
64 minute read
Fish Health and Welfare
Duty of care

Looking after fi sh wellbeing is an imperative for the industry



Fish welfare is in the news. The aquaculture industry has come under fi re, ini� ally a� er a report from Compassion in World Farming (CIWF) – accompanied by undercover video footage showing dead and injured fi sh – alleged poor prac� ce in the Sco� sh salmon farming industry. Then, a controversial documentary shown on Ne� lix – Seaspiracy – took aim at the fi shing industry as a whole, including aquaculture.
The allega� ons and conclusions in both the CIWF report and Seaspiracy are contested by the industry. Responsible fi sh farm operators take the welfare of their stock seriously, not only for economic reasons but also because the industry needs to do the right thing – and to be seen to do it.
Governments are also serious about fi sh welfare and they have also challenged the industry recently. In Norway, a government study of premature mortali� es in salmon farming has found that 2020 was one of the worst years for mortali� es in the country’s farms. The Norwegian Veterinary Ins� tute has called for “new thinking” to address the ma� er (see Vince McDonagh’s ar� cle, page 34 of this issue).
In Scotland, the Farmed Fish Health Framework under Government agency Marine Scotland has set out a clarifi ed set of priori� es: addressing the causes of fi sh mortality, understanding the impact of climate change and the development of treatments.
The steering group for the 10 year framework, a collabora� on between the aquaculture sector and Sco� sh Government and its advisers, will be chaired by The Sco� sh Government’s Chief Veterinary Offi cer Dr Sheila Voas.
The key priori� es for the framework will be: • developing a consistent repor� ng methodology for collec� on of informa� on on the causes of farmed fi sh mortality, and providing survival data; • addressing the impact of climate change and ocean acidifi ca� on, including real-� me monitoring of plankton and mapping clima� c condi� ons around Scotland’s coasts; and • encouraging the development of new medicines with the aim of increasing treatment fl exibility.
As from 29 March, repor� ng on sea lice numbers is now mandatory for fi sh farmers in Scotland. While previous arrangements required repor� ng only where specifi c levels were met or exceeded (a weekly average of two adult female sea lice per fi sh), sea lice numbers will now need to be reported weekly, one week in arrears, to Sco� sh ministers irrespec� ve of the count. Where no count is conducted the reason must be given.
Meanwhile the industry itself con� nues to fi nd ways to be� er protect its stock. Vaccina� on has long been a game-changer against many biological threats, but since fi sh are very sensi� ve to handling, automated and semi-automated machines need to ensure that vaccines are delivered effi ciently, accurately and in a way that has least impact on the fi sh.
Aquaculture medical group PHARMAQ has been providing vaccina-

“Repor� ng on sea lice numbers is now mandatory” LET AutoTend TAKE CARE OF YOUR EGGS! LET AutoTend TAKE CARE OF YOUR EGGS! LET AutoTend TAKE CARE OF YOUR EGGS! LET AutoTend TAKE CARE OF YOUR EGGS!
AutoTend is the first robot of AutoTend is the first robot of its kind designed for automatic egg and fry tending. AutoTend is the first robot of its kind designed for automatic egg and fry tending. its kind designed for automatic egg and fry tending. AutoTend is the first robot of its kind designed for automatic egg and fry tending.
Top: Nofi ma found hydrolysed krill in feed led to improved skin quality for smolts Above: Dr Sheila Voas
Advanced machine vision which Careful handling of roe and Careful handling of roe and fry using suction to extract, and Advanced machine vision which allows for a dual evaluation of the Careful handling of roe and fry using suction to extract, and slight pressure to move the upper layers of roe and fry for access to the lower layer. Advanced machine vision which allows for a dual evaluation of the object before it is removed. This means that no healthy eggs or fry will be accidentally discarded. Careful handling of roe and fry using suction to extract, and slight pressure to move the upper layers of roe and fry for access to the lower layer. Advanced machine vision which allows for a dual evaluation of the object before it is removed. This means that no healthy eggs or fry will be accidentally discarded. fry using suction to extract, and slight pressure to move the upper layers of roe and fry for access to the lower layer. allows for a dual evaluation of the object before it is removed. This means that no healthy eggs or fry will be accidentally discarded. object before it is removed. This slight pressure to move the means that no healthy eggs or fry will be accidentally discarded. upper layers of roe and fry for access to the lower layer. WATCH THE WATCH THE VIDEO! WATCH THE VIDEO!
VIDEO!
See AutoTend in action at See AutoTend in action at See AutoTend in action at alvestad.com/en/produkter/autotend/ alvestad.com/en/produkter/autotend/ WATCH THEalvestad.com/en/produkter/autotend/ VIDEO!
For more information please contact us today For more information please contact us today
See AutoTend in action at alvestad.com/en/produkter/autotend/ For more information please contact us today alvestad.com/en alvestad.com/en alvestad.com/en

� on machines since its acquisi� on of Nordland Fishtech and Nordland Se� vaks in 2017. PHARMAQ’s NFT semi-automa� c vaccina� on machines come in three models to deliver diff erent vaccina� on regimes. All are capable of vaccina� ng 2.4 fi sh per second, 8,500 per hour.
Aqualife, located in Scotland, Norway and Portugal, has also developed a semi-automa� c vaccina� on system. The Aqualife SAIB machines are designed so that they can be adapted to deliver for a range of species – not just salmon, but, for example, � lapia or pangasius. The model was developed and patented in 2020, and is expected to be commercially available from July this year.
The Aqualife system requires less than half the operators of a tradi� onal manual vaccina� on team and requires li� le training as fi sh are simply fed into it. It has been designed to be simple, small, light and extremely quiet when compared to other systems on the market and can quickly and easily switch between vaccina� on strategies and species at the touch of a bu� on.
Vaccina� ng fi sh – and the handling that this requires – can be a tricky task. MSD Animal Health UK not only provides the vaccines themselves but also monitors the staff delivering the vaccines to see whether addi� onal training or guidance is required. Because ge� ng monitors out to a remote loca� on can be problema� c, MSD has developed “smart glasses” that enable its veterinary experts to oversee the vaccina� on process and fl ag up anything that needs a� en� on. The glasses can be worn and controlled hands-free by the user, and provide a visual link for the MSD expert (see page 42 for more on how this has been applied in prac� ce).
Feed is also an important factor for fi sh health. Two recent studies from the Norwegian Ins� tute of Food, Fisheries and Aquaculture Research Nofi ma looked at the impact of feeding regimes. One, recently reported in this magazine (Fish Farmer, March 2021), found that giving the wrong feed to ballan wrasse – an important cleaner fi sh species used to control sea lice – could have an adverse eff ect on their health. The study found that young fi sh fed on extruded feeds – where the feed materials are processed at high temperatures – are more likely to develop skeletal deformi� es. (For more on cleaner fi sh health see “Looking a� er lumpfi sh”, on page 48 of this issue).
Another project found that salmon smolt given feed that includes hydrolysed krill grow faster and are more likely to survive their introduc� on to the sea, according to researchers at Nofi ma.
The study aimed to fi nd ways to reduce smolt mortality and improve growth for the cri� cal





fi rst year at sea. The study, carried out in collabora� on with krill producer Rimfrost, used a hydrolysed krill-based protein, OlyPep.
The researchers found that even small quan� � es of the krill protein had a posi� ve impact on feed intake and growth. Senior Researcher Dr Sissel Albrektsen says: “In our experiments we observed that the rela� vely small quan� � es of hydrolysed krill added to the feed produced great results. We no� ced that the smolt quickly start feeding and we saw a greater increase in weight. Larger, more robust fi sh can quickly improve the economic situa� on of fi sh farmers.”
The study found that the krill feed also helps the smolt develop healthy skin. Dr Albrektsen explains: “By using an advanced fi sh skin analysis tool in a follow-up experiment involving salmon being released into the sea, we found fewer incidences of injuries and dark pigments in the skin of fi sh which had hydrolysed krill in their feed. This shows that fi sh which eat and grow well develop thicker skin at an earlier stage compared to fi sh that start feeding at a later stage. Be� er quality fi sh skin means more robust fi sh which are less prone to injury and disease. Addi� on of krill hydrolysate can help to improve fi sh health and the economic situa� on of fi sh farmers.”
Meanwhile a study commissioned by feed company Tecnovit has found that a food supplement based on natural ingredients can help protect farmed gilthead sea bream against a common parasite, Sparicotyle chrysophrii.
AROTEC-G® is a microencapsulated func� onal product composed of a blend of garlic essen� al oil, carvacrol (found naturally in the herb oregano) and thymol (derived from thyme and other plants).
The research revealed that fi sh fed with the supplement gained addi� onal protec� on for their skin and gills against the parasite, and their mucus was also more resistant to several strains of bacteria (more details on page 50).
Biological challenges are among the biggest headaches for the aquaculture industry – so staying up to date with the latest science is a must. FF

