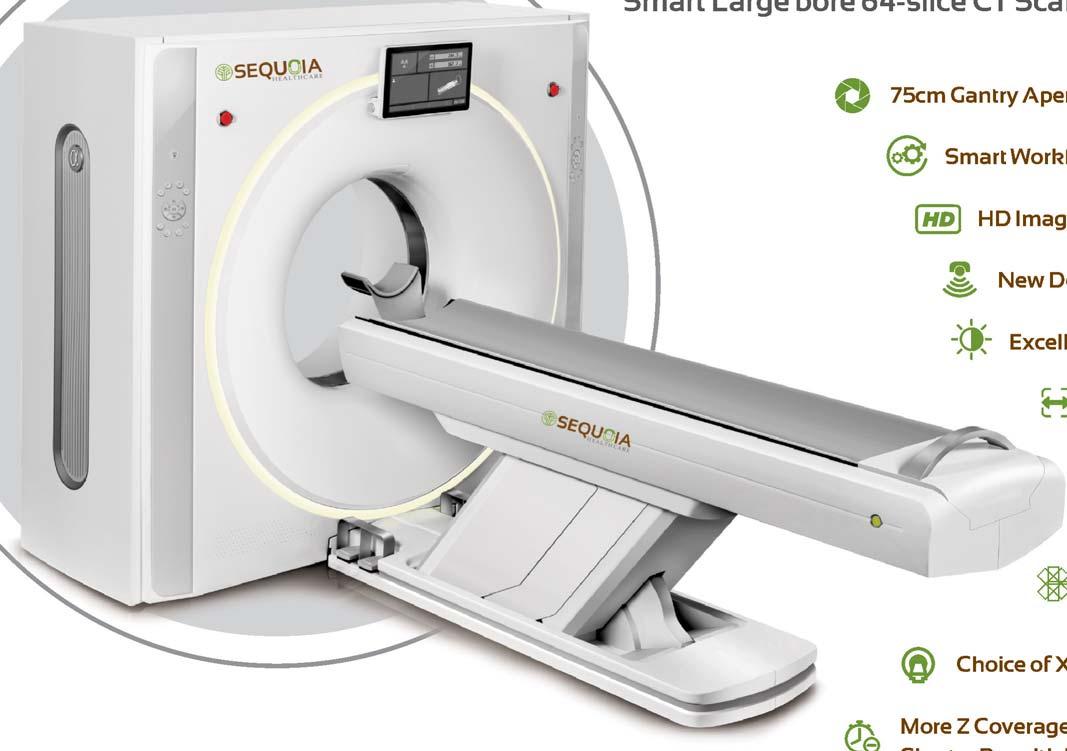
TheReliable FloorMount DigitalRadiography Solution

32/40/50kWX-RayGenerator RotatingAnodeX-RayTube

17”x17”LargeIGZOPanel MobileTableWithTransparentRadiolucentTabletop andFrontwheellocking DigitalControlPanel,FullDICOM3.0Connectivity, HIS/RIS/PACS/LaserImagerCompatible
Alsoavailablein HRAD50/40/32Series-DR2Prime &XRAD500/300SeriesDR-1Prime
ISO13485:2016CERTIFIEDCOMPANY
ISO9001:2015CERTIFIEDCOMPANY

NowPoweredby…
PremiumSeriesDetector QX PLATFORM
BPLMEDICALTECHNOLOGIESPRIVATELIMITED
Regd.Office: 11th KM,BannerghattaRoad, Arakere,Bangalore-560076,India.
TollFree:1800-4252355
Website:www.bplmedicaltechnovlogies.com
ForEnquiries:sales.medical@bpl.in
CIN:U33110KA2012PTC067282









TRIAGE
•Vitalsignsin triagedonot meetSIRScriteria
•Standardlab workiscollected andsenttolab

Temperature: 37.5ºC
Pulse:103
BloodPressure: 105/80
Respiratory Rate:19
DIAGNOSE
•Furthertesting isperformed
•Automaticreflexto additionaltestsvia REMISOLAdvance middleware*
Patientstartedon ceftriaxone,abroadspectrumantibiotic.
8:32AM
39-yearoldfemalepatientheadstothe emergencyroomwithchiefcomplaint ofpainonherrightside.
11:03AM
DxC700AUAnalyzer
DxI9000Access
Immunoassay Analyzer
ELEVATEDCRP
ELEVATEDPCT
ELEVATEDIL-6
SUSPECT
•Bloodcollected forCBC-Diff
•Urinesample collected
•Automaticreflexto additionaltestsvia REMISOLAdvance middleware*
Patientworsensand istransferredtothe intensivecareunit. Sepsissuspected.
CONFIRM
•Microbialanalysis identifiesESβL producingE.coli
•Patientswitchedto meropenem Patient’scondition improvesandshe ismovedtononcriticalcareunit.
Patientisdischarged homewithina fewdays.
9:46AM
HEMATOLOGY
DxH900Analyzer
ELEVATEDWBC
ELEVATEDMDW
URINALYSIS
DxUIrisAnalyzer
ELEVATEDWBC
ELEVATEDBacterialCount
MICROBIOLOGY
DxMMicroScanWalkAway
ID/ASTSystem
PathogenIdentifiedasan ESβL-producing E.coli
Nowisthetimeto SUSPECT, DIAGNOSE,and CONFIRM sepsis withthehelpofBeckmanCoultersolutionsinyourlaboratory.
FindoutmoreaboutBeckmanCoultersolutions forsepsispatientmanagement.
*REMISOLAdvanceisatrademarkorregisteredtrademarkofNormand-InfoSASintheUnitedStatesandothercountries.Usedunderlicense. ©2023BeckmanCoulter,Inc.Allrightsreserved.BeckmanCoulter,thestylizedlogo,andtheBeckmanCoulterproductandservicemarksmentionedherein aretrademarksorregisteredtrademarksofBeckmanCoulter,Inc.intheUnitedStatesandothercountries.ForBeckmanCoulter’sworldwideofficelocations






































MEDTECH
Chairman of the Board
ViveckGoenka
Sr.Vice President-BPD
Neil Viegas
Vice President-BPD
Harit Mohanty
Editor
Viveka Roychowdhury*
Editorial Team
Lakshmipriya Nair
Kalyani Sharma
DESIGN
Art Director
Pravin Temble
Senior Designer
Rekha Bisht
Senior Artist
Rakesh Sharma
Marketing Team
Rajesh Bhatkal

Ashish Rampure
Debnarayan Dutta
Production Co-ordinator
DhananjayNidre
Scheduling & Coordination
Pushkar Waralikar CIRCULATION
Mohan Varadkar
START-UPS
P15: INTERVIEW
Pg
COVER
P12: INTERVIEW ATULKURANI Vice President, Global Head - Medical Practice & IoT, Capgemini Engineering
23 DPDPACT,2023 AND ITS ROLE IN ELEVATING MEDICALRESEARCH IN INDIA

VEENAREDDY Mission Director, USAID/India

28 BREAKING BARRIERS TO NOVEL TREATMENT: UNRAVELING FINANCIALENIGMA FOR CANCER PATIENTS IN INDIA
P24: INTERVIEW
DR RASHMI SALUJA Executive Chairperson, Religare Enterprises

(P) Ltd.All rights reserved throughout the world. Reproduction in anymanner,electronic or otherwise,in whole or in part,without prior written permission is prohibited.

The deep criticism of the National Medical Commission Registered Medical Practitioner (Professional Conduct) Regulations, 2023, now held in abeyance, has yielded one positive: the government has started a weekly redressal/ public hearing session for grievances/suggestions/query resolution, each Thursday, from 11 am - 1 pm.
As per a public notice from Joint Secretary (Medical Education) Dr Vipul Agarwal, who also officiates as Secretary, National Medical Commission (NMC), the first session would be on September 7, with an aim to ‘’to promptly resolve the grievances of students and other stakeholders.” The notice goes on to state that while efforts will be made to resolve the issue on site, NMC will try to ensure the written response is sent within a maximum seven days. The request needs to be emailed at least two days in advance and once they receive confirmation of the date, two representatives per request can schedule a visit.
Building trust between various stakeholders is a good idea and public hearings are a good way to encourage a healthy transparent discourse. However, it remains to be seen if two hours per week will suffice.
Response to Dr Agarwal’s LinkedIn post indicates the plethora of sore points. Several students of the 1000-odd 2021 batch of medical students from medical colleges in Ukraine are caught in limbo, with the Russia-Ukraine war on one side and NMC regulations on the other. They have been pleading for transfer to other NMC-recognised countries/institutes like their peers of other batches.
Similarly, the decision of the NMC to drop three departments (respiratory medicine, physical medicine and rehabilitation, and emergency medicine) from the Minimum Standard Requirements for UG medical education is worrisome. Experts point out that post-COVID, training medical students to recognise and treat respiratory infections is even more crucial. Given that Prime Minister Modi himself is closely involved in the End TB campaign, this was a surprising move.
While the NMC’s move to drop emergency medicine was reportedly triggered by medical colleges finding it difficult to recruit faculty for this department, experts point out that expecting different departments to handle emergency medicine in rotation 24x7 is not optimal for patients or medical students. The physical medicine and rehabilitation department is important to treat the physically handicapped as well as other cases needing rehab care. All in all, these are critical departments and leaving it to medical colleges to include them or not, will deprive future generations of doctors of these vital skills.
But while medicos air their grievances, one hopes that they introspect on the issues exposed once again by the opposition to the National Medical Commission Registered Medical Practitioner (Professional Conduct) Regulations, 2023.While the rules and fines governing might be diluted in the final rules, there is no doubt that the doctor-pharma company relationship will be continue
to come under even sharper scrutiny. The debate throws up important issues, which have become flashpoints in the past. While doctors (represented by the IMA) are comparing the NMC’s push towards promotion of generics as “running trains without tracks’’, they point out that prescribing generics gives chemists/ pharmacists the power to fill/substitute their prescription with whichever pharma companies/ distributors /brands give them better margins. Moreover, they reason that it is the doctors and not pharmacists who know a particular patient’s medical history so this will impact health outcomes. This is clearly a tug of war, motivated by patient mindshare as well as monetary considerations.
There are divisions within the ranks of medicos too. The Alliance of Doctors for Ethical Healthcare (ADEH) urged the NMC to bring back the directive, with modifications, suggesting a “fresh, improved directive mandating doctors to write prescriptions in generic name only with the company name in brackets if any RMP desires to”. Once the government can guarantee the quality of all medicines, ADEH advocates dropping all brand names. It has also urged the government to ban irrational fixed dose combinations, another long standing demand.
Thus, it is clear that doctors are not immune to conflict of interests. Some of the NMC’s regulations seek to prohibit doctors and their families from accepting gifts, fully-paid trips to medical conferences, etc. While the Uniform Code for Pharmaceuticals Marketing Practices (UCPMP) is supposed to be followed by all pharma companies, it is very evident that there are many loopholes.
For instance, while some medical conferences were postponed till clarity was received on the NMC diktat, doctor associations have interpreted the proposed regulations to suit their means. For example, pharma/ diagnostic/medical device companies can route their sponsorships to associations as this would not be directly influencing individual doctors.
No one doubts that the intent behind the National Medical Commission Registered Medical Practitioner (Professional Conduct) Regulations, 2023 is sound: unbiased professional conduct of doctors.But who will maintain this across the rest of the patient’s wellness journey: chemists/pharmacists and pharma companies, hospitals and beyond?
Every regulation is only as strong as its implementation. Thus, the government will have to carefully plan the roll out any policies, and the impact on the various stakeholders like doctors, chemists, pharma companies and patients. That is Utopia. Until we reach there, patients have to stay alert, become more aware, and educate themselves on their choices.
VIVEKA ROYCHOWDHURY, Editor viveka.r@expressindia.com viveka.roy3@gmail.com
While medicos air their grievances,one hopes that they introspect on the issues exposed once again by the opposition to the National Medical Commission Registered Medical Practitioner (Professional Conduct) Regulations, 2023




What specific areas within the medtech industry is Capgemini currently focusing on in terms of innovation and intelligent solutions?
Capgemini is involved with medtech companies in their journey to leverage digital technologies to streamline operations, improve clinical outcomes, and enhance customer experiences. This could involve adopting IoT solutions, data analytics, digital health platforms, AI/ML and smart connected devices to gather valuable insights and improve decision-making.
We are involved in assisting companies in developing innovative medical devices, leveraging advanced manufacturing techniques including technologies like 3D printing, designing and implementing solutions for remote patient monitoring and telehealth services, which have gained significant importance post the COVID-19 pandemic and the growing demand for remote healthcare options.
Capgemini is also involved in assisting companies in developing innovative digital solutions like SAMD (software as a Medical Device) and digital transformation that complement or replace traditional medical treatments.
We extensively support clients in their entire product lifecycle management spanning services extending from technology refresh/migration of their legacy systems, value engineering of products for emerging markets to ongoing product sustaining involving
design optimisation, obsolescence, enhancements, compliance management, etc.
Capgemini is also involved in development of intelligent manufacturing and operations which involve the implementation of smart factory, intelligent supply chain, and insight driven operations.

