

Be�er Health and a better wayoflife.




Be�er Health and a better wayoflife.


Howpatientsreceivecare,hasbeenshifted
fromhospitalstothecomfortoftheirhomes. Today,arisingnumberofpeoplepreferto maintaintheirsenseofautonomyandindependence bytakinggreaterresponsibilityfortheirhealth,rather thanhandingitovertodoctors.Technological advancementssuchastheuseofmedicaldevices todayareprovingtosupportthesehealth-conscious individualsintheirjourneyofhealth.Forinstance, insteadofwaitinglonghoursatthedoctor’sclinic, patientscouldkeeptrackoftheirdiseasessuchas diabetesorhypertensionwhichenablesthemtomake lifestylechoicesaccordingly.
Someassistivemedicaldevicesareinuseforalong time,suchasdosingcupsfortheadministrationof medications,firstaidequipmentsuchas thermometers,heatingpads,andprostheticdevices suchasartificialarmsorlegs,andotherrelated.But recently,newermedicaldevicesarestartingtomigrate there.
Theyarepresentingneweropportunitiesforboth healthcareprofessionalsaswellasconsumersto deliverandseekhealthinasafe,usable,andcosteffectivemanner.

Thegrowingsophisticationofmedicalequipmenthas significantlyimprovedthehealthoftheindividualand society.Ithasimprovedindividualsurvivabilityinthe faceofdiseaseorinjury,greatlyenhancingpatients' lifethroughanaccuratediagnosisandtherapeutic results.Butitisnottheonlybenefitthatthe advancementhasbrought.Withtheclimbingcostsof healthcarefacilitiesaswellasthelackofenough healthcarestaffwhocanattendtoeveryminute concernofthepatient,themedicaldevicesacthas easedthetensionandimprovedhealthcaresustainably forboththepatientsandhealthcareproviders.Inthe followingeditionofInsightsCare,“Europe'sTop5 MedicalDeviceCompaniestoWatchin2023,”we presentyouthosecompanieswhohavelifted
healthcaretoawholeanotherlevel,throughtheir innovativeserviceswithinthemedicaldevice industry.Ifyouareenthusiasticaboutmedical devices,thenthefollowingcompanieswillnotonly inspireyoubutalsoprovideyouwithmeaningful insightsthathaveledthemtothepositiontheyarein today.
We hope you have an insightful read!
Abhishek Joshi




DIVE Medical company has been dedicated to enable precise and objective exploration of the visual functions not only in children but in all noncollaborative patients.
Injeq is a company that founded a solution to to make lumbar punctures safer and more precise through its Injeq IQ-Tip®, a spinal needle that detects gives out an audio alarm to the physician after detecting spinal fluid.
The company is known for its medical device MOWOOT, which is a non-laxative solution against chronic constipation.
Palliare, a company that is innovating in the insufflation space, is working toward creating a safer operating environment that provides better physician control and patient outcomes

Palliare's vision is to become the world technology leader in advanced insu�ation by innovating to meet the needs of minimally invasive procedures.





