Aquaculture
journal homepage: www.elsevier.com/locate/aquaculture
Gum
Arabic-enriched diet modulates growth, antioxidant defenses, innate immune response, intestinal microbiota and immune related genes expression in tilapia fish
Mohammed A.E. Naiel a, * , Samah A.A. Abd El-hameed b , Ahmed H. Arisha d, e , Samar S. Negm c
a Department of Animal Production, Faculty of Agriculture, Zagazig University, Zagazig 44519, Egypt.
b Fish Health and Diseases Department, Central Lab for Aquaculture Research, Abbassa, Abu Hammad, Agriculture Research Center, Giza, Egypt
c Fish Biology and Ecology Department, Central Lab for Aquaculture Research, Abbassa, Abu Hammad, Agriculture Research Center, Giza, Egypt
d Department of Animal Physiology and Biochemistry, Faculty of Veterinary Medicine, Badr University in Cairo (BUC), Badr City, Cairo, Egypt
e Department of Physiology, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt
ARTICLE INFO
Keywords:
Gum Arabic
Hematology
Antioxidant
Innate immunity
Intestinal microbiota
ABSTRACT
This study was designed to assess the influence of dietary supplementation with Gum Arabic at various levels in Nile tilapia fish, Oreochromis niloticus For 56 days feeding period, a total of 200 juvenile fish were allocated into 20 aquariums and divided into four equal groups, five replicates each, with Gum Arabic (GA) dietary inclusion as follow; the fish group was given a basal diet (CTR 0%); fish group fed a diet supplemented with GA 0.25%; fish group fed a diet supplemented with GA 0.5%; and fish group fed a diet supplemented with GA 1%. Dietary supplementation with 0.5 or 1% GA significantly improves final fish biomass, weight gain, weight gain percent, consumed feed, feed conversion ratio, innate immune response and antioxidant status compared to other treatments. The fish fed elevated levels of GA (1% GA) exhibited higher lipase and amylase activities as well as total protein, albumin, and globulin levels and lower uric acid, AST, and ALT levels. Furthermore, 1% GA enriched tilapia diets significantly up-regulated IL-1β and IL10, whereas downregulated TNFɑ mRNA expression in kidney and spleen tissues. Moreover, highly-AG enriched diets were associated with stronger antibacterial efficacy against pathogenic bacterial populations, together with increased counts of grown Bacillus in the mid gut. Overall, these findings suggest that dietary inclusion of 1% GA could be beneficial and should be explored as a functional feed supplement for Nile tilapia farming.
1. Introduction
Worldwide, tilapia is ranked the second-largest farmed cichlid aquatic species, behind only carp (Negm et al., 2021). Regarding FAO global production statistics, total cultured Nile tilapia output accounted for 71.2% of all cultivated fish species (FAO, 2018). Compared with traditional culture systems, the intensive culture system is known for higher stocking rates, feed input levels, and management techniques (Soliman and Yacout, 2016). Meanwhile, the elevated stocking density of reared farmed fish induced significant stress and suppressed fish immunological and antioxidant activity leading to increased fish susceptibility to pathogenic infection (Alam et al., 2021; Raz et al., 2021). Until now, antibiotics have been supplemented to prevent infection in cultured fish, although expensive, difficult to apply on a wide scale, and threaten the aquatic environment and consumer health (Naiel et al.,
* Corresponding author.
E-mail address: mohammednaiel.1984@gmail.com (M.A.E. Naiel).
https://doi.org/10.1016/j.aquaculture.2022.738249
2021d). Thus, promoting fish immunological and antioxidant activity is a very safe and beneficial approach for eliminating stress and consequently limits the development of any infectious diseases (Abdelghany et al., 2020; Naiel et al., 2020).
Several recent studies have proven that many herbs might be incorporated into fish diets, act as immunostimulants and possess antibacterial properties (Shafique et al., 2021). Moreover, many herbs and their bioactive molecules indicated numerous favorable impacts on fish performance, production, innate immune responses, antioxidant activity against pathogens or environmental stressors such as turmeric (Curcuma longa) (Abd El-Hakim et al., 2020); lemongrass (Cymbopogon citratus) (Al-Sagheer et al., 2018), Moringa oleifera (Zhang et al., 2020), rosemary (Rosmarinus officinale) (Naiel et al., 2019), Eruca sativa (Ibrahim et al., 2019), thyme (Thymus vulgaries) (Abd El-Naby et al., 2020; Alagawany et al., 2021).
Received 22 January 2022; Received in revised form 14 March 2022; Accepted 8 April 2022
Availableonline13April2022
0044-8486/©2022ElsevierB.V.Allrightsreserved.
Haemato-biochemical indices, antioxidant defenses, and intestinal microbial population are the leading indicators of general fish health status in farmed fish (Naiel et al., 2021a). Remarkably, a significant link was suggested between enriching fish diets with several types of herbs or their bioactive molecules and boosting haemato-biochemical indices, antioxidant activity, and immunological responses against pathogens (Abd El-hameed et al., 2021; Naiel et al., 2021b). Some natural compounds, such as aloe, roots, and propolis, have also been exhibited to be effective immunostimulants in cultured fish (Libanori et al., 2021).
Gum arabic (GA) is the gum secreted by some trees, such as the Acacia senegal or Sengalia Senegal (Bakhoum et al., 2018). Also, GA is an important natural molecule that possesses powerful immunostimulatory, antioxidant activities and anti-inflammatory properties in mammals (Fouda et al., 2019). To the best of our knowledge, there is no scientific evidence on the possible use of GA dietary supplementation in farmed tilapia fish. Thus, this feeding trial was conducted to evaluate the expected beneficial impacts of dietary supplemented GA on Nile tilapia production and health as well as the GA effects on blood haematobiochemical indices, antioxidant activity, innate immune responses and intestinal microbial modulation in farmed tilapia fish
2. Material and methods
2.1. Experimental design and rearing managements
Two hundred apparent healthy Nile tilapia (Oreochromis niloticus) juveniles (22.4 ± 0.28) g were obtained from a private hatchery in Small Abbassa and transported using air-bags to the indoor wet Lab., which is part of the Fish Health and Diseases Department, Central Laboratory for Aquaculture Research (CLAR), and maintained in a fiberglass tank for two weeks for acclimation to laboratory conditions. Fish were randomly assigned into four equal groups in twenty glass aquaria (five replicates for each group) at the rate of 10 fish per 120 L. All aquaria were supplemented with compressed air via air stones and aquarium air pumps. During the acclimation period, fish were fed on a formulated basal diet with no supplementation at a rate of 3% of the biomass, which was provided equal rations at 09:00 h and 17:00 h to adapt to the prepared diet and the trial conditions.
For 8 weeks, a basic diet was offered to tilapia fish (Table 1); the formulated basal diet was considered as a control diet and to prepare experimental diets, the basal formulation was supplemented with variable levels of GA as follow; CTR; the fish group fed on the commercial basal diet without any supplements, GA 0.25%; the fish group fed fortified diet with 0.25% Gum Arabic, GA 0.5%; the fish group fed fortified diet with 0.5% Gum Arabic, GA 1%; the fish group fed fortified diet with 1% Gum Arabic.
Throughout the experiment, water was replaced (50%) with clean, and well-aerated water in daily manner and water quality indices were monitored weekly. Water quality parameters were adjusted to meet the needs of the fish as described by Boyd and Tucker (2012) (27.2–29.8 ◦ C water temperature, 7.89 ± 0.46 mg/L for dissolved oxygen (DO), 7.98–8.39 for the pH value, and 0.11 ± 0.03 mg/L for un-ionized ammonia).
2.2. Processed diets and their chemical analysis
Gum Arabic (GA) was obtained from a local market. Before adding a suitable quantity of water to the mixture, the raw ingredients were properly blended with the tested levels of additives. Pelletizing the prepared diets was accomplished using a pellet mill grinder. While, the chemical analysis of diet samples have been estimated and calculated moisture, dry matter, crude protein, crude fat, and ash content using Thiex et al. (2012) standard methods. The nitrogen-free extract (NFE) was determined statically using the following formula; NFE (g kg 1) = 1000 – (crude protein + crude lipids+ ash + crude fiber). Moreover, the gross energy (GE) was calculated based on the values
Table 1
Ingredient and chemical composition of the experimental diet (%). Ingredient
a Premix Composition (level per each kg of product): folic acid, 370 mg; pantothenic acid, 3900 mg; biotin, 40 mg; copper, 740 mg; choline, 75 g; iron, 7500 mg; inositol 10 g; iodine, 43 mg; manganese, 7600 mg; niacin, 8800 mg; selenium, 38 mg; vitamin A, 780,000 IU; vitamin B1, 1400 mg; vitamin B12, 1900 μg; vitamin B2, 1450 mg; vitamin B6, 1400 μg; vitamin C, 19.5 g; vitamin D3, 160,000 IU; vitamin E, 14,800 IU; vitamin K3, 480 mg; zinc 1400 mg.
b Butylated hydroxytoluene.
c Nitrogen free extract (NFE) = 100 – (crude protein + moisture+ crude lipids+ ash + crude fiber).
d Calculated using the factors 5.64 kcal 100 g-1, 9.45 kcal 100 g-1, and 4.11 kcal 100 g-1protein, fat and carbohydrate, respectively.
for protein, lipid and carbohydrate as 23.6, 39.5 and 17.2 Kcal/g, respectively, as presented in Table 1 All analyses are performed in five replicates.
2.3. Growth performance and utilization of feeds
During eight weeks, experimental diets were provided twice daily (9.00 and 17.00 h) until apparent satiation. The satiation level was confirmed based on apparent visual satiety. Feed was carefully supplied, ensuring that no feed remained on the aquarium bottom. Unconsumed feed was siphoned carefully from each aquarium for 30 min after begin of feeding at each meal every day. Once we measured the dried mass of siphoned food, we deducted it from total feed consumption (FC) to calculate actual feed intake. In all experimental groups, feed allowance of fish was re-adjusted every day according to the feed intake of fish in the control group the previous day. Moreover, the survived fish were weighed every two weeks for estimating consumed diets and calculated growth. The growth, efficiency of feeds, and survival rate of tilapia juveniles were estimated at the end of the feeding trial using the following formulas:
Weight gain (WG) = W 2 W 1
Weight gain percent, WGP (%) = 100 x (WG/IW)
where W1 and W2 are the initial weight and final biomass, respectively.
Total Feed intake, TFI (g feed/fish) = FI n
where, FI is the consumed diets during the feeding trial and n is the total fish number.
Feed conversion ratio, FCR = TFI WG
Protein intake, PI = TFI × crude protein fish number
Protein efficiency rate, PER = WG ÷ PI
Survival, SR (%) = (final survived fish number / initial fish number) x 100.
2.4. Blood and tissue sampling
At the end of the experiment, three fish were randomly sampled from each aquarium (15 per treatment) and anesthetized with 120 mg/L amino-benzoic acid (Sigma–Aldrich). Blood samples were collected from caudal vein using sterile syringe contain small amount of dipotassium salt of EDTA. The collected blood samples were directly centrifuged at 1500 xg for 15 min to obtain plasma. The plasma was stored at 4 ◦ C for further biochemical, antioxidant and immunity parameters analysis. Also, kidney and spleens were collected and kept at 80 ◦ C until the extraction of total RNA for gene expression. Finally, the midguts of three fish from each group were randomly selected for bacterial population detection.