Capgemini’s Intelligent Industry framework encompasses an end-to-end vision of a fully connected product lifecycle and supply chain made possible by various technology enablers. This connectivity drives new business models and sales growth, operational efficiencies, and an unprecedented ability to connect with and respond to customers and their changing needs.
Capgemini also helps medtech companies navigate the complex regulatory landscape to ensure their products meet safety, quality, and compliance standards. We provide regulatory, compliance and quality (RCQ) consulting, help build intelligent RCQ solutions and manage RCQ as a service for our clients.
Another key area of focus is the data driven solutions and AI to industrialize data driven approaches across the full R&D Value chain including decentralized clinical trial enablement and smart connected labs for medtech and pharma industry.
All these services are being delivered, cross leveraging skills from our new age technology COEs in areas like Generative AI, 5G technologies,
Capgemini is also involved in development of intelligent manufacturing and operations which involve the implementation of smart factory,intelligent supply chain,and insight driven operations
Data and Insights, Digital Twins, MBSE, etc. to bring in the appropriate efficiencies in the operations and improvement of clinical outcomes.
What emerging technologies do you see as particularly impactful for the future of manufacturing and operations in medtech?
Additive Manufacturing: 3D printing has the potential to revolutionise medtech by enabling the rapid and customised production of medical devices, implants, prosthetics, and even complex anatomical models for surgical planning. This technology can reduce production costs, lead times, and waste while allowing for intricate and patient-specific designs.

Advanced robotics and automation systems have the potential to enhance precision and efficiency in manufacturing processes.
Robotic systems can perform tasks such as assembling intricate medical devices, conducting quality control inspections, and handling hazardous materials with a high degree of accuracy.
The integration of IoT and sensor technologies into medical devices to make them smart and connected can provide real-time monitoring, data collection, and communication capabilities. This connectivity enables remote patient monitoring, digital health platforms, predictive maintenance for medical equipment, and the collection of valuable data for process optimisation.
AI and machine learning algorithms can analyse large volumes of medical data to optimise manufacturing processes, predict equipment failures, and improve the overall quality of medical devices. These technologies can also be used for image analysis, diagnostic support, and drug discovery.
Bioprinting involves the layer-by-layer assembly of living cells and biomaterials to create functional tissues and even organs. In the context of medtech, bioprinting can be used to produce custom
implants, tissue scaffolds, and other regenerative medicine solutions. Thus development of new materials, such as smart materials, biocompatible polymers, and self-healing materials, can have a substantial impact on the design functionality and manufacturing of medical



devices, implants, and wearables and on the overall manufacturing process involved.
AR/VR: The design and development of medical equipment is one of the major areas where AR/VR is making a difference. Multiple iterations and prototypes are common in
traditional design procedures, which increases the cost and adds time to the process. With AR/VR technology, designers may now create, visualise, and alter medical devices in a three-dimensional world virtually. Manufacturers can then evaluate the viability of designs, spot any potential
problems, and make the required corrections before the manufacturing process begins. Manufacturers can reorganise their processes, boost productivity, and maximise resource use with the aid of these technologies. AR allows workers to receive real-time instructions,
www.hmdhealthcare.cominfo@hmdhealthcare.com

data, and visual assistance by superimposing digital information onto physical workstations, increasing productivity and decreasing errors. Virtual reality (VR) provides life-like simulations that let manufacturers evaluate assembly procedures, spot bottlenecks, and make adjustments without affecting the production line. This ensures improved product quality, this also results in less downtime and higher output.
Nanotechnology can offer breakthroughs in areas such as drug delivery, biomaterials, and diagnostics. Nano-sized materials and devices can interact with biological systems at the cellular and molecular levels, leading to innovative medical solutions.
Personalised medicine: leveraging AI Algorithms we can design custom prosthetics or implants tailored to an individual's anatomy. By analysing medical images and other data, AI can generate designs that optimise fit and functionality for a patient, with minimal intervention from surgeons. Leveraging new age technologies like additive printing we can build such customised implants. Thus, we are seeing a shift towards small-volume batches for personalised medicines and NextGen therapies.
What are the key challenges in accelerating the pace of technology evolution in healthcare?
Regulatory and compliance needs in healthcare demand
strict adherence to safety, efficacy, and patient privacy, making the integration of new technologies into clinical practice a lengthy and expensive process. Alongside this, there are major concerns regarding Data privacy and security, especially with sensitive patient data involved, necessitating compliance with acts like HIPAA (Health Insurance Portability and Accountability Act). Additionally, the healthcare ecosystem faces challenges in interoperability due to its diverse range of systems, platforms, and devices. Inconsistent standards and protocols make integration challenging, while cost considerations, especially for smaller organisations, and unclear reimbursement models deter the adoption of innovative technologies.
Moreover, the introduction of technologies such as AI, genetic testing, and telemedicine bring forth ethical and legal dilemmas around patient autonomy, consent, and equitable access, coupled with concerns over job displacement and biased algorithms. Clinical Validation demands robust evidence, which means conducting rigorous trials, which can be resource intensive. The Lengthy Cycle of Developments, particularly in medical devices and pharmaceuticals, further adds to the time and effort.
Addressing these challenges requires a collaborative effort involving
healthcare providers, technology developers, policymakers, regulatory agencies, patients, and other stakeholders. Therefore, it's important to strike a balance between innovation and the careful consideration of the potential risks and benefits associated with the adoption of new technologies in healthcare.
In your opinion, what are the most exciting opportunities for technology-driven transformation in the healthcare sector in the coming years?

Telemedicine and remote care have expanded to offer services to patients everywhere, facilitated by video consultations, remote monitoring devices, and digital health platforms. This allows for timely care and monitoring in the home environment. Simultaneously, AI and Machine learning algorithms, particularly Generative AI, are significantly enhancing diagnostic capabilities. They can analyse medical images, detect patterns in vast data sets, and identify diseases early. This subset of AI, which can generate new content from existing data, allows systems to adapt autonomously to new situations while ensuring safe diagnosis and treatment. Additionally, AI's role in drug discovery, through the analysis of extensive data sets to predict potential drug candidates, is streamlining the introduction of new drugs to the market. Wearable devices and sensors have emerged as
continuous health monitors, aiding in preventive care, managing chronic diseases, and facilitating early intervention. Robotics is transforming surgery, allowing for precise, less invasive procedures and shortened recovery times while VR and AR technologies provide immersive training environments for healthcare professionals, with surgeons practicing procedures virtually and students exploring medical concepts interactively.
Digital therapeutics, as software-based interventions, address conditions like diabetes and mental health using interactive platforms. 3D bioprinting, with the potential to create functional tissues and organs, promises to address the donor organ shortage.
Also, advances in precision medicine, fueled by genomics, molecular profiling, and data analytics, make healthcare more personalised, targeting treatments to an individual's genetics, lifestyle, and history. Neurotechnology and braincomputer interfaces hold potential for treating neurological disorders and cognitive impairments by facilitating communication between the brain and external devices. Finally, nanotechnology's breakthroughs, from drug delivery to diagnostics, are revolutionising medicine by interacting with biological systems on a cellular and molecular scale.
Can you provide examples of
where Capgemini's solutions have improved operational efficiency and quality control in medtech manufacturing?
Capgemini collaborates with medtech manufacturers to implement smart factory solutions by deploying sensors and IoT devices while building data analytics platforms on the manufacturing floor. This realtime data collection facilitates analysis that helps in identifying inefficiencies, predicting maintenance, and streamlining production workflows. Additionally, Capgemini aids in enhancing a medtech company's quality management system (QMS) by digitising processes, ensuring regulatory adherence, and enhancing traceability, especially aligning with new EU MDR requirements. Capgemini also assists medtech manufacturers in creating digital twins of manufacturing processes and equipment. By simulating various scenarios, the manufacturer can optimize operations, test new production strategies, and identify bottlenecks before implementing changes on the factory floor. Other than that, Capgemini supports medtech companies to integrate automation and robotics solutions into their manufacturing processes. This can lead to reduced manual labor, improved precision, and higher consistency in product quality.
Kalyani.sharma@expressindia.com journokalyani@gmail.com
Recognising the gaps in health systems,Sustainable Access to Market and Resources for Innovative Delivery of Healthcare (SAMRIDH),a blended finance (BF) facility,supported by USAID and implemented by IPE Global,was initiated amidst the pandemic. Veena Reddy, Mission Director,USAID/India and Himanshu Sikka, Project Director,SAMRIDH and Chief Strategy and Diversification Officer,IPE Global in an interaction with Kalyani Sharma talks about this facility and its mission
What prompted the creation of SAMRIDH and what is its underlying purpose and mission?
Reddy: When the COVID-19 pandemic hit, there was an urgent need for healthcare solutions in the hardest-hit, and hard-to-reach communities. That’s where the SAMRIDH Blended Finance Facility was born. The U.S. Agency for International Development (USAID) worked with public and private sector partners across India to build sustainable and responsive solutions. By increasing researchers’ and incubators’ access to markets and funding streams, SAMRIDH helped to scale their life-saving initiatives. SAMRIDH is a story of collaboration and innovation in the face of adversity, and the solutions developed here in India have the potential to serve as best practices across the world. We’re proud to say that SAMRIDH innovations have already reached millions, and are ready to be taken globally.
Why did USAID choose to partner with IPE Global for this initiative? Please explain the organisation’s role under this initiative.
Reddy: When we recognised the scope of the challenges posed by the pandemic, IPE Global stood out as an ideal partner, with the experience and expertise to create a sustainable environment for health innovation and to scale up solutions to maximise impact across India. Together,
we engaged India’s dynamic start-up ecosystem and collaborated with stakeholders from the government, academia, and the private sector to develop SAMRIDH.
What is blended financing and how does it help in the last mile reach of affordable and quality healthcare?
Reddy: Blended financing combines public and philanthropic funds with commercial capital to fund innovative solutions at all stages of the process, from program design to implementation at a widespread scale. As the needs of any single entrepreneur will vary, using blended finance tailors financing or support to match individual needs.
Through SAMRIDH, we were able to rapidly support and scale innovative healthcare solutions, reaching India’s most vulnerable populations during the COVID-19
pandemic, including poor and tribal areas, tier 2 and 3 cities, and urban areas that struggle to access quality healthcare facilities and services.
What are some of the interventions implemented by SAMRIDH that make sure healthcare is reaching the hard-to-reach populations?
Sikka: Through SAMRIDH, IPE Global and USAID have helped develop a range of innovative healthcare solutions that have increased access to primary healthcare for even the hardest-to-reach communities. For example, a key challenge is delivering healthcare services and devices to remote populations and populations that don’t always have access to a reliable power supply. To overcome this, we worked with partners to redevelop key diagnostic and life-supporting medical devices to be portable, battery-powered, and reasonably priced. This has revolutionised the ability to detect and address health problems early through diagnostic tools, including RT LAMP-based COVID-19 testing, genome sequencing, artificial intelligence-enabled platforms, and even a portable ECG monitor that has dramatically increased patients’ chances of survival by allowing doctors to intervene early, which has been deployed in 1,000 clinics and small hospitals to date.
How can philanthropic investment be used to
improve healthcare challenges?
Sikka: As Veena has articulated, the blended financing model helps channel private investment to healthcare enterprises with innovative and catalytic ideas.
Philanthropic investment unlocks access to private capital through financial instruments like partial risk guarantees, social success notes, interest subvention and returnable grants. This helps mitigate the risks inherent to commercial investments and incentivise healthcare enterprises by unlocking greater pools of capital.
Philanthropic capital can also help to establish or strengthen a social enterprise's credibility and reputation, which in turn can increase their eligibility for other funding opportunities.
So far, how has SAMRIDH helped strengthen the
healthcare systems in India?

Reddy: SAMRIDH has leveraged $300 million to support over 60 social entrepreneurs, and counting. The high-impact solutions they’ve developed through this support have cumulatively served more than 25 million people across India, improving access to quality and affordable healthcare services for those who need it the most.

