
Rajat Ghai Business Head Enterprise Accounts & Solutions, GE HealthCare South Asia
DIAGNOSTICS
Unveiling the genetic complexity of neurological disorders

VOL.17 NO 5 PAGES 40 www.expresshealthcare.in
MEDTECH
FOREMOSTHEALTHCARE MAGAZINE SINCE 2000JUNE 2024,`50
INDIA'S


















































Chairman of the Board
ViveckGoenka
Sr.Vice President-BPD
Neil Viegas
Vice President-BPD
Harit Mohanty
Editor Viveka Roychowdhury*
Editorial Team
Lakshmipriya Nair
Kalyani Sharma
Kavita Jani
Neha Aathavale
DESIGN
Art Director
Pravin Temble
Senior Designer
Rekha Bisht
Senior Artist
Rakesh Sharma
Marketing Team
Rajesh Bhatkal
Douglas Menezes
Ashish Rampure
Debnarayan Dutta
Production Co-ordinator
DhananjayNidre
Scheduling & Coordination
Pushkar Waralikar
CIRCULATION
Mohan Varadkar
CONTENTS

INTERVIEW

P10: RAVINDRAKUMAR CEO, Lupin Diagnostics
PUBLIC HEALTH

18 HOWPOC TEST KITS COULD HELP END TB BY2030

20 ONE HEALTH APPROACH KEYTO PREVENTH5N1 BIRD FLU SPILLOVER FROM ANIMALS TO HUMANS
12


MEDTECH

P21:
INTERVIEW RAJATGHAI
Business Head Enterprise Accounts & Solutions, GE HealthCare South Asia
STRATEGY

26 HOWNURSES CAN EDUCATE COMMUNITIES ABOUTEYE HEALTH
Regd.With RNI No.MAHENG/2007/22045.Postal Regd.No.MCS/162/2022 - 24.Printed and Published byVaidehi Thakar on behalf of The Indian Express (P) Limited and Printed at The Indian Express Press,Plot No.EL-208,TTC Industrial Area,Mahape,Navi Mumbai-400710 and Published at Mafatlal Centre,7th floor,Ramnath Goenka Marg,Nariman Point,Mumbai 400021.
Editor: Viveka Roychowdhury.* (Editorial & Administrative Offices: Mafatlal Centre,7th floor,Ramnath Goenka Marg,Nariman Point,Mumbai 400021) * Responsible for selection of news under the PRB Act.Copyright © 2017.The Indian Express (P) Ltd.All rights reserved throughout the world.
Reproduction in anymanner,electronic or otherwise,in whole or in part,without prior written permission is prohibited.
June 2024 EXPRESS HEALTHCARE 7
Express Healthcare®
Pg
Will Modi 3.0 mean a revamp
or more of the same?
Now that the results of the Lok Sabha General Elections 2024 are in, with the BJP-led National Democratic Alliance (NDA) set for a third term, one hopes that the new government heeds the advice being handed out by healthcare experts on their expectations. The hope is that with no regime change, healthcare policies and schemes set in motion during the previous two terms will continue on the same path.
While Prime Minister Modi fully anticipated a third term and had already drawn up 100 day work plans for key areas, as the BJP does not have the overwhelming majority that it hoped for, coalition politics might call for a dilution and diversification of previous policies. Hence there will be a period of flux while the new government settles in.
This could mean that the BJP might be pressurised by coalition partners to make changes even in flagship policies and schemes. There is no doubt that the experience of the past few years has proved that implementation might not have lived up to intent and some schemes might need tweaking. Will Modi 3.0 mean a revamp or more of the same?
For instance, the flagship Ayushman Bharat scheme will not help the country achieve universal health coverage (UHC) if certain glaring gaps are not plugged. Also, the private sector remains lukewarm and might withdraw support as there is not much incentive to join a scheme where reimbursements are delayed to the point of making operations unsustainable. The digitisation of medical records, moving to Electronic Health Records (EHR) in the Ayushman Bharat Digital Mission is also a task left half done which needs to be completed.
The health ministry also needs to resolve the sticky issue of getting private hospitals to agree to standardisation of rates, a process started in the previous term but left incomplete. The centre will need to follow up with state governments to ensure that a dialogue with private hospitals results in some baseline rate discussions. Optimising public spend on healthcare should also be a priority, by integrating schemes and pooling resources where necessary.
The new government will also have to balance the interests of various stakeholders. On one hand, India's medtech entrepreneurs are encouraged to Make in India to reduce imports of medical equipment, as part of the Atmanirbhar Bharat initiative. This is a matter of health security for the nation.
On the other hand, the government has changed rules to allow global medtech players to import refurbished/ pre used High-End And High-Value (HEHV) medical equipment up to 7 years old, adding two years to the previously stipulated term of five years.

Besides balancing the interests of various stakeholders, the new government will have to tighten healthcare regulations, putting patient safety and cost high on its agenda
Besides being caught in the turf war between global medtech majors and indigenous manufacturers, the new government will have to tighten the regulations, putting patient safety and cost high on its agenda.
For instance, a recent PIL filed by Patient Safety and Access Initiative of India Foundation (PSAIIF), a Delhi-based not-for-profit organisation, alleges that India is being used as a 'dumping ground' for the 'rampant illegal import' of HEHV medical equipment into India, which may be considered obsolete in their country of origin, without obtaining the prior approval of the Ministry of Environment, Forest and Climate Change (MoEFCC}.
The PIL cites data available on the website of the MoEFCC, showing that the Expert Committee of the MoEFCC has granted approvals for the import of robotic-assisted surgical systems on eight occasions for the import of 19 robotic-assisted surgical systems.
However, data available on the website of the Trade Vision portal for Export-Import data, shows that in the last five years (i.e. from January1, 2019 to March 31, 2024), just one company has imported as many as 845 items of such used/refurbished/reconditioned robotic-assisted surgical systems, including its components and accessories worth over Rs 250,00,00,000 into India. The PIL points out that various hospitals are extensively advertising the introduction of robotic-assisted surgical systems without disclosing that these are used and second-hand, which poses a risk to patients, while also increasing the cost of healthcare.
The PIL admits that due to the limited resources available, PSAIIF has not been able to look at and unearth similar data for the other 49 categories of refurbished HEHV medical equipment. However, they believe that various other entities may also be involved in similarly importing refurbished HEHV medical equipment into India without the mandatory prior approval.
The PIL makes the case that the Medical Device Rules, 2017 does not cover used/refurbished medical devices, let alone its import. This is one more gap which needs to be plugged.
These are but a few of the pending health policy issues that the incoming administration will have to work on. Will a BJP without the expected overwhelming majority be a better bet for India's health sector? Maybe having checks and balances are key to ensuring equitable access. Only time will tell.
VIVEKA ROYCHOWDHURY, Editor viveka.r@expressindia.com viveka.roy3@gmail.com
EDITOR’S NOTE EXPRESS HEALTHCARE June 2024 8














INTERVIEW
Immense need for diagnostic labs regulations to be more transparent
Ravindra Kumar ,CEO,Lupin Diagnostics in an interaction with Kalyani Sharma talks about the future of diagnostics in tier-II and III cities,role of technology and more

The seamless integration of AI-driven diagnostics is not only boosting operational efficiency in diagnostic labs but is also offering patients a more accessible,convenient, and transparent healthcare journey
What are your thoughts on the future of diagnostics in tier-II and III cities? How do you see the landscape evolving in terms of accessibility, affordability, and quality of diagnostic services in these regions?
As per the industry estimates, the Indian diagnostic services market was valued at $16.23 billion in 2023, and a recent report indicates this will touch $43.57 billion by FY32, driven by factors such as rising incomes, easier access to quality healthcare, greater awareness of personal health, and increasing penetration of health insurance.
70 per cent of the Indian population resides in tier-II & tier-III cities, and still, the diagnostics testing is very limited in these cities. Healthcare reach is still minimal for the residing population. Handling NCD patients is a big challenge in these cities today. We all know that quality diagnostics assist in 70 per cent of medical treatment for patients. The government's focus on providing quality yet affordable healthcare for all, along with advancements in health technology, has contributed to the rapid expansion of the diagnostics industry. Initiatives like PMJAY are also driving the adoption of insurance, solving the affordability of care for large sections of society and leading to increased demand for quality healthcare in tier II and III cities. Patients in these cities are also increasingly seeking comprehensive diagnostics packages, driving the shift towards preventive healthcare.
The diagnostics industry is witnessing rapid advancements in technology. Could you discuss some of the key technological trends that are shaping the industry, such as AI-driven diagnostics, portable testing devices, and telemedicine integration?
From the digitisation of patient records to the emergence of advanced diagnostic tools,
technology is revolutionising the patient experience. The seamless integration of AI-driven diagnostics is not only boosting operational efficiency in diagnostic labs but is also offering patients a more accessible, convenient, and transparent healthcare journey.
At the forefront, we witness groundbreaking advancements in molecular diagnostics and genetic testing. These technologies enhance diagnostic accuracy, empowering doctors with timely insights for better patient care. As AI takes on a larger role in diagnostics, patients stand to benefit from faster and more accurate assessments, leading to earlier detection and treatment of health conditions. Furthermore, the reduced human intervention in the sample testing process minimises and enhances overall healthcare outcomes and patient satisfaction.
How do you anticipate the regulatory landscape to evolve in response to technological advancements in diagnostics? The organised sector of the diagnostics industry in India has historically strived to maintain the highest quality and safety standards. Regulatory compliance, including adherence to environmental health standards, is also becoming increasingly important. However, in India, only about 3 per cent of diagnostic laboratories are NABL accredited.
The pandemic has underscored the importance of reliable test results, and as patients become more aware of the importance of getting tested by accredited labs, an increasing number of labs will opt to undergo rigorous accreditation processes. There is an immense need for diagnostics laboratory regulations to be more transparent as well as stringent and the process for running diagnostics laboratories in India.
Lupin's Diagnostics has recently
EXPRESS HEALTHCARE June 2024 10
strengthened its presence in South India and other markets by launching labs in Mumbai, Pune, Kolkata, Vijayawada, Bengaluru, and Chennai. Could you discuss the impact of these expansions on enhancing healthcare accessibility in these regions and improving patient outcomes?
With an extensive network comprising 40+ laboratories and over 700+ collection centres across India, Lupin Diagnostics ensures accessibility and convenience for patients across key cities like Mumbai, Pune, Kolkata, Vijayawada, Bengaluru, Hyderabad, and Chennai. We prioritise accuracy and quality by following stringent quality control protocols, ensuring the consistent integrity and quality of each sample, which is demonstrated by 50 per cent+ lab being NABL accredited.
By establishing state-ofthe-art laboratories equipped with cutting-edge diagnostic technologies and staffed with highly qualified clinical experts, Lupin Diagnostics is able to offer a wide range of reliable and high-quality diagnostic services. These services include molecular diagnostics, cytogenetics, flow cytometry, cytology, microbiology, serology, haematology, immunology, routine biochemistry, and more, catering to both routine, specialised and super-specialized testing needs.
Moreover, Lupin Diagnostics enhances patient convenience through a robust home collection service team and network, enabling real-time tracking of assigned phlebotomists. We also offers value-added services such as dynamic smart reports, health monitoring tips, and historical trend graphs, further empowering patients to take charge of their health. Thus, the expansion underscores Lupin Diagnostics' Laboratories a relentless dedication to
patient-centricity and its mission to be a catalyst for healthier lives.
Could you elaborate on Lupin's Diagnostics' approach to leveraging data analytics and digital technologies to improve diagnostic accuracy and
efficiency?
The future of diagnostics lies in leveraging data analytics and digital technologies to offer personalised, transparent, and efficient healthcare solutions. In the rapidly evolving landscape of healthcare, these tools are becoming essential in driving
personalized medicine and reshaping the diagnostic process.
We at Lupin Diagnostics recognise the transformative potential of data analytics and digital technologies in enhancing diagnostic accuracy and efficiency, and by embracing these
advancements, we aim to contribute to the ongoing evolution of the diagnostics industry towards more personalised, transparent, and patient-centric healthcare solutions.
Kalyani.sharma@expressindia.com journokalyani@gmail.com