Far left: This is the kind of image nobody in the industry wants to see
(photo: Compassion in World Farming) Left:: MSD vaccina� on Above left: Dr Sissel Albrektsen, Nofi ma
Above right: The Aqualife SAIB machine Right: Sea louse
How Foover can help
A new system can help remove mortalities quickly and effi ciently

Standard livestock farming systems have been developed to be effi cient at growing animals, but as in nature there is always a proportion which won’t make it through to harvest. Although this is a subject which many fi nd unpalatable, mortality is a fact of farming and dealing with it is an important part of animal husbandry.
The salmon farming sector has come in for signifi cant criticism over mortality levels in recent years, but understanding mortality and reducing its occurrence is one of the key measures in the 10year farmed fi sh health strategy.
The industry standard, and best practice, is to remove any dead fi sh on a daily basis. This can be challenging, but the Foover mortality recovery system developed by UCO provides a fast and effi cient way of recovering and verifying that all mortalities have been collected. This is benefi cial to stock health by reducing both the viral load in the pen and the potential for issues to escalate and spread, which is in line with the principles of the Scottish Code of Good Practice.
Predator attacks by seals can also have a devastating effect on the welfare of farmed salmon, and removing potential food sources like mortalities at the earliest opportunity can reduce the potential for attacks.
At Underwater Contracting, we have found that using the Foover system ensures that all pens can be verifi ed mortality-free on a daily basis, reducing the potential for fi sh health issues and maintaining the health and welfare of the stock, which makes fi nancial sense.
www.underwatercontracting.com


Above: Atlan� c salmon Left: The Underwater Contrac� ng Offi ce

The jab that’s just the job

The new generation of vaccination machines are safe, effective and economical
BY JAN OPPEN BERNTSEN, PHARMAQ FISHTEQ AS AND CHRIS MITCHELL PHARMAQ LTD
Immunisation of stock is now a standard practice in salmonid aquaculture and has been so for nearly three decades. In some countries it is actually illegal to transfer unvaccinated smolts to sea. The main routes of vaccine delivery are either through immersion or injection, and the methodology of the latter is developing rapidly. Whereas only a few years ago the vast majority of injections were delivered by hand into the fish’s peritoneum (IP), recent developments in both vaccine and vaccination technologies have seen some vaccines now requiring intramuscular delivery (IM). Modern vaccination machines must therefore be capable of both delivery routes. In Norway, the proportion of transferred smolts that have been machine-vaccinated is now more than two-thirds of the total and growing.
This important trend was largely behind the acquisition of Nordland Fishtech and Nordland Settvaks by PHARMAQ, part of Zoetis in 2017. At that time, these two Nordland sister companies were fledgling players in the growing vaccination sector of Norwegian aquaculture and had already developed the semi-automatic NFT fish vaccination and sorting machines. PHARMAQ saw this as an interesting opportunity to bring mechanised vaccination to the global aquaculture industry through its established international network.
The NFT machines are developed and fabricated in the heart of the Norwegian salmon farming area – Helgeland in Nordland county – very close to many end users. These fish farmers have been instrumental in contributing their knowledge, experience and expertise to the NFT team who have now developed three different models from the original NFT concept.
“At Pharmaq we focus on enhancing our customer’s experience by understanding their needs which we recognize vary between customers and which is why we have developed three different NFT models. It is the customer’s vaccination regimen – i.e. what combination of vaccine formulations are used to immunise their fish – that decides the appropriate model for their circumstances,” says Managing Director of PHARMAQ Fishteq Dagfinn Strømme.
The NFT Fish Vaccination Machines Models
The machines are compact and robust, and occupy a small footprint compared to similar machines on the market. In addition, capacity can be upscaled to suit bigger operations by joining two machines over a common assembly table. PHARMAQ Fishteq currently produces three models, the NFT 20, NFT 25 and NFT 30. All models are designed to deliver different combinations of all commercially available fish vaccines according to the following:


Left: Dagfinn Strømme Above: The NFT Fish Vaccination Machine Top right: The sorting module splits vaccinated fish into three sizes which can be selected by the operator. If, occasionally, a fish has not been vaccinated it is sent down the fourth channel

• NFT 20: Delivers one IP vaccine. • NFT 25: Delivers two IP vaccines through one needle, simultaneously.
The vaccines can be of different formulations, such as water-based and oil-based. It comes with a specially developed duo-adapter that ensures that all individual doses are perfect mixes of the component vaccines. For special requirements, the NFT 25 can also be reconfigured to deliver three vaccines simultaneously in a single injection. • NFT 30: Delivers one or two IP vaccines. In addition, it has a special
DNA intramuscular (IM) module that is capable of delivering vaccines that have been formulated to be administered through this route.
All the models are capable of vaccinating 2.4 fish per second at full capacity, 8 500 fish per hour. This eliminates extra handling of the fish so shortening the time spent out of water and ultimately leading to better fish welfare and production economics. Less stress for both the fish and the operators!
Facts about the NFT Vaccination Machines
Currently, the NFT machines can be used to vaccinate the following fish species: • Salmon • Trout • White fish • Arctic charr • Sea bass • Tilapia
The machines handle and vaccinate fish ranging in size between 120 mm and 250 mm (20-150 grams). They use a Machine Vision System to determine the inoculation site on each fish. This is achieved through image recognition software that has been specially developed to account for size and species in determining where the needle should be placed for each injection. Dose accuracy is an impressive +/- 2 %.
Vaccinated fish can be sorted into three different size categories which can be set by the operator prior to vaccination. In addition, the machine has a channel for misplaced, undersized or rejected fish. All data pertaining to the number of vaccinates, size and distribution can be easily exported from the machine by e-mail.
In addition, the machines have built-in warning and reminder systems to alert operators as to when important component changes are required as well as if there has been a fall in the pressure required to correctly inject the vaccines.
The operational requirements of the NFT machines include a supply of water, electricity and dry, compressed air. Internet is also needed for access to software updates and on-line support. FF

KEY BENEFITS
So, what are the key benefits of moving away from manual vaccination and towards using the NFT suite of vaccination machines?
• Two NFT machines sharing a common fish table and four operators, have the capacity to vaccinate 17,000 fish per hour.
This allows for fewer people on-site over a shorter period and, ultimately, better biosecurity. The high capacity also caters for large tanks that can be vaccinated in a single day – so there is less starve time required, less downtime and improved fish welfare. • Training of operators to become “super users” can be done without compromising the quality of injections; the machines also eliminate the risk of self-injection. • The machine can deliver up to three vaccines in the same operation, both intraperitoneal and intramuscular vaccines.
Auto-sorting of fish in up to three sizes can be achieved in the same operation, eliminating extra handling of the fish, shortening the time spent out of water and ultimately leading to better fish welfare and production economics. • The high accuracy of both dose volume (+/- 2%) and injection site (+/- 0.3 mm) ensures consistently high-quality vaccination hour after hour, the whole day through.
NFT MACHINES
E cient and precise vaccination with comprehensive quality control features
For more information, please contact Pharmaq Fishteq: dagfinn.stromme@zoetis.com | +46 23 29 85 00 | pharmaq.com



Far sighted strategy





Remote technology means that vaccination on fi sh farms can be assessed even when in-person audits are restricted