Improvingpatientcarewithnewtechnologiesiscritical
tocontinuetoimproveproceduraloutcomesand patientqualityofcare.Inrecentyears,therehasbeen significantevolutioninmanyspaceswithinthemedical fieldthathavebenefitedbothpatientoutcomesaswellas providers’abilitytodelivertopcare.
Manyoftheseadvancementshavecomewithagreater focusonintegratingtechnologyintomedicine.
Palliare,acompanythatisinnovatingintheinsufflation space,isworkingtowardcreatingasaferoperating environmentthatprovidesbetterphysiciancontroland patientoutcomes.
WehadtheopportunitytospeakwithPalliareCEOJohn O’Deatolearnmoreaboutthiscutting-edgecompanyand themanbehinditsvision.
Palliareisanorganizationthathasbeenahighlightedname inthehealthcaresector.CEOJohnO’Deahasalegacyand reputationforbeingaleaderincreatingsolutionsto challengesthatmedicalprofessionalsfaceduringsurgical andendoscopicprocedures.
BeforePalliare,JohnfoundedacompanycalledCrospon, whichoperatedintheendoscopyspaceandwaslater acquiredbyMedtronic(NYSE:MDT)in2017.Duringthis period,hespentampletimeobservingadvancedendoscopic procedures.Inthelasttwodecades,heobservedtheshiftin endoscopyfrombeingpurelydiagnostictoproviding surgicaltreatments.
Insufflation,wheregasesareinjectedintothesurgical cavity,hadbeenleftuntouchedandwasnotbeingevolved tomeettheneedsofthesemorecomplexandlonger endoscopicandlaparoscopic.
Regardingthisconcern,Johnsays, “In the same period, we have seen the evolution of laparoscopic surgery to robotic surgery. Similarly, insufflators have only started to evolve to meet the emerging needs of these procedures — particularly concerning managing smoke evacuation and leaks from trocars.”
Fromthisobservation,anopportunitywasborntoinnovate intheinsufflatorspaceandprovideasaferenvironmentand
bettercontrolforphysiciansconductingthesecases.The companycreatedacompactinsufflatortailoredtothe evolvingneedsofendoscopic,laparoscopic,androbotic surgery.
Anengineerbytrade,Johnspenthisearlycareerworking forPuritanBennett,wherehedesignedICUventilators. Later,in1998,heCo-foundedCaradyne,whichdeveloped non-invasiveventilationproductsforERandNICU settings.Thecompanywasacquiredin2004byRespironics (NASDAQ:RESP).
In2007,hefoundedCrospon,whichdevelopedanovel diagnosticproductcalledEndoFLIP.Thisdeviceassesses sphincterperformanceandmotilityinthegastrointestinal tract.ThiscompanywasacquiredbyMedtronicin2017 (NYSE:MDT).
Johnshares, “In 2018, a year after Medtronic acquired Crospon, I founded Palliare. We received FDA clearance for its flagship insufflator device, EVA15, in 2020. We offer two leading solutions: the EVA15 and the EndoTrap, and our vision is to continue to evolve the EVA platform to bring more innovations in surgical and endoscopic insufflation to market.”
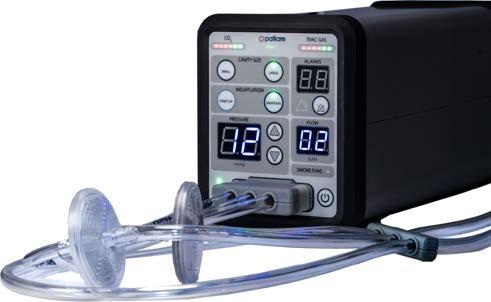
TheEVA15insufflatorandsmokeevacuationsystem providesbest-in-classlaparoscopic,robotic,andsurgical endoscopicinsufflation.ItmeetsAORNguidelineson surgicalsmokeevacuation,whichisimportanttosupport thesafetyoftheORandendoscopysuitestaffandadhereto arecentlegislativedrivetobansurgicalsmokeintheOR.
Duringthepandemic,John’swell-knownlegacyof innovationbroughthimandPalliareanewopportunity.He shares, “In 2020, during the COVID-19 crisis, we were selected by the European Commission to design devices to reduce potentially contaminated gases emitting from the patient during surgery and endoscopy. The project, called PORSAV (Protecting OR Staff From Aerosolized Virus), was completed in July 2022. Two devices, EndoTrap and LeakTrap, were developed by the project consortium for which Palliare was the technical lead.”
EndoTrapwasbornduringCOVID-19butcontinuesto haveastrongrolebeyondit.Thedeviceisdesignedto
improvestaffsafetybyreducingexposuretoairborne particlesduringupperendoscopyandbronchoscopy procedures.Aprotectivebarrieriscreatedaroundthe patient’snose,mouth,andendoscopeusingasingle-port anesthesiamaskandviralfilter,alongwiththeEndoTrap, whichconsistsofaT-piece,avalve,andaprotectivesleeve. EndoTrapisintendedtobeusedduringhigh-risk bronchoscopyandupperGIendoscopyprocedures.
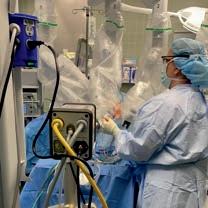
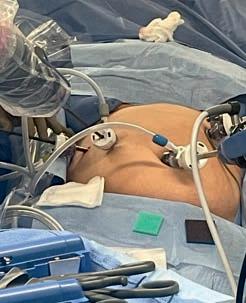

Thecompany’smainproduct,EVA15,isaninsufflatorwith abuilt-insmokeevacuationsystem.Anysurgicalsmoke removedfromthepatientisfilteredthroughanultra-low particulate(ULPA)filter,whichcantrapparticlesdownto 0.04umindiameter.ThisisimportanttoprotectORstaff fromsmokeproducedduringtheseprocedures.