2.5. Blood hematological parameters
Fresh blood samples were used to assess hemoglobin (Hb) concentration u a commercial kit (Diamond Diagnostic, Egypt). A manual method was applied for counting total erythrocyte (RBCs) and leukocytes (WBCs) immediately with a hemocytometer after dilution using Natt and Herrick’s solution (Maxham et al., 2016). Hematocrit (Ht %) was determined by the microhematocrit method described by Goldenfarb et al. (1971) and RBC, Ht, and Hb values were used to calculate the red cell indices such as mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) according to Sarma (1990) formulas:
MCV ( μm3 cell 1 ) = Ht (v/v ratio) x 1000/RBC ( 106 cell μl 1 )
MCH ( pg cell 1 ) = Hb ( g dl 1 ) x 10/RBC ( 106 cell μl 1 )
MCHC = Hb ( g dl 1 )/Ht (v/v ratio)
2.6. Blood biochemical analysis
Biochemical parameters such as total protein (TP) and albumin (ALB) in the blood were measured using a colorimetrical procedure with a spectrophotometer (Opti Zen POP UV/VIS). At the same time, globulin (GB) was calculated mathematically by subtracting the albumin value from the total protein value. Also, Spectrophotometric estimation of parameters related to kidney function such as creatinine (CR) and uric acid (UA) was performed using special commercial kits (BioMed, diagnostic, Egypt) in accordance with the Folin and Wu (1919) protocol. Commercial kits (ALT or AST Assay Kit, ABACM241035) have evaluated liver enzyme activities such as ALT and AST, as designated by Wilkinson et al. (1972)
2.7. Blood digestive enzymes assays
Amylase and lipase activities were quantified spectrophotometrically at A540 and A714 wave-lengths as described by Wang et al. (2019) Where, protease activity was estimated using casein as a substrate following the assay of Sigma’s non-specific protease activity (CuppEnyard, 2008).
2.8. Immunological assays
Ai (1990) procedure was used to estimate the plasma lysozyme level using microwell plates. The fish complement 3 (C3) (ELISA) Kits (MyBioSource Co., USA, No: MBS1601750) had been applied for estimating COMPC3 levels as illustrated by manual guidelines. The immunoglobulin M (IgM) and immunoglobulin A (IgA) were measured calorimetrically according to Granfors (1979) procedure using Fish immunoglobulin (ELISA) kits (Cusabio, Bio-tech Co., Ltd., Wuhan, China, No: MBS282651).
Blood antioxidant defenses The antioxidant activities were detected using specific colorimetric kits obtained from Bio-diagnostic Co., Cairo, Egypt, following Nishikimi et al. (1972) for superoxide dismutase (SOD), Aebi (1984) for catalase (CAT), Beutler (1963) for glutathione peroxidase (GSH) and Draper and Hadley (1990) for the lipid peroxidation marker; malonaldehyde (MDA) detection.
2.9. Intestinal microbial estimation
Preparation of the media and bacterial isolation were done according to Esakkiraj et al. (2009) procedure. Sterilization of the media was done by autoclaving at 121 ◦ C for 15 min. One gram of midgut content was diluted in 9 mL of 0.85% sterile sodium chloride (w/v) and was then vortexed. Ten-fold serial dilutions were carried out for bacterial detection. Afterward, 0.1 mL aliquot was cultured on nutrient agar (Lab M, Lancashire, UK) according to the plate count method for aerobic colony counting. The same method and media were used for anaerobic but under anaerobic conditions (candle jar). Also, 0.1 mL aliquots were cultured on MRS agar for Bacillus, Muller and Hinton for Pseudomonas, MacConkey agar for coliform and EMB agar for E. coli counts. All samples were incubated at 37 ◦ C for 24–48 h and counts were expressed as log cfu/g as described by Al-Harbi and Uddin (2005) and Espírito Santo et al. (2007)
2.10.
RNA extraction and cDNA synthesis
For total RNA extraction, the spleen and kidney tissue samples (30 mg) were directly homogenized with the Eastep® Super Total RNA Extraction Kit (Promega, Shanghai, China; Cat. No. LS1040). To avoid RNA contamination, 2 μL of extracted RNA (10 mg/ml, Fermentas) was blended with 20 μL of DNA before being dissolved in TE buffer (Tris–EDTA, pH = 8.0) then incubated at 37 ◦ C for 3–4 h. The quality of extracted RNA was evaluated using gel electrophoresis (contains 1.5% agarose gel) and the A260/280 absorbance ratio on a Nano-Drop 2000 (Thermo Scientific, USA). Then, Reverse transcription of 1 g of total RNA using GoScriptTM Reverse Transcription Mix (Promega, Shanghai, China; Cat. No. LS2054) was performed following the manufacturer’s protocol.
2.11. Quantitative real-time PCR
The primer sequences for interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interleukin-10 (IL-10) are illustrated in Table 2 (Abu Zeid et al., 2021). Briefly, the total reaction volume (20 μL) containing 10 μL of 2 Go Taq® qPCR Master Mix, 2 μL of cDNA, 0.4 μL of each forward and reverse primer and 7.2 μL of nuclease-free water. The following qRT-PCR thermocycling limits were applied: 95 ◦ C for 2 min, then 40 cycles of 95 ◦ C for 15 s., 60 ◦ C for 1 min, followed by melting phase was from 60 ◦ C to 95 ◦ C. Each real-time PCR reaction (including cDNA synthesis) was repeated three times to approve the accuracy of the obtained results. The GAPDH gene was applied as an endogenic reference to quantify the relative mRNA transcription levels. The product’s dissociation curve analysis and agarose gel electrophoresis were used to confirm the q-RT-PCR technique. The relative gene-transcription levels were estimated using the 2ʌʌCt formula after determining the threshold cycle (Ct) values for each examined sample (Livak and Schmittgen,
M.A.E. Naiel
Table 2
The sequence of primers designed to detect target genes using real time PCR technique. Primer
2.12. Statistical procedures
The calculated and collected data were firstly tested for normality and homogeneity followed by one-way analysis of variance (ANOVA) followed by tukey post hoc test to identify whether the estimated measurements were significantly influenced by GA levels in fish diets. P < 0.05 was considered significant. The data are illustrated in tables and figures as mean ± SE.
3. Results
3.1. Performance indices
0.5 or 1% GA-enriched fish diets with presented higher performance in terms of final biomass (p value: <0.001), weight gain (p value: <0.001), weight gain percent (p value: <0.001), compared to the fish group fed GA free diet (Table 3). Compared to the control group, the fish group fed diets supplemented with GA 0.5 or 1% showed a significant (p value: <0.001) decrease in consumed feed, feed conversion ratio, protein intake, and protein efficiency ratio. During the feeding trial period, no mortality in either of the experimental groups was noticed.
3.2. Hematological measurements
Fish supplemented diets fortified with GA 1% showed a greater
Table 3
Growth performance, feed efficiency and survival rate of Nile tilapia fish fed on diets containing several levels of Gum Arabic for 56 days.
Parameters Treatments
±
<
increase in hemoglobin and WBCs levels than the other treatment groups, followed by the fish fed diets supplemented with GA 0.5%. In addition, fish supplemented with GA 1% presented a higher MCV, MCH and MCHC levels (p value: 0.001, 0.014 and 0.039, respectively) than those fed with the concentration of GA 0.25 or 0.5% (Table 4). On the other hand, feed supplements had no effect on RBC and hematocrit levels.
3.3.
Biochemical parameters
Supplementation with different levels of Gum Arabic significantly improved the blood biochemical parameters of tilapia fish (Table 5). The fish group fed enriched diets with GA 1% exhibited higher total protein, albumin and globulin values than the control group. Also, dietary inclusion of GA 1% within fish feed showed a significant decrease of uric acid, AST, and ALT concentrations compared with the fish group fed free-GA diet. Meanwhile, the dietary supplementation of various amounts of GA did not alter the blood creatinine level.
3.4. Digestive enzymes
The analysis of digestive enzymes indicated that increasing the amount of GA in fish feed remarkably improved lipase and amylase activity (p values: 0.023 and 0.001, respectively), but did not affect protease levels (Table 6). The fish group fed the diet supplemented with GA 1% reported the highest levels of lipase and amylase followed by GA 0.5 fish group then GA 0.25% fish group.
Table 4
Blood hematological parameters of Nile tilapia fish fed on diets containing several levels of Gum Arabic for 56 days.
<0.001
PI (g) 15.35 ± 0.20a 14.68 ± 0.16a 13.69 ± 0.21b 13.049 ± 0.07b <0.001
PER (g/g) 0.42 ± 0.001a 0.39 ±
±
±
<0.001
CTR, control group fed basal diet; GA0.25%, fish group fed enriched diets with 0.25% Gum Arabic; GA0. 5%, fish group fed enriched diets with 0.5% Gum Arabic; GA1%, fish group fed enriched diets with 1% Gum Arabic. IW, initial weight (g); FW, final weight (g); WG, weight gain (g); FI, feed intake (g); WGP %, weight gain percentage; FCR, Feed conversion ratio (g/g); PI, Protein intake; PER, Protein efficiency rate; SR, survival rate. Different superscript letters at the same raw indicate the significance of experimental groups (p ˂0.05). All data were presented as the mean ± standard error.
CTR, control group fed basal diet; GA0.25%, fish group fed enriched diets with 0.25% Gum Arabic; GA0. 5%, fish group fed enriched diets with 0.5% Gum Arabic; GA1%, fish group fed enriched diets with 1% Gum Arabic. RBCs, Red blood cell; WBCs, White blood cell; Hb, Hemoglobin; HT, Hematocrit; MCV: Mean corpuscular of red blood cells; MCH, Mean corpuscular hemoglobin values and MCHC, Mean Corpuscular Hemoglobin Concentration. Different superscript letters at the same raw indicate the significance of experimental groups (p ˂0.05). All data were presented as the mean ± standard error.
M.A.E. Naiel
Table 5
Blood biochemical parameters of Nile tilapia fed on diets containing several levels of Gum Arabic for 56 days.
Parameters
TP (g/dL)
GLOB (g/ dL)
±
±
±
CTR, control group fed basal diet; GA0.25%, fish group fed enriched diets with 0.25% Gum Arabic; GA0. 5%, fish group fed enriched diets with 0.5% Gum Arabic; GA1%, fish group fed enriched diets with 1% Gum Arabic. TP, total protein; ALB, albumin; GLOB, globulin; UA, uric acid; CR, creatinine; AST, Aspartate aminotransferase ALT, alanine aminotransferase.
Different superscript letters at the same raw indicate the significance of experimental groups (p ˂0.05). All data were presented as the mean ± standard error.
Table 6
Blood digestive enzymes of Nile tilapia fed on diets containing several levels of Gum Arabic for 56 days.
Parameters
Protease (ng dl 1)
CTR, control group fed basal diet; GA0.25%, fish group fed enriched diets with 0.25% Gum Arabic; GA0. 5%, fish group fed enriched diets with 0.5% Gum Arabic; GA1%, fish group fed enriched diets with 1% Gum Arabic. Different superscript letters at the same raw indicate the significance of experimental groups (p ˂0.05). All data were presented as the mean ± standard error.
3.5. Blood antioxidant defenses, lipid peroxidation and non-specific immune responses
A significant increase in GSH, CAT, and SOD levels were reported in fish groups given 1% GA when compared to the control group. However, GA feeding in fish diets significantly decreased MDA levels compared to the control group (Table 7). With respect to immunological parameters, the highest concentration of GA in fish diets (0.5 or 1%) significantly improved all innate immunity parameters levels (lysozyme, COMPC3, IgM and IgA) (Table 7). Furthermore, the maximum lysozyme, COMPC3, IgM, and IgA concentrations were found mainly in the GA 1% supplemented group.
