Clinical symptoms associated with exposure to mycotoxins
Mycotoxins, alkaloids, certain lactones, and coumarins, as well as eicosanoic carboxylic esters are not degraded (at least, not completely) by the rumen microbiota and can produce clinical signs (Fink-Gremmels, 2008b).
Specific toxins affect specific organs or tissues such as the liver, kidney, oral and gastric mucosa, brain, or reproductive tract (Fink-Gremmels, 2008b).
Mycotoxins can cause acute episodes when animals consume critical quantities. In acute mycotoxicosis, the signs of disease are often marked and directly referable to the affected target organs.

However, at lower concentrations (chronic exposure), the effects of mycotoxins are more variable.
They reduce the growth rate of young animals and some interfere with the mechanisms of disease resistance and impair immune responsiveness, making the animals more susceptible to infections (Pier et al., 1980).
In these circumstances is frequent to see chronic issues, including a higher incidence of disease, poor reproductive performance, or suboptimal milk production (Whitlow and Hagler Jr, 2010).
7
Symptoms associated with exposure to Aflatoxins
Based on field experience, the main clinical findings of aflatoxicosis in ruminant animals are:
Sudden death (Image 1)
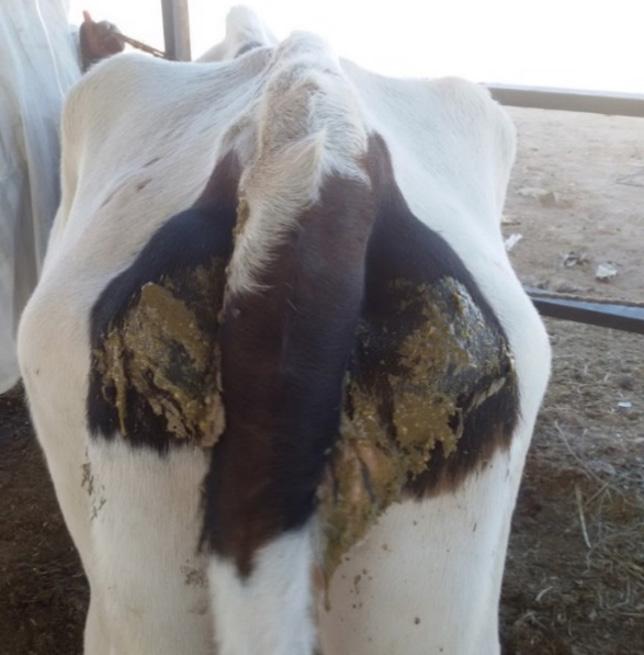
Diarrhea (Image 2)
Anorexia
Rough coat
An outbreak of aflatoxicosis in cattle demonstrated that depression and inappetence were the predominant clinical findings (Elgioushy et al., 2020).
Van Halderen et al. (1989) described a field outbreak mortality of 7/25 calves fed rations containing maize with 11.790 µg of Aflatoxins/kg.
Clinical signs included loss in body mass, rough coat, diarrhea, and rectal prolapse.
In Australia, acute aflatoxicosis that caused mortality of 12/90 drought-stricken calves fed on peanut hay containing of 2230 µg AF/kg was reported (McKenzie et al., 1981).

In Pakistan, 45 field cases of aflatoxicosis on a local farm in Okara were recorded (Sohooa et al., 2015).
The cows were fed corn-rich forage with 33.500 µg AF/kg and the clinical signs observed were anorexia, depression, photosensitization, and diarrhea, which resulted in the death of 15 animals.
8
Image 1. Sudden death in a cow due to aflatoxicosis.
Image 2. A cow with signs of diarrhea due to aflatoxicosis.
Experimental aflatoxicosis has also been associated with clinical signs of reduced feed intake and feed conversion, reduced milk production, reduced reproduction capacity, lameness, immunosuppression, hepatotoxicity, and nephrotoxicity (Gonçalves et al., 2015).
A significant decrease in milk production in cattle fed 13 mg AFB1/day for 7 days was reported (Applebaum et al., 1982).
A numerical drop in milk yield in cows fed 75 µg/kg Dry Matter Intake (DMI) (1725 µg/head per day) for 5 days was also reported (Ogunade et al., 2016) and (Jiang et al., 2018).

