Large Animal Review
ORIGINAL ARTICLES
BOVINE

ISSN: 1124-4593
LARGE ANIMAL REVIEW is ranked in Citation Index (SciSearch®) Journal Citation Reports/Science Edition and CAB ABSTRACTS
• Impacts of subclinical mastitis on milk quality, clotting ability and microbial resistance of the causative Staphylococci
• Saccharomyces cerevisiae diet supplementation influences haematological parameters in healthy steers
• Comparison of Some Biometric Index Values in Anatolian Black Cattle Calves Raised in Different Locations
OVINE
• Investigation of the availability of vaginal electrical resistance during estrus synchronization in ewes
• The effect of 2PGF2α and P4eCG protocol of estrus synchronization on reproductive performance of nulliparous Ghezel ewes
CAPRINE
• Images of Normal Ocular Fundus in Saanen Goats


• The effect of iodine drenching during late pregnancy on thyroid hormones and biochemical parameters of black goats and their kids performance
EQUINE
• Effectiveness of the extract of Olea europaea and Griffonia simplicifolia on horses affected by equine gastric ulcer syndrome
Bimonthly, Year 29, Number 3, June 2023
LAR
03/23
SOCIETÀ
ASSOCIAZIONE
ITALIANA VETERINARI PER ANIMALI DA REDDITO
FEDERATA ANMVI
1Agro-foodDepartment,FacultyofNaturalandLifeScience,UniversityofSaadDahlabBlida1PB:09000, Blida,Algeria
2LaboratoryofBiotechnology,EnvironmentandHealth,UniversityBlida1PB:09000,Blida,Algeria
3InstituteofVeterinaryScience,UniversityofSaadDahlabBlida1PB:09000,Blida,Algeria
4LaboratoryofAnalyticalBiochemistryandBiotechnology,UniversityofMouloudMammeri,PB17RP15000, Tizi-Ouzou,Algeria
SUMMARY
Bovine mastitis is one of the most problematic diseases and continues to be a leading cause of heavy economic losses in the dairy industry and a public health hazard globally. To understand the characteristics of subclinical mastitis (SCM) in lactating cows and their associated effects on milk quality, protein composition, and milk clotting ability, 240 quarter-milk samples were collected and tested by California Mastitis Test (CMT). Milk composition was analyzed using LactoScope FT-A and separation of protein fractions was performed by PAGE-SDS electrophoresis. We also measured the time from rennet addition to milk gelation (RCT) as a traditional milk coagulation trait. Samples with SCM were analyzed bacteriologically, and Staphylococci isolates were tested for antibiotic susceptibility. Higher values of conductivity and pH were recorded from CMT-positive milk samples. Overall, 50/240 (20.83%) quarters suffered from SCM, whose 64% (32/50) infected with Staphylococci. On the 36 tested Staphylococci, resistance to penicillin and erythromycin represented 83.3%, and 61.1% respectively. Resistance to cefoxitin was linked to three isolates while 77.7% were multi-drug resistant, but in proportion that differ between S. aureus (88.8%) and non-aureus Staphylococci (74.1%). Physico-chemical analysis indicated that, quarters with SCM had lower milk-fat content and mineral content compared with quarters without SCM. The profiles of total proteins electrophoresis revealed degradation of casein fractions in milk with SCM. Milk samples subclinically infected with Staphylococci exhibited longer coagulation time (1093.9±781.9 seconds) and weaker clotting activity (2.55±1.49 RU) than milk samples collected from healthy quarters which showed 325.3±177.5 seconds and 7.80 ± 4.46 RU. The increase in conductivity due to intramammary infection, was highly associated with an elongation in RCT. Moreover, clotting activity was inversely proportional to conductivity. Due to its impacts on milk composition, proteins integrity and clotting ability, SCM still a major concern in dairy industry which needs efficient measures to control their occurrence in dairy herds.
KEY WORDS
Antibiotic susceptibility, clotting ability, milk composition, Staphylococci, subclinical mastitis.
INTRODUCTION
Achievement of high yield and quality products in the dairy processing industry depends on the quality of raw milk (1). The latter is still affected by several environmental and individual factors, including cow’s health status (2). Indeed, nutritional values and content of milk which are important for human nutrition, may be depreciated with a systemic or mammary gland infection of host animals. Bovine mastitis is a complex disease, mainly caused by a variety of pathogens, with substantial differences in infection patterns with no simple model encompassing all possible facets of the disease (3). Antibiotic therapy is commonly implemented for prevention and control of
Corresponding Author: Bentayeb Lamia (bentayeb_lamia@univ-blida.dz lamius-belius@hotmail.fr)
mastitis; unfortunately, despite the best possible antimicrobial treatments available, bacteriological cure failure is common, especially of intramammary infections (IMI) associated with S. aureus (4). This situation could lead to the persistence and transmission of multidrug-resistant bacteria in dairy farms. Mastitis represents one of the most economically important health traits for milk production which makes it among the major concerns for the livestock sector (5). In cases of subclinical mastitis (SCM), no visible abnormalities of the milk or udder can be observed, and tests are needed to detect the inflammatory responses following IMI. For their detection, methods such as the California mastitis test (CMT), Somatic Cells Count (SCC), certain biochemical methods, bacteriological examination of milk and electrical conductivity have been suggested (6). SCM usually leads the clinical form as it is of longer period, difficult to diagnose, adversely affects milk production and quality and comprises a reservoir of pathogens affecting other animals within the herd (3,7). Increased SCC (higher than
LAMIA BENTAYEB1,2*, MADJID AKKOU3, SALIHA SI-AHMED ZENNIA4, YACINE TITOUCHE4, AMEL DOUMANDJI1, SMAIN MEGATELI1
L.Bentayebetal.LargeAnimalReview2023;29:105-111105
Impacts of subclinical mastitis on milk quality, clotting ability and microbial resistance of the causative Staphylococci N
200000cells/mL) in milk is commonly used as indicator of SCM and reflects the onset of an immune response to the presence of IMI. High SCC in milk reduces the quality of milk and dairy products, affects shelf life and flavor of milk, and deteriorates the physicochemical properties and cheese-making traits of milk (2,8,9). Besides, a very low SCC (lower than 150000cells/mL) was also reported to have a negative effect on some milk technological traits, which could be associated with an ineffective response to an undetectable mastitis event (2). Therefore, the objectives of this current study were to characterize SCM in lactating cows and their relationship with milk quality, protein composition, and milk clotting ability.
MATERIAL AND METHODS
Study area and cows
Animals were selected from eight dairy herds located in the department of Tizi-Ouzou (Algeria). Sixty lactating cows were enrolled in the present study. The average herd size was 7.5 ranging from 5 to 17 cows /herd. Breeding management followed an extensive and sometimes intensive mode. Cows included in this study were selected randomly within herds’ accessibility of the breeders. Age, lactation stage and parity were not considered in the choice of animals.
Screening for mastitis
After obtaining permission from farms owners, udders of the cows were first examined by visual inspection and palpated for the presence of any lesion, pain, heat and swelling. Any abnormality in color or consistency of milk collected from each quarter was checked. CMT was performed on the clinically healthy udder-quarters at the post-colostral period to determine the presence of SCM (10). Milk samples from 240 udder-quarters were collected from the 60 cows and tested using CMT. When a quarter showed no visible signs of clinical mastitis but revealed positive to CMT, it is considered impaired with SCM.
Samples collection from cows
Two different milk samples were collected before the morning milking according to National Mastitis Council (NMC) guidelines (11). For bacteriological analysis, teat ends were cleaned externally with commercial pre-milking disinfectants, dried with individual towels and cleaned again with alcohol. After discarding the first streams of foremilk, approximately 10mL of milk from each quarter was collected in sterile tubes. Immediately after aseptic collection of milk samples, approximately 100mL of milk was manually collected from each CMT-positive and CMT-negative quarters. The latter sample was dedicated for the analysis of milk composition and cheese-making traits. All quarter-milk samples individually collected were stored at 4°C, and submitted to the laboratory within 24 hours.
Bacteriological analysis
Bacteriological analysis was performed exclusively on CMTpositive quarter-milk samples according to NMC standards (11). Briefly from each sample, 0.01mL of milk was plated both on mannitol salt agar medium and blood agar. Cultures were examined after being incubated aerobically for 24 h or 48 h at 37°C. A mammary quarter was considered culture positive when
the growth of at least one colony was detected on the streaks. Samples yielding more than two different bacterial species were considered to be contaminated. Bacteria were identified based on colony morphology and biochemical tests. Catalase tests with hydrogen peroxide 3% were used to differentiate between catalase positive Staphylococci and catalase negative cocci. Coagulase tests were carried out using sterile rabbit plasma to distinguish S. aureus from non-aureus Staphylococci.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was carried out with equivalence of 0.5 McFarland turbidity standards by agar disc diffusion method on Mueller-Hinton agar plates following the guidelines of Clinical and Laboratory Standards Institute (12). The reference strain (S. aureus ATCC 25923) was used as a control for the disc diffusion technique. The isolated strains were tested for their susceptibility to cefoxitin (indicative for methicillin resistant Staphylococci (MRS)), oxacillin, penicillin, tetracycline, spiramycin, erythromycin, clindamycin, vancomycin, fusidic acid and chloramphenicol.
Milk composition analysis and proteins separation
All analytical evaluations have been completed in duplicate for each quarter-milk sample. Milk was tested within 24h of collection for fat, solids non-fat, protein, lactose (%), and mineral substances using a LactoScope FT-A Results Plus (PerkinElmer, Inc; Connecticut, U.S.A). This tool also recorded electrical conductivity (EC), density, and pH. This latter was compared with the milk pH recorded at room sample temperature using a pH-meter (HANNA Instruments, Lingolsheim, France). Milk protein fractions were separated in the presence of Sodium Dodecyl Sulfate (SDS 10%, w/v) and 2-mercaptoethanol (4%, v/v) by PAGE-SDS electrophoresis.
Milk clotting aptitude determination
We measured under standardized conditions, the rennet coagulation time (RCT) as a traditional milk coagulation trait (13). Coagulation was observed on a thin sheet of milk in a rotating tube set on a black background. By reproducing the clotting protocol performed in small-scall industries, we have prepared a reference sample to which we have compared the results of the samples tested. The method is to add 1µL of enzyme solution to 2mL of standard substrate (reconstituted milk powder) at 38°C and then record the coagulation time. The procedure was repeated twice in order to achieve more reliable results.
The preparation of the enzymatic solution consists in dissolving 22mg of the coagulating enzyme: recombinant bovine chymosin in 5mL of distilled water, followed by magnetic stirring. The preparation of the standard substrate consists of dissolving milk powder of the low heat at 0% (w/v) in a solution of CaCl2 (0.01 M) and adjusting the pH to 6.5 by adding a solution of NaOH (0.1). The standard substrate is then divided into 2 test tubes (2mL/tube). The addition of the coagulant extract was performed within 10µL/2mL of the standard substrate. Immediate and rapid homogenization is done. In the Bain Marie, the three successive reversals of the mixture after 30 seconds correspond to time zero. The selected healthy and mastitis milk samples were also distributed in test tubes within 2mL each and 10µL of the enzyme solution were added respectively. Before being placed in the Bain Marie 38°C, a quick and immediate homogenization was achieved. RCT was recorded by a stop-
106Impactsofsubclinicalmastitisonmilkquality,clottingabilityandmicrobialresistanceofthecausativeStaphylococci
watch and clotting activity was calculated with the following formulas:
Clotting Activity =10 x Standard Milk Volume / Enzyme Volume x RCT
Clotting Activity: Rennet Unit (RU)
Standard Milk Volume: 2 mL
Enzyme Volume: 0.01 mL
RCT: Rennet addition to milk gelation (seconds)
Statistical analysis
Raw data were entered to Microsoft Excel for Windows (2010; Microsoft Corp., Redmond, WA, USA) and imported to SPSS software version 20.0 (IBM Corp., Armonk, NY, USA) for statistical analysis. Initial descriptive statistics were done to summarize data while comparisons between averages were performed using Student test. Pearson’s correlation analysis was used to establish the relationship between conductivity and coagulation characteristics in milk. A p-value of 0.05 was used to determine the significance level.
RESULTS
Prevalence of subclinical mastitis
Mastitis in its clinical and subclinical forms was diagnosed in 28 lactating cows. While moderate clinical (one quarter) and subclinical (three quarters) mastitis co-existed in one cow, one quarter was affected with moderate clinical form in another cow. Clinical mastitis wasn’t be considered for further analysis.
From SCM prevalence stand point, all herds experienced SCM with 45% (27 of 60) affected cows and 20.83% (50 of 240) affected quarters. Most of the cows with SCM (13 of 27; 48.1%) had only one affected quarter, whereas 29.6 % (8 of 27) of the cows were diagnosed with SCM in two quarters, 11.1% (3 of 27) had three quarters affected, and 11.1% (3 of 27) had SCM in all four quarters (Table 1).
Bacterial analysis and intramammary infection
Only CMT-positive quarter-milk sampleswere analyzed bacteriologically. At least one bacterial species was isolated from 92% (46/50) of the cultured CMT-positive milk samples. Fiftytwo isolates (one to two bacterial strains recovered after culture of CMT-positive samples on blood agar) were recovered from 46 positive milk samples. Samples showing two mixed bacteria species represented 12% (6 of 50). The most commonly isolated udder pathogen was Staphylococcus within 64% (32 of 50) of the CMT-positive milk samples, giving a quarter prevalence of 13.33% (32 of 240). The frequencies of Non-aureus Staphylococci (NAS) and S. aureus isolation in milk samples represented 50% and 18% respectively (Table 2).
Antibiotic susceptibility of Staphylococci
Antibiotic resistance determination revealed that only 8.3% of the Staphylococcal isolates (3/36) were susceptible to all the tested antibiotics. Higher levels of resistance were associated with penicillin, and erythromycin with 83.3% and 61.1% respectively. Resistance to cefoxitin was observed from three isolates. Multi-drug resistance was observed in 77.7% of the tested isolates, but in proportion that differ between S. aureus (88.8%)
Farms88(100%)/1(12.5%)0/ Cows6027(45%)/2(3.32%)*32(53.33%)/
Quarters24050(20.83%)/2(0.41%)188(78.33%)/
Analyzed milk samples
BacteriologicalanalysisofCMT-positives5050(100%)0/
Physico-chemicalanalysis7449(66.21%)25(33.78%)/
Milkclottingaptitudeanalysis3117(54.83%)14(45.16%)/
NB. *:Onecowshowed3quarterswithSCMandonequarterwithCM
L.Bentayebetal.LargeAnimalReview2023;29:105-111107
Physico-chemical analysis Mean±sdMean±sdMean±sdP-Value Milk features pH6.53±0.246.56±0.23a6.44±0.22bP<0.001 Conductivity5.27±0.035.47±0.62c4.93±0.38dP<0.001 Density33.12±4.6733.15±5.4932.90±3.07P=0.75 Milk components (g/100g) Fat3.65±0.583.58±0.643.74±0.43P=0.08 Totalproteins3.05±0.483.06±0.553.01±0.36P=0.52 Lactose4.61±0.694.61±1.54.59±0.43P=0.9 Mineralsubstances0.70±0.230.65±0.24a0.77±0.19bP=0.001 Solidsnon-fat8.33±1.198.36±0.14b8.24±0.79cP<0.001
Mastitis screening TotalSCM/CMHealthyP-value sd:Standarddeviation;SCM:Subclinicalmastitis:CM:Clinicalmastitis;
Table 1 -Screeningforsubclinicalmastitisandassociatedmilkcharacteristics.
**:CultureswithtwodistinctcoloniesforNAS;Isolates:bacterialisolates
and NAS (74.1%). Of the twenty-four characterized patterns of resistance, a high diversity of profiles was detected among the NAS with 17 patterns than S. aureus isolates with seven different patterns (Table 3).
Physicochemical characteristics
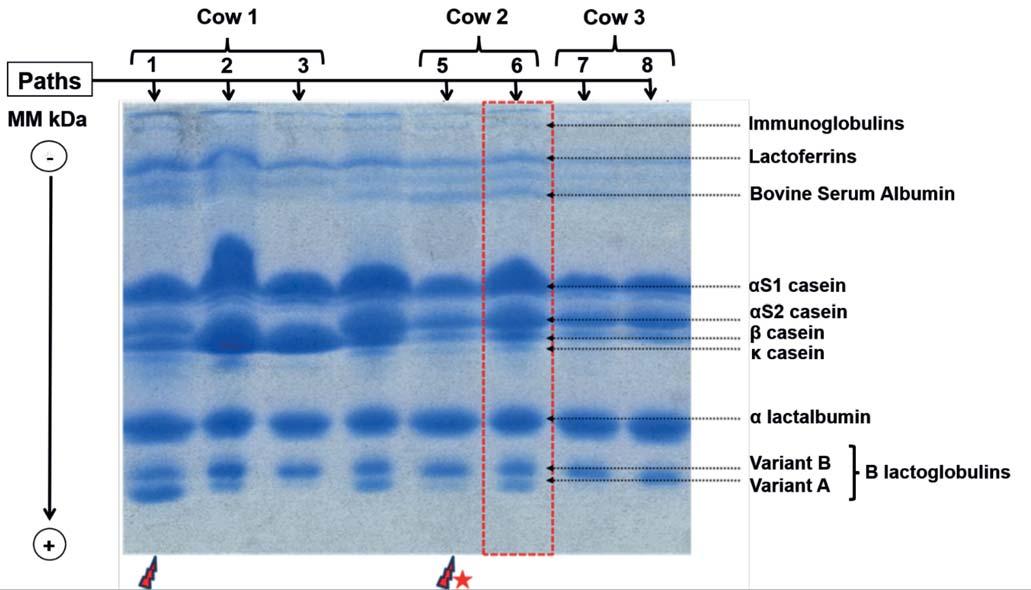
Milk composition results were obtained from 74 quarter-milk samples. The overall means were as follows: 4.61% (± 0.69) lactose, 3.05% (± 0.48) protein, 3.65% (± 0.58) fat, 8.33% (± 1.19) nonfat solids, and 11.98% (±1.77) total solids contents in all samples. CMT-positive milk samples showed higher values of conductivity (p<0.001) and pH (p<0.001). Moreover, SCM increased solids non-fat contents (SNFC: p<0.001) but reduced mineral substances (p=0.001). No difference was found in milk fat content (MFC: p=0.08), total proteins (p=0.52) and lactose (p=0.9) between infected and uninfected quarters (Table 1). In opposite to milk samples from healthy quarters, milk samples with SCM showed well focused and more intense Igs, lactoferrins and BSA bands and less intense and non-focused bands for α-lactalbumin and β-lactoglobulin. The behavior of caseins fractions varied from one mastitic-quarter to another depending on the severity of the infection. Indeed, degradation of caseins in milk with SCM was mostly high, especially for κ-CN>> β-CN > α S2-CN. This latter was characterized by un-
focused bands in form of patches losing their properties of migration (Figure 1).
Milk clotting ability
Thirty-one milk samples were subjected for milk coagulation trait analysis. The mean RCT recorded from mastitis quarter milk samples (1093.9±781.9 seconds) was higher (p<0.001) than that of the healthy (325.3±177.5 seconds) milk samples. Furthermore, the clotting activity was lower (2.55±1.49 RU) from mastitis milk samples than the healthy milk samples (7.80±4.46 RU). The clotting activity recorded upon the low heat 0% MFC control was higher from that recorded from mastitis and healthy milk. The increase in conductivity due to IMI, was highly associated with an elongation in RCT (R=0.69: p<0.001). Moreover, clotting activity was inversely proportional to conductivity (R=-0.38: p=0.03) (Table 4).
DISCUSSION
Udder health
At least one mastitis case was diagnosed from the entirely investigated herds in the present study, the detected prevalence of SCM at the quarter level was 20.83%. This relatively high
6antibiotics25.51{P,FX,E,VA,FA,CD}1{P,OX,FX,E,FA,CD}
5antibiotics38.30-3{P,OX,TE,E,CD},{P,TE,E,FA,CD},{P,SP,TE,E,CD}
4antibiotics513.80—5{P,E,FA,CD},{P,TE,E,CD},{P,C,E,CD}
3antibiotics1130.53{P,TE,E},{P,C,CD}8{P,FX,OX},{P,TE,E},{P,E,CD},{P,TE,FA},{P,E,FA}, {TE,E,CD}
2antibiotics719.44{P,E},{P,C},{P,SP},{P,TE}3{P,E}
1antibiotic513.80—5{P},{E},{C}
SENSBLE38.31—2—
108Impactsofsubclinicalmastitisonmilkquality,clottingabilityandmicrobialresistanceofthecausativeStaphylococci
Quartermilkscreening240——— SamplespositivetoCMT5010020.83— NegativeculturesforCMT-positivesamples481.660
Cultureswithanundeterminedbacterium16326.6616 Cultureswith S. aureus 9183.759 CultureswithNAS(Non-aureusStaphylococci)25**5010.41 27
PositiveculturesforCMT-positivesamples469219.1652 Positivecultureswithonetypeofcolonies408016.6640 Positivecultureswithtwotypesofcolonies6122,512
Analysis No. of samplesFrequency %Prevalence %No. of isolates
Table 2 -Prevalenceandbacterialisolationfrequenciesfrombovinemastiticmilksamples.
Total 36—9No.=727No.=17
Staphylococci S. aureus NAS ResistanceNRateNo.Patterns of resistance No.Patterns of resistance
Table 3 -AntibioticresistancepatternsofStaphylococciinvolvedinbovinesubclinicalmastitis.
Drug
P. Penicillin; OX. Oxacillin; FX. Cefoxitin; E. Erythromycin; TE. Tetracyclin; CD. Clindamycin; FA. FusidicAcid; VA. Vancomycin; SP. Spiramycin; C.Chloramphencol.
CMT-Positive :1(RAQ);5(RPQ);7(RPQ)
CMT-Negative:2(LAQ);3(RPQ);6(LPQ);8(LPQ)
RAQ:RightAnteriorQuarter; RPQ:RightPosteriorQuarter; LAQ:LeftAnteriorQuarter;
prevalence of bovine SCM was similar to the results obtained in other previous Algerian studies (14). The increased incidence of SCM in dairy livestock could be due to a lack of implementation of regular mastitis prevention and/or control strategies other than treating clinical cases. Although CMT is still used in most of studies due to its convenience and had been validated in field applications for SCM detection (6), none of the farmers were doing CMT or other tests routinely to screen their cows for SCM. Milk conductivity is considered an indicator of udder health as a result of changes in the ion balance associated with the inflammatory response to IMI (15). We observed an increase in milk conductivity and pH in quarters positive to CMT.
Contagious mastitis is considered of fairly vital significance to the public health as it is linked with many zoonotic diseases in which milk turns as a vehicle for the infectious agents (16,17). This highlights the importance of hygiene and managemental practices inside dairy farms. Moreover, it would be a serious hazard for public health because that mastitic milk is usually added into a bulk milk tank, especially in populations where some people could consume raw milk or non-heat-treated dairy products like yogurt or cheese (18). According to the microbiological finding of the study, Staphylococci were blamed from 64% of mastitic-quarters accounting 18% of S. aureus and 50 % of NAS. Consequently, co-infection with Staphylococci was detected in two quarters. S. aureus is considered as the main contagious mastitis causative agent with high ability to persist inside the udders. Its pathogenicity is based on the presence of important mechanisms such us its ability to form biofilms, polysaccharide capsule small colony variants, and their ability to invade professional and nonprofessional cells, which will protect S. aureus persistence from the innate and the adaptive
immune response of the cows, and from antibiotics (19). The preponderance of NAS species in the study animals has also been observed in many other studies. Indeed, NAS have become the most common bovine mastitis isolate in many countries and could therefore be described as emerging mastitis pathogens (20). The dominance of this group of pathogens is possibly as a result of poor milking hygiene. NAS commonly colonize the teat end and teat canal only and are difficult to associate with clinical mastitis; under some circumstances however, they may lead to raised somatic cell counts and subclinical mastitis (20). Since they are a contagious and common colonizer of the teatend and teat canal, the use of dry cow therapy and post-milking teat disinfectants are of great value in controlling the disease. These control measures however, were not used by most of farmers that participated in the study.
Antibiotic resistance has increased among various bacterial pathogens which is considered an emerging problem with a major public health concern due to the risk of resistance transmission to human as well as its influence on the effectiveness of the current antibiotic therapy (17,21). From bovine mastitis standpoint, the failure in the treatment always occurred due to: chronic infection accompanied with fibrosis, inadequate dose of antibiotics, and emergence of multidrug-resistant bacterial pathogens (22). We reported herein, high rates of antimicrobial resistance among Staphylococci stains involved in bovine mastitis, especially for penicillin and erythromycin with 83.3%, and 61.1% respectively. Similar levels of resistance have been reported previously, to the first-line treatment with penicillin in Staphylococci strains associated with bovine mastitis in Algeria (23). Additionally, cefoxitin resistance was used to determine the methicillin-resistant Staphylococcus isolates, and antimicrobial susceptibility testing showed three strains were
L.Bentayebetal.LargeAnimalReview2023;29:105-111109
Figure 1 -ElectrophoreticprofilesoftotalproteinsinPAGE-SDS.
LPQ:LeftPosteriorQuarter; MM:MolecularMass
resistant to cefoxitin. For technical considerations, PCR have not been used for detection of mecA and/or mecC genes in the present study. The tested isolates showed 77.7% of multi-drug resistance, but in proportion that differ between S. aureus (88.8%) and NAS (74.1%). The relatively high resistance spectrum of Staphylococci involved in bovine mastitis is likely due to frequent and long-term use of antibiotics in therapeutics.
Milk quality and transformation ability
Variations in milk composition due to mastitis may impair the transformation process and the quality of dairy products (8). Our data from naturally occurring SCM cases in lactating cows, indicated that IMI affects negatively quarter milk composition. It has been shown that the degree of changes depends on the inflammatory response, bacterial pathogenicity as well as the severity and amount of affected tissue in the mammary gland (24). In cases of SCM, increased plasmin activity results impaired functional and secretory capacity of the mammary gland’s epithelial cells (8,25), leading to a decreased MFC in milk (26). However, despite the well-established negative effect of plasmin on mammary epithelial cells’ synthetic and secretory activity during IMI, literature results on the effect of SCM on MFC are contradictory. The latter supports the findings of the present study where impaired quarters by SCM produced milk with non-significant decreased MFC. Hence, the MFC increases due to the decreased milk volume in the infected glands
(27).
Our protein content analysis was based mainly on the total protein value. It has been observed that milk samples from infected quarters have higher total protein and whey protein values but lower casein content when compared to milk samples from healthy quarters (1). The percentages of casein and whey protein were not calculated in the present study. Indeed, the disruption of the mammary gland’s epithelium increased permeability of the milk barrier and facilitates the passage of serum proteins into the milk (25). Meanwhile, proteinases originating from bacteria and leucocytes in the mastitic milk cause the proteolysis of caseins, leading to a decrease in the casein content of milk (25,28). It seems herein that the decrease in the casein content was enough to compensate for the increase in the milk whey protein content which finally results in non-significant effect of SCM on total protein content in milk.
The levels of fat and protein contents evidenced good nutritional and cheese making quality of milk. Thereby, the coagulation process of milk starts with hydrolysis of κ-CN by the chymosin of rennet followed by the aggregation of casein micelles which form a reticulum entrapping the soluble phase and fat globules (29). The number of secondary interactions within the curd increases over time leading to its syneresis and partial expulsion of whey. In the present study, milk samples subclinically infected exhibited longer coagulation time and weaker clotting activity than milk samples collected from healthy
110Impactsofsubclinicalmastitisonmilkquality,clottingabilityandmicrobialresistanceofthecausativeStaphylococci
Standard T1852 control T2878 865 2.31 S. aureus Positive ConductivityRCT(s)CA (RU)NegativeConductivityRCT(s)CA (RU) controlcontrol V4.PD+5.4116181.23V.AG-5.0914613.69 KV5.PG+6.5035360.56KV4.AG-5.515113.91 O1.PD+4.94254.70O2.PD-5.273654.87 O’1.PG+4.344264.16O’1.PD-4.352777.22 O’3.AG+A4.603365.95O’3.AD-4.502527.93 O’4.AG+A4.9711431.74O’4.PD-4.752647.57 8.AG+5.137952.519.AD-5.176932.88 T1.PG+5.4314341.39T2.PD-4.932787.19 NAS O6.PD+5.6512451.60O7.PD-4.2711517.39 V1.PD+5.399302.15V1.PG-4.751727.35 KV1.AG+4.446563.04KV1.PD-5.463036.60 6.AG+4.865513.626.PD-5.6513414.92 V2.AD+6.7918981.05V2.AG-4.615943.35 O4.AD+5.656372.27O4.PG-5.14504.44 O’4.PG+4.7711051.80---O2.PG+5.314704.25KV1.AD+5.2213921.43---Mean5.25 1093.9 2.55 Mean4.95 325.3 7.80 Sd 0.65 781.9 1.49Sd0.43 177.5 4.46
/CodeRennet Clotting TimeMeanCA (RU)
Table 4 -Clottingabilitycomparisonbetweenmastiticandnon-mastiticmilksamples.
T1:Standardcontrollowheat:MFC:0%.
quarters. Deteriorating of coagulation properties could be attributed mainly to the higher milk pH and the degradation of casein fractions. Indeed, greater casein breakdown has a relevant effect on the technological behavior of bovine milk; while higher milk pH causes a decrease in the enzyme activity involved in milk clotting which negatively affects both traditional and modeled coagulation properties (9,30).
CONCLUSION
Our study identified that milk components and clotting ability features were sullied by SCM. The high prevalence of SCM in cows and multi-drug resistance of the incriminated Staphylococci highlight regular monitoring of the disease at farm level. Every farm must have determined critical points of fresh milk production chain in their conditions. By continuous control of these critical points the possible hazards can be prevented, so the milk quality can be improved and maintained for the consumer confidence.
ACKNOWLEDGEMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare that they have no conflict of interest
References
1. Forsbäck L., Lindmark-Mansson H., Andrén A., Svennersten-Sjaunja K., (2010). Evaluation of quality changes in udder quarter milk from cows with low-to-moderate somatic cell counts. Animal, 4: 617-626. https://doi.org/10.1017/S1751731109991467
2. Bobbo T., Ruegg P.L., Stocco G., Fiore E., Gianesella M., Morgante M., Pasotto D., Bittante G., Cecchinato A., (2017). Associations between pathogen-specific cases of subclinical mastitis and milk yield, quality, protein composition, and cheese-making traits in dairy cows. J Dairy Sci, 100: 4868-4883. https://doi.org/10.3168/jds.2016-12353
3. Reshi A.A., Husain I., Bhat S.A., Rehman M.U., Razak R., Bilal S., Mir M.R., (2015). Bovine mastitis as an evolving disease and its impact on the dairy industry. Int J Cur Res Rev, 7: 48-55.
4. Barkema H.W., Schukken Y.H., Zadoks R.N., (2006). Invited review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J Dairy Sci, 89: 1877-1895. https://doi.org/10.3168/jds.S0022-0302(06)72256-1
5. Martins L., Barcelos M.M., Cue R.I., Anderson K.L, Santos M.V., Gonçalves J.L., (2020). Chronic subclinical mastitis reduces milk and components yield at the cow level. J Dairy Res, 87, 298-307. https://doi.org/10.1017/S0022029920000321
6. Kaikçi G., Çetin Ö., Bingöl E.B., Gündüz M.C., (2012). Relations between electrical conductivity, somatic cell count, California mastitis test and some quality parameters in quality parameters in the diagnosis of subclinical in dairy cows. Turk J Vet Anim Sci, 36: 49-55. https://doi.org/10.3906/vet1103-4
7. Ruegg P.L., (2017). A 100-Year Review: Mastitis detection, management, and prevention. J Dairy Sci, 100: 10381-10397. https://doi.org/10.3168/jds.2017-13023.
8. Le Maréchal C., Thiéry R., Vautor E., Le Loir Y., (2011). Mastitis impact on technological properties of milk and quality of milk products-A review. Dairy Sci Technol, 91: 247-282. https://doi.org/10.1007/s13594-0110009-6
9. Pegolo S., Tessari R., Bisutti V., Vanzin A., Giannuzzi D., Gianesella M., Lisuzzo A., Fiore E., Barberio A., Schiavon E., Trevisi E., Piccioli Cappelli F., Gallo L., Ruegg P., Negrini R., Cecchinato A., (2021). Quarter-level analyses of the associations among subclinical intramammary infection and milk quality, udder health, and cheese making traits in Holstein cows. J Dairy Sci, 105: 3490-3507. https://doi.org/10.3168/jds.2021-21267
10. Quinn P.J., Carter M.E., Markey B., Carter G.R., (1994). Clinical Veterinary Microbiology. Wilfe Publishing, London, UK.
11. NMC., 1990. Microbiological procedures for the diagnosis of udder infection-3rd Edition. National Mastitis Council, Arlington, VA, USA
12. CLSI., 2007. Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute, Wayne, PA, USA.
13. Jeantet R., Croguennec T., Garric G., Brule G., (2017). Initiation à la technologie fromagère. 2ème Ed., Editions TEC & DOC, Lavoisier, Paris. France.
14. Akkou M., Bouchiat C., Antri K., Bes M., Tristan A., Dauwalder O., Martins-Simoes P., Rasigade J.P., Etienne J., Vandenesch F., RamdaniBouguessa N., Laurent F., (2018). New host shift from human to cows within Staphylococcus aureus involved in bovine mastitis and nasal carriage of animals’ caretakers. Vet Microbiol, 223: 173-180. https://doi.org/10.1016/ j.vetmic.2018.08.003.
15. Kandeel S.A., Megahed A.A., Ebeid M.H., Constable P.D., (2019). Ability of milk pH to predict subclinical mastitis and intramammary infection in quarters from lactating dairy cattle. J Dairy Sci, 102: 1417–1427. https://doi.org/10.3168/jds.2018-14993
16. Galal Abdel Hameed K., Sender G., Korwin-Kossakowska A., (2006). Public health hazard due to mastitis in dairy cows. Anim Sci Pap Rep, 25: 7385.
17. Titouche Y., Akkou M., Houali K., Auvray F., Hennekinne J.A., (2022). Role of milk and milk products in the spread of methicillin-resistant Staphylococcus aureus in the dairy production chain. J Food Sci, 87: 3699-3723. https://doi.org/10.1111/1750-3841.16259
18. Awad A., Ramadan H., Nasr S., Ateya A., Atwa S., (2017). Genetic characterization, antimicrobial resistance patterns and virulence determinants of Staphylococcus aureus isolated from bovine mastitis. Pak J Biol Sci, 20: 298-305. https://doi.org/10.3923/pjbs.2017.298.305
19. Zaatout, N., Ayachi, A. and Kecha, M., 2020. Staphylococcus aureus persistence properties associated with bovine mastitis and alternative therapeutic modalities. J Appl Microbiol, 129: 1102-1119. https://doi.org/10.1111/jam.14706
20. Pyörälä S., Taponem S., (2009). Coagulase-negative staphylococciEmerging mastitis pathogens. Vet Microbiol, 134: 3-8. https://doi.org/10.1016/j.vetmic.2008.09.011
21. Ye Q., Wu Q., Zhang S., Zhang J., Yang G., Wang H., Huang J., Chen M., Xue L., Wang J., (2017). Antibiotic-resistant extended spectrum ß-lactamase- and plasmid-mediated AmpC-producing Enterobacteriaceae isolated from retail food products and the pearl river in Guangzhou, China. Front Microbiol, 8 :96. https://doi.org/10.3389/fmicb.2017.0009
22. Seegers H., Fourichon C., Beaudeau F., (2003). Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet Res, 34: 475-491. https://doi.org/10.1051/vetres:2003027
23. Akkou M., Antri K., Bachtarzi M.A., Bes M., Tristan A., Dauwalder O., Kaidi R., Meugnier H., Tazir M., Etienne J., Laurent F., RamdaniBouguessa N., (2016). Phenotypic and genotypic characterization of Staphylococcus aureus strains associated with bovine mastitis and nasal carriage of workers in contact to animals in Algeria. Pak Vet J, 36: 184188. http://www.pvj.com.pk/archive/Volume_36_Issue_2_2016.htm
24. Pyörälä S., (2003). Indicators of inflammation in the diagnosis of mastitis. Vet Res, 34: 564-578. https://doi.org/10.1051/vetres:2003026.
25. Ogola H., Shitandi A., Nanua J., (2007). Effect of mastitis on raw milk compositional quality. J Vet Sci, 8: 2374-2. https://www.ncbi.nlm.nih.gov/ pmc/articles/PMC2868129/
26. Auldist M.J., Coats S., Rogers G.L., McDowell G.H., (1995). Changes in the composition of milk from healthy and mastitic dairy cows during the lactation cycle. Aust J Exper Agricul, 35: 427-36. https://doi.org/10.1071/ EA9950427
27. Bansal B.K., Hamann J., Grabowski N., Singh K.B., (2005). Variation in the composition of selected milk fraction samples from healthy and mastitis quarters, and its significance for mastitis diagnosis. J Dairy Res, 72: 144-152. https://doi.org/10.1017/s0022029905000798
28. Le Roux Y., Laurent F., Moussaoui F., (2003). Polymorphonuclear proteolytic activity and milk composition change. Vet Res, 34: 629-45. https://doi.org/10.1051/vetres:2003021
29. Guinee T.P., (2003). Role of protein in cheese products. Pages 1083-1159 in Advanced Dairy Chemistry, Vol. 1B: Proteins: Basic Aspects. 3rd ed. P. F. Fox and P. L. H McSweeney, ed. Sprin
30. Stocco G., Cipolat-Gotet C., Cecchinato A., Calamari L., Bittante G., (2015). Milk skimming, heating, acidification, lysozyme, and rennet affect the pattern, repeatability, and predictability of milk coagulation properties and of curd-firming model parameters: A case study of Grana Padano. J Dairy Sci, 98: 5052-5067. https://doi.org/10.3168/jds.2014-9146
L.Bentayebetal.LargeAnimalReview2023;29:105-111111

