MASTERMINDS


Transforming Brain Disease Research 2025 BOLD FORWARD THINKING




Transforming Brain Disease Research 2025 BOLD FORWARD THINKING

WELCOME TO THE 2025 EDITION OF MASTERMINDS
Reflecting on our last trip around the sun, I marvel at the people and events that have impacted the faculty, trainees and staff of the Florida Atlantic's Stiles-Nicholson Brain Institute (SNBI) this past year.
Corrine Lasmézas, DVM, Ph.D., joined us as the inaugural director of the David and Lynn Nicholson Center for Neurodegenerative Disease Research. She will host internationally recognized scientist Sir John Hardy, Ph.D., for grand opening ceremonies in March, formally launching a collective fight against Alzheimer’s disease and other devastating brain disorders. With a $2 million philanthropic gift, this visionary center is poised to make great strides in moving from molecule to medicine.
At the heart of this initiative is an interdisciplinary approach bringing together world-class scientists, clinicians and engineers, to unravel the complex mysteries of neurodegenerative diseases and overcome barriers to cures. The work at the Nicholson Center follows a path blazed by decades of research yet charts its own course, embracing innovative hypotheses and technologies to change the landscape of brain health for those dealing with disorders that rob too many people of movements and memories.
One promising frontier for treatment of brain disorders is an implementation of MRI-couple low intensity, focused ultrasound aimed at permitting greater brain access of novel therapeutics, otherwise rebuffed by the fortress walls of the blood-brain barrier. In partnership with Insightec, a medical device company, researchers from the Institute, including Gregg Fields, Ph.D., vice president of research, are racing against time to open new paths for improved treatments.
Other new technologies are transforming our scientists’ work, including the addition of a Miltenyi Ultramicrope Blaze platform that
allows for full 3D scanning at high-speed of intact animal brains, using advanced lightsheet imaging technology. A second addition to the core is a Bruker GeoMx Spatial Profiler, which allows the messages of the genome to be sampled at single-cell resolution without removing these cells from their native environment. Finally, the arrival of a GE 3T Premier MRI Scanner, housed at the new Florida Atlantic MRI Research and Discovery Center, Boca Raton campus, helps scientists understand the complexities of the human brain, at rest and in action.
for students living and working here, and we are thankful for the supplemental support generously provided by Anna Ewing and John Capotosto, as well as from the June Jones Foundation, to offset these costs and minimize time away from research to teach.


Mature (ahem) neuroscientists recognize the spirit, energy and insights of younger scientists. As we welcomed the third wave of aspiring brain scientists to the Neuroscience Graduate Program (NGP) and now recruit the fourth, it is exciting to see a rich pool of talent join the battle to reveal the mysteries of the mind.
Two trainees also became the first NGP students to defend their dissertation proposals, and are now racing ahead to make discoveries that will change lives. Being a graduate student in Palm Beach County represents a significant economic challenge
In addition, MobileMinds, launched by SNBI’s ASCEND Program (Advancing Science to Community Engagement through Neuroscience Discovery), brought brain science and health lessons to school systems, community centers and even the local mall. Supported by a gift from the Per an Astrid Heidenreich Foundation, as well as funds from the Sharron and Joseph Ashby Hubert Fund of the Community Foundation of Broward, our team is turning on the lights for youth just now thinking about their future.
Lastly, Brainy Days, a program funded by Palm Health Foundation, hosted Insights at the Institute: Creativity and the Bipolar Brain, an art exhibition donated by Dusty and Joyce Sang, co-directors of the Ryan Licht Sang Bipolar Foundation, aimed at reducing the stigma that keeps so many in the shadows, undetected and untreated.
The SNBI team is excited for the future, and as we continue to advance brain science and education at Florida Atlantic, we gather the insights and inspiration needed to go “once more unto the breach.” I hope you will enjoy the stories of our efforts and wish upon us success for the coming year.
Randy D. Blakely, Ph.D.
Executive Director, Florida Atlantic
Stiles-Nicholson Brain Institute
Director, Neuroscience Ph.D. Program
David J. S. Nicholson Distinguished Professor in Neuroscience
Professor of Biomedical Science,
Charles E. Schmidt College of Medicine
Gregg Fields, Ph.D.
Vice President, Research, Executive Director, Florida Atlantic Institute for Human Health and Disease Intervention Professor, Department of Chemistry and Biochemistry, Florida Atlantic Charles E. Schmidt College of Science fieldsg@fau.edu
Randy D. Blakely, Ph.D.
Executive Director, Florida Atlantic Stiles-Nicholson Brain Institute Professor, Biomedical Science
Charles E. Schmidt College of Medicine rblakely@health.fau.edu
David J. S. Nicholson
Distinguished Professor in Neuroscience
Managing Editor
Cammi Clark, Ph.D.
Assistant Managing Editor Chelsey Matheson
Contributing Writers and Editors
Polly Burks, Jeff Brooks-Gillies, Cammi Clark, Chelsey Matheson, Judy Gelman Myers, Wynne Parry, Alexandra Paz, Ph.D.
Photography and Images
Paige Arriola, Katarzyna Bytnar, Alex Dolce, Getty Images, iStock.com, Chelsey Matheson, Carrie Rodman Photo
Design and Graphics
Craig Korn
Illustrations
Sam Falconer

Notice: Reasonable requests should be sent to the Division of Research at least 20 days in advance via dorcommunications@fau.edu.

BY Chelsey Matheson
Advanced neuroscience research requires cutting-edge tools that allow scientists to test more complex research questions and explore new perspectives of the brain.
Florida Atlantic University recently acquired a novel digital technology (proprietary of NanoString, a Bruker company) that provides researchers a more comprehensive image of brain cells and how they respond to certain changes in their environment. The GeoMX Digital Spatial Profiler (DSP) allows researchers to profile changes on a molecular level in unique cellular populations. Tissue is often made up of more than one cell type and understanding how each type of
The GeoMX Digital Spatial Profiler (below) is part of the Advanced Cell Imaging Core, located on Florida Atlantic’s John D. MacArthur Campus in Jupiter.
The technology is open to external use for scientific research.

cell changes in response to a mutation, a drug or an environmental stimulus can aid scientists’ ability to target specific cell populations that are implicated in the development of diseases.
“The faculty at the Stiles-Nicholson Brain Institute are interested in delineating how specific mutations, as well as inflammation and drugs, can alter how a cell functions within the brain,” said Paula GajewskiKurdziel, Ph.D., a research assistant professor and project manager of the GeoMX DSP.
The technology allows a user to select up to three different cell types within
Paula Gajewski-Kurdziel, Ph.D.
a tissue to visualize and collect samples from. These samples contain macromolecules called ribonucleic acid (RNA) from the inside of the cells, which can indicate the types of changes the cells are making and be used to find novel, targetable molecular structures that could provide new avenues for treatment of various conditions and diseases.
“Our faculty and researchers are using this information to assess the impact of inflammation in distinct brain regions, as well as mutations related to autism spectrum disorder,” GajewskiKurdziel said.
BY Polly Burks
Digital physics, chatbot epistemology and the future of Artificial General Intelligence took center stage at the second annual Mindfest, hosted by Florida Atlantic’s Center for the Future Mind.
Keynote speakers included:
• Stuart Hameroff, co-founder, director, Center for Consciousness Studies and Professor Emeritus, departments of anesthesiology and psychology, University of Arizona
• Sara Imari Walker, theoretical physicist and deputy director of the Beyond Center, Arizona State University
• Scott Aaronson, OpenAI/David J. Bruton Jr. Centennial Professor of computer science at the University of Texas at Austin
• Hartmut Neven, vice president of engineering at Google and founder and head of Google’s Quantum Artificial Intelligence Lab
• Thomas Pike, dean, Oettinger School of Science and Technology, National Intelligence University, Washington, D.C.
• Michael Patrick Lynch, provost professor of the Humanities and Board of Trustees Distinguished Professor of Philosophy
• David Chalmers, New York University

Next: Mindfest 2025, March 12 and 13
For more information, visit fau.edu/future-mind. The conference was organized by Susan Schneider, the William F. Dietrich Distinguished Professor at Florida Atlantic, former NASA chair and distinguished scholar at the Library of Congress. The center is based within Florida Atlantic’s Stiles-Nicholson Brain Institute, as well as the Dorothy F. Schmidt College of Arts and Letters.
Conference sessions included:
• Is Your Brain a Quantum Orchestra? The ‘Orch OR’ Theory of Consciousness
• Putting Ourselves Back in the Equation: Why Physicists Are Studying Human Consciousness and AI to Unravel the Mysteries of the Universe
• The Problem of Human Specialness in the Age of AI
• Democratic AI: The Benefits and Dangers of the Complex System Driving AI
• Are We in a Computer Simulation, and If So, Why Would it Matter?
Dan Nemeth, Ph.D., postdoctoral fellow, Charles E. Schmidt College of Medicine and Stiles-Nicholson Brain Institute, recently co-authored a study that reveals specific neural circuitries responsive to immune signaling. The article was published in the Journal of Neuroinflammation.
“Interleukin-1 Receptor 1 (IL-1R1) is known as the primary conductor of inflammatory processes; however, recent research shows that it is more involved in our day-to-day brain function than previously thought. IL-1R1 signaling has been implicated in normal brain functions like memory, sleep and cognition and, when excessive IL-1R1 signaling is present, it is thought to lead to mood, affective and memory disorders. It has yet to be discovered how IL-1R1 may control or modify normal brain function,” Nemeth said.
The study aimed to provide a more detailed analysis of which cells express IL-1R1 in the brain.
Using state-of-theart genetic techniques, the researchers discovered that distinct neurons throughout the brain express IL-1R1. While we found neuronal IL-1R1 in brain regions related to mood, affect and cognition, an unexpected finding is that IL-1R1 is expressed in neurons in the sensory system.

“This new discovery opens questions about whether immune signals influence our sensory processing and whether IL-1R1mediated alterations of sensory signals contribute to cognitive issues, anxiety or depression,” he said. “Furthermore, this study shows that neurons do not signal the same way other IL-1R1-expressing cells do. Using high-tech spatial transcriptomics, we identified that neuronal IL-1R1 regulates synapse organization without causing inflammation. This suggests that IL-1R1 has a role in synaptic formation and can modify neural circuits and their function.”
Launching the New MRI Research and Discovery Center
Nearly 100 people recently gathered to celebrate the grand opening of Florida Atlantic’s new MRI Research and Discovery Center, supported by the university’s StilesNicholson Brain Institute and Institute for Human Health and Disease Intervention.
The institutes also announced the first round of pilot grants for use of human studies tapping into the resources of the new MRI center, led by Andrew Newberg, MD, medical director.
Take a look at the projects on the next page.




