Haematological Conditions in General Practice
Chair: A/Prof James Morton AM
Director of Haematology
Icon Group





Chair: A/Prof James Morton AM
Director of Haematology
Icon Group





Lymphoma
With a network of more than 40 clinical haematologists, day oncology centres across the state, outreach clinics in remote and regional areas and telehealth services, Icon Cancer Centre is committed to delivering the best possible care for all patients in Queensland.
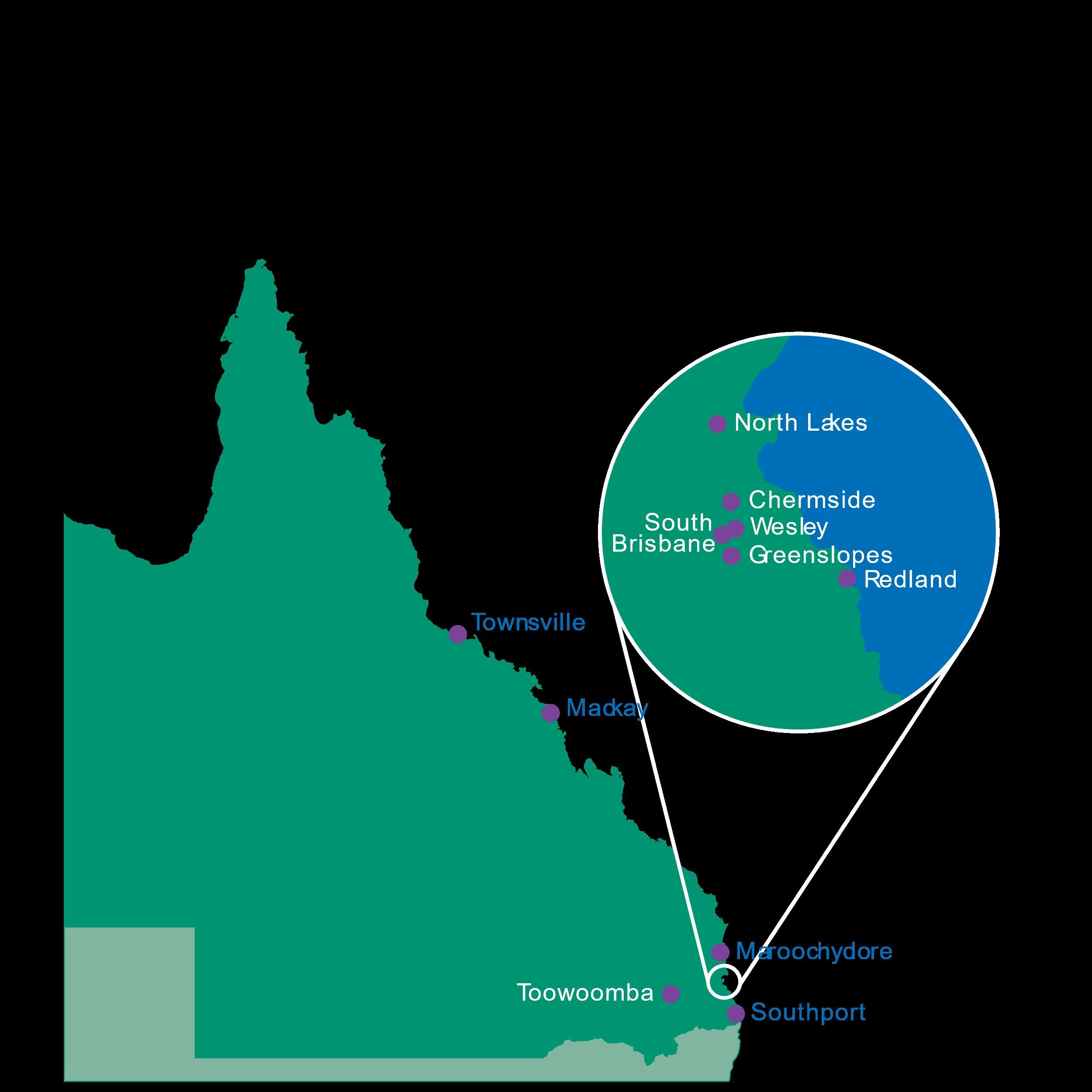
Icon Cancer Centre sites
• Townsville
• Mackay
• Maroochydore
• Toowoomba
• North Lakes
• Chermside
• Wesley
• Greenslopes
Satellite and outreach clinics
• Cairns
• Rockhampton
• Bundaberg
• Hervey Bay
• Maryborough
• Warwick
• Tarragindi
• Sunnybank
• Redland
• South Brisbane
• Southport
• Ipswich
• Springfield Central
• Benowa
• Tugun

IconDoctorApp.com is a dedicated secure referral and online education platform created exclusively for GPs and specialists.
Accessible via desktop and mobile, IconDoctorApp.com provides:
• Expedited secure referrals
• Haematology and oncology RACGP accredited and self-directed online learning
• Event registration and CPD records
• Diagnosis and treatment pathways for referral decision making
To learn more about our new online platform and create your account, visit IconDoctorApp.com

Karthik Nath
MBBS (Hons) BSc FRACP FRCPA PhD
Clinical Haematologist
Deputy Director of Cellular Therapy
Icon Cancer Centre South Brisbane


• Overview of CAR T-cell therapy
• Indications for CAR T-cell therapy in blood cancers
• Unique Toxicities of CAR T-cell therapy
• Future Developments – Immune-effector cell (IEC) therapies
Cappell, Kochenderfer,


CD3 “T-cell marker”

PAX-5 “Lymphoma marker”

CD4 “T-cell marker”

CD8 “T-cell marker”

• Overview of CAR T-cell therapy
• Indications for CAR T-cell therapy in blood cancers
• Unique Toxicities of CAR T-cell therapy
• Future Developments – Immune-effector cell (IEC) therapies
CAR T-cell therapy is approved for use in relapsed/refractory blood cancers.