In another study (Sulzberger et al., 2017), depression in milk yield and feed conversion at 100 µg AFB1/kg of DMI was recorded.
In contrast, some studies on low levels of aflatoxins have shown a nonsignificant decrease in milk production (Kutz et al., 2009; Masoero et al., 2007; Sumantri et al., 2012).
Exposure to aflatoxins also affects rumen fermentation, reducing the utilization of nutrients and eventually affecting animal productivity (Kemboi et al., 2020).
For example, reduced gas production, dry matter digestibility, and ammonia-N concentrations caused by AFB1 in vitro have been reported (Mojtahedi et al., 2013).
In another study, AFB1 reduced ammonia-N and volatile fatty acid (VFA) concentrations without reducing dry matter digestibility or affecting VFA patterns (Jiang et al., 2012).
9
Symptoms associated with exposure to Deoxynivalenol
DON has been found to affect ruminal fermentation and can cause reduced milk yield (Whitlow and Hagler, 2010).
In a study on the effect of a diet contaminated with DON in non-lactating Holstein dairy cows fed at a dose of 1.5 mg DON/kg and 6.4 mg DON/kg of feed for 6 weeks, there was a slight decline in feed consumption following the change from the low dose (1.5 mg DON/kg) to the high dose (6.4 mg DON/kg) (Trenholm et al., 1985)
Symptoms associated with exposure to Fumonisins
Ruminants are more resistant to fumonisin toxicity than monogastric animals (Mathur et al., 2001).

However, feeding trials with 75 mg FB1/ kg, 94 mg FB1/kg, and 105 mg FB1/ kg for 14 days, 253 days, and 31 days, respectively, have been reported to cause reduced milk yield, reduced feed intake, hepatotoxicity, nephrotoxicity, and reproduction problems (Richard et al., 1996).
In calves, severe liver failure in 2–3-month-old calves with no functioning rumen induced by 1.13 mg DON/kg feed was reported (Valgaeren et al., 2019).
Experimental intravenous administration of 1 mg FB1/kg to calves for 7 days caused lethargy, loss of appetite, hepatotoxicity, and nephrotoxicity (Mathur et al., 2001).
In a study with milk-fed male Holstein calves aged 7 to 14 days treated daily with 1 mg FB1/kg (iv) until euthanasia on day 7, treated calves were lethargic and had decreased appetite from day 4 onward (Mathur et al., 2001).
10
Ochratoxicosis is rarely reported in cattle, which is attributed to the ability of the rumen microbiota to easily degrade OTA to non-toxic forms as demonstrated (Richard et al., 1996).

However, anorexia, diarrhea, difficulty in rising and cessation of milk production with recovery on the 4th day in cattle fed a high single dose of OTA (13.3 mg DON/ kg) has been recorded experimentally, whereas in the field low doses (0.2 mg DON/kg, 0.75 mg DON/kg, and 1.66 mg DON/kg) for 5 days produced no clinical disease (Ribelin et al., 1978).
In dairy cattle, T-2 toxin has been associated with hemorrhagic gastroenteritis (Whitlow and Hagler Jr, 2010), feed refusal, and gastrointestinal lesions (Weaver et al., 1980).
Weaver et al. (1980) reported severe depression, hindquarter ataxia, knuckling of the rear feet, listlessness, and anorexia in a calf fed 0.6 mg T-2 toxin/kg for seven consecutive days.
Reduction in milk yield and the absence of estrus have also been associated with T-2 (Whitlow and Hagler, 2010).
11
Ochratoxins
associated with exposure to T-2 toxin
Symptoms associated with exposure to
Symptoms
Symptoms
Early abortion in cattle feeding on hay containing 10 mg ZEN/kg has been recorded (Kallela y Ettala, 1984).
Abnormal estrus cycle, vaginitis, behavioral estrus in gestating animals, mammary development in prepuberal heifers, and sterility have also been reported in cattle fed 1.5 mg ZEN/kg feed (Gupta, 2012).
Experimental studies using 500 mg and 250 mg of 99% purified Zearalenone in a gelatin capsule administered orally to lactating dairy cattle and virgin heifers, respectively, showed no effects except for depression in the conception rate in the virgin heifers (Weaver et al., 1986a, b).