How can SAMRIDH be sustained beyond USAID support, and what measures are you taking to ensure the same?
Sikka: As the first blended finance facility of its kind, SAMRIDH has gained momentum and recognition not just in India, but around the world. The initiative won the prestigious P3 Impact award, and was recognised by the World Economic Forum as one of the world’s top 50 COVID-19 last mile responders. And, this is just the beginning, with tremendous potential for scale. We can realise this potential by raising more grant capital for programs in India; using SAMRIDH as a model to establish similar structures in low-resource locations in Africa and Asia; and expanding the platform for use in other areas – like education and engagement on the climate crisis – where technology can expand access to life-changing resources.
Kalyani.sharma@expressindia.com
journokalyani@gmail.com

Collaborative efforts and innovation are crucial to strengthening India's diagnostic landscape for Emerging Infectious Diseases





 ByKalyani Sharma
ByKalyani Sharma
Emerging Infectious Diseases (EIDs) are those that have recently appeared in a population or are rapidly increasing in incidence or geographic range. These diseases pose a significant threat due to their unpredictable nature, potential for rapid spread, and the ability to overwhelm healthcare systems. Recent examples, such as the COVID-19 pandemic, have underscored the importance of early detection and containment.
India, with its vast population, high population density, and diverse ecosystems, is particularly vulnerable to the emergence of new infectious diseases. The country has experienced outbreaks of diseases like Nipah virus, Zika virus, and the reemergence of diseases like dengue and tuberculosis in drugresistant forms. Therefore, a robust and proactive approach to disease surveillance and diagnostics is essential.
The role of diagnostics in surveillance and early detection is pivotal in mitigating the impact of these diseases. The diagnostics industry's innovation, collaboration, and technological advancements have played a pivotal role in identifying and managing these diseases before they escalate into public health crises.
As the country continues to invest in this critical area, it can hope to better protect the health and well-being of its population and contribute to global efforts to prevent and manage EIDs.
Explaining about EIDs, Sudhanshu Srivastava, Chief Business Officer, TRUSTlab said, “According to CDC & the National Institute of Allergy and Infectious Diseases, EID can be defined as outbreak of novel infections which has not been reported in last 3 decades viz. HIV, SARS, MERS Lyme disease, E. coli, hantavirus, dengue fever, Ebola, West Nile virus, and the Nipah, Zika virus, & the latest one SARS-CoV2. Though WHO in 2007 warned about the substantial increase in instances as well as unique strains pathogens, and isolated & assorted steps were taken by individual government which not suffice the need.”
POCTdirectly impacts the overall coverage,convenience,and individual inclination to cooperate for testing, helps to achieve higher success rate of objective to provide security to entire community
Sudhanshu Srivastava Chief Business Officer, TRUSTlabThe landscape of disease detection and management has witnessed a revolutionary transformation in recent years,largely owing to the rapid progress in technology
Raghavendra Goud Vaggu Global CEO, Empe Diagnostics

Leveraging the capabilities of mass spectrometry imaging,metagenomics analysis,and advanced biomarker platforms,these tools are advancing our understanding of diseases,enabling early interventions,and guiding the development of targeted therapies
Dr Gunisha Pasricha Principal Scientist, Infectious Disease Expert, MedGenome
Stressing on the landscape of disease detection, Raghavendra Goud Vaggu, Global CEO, Empe Diagnostics said, “The landscape of disease detection and management has witnessed a revolutionary transformation in recent years, largely owing to the rapid progress in technology. From traditional methods of disease diagnosis that took days or even weeks to yield results, we have transitioned to a realm where real-time data analysis can provide insights in a matter of hours. This acceleration has proved
invaluable in the early detection of EIDs, as timely information can prevent their escalation into full-blown public health crises.” Sanjeev Bhatt, Senior Vice President, Meril Life Sciences said, “In the realm of emerging infectious diseases, India's diagnostic industry takes center stage, providing a critical shield through surveillance and early detection. Fueled by innovation, collaboration, and cutting-edge technology, this sector serves as a guardian, identifying and managing potential health threats
before they escalate into crises. Utilising state-of-the-art tools like POCT and IVDs, healthcare professionals are equipped with preemptive insights.”
“This endeavor gains further significance against the backdrop of a challenging reality: a resource gap of over 4 million health workers in the country. With nearly60 per centof existing personnel situated in urban areas and 70 per cent of our population residing in rural regions, the delivery and maintenance of healthcare facilities
become a formidable challenge in resource-limited settings. POC diagnostic devices emerge as a crucial preliminary step in clinical diagnosis, effectively alleviating the strain on our advanced medical ecosystem. As we journey forward, our unwavering commitment to the nation's health and well-being remains steadfast.”
Diagnostics form the foundation of disease surveillance and early detection. In the context of EIDs, diagnostics involve the identification of pathogens, characterisation of their genetic makeup, and the development of tests that can rapidly and accurately detect these pathogens in patients or within the environment. Diagnostics also encompass the development of tools for tracking the spread of diseases and monitoring their evolution.
On this, Animesh Roy, Chief of Healthcare Operations, Even Healthcare shares, “India, though rapidly growing, still has a long way to go before being immune to any future EIDs. Diagnostic companies are expanding fast to remote areas to make tests more accessible, but only ~40 per cent of pin codes are currently operational and capable of doing these tests. Making the existing tests available and more accessible to wider regions would be the first win against EIDs. Fair to say we are on the right track. Compared to other developing countries, India is doing far better, evidenced by our commendable efforts during the last pandemic.”
Dr Avinash Phadke, President & Mentor, Agilus Diagnostics said, “India's diagnostic industry has played a vital role in the surveillance and early detection of EIDs such as H1N1, Zika, and Nipah virus. Through nationwide screening programs, advanced molecular diagnostics, and specialised virology labs, the industry has enabled rapid containment and treatment. Collaborative efforts with global health organisations have further
strengthened India's ability to respond to outbreaks, ensuring adherence to international standards and facilitating the exchange of vital information. The integration of epidemiological data with diagnostic insights has enhanced disease monitoring and containment strategies, contributing to global health security.”
Through a combination of cutting-edge technologies, extensive networks, R&D investments, and collaborative efforts, the sector has become a vital component of the country's public health response. As India continues to face the challenges of EIDs, its diagnostic industry will undoubtedly play a crucial role in protecting the health and wellbeing of its population.
According to the Indian Brand Equity Foundation (IBEF), the Indian diagnostics market was valued at $8.4 billion in 2020, with an impressive compound annual growth rate (CAGR) of 20.4 per cent over the past decade. Dr Shivani Sharma, Vice-President of Pathology Services & Lab Director, CORE Diagnostics consider this growth as a testament to the industry's commitment to innovation and its critical role in disease surveillance.
She adds, “The impact of India's diagnostic industry extends beyond the laboratory doors. Timely and accurate diagnoses empower public health interventions that are both targeted and effective. By identifying the presence of pathogens at an early stage, authorities can implement swift containment measures, preventing the spread of diseases and saving resources. A case in point is the successful containment of the Nipah virus outbreak in Kerala in 2018. Early detection and rapid response, facilitated by diagnostic tests, played a crucial role in preventing a widespread epidemic.”

Techniques like POCT and IVDs play pivotal roles in tackling EIDs by enabling rapid and
Investments in research and development,strengthening healthcare infrastructure,and promoting public-private partnerships will be crucial in enhancing India's diagnostic capabilities for EID surveillance and early detection
Dr Shivani Sharma Vice-President of Pathology Services & Lab Director, CORE Diagnostics
accurate diagnosis, surveillance, and management of these diseases. Their contributions are especially crucial in the context of EIDs, which demand swift and effective responses to prevent their spread and mitigate their impact.
POCT and IVDs are indispensable tools in the battle against EIDs. Their combined capabilities for rapid diagnosis, surveillance, and monitoring are crucial in curbing the spread of EIDs, protecting public health, and guiding effective response
strategies. As the field of diagnostics continues to advance, these technologies will play increasingly vital roles in safeguarding global health security.
Srivastava highlights, “POCT directly impacts the overall coverage, convenience, and individual inclination to cooperate for testing, helps to achieve higher success rate of objective to provide security to entire community, Population. IVD is the term used for techniques & methods deployed for early detection by PCR, & Rapid Antigen &

surveillance via antibody mapping.”
POCT devices are designed to provide diagnostic results quickly, often within minutes, at or near the patient's location. This rapid turnaround time is invaluable for EID detection, as it allows healthcare professionals to promptly identify infected individuals and initiate appropriate treatment or isolation measures.
POCT can be deployed in a wide range of settings, including remote or resource-limited
areas, where access to central laboratories is limited. This capability is critical for early detection and surveillance of EIDs, as it ensures that even underserved populations can be quickly tested and monitored.
Traditional IVDs, used in centralised laboratories, also offer accurate diagnosis but may have longer turnaround times compared to POCT. However, they remain essential for confirming diagnoses, conducting in-depth analysis, and monitoring disease progression.
Roy shares that POCT is a good weapon against EIDs. He said, “since they require minimal investment and effort. POCT can massively help in segregating cases, thereby reducing loads on medical institutions. However, its availability poses a concern. That said, POCTs are generally developed later than IVDs for an emerging pathogen. Therefore, their role in “reducing an EID” is less preventive and more detective.”
Technological advancements are playing a significant role in driving the early detection of EIDs in India. These advancements have improved the speed, accuracy, and accessibility of diagnostic tools, allowing for more rapid and effective responses to disease outbreaks.
Experts talks about some key technological advancements and emerging tools that are shaping early detection efforts in India.
Phadke shares, “Technological advancements such as NextGeneration Sequencing (NGS) for HIV drug resistance monitoring, AI-driven platforms for TB radiological interpretations, and genomic assays for drug-resistant Typhoid are revolutionising early detection. These technologies provide detailed insights into the nature and behavior of infectious agents, enabling targeted interventions. The integration of these advanced tools ensures a more proactive and precise approach to infectious disease
India,though rapidly growing,still has a long way to go before being immune to any future EIDs
Animesh Roy Chief of Healthcare Operations, Even Healthcare
The integration of epidemiological data with diagnostic insights has enhanced disease monitoring and containment strategies,contributing to global health security
Dr Avinash Phadke President & Mentor, Agilus Diagnostics
management, contributing to public health resilience.”
Dr Kirti Chadha, Chief Scientific Officer, Metropolis Healthcare also believes that NGS and Polymerase Chain Reaction (PCR) are emerging as powerful technologies in the surveillance and early identification of EIDs. NGS enables rapid and thorough genetic sequencing of pathogens, allowing for the identification and characterisation of new bacteria. PCR, on the other hand, allows for the highly sensitive detection of specific DNA or RNA sequences, which aids in quick pathogen identification. These techniques enable scientists to quickly identify and track EIDs, allowing them to take proactive efforts to limit their spread. Furthermore, serological techniques that detect antibodies in blood samples help to monitor immunity and estimate the incidence of EIDs. By enabling precise and prompt reactions, the integration of these new diagnostic technologies improves worldwide readiness against the threat of EIDs.
Dr Sharma considers Rapid antigen tests and multiplex PCR panels as the emerging powerful tools for EID surveillance and early detection. She added, “Rapid antigen tests offer quick results and are suitable for mass screening, while multiplex PCR panels allow simultaneous detection of multiple pathogens, saving time and resources during outbreaks.”
Talking about Cutting-edge tools, Dr Chadha said, “The substantial developments in surveillance and diagnostics that have been achieved demonstrate that the incidence of EIDs and REIDs has increased considerably over the last few decades. These developments improve the detection of outbreaks and facilitate the early implementation of response measures. Certain geographic areas such as Asia, tropical Africa, and Latin America are more likely to experience EID and REID events. EID and REID events have diverse potential to give rise to epidemics and pandemics, and their association with critical illness, adverse health outcomes, and the need
for isolation measures is variable.”
Chander Shekhar Sibal - Senior Vice President & Head of Healthcare Division,FUJIFILM India opines that by harnessing modern technologies to create sophisticated surveillance systems, India’s diagnostic industry has laid a solid foundation for a robust response to emerging infectious diseases. He added, “effective disease surveillance forms the bedrock of robust public health responses. India's diagnostic sector acknowledges this imperative and is investing in the enhancement and expansion of surveillance systems so that potential outbreaks can be swiftly identified and addressed, thereby preventing their escalation. Advancements in current technologies have given rise to novel applications like point-ofcare, direct-to-consumer testing, and over-the-counter testing. This fusion of cutting-edge technology, collaborative partnerships, and pioneering research positions India's diagnostic
Chander Shekhar Sibal Senior Vice President & Head of Healthcare Division, FUJIFILM India
sector at the forefront of the global fight against impending challenges.”
Vaggu opines, “India has recognised the potential of technological advancements in disease surveillance and the diagnostics industry in India has responded to this challenge with remarkable innovation and collaboration. Advanced molecular techniques, like PCR and gene sequencing, have revolutionised the identification of pathogens. These techniques allow scientists to quickly analyse genetic material, enabling the accurate detection and classification of infectious agents. Moreover, the integration of data science and Artificial Intelligence (AI) into disease surveillance has been a game-changer. Through the analysis of vast datasets, these technologies can identify patterns and anomalies that might not be evident to the human eye. This not only expedites the identification of potential outbreaks but also aids in predicting disease spread and severity,
enabling proactive measures.”
Dr Gunisha Pasricha, Principal Scientist, Infectious Disease Expert, MedGenome also shares, “Many innovative technologies, ranging from liquid biopsies and single-cell sequencing to AIdriven diagnostics and CRISPRbased tests, offer unprecedented insights into disease detection, biomarker discovery, and personalised treatment. Leveraging the capabilities of mass spectrometry imaging, metagenomics analysis, and advanced biomarker platforms, these tools are advancing our understanding of diseases, enabling early interventions, and guiding the development of targeted therapies.”
“One of these important diagnostic tool Liquid biopsies involves the analysis of circulating biomolecules such as DNA, RNA, and proteins found in bodily fluids like blood. They offer a minimally invasive way to detect and monitor diseases, including cancer, by identifying genetic mutations, tumour markers, and other relevant biomarkers.”
The diagnostic landscape for EIDs in India faces several significant challenges that impact the early detection, monitoring, and management of these diseases. Addressing these challenges is crucial to strengthening the country's preparedness and response to EIDs.
Limited access to advanced diagnostics, resource constraints, including shortages of skilled personnel, laboratory infrastructure, and funding, pose significant challenges. Many healthcare facilities in India lack the necessary resources to establish and maintain advanced diagnostic capabilities.
Dr Sharma opines that despite advancements, challenges persist. She added, “limited access to advanced diagnostics in rural areas, regulatory hurdles, and the need for continuous innovation are among the challenges faced by the diagnostic industry. Looking ahead, investments in research and development, strengthening healthcare infrastructure, and promoting public-private partnerships will be crucial in enhancing India's diagnostic capabilities for EID surveillance and early detection.”
Talking about the journey ahead, she said, “As the world grapples with the ever-present threat of EIDs, India's diagnostic industry stands as a beacon of hope and progress. The sector's ability to harness technology, leverage data, and drive targeted interventions has positioned it as a global leader in disease surveillance and early detection. The remarkable growth trajectory, coupled with the tangible impact on public health outcomes, underscores the industry's indispensable role in safeguarding communities,
Phadke shares, “Challenges in the diagnostic landscape include disparities in rural healthcare infrastructure affecting diseases like tuberculosis, regulatory complexities in diagnostic approvals for emerging infections, and skilled workforce shortages in specialised areas like virology. However, national