Inpatient (Kite, Gilead)
Inpatient (Kite, Gilead)
Outpatient (Novartis)
Outpatient (BMS)
Inpatient (BMS)
Outpatient (J&J)

• r/r CLL or MCL ≥ 2 prior lines (2024)
MM after ≥ 2 lines of therapy (2024)
MM after ≥ 1 line of therapy (2024)
Cappell, Kochenderfer, Nat Revs Clin Oncol, 2024 (modified)

BCMA-directed CAR T-cell therapy
Trial No. of patients
ORR %

• Overview of CAR T-cell therapy
• Indications for CAR T-cell therapy in blood cancers
• Unique Toxicities of CAR T-cell therapy
• Future Developments – Immune-effector cell (IEC) therapies


• Phase 1. CAR T-cell trafficking to tumor site
• Phase 2. CAR T-cell activation/proliferation; cytokine production; activation of endogenous immune cells
• Phase 3. ↑ cytokines → systemic inflammatory response
• Phase 4. Diffusion of CAR-T, cytokines, activated monocytes into CSF (ICANS)
• Phase 5. Resolution phase

Nature Reviews, 2022


• Overview of CAR T-cell therapy
• Indications for CAR T-cell therapy in blood cancers
• Unique Toxicities of CAR T-cell therapy
• Future Developments – Immune-effector cell (IEC) therapies
Gastric adenocarcinoma Auto CAR T-cell Claudin 18.2
Renal cell carcinoma Allo CAR T-cell CD70
Prostate cancer Auto CAR T-cell PSMA
Gastric adenocarcinoma Allo NK T-cell -
Synovial sarcoma TCR T-cell -
Melanoma TIL -
Melanoma + lung cancer IL15 secreting TIL -
Mesothelioma Auto CAR T-cell Mesothelin
Auto, autologous; allo, allogeneic; TCR, T-cell receptor; TIL, tumor infiltrating lymphocyte














Largest provider of ASCT in Queensland Favorable survival outcomes with ASCT compared to national registry data





ASCT, autologous stem cell transplant
Icon Cancer Centre
Ian Irving
James Morton
Kerry Taylor
Jason Butler
Simon Durrant



Icon Cellular Therapy Lab
Debra Taylor
Icon Cancer Foundation
Leanne Hardymann
Icon Group – Research
Icon Pharmacy
Memorial Sloan Kettering Cancer Center, NYC
Jae Park
Sham Mailankody
Translational Research Institute
Maher Gandhi
QIMR Berghofer
Steven Lane




Dr. Ross Tomlinson – Hematologist
Icon Cancer Centre Wesley
Icon Cancer Centre Chermside




Outline the differences between plasma cell disorders


Classify plasma cell and platelet disorders through clinical diagnosis Implement evidence-based patient management of plasma cell disorders

Summarise the relationship between monoclonal gammopathy of undetermined significance (MGUS) and blood cancers or other serious diseases


Progressive fatigue
Constipation
Progressive lower back pain for approximately 3 months




Pre malignant monoclonal gammopathies
• MGUS
Malignant monoclonal gammopathies
• Smouldering multiple myeloma (MM)
• Multiple myeloma/Plasma cell leukaemias
• Solitary plasmacytomas

• Waldenstrom macroglobulinemia










Signs
Unexplained anaemia
Renal impairment
Neuropathy
Restrictive heart failure
Lytic lesions on imaging
Unexplained osteoporosis
Lymphocytosis
Other cytopenias
Symptoms
Fatigue Increasing bone pain (pelvis/hips/lower back)
Weight loss
Fevers Increased infections (e.g. sinus, pneumonia
Surprisingly very little!
Occasionally pts will have hepatosplenomegaly
“Classic signs” of amyloidosis
• Macroglossia
• Periorbital ecchymosis


Serum protein electrophoresis (should include an immunofixation)
• Will detect whole immunoglobulin e.g. an IgG, IgA etc.
Necessary companion tests
• Serum free light chains
• Immunoglobulins
⚬ Will allow the measuring of normal/residual globulins (can be helpful if associated hypogammaglobulinemia)

GP Driven investigations
• FBC, chem20, coagulation studies
• SPEP, SFLC, immunoglobulins
• Urine not essential unless thinking about amyloidosis (e.g. weight loss, fluid retention, cardiac sx)
Haematologist driven investigations
Skeletal imaging:
• Low dose whole body CT scan OR whole body MRI OR CT/PET
BMAT:
• useful for quantifying plasma cells/detection of amyloidosis

Mr. AM








MSKCC – Multiple Myeloma information


What is the significance of monoclonal gammopathy of undetermined significance? Catherine Atkin, Alex Richter, Elizabeth Sapey Clinical Medicine Oct 2018, 18 (5) 391-396; DOI: 10.7861/clinmedicine.18-5-391



Approx 5% over the age of 60
Increases as age increases ~10% over age 85
Can be found incidentally Needs risk stratification and appropriate referral May need further/companion tests (e.g. BMAT, imaging)

Rajkumar SV et. al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005 Aug 1;106(3):812-7. doi: 10.1182/blood-2005-03-1038. Epub 2005 Apr 26. PMID: 15855274; PMCID: PMC1895159.
B symptoms
• fevers, night sweats, weight loss
Significant bone pain
Peripheral neuropathy
Amyloidosis related sx:
• Sx of cardiac failure (e.g. lower limb oedema, decreasing exercise tolerance, easy bruising)

IgG paraproteins
• if <10g/L – I will generally observe and NOT BMAT or skeletal image
• if >10g/L – I will BMAT and skeletal image
IgM paraproteins
• more often associated with lymphomas, than myelomas
• BMATs are informative –will pick up a B cell lymphoma
• PET scan often better to image for adenopathy/liver/splenomegaly
IgA PP
• I will always image and BMAT as level poorly correlates with disease burden
Stern S et al. the Haemato-Oncology Task Force of the British Society for Haematology and the UK Myeloma Forum. Investigation and management of the monoclonal gammopathy of undetermined significance. Br J Haematol 2023; 202(4): 734–744. https://doi.org/10.1111/bjh.18866

At the start I check q3-4 months to get a feel for its tempo
If after 1 year its stable – can go to checking it q6-12/12
Frequency of monitoring can change if there is a change in protein (e.g. more frequently if paraprotein increases)

Any concern re: development of symptoms should trigger a review by Haematologist


Malignant condition
Significant plasma cell burden but no SLIM-CRAB criteria
Requires closer observation than MGUS
Approx 0.5% of adults >40
Prevalence increases with age
• 1.08% over age 70
• 1.59% over age 80
Hughes D et. al. Diagnosis and management of smouldering myeloma: A British Society for Haematology Good Practice Paper. Br J Haematol. 2024 Apr;204(4):1193-1206. doi: 10.1111/bjh.19333. Epub 2024 Feb 23. PMID: 38393718.