June 2024 EXPRESS HEALTHCARE 11 DispojektSingleUseSyringe withsafetyneedle www.hmdhealthcare.com Scanfor 3DVideo Scanfor ProductFilm Scantowatch thetutorial NewlyLaunch

EXPRESS HEALTHCARE June 2024 12 cover )



POC diagnostic hold immense potential in transforming healthcare accessibility in India, provided there are clear regulatory pathways, optimal infrastructure investment,and stringent quality control measures
ByKalyani Sharma
June 2024 EXPRESS HEALTHCARE 13
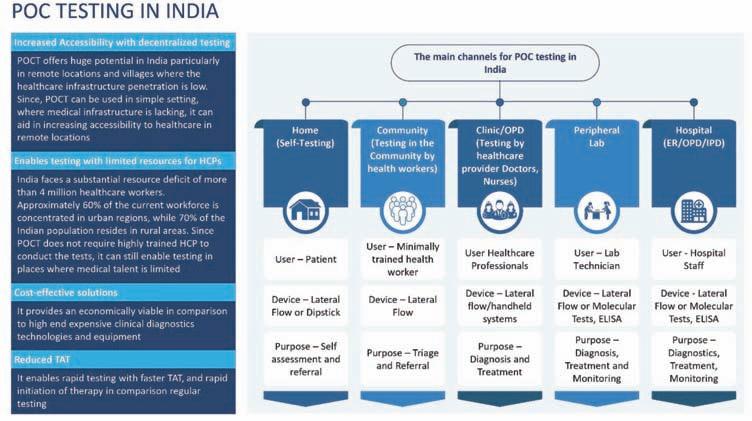
In recent years, the healthcare landscape in India has witnessed a paradigm shift towards more accessible, efficient, and patient-centricdiagnosticsolutions. Among these advancements, Point-of-Care (POC)diagnosticsstands out as a transformative technology with the potential to revolutionise healthcare delivery across the country.
Current state of POCdiagnosticsin India
Markets and Markets report says, “The size of global point of care diagnostics market in terms of revenue was estimated to be worth $49.7 billion in 2023 and is pois to reach $77.8 billion by 2028, granting at a CAGR of 9.4 per cent from 2023 to 2028. The key factors driving market growth include the rising incidence of infectious diseases such as influenza, HIV, tuberculosis, moreover, favourable government policies promoting the adoption of POC testing, rising CLIA-waived point of care tests, and shift towards healthcare decentralisation are some of the major factors contributing towards the growth of market during the forecast period.”
“However, the rising pricing pressure on these devices due to reimbursement cuts, and the stringent regulatory approval processes for commercialising POC products is expected to restrain market growth during the forecast period. Point of Care Diagnostics Market.”
Talking about the current state of POC diagnostic market, Pritam Kumawat, Founder and CEO, Sanskritech Smart Solutions said, “The current state of POC diagnostics in India reflects a promising landscape marked by increasing adoption of new technologies. There is a noticeable shift towards POC testing, especially in remote and underserved areas. The demand for rapid and on-the-spot diagnostics is driving the increased adoption of Point-of-Care Testing (POCT).”
Venkata Suman Cherukuri, MD and Chairman, TRUSTlab Diagnostics also shares, “The current state of POC diagnostics




The current state POC diagnostics in India reflects a promising landscape marked by increasing adoption of new technologies
Pritam Kumawat Founder and CEO,Sanskritech Smart Solutions
POC diagnostics have gained traction due to their ability to provide rapid, accurate test results at or near the site of patient care
Venkata Suman Cherukuri MD and Chairman,TRUSTlab Diagnostics
By conducting prompt and accurate diagnostic procedures at the point of care,POC diagnostics eliminate the need for patients to travel from remote locations to centralised facilities, thus enhancing healthcare accessibility,particularly in rural and underserved areas
Dr Nilesh Shah
President & Chief of Science & Innovation, Metropolis Healthcare
While the use of POCs has gained momentum in India,the country provides a huge market potential for POC diagnostic devices,particularly in the future,particularly in the case of non-communicable and infectious diseases
Dr Sohini Sengupta
Medical Laboratory Director,Redcliffe Labs and member of POCTCommittee of the International Federation of Clinical Chemistry & Laboratory Medicine (IFCC)
in India reflects a growing adoption of this technology, particularly in addressing the challenges of accessibility and timely healthcare delivery. POC diagnostics have gained traction due to their ability to provide rapid, accurate test results at or near the site of patient care. This is particularly crucial in India, where remote and underserved communities often face barriers to accessing centralised healthcare facilities. While progress has been made in deploying POC tests for diseases like HIV, malaria, and tuberculosis, challenges such as infrastructure limitations, regulatory hurdles, and ensuring quality assurance remain areas of focus for further development and expansion of POC diagnostics in India.”
Dr Nilesh Shah, President & Chief of Science & Innovation, Metropolis Healthcare opines that one of the remarkable aspects of POC diagnostics is its ability to transcend geographical barriers. It enables testing in locations where traditional lab setups are impractical or unavailable, ensuring broader access to diagnostic services. This ease of adoption has fueled a surge in demand for POC testing within the diagnostic industry, making it an increasingly pivotal field today. In this transformation, POC diagnostics play a crucial role as key facilitators. By bringing testing closer to patients, they reduce turnaround times and enhance patient outcomes, thus reshaping the landscape of healthcare delivery.
Sharing some stats, Dr Sohini Sengupta, Medical Laboratory Director, Redcliffe Labs and member of POCT Committee of the International Federation of Clinical Chemistry & Laboratory Medicine (IFCC) emphasises, “With its huge population, India has a resource gap of over 4 million health workers. About 60 per cent of the existing personnel work in urban areas, whereas 70 per cent of the Indian population lives in rural areas. This scenario makes accessibility of diagnostics challenging in resource-limited rural areas. This is where POCT can help India by reducing the burden on
EXPRESS HEALTHCARE June 2024 14 cover )
the medical ecosystem. With a rapid increase, the POC diagnostics market was estimated to advance at a compound annual growth rate (CAGR) of 9.3 per cent from 2013 to 2018 and is expected to grow at 11.9 per cent CAGR during 2018–2023 worldwide. While the use of POCs has gained momentum in India, the country provides a huge market potential for POC diagnostic devices, particularly in the future, particularly in the case of noncommunicable and infectious diseases.”
“If we talk about sales, the fiscal year 2020-21 has been a boom so far. People now have increased awareness, and the market is expected to grow by a CAGR of 17.3 per cent between 2022 and 2027, which will bring the evaluation of the POCT market in India closer to approximately 11,000 crores.”
Significance of POC diagnostics in improving healthcare access
The significance of POC diagnostics can be understood through various dimensions including accessibility and convenience, rapid results and early intervention, cost-effectiveness, empowerment of healthcare providers, support for national health initiatives and integration with digital health solutions.
Through POC diagnostics, testing is done at the patient’s location such as a remote village, community health center or even a patient’s home. By reducing the distance that patients have to travel for medical assistance, POC diagnostics can greatly improve access to healthcare.
Minimising expensive laboratory infrastructure and reducing the number of hospital visits are the ways through which POC diagnostics help to cut down healthcare costs. This is very useful for low-income populations who may refuse to go for necessary tests due to financial problems. Furthermore, early detection and treatment of diseases can avert complications hence decreasing long term health care expenses.




Given the scarcity of healthcare professionals relative to the growing population,the development of POCT devices is paramount
Deepak Sahni Founder, Healthians
POCTdevices are becoming smaller and more precise thanks to technological advancements including better instrumentation and the downsising of electronics
Dr Nanda Kachare
Head of Department of Pathology & Laboratory Medicine, Jupiter Hospital,Pune
Sharing his views on this, Dr Shah said, “The significant increase or improvement in access is most noticeable in tier 3, tier 4, or rural areas when utilising POC diagnostics. By conducting prompt and accurate diagnostic procedures at the point of care, POC diagnostics eliminate the need for patients to travel from remote locations to centralised facilities, thus enhancing healthcare accessibility, particularly in rural and underserved areas.
For instance, in Gujarat, where numerous pockets exhibit a high prevalence of sickle cell anemia, POC diagnostics have facilitated the screening of over a crore population. This achievement, which would have been unfeasible with traditional lab tests, underscores the reliability and utility of POC diagnostics for mass screening initiatives.”
Advancements in biosensors and bioanalytical techniques are revolutionising POCTdevices,promising improved accuracy,and reliability in diagnostics
Dr Divya Kant Consultant –Radiologist, Asian Hospital Faridabad
The regulatory framework is a little complex and can cause delays in approvals.However,with the rise in the demand and more awareness processes are getting established
Dr Harpreet Kaur
Senior Consultant & HOD-Lab Services & Blood Centre, Aakash Healthcare,New Delhi
Rapid result delivery by POC diagnostics hastens decisionmaking and early intervention. This is crucial for acute ailments and communicable diseases where prompt diagnosis can remarkably improve patient outcomes, as well as halt disease propagation. For instance, rapid testing for diseases like malaria, tuberculosis, leprosy, and COVID-19 has been instrumental in managing outbreaks and giving instant care to the concerned populations.
Also, POC diagnostic tools put right into the hands of local health care providers, such as community health workers and nurses, the ability to carry out fundamental diagnostic tests. This decentralisation of diagnostic services strengthens the primary healthcare systems for wider coverage and better resource utilisation in public health programmes
On this, Deepak Sahni, Founder, Healthians shares, “Given the scarcity of healthcare professionals relative to the growing population, the development of POCT devices is paramount. These devices empower non-specialised personnel and individuals to conduct essential diagnostic tests swiftly, easing the burden on healthcare professionals and infrastructure.”
June 2024 EXPRESS HEALTHCARE 15
Latest technological innovations driving the evolution of POC diagnostics in India Technology integration enable collecting data in real-time, remote monitoring, telemedicine consultations, extending healthcare services and enabling continuous patient management. These types of innovations have great potential to improve rural healthcare delivery (e.g., portable diagnostic devices, telemedicine systems).
Dr Nanda Kachare, Head of Department of Pathology & Laboratory Medicine, Jupiter Hospital, Pune stresses, “POCT devices are becoming smaller and more precise thanks to technological advancements including better instrumentation and the downsising of electronics. To increase comfort, speed, and accuracy, some examples of state-ofthe-art POCT techniques include blood gas analysis, microneedles, urine strip tests, and microfluidics. “
“There are several recommendations available for different subsets of POCT, such as the World Health Organization's (WHO) ASSURED recommendations. WHO has suggested that affordable, sensitive, specific, user-friendly, robust, equipment-free, and delivered (to the end user) referred to by the abbreviation ASSURED are the essential elements of an effective POCT. “
Dr Divya Kant, Consultant –Radiologist, Asian Hospital Faridabad, “Advancements in biosensors and bioanalytical techniques are revolutionising point-of-care testing (POCT) devices, promising improved accuracy, and reliability in diagnostics. These novel biosensors, offer highly sensitive and specific detection of analytes, facilitating rapid and precise diagnostics at the point of care.”
Kumawat shares that the integration of biosensors and nanotechnology has further enhanced the capabilities of POCT devices, enabling rapid and sensitive detection of biomarkers associated with various diseases. Biosensors utilise biological molecules or synthetic receptors to




