ANNUAL RESEARCH REPORT 2023 1 Annual Research Report 2023

Delivering the best care possible, to as many people as possible, as close to home as possible.”




I’m pleased to present Icon Group’s 2023 Annual Research Report, which highlights Icon’s commitment to advancing cancer treatment and technology through our leading global clinical trials program.
As a leading integrated cancer care provider, we know the significant impact cancer has on patients, their families, and communities around the world.
Our mission is to bring the best care possible, to as many people as possible, as close to home as possible. And with expected increases in cancer cases, we’re committed to investing in research and continuing to be at the forefront of new and emerging treatments that benefit people and communities and help reduce the global cancer burden.
With a growing global reach, Icon delivers Australia’s largest private cancer clinical trials program, bringing together more than 35 years’ experience and dedication, across medical oncology, haematology, radiation oncology, and precision medicine, as well as unique Icon-led trials.
2023 was a significant year for our 3,500 dedicated healthcare professionals with the opening of new cancer centres, introduction of new technologies and treatment techniques and expansion into new geographies.
We entered the United Kingdom with the signing of a strategic partnership with Nuffield Health, which will see us working together to advance access to cancer treatment in the UK. Our new joint venture with Sunsuria Healthcare established Icon in Malaysia, building on our commitment to enhancing cancer care in the ASEAN region.
Throughout the year, our research team and growing network of clinicians showcased their expertise, experience and drive, keeping Icon at the forefront of advancements in oncology research and beyond.
Together, our team built on long-term industry partnerships, recruited record numbers of patients to our in-house trials, introduced clinical trials in regional Australia, and expanded Singapore’s trial program to deliver more research opportunities for our clinicians and most importantly, our patients.
Our research offering will continue to succeed under experienced directorship and a proven model that will see us reach more people and deliver new and innovative therapies to improve the future of healthcare. I eagerly anticipate the same time next year when we can once again showcase Icon’s leading research and service capabilities that are transforming lives every day.
 Mark Middleton OAM Icon Group Chief Executive Officer
Mark Middleton OAM Icon Group Chief Executive Officer
2023 was a significant year for our 3,500 dedicated healthcare professionals with the opening of new cancer centres, introduction of new technologies and treatment techniques and expansion into new geographies.”
ANNUAL RESEARCH REPORT 2023 5

Foreword
Executive Manager –Research

ANNUAL RESEARCH REPORT 2023 6
2023 welcomed further growth and developments in our research, driving forward Icon’s regional presence, new research in precision medicine, and global reach.
Following the retirement of our former Director of Research, Dr John Bashford, we welcomed Dr Craig Gedye as Director of Research (Medical Oncology and Haematology).
Craig is an exceptional clinician and dedicated researcher with a wealth of knowledge and endless dedication to advances in cancer treatment. Craig’s knowledge of the research landscape will confidently drive forward John’s legacy and evolve our research capabilities into the future. Having only been here a few months, Craig’s contribution is already seeing growing research interest from Icon’s network of doctors and he continues to work closely with our Director of Research (Radiation Medicine and Theranostics), A/Prof Dr Jonathan Ramsay to move the dial on research opportunities.
I’m incredibly proud of our research team’s achievements and how we’ve contributed to our mission through the following key areas:
• Introduced a full trials program at Icon Cancer Centre Townsville to deliver access to research in a regional community.
• Built the foundations of our precision medicine research partnerships and programs.
• Realigned Icon Cancer Foundation and the strategy of the Investigator Initiated Trials portfolio.
• Growth of our research presence in Southeast Asia through maturing our Singapore clinical trials portfolio.
Through 2024 and beyond, we’ll set our sights on entering new geographies to bring more trials to more people, establishing a global Theranostics research program, and delivering a global radiation medicine research strategy.
With a global lens, Icon’s research will continue to grow in Singapore, Australia and New Zealand and broaden into the UK and Malaysia as the group brings trials closer to home for more people.
Together we’re taking the next steps in research and stretching our capabilities to meet future cancer rates and lead evolutions in cancer treatment and outcomes.
I would like to thank my senior leadership team, our entire research team, our collaborative partners, dedicated doctors, investigators and patients for your tireless efforts in advancing the role of clinical trials. You are an inspiration, thank you for your commitment.
Sophie Mepham PhD Executive Manager – Research
With a global lens, Icon’s research will continue to grow in Singapore, Australia and New Zealand and broaden into the UK and Malaysia.”
ANNUAL RESEARCH REPORT 2023 7
Foreword Directors of Research

A word from Dr
Jonathan Ramsay
Group Director of Research (Radiation Medicine and Theranostics)
2023 saw the ongoing growth of our Investigator Initiated Trials (IITs) across Icon’s cancer centres in Australia, with stellar performance in our radiation oncology trials and strong beginnings towards our precision medicine future.
Significantly, 53% of our treatment centres and 43% of our Radiation Oncologists are actively engaged in research. Currently, we have 420 Icon patients participating in radiation medicine studies, the majority enrolled in IITs. And since January 2023, we’ve welcomed 120 new patients onto IITs and 21 patients to Collaborative Group Studies.
In 2023, we initiated two randomised studies: CONSOLE, which compares standard versus stereotactic radiotherapy for symptomatic non-spinal bone metastases, and ROSEND, a pioneering study investigating VMAT radiation therapy for severe rosacea versus standard care.
Looking ahead to 2024, we plan to launch three new IITs:
• PUMA, a randomised study examining a one-week radiation therapy schedule for early breast cancer.
• POCAHONTAS, utilises stereotactic ablative radiation therapy (SABR) for PSMA positive metastases in prostate cancer.
• ERASE, combining SABR with immunotherapy in metastatic melanoma.
In addition to our radiation oncology focus, we’re excited to begin studies with Atomic Oncology to explore a novel method of assessing tumour radiosensitivity in patients. Alongside this, Icon also launched its first Theranostics service at Icon Cancer Centre North Lakes, in Brisbane’s northern suburbs. This will see an expansion into Theranostics research in 2024, collaborating with biomedical companies to provide our patients access to new agents for more cancers.
Significantly, 53% of our treatment centres and 43% of our Radiation Oncologists are actively engaged in research.”
Finally, 2023 also saw another milestone with the relaunch of Icon Cancer Foundation emphasising a renewed focus on fundraising for trials that will impact patient outcomes, particularly within radiation oncology.
I’d like to extend my gratitude to Icon Group and Icon Cancer Foundation for their financial support of our IIT program, along with our commercial sponsors.
Lastly, none of this would be possible without the courage of our patients and their families. From our team at Icon – our gratitude and thanks go to you.
ANNUAL RESEARCH REPORT 2023 8

A word from Dr Craig Gedye
Group Director of Research (Medical Oncology and Haematology)
I’d like to begin by recognising the amazing career and impact of Dr John Bashford, the inaugural Icon Group Director of Research. His retirement in 2022 marked the beginning of my journey with Icon with big shoes to fill. I’m inspired by the incredible legacy John has left here at Icon and share with him a deep and enduring commitment to research.
As we read through the pages of our Annual Report, I’m filled with a sense of gratitude for our patients, clinicians and research team. It reflects the remarkable strides we have made in research and our continued investment in trial programs and our doctors to advance the future of cancer care.
In my first year, I’ve focused on getting to know Icon’s research systems and doctor network, and expanding our research team and maturing processes to better enhance clinician engagement and experience.
Our doctor network continues to grow year on year. This is reflected in the number of our principal investigators, and with the number of our female principal investigators almost at parity.
We continue concerted efforts to streamline processes, enhance communication, and deliver meaningful support to our network so we can provide more research opportunities for clinicians and our patients. This effort has broadened our reach and is fostering a collaborative and truly integrated approach to research and clinical trials.
In my first year, I’ve focused on getting to know Icon’s research systems and doctor network, and expanding our research team and maturing processes to better enhance clinician engagement and experience. ”
In the coming years, we look forward to seeing Icon’s mark on research extend globally and to continue building a truly integrated and engaged network of doctors and clinicians who bring collective knowledge and expertise, allowing us to deliver better care today, and innovate for the future.
There is no progress without research and there is no research without our kind and generous patients. To every patient volunteering in a clinical trial – I thank you for your contribution, you make a difference in the lives of many.
ANNUAL RESEARCH REPORT 2023 9

ANNUAL RESEARCH REPORT 2023 10 Contents Our Mission 13 Our Global Impact 14 A year in review 16 2023 Highlights 19 Research Impact in 2023 20 Welcoming Dr Craig Gedye 22 Exploring what’s next in Phase I and Antibody – Drug Conjugates 24 Ian’s story – finding a trial on the sunny Gold Coast 26 Bringing research to North Queensland 28 Building on a 30-year legacy at Wesley 30 Integrating research into your practice 32 Carol’s story – a trial first offering new hope 34 Technical research and precision medicine 36 Amy’s story – a career in research 38 Pharmacy update 40 Compounding update 42 Singapore research update 44 Allan’s story – a generous heart through trying times 46 Icon and Omico Collaboration 48 Governance and Quality update 51 Future strategy 52 Relaunching Icon Cancer Foundation 54 Update to Icon’s Clinical Research Service 56 Acknowledgements 58 Appendices 61 Research partners 62 Principal Investigators 68 2023 Publications 80 Clinical Trials Registry 91


ANNUAL RESEARCH REPORT 2023 11

ANNUAL RESEARCH REPORT 2023 12
Our Mission
Icon Group’s mission is to deliver the best care possible, to as many people as possible, as close to home as possible. We deliver world-leading integrated healthcare while working to address the global cancer burden.
Patient focused and doctor led, Icon provides personalised care. This included choice of clinician, treatment and timing, the supply of lifesaving products, offering the latest treatment and technologies, investing in clinical research and innovation and striving to improve cancer care and outcomes for patients and communities.
Our full spectrum of cancer care includes haematology, medical oncology, radiation oncology, Theranostics, chemotherapy compounding, pharmacy management and clinical research.
Across our international network, Icon delivered 3.5 million patient interactions in 2023 and continues to focus on increasing our reach to ensure more people have access to the best possible care.
Our vision for research
Icon’s research team is essential to the evolution of healthcare, continuing to disrupt and innovate the way we manage and treat cancer. Our goals is to provide more patients with care opportunities previously not available, by bringing tomorrow’s treatments closer to today.
With more than 35 years’ experience, research is part of Icon’s DNA. Our commitment is backed by international industry partnerships, investment in dedicated research teams and clinical leadership from highly respected researchers and specialists. Icon’s international network of clinicians believe clinical trials are critical to the provision of exceptional cancer care and continue to confidently lead the way in operating Australia’s largest private cancer clinical trials program, while growing the program globally.
ANNUAL RESEARCH REPORT 2023 13
Five cancer centres and one management service agreement Nuffield Health partnership
Our Global Impact
Comprehensive Cancer Care
Research at Icon forms an integral part of delivering true end-to-end care and providing our patients and clinicians access to new and emerging treatments and remaining at the forefront of cancer care.
Over 50 cancer centres globally
3.5 million patient interactions in 2023
Australia’s largest private cancer clinical trials program
Delivers 1.5 million infusions annually
A network of 300+ internationally recognised specialists
Management of 70+ pharmacies across hospital and retail
KINGDOM
MAINLAND UNITED
ANNUAL RESEARCH REPORT 2023 14
HONG KONG
Two cancer centres
Two specialist centres
Sunsuria Healthcare partnership
MALAYSIA
SINGAPORE
One cancer centre
Management of 70+ pharmacies
38 cancer centres
AUSTRALIA
Seven cancer centres including flagship Mt Alvernia integrated centre
Three centres delivering research
One health screening centre
Drug stability and research lab
Four TGA licensed compounding facilities
23 centres delivering research Research capabilities
NEW ZEALAND
One cancer centre
One GMP licensed compounding facility
MAINLAND CHINA
ANNUAL RESEARCH REPORT 2023 15
Icon Group provides the full spectrum of cancer care including haematology, medical oncology, radiation management and research. Delivering Australia’s largest private cancer clinical trials program with true end-to-end model of care including a long-term investment and commitment to increasing access The following is a snapshot of our research impact in 2023.
60
New clinical trials/ research projects
Compounding and pharmacy active trials including trials external to Icon Group
Icon Cancer delivering project opportunities
New trials/research projects selected for participation and undergoing start-up
40 151
Patients were recruited to Investigator Initiated research projects
Clinical trials open to recruitment in 2023
Active principal investigators of clinical trials/ research projects
279
Patients recruited to clinical trials/ research projects
108 59 10 21 16 65
patients recruited to phase I clinical trials
patients recruited to phase I-II clinical trials
patients recruited to phase II clinical trials
patients recruited to phase II-III clinical trials
patients recruited to phase III clinical trials
patients recruited to phase IV trials and registries
500+ 166 71 27 105
18% 11% 11% 10% 9% 8% 8% 5%
ANNUAL RESEARCH REPORT 2023 16
A year in review
radiation oncology, compounding, pharmacy with a growing global reach, our centres offer a access to trials and research projects.
760 Cancer Centre sites delivering clinical trials/research opportunities
Patients active on clinical trials of which 279 were newly recruited
Clinical trials/research projects closed to recruitment but still with workload
of active principal investigators of clinical trials/research projects are women
Prostate cancer
Pancreatic cancer
Ovarian cancer
Breast cancer
Lung cancer
Endometrial cancer
Gastrointestinal cancer
CLINICAL TRIALS
Sarcoma 79%
Research Locations
Adelaide (Kurralta Park), SA
Auchenflower, QLD
Chermside, QLD
Concord, NSW
East Melbourne (Freemasons), VIC
Farrer Park, SG
Gleneagles, SG
Gold Coast Private, QLD
Gold Coast University, QLD
Gosford, NSW
Greenslopes, QLD
Hobart, TAS
Maroochydore, QLD
Mount Alvernia, SG
Mount Elizabeth, SG
Moreland, VIC
Mulgrave, VIC
North Lakes, QLD
Revesby, NSW
Richmond, VIC
South Brisbane, QLD
Southport, QLD
Toowoomba, QLD
Townsville, QLD
Wahroonga, NSW
Wesley, QLD
Windsor Gardens, SA
New Research Locations in 2023
Wellington, NZ
First Trials Initiated in 2023
Auchenflower, QLD
Revesby, NSW
Townsville, QLD
Wahroonga, NSW
166
19
Phase 1 Medical Oncology Radiation Medicine Haematology
Phase 1 Medical Oncology Radiation Medicine Haematology
Phase 1 Medical Oncology Radiation Medicine Haematology
ANNUAL RESEARCH REPORT 2023 17




2023 Highlights
ANNUAL RESEARCH REPORT 2023 20 Research
279 23 Total Research Accrual Total
Collaborative Pharmaceutical Icon Phase I Phase I/II Phase II Phase II/III Phase III Phase IV Non-Trial
Impact in 2023
Clinical Accrual Monthly Mean
Haematology
Medical Oncology
Radiation Medicine
ANNUAL RESEARCH REPORT 2023 21 20 15 5 10 0 JAN MAR MAY JUL SEP NOV FEB APR JUN AUG OCT DEC Research Accrual by Treatment – Jan to Dec 2023 20 15 5 10 0 JAN MAR MAY JUL SEP NOV FEB APR JUN AUG OCT DEC Research Accrual by Sponsor Type – Jan to Dec 2023 15 5 10 0 JAN MAR MAY JUL SEP NOV FEB APR JUN AUG OCT DEC Research Accrual by Phase of Trial – Jan to Dec 2023
Welcoming Dr Craig Gedye
Following the retirement of Dr John Bashford, in July 2023 Icon proudly announced the appointment of Dr Craig Gedye as the new Group Director of Research for Medical Oncology and Haematology, marking the next chapter of growth for Icon’s research team.
Dr Gedye brings with him a wealth of experience and expertise in cancer research, making him an invaluable addition to Icon’s strong clinical leadership team. With an extensive background in both public and private hospitals in Australia, New Zealand and Canada, Dr Gedye has held key leadership roles that have significantly contributed to the advancement of cancer care globally.
Read on to learn more about Craig and how he finds daily inspiration through research and his care for patients.
Choosing the Path of Oncology
Craig was raised in a supportive family and attended a public school with excellent teachers. Despite having no family in medicine or science, Craig gravitated towards the field, earning an undergraduate degree in chemistry and biochemistry. Finding pure research wasn’t fulfilling enough, he was lucky to be able to attend medical school, and contemplated various medical specialties, including cardiology, intensive care, and family practice but landed on oncology.