William Alexander, Ph.D.
Foraging is a ubiquitous activity across species and has been widely used to investigate the neural correlates of high-level decision-making in humans and animals. While research on foraging has focused on the high-level patch-leaving decision, foraging in the real world requires the coordination of behaviors across a range of temporal and spatial scales. This proposal, using fMRI, behavioral studies and computational modeling to investigate whether foraging behavior is optimal when low-level behaviors must be optimized alongside high-level choices, and which regions of the brain represent decision variables necessary for achieving this joint optimization.


(Sammy) Hong, Ph.D., and Robert Stackman, Ph.D.
The proposed research aims to elucidate the contribution of visual imagery to working memory and episodic memory in subjects with aphantasia and age-matched controls. Aphantasia is a natural condition characterized by impairments in mental imagery, especially visual imagery. Using fMRI in conjunction with computational modeling, the investigators will investigate mental representations of visual working memory and visual imagery. The project seeks to determine whether aphantasia stems from difficulties in generating mental imagery, or from a dysfunction in the conscious awareness of the neural representation produced by mental imagery.


Corinne Lasmézas, DVM, Ph.D., and Michael R. Dobbs, MD
Measurement of Brain NAD Levels in Alzheimer’s Patients and Control Subjects by 1H-MRS
Alzheimer’s disease is an agerelated neurodegenerative disease linked to the accumulation in the brain of misfolded protein aggregates inducing a cascade of cellular toxic gain of function events eventually leading to neuronal demise. Notably, experimental models have shown reductions of the cell essential metabolite nicotinamide adenine dinucleotide (NAD) as well as the promise of NAD elevating interventions. This study will obtain pilot data related to the potential use of brain NAD as a biomarker for disease progression and/or a biomarker to identify classification criteria for patients with the lowest NAD levels who would most benefit from NAD elevating strategies currently under preclinical development. (continued)


Beth Pratt, Ph.D., and Cheryl Krause-Parello, Ph.D.
Feasibility and Acceptability of Magnetic Resonance Imaging
Biomarkers to Explore the Effects of Canines in Veterans with Posttraumatic Stress Symptoms
A large number of U.S. veterans battle post-traumatic stress disorder, yet current treatment options demonstrate suboptimal symptom improvement. Given the significant consequences of Post-traumatic Stress Syndrome in veterans’ lives, it is imperative to identify complementary and integrative approaches to promote their health and well-being. Using a within-participants design, the investigators will evaluate the feasibility, acceptability and suitability of utilizing structural MRI (sMRI) and resting-state functional MRI (rs-fMRI) as neurobiological imaging biomarkers in an existing dog adoption and training intervention project among veterans with Post-traumatic Stress Syndrome.

White matter fiber tracts in the brain, identified using diffusion MRI, reveal the pathways of microscopic water movement. The fibers are color-coded according to the direction of water diffusion: Blue represents diffusion in the up-down (inferior-superior) direction, green indicates diffusion in the front-back (anteriorposterior) direction, and red shows diffusion in the left-right direction. The MRI data were acquired from a healthy volunteer at Florida Atlantic University’s MRI facility. Magnetic resonance imaging (MRI) is a non-ionizing medical imaging technique that uses magnetic fields to generate detailed images. In this case, MRI was employed to capture a series of brain images, each sensitive to water movement along a specific direction. Mathematical models were then used to compute the direction and magnitude of the brain fibers. A tracing technique was applied to identify the white matter fiber tracts, with the visualization color-coding the fibers based on the direction of diffusion.
by Refaat Gabr, Ph.D., director of Florida Atlantic’s new MRI Research and Discovery Center, earned an honorable mention in the Division of Research Art of Science photography and videography contest. See more winners on page 29.
BY Chelsey Matheson
To accelerate progress in brain health and science, Palm Health Foundation has launched a moonshot aspiration called the Brain Coast Vision to create a hub of innovation in Palm Beach County.
Some of humankind’s most difficult conditions to understand and treat are related to brain function. Unlocking the brain’s innermost secrets has proven more difficult than putting an astronaut on the moon. As one of the foremost neuroscience research institutions in the region, Florida Atlantic’s Stiles-Nicholson Brain Institute has signed on as a partner in Palm Health Foundation’s important new endeavor.
“We are poised to realize a new vision, The Brain Coast,” said Patrick J. McNamara, president and CEO of Palm Health Foundation. “Our next frontier is right here on Earth with the aim to accelerate the progress of innovation to diagnose, treat and cure a wide range of neurodegenerative diseases, psychiatric disorders and other brain conditions that affect eight in 10 Americans.”
On Oct. 24, 2024, Palm Health Foundation presented The Brain Coast Vision during one of its Train the Brain events held at the Brain Institute. Attendees heard first-hand from McNamara and Randy D. Blakely, Ph.D., executive director of the Brain Institute, about the scope and purpose the vision.


The initiative is comprised of three pillars:
• Deepening brain science, education and resiliency through research
• Engaging the community
• Spurring innovation to improve brain health mental health and community resilience
“Thousands of brain scientists, trainees and community organizations oriented toward brain health work tirelessly up and down the Atlantic Coast of Florida to tackle the origins and impact of brain disorders,” Blakely said. “The Stiles-Nicholson Brain Institute, with research faculty on Florida Atlantic’s Harbor Branch, Jupiter, Boca Raton and Davie campuses, reflects a decade-long, multimillion dollar investment in discovery, basic and clinical research into the mysteries of the brain and its diseases.”
An example of Palm Health Foundation’s commitment to the Brain Coast Vision is its investment in the field of computational neuroscience at Florida Atlantic. Through this highly specialized discipline, scientists can push the boundaries of traditional biological research and create novel connections among big data sets produced
For more information, visit palmhealthfoundation.org/our-work-advancing-brain-health.
From left: LeeAnne LaBanz, Stiles-Nicholson Foundation, Patrick McNamara, Palm Health Foundation, David J. S. Nicholson, Randy D. Blakely, Ph.D.
Next Steps:
Gather the talent of the existing richness in the research, education, industry and community service organizations into what McNamara labeled as the “Brain Coast Alliance,” the collaborative body to put the vision into practice. Already, two other research powerhouses in brain research, the Max Planck Florida Institute for Neuroscience and the Herbert Wertheim UF Scripps Research Institute have joined the alliance.
in neuroscience labs, exponentially accelerating the rate of scientific discovery and translation to advance human health.
During the Train the Brain event, attendees heard from faculty and trainees who have benefited from Palm Health Foundation’s computational neuroscience funding and how their work is helping to unravel complicated neurological dysfunctions related to conditions like Alzheimer’s disease, Huntington’s disease, depression and autism.

“This is an amazingly visionary gift,” said Blakely, who is also the David J. S. Nicholson Distinguished Professor in Neuroscience and a professor of biomedical science in the Charles E. Schmidt College of Medicine. “Palm Health Foundation has been on the forefront of challenging its community to invest in the future of health and welfare of its community. We are very proud to be a contributor to the timely and transformative vision of The Brain Coast.”
BY Judy Gelman Myers

For his doctoral thesis at Florida Atlantic University, Juan Lopez, Ph.D., needed a swarm of genetically modified flies. In order to conduct myriad experiments investigating synaptic activity in Drosophila neurons, he had to laboriously crossbreed mutants with mail-ordered modified animals — 10,000 to 15,000 because only 2 to 3% would survive into adulthood.
Today, as a postdoctoral researcher in the Charles E. Schmidt College of Science and the Stiles-Nicholson Brain Institute, under the mentorship of Rodrigo Pena, Ph.D., Lopez is building a computational model that can replace his painstaking work with live animals.
“If the model is successful, it’ll let us perform experiments modeling flies that have an incredibly low survival rate,” he said.
Lopez’s model replicates a giant neuron that enables escape behavior in the fly. He mathematically formulates physiological parameters of the neuron, such as size, diameter and ion conductance, and divides them into four “compartments”
representing different segments of the neuron and postsynaptic partner.
“The compartments are chained together to mimic an action potential traveling down the Giant Fiber’s length,” he said.
Lopez and his team can assess the effect of the mutations they introduced into the fly by measuring whether it produced an action potential on the other side of the synapse.
Lopez’s novel approach makes his model an effective tool for both research and teaching. “It lets you do all sorts of things faster than it would take with a fly, and it can be used with undergrads, grad students — even people who’ve never been in the lab,” he said.
Moreover, the “compartments” can be programmed to represent any part of the neuron, giving the model flexibility and breadth. “Not every compartment has to be the same. And just because right now it’s a Drosophila model doesn’t mean that it can’t be applied to other neurons as well,” he said.
BY Jeff Brooks-Gillies

Andrea Cippitelli, Ph.D., who brought his expertise in animal modeling of drug abuse disorders to Florida Atlantic University in 2018, will continue that work as an assistant professor in the Department of Biomedical Science at the Charles E. Schmidt College of Medicine.
Cippitelli completed his graduate work in pharmaceutical sciences at University of Camerino, Italy, before coming to the United States for a postdoctoral fellowship at the National Institute on Alcohol Abuse and Alcoholism in Bethesda, Maryland.
There, he joined the lab of Markus Heilig, MD, Ph.D., a prolific scholar of the neurobiology of alcoholism, and learned about techniques to study alcoholism in animal models.
Cippitelli came to Florida Atlantic in 2018 as a research assistant professor in the Department of Biomedical Science. Since then, he’s been the principal investigator or co-investigator on several grants awarded by the National Institutes of Health, the Department of Defense and other sources. His recent funding awards have supported work exploring therapies for methamphetamine use disorder, cocaine use disorder and alcoholism comorbid with post-traumatic stress disorder.
A primary example of this work is a 2022 study on PPL-138, a recently identified compound with high affinity for opioid receptors and fewer side effects than other opioid analgesics. Cippitelli used an animal model that mimics the binge pattern typical of human cocaine use to show that the compound reduces self-administered cocaine intake in rats. PPL-138 also lowered cocaine seeking in an animal model of relapse. He’s now exploring the safety of the compound, as well as its therapeutic potential for methamphetamine and alcohol use disorders.
“This compound is very promising,” he said. “It is pretty interesting and is a very effective drug in decreasing cocaine use disorder.”
Cippitelli also has experience with cannabinoid research from his doctoral dissertation. More recently, he was awarded a grant to study the effect of cannabidiol in an animal model of migraines. These intense headaches and their associated symptoms are a leading cause of disability but are still poorly understood.
For that study, the results of which were published in the journal Pain, Cippitelli and his collaborators came up with a relatively new model of chronic migraine that worked well in female mice. Their findings suggest

that cannabidiol can be effective both as a preventive tool and as a treatment for headaches, as well as effective in preventing chronic migraine.
That funding was provided by the Consortium for Medical Marijuana Clinical Outcomes Research, which awarded Cippitelli another grant in 2021 to study the mechanism of action of cannabidiol in decreasing migraine disorder.
Despite his recent forays into cannabinoid and migraine studies, Cippitelli sees himself as a drug addiction researcher.
“I work with virtually all drugs of abuse,” he said. “I am deeply involved in this research of modeling and finding new therapies for these disorders.”