MELISSA PENNISI1, FRANCESCA ARFUSO1*, ELISABETTA GIUDICE1, CLAUDIA GIANNETTO1, GIUSEPPE BRUSCHETTA1, GIUSEPPE PICCIONE1, ENRICO FIORE2
1DepartmentofVeterinarySciences,UniversityofMessina,PoloUniversitariodell’Annunziata,98168 Messina
2DepartmentofAnimalMedicine,ProductionsandHealth(MAPS),UniversityofPadua,Vialedell’Università, 35020,Legnaro(PD),Italy
SUMMARY
Intensive farm conditions, overcrowding and limited individual space, high grain feed, transportation, exposure to pathogens and high productivity are several stressors that can threaten animal welfare and the search for different tools to help maintain the balance between high farm productivity and animal welfare is increasingly well established. The effects of yeast Saccharomyces cerevisiae diet supplementation on cattle growth performance were widely investigated, but few studies debated about the health status of steers. For this purpose, two groups of Charolaise steers were equally divided according to the type of administered food: the control group (CG), which received the base diet without yeast supplement and the treatment group (YG), which each animal received the base diet with 5g of yeast Saccharomyces cerevisiae supplementation (YS) per day. From each group, blood samples were collected at three different time point, before (t0), after 21 (t1) and 42 (t2) days of the start of the study to evaluate changes on haematological parameters, including red blood cells (RBC), haematocrit (HCT), haemoglobin (HGB), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), mean corpuscular volume (MCV), white blood cells (WBC), neutrophils (NEU), lymphocytes (LYM), monocytes (MONO), eosinophils (EOS), basophils (BASO), and platelets (PLT). According to two-way analysis of variance (ANOVA), some haematological parameters including RBC (P<0.01; F(2,116) = 9.08), HGB (P<0.001; F(2,116) = 16.17), HCT (P<0.001; F(2,116) = 9.67), MCV (P<0.05; F(2,116) = 29.42), MCH (P<0.001; F(2,116) = 43.90), MCHC (P<0.05; F(2,116) = 44.27), MONO (P<0.001; F(2,116) = 15.34), EOS (P<0.001; F(2,116) = 8.24), BASO (P<0.01; F(2,116) = 43.15) and PLT (P<0.001; F(2,116) = 15.76) showed a significant effect of time and group. Results gathered in the current study suggest that Saccharomyces cerevisiae diet supplementations do not have a significant impact on the health status of cattle.
KEY WORDS
Live yeast; growth; haematological parameters; steers.
INTRODUCTION
Nowadays, the livestock systems aim to enhance animal growth and productivity to maximize profit in a rather short time. At this purpose, beef steers are feeding with minimum of roughage and high amount of concentrate with detrimental impacts on animal health (1). Certainly, highly fermentable substrates can lead to the imminent rumen dysfunction by an alteration of pH with negative impact on ruminal microbial ecosystem, ruminal inflammation and metabolism disorder, such as acidosis (2). The yeast supplement can help to minimize the negative effects of altered ruminal fermentation pattern, in order to keep a good health status and welfareof the animal (3). In particular, yeast supplementation improves sta-
Corresponding Author: Francesca Arfuso (farfuso@unime.it)
bility of ruminal pH, digestibility of organic matter and fiber by modifying the microflora of the host’s digestive tract and (4). Moreover, yeast supplementation is widely used in intensive dairy cattle farm to support milk production and feed conversion efficiency (5) and in beef cattle farm to improve the digestion of fiber and utilize the lactate by bacteria and to improve growth performance and safeguard liver health (6-9). In spite of that, other authors found similar or reduced growth rate between group feeding with yeast supplementation and control group (10, 11). These controversial results on yeast supplementation effects may depend on diet composition and yeast dose used (12). In livestock system, animals are usually submitted to a high amount of stressors caused by several factors such as high productivity, overcrowded and limited individual space, transportation, vaccination, exposure to pathogens and poor quality nutrition (13). These aspects have a negative impact on immune system and animals become susceptible to several pathogens, hence an extensive use of antibiotics for prophylactic purpose in farms is common.Yeasts have been em-
M.Pennisietal.LargeAnimalReview2023;29:113-117113
Saccharomyces cerevisiae diet supplementation influences haematological parameters in healthy steers
N
ployed as replacement of antibiotics used as growth promotans and interact directly with immune cells, modifying their blood concentration (8, 14).
In view of such consideration, the aim of the present study was to evaluate the effect of time and of treatment on haematological parameters measured in steers feed with a diet supplemented with Saccharomyces cerevisiae

MATERIALS AND METHODS
2.1 Animal and experimental design
Sixty Charolaise steers, 10 months of age, initial body weight 518±16.24, were selected from a farm located in the Northeast of Italy (45° 24’ N: 11° 52’ E, 12 m above sea level) and were enrolled in the study. All animals are clinically healthy and free from external and internal parasites. Their status was evaluated based on rectal temperature, heart and respiratory rate, fecal consistency and haematochemical profile. Animals were kept into pens and had free access to water.
Animals were divided into two equal groups, 30 animals each: the treatment group (YG) received the base diet with 5g of Saccharomyces cerevisiae supplementation; the control group (CG) received the base diet without yeast supplementation. The viable cells of Saccharomyces cerevisiae were a strain (NCYC Sc 47) produced by batch fermentation in a growth medium typical of those used for the industrial production of yeasts and with guaranteed concentration of 1010 CFU/g.
All treatments, housing and animal care were carried out in accordance with the standards recommended by the EU Directive 2010/63 EU for animal experiments.
2.2 Sampling and laboratory analysis
Blood samples were collected by jugular venepuncture, from both groups, into vacutainer tubes with EDTA anticoagulant agent, at day 1 (t0) and at 21 (t1) and 42 (t2) days of the start of experimental period. The sampling was carried out by qualified and experienced personnel, avoiding unnecessary injuries and stress to the animals. EDTA whole blood samples were processed in the laboratory within 2 hours by means of an au-
Figure 1 -Meanvalues±SDofhaematologicalparameters,togetherwithdifferencesrelatedtotime,measuredinsteersfeedingwiththe basedietwith5gofSaccharomycescerevisiae(YG)andincontrolsteers(CG)feedingwiththebasedietwithoutyeastsupplementation.
2
Saccharomyces cerevisiae dietsupplementationinfluenceshaematologicalparametersinhealthysteers
Table 1 -Meanvalues±SDofhaematologicalparametersmeasuredinsteersfeedingwiththebasedietwith5gofSaccharomyces cerevisiae(YG)andincontrolsteers(CG)feedingwiththebasedietwithoutyeastsupplementation.
tomated hematology analyzer (HeCo Vet C; SEAC, Florence, Italy) for the evaluation of complete blood count including RBC, HCT, HGB, MCH, MCHC, MCV, WBC, NEU, LYM, MONO, EOS, BASO, PLT. Leukocyte identification and counting was performed on all whole blood samples by manual analysis.
2.3 Statistical analysis
The obtained data were expressed as mean ± standard deviation (SD).
For each group, a separate analysis of variance (ANOVA) with repeated measures was applied to determine the influence of feed supplementation and of time (t0, t1, t2) on haematological parameters in both groups. Bonferroni multiple comparison tests were applied for post hoc comparisons. All the statistical analyses were performed using the Statistica 8 software (Statsoft Inc., Tulsa, OK, USA). P values<0.05 were considered statistically significant.
RESULTS
Table 1 showed mean values ± SD and significant effect of groups of the blood haematological parameters (RBC: F(1,116)=7.29; MCV: F(1,116)=4.72; MCHC: F(1,116)=5.92; MPV: F(1,116)=5.35; BASO: F(1,116)=11.9). As showed in Fig. 1, two-way
analysis of variance (ANOVA) showed a significant effect of time on RBC (F(2,116)=9.08), HGB (F(2,116)=16.17), HCT (F(2,116)=9.67), MCV (F (2,116) =29.42), MCH (F (2,116) =43.90), MCHC (F(2,116)=44.27), MONO (F(2,116)=15.34), EOS (F(2,116)=8.24), BASO (F(2,116)=43.15), PLT (P<0.001; F(2,116)=15.76) and HCT (P<0.01; F(2,116)=9.67). No significant effect of time (P>0.05) was observed on WBC, NEU and LYM in both groups throughout the experimental study.
DISCUSSION
A lot of works has demonstrated that yeast and its product fermentation supplementation improved ruminal fermentation, feed efficiency, energy status and body weight of cattle and the organism reaction for inflammation due to feed high grain rations (15-18).The yeast supplementation can provide favourable effects in terms of profitability, but there are few studies regarded effects on health status of steers (19, 20). Piccione et al (21) found a significant lower acute phase response in treated group respect to control group during the finishing phase.Moreover, Idowu et al (16) found a positive effect on health, during and after administration of yeast fermentation products, related to their ability to reduce inflammatory stress. Other authors suggest that cattle resulted better prepared for exposure to a
M.Pennisietal.LargeAnimalReview2023;29:113-117115
RBC(x106cells/µL)CG10.31±1.199.42±1.119.42±0.99 YG9.64±1.149.20±0.709.35±0.68 HGB(g/dL)CG12.71±1.2212.10±1.3113.23±1.15 YG11.92±1.17 ** 12.21±0.7713.21±1.17 HCT(%)CG33.80±3.3631.70±3.4433.99±2.95 YG31.66±2.97 ** 32.04±1.8034.39±2.94 MCV(fL)CG32.95±2.6633.80±2.8636.30±3.09 YG33.02±2.5434.93±2.0536.79±1.83 MCH(pg)CG12.39±1.0112.91±1.1314.13±1.17 YG12.44±0.8913.29±0.8014.13±0.74 MCHC(g/dL)CG37.60±0.6638.20±0.5038.93±0.58 YG37.69±0.7038.08±0.7738.41±0.66 ** WBC(x103cells/µL)CG10.02±1.919.27±1.5310.05±1.82 YG10.28±2.359.37±1.579.77±1.54 NEU(x103cells/µL)CG2.44±0.942.49±0.802.95±0.90 YG2.76±1.822.70±1.042.70±0.74 LYM(x103cells/µL)CG6.10±1.205.68±1.045.69±1.19 YG5.98±1.385.62±1.305.79±1.18 MONO(x103cells/µL)CG0.68±0.180.57±0.190.56±0.19 YG0.73±0.220.55±0.240.50±0.14 EOS(x103cells/µL)CG0.59±0.480.40±0.260.71±0.51 YG0.61±0.550.37±0.260.65±0.37 BASO(x103cells/µL)CG0.08±0.040.08±0.030.04±0.02 YG0.07±0.030.07±0.020.04±0.01 PLT(x103cells/µL)CG467.79±159.25406.65±133.57350.27±106.39 YG446.49±138.82321.89±158.23 * 345.45±131.24
ParametersGroupt0t1t2 Significant effect of group: *P<0.05 and ** P<0.01
pathogen hereafter previous immune stimulation by feed supplementation with a yeast fermentation product (22). Regarding haematology results, a significant decrease of RBC from t2 and t1 compared to t0 was observed in CG but no change was observed in YG. Results concerning HGB, suggest that its concentration showed an increase during the experimental study as a consequence of a continuous increase in muscle mass of steers (23). HGB, HCT and PLT concentrations values were result higher in CG respect YG during t0, probably for transient hydration differences between groups (21). Likewise, a significant decrease of MONO concentrations from YG and from CG were observed and it can be partially attributed to a reduction in inflammatory stress throughout experimental study.A study carried out on growing beef cattle showed that supplementation of hydrolysed yeast did not influence erythrocytes parameters, WBC, lymphocytes, or eosinophils, while neutrophils and monocytes were increased with hydrolysed yeast supplementation (24). Similarly, Adili et al. (25) reported that neutrophils were increased by the addition of hydrolysed yeast to dairy cows. Neutrophils can protect livestock against the most common infectious diseases (26). Kim et al. (27) observed that Holstein calves fed hydrolysed yeast showed enhanced neutrophils. Similarly, Wang et al. (28) indicated that live yeast increases the expression of genes that improve the function of neutrophils, especially those that code for the IL-4 receptor and IL1B in dairy cattle. Pedro et al. (29) found that Dectin-1 activation increases the expression of pro-inflammatory cytokines in monocytes in response to -glucan in yeast products. In addition, modulation of monocyte activation has also been related to bovine neutrophil degranulation (30).These results indicate that the addition of yeast to the cattle has the possibility of reducing inflammatory factors via enhanced neutrophils and monocytes in growing beef cattle.
CONCLUSION
The results gathered in the current study suggest that the base diet with addition of 5gr yeast (Saccharomyces cerevisiae NCYC Sc 47) supplementation did not negatively affect the overall health status of steers as suggested by haematological changes herein found. However, further studies are needed in order to evaluate the impact of a higher concentration of yeast supplement on steers wellness at all stages of their farming life.
References
1.Dias A.L.G., Freitas J.A., Micai B., Azevedo R.A., Greco L.F., Santos J.E.P. (2018). Effect of supplemental yeast culture and dietary starch content on rumen fermentation and digestion in dairy cows. J Dairy Sci, 101: 201221.
2.Nagaraja T.G., Chengappa M.M. (1998). Liver abscesses in feedlot cattle: a review. J Anim Sci 76: 287-298.
3. Adams D.C., Galyean M.L., Kiesling H.E., Wallace J.D., Finkner M.D. (1981). Influence of viable yeast culture, sodium bicarbonate and monensin on liquid dilution rate, rumen fermentation and feedlot performance of growing steers and digestibility in lambs. J Anim Sci, 53: 780-788.
4. Shen Y., Wang H., Ran T., Yoon I., Saleem M.A., Yang W. (2018). Influence of yeast culture and feed antibiotics on ruminal fermentation and site and extent of digestion in beef heifers fed high grain rations. J Anim Sci, 96(9): 3916-3927.
5. Poppy G.D., Rabiee A.R., Lean I.J., Sanchez W.K., Dorton K.L., Morley P.S. (2012). A meta-analysis of the effects of feeding yeast culture produced
by anaerobic fermentation of saccharomyces cerevisiae on milk production of lactating dairy cows. J Dairy Sci, 95:6027-6041.
6. Wiedmeier R.D., Arambel M.J., Walters J.L. (1987). Effect of yeast culture and aspergillus oryzae fermentation extract on ruminal characteristics and nutrient digestibility. J Dairy Sci, 70: 2063-2068.
7. Callaway E.S., Martin S.A. (1997). Effects of a Saccharomyces cerevisiae culture on ruminal bacteria that utilize lactate and digest cellulose. J. Dairy Sci. 80:2035- 2044.
8. Broadway P.R., Carroll J.A., Sanchez N.C. (2015). Live Yeast and Yeast Cell Wall Supplements Enhance Immune Function and Performance in FoodProducing Livestock: A Review. Microorganisms. 3(3): 417-27.
9. Alberghina D., Fiore E., Piccione G., Marafioti S., Morgante M., Gianesella M (2016). Evaluation of hepatic markers and body weight gain in growing and finishing steers. Comp Clin Pathol, 25: 721-725.
10.Tripathi M.K., Karim S.A., Chaturvedi O.H. Verma D.L. (2008). Effect of different liquid cultures of live yeast strains on performance, rumen fermentation and microbial protein synthesis in lambs. J Anim Physiol Anim Nutr, 92: 631-639.
11.Armato L., Gianesella M., Fiore E., Arfuso F., Rizzo M., Zumbo A., Giudice E., Piccione G., Morgante M. (2016). Effect of live yeast & yeast cell wall Saccharomyces cerevisiae diet supplementation on faeces chemical composition and growth performance in growing and finishing beef steers. LAR, 22: 203-210.
12.Lòpez-Soto M.A., Valdès-Garcìa Y.S., Plascencia A., Barreras A., CastroPerez B.I., Estrada-Angulo A., Rios F.G., Gòmez-Vazquez A., Corona L., Zinn R.A. (2013). Influence of feeding live yeast on microbial protein synthesis and nutrient digestibility in steers fed a steam-flaked corn-based diet. Acta Agric Scand, AAnim Sci, 63, 39-46
13.Lynch E., McGee M., Earley B. (2019). Weaning management of beef calves with implications for animal health and welfare. J App Anim Res, 47:167175.
14.Ran T., Shen Y.Z., Saleem A.M., AlZahal O., Beauchemin K.A., Yang W.Z. (2018). Using ruminally protected and nonprotected active dried yeast as alternatives to antibiotics in finishing beef steers: growth performance, carcass traits, blood metabolites, and fecal Escherichia coli. J Anim Sci, 96(10): 4385-4397. Erratum in: J Anim Sci, 96(12): 5345.
15.Armato L., Gianesella M., Morgante M., Fiore E., Rizzo M., Giudice E., Piccione G. (2016). Rumen volatile fatty acids × dietary supplementation with live yeast and yeast cell wall in feedlot beef cattle, Acta Agric Scand, AAnim Sci, 66(2): 119-124.
16.Idowu M.D., Taiwo G., Pech Cervantes A., Bowdridge S.A., Ogunade I.M. (2022). Effects of a multicomponent microbial feed additive containing prebiotics and probiotics on health, immune status, metabolism, and performance of newly weaned beef steers during a 35-d receiving period. Transl Anim Sci, 6(2):txac053.
17.Adeyemi J.A., Harmon D.L., Compart D.M.P., Ogunade I.M. (2019). Effects of a blend of Saccharomyces cerevisiae-based direct-fed microbial and fermentation products in the diet of newly weaned beef steers: growth performance, whole-blood immune gene expression, serum biochemistry, and plasma metabolome1. J Anim Sci, 97(11):4657-4667.
18.Ogunade I.M., McCoun M., Idowu M.D., Peters S.O. (2020). Comparative effects of two multispecies direct-fed microbial products on energy status, nutrient digestibility, and ruminal fermentation, bacterial community, and metabolome of beef steers. J Anim Sci, 98(9):skaa201.
19.Burdick Sanchez N.C., Young T.R., Carroll J.A., Corley J.R., Rathmann R.J., Johnson B.J. (2013). Yeast cell wall supplementation alters the metabolic responses of crossbred heifers to an endotoxin challenge. Innate Immun, 20: 104-112.
20.Shen Y., Davedow T., Ran T., Saleem A.M., Yoon I., Narvaez C., Mcallister T.A., Yang W. (2019). Ruminally protected and unprotected Saccharomyces cerevisiae fermentation products as alternatives to antibiotics in finishing beef steers1. J Anim Sci, 97(10): 4323-4333.
21.Piccione G., Badon T., Bedin S., Giannetto C., Morgante M., Giudice E., Gianesella M., Fiore E. (2021). Evaluation of yeast supplementation in steers housed under suitable temperature-humidity index, Biol Rhythm Res, 52(9), 1313-1321.
22.Burdick Sanchez N.C., Carroll J.A., Broadway P.R., Edrington T.S., Yoon I., Belknap C.R. (2020). Some aspects of the acute phase immune response to a lipopolysaccharide (LPS) challenge are mitigated by supplementation with a Saccharomyces cerevisiae fermentation product in weaned beef calves. Transl Anim Sci, 24,4(3):txaa156.
23.Owens F.N., Gill D.R., Secrist D.S., Coleman S.W. (1995). Review of some aspects of growth and development of feedlot cattle. J Anim Sci, 73(10): 3152-72.
116 Saccharomyces cerevisiae dietsupplementationinfluenceshaematologicalparametersinhealthysteers
24.Gunun N., Sanjun I., Kaewpila C., Foiklang S., Cherdthong A., Wanapat M., Polyorach S., Khota W., Kimprasit T., Kesorn P., Milintawisamai N., Gunun P. (2022). Effect of Dietary Supplementation of Hydrolyzed Yeast on Growth Performance, Digestibility, Rumen Fermentation, and Hematology in Growing Beef Cattle. Animals, 12, 2473.
25.Adili S., Sadeghi A.A., Chamani M., Shawrang P., Forodi F. (2020). Autolysed yeast and yeast extract effects on dry matter intake, blood cells counts, IGG titer and gene expression of IL-2 in lactating dairy cows under heat stress. Acta Sci Anim Sci, 42: e48425.
26.Bassel LL., Caswell J.L. (2018). Bovine neutrophils in health and disease. Cell Tissue Res, 371: 617-637.
27.Kim E.T., Lee H.G., Kim D.H., Son J.K., Kim B.W., Joo S.S., Park D.S., Park Y.J., Lee S.Y., Kim M.H. (2020). Hydrolyzed yeast supplementation in calf
starter promotes innate immune responses in Holstein calves under weaning stress condition. Animals, 10: 1468.
28.Wang Y.Q., Puntenney S.B., Burton J.L., Forsberg N.E. (2009). Use of gene profiling to evaluate the effects of a feed additive on immune function in periparturient dairy cattle. J Anim Physiol Anim Nutr, 93: 66-75.
29.Pedro A.R.V., Lima T., Fróis-Martins R., Leal B., Ramos I.C., Martins E.G., Cabrita A.R.J., Fonseca A.J.M., Maia M.R.G., Vilanova M., Correia A. (2021). Dectin-1-mediated production of pro-inflammatory cytokines induced by yeast -glucans in bovine monocytes. Front Immunol, 12: 689879.
30.Hussen J., Koy M., Petzi W., Schuberth H.J. (2016). Neutrophil degranulation differentially modulates phenotype and function of bovine monocyte subsets. Innate Immun., 22: 124-137.
M.Pennisietal.LargeAnimalReview2023;29:113-117117