For POC diagnostics to be widely and successfully adopted in the medical system of India,efforts in the areas of setting up the infrastructure, designing policies,and technology are necessary
Dr Aakaar Kapoor CEO and Lead Medical Advisor, City X- Ray & Scan Clinic
There should be norms for test validation and compatibility with existing lab-based technologies.Aligning and establishing the medical decision limits on POC requires larger comparative studies with already established lab based technologies
Dr Rajesh Bendre National Technical Head & Chief Pathologist Apollo Diagnostics,Mumbai
Collaboration among accreditation bodies,manufacturers,and healthcare professionals is essential
Dinesh Chauhan CEO, CORE Diagnostics
POC diagnostics have the potential to transform healthcare in India by providing a path to increased access, cost,and treatment quality
Manav Teli Executive Director, Lord’s Mark Industries
detect specific analytes in biological samples, offering high sensitivity and specificity. Nanotechnology-based assays leverage nanoparticles' unique properties to enhance diagnostic test performance, such as amplifying signal detection or enabling multiplexed analysis of multiple targets simultaneously. Recent advancements in the field of biosensing technology, microfluidics, and paper-based diagnostics will improve the quality and efficiency of diagnostics.
Challenges hindering the widespread adoption of POC diagnostics in India
While POC diagnostics hold great potential for improving healthcare access in India, several challenges hinder their widespread adoption.
The regulatory landscape for medical devices and diagnostics continues to change in India. Also, complicated regulatory requirements can prevent new POC diagnostics tools from getting their approval and entry into the market.
Dr Harpreet Kaur, Senior Consultant & HOD-Lab Services & Blood Centre, Aakash Healthcare, New Delhi explains, “The regulatory framework is a little complex and can cause delays in approvals. However, with the rise in the demand and more awareness processes are getting established. Streamlining regulatory processes, investing in healthcare infrastructure, and training programs, promoting quality assurance mechanisms, and exploring innovative financing mechanisms to improve affordability and access to POC testing services is the need of the hour.”
Dr Shah believes that maintaining quality assurance standards in POC diagnostics poses a significant challenge.
He said, “Variations in testing conditions and operator skills can compromise the reliability of results. However, depending on the adopted tool, the reliability level of POCT is notably high. While using POCT solely as a screening tool, concerns arise regarding false negatives, but false positives are deemed acceptable
EXPRESS HEALTHCARE June 2024 16
cover )
as they can be confirmed through further testing. This quality concern is one of the primary challenges faced.”
In many parts of India, especially rural and remote areas, there is inadequate infrastructure to support the use of advanced POC diagnostic tools. Issues such as unreliable electricity supply, lack of internet connectivity, and insufficient healthcare facilities can impede the effective deployment and use of these technologies.
According to Dr Aakaar Kapoor, CEO and Lead Medical Advisor, City X- Ray & Scan Clinic, While POC diagnostics have positively impacted healthcare, issues related to equitable access, energy sustainability, and scalability across diverse populations and geographies need to be addressed. For POC diagnostics to be widely and successfully adopted in the medical system of India, efforts in the areas of setting up the infrastructure, designing policies, and technology are necessary.
Dr Rajesh Bendre, National Technical Head & Chief Pathologist Apollo Diagnostics, Mumbai also shares, “The challenges hindering the widespread adoption of POC diagnostics in India are multifaceted and deeply rooted. Regulatory hurdles, such as lengthy approval processes and complex compliance requirements, often act as a barrier for manufacturers looking to introduce new technologies into the market. Infrastructural constraints, including inadequate healthcare facilities, limited access to electricity, and a lack of trained personnel to operate POC devices cause further issues. There should be norms for test validation and compatibility with existing lab-based technologies. Aligning and establishing the medical decision limits on POC requires larger comparative studies with already established lab based technologies.”
Effective use of POC diagnostics requires trained personnel who can operate the devices, interpret results accurately, and maintain the equipment. There is a significant gap in training

and skill development among healthcare workers, particularly in rural areas. Continuous education and training programs are necessary to ensure that healthcare providers can effectively utilise POC diagnostics?. Collaborative efforts at each level of the ecosystem is crucial to overcome the current challenges. Highlighting the role of collaboration for better quality assurance and standardisation, Dinesh Chauhan, CEO, CORE Diagnostics said, “Collaboration among accreditation bodies, manufacturers, and healthcare professionals is essential. Robust quality control mechanisms for POC devices, along with standardised protocols for calibration, maintenance, and performance evaluation, ensure accurate
and reliable results. Trust in POC diagnostics builds confidence among patients and clinicians.”
He added, “NGOs, community health workers, and educational institutions can collaborate to raise awareness about POC benefits. Emphasising early diagnosis and timely treatment through POC devices is crucial. Proper training of healthcare workers ensures effective utilisation of these technologies.”
Sahni also believes that, “Quality assurance concerns outlined by ISO 22870 underscore the necessity for rigorous standards in method performance verification, staff competency, and continuous improvement. Addressing these barriers requires collaborative efforts
from policymakers, healthcare professionals, and technology providers to ensure effective integration and utilisation of POC diagnostics in India's healthcare ecosystem. Overcoming these challenges is essential to realise the potential benefits of POC diagnostics in improving healthcare access and outcomes nationwide.”
According to Cherukuri, “Government-industry partnerships can streamline regulatory processes and incentivise innovation. Healthcare provider involvement is crucial for integrating POC technologies into existing workflows and ensuring quality assurance. Collaboration with academic institutions can drive research and development of affordable and accurate POC
devices tailored to India's needs. Additionally, partnerships with non-governmental organisations can extend POC testing to underserved communities. To capitalise on opportunities, stakeholders must invest in infrastructure, training, and public awareness campaigns. Leveraging digital platforms for telemedicine and data management can enhance accessibility and efficiency. “
Future outlookof POC diagnosticsin India
Experts believes that the future outlook for POC diagnostics in India is promising
According to Manav Teli, Executive Director, Lord’s Mark Industries, the possibilities for POC diagnostics in India are good. As healthcare costs and life expectancy increases, there will be a greater demand for accessible and affordable diagnostic options. Rising income levels and knowledge of preventative testing are likely to drive up demand, especially in underprivileged areas. Furthermore, technological breakthroughs like as AI and IoT will drive industry innovation, making POC diagnostics more precise and efficient than ever before.
Finally, POC diagnostics have the potential to transform healthcare in India by providing a path to increased access, cost, and treatment quality. By solving implementation issues and impediments and leveraging technology breakthroughs, we may realise the full promise of POC diagnostics, altering the healthcare landscape for future generations. As we welcome these advancements, let us maintain our commitment to guaranteeing fair access to healthcare for all, regardless of geography or socioeconomic background.”
POC diagnostic hold immense potential in transforming healthcare landscape in India, provided there areclear regulatory pathways, optimal infrastructure investment, and stringent quality control measures.
Kalyani.sharma@expressindia.com journokalyani@gmail.com
June 2024 EXPRESS HEALTHCARE 17
Source: FROST & SULLIVAN
PUBLIC HEALTH
HowPOC test kits could help end TB by2030
Enabling same day test and treat strategies,using WHO recommended point-of-care (POC) TB screening and diagnostic tools emerged as a key recommendation at a recent meeting of national TB programme managers from 34 high TB burden countries. Viveka Roychowdhury reports on how a combo of POC Xrays and molecular test kits,designed and made in India,is helping these countries make up for lost time in the race to end TB by 2030