In an experimental study, Diplodiosis, a type of neuromycotoxicosis that mainly affects cattle, characterized by ataxia, paresis and paralysis, was induced in 13 heads of cattle, 16 sheep and 3 goats by dosing them with Diplodia maydis (Kellerman et al., 1985).

In Australia, Acute Bovine Liver Disease (ABLD) was recorded in 45 naturally affected cattle from 13 outbreaks occurring from 2010 to 2019.
Clinical signs commonly included a combination of mild photosensitization, progressive neurologic signs, and hypogalactia, which preceded death in less than 48 hours (Manthorpe et al., 2021).
12
associated with exposure to Zearalenone Symptoms associated with other fungal related infections
Lesions associated with exposure to mycotoxins
Aflatoxin-induced lesions
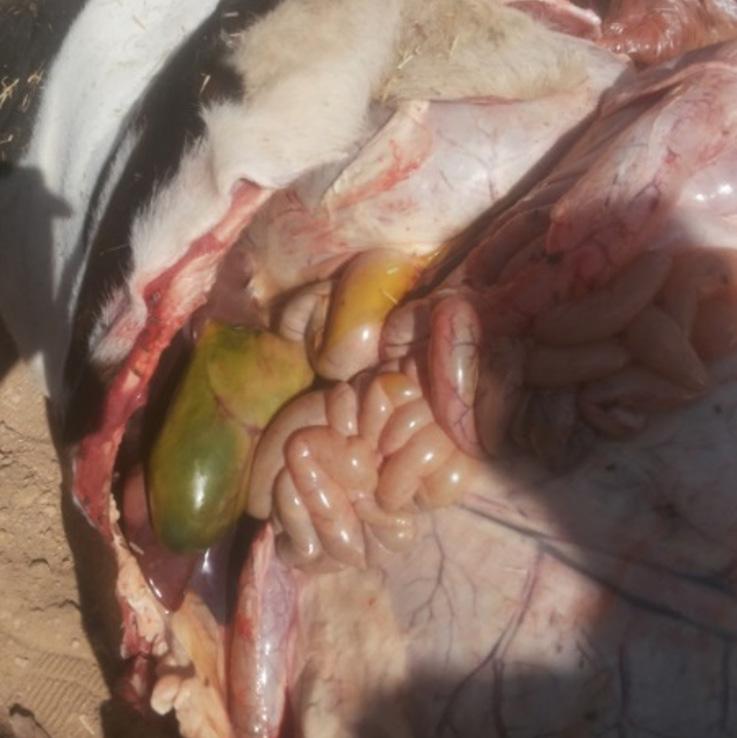
Based on field experience, the gall bladder, liver, and kidneys are the most commonly affected organs in cases of aflatoxicosis (Images 3, 4 and 5).


Acute aflatoxin toxicity has been found to provoke major signs of liver lesions, leading to congestion and bleeding (Pier, 1992; Pier and Richard, 1992).
Aflatoxicosis also causes fatty acid accumulation in the liver, kidneys, and heart, and may be responsible for encephalopathies and oedemas (Pfohl-Leszkowicz, 2000).
However, chronic aflatoxicosis is more common and, in this case, the liver is the main target.
Aflatoxins act as DNA intercalating agents, binding to guanine bases and leading to cell death or tumor formation (Pfohl-Leszkowicz, 2000).
13
Image 3
Enlarged gall bladder in cow dead affected by aflatoxicosis.
Image 4. Congested kidney in cattle afflicted with aflatoxicosis.
Image 5 Liver with necrotic areas from a cow with aflatoxicosis.
Fumonisin-induced lesions
Oral administration of a diet containing FB1 (2.36 mg FB1/kg/day increased to 3.54 mg FB1/kg/day) to calves for 239 to 253 days showed elevated sphinganine/ sphingosine ratios with mild hepatocellular morphological changes accompanied by mild bile duct epithelial changes (Baker and Rottinghaus, 1999).
Lesions associated with other fungal related infections

Sphinganine and sphingosine concentrations in liver, kidney, lung, heart, and skeletal muscle were increased in milk-fed male Holstein calves aged 7 to 14 days treated with at 1 mg/ kg (iv), daily until euthanized on day 7.
In this case, the hepatic lesions were characterized by disorganized hepatic cords, varying severity of hepatocyte apoptosis, hepatocyte proliferation, and proliferation of bile ductular cells.