BY Chelsey Matheson
Florida Atlantic’s Stiles-Nicholson Brain Institute (SNBI) welcomed eight students to the Neuroscience Graduate Program (NGP) in the Fall 2024 Semester.
“This year’s incoming class is comprised of a distinguished group of neuroscience trainees marked by their excellent academic records and substantial hands-on research experience,” said Randy D. Blakely, Ph.D., executive director of SNBI, David J. S. Nicholson Distinguished Professor of Neuroscience and professor of biomedical science in the Charles E. Schmidt College of Medicine.

The NGP is an interdisciplinary program, immersing students in hands-on research that spans the breadth of neuroscience inquiry and includes opportunities to train with worldclass scientists at Florida Atlantic and Max Planck Florida Institute for Neuroscience. Research experience begins during the first year, as students rotate through three research laboratories before they decide which field to pursue for their doctoral studies.
Seven students in the class of 2024 were awarded Ewing/Capotosto Fellowships, funded through Anna Ewing and John Capotosto. One student also
received a Graduate College Dean’s Fellowship. These students were selected based on academic performance and high potential for success as graduate students.
“These fellowships help promising young scientists shed some of the financial burden of pursuing their doctorates,” Blakely said, “so they can focus on developing their knowledge and skills and take full advantage of the world-class research opportunities available to them at FAU. As we are now attracting superior applicants from around the country, we face intense competition from other fine programs, so this support greatly enhances our ability to recruit them.”


THE COHORT


Martin earned his bachelor’s degree in biomedical sciences with a focus in neuroscience from the University of Central Florida. During his undergraduate studies, he investigated the effects of Vitamin C and oxidative stress in neural progenitor cells to understand how molecular changes in brain cells may influence behavior. After graduation, Martin joined the lab of Matthew Disney, Ph.D., at the Wertheim UF Scripps Institute for Biomedical Innovation and Technology on the Florida Atlantic Jupiter Campus as a postbaccalaureate fellow studying the SARS-CoV-2 genome.

McDonald earned a bachelor’s degree in cell biology from the University of California Davis where he studied how genetic information is exchanged during meiosis. After graduation, he worked as a research assistant at the Salk Institute for Biological Studies in San Diego to support research characterizing the molecular signature of memory in the dentate gyrus, a region of the brain important to learning, memory and emotional control.


Murphy earned a bachelor’s in psychology from the University of Indianapolis and a master’s in experimental psychology from Nova Southeastern University. During her graduate work, she studied a variation in the COMT gene (a gene that breaks down neurotransmitters such as dopamine) and the subsequent stress responses. She also worked as a research assistant, employing molecular biology methodologies to explore stress, inflammation and cognitive function in human participants.


Nott earned a bachelor’s degree in biology and chemistry from the University of Charleston, West Virginia. During her undergraduate studies, she investigated innovative brain imaging technologies. She contributed to research that culminated in achieving a brain scan with free movement in a virtual reality setting. These results highlighted the potential of portable, affordable neuroimaging devices to improve healthcare access in underserved areas.


Origlio earned a bachelor’s degree with a concentration in biopsychology/neuroscience from New College of Florida where she studied white matter pathways in cetaceans (dolphins and whales). Her research investigated the neurological basis of complex social patterns by examining the brains of multiple cetacean species, which revealed patterns between underlying neural connectivity and observable social behaviors.


Patel earned a bachelor’s degree in psychology with a minor in biology from East Tennessee State University. Her undergraduate research contributed to projects relating to adolescent alcohol use disorder, flavored conditioned cues associated with alcohol in adulthood, associative learning models and overall behavioral neuropharmacology.


Sankey earned a bachelor’s in behavioral neuroscience with a minor in psychology from Florida International University. Her undergraduate research was split between neurocircuitry/cognition and Parkinson’s disease. In addition, she conducted a research project that compared autophagy (the natural recycling process of cells) in two types of brain cells called astrocytes and microglia to help understand cellular/molecular pathways in neurodegenerative diseases.


Smith earned a bachelor’s degree in neuroscience from Indiana University. As an undergraduate research assistant, she investigated how dysregulation in endocannabinoid signaling in the cerebellum is associated with neurodevelopment disorders like autism spectrum disorder. In the cannabinoid field, she concentrated on cellular, molecular and behavioral neuroscience.
Milestone:
Valorie Wiseman (mentors: Summer Sheremata, Ph.D., and Sang ‘Sammy’ Hong, Ph.D.) and Kaylee Biegler (mentor: Patrick Grant, Ph.D.) became the first of the Neuroscience Graduate Program trainees to defend their dissertation proposals.

Valorie Wiseman
Kaylee Biegler

BY Chelsey Matheson
This year, four graduate students studying at Florida Atlantic’s Stiles-Nicholson Brain Institute were appointed as June Jones Scholars. The new program provides one semester of stipend support to assist the students in focusing on their research instead of having to receive their stipend through undergraduate teaching. This frees up 20 hours of their week to devote to innovation and discovery in the lab. An additional two students are set to be awarded the scholarship during the Summer 2025 semester. The program is made possible by funding from the June Jones Foundation.
Neuroscience Graduate Program
Mentor: Henriette van Praag, Ph.D.

Alvarez’s current research investigates vestibular function (the sensory system in the inner ear that helps maintain balance and spatial orientation) in Parkinson’s disease (PD) using artificial intelligence technology to obtain various movement metrics to assess both gait and balance. PD symptoms are predominantly characterized by deficits to motor function, which correlates to deficits seen in vestibular function, both of which have been associated with an increased fall risk for PD patients. Some work has shown that proper characterization of vestibular function can predict fall risk in patients and therefore can potentially be used to contribute
to preventative measures. Recent work has also linked vestibular function to cognitive disorders and quality of life in patients. The research on this in PD is limited and further investigation is needed to delineate the relationship between cognitive function and the vestibular system. Therefore, Alvarez said, the goal of her work is to further characterize vestibular function and its possible correlations to cognitive decline in the PD population.
Neuroscience Graduate Program
Mentor: Rodrigo Pena, Ph.D.

Krubitski's research focuses on understanding the dynamics of co-transmission, a process where neurons release multiple neurotransmitters simultaneously. Co-transmission introduces a level of precision in synaptic signaling that differs from single neurotransmitter transmission that has been widely studied previously. There is little known about co-transmission, particularly how it contributes to synaptic integration and its role in normal and pathological conditions, which is what I aim to study.
Specifically, Krubitski is currently studying the co-transmission of glutamate and GABA, which produces biphasic activity patterns that depend on temporal and amplitude differences in excitatory and inhibitory signals. This biphasic activity also determines how they are integrated in the postsynaptic neurons, which affects spiking dynamics and neural communication.
Her work uses modeling techniques to explore how dendritic filtering and temporal integration shape these dynamics. In simulating different combinations of temporal and amplitude differences, we are able to identify different summation patterns in the postsynaptic neuron, such as high-pass, band-pass and low-pass filtering, which affects how the neuron integrates and propagates the incoming signal. Additionally, she examines different currents such as persistent sodium and hyperpolarization-activated currents and their roles in enhancing excitatory summation and inhibitory filtering in co-transmission as opposed to synaptic transmission.

Mentor: Randy D. Blakely, Ph.D. Walsh’s research specifically emphasizes the role of the presynaptic serotonin transporter (SERT) and neuroinflammation. Her current studies investigate the molecular and genetic mechanisms underlying neurodevelopmental and neuropsychiatric disorders, with a special emphasis on how altered serotonin signaling contributes to these conditions. The goal of this work is to uncover insights that could lead to better treatments for disorders by understanding the interaction between serotonin signaling and neuroinflammation, she said.

Mentor: Summer Sheremata, Ph.D. Wiseman’s research explores how the two brain hemispheres process visual information differently, particularly during a phenomenon called visual crowding. Visual crowding occurs when a viewer can identify a target, such as a letter, when it is alone, but cannot identify the same target when it is surrounded by distractors, such as within a word. Visual crowding is a fundamental limit of attention and is highly associated with dyslexia, a common learning disability. While crowding has long been investigated, the allocation of attentional resources and lateralization during crowding is limited. The goal of Wiseman’s research, she said, is to better understand how contralateral biases in behavioral and neural data impact common human behaviors, such as reading, and to benefit future dyslexia research.

Florida Atlantic University is opening a bold new frontier in the fight against neurodegenerative diseases with the launch of the David and Lynn Nicholson Center for Neurodegenerative Disease Research.
Armed with a $2 million philanthropic gift, this visionary center unites top-tier scientists, clinicians and engineers in a concerted effort to unravel the mysteries of Alzheimer’s, Parkinson’s, ALS and other debilitating brain disorders.

Positioned at the cutting edge of groundbreaking research, the center aims to revolutionize how we diagnose, treat and ultimately prevent these diseases, offering new hope for millions affected by them.
With a dynamic, interdisciplinary approach and a focus on pioneering therapies, the Nicholson Center is set to reshape the future of brain science and ignite a new era of medical innovation.

BY Wynne Parry

Though scientists have known for decades that ultrasound waves can be used for medical purposes, recent technological advances have brought ultrasound to the forefront of medical innovation. Clinicians, engineers and researchers are now using MRI to guide focused ultrasound waves in the treatment of various brain disorders — including Alzheimer’s and Parkinson’s disease, essential tremor, neuropathic pain and even brain cancer.
Florida Atlantic is part of this medical breakthrough, entering a new phase of its long-standing collaboration with Insightec, a pioneer in focused ultrasound. The two institutions have signed a Memorandum of Understanding to advance academic research in the area. Insightec will provide the ultrasound technology; Florida Atlantic will provide the MRI and clinical research.
“Ultrasound has the potential to be a game changer. We’re incredibly excited to be partnering with Insightec,” said Gregg Fields, Ph.D., vice president for research, who will oversee clinical research for the Florida Atlantic/Insightec collaboration. Fields is the principal investigator on a State of Florida grant that applies magnetic resonance-guided ultrasound to treat Alzheimer’s disease patients and develops approaches to monitor the effectiveness of ultrasound treatments using blood drawn from patients.
A burgeoning scientific understanding of the changes in the brain that drive neurodegenerative disorders — Alzheimer’s, Parkinson’s, and others like them — suggests that the field is entering a new era, one that could bring new ways of interfering with their progression and one day eliminating them at their origins.