3.6. Immune-related genes transcription
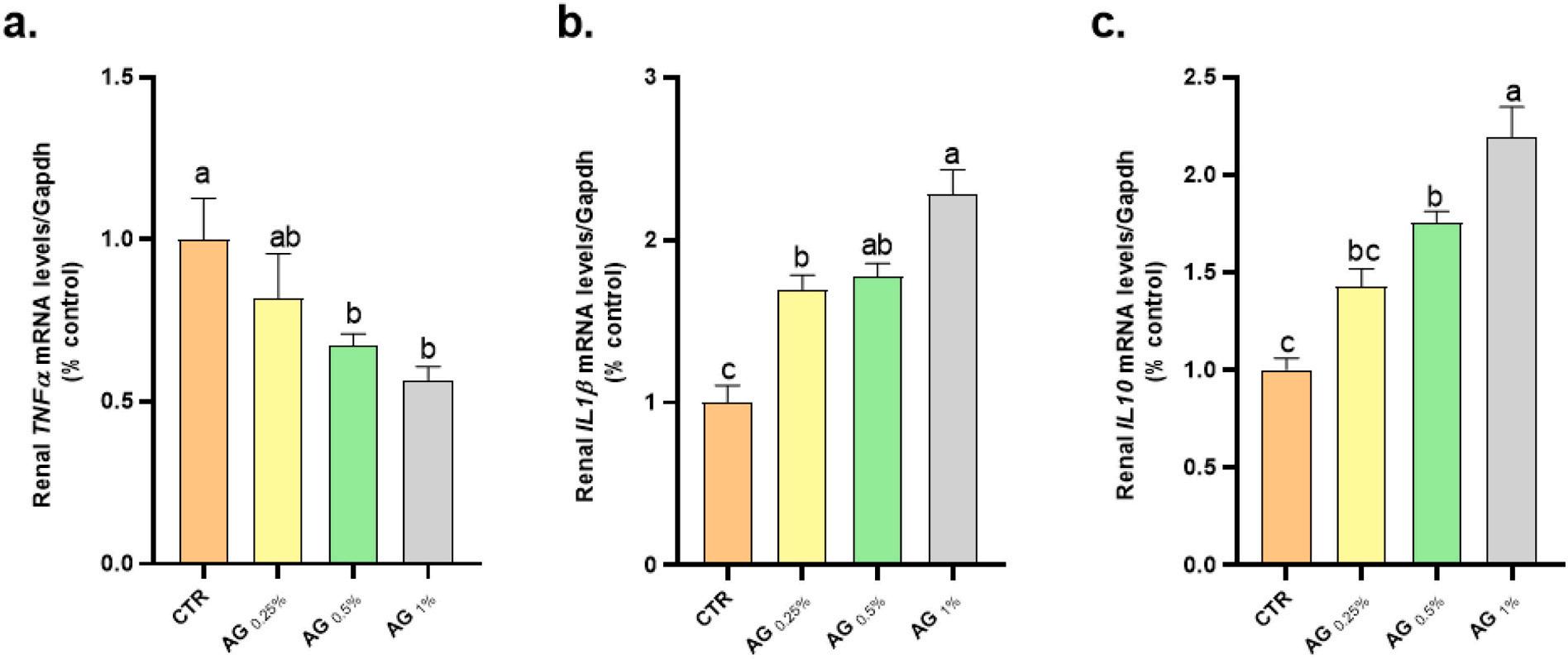
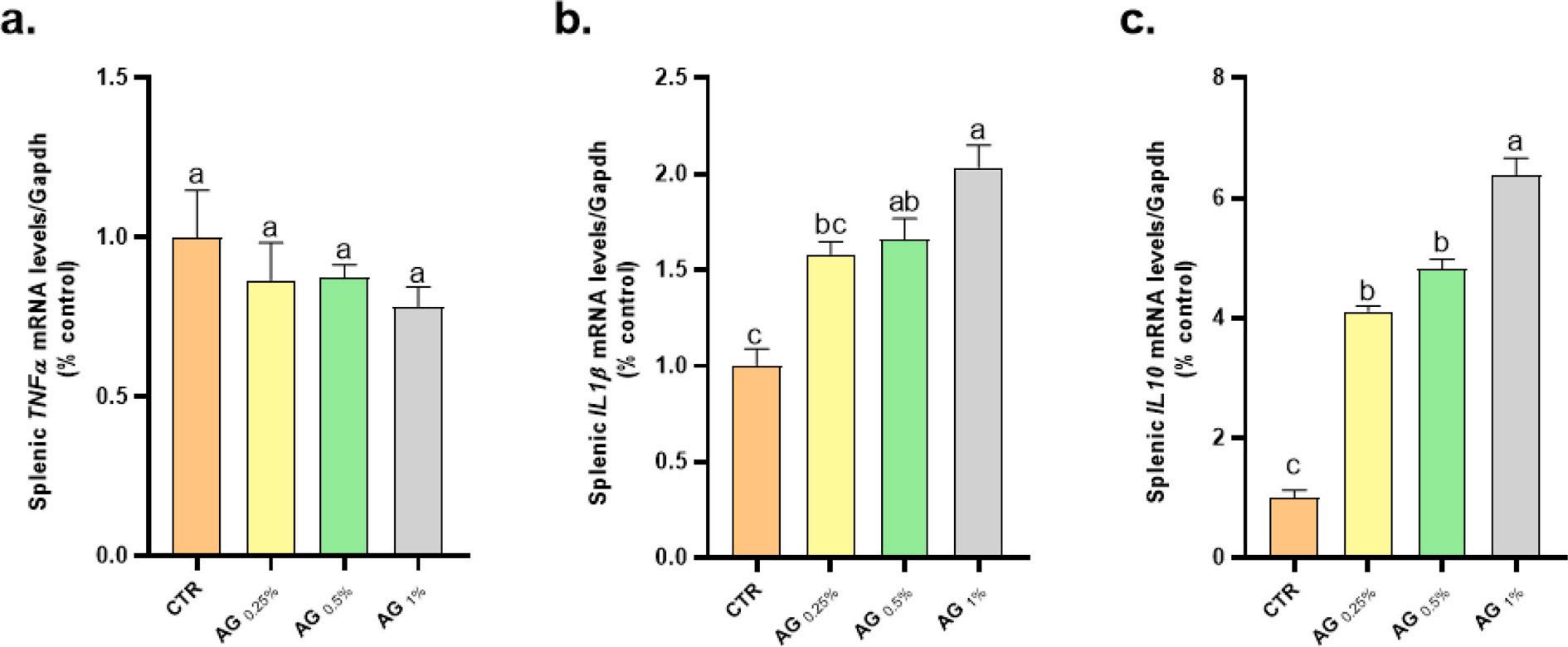
In reference to the fold change values of immune-related genes transcription in both spleen and kidney tissues, fortified tilapia diets with graded levels of GA showed up-regulated the mRNA expression of both IL-1β and IL-10 genes, but not TNF-a, as shown (Figs. 1 and 2). The fish group fed diets supplemented with GA 1% exhibited elevated IL-1β and IL-10, but not TNF-a, mRNA expression, compared with the other treatments.
Table 7
Antioxidative defenses and non-specific immune activities of Nile tilapia fish fed on diets supplemented with graded levels of Gum Arabic for 56 days.
Parameters Treatments
Innate immune responses
Antioxidant defense markers and lipid peroxidation
CTR, control group fed basal diet; GA0.25%, fish group fed enriched diets with 0.25% Gum arabic; GA0. 5%, fish group fed enriched diets with 0.5% Gum arabic; GA1%, fish group fed enriched diets with 1% Gum arabic.
LYZ, lysozyme; COMP C3, complement C3; IgM, immunoglobulin M; IgA, immunoglobulin M; MDA, malonaldehyde; GSH, glutathione; CAT, catalase; SOD, super oxide dismutase.
Different superscript letters at the same raw indicate the significance of experimental groups (p ˂0.05). All data were presented as the mean ± standard error.
3.7. Microbiota biodiversity
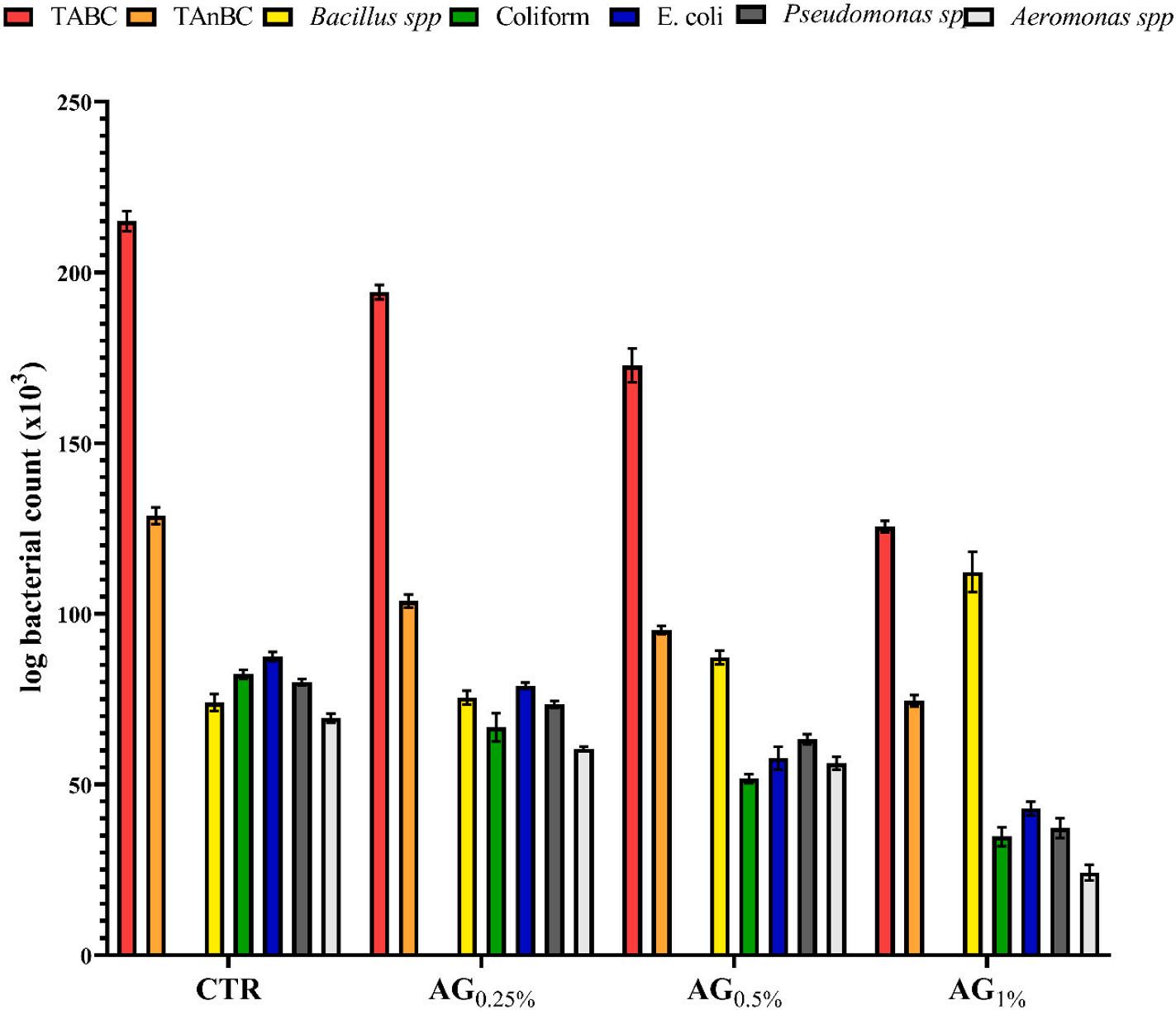
concerning the total counts of aerobic (TABC) and anaerobic bacterial (TAnBC), the fish group supplemented with a diet fortified with a high level of GA (GA 1%) had greater antibacterial properties against both intestinal TABC and TAnBC counts when compared to the other treatments (Fig. 3). Furthermore, the fish groups supplemented with GA 0.5% and GA 1% showed a greater potential to promote Bacillus spp. growth when compared with other treatments (Fig. 3). In contrast, fish treated with Gum Arabic at either 0.5 and 1% lowered the coliform, E. coli, Pseudomonas spp., and Aeromonas spp. counts in mid intestine samples compared to the control group.