Dr Kuldeep Singh Sachdeva,former Head of India’s National TB Elimination Programme (former DDG-TB) and India’s NACO (former DDG-NACO); Former South-East Asia Regional Director of International Union Against Tuberculosis and Lung Disease (The Union); and currentlyserving as President-CMO,Molbio Diagnostics moderates a panel discussion on ·"United Against TB - Accelerated Action Towards Achieving SDG on Tuberculosis”.The panellists were Prof Moses Joloba,Director,National TB Reference Laboratory,Uganda; Dr Md.Mahafuzer Rahman Sarker,Line Director,National TB Control Programme,Ministryof Health,Bangladesh; Dr YewulsewKassie,USAID Ethiopia; Tarit Chakraborty,TB survivor and leader of TB People (India); Blessina Kumar,TB survivor and CEO of Global Coalition of TB Advocates (GCTA) (virtually); Tariro Kutadza,TB survivor and founder-leader of TB People (Zimbabwe) and member, Union CommunityAdvisoryPanel (UCAP); Dr Lalaine Mortera,FHI 360,Philippines; Sumit Mitra,President (Global Sales and Marketing),Molbio Diagnostics and Shobha Shukla,founder and Executive Director,CNS
Representatives anchoring the national TB programmes of 34 high TB burden countries, spanning Asia, Africa and Latin America, met recently in Goa to discuss ways to accelerate action towards achieving the TB-related UN Sustainable Development Goals (SDGs) and ending TB by 2030.
The discussions centered around how they could accelerate progress to achieve the commitments given by political representatives at the UN High-Level Meeting (UNHLM) in 2023. Several world leaders had committed that by the 2027 UNHLM on TB, they would achieve at least 80 per cent reduction in TB incidence by 2030, 90 per cent reduction in TB deaths by 2030, and zero catastrophic costs for TB-affected families. However,
progress on these and other goals has been patchy, barring a few noteworthy projects.
The discussions and recommendations from the meeting resulted in the Goa Declaration, a joint accelerated action plan for TB elimination, which is to be submitted to WHO for consideration at the UN HighLevel Meeting (UNHLM).
One of the key recommendations was prioritising of taking WHO recommended pointof-care TB screening and diagnostic tools to the people’s doorstep, over optimising existing centralised or semi-centralised or lab-dependent tools or models which often have access barriers, result in diagnostic delays, catastrophic costs, and drop out of people from the care cascade.
Another recommendation reads: Screening everyone for
TB regardless of symptoms in high burden settings. With an alarming number of TB prevalence surveys showing that a large number of people with TB are asymptomatic, it is imperative to find all people with TB early enough by deploying evidence-based tools like ultraportable x-rays for populationwide screening (regardless of symptoms) and confirming those with presumptive TB on molecular test upfront. Linking all of them to care will help stop the infection-spread. Increasing use of mobile/ outreach clinic for population-based screening will help. Where appropriate, intensified TB screening of everyone (regardless of symptoms) should be done in private sector facilities too.
Significantly, the Declaration recommends making same
day test and treat a reality for everyone with TB including key vulnerable populations. A test which confirms TB while the patient is still in the clinic allows health authorities a better chance to treat confirmed patients, reducing chances of TB infection within the community.
Presentations and discussions over the three day meet, shared data and experiences of the use of some such systems, which were cited in the Declaration as examples that 'should be considered for a wider scale up in appropriate contexts –along with all other evidenceand science-based approaches to find, treat and p revent all TB.'
All examples featured Goabased Molbio Diagnostics' Truenat kit, a point of care (POC) micro-PCR based diagnostic
kit, designed and Made in India, used in combination with Molbio's portable digital X-ray unit, or similar POC interventions from medtech majors.
Endorsed by the WHO in July 2022, as initial tests to identify TB and detect rifampicin resistance in updated policy guidelines, Molbio's Truenat system has been deployed in India and other high TB burden countries with similar poor/low resource settings.
As Sriram Natarajan, Founder and CEO, Molbio Diagnostics explains, Molbio's Prorad ultraportable handheld digital X-ray device is being used as a mass screening, population level TB screening tool. Presumptive positive cases are then tested on Molbio's battery operated Truenat, a real time quantitative micro-PCR system, which gives test results
EXPRESS HEALTHCARE June 2024 18
within the hour, at the point of testing, which could be a remote public health centre. Thus it allows health officials to test, report via a real-time data transfer capability through SMS/E-mail/data push and start treatment of TB patients based on the results on the same day.
Globally, the company has over 10,000 installations in over 80 countries, across both public and private sectors, of which India accounts for 6000+ devices. In India, Natarajan says that the company's Truenat assay is now available at block level TB testing in some states. While Goa became the first state in the country to completely replace microscopy with upfront molecular testing from December 2020, other larger states using Truenat kits include Andhra Pradesh, Orissa, Uttarakhan, Tamil Nadu and Maharashtra.
The public health impact
The Goa Declaration lists several examples where the Truenat has helped health authorities 'reach out to the unreached'. For instance, the Prorad ultraportable x-ray and Truenat molecular test machines in a backpack of motorcycle riders are reaching difficult to access areas in Delhi and Haryana.
There are early signs that the same day test and treat strategy is paying off. As per the India TB Report 2024, the estimated incidence of TB in 2023 increased slightly to 27.8 lakh from the previous year’s

estimate of 27.4 lakh. The actual number of cases reported was 25.5 lakhs. Thus the gap between the estimated incidence and reported cases has reduced. Experts are attributing this reduction to better diagnosis, thanks to increased screening under the government’s Ni-kshay portal tracking all TB patients and the deployment of more accurate and faster POC tests like Molbio's Truenat assays.
The data from other countries also seems promising. The Declaration quotes examples from the Philippines, Uganda, Bangladesh, Nigeria, Ethiopia, Tanzania, Zambia and Zimbabwe, where the percentage of TB patients missed in previous testing rounds reduced significantly when molecular tests were used. Data has shown that as new TB case notifications rise with the deployment of POC molecular tests, more people get treated and TB treatment success rates also rise in subsequent years.
For instance, consider the Philippines, where Fujifilm ultraportable x-ray and Molbio’s Truenat are being taken from islet to islet in Bantayan municipality in pump boats, to screen, diagnose and treat people with TB. The Goa Declaration document cites that as a result, TB screening of people with presumptive TB went up from 180 in 2021 (before new tools were deployed) to 3153 in 2024; new TB case notification rose from 110 in 2021 to 341 in 2023; and TB treatment success rate was 98 per cent in 2023. Philippines national treatment success rate was 80 per cent as per WHO Global TB Report 2023. TB services have also been made an integral part of a broad range of healthcare services provided by the Bantayan Rural Health Unit, such as, HIV, NCDs, mental health, immunisation, etc.
Beyond TB
While the benefits of same day screening, testing, report-
ing of TB can fast track the End TB programme and be a game changer, Natarajan reminds us that the Truenat system has already proved its worth in health emergencies, citing its extensive use during the COVID pandemic. In fact he claims that the Truenat kit helped p revent a pandemic, thanks to the quick detection of the Nipah virus in Kerala, allowing authorities to quickly quarantine the initial few patients and prevent the spread.
Summing up, hebelieves that from a long term capex point of view, the Truenat system is a good investment of public health funding, as the system can be used for multiple diseases. States like Andhra Pradesh and Orissa are going far beyond TB and deploying the same Truenat platform to test for infectious diseases like hepatitis B, hepatitis C, and H1N1.He says the Truenat real-time PCR platform will continue to ex-
pand its basket of tests, beyond infectious diseases to non-communicable diseases such as breast cancer, sickle cell disease, diabetes, and more.
POC devices like hand-held X-ray machines and battery powered micro-PCR kits are evidence of the frugal engineering prowess of India's medtech entrepreneurs. Theirefforts have democratised access to diagnostics tools previously restricted to metros. However, the speed of their roll out in India could be faster. Better coordination and integration of different health schemes will possibly result in a larger pool of funds, as the same infrastructure can be optimally used across diseases.
(The author attended part of the United Against TB meeting in Goa on the invitation of Molbio Diagnostics)
viveka.r@expressindia.com
viveka.roy3@gmail.com

June 2024 EXPRESS HEALTHCARE 19
PUBLIC HEALTH
One Health approach keyto prevent H5N1 bird flu spillover from animals to humans
In May,Australia reported its first case of H5N1 bird flu strain.This development comes on the heels of “an enormous concern" expressed by the World Health Organisation (WHO) regarding the increasing transmission of H5N1 bird flu to various species,including humans.According to recent WHO reports,since January 2003,a total of 248 cases of human infection with avian influenza A(H5N1) virus have been reported within the Western Pacific Region,resulting in 139 fatalities.To shed more light on this pressing issue, Professor Michael Ward,Chair of Veterinary Public Health and Food Safety at The University of Sydney engages in a conversation with Neha Aathavale,recommending that early detection is key,and surveillance technologies are needed to prevent H5N1 bird flu transmission to other species INTERVIEW
What are the pathways through which the H5N1 avian flu virus can infect humans?
Generally, the pathway is considered to be aerosols and droplets via the respiratory route or conjunctiva. So close contact is required, and in the past most infections have been linked to working in chicken farms, or during the process of backyard slaughter. There is a possibility of food-borne transmission, but the evidence for that is not as strong.
Could you outline the early symptoms of avian flu in humans that should prompt immediate testing and containment measures?
The symptoms are of the flu, but might be more severe than seasonal flu. The medical practitioner would need to be asked this question (not a
veterinarian).
What specific surveillance measures should be adopted by healthcare organisations to monitor and detect avian flu outbreaks more effectively, especially in the regions with significant poultry populations?
In general, the most sensitive surveillance is close monitoring of domestic poultry flocks for disease. In the case of Highly Pathogenic Avian Influenza the signs of disease occur very quickly and are very difficult to miss. So, the issue needs to be reported by poultry owners and managers to the authorities.
Monitoring wild birds is also important, either for signs of disease (passive surveillance) or taking samples (active surveillance), either directly from captured wild birds or from their environment.

How can healthcare providers encourage and facilitate timely reporting of avian flu cases?
The presence of HPAI is much more likely to be detected in domestic poultry first. But reporting from the human medical system helps by establishing that spillover from poultry has occurred.
What emerging technologies and research areas should the healthcare sector focus on, to improve the detection, treatment and prevention of avian flu?
Early detection is key, so surveillance technologies are needed. One is the simple monitoring of the health status of a flock. In human medicine the use of antivirals and vaccines might be considered, but need to consult with a human medical expert.
How can these innovations be integrated into current healthcare practices?
On the veterinary side, daily recording of health status is needed. The first induction might be a small rise in mortality rate. Setting threshold (tolerance) levels is needed so that the authorities can be alert early.
From a public health perspective, what coordinated strategies should be in place between the healthcare sector, government and poultry farmers to mitigate the flu outbreaks? There needs to be communication-farms and animal health authorities, and between animal and human health authorities. This is called a One Health approach, which is based on the sharing of information. The response to an outbreak also requires a One Health approach in which personnel from both animal and human health work together with common resources to control an outbreak. The aims included minimising animal welfare impacts and preventing spillover events from animals to humans.
nehaaathavale75@gmail.com

EXPRESS HEALTHCARE June 2024 20
MEDTECH
INTERVIEW
From imports to innovation: The ‘Self-Reliant India’paradigm shift
Rajat Ghai ,Business Head Enterprise Accounts & Solutions,GE HealthCare South Asia in an interaction with Express Healthcare talks about dominating medtech trends in India
What have been some of the dominating trends in medtech in India?
Healthcare is at a tipping point. We are witnessing a seismic shift in the way healthcare is perceived and delivered. From technological breakthroughs making way for a more personalised, ubiquitous, and precision-led care to integration of AI and ML in every aspect of healthcare, the transformation is remarkable. Catalysed by growing awareness among patients and multiple driving factors, the need for preventive, valuebased care is making way for more innovations. This, in turn, has spurred the demand for medtech devices, propelled by many other significant drivers.
◆ Strengthening of the medtech infrastructure: Catapulted by demographic needs, increasing insurance penetration, and lifestyle changes, investments are the booming in the healthcare market both in private sector and public infrastructure.
Hospital chains are adding new hospitals while adding beds to existing ones.1
Similarly in public space, we are witnessing establishment of 16 new All India Institutes of Medical Sciences (AIIMS) across many States. There is a focus on strengthening of primary healthcare, which takes care of healthcare needs at the bottom of the pyramid. We are surely in for a decade of unprecedented growth.
◆ Leveraging technology to make healthcare affordable and accessible: The government is focussing on strengthening the domestic