The Covid-19 pandemic put a halt to the majority of farm visits last year. This spurred MSD Animal Health UK to develop new strategies to provide addi� onal support and ensure that best prac� ce could be applied when carrying out vaccina� ons, despite the restric� ons.
A� er undergoing a rigorous trialling and tes� ng period, Claudia Marin, Aquaculture Field Technician at MSD Animal Health UK and Emily Underhill, Senior Technician and Fish Health Coordinator at Grieg Seafood, describe their experience of using Smart Glasses to carry out virtual vaccina� on assessments.
First, why were Smart Glasses developed?
“Even before the pandemic hit, we had been thinking about addi� onal tools and resources we could add to our portfolio to be� er support customers,” explains Claudia.
“We s� ll believe face-to-face contact is extremely important, but we recognised that remote tools would prove benefi cial as well. This is due to recircula� on aquaculture systems having high biosecurity standards, meaning frequent visitors can pose a disease risk. Addi� onally, many hatcheries are in very remote areas, so it can take upwards of fi ve hours for us to reach them,” explains Claudia.
“Usually, we would visit sites at least once during a vaccina� on event, so any tool we developed needed to allow us to maintain this level of support.”
Smart Glasses are a hands-free, wearable device, which are controlled via voice command. Once the glasses are set-up, the user, whether that be a farmer, manager or vet, wears the device during vaccina� on events.
These are connected to the MSD Animal Health team who can see, on their computer screen, exactly what the user is looking at. The team can write messages in a ‘chat’ func� on to the user which the device will then read out verbally through an earpiece. The MSD Animal Health team can hear exactly what the user says, meaning responses can be spoken rather than wri� en.
“Normally, we’d be next to the farmers checking the fi sh to assess vaccina� on accuracy and carry out internal inspec� ons to see if the vaccine has been deposited correctly. With Smart Glasses, we simply see exactly what the user sees, they will then carry out the dissec� on and we will view this over the