Corinne Lasmézas, DVM,
Ph.D.


We need new methods to diagnose patients earlier in the course of disease and new therapeutics to interfere with the mechanisms that drive it.I believe we — the field and FAU — are ready to develop them.”
- Corinne Lasmézas, DVM, Ph.D.
The University’s new David and Lynn Nicholson Center for Neurodegenerative Disease Research at Florida Atlantic’s Stiles-Nicholson Brain Institute is poised to help usher in these advancements. Established with a $2 million gift from philanthropist and wealth manager David J. S. Nicholson, the center brings together researchers and resources from across the University in a coordinated attempt to promote innovative research and education.
“We need new methods to diagnose patients earlier in the course of disease and new therapeutics to interfere with the mechanisms that drive it,” says Corinne Lasmézas, DVM, Ph.D., the center’s inaugural director. “I believe we — the field and FAU — are ready to develop them.”
Much of this work is already well underway, according to Randy D. Blakely, Ph.D., executive director of the Brain Institute. He notes that faculty members across the University’s campuses, colleges and departments are already working on these conditions and related topics. Moreover, a significant number of doctoral trainees in the recently initiated Neuroscience Graduate Program are planning to pursue careers in neurodegenerative disease research, a welcome sign to the center’s newly recruited scientists.
“We have built up gained considerable momentum in this area in just a few years,” Blakely said. “This is why we need a center: To bring it all together and make the whole more than the sum of its parts.”

Lynn and David Nicholson
In Alzheimer’s, Parkinson’s, Huntington’s, amyotrophic lateral sclerosis (ALS) and scores of other neurodegenerative diseases, neurons – the message-sending cells in the nervous system –become damaged, stop functioning or die. Their loss leads to deterioration in the faculties we otherwise take for granted, such as moving smoothly or maintaining a posture or a train of thought. Over time, these changes grow more debilitating, eventually becoming fatal.
“Fifteen years ago, many researchers would have told you, ‘we have no idea what causes these diseases,’” said Lasmézas, who is also a professor in the Department of Clinical Neurosciences in the Charles E. Schmidt College of Medicine and has joint appointments with the Brain Institute and the Institute for Human Health and Disease Intervention. “We’re now at a point where the scientific community has a lead on the mechanisms that lead brain cells to die.”
One significant advancement came when researchers realized that neurodegenerative disorders share a common, underlying biology: They are driven by malformed proteins that form clumps. These clumps, or aggregates, damage neurons and propagate from cell to cell.
Earlier in her career, Lasmézas’ research focused on a rare variety of rapidly fatal, degenerative, neurological diseases: those caused by infectious proteins known as prions. In these conditions, which
By fostering collaboration across the university, the new Center for Neurdegenerative Disease Research at Florida Atlantic University unites researchers to combat these devastating conditions.

include Creutzfeldt-Jakob disease and mad cow disease, prions form these toxic aggregates. The scope of her research gradually expanded to the more common variety of neurodegenerative diseases as studies showed the prion-like behavior in the proteins responsible for them, such as alpha-synuclein in Parkinson’s, SOD1 or TDP-43 in ALS, and amyloid-beta and tau in Alzheimer’s.
Since then, research in her lab, which she moved to Florida Atlantic from the Herbert Wertheim UF Scripps Institute for Biomedical Innovation and Technology, uncovered new types of toxic protein clumps and found that these impair the brain’s ability to produce energy, contributing to neuron’s death in a handful of these conditions. The team also identified compounds that could interfere in this process. Lasmézas has founded a biotech company, Vova Ida Therapeutics, to develop therapies based on this research.
“The Center for Neurodegenerative Research will help us and our colleagues to bridge the gap to the clinic,” Lasmézas said.
The center, which will officially celebrate its inauguration in March 2025, was funded as part of $10 million Nicholson
gave to the University to fund neuroscience research and education initiatives. This gift also supported construction of the 60,000-square-foot Stiles-Nicholson Brain Institute research facility on the John D. MacArthur Campus in Jupiter, where the center’s offices are now located.
Although headquartered here, the center will have connections across the university and its disciplines. Some of its faculty members, including Lasmézas and Blakely, explore fundamental biological questions. Others have complementary expertise such as clinical research, pharmacology, advanced imaging, chemistry, engineering, and biostatistics. The center’s membership will include faculty from the Charles E. College of Science, the Charles E. College of Engineering and Computer Science, the College of Medicine and the Christine E. Lynn College of Nursing, which cares for patients with Alzheimer’s and other memory disorders, through the memory clinic and the Louis and Anne Green Memory and Wellness Center. Lasmézas also anticipates involving the College of Social Work and Criminal Justice.
The center’s creation capitalizes on work already underway, according to Blakely. Biochemistry and chemistry faculty have been conducting numerous studies related to neurodegenerative
disorders, for example. Likewise, in the College of Medicine, philanthropic gifts from Ann and John Wood and the Carl Angus DeSantis Foundation are supporting research and improvements to care for patients.
Through Nicholson’s gift, Florida Atlantic has recruited two additional faculty: Srinivasa (Srini) Subramaniam, Ph.D., who studies Huntington’s disease, and Alzheimer’s disease researcher Qi Zhang, Ph.D. In addition, the gift has provided pilot grants to faculty interested in pursuing related research and the purchase of new equipment, which will allow researchers to analyze individual cells’ gene expression from within whole tissue.
“The Center for Neurodegenerative Disease Research will wave the flag broadly across our campuses for this kind of research and draw investments that stimulate further research and education in this area,” Blakely said.
As the center gets off the ground, Lasmézas has received funding for an initiative building off her lab’s work on how clumps of abnormal protein impair brain cells’ ability to produce energy. So far, these studies have been conducted in cells and animal models. Now, with help from Florida Atlantic colleagues, she plans to look for similar deficits in the brains of patients with Alzheimer’s, with the ultimate goal of aiding drug development.
The researchers plan to recruit patients through the Memory and Wellness Center and examine them with magnetic resonance imaging at Florida Atlantic’s Clinical Research Unit. Other faculty in the College of Medicine and College of Nursing are also part of the project.
Because this disruption to energy production appears common in neurodegeneration, the teams’ findings could be applicable not just to Alzheimer’s but to other conditions as well. The same is likely true for other avenues of research related to protein misfolding or neuro-inflammation, also common features of these diseases, according to Lasmézas.
“My personal vision is that, by better understanding these diseases and the mechanisms they share, we can tackle several of them at the same time,” she said.


Gregg B. Fields, Ph.D., a faculty member of Florida Atlantic’s Stiles-Nicholson Brain Institute, is now the university’s vice president for research, a role he previously served as interim, and pursues research in Alzheimer’s disease and glioblastoma.
In the realm of innovative medical science, few researchers are as distinguished as Fields, whose career seamlessly blends biology, chemistry and groundbreaking treatment strategies. His extensive background in chemistry, which includes both bachelor’s and doctoral degrees, helps advance specialization in pharmaceutical chemistry. Fields is highly regarded for developing therapeutic agents, and his research has significantly advanced the understanding of disease progression and treatment.
In addition, Fields was recently appointed a Fulbright Specialist Program grantee following a competitive application process, selected by the United States Department of State’s Bureau of Educational and Cultural Affairs (ECA). The Fulbright Specialist Program, part of the larger Fulbright Program, was established in 2001 by the ECA. The program pairs highly qualified U.S. academics and professionals with host institutions abroad to share their expertise, strengthen institutional linkages, hone their skills, gain international experience, and learn about other cultures while building capacity at their overseas host institutions.
Fields’ extensive experience in research, education and international collaboration drives his unique expertise and dedication to advance medical science and contribute to global scientific and educational communities, sharing his knowledge and fostering international partnerships in the quest to improve disease discovery and treatment.
BY Jeff Brooks-Gillies


The State of Florida’s premier Alzheimer’s disease grant program awarded members of Florida Atlantic University’s Stiles-Nicholson Brain Institute three grants totaling $1 million for their novel approaches to care and treatment of the disease.
The funding will support two projects investigating the role of relatively unexplored facets of brain chemistry in Alzheimer’s onset and progression. The third award will fund the development of a simple screening test to help inform an individual with memory concerns whether they can safely drive a car.
A full 20% of Florida’s population is made up of adults aged 65 and older, the age range at the greatest risk of developing Alzheimer’s. The state is second only to California in the number of adults aged 60 and older. By 2045, the number of adults 60 and older is expected to grow to 30% of the state’s
population. As this population grows, so too will the number of Floridians diagnosed with Alzheimer’s disease.
The Ed and Ethel Moore Alzheimer’s Disease Research Program, created by the Florida Legislature in 2014 to address the growing impact of the disease in the state, has awarded three $350,000 grants to Florida Atlantic researchers.
Among the priority research areas for the grant program is the elucidation of the basic science related to Alzheimer’s disease. Much of that research has historically focused on proteins and genes, but new evidence suggests that brain lipids such as cholesterol play an important role in neurodegeneration, according to Qi Zhang, Ph.D., an associate professor in the Department of Chemistry and Biochemistry at the Charles E.


Qi Zhang, Ph.D.


Cudic, Ph.D.
Schmidt College of Science and the Advanced Cell Imaging Core scientific director for the Brain Institute.
Zhang’s research team was awarded a $350,000 Moore Grant to test the hypothesis that Alzheimer’s risk factors come together to disrupt brain cholesterol processes, triggering synaptic dysfunction. Their methods will include new imaging tools for brain cholesterol. The project could lead to new insight into brain cholesterol and potentially new therapeutic strategies for Alzheimer’s disease.
Another brain chemistry project funded by the grant program could provide a new avenue to explore the mounting evidence that neuroinflammation plays a key role in the development and progression of Alzheimer’s disease.
Mare Cudic, Ph.D., associate professor in the Department of Chemistry and Biochemistry, previously studied the role of glycosylation in cancer progression and metastasis. Recent research has shown that the same type of glycosylation she studies in cancer is also present in the amyloid beta protein. In Alzheimer’s disease, this protein is cleaved by enzymes to produce smaller peptides that build up the amyloid plaques in the brain.
“That caught my attention. In particular, we were intrigued by the presence of the glycans in the proximity to the sites cleaved by enzymes and wonder if this may affect the buildup of the amyloid plaques in the brain,” said Cudic, who will lead the project. “We were very interested to look into how glycosylation affects inflammation in the brain, and we already have some promising preliminary results.”


