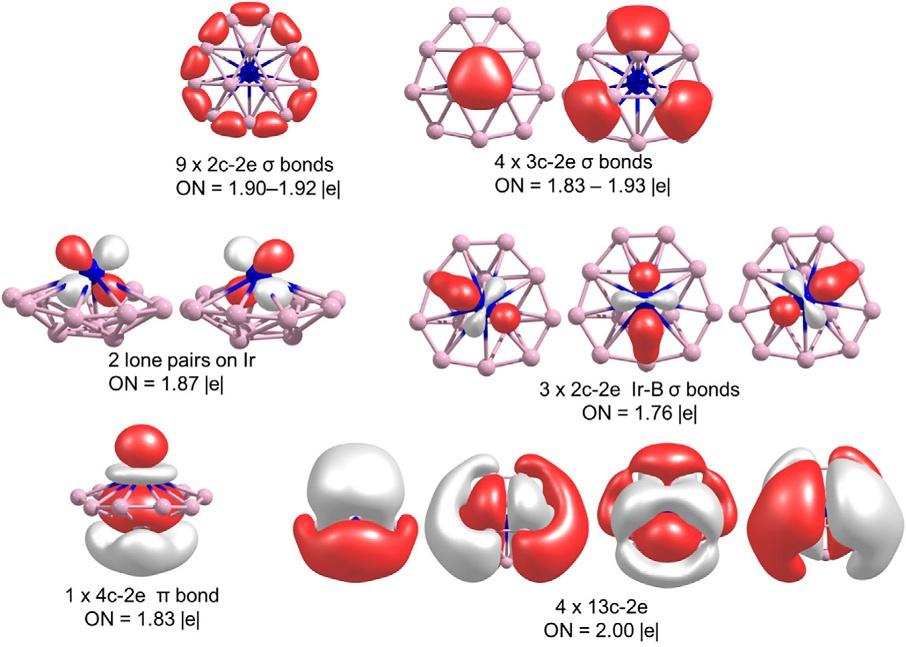
Returningtosmall2Dboronclusters,theoreticalstudiesshowhowthetransformationofarigidsystemsuchastheB12 cluster,whichhasahighrotationalenergybarrierintheinnerring,toadynamicWankelmotorsystemispossiblethrough theincorporationofcertaintransitionmetals,mainlyIr,whichlowerstherotationalbarriersignificantly,transforming MB12 clustersintoWankelMotor [65]
TheglobalminimumofIrB12 isasymmetricbowl-shapedstructureinwhichtheIratomislocatedontheconcavesideof bowl,similartoitslightercongenersCoB12 andRhB12 .AlthoughalltheseMB12 clustersshowadynamicalbehavior
FIG.4 SigmabondsdetectedbyAdNDPforgroundstatesandtransitionstatesofB13 + andB15 systems. (PictureobtainedfromS.Jalife,L.Liu,S.Pan, J.L.Cabellos,E.Osorio,C.Lu,etal.,Dynamicalbehaviorofboronclusters,Nanoscale8(2016)17639–17644. https://doi.org/10.1039/c6nr06383g with thepermissionofRoyalSocietyofChemistry.)
analogoustotheso-called“Wankelmotors,”rotationofinnerB3 ringaroundofperipheralB9 ring,theenergybarrierislower forIrB12 system(5.0kcalmol 1).AlowinteractionenergybetweenB3 andMB9 fragmentsisthemainreasonwhy therotationalenergybarrierislowerforIrB12 thanforCoB12 andRhB12 clusters.Thechemicalbondingschemefor IrB12 obtainedbyAdNDPanalysisispresentedin Fig.7 andlocatestwolonepairsonIratom,nine2c-2elocalized s bonds intheperipheralB9 ring,andone3c-2edelocalized s bondininnerB3 ring.Oneofthemostimportantresultsisthatinner B3 andouterB9 ringsareconnectedbythreedelocalized s bondsof3c-2etypeandtherearenolocalizedbondsbetweenthe
FIG.5 AdNDPanalysisforB182 (PictureobtainedfromD.Moreno,S.Pan,L.L.Zeonjuk,R.Islas,E.Osorio,G.M.Guajardo,etal.,B182 :axquasiplanarbowlmemberoftheWankelmotorfamily,Chem.Commun.50(2014)8140–8143. https://doi.org/10.1039/c4cc02225d withthepermissionofRoyal SocietyofChemistry.)