glasses providing our assessment and feedback in real-� me but remotely,” says Claudia.
Emily Underhill, senior technician and fi sh health coordinator at Grieg Seafood, explains that when she was ini� ally approached by the MSD Animal Health team about whether she wanted to trial the Smart Glasses, she felt it was an opportunity worth taking.
“Due to the restric� ons and our remote loca� on in Shetland, it’s been really challenging to get people into the hatchery. So the opportunity to carry out remote audi� ng, s� ll to the high level of face-to-face audits, really appealed to us. The camera sees exactly what you’re seeing, so the auditor can view the fi sh in great detail and thoroughly assess vaccina� on effi cacy.”
Emily feels that using modern technology is a great way for the aquaculture industry to con� nuously develop, and once you understand how this par� cular device works, it’s really easy to use.
“Ini� ally, Claudia visited us to correctly setup the glasses and carry out training on how to use them. We were then able to take the support she gave us and use that prac� cally. The MSD Animal Health team has been very thorough in making sure we fully understand the technology before using it. This included carrying out a prac� ce audit before the actual event to make sure that when the � me came, the vaccina� on event ran smoothly.”
As fi sh health and welfare is a key priority for Grieg Seafood, using Smart Glasses has enabled the team to maintain their high standards throughout these challenging � mes.
“One of the great benefi ts of the Smart Glasses is that you are able to access immediate support from the MSD Animal Health technical team. In the past, it would take the best part of a day for someone to reach us, whereas with the Smart Glasses someone is able to connect with us within an hour and help us resolve an issue.”
Looking to the future, when hopefully restric� ons are eased, Emily plans to con� nue using Smart Glasses alongside face-to-face support.
“Although I don’t think Smart Glasses completely remove the importance of having someone present on-site, they do work really eff ec� vely and so are a good tool to use alongside in-person support. The fact that we have s� ll been able to carry out vaccina� on and pre-transfer audi� ng despite on-farm site visits not being possible has been really important.
“For us, this has defi nitely shown what’s possible in the future and how we can make vaccina� on events easier despite being in such a remote loca� on.”
Claudia explains that MSD Animal Health is planning to roll the device out to more customers who would like to u� lise this remote tool.
“To use Smart Glasses it’s really essen� al to have good connec� vity, so if customers want to use the device, we will carry out a site visit fi rst to pilot the glasses and check the connec� vity is good enough. Once we’ve checked this, we will then carry out a face-to-face training session with the customer to set-up the device making sure it is ready to go.”
For more informati on about Smart Glasses, look out for the latest episode of the Producing Healthy Salmon podcast series. If you’re interested in using the device, contact your local MSD Animal Health representati ve. FF
Opposite: Claudia Marin Above Emily Underhill
Update: Novel spinal deformities in Norway Update: Novel spinal deformities in Norway
In response to a complaint regarding an advertorial titled, “Vaccine Breakthrough” that Elanco placed in Fish Farmer Yearbook 2021, we wish to clarify that all the data and conclusions written in that advertorial are founded solely on experience in Norwegian salmon operations.
One of the main references used for the advertorial was a review commissioned by the Norwegian Ministry of Trade, Industry and Fisheries from the Norwegian Veterinary Institute. The objective of the review was to verify one of the allegations made by various Norwegian farmers against compulsory PD vaccination in light of the side effects, in the form of cross stitch spinal deformities, they had experienced following the use of two oil-adjuvanted PD vaccines. The certified translation of the letter summarising the review is hereby presented in full:
Introduction
In a letter dated 27 May 2020 from the Norwegian Ministry of Trade, Industry and Fisheries (NFD) to the Norwegian Veterinary Institute (VI), the NFD writes the following: “With regard to instructions concerning the vaccination of fish against PD, it has emerged that the incidence of adverse events may be higher than previously thought. This has caused the Ministry to request a technical assessment from the Norwegian Veterinary Institute. We make reference to the discussions we had with the Veterinary Institute regarding an introductory study to determine the incidence of adverse events and impacts on fish welfare (dive reference 19112399). We have attached to Veterinary Institute’s project outline” Reference is made to a project outline for the analysis of data from the aquaculture industry and vaccine companies dated 15 May 2020 from the Veterinary Institute. On the basis of one such analysis, the NFD asks the VI to assess how the vaccination order (the PD regulation, Section 7) will affect the welfare of vaccinated fish. The NFD also writes that the assessment should include results from other relevant research projects, including an ongoing project led by NOFIMA. A project group at the VI was established for the assignment on 24 June and the group has carried out a survey of existing information/data. All fish placed in the sea in Norway are vaccinated against the most common bacterial diseases. Most available PD vaccines are 1-component and therefore a different vaccine (typically 6-component) will always additionally be used. As of now, the following PD vaccines are available on the Norwegian market: MSD Animal Health • Norvax Compact PD vet (1-component, inactivated, oil-adjuvanted). Marketing authorisation (MA) August 2011, but on sale from 2009 under special registration exemption. • Aquavac PD7 vet (7-component, inactivated, oil-adjuvanted). MA February 2015, on sale from July 2015. Pharmaq • Alphaject micro 1 PD (1-component, inactivated, oil-adjuvanted). MA November 2015, market access from April 2017 due to patent. Elanco • Clynav (1-component, DNA vaccine, nonadjuvanted). MA June 2017, on sale from
January 2018 The project outline dated 15 May 2020 from the VI describes an arrangement where the VI has access to raw data from external stakeholders and processes these statistically. It became clear that most stakeholders are unwilling to obtain and hand over raw data for this purpose. Raw data is hard to derive meaning from compared to a report or presentation. Since adverse events in the form of spinal deformations are mostly found in harvest results, it can require a lot of resources to trace back to information on the vaccine used and the number of affected fish. Only one aquaculture company was willing to provide raw data that included all exposures over a given period using different vaccines. From the other companies we have processed data and the stakeholders’ own conclusions. A summary of the information we received, sorted by source, is given below:
Norwegian Medicines Agency (NoMA):
Manufacturers of medicinal products are obliged to report serious events to the NoMA within 15 days of the event. Non-serious events are reported in regular reports (PV/AE cases), and for products which have been on the market for more than 5 years, such PV/AE cases are sent to the NoMA every 3 years. The Norwegian Medicines Agency summarizes the situation as follows: “The Norwegian Medicines Agency recorded an increased incidence of adverse events for a new type of spinal deformity (“cross-stitch vertebrae” +/- connective tissue formation and melanin along the spine) in relatively large/harvestready salmon for the vaccines Aquavac PD7 and Alpha Ject Micro 1PD especially. On this basis, the Norwegian Medicines Agency contacted the manufacturers of these two vaccines for a more detailed assessment of the incidence and causation of these adverse events. (...) the information we have received indicates that PD vaccines may be involved in these “adverse events”, but that there are most likely to be multifactorial causes. According to Pharmaq, no deformities similar to those seen in Norway have been reported for their vaccine in the UK and IE. We have received a notification of adverse events for Clynav describing deformities, but the notification does not contain enough information to indicate whether this refers to “cross-stitch vertebrae”. Clynav was introduced onto the market in January 2018 and there is currently a limit to how many Clynav-vaccinated fish are harvested. “ “The Norwegian Medicines Agency cannot draw a final conclusion on the causal relationship between spinal deformities and PD vaccines. However, we believe it is relevant that, as of today, information in the adverse events section of the summaries of product characteristics, if any, may indicate a causal relationship between the PD vaccines and “cross-stitch vertebrae”. New text for the adverse events section for Aquavac PD7 has been completed. Please refer to the attached updated SPC. The case for Alpha Ject micro 1PD is under consideration”.
The VI has sent all the notifications from the NoMA’s records regarding PD vaccines from 01.01.2016 up to and including 13.08.2020, a total of 108 cases. The reports were received in PDF format and a lot of information is available as free text. To get an overview, we have counted the number of cases that contain information about “spinal deformations”, also called “spinal deformities” and “cross-stitch vertebrae”. There are several types of spinal deformities and the most accurate classification is achieved by means of x-ray analysis. The diagnosis of cross-stitch vertebrae is made by means of x-ray analysis and was registered for the first time at the NoMA in the course of 2017. In one of the first cases, the diagnosis was unknown and terms such as “new type of deformity” and the like were used. Some of the cases from 2018-2020 lack information on cross-stitch vertebrae and perhaps no x-ray analysis was performed. This means that the total number of cases of cross-stitch vertebrae may be higher. If one assumes that the medicinal product manufacturers have met their obligations and have the same threshold for reporting suspected adverse events to the Norwegian Medicines Agency, the number of cases for each product may give an indication of the scope. Of a total of 108 cases reported in relation to PD vaccines, 76 adverse event cases concern spinal deformities, of which 46 cases are recorded with findings of cross-stitch vertebrae (see Table 1).
Table 1. Number of adverse events per PD vaccine, reported to the Norwegian Medicines Agency relating to spinal deformities or cross-stitch vertebrae, in the period 1.1.2016-13.08.2020.
Vaccine Cases of spinal deformity
Cases of cross-stitch vertebrae
AQUAVAC PD7 59 37 ALPHA JECT 11 3 micro 1 PD
CLYNAV 0* 0
NORVAX COMPACT PD
Total 6 6
76 46
*One case that is not conclusive, possible findings of spinal deformities.
Most recorded cases were for Aquavac PD7 (MSD Animal Health) and this is also the first of the newer PD vaccines and was made available in the summer of 2015. Alphaject micro 1PD (Pharmaq)
came on sale in Norway in the summer of 2017 and Clynav (Elanco Europe Ltd) came on sale in the winter of 2018. Norvax Compact PD is the oldest PD vaccine and has been on sale since 2008. Adverse events in the form of spinal deformities are mainly recorded on salmon over 3 kg, often during quality control at the slaughterhouse. When reviewing the cases, in many cases it is challenging for the manufacturer of the medicinal product to obtain information on the proportion and number of affected individuals. In some cases, estimates are based on a reported decrease in % “superior” salmon, and information from the so-called “100-fish control”, where the reason for down-classification is registered (spinal deformations may be a category). At other times, private visits are made to the slaughterhouse with counts and the taking of sample for x-ray analysis, and in some cases it has not been possible to collect data or samples, and estimates have been based on oral information. The extent of spinal deformities varies from 70% (conservative estimate) to 1% of PD-vaccinated salmon in a slaughter pen, and the spread is greatest for cases with Aquavac PD7 (a total of 59 cases). In the 11 cases registered for Alphaject micro 1PD, there are two cases with >25% spinal deformities, while the remaining are 2-8%. For the 6 cases registered for Compact PD, there is 1 case with a maximum of 14% spinal deformities (here a low incidence of crossstitch vertebrae is indicated), in the other cases the incidence is 2.5-6%. For Compact PD, which had a monopoly in the PD vaccine market from around 2008 to 2015, no cross-stitch vertebrae were registered in 2017. Whether this is due to changes in production conditions in the last few years, or an increased awareness of spinal deformations and PD vaccination, is unknown. The vaccine manufacturer MSD Animal Health has recently updated its Aquavac PD7 package leaflet. Increased risk of adverse events in the form of cross-stitch vertebrae, especially when vaccinating fish under 1 year old (autumnrelease), is now included. It is referred to as a “common” adverse event, i.e. occurs in more than 1 but fewer than 10 in a hundred individuals. The vaccine manufacturer Pharmaq is in process of updating its package leaflet for Alphaject micro 1 PD. The fact that both manufacturers are changing their package leaflets indicates that they accept that the products are associated with the adverse event of cross-stitch vertebrae.