Additionally, renal lesions consisted of vacuolar changes, apoptosis, karyomegaly, and proliferation of proximal renal tubular cells, as well as dilation of proximal renal tubules (Mathur et al., 2001).
Moreover, signiicant increases in serum liver enzyme activity with mild microscopic liver lesions and eventual impairment of lymphocyte blastogenesis were described in two calves fed at the highest Fumonisin level (Osweiler et al., 1993).
In an experimental study dosing cattle, sheep, and goats with Diplodia maydis, an extensive laminar subcortical status of spongiosis was evident in the cerebrum and cerebellum of a sheep that had been long paralyzed and a steer that had permanent locomotory disturbance (Kellerman et al., 1985).
In Australia, in cases of Acute Bovine Liver Disease (ABLD) the main histological lesions were severe periportal hepatocellular coagulative necrosis and erythrocyte pooling, which often extended to massive necrosis.
14
REFERENCES
Albonico, M., Schutz, L.F., Caloni, F., Cortinovis, C., Spicer, L.J., 2017. In vitro effects of the Fusarium mycotoxins fumonisin B1 and beauvericin on bovine granulosa cell proliferation and steroid production. Toxicon 128, 38-45.
Alonso, V., Díaz Vergara, L., Aminahuel, C., Pereyra, C., Pena, G., Torres, A., Dalcero, A., Cavaglieri, L., 2015. Physiological behaviour of gliotoxigenic Aspergillus fumigatus sensu stricto isolated from maize silage under simulated environmental conditions. Food Additives & Contaminants: Part A 32, 236-244.
Antonissen, G., Martel, A., Pasmans, F., Ducatelle, R., Verbrugghe, E., Vandenbroucke, V., Li, S., Haesebrouck, F., Van Immerseel, F., Croubels, S., 2014. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 6, 430-452.
Applebaum, R.S., Brackett, R.E., Wiseman, D.W., Marth, E.H., 1982. Responses of dairy cows to dietary aflatoxin: Feed intake and yield, toxin content, and quality of milk of cows treated with pure and impure aflatoxin. Journal of Dairy Science 65, 1503-1508.
Awad, W.A., Hess, C., Hess, M., 2017. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins 9, 60.
Baker, D.C., Rottinghaus, G.E., 1999. Chronic experimental fumonisin intoxication of calves. Journal of Veterinary Diagnostic Investigation 11, 289-292.
Bouslimi, A., Bouaziz, C., Ayed-Boussema, I., Hassen, W., Bacha, H., 2008. Individual and combined effects of ochratoxin A and citrinin on viability and DNA fragmentation in cultured Vero cells and on chromosome aberrations in mice bone marrow cells. Toxicology 251, 1-7.
Caloni, F., Ranzenigo, G., Cremonesi, F., Spicer, L.J., 2009. Effects of a trichothecene, T-2 toxin, on proliferation and steroid production by porcine granulosa cells. Toxicon 54, 337-344.
Chain, E.P.o.C.i.t.F., 2012. Scientific Opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA Journal 10, 2605.
Changwa, R., Abia, W., Msagati, T., Nyoni, H., Ndleve, K., Njobeh, P., 2018. Multi-mycotoxin occurrence in dairy cattle feeds from the gauteng province of South Africa: A pilot study using UHPLC-QTOF-MS/MS. Toxins 10, 294.
Donkor, O., Ramchandran, L., Vasiljevic, T., 2016. Techniques 8 and Control for of Mycotoxins Detection, Quantification in Dairy Products. Microbial Toxins in Dairy Products 7, 201.
Elgioushy, M.M., Elgaml, S.A., El-Adl, M.M., Hegazy, A.M., Hashish, E.A., 2020. Aflatoxicosis in cattle: clinical findings and biochemical alterations. Environmental Science and Pollution Research 27, 35526-35534.
Fink-Gremmels, J., 2008a. The role of mycotoxins in the health and performance of dairy cows. Vet J 176, 84-92.
Fink-Gremmels, J., 2008b. The role of mycotoxins in the health and performance of dairy cows. The Veterinary Journal 176, 84-92.
Frisvad, J.C., Rank, C., Nielsen, K.F., Larsen, T.O., 2009. Metabolomics of Aspergillus fumigatus. Medical Mycology 47, S53-S71.