Currently,most components, such as displays,sensors,and detectors used in radiology and imaging devices,are imported due to a lack of economy of scale,inverted import duty structures and limited incentives
market with policies and schemes that cater to the remotest parts of the country. In the recent years, we have seen a strong government push for developing wellness centres at the public healthcare centre (PHC) or community health centre (CHC) level to improve accessibility. Given the magnitude of healthcare needs, both Central and State Governments are inviting private players by way of Public-private partnerships (PPPs) to deliver healthcare both at district hospitals and medical colleges. At Wipro GE Healthcare, we have partnered with the States and the Centre for diagnostic Imaging PPPs, which is our area of expertise. We have had the privilege of partnering with more than 20 States and have 300+ equipment installed under PPP. PPPs may range from 16Slice CT machine in a District hospital to more advanced 3T/1.5T MRIs and 128Slice CTs in Medical colleges. We have seen PPPs evolve in care areas such as cancer care, cardiac, etc.
To make healthcare affordable, medical device companies will have to bring down the cost of the equipment through enhanced localisation. Now, when we speak about the impact of localisation, at Wipro GE Healthcare, we have established four factories with the last one under the government’s Production Linked Initiatives (PLIs). We have also significantly localized the manufacturing of products such as PET CT
scans, 16-slice and 128-slice CT machines, CathLabs, and ultrasound machines.
Localization at the component and sub-component levels will further enhance affordability.
◆ Digitisation of healthcare: India's healthcare system is experiencing a digital revolution, marked by the rising popularity of telemedicine consultations and electronic medical records (EMRs). This digitization has expanded access to healthcare, particularly in remote areas.
The government’s Ayushman Bharat Digital Missioni has further advanced the goal of enhancing accessibility and equity of health services, to ensure a continuum of care where citizens own their data. Additionally, by leveraging IT and associated technologies, it supports existing health systems through a holistic, citizen-centric approach.
At Wipro GE Healthcare, we are leveraging digitization to promote remote healthcare. For instance, we inaugurated Tele-ICU solutions, in collaboration with Medanta Group of hospitals to enhance the critical care practices. Secondly, we also recognize that intelligent technology applications can help improve efficiencies that then help to improve patient care and the lives of healthcare providers. Our vision is to be the leading innovator in precision health, elevating our customers’ ability to deliver on their mission of providing the best patient care possible.
The NMDP has laid out a promising charter for Indian medtech for 2047.
June 2024 EXPRESS HEALTHCARE 21
MEDTECH
Are there any challenges that you see in the existing system?
The National Medical Devices Policy 2023 (NMDP 2023) outlines a roadmap for the accelerated growth of the medical devices sector to achieve accessibility, universality, affordability, patient-centered quality care, preventive and promotive health, research, and innovation. This policy aims to enhance our competitiveness and self-reliance, targeting a 10-12 percent share of the global manufacturing marketii. At Wipro GE Healthcare, we are manufacturing many of the devices mentioned in the NMDP, such as the 128-slice CT machine and CathLab.
The public healthcare system primarily consists of secondary and tertiary care institutions in key cities, focusing on providing basic healthcare facilities through Primary Healthcare Centers (PHCs) in rural areas. The private sector, concentrated in metros, tier-I, and tier-II cities, provides most of the secondary, tertiary, and quaternary care institutions.
In the Interim Union Budget 2024-25, the government allocated Rs. 90,659 crore (US$ 10.93 billion) to the Ministry of Health and Family Welfare (MoHFW)iii. While this paves way for progress within the sector, for a longerterm impact, it is essential to incentivize private sector healthcare providers to purchase Made in India products, potentially through rebates or subsidies.
Now, when we speak about India’s import dependency, it is important to understand the ‘why’. Currently, most components, such as displays, sensors, and detectors used in radiology and imaging devices, are imported due to a lack of economy of scale, inverted import duty structures, and inadequate incentives. Hence, as the domestic manufacturing footprint expands, a strategic shift from assembly manufacturing to researchbased indigenous manufacturing is necessary.
A step in this direction would be to ensure a level playing field for local manufacturers.
◆ Promote locally manufactured devices in public and private procurement: Seamlessly integrating locally manufactured medical devices into public procurement and offering a 20 per cent rebate for private procurement in underserved areas will ensure market access and encourage the use of domestically produced devices.
◆ Exemption of multiple Quality Control Orders (QCOs) and Compulsory Registration Scheme (CRS) for imports: Current regulations on imports of components, accessories, and spares for medtech manufacturing add to local manufacturing costs, affecting global competitiveness. Exemptions from these multiple QCOs and CRS, which do not apply to imported medical devices, are necessary.
◆ Address predatory pricing and dumping: Implementing duty arbitrage through QCOs and CRS will combat predatory pricing and dumping by medical device companies from border countries, ensuring a fair competitive environment for domestic manufacturers.
How does GE HealthCare plan to address challenges in the Indian healthcare landscape, such as accessibility and affordability?
India’s public expenditure on healthcare reached 2.1 per cent of GDP in FY23, up from 1.6% in FY21, according to the Economic Survey 2022-23iv. Despite efforts to localize manufacturing, healthcare expenses remain a significant burden. There is a seismic shift in the way the government is addressing the infrastructural challenges with initiatives like Ayushman Bharat, the Pradhan Mantri Swasthya Suraksha Yojana and more. In fact, the hospital industry in India, accounting for 80 per cent of the total healthcare market, is
attracting huge investments. The diagnostics industry in India is currently valued at $4 Bnv. While challenges exist, it’s time to look at the opportunities that exist within the sector.
Here are a few ways we address the challenges and capitalize on the opportunities that exist within the sector:
◆ Public-Private Partnerships (PPP): PPPs have been the cornerstone of accessible quality care across geographies. Case in point— the government undertook the FDS or Free Diagnostics Scheme on PPP basis in 2015 to provide access to CT scans, basic lab tests and dialysis facilities in every district in the country. Phase 1 was to install such facilities in around 150 out of the approximately 750 districts in the country.
In Assam, we advocated and worked closely with the government as well as National Health Systems Resources Centre (NHSRC), based in New Delhi, and took a lead in Free Diagnostic Scheme implementation. A CT machine was placed at each of the 28 districts with tele-reporting solutions by Spandan Diagnostics, our healthcare partner, to provide affordable healthcare across the State. Wipro GE Healthcare has partnered with the states to establish more than 200 CT machines across 15 states along with our clinical partners largely in district hospitals but also in medical colleges.
In alignment with the General Financial Rules (GFR) 144vi, GE HealthCare ensures strict compliance in our procurement and partnership processes. GFR 144, which restricts procurement from countries sharing a land border with India unless registered with the competent authority, is critical to maintaining national security and integrity. As one of the leading medtech players in the country, we verify the origins of our equipment manufacturers and service providers to comply with these regulations. This ensures that our projects are
not only effective in delivering quality healthcare but are also secure and compliant with government standards. This compliance is integral to our operations, especially in PPP projects, to guarantee that all stakeholders adhere to the necessary guidelines, thus fostering a transparent and secure partnership environment.
◆ Incentivising domestic production: The government’s proactive steps, such as the Production Linked Incentive (PLI) scheme and the Promotion of Research & Innovation in the Pharma sector (PRIP), aim to boost domestic manufacturing and attract substantial investment in the medical devices sector.
Wipro GE Healthcare has been at the forefront of supporting the ‘Make in India’ initiative, investing heavily in manufacturing, R&D, and innovation over the last three decades. We have established four joint venture manufacturing facilities in Bangalore, including one with PSU Bharat Electronics Ltd and three with Wipro Enterprises Limited India. Our recent PLI greenfield entity, Wipro GE Medical Device Manufacturing, further strengthens this commitment. Our local content has grown from 20-25 per cent to an impressive 4045 per cent this year.
◆ Investment in R&D: We were among the first healthcare companies to start healthcare technology R&D in India over three decades back. With 1100+ filed patents and the delivery of 125+ innovations, our R&D efforts have been significant. We also established GE HealthCare’s 5G Innovation Lab in JFWTC, Bengaluru – the first for us, globally. Through a concerted effort supported by the government, GE HealthCare aims to make significant strides in improving the accessibility and affordability of healthcare in India.
The Department of Pharmaceuticals and CII recently launched Meditech Stackathon 2024 to boost the MedTech sector by
analyzing the value chain. The policy environment itself has been quite dynamic - What is your view on the recent policies?
The Department of Pharmaceuticals (DoP) and the Confederation of Indian Industry (CII) recently launched the Meditech Stackathon 2024 to boost the MedTech sector by analyzing the value chain. The Stackathon segregated the sector into eight categories: imaging devices, critical care, cancer therapy, assistive medical devices, body implants, surgical & hospital equipment, consumables & disposables, and IVD diagnostics & reagents. This structured approach is the first of its kind and aims to foster collaboration among competing companies to collectively map and analyse the value chain of specialized technology platforms, components, sub-component, and raw materials.
The objective of the Stackathon was to generate recommendations that will drive the growth of innovationbased, value-added local manufacturing. Wipro GE Healthcare led the imaging devices segment alongside other multinational and domestic players. This initiative will bring in immense value to bridge existing gaps, strengthen value chain analysis to PLI and researchlinked incentive schemes.
In my view, the recent policies, including the Meditech Stackathon 2024, reflect a dynamic and proactive approach to fostering growth in the MedTech sector. By creating a collaborative platform for industry stakeholders to innovate and enhance local manufacturing capabilities, these policies are setting the stage for significant advancements in healthcare technology in India. The focus on segment-wise value chain analysis and the implementation of targeted incentive schemes demonstrates a comprehensive strategy to make India a global hub for high-quality medical devices.
EXPRESS HEALTHCARE June 2024 22
DIAGNOSTICS
Unveiling the genetic complexityof neurological disorders
Dr Anup Rawool,Associate Director,Medical Genetics and Head,Scientific and Medical Affairs, MedGenome provide a comprehensive analysis of the genetic determinants,clinical manifestations,diagnostic modalities,and therapeutic implications of neurological disorders, with a focus on recent advances in the field of medical genetics
Neurological disorders represent a formidable challenge in clinical medicine, characterised by a diverse spectrum of conditions affecting the central and peripheral nervous systems. These disorders, ranging from neurodegenerative diseases like Alzheimer's and Parkinson's to rare genetic syndromes such as Huntington's disease and CADASIL, pose significant clinical and scientific dilemmas. Understanding the genetic architecture underlying these disorders is paramount for elucidating their etiology, facilitating precise diagnosis, and paving the way for targeted therapeutic interventions.
Genetic landscape and pathogenesis
Neurological disorders often arise from perturbations in genetic pathways governing neural development, function, and maintenance. While some disorders exhibit Mendelian patterns of inheritance, others involve complex interactions between genetic susceptibility factors and environmental triggers.
◆ Familial Alzheimer's disease, for instance, is characterized by mutations in genes encoding proteins involved in amyloid processing, such as presenilin-1 (PSEN1), presenilin-2 (PSEN2), and amyloid precursor protein (APP).
◆ Similarly, Huntington's disease is caused by an expansion of CAG repeats within the huntingtin (HTT) gene, leading to aberrant protein aggregation and neurotoxicity.
◆ While multiple genes are linked to Parkinson’s Disease,