Comparison of Some Biometric Index Values in Anatolian
Black Cattle Calves Raised in Different Locations
ÇAĞRI MELİKŞAH SAKAR1*, İLKER ÜNAL1, YASİN ERGİDEN1, UĞUR ZÜLKADİR2
1InternationalCenterforLivestockResearchandTraining,Mamak,Ankara,Turkey
2SelçukUniversity,FacultyofAgriculture,DepartmentofAnimalScience,Konya,Turkey
SUMMARY
In this study, some biometric index values of locally adapted Anatolian Black cattle raised in two different regions here defined as institute and village. The measurements were taken from the total of 829 animals in different growth periods such as birth (n=220), 3rd (n=208), 6th (n=206) and 12th month (n=195) of ages. In the study, a total of seventeen biometric indices were used to assess the general conformation of the animals as well as the development in the different regions. Examined biometric indexes were consist of Massiveness Index (MI), Area Index (AI), Height Index (HI), Height Slope Index (HS), Lateral Body Index (LBI), Pectoral Index (PI), Thoracic Index (TI), Length Index1 (LI1), Length Index2 (LI2), Chest Depth Index (CDI), Under Sternum Index (USI), Conformation Index (CI), Thoracic Development Index (TDI), Cannon Bones Index (CBI), Dactylo-Thoracic Index (DTI), Dactyl Costal Index (DCI) and Cannon Bone Load Index (CBL). Mostly of these values (MI, AI, LI2, CDI, PI, HS, CI, TDI) increase with the age of the animals, while some of them (LBI, USI, BI, DTI, DCI, CBL) decrease, and also there are also values (HI, TI, LI1) that are generally linear. In all examined periods, biometric indexes such as MI, AI, LI1, LI2, USI, HS, TDI, BI, DTI and DCI were found significantly higher in animals raised at the institute, while indexes like HI, LBI, TI, CDI, PI, CI, CBL were found significantly higher in animals raised at the village. The highest positive correlations were found between MI&AI, MI&TDI, PI&CDI, DTI&DCI and DTI&CBL values. On the other hand, the highest negative correlations were determined between MI&CBL, AI&DTI, AI&CBL, HI&HS, LBI&LI2, PI&USI, CDI&USI values. As a result, the indexes representing area and size were found to be higher in animals raised under Institute conditions, while the indexes determining long walks in mountainous and rough terrains were found to be superior in cattle raised in villages.
KEY WORDS
Biometric index, Anatolian Black Cattle, body measurement, conservation.
INTRODUCTION
The steady increment in the world population lead to increase importance of animal production1. In Turkey, cattle breeding has an important place in animal husbandry. While there are approximately 18 million cattle in Turkey, approximately 8% of these belong to local breeds. Domestic cattle are raised in rural areas, which is especially important for the evaluation of weak pasture areas. Among the domestic cattle breeds in Turkey, Anatolian Black cattle has the widest living area. This breed, which is mostly bred in rural mountainous lands in the Central Anatolian Region of Turkey, is known as a low-yielding breed. In general, meat and a little milk yield are used. They have adapted to unfavourable conditions in the regions where they are grown, and have gained resistance to harsh winters, drought, hunger, thirst and diseases3,4
Corresponding Author: Çağrı Melikşah Sakar (melikksahi@gmail.com).
Considering the phenotypic characteristics of the Anatolian Black breed grown under these conditions, studies that reveal the descriptive and actual yields of the breed are insufficient. The need to characterize and document local animal populations has gradually gained global importance5. Since these animals are adapted to these regions, their characterization studies should be performed and evaluated. Zootechnical indexes provide information about the functional characteristics of animals, the definition of structure and proportions, and the breed, ability and production performance of the animal5 Body measurements of the cattle represent by different body conformation that important for selection criteria6. In addition, the body dimensions of the animal are important criteria in the selection of quality animals. Body indices are used to determine aptitude for certain services such as velocity, resistance and traction7. Indices such as Conformation Index, Body Ratio, Height Slope Index are relatively easy-to-measure indicators of skeletal development and these related to the health and resilience of animals.
In this study, it was aimed to determine the biometric index values of Anatolian Black cattle raised under the breeder con-
Ç.M.Sakaretal.LargeAnimalReview2023;29:119-127119
N
VillageFemale51504847196 Male56545450214
Total220208206195829
ditions and at the institute conditions at birth, 3, 6 and 12 months and also to compare the values between regions.
MATERIAL AND METHODS
Animal Material
The animal material of this study consisted of Anatolian Black (AB) cattle grown under protection in two different regions. These places are the “International Center for Livestock Research and Training (39°97 N, 33°10 E; elevation 826 m)” and “Osmansin village of Çamlıdere district of Ankara (40°43 N, 32°24 E; elevation 1175 m)”. This breed has been conserved within the scope of the project “Conservation of Domestic Genetic Resources and Sustainable Use” conducted by the General Directorate of Agriculture Research and Policies (TAGEM).
Anatolian Black calves are raised with their dams from birth and they are allowed to suckle their dams freely. ABCs are not milked in the farm. Feeding of ABC breeds cows are two meals a day, morning and evening, ad libitum in the form of total mixed feed. 80% barley bales and 20% dry meadow grass as roughage are given to the AB cows.
Data Set
In the study, measurements were taken from a total of 829 AB calves born between 2015-2020 and these are shown in Table 1 in detail. Measurements made in the village were only taken in 2018. These measurement periods were birth, 3, 6 and 12 months of age. All animals measured in the herd were also recorded regularly information such as birth date, sex, maternal ear tag ID number and age.
Then, between the specified periods biometric indices were determined by means of linear statistics. Also, the calculations of the indexes obtained from this growth and development are shown in Table 25, 8-11 .
Statistical analysis
The analyses of data were used Minitab 16 package programme12. The test of Tukey provided by Minitab was realized for multiple comparisons. All indexes were analyzed by using the following General Linear Model (GLM) procedure. The difference between the averages was tested by the «Tukey Multiple Comparison» test. The relationship between body indices was determined by «Pearson Correlation». This (GLM) formula; Yijklmn =µ + ai + bj + ck + eijkl
Where; Yijkl : observed data;
µ: Overall mean;
ai: i. effect of region (1:institute, 2:village);
bj: j. effect of calf’s sex (1:female, 2:male);
ck: k. effect of dam age (2-3, 4-7, 8-10, 11+);
eijkl: random error.
RESULTS
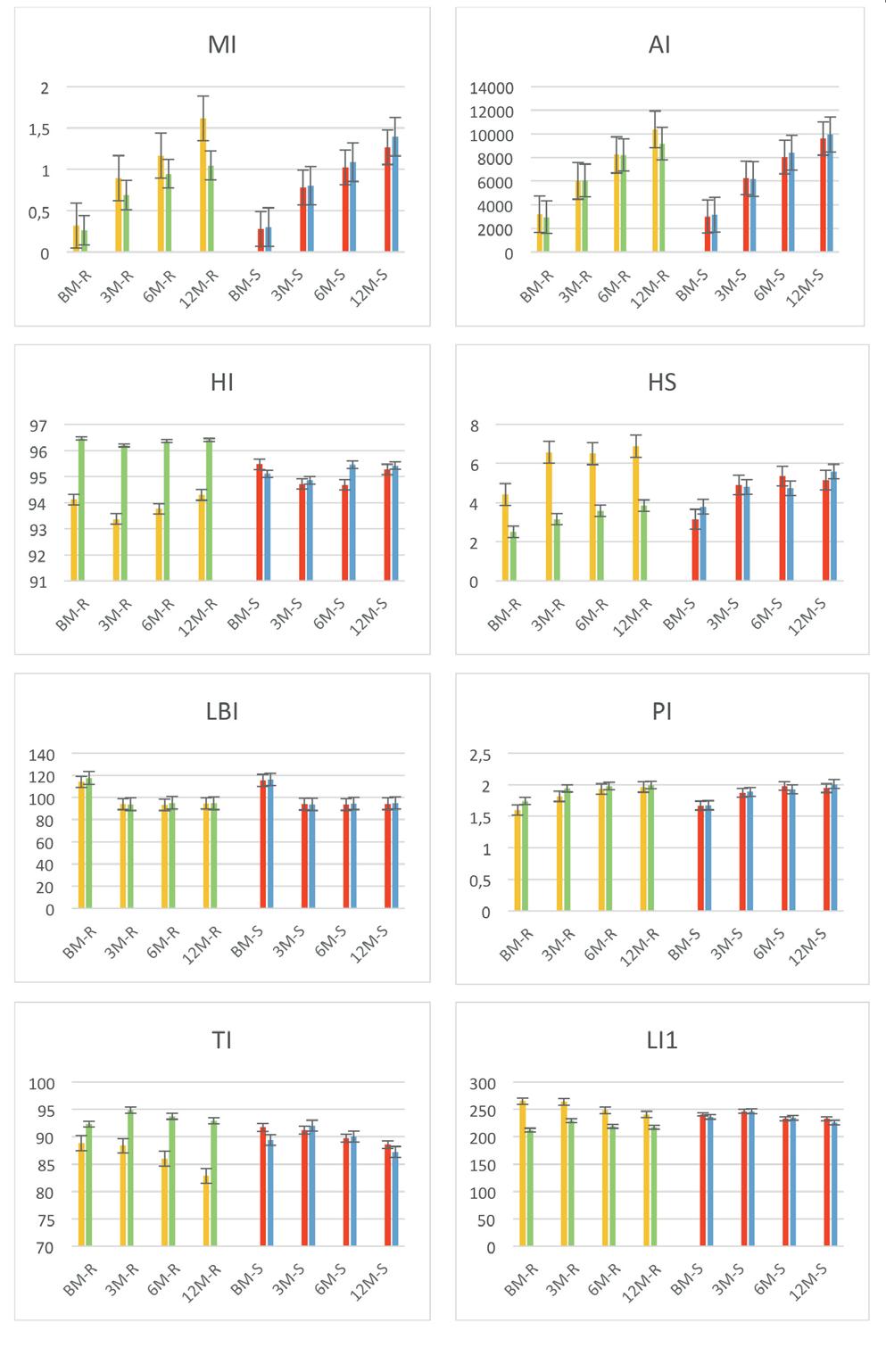
In the study, a total of seventeen biometric indices were used to assess the general conformation of the animals as well as the development in the different regions. Biometric index values and P values in Table 3 were presented at BM, 3M, 6M and 12M in AB Cattle. Mostly of these values (MI, AI, LI2, CDI, PI, HS, CI, TDI) increase with the age of the animals, while some of them (LBI, USI, BI, DTI, DCI, CBL) decrease, and also there are also values (HI, TI, LI1) that are generally linear. The statistical differences between the biometric index values in the growth periods of the animals were generally found to be significant between the institute and the village, but insignificant between the females and the males with dam age values.
In the study, biometric index values according to regions and gender were prepared in graphics and presented in Figure 1. It has been explored to enable earlier ages assessment of animals and, the comparison of the breed by establishing a standard format. Then, the value of each index determined type and function.
In the study, phenotypic correlations between biometric indices values are presented in Table 4. Correlations were prepared as a single correlation, taking into account the data of a feature in all periods (BM, 3M, 6M and 12M). A total of 136 correlations were estimated using 17 biometric indices examined in AB’s. Of these correlations, 123 (118 P<0.001, 5 P<0.05) were found to be significant, and 13 of them were found to be insignificant. Of these significant correlations, 58 were positive and 65 were negative.
DISCUSSION
The general body shape of animals is called conformation, and although environmental factors help shape the animal’s body, it is mainly the result of many hereditary traits7. Most of the traits examined in study were not affected by maternal age. This may be due to the fact that AB calves raised both in the institute and in the village are kept free with all other calves from birth along with their mothers and the maternity ability is high in AB. In addition, it may be a factor that births in AB’s are later than the culture breeds, so that the mother candidates complete the necessary size development.
In this study, the MI value of the animals in the institute was higher than the animals in the village and the values of the males were higher than the females in all periods. This may be an indication that the live weights of the animals and male animals in the institute and that their meat abilities are at a higher level. This value was found to be higher in males than females in Borgou cattle (2.43, 2.21; P<0.01) and in Ecuadorian Criollo Santa Elena Peninsula cattle (433.06, 310.35; P<0.0001) as in this study13,7. The same value was found to be higher similarly in males (2.92, 2.46; P<0.001 and 2.51, 2.25; P<0.01) in studies with Gudali cattle10,9
The AI value, like the MI value, appears to be higher in institute and male animals. This is an indication that these animals are larger in size. This value was found to be higher in males than in females in Wonosobo (5393, 4384) and Batur (5178, 4374) sheep14
The HI value was found to be less than 1.0 in both the institute and the village animals in all periods, this is due to the fact that the withers of the animals are lower than the rump. This
120ComparisonofSomeBiometricIndexValuesinAnatolianBlackCattleCalvesRaisedinDifferentLocations
InstituteFemale48444438174 Male65606060245
Table 1 -NumberofAnimalsExaminedbyRegionandSex. RegionSexBM3M6M12MTotal
Table 2 -IndexesandTheirFormulasUsedintheStudy. Index NameAbb.Index
MassivenessIndex(RelativeMIliveweight/withersheightAsthevaluesincrease,meat-typecharacteristicof WeightIndex,Compacttheanimalincreases.(Increasesasthecalfgrow). Index,IndexofBodyWeight)
AreaIndexAIwithersheight×bodylengthThegreatertheindex,thelargertheanimal. (Increasesasthecalfgrow).
HeightIndex(BodyRatio)HIwithersheight/rumpheight×100Ifthewithersarelowerthantherumptheanimalis lowinthefrontandviceversa.
HeightSlopeIndexHSrumpheight-withersheightPositive:healthyposturequality
LateralBodyIndexLBIwithersheight/bodylength×100 (Proportionality)
PectoralIndexPI(withersheight+rumpheight)/2)/Whenthebackheightislessthanthespaceunder sternumheighttheanimalisconsidered“farfromground”,this beingatraitthatfavorsduetorelativelylonglegs.
ThoracicIndexTIbodylength/chestgirthTI>0.90:longilinealanimal (thoraxperimeter)×100TIbetween0.85and0.89:mediolinealanimal TI<0.85:brevilinealanimal
LengthIndex1LI1bodylength/chest(thorax)depthx100
LengthIndex2(RelativeLI2bodylength/withersheight×10090>LI2<110:squarebodyshape BodyIndex,BodyLI2>110:oblongbodyshape LengthIndex)
ChestDepthIndex(RelativeCDIchestdepth/withersheight×100 ThoraxDepthIndex)
UnderSternumIndexUSIsternumheight/withersheight×100
ConformationIndex(Baron&CIchestgirth2/withersheightThegreatertheindex,themorerobusttheanimal. Crevat,AnamorphosisIndex)(Increasesasthecalfgrow).
ThoracicDevelopmentIndexTDIchestgirth/withersheightDT>1.2:indicatinganimalswithgood.
CannonBonesIndex(RelativeCBIcannoncircumference(shinboneAnimalrobustness CannonBoneThicknessIndex)perimeter,frontwristgirth)/ withersheight×100
Dactylo-ThoracicIndexDTIcannoncircumference/chestgirth×100Notexceed10.5inlightanimals (BoninessIndex,ChestDactylDTI>10.8inintermediateanimals Index)DTI>11.00inslightlymeatanimals DTI>11.5inheavymeatanimals
DactylCostalIndexDCIcannoncircumference/bodylength×100
CannonBoneLoadIndexCBLcannoncircumference/liveweight×100
value was found to be 1.0 in Borgou cattle5 and 0.994 in Pantaneiro horses7. It is desirable that the HI value is close to 1, as it is an indication that a balanced animal has better production and better health, especially in rough terrain. The fact that the value of the animals in the village is higher than the ones in the institute indicates that these animals are genetically more resistant to long walks. Imbalance in this index may indicate a susceptibility to problems in the joints in the anterior and posterior limbs of the animal, thereby damaging the skeleton7
While the HS value of the animals in the institute was higher than those in the village in all periods, it is seen that the values of the female and male animals are close to each other. The fact that this value is close to zero is important for a healthy
posture quality and may indicate that the animals in the village are more suitable for walking in rough terrain conditions. This value was reported as 3.79 in Pasundan cattle6
LBI value was found close to each other in general, both between regions and between genders. This value was found to be 91.51 and 86.40 in Guaymi Creole cattle11,15. The lower this value, the closer it is to the rectangle, which is a dominant shape in meat-producing animals.
While the PI value of the animals in the village was found to be higher than those in the institute in all periods, it is seen that the values of the female and male animals are close to each other. This may be due to the fact that the animals in the village have higher legs, just like the USI values. When the back height is less than the space under the animal is considered “far from
Ç.M.Sakaretal.LargeAnimalReview2023;29:119-127121
FormulaSignificance
P1:Region,P2:Sex,P3:DamAge; ***:P<0.001,**:P<0.01,*:P<0.05,NS:non-significant CV:Coefficientsofvariance,R2:Coefficientofdetermination
MIBM0.290±0.0034********38.3317.94 3M0.790±0.0115***NS*38.9721.00 6M1.055±0.0140*****NS34.7218.57 12M1.330±0.0258*****NS52.5427.56 AIBM3066±29.92*******16.9513.34 3M6154±63.52***NS6.3312.71 6M8215±80.15NS****0.0011.86 12M9769±111.0***NSNS22.4713.58 HIBM95.28±0.156***NSNS28.692.40 3M94.78±0.185***NSNS29.862.75 6M95.06±0.142*******43.952.30 12M95.35±0.171***NSNS25.312.18 LBIBM115.90±0.802**NSNS4.168.58 3M93.90±0.667NSNSNS0.008.14 6M94.16±0.543*NSNS1.766.81 12M94.67±0.582NSNSNS0.006.33 TIBM90.57±0.581****NS6.678.20 3M91.62±0.569***NSNS19.137.86 6M89.89±0.532***NSNS28.638.16 12M87.89±0.511***NSNS47.438.54 LI1BM238.8±2.753***NSNS39.9418.24 3M246.6±2.404***NSNS29.8713.72 6M233.7±2.386***NSNS21.3012.98 12M229.2±2.643***NSNS14.2512.36 LI2BM87.95±0.614***NSNS5.668.77 3M108.59±0.773NSNSNS0.008.18 6M107.49±0.673**NS*4.777.10 12M106.67±0.647NSNSNS0.006.27 CDIBM38.41±0.325***NSNS40.3313.32 3M44.94±0.345***NS*35.0110.57 6M47.14±0.376***NSNS12.299.79 12M47.83±0.464***NS5.5210.18 USIBM62.16±0.313***NSNS43.648.31 3M55.51±0.322***NS*35.018.74 6M53.49±0.365***NSNS19.208.78 12M52.98±0.445***NSNS13.489.29 PIBM1.672±0.0088***NSNS35.047.79 3M1.870±0.0128***NS*21.148.52 6M1.954±0.0147NS*NS5.938.52 12M1.979±.0.0189NS*NS1.629.33 HSBM3.461±0.125*****NS30.7251.20 3M4.852±0.181***NSNS37.3052.83 6M5.039±0.147******45.4746.72 12M5.362±0.208***NSNS30.6848.86 CIBM164.57±2.922***NSNS89.7565.51 3M245.40±2.817***NSNS94.1053.89 6M308.31±2.993***NSNS96.2455.42 12M370.97±3.341****NS97.6055.71 TDIBM0.975±0.0046***NSNS30.446.79 3M1.192±0.0068***NSNS26.257.70 6M1.205±0.0065***NSNS47.278.14 12M1.224±0.0062***NSNS58.708.01 CBIBM13.60±0.087******NS42.249.95 3M13.45±0.087******NS26.678.64 6M12.81±0.087***NS*32.048.82 12M12.44±0.074*********55.858.53 DTIBM13.96±0.073******NS20.617.02 3M11.34±0.079NS***NS9.427.75 6M10.66±0.062NS***NS4.206.58 12M10.23±0.068NS****9.206.61 DCIBM15.66±0.124******NS22.6510.60 3M12.49±0.082******NS22.978.36 6M11.96±0.076****NS23.918.15 12M11.74±0.092********39.139.67 CBLBM50.06±0.642***NS**11.3316.07 3M18.73±0.299***NSNS17.3118.55 6M13.09±0.202**NSNS3.2316.44 12M10.39±0.182***NSNS37.6721.31
122ComparisonofSomeBiometricIndexValuesinAnatolianBlackCattleCalvesRaisedinDifferentLocations
FactorPeriodGeneralP ValuesR2 (%)CV P1P2P3
Table 3 -BiometricIndexValuesandPValuesinAnatolianBlackCattle.
ground”, this being a trait that favours due to relatively long legs7 This value was found to be 0.572 and 0.584 in males and females, respectively, in Pantaneiro horses7. The TI is a measure of the proportionality of a breed7. While this value was found to be higher in the animals in the village
than the animals in the institute in all periods, the values of females and males were found close to each other. In the study, while the animals in the village were found to be longilineal (BI0.90) at all periods, the animals in the institute were found to be mediolineal (BI between 0.85 and 0.89) at the BM, 3M

Ç.M.Sakaretal.LargeAnimalReview2023;29:119-127123
******:P<0.001**:P<0.01*:P<0.05NS:non-significant
Table 4 -PhenotypiccorrelationsbetweenbodyindicesinAnatolianBlackcattle.
124ComparisonofSomeBiometricIndexValuesinAnatolianBlackCattleCalvesRaisedinDifferentLocations
Figure 1. Cont.

Ç.M.Sakaretal.LargeAnimalReview2023;29:119-127125
Figure 1. Cont.
and 6M periods, and brevilineal (BI0.85) at the 12M period. In addition, female and male animals were found to be longilineal in the first 3 periods and mediolineal in the 12M period. These findings indicate that the animals in the village are more suitable for speed and walking, and the animals in the institute are more suitable for strength. This value is found 0.77 in both males and females in Borgou cattle5, 80.52 in Guaymi Creole cattle11, 116.15 in females and 74.31 in males in Santa Elena cattle13.
The LI1 value in all periods was found to be higher for the animals in the institute than for the animals in the village and the males than the females. In this case, it can be said that the animals in the institute have longer bodies. This value has been reported as 100.85 at 42 months of age and 100.05 at 60 months of age in beef cattle8
The LI2 value was generally found to be close to each other, both between regions and between genders. While this value was found to be lower than 90 in calves during the birth period, it was found to be close to 110 in the other 3 periods. This is an indication that calves have a shorter body in BM and their body lengthens proportionally with advancing age. This value was reported as 91.51 in Guaymi Creole cattle [11] , 97.95 at 42 months of age in beef cattle and 98.85 at 60 months of age [8]
While the CDI value was found to be higher in village animals in all periods, it was found to be close to each other in males and females. In Guaymi Creole cattle 54.7311, in Ecuador native cattle 51.52 in females, 47.74 in males (P=0.0229)13, in Goudali cattle 48.8 in females, 48.3 in males9 reported as. The mean CDI value, especially in females, is an indicator of skeletal thinness and its relationship with its suitability for milk production13. This value indicates that the population in the village has a dorsolumbar line with an increasing caudal slope that supports movement over rough terrain.
The USI value was found to be opposite to the CDI value and was higher in the animals in the institute, while it was found close to each other in females and males. This value was found to be close to each other in males (51.7) and females (51.2) in Goudali cattle9
While the CI value was found to be higher in all periods in the village animals than in the institute, it is seen that it is close to
Abbreviations:
BM-R:Birth month-region, 3M-R:3 monthregion, 6M-R:6 month-region, 12M-R:12 month-region.
BM-S:Birth month-sex, 3M-S:3 month-sex, 6M-S:6 month-sex, 12M-S:12 month-sex.
The first four group; 1. bar=Institute, 2. bar = Village.
The second four group; 1. bar=Female, 2. bar = Male.
each other in female and male animals. This value is related to the health and resistance of the animals, which indicates that the animals in the village have a more robust conformation. While it was found 206.02 in males and 195.93 in females in Borgou cattle5, gender differences were significant (P<0.01) in Pantaneiro horses7.
TDI value, while the values of animals in the institute were higher than those in the village in all periods, it is seen that the values are close to each other in female and male animals. However, since the animals in the institute have TDI values below 1.2 in the BM and 12M periods, and the animals in the village in all periods, it cannot be said that the calves are in good thoracic development visually. In animals, a small thorax is associated with a lack of musculature and deficiencies in the cardiovascular system7. This value was found to be 1.29 in females and 1.31 in males in Borgou cattle5
The CBI value was found to be higher in the institute and male animals than the others. This is an indication that these animals are more robust. This value was found to be higher (P=0.0001) in males (14.91) and females (12.53) in Ecuadorian native cattle13.
While DTI values in all periods were found to be close to each other between regions, the values of male calves were found to be higher than females. While the values of the animals in the institute and in the village were high in the UN, they decreased towards the age of 12 months and fell below 10.5. This may be an indication that AB animals have insufficient meat skills. This value, as in this study, was found to be higher in males (P=0.0001, P=0.01) in Ecuadorian native cattle (11.10, 9.88) and Goudali cattle (11.08, 10.46)13,9, it was higher (P=0.01) in females (11.92, 11.29) in Borgou cattle5
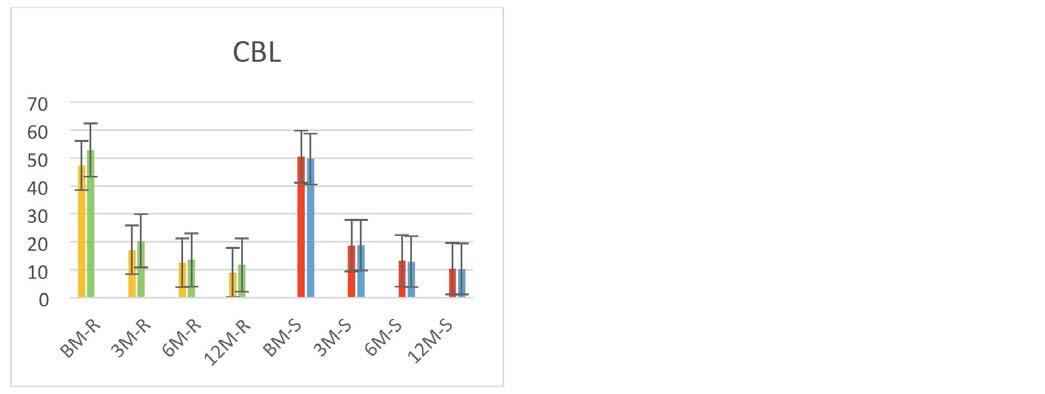
DCI value was found to be higher than the others in the institute and male animals. This value is an indication that these animals have a higher value cannon circumference. This value, as in this study, were found higher (P=0.0001) males (44.60) than females (21.77) in Ecuadorian native cattle13
While the CBL value was higher in the animals in the village than the animals in the institute, it was found close to each other in males and females in all periods. This value was found to be higher (P=0.0001) in females (4.05) than males (3.39) in Ecuadorian native cattle13.
126ComparisonofSomeBiometricIndexValuesinAnatolianBlackCattleCalvesRaisedinDifferentLocations
Figure 1 -Displayofbiometricindexvaluesbyregionandgender.Errorbarshowsthestandarderrormeanofthevalues.
Correlation Between Biometric Indices
Relationships between body measurements and live weight may vary depending on many factors, including the animal’s age, breed and nutritional level [16]. For this reason, separate correlation values can be prepared for cattle breeds grown in different regions. In the study, the low level of correlation between the analyzed variables is indicative of the high underlying variability in this population13. This may be due to animals being raised using inappropriate selection criteria and breeding programs.
The high correlations are due to the low coefficient of variation for the traits measured and may be related to individual preferences of the technicians who took the measurements7. In the study, the highest positive correlation values were found between MI-AI, MI-TDI, PI-CDI, DTI-DCI and DTICBL. This is an indication that the indices determining the characteristics such as meat type, area coverage and size in AB cattle effect each other positively. When the literature data is examined, were found to be negative relations between TI-LI2 (0.72), CI-MI (0.62), DTI-Pelvic Index (0.61)7, Longitudinal Pelvic Index-Rump Length Index (0.92), TDI-CI (0.90) and Transverse Pelvic Index-Witdh Slope (0.86)6
The highest negative correlation values also were found between MI-CBL, AI-DTI, AI-CBL, HI-HS, LBI-LI2, PI-USI and CDIUSI. This situation arises from the numerator/denominator relationship, which is generally used in the calculation of the index values of body measurements, and while a feature increases the value of an index, it can decrease its value due to being in the denominator of another feature. In the literature were found to be negative relations between LBI-LI2 (-0.99), Pelvic IndexRump Length Index (-0.78)7, HI-HS (-0.99), LBI-LI2 (-0.99) and TDI-Body Index (-0.82)6.
CONCLUSION
Some biometric indexes of populations raised in two different regions were evaluated in this study, which was carried out with Anatolian Black cattle. According to the findings obtained, the animals raised in the Institute were found to have higher values in terms of features such as area, size and size, it can be said that the meat production abilities of these animals are at a higher level than those in the village. The cattle raised in the village, on the other hand, were found to be superior in terms of long walks in mountainous and rough terrain. According to the biometric index values, it cannot be said that the herd raised both in the institute and in the village is sufficient in terms of dairying characteristics. These results show that selection through biometric traits is possible in Native Black cattle and measures can be taken to increase the genetic potential of the breed.
Acknowledgements
We would like to thank “Turkey’s Ministry of Agriculture and Forest, General Directorate of Agricultural Research and Policies” who have given the necessary permission to provide the animal material that used in the study.
Conflict of Interest
The authors declare no conflict of interest.
References
1.Demir Y., Keskin S. (2021). Examination of OECD Countries for the Presence of Livestock by Non-Metric Multidimensional Scaling. Livest Stud, 61 (2): 46-54. DO: 10.46897/livestockstudies.610202
2.TUIK. (2021). Number of cattle in Turkey. https://data.tuik.gov.tr/Bulten/Index?p= Hayvansal-Uretim-Istatistikleri-Aralik-2021-45593 (accessed 04 May 2022).
3.Sakar ÇM., Zülkadir U. (2022). Determination of some growth and development characteristics between birth and twelve months age in Yerli Kara cattle. J Agr Sci, 28 (1): 33-39. DOI: 10.15832/ankutbd.720072
4.Ünal İ., Tuncer HI., Sakar ÇM., Ünay E. (2019). The effect of maternal age on some body measurements in Anatolian Black Calves. Black Sea J Agr. 2 (1): 47–50.
5.Worogo HSS., Offoumon TOTL., Alabi CDA., Tchokponhoue U., Idrissou Y., Assani AS., Soule F., Iwaka C., Traore IA. (2022). Zoometric index analysis in borgou cattle breed reared on station in northern benin. J Anim Health Prod, 10 (1): 129-134. DO: 10.17582/journal.jahp/2022/10.1.129.134
6.Putra WPB., Syahruddin S., Arifin J. (2020). Principal component analysis (PCA) of body measurements and body indices in the Pasundan cows. Black Sea J Agr, 3 (1): 49-55.
7. Mcmanus CM., Santos SA., da Silva JA., Louvandini H., de Abreu UGP., Sereno JRB., Mariante ADS. (2008). Body indices for the pantaneiro horse. Braz J Vet Res Anim Sci, 45 (5), 362-370. DO: 10.11606/issn.16784456.bjvras.2008.26677
8. Alderson GLH. (1999). The development of a system of linear measurements to provide an assessment of type and function of beef cattle. Animal Genetic Resources/Resources génétiques animales/Recursos genéticos animales, 25: 45-55. DO: 10.1017/S1014233900005782
9. Crimella C., Barbieri S., Giuliani MG., Zecchini M. (2003). Body measurements and morphological indexes of a cattle population in the Adamawa region (Cameroon). Ital J Anim Sci, 2 (sup1): 243-253, 2003. DOI: 10.4081/ijas.2003.s1.340
10. Nsangou AS., Soh BG., Kıngsley MT., Felix M. (2022). Metric characteristics of the zebu (Bos indicus) Gudali variety Banyo in the high Guinean savannah area of Cameroon. Black Sea J Agr, 5 (2): 58-68. DO: 10.47115/bsagriculture.1011651
11. Villalobos-Cortés A., Carbonó M., Rodríguez A., Arosemena E., Jaén M. (2021). Phenotypic characterization of the Guaymi breed in conservation centers of Panama. Afric J Agr Res, 17 (6): 907-915. DO: 10.5897/AJAR2021.15495
12. Minitab I. (2010). Minitab 16 statistical software. Minitab Inc. State College, Pennsylvania, USA.
13. Cabezas Congo R., Barba Capote C., González Martínez A., Cevallos Falquez O., León Jurado JM., Aguilar Reyes JM., García Martínez A. (2019). Biometric study of Criollo Santa Elena Peninsula cattle (Ecuador). Revista mexicana de ciencias pecuarias, 10 (4): 819-836, 2019. DO: 10.22319/rmcp.v10i4.4850
14. Ibrahim A., Budisatria IGS., Baliarti E., Putra WPB. (2022). Factor and discriminant analyses in the morphostructure of Batur and Wonosobo sheep breeds. Ind J Anim Res, 56: 1-7. DO: 10.18805/IJAR.BF-1455
15. Rodríguez M., Fernández G., Silveira C., Delgado JV. (2001). Estudio étnico de los bovinos criollos del Uruguay: I. Análisis biométrico. Archivos de zootecnia, 50 (190): 113-118, 2001.
16. Ozkaya S., Bozkurt Y. (2009). The accuracy of prediction of body weight from body measurements in beef cattle. Archiv Tierzucht, 52 (4): 371-377. DO: 10.5194/aab-52-371-2009.
Ç.M.Sakaretal.LargeAnimalReview2023;29:119-127127