both in India and beyond.”
EIDs pose ongoing challenges that demand a strategic approach to manage spread of such diseases.
Dr Kirti Chadha Chief Scientific Officer, Metropolis Healthcare
India's diagnostic sector is investing in the enhancement and expansion of surveillance systems so that potential outbreaks can be swiftly identified and addressed,thereby preventing their escalation
initiatives and public-private partnerships are fostering innovation and collaboration to overcome these challenges. The journey ahead is promising, with continued technological advancement, collaboration, and a focus on quality and patient-centric care. The diagnostic industry's commitment to these principles ensures that we are well-positioned to face future challenges and continue to make significant contributions to global health.”
Effective data management and reporting systems are essential for monitoring disease trends and outbreaks. However, India faces challenges in data integration, real-time reporting, and data sharing among healthcare facilities and public health authorities.
Emphasising on the management of vast data, Dr Pasricha said, “Challenges encompass the management of vast data that is generated, requires robust storage capacity, and skilled resources and finances to extract meaningful insights while ensuring data protection. However, looking forward, the journey entails ad-
vancing precision medicine, leveraging population genomics for public health, harnessing AI and data science, enhancing early detection and prevention, innovating in therapeutics, and empowering patients with their genetic information. By seizing these opportunities, we can reshape healthcare with a profound understanding of genetics on health through cutting-edge diagnostics, thus charting a transformative path forward.”
Public awareness of the importance of early diagnosis and the existence of diagnostic facilities is often low, particularly in rural areas. Additionally, stigma associated with certain EIDs can deter individuals from seeking testing and treatment, hindering early detection efforts.
Collaboration between various stakeholders, including government agencies, research institutions, private diagnostic companies, and international organisations, is essential for a comprehensive response to EIDs. Ensuring effective coordination can be challenging due to bureaucratic hurdles and differing priorities.
● Express Healthcare accepts editorial material for the regular columns and from pre-approved contributors/columnists.
● Express Healthcare has a strict non-tolerance policy towards plagiarism and will blacklist all authors found to have used/referred to previously published material in any form, without giving due credit in the industryaccepted format.
● As per our organisation’s guidelines,we need to keep on record a signed and dated declaration from the author that the article is authored by him/her/them,that it is his/her/their original work,and that all references have been quoted in full where necessary or due acknowledgement has been given.The declaration also needs to state that the article has not been published before and there exist no impediment to our publication.Without this declaration we cannot proceed.
● If the article/column is not an original piece of work,the author/s will bear the onus of taking permission for re-publishing in Express Healthcare.The final decision to carry such republished articles rests with the Editor.
Dr Chadha mentions, “EIDs pose ongoing challenges that demand a strategic approach to manage spread of such diseases. The response should be based on a multifaceted approach, which integrates different disciplines and sectors, including veterinary medicine, biology, epidemiology, immunology, human medicine, public health, behavioral and communication science, anthropology, sociology, psychology, education, and others. Focusing on preventive strategies and policies, especially in developing countries where resources are limited, along with strengthening surveillance, rapid risk assessment, and risk communication are of paramount importance.”
“Newer epidemiological surveillance tools, such as artificial intelligence and wastewater surveillance, the evolution of rapid, multiplex, and easy to use diagnostics, and the prompt development and evaluation of novel therapeutics will help. In addition, speed in producing safe and effective vaccines against as during recent pandemic for the novel virus can save more lives.”
The rapid evolution of
pathogens and the emergence of new variants, as seen with COVID-19, can pose challenges for diagnostics. Ensuring that existing diagnostic tests remain effective against new strains requires ongoing research and development.
Also, deciding where and how to allocate limited resources for diagnostics during an EID outbreak can be a complex task. Balancing the need for widespread testing with resource constraints is a continuous challenge.
Talking about the collaborative nature of modern diagnostics, Vaggu said, “Public health agencies, research institutions, and private sector entities are joining forces to pool their expertise and resources. This interdisciplinary approach has facilitated the quick development of diagnostic tools, such as rapid antigen tests and point-of-care devices, which can provide results within minutes. Such tools are particularly vital in resourcelimited settings, where timely detection can make a substantial difference.”
“Furthermore, the diagnostics industry's commitment to continuous research and devel-
● Express Healthcare’s prime audience is senior management and professionals in the hospital industry.Editorial material addressing this audience would be given preference.
● The articles should cover technology and policy trends and business related discussions.
● Articles by columnists should talk about concepts or trends without being too company or product specific.
● Article length for regular columns: Between 1300 - 1500 words.These should be accompanied by diagrams,illustrations,tables and photographs,wherever relevant.
● We welcome information on new products and services introduced by your organisation for our Products sections.Related photographs and brochures must accompany the information.
● Besides the regular columns,each issue will have a special focus on a specific topic of relevance to the Indian market.You may write to the Editor for more details of the schedule.
● In e-mail communications,avoid large document attachments (above 1MB) as far as possible.
● Articles may be edited for brevity,style,
opment has led to the creation of innovative surveillance systems. These systems amalgamate data from various sources, including clinical laboratories, hospitals, and even social media, to detect early signs of disease outbreaks. By identifying unusual spikes in cases or symptoms, these systems offer an invaluable tool for early intervention. As the world grapples with the complexities of global health security, the strides made by the Indian diagnostics sector stand as a beacon of hope, ushering in an era where datadriven early detection is a formidable shield against the threat of EIDs"
Addressing these challenges requires a multi-pronged approach that involves investment in healthcare infrastructure, workforce training, quality control measures, and public health education. Collaborative efforts among government agencies, healthcare institutions, researchers, and international partners are crucial to strengthening India's diagnostic landscape for EIDs.
Kalyani.sharma@expressindia.com journokalyani@gmail.com
relevance.
● Do specify name,designation,company name, department and e-mail address for feedback,in the article.

● We encourage authors to send a short profile of professional achievements and a recent photograph,preferably in colour,high resolution with a good contrast.
Email your contribution to: viveka.r@expressindia.com viveka.roy3@gmail.com Editor, Express Healthcare
The country has faced several outbreaks,including Nipah virus,Zika virus,and the more recent COVID-19 pandemic. Ashish Gupta,Sales Head - South West Asia,Beckman Coulter Diagnostics talks about the challenges posed by emerging infectious diseases in India and discusses strategies for prevention and control
In recent decades, the world has witnessed the rapid emergence and spread of infectious diseases that pose significant threats to global health and economies. India, with its dense population, diverse ecosystems, and urbanisation, is particularly vulnerable to the emergence of new infectious diseases. The country has faced several outbreaks, including Nipah virus, Zika virus, and the more recent COVID-19 pandemic. Emerging infectious diseases (EIDs) are caused by novel or re-emerging pathogens that infect humans and other species, often leading to outbreaks or epidemics. Factors such as urbanisation, deforestation, climate change, and increased international travel facilitate the transmission of these diseases. In India, the coexistence of urban and rural populations, along with inadequate healthcare infrastructure in certain regions, creates fertile ground for the emergence and rapid spread of infectious diseases.
Notable emerging infectious diseases in India: The Nipah virus outbreak in 2018 in Kerala highlighted the potential dangers of zoonotic diseases, where pathogens are transmitted from animals to humans. In 2016, cases of Zika virus infection were reported in India. The virus is primarily transmitted by Aedes mosquitoes and can lead to birth defects in newborns if pregnant women are infected. The most significant and ongoing global health crisis, the COVID-19 pandemic caused by the novel coronavirus (SARS-CoV-2), has exposed the vulnerabilities in India's healthcare infrastructure.
India faces several challenges in effectively managing and preventing emerging infectious diseases: Lack of inadequate healthcare infrastructure, limited/ weak disease surveillance systems hinder early detection and response to outbreaks. Many emerging diseases, such as Nipah virus and avian influenza, are zoonotic in nature, making it challenging to predict and control their transmission.
India's diagnostic industry: Akeyplayer

India's diagnostic industry has evolved significantly over the years, witnessing a rapid growth in innovation and infrastructure. This industry encompasses a wide array of services, including medical laboratories, pointof-care testing, imaging, and molecular diagnostics. The industry's agility and adaptability have enabled it to respond swiftly to emerging threats. In the context of
EIDs, diagnostic tools empower healthcare professionals to swiftly detect outbreaks, formulate targeted interventions, and prevent the further spread of infections.
Surveillance through rapid testing: This speed is es-
sential in identifying and isolating individuals carrying infectious agents, thereby containing outbreaks.
Early detection saves lives: Early detection is a critical factor in minimising the impact of EIDs. India's diagnostic industry has harnessed advancements in genomics, proteomics, and bioinformatics to develop tests that detect even the subtlest signs of infection. This proactive approach allows healthcare professionals to intervene before infections spiral out of control, ultimately saving lives.
Scaling up for pandemic preparedness: The COVID19 pandemic has underscored the importance of a robust diagnostic infrastructure. India's diagnostic industry swiftly pivoted to develop and deploy tests for SARS-CoV-2, the virus responsible for COVID-19. This massive effort showcased the industry's ability to scale up production, distribute tests nationwide, and collaborate with research institutions.
Empowering healthcare professionals: Beyond technological advancements, the diagnostic industry plays a vital role in training healthcare professionals. Equipping doctors, nurses, and laboratory technicians with the skills to use diagnostic tools effectively enhances the accuracy of disease detection and the overall quality of patient care.
Emerging technologies like Liquid Biopsies, Nanotechnology, Artificial Intelligence (AI): AI-driven algorithms analyse complex medical data, from medical images to genetic sequences, to identify patterns and trends that can aid in diagnosis, risk assessment, and treatment selection.
Point-of-Care molecular tests: Advancing surveillance on the frontlines with Point-of-Care molecular tests allow healthcare professionals to detect pathogens at the site of care, speeding up diagnosis and response times during outbreaks.
Conclusion
In the ongoing battle against Emerging Infectious Diseases, these emerging diagnostic tools are proving to be invaluable assets. From rapidly diagnosing pathogens at the point of care to decoding their genetic makeup, these technologies are arming healthcare professionals with the tools they need to detect and respond to outbreaks with unprecedented speed and accuracy. As these tools continue to evolve, they hold the potential to transform our approach to EID surveillance, early detection, and ultimately, the protection of public health.
Early detection is a critical factor in minimising the impact of EIDs.India's diagnostic industry has harnessed advancements in genomics, proteomics,and bioinformatics to develop tests that detect even the subtlest signs of infection