metalIrandinnerB3 ring.Additionally,AdNDPanalysisshowsinteractionsbetweenIrandtheB12 moietythroughthree s andone p bonds.Furthermore,fourfullydelocalizedorbitalsalsocontributetotheinteractionbetweenMatomandB12 moiety.
3.3Designofsandwichstructures
ThediscoveryofferroceneFe(C5H5)2 in1951attractedcountlessinterestinfundamentalresearchatthetimeduetoits unusualstructureandbondingcharacteristics,resultsthatledtonumerousapplicationsinmaterialssciencesuchasdissolutionofmetalions,catalysis,andbiologicalresponse;amongothers [66,67].Oneofrulesforstructuraldesignofthese systemsinvolvesligandsthatcancoordinatewithtransitionmetalatomsthroughinteractionsbetweenthedelocalized p MOsofligandsandpartiallyoccupieddorbitalsoftransitionmetals.
FIG.6 ChemicalbondingschemeforCB18 reportedbyAdNDP. (PictureobtainedfromF.Cervantes-Navarro,G.Martinez-Guajardo,E.Osorio, D.Moreno,W.Tiznado,R.Islas,etal.,Stoprotating!OnesubstitutionhaltstheB19 motor,Chem.Commun.50(2014)10680–10682. https://doi. org/10.1039/c4cc03698k withthepermissionofRoyalSocietyofChemistry.)
FIG.7 AdNDPanalysisforIrB12 (PictureobtainedfromL.Liu,D.Moreno,E.Osorio,A.C.Castro,S.Pan,P.K.Chattaraj,etal.,Structureand bondingofIrB12-:convertingarigidboronB12platelettoaWankelmotor,RSCAdv.6(2016)27177–27182. https://doi.org/10.1039/c6ra02992b with thepermissionofRoyalSocietyofChemistry.)
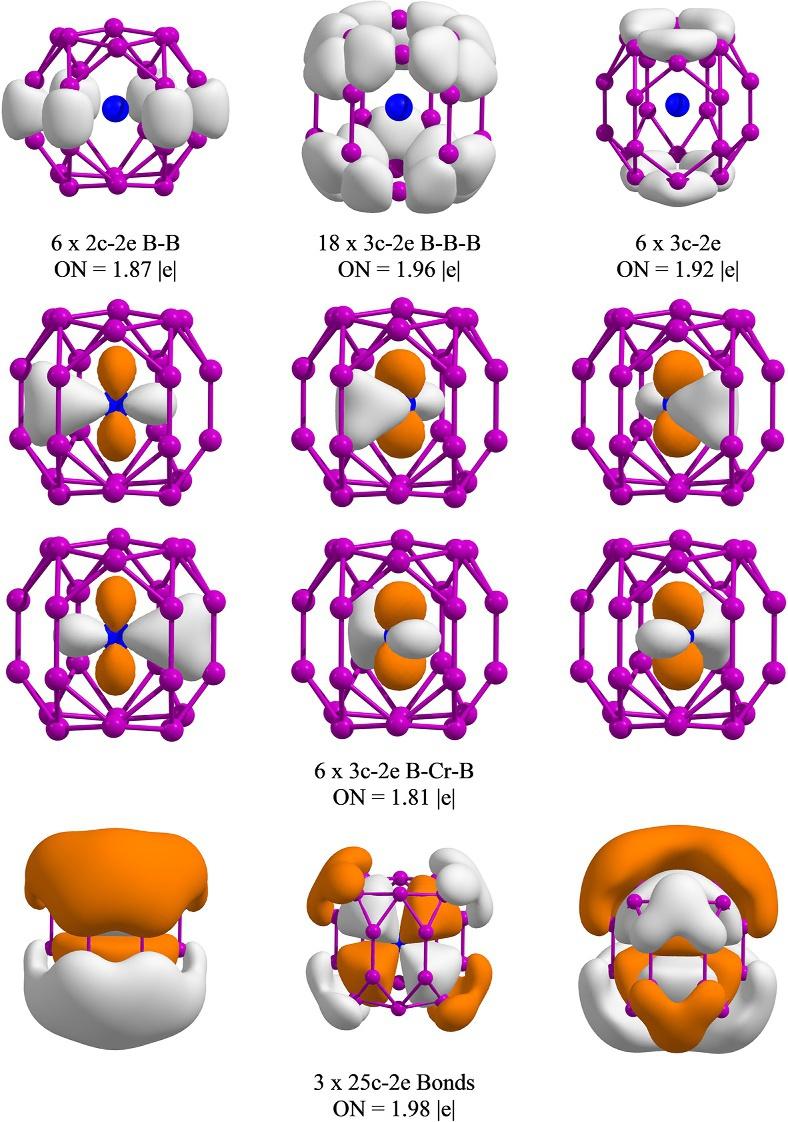