Fushimi, Y., Takagi, M., Monniaux, D., Uno, S., Kokushi, E., Shinya, U., Kawashima, C., Otoi, T., Deguchi, E., Fink-Gremmels, J., 2015. Effects of Dietary Contamination by Zearalenone and Its Metabolites on Serum Anti-Müllerian Hormone: Impact on the Reproductive Performance of Breeding Cows. Reprod Domest Anim 50, 834-839.
Gallo, A., Giuberti, G., Bertuzzi, T., Moschini, M., Masoero, F., 2015. Study of the effects of PR toxin, mycophenolic acid and roquefortine C on in vitro gas production parameters and their stability in the rumen environment. The Journal of Agricultural Science 153, 163-176.
Gao, Y., Meng, L., Liu, H., Wang, J., Zheng, N., 2020. The compromised intestinal barrier induced by mycotoxins. Toxins 12, 619.
Gonçalves, B.L., Corassin, C.H., Oliveira, C.A.F.d., 2015. Mycotoxicoses in dairy cattle: a review. Asian Journal of Animal and Veterinary Advances 10, 752-760.
Guerre, P., 2020. Mycotoxin and gut microbiota interactions. Toxins 12, 769.
Gupta, R.C., 2012. Veterinary toxicology: basic and clinical principles. Academic press.
Hartinger, T., Kröger, I., Neubauer, V., Faas, J., Doupovec, B., Schatzmayr, D., Zebeli, Q., 2023. Zearalenone and Its Emerging Metabolites Promptly Affect the Rumen Microbiota in Holstein Cows Fed a Forage-Rich Diet. Toxins (Basel) 15.
Jiang, Y., Ogunade, I., Kim, D., Li, X., Pech-Cervantes, A., Arriola, K., Oliveira, A., Driver, J., Ferraretto, L., Staples, C., 2018. Effect of adding clay with or without a Saccharomyces cerevisiae fermentation product on the health and performance of lactating dairy cows challenged with dietary aflatoxin B1. Journal of dairy science 101, 3008-3020.
15
Jiang, Y., Yang, H., Lund, P., 2012. Effect of aflatoxin B1 on in vitro ruminal fermentation of rations high in alfalfa hay or ryegrass hay. Animal feed science and technology 175, 85-89.
Jovaišienė, J., Bakutis, B., Baliukonienė, V., Gerulis, G., 2016. Fusarium and Aspergillus mycotoxins effects on dairy cow health, performance and the efficacy of Anti-Mycotoxin Additive. Pol J Vet Sci 19, 79-87.
Kallela, K., Ettala, E., 1984. The oestrogenic Fusarium toxin (zearalenone) in hay as a cause of early abortions in the cow. Nordisk veterinaermedicin 36, 305-309.
Kellerman, T.S., Rabie, C.J., van der Westhuizen, G.C., Kriek, N.P., Prozesky, L., 1985. Induction of diplodiosis, a neuromycotoxicosis, in domestic ruminants with cultures of indigenous and exotic isolates of Diplodia maydis. Onderstepoort J Vet Res 52, 35-42.
Kemboi, D.C., Antonissen, G., Ochieng, P.E., Croubels, S., Okoth, S., Kangethe, E.K., Faas, J., Lindahl, J.F., Gathumbi, J.K., 2020. A review of the impact of mycotoxins on dairy cattle health: Challenges for food safety and dairy production in sub-Saharan Africa. Toxins 12, 222.
Korosteleva, S.N., Smith, T.K., Boermans, H.J., 2009. Effects of feed naturally contaminated with Fusarium mycotoxins on metabolism and immunity of dairy cows. J Dairy Sci 92, 1585-1593.
Kurtz, R.S., Czuprynski, C.J., 1992. Effect of aflatoxin B1 on in vitro production of interleukin-1 by bovine mononuclear phagocytes. Veterinary immunology and immunopathology 34, 149-158.
Kutz, R., Sampson, J., Pompeu, L., Ledoux, D., Spain, J., Vazquez-Anon, M., Rottinghaus, G., 2009. Efficacy of Solis, NovasilPlus, and MTB-100 to reduce aflatoxin M1 levels in milk of early to mid lactation dairy cows fed aflatoxin B1. Journal of dairy science 92, 3959-3963.