On 11 August 2023, the President of India provided assent to the Digital Personal Data Protection, Act 2023 (also known as DPDPA or DPDP Act), paving the way for the growth of privacy and data protection measures in India. This is a landmark movement and will lead to the growth and proliferation of India’s digital economy. While numerous facets of this Act will be subjects of debate across various sectors, one of the deepest impacts will resonate within the Life Sciences and Healthcare (LSHC) industry in India.
India has a very complex healthcare history characterised by the co-existence of numerous medicine systems, where Ayurveda, Unani, Siddha, and other traditional systems of medicine co-exist with modern allopathic medicine and innovative surgical methods. Presently, India has the largest public health insurance program under Ayushman Bharat and continues to advance in key primary health indicators.
The next stage of the evolution of the Indian healthcare industry would totally depend on the drive to enhance medical research in the country. Despite notable advancements in various domains, the expansion of medical research remains a challenge. The Indian Council of Medical Research (ICMR) was established in 1911 and is one of the oldest biomedical research organisations in the World. Today, ICMR has 31 centers, and more than 800 scientists work with ICMR. Aside from a handful of government institutions, India has been lagging in the field of medical research.
One of the key reasons is the distinct genotype and phenotype found in India, necessitating research conducted within the country to identify novel therapies that take into consideration our unique parameters.
Additionally, disease prognosis
significantly depends on factors like nutrition and climate, which are unique to our country. Currently, a majority of the research takes place in Western countries, leading us to conduct bridging studies to assess the effectiveness of these therapies in an Indian context.
As per media report, in 2021, India had filed 394
patents in healthcare, making it eighth in the global patents list-the highest being the US with 1933 patents filed. China filed 792 patents in the same year.
A crucial aspect that the Act will ensure is fostering collaboration within the life sciences and healthcare ecosystem. This collaboration isn’t just for innovative therapies but is also important for reducing procedure costs. Innovative procedures, backed by research in India and our own clinical data, will contribute to cost reduction. Ultimately, this will ensure the timely accessibility of these therapies to all citizens and expand the market for healthcare services in India.
Howwill the DPDPAct help?
The Act undoubtedly presents a first step towards promoting research in India. Prior to its enactment, the guidelines governing the generation, storage, utilisation, and exchange of data were outlined in the 2000 IT Act.
The DPDP Act clearly lays down the guard rails for the treatment of personal data and with these controls in place, significant interest from the large private sector in healthcare and medical research will commence. This aligns with the establishment of the National Research Foundation, which commenced with a dedicated budget of Rs 50,000 crores in the 2021-22 budget.
In the last twenty years, substantial research has been carried out in medical devices and biomedical studies with a growth rate of 8 per cent. The Act is positioned to accelerate this advancement, providing the private sector with an ap-
propriate structure to initiate the monetisation of its data assets. The crucial steps that organisations would need to consider include:
◆ Identify and classify the various forms of clinical data within the organisation
◆ Develop a structured approach for consent management, establishing a procedure for obtaining consent during data collection, and determining strategies for utilising preexisting data
◆ Deploy mechanisms to leverage the data effectively in areas such as clinical research, training AI, and other relevant areas with the right security and privacy controls in place
◆ Maintain legitimate contracts with other data processors to ensure compliance and secure data handling
While these are some of the key highlights, it’s evident that this marks just the beginning of the data-driven research journey for the majority of life sciences and healthcare organisations.
Having said that, the DPDP Act marks only the commencement. It will be interesting to witness the clauses within the Digital India Act. The next step involves fostering a culture of research and development in the country. Moreover, establishing an ecosystem for research that includes talent, process, and technology along with governance and avenues for research monetisation is vital. Undoubtedly, the journey ahead is long, yet it begins with the first step. Despite numerous challenges, the Act has successfully become a reality, positioned to shape the future of healthcare in India.
In the last twenty years, substantial research has been carried out in medical devices and biomedical studies with a growth rate of 8 per cent.The Act is positioned to accelerate this advancement,providing the private sector with an appropriate structure to initiate the monetisation of its data assets
The Government of Arunachal Pradesh (GoAP),Sir Ganga Ram Hospital (SGRH) and Religare Enterprises sign an MoU to support the development of the state’s healthcare services. Dr Rashmi Saluja ,Executive Chairperson,Religare Enterprises in an interaction with Kalyani Sharma talks about the objective behind this MoU and partnership’s long term impact on the state

Can you elaborate on the key objectives and motivations behind the Memorandum of Understanding (MoU) signed between the Government of Arunachal Pradesh and your organisations?
As an organisation Religare through the Religare Care Foundation (RCF) is committed to the cause of healthcare, education, and sports. Our MoU with the Government of Arunachal Pradesh and Sir Ganga Ram Hospital (SGRH) is rooted in this commitment.The broad objectives encompass strengthening tertiary care facilities, addressing the healthcare skills gap, and ensuring affordable and equitable healthcare for the people of Arunachal Pradesh.
The agreement and its goals are in line with the vision of Hon'ble Prime Minister Shri Narendra Modi Ji to enhance the healthcare infrastructure in the Northeast. This endeavour will not only bolster healthcare initiatives in the region but also improve social infrastructure.
The first initiative under the MoU is to establish a "Centre of Excellence for Renal Sciences" at the Tomo Riba Institute of Health & Medical Sciences (TRIHMS). The objective is to bring advanced medical care closer to residents of the State, thereby reducing the need to
travel to other cities to avail of medical services related to renal health problems.
We are thankful to the Government of Arunachal Pradesh for choosing RCF and SGRH as esteemed partners in its ambition to develop the healthcare infrastructure and services in the State.
What do you envision as the long-term impact of this partnership on the healthcare landscape of Arunachal Pradesh?
The partnership is poised to have a transformative and lasting impact on the healthcare sector in the state. The establishment of the Centre of Excellence for Renal Sciences marks the beginning of a comprehensive healthcare transformation, potentially leading to the creation of additional specialised medical facilities. This approach will significantly increase access to advanced healthcare services for state residents, reducing disparities and financial burdens associated with seeking specialised care outside the state.
Religare has onboarded SGRH as a technical partner in the agreement. SGRH is a leading healthcare service provider in the country. The institution will play a crucial role in providing necessary advice to the relevant health agencies of the State. It will
Continued on Page 26
The COVID-19 pandemic has decisively accelerated the push towards seamless health and wellbeing of citizens, away from the clutches of fragmented and episodic healthcare. From the inception of the Universal Health Coverage (UHC) initiative Ayushman Bharat Pradhan Mantri Jan Arogya Yojana (AB-PMJAY) in 2018 to the ongoing implementation of Ayushman Bharat Digital Mission (ABDM), India has certainly made great strides towards nationwide health system transformation. However, a highly fragmented system across diverse geographies and demographies, lack of awareness of these welfare initiatives and also trust amongst citizens besides low education levels amongst the BOP segments, continuing high out of pocket expenditure and finally lack of integration between government’s various initiatives pose critical challenges towards optimising the overall impact case.
On the brighter side, the ever-expanding coverage of mobile phones and internet is fast percolating to the bottom rungs, and this holds the potential of offering disruptive solutions towards a seamless care continuum. World over, technology indeed is a major driver of such paradigm shift in healthcare delivery. However, technology even when done right needs to synergise with major policy level interventions and continuing stakeholder engagement to achieve key strategic imperatives of such health system transformation
In this context, Adam Boehler’s 4 ‘P’s i.e., ‘an empowered Patient’, ‘an accountable Provider’, ‘Paying for outcomes than for services’ (the
Payer context) and finally ‘Prevention over episodic care’ serve as foundational tenets of a comprehensive and value-based health system transformation spanning across the public and private healthcare domains. This approach can deliver progressively better health outcomes while also lowering costs to help optimise system wide efficiencies. Further, such health system transformation must also ultimately deliver on the triple aim i.e., enhanced access, affordability and of course better quality of care.
The various initiatives under the Ayushman Bharat umbrella, from transforming primary care through HWC initiatives, the PMJAY insurance scheme to cover the hospitalisation expenses for the BOP households of India and now the big push for a connected digital ecosystem through the ongoing ABDM implementation aim to deliver on these strategic objectives. Creating a strong and sustainable buy-in from all three principal stakeholders of care delivery: the patient, the provider and the payer is key. Such system wide transformation and integration rest on following pillars:
◆ Stakeholder awareness and engagement
◆ Stakeholder incentives
◆ UHC and the payor
◆ Capacity building
◆ Technology and innovation
◆ Policy and governance
Stakeholder awareness and engagement: Boosting citizen level awareness of HWCs, PMJAY and ABDM initiatives and expanding the coverage of these schemes can be achieved by identifying and engaging with more grassroot level community workers and NGO partners. Besides Pradhan Mantri Aarogya Mitras (PMAMs) and ASHAs, a host
of other non-profit organisations like the SWASTH Alliance and its partners can be roped in. Engaging with microfinance organisations and their Village Level Entrepreneurs, VLEs that are active across many rural districts can also play a pivotal role. These community workers can become the first point of contact for patients seeking healthcare services and also navigation through different levels of health system from OP to IP to post-IP journeys.
On the other hand, partners like PATH and CHAI are already working on creating nationwide microsites to boost ABDM adoption through their focus on health professionals’ outreach. Besides, multilingual technology solutions that leverage smartphone penetration to boost stakeholder awareness, education and trust can play pivotal role. Further, focused IEC campaigns leveraging district community radios and mobile phones will greatly boost technology adoption.
Stakeholder incentives: Incentivising the grassroot workers, NGOs and other partners based on schemes’ uptake and utilisation by citizens in near term can go a long way. Beyond this, strategies to incentivise health and wellness promotion and preventive care based on im-