4. Discussion
Growth performance and feed utilization parameters are essential indicators of fish health and productivity. According to our feeding trial results, tilapia fish diets supplemented with 0.5 or 1% GA had the best growth performance indices in terms of feed intake, feed conversion, final biomass, weight gain, and specific growth rates, when compared to the control group, which was found to be consistent with previous research findings using various prebiotics or natural products. For instance, El-faki (2016) exhibited that tilapia fish fed diet supplemented with 3% GA had better performance and feed efficiency measurements but still had a lower survival rate than the un-supplemented fish group. Also, Faggio et al. (2015) reported that fed Mugil Cephalus supplemented diets with 12% GA had no adverse effects on growth compared with the control group. Differences in fish growth response to varied GA concentrations may be attributed to supplemented form, fish feeding habitats, fish species, or rearing conditions.
Besides, GA could be categorized as a natural prebiotic source (Calame et al., 2008). Prebiotics contains fibers and natural simple structure sugars that boost the performance and encourage the development of beneficial bacterium within the intestinal microbiota particularly probiotic bacteria (Naiel et al., 2021c). Also, GA contains neutral
M.A.E. Naiel et al.
Fig. 1. Influences of supplemented tilapia diets with graded levels of Gum arabic (0.0, 0.25, 0.5 or 1%) for 56 days on mRNA expression folds of kidney (a) TNF-2a (Tumor necrosis factor-2a), (b) IL-1β (interleukin 1β) and (c) IL-10 (interleukin 10) genes. The different letters were applied to identify the significant differences (p < 0.05) between the experimental treatments.
Fig. 2. Influences of supplemented tilapia diets with graded levels of Gum arabic (0.0, 0.25, 0.5 or 1%) for 56 days on mRNA expression folds of spleen TNF-2a (Tumor necrosis factor-2a), IL-1β (interleukin 1β) and IL-10 (interleukin 10) genes. The different letters were applied to identify the significant differences (p < 0.05) between the experimental treatments.
sugars (rhamnose, arabinose, and galactose), glucuronic acid, polysaccharides and minerals responsible for performance stimulation through the development of beneficial bacteria (Al-Baadani et al., 2021). Previous reports showed that GA acts as a prebiotic when supplemented to diets, boosting growth and stimulating digestive tract secretion and intestinal health status by interacting with the tract epithelium, mucus, and tract microbiota (Hu et al., 2014; Celi et al., 2018; Xia et al., 2020). Therefore, enriched diets with GA might enhance digestive tract functioning (Calame et al., 2008). Also, GA is a welltolerated dietary fiber with bifidogenic characteristics that improve gut health against enteric infections (Cherbut et al., 2003).
Moreover, the fish group fed a high concentration of GA (1%) significantly increased blood lipase and amylase, indicating elevated secretion in the gastrointestinal tract. Rather of being degraded into their main constituent in the intestines, the entero-pancreatic enzyme might be re-cycled by being absorbed into the blood, re-accumulated by the pancreas, and re-utilized (Rothman et al., 2002). Thus, it was shown that there is a strong correlation between high blood levels of digestive enzymes and high levels of digestive enzymes in the digestive tract.
These results were similar to Nasif et al. (2011) findings that gum combined with aspirin caused a remarkedly increase in intestinal amylase, lipase secretions compared with the control group in rats. Such an increase in digestive enzyme secretions in addition to the various beneficial characteristics of GA to the intestinal mucosal layers, leads to the inhibition of the growth of harmful microorganisms, therefore improving gastrointestinal digestive properties (Abd El-Mawla and Osman, 2011).
Blood hematological parameters are essential for evaluating diet quality, general health status, aquatic environment toxicity, and disease diagnosis (Ismael et al., 2021). Among the blood parameters measured, hemoglobin MCV, MCH, and MCHC values were significantly improved when the level of GA in the diet was increased (up to 1% GA). Similar results were reported by Francesco et al. (2012) in mullets and Satheeshkumar et al. (2012) on wild marine teleost fish. While, Faggio et al. (2015) indicated that when 12% GA-pellets were supplemented to mullet fish for a short period of time, no significant change in WBC levels was observed. This study discovered that not only the amount of GA supplemented in the feeding diet, but also the period of supplementation
M.A.E. Naiel
and fish species affect the fish’s hematopoiesis, however, the exact mechanisms by which this happens is unknown and requires further investigation.
Concerning the WBC count, fish fed a diet containing 1% GA had a considerably higher leucocytic count than fish fed basal diet, provides a higher capability to sustain homeostasis, as leukocytes are defensive system cells that initiate the immune response, and lymphocytes are critical cells for humoral and cell-mediated immunity in fish. This rise in the WBCs count was also confirmed by Azevedo et al. (2016), who reported higher total leukocytic count in Nile tilapia during the fall season, assert that a high WBCs count in the blood is indicative of boosted immunological activity.
Biochemical blood indices reflect the health condition of fish when they are subjected to numerous feed supplements (Fazio, 2019; Alandiyjany et al., 2022). In terms of blood constituents, fish offered a diet supplemented with GA 1% showed remarkedly higher levels of total protein, albumin, and globulin as compared to fish fed GA 0.25 or 0.5%, which demonstrated an improved ability to maintain overall health status. Besides, in comparison to other treatment groups, dietary inclusion with a high level of GA (1%) resulted in decreased AST, ALT, and uric acid concentrations. Our results in tilapia fish were found to be in line with findings in other mammals where rabbit supplemented diets with 2–20 g GA per kg diet significantly increased total protein, albumin, and glucose concentrations and remarkedly declined liver and kidney enzyme activities (Amber et al., 2017). Moreover, Nasir et al. (2016) reported that mice supplemented 100 g GA per liter in drinking water reduced uric acid and glucose levels. Moreover, Al-Fadil et al. (2013) reported that supplemented broiler chicken diets with 20, 40, and 60 g GA/kg significantly reduced uric acid levels in the blood. The enrichment of tilapia diets with GA as a prebiotic might be responsible for the production of short-chain fatty acids and ammonia in the digestive tract, as well as the creation of a suitable environment for beneficial microbiota, resulting in higher nitrogen secretion and lower
serum urea nitrogen concentration (Al-Baadani et al., 2021).
Glutathione, superoxide dismutase, and catalase have been discovered to play a very important role function in the phase II detoxification of lipid peroxides process and to have an anti-free radical effect in cells under oxidative stress (Ziki´ c et al., 2001; Rudneva et al., 2010; Sandamalika et al., 2021). Moreover, increased malondialdehyde (MDA) levels are often assumed to be an indication of lipid peroxidation induced by oxidative stress generated by fish exposure to suboptimal environmental conditions (Garcia et al., 2020). Concerning our antioxidant defense markers results, higher GSH, SOD, and CAT values, as well as lower MDA concentrations, were observed in association with increasing the dietary inclusion level of GA within the feeding diet. Several studies have already shown GA’s antioxidant properties in mice (Ahmed et al., 2016), broiler (Alzawqari et al., 2016) and Mugil cephalus (Faggio et al., 2015) Musa et al. (2013) reported that GA could promote the antioxidant activity of the hepatocytes via modulating the oxidative stress-related genes expression. Moreover, GA has been shown to have a high concentration of phenolic chemicals, which may be responsible for its strong antioxidant action (Mirghani et al., 2018).
Lysozyme activity is a key indicator of fish adaptive immunity (Saurabh and Sahoo, 2008). Furthermore, the complement activity is the main factor of the innate immune response and plays a vital role in destroying pathogenic bacteria, phagocytosis, anti-inflammatory effects, complex immune clearance, and production of antibodies (Holland and Lambris, 2002). Additionally, IgM and IgA support both the innate and adaptive immune systems in fish, with functional activities such as complement pathways activation, pathogen phagocytosis and removal, and cellular cytotoxicity (Mashoof and Criscitiello, 2016). Furthermore, the spleen and head kidney are well-known lymphoid organs of teleost fish that are discovered to form at 2 days post-hatching in Asian seabass and are generally rich in melano-macrophage clusters, indicating their immune-related activities (Jiang et al., 2014). In this investigation, the fish fed high amounts of GA (1%) had the greatest
Fig. 3. Effects of enriched tilapia diets with several level of Gum arabic (0.0, 0.25, 0.5 or 1%) for 56 days feeding trial on mid intestinal aerobic (TABC), anaerobic bacterial (TAnBC), Bacillus spp, coliform, E. coli, Pseudomonas spp and Aeromonas spp counts. All of the data that was represented was mean ± SE.
M.A.E. Naiel
levels of Lysozyme, COMPC3, and immunoglobulins, followed by fish given diets supplemented with 0.5% GA, when compared to other treatment groups. The obtained results found to be similar to Soleimani et al. (2012) findings that enriched Caspian roach (Rutilus rutilus) fry diets with 10, 20 or 30 g fructooligosaccharide (FOS) per kg enhance the immunoglobulins, complement and lysozyme levels and promote the innate immune responses. Also, diets supplemented with 2 g GA per kg found to be significantly increased immunoglobulins levels (IgG, IgM, and IgA) in rabbits (El-Ratel et al., 2019) and rats (Ali et al., 2013). The obtained results proposed that GA could indirectly stimulate the fish immune system via altering the production of the pro-inflammatory cytokines on intestinal mucosa (Patel and Goyal, 2015) or stimulate the activation of interleukins, which influence immunological responses (Blanco et al., 2008). Dendritic cells are a special type of antigenpresenting cell (APC) that showed a vital function in the innate immune responses. Ahmed (2018) supported that GA dietary supplementation could promote dendritic cells production. Also, Xuan et al. (2010) exhibited that GA (5 g per liter) has remarkable immunological influences via modulating the properties of intestinal dendritic cells through direct binding with the intestinal lumen cells as APC and thus providing a potential impact in the immune response regulation process by improving the cytokine genes (IL-1, IL-10, and TNF-α) production. The findings indicated that GA might indirectly boost the expression of immune related genes/cytokines in a variety of tissues (Abdel-Rahman et al., 2006; Patel and Goyal, 2015).
