
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 12 | Dec 2024 www.irjet.net p-ISSN: 2395-0072


International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 12 | Dec 2024 www.irjet.net p-ISSN: 2395-0072
Beena Kumari I P1 , Aruldev A2 , Aswathi P3, Sarath Krishna S4 , Aleena S Kumar5
1 Asst. Professor, Dept. of Civil Engineering, Ahalia School of Engineering and Technology, Kerala, India.
2UG Scholar, Dept. of Civil Engineering, Ahalia School of Engineering and Technology, Kerala, India.
3UG Scholar, Dept. of Civil Engineering, Ahalia School of Engineering and Technology, Kerala, India.
4UG Scholar, Dept. of Civil Engineering, Ahalia School of Engineering and Technology, Kerala, India.
5UG Scholar, Dept. of Civil Engineering, Ahalia School of Engineering and Technology, Kerala, India.
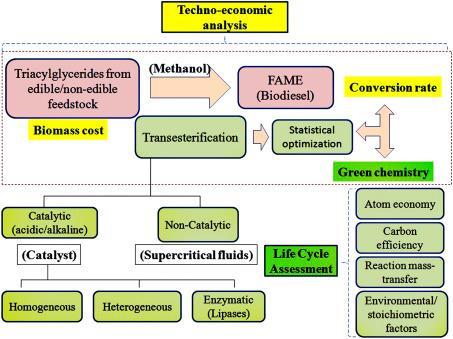
Abstract - The growing demand for renewable energy and rising environmental concerns have fueled increasedresearch into producing biodiesel from waste cooking oils (WCOs).This review offers an in-depth examination of methods for producing both biodiesel and glycerin from WCOs from 2020 to 2024. It evaluates various transesterification approachessuch as alkaline, acidic, enzymatic,andsupercriticalmethodsby assessing yield, purity, and economic feasibility. The study also explores glycerin processing, a key byproduct, and its diverse applications. In addition, it highlights the challenges and future outlook in biodiesel production from WCOs, emphasizing the critical role of sustainable practices and technological advancements. By addressing these factors, the review underscores the potential for WCO-basedbiodieseland glycerin to make meaningful contributions to renewable energy solutions and environmental sustainability by addressing these factors
Key Words: WCOs, FAMEs, FFAs, Transesterification, NaOH, Methanol, Glycerine, Biodiesel.
Fossilfuelshavelongbeenthedominantsourceofenergy worldwide.However,theiradverseenvironmentalimpacts, suchasthereleaseofgreenhousegasesandairpollutants, haveledtogrowingconcernsovertheirsustainability.These environmental challenges have prompted an accelerated global shift toward renewable energy sources, which are seenasessentialtomitigatingclimatechangeandensuring energy security in the future. Among these renewable alternatives,biodieselhasemergedasapromisingsubstitute forconventionaldieselfuel.Derivedfromvegetableoilsand animal fats, biodiesel is not only renewable and biodegradablebutalsoreducesdependenceonfossilfuels.It has been widely regarded as an eco-friendly solution to reduce emissions and improve air quality (Knothe et al., 2005).
Wastecookingoils(WCOs),whichareabundantlyavailable and inexpensive, have gained considerable attention in recent years as a potential feedstock for biodiesel production.WCOs,whicharetypicallydiscardedafterusein households,restaurants,andfoodprocessingindustries,are asignificantsourceofwasteinmanyregions.Thereuseof
***
these oils for biodiesel production offers a dual benefit: it reduces waste disposal challenges while simultaneously contributing to the production of a sustainable energy source. Compared to virgin vegetable oils, WCOs offer multipleadvantages,includinglowerrawmaterialcostsand a reduced environmental footprint. The process of convertingwasteintoenergyfurtherenhancestheecological benefits, making WCO-based biodiesel a more sustainable andcost-effectivealternativetotraditionalbiodieselsources (Diasetal.,2011).
TheproductionofbiodieselfromWCOstypicallyinvolvesa chemicalreactionknownastransesterification.Thisprocess involvestheconversionoftriglyceridespresentintheoils intofattyacidmethylesters(FAMEs),whicharetheprimary component of biodiesel, and glycerine as a by-product. Transesterification has become the most commonly employed method for biodiesel production due to its efficiency and established protocols in the industry. This chemicalreactionallowsforthetransformationoftheraw WCOmaterialintoahigh-qualitybiodieselfuelsuitablefor variousapplications(Freedmanetal.,1984).Overthepast fewyears,anumberofadvancedtransesterificationmethods and modifications have been introduced to improve biodiesel yields, reduce the need for costly reagents, and increaseoverallefficiency.