“I ended up specialising in oncology because I found it was a perfect blend of family practice and scientific research. I had the opportunity to help people and progress science,” Craig explained.
The Humility of Oncology
One of the unique aspects of oncology, according to Craig, is the need for humility and to be honest with patients about the uncertainties associated with treatment while providing the best cancer care possible.
“Many current cancer treatments are really helpful, but all of our current treatments could be better. We need to keep improving and working with our patients to find treatments that will help in any way we can.”
Contributing to best care possible through research
The desire to improve cancer treatments and patients’ outcomes drives Craig’s focus on research. Two milestones have shaped his approach to patient care and fuelled his research work.
ANNUAL RESEARCH REPORT 2023 22
The first was when he happened to be one of the first doctors in the world to prescribe pembrolizumab (Keytruda), a groundbreaking cancer drug which today is commonly used to treat many types of cancer.
“Being part of the very first clinical trial for Keytruda was such an enormous gift. That’s a once in a lifetime moment because it’s rare that a Phase I trial will have such a huge impact on people’s lives, I was very aware of that special moment, and I still am to this day.”
The second was his collaboration with a patient to develop a novel treatment strategy, leading to multiple clinical trials.
“I treated a person with cancer who had had quite bad side effects to chemotherapy and didn’t really want to go through that again. We agreed a different plan, shorter treatments and longer breaks. He suffered less side effects and the chemotherapy worked for much longer than expected.
“He experienced over two years of benefit from a drug that usually works on average for just over two months.
“The results of his treatment made me focus on the idea of adaptive therapy and encouraged me to seek funding for a clinical trial. We’re now on our third funded clinical trial testing the idea of stopping and starting treatment to spread out the benefit and hopefully help people live longer.”
Promising Developments in Cancer Research
Craig highlights several promising developments in cancer research, including immunotherapy, “intravenous radiation treatment” (Theranostics), and personalised treatments. He believes that these advancements hold great potential.
“Icon recently launched their Theranostics program, a completely new type of cancer treatment, showing promising results in some cancers. It will be exciting to see how we can contribute to better patient outcomes.”
For Craig, cancer care and good research is about teamwork. It’s clinicians working with clinical teams, patients, and industry partners to create a truly integrated network of care.
“I joined Icon at just the right moment, and I’m so grateful for the enormous opportunity to be part of a global, integrated oncology network,” he explained.
“I’ve come on board with a priority to understand what matters most to the doctors and healthcare professionals in our network and how we can provide more research opportunities for the patients we care for.
“Icon’s values are exceptional, and I know I’ve joined a place with endless opportunity for us to grow and innovate and ultimately deliver better care.”
I’ve come on board with a priority to understand what matters most to the doctors and healthcare professionals in our network and how we can provide more research opportunities for the patients we care for.”
ANNUAL RESEARCH REPORT 2023 23
Exploring what’s next in Phase I and Antibody-Drug Conjugates
Medical Oncologist and Icon’s Medical Oncology
Research Committee Chair, A/Prof Jim Coward shares his insights on the future of cancer treatment, focusing on the promising developments in Phase I trials and how Antibody-Drug Conjugates (ADCs) are forging new hope for more cancer patients.
Exploring ADCs
ADCs are a relatively old concept but in recent years, researchers and oncologists are learning more about the benefits of ADCs and how they can be used to treat a wider range of tumours and cancer types. Currently, ADCs are commonly used in Phase I cancer clinical trials and have recently seen a significant increase in new ADCs being studied in a Phase I setting.
A/Prof Coward who leads Icon’s Phase I unit in Queensland, Australia explains the strength of these targeted therapies.
“An ADC is essentially two for the price of one – you’re getting chemotherapy which is linked to an antibody which is focusing on targeting a protein or receptor which is integral to a cancer’s aggressive behaviour, hence a very targeted approach,” said Jim.
“We’re currently seeing some really promising results in patients with chemo-resistant disease and in diseases which are resistant to immunotherapies and other novel targeted therapies.”
Overcoming Resistance to Immunotherapy
A/Prof Coward also highlighted the ongoing efforts to overcome resistance to immunotherapy.
“We’re looking at using really novel combinations of already pre-existing chemotherapy agents targeting smaller immune mediators responsible for resistance to those immunotherapies.”
ADCs are administered intravenously and are gaining significant traction in the treatment of gynaecological cancers, particularly breast and ovarian.
“In the past 20 years, breast cancer patients with high levels of HER2+ have been treated with targeted drugs like Herceptin and Perjeta,” explained Jim.
“However, those with lower HER2 levels weren’t eligible for such treatments until recently - now, new therapies are available that use antibodydrug conjugates for tumours which don’t necessarily over express HER2+.
ANNUAL RESEARCH REPORT 2023 24
It means that we’re really forging new horizons for patients who previously would have been transferred to palliative care because there were no other suitable treatment options.”

“These therapies, which link an antibody to HER2+ with chemotherapy, have shown impressive results, not only resistant to chemotherapy but also resistant to prior HER2 directed therapies.”
Similarly, in ovarian cancer, particularly in cases resistant to chemotherapy, favourable results have been seen with antibodies targeting proteins linked to chemotherapy resistance.
Implications for Current and Future Patients
Discussing the implications for current and future patients, A/Prof Coward is optimistic.
“In ovarian cancer alone, there are more than 15 ADCs currently under preclinical investigation, some with really positive preliminary results,” he explained.
“It means that we’re really forging new horizons for patients who previously would have been transferred to palliative care because there were no other suitable treatment options.
Phase I trials at Icon
We’re seeing incredible survival benefits in patients who otherwise wouldn’t have had no other systemic treatment available.”
Looking Ahead
ADCs, immunotherapy, and fibroblast growth factor receptors (FGFR) inhibitors are some of the most promising treatments for cancers.
“Patients with certain mutations, for example FGFR mutation will often have very aggressive disease and exhibit early resistance to chemotherapy, however targeted therapies are resulting in positive results and prolonged survival rates.”
“Some of them can have severe side effects, so managing these while maintaining the treatment’s effectiveness is proving to be our current challenge.
“Overall ADCs and agents targeting immunoresistant pathways and targeted therapies for specific gene mutations are really encouraging for future outcomes, and that’s why I focus on incorporating trials in my practice – to learn more and help extend people lives.”
Icon is proud to operate Australia’s largest private Phase I clinical trials program and leads the way in first in-human trials helping to evolve cancer treatments. Phase I makes up approximately 30% of Icon’s trial portfolio.
ANNUAL RESEARCH REPORT 2023 25
Ian’s story
Finding a trial on the sunny Gold Coast
Having worked outdoors in the Northern Territory all his life, Ian has always been vigilant about having regular skin checks due to being in the sun each day.


ANNUAL RESEARCH REPORT 2023 26
“It was normal for me to get an annual skin check and the odd cancer removed,” said Ian. When Ian had a squamous cell carcinoma (SCC) removed from his neck in January 2022, he didn’t think much of it.
Six months later, Ian noticed a lump “the size of a pea” growing on the lower part of his neck.
“I went to the doctor and numerous blood tests didn’t pick anything up. It wasn’t until I was referred to an ear, nose and throat specialist that an internal biopsy was conducted, and it was confirmed to be cancer. A PET scan followed and that found three other cancers inside my neck on my lymph nodes.
“I consider myself lucky that one of the cancers formed a lump, which raised the red flag. Who knows how far the cancer could’ve spread if it went undetected.”
Three weeks after his diagnosis, Ian was booked in for surgery. During those three weeks, the lump on Ian’s neck grew to the size of a golf ball.
Ian had 20 lymph nodes removed during his eight-hour surgery. Biopsy results from the surgery revealed the cancer had spread to other parts of Ian’s body, including his lungs.
“Thankfully, I had a proactive oncologist who took it upon himself to look deeper into my cancer,” says Ian.
“He told me that my form of cancer was rare and aggressive, and that chemotherapy would be a waste of time.”
When Ian’s oncologist started looking into clinical trial options, he discovered a suitable trial for patients like Ian with high-risk cutaneous squamous cell carcinoma (cSCC), called C-POST.
“The Northern Territory is the only state/territory where this trial isn’t run, so my oncologist gave me a list of options and I chose to participate in the trial through Icon Cancer Centre Southport,” says Ian.
“In January 2023 I commenced the trial and since then, I have travelled to the Gold Coast every three weeks for treatment.”
Ian, who has always maintained a positive attitude throughout his cancer journey, says he feels lucky to have access to the clinical trial.
“I’ve worked all my life and everything changed following my cancer diagnosis,” says Ian.
“Of course, it’s been challenging but it has also been a good opportunity for me to stop and smell the roses.
“I don’t mind the travelling at all. I enjoy visiting (the Gold Coast) and spending time in a place that is vibrant and cheery.”
So far Ian has had promising results on the trial, with active treatment scheduled to finish in early 2025.
Ian is already planning a return to work upon finishing the trial.
Thankfully, I had a proactive oncologist who took it upon himself to look deeper into my cancer.”
ANNUAL RESEARCH REPORT 2023 27
Bringing research to North Queensland
2023 saw the launch of a clinical trials program at Icon Cancer Centre Townsville in North Queensland.

ANNUAL RESEARCH REPORT 2023 28
Icon Cancer Centre Townsville Site Manager, Georgie Whelan, Medical Oncologist, Dr Abhishek Joshi and Clinical Research Coordinator Page Whibley.
Every year, more than 150,000 Australians are diagnosed with cancer, including 32,000 Queenslanders. More than 70% now survive cancer for five years or more and enjoy a higher quality of life after cancer. However, these outcomes are not experienced equally by all, especially those living in regional and remote areas.
Research shows poorer survival rates across all cancers for regional, rural, and remote Queenslanders.
The introduction of clinical trials to Icon Townsville is a significant step towards closing care gaps. It allows our patients to access the latest treatments without being disadvantaged due to their geographical location.
Barriers and Solutions for Regional Trials
In order to introduce a full research program at Icon Townsville, the team needed to set up a research coordinator and program that suited a regional centre.
Queensland State Clinical Research Coordinator Manager, Meghan Leigh said the team’s national experience made introducing trials in a regional centre possible.
“We were able to use our network to deliver training and research setup onsite and ensure we could start a successful clinical trial program where traditionally it can be very difficult to set up,” said Meghan.
A major barrier was the recruitment of a Clinical Research Coordinator (CRC) and establishing relationships to prove the team can deliver safe and effective trials.
“Being regional is always a limiting factor –however, as we establish ourselves, research staff are often attracted to working in a regional setting, knowing they have the support of a larger network,” said Meghan.
“The regional nature of a site like Townsville also makes it harder to be eligible to run certain trials, often due to the limitations with external vendors. But with our strong governance through the first year we were able to launch three trials with two global first patients screened and one global first patient treated.”
Future of Clinical Trials in Townsville
The team continue building the research portfolio to offer more patients access to trials they may not have received, or had to travel away from their home and support networks, to access.
“We plan to broaden into the haematology space, grow our research team, and be recognised as a leader for clinical trials in North Queensland with a focus on introducing a tele-trials model,” explained Meghan.
Improving Access to Research in Regional Communities
In line with Icon’s mission, Icon Townsville’s research team don’t want patients missing out on treatment options due to where they live.
Medical Oncologist and researcher, Dr Abishek Joshi explains the importance of investing in clinical trials for regional communities.
“Regional and remote communities are one of the most vulnerable groups when it comes to equity in cancer care delivery and outcomes –due to barriers like long travel times and inability to leave their jobs and homes,” said Dr Joshi.
Clinical trials are now considered standard of care treatment across many cancers and it’s important these are provided to our regional community in North Queensland to achieve quality care similar to metropolitan cities.”
“We’re fortunate to have a team dedicated to increasing research in the region and looking at options like tele trials to close the care gap and increase access to potentially life-saving trials here in Townsville.”
ANNUAL RESEARCH REPORT 2023 29

Building on a 30-year legacy at Wesley
In 2023, Icon Cancer Centre Wesley in Brisbane, Queensland experienced their highest year of patient recruitment and trial site activation since beginning a strong trials program over 30 years ago.
Icon Wesley, alongside Icon’s South Brisbane location, is the birthplace of Icon Group’s day hospital network and boasts a long-term commitment to research driven by the site’s doctors and collaboration with Wesley Hospital’s wider research team.
ANNUAL RESEARCH REPORT 2023 30
Icon Cancer Centre Wesley: Grace Knight, Deanne Mazzer, A/Prof Paul Vasey, Amy Morrow and Stuti Kapadia.
Significant Growth
2023 recorded the site’s greatest growth for three years with 30 patients recruited to a combination of medical oncology and haematology clinical trials. Almost half of this recruitment was from two phase II trials under Principal Investigators A/Prof Jeffrey Goh and A/Prof Paul Vasey, specifically the SKB264-II-06 and the ALKS4230-007 clinical trials.
A/Prof Jeffrey Goh joined the Icon Wesley team in 2023 bringing with him his special interest in early phase clinical trials and treatment of patients diagnosed with gynaecological and genitourinary cancers. For the first time, the centre also participated in two first in human clinical trials (RC198-G001 and OP-1250-002 trials) under the well-experienced Principal Investigators, A /Prof Paul Vasey and A/Prof Nicole McCarthy.
Future Potential and Collaborative Group Trials
Thirteen of the clinical trials activated in 2023 across all of Icon’s research network were at Icon Cancer Centre Wesley, well above the rate of previous years. Similarly, the team contributed to Icon’s research through the high recruitment of patients to collaborative group trials for pancreatic cancer and acute myeloid leukaemia. In addition to clinical trials, Icon Wesley places high value on academic data collections and participated strongly in Monash University’s Myeloma and Related Diseases Registry.
Expansion of Radiation Therapy Services
Radiation therapy services have also become more accessible to patients in Brisbane with the Icon Cancer Centre at Auchenflower opening in 2023 across the road from Icon Wesley. This facility has attracted some of Icon’s most experienced radiation oncologists and will not only provide greater access and availability to radiation therapy, but also opportunities to participate in combined modality clinical trials between the two centres.
A/Prof Paul Vasey says the team will continue to expand research capabilities to benefit more people.
“With a strong team of clinicians and researchers both onsite and across the Icon network, coupled with Wesley’s state-of-the-art equipped pharmacy, research sponsors and referrer network, our team will continue contributing to advancing cancer care for more people,” said Paul.
ANNUAL RESEARCH REPORT 2023 31
Integrating research into your practice
Medical Oncologist, Dr Chun Loo Gan shares his experience.