Feed supplements are one of the numerous factors that affect the gastric microbial population of fish (Egerton et al., 2018). Prebiotics modulate the entire gastric environment of the fish through modulating microbial biodiversity and enhancements the epithelium layer structure, which regulates the interaction of the gut microbiota and the host (Vargas-Albores et al., 2021). In addition, prebiotics may benefit fish health by selectively colonizing the gastrointestinal tract with favorable microorganisms such as, lactobacillus, bifidobacteria, Saccharomyces sp. and some non-pathogenic strains of E. coli (Buentello et al., 2010). Beneficial bacterial strains that predominate the intestinal tract enhance the enteric environment by releasing bacteriocins, which suppress the development of some pathogenic bacterial strains and, therefore, undoubtedly alter the host’s microbiota diversification (Reza et al., 2009). In the present research, fish-fed diets supplemented with 1% GA had the highest bacillus spp. counts when compared to other treatments. In addition, increasing the amount of GA in tilapia diets remarkedly reduced total aerobic and anaerobic bacterial counts, as well as Pseudomonas spp., Aeromonas spp., and E.coli numbers in the digestive tract. These results were similar to Alarifi et al. (2018) findings that dietary supplementation of 10 and 20 g GA per kg had the same prebiotic Fructooligosaccharide (FOS) effects caused by an increase in Lactobacillus spp. and Bifidobacterium spp. Moreover, Lham et al. (2015) and Hu et al. (2016) reported that diets supplemented with 2.5 up to 20 g GA per kg diet exhibited higher antibacterial properties against the development of Escherichia coli, Streptococcus Pneumonia, Streptococcus Pyogenes, Salmonella Typhi, Pseudomonas Aeruginosa, and Staphylococcus Aureus. The higher antimicrobial efficiency of GA may be attributed to the existence of elevated levels of tannins and saponins molecules (Ahmed, 2018).
5. Conclusion
Taken together, findings in this feeding trial demonstrate that supplementing tilapia diets with Gum Arabic (1%) boosts performance, antioxidant status, immunological response and modulates intestinal microbiota. GA 1% enriched tilapia diet is a potential feed additive in aquaculture and may improve fish production practices in the future.
Funding
Not applicable.
Ethics approval
The Local Experimental Animal Care Committee requirements were followed, and the Institutional Animal care and use Committee (IACUC), Zagazig University, endorsed the research.
CRediT authorship contribution statement
Mohammed A.E. Naiel: Conceptualization, Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Samah A.A. Abd El-hameed: Methodology, Investigation, Validation, Visualization, Writing – review & editing. Ahmed H. Arisha: Methodology, Investigation, Validation, Visualization, Writing –review & editing. Samar S. Negm: Methodology, Formal analysis, Data curation, Investigation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The all-authors state that they have no conflicting interests that may have influenced the results described in this manuscript.
Acknowledgment
All authors would like to thank Dr. Abdullah Ibrahim El-Kholy, Pharmaceutical Technology Unit, National Institute of Laser Enhanced Sciences (NILES), Cairo University, for his valuable and constructive english and grammatical edits to this manuscript. His generosity in donating his time and effort has been much appreciated.
References
Abd El-Hakim, Y.M., El-Houseiny, W., Abd Elhakeem, E.M., Ebraheim, L.L., Moustafa, A. A., Mohamed, A.A.R., 2020. Melamine and curcumin enriched diets modulate the haemato-immune response, growth performance, oxidative stress, disease resistance, and cytokine production in Oreochromis niloticus Aquat. Toxicol. 220, 105406
Abd El-hameed, S., Negm, S.S., Ismael, N.E., Naiel, M.A., Soliman, M.M., Shukry, M., Abdel-Latif, H.M., 2021. Effects of activated charcoal on growth, immunity, oxidative stress markers, and physiological responses of Nile tilapia exposed to sublethal Imidacloprid toxicity. Animals. 11, 1357
Abd El-Mawla, A.M., Osman, H.E.H., 2011. Effects of gum acacia aqueous extract on the histology of the intestine and enzymes of both the intestine and the pancreas of albino rats treated with meloxicam. Pharm. Res. 3, 114
Abd El-Naby, A.S., Al-Sagheer, A.A., Negm, S.S., Naiel, M.A., 2020. Dietary combination of chitosan nanoparticle and thymol affects feed utilization, digestive enzymes, antioxidant status, and intestinal morphology of Oreochromis niloticus. Aquaculture. 515, 734577
Abdelghany, M.F., El-Sawy, H.B., Abd El-Hameed, S.A., Khames, M.K., Abdel-Latif, H.M., Naiel, M.A., 2020. Effects of dietary Nannochloropsis oculata on growth performance, serum biochemical parameters, immune responses, and resistance against Aeromonas veronii challenge in Nile tilapia (Oreochromis niloticus). Fish & Shellfish Immunol. 107, 277–288
Abdel-Rahman, A.M., El-Sahrigy, S.A., Bakr, S.I., 2006. A comparative study of two angiogenic factors: vascular endothelial growth factor and angiogenin in induced sputum from asthmatic children in acute attack. Chest. 129, 266–271
Abu Zeid, E.H., Khalifa, B.A., Said, E.N., Arisha, A.H., Reda, R.M., 2021. Neurobehavioral and immune-toxic impairments induced by organic methyl mercury dietary exposure in Nile tilapia Oreochromis niloticus Aquat. Toxicol. 230, 105702
Aebi, H., 1984. (13) Catalase in vitro. Methods Enzymol. 105, 121–126
Ahmed, A.A., 2018. Health Benefits of Gum Arabic and Medical Use. Academic Press, pp. 183–210
Ahmed, A.A., Fedail, J.S., Musa, H.H., Musa, T.H., Sifaldin, A.Z., 2016. Gum Arabic supplementation improved antioxidant status and alters expression of oxidative stress gene in ovary of mice fed high fat diet. Middle East Fertility Soc J. 21, 101–108
Ai, Ellis, 1990. Lysozyme assays. Techniques in Fish Immunol. 1, 101–103
Alagawany, M., Farag, M.R., Abdelnour, S.A., Elnesr, S.S., 2021. A review on the beneficial effect of thymol on health and production of fish. Rev. Aquac. 13, 632–641.
Alam, R.T.M., Abu Zeid, E.H., Khalifa, B.A., Arisha, A.H., Reda, R.M., 2021. Dietary exposure to methyl mercury chloride induces alterations in hematology, biochemical parameters, and mRNA expression of antioxidant enzymes and metallothionein in Nile tilapia. Environ. Sci. Pollut. Res. 28, 31391–31402
Alandiyjany, M.N., Kishawy, A.T.Y., Abdelfattah-Hassan, A., Eldoumani, H., Elazab, S.T., El-Mandrawy, S.A.M., Ibrahim, D., 2022. Nano-silica and magnetized-silica mitigated lead toxicity: their efficacy on bioaccumulation risk, performance, and
M.A.E. Naiel
apoptotic targeted genes in Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 242, 106054
Alarifi, S., Bell, A., Walton, G., 2018. In vitro fermentation of gum acacia–impact on the faecal microbiota. Int. J. Food Sci. Nutr. 69, 696–704
Al-Baadani, H.H., Al-Mufarrej, S.I., Al-Garadi, M.A., Alhidary, I.A., Al-Sagan, A.A., Azzam, M.M., 2021. The use of gum Arabic as a natural prebiotic in animals: a review. Anim. Feed Sci. Technol. 114894
Al-Fadil, S., Mukhtar, M.A., Tabidi, M.H., 2013. Response of broiler chicks to diets containing gum arabic as a natural prebiotic. J. Curr. Res. Sci. 1, 247–253
Al-Harbi, A.H., Uddin, N., 2005. Bacterial diversity of tilapia (Oreochromis niloticus) cultured in brackish water in Saudi Arabia. Aquaculture. 250, 566–572
Ali, B.H., Beegam, S., Al-Lawati, I., Waly, M., Al Za’abi, M., Nemmar, A., 2013. Comparative efficacy of three brands of gum acacia on adenine-induced chronic renal failure in rats. Physiol. Res. 62, 47–56.
Al-Sagheer, A., Mahmoud, H., Reda, F., Mahgoub, S., Ayyat, M., 2018. Supplementation of diets for Oreochromis niloticus with essential oil extracts from lemongrass (Cymbopogon citratus) and geranium (Pelargonium graveolens) and effects on growth, intestinal microbiota, antioxidant and immune activities. Aquac. Nutr. 24, 1006–1014
Alzawqari, M., Al-Baddany, A., Al-Baadani, H., Alhidary, I., Khan, R.U., Aqil, G., Abdurab, A., 2016. Effect of feeding dried sweet orange (Citrus sinensis) peel and lemon grass (Cymbopogon citratus) leaves on growth performance, carcass traits, serum metabolites and antioxidant status in broiler during the finisher phase. Environ. Sci. Pollut. Res. 23, 17077–17082
Amber, K., Abd El-Nabi, F.M., Morsy, W., Morsy, S.H., 2017. Gum Arabic as prebiotic in growing rabbits diet. Global Vet. 19, 465–471
Azevedo, T., Albinati, R., Guerra-Santos, B., Pinto, L., Lira, A., Medeiros, S., Ayres, M., 2016. Reference values of hematological parameters of Oreochromis niloticus (Linaeus, 1758) cultivated in net cages in Paulo Afonso, state of Bahia, Brazil Brazilian. J. Aquatic Sci. Technol. 20, 63–74
Bakhoum, N., Fall, D., Fall, F., Diouf, F., Hirsch, A., Balachandar, D., Diouf, D., 2018. Senegalia senegal (synonym: Acacia senegal), its importance to sub-Saharan Africa, and its relationship with a wide range of symbiotic soil microorganisms. South African J. Botany. 119, 362–368
Beutler, E., 1963. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 61, 882–888
Blanco, P., Palucka, A.K., Pascual, V., Banchereau, J., 2008. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 19, 41–52.
Boyd, C.E., Tucker, C.S., 2012. Pond Aquaculture Water Quality Management. Springer Science & Business Media
Buentello, J.A., Neill, W.H., Gatlin, I., Delbert, M., 2010. Effects of dietary prebiotics on the growth, feed efficiency and non-specific immunity of juvenile red drum Sciaenops ocellatus fed soybean-based diets. Aquac. Res. 41, 411–418
Calame, W., Weseler, A.R., Viebke, C., Flynn, C., Siemensma, A.D., 2008. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. British J. Nutr. 100, 1269–1275
Celi, P., Verlhac, V., Fru-Nji, F., Kluenter, A., Steinert, R., Cowieson, A., 2018. Gastrointestinal functionality in animal nutrition and health. AFMA Matrix. 27, 24–31
Cherbut, C., Michel, C., Raison, V., Kravtchenko, T., Severine, M., 2003. Acacia gum is a bifidogenic dietary fibre with high digestive tolerance in healthy humans. Microb. Ecol. Health Dis. 15, 43–50
Cupp-Enyard, C., 2008. Sigma’s non-specific protease activity assay-casein as a substrate. J. Vis. Exp. e899
Draper, H.H., Hadley, M., 1990. (43) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 186, 421–431
Egerton, S., Culloty, S., Whooley, J., Stanton, C., Ross, R.P., 2018. The gut microbiota of marine fish. Front. Microbiol. 9, 873
El-faki, F.E.-M., 2016. Evaluation of the additions gum Arabic on the growth performance indices of mix-sex Tilapia O. niloticus fingerlings. Sudan Univ. Sci. & Technol. 1–74.