its genetics are complex. Genetic testing, still in the research stages, is available for some rare types.
◆ The genetic neurological condition for CADASIL is characterized by migraines, stroke-like episodes, cognitive issues, and dementia, and is linked to the NOTCH3 gene. Understanding the molecular mechanisms underlying these disorders provides crucial insights into their pathogenesis and informs therapeutic strategies aimed at modulating disease progression.
Genetic determinants and clinical manifestations
The clinical manifestations of neurological disorders often reflect the underlying genetic defects and the affected neural circuits. Familial forms of Alzheimer's disease typically present with early-onset cognitive decline and memory impairment, whereas Huntington's disease is characterised by chorea (Involuntary jerking or writhing movements), cognitive decline, and psychiatric symptoms. Parkinson's disease, on
the other hand, manifests with bradykinesia (slowness of movement and speed), rigidity, tremor, and postural instability, reflecting the degeneration of dopaminergic neurons in the substantia nigra. CADASIL, a hereditary cerebral small vessel disease, presents with recurrent strokes, migraine headaches, and cognitive impairment. Elucidating the genetic determinants of these disorders not only aids in accurate diagnosis but also facilitates prognostication and personalised treatment planning.
Diagnostic modalities:
From conventional to Next-Generation
Sequencing (NGS)
Advances in genetic testing technologies have revolutionised the diagnostic approach to neurological disorders, enabling precise identification of pathogenic variants and genotype-phenotype correlations. Conventional molecular genetic testing methods, such as Sanger sequencing and polymerase chain reaction (PCR), remain invaluable for targeted analysis of known disease-causing genes. However, NGS technologies, including whole exome sequencing (WES) and whole genome sequencing (WGS), have emerged as powerful tools for comprehensive genomic analysis, facilitating the discovery of novel disease-associated genes and variants. These high-throughput sequencing platforms enable simultaneous interrogation of thousands of genes, providing unprecedented insights into the genetic basis of neurological disorders and guiding personalised treat-
ment strategies.
Therapeutic implications and future directions
Despite significant advancements in our understanding of the genetic basis of neurological disorders, therapeutic options remain limited, particularly for neurodegenerative diseases. Current treatment modalities focus primarily on symptomatic management and disease modification, with few disease-specific therapies available. However, emerging therapeutic approaches, such as gene therapy, RNA-based therapies, and small molecule inhibitors, hold promise for targeted intervention and precision medicine. Additionally, ongoing research efforts aimed at unraveling the molecular mechanisms underlying these disorders and identifying novel therapeutic targets are poised to revolutionise the field of neurogenetics. By harnessing the power of genetics, molecular biology, and translational research, we can envision a future where personalised therapies tailored to individual genetic profiles transform the landscape of neurological care.
The genetic complexity of neurological disorders presents both challenges and opportunities for clinicians and researchers alike. By unraveling the intricate genetic architecture of these disorders and translating genetic insights into clinical practice, we can revolutionise diagnostic accuracy, prognostication, and therapeutic efficacy, ultimately improving outcomes for patients affected by these debilitating conditions.
June 2024 EXPRESS HEALTHCARE 23
DIAGNOSTICS
Unlocking the power of NGS for pandemic preparedness and beyond
Dr Somesh Kumar,Country Director,Jhpiego-India,Senior Director,Technical
Leadership & Innovations- US highlights the importance Next-Generation Sequencing (NGS) in disease surveillance
Disease surveillance acts as our vigilant guardian, providing us with the intelligence needed to respond with precision during pandemics and customise strategies to the distinctive traits of each outbreak. It serves as the wellspring of vital data, guiding our decisionmaking and the efficient allocation of resources, thereby bolstering our resilience against the ever-looming specter of public health threats. With the world's concerted move towards unlocking the untold potential of genomics, our surveillance capabilities take a giant leap forward, offering us the swiftness required to spot emerging diseases on the horizon. This, in turn, grants us the invaluable time needed to deploy robust response measures with agility and effectiveness. Amid the turbulent waves of the COVID-19 pandemic, the birth of the Indian SARS-CoV-2 Genomic Consortium (INSACOG) marked a transformative milestone. It swiftly expanded its network of Next-Generation Sequencing (NGS) sites across the nation, forming an omnipresent sentinel for scrutinising the genetic essence of the virus during this relentless battle. NGS, a powerful tool, unlocks the secrets hidden within a pathogen's genetic code. It unveils the origins, maps the mutations, and anticipates potential variants, offering a compass through the uncharted territory of viral evolution.
The episodes of the highly contagious Delta and Omicron variants that unfurled in 2021 and 2022 serve as compelling testaments to NGS's pivotal role. It played the role of a swift investigator, enabling timely identification and thorough understanding of these rapidly

evolving viral strains. This achievement was made possible by the extensive network of INSACOG. In the face of these unpredictable twists in the viral saga, Indian health authorities were equipped with the knowledge needed to adapt public health measures and vaccination strategies, thus charting a more effective course for safeguarding public health.
NGS is a revolutionary technology that has the potential to transform pandemic preparedness and response through its astounding capabilities of detecting the range of pathogens, sequencing the genomes of pathogens, and tracking the spread of pathogens. NGS detects and sequences a wide range of pathogens, including viruses, bacteria, fungi, and parasites, with high accuracy and sensitivity. This information can be used to identify new and emerging pathogens, including Disease X. "Disease X"
is a term used to refer to a hypothetical, unknown, or novel disease that could emerge in the future, potentially causing a global pandemic. It is a placeholder term used by health authorities and experts to highlight the unpredictability of infectious diseases. In essence, Disease X represents the idea that new diseases can appear unexpectedly, with the potential for rapid transmission and severe health impacts. By acknowledging the existence of such an unknown or unexpected disease, the global health community aims to emphasise the importance of preparedness and the need for robust response strategies to address emerging health threats. By sequencing the genomes of pathogens from different patients and locations, NGS can help scientists to identify new pathogens, track their spread, and monitor their evolution. This information can be used to develop and imple-
ment effective prevention and control measures, thus guiding the development of diagnostic tests and treatments; track pathogen spread thus facilitating public health interventions such as contact tracing and quarantine; and monitor pathogen evolution thus aiding in the development of new vaccines and treatments.
NGS can also be used to sequence the genomes of pathogens from animals and the environment. This can help to identify new pathogens that may have the potential to spill over from animals to humans or from the environment to humans. One Health is an approach to health that recognises the interconnectedness of human, animal, and environmental health. NGS is a powerful tool for supporting the One Health approach to pandemic preparedness and response by enabling the detection and surveillance of pathogens in both animals and humans.
The emergence/re-emergence of newer pathogens/variants has been associated with the increase in Antimicrobial Resistance (AMR) and Multidrug Resistant (MDR) pathogens. In India, the National Antimicrobial Resistance Research and Surveillance Network (NARS Net) is using NGS to identify and characterise AMR genes and mutations in pathogens from both human and animal sources, facilitating the tracking of AMR spread, identification of risk factors, and the formulation of strategies for AMR prevention and control.
Beyond its application in pandemic preparedness and response, NGS also has the potential to revolutionise disease detection and surveillance across the spectrum. For example, NGS can be used to identify the causative agents of
rare and emerging diseases, detect pathogens in low-abundance clinical specimens, monitor the response of pathogens to treatment, track the spread of antimicrobial resistance, and develop personalised treatment plans for patients with communicable as well as noncommunicable diseases.
While NGS is a groundbreaking advancement, several challenges must be addressed before it becomes universally accessible in public health settings. Cost remains a significant barrier, but the decreasing expense of NGS offers hope for wider adoption. Diversifying the use-cases for NGS in public health can potentially also decrease the cost for the manufacturers. Additionally, the need for trained personnel to operate and interpret NGS data underscores the importance of investing in staff training within public health laboratories.
Thus, despite these challenges, NGS is a beacon of hope in the fight against infectious diseases. Its potential to identify and track pathogens, unveil emerging variants, and monitor AMR is unparalleled. INSACOG and NARS Net's utilisation of NGS in support of a One Health approach exemplifies the crucial role this technology plays in our collective endeavor to prevent and control future pandemics.
As NGS technology continues to evolve and become more accessible, its impact on public health is poised to be profound. NGS has the potential to enable earlier and more accurate diagnosis of infectious diseases, guide the development of more effective treatments, and inform targeted public health interventions, thereby increasing the responsiveness and resilience of our health systems.
EXPRESS HEALTHCARE June 2024 24
DIAGNOSTICS
Diagnostic lab accreditation: Ensuring qualityand standardisation in healthcare
Dr Shivali Ahlawat,Director-Technical Operations,Oncquest Laboratories explains how accreditation serves as a mechanism to establish and maintain quality standards within diagnostic laboratories
70 per cent of medical decisions are based on laboratory investigations, hence their accuracy and reliability are critical for patient health. The best way to ensure that labs follow uniform, structured quality processes is for them to get accreditation based on ISO 15189 and NABL 112 standards. This ensures patient results pass through various checks, assisting in correct diagnostics and better patient outcomes. Accreditation also builds trust in customers by recognising the competence of the lab. Public awareness about lab tests, especially during the COVID pandemic, has increased, highlighting the importance of accreditation in maintaining patient safety and enhancing patient experiences.
Need and significance
Quality benchmarks are essential for affordable and high-quality healthcare services. Accreditation recognises labs' commitment to excellence and high professional standards, with the main aim of providing affordable healthcare to the last mile.
NABL (National accreditation Board for Laboratories) is a voluntary body established to ensure lab quality. To encourage smaller labs to go in for accreditation, the NABL had started Medical Entry Level Testing (MELT) Labs Program a few years ago to sensitise medical testing laboratories to quality practices and provide access to quality healthcare for the majority of citizens, even those residing in villages and small towns. In the recent years, automation is also playing a major role in accreditation, with NABL implementing online visits and geotagging to ensure lab presence and infrastructure.
Within the complex healthcare network, where accurate diagnosis is the cornerstone of ef-
fective treatment, diagnostic laboratories serve as silent heroes, unravelling the mysteries of health conditions through meticulous testing. However, in a landscape teeming with complexities and variables, ensuring the consistency and accuracy of diagnostic test results presents a formidable challenge. In response to this challenge, the concept of laboratory accreditation, offers a framework for quality assurance and standardisation in diagnostic healthcare.
Accreditation bodies must evolve to handle future requirements and meet the evolving needs of the healthcare industry.
Accreditation bodies and certifications
Several accreditation bodies oversee the accreditation process for diagnostic laboratories worldwide, including the NABL in India, which sets the standards and guidelines that laboratories must meet to achieve accreditation. The NABL, like organizations in other countries, conforms to the International standard 1S0 15189.
ISO 15189 certification focuses on various aspects of laboratory management, including quality management systems, personnel competence, equipment calibration, and customer satisfaction. Laboratories that achieve ISO 15189 certification demonstrate their dedication to providing high-quality diagnostic services while adhering to internationally recognised standards.
Challenges and opportunities
hile diagnostic lab accreditation offers numerous benefits, challenges remain in its widespread adoption, particularly in resource-limited settings. Limited financial resources, inadequate infrastructure, and a shortage of trained personnel can pose sig-