Thisreviewaimstocomprehensivelyexaminethemethods used for biodiesel and glycerine production from WCOs between2020and2024.Thefocuswillbeonevaluatingthe effectiveness of these methods in terms of biodiesel yield, fuel purity, and economic feasibility. By reviewing and comparing recent advancements, this paper provides an updated understanding of the most promising techniques andtheirpotentialforlarge-scaleimplementation.Through this analysis, the review will contribute to the ongoing developmentofbiodieselproductionstrategies,addressing bothenvironmentalandeconomicconcerns
Severalmethodshavebeenutilizedforbiodieselproduction from WCOs, each with its own advantages and disadvantages.Thissectiondelvesintothemostcommonly

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 12 | Dec 2024 www.irjet.net p-ISSN: 2395-0072
employedtechniques,focusingontheireffectivenessduring theperiod2020to2024
Alkaline transesterification is one of the most commonly usedmethodsforbiodieselproduction,utilizingsodiumor potassium hydroxide as the catalyst. It is favored for its simplicity,highyield,andrelativelylowcost,whichmakeit an attractive option for large-scale biodiesel production (Vicenteetal.,2004).Themethodinvolvesthereactionof triglycerides(fatsoroils)withalcohol(usuallymethanolor ethanol),producingbiodiesel (methyl orethyl esters)and glycerol as a by-product. Despite its widespread use, this methodhasakeylimitation:itssensitivitytofreefattyacids (FFAs)presentinwastecookingoils(WCOs).
Challenges with FFAs in Alkaline Transesterification The presence of FFAs in WCOs can significantly hinder the alkalinetransesterificationprocess.WhenFFAsarepresent in the oil, they react with the alkaline catalyst (such as sodium hydroxide) to form soaps (sodium salts of fatty acids). Soap formation leads to a reduction in the overall biodieselyield,assomeofthefattyacidsare"lockedup"in thesoapratherthanconvertingintobiodiesel.Additionally, soapformationcomplicatesthepurificationandseparation processes during downstream processing, making it more difficult to separate the biodiesel from the glycerol and residualcontaminants.
StrategiestoOvercomeFFASensitivityInresponsetothis challenge,variousstrategieshavebeendevelopedinrecent years to address the issue of FFAs in WCOs while still utilizingthealkalinetransesterificationmethod.Oneofthe most widely explored approaches, particularly during the period from 2024 to 2020, involves two-stage transesterification.
Two-StageTransesterificationProcess:Thisprocessinvolves twodistinctstepsaimedatremovingorneutralizingFFAs before proceeding with the alkaline transesterification reaction.Inthefirststage,FFAsareremovedorconverted into esters. Typically, this is done by esterification, where methanolandanacidiccatalyst(e.g.,sulfuricacid)areused TheacidcatalyzedesterificationeffectivelyreducestheFFA content by converting the FFAs into biodiesel (methyl esters). In the second stage, the alkali-catalyzed transesterification process is carried out as usual, with methanolandabasecatalyst(suchassodiumhydroxide)to producebiodiesel.Thistwo-stageprocesshelpsreducesoap formation,astheFFAcontenthasalreadybeenminimized. Studieshaveshownthatthisdualapproachleadstohigher yieldsofbiodiesel,asitminimizestheissuesassociatedwith soapformationandmakesthedownstreamprocessingmuch simpler(Islametal.,2020).Theacid-catalyzedesterification stepensuresthatevenoilswithahighFFAcontentcanstill beconvertedintobiodieselefficiently.
OtherModificationsandInnovationsInadditiontothetwostageprocess,variousothermodificationsandinnovations havebeenexploredinrecentyearstofurtherimprovethe effectivenessofalkalinetransesterification, particularlyin dealing with WCOs and other feedstocks with high FFA content.Someoftheseinclude:
i.Pre-treatmentwithActivatedCarbonorClay:Researchhas exploredtheuseofadsorbentslikeactivatedcarbonorclay toremoveFFAsfromtheoilbeforetransesterification.This helps lower the FFA concentration, thus reducing the likelihood of soap formation during the alkaline transesterification.
ii.UltrasoundandMicrowave-AssistedTransesterification: These technologies have been shown to improve the efficiency of the transesterification reaction by increasing themasstransferandreactionrates.Thesemethodscanbe particularlyeffectivewhenusingWCOs,astheycanreduce therequiredreactiontimeandenergyinput.
iii.OptimizedCatalystSystems:Somestudieshavefocused on developing more effective catalyst systems that can tolerate higher levels of FFAs, reducing the need for extensive pre-treatment. For example, mixed metal oxide catalystsorenzymaticcatalystshavebeeninvestigatedfor theirabilitytocatalyzetransesterificationreactionsevenin thepresenceofFFAs.
vi.SupercriticalFluidTransesterification:Anotheradvanced approach involves conducting the transesterification reactionunder supercritical conditions (high temperature and pressure). This method has been shown to improve biodiesel yields from oils with high FFA content, but it is generallymoreenergy-intensiveandcostly.
