MAY2023,` 40


Express Pharma to host the fourth edition of Vizag Pharma Summit












FDD Conclave 2023: Making India an epicentre for FR&D

INTERVIEW
Dr Samir Vyas CountryGeneral Manager,India, Agilent
VOL.18 NO.6 PAGES 68 www.expresspharma.in
EVENTS












NIPRO PHARMAPACKAGING INTERNATIONAL Blokhuisstraat 42, 2800 Mechelen, Belgium | pharmapackaging@nipro-group.com | www.nipro-group.com with anti-counterfeiting technology GLASS AMPOULES Additional feature to prove drug product authenticity • Invisible to the naked eye • Compatible with existing ampoule types • Low regulatory impact • Economical solution

www.lbbohle.com Developingbeyondyourexpectations! TabletCoatingTakentotheNextLevel Functionalcoating,delayedrelease products,entericcoating,APIcoating Visitour InnovationCenter inHyderabad fortestsandtrials







Chairman of the Board
ViveckGoenka
Sr.Vice President-BPD

Neil Viegas
Vice President-BPD
Harit Mohanty
Editor
Viveka Roychowdhury*
Editorial Team
Lakshmipriya Nair
Kalyani Sharma
DESIGN
Art Director
Pravin Temble
Senior Designer
Rekha Bisht
Senior Artist
Rakesh Sharma
Marketing Team
Rajesh Bhatkal
Ashish Rampure
Debnarayan Dutta
Production Co-ordinator
DhananjayNidre
Scheduling & Coordination
Pushkar Waralikar
CIRCULATION
Mohan Varadkar
CONTENTS
Charting India Pharma Inc’s growth path
Pharma leaders and experts highlight opportunities in the life sciences industry, address critical issues and explore development strategies for India Pharma Inc's progress at Ahmedabad Pharma
Summit 2023 | P20
EVENTS STRATEGY HR
14 EXPRESS PHARMA TO HOSTTHE FOURTH EDITION OFVIZAG PHARMA SUMMIT
15 FDD CONCLAVE 2023: MAKING INDIAAN EPICENTRE FOR FR&D

PACKAGING
31 PHARMACY EDUCATION IN INDIA: STRATEGIES FOR ABETTER FUTURE
32 MITIGATING FILL, FINISH AND CCI CHALLENGES FOR INJECTABLES

P16: INTERVIEW DR SAMIR VYAS Country General Manager, India,Agilent


Mumbai-400710 and Published at Mafatlal Centre,7th floor,Ramnath Goenka Marg,Nariman Point,Mumbai 400021.
Editor: Viveka Roychowdhury.* (Editorial & Administrative Offices: Mafatlal Centre,7th floor,Ramnath Goenka Marg,Nariman Point,Mumbai 400021)
* Responsible for selection of news under the PRB Act.Copyright © 2017.The Indian Express (P) Ltd.All rights reserved throughout the world. Reproduction in anymanner,electronic or otherwise,in whole or in part,without prior written permission is prohibited.
May2023 EXPRESS PHARMA 9 Express Pharma® Regd.With
No.MAHENG/2005/21398.Postal Regd.No.MCS/164/2022 - 24.Printed and Published byVaidehi Thakar on behalf of The
Express
Industrial Area,Mahape,Navi
RNI
Indian
(P) Limited and Printed at The Indian Express Press,Plot No.EL-208,TTC
Why Mankind Pharma’s IPO could be a milestone
Mankind Pharma's successful IPO raises hope for IPO-wannabes but the real hard work starts after the IPO party ends. Most companies face an IPO hangover, with share prices dipping post the IPO as they struggle to meet raised investor expectations. Will Mankind Pharma buck this trend?
Mankind Pharma's IPO was oversubscribed 15.32 times, thanks largely to qualified institutional buyers (QIBs). The response of retail investors was slightly more circumspect, bidding for 92 per cent of the allotted 35 per cent shares.
In spite of this dampener, Mankind Pharma's IPO holds out hope to other companies, pharma and non-pharma, planning for their IPOs. But only if the pricing is right and the company's financials pass muster.
Mankind Pharma ticks all the right boxes. It has a good product mix of consumer and Rx brands with most leading or in the top five brands in their categories. It has a strong differentiated pipeline, proven leadership, healthy CAGRs, etc.
But like most of its pharma peers, it also faces challenges. Regulatory oversight is a major concern, especially as most pharma companies are turning to third-party manufacturers to keep costs down and be more flexible. Pharma companies in India are set to face higher scrutiny, by global as well as national regulators, thanks to recent high-profile cases of violations of good manufacturing practices (GMP).
For instance, the Drug Controller General of India (DCGI) along with state drug control heads, has been on a clean-up drive since March. As per reports, around 200 companies suspected of faulty manufacturing practices are on the radar. 76 pharma companies in 20 states have faced such inspections, resulting in 26 pharma companies receiving show cause notices. Thus the GMP bar will only rise further.
Secondly, increasing price control in the domestic market impacts Mankind Pharma more than some of its peers, as approximately 97 per cent of its revenues (past three years + nine months ending December 31, 2021) are from India. In the latest recalibration of prices of medicines in the National List of Essential Medicines (NLEM), the National Pharmaceutical Pricing Authority (NPPA) has capped the prices of 651 of the 870 drugs listed in the NLEM. While a seven
per cent reduction in costs of these medicines is welcome news for patients, this will impact the balance sheets and strategies of pharma companies in India.
Thirdly, while pharma companies in India might benefit from the China+1 policy, companies like Mankind Pharma are still dependent on China and other countries for certain raw materials.
These challenges force smaller pharma companies to cut R&D expenses. Industry reports estimate that five of the top pharma companies in India account for two-thirds of the R&D spends. This is not a good sign as it will translate into a thin product pipeline, with few to none differentiated new products.
This cautious investor sentiment in pharma is also reflected in the recently released India Private Equity Report 2023 from Bain and Indian Venture and Alternate Capital Association (IVCA), titled 'Trial by fire: Indian PE ecosystem stays resilient in a globally challenging year'. As per the report, pharma was one of the sectors which saw a slowdown in PE activity in 2022.
The Bain-IVCA report points out that the long-term outlook for the pharma sector in India is positive, led firstly by a deep pharma ecosystem with consistent improvement in quality, compliance, reliability and cost. Will pharma companies continue to invest in quality systems and upskill their employees to work on such systems?
Secondly, regulatory enablers are boosting manufacturing, such as PLI 2.0 being expanded to high-value goods, and government investment in bulk drug parks to boost local API manufacturing. Finally, tailwinds from global sourcing diversification, and the opportunity for generics where India has the largest share in global supply by volume, make the sector a long-term bet, as per the Bain-IVCA report.
While Mankind Pharma’s IPO re-validates the long-term potential of the India Pharma Inc narrative, it won’t take much for the story to unravel. Pharma leadership will thus need to strategise carefully on the most efficient way forward.
VIVEKA ROYCHOWDHURY, Editor viveka.r@expressindia.com viveka.roy3@gmail.com

EXPRESS PHARMA May2023 10 EDITOR’S NOTE
While Mankind Pharma’s IPO revalidates the long-term potential of the India Pharma Inc narrative,it won’t take much for the story to unravel










Express Pharma to host the fourth edition of Vizag Pharma Summit
The event will also showcase the strengths and advantages that Vishakhapatnam brings to the table,as India Pharma Inc's charts its blueprint for progress
Express Pharma is organising a series of “Pharma Summits” across the nation's pharma centres to strategically leverage opportunities as well as find and apply new operating business models that support a value-driven approach to foster business growth. The overarching theme for this year's “Pharma Summits” is "Volume to Value Leadership: Opportunities and Challenges for India Pharma Inc."
One of them in this series is the Vizag Pharma Summit. It will be held on May 19, 2023, at The Gateway Hotel, Vishakahapatnam.
Experts and veterans of the industry will address critical
CONTRIBUTOR’S CHECKLIST
issues and discuss development strategies to embrace the challenges in this transitory environment and turn them into competitive advantages, so that the scales could be tilted in its favour and Brand India Pharma can re-emerge
❒ Express Pharma accepts editorial material for regular columns and from pre-approved contributors / columnists.
❒ Express Pharma has a strict non-tolerance policy of plagiarism and will blacklist all authors found to have used/refered to previously published material in any form, without giving due credit in the industryaccepted format.All authors have to declare that the article/column is an original piece of work and if not,they will bear the onus of taking permission for re-publishing in Express Pharma.
❒ Express Pharma's prime audience is senior management and pharma professionals in the industry.Editorial material addressing this audience would be given preference.
❒ The articles should cover technology and policy trends and business related discussions.
❒ Articles for columns should talk about concepts or trends without being too company or product specific.
❒ Article length for regular columns: Between 1200 - 1500 words.These should be accompanied by diagrams,illustrations,tables and photographs,wherever relevant.
❒ We welcome information on new products and services introduced by your organisation for our various sections: Pharma Ally (News,
stronger than ever before.
The event will also showcase the strengths and advantages that Vishakhapatnam brings to the table, as India Pharma Inc's charts its blueprint for progress. It will highlight how Vishakhapatnamis well-positioned, to be
an ideal location forpharma with its favourable business location, a skilled workforce, and a supportive regulatory environment.
Topics to be covered
◆ Emerging trends and challenges in thepharma industry
◆ Identifying collaborative models for drug development and commercialisation
◆ Designing strategies for enhancing drug discovery and development processes
◆ Elevatingpharmaperformance: Regulatory compliance and quality assurance
◆ Mapping innovation and building a cost leadership strategy
Products,Value Add),Pharma Packaging and Pharma Technology Review sections.Related photographs and brochures must accompany the information.
❒ Besides the regular columns,each issue will have a special focus on a specific topic of relevance to the Indian market.
❒ In e-mail communications,avoid large document attachments (above 1MB) as far as possible.
❒ Articles may be edited for brevity,style,and relevance.
❒ Do specify name,designation,company name,department and e-mail address for feedback,in the article.

❒ We encourage authors to send their photograph.Preferably in colour,postcard size and with a good contrast.
◆ Developing a unified business process to reduce compliance risk
◆ Building future-proof supply chain management and logistics
Who should attend CXOs, Plant Heads, Department Heads, Functional Heads and Senior Professionals from:
◆ Operations
◆ QA/QC
◆ IT
◆ Packaging
◆ Production
◆ R&D/Laboratories
◆ Supply chain/Procurement
Venue: Hotel Taj Gateway, Vishakhapatnam
Date: May 19, 2022
Timing: 03:30 pm to 08:00 pm (followed by networking dinner)
EXPRESS PHARMA May2023 14 EVENTS
Experts and veterans of the industry will address critical issues and discuss development strategies to embrace the challenges in this transitory environment and turn them into competitive advantages
your contribution to: The Editor, Express Pharma, Business Publications Division,The Indian Express (P) Ltd, Mafatlal Centre,7th floor,Ramnath Goenka Marg, Nariman Point,Mumbai 400021 viveka.r@expressindia.com viveka.roy3@gmail.com
Email
FDD Conclave 2023: Making India an epicentre for FR&D
FDD Conclave 2023 will discuss how India Pharma Inc needs to leverage its existing strengths and build new capabilities to develop of innovative and differentiated pharma products,complex drugs or biologics and biosimilars across a range of indications for its next phase of growth
Organised by Express Pharma, Pharma Formulation and Drug Delivery (FDD) Conclave 2023 will be held this year on July 21-22, 2023 at Park Hyatt, Hyderabad It is 'the' platform for leaders, experts and veterans of FR&D to come together conferand converse, on the current and future trends in the industry, their growth drivers and the challenges to tackle them as well as form meaningful alliances to fast-track progress.
FDD Conclave 2023 will bring the FR&D community together to discuss how India Pharma Inc needs to leverage its existing strengths and build new capabilities to develop of innovative and differentiated pharma products, complex drugs or biologics and biosimilars across a range of indications for its next phase of growth.
FR&D leaders will highlight evidence-based strategies and approaches to develop novel formulations and drug delivery systems which will help create intellectual property, enhance life-cycle management, and amplify cost and market differentiation.