NOFIMA in the person of Dr. Grete Bæverfjord:
Nofima is responsible for the ongoing FHF [Norwegian Seafood Research Fund] project 901430 “Prevention of cross-stitch vertebrae in farmed salmon”, together with INAQ, Pharmaq AS, Pharmaq Analytic and NMBU. The project started in August 2017 and will be completed in December 2020. Dr. Bæverfjord is leading the project and clearly states that there is a connection between some PD vaccines and crossstitch vertebrae. We have had access to 2 project presentations, 1 partial report from INAQ, and 2 scientific publications related to the project. • Field data and sample materials - analysis of risk factors (responsible party: INAQ) Production data from various salmon producers have been collected to identify risk factors for spinal deformations and the development of cross-stitch vertebrae. We have had access to a partial report, which includes salmon smolt released in the period 2015-2017. There is no indication how many aquaculture enterprises are included or which geographical areas the data are obtained from, but since only 2 vaccine types are included (Norvax Compact PD (mono PD) and Aquavac PD7 (7-valent PD)), this indicates that the facilities are from a SAV3-endemic region where PD vaccination is common. When it comes to delimitations in method, the report says the following: “In order to identify possible links between production factors and the development of crossstitch vertebrae, it was necessary to determine whether or not fish had developed cross-stitch vertebrae. The development of cross-stitch deformation is shown to be difficult to detect from external observation and there is no simple diagnostic test available. (...) However, fillet analysis at a slaughterhouse, where one has growth of connective tissue in the fillet, as well as malformation in the spinal column will be indicative that spinal deformation may be due to cross-stitch vertebrae. “
To determine whether fish had developed malformations of the spine, information from 3 different sources was used: 1) Quality reports from slaughterhouses with further processing (N= 122 357 fish, 105 cages, 60 autumn release, 45 spring release), 2) Fillet analyses at the slaughterhouse under the direction of the project (N= 360 fish, 20 cages, 17 autumn release and 3 spring release) and 3) X-ray analysis under the auspices of the project (N=393 fish, 28 cages, 24 autumn release and 4 spring release). All 3 data sources show a significant relationship between vaccine type and malformations of the spine. The quality reports from processing facilities were well distributed between fish released in spring and fish released in autumn, and the analyses showed the timing of release had an obvious impact: “The predictions show that one can expect about 6% of fish to develop spinal deformation, if the fish is vaccinated with PD 7 and released into the sea in autumn. There is significant variation in the predictions associated with random impacts on the fish group, from in the region of 0 to 64% for autumn-release fish vaccinated with PD 7. There was also a negative impact from the interaction between spring-release fish and the PD 7 vaccine, suggesting that the PD 7 vaccine is associated with significantly less risk of developing spinal deformation in spring-release fish. Predictions for fish vaccinated with Norvax PD in addition to other vaccines showed a lower risk of developing spinal deformation compared to fish vaccinated with PD 7 , both for springrelease and autumn-release fish” (...) No link was found between the development of spinal deformation and the type of salmon strain, the number of non-medicinal lice treatments, or whether the feed contained glucan (used in immunostimulating feed). • Experimental studies - verification of risk factors and mechanisms (responsible Nofima and Pharmaq). This work is still ongoing but it was orally communicated that the incidence of cross-stitch vertebrae in this study has been low. • By analysing vertebrae from salmon ready for harvesting, already categorised as “crossstitch” using “computed tomography” (CT), histology and scanning electron microscopy, injuries have been identified in an area of the bone tissue that may correspond to the growth zone at the time of smoltification (Holm et al 2020). It may coincide with the timing of vaccination, but primary and secondary causes of changes in growth at this stage are not discussed further in this work, and not at all in a similar article from the same project (Trangerud et al 2019)
Aquaculture enterprises
We have been in contact with a selection of aquaculture enterprises and requested access to data on the mapping of adverse events related to PD vaccination. The first priority has been the companies Lerøy Seafood, Mowi and SalMar, which in letters and meetings with the NFD expressed concern about fish welfare during the mandatory PD vaccination. None of these companies has provided access to raw data, but SalMar and Mowi have submitted processed data in the form of summaries from field studies comparing harvested or harvest-ready, PDvaccinated and non-PD-vaccinated salmon: One company sent information from 3 facilities where they have held vaccination trials with a socalled “mark & mix” setup, i.e. the vaccine groups are labelled and mixed at the time of release into the sea and exposed to identical environmental conditions in the same cage unit. All 3 trials have been carried out fish under one year old (autumn-release fish). In facilities 1 and 2, there were two cages with a “mark & mix” setup, respectively with a 75:25 and 50:50 mixing of AquaVac PD7 (PD vaccine) and Alphaject micro 6 (non-PD vaccine). In total, findings of crossstitch vertebrae were recorded in 14.6% and 16.2% of sampled fish in the PD7-vaccinated group, compared with 6.7% and 0% in sampled fish in the Alphaject micro 6-vaccinated group. Level of severity is not indicated. In facility 3, the “mark & mix” setup comprised approx. 80:20 mixing of Alphaject micro 1PD (PD-vaccinated) vs. Alphaject micro 6 (non-PD vaccine) in two cages. Removal of 50 random fish/vaccine/ cage for x-ray analysis showed an average of 15% affected by cross-stitch vertebrae in the Alphaject micro 1PD-vaccinated group, compared to 3% in the Alphaject micro 6 group. Severe deformations were found in 10% and 2% of sampled fish in the micro 1PD group, and 0% and 2% of the micro 6 group. In addition, we have obtained information from 1 facility (2017 generation, autumn-release), where 2 cages of Aquavac PD7-vaccinated salmon and 7 cages without PD vaccine (unnamed vaccine) have been compared. The cages with AquaVac PD7 had a lower superior proportion, and during filleting, cartilage was recorded in 46% and 50% of the PD7-vaccinated fish (0-5% in the others). Cross-stitch vertebrae were confirmed in nine out of ten affected PD7-vaccinated individuals, and the level of severity was high. Two major aquaculture enterprises with experience of PD vaccination in the SAV3 zone have been asked to share data. We received the following statement by email from one enterprise: “Our company (anonymised) experienced major problems with skeletal deformities and growth in 2015, 2016 and 2017 G in the sea (...) Main findings: Fish with the Aquavac PD7 vaccine had on average a risk of deformity 26%
greater than fi sh with the other vaccine. The more days between vaccination and release, the less deformities. The higher the seawater temperature in the fi rst 60 days in the sea, the more deformities. This is consistent with the hypothesis that autumn-release fi sh have the highest risk of skeletal deformities when using the PD7 vaccine”. The second enterprise sent a summary of fi ndings after harvesting two autumn-2017 releases, vaccinated with Alphaject micro 1PD. The analyses showed that 7-8% of the fi sh had deformations associated with cross-stitches, but the damage was relatively limited. Aquaculture enterprises with experience of PD vaccination in the SAV2 zone (Trondelag) have also been invited to share data/information. One enterprise has been willing to share raw data in the form of % spinal deformities during harvesting control of all production units (cages) harvested in the period 2017 to the current date (from Autumn 2015 release). This has given the Norwegian Veterinary Institute the opportunity to analyse data from a total of 202 cages. The percentage of spinal deformities was based on sampling 2x100 fi sh per day for quality control and categorisation of reasons for downgrading. No data are available on further classifi cation of deformities using x-ray analysis. The model demonstrated a clearly signifi cant association between PD vaccination and spinal deformities (p<0.001). The dataset was unbalanced in respect of diff erent PD vaccines, e.g. only 3 units with Clynav. Therefore, the association between diff erent types of PD vaccine and deformities was inconclusive, for example, it cannot be concluded from these data that vaccination with Clynav resulted in an increased risk of spinal deformity.
Figure 1. (Below) Proportion of fi sh with spinal deformities per vaccine type shown as a “Boxplot”. The line in the centre of the box is the median (the most commonly occurring % spinal deformity value), and the upper and lower wall of the box shows the area where 50% of the values are located. The 25% lowest values are located along the line under the lower wall and the 25% highest values are located along the line above the upper wall. Some of the boxes have N only equal to 2 or 3 and therefore have little information value.
When comparing PD-vaccinated fi sh with non-PDvaccinated fi sh, the likelihood of spinal deformities when harvesting PD-vaccinated fi sh is 4.4 times greater. The model also indicated a positive signifi cant association between spinal deformities and average weight at harvest, number of harvested fi sh per cage and smolt supplier, but the eff ect was much less than PD-vaccination. There was no signifi cant association between spinal deformations and timing of release (autumn or spring) as opposed to what was shown in the FHF project. One explanation might be that the FHF project mainly had harvest data from the SAV3 area, where there may be greater temperature diff erences between autumn and spring than further north. Modelling based on data from the SAV2 area predicted that approx. 4% of PD-vaccinated salmon and approx. 1% of non-PD vaccinated salmon can be expected to have spinal deformations at the time of harvesting. The FHF project did not include data from salmon without a PD vaccine, but predicted approx. 6% spinal deformations for Aquavac PD7-vaccinated salmon, with slightly greater variation than in our analyses. This shows that it is worthwhile collecting and analysing data from geographical and/or production-relevant areas where specifi c measures are implemented. In addition, we have obtained summaries of analyses carried out after complaints after harvesting of 1 site vaccinated with Alphaject micro 1PD from another enterprise in the Trondelag. In this case, a prevalence of 5-8% of fi sh with spinal deformities and connective tissue/cartilage was assumed, and the crossstitch pathology was verifi ed at the harvest line using x-ray analysis of three selected individuals with deformities.
Pharmaceutical companies