EffectivenessandFutureOutlook(2024-2020)Theperiod from2024to2020hasseensignificantprogressinrefining thealkalinetransesterificationprocessforWCOswithhigh FFA content. Research has continuously focused on improving the yield and quality of biodiesel while minimizing the negative impact of FFAs. By incorporating modifications like two-stage transesterification, the productionofhigh-qualitybiodieselfromWCOshasbecome morefeasibleandeconomicallyviable.Lookingforward,itis likely that further improvements in catalyst design, pretreatmentprocesses,andreactionconditionswillcontinueto enhance the efficiency of alkaline transesterification. The developmentofmoresustainableandcost-effectivemethods to deal with FFAs and other impurities in WCOs will be crucialinmakingbiodieselproductionfromwasteoilsmore widespreadandeconomicallyviable.
Acidic transesterification is a catalytic process that uses strongacids(typicallysulfuricacidorhydrochloricacid)to converttriglyceridesinvegetableoilsorwastecookingoils (WCOs)intobiodiesel(methylorethylesters).Thismethod

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 12 | Dec 2024 www.irjet.net p-ISSN: 2395-0072
isparticularlybeneficial for feedstocks withhighlevels of freefattyacids(FFAs),whichwouldotherwiseinterferewith the alkaline transesterification process. Acidic transesterificationcircumventstheissueofsoapformation, whichoccursinalkalinecatalysiswhenFFAsreactwiththe basetoformsoap,leadingtodecreasedbiodieselyieldand morecomplexdownstreamprocessing.
Challenges with High FFA Content: One of the primary challengesinbiodieselproductionfromoilswithhighFFA content (such as WCOs) is the formation of soaps during alkaline transesterification. These soaps not only reduce biodiesel yield but also complicate the separation of biodieselfromglycerolandothercontaminants,increasing operational costs. In contrast, acidic transesterification addressesthischallengebyconvertingFFAsintobiodiesel before the main transesterification step, thus preventing soapformation.
The process of acidic transesterification typically follows two-stepprocedure:Thefirststepinvolvesesterificationof the FFAs present in the feedstock. Methanol or ethanol is reacted with the FFAs in the presence of a strong acid catalyst,suchassulfuricacid.Thereactionproducesmethyl orethyl estersand reduces theFFAcontentintheoilto a level that can be effectively processed through alkaline transesterification. This step is crucial for reducing soap formation and improving biodiesel yield in subsequent reactions.Followingesterification,theoilundergoesalkaline transesterification,typicallyusingsodiumhydroxide(NaOH) or potassium hydroxide (KOH) as catalysts. In this step, methanolisusedtoconvertthetriglyceridesintobiodiesel andglycerol.Thealkalicatalystreactswiththetriglycerides, yieldingbiodieselandproducingglycerolasaby-product.
Thistwo-stepapproachallowsfortheeffectiveconversionof oilswithhighFFAcontent,ensuringahigh-qualitybiodiesel productwithminimalsoapformation.
InnovationsandModifications:Severalmodificationshave been introduced in recent years to optimize acidic transesterification,including:
i.SolidAcidCatalysts:Theuseofsolidacidcatalysts,suchas heteropolyacidsorion-exchangeresins,hasgainedattention due to their ability to catalyze esterification reactions without generating significant waste. These catalysts are reusable, reducing the overall cost and environmental impactoftheprocess.(Guoetal.,2023)
ii. Enzyme-Assisted Acidic Transesterification: Enzymatic catalystshavebeencombinedwithacidictransesterification to create a hybrid approach. Enzymes such as lipases can catalyze the esterification of FFAs under mild conditions, enhancingtheefficiencyofthereactionandreducingenergy consumption.
iii. Optimization of Reaction Conditions: Research has focused on improving reaction parameters, such as
temperature, pressure, and methanol-to-oil ratio, to maximize biodiesel yield while minimizing environmental impact. The development of continuous flow reactors and theintegrationofco-solventshavealsoshownpromisein reducingtheoverallreactiontime.