Diagnostic lab accreditation plays a crucial role in strengthening healthcare preparedness and response capabilities in the context of global health obstacles,such as infectious disease outbreaks and antimicrobial resistance
nificant barriers to accreditation for many laboratories. Moreover, the accreditation process can be time-consuming and resourceintensive, requiring dedicated efforts from laboratory staff and management.
However, despite these challenges, accreditation presents significant opportunities for im-
proving healthcare standards and patient outcomes. Governments, healthcare organisations, and international agencies can strengthen healthcare systems' resilience and capacity to respond to emerging health threats by investing in accreditation programs and supporting laboratories in meeting accreditation requirements.
Advantage to healthcare
Accreditation serves as a mechanism to establish and maintain quality standards within diagnostic laboratories. It involves a rigorous evaluation process conducted by independent accrediting bodies to assess a laboratory's compliance with predefined quality benchmarks. These benchmarks encompass various aspects of laboratory operations, including personnel competency, equipment calibration, quality control procedures, and adherence to standardised testing protocols.
Moreover, accreditation instils confidence and trust among patients and healthcare providers alike. Patients can rest assured knowing that they are receiving diagnostic services from a facility that meets internationally recognised quality standards. Healthcare providers, on the other hand, can rely on accredited laboratories to deliver reliable results, enabling them to make well-informed clinical decisions.
Addressing global health obstacles
Diagnostic lab accreditation plays a crucial role in strengthening healthcare preparedness and response capabilities in the context of global health obstacles, such as infectious disease outbreaks and antimicrobial resistance. Accredited laboratories are better equipped to detect and diagnose emerging infectious diseases, enabling early contain-
ment and mitigation efforts. Moreover, accreditation ensures the reliability and comparability of diagnostic data, facilitating surveillance, monitoring, and epidemiological investigations.
Accreditation also contributes to international collaboration and harmonisation efforts in public health. By aligning with internationally recognised standards and guidelines, accredited laboratories promote interoperability and data exchange, enhancing global health security and resilience. Furthermore, accreditation fosters trust and confidence among international partners, facilitating the sharing of resources, expertise, and best practices in disease control and prevention.
Besides, as the healthcare landscape continues to evolve, the importance of diagnostic lab accreditation cannot be overstated. By investing in accreditation programs and supporting laboratories in meeting accreditation requirements, stakeholders can strengthen healthcare systems' capacity to deliver highsuperior diagnostic services, ultimately leading to better health outcomes for individuals and communities worldwide.
Conclusion
Thus, diagnostic lab accreditation is not merely a stamp of approval but a commitment to excellence in healthcare delivery. Looking ahead, diagnostic lab accreditation must evolve to address emerging trends and challenges in healthcare, including new technologies, expanding diagnostic services, and global health threats. By embracing innovation, collaboration, and continuous improvement, accreditation can further enhance its impact on healthcare standards and patient outcomes, ultimately advancing the goal of universal access to high-quality healthcare for all.
June 2024 EXPRESS HEALTHCARE 25
STRATEGY
Hownurses can educate communities about eye health
Dr Rishi Raj Borah,Country Director,Orbis India stresses that through targeted educational initiatives,nurses can equip individuals and families with the information they need to protect their vision and prevent avoidable blindness
The World Health Organization (WHO) estimates that nearly 2.2 billion people have a vision impairment, with at least one billion having a vision impairment that could have been prevented or treated. In many developing countries, including India, a lack of awareness about eye health and limited access to preventive care contribute significantly to childhood blindness. Here, nurses, as trusted healthcare professionals, are uniquely positioned to bridge the knowledge gap and empower communities to prioritise eye health. Through targeted educational initiatives, nurses can equip individuals and families with the information they need to protect their vision and prevent avoidable blindness.
Nurses as educators: Building capacityin communities
Nurses have an added advantage in the community as they work closely with patients and connect with them differently by being in the know of things beyond a patient’s medical
record. By organising educational workshops, school screenings, and community awareness campaigns, nurses can effectively disseminate essential information on eye health. These educational programs can address topics such as the importance of regular eye checkups. Eye examinations, even for those without noticeable vision problems, are crucial for detecting and managing eye diseases at an early stage. Besides, it is equally important to educate communities about common eye conditions like cataracts, Diabetic Retinopathy, glaucoma, and refractive errors as an informed person would be better able to identify early warning signs and seek timely medical attention.
Moreover, there is still a lack of awareness on how some easy-to-follow practices can go a long way toward healthy vision. Simple practices like maintaining good hygiene specially hand hygiene, eating a balanced diet, keeping an eye on screen time, ensuring proper lighting while engaging in activities like read-
CONTRIBUTOR’S CHECKLIST
● Express Healthcare accepts editorial material for the regular columns and from pre-approved contributors/columnists.
● Express Healthcare has a strict non-tolerance policy towards plagiarism and will blacklist all authors found to have used/referred to previously published material in any form,without giving due credit in the industry-accepted format.
● As per our organisation’s guidelines,we need to keep on record a signed and dated declaration from the author that the article is authored by him/her/them, that it is his/her/their original work,and that all references have been quoted in full where necessary or due acknowledgement has been given.The declaration also needs to state that the article has not been published before and there exist no impediment to our publication.Without this declaration we cannot proceed.
● If the article/column is not an original piece of work, the author/s will bear the onus of taking permission for re-publishing in Express Healthcare.The final decision to carry such republished articles rests with the Editor.
● Express Healthcare’s prime audience is senior management and professionals in the hospital industry. Editorial material addressing this audience would be given preference.

ing and writing, and protecting eyes from UV rays can significantly reduce the risk of eye problems. Nurses can guide communities in incorporating these practices into their daily routines.
Effective communication strategies for maximum impact
For educational initiatives to be effective, nurses must tailor their communication strategies to the specific needs of the community. For instance, using the local language and incorporating culturally rele-
Nurses
have an added advantage in the community as they work closely with patients and connect with them differently by being in the know of things beyond a patient’s medical record
vant examples will resonate better with the target audience. In addition, they can make the learning process interactive by employing activities, demonstrations, role plays and visual aids to enhance engagement and knowledge retention. Besides, partnering with community leaders, educators, and other healthcare professionals can amplify the reach and impact of educational programs.
Investing in nurses: A catalyst for change Nurses are the backbone of our healthcare system, and their role in promoting eye health education is invaluable.
● The articles should cover technology and policy trends and business related discussions.
● Articles by columnists should talk about concepts or trends without being too company or product specific.
● Article length for regular columns: Between 1300 - 1500 words.These should be accompanied by diagrams,illustrations,tables and photographs, wherever relevant.
● We welcome information on new products and services introduced by your organisation for our Products sections.Related photographs and brochures must accompany the information.
● Besides the regular columns,each issue will have a special focus on a specific topic of relevance to the Indian market.You may write to the Editor for more details of the schedule.
● In e-mail communications,avoid large document attachments (above 1MB) as far as possible.
● Articles may be edited for brevity,style,relevance.
● Do specify name,designation,company name, department and e-mail address for feedback,in the article.
● We encourage authors to send a short profile of professional achievements and a recent photograph, preferably in colour,high resolution with a good contrast.
By investing in nurses through training workshops including patient communication, equipping them with educational resources, and recognising their contributions, healthcare institutions can enable them to become effective educators and advocates for eye health in their communities.
The path towards preventing childhood blindness and ensuring healthy vision for all requires a multi-pronged approach. By empowering communities with knowledge through targeted educational initiatives led by nurses, we can create a future where everyone can see the world clearly.
Email your contribution to: viveka.r@expressindia.com viveka.roy3@gmail.com Editor, Express Healthcare

EXPRESS HEALTHCARE June 2024 26



FEATURES
JMSMedi-Tapecomeswithalidtape. Betteradhesiononanypartofbody. Latex-free&Hypo-allergenicMicroporous papertape.Easytorewindandeasyto findedge.
Doesnotcomeoffeasilyevenafter wettingorbathing.























RepresentingJMSin
JMSMediTape hemantsurgical.com ForMoreInformation
Indiasince1987
+918425840446 BUSINESS AVENUES EXPRESS HEALTHCARE EXPRESS HEALTHCARE June 2024 27









































































www.indiahealth-exhibition.com
EMBASSYOFHUNGARY
Followuson: forthelatestupdates BUSINESS AVENUES EXPRESS HEALTHCARE June 2024 EXPRESS HEALTHCARE 28
























CovertheLengthand BreadthofIndia
India's Largest & No.1 Healthcare Event & Hospital needs Exhibition 234 38th Edition| AUG2024 CHENNAINEWDELHI 39th Edition| OCT2024 567 BUSINESS AVENUES EXPRESS HEALTHCARE EXPRESS HEALTHCARE June 2024 29
58,000sqmtofexhibitionspace Forenquiries:+917305789789|www.medicall.in|info@medicall.in









SILICONETRANSPARENTTUBING M.K.SiliconeProductsPvt.Ltd. fortheQualityConscious…. INDIA QM002 AnISO9001-2015COMPANY Quality Products Since 1997 CERTIFIED CLEA N ROOM IS08 BlueHeaven 208,HillViewIndustrialPremises,
BUSINESS AVENUES EXPRESS HEALTHCARE June 2024 EXPRESS HEALTHCARE 30 To Advertise in Business Avenues
AmrutNagar,Ghatkopar(W),Mumbai-400086,India. Mob.:9321965968/9869412342 E-mail:sales@mksilicone.com
Email: rajesh.bhatkal@expressindia.com rbhatkal@gmail.com