A key aspect of the EVA15 design is that when smoke is evacuated from the surgical cavity, fresh gas is injected into the cavity so that the surgeon experiences no collapse or reduced visibility. Pressure is sampled 100 times per second so that the response to evacuation is almost instantaneous and there is no perceived change in the pressure.
WhiletalkingabouttheEVA15,Johnadds, “A key aspect of the EVA15 design is that when smoke is evacuated from the surgical cavity, fresh gas is injected into the cavity so that the surgeon experiences no collapse or reduced visibility. Pressure is sampled 100 times per second so that the response to evacuation is almost instantaneous and there is no perceived change in the pressure.”
Inthecomingyears,Palliareisfocusedonmovingthe insufflationfieldforwardwithexcitingdevelopmentsin intelligentinsufflation,suchasadvisorycapabilitiesto guidesurgeonstotheoptimuminsufflationpressure,thus reducingtherisksassociatedwithhyper-insufflation.
StatingtheprimarygoalofPalliare,Johnsays,“We will be exploring these techniques with the anesthesiologist community, not only for the perioperative phase of a procedure but also with a focus on exploring whether or not smart insufflation can reduce postoperative pain for patients. The company has a lot of exciting innovation goals that will make procedures safer for the medical community and help improve patient outcomes.”
Thecompanyisseekingtobringtomarkettheworld’sfirst bi-levelinsufflatortoallowinsufflationpressurestobe quicklychangedintra-procedurally,afeatureofcentral importanceinnew,emergingendoscopicsurgery procedures.
Thereisastronglegislativeagenda,particularlyintheU.S., tobansurgicalsmokeintheoperatingroom.Many insufflatorscan’tkeepthepressureconsistentwhengasis extractedbystandalonesmokeevacuation.Asaresult, surgeonsexperienceacompromisedsurgicalfielddueto decreasedvisibilitywhenthegasisremoved.
ThistechnicalissuehascausedmajorconcernintheOR. Everyoneagreessurgicalsmokeisdangerous,butsois forcingsurgeonstooperatewithsub-optimalvisibility duringaprocedure.
Anewerinsufflationtechnology,likeEVA15,eliminates thiscompromiseandfacilitatesbothasaferenvironmentfor thesurgicalstaffwithpropersmokeremovalandasafer proceduralenvironmentforthephysicianandpatient.
Itdoesthisbyconsistentlydeliveringthecorrectpressure, thuskeepingclearvisibilityduringtheprocedure.
Johnadds, “We have worked with AORN to produce an accredited CE course, Understanding the Challenges of Laparoscopic Insufflation, to educate the OR community on how the smoke evacuation and insufflation systems can be designed to operate to meet the needs of all staff in the OR.”
Manysmokeevacuatorsoperatecontinuously,regardlessof whetherornotsmokeisbeinggenerated.Thisleadsto continuousCO2beingpumpedintotheOR.
AsPalliarelookstothefuture,itisseekingtomakethisa saferenvironmentforallinvolved.Johnadds, “We are working on the way to have a surgical robot or energy system inform the insufflator when it needs to turn on and off smoke evacuation to reduce the unnecessary CO2 produced and emitted during a procedure.”
“As the company’s founder, it is my focus to continuously develop, innovate, and advance the insufflation space,” Johnsays.Asanexample,Palliarebelievesthatinsufflators shouldhavealayerofcommunicationwithother
We o�er two leading solutions: the EVA15 and the EndoTrap, and our vision is to continue to evolve the EVA platform to bring more innovations in surgical and endoscopic insu�ation to the market.
Over the past decade, there has been li�le innovation in insu�ation technologies designed to inject gas into the surgical cavity. In particular this technology has not evolved to meet the needs of more complex, longer endoscopic procedures.

Similar to how we saw laparoscopic insulation needing to evolve to address the needs of laparoscopic robotic surgery, we see a need to move forward insu�ation technology in the emerging fields of endoscopic and robotic endoluminal surgery. We are now working with a number of emerging leaders in both the laparoscopic and endoluminal robotic surgery spaces.
instrumentsintheORandhasembeddedanAPIinthe EVA15.Thisallowsmanufacturersoflaparoscopic, endoscopic,androboticsystemstoremotelycontrolthe insufflator.
WordsofWisdom

Palliarehasalwayshadtheinterestsofpeople–boththe patientsandthecliniciansdeliveringthecare–atheart. John’sbestadvicetoanymedicaltechnologyordevice developeristobe“needs-driven”insteadof“technologydriven.”

Heshares“WeareexcitedtohaverecentlyobtainedaU.S. patentforenergy-activatedsmokeevacuationincontinuous pressureinsufflators.Thisisimportantbecauseitmeansthat CO2wasteisreduced,andlessgasisneedlesslyevacuated fromthesurgicalcavity.Withmostcompetitors,smoke evacuationiscontinuouslyactivatedfortheentire procedure.”














Inthecurrenttimes,earlydetectionofvisualproblems ininfants,childrenbelowtheageofthree,andpatients withneurocognitivedisorderremainasignificant challenge.Thestandardvisualtestsaremajorlydesigned foradultpatientswithnormalcognitivedevelopment.Itis hardlypossibletousethesametestsinyoungerchildrenor patientswithdevelopmentalissues.
Forthisreason,theDIVEMedicalDevicehasbeen developedtoenablepreciseandobjectiveexplorationofthe visualfunctionsnotonlyinchildrenbutinallnoncollaborativepatients.
Withamissiontoprovideuniversal,affordable,and effectivemeanstopreventvisionimpairment,DIVE Medical'sChiefTechnologyOfficerandCo-founder, MartaOrtin,believesinthevisionofensuringearlyvision diagnosisandtreatment.
Inaninterviewwith Insights Care,MartatalksaboutDIVE andhowthedeviceishelpingdoctorsintheearlydiagnosis andtreatmentofvisualproblemsinsmallchildrenand babies.
Below are the excerpts from the interview:
PleasetellusaboutDIVEMedicalindetail.
DIVEbeganasacollaborativeresearchprojectbetween ophthalmologistsandopticiansfromtheAragonHealth ResearchInstituteandengineersfromtheUniversityof Zaragoza(Spain).Whenwepresentedourresultsat congresses,professionalsinthefieldexpressedalotof interestinourproject.
Theyoftentoldusthattheywouldliketobeabletouseour deviceintheirpracticeandthattheythoughtitwas somethingusefulthatcouldbenefitmanypeople. Encouragedbythegoodreception,westartedthis entrepreneurialadventureandfoundthecompanyDIVE Medicalinearly2020.
Fromthenon,severalofthepeoplewhohadworkedinthe researchofthedevicewithintheinstitutionsjoinedthe company,inwhichwearealreadysevenemployees.
Pleasegiveusabriefoverviewofyourjourneyinthe healthcareniche,highlightingtheUSPsofDIVE Medical.
DIVEisadevicethatallowsustoexplorethevisual functionofanypatient,eventhosewithwhomdoctors cannotcommunicate(infants,youngchildren,orpeople withneurocognitiveproblems)andwhoaremoredifficult toexamine(suchaspatientswithlowvision).
Itisagreatchallengeforprofessionalstoexplorethese patients,andtheresultstheycanobtainareoftensubjective andinaccurate.
DIVEisabletoperformacompletevisualexaminationof thesepatientsandprovideobjectiveandaccuratemeasures oftheirvisualfunction.Inthisway,wehelpmakeanearlier andmorereliablediagnosisandfacilitatethefollow-upof pathologiesandvisualtherapies.
WhatarethecorevaluesuponwhichDIVEMedicalis built?Whatisitsvisionandmission?