EffectivenessandFutureOutlook(2024-2020):From2020 to2024,significantimprovementsintheefficiencyofacidic transesterificationhavebeenachieved,particularlythrough the use of solid acid catalysts and optimization of process conditions. These innovations have reduced the environmentalimpactofacidictransesterificationandmade it more commercially viable. Going forward, the developmentofmoreefficientandsustainableacidcatalysts, aswellastheintegrationofthisprocesswithotherbiodiesel production technologies, will likely drive further improvements in the cost-effectiveness and scalability of acidictransesterification.Additionally,aswastecookingoils becomeamorewidelyusedfeedstock,thedemandforacidic transesterificationwillcontinuetorise,promptingfurther researchintooptimizingthismethod.
Enzymatictransesterificationisanenvironmentallyfriendly and sustainable method for producing biodiesel. This method utilizes enzymes, most commonly lipases, as catalyststoconverttriglyceridesintobiodiesel.Theprocess iscarriedoutundermildconditions(lowertemperatureand pressure),whichleadstolesswasteproductionandreduced energy consumption compared to traditional chemical methods. Despite these benefits, enzymatic transesterificationfaceschallenges,suchashigherenzyme costs,lowerreactionrates,andsensitivitytoimpuritiesin thefeedstock.
ChallengeswithEnzymeCostandEfficiency:Thehighcostof enzymes remains one of the significant drawbacks of enzymatictransesterification.Enzymes,particularlylipases, areexpensivetoproduceandmayneedtobereplacedafter each batch, making the process less cost-effective than chemicalmethods.Additionally,enzymescanbesensitiveto various impurities in feedstocks, including water, alcohol, andFFAs,whichcanreducetheircatalyticactivity.However, innovations in enzyme immobilization and reactor design have improved the cost-efficiency and robustness of enzymatictransesterification.
InnovationsandModifications:Severalstrategieshavebeen explored in recent years to enhance the performance of enzymatictransesterification,including:
i. Enzyme Immobilization: Enzyme immobilization techniques,suchasadsorptiononsolidsupports(e.g.,silica, activatedcarbon,magneticnanoparticles),havesignificantly improvedthestability,reusability,andcatalyticefficiencyof lipases.Immobilizedenzymescanbereusedmultipletimes, reducing the overall operational costs and making the processmoreeconomicallyviable.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 12 | Dec 2024 www.irjet.net p-ISSN: 2395-0072
ii.Co-SolventsandCo-Catalysts:Theadditionofco-solvents orco-catalystshasbeenstudiedtoimprovethesolubilityof feedstocks and methanol in the reaction medium. Cosolventssuchasacetone,ethanol,andhexanecanenhance the interaction between the enzymes and the substrates, improvingreactionratesandbiodieselyield.
iii. Thermal and pH Optimization: Enzymes are often optimizedforuseathighertemperaturesandmoreextreme pHconditions,whichhelpsenhancetheircatalyticefficiency and stability. Lipases that function under non-ideal conditionsallowforgreaterflexibilitywhenprocessingoils withhigherFFAcontentorvaryingviscosity.
EffectivenessandFutureOutlook(2020-2024):Enzymatic transesterificationhasgainedsubstantialattentionduetoits environmentalbenefits,especiallyintermsofreducedwaste productionandtheuseofmildreactionconditions.However, therelativelyhighcostofenzymesandtheslowerreaction rateshavehindereditswidespreadadoption.From2020to 2024, significant progress has been made in overcoming thesebarriers.Advancesinenzymeimmobilizationhaveled to more cost-effective solutions, and hybrid approaches using enzymatic catalysts in combination with chemical methods are becoming more common. The future of enzymatic transesterification will likely involve the development of low-cost, robust enzymes and optimized reactor systems that further reduce the overall cost of biodiesel production. Additionally, the integration of this method with other technologies (e.g., ultrasonic or microwave-assistedtransesterification)couldimprovethe efficiencyandscalabilityofenzymaticbiodieselproduction.
Supercritical methanol transesterification is a highefficiency,non-catalyticmethodforbiodieselproduction.In this process, methanol is used in its supercritical state, where it exhibits both solvent and catalytic properties, allowing for faster transesterification of triglycerides into biodiesel.Theprocessoccursunderhightemperatureand pressureconditions,typicallyabovethecriticaltemperature (240°C)andpressure(80atm)ofmethanol.Thismethodis advantageous for its high biodiesel yield and ability to processfeedstockswithhighFFAcontent,makingitidealfor wasteoilssuchasWCOs.
Challenges with High Energy Consumption: The primary challenge associated with supercritical methanol transesterification is its high energy consumption. Maintaining supercritical conditions requires specialized equipment,suchashigh-pressurereactors,andsubstantial energyinput.Thismakestheprocessenergy-intensiveand costlycomparedtoconventionalcatalyticmethods.Thehigh capitalandoperationalcostsaresignificantbarrierstothe widespread adoption of supercritical methanol transesterification in large-scale biodiesel production. Despite these challenges, researchers are continuously exploring ways to optimize this process to improve its economicfeasibility.