Lloyd, W., 1980. Citrinin and ochratoxin toxicoses in cattle in the United States. In: Proceedings of the 2nd International Symposium of Veterinary Laboratory Diagnosticians, June 24-26 1980, Lucerne, Switzerland, IV., pp. 435-439.
Manthorpe, E.M., Jerrett, I.V., Rawlin, G.T., Woolford, L., 2021. Clinical and pathologic features of acute bovine liver disease in Australia. Journal of Veterinary Diagnostic Investigation 33, 875-883.
Masoero, F., Gallo, A., Moschini, M., Piva, G., Diaz, D., 2007. Carryover of aflatoxin from feed to milk in dairy cows with low or high somatic cell counts. Animal 1, 1344-1350.
Mathur, S., Constable, P.D., Eppley, R.M., Waggoner, A.L., Tumbleson, M.E., Haschek, W.M., 2001. Fumonisin B1 is hepatotoxic and nephrotoxic in milk-fed calves. Toxicological Sciences 60, 385-396.
McKenzie, R., Blaney, B., Connole, M., Fitzpatrick, L., 1981. Acute aflatoxicosis in calves fed peanut hay. Australian Veterinary Journal 57, 284-286.
Mojtahedi, M., Mesgaran, M.D., Vakili, S.A., Hayati-Ashtiani, M., 2013. Effect of aflatoxin B1 on in vitro rumen microbial fermentation responses using batch culture. Annual Research & Review in Biology, 686-693.
Njobeh, P.B., Dutton, M.F., Tevell Åberg, A., Haggblom, P., 2012. Estimation of multi-mycotoxin contamination in South African compound feeds. Toxins 4, 836-848.
O’Brien, M., Nielsen, K.F., O’Kiely, P., Forristal, P.D., Fuller, H.T., Frisvad, J.C., 2006. Mycotoxins and other secondary metabolites produced in vitro by Penicillium paneum Frisvad and Penicillium roqueforti Thom isolated from baled grass silage in Ireland. Journal of Agricultural and Food Chemistry 54, 9268-9276.
Ogunade, I., Arriola, K., Jiang, Y., Driver, J., Staples, C., Adesogan, A., 2016. Effects of 3 sequestering agents on milk aflatoxin M1 concentration and the performance and immune status of dairy cows fed diets artificially contaminated with aflatoxin B1. Journal of dairy science 99, 6263-6273.
Oswald, I.P., Marin, D., Bouhet, S., Pinton, P., Taranu, I., Accensi, F., 2005. Immunotoxicological risk of mycotoxins for domestic animals. Food additives and contaminants 22, 354-360.
Osweiler, G., Kehrli, M., Stabel, J., Thurston, J., Ross, P., Wilson, T., 1993. Effects of fumonisin-contaminated corn screenings on growth and health of feeder calves. Journal of animal science 71, 459-466.
Perego, M.C., Morrell, B.C., Zhang, L., Schütz, L.F., Spicer, L.J., 2020. Developmental and hormonal regulation of ubiquitin-like with plant homeodomain and really interesting new gene finger domains 1 gene expression in ovarian granulosa and theca cells of cattle. J Anim Sci 98.
16
PFOHL-LESZKOWICZ, A., 2000. Risques mycotoxicologiques pour la santé des animaux et de l’homme. Cahiers de nutrition et de diététique 35, 389-397.
Pier, A., 1992. Major biological consequences of aflatoxicosis in animal production. Journal of Animal Science 70, 3964-3967.
Pier, A., Richard, J., 1992. Mycoses and mycotoxicoses of animals caused by Aspergilli.
Pier, A.C., Richard, J.L., Cysewski, S.J., 1980. Implications of mycotoxins in animal disease. J Am Vet Med Assoc 176, 719-724.
Ribelin, W., Fukushima, K., Still, P., 1978. The toxicity of ochratoxin to ruminants. Canadian Journal of Comparative Medicine 42, 172.
Richard, J., Meerdink, G., Maragos, C., Tumbleson, M., Bordson, G., Rice, L., Ross, P., 1996. Absence of detectable fumonisins in the milk of cows fed Fusarium proliferatun (Matsushima) Nirenberg culture material. Mycopathologia 133, 123-126.
Silva, L.A., de Mello, M.R.B., Oliveira Pião, D., Silenciato, L.N., de Quadros, T.C.O., de Souza, A.H., Barbero, R.P., 2021. Effects of experimental exposure to zearalenone on reproductive system morphometry, plasma oestrogen levels, and oocyte quality of beef heifer. Reprod Domest Anim 56, 775-782.