FBC, chem20, SPEP, SFLC, immunoglobulins
Skeletal imaging
• Low dose whole body CT, MRI, or CT-PET
Bone marrow aspirate and trephine
• Accompanied cytogenetic evaluation and flow cytometry

Multiple risk scores available
• Mayo 20-2-20
• IWMG 20-2-20 with FISH
Risk is generally based on plasma cell burden, size of paraprotein, and FLC assay results
Consideration given to reevaluating patients over time
Skeletal imaging repeated for monitoring

These patients require ongoing haematologist follow up


Increasing age of population
Pts with myeloma are living longer
Average life expectancy gone from 3 years >10 years
Same workup for previous states
Extra components are prognostic (e.g. cytogenetics)

Treatment should be aimed at
• Maximizing depth and duration of response/remission
• Minimizing toxicity
• Improving QoL and lengthen overall survival
• Alleviate symptoms
• Prevent further organ damage
Sive J et. al. British Society of Haematology. Guidelines on the diagnosis, investigation and initial treatment of myeloma: a British Society for Haematology/UK Myeloma Forum Guideline. Br J Haematol. 2021 Apr;193(2):245-268. doi: 10.1111/bjh.17410. Epub 2021 Mar 21. PMID: 33748957.

Adjustment for frailty/frailty assessment
• Disease of the elderly
• Myeloma therapy doses can, and should, be adjusted depending on age/frailty scores
Transplant eligibility
• Recommended for younger, fitter pts
• Selected pts >70 can be considered for autologous transplantation
Patient and clinician preference
• Variability and breadth of choice for oral therapies, subcutaneous and IV therapy based on line of therapy/stage of disease

Treatment paradigms
• 2 drugs better than 1, 3 drugs better than 2
• Always looking to combine therapies
• First remission is always the longest, with then diminishing returns
Fit patients
• Velcade based chemotherapy (VRD)
• Stem cell transplantation
• Maintenance lenalidomide
Frail/Less fit pts
• Velcade based treatment (e.g. VRD-lite, VCD) or Imid based therapy

Choose the right therapy for the right patients – very individualised care.
Generally progress through available options depending on patient risk, disease presentation, comorbidities etc.

Options:
• Proteosome inhibitors (Bortezomib, Carfilzomib)
• Immunomodulatory medications (e.g. Lenalidomide, Pomalidomide)
• Monoclonal antibodies (e.g. Daratumumab, Elotuzumab)
Future of myeloma is bright – but not cured yet
• bacteria, viruses etc.
Prophylactic antibiotics for PCP (resprim) and shingles (Valtrex)
Monthly IVIG for hypogammaglobulinemia
Secondary cancers skin cancers, breast, bowel, colon etc.
Bone health
1-3 monthly zometa
Metabolic complications
Sive J et. al. British Society of Haematology. Guidelines on the diagnosis, investigation and initial treatment of myeloma: a British Society for Haematology/UK Myeloma Forum Guideline. Br J Haematol. 2021 Apr;193(2):245-268. doi: 10.1111/bjh.17410. Epub 2021 Mar 21. PMID: 33748957.


Vaccinations – COVID-19, pneumococcal, shingles, influenza
Mgmt of chronic comorbidities
Skin checks
Survivorship – depression/anxiety
Metabolic issues – obesity, T2DM, cholesterol, etc
Can call anytime to discuss cases










Prof Morie Gertz –Mayo Clinic Rochester

Amyloidosis is a group of diseases caused by abnormal deposition in the body of amyloid fibrils, made up of misfolded proteins
Amyloid is derived from the word ‘amylon’ in Greek or ‘amylum’ in Latin, means cellulose or “starch like”



Our genes and our DNA code for the manufacture of small molecules called proteins
Proteins help life’s biological processes antibodies as part of our immune responses to infection hormones that regulate our body’s growth, regulation and organ function
Once produced, proteins naturally fold into a particular shape - The way a protein folds allows for its specific function

When proteins are folded properly, they work as they should - and we enjoy good health
When proteins “kink” or misfold, they may be able to make amyloid fibrils
As we age, protein stability can start to falter
Proteins may kink more easily
If we inherit genetic variation, some proteins mis-fold more easily
If we have inflammation for many years, some proteins mis-fold more easily
Usually our bodies can identify & remove abnormally folded proteins
But sometimes…. we produce too much abnormal protein for our body to handle or we are not able to break down and clean up the abnormal protein at all
Amyloidosis is a protein folding disorder


The misfolded protein (amyloid) takes on a shape that makes it difficult for the body to break down
These amyloid proteins link together to form rigid, linear “fibrils”
- accumulate in organs and tissues
- causing damage and organ failure with time


Fragments

Normal TTR Tetramer


TTR circulates as a “4 leaf clover” or a “flower”

Individual proteins misfold

“Leaves” fall off the flower as we age “Leaves” mis fold or kink
Quintas et al. J Biol Chem 2001;Johnson et al. JMB 2012; Marcoux et al. EMBO Mol Med 2015; Bulawa et al. PNAS 2012
Stick together Aggregate abnormally


Misfolding allows the leaves to stick together
Then they arrange into very hard amyloid fibrils that deposit between cells and tissues abnormally

“Rare” condition
~300 Australians are estimated to develop AL each year
Up to 3000 Australians are thought to have TTR
We have been under diagnosing
New tools (modern echo with strain imagining) and cardiololgist awareness
2023: QLD Amyloidosis Centre are getting 400+ referrals of confirmed Amyloidosis

Number of TTR referrals to VTAS since mid-2014
Production of excessive amount of immune light chains
eg. MGUS or Multiple myeloma – AL
Family history of amyloidosis
ie. genes produce proteins that more easily misfold – Hereditary
Chronic infection/inflammatory disease
eg. Crohn’s disease, recurrent infections – AA
Ageing - TTR


Lousada et al. Adv Ther. 2015;32(10):920-8.
Common sites include
Bladder
Airway (trachea, lungs)
Eye
Gastrointestinal tract
Skin
Breast
Localised amyloid deposits are usually made up of light chains
Similar to systemic AL amyloidosis


However the abnormal light chains are only in the affected tissue, not the bone marrow
Typically an excellent prognosis – no treatment needed in most cases!
Diagnosed ONLY after thoroughly excluding systemic disease
DOES NOT require chemotherapy / transplant
Accounts for ~20-30% of new diagnoses
Begins in the bone marrow the “blood factory” of haematopoiesis
Plasma cells normally produces “antibodies” that protect us from infection

These antibody proteins are made up of light chains and heavy chains


In AL, too many mis-folded light chains are being made
These ‘free light chains’ cannot be broken down efficiently
They bind together to form amyloid fibrils that build up in organs and tissues
Organs involved can be: Kidneys, heart, liver, nerves, intestines, skin, tongue and blood vessels