InnovationsandModifications:Recentresearchhasfocused on improving the efficiency and reducing the energy consumptionofsupercritical methanol transesterification. Innovationsinclude:
i. Microwave-Assisted Supercritical Methanol Transesterification:Microwaveheatinghasbeenemployed toenhancetheheatingefficiencyinsupercriticalmethanol transesterification.Theuseofmicrowavesreducesreaction timeandimprovesenergyefficiencybyprovidingrapidand uniform heating. This approach significantly cuts down energyconsumptionwhilemaintaininghighbiodieselyields.
ii.UseofCo-SolventsandAdditives:Theincorporationofcosolvents,suchasethanolorwater,hasbeeninvestigatedto reducethetemperatureandpressurerequiredtoreachthe supercritical state of methanol. This reduces the energy demand and makes the process more economical. Additionally, the use of additives can help increase the solubility of triglycerides and methanol, enhancing the efficiencyofthetransesterificationreaction.
iii.ContinuousFlowReactors:Movingfrombatchreactorsto continuousflowreactorshasbeenexploredtoimprovethe scalability of supercritical methanol transesterification. Continuousflowreactorsallowforhigherthroughputand moreefficientheattransfer,improvingtheoverallefficiency oftheprocessandreducingthedowntimebetweenbatches.
EffectivenessandFutureOutlook(2020-2024):From2020 to2024,supercriticalmethanoltransesterificationhasseen promising developments, particularly in terms of process optimization and energy efficiency. The integration of microwave-assisted heating and continuous flow reactors hasimprovedtheeconomicfeasibilityoftheprocess.
However, the high energy demand remains a major challengethatneedstobeaddressedforthemethod tobe more widely adopted. Future research will likely focuson reducingenergyconsumption,enhancingreactordesign,and exploring new catalyst-free methods to lower operational costs. As the technology matures, supercritical methanol transesterificationhasthepotentialtobecomeakeymethod forlarge-scalebiodieselproduction,particularlyfromwaste oilsandlow-qualityfeedstocks.
Eachmethodoffersdistinctadvantagestailoredtodifferent feedstocksandproductionneeds:alkalinetransesterification isidealforlow-FFAoils,acidictransesterificationworkswell forhigh-FFAoils,enzymatictransesterificationprovidesan environmentally friendly and high-quality output, and supercritical methanol transesterification achieves rapid reactions and high yields without catalysts. As research progresses,thesemethodscontinuetoberefinedtoimprove their efficiency, sustainability, and economic viability, contributing to the growing potential of biodiesel as a renewableenergysource.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 12 | Dec 2024 www.irjet.net p-ISSN: 2395-0072
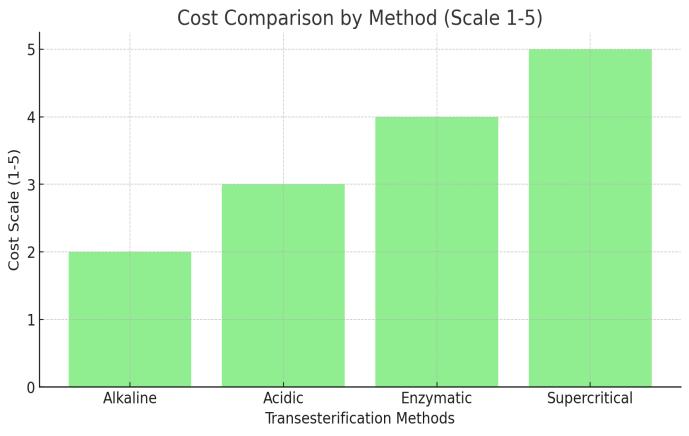
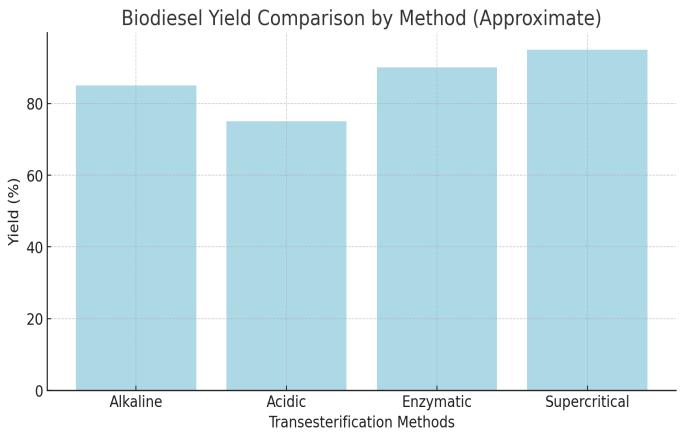
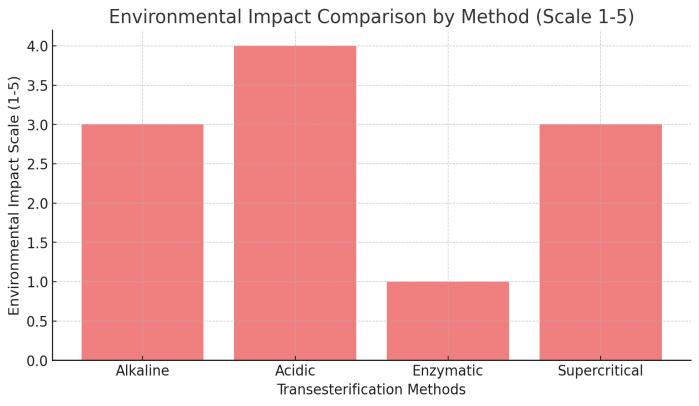
Aspect
Catalyst Type
Yield and Purity
Reaction Conditions
Cost and Scalability
Environmental Impact
Advancements (2024-2020)