Another research priority for the Ed and Ethel Moore Program is a focus on the social and behavioral aspects of caring for people with Alzheimer’s Disease. One of the first major challenges for Alzheimer’s caregivers is the question of whether a person in the early stages of dementia can still safely drive a car.
Ruth Tappen, Ed.D., RN, FAAN, professor and Christine E. Lynn Eminent Scholar at Atlantic’s Christine E. Lynn College of Nursing, previously developed Fit2Drive, an online screening test that can help predict an older driver’s ability to pass an on-road driving exam.
That research narrowed down a field of cognitive assessments to two well-known and relatively brief tests that, along with an algorithm developed by Tappen and her colleagues, could predict an aging driver’s road test results with over 90% accuracy.
Fit2Drive is web browser-based and requires the user to input the results of the two tests that, while short, still need to be administered by a trained technician. With support from an Ed and Ethel Moore Program grant, the Fit2Drive team will now begin development of a tablet app version of the tool that users can complete on their own in a primary care office setting.
“That’s what this is all about: What can we do accurately on a tablet without a psychometrician administering these tests,” Tappen said.
BY Jeff Brooks-Gillies

An intriguing gene that brain scientists have tracked for 10 years from roundworms to rodents has been shown for the first time to control key aspects of basic cell physiology, according to new research led by Florida Atlantic University.
The mechanism by which the enigmatic gene sustains energy production, suppresses oxidative stress, constrains neuronal hyperactivity, and wards off age-dependent neurodegeneration was revealed by a team of scientists headed by Randy D. Blakely, Ph.D., executive director of Florida Atlantic’s StilesNicholson Brain Institute.
The gene, known as swip-10 in the roundworms in which it was first discovered, carries out a key enzymatic reaction to support the function of mitochondria, the cell’s energetic powerhouse.
depends on the gene’s regulation of a biologically active form of the micronutrient copper. Without the protein encoded by the gene, the health of neurons suffers and other cells show significant signs of stress.
As Blakely and collaborators first elucidated the identity and function of the gene after its discovery in 2015, geneticists
found that a reduction in the human version of the protein led to increased risk for a common form of Alzheimer’s disease. The chemical pathway described in the new research may therefore provide new opportunities for the development of new medications to treat Alzheimer’s disease, as well as other neurodegenerative disorders such as Parkinson’s disease, that display alterations in mitochondrial function.
This breakthrough, recently published in the Proceedings of the National Academy of Sciences, “provides a cogent example of how research in a simple organism can lead to discoveries with relevance to human disease, owing to the conservation of genes and biochemical pathways across millions of years of evolution,” Blakely said.
“Although roundworms appear at first glance to have little in common with humans, their simple nervous system expresses many of the same genes that build the marvelously complex brain we use to think, feel and dream,” he said. “Stepwise, we figure out how genes work in simple systems and then pursue the actions of related genes in more complex organisms like mice and rats whose nervous systems are more like ours, ultimately gaining insights into how the human brain works, and why sometimes, it fails.”
The identification of this gene began with investigations into how another powerful brain chemical, dopamine, is controlled. Blakely grasped the power of a genetic model of the roundworm Caenorhabditis elegans (C. elegans) — a common tool of neuroscientists, including multiple Nobel laureates — to illuminate fundamental mechanisms that support neural signaling and health. Worms are known to use dopamine to control movement, reminding Blakely that loss of dopamine in the human brain leads to impaired movement associated with Parkinson’s disease.
Blakely and postdoctoral fellow Lankupalle Jayanthi, Ph.D., now associate professor at Virginia Commonwealth University, first cloned the C. elegans gene that encodes the dopamine transporter protein. The human version of this protein is well known to neurobiologists as responsible for the addictive actions of cocaine.
Next, Blakely lab postdoctoral fellow Richard Nass, Ph.D., associate professor at Indiana University School of Medicine, hooked a fluorescent molecule to the worm transporter gene.
Through the worm’s transparent body, scientists could for the first time visualize dopamine neurons in a freely moving animal.
“These early steps, taken more than 20 years ago, were very exciting,” Blakely said. “But we saw a bigger prize ahead if we could come up with an easy way to identify new genes whose loss would disrupt the function or health of dopamine neurons. Then we might have new leads on human disorders where dopamine function is disrupted as in Parkinson’s disease or addiction.”
Later, Shannon Hardie, Ph.D., a former Blakely Lab member and current interim dean at the Kinkaid School, discovered that worms lacking the dopamine transporter are almost immediately paralyzed when they are put into water, whereas normal worms can swim vigorously for an hour. The team called this loss of swimming capacity “Swip,” named for their swimming-induced paralysis.

Neurons and other cells in the brain harbor secrets that will take us hundreds of years, if not longer, to sort out, and there are surprises all along the way that can be targets for new interventions.”
- Randy D. Blakely, Ph.D.

Just as important, Hardie found that swimming paralysis could be entirely reversed if normal dopamine signaling was restored.
Swip was the simple, dopamine-dependent behavior Blakely had been looking for as a means to identify genes that support the signaling and health of dopamine neurons. Former lab member Andrew Hardaway, now assistant professor at University of Alabama-Birmingham, then screened mutant worms for emergence of Swip, identifying a numbered collection of genes whose mutation caused the production of altered proteins, including one labeled simply as SWIP-10.
When analyzing the SWIP-10 protein, Hardaway and Blakely found it was highly related to another protein known to carry a particular fold in its structure. Notably, the alterations in SWIP10 found by Hardaway to cause paralysis were found in this same fold.
When Blakely’s graduate student Chelsea Gibson, now at Oak Ridge National Laboratory, found that SWIP-10 mutant worms lost several classes of neurons as they age, including those that
make dopamine, the idea that the human equivalent known as MBLAC1 might underlie some forms of neurodegenerative disease seemed likely. She also observed the telltale signs of oxidative stress, a feature thought by many to cause or accelerate human neurodegenerative disease.
“Finding out that MBLAC1 was a risk factor for Alzheimer’s disease really got the motor running and has shifted a significant fraction of our work to pursuing SWIP-10 and MBLAC1 in parallel to gain further insights into neurodegenerative disease,” Blakely said.
For example, Blakely’s group has created mice lacking MBLAC1. Preliminary studies have found that their brains exhibit an elevated level of beta-amyloid plaques — a major molecular hallmark of Alzheimer’s — when the mice are engineered to carry other mutations found in the disease.
“This finding is quite promising, as many risk factors for lateonset Alzheimer’s disease are not believed to cause the disorder themselves, but rather to accelerate or magnify pathological events triggered by other changes,” he said.
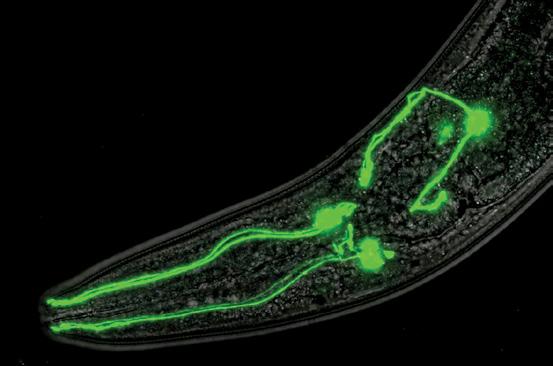
The brain of the tiny roundworm Caenorhabditis elegans is simple but can still provide insights into human neurological conditions.
What can a tiny worm reveal about Alzheimer’s and other human neurological disorders? A lot, as it turns out, according to Randy D. Blakely, Ph.D., executive director of Florida Atlantic University’s Stiles-Nicholson Brain Institute. Eighty-three percent of the proteins in Caenorhabditis elegans have a human counterpart. Manipulating the genome in this worm is simpler and faster than in many other model organisms, and yields crucial insights into the mechanisms of human disease. The model is so important that its use has fueled the work of eight Nobel laureates, including two in physiology or medicine in 2024.
Read more in Nature nature.com/articles/d42473-024-00302-0
So how does loss of SWIP-10 (and presumably MBLAC1) lead to neurodegeneration? Blakely and recently graduated doctoral student Peter Rodriguez reasoned that the enzymatic action of MBLAC1 must hold clues. They connected the dots between two seemingly unrelated studies from other labs.
The first described an enzymatic activity for MBLAC1. The second revealed a surprising path from the product of that enzymatic activity to a form of the essential micronutrient copper. The Florida Atlantic team reasoned that if MBLAC1 could be linked to the regulated balance of the levels of two copper ions in the body, then worms lacking SWIP-10 should follow suit.
Indeed, Bakely Lab experiments showed these animals demonstrated significantly lower levels of the beneficial copper ion, diminished mitochondrial function, lowered ATP levels, and increased oxidative stress. Importantly, these functions, as well as the neurodegeneration seen in prior studies, were restored by genetically augmenting SWIP-10 mutant worms with copies of functional SWIP-10, and by pharmacologically elevating levels of the beneficial copper ion.
This new understanding of the mechanism between SWIP10, MBLAC1 and copper has shown the researchers points in a chemical pathway that may prove to be therapeutically beneficial, Blakey said. The work has led to an international patent application by Blakely’s team in hopes of securing the investments needed to expand and translate the SWIP-10 and MBLAC1 findings into improved diagnostics or therapeutics for neurodegenerative disease.
“Neurons and other cells in the brain harbor secrets that will take us hundreds of years, if not longer, to sort out, and there are surprises all along the way that can be targets for new interventions,” Blakely said. “I am certain that there will be a back and forth between the worm and the rodent and the human brain as we capture the opportunities that each gives us to help people who suffer from devastating brain disorders.”