Integrating research into your practice
Driven by the desire to transform scientific discoveries into life-changing treatments, Icon Cancer Centre Medical Oncologist
Dr Chun Loo Gan (above) chose to integrate research and clinical trials into his medical oncology career from the start, recognising this path as a powerful way to impact cancer care and improve patient outcomes.
A career in clinical research
As an advanced medical oncology trainee, Dr Gan discovered his passion for clinical research, realising the significant benefits it could have on patient outcomes.
“I witnessed the transformative impact of immunotherapy on patients, seeing firsthand how it prolonged survival and reduced morbidity,” Chun said.
“The ability to bridge the gap between science and medicine and contribute to advancements that can significantly improve patients' lives fuels my passion for clinical research.”
After choosing to incorporate research and trials into his work, Chun completed further medical training – with clinical trial and genitourinary oncology research fellowships across Australia and Canada and a Master's in Cancer Sciences.
“Further clinical research and trials education provided me with in-depth knowledge of clinical research methodology and has been crucial in equipping me with the knowledge needed to shape my career, becoming a resourceful clinician,” he explained.
Accelerating new and emerging treatments
Integrating clinical research into his practice as a medical oncologist has allowed Chun to provide the best care possible to his patients.
“It’s an immensely gratifying experience to provide patients with state-of-the-art treatment, which was otherwise unavailable without the opportunity provided by clinical trials,” he said.
“I recently had a patient with Stage 4 bladder cancer, and I knew a more advanced treatment was available, but it was not yet available under the Pharmaceutical Benefits Scheme (PBS), making it quite costly for the patient.
“Instead, I was able to enroll the patient in one of our clinical trials, offering access to this cuttingedge treatment, and after just three months, they achieved almost complete remission.”
ANNUAL RESEARCH REPORT 2023 32

Addressing gaps in care
Through his work in research and clinical trials, Dr Gan is helping to bridge the care gap still present with various cancer types.
“Despite the remarkable advancements in oncology, a substantial gap in care persists, particularly for individuals with gall-bladder cancer, pancreatic cancer, and gastrooesophageal cancer,” he said.
“However, I remain optimistic about the ongoing progress in drug development and the initiation of clinical trials, especially in immuno-oncology and its broad application in combating various malignancies, aimed at improving outcomes for these patients.”
Navigating the challenges of clinical trials
Clinical trials can bring great successes, but Dr Gan acknowledges the challenges.
“Unfortunately, it's not always smooth sailing navigating the landscape of clinical trials, which presents its share of challenges – especially when a trial doesn’t work out how we hoped,” he explained.
“You also need to be vigilant in monitoring patients to manage new toxicities associated with treatments and support patients and their families through disappointment, providing ongoing emotional support.
“Though this can be difficult, trials that don't work can help us find treatment options that do, providing more hope for more patients.”
Introducing research to clinical practice
Dr Gan believes getting involved in clinical trials is essential for other doctors to improve patient outcomes in the future.
“Previously, I had a perception that clinical trials were tedious and often yield little results for patients, but my experiences have drastically shifted this perspective," Chun said.
There
are many options and support networks that help clinicians introduce trials into their practice and I would encourage others to see research as a positive addition to clinical work, rather than a burden, particularly knowing you can have a trial team supporting you.”
"I now view clinical trials as pathways brimming with potential for our patients, and while some trials may yield disappointing results, others have the power to be transformative.
"Through these trials, we can positively impact patient care and outcomes now and in the future.”
ANNUAL RESEARCH REPORT 2023 33
Icon Cancer Centre Chermside team: Amy Goh, Leila Warnes, Jesse Peet and Dr Chun Loo Gan.
Carol’s story A trial first offering new hope
In an Australian first, Icon Cancer Centre has launched the TREMOR phase two clinical trial, which aims to study the effectiveness of advanced stereotactic radiation therapy in the treatment of essential or Parkinson’s related tremor.
Conventional treatment for tremor within the clinical trial setting involves deep brain stimulation, an invasive surgical procedure that places electrodes or needles into the central part of the brain and a stimulator into the chest wall.
With the launch of TREMOR at Icon Cancer Centre Richmond, located within Epworth Richmond Hospital, up to 30 eligible Australians with essential tremor or tremor dominant Parkinson’s can access this cutting-edge, non-invasive radiation therapy clinical trial.
Improving quality of life
Retired nurse, Carol (right) has suffered from essential tremor for the past five years, with her condition gradually deteriorating before accessing the TREMOR clinical trial in November 2023.
Being able to access this ground-breaking clinical trial has provided new hope and opportunities for Carol.
“I was still working as an aged care nurse when I first developed symptoms. My right hand started shaking first,” said Carol.
“Initially I worked around it, but gradually it got worse and eventually my left hand started to shake as well. It got to a point where I couldn’t even carry a cup of tea to my patients.
“I went through a lengthy diagnosis process and when I was eventually diagnosed, we couldn’t find medication that worked for me. I was at a loss on what I could do and I thought I would have to live with it, which was devastating. I lost my ability to write. I couldn’t even sign my name.”
I went into the trial with the attitude that any improvement would be a blessing.”

ANNUAL RESEARCH REPORT 2023 34
Providing hope
Carol is the primary carer for her daughter, who suffers from chronic health issues. Carol visits her daughter each day to assist with cooking and cleaning. This became increasingly harder for Carol as her essential tremor symptoms progressed.
“I went into the (TREMOR) trial with the attitude that any improvement would be a blessing,” says Carol.
“I love writing, so to be able to write again would be wonderful.
“One of my hobbies is carpentry. I enjoy making furniture, but I can’t even measure items now, let alone hold a drill or cut anything, so unfortunately that hobby has gone by the wayside since my essential tremor diagnosis.
“I’d love to get back to doing the things I enjoy.”
An avid travelling, Carol is now planning a cruise in January 2025.
“My goal is to be able to use a knife and fork to eat meals without spilling food on myself,” says Carol.
“It’s a basic function that many people take for granted but for me, it would make a big difference.”
“I’m noticing small improvements in many tasks I couldn’t do before taking part in the TREMOR trial. Although they may be small improvements, they are making a big difference in my life.”
Funding for the TREMOR clinical trial was provided by the Epworth Medical Foundation, Icon Cancer Foundation and supported in kind by Icon Cancer Centre.


ANNUAL RESEARCH REPORT 2023 35


Technical research and precision medicine
Through Icon’s centralised Clinical Care Team, the group can access a wide range of resources and training that aid in the development of quality researchers and research programs.
As a comprehensive cancer care organisation, Icon also conducts technical research including projects led by nurses, medical physicists, radiation therapists, pharmacists and other medical professionals.
Our clinical teams also published seven peer-reviewed publications and continued to mature its governance to deliver more technical research and provide unique opportunities to incorporate research into day-to-day practice.
ANNUAL RESEARCH REPORT 2023 36
Theranostics update
In 2023, the team focussed on the successful launch of Icon’s first Theranostics program and delivered record numbers of research education sessions, including remote sessions for Icon’s Singapore teams.
In 2023, Icon’s research and molecular oncology team developed a comprehensive Theranostics program to support clinical trials and future direction. This achievement sets us apart in the industry and positions us for future growth in precision medicine research.

Appointment of A/Prof David Macfarlane
A/Prof David Macfarlane (left) joined Icon in August 2023. With over 30 years of experience in nuclear medicine therapy, he is committed to making this treatment more widely available to patients, especially through the introduction of clinical trials. A/Prof Macfarlane will serve as the Theranostics Research Committee Chair, starting in 2024.
Research Potential and Future State
Theranostics at Icon is attracting significant pharmaceutical and biotech investment, driving continued research and expansion. The team attended the European Association Nuclear Medicine EANM Congress 2023 to discuss opportunities for sponsored clinical trials and offering clinical research services to smaller biotech companies.
Key Focus Areas for 2024
Expansion in Singapore
In 2023 a second clinical trials headquarters was established at the newly opened Icon Cancer Centre at Mount Alvernia Hospital, Singapore’s first fully integrated private cancer centre. In 2024, this program will see the continued growth of both Icon’s wider clinical trials portfolio and Theranostics research.
New Innovations and research
An internal program, Theranostics Research Enabler, is underway to help promote collaboration within the Theranostics market and provide access to sponsored clinical trials and utilise Icon’s clinical research services. 2024 will see a focus on securing new partnerships that will grow Icon’s Theranostics research and provide patients with access to clinical trials.

Nuclear medicine growth
Our focus on expanding our nuclear medicine capabilities will continue, bolstered by the appointment of Dr Nat Lenzo (left). Dr Lenzo joined Icon in April 2024 as Clinical Director, Molecular Imaging and Nuclear Oncology (Asia Pacific). His expertise and international recognition, alongside collaboration with A/Prof Macfarlane will be instrumental in propelling Icon’s precision medicine capabilities not only in Australia, but globally.
Training and Development
As we increase the number of clinical trials and focus on patient recruitment, we’re committed to upskilling our people to enable them to keep improving patient care. 2024 and beyond will continue a focus on strong education and training programs across Icon’s network.
ANNUAL RESEARCH REPORT 2023 37
Amy’s story A career in research
From student to team lead: How Amy Morrow (right) propelled her research career in three years
Progressing from Icon research’s Student Placement Program to Clinical Research Coordinator Team Lead in just three years, Amy Morrow is dedicated to a career in clinical research and creating better outcomes for people diagnosed with cancer.


Discovering a passion for research
Choosing a nursing career pathway after school, Amy Morrow soon discovered it wasn’t for her. However her hunger to work with patients and make a difference, Amy found her calling in research and oncology.
“It was my student placement at Icon that was pivotal in my journey, solidifying not just my love of research but the direction I wanted to take my research career,” Amy said.
“A placement at Icon allowed me to explore and experience the world of clinical oncology research –learning the required skills, completing my Good Clinical Practice (GCP) training, connecting me with industry experts, and ultimately landing me a job with Icon.”
Curating a research career with Icon
Amy joined Icon Cancer Centre Wesley, Queensland, in 2021, moving across our Brisbane centres while progressing with Icon. Over her short time with Icon, she progressed from Clinical Research Assistant to Team Lead.
“I have had some great mentors at Icon, including Dr Jeffrey Goh, Dr Jim Coward and the Icon research team, who helped me excel,” Amy said.
“They gave up their time to teach me – having one-on-ones, taking me into patient consults and inviting me to conferences – anything to help me learn and grow.”
With a research career at Icon, Amy can provide better patient outcomes.
“I know each day I’m making a difference in the lives of patients - even if the trial didn’t work this time, we know it is creating a better future of cancer care,” Amy said.
“Icon has an incredible research portfolio and robust governance in place, allowing CRCs and doctors to conduct quality research and ensure patients have access to groundbreaking trials.”
ANNUAL RESEARCH REPORT 2023 38
Making an iconic clinical researcher
Amy believes managing emotions and a willingness to learn is crucial as a clinical researcher.
“Find ways to self-regulate your emotions, whether debriefing with a close colleague or loved one, going for a walk or just some form of self-care,” Amy said.
“This will ensure you are fully available for the patient and can continue to provide the best care possible to them.”
Contributing to future cancer care
Looking towards the future of cancer care, Amy has seen the move towards genetically driven cancer treatment over the past few years.
“Genetically driven treatments allow us to provide more targeted care to the individual and their specific diagnosis,” Amy explained.
“This is a good step towards a better future for cancer patients and will hopefully lead to better outcomes for more patients with different types of cancers.”
Amy said we will continue to make these new treatment options more accessible to everyone.
“As we get more targeted treatments and panels, Medicare doesn’t cover treatment, which means finding sponsors willing to help,” Amy explained.
“Our current sponsors are impressed by our governance and mature clinical trials program, and we’re always looking to find more organisations to partner with to ensure more people can access this level of care.
“We’ll also continue to work with more doctors across our global network, keen to expand their research, so patients coming to Icon know they can participate in clinical trials.”
ANNUAL RESEARCH REPORT 2023 39
Amy Morrow (centre), with Icon Cancer Centre Wesley team members.
Pharmacy update
Icon Group’s national pharmacy services and governance structures continue to contribute to clinical trials and research, providing quality care and pharmacy management of trials within Icon’s network, as well as with hospital partners and external providers. The team deliver best-in-class pharmacy services, including supporting the safe delivery of specialised clinical trials and handling of trial medications. This performance is underpinned by a deep and ongoing commitment to quality, governance and continual improvement.
Highlights of 2023
Re-establishing the Clinical Trials Pharmacy Audit
After a COVID hiatus, the Clinical Trials Audit Program was re-established. The goals of the audit program are to ensure that all pharmacies providing clinical trials services are doing so in accordance with Good Clinical Practice (GCP), have the appropriate regulatory arrangements in place, and have completed the required business processes within suitable timeframes. In 2023, the program audited 18 pharmacy sites, across eight different domains, leading to the development of new training resources, workflow guidelines, and data registers.
Pharmacy Practice Unit (PPU) renamed Quality and Medication Safety Unity (QMSU)
In 2023, the well-established PPU underwent an audit and restructure and in March 2024 was renamed to the Quality and Medication Safety Unit (QMSU) to better reflect its extensive function. The QMSU leads the clinical governance, quality, and safety of Icon Group Pharmacy services in Australia.
Expansion of the Quality Medication Safety Units (QMSU) Central Clinical Trial Team
In 2023, the QMSU Central Clinical Trial Team expanded its role and centralised all clinical trial startup activities for over 20 pharmacy sites. This resulted in the startup of 110 new clinical trials Australia wide and the establishment of clinical trial pharmacy services at three new locations – Slade Pharmacy Fitzroy (Victoria), Icon Pharmacy Townsville (Queensland) and Epic Pharmacy Midland (Western Australia).
The release of the Australian Commission on Safety and Quality in Health Care National Clinical Trials Governance Framework triggered an in-depth review by the Central Clinical Trial Team of Icon’s pharmacy processes, ensuring we continue to comply with all standards. This central support ensures consistency across clinical trials, while removing the specialised administrative role on frontline pharmacy staff who need the time to focus on patient care. It also continues to embed strong governance framework across a wide geographical remit.
ANNUAL RESEARCH REPORT 2023 40
Total activity across Icon Group Pharmacy Services for 2023
486 Sponsored Non-Sponsored Trials opened in 2023 410 76 110
Other 95 347 42 active trials
Phase of trial Disease Category
Phase 1: 127
Phase 2: 108
Phase 3: 240
Phase 4: 7 Not listed: 4
Haematology Oncology
9000+ number of medications dispensed to clinical trial patients
310 93 trials requiring aseptic compounding of investigational products trials with compounding performed by external vendors
217 trials with compounding performed by pharmacy teams onsite
ANNUAL RESEARCH REPORT 2023 41

Compounding update
Icon Group’s compounding arm, Slade Health, assists hospitals with clinical trials by providing compounding services from our TGA/Medsafe-licensed facilities. Currently supporting more than 200 clinical trials, our experience covers Phase I-IV studies, primarily in oncology and haematology.
Slade Health’s clinical trials service includes national clinical trial coordination to ensure relevant criteria is met for the compounding of investigational products. Each compounding facility has dedicated clinical trials team members who have complete oversight over the management and segregated storage of clinical trial drugs in validated and monitored equipment.
Slade Health also supports the administrative work required in clinical trials by providing relevant manufacturing records and maintaining training documentation, guaranteeing compliance to Good Clinical Practice (GCP) and Good Manufacturing Process (GMP).
With validated shippers, the Slade team can assure the safe and secure cold chain or ambient transport of clinical trial products to hospitals on time.
ANNUAL RESEARCH REPORT 2023 42
7420
320
Clinical Trials services across Slade’s Australia and New Zealand compounding facilities
85% ~1500 trial products compounded 70% 30% industrysponsored Collaborative Research Group/ Investigator-Led Studies or trials supported are Phase II - 111
clinical trial patients reached
Investigational products handled include injectable monoclonal antibodies, small drug molecules, antibody-drug conjugates, cytotoxic chemotherapy, mRNA vaccines, oligonucleotides
Slade’s Drug Stability Testing and Research Laboratory continued vital research and have increased medication shelf-life by several days providing greater access to specialised care for patients undergoing breast, skin and gastrointestinal cancers, and multiple sclerosis
ANNUAL RESEARCH REPORT 2023 43

Singapore research update
2023 saw the continued growth of trials in Singapore including the set-up of a second head office for clinical trials at the recently opened Icon Cancer Centre at Mount Alvernia Hospital, Singapore’s first integrated cancer care centre delivering all aspects of treatment under one roof.
2024 and beyond will see a focus of increasing the clinical trial portfolio including new therapies, such as Theranostics, and expanding partnership and collaborations with trial sponsors and CROs.
I was recommended by my oncologist to participate in a clinical trial. After thorough discussions with my oncologist, I decided to participate in the clinical trial as it would not only be beneficial for me, but also for future patients. By doing so, I can play a part in the development and improvement of drugs to fight cancer.”
Ong Guat Eng, trial patient

ANNUAL RESEARCH REPORT 2023 44

Highlights of 2023

Leading Recruitment
The team achieved a few notable firsts, including first in Singapore to recruit patients to HERTHENA and STELLAR 303 trials, and activated their first Phase I clinical trial, RELATIVITY 106-BMS.