Sodiumorpotassium hydroxide
Highyieldbutaffected byfreefattyacid(FFA) content,causingsoap formation
Moderatetemperature andpressure,but sensitivetoimpurities
Cost-effective,simple, butrequiresFFApretreatment
Generatessoapwasteif notoptimized
Two-stageprocesses forbetterFFAhandling andhigheryields
Sulfuricacid, hydrochloricacid Lipases(enzymes)
EffectiveforhighFFA contentoils,but slowerandproduces waste
Requiresharsh conditionsandlonger reactiontimes
Moreexpensiveand lessefficientforlarge scale
Highenvironmental impactduetowaste andenergyuse
Useofsolidacid catalystsderivedfrom wastetoreduce impact
High-qualitybiodiesel, environmentally friendly
Mildconditions,lower temperatures,and environmentally friendly
Highcostdueto enzymeuse,with researchimproving enzymereusability
Lowwasteproduction, environmentally friendly
Immobilizationof enzymesforreusability andcostreduction
None(uses supercritical methanol)
Veryhighyieldsand reducedseparation issues
Hightemperatureand pressure,energyintensive
Highinitialinvestment forspecialized equipment,lesscosteffective
Energy-intensivebut nocatalystwaste
Microwave-assisted methodstoreduce energyuseand increasespeed
Comparison

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 12 | Dec 2024 www.irjet.net p-ISSN: 2395-0072
GlycerineRecoveryMethodsinBiodieselProduction
The recovery of glycerin from biodiesel production is essential for ensuring the sustainability of biodiesel processes.Glycerine,abyproductoftransesterification,must be separated from biodiesel efficiently to be purified and reused. Common methods for glycerine recovery include decantation,filtration,andcentrifugation,eachwithitsown advantages and limitations depending on the mixture’s characteristicsandtherequiredpurityofglycerine
i.Decantation: Thisisasimpleandcost-effectivemethodthat relies on the density difference between glycerine (1.26 g/cm³)andbiodiesel(0.88–0.90g/cm³).Glycerinesettlesat the bottom of the mixture, making it easy to separate. However, decantation may not effectively remove smaller impuritieslikeresidualcatalystsormethanol.
ii.Filtration: Filtrationremovesparticulatematter,suchas finesolidsandtracesofcatalysts,afterdecantation.Ithelps furtherpurifytheglycerinbutislesseffectiveatremoving liquidimpuritieslikemethanolorotherresidualchemicals.
iii.Centrifugation:Centrifugationuseshigh-speedspinningto createcentrifugalforcesthatseparateglycerinfrombiodiesel more effectively, particularly in emulsified mixtures. This method can achieve higher purity than decantation and filtration but requires more energy and has higher operationalcosts.
Thechoiceofmethoddependson:
a)Physicalproperties:Ifglycerineandbiodieseldiffergreatly indensityorviscosity,decantationorfiltrationcanbemore effective.
b)Purityneeds:Forhigh-purityglycerine,advancedmethods like centrifugation or a combination of techniques may be necessary.
c)Scaleofoperation:Large-scalebiodieselplantsoftenrely oncentrifugationorfiltrationforefficientglycerinerecovery.