The leaders will also examine the impact of recent government initiatives, regulations and policies to help promote innovation in pharma R&D and help India secure and sustain a key position in the global pharma R&D landscape.
Topics/Themes to be discussed
◆ Incentivising pharma FR&D in India: Policies, regulations & initiatives
◆ Fuelling innovation in biopharma FR&D
◆ Building India's FR&D talent pool: Approaches & Techniques
◆ Developing cost-effective drug formulations and delivery systems: Opportunities & Challenges
◆ FR&D for continuous manufacturing
◆ Predictive approaches for
solid formulation
◆ Potential of emerging vaccine technologies
◆ Developments in injectable drug delivery
◆ Impact of additional budget-
ary allocation for R&D
◆ Role of emerging technologies in drug development
May2023 EXPRESS PHARMA 15
STRATEGY
At Agilent,we believe that sustainability, productivityand efficiencycan co-exist in a lab without compromising on ROI
Dr Samir Vyas ,Country General Manager,India,Agilent shares insights about the importance of sustainability,challenges that hinders sustainability initiatives and endeavours taken by Agilent towards sustainability,in an exclusive interview with Viveka Roychowdhury

What are the top three to five sustainability challenges in today’s pharma labs?
Several sustainability challenges must be addressed in today's pharma laboratories to minimise environmental impacts and support a more sustainable future.
One of the most difficult challenges in pharma labs today is that they generate a significant amount of waste, which includes both hazardous and non-hazardous materials, which can have serious environmental consequences. Aside from this, improperly disposed of unused medications by consumers can contribute to environmental contamination and potentially harm wildlife. Whereas proper disposal of unused medications, as well as initiatives to reduce packaging waste, chemical waste, and improve recycling programmes, will aid in proactively addressing this challenge.
Like many other industries, pharma labs contribute to greenhouse gas emissions and carbon footprints through their energy use, transportation, and waste management practices. The day-to-day operation of these laboratories requires a substantial amount of energy and water, and many still rely on fossil fuels for energy. Overall, pharma labs must
reduce their consumption of water and transition from fossil fuel-derived to renewable energy sources to mitigate significant environmental impacts.
According to one of our global customer surveys, 85 per cent of pharma labs and
organisations already have sustainability goals in place, and 83 per cent of pharma lab leaders believe their current workflow needs to be optimised to meet their sustainability goals.1
What are the quick ways to
diagnose/find and address these sustainability issues?
Especially those related to excessive water and energy consumption, waste generation by biopharma labs etc.?
To meet sustainability objectives and comply with regulations, biopharma laboratories can adopt sustainable practices such as waste reduction, energyefficient processes, and environmentally friendly operations. The laboratories can utilise equipment and instruments designed to be environmentally friendly. This can include equipment with low energy consumption, energy-efficient HVAC systems, and eco-friendly materials. By monitoring these practices, they can identify areas for improvement and make timely adjustments.
In addition, these laboratories can create active pharma ingredients (APIs) and finished products that are designed to be more sustainable and environmentally friendly. This may involve the use of ecofriendly raw materials, green manufacturing processes, and eco-friendly packaging. Furthermore, they can reduce the pollution caused by their products and processes by maximising the use of raw materials and minimising waste production.
What have been the
outcomes of Agilent’s commitment to sustainable lab practices? What did it cost to achieve these outcomes?
At Agilent, sustainability is a top priority for our product development and manufacturing processes. In 2020, we began partnering with My Green Lab to advance our sustainability efforts. This partnership started with select Agilent instruments being independently audited for the organisation’s Accountability, Consistency, and Transparency (ACT) Environmental Impact Factor Label. Since then, our collaboration has expanded to include participation in the My Green Lab Certification programme, and we are proud to have achieved the highest level of sponsorship, known as the "Angel" level. Our commitment to sustainability through collaboration with My Green Lab demonstrates our dedication to promoting environmentally responsible practices in the scientific community.
Can you give details about Agilent’s partnership with My Green Lab and ACT and the objectives?
Agilent is committed to promoting environmental sustainability and as a key example, has formed a partnership with My Green Lab to help accomplish this
EXPRESS PHARMA May2023 16
INTERVIEW
Pharma labs must reduce their consumption of water and transition from fossil fuel-derived to renewable energy sources to mitigate significant environmental impacts
objective. Initially, we began by partnering with My Green Lab on the organisation’s ACT Environmental Impact Factor Label programme. The ACT label provides information about the environmental impact of manufacturing, using, and disposing of a product and its packaging, enabling purchasers to make better informed, sustainable choices.
In 2021, we became a proud sponsor of the My Green Lab Certification programme, the gold standard for best practices in laboratory sustainability. Our sites in Waldbronn, Germany, Cheadle, United Kingdom, and Santa Clara, California, have achieved the highest level of certification – ‘green’. This certification demonstrates our dedication to enhancing the environmental sustainability of our global internal laboratory operations.
What are some of the basis sustainable practices that can be adopted at pharma manufacturing plants?


Energy efficiency:
Improving energy efficiency by using energy-saving equipment, optimising processes, and utilising renewable energy sources, such as solar power, can reduce carbon emissions and help lower energy costs.
Water conservation: Implementing water-efficient equipment and processes, and recycling and reusing water can help conserve water resources, reduce wastewater discharge, and lower water costs.
Waste reduction: Adopting a zero-waste approach by reducing, reusing, and recycling waste, and properly disposing of hazardous waste can significantly reduce the environmental impact of pharmaceutical manufacturing.
Sustainable sourcing: Choosing sustainable and environmentally friendly materials, ingredients, and suppliers can help promote sustainable practices and
reduce the environmental impact of pharmaceutical manufacturing.



Green chemistry: Using green chemistry principles, which focus on reducing or eliminating the use and generation of hazardous substances, can help promote sustainable manufacturing

practices. Life cycle analysis: Conducting life cycle analyses to identify the environmental impact of a product throughout its life cycle can help pharma companies make informed decisions and promote sustainable practices.
Adopting sustainable practices at labs or manufacturing plants comes at a cost. How can pharma companies justify these costs as investments with ROI beyond it being the right thing to do for future generations on this planet? Multiple reports suggest that
Noeffort. Nogaps. Noworries.
research laboratories consume up to 10x more energy and 4x more water than office spaces, which can impact not only the efficiency but also the productivity of the laboratory.2, 3 To combat the same, most pharma and


Continued on Page 19
testo174series
Minidataloggersfortemperatureandhumiditymeasurement
l
l Largedisplaywithhighdatasecurity



Measurementdatamemoryforupto16,000readings
l Nodatalosseswhenthebatteryrunsoutorischanged l


testo175series








Compactdataloggersforlongtermmonitoringoftemperature l andhumidity
Measurementdatamemoryfor1millionmeasurementvalues l
Highlevelofdatasecurity l
EasytransferofmeasurementdataviaUSBcableorSDcard l
testo176series
Highprecisionmeasurementswithmultiplechannelsfor l simultaneousmeasurementatsites
RobustSShousingtocomplywithpharmaregulations l Memoryforupto2millionreadings l
l
Largedisplayforclearerview
May2023 EXPRESS PHARMA 17
Designedin GERMANY +912025920000 info@testo.in TestoIndiaPvtLtd
Howwill green chemistrytransform the future of pharma manufacturing
Implementing green chemistry in pharma manufacturing can reduce waste and pollution, improve efficiency,and promote sustainability and social responsibility,highlights
Naveen Kulkarni,CEO,Quantumzyme

The idea of green chemistry was initially developed as a response to the Pollution Prevention Act of 1990, which declared that US national policy should eliminate pollution by improved design including cost-effective changes in products, processes, use of raw materials, and recycling instead of treatment and disposal.
The 12 Principles of Green Chemistry were published in 1998, providing the new field with a clear set of guidelines for further development.
In the last 10 years, national and international networks have proliferated, special issues devoted to green chemistry have appeared in major journals, and green chemistry concepts have continued to gain traction. A clear sign of this was provided by the citation for the 2005 Nobel Prize for Chemistry awarded to Chauvin, Grubbs, and Schrock, which commended their work as “a great step forward for green chemistry”.
In recent years, pharma industry has been exploring the potential of green chemistry in drug manufacturing. This sector is responsible for producing life-saving drugs and has the potential to make a significant impact on global sustainability efforts by adopting green chemistry practices. Thus, implementing green chemistry in pharma manufacturing can reduce waste and pollution, improve efficiency, and promote sustainability and social responsibility.
Furthermore, recent developments in adopting green catalysts, enzymes, have provided breakthrough advantages over traditional chemistry by being
able to selectively catalyse the reaction, save costs and time as well as reducing impurities and waste, hazardous byproducts.
It is noteworthy that the Nobel prize in chemistry in 2018 was given to enzyme engineering highlighting the importance of its role in reducing pollution and environmental impact.
Hence, as the demand for sustainable pharma continues to grow, green chemistry and more specifically, enzymes will play an increasingly important role in the future of pharma manufacturing.
Here's how green chemistry will transform the future of pharma manufacturing:
◆ Reducing waste and pollution: Pharma manufacturing is energy- and resource-intensive, often resulting in large amounts of waste and pollution. Traditional methods of drug synthesis typically rely on hazardous chemicals, which can pose a significant risk to both human health and the
environment. Thus, green chemistry offers an alternative approach, utilising safer and more sustainable materials and processes. Using enzymes as catalysts instead of typical chemical catalysts is one example of a green chemistry method in pharma manufacturing. Enzymes are biodegradable and pose minimal environmental impact, while increasing drug production selectivity and efficiency. Hence, green chemistry can dramatically minimise the environmental effect of pharma manufacturing by lowering the usage of hazardous chemicals and minimising waste.
◆ Improving efficiency and reducing costs: Green chemistry can enhance the effectiveness of pharmaceutical manufacturing processes and decrease waste as well as pollution. It can simplify drug synthesis and reduce production time and cost by utilizing new technologies and innovative
methods. Furthermore, continuous flow chemistry is an example of a green chemistry technology that enables faster and more efficient drug manufacturing. It uses small reactors to conduct continuous reactions, resulting in shorter reaction times, higher yields, and less waste. Thus, green chemistry can help make drugs more accessible and affordable for patients by lowering the time and cost of drug manufacturing.
Promoting sustainability and social responsibility
Green chemistry in pharma manufacturing benefits not only the environment and industry, but can also encourage social responsibility and sustainability. In the modern-day world, as consumers are becoming more aware of the environmental impact of products, they are seeking more environmentally friendly alternatives. Thus, by adopting green chemistry practices, pharma companies can demonstrate their commitment to sustainability and responsible manufacturing. Furthermore, green chemistry can aid in promoting sustainability throughout the supply chain. Therefore, pharma businesses can encourage suppliers to adopt more sustainable practices by employing more sustainable materials and minimising waste. This has the potential to spread positivity throughout the sector, boosting sustainability and social responsibility.
◆ Challenges and opportunities: While chemistry offers many benefits to pharma manufacturing, there are also challenges to its adoption. One significant challenge is the need
for investment in new technologies and equipment. Green chemistry requires new and innovative technologies, which can require high upfront costs. However, as the demand for sustainable pharma grows, the long-term benefits of green chemistry will outweigh the initial investment.
Another challenge is the need for regulatory approval. Green chemistry practices and technologies may require regulatory approval before being implemented in pharma manufacturing. This can slow down the adoption process, but it is necessary to ensure the safety and efficacy of pharma products.
Green chemistrypaves the wayfor sustainable pharma manufacturing! Green chemistry represents an exciting and innovative approach to pharma manufacturing. The adoption of green chemistry practices can lead to a reduction in waste and pollution, improvements in efficiency, and promotion of sustainability and social responsibility throughout the industry. Even though there are challenges to its adoption, the long-term benefits of green chemistry outweigh the initial costs. Furthermore, as the demand for sustainable pharma continues to grow, the pharma industry can significantly impact global sustainability efforts by adopting green chemistry practices. Thus, the transformation of pharma manufacturing towards green chemistry is essential for meeting sustainability goals and improving public health by ensuring the production of safe and effective medicines.
EXPRESS PHARMA May2023 18 STRATEGY
At Agilent,we believe that sustainability...
Continued from Page 17
life sciences companies have their own sustainability goals in motion. At Agilent, we believe that sustainability, productivity, and efficiency can co-exist in a lab without compromising on ROI.
In addition to basic costsaving measures such as turning off lights and machinery when not in use, lab spaces can benefit from new energy and waste reduction methods and systems that are already available. Although adoption of these technologies can be slow in regulated industries such as pharmaceuticals, it is crucial for companies to transition as soon as possible to reduce the industry's
overall environmental impact.
One way for companies to address sustainability concerns is by conducting independent audits through external organisations, such as My Green Lab, to track and
manage the environmental footprint of their labs. Such audits will encourage other labs to adopt more sustainable practices and promote the industry's collective commitment to reducing its
environmental impact.
Pharma companies can also choose to purchase more environmentally friendly instrumentation, which have been assessed for their environmental impact and do
not compromise on quality results. Also, they can gain a better understanding of the utilisation of their instrument fleet with digital tools like asset management, advanced automation, and internet of things (IoT) technology in order to maximise efficiency without necessarily purchasing more instruments, reducing both cost and carbon footprint.
References:

1The 2050 Lab of the Future: Sustainability (theanalyticalscientist.com)
2TCIN Case Study (mygreenlab.org)

3Clinical Labs: Making the Switch to Green | AACC.org viveka.r@expressindia.com viveka.roy3@gmail.com
+919900674407|info@srico-labworld.com|www.srico-labworld.com

May2023 EXPRESS PHARMA 19 STRATEGY
At Agilent,sustainability is a top priority for our product development and manufacturing processes.In 2020,we began partnering with My Green Lab to advance our sustainability efforts.This partnership started with select Agilent instruments being independently audited for the organisation’s Accountability,Consistency,and Transparency (ACT) Environmental Impact Factor Label

EXPRESS PHARMA May2023 20 cover )
Pharma leaders and experts highlight opportunities in the life sciences industry, address critical issues and explore development strategies for India Pharma Inc's progress at Ahmedabad Pharma Summit 2023

 By Lakshmipriya Nair
By Lakshmipriya Nair
May2023 EXPRESS PHARMA 21
India Pharma Inc, hailed for its prowess in the production of high-quality generic medicines, now needs to shift from a conventional, volume-based business strategy to a value-based approach to keep it up its growth momentum. In this context, finding the right opportunities and understanding the challenges has become crucial.
Hence, ExpressPharma is organising a series of “PharmaSummits”across the country’s pharma hubs to strategically leverage opportunities as well as find and apply new operating business models that support a valuedriven approach to foster business growth. The overarching theme for this year'sPharmaSummitsis
Gujarat is a leader in pharma manufacturing and accounts for 33 per cent of the country's sector turnover and 28 per cent of exports
Dr Hemant Koshia Commissioner, FDCA- Gujarat



Innovation and differentiation will be key to the progress of India Pharma Inc.All stakeholders should work to optimise India's natural resources, scientific capabilities to spur growth
promote good regulatory practices, enforce drug quality, facilitate consistent and accurate data documentation, etc.
Value leadership requires innovation and differentiation
"Volume
to Value Leadership: Opportunities and Challenges for IndiaPharmaInc."
The first one of this series in FY2023-24 was the AhmedabadPharmaSummit.Held on April 21,2023, at the DoubleTree by Hilton, Ahmedabad, the maiden edition of this eventemerged as a platform for experts, thought leaders and veterans from the industry and regulatory bodies to assess the potential growth areas in the life sciences industry and explore opportunities for businesses in this sphere to create value and long-term sustainability.
Here is a summary of the lessons learnt from the views and insights shared by the distinguished speakers at Ahmedabad Pharma Summit 2023.
Technologycan aid regulators to enable good governance, compliance
Dr Hemant Koshia, Commissioner, FDCA-Gujarat gave a very insightful key note address on the topic, Ahmedabad: A go-to destination for pharma manufacturing capabilities. In this session, he not only established the relevance and importance of Ahmedabad as a major pharma man-
Dr Viranchi Shah National President, IDMAand Director, Saga Laboratories Performance management, leadership,people,culture,continous improvement,risk and quality management etc are some of the key enablers of operational excellence
Dr Sanjay Kumar Jain President - India Operations, Amneal Pharmaceuticals
After emerging as a key supplier of generics medicines globally and being the “Pharmacy of the World”, India now has to prove its mettle as a value-driven leader in pharma to continue its growth momentum. Union Health Minister Mansukh Mandaviya too gave the call for a shift from Volume’ to ‘Value’ leadership to capture global pharma markets in the recent past as part of Vision 2047, a road map that envisages India as a developed nationby the year 2047.
So, a special address from Dr Viranchi Shah,National President, IDMA and Director, Saga Laboratories on ‘Indian pharma - Towards value leadership’ at the Ahmedabad Pharma Summit was very timely and pertinent. He informs that the goal for the Indian pharma sector by 2047 is to become a $500 billion industry and this is possible only through value leadership. To meet this ambitious target, he emphasised on the importance of adopting global best practices in pharma R&D and manufacturing. He also stressed that innovation and differentiation will be of utmost importance to accelerate production, increase global footprint, improve clinical outcomes and drive patient-centricity.
ufacturing hub, but also showcased how Gujarat is very relevant and key to India Pharma Inc’s growth trajectory. Highlighting Gujarat’s contributions in the life sciences sector, he informed that the state accounts for >32 per cent of national pharma production ($6.9 billion) and >28% of national pharma exports ($6.6 billion). It has over 110 USFDA approved sites, 50 EUGMP approved
sites, 1081 WHO GMP approved units. Speaking on FDCA’s efforts, as a regulatory agency, to help build and sustain and healthy and thriving pharma sector in the state, Dr Koshia detailed how technology was leveraged to implement e-governance, and various advanced tools of enforcement were utilised to enable quality enforcement in the state by the regulatory officials.
A key learning from his session was that as the life sciences sector evolves and market demands change, traditional approaches to enable compliance must make way for newer and more effective models. Adoption of digital technologies can help regulatory agencies to drive and maintain compliance by improving their capabilities to analyse data, detect compliance issues, identify risks,
One of the most important takeaways from his session was that all pharma stakeholders should contribute and strive collectively towards building a robustecosystem through scientific advancements, advanced skills, progressive regulatory changes, and use of data and emerging technologies.
Right processes and practices enable and empower strategies
An insightful session on
EXPRESS PHARMA May2023 22 cover )
“Enabling and optimising operational excellence,” by Dr Sanjay Kumar Jain,President - India Operations, Amneal Pharmaceuticals accentuated how rising operational costs can lead to diminishing returns on products and affect the growth of life sciences organisations. He emphasised that operational excellence is essential in the pharma industry to balance cost and product excellence through optimised processes and best practices.
Dr Jain outlined a five-step strategy for operational excellence which involves assembling a cross-functional team to create a multi-dimensional plan, clearly defining the benchmark, strategy and performance goals for the endeavour at hand, aligning the organisation’s growth with a digital transformation journey, prioritising the implementation of this strategy by balancing risk, cost and performance, and reviewing the lessons learnt to continuously optimise the plan. He explained about the role of performance management, leadership, people, culture, risk and quality management etc. in enabling operational excellence.
Dr Jain also outlined and detailed the methods or approaches that can help achieve operational excellence such as Kaizen, Lean Manufacturing, Siz Sigma, Process Mapping, 5S implementation, X Matrix, Automation/ Digitisation, Poka Yoke etc.


A vital lesson from his session was that operational excellence is pivotal to maximise productivity, add value and offer more differentiation in products, eliminate risks, and identify growth opportunities, and safeguard quality.
Driving a culture change is imperative to achieve value leadership
Another important session at Ahmedabad Pharma Summit was on ‘Manufacturing Science and Technology: A road map for value-driven, hybrid business model’. The speaker
for this session, Gouri Prasad Nanda, GM - MS&T, Zydus Group, enlightened the audience about some common lapses and errors in the pharma sector with a few hard-hitting questions and observations. His session highlighted that as the industry undergoes a shift from a volume-based to a value-based business model, it should learn to effectively balance product quality, cost-effectiveness and patient-centricity to achieve long-term success.
He also explained how manufacturing science and
The volume to value business model represents a major shift in the pharma industry.It seeks to improve product quality,enhance patient outcomes and contain costs.MS&Tcan help improve quality and drive value
abad Pharma Summit was on ‘Building future ready pharma facilities,’ a relevant topic since infrastructure is of the essence to sustain progress in the pharma industry. Sanjeev Mahajan, VP-Quality, Cadila Pharmaceuticals, explained that automation and digitalisation, modular design, flexible manufacturing platforms, sustainability, regulatory compliance, robust quality management and effective talent management are some of the facets of future-ready pharma facilities.
Future ready facilities will be pivotal to India Pharma Inc's progress.Pharma facilities of the future need effective integration of technologies such as MI,AI and robotics; modular design; flexible manufacturing platforms and sustainable practices
Future-ready facilities will enable cost effectiveness and manufacturing efficiencies, reduce probability of errors, enhance manufacturers’ confidence on products and processes and generate more confidence and credibility among regulatory agencies.
Thus, a major take away from this session was that pharma facilities have to adapt to a rapidly evolving healthcare landscape and meet the need for innovative therapies. Focussing on implementation of future-ready facilities is the need of the hour to deliver safe and cost effective pharma products and meet the challenges of an evolving pharma industry.
Facilitating progress
technology (MS&T), a relatively new function can help and support key activities such as risk management and troubleshooting, monitoring and analysis of process data, lead technical transfer activities, process change control management, technology strategy and innovation etc to ensure that products are in a constant state of control. It can also aid the implementation of a people and capability development strategy to guarantee the right talent and skill-set across the organisation to achieve its goals.
One of the major learnings from this session was that since quality equates to value, each pharma organisation must tackle behavioral issues that threaten meaningful change. Building and nurturing a culture of quality and compliance is a must to make products and processes more robust, efficient and effective.
Healthcare’s transforming landscape necessitates future-readypharma facilities
The last session at Ahmed-
Thus, the Ahmedabad Pharma Summit very clearly brought out that India Pharma Inc needs to continuously evolve and transform with the help of new-age technologies, novel approaches, inn ovative techniques, skilled and trained resources, and a growth-oriented mindset to prepare for an illustrious future. Express Pharma will continue to cover and chronicle learnings from experts and leaders of various pharma hubs for our readers. Stay tuned for updates and coverage about our upcoming Pharma Summits. The next one, Vizag Pharma Summit 2023, will be held in Visakhaptanam on May 19, 2023.
lakshmipriya.nair@expressindia.com laxmipriyanair@gmail.com
May2023 EXPRESS PHARMA 23
Sanjeev Mahajan VP-Quality, Cadila Pharmaceuticals
Gouri Prasad Nanda GM - MS&T, Zydus Group
India Pharma Inc needs to continuously evolve and transform with the help of new-age technologies,novel approaches,innovative techniques,skilled and trained resources,and a growth-oriented mindset to prepare for an illustrious future








New-age solutions for pharma and life sciences

Ahmedabad Pharma Summit 2023 comprised sessions and presentations about products and


to life sciences industry.Here is a summation of those sessions at the event,recently hosted by ExpressPharma
VENTILUS: The Swiss knife tool you want in your pharma plant
Polymeric solutions for pharma,biopharma and medical industry
Science behind Pure Water.The NXXTis here!!
Bernat Rodriguez Sales Director Romaco Innojet GmbH gave an overview about his organisation’s solutions for the pharma industry and explained that it seeks to be a one-stop solution for processing and packaging equipment. He went on to give details about one interesting solution called Romaco Innojet VENTILUS, and said that it operates like a Swiss knife i.e. it is multifunctional. He explained that it is a multi-processor for drying, granulation and coating with advanced, multi-
purpose FB technology. Detailing the various USPs of the Innojet, he explained how it is a high performance dryer and aids in the implementation of an optimal tablet coating process. He informed that the solution enables estimated operational costs saving of 25 per cent.
Prabhat Balyan, Assistant Manager, Ami Polymer and Dhruv Borda, Assistant Manager Sales & Marketing, Ami Polymer spoke on polymer solutions for drug manufacturing process at Ahmedabad Pharma Summit 2023. Sharing information about different types of tubings and hoses and their applications in the life sciences industry, the speakers explained how different drugs call for different kinds of solutions. The speakers also explained how polymeric products can ensure regulatory compliance. The speakers concluded the presentation by reiterating that AMI Polymer can help the pharma industry with the right polymer solutions for their drug manufacturing process.
Meenu Bansal, Field Marketing Expert, Merck Life Sciences gave an informative presentation on Merck’s new product Milli-Q at Ahmedabad Pharma Summit 2023. She educated the audience on Merck's innovative solutions to help the pharma industry handle its everyday needs for purified water in their operations. Giving an over view on Merck Life Sciences' Milli-Q Lab Water portfolio, she spoke about the company’s wide range of sustainable pure and ultrapure water purification systems for pharma, clinical, academic, industrial, research, and government laboratories. She
informed that they can be adapted to every user’s application requirements and comes with unique delivery design to dispense water in an optimal way.

May2023 EXPRESS PHARMA 25
Bernat Rodriguez,Sales Director,Romaco Innojet GmbH
DhruvBorda,Asst.Manager – Sales & Marketing, Ami Polymer
Prabhat Balyan,Asst.Manager – Business Development, Ami Polymer
Meenu Bansal,Field Marketing Expert,MerckLife Science
Romaco Innojet VENTILUS can enable an optimal tablet coating process and provide estimated operational costs saving of 25 per cent
Milli-Q ultrapure water solutions are designed to produce consistent ultrapure water quality that can be adapted to users' specific application requirements and comes with a range of intelligent design features
solutions which are relevant
Role of active packaging in drug development
Enhancing pharma manufacturing capabilities
High-resolution measurement in particle size analysis - Reliablyspot small differences!
Dhairy Sharma, Technical Sales, Cilicant, gave an instructive presentation at Ahmedabad Pharma Summit 2023 which highlighted packaging’s critical responsibility in drug development. He spoke on active packaging and its ability to enhance the shelf life of products. He shared more details about his company’s active packaging solutions to help pharma companies protect and preserve their products. He particularly focused on Accuflip, an equilibrium relative humidity


Dr Abhijit V Gothoskar, Formulation Expert, Sigachi Industries began his presentation by elucidating what productivity, effectiveness and efficiency entails and how they can be achieved. Then he highlighted that reducing number of unit operations in drug manufacturing can improve productivity. Furthermore, he spoke on how multi-functional excipients can enhance productivity, effectiveness and efficiency in drug
Anuj Jindal,Market Development Specialist,Beckman Coulter Life Sciences
Anuj Jindal, Market Development Specialist, Beckman Coulter Life Sciences spoke on the importance of particle size in pharma industry and how it impacts product effectiveness as well as production processes, thereby making it important to characterise

LS13320XR from Beckman Coulter is a laser diffraction particle size analyzer with PIDS technology.It can accurately measure samples,both in nano as well as micron range,from 10nm to 3500 micrometers
(ERH) regulator which can avert over-desiccation of capsule cells and safeguard pharma products’ efficiency and efficacy.
development and manufacturing. Pointing out that coprocessed excipients offer benefits not offered by single excipients alone, he explained how co-processing excipients enable greater functionality, balanced properties, convenience, consistent performance and Quality by Design. He also informed that Sigachi has a wide range of co processed excipients and can be a reliable formulation partner to the pharma industry.
both active components and excipients. Then, he outlined the importance of laser diffraction and its advantages in detecting particle sizes. He explained how Beckman Coulter’s solution that allows laser diffraction plus advanced polarisation intensity differential scattering (PIDS) technology enables high-resolution measurement and reporting of real data down to 10 nm, and provides accurate, reliable detection of multiple particle sizes in a single sample.
EXPRESS PHARMA May2023 26 cover )
DhairySharma,Technical Sales,CILICANT Dr Abhijit VGothoskar,Formulation Expert, Sigachi Industries
Reducing number of unit operations can improve productivity. Co-processed excipients are more effective than single excipients.Sigachi can be the right partner to pharma formulators
ACCUFLIPfrom Cilicant has been designed to regulate Equilibrium Relative Humidity (ERH) within a specific range in order to protect medications from overdesiccation.It does this through a specialised sorbent that has the ability to absorb and desorb
GLIMPSES OFAHMEDABAD PHARMASUMMIT2023









May2023 EXPRESS PHARMA 27
GLIMPSES OFAHMEDABAD PHARMASUMMIT2023








EXPRESS PHARMA May2023 28 cover
)






May2023 EXPRESS PHARMA 29
PHARMASUMMIT2023
GLIMPSES OFAHMEDABAD

Pharmacyeducation in India: Strategies for a better future
Dr Guntupalli Chakravarthi,Principal,College of Pharmacy,KLDeemed to be University emphasises that institutions providing pharmacy education should focus on providing vocational training,collaborate with business professionals,and give their students access to up-to-date curriculum

The Indian pharma industry plays a crucial role in enhancing the Indian economy. It holds the third-highest rank worldwide in terms of production by volume. Furthermore, India has also become the world's largest provider of generic medicines, with a 20 per cent share by volume. In addition, it is also home to more than 3,000 pharma companies, 10,500 manufacturing facilities, and the highest number of US FDA-compliant pharma plants outside of the US, according to the National Investment Promotion and Facilitation Industry.
As we witness the tremendous growth of the pharmacy industry, we also require talented pharmacists who can take the industry to a whole new level of growth and success. To secure this idea, pharmacy education in India needs to transform so that it creates further opportunities while also solving challenges in the industry.
Need to enhance education
According to government estimates, there are approximately 17 lakh registered pharmacists in India. These professionals are vital in delivering healthcare in the country owing to their in-depth knowledge of medicines, their formulation, and their usage. They will define the future of holistic patient care in India. Therefore, the quality of education they receive has a direct impact on the quality of healthcare the common people get.
We have also observed that technical education has surged in India, especially in the pharma sector. However, not
everyone has exposure to this quality education. For India to grow as a hub of pharmacy and meet global standards and expectations, we need a change in education.
Updating the curriculum
Dr Bharati Pravin Pawar, Minister of State for Health and Family Welfare while inauguratingPharma Anveshan 2023 said that the pharma industry is in dire need to adopt a focused approach and upgrading the curriculum according to the latest developments in the industry. She also emphasised the importance of offering thorough training in the use of specialised medications to increase the reach of pharmacists across the nation.
The last updated curriculum in 2020 included subjects such as Social Pharmacy and Pharmacotherapeutics to enhance students' knowledge in areas of pharma care and preventative healthcare. In addition to that, there is also a pressing need to include the latest subjects such as pharmacovigilance (PV) and medical writing (MW) in the curriculum. Pharmacovigilance (PV) will help students understand and monitor the after-effects of drugs after they are licensed to use. Furthermore, medical writing will help in creating clinical trial documents, and structured documents which will lead to clarification of research results, product use and other medical-related information.
More emphasis on vocational education
For professionals in the healthcare sector, vocational and specialised skills are necessary. However, when we compare it to the pharma sector, there still lies a significant gap that needs to be bridged. The industry already has several back-end and front-end operations such as manufacturing, R&D, sales, TQM and more. Hence there is a pressing need that students to cultivate the necessary skills as they are a pragmatic approach to hands-on jobs. Vocational training is the key to cultivating sophisticated skills which are required in the industry. Hence, much emphasis must be given to providing an industrial and practical approach to the students. Furthermore, clinical training must be given significant importance and made part of the curriculum. A better way to do it is by implementing researchoriented learning which is way more effective than traditional methods. Furthermore, National Educational Policy (NEP 2020) in India has also emphasised the importance of amalgamation of vocational with general education which can be beneficial for the development of the students.
Developing industryties
Another aspect that needs to be focused on is the industryacademia ties. This coordination and collaboration will further sharpen the wedge between the skills learned by students and the skills that need to be applied on a job. The regular interaction between the two domains ensures that the students stay abreast of all
the industrial developments. Furthermore, this will also help students to understand and observe the first-hand industry developments which will further guide them to adapt to the changing needs.
There is also a need to create programs which cater to these practical learnings by bringing on board some of the seasoned leaders from the industry. These leaders can further guide on what skills will be needed to flourish in the sector. Colleges and universities can further partner to create a system that is beneficial for students to experience how the industry works.
All things considered
We can witness a myriad of transformations happening in the pharmacy industry while there is a dire need to change education as well to cope. It is significantly moving from volume towards a value-based industry. To keep its promise of being a high-quality, dependable supplier of medicines to the world, the industry will need to constantly invest in upgrading standards. To be a part of this value addition, there is a dire need to cultivate a pool of skilled talent. This skilled and agile talent can leverage opportunities and negate challenges in this post-COVID era.
The goals can be achieved effectively if educational institutions focus on providing vocational training, collaborate with business professionals, and also give their students access to up-to-date curriculum. This will guarantee that new pharmacy industry aspirants have a bright future and will further propel the sector to new heights.
May2023 EXPRESS PHARMA 31 HR
PACKAGING
Mitigating fill,finish and CCI challenges for injectables


Primum non nocere. First, do no harm.
The often-official motto of healthcare providers, organisations, and associations around the world sets the bar for the global pharma industry. Patient safety should always come first. However, this philosophy isn’t just relegated to guiding the practices of medical professionals and drug developers. It extends all the way down to the healthcare sector’s expectations for every component, instrument, and process orbiting the development and manufacture of medicine—especially parenteral drugs. Injectable therapies are some of the most highly sensitive drugs that require the most rigorous protective measures to ensure no particles, microbes or oxidisation jeopardises formulation integrity.
These high standards aren’t reachable without serious commitments to Container Closure Integrity (CCI). CCI is the ability of a container closure system to maintain the sterility of final pharma, biological and vaccine products throughout their shelf life. It is also a regulatory requirement upon which to qualify closure designs. In the case of parenteral drugs, CCI aims to avoid adulteration of the drugs packaged in vials, syringes, and cartridges. Even though these types of packaging systems are sealed in a hermetic manner, there are still many risks to mitigate.
What factors threaten CCI?
CCI is subject to many threats from the ambient environment. Vials, cartridges, and syringes are often packaged one of two ways. The industry preference is with terminal sterilisation wherein the entire packaging system is sterilised. Alternatively, drug manufacturers can package drugs with
components that have been sterilised individually, assembled, then filled aseptically by filtering the drug product through a 0.2-micron filter. However, drugs filled and packaged using either method are still subject to a few potential risks around leaks due to CCI failure:
1. Loss of aqueous solvent due to vaporisation: If the drug is in an aqueous medium, any hole in the vial can accelerate vaporisation. That can translate to a loss of solvent. Not only can that issue compromise the formulation and patient health, but the disappearance of a labeled ingredient can land drug manufacturers on the wrong side of the law and potentially find professionals close to the product manufacture in jail. This is because it is illegal to sell a mislabeled drug in most countries around the world.
2. Oxidisation: Oxygen is, obviously, a potent oxidiser that must be excluded from the packaging system of parenteral drugs. Otherwise, the presence of oxygen can lead to the breakdown of fats, lipids, proteins, and other ingredients, compromising the drug formulation
and impact of the dosage. For example, in a 5mL vial, there are typically 3 mLs of liquid drug product and 2 mLs of gas that sits above it. Drug manufacturers blanket their product with either nitrogen or argon to exclude any oxygen from the package. A leak would cause equilibration between the contents of the container and the outside environment. The oxygen-rich environment outside the vial will try to seep into the vial interior and equilibrate so it’s 18 per cent oxygen outside and inside the vial, achieving the same barometric pressure. This can lead to a loss in the active pharma ingredient and prevent the delivery of the therapeutic dose.
3. Inadvertent introduction of microbes: A leak in the vial of a parenteral drug package can let in more than just oxygen. There can still be the threat of microbe introductions, which can be highly dangerous depending on the microbe and the condition of the already compromised immune system of the patient. Even the pressure change of a storm could create the conditions to not just pull out, but also push in contents from the vial if there is a leak.
What can drug manufacturers do to minimise these risks?
First, we must remember that every small component, like the stoppers, plungers, and caps, have critical roles to play in preserving drug integrity as part of the parenteral drug packaging system. Stoppers are placed at the top of syringes, vials, and cartridges to seal the barrels of these containers. Plungers glide through the barrel of syringes to deliver injectable drugs smoothly and effectively. Caps often top off vials and are comprised of both metal and rubber components. All must
EXPRESS PHARMA May2023 32
Eugene Polini,Technical Key Account Manager,Datwyler points out that injectable therapies are some of the most highly sensitive drugs that require the most rigorous protective measures to ensure no particles,microbes or oxidisation jeopardises formulation integrity
Every small component,like the stoppers,plungers,and caps, have critical roles to play in preserving drug integrity as part of the parenteral drug packaging system
be designed with painstaking care to ensure compatibility with the drug product they interact with. Once that bar is cleared, there are many assessments, practices, and technologies available to help drug manufacturers enhance CCI:

1. It starts with a paper analysis. In this process, analysts compare drawings of components like stoppers and plungers to the drawings of the vials and syringe barrels to ensure that there is enough compression and interference between the elastomer and the glass or plastic package to create a seal. In addition, they check to make sure there isn’t too much interference or compression, which can compromise machinability during insertion. For syringes, they must also check the activation of plunger movement for the same factors. The fit can’t be too tight or too loose. It must be just right. We typically aim for a couple percentage points of compression between the rubber and the walls of the package, but optimal results may vary. For a stopper, more compression is desirable. A plunger requires less to ensure mobility up and down the barrel of the vial. It’s also important at this stage to account for blowback tolerances.
Many vials are now made with blowback features, small, recessed rings inside the neck of the vial so the rubber can relax into that recess so that a little bit of back pressure isn’t going to pop it off. A corollary feature can be added to the stopper – like a protuberance that can snag into the recess in the neck of the vial. However, problems can arise when the blowback feature is mismatched with the stopper design, exacerbating the issue. More recently, there was a movement to make blowback tolerances and dimensioning for these features clearer at the specification process which enables drug manufacturers to better identify potential mismatches at the paper analysis stage.
2. Next comes dry lab (or exploratory developmental) work wherein a series of practical physical tests are
conducted. The pop-up test is one example. In this test, a vial is filled with water and a stopper is placed loosely on top of the vial. The tester freezes the whole system overnight. The next morning, they bring it out and insert that stopper to observe whether it pops out as the system warms up due to positive pressure inside. That cold gas in the headspace after it’s sealed and starts to warm up, goes to a higher pressure and can pop the stopper off. The test offers an effective way to assess for stopper and vial compatibility.
3. Following the practical tests, CCI testing is conducted with more advanced instrumentation
and, typically, placebos (drug product without the active ingredient) to ensure the package is robust and shows no leakage over the temperature range that the drug product will experience through its lifecycle. This may include cold chain testing for cryogenic or cold storage, often a very challenging barrier to success. Some drugs must stay at liquid nitrogen temperatures –185 degrees Celsius to maintain viability. However, rubber and plastic materials take on the attributes of glass at those temperatures, making it difficult to achieve an adequate seal, and requiring alternative approaches. Very careful scrutiny must be paid to CCI
testing—especially with cryogenic and cold storage.
4. Residual Seal Force testing can help testers understand the residual spring left in the elastomeric closures flange. The flange compresses as vertical force is applied to it during capping. By locking the skirt in that metal furl during sealing, the energy in that rubber flange is captured and causes the rubber to be in a state of compression with the glass crown finish which ensures a great seal during the product lifespan. By measuring Residual Seal Force, testers can understand if the capping force is too great and liable to create a wrinkle and fold, and ultimately, a product leak.
This testing can help drug manufacturers find their sweet spot for capping force as a preventative measure to maximise seal integrity.
5. Once the product is made, the shelf studies begin. These include repetitive CCI testing at frequent intervals throughout the proposed shelf life of the drug. Typically, the manufacturer will put up three lots of drug product in its field packaging and at periods of 0, 1, 2, 3, 6, 12 and 18 months, they’ll pull samples and test them for, among other things, container closure integrity. This assessment gives drug manufacturers a good idea of how the drug will fare over time on the shelf.
Working toward CCI around the world!
No matter where drugs are manufactured, pharma companies are still subject to the CCI standards of the markets they serve. This can make the whole process complicated as pharmacopoeias vary with regions. For example, markets like Japan have had extremely strict regulations around sterility for closures compared to other markets that took significant R&D to develop elastomeric closures that met the standard. In the US, the Food and Drug Administration (FDA) prefers the deterministic CCI testing versus the probabilistic CCI testing of the past. The FDA asks drug manufacturers to use deterministic CCI testing instrumentation and technology because probabilistic methods are more subjective and contain more qualitative methodology while deterministic methods are quantitative and nondestructive while giving actionable insights. There is greater certainty in the deterministic testing methodology.
It is critical that drug manufacturers comprehend the differences between the markets they serve and work closely with their suppliers who understand the nuances of different regional regulations, and of course work toward the highest standards to do no harm.
May2023 EXPRESS PHARMA 33
Tech and policyevolution of India’s pharma supplychain
Dinesh Tarachandani,Head – Global Logistics,DPWorld Subcontinent points out that as the pharma industry accelerates its digital transformation journey,the supply chain services providers are focussing on driving innovations backed by data to strengthen pharma and medical logistics

The pharma industry is one of the critical pillars of India’s economy, and supply chain is what gives it enduring strength. For the longest time, however, the pharma supply chain was unorganised. This is changing now, with the entry of big players and retail giants. We are also seeing a shift away from the old ways when patients used to go to pharmacists to pick up the medicines they needed. Today the pharma, healthcare, and retail industries offer not just products, but personalised experiences and value-added services to patients. Products are delivered to people’s doorsteps in record time, that too with discounts and offers.
India is the world’s third largest producer of pharma products and has earned the sobriquet of “the pharmacy to the world”. Indian pharma companies have a substantial share in the prescription market in the US and the EU. India also has the largest number of FDA-approved plants outside the US.
To continue driving growth for the industry, we need modern, smart supply chains that are compliant with both domestic and international regulations; follow robust standard operating procedures; have multimodal capabilities across air, land, and water; provide end-to-end visibility and control; and use the latest technologies for competitive advantage. Let us examine some of the recent developments and emerging trends on the policy, consumer, and technology fronts that are shaping the evolution of India’s
pharma supply chain.
Policy-aided modernisation and growth
The supply chain in postCovid times is being shaped, in part, by government policies. In September 2022, the Central Government announced the National Logistics Policy with the objective of lowering the cost of logistics, increasing the competitiveness of Indian products in domestic and international markets, and improving the efficiency of industries.
India’s pharma MSMEs will
benefit from the reduction in logistics costs. The establishment of multi-modal logistics parks, as proposed in the policy, will improve last-mile connectivity, and strengthen the cold storage and warehousing infrastructure across the country. It will also allow for inventory buffers, thus lessening our dependence on other countries for raw materials.
In the pharma supply chain, products are usually sent to C&F agents, then to city-level distributors, and ultimately to retailers. Since much of this happens at a
local level, it can cause compliance issues, especially when it concerns medicines that need to be transported and stored at specific temperatures. This is because, in India, temperatures can vary widely across states, and even within cities in the same state. The newly proposed storage and warehousing policies will guide the ongoing efforts of pharma and logistics companies in addressing such issues by increasing the accessibility of the cold chain infrastructure and enhancing the connectivity to the storage areas through multimodal connectivity.
Building robust cold chain and reverse logistics capabilities
Cold chain is one of the toughest aspects of logistics to get right, and the demand for it is on the rise. Pharma and healthcare companies often ship products like vaccines, blood products, and medicines, which need to be maintained at certain temperatures to preserve their potency and validity. This can be achieved through temperature-controlled warehousing and transportation, with IoT sensors to continuously monitor the temperature. Furthermore, cold chain systems must have inbuilt protocols for mitigating risk and loss, and strong capabilities for dealing with contingencies.
Reverse logistics capability is yet another crucial aspect of pharma supply chain. There could be times when certain products need to be withdrawn from the market. This entails picking up the product from every nook and
corner of the country and bringing it to the company’s warehouses or to their country of origin. There’s a need for more investments in setting up dedicated centres that perform quality checks, re-labelling, and dispatch of the returned products to the warehouses. Technology will help in developing a robust control environment to prevent fraud and tampering in both forwar d and reverse logistics.
The role of Blockchain
Spurious drugs are a serious issue across the world, but a technology-enabled, transparent supply chain can help in addressing it. In Africa, improvements in pharma supply chain have reduced the prevalence of spurious and failed drugs from almost 45 per cent to less than 10 per cent in the past 10-15 years.
Supply chain visibility, speed and coordination are critical to the safe and timely delivery of pharma products, and Blockchain technology can be of great help in several areas. These include monitoring the cold chain; identifying contamination of high-value, temperature-sensitive products; ensuring the authenticity of products; improving time and cost efficiencies; addressing regulatory requirements for drug tracking, and more. Blockchain can help in accurately recording price, date, location, quality, certification, and other relevant information. This improves supply chain transparency and traceability and reduces administrative costs. Blockchain can help pharma companies build trust and
EXPRESS PHARMA May2023 34
LOGISTICS
optimise the value chain through secure and tamperevident data-sharing; product provenance; and digital asset tracking. Blockchain technology can give a clear picture of every capsule, every drop of a product from the manufacturer to the consumer, and nobody can manipulate the data.


Predictive supplychain with Big Data,AI,and ML
Pharma businesses that invest in Big Data Analytics, or work with logistics partners who do so, gain a great competitive advantage, as they can make more informed decisions, optimise capacity utilisation, reduce risk, and improve customer experiences. Dynamic, real-time route optimisation through the correlation of multiple data streams (such as shipments, weather, and traffic) can enable more efficient scheduling of consignments, optimisation of load sequences, and accurately predict the time of arrival. Smarter forecasting of demand, capacity, and labour can significantly optimise planning and resource utilisation and reduce supply chain costs. Big Data can be used to mitigate risks by detecting, evaluating, and providing alerts on potential disruptions caused by unexpected events, man-made or natural. This can be further enhanced with the integration of data from IoT devices.
Pharma businesses that invest in Big Data Analytics,or work with logistics partners who do so,gain a great competitive advantage,as they can make more informed decisions,optimise capacity utilisation,reduce risk,and improve customer experiences.Dynamic,real-time route optimisation through the correlation of multiple data streams (such as shipments,weather,and traffic) can enable more efficient scheduling of consignments,optimisation of load sequences, and accurately predict the time of arrival
Big Data, together with Artificial Intelligence (AI) and Machine Learning (ML), can also help pharma companies predict prescription trends and align their operations accordingly, well in advance. Geography-based market trends, customer behaviours, and healthcare trends can be used to predict orders. For instance, Rajasthan might have higher prevalence of a different set of illnesses as compared to Karnataka. The demand for medicines will accordingly differ. By studying the demand data, AI will be able to predict the prescription trends, and enable pharma companies and retailers to be ready with adequate quantities of the required products at the distribution centres
that are closest to the most likely customers. This can help pharma retailers to meet heavy demand and offer quick home deliveries. With pharma industry accelerating its digital transformation journey, the supply chain services providers have also increased their focus on driving innovations backed by data to strengthen the pharma and medical logistics.
For instance, DP World’s production management and business intelligence tools provide enhanced visibility and control across the supply chain, thereby supporting optimum decision making. Furthermore, company’s suite of digital technologies is helping our customers identify bottlenecks in their supply chains and smooth the
flow of medical supplies across borders.
Looking ahead
The era of the metaverse is dawning, and although it’s still early days, we can expect the metaverse to find a wide range of use cases that enable pharma companies to improve their experiential offerings. The metaverse will also play a vital role in applications such as simulating terminal operations; carrying out container and vessel inspections; conducting immersive, online safety trainings; and creating Digital Twins where a virtual representation of real-world processes is created, which can help in addressing industrial and supply chain challenges and achieving substantial time
and cost savings.
Metaverse can help a single operator monitor terminals from different parts of the world in real time through virtual reality. It can also enable pharma companies to virtually check out the facilities and capabilities of prospective logistics partners. From a user benefit standpoint, Metaverse can be used to demonstrate the correct way of administering a certain medicine.
In coming years, we could see specialisations emerging within the pharma supply chain. Clinical trials, for instance, require a specialised supply chain because the samples are highly sensitive and need careful management and handling.

Meanwhile, as the costs and complexities of healthcare and pharma logistics continue to rise, pharma companies need logistics partners who can mitigate any adverse impacts on their business and on patients. The ability to leverage relationships, network, experience and skills is crucial to ensure that access to quality healthcare remains equitable across the globe.
Partnering with logistics providers can yield significant benefits for pharma companies on both the business and the consumer health fronts. With the health of millions of humans at stake, there is no room for compromise or laxity.
May2023 EXPRESS PHARMA 35
Cloud enhancing data sets for real-world clinical trials
Software highlights how
initiatives to improve patient outcomes

Real-world data can help researchers make important decisions about clinical trials. However, that data must be organised and accessible. The volume of data being collected in the healthcare industry is increasing rapidly. If researchers and healthcare organisations can access quality data sets, they could unlock new insights to drive improved health outcomes.
This is especially useful in clinical trials, where pharma companies and researchers actively work toward new treatments. Real-world data (RWD) and real-world evidence (RWE) can help scientists to make important decisions for clinical trials.
Making effective use of RWD also goes beyond clinical trials, as it can help payers, regulatory bodies, and policymakers. However, to access large patient data sets, organisations often need to rely on the cloud for its scalability and flexibility. To use RWD and RWE, it’s important for healthcare stakeholders to understand what they are, how they relate to clinical trials, and how the cloud can support data initiatives to improve patient outcomes.
Real-world data can give researchers a comprehensive and up-to-date understanding of a disease, its treatment, and its impact on patients. This data can then be used to make informed decisions about which patients should be included in a study, which treatments to evaluate, and which outcomes should be monitored. It can also provide insight into how specific treatments are used in clinical practice and impact patient outcomes. Such data can be invaluable for clinical trial planning and design, ensuring that the results from
a trial will be relevant to the real world.
Cloud enhancing data sets for real-world clinical trials
The cloud provides a secure and scalable platform for data storage and analytics. It also offers an expansive, low-cost infrastructure for organisations to store, analyse, and share large datasets. It provides data secu-
rity, scalability, reliability, and access to powerful analytics tools that can be used to gain insights and make better decisions. Additionally, the cloud can be used to power AI and machine learning applications to improve accuracy and speed in data analysis.
Real-world clinical trial data sets contain a wealth of information about the patients enrolled
in the study, the treatments they receive, and their outcomes. Analysing and interpreting these data sets to drive greater clarity, structure, and context is now the need of this hour. Data sets often contain large amounts of data that are difficult to understand, and the relationships between variables are only sometimes transparent. Cloudenhancing data sets can help bridge this gap.
Many organisations rely on the cloud to bring real-world data together. These real-world data platforms leverage cloud computing technologies such as cloud storage, flexible computing, geographic reach, and AI with machine learning (ML) to bring varied real-world data sources together, which is especially important when it comes to clinical trials. In addition to public databases, researchers or healthcare organisations can create in-house databases or aggregate data from several public databases and apply an artificial intelligence (AI) algorithm to enhance real-world data analysis with fewer mistakes compared with a human. Cloud computing and ML models can help healthcare organisations break down data silos and digest information to be accurate, relevant, and actionable. This allows organisations to focus on patient care while cloud technology automatically normalises, indexes, structures, and analyses the data for them. These data sets can be organised and contextualised to facilitate a better understanding of the data. Rich visualisations and machine learning algorithms can better simplify these data sets. Cloud computing also allows faster and more efficient data processing and analysis. As cloud computing continues to
grow, the potential of cloud-enhancing data sets for real-world clinical trials is also increasing. These data sets can provide valuable insights into patient outcomes and treatments, helping improve patient care and facilitate better decision-making.
Looking at the future
Today, we are seeing a wave of healthcare organisations moving to the cloud, enabling researchers to aggregate and harmonise research and development data with information from across the value chain while benefitting from compute and storage options that are more cost-effective than onpremises infrastructure. Cloudbased hyper-scale computing and ML enable organisations to collaborate across data sets, create and leverage global infrastructures to maintain data integrity, and more easily perform ML-based analysis to accelerate discoveries and de-risk candidates faster.
The future of real-world data in healthcare is very promising. Real-world data (RWD) is derived from sources outside the traditional clinical trial setting, such as patient electronic health records, insurance claims, and patient-reported outcomes. It can be used to improve patient care, inform health policies, and demonstrate medical products' safety and effectiveness. RWD can also provide insights into healthcare costs, utilisation, adherence, and patient outcomes. The data will continue to inform healthcare decisions and improve the quality of care. Additionally, using artificial intelligence (AI) and machine learning (ML) will become increasingly important in analysing RWD, allowing for more accurate and timely insights.
EXPRESS PHARMA May2023 36 IT@PHARMA
Shivajyoti Bhattacharjee,VP– Healthcare & Life Sciences,Cybage
RWD and RWE are pivotal to clinical trials,and how the cloud can support
Real-world clinical trial data sets contain a wealth of information about the patients enrolled in the study,the treatments they receive,and their outcomes.
Analysing and interpreting these data sets to drive greater clarity, structure,and context is now the need of this hour










BUSINESS AVENUES EXPRESS PHARMA EXPRESSPHARMA May2023 37

BUSINESS AVENUES EXPRESS PHARMA May2023 EXPRESSPHARMA 38
ZONE1 REMOTEMONITOR
ARemoteMonitorsuitableforusein
Zone1,Zone2Gasgr.I,IIA&IIBhazardousareas
rPC1200
PRODUCTFEATURES
Zone1Certified Workstation

IntrinsicallySafe Keyboard
Supports VNCServer

CORPORATEOFFICE
SAP,MES,SCADAcanbe OperatedonShopFloor
USFDA21
CFRPart11Compliant
ReplacesNon-compliant/ FirmwareHMI
POLMONINSTRUMENTSPVT.LTD. PolmonHouse,NizampetRoad,Kukatpally,Hyderabad-500085TelanganaIndia T:+914023057308/3046info@polmon.comwww.polmon.com
YourtrustedpartnerforProcess&AnalyticalInstrumentation


POLMON’SCAPABILITYMATRIX
•INSTRUMENTS•AUTOMATION
•HEATTRANSFERSYSTEMS
•MEDICALDEVICES•SERVICES
AuthorisedSystemIntegrator &GalaxyPartnerSince2001
BUSINESS AVENUES EXPRESS PHARMA EXPRESSPHARMA May2023 39




















BUSINESS AVENUES EXPRESS PHARMA May2023 EXPRESSPHARMA 40

BUSINESS AVENUES EXPRESS PHARMA EXPRESSPHARMA May2023 41

BUSINESS AVENUES EXPRESS PHARMA May2023 EXPRESSPHARMA 42

BUSINESS AVENUES EXPRESS PHARMA EXPRESSPHARMA May2023 43

































BlueHeaven E-mail:sales@mksilicone.com 208,HillViewIndustrialPremises, AmrutNagar,Ghatkopar(W),Mumbai-400086,India. Tel.:022-25004576Mob.:9321965968 SILICONETRANSPARENTTUBING M.K.SiliconeProductsPvt.Ltd. fortheQualityConscious…. INDIA QM002 AnISO9001-2015COMPANY Quality Products Since 1997 CERTIFIED CLEA N ROOM IS08 BUSINESS AVENUES EXPRESS PHARMA May2023 EXPRESSPHARMA 44 To Advertise in Business Avenues Email: rajesh.bhatkal@expressindia.com rbhatkal@gmail.com

BUSINESS AVENUES EXPRESS PHARMA EXPRESSPHARMA May2023 45
Productionof Transdermal&Oral FilmSystems

Coating,DryingandLaminatingallperfectlycoordinated
MATHISAGdevelopsandmanufacturesawiderangeofsystemsandplantsfor useincleanroomsandnormalatmospheres












Thesenaturallycomplywithallapplicablestandards,regulationsandspecific customerrequirements













Wewillshowyouhowtoachievetheoptimalperformanceofoursystems
MarketleadersareusingMathistechnology
BUSINESS AVENUES EXPRESS PHARMA May2023 EXPRESSPHARMA 46




BUSINESS AVENUES EXPRESS PHARMA EXPRESSPHARMA May2023 47






















DataIntegrity,ImportanceandCompliance DataAcquisition PasswordEncryption GraphicalAnalysissdisplay RealTimeAnalysis SecuredAuditTrail(User, Equipment.AlarmSMS,Events) Sterile & Non-Sterile Latex GLOVES 16” Sterile&Nonsterilelatexcleanroomcompatible 100%naturallatex&Powder-free Ambidextrous Texturedpalm-ngerswithBeadedcuff Double-donnable&ExcellentFit GammaIrradiated +912240787979|info@june4gmp.com|www.june4gmp.com care@jeclin.co|www.jeclin.co ™ AvailablewithSterilityTestingDocuments BUSINESS AVENUES EXPRESS PHARMA May2023 EXPRESSPHARMA 48

BUSINESS AVENUES EXPRESS PHARMA EXPRESSPHARMA May2023 49
OsmoTECH®XTSingle -SampleMicro-Osmometer





Nowavailable!
Best-in-classosmolalityperformance, designedwithyouinmind.

HIGHLIGHTEDFEATURES:

Offersthewidestrangeofosmolalitytesting(0–4000mOsm/kgH2O) Supports21CFRpart11,GMPandEUAnnex11compliance


MeetsPharmacopeiaosmolalitytestingguidelines

3Leveluseraccessandpasswordprotection
Storage:unlimiteddatastorageforaccess
Audittrail:Preserveunlimitedresultsandevents
Databasebackup,protectsyourdatawithautomaticormanualbackup
No.127,BussaUdyogBhavan,TokershiJivrajRoad,SewriWest,Mumbai-400015, Maharashtra,Landline:+91022-24166630Mobile:+919833286615
® ® ®
BUSINESS AVENUES EXPRESS PHARMA May2023 EXPRESSPHARMA 50

BUSINESS AVENUES EXPRESS PHARMA EXPRESSPHARMA May2023 51






























BUSINESS AVENUES EXPRESS PHARMA May2023 EXPRESSPHARMA 52
PHARMA PULSE
L.B.Bohle – The tablet coating experts
As a coating process consists of simultaneous spraying,mixing and drying processes,a good coating uniformity can only be achieved with the choice of the proper parameters.L.B.Bohle's tablet coaters are technology leaders in this field with the shortest process times
When it comes to fast, problem-free, and efficient coating of tablets, the tablet coaters from L.B. Bohle Maschinen und Verfahren GmbH (Germany) have been defining the standard for years.
Nowadays, pharma film coating represents an important process step in the pharma oral solid dosage production. Most coating processes are performed for drug release modification, drug stability improvement against light or moisture and taste masking. Furthermore, patient compliance issues play an important role, as swallowability improvement or a simpler identification due to a different color.
Finally, API coating is gaining more and more importance since it enables fixed dose combinations or the combination of incompatible drugs. Also, different drug release characteristics can be realized by applying for example sustained release coatings in addition to immediate release coating layers. Such formulations sometimes consist of up to four coating layers, which leads to long processing times. To successfully develop and produce such formulations coating uniformity is a decisive prerequisite and a quality attribute.
As a coating process consists of simultaneous spraying, mixing and drying processes, a good coating uniformity can only be achieved with the choice of the proper parameters. L.B. Bohle's tablet coaters are technology leaders in this field with the shortest process times. Furthermore, they ensure best production results and offer the best combination of the three process operations on the market:


◆ Mixing: It is essential that the tablet cores move evenly underneath the spray cones without mechanically stressing and damaging the tablet cores. The perfect solution for this is an extended coating drum (length to diameter (L/D) > 1) with L.B.
Bohle's patented mixing spirals. The mixing spirals guarantee a constant and gentle mixing of the flat tablet bed. This reduces the mass pressure within the tablet bed. The rotary movement of the coater drum produces radial mixing, resulting in a dead-zone-free tablet bed. Due to continuous guidance by means of the double spirals, the tablets do not experience strong acceleration, so that tablet breakage or even twinning does not occur.
◆ Spraying: Due to the drum geometry, the largest possible spray area is achieved in the moving tablet bed. This allows the use of a large number of spray nozzles. Thus, L.B. Bohle Coaters reduce the process time by approximately 50 per cent compared to conventional
coaters on the market with L/D ratios < 1 due to higher spray rates. In addition to the suspension, the nozzle type, the number of nozzles and the distance between them and the tablets are particularly important. L.B. Bohle offers various solutions for adjusting the nozzle-to-tabletbed distance, the spray angle of the nozzles and the pressure parameters for atomisation.
◆ Drying: L.B. Bohle tablet coaters guarantee the best energy and mass transfer through a modified air flow. The process air is fed directly into the tablet bed. The air flows directly and smoothly into the tablet bed and ensures fast drying of the sprayed suspension. The spray nozzles are not exposed to the supply air flow for the entire duration of the process, so that
they remain cool during the spraying process. As a result, spray drying effects are reduced to a minimum and coating efficiencies of >95 per cent are achieved. This advantage is also particularly important in the coating of active ingredients, as less spraying loss and more uniform tablet coatings are produced.
Coating machine for any application
The BFC tablet coater is the high-end version of all L.B. Bohle tablet coaters, featuring high efficiency, optimum performance, and lowest spray losses. The BFC reduces process time by approximately 50 per cent compared to standard coaters while providing the best coating

May2023 EXPRESS PHARMA 53
uniformity. The high-end tablet coater achieves RSD < 3 per cent for all processes. In addition to this result, the BFC also guarantees perfect results in color coating accuracy (< 0.5 deltaE measurements). The integrated high-pressure washing system guarantees Cleaning-In-Place (CIP) with first-class results.
The BFC coaters realise batch sizes of 50-980 liters. Due to geometric similarities, a scale-up, as with all L.B. Bohle Coaters, possible without problems.
The BTC is the economical alternative for all coating applications. With the BTC, L.B. Bohle offers a cost-saving system for more efficient and longer running processes in pharmaceutical production.
Major advantages of the BTC include its simple design for
"through-the-wall" installation, the built-in control cabinet, and a simple, functional housing. In addition, the Bohle Tablet Coater supplies all nozzles with suspension fluid from a single pump head.
The BFC Tripan is a particularly versatile system that can be operated with three drums. Thus, batches of 7-75 liters can be realised. The drums can be changed easily and quickly by using a lifting device.
◆ L.B. Bohle also has the perfect solution for research and development: The BFC 5 laboratory coater is designed as a mobile stand-alone unit. The BFC 5 can be operated with two different drums. The smaller pan can also be separated with a divider plate. This flexibility allows a batch size range of 2-13
liters. With a divider plate option batch size range of 0.8-13 liters can be achieved. A multi-panel touch panel visualisation is attached to the coater.
L.B. Bohle meets the increased demand for continuous manufacturing with the semicontinuous coater KOCO®. The KOCO® is based on the proven, patented design of all L.B. Bohle tablet coaters. The auto coater is charged from the top and the coated tablets are discharged though a corresponding opening at the front of the coater door. The process analysis is carried out via an integrated Raman probe.
Containment - no problem!
The number and scope of containment applications in pharmaceutical production has in-
creased significantly in the last few years. L.B. Bohle has thus frequently implemented containment solutions in the coating sector in all toxicity classes and for all production scopes. A R&D version of a containment coater is also available. The containment coaters impress with e.g. an automatic nozzle adjustment, infrared product temperature measurement, the connection for cleaning and drying as well as the control integration of the containment valves or side doors with inflatable seals.
Slotted coating pan for mini tablets
Mini tablets offer the advantage that several tablets can be combined for the application of the required single dose. In a specially slotted coating drum,
which is provided with a corresponding perforation, not only normal-sized cores but also small cores with a diameter of 1.5 millimeters can be filmed.
Trial availabilityin India
Starting in May, a BFC 5 EX laboratory autocoater will be available for trials and process optimisation at our Innovation Center in Hyderabad. Meet our team at the Innovation Center and convince yourself of the high quality of the machine. All L.B. Bohle will help you to optimise your production process, reliability, and efficiency.
Contact:
Parag Radia Director L.B. Bohle India p.radia@lbbohle.com +91 987 959 8784
EXPRESS PHARMA May2023 54
PHARMA PULSE
E m a i l : r a j e s h b h a j n i k @ e x p r e s s i n d i a c o m ■ C o n t a c t N o 9 8 6 7 1 4 5 0 2 8 C o m p a n y N a m e - T h e I n d i a n E x p r e s s ( P ) L t d , C o m p a n y A d d r e s s - M a f a t l a l C e n t r e , 7 t h F l o o r , R a m n a t h G o e n k a M a r g , N a r i m a n P o i n t , M u m b a i - 4 0 0 0 2 1 B a n k N a m e - H D F C B a n k L t d ● B a n k A d d r e s s - C - 5 / 3 2 , S a f d a r j u n g D e v e l o p m e n t A r e a ( S D A ) , N e w D e l h i - 1 1 0 0 1 6 ● A c c o u n t - 0 0 3 2 8 6 3 0 0 0 0 0 7 5 ● S w i f t C o d e - H D F C I N B B ● I F S C - H D F C 0 0 0 0 0 3 2 A c c o u n t T y p e - C u r r e n t
PROSOLV® SMCC 50 - Ahigh-functional excipient from JRS Pharma
PROSOLV® SMCC provides solutions to the problems often encountered by formulation scientists while using conventional diluents having low bulk density (BD),poor flow,low compactability or loss of compactability during processing,sticking and sensitivity to lubricant(s)
The development of coprocessed, multi-functional excipients has enabled formulators to address multiple challenges with a single excipient, resulting in enhanced production and better finished product quality.
WhyPROSOLV® SMCC?
Attaining good hardness at low compaction forces is quite essential while considering suitability of excipient for tablet formulation. Microcrystalline cellulose is one of the widely used and accepted excipients in tablet dosage form. Compactability of microcrystalline cellulose (MCC) is of prime importance during compression.
PROSOLV® SMCC is a novel high-functionality tableting excipient. The material is manufactured by co-processing MCC with colloidal silicon dioxide (CSD) and can be used to improve flow, lubricant sensitivity and tablet strength. The addition of CSD in MCC helps to improve compactability [1][2]
It has been reported that silicification appears to have no apparent effect on the primary chemical and polymorphic characteristics of MCC. This suggests that bulk modification of MCC does not occur during silicification and that the CSD, either by providing surface modification or by modifying strengthening interactions, is primarily responsible for the improvements in functionality, in particular tablet strength. This may be solely due to a morphological property or some other silicon dioxide MCC interfacial interaction. Based on scanning electron microscopy studies together with electron microprobe analysis, it was stated that silicon dioxide is primarily located at the
surface of the SMCC particles. While certain amounts of silicon dioxide were detected in the internal regions of some particles, the colloidal silicon dioxide particles present at the surfaces of the SMCC particles are shown to be uniformly distributed[2][4]
properties, applications and advantages of PROSOLV® SMCC 50 & PROSOLV® SMCC 50 LD.
These grades offers several benefits to formulation development scientist(s) during development and production at later stage. These benefits
PROSOLV® SMCC is available in following grades[3] –
PROSOLV® SMCC 50 LD Best in class binder (improves tabletability)
PROSOLV® SMCC 50 Formulas in which optimal compaction and decent floware required
PROSOLV® SMCC 90 Formulas in which a balance of flow and compaction are required
PROSOLV® SMCC HD 90 Formulas in which optimal flowand consolidation are required
PROSOLV® SMCC 90 LM Equivalent to PROSOLV® SMCC 90, with lower moisture content
PROSOLV® SMCC provides solutions to the problems often encountered by formulation scientists while using conventional diluents having low bulk density (BD), poor flow, low compactability or loss of compactability during processing, sticking and sensitivity to lubricant(s). This article will focus on physico-chemical
along with a few case studies will be elaborated in next section of this article –
Benefits of PROSOLV® SMCC 50 and PROSOLV® SMCC 50 LD in Roller Compaction Applications [3][4]:
As stated above,Silicification of Microcrystalline cellulose results in an increase in surface area. This increase in

PROSOLV® SMCC 50 LD is the Lowdensitygrade from PROSOLV® familyof excipients having belowcharacteristics –
surface area increases binding interaction between the individual particles by reducing the interparticulate cohesion. As a result, silicification improves both the flowability and re-compaction properties of compacts.
PROSOLV® SMCC 50 LD is a low-density PROSOLV® SMCC grade, it is having benefits like it improves the tablet hardness and re-compactability issues after roller compaction. PROSOLV® SMCC 50 LD can be first choice.
PROSOLV® SMCC 50 can be useful when both tablet hardness and powder flow are required.
Following are the case studies to evaluate the effect of physiochemical properties of PROSOLV® SMCC 50 and PROSOLV® SMCC 50 LD on tablet dosage form.
Roll compaction is necessary to improve bulk density, flowability, compactability & to improve content uniformity in the tablet dosage form.
In this study the comparison of MCC 101, PROSOLV SMCC 50 & PROSOLV SMCC 50 LD was studied & evaluated in roller compaction. The Pow-
der blends used consisted of 75% of the Cellulosic compound (either MCC or SMCC) and 25% Dicalcium phosphate dihydrate.Material were blended for 10 mins.Different roller compaction forces were adjusted and the compacts were milled. The resulting powder was tested for particle size distribution and flowability. Furthermore, placebo tablets containing 0.5% of the lubricant, Sodium Stearyl fumarate (PRUV®), were compressed (Tablet weight- 400 mg, round, 13mm diameter) and hardness as well as disintegration time of compressed tablets was analyzed.
Compacts comparison
In this study, the comparison of compacts at 25 KN was evaluated. After roller compaction compacts density plays an important role to get desired Granules: Fines ratio. This is important step for the flow & content uniformity point of view.
Observation: MCC 101, PROSOLV® SMCC 50 and PROSOLV® SMCC 50 LD yield nicely shaped hard compacts at
PROSOLV® SMCC 50 is the grade from PROSOLV® familyof excipients having belowcharacteristics –
May2023 EXPRESS PHARMA 55 PHARMA PULSE
Grade Functionality
Particle size (d50) Bulkdensity Around 50 μm 0.20-0.30
g/cc
Particle size (d50) Bulkdensity Around 65 μm 0.25-0.37 g/cc Ingredients Percentage PROSOLV® SMCC or MCC 75 % DCP(EMCOMPRESS®) 25 % PRUV®
MCC 101 PROSOLV® SMCC 50 PROSOLV® SMCC 50 LD
(Extra granular) 0.5 %
PHARMA PULSE
25kN compaction force.From above picture we can conclude that PROSOLV® SMCC 50 LD yields dense compacts compare to Microcrystalline cellulose.
Flowability
In this study the flow of
lubricant sodium stearyl fumarate (PRUV®) & compressed in to tablets.
Compacts based on PROSOLV® SMCC 50LD yield 30% harder tablets. Compacts based on MCC 101 and PROSOLV® SMCC 50 have
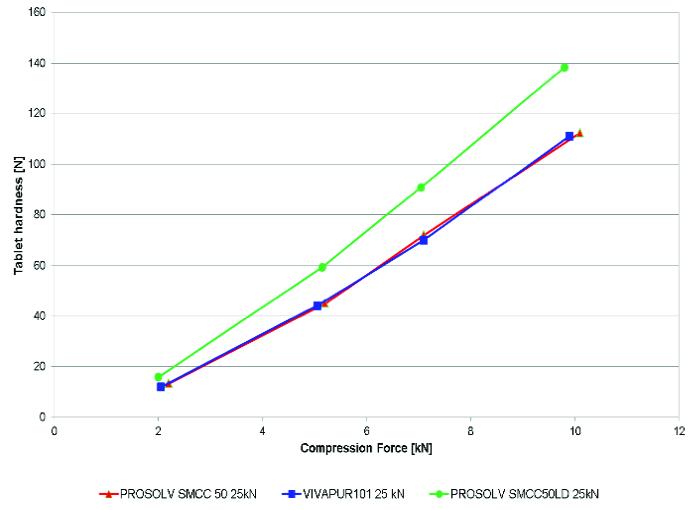
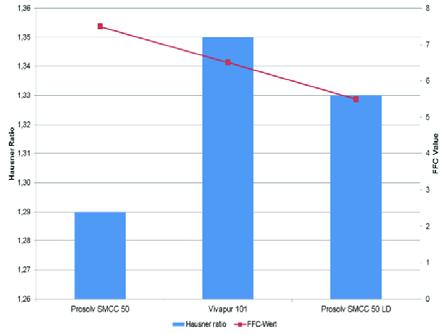
nearly identical tablet hardness profiles over the entire range of compression forces.
Conclusion
The silicification of MCC has been shown to significantly influence the hardness and the flowability of compacts in roll compaction process, as well as the hardness of tablets made from granules obtained from the milled compacts. If the overall target in tablet production is the highest possible tablet hardness, the use of PROSOLV® SMCC 50 LD recommended [3][5][6]
If both, the particle flow and the tablet hardness is the target PROSOLV® SMCC 50 is the best choice [3]
REFERENCES
[1] Tobyn, M.J., McCarthy, G.P., Staniforth, J.N., Edge, S. (1998) Physicochemical comparison between microcrystalline cellulose and silicified microcrystalline cellulose. Int. J.Pharm. 169, 183-194.
[2] Sheskey, P.J., Cook, W.G., Cable. C.G. (2017) Handbook of Pharmaceutical Excipients, 8th edition, Pharmaceutical Press, 1007-1009.
[3] PROSOLV® SMCC (jrspharma.com) (https://www.jrspharma.com/ph arma_en/products/excipients/pro solv-smcc.php)

milled compacts was evaluated.
Observation: Compacts were milled. It was found that PROSOLV® SMCC 50 exhibit best flowability followed by PROSOLV® SMCC 50 LD and MCC 101.
Tablet Hardness
In this study the effect on Tablet harness of roller compacted granules was evaluated.
Observation: Tablets were manufactured by using
CONTRIBUTOR’S CHECKLIST
❒ Express Pharma accepts editorial material for regular columns and from pre-approved contributors / columnists.
❒ Express Pharma has a strict non-tolerance policy of plagiarism and will blacklist all authors found to have used/refered to previously published material in any form,without giving due credit in the industry-accepted format.All authors have to declare that the article/column is an original piece of work and if not,they will bear the onus of taking permission for re-publishing in Express Pharma.
❒ Express Pharma's prime audience is senior management and pharma professionals in the industry.Editorial material addressing this audience would be given preference.
❒ The articles should cover technology and policy trends and business related discussions.
❒ Articles for columns should talk about concepts or trends without being too company or product specific.
❒ Article length for regular columns: Between 1200 - 1500 words.These should be accompanied by diagrams,illustrations,
tables and photographs,wherever relevant.
[4] Sherwood, B.E., Becker, J.W. (1998) A new class of high functionality excipients: silicified microcrystalline cellulose. Pharm. Tech. 22, 78-88.
[5] Staniforth, J. N., Tobyn, M. J. (1996) towards a new class of high functionality tablet binders. III: Physical characteristics and particle morphology of silicified microcrystalline cellulose (SMCC). Proc. AAPS Conf. PT6162
[6] Sherwood, B. E., Hunter, E. A., Staniforth J. N. (1996) Silicified microcrystalline cellulose (SMCC): a new class of high functionality binders for direct tableting. Proc. AAPS Conf. PT 6164
❒ We welcome information on new products and services introduced by your organisation for our various sections: Pharma Ally (News,Products,Value Add),Pharma Packaging and Pharma Technology Review sections.Related photographs and brochures must accompany the information.
❒ Besides the regular columns,each issue will have a special focus on a specific topic of relevance to the Indian market.
❒ In e-mail communications,avoid large document attachments (above 1MB) as far as possible.
❒ Articles may be edited for brevity,style,and relevance.
❒ Do specify name,designation,company name,department and e-mail address for feedback,in the article.

❒ We encourage authors to send their photograph.Preferably in colour,postcard size and with a good contrast.
AUTHOR
Prashant Bhangdiya

Business Development Manager – Pharma
Rettenmaier India Private Limited Prashant.bhangdiya@jrsindia.com
Email your contribution to: The Editor, Express Pharma,Business Publications Division,The Indian Express (P) Ltd, Mafatlal Centre,7th floor,Ramnath Goenka Marg,Nariman Point,Mumbai 400021 viveka.r@expressindia.com viveka.roy3@gmail.com
EXPRESS PHARMA May2023 56
PRIME NEO: Multi-composite,high speed doors for clean rooms from Gandhi Automations


When it comes to pharma facilities and laboratories, clean rooms’ hygiene and protection from environmental contamination are the most important factors to consider.
Gandhi Automations’ PRIME NEOhigh speed doors are designed to provide superior sealing and resistance to pressure differences for clean rooms.
And the added advantage is that they are absolutely washable.
PRIME NEOhigh speed doors assure:
◆ Minimised contamination: The reinforced polymer material is smooth-surfaced and joint-free. The door structure is designed with full accessibility for water cleaning. Contamination risks are minimal, making
it fully compliant with the requirements of clean environments.
◆ Just-in-time opening cycle: Its smart design and German technology prevent delays in operation. Opening and closing on time reduces exposure time to a minimum, resulting in reduced energy cost and risks of airborne contamination.
◆ Sealing and resistance to
pressure differences: The door has a flexible curtain with horizontal FRP stiffeners guided in vertical guides. Pressure is evenly distributed over the whole curtain, pushing it against the guides giving a perfect sealing. This design allows the door to operate even under pressure differences up to 50 Pa for a controlled room environment.
◆ Safety: PRIME NEOreduces the risk of accidents and damage. The free, flexible and soft bottom edge minimises hitting impact. The radar detector and inbuilt photocells reopen the door at the slightest impact.
◆ Windows: The transparent material allows people to see coming traffic. Long term
transparency is assured, as the material is anti-fatigue PVC fabric.
◆ Multi composite structure: The toughness of side guides and their ability to absorb impact avoid high repair costs. PRIME NEO is anti-corrosion and paint free. This ensures durability in corrosive and aggressive environments.
◆ Auto-reset: The door is equipped with auto reset technology. In case of an accidental crash, the curtain sets itself again. This unique system avoids time loss and reduces the risk of high repair costs.
To know more aboutPRIME NEO, write tosales@geapl.co.in OR call 022 6672 0200 / 0300
May2023 EXPRESS PHARMA 57 PHARMA PULSE
Gandhi Automations presents multi composite high-performance door PRIME NEO for clean environments that are completely washable,provide greater sealing and are pressure resistant
ospVFFS: Pre-engineered solution for Vertical Form,Fill and Seal machine byB&R
With ospVFFS solution, B&R India has brought about a revolution in VFFS machine building, enabling machine builders to reduce machine development time, improve efficiency, minimize maintenance and benefit from a cost-effective solution. This solution enables builders to configure a VFFS machine within 30 minutes with various possibilities of packet sizes.
This not only improves machine performance but also enables a shorter timeto-market. In addition, the configurable solution is Industry 4.0 ready with webbased diagnostics and secure IT connectivity over inbuilt OPC UA.

B&R’s out-of-the-box ospVFFS solution enables rapid machine development and testing of VFFS machine with minimal time to market and effort. With the availability of such solution, OEMs achieve

longer availability, better performance, and higher quality, which boosts return on investment (ROI).
The product offers easy and secure remote maintenance coupled with predictive maintenance that helps OEMs to access their machine securely from anywhere across the globe. This provides best-in-class service and support to on-site maintenance teams reducing fault diagnostic times and minimising downtimes.
Contact B&R Industrial Automation (A member of the ABB Group), Pune
Email: marketing.in@ br-automation.com Tel: 020 41478999
EXPRESS PHARMA May2023 58 PHARMA PULSE
B&R’s out-of-the-box ospVFFS solution enables rapid machine development and testing of VFFS machine with minimal time to market and effort
ospVFFS solution offers easy and secure remote maintenance coupled with predictive maintenance that helps OEMs to access their machine securely from anywhere across the globe


REGD.WITH RNI NO.MAHENG/2005/21398,POSTAL REGD.NO.MCS/164/2022 – 24,PUBLISHED ON 5TH EVERY MONTH, POSTED ON 9TH,10TH,AND 11TH EVERY MONTH POSTED AT MUMBAI PATRIKA CHANNEL SORTING OFFICE,MUMBAI – 400001













































































 By Lakshmipriya Nair
By Lakshmipriya Nair





























































































































































