BY Jeff Brooks-Gillies
It’s one of the most fraught and dreaded experiences for aging drivers, their families and caregivers: the realization that a loved one’s cognitive decline has left them unsafe behind the wheel of a car.
Even when working through this transition with compassion and concern for the health and safety of the driver and other motorists, these conversations can easily give over to anger and frustration.
“That set of keys is our ticket to being independent adults in so many ways,” said Ruth Tappen, Ed.D., RN, FAAN, professor and Christine E. Lynn Eminent Scholar, Florida Atlantic’s Christine E. Lynn College of Nursing.
“It’s life-changing to give up your keys,” said Tappen, who has decades of experience studying care of individuals with Alzheimer’s disease and related dementias. “Many families say it’s the hardest thing they had to deal with as things were changing. It was easier to give up work.”
An online tool developed by Tappen and her colleagues aims to ease that decision. Called Fit2Drive, it’s a screening test that can help predict an older driver’s ability to pass an on-road
driving exam. It’s simple enough to administer in a primary care doctor’s office and is 91.5% accurate in predicting on-road results, according to a recently published article describing the tool in the Journal of the American Medical Directors Association.
The primary care office is one of the places where Fit2Drive may be most helpful, Tappen said. It’s among the first places that patients and caregivers take their concerns about driving, but it’s difficult for providers to make an informed decision on driving ability within the confines of a regular visit.
“It’s really hard to judge when you’re in a clinic or a medical office,” Tappen said. “Many cognitive batteries can last an hour or two. In a primary care office, you don’t have time for that.”
The online Fit2Drive tool requires users to input scores from just two common, relatively brief cognitive assessments that Tappen and her team found to be the best predictors of success in an on-road evaluation: the Mini Mental State Exam and the Trails Making Test. An algorithm converts those scores into a probability that the driver would pass a road test.
“Then we have some objective data to help make the decision, to guide people,” Tappen said.
Driving hadn’t been Tappen’s focus when it came to dementia research, but it was an issue the Florida Department of Transportation wanted to understand better. Tappen and her collaborators spoke with Alzheimer’s support groups around the state, gathering stories from people with memory concerns and their caregivers.
“People told us stories that if we had just done a survey or sent out a questionnaire, we would not have heard,” she said. “People had some fascinating, some heart-breaking stories. Those are not stories that you forget. And you understand that this is a big issue in people’s lives.”
That experience led Tappen to develop a driver evaluation program as part of her work in founding and directing the Louis and Anne Green Memory and Wellness Center. The center is a component of the College of Nursing with a mission to meet the complex needs of patients with memory disorders and their families through care, research and education.
The center’s driver program includes several established assessments of relevant cognitive areas (like concentration,
visual-spatial abilities and problem solving) and an on-the-road test with a trained instructor.
Many participating drivers choose to share their results with the center’s researchers, creating a data pool that proved integral to developing and calibrating the Fit2Drive algorithm. The team also recruited drivers without known memory issues to complete the same cognitive assessments and a road test. Adding this data to the calculations significantly improved the algorithm’s predictive accuracy, Tappen said.
The tool is complete and available online at Fit2Drive.org, but the work isn’t done. Tappen received a $350,000 grant from the Florida Department of Health’s Ed and Ethel Moore Alzheimer’s Disease Research Program to develop a tablet app version of the tool that would allow users to complete an assessment and see their probability of passing a road test on a single device.
“The idea is to put a short set of cognitive tests on a tablet that will predict as well as the current Fit2Drive, or hopefully even better,” she said.


It’s life-changing to give up your keys. Many families say it’s the hardest thing they had to deal with as things were changing. It was easier to give up work.”
- Ruth Tappen, Ed.D.

BY Judy Gelman Myers

As amyloid proteins malfunction, they accumulate in various organs, such as the kidney, heart and brain, causing conditions like renal amyloidosis, stroke, and Alzheimer’s disease. To combat amyloidosis head-on, Florida Atlantic University’s Schmidt College of Medicine held the first Amyloid-related Diseases Summit, established and funded by philanthropist Ann Wood and the Fairfaxwood Foundation.
The summit was chaired by Michael R. Dobbs, MD, the first endowed FairfaxWood Chair of Clinical Neurosciences, chair and professor of the Clinical Neurosciences Department and associate dean of clinical affairs within Florida Atlantic’s Charles E. Schmidt College of Medicine. It was the first of many projected events aimed at discovering a cure for this currently incurable disease.
“Our initial summit focused on basic research in amyloid-related diseases. Future summits will bring together people who are making the discoveries and experts who can apply their findings. That’s where new treatments are made — that’s where the magic happens,” Dobbs said.
The summit, called Breaking the Ice, gathered scientists from Florida Atlantic and across the globe, representing a broad cross-section of disciplines, including neuroscience, chemistry,

molecular biophysics, pharmacy, and structural bioinformatics. After sharing their research findings, participants were given unstructured time to simply interact.
“It was a test to see what would happen when we put these individuals in a room together,” Dobbs said. “I was surprised by how enthusiastic they were and how much they liked the format. They uniformly said they rarely get to have that kind of interaction, because conferences are usually very structured. At our summit, new relationships were forged, including pending research collaborations between people who had never met each other before.”
Wood attended the summit both days. Her funding followed a $28 million scholarship gift in 2022 to support medical education at Florida Atlantic. Wanting to see the event for herself,” she said. “We put them in a room and gave them all the time they needed. They’ll figure out a cure.”
The next summit is slated for spring 2025. It will focus on the common threads between amyloid deposition in the heart and the brain, with double the participants, and — naturally — lots of unstructured time.
BY Chelsey Matheson
Great advancements in microscope technology enable leaps in neuroscience discovery – and they give us an inside view of the remarkable beauty of the brain.
Marianne Charlene Monet, a doctoral student at Florida Atlantic’s Stiles-Nicholson Brain Institute under the mentorship of Ning Quan, Ph.D., professor, Charles E. Schmidt College of Medicine, studies how the immune system affects the brain during childhood, and how these effects can influence behavior and choices later in life.
“In our research, we’ve discovered that multiple brain regions express the neuronal IL-1R1 protein,” Monet said. “We also identified that neuronal IL-1R1 plays a crucial role in social interaction deficits and stress exposure in early life.”
If a child faces frequent or long-term immune challenges, it activates the immune system, which can disrupt normal brain development. This disruption may affect brain areas essential for social behaviors, like forming and maintaining friendships, potentially making these social interactions more challenging as they grow older, Monet said.
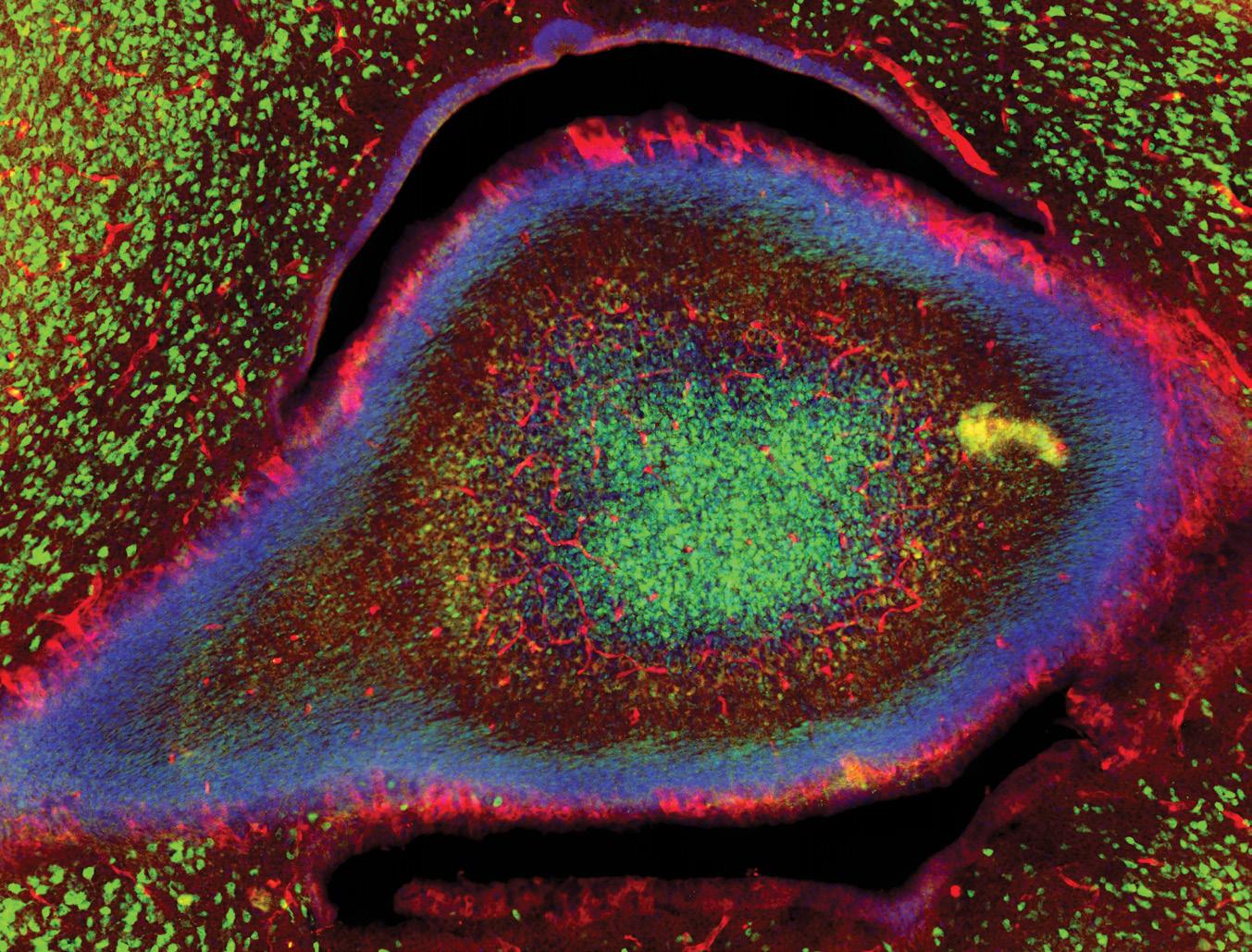
It was during an early phase of this project that Monet captured the image that earned her third place in this year’s Art of Science contest.
“The first step of my project was to identify which brain regions and cell types express neuronal IL-1R1,” she said. “To do this, I mapped IL-1R1 expression across different brain areas in developing mice. During this initial mapping, I came across an
eye-catching structure expressing IL-1R1 and captured the image.”
“Eye-catching” is the operative phrase. Using an epi-fluorescence microscope, the blood vessels and neurons that contain 1L-1R1 appear as a red, oblong shape surrounded by green-pigmented neurons that lack the target protein. The result looks uncannily like an eye staring back at the viewer from the inner reaches of a young mouse cerebellum.
“As a neuroscientist, I’m constantly fascinated by images of the brain,” Monet said. “This image showcases the beauty of the brain. It’s a remarkable organ capable of performing millions of functions simultaneously in such a short period of time.”
The epi-fluorescence microscope was essential to Monet’s investigation because it allowed her to quickly focus and scan brain regions, saving valuable time during imaging sessions. Its high-resolution magnification can capture both broad and detailed views of brain structures – as exemplified by her awardwinning submission. And it can precisely focus on different depths within a tissue, “which is important for imaging complex, three-dimensional structures like the brain,” she said.
As her project progressed, Monet’s focus shifted to the hippocampus, so she didn’t have the opportunity to investigate the unique cerebellar structure featured in her image.
“I thought it was worth sharing, hoping it would spark curiosity and excitement in others about the wonders of the brain,” she said.