Accreditation Milestone
In 2023, Icon Singapore’s research services were evaluated for the first time under the Australian Council on Health Standards International (ACHSI) and included as part of Icon’s wider accreditation processes. Accreditation was achieved in the first quarter of 2024 and highlights our dedication to upholding quality standards in our research practices, ensuring the implementation of ethical policies and guidelines for the safety and well-being of our patients.

Networking opportunities
The team focussed on building relationships and securing more partners and referrals and held the first Clinical Trial Open House at Icon Cancer Centre Mount Alvernia. These proceedings included presentations by Group Executive Manager Research, Sophie Mepham, Icon ASEAN CEO, Serena Wee, Director of Icon Singapore Clinical Trials, Joanne Chio and Singapore Research Committee Chair, Dr Hsieh Wen-Son and Group Director Molecular Oncology, Julie Crouch.
ANNUAL RESEARCH REPORT 2023 45
Allan’s story A generous heart through trying times
When Hobart resident, Allan (right) was diagnosed with stage 4 bowel cancer in 2022, his first thought wasn’t about himself.
Instead, the 64-year-old immediately thought of his family.
“When I was told of my diagnosis, I knew it wasn’t good, but I was determined to beat it,” says Allan.
“I’ve been married to my wife, Jeanette for 45 years and we have two children and four grandchildren. From the start, I’ve been determined to beat the cancer for them.
“When I received my diagnosis, I was told I had three to five years to live. I said I wouldn’t accept that. I want at least another ten.”
Allan first noticed symptoms when his bowel movements became more frequent, leading him to take a bowel cancer test.
Following his diagnosis and surgery to remove some of the cancer, Allan commenced chemotherapy at Icon Cancer Centre Hobart under the care of medical oncologist, A/Prof Louise Nott.


ANNUAL RESEARCH REPORT 2023 46
My message to people is, when you get them, use them. It’s a simple test that could save your life.”
“Louise has been amazing. I had a trip to Melbourne during my treatment and while I was away, I experienced bleeding. I called Louise on the Saturday, and she contacted me on the Sunday and arranged to see me first thing on Monday morning. Louise and the team at Icon are incredible and go above and beyond,” says Allan.
It's for that reason, Allan and Jeanette made the generous decision to donate to the Icon Cancer Foundation, a not-for-profit charity that raises funds to support Icon Cancer Centre’s network of doctors and healthcare professionals to do vital independent cancer research.
“The team at Icon in Hobart have given so much to me, so I wanted to find a way to give back to them. It was a great feeling when we signed the cheque,” says Allan.
“I’ve had a good life and I want to be able to help others who are going through cancer.”
While Allan has shared his giving nature with the Icon Hobart team, he has also shared his vibrant personality.
During his treatment, Allan has worn a series of brightly coloured shirts, bringing smiles to the faces of patients and staff at the centre.
“Any kind of shirt I can find that’s bright, I wear to treatment. It’s a way of being positive. There’s a lot of negativity at a cancer centre, so I wanted to make people smile,” says Allan.
“My daughter is a hairdresser, so she has also given me brightly coloured hair. The reactions have been lovely and a good talking point for people.”
Allan is now determined to spread the bowel cancer awareness message.
“I had the (bowel cancer screening) kits sitting in the drawer at home for a while and didn’t use them until I experienced symptoms,” says Allan.
“My message to people is, when you get them, use them. It’s a simple test that could save your life.”
ANNUAL RESEARCH REPORT 2023 47
Icon and Omico A national collaboration to transform cancer treatment pathways in Australia
In early 2023, Icon joined a network of key cancer organisations and stakeholders across Australia in a unique collaboration with Omico to change the way we fight cancer.

Omico (trading name of The Australian Genomic Cancer Medicine Centre), is a national, independent, not-for-profit organisation leading the use of ‘precision oncology’ to improve outcomes for people with cancer in Australia. It brings together a unique network of stakeholders to accelerate access to genomic/ molecular profiling and facilitate the delivery of genomic cancer medicine clinical trials to thousands of Australians.
Introducing PrOSPeCT – the largest oncology genomics initiative in Australia
At the heart of this collaboration is PrOSPeCT (Precision Oncology Screening Platform Enabling Clinical Trials), fuelled by public-private funding and partnerships totalling over $185M, including $61.2M grant funding from the Australian Government.
Genomic medicine has redefined the landscape of oncology, offering tailored treatments based on individual genetic profiles.”
By the end of 2025, PrOSPeCT aims to provide free comprehensive genomic profiling (CGP) to more than 23,000 Australians with advanced or incurable cancers and identify potential matches for patients to clinical trials of new targeted therapies. Importantly, Omico is proactively increasing the number of precision oncology trials conducted in Australia to ensure that patients have accelerated access to new, personalised therapies.
ANNUAL RESEARCH REPORT 2023 48
Federal Member for Dobell, NSW, Emma McBride speaking at the launch of PrOSPeCT.
Enhancing Clinician and Patient Access to Precision Oncology
PrOSPeCT allows clinicians to access CGP for their patients with advanced or incurable cancer, at no cost to the patient. Patients can be referred at any stage of their cancer journey. The additional CGP information, provided to the clinician along with treatment recommendations in a report from Omico’s Molecular Oncology Board (MOB), can assist to identify new treatment pathways for the patient.
As of March 2024, over 700 clinicians have referred patients to the Cancer Screening Program (CaSP) of PrOSPeCT. More than 4,084 patients have consented to participate in PrOSPeCT, with 39% hailing from remote, rural, or regional areas, including Indigenous communities. Importantly, 78% of patients who received a MOB report containing their CGP results were recommended a matched therapy, underscoring the benefits of precision oncology in identifying potential new treatment options.
Driving Jobs and Economic Growth
As well as health benefits, PrOSPeCT is also creating skilled jobs, building Australia’s’ capabilities and infrastructure in cancer research and care, while strengthening our position as a premier destination for precision oncology trials. By December 2023, it created 99 direct jobs and an estimated 590 indirect jobs across Australia, spanning medical, scientific, administrative, and pharmaceutical sectors. Moreover, it has stimulated over $102 million in foreign investment into locally conducted oncology clinical trials, amplifying patient access to innovative therapies while delivering significant savings to the healthcare budget.
Building Capacity and Capability
Omico has established a nationally coordinated approach to embed company-sponsored trials across 43 sites, encompassing metropolitan and regional areas.
Notably, 40 traineeships in clinical trial research skills, with a strong emphasis on regional representation, underscore the commitment to fostering talent diversity and skill development.
PrOSPeCT has expanded Australia's capability in genomic profiling, with five pathology providers now accredited to deliver these services locally, marking a pivotal shift from offshore reliance.
PrOSPeCT is also actively building rich real-world data collection, including linkage to external datasets and data enhancements, and working closely with industry and research partners to provide data to support clinical trials and realworld evidence studies.
A Shared Vision for Cancer Care
Genomic medicine has redefined the landscape of oncology, offering tailored treatments based on individual genetic profiles. Icon and Omico share a commitment to improving treatment options and outcomes for patients with cancer, including those with rare and less common cancers, as well as for people living in geographically isolated areas.
Icon is proud to be involved in this collaboration, which is not only transforming the landscape of cancer care but also instilling hope for a future where personalised therapies offer newfound avenues for improving outcomes and survival.


ANNUAL RESEARCH REPORT 2023 49
Professor David Thomas at the launch of PrOSPeCT.


ANNUAL RESEARCH REPORT 2023 50

Governance and Quality update
In 2023, the Icon Governance team underwent a transformation with the alignment of Quality into the team structure. This provided greater efficiency and support to Icon’s research locations, increasing their ability to activate more trials.
Working closely with our South Australian sites, we were involved in the first site Accreditation against the National Clinical Trials Governance Framework (NCTGF). This was not only a first for Icon but we were the first site within Australia to undergo Accreditation for our clinical trials program under the Short Notice Assessment (SNAP) framework. Through this assessment our program was measured against a maturity scale rating, with our South Australia sites receiving the highest rating of ‘Mature’ in all areas we were assessed against. This was an exciting opportunity to demonstrate the integration of clinical trials within our clinics and our strong commitment to Patient Safety and Quality standards across Icon Group.
Building on the group’s commitment to quality, the team introduced the Standard Operating Procedures Working Group, bringing together core team members across research to help build improvements within our site SOPs. We’re excited to be rolling these out into 2024.
The growth of our Global Office of Research Governance, Implementation and Quality team in 2023 will ensure continued growth of Icon’s trials program via efficient study start-up well into 2024.
Our South Australia site received the highest rating of ‘Mature’ in all
areas we were assessed against.”
ANNUAL RESEARCH REPORT 2023 51
Future Strategy
As we move forward into 2024 and beyond, Icon’s vision is set on global scalability. Our strategic focus is on expanding our reach into new geographies, underpinned by the establishment of a global Theranostics research program and a global radiation medicine research strategy.
Our future strategy is rooted in sustainable expansion, innovative research programs, and a focus on global governance to deliver a truly comprehensive clinical trials program. The team look forward to progressing research and are committed to making a significant impact on global health outcomes.
ANNUAL RESEARCH REPORT 2023 52
Global Theranostics Research Program
Our global theranostics research program will be at the forefront of our expansion. This program will aim to deliver more theranostics programs across Icon’s network, with a focus on research opportunities across Australia, New Zealand, Singapore and the UK, providing greater access to new and emerging personalised treatments and trials for more cancer types and people.
Global Radiation Medicine Research Strategy
In parallel, our global radiation medicine research strategy will seek to advance the field of radiation therapy through investment in significant trials. Through this strategy, our team will focus on developing new and innovative techniques and technologies that can enhance the precision and effectiveness of radiation treatment and increase access to research on a global scale.
Geographical Expansion
In 2024, we have already seen significant wins for our growth in New Zealand, with the Bowen Icon Cancer Centre recruiting their first three trial patients. Alongside Icon Group’s expansion, the research team is planning to begin implementing trials into the United Kingdom and Malaysia.
ANNUAL RESEARCH REPORT 2023 53
Relaunching Icon Cancer Foundation
Icon Cancer Foundation (ICF), an independent not-for-profit charity, was relaunched in 2023, reflecting a continued commitment to grow Icon’s Investigator Initiated research program. ICF works with Icon Cancer Centre’s national network of doctors and healthcare professionals to fund, promote and support cutting-edge research that aims to advance cancer treatments.
2023 was a transformative year for ICF with a new strategic focus on increasing its research program in Australia, funding innovative studies and clinical trials that focus on patients’ unmet treatment needs.
New Community and Partnership Manager
ICF welcomed Community and Partnerships Manager, Leanne Hardyman. Leanne plays a key role in developing and managing ICF. Her focus on fundraising and stakeholder engagement drives the success of the foundation to ensure more patients have access to new and emerging treatments through research and clinical trials.
Strategic Plan
In line with a new strategic vision, ICF’s Board of Directors approved a three-year Strategic Plan aligning the foundation’s vision, mission and research focus and detailing fundraising goals. It focuses on ICF’s strategic pillars:
• Patient connection
• Engaged stakeholders
• Purpose driven partnerships
• Sustainable growth
• Facilitate research; and
• Foundation awareness.
This Plan, alongside ICF’s governance, ensures all research and clinical trials supported by ICF are assessed by independent ethics committees. View ICF’s Strategic Plan by visiting iconcancerfoundation.org.au/about-us/ governance
Patient-centred research
ICF supports clinical trials that focus on finding better and innovative treatments, understanding diseases more deeply and improving how we detect and diagnose medical conditions.
2024 and beyond will see a focus on research projects looking at new treatments and the use of technology in Theranostics, radiation therapy, immunotherapy and chemotherapy to target breast, prostate, skin and brain cancers. The focus will be on patient-centred research that can help advance cancer treatment as well as exploring other common health conditions, such as Parkinson’s Disease.
Learn more about Icon Cancer Foundation at


ANNUAL RESEARCH REPORT 2023 54


We can’t evolve the way we treat cancer without clinical trials. Trials cost money to run...and often we don’t have the support of pharmaceutical company sponsors for radiation medicine trials, so we rely on community support. Your support will help us help patients today and far into the future.”
Dr Andrew See Radiation Oncologist and researcher Icon Cancer Centre, Victoria
What is an Investigator Initiated Trial?
An Investigator Initiated Trial (IIT) is a clinical trial or study where the research idea, design and implementation is led by a researcher or clinician, rather than a pharmaceutical company or medical device manufacturer. IITs are important as these trials explore new uses for existing treatments, study rare conditions and investigate innovative approaches to treatment that may not be a priority for commercial entities.
IITs are usually sponsored by an academic or hospital institution, a not-for-profit entity, a scientific group, or a clinical investigator. At Icon Group, this type of research is usually sponsored by Icon Cancer Foundation. Icon Group provides support to start up and run IITs at Icon Cancer Centre sites.
What is a commercial trial?
A commercial clinical trial is sponsored, initiated and funded by an independent commercial entity, most commonly a pharmaceutical or medical device/technology company. At Icon Group, these trials form the majority of our global research program.
ANNUAL RESEARCH REPORT 2023 55
Update to Icon’s Clinical Research Service
In 2023, the Investigator-Initiated trials (IITs) team was rebranded as the Icon Clinical Research Service (CRS). This change reflects the scope of the CRS, which is to provide end-to-end service to Icon clinicians for investigator-led studies and external clients for the development and management of commercial trials. The team currently manages a portfolio of 15+ IITs, a growing number of retrospective investigator led studies, as well as engaging with commercial clients to develop research studies which can be implemented through Icon.
2023 Highlights
Record Recruitment
2023 was the first year where over 100 participants were recruited to Icon IITs, highlighting continued year-on-year growth and investment in Icon investigator-led research.
LIBERATE Study
The 100th patient was recruited to the LIBERATE registry with the study being extended to 150 participants to support this important clinical technique for treatment of men with early-stage prostate cancer. This study based in Victoria is led by Radiation Oncologist, Dr Andrew See in conjunction with Alfred Health Urologist, A/Prof Jeremy Grummet.