MSD markets the inactivated oil-adjuvanted vaccines Norvax Compact PD and Aquavac PD7. From MSD, the VI was sent a presentation shown at the specialist conference “PD TriNations 2019”. In the presentation from MSD, a selection of fi eld observations is shown with samples at the time of harvest. There is a large variation in the incidence of diff erent types of spinal deformations between diff erent fi sh groups, also when it comes to autumn-releases vaccinated with PD7 (in accordance with the FHF project). This indicates
20
Spinal deformities (%)
15
10
5
0
Alpha Ject Micro 6 (N=62) Pentium Forte Plus (N=3)
MSD Aquavac 6 (N=2) Mixed no-PD (N=11) Alpha Ject Micro 1 PD (N=74) Aquavac PD7 vet (N=38) Clynav Mixed PD (N=3) (N=9) other unknown (production) factors that aff ect the risk of deformities developing, i.e. the problem is multifactorial. Furthermore, the following is summarised from 1 controlled study, testing diff erent vaccines with and without a PD component on 1 autumn release in 2017: • Various categories of spinal deformations are recorded for all fi sh, irrespective of vaccine • Cross-stitch vertebrae were only observed in groups where the PD component was included in the vaccine • The cross-stitch vertebrae were not visible on the X-ray analysis until 2.5 months before harvesting • The addition of B-glucan in feed has no eff ect on development of cross-stitch vertebrae Pharmaq markets the inactivated oil-adjuvanted vaccine Alphaject micro 1 PD Pharmaq provided information that the company is in an ongoing process with NoMA of updating the package leafl et. They have informed their customers of the risk of adverse events when vaccinating autumn-release smolt. Otherwise, please refer to information provided to the VI in November 2019 under a confi dentiality agreement. This information does not address adverse events to any signifi cant extent and is consistent with information received from the NoMA. Otherwise, Pharmaq confi rms: For the regions south of Hustadvika, the usage pattern for PD vaccines is roughly as follows: • Fish vaccinated with Clynav do not appear to be aff ected by spinal deformities and Clynav is the vaccine most used on fi sh less than 1 year old. • The oil-based, inactivated vaccines are most commonly used on 1-year-olds that are not at risk of the new type of adverse events. • Overall, approx. 98 % of all fi sh are vaccinated against PD in these areas. This is done on a voluntary basis without any instruction from the authorities. Assessment of the risk profi le when using PD vaccines in the SAV 2 area must be seen in this context. • Several of the major producers who may be going to vaccinate in the SAV2 area have successively used the same PD vaccines in the
SAV3 area on a voluntary basis. Elanco markets Clynav, monovalent DNA vaccine Approx. 18 million doses were sold in 2018 and 56 million in 2019. Elanco provides information that, so far, they have had 1 adverse event at the Norwegian Medicines Agency, but this is not yet conclusive. The VI has received a report from NOFIMA summarising the x-ray of 477 fi sh from 2 groups of autumn smolt vaccinated with Aquavac PD 7 and Clynav + AlphaJect micro 6 respectively: 22% of Aquavac PD 7-vaccinated fi sh had crossstitching and 13% had severe disorder. The corresponding fi gure for the fi sh vaccinated with Clynav + AlphaJect micro 6 was 4% and 1% had severe disorder. The study cannot determine whether the impact of cross-stitching in the Clynav-vaccinated group is due to the PD vaccine or AlphaJect micro 6. This is because salmon vaccinated with standard 6-component vaccines may also have an incidence of spinal deformities with fi ndings of cross-stitch vertebrae at the time of harvesting, but prevalence and severity appear to be low.
The Norwegian Food Safety Authority
The Food Safety Authority has received exemption applications from Måsøval, Mowi, Lerøy, AquaGen and SalMar. In addition to their own arguments, many of the exemption applications are also supported by the letter “Statement from the Aquaculture Working Group in P06, the fish-health group” sent to the NFD on 15.01.2020. Key arguments include the lack of assessment of adverse events, the lack of a cost-benefit assessment, the PD vaccines are not approved for broodstock, it is not known how existing vaccines work against SAV2, technical and adverse event problems if one also wants to vaccinate against yersiniosis, and that the vaccination instruction is late in arriving, making it difficult to change vaccination programs in time. The VI makes the following comments: • It is true that there was little focus on the adverse events of the vaccines during the hearing process, but this was also not a major issue in any of the hearing bodies. To a greater extent, efficacy was demonstrated with the use of new vaccines and the possibility of combating PD. • It is true that no cost-benefit assessment has been carried out. • It is true that the current PD vaccines are not approved for broodstock, but neither are there any other vaccines with which to vaccinate broodstock in the meantime, and the vaccines are then used “off label” by the prescribing fish-health personnel making their own assessment of their use. • Information is available on how existing vaccines work against SAV2 in the form of presentations at various technical meetings, such as PD TriNation, Dublin 2015), but the data are not published in scientific journals.
There are documented immunological cross-reactions between the different genotypes (Graham et al. 2014), and the type classification for SAV is based on minor genetic differences which are not necessarily reflected in differences that affect vaccine efficacy. For example, Clynav is based on the genotype SAV2, but the efficacy of the vaccine has been documented against SAV3. It is not unreasonable to assume that Clynav works as well against SAV2 as against SAV3. • No information is available on whether
PD vaccination may affect the efficacy or adverse events of the yersiniosis vaccine (1-component). Apart from the specific
“cross-stitch” adverse event, the general adverse event problems, but also the level of protection against yersiniosis, are likely to be more affected by the complex multivalent vaccine that will be administered at the same time as the yersiniosis component.
However, it is possible that from a purely technical standpoint, it may be difficult to triple vaccinate against PD, yersiniosis and the remaining diseases in a single operation.
Summary of information received, the Veterinary Institute’s assessment:
• The database available to us indicates serious adverse events in the form of spinal deformities of the type “cross-stitch vertebrae” in salmon vaccinated with inactivated PD vaccines. These are Aquavac PD7 (MSD Animal
Health) and Alphaject micro 1 PD (Pharmaq) which to a greater extent are associated with this type of adverse event. Other PD vaccines in the analysis are not associated or associated to a limited extent with spinal deformities. • However, the data material has weaknesses.
In general, existing data and information from the authorities and some relevant players in the industry. The sources are the Norwegian
Medicines Agency, a research project funded by the FHF, and data collected in various ways by aquaculture enterprises and pharmaceutical producers. Only one aquaculture enterprise has granted access to production data which has facilitated its own statistical analyses.
This material can be used to indicate which variables in the dataset can be associated with spinal deformities and the strength of this association. However, we cannot say anything about causal relationships or risk factors other than those included in the submitted dataset. This indicates that the conclusions are uncertain and caution must be exercised. • The Norwegian Medicines Agency has often made available all relevant adverse event reports from 2016 up to the present day and this information has been decisive for the
VI’s conclusions in the case. In the process involving MSD Animal Health and Pharmaq, agreement has been reached on updating the package leaflets of Aquavac PD7 and Alphaject micro 1 PD. In its conclusion sent to use by email, the Norwegian Medicines Agency says: “The Norwegian Medicines Agency cannot draw a final conclusion on the causal relationship between spinal deformities and PD vaccines. However, we believe it is relevant that, as of today, information in the adverse events section in the summaries of product characteristics may indicate a causal relationship between the PD vaccines and “cross-stitch vertebrae”. New text for the adverse events section for Aquavac PD7 has been completed; please refer to the attached updated SPC. The case for Alpha Ject micro 1PD is under consideration”. • Calculations carried out in the FHF project 901430 show that it can be expected that approx. 6% of fish under 1 year old, vaccinated with Aquavac PD7, will develop spinal deformations, but that a significant variation can also be expected. Springrelease, Aquavac PD7-vaccinated smolt have a significantly lower risk. • FHF project 901430 has not conducted similar calculations for salmon vaccinated with
Alphaject micro 1 PD. Information from several different sources, as well as own analyses from the SAV2 zone, indicates that the incidence of spinal deformations in salmon vaccinated with Alphaject micro 1 PD, is probably on a par with, or somewhat lower than, that for
Aquavac PD7. • Salmon vaccinated with standard 6-component vaccines may also have an incidence of spinal deformities with findings of cross-stitch vertebrae at the time of harvesting, but prevalence and severity appear to be low. • Our own analysis of data from the SAV2 zone shows no difference in the risk of spinal deformities at the time of harvesting between autumn-release and spring-release fish. This inconsistency with the results of the FHF
project may be due to data being taken from different parts of the coast. • Adverse events have been used as a basis for applications for exemption from the vaccination order. Extensive administration of exemptions will weaken the purpose of the order and a high vaccination level in the area is necessary if vaccination is to contribute to limiting further the spread of PD. • A high vaccination level is particularly important as the PD vaccines on the market are believed to have moderate efficacy in the field. For example, there are a large number of PD detections in the SAV3 zone each year, even though the area has a high proportion of
PD-vaccinated fish. Therefore, it is important that vaccination does not replace, but supplements, other biosafety measures. There is still too little experience in the field and field data to conclusively prove the effect of Clynav. • If Aquavac PD and/or Alphaject micro 1 PD is considered to have too high a risk of adverse events, especially for autumn-release smolt (under 1 year old), other PD vaccines are still available on the market and can be used.
Norvax Compact PD has been associated with cross-stitch vertebrae, but to a much lesser extent than the above-mentioned vaccines (cf. FHF Project 901340). And apart from 1 adverse event case which is still not conclusive, no serious degree of spinal deformities has been reported in salmon vaccinated with
Clynav. • Therefore, it should be possible to adhere to the vaccination instruction without adversely affecting the welfare of the fish to an extent greater than a normal vaccination with 6-component vaccines.
(Norwegian Veterinary Institute signatories)
Edgar Brun Dept. Director, Department of fishing health and fish welfare
Eirik Biering Head of Section, Section for aquaculture, wild fish and welfare
References
Holm, H., Ytteborg, E., H0st, V., Reed, A. K., Dalum, A. 5., & Bæverfjord, G. (2020). A pathomorphological description of cross-stitch vertebrae in farmed Atlantic salmon (Salmo salar L. ). Aquaculture, 735382. Trangerud, C., Bjørgen, H., Koppang, E. 0., Grøntvedt, R. N., Skogmo, H. K., Ottesen, N., & Kvellestad, A. (2020). Vertebral column deformity with curved cross-stitch vertebrae in Norwegian seawater-farmed Atlantic salmon, Salmo salar L. Journal of Fish Diseases, 43(3), 379-389. Graham, D. A., Rowley, H. R., & Frost, P. (2014). Cross-neutralization studies with salmonid alphavirus subtype 1-6 strains: results with sera from experimental studies and natural infections. Journal of fish diseases, 37(8), 683-691.
Clynav, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates. All other product and company names are trademarks of their respective owners. © 2021 Elanco PM-UK-20-0301