Requires plasma cell directed therapy:
Daratumumab, Bortezomib, Cyclophosphamide, Dexamethasone
6 cycles / months of therapy
Autologous Bone Marrow transplantation
Must not have significant cardiac involvement and be eligible for transplant
Amyloid resorption antibodies in clinical trials (CAEL101)
Most critical is to support the organs effected:
AVOID B-blockers and ACE inhibitors / ARB
VTE prophylaxis
Nutrition and IV albumin
Immunosuppression prophylaxis: antibiotics (Doxycycline and Ig replacement)
The amyloid-forming protein is “transthyretin” (TTR)
TTR is a produced by the liver
TTR helps to transport thyroid hormone and vitamin A around the body
TTR Amyloidosis can be…
Acquired - 90%
Usually >70 years
Much more common in men
Usually carpal tunnels involved ~10-15 years before the heart is involved
Hereditary - 10% (aka variant-TTR)
Affects men and women equally


>100 known mutations of TTR gene can produce more unstable variant TTR proteins
More easily misfold and aggregats into amyloid fibrils
Carpal tunnels, nerves and heart are usually affected
Much more common than previously recognized
Up to 30% of patients with heart failure despite normal pumping of the heart


Diflunisal (off label anti-inflammatory agent)
Requires normal renal function and surveillance for NSAID complications
Useful for TTR neuropathy
ECGC green tea extract
Yes it actually works – but has a modest benefit
Tafamidis for cardiac TTR has been recommended for PBS – listing expected very soon
Oral
Patiseran - snRNA inhibitors for hereditary TTR with neuropathy
Knocks down TTR protein production and therefore TTR amyloid production
IV medication 3 weekly infusion
Cost >$400000/y - Patiseran pending PBS approval



Symptoms often are non-specific and easily missed -> can mimic other more common conditions
Common vague symptoms at presentation:
New dyspea or pedal ankles
Neuropathy: Numbness/pain in fingers and toes
Autonomic neuropathy: Diarrhea/bloating
Postural hypotension: Light headedness when standing up
Loss of appetite and/or weight loss
Proteinuria in the urine
Screening Echo with “Thickened heart”
New atrial fibrillation
Take a biopsy
“Gold standard” is to detect positive Congo red amyloid staining on a biopsy
Biopsies usually taken from the organ in question, eg. kidney
Blood and urine tests
To measure if there is abnormal production of serum free light chains
Can identify >98% of patients with AL amyloidosis
Bone marrow biopsy
To help confirm the AL type and to access medications
PYP Cardiac “bone” scan
Genetic tests
To exclude hereditary disease



Bone scan showing amyloid in the heart

Use a multidisciplinary team with experience treating amyloidosis
Co-ordinating amyloidosis canter / specialist
Local Haematologist, Cardiologist, Nephrologist, Neurologist, Gastroenterologist, Nursing staff…
Goal
Eliminate the supply of amyloid protein to improve organ function and survival
Manage symptoms to promote patient’s well being and quality of life
General treatment approaches
Decrease production of the amyloid-forming protein
Stabilise the normal structure of the amyloid-forming protein - thus preventing it from misfolding into amyloid
Target amyloid deposits directly destabilizing the amyloid fibrils if possible
Support the organs affected by amyloid deposits

Dr Michelle Spanevello | Haematologist
Bphty(Hons), MBBS(Hons), FRACP, FRCPA Icon Cancer Centre Chermside

Case reports in 1950s linking long-haul flights to thrombosis
Jacques mentions phlebitis first in 3 reports from 1951
Attributed to Homans 1954 reporting DVT in a doctor after a long-haul flight
Homans cases not confined to air travel
2 long-haul flights, 2 long ‘automobile’ trips, 1 crossed leg sitting at theatre
Homans links prolonged dependency stasis to DVT
Reports of PE during the London ‘Blitz’ (air raid shelters) in 1940s
Causality not able to be established

Czuprynska et al, BJH 2020
Cannegieter et al, Travel Medicine Chpt 52 2019
Appears in literature in 1977
Ongoing reports of events with air and overland travel through 1980s and 1990s
Commercial aircraft more regular undertaking
Cramped seating conditions
Variety of modes of travel

Czuprynska et al, BJH 2020 Cannegieter et al, Travel Medicine Chpt 52 2019



One a month dies from DVT at Heath row | Daily Mail On line
British Travel Health Association reports 2500 flying related PE per year


House of Lords Science and Technology Committee inquiry 5th report
2000, funded by Department of Transport
Travel documented as a risk factor for VTE in 2000
Cohort studies, RCTs, Cross-sectional studies
Not without controversy according to correspondence in the Lancet 2004
2001 Giangrande encourages this encompassing terminology

Kestevan, Thorax 2000, Giangrande, J Trav Med 2000
Correspondence, Lancet 2004
Ansari et al, J Trav Med 2005, Czuprynska et al, BJH 2020
Cannegieter et al, Travel

Immobility is a key factor
Risk of VTE doubles after long-haul flight > 4h
This occurs for other forms of travel with seated
immobility
Longer trip / multiple trips increases risk

Risk is accentuated by presence other VTE risk factors
Risk is accentuated in obesity, extremes of height, COCP and thrombophilia
Absolute risk is 1 in 6000
Some flight specific factors may exist
Study results released on travel and blood clots (who.int)
26 172 enrolled as of May 2009
2% developed VTE in association with recent travel
Travellers with VTE were Younger
Previous VTE
BMI slightly higher (28 vs 27)
Using hormonal contraception
Positive thrombophilia test

Tsoran et al, Throm Res 2010

Large population-based case-control study extension of MEGA
1906 patients with index VTE (<70 yo) matched to controls
12% (233) had > 4 h travel in preceding 8 weeks compared to 9.5%
Travel associated VTE OR 2.1
Similar for travel by car, bus, train but more pronounced with air travel
Risk highest in first week after travel
Increased risk with additional risk factors
Kuipers et al, Blood 2009
Czuprynska et al, BJH 2020
Data for arrivals of international flights 1981-1999
Over that time, 4.8M Australian and 4.6M non-Australian citizen arrivals –main analysis on Australian travellers
5408 out of 16205 patients admitted to WA hospital with VTE could be linked to a record of international flight arrival
153 Australian citizens and 438 non-Australian cases of VTE within 100 days of arrival of international flight
Incidence rate highest in first 2w of arrival (“hazard period”)

Keman et al, BMJ 2003
Prospective study, average flight duration 12.4h (10-15h)
355 Low Risk travellers
Exclusions: artificial valves, PPM, chronic conditions, mothers travelling with children less than 2, surgery within 6m, severely obese, previous DVT, coagulation disorders, immobility, neoplastic disease within 2y, large varicose veins
389 High Risk travellers
USS done at 24h on disembarkation
Incidence of 1.4% Asymptomatic DVT only in high risk travellers
Greatest frequency of VTE in non-aisle seats (18 of 19 thromboses, 94.7%)

