4 minute read
S.Catalini Self Assembling of Lysozyme: Structural and Elastic Investigations
Self-Assembling of Lysozyme: Structural and Elastic Investigations
S.Catalini1, M. Paolantoni2 ,L. Comez3, P. Sassi2 , A. Morresi2 , A. Taschin1,4, P. Bartolini1, P. Foggi1,2 and R. Torre1,5 1European Laboratory for Non-Linear Spectroscopy (LENS), Via Nello Carrara 1, 50019, Sesto Fiorentino, Italy 2Dipartimento di Chimica, Biologia e Biotecnologie, Università di Perugia, Via Elce di sotto 8, 06123, Perugia, Italy 3IOM-CNR c/o Dipartimento di Fisica e Geologia, Università di Perugia, via Pascoli, 06123 Italy 4ENEA centro di ricerche Frascati, Via E. Fermi 45, 00044, Frascati, Italy 5Dipartimento di Fisica ed Astronomia; Università di Firenze, Via G. Sansone, 1, 50019, Sesto Fiorentino, Italy
Advertisement
Abstract: The relation between structural and elastic properties
of a crowded lysozyme aqueous solution is presented. Local structures of the system it has been investigated by Infrared absorption, while the viscoelastic response was explored through Transient Grating spectroscopy. A broad band THz investigation by means of time-domain spectroscopy is planned. One of the challenges in soft matter science is to relate the molecular interactions and the local structures with the macroscopic properties of the soft material, in particular with viscoelastic dynamic. Our results highlight the direct connection between the conformational variations of the lysozyme protein and the propagation of acoustic waves in the crowded water solution; the protein self-assembling phenomena determines several macroscopic features including the damping/velocities of elastic waves. Moreover, the environmental conditions (temperature and acidity) control the extension of molecular aggregation; from local clusters (crowed solutions) to percolating networks (hydrogel).
I. INTRODUCTION A wide range of molecular condensed matter can be classified as Soft Matter (SM); these are characterized by a structural self-organization, which defines several macroscopic features. The relationship that exists between the molecular interactions and the environmental changes, leads to the complex behavior of SM [1]. SM based on proteins represents an even more challenging system; in particular, the globular proteins are very relevant in this topic, due to their importance in life science [2]. One of the major goal is to relate the intra- and inter-molecular interactions, that govern the structure of a single protein and its aggregates with the viscoelastic properties of the whole system. Studies of crowded protein solutions are necessary for the understanding of the physics that governs the behavior of the living systems but also in industrial and pharmaceutical fields [3]. Our work is aimed to relate the structural and viscoelastic properties of an aqueous solution of Hen Egg White Lysozyme (HEWL), at low pH and high concentration, by means of spectroscopic techniques. Indeed, the system it has been investigated by Fourier Transform Infrared (FTIR) and Heterodyne Transient Grating (HD-TG) spectroscopies [4, 5], in order to relate pro ein s structural features with the viscoelastic properties of the entire system. We will focus on the identification of any phonon-like resonance within HEWL hydrogels using THz Time-Domain spectroscopy.
II. RESULTS
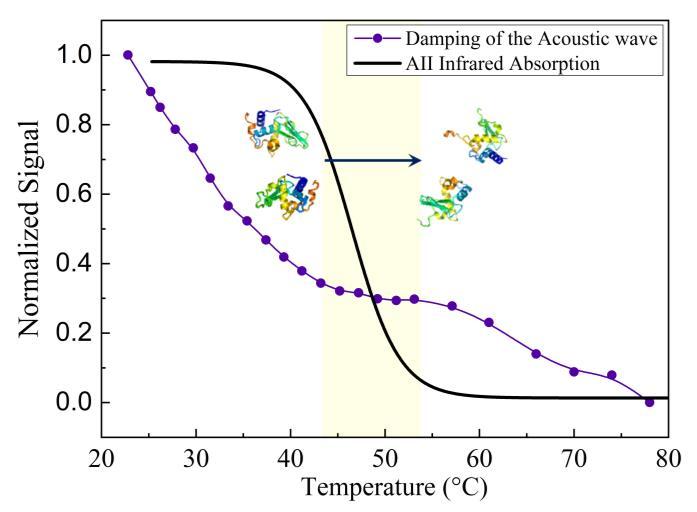
HEWL thermal behavior it has been explored taking into account the damping rate of the acoustic wave, estimate using HD-TG measures, and the absorbance value of the Amide II signal, through FTIR spectra. Both variables trend are reported in Fig. 1 for comparison.
Fig. 1. Acoustic wave damping trend and Amide II absorbance trend, of a concentrated acid HEWL solution, as a function of temperature.
The trend of the acoustic damping rate with temperature shows a clear variation in the range 45-55°C. A comparison with experimental results from FTIR techniques suggests the origin of this elastic phenomenon: the transition of proteins from folded to unfolded state. In fact, the absorbance signal of the Amide II confirms that breaking of the intra-molecular bonds, i.e the proteins denaturation, takes place in an overlapping temperature range. This causes the exposition of the hydrophobic residues of protein, which modulate the aggregation phenomena.
REFERENCES
[1] an der G ch J. Grand challenges in sof ma er ph sics , Frontiers in Physics. 2018 Aug 22;6:87. [2] Munishkina LA, Cooper EM, Uversky VN, Fink AL. The effect of macromolecular crowding on protein aggregation and amyloid fibril forma ion , Journal of Molecular Recognition. 2004 Sep;17(5):456-64. [3] Gao M, Held C, Pa ra S, Arns L, Sado ski G, Win er R. Cro ders and cosolvents major contributors to the cellular milieu and efficient means to co n erac en ironmen al s resses , ChemPhysChem. 2017 Nov 3;18(21):2951-72. [4] Torre, R., Time-Resolved Spectroscopy in Complex Liquids, An E perimen al Perspec i e , Springer US, Boston, MA, 2008 [5] Catalini S, Taschin A, Bartolini P, Foggi P, Torre R. Probing Globular Protein Self-Assembling Dynamics by Heterodyne Transient Grating E perimen s , Applied Sciences. 2019 Jan;9(3):405.
081




083