Investigation of the availability of vaginal electrical resistance during estrus synchronization in ewes
KOLDAŞ ÜRER ECE1*, KÖSE AYŞE M.1,
l
SUMMARY
Vaginal electrical resistance (VER) value measurement, which is a noninvasive method, is used to determine the estrus, appropriate insemination or mating time in sheep. The aim of this study was to investigate the availability of vaginal resistance values to evaluate the success of estrus synchronization program and to estimate early pregnant and returning ewes. Besides, it was also aimed to reveal the pregnant and non-pregnant ewes during the first trimester of pregnancy. Thirty-four healthy Awassi ewes were used in the study. Intravaginal sponges were inserted to ewes for 11 days and 0.075 mg d-cloprostenol was injected intramuscularly at the day of sponge withdrawn. Ewes were mated with fertile rams in estrus. Key times of the study were the day sponge insertion (D0), the day of estrus (DE), sixty hours after sponge removal (h60), eighteen days after estrus (D18pe), thirtyfive days (D35pe) and fifty days after estrus (D50pe). VER value was measured on D0, DE, h60, D18pe, D35pe and D50pe. Progesterone concentration was measured from blood samples of D0, DE and D18pe. The change in VER value and progesterone concentration on different days (D0, DE, D18pe) of the estrus synchronization protocol was significant (P<0.001). While VER measurement was not significant in the early diagnosis of pregnancy (P>0.05) on D18pe, it was found to be significant (P<0.05) in identification of returning ewes. VER cut-off value was found to be <255 ohm for determination of returning ewes. There was no significant (P>0.05) difference in VER values of pregnant and non-pregnant ewes at D35pe or D50pe. As a result, VER value measurement in the first trimester of pregnancy does not seem to be useful in determining both of the presence and the day of pregnancy. On the other hand, VER measurement can be useful in evaluating synchronization success and determining the first spontaneous estrus after induced estrus in the absence of teaser rams under field conditions.
KEY WORDS
Vaginal impedance, pregnancy, returning ewe.
INTRODUCTION
Sheep are seasonal polyestrous animals that cycling every 1618 days during the breeding season. Awassi sheep may not be considered a prolific breed due to low litter size, approximately 1.08 1. Therefore, it can be said that there is a high demand for fertility programs, whether professional or conventional breeding models. The average duration of estrus is 35-45 hours2, and it’s difficult to detect as in other sheep breeds. The importance of estrus detection increases with the intensive applies of estrus synchronization and artificial insemination in sheep. The only accurate indication on the field that a sheep stands estrus is acceptance of a ram in mounting. Techniques have been developed by using this indicator such as vasectomized teaser males3, marker crayon on the male4, electronic mount detectors5 and video tracking of behavioral patterns6. However, there are difficulties in applying each method in field conditions associated with increased herd size5. It has been suggested that the general condition of the rams, the ratio of rams to ewes
Corresponding Author: Ece Koldaş Ürer (ecekoldas@gmail.com, ecekoldas@mku.edu.tr).
in the herd, the competition between rams for estrus ewes may change the reliability of the use of teaser rams in detecting estrus7.
The most informative method to evaluate female’s fertility is to measure steroidal hormones in the bloodstream8. However, it’s rarely applied due to the individual handling of each animal, the extra cost of analysis, and the need for professional staff. Therefore, it seems more advantageous to use noninvasive methods following the reflection of steroidal hormones in the detection of estrus. During estrus, the NaCl level of the vaginal mucus increases with the effect of estrogen, adrenocorticotropic hormone and aldosterone, which increases the vaginal electrical resistance (VER)9. VER value was frequently used to detect estrus or ovulation and proper time of insemination or mating in many species10-13.
We hypnotized that the VER measurement, which is an available method in the field conditions, can be used in many stages of the estrus synchronization program. Therefore, three consecutive phases of a single synchronization program were evaluated for the purposes of the study: i) to determine the mean VER values and serum progesterone (P4) concentrations of ewes on the key times of the estrus synchronization protocol, ii) to investigate the use of VER values in the detection of pregnant,
GÖZER AHMET1, BAHAN ONUR1
1DepartmentofObstetricsandGynecology,FacultyofVeterinaryMedicine,MustafaKemalUniversity, Hatay,Turkey
Koldaş ÜrerEceeetal.LargeAnimalReview2023;29:129-134129
non-pregnant and estrus ewes before the ultrasound imaging, and iii) to compare the VER values of ewes in first trimester of pregnancy with non-pregnant ewes. In other words, it was aimed to demonstrate the effectiveness of VER measurements in evaluating the success of estrus synchronization, in the early diagnosis of ewes that conceive or not after synchronization, and in revealing pregnant ewes on the first trimester and nonpregnant ewes.
MATERIALS AND METHODS
This study was conducted in Belen, Hatay province in eastern Turkey, in the breeding season (late June to July). The study was approved by the local animal research ethics committee of Hatay Mustafa Kemal University, with the approval number of 2021/06-07.
Animals and Management
Thirty-four healthy Awassi ewes with a mean body weight of 42-47 kg, aged 3-5 years were used. Ewes were housed in a semienclosed shelter with open air ventilation system, away from rams and allowed 8-12 hours of natural grazing. Ewes were received regular antiparasitic treatments (Okzan®, Ceva, Turkey; Blotic %7®, Topkim, Turkey) and vaccinations (Biocan-R®, Interhas, Turkey; Supervac-9®, Vetal, Turkey; Brudoll-M®, Dollvet, Turkey; Poxvac®, Vetal, Turkey).
Study Design
Estrus was synchronized with Medroxyprogesterone acetate (MAP) impregnated intravaginal sponges in 34 ewes. After the sponge withdrawal, rams joined the flock for 3 days. Blood samples were collected from all ewes for serum P4 measurement at the time of sponge insertion (D0) and on the day of estrus (DE). VER values of ewes were measured and noted at D0, DE and 60 hours after sponge removal (h60). 18th days following the estrus (D18pe), blood samples were collected from all ewes and VER values were also measured and noted on the same day. Subsequently, at 35 days after estrus (D35pe) and 50 days after estrus (D50pe), ultrasound imaging for pregnancy diagnosis was performed and VER values were measured. Estrus synchronization protocol and whole study design are given in Fig. 1. To illuminate the points where VER measurement can be used in the synchronization protocol, the experiment was designed as follows;
Evaluation of the success of estrus synchronization: Key times
were considered D0, DE, and h60. The variation of mean serum P4 concentrations (on D0, DE, and D18pe) and VER values (on D0, DE, h60, and D18pe) of ewes were evaluated.
Identification of returning ewes after synchronization: The usability of the VER value in determining the returning ewes was investigated. Key time was considered D18pe. VER and P4 concentrations were evaluated. Ewes were divided into two groups according to serum P4 levels; Group E (n=17) with serum concentrations below 1ng/ml and Group L (n=17) with serum concentrations above 1ng/ml. Group E was assumed to be in estrus, while Group L was assumed to have a luteal structure in the ovary. A cut-off value was calculated using the estrus variable. VER values measured below the cut-off value was indicated ewes stood estrus, while values above the cut-off value was indicated ewes did not stand estrus. False positive and false negative estrus detection rates were calculated. Accordingly VER>cut-off value and P4<1 ng/ml were considered false negative, VER<cut-off value and P4>1 ng/ml were considered false positive.
Early identification of pregnant ewes: The usability of the VER value in determining the early pregnancy diagnosis was investigated. D18pe, D35pe, and D50pe were considered key times. Pregnancy diagnosis results at D35pe and D50pe and VER value in D18pe were examined. A cut-off value was calculated using the pregnancy variable. Values below the VER cut-off were used to identify ewes that were not pregnant, and values above the cut-off were used to identify ewes that were pregnant. Monitoring the VER value in the first trimester of pregnancy and comparing it with non-pregnant ewes: Key times were D35pe and D50pe. Ewes were divided into two groups according to pregnancy results regardless of serum progesterone concentration in D18pe; Group NP (n=17) non-pregnant ewes in both examinations and Group P (n=17) pregnant ewes in both examinations. VER values of Group NP and Group P were measured and the relationship between the groups and within groups was evaluated.
Estrus Synchronization
Ewes were treated with intravaginal sponges containing 60 mg of MAP (Esponjavet, Hipra, Spain) for 11 days. At the time of sponge removal, intramuscular 0.075 mg d-cloprostenol (Senkrodin®, Veta, Turkey) was administered. Ewes in estrus after sponge removal were mated to five fertile rams naturally. The rams were joined to the flock twice daily for two hours (early morning and late evening), for three days, starting from 24 hour after the sponge’s removal. All ewes were exposed to
130Investigationoftheavailabilityofvaginalelectricalresistanceduringestrussynchronizationinewes
Figure 1 -Estrussynchronizationprotocolandparametersmonitoredduringandaftertheprotocol.
Table 1 -VERvaluesandP4concentrationsofewesduringestrussynchronizationprotocol.Differentsuperscriptsinsamelineshowsignificantdifferences(P<0.05).
rams. The day when at least half of the ewes stood still during mating with ram was considered the estrus day of the flock (DE). 3 days (72 hours) after sponge removal, rams left the flock and did not come into contact with the ewes again.
Ultrasound imaging
Transabdominal ultrasonography was performed in the ewes by real-time ultrasound device with 6-8 MHz probe (Falco 100, Pie Medical, Netherlands). All examinations were performed by the same veterinarian. In the diagnosis of pregnancy, visualizing of vesicle, viable embryo with heartbeat at D35pe and placentomes, fetal heartbeat and ossification, vesicles of embryo at D50pe were accepted pregnancy criteria.
Determination of vaginal electrical resistance
Values of VER were determined with a heat detector (Draminski Electronics in Agriculture, Poland) according to method previously descripted 14. Before the determination of VER, vulva of ewes was cleaned by a mild antiseptic solution containing 1% potassium permanganate. Then heat detector put in place with vagina, and the average value of three consecutive measurements was recorded.
Blood sampling and analyzes
Blood samples were taken from v. jugularis into tubes without
coating anticoagulant. The blood samples were centrifuged at 3000 rpm for 5 minutes; their serum was removed and stored at -20 ºC until the analysis of P4 by Direct Chemiluminescence method (ADVIA Centaur® XP Immunoassay System ReadyPack, Progesterone (PRGE), REF 01586287, Siemens, USA).
Statistical analysis
All statistical analyses were performed using the SPSS software package (SPSS 23.1.0.). The variation of P4 and VER values during estrus synchronization analyzed with repeated measures ANOVA. Similarly, relationship between the VER values of pregnant and non-pregnant ewes analyzed with repeated measures ANOVA. Greenhouse Geisser’s test was used to adjust the degrees of freedom in case of the violation of the normality assumption. ROC (Receiver Operating Characteristic) analyzes was done to determine the cut-off value of VER for estrus detection and early pregnancy diagnosis.
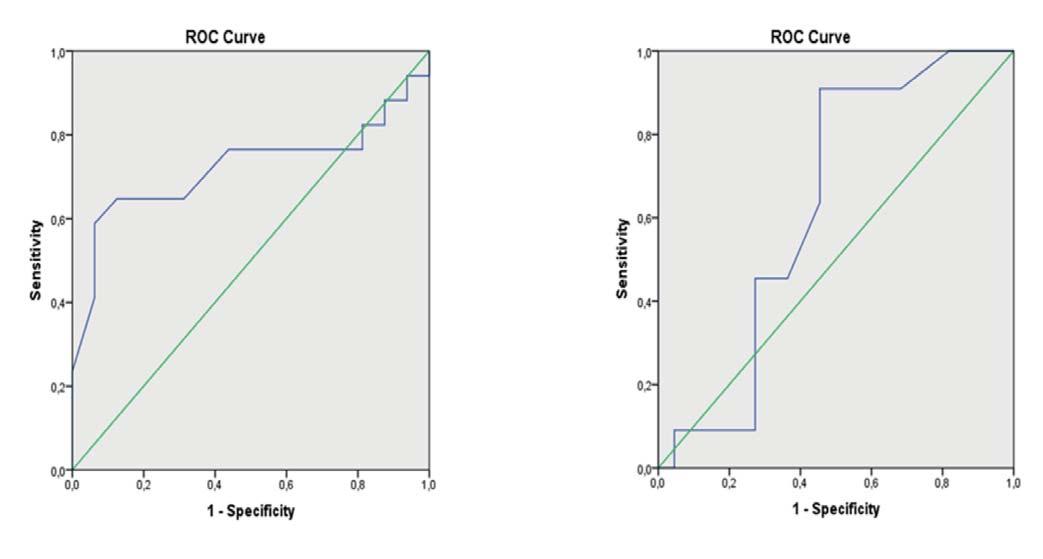
RESULTS
The change of VER value and P4 concentrations during estrus synchronization is given in Table 1. The cut-off for VER value was found to be <255 ohm (AUC: 0,721, 95% CI: 0.5320.909) on the determination of returning ewes (P=0,031) with 54.8% sensitivity and 93.8% specificity (Fig. 2). In detection
Koldaş ÜrerEceeetal.LargeAnimalReview2023;29:129-134131
VER(ohm,)485,45±21,12a295,15±10,65b322,73±16,86b337,58±21,38b<0,001 Progesteron(ng/ml)0,68±0,18b0,28±0,02b2,42±0,47a<0,001
Figure 2 -TheROCcurveatD18peina)estrusdetectionandb)earlypregnancy.
D0 DE h60 D18 pe P D0:thedayofthespongeinsertion,DE:estrus,48hoursafterspongewithdrawn,h60:60thhoursafterspongewithdrawn,D18pe:18thdayspost-estrus ab
of estrus, 20.58% (7/34) false negative (VER>255 and P4<1 ng/ml) and 2.94% (1/34) false positive (VER<320 and P4>1 ng/ml) diagnosis were obtained according to VER measurement on D18pe.
A separate cut-off value was calculated for early pregnancy diagnosis, considering that there may be ewes who could not maintain their pregnancy until ultrasound imaging, even if they were on luteal phase (serum P4 value>1ng/ml) at D18pe. Early pregnancy diagnosis related cut-off value of VER was found insignificant (P=0.229) (with 90.9 % sensitivity; 95% CI, 23.5 - 83.1 and 54.5% specificity; 95% CI, 28.8 - 96.7), (<270 ohm for non-pregnant ewes; AUC: 0.630, 95% CI: 0.440-0.821), (Fig. 2). When the retrospective records of the pregnant ewes were examined after the ultrasound examination, it was determined that 70.6% (12/17) of the ewes in Group L and 29.4% (5/17) of the ewes in Group E were pregnant.
Values of VER were found to be similar between pregnant and non-pregnant ewes in D35pe and D50pe (P>0.05), while the change of VER values was statistically significant in both Group P and Group NP (P<0.05), (Table 2).
DISCUSSION
Although the breeding season of Awassi sheep varies according to the environmental factors such as region or climate, it starts in April and continues until September 15. In regions closer to the equator, such as Jordan, the breeding season may not begin until mid-July 16. In the Mediterranean region, where we also conducted this study, June-July is considered the breeding season 17. It was reported that mean P4 concentrations before mating were 0.19±0.13 ng/ml (0.62±0.44 nmol/L) and during the luteal phase in non-pregnant ewes was 3.4±1.03 ng/ml (11.0±3.3 nmol/L)18. On the other hand, it is known that during the first estrus cycle of transition to the breeding season, the maximal serum P4 concentrations are lower compared to the mid-breeding season in sheep19. The mean serum P4 concentration of ewes was 0.68±0.18 ng/ml at the time of sponge insertion (D0) in this study, indicating that some ewes may still be in the transition period considering previous studies 16. The P4 concentration of ewes decreased during estrus (0.28±0.02 ng/ml) and increased sharply after 18 days of estrus (2.42±0.47 ng/ml). This fact indicates that estrus was successfully induced by estrus synchronization program.
A positive correlation was notified between VER values and blood serum P4 concentrations 20. Although the P4 concentration was low, the highest VER value of the study (485.45±21.12 ohm) was measured (P<0.001) on D0, probably due to the absence of estrogen. VER values decreased to the lowest value (295.15±10.65 ohm) during estrus. Since the earlier studies examining the efficacy of VER measurement in sheep 21, it is known that lower vaginal electrical impedance values
were observed in the follicular phase compared to the other phases. However, a single VER value that would indicate estrus in sheep seems unfeasible. Some variations of VER values are possible at the same stage of different cycles of the same sheep 14. In a recent study, the VER value was measured just before the artificial insemination and it was reported that the VER value of the ewes that would become pregnant was lower than that of the ewes that would not become pregnant, and the VER value of the ewes that would become pregnant was <300 ohm22. Since the VER increased at the 60th hour of sponge withdrawal (322.73±16.86) compared to estrus, it can be thought that most ewe’s estrus stage was already ends. The mean serum P4 value (2.42±0.47 ng/ml) of the ewes on D18pe indicates that some ewes have a luteal structure on their ovaries. Similarly, VER value on the same day (337.58±21.38) may indicate that ewes are in the luteal phase rather than the follicular phase. As a result, it was concluded that the VER value can be used to reveal the success of estrus synchronization, even if it is measured in key days of synchronization. However, measurement of VER in the transition period is not recommending since there will be individual differences among the herd.
The common practice in breeding herds of sheep is to mate under controlled conditions after estrus has been detected during the breeding season23. It is known that the main purpose of synchronization is to ensure the effective planning of the lambing season and to ensure homogeneous lambing in terms of matching the supply and demand in the market24. However, pregnancy rate obtained with the breeding program may vary depending on management factors and individual differences such as age, feeding and housing25. Ewes that do not conceive after an estrus synchronization protocol mate with rams in the subsequent estrus. However, if the number of nonpregnant sheep is higher than admissible, the basic homogeneity target may be impaired in farms where the sheep/ram ratio may be insufficient or where the rams are unavailable. Accordingly, it is necessary to identify non-conceived females as early as possible and to implement a new fertility program. Earliest studies reveal that the P4 concentration in non-pregnant animals is below 1 ng/ml at 18-22 days after mating in sheep26. Considering that the mean estrus cycle duration in Awassi ewes is 16-18 days15, we hypothesized that ewes without luteal structure in their ovaries should be in estrus again simultaneously 18 days after induced estrus. Serum P4 concentration was used to reflect the presence of luteal structure. It was assumed that if the serum P4 concentration is above 1ng/ml, luteal structure is present, if it is below 1ng/ml, there is no luteal structure, and the ewe was stand estrus. Ewes at re-estrus on D18pe had a VER cut-off value <255 ohm. However, it should be considered that cut-off success is approximately 59% in identifying estrus, and 94% in identifying non-estrus ewes. Therefore, it is not surprising that there were 20.58% (7/34) false negative estrus (VER>255 and P4<1 ng/ml) and 2.94% (1/34) false positive
132Investigationoftheavailabilityofvaginalelectricalresistanceduringestrussynchronizationinewes
GroupsD35peD50peVERVERxPregnancyPregnancy Pregnancy GroupNP440,59±28,84331,88±24,050,0020,8610,528 GroupP451±50,27361,25±18,71
VERP D35
Table 2 -VERvaluechangeatD35peandD50peinpregnantandnon-pregnantewes.
pe:35daysafterestrusD50pe:50daysafterestrus.
estrus (VER<255 and P4>1ng). It is thought that false negative diagnosis may be due to the presence of acyclic animals. When the individual records of these animals were examined, it was seen that 6 out of 7 ewes were exhibited a similar profile at D0 and DE, and did not conceive. On the other hand, false positive diagnoses may be caused by measurement error. It is thought that the rather low rate of false positive estrus diagnosis can be ignored if mating used as key method for conception on post-synchronization re-estrus. However, measuring only the VER value to decide on artificial insemination may be very erroneous practice.
The cut-off value which may indicate early pregnancy at D18pe was not significant (P>0.05). Measuring progesterone concentration of sheep 16-18 days after mating is recommended as an early pregnancy indicator 27. However, it was reported that the test had sensitivity as low as 60%28. In this study, it was determined that 29.4% (5/17) of ewes with P4 concentration <1ng/ml in D18pe were pregnant on ultrasound examination. While 4 of these 5 ewes were diagnosed estrus (p4<1ng/ml and VER<255 ohm) in both VER and progesterone measurements at D18pe, one of them (1/5) was diagnosed with false negative estrus (p4<1ng and VER>255 ohm). A possible explanation of this hormonal profile could be the measurement error. However, in this study, where we were aimed to reveal usability of VER measurement in field conditions, we considered measuring serum progesterone values as the gold method and approve the VER values reflect steroid concentration in bloodstream. On the other hand, 29.4% of non-pregnant animals were found in pregnancy examination although serum progesterone concentration is >1ng/ml on D18pe. We think that there may be early embryonic death which would expect in any flocks. The cutoff value of VER was significant in identifying the returning ewes (<255 ohm) while was not significant in identifying early pregnancies (<270 ohm). It is difficult to say that the statistical significance and numerical difference between the cut-off values can only be caused by early embryonic death. We think that this result may be caused by variables such as the presence of acyclic sheep, early embryonic losses, measurement error and many other possible failures that were not identified in this study. As a result, VER value (<255 ohm) measurement 18 days after mating can be used in detecting re-estrus but is not as successful as the use of teaser rams and does not indicate early pregnancy. However, we consider it usable when rams are not available as it can provide a preliminary information and estimate of the average number of ewes returning in a synchronized flock. It is also recommended that similar studies be carried out in larger flocks in the middle of the breeding season. It is thought that specificity and sensitivity rates may be higher in these herds as individual variations can be minimized. There are few studies investigate the variation of VER value after pregnancy diagnosis. In a human study, it was revealed that cervical stromal impedance was higher in pregnant women than in non-pregnant women, but this difference was reported to be particularly remarkable in the last trimester of pregnancy29 In goats, it was reported that the VER value was the lowest in estrus and the highest in the luteal phase, but there was no difference between diestrus and pregnancy30. We hypothesized that non-pregnant ewes would stand estrus again on the days corresponding to the 3th and 4th cycles after induced estrus; therefore, VER values in nonpregnant ewes would be lower than pregnant ewes on luteal phase. However, the difference in VER value between Group P and Group NP was not significant
(P>0.05). It can be thought that some ewes had a luteal structure in their ovaries although they were not pregnant or may be acyclic ewes in Group NP. The estrus cyclicality after synchronization may also have changed due to individual differences. However, since the presence of CL and serum P4 levels were not revealed in this study, it would be speculation to assume that this is the case. Nevertheless, our result corresponds well with that previous reports29,30. The VER value in the first trimester of pregnancy in ewes does not indicate a pregnancy-related point.
In this study, the availability of VER measurement in the estrus synchronization program was investigated and it was concluded that it could be useful in determining the first estrus after induced estrus in field conditions. It can also be used to evaluate synchronization success in the herd but failed to identify first trimester pregnancies. It is recommended to measure the VER value 18 days after estrus, to detect ewes that were likely not conceived, if rams are not available. This practice can significantly increase the rate of homogeneous lambing in synchronized herds.
ACKNOWLEDGEMENT
This study is dedicated to our colleagues and friends Dr. Pınar Ambarcığlu Kısaçam, Dr. Mehmet Ali Kısaçam, Dr. Erhan Tek, and Assoc Prof İbrahim Ozan Tekeli, who passed away on February 6, 2023 in Turkey in a greater earthquake, and we wish for them to find eternal peace in paradise. Particularly, we wish to thank Dr. Pınar Ambacıoğlu Kısacam for her valuable contributions on statistical analysis in this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest
References
1.Kridli R.T., Abdullah A.Y., Al-Smadi N.M. (2007). Reproductive performance and milk yield in Awassi ewes following crossbreeding. Small Rumin Res, 71(1-3): 103-108. Doi: 10.1016/j.smallrumres.2006.05.007
2.Alkass J.E., Hermiz H.N., Baper M.I. (2021). Some aspects of reproductive efficiency in Awassi ewes: A review. Iraqi J Agric Sci, 52(1): 20-27. doi: 10.36103/ijas.v52i1.1232
3.Chenoweth P.J., Landaeta-Hernández A.J., Flöercke C. (2014). Reproductive and Maternal Behavior of Livestock. Genet Behav Domest Anim, 5: 159194. doi: 10.1016/B978-0-12-394586-0.00005-6
4.Radford H.M., Watson R.H., Wood G.F. (1960). A crayon and associated harness for the detection of mating under field conditions. Aust Vet J, 36: 57-66. doi: 10.1111/j.1751-0813.1960.tb03764.x
5.Alhamada M., Debus N., Lurette A., Bocquier F. (2016). Validation of automated electronic oestrus detection in sheep as an alternative to visual observation. Small Rumin Res, 134: 97-104. doi: 10.1016/j.smallrumres.2015.12.032
6.Díaz A., Orihuela A., Aguirre V. Clemente, N., Pedernera, M., Flores-pérez, I., Vázqueza R., Ungerfeld R. (2021). Ewes prefer subordinate rather than dominant rams as sexual partners. Appl Anim Behav Sci, 238: 105306. doi: 10.1016/j.applanim.2021.105306
7.Kohno H., Okamoto C., Iida K., Takeda T., Kaneko E., Kawashima C., Miyamoto A., Fukui Y. (2005). Comparison of estrus induction and subsequent fertility with two different intravaginal devices in ewes during the non-breeding season. J Reprod Dev, 51(6): 805-812. doi: 10.1262/jrd.17037
8.Hodges K., Brown J., Heistermann M. (2010): Endocrine monitoring of reproduction and stress. In: Wild mammals in captivity: principles and techniques for zoo management, Eds. Kleiman D.G., Thompson K.V., Baer C.K., 447-468, University of Chicago Press, Chicago, IL.
9.Fehring R.J. (1996). A comparison of the ovulation method with the CUE ovulation predictor in determining the fertile period. J Am Acad Nurse
Koldaş ÜrerEceeetal.LargeAnimalReview2023;29:129-134133
134Investigationoftheavailabilityofvaginalelectricalresistanceduringestrussynchronizationinewes
Pract, 8:461-466. doi: 10.1111/j.1745-7599.1996.tb00604.x
10.Theodosiadou E., Amiridis G.S., Tsiligianni T. (2014). Relationship between electrical resistance of cervical mucus and ovarian steroid concentration at the time of artificial insemination in ewes. Reprod Biol, 14(3): 234-237. doi: 10.1016/j.repbio.2014.03.001
11.Murtaza A., Khan M.I.U.R., Abbas M., Hameed N., Ahmad W., Mohsin I., Tahir M. Z. (2020). Optimal timing of artificial insemination and changes in vaginal mucous characteristics relative to the onset of standing estrus in Beetal goats. Anim Reprod Sci, 213:106249. doi: 10.1016/j.anireprosci.2019.106249
12.Zuluaga J.F., Saldarriaga J.P., Cooper D.A., Cartmill J.A., Williams G.L. (2008). Evaluation of vaginal electrical resistance as an indicator of follicular maturity and suitability for timed artificial insemination in beef cows subjected to a synchronization of ovulation protocol. Anim Reprod Sci, 109(1-4): 17-26. doi: 10.1016/j.anireprosci.2007.10.002
13.Gürler H., Koldaş E., Binli Önyay F., Akçay A. (2018). Efficiency of vaginal electrical impedance to determine the stage of the reproductive cycle in bitches. Med Weter, 74(3):179-181. doi: 10.21521/mw.6080
14.Talukder M.R.I., Hasan M., Rosy T.A., Bari F.Y., Juyena N.S. (2018). Monitoring vaginal electrical resistance, follicular waves, and hormonal profile during oestrous cycle in the transition period in Bangladeshi sheep. J Vet Res, 62(4): 571-579. doi: 10.2478/jvetres-2018-0080
15.Talafha A.Q., Ababneh M.M. (2011). Awassi sheep reproduction and milk production: Review. Trop Anim Health Prod, 43(7): 1319-1326. doi: 10.1007/s11250-011-9858-5
16.Husein M.Q., Kridli R.T. (2003). Effect of progesterone prior to GnRHPGF2α treatment on induction of oestrus and pregnancy in anoestrous Awassi ewes. Reprod Domest Anim, 38(3): 228-232. doi: 10.1046/j.14390531.2003.00411.x
17.Canooğlu E., Saribay M.K. (2015). Morphology of the reproductive tract and reproductive physiology [Turkish]. In: Obstetrics and infertility in farm animals, Ed. Semacan A., Kaymaz M., Fındık M., Rişvanlı A., Köker A., 467-490, Medipres, Malatya, Turkey.
18.Zarkawi M. (1997). Monitoring the reproductive performance in Awassi ewes using progesterone radioimmunoassay. Small Rumin Res, 26(3): 291-294. doi: 10.1016/S0921-4488(97)00011-4
19.Bartlewski P.M., Beard A.P., Rawlings N.C. (1996b). Ovarian function in ewes at the onset of the breeding season. Anim Reprod Sci, 57(1-2): 67-
88. doi: 10.1016/s0378-4320(99)00060-3
20.Theodosiadou E., Tsiligianni T. (2015). Determination of the proper time for mating after oestrous synchronisation during anoestrous or oestrous by measuring electrical resistance of cervical mucus in ewes. Vet Med, 60(2): 87-93. doi: 10.17221/7982-VETMED
21.Bartlewski P.M., Beard A.P., Rawlings N.C. (1999a). The relationship between vaginal mucous impedance and serum concentrations of estradiol and progesterone throughout the sheep estrous cycle. Theriogenology, 51(4): 813-827. doi: 10.1016/s0093-691x(99)00029-1
22.Rahman M.M., Naher N., Isam M.M., Hasan M., Naznin F., Bhuiyan M M.U., Bari F.Y., Juyena N.S. (2020). Natural vs synchronized estrus: determinants of successful pregnancy in ewes using frozen-thawed Suffolk semen. J Anim Reprod Biotechnol, 35(2): 183-189. doi: 10.12750/ JARB.35.2.183
23.Dursun Ş. (2019). Effect of different short term synchronization protocols on estrus and fertility in non-pregnant ewes during the breeding season. J Hell Vet Med, 70(2): 1461-1466. doi:10.12681/jhvms.20813
24.Nozieres-Petit M.O., Moulin C.H. (2021). The management of lamb heterogeneity is a tool for farmers’ marketing strategies. Animals, 11(2): 551. doi: 10.3390/ani11020551
25.Özyurtlu N., Bademkıran S.(2010). Estrus synchronization and ınduction of estrus methods in sheep [Turkish]. Dicle Üniv Vet Fak Derg, 1(1):17-22.
26.Shemesh M, Ayalon N, Mazor T.(1976). Early pregnancy diagnosis in the ewe, based on milk progesterone levels. Reproduction, 56(1): 301-304. doi: 10.1530/jrf.0.0560301
27.Karen A., Beckers J.F., Sulon J., de Sousa N.M., Szabados K., Reczigel J., Szenci O. (2003). Early pregnancy diagnosis in sheep by progesterone and pregnancy-associated glycoprotein tests. Theriogenology, 59(9): 1941-1948. doi: 10.1016/S0093-691X(02)01289-X
28.Gvozdic D., Ivkov V. (1994). Early pregnancy diagnosis in ewes. Acta Vet Belg, 44(1994): 215-219.
29.Gandhi S.V., Walker D., Milnes P., Mukherjee S., Brown B.H., Anumba D.O. (2006). Electrical impedance spectroscopy of the cervix in non-pregnant and pregnant women. Eur J Obstet Gynecol Reprod Biol, 129(2): 145149. doi: 10.1016/j.ejogrb.2005.12.029
30.Nain S., Kumar D., Prakash B., Purohit G. (2020). Vaginal electrical resistance (VER) measurements in goats in different reproductive states. Int J Livest Res, 10(6): 122-126. doi: 10.5455/ijlr.20200329032847
ABOLFAZL HAJIBEMANI1*, YOUNES HEYDARI1, REZA ASADPOUR1, EZZATOLLAH FATHI1
1DepartmentofClinicalSciences,FacultyofVeterinaryMedicine,UniversityofTabriz,PostalBox 416379828,Tabriz,Iran
SUMMARY
The hormonal protocol is used to increase reproductive efficiency in ewes. The aim of the study was to investigate the effects of 2 prostaglandin F2 alpha (2PGF2α) and compared it with progesterone+ equine chorionic gonadotropin (P4eCG) in nulliparous Ghezel ewes. A total of 132 nulliparous ewes were randomly divided into three groups. The control group (n=42) did not receive any hormonal treatment. The P4eCG group (n=48) used of intravaginal sponge for a 12-day and an injection of 400IU of eCG at the time of sponge removal. The 2PGF2α group (n=42) received the double injection of PGF2α at a 9-day interval. For measurement of the progesterone (P4) concentration, three times (at the start of the experiment, at the time of withdrawal the vaginal sponge and the second injection of PGF2a and on days 20 after mating), blood samples were taken from experimental groups ewes. Pregnancy detection was done on the 30-35 days of pregnancy using B-mode ultrasonography. The estrous rate was obtained 100% in all groups. The highest number of nulliparous ewes were in estrus 48 h after the end of the synchronization program (control: 85.8%, 2PGF2α: 85.7%, P4eCG groups: 58.4%). No significant difference was observed in serum P4 concentration at the time 1 and 3 between the groups. The pregnancy and lambing rates were significantly higher (P<0.05) in the P4eCG (85.4% and 85.4%, respectively) and 2PGF2α (83.3% and 83.3%, respectively) groups than in the control group (28.6% and 23.8%, respectively). The litter size and twin rates in the P4eCG treatment group (1.19 and 19.5%, respectively) were significantly higher (P<0.05) compared to the 2PGF2α (1 and 0%, respectively) and control groups (1 and 0%, respectively). The fecundity rate was no statistically significant difference between the P4eCG (1.02±0.08) and 2PGF2α (0.83±0.05) groups (P>0.05). Based on the results, it was concluded that 2PGF2α injection can be employed to synchronize nulliparous estrus ewes in the late breeding season and is a suitable alternative for the P4eCG protocol.
KEY WORDS
Nulliparous ewes, PGF2α, Progesterone, Estrussynchronization.
INTRODUCTION
Ewes show seasonal reproductive activity. In the Northern hemisphere, the breeding season of ewes starts in early autumn and continues until early winter. The non-breeding season also begins at the end of winter and continues until mid-autumn (1). Today, numerous hormonal protocols are used to increase reproductive efficiency during the breeding season and induce estrus during the non-breeding season in ewes. Using vaginal progestogen compounds with equine chorionic gonadotropin (eCG) is the most common technique for estrus induction and synchronization (2).
The use of intravaginal progestogens causes problems such as vaginitis, environmental impact, affects fertility and animal welfare (2,3). Also, there is a possibility of the sponge falling and foul-smelling discharge following the removal of intravaginal sponges (4). Manes et al. (5) showed that sperm function and viability could be negatively affected by cervical discharge in ewes treated with intravaginal sponges. Moreover, the treatment
Corresponding Author:
Abolfazl Hajibemani (a.hajibemani@tabrizu.ac.ir)
lcost of the intravaginal progestogens (e.g., sponge and controlled internal drug releasing (CIDR)), as well as eCG, are expensive (6,7).
Induction of luteolysis and estrus synchronization using prostaglandin F2α (PGF2α) is one of the alternative ways to manage herd reproduction, especially during the breeding season. It does not interfere negatively with the reproductive response, does not cause reproductive disorders (e.g., vaginitis), and is effective, decreased cost and easy to use (7,8). Two injections of PGF2α in cyclic ewes at 9-12 days interval induces estrus in 72 h after the second PGF2α injection in 95% of the treated ewes (9). Most ewe is in the mid-luteal phase at the time of the second PGF2α injection, therefore, they responded to PGF2α injection (1). The injection of two doses of PGF2α to estrus synchronization is more economical than progesterone sponges in the breeding season. However, this method has shown different fertility rates in various studies (3,6,7).
Therefore, the aim of the present study was to investigate the effects of two injections of PGF2α with intervals of 9 days and progesterone sponge + eCG (P4eCG) in nulliparous Ghezel ewes at the breeding season.
A.Hajibemanietal.LargeAnimalReview2023;29:135-140135
The effect of 2PGF2α and P4eCG protocol of estrus synchronization on reproductive performance of nulliparous Ghezel ewes
MATERIALS AND METHODS
Animal
All procedures used in the present study were licensed by the Research Committee of the Department of Veterinary Medicine, University of Tabriz, Tabriz, Iran (99.02.6, 1399/05/01). This study was conducted in Tabriz (East Azarbaijan province, Iran). The Tabriz is located at latitude: 38o448N; 46o448E altitude of 1351 m above sea level. The climate is hot and humid during the summer and temperate during spring and autumn, while the winters are cold with a minimum temperature of - 10 °C. The experiment was performed in nulliparous Ghezel ewes during the breeding season, in the month of November, to achieve the lambing in May. A total of 132 healthy, nulliparous Ghezel lamb-ewe with an average body weight of 51.2 ± 1.5 kg and a body condition score (BCS) of 2.25±0.05 (score 1 for too thin ewes and a score of 5 for too fat ewes), were used in this study. BCS of nulliparous ewe was evaluated by palpation of back vertebral (10). Lamb-ewes were from the same farm.
Feeding experimental ewes
Lamb-ewes were managed as a single group. The ewe was fed from the natural pastures in the spring and summer seasons. Lamb-ewes were 10 hours/day at pasture. The ewes were manually fed with grass hay, barley grain, corn silage, bran, soybean meal and mineral and vitamin supplements in the autumn and winter seasons. The quantity of supplementation was 400gr/ewe/day. The water was access ad libitum.
Experimental design
After evaluation of the age, body weight, and BCS, the ewes were randomly divided into three groups. The first group (control group, n=42), received no any hormonal treatment. The second group (P4+eCG: P4eCG group, n=48) used of intravaginal sponge for a 12-day (Esponjavet, HIPRA, Spain) and intramuscular injection of 400IU of eCG (Gonaser, HIPRA, Spain) at the time of sponge removal. The third group (PGF2α+PGF2α: 2PGF2α group, n=42) received the double intramuscular injection of PGF2α (Vetaglandin, Aburaihan, Iran) with a dose of 75 g at a 9-day intervals.
Oestrus detection
24 h after the sponge removal or the second injection of PGF2α, nulliparous Ghezel ewes were exposed to the twenty-five fertile rams to observe oestrus behaviors. The oestrus was detected every 8 hour for four days. Ewes with standing heat were considered oestrus ewes. In addition to, behaviors including restlessness, teasing the ram and flehmen response was noted (11).
Mating management
The reproductive health of the rams was examined before inserting into the herd. Rams were separated at least one month from the herd before the beginning of the study. After 24 h of the removal of the sponge and the injection of the second PGF2α, one healthy and fertile ram was added to the herd for every five nulliparous ewes for mating (ram/ewe ratio of 1:5). This is an average ratio.
Blood sampling
For measurement of the P4 concentration, three times, blood samples were taken from experimental groups ewes. The first time blood sampling was collected at the start of the experi-
ment, the second time blood sampling was collected at the end of the estrous synchronization protocol (at the time of withdrawal the vaginal sponge and the second injection of PGF2a), and the third time blood sampling was taken 20 days after mating. Blood samples were taken by a venoject tube from the jugular vein. Serums of blood samples were separated by centrifugation at 1500 rpm for 15 min. After centrifugation, serums were kept at -20°C until the evaluation of P4 concentration. Serum progesterone concentration was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Progesterone ELISA, Monobind, USA). The sensitivity of this kit is 0.1 ng/mm, and the intra-assay and inter-assay CV were 3.8% and 7.5%, respectively. The ewes with P4 concentrations>1 ng/mL were considered cyclic ewes.
Pregnancy diagnosis
Pregnancy detection was done on the 30-35 days of pregnancy using a B-mode ultrasonography machine (SIUI 800V, China) was equipped with a 5 and 7.5 MHZ linear, rectal transducer. Ewes were restrained in a standing position. For pregnancy diagnosis, the probe was placed at the inguinal region and also inserted into the rectum. The probe was covered with ultrasonography gel before using for pregnancy diagnosis. Pregnancy was determined based on the detection of viable embryonic vesicle within the uterine horn (12).
Evaluation of reproduction indicators
Reproductive performance was statistically analyzed for each experimental group. Variable were included: Estrous rate = (number of ewes showing estrus behaviors/total number of ewes in each group) × 100, End treatment-onset estrus interval (Estrus onset time) = the time between sponges removal or after the second injection of PGF2α and first expression of standing heat), pregnancy rate (number of pregnant ewes/number of ewes which were introduced to a ram in each group), lambing rate (number of lambed ewe/number of ewes which were introduced to a ram in each group), fecundity rate (number of lambs/number of ewes which were exposed to a ram in each group), litter size (number of lambs/number of a lambed ewe), and the twin rate.
Statistical analysis
Statistical analysis of data was performed using SPSS software (Version 22.0, SPSS Inc, Chicago, Illinois). Data were shown as mean ± SEM and percentage. The Chi-square test was used to compare factors of estrous rate, pregnancy and lambing rates, fecundity rate and the gender of the lambs between the studied groups. The Fisher’s exact test was used to compare factors of twin rate, and the female rate between the studied groups. The results of the end treatment-onset estrus interval, the concentration of progesterone, the gestation length, and the litter size were statistically analyzed using one-way analysis of variance (ANOVA) and Turkey HSD test as the post hoc test were used. The P-value of < 0.05 was considered significant.
RESULTS
Serum progesterone concentration
The results of serum progesterone concentration of the studied groups are shown in figure 1. No significant difference was observed in serum progesterone concentration between the
2Theeffectof2PGF2α andP4eCGprotocolofestrussynchronizationonreproductiveperformance
Control42/42(100%)45.4±2.9a 2PGF2α 42/42(100%)44.8±2.1a P4eCG48/48(100%)54.00±2.5B
Groups Estrous rate (%)End treatment-onset estrus interval (h) a,bDifferentsuperscriptletterswithineachcolumnindicatesignificantdifferences(P<0.05).
groups when starting the synchronization program (P>0.05). At the end of the synchronization programs, the serum progesterone concentration in the 2PGF2α injections treatment group was significantly higher than in the P4eCG treatment group (P<0.05). In the third time of serum progesterone concentration (day 20 after mating), no statistically significant difference was observed between the studied groups (P>0.05).