OurvisionistogrowDIVEtobecomethenationaland internationalstandardforexploringthevisualfunctionin allpatientsandbecomeareferenceinvisualtest development.Ourmissionistouniversalizeeyecareby offeringnewtechnologiesthatallowtheearlydetection, diagnosis,andfollow-upofvisualpathologiesinalltypes ofpatients.Ourvaluesareintegrity,effectiveness, accountability,collectivetalent,andthepursuitof excellence.
Whatisyouropiniononthenecessityforthehealthcare industrytoleveragetheemergingtrendsoftechnology concerningfulfillingyourclient'srequirementsinthe healthcareindustry?
Theintroductionoftechnologyinthehealthcareindustry bringstremendouspotentialapplications.Inparticular,in
theophthalmologyfieldinwhichwework,withoutusing technology,itisverychallengingtoexplorecertainpatients (includingbabies,patientswithneurocognitivedisorders,or theelderlypopulation),withresultsbeingverysubjective andnon-repeatable,andwithsomevisualfunctionsbeing impossibletomeasure.
Byintroducingtechnology,thesechallengescanbe overcome.Inparticular,DIVEhasascreenonwhichwe displaystimulidesignedtoevaluatedifferentaspectsofthe visualfunction.Inaddition,weincorporateeye-tracking technology,whichallowsustoknowthepointonthe screenwherethepatientislookingatanygivenmoment, anddeeplearningforanautomatizedanalysisofthe outcomes.
Whatwasyourinspirationandcommitmentbehind developinginnovativehealthcareproductstoimprove people'slives?
Westartedworkingonthisprojecttosolvetheneedthe ophthalmologistsofourresearchteamhadeverydayduring theirclinicalpractice.Assoonaswestartedworkingonthe developmentofDIVE,wewereabletoseetheimpactof ourworkinaverycloseandimmediateway.Ourbiggest motivationisDIVE'stangiblebeneficialimpactonmany patients;thisisthemostgratifyingthingforus.
Asanestablishedindustryleader,whatwouldbeyour advicetothebuddingentrepreneursandenthusiasts aspiringtoventureintothehealthcareindustry?
Iwouldtellthemtohaveaclearobjectiveandhigh motivation.Thisisacomplexsectorthatconstitutesmany paperworkrequirementsandaprolongedentryprocess.In turn,itisveryrewardingtoseehowsomethingyouhave workedonforyearshelpsimprovethelivesofpeople.
Whatareyourfuturegoals,andhowdoyouenvision furtherstrengtheningthestrongholdofDIVEMedical inthehealthcaresector?

Wearecurrentlyfocusingonapplyingourtechnologyfor visualexploration,butithasalreadyopenedupalotof excitingopportunities.Besidesincorporatingnewtestsinto ourexistingDIVEdevice,wearealsoplanningon developingvisualtherapyexercisesandsystemsforthe automaticearlydetectionofneurologicalpathologiesthat affectvision,suchasParkinson's,Alzheimer's,orautism.
Pleasegiveusafewtestimonialsofyour clients/customersandalistofawards/recognitionsthat accuratelyhighlightDIVEMedical'spositionasa boomingcompanyinthehealthcaresector.
Testimonialsfromophthalmologistswithwhomwerana researchproject:
"DIVEisatoolthatputstechnologyattheserviceof clinicalneeds.Itprovidestheophthalmologistwithvery valuableinformationbecauseitiscapableofanalyzingand quantifyingvisualfunctionsasithadneverbeenachieved before.Itisespeciallyusefulforpediatricophthalmology, whereexplorationscanbedifficult.DIVEcaptures children'sattentionfromthebeginning,andtheir collaborationismuchbetter."
- Teresa Pérez Roche. Pediatric Ophthalmologist. Hospital Universitario Miguel Servet, Zaragoza (Spain).
"Itisaveryusefuldevicefordecisionmaking.Experience andthesubjectiveimpressionofthespecialistareoften neededtomakeadiagnosis.DIVEfacilitatesdecisionmakingsinceitprovidesobjectiveandquantitativedata.It isalsousefulforpatientfollow-up,allowingtoseetheir evolutionandtransmittheresults.DIVEmakestheoffice visitseasier."
- Sandra de Fernando. Ophthalmologist. Hospital Universitario Cruces, Bilbao (Spain).
"Interestingdeviceforadeeperanalysisofocularand saccadicmovements.Inchildrenwithneurological problems,itis[traditionally]morecomplicatedtoevaluate thisfunction.Exploringcontrastsensitivityandcolor perceptionisalsoveryinterestingforchildrenwhose diagnosisismorecomplex.Traditionaltestsaremore boring,butthroughgames[inDIVE]itismoreenjoyable forchildren.Goodexperiencewithchildrenthathave syndromesorneurologicalproblems.Usefulforyoung children,withwhomdoubtsarisewhenmakinga diagnosis."
- Marta Galdós. Ophthalmologist. Hospital Universitario Cruces, Bilbao (Spain).