Introducing IITs in New South Wales
Two studies were activated for the first time in New South Wales. The CONSOLE trial was activated at Icon Cancer Centre Wahroonga (led by Radiation Oncologist, Dr Angela Yates) and seeks to improve pain control for patients receiving radiation therapy to painful bony metastases.
The ROSEND trial was introduced at Icon Cancer Centre Revesby (led by Radiation Oncologist, Prof Gerald Fogarty) and is the first-of-its-kind in the world, focusing on the use of VMAT radiation therapy to treat Rosacea, a chronic benign condition affecting the skin.
ANNUAL RESEARCH REPORT 2023 56


Future state
2024 and beyond will see continued promotion of Icon’s Clinical Research Service to provide bespoke support and tailored service to develop and manage studies for commercial clients. The service will connect Icon clinicians with commercial clients who are developing new and innovative treatment and diagnostic strategies to manage cancer patients in the fields of radiation medicine, medical oncology, haematology and Theranostics.
Under Icon Group and Varian Medical Systems’ Research Framework Agreement, a number of IITs are under development and set to launch in the third quarter of 2024. This will include ERASE (led by A/Prof Matthew Foote and A/Prof Mark Pinkham) and POCAHONTAS (led by Dr Andrew See, A/Prof Louise Nott and A/Prof Shankar Siva).
These two trials will be the first IITs activated under the Varian Research Framework agreement and demonstrates our commitment to partnering with leading industry organisations to bring improved and advanced treatments and technologies to our patients.
There is no doubt in my mind that as a result of the treatment, my life has been extended far beyond what I could have expected. My quality of life has been excellent.”
Ian, prostate cancer patient, clinical trial participant
ANNUAL RESEARCH REPORT 2023 57
Annual IIT recruitment numbers 2023 2022 2021 2020 2019 2018
Acknowledgements
Icon would like to warmly thank all the doctors and research team members for their dedication to the promotion and delivery of clinical trials.
Thank you to our sponsors and partners for continuing to work collaboratively with us to increase access to clinical trials and help reduce the growing global cancer burden. We extend our special thanks and best wishes to our patients, whose participation in clinical trials makes it possible to discover groundbreaking new treatments. We also warmly acknowledge the valued support of donors to Icon Cancer Foundation (ICF), an independent not-forprofit registered charity whose mission is to promote, initiate and support clinical trials and research, striving towards a brighter future for cancer patients and communities. ICF is committed to improving patient access to new and emerging treatments by raising awareness of and sponsoring Investigator Initiated Trials (IITs).
Community fundraising is crucial to the delivery of IITs as they are not sponsored by large pharmaceutical companies and involve ongoing costs and resources to deliver vital and often unique research to advance medicine.
All funds raised for ICF support the delivery and execution of IITs including trial management, recruitment, data collection and publication, and ongoing trial patient support including access to transport, accommodation and clinical research coordinators and allied health professionals.
Together we continue to provide hope and opportunity to patients today and tomorrow.

We extend our special thanks and best wishes to our patients, whose participation in clinical trials makes it possible to discover groundbreaking new treatments.”
ANNUAL RESEARCH REPORT 2023 58


ANNUAL RESEARCH REPORT 2023 59


Appendices
Research partners


Icon is proud to work with over 200 collaborators including Clinical Research Organisations (CROs), commercial and non-commercial organisations (including the pharmaceutical industry, hospitals, and universities), industry vendors and service providers. We understand that making a difference in reducing the global cancer burden is a collaborative effort and continue to partner with leading organisations to contribute to the future of cancer care.
ANNUAL RESEARCH REPORT 2023 62

Collaborator
BioCollab Pty Ltd
Cancer Council
Concord Hosiptal
Epworth HealthCare
Gallipoli Medical Research Foundation
Ghent University
Griffith University
London Regional Cancer Program
Mater Health
Mater Research
Monash University
Omico
OncoShot
Peter MacCallum Cancer Institute
Prospection
QIMR Berghofer Medical Research Institute
Queensland University of Technology
Translational Research Institute
UnitingCare Health
University of Queensland
University of Sydney
ANNUAL RESEARCH REPORT 2023 63
Sponsor/CRO
Abbisko Therapeutics Australia Pty Ltd
Abbvie
Accendatech
Acerta Pharma LLC
Adagene
Agenus Inc
Akeso BioPharma Co
Alexion Pharmaceuticals
Alkermes, Inc.
ALX Oncology Holdings
Amgen Inc
Antengene Corp
Anwita Biosciences Australia Pty Ltd
Arcus Biosciences
Ascendis Pharma
Ascentage Pharma Pty. Ltd
Astellas Pharma Inc
AstraZeneca
Atomic Oncology Pty Limited
Atridia Pty Ltd
Australasian Gastro-Intestinal Trials Group (AGITG)
Australasian Leukaemia & Lymphoma Group (ALLG)
Australasian Myeloma Research (AMaRC)
Australia New Zealand Gynaecological Oncology Group (ANZGOG)
Australian and New Zealand Urogenital and Prostate Cancer Trials Group (ANZUP)
Avance Clinical
AVEO Pharmaceuticals, Inc
Ayala Pharmaceuticals Inc.
Bayer AG
BeiGene
BicycleTx Limited
BioAtla Inc
Biosplice Pacific Pty Ltd.
Boehringer Ingelheim
Boston Scientific
Breast Cancer Trials (BCT)
Bristol Myers Squibb (BMS)
Cascadian Therapeutics, Inc
Catalyst Clinical Research APAC Pty
Celcuity Inc.
Celgene Corporation
Checkmate Pharmaceuticals, Inc.
ChemoCentryx, Inc.
Chia Tai Tianqing Pharmaceutical Group
Clinical Network Services (CNS) Pty. Ltd
Clinipace
Clintec
Constellation Pharmaceuticals
CSL Limited
CStone Pharmaceuticals
Daiichi Sankyo Company, Limited
Debiopharm Group
Deciphera Pharmaceuticals, LCC
DrugDev, Inc.
Eisai Co., Ltd
Eli Lilly and Company
Eucure (Beijing) Biopharma Co., Ltd
Exelixis, Inc
F. Hoffmann-La Roche AG (Roche)
Five Prime Therapeutics, Inc.
Fortrea Australia Pty. Ltd.
Fusion Pharmaceuticals, Inc.
Genentech, Inc
Genmab A/S
Genomics for Life
Genzyme
George Clinical
ANNUAL RESEARCH REPORT 2023 64
Gilead Sciences, Inc.
GlaxoSmithKline plc (GSK plc)
GreenLight Clinical
Hangzhou TigerMed
Hinova Pharmaceuticals Inc
Hummingbird Bioscience Australia Pty Ltd
Hutchison MediPharma Limited
ICON plc
Idera Pharmaceuticals, Inc.
ImmuneOnco Biopharmaceuticals (Shanghai) Inc.
ImmunoGen, Inc
Immunomedics
INC Research
Incyte Corporation
INmune Bio Inc
IQVIA
Janssen Pharmaceuticals
Jiangsu Alphamab Pharmaceuticals Co., Ltd.
Jiangsu Hengrui Medicine
Johnson & Johnson
Kartos Therapeutics
Karyopharm Therapeutics Inc.
Kazia Therapeutics Limited
KCR CRO Pty Ltd
Kinnate Biopharma Inc.
Labcorp
Laboratoires Servier (Servier)
LaNova Australia Pty Ltd
Loxo Oncology, Inc.
MacroGenics, Inc
MaxiNovel Pharmaceuticals Co.,Ltd
Medicenna Therapeutics Corp
MedImmune, LLC
Medivation, Inc.
Medpace Holdings, Inc.
Melanoma and Skin Cancer Trials (MASC Trials)
Merck KGaA
Merck Sharp & Dohme (Australia) Pty. Ltd (MSD)
Mersana Therapeutics, Inc
Metagone Biotech Inc.
Microba Life Sciences
Millenium Pharmaceuticals
Mirati Therapeutics Inc
Molecule2Market
MorphoSys AG
Morphotek, Inc.
Multitude Therapeutics Inc.
Myeloid Therapeutics
Myovant Sciences
Nektar Therapeutics
Novartis AG
Novogen Research Pty Ltd
Novotech (Australia) Pty Limited
Nucleus Network
Olema Oncology Australia Pty Ltd
OncoC4, Inc.
Onconova Therapeutics Inc.
Panbela Therapeutics Pty. Ltd.
Parexel International
Pfizer, Inc.
Pharmaceutical Product Development, Inc. (PPD)
Pharmaceutical Solutions Limited (PharmaSols)
Pharmacyclics LLC
Pharmaxis Ltd.
Pharm-Olam LLC
Pharos I&BT Co., LTD.
PPD Australia Pty Ltd
PRA Health Sciences
Prothena Biosciences Inc
PSI CRO AG
ANNUAL RESEARCH REPORT 2023 65
Regeneron Pharmaceuticals
Relay Therapeutics, Inc.
RemeGen Biosciences
Revolution Medicines Inc.
Sanofi S.A.
Seagen Inc.
Shanghai Haihe Pharmaceutical Co., Ltd
Shanghai Henlius Biotech, Inc.
Shanghai Junshi Biosciences Co., Ltd
Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd
Sierra Oncology, Inc.
Silence Therapeutics
SonnetBio Pty Ltd
Sumitomo Pharma Oncology, Inc
Supportive Therapeutics, LLC
Suzhou Sinovent Pharmaceuticals Co., Ltd. (Sinovent)
Symvivo Corporation
Syneos Health
Takeda Oncology
Telios Pharma, Inc.
Telix International Pty Ltd
TG Therapeutics Inc.
Tigermed Australia Pty Ltd
Translational Research In Oncology
Trans-Tasman Radiation Oncology Group (TROG)
Turning Point Therapeutics, Inc.
Varian
Verastem, Inc.
Vincerx Pharma Pty. Ltd
Vividion Therapeutics, Inc.
Worldwide Clinical Trials
Y-mAbs Therapeutics Inc
Zentalis
Ziopharm Oncology, Inc.
There is no progress without research and there is no research without our kind and generous patients.”

ANNUAL RESEARCH REPORT 2023 66
Vendor
Advara Heartcare
Ashford Cardiac Clinic
Australian Clinical Labs
Bellberry Limited
Chemtronics Biomedical
ClinPath Pathology
ClinTrial Refer
Conrad Eye Care
CSK Group
DocuSign
Grace Records Management
Heartcare Queensland
Hobart Pathology
I-MED Radiology Network
Iron Mountain
Isothermal Service Pty Ltd
Jones Radiology
Lucence
Lumus Imaging
Melbourne Pathology
North Eye Specialists
Outlook Eye Centre
Qscan
Queensland Cardiovascular Group
Queensland Eye Institute
Queensland X-Ray
RealTime Software Solutions, LLC
SA Pathology
Sonic HealthCare
South Coast Radiology
Sullivan Nicolaides Pathology
Translationz
TrialDocs International Pty Ltd
TriNetX, LLC
Valley Eye Specialists

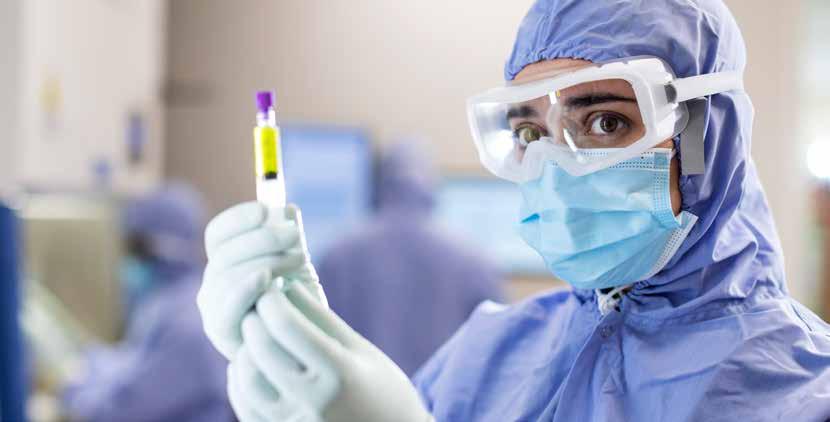
ANNUAL RESEARCH REPORT 2023 67
Principal Investigators
This report features the significant work of our experienced and dedicated Principal Investigators. Icon is fortunate to have over 70 active Principal Investigators bringing a wealth of experience, knowledge, and dedication to their respective fields. Their research plays a critical role in the advancement of cancer care and our shared mission to bringing the best possible care to more people.



Visit our Icon Cancer Centre websites to view doctor profiles: Australia New Zealand Singapore ANNUAL RESEARCH REPORT 2023 68

Queensland
Medical Oncology
Principal Investigator
Dr Vladimir Andelkovic MD, FRACP
Dr Adeola Ayoola
MBBS, FRACP, MBA (IC London)
A/Prof Jim Coward
BSc (Hons), MBBS, MRCP (UK), FRACP, PhD
A/Prof Simon Durrant
MBBS, MRCS, LRCP, FRACP, FRCPath
Dr Paul Eliadis AM
BSc, MBBS, Hon.DLitt (QLD), DUniv Griff., FRACP, FRCPA
A/Prof Jeffrey Goh
MBBS (Monash), FRACP
Dr David Grimes
MBBS (Qld), FRACP
Dr Keith Horwood
MBBS (Hons) FRACP
Dr Mohammed Islam
MBBS, FRACP
Dr Abhishek Joshi
MBBS, MD, DM, FRACP
Special Interest/s
Breast, Liver, Sarcoma
Location
South Brisbane
Breast, Colorectal, Prostate, Melanoma Townsville
Breast, Ovarian, Gynaecological, Gastrointestinal, Lung
South Brisbane, Chermside, Southport
Immunohaematology, Leukaemia, Lymphoma Wesley, Chermside, North Lakes
Colorectal, Gastrointestinal, Leukaemia, Lymphoma, Stem cell transplantation Wesley
Colorectal, Ovarian, Prostate, Gynaecological
Chermside, Greenslopes, Wesley
Breast, Gastrointestinal, Genitourinary, Lung Wesley, North Lakes
Breast, Gastrointestinal, Lung, Ovarian Southport
Colorectal, Genitourinary, Head and Neck, Lung, Prostate Southport
Breast, Colorectal, Lung, Melanoma, Prostate Townsville
ANNUAL RESEARCH REPORT 2023 69

Queensland
Medical Oncology
Principal Investigator
Dr Chun Loo Gan
MBBS, MCncrSc, FRACP
Dr Agnieszka Malczewski
MBBS, FRACP, MSc
A/Prof Nicole McCarthy
MBBS (Hons), MHSc, FRACP
A/Prof Michael Slancar MD, FRACP
Dr Adam Stirling
MBBS(Hons), FRACP
A/Prof Paul Vasey
MBChB, MSc(Clin.Pharm), MD, MRCP(UK), FRACP
Nuclear Medicine
Principal Investigator
A/Prof David Macfarlane MBBS, FRACP, FAANMS
Special Interest/s
Location
Breast, Gastrointestinal, Genitourinary, Lung, Melanoma Chermside, Greenslopes
Gastrointestinal, Melanoma, Pancreatic, Prostate Wesley
Breast, Colorectal, Gynaecological, Sarcoma Wesley
Breast, Colorectal, Gynaecological, Lung, Prostate Southport
Breast, Colorectal, Genitourinary, Lung, Melanoma Wesley
Genitourinary, Gynaecological, Lung, Ovarian Wesley
Special Interest/s
Location
Neuroendocrine tumours, Nuclear medicine, Prostate, Theranostics Greenslopes, Redland
ANNUAL RESEARCH REPORT 2023 70