El-Ratel, I., Ismail, R.F., Fouda, S.F., 2019. Productive performance, carcass traits, lipid profile, antioxidants and immunity of growing rabbits treated with gum Arabic under Egyptian summer condition. Egy. J. Nutr. Feeds. 22, 383–394
Esakkiraj, P., Immanuel, G., Sowmya, S., Iyapparaj, P., Palavesam, A., 2009. Evaluation of protease-producing ability of fish gut isolate Bacillus cereus for aqua feed. Food Bioprocess Technol. 2, 383–390
Espírito Santo, M.L.P., Vivian, V., Mirapalheta, T., Carbonera, N., Coelho, G., Damian, C., 2007. Chemical, physical and microbiological changes in tilapia (Oreochromis niloticus) during marination. Alim. Nutr. 18 (1), 1–5
Faggio, C., Fazio, F., Marafioti, S., Arfuso, F., Piccione, G., 2015. Oral administration of gum Arabic: effects on haematological parameters and oxidative stress markers in Mugil cephalus Iran. J. Fish. Sci. 14, 60–72
FAO, 2018. Food and agriculture organization of the united nations. In: Global Aquaculture Production. Contributing to Food Security and Nutrition for All, Rome, 2018, p. 200
Fazio, F., 2019. Fish hematology analysis as an important tool of aquaculture: a review. Aquaculture. 500, 237–242
Folin, O., Wu, H., 1919. A system of blood analysis. J. Biol. Chem. 38, 81–110
Fouda, A., El-Aziz, Abd, Mabrouk, N., 2019. Effects of arabic gum on cardiomyopathy in a rat model of type II diabetes. Al-Azhar Med. J. 48, 29–42
Francesco, F., Satheeshkumar, P., Senthil Kumar, D., Caterina, F., Giuseppe, P., 2012. Comparative study of hematological and blood chemistry of Indian and Italian Grey mullet (Mugil cephalus Linneaus 1758). HOAJ Biol. 1–5
Garcia, D., Lima, D., da Silva, D.G.H., de Almeida, E.A., 2020. Decreased malondialdehyde levels in fish (Astyanax altiparanae) exposed to diesel: evidence of metabolism by aldehyde dehydrogenase in the liver and excretion in water.
Ecotoxicol. Environ. Saf. 190, 110107
Goldenfarb, P.B., Bowyer, F.P., Hall, E., Brosious, E., 1971. Reproducibility in the hematology laboratory: the microhematocrit determination. Am. J. Clinical Pathol. 56, 35–39
Granfors, K., 1979. Measurement of immunoglobulin M (IgM), IgG, and IgA antibodies against Yersinia enterocolitica by enzyme-linked immunosorbent assay: persistence of serum antibodies during disease. J. Clin. Microbiol. 9, 336–341
Holland, M.C.H., Lambris, J.D., 2002. The complement system in teleosts. Fish & Shellfish Immunol. 12, 399–420
Hu, Y., Dun, Y., Li, S., Zhao, S., Peng, N., Liang, Y., 2014. Effects of Bacillus subtilis KN-42 on growth performance, diarrhea and faecal bacterial flora of weaned piglets. AsianAustralasian J Ani. Sci. 27, 1131.
Hu, Q., Gerhard, H., Upadhyaya, I., Venkitanarayanan, K., Luo, Y., 2016. Antimicrobial eugenol nanoemulsion prepared by gum arabic and lecithin and evaluation of drying technologies. Int. J. Biol. Macromol. 87, 130–140
Ibrahim, R.E., El-Houseiny, W., Behairy, A., Abo-Elmaaty, A., Al-Sagheer, A.A., 2019. The palliative role of Eruca sativa leaves dietary supplementation against oxidative stress, immunosuppression, and growth retardation in temperature-stressed Oreochromis niloticus J. Thermal Biol. 84, 26–35
Ismael, N.E., Abd El-hameed, S.A., Salama, A.M., Naiel, M.A., Abdel-Latif, H.M., 2021. The effects of dietary clinoptilolite and chitosan nanoparticles on growth, body composition, haemato-biochemical parameters, immune responses, and antioxidative status of Nile tilapia exposed to imidacloprid. Environ. Sci. Pol. Res, 1–16
Jiang, J., Miyata, M., Chan, C., Ngoh, S.Y., Liew, W.C., Saju, J.M., Chang, S.F., 2014. Differential transcriptomic response in the spleen and head kidney following vaccination and infection of Asian seabass with streptococcus iniae PLoS One 9, e99128
Lham, A.B., NKK, Hindi, Jebur, M.H., Mahdi, M.A., 2015. In VitroAntimicrobial activity of gum Arabic (Al manna and Tayebat) prebiotics against infectious pathogens. Human J. 3, 1–9
Libanori, M., Santos, G., Pereira, S., Lopes, G., Owatari, M., Soligo, T., Mourino, J., 2021. Dietary supplementation with benzoic organic acid improves the growth performance and survival of Nile tilapia (Oreochromis niloticus) after challenge with Streptococcus agalactiae (Group B). Aquaculture. 545, 737204.
Livak, K.J., Schmittgen, T.D., 2001. Analysis of relative gene expression data using realtime quantitative PCR and the 2 ΔΔCT method. Methods. 25, 402–408 Mashoof, S., Criscitiello, M.F., 2016. Fish immunoglobulins. Biol. 5, 45
Maxham, L.A., Forzan, M.J., Hogan, N.S., Vanderstichel, R.V., Gilroy, C.V., 2016. Hematologic reference intervals for Xenopus tropicalis with partial use of automatic counting methods and reliability of long-term stored samples. Vet. Clin. Pathol. 45, 291–299
Mirghani, M.E., Elnour, A.A., Kabbashi, N., Alam, M.Z., Musa, K.H., Abdullah, A., 2018. Determination of antioxidant activity of gum arabic: an exudation from two different locations. Sci. Asia 44, 179–186
Musa, K.H., Abdullah, A., Kuswandi, B., Hidayat, M.A., 2013. A novel high throughput method based on the DPPH dry reagent array for determination of antioxidant activity. Food Chem. 141, 4102–4106
Naiel, M.A., Ismael, N.E., Shehata, S.A., 2019. Ameliorative effect of diets supplemented with rosemary (Rosmarinus officinalis) on aflatoxin B1 toxicity in terms of the performance, liver histopathology, immunity and antioxidant activity of Nile tilapia (Oreochromis niloticus). Aquaculture. 511, 734264
Naiel, M.A., Ismael, N.E., Negm, S.S., Ayyat, M.S., Al-Sagheer, A.A., 2020. Rosemary leaf powder–supplemented diet enhances performance, antioxidant properties, immune status, and resistance against bacterial diseases in Nile Tilapia (Oreochromis niloticus). Aquaculture. 526, 735370
Naiel, M.A., Negm, S.S., Abd El-hameed, S.A., Abdel-Latif, H.M., 2021a. Dietary organic selenium improves growth, serum biochemical indices, immune responses, antioxidative capacity, and modulates transcription of stress-related genes in Nile tilapia reared under sub-optimal temperature. J Thermal Biol. 99, 102999.
Naiel, M.A., Khames, M.K., Abdel-Razek, N., Gharib, A.A., El-Tarabily, K.A., 2021b. The dietary administration of miswak leaf powder promotes performance, antioxidant, immune activity, and resistance against infectious diseases on Nile tilapia (Oreochromis niloticus). Aquacul. Rep. 20, 100707
Naiel, M.A., Farag, M.R., Gewida, A.G., Elnakeeb, M.A., Amer, M.S., Alagawany, M., 2021c. Using lactic acid bacteria as an immunostimulants in cultured shrimp with special reference to Lactobacillus spp. Aquac. Int. 29, 219–231
Naiel, M.A., Gewida, A.G., Merwad, A.-R.M., Abdel-Hamid, E.A., Negm, S.S., Alagawany, M., Farag, M.R., 2021d. The effects of various organic fertilizers with or without adsorbents on the productivity, antioxidant status and immune responses of Nile tilapia raised in cement ponds. Aquaculture 737593
Nasif, W., Lotfy, M., Mahmoud, M., 2011. Protective effect of gum acacia against the aspirin induced intestinal and pancreatic alterations. Eur. Rev. Med. Pharmacol. Sci. 15, 285–292
Nasir, O., Babiker, S., Salim, A.-M.M., 2016. Protective effect of gum Arabic supplementation for type 2 diabetes mellitus and its complications. Int J Multidiscip Curr Res. 4, 288–294
Negm, S.S., Ismael, N.E., Ahmed, A.I., El-Asely, A.M., Naiel, M.A., 2021. The efficiency of dietary Sargassum aquifolium on the performance, innate immune responses, antioxidant activity, and intestinal microbiota of Nile Tilapia (Oreochromis niloticus) raised at high stocking density. J. Appl. Phycol. 33, 4067–4082
M.A.E. Naiel
Nishikimi, M., Rao, N.A., Yagi, K., 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46, 849–854
Patel, S., Goyal, A., 2015. Applications of natural polymer gum arabic: a review. Int. J. Food Prop. 18, 986–998
Raz, S.H., Abdelnour, S.A., Alotaibi, M.A., AlGabbani, Q., Naiel, M.A., Shokrollahi, B., Alagawany, M., 2021. MicroRNAs mediated environmental stress responses and toxicity signs in teleost fish species. Aquaculture 737310
Reza, A., Abdolmajid, H., Abbas, M., Abdolmohammad, A.K., 2009. Effect of dietary prebiotic inulin on growth performance, intestinal microflora, body composition and hematological parameters of juvenile beluga, Huso huso (Linnaeus, 1758). J. World Aquacul. Soci. 40, 771–779
Rothman, S., Liebow, C., Isenman, L., 2002. Conservation of digestive enzymes. Physiol. Rev. 82 (1), 1–18.
Rudneva, I.I., Kuzminova, N.S., Skuratovskaya, E.N., 2010. Glutathione-S-transferase activity in tissues of Black Sea fish species. Asian J. Exp. Biol. Sci. 1, 141–150 Sandamalika, W.G., Kwon, H., Lim, C., Yang, H., Lee, J., 2021. The possible role of catalase in innate immunity and diminution of cellular oxidative stress: insights into its molecular characteristics, antioxidant activity, DNA protection, and transcriptional regulation in response to immune stimuli in yellowtail clownfish (Amphiprion clarkii). Fish & Shellfish Immunol. 113, 106–117
Sarma, P.R., 1990. Red Cell Indices; Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed. Butterworth Publishers
Satheeshkumar, P., Ananthan, G., Senthilkumar, D., Khan, A.B., Jeevanantham, K., 2012. Comparative investigation on haematological and biochemical studies on wild marine teleost fishes from Vellar estuary, southeast coast of India. Comp. Clin. Pathol. 21, 275–281
Saurabh, S., Sahoo, P., 2008. Lysozyme: an important defence molecule of fish innate immune system. Aquac. Res. 39, 223–239
Shafique, L., Abdel-Latif, H.M., Hassan, F.-u., Alagawany, M., Naiel, M.A., Dawood, M.A., Liu, Q., 2021. The feasibility of using yellow mealworms (Tenebrio molitor): towards a sustainable aquafeed industry. Animals. 11, 811
Soleimani, N., Hoseinifar, S.H., Merrifield, D.L., Barati, M., Abadi, Z.H., 2012. Dietary supplementation of fructooligosaccharide (FOS) improves the innate immune response, stress resistance, digestive enzyme activities and growth performance of Caspian roach (Rutilus rutilus) fry. Fish & Shellfish Immunol. 32, 316–321
Soliman, N.F., Yacout, D.M., 2016. Aquaculture in Egypt: status, constraints and potentials. Aquac. Int. 24, 1201–1227
Thiex, N., Novotny, L., Crawford, A., 2012. Determination of ash in animal feed: AOAC official method 942.05 revisited. J. AOAC Int. 95, 1392–1397
Vargas-Albores, F., Martínez-C ´ ordova, L.R., Hern´ andez-Mendoza, A., Cicala, F., LagoLest ´ on, A., Martínez-Porchas, M., 2021. Therapeutic modulation of fish gut microbiota, a feasible strategy for aquaculture? Aquaculture 737050
Wang, W., Ishikawa, M., Koshio, S., Yokoyama, S., Dawood, M.A., Hossain, M.S., Moss, A.S., 2019. Effects of dietary astaxanthin and vitamin E and their interactions on the growth performance, pigmentation, digestive enzyme activity of kuruma shrimp (Marsupenaeus japonicus). Aquac. Res. 50, 1186–1197.