Theimplementationofdigitalhealthstrategies
andserviceshasundergoneaconsiderable revolutionasaresultoftheparadigmshift fromprovider-centeredhealthcaretopatient-centered healthcare.Eachnation'shealthcareregulatory agenciesplayacrucialroleincreatingthebest systems,strategies,andprocedurestooptimizecare deliveryandprovidepatientswiththebestpossible treatment.
ThemajorityofWesternEuropeannationscurrently havepoliciesorstrategiesthatoutlinethegoalsof eHealthimplementation,theobjectivesofeHealth, andafutureroadmapforexpandingandintroducing additionalhealthcareservices.Next-generationhealth informaticssolutionsarebeingintroducedinnations withmoredevelopedhealthcareICTinfrastructure, suchastheUnitedKingdom,Germany,the Netherlands,Sweden,Denmark,Norway,andFinland, toenhancepatient-centeredtreatment.

Below are some of the aspects that have empowered innovations in Europe’s Medical Device Industry.
Moreproductsarebeingsuppliedthroughremotely hosteddeploymentsratherthanon-premisededicatedserver modelsasthemedicalsoftwaremarketevolves.Asaresult ofthisinescapabletransitiontocloud-baseddigitalhealth services,mostproviderswillnolongerneedtomaintain expensiveandunsafedatacenters.Theindustryis anticipatedtogrowoverthenexttwotothreeyearsas providersinWesternEuropemigratetocloud-basedcare managementsystemslikehospitalinformationsystems (HIS),ambulatorycaremanagementsystems,patient managementsystems(PMS),electronicmedical/health records(EMR/EHR),andelectronicprescribing.Afew providersalsoprovidehybridcloudmodelswithbenefits includingremotebackup,disasterrecovery,highresource availability,andsecurityandsupportformobiledevices.


WesternEuropeispredictedtoexperiencebriskexpansion inthemarketforhealthcaresystemsintegration.Many applications,includingmedicalrecordsystems,medical deviceintegration,laboratoryintegration,radiology integration,hospitalsandclinicsintegration,telehealth portals,homehealth,andotherapplications,heavilyrelyon integrationandinteroperabilityservices.Fasthealthcare InteroperabilityResource(FHIR)andSMARTonFHIR, theorganization'snext-generationstandardframeworksthat makeuseofthemostrecentWebstandardsandplacea strongemphasisonimplementations,haveemergedto addressthechallengesofclinicalandadministrativedata interoperabilityintherealworld.
Inhighacuitycareunits,theintegrationofmedicaldevice datawithpatientmedicalrecordsimprovespatient monitoringandsurveillance.Deviceconnectivityhasthe abilitytodecreasehumanerrorwhileboostingoperational effectiveness,whichisadvantageoustobothpatientsand clinicians.Alongwithdeviceintegration,otherserviceslike clinicalalarmsbasedonEarlyWarningScores(EWS), predictiveanalytics,virtualcriticalcareunits,andwarning systemsforemergencyresponseteamscanassistdoctors practisepreventivecareandenhanceresults.Inhighacuity
careunits,theintegrationofmedicaldevicedatawith patientmedicalrecordsimprovespatientmonitoringand surveillance.Deviceconnectivityhastheabilitytodecrease humanerrorwhileboostingoperationaleffectiveness, whichisadvantageoustobothpatientsandclinicians.
Bring-your-own-device(BYOD)andbring-your-ownapplication(BYOA)solutionsarebecomingmoreandmore popularinthehealthcareindustryasaresultofthe convergenceofmobilesolutionsandhealthcare technologies.Thegrowthofthisindustryisprimarily attributabletotheincreasedadoptionofsmartphones, connectedmedicaldevices,healthwearables,mHealthapps forthemanagementofchronicdiseases,androbust3Gand 4Gnetworkpenetrationthatprovidesuninterruptible connectivityfordigitalhealthcareservices.
ThehealthcareindustryinWesternEuropehaslongbeen regardedasbeingunreachableorlessdesirableasatarget forITadvancements.Thisisunquestionablynolongerthe case,andthefutureintheevolvinghealthcareindustry holdsmanywonderfulpossibilitiesforfreshchances.The installationofasafeandinter-institutionalnetworked infrastructurebyprovidersseekingtoimprovehealthcare deliveryisthemaindriveroftheenormousdevelopment seenintheEuropeanhealthcareITsector.Bygradually replacinglegacyandnon-interoperablehealthcareIT systemswithsystemsthatsupportopenstandards-based datainterchange,theaimistofurtherreduceadministrative expenses,avoidhumanerrors,optimizetheuseofmedical data,andimprovethequalityofservices.
ThebeststrategytoensurethatWesternEuropeancountries supplyhigh-qualityhealthcaretotheircitizensisto encourageeHealthadvancesofmedicaldevicesinthe healthcareindustry.Thehealthsystemscanbenefitfrom increasedpatientempowerment,workflowoptimization throughdigitalmeans,amorecompetentworkforce,more effectiveandsustainablehealthandcaresystems,and responsivegovernmentaladministrations.
-Anish Miller
Healthcareadvancementsthatweremereideas
decadesagoarecomingtofruition,provingtobe muchmorevaluabletosavinghumanlivesand enhancingthequalityoflife.Medicaldeviceshavebeena vitalpartoftheseadvancementsandcanenabletechnology foritsapplicationstohelpabettercause,thusisthestoryof Injeq
Injeqwasfoundedwhenagroupofexpertsinthefieldsof technologyandmedicinesawthatblindpunctureswere problematicastheyintroducedleukemiacellsfromthe bloodtothecentralnervoussystem.Thecompanyfounda solutionthroughamedicalneed:tomakelumbarpunctures saferandmoreprecise.ItsInjeqIQ-Tip®isaspinal needlethatfacilitateslumberpunctures.Itgivesoutan audioalarmtothephysicianwhentheneedletipdetects spinalfluidbasedonbioimpedancedetection.Ina conversationwithMaaritForsten,theClinical ApplicationManagerofInjeq,shetalksmoreindepth abouttheorganization’sclinicallyprovenproduct.
Below are the highlights of the interview:
Pleasebriefusaboutyourcompanyandwhatideasand developmentsledtoitsinceptioninthemedicaldevice niche.
Injeqwasfoundedbasedontheproblemsofblind punctures,i.e.,puncturesduringwhichthedoctor
performingtheprocedurecannotseewherethetipofthe needleis.Untilnow,theadvancementoftheneedlewas basedonthehapticfeelingintheirfingertipsand knowledgeofthehumananatomy.Forinstance,avoiding nervesandfindingcavitieslikethespinalcavityfor cerebrospinalfluidhavebeenbasedontheprofessional experienceofthemedicalpractitionerssofar.
Whatareyourkeyproductsandservices?Howareyou revolutionizingthemarketwiththem?
Wearemanufacturingbioimpedancespectroscopy-based needleswithtissuerecognition.Ourcurrentsolutionisa spinalneedlethatrecognizescerebrospinalfluidonthe needletip.Forthelast100years,nothingparticularlynew hashappenedwithspinalneedles.Nowwehavethis innovativeneedlethat,afterdetectingcerebrospinalfluid, givesanalarmtothephysicianperformingthelumbar puncture.Thishasturnedouttobeveryefficientin avoidingthetypicalandsometimescriticalcomplications relatedtolumbarpunctures.
Pleasebriefusonthecompany’sprofessionalmilestones sofar.
Theproblemofblindpuncturesandtissuerecognitionwas foundbyProfessorRittaSeppänen-Kaijansinkkobasedon allthepatientinjuryreportsshehadreceivedwhileworking inthispatientinjuryboard.
Until now, the advancement of the needle was based on the haptic feeling in their fingertips and knowledge of the human anatomy. But now we have this innovative needle, the Injeq IQ-Tip®, that, after detecting cerebrospinal fluid, gives an alarm to the physician performing the lumbar puncture. This innovation has the potential to improve the safety of all blind punctures and decrease the incidence of related complications, reducing the overall cost of health care in the process.