Belcaro et al, Angiology 2001

Prospective, public advertisement – volunteers with > 10h flight exposure
917 eligible, excluded high risk travellers (previous VTE, on anticoagulation, recent surgery)
Preflight D dimer and then serial up to 3m (excluded those with abnormal preflight D dimer)
112 suspected cases of VTE went onto USS / CTPA / V/Q scan
9 confirmed, 7 had additional risk factors
Frequency of VTE was 1% (compression stockings used in about 1 in 6)
Concluding a positive association between multiple long-distance flights and VTE
Absolute risk per flight > 4h is 1 in 4500-4600 (1 in 6000 in low risk)
Incidence of PE in air travel low 0.4 per million flyers
Incidence PE increases with distance travelled and longer flight
duration
4.8 cases per million for > 10 000km
0.01 cases per million < 5 000km
Consistent increase in risk with additional risk factors such as prior VTE

Lapostolle et al, 2001, Kuipers et al, 2007
Kahn et al, CHEST 2012, Czuprynska et al, BJH 2020
McKerrow Johnson et al, Wild Env Med 2022

Annual incidence of all cause VTE is 1 in 1000
1 in 100 for elderly, 1 in 10 000 for younger population
Slow and steady increase over recent decades
Up to threefold increase with long haul flights (weak)
Incidence 4.0/1000 persons per year compared with 1.2/1000 per year in non-exposed time (retrospective cohort)
Incidence of symptomatic VTE 0.3%
Firkin et al, Aus Prescr 2009, Ringwald et al, Trav Med ID 2014,
Cannegieter et al, Travel Medicine Chpt 52 2019, Czuprynska et al, BJH 2020
Heterogenous studies
Range of outcome measures – some of uncertain clinical significance
Case-control studies suggest a causative association – some do not
Volunteer bias
Recall bias
Survivor bias
Healthy Traveller Effect

Ansari et al, J Trav Med 2005
Czuprynska et al, BJH 2020
Cannegieter et al, Travel Medicine Chpt 52 2019

October 1821 – 5 September 1902
German physician, anthropologist, pathologist, prehistorian, biologist, writer, editor, and politician
Known as "the father of modern pathology“, the founder of social medicine, and to his colleagues, the "Pope of medicine“
Rudolf Virchow – Wikipedia, accessed 17/9/24

Described and named disease processes such as leukaemia, embolism and thrombosis
Developed the first systematic method of autopsy
Introduced hair analysis in forensic investigation

…But perhaps not the Pope of Medicine
Plagiaristic work in Cellular Pathology (1858), third dictum in cell theory: Omnis cellula e cellula (“All cells come from cells”)
Opposed the germ theory of disease
Anti-Darwinist describing Neanderthal man as a deformed human
Various eponymous syndromes attributed to him such as Virchow’s node, Virchow-Robin spaces, Virchow-Seckel Syndrome and Virchow’s triad

Virchow described mechanisms of thrombosis but not a triad
He published on the pathology of pulmonary embolism in 1856 He did not include endothelial injury
Consensus and mention of this terminology appeared in the 1950s Modern understanding is similar to the description from Virchow


Postulated Venous Stressors of Travel
• Immobility
• Cramped Seat Position
• Dehydration
• Hypobaric hypoxia (air)
Immobility: Duration of Travel is Important


Risk increases with increasing duration of flight (4h+)
Risk also increases with several flights in a short time frame
Frequency of severe PE if air travel > 6h is 150 times higher if <6h
Peak risk travel > 8h
Sandor et al, Pathophysiology 2008
Silverman et al, Lancet 2009
Cannegieter et al, Travel Medicine Chpt 52 2019
Firkin et al, Aust Prescr 2009


Pitch in Economy
1980s Boeing 747: 81.3cm (32in)
2000s BA 78.75cm (31in), AA 86.4cm (34in), QANTAS 81.3cm (32in)
Current QANTAS/UA 78.7cm-81.3cm (31-32in), Singapore/Cathay/Emirates 81.3cm (32in), Delta 78.7cm (31in), JAL 83.8cm, Jetstar 76.2cm (30in)
Civil Aviation Authority recommends minimum seat-pitch 71.6cm (28.2in)
Anthropometric growth over time
Silverman et al, Lancet 2009
Best economy seats on international flights out of Australia (smh.com.au) Long-haul Economy Class Comparison Charti - SeatGuru
LONFLIT showed greatest frequency of VTE in non-aisle seats
MEGA study showed associations with extremes of height and obesity
Height > 1.9m after any travel OR 4.7
Height < 1.6 after air travel only OR 4.9
Obese BMI > 30 increased risk
BEST study 2003: No difference between Business and Economy Class

Rage / Knee Defenders / Pre-reclined seats
Jacobson et al, S Afr Med J 2003, Belcaro et al, Angiology 2001, Kuipers et al, Blood 2009, Mendoza, Int J Healthcare M 2018


Limited information on seat pitch Oedema develops in 4 hours sitting Twofold increase in long distance travel by any mode OR 2.7 any travel vs OR 3.0 air travel
Travel thrombosis more frequent after car and bus travel (70%) then air flights (30%)
Dutch pilot study 2014: 2630 Male pilots, 20 420 person years, no difference with general population
Ferrari et al, Chest 1999, Sandor et al, Pathophysiology 2008, Firkin et al, Aus Presc 2009, Kuipers et al, J Thom Haemost 2014
Planes, Trains & Automobiles (1987) - IMDb
No thrombophilia
Random exposure to hypobaric hypoxia and normobaric normoxia
No significant changes in TAT, PT fragment, D dimer and ETP
No evidence of platelet activation
No evidence of endothelial activation
No significant differences in RCC, WCC, Plt, Hct
High responders: Fvl + COCP

Comparison 8h flight, 8h movie marathon, 8h usual daily activities
TAT increased by 30% with flight
D dimer rose to 216ug/L flight, 180ug/L movies
Toff et al, JAMA 2006 Schreijer et al, Lancet 2006
Quiet sitting
Rise in Hct
Rise in protein concentrations
Simulated 8h flight

Reduced humidity
Increased mean plasma osmolality
Increased mean urine osmolality
May reduce fibrinolytic activity
Mendis et al, Bulletin of WHO 2002





Flow:
Lower limb linear blood velocity reduced by ⅔ in sitting, ½ standing cf recumbency
Stasis due to pressure or position eg crossed legs
Window seating and relative immobility / inertia
Hypobaric hypoxia and vessel wall relaxation
Release of nitric oxide, low oxygen pressure