Theincreasingproductionofbiodieselhasledtoasurgein the availability of glycerin. Consequently, researchers and industries are actively exploring new and diverse applicationsforglycerintoenhanceitseconomicvalue.
a) Cosmetics and Personal Care: Glycerine's moisturizing propertiesmakeitawidelyusedingredientincosmeticsand personalcareproducts,suchaslotions,creams,andsoaps.It actsasahumectant,attractingandretainingmoistureinthe skin, leading to improved hydration and smoother skin texture(Singh&Sharma,2012).
b)Pharmaceuticals:Glycerineisemployedinpharmaceutical formulations as a solvent, humectant, and sweetener. It is used in various drug delivery systems, including syrups, tablets,andointments.Itsnontoxicnatureandcompatibility

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 12 | Dec 2024 www.irjet.net p-ISSN: 2395-0072
with a wide range of drugs make it a suitable excipient in pharmaceuticalformulations(Pateletal.,2012).
c)FoodIndustry:Glycerine isa versatileingredientinthe food industry, serving as a sweetener, humectant, and stabilizer. It is used in various food products, including bakedgoods,confectionery,andbeverages(Pagliaro&Rossi, 2010).
FeedstockAvailabilityandQuality:TheconsistencyofWCO supply and its variable quality can pose challenges for biodieselproduction.Thepresenceofimpurities,likeFFAs andwater,canaffecttheefficiencyofthetransesterification process.Therefore,efficientpretreatmentmethodsforWCO arecrucial.
Catalyst Optimization: Developing cost-effective and environmentallyfriendlycatalystsisessentialforimproving the sustainability of biodiesel production. Research on heterogeneouscatalystsderivedfromrenewablesourcesis progressing,butfurtheroptimizationisneeded.
GlycerineValorization:Despitetheincreasingapplicationsof glycerine,itsmarketvalueisstillrelativelylowcomparedto theotherbiodieselco-products.Exploringnewanddiverse applicationsforglyceriniscrucialtomaximizeitseconomic potential.
Energy Consumption: The transesterification process requires energy for heating and stirring, impacting the overallenergyefficiencyofbiodieselproduction.Developing energy-efficient processes is vital for minimizing environmentalimpact.
Economic Viability: The economic feasibility of biodiesel production from WCOs is influenced by various factors, includingfeedstockcost,catalystcost,andproductdemand. Ensuring the long-term economic viability of biodiesel productionrequirescarefullyconsideringthesefactors.
Advancements in technologies like membrane separation, microwave-assisted processes, and supercritical fluid extractioncan enhancethe efficiencyandsustainability of biodieselproduction.Integratingbiodieselproductionwith otherindustries,suchaswastewatertreatmentandbiogas production,canenhanceresourceutilizationandreducethe overallenvironmentalfootprint.
Implementing the biorefinery concept, where various valuableproductsareextractedfromWCOs,canmaximize theeconomicandenvironmentalbenefitsofthisfeedstock. Implementing supportive policies and incentives can encourage the adoption of biodiesel and stimulate further researchanddevelopmentinthisfield.(Zhangetal.,2023)
Biodiesel production from WCOs has emerged as a promising pathway for renewable energy generation and waste management. The period from 2020 to 2024 witnessed significant progress in understanding and optimizingthedifferentmethodsforbiodieselandglycerin production, with a focus on enhancing efficiency, sustainability,andeconomicviability.
Alkaline transesterification remains the most popular method,butmodificationsliketwostagetransesterifications are being implemented to address challenges associated with FFA content. Enzymatic and supercritical transesterification are gaining traction due to their environmentally friendly nature and potential for highquality biodiesel. Moreover, efforts are being made to optimize glycerin recovery and purification methods to maximizeitseconomicvaluethroughdiverseapplications.
Despite the advancements, several challenges remain, including feedstock availability, catalyst optimization, and glycerinvalorization.Addressingthesechallengesthrough continuous technological innovation, coupled with supportive policies and incentives, will be crucial for the long-termsuccessofthebiodieselandglycerinindustryfrom WCOs.
[1] Gahlot,K.,Suyambulingam,I.,Sanjay,M.R.,Divakaran, D., & Siengchin, S. (2024). Progress and facts on biodieselgenerations,productionmethods,influencing factors, and reactors: A comprehensive review from 2000to2023. Energy Conversion and Management, 302, 117907.
[2] Guo,Y.,Delbari,S.A.,Namini,A.S.,Le,Q.V.,Park,J.Y., Kim, D., Varma, R. S., Jang, H. W., T-Raissi, A., & Shokouhimehr,M.(2023).Recentdevelopmentsinsolid acid catalysts for biodiesel production. Molecular Catalysis, 547,113700.