Howhastechnologyaidedyourgoalofinnovatinginthe medicaldeviceindustry?Whatareyourkeyadaptations oftechnologyinyourcompany?
Ourcurrentmainsolutioniscerebrospinalfluiddetection forchallengingand,eventually,foralllumbarpunctures. Generally,thisinnovationhasthepotentialtoimprovethe safetyofallblindpuncturesanddecreasetheincidenceof relatedcomplications,thiswayreducingtheoverallhealth carecostsalsoremarkably.
Whatarethechallengesthatyouhavefacedtillnow? Howhaveyouinfusedyourleadershipskillstoturn themintoopportunitiesforyourcompanyaswellasfor theindustry?
ThefirstchallengewastogetCEapprovalforInjeq’s medicaldevice,whichrepresentsthehighestriskclass3
accordingtothenewMedicalDeviceRegulation.CEmark wasgainedinDecember2021.Afterthismarketing approval,thefirstdistributorcontractsweresigned,andthe salestothebiggestEuropeanmarketswereaimedtostart. Thenextchallengewasthepriceofourdevice,whichinthe beginningwastentimesmoreexpensivecomparedtothe conventionalwayofmakinglumbarpunctures.However, byimprovingourmanufacturingprocesseswewereableto remarkablyreducetheinitialprices(almosthalfofthe original).Inaddition,wehavegoodclinicalevidenceofthe benefitsachievedwithInjeqIQTipsystem.

Asanexperiencedleader,whatwouldbeyouradviceto buddingentrepreneurswishingtoenterthisindustry?
First,consulttheclinicalworldaboutwhattheyneed, whichmeans—donotmaketheinnovationfirstandthengo tomarket,butviceversa.Second,choosethelowest possibleriskgroupforyourmedicaldevice;choosethe indicationareanothavingcontactwiththecentralnervous system,heart,orbigbloodvessels,whichautomatically makesyourdeviceclass3.Third,choosethematerialsand manufacturingprocessessothattherearenoriskymaterials likenickelorprocessedchemicalsthatcauseallergies,toxic
reactions,orotherharmtotheuserorpatient.Lastly,listen tothefeedbackallthewaythrough.
Whatarethefuturegoalsofyourcompany?Howdo youenvisionscalingupitsgrowthandoperationsin 2022andbeyond?
Weareplanningtofindotherbeneficialapplicationareas forbioimpedance-basedtissuerecognition.Thegoalisto maketheearlierpassiveneedlesactivetoimprovesafety, efficiency,andpatientexperienceandreduceoverall healthcarecosts.