FIG.8 AdNDPanalysisofendohedralstructure(I)ofCrB24. (PictureobtainedfromL.Liu,D.Moreno,E.Osorio,A.C.Castro,S.Pan,P.K.Chattaraj, etal.,StructureandbondingofIrB12-:ConvertingarigidboronB12platelettoaWankelmotor,RSCAdv.6(2016)27177–27182. https://doi.org/10. 1039/c6ra02992b andL.Liu,E.Osorio,T.Heine,Theimportanceofdynamicsstudiesonthedesignofsandwichstructures:aCrB24case,Phys.Chem. Chem.Phys.18(2016)18336–18341. https://doi.org/10.1039/c6cp02445a withthepermissionofRoyalSocietyofChemistry.)
StudiesreportedbyLi’sgroupshowhowboronclusterscanbeusedforthedesignofsandwichstructuresusingcertain transitionmetalatoms.Inthislight,Lietal.reportedhowCrB24 clustercanbeconstitutedbyakindofsandwich-type structureinwhichthechromiumatomislocatedbetweentwoB12 sheets,paralleltoeachother [68].Intuitively,the CrB24 sandwichcomplexmightnotnecessarilybethemostthermodynamicallystablestructure,sincetwoB12 units areweaklycoordinatedthroughachromiumatom.InvestigationsfocusingonBO-MDsimulationsshowedhowthe sandwich-likeCrB24 structurehasextremelypoordynamicstability:theconformationcollapsesresultinginahighlysymmetricendohedralstructurewithachromiumatominthecenterofaB24 cage [69].TheAdNDPanalysisforCrB24 systemis presentedin Fig.8 andshowshowtheendohedralCrB24 complexisstabilizedduetopresenceofsix3c-2e s bondsdelocalizedbetweenboronatomsinthecentralpartofboxandthecentralchromiumatom.Thesebondscorrespondtoamixing betweenpz orbitalsofboronatomsanddorbitalsofchromiumatom.Finally,thewholesystemisstabilizedbypresenceof three25c-2e p bondsdelocalizedoverthewholebox.
Althoughthesmallboronclusterspreferplanar(2D)conformationstomaximizethenetworkofmulticentertwoelectronbonds,thepresenceofametalwithsuitablecharacteristicscanreshapethemorphologyofclusterandformsystems ofboronlikeNanowheel,inwhichthemetalislocatedinthecenterofit.Theoreticalandphotoelectronspectroscopy