provements in population health metrics in the long run can deliver on the shift to value based care.
From ABDM perspective, incentivising district verifiers to speedily onboard more facilities especially private ones across states and UTs can boost the Health Facility Registry, HFR uptake and health records linking. Similarly, registered health professionals like doctors and allied health professionals can be incentivised for creating more ABHAs and linking health records of their patients. Democratising the Digital Health Incentives Scheme (DHIS) can hugely boost private sector involvement for both health facilities and digital solution providers. Startups like KARKINOS are well poised to reap early benefits of DHIS and evangelise the adoption of ABDM amongst its provider networks.
UHC and the payor: The ABDM initiative holds the potential for generating massive amounts of population health data through implementing interoperability standards between health facilities and allowing data mining for research and insurance product innovation. Also, such population level health data mining will enable incumbents and startups in the insurance sector to eventually offer much cheaper health plans while cutting underwriting risks to target the pressing need-gap to cover the case of ~400 million “missing middle” who are currently neither covered by PMJAY nor can afford the prevalent high health plan premiums. The Health Claims Exchange (HCX) currently in the works will greatly enhance the accuracy and efficiencies of claim settlements very soon.
Insurtech and virtual first
comprehensive-primaryhealthcare focused startups like Clinikk especially targeting the gig/ blue-collared workers are fast disrupting this space by offering comprehensive plans that cover wellness, OP and also IP patient journeys. The availability of more affordable and holistic health plans will pave the way for policymakers to usher in mandatory health insurance for all its citizens and thus move decisively towards comprehensive UHC for the nation.
Capacity building: Capacity building that focuses on infrastructure as well as human resources training and upscaling is a key cornerstone. Over the last couple of years, the larger organized private sector providers have made considerable inroads into tier 2 and tier 3 cities and towns building and enhancing quality secondary care facilities at these places as part of their growing hub & spoke model. A governmental boost in viability gap funding for these players to further expand their hinterland outreach, as already envisaged as part of healthcare spending budgets can further boost capacity building efforts.
Parallelly, the COVID pandemic has pushed most state governments to build and operationalise teaching and tertiary referral hospitals/ medical colleges covering an ever-increasing number of districts to churn out more doctors. Further, human resources training and upscaling at the grass roots level focusing on strengthening the lower cadres of allied health professionals on the lines of LVPEI’s task shifting model holds tremendous potential. A technology boost to such endeavors by startups like Noora Health can prove to be a
Dr Arindam Basu,a physician and management consultant in public health and health technology shares his analysis on Ayushman Bharat and highlights that creating a strong and sustainable buy-in from all three principal stakeholders of care delivery: the patient,the provider and the payer is key
game-changer. Technology and innovation: Integration of various union government run public health programs like Nikshay and those for NCDs and cancers and also the PMJAY scheme into the ABDM ecosystem is gathering steam. New usecases like scan-and-share are fast changing the digital health landscape. The increasing participation of laboratory, diagnostics and pharmacies besides hospitals and nursing homes holds the promise of providing muchneeded opportunities for ABDM compliant digital health startups to test, improvise and upscale their offerings through large scale pilots leveraging the captive ABDM ecosystem users.
However, data quality and usability, ease of interoperability, APIs’ performance and data mining through anonymisation for research and analytics along with private sector engagement especially on the providers front remain difficult challenges for the orchestrators of ABDM. The much-touted UHI along the lines of UPI promises to transform healthcare service provider discoverability and ease health system navigation for end-users, patients and their families. However, the endeavor in its nascent stages currently does not provide health workers, providers, data analysts and policymakers the much-needed holistic view of patient movement through an end-to-end patient
journey. Policy and governance: Policy-making and its periodic review has become critical to keep up with the fast pace of technology permeation into and digitisation of India’s healthcare system. The ‘Clinical Establishments Act’ that has been implemented across over 20 states and UTs over the past few years is a classical case in point. The Act does not recognise the fast-expanding role of new age virtual first/ virtual only telemedicine providers as ‘clinical establishments’ that can be registered and further regulated under the purview of the Act. The same is reflected in ABDM’s ambiguity to register these entities as ‘facilities’ as part of the Health Facility
Registry. On another front, the delay in legislating on the digital personal data protection bill has impacted the ongoing digitisation of citizens’ health records due to the lack of clarity on health data protection, retention and exchange related policies.
Further making some level of NABH quality accreditation mandatory for healthcare providers across the physical and digital spectrum will go a long way in ensuring minimum care quality standards and safety for patients across states and UTs. The shifting of ‘health’ from the current States’ List to Concurrent List under the Constitution as envisaged in the Fifteenth Finance Commission’s report will further boost uniformity
across care access and quality standards and importantly an efficient oversight mechanism.
In conclusion, various ambitious welfare initiatives like the ones on UHC and a nationwide uniform digital health platform through ABDM must be accompanied with policy level evolution to achieve convergence. A 30,000-foot holistic view is much required to put together the various pieces of the ongoing health system transformation. Thinktanks and advisory services that deliver on connecting the dots through the fast-evolving phygital ecosystem can ensure various initiatives talk to each other and thus lead to a comprehensive and all-levels integrated future ready Ayushman Bharat.
Continued from Page 24
also support by providing training and sharing medical protocols with the healthcare staff of Arunachal Pradesh.
Over the long term, this partnership will work towards making Arunachal Pradesh a hub for specialised medical care in the Northeast, attracting patients from neighbouring regions and contributing to the state's economic development.
Additionally, the enhanced healthcare infrastructure could drive medical research and innovation within the state, propelling advancements in healthcare practices.
The positive spill-over effect may extend to medical education, as improved healthcare facilities often attract educators, students, and professionals, potentially fostering the growth of medical education institutions within the state.
This partnership, driven by a commitment to affordable and equitable healthcare, can potentially bring about a
comprehensive healthcare evolution, positively impacting the health and wellbeing of citizens in the region and solidifying the state's position as a leading healthcare destination.
We share a vision with the State to develop it as a hub for medical services in the region, and all our initiatives under this agreement will be for achieving this vision. Moreover, it will also make the state’s healthcare system future-ready to tackle COVIDlike pandemics.
How do you believe this collaboration will further contribute to closing the skills gap? Can you provide examples of the training and skill development programs that will be implemented to elevate the capabilities of healthcare professionals in the state?
This collaboration holds great potential to significantly contribute to closing the skills gap in the state's healthcare sector. The technical advisory support from SGRH will play a pivotal role in enhancing the
capabilities of local healthcare professionals. By sharing best practices, advanced medical knowledge, and specialized clinical techniques, this partnership can empower local doctors, nurses, and other healthcare professionals to offer a higher standard of care.
Possible examples of training and skill development programs that will be implemented under this collaboration include specialised medical workshops. These workshops could cover a range of critical areas, such as advanced surgical techniques, diagnostic procedures, and patient management protocols.
Additionally, mentorship programs led by experienced medical professionals from SGRH could provide invaluable guidance to local healthcare practitioners, helping them navigate complex cases and stay updated on the latest medical advancements.
Furthermore, capacitybuilding efforts might involve
improving the knowledge and application of cutting-edge medical equipment and technologies, ensuring that healthcare professionals in Arunachal Pradesh are wellequipped to handle diverse treatment scenarios.
What challenges do you anticipate encountering during the execution of these healthcare projects, and what strategies have been put in place to overcome them?
We don’t see any significant challenge in executing healthcare initiatives under this partnership. Our confidence is based on the strong commitment we have been witnessing right from the start of the Government of Arunachal Pradesh and its officials towards improving healthcare services in the State.
Additionally, we have a very strong technical partner in SGRH, which is an institution of global repute. The hospital has dedicated its resources, including doctors, to this work.
However, Arunachal Pradesh is a large state with a dispersed population. Providing universal access to quality healthcare will involve innovative use of technology and resources. Already the government has deployed drones for supplying medicines to remote areas in the state. This initiative can be further augmented through the use of telemedicine, which will allow quality medical advice to reach the remotest corners of the state in a costeffective way. Such interventions can be planned with the backing of the government machinery. We are confident that with the support of the government and our technical partner, we will be able to register progress. Also, quality healthcare is dependent on quality medical staff. We have identified this as a gap and are already starting work on that in the form of training and skilling.

India finds itself in the midst of a significant demographic, epidemiological, and environmental transition, exacerbating the challenge of a triple disease burden: RMNCHA+N, communicable including emerging/remerging infectious diseases and increasing noncommunicable diseases. The spectre of climate change further compounds this complexity by contributing to the morbidity and mortality.
In addressing these issues, there exists a trinity of approaches: improved services, health education, and regulatory measures. While health education remains an ideal solution, there are times when a regulatory approach becomes imperative, given the gravity of the situation.
The Government of India is making all efforts for a resilient and strengthened health care system with an aim to achieve Universal health care. The Constitution of India also charges every state for the improve ment of public health among its primary duties. However, to strengthen the health care system and achieve India’s international commitments under various instruments, a focused legislatory approach, called the ‘Public Health Legislation’, is a pre-requisite.
The imperative of legislating public health has long been recognised, evidenced by the presence of 124 direct and incidental laws related to public health. Among these, 67 Acts are administered by various ministries. There are incidental laws like, Births, Deaths and Marriages Registration Act, 1886; Epidemic Diseases Act, 1897; Indian Red Cross Society Act, 1920;
Drugs (Control) Act, 1950; and Consumer Protection Act, 1986, etc., which are not specific to any hazard or an entry point - but relevant for containment and mitigation of disease outbreaks.
The central government attempted in 1955 and 1987 to persuade states to pass legislations, based on what it called the ‘Model Public Health Act’. However, only 8 states currently have their own public health laws. Further attempts have also been made by the government to ensure good governance in welfare states however, the expected level of success and implementation could not be achieved.
Standing at the crossroad, the Government of India earnestly realised its constitutional duty of improving public health and passed the Disaster Management Act in 2005 with the primary objective of preparedness, p revention and early planning towards disaster. Although the act undoubtedly filled a huge gap in the scheme of governmental actions towards deal-
ing with disasters, it did not identify ‘disaster- prone zones. The Act portrays every disaster as a sudden occurrence and falls way short in recognizing their progressive nature. Added to that, delayed response, inappropriate implementation of plans and policies, and procedural lags plague the disaster management scheme in India.
With the International Health Regulations, 2005 coming into force in 2007 as a soft international health law, India acted proactively and drafted the National Health Bill, 2009. Though the Bill provides for protection and fulfilment of the right to health and wellbeing, health equity and justice, and a robust health care system, it entailed significant financial expenditure and was soon discontinued. Further, health being a ‘State subject’, there was limited adoption by the States of the other model laws. Also, the need for an enforceable statutory structures to ensure intersectoral convergence on social determinants of health at national, state and district levels remained unfulfilled.
The outbreak of the novel coronavirus (COVID-19) stimulated the Indian government to re-evaluate its public health strategy. The Ministry of Health & Family Welfare, Government of India, invoked the provisions of the antiquated Epidemic Diseases Act of 1897. However, it soon became evident that this archaic legislation was inadequate for comprehensive public health surveillance and response. A more robust framework was needed to address epidemics and disasters effectively. The government was compelled to
clamp the 125-year-old Epidemic Diseases Act, 1897, in March 2020 to enforce social distancing and public curfew norms in the country.
However, legislating during pandemic has a major risk of focussing only on emergency provisions, while omitting the requirement to regulate ‘essential public health functions’ to be performed by various public health agencies with commensurate duties of citizens.
Thus, the answer to implementing India’s international commitments and obligations towards the subject of ‘health’ and to combat the aforenoted limitations as well as addressing the overall concerns on public health, would be National Public Health Legislation drafted under Entries 13 and 14 of List of Schedule VII and Article 253 of the Constitution of India to ensure nation-wide application.
Taking a global view, countries like Australia and Canada have championed Public Health Acts, demonstrating the transformative
power of coherent legislation. Their experiences reflect the potential of proactive public health policies in safeguarding populations.
Thus, the act should aim towards One Health approach, Protecting and promoting public health, Controlling the risk to public health, making provisions for social determinants of health such as water, environment, sanitation, waste management and inclusion of mental health, among others. A four-tier governing structure at the National, State, District, and Block levels would facilitate effective implementation.
Public health is an intricate web where individual actions ripple through societies, necessitating legal foundations at central level to uphold the greater good. Critics might argue that legislation encroaches upon personal freedom, yet we must recognize that a collective approach is vital to combat any impending health crises. Another argument may point towards ‘Health’ being a state subject but learnings from COVID-19 have taught us that we can ill afford another ‘Model Public Health Act’ with no adoption.
Thus, enacting the National Public Health Act isn't just a call; it's a commitment to safeguard the health and dignity of every citizen. With this legislation, we signal our allegiance to an inclusive, healthier India, upholding the ideals of unity, progress, and collective effort (Sabka Saath, Sabka
Vikas, Sabka Vishwas, Sabka Prayaas, Sabka Swasthya)It's time to raise the curtain on this pivotal Act, scripting a healthier future for all.

Vasudha
that enacting the National Public Health Act isn't just a call; it's a commitment to safeguard the health and dignity of every citizenVasudha Khanna Maj Gen (Prof) Atul Kotwal


Cancer is a devastating disease that affects millions of people around the world. In India, the number of diagnosed cancer patients is projected to increase to 29.8 million by 2025; a drastic increase from the 26.7 million people in 2021.1 In the past, cancer patients were typically given the option to undergo chemotherapy, radiation, surgery, or a combination of the three. That is until immunotherapy became the safer and more effective option. Immunotherapy is tasked with utilising the patient's own immune system in order to recognise and attack abnormal cells. In doing so, the healthy cells are left alone, yielding a treatment plan with much fewer adverse side effects. Immunotherapy allows the patient to have a long-term positive response to the treatment while improving their quality of life because of the reduced toxicity. It can be used alone or as a combinatorial approach with chemotherapy. However, with the lower physiological toxicity, comes an increase in the financial toxicity. Being such a novel treatment comes at an incredibly high price. Drugs that have heretofore been approved by the US Food and Drug Administration (FDA) are patented by American corporations, making them available at a premium. Furthermore, only a minority have substantial health insurance in India, and out of those, only a few insurance agencies cover immunotherapy. This report will address the factors that currently affect a patient’s access to intravenous (IV) immunotherapy, the effects of financial toxicity on the patient’s life, and potential avenues for financial relief.
In India, the cost of immunotherapy for each cycle can fall anywhere between rupees 1 lakh to 3 lakhs, with the average falling at around 2 lakh rupees per cycle, after taking into consideration the robust patient assistance programs. With this form of biological therapy, there are various other costs that must also be taken into account before a patient can opt for this treatment. The primary cost is for the drug itself. Four of the most common intravenous immunotherapy drugs currently on the market are Pembrolizumab, Nivolumab, Durvalumab, Atezolizumab, and Avelumab. These drugs are most commonly used to treat Head and Neck Cancer, Lung cancer, Breast cancer, Gastrointestinal cancer, Genitourinary cancer, and Melanoma.2 They are used in advanced stages of these cancers, where the treatment is absolutely necessary and must be administered indefinitely until the disease progresses. Immunotherapy is also being used in the early stages of certain cancers like breast, lung, and gastrointestinal and has shown promising results. The