BUSINESS AVENUES EXPRESS HEALTHCARE EXPRESS HEALTHCARE June 2024 31
HEALTHCARE TRACKER
Polymed: Pioneering innovations in dialysis medical devices
Arnab
Basu Mallik,Vice President- Renal Business & Government
Affairs,Polymed explains how
the company is emerging as a leader in the dialysis medical devices sector with a suite of groundbreaking products
In recent years, the incidence of Chronic Kidney Disease (CKD) and EndStage Renal Disease (ESRD) has been on the rise in India. This alarming trend underscores the urgent need for innovative and reliable dialysis solutions to meet the growing demand for renal care. In response to this critical healthcare challenge, Polymed has stepped up to the plate, emerging as a leader in the dialysis medical devices sector with a suite of groundbreaking products.
Traditional dialysis equipment and consumables have often been imported, resulting in high costs and limited accessibility for many patients. Recognising these challenges, Polymed has committed to revolutionising the dialysis space with affordable, highquality, and locally manufactured products.
One of Polymed's most notable achievements is the development of the first BIS certified made in India Dialysis Machine. This certification not only ensures compliance with stringent quality and safety standards but also signifies a major leap forward towards delivering reliable dialysis equipment.
Most importantly, POLYMED Dialysis machine offers advanced feature like SLED (Slow long efficiency Dialysis) which opens a large horizon of therapy at economy. This feature provides accessibility to next line of dialysis care to patients.
Polymed's commitment to renal care extends beyond just dialysis machines. The company offers a comprehensive range of dialysis products designed to meet the diverse needs of patients and health-

By providing cost-effective and high-quality dialysis solutions,Polymed is helping to alleviate the burden of kidney disease on patients and the healthcare system
care providers. Polymed provides a wide variety of dialyzers, which has revolutionised the therapy delivery by offering Mid Flux & High Flux range, thereby maximising clearance, better patient outcomes & QoL.
As a comprehensive Dialysis Solution, POLYMED offers high quality disposables which includes AVF, which ensures smooth insertion, & universal Blood line, which can be used across all HD machines.
Company also provides comprehensive catheter kits with nitinol guidewires designed for ease of use and optimal performance in various dialysis procedures. These dialysis products are a testament to the com-
pany's unwavering commitment to quality and innovation. By leveraging advanced technology and adhering to rigorous manufacturing standards, Polymed ensures that its products meet the highest levels of safety and efficacy. This dedication to excellence has enabled Polymed to earn the trust of healthcare professionals and patients alike.
The introduction of Polymed's dialysis medical devices has had a significant impact on the healthcare landscape in India. By providing cost-effective and high-quality dialysis solutions, Polymed is helping to alleviate the burden of kidney disease on patients and the healthcare system. The availability of locally manufactured dialysis machines and consumables also reduces dependence on imports, fostering greater self-reliance in the healthcare sector.
As the demand for dialysis continues to grow, Polymed remains at the forefront of innovation in renal care. The company's ongoing research and development efforts are focused on enhancing existing products and exploring new solutions to further improve patient outcomes. With a strong foundation built on quality, innova tion, and patient-centric care, Polymed is poised to continue making significant contributions to the field of dialysis and beyond.
Polymed's dialysis medical devices vertical represents a beacon of hope for the millions of patients affected by kidney disease in India. Through its pioneering products and steadfast commitment to excellence, Polymed is transforming renal care and ensuring a brighter, healthier future for all.
EXPRESS HEALTHCARE June 2024 32
Beyond The Microscope : Flowcytometry in clinical settings
Vikram Patil,Deputy Product Manager – Flow Cytometry,Sysmex India Pvt.Ltd talks about the ways to address the hidden challenges when implementing flow cytometry in hospitals
In the field of modern healthcare, Flow cytometry plays a crucial role in clinical diagnosis. Flow cytometry, at its core, is a technique that allows for the measurement of multiple characteristics of individual cells or particles as they flow through a laser beam. This technology uses the principles of optics, fluid dynamics, and immunology to provide detailed insights into cellular composition.
In hemato-oncology, flow cytometry serves as an irreplaceable tool for the diagnosis and monitoring of blood disorders like leukemia and lymphoma. It enables the classification of hematological malignancies, helping clinicians tailor treatment strategies for patients. Additionally, flow cytometry plays a crucial role in assessing minimal residual disease (MRD), a factor that directly impacts the prognosis and treatment decisions for patients in remission.
Beyond hemato-oncology, flow cytometry is an essential asset in the field of transplantation. It facilitates the crossmatching of donor and recipient tissues, ensuring compatibility and minimising the risk of graft rejection.
CD4 cell counting is another application where flow cytometry is irreplaceable. In patients with HIV/AIDS, monitoring CD4 counts is essential in evaluating the progression of the disease and guiding antiretroviral therapy. Accurate CD4 cell counts are required for assessing immune status and predicting the risk of opportunistic infections, making flow cytometry an essential component of HIV care.
Flow cytometry is essen-
tial for quantifying hematopoietic stem cells, a crucial component in bone marrow and stem cell transplants, which is essential to ensure successful engraftment and reconstitution of the patient's immune system.
Extending even further, it is also used in immunology research, cancer immunotherapy development, and pharmaceutical drug discovery. I
Pain points of flow cytometry
◆ Despite the numerous benefits, this technique is not without its challenges. Firstly, the cost of equipment and reagents can be prohibitive, particularly for smaller healthcare facilities.
◆ Flow cytometers are complex instruments, and their maintenance and operation require specialised training.
◆ Interpretation of flow cytometry data requires skilled professionals who understand immunophenotyping.
◆ Inaccurate sample preparation can lead to misinterpretation of results and unreliable data. Proper handling and labeling of samples are essential to avoid false-positive or false-negative results.
Factors affecting flow cytometryassays
◆ Sample quality - contaminated or degraded samples can lead to unreliable results.
◆ Instrument calibration and standardisation are of paramount importance to ensure reproducible results. Variability in instrument performance can lead to reduced reproducibility.
◆ Choice of fluorochromes, antibodies & clones is another critical consideration. Selecting the appropriate combinations that target specific

markers of interest while minimizing spectral overlap is essential.
These challenges - high costs, the need for specialised training, experiment design-
flow cytometry continues to grow, and as we address these pain points and optimise its applications.
Sysmex’s XF-1600 Flow cytometer offers a comprehensive package that can address some of the pain points associated with flow cytometry.
◆ Its fluidic system is built on the proven XN series hematology analyzers which provides stability and precise beadles cell counting. This ensures the instrument maintains consistent fluidics performance, reducing the risk of fluctuations in results due to fluidic issues, a common pain point in flow cytometry.
◆ With a maximum acquisition speed of 50,000 cells per second, the XF-1600 stands as the fastest analyzer on the

ing, sample preparation, instrument maintenance, data interpretation skills cannot be underestimated. It is essential for healthcare institutions and research laboratories to invest in the necessary resources, training, and quality control measures to harness the full potential of this technology. The significance of
market. This speed is vital, especially when dealing with large sample populations for rare event detections. It addresses the pain point of slow data acquisition.
◆ The instrument's ability to perform an automated background check during start-up, without running any sample or QC material, reduces the
risk of errors in data analysis, which can arise when background noise is not adequately accounted for.
◆ The XF-1600 comes equipped with an in-built autoloader which reduces the manual labor and minimising the risk of human error during the loading process. The autoloader addresses one of the pain points in flow cytometry, which is sample handling efficiency.
◆ The Venturi One is a powerful data analysis software package with an ability to merge up to ten FCS files, each containing up to 5,000,000 events, is essential for applications such as minimal residual disease (MRD) detection. This allows clinicians to work with larger data files efficiently without any software lag. This addresses the pain point of investing in expensive third-party software.
◆ XF-1600's can be integrated with Sysmex’s cell washer centrifuge which automates the cell washing steps (centrifugation, decanting & resuspension of cells). This takes care of any manual errors & sample degradation due to incorrect handling.
In summary, the XF-1600 instrument model incorporates a range of features that directly address some of the key pain points associated with flow cytometry. From ensuring stability and precision in fluidics to addressing issues related to data acquisition speed, sample handling, background checks, powerful data analysis, and compatibility with other essential equipment, this instrument is designed to enhance the accuracy, efficiency, and ease of use of flow cytometry in hospitals.
June 2024 EXPRESS HEALTHCARE 33
Kegal balls and its applications
Parimita Barik,Domestic Marketing Assistant Manager,Ami polymer pvt ltd talks
about
Kegal balls
What is Kegel ball
Kegel balls are known as pelvic exercise balls. These are the Medical grade silicone rubber and alloy steel weight balls specially designed to help strengthen the pelvic floor muscles. These balls are also known for sexual pleasure and being called as orgasm balls, jiggle balls, pleasure balls etc.
Feature of Kegel balls
◆ This ball is round spherical shape and each weight ball is equipped with a silicone thread.
◆ Its made of alloy steel ball with glass filled nylon and after covering with silicone.
◆ These are reusable, BPA free and travel friendly

◆ These are available in 3 variants 38g, 44g and 62g from lower weighted to higher
Applications
◆ Kegal balls are helpful to
strengthen the pelvic floor muscle in lower abdominal area and enhances vagina health by simple kegel exercise process
◆ It helps in reducing the risk
of pelvic organ prolapse.
◆ Kegel balls are helpful for urinary control.
◆ It offers vaginal muscle exercise and improves sexual experiences for female.
◆ These kegel balls also has potential to make sex more pleasurable.
Howto use kegel balls
◆ After washing hands properly, we need to insert the balls into the vagina and then contract and relax our pelvic floor muscles to hold the balls in place. Follow 3 sets with 10 repetitions.
◆ We can do this while sitting , standing or walking but long walk is not recommended.

Author:Parimita Barik
Domestic Marketing Asst. Manager Ami polymer pvt ltd.


EXPRESS HEALTHCARE June 2024 34
TRACKER
HEALTHCARE
Ami polymer pvt ltd. 1. Food Grade Tested- FDA 21 CFR 177.2600 2. ROHS compliant 3. BPA free 4. Phthalate free 5. TSE/BSE free
Certifications provided by

Advanced.Seamless.Integrated.
Aplioaintegratesindustry-leadingimaging,advancedapplications, andsmartworkflowsintoasmall,lightweightpackage. Thesystemcanbescaledforawidevarietyofclinicalportfolios fromsharedservicestodedicated,specializedapplicationsstrengtheningyourclinicalconfidenceevenforthemost demandingcases.

©CanonMedicalSystemsCorporation2022.Allrightsreserved. ERBISENGINEERINGCOMPANYLIMITED 39SecondMainRoad,RajaAnnamalaipuram,Chennai-600028.Tel:04442961 400MailID: info@erbismedical.com
https://global.medical.canon/
REGD.WITH RNI NO.MAHENG/2007/22045,POSTAL REGD.NO.MCS/162/2022 – 24,PUBLISHED ON 8TH EVERY MONTH, POSTED ON 14TH,15TH,16TH EVERY MONTH,POSTED AT MUMBAI PATRIKA CHANNEL SORTING OFFICE,MUMBAI – 400001
















































































































































