Endothelial wall:
Position / kinking of popliteal veins (tall, short, elderly, obese)
Transverse folds and rings form and fix with age
Localised fibrosis and sclerosis of the intima and media
Hypobaric hypoxia and vessel wall relaxation
Release of nitric oxide, low oxygen pressure
Hypercoagulability:
Low humidity and dehydration, haemoconcentration, hyperviscosity
Activation of coagulation and reduced fibrinolysis present prior to travel (inconsistent)
Additional risk factors

Cramped positions, Relative immobility, ?Hypobaric hypoxia

Direct pressure
?Hypobaric hypoxia
Localised fibrosis / sclerosis
Age
Endothelium
Flow
?Dehydration
?Hypobaric hypoxia
?Low humidity
?Fibrinolysis/?Activation
Additional RF
Coagulability













3-4 minutes every hour





Avoid dehydration
Avoid alcohol
Avoid caffeine
Avoid immobility
Exercise legs regularly (Homans 1954, indirect)
Avoid sedatives

Czuprynska et al, BJH 2020
Watson et al, BJH 2010
Silverman et al, Lancet 2009
Firkin et al, Aus Prescr 2009
Cochrane database 2021
12 RCTs combined (8 LONFLIT)
Below knee to hip, 10-30mmHg
Asymptomatic clots reduced if wearing GCS
CCL I, OR 0.10
Extrapolated benefit
Low risk
4.5 fewer symptomatic DVT per 10 000, 24 fewer
PE per 1 000 000
High risk
16.2 fewer symptomatic DVT per 10 000, 87 fewer
PE per 1 000 000

Czuprynska et al, BJH 2020, Clarke et al, CD 2016 and 2021, Kahn et al, CHEST 2012

Asymptomatic DVT rates in 249 ‘high-risk’ subjects
Clexane 1mg/kg 2-4h before travel, ASA 400mg daily for 3 days commencing 12h prior, no prophylaxis
LMWH 0% vs Aspirin 3.6% vs no prophylaxis 4.8%
et al, Angiology 2002
Silverman et al, Lancet 2009
Kahn et al, CHEST 2012
Czuprynska et al, BJH 2020
If chemoprophylaxis is considered appropriate, anticoagulants should be used over anti-platelet drugs

Silverman et al, Lancet 2009
Watson et al, BJH 2010
Kahn et al, CHEST 2012
Cannegieter et al, Travel Medicine Chpt 52 2019
Czuprynska et al, BJH 2020


https://www.janssenscience.com/products/xarelto/medical-content/use-ofxarelto-in-vte-prevention-for-long-distance-travel , accessed 22/9/2024 Ringwald et al, Trav

Survey of delegates of the XXth ISTH Congress, the 15th ISDB Congress and the 13th Cochrane Colloquium
All taking place in Australia 2005
2089 responses
80% had used preventative measures
ISTH delegates used LMWH and VKA
Medical doctors used more pharmacological prophylaxis than research and non-clinical attendees
High usage in those with no risk factors (77%, 90% in high risk group)
Kuipers et al, J Thromb Haemost 2006

Avoid the window seat
Don’t be too tall or too short or too obese
(No studies address utility of exercises / mobilising)
(No strong recommendation on alcohol)
(No strong evidence for chemoprophylaxis)
(Stockings could reduce oedema/asymptomatic DVT)
Czuprynska et al, BJH 2020 Sandor, Pathophysiology 2008





Travel is a weak provoking risk factor for thrombosis
More relevant in those with additional risk factors
Association is with duration of travel more than mode
Limited evidence for any prophylactic intervention
Assess risk for / benefit of compression / chemoprophylaxis on an individual basis
May benefit from GCS or short period of LMWH
Firkin et al, Aus Prescr 2009
Czuprynska et al, BJH 2020





A/Prof James Morton AM Icon Group Director of Haematology & Clinical Haematologist
Cumulative Full Blood Examination

Cumulative Iron Studies





• Cellular storage iron
• Minute quantities released blood
o Mostly apoferritin
o Proportional to stored iron
• Increased
o 10% iron overload: each 1ug/l = 10mg stored iron
o 90% non iron overload
o NB: alcohol - especially beer (>2/day)

Increased demand
• Rapid growth
• Pregnancy, breast feeding (total 1000mg iron)
• EPO treatment
Increased loss
• Bleeding: Gastrointestinal (GI), renal, menstrual, telangiectasia
• Blood donor
• Venesection: HFE, PRV
Reduced absorption
• Dietary deficiency
• Malabsorption disease: CD, IBD, autoimmune gastritis, PPI
• Malabsorption surgery: Gastrectomy, gastric bypass



*Note:
TIBC: Total Iron Binding Capacity
TF Satn: Transferrin Saturation
sTfR: Soluble Transferrin Receptor

Iron Supplement
• Ferric Citrate
• Ferric maltol
• Ferrous fumurate
• Ferrous gluconate
• Ferrous sulphate
• Ferrograd + C
• Maltofer
• Enteric costed or SR

1gm = 210mg elemental iron
30mg = 30mg elemental iron
325mg = 106mg elemental iron
325mg = 36mg elemental iron
325mg = 65mg elemental iron
Vit C 500mg, elemental iron 105mg
100mg elemental iron
Poor absorption due to iron release beyond upper jejenum (DMT1 duodenum and upper jejenum)
• Haem Iron
50% absorbed; minimal GI: very expensive: supplement rather than replacement
1. Daily or alternate day
• No role bd: hepcidin induction and reduced absorption
2. Optimal empty stomach
• Especially avoid milk, tea, coffee, eggs, fiber, cereals
• Impact meat vs. non-meat protein
3. Vitamin C improves absorption
• NB: Little benefit above 80mg
4. Maximum absorbed per day 25mg
Control 21%, 80mg vit C 27%, 500mg VitC 31%, Coffee 10%, Breakfast 7%
absorbed
Elderly: 15 v 50 v 150 mg elemental iron: No difference rise ferritin or Hb but increased GI

Gastrointestinal (GI) Side Effects
• Metallic taste, nausea, wind, constipation or diarrhea
• Reduce with alternate day and reduced dose of elemental iron

Response
• Pagophagia 24hr, Restless Legs 72hr, sense wellbeing 72hr
• 5 days ↑ retics, 2 weeks ↑ Haemoglobin (Hb) 1g/dl (dependent severity anaemia):
Failure: ↑ Hb 1g/dl at 4 weeks
• Papillation tongue: 4 weeks
Duration
• 3 months after normalisation of Hb
Ferric