Companiesinthemedicaldevicebusiness(also
knownasMedTech),whichisunderincreasingprice pressurefromhealthcareprovidersandcontending withdecliningreturnsfrommaturetechnologies,mustnow lookfornovelmethodstoboosttheirbottomlines.For medicalequipmenttocontinuetobecost-competitiveinthe future,supplychainsmustbedigitalized.
Businessexecutivescontinuetoexpressincreasingconcern aboutthetoughpricecompetitionanddownstreampressure intheMedTechsector.Lessexpensivetechnology,valueandoutcome-basedpaymentmethods,andstricterregulatory proceduresaredemandedbypayers,providers,and governments.Additionally,inatimeofrisingout-of-pocket (OOP)expenses,patientshaveevolvedintovalue-conscious shoppers.Patientsareincreasinglypricesensitiveandprone toskippingtreatmentwhenfacedwithhighdeductibles, evenwhiletheyarewillingtopayoutofpocketforhighqualitypremiumservices.PricesforMedTechdeviceshave fallenfurtherinthefaceofthesechallenges.Theseplayers mustnotonlyofferexceptionalfeaturesbutalsooutstanding valuetopatientsandproviderswhileminimisingexpensesin ordertocounteractdroppingpricing.
Below are the transforming approaches adopted within the leading medical device companies.
Weadvisea"dreamlarge,startsmall,actquickly"strategy becausedisruptivedigitalmedicaldevicesanddigitalsupply
networksmayhavebroadrepercussions.Thefirststageisto createafuture-stateimageofasupplychainthatis optimisedfromsupplierstoproviderstopatients.Inorder toreducetherisksandshowearlysuccesstostakeholders, startwithsmalladjustments.Inordertogainswiftvictories, medtechcompaniesshouldfocusonissuesthathavethe potentialtounleashvalueatseveralstages.Buildingon earlyachievements,businessescancontinuetocreateDSNs inareaswheredoingsomakesbothstrategicand economicalsense.
ToeffectivelyutiliseDSN'spotential,MedTechbusinesses mustscheduletimeandresources,traintheirworkforces, andtrainthemselves.Ingeneral,ratherofconcentratingon department-specificoperations,theworkforceshould becomemoretech-savvyandabletohandlemultipleduties.
Therearethreereasonswhyit'scrucialfortheworkforceto beknowledgeableaboutdigitalsolutions:

• Theusersofdigitalsolutionsmustbeabletoderive valuefromthemasconsumersoftheunderlying technology.
• Theworkforceneedstobeeducatedonthetoolsand processesbeingdeployed,theorderofnew technologiestobeadopted,andtheirexpectedimpact ascustomersofdigitalsolutions.
• Teamsneedtobereadyforachangedworking relationshipwithITastheyimplementdigitalsolutions sinceindividualteamsmayreplaceITastheprincipal ownersoftechnology.
Toachievesignificantchangeacrossthebusiness,MedTech organizationsmustadoptanagilestrategytoDSN developmentanddeployment.Withthismethod, requirementscanbedevelopedincrementallyratherthanall atonce.Adopterscanmodifydevelopmentbasedon constantlychangingaimswithenhancedconfidenceinthe initiative'sultimatesuccessthankstoadopters'agilityin refiningandmodifying.
MedTechcompaniescanconcentrateonnewtechnologies andframeworksasawayoffendingoffthreatsand favourablyimpactingtheirbottomline,despitethe difficultiesthatmayappearinsurmountable.Usingdigital supplynetworks(DSNs)toimproveoperationalefficiency andgivetheircustomersmorevaluewouldbeagoodstart.

SomeoftheareaswhereMedTechcompaniescanseek benefitswhenadoptingdigitaltechnolgiesinvolve: Inventorymanagement,Logisticsanddistribution,device maintenance,Productdevelopment,andwarehouse operations.
Companiesmayincreasesupplychainvisibility,lower maintenanceandR&Dcosts,boostcustomerhappiness, andenhancepatientexperiencesbyimplementing digitalizationintheseareas.