Reproductive performance
The results regarding the estrous rate in the experimental groups are shown in Table 1. The estrous rate was obtained 100% (132/132) in all groups. All the nulliparous ewes were estrus within four days after the end of the synchronization program in the studied groups. The end treatment-onset estrus interval was significantly lower in the 2PGF2α treatment group and the control group than in the P4eCG treatment group (P<0.05) (Table 1). Figure 2 shows the distribution of estrus after the end of synchronization program in the studied nulliparous ewes. The number of ewes in estrus was lower in the P4eCG group compared to the control and 2PGF2α groups after 48 h (P<0.05). (control: 85.8%, 2PGF2α:85.7%,P4eCG groups: 58.4%).
There was no statistically significant difference in the gestation length between the treatment groups (Table 2) (P>0.05). The
reproductive performance of experimental groups is presented in Table 2. As shown in Table 2, the pregnancy and lambing rates were significantly higher in the P4eCG and 2PGF2α treatment groups than in the control group (P<0.05).
The litter size and twin rates in the P4eCG treatment group were significantly higher (P<0.05) compared to the 2PGF2α treatment and control groups. The fecundity rate was significantly higher in the P4eCG and 2PGF2α treatment groups than in the control group (P<0.05).
DISCUSSION
The results of the present study showed that the use of 2PGF2α and P4eCG treatment protocol improved reproductive performance in the nulliparous Ghezel ewes, as has been previously reported in ewes (8,13-16).
In this study, it was shown that the estrus rates were 100% in the studied groups. Estrous response was found in the all groups. Lamb-ewes of the three treatments were managed as one group, therefore could consider that animals of the control group were influenced by the estruses of the animals receiving treatments. In previous studies, the estrus rate was reported after the injection of 2PGF2α and P4eCG at 70.91% and 95.97% (17), 85% and 80% (18), 77.8% and 100% (19), 37% and 100% (14), and 83.3% and 75% (20), respectively. The estrus rate in the present study was similar or better to the reference values. The results of a similar this study conducted by Wei et al. (17) revealed that the intervals between the end of treatment and the onset of estrus in the 2PGF2α and P4eCG hormonal programs were 45.35±6.16 h and 50.46±7.15 h, respectively, which was significantly higher in the P4eCG group. This is likely because the higher P4 at the time of sponge removal causes that the beginning of preovulatory surge delay and also, the progesterone analogue suppresses secession of luteinizing hormone (LH) and
-Serumprogesterone(Mean±SE)concentrationsofnulliparousewesincontrolandtreatmentgroupsinfourdifferenttimes.Time 1:atthestartoftheexperiment,time2:atthetimeofwithdrawalofthevaginalspongeandthesecondinjectionofPGF2a,time3:20days aftermating(a,bP<0.05).
A.Hajibemanietal.LargeAnimalReview2023;29:135-140137
Figure 1
Table 1 -Estrousrate(%)andEndtreatment-onsetestrusinterval (h)ofnulliparousewesincontrolandtreatmentgroups.
gonadotropin-releasing hormone (GnRH) more (21,22). Inconsistent with the findings of the present study, some studies indicated that the interval between the end of treatment and the onset of estrus was higher in the 2PGF2α treatment group than in the P4eCG treatment group (19,20). However, in these reports, the amount of end treatment-onset estrus interval in the 2PGF2α treatment group (38-44 h) was similar to the result of the 2PGF2α group in the current study (42-48 h). In the studies by Ayoub et al. (20) and Danjuma et al. (23) the end treatment-onset estrus interval was insignificant between the two treatment groups. However, they were reported that the duration of the end treatment-onset estrus interval was 44-54 h in the treatment groups, which was similar to data in the current study. In agreement with the present study, the other studies reported a higher percentage of ewes were estrus between
40 and 48 h after the end of treatment (24,25).
These results showed that the use of 2PGF2α hormone treatment could cause a pregnancy rate similar to P4eCG hormone treatment and was higher than that in the control group. No negative effects of 2PGF2α hormone treatment was presented, because the pregnancy rates were similar in both treatments. In agreement with the results of our study, the findings of some studies showed that the pregnancy rate was similar in the 2PGF2α and P4eCG treatment groups in Awassi (55% and 47%, respectively) (18) and tropical hair (53.33% and 60%, respectively) (7) and Lacaune ewes (66.7% % and 76.5%, respectively) (19). These studies showed that 2PGF2α hormone treatment could be used to synchronize estrus with an acceptable pregnancy rate. Wei et al. (17) showed that the pregnancy rate in the 2PGF2α injection group with a dose of 0.24 mg (92.86%) in some herds
Femalerate20.0%(2/10)
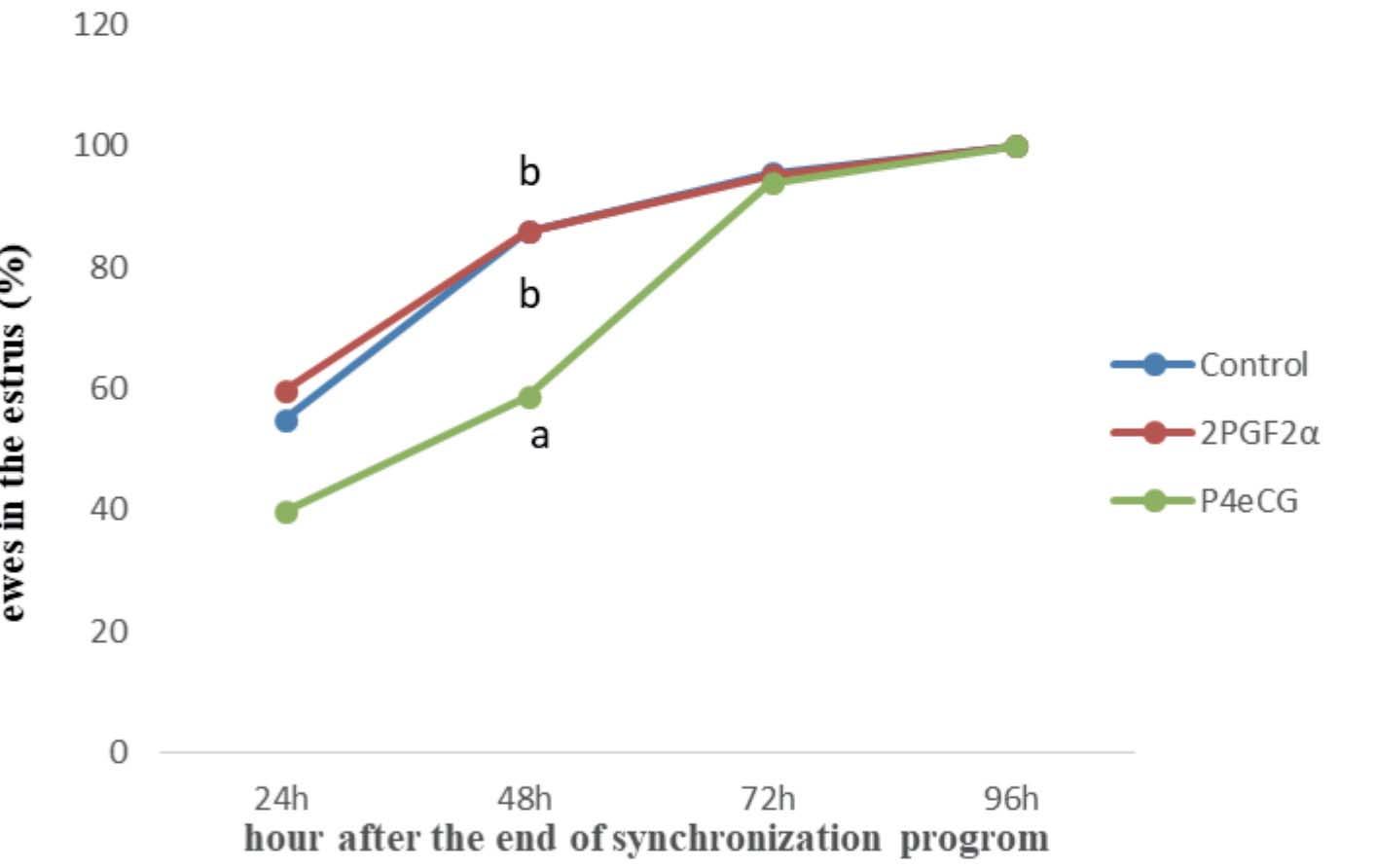
138Theeffectof2PGF2α andP4eCGprotocolofestrussynchronizationonreproductiveperformance
Gestationlength(days)149.47±1.57150.17±0.46147.04±3.6 Pregnancyrate28.6%(12/42)a83.3%(35/42)b85.4%(41/48)b Lambingrate23.8%(10/42)a83.3%(35/42)b85.4%(41/48)b Littersize1±0a1±0a1.19±0.06b Fecundityrate0.30±0.05a0.83±0.05b1.02±0.08b
a0%(0/35)a19.5%(8/41)b
Figure 2 -Distributionofestrusaftertheendofthesynchronizationprogramincontrolandtreatmentgroups(a,bP<0.05).
Twinrate0%(0/10)
Singlerate100%(10)100%(35)80.5%(33)
a57.1%(20/35)b54.2%(26/48)b
Malerate80.0%(8/10)42.9%(15/35)45.8%(22/48)
α P4eCG
Table 2 -Reproductiveperformanceofnulliparousewesincontrolandtreatmentgroups.
ParametersControl2PGF2
a,bDifferentsuperscriptletterswithineachcolumnindicatesignificantdifferences(P<0.05).
had no statistically significant difference compared to the sponge treatment group with 400 IU eCG at the time of sponge removal (92.31%) which was in agreement with the results of the present study (17). Cueto et al. (26) reported that 2PGF2α hormonal protocol with a long interval of 14 days caused a significantly lower pregnancy rate (52.0%) than in the P4-eCG group (76.4%), which was inconsistent with our results. Moreover, in their study, the pregnancy rate was lower in the 2PGF2α group than in the present study. The results of the present study showed that the pregnancy rate in the 2PGF2α group was better than some previous studies. Differences in pregnancy rates between studies could be due to differences in the season of implementing the hormonal protocol, dose and type of drug and the sheep breed.
In the current study, acceptable lambing rate of ewes was obtained in the 2PGF2α and P4eCG treatment groups. The findings of the present study showed that P4eCG treatment improved the litter size and twin rate. In other similar studies conducted, litter size was significantly higher in the P4eCG treatment group than in the 2PGF2α treatment group (14,17) that were similar to the current study; nevertheless, in other studies conducted, this difference was not significant (6,20). The findings of some studies showed that the use of P4 without eCG injection has similar results to PGF2α injections regarding the litter size and twin rate. It has been reported that the injection of eCG with P4 improved multiple births and litter size. Use of eCG at the end of progesterone treatments stimulate follicular growth and improves the rate ovulation response (3,6,9). The results of Fierro et al. 36 study showed that litter size was significantly higher in the P4eCG treatment group than in the treatment group with 2PGF2α injections at a 13-day interval; however, in the treatment group with 2PGF2α injections at 12, 14, 15, and 16 days intervals, it was similar to the P4eCG treatment group. Therefore, based on their study, 2PGF2α injection interval can be effective. Overall, treatments with P4eCG improve the litter size.
The fecundity rate is the most critical reproductive index to assess the herd’s reproductive performance, and it was the same rate in the two treatment groups in the present study. Therefore, the results of the fecundity rate showed that using of the 2PGF2α hormonal protocol can be a good alternative for the P4eCG protocol with acceptable economic success in the breeding season. But, based on the findings of some studies, the 2PGF2α hormonal protocol with an interval of 9, 12, 13, and 14 days significantly could reduce the fecundity rate compared to the P4eCG group; nonetheless, the 2PGF2α hormonal protocol with a longer interval had the similar fecundity rate as the P4eCG hormonal program (14,27). In general, the difference in reproductive performance in different studies can be attributed to differences in breed, geographical conditions, nutrition and management, the season conducting the experiment, drug dosage and the duration of P4 treatment and interval of 2PGF2α treatment.
In the present study, the serum concentration of progesterone was higher than 1 ng/ml in all treatment groups at the beginning of the program, which indicated that the nulliparous ewes were cyclic (28). According to the results of studies, a new follicular wave developed a few days later the first PGF2α injection and then followed the formation of an active corpus luteum and secreted and increased progesterone hormone (7,29). On the other hand, when the sponge is placed in the vagi-
na the level of progesterone gradually begins to decrease one to two days later. Also, the level of progesterone is low at the time of sponge removal (7,30) which confirmed the results of the present study. The serum level of progesterone increased in the third period, which was 19 days after mating, which is due to the pregnancy and the presence of the mature corpus luteum that produced a large amount of progesterone (16).
CONCLUSION
In conclusion, the use of 2PGF2α and P4eCG hormonal protocol to synchronize nulliparous Ghezel ewes improved reproductive performance compared to the nulliparous ewes that no hormonal protocol were used. Furthermore, the 2PGF2α injection protocol induced pregnancy, lambing and fecundity rates similar to the P4eCG protocol. The 2PGF2α injection protocol can be advised for nulliparous Ghezel ewes due to its short treatment period, lower drug price, high reproductive performance and easy application. Consequently, the 2PGF2α injection with a 9-day interval protocol can be employed to synchronize estrus nulliparous Ghezel ewes in the late breeding season and is a suitable alternative for the P4eCG protocol.
Acknowledgment
The authors thank the University of Tabriz. The authors also thank the farm manager at Bostan abad, for their kind cooperation.
References
1.Abecia J., Forcada F., González-Bulnes A. (2012). Hormonal control of reproduction in small ruminants. Anim Reprod Sci,130: 173-179.
2.Gonzalez-Bulnes A., Menchaca A., Martin G.B., Martinez-Ros P. (2020). Seventy years of progestagen treatments for management of the sheep oestrous cycle: Where we are and where we should go. Reprod Fertil Dev,32: 441-452.
3.Viñoles C., Paganoni B., Milton J., Driancourt M., Martin G. (2011). Pregnancy rate and prolificacy after artificial insemination in ewes following synchronisation with prostaglandin, sponges, or sponges with bactericide. Anim Prod Sci,51: 565-569.
4.Greyling J., Erasmus J., Taylor G., Van der Merwe S. (1997). Synchronization of estrus in sheep using progestagen and inseminating with chilled semen during the breeding season. Small Rumin Res,26: 137-143.
5.Manes J., Ríos G., Fiorentino M.A., Ungerfeld R. (2016). Vaginal mucus from ewes treated with progestogen sponges affects quality of ram spermatozoa. Theriogenology,85: 856-861.
6.Hasani N., Ebrahimi M., Ghasemi-Panahi B., HosseinKhani A. (2018). Evaluating reproductive performance of three estrus synchronization protocols in Ghezel ewes. Theriogenology,122: 9-13.
7.Alavez Ramírez A., Arroyo Ledezma J., Montes Pérez R., Zamora Bustillos R., Navarrete Sierra L.F., Magaña Sevilla H. (2014). Estrus synchronization using progestogens or cloprostenol in tropical hair sheep. Trop Anim Health Prod,46: 1515-1518.
8.Fierro S., Gil J., Viñoles C., Olivera-Muzante J. (2013). The use of prostaglandins in controlling estrous cycle of the ewe: A review. Theriogenology,79: 399-408.
9.Olivera-Muzante J., Gil J., Rojas N., Vinoles C., Espejo L., Soca F., Fierro S. (2012) Inclusion of a GnRH analogue to improve a prostaglandin F2 alpha-based protocol for timed artificial insemination (TAI) in sheep. Reprod Domest Anim,47: 422–422.
10.Pugh D., Baird A. (2002). Theriogenology of sheep and goats. Sheep and Goat Medicine first edition, 173, Saunders, Philadelphia, USA.
11.Ucar O., Kaya M., Yildiz S., Onder F., Cenesiz M., Uzun M. (2005). Effect of progestagen/PMSG treatment for oestrus synchronization of Tuj ewes to be bred after the natural breeding season. Acta Veterinaria Brno,74: 385-393.
12.Aziz D., Lazim E. (2012). Transabdominal ultrasonography in standing
A.Hajibemanietal.LargeAnimalReview2023;29:135-140139
position for pregnancy diagnosis in Awassi ewes. Small Rumin Res,107: 131-135.
13.Hajibemani A., Sheikhalislami H., Shahrbabak M.J.B., Jozani R.J., Ommati M.M. (2022). Effect of PGF2α and GnRH administration on reproductive performance in Ghezel ewes. Prostaglandins Other Lipid Mediat,161: 106640.
14.Safdarian M., Kafi M., Hashemi M. (2006). Reproductive performance of Karakul ewes following different oestrous synchronisation treatments outside the natural breeding season. S Afr J Anim Sci,36: 229-234.
15.Hameed N., Khan M.I.-u.-R., Zubair M., Andrabi S.M.H. (2021). Approaches of estrous synchronization in sheep: Developments during the last two decades: A review. Trop Anim Health Prod,53: 1-10.
16.Mirzaei A., Mohebbi-Fani M., Omidi A., Boostani A., Nazifi S., Mahmoodian-Fard H., Chahardahcherik M. (2017). Progesterone concentration and lambing rate of Karakul ewes treated with prostaglandin and GnRH combined with the ram effect during breeding and non-breeding seasons. Theriogenology,100: 120-125.
17.Wei S., Chen S., Wei B., Liu Z., Bai T., Lin J. (2016). Estrus synchronization schemes and application efficacies in anestrus lanzhou fat-tailed ewes. J Appl Anim Res,44: 466-473.
18.Alnimer M., Tabbaa M.J., Amasheh M., Alzyoud H. (2005). Hormonal treatments and the ram effect on synchronised oestrus in Awassi ewes at the beginning of the breeding season. New Zealand J Agric Res,48: 473-480.
19.Lombardo H.N.S., Monteiro C.A.S., Delgado K.F., Pinna A.E., de Paula Vasconcelos C.O., Nogueira L.A.G., Brandão F.Z., Balaro M.F.A. (2020). Hormonal Protocols for the Synchronization and Induction of Synchronized Estrus in Dairy Ewes Kept under Tropical Conditions. Acta Sci Vet,48: 1751.
20.Ayoub M., Tharwat R., Yaseen M., Soliman E. (2020). Using Progesterone and Prostaglandin F2α for Ewes Estrus Synchronization during Summer Season in Egypt. J Anim Poult, Fish Prod,9: 1-8.
21.Jeffcoate I., Rawlings N., Rieger D. (1984). Progesterone and the surge re-
lease of gonadotropins in the ewe. Domest Anim Endocrinol,1: 309-317.
22.Menegatos J., Chadio S., Kalogiannis T., Kouskoura T., Kouimtzis S. (2003). Endocrine events during the periestrous period and the subsequent estrous cycle in ewes after estrus synchronization. Theriogenology,59: 15331543.
23.Danjuma F.A., Bawa E., Nwannenna A. (2015). Prostaglandin versus progestagen protocols in oestrus synchronization in the Yankasa ewe. Sci J Anim Sci,4: 97-102.
24.Knights M., Hoehn T., Lewis P., Inskeep E. (2001). Effectiveness of intravaginal progesterone inserts and FSH for inducing synchronized estrus and increasing lambing rate in anestrous ewes. J Anim Sci,79: 11201131.
25.Ramírez A.A., Villalvazo V.M.M., Arredondo E.S., Ramírez H.A.R., Sevilla H.M. (2018). D-Cloprostenol enhances estrus synchronization in tropical hair sheep. Trop Anim Health Prod,50: 991-996.
26.Cueto M.I., Bruno-Galarraga M.M., Fernandez J., Fierro S., Gibbons A.E. (2020). Addition of eCG to a 14 d prostaglandin treatment regimen in sheep FTAI programs. Anim Reprod Sci,221: 106597.
27.Fierro S., Viñoles C., Olivera-Muzante J. (2017). Long term prostaglandin based-protocols improve the reproductive performance after timed artificial insemination in sheep. Theriogenology,90: 109-113.
28.Zarkawi M. (2001). Oestrous synchronisation and twinning rate of Syrian Awassi ewes treated with progestagen and PMSG during the breeding season. New Zealand J Agric Res,44: 159-163.
29.Wolfenson D., Thatcher W., Savio J., Badinga L., Lucy M. (1994). The effect of a GnRH analogue on the dynamics of follicular development and synchronization of estrus in lactating cyclic dairy cows. Theriogenology,42: 633-644.
30.Texeira T.A., Da Fonseca J.F., de Souza-Fabjan J.M.G., de Rezende Carvalheira L., de Moura Fernandes D.A., Brandão F.Z. (2016). Efficiency of different hormonal treatments for estrus synchronization in tropical Santa Inês sheep. Trop Anim Health Prod,48: 545-551.
140Theeffectof2PGF2α andP4eCGprotocolofestrussynchronizationonreproductiveperformance
Images of Normal Ocular Fundus in Saanen Goats j
OSMAN BULUT1,*, BÜŞRA KIBAR KURT2, ZEYNEP BILGEN ŞEN2, ZEYNEP BOZKAN2
PhDDVM
1,*DepartmentofSurgery,FacultyofMilasVeterinaryMedicine,MuglaSitkiKocmanUniversity,Mugla, Turkey
2DepartmentofSurgery,VeterinaryFaculty,AydinAdnanMenderesUniversity,Aydin,Turkey
SUMMARY
Imaging of the fundus during ophthalmological examination of the eye allows the evaluation and diagnosis of many diseases. Traditionally, eye examination is performed by using an ophthalmoscope. Indirect ophthalmoscopy is also used for visualization of the posterior segment in animals. By this way, posterior segment structures of the eye such as optic disc, retina, tapetal and nontapetal regions, retinal vessels and choroidea can be examined. Ultrasonography and special camera systems are also used currently to image for the ocular fundus.The structure of the fundus can vary between species, as well as between races within the same species. No study on fundus imaging of Saanen goats were found among the ophthalmological studies performed in goats.The ClearView fundus camera was used by holding it towards the goat’s pupils, and both eyes were examined.Data on the tapetal region, optic disc in the nontapetal region, retina and retinal vessels were obtained. All goats tapetal regions contained predominantly blue or green (most frequently). The nontapetal region was predominantly dark brown. The optic disc was located at the tapetal-nontapetal region border mostly located in the tapetal region. The form of the optic disc was oval and round. A gray spot in the center of the disc represents the remnant of the hyaloid artery or Bergmeister’s papilla. Bergmeister papillae were found in 29 animals in total, and in only one eye of 10 animals. The tapetal fundus in goats had a mild uniform stippling at the end of the capillaries called stars of Winslow. These stars are characteristic, giving these species a singular ophthalmoscopic aspect. While Winslow’s stars were observed in 35 animals in total, they were found in only one eye in seven goats. Our study aimed to obtain the normal reference values of the fundus of Saanen goats and to contribute to the literature.
KEY WORDS
Caprine; optic disc; retina; tapetal; small ruminant.
INTRODUCTION
The eye is an organ of the visual system and a structure that can reflect the symptoms of diseases of the vascular and central nervous systems. Therefore, all kinds of information obtained from the eye examination is very important.1
Saanen goats are known to originate from the Saanen Valley of Switzerland and have the largest breed characteristics among goat breeds in Switzerland. 2 Eye problems in goats can cause serious economic losses as with all farm animals.3 Ophthalmic studies in goats are generally encountered infrequently. However, goats are often preferred for practicing manipulative approaches to the eye in human ophthalmology.4
Imaging of the fundus during the ophthalmological examination of the eye allows the evaluation and diagnosis of many diseases. However, when the normal anatomical components of the ocular fundus are not fully known, it is impossible to fully evaluate the pathological changes.5
Corresponding Author: Bulut Osman (obulut@mu.edu.tr).
Traditionally, eye examinations are performed by using an ophthalmoscope. Indirect ophthalmoscopy is also used to visualize the fundus and posterior segment structures such as optic disc, retina, tapetal and nontapetal regions, retinal vessels and choroidea.6 Anatomical and pathological information about these structures can be accessed by imaging the fundus, and the animal can be identified simultaneously.7,8 For this purpose, various fundus imaging techniques can be used to reveal data on these structures following dilation of the pupil with mydriatics.9 Today, ultrasonography, fluorescein angiography and special camera systems are also used for this purpose.10 The structure of the fundus can vary between species, as well as between races within the same species. No studies were found on fundus imaging of Saanen goats among the ophthalmological studies conducted in goats. Therefore, our study aimed to obtain the normal reference values of the fundus of Saanen goats and to contribute to the literature.
MATERIAL AND METHODS
The material of the study consisted of 54 healthy adult female
O.Bulutetal.LargeAnimalReview2023;29:141-145141
Saanen goats. No ophthalmic abnormalities were present. Preclinical examinations of all goats included in the study were performed before the procedure. Body temperatures were measured, heart and respiratory sounds were listened to in order to determine any abnormalities. Goats with normal physiological values were included in the study and then ophthalmological examinations were carried out. Reflex examinations such as pupillary light reflex, palpebral reflex, menace reflex and dazzle reflex were performed. As a result of the reflex examinations, reflexes were scored as present/weak/absent. The eyelids, conjunctiva, cornea, iris and lens were examined under direct ophthalmoscopy to determine if there were any eye problems. Tear production and intraocular pressure were assessed. Tear production values (10.2±3.4 mm/min) and intraocular pressure (16.4±5.75 mmHg) were found. And these finding were considered normal. All healthy goats were included in the fundus examination as part of the study.
Inspections of all goats was carried out in a semi-dark environment. For this purpose, the goats were brought to the examination area five minutes before the procedure, so that their eyes could become accustomed to the environment and the goats could be calmed down. Sedation and local or parenteral anesthesia were not administered to the goats. In addition, local midratics were not administered to any of the goats before or during the procedure.
A ClearView Fundus (Kruuse / Langeskov - Denmark) camera was used by holding to the goats’ pupil, and both eyes were examined. Fundus examination in goats was performed while the animals were standing with their heads held in a natural position to maintain the natural position of the eyeball. The images were then transferred and recorded in a computer environment. Following the adaptation of the goats to the semi-dark environment, the examination was started without general or local anesthesia and any mydriatic drops. The camera was held at a 90-degree angle to the horizontal plane of the goat’s eyes while they were standing. The camera lens was approximately 1 cm away from the cornea. This position was attempted to be maintained in all fundus examinations performed in goats. Fundus images were taken while the optic disc located in the center (Figure 1). In addition, this position contributed to the accurate visualization and interpretation of the retinal vascular system. The color of the tapetal area during fundoscopy was evaluated on the images in which the optic disc was fully visible in order to prevent different reflections of light. Data on the tapetal region, optic disc in the nontapetal region, retina and retinal ves-
sels were obtained.
The pupils of the goats were observed to be rectangular shape and extended horizontally. Fundus findings in goats were analyzed, such as tapetal region, nontapetal region and optic disc. Additionally, differences in the retinal vessels were revealed. The color, shape, and homogeneity of the tapetal region, the border of the tapetal-nontapetal region, the color of the nontapetal region, the border, location and characteristics of the optic disc, and retinal vessels were determined.
GraphPad Prism7 (Carlsbad, CA, USA) was used to compare the significance levels between fundus differences amongst goats. The chi-square method conducted for evaluation of data with an accepted significance level as p<0.05.

RESULTS
All goats’ tapetal regions contained predominantly blue or green regions, however, green (81.48%) was observed most frequently. Apart from this, there was 3 goats (5.55%) with green-orange, green-blue and purple-blue tapetal region color. The pigment ratio in the tapetal regions was more intense in the temporonasal region.
The nontapetal region was predominantly dark brown and heterogeneous due to the pigment density in 51 goats (94.44%), green-brown in 2 goats (3.70%), and blue in 1 goat (1.85%).
The optic disc was located at the border of the tapetal and nontapetal regions, mostly in the tapetal region. The form of the optic disc was oval and round. The optic disc structure was different in the right and left eyes of 5 goats (9.25%). The optic disc was mostly light orange (82.40%), while in some goats it appeared in a faint orange contrast (15.74%) due to differences in the homogeneity of the papillae. Brown areas were observed in the optic disc of one eye of 2 goats (3.70%) (Table 1). The optic disc was partially or completely surrounded by oval-shaped, pigmented rings. Oval pigmented Bergmeister papillae have been found in goats due to myelination of the optic nerve head. Due to the myelination of the disc, orange or grayish pigment rings were evident in the temporal and medial regions of the optic disc. It was accepted that the remnant of the hyaloid vessel appears frequently in congenital vascular anomalies in goat and sheep.
A gray spot in the center of the disc represents the remnant of the hyaloid artery or Bergmeister’s papillae. It was observed that these papillae created bumps that resembled fingers on the optic nerve head and their color changed depending on the degree of vascularization. In the study, Bergmeister papillae were found in 29 goats (53.70%). In 10 of these goats, it was found in a single eye (18.51%) (Figure 2). In 15 animals (27.77%), Bergmeister papillae could not be observed in either eyes (Table 2).
The tapetal fundus in goats had a mild uniform stippling at the end of the capillaries called stars of Winslow. Winslow’s stars surrounding the optic disc were usually a pale, yellowish color (Figure 3). While Winslow’s stars were observed in 35 animals (64.81%) in total, they were found in only one eye in 7 goats (12.96%). In 12 goats (22.22%), Winslow’s stars could not be detected in either eyes (Table 2).
Dominant retinal vessels were visible in all goats. The vascular structure in the retina was homogeneous. Vascular patent was similar to other ruminates. Three types of vessels emerging from the optic disc were observed; the first of these was the veins which
2ImagesofNormalOcularFundusinSaanenGoats
Figure 1 -FundoscopyviaClearViewfunduscamera.Opticdisc canbeseenonscreenatthecenterofthefundusimage.
were the largest in diameter and the darkest in color. The second one was determined to be arteries, with a lighter color and smaller diameter compared to the veins, and originates more peripherally on the disc. The third vessel was determined as capillaries, which have a much smaller diameter than arteries and veins. The capillaries originate from the disc and disappear 1 or 2 disc diameters distance away from the disc (Figure 4). The measurements in these vessels were evaluated visually.
Generally, the numbers of veins, arteries and capillaries displayed in the optic disc area varies in animals. Indeed, a total of 3-6 retinal arteries and 2-5 retinal veins were found in goats. We observed that most of the goats had 4 retinal arteries (38.8%). This was followed by 3, 5 and 6 arteries, respectively. Additionally, considering the number of retinal veins, goats with 2 retinal veins were predominant (63.8%). This was followed by 3, 5 and 4 veins. In a total of 51 eyes (47.22%), the number of arteries extending dorsally was higher than those extending ventrally. In 14 eyes (12.96%), the number of ventral arteries was higher. In 43 eyes (40.95%), the numbers of dorsal and ventral arteries were equal.

epithelium.