cost of these drugs alone is more than what a majority of cancer patients in India can afford. For the administration of immunotherapy drugs, in most situations, the patient must also get a PD-L1 test done to gauge whether this form of treatment is even the right path to go down.
Targeted therapies are another form of treatment that has become increasingly efficient in the last five years. They work by interfering with certain molecules or pathways and thereby prevent the spread of cancer. This type of treatment is a lot more precise and effective, with fewer adverse side effects as compared to traditional chemotherapy. The principle of targeted therapy is that the target inhibitor blocks a specific intracellular function. This local attack doesn’t damage normal cells like cytotoxic chemotherapy does, making it a less risky form of treatment.3 However, oral targeted therapy, like immunotherapy, can be extremely expensive. The cost of drugs such as Olaparib, Alectinib, Osimertinib, etc. is upwards of 1 lakh rupees after
consideration of patient assistance programs. Additionally, patients need to undergo biomarker testing to identify which targeted therapy they need to be given. An example of this is the test done for EGFR, ALK, or ROS1.4 If these tests yield a positive result for any biomarkers, the treatment options open up to include targeted therapies. Thus, also increasing the cost.
Most insurance companies reject claims for oral targeted therapies.
For patients without health insurance, it is an enormous financial burden if they opt for immunotherapy. There are few options– all within similar price ranges– making it seem like an impossible choice. The only significant relief patients get in regard to expensive medications is if they have access to biosimilars, which are alternatively known as subsequent-entry biologics. In India, several biosimilars are being made available to cancer patients, and their efficacies are almost identical to their reference drugs. Being homegrown medications, the cost is lowered exponentially. How-
ever, there is a major obstacle to the advent of these biologics. In the US, immunotherapy drugs are held under a patent, which allows the proprietor to be the sole producer of a certain drug. While this is great news for large pharmaceutical corporations, it is not for cancer patients without health insurance. Under these circumstances, biosimilars can only be utilised once the patent period has expired. For drugs such as Pembrolizumab and Nivolumab, the patent term expires only in 2028,5 which means that these drugs are not going to get any more affordable for at least a halfdecade. Another issue is that of “patent thickets”, where overlapping patents can prevent competitors from producing biosimilars for a longer period of time.6 So while India is one of the forerunners in the biosimilar market, there are currently no avenues for cancer patients to pursue if they are looking to avail immunotherapy biosimilars. This ultimately does nothing to alleviate the financial burden put on patients who opt for immunotherapy treatments.
Many a time, the lives of lowincome patients are affected detrimentally and it starts to impede on their everyday necessities. We talked to several patients under our care, all of whom come from different financial backgrounds. Households that earned more than 10 lakhs per year and/or had private health insurance were able to afford immunotherapy on a regular basis. The patients with private insurance had health coverage ranging from 40 lakh rupees to
Aditi Iyengar1,Muthulingesh Kumar2,Vishwanath Sathyanarayanan3 talks
factors that currently affect a patient’s access to Intravenous (IV) immunotherapy,the effects of financial toxicity on the patient’s life,and potential avenues for financialAditi Iyengar Muthulingesh Kumar Vishwanath Sathyanarayanan
unlimited. This meant that they only paid out-of-pocket for the investigative tests and some hospital charges. On the other hand, households that earned less than 5 lakh rupees per annum or had private health insurance up to 10 lakh rupees, or were uninsured, were affected the most by the high cost of treatment. Many of them either could not start immunotherapy or had to stop mid-course due to their financial struggles. Their families struggled to keep up with daily expenditures and they worried for their quality of life outside of the hospital. Certain patients who had government health insurance, especially from central govt/public sector undertakings were also able to get immunotherapy approvals and able to continue immunotherapy. Based on several testimonies from our patients, it was apparent that middle and lower-income patients are impacted detrimentally. But even so, there are only a few avenues that offer some assistance. With immunotherapy, some patients get assistance programs for immunotherapy drugs approved by The Central Drug Standard Control Organization (CDSCO) under the Directorate General of Health Services.7 For oral targeted therapies, some companies offer a free lifetime supply for patients who are economically underprivileged. After surveying patients under our care, the general consensus is that there need to be more avenues of financial relief available to patients for targeted therapies and immunotherapy.
The one concern all of our patients had, regardless of their insurance status, was how much financial stress was put on them because immunotherapy was not covered under their insurance. Most insurance companies do not cover immunotherapy to begin with. This makes it so that the patients are forced to pay for such an expensive drug out-ofpocket. Or worse yet, they are forced to opt for a different treatment that may not yield similar efficacy. Insurance companies need to cover immunotherapy and all the associated diagnostics so as to give the patient the best chance at fighting their cancer. It would help a lot if there were separate packages that covered oncological care specifically since they are inherently more expensive than standard healthcare. These agencies also need to cover more outpatient procedures, rather than just the ones done after admission. By doing so, patients can undergo the elaborate testing protocols required prior to and during treatment, without the burden of wondering how to pay for it.
Healthcare policymakers should be tasked with devising plans to find out how they can reduce the cost for the patients. For those without insurance, this would make immunotherapy a more viable treatment, where it might have been out of reach prior to this. But even for insured individuals, the drugs become a lot more affordable and accessible.
Another way to decrease the financial toxicity associated
with immunotherapy is by giving patients very low doses of the drug. A study conducted by the Tata Memorial Centre in Mumbai found that administering doses that are 1/10th of the usual amount of Nivolumab in addition to the metronomic chemotherapy regimen, showed positive results in patients with Head and Neck cancers. This form of treatment using Nivolumab demonstrated a significant reduction in the financial toxicity, bringing it to about a tenth of its retail price for a full dose. The results were remarkable. The group that was given immunotherapy as opposed to the standard treatment, showed better survival rates and demonstrated an overall better response. The article also emphasised that while this may not be applicable in all countries due to the differences in the causes and treatments, it opens up more opportunities for lower-income patients in countries like India.8
The most effective change that can be made comes straight from the pharmaceutical drug market. As we’ve explored before, the advent of biosimilars has drastically decreased the cost of drugs in India. But the matter of patents still remains a huge obstacle. For immunotherapy drugs such as pembrolizumab or nivolumab, whose patent periods expire in 2028, finding a way to create biosimilars today would significantly increase the accessibility and affordability for patients in India. Organisations like the European Medicines Agency (EMA) should review biosimilars at
least several years prior to the expiry of their reference’s patent. Companies that develop these subsequent-entry drugs should be allowed to begin clinical trials in advance and their documented proof of efficacy should be used to approve their entrance into the market. This would make even more sense because it makes life-saving drugs more accessible. Without these medications, patients are forced to opt for more traditional forms of treatment, which are not nearly as effective. By doing this, not only would this relieve some of the pressure put on hospitals to provide affordable care, but it would also readily allow lowerincome patients to access their treatment.
References
1. Kulothungan, Vaitheeswaran, et al. “Burden of Cancers in India - Estimates of Cancer Crude Incidence, YLLs, YLDs and DALYs for 2021 and 2025 Based on National Cancer Registry Program.” BMC Cancer, vol. 22, no. 1, BioMed Central, May 2022, https://doi.org/10.1186/s12885-02209578-1.
2. Immunotherapy for Advanced Non–Small Cell Lung Cancer: A Decade Of ..., ascopubs.org/doi/full/10.1200/EDB K_321483. Accessed 30 July 2023.
3. Carrington, Christine. “Oral Targeted Therapy for Cancer.” Australian Prescriber, Oct. 2015, www.ncbi.nlm.nih.gov/pmc/articles/PMC4657306/.
4. Graig, Laurene A. “Introduction.” Biomarker Tests for Molecularly Targeted Therapies - NCBI Bookshelf, 30 June 2016, www.ncbi.nlm.nih.gov/books/NBK
379335.
5. Letter, Pharma. “Establishing a Strong Presence in Biosimilars Is the Next Frontier for Indian Life Sciences Companies. With Biosimilars Expected to Generate Major Traction in Global Markets Over the Next Decade, Growing From $12 Billion in 2020 to at Least $36 Billion by 2025 at a CAGR of 24%, Studies Indicate India Does Have the Potential to Supply $5 Billion Worth of Biosimilars Globally in the Next Decade if It Overcomes Some Challenges, Reports the Pharma Letter’s….” Thepharmaletter, www.thepharmaletter.com/article/make-in-india-to-fuel-four-foldgrowth-in-biosimilars-and-vaccine s-by-2026-report.
6. Goode, Rachel W., and Bernard Chao. “Biological Patent Thickets and Delayed Access to Biosimilars, an American Problem.” Journal of Law and the Biosciences, vol. 9, no. 2, Oxford UP, July 2022, https://doi.org/10.1093/jlb/lsac022.
7. Functions www.cdsco.gov.in/opencms/opencm s/en/About-us/Functions.
8. Low-Dose Immunotherapy in Head and Neck Cancer: A Randomized Study, 20 Oct. 2022, ascopubs.org/doi/10.1200/JCO.22.0 1015.
About authors
1. Intern, Department of Medical Oncology, Apollo Hospitals, Bangalore; and Undergraduate Student, Baylor University, Waco, Texas
2. DNB resident, Department of Medical Oncology, Apollo Hospitals, Bangalore
3. Senior Consultant and Academic Advisor, Department of Medical Oncology, Apollo Hospitals, Bangalore*
*Compiled under the guidance of Dr Vishwanath Sathyanarayanan
Today nuclear medicine is one of the fastest growing and most promising segments of the medical imaging industry. Nuclear medicine comprises of diagnostic and therapeutic procedures with safe, painless, and cost-effective techniques. The technology provides functional as well as anatomical information, and is found highly beneficial in diagnosis of organ function abnormalities, cancerous growths, cardiac diseases, neurological disorders, blood flow blockages, and dysfunction of any major organ. Nuclear medicine has an edge on all other diagnostic techniques as it determines the presence of disease based on metabolic changes rather than changes in organ structure.
An exponential growth of nuclear medicine theranostics is seen in India for the past few years. What started with 131I in Graves’ disease and thyroid cancer has today evolved to the era of targeted radiopharmaceutical therapy (RPT) using beta and alpha emitters in prostate cancer, neuroendocrine tumours among others. The indigenous supply chain ranges from 99Mo/99mTc- generators, radio-iodine, 177Lu, 153Sm and cold kits for skeletal, renal, liver, hepatobiliary and thyroid scintigraphy studies. However, due to increased demands of these radiopharmaceuticals in tertiary care public and private medical hospitals, import dependency of 99Mo/99Tc generators is on the rise for many years. 177Lu-PSMA and 177Lu-DOTATATE supplied by BRIT for treatment of prostate and metastatic neu-
roendocrine tumours respectively has been phenomenal making these treatment affordable to a large section of the patients in different regions of the country. Still many larger centres are dependent on import supply of these two therapeutic radiopharmaceuticals. 68Ge/68Ga generators, Thallium -201 and Alpha emitters are not pro-
duced in India and are therefore totally imported for their extensive use both in PET/SPECT imaging and therapy. Their impact on the management of prostate and NETs has already been proven in several studies coming out of the premiere institutions in India. The cutting -edge research from India in the area of effective

treatment of advanced staged prostate and NETs using both beta and alpha emitters have been recognised at international platforms. FDA have taking note of these pioneering research from India which will pave the way for granting its approval for alpha therapy in prostate and metastatic NETs.
In a significant development, Russia has increased the supply of radioactive isotopes to India, which is poised to give a tremendous boost to nuclear medicine treatment in the country.
The Ga-68 isotope, specifically used in nuclear medicine, plays a pivotal role in diagnosing a wide range of oncological diseases (cancer) using positron emission tomography (PET) scanners. Globally, Ga-68 provides highprecision diagnostics for a wide range of nosologies.
Nuclear Medicine is one of the truly inter and interdisciplinary field of medicine, resulting out of amalgamation of all the pure branches of science i.e., physics, chemistry, biology and of course mathematics. As the field of Theranostics grows, it is essential to maintain coherence between different fast evolving facets of the field, which, if not orchestrated properly, may lead to loss of efficiency and efficacy of potentially life changing Nuclear Medicine treatment and diagnostic methods.
The emergence and advancement in Nuclear Medicine with high-resolution hybrid imaging technologies (SPECT/CT, PET/CT, PET/MR) and the development of newer and targeted radiotracers for both diagnosis as well as therapy have
paved the way for the exponential growth of this speciality around the World.
The community needs to brace itself for exponential increase in demand of clinically useful diagnostic and therapeutic radionuclides, PET/CT, SPECT/CT and cyclotron installations in the coming years due to the expansion of their applications in many more cancer entities. This expansion needs huge investments in terms of both expert manpower and economics. The Govt. of India, the Department of Atomic Energy is making immense efforts to escalate the indigenous production of these diagnostic and therapeutic radionuclides.
India is a reckoned entity in the field of nuclear medicine science. With over 442 centres of excellence of nuclear medicine spread over more than 60 cities and with about 359 PET centres, India has only 5.7 per cent share of the global Nuclear Medicine Market. A larger chunk of healthcare in nuclear medicine remains untapped in our country. Owing to the increased focus on healthcare by the Government of India, there are strong opportunities for the Overseas Nuclear Medicine related companies to invest in expanding Indian nuclear medicine market both in imaging technologies and the emerging radionuclide alpha and beta therapies. Formalisation of such ties at the governmental levels between the participating countries will facilitate accessibility and affordability of emerging radionuclide therapies (which are not available in India) to the un-affording patients of our country.
Prof.Baljinder Singh
discusses how nuclear medicine has an edge on all other diagnostic techniques as it determines the presence of disease based on metabolic changes rather than changes
The community needs to brace itself for exponential increase in demand of clinically useful diagnostic and therapeutic radionuclides,PET/CT,SPECT/CT and cyclotron installations in the coming years due to the expansion of their applications in many more cancer entities






















