Thefutureofhealthcareisshapingupinfrontofour veryeyeswithadvancesindigitalhealthcare technologyandnanotechnology.Thisrestsin workinghand-in-handwithtechnology,andhealthcare workershavetoembraceemerginghealthcaretechnologies tostayrelevantinthecomingyears.
Inmedicineandhealthcare,digitaltechnologycouldhelp transformunsustainablehealthcaresystemsintosustainable ones,equalizetherelationshipbetweenmedical professionalsandpatients,andprovidecheaper,faster, moreeffectivesolutionsfordiseases.Technologiescould simplyleadtohealthierindividualslivinginhealthier communities.
Leveraging the latest advancements,MOWOOT,agrowthstagemedicaldevicecompanyhasdevelopedamedical devicethatstimulatesthelargeintestineinanon-invasive way.Itisalsomarketinganovel,purelyphysical,non-drug, andnon-invasivesolutiontochronicintestinaltransit disorders,suchasneurogenicboweldisorders(NBD), opioid-inducedconstipation(OIC)orchronicidiopathic constipation(CIC).
UndertheproficientleadershipofDrMarkusWilhelms, CEOandCo-founder, MOWOOThasreceivedthe'FDA BreakthroughDeviceDesignation'forthecriticalimpacton patients'qualityoflife,whichopensthedoortothelarge USmarket.
Inaninterviewwith Insights Care,DrMarkusWilhelms shedslightontheinnovationsempoweredbyMOWOOTin thehealthcareindustry.
DrWilhelms,pleasetellusaboutMOWOOT,itsUSPs andwhatmakesitasuccessfulcompanyinthe healthcaresector.
ThemultidisciplinaryfounderteamofMOWOOTmetin 2013inthedHealthBarcelonaprogramfor'needs-driven innovation,'aEuropeanversionofStanfordUniversity's Biodesignprogram.
Applyinginnovationmethodologieslikedesign-thinking andbasedonover500patientinterviews,theteam developedasolutiontoasignificantunmetneedinbowel managementincollaborationwithleadingneurorehabilitationhospitalInstitutGuttmannandconstitutedthe companyinNovember2014.TheMOWOOTdeviceisnow alreadyavailableinseveralEuropeanmarketsandiseven recommendedinvariousclinicalguidelines.
WhatarethecorevaluesuponwhichMOWOOTis built,andWhatisitsvisionandmission?
Sincethebeginning,theMOWOOTteamhasbeenguided bydirectcontactwithpatientsandcaregiverstodevelop andmarketoursolutiontosolveanunmetneedand improve the quality of life in patients with intestinal transit disorders.
Whatisyouropiniononthenecessityforthehealthcare industrytoleveragetheemergingtrendsoftechnology tofulfilyourclient'srequirementsinthehealthcare industry?
Firstly,I'dstateunderstandingtheunmetneed,includingall relevantstakeholders,andfindingthesimplestpossible solutioniscrucial.Simplicityiselegance.Innovationdoes notnecessarilyneedtobecomplicated.However,ifa simpleandelegantapproachincludesan'emergingtrend,' thengoforit.
Whatwasyourinspirationandcommitmentbehind developinginnovativehealthcareproductstoimprove people'slives?
Ourfounderteamconsistsoffourpeoplewithdifferent professionalbackgrounds.Whatwehaveincommonisthat wewanttohaveapositiveimpactonthepatientsandalso onsociety.
MOWOOT team has been guided by direct contact with patients and caregivers to develop and market our solution to solve an unmet need and improve the quality of life in patients with intestinal transit disorders.
Wefirmlybelievethattodoso,toreallyreachasmany peopleaspossible,younotonlyneedtohaveaninnovative productbutalsoimplementitintoaviablebusinessmodel. Innovatinginhealthcareisoneofthemostdifficulttypesof entrepreneurship,butweacceptedthechallenge!
Asanestablishedindustryleader,whatwouldbeyour advicetothebuddingentrepreneursandenthusiasts aspiringtoventureintothehealthcareindustry?
First,startwithunderstandingtheunmetneedandits environment/stakeholdersanddolotsofinterviewstolearn aboutthis.Youwillhavetodevelopaquantifiablevalue propositionforyoursolution,takingallstakeholders'needs intoaccount(includingpayors,regulators,etc.)Applythe "scientificmethod"tobusinesscreation:build>measure> learn(repeat)
Nowadays,thereareverywell-developedtoolsavailableto guidenoviceentrepreneursintheirhealthcareadventure, forexample,theGAITSsystembyCIMITinBoston.I wishwewouldhavehadaccesstothistypeoftoolwhenwe started—highlyrecommended!
Whatareyourfuturegoals,andhowdoyouenvision furtherstrengtheningthestrongholdofMOWOOTin thehealthcaresector?
Weplantoenterthepublichealthcarereimbursementin severalEUmarketsregardingbusinessdevelopment. Moreover,marketentryintheUSAisthenextnaturalstep forMOWOOT.Regardingproducts,weareworkingona pediatricversionofourdevicetoansweramarketpulledby manyparentsandcaregiverswhocontactedus.
Pleasegiveusalistofawards/recognitionsthat accuratelyhighlightyourorganization'spositioninthe market.
Among23companies,inanextraordinaryall-digital RedefiningEarly-StageInvestments-RESI-conference edition,MOWOOTwasvotedasthe'Best Medical Device Company'oftheeventandachievedsecondplaceinthe overallratingofthemostvotedcompany.

MOWOOTreceivedthesealofapproval"ImpactBusiness" forstart-upswithsocialimpactafterathoroughanalysisby theShip2Bfoundationintheframeworkoftheir Tech4Healthaccelerationprogram.

MOWOOTisthewinneroftheBestOverallCompany st Award(1 place)asthecompanywiththemostvotesofall threecategories(MedTech,biotechanddigitalhealth)of theEuropeanHealthCatapultattheEITHealthSummitin London.
EuropeanHealthCatapultisanaccelerationprogramand start-upcompetition.Itawardsthebest-of-the-best innovationconceptsfromMedTech,biotech,anddigital healthacrossEurope.MOWOOTisthewinnerofthe st AudienceAward(1 place)intheMedTechcategoryofthe EuropeanHealthCatapultattheEITHealthSummitin London.
MOWOOTalsoachievedsecondplaceinthe Healthcare Professional Jury awards intheMedTechcategoryofthe EuropeanHealthCatapultattheEITHealthSummitin London.