Looking after lumpfish






An easy-to-use scoring system will help fi sh farmers assess and safeguard the welfare of their cleaner fi sh
BY DR SARA BARRENTO
With a plump body and a unique appearance, the lumpfi sh is rarely seen in markets or shops outside Norway or Iceland. In Europe “lumpfi sh caviar” can be purchased from most supermarkets, but the species has also gained fame in the aquaculture industry in recent years as a cleaner fi sh to control sea lice in salmon farms.
Every year 50 million lumpfi sh are used by salmon farmers in Europe to eat sea lice. Sea lice feed on the skin and mucus of the Atlan� c salmon, reducing their growth, impairing their health, and compromising their welfare. The losses caused by sea lice are enormous and amount to millions of pounds every year. Lumpfi sh are an effi cient cleaner fi sh and can reduce the use of toxic an� -parasi� c drugs by 80%.
Work carried out by our research group at the Centre for Sustainable Aqua� c Research (CSAR) since 2015 has made it possible to culture millions of lumpfi sh in cap� vity, but there is a need to develop tools to benchmark and improve their welfare.
Lumpfi sh welfare ma� ers
Studies suggest that between 33% and 50% of lumpfi sh may die following deployment in salmon cages. Emacia� on, stress, diseases, salmon cages. Emacia� on, stress, diseases, and poor knowledge of their specifi c nutri� onal and habitat requirements are the principal challenges for lumpfi sh welfare (Gu� errez Rabadan et al 2021). The public and retailers generally support the use of lumpfi sh for controlling sea lice, but only if the welfare of cleaner fi sh is not compromised. The development of a suitable method for assessing ment of a suitable method for assessing lumpfi sh welfare is important, not only for lumpfi sh welfare is important, not only for iden� fying those ac� vi� es that compromise it,



















Above: Sara Barrento Left: Lumpfi sh in hatchery tank at the Centre for Sustainable Aqua� c Research, Swansea University (photo @CSAR) Top right: Lumpfi sh Opera� onal Welfare Score Index explained (source: CSAR) Right: Lumpfi sh at the Centre for Sustainable Aqua� c Research, Swansea University (photo @CSAR)


but also for quality assurance, and for restoring public confi dence in the salmon farming industry and its ability to tackle the threat posed by sea lice.
Although some welfare indicators exist for lumpfi sh, not all can easily be used by fi sh farmers. Our research group has recently developed and validated a rapid Lumpfi sh Opera� onal Welfare Score Index (LOWSI) in collabora� on with salmon and lumpfi sh farmers.
“To be eff ec� ve, welfare indicators need to be prac� cal and easy to use, or they will not be used by fi sh farmers. The Opera� onal Welfare Indicators we developed for lumpfi sh were designed with farmers in mind: this index is rapid, prac� cal and easy to score”, notes Carolina Gu� errez-Rabadan, the lead author of the study.
The welfare score index is based on the assessment of four visual indicators: skin damage, fi n damage, eye condi� on and sucker deformi� es, and a fi � h indicator based on the body mass index (BMI) also known as rela� ve weight. The LOWSI uses a simple three-point Likert type score (this is a ra� ng scale o� en used in surveys) for each of the fi ve opera� onal welfare indicators, with their sum ranging from 0 (best) to 10 (worst). Lumpfi sh can then be classifi ed into three welfare classes depending on the LOWSI values: (A) Good welfare (< 3 points), (B) Moderately compromised welfare (3–5 points), and (C) Severely compromised welfare (> 5 points).
Making the score index even easier to use
The score index was validated and published in the journal Aquaculture and it is freely available online. Scien� fi c publica� ons are essen� al to validate a new procedure but are not the best way to show farmers how to implement it. So, our team is now developing The Lumpfi sh Welfare Watcher, a free web-based applica� on that will calculate the BMI (rela� ve weight) of lumpfi sh, based on the wet weight and total length entered by the fi sh farmer, and determine the propor� on of fi sh that are emaciated, underweight, and normal, as well as providing recommenda� ons for ac� on.
The applica� on will also calculate the Lumpfi sh Opera� onal Welfare Score Index (LOWSI) based on the four visual indicators and the rela� ve weight. It will also calculate the probability of escape from salmon net pens with nets of various mesh sizes.
The Lumpfi sh Welfare Watcher applica� on will be accessible via the user’s web browser. It will be accompanied by a user manual and an e-training course that will be disseminated via webinars and training sessions in the Autumn of 2021.
Professor Carlos Garcia de Leaniz, CSAR Director says: “The Lumpfi sh Welfare Watcher will provide a rapid assessment of lumpfi sh welfare and recommend a course of ac� on. This will help overcome an important knowledge gap, improve the welfare of cleaner fi sh, and reduce the problem posed by sea lice in salmon farming.”
Dr Sara Barrento is Science Communica� on and Stakeholder Engagement Manager with the Centre for Sustainable Aqua� c Research,
Swansea University. FF
This work is funded by the UK Seafood Innovati on Fund, Administered by the Centre for Environment, Fisheries and Aquaculture Science (Cefas) on behalf of the Department for Environment, Food and Rural Aff airs (Defra), and Access2Sea: New Opportuniti es for More Competi ti ve and Sustainable Blue Growth; funded by European Regional Development Fund (ERDF) under the umbrella of INTERREG Atlanti c Area with the project identi fi cati on code EAPA_1059/2018 – ACCESS2SEA and SMARTAQUA: aquaculture beyond food is supported by the Welsh Government and the European Regional Development Fund. This work was developed at The Centre for Sustainable Aquati c Research in collaborati on with The Scotti sh Salmon Company; MOWI Scotland; The Cleaner Fish Company; Ocean Matt ers; and Three Sixty Aquaculture.
For more informati on see www.swansea.ac.uk/bioscience/csar/projects/lumpfi sh/
More than a MICROCAPSULE

AROTEC-G® offers an effective and sustainable strategy for the control of the ectoparasite Sparicotyle chrysophrii in gilthead seabream farming