[3] Zhang,L.,Wu,Y.,&Li,H.(2023).Membranetechnology for glycerin purification in biodiesel production: A review. Separation and Purification Technology, 300, 122132.
[4] Yuan,Q.,Zhang,Z.,Li,Y.,&Wang,Y.(2022).Microwaveassisted supercritical methanol transesterification for biodiesel production from waste cooking oil. BioresourceTechnologyReports,19,101001.
[5] Ma, Y., Zhang, W., Li, Z., Tian, X., & Xu, Z. (2021). Biodieselproductionfromwastecookingoilusingsolid acidcatalystderivedfromagriculturalwaste.Fuel,292, 120208.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 11 Issue: 12 | Dec 2024 www.irjet.net p-ISSN: 2395-0072
[6] Gao, X., Chen, J., Li, L., Yan, S., & Zhang, X. (2021). Immobilizationoflipaseonmagneticnanoparticlesfor biodieselproductionfromwastecookingoil. Bioresource Technology, 319,124211.
[7] Gao, X., Chen, J., Li, L., Yan, S., & Zhang, Q. (2021). Immobilizationoflipaseonmagneticnanoparticlesfor biodiesel production from waste cooking oil. BioresourceTechnology,336,125295.
[8] Naji,S.Z.,Tye,C.T.,&Abd,A.A.(2021).Stateoftheart of vegetable oil transformation into biofuels using catalyticcrackingtechnology:Recenttrendsandfuture perspectives. Process Biochemistry, 109,84-96.
[9] Islam,M.T.,Amin,M.S.,&Karim,A.(2020).Biodiesel production from waste cooking oil using two-stage transesterification process with optimization. RenewableEnergy,151,10601069.
[10] Chang,F.,Zhou,Q.,Pan,S.X.,&He,Y.(2020).Catalytic upgradingofglycerol,apromisingbiodieselcoproduct. Biomass, Biofuels, Biochemicals,1-32
[11] Sharma, Y. C., Singh, B., & Upadhyay, S. N. (2016). Biodieselfuelproductionthroughvarioustechnologies: Areview.RenewableandSustainableEnergyReviews, 53,1172-1184.
[12] Uprety,B.K.,Chaiwong,W.,Ewelike,C.,&Rakshit,S.K. (2016). Biodiesel production using heterogeneous catalysts including wood ash and the importance of enhancingby-productglycerolpurity. EnergyConversion and Management, 115,191-199.
[13] Atadashi,I.M.,Aroua,M.K.,Aziz,A.A.,&Sulaiman,N.M. N.(2013).Areview onthefeasibilityof biodiesel asa renewableenergy.RenewableandSustainableEnergy Reviews,17,61-79.
[14] Patel, R., Kumar, V., & Patel, R. V. (2012). Glycerol: A promising pharmaceutical additive. International Journal of Pharmaceutical Sciences and Nanotechnol, 5(2),2414-2421.
[15] Singh,S.,&Sharma,D.(2012).Applicationsofglycerolin personal care and cosmetic products. International JournalofPharmaceuticalSciencesandDrugResearch, 4(2),112116.
[16] Dias,A.K.,Alves,M.J.,Mota,J.,Amaral,L.,&Reis,A.M. (2011).Optimizationofbiodieselproductionfromwaste cooking oil using response surface methodology. Fuel ProcessingTechnology,92(12),2266-2271.
[17] Zhang,J.,Chen,S.,Yang,R.,&Yan,Y.(2010).Biodiesel productionfromvegetableoilusingheterogeneousacid andalkalicatalyst. Fuel, 89(10),2939-2944.
[18] Demirbas,A.(2009).Biodieselfuelsfromvegetableoils via transesterification and other methods: A survey. EnergyConversionandManagement,50(1),278-282.
[19] Pagliaro,M.,Ciriminna,R.,Kimura,H.,Rossi,M.,&Della Pina,C.(2007).Fromglyceroltovalue-addedproducts. Angewandte Chemie International Edition, 46(24), 4434-4440.
[20] Vicente,G.,Martínez,M.,&Aracil,J.(2004).Supercritical biodiesel production: Process optimization and comparison with conventional methods. Journal of SupercriticalFluids,30(2),119-127.
[21] Shimada, Y., Sema, D., Sugihara, A., & Nakajima, M. (2002).Biodieselproductionfromvegetableoilsusing immobilized lipase. Journal of the American Oil Chemists'Society,79(11),1123-1128.
[22] Freedman, B., Pryde, E. H., & Mounts, T. L. (1984). Variables affecting the yields of fatty esters from transesterifiedvegetableoils.Journal oftheAmerican OilChemists'Society,61(10),1638-1643.