Queensland
Clinical Haematology
Principal Investigator
Dr Raymond Banh
MBBS, FRACP, FRCPA
Dr Jason Butler
MBBS, M.MED Sci (CLIN EPID), FRACP, FRCPA
Dr Joseph Clarey
MBBS, MClinTRes, FRACP, FRCPA
A/Prof Simon Durrant
MBBS, MRCS, LRCP, FRACP, FRCPath
Dr Paul Eliadis AM BSc, MBBS, Hon.DLitt (QLD), DUniv Griff., FRACP, FRCPA
Dr Stephen Fanning
MBBS, MSc, FRACP, FRCPA
Dr Robert Hensen
MBBS, FRACP, FRCPA
Dr Matthew Hourigan
MBBS (Hons), FRACP, FRCPA
Dr Ian Irving
MBBS, FRACP, FRCPA
A/Prof Kerry Taylor
MBBS(Hons), FRACP, FRCPA
Special Interest/s
Leukaemia, Lymphoma, Multiple Myeloma, Myeloproliferative neoplasms
Leukaemia, Lymphoma, Multiple Myeloma, Stem cell transplantation
Leukaemia, Lymphoma, Multiple Myeloma, Myelodysplasia
Immunohaematology, Leukaemia, Lymphoma
Colorectal, Gastrointestinal, Leukaemia, Lymphoma, Stem cell transplantation
Leukaemia, Lymphoma, Multiple Myeloma, Myelodysplasia
Leukaemia, Lymphoma, Multiple Myeloma, Myeloproliferative neoplasms
Leukaemia, Lymphoma, Multiple Myeloma, Myeloproliferative neoplasms
Lymphoma, Multiple Myeloma, Myeloproliferative neoplasms, Stem cell transplantation
Leukaemia, Lymphoma, Multiple Myeloma, Myelodysplasia, Myeloproliferative neoplasms
Location
South Brisbane, Wesley
South Brisbane, Chermside
Wesley
Wesley, Chermside, North Lakes
Wesley
Wesley, Chermside, South Brisbane
Wesley, Chermside, North Lakes
Wesley, Redland, Greenslopes
Wesley, Mackay
South Brisbane
ANNUAL RESEARCH REPORT 2023 71

Queensland
Radiation Oncology
Principal Investigator
Dr Joanne Castelli FRANZCR, MBBS (Hons), BSc (Biomed)
A/Prof Matthew Foote
BSc, MBBS (Hons), FRANZCR
A/Prof Jim Jackson
MBBS, FRANZCR, PhD
Dr Eric Khoo MBBS, FRANZCR
Dr Luke McGhee BSc, MBBS (Hons)
Dr Lekshmi Nair
MBBS, FRANZCR
Dr Andrew Oar BSc, MBBS, MIPH, FRANZCR
A/Prof Mark Pinkham
BM, BCh, MA(Hons)(Oxon), FRANZCR
Dr Vivien Tse
MBBS, MSc, MRCP (UK), FRCR (UK), FRANZCR
Special Interest/s Location
CNS, Breast, Lung, Stereotactic RT Maroochydore
CNS, Head and Neck, Skin, Stereotactic RT
CNS, Breast, Head and Neck, Lung, Prostate, Stereotactic RT
CNS, Head and Neck, Skin, Stereotactic RT, Palliative care
Greenslopes, Redland, Springfield
Gold Coast Private, Gold Coast University Hospital, North Lakes, Greenslopes
Gold Coast Private Hospital
Breast, Genitourinary, Prostate, Skin Cairns (Liz Plummer)
Breast, Colorectal, Gastrointestinal, Genitourinary, Lung, Skin Toowoomba
Breast, Gastrointestinal, Lung, Skin, Stereotactic RT
CNS, Lung, Melanoma, Palliative and Stereotactic RT
Gold Coast Private Hospital
North Lakes, Greenslopes, Auchenflower
CNS, Colorectal, Genitourinary, Gynaecological, Lung Toowoomba
ANNUAL RESEARCH REPORT 2023 72

New South Wales
Radiation Oncology
Principal Investigator
Prof John Boyages AM MBBS (Hons), PhD, FRANZCR
Prof Gerald Fogarty
BSc, MBBS, PhD, FRANZCR
Dr Andrew Fong
BSc (Med), MBBS, MeH, FRANZCR
Dr Amy Yuen Meei Teh
BSc (Med), MBBS (Hons), FRANZCR
Dr Angela Yates
BSc, BA, MBBS, MMed (clin epi), FRANZCR
Special Interest/s
Location
Breast, Lymphoedema Wahroonga, Gosford
Skin
Revesby, Gosford, Wahroonga
Breast, Head and Neck, Lung, Stereotactic RT Wahroonga
Breast, Prostate Wahroonga
CNS, Breast, Gastrointestinal, Head and Neck, Lung, Skin Wahroonga
Australian Capital Territory
Radiation Oncology
Principal Investigator
Dr Andrew Lee
FRANZCR, MBBS, B Med Sci (Adv)
Special Interest/s
Location
CNS, Breast, Gynaecological, Lymphoma, Prostate Canberra
ANNUAL RESEARCH REPORT 2023 73

Victoria
Radiation Oncology
Principal Investigator
Dr Patrick Bowden MBBS, FRANZCR
Dr Neda Haghighi MD, FRANZCR
Dr Andrew See MBBS, FRANZCR
Special Interest/s
CNS, Gastrointestinal, Genitourinary, Prostate, Oligometastatic
CNS, Oligometastatic, Stereotactic RT
Breast, Gastrointestinal, Genitourinary, Prostate, Skin, Stereotactic RT
Location
Epworth Richmond, Epworth Freemasons, Moreland, Canberra, Mulgrave, Holmesglen
Epworth Richmond, Holmesglen
Epworth Freemasons, Epworth Richmond, Mulgrave, Moreland, Mildura, Holmesglen
Dr Kevin So MBBS, BMedSc(Hons), FRANZCR
CNS, Breast, Gastrointestinal, Lung, Skin
Epworth Freemasons, Epworth Richmond, Mulgrave, Moreland, Holmesglen
ANNUAL RESEARCH REPORT 2023 74

South Australia
Medical Oncology
Principal Investigator
Dr Carolyn Bampton
MBBS, FRACP
Dr Sarwan Bishnoi
MBBS, MD, FRACP
Dr Kerry Cheong
MBBS(Hons), FRACP
Dr Craig Gedye
BSc(Hons), MBChB, FRACP, PhD
Dr Amy Hsieh
MBBS, FRACP
Dr Anna Mislang
MBBS, FRACP
Dr Meena Okera
MBBS(Hons), FRACP
Dr Dainik Patel
MBBS, FRACP
Dr Nimit Singhal
MBBS, FRACP
Dr Annabel Smith
MBBS, FRACP
Dr Brian Stein
MBBS(Hons), FRACP
Dr Hsiang Tan
MBBS, FRACP
Special Interest/s
Location
CNS, Breast, Gastrointestinal, Lung, Prostate Kurralta Park
Colorectal, Head and Neck, Lung, Prostate
Breast, Gastrointestinal, Lung
Bladder, CNS, Genitourinary, Melanoma, Prostate
Kurralta Park
Kurralta Park
Kurralta Park, Windsor Gardens
Breast, Genitourinary, Lung, Melanoma Windsor Gardens
Breast, Gastrointestinal, Genitourinary, Lung, Melanoma
Breast, Gynaecological
Breast, Gastrointestinal, Genitourinary, Lung, Neuroendocrine
CNS, Gastrointestinal, Genitourinary, Lung, Sarcoma
Kurralta Park
Kurralta Park
Kurralta Park, Windsor Gardens
Kurralta Park
Gastrointestinal, Genitourinary, Lung, Melanoma Kurralta Park
Head and Neck, Lymphoma, Melanoma, Geriatric oncology
Genitourinary, Prostate
Kurralta Park
Kurralta Park, Windsor Gardens
ANNUAL RESEARCH REPORT 2023 75

South Australia
Clinical Haematology
Principal Investigator
Dr Stanley Cheung
MBBS, PhD, MRCP, FRACP, FHKCP, FHKAM, FRCP (Glasg), AFRACMA
Dr Akash Kalro
MBBS, MD, FRCPA, FRACP
Special Interest/s
Leukaemia, Lymphoma, Multiple Myeloma, Thromnbosis and haemostasis
Lymphoma, Multiple Myeloma, Stem cell transplantation
Location
Kurralta Park, Windsor Gardens
Kurralta Park
ANNUAL RESEARCH REPORT 2023 76

Tasmania
Medical Oncology
Principal Investigator
Dr Cristina Moldovan MBBS, FRACP
A/Prof Louise Nott
BMedSci, MBBS(Hons), FRACP
Radiation Oncology
Principal Investigator
Dr Raef Awad
BSc, MBBS, MD, FRANZCR
A/Prof Michael Jones
BSc, BE (Hons), MBBS, MPHTM, PhD, FRANZCR
Special Interest/s
Location
Gastrointestinal, Genitourinary, Gynaecological, Head and Neck, Skin Hobart
Breast, Colorectal, Lung, Prostate, Skin Hobart
Special Interest/s
Location
Breast, Genitourinary, Lung, Prostate Hobart
CNS, Breast, Gastrointestinal, Lung, Prostate Hobart
ANNUAL RESEARCH REPORT 2023 77

New Zealand
Medical Oncology
Principal Investigator
Dr Catherine Barrow
MBChB, FRACP
Dr David Okonji
MBBCh(UK) MRCP(UK) MRCPS(Glas)
DTM (R.C.P. & S. Irel.) FRACP
Radiation Oncology
Principal Investigator
Dr David Anderson
MBChB, FcRadOnc (SA)
Special Interest/s
Breast, Lymphoma, Melanoma
Breast, Genitourinary, Melanoma, Prostate
Location
Bowen
Bowen
Special Interest/s
Breast, Lung, Prostate, Oligometastatic, Stereotactic RT
Location
Bowen
ANNUAL RESEARCH REPORT 2023 78