Art of Science, Honorable Mention
Tessa Dallo, Charles E. Schmidt College of Science, captured this image of a developing zebrafish. She used Zebrabow, a genetic tool with a diverse color profile, to fluorescently label its cells as they reproduce and mature.
Injecting specific RNA into zebrafish embryos at the one-cell stage results in a range of colorful hues. After a few days, this rainbow overlay of different cells appears. Since cells with the same origin will have the same color, this is a valuable tool for Dallo’s research team to conduct cell lineage analyses.
6TH
Art of Science, Honorable Mention
Tyler Sarovich captured this image that shows induced pluripotent stem cells (iPSCs) derived from a Huntington’s disease (HD) patient. Huntington’s disease is an inherited brain disorder that causes nerve cells to break down over time. No treatment or cure can slow or stop its progression. Researchers are using iPSCs to investigate the molecular mechanisms surrounding the mutated protein that causes HD. Sarovich imaged these cells using fluorescent microscopy at the Charles E. Schmidt College of Medicine.
Note: Both of these honorable mentions winnerss are doctoral students in the Florida Atlantic Neuroscience Graduate Program (NGP), administered by the Stiles-Nicholson Brain Institute. NGP immerses students in hands-on research that spans the breadth of neuroscience.



BY Jeff Brooks-Gillies
Amid all the galaxy-traversing technology in the Star Wars series, one of the greatest scientific advancements on display passes quickly in a seconds-long scene in a doctor’s office. A medical droid pokes Luke Skywalker’s new robotic hand, and it flinches, suggesting it not only sends realistic pain sensations, but also reacts naturally to motor control signals that the brain sends in response.
Here on Earth, recreating this seamless two-way communication between the brain and robotic devices is a “grand challenge” in the field of robotics and neuroscience, according to Erik Engeberg, Ph.D., a professor in Florida Atlantic University’s Department of Ocean and Mechanical Engineering at the College of Engineering and a member of the Florida Atlantic’s Stiles-Nicholson Brain Institute.
Ideally, an artificial limb would respond to the brain’s signals for movements while also providing realistic signals that mimic the full spectrum of sensations related to touch.
“They are two problems that interact with each other and compound upon one another,” Engeberg said. “One is motor control, and one is on sensation and feedback, and both of those need to be working really well to enable high functionality.”
While scientists have made great advancements in brain-machine interfaces that communicate through the electrical signals that travel through our nerves, there are still obstacles. Those include the costs, risks and regulatory requirements. A new research platform developed by Engeberg and his collaborators could help overcome those hurdles.

The neurons are feeling, in a somewhat realistic way, what the robotic hand is feeling if it were all one biological organism, like a human or an animal.”
- Erik Engeberg , Ph.D.
The platform pairs a robotic, sensor-equipped hand with a biological neural network — neurons cultured in a multichannel microelectrode array — along with electrodes that record electrical activity. According to an article recently published in the journal Biomimetics, the platform shows the neural network was able to differentiate between two categories of modeled human touch receptors while also exhibiting motor control of the robotic hand.
“I’m trying to find a way to accelerate progress through this new interface and platform,” said Engeberg, who is also affiliated with the Department of Biomedical Engineering, Department of Electrical Engineering and Computer Science, and the Center for Complex Systems and Brain Sciences.
“There is good potential to reduce risk during the technology and hardware evaluation phase of experiments, and also for algorithm development with these kinds of interfaces,” he said.
Even top-of-the-line assistive devices have limited functionality because they don’t speak the same language as our nervous system, Engeberg said. The new study builds on previous findings from his research group on translating robotic sensations into a code that nerves are able to understand and convert into a plan for action.
In the nervous system, information is transmitted in spikes called action potentials or nerve impulses. The robotic hand has a sensor that measures touch-related parameters like force and slippage. The platform converts those digital measurements into spikes that are quite similar to those produced naturally in a human, Engeberg said.
That signal is then sent to electrically stimulate the cells in the biological neural network, which Engeberg refers to as a brain on a dish.
“The neurons are feeling, in a somewhat realistic way, what the robotic hand is feeling if it were all one biological organism, like a human or an animal,” he said.
The platform then records the electrical activity from the neurons that is evoked by the tactile touch signal from the hand, which is then decoded and converted into another signal that can be used to control the hand.
“It forms a loop,” Engeberg said. “The signal comes from the hand to the neurons, and then from the neurons to the hand.”

For the study published in Biomimetics, the researchers explored how the platform would respond to mathematical models that mimic the sensations associated with two different categories of biological mechanoreceptors. Both types of receptors — rapidly adapting and slowly adapting — detect elements of touch like pressure and vibration. The rapidly adapting sensors respond briefly to a stimulus, while the slowly adapting receptors continue to respond as long as the stimulus is present.
When the research team stimulated the biological neural network using those models, the neurons responded and controlled the robotic system differently, Engeberg said. They were able to detect and classify these signatures in the brain using a convolutional neural network, a type of artificial intelligence used for image recognition.
That shows that the closed-loop robotic hand system is responsive to different sensations of touch, and that researchers can quantify the outcomes that different encoding methods produce, Engeberg said.
“It’s like a proof of concept,” he said. “We actually are able to, with some precision and fidelity, tease out the details and understand what the potential ramifications are when we’re trying to remap someone’s sensation of touch.”


A novel biohybrid neuro-prosthetic research platform will help to refine control of artificial hands and lead to a better understanding of the complex sensation of touch.
This research was supported by the National Institutes of Health and the National Science Foundation. Study co-authors are Craig Ades and Moaed A. Abd, Ph.D., students in Florida Atlantic’s Department of Ocean and Mechanical Engineering; Douglas T. Hutchinson, M.D., a professor in the Department of Orthopedics, University of Utah; the late Emmanuelle Tognoli, Ph.D., research professor, Center for Complex Systems and Brain Sciences, Florida Atlantic’s Charles E. Schmidt College of Science; Sarah E. Du, Ph.D., associate professor, Florida Atlantic’s Department of Ocean and Mechanical Engineering; and Jianning “Jenny” Wei, Ph.D., associate professor of biomedical science, Florida Atlantic’s Charles E. Schmidt College of Medicine.
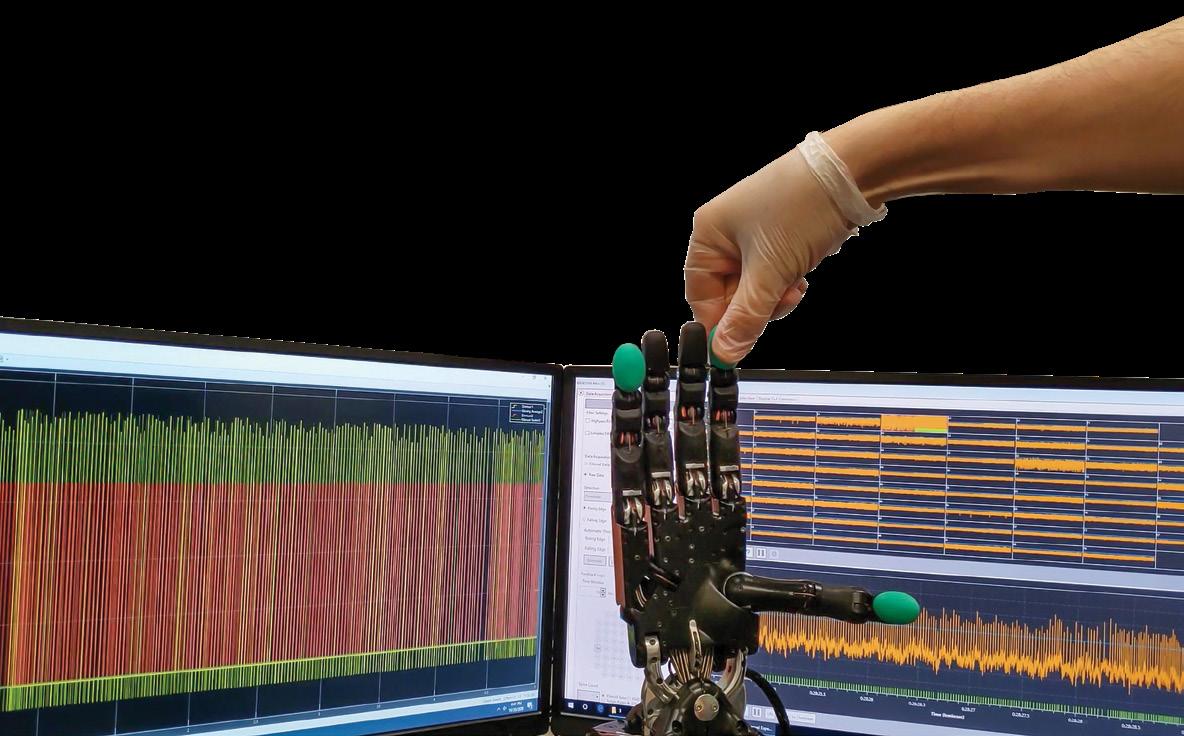




BY Judith Gelman Myers

When scientists first observed that offspring of women who abused drugs during pregnancy were more likely to become abusers themselves, they wondered whether the cause was environmental or genetic. Extensive research showed that both the environment and genetics had a strong effect, but no one was able to identify the origin of addiction or explain how it worked.
Now, a team of scientists at Florida Atlantic University’s Stiles-Nicholson Brain Institute suggest that adults born of women who abused drugs during pregnancy do not have a higher risk of addiction because of their genes; it’s because amphetamines revise the expression of proteins their genes produce. The scientists’ findings may indicate a new pathway to treatment for addiction.