Anup Kumar Ramachandran ,Business Head-Ultrasound,GE HealthCare South Asia in an interaction with Express Healthcare emphasizes that technology innovations in imaging are breaking new frontiers.Among the many of them,4D imaging,quantification tools and AI technology are the three leading ones.Innovations in imaging may come with applicability in ensuring better diagnostics
What are various ultrasound technology devices used for diagnostics and care of cardiovascular diseases? Which advanced equipment does Wipro GE Healthcare have in this category for the Indian market?
Statistics reveal that cardiovascular diseases (CVDs) such as ischaemic heart disease and cerebrovascular such as stroke account for 17.7 million deaths. 1 In accordance with theWorld Health Organization, India accounts for one-fifth of these deaths worldwide especially in younger population. Now, let’s understand the role of technology in addressing this burden.
CVDs comprising heart and vascular disorders like coronary heart disease, hypertension, dyslipidemia, and myocarditis are often diagnosed by their symptomatology such as dyspnea or breathlessness, cyanosis or blue discoloration due to lack of oxygen, and palpitations. Confirmation is often done with ECG and cardiovascular imaging techniquesEchocardiography which examines heart structure, lesions, and progression of
the disease. With advanced imaging technology, there could be more accuracy in diagnosis, better observation of cardiac morphology and quick diagnosis of pathologies of all kinds to ensure critical intervention and treatment.
Moreover, precision is changing the rules of the game. Powered by AI, ultrasound imaging is now working with robust/dependable algorithms, accelerating diagnosis time, faster delivery of care andhelping reduce tedious tasks and inter-observer variability. Ergonomically structured machines and the latest XDclear™ probes offer exceptional imaging with a wide range of functionalities.
At Wipro GE Healthcare, our Vivid ultrasound systems are designed to excel in 2D and 4D cardiac imaging. The Vivid E95 is GE HealthCare (GEHC) cardiovascular ultra-sound’s leadership scanner which offers advanced features such as indepth visualisation of cardiovascular anatomy and seamless workflow, for any kind of interventional minimally invasive procedures. Designed based on intuitive ergonomics with easy-to-use navigation tools,
the Vivid system may be adapted to each procedure’s specific needs, providing precise quantification, enhanced view of spatial relationships between flow and the surrounding structures, and simplified live image guidance.
Detection of structural heart diseases may become easier with:
◆ Clear image quality with cSoundTMsoftware beamformer
◆ Photorealistic visualisation of the anatomy with FlexiLight and HD Color flow rendering technique for semi-transparent visualisation of origin and size of high-velocity jets
◆ Simplified live guidance and improved quality of communication within the Heart Team with CT Fusion Live, 4D Markers, FlexiSlice and View-X
What are the top 3 promising technology innovations in the Indian cardiovascular devices market? Any specific development by GE HealthCare considering its sustainability & driven by patient care?
Technology innovations in imaging are breaking new frontiers. Among the many of
At Wipro GE Healthcare,we ensure that the machines are understood by clinicians, aligning with their workflow, reproducible results,and simplification of tasks

them,4D imaging, quantification tools and AI technologyare the three leading ones. Innovations in imaging may come with applicability in ensuring better diagnostics. In such scenarios, the cSound™ ADAPT technology with Vivid E95 both in 2D and 4D imaging is intended to provide exceptional image quality to give a conclusive diagnosis. The clarity in understanding pathologies using cardiac auto doppler and AI auto-measure 2D helps improve workflow and makes tasks less cumbersome for clinicians. These AI tools help in reproducible results, reduce inter-observer variability and enhance speckle imaging.
The machine also has oneclick AFI for diagnosis which can be incorporated into daily clinical practice. AFI can help
to quantify heart failure with preserved ejection fraction, AFI is a predictor of early and long-term outcomes after therapeutic Cardiac surgery and is useful for follow-up evaluations of therapeutic results.
Are there an adequate number of people trained to use these devices and interpret results for cardiovascular care? Any changes/ improvements that are happening on this front in India?
At Wipro GE Healthcare, we ensure that the machines are handled and understood by imaging clinicians, aligning with their workflow, reproducible results, and simplification of tasks. Our dedicated team of cardiovascular application specialists are specially trained in the application of
vivid ultrasound, to ensure that cardiologists can utilise the system for better patient care, diagnosis and management at all times.
The machines powered by AI are equipped with the digital expert application which is remotely managed by the GE HealthCare application team for troubleshooting any problems that may arise. This also helps the team collaborate with clinicians on the use and versatility of various tools across different kinds of pathologies.
Virtual workshop using 2D/4D Echopac has been conducted to enable clinicians to use various tools for accurate diagnosis. The virtual platform enables hands-on sessions to give real-life clinical scenarios, for better application of the tools for diagnosis.
How do you see the Indian cardiovascular devices market evolving over the next 5-10 years, and what role will ultrasound technology play in this regard?
With cardiovascular cases becoming more complex in diagnosis, multi-systemic pathologies and case numbers are only growing, it has become critical to expand the need of ultrasound imaging for detection, intervention and always monitoring minutely and consistently.
Ultrasound technology is non-invasive, accessible and tools such as speckle tracking imaging can go a long way in the early detection of heart failure, prognosis in oncology patients, valvular heart disease and CAD- coronary heart disease.
AI gives reproducible results with pre-trained insights, thereby reducing interoperate variability of the data analysed. This is a huge boost to the reliability and dependability of the machine for quick intervention without unnecessary delays.
We look forward to the next few years in seeing more evolved machines providing better service and digital expertise, more precise and varied implementation of the application/tools of the system, and all of it towards better diagnostic treatment and positive prognosis with early intervention and management.
References
1. https://www.ncbi.nlm.nih.gov/ pmc/articles/PMC6994761/#bib1
Anil

The “Sysmex Way”, the corporate philosophy of the Sysmex Group, defines our mission of “Shaping the advancement of Healthcare”. Sysmex India is continuously increasing its horizon and today, Sysmex India deliver total solutions in the field of clinical laboratory testing, including haematology, haemostasis, urinalysis, clinical chemistry, flowcytometry, Life science and scientific services.
In the anticipation of increased demand for testing in India’s high growth market, Sysmex India has decided to construct the new manufacturing base in Sanand Ahmedabad, in order to ensure a stable supply of products in the medium to long term through maintenance and expansion of the existing manufacturing structure. Its products and solutions continuously help improve patient clinical services and efficiency advances resulting in cost savings, which in turn translate to the delivery of better patient care.
Globally, Sysmex has a presence in over 190 countries and supplies its products and services. With the tagline “Together for a better healthcare journey” Sysmex is committed towards better patient care.
Sysmex operates in the healthcare domain, which continues to evolve and develop, constantly changing its form as new needs are created by advances in science and society. Within the healthcare domain, the value of diagnostics is also increasing. Development is a way of life in Sysmex and despite being leaders in haematology, Sys-
with corporate
sources for future generations. At Sysmex, sustainability is associated with our organisation’s holistic approach, considering everything from manufacturing to logistics to customer services. Blood donation camps, tree plantations and assistance for under privileged kids are routing activities done at Sysmex India level.
The Indian market is diverse at the level of socio-economic factors, various languages as well as healthcare systems. Expanding and advancing our business foundation is the core of Sysmex’s strategy for India. Sysmex is a market leader in the hematology field, and we are constantly improving and innovating so that in the coming years we will be ready to take a major step towards “Make in India”. In the past few years, we have grown considerably the field of Urinalysis and are aiming to establish our self as market leaders even this field and we take pride in the fact that we are already established as the numero uno market position in the field off Hematology. While we have achieved a lot there is still a lot more we have to do. We are halfway through of FY 23 now and aiming to:
◆ Create innovative diagnostics values as a top IVD company.
Clinical Flow Cytometry solutions and Life science like XF-1600 (Multi color Flow Cytometry analyzer), PS-10 (Flow cytometry sample preparation system), FISH probes and NGS products.
◆ Great place to work with equal opportunities for employees. Enrich the talent portfolio, which contributes to strategy execution, and creates an attractive climate that leverages diverse talent. One of key strategies in FY 23 is to bring focus on our new launches. We are a global leader in multiple IVD category including hematology, urinalysis and hemostasis and a new entrant in automation workflow. Sysmex now is introducing ‘A next level of automation of flow cytometry”. Our Flow cytometry system comprises PS-10 sample preparation system, Rotolavit-II-S Automatic Cell Washing Centrifuge, XF-1600 Flow Cytometer, Abs portfolio, ancillary reagents, and VenturiOne data analysis software. With this, highly trained operators are no longer needed and can save their hours spent manually pipetting, thereby using their valuable time for more complex analytical activities with Sysmex next level of automation of Flow cytometers.
mex is working to distribute new screening equipment to continually meet customers’ expectations.
Sysmex India continuously gives back to society through corporate social responsibility
and has cultivated a culture of sustainability at its Mumbai corporate office and Baddi Manufacturing plant. Sustainability improves the quality of our lives, protects our ecosystem, and preserves natural re-
◆ Expand and achieve high growth through proactive investment in the key field of our conventional IVD domain in the field of Hematology, Urinalysis and Hemostasis.
◆ Introduce new business to achieve dynamic growth via the launch of
Going forward, Sysmex aims to continue to strengthen its sales and support network and provide excellent services to client as it strives to contribute to the development of healthcare in India and maintain its leadership position in Haematology, Coagulation and Urinalysis.
Prabhakaran,Managing Director,Sysmex India Pvt Ltd emphasises that
philosophy of the Sysmex Group,shaping the advancement of healthcare,we are trying to improve the quality of life of people around the world at each stage of their healthcare journey with optimising healthcare and cost
Sysmex operates in the healthcare domain,which continues to evolve and develop, constantly changing its form as new needs are created by advances in science and society
The world of physiotherapy is at an exciting crossroads, where cutting-edge technology and innovative equipment play a pivotal role in shaping the future of healthcare. Medikabazaar, can be your perfect partner in this transformation. With a diverse range of physiotherapy products, Medikabazaar simplifies the process of setting up your center, propelling you towards growth and excellence.

Medikabazaar recognises this vision and offers a comprehensive range of products that align with your aspirations. From advanced rehabilitation machines to essential supplies, every piece in their collection is curated to support your
journey towards excellence. In a rapidly evolving field like physiotherapy, staying at the forefront of innovation is crucial. Medikabazaar offers a carefully curated selection of products backed by the latest advancements. With everything conveniently available under one roof, Professionals can focus your energy on developing cutting-edge treat-
ment protocols rather than navigating a maze of suppliers. They thrive to complements your proficiency by providing tools that amplify your skills. From state-of-the-art electrotherapy devices to ergonomic exercise equipment, their range empowers you to tailor treatments that are both effective and efficient. Medikabazaar's portfolio extends be-
yond machines to encompass essentials like disposables, mobility aids, and diagnostic tools. Their platform simplifies the entire procurement process, allowing you to order equipment with just a few clicks. This streamlined journey not only saves time but also enhances your center's impact by enabling you to focus on what truly matters – your patients.
The future of physiotherapy is bright, and India can be its architects. Medikabazaar stands as your partner, providing a range of products that will help you design a center that embodies growth, excellence, and inn ovation. As y ou embark on this transformative journey, remember that every step you take towards simplifying your setup paves the way for a more impactful, efficient, and holistic approach to healthcare. Let Medikabazaar's diverse product range be your compass as you navigate the exciting landscape of designing futures in physiotherapy.
With a diverse range of physiotherapy products,Medikabazaar simplifies the process of setting up your center,propelling you towards growth and excellence
From state-of-the-art electrotherapy devices to ergonomic exercise equipment,their range empowers you to tailor treatments that are both effective and efficient
Aplioaintegratesindustry-leadingimaging,advancedapplications, andsmartworkflowsintoasmall,lightweightpackage. Thesystemcanbescaledforawidevarietyofclinicalportfolios fromsharedservicestodedicated,specializedapplicationsstrengtheningyourclinicalconfidenceevenforthemost demandingcases.