O.Bulutetal.LargeAnimalReview2023;29:141-145143
Tapetal region color Green Blue Green-Orange Purple-Blue Green-Blue 88(81.48%) 14(12.96%) 2(1.85%) 2(1.85%) 2(1.85%) 44(81.48%) 7(12.96%) 1(1.85%) 1(1.85%) 1(1.85%) 0.010 Nontapetal region color Brown Green-Brown Blue-Brown 102(94.44%) 4(3.70%) 2(1.85%) 51(94.44%) 2(3.70%) 1(1.85%) 0.012 Optic disc morphology Oval Round Oval-Round 69(63.88%) 39(36.11%) 0(0%) 32(59.25%) 17(31.48%) 5(9.25%) 0.064 Optical disc color Orange PaleOrange Brown-Orange Orange-LightOrange 89(82.40%) 17(15.74%) 2(1.85%) 0(0%) 0(0%) 41(75.92%) 6(11.11%) 2(3.70%) 5(9.25%) 0(0%) 0.078 Bergmeister papillae Unilateral Bergmeister papillae 68(62.96%) 0(0%) 29(53.70%) 10(18.51%) 0.036 Winslow’s stars Unilateral Winslow’s stars 77(71.29%) 0(0%) 35(64.81%) 7(12.96%) 0.028 Number of eyes (n=108)Number of animals (n=54) p-value
Table 1 -Fundusdifferencesamonggoats.
Figure 2 -Bergmeisterpapillaeprojectsfromthecentralportionof theopticdiscintothevitreousbody.
Figure 3 -Winslow’sstarsthatsurroundedbyayellowisharea(arrows),showdifferentcolor,sizeandmorphology.Winslow’sstars arevisiblethroughoutthetapetalfundusandrepresentend-onchoroidalcapillariesextendingvitreadthroughtheretinalpigment
While the number of veins extending towards the dorsally was high in 34 eyes (31.48%), the number of veins towards the ventrally was high in 5 eyes (4.62%). In 69 animals (57.40%), the number of veins extending both dorsally and ventrally were equal. The number of arteries in both eyes was equal in 23 (42.59%) of the goats and the number of veins was equal in 28 (51.85%) (Table 2). It was observed that the vessels extended to the middle part of the optic disc. No signs of diseases such as active chorioretinal disease, retinal detachment, or scarring were found in any of the goats. It was determined that the ophthalmoscopic fundus images of goats were different from those of cattle and sheep.
There’s no significance between optic disc morphologies (p>0.05). The most frequency amongst tapetal region color is green (44) and brown (51) is most frequent color amongst nontapetal region. There is also small significance (p 0,036) between Bergmeister papillae- unilateral Bergmeister papillae and Winslow’s stars- unilateral Winslow’s stars.
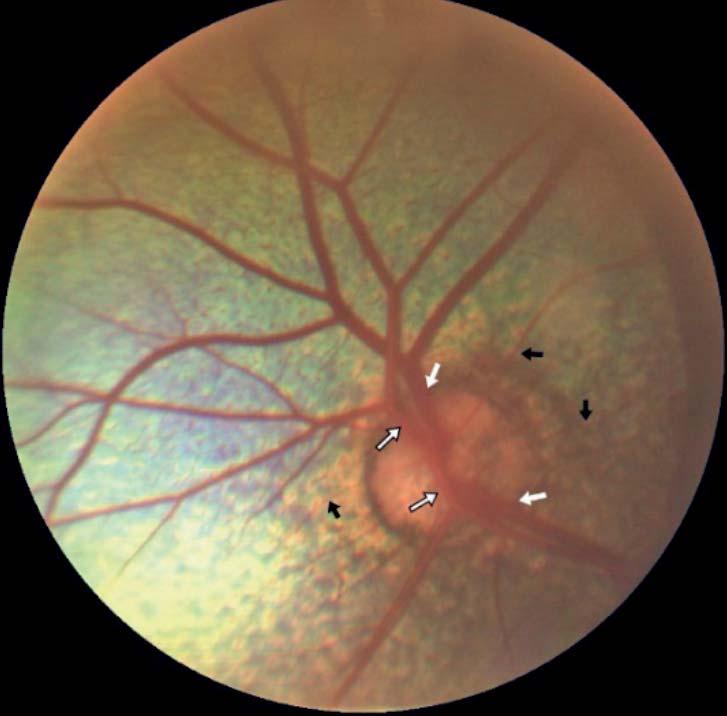
DISCUSSION
There are several methods of evaluating the posterior segment,
such as ocular ultrasonography, retinal optical coherence tomography, fluorescein angiography, fundus photography and direct and indirect ophthalmoscopy. Whereas direct and indirect ophthalmoscopy and fundus photography are often used for fundus imaging.9,11
Fundus structures may differ between animals and between species. Currently, no study has been conducted to reveal ophthalmological data using fundus imaging in Saanen goats. Our study aimed to obtain the normal reference values of the fundus of Saanen goats and to contribute to the literature. In the presented study, a ClearView fundus camera was used to take fundus images of the posterior segment of a total of 54 Saanen goats.
In the presented study, no mydriatic drug was applied locally to the eye. Some structures of the fundus were evaluated, likely due to the rectangular shape of the pupils of goats which allows for examination. Winslow’s stars could not be detected in both eyes of all goats. It is thought that the differences in the imaging angle and the lack of mydriatic medication during the fundus images caused the Winslow’s stars not to be seen sometimes. 10 The different angles of the images taken during fundoscopy were considered as an individual error. It is necessary to look at different angles to view the entire fundus, and it was thought that the shape of the pupil may have influenced these angles. The lack of visualization of structures such as Winslow’s stars in some animals is thought to be due to the insufficient mydriasis. 10
The most dominant color of the tapetal regions of goats was determined as green. While green tapetal color was dominant in 44 goats (81.48%), blue tapetal color was observed in 7 goats (12.96%). Galán et al. 10 reported that the dominant tapetal color in goats is blue, and Sengoz Sirin 12 found a higher rate of mixed tapetal colors, including yellow, blue and green.
The nontapetal region was predominantly dark brown in 51 goats (94,44%) and was heterogeneous due to the pigment density. However, Galán et al. 10 and Sengoz Sirin 12 reported that nonpigmented nontapetal regions are more dominant.
The location of the optic disc was mostly observed in the tapetal region, and less at the border of the tapetal-nontapetal region.
While Rambabu and Ramani 11 detected the optic disc in the tapetal region in goats, Galán et al. 10 and Sengoz Sirin 12 observed the optic disc at the tapetal-nontapetal region border in goats. Aksoy et al. 13 found the optic disc mostly in the tapetal region in dogs, while Shinozaki et al 14 found the optic disc is in the ventral of the tapetal region in horses. In our study, optic disc localization was observed at the border of the tapetalnontapetal region similar to the studies of Galán et al. 10 and Sengoz Sirin 12 .
144ImagesofNormalOcularFundusinSaanenGoats
Number of Arteries 3 4 5 6 37(34.25%) 42(38.88%) 26(24.07%) 3(2.85%) 2/1=261/2=11 2/2=413/1=1 3/2=232/3=3 4/2=13/3=2 Number of Veins 2 3 4 5 69(63.88%) 36(33.33%) 1(0.92%) 2(1.85%) 1/1=69 2/1=311/2=5 3/1=1 3/2=2 Number of eyes (n=108)Dorsal/Ventral ratio
Table 2 -Numberofvesselsdeterminedingoats.
Figure 4 -Whitearrows:Veins.Black-whitearrows:Arteries.Black arrows:Smallvessels.Theveinsarelargerthanarteriesanddarkest.Thevesselshavethesmallestdiameterandoriginatefromthe opticdisc.
The shape of the optic disc was found as horizontal in deer 1 , oval in dogs and horses 13, 15. In other studies conducted in goats, Broadwater et al. 4 found the optic disc to be oval, Rambabu and Ramani 11 found it to be round, Sengoz Sirin 12 and Ledbetter and Gilger 16 found it to be oval and round. In our study, the optic disc was observed in both oval and round forms in goats, and the dominant form was oval (63.88%). Additionally, in 5 goats (9.25%), it was observed that the form and shape differed between in the right and left eyes. The color of the optic disc was found to be orange light (82.40%) as stated by Galán et al. 10 and Sengoz Sirin 12 .
Oval pigmented Bergmeister papillae have been found in goats due to myelination of the optic nerve head. It has been observed that these papillae create finger-like bumps on the optic nerve head and appear in varying colors depending on the degree of vascularization. While Bergmeister papillae were observed in 29 animals (53.70%) in total, it was not found in one eye of 10 animals (18.51%) and in both eyes of 15 animals (27.77%). Galán et al. 10 and Sengoz Sirin 12 also detected Bergmeister papillae in their study in goats and these results consistent with our study.
Nasisse 17 suggested that Winslow’s stars are red only when the tapetal region is yellow in their study of foals. Ledbetter and Gilger 16, stated that the color of Winslow’s stars is independent of the color of the tapetal region. In the present study, Winslow’s stars surrounding the optic disc were seen bilaterally (64.81%) in 35 goats and unilaterally in 7 goats (12.96%), appearing as light yellowish in color. In 12 goats (22.22%), Winslow’s stars could not be detected in both eyes because of the mydriatic was not applied. Galán et al. 10 and Sengoz Sirin 12 stated that the color of Winslow’s stars is independent of the color of the tapetal region and that the yellowish area around the optic disc may originate from capillaries that cross the tapetum. In this study, it has been determined that the Winslow’s stars can appear in different colors and shapes.
In goats, three recognizable retinal vessels originating from the optic disc were observed. These are arteries, veins and capillaries. In our study, it was observed that the number of arteries ranged from 3 to 6 and the number of veins ranged from 2 to 5. It was observed that the arteries were mostly extended dorsally (47.22%), while the veins were mostly located equally in both the dorsal and ventral directions (57.40%). Sengoz Sirin 12 detected 3-6 arteries and 5-8 veins. Galán et al. 10 found3-6 arteries and 2-3 veins in goats. They determined the ratio of the number of arteries extending to the dorsal area to be 1/3. Also they found the ratio of the number of veins to the dorsal and ventral areas to be half.
CONCLUSION
In conclusion, this study of 54 Saanen goats provides valuable information on ophthalmic examination findings in these animals. No signs of diseases such as active chorioretinal disease, retinal detachment, and scarring were found in any of the goats. It was determined that the ophthalmoscopic fundus images of goats were distinct from those of cattle and sheep. The limitations of this study were that lack of mydriatic usage and the angles of imaging affected the images. However, the pupillary structure of goats allowed examination without the use of mydriatics. Examination was possible without the mydriatic, but this limited the visibility of the Winslow’s stars. The
angle of the fundus camera may cause color differences in the tapetal area; however, these differences were minimized as the color of the tapetal area was evaluated in the images where the optic disc was in the center.
Ocular fundus images results provide normal means and ranges to help identify clinical cases of ophthalmic disease in Saanen goats
Conflict of Interest Statement
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Ethical Approval
The ethical approval of the study was provided by the University’s Institutional Animal Care and Use Committee (approval number:64583101/2021/121). In this study, a signed information confirmation form was obtained from the patient owners.
Funding Information
No funding was received to assist with the preparation of this manuscript.
References
1.Dukes T.W. (1969). The Ocular Fundus of Normal White Tailed Deer (Odocoileus virginianus). J Wildl Dis, 5: 16-17.
2.Shelton M. (1978). Reproduction and breeding of goats. Int J Dairy Sci, 61: 994-1010.
3.Rajathi S. (2021). Morphological and Morphometrical Studies of Eyeball and Retina in Goats. IJVA, 33: 17-19.
4.Broadwater J.J., Schorling J.J., Herring I.P., Pickett J.P. (2007). Ophthalmic Examination Findings in Adult Pygmy Goats (Capra hicus). Vet Ophthalmol, 10: 269-273.
5.Sini K.R., Kelawala D.N., Patil D.B., Parikh P.V., Parulekar E.A. (2016). Ocular Funduscopy of Different Canine Breeds in ndia - A Clinical Study of 70 Normal Dogs. Intas Polivet, 17: 328-333.
6.Kanemaki N., Inaniwa M., Terakado K., Kawarai S., Ichikawa Y. (2017). Fundus Photography with A Smartphone in Indirect Ophthalmoscopy in Dogs and Cats. Vet Ophthalmol, 20: 280-284.
7.Alina D., Muste A., Beteg F., Briciu R. (2008). Morphological Aspect of Tapetum Lucidum at Some Domestic Animals. Vet Med, 65: 1843-5378.
8.Rojas-Olivares M.A., Caja G., Carné S., Salama A.A.K., Adell N., Puig P. (2012). Determining the optimal age for recording the retinal vascular pattern image of lambs. J Anim Sci, 90: 1040-1046.
9.Barron U.G., Corkery G., Barry B., Butler F., McDonnell K., Ward S. (2008) Assessment of retinal recognition technology as a biometric method for sheep identification. Comput Electron Agric 60: 156-166.
10.Galán A., Martín-Suárez E.M., Granados M.M., Gallardo J.M., Molleda J.M. (2006). Comparative fluorescein angiography of the normal sheep and goat ocular fundi. Vet Ophthalmol, 9: 7-15.
11.Rambabu K., Ramani C. (2017). Normal Ocular Fundus Imaging of Domesic Goat (Capra hircus). Intas Polivet, 18: 509-510.
12.Sengoz Sirin O. (2020). Normal Ocular Fundus Imaging with Smartphone Ophthalmoscope in Honamlı Goat Breed. Dicle Üniv Vet Fak Derg, 13: 65-69.
13.Aksoy O., Gungor E., Kirmizibayrak T., Saroglu M., Ozaydin I., Yayla S. (2011). Identification of Normal Retina’s Variations in Kars Shepherd Dogs via Fundoscopic Examination. Kafkas Univ Vet Fak Derg, 17: 167170.
14.Shinozaki A., Takagi S., Hosaka Y.Z, Uehara M. (2013). The Fibrous Tapetum of The Horse Eye. J Anat, 223: 509-518.
15.Barnett K.C. (1972) The Ocular Fundus of The Horse. Equine Vet J, 4: 17-20.
16.Ledbetter E.C., Gilger B.C. (2013). Diseases and surgery of the canine cornea and sclera. In: Veterinary ophthalmology, Ed. Gelatt K.N., Gilger B.C., Kern T.J., 976-1049, Oxford Wiley-Blackwell.
17.Nasisse M.P. (1991). Feline ophthalmology. In: Veterinary Ophthalmology, Ed. Gelatt K.N. 529-575, Lea & Febiger, Philadelphia.
O.Bulutetal.LargeAnimalReview2023;29:141-145145
GUIDELINES FOR AUTHORS LAR
Large Animal Review
Large Animal Review is a bimonthly magazine published by SIVAR (Italian Society of Farm Animals Veterinary Practitioners) for scientific updating of veterinarians who deal with animals in livestock production and the supply chain control in the production of food industry. The topics of main interest for the journal are those of internal medicine, surgery, obstetrics, animal nutrition, zootechnics, infectious and parasitic diseases, food safety and security, animal welfare, prevention and management.
■ MANUSCRIPTS
Large Animal Review publishes manuscripts in the form of reviews, original articles and case reports; manuscripts must comply with the guidelines below.
Review - This is a complete coverage of a specific topic accompanied by a detailed and updated bibliography. Authors interested in writing a review should contact the editor of Large Animal Review. The text should not have more than 48.000 characters (including spaces) and not be accompanied by more than 15 figures or tables.
Original Article - The papers published in Large Animal Review are short or full-length research articles related to the topic of the journal. The full text article should not exceed 32.000 characters (including spaces) and should not be accompanied by more than 10 figures or tables. Manuscripts in the form of short articles should not exceed 16.000 characters and no more than 4 figures or tables.
Case Report - Single clinical or herd case report should be presented in Large Animal Review. The manuscript must not exceed 10.000 characters (including spaces) and no more than 4 figures or tables.
■ FORMAT
All manuscripts (review, original articles and case reports) must have the following structure.
Language - English or Italian.
Title - The title of the manuscript has to be short and explicative and written on the front of the first page. Under the title, names of authors should be given indicating the surname and the name (e.g., Smith Tom). Institutional addresses are displayed below the author names; footnotes referring from author names to displayed addresses should be numbered. The full name, mailing address, phone number, and e-mail address of the corresponding author should appear directly below the affiliation lines on the title page. The corresponding author will be identified by a symbol footnote (e.g., Smith Tom*) and e-mail address below the affiliation lines on the first page of the published article (e.g., *Corresponding author: Smith Tom, University of …).
Abstract - Abstract has to be placed on the second page of the manuscript and written in English. Abstracts should be limited from 300 to 500 words. The abstract disseminates scientific information through abstracting journals and is a convenience for readers. Exclude references, figure or table.
Official scientific journal of SIVAR
Key Words - After the abstract, list 3 to 5 key words have to be placed. In case of manuscripts written in Italian, the key words have also be translated into English.
Body of the Paper - The manuscript of the original articles must show the following outline: introduction, materials and methods, results, discussion, conclusions, acknowledgments and bibliography. Regarding the reviews, the outline is not expected, but the topic must be clearly argued and divided in chapters.
The text should be typed in Microsoft Word preferably, or OpenOffice or Rich Text Format, with lines and pages numbered consecutively, using Times New Roman font at 12 points. All margins should be at least 2 centimeters, single spacing and cannot exceed the number of characters (including spaces) indicated in the previous section for each type of manuscript.
Tables and Figures - Tables, graphs and images must be included in the manuscript text and numbered (Arabic numerals). The titles or captions should describe concisely the data shown, sufficiently detailed and comprehensible to the reader. Tables and figures should be placed in separate sections at the end of the manuscript.
References - The references must be selected by the authors (not more than 30, except in a review) and should be cited in the text with a serial number in round brackets and listed in the same numerical order in the bibliography.
For articles from journals you should indicate: surname and first initial of the author and co-author/s names, year of publication, article title, abbreviated indication of the magazine (Index Medicus), volume number, number of first and last pages. For citations to articles or chapters contained in textbooks, you should indicate: surname and first initial of the Author and co-author/s names, year of publication, chapter’s title, book title, volume number (if more than one volume) editors, edition, first and last page of the chapter, publishing house and its location.
Examples:
– Journals - Galey F.D., Terra R., Walker R., Adaska J., Etchebarne M.A., Puschner B., Fisher E., Whitlock R.H., Rocke T., Willoughby D., Tor E. (2000). Type C botulism in dairy cattle from feed contamined with a dead cat. J Vet Diagn Invest, 12: 204-209.
– Books - Gustafson D.P. (1986). Pseudorabies. In: Diseases of swine, Ed. Dunn H.W., 5th ed., 274-289, Iowa State University Press, Ames, IA.
– Conferences - Barbano D. M. (1996). Mozzarella cheese yield: Factors to consider. Page 29 in Proc. Wisconsin Cheese Makers Mtg., Madison. Ctr. Dairy Res., Univ. Wisconsin, Madison.
■ SUBMISSION OF MANUSCRIPTS
The manuscripts have to be submitted exclusively on the following link: www.largeanimalreview.com
Informations: Dr. Enrico Fiore - Technical Editor largeanimalreview@sivarnet.it
- I TALIAN A SSOCIATIONOF F ARM A NIMAL P RACTITIONERS Centro Studi EV - Cremona (I) - Tel. +39 0372 403539 - info@sivarnet.it - www.sivarnet.it
The Effect of Iodine Drenching During Late Pregnancy on Thyroid Hormones and Biochemical Parameters of Black Goats and Their Kids Performance
HAWAR M.H. ZEBARI* AND HARBI S. SALIH
DepartmentofFoodScienceandTechnology,CollegeofAgriculturalEngineeringSciences,Universityof Duhok,ZakhoStreet38,42001DuhokCity,KurdistanRegion,Iraq
SUMMARY
The objective of the current experiment was to evaluate the influence of Iodine drenching during late pregnancy on thyroid hormones and biochemical parameters in Black goats and their kids performance. Pregnant black goats (n=24) were used in this study. Goats were drenched with potassium iodide (KI) based on 76% of Iodine with 0 mg KI/day (control; C), 0.50 mg KI/day (treatment one; T1) as low iodine group and 1.00 mg KI/day (treatment two; T2) as high iodine group. Blood was collected weekly from goats at week six before parturition and to parturition and 1st week of age from kids. Serum was separated from blood samples and analyzed for serum content of iodine, thyroid stimulating hormone (TSH), triiodothyronine (T3) and thyroxine (T4) and biochemical parameters of goats and their kids. The concentration of TSH was higher (p<0.019) in C group. Both T3 and T4 concentration were higher (p<0.001) in T1 and T2. There was no effect of KI drenching on TSH, T3 and T4 in kids. Total protein and glucose were significantly higher in T2 group compared with C group, while triglyceride was significantly more in C group compared with T1 and T2 groups. Higher level of glucose was recorded in T2 group, while higher level of cholesterol was recorded in C group of kids compared with other groups. Kids body weight were higher (P<0.001) in T2 groups compared with T1 and groups C. In conclusion, in response to KI drenching TSH level declined in T1 and T2, while remained higher in C group of late pregnant goats. Iodine drenching can improve total protein and glucose and also regulate T3 and T4 in the goat. Iodine drenching of pregnant goat can also increase new born kids body weight.
KEY WORDS
Iodine drenching; Late pregnancy; Black goats; Milk; Biochemical.
INTRODUCTION
Iodine is one of the essential components of thyroidal hormones for the synthesis of the thyroid hormones thyroxine (T4) and triiodothyronine (T3; Nudda et al., 2009) and through which it is involved in a number of biological functions of the animals (Dušová et al., 2014). There exists a great deal of evidence that it is the free or unbound portion of the circulating thyroid hormone that is accessible to the tissues of the animal body and its effect on their metabolism and functions (Todini, 2007). The T4 and T3 hormones contribute to the maintenance of protein and energetic metabolism homeostasis in the animal body; they influence body growth, thermoregulation and biochemical metabolites (Huszenicza et al., 2002). Low iodine concentrations in livestock feeds of high altitude area causes a longterm deficiency of iodine in traditional animal farms and their new born kids’ performance (Dušová et al., 2014). The deficiency of I is more prevalent primarily in high moun-
tain regions and the area that were covered with Ice of the world such as the Himalayas mountain (Nyström et al., 2016; Pachuri, 1981), the European Alps, and the Andes, this may be due to the fact that the I has been washed away by glaciations, soil erosion and flooding as a result of high raining levels (Mercer, 2006). A deficiency of iodine also leads to hypothyroidism, which causes reduced immunity and inhibits physiological status of animal such as an effect on milk components, hormones and blood biochemical parameters (Sokkar et al., 2000; Nudda et al., 2009). Iodine deficiency has a significant impact on the unborn’ mental and physical development; severe I deficits can lead to cretinism (Hetzel, 1983). As a result of insufficient food availability during late pregnancy and minerals such as I, does may lose weight, in active immunity (Osuagwuh, 1992), low growth rate and reproductive consumption such as an increase in the rat of abortion and neonatal mortality due to low birth weight of kid (Cappai et al., 2019).
The iodine requirement is usually higher in goats than in other ruminant animals: a dietary iodine concentration considered inadequate for goats (Meschy, 2000; Oramari et al., 2014). Due to its browsing behavior and lower soil consumption compared with other grazing animals, the goat is regarded
H.M.H.Zebarietal.LargeAnimalReview2023;29:147-154147
Corresponding Author: Hawar M. H. Zebari (hawar.mikahil@uod.ac).
j
as an indicator species of I deficiency (Smith and Sherman, 2009) because goats as browsers breeds are preferring to eat leaves, twigs, vines and shrubs (Lovreglio et al., 2014). Even though the use of an iodine supplement in goats should be a common practice, a large database on iodine requirements in goats is not available. In accordance with NRC (2007) the recommended concentration of dietary iodine is 0.5 mg/kg of DM of the diet in growing and non-lactating goats and 0.8 mg/kg of DM for goats; however, these recommendations are based on a limited number of data base (Nudda et al., 2009). Black goats are mainly raised as a double purpose animal for meat and milk, in addition to secondary importance for hair production (Zebari et al., 2013; Juma and Al Kass, 2005). There are limited published study on the effect of Iodine drenching on black goats and their new born kids raised under traditional conditions. Therefore, the objective of the present study was to evaluate the effect of Iodine drenching during late pregnancy on thyroid hormones and serum biochemical parameters in black goats and their new born kids performance.
MATERIALS AND METHODS
The present study was conducted between 15th of November 2021 and 20th of January 2022 at one of the traditional goat farms, Zawita, Duhok, Kurdistan Region-Iraq. The Research Ethics Committee of Animal Production Department, College of Agricultural Engineering Sciences, University of Duhok approved the research protocol.
Experimental animal, housing and management
Pregnant black goats (N=24) with body weight (34.81±2.4kg) and ages (3.15±1.2 years) were used in the present study from six weeks before and one week after parturition at one of the traditional goat farm, Zawita, Duhok, Kurdistan Region-Iraq. At the start of the study, the goats were submitted for detection of any disease. The goats were kept with the main flock. The goats were put out to graze during the day from 06:00 am to 06:00 pm and housed in a free stall yard during the night. During housing, total mixed ration (TMR; hay, barley and wheat barn) was provided daily, sufficient for ad libitum availability. Nutrient content of the total mixed ration were Dry matter (DM) (88.79%), CP (11.16% DM), CF (23.4% DM), Fat (2.1% DM), NFE (46.34% DM), ash (5.79% DM), ME (2196.91 kcal/kg) and moisture (11.21%). Water was also provided ad libitum from water troughs at the free stall yard.
Pregnancy Diagnoses and Treatments
At the start of the study, pregnant goats were selected from the main herd using Veterinary Ultrasound Scanner (CD66V; Zhuhai Carellfe Medieal Technology Co., Ltd, China). The goats were randomly divided into three experimental homogeneous groups (n=8 per group) on the basis of live body weight (35.66±2.1 kg), (34.66±2.1 kg) and (34.11±2.1 kg). Each goat was supplemented with potassium iodine (KI; Chem-Lab NV, Industriezone, B-8210 Zedelgem, Belgium) with 0 mg I/day (Control), 0.50 mg I/day (Treatment one; T1) as low iodine group and 1.00 mg I/day (Treatment two; T2) as high iodine group. The dose of KI was dissolved in water and then orally drenched daily each goat at 4:00 pm using a manual syringe gun six weeks before parturition
(late pregnancy period) until parturition.
Blood Collection and analytical techniques
Blood samples were collected from goats weekly from week six (6±0.7 SD) pre-partum. Blood collected from goats by jugular venipuncture using a 20 G needle syringe into 10ml vacuutainer tubes (Medicalet, Pingshan New District, Shenzhen City 518118, China). Blood samples were also taken from their new born kids (n=26) with a body weight of (4.25±0.6 kg). Blood samples were centrifuged at 6000g for 12 min using a SIGMA centrifuge (SIGMA Osterode am Harz, Germany). Serum was separated and stored at -20°C until analyzed for hormonal and biochemical parameters analysis. Serum T3, T4 and TSH and biochemical parameters (total protein, glucose, cholesterol, triglyceride, HDL and LDL) of goats and their newborn kids were analyzed by cobas 6000 (Hitachi High-Technology Corporation, Tokyo, Japan).
STATISTICAL ANALYSES OF DATA
The data were statistically analysed using Genstat statistical analysis software package (Genstat V 14th.19.1.14713 provided by VSN International Ltd, UK). Repeated measures ANOVA was used to analyze the data of serum TSH, T3, T4 and T3/T4 ratio and biochemical parameters of goats and to compare between treatments. Factorial one-way ANOVA analysis were used to compare between the datasets of serum TSH, T3, T4 and T3/T4 ratio, body weight and biochemical parameters of the newborn kids. The comparison between C, T1 and T2 was analyzed by Tukey test. Differences were reported as significant at P<0.05 and trends were reported when P-values were between <0.1 and >0.05.
RESULTS AND DISCUSSIONS
Effect of KI drenching on Thyroid stimulating hormone (TSH) in goats
There was a significant influence of time (p<0.019) on serum TSH concentration in goats during the study period (Figure 1). There was a significant increase in TSH from week three of treatment (W-4 before parturition) in groups C with progress in pregnancy period. These results are in constant with previous studies conducted by Bhardwaj (2018) and Singh et al. (2002). There was also a significant impact (p<0.018) of KI supplementation on serum TSH concentration. Significantly higher TSH (mean±SEM) concentration was recorded in C group (0.093±0.02 IU/mL) compared with T1 (0.062±0.01 IU/mL) and T2 (0.033±0.01 IU/mL) group of goats. The results of the present study agree with those reported by Davoodi et al. (2022) who found higher serum TSH levels in the iodine deficiency group of goats compared with the treated group of goats. Previously, it has been studied levels of TSH in the goats with hypothyroidism by Kadum and Luaibi (2017) and observed that levels of TSH were significantly lower in iodide treated group compared with control group. The level of TSH concentrations has been used as an indicator that reflects the deficiency of the dietary iodine intake by animals, with higher concentrations of TSH being revealing of lower iodine intake. While there was time and treatment interaction effect (p=431) on TSH levels.
148TheEffectofIodineDrenchingDuringLatePregnancyonThyroidHormones
The higher concentration of TSH in groups C may be due to that the iodine deficiency in the body leads to the overproduction of the TSH from the pituitary gland and the attempt of the thyroid to compensate for the deficiency in the thyroid hormones (Singh et al., 2002; Davoodi et al., 2022). Inadequate amount of I in the thyroid gland lead to production of un-iodinated inactive prehormone compound instead to T4 which results the stimulation of pituitary gland to secret more TSH (Bhardwaj, 2018).
Effect of KI drenching on T3 and T4 hormone and T3/T4 ratio in goats
The data of serum T3 and T4 concentration and T3/T4 ratio are reported in Table 1. Regarding serum T3 concentration, there was a significant impact (p<0.001) of time on serum T3 hormone concentration during late pregnancy period of goats. There was an increase in serum T3 hormone level in both T1 and T2 groups during experiment progress. Similarly, an increase in T3 levels was found with progress in weeks of sampling in wool goats fed supplemented with Iodine (Pattanaik et al., 2004). However, in contrast to the present results Todini (2007) and Nudda et al. (2013) found a slightly decrease in T3 concentration with advanced sampling weeks in lactating goats. These may be due to that Todini (2007) and Nudda et al. (2013) used goats during the lactating period rather than pregnant goats. While there was a fluctuation in the level of serum T3 hormone in C group. There was also a significant effect (p=0.042) of KI treatment on the level of T3 hormone, serum T3 level was higher in T1 and T2 groups compared with