“For a long time, scientists focused on finding the genes responsible for addiction. Our study shows that it’s not the genes but the epigenetics — what the drug does to the expression of the gene,” said Lucia Carvelli, Ph.D., the study’s senior author and associate professor of neuroscience, Harriet L. Wilkes Honors College, Brain Institute investigator and associate professor of biomedical science, Charles E. Schmidt College of Science, which was published in the International Journal of Molecular Sciences.
Genes are bits of DNA strung together into a long strand wrapped around a “spool.” The “spool” is made of proteins called histones, which keep the strand wrapped by forming reversible interactions with the DNA. The tightness of the wrap


affects which genes are turned into proteins — molecules that carry out specific functions within the cell — and which are not. Carvelli and her team discovered that amphetamine changes how tightly the DNA is wound around the spool and, therefore, which proteins are produced.
In this paper, Carvelli and her team focused on the production of two proteins involved in addiction — one that synthesizes dopamine and one that packages dopamine into special containers called vesicles inside the cell. In a previous paper, the team identified a third protein that retrieves the dopamine and brings it back inside the cell. Dopamine sends chemical messages that, among other things, create goal-directed and reward-seeking behaviors.
Working with C. elegans — a tiny worm that responds to dopamine the same way humans do — the team found that the change in gene expression caused by amphetamines led to a buildup of dopamine in adult animals exposed to amphetamine during embryogenesis.

For a long time, scientists focused on finding the genes responsible for addiction. Our study shows that it’s not the genes but the epigenetics — what the drug does to the expression of the gene.”
- Lucia Carvelli, Ph.D.
A higher level of dopamine didn’t change the worms’ behavior in normal conditions, said Carvelli, comparing worms that had been exposed to amphetamines during embryogenesis and worms that had not. She explained that while amphetamine exposure during embryogenesis creates adult animals with a higher basal level of dopamine, they’re able to adapt to the higher level they’ve had since they were embryos.
The problem arose, they found, when the animals that had been exposed to amphetamine as embryos were presented with amphetamines as adults. These animals exhibited extreme hypersensitivity to the drug.
“Besides reward, amphetamines increase risk-taking behaviors,” said Carvelli. “For addiction, that’s not good, because it makes a person more likely to risk taking the drug — which is highly addictive — and thus more likely to develop addiction.”
Their findings may have clinical implications. Using a technique that investigates the interaction between histones and DNA, Carvelli and her colleagues discovered that amphetamines changed gene expression by adding a molecule called a methyl group at various points on the histones. This job is done by a protein called methyl transferase. If its activity is blocked, amphetamines can’t change gene expression.
Several medications approved by the United States Food and Drug Administration, used primarily to treat leukemia, block the activity of methyl transferase. That’s the good news. The bad news: When it’s blocked, it’s blocked everywhere in the body, including places where it may perform a beneficial function.
Carvelli and her team are broadening the scope of their research, investigating whether the effect of amphetamine is maintained transgenerationally. “We saw that the behavioral impacts of amphetamine exposure during embryogenesis extends to the second and third generation, but we haven’t yet done any studies to see if this is also true for the changes in histone modifications and gene expression,” Carvelli said. Co-authors Katie E. Poquette and Sophia L. Amro Gazze, Florida Atlantic undergraduate students at the Wilkes Honors College, are testing other enzymes that may remove the methyl groups and whether they’re affected by amphetamines.
“For many years scientists have studied how amphetamine produces addiction, but we still don’t know each step of this process. That means that what is being identified so far is not the full picture,” Carvelli said. “We’re using C. elegans to see if we can find the missing pieces, but there’s still a lot to do.”

6TH ANNUAL ART OF SCIENCE
Katie Poquette, an undergraduate student in the Harriet L. Wilkes Honors College, captured this photograph of tiny worms called C. elegans. These worms develop outside the uterus, making them ideal subjects for studying how substances and conditions affect embryos – without maternal influence.
Poquette investigated the long-term effects of embryonic amphetamine exposure. Her study found that continuous exposure to amphetamine during embryogenesis altered the expression of the key dopaminergic proteins tyrosine hydroxylase (TH) and vesicular monoamine transporter (VMAT) in adult worms, leading to hypersensitivity to the drug.

BY Chelsey Matheson

Access to high-quality education in science, technology, engineering and math (STEM) is crucial for allowing children the opportunity to flourish in the 21st Century. Students who complete grade school without it face an uphill battle with college admission, career opportunities and breaking the cycle of poverty.
Florida Atlantic’s Stiles-Nicholson Brain Institute introduced its MobileMinds program to help close the Title I STEM gap in Palm Beach County schools by taking science and professional scientists directly to the middle and high schools that are falling behind in this area. A $400,000 gift from the Sharron and Joseph Ashby Hubert Fund of the Community Foundation

MobileMinds in the Community Look for MobileMinds at local community events, including:
• Community festivals
• National Alliance on Mental Illness annual NAMIWalk fundraising team
• Science fairs
• Health expos and more
of Broward included funding to expand MobileMinds south with a brand-new van and additional resources to reach more students than ever before.
“We are thrilled to reach a new community, providing STEM opportunities to underserved students and promoting science literacy,” said Nicole Baganz, Ph.D., director of the Advancing STEM-community Engagement Through Neuroscience Discovery (ASCEND) program at the Brain Institute, which runs the MobileMinds initiative. “As the second most populous county in Florida and home to 198 Title I schools, Broward County is crucial to our mission. The Community Foundation of Broward’s support makes this possible.”
The Broward program will provide the same wide range of activities and opportunities as are currently available, including brain dissections, neurosciencethemed crafts and educational games.
Though the new van didn’t hit the streets until late 2024, the ASCEND team began providing services to three Broward County schools during the Fall semester.
Kylie Kealoha, a student in Florida Atlantic’s Integrative Biology-Neuroscience graduate program, was tapped to become the new Broward County program coordinator. Kealoha has worked as a volunteer for MobileMinds since 2022.
“With our presence in Broward, we will be better able to recruit students on FAU’s Boca Raton campus,” Baganz said. “While Kylie will be supported by our existing team, her presence on the southern campus will also be key to expanding our support network and our community outreach and partnership development in the county.”
MobileMinds offers students a unique window into real science at work and the importance of honing curiosity to explore questions that could lead to life-changing discoveries. By interacting directly with neuroscientists and their trainees, children can dispel some of their fears of science and math and learn that careers in these fields are attainable.
To sustain and grow this important program, more funding is needed. Gifts from businesses, foundations and philanthropic organizations could help:
• Support scientist trainee volunteers by covering the costs of their time and travel to events and schools
• Purchase essential supplies and equipment
• Cover operational costs, including gas and maintenance of the van
“Our vision extends beyond Broward County,” Baganz said. “As we look to grow our impact, we’re seeking opportunities to expand into Miami/Dade County. Support for additional resources and program personnel will be essential.”


BY Alexandra Paz, Ph.D.
Florida Atlantic’s Stiles-Nicholson Brain Institute (SNBI) achieved another year of growth and expansion in its community engagement efforts.
Brainy Days 2024, the Institute’s annual celebration of brain science, drew a record crowd of more than 1,000 attendees across a series of events. Here’s a look at some of the keynotes they came to hear:
• The speaker lineup kicked off at SNBI, located on Florida Atlantic’s Jupiter campus, with John Cryan, Ph.D., from University College Cork in Ireland, exploring the link between brain health and the gut microbiome, revealing how people’s diet shapes who they are
• The Cox Science Center and Aquarium hosted Anjan Chatterjee, Ph.D., of the University of Pennsylvania, delving into the neuroaesthetics, uncovering the neural processes that drive perceptions of beauty
• And, David Richardson, Ph.D., founder director of the Center for Healthy Minds at the University of Wisconsin-Madison, a renowned expert with decades of research experience, sharing insights on the transformative power of meditation on brain health and overall well-being
Each talk was followed by a reception and book signing, providing attendees with a unique opportunity to connect with the speakers and like-minded individuals.




In addition to the Briany Days guest speaker series, the Institute hosted a range of events that showcased the intersection of art, science and community. The Ryan Licht Sang Foundation’s INSIGHTS VI exhibit transformed the SNBI auditorium into a vibrant art museum, featuring poignant artworks created by artists living with bipolar disorder. This display sparked important conversations and raised awareness about mental health, providing a unique platform for artistic expression.
In addition, the Brain Sparks event at the Cox Science Center drew more than 500 attendees for a day filled with neuroscience activities, inspiring the next generation of neuroscience explorers.
Additionally, the Institute partnered with the Mind, Music and Movement Foundation (M3F) for its Rhythms for the Brain event at the Kravis Center in West Palm Beach, a captivating event that combined talks, dance demonstrations and performances by the Voices of Parkinson’s Chorus.







The SNBI and the Jupiter Life Science Initiative also teamed up to host Neural Nexus: Fusing Science, Art and Creativity at SNBI, which brought together internationally renowned artist and neuroscientist Greg Dunn, Ph.D,. and author Susan Nalbantian, Ph.D., for the fusion of science, art and creativity, followed by a post-event reception.
“Through events like Brainy Days, we foster a culture of collaboration, creativity and inclusivity, ultimately driving innovation and improving lives,” said Nicole Baganz, Ph.D., director of community engagement and programming at SNBI.
Community engagement efforts continued with a special pop-up Brain Sparks event at the Gardens Mall, bringing neuroscience fun to a new audience, as well as the Advancing STEM Community Engagement through Neuroscience Discovery (ASCEND) after-school program, NeuroExplorers returning, allowing 18 students to embark on a semester-long journey to becoming junior neuroscientists and explore the intricacies of the brain.
In culmination, the MobileMinds program made significant strides in bridging the STEM gap in Title I schools, engaging more than 6,000 students across four South Florida counties with interactive neuroscience
March 3
The Power of Arts and Science for Resiliency in Aging
Presented by Mind, Music and Movement Foundation
Osher Lifelong Learning Institute, Jupiter
March 10
Ribbon Cutting - David and Lynn Nicholson Center for Neurodegenerative Disease Research
Sir John Hardy, Ph.D., Keynote Speaker
Topic: The Genetic Origins of Alzheimer’s Disease
Stiles-Nicholson Brain Institute, Jupiter
March 18
Michael C. Burton, Ph.D.
Topic: Nervous System and Immune System Communication
Stiles-Nicholson Brain Institute, Jupiter
experiences. The Institute also received a grant from the Sharron and Joseph Ashby Hubert Fund of the Community Foundation of Broward, which included funding to purchase an additional ‘brain van,’ allowing MobileMinds to extend its reach into Broward County.
“By bridging the gap between our neuroscience community, researchers, educators and the public, we can unlock a deeper understanding of the human brain and its many mysteries,” Baganz said.
Formally known as C. elegans, the tiny, inauspicious roundworm is a favorite of neuroscientists seeking to identify and manipulate genes that impact neural signaling and health.