- 15%
– 50%
Improvements
- 5%
– 20%
Prolonged Release (Ferrograd) Polymaltose (Maltofer)
*In general: Ferrous S better absorbed and more rapid increase Haemoglobin (Hb),
Ferric Polymaltose better tolerated.
Iron Infusion
• Ferric Carboxymaltose (Ferinject, Ferrosig)
• Ferric Derisomaltose (Monofer)
• Iron Sucrose (Venofer)
Intravenous (IV) Iron dosing
• Ganzoni
o Dose (mg) = wt x (target – current Hb) x 0.24 + 500mg
• Simplified Hb Wt

• Oral iron: compliance / patience / intolerance / failure
• Blood loss exceeds capacity oral iron to meet needs
• Co-existing inflammatory state (Hepcidin block)
• Chronic Kidney Disease (CKD)
• Inflammatory Bowel Disease (IBD)
• Heart Failure
• Athletes
• Pregnancy

• Dose: See Ganzoni
• Site: Forearm, Gravity Feed
• Pre-meds: No*
o *Inflammatory arthritis: Methyl pred 125mg pre, Prednisone 1mg/kg x 4 days post
• Ferinject (FCM): 1gm: 15min, repeat dose 7 days
• Monofer: 1500mg, 30 mins
• Reactions
o Non-Allergic: 1/200: Fishbane reaction: CARPA: labile free iron
• Stop until settled: restart
• Fails to settle 30min: hydrocortisone: restart 30min after settled
o Allergic: serious: 1/200000
• Hypophosphatemia:
o At risk: low Pi (IBD, bariatric surgery, EtOH, Diabetes Mellitus)
o Severe / symptomatic: 11%: 4-14 days: Fatigue, Myalgia
o FCM 50% overall, perists at 5 weeks 30%: Monofer 8%, mild
o Risk Osteomalacia




• Prevalence: 30% stable and 50% unstable patients
• Mechanism: nutrition, malabsorption (hepcidin, mucosal oedema)
• Impact; not Hb mediated (EPO studies): ? myocardial muscle iron
• Treat: Ferritin < 100 or Ferritin < 300 and Tsat < 20%
• Meta-analysis Ferric carboxymaltose (FCM)
o N = 839 (mostly FAIR-HF and CONFIRM-HF studies)
• Improved PS and NYHA score, reduced hospitalisation and all-cause mortality (HR 0.60)
• Benefit limited those with Tsat < 20% and especially < 13%
• TDI: TSAT > 70% ROS: cardiac toxicity
Eur J Heart Failure, 2018:20: 125-133
• Serology Coeliac Disease negative
• G/Colon: Crohns Disease
• Ferinject 1gm: well-tolerated


Dr Ian Irving Haematologist, Icon Cancer Centre Wesley Chief Medical Officer, Icon Group








• 65 year-old woman
• Works in Real Estate
• History of Hypothyroidism
- On thyroxine
• FBC as part of monitoring thyroid replacement therapy

• 65 year-old woman
• Works in Real Estate
• History of Hypothyroidism
- On thyroxine




• Infection – especially viral infections
• Splenomegaly / Hypersplenism
• Autoimmune disease
• Nutritional deficiencies
• Drugs / Medications
• Bone marrow pathology
• Other – including benign and chronic idiopathic neutropenia

• Often the only abnormality on the FBC
• Note reference range differences between labs
• Benign neutropenia often transitory
• Chronic idiopathic neutropenia often a diagnosis of exclusion
• History of infection important in planning management
• Note Benign Ethnic Neutropenia





• 25 year-old woman
• Psychologist
• No significant medical history
• No regular medications

• Examination – no splenomegaly, BMI 32

• 25 year-old woman
Psychologist • No PMHx • No regular medications

• 25 year-old woman
Psychologist • No PMHx • No regular medications

Relatively common finding on FBC
• Generally reactive (especially in absence of other abnormalities)
Wide differential diagnosis
• Inflammatory states, infections
• Role of obesity, smoking
• Rarely primary haematological malignancy
• Can often be observed
88 year old man
Retired property developer
Presentation with Cough
Mild fatigue
No fever or sweats


88 yo man
Retired developer
Presentation with Cough
Mild fatigue
No fever or sweats


88 yo man
Retired developer
Presentation with Cough
Mild fatigue
No fever or sweats


• B12 / Folate deficiency
• Medications
• Alcohol excess / Chronic Liver Disease
• Hypothyroidism
• Reticulocytosis
• Bone Marrow disorders

88 yo man
Retired developer
Presentation with Cough
Mild fatigue
No fever or sweats







• Pre – leukaemia
• Is a continuum to Acute Myeloid Leukaemia (AML)
• Disease of aging in particular
• Myelodysplastic Syndrome vs Myeloproliferative Neoplasms (MPN)
• Mutations now described and pre-MDS state of Clonal Haematopoiesis (CH)
• Next Generation Sequencing (NGS) panels now available

Hypertension - perindopril
Diabetes Mellitus - diet
Reflux oesophagitis
Well
Routine annual FBC

Hypertension - perindopril
Diabetes Mellitus - diet
Reflux oesophagitis
Well
Routine annual FBC





• Artefactual
• Infections - eg. viral, sepsis, DIC
• Medications – drug induced immune thrombocytopenia, alcohol, chemotherapy
• ITP
• Hypersplenism
• B12 / Folate deficiency
• BM disorders
• Familial thrombocytopenia / Other eg. TTP, HUS







• Bleeding history is important
• Isolated thrombocytopenia vs abnormal FBC
• Clinical examination findings
• Level of thrombocytopenia
• Observation and repeat testing

Felt quite well
Elevated WCC


Felt quite well
Elevated WCC


Felt quite well
Elevated WCC


Felt quite well
Elevated WCC


• Basophilia
• Leuco-Erythroblastic blood film Vs
• Isolated neutrophilia

Dr Jason Restall | Haematologist
Icon Cancer Centre Wesley
Icon Cancer Centre Chermside
Icon Cancer Centre North Lakes



Normal range: 0.8 to 4.0
Lymphocytosis may be due to a rise in
-Lymphoblasts
-B lymphocytes
-T lymphocytes
-NK cell
-Or a combination of the above


1. Clonal
2. Non-Clonal
Infection e.g. CMV, EBV, cat scratch disease, HIV, HCV, HBV
Inflammation e.g. autoimmune disease
Hypothyroidism
Smoking
Medications
Hypo/asplenism

Physical stress

Lymphocyte subsets
-Lymphocyte subsets will give a breakdown of the different types of lymphocytes, it does not look for clonality
-It may be useful to determine which subgroups are elevated
-Often if multiple subgroups are elevated it is more likely to reflect reactive lymphocytosis


Surface marker studies are designed to look for clonality
They determine the presence of different markers on the surface of the lymphocytes
This allows a quantitative estimation of the abnormal lymphocytes