Smith, B.P., 2014. Large animal internal medicine-E-Book. Elsevier Health Sciences.
Sohooa, R., Khana, A.U., Ameena, K., Rafia-Munire, A., Saleemb, F., 2015. Outbreak of aflatoxicosis on a local cattle farm in Pakistan. Veterinaria 3, 13-17.
Średnicka, P., Juszczuk-Kubiak, E., Wójcicki, M., Akimowicz, M., Roszko, M., 2021. Probiotics as a biological detoxification tool of food chemical contamination: A review. Food and Chemical Toxicology 153, 112306.
Storm, I., Sørensen, J.L., Rasmussen, R.R., Nielsen, K.F., Thrane, U., 2008. Mycotoxins in silage. Stewart Postharvest Rev 4, 1-12.
Sulzberger, S., Melnichenko, S., Cardoso, F., 2017. Effects of clay after an aflatoxin challenge on aflatoxin clearance, milk production, and metabolism of Holstein cows. Journal of dairy science 100, 1856-1869.
Sumantri, I., Murti, T., Van der Poel, A., Boehm, J., Agus, A., 2012. Carry-over of aflatoxin B1-feed into aflatoxin M1-milk in dairy cows treated with natural sources of aflatoxin and bentonite. Journal of the Indonesian tropical animal agriculture 37, 271-277.
Sutton, P., Waring, P., Müllbacher, A., 1996. Exacerbation of invasive aspergillosis by the immunosuppressive fungal metabolite, gliotoxin. Immunology and cell biology 74, 318-322.
Tola, M., Kebede, B., 2016. Occurrence, importance and control of mycotoxins: A review. Cogent Food & Agriculture 2, 1191103.
Trenholm, H., Thompson, B., Martin, K., Greenhalgh, R., McAllister, A., 1985. Ingestion of vomitoxin (deoxynivalenol)-contaminated wheat by nonlactating dairy cows. Journal of Dairy Science 68, 1000-1005.
Valgaeren, B., Théron, L., Croubels, S., Devreese, M., De Baere, S., Van Pamel, E., Daeseleire, E., De Boevre, M., De Saeger, S., Vidal, A., 2019. The role of roughage provision on the absorption and disposition of the mycotoxin deoxynivalenol and its acetylated derivatives in calves: From field observations to toxicokinetics. Archives of toxicology 93, 293-310.
Van Halderen, A., Green, J., 1989. A field outbreak of chronic aflatoxicosis in dairy calves in the Western Cape Province. Journal of the South African Veterinary Association 60, 210-211.
Wang, J., Liu, Z., Han, Z., Wei, Z., Zhang, Y., Wang, K., Yang, Z., 2020. Fumonisin B1 triggers the formation of bovine neutrophil extracellular traps. Toxicology letters 332, 140-145.
Weaver, G., Kurtz, H., Behrens, J., Robison, T., Seguin, B., Bates, F., Mirocha, C., 1986a. Effect of zearalenone on dairy cows. American Journal of Veterinary Research 47, 1826-1828.
Weaver, G., Kurtz, H., Behrens, J., Robison, T., Seguin, B., Bates, F., Mirocha, C., 1986b. Effect of zearalenone on the fertility of virgin dairy heifers. American Journal of Veterinary Research 47, 1395-1397.
Weaver, G., Kurtz, H., Mirocha, C., Bates, F., Behrens, J., Robison, T., Swanson, S., 1980. The failure of purified T-2 mycotoxin to produce hemorrhaging in dairy cattle. The Canadian Veterinary Journal 21, 210.
Whitlow, L., Hagler Jr, W., 2010. Mycotoxin effects in dairy cattle. Mid-South Ruminant.
Whitlow, L., Hagler, W., 2010. Mold and mycotoxin issues in dairy cattle: effects, prevention and treatment. Adv Dairy Technol 20, 195-209.
17


 Sabry El-khodary Professor of Internal Medicine and vice dean for post graduate studies and research affair, Faculty of Veterinary Medicine, Mansoura University, Egypt
Sabry El-khodary Professor of Internal Medicine and vice dean for post graduate studies and research affair, Faculty of Veterinary Medicine, Mansoura University, Egypt