C group of goats. These results are agreed with those reported by Nudda et al. (2013) who found higher (p<0.001) T3 serum concentration in lactating goats fed diet with high iodine (0.90 mg of KI/day) compared with low iodine (0.0 mg of KI/day). Concerning the data related to serum T4 hormone, time had a significant effect (p<0.001) on the serum T4 hormone throughout the experiments period. There was also an effect (p=0.035) of KI drenching on the level of serum T4 hormone. Higher levels of serum T4 hormone were recorded in T2 group of goats that were supplemented with 1.0 mgKI/day compared with C group of goats. Similarly, Zarbalizadeh-Saed et al. (2020) found a higher (p<0.05) serum concentration of T4 in ewes supplemented with 0.4 mg of I per day compared with zero level of I supplementation. Nudda et al. (2013) also found a higher (p=0.059) serum concentration of T4 in lactating goats supplemented with KI compared with the control group. While, there was no significant different between T1 and T2 group of goats. Higher level of T4 concentration in T2 groups of goats received 1.0 mg/KI/day may be due to increased iodine titration for T4 synthesis in thyroid gland. There was no significant effect of time (p=0.398) and KI treatment on the T3/T4 ration in serum of goats during late pregnancy. There was also no impact of time and treatment interaction on the serum T3 (p=0.541) and T4 (p=0.803) hormone and T3/T4 ration (p=0.399) of goats during late pregnancy. These results are in constant with those reported by Zarbalizadeh-Saed et al. (2020) who observed no significant difference (p>0.05) in T3/T4 ratio between (0.0 mg I/d) and (0.4 mg I/d) supplemented to ewes during pregnant period.
Figure 1 -TheeffectofIdosesonserumthyroidstimulatinghormone(TSH)ofblacknativegoats.Tr.=treatment,T.=time,Tr.xT.=treatment andtimeinteraction,C=control(noIsupplementation;0mgZn/day),T1=Treatmentone(0.50mgI/day),T2=Treatmenttwo(0.1mgI/day),ErrorbarsindicateSEM.AB__Letterswithsuperscriptissignificantlyregardingtheeffectoftreatments.abc__Letterswithsuperscriptissignificantlyregardingtheeffectoftime/weekoftreatments.
H.M.H.Zebarietal.LargeAnimalReview2023;29:147-154149
ParametersTr.OverallTime/weeksP Value means-6-5-4-3-2-1Tr.T.Tr.
Tr.=treatment,T.=time,Tr.xT.=treatmentandtimeinteraction,T3=Triiodothyronine,T4=Thyroxin,C=control(noIsupplementation;0mgZn/day),T1=Treatmentone(0.50mgI/day),T2=Treatmenttwo(0.1mgI/day).Overallmeanswithdifferentsuperscriptlettersdiffer(p
However, Nudda et al. (2012) found a significantly higher (P<0.001) T3/T4 in lactating goats supplemented with KI compared with control group, this may be due to that Nudda et al. (2013) used goat during different physiological status.
Effect of KI drenching on serum biochemical parameters of black native goats
The effect of KI drenching on serum biochemical parameters are illustrated in Table 2. Potassium Iodine drenching had a significant effect (p=0.045) on serum total protein (g/dl). Significantly higher total protein was recorded in T2 group of goats compared with C group, while there were no differences between T2 and T1 groups. These results agree with those reported by Abozed et al. (2020) who found that the treated group of
ewes had a significantly higher concentration of serum total protein (6.43 ± 0.13 g/dl) compared with control group (5.65 ± 0.20 g/dl). Likewise, previous studies also found higher serum total protein in I supplemented group of sheep compared with the control group (Zeedan et al., 2010; Dušová et al., 2014). The higher levels of total protein in KI drenching groups of goats during late pregnancy may be due to that I supplementation increased the level of thyroid hormones both T3 and T4 hormones which in turn the thyroid hormones participate in the maintenance of the level of total protein (Huszenica et al., 2002). However, Pattanaik et al. (2011) found no significant effect of iodine (0.1 mg I/day) supplementation on total protein concentration (8.98 g/dl) in serum compared with control group (8.65 g/dl) of indigenous adult goats. This may be due to fact that in the present study were used goats that were in the late
Table 2 -Serumbiochemicalparameters(Means±SEM)inresponsetoPotassiumIodine(KI)drenchingfromweek-6toparturitioninblack nativegoats. SEM=standarderrorofmeans,C=control(0mgI/day),T1=treatmentone(0.5mgI/day),T2=treatmenttwo(1.0mgI/day),W=week,Tr.=treatmentsandT=time.Meanswithdifferentsuperscriptlettersinoverallmeanscolumnsdiffer(p<0.05).
150TheEffectofIodineDrenchingDuringLatePregnancyonThyroidHormones
Table 1 -TheeffectofKIdosesonserumT3andT4hormoneandT3/T4raioofblacknativegoats.
<0.05). T3 (nmol/L)C 2.3±0.2b2.4±0.32.7±0.22.2±0.42.3±0.52.4±0.42.0±0.4 T1 2.5±0.3a2.1±0.22.5±0.42.5±0.32.6±0.52.7±0.22.8±0.30.042<0.0010.541 T2 2.6±0.3a2.3±0.22.4±0.42.5±0.32.6±0.22.8±0.72.9±0.3 T4 (nmol/L)C 101.2±11.3b112.5±9.4105.4±13.894.3±9.9108.7±9.896.1±12.389.9±12.8 T1 106.1±11.8ab97.6±14.698.1±5.599.7±8.3110.4±33.8114.3±22.6116.3±10.00.035<0.0010.803 T2 114.4±12.3a100.2±12.8103.1±14.7114.1±12.9112.6±13.2127.7±21.2128.7±9.4 T3 and T4 (%)C 0.023±0.0020.021±0.0030.023±0.0030.021±0.0010.022±0.0030.024±0.0020.026±0.004 T1 0.023±0.0020.021±0.0040.023±0.0020.023±0.0030.023±0.0030.025±0.0020.025±0.0010.3980.3310.399 T2 0.23±0.0020.021±0.0020.023±0.0020.024±0.0020.024±0.0040.024±0.0020.022±0.003
x T.
Totalprotein(g/dl)C6.6±0.2b6.4±0.46.9±2.06.7±0.46.7±0.46.6±0.56.4±0.2 T17.0±0.2ab7.4±0.37.1±0.36.8±0.26.9±0.46.8±0.36.8±0.20.0480.3630.547 T27.4±0.2a7.7±0.47.4±0.57.5±0.27.4±0.17.3±0.27.2±0.3 Glucose(mg/dl)C56.2±1.8b58.2±1.956.3±3.356.5±4.154.5±2.957.8±5.753.7±2.8 T159.9±2.3a58.3±2.757.0±3.159.8±5.657.0±3.860.5±7.862±2.30.0370.1370.446 T260.6±1.7a61.7±5.958.2±3.261±3.359.5±1.260±6.061.2±4.1 Cholesterol(mg/dl)C85.5±9.370.5±10.983.8±20.281.2±9.786.8±6.991.2±14.899.2±8.1 T184.6±10.566±9.883.3±13.090.8±14.184.2±10.685.7±10.897.5±11.30.894<.0010.349 T281.8±9.066.5±12.584±14.879±7.181.2±9.486.8±12.093.2±8.6 Triglyceride(mg/dl)C24.0±6.3b15.3±6.919.3±13.521.2±9.227.2±9.030±4.430.8±16.7 T124.3±5.8b20.5±1.917.5±10.421.7±12.723.83±9.929±6.633±8.00.015<.0010.644 T231.6±6.5a26.8±12.625.3±5.829±5.429.7±13.736.7±15.942.3±9.6 HDL(mg/dl)C52.5±4.645.17±5.452.8±9.849.8±5.052.8±6.556.5±5.657.7±4.1 T152.0±4.645.33±6.050.2±5.557±7.849.2±5.653.7±4.356.8±5.00.172<.0010.623 T255.1±8.746.0±8.750.7±7.359.7±10.256.2±9.663.3±9.171.8±10.2 LDL(mg/dl)C30.1±5.220.7±3.929.7±7.730.7±3.030.7±7.232±6.436.5±7.7 T133.9±6.124±12.131.2±4.233.7±5.834.5±5.837.8±11.942±5.70.721<.0010.352 T234.4±6.727±8.032.3±14.331.3±11.932±4.737.5±4.446.3±3.0 ParametersTr.OverallTime/weeksP Value meansW-6W-5W-4W-3W-2W-1Tr.T.Tr. x T.
pregnancy period, while Pattanaik et al. (2011) used adult nonpregnant goats.
Glucose was significantly (p=0.037) higher in group T2 (60.6±1.7 g/dl) compared with group C (56.2±1.8 g/dl), while there was no significance different between T2 and T1 (59.9±2.3 g/dl) groups. Similar to the finding of the present study, it has been reported that the concentration of serum glucose increased with iodine supplementation. Zeedan et al. (2014) found that glucose concentration was significantly (p<0.05) higher with iodide supplementation in Buffalo. Furthermore, El-Salaam et al. (2018) reported higher glucose levels in supplemented KI of pregnant camels during pre and post-partum periods compared with control groups. An increase of serum concentration of total protein glucose and their fractions may be attributed to increasing thyroid hormones, which leads to stimulate the basal metabolic rate through regulation of the carbohydrates and proteins metabolism in the animal body (Lawrence and Fowler, 1997). Dandan Wang et al., (2021) reported a relationship between iodine status and blood glucose. There was also no significant effect of KI drenching on cholesterol (mg/dl). In contrast to the results of the present study, Pattanaik et al. (2011) reported a significantly lower serum cholesterol concentration in I supplemented group (149.04 mg/dL) compared with control group (132.55 mg/dL). However, the period of treatment of KI drenching had a significant effect (p<0.001) on cholesterol; the higher concentration of cholesterol was recorded in week -1 before parturition in group C. Kaneko (1997) reported a negative relationship between iodine (thyroid) status and serum cholesterol concentration.
Triglyceride (mg/dl) was higher (p=0.015) in T2 (31.6±6.5 mg/dl) in comparison to groups C and T1. Significantly higher concentrations of triglyceride were recorded in week1 before parturition (42.3±9.6 mg/dl) in group T2 compared with other weeks of other groups. Previously, similar to the present results, Xia et al. (2013) observed a significant increase in the serum triglyceride level in I drenching mice group compared with control group. Xia et al. (2013) also found a positive association by the correlation of the dose dependent increase of serum triglyceride content (r00.498, p<0.01) and serum triglyceride concentration in iodine-loaded groups. An increase in serum triglyceride may be due to that the liver playing a pivotal role in systemic lipid homeostasis (Xia et al., 2013). The increased triglyceride concentrations observed in the serum of goats drenched with high iodine (1.00 mg KI/d) group could result from the significant up-regulation of the sterol regulatory element-binding protein 1c (SREBP-1c) and its target gene fatty acid synthase (FAS) which are involved in the synthesis of triglycerides. The SREBP- 1c is one of the three isoforms of the SREBP family. SREBP- 1c is involved in the regulation of triglyceride metabolism in the liver as a transcription factor. It stimulates transcription of the genes associated with fatty acid biosynthesis, such as acetyl CoA carboxylase and FAS and plays a crucial role in the development of fatty liver (Ahmed, 2007; Shimano et al., 2013)


Serum HDL (mg/dl) was not significantly affected by KI drenching comparing the overall means. While the period of KI drenching had a significant (p<0.001) effect on HDL concentration, the higher concentration of HDL in serum was recorded in week -1 before parturition in group T2 (71.8±10.2 mg/dl) in comparison to other weeks of different groups of the studied goats. There was also no significant effect of KI drenching on LDL concentration (mg/dl) comparing the overall means. The sig-
nificantly higher serum LDL was recorded at week -1 (42±5.7 mg/dl) and (46.3±3.0 mg/dl) before parturition in T1 and T2 groups, respectively in comparison to other weeks of different groups of goats. These results agree with those reported previously by Barcelos et al. (2022) who found that HDL and LDL were increased with an advance in time of mineral and vitamin E supplementation in goats. An increase in LDL at the last week of treatment may be due to that triglyceride could be stored as lipid droplets in the liver of the pregnant goats and secreted a triglyceride-rich lipoprotein known as very low density lipoprotein (Davis, 1999). There looks to be a compensatory response in the liver. Due to the increased levels of triglyceride in the liver, the more triglyceride is converted into very LDL and secreted into the animal blood (Xia et al., 2013). There was no significant effect of treatment and time interaction on all serum biochemical parameters.
Effect of KI drenching in black goats during late pregnancy TSH, T3 and T4 hormone and T3/T4 ratio of newborn kids
The data related to newborn kid’s hormone which include TSH, T3, T4 hormone and T3/T4 ratio are shown in Figure 2, 3, 4
mone(TSH)ofnewbornkids.C=control(noIsupplementation;0mg Zn/day),T1=Treatmentone(0.50mgI/day),T2=Treatmenttwo(0.1 mgI/day),ErrorbarsindicateSEM.
newbornkids.C=control(noIsupplementation;0mgZn/day), T1=Treatmentone(0.50mgI/day),T2=Treatmenttwo(0.1mgI/day), ErrorbarsindicateSEM.
H.M.H.Zebarietal.LargeAnimalReview2023;29:147-154151
Figure 2 -TheeffectofIdosesonserumthyroidstimulatinghor-
Figure 3 -TheeffectofIdosesonserumtriiodothyronine(T3)of
and 5, respectively. Although, significantly higher serum concentrations of TSH were recorded in C group of goat and significantly higher concentration of T3 and T4 were recorded in supplemented groups of goat, while no significant effect of KI treatments on TSH (P=0.359), T3 (p=0.1221) and T4 (p=0.372) were recorded in newborn kids of different groups of studied pregnant goats. This may due to that there is no or lack transition of TSH, T3 and T4 hormones from mother through the placenta to the embryo (Zarbalizadeh-Saed et al., 2020).


There was also no significant different (p=0.700) in T3/T4 ratio between newborn kids of different treated groups of goats. Previously, Zarbalizadeh-Saed et al. (2020) also found no significant (p<0.05) effect of 0.4 mg I/d on T3 and T3/T4 ratio compared with zero (0.0 mg I/d) level of iodine in newborn lams, while he found a significantly (p<0.05) higher concentration of T4 in newborn lambs received 0.4 mg I/d compared with zero level. This may be due to that ZarbalizadehSaed et al. (2020) used selenium with iodine rather than KI. Previously, it has been reported that selenium has the most important role in the metabolism of thyroid hormones after io-
dine, and there is a positive relationship between the amount of selenium in the animal body and activity of the thyroid hormones (Köhrle, 1999).
Effect of KI drenching in back goats during late pregnancy on newborn kids
Serum biochemical parameters
The effect of KI supplementation in black native goats during late pregnancy on their new born kids biochemical parameters are shown in Table 3. Serum total protein was not significantly affected by I drenching. The significantly higher (p=0.038) glucose (mg/dl) was found in group T2 compared with C and T1 groups of kids, while there was no significant different between C and T1 groups at the first week after parturition. Similarly, Kerslake et al. (2010) found that new born lambs from iodine-supplemented ewes had higher levels of plasma glucose concentration compared with new born lambs from non-supplemented ewes. This may due to that the glucose from the high iodine intake groups of goat defused to fetal by placenta, because significantly higher levels of serum glucose were recorded in iodine supplemented group of maternal goats during late pregnancy in the current study and maternal glucose is the primary source of energy for fetal and placental tissues (Bell and Bauman, 1997).
However, serum cholesterol (mg/dl) was significantly higher (P=0.053) in C group (54.6±11.7 mg/dl) compared with kids of treated groups of goats. While lower serum triglyceride was recorded in T2 (88.4±5.3 mg/dl) compared with other groups of kids. The lower concentration of serum cholesterol of newborn kids of iodine drenched groups at late pregnancy is unclear.
There was no significant effect of KI drenching on triglyceride (mg/dl). The results of the present study also agree with the results of the present study, Xia et al. (2013) reported lower concentrations of serum triglyceride in I supplemented group of animals. There was also no significant effect of KI drenching HDL (mg/dl) and LDL (mg/dl) in new born kids of the supplemented goats during late pregnancy. Likewise to the current results, Vahedi et al. (2021) found no significant effect of macro algae (Azolla pinnata) on blood HDL and LDL level in lambs.
Effect of KI drenching in back goats during late pregnancy on body weight (Kg) of newborn kids
The data regarding newborn kids body weight are shown in Figure 6. Newborn kids body weight (Kg; mean±SEM) were significantly higher (p<0.001) in groups T2 (4.12±0.22 Kg) of goats in comparison to T1 (3.25±0.17 Kg) and C (3.23±0.17 Kg) groups of studied goats. These results agree with results reported by Zarbalizadeh-Saed et al. (2020) who showed a significantly higher lambs performance of ewes supplemented during late pregnancy with iodine (0.4 mg/d) compared with control (0.0 mg/d) group. In agreement with the findings of the present study, Aghwan et al. (2013) found significantly higher daily body weight of iodine-fed diets growing male kids. The significant higher total body weight of Kids of the high I group (1.00 mg KI/d) of goat in the present study may be due to higher concentrations of T3 and T4 hormones in their blood, and improved their metabolic process (Aghwan et. At., 2013), which in turn increased the kid’s body weight. In contrast to the re-
152TheEffectofIodineDrenchingDuringLatePregnancyonThyroidHormones
Figure 4 -TheeffectofIdosesonserumthyroxine(T4)ofnewbornkids.C=control(noIsupplementation;0mgZn/day),T1=Treatmentone(0.50mgI/day),T2=Treatmenttwo(0.1mgI/day),Error barsindicateSEM.
Figure 5 -TheeffectofIdosesonserumT3/T4ratioofnewborn kids.C=control(noIsupplementation;0mgZn/day),T1=Treatment one(0.50mgI/day),T2=Treatmenttwo(0.1mgI/day),ErrorbarsindicateSEM.
Figure 6 -TheeffectofIdosesonbirthweight(Kg)ofnewborn kids.C=control(noIsupplementation;0mgZn/day),T1=Treatment one(0.50mgI/day),T2=Treatmenttwo(0.1mgI/day),ErrorbarsindicateSEM.

