4 minute read
development of pharmaceutical granules
This equipment works with small amounts of raw material (15 to 20 grams), which are allocated in a mixing compartment. Two rotating blades, coupled to a signal transducer, will homogenize the sample. So that it will be possible to obtain a torque value, measured and calculated by the CalevaMTR ® software, through one of three different analytical methods: Consistency (used to check properties of powders produced in large-scale granulation systems and can be used as a quality control tool), Variable Mixed Time (provides an indication of the torque response in relation to the binder ratio and mixing time) and Multiple Addition (used to study the best granulation condition and determine its endpoint). In addition, the
F e b . d e 2 0 2 0 | V o l . 8
Advertisement
Multiple Addition method, in combination with statistical planning for the formulation of pharmaceutical granulates, allows the optimization of the multiparticulate formulations development, making it possible to choose the best proportions of active pharmaceutical ingredient (API) and excipients for the composition of the formulation. Thus, the formulator acquires full knowledge of the formulation and its processing parameters, guaranteeing the quality of the product from the first stages of development, as provided by Quality by Design (QbD) (MISHRA; ROHERA, 2016).
As a practical example, there is caffeine, an API that has poor flow, poor compressibility index and can be used in high doses as an energy supplement. In this context, the production of caffeine-containing tablets is conditioned to its granulation.
In order to choose the best formulation containing caffeine and determine its endpoint, a 3 3-1 fractional factorial design, resulting in 9 formulations, was planned and evaluated on the MTR under the following conditions: 60-second mix time, blade rotation at 50 rpm and log time of 20 seconds. After evaluating the results of the 9 formulations, the one with the highest maximum torque value was chosen, consisting of 30% caffeine, 1% carrageenan and Avicel ® 101 in sufficient quantity to complete 15 grams of sample.
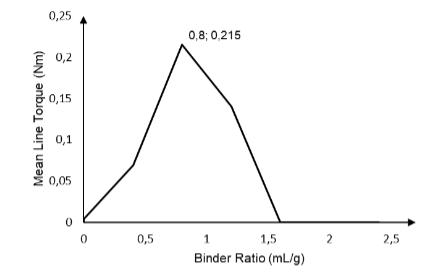
Then, through the rheological profile of this formulation (Figure 3), it was possible to determine the granulation endpoint of. It is important to emphasize that, as presented by Belem and Ferraz (2020), the ideal final stage still a contradictory topic among several authors. Some defend the choice of a point between the pendular and funicular stages, others defend a point between the funicular and capillary and, finally, there are those who follow the line that the capillary stage can be considered a good approximation of the granulation endpoint. In the present study, we opted to follow this last research line, so that the capillary stage (maximum torque) was adopted as the granulation endpoint. According to Figure 3, the liquid / solid ratio required to achieve the highest torque value is 0.8 mL / g. So to produce 200 grams of this granulate, is necessary to add 160 mL of the binder liquid to the solid mixture, thus defining the granulation endpoint.
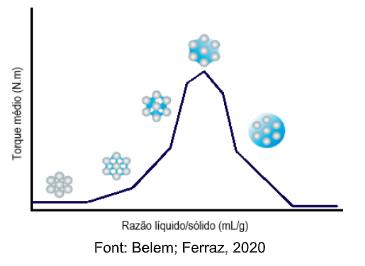
Figure 2. Intensity curve of the average torque as a function of the liquid / solid ratio.
Figure 3. Intensity curve of the average torque as a function of the amount of binder added to a mixture of 15 grams containing caffeine (30%), carrageenan (1%) and Avicel® 101 (q.s.p)
F e b . d e 2 0 2 0 | V o l . 8
The work in question was carried out at the Pharmaceutical Development and Innovation Laboratory of the Faculty of Pharmaceutical Sciences of the University of São Paulo (Deinfar FCF / USP), under the coordination of Prof. Dr. Humberto Gomes Ferraz. Deinfar was the first laboratory in Brazil to invest in an wet powder rheometer and, since its acquisition, several development projects for formulations of solid pharmaceutical dosage have been carried out employing several active ingredients. In addition, the laboratory has presented the technique and its benefits in different courses and academic works, fulfilling its role in the spread of knowledge and innovation in the Brazilian pharmaceutical industry.
References
BELEM, B. R.; FERRAZ, H. G.. Rheological profile in mixer torque rheometer of samples containing furazolidone and different binders. Chemical Engineering Research And Design, [S.L.], v. 160, p. 533-539, ago. 2020. Elsevier BV. http://dx.doi.org/10.1016/j.cherd.2020.06.022.
ENNIS, B. . Theory of granulation: a engineering perspective. In: PARIKH, D. M. (Ed.). Handbook of pharmaceutical granulation technology. 3. ed. CRC Press, 2016. p.676.
HANCOCK, B. C.; YORK, P.; ROWE, R. C. An assessment of substrate-binder interactions in model wet masses. 1: Mixer torque rheometry. International Journal of Pharmaceutics, v. 102, n. 1–3, p. 167–176, 1994.
MISHRA, Saurabh M.; ROHERA, Bhagwan D.. An integrated, quality by design (QbD) approach for design, development and optimization of orally disintegrating tablet formulation of carbamazepine. Pharmaceutical Development And Technology, [S.L.], v. 22, n. 7, p. 889-903, 27 jun. 2016. Informa UK Limited. http://dx.doi.org/10.1080/10837450.2016.1199566.
SAKR, W. F.; IBRAHIM, M. A.; ALANAZI, F. K.; SAKR, A. A. Upgrading wet granulation monitoring from hand squeeze test to mixing torque rheometry. Saudi Pharmaceutical Journal, v. 20, n. 1, p. 9–19, 2012.
SURESH, P.; SREEDHAR, I.; VAIDHISWARAN, R.; VENUGOPAL, A. A comprehensive review on process and engineering aspects of pharmaceutical wet granulation. Chemical Engineering Journal, v. 328, p. 785–815, 2017.
ZHANG, S.; LAMBERTO, D. J. Development of new laboratory tools for assessment of granulation behavior during bulk active pharmaceutical ingredient drying. Journal of Pharmaceutical Sciences, v. 103, n. 1, p. 152–160, 2014.