Singapore
Medical Oncology
Principal Investigator
Dr Daniel Chan Boon Yeow
MBBS (Hons), MRCP (UK), FAMS (Medical Oncology), FRCP (UK)
Dr Hsieh Wen-Son
BSc (USA), MD (USA), Internal Medicine (USA), Medical Oncology (USA), Diplomate Internal Medicine and Medical Oncology ABIM (USA)
Dr Patricia Kho Sunn Sunn
MBBS (UK), FRACP (Australia), MPhil (Sydney)
Dr Lee Guek Eng
BSc, MBBS, MRCP, MMed
Dr Robert Lim
MChB, ABIM (Int Med), ABIM (Med Onc), ABIM (Heme), FRCP (Edin)
Dr Tan Yew Oo
MBBS, FRCP (Canada), FACP (USA), FRACP (Aus), FRCP (Edin), FRCP (Glasg), FAMS (Medical Oncology), Diplomate of American Board of Internal Medicine, Haematology and Medical Oncology
Dr Karmen Wong
MBBS (AUS), MRCP (UK), FRCP (Glasg), MMed (Int Med), FAM
Special Interest/s
Breast, Colon, Lung, Lymphoma, Sarcoma
Lymphoma, Head and Neck, Lung, Hepatobiliary
Location
Mount Elizabeth, Mount Alvernia
Mount Elizabeth, Farrer Park, Mount Alvernia
Lung, Head and Neck, Gastrointestinal, Breast Mount Alvernia, Novena
Breast, Gynaecological
Colorectal, Gastric, Liver, Pancreatic, Oesophageal, Biliary tract
Farrer Park, Mount Alvernia, Novena
Farrer Park, Mount Elizabeth, Mount Alvernia
Breast, Gastrointestinal, Liver, Lung, Lymphoma
Farrer Park, Mount Alvernia
Breast, Gastrointestinal, Gynaecological
Gleneagles, Mount Alvernia
ANNUAL RESEARCH REPORT 2023 79
2023 Publications
1. Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. Turner NC, Oliveira M, Howell SJ, Dalenc F, Cortes J, Gomez Moreno HL, Hu X, Jhaveri K, Krivorotko P, Loibl S, Morales Murillo S, Okera M, Park YH, Sohn J, Toi M, Tokunaga E, Yousef S, Zhukova L, de Bruin EC, Grinsted L, Schiavon G, Foxley A, Rugo HS; CAPItello-291 Study Group. N Engl J Med. 2023 Jun 1;388(22):2058-2070. doi: 10.1056/ NEJMoa2214131.
Icon Authors: Meena Okera, Yeon Hee Park, Joohyuk Sohn, Masakazu Toi, Eriko Tokunaga, Samih Yousef, Hope S Rugo
2. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): an international, openlabel, randomised, phase 3 trial. Sweeney CJ, Martin AJ, Stockler MR, Begbie S, Cheung L, Chi KN, Chowdhury S, Frydenberg M, Horvath LG, Joshua AM, Lawrence NJ, Marx G, McCaffrey J, McDermott R, McJannett M, North SA, Parnis F, Parulekar W, Pook DW, Reaume MN, Sandhu SK, Tan A, Tan TH, Thomson A, Vera-Badillo F, Williams SG, Winter D, Yip S, Zhang AY, Zielinski RR, Davis ID; ENZAMET trial investigators and Australian and New Zealand Urogenital and Prostate Cancer Trials Group. Lancet Oncol. 2023 Apr;24(4):323-334. doi: 10.1016/S14702045(23)00063-3.
Icon Authors: Francis Parnis
3. Response from Batumalai V et al. Batumalai V, Descallar J, Gabriel G, Delaney GP, Oar A, Barton MB, Vinod SK. Asia Pac J Clin Oncol. 2023 Jun;19(3):415. doi: 10.1111/ajco.13891. Epub 2022 Oct 27.
Icon Authors: Andrew Oar
4. Management of Recurrent HPV-Positive Oropharyngeal Squamous Cell Carcinoma: a Contemporary Review. Dowthwaite S, Jackson J, Dzienis M, Khoo E, Cronin M, Guazzo E. Curr Oncol Rep. 2023 May;25(5):501-510. doi: 10.1007/s11912-02301386-5. Epub 2023 Mar 7.
Icon Authors: Jim Jackson, Eric Khoo
5. Merkel cell carcinoma: a forty-year experience at the Peter MacCallum Cancer Centre. Wang AJ, McCann B, Soon WCL, De Ieso PB, Bressel M, Hui A, Chua M, Kok DL. BMC Cancer. 2023 Jan 7;23(1):30. doi: 10.1186/s12885-022-10349-1.
Icon Authors: Paul De Ieso
6. Emergent radiotherapy for spinal cord compression/impingement-a narrative review. Zaki P, Barbour A, Zaki MM, Tseng YD, Amin AG, Venur V, McGranahan T, Vellayappan B, Palmer JD, Chao ST, Yang JT, Foote M, Redmond KJ, Chang EL, Sahgal A, Lo SS, Schaub SK. Ann Palliat Med. 2023 Nov;12(6):1447-1462. doi: 10.21037/apm-23342. Epub 2023 Sep 22.
Icon Authors: Matthew Foote
7. Activity and outcomes of autologous stem cell transplantation in the private sector in Australia. Nath K, James Y, Taylor D, Gardner R, Rai N, Ware RS, Taylor K, Morton J, Durrant S, Irving I, Bashford J Intern Med J. 2023 Jun;53(6):923-929. doi: 10.1111/imj.15754. Epub 2022 Sep 4.
Icon Authors: Karthik Nath, Yvette James, Raeina Gardner, Kerry Taylor, James Morton, Simon Durrant, Ian Irving, John Bashford
8. A multi-centre survey of New Zealand cancer patients’ preferences for radiation treatment information. Flockton A, Leong A, Gilfillan D, Larsen P. J Med Radiat Sci. 2024 Mar;71(1):9199. doi: 10.1002/jmrs.745. Epub 2023 Dec 22.
Icon Authors: Aidan Leong
ANNUAL RESEARCH REPORT 2023 80
9. Acquired HbH disease diagnosed by HbA1c capillary electrophoresis. McKeague S, Peake N, Lovelock D, Chow J, Benson R, Fanning S. Br J Haematol. 2023 Mar;200(6):687. doi: 10.1111/bjh.18587. Epub 2022 Dec 14.
Icon Authors: Stephen Fanning
10. Global reported impacts of COVID-19 on lymphoma patients and the emerging clinical management approaches in response to the ongoing pandemic. Elliott EK, Hensen R, Haupt LM, Griffiths LR. Eur J Haematol. 2023 May;110(5):457-469. doi: 10.1111/ejh.13926. Epub 2023 Jan 30.
Icon Authors: Robert Hensen
11. Rectal cancer treatment: an embarrassment of riches? Glyn T, Oar A, Vatandoust S, Heriot A, Jain A. ANZ J Surg. 2023 Oct;93(10):22932294. doi: 10.1111/ans.18634. Epub 2023 Jul 28.
Icon Authors: Andrew Oar
12. Emergent radiotherapy for brain and leptomeningeal metastases: a narrative review. Barbour AB, Zaki P, McGranahan TM, Venur V, Vellayappan B, Palmer J, Halasz LM, Yang JT, Blau M, Tseng YD, Chao ST, Suh JH, Foote M, Redmond KJ, Combs SE, Chang EL, Sahgal A, Lo SS. Ann Palliat Med. 2023 Nov;12(6):1405-1419. doi: 10.21037/apm-221276. Epub 2023 Jun 27.
Icon Authors: Matthew Foote
13. A phase 1a/1b first-in-human study (COMPASSION-01) evaluating cadonilimab in patients with advanced solid tumors. Frentzas S, Gan HK, Cosman R, Coward J, Tran B, Millward M, Zhou Y, Wang W, Xia D, Wang ZM, Li B, Xia M, Desai J. Cell Rep Med. 2023 Nov 21;4(11):101242. doi: 10.1016/j.xcrm.2023.101242. Epub 2023 Oct 17.
Icon Authors: Jim Coward
14. Outcomes for International Metastatic Renal Cell Carcinoma Database Consortium Prognostic Groups in Contemporary First-line Combination Therapies for Metastatic Renal Cell Carcinoma. Ernst MS, Navani V, Wells JC, Donskov F, Basappa N, Labaki C, Pal SK, Meza L, Wood LA, Ernst DS, Szabados B, McKay RR, Parnis F, Suarez C, Yuasa T, Lalani AK, Alva A, Bjarnason GA, Choueiri TK, Heng DYC. Eur Urol. 2023 Jul;84(1):109-116. doi: 10.1016/j. eururo.2023.01.001. Epub 2023 Jan 26.
Icon Authors: Francis Parnis
15. A tailored approach to horizon scanning for cancer medicines. Soon JA, To YH, Alexander M, Trapani K, Ascierto PA, Athan S, Brown MP, Burge M, Haydon A, Hughes B, Itchins M, John T, Kao S, Koopman M, Li BT, Long GV, Loree JM, Markman B, Meniawy TM, Menzies AM, Nott L, Pavlakis N, Petrella TM, Popat S, Tie J, Xu W, Yip D, Zalcberg J, Solomon BJ, Gibbs P, McArthur GA, Franchini F, IJzerman M. J Cancer Policy. 2023 Dec;38:100441. doi: 10.1016/j.jcpo.2023.100441. Epub 2023 Nov 20.
Icon Authors: Matthew Burge, Louise Nott, John Zalcberg
16. Radiation Therapy and Indigenous Peoples in Canada and Australia: Building Paths Toward Reconciliation in Cancer Care Delivery. Chan J, Griffiths K, Turner A, Tobias J, Clarmont W, Delaney G, Hutton J, Olson R, Penniment M, Bourque JM, Brundage M, Rodin D, Slotman B, Yap ML. Int J Radiat Oncol Biol Phys. 2023 Jun 1;116(2):421-429. doi: 10.1016/j. ijrobp.2022.09.085. Epub 2023 Mar 21.
Icon Authors: Jonathon Hutton, Michael Penniment
ANNUAL RESEARCH REPORT 2023 81
2023 Publications
17. Individualized breathing trace quality assurance for lung radiotherapy patients undergoing 4DCT simulation. Rijken J, Hu Y, Hiscoke K. J Appl Clin Med Phys. 2023 Jun;24(6):e13929. doi: 10.1002/acm2.13929. Epub 2023 Feb 20.
Icon Authors: James Rijken, Yunfei Hu, Kelvin Hiscoke
18. PEACE V-Salvage Treatment of OligoRecurrent nodal prostate cancer Metastases (STORM): Acute Toxicity of a Randomized Phase 2 Trial. Ost P, Siva S, Brabrand S, Dirix P, Liefhooghe N, Otte FX, Gomez-Iturriaga A, Everaerts W, Shelan M, Conde-Moreno A, López Campos F, Papachristofilou A, Guckenberger M, Scorsetti M, Zapatero A, Villafranca Iturre AE, Eito C, Couñago F, Muto P, Van De Voorde L, Mach N, Bultijnck R, Fonteyne V, Moon D, Thon K, Mercier C, Achard V, Stellamans K, Goetghebeur E, Reynders D, Zilli T. Eur Urol Oncol. 2023 Oct 9:S2588-9311(23)00199-2. doi: 10.1016/j.euo.2023.09.007. Online ahead of print.
Icon Authors: Shankar Siva, Kristian Thon
19. Wide-field radiation therapy for skin cancerisation - Have we forgotten what we learned? Daly T, Veness M, Poulsen M, Muir J, De’Ambrosis B, Kennedy D. J Med Imaging Radiat Oncol. 2023 Feb;67(1):128-131. doi: 10.1111/1754-9485.13489. Epub 2022 Oct 31.
Icon Authors: Michael Poulsen
20. A comprehensive study of the epidemiology of haematological malignancies in North Queensland. Nath K, Boles R, Emeto TI, Adegboye OA, Castellanos ME, Alele FO, Pearce J, Ewart B, Ward K, Lai HC, Morris E, Hodges G, Irving I. Intern Med J. 2023 Apr;53(4):540-549. doi: 10.1111/ imj.15594. Epub 2022 Aug 15.
Icon Authors: Karthik Nath, Hock C Lai, Edward Morris, Georgina Hodges, Ian Irving
21. Advanced fumarate hydratase-deficient renal cell carcinoma responding to combination immune checkpoint inhibitors. Howells E, Wigston L, Mackie G, Tran B, Nott L. Can J Urol. 2023 Jun;30(3):11558-11561.
Icon Authors: Louise Nott
22. Breast lymphedema following breastconserving treatment for breast cancer: current status and future directions. Brunelle CL, Boyages J, Jung AW, Suami H, Juhel BC, Heydon-White A, Mackie H, Chou SS, Paramanandam VS, Koelmeyer L, Taghian AG. Breast Cancer Res Treat. 2024 Apr;204(2):193-222. doi: 10.1007/s10549-02307161-1. Epub 2023 Dec 15.
Icon Authors: John Boyages
23. Efficacy and safety of cosibelimab, an antiPD-L1 antibody, in metastatic cutaneous squamous cell carcinoma. Clingan P, Ladwa R, Brungs D, Harris DL, McGrath M, Arnold S, Coward J, Fourie S, Kurochkin A, Malan DR, Mant A, Sharma V, Shue H, Tazbirkova A, Berciano-Guerrero MA, Charoentum C, Dalle S, Dechaphunkul A, Dudnichenko O, Koralewski P, Lugowska I, Montaudié H, Muñoz-Couselo E, Sriuranpong V, Oliviero J, Desai J. J Immunother Cancer. 2023 Oct;11(10):e007637. doi: 10.1136/jitc-2023007637.
Icon Authors: Jim Coward
24. Clinical assessment of a novel machinelearning automated contouring tool for radiotherapy planning. Hu Y, Nguyen H, Smith C, Chen T, Byrne M, ArchibaldHeeren B, Rijken J, Aland T. J Appl Clin Med Phys. 2023 Jul;24(7):e13949. doi: 10.1002/ acm2.13949. Epub 2023 Mar 4.
Icon Authors: Yunfei Hu, Huong Nguyen, Claire Smith, Tom Chen, Mikel Byrne, Ben Archibald-Heeren, James Rijken, Trent Aland
ANNUAL RESEARCH REPORT 2023 82
25. Response to: Radiation Oncology Viewpoint: Wide-field radiation therapy for skin cancerisation-Have we forgotten what we learned? Fogarty GB, Paton EJ, Schlect D, Christie D, Ha T, Ng E, Wong B, Potter A. J Med Imaging Radiat Oncol. 2023 Jun;67(4):463-464. doi: 10.1111/17549485.13531. Epub 2023 Apr 29.
Icon Authors: Gerald Fogarty
26. Single-Fraction Versus Fractionated Preoperative Radiosurgery for Resected Brain Metastases: A PROPS-BM International Multicenter Cohort Study. Prabhu RS, Akinyelu T, Vaslow ZK, Matsui JK, Haghighi N, Dan T, Mishra MV, Murphy ES, Boyles S, Perlow HK, Palmer JD, Udovicich C, Patel TR, Wardak Z, Woodworth GF, Ksendzovsky A, Yang K, Chao ST, Asher AL, Burri SH. Int J Radiat Oncol Biol Phys. 2024 Mar 1;118(3):650-661. doi: 10.1016/j. ijrobp.2023.09.012. Epub 2023 Sep 16.
Icon Authors: Neda Haghighi
27. Prospective Surveillance with Compression for Subclinical Lymphedema: Symptoms, Skin, and Quality-of-Life Outcomes. Dietrich MS, Gaitatzis K, Koelmeyer L, Boyages J, Abramson VG, McLaughlin SA, Ngui N, Elder E, French J, Hsu J, Hughes TM, Stolldorf DP, Shah C, Ridner SH. Lymphat Res Biol. 2023 Jun;21(3):304-313. doi: 10.1089/ lrb.2022.0020. Epub 2022 Sep 20.
Icon Authors: John Boyages
28. Risk Factors for Progression and Toxic Effects After Preoperative Stereotactic Radiosurgery for Patients With Resected Brain Metastases. Prabhu RS, Akinyelu T, Vaslow ZK, Matsui JK, Haghighi N, Dan T, Mishra MV, Murphy ES, Boyles S, Perlow HK, Palmer JD, Udovicich C, Patel TR, Wardak Z, Woodworth GF, Ksendzovsky A, Yang K, Chao ST, Asher AL, Burri SH. JAMA Oncol. 2023 Aug 1;9(8):10661073. doi: 10.1001/jamaoncol.2023.1629.
Icon Authors: Neda Haghighi
29. Closing the gap in Indigenous Australian cancer care: Initiatives to foster cultural safety and improve access to radiation therapy. Summers A. J Med Imaging Radiat Sci. 2023 Dec;54(4S):S38-S43. doi: 10.1016/j. jmir.2023.07.016. Epub 2023 Aug 6.
Icon Authors: Amber Summers
30. Patient-reported Outcomes in Men with Metastatic Castration-resistant Prostate Cancer Harboring DNA Damage Response Alterations Treated with Talazoparib: Results from TALAPRO-1. Saad F, de Bono J, Barthélémy P, Dorff T, Mehra N, Scagliotti G, Stirling A, Machiels JP, Renard V, Maruzzo M, Higano CS, Gurney H, Healy C, Bhattacharyya H, Arondekar B, Niyazov A, Fizazi K. Eur Urol. 2023 Apr;83(4):352-360. doi: 10.1016/j. eururo.2022.05.030. Epub 2022 Jun 22.
Icon Authors: Adam Stirling
31. Patterns of curative treatment for nonsmall cell lung cancer in New South Wales, Australia. Batumalai V, Descallar J, Gabriel G, Delaney GP, Oar A, Barton MB, Vinod SK. Asia Pac J Clin Oncol. 2023 Apr;19(2):e149-e159. doi: 10.1111/ajco.13811. Epub 2022 Jul 17.
Icon Authors: Andrew Oar
32. Pamiparib in combination with tislelizumab in patients with advanced solid tumours: results from the dose-expansion stage of a multicentre, open-label, phase I trial. Friedlander M, Mileshkin L, Lombard J, Frentzas S, Gao B, Wilson M, Meniawy T, Baron-Hay S, Briscoe K, McCarthy N, Fountzilas C, Cervantes A, Ge R, Wu J, Spira A. Br J Cancer. 2023 Sep;129(5):797-810. doi: 10.1038/s41416-023-02349-0. Epub 2023 Jul 20.
Icon Authors: Nicole McCarthy
ANNUAL RESEARCH REPORT 2023 83
2023 Publications