Wilkinson, J., Baron, D., Moss, D., Walker, P., 1972. Standardization of clinical enzyme assays: a reference method for aspartate and alanine transaminases. J. Clin. Pathol. 25, 940
Xia, Y., Wang, M., Gao, F., Lu, M., Chen, G., 2020. Effects of dietary probiotic supplementation on the growth, gut health and disease resistance of juvenile Nile tilapia (Oreochromis niloticus). Ani. Nutr. 6, 69–79
Xuan, N.T., Shumilina, E., Nasir, O., Bobbala, D., Gotz, F., Lang, F., 2010. Stimulation of mouse dendritic cells by gum Arabic. Cell. Physiol. Biochem. 25, 641–648
Zhang, X., Sun, Z., Cai, J., Wang, J., Wang, G., Zhu, Z., Cao, F., 2020. Effects of dietary fish meal replacement by fermented moringa (Moringa oleifera Lam.) leaves on growth performance, nonspecific immunity and disease resistance against Aeromonas hydrophila in juvenile gibel carp (Carassius auratus gibelio var. CAS III). Fish & Shellfish Immunol. 102, 430–439
Zikic, R., Stajn, A.S., Pavlovic, S.Z., Ognjanovic, B.I., Saicic, Z., 2001. Activities of superoxide dismutase and catalase in erythrocytes and plasma transaminases of goldfish (Carassius auratus gibelio Bloch.) exposed to cadmium. Physiol. Res. 50, 105–111
M.A.E.
Other documents randomly have different content
consider themselves citizens, and think worthy thoughts. So that in a short time these forces became the best in the North.
By order of the regency, General Steinbock put himself at the head of 8,000 veteran troops and 12,000 recruits, to pursue the Danes, who were ravaging all the country round Elsingburg, and had already put some places far inland under contribution.
There was neither time nor money to get uniforms for the soldiers; most of the country labourers came dressed in their linen smocks, with pistols tied to their girdles by cords. Steinbock, at the head of this extraordinary army, came up with the Danes three leagues from Elsingburg, on the 10th March, 1710. He intended to rest his troops some days, to entrench, and to give his raw recruits time to get accustomed to the enemy; but the peasants clamoured to fight directly they arrived.
Some officers who were there told me that they saw them almost all foaming with rage, so great is the Swede’s hatred of the Dane. Steinbock took advantage of this disposition, which is almost as effective in war as military discipline. The Danes were attacked, and the strange sight was seen—of which there are, perhaps, no two other instances—of raw forces equalling in bravery a veteran corps at the first attack. Two regiments of these undisciplined peasants cut the Danish army to pieces, and left only ten survivors.
The Danes, entirely routed, retreated under the cannon of Elsingburg. The passage from Sweden to Zeeland is so short that the King of Denmark heard of the defeat of his army in Sweden the same day at Copenhagen, and sent his fleet to bring off the remnant of his army. The Danes hastily left Sweden five days after the battle, but, being unable to bring away their horses, and not wishing to leave them to the enemy, they killed them all and fired their provisions, burning their corn and baggage, and leaving 4,000 wounded in Elsingburg. The majority of these died from the infection from the large number of dead horses, and from lack of food, which even their own countrymen deprived them of, lest they should fall into Swedish hands.
At the same time the peasants of Delecarlia, having heard in the depths of their forests that the King was prisoner in Turkey, sent a deputation to the Regency at Stockholm, offering to go, at their own expense, to rescue their master from the enemy’s hands with a force of 20,000 men. This proposal, useless as it was, was heard with pleasure, because it proved the courage and loyalty of the proposers, though it was rejected; and they gave the King an account of it, when they sent him word about Elsingburg. King Charles received this cheering news in his camp near Bender, in July 1710, just after another event which confirmed him in his hopes.
The Grand Vizir Couprougli, who was opposed to his plans, was turned out of office after he had been in the ministry two months. Charles XII’s little Court, and his adherents in Poland, boasted that he made and removed vizirs, and was governing Turkey from his retreat at Bender. But he had no hand in the ruin of this favourite.
The rigid justice of the Vizir, it was said, was the only cause of his fall; his predecessor had been accustomed to pay the janissaries, not out of the Imperial treasury, but from the money he got by extortion. Couprougli, on the other hand, paid them from the treasury. For this Achmet accused him of putting the subjects’ interest before that of the Emperor. “Your predecessor, Chourlouli,” he said, “managed to find other ways of paying my troops.” The Grand Vizir replied, “If he had the art of enriching your Highness by theft, it is an art of which I am proud to be ignorant.”
The great secrecy observed in the seraglio rarely lets such stories leak out, but this got known at the time of Couprougli’s fall. The Vizir’s courage did not cost him his head, because real goodness often forces even those whom it offends to respect. He had leave to retire to the island of Negropont.
After this the Sultan sent for Baltagi Mahomet Pasha of Syria, who had been Grand Vizir before Chourlouli. The Baltagis of the seraglio, so called from balta, meaning an axe, are slaves employed to cut wood for the use of princes of the blood and the Sultana. This Vizir had been baltagi in his youth, and had always retained the name, according to the custom of the Turks, who are not ashamed to
bear the name of their first profession, their father, or their birthplace. While Baltagi was a servant in the seraglio he was fortunate enough to do Prince Achmet some trifling service, that Prince being then a prisoner of State in the reign of his brother Mustapha. Achmet gave one of his female slaves, of whom he had been very fond, to Baltagi Mahomet, when he became Sultan. This woman made her husband Grand Vizir by her intrigues; another intrigue deposed him, while a third made him Grand Vizir again. Baltagi had no sooner received the seal of the Turkish empire than he found the party of the King of Sweden dominant in the seraglio. The Sultana Valida, the Sultan’s favourite, the chief of the black eunuchs, and the aga of the janissaries, were all in favour of war against the Czar. The Sultan had decided on it, and the very first order he gave the Grand Vizir was to go and attack the Russians with 200,000 men. Baltagi had never been in the field, but was no idiot, as the Swedes, out of pure malice, have represented him to be. When he received from the Sultan a sabre set with precious stones, “Your Highness knows,” he said, “that I have been brought up to use an axe and fell wood, and not to wield a sword, or to command armies. I will do my best to serve you; but if I fail, remember that I have begged you not to lay it to my charge.” The Sultan assured him of his favour, and the Vizir prepared to carry out his orders. The Ottoman Porte’s first step was to imprison the Russian ambassador in the castle of seven towers.
It is the custom of the Turks to begin by seizing those ministers against whom they declare war. Strict observers of hospitality in every other respect, in this they violate the most sacred of international laws. They act thus unfairly under the pretext of fairness, persuading themselves and trying to persuade others that they never undertake any but a just war, because it is consecrated by the approbation of their Muphti. Thus they look upon themselves as armed to chastise the violation of treaties (which they often break themselves), and argue that the ambassadors of kings at variance with them are to be punished as accomplices of their masters’ treachery. Besides this, they affect a ridiculous contempt towards Christian princes and their ambassadors, whom they regard as only consuls and merchants.
The Kan of Crimean-Tartary had orders to be ready with 400,000 Tartars. This Prince rules over Nagai, Bulziac, part of Circassia and all the Crimean district called by the ancients the Tauric Chersonese, whither the Greeks carried their commerce and their arms, building large cities there; and whither the Genoese afterwards penetrated, when they were masters of the trade of Europe.
In this country there are the ruins of Grecian cities, and some Genoese monuments still subsisting in the midst of desolation and savagery. The Kan is called Emperor by his own subjects, but in spite of this grand title he is a mere slave to the Porte. The fact that they have Ottoman blood in their veins, and the right they have to the Turkish Empire on the extinction of the race of the Sultan, make their family respected and their persons formidable even to the Sultan himself: that is why the Sultan dare not destroy the race of the Kans of Tartary; but he hardly ever allows them to continue on the throne to an advanced age. The neighbouring pashas spy on their conduct, their territories are surrounded by janissaries, their wishes thwarted by the Grand Vizir, and their designs always suspected. If the Tartars complain of the Kan, this is an excuse for the Porte to depose him; if he is popular among them it is regarded as a crime, for which he will be even more readily punished. Thus all of them leave the throne for exile, and finish their days at Rhodes, which is generally both their place of exile and their grave.
The Tartars, their subjects, are the most dishonest folk in the world; yet, at the same time (inconceivable as it seems), the most hospitable. They go a fifty leagues’ journey to fall upon a caravan and to destroy towns, but if any stranger happens to pass through their country, he is not only received and lodged everywhere, and his expenses paid, but everywhere the inhabitants strive for the honour of having him as guest.
The master of the house, his wife and daughters vie with one another in his service. Their ancestors, the Scythians, transmitted to them this inviolable regard for hospitality; and they still retain it, because the scarcity of strangers in their country, and the cheapness of provisions, makes this duty in no way burdensome to them. When the Tartars go to war with the Ottoman army they are maintained by
the Sultan, but receive no other pay but their booty; this makes them more ardent at pillage than at regular warfare.
The Kan, bribed by the presents and intrigues of the King of Sweden, got permission to have the general rendezvous of troops at Bender, under the King’s eye, that he might realize that the war was being made for him. The new vizir, Baltagi, not being bound in the same way, would not flatter a foreign prince so far. He countermanded the order, and the great army was collected at Adrianople.
The Turkish troops are not so formidable now as they were when they conquered so many kingdoms in Asia, Africa and Europe. Then they triumphed over enemies less strong and worse disciplined than themselves by physical strength, courage and the force of numbers. But now that Christians understand the art of war better, they seldom failed to beat the Turks in a drawn battle, even when their forces are inferior in number. If the Ottoman empire has lately gained some success, it is only in a contest with the Republic of Venice, reputed more wise than warlike, defended by strangers, and ill supported by Christian princes, who are always divided among themselves.
The janissaries and spahis attack in disorder, and are incapable of action under command, or of a rally; their cavalry, which should be excellent, considering the good breed and agility of their horses, is unable to sustain the shock of German cavalry; their infantry were not yet able to use the fixed bayonet; besides this, the Turks have had no great general since Couprougli, who conquered Candia. A slave brought up in the idleness and the silence of the seraglio, made a vizir through favouritism, and a general against his own inclinations, headed a raw army, without experience and without discipline, against Russian troops, with twelve years’ experience in war, and proud of having conquered the Swedes.
The Czar, according to all appearances, must have vanquished Baltagi, but he made the same mistake with regard to the Turks as the King of Sweden was guilty of in his own case; that is, he had too poor an opinion of his enemy. Upon the news of the Turkish preparations he left Moscow; and having given orders to change the
siege of Riga into a blockade, he drew up his army of 24,000 men on the Polish frontier. With this army he marched to Moldavia and Wallachia, formerly the country of the Daci, but now inhabited by Greek Christians, tributary to the Sultan.