BY JOANA P. FIRMINO AQUACULTURE@FARMFAES.COM

AROTEC-G® is a microencapsulated func� onal product composed of a TEC-G®. The compound did not aff ect fi sh growth blend of garlic essen� al oil, carvacrol and thymol with numerous pro- performance or gut microbiota diversity, although duc� ve, welfare and environmental advantages. These widely recog- subtle varia� ons in microbiota composi� on and nised compounds are well-known for their an� parasi� c, an� microbial, func� onality were suggested to par� cipate in the an� -infl ammatory and an� oxidant proper� es, without any detrimental eff ect modula� on of the intes� ne immune and an� oxifor the fi nal consumer, or the environment. Besides, the exclusive microencap- da� ve profi le observed in fi sh fed with AROTEC-G®. sula� on technology ensures the stability of the compounds and a sustained re- Gilthead seabreams fed with the AROTEC-G® suplease throughout the gastrointes� nal tract. The effi ciency of AROTEC-G® against plemented diet did not show any intes� ne histobacterial and parasi� c challenges has been scien� fi cally proven through both in pathological altera� ons, proving that AROTEC-G® vitro and in vivo experimental studies. administra� on is safe to be included in func� onal
A trial with juvenile gilthead seabream fed with an AROTEC-G® supplemented diets to improve fi sh mucosal health. diet for two months was performed in order to discover its eff ects upon the Since the tradi� onal use of chemotherapeu� c pathogen´s main portals of entry, i.e., mucosal � ssues, such as gills, skin, and agents to prevent and control fi sh diseases in aqintes� ne. uaculture has been heavily cri� cised, the overall re-
Gills were par� cularly studied to evaluate the protec� ve eff ect of sults of AROTEC-G® supplementa� on demonstrated AROTEC-G® against the ectoparasite Sparicotyle chrysophrii, one of the main promising poten� al for this product to be used as pathogenic agents aff ec� ng gilthead seabream farming in the Mediterra- a sustainable prophylac� c tool in the health mannean. In this regard, AROTEC-G® inclusion in the diet promoted the modula- agement for aquaculture farms. FF � on of genes involved in immunity routes, redox processes, and metabolism in the gills. In par� cular, processes mediated by cells of the innate immune system, such as granulocytes, were preponderant. This poten� al immunoprotec� ve eff ect of AROTEC-G® in gills was corroborated by an in vivo cohabita� on challenge, in which fi sh fed with the product were subsequently exposed to S. chrysophrii for another month of feeding. The administra� on of AROTEC-G® decreased by 78% the total prevalence of the ectoparasite in gills, par� cularly aff ec� ng adult, juvenile and egg stages of the parasite (Figure 1).
The eff ect of AROTEC-G® upon the skin was also studied. Results obtained from the analysis of the epidermal mucus revealed a signifi cant decrease in stress biomarkers, mainly cor� sol, when animals were fed with AROTEC-G®, indica� ng an improvement in overall fi sh health condi� on and welfare. Moreover, the mucus from fi sh fed with AROTEC-G® also signifi cantly inhibited the Figure 1 in vitro growth of pathogenic bacteria, par� cularly fi sh pathogens such as Vibrio anguillarum and Pseudomonas anguillisepti ca (Figure 2). Those results were supported by the modula� on of genes related with immunity and secretory responses in skin. Similarly, according to the intes� ne analysis, the ac� va� on of granulocytes was also suggested to be the main actor of the mucosal immune response promoted by ARO-

Figure 1: Number of S. chrysophrii parasites per fi sh fed with the AROTEC-G® and control experimental diets (mean ± standard devia� on). Asterisks (*) indicate signifi cant diff erences between dietary groups (p < 0.05). Adapted from Firmino et al. 2020 (h� ps://doi. org/10.1038/s41598020-74625-5). Figure 2: V. anguillarum, P. anguillisepti ca, and Escherichia coli growth inhibi� on on skin mucus of gilthead seabream fed with a diet supplemented with AROTEC-G® and a control diet. Asterisks (*) indicate signifi cant diff erences in bacterial growth between dietary groups (p < 0.05). Adapted from Firmino et al. 2021 (h� ps:// doi.org/10.3389/ fi mmu.2021.633621).

Smart ideas

From net-cleaning ROVs to cleaner fi sh hides, AKVA can help keep your stock safe
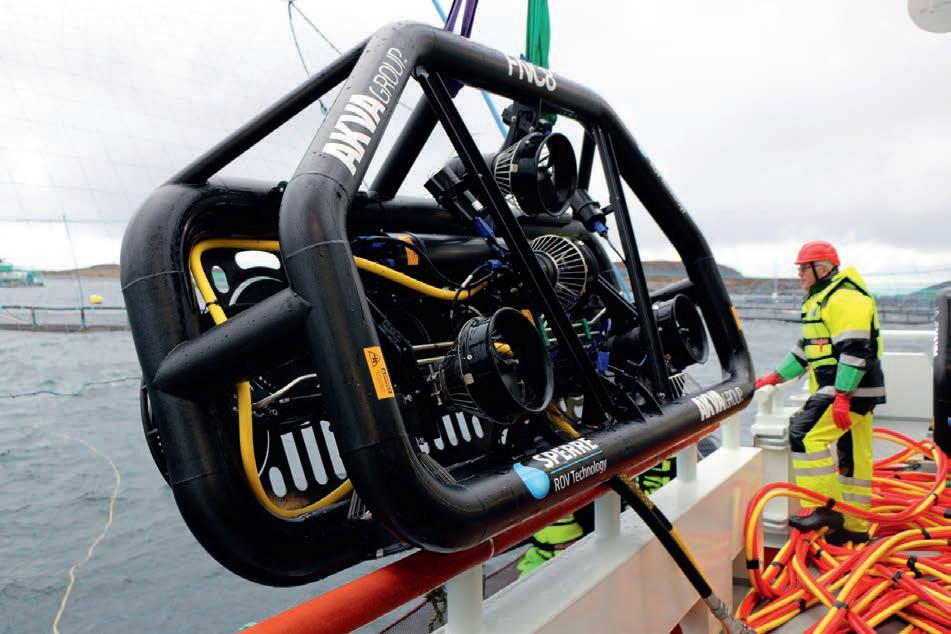
Five years ago, AKVA group and the ROV (remotely operated vehicle) manufacturer Sperre launched the fi rst remote free-fl ying net cleaning system.
At the � me it was not uncommon to apply whatever pressure was available from the HP pumps onboard the service vessels to the nets, in many cases well over 200 bar. As it turned out, however, the combina� on of net cleaners using belts as propulsion crawling on the nets, and high pressure applied to the nets, resulted in signifi cant wear and tear on the nets and net mesh. Today we know be� er.
Remotely operated free-fl ying net cleaning systems were fi rst introduced with the launch of the FNC8 (Flying Net Cleaner) at the end of 2016. It was the fi rst of its kind in the world and has radically changed our en� re approach to net cleaning.
With low running costs of £100-£150 per day for 12 hours of cleaning and quick cleaning � mes the FNC8 is also helping to reduce the stress on fi sh during the cleaning process. We are currently tes� ng a system for the collec� on of loose fouling par� cles caused by the net cleaning process and we are confi dent that we will have a good solu� on to this problem soon. This will enable the system to both clean nets effi ciently and enhance fi sh welfare during the cleaning process.
AKVA group and OK Marine
OK Marine, partnered with AKVA since 2015, is a world-leading supplier of cleaner fi sh equipment which can be used to enhance the welfare of all farmed fi sh. While cleaner fi sh can help to reduce the lice burden on salmon, improving welfare, it is important to remember that they also have their own welfare needs. OK Marine provide a full range including hides and feeding equipment through their GOOD concept.
Feeding is necessary to ensure working and healthy cleaner fi sh. The
Above: The FNC8 is easy to deploy and reduces stress on both nets and fi sh Top right: A typical installa� on on the barge for a FNC8 cleaning sta� on Right: OK Marine cleaner fi sh hides provide an excellent place for cleaner fi sh to rest and thrive GOOD range off ers both surface and sub-surface feeding op� ons to ensure that the correct nutri� on is reaching the cleaner fi sh, and this ensures both top health and high welfare standards. Feeding of cleaner fi sh is tricky and providing several methods ensures that every fi sh has a chance to get the nutri� on it needs to thrive.
To protect cleaner fi sh from danger, it is cri� cal to provide good opportuni� es to return to safe feeding grounds and hides. The GOOD concept is based on solid competence and extensive experience and has been developed in close collabora� on with fi sh farmers. This also includes recapture tools that enable op� mal handling of cleaner fi sh during opera� ons which could compromise the welfare of the cleaner fi sh.
To discuss any of these products, please contact Donald Fowler, Commercial Manager, AKVA group Scotland at d.fowler@akvagroup.com or 07766367433 FF