33. Can (68) Ga-PSMA positron emission tomography and multiparametric MRI guide treatment for biochemical recurrence after radical prostatectomy? Khanna Y, Chinni V, Gnanasambantham K, O’Sullivan R, Ballok ZE, Ryan A, Ramdave S, Sivaratnam D, Bowden P, Guerrieri M, Ranasinghe WKB, Frydenberg M. BJU Int. 2023 Sep;132(3):321328. doi: 10.1111/bju.16037. Epub 2023 May 16.
Icon Authors: Patrick Bowden
34. Guidelines for safe practice of stereotactic body (ablative) radiation therapy: RANZCR 2023 update. Liu HY, Hardcastle N, Bailey M, Siva S, Seeley A, Barry T, Booth J, Lao L, Roach M, Buxton S, Thwaites D, Foote M. J Med Imaging Radiat Oncol. 2023 Dec 31. doi: 10.1111/1754-9485.13618. Online ahead of print.
Icon Authors: Howard Yu-Hao Liu, Matthew Foote, Shankar Siva
35. Factors associated with weight gain after breast cancer: Results from a communitybased survey of Australian women. Ee C, Cave A, Vaddiparthi V, Naidoo D, Boyages J. Breast. 2023 Jun;69:491-498. doi: 10.1016/j. breast.2023.01.012. Epub 2023 Jan 25.
Icon Authors: John Boyages
36. Liposuction for Advanced Lymphedema in a Multidisciplinary Team Setting in Australia: 5-Year Follow-Up. Karlsson T, Mackie H, Koelmeyer L, Heydon-White A, Ricketts R, Toyer K, Boyages J, Brorson H, Lam T. Plast Reconstr Surg. 2024 Feb 1;153(2):482-491. doi: 10.1097/PRS.0000000000010612. Epub 2023 Apr 28.
Icon Authors: John Boyages
37. The safety, feasibility, and efficacy of an 18week exercise intervention for adults with primary brain cancer - the BRACE study. Sandler CX, Gildea GC, Spence RR, Jones TL, Eliadis P, Walker D, Donaghue A, Bettington C, Keller J, Pickersgill D, Shevill M, Biggs V, Morrison B, Jonker F, Foote M, Bashford J, Hayes SC. Disabil Rehabil. 2023 Jun 13:1-10. doi: 10.1080/09638288.2023.2221041. Online ahead of print.
Icon Authors: Paul Eliadis, Matthew Foote, John Bashford
38. Management of rectal cancer in the era of total neoadjuvant therapy and watch and wait: A multidisciplinary team discussion at the Australasian Gastro-Intestinal Trials Group (AGITG) Annual Scientific Meeting 2022. Jain A, Gormly KL, Glyn T, Sammour T, Koay EJ, Oar A, Jameson MB, Smyth EC, Vatandoust S. Asia Pac J Clin Oncol. 2023 Jun 21. doi: 10.1111/ajco.13974. Online ahead of print.
Icon Authors: Andrew Oar
39. Axillary Treatment and Chronic Breast Cancer-Related Lymphedema: Implications for Prospective Surveillance and Intervention From a Randomized Controlled Trial. Boyages J, Vicini FA, Manavi BA, Gaw RL, Koelmeyer LA, Ridner SH, Shah C. JCO Oncol Pract. 2023 Dec;19(12):1116-1124. doi: 10.1200/OP.23.00060. Epub 2023 Oct 10.
Icon Authors: John Boyages
ANNUAL RESEARCH REPORT 2023 84
40. Patient-reported outcomes in high-risk HR+ /HER2- early breast cancer patients treated with endocrine therapy with or without palbociclib within the randomized PENELOPE(B) study. García-Sáenz JA, Marmé F, Untch M, Bonnefoi H, Kim SB, Bear H, McCarthy N, Gelmon K, Martin M, Kelly CM, Reimer T, Toi M, Law E, Bhattacharyya H, Gnant M, Makris A, Seiler S, Burchardi N, Nekljudova V, Loibl S, Rugo HS. Eur J Cancer. 2024 Jan;196:113420. doi: 10.1016/j. ejca.2023.113420. Epub 2023 Nov 5.
Icon Authors: Nicole McCarthy
41. Clinical Oncology Society of Australia Position Statement: 2022 update to the safe handling of monoclonal antibodies in healthcare settings. Ryan M, Lam N, Wright K, Siderov J. Asia Pac J Clin Oncol. 2023 Dec;19(6):723-730. doi: 10.1111/ajco.13943. Epub 2023 Mar 10.
Icon Authors: Neil Lam
42. ESTRO-ACROP guideline for positioning, immobilisation and setup verification for local and loco-regional photon breast cancer irradiation. Mast ME, Leong A, Korreman SS, Lee G, Probst H, Scherer P, Tsang Y. Tech Innov Patient Support Radiat Oncol. 2023 Sep 12;28:100219. doi: 10.1016/j. tipsro.2023.100219. eCollection 2023 Dec.
Icon Authors: A Leong
43. Patterns of Relapse in Australian Patients With Clinical Stage 1 Testicular Cancer: Utility of the Australian and New Zealand Urogenital and Prostate Cancer Trials Group Surveillance Recommendations. Conduit C, Lewin J, Weickhardt A, Lynam J, Wong S, Grimison P, Sengupta S, Pranavan G, Parnis F, Bastick P, Campbell D, Hansen AR, Leonard M, McJannett M, Stockler MR, Gibbs P, Toner G, Davis ID, Tran B, Kuchel A. JCO Oncol Pract. 2023 Nov;19(11):973-980. doi: 10.1200/OP.23.00191. Epub 2023 Jun 16.
Icon Authors: Aaron Hansen, Francis Parnis
44. Intrafraction Motion and Margin Assessment for Ethos Online Adaptive Radiotherapy Treatments of the Prostate and Seminal Vesicles. Byrne M, Teh AYM, Archibald-Heeren B, Hu Y, Rijken J, Luo S, Aland T, Greer P. Adv Radiat Oncol. 2023 Nov 4;9(3):101405. doi: 10.1016/j. adro.2023.101405. eCollection 2024 Mar.
Icon Authors: Mikel Byrne, Amy Yuen Meei Teh, Ben Archibald-Heeren, Yunfei Hu, James Rijken, Trent Aland
45. Australia and New Zealand Transplant and Cellular Therapies (ANZTCT) position statement: COVID-19 management in patients with haemopoietic stem cell transplant and chimeric antigen receptor T cell. Perram J, Purtill D, Bajel A, Butler J, O’Brien T, Teh B, Gilroy N, Ho PJ, Doocey R, Hills T, Perera T, Douglas G, Ramachandran S, Chee L, Trotman J, Weinkove R, Keogh S, Fraser C, Cochrane T, Watson AM, Diamond P, Latimer M, Irving I, Blyth E, Cheah C, Cole T, Milliken S, Yang H, Greenwood M, Bardy P, Kennedy G, Larsen S, Conyers R, Hamad N. Intern Med J. 2023 Jan;53(1):119-125. doi: 10.1111/imj.15978.
Icon Authors: Jason Butler, Ian Irving
46. Definitive Stereotactic Body Radiation Therapy in Early-Stage Solitary Hepatocellular Carcinoma: An Australian Multi-Institutional Review of Outcomes. Liu HY, Lee YD, Sridharan S, Wang W, Khor R, Chu J, Oar A, Choong ES, Le H, Shanker M, Wigg A, Stuart K, Pryor D. Clin Oncol (R Coll Radiol). 2023 Dec;35(12):787-793. doi: 10.1016/j. clon.2023.08.012. Epub 2023 Sep 5.
Icon Authors: Andrew Oar, Yoo-Young Dominique Lee, Howard Liu, Mihir Shanker, Andrew Oar, David Pryor
ANNUAL RESEARCH REPORT 2023 85
2023 Publications
47. Corrigendum to “Outcomes for International Metastatic Renal Cell Carcinoma Database Consortium Prognostic Groups in Contemporary First-line Combination Therapies for Metastatic Renal Cell Carcinoma”. Ernst MS, Navani V, Wells JC, Donskov F, Basappa N, Labaki C, Pal SK, Meza L, Wood LA, Ernst DS, Szabados B, McKay RR, Parnis F, Suarez C, Yuasa T, Lalani AK, Alva A, Bjarnason GA, Choueiri TK, Heng DYC. Eur Urol. 2023 Jun;83(6):e166-e167. doi: 10.1016/j.eururo.2023.03.003. Epub 2023 Mar 25.
Icon Authors: Francis Parnis
48. Universal evaluation of MLC models in treatment planning systems based on a common set of dynamic tests. Saez J, BarDeroma R, Bogaert E, Cayez R, Chow T, Clark CH, Esposito M, Feygelman V, Monti AF, Garcia-Miguel J, Gershkevitsh E, Goossens J, Herrero C, Hussein M, Khamphan C, Kierkels RGJ, Lechner W, Lemire M, Nevelsky A, Nguyen D, Paganini L, Pasler M, Fernando Pérez Azorín J, Ramos Garcia LI, Russo S, Shakeshaft J, Vieillevigne L, Hernandez V. Radiother Oncol. 2023 Sep;186:109775. doi: 10.1016/j.radonc.2023.109775. Epub 2023 Jun 28.
Icon Authors: John Shakeshaft
49. Radiation necrosis and therapeutic outcomes in patients treated with linear acceleratorbased hypofractionated stereotactic radiosurgery for intact intracranial metastases. Zhang Q, Hamilton D, Conway P, Xie SJ, Haghighi N, Lasocki A. J Med Imaging Radiat Oncol. 2023 Apr;67(3):308-319. doi: 10.1111/17549485.13519. Epub 2023 Feb 27.
Icon Authors: Paul Conway, Neha Haghighi, Qichen Zhang
50. A first-in-human phase 1 study of nofazinlimab, an anti-PD-1 antibody, in advanced solid tumors and in combination with regorafenib in metastatic colorectal cancer. Day D, Park JJ, Coward J, Markman B, Lemech C, Kuo JC, Prawira A, Brown MP, Bishnoi S, Kotasek D, Strother RM, Cosman R, Su R, Ma Y, Yue Z, Hu HH, Wu R, Li P, Tse AN. Br J Cancer. 2023 Nov;129(10):1608-1618. doi: 10.1038/s41416-023-02431-7. Epub 2023 Sep 20.
Icon Authors: Jim Coward, Sarwan Bishnoi, Dusan Kotasek
51. Patterns of practice regarding use of radiation therapy following radical prostatectomy: A survey of Radiation Oncologists and Urologists in Australia and New Zealand. Lawless A, Kneebone A, Armstrong S, Chander S, Christie D, Finnigan R, Hayden A, Higgs B, Knox M, Sasso G, Sidhom M, Shakespeare TP, Holt T. J Med Imaging Radiat Oncol. 2023
Aug;67(5):556-563. doi: 10.1111/17549485.13552. Epub 2023 Jun 21.
Icon Authors: Renee Finnigan, Braden Higgs, Tanya Holt
52. Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): extended follow-up from the phase 3, randomised, open-label study. Choueiri TK, Eto M, Motzer R, De Giorgi U, Buchler T, Basappa NS, Méndez-Vidal MJ, Tjulandin S, Hoon Park S, Melichar B, Hutson T, Alemany C, McGregor B, Powles T, Grünwald V, Alekseev B, Rha SY, Kopyltsov E, Kapoor A, Alonso Gordoa T, Goh JC, Staehler M, Merchan JR, Xie R, Perini RF, Mody K, McKenzie J, Porta CG. Lancet Oncol. 2023 Mar;24(3):228-238. doi: 10.1016/S14702045(23)00049-9.
Icon Authors: Jeffrey Goh
ANNUAL RESEARCH REPORT 2023 86
53. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): a double-blind, randomised, phase 3 trial. Motzer RJ, Russo P, Grünwald V, Tomita Y, Zurawski B, Parikh O, Buti S, Barthélémy P, Goh JC, Ye D, Lingua A, Lattouf JB, Albigès L, George S, Shuch B, Sosman J, Staehler M, Vázquez Estévez S, Simsek B, Spiridigliozzi J, Chudnovsky A, Bex A. Lancet. 2023 Mar 11;401(10379):821-832. doi: 10.1016/S01406736(22)02574-0. Epub 2023 Feb 9.
Icon Authors: Jeffrey Goh
54. Summary of Research: Adjuvant Nivolumab Plus Ipilimumab Versus Placebo for Localized Renal Cell Carcinoma After Nephrectomy (CheckMate 914): A Double-Blind, Randomized, Phase 3 Trial. Motzer RJ, Russo P, Grünwald V, Tomita Y, Zurawski B, Parikh O, Buti S, Barthélémy P, Goh JC, Ye D, Lingua A, Lattouf JB, Albigès L, George S, Shuch B, Sosman J, Staehler M, Vázquez Estévez S, Simsek B, Spiridigliozzi J, Chudnovsky A, Bex A. Target Oncol. 2023 Sep;18(5):639-641. doi: 10.1007/s11523-023-00987-1. Epub 2023 Sep 2.
Icon Authors: Jeffrey Goh
55. Phase 3 CLEAR study in patients with advanced renal cell carcinoma: outcomes in subgroups for the lenvatinib-pluspembrolizumab and sunitinib arms. Grünwald V, Powles T, Eto M, Kopyltsov E, Rha SY, Porta C, Motzer R, Hutson TE, Méndez-Vidal MJ, Hong SH, Winquist E, Goh JC, Maroto P, Buchler T, Takagi T, Burgents JE, Perini R, He C, Okpara CE, McKenzie J, Choueiri TK. Front Oncol. 2023 Aug 16;13:1223282. doi: 10.3389/ fonc.2023.1223282. eCollection 2023.
Icon Authors: Jeffrey Goh
56. Corrigendum: Phase 3 CLEAR study in patients with advanced renal cell carcinoma: outcomes in subgroups for the lenvatinibplus-pembrolizumab and sunitinib arms.
Grünwald V, Powles T, Eto M, Kopyltsov E, Rha SY, Porta C, Motzer R, Hutson TE, Méndez-Vidal MJ, Hong SH, Winquist E, Goh JC, Maroto P, Buchler T, Takagi T, Burgents JE, Perini R, He C, Okpara CE, McKenzie J, Choueiri TK. Front Oncol. 2024 Mar 1;13:1343027. doi: 10.3389/ fonc.2023.1343027. eCollection 2023.
Icon Authors: Jeffrey Goh
57. Dosimetry implications of VMAT delivery angles and intra-fraction motion for SBRT prostate treatments. Dimiskovski D, Aland T. Med Dosim. 2023 Winter;48(4):249-255. doi: 10.1016/j. meddos.2023.05.004. Epub 2023 Jun 30.
Icon Authors: Dimitrij Dimiskovski, Trent Aland
58. A multi-centre survey of New Zealand cancer patients’ preferences for radiation treatment information. Flockton A, Leong A, Gilfillan D, Larsen P. J Med Radiat Sci. 2024 Mar;71(1):9199. doi: 10.1002/jmrs.745. Epub 2023 Dec 22.
Icon Authors: Alannah Flockton, Aidan Leong
59. Field cancerisation and radiotherapy: a case of treatment complications. Fogarty GB, Paton EJ, Christie D. Med J Aust. 2023 Oct 2;219(7):335. doi: 10.5694/mja2.52086. Epub 2023 Aug 21.
Icon Authors: Gerald Fogarty
60. Systemic treatment of advanced and metastatic urothelial cancer: The landscape in Australia. Gurney H, Clay TD, Oliveira N, Wong S, Tran B, Harris C. Asia Pac J Clin Oncol. 2023 Dec;19(6):585-595. doi: 10.1111/ ajco.14001. Epub 2023 Sep 20.
Icon Authors: Tim Clay
ANNUAL RESEARCH REPORT 2023 87
2023 Publications
61. Evaluation of HyperArc™ using film and portal dosimetry quality assurance.
Kamst O, Desai P. Phys Eng Sci Med. 2023 Mar;46(1):57-66. doi: 10.1007/s13246-02201197-1. Epub 2022 Dec 1.
Icon Authors: Onno Kamst, P Desai
62. LOREALAUS: LOrlatinib REAL-World AUStralian Experience in Advanced ALKRearranged NSCLC. Alexander M, Wei J, Parakh S, John T, Kao S, Nagrial A, Bowyer S, Warburton L, Moore M, Hughes BGM, Clay TD, Pavlakis N, Solomon BJ, Itchins M. JTO Clin Res Rep. 2023 Feb 26;4(4):100490. doi: 10.1016/j.jtocrr.2023.100490. eCollection 2023 Apr.
Icon Authors: Tim Clay
63. Safety and feasibility of hyperthermic intraperitoneal chemotherapy during interval cytoreductive surgery in patients with advanced high-grade serous ovarian, fallopian tube, peritoneal cancer in an Australian context. Samoylovich A, Jennings B, Shannon C, Coward JI, Lourie R, Riordan J, Lai NA, van Driel WJ, Cabraal N, Jagasia N, Chetty N, Naidu S, Perrin LC, Barry SC. Aust N Z J Obstet Gynaecol. 2023 Oct;63(5):702-708. doi: 10.1111/ajo.13694. Epub 2023 May 31.
Icon Authors: Jim Coward
I’m passionate about our involvement in research. All the improvements and outcomes in cancer treatment you see today are a result of the research efforts that have gone on for the last 50 years. Through research we are constantly moving forward and changing outcomes for people today and in the future, and as clinicians we get to see the tangible benefits to real people every day.”
Dr Hsieh Wen-Son Icon Singapore Research Committee Chair

ANNUAL RESEARCH REPORT 2023 88



ANNUAL RESEARCH REPORT 2023 89

ANNUAL RESEARCH REPORT 2023 90

Clinical Trials Registry
For a list of our current clinical trials, scan the QR codes below:
Australia
Singapore
ANNUAL RESEARCH REPORT 2023 91

ANNUAL RESEARCH REPORT 2023 92 ICON GROUP HEAD OFFICE Level 1, 22 Cordelia Street South Brisbane QLD 4101 Australia T +61 7 3737 4500 E research@icon.team icongroup.global Follow Icon Group on LinkedIn GLB GRP 113260_2406







 Mark Middleton OAM Icon Group Chief Executive Officer
Mark Middleton OAM Icon Group Chief Executive Officer