Moldavia was then governed by Prince Cantemir, a Greek by birth, who had the talents of the ancient Greeks together with a knowledge of letters and of arms. He was reputedly descended from the famous Timur, famous under the name of Tamberlain: this genealogy seemed more distinguished than a Greek one. They proved it from the name of the conqueror; Timur, they said, is like Temir: the title Kan, which Timur had before his conquest of Asia, appears again in the name Cantemir: thus Prince Cantemir is a descendant of Tamberlain; that is the sort of basis on which most genealogies are built.
To whatever house Cantemir belonged, he owed all to the Ottoman Porte. Scarcely had he been invested with his principality than he betrayed the Emperor his benefactor for the Czar, from whom he had greater expectations. He believed that the conqueror of Charles XII would easily triumph over an obscure vizir, with no military experience, who had appointed as his lieutenant the chief customs officer of Turkey; he reckoned on all Greece joining his faction, and the Greek priests encouraged him in his treachery. The Czar made a secret treaty with him, and having received him into his army, marched up country, and arrived in June 1711 on the northern side of the river Hierasus, now Pruth, near Jazy, the capital of Moldavia.
As soon as the Grand Vizir heard that Peter had arrived, he left his camp at once, and following the course of the Danube, was going to cross the river on a bridge of boats near Saccia, at the same spot where Darius had built the bridge that bore his name. The Turkish army marched so rapidly that they soon came in sight of the Russians, with the river Pruth between them.
The Czar, sure of the Prince of Moldavia, never expected that the subjects might fail him; but the Moldavians often oppose their interests to those of their masters. They liked the Turkish rule, which
is never fatal except to the grandees, and pretends a leniency to its tributaries; they were afraid of the Christians, especially the Russians, who had always used them ill.
Those who had undertaken to furnish the Russians with provisions made with the Grand Vizir the same bargain they had made with the Czar, and brought all their provisions to the Ottoman army. The Wallachians, neighbours of the Moldavians, showed the same care for the Turks, for to such a degree the remembrance of former cruelties had alienated their minds from the Russians.
The Czar, thus frustrated of his hopes, which he had perhaps indulged too readily, found his army suddenly destitute of food and without forage.
In the meantime the Turks crossed the river, cut off the Russians, and formed an entrenched camp in front of them.
It is strange that the Czar did not dispute the passage of the river, or at least repair this fault by engaging the Turks at once, instead of giving them time to tire out his army with fatigue and famine. But that Prince seems, in this campaign, to have acted in every way for his own ruin; he was without provisions, with the river Pruth behind him, and about 4,000 Tartars continually harassing him to right and left. In these extremities he said publicly, “I am at least in as bad a case as my brother Charles at Pultawa.”
Count Poniatowski, indefatigable agent to the King of Sweden, was in the Grand Vizir’s army with some Poles and Swedes, who all thought the Czar’s ruin inevitable.
As soon as Poniatowski saw that the armies must inevitably meet, he sent word to the King of Sweden, who, eager for the pleasure of attacking the Russian Emperor, started that moment from Bender, with forty officers. After many losses, and several destructive marches, the Czar was driven back on Pruth, and had no cover left but some chevaux de frise and some wagons. A party of the janissaries and spahis fell immediately on his army in that defenceless condition, but they attacked in disorder, and the
Russians defended themselves with an energy inspired by the presence of their Prince and despair.
The Turks were twice driven back. Next day M. Poniatowski advised the Grand Vizir to starve out the Russians, for they lacked all necessaries, and would be obliged to surrender at discretion in one day.
The Czar has since then repeatedly acknowledged that he never felt anything so acutely as the difficulties of his position that night: he turned over in his mind all that he had been doing for so many years for the glory and good of his people, so many great plans, always interrupted by war, were perhaps about to perish with him, before having reached completion. He must either die of hunger or attack nearly 200,000 men with feeble troops, reduced by half from their original number, a cavalry with scarcely a horse between them, and infantry worn out by hunger and fatigue.
He called General Czeremetoff at nightfall, and ordered him peremptorily to have all ready by daybreak for an attack on the Turks with fixed bayonets.
He gave strict orders also that all baggage should be burned, and that no officer should keep more than one wagon, so that in case of defeat the enemy might not have the booty they expected.
Having made all arrangements with the general for the battle, he withdrew into his tent overcome by grief, and seized with convulsions, to which he was subject, and which worry brought on with redoubled violence. He forbade any one to enter his tent during the night on any pretext whatever, not wanting to receive remonstrances against a desperate but necessary resolve, and much less that any should witness the wretched state he was in. In the meantime they burned the greater part of the baggage as he had ordered; all the army followed this example with much regret, and some buried their most cherished treasures. The generals had already given orders for the march, and were trying to give the army the confidence which they did not feel themselves; the men, exhausted by fatigue, and starving, marched without spirit or hope. The women, of whom there were too many in the army, uttered cries
which further unnerved the men; every one expected that death or slavery would be their portion next morning. This is no exaggeration, it is the exact account of officers who served in the army.
There was at that time in the Russian camp a woman as extraordinary as the Czar himself. She was then known only by the name of Catherine. Her mother was an unfortunate country woman called Erb-Magden, of the village of Ringen in Estonia, a province held in villeinage, which was at that time under the rule of Sweden. She had never known her father, but was baptized by the name of Martha. The priest of the parish brought her up out of pure charity till she was fourteen, then she went into service at Mariemburg, in the house of a Lutheran minister whose name was Gluk.
In 1702, at the age of eighteen, she married a Swedish dragoon. The day after her marriage part of the Swedish troops were beaten by the Russians, and the dragoon was in the action. But he never returned to his wife, and she could never learn whether he had been taken prisoner, nor later could she get any news of him.
Some days after she was taken prisoner herself, and was servant to General Czeremetoff, who gave her to Menzikoff, a man who had known fortune’s extremes, for he had become a general and a prince from being a pastry-cook’s boy, and then was deprived of everything and banished to Siberia, where he died in misery and despair. The Czar was at supper with this prince when he first saw her and fell in love with her. He married her secretly in 1707, not fascinated by womanly charms, but because he found that she had the strength of mind to second his designs, and even to continue them after him. He had long since put away his first wife Ottokefa, daughter of a boyard, on a charge of opposition to certain political reforms he had made.
This was the greatest of all crimes in the Czar’s eyes. He would have none in his family who differed from him. In this foreign slave he expected all the qualities of a sovereign, though she had none of the virtues of womanhood. For her sake he scorned the petty prejudices which would have hampered an ordinary man, and had her crowned Empress. The same capacity which made her Peter’s wife gave her the empire after her husband’s death. Europe was
amazed to see a bold woman, who could neither read nor write, supply her lack of education and her weakness by spirit and courage, and fill the throne of a legislator gloriously.
When she married the Czar she left the Lutheran faith for that of the Russian Church; she was baptized again according to the Russian rite, and instead of the name of Martha she took that of Catherine, by which she has been known ever since. This woman was in the camp at Pruth, and held a private council with the generals and the Vice-Chancellor while the Czar was in his tent.
They agreed that it was necessary to sue for peace, and that the Czar must be persuaded to this course. The Vice-Chancellor wrote a letter to the Grand Vizir in his master’s name, which the Czarina, in spite of the Emperor’s prohibition, carried into the tent to him, and after many prayers, tears and argument, she prevailed on him to sign it; she then took all her money, all her jewels and valuables, and what she could borrow from the generals, and having collected by this means a considerable present, she sent it with the Czar’s letter to Osman Aga, lieutenant to the Grand Vizir.
Mahomet Baltagi answered proudly, with the air of a vizir and a conqueror, “Let the Czar send me his first minister, and I will see what can be done.” The Vice-Chancellor came at once, loaded with presents, which he offered publicly to the Grand Vizir; they were large enough to show they needed his help, but too small for a bribe. The Vizir’s first condition was that the Czar, with all his army, should surrender at discretion. The Vice-Chancellor answered that the Czar was going to attack him in a quarter of an hour, and that the Russians would perish to a man, rather than submit to such shameful conditions. Osman seconded him by remonstrances.
Baltagi was no soldier. He knew that the janissaries had been repulsed the day before, and was easily persuaded by Osman not to risk certain advantages by the hazard of a battle. He therefore granted a suspension of hostilities for six hours, during which the treaty could be arranged.
During the discussion an incident occurred, proving that the word of a Turk is often more reliable than we think.
Two Italian noblemen, related to a M. Brillo, colonel of a regiment of grenadiers in the service of the Czar, going to look for forage, were taken by the Tartars, who carried them off to their camp, and offered to sell them to an officer of the janissaries. The Turk, enraged at such a breach of the truce, seized the Tartars and carried them before the Grand Vizir, together with the two prisoners. The Vizir sent them back at once to the Czar’s camp, and had the two Tartars who had carried them off beheaded. In the meantime the Kan of Tartary opposed the conclusion of a treaty which robbed him of all hopes of pillage. Poniatowski seconded him with urgent and pressing reasons. But Osman carried his point, notwithstanding the impatience of the Tartar and the insinuations of Poniatowski.
The Vizir thought it enough for his master the Sultan to make an advantageous peace; he insisted that the Russians should give up Asoph, burn the galleys that lay in that port, and demolish the important citadels on the Palus-Mæotis; that all the cannon and ammunition of those forts should be handed over to the Sultan; that the Czar should withdraw his troops from Poland; that he should not further disturb the few Cossacks who were under Polish protection, nor those that were subject to Turkey, and that for the future he should pay the Tartars a subsidy of 40,000 sequins per annum—an irksome tribute which had been imposed long before, but from which the Czar had delivered his country.
At last the treaty was going to be signed, without so much as a mention of the King of Sweden; all that Poniatowski could obtain from the Vizir was the insertion of an article by which the Russians should promise not to hinder the return of Charles XII, and, strangely enough, that a peace should be made between the King and the Czar if they wished it, and could come to terms.
On these terms the Czar got liberty to retreat with his army, cannon, artillery, colours and baggage. The Turks gave him provisions, and there was plenty of everything in his camp within two hours of the signing of the treaty, which was begun on the 21st July, 1711, and signed on the 1st of August.
Just as the Czar, rescued from his dangerous position, was drawing off with drums beating and colours flying, the King of Sweden, eager to fight, and to see the enemy in his hands, came up; he had ridden post haste about fifty leagues from Bender to Jazy, and alighting at Count Poniatowski’s tent, the Count came up to him sadly and told him how he had lost a chance which would perhaps never recur.
The King, beside himself with rage, went straight to the tent of the Grand Vizir, and with flushed face reproached him for the treaty he had just made.
“I have authority,” said the Grand Vizir, calmly, “to make peace and to wage war.”
“But,” answered the King, “had you not the whole Russian army in your power?”
“Our law,” said the Vizir solemnly, “commands us to grant peace to our enemies when they implore our mercy.”
“Ah,” replied the King, in a rage, “does it order you to make a bad treaty, when you can impose the terms you please? Was it not your duty to take the Czar prisoner to Constantinople?”
The Turk, thus nonplussed, answered slyly, “And who would govern his empire in his absence? It is not fitting that all kings should be away from home.”
Charles replied with an indignant smile, and then threw himself down on a cushion, and, looking at the Vizir with resentment mingled with contempt, he stretched out his leg towards him, and, entangling his spur with his robe, tore it; then jumped up, mounted, and rode to Bender full of despair.
Poniatowski stayed some time longer with the Grand Vizir, to see if he could prevail on him by gentler means to make some better terms with the Czar, but it was prayer-time, and the Turk, without one word in answer, went to wash and attend to his devotions.