Industry Sagacity

The Impact of Emerging Technologies on The Pre-Clinical CRO Market

Redefining Advancements
Role of CROs in Shaping the Future of Clinical Research
Most
 Dr Ibrahim Farr Chairman and CEO Pivotal
Dr Ibrahim Farr Chairman and CEO Pivotal
A Name Synonymous to Innova�ons
RESEARCHING and Educating to SAVE lives.


Editor’s Note

The Augmenta�ons for Perpetual Be�erments
DuringtheCOVID-19pandemic,ifclinicians,
healthcareservice-providingstaff,nurses,and doctorswerebattlingattheforefront,thenthe ClinicalResearchOrganizations(CROs)havebeen workinghardinthebackgroundtodevelopeffective vaccinestofightthisnovelcoronavirus.
Withmillionsofcasesemergingeverydayglobally,the demandforquickvaccinationraisedtheexpectationsfrom theCROstorisetotheoccasionandintensifytheirvaccine research,clinicaltrials,testinganddevelopmentalefforts, whichtheydidinarecordtime.
Becauseoftheirtremendousefforts,weasaglobalunit cameupwithnearlyadozenvaccinesalreadybeing deployedworldwide,withoversixty-eightpercentglobal populationvaccinatedatleastonce.
ThesearetremendousachievementswhiletheseCROsare furtheradvancingtheirforcestofindmorepossibleways throughnoveltechnologiestomitigatethevirusspreading andboosttheglobalpopulation’simmunityfurther
Themomentumwillbecarriedforwardaspredictedbythe Reportlinker’sreport.Accordingtothereport,theglobal CROmarketisprojectedtogrowataCAGRof10.06% during2022-26andreach$40billionbytheendof2026. Thereportfurtherstatesthatthemarketwillbefurther drivenbythegrowingbiopharmaceuticalindustry, increasedglobalR&Dexpenditureandinvestment, regulationsofclinicaltrials,andtechnologicalinnovations inthehealthcareindustry,particularlyintheCROend-user marketsegmentandgeographicallandscape.
Tograsptheseverypositivescenariosforthepresentand futureoftheCROindustry,InsightsCare’steamunveiled thisexclusiveeditionofthe‘10 Most Innovative CROs To Watch In 2023.’ThesetenCROsareamongthedisruptive innovatorswhenitcomestoclinicaltrials,R&D,and assistingthevaccinedevelopmentdrive.Alsoincludedin thiseditionaretwotrendyarticlesourin-houseeditorial teamcuratedtogiveyouaholisticviewoftheentire scenario.

FlipthroughthefollowingpagesandwitnessCROs’ futuristicleapintoahealthiertomorrow;enjoy!
-Anish Miller

CoverSto A Name Synonymous to Innovations 08 PIVOTAL C O



Articles eImpactofEmergingTechnologies onePre-ClinicalCROMarket Industry Sagacity 16 RoleofCROsinShapingtheFuture ofClinicalResearch Redefining Advancements 28 20 24 32 Providing Synergic Solutions for win-win Outcomes CLINERGY HEALTH RESEARCH Blend of Innovations Assuring Adaptability OnQ Research GENE THERAPY AND CANCER TREATMENT CXO N T E N T S






Co-designer
Art & Picture
Deshmukh Art & Design
Editor-in-Chief Pooja
Managing
Joshi Senior
Visualiser
Research Analyst
Business Development Executives
Business Development
Sales
SME-SMO Executives Gemson Digital Marketing Manager Alina
Technical Consultants David,
Technical
Marketing
Assistant Technical
Assistant Digital Marketing Manager Renuka Kulkarni Assisting Editors Saloni, Drishti Sonia
Circulation Manager Tanaji Fartade Copyright © 2023 Insights Success Media and Technology Pvt. Ltd., All rights reserved. The content and images used in this magazine should not be reproduced or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior permission from Insights success. Reprint rights remain solely with Insights Success Media and Technology Pvt. Ltd. Insights Care is powered by Insights Success Media and Technology Pvt. Ltd. January, 2023 Follow us on : www.facebook.com/InsightsCare/ https://twitter.com/Insightscare Insights Success Media Tech LLC 555 Metro Place North, Suite 100, Dublin, OH 43017, United States Phone - 302-319-9947 Email: info@insightscare.com For Subscription: www.insightscare.com Insights Success Media and Technology Pvt. Ltd. Office No. 22, Rainbow Plaza, Shivar Chowk, Pimple Saudagar, Pune, Maharashtra 411017 Phone - India: 7410033802, 74100058552 Email: info@insightscare.com For Subscription: www.insightscare.com sales@insightscare.com Contact Us:
Paul Belin
Editor Mrunalinee
Head
Bansal
Editor Abhishek
Editor Anish Miller
David King
Eric Smith Sarah Wilson, John Smith, Alex Vincent
Amy Jones
Manager
Executives Kelli, Bill, Anna
Sege
Robert
Head Jacob Smile
Manager John Smith
Head Amar Sawant
Raizada
10 MOST INNOVATIVE CROs TO WATCH IN 2023
COMPANY
BRIEF FEATURING
1MED SA 1med.ch
Biomapas biomapas.com
CLINERGY Health Research clinergyhealth.com
Ino�v ino�vco.com
Link Medical Research linkmedical.eu
Enrico Perfler CEO
1MED is a Swiss-based regulatory and quality consultancy company and full service CRO
Audrius Sveikata CEO Biomapas is a func�onal and full outsourcing solu�on provider to the global life science industry
CLINERGY was created to fill gaps le� by the tradi�onal outsourcing model and to leverage opportuni�es for the healthcare industry and clinical sites on a cost-effec�ve way, so that health innova�on reaches pa�ents faster
Bob Leasure CEO
Through scien�fic leadership and ongoing investments, Ino�v delivers a comprehensive range of nonclinical and analy�cal services that will exceed your expecta�ons.
Ola
CEO
LINK Medical is a full-service Nordic CRO providing product development services for the pharmaceu�cal and medical device industries across Northern Europe.
NDA Regulatory Science (NDA Group) ndareg.com
Chris�ne Lind Vice President Commercial
NDA is a leading regulatory drug & device development consultancy, suppor�ng companies with their regulatory, pharmacovigilance, quality and strategic planning.
OnQ Research onqsa.co.za

Catherine Lund Founder and Managing Director
OnQ Research is a South African contract research organisa�on that offers services to assist with the en�re con�nuum of clinical research.
Orphan Reach orphan-reach.com
P.R.I.S.M.A.- CRO GmbH prisma-cro.com
PIVOTAL pivotalcr.com
Thomas
CEO Orphan Reach is a full-service CRO with clinical opera�ons in place since 2002.
Gernot
P.R.I.S.M.A. is a German Clinical Research Organiza�on (CRO) and dedicated to support the European por�on of the pharmaceu�cal and biotech industries’ clinical development programs.
Pivotal is a Contract Research Organiza�on that weaves together the scien�fic insight, the technology and the resources to help customers address both current and future needs.
Tiago M D da Silva Managing Director
Gudmundsen
Ogorka
Cremer MD
Dr Ibrahim Farr Chairman and CEO
A Name Synonymous to Innova�ons


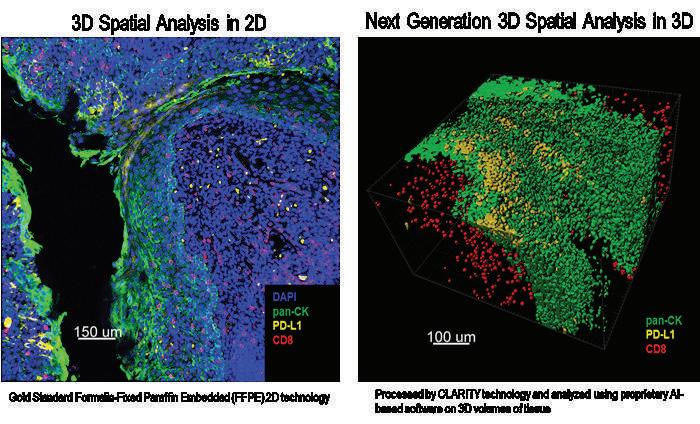
Pivotal’s team is proud of being involved in the ac�va�on, conduc�on and the execu�on of discrete full ac�vi�es of nearly 500 different clinical research projects in quite dis�nct therapeu�c areas and phases and with many diverse inves�ga�onal compounds, devices and products.