sults of the present study, Aumont et al. (1989) found no significant effect of I intake on the lamb’s birth weight between three different groups of ewes fed diet with 0.13 mg/kg DM, 0.22 mg/kg DM and 10.77 mg/kg DM of iodine content, this may be due to that Aumont et al. (1989) used ewes in his study rather than pregnant goats during late pregnancy.
CONCLUSIONS
The concentration of TSH remained higher in C group, while TSH levels declined in T1 and T2 in response to KI drenching of late pregnant goats. Iodine drenching can improve total protein and glucose and also regulate the levels of both T3 and T4 in the goat during late pregnancy. The concentration of triglyceride was higher in C group without adverse effects on LDL and HDL. Regarding new born kids, the levels of serum glucose were increased in T2 group, while the levels of cholesterol were increased in C group in response to KI drenching in late pregnant goats. Iodine drenching of pregnant goat can also increase new born kids body weight.
Conflicts of interest
The authors declare that they have no conflict of interest.
Authors Contributions
All Authors who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the conception and design of this work or the analysis and interpretation of the data, as well as the writing of the manuscript, to take public responsibility for it. Authors believe the manuscript represents valid work. Furthermore, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication.
References
Abozed, G., Saleh, A.A.K., El-Sayed, E.H., Abdel Khalek, T.M.M. and Hamdon, H., 2020. Effect of Iodine Supplementation on Physiological Responses and Metabolic Rate of Saidi Pregnant Ewes and the Performance of their Lambs. Journal of Animal and Poultry Production, 11(8), pp.303-307.
Aghwan, Z.A., Sazili, A.Q., Alimon, A.R., Goh, Y.M. and Hilmi, M., 2013. Blood haematology, serum thyroid hormones and glutathione peroxidase status in Kacang goats fed inorganic iodine and selenium supplemented diets. Asian-Australasian Journal of Animal Sciences, 26(11), p.1577.
Ahmed, M.H. and Byrne, C.D., 2007. Modulation of sterol regulatory element
binding proteins (SREBPs) as potential treatments for non-alcoholic fatty liver disease (NAFLD). Drug discovery today, 12(17-18), pp.740-747.
Aumont, G., Levieux, D., Lamand, M. and Tressol, J.C., 1989. Iodine nutrition in ewes. 2. Effects of low to high iodine intake by ewes on the I content of biological fluids and plasma immunoglobulins G in newborn lambs. Reproduction Nutrition Development, 29(2), pp.203-217.
Barcelos, B., Gomes, V., Vidal, A.M.C., de Freitas Júnior, J.E., de Araújo, M.L.G.M.L., Alba, H.D.R. and Netto, A.S., 2022. Effect of selenium and vitamin E supplementation on the metabolic status of dairy goats and respective goat kids in the peripartum period. Tropical Animal Health and Production, 54(1), pp.1-13.
Bell, A.W. and Bauman, D.E., 1997. Adaptations of glucose metabolism during pregnancy and lactation. Journal of mammary gland biology and neoplasia, 2(3), pp.265-278.
Bhardwaj, R.K. 2018. Iodine deficiency in goats. In Goat science. Rijeka: IntechOpen.
Cappai, M.G., Liesegang, A., Dimauro, C., Mossa, F. and Pinna, W., 2019. Circulating electrolytes in the bloodstream of transition Sarda goats make the difference in body fluid distribution between single vs. twin gestation. Research in veterinary science, 123, pp.84-90.
Davis, R.A., 1999. Cell and molecular biology of the assembly and secretion of apolipoprotein B-containing lipoproteins by the liver. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1440(1), pp.1-31.
Davoodi, F., Zakian, A., Rocky, A. and Raisi, A., 2022. Incidence of iodine deficiency and congenital goitre in goats and kids of Darreh Garm region, Khorramabad, Iran. Veterinary Medicine and Science, 8(1), pp.336-342.
Dušová, H., Trávníek, J., Peksa, Z., Šimák-Líbalová, K., Šimková, A., Falta, D. and Švejdová, K., 2014. The influence of high iodine intake on chosen blood parameters of sheep. Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis, 62, p.8.
El-Salaam, A., El-Tahan, A.A.H. and Bakr, A.A., 2018. Impact of dietary iodine supplementation on productive and reproductive performance of Maghrebian She-camels.
Hetzel, B., 1983. Iodine deficiency disorders (IDD) and their eradication. The Lancet, 322(8359), pp.1126-1129.
Huszenica, G. Y., Kulcsar, M. and Rudas, P. 2002: Clinical endocrinology of thyroid gland in ruminants. Vet. Med.- Czech, 47, 7: 199–200.
Huszenicza, G.Y., Kulcsar, M. and Rudas, P., 2002. Clinical endocrinology of thyroid gland function in ruminants. VETERINARNI MEDICINAPRAHA-, 47(7), pp.199-210.
Juma, K.H. and I.E. Alkass. 2005. Native goats of Iraq: A review. Dirasat, Agric. Sci. 32: 180-188.
Kadum, N.B. and Luaibi, O.K., 2017. Clinical study hypothyroidism in goats and treatment by iodine compounds. Journal of entomology and Zoology Studies, 5, pp.1956-1961.
Kaneko, J.J., Harvey, J.W. and Bruss, M.L. eds., 2008. Clinical biochemistry of domestic animals. Academic press. pp. 571–588.
Kerslake, J.I., Kenyon, P.R., Stafford, K.J., Morris, S.T. and Morel, P.C.H., 2010. Can maternal iodine supplementation improve twin-and triplet-born lamb plasma thyroid hormone concentrations and thermoregulation capabilities in the first 24–36 h of life?. The Journal of Agricultural Science, 148(4), pp.453-463.
Köhrle, J., 1999. The trace element selenium and the thyroid gland. Biochimie, 81(5), pp.527-533.
Lawrence, T.L.J. and V.R. Fowler. 1997. Growth of farm animals. CAB International. Wallingford, Oxon OX10 8DE, UK. 114.
Lovreglio, R., Meddour-Sahar, O. and Leone, V., 2014. Goat grazing as a wildfire prevention tool: a basic review. Iforest-Biogeosciences and Forestry, 7(4), p.260.
Mercer, L.P. 2006, September. International iodine deficiency. In Forum on Public Policy: A Journal of the Oxford Round Table. Forum on Public Policy.
Meschy, F., 2000. Recent progress in the assessment of mineral requirements of goats. Livestock Production Science, 64(1), pp.9-14.
National Research Council (US). Committee on Nutrient Requirements of Small Ruminants, National Research Council, Committee on the Nutrient Requirements of Small Ruminants, Board on Agriculture, Division on Earth and Life Studies, 2007. Nutrient requirements of small ruminants: sheep, goats, cervids, and new world camelids.
Nudda, A., Battacone, G., Bomboi, G., Floris, B., Decandia, M. and Pulina, G., 2013. Effect of dietary iodine on thyroid hormones and energy blood metabolites in lactating goats. Animal, 7(1), pp.60-65.
Nudda, A., Battacone, G., Decandia, M., Acciaro, M., Aghini-Lombardi, F., Frigeri,
H.M.H.Zebarietal.LargeAnimalReview2023;29:147-154153
M. and Pulina, G., 2009. The effect of dietary iodine supplementation in dairy goats on milk production traits and milk iodine content. Journal of Dairy Science, 92(10), pp.5133-5138.
Nyström, H.F., Brantsæter, A.L., Erlund, I., Gunnarsdottir, I., Hulthén, L., Laurberg, P., Mattisson, I., Rasmussen, L.B., Virtanen, S. and Meltzer, H.M., 2016. Iodine status in the Nordic countries–past and present. Food & nutrition research, 60(1), p.31969.
Oramari, R.A., Bamerny, A.O. and Zebari, H.M., 2014. Factors affecting some hematology and serum biochemical parameters in three indigenous sheep breeds. Advances in Life Science and Technology, 21, pp.56-63.
Osuagwuh, A.I.A., 1992. Effects of strategic feed supplementation during pregnancy on birthweight and perinatal survival of West African Dwarf kids. The Journal of Agricultural Science, 119(1), pp.123-126.
Pachuri, S.P. 1981. Clinical Studies on Endemic Goiter in Goats in Tarai Status Report.Pantnagar, India: Department of Medicine, COVSc, G.B. Pant University of Agricultureand Technology.
Pattanaik, A.K., Khan, S.A. and Goswami, T.K., 2011. Iodine supplementation to a diet containing Leucaena leucocephala leaf meal: consequences on nutrient metabolism, clinical chemistry and immunity of goats. Animal Production Science, 51(6), pp.541-548.
Pattanaik, A.K., Khan, S.A., Mohanty, D.N. and Varshney, V.P., 2004. Nutritional performance, clinical chemistry and semen characteristics of goats fed a mustard (Brassica juncea) cake based supplement with or without iodine. Small Ruminant Research, 54(3), pp.173-182.
Shimano, H., Yahagi, N., Amemiya-Kudo, M., Hasty, A.H., Osuga, J.I., Tamura, Y., Shionoiri, F., Iizuka, Y., Ohashi, K., Harada, K. and Gotoda, T., 1999. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. Journal of Biological Chemistry, 274(50), pp.35832-35839.
Singh, J.L., Sharma, M.C., Kumar, M., Rastogi, S.K., Gupta, G.C., Singh, S.P., Sharma, L.D. and Gandhi, V.K., 2002. Assessment of therapy in goitrous goats through some cardiac function tests. Small ruminant research, 44(2), pp.119-124.
Smith, M.C. and D.M. Sherman. 2009. Goat medicine. John Wiley and Sons. Sokkar, S.M., Soror, A.H., Ahmed, Y.F., Ezzo, O.H. and Hamouda, M.A., 2000. Pathological and biochemical studies on experimental hypothyroidism
in growing lambs. Journal of Veterinary Medicine, Series B, 47(9), pp.641652.
Todini, L., 2007. Thyroid hormones in small ruminants: effects of endogenous, environmental and nutritional factors. Animal, 1(7), pp.997-1008.
Vahedi, V., Hedayat-Evrigh, N., Holman, B.W. and Ponnampalam, E.N., 2021. Supplementation of macro algae (Azolla pinnata) in a finishing ration alters feed efficiency, blood parameters, carcass traits and meat sensory properties in lambs. Small Ruminant Research, 203, p.106498.
Wang, D., Wan, S., Liu, P. Meng, F., Zhang, X., Ren, B., Qu, M., Wu, H., Shen, H. and Liu, L., 2021. Relationship between excess iodine, thyroid function, blood pressure, and blood glucose level in adults, pregnant women, and lactating women: A cross-sectional study. Ecotoxicology and Environmental Safety, 208, p.111706.
Xia, Y., Qu, W., Zhao, L.N., Han, H., Yang, X.F., Sun, X.F., Hao, L.P. and Xu, J., 2013. Iodine excess induces hepatic steatosis through disturbance of thyroid hormone metabolism involving oxidative stress in BALB/c mice. Biological trace element research, 154(1), pp.103-110.
Zarbalizadeh-Saed, A., Seifdavati, J., Abdi-Benemar, H., Salem, A.Z., Barbabosa-Pliego, A., Camacho-Diaz, L.M., Fadayifar, A. and Seyed-Sharifi, R., 2020. Effect of slow-release pellets of selenium and iodine on performance and some blood metabolites of pregnant Moghani ewes and their lambs. Biological trace element research, 195(2), pp.461-471.
Zebari, M.H., Buti, E.T.S. and Hamo, R.A.H., 2013. Some blood biochemical parameters of meriz does during different physiological status. Sovetskii Vrachebnyi Sbornik, 18, pp.190-194.
Zeedan, K.I., El-Malky, O.M., Mousa, K.M., El-Giziry, A.A. and Etman, K.E.I., 2010. Nutritional studies on some different sources of iodine on productive performance, ruminal fermentation and blood constituents of Buffalo. 1-Effect of two different iodine levels on productive and reproductive performance of buffalo cows. J. Am. sci, 6, pp.1090-1106.
Zeedan, Kh II, Weld Abd-Elkader, S.I., Kh Mousa, M.M. and Etman, K.E.I. 2014. Nutritional studies on some different sources and levels of iodine on productive performance, ruminal fermentation and blood constituents of buffalo. 3-Effect of different levels of iodine on productive performance of buffalo calves. Journal of Animal and Poultry Production 5, pp.143-156.
154TheEffectofIodineDrenchingDuringLatePregnancyonThyroidHormones
SARA BUSECHIAN1*, CHIARA CATALANO2, PAOLA STRATICÒ3, ALESSANDRO GRAMENZI2,3, LUCIO PETRIZZI3, SIMONA ORVIETO4, FABRIZIO RUECA5
1DepartmentofVeterinaryMedicine,ViaSanCostanzo4,06126,Perugia,Italy
2EquineLineConsultant,NBFLanesSrl,CorsodiPortaVittoria14,20122,Milano,Italy
3DepartmentofVeterinaryMedicine,localitàPianod’Accio,64100,Teramo,Italy
4Privatepractitioner,Perugia,Italy
5SportHorseResearchCenter,DepartmentofVeterinaryMedicine,ViaSanCostanzo4,06126,Perugia,Italy
SUMMARY
Equine gastric ulcer syndrome (EGUS) is a worldwide disease, found especially in racehorses, although it can affect different categories of animals with difference in prevalence. Recently, two different diseases have been recognized, the first affecting the squamous mucosa (Equine Squamous Gastric Disease, ESGD) and the other the glandular mucosa (Equine Glandular Gastric Disease, EGGD), with different pathophysiology, risk factors and management. To date, the main strategy for the treatment involves acid-suppressive therapy with omeprazole, often associated with sucralfate; yet the total safety and absence of side effects of this treatment have recently been questioned. Recently, an increasing number of studies have been investigating the use of complementary feeds to manage of these health issues. The purpose of this study is to evaluate the effectiveness of a complementary feed administered for 28 days for EGUS control. A gastroscopy (T0) was used to select sixteen horses with lesions on the squamous mucosa, graded between 0/4 and 4/4. A second gastroscopy was performed at the end of the supplementation (T1) to assess any changes in the lesions score. The data obtained did not reveal statistically significant differences that might enable us to objectively establish whether the product is effective in the case of these gastric pathologies. However, gastroscopic images showed a clear improvement in the lesions and in the appearance of the mucosa even in the absence of score variations, suggesting that 28 days of administration may not be sufficient for complete healing. During the trial the treated subjects displayed an improvement in behavior and appeared to be more relaxed and inclined to work. The results allow us to affirm that, despite not being statistically relevant, the feed supplement improved not only the appearance of the gastric mucosa, especially of the squamous mucosa, but also the well-being of the horses, making them more willing to work. Further studies are needed, with an increased number of horses and a longer length of supplementation period.
KEY WORDS
Griffonia simplicifolia; Olea europaea; ESGD; EGGD; gastric ulcers.
INTRODUCTION
Equine Gastric Ulcer Syndrome (EGUS) is a multifactorial pathology characterized by the presence of erosive and ulcerative lesions in the gastric mucosa. Recently [1-5], EGUS has been divided into two different diseases: Equine Squamous Gastric Disease (ESGD) and Equine Glandular Gastric Disease (EGGD) in relation to the location of ulcers in the squamous or glandular portion of the stomach, respectively. Prevalence varies with breed and training level and different incidence between ESGD and EGGD has been found [1-13]. All ages and breeds are susceptible to EGUS, yet factors such as diet and management play a pivotal role in the ulcer formation and maintenance [1,2,5,14]. The highest prevalence is found in sport hors-
Corresponding Author:
Sara Busechian (sarabusechian@gmail.com).
es, with percentages ranging from 66-91% in thoroughbred horses and with increases of up to 80% -100% when in full activity [1-5,7,15]. A prevalence of 40% has been reported in Quarter Horses [1,2,5,16,17]. In addition to intense exercise and competitions, the risk factors include nutrition and stress [15,12,18,19]. The reported clinical signs appear to be varied and nonspecific and include not only anorexia or disorexia, a poor body condition score and weight loss, chronic diarrhea, a dull, shaggy coat, bruxism, behavioral changes (including aggression and nervousness) and recurrent or acute colic but also reduced performance [1-5,14,17,20]. The treatment is based on the maintenance of a gastric pH above 4 to allow the mucosa to initiate the reparative processes. Proton pump inhibitors and H2 receptor antagonists are the most commonly used drug classes in horses. In the first class, omeprazole is the most widely studied [1-5,17,20]. However, these treatments entail several disadvantages: they are expensive and require a veterinary prescription, if discontinued they may lead to relapses, and, if administered for prolonged periods of time they may increase the
S.Busechianetal.LargeAnimalReview2023;29:155-162155
Ó
Effectiveness of a complementary feed containing extract of Griffonia Simplicifolia and Olea Europaea on horses affected by equine gastric ulcer syndrome
gastric pH excessively, leading thus to inadequate digestion [17,21,22]. In two recent papers, Sykes sought to assess whether the use of omeprazole was correctly deemed as safe and devoid of both short-term and long-term adverse effects. In the author’s view there may be a correlation between the use of omeprazole and gastric hyperacidity due to a rebound effect, a decrease in calcium absorption and the destruction of normal balance and activity in the large intestine [21,22]. Furthermore, omeprazole-based pharmaceutical products are not permitted in some competitions [17]. For this reason, there has been a growing interest in evaluating valid alternatives based on the use of complementary feeds [14,17,23-27].
Griffonia simplicifolia is an African plant, the seeds of which are rich in 5-hydroxy-l-tryptophan (5-HTP), a precursor of serotonin (5-HT) [28]. This is a monoamine that has a role as a neurotransmitter in the central nervous system, including a role in the control of mood and memory. Its depletion is responsible for anxiety, depression, schizophrenia, etc., but the administration of 5-HTP orally increases the concentrations of 5-HP in the central nervous system. In human medicine, this plant is used successfully to treat migraine, depression, anxiety, weight loss and insomnia [29-32].
Olea europaea is a fruit tree used mainly for nutritional purposes. In phytotherapy, it is used to prevent diabetes, cardiovascular diseases, cancer and malaria. Fruits and leaves, rich in polyphenoles, especially oleuropein and hydroxytyrosol, have various effects, e.g. antioxidant, hypoglycaemic, anticancer, hypotensive, etc. [33-36]. On gastric diseases, Olea europaea extracts, from both fruits and leaves, have demonstrated a positive effect, with a reduction in the acidity of the stomach content and the prevention of the development of gastritis and gastric ulcers in rats [33,34,37]. Furthermore, the leaf extracts have the ability to block calcium channels, reducing the secretion of histamine and the production of gastric acid [38].
Considering the properties of the extracts of G. simplicifolia and O. europaea, and the need for effective nutraceutical products for the treatment and prevention of gastric ulcers in horses, aim of this study was to investigate the use of a novel commercial feed supplement (Gastro Horse Relax, NBF lines, Milan, Italy) to manage of gastric ulcers in Quarter Horses.
MATERIALS AND METHODS
Animals
The study was carried out in collaboration with the University of Teramo and the University of Perugia and authorized by the Ethical Committee of the University of Teramo (CEISA) under protocol No. 15/2019. American-riding trained adult horses were included, with no restriction of breed or sex. Horses were excluded if they presented signs of systemic diseases or were treated with any kind of medication, especially those of the stomach and the gastrointestinal system. Animals could be excluded at any time at the owners’ request.
Sixteen horses, were enrolled, aged between 3 and 16 (mean 9, s.d 5). All were used for Reining or Performance training. Most were Quarter Horses (9/16, 56%), 2/16 (13%) were Paint, 2/16 (13%) were mix breed, 1/16 (6%) was Thorougbred, 1/16 (6%) Appaloosa and 1/16 (6%) Italian Saddlebred. Based on the sex, 6/16 (38%) were geldings, 1/16 (6%) were males and 9/16 (56%) were females (Table 1).
Before the start of the study, a questionnaire was given to the
owners, with questions about the management and feeding regimen of the horses (e.g., type of housing, the access to a paddock, type of food and diet, the potential use of additional complementary feeds, the level of exercise carried out daily/weekly, whether the horse had been moved in the months prior the study and the presence of any previous or current systemic pathologies that may be associated with therapeutic treatments). The owners were interviewed to inquire whether they had noticed symptoms or behaviors related to EGUS (weight loss, recurrent colic, reduced performance, behavioral changes, etc.[1-5]). According to the questionnaires, management was similar for all horses: all the animals were housed in boxes with access to a sand paddock a few times a week and were trained for one hour every day. The diet involved the administration of 8-10 kg of mixed hay (with alfalfa) and an average of 4 kg of concentrate per day. Only 1/16 (Table 2 n.5) had been moved to a different stable the month before the study began and consequently both food and work management had changed. According to the owners, none of the horses showed any sign of gastric ulcers,
Gastroscopic examination
Before each gastroscopy, a physical examination was carried out and the weight of the animal was measured with a metric tape [39].
Endoscopy was performed according to bibliography [2,3], the animals were fasted for 16-18 hours and were sedated with alpha-2-agonist drugs (xylazine, 0.6-1 mg / kg IV). A portable processor (Tele Pack Vet X Led, Karl Storz, Germany) and an endoscope 3m long and 10.4mm in diameter (60130PKS; Karl Storz, Germany) were used. The scope entered through the lower nasal meatus, the nasopharynx and the esophagus to reach the stomach. The organ was distended with air to allow a clear view and the mucosa was rinsed with water if it was covered
156Effectivenessofacomplementaryfeedcontainingextractof
onhorses
Griffonia Simplicifolia and Olea Europaea
1 QHG3 2 QHG13 3 QHM16 4 QHF8 5 QHG4 6 PaintF3 7 QHF4 8 AppaloosamixF14 9 PSIG15 10 QHF10 11 QHG4 12 AppaloosaG10 13 PaintF6 14 SCNF14 15 SIF10 16 QHF6
Table 1 -Listofhorsesenrolledinthestudy,QH:quarterhorse, SCN:mixedbreed,SI:Italiansaddlebred,G:gelding,M:male,F: female.
BreedGenderAge
Maltodextrin24,75
Dextrose10,75
Spraydriedmaltpowder8
Lecithinpowder4
Guargum4
Kaoliniticclay4
Pectinpowder4
Alfalfaleaffinepowder4
Carrotsfinepowder4
Magnesiumhydroxide2
Olea europaea leafextract2
Griffonia simplicifolia (98%)1
Precipitatedsilicicacid1
Magnesiumoxide0,80
Appleflavoringpowder0,10
Saccharomyces cerevisiae 0,08
with foam and/or residual food material. The feed supplement was added to the standard diet of the horses for 28 days. At the end of this period (T1), a complete physical examination, the recording of the weight with a metric tape and a control endoscopy were performed. At the same time, the owners were asked whether they had noticed any improvement in the horse’s
clinical situation, including its willingness to work. At the end of the trial, gastroscopy recordings were reviewed and the grading score was assigned by a veterinarian who was unaware of the time (T0 or T1) when the investigations had been carried out. Ulcers in the squamous portion were graded using the scoring system by the ECEIM Consensus Statement. As bibliography lacks a standard scoring system to classify the severity of lesions for EGGD, the horse was considered positive or negative only on the basis of the presence or absence of lesions, regardless of their severity [1,2].
Feed supplement
The feed supplement (Gastro Horse Relax, NBF Lanes Milano) introduced into the diet is a product containing Griffonia simplicifolia and Olea europaea extracts associated with other nutraceutical components (Table 2). It was administered orally in quantities of 30 g twice daily for 28 days, mixed with the feed. During this period the daily management of the animal remained unchanged.
Statistical Analysis
The data was collected in a Microsoft Excel for the subsequent descriptive statistical evaluation. The difference between the grade for ESGD, the presence of EGGD and weight estimation before and after treatment was assessed using paired sample sign test with significance set to p < 0.05. Statistical analysis was performed using R commander package (R Core Team, 2020).
RESULTS
Before supplementation, 14/16 (88%) animals had ESGD with a score between ¼ and 4/4, whereas 5/16 (31%) were positive for EGGD, as shown in Table 3 and pictures 1 and 2.
Table 3 -PresenceofESGD(grade0-4/4)andEGGD(0:negative,1:positive)andweightestimationbeforeandafter28daysof administrationofthefeedsupplement(GastroHorseRelax,NBFLanesMilano)[2,39].
S.Busechianetal.LargeAnimalReview2023;29:155-162157
Soyashellpowder25,52
IngredientsPercentage 1 1100477477 2 1001477484 3 2210514530 4 2100522545 5 2400484484 6 2211393407 7 1200407448 8 2110463463 9 0100470507 10 4401470484 11 4400430477 12 4100448470 13 1100470463 14 0000593585 15 1110570600 16 4210455470
Table 2 -Compositionofthefeedsupplement(GastroHorse Relax,NBFLanesMilano)usedinthestudy.
HorseT0 ESGDT1 ESGDT0 EGGDT1 EGGDT0 WeightT1Weight
After supplementation 5/16 (31%, Table 3 nos.2-4-8-12-16) horses showed a score improvement for ESGD, whereas 8/16 (50%, Table 3 nos. 1-3-6-10-11-13-14-15) showed no change and 3/16 (19%, Table 3 nos.5-7-9) displayed a higher score (figure 1).
While 4/5 horses (80%, Table 3 nos. 3-8-15-16) positive for EGGD no longer showed glandular lesions at T1, 1/5 (20%, Table 3 nos.6) still tested positive. 2/11 horses (18%, Table 3 nos. 2-10) that were negative for EGGD at T0 showed lesions in the glandular mucosa after the treatment (fig. 3).
After supplementation, weight had increased in 11/16 horses (69%, Table 3 nos. 2-3-4-6-7-9-10-11-12-15-16), decreased in 2/16 (12%, Table 3 nos. 13-14) and did not change in 3/16 (19%, Table 3 nos. 1-5-8).
ESGD scores, the presence of EGGD and weight did not show statistically significant differences before and after supplementation (fig 1, 3 and 4).
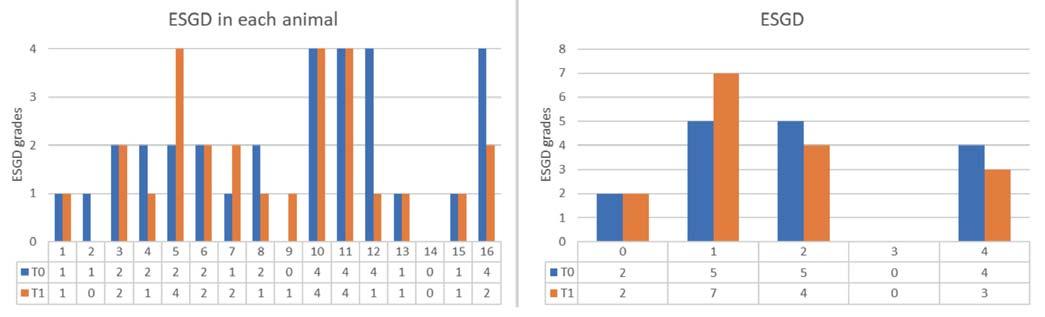
All owners, however, noticed an improvement in the horse’s behavior: the animal was more willing to work and displayed a relaxed attitude during the training session.
DISCUSSION
Although all ages and breeds are susceptible to EGUS, diet and management appear to be predisposing factors and play a key role in ulcers formation and maintenance [1,2,4,5,12,18,20]. Therefore, interventions acting on these factors may help to limit the risk of gastric lesions [1,2,4,12,14,17]. A recent study by Luthersson et al [40] highlighted the importance of specially formulated diets to maintain adequate gastric function and decrease the risk of relapse after treatment with omeprazole. The most widely used drug therapies to treat ulcers aim to decrease gastric secretions by increasing the pH, in order to create a more favorable environment for the healing of mucosal lesions [1,2,5,14,17,20]. The drug recommended is omeprazole possibly associated, in presence of EGGD, with sucralfate [1,2,4,17].
Recent interest in the use of plant-based products, nutraceuticals, oils and minerals has been increasingly growing as drugs are expensive, they can be considered as doping in certain competitions, they require a veterinary prescription and long-term therapies may lead to an excessive increase in pH with subsequent problems to digestion and absorption [1,2,4,14,17,21,22].
Given this growing trend to seek therapeutic alternatives, the aim of the study was to evaluate whether the use of a feed supplement (Gastro Horse Relax, NBF Lanes Milano) containing extracts of Griffonia simplicifolia and Olea europaea may be a beneficial support tool in the case of gastric ulcers in horses. A study by Jan in 2010 [34] assessed the effect of the administration of Olea europaea on the volume of gastric secretions and on the acidity in rabbits after induction of gastric secretion with carbachol, obtaining positive data on both its efficacy and its safety of use. The 13% leaf extract has been shown to have the ability to block calcium channels (Ca ++) [33,37,38]. Agents that have this action are mainly used for treatment of cardiovascular diseases as they inhibit muscle contraction. Gastric motility and secretion, however, depend on the concentration of calcium ions: the release of histamine by the peritoneal mast cells is closely linked to the extracellular concentration of Ca ++, and a decrease in its levels would therefore lead to a reduction in the effects of histamine with less production of gastric acids [34,38].Thanks to its antioxidant activity and ability to control the inflammatory process, oleuropein contained in the olive leaves can be used as a natural therapy for gastritis [33,37].
Griffonia simplicifolia seeds are very rich in 5-hydroxy-tryptophan (5-HTP), a direct precursor of serotonin [29-32]. 5-HTP is able to quickly cross the blood-brain barrier, increasing its concentration in the central nervous system. Its therapeutic potential, both as an anxiolytic-like product and as a support tool in pathologies linked to serotonin depletion, has been investigated in several studies with positive results. [29,30]. To date, the seed extract of this plant is commonly used to treat anxiety, depression, insomnia, migraines and headaches [29-32]. Tests carried out on herbal preparations or nutraceuticals to treat EGUS have yielded dissimilar and not always positive results [14,17,23-27,41]. The data revealed by the study presented in this manuscript, indicates that the supplement (Gastro Horse Relax, NBF Lanes Milano) used, does not show a statistically relevant effectiveness in preventing or healing ulcers already present. It should be noted, however, that many horses (13/16, 81%) which were administered the product showed an improvement or at least a score stability for ESGD and it was effective in the majority of horses affected by EGGD (4/5, 80%). Even individual animals which did not display a change in the ESGD score (8/16, 50%) exhibited a noticeable improvement in lesion size and depth (Fig. 2). A tendency to ulcer healing
158Effectivenessofacomplementaryfeedcontainingextractof Griffonia Simplicifolia and Olea Europaea onhorses
Figure 1 -Distributionofthelesionsofthesquamousmucosa(ESGD)before(T0)andafter(T1)28daysofsupplementation;ontheleftresultsforeachanimal,ontherightdistributionofthelesionsinthepopulation.
ng themargoplicatus,BESGD4atT1,theareasofdeepulcerationarestillpresentalongthemargoplicatusonthelessercurvature,butthe lesionsaresmallerindiameterandlesserinnumber;
Middle:Horseno.12CESGD4atT0,presenceofdeepareasofulcerationalongthelessercurvature,alongthemargoplicatus,DESGD1 atT1,presenceofyellowingofthemucosa(hyperkeratosis),alongthelessercurvature;
Lower:Horseno.16:EESGD4atT0,presenceofadeepareaofulceration(arrow)onthelessercurvature,FESGD2atT1,presenceofsmallerareasoferosionalongthelessercurvature,noareasofdeepulcerationarevisibleaftertreatment.
was visible in many gastroscopy images and an improvement in the general appearance of the mucosa was noted (Fig. 2). In addition, the horse that had undergone a change of management (Table 3 subject No. 5) due to the move from a different stable in the month prior to the study was the only one to show a significant worsening of the ESGD score, rising from 2/4 to 4/4. This may be linked to an increase in the risk class
for ulcer development: changes in management, movement and variations in the social dynamics of horses are among the major determinants of the development of gastric lesions; furthermore, the animal was subjected to a higher workload, which is also associated with an increased risk of presenting gastric ulcers [1,2,12,18]. One other horse (table 3, number 7) showed an increase in the score for ESGD, but this was not as
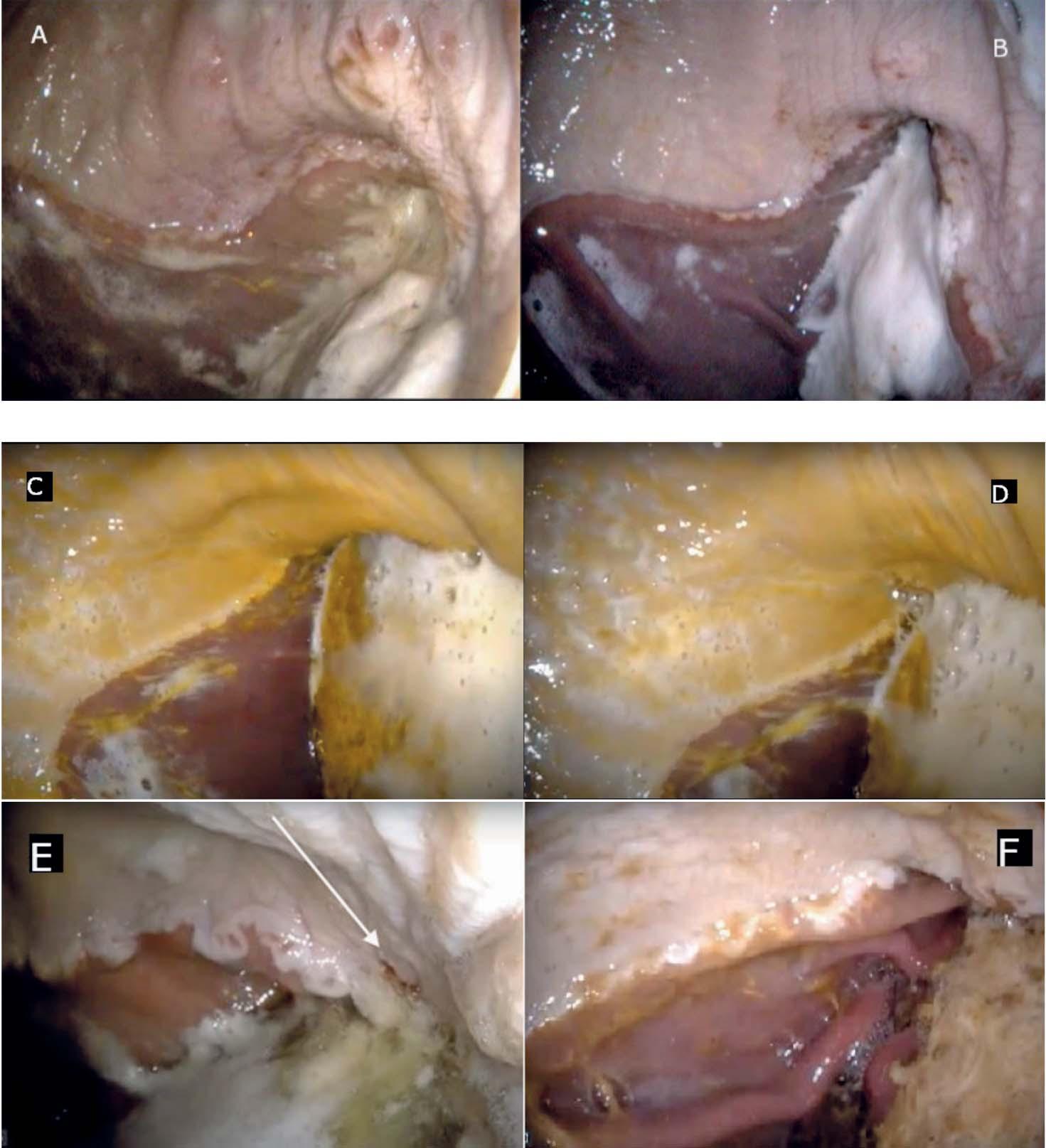
S.Busechianetal.LargeAnimalReview2023;29:155-162159
Figure 2 -Upper:Horseno.10AESGD4atT0,presenceofdeepareasofulcerationonthelessercurvature,nexttothecardiasandalo
marked, going from grade 1 to grade 2. In recent years, the clinical significance of grade 2 has been questioned, since many horses with ESGD this severe do not show clinical signs and these lesions could heal spontaneously more easily than other grades [1,2,4,5,13,18].
Many of the animals gained weight (11/16, 69%, Table 3), but the increase was not statistically significant. This could be related to a better digestion of the feed, due to an improvement in the stomach environment, secondary to the start of the healing process of the gastric lesions [1,2,5,13].

During the study, the owners reported an improvement in attitude and willingness to work, in some cases even a calmer demeanor. These improvements are consistent with the findings of various authors: reduced performance and behavioral changes may be caused by the presence of gastric diseases
[1,2,4,5,14,17,20,42] and an improvement in the demeanor of the horse could be related to a decreased discomfort. Furthermore, the increase in serotonin caused by the intake of 5HTP contained in Griffonia simplicifolia leaf can be responsible for the calmer attitude of the animals [29,30].
The analysis of the questionnaires completed by the owners allowed us to observe that the animals’ management and diet shared many similarities, even though they were housed in two different stables. For this reason, the presence of ulcers and/or the unchanged score of some subjects may possibly be due to an individual predisposition or to a different degree/type of training.
The study shows a very high prevalence of ESGD in this population (14/16, 88%), similar to the percentages observed in sport horses [1,2,4,5,16], although higher than those identi-
160Effectivenessofacomplementaryfeedcontainingextractof Griffonia Simplicifolia and Olea Europaea onhorses
Figure 3 -Distributionofthelesionsoftheglandularmucosa(EGGD)before(T0)andafter(T1)supplementation;0:negative,1:positi ve;on theleft,resultsforeachanimal,ontheright,distributionoflesionsinthepopulation.
Figure 4 -Weightofthehorsesbefore(T0)andafter(T1)supplementation.
fied for the Quarter Horse breed [16] which is generally used for this type of activity. This could be related to the different management of the horses in this population compared to those in the literature, but could also be caused by the different breeds included in the study.
CONCLUSIONS
The feed under study did not lead to a resolution or improvement of gastric lesions in the group of horses evaluated. However, this may be related to the small number of animals investigated and to the great variability of score at inclusion, with the presence of high-grade lesions. Nevertheless, this research has revealed a change in the appearance of mucosa with a tendency to a reduction in the number and size of lesions, even in the most severe cases. This suggests that 28 days of therapy may not be sufficient to achieve improvement in the majority of the treated horses and that more significant results may be obtained by administering the nutraceutical for a longer period. The feed supplement enabled improved behavior of the treated individuals, which were more relaxed and willing to work. This outcome may be ascribed to the increased release of serotonin due to the presence of Griffonia simplicifolia seed extract in the nutraceutical. This trial is to be considered as a preliminary study that deserves further research, also in light of both the high prevalence of ESGD and EGGD in horses and the increasing request for complementary feeds to support drug treatments.
References
1.Rendle D, Bowen M, Brazil T, Conwell R, Hallowell G, Hepburn R, et al. (2018) Recommendations for the management of equine glandular gastric disease. UK-Vet Equine;2:2-11. doi:10.12968/ukve.2018.2.s1.3.
2.Sykes BW, Hewetson M, Hepburn RJ, Luthersson N, Tamzali Y. (2015) European College of Equine Internal Medicine Consensus Statement-Equine Gastric Ulcer Syndrome in Adult Horses. J Vet Intern Med;29:1288-99. doi:10.1111/JVIM.13578.
3.Sykes BW, Jokisalo JM. (2014) Rethinking equine gastric ulcer syndrome: Part 1 - Terminology, clinical signs and diagnosis. Equine Vet Educ;26:5437. doi:10.1111/EVE.12236.
4.van den Boom R (2022). Equine gastric ulcer syndrome in adult horses. Vet J;283-284:105830. doi:10.1016/J.TVJL.2022.105830.
5.Sanchez LC. (2018) Disorders of the Gastrointestinal System. In Equine Internal Medicine, Ed. Reed S.M., Bayly W.M., Sellon D.C., 4th Ed., , p. 709842, Elsevier, St. Louis, Missouri. doi:10.1016/B978-0-323-443296.00012-7.
6.Begg LM, O’Sullivan CB. (2003) The prevalence and distribution of gastric ulceration in 345 racehorses. Aust Vet J;81:199-201. doi:10.1111/j.17510813.2003.tb11469.x.
7.Bell RJW, Kingston JK, Mogg TD, Perkins NR. (2007) The prevalence of gastric ulceration in racehorses in new zealand. N Z Vet J;55:13-8. doi:10.1080/00480169.2007.36729.
8.Niedwied A, Kubiak K, Nicpo J. (2013) Endoscopic findings of the stomach in pleasure horses in Poland. Acta Vet Scand;55:45. doi:10.1186/17510147-55-45.
9.le Jeune SS, Nieto JE, Dechant JE, Snyder JR. (2009) Prevalence of gastric ulcers in Thoroughbred broodmares in pasture: A preliminary report. Vet J;181:251-5. doi:10.1016/J.TVJL.2008.03.020.
10.Tamzali Y, Marguet C, Priymenko N, Lyazrhi F. (2011) Prevalence of gastric ulcer syndrome in high-level endurance horses. Equine Vet J;43:1414. doi:10.1111/j.2042-3306.2010.00129.x.
11.Luthersson N, Nielsen KH, Harris P, Parkin TDH. (2009) The prevalence and anatomical distribution of equine gastric ulceration syndrome (EGUS) in 201 horses in Denmark. Equine Vet J;41:619-24. doi:10.2746/042516409X441910.
12.Busechian S, Sgorbini M, Orvieto S, Pisello L, Zappulla F, Briganti A,Nocera I, Conte G, Rueca F. (2021) Evaluation of a questionnaire to detect the risk of developing ESGD or EGGD in horses. Prev Vet Med;188. doi:10.1016/j.prevetmed.2021.105285.
13.Busechian S, Turini L, Sgorbini M, Bonelli F, Pisello L, Pieramati C, Orvieto S, Rueca F.(2022)Body Condition Score Is Not Correlated to Gastric Ulcers in Non-Athlete Horses. Anim, Vol 12, Page 2637 2022;12:2637. doi:10.3390/ANI12192637.
14.Andrews FM, Larson C, Harris P. (2017) Nutritional management of gastric ulceration. Equine Vet Educ;29:45-55. doi:10.1111/EVE.12495.
15.Cate RE, Nielsen BD, Spooner HS, O’Connor-Robison CI, Schott HC. (2012) Prevalence of gastric ulcers and relationship to other parameters in Standardbred racehorses. Comp Exerc Physiol;8:47-52. doi:10.3920/CEP12009.
16.Bertone J. (2002) Prevalence of Gastric Ulcers in Elite, Heavy Use Western Performance Horses. in 48th Annu. AAEP Conv., vol. 48,, p. 256-9.
17.Zavoshti FR, Andrews FM. (2017) Therapeutics for Equine Gastric Ulcer Syndrome. Vet Clin Equine Pract;33:141-62. doi:10.1016/ J.CVEQ.2016.11.004.
18.Sykes BW, Bowen M, Habershon-Butcher JL, Green M, Hallowell GD. (2019) Management factors and clinical implications of glandular and squamous gastric disease in horses. J Vet Intern Med;33:233-40. doi:10.1111/JVIM.15350.
19.Malmkvist J, Poulsen JM, Luthersson N, Palme R, Christensen JW, Søndergaard E. (2012) Behaviour and stress responses in horses with gastric ulceration. Appl Anim Behav Sci;142:160-7. doi:10.1016/J.APPLANIM.2012.10.002.
20.Videla R, Andrews FM. (2009) New perspectives in equine gastric ulcer syndrome. Vet Clin North Am Equine Pract;25:283-301. doi:10.1016/J.CVEQ.2009.04.013.
21.Sykes BW. (2019) Courses for horses: Rethinking the use of proton pump inhibitors in the treatment of equine gastric ulcer syndrome. Equine Vet Educ;31:441-6. doi:10.1111/EVE.12894.
22.Sykes BW. (2021) A free ride: Is long-term omeprazole therapy safe and effective? Equine Vet Educ;33:556-60. doi:10.1111/EVE.13458.
23.Stucchi L, Zucca E, Serra A, Stancari G, Ceriotti S, Conturba B, Ferro E, Ferrucci F (2020). Efficacy of the Administration of a Natural Feed Supplement in the Management of Equine Gastric Ulcer Syndrome in 7 Sport Horses : A Field Trial. Am J Anim Vet Sci;12:104-10. doi:10.3844/AJAVSP.2017.104.110.
24.Bonelli F, Busechian S, Meucci V, Caporrino G, Briganti A, Rueca F,Zappulla F, Ferini E, Ghiandai L, Sgorbini M. (2016) pHyloGASTRO in the Treatment of Equine Gastric Ulcer Lesions. J Equine Vet Sci;46. doi:10.1016/j.jevs.2016.06.069.
25.Sykes BW, Sykes KM, Hallowell GD. (2014) Efficacy of a Combination of Apolectol, Live Yeast (Saccharomyces cerevisiae [CNCM I-1077]), and Magnesium Hydroxide in the Management of Equine Gastric Ulcer Syndrome in Thoroughbred Racehorses: A Blinded, Randomized, PlaceboControlled Clinical Trial. J Equine Vet Sci;34:1274-8. doi:10.1016/ J.JEVS.2014.09.006.
26.Venner M, Lauffs S, Deegen E. (1999) Treatment of gastric lesions in horses with pectin-lecithin complex. Equine Vet J Suppl:91-6. doi:10.1111/J.2042-3306.1999.TB05178.X.
27.Bush J, van den Boom R, Franklin S. (2018) Comparison of aloe vera and omeprazole in the treatment of equine gastric ulcer syndrome. Equine Vet J;50:34-40. doi:10.1111/EVJ.12706.
28.Fellows LE, Bell EA. (1970) 5-hydroxy-l-tryptophan, 5-hydroxytryptamine and l-tryptophan-5-hydroxylase in Griffonia simplicifolia. Phytochemistry;9:2389-96. doi:10.1016/S0031-9422(00)85745-3.
29.Carnevale G, Di Viesti V, Zavatti M, Benelli A, Zanoli P. (2011) Influence of Griffonia simplicifolia on male sexual behavior in rats: Behavioral and neurochemical study. Phytomedicine;18:947-52. doi:10.1016/ J.PHYMED.2011.02.009.
30.Carnevale G, Di Viesti V, Zavatti M, Zanoli P. (2011) Anxiolytic-like effect of Griffonia simplicifolia Baill. seed extract in rats. Phytomedicine;18:848-51. doi:10.1016/J.PHYMED.2011.01.016.
31.Sommer C. (2006) Is serotonin hyperalgesic or analgesic? Curr Pain Headache Rep;10:101-6. doi:10.1007/S11916-006-0020-4.
32.Esposito M, Ruberto M, Pascotto A, Carotenuto M. (2012) Nutraceutical preparations in childhood migraine prophylaxis: effects on headache outcomes including disability and behaviour. Neurol Sci;33:1365-8. doi:10.1007/S10072-012-1019-8.
33.Al-Quraishy S, Othman MS, Dkhil MA, Abdel Moneim AE. (2017) Olive (Olea europaea) leaf methanolic extract prevents HCl/ethanol-induced
S.Busechianetal.LargeAnimalReview2023;29:155-162161
162Effectivenessofacomplementaryfeedcontainingextractof Griffonia Simplicifolia and Olea Europaea onhorses
gastritis in rats by attenuating inflammation and augmenting antioxidant enzyme activities. Biomed Pharmacother;91:338-49. doi:10.1016/J.BIOPHA.2017.04.069.
34.Jan M. (2010) Effects of “Olea europaea” extract on volume and acidity of carbachol induced gastric secretion, liver and kidney function in rabbits | Request PDF. J Ayub Med Coll;22:113-5.
35.Acar-Tek N, Aagündüz D. (2020) Olive Leaf (Olea europaea L. folium): Potential Effects on Glycemia and Lipidemia. Ann Nutr Metab 2020;76:105. doi:10.1159/000505508.
36.González-Hedström D, García-Villalón ÁL, Amor S, de la Fuente-Fernández M, Almodóvar P, Prodanov M, Priego T, Martin AI, Inarejos-Garcia AM, Granado M. (2021) Olive leaf extract supplementation improves the vascular and metabolic alterations associated with aging in Wistar rats. Sci Rep;11:8188. doi:10.1038/S41598-021-87628-7.
37.Alirezaei M, Dezfoulian O, Neamati S, Rashidipour M, Tanideh N, Kheradmand A. (2012) Oleuropein prevents ethanol-induced gastric ulcers via elevation of antioxidant enzyme activities in rats. J Physiol
Biochem;68:583-92. doi:10.1007/S13105-012-0177-8/FIGURES/6.
38.Rauwald HW, Brehm O. (1991) Screening of some medicinal plants for their possible calcium-antagonistic activity. Planta Med;57:A59-60. doi:10.1055/S-2006-960320/BIB.
39.Ellis JM, Hollands T. (1998) Accuracy of different methods of estimating the weight of horses. Vet Rec;143:335-6. doi:10.1136/VR.143.12.335.
40.Luthersson N, Bolger C, Fores P, Barfoot C, Nelson S, Parkin T,Harris P. (2019) Effect of Changing Diet on Gastric Ulceration in Exercising Horses and Ponies After Cessation of Omeprazole Treatment. J Equine Vet Sci;83. doi:10.1016/J.JEVS.2019.05.007.
41.Munsterman AS, Dias Moreira AS, Marqués FJ. (2019) Evaluation of a Chinese herbal supplement on equine squamous gastric disease and gastric fluid pH in mares. J Vet Intern Med;33:2280-5. doi:10.1111/JVIM.15603.
42.Millares-Ramirez EM, Le Jeune SS. (2019) Girthiness: Retrospective Study of 37 Horses (2004-2016). J Equine Vet Sci;79. doi:10.1016/ j.jevs.2019.05.025.






