The pattern (immunophenotype) of the clonal population may also help in diagnosis of the subtype of leukaemia/lymphoma present

Three populations were analysed:
1. Lymphocytes comprise 71%.
T–cells 14% CD4/CD8: 1.3
B–cells 83% kappa/lambda: >100
NK–cells 2%
A monoclonal B–cell population comprises 83% of lymphocytes The phenotype is: CD5 +ve
CD10 –ve CD19 +ve CD20 +ve (weak) CD23 +ve CD79b weak CD200 +ve CD43 weak SMIg Kappa (weak)
2. Myeloid cells: Neutrophil lineage comprise 25% Monocyte lineage comprise 2%
3. Blasts (CD34+) comprise <0.1%
The majority of clonal mature lymphocytosis will be B- cell Non-Hodgkin Lymphoma, usually with an indolent presentation

Types include:
- B-CLL
- Mantle cell lymphoma
- Lymphoplasmacytic lymphoma
- Splenic marginal zone lymphoma
- Marginal zone lymphoma
- Hairy cell leukaemia
More aggressive B-Cell Non-Hodgkin lymphoma may be detected in the peripheral blood, however it is less common

Types include:
- DLBCL - Follicular lymphoma
- Burkitts lymphoma
NB – Hodgkins lymphoma is not usually detected in the peripheral blood and negative surface marker studies do not exclude a diagnosis

Often low grade B-NHL may be monitored
Recommended baseline testing includes -FBC
Looking for the presence of lymphocytosis and cytopenias
Cytopenias may be due to marrow infiltration, sequestration, or autoimmunity e.g.
AIHA/ITP -ELFTs
In particular for elevated LDH, hypercalcemia, or evidence of hepatic or renal dysfunction
Surface marker studies to aid categorisation and to confirm clonality

Protein studies – serum EPP with immunofixation, serum FLC, immunoglobulins
Biopsy – core biopsy or nodal excision is preferred; FNA only confirms the presence of NHL, it does not allow subtyping and would need to proceed to core or excision
CT n/c/ap +/- PET
-Used more for staging rather than for diagnosis
-Can be used to aid prognostication/guide treatment with availability of cytogenetic and FISH studies

Newer testing
- Next generation sequencing panels
Newer therapies are becoming available for treatment of lymphoma

BCL2 inhibitors – venetoclax
BTK inhibitors – ibrutinib, acalabrutinib, Zanubrutinib
Monoclonal antibodies – rituximab, Obinutuzumab
Bispecific antibodies – epcoritamab, glofitamab
Venetoclax
-Approved for usage in B-CLL (also used in AML)
-Recently approved in combination with ibrutinib as an all oral regimen for treatment of previously untreated B-CLL

Predominant issues:
-Tumour lysis at commencement of therapy
-Nausea
-Neutropenia
Approved for usage in B-CLL, mantle cell NHL, Waldenstroms
Macroglobulinemia
-Include ibrutinib, acalabrutinib, Zanubrutinib
Issues include:
-Nausea
-Chest infection
-Atrial fibrillation
-Strong antiplatelet effect – need to be withheld prior to procedures (often as strong/stronger effect than clopidogrel)


Dr Jacquie Taylor |Clinical Haematologist
Icon Cancer Centre South Brisbane
Icon Cancer Centre Redland


39yr lady – referred for opinion regarding recurrent iron deficiency
Ferinject 2015, 2017
Mirena inserted 2018
FOBT negative
Coeliac screen negative
Normal diet, intolerant of oral iron
“debilitating fatigue” , affecting her mood
GP organised another ferinject in the interim.





Differential diagnosis
iron deficiency
bleeding inflammation/infection
Myeloproliferative disorder
? ET/PV/PMF/CML



Polycythaemia vera - PV
Essential thrombocythaemia - ET
Primary myelofibrosis - PMF




Kiladjian JJ. Hematology 2012;2012:561–566


Polycythaemia Vera – features
Increased red cell production
Virtually all have JAK2 V617F mutation +/- leucocytosis and thrombocytosis
JAK2 Exon 12 mutation often isolated erythrocytosis
Can be masked by iron deficiency In 20% of cases presentation is at the time of unexpected vascular event
Venous – DVT/PE, Sagittal vein thrombosis, especially portal vein, mesenteric, splenic vein
Arterial MI, CVA




Major criteria
Elevated Hb or HCT
Men Hb > 165, HCT > 49%
Women Hb > 160, HCT >48%
Bone marrow biopsy morphology
hypercellularity with panmyelosis, including prominent erythroid, granulocytic, and megakaryocytic proliferation with pleomorphic, mature megakaryocytes
Presence of JAK2 p.V617F or JAK2 exon 12 mutation
Minor criteria
Subnormal EPO level
Diagnosis of PV requires all 3 major criteria or the first 2 major + minor criterion

Most common
Fatigue
Pruritus (frequently aquagenic and worsened by bathing)
Night sweats
Other
Driven by high red blood cell mass
headaches (including migraines)
difficulties with concentration less commonly bone pain or splenomegaly-related symptoms.
Physical signs – plethora, splenomegaly, rarely hepatomegaly

Thrombotic events are the leading cause of preventable death in PV

1. Control of red cell mass = phlebotomy and/or cytoreductive therapy
Venesections
Hydroxyurea – Hydrea – Hydroxycarbamide – oral tablets
Peginterferon – Pegasys – Weekly subcut injection
Target HCT <0.45
2. Symptom relief – aspirin, and/or cytoreductive therapy
3. Prevent cardiovascular complications of PV – aspirin, quit smoking, treat diabetes and HTN.
Polycythaemia Vera
LOW risk
Age < 60 and NO prior history of thrombosis
HIGH risk
Age > 60 OR a prior thrombosis


Indolent disease
Does impact negatively on survival compared with age/sex matched population
20% of PV patients evolve to post-PV-myelofibrosis over time
Leukaemic transformation occurs in 2.3-14.4 % of patients with PV within 10yrs and 5.5-18.7% within 15 years.
Risk factors for leukaemic transformation include age, leucocytosis, cytogenetic abnormalities and some mutations.

Initially venesection + hydrea + aspirin
Hydrea + aspirin alone for last 1 yr



If there are unexplained elevations in plts/WCC/Hb – may be a myeloproliferative neoplasm
PV or MF or very rarely CML can present with thrombocytosis not just ET
Iron deficiency is often required to control polycythaemia vera
Do not supplement iron in PV patients
Patients with MPNs are at increased risk of thrombosis
If there is an unexpected or unusual site VTE consider JAK2 testing