,
10 Most Innova�ve CROs To Watch In 2023







 Dr Ibrahim Farr Chairman and CEO
Pivotal
Dr Ibrahim Farr Chairman and CEO
Pivotal
Pharmaceuticalcompanieshavebeenincreasingly
usingoutsourcingtobemoreefficientandeffective indrugdevelopment,helpreducetime-to-market andboosttheongoingcommercialsuccessoftheir products.ThisshiftisacceleratingM&Aacrossmany segmentsofoutsourcedpharmaceuticalservices.Acrossthe pharmaceuticalvaluechain,outsourcedservicesaregaining traction,fromdrugdiscovery,contractresearchandcontract manufacturingtocommercializationservices,safetyand riskmanagementandpharmaceuticalIT.
CROsgenerallyemployanexpertise-driven,project-based consultingmodelthatrequiresstrategyandcapability aroundmanagingcomplextrials.Mostimportantly,while somelargeserviceprovidersexistinthissegment, significantconsolidationopportunitiesexist.Incomparison, thepreclinicalCROmarketismorematureand consolidated.Overtheyears,manylargepharmaceutical manufacturershavemovedfromworkingwithasetof preferredproviderstoformingstrategicpartnershipswith onlyoneortwolargeCROs.
Nevertheless,otherpharmaand,preferably biotechnological,companiesdostillprefertoworkwitha setofqualifiedmid-sizeCROsinordertoprovidethem withmorepersonalizedfocusedspecializedservices.
Withtheobjectiveofbecomingareliablefull-serviceCRO, Pivotalwasbornbackin2001ontheprinciplethat strategicscientificandmedicaladviceandsupportshould bethebackboneofallclinicaltrialswithproperand sufficientresourcestocovernotonlytheexecutionofall essentialaspectsandstagesofclinicaltrialsfromsetupto executiontotermination,includingclinicalmonitoringand projectmanagement.

UndertheleadershipofDrIbrahimFarr,Chairmanand CEO,Pivotalalsooverseesothercomplementaryactivities suchasmedicalmonitoringandwritingsupport,or pharmacovigilanceanddata-managementandbiostatistical services,i.e.,almostallthatittakestocompleteaclinical trial,whilejustleavingsomeveryspecializedtasks(e.g., drugdepotservicesorlab.analytics)inthehandsofvery specializedvendorsindependentfromthecompany.
TheProfoundLegacy
Now,almost21yearsfromitsfoundation,Pivotal’steamis proudofbeinginvolvedintheactivation,conduction,and theexecutionofdiscretefullactivitiesofnearly500 differentclinicalresearchprojectsinquitedistinct therapeuticareasandphasesandwithmanydiverse investigationalcompounds,devicesandproducts.
Withsomemoredetail,Pivotalhasbeenengaged,upto date,inatotalof361clinicaltrials–fromphase1tophase 4-thatinvolvedthemanagementofalmost6,000 investigationalsitesand>40,000patientsandincloseto 115non-interventionalstudies(NIS)whichinvolved,in turn,thehandlingof>9,000sitesand>62,000patients.Or in“bignumbers”:anaccumulatedexperienceresulting fromworkingwith>15,000sitesand>100,000patients–a heavybackpacktosupportitsexpertiseandknowledge.


Pivotal’steamgenuinelybelievethisallhasonlybeen possiblebecauseoftheirprovenexperienceinanddeep knowledgeoftheclinicalresearchlandscapeandbecauseof thehighqualitywithwhichPivotal´sassociatesfaceand manageallclinicalprojectsthathavebeenentrustedwith–alloftheseaspectsatrademarkofPivotalandwhichhave warrantedanessentialnumberofclientsrepeatingbusiness withus(almost75%ofrepeatbusiness),andothersturning toPivotaltoassumetherescueoftheiron-goingtrialswhen “notflowing”asplanned(some32clinicaltrialstodate).
Yet,andasidefromallthosemoregeneralandtechnical featuresdefiningPivotal,theteambelievesthatitsmost essentialstrengthscomenotonlyfromthemselvesbutalso
,Pivotal’s team genuinely believe this all has only been possible because of their proven experience in and deep knowledge of the clinical research landscape.
fromitsinternalcultureandauniquewayofapproaching theirclientsandtheirconcerns, always from a personal perspective and commitment to deliver high-quality results.
TheContinuumofSuccess
Overtheyears,Pivotalhassucceededinattracting–and retaining-exceptionalassociates,allcommittedtoand passionateaboutclinicalresearchanddeliveringmedical solutionsmuchclosertopatients.Such“exceptionality”can befoundatalloperationallevels,anditsstaff–particularly itsseniormanagementteams-isabsolutelyapproachable anddirectly–andpersonally-involvedinallprojectsthatthe companycollaborateswith.

Pivotalanditsassociatesareprettyawareofhowcostlyand crucialeverysingleprojectmightbefortheclients.They feelunabletogetintoanyclinicalstudy–ampleor “tiny”-withoutputtingthecompany’sexperienceand knowledgeintothem.
WhenworkingwithPivotal,clientsdonotsimplywork with“individuals”butwitharobustteamdrivenbya“oneteam”attitude,commitment,andintegrity.Pivotaltriesto keepitscustomersinthecenterofitswork,listentotheir
inputs,andputitselfintheirshoestobettercomprehend theirneedstobecomeanextensionoftheirteams.
Last,itisimportanttomentionthatapartwithitsclients, Pivotalaimstostayconnectedwiththe“ecosystem”–namely,thewholeclinicalandinnovationcommunities-to warrantin-timeaccesstobreakthroughscience,bestsites, talent,andtechnology—deliveringdailytoitsclients,and makingsureeverydeliverable,document,slide,ormessage isclear,addsvalueand,eventually,reducestimetomarket. Thisalliswhat,theteambelieves,hasmadePivotala favoritepartnerforahighnumberofBiotechandPharma companies.
Adaptingtothepost-COVID-19ClinicalTrialsScenario
Itwasundoubtedlyunthinkablebackin2019thata “catastrophe”likewhattheCOVID-19pandemichas representedcouldsimplyhappenand,muchlesspredictall theeffectsitbroughtalongside–atagloballeveland, obviously,atanysmallersinglecompartmentofhuman lives,includingtheclinicalresearchecosystem.
AnalyzinghowtheCOVID-19hasimpactedtheclinical trials–andmayimpactinthefuture-isnota




straightforwardquestionbutratherapolyhedriccomplex problemrequiringsimultaneouslooksfromquitedifferent butcomplementaryangles.
Firstly,ithasaffectedallcompaniesdevelopingnew medicalsolutionsforpatients,includingBiotechand Pharmaandothersupporting-servicescompanieslike clinicalCROs.Then,undoubtedly,ithasimpactedthe organizationofinvestigatorsatinvestigationalsiteswho hadhadtoadapt,fromonedaytothenextone,toa differentwaytohandleandmanagetheirclinicalresearch activitiesandtheirpatients–withpatientsnotabletoattend theirvisitsatthesites.
Andlast,likelythemostcriticallinkofthechain,ithashad animpactonthousandsofpatientsbeingtreatedinthe contextofclinicaltrials,frightenedthatthehopestheyput ineverynewtreatmenttheywerehelpingtodevelopfor themselvesandforothersmaysimplyflyaway.However, despitethepotentiallyterriblescenariothatCOVID-19may haveresultedin,allactorsinvolvedinclinicalresearch havefoundawaytomoveforwardandovercomeall hurdlesencountered.
Indeed,theCOVID-19pandemicandalllessonslearned willconditiontheclinicaltrialsarenainthefutureandhow allactorsinvolvedplaytheirrespectiveroles.Andpotential newclinicalresearchscenariosandruleswillneedtobe considered,yetstillmaintainingpatientsattheverycore, warrantingtheirrights,hopesandillusionsandhavingthem reassuredateverymoment–mayotherpandemicburstout (oranyothersimilarevent)thatthey,patients,willbe treatedasneededanddeserved.
Amongthelessonstakenfromthe“COVID-19experience, Pivotalhaslearnedthattherearealternativestotheclassical face-to-facevisitstositesandthatdatacanstillbecollected andverifiedremotelyasmuchaspossible.Butalso,others bysimplyusingonlinetechnologiesthatallowpatientsto transmitoutcomes,asperthecorrespondingprotocol.Orin otherwords,theCOVID-19pandemichasprobablyopened upthedoortothatnewtypeofdecentralizedclinicaltrials (DCTs).Advantages,disadvantagesandchallengesofDCTs vs.centralizedtrialswillrequire,ontheotherhand,deep discussioninanotherarticleandthisisbeyondthetopics thatpretendtobenowcovered.

PivotalalsoexperiencedthatduringthisCOVID-19period, thestudytreatment–commonlyadministeredateach investigationalsite-canbeefficientlydeliveredtopatients bypostorcouriertothesafetyoftheirhomesforselfadministration.Andthatevenformorecomplex administrationroutesrequiring,forinstance,injections, specializedstaffmayvisitpatientsattheirplacesfordosing –and,whynot,tocollectsamplesasneeded.

Nevertheless,anddespitethealternativesjustpointedout andappliedbyPivotal,therewillsurelybemany procedures,trialactivitiesandmeasuresortrialcompounds –e.g.,particularlyunstable-thatwillnotallowaneasyshift tothispotentialnewmodelofmakingclinicalresearch home-friendly
Beasitmay,the“take-home”messagefromtheCOVID-19 outbreakisthatalternativewaysofdoingclinicalresearch arepossibleandthat“rethinking”clinicaltrialdesignsand operationalapproachesaremuchmorethanrecommended. Allparties–fromsponsorstoinvestigatorsandsitesto patientsandregulators-mustchangetheirmindsetsand lookatthesealternativeswithinterestsincetheymayalso helptoreducetheoverallcostsofclinicalinvestigations. AndPivotalandotherclinicalCROs,ontheirside,will havetoadapttheiroperationalprocedurestothisnew clinicalresearch(remote)environment–surely,alongway togoahead,butmuchmotivating.

,Pivotal definitely wants to play the new game of DCTs but always remains true to its clientoriented and pa�ent-centric a�tude, its commitment, and strong belief in science and quality as the basis to build on medical discoveries and advances.
Innovationandnewtechnologieswillbethebasisofthis potentialtransformation,whichisalreadyinPivotal’s DNA.Thus,thecompanymeetsthefirst“inclusion criterion”forthisnewenvironmentandparadigmshift.
Andforthefuture,thecompanyconsiders,ifnotentirely shiftingtoDCT-likeapproaches,atleasttostartwalking thatnewpathwayparalleltotheclassicalone.Pivotal definitelywantstoplaythenewgamebutalwaysremains truetoitsclient-orientedandpatient-centricattitude,its commitment,andstrongbeliefinscienceandqualityasthe basistobuildonmedicaldiscoveriesandadvances.
EmbracingtheFutureRoadmap

TheCROmarketisconstantlymoving,andonthebasis thereof,“M&A”processesrelyon.Andasaconsequence ofthis,newgiantactorsemergeeveryyearwhilesmaller CROssuffer,ifnotliterallyclosethebusiness.Niche specializationorshiftingtonewtargetsponsors–e.g., biotech-mayhelpthemtosurviveandthrive,though.
Thesearecertainlythemarketrulesbutareatrendthatmay deprivethesmallerinnovatorsBiotechofpartneringwith theright-sizeyetexperiencedCROsifnootheractorscan befoundoutthere.Notmuchtodotostopthemarket’s hunger,butjustsendingamessagetosmallerinnovators andsharingthethoughtthatrightsizingisstillavalid principleforsponsorstoconsiderwhenselectingtheir partnerCRO–hence,mid-sizeCROssuchasPivotalwill surelybemuchmoreagileandwillputtheirclients´ projectsamongthosemuchmoreimportantfortheclient.
Mid-sizeandsmallerfocusedCROsarelikelydifferent frommergedgiantsinthattheymaybejustonemoreteam memberoftheirclientsandworkincrediblyclosetothe properclients´internalteams.Andatleast,thisiswhatthe companyhasbeendoingandpretendingatPivotaland what has been the basis for its success story to date.
Thefuturestartstoday,actually,andfor2023Pivotal intendstokeepgrowingwhileremainingfaithfultothe “organicgrowth”policyithasbeenfollowingsince inception–ratherthanbasingitsexpansionsolelyon “M&A”operations-.Therefore,thecompanyplanstooffer somethingelsetoenticeoldandnewclients.COVID-19 outbreakhaspointedoutthenewpathwaytofollow-DCTs, digitalization,technology-and,asalreadymentioned,thisis amaneuverinwhichthecompanyhasalreadybeen investingtogetthere.Butintheshortterm,Pivotalplansto
worktowardsgivinganimpulsetothesciencebehind clinicalresearch.
Withthisobjectiveinmind,thecompanyhasonboarded newtalentforgedinaresearch-makingenvironmentwitha provencapacityforplanningandconductingexperiments and,importantly,foranalyzing,interpreting,andpublishing results.
Pivotal’sultimateideaistoprovidesuchstrategicscientific analysisandadvicetoitsclientsineverynewprojectitis awarded,inreal-time,sothatrapidbutrobustdecisionson howtoprogressforwardcanbemade,avoidingthe compromisingofatotalbudgetinaprojectthatmayhave lostsenseorwhichmaybenefitfromre-focusing.

AnindependentLookatPivotal
Pivotalisproudofbeingthefoundationitistoday,andits teambelievesin“we-are-Pivotal;”henceitrunstheriskof actingsubjectivelywhengivingmarkstothecompany Externalindependentopinionsmayhelp,though.These wordsofwisdomshowcasethestatusquoof Pivotal—beingoneoftheinnovativeCROsinthe healthcareindustry.Detailedquotesaredepictedinour websiteatwww.pivotalcr.comandatourpatientjourney armdivisionatwww.pivotalpatientjourney.comandbelow isanexampleofthesequotes:
“PivotalcontinuestobemyCROofchoice.They complementourexistingteambyprovidingfunctionalarea expertisethatwedonotcurrentlypossess.Theirpersonnel arehighlyengaged,experienced,andproficientinwhat theydo.Pivotal’sleadershipteamisexperiencedandmore importantly,incrediblyengagedthroughouttheentirestudy. ThesearesomeofthereasonsPivotalcontinuestobea trustedpartnerforus.” -Chief Operating Officer, at a USbased Medical Device Co
“IhaveworkedwithPivotalontwostudiesandhavebeen particularlyimpressedwiththecaretheygivestudies,their personalattentiontotheneedsofsponsors,andtheir dedicationtogettingthingsright.Pivotalisaclosely-knit group.Thereisnobureaucracy,simplythededicationto servingtheneedsoftheirbiotechclients.Theyarethe closestthingIcanfindtohavingmycompanypersonnelon thegroundinSpainandEurope.”-ChiefMedicalOfficer,at aUS-basedBiotechCo
“TheteamatPivotaloperatesasaseamlessextensionof ourinternalteam,buildingtrustwithourEuropeanclinical trialsitesandourconfidenceinourabilitytoexecutehighqualityclinicaltrials.ThePivotalmanagementteamactsas atruepartner,equallyfocusedonourobjectivestodevelop innovativetherapeutictreatmentsforpatientswithserious unmetmedicalneeds.” -Group Vice President, Development Sciences Operations at a Mid-Size US Pharma Co

“AsanindependentinvestigatorandheadofaCooperative Group,IhavebeenworkingwithPivotalsince2014onsix projectsinGUtumorsandwhatIhaveencounteredisa dedicatedteamtoclinicalresearch,verymuchattentiveto thecomplexneedsofindependentinvestigators, responsivenessandquality-mindedthroughout.
Theteamgetsitrightthefirsttimeinalltheservices, includingregulatoryandclinicaloperations,medicaland safetymonitoring,datamanagement,andbiostats.Pivotalis myfavoriteCROstoworkwith,andIhavenohesitationin recommendingitforanynewtrialsinOncologyand beyondinEurope.”-Senior Researcher PSMAR/IMIM Hospital del Mar, Barcelona, Spain, and Associate Professor of Medicine Harvard Medical School
“IhaveworkedwithPivotaloninternationalclinicaltrials bothofanICUtherapeuticandacancerscreening device/diagnostic.Icannotthinkofabetterpartner Acosteffectivecollaboratorwithdeepfunctionalexpertise, particularlyindatamanagement,biostatistics,medical monitoring,pharmacovigilance,andprojectmanagement, butevenmoreimportantly,acompanywithacultureof problem-solvingandsharedresponsibility
WorkingwithPivotalisnever“us”and“them”;itisone sharedmissionwithagroupofprofessionalswhosimplydo whateverittakestoaccomplishthegoal.Thisstartsatthe top,withthecredible,expert,andever-presentleadershipof theCEO,himselfaformerseniorPharmadrugdeveloper, andfiltersthrougheverylayeroftheorganization.”
-CEO, at a US-based Biotech Co.
“Thepatientrecruitmentprocesshasbeenawe-inspiring, andIhavelearnedalotthroughyourcompanyandyourCLyspatientplatform.Youaremakingtheworldofresearch abetterplacewithyourcompanyandyourways.”
-Ophthalmologist and PI and Med. Monitor lead in FDAmonitored clinical trials.


“Andalloftheaboveseemstobesupportedbythefactthat Pivotalwasselectedasoneofthefewfirmsthatattracted recognitionfromthePharmaIQnetworkasaRISING STAR.”
Theresearchbasecomprisedinternationalparticipantsfrom mainlybigPharmaorBiotechs,SMEspharmaand consultants,governmentbodies,medicaldevice manufacturersandpublichospitals.Wehavekeptdoingas goodasthen,soweareconfidentwewillsurelybe perceivednowaswethenwere.Clientshavethelastword, butbusinesscominginalignswiththisfeeling.
,Pivotal has succeeded in a�rac�ng – and retaining- excep�onal associates, all commi�ed to and passionate about clinical research and delivering medical solu�ons much closer to pa�ents.












tssuccess tscare.com Nev er Miss An Issue Subscribe Today Corporate Office Insights Success Media Tech LLC 555 Metro Place North, Suite 100, Dublin,OH 43017, United States Phone - (614)-602 - 1754,(302)-319-9947 Email: info@insightscare.com For Subscrip�on : www.insightscare.com Check should be drawn in favor of : INSIGHTS SUCCESS MEDIATECH LLC www.insightscare.com























Industry
www.insightscare.com JANUARY | 2023 16
Sagacity
The Impact of Emerging Technologies on the Pre-Clinical CRO Market
Technologyischangingthehealthworld’sfuture, whilefuturetechnologyisreshapingthepresent worldofhealth.Alreadyemergingfromthe turbulentoceansofchangingtimesarenovelwaysof carryingoutorperformingpreclinicalresearchand conductingtrials.
ManygiantClinicalorContractResearchOrganizations (CROs)havealreadystartedintegratingtheirpreclinical research,testing,andtrialswithnotonlydigital,virtual, andremotetechnologiesbutarealsoimplementing ArtificialIntelligence(AI),CloudComputing,and MachineLearning(ML)technologiestoefficiently conduct,perform,orcarryoutsuchexperimentsin increasinglyenhancedenvironments.


Theprocessofdevelopingamedicinalproductisalong one,wheretherearemanysubprocesseslikeideaor conceptgeneration,framing,discoveringanddeveloping thatideafurther,thenthepre-clinicalresearchstagethen comestotheclinicaltrialstage,andfinally,theFDA assessmentandreviewstagewhichapprovesor disapprovestheproductbasedonitscriteria.
ThePowerofModernTech
Althoughallthesesubprocessesareequallyimportant,the stagewherepre-clinicalresearchisconductedisthemost crucialoneastheprospectiveproductideasorconcepts
www.insightscare.com JANUARY | 2023 17
haveastrongchanceoffailing.Thisiswherepreclinical CROsplayaverymajorroleinoptingforadvanced technologiessotheycouldresearchtillthelastelementof eachideaandconceptfromeverypossibleangle,andfind outthepossibilities,andprobabilitiesofthesuccessandthe failureofthedrugormedicineindevelopment.
Moderntechnologiesarenotonlygivingmorepowertothe preclinicalCROstolookatthepreclinicaltrialprocesswith aholisticviewincreasingtheprobabilityofthedrugor medicinemovingtothefurtherstagebutarealsohelping theminsavinghugecosts.
Letuslookindetailatthesereasonsanddigourselves deepertogainabroaderperspective.
AHolisticTechPerspective:
AdvanceTechnologicalImplementationlowersthe Uncertaintyofthepreclinicalexperimentsbystreamlining andoptimizingdetaileddatacollection,easingdownthe pre-clinicaltrials’subjectselection,decreasingthetime durationofresearch,andreducingfinancialcosts.Research, datacollection,testsubjectselectionandcontinuous analysisareanintegralpartofdrugresearchandmedicine development.However,withincreasingintricacyinthe entireprocess,navigatingthroughthecontinuousstreamof incomingdatagetsimpossibleforthehumanresearcher
Thisiswhereadvancedtechnologieslikeautomation,AI, cloudcomputing,BigDataAnalysis,ML,etc.providenot onlymonitoring,tracing,tracking,andcollectingdatabut alsodeepandmachinelearningoftherecordeddatatooffer preciseanalysis,interpretation,flow,patternwhile matchingthesampleswithearlieravailableones.

Live COVID-19 Example:
Itisbecauseofusingsuchnoveltechnologiesthatinjusta yearandahalf,CROsacrosstheworldcouldcomeupwith morethanadozencoronavirusvaccinesincludingSerum InstituteofIndia’sCOVOVAXwhichhasbeendeveloped throughthreetrialsinonecountryandapprovedinfive countries,COVISHIELD(OxfordAstraZeneca Formulation)developedthroughfourtrialsinonecountry andapprovedin49countries;Novavax’sNUVAXOVID whichhasbeendevelopedthrough17trialsin13countries andapprovedin38countries;Oxford/AstraZeneca’s VAXZEVRIAdevelopedthrough66clinicaltrialsin31 countriesandapprovedin140countries;andsoonandso forth.
Duetothisrapidvaccinedevelopment,accordingtoThe NewYorkTimes’COVIDvaccinetracker,asofJuly2022 over5.23billionpeopleor68.2%oftheworldpopulation havereceivedadoseofaCOVID-19vaccine.
FuturisticTechnologiesistheFuture
Clinicaltrialresearchersalsogetbenefittedbygaining actionableinsightsintothedetailedreportscreatedbyusing digitaltechnologies.Forexample,AIandMLarenow widelyusedinthemostappropriatesamplegroupselection thatrespondsmoretothepre-clinicaltestsandtrials.



Thefirstmajoradvantageofusingthesetechsisthe automatedcellularlevelselection,datagenerationand analysis.Thesefuturistictechnologiesmoderncell-based selectionoffersanearly-stageissueidentificationand problemdetectionwithpotentialdrugdevelopmentsand testing.Itaidsinreducingsamplewastage,time,andeffort, andstreamlinestheentireprocessofresearchand development.Forexample,datasamplescollectedduring theresearchusingAIcanalsobeutilizedforthemost suitablepatientmatchingduringtheclinicaltrialstesting process.
Preclinicalimage,graphics,andsamplematchingand analysisprocessautomationarealsofeasibleusingtechs suchasAIandML.CROsarenowincreasinglyusingthese technologiesforautomatedtestingsampleanalysis, identificationofmolecularcompounds,andanalysepatterns fordrugdiscovery

ForExample,AIisnowbeingusedbytheInstituteof CancerResearchformakingpredictionsregardingcancer drugs’novelprospectsandperformingrepetitivetaskslike researchrecordup-gradationanddataextraction.Likewise, scientistshavesuccessfullydevelopedEve,anAIRobotto helpresearchersinspeedingupthedrugdiscoveryprocess.
-Anish Miller
www.insightscare.com JANUARY | 2023 18


TheHealthcareindustryusuallyfacesproblemsto
bridgethegaptosolvetheproblemspromptly,and isn'titsomethingthatshouldbetakencareof?
Acknowledgingtheseconcernsoffillinguptheoperational gapsandsolvingtheproblemsinatimelymanner, ClinergyHealthResearchhassolutionstoavarietyof managerialissueswhilealsoprovidingafullrevampofall thehealthcareprocesses.
Havingthisproblem-solvingapproachandabundant experience,TiagoMDdaSilva,ManagingDirector,has scientificandmanagementknowledgetoenhancethe operationalexperienceanddeliversuperiorresults.
Inaninterviewwith Insights Care,Mr.daSilvatalksabout Clinergyrolesandhowitismakingasignificantdifference throughitssolutionsintothehealthcareniche.
PleasebriefouraudienceaboutClinergyHealth Research,itsUSPs,andhowitispositionedasareliable nameintheCROsector?
ClinergyisaCRO,butwealwaysliketohighlightthatour "C"standsfor"Collaborative"ratherthan"Contract,"and, whileforsomepeople,thatmightlooklikeasmalldetail,it makesallthedifference.
BeingaCollaborativeResearchOrganizationmeansthat ourstructure,processes,values,andpeoplearefully focusedonlong-termvalue-addingpartnershipsratherthan onshort-termtransactionalrelationshipswithclients. Suchamindsetallowsustotrulyembraceourclients´ visionandwaysofworking.Weactasanextensionoftheir team,focusingonwhatmatterstothemwhilefeeling empoweredto"runtheextramile"todeliveroperational excellence.
Thisisnowmoreimportantthaneverasweseewhathas beenhappingtotheCROenvironmentoverthepasttwo decades,withsomanymergersandacquisitionsresultingin hugeCROswithinflatedstructures(andcosts)thatsurely canstillserveasetofsubstantialclientsbutnolongerfitsto servesmallbiotechs,pharmacompaniesandhealthcare start-upsgiventheirveryparticularneedsintermsofcosts, flexibility,senseofurgencyandattention.
UnlikemostCROs,ourkeydriverisnotrelatedtoshorttermfinancialgoals.However,long-termcustomer satisfactionandwebringthattodailylifethroughtheway weshapeourKPIs,qualitycontrolstrategies,andriskbasedthinkingineverythingwedo.Anotherelementthat bringsouruniquemindsettolifeisthefactthatwehave madeaconsciousdecisionnottohaveabusiness development/salesteam.
Wheneverclientsreachouttouslookingforourservices, theygettotalkdirectlywithourteamofmedicaldoctors, pharmacists,nurses,biologistswhowillbedirectly involvedintheclient´sclinicaltrial/project,mostofwhom havebeenonclinicaldevelopmentfor15,20+years.
Attheendoftheday,sciencecomesfirstineverythingwe dosothatwecanachieveourmissionof"helping health innovation reaching patients faster."
Shedsomelightonyourofferingsandhowtheyimpact theCROindustryaswellasyourclients.Howyour companyprovidesresearchservicestovarious organizations?
Thevalueweaddasstrategicpartnerstoourclientsis muchmorearesultofourfocusonthe"how"ratherthanon the"what."Weareequippedtoprovideend-to-endplanning solutions,conductingandreportingclinicaltrialsfromsite
10 Most Innova�ve CROs To Watch In 2023 www.insightscare.com JANUARY | 2023 20
Tiago M D da Silva Managing Director

feasibilityandselectiontoprojectmanagement,regulatory submissions,risk-basedmonitoring,datamanagement, statistics,medicalwriting,amongothers.However,the meansweutilizetodeliverareessential.
Weareverymeticulouswhenitcomesdownto understandingwhatdeliverymethodscanfiteachclient bettersothatwecanoffercost-effectivesolutionstailored toeachofthemwithouthavingtochoosebetweencostand efficiencyandwithouttryingtopush"overthetop" solutionsonlytojustifywehavethem.
Our values are built upon elements that are key to delivering operational excellence with a high focus on the human aspects that make a difference in how we deliver it daily.
www.insightscare.com JANUARY | 2023 21
Whilewerelyonstate-of-the-arttechsolutionstoplanand managetrialsefficiently,oursecretsauceisourpeople,no questionaboutit.Asweliketosay,Clinergyisallabout "humanologyandtechnologyforhigh-performanceclinical development."
WhatarethecorevaluesuponwhichClinergyHealth Researchisbuilt?Whatisthevisionandmissionofyour organization?
Ourvaluesarebuiltuponelementsthatarekeyto deliveringoperationalexcellencewithahighfocusonthe humanaspectsthatareeffectiveinhowwedeliveritdaily
Theyareasenseofurgency,entrepreneurship,efficiency, caringforothers,integrity,andpassion.
Weallknowhowhardandcostlyitistoputhealth innovationintothehandsofthosewhoneededit,and,as scientists,thereisnothingmoredisappointingthan inefficienciesplayingaroleinthat.Ourmissionof"helping health innovation reaching patients faster"remindsusthat weshouldalwaysbeactingascatalystssothatweaddreal valuetotheinnovationchain.
MrTiago,pleasetellusaboutyourprofessionaltenure intheCROIndustry.
BeforefoundingCLINERGY,Ihavefulfilleddifferent clinicaloperationsroles,includingleadingtheGlobal ClinicalResearchOperationsformedicinalproductswithin aUK-basedFTSE100consumerhealthcompany

Ihadthepleasuretospendthelast17yearsdrivingand deliveringstrategicprojectsforcompaniessuchas Novartis,Abbott,EliLilly,andReckittBenckiser,helping patientshaveaccesstovalue-addingmedicinesandhealth innovationacrossmultiplegeographiclocations.
Havinglivedandworkedinbothhemispheresoneitherside oftheAtlantic,Ihaveacquiredanintimateappreciationof theculturalandregulatorydifferencesthattrulyimpact globalclinicalresearch.
Beinganexperiencedleader,shareyouropiniononhow moderntechnologieshaveimpactedtheCROsector. HowhasClinergyHealthResearchincorporatedsuch technologiesintoitsdailyoperations?
Clinicaldevelopmenthasbenefitedagreatdealfromnew techsolutionsoverthepast15years,mostnotablyoverthe pastdecade.CROshaveakeyroleinmakinginnovative solutionsavailabletotheirclientsbyutilizingdata-driven solutionstoreduceoperationalcomplexity,makebetter-
informeddecisionsaroundstudydesign,monitorworkforce assignment,andconductremotetrials.
AtClinergy,wecanembedvariouslevelsoftechnology accordingtoeachtrial´needs,fromeTMFsthatcounton machinelearningtoautomatedocumentfillingtohigh-tech risk-basedstudyexecutionsolutionsthatcavesaveupto 40%ofmonitoringcosts.
Whatwouldbeyouradvicetobuddingentrepreneurs whoaspiretoventureintotheCROspace?
Settingyourselftobeabletoactasapartnertocompanies thatoperateinoneofthemostregulatedenvironmentsout thereisnoeasytask;peopleshouldknow Themost importantpointIwouldhighlighttothoseconsidering enteringthisspaceis:tomakesurethecorevalueswhich youbelievewillsetourcompanyapartfromotherplayers aregenuinelyembeddedindailyoperations.Alltherestis easilyavailabletoanyoneelse.Inanexpensiveand complexmarketsuchasclinicaldevelopment,thecostis certainlynotpartofthetopthreedecisionfactorsformost companiesastheyknowhowmuchmoreexpensiveitcould betohaveanentireclinicaltrialfailingduetooperational flawscomparedtopayingabitmoretohaveapartnerwho candeliverhighstandardsbytheirside.
Howdoyouenvisionscalingyourorganization's operationsandofferingsin2022andbeyond?
Wehaveaclearviewwhenitcomesdowntoourgrowth expectations.Althoughthedemandisveryhigh,wehave nointerestingrowingatanycostasweknowthedangers thatcomewiththat.Wesuffernoexternalpressureto deliverbetterfinancialresultseveryquarterasaprivately heldcompany.Thatallowsustoentirelyfocusoncustomer satisfactionbasedonhigh-qualityandhigh-performance outputaskeydrivers.Wehavebeengrowingsteadily,and weplantokeepacloseeyeonthebalancebetweengrowth andexcellence.
www.insightscare.com JANUARY | 2023 22
 Oscar Juan MD, PhD, Medical Oncologist, Senior Medical Manager
Oscar Juan MD, PhD, Medical Oncologist, Senior Medical Manager
www.insightscare.com JANUARY | 2023 24
Pivotal S.L
Gene THERAPY and Cancer Treatment
Genetherapyhasevolvedfrombeingsciencefiction
tobecomingarealitywiththepossibilitynotonly fortreatinghereditarydiseasesbutalsofortreating geneticallycomplexdiseases,suchascancer
Genetherapycanbedefinedasthesetoftechniquesthat usenucleicacids(DNA,RNA,ortheirvariants)toreplace, correct,orinhibitanendogenousgeneinthecellsofthe organismor,actingindependentlyofthegene,useanucleic acidtosynthesizeproteinsorenzymesthatprovidenew biologicalpropertiestocellsortissues.Theobjectiveof genetherapyistotreatoralleviateadisease.
Dependingonthetargetcellsonwhichwe´dwanttoact, wecandifferentiatetwokindsofgenetherapy:
•Germgenetherapy,whichistheinsertionofthe therapeuticgeneingermcells,suchaseggcellsinfemales andspermcellsinmales.Sincethistherapyaltersthegerm cells,finally,thechangesmoveontothenextgeneration. Thistypeoftherapycouldbeusedtodefinitivelycorrect congenitaldiseases.However,thishasnotbeendonein humansduetoitsgreatercomplexityand,especially,dueto ethicalconsiderations.
•Somaticgenetherapy,theobjectiveofthistherapyisto introduceoraltergenesinsomaticcells,i.e.,thecellsthat makethebodytissuesofmulticellularorganisms.These changesarenottransferredtoaperson´soffspring.
In Vivo and Ex Vivo GeneTherapy
Dependingonhowwemanagegenes,wecandifferentiate twotypesofsomaticgenetherapy:
Ÿ In vivo gene therapy:thetherapeuticnucleicacidsare introduceddirectlyintothepatient’sbody Thevectorgeneconstructisintroducedbycell-specificdirect injectionintotissueinneed.Onceinsidethebodyand incontactwiththetargetedcells,thegeneis incorporatedintothetissue’scells,encodingthe productionoftheneededprotein. In vivo genetherapyis lesscomplexthan in vitro genetherapy.However,in general,thereislesscontroloverthetransfectionofthe geneanditsefficacy.
Ÿ Ex vivo gene therapy involvesextractingthecellsfrom thesystem,isolatingthem,growingtheminaculture, andinsertingthegenes.Afterwards,cellsarereturnedto thebodyinasimilarproceduresuchasthoseusedin hematopoieticstem-celltransplantation. Ex vivo gene therapyhastheadvantagesofallowingtochoosethe celltype,havingmorecontrolovertheprocedure,and havinggreaterefficacyingenetransfection.However, itscomplexityishigherthan in vivo genetherapy,has anincreasedcost,andcanonlyusecellsthatcangrow inculture.
Deliverystrategiesfortherapeuticapplications
Thegoalofgenetherapyistoachieveadurableexpression ofthegenetransfectedtocorrectthegenealterationthat causesthedisease.Asafe,effective,andcontrollable deliverysystemiskeyingenetherapy.Therearetwotypes ofvectors:viralvectorsandnon-viralvectors.
Viral vectors
Virusesare,sofar,themostuseddeliverystrategy The advantagesofviralvectorsaretheavailabilityofwellestablishedprotocols,hightransductionefficiencies,and
www.insightscare.com JANUARY | 2023 25
About the Author
Oscar Juan, MD, PhD, Medical Oncologist, Senior Medical Manager at Pivotal S.L.U.

Oscar is MD from Universitat de València, Spain (1991) and trained in Medical Oncology and Haematology at Hospital Clínico Universitario de Valencia, Spain (1995). He also holds a PhD Cum laude from Universitat de València (2002).
He has been working as Medical Oncologist since 1996 in various hospitals in Spain and most recently at Hospital Universitari i Politècnic La Fe in Valencia, Spain.
He has participated as Principal Investigator in more than 100 international trials including pivotal trials than have changed the clinical practice and paradigms in lung cancer treatment.
As a member of the Biomarker and Precision Medicine Unit, he was focused on metabolomics, epigenetic and genomic biomarkers in EGFR-mutated lung cancer patients and patients receiving immunotherapy
He joined Pivotal in January 2020 as Senior Medical Manager in Oncology. He focuses on supporting the strategic clinical development of new molecules and acts as the medical reference and medical monitor for several innovative early phases trials and provide guidance to the Clinical Project team members at Pivotal to ensure adherence to study objectives and timelines.
www.insightscare.com JANUARY | 2023 26
havingmechanismsforintroducinggeneticmaterialinto cellssystematically.Viralvectorsincluderetrovirus, lentivirus,adenovirus,andadeno-associatedvirus(AAV). Retroviralvectorwastheearliestusedandhasaminimal riskofcausinginsertionmutations.
Non-viral vectors
Non-viralvectorscancarryahighergeneticload,aresafe, andgenerallyinducealowerimmuneresponse.The liposomeisoneofthemostattractivenon-viralvectorsfor genetherapeutics.Liposomehasnoinhibitionandno significantdamagetonormaltissuesandcellsaroundthe targetcells,enablingthetargetgenetobefullytransfected intothetargetcells.AfterthesuccessofSARS-CoV-2 vaccines,lipidnanoparticleshavebecomeawidelyused vectorforgenetherapy
Genetherapyinmonogenicdiseases
Genetherapywasinitiallyconceivedasatreatmentof geneticdiseasescausedbymutationofasinglegene (monogenic).Hereditarydiseasesincludevariousdisorders inwhichadefectivegenedeterminesthataproteinisnot synthesizedoriselaboratedabnormally,causingmany differentclinicalmanifestationsdependingonthestructural orenzymaticfunctionofthatparticularprotein(e.g., hemophiliaA).Theseproblemscouldbesolvedwithgene therapybysupplyinganormalcopyofthedefectivegeneto theaffectedtissues.
ApproachesinCancergenetherapy

Canceroccursduetothedisruptionofnormalcell proliferationandapoptosisprocesses.Theaimofgene therapyincancerisdifferentfromthetreatmentof monogenicdiseasesinwhichthegoalistocorrectaspecific geneticdefect.
Severalgenetherapyapproacheshavebeendevelopedfor cancermanagement,suchus:
Ÿ OncolyticVirotherapy(OV)isoneofthemost promisingapproachesfortumorimmunotherapy.OV introducewild-typetumorsuppressorgenesintocells thatlackthetumorsuppressorgeneleadingtotumor cells’lysisinducingananticancerimmuneresponse (e.g.,p53gene).
Ÿ GenesilencingtherapyusesanRNAinterference (RNAi)thatinducesequence-specificdegradationof complementarymRNA.RNAiisusedindifferentareas
ofmedicine,suchasneurodegenerativeand cardiovasculardiseases,viralinfections,andcancer Sincecanceristheaccumulationofvariousmutation typesthatregulategenenetworkspromotingcell proliferation,RNAiisanattractiveapproachtotreating tumors.
Ÿ Suicidegenetherapyconsistsinintroducingsuicide genestoexpressenzymesorproteinsthatdirectly triggerthedeathoftumorcells(thegeneencodesa proteinthatiscytotoxic)orindirectly(thegene expressesenzymesthataresensitivetoadrugthatis administeredconcomitantly).
Ÿ Chimericantigenreceptor(CAR)-Tcelltherapyisa slightlydifferentprocesswithrespecttothemoredirect formsofgenetherapy.CAR-Tcellsarealab-generated fightercellscustomizedforeachindividualpatient. TheyaremadebycollectingTcellsfromthepatientand re-engineeringtheminthelaboratoryaddinganti-cancer geneticcodetoproduceproteinsontheirsurfacecalled chimericantigenreceptors,orCARs.TheseCARs recognizeandbindtospecificproteins,orantigens,on thesurfaceofcancercells.
CAR-Tcelltherapiesarethemostcommontechnology usedinpotentialcancertherapyandrepresent49%ofthe geneticallymodifiedcelltherapiespipeline.However, CAR-Tcelltherapiescancauseseveresideeffectsbeingthe mostfrequentandseriousonethecytokinerelease syndrome(CRS).
Todate,theFDAhasapprovedseveralCAR-Tcell therapiesforhematologicaldiseases.However,challenges remainfortheuseofCAR-Tcelltherapytotreatsolid tumorsduetotheirheterogeneityandlocalizationsinthe humanbody.
ConclusionsDrawn
Genetherapyrepresentsanoveltherapeuticapproachfor managingdiseaseswithdifficulttreatment.Monogenic diseaseshavebeentreatedsuccessfullywithgenetherapy, butcancerisacomplexprocessinwhichcellshavealtered theirnormalproliferationandapoptosis.Recentprogressin developingsafeandeffectivevectorsforgenedelivery, understandinggeneediting,andtheadvancesinadoptive immunotherapywithCAR-Tcellshasopenedupnew avenuesandstrategiesfortreatingintractablediseases.
www.insightscare.com JANUARY | 2023 27
Role of CROsin Shaping the of Clinical Research Future

PostCOVID-19pandemicworldhas
completelychanged.Theimpactismost severeonthehealthcareindustry,especially ontheclinicalresearchandtrialsactivitiesacrossthe globe.Thelifescienceindustrymetunprecedented challengeswhichforcedittoaccelerateinnovation, revampitstraditionalmindsetandembracedigital, virtualandonlinecapabilitiestoensureservice continuance.
Traditionally,theIndustrywasverycautious,slow andreticentinadoptingdigitalandvirtualclinical trialpractices.Itwascomprehensible,evidentand expectedsincemanyofthedevice-dependent diagnosisandtreatmentdriventherapeuticareas addedmuchmorecomplexitytothedigital,virtual, andremoteofferingevolution.Further,already established,proven,andexistingdecadeoldpractices couldnotbeabandonedorreplacedorshiftedtothe digital,virtualorremotemode.
Thisemergedasthemajorconcerninthecontinuance ofclinicalresearchandtrialniche,leadingtoalmost 87%reductioninclinicalresearchinEnglandduring thepeaktimeoffirstwave,aslaterfoundoutby UniversityCollegeLondonstudy Answeringthe questionofavoidance,theindustryopenedupits mindsettoacceptthechangingrealitiesofclinical trialsandshiftingnatureofclinicalresearchinthe post-pandemicworld.
www.insightscare.com JANUARY | 2023 28

Redefining Advancements www.insightscare.com JANUARY | 2023 29
TheChangingDynamicsofClinicalResearchIndustry

Arapidlygrowingindustry,clinicalresearchandtrialsis advancingduetonewstudiesconductedinrecordnumbers, ever-increasingpatientparticipationexpandingtheclinical trialsubjectpool,numerousresearchsitesofexcellent quality,andahugevolumeofsuccessfultrialsoverthe yearscontributingtotheexpertiseandvalues.Further developmentoftechnicalmodulesandvariousmodalities couldimproveclinicaltrialcontinuanceanddrive innovationandchangetothehigherlevel.
However,allthesemodalitiesandmodulesmustmeetthe standardcriteria’sandbenchmarksofexcellencesothatthe safetyandsecurityofthetrialscouldbemaintainedor enhanced.Thisiswheredigital,virtual,andremote technologiescouldaidinoptimizingvariousprocesseslike sitefeasibility,pre-screeningofpatients,selectionand clinicaltrialprovidence.
Byreviewingitsowntrends,changes,pressures,upheavals, successesandfailuresduringthelasttwoandahalfyears, theclinicaltrialindustrymanagedtocomeupwithmore thanadozencoronavirusvaccines,thuspushingforward theinnovationdrivenmomentumandacceleratingthe renewedfocusbyconsideringkeyfactorsofsuccess.
Tech-Focus:
Withmanyoftherestrictionsstillbeingineffect,industry’s digitalmindednessiscontinuing.Simply,theindustryhas nowfullyadoptedanentiredecentralizedvirtualmodel attitudeorahybridapproach.Inthisnewapproach, organizationsarecompelledbythevirustocontinuewith theclinicaltrialmodalitieswhichcanofferat-homeor remotetestingfacilitiesalongwithonlinepracticesand digitaltechniques.Itwillensureclinicaltrialcontinuance forpatientsinamorecomfortable,convenient,safeand securemanner Thiswillbedonewithoutanynegative impactontheclinicalinvestigatorsparticipation.
Duringthelasttwoandahalfyearsremotepre-screening tests,e-consentpracticesandmonitoringapprovalby patientsandcliniciansisontherise.Sincetheyarenow establishedaspreliminarybenchmarksforfuture modalities,theindustrycouldfurtherleverage advancementsinpracticesbestsuitableandfeasibleacross theentirespectrumoflifescienceindustrytofurtherdrive safe,secure,flexible,patient-centric,comfortable, convenient,andefficientclinicalresearchandtrial
modalitieswhichwillconstantlykeepimprovingthefuture performanceofclinicaltrials.
Itwillprovideaprimaryfoundationforthebothhybrid modelledandfullyvirtualresearchandtrialenvironments whichwillbecompletelyequippedwithprecisetools, adequatetechniques,robustandseamlessinfrastructure,a well-integratedecosystem,andbig-dataanalytical methodologies.
CreatingStreamlinedEcosystem:
Whencross-functionalentitiesworkjointly,alltherelated stakeholderscouldparticipateinofferinginputs,gaining information,andmutuallyassessingthedevelopmental approachestoincreasetheresultacceptanceamidstthe changingmarketdynamics.Thisiscrucialwhenderiving digital,virtualandremoteclinicaltrialeffectiveness assessment.
Astheyhaveeasyaccesstopatients,clinicalinvestigators, sites,andsponsors,partnersofClinicalResearch Organizations(CROs)canpromptlyfacilitatefurther enhancementsindigital,virtualandremoteclinicaltrials conductionssuccess.
FuturisticCROsTransformingtheClinicalResearch
Beforethepandemichappenednotanyexpertwouldhave beenabletopredictthefutureofhealthcareindustryforget ofclinicalresearchandtrialindustry Althoughthe pandemicisunprecedentedinitsdevastation,onethingit didgoodisthewayitdivertedtheentireprogresspathof sectorworldover.
Thepossibilities,opportunities,andprobabilitiesithas openedupisanunimaginablypositivefutureinitself.The journeyhasmerelystarted.Wecouldpushtheboundaries ofnowestablishedinnovationfurther Thiswaywecould exploresomeunexploredareasintheclinicaltrialswith manyCROscomingtogetherandcreatinganupbeat ecosystemwhichwilloperatelikeanindustryorganwhich willfacilitateafuturewheregloballyinnovativemodalities couldbeestablished,enhancedandadvancedsothat wheneveranyunprecedentedcrisissituationwillarrivein thefuture,theindustryasawholeandtheworldasunited willbeabletocombat.
-Anish Miller
www.insightscare.com JANUARY | 2023 30

OnQ Research
Blend of Innova�ons Assuring Adaptability
Clinicaltrialsarecarriedoutbycontractresearch
organizations(CROs)forthepharmaceutical, medicaldevice,andbiotechnologybusinessesas wellasforacademicinstitutions,governmentalagencies, andfoundations.UsingaCROforoutsourcingreducesthe amountoftimeneededforclinicaltrialsandproduct development.

Whencomparedtoconductingthetrialinternally,working withaCROfrequentlyresultsinsignificanttimesavings. Thetoolsandresourcesneededarealreadyavailableto CROs,andtheyalsohaveastaffofinternalexpertswith experienceinallfacetsofclinicaltesting,development,and compliance.
Withmorethan20yearsofexperience,OnQResearchisa full-servicecontractresearchorganization(CRO).Itis dedicatedtoofferingpharmaceutical,biotech,otherCROs, andalliedbusinessesinnovative,affordable,anduseful clinicalresearchservices.OnQResearchwasestablishedin 1999andhassincecarriedoutmorethan500clinicaltrials insouthernEuropeandmorethan10Africannations.
WithoutthechancetoincorporateotherAfricannations, SouthAfricacannotbeviewedinisolation.Mozambique, Botswana,Rwanda,Ghana,Uganda,Kenya,andTanzania arejustafewoftheAfricannationswhereOnQResearch hasconductedextensiveresearch.Thisentailsassessinga newregionandassistinginthecreationoffull-fledged, qualifiedclinicalresearchsitesthroughsiteevaluationand resourceandcapacitybuilding.Successinallclinicaltrials ontheAfricancontinentisguaranteedbyitsdistinctive ClinicalMonitoringTeamconceptandclinicalstudy approach.Thecompanyisknownforproducing high-qualityresearchquicklyandeffectively
ClientscancentralizeresearchacrossAfricawiththehelp ofOnQ.Duetoitsextensiveexpertiseandexperience,it canprovideexcellentpatientcare,lowerrisk,andsuperior datamanagement.OnQ'sclinicalresearchteamisspread acrossallofthemajorcitiesineachnation,allowingitto beflexibleanddeployresourcesasneeded.
UndertheadeptleadershipofCatherineLund,the FounderandManagingDirector,OnQResearchoffers
TheAdeptLeader
Catherine started her clinical research journey as a study coordinator for an RSV trial. She then joined IQVIA(then Quintiles) as a clinical trial assistant in 1996 and trained to become a CRA. She started consulting in 1999 and gradually evolved the business into a CRO. She describes herself as an accidental entrepreneur and grew the team and processes as the demand grew
Catherine Lund is the Founder and Managing Director of OnQ Research. She is, by training, a registered neonatal nurse who entered the clinical research arena as a research nurse, followed by her formal clinical research training at Quintiles. She left Quintiles to pursue a career in contracting.
Through collaborations, she then established OnQ Research, initially as a consultancy, which evolved into a full-service, SouthAfrican-based CRO in 1999. Catherine Lund has served on the SACRA(SouthAfrican Clinical Research Association) Exco for a period of three years. She was the Vice Chair for the SouthAfrican Chapter ofACRP(Annual Review of Competency Progression) in 2003.
Catherine Lund is a registered nurse with a diploma in general, midwifery, community health, and psychiatry, a certificate in neonatal intensive care, and a BAin diplomacy and political studies from B.G.Alexander Nursing College and UNISA, respectively
10 Most Innovative CROs to Watch in 2023 www.insightscare.com JANUARY | 2023 32
 Catherine Lund Founder and Managing Director OnQ Research
Catherine Lund Founder and Managing Director OnQ Research
top-notchclinicaltrialmanagementandcoordination throughCRAswithregionalbasesinallmajorcitiesinthe nationsitoperatesin.Thisensuresclosesitemanagement andreducescostlytravelexpenses.Itisperfectforallofits clinicaltrialmanagementneedsduetoitssignificant experienceincarryingoutsuccessfultrials.
Below are highlights of the interview that emphasize OnQ’s services to assist with the entire continuum of clinical research.
Catherine,pleasebriefouraudienceaboutyour company,itsUSPs,andhowitispositionedasareliable nameintheCROsector?
OnQResearchisaSouthAfrican-based,organicallygrown CROservicingprimarilytheAfricancontinent.OnQ Researchpridesitselfonofferingitsclientsablendof innovation,adaptabilitywhileaddingrealvaluewithour highlytrainedstaff.Weusescienceasthebasicpremisefor ourserviceswithoutcompromisingonquality.
OnQResearchdoesnothavea“onesizefitsall”approach.’ Thisappliestoallaspectsoftheclientrelationshipand interface,frombudgetstosizesofteams,systemsand processesoffered,andlevelsofengagement.
Shedsomelightonyourofferingsandhowtheyimpact theCROindustryaswellasyourclients.Howyour companyprovidesresearchservicestovarious organizations?
Weofferassistancewithprotocolinceptionanddesign, liaisingandobtainingappropriateHealthAuthorityandIRB approvals,selectinghighqualitysitesthroughourextensive networks,projectmanagement,monitoring,data management,andmedicalwriting.
Whatarethecorevaluesuponwhichyourorganization isbuilt?Whatisthevisionandmissionofyour organization?

OurcorevaluesareEmpowerment:Weempoweremerging biotechandpharmacompanieswithtools,resources,and
expertiseforclinicaltrialexecution.Internally,wealso applythesamevalue.Wearepassionateaboutcareer growthandofferingpotentialcandidatesopportunitiesto addvaluetoourorganization.Collaboration:OnQ collaborateswithsponsorsandclientstodrivecosteffectivedeliveryofclinicaltrials.Quality-focused.Weare committedtotheethicalconductofclinicaltrialsand deliveringqualitydatafordrugregistration.
Beinganexperiencedleader,shareyouropiniononhow moderntechnologieshaveimpactedtheCROsector. Howhasyourcompanyincorporatedsuchtechnologies intoitsdailyoperations?
Wemakeuseofcarefullyselected,fit-for-purposesystems forday-to-dayclinicaltrialmanagement.WeuseaCTMS forprojectmanagementtrackingandareintheprocessof implementingalocallydevelopedlearningmanagement systemandqualitymanagementplatform.Asourcompany grows,theimplementationofnewtechnologiesiscrucialto improvingefficiencies.Still,itrequirescarefulrisk-benefit considerationstoensurethatweremaincapableof deliveringahigh-qualityproductatacompetitiveprice.
Whatwouldbeyouradvicetobuddingentrepreneurs whoaspiretoventureintotheCROspace?
AlthoughIam,ofcourse,biased,thisisoneofthemost excitingindustriesinwhichtoventure.Networkingiskey. Attendingasmanyconferencesasyoucanandaccessingas muchinformationasyoucan.Understandwhatpartofthe CROspaceexcitesyouandwhereyoucanseeyourfuture path.
Howdoyouenvisionscalingyourorganization’s operationsandofferingsin2023andbeyond?
Ourcompanyisverydeliberateinitsfocusonbeingthe premierCROinAfrica.Ourplantoachievethisistobe intentionalaboutinvestinginourstaffandunderstanding thatallourclientsrequireabespokeanduniqueservice.
Pleasegiveusafewtestimonialsofyour clients/customersandalistofawards/recognitionsthat accuratelyhighlightyourorganization’spositioninthe market.(Ifavailable/permissible)
Ourclientsconsistentlyoffersimilarfeedbackinthatthey enjoyworkingwithourorganization,whichisflexible, adaptable,andupforanychallenge.Ithasbeenbroadly indicatedthatwehavea“can-do”problem-solving approachtoconductingclinicalresearchwithout compromisingonqualityoutcomes.
www.insightscare.com JANUARY | 2023 34
OnQ Research prides itself on offering its clients a blend of innova�on, adaptability while adding real value with its highly trained staff.




 Dr Ibrahim Farr Chairman and CEO Pivotal
Dr Ibrahim Farr Chairman and CEO Pivotal



























 Dr Ibrahim Farr Chairman and CEO
Pivotal
Dr Ibrahim Farr Chairman and CEO
Pivotal


























































 Oscar Juan MD, PhD, Medical Oncologist, Senior Medical Manager
Oscar Juan MD, PhD, Medical Oncologist, Senior Medical Manager






 Catherine Lund Founder and Managing Director OnQ Research
Catherine Lund Founder and Managing Director